Note: Descriptions are shown in the official language in which they were submitted.
~6~7~
-- 1 --
This invention relates to the provision of intermediates
useful in the preparation of 4~5)-substituted imidazole
derivatives and their non-toxic salts, and to the 4(5)-
substituted imidazole derivatives so formed.
The application is a division of application Serial No.
495,608 filed November 18, 1985.
Adrenergic receptors deno-te physiologically-active binding
sites which are specific to noradrenaline and adrenaline and are
located on the surface of -the cell membrane. The adrenoceptors
of the sympathetic nervous system have been classified into two
different subtypes, namely, alpha- () and beta- (~) recep-tors
which can both be further divided into two subgroups i.e. ~1 and
~2 as well as ~1 and ~2~ Of these receptor types, ~,, B2 and
are mainly located postsynaptically on the surface of, e.g.,
smooth muscles and thus mediate, e.g., smooth muscle contraction
or relaxation, whereas ~2 receptors are mainly located presyn-
aptically on the terminals of noradrenergic nerves. ~f ~2
receptors are stimulated by noradrenaline under physiological
conditions, noradrenaline release is blocked this is a negative
feed-back phenomenon.
Except by noradrenaline, this negative feed-back phenomenon
may be induced by certain ~2-agonists, e.g., detomidine ~compound
A hereinbelow) and some of its near deriva-tives. The primary
pharmacodynamic effects of detomidine, e.gO, sedation, have also
been proved to be due to its ability to stimulate ~-receptors
~:;
`~Y~
, ' ' `'
, ; ~, . . ... .
,~ ' ', .
.
'
i268770
- la -
(see, Virtanen et al)., Progress in Neuro-Phychopharmacology and
Biological Psychiatry, suppl. 1983, p. 308).
Many sedatives and analgetics are used in veterinary
1268770
medicine. ~ c].ass of such ~eterinary medicines includes, e.g.
detornidine (compound ~) and near cleri~ati~es thereof.
3 ~CH
Compound A
(detomidine)
Compound ~ has been disclosed in e,g, Eur, Pat, ~ppl, 24829,
Detomidine is used in veterinary medicine, especially
in the handling of horses and cattle (pharmacological
restraint), whereby the animal is sedated before in~estigation,
treatment and difficult medical operations. E~en a small
surgical operation cannot be carried out without the use of a
sedati~e agent.
When the treatment carried out during the action of
detomidine is finished, it is for practical reasons desirable
to interrupt and restrain the effect by a specific antagonist
or anticlote. The animal can then immediately be transported
away from the surgery, whereby expensi~e awakening rooms are
not required. The ability of the animal to control its
mo~ements and co-ordination after awakening is impro~ed.
When treating animals in cold surroundings this is
absolutely necessary, because otherwise the animal will remain
lying still for too long a time. Because of the awakening
agent, the feeding of cattle can start more rapidly than
-- 2
12~i8770
otherwise. ~n interruption in feeding causes disturbances in
production.
The use of an awakening agent in connection with the
use of detomidine sa~es time for the ~eterinarian as well as
for the owner of the ani.mal. The anti.dote enables the use of
higher doses of detomidine, which induce a stronger analgetic
effect. lhus, the safety of the treatment of large ani.mals is
increased. Without any awakening agent, detomidine cannot be
used in some cases, as it is often not possible to wait until
the animal has reco~ered from the influence of detomidine.
~ selecti~e u2-antagonist may also be predicted to
be of use in some diseases which are believed to be connected
with deficiency of noradrenalin auailable in the postsynaptic
adrenoceptors of the central and/or peripheral nervous system.
These diseases include e.g. endogenic depression and asthma.
Glucose and lipid metabolisms are regulated by an
inhibitory mechanism in~ol~ing ~2-receptors. Thus
a2-antagonists may be significant in the treatment of
metabolic diseases, e.g. diabetes and obesity.
Presynaptic Q2-receptors also take part in platelet
aggregation. It has been shown the a2-agonists acti~ate and
antagonists inhibit human platelet aggregation (Grant ~
Schutter, Nature 1979, 277, 659). Thus Q2-antagonists may be
useful clinica~ly in pathogenic states inuol~ing increasing
aggregation, e.g. migraine. The acute effects of ergotamine, a
classical compound against migraine, are regarded as being due
to its ai-agonist effect. Thus compounds with both
antagonist effects of a2-receptOrS and agonist effects of
-- 3 --
~6l377~
-- 4
postsynaptic I-receptors may have great advantages in acute and
preventive treatment of migraine.
The invention disclosed and claimed in the parent
application Serial No. 495,608 filed November 18, 1985 taught the
provision of certain 4(5)-substituted imidazole derivatives, and
their non-toxic pharmaceutically-acceptable, acid-addition salts,
and mixtures thereof, which have been found to have valuable
properties as antagonists to ~2-recePtors.
The present invention provides intermediates for the
preparation of such 4~5)-substituted imidazole derivatives, and
such intermediates.
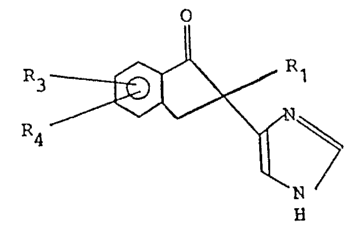
Thus, by a broad aspect of this invention, an intermediate
compound is provided of the Formula:
R3 ~ R
R4 ~ N ~
in which Rl is H, alkyl of 1 to 4 carbon atoms, alkenyl of 2 to 4
carbon atoms, OCH3 or OCH2CH3; R3 is H, CH3, CH2CH3, OCH3 or Hal
and R4 is H, CH3, CH2CH3, OCH3 or Hal, and Hal is halogen. In
such compound R1 may be hydrogen, and R3 and R4 may be hydrogen.
~268770
- 4a -
By aspects of -the invention provided by the parent appli-
cation Serial No. 495,608, alternative processes have been
provided for the preparation of substituted imidazoles of the
general Formula ~I)
R3 R2
~ H
or their non-toxic, pharmaceutically-acceptable, acid-addition
salts, wherein X is -CH2-, -CH2CH2- or -O-; R~ is H, lower alkyl
having 1 to 4 carbon atoms, lower alkenyl having 2-4 carbon
atom~, OCH3 or OCH2CH3; R2 is H, CH3, CH2CH3, OCH3 or OH; R3 is H,
CH3, CH2CH3, OCH3 or Hal; R4 is H, CH3, CH2CH3, OCH3 or Hal; and
Hal is halogen. The alternative processes are a~ follows:
a) halogenating a compound of the Formula
.
R2
N~ J~, O
~68770
-- 5
wherein Rz~ R3 and R~ are as defined above, to give a compound of
the Formula
R~ , ~
wherein Hal is Cl or Br; then reacting that compound with
formamide to give a compound of the Formula
R4 ~ ~ (
N
H
then catalytically hydrogenating that compound to give a compound
of Formula (I), where R1 is hydrogen, R3-and R4 are as defined
above and Rz i~ hydrogen, methyl or ethyl;
or b) reacting a compound of the Formula
~ C - H
iZ68770
-- 6
wherein X, R,, R2, R3 and R4 are as defined above, and wherein
R5, R6, R, and R~3, may be at least one of the following atoms or
atom groups, namely, hydrogen, hydroxy, halogen, amino, -O-alkyl
containing 1 to 7 carbon atoms, or
o
-O-C-Rg
wherein Rg is an alkyl radical containing 1 to 7 carbon atoms, or
an aryl radical containing 6 to 10 carbon atoms, and wherein R5
and R, can be combined to form a keto group, or wherein R6 and Rt3
can be combined to form a keto group, with formamide to give a
compound of Formula ~I) as defined above;
or c) halogenating a compound of the Formula
R2
R4 \N
wherein X, Rl and R2 are as defined above, to give a compound of
Formula I, wherein R3 is halogen and R4 is H or both R3 and R4
are halogen;
or d) brominating a compound of the Formula
~ ¢ - C - CH3
iz68~70
wherein X, R2, R3 and R4 are defined above, to give a compound of
the Formula
R2 B r
C--CH2Br
then reacting such compound with formamide to give a compound of
the Formula R2
R
and then hydrogenating such compound to a compound of Formula
(I), wherein Rl is hydrogen, R3, R4 and X are as defined above
and R2 is hydrogen, methyl or ethyl;
or e) reacting a compound of the Formula
~ CH2-- H~
~26877~
-- 8
wherein R3 and R4 are as defined above r and R is a benzyl group,
with thionyl chloride to give a compound of the Formula
~ - CH CH ~ ~ ,
then reacting such compound with sodium cyanide to give a
compound of the Formula
R CN
~ CH2 CH ~ ~
then hydrolysing such compound in alkaline solution to give a
compound of the Formula
~ C~ - CH
~2687710
- 9 -
then reacting ~uch compound with polyphosphoric acid to give a
compound of the Formula
R3 ,R
R4
and then either:
i) hydrogenating such compound with hydrogen using
palladium-in-carbon as catalyst to gi.ve a compound of the Formula
~ and then reducing ~uch compound with NaBH4 to give a compound of
the Formula
OH R
12687~0
-- 10 --
or ii) reducing ~uch compound with NaBH~ to give a compound
of the Formula
4 ~ \
OH
and then hydrogenating ~uch compound with hydrogen using
palladium-in-carbon as catalyst to give a compound of the Formula
R~
or f) halogenating and reacting a compound of the Formula
R~ ~0 ~ -CH3
~2687~'0
-- 11 --
wherein R1, R3, and R4 are as defined above, with formamide to
give a compound of the Formula
R~
and then either: ~i) hydrogenating that compound with hydrogen
using Pd/C as catalyst to give a compound of the Formula
~'
or ~ii) reacting that compound wlth NaBH4 to give a compound of
the Formula
H
or g) hydrogenating a compound of the Formula
1268770
- 12 -
wherein R~, R3 and R4 are as defined above, with hydrogen using
Pd/C a~ cataly~t to give a compound of the Formula
'~ , "; ~
or ~h) halogenating and reacting a compound of the Formula
< RCl C~3
wherein Rl, R3 and R4 are a~ defined above, with formamide to
give a compound of the Formula
and then hydrogenating that compound with hydrogen u~ing Pd/C as
cataly~t to give a compound of the Formula
~Z6877~
- 13 -
Any of the above-described processes may include the final
step o converting that compound of Formula ~I) to one or more of
its non-toxic, pharmaceutically-acceptable, acid-addition salts.
In sub-process ~a) above, the halogenation may be performed
by reaction with a halogen in methylene chloride or in diethyl
ether, the halogen preferably being bromine, and the bromination
being performed with stirring at a temperature of 10-C. In
addition, in sub-process ~al above, the halogenated product and
formamide may be heated at 130-200C for 3-8 hours. Furthermore,
in sub-process ~a) above, the catalytic hydrogenation may be
performed in acidic water-ethanol mixture at a temperature of
10C at normal or elevated pressure, the hydrogenation preferably
being carried out using Pd/C as catalyst.
In sub-process ~b) above, the starting compound of that
formula may be one of the following:
R3 R2 ~ R3 R2
~ j ~ C-CH2~QI . , ~ CH - C - H
R4 R4
'1 Z687'70
-- 14 --
R3 R2 R3 R2
~ ~ CH2OU , ~ U-c u or
R3 R2
OH /OR
~ /~ CH -CH
R4
In this sub-process (b), the halogenation may be performed
by reaction with a halogen in methylene chloride or in diethyl
ether, the halogen preferably being bromine, and the bro~ination
preferably being performed with stirring at a temperature of
lO~C. In such sub-process ~b) above, the halogenated product and
formamide are preferably heated at 130-200-C for 3-8 hours.
In one further sub-generic process of sub-process ~b)
above, a compound of the following Formula
12687~0
- 15 -
~ ~-C~3
may be halogenated to provide a compound of the Formula
~-cH2HBl ,
preferably where the halogen ~Hal) is bromine, then that compound
may be reacted with formamide to provide a compound of the
Formula
R~N~
R4 H
In all cases, R , Rz, R3 and R4 are as previously defined.
In another sub-generic process of sub-process (b) above, a
compound of the Formula
R3 52
1~
1~68'~70
- 16 -
i~ reacted with a Grignard reagent of the Formula
_ _
8 /C2 5
Mg CN-C - CH
OC2Hs
to provide a compound of the Formula
~ ~ 11 / 2 5
R4
that compound is then reduced to a compound of the Formula
R~3 R2
J,~ CH-CH
and that compound i~ then reacted with formamide to provide a
compound of the Formula
~ N ~
In all caAe~, X, Rl, R2, R3 and R4 are a~ defined above.
~268770
- 17 -
The Grignard reaction is preferably carried out in
tetrahydrofuran or in diethyl ether at room temperature. The
reduction step is preferably performed with sodium borohydride in
ethanol at room temperature. The halogenated product and
formamide are preferably heated at 130-200C for 3-8 hour~.
In sub-process ~c) above, the halogenation is preferably
carried out with bromine in acidic water at a temperature of
10c. In sub-process (d~ above, the halogenation is preferably
performed by reaction with a halogen in methylene chloride or
diethyl ether. In either case, the halogen is preferably
bromine, and the bromination is preferably performed with
stirring at a temperature 10 D C .
In sub-process (d) above, the halogenated product and
formamide are preferably heated at 130-200-C for 3-8 hours. In
sub-process ~d~ above, the catalytic hydrogenation is preferably
performed in acidic water-ethanol mixture at lO~C at normal or
elevated pressure, the hydrogenation preferably being carried out
with hydrogen using Pd~C catalyst.
In sub-processes (e) ~i) or ~e)~ii) above, the catalytic
hydrogenation is preferably performed in acidic water-ethanol.
The compounds prepared by any of the processe~ described
above can thereafter be converted to their non-toxic, pharma-
ceutically-acceptable, acid-addition salts, since they can form
acid-addition salts with both organic and inorganic acias. They
can thus form many pharmaceutically-usable acid-addition salts,
1261~3770
- 18 -
e.g. chlorides, bromides, .~ulfates, nitrates, phosphates,
sulfonates, formates, tartrates, maleates~ citrates, benzoates,
salicyla-tes, ascorbates, and the like.
In more general terms, the compounds of the Formula (I) can
be prepared according to the following processes:
(A) R2 R2
y~ C-cH3 ha~ogenation~ ~ 1OC-CH2Ha~
R2 R2 .
O R ¦ cata~ytic R3 ~ ~ N~
hydrogen1cion~ ~ y
(B) y ~ h
wherein R5, Rfi, R7 and R~ can be at least one of the following
atoms or atom groups: hydrogen, hydroxy, halogen, aMino, -O-alkyl
containing 1 to 7 carbon atoms, or
O
-O-C-R9
1 268770
-- 19 --
wherein R9 is an alkyl radical containing 1 to 7 carbon atoms, or
an aryl radical containing 6 to 10 carbon atoms, and wherein R5
and R, can be combined to form a keto group, or wherein R~ and R8
can be combined to form a keto group.
In process (B) above, for example, the following compounds
can be used as starting materials:
R3 R2 R3 R2
~c-c~2~ c-l~
~ R4 R4
R3 R2 R3 R2
~ C-CU20~ , ~ C~ C/1~ or
¦ ~4 R4
R3 R2
~ CH-C
R4
~268770
- 20 -
A particularly convenient way to perform process (s~ above
is the following proces~ (B1):
(Bl)
R3 R2 halogenation R2
~ 3 ~ C~2Ha]
R4
O R ~ ~
Another advantageous adaptation of process ~B) above is the
following proce~s (B2):
~B2)
Br ~ ~ IC-~OcZuS
U / 5 ~i~R ~Rl
red~ctio ~ OC2U5 ~ X ~ R ~
~2687~
- 21 --
~C)
h~o~en~tion ~ N
H H
wherein R3 i~ a halogen ato~ and R4 is ~, or both R3 and R~ are
halogens.
~D)
.
~ dt2 ,7 ~-C'd23r
R4 E~
hydrogen4t ion ~>
H
~268770
--., 22 --
( E )
ON~ 50C12 ~ Cl~
R3 R R3 R
~_CH2--CH~ H
M ~ C
oD `bH
N~B~H~ lYal~14
R3 R3
R~
Pd/C~ H2 ~ H
~ /
R4
i2~
-23-
D.
R3 R2 R3 R2
~ Br2 ` ~ CH2 Br
R4 ~
.
O ~ hydrogen-tion ~N
H
1268770
--24--
wherein R i~3 a benzyl group.
(F) O
R3 6~ Rl R3 ~ Rl H-C-~H
haloqenat~ ~ ~0 1 X 2
R~/ \~-C~3 R~/ ~ CH2
R~ Na8H
H(3 lH2, Pd/C ~'/", H(~)
R~
(G)R3 R~ 2
~ C CH 2 ) H - -I IH2 \¢~
R
~268~70
- 25 -
In process ~A) above the halogenation step can be performed
by reaction with, e.g. bromine, in methylene chloride or in
diethyl ether by stirring at 10 C.
In the second step, the halogenated product and formamide
are heated at 130-200 C Eor 3 to 8 hours.
The catalytic hydrogenation is preferably performed in an
acidic water-ethanol mixture at 70 C. at normal or elevated
pressure with hydrogen ~Ising, e.g. Pd/~ as catalyst.
In process (B1), the firs-t and second steps may be performed
in the same way as the corresponding s-teps in process (A).
In process (B2), the Grignard reaction is carried out in,
e.g. tetrahydrofuran or diethyl ether, at room temperature.
The reduction step is performed with, e.g. sodium
borohydride in ethanol at room temperature. The reaction with
formamide is carried out as in (A) and (B1), namely, by heating
at 130 C.-200 C. for 3 to 8 hours.
In process (C), the halogenation is carried out with, e.g.
bromine in acidic wa-ter at 10 C.
~Z68~70
- 26 -
According to another aspect of the invention of the above-
identified parent application, new imidazole derivatives are
provided which are new potent and selec-tive ~2-recePtor
antagonists and which have the general Formula
~ ~ ,N ~
or the non-toxic, pharmaceutically-acceptable, acid-addition
salts thereof, or mixtures thereof, wherein:
X is -CH2-, -CH2CH~- or -O-;
Rl is H, a lower alkyl having 1 to 4 carbon atoms, a lower
alkenyl group having 2 to 4 carbon atoms, OCH3 or OCH2CH3;
R2 is H, CH3, CH2CH3, OCH3 or OH;
R3 is H, CH3, CH2CH3, OCH3 or Hal;
R4 is H, CH3, CH2CH3, OCH3 or Hal; and
Hal is halogen.
~ pecific compounds provided by specific aspects of the
invention of the above-identified parent application, are the
following:
(a) 4(5)-(2,3-dihydro-lH-inden-2-yl)imidazole, or its
hydrochloride salt;
(b) 4(5)-(2,3-dihydrobenzofuran-2-yl)imidazole, or its
hydrochloride salt;
(c) 4(5~-(5-bromo-2,3-dihydrobenzofuran-2-yl)imidazole, or
its hydrochloride salt;
~268';P70
- 26a -
(d) cis-4(5)-(2,3-dihydro-1-methyl-lH-inden-2-yl)imidazole,
or its hydrochloride salt;
(e) cis-4~5)-(2,3-dihydro-1,6-dimethyl-lH-inden-2-yl)-
imidazole, or its hydrochloride salt;
~268770
-27-
~f) ~i~-4~5)-(2,3-dihydro-1,4-dimethyl-lH-inden-2-
yl)imidazole, or its hydrochloride salt;
(g) 4(5)-(2,3-dihydro-2-methyl-lH-inden-2-yl)imidazole, or
its hydrochloride salt;
(h) 4(5)-(2,3-dihydro-5-methyl-lH-inden-2-yl)imidazole, or
its hydrochloride salt;
(i) 4(5)-(2,3-dihydro-2-ethyl-5-methyl-1~-inden-2-
yl)imidazole, or its hydrochloride salt;
(j) 4(5)-(2,3-dihydro-2-ethyl-lH-inden-2-yl)imidazole, or
its hydrochloride salt;
(k) 4~5)-(2,3-dihydro-2,5-dimethyl-lH-inden-2-yl)imidazole,
or its hydrochloride salt;
(1) 4(5)-(1,2,3,4-tetrahydronaphth-2-yl)imidazole, or its
hydrochloride salt;
(m) 4(5)-~2,3-dihydro-1,4-dimethyl-lH-inden-2-
yl)imidazole;, or its hydrochloride salt
(n) 4~5)-~2-ethyl-1,2,3,4-tetrahydronaphth-2-yl)imidazole,
or its hydrochloride salt;
(o) 4(5)-(2,3-dihydro-2-ethyl-1-methyl-lH-inden-2-yl)-
imidazole, or its hydrochloride salt;
(p) (5)-(2,3-dihydro-2-n-propyl-lH-inden-2-yl)imidazole, or
its hydrochloride salt;
(q) 4(5)-~2,3-dihydro-2-n-butyl-lH-inden-2-yl)imidazole, or
its hydrochloride salt;
(r) 4(5)-~2,3-dihydro-5-methyl-1~-inden-2-yl)imidazole, or
its hydrochloride salt;
12687~'0
-2~-
(q) 4(5)-(2,3-dihydro-2-ethyl-1-hydroxy-lH-inden-2-yl)-
imidazole, or its hydrochloride salt;
~t) 4(5)-(2,3-dihydro-2-ethyl-1~-inden-2-yl)imidazole, or
its hydrochloride saltr
(u) 4(5)-(2,3-dihydro-1-methyl-lH-inden-2-yl)imidazole, or
its hydrochloride salt;
(v~ 4(5)-(2,3-dihydro-1,6-dimethyl-1~-inden-2-yl) i~idazole,
or its hydrochloride salt;
(w) 4(5)-(5-chloro-2,3-dihydro-lH-inden-2-yl)imidazole, or
its hydrochloride salt;
(x) 4(5)-(4-chloro-2,3-dihydro-1~-inden-2-yl)imidazole, or
its hydrochloride salt;
~y~ 4tS)-(4-bromo-2,3-dihydro-lH-inden-2-yl)imidazole, or
its hydrochloride salt;
(z) 4(5)-(5-bromo-2,3-dihydro-lH-inden-2-yl)imidazole, or
its hydrochloride salt;
(aa) 4(5)-(4-methyl-2,3-dihydro-lH-inden-2-yl)imidazole, or
its hydrochloride salt;
(bb) 4(5)-(2,3-dihydro-1-hydroxy-1~-inden-2-yl)imidazole, or
its hydrochloride salt;
(cc) 4~5)-(2,3-dihydrobenzofuran-2,4-dimethylbenzofuran-2-
yl)imidazole, or its hydrochloride salt;
(dd) 4(5)-(2,3-dihydro-5-methylbenzofuran-2-yl)imidazole, or
its hydrochloride salt;
(ee) 4(5)-(2,3-dihydrobenzofuran-2,4-dimethylbenzofuran-2-
yl)imidazole, or its hydrochloride salt;
12~87~0
- 29 -
(ff) 4(5)-~2,3-dihydro-4-methyl-1H-inden-2-yl)imidazole, or
its hydrochloride salt;
(gg) 4(5)-(5-chloro-2,3-dihydrobenzofuran-2-yl)imidazole or
its hydrochloride salt;
(hh) 4(5)-(2,3-dihydro-2-methylbenzofuran-2-yl)imidazole or
its hydrochloride salt.
The invention disclosed and claimed in the parent
application Serial No. 495,608 filed November 18, 1985 also
provides, as a novel pharmaceutical composition, an effective
. amount of a substituted imidazole of the general Formula (I)
_ .
~'~`J' j~;~ (1)
H
or the non-toxic, pharmaceutically-acceptable, acid-addition
salts thereof, or mixtures thereof, wherein:
X is CH2, CH2CH2 or O;
R1 is H, a lower alkyl having 1 to 4 carbon atoms, a lower
1268770
- 29a -
alkenyl group having 2 to ~ carbon atoms, OCH3 or OCH2CH3;
R2 is H, Cl-13, Cl-12C~13, OCI13 or OH;
R3 is H, CH3, CH2CH3, OCH3 or Hal;
R~, is H, CH3, Cl12CH3, OCH3 or Hal; and
Hal is halogen; or the non-tox:ic, pharmaceutically-acceptable,
acid-addition salts thereof, or mixtures thereof, and a
pharmaceutically-acceptable carrier. That pharmaceutical
composition can contain, as a specific pharmaceutically-active
compound, any of compounds (a) - (hh) disclosed above.
1268770
-30-
Compound I
q t5)-(2, 3-dihydro-lH-ind~n-2-yl)-imidazole
~?
.
Compound II
4(5)-(2,3-dihydrob~nzofuran-2-yl)imi.daole
H
Compound III
4(5)-(5-bromo-2,3-dihydrobenzofuran-2-yl)imidazole
~;2687~)
-3].-
Compound IV
4(5)-~2,3-dihydro-1-methyl-1~-inden-2-yl)imidazole
c~3
Compound V
4(5)-(2,3-dihydro-1,6-dimethyl-lH-inden-2-yl)imidazole
CH3 cH3
Compound VI
4(5)-(2,3-dihydro-1,4-dimethyl-lH-inden-2-yl)imidazole
CH3
~9
C H
~X6B770
-32-
Compound ~II
4(5)-(2,3-dihydro-2-methyl-lH-inden-2-yl)i.midazole
cH3
Compound ~III
4(5)-(2,3-dihydro-5-~methyl-lH-illden-2-yl)imidazole
C~
H
'
! compound IX
4(5)-(2,3-dihydro-2-ethyl-5-methyl-lH-inden-2-yl)imidazole
CH3 CH2-CH3
~N
~1 2687 ~0
-33-
Compound X
4(5)-(2,3-dihydro-2-~thyl-1H-ind~n~2-yl)imidazole
ICH3
cH2
~1'~
Compound XI
4(5)-(2,3--dihydro~2,5-dimethyl-1H-ind~n-2-yl)imidazol~
CH3 cH3
~k~?
.
Compound XII
4(5)-(1,2,3,4-tetrahydronaphth-2-yl)imidazole
lZ68770
-34-
Compound XIII
4(5)-(5-chloro-2,3-dihydro-lH-inden-2--yl)i.midazol~
Cl~
Compound XI~
4(5)-(4-chloro-2,3-dihydro-lH-ind~!n-2-yl)imidazole
Cl H
-
Compound X~
4(5)-(4-bromo-2,3-dihydro-lH-inden-2-yl)imidazole
.
-3s 1268770
Compound XUI
4(5)-(5-bromo-2,3-dihydro-1H-inden-2-yl)imidazole
~r
Compound X~II
4(5)-(4-methyl-2,3-dihydro-111-inden-2-yl)imidazole
3 H
_ .. . . .. _
Compound XUIII
4(5)-(2,3-dihydro-1-hydroxy-lH-inden-2-yl)imidazole
OH
H
.. ..
The pharmacological acti~ity of the compounds of
aspects of the present in~ention was determi.ned as follows:
1. ~ -antagonism in ~itro
~ 2-antagonism was determined by means of isolated,
electrically stimulated mouse ~as deferens preparation
(Marshall et al., Br. J. Pharmac. 62, 147, 151, 1978). In this
model, Q -antagonist is seen by administering it prior to the
agonist and by detemining its P~2 ~alue. Known
~2 -antagonists ].ike yohimbine and rauwolscine were used as
1268770
-36-
reference substances.
To obtain information on the selecti.uity of the
antagonist between Ql-and n2-receptors its ability to
inhibit or stimulate.Q1~receptors was determined by means of
isolated anococcygeus muscle (rat). The reference substances
were phenylephrine a known a.l ag~nist , and prazosin a
known a 1-antagonist To cletermine a-alltagonism muscular
contraction was induced by phenylepllrine and the P~2 ~alue of
the studied compound was deterrnined as above. a l-agonist
effect is presented as the PD2 ualue (negati~e logarithm of
the molar concentration of the compound producing 50 per cent
of maximal contraction). Examples of the results are gi~en in
Table 1.
1;268770
Table 1
1l2-antagonism Q2 -anta90nisnl Q l-agonism
(p~ vs detomidine) (P~2 us phenyl- (pD2)
mouse uas deferens ephrine) rat
rat anococcygeus anococcygeus
Compound I 8.8 - 6.5
Compound II 7.5 - 5.5
Compound III 6.2 - 4.5
Compouna IV 8.7 - 6.5
Compound V 7.6 5.9
Compound VI 7.6 - 5.5
Compound VII 8.1 - 6.5
Compound VIII 7.7
Compound IX 6.6
Compound X 8.3
Compound XII 7.7 - 6.0
Yohimbine 8.1 6.6
Rouwolscine 8.1 6.3
Prazosin <5 9.0
Phenylephrine - - 6.5
2. n2-antagoni ~m i n vivo
The central 2-blocking effect of the studied substances at in
vivo conditions wa~ ~tudied u~ing two method~. Fir~t, it is
1268770
-3~3-
known that in the rat c2-agonists induce dilation o~ the pupil
(mydriasis) which effect is transmitted via ~2-receptors of the
central nervous system. In anaesthetized
rat, a standard dose of detomidine was administered
intra~eno~lsly. Thereafter, increasing doses of the studied
antagonist were injected intra~eneously and the reuersal of
detomi.di.ne-induced mydriasis was followed. The Ed50 value of
the antagonist, i.e. the dose producing a 50 per cent re~ersal,
was determined. Example of the results of this test are
presented in Table 2.
Compound Ed50
( g/kg i~)
I 3
II 70
III 320
IU 100
V 100
UI 100
UII 3
UIII 20
X 6
Yohimbine 200
Phentolamine 1000
Prazosin 1000
-antagonism in the central ner~ous system was secondly
studied by following the ability of the antagonist to inhibit
detornidine induced sedation in the mouse. This was done by
lZ68770
-39-
measuring the increase of harbiturate sleeping ti.me induced by
detornidine. This eFfect of detomidine is known to be induced
through ~2-receptor acti.~ati.on. The antagonist can be
studied by administering it prior to detom-idine. The results
of the selected cornpounds are shown in Table 3.
Table 3. Effect of different antagonists (+ per cent of
. _
controls) on detomidine (150 g/kg ip)-inducecl potentiation of
the barbiturate sleeping ti.me i.n mice.
-
Dose Compound I Cornpound II yohimbine Prazosin
mg/kg
o.l -20 -5 0 0
0.3 -60 -30 -18 0
1 -100 -60 -64 0
3 not measured -70 -70 +16
rot measured -as -loo +18
)
1,
~;~6~
--~o--
In the examples below, where lH nnd 13C NMR spectrum 6hifts are
pre~ented, the NMR spectra were determined with a Bruker WB 80
DS spparatus using an internal tetrsmethylsilane standard, from
which the presented chemical ~hifts ~ ~, ppm) nre tabulated. The
letters ~, d, t flnd m are used to indicate a singlet, doublet,
triplet or multiplet, respectively. In the same connection, the
number of hydrogen atoms is also stated. The compounds which are
indicated as bases are tested in deuterium methanol, deuterium
acetone or deuterium chloroform, while the values for compounds
which are indicated as hydrochlorides were determined in
deuterium oxide or deuterium methanol. The mass spectra were
determined with a Kratos MS ~0 Autoconsole apparatus
Example 1
4(5)-(2,3-Dihydro-lH-inden-2-yl)imida~ole
The 1-(2,3-dihydro-lH-inden-2-yl)ethanone used as the starting
material can be obtained according to the publication (Carlson,
G. L. B., Quina, F. H., Zarnegar, B. M. and Whitten, D. G., J.
Am. Chem. Soc. _ (1975) 347).
a) 2-Bromo-1-(2,3-dihydro-lH-inden-2-yl)ethanone
Bromine (6.8 g) is slowly added to a stirred solution of
1-(2,3-dihydro-lH-inden-2-yl)ethanone (6.8 g) in 200 ml of dry
ether, while keeping the temperature at + lO~C. The rate of the
addition of bromine is controlled 80 that the colour due to one
added portion of bromine has been discharged before another
portion is added. When the addition is complete, the ethereal
solution is washed four times with 3 M sodium carbonate
solution, and is then washed three times with water. The
ethereal solution is dried with anhydrous magnesium sulphate.
After removal of the solvent the solid 2-bromo-1-(2,3-dihydro-
lH-inden-2-yl)ethanone is obtained.
MS (m/z, % the relative intensity): 240 and 238 (8 and 12,
M ), 159 (47, M-Br), 145 (~1, M-CH2Br), 117 (73, M-COCH2Br),
116 (78), 115 (100, ~ ~ ~)
i268770
41-
b) 4(5)-(2,3-Dihydro-lH-inden-2-yl)imidazole
A mixture of 2-br~mo-1-(2,3-dihydro-lH-inden-2-yl)ethanone
(9.4 g) and formamide (140 ml) i6 heated at 170-180C for 4
hours. Then the reaction mixture is allowed to cool to ambient
temperature and poured into ice-cold, dilute hydrochloric acid
solution. The mixture is washed twice with toluene. Then the
aqueous layer is made alkaline with ammonia and extracted
eeveral times with ethyl acetate. The combined organic layers
are dried over anhydrous magnesi~ sulfate and evaporated to
dryne6s under reduced pressure. The oily residue, which contains
the crude product of 4(5)-(2,3-dihydro-lH-inden-2-yl)imidazole
i9 purified by flash chromatography (solvent system: methylene
chloride - methanol 9.5:0.5). The 4(5)-(2,3-dihydro-lH-inden-2-
yl)imidazole thus obtained is converted to its hydrochloride
salt. The base is dissolved in ethyl acetate. Dry hydrogen
chloride in ethyl acetate is added. The hydrochloride is pre-
cipitated with dry ether.
4(5)-(2,3-Dihydro-lH-inden-2-yl)imidazole hydrochloride:
MS: 184 (100 M+ ), 183 (71, M-H), 169 (89, M-CU3), 156 (32), 150
(10), 147 (12), 142 (17), 141 (10), 139 (18), 129 (2~0), 128
(24), 127 (15), 119 (12), 116 (23), 115 (36), 111 (10), 91 (25),
77 (8), 69 (20)
lH NMR (80 MHz, MeOH-d4): 2.93-3.83 (5H, m, H21, H2 and H23),
7.08-7.27 (4H, m, aromatic), 7.35 (lH, dd, im-5(4)), 8.83 (lH,
d, 4J 1.37 Hz, im-2)
13C NMR (20 MHz, MeOH-d4): 36.80 (OFR d, C2), 39.71 (2t, Cl and
C3), 115.96 (d, im-5(4)), 125.32 (2d, aromatic), 127.86 (2d,
aromatic), 134.85 (d, im-2), 138.76 (8, im-4(5)), 142.42 (26,
and Cg)
1268~70
-42-
Example 2
4(5)-(2,3-Dihydrobenzofuran-2-yl)imidnzole
a) l-(Benzofuran-2-yl)-2-bromoethAnone
BenzofurAn-2-yl methyl ketone (20 g) i8 di~solved in 100 ml of
methylene chloride and 3.2 ml of bromine inmethylene chloride is
added at 5-10C. Then the reaction mixture is stirred at + 15~C
for 2 hours. Then it is washed with water, with diluted sodium
bicarbonate solution and again with water. The organic phsse is
dried and evaporated to dryness to give crude 1-(benzo~uran-2-
yl)-2-bromoethanone.
b) 4(5)-(Benzofuran-2-yl)imidazole
The crude product from step a) (12,1 g) and formamide (60 ml)
are combined and heated at 170C for 5 hours. The reaction
mixture is poured in water and concentrated hydrochloric acid
added to make the mixture acidic. It is then washed with
methylene chloride and the aqueous phase is made alkaline with
sodium hydroxide. The product is extracted into methylene
chloride which thereafter is washed with water, dried with
sodium sulfate and evaporated to dryness. The residue consisting
of crude product is converted to its hydrochloride salt in ethyl
~cetate. M.p. 229-235~C.
lH NMR (80 MHz, D20): 4.96 (2H, 8), 6.77 (lH, A), 7.16-7.49 (6H,
m), 8.46 (lH, 8)
c) 4(5)-(2,3-Dihydrobenzofuran-2-yl)imidazole
The product from step b) (5 g) is dissolved in water (60 ml) and
ethanol (30 ml) and concentrated hydrochloric acid (9 ml) is
added. Then the reaction mixture is hydrogenated at 60~C with
10 % palladium on carbon a8 cAtAlyst until no more hydrogen is
consumed.
_~3_ ~268770
Then the catAlyst is filtered And ethanol is distilled off. The
aqueous ~olution is washed with methylene chloride and made
alkaline with sodium hydroxide. The product i8 extracted into
toluene. Tlle toluene i8 w~shed with water snd evaporated. The
residue is crystallized from toluene-i~opropanol flnd is then
converted to its hydrochloride salt in isopropanol-ether. The
yield is 1.3 g, m.p. 177-178C.
MS: 186 (46 %), 185 (13 %), 170 (15 %), 169 (100 %), 159 (5 X),
158 (8 %), 157 (7 %), 146 (16 ~), 142 (43 X), 131 (11 X), 130
(20 X), 103 (lO X)
Example 3
4(5)-(5-Bromo-2,3-dihydrobenzofuran-2-yl)imidazole
4(5)-(2,3-dihydrobenzofuran-2-yl)imidazole (0.6 g) and water (8
ml) are combined. Concentrated hydrochloric acid is added until
the solution i6 acidic. Bromine (0.52 g) is added dropwise at
10C and the mixture is stirred at this tempersture for
another half an hour. The precipitated product i8 filtered off
and washed with water. The crude product is dis601ved in warm
water and the undisaolved material filtered off. The filtrate i6
made alkaline with sodium hydroxide and the precipitate is
filtered off. The product is converted to its hydrochloride sAlt
in isopropanol-ether. The yield of 4-(5-bromo-2,3-dihydrobenzo-
furan-2-yl)imidazole hydrochloride is 0.4 g, m.p. 202-204C.
M.p. of the ba~e is 187-188C.
Example 4
cis- 4(5)-(2,3-Dihydro-l-methyl-lH-inden-2-yl)imidflzole
The cis-2,3-dihydro-1-methyl-lH-indene-2-carboxylic acid used as
the starting material can be obtained according to the liter~-
ture (for example Shadbolt, R. S., J. Chem. Soc. (C), (1970)
920).
68770
a) cis-2,3-Dihydro-l-metlIyl-llI-indene-2-carboxylic ncid chloride
cis-2,3-Dihydro-l-methyl-lH-indene-2-cArboxylic acid (52.6 g) iB
converted to its acid chloride by treatment with thionyl
chloride (130 ml). Excess thionyl chloride is di~tilled off ~nd
the acid chloride is distilled, b.p. 86-89'C/0.45 mmHg. The
yield i8 47.9 g, 83 %.
b) cis-1-(2,3-Dihydro-l-methyl-lH-inden-2-yl)ethanone
Ci5-1-(2~3-DihydrO-l-metllyl-lH-inden-2-yl)ethanone i8 prepared
by the treatment of ci6-2,3-dihydro-l-methyl-lH-indene-2-
carboxylic acid chloride with ethoxymflgnesiummalonic flcid ethyl
ester in dry ether flnd thereafter by the treAtment of sulfuric
acid according to the publication (Reynolds, G. A. and Hfluser,
C. B., Org. Synth. 30 (1957) 70). The yield is 92 %.
ci~-1-(2,3-dihydro-1-methyl-lH-inden-2-yl)ethanone:
MS : 174 (31, M+ ), 159 (71, M-CH3), 131 (38, M-COCH3), 130
(100), 129 (27), 128 (21), 116 (24), 115 (54), 91 (33), 43 (16,
cocH3 )
lH NMR (80 MHz, CDC13): 1.36 (3H, d, J 6.67 Hz, ~CHC~ ), 2.24
(3H, ~, COCH3), 2.79-3.64 (4H, m, Hl, H2 and H23 of the indflne
ring), 7.17 (4H, 8, aromatic)
13C NMR (20 MHz, CDC13): ~ 19.60, 28.99, 34.80, 41.58, 61.05,
123.17, 124.13, 126.65, 126.71, 140.48, 146.38, 208.99
_~5 ~ 2 ~ 8 7 7 0
c) 2-Bromo-1-(2-bromo-2,3-dihydro-1-methyl-lH-inden-2-yl)-
ethanone
Bromine in methylene chloride is slowly added to a stirred
solution of cis-1-(2,3-dihydro-1-methyl-lH-inden-2-yl)ethanone
(34.8 g) in methylene chloride (835 ml), while keeping the
tempersture flt ~ 10 C. The reaction is followed by GLC. The
first products are the isoTners of 1-(2-bromo-2,3-dihydro-1-
methyl-lH-inden-2-yl)ethanone and cis-2-bromo-1-(2,3-dihydro-1-
methyl-lH-inden-2-yl)ethanone. ~en the added amount of bromine
is 0.3 mol, only the ~inal product, 2-bromo-1-(2-bromo-
2,3-dihydrol-methyl-lH-iTlden-2-yl)ethanone is visible in the
chromatogram and the mono bromo products cannot be seen in the
chromatogram any more. The methylene chloride solution i6 washed
with water, then several times with the diluted NaHC03 Solution
and finally with water. The solvent is dried with Na2so4 and
evaporated to dryness.
2-bromo-1-(2-bromo-2,3-dihydro-1-methyl-lH-inden-2-yl)ethanone:
MS of the isomer a : 334, 332, 330 (0.5, 1, 0.5, M~'), 253 and
251 (65 and 68, M-Br), 211 and 209 (1 and 1, M-COCIT2Br), 172
(11), 157 (28), 148 (26), 131 (15), 130 (80), 129 (9,3), 128
(79), 127 (30), 123 (22), 121 (23), 115 (100, ~ \~ +), 102
(10), 95 (14), 93 (14), 77 (11).
MS of the isomer b : M~ invisible, 253 (65), 251 (69), 209 (1),
211 (1), 172 (17), 157-(38), 143 (28), 131 (14), ]30 (69), 129
(100), 128 (85), 127 (35), 123 (18), 121 (18), 115 (95), 102
(11), 95 (13), 93 (14), 77 (12).
cis-2-bromo-1-(2,3-dihydro-1-methyl-llT-inden-2-yl)ethanone:
If the products are isolated, when 0.2 mol of bromine (instead
of 0.3 mol) has been added, the following mixture of
-46- 1 2 6 8 ~ o
products is obtained: 1-(2-bromo-2,3-dihydro-1-methyl-lH-inden-
2-yl)ethanone, cis-2-bromo-1-(2,3-dihydro-1-methyl-lH-inden-2-
yl)ethnnone and 2-bromo-1-(2-bromo-2,3-dihydro-1-methyl-lH-
inden-2-yl)ethsnone. Also a little amount of the 0tarting
compound can be seen in the chromatogram.
MS : M invisible, 173 (100, M-Br), 155 (12), 145 (26), 143
(10), 131 (31, M-cocH2Br)~ 130 (16), 129 (29), 128 (26), 127
(14), 116 (29), 115 (59), 91 (28).
d) 4(5)-(1-Methyl-inden-2-yl)imidazole
2-Bromo-1-(2-bromo-2,3-dihydro-1-methyl-lH-inden-2-yl)ethsnone
(34.0g) and formamide (520 ml) are combined and the mixture is
heated with stirring at 170C for 3 hours. The reaction
mixture i8 cooled, then poured into water, made acidic with
hydrochloric acid and wa6hed with methylene chloride. The
aqueous layer i~ then made alkaline with sodium hydroxide and
the mixture is extracted with ethyl acetate. The organic
extracts are washed with water and dried and evaporated to
dryness. The re6idue, which consists of the crude product, is
converted to the hydrochloride salt in ethyl acetate. After the
recrystallization of the hydrochloride from isopropanol-ethanol
the yield of the product is 11.4 g, 48 ~ (m.p. 265 - 268~C).
The hydrochloride salt of 4(5)-(1-methyl-inden-2-yl)imidazole:
MS : 196 (100, M+'), 195 (44, M-H), 181 (30, M-CH3), 168 (10),
167 (10), 141 (12), 139 (9), 127 (12), 115 (10), 98 (8), 97 (9).
H NMR (80 MHz, MeOH-d4): ~ 2.34 (3H, t, 5J 2.22 Hz, CH3), 3.75
(2H, q, 5J 2.22 HZ~cH2)~ 7.16 - 7.55 (4H, m, aromatic), 7.71
(lH, d, im-5(4)), 8.97 (lH, d, 4J 1.37 Hz, im-2).
!; -47- i268~
3C NMR (20 MHz, MeOH-d4): ~ 12.10 (OFR q), 40.16 (t), 116.81
(d), 120.75 (d), 124.56 (d), 126.04 (8), 127.19 (d), 127.74 (d),
131.49 (8), 134.79 (d), 141.06 (8), 143.30 (8), 146.63 (8).
e) 4(5)-(2,3-Dihydro-l-methyl-lM-inden-2-yl)imidazole
The crude product of 4(5)-(1-methyl-inden-2-yl)imidazole (3.3 g)
i8 dissolved in water (40 ml)-ethanol (20 ml)- concentrated
hydrochloric acid (6 ml) solution. Then 0.33 g of 10 ~ Pd/C i9
added and the mixture is stirred vigorously under a hydrogen
fltmo6phere at 60C until no more hydrogen i8 con6umed. The
reaction mixture is then filtered and the filtrate is evaporated
to a smsller volume. The acidic solution is washed with
methylene chloride. The aqueous phase is then made alkaline and
extracted with methylene chloride. The organic extracts are
dried and evaporated to dryness. The crude cis-4(5)-(2,3-
dihydro-l-methyl-lH-inden-2-yl)imidazole is purified by
converting it into the hydrochloride salt in acetone-ethyl
acetate. The melting point of the hydrochloride is 192 - 194 C.
The hydrochloride salt of ci8-4(5)-(2,3-dihydro-l-methyl-lH-
inden-2-yl)imidazole:
MS : 198 (100, M~ ), 197 (27, M-H), 183 (78, M-CH3), 170 (14),
169 (43), 156 (17), 154 (18), 142 (11), 130 (36), 129 (24), 128
(27), 127 (15), 117 (14), 116 (12), 115 (44), 91 (25), 82 (17),
81 (30), 77 (11).
lH NMR (80 M}lz, MeO13-d4): ~ 0.94 (3H, d, 3J 7.01 Hz, CH3), 3.23
- 4.03 (4H, m, Hl, H2 and H23), 7.19 - 7.25 (5H, m, aromatic and
im - 5(4)), 8.85 (lH, d, 4J 1.37 Hz, im-2).
3C NMR (20 MHz, DMSO-d6): ~ 16.34 (OFR q), 34.41 (t), 39.23
(d), 41.95 (d), 115.73 (d), 123.66 (d), ]24.08 (d), 126.50 (2d),
133.28 (d), 133.77 (6), 140.49 (8), 147.00 (8).
~- 1268770
Exnmple 5
cis-4(5)-(2,3-Dihydro-1,6-dimethyl-lH-indenr2-yl)imidazole
a) ~ -Acetyl-4-methylbenzenepropnnoic acid ethyl e~ter
The starting material, ~ -acetyl-4-methylbenzenepropsnoic acid
ethyl ester can be prepMred for example according to the
publication by L. Borowiecki and A. Kszubski (Pol. J. Chem. 52
(1978) 1447). Yield 60 X, b.p. 120 - 150 C/0.15 mmHg.
-Acetyl-4-methylbenzenepropanoic acid ethyl ester:
111 NMR (80 ~lz, cDcl3): ~ 1.20 (3H, t, J 7.18 Hz, CH2cH3), 2.17
(3H, 8, CH3C0 or ArCH3), 2.29 (3H, 8, ArCH3 r CH3C0), 3.11 (2H,
distorted d, Jab 7.58 Hz, ~ CHC1~2-), 3.74 (lH, distorted t, Jab
7.58 Hz,~C CH2-), 4.14 (211, q, J 7.18 Hz, CH2CH3)~ 7.06 (4H, 8,
aromatic).
13C NMR (20 MHz, cDcl3): ~ 13.91, 20.88, 29.35, 33.56, 61.23,
61.35, 128.49 (2), 129.10 (2), 134.97, 136.00, 169.00, 202.15.
b) l,6-Dimethyl-indene-2-carboxylic acid
The 1,6-dimethyl-indene-2-carboxylic acid can be prepared by the
treatment of C~-acetyl-4-methylbenzenepropanoic acid ethyl ester
with sulfuric acid (Shadbolt, R.S., J. Chem. Soc. (C), (1970)
920). Recrystallization from ethanol, m.p. 174 - 182 C. Yield
59 %.
1,6-dimethyl-indene-2-carboxylic acid:
lH NMR (80 MHz, DMSO-d6): ~ 2.38 (3H, 8, ArC_3), 2.46 (3H, t,
5J 2.39 Hz, = Cl-cH3)~ 3.53 (2H, q, 5J 2.39 Hz, CH2), 6.68 (lH,
broad 8, C00_), 7.11 - 7.44 (3H, m, sromatic).
9- 126877~
3C NMR (20 MHz, DMSO-d6) ~ 11.95 (OFR q), 20.97 (q), 38.14
(t), 121.33 (d), 123.51 (d), 128.20 (d), 130.59 (8), 135.59 (8),
140.07 (8), 145.06 (9), 149.60 (8), 166.49 (8).
c) 1,6-Dimethyl-indene-2-carboxylic acid chloride
1,6-Dimethyl-indene-2-cflrboxylic acid (37.3 g) iB converted to
its acid chloride by treatment with thionyl chloride (580 ml).
Excess thionyl chloride is distilled off. Yield 40.5 g, 99 %.
d) l-(l,6-nimethyl-inden-2-yl)eth~none
1-(1,6-Dimethyl-inden-2-yl)ethanone i8 prepared by the same
procedure 86 cis-1-(2,3-dihydro 1-methyl-lH-inden-2-yl)ethanone
in Example 4b. A mixture of dry ether and tetrahydrofuran i8
used as solvent. Yield 84 %.
1-(1,6-Dimethyl-inden-2-yl)ethanone:
MS: 186 (68, M~ ), 171 (35, M-CH3), 144 (39), 143 (100,
M-COCH3), 142 (13), 141 (32), 129 (17), 128 (71), 127 (16), 115
(26), 43 (77, CocH3).
lH NMR (80 MHz, CDC13): ~ 2.41 (3H, 8, ArCH3 or COC _3), 2.42
(3H, 8, COC_ 3 or ArCH3), 2.51 (3H, t, 5J 2.39 Hz, 5 C~l-CH3)~
3.61 (2H,q, 5J 2.39 Hz, CH2), 7.11 - 7.41 (3H, m, aromatic).
3C NMR (20 MHz, CDC13): ~ 12.85 (OFR q), 21.39 (q), 30.02 tq),
38.80 (t), 122.02 (d), 123.62 (d), 129.01 (d), 136.36 (8),
137.82 (8), 140.24 (8), ~45.60 (6), 149.87 (8), 196.4~ (8).
~ ! --5 O-- 12~8';P7~
e) 2-Bromo-1-(1,6-dimethyl-lH-inden-2-yl)ethsnone
Bromine (2.80 g) is added to 1-(1,6-dimethyl-inden-2-yl)ethanone
(3.00 g) in dry ether (30 ml), while keeping the temperature st
+10C. The mixture i8 extracted with water, Meveral times with
the diluted NaHC03 solution, again with water, dried and
evaporated under reduced pressure to afford the product (2.54
g, 59 X).
2-bromo-1-(1,6-dimethyl-lH-inden-2-yl) ethanone:
MS: 266 and 264 (13 and 13, M+ ), 185 t4, M-Br), 171 (100,
M-CH2Br), 157 (13), 143 (49, M-COCH2Br), 142 (18), 141 (32), 128
(27), 115 (18).
f) 4(5)-(1,6-Dimethyl-lH-inden-2-yl)imidazole
4(5)-(1,6-Dimethyl-lH-inden-2-yl)imidazole is prepared by the
reaction of 2-bromo-1-(1,6-dimethyl-lH-inden-2-yl)ethanone (14.3
g) with formamide (130 ml) as descrihed earlier in Example 4d.
The crude bAse is purified by flash chromatography (solvent
system: methylene chloride - methanol 9.5:0.5). The 4(5)-(1,6-
dimethyl-lH-inden-2-yl)imidazole thus obtained is conve,rted to
its hydrochloride salt. The base is dissolved in ethyl acetate.
After dry hydrogen chloride in ethyl acetate is added the hydro-
chloride salt precipitates.
The base of 4(5)-(1,6-dimethyl-lH-inden-2-yl)imidazole:
lH NMR (80 MHz, MeOH-d4): ~ 2.28 (3U, t, 5J 2.05 Hz, ~Cl-CH3),
2.38 (3H, 8, ArCH3), 3.64 (2H, q, 5J 2.05 Hz, CU2), 6.91-7.33
(4H, m, aromatic and im-5(4)), 7.73 (lH, d, 4J 0.86 Hz, im-2).
-51- ~2~0
The hydrochloride salt of 4(5)-(1,6-dimethyl-inden-2-yl)-
imidazole:
lH NMR (80 ~Iz, MenH-d4): S 2.33 ~3H, t, 5J 2.22 Hz, = ICl-cH3)~
2.41 (311, B, ArC113), 3.71 (2H, q, 5J 2.22 Hz, CH2), 7.05-7.43
(3H, m, aromatic), 7.70 (111, d, im-5(4)), 8.96 (lH, d, 4J 1.37
Hz, im-2).
g) ci6-4(5)-(2,3-~ihydro-1,6-dimethyl-lH-inden-2-yl)imidazole
The hydrocl~loride 6alt of 4(5)-tl,6-dimethyl-inden-2-yl)-
imidazole (0.55 g) is dissolved in wflter (6 ml)-ethanol (3 ml)-
concentrated hydrochloric acid (4 ml) solution. Hydrogenation i6
performed a6 it i6 described in Example 4e. The Cl6-4(5)-(2,3-
dihydro-1,6-dimethyl-lH-inden-2-yl)imidszole is converted into
the hydrochloride salt in ethylacetflte. The melting point of the
hydrochloride salt is 192-196 DC.
The hydrochloride 6alt of cis-4(5)-(2,3-dihydro-1,6-dimethyl-lH-
inden-2-yl)imidazole:
MS: 212 (100, M+'), 211 (25, M-H), 197 (73, M-CH3), 183 (41),
170 (14), 168 (14), 144 (25), 141 (10), 131 (22), 129 (19), 128
(20), 115 (14), 98 (12), 91 (15).
lH NMR (80 MHz, MeOH-d4): ~ 0.93 (3H, d, J 7.01 Hz, ~CHCH3),
2.31 (3H, 6, ArCH3), 3.16-4.00 (4H, m, Hl, H2 and H23), 6.96-
7.12 (3H, m, aromatic), 7,24 (lH, broad 6, im-5(4)), 8.85 (lH,
d, 4J 1.37 Hz, im-2).
3C NMR (20 MHz, MeOH-d4): ~ 16.49 (OFR q), 21.39 (q), 36.17
(t), 41.83 (d), 43.98 (d), 117.17 (d), 125.14 (d), 125.38 (d),
128.74 (d), 134.63 (d), 136.55 (s), 137.82 (8), 138.70 (8),
]48.14 (8).
-52- 1 ~ ~ ~ o
Example 6
cis-4(5)-(2,3-Dihydro-1,4-dimethyl-lH-inden-2-yl)imidazole
Method A:
a) ~ -Acetyl-2-methylbenzenepropanoic acid ethyl ester
The starting materiall ~ -acetyl-2-methylbenzenepropsnoic acid
ethyl ester can be prepared for example according to the
publication by L. Borowiecki and A. Kazubski (Pol. J. Chem. 52
(1978) 1447). Yield 64 %, b.p. 142 - 152 C/1.5 mmHg.
G~-acetyl-2-methylbenzenepropanoic acid ethyl ester:
lll NMR (80 MHz, CDC13): ~ 1.19 (3H, t, J 7.18 Hz, cH2c~l3)~ 2.18
(3H, 8, CH3C0 or ArCH3), 2.32 (3H, 8, ArCH3 r C~3C0), 3.17 (2H,
distorted d, Jab 7.58 Hz, ~CIIC112-)~ 3.76 (IH, distorted t, Jab
7.58 Hz, ~ CHCH2-), 4.14 (2H, q, J 7.18 Hz, CH2cH3)l 7.10 (4H,
8, aromatic).
b) l,4-Dimethyl-indene-2-carboxylic acid
The 1,4-dimethyl-indene-2-carboxylic acid can be prepared by the
treatment of C(-acetyl-2-methylbenzenepropanoic acid ethyl ester
with ~ulfuric acid (Shadbolt, R.S., J. Chem. Soc. (C), (1970)
920). Recrystallization from ethanol, m.p. 190 - 193 C. Yield
61~.
1,4-dimethyl-indene-2-carboxylic acid:
lH NMR (80 MHz, DMSO-d6): ~ 2.33 (3H, 8, ArCH3), 2.46 ~3H, t,
5J 2.39 Hz = ICl-CII3), 3.48 (2H, q, 5J 2.39 Hz, CH2), 7.09 - 7.42
(4H, m, aromatic and -COOH).
~53- ~2~0
3C NMR ~20 Mllz, DMSO-d6): ~ 12.04 (OFR q), 17.86 (q), 37.44
(t), 118.51 (d), 126.68 (d), 128.26 (d), 130.11 (s), 132.71 (8),
141.67 (8), 144.42 (8), 149.81 (8), 166.43 (9).
c) l,4-Dimethyl-indene-2-carboxylic scid chloride
1,4-Dimethyl-indene-2-carboxylic acid i~ converted to its acid
chloride by treatment with thionyl chloride. Yield ln0 %.
d) l-(1,4-Dimethyl-inden-2-yl)ethanone
1-(1,4-Dimethyl-inden-2-yl)ethanone is prepared by the same
procedure as l-(l ,6-dimethyl-inden-2-yl)ethanone in Example 5d.
Yield 75 %.
1-(1,4-Dimethyl-inden-2-yl)ethanone:
MS: 186 (60, M+' ), 171 (29, M-CH3), 144 (33), 143 (100,
M-COCH3), 141 (27), 129 (18), 128 (66), 127 (15), 115 (28), 43
(60, CoCH3).
lH NMR (80 MHz, CDC13): ~ 2.38 (3H, 8, ArC113 or CoCH3), 2.46
(3H, 8, COCH3 r ArC~3)~ 2.53 (3H, t, 5J 2.39 H~,= Cl - CH3),
3.55 (2H, q, 5J 2.39 Hz, CH2), 7.10 - 7.46 (3H! m, aromatic).
13C NMR (20 MHz, CDcl3): ~ 13.13 (OFR q), 18.33 (q), 30.14 (q),
38.13 (t), 119.23 (d), 127.13 (d), 129.10 (d), 133.25 (8),
137.55 (c), 141.84 ( B), 145.14 ( 8), 150.17 ( B), 196.46 ( 9) .
e) 2-Bromo-1-(1,4-dimethyl-inden-2-yl)ethanone
2-Bromo-1-(1,4-dimethyl-inden-2-yl)ethanone i6 prepared by the
same procedure as 2-bromo-1-(1,6-dimethyl-inden-2-yl)ethanone in
~xample 5e. Yield 45 %.
2-bromo-1-(1,4-dimethyl-inden-2-yl)ethanone:
MS: 266 and 264 (14 and 15,1 M+ ), 185 (3, M-Br), 171 (100,
M-CH2Br), 157 (13), 143 (M-COCH2Br), 142 (18), 141 (33), 128
(32), 115 (21).
_5~_ lZ6~0
f) 4(5)-(1,4-Dimethyl-inden-2-yl)imidazole
4(5)-(1,4-Dimethyl-inden-2-yl)imidazole is prepared by the
reaction of 2-bromo-1-(1,4-dimethyl-inden-2-yl)ethanone (8.7 g)
with formamide (330 ml) as de~cribed earlier in Example 4d. The
product as base is extracted into methylene chloride. The yield
of the base product i8 3.0 g, 44 %.
g) cis-4(5)-(2,3-Dihydro-1,4-dimethyl-111-inden-2-yl)imidazole
The crude product of 4(5)-(1,4-dimethyl-inden-2-yl)imidazole
(3.0 g) i8 dissolved in water (35 ml) -ethanol tl8 ml)-
concentrated hydrochloric acid (17.4 ml) solution. Then 0.30 g
of 10 % Pd/C i8 added and the mixture is stirred under a
hydrogen athmosphere st 60 C until no more hydrogen is
consumed. Work-up of the reaction mixture is as before in
Example 4e. The crude imidazole derivative is purified by flash
chromatography (solvent system: methylene chloride/methanol
9.5/0.5). The cis-4(5)-(2,3-dihydro-1,4-dimethyl-lH-inden-2-yl)-
imidazole is converted into its hydrochloride salt in iso-
propanol/ethyl acetate and ether is added to precipitate the
salt, m.p. 135-140 C.
The hydrochloride salt of cis-4(5)-(2,3-dihydro-1,4-dimethyl-
lH-inden-2-yl)imidazole:
MS: 212 (100, M ), 211 (30, M-CH3), 197 (80), 184 (13), 183
(34), 182 (11), 170 (13), 168 (16), 144 (35), 143 (10), 141
(11), 131 (14), 129 (15), 128 (12), 127 (10), 115 (17), 98 (16),
91 (15).
lH NMR (8n MHz, MeoH-d4): ~ O.92 (3H, d, 3J 6.84 Hz, CH3CH ~ ),
2.31 (3H, 8, ArCH3), 3.14-4.01 (4H, m, Hl, H2 and H23),
6.98-7.09 (3H, m, aromatic), 7.28 (lH, broad 8, im-5(4)), 8.83
(lH, d, 4J 1.37 Hz, im-2).
_55- lZ68~0
3C NMR (20 MHz, MeOll-d4): ~ 16.79 (OFR q), 19.06 (q), 34.95
(t), 41.13 (d~, 44.16 (d), 117.20 (d), 122.14 (d), 128.25 (d),
128.77 (d), 134.67 (d), 134.88 (~), 136.49 (8), 140.30 (8),
147.l37 (8).
Method B:
a) _ -2,3-Dihydro-1,4-dimethyl-1ll-indene-2-carboxylic acid
1,4-Dimethyl-indene-2-carboxylic acid (35.5 g) iG hydrogenated
in ethanol-wflter (700 ml - 70 ml) over 10 X palladium on carbon
at ambient temperature. After filtration ethanol i~ evaporated.
Water is added and the precipitated cis-2,3-dihydro-1,4-
dimethyl-lH-indene-2-carhoxylic acid i8 filrated. Yield 33.3 g,
93 % M.p. 132 - 135 ~C.
cls-2,3-Dihydro-1,4-dimethyl-lH-indene-2-carboxylic acid:
lH NMR (80 MHz, DMSO-d6): ~ 1.08 (3H, d, J 6.78 Hz, CH3CH ~ ,
2.20 (3H, 9, ArCH3), 2.70 - 3.67 (4H, m, Hl, H2 and H23), 6.88 -
7.08 (3H, m, aromatic), 12.15 (lH, broad 8, -COOH).
3C NMR (20 MHz, DMSO-d6): ~ 16.95 (OFR q), 18.49 (q), 31.39
(t), 41.01 (d), 47.43 (d), 120.60 (d), 126,44 (d), 127-14 (d),
133.01 (8), 139.64 (8), 146.45 (8), 174.33 (8).
b) cis-2,3-Dihydro-1,4-dimethyl-lH-indene-2-carboxylic acid
chloride
cis-2,3-Dihydro-1,4-dimethyl-lH-indene-2-carboxylic acid i6
converted to its scid chloride by treatment with thionyl
chloride. Yield 92 ~.
cis-2,3-dihydro-1,4-dimethyl-lH-indene-2-carboxylic acid
chloride:
-5~- 1 26~
111 NMR (80 MHz, CDC13): ~ 1.44 (3H, d, J 6.67 Hz, CH3cH ~ )~
2.25 (3H, ~, ArC~ ), 2.84 - 4.02 (4H, m, Hl, H2, H23), 6.92 -
7.11 (311, m, aromatic).
c) cifi-1-(2,3-Dihydro-1,4-dimethyl-111-inden-2-yl)ethanone
1-(2,3-Dihydro-1,4-dimethyl-lH-inden-2-yl)ethanone i8 prepared
by the ~ame procedure as 1-(2,3-dihydro-1-methyl-lH-inden-2-yl)-
ethanone in Example 4b. B.p. 181-182 C/l m~lg. Yield 55 %.
cis-1-(2,3-dillydro-1,4-dimetllyl-lH-inden-2-yl)ethanone:
lH NMR (80 MHz, CDC13): ~ 1.37 (3H, d, 3J 6.65 Hz, C~3CH ~),
2,26 (6H, 2s, COCH3 and ArCH3), 2.85-3.72 (4H, m, Hl, H2 and H23
of the indane ring), 6.88-7.16 (3H, m, aromatic).
13C NMR (20 MHz, CDC13): ~ 18.82 (OFR q), 19.91 (q), 28.99 (q),
33.44 (t), 41.85 (d), 60.65 (d), 120.56 (d), 127.04 (d), 127.56
(d), 133 55 (B), 13g.30 (fi), 146.17 (s), 209.14 (s).
d) 2-Bromo-1-(2-bromo-2,3-dihydro-1,4-dimetllyl-111-inden-2-yl)-
ethanone
Bromination of cis-1-(2,3-dihydro-1,4-dimethyl-lH-inden-2-yl)-
ethanone (11.78 g) i8 performed with bromine (10.00 g)/methylene
chloride (4n ml) in methylene chloride (120 ml) as in the case
of l-(1,6-dimethyl-inden-2-yl)ethanone in Example 5e Work-up of
the reaction mixture gives the light yellow oil, which contains
two isomers (a and b) of 2-bromo-1-(2-bromo-2,3-dihydro-1,4-
dimethyl-lH-inden-2-yl)ethanone.
2-bromo-1-(2-bromo-2,3-dihydro-1,4-dimethyl-1H-inden-2-yl)-
ethanone:
~57~ 1 Z 6 8 7 7
MS of the isomer a: 348, 346, 344 (0.3, 0.5, 0.1, M+-), 267 and
265 (77 and 77, M-Br), 186 (10), 171 (18), 157 (18), 144 (64),
143 (74), 141 (23), 129 (74), 128 (100), 127 (29), 123 (16), 121
(16), 115 (24), 43 (13).
MS of the i~omer b: 348, 346, 344 (all invisible, M+'), 267 and
265 (71 and 78, M-Br), 186 (18), 185 (16), 171 (38), 157 (24),
144 (52), 143 (91), 141 t32), 129 (73), 128 ~100), 127 (30), 123
(12), 121 (13), 115 (36), 43 (15).
e) 4(5)-(1,4~Dimethyl-inden-2-yl)imidazole
4(5)-(1,4-Dimethyl-inden-2-yl)imidazole is prepared from 2-
bromo-1-(2-bromo-2,3-dihydro-1,4-dimethyl-lH-inden-2-yl)ethanone
and formamide as deacribed for 2-bromo-1-(2-bromo-2,3-dihydro-1-
methyl-lll-inden-2-yl)ethanone in Example 4d.
f) _ -4(5)-(2,3-Dihydro-1,4-dimethyl-lH-inden-2-yl)imidazole
cis-4(5)-(2,3-Dihydro-1,4-dimethyl-lH-inden-2-yl)imidazole i6
obtained in a similar manner a8 in method Ag.
Example 7
4(5)-(2,3-Dihydro-2-methyl-lU-inden-2-yl)imidazole
a) 2,3-Dihydro-2-methyl-lH-indene-2-carboxylic acid
2,3-Dihydro-2-methyl-lH-indene-2-carboxylic acid can be prepared
for example by the procedure of Huebner, C.F., Donoqhue, E.M.,
Strachan, P.L., Beak, P. and Wenkert, E. (J Org. Chem. 27
(1962) 4465) or by the reaction of lithium N-i60propylcyclo-
hexylamide and methyl iodide (Rathke, M.V. and Lindert, A., J
Am. Chem. Soc. 93 (1971) 2318) with 2,3-dihydro-lH-indene-2-
carboxylic acid methyl ester (prepared by the methylation of
2,3-dihydro-lH-indene-2-carboxylic acid in the presence of
~ulphuric acid) followed by hydrolysi6.
1268770
-5f3-
2,3-dihydro-2-metllyl-1}1-indene-2-cflrboxylic acid:
11~ NMR (80 MHz, CDC13): ~ 1.40 (3TI, s, CH3), AB quartet: ~A
2,84, ~ B 3.52, J~ , 15.73 llz (4H, 2 x CH2), 7.17 (4H, 6,
aromatic), about 9.3 (lH, broad 8, COOII).
3C NMR (20 Mllz, CDC13): ~ 24.84 (OFR q, CH3), 43.94 (2 t, Cl
and C3), 49.48 (6, C2), 124.62 (2 d, aromatic), 126.62 (2 d,
aromatic), 141.06 (2 8, C8 And C9), 183.65 (8, CO).
b) 2,3-Dihydro-2-methyl-111-indene-2-cArboxylic acid chloride
A stirred mixture of 2,3-dihydro-2-methyl-lH-indene-2-carboxylic
acid (6.70 g) and thionyl chloride (70 ml) is heated under
reflux for 14 hr. The exce66 of thionyl chlorid~ is removed ~nd
the acid chloride i6 di6tilled. Yield 5.35 g, 72 Z, bp. 93-98
~C/3 mml-lg.
2,3-dihydro-2-methyl-111-indene-2-carboxylic acid chloride:
H NMR (80 MHz, CDC13): ~ 1.51 (3H, 8, CH3), AB quartet:
A 2.91, ~ B 3.60, J ~a 15.90 Hz (4H, 2 x CH2), 7.19 (4H, 8,
aromatic).
c) 1-(2,3-Dihydro-2-methyl-111-inden-2-yl)ethanone
1-(2,3-Dihydro-2-methyl-lH-inden-2-yl)ethanone is prepared from
2,3-dihydro-2-methyl-111-indene-2-carboxylic acid chloride in the
same way as it i~ described in Example 4b. Yield 75 %.
1-(2,3-dihydro-2-methyl-lH-inden-2-yl)ethanone:
lH MMR (80 MHz, CDC13): ~ 1.32 (3H, 8, / CCH3), 2.20 (3H, 8,
CocH3)~ AB quartet: ~ A 2.76, ~ B 3 39- JAB 15.73 Hz (4H, 2 x
CH2), 7.17 (411, 8, aromatic).
1268~70
-59-
d~ 2-Bromo-1-(2,3-dihydro-2-methyl-111-inden-2-yl)ethanone
l-(2~3-Dihydro-2-methyl-lll-inden-2-yl)ethsnone (3.69 g) in
methylene chloride (40 ml) i~ stirred and cooled at 10 C during
the dropwise addition of bromine (2.82 g)/methylene chloride (10
ml). Work-up of the resultant solution give6 2-bromo-1-(2,3-di-
hydro-2-methyl-lH-inden-2-yl)ethanone.
2-bromo-1-(2,3-dihydro-2-methyl-lH-inden-2-yl)ethanone:
MS: 254 and 252 (2 and 2, M~), 239 and 237 (0.5 and 0.5,
M-CH3), 173 (100, M-Br), 159 (39, M-CH2Br)~ 155 (13), 145 (30),
143 (10), 131 (97, M-COCH2Br)~ 130 (30), 12S (40), 128 (34), 127
(19), 116 (29), 115 (69), 91 (50), 77 (12), 63 (10), 43 (22).
e) 4(5)-(2,3-Dihydro-2-methyl-lH-inden-2-yl)imidazole
4(5)-~2,3-Dihydro-2-methyl-lH-inden-2-yl)imidazole is prepared
by the reaction of 2-bromo-1-(2,3-dihydro-2-methyl-lH-inden-2-
yl)ethanone (2.04 g) with formamide (60 ml) a8 described in
Example 6, method Af. Purification of the crude base via fla~h
chromfltography (methylene chloride/methanol 9.75/0.25) gave pure
4(5)-(2,3-dihydro-2-methyl-lH-inden-2-yl)imidazole. M.p.~ of the
base 167-170 C.
The base of 4(5)-(2,3-dihydro-2-methyl-lH-inden-2-yl)imidazole:
MS: 198 (44, M+ ), 197 (13, M-H), 183 (100, M-CH3), 129 (14),
128 (18), 115 (22), 91 (28), 77 (11).
lH NMR (80 Mllz, CnC13): ~ 1.48 (3H, 8, CH3), AB quartet:SA
2.98, SB 3.32, JAB~ 15.39 Hz (4H, 2 x CH2), 6.78 (lH, 8, im-
5(4)), 7.16 (411, fi, aromatic), 7.54 (lH, 8, im-2), 8.74 (lH, 8,
~NH).
; -~o- ~268770
J
Example 8
4(5)-(2,3-Dihydro-lH-inden-2-yl)imidnzole
a) 2-~romo-1-(2-bromo-2,3-dihydro-1}1-inden-2-yl)ethanone
The procedure of Example 1 a) i9 repeated, except that the
amount of bromine i8 doubled. After removal of the ~olvent the
crude product i9 used as such in step b).
b) 4(5)-(lH-Inden-2-yl)imidazole
The procedure of Example 1 b) i6 repeated. The product i6 re-
crystallized from methylene chloride.
c) 4(5)-(2,3-Dihydro-lH-inden-2-yl)imidazole
The procedure of ExRmple 2c is repeated except that 4(5)_(1H_
inden-2-yl)imidazole i8 used in place of 4(5)-(2,3-dihydrobenzo-
furan-2-yl)imidazole. When the uptake of hydrogen ceases, the
reaction mixture is filtered and the filtrate i8 made alkaline
with sodium hydroxide. The separated oil i3 extracted into
methylene chloride. The combined extracts are washed with water,
dried over Na2S04, and evaporated to dryness. The crude product
i6 purified by converting it into the hydrochloride in ethyl
acetate. M.p.: 184 - 191 C.
Example 9
4(5)-(2,3-nihydro-5-methyl-lll-inden-2-yl)imidazole
The procedure of Example 8 i8 repeated except that,in place of
1-(2,3-dihydro-lH-inden-2-yl)ethanone, 1-(2,3-dihydro-5-methYl-
lH-inden-2yl)ethanone is used. M.p. (HC1): 171- 175 C.
lR NMR (80 ~Iz, cDcl3~ a6 base): 2.3 (6, 3H), 2.8-3.8 (m, 5H),
6.8 (6, lR), 7.0-7.1 (m, 311), j7.5 (6, lH), 9.9 (s, 111).
-6L- ~68~
Example 10
4(5)-(2,3-Dihydro-2-ethyl-5-methyl-111-inden-2-yl)imidazole
The procedure of Example 1 iB repeated except that,in place of
1-(2 3-dihydro-lH-inden-2-yl)ethanone~ 1-(2,3-dihYdr-2-ethY
methyl-lH-indene-2-yl) is used. M.p. 54-S7 C as base.
MS: 226 (40 %), 211 (12 %), 197 (100 %), 182 (7 %), 128 (12 %),
98 (17 %), 84 (15 %).
Ex&mple 11
4(5)-(2,3-Dihydro-2-ethyl-lH-inden-2-yl)imidazole
The compound i6 prepared according to the procedure of Example 7
using 2,3-dihydro-lU-indene-2-carboxylic acid methyl ester and
ethyl bromide a6 starting materials. M.p. (HCl): 211-215 C.
111 NMR (B0 MHz, CDC13, a6 base): 0.78 (t, 3H), 1.88 (q, 2H),
3.17 (q, 4H), 6.75 (8, lH), 7.13 (s, 4H), 7.53 (s, lH), 10.01
(B, lH).
Example 12
4(5)-(2,3-Dihydro-2,5-dimethyl-lH-inden-2-yl)imidazole
The procedure of Example 1 i8 repeated except that, in place of
1-(2,3-dihydro-lH-inden-2-yl) ethanone, 1-(2,3-dihydro-2,5-dimethyl-
lH-inden-2-yl)ethanone is used.
M.p.: 148-151 ~C a6 base.
lH NMR (80 MHz, CDC13, as hydrochloride): 1.51 (8, 3H), 2.27 (6,
3H), AB quartet ~ A 3-04- ~ B 3.24 JAB 15.45 Hg (4H, 2 x
CH2), 6.87-6.99 (m, 4H), 9.04 (6, lH), 14 (broad band, 2H).
` ~68~70
-~,2-
Exnmple 13
4(5)-(2,3-Dihydro-lll-inden-2-yl)imidazole
a) 2,3-Dihydro-lH-inden-2-yl glyoxal diethyl acetal
0.73 g of magnesium turnings are covered with 90 ml of dry
diethylether. To thMt mixture i6 then added 6 g of 2-bromoindane
in 20 ml of dry diethylether at such a rate that a gentle
boiling is maintained. When the magnesium turnings have reacted
the solution containing the Grignard reagent is cooled to room
temperature~ The reaction mixture i6 then added dropwi6e, over a
period of 3 hours, to a cooled (0 - 5 C) solution of diethoxy-
acetic acid piperidinyl amide (6.4 g) in 20 ml of dry diethyl-
ether. After the ~dition is complete, the reaction mixture is
stirred for two hours at 5 C. The mixture is then poured
into a cold 2 % sulfuric acid 301ution (50 ml). The solution i9
extracted with ether and the combined ether extracts are washed
witl- water and evsporsted to dryness to give a residue of crude
product, which is u6ed without purificstion in step b).
b) l,l-Diethoxy-2-hydroxy-2-(2,3-dihydro-1H-inden-2-yl)ethane
4 g of crude 2,3-dihydro-lH-inden-2-yl glyoxal diethyl acetal is
dissolved in 20 ml of ethanol and 3.~ R of sodium borohydride is
added in smsll portions at a tempersture below 30 C. After the
addition is complete, the mixture is stirred overnight at room
temperature. About 15 ml of ethanol i6 distilled off and 30 ml
of water is added. The solution is extracted with methylene
chloride. The combined methyle chloride extracts are washed with
water, dried with sodium sulfate, and evaporated to dryness. The
yield is 4 g of oil, which is used directly in 6tep c).
c) 4(5)-(2,3-Dihydro-lll-inden-2-yl)imidazole
-63- 1268~0
4 ~ of the oil from the preceding step and 15 ml of formamide
are combined and stirred at 150 C while passing ammonia gas
into the solution for 6 hours. The mixture is cooled to room
temperature and 40 ml of wflter is added. Concentrated hydro-
chloric acid is added with cooling until the p~l is 3-4.
The solution i6 washed with toluene, cooled and the p~l i8
adju6ted to 10 - 12 with 20 % ~odium hydroxide solution. The
mixture is extracted with methylene chloride and the combined
methylene chloride extracts nre extracted with 10 % acetic acid
solution. l`he combined acetic acid extracts are made alkaline
(pH 10 - 12) with 20 % sodium hydroxide solution. The product i8
extracted into chloroform, and the combined chloroform extracts
washed with water and dried with sodium sulfate. The solution is
evaporated to dryness to give the product as base.
The hydrochloride is prepared by dissolving the base in ethyl
acetate and adding llCl-ethyl scet~te until pll is 4. The
mixture is cooled and filtered nnd the filter cake washed with fl
small arnount of ethyl acetate. M.p. 185 - 193 C.
Example 14
4(5)-(1,2,3,4-Tetrahydronaphth-2-yl)imidazole
The starting material, 1-(1,2,3,4-tetrahydronaphth-2-yl)ethanone
can be prepared from l,2,3,4-tetrahydro-2-naphthoic acid chlori-
de for example by the procedure of Newman, M.S. and Mangham,
J.R. (J. Am. Chem. Soc. 71 (1949) 3342) or as described in this
application for many acid chlorides to afford the acetyl derivative6.
a) The mixture of 2-bromo-1-(1,2,3,4-tetrahydronaphth-2-yl)etha-
none and 2-bromo-1-(2-bromo-1,2,3,4-tetrahydronaphth-2-yl)etha-
none
1268770
-6~-
Bromination of 1-(1,2,3,4-tetrahydronnphth-2-yl)etllflnone (3.00
g) in methylene chloride with bromine (2.75 g)/methylene
chloride (10 ml) by the normal procedure described for example
in Example 5 affords the mixture of 2-bromo-1-(1,2,3,4-tetra-
hydronaphth-2-yl)etllanone and 2-bromo-1-(2-bromo-1,2,3,4-tetra-
hydronaphth-2-yl)ethanone.
2-bromo-1-(1,2,3,4-tetrahydronapllth-2-yl)ethanone:
MS: 254 and 252 (14 and 14, M+ ), 173 (54, M-Br), 159 (21,
M-CH2Br), 150 (11), 145 (25), 131 (46, M-COCH2Br), 130 (23), 129
(100), 128 (27), 127 (12), 116 (12), 115 (26), 91 (16).
2-bromo-1-(2-bromo-1,2,3,4-tetrahydronaphth-2-yl)ethanone:
MS: 334, 332 and 330 (invisible, M+'), 253 and 251 (96 and 100,
M-Br), 172 (32, M-Br-Br), 157 ~11), 153 (14), 130 (25,
M-Br-COC1l2Dr)~ 129 (81), 128 (56), 127 (22), 115 (20).
b) 4(5)-~1,2,3,4-Tetrahydronaphth-2-yl)imidazole
The mixture of 2-bromo-1-(1,2,3,4-tetrahydronaphth-2-yl)ethanone
and 2-bromo-1-(2-bromo-1,2,3,4-tetrahydronaphth-2-yl)ethanone is
heated with formamide as in the case of Example 4d to ~sfford a
mixture of 4(5)-(1,2,3,4-tetrahydronaphth-2-yl)imidazole and
probably both 4(5)-(1,4-dihydronaphth-2-yl)imidazole and 4(5)-
(3,4-dihydronaphth-2-yl)imidazole This mixture is directly
hydrated at 70 C as in Example 4e to provide crude 4(5)-
(1,2,3,4-tetrahydronaphth-2-yl)imidazole. The product as base is
purified by flash chromatography (solvent system: methylene
chloride/methanol 9.5/0.5). M.p. of the hydrochloride salt of
4(5)-(1,2,3,4-tetrahydronaphth-2-yl)imidazole lfi8-177 C.
The hydrochloride salt of 4(5)-(1,2,3,4-tetrshydronaphth-2-yl)-
imidazole:
12613770
-65-
MS: 198 (lOt), ~'), 197 (64), 183 (31), 170 (22), 169 (30), 130
(22), 129 (18~, 128 (23), 117 (16), 116 (10), 115 (30), 104
(77), 103 (23), 98 (12), 95 (12), g4 (12), 91 (1~), 82 (30), 81
(1.~) .
lH NMR t80 Mllz, MeOIl-d4): c~ 1.66-2.46 (211, m, -cH2cH2cH ),2.86-3.13 (511, m, 2 x ArCH2 and -Cl12ClIC112), 7.11 (411, 8, aroma-
tic), 7.34 (111, m, im-5(4)), 8.85 (111, d, 4J 1.54 Hz, im-2).
3C NMR (20 Mllz, MeOH-d4): ~ 29.23 (OFR t), 29.63 (t), 32.53
(d), 35.50 (t), 115.84 (d), 126.89 (d), 127.19 (d), 129.92 (2
d), 134.70 (d), 135.43 (6), 136.52 (8), 139.45 (6).
Example 15
4(5)-(2-Ethyl-1,2,3,4-tetrahydronaphth-2-yl)imidazole
a) 2-Ethyl-1,2,3,4-tetrahydro-2-nsphthoic acid methyl ester
1,2,3,4-Tetrahydro-2-napthoic acid methyl ester (prepared by
the methylation of 1,2,3,4-tetrahydro-2-naphthoic acid) is
converted to 2-ethyl-1,2,3,4-tetrahydro-2-naphthoic acid methyl
ester by the procedure of Rathke, M.V. and Lindert, A.,(J. Am.
Chem. Soc. 93 (1971) 2318). B.p. 90-95C/0.3 mmHg. Yield 88%.
1,213,4-Tetrahydro-2-naphthoic acid methyl ester:
lH NMR (80 MHz, CDC13): ~ 0.88 (3H, t, J 7.69 Hz, -CH2CH3),
1.53-3.34 (8H, m, -CH2CH3 and the methylene protonfi of the
ring), 3.64 (3H, 6, CoocH3), 7.07 (4H, 5, aromatic).
13c NMR (20 MHz, cDcl3): ~ 8.77 (OFR q), 26.26 (t), 30.23 (t),
31.05 (t), 36.83 (t), 46.09 (8), 51.51 (q), 125.62 (2d), 128.52
(d), 129.07 (d), 134.91 (8), 135.37 (6), 176.66 (~).
~26~770
-66-
b) 2-Ethyl-1,2,3,4-tetrallydro-2-naphtl)oic acid
The mixture of 2--ethyl-1,2,3,4-tetrahydro-2-naphthoic acid
methyl ester (32.3 g), sodium hydroxide (32.3 g), ethanol
~450 ml) and w~ter (323 ml) is refluxed for 8 hr. Ethanol is
largely distilled in vacuo, the residue diluted with water and
washed with ether. The aqueous solution gives an acidification
with hydrochloric acid the de6ired 2-ethyl-1,2,3,4-tetrahydro-2-
nsphthoic acid. The product i8 filterd. Yield 22.2 g, 73 %.
2-Ethyl-1,2,3,4-tetrahydro-2-naphthoic acid:
H NMR (80 Mllz, CDC13): d 0.83 (3H, t, J 7.69 Hz, -CH2C 3),
1.55-3.33 (8H, m, -C112CU3 and the methylene protons of the
rin~), 7.01 (3H, 8, aromatic), 11.45 (lH, broad 8, -COOH).
3C NMR (20 MHz, CDC13): ~ 8.75 (OFR q), 26,19 (t), 29.94 (t),
30.91 (t), 36.54 (t), 45.89 (8), 125.75 (2d), 128.63 (d), 129.14
(d), 134.68 (s), 135.35 (8), 183.06 (8).
c) 2-Ethyl-1,2,3,4-tetrahydro-2-naphthoic acid chloride
A mixture of 2-ethyl-1,2,3,4-tetrahydro-2-naphthoic acid
(22,0 2) and thionyl chloride is boiled for 5 days. The acid
chloride is distilled. B.p. 110-115 C/0.2 mm11~. Yield 21.6 g,
90 %.
2-Ethyl-1,2,3,4-tetrahydro-2-naphthoic acid chloride:
H NMR (80 Mhz, CDC13): ~ 0.97 (3H, t, J 7.69 Hz, -CH2C 3),
1.67-3.38 (811, m, -CH2CH3 snd the methylene protons of the
ring), 7.10 (4H, 8, aroma~tic).
~2~87~
-67-
13C NMR (20 Mllz, Cl)C13): ~ 8.36 (OFR q), 26.00 (t), 30.70 (t),
30.79 (t), 37.11 (t), 56.67 (6~, 126.05 (d), 126.24 (d), 128.66
(d), 129.08 (d), 133.38 (6), 134.65 (s), 178.46 (s).
d) 1-(2-Ethyl-1,2,3,4-tetrahydronaphtll-2-yl)ethanone
2-Ethyl-1,2,3,4-tetrahydro-2-naphthoic acid chloride is con-
verted to 1-(2-ethyl-1,2,3,4-tetrahydronaphth-2-yl)ethanone by
the procedure described for example in Example 4b.
e) 2-Bromo-1-(2-ethyl-1,2,3,4-tetrahydronaphth-2-yl)ethanone
Bromination of 1-(2-ethyl-1,2,3,4-tetrahydronaphth-2-yl)ethanone
by the procedure of Example 7d yields 2-bromo-1-(2-ethyl-
1,2,3,4-tetrahydronaphth-2-yl)ethanone.
2-Bro-no-1-(2-ethyl-],2,3,4-tetrahydronaphth-2-yl)ethanone:
MS: 282 and 280 (4 and 4, M~), 253 and 251 (8 and 8, M-CH2CH3),
201 (20, M-Br), 187 (28, M-CH2Br), 159 (22, M-COCH2), 157 (12),
145 (30), 131 (10), 130 (12), 129 (50), 128 (32), 127 (14), 117
(100), 115 (30), 91 (21), 77 (10), 43 (24).
f) 4(5)-(2-Ethyl-1,2,3,4-tetrahydronaphth-2-yl)imidazole
2-Bromo-1-(2-ethyl-1,2,3,4-tetrahydronaphth-2-yl)ethanone is
converted to 4(5)-(2-ethyl-1,2,3,4-tetrahydronaphth-2-yl)-
imidazole by the procedure of Example 7e. M.p. of the hydro-
chloride salt 148-156DC.
The hydrochloride salt of 4(5)-(2-ethyl-1,2,3,4-tetrahydro-
naphth-2-yl)imidazole:
MS: 226 (63, M~), 211 (17, M-CT13), 198 (25), 197 (100,
M-CH2CH3), 195 (17), 129 (15), 128 (12), 115 (13), 104 (20), 98
(14), 82 (19), 81 (30), 69 (11).
i
~ 1268770
-68-
lH N~ (80 MHz, MeOII-d4): ~ 0.79 (3H, t, J 7.52 Hz, -CH2CH3),
1.63-3.34 (8H, m, -CH2CH3 and the methylene protons of the
ring~. 7.02-7.14 (5H, m, aromatic and im-4), 8.74 (lH, d, 4J
1.37 Hz, im-2).
13C NMR (20 MHz, MeOll-d4): d 8.48 (OFR q), 26.70 (t), 33.30
(t), 34.00 (t), 38.54 (8), 39.48 (t), 117.91 (d), 126.99 (d),
127.11 (d), 129.66 (d), 130.14 (d), 135.02 (8), 135.20 (d),
136.20 (8), 140.40 (8).
Example 16
4(5)-(2,3-Dihydro-2-ethyl-1-methyl-lH-inden-2-yl)imidazole
a) ci6-2,3-Dihydro-l-methyl-lH-indene-2-carboxylic acid methyl
ester
_ -2,3-Dihydro-l-methyl-lH-indene-2-carboxylic scid methyl
ester i8 prepared from cis-2,3-dihydro-1-methyl-lH-indene-2-
carboxylic acid (see ~xample 4) by the standard procedures using
methanol and concentrated sulphuric acid. Yield 91 ~.
cis-2,3-Dihydro-l-methyl-lH-indene-2-carboxylic acid methyl
ester:
lH NMR (80 MHz, CDCI3): ~ 1.14 (3H, d, J 6.84 Hz, CHCH3),
2.76-3.66 (4H, m, Hl, H2 and H23 of the indane ring), 3.72 (3H,
a, -COOCH3), 7.17 (4H, 8, aromatic).
13C NMR (20 MHz, CDC13): ~ 17.01 (OFR q), 33.21 (t), 41.93 (d),
48.53 (d), 51.37 (q), 123.481 (d), 124.45 (d), 126.66 (d),
126.81 (d), 140.92 (8), 146.76 (8), 173.98 (8).
lZ6~37'70
-69-
b) 2,3-Dihydro-2-ethyl-1-methyl-lH-indene-2-carboxylic acid
methyl ester
2,3-Dihydro-2-ethyl-1-methyl-lH-indene-2-carboxylic acid methyl
e6ter is prepared by the procedure of Bathke, M.V. snd Lindert,
A. (J. Am. Chem. Soc. 93 (1971) 2318). B.p. 90-95 C/0.3 mmHg.
Yield 51 ~. The product i6 probably the mixture of two i60mers
(cis the major isomer, tran6 the minor i60mer).
2,3-Dihydro-2-ethyl-1-methyl-lH-indene-2-carhoxylic acid methyl
ester (the ci6-i60mer):
H NMR (RO Mllz, CDC13): ~ 0.86 (3H, t, J 7.18 Hz -CH2CH3),
1.122 (3H, d, J 7.18 Hz, ,CHCH3), 1.25-2.18 (2H, m, -CH2CH3),
3.10 (lH, q, J 7.18 Hz, ~ CHCH3), AB quartet: DA 2.82,
DB 3.52, JAB 16.41 Hz (211, H23 of the indane ring), 3.70
(3H, 6, C~OCR3), 7.15 (4H, 6, aromatic).
13C ~R (20 MHz, CDC13): ~ 9.84 (OFR q), 17.53 (q), 30.76 (t),
36.99 (t), 49.77 (d), 51.28 (q), 59.27 ~8), 123.60 (d), 124.60
(d), 126.45 (d), 126.63 (d), 140.74 (6), 146.52 (6), 175.52 (8).
c) 2,3-Dihydro-2-ethyl-1-methyl-lH-indene-2-carboxylic ocid
2,3-Dihydro-2-ethyl-1-methyl-lH-indene-2-carboxylic acid i6
synthe6ized by the pro~edure of Example 15b. Yield 97%.
2,3-Dihydro-2-ethyl-1-methyl-lH-indene-2-carboxylic acid (the
ci6-i60mer):
1H NMR (80 MHz, CDC13): ~ 0.93 (3H, t, J 7.18 Hz -CH2CH3),
1.23 (3H, d, J 7.18 Hz, ` CHCH3), 1.32-2.23 (2H, m, -CH2CH3),
3.13 (lH, q, J 7.18 Hz, ~ CHCH3), AB quartet: DA 2.83,
DB 3 49~ JAB 16.21 Hz (2H, H23 of the indane ring), 7.15
(4H, a, aromatic), 10.70 (lH, broad 6, -COOH).
3770
,
13C NMR (20 MRz, CDC13): S 9.81 (OFR q), 17.2h (q), 30.64 (t),
36.90 (t), 49.59 (d), 59.12 (8), 123.57 (d), 124.57 (d), 126.54
(d), 126.72 (d), 140.59 (8), 146.25 (8), 181.79 (8).
d) 2,3-Dihydro-2-ethyl-1-methyl-lH-indene-2-carboxylic acid
chloride
2,3-Dihydro-2-ethyl-1-methyl-lH-indene-2-carboxylic acid
chloride i5 prepnred by the 6tandard procedure using thionyl
chloride and has the boiling point 105C/0.3 mmHg. Yield 94 Z.
2,3-Dihydro-2-ethyl-l~methyl-lH-indene-2-carboxylic acid
chloride (the cis-isomer):
H NMR (80 MHz, CDC13): ~ 0.95 (3H, t, J 7.18 Hz -CH2CH3), 1.28
(3H, d, J 7.01 Hz,~ CHCH3), 1.40-2.31 ~2H, m, -CH2CH3), 3.18
(lH, q, J 7.01 Hz, ~CHCH3), AB quartet: DA 2.92, DB 3 50-
JAB 16.24 Hz (2H, R23 of the indane ring), 7.17 (4H, 8,
aromatic).
13C NMR (20 MHz, CDC13): cS 9.38 (OFR q), 17.86 (q), 30.88 (t),
36.60 (t), 49.74 (d), 68.75 (8), 123.75 (d), 124.87 (d), 127.02
(2d), 139.07 (8), 145.61 (8), 177.21 (8).
e) 1-(2,3-Dihydro-2-ethyl-1-methyl-lH-inden-2-yl)ethanone
1-(2,3-Dihydro-2-ethyl-1-methyl-lH-inden-2-yljethanone is
synthesized by the procedure of Example 4b.Yield 69~.
1-(2,3-Dihydro-2-ethyl-1-methyl-lH-inden-2-yl)ethanone (the cis-
isomer):
1~68770
-71
H NMR (80 MHz, CDC13): ~ 0.81 (311, t, J 7.18 Hz -CH2C113), 1.06
(3H, d, J 7.18 Hz, ~CHCH3), about 1.2-2.2 (211, m, -CH2CH3), 2.10
(3H, 8, OOCI13), 3.10 (lH, q, J 7.18 llz, ~CHCH3), AB quartet:
DA 2.75, DB 3 45~ JAB 16.41 Hz (2H, H23 of the indane
ring), 7.15 (411, 8, ~romatic).
13C NMR (20 MHz, CDC13): ~ 9.60 (OFR q), 17.35 (q), 27.55 (q),
29.82 (t), 35.33 (t), 49.04 (d), 64.93 (8), 123.60 (d), 124.87
(d), 126.60 (d), 126.75 (d), 140.80 (6), 146.67 (s), 210.85 (8).
f) 2-Bromo-1-(2,3-dihydro-2-ethyl-1-methyl-1}1-inden-2-yl)-
ethanone
2-Bromo-1-(2,3-dihydro-2-ethyl-1-methyl-lH-inden-2-yl)ethAnone
i~ prepared from 1-(2,3-dihydro-2-ethyl-1-methyl-lH-inden-2-yl)-
ethsnone (21.6 g) by treatment with bromine (17.6 g) in
methylene chloride (300 ml). Yield 65 %.
g) 4(5)-(2,3-Dihydro-2-ethyl-1-methyl-lH-inden-2-yl)imidazole
The procedure of Example lb i9 used to synthe6ize 4(5)-(2,3-
dihydro-2-ethyl-1-methyl-lH-inden-2-yl)imidazole. Yield 28 %.
The b~se obt~ined i6 converted to it3 hydrochloride ~al~t in dry
ether. The hydrochloride salt is recrystallized from ethyl
acetate - petroleum ether. The product is the mixture of two
isomers, ci6 85 ~ and tran6 15 %. The melting point of the
hydrochloride 6alt ifi 154-158C.
The hydrochloride salt of 4(5)-(2,3-dihydro-2-ethyl-1-methyl-
lH-inden-2-yl)imidazole (the mixture of the ci6- and trans-
i60mer, 85 % and 15 %):
MS: 226 (30, M~), 211 (15, M-CH3), 197 (100, M-CH2CH3), 182
(10), 129 (10), 128 (10), 115 (10), 91 (12).
12~;8770
--72--
lH NMR (80 MHz, MeOll-d4): ,~ 0.79 (3H, distorted t, 3J 7.35 Hz
-CH2C113), 0.95 (3H, d, 3J 7.18 Hz, ~CHCH3, the cis-ifiomer),
1.28 (d, J 7.18 Hz, ~CHCH3, the trsns-isomer), 1.47-2.27 (2H, m,
-CH2CH3), 2.99-3.48 (311, m, Hl and H23 protons of the indane
ring). 7.14-7.31 (511, m, ~romatic and im-4(5)), 8.90 (11l, d, 4J
1.54 Hz, im-5).
The cis-isomer 13C NMR (MeOIl-d4): ~ 9.72 (OFR q), 16.47 (q),
31.91 (t), 40.69 (t), 51.31 (d), 52.49 (s), 118.24 (d), 124.75
(d), 125.48 (d), 128.02 (2d), 135.08 (d), 138.B3 (s), 141.10
(~), 147.70 (~).
Example 17
4(5)-(2,3-Dihydro-2-n-propyl-lH-inden-2-yl)imidazole
4(5)-(2,3-Dihydro-2-n-propyl-lH-inden-2-yl~imidazole i6 prepared
according to the procedure of Example 7 using 2,3-dihydro-lH-
indene-2-carboxylic acid methyl ester and n-propyl bromide as
Ytarting m~terials. M.p. of the hydrochloride salt: 169-171C.
The hydrochloride salt of 4(5)-(2,3-dihydro-2-n-propyl-lH-inden-
2-yl)imidazole:
MS: 226 (25, M~), 197 (17, M-CH2CH3), 183 (100, ~CH2CH2CH3),
115 (13), 91 (17).
lH NMR (80 MHz, MeOH-d4): ~ 0.79-1.31 (5H, m, 0.88 distorted t,
CH2CH3), 1.79-1.99 (2H, m, _2CH2CH3), AB quartet: DA ' DB
3.23, JAB 16 4 Hz (4H, H21 and H23 of the indane ring), 7.05-
7.25 (4H, m, aromatic), 7.31 (lH, d, 4J 1.4 Hz, im-5(4)), 8.82
(lH, d, im-2, 4J 1.4 Hz).
126~770
-73
Ex~lmple 18
4(5)-(2,3-Dihydro-2-n-butyl-111-inden-2-yl)imidazole
4(5)-(2,3-Dihydro-2-n-butyl-111-inden-2-yl)imidazole i6 prepared
according to the procedure of Example 7 using 2,3-dihydro-111-
indene-2-carboxylic acid methyl ester and n-butyl bromide as
starting materials. M.p. of the hydrochloride salt: 129-132 C.
The hydrochloride salt of 4(5)-(2,3-dihydro-2-n-butyl--lH-inden-
2-yl)imidazole:
MS: 240 (22, Mh), 197 (12, ~C1l2CH2CH3), 183 (100, M-CH2CH2CH2-
CH3), 170 (24), 141 (23), 129 (10), 128 (10), 115 (15), 97 (11),
91 (17), 81 (16), 77 (38), 69 (16), 57 (18), 55 (17), 51 (10).
H NMR (8n MHz, MeOH-d4): ,~ 0.86 (3H, distorted t, CH3), 1.00-
1.50 (4H, m, CH2CH2C113), 1.81-2.()O (2H, m, CH2CH2CH2CH3), AB
quartet: DA = DB 3.23, JAB 16.4 Hz (4H, H21 and H23
protons of the indane ring), 7.05-7.25 (4H, m, aromatic), 7.31
(lH, d, 4J 1.4 Hz, im-5(4)), 8.81 (111, d, 4J 1.4 Hz, im-2).
Example 19
4(5)-(2,3-Dihydro-2-eth~yl-1-hydroxy-lH-inden-2-yl)imidazole
a) 4(5)-(2,3-Dihydro-2-ethyl-1-oxo-lH-inden-2-yl)imidazole
2-Acetyl-l-indanone (Liebigs Ann. Chem. 347 (1906) 112) is alkylated
with ethylhromide in acetone in the presence of sodiumcarbonate to 2-
acetyl-2-ethyl-1-indanone. Acetyl is brominated with bromine in methanol
and condensed to imidazole by heatin~ in formamide as before. The meltin~
point of the product as base is 126-127C (from ethyl acetate).
b) 4(5)-(2,3-Dihydro-2-ethyl-1-hydroxy-lH-inden-2-yl)imidazole
The carbonyl group of oxo inden imidazole from the step a) is reduced to
the alcohol group with sodium borohydride in ethanol. The product is the
iZ68~70
_ 7 D~-- i
mixture of cis-trans stereoisomers, the purification of which is
accomplished by liquid chromatographically.
cis-isomer as hydrochloride (m.p. 184-185C):
lH NMR (80 MHz, MeOH-d4): 0.73 (3H, t), 1.36 (2H, m), 3.36 (2H, m),
3.61 (3H, s), 5.15 (lH, s), 7.06 (lH, d), 7.2-7.4 (4H, m), 8.69 (lH, d)
trans-isomer as hydrochloride:
lH !lMR (80 MHz, MeOH-d4): 0.80 (3H, t), 1.84 (2H, m), 3.15 (2H, m),
3.24 (3H, s), 5.15 (lH, s), 6.87 (lH, d), 7.2-7.4 (4H, m), 8.54 (lH, d)
Example 20
4(5)-(2,3-Dihydro-2-ethyl-lH-inden-2-yl)-imidazole
The oxo derivative prepared in the example 19 (step a) or the hydroxy
derivative (step b) is hydrogenated in 2 N hydrochloric acid in the
presence of 10 % palladium on carbon at 70C. When the uptake of hydro~en
ceases the reaction mixture is filtered and made alkaline. The product
is extracted to methylene chloride which is washed with water, dried and
evaporated to dryness. From the residue, which is the product as base, is
made hydrochloride in ethyl acetate with dry hydroqen chloride.
M.p.: 211-215C.