Note: Descriptions are shown in the official language in which they were submitted.
1301~!352
.
~EDICAL ELECTRODE WITH RE~SABLE CONDUCTOR
Summary of the Invention
The present invention relates to medical electrodes for
trans;itting electrical signals between the skin of a subject,
such as a medical patient, and peripheral equipment for monitoring
signals derived from the skin of the subject. This invention also
relates to medical electrodes for applying stimulation signals to
the skin of a subject.
There is a continuing need for high quality but
inexpensive medical electrodes for ECG and related uses that
reliably transmit signals to enable traces to be obtained that
accurately represent signals produced~by a patient's heart. For
purposes of convenience and safety, such electrodes shoul(l be so
inexpensive that it is practical to dispose of them after only one
use. Accordingly, a primary object of this invention is to
provide an inexpensive, high quality medical electrode.
One approach to providing inexpensive monitoring or ECG
electrodes has been to provJide a disposable electrode pad
including an electrolyte and a carrier therefor and a reusable
electrode conductor which is attached to a cable or lead wire used
for connection to external monitoring equipment. It is generally
recognized that, in order to obtain high quality traces, the
portion of the electrode conductor engaged with the electrolyte
should be a substantially pure metal, either substantially pure
silver or a silver coated conductive plastic being preferred for
ECG's taken with the patient at rest. For electrode applications
in which signals are to be transmitted to the skin from external
equipment, such as transcutaneous nerve stimulation (TENS)
electrodes, the quality requirements of the conductor are not so
13~1852
high. For example, they may be made from conductive plastic or
from 1 esser expensive metals such as stainless steel.
Nevertheless, electrode conductors usually comprise the most
expensive part of a medical electrode. By providing a reusable
electrode conductor, substantial economies may be had because the
more expensive conductor may be reused many times while the less
expensive electrode pad is discarded after each use. This
invention takes advantage of this approach and it is a further
object of this invention to provide an improved, high quality
electrode having an inexpensive and disposable electrolyte pad and
reusable electrode conductor.
One of the important considerations in the construction
of an electrode of the type having a reusable conductor is the
manner in which the conductor is attached to the electrode pad.
In practice, the electrode pads are adhered to the skin of a
patient and the electrode conductors are thereafter connected to
the pads. Such connections, and subsequent disconnections, should
be readily made without causing cliscomfort to the patient. For
~ong term monitoring applications, the electrodes should have a
low profile to minimize the patient's discomfort and to enable the
patient to roll over in bed with little li~elihood of accidentally
pulling off the electrode or disrupting the connection between the
electrode lead wire and the electrolyte gel.
To obtain high quality traces, the connection should be
sufficiently secure that the electrode conductor is held firmly
engaged with the electrolyte. This is a particularly difficult
problem with electrodes used for long term monitoring and also
with electrodes used for stress testing wherein the patient is
physically quite active. Therefore, it is a further object of
this invention to provide a medical electrode of the type
. -2-
13018~;2
comprising a reusable conductor and a dispos;ble electrode pad
having an improved connection between the conductor and the
electrode pad whereby the conductor can be easily and securely
engaged with the electrolyte. A related object is to provide an
inexpensive electrode that has a low profile with an electrode
conductor-to-electrolyte connection sufficiently secure that the
electrode may be satisfactorily and comfortably used for long term
monitoring and stress testing applications as well as for less
demanding applications.
In accordance with this invention, a medical electrode is
provided having an electrode pad comprising a laminated assembly
of a flexible, electrically non-conductive, foam plastic body or
frame with a patient-contacting adhesive layer on its lower
sur-face. The foam frame has a bore filled with an electrolyte gel
matrix, preferably a conductive adhesive, a urethane hydrogel
being t~e material of choice. The electrode pad further comprises
an electrically non-conductive socket p~.te overlying the gel
matrix and the foam body to which it is .,dhesively secured. The
socket plate is provided with a socket for connection of an
electrode conductor to external monitoring equipment. The socket
preferably comprises a bore centrally located over the gel matrix.
For reasons to be described, the socket preferably has a release
coating on its top surface.
The electrode conductor has a low profile and is provided
with a short shank adapted to be inserted into the bc,re in the
socket plate. The conductor further includes a disc-like top
plate adapted to overlie the portion of the socket plate
surrounding the bore, so that it may be inserted into the bore of
the socket plate and project only slightly above it. A reusable
3~ ~ea~ wire ~a~7~ a ~ack ~ar con~ection to externa~ ~onitoling
equipment is attached to the top plate.
The lead wire may be adhered to or embedded in the top
-3-
130~85Z
plate of the electrode conductor. For monitoring purposes, the
entire electrode conductor may be made from a cond~ctive plastic
and coated with a silver paint or plating. A particularly low
profile may be obtained by embedding the end of the lead wire into
the edge of the conductor top plate. Rather than silver coating
the entire conductor, a small silver plated plastic plug may be
press fit within a bore in the conductive plastic body of the
electrode conductor. For an electrode intended to be used for
stimulation purposes, an uncoated conductive plastic electrode
conduc~or is preferred. This would usually have a larger
skin-facing area than a monitoring electrode.
Further in accordance with this invention, the laminated
assembly forming the electrode pad includes an electrode conductor
and lead wire clamp plate that comprises a flexible, electrically
non-conductive, foam plastic sheet or body with a pressure
sensitive adhesive layer on its lower surface. Part of the clamp
plate is strongly adhered to the frame and the rest of the clamp
plate is adhered to the release coated top of the socket plate.
In use, the electrode pad is applied to the skin of a subject, the
part of the clamp plate engaging the release coated socket plate
is peeled away from the socket plate, the electrode conductor is
inserted into the bore of the socket plate, and the clamp plate
re-adhered to the release coated top of the socket plate in
covering relation to the electrode conductor and the end of the
lead wire attached thereto. Accordingly, the lead wire and the
electrode conductor are securely held in place relative to the
electrolyte.
The shank of the electrode conductor may be slightly
oversized with respect to the bore of the socket plate and have
ridges so constructed that the portions of the soc~et plate
surrounding the bore tightly engage the conductor between the
ridges to enhance the security of the connection between the
1301~152
electrode conductor and the socket p~ate. As an alternativer the
shank may snugly fit within the bore of the socket plate and the
electrode conductor will be held in place primarily by the.clamp
plate.
Other objects and advantages of this invention will
become apparent from the following description and the drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
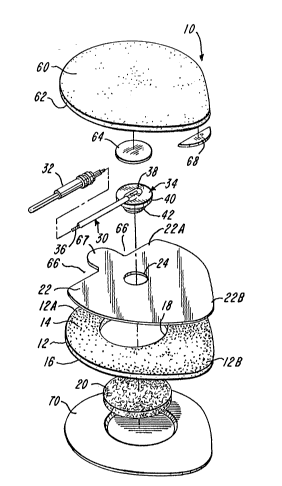
FIG. 1 is an exploded perspective view of a medical
electrode pad of this invention mounted on a release liner, a lead
wire cable thereforr and an electrode conductor.
FIG. 2 is a perspective view of the electrode pad ~nd the
release liner of FIG. 1 prior to use.
FIG. 3 is a perspective v'iew of the,electrode pad of FIG.
1 shown with the clamp plate peeled back to permit attachment of
the lead wire and the electrode conductor to the socket plater the
the lead wire being shown in fragmentary perspective and the
electrode conductor being shown exploded from its socket.
FIG. 4 is a perspective view of the electrode pad of FIG.
1 as it would appear during user with the the lead wire being
shown in fragmentary perspective
FIG. 5 is an enlargedr cross-sectional view of the
medical electrode pad and the release liner taken on line 5-5 of
FIG. 2.
FIG. 6 is an enlarged cross-sectional view of the medical
electrode p~ad, a fragment of the lead wirer and the electrode
conductor taken on line 6-6 of FIG. 4.
FIG. 7 is a greatly enlarged cross sectional view of the
electrode conductor and a fragment of the lead wire.
FIG. 8 is an exploded perspective view of the electrode
1301852
conductor and a fragment of the lead wire of a modification.
FIG. 9 is an enlarged cross sectional view of the
electrode conductor and a fragment of the lead wire forming the
modification of FIG. 8.
FIG. 10 is an exploded perspective view of a second
embodiment of a medical electrode pad of this invention, a
fragment of a lead wire therefor, and an electrode conductor.
FIG. 11 is a fragmentary cross-sectional view of a
medical electrode pad, a fragment of the lead wire, and a third
embodiment of an electrode conductor.
DESCRIPTION OF THE PREFERRED El`IBODIMENTS
With reference to FIGS. 1 to 7, a medical electrode
according to the present invention includes a disposable
electrolyte carrier or electrode pad generally designated 10 which
comprises a laminated assembly of a flexible, generally
ring-shaped body or frame 12 having adhesive layers 14 and 16 on
its upper and lower faces, respectively. The frame 12 is made
from electrically non-conductive foam material and may comprise
any of a wide variety of closed cell thermoplastic foams which are
well known in the art, the material of choice for the present
invention being a polyethylene foam. Adhesive layers 14 may be
any suitable adhesive usable with the frame 12 and the layer 16
may comprise any conventional, electrically non-conductive
pressure sensitive adhesive of the type generally known as
"patient contact" adhesives which may be safely used to affix the
electrode pad 10 to the skin of a patient.
The frame 12 has a circular bore 18 filled with a
generally cylindrical matrix 20 of electrolyte gel, the gel matrix
20 preferably comprising a conductive adhesive and having a
130~852
thickness greater than the frame 12. Various conductive adhesive
materials may be used depending upon the applicàtion for which the
electrode is intended. The l;laterial of choice for the present
application is a urethane hydrogel which is of a gelatinous
consistency and which contains an electrolyte in an amount
sufficient to render it electrically conductive. The electrolyte
comprises an ionizable salt compatible with the metal used to form
the electrode conductor which will be described below. These are
well known in the art; examples are the use of sodium chloride
L0 when the conductor is made from or coated with silver, as is
presently preferred for monitoring purposes, and the use of sodium
sulfate with stainless steel.
Alternate materials that may be used for the electrolyte
include a commercially available conductive adhesive composition
comprising karaya gum modified with sodium chloride, available
from LecTec Corporation, 120 South Crosstown Circle, Eden Prairie,
Minnesota. Various other conductive adhesive compositions that
may be usable are described in the following United States
Patents: Marks et al. No. 3,357,930; Kater No. 3,993,049: Berg
No. 4,066,078: ~ymes No. 4,125,110: Cross et al. No. 4,141,366:
and Hymes No. 4,274,420. Whatever the composition of the
conductive adhesive, it should be of the type which will adhere to
the skin of a patient and will have a cohesive strength sufficient
to substantially maintain its shape and to permit it to be peeled
from the skin to which it is attached without leaving any
appreciable residue.
The shape of the frame 12 is not critical to its
function, except that, for most applications, the gel receiving
bore 18 is preferably generally centrally located and completely
surrounded by the frame 12 to avoid drying out of the electrolytiç
~3~1~352
gel. The frame 12 shown in the drawing has a generally
semi-circular "rear" portion l~A and a rounded triangular "front"
portion 12B. This particular configuration is use~ul for pulposes
which will be described below.
Overlying the gel matrix 20 and the frame 12 is a socket
plate 22 adhered to the frame 12 by the adhesive layer 14 on the
upper surface of the frame 12. Socket plate 22 performs the
functions of maintaining the shape of the electrode pad 10, of
providing a covering for the gel matrix 20, and of providing a
socket for connection of the electrode pad 10 to external
monitoring equipment. The socket is in the form of a circular
bore 24 extending through the socket plate 22 for the attachment
of a reusable electrode conductor and lead wire as will be
described below. Socket bore 24 has a substantially smaller
diameter than the gel-receiving bore 18 in the frame 12. It is
generally centrally located within the socket plate 22 and is
coaxial with the center of the gel matrix 20. The socket plate 22
comprises a relatively stiff sheet of electrically non-conductive
thermoplastic material such as styrene, vinyl, or polyethylene
terephthalate *(Mylar). In general, the socket plate 22 should be
resiliently flexible but sufficiently stiff that it will firmly
hold the electrode conductor within the socket 24 as will be
described below. Mylar sheet having a thickness of approximately
4 or 5 mils or vinyl sheet of approximately 5 or 6 mils are
examples of materials from which the socket plate 22 could be
made. The top surface of the socket plate 22 has a release
coating, such as a silicone, to render it partly resistant to
adhesives for reasons which will become apparent.
A reusable lead wire, generally designated 30, is
proviced, having a jack 32 at one end and an electrode conductor
~c , -8-
'~ *Trade Mark
130~852
34 at its other end, and functions to transmit signals to or from
the skin of a subject. Thus, the lead wire 30 has a conductive
wire 36 jacketed by an insulating sleeve preferably made from
silicone rubber or other adhesive-resistant material. A bare end
of the wire 36 opposite the jack 32 is fixedly attached, as by an
epoxy adhesive 38, to the electrode conductor 34. In the
contemplated use of this invention, the electrode pad 10 will be
discarded after each use, but the lead wire 30 and the electrode
conductor 34 will be repeatedly used with like electrode pads.
With reference to FIG. 7, the electrode conductor 34
comprises a one piece body having a circular, disc-like top plate
40 and a short, generally cylindrical shank 42 depending centrally
therefrom. Shank 42 is adapted to extend through and be retained
by the socket plate bore 24 so that it becomes lodged in the gel
matrix -20 as shown in FIG. 6. There it will be noted that the
length of the shank is approximately the same as, or less than,
the combined thicknesses of the frame 12 and the socket plate 22.
For monitoring purposes, it is preferred that the
electrode conductor 34 comprise substantially pure silver or be
plated or coated with substantially pure silver and that its outer
surface be chlorided. Since low cost is important, the presently
preferred electrode conductor for monitoring purposes comprises a
nylon rendered conductive by inclusion of carbon that preferably
has a silver coating, which may be painted or plated, at least
along a portion of its surface that engages the gel matrix 20. It
would also be possible to use a silver plated non-conductive
plastic, such as ABS, but it is believed that such an electrode
conductor may have a sufficient portion of its silver plating
removed by abrasion resulting from repeated applications to the
electrode pads that the continuity of the silver plating may be
lost so quickly that the useful life of the electrode conductor
13018S2 ~
would be unsatisfactorily limited. Other metals, such as
stainless steel, could be used for short term monitoring, but
silver provides the highest quality traces. A conductive plastic,
such as the nylon material described above, could be used for
stimulation electrodes by which signals are transmitted from
external equipment to the skin of a patient.
Referring again to FIG. 7, the cylindrical shank 42 of
the electrode conductor 34 illustrated therein has a concave
bottom surface 44, a beveled lower outer wall 46 in the form of an
inverted, truncated cone, sloping upwardly and outwardly from the
bottom surface 44 that terminates at its upper end in a circular
ridge or shoulder 48. Above the ridge or shoulder 48, there are
plural additional circular ridges or shoulders 50 formed at the
upper ends of each of plural body sections that also are in the
form of inverted, truncated cones but which have a lesser height
than the lower wall surface 46. Accordingly, the entire length of
the shank 42 is provided with plural, closely-spaced, parallel
ridges or shoulders 48 and 50 separated by plural circular
grooves, designated 52.
The ridges or~shoulders 48 and 50 have a diameter
slightly greater, on the order of .010 to .020 inch, than the
diameter of the socket bore 24. As an example, an electrode pad
having a socket bore diameter of approximately .344 inch may be
used with an electrode conductor having an outermost ridge
diameter of approximately .355 inch.
Referring to FIGS. 1 to 5, the laminated assembly
forming the electrode pad 10 further comprises a flexible top or
clamp plate 60 having an adhesive layer 62 on its bottom surface.
A release paper disc 64 is adhered to the center bottom of the
clamp plate 60 in coaxial alignment with the bores 18 and 24. The
-10-
~L30~852
clamp plate 60 is made from electrically non-conductive foam
material and, or economy of manufacture, preferably comprises the
same material from which the frame 12 is made. Clamp plate 60
preferably has the same size and t`l~e same outer marginal
configuration as the frame 12 and is positioned on top of the
socket plate 22 and the frame 12 so that its outer margin is
coextensive or common with the outer margin of the frame 12O The
socket plate 22 is somewhat smaller than the frame 12 and the
clamp plate 60r so that parts of the frame 12 and the clamp plate
60, or more precisely their adhesive layers 14 and 62, are
strongly adhered to one another.
In the particular embodiment illustrated in FIGS. 1-5,
the outer margin of the front portion, designated 22B, of the
socket plate 22 is coextensive or common with both the frame front
portion 12B and the corresponding portion of the clamp plate 60, -
but the outer margin of its rear portion, designated 22A is
constructed to be spaced inwardly from, i.e., closer to the axis
of the bores 18 and 24, than the corresponding outer margins of
the frame rear portion 12A and the corresponding portion of the
clamp plate 60. Accordingly, the extreme rear portions of the
frame 12 and the clamp plate 60 are strongly adhered to one
another. The shape of the socket plate 22 is not critical, but it
is important that a substantial portion of the socket plate 22
extend to the common outer margin of the frame 12 and the clamp
plate 22 to separate them, and that a portion, usually of lesser
area than the latter portion, be effectively cut away so that the
frame 12 and the clamp plate 60 are directly adhered to one
another.
The rear portion 22A of the socket plate 22 is formed
with notches 66 uncovering a substantial portion of the rear
1;~011~52
portions of the frame 12 and the clamp plate 60. The notches 66
are separated by a boss 67 that does not extend to the outer
margin of the frame 12 and the clamp plate 60 so that the entire
rear portions of the frame 12 and the clamp plate 60 are strongly
adhered to one another. A small paper finger tab 68 is adhered to
the extreme front end of the adhesive layer on the bottom of the
clamp plate 60 as a convenience in lifting the clamp plate 60 from
the socket plate 22, as described below.
Referring to FIGS. 2 and 5, the electrode pad 10 is
mounted, as is conventional, on a release liner 70 that covers the
adhesive layer 16 and the lower surface of the gel ~atrix 22 and
from which the electrode pad 10 would be removed immediately prior
to use. The release liner 70 may comprise a sheet of silicon
coated paper, styrene, or the like, formed to the same outer
marginal shape as the frame 12. The assembled electrode pad 10
and release liner 70 may be packaged along with several other
electrode pads 10 mounted on release liners 70 for shipment and
storage in a substantially air and moisture vapor impervious
package or envelope, which may comprise a conventional plastic and
metal foil laminate.
I~hen an electrode pad 10 of this invention is to be used,
it is stripped o~f the release liner 70 and pressed onto the skin
of a patient to which it is adhered, primarily by the adhesive
layer 16 on the bottom of the frame 12 and also by the inherent
tackiness of the gel matrix 20 The clamp plate 60 is then peeled
upwardly and rearwardly away from the socket plate 22, as shown in
FIG. 3, to expose the socket bore 24 and to thereby permit
insertion of the shank 42 of the electrode conductor 34 into the
the area occupied by the gel matrix 20. Of importance at this
time is the adhesion between the mutually contacting portions of
:
~301852
the socket plate 22 and the clamp plate 60, which strongly resists
complete removal of the clamp plate 60 from the frame 12. As a
result, the clamp plate 60 bends or hinges where it overlies the
rear edge portions of the socket plate 22 and remains adhered to
the frame 12 as it is peeled back to expose the socket bore 24.
After the clamp plate 60 is peeled back as shown in FIG.
3, the electrode conductor 34 is inserted into the bore 24 of the
socket plate 22 and the clamp plate 60 is then returned into
overlying engagement with the front portion of the socket plate
22. The parts will then appear as shown in FIGS. 4 and 6, with
the clamp plate f.0 overlying the electrode conductor 34 and the
adjacent end of the lead wire 30, which are effectively clamped
between the socket plate 60 and the clamp plate 22. The rele.;e
paper disc 64 is sufficiently large that it, and not the adhesive
layer 62, engages the top of the el-~ctrode conductor 34. Here it
may be noted that FIG. 6 shows the hank 42 inserted into the bore
24 of the electrode pad 10, but the electrode pad 10 is not shown
applied to the skin of a patient. In practice, such would
ordinarily not be done.
When the electrode is no longer needed, the clamp plate
60 is again peeled back so that the electrode conductor 34 may be
removed from the socket bore 24. The electrode pad 10 may then be
removed from the subject and discarded.
For proper operation, the clamp plate 60 should adhere to
the socket plate 22 with a sufficient bond that the electrode
conductor 34 and the adjacent end of the lead wire 30 will be
firmly clamped between the socket plate 22 and the clamp plate 60
throughout the duration of the monitoring procedure. On the other
hand, it should be reasonably easy to peel the clamp plate 60 off
the socket plate 22. Those familiar with adhesives of the type
used with medical electrodes will be aware that the adhesive layer
62 on the bottom surface of the clamp plate 60 and the release
130~852
coating on the top surface of the ~ocket plate ~2 can be
formulated and applied to achieve the desired adhesive strength
between the socket plate 22 and the clamp plate 60. Also, the
adhcsive layer 62 should readily peel away from the underlying
portion of the lead wire, which it will do if the lead wire jacket
is made from silicone rubber or the like.
With reference to FIGS. 6 and 7, because the lowest ridge
or shoulder 48 of the electrode conductor shank 42 has a diameter
greater than the bore 24, the beveled lower outer wall 46 of the
shank 42 pushes the margins of the socket bore 24 downwardly as
the shank 42 is inser'ed therein. This is feasible because the
socket plate 22 is resiliently flexible and because the electrode
conductor shank 42 is only minimally larger than the bore 26.
Ultimately, the ridge 48 passes the bore 24 and, due to the
resiliency of the socket plate 22, the margin of the bore 24 is
biased to enter the groove 52 immediately above the lowest
shoulder 48. As shown in FIG. 6, when the electrode conductor 34
passes through the bore 24, it becomes intimately engaged with the
gel matrix 20. The bottom surface 44 of the shank 42 is made
concave to provide a pocket for receiving the gel. Accordingly,
the distance by which the gel matrix 20 is displaced downwardly
upon connection of the lead wire 30 in the electrode pad 10 is
minimized.
It will be appreciated that the electrode assembly shown
in FIGS. 4 and 6 has an extremely low profile, adding to the
thickness of the electrode pad only the thickness of the conductor
top plate 40, the lead wire 30 and the epoxy adhesive 38.
Further, it is seen that the electrode assembly of FIGS. 4 and 6
meets all of the objects of the invention, and in general
constitues an inexpensive, high quality electrode that is easily
assembled and dissasembled, comfortable to use, and, because of
its low profile and the clamping of the conductor 34 and the
-14-
130~852
adjacent end of the lead wire 30, may reliably be used for both
long term and stress monitoring applications and for other
applications as well.
In the modification of FIGS. 8 and 9, a lead wire
assembly 72 has a conducive wire 74 fixedly attached to an
electrode conductor 76 by an insert molding process that embeds
the end of the wire 74 in the edge of the top plate 78 of the
electrode conductor 76. The electrode assembly of FIGS. 8 and 9
may be used with the electrode pad 10 of FIG5 1-5. As apparent,
the profile of this construction is even lower than the first
described embodiment.
FIGS. 8 and 9 show another variation, in which the
electrode conductor 76 comprises a hollow, generally cylindrical
body made from conductive plastic in the bore of which a silver
plated,' non-conductive plastic plug 80 is inserted, the plug 80
providing proper contact to the gel matrix of the electrode pad.
FIG. 10 shows a medical electrode construction
particularly adapted to transmit stimulation signals to the skin
of a patient rather than transmit signals from the skin. Thus,
the pad 90 has a frame 92 with a bore 94 filled with an
electrolyte gel matrix 96 and a socket plate 98 with a bore 100
adapted to receive the shank'of an electrode conductor 102
attached to a lead wire assembly 104. Overlying the socket plate
98 is a clamp plate 106 provided with a centrally located release
paper member 108 and a paper finger tab 110. Prior to assembly,
the electrode pad 90 may be mounted on a release liner 112.
Except for the electrode conductor 102, the various parts of this
embodiment may be made from the same materials and they cooperate
in the same manner as the corresponding parts of the embodiment of
FIGS. 1-7. One difference between this embodiment and the
-15-
1301852
embodiment of FIGS. 1-7 is that the diameter of the shank of the
electrode conductor 102 is substantially greater than the shank 42
of the first embodiment so that the electrode may be used to apply
stimulation signals to a relatively large area of the patients
skin. The bores 94 and 100, and the gel matrix 96 are
correspondingly greater in diameter. In general, the entire pad
90 may have a larger skin-facing area to accomodate the larger
electrode conductor. Another difference between this embodiment
and the first embodiment is that the electrode conductor 102 may
be made from a conductive plastic or from stainles steel or some
other relatively inexpensive material, since the reliablity of a
silver conductor is not needed.
FIG. 11 shows another ~mbodiment of an electrode in
accordance with this invention which may be construct~d as either
a monitoring or a stimulation electrode. Again, corresponding
parts may be the same as previously described. Thus, the
electrode of FIG. 11 comprises an electrode pad 120 having a frame
122, a gel matrix 124, a socket plate 126 with a bore receiving an
electrode conductor 130 having a top plate 132 and a shank 134~
The electrode conductor 130 is attached to a lead wire 138, such
as by an adhesive 140. A socket plate 142 overlies the electrode
conductor 130, the adjacent end of the lead wire 138 and the
socket plate 122, and cooperates therewith in the same manner as
described above in reference to FIGS. 1-5. The difference is that
the electrode conductor shank 134 of this embodiment has a smooth,
cylindrical configuration and simply snugly fits within the bore
of the socket plate 126 so that, in use, the electrode conductor
130 will be held in place almost solely by the clamp plate 142.
From the foregoing description, it may be seen that
inexpenSive electrodes are provided that may be used for long term
-16-
130185Z
or short term monitoring applications, for stress testing, for
rest testing, and, with modifications, for stimulation purposes.
The electrodes may be also be made in small sizes for neonatal
monitoring. Thus, this invention provides a medical electrode
S construction with nearly universal applications.
Although the presently preferred embodiment of this
invention has been described, it will be understood that various
changes may be made within the scope of the appended claims.