Note: Descriptions are shown in the official language in which they were submitted.
~~'4JQ~~
F-5823
~BQ.~u~~lQ~1_Q.~_.~~5.~_Y~~~Q~~~y_~~~~~_L~I~~.I~~~~~
Mineral oil based lubricants are conventionally
produced by~ a separative sequence carried out in the
petroleum refinery which comprises fractionation of a
paraff inic crude oil under atmospheric pressure followed
by fractionation under vacuum to produce distillate
fractions (neutral oils) and a residual fraction which,
after deasphalting and severe solvent treatment may also
be used as a lubricant basestock usually referred to as
bright stock. Neutral oils, after solvent extraction to
remove low viscosity index (V.I.) components are
conventionally subjected to dewaxing, either by solvent or
catalytic dewaxing processes, to the desired pour point,
after which the dewaxed lubestock may he hydrofinished to
improve stability and remove color bodies. This
conventional technique relies upon the selection and use
of crude stocks, usually of a paraffinic character, which
produce the desired lube fractions of the desired
qualities in adequate amounts. The range of permissible
crude sources may, however, be extended by the lube
hydrocracking process which is capable of utilizing crude
stocks of marginal or poor quality, usually with a higher
aromatic content than the best paraffinic cruder. The
lube hydrocracking process, which is well established in
the petroleum refining industry, generally comprises an
initial hydrocracking step carried out under high pressure
in the presence of a bifunctional catalyst which effects
partial saturation and ring opening of the aromatic
-2-
2045006
components which are present in the feed. The
hydrocracked product is then subjected to dewaxing in
order to reach the target pour point since the products
from the initial hydrocracking step which are paraffinic
in character include components with a relatively high
pour point which need to be removed in the dewaxing step.
Current trends in the design of automotive
engines are associated with higher operating temperatures
as the efficiency of the engines increases and these
higher operating temperatures require successively higher
quality lubricants. One of the requirements is of higher
viscosity indices (V.I.) in order to reduce the effects of
the higher operating temperatures on the viscosity~of the
engine lubricants. High V.I. values have conventionally
been attained by the use of V.I. improvers, e.g.
polyacrylates, but there is a limit to the degree of
improvement which may be effected in this way. In
addition, V.I. improvers tend to undergo degradation under
the effects of high temperatures and high shear rates
encountered in the engine, the more stressing conditions
encountered in high efficiency engines resulting in even
f aster degradation of ails which employ significant
amounts of V.I. improvers. Thus there is a continuing
need for automotive lubricants which are based on fluids
of high viscosity index and which are stable to the high
temperature, high shear rate conditions encountered in
modern engines.
Synthetic lubricants produced by the
polymerization of olefins in the presence of certain
catalysts have been shown to possess excellent V.I.
values, but they are expensive to produce by the
conventional synthetic procedures and usually require
-3-
2045096
expensive starting materials. There~is therefore a need
for the production of high V.I. lubricants from mineral
oil stocks which may be produced by techniques comparable
to those presently employed in petroleum refineries.
In theory, as well as in practice, lubricants
should be highly paraffinic in nature since paraffins
possess the desirable combination of low viscosity and
high viscosity index. Normal paraffins and slightly
branched paraffins, e.g. n-methyl paraffins, are waxy
materials which confer an unacceptably high pour point on
the lube stock and are therefore removed during the
dewaxing operations in the conventional refining process
described above. It is, however, possible to process waxy
feeds in order to retain many of the benefits of their
paraffinic character while overcoming the undesirable pour
point characteristic. A severe hydrotreating process for
manufacturing Iube oils of high viscosity index is
disclosed in B~y~~Qpm~~~~_j<D_y~~y3c~~~og, PD 19(2), 221-
228, S. Bull et al, and in this process, waxy feeds such
as waxy distillates, deasphalted oils and slack waxes are
subjected to a two-stage hydroprocessing operation in
which an initial hydrotreating unit processes the feeds in
blocked operation with the first stage operating under
higher temperature conditions to effect selective removal
of the undesirable aromatic compounds by hydrocracking and
hydrogenation. The second stage operates under relatively
milder conditions of reduced temperature at which
hydrogenation predominates, to adjust the total axomatic
content and influence the distribution of aromatic types
in the final product. The viscosity and flash point of
the base oil are then controlled by topping in a
subsequent redistillation step after which the pour point
-4- ,
~o4soss
of the final base oil is controlled by dewaxing in a
solvent dewaxing (MAR-toluene) unit. The slack waxes
removed from the dewaxer may be reprocessed to produce a
base oil of high viscosity index.
Processes of this type, employing a waxy feed
which is subjected to hydrocracking over an amorphous
bifunctional catalyst such as nickel-tungsten on alumina
or silica-alumina are disclosed, for example, in GB-A-
1,429,494, 1,429,291 and 1,493,620 and US-A-3,830,273,
3,776,839, 3,794,580 and 3,682,813. In the process
described in GB 1,429,494, a slack wax produced by the
dewaxing of a waxy feed is subjected to hydrocracking over
a bifunctional hydrocracking catalyst at hydrogen
pressures of 2,000 psig (13881 kPa) or higher, followed by
dewaxing of the hydrocracked product to obtain the desired
pour point. Dewaxing is stated to be preferably carried
out by the solvent process with recycle of the separated
wax to the hydrocracking step.
In processes of this kind, the hydrocracking
catalyst is typically a bifunctional catalyst containing a
metal hydrogenation component on an amorphous acidic
support. The metal component is usually a combination of
base metals, with one metal selected from the iron group
(Group VIII} and one metal from Group VIB of the Periodic
Table, for example, nickel in combination with molybdenum
or tungsten. Modifiers such as phosphorus or boron may be
present, as described in GB 1,350,257, GB 1,342,499, GB
1,440,230, FR 2,123,235, FR 2,124,138 and EP 199,394.
Boron may also be used as a modifier as described in GB
1,440,230. The activity of the catalyst may be increased
by the use of fluorine, either by incorporation into the
catalyst during its. preparation in the form of a suitable
-5-
~0450~6
fluorine compound or by ~~_~~tg fluoriding during the
operation of the process, as disclosed in GB 1,390,359.
Although the process using an amorphous catalyst
for the treatment of the waxy feeds has shown itself to be
capable of producing high V.I.,lubricants, it is not
without its limitations. At best, the technique requires
a significant dewaxing capability,. both in order to
produce the feed as well as to dewax the hydrocracked
product to the desired pour point. The reason for this is
that although the amorphous catalysts are effective for
the saturation of the aromatics under the high pressure
conditions which are typically used (about 2,000 psig)
their activity and selectivity for isomerization of the
paraffinic components is not as high as might be desired;
the relatively straight chain paraffins are not,
therefore, isomerized to the Less waxy isoparaffins of
relatively high viscosity index but with low pour point
properties, to the extent required to fully meet product
pour point specifications. The waxy paraffins which pass
through the unit therefore need to be removed during the
subsequent dewaxing step and recycled, thus reducing the
capacity of the unit. The restricted isomerization
activity of the amorphous catalysts also limits the
single-pass yields to a value below about 50 percent, with
the corresponding waxy conversion being about 30 to 60$,
even though higher yields would obviously enhance the
efficiency of the process. The product V.I. is also
limited by the isomerization activity, typically to about
145 at OoF (-lBoC) pour point in a single pass operation.
The temperature requirement of the amorphous catalysts is
also relatively high, at least in comparison to zeolite
catalysts, typically being about 700 to 800oF (371 to
-6-
427oC) .
2045096 '
Another approach to the upgrading of waxy feeds
to high V.I. lubricant basestocks is disclosed in US-A-
4,919,788 and 4,975,177, in which a waxy feed, typically a
waxy gas oil, a slack wax, or a deoiled wax, is
hydroprocessed over a highly siliceous zeolite beta
catalyst. Zeolite beta is known to be highly effective
for the isomerization of paraffins in the presence of
aromatics, as reported in.US 4,419,220, and its
capabilities are effectively exploited in the process of
US 4,919,788 and 4,975,177 in a manner which optimizes the
yield and viscometric properties. of the products. The
zeolite beta catalyst isomerizes the high molecular weight
paraffins contained in the back end of the feed to less
waxy materials while minimizing cracking of these
components to materials boiling outside the lube range.
The waxy paraffins in the front end of the feed are
removed in a subsequent dewaxing step, either solvent or
catalytic, in order to achieve the target pour point. The
combination of paraffin hydroisomerization with the
subsequent selective dewaxing process on the front end of
the feed is capable of achieving higher product V.I.
values than either process on its own and, in addition,
the process may be optimized either for yield efficiency
or for V.i. efficiency, depending upon requirements.
While this zeolite-catalyzed process has shown
itself to be highly effective fox dealing with highly
paraffinic feeds, the high isomerization selectivity of
the zeolite beta catalysts, coupled with its lesser
capability to remove low quality aromatic components, has
tended to limit the application of the process to feeds
which contain relatively low quantities of aromatics: the
- 7 - o
X045096
aromatics and other polycyclic materials are less readily
attacked by the zeolite with the result that they pass
through the process and remain in the product with a
consequent reduction in V.I. The lube yield also tends to
be constrained by the low wax isomerisation selectivity at
low conversions and by wax cracking out of the lube
boiling range at high conversions: maximum lube yields are
typically obtained in the 20 to 30 weight percent
conversion range (650oF+ conversion). It would therefore
be desirable to increase isomerization selectivity and
simultaneously to reduce hydrocracking selectivity in
order to improve lube yield while retaining the high V.I.
numbers in the product.
In summary, therefore, the processes using
amorphous catalysts can be regarded as inferior in terms
of single pass conversion and overall yield because the
amorphous catalysts are relatively non-selective for
paraffin isomerization but have a high activity for
cracking so that overall yield remains low and dewaxing
demands are high. The zeolite-catalyzed process, by
contrast, is capable of achieving higher yields since the
zeolite has a much higher selectivity for paraffin
isomerization in the presence of polycyclic components but
under the moderate hydrogen pressures used in the process,
the aromatics are not effectively dealt with in lower
quality feeds and operation is constrained by the
differing selectivity factors of the zeolite at different
conversion levels.
We have now found that high quality, high
viscosity index (V. I.) lubricants can be readily produced
by a wax hydroisomerization process, using zeolite
catalysts of controlled low acidity at high pressures,
-8- 2045096 '
which is capable of being operated with feeds of varying
composition to produce high quality lube basestocks in
good yield, producing low pour point products with very
high viscosity indices. Although the product V.I. is
dependent upon the composition of the feeds, especially
its wax content, high V.I. values typically above about
140, usually in the range of 140 to 155, may be obtained
with the preferred slack Wax feeds with values of 143 to
147 being typical. Compared to the process using
amorphous catalysts, yields are higher and~the dewaxing
requirement for the product is markedly lower due to the
effectiveness of the process in converting the waxy
paraffins, mainly linear and near linear paraffins, to
less waxy isoparaffins of high viscosity index.
According to the present invention a process for
producing a high viscosity index lubricant having a
viscosity index of at Least 125, from a petroleum wax feed
having a paraffin content of at least 40 weight percent,
comprises isomerizing waxy paraffins present in the feed
in the presence of hydrogen at a hydrogen partial pressure
of at least 6991 kPa (1000 psig) and in the presence of a
low acidity zeolite isomerization catalyst having an alpha
value of not more than 20 and comprising a noble metal
hydrogenation component on a porous, zeolite support
material, to isomerize waxy paraffins to less waxy
isoparaffins. The feed typically comprises a petroleum
Wax having a wax content of at least 60 weight percent and
an aromatic content of from 5 to 20 weight percent, such
as a slack wax having an aromatic content of from 8 to 12
weight percent. Deoiled waxes and solvent refined
raffinates may also serve as feed.
The isomerization catalyst preferably comprises
-9-
X045096
a zeolite beta isomerization catalyst having an alpha
value not greater than 10, advantageously not greater than
5. Suitable catalysts include boron-containing zeolite
beta in which the boron is present as a framework
component of the zeolite beta. The zeolite is usually
composited with a matrix material, and in a favored
embodiment comprises from 0.5 to 2. weight percent
platinum.
The hydroisomerization may be carried out at a
hydrogen partial pressure of 10451 to 17326 kPa (1500 to
2500 psig), the conversion to 343oC- (650oF-) product of
not more than 30 weight percent, based on the feed to the
isomerization step, suitably from 10 to 20 weight percent
based on the feed to the isomerization step. The
temperature at which the isomerization step is carried out
is preferably not greater than 427oC (800oF), preferably
from 316 to 427oC) (600 to 800oF).
The hydroisomerized product may be subaected to
a dewaxing to achieve a target pour point, with a loss
during the dewaxing of not more than I5 weight percent.
The product generally has a V.I. of 130 to 150.
In the process, the paraffins present in the
feed are selectively converted to iso-paraffins of high
V.I. but lower gour point so that a final Tube product of
good viscometric properties is produced with a minimal
degree of subsequent dewaxing. A low acidity zeolite
hydroisomerization catalyst is employed, in which the
zeolite component is zeolite beta in one of its low
acidity forms. A noble metal, preferably platinum, is
used to provide hydrogenation-dehydrogenation
functionality in this catalyst in order to promote the
desired hydroisomerization reactions. The process is well
-10-
~o~5o~s
suited for upgrading waxy feeds such as slack wax with
aromatic contents greater than about 15 weight percent to
high viscosity index lubricating oils with high single
pass yields and a limited requirement for product
dewaxing.
The yield benefits associated with the use of
the low acidity hydroisomerization_catalysts at the high
hydrogen pressure used according to the invention are
unexpected since the use of high hydrogen pressures with
catalysts of higher acidity has been shown~to result in
lower isomerization selectivity.
~~ a~3D9~
In the accompanying drawings Figures 1 and 2 are
graphs illustrating the results of wax hydroprocessing
experiments reported in the Examples.
The invention is capable of operation with a
wide range of feeds of mineral oil origin to produce a
range of lubricant products with good performance
characteristics, especially of low pour point and high
viscosity index. The quality of the product and the yield
in which it is obtained is dependent upon the quality of
the feed and its amenability to processing by the present
catalysts; products of the highest V.I, are obtained by
using the preferred wax feeds described below but products
with lower V.T. values may also be obtained from other
feeds which contain a lower initial quantity of waxy
components which are converted into high V.I. iso-
paraffins by the isomerization catalyst. The use of feeds
with lower wax contents may also result in lower yields,
particularly if the feed preparation or processing is
-11-
2045096
carried out under conditions to maximise the V.I. since
then it is necessary to remove the lower quality
components at some point or another, with the concomitant
effect on yield.
The feeds which may be used should have an
initial boiling point which is no lower than the initial
boiling point of the desired lubricant. Because this is
usually about 650oF (about 343oC) or higher, the feed will
normally be a 650oF+ (about 343°C+) fraction. Feeds of
this type which may be used include vacuum~gas oils as
well as other high boiling fractions such as distillates
from the vacuum distillation of atmospheric resids, and
raffinates from the solvent extraction of such distillate
fractions.
The feed may require preparation in order to be
treated satisfactorily in the hydroisomerization step.
The preparation steps which are generally necessary are
those which remove low V.I. components such as aromatics
and polycyclic naphthenes. Removal of these materials
will result in a feed for the hydroisomerization step
which contains higher quantities of waxy paraffins which
are then converted to high V.I., low pour point iso-
paraffins. In order to produce the highest quality lubes,
i.e, materials having a V.I. above 140, the feed to the
hydroisomerization step should have a V.I. of at least
130, although lower quality products may be produced by
the use of feeds which have lower V.I. values.
Suitable pre-treatment steps for preparing feeds
for the hydroisomerization are those which remove the
aromatics and other low V.I. components from the initial
feed. Solvent extraction using a solvent such as
furfural, phenol or N,N-dimethylformamide is suitable for
-12- ~:Q~ io~fi '
this purpose, as is hydrotreatment, especially at high
hydrogen pressures which are effective for aromatics
saturation, e.g. 1500 psig (about 10,441 kPa) or higher.
Hydrotreatment may be preferred over solvent extraction in
view of the losses which take place during the extraction
process.
The preferred gas oil and distillate feeds are
those which have a high wax content, as determined by ASTM
D-3235, preferably over about 50 weight percent. Feeds of
this type include, for example, certain South-East Asian
and mainland China oils. These feeds usually have a high
paraffin content, as determined by a conventional P/N/A
analysis. The properties of typical feeds of this~type
are set out in Tables 1 and 2 below.
TABLE 1
1~1~1I1~~_S~a~_Q~.1
Nominal boiling range, oC (oF) 345-540 (650-1000)
API Gravity 33.0
Hydrogen, wt% I3.6
Sulfur, wt% 0.07
Nitrogen, ppmw 320
Basic Nitrogen, ppmw I60
CCR 0.04
Composition, wt% 60
Paraffins 23
Naphthenes 23
Aromatics 17
Bromine No. 0.8
RV, 100oC, cSt 4.18
Pour Point, oC (oF) 46 (115)
95% TBP, oC (oF) 510 (950)
-13° ,
%045096
TABLE 2
$~~ ~.3~ a~_~ egg
Nominal boiling range, oC, (oF) 345-510 (650-950)
API Gravity 38.2
H, wt% 14.65
S, wt% 0.02
N, ppmw 16
Pour Point, °C (°F) 38 (100)
RV at 100oC, cSt 3.324
P/N/A wt%
Paraffins 66
Naphthenes 20
Aromatics 14
The preferred feeds for producing the products of the
highest viscosity index are petroleum waxes which contain
at least 50% wax, as determined by ASTM Test D-3235. In
these feeds of mineral oil origin, the waxes are materials
of high pour point, comprising straight chain and slightly
branched chain paraffins such as methylparaffins.
Petroleum waxes, that is, waxes of paraffinic
character are derived from the refining of petroleum and
other liquids by physical separation from a wax-containing
refinery stream, usually by chilling the stream to a
temperature at which the wax separates, usually by solvent
dewaxing, e.g., MER/toluene dewaxing or by means of an
autorefrigerant process such as propane dewaxing. These
waxes have high initial boiling points above about 650oF
(about 343oC) which render them extremely useful for
processing into lubricants which also require an initial
boiling point of at least 650oF (about 343oC). The
2~~JO~Ei '
-14-
presence of lower boiling components is not to be excluded
since they will be removed together with products of
similar boiling range produced during the processing
during the separation steps which follow the
characteristic processing steps. Since these components
will, however, load up the process units they are
preferably 'excluded by suitable choice of feed cut point.
The end point of wax feeds will vary according to the
characteristics of the stream from which the wax has been
removed, with distillate (neutral) streams~usually giving
waxes with end points of not more than about 1050oF (about
565oC) but higher boiling wax feeds such as the petrolatum
waxes, i.e, waxes separated from bright stock may also be
employed, these waxes typically having end points up to
about 1300oF (about 705oC).
The wax content of the preferred feeds is high,
generally at least 50, more usually at least 60 to 80,
weight percent with the balance from occluded oil
comprising iso-paraffins, aromatics and naphthenics. The
non~-wax content will normally not exceed about a0 weight
percent of the wax and preferably will not exceed 25-30
weight percent. These waxy, highly paraffinic wax stocks
usually have low viscosities because of their relatively
low content of aromatics and naphthenes although the high
content of waxy paraffins gives them melting point and
pour points which render them unacceptable as lubricants
without further processing.
The preferred type of wax feeds are the slack
waxes, that is, the waxy products obtained directly from a
solvent dewaxing process, e.g, an MEK or propane dewaxing
process. The slack wax, which is a solid to semi-solid
product, comprising mostly highly waxy paraffins (mostly
-15- :045096
n- and mono-methyl paraffins) together with occluded oil,
may be used as such or it may be subjected to an initial
deoiling step of a conventional character in order to
remove the occluded oil (Foots Oil) so as to form a
harder, more highly paraffinic wax which may then be used
as the feed. The Foots Oil contains most of the
aromatics present in the original slack wax and with these
aromatics, most of the heteroatoms. The deoiling step is
desirable, therefore, because it removes the undesirable
aromatics and heteroatoms which would otherwise pass
through the hydroisomerization step and reduce the V.I. of
the final product. The oil content of de-oiled waxes
maybe quite low and for this purpose, measurement of the
oil content by the technique of ASTM D721 may be required
for reproducibility, since the D-3235 test referred to
above tends to be less reliable at oil contents below
about I5 weight percent. At oil contents below about 10
percent, however, the advantage of the present zeolitic
catalysts may not be as marked as with oil contents of
from about 10 to 50 weight percent and for this reason,
wax feeds conforming to this requirement will normally be
employed.
The compositions of some typical waxes are given
in Table 3 below.
TABLE 3
YL~75_S:gID852~~~~QD_~_~~~_?r~9b~_5:~115~.~
__E~,_ __8__ __~__ __~?__
Paraffins, wt% 94.2 81.8 70.5 51.4
Mono-naphthenes, wt% 2.6 11,.0 6.3 16.5
Poly-naphthenes, wt% 2.2 3.2 7.9 9.9
Aromatics, wt% 1.0 4.0 15.3 22.2
-16- 2o4so~s
A typical slack wax feed has the composition
shown in Table 4 below. This slack wax is obtained from
the solvent (MEK) dewaxing of a 300 SUS (65 cSt) neutral
oil abtained from an Arab Light Crude.
TABLE 4
$~S$_ld~X_Er..QB~''.I~~,.~ag
API 39
Hydrogen, wt% 15.14
Sulfur, wt% 0.18
Nitrogen, ppmw 11
Melting point, (F) 57 (135)
oC
KV at 100oC', 5.168
cSt
PNA, wt%:
Paraffins 70.3
Naphthenes 13.6
Aromatics 16.3
Simulated Distillation:
1QE1
375 (710)
413 (775)
30 440 (825)
50 460 (860)
70 482 (900)
90 500 (932)
95 507 (945)
Another slack wax suitable for use in the
present process has the properties set out in Table 5
below, This wax is prepared by the solvent dewaxing of a
450 SUS (100cS) neutral raffinate:
-17- X045096 '
TABLE 5
~~~Sr~5~01~.1~~QE.g.~~3~~
Boiling Range, of (oC) 708-1053 (375-567)
API 35.2
Nitrogen, basic, ppmw 23
Nitrogen, total, ppmw 28
Sulfur, wt% 0.115
Hydrogen, wt% 14.04
Pour point, of (oC) 120 (50)
KV (100~C) 7.025
KV (300oF, 150oC) 3.227
Oil (D 3235) 35
Molecular wt. 539
P/N/A:
Paraff ins -
'Naphthenes -
Aromatics 10
The paraff inic componentspresent in the
original wax feed possesses good V.T. characteristics but
have relatively high pour points as a result of their
paraffinic nature. The objective of 'the
hydroisomerization is, herefore, to effect a selective
transformation of these paraffinic components to iso-
paxaffins which, while possessing good viscometric
properties, also have higher pour points. This enables
the pour point of the final product to be obtained without
an excessive degree of 8ewaxing following the
hydroisomerizati,on.
The catalyst used in the hydroisomerization is
one which has a high selectivity for the isomerization of
waxy, linear or near linear paraffins to less waxy,
isoparaffinic products. Catalysts of this type are
-1 s- ~:U45096 '
bifunctional in character, comprising a metal component on
a large pore size, porous support of relatively low
acidity. The acidity is maintained at a low level in
order to reduce conversion to products boiling outside the
lube boiling range during this stage of the operation. In
general terms, an alpha value below 20 should be employed,
with preferred values below 10, best results being
obtained with alpha values below 5.
The alpha value is an approximate indication of
the catalytic cracking activity of the catalyst compared
to a standard catalyst. The alpha test gives the relative
rate constant (rate of normal hexane conversion per volume
of catalyst per unit time) of the test catalyst relative
to the standard catalyst which is taken as an alpha of 1
(Rate Constant = 0.016 sec -1). The alpha test is
described in US-A-3,354,078 and in ,~,~_~,~~~~,y~~,~, 4, 527
(1965); ~, 278 (I966); and ~],, 395 (1980). The
experimental conditions of the test'used to determine the
alpha values referred to in this specification include a
constant temperature of 538oC and a variable flow rate as
described in detail in ,~i_~~~~~y~~~, ~1, 395 (1980).
A preferred hydroisomerization catalyst for the
second stage employs zeolite beta as a,support since this
zeolite has been shown to possess outstanding activity for
paraffin isomerization in the presence of aromatics. The
low acidity forms of zeolite beta may be obtained by
synthesis of a highly siliceous form of the zeolite, e.g.
with a silica-alumina ratio above about 50:1 or, more
readily, by steaming zeolites of lower silica-alumina
ratio to the requisite acidity level. Another method is
by replacement of a portion of the framework aluminum of
the zeolite with another trivalent element such as boron
~04509~
-19- ,
which results in a lower intrinsic level of acid activity
in the zeolite. The preferred zeolites of this type are
those which contain framework boron, and normally at least
0.1 weight percent, preferably at least 0.5 weight
percent, of framework boron is preferred in the zeolite.
In zeolites of this type, the framework consists
principally.of silicon tetrahedrally coordinated and
interconnected with oxygen bridges. A minor amount of a
trivalent element (alumina in the case of alumino-silicate
zeolite beta) is usually also coordinated and forms part
of the framework. The zeolite also contains material in
the pores of the structure although these do not form part
of the framework constituting the characteristic structure
of the zeolite. The term "framework" boron is used here
to distinguish between material in the framework of the
zeolite which is evidenced by contributing ion exchange
capacity to the zeolite, from material which is present in
the pores and which has no effect on the total ion
exchange capacity of the zeolite.
Methods for preparing high silica content
zeolites containing framework boron are known and are
described, for example, in US-A-4,269,813 and 4,672,049.
As noted there, the amount of boron contained in the
zeolite may be varied by incorporating different amounts
of borate ion in the zeolite forming solution, e.g., by
the use of varying amounts of boric acid relative to the
forces of silica and alumina.
In low acidity zeolite beta catalysts, the
zeolite should contain at least 0.1 weight percent boron.
Normally, the maximum amount of boron will be about 5
weight percent of the zeolite and in most cases not more
than 2 weight percent of the zeolite. The framework will
;~o~~o~s
normally include some alumina and the silica:alumina ratio
will usually be at least 30:1, in the as-synthesized
conditions of the zeolite. A preferred zeolite beta
catalyst is made by steaming an initial baron-containing
zeolite containing at least 1 weight percent boron (as
B203) to result in an ultimate alpha value no greater than
about 10 and preferably no greater than 5.
The steaming conditions should be adjusted in
order to attain the desired alpha value in the final
catalyst and typically utilize atmosphereslof 100 percent
steam, at temperatures of from~about 800 to about 1100oF
(about 427 to 595oC). Normally, the steaming will. be
carried out for about 12 to 48 hours, typically about 24
hours, in order to obtain the desired reduction in
acidity. The use of steaming. to reduce the acid activity
of the zeolite has been found to be especially
advantageous, giving results which are not achieved by the
use of a zeolite which has the same~acidity in its as-
synthesized condition. It is believed that these results
may be attributable to the presence of trivalent metals
removed from the framework during the steaming operation
which enhance the functioning of the zeolite in a manner
which is not fully understood.
The zeolite will usually be composited with a
matrix material to form the finished catalyst and for this
purpose conventional non-acidic. matrix materials such as
alumina, silica-alumina and silica are suitable with
preference given to silica as a non-acidic binder,
although non-acidic aluminas such as alpha boehmite (alpha
alumina monohydrate) may also be used, provided that they
do not confer any substantial degree of acidic activity on
the matrixed catalyst. The use of silica as a binder is
-2I- '
2Q450~~
preferred since alumin,a, even if non-acidic in character,
may tend to react with the zeolite under hydrothermal
reaction conditions to enhance its acidity. The zeolite
is usually composited with the matrix in amounts from
80:20 to 20:80 by weight, typically from 80:20 to 50:50
zeolite:matrix. Compositing may be done by conventional
means including mulling the materials together followed by
extrusion or pelletizing into the desired finished
catalyst particles. A preferred method for extruding the
zeolite with silica as a binder is disclosed in US-A-
4,582,815. If the catalyst is to be steamed in order to
achieve the desired low acidity, it is performed after the
catalyst has been formulated with the binder, as is
conventional.
The isomerization catalyst also includes a metal
component in order to promote the desired
hydroisomerization reactions which, proceeding through
unsaturated transitional species, require mediation by a
hydrogenation-dehydrogenation component. In order to
maximize the isomerization activity of the catalyst,
metals having a strong hydrogenation function are
preferred and for this reason, platinum and the other
noble metals such as palladium are given a preference.
The amount of the noble metal hydrogenation component is
typically in the range 0.5 to 5 weight percent of the
total catalyst, usually from 0.5 to 2 weight percent. The
platinum may be incorporated into the catalyst by
conventional techniques including ion exchange with
complex platinum canons such as platinum tetraammine or
by impregnation with solutions of soluble platinum
compounds, for example, with platinum tetraammine salts
such as platinum tetraamminechloride. The catalyst may be
~o4so9~
-22-
subjected to a final calcination under conventional
conditions in order to convert the noble metal to the
oxide form and to confer the required mechanical strength
on the catalyst. Prior to.use the catalyst may be
subjected to presulfiding, by established techniques.
The conditions for the hydroisomerization are
adjusted to achieve the objective.of isomerizing the waxy,
linear and near-linear paraffinic components in the feed
to less waxy but high V.I, isoparaffinic materials of
relatively Lower pour point while minimizing conversion to
non-lube boiling range products (usually 650oF- (345°C-)
materials). Since the catalyst used has a low acidity,
conversion to lower boiling products is usually at~a
relatively Low level and by appropriate selection of
severity, the operation of the process may be optimized
for isomerization over cracking. At conventional space
velocities of about 1, using a Pt/zeolite beta catalyst
with an alpha value below 5, temperatures for the
hydroisomerization will typically be in the range of about
600 to about 780oF (about 315 to 415oC) with 650oF+
(343°C+) conversion typically being from about 10 to 40
weight percent, more usually 12 to 30 weight percent, of
the waxy feed. However, temperatures may be used outside
this range, for example, as low as about 500oF (260oC) and
up to about 800oF (about 425oC) although the higher
temperatures will usually not be preferred since they will
be associated with a Lower isomerization selectivity and
the production of less stable tube products as a result of
the hydrogenation reactions being thermodynamically less
favored at progressively higher operating temperatures.
Space velocities will typically be in the range of 0.5 to
2 LHSV (hr-1) although in most cases a space velocity of
2045096
-23-
about 1 LHSV will be most favorable.
The hydroisomerization is operated at hydrogen
partial pressures (reactor inlet) of at least 1000 prig
(6991 kPa), usually 1000 to 3000 psig (6991 to 20771 kPa~)
and in most cases 1500-2500 psig (10451 to 17326 kPa).
Hydrogen circulation rates are usually in the range of
about 500 to 5000 SCF/Bbl (about 90 to 900 n.l.l.-1).
Since some saturation of aromatic components present in
the original feed takes place in the presence of the noble
metal hydrogenation component on the catalpst, hydrogen is
consumed in the hydroisomerization even though the desired
isomerization reactions are in hydrogen balance; for this
reason, hydrogen circulation rates may need to be adjusted
in accordance with the aromatic content of the feed and so
with the temperature used in the hydroisomerization since
higher temperatures will be associated with a higher level
of cracking and, consequently, with a higher level of
olefin production, some of which will be in the tube
boiling range so that product stability will need to be
assured by saturation. Hydrogen circulation rates of at
least 1000 SCF/Bbl (about 180 n.l.l.-1) will normally
provide sufficient hydrogen to compensate for the expected
hydrogen consumption as well as to ensure a low rate of
catalyst aging.
The relatively low temperature conditions which
are appropriate for the paraffin isomerization disfavor
cracking reactions but are thermodynamically favorable for
the saturation of any lube range olefins which may be
formed by cracking, particularly in the presence of the
highly active hydrogenation components on the catalyst.
Because of this, the hydroisomerization is also effective
far hydrofinishing the product so that product stability
-24-
20450~6
is improved, especially stability to ultraviolet
radiation, a property which is frequently lacking in
conventional hydrocracked lube, products. The isomerized
product may therefore be subjected simply to a final
dewaxing step in order to achieve the desired target pour
point and usually there will be no need for any further
finishing steps since a low unsaturates content, both of
aromatics and of lube range olefins, results from the
optimized processing in the two functionally separated
steps of the process. The product may be~subjected to a
final fractionation to remove lower boiling materials,
followed by a final dewaxing step in order to achieve
target pour point fox the product.
Although a final dewaxing step will normally be
necessary in order to achieve the desired product pour
point, it is a notable.feature of the present process that
the extent of dewaxing required is relatively small.
Typically, the loss during the final dewaxing step will be
not more than 15-20 weight percent of the dewaxer feed and
may be lower. Either catalytic dewaxing or solvent
dewaxing may be used at this point and if a solvent
dewaxer is used, the removed wax may be recycled to the
hydroisomerization for a second pass through the
isomerization step. The demands on the dewaxer unit for
the product are relatively low and in this respect the
present process provides a significant improvement over
the process employing solely amorphous catalysts where a
significant degree of dewaxing is required. The high
isomerization selectivity of the zeolite catalysts enables
high single pass wax conversions to be achieved, typically
about 80% as compared to 50% for the amorphous catalyst
process so that unit throughput is significantly enhanced.
-25-
;~t~~~096
The products from the process are high V.I., low
pour point materials which are obtained in excellent
yield. Besides having excellent viscometric properties
they are also highly stable, both oxidatively and
thermally and to ultraviolet light. V.I. values in the
range of 125 to 150 are typically obtained with the
preferred wax feeds to the process and values if at least
140, typically 143 to 147, are readily achievable with
product yields of at least 50 weight percent, usually at
least 60 weight percent, based on the original wax feed,
corresponding to wax conversion values of almost 80 and 90
percent, respectively.
~x~bPL.~.~
The following examples are given in order to
illustrate various aspects of the present process.
Examples 1 and 2, directly following, illustrate the
preparation of low acidity Pt/zeolite beta catalysts
containing framework boron.
~x~~81~_1
A boron-containing zeolite beta catalyst was
prepared by crystallizing the following mixture at 285oF
(140oC) for 13 days, with stirring:
Boric Acid, g. 57.6
NaOH, 50~, ml. 66.0
TEABr, ml. 384
Seeds, g. 37.0
Silica, g. 332
Water, g. 1020
l~Ig~~.S s
1. TEABr = Tetraethylammonium bromide, as 50$ aqueous
solution.
-26_ ~~45~9'~; .
2, Silica = Ultrasil (trademark)
The calcined product had the following analysis
and was confirmed to have the structure of zeolite beta by
x-ray diffraction:
Si02 76.2
A1203 0.3
B 1.08
Na, ppm 1070
N 1.65
Ash 81.6
E~s~~3~~,~_2
An as-synthesized boron-containing zeolite beta
of Example 1 was mulled and extruded with silica in a
zeolite:silica weight ratio of 65:35 dried and calcined
at 900oF (480oC) for 3 hours in nitrogen, followed by
1000oF (540oC) in air for three hours. The resulting
extrudate was exchanged with lN~ammonium nitrate solution
at room temperature for 1 hour after which the exchanged
catalyst was calcined in air at 1000oF (540oC) for 3
hours, followed by 24 hours in 100 percent steam at 1025oF
(550oC). The steamed extrudate was found to contain 0.48
weight percent boron (as B203), 365 ppm sodium and 1920
ppm A1203. The steamed catalyst was then exchanged for 4
hours at room temperature with IN platinum tetraammine
chloride solution with a final calcination at 660oF
(350oC) far three hours. The finished catalyst captained
0.87 weight percent platinum and had an alpha value of 4.
-27- ~0450~~;
A sample of an aluminosilicate zeolite beta with
a bulk Si02/A1203 ratio of 40 was extruded with alumina to
produce a 65% zeolite/35% A1203 (by weight) cylindrical
extrudate. This material was then dried, calcined and
steamed to reduce the alpha to 55. Platinum was
incorporated by means of ion exchange using Pt(NH3)4C12,
to a final Pt loading of 0.6 weight percent.
.E.~~~B~~_4
This Example illustrates a wax
hydroisomerization process using a low acidity zeolite
beta hydroisomerization catalyst. The process was
operated under both low (400 psig/2860 kPa) and high (1750
psig/12170 kPa) conditions.
A low acidity silica-bound zeolite beta catalyst
prepared by the method described in Example 2 above was
charged to a reactor in the form of~30/60 mesh (Tyler)
particles and then sulfided using 2% H2S/98% H2 by
incrementally increasing the reactor temperature up to
750oF (400oC) at 50 psig (445 kPa abs). The feed was a
slack wax having the properties set out in Table 6 below.
TABLE 6
E~gB.~~~.i~~_Qf_S.lsa~$_ldsalcY_~~~~_1~Q,~3~$_Q.ill
APi Gravity 34.4
Hydrogen, wt% 14.45
Nitrogen, ppm 32
Sulfur, wt% 0.125
Water, ppm 44
~o4~oss
-28- ,
.~.i~~a~~~s~_~?~,~.il~ti~~ E.~lg~l
0.5% 731 (388)
791 (422)
821 (438)
854 (457)
877 (469)
899 (482)
919 (493)
940 (504)
964 (518)
989 (532)
1019 (548)
1040 (560)
99.5 1084 (584)
Unrecovered Amt. 0.0
The slack wax feed was charged directly to the
catalyst in concurrent downflow with hydrogen under the
following conditions:
LHSV, hr'1 0.5
H2, Flow Rate, SCFJBbl
(n.l.l.-1) 2500 (455)
Total Pressure, ps.ig
(kPa abs) 400 and 1750 (2857 and 12159)
The temperature was varied in the range from 700
to 780oF (about 370 to 415oC) to give differing levels of
wax conversion from l0 to 30 percent, as discussed below.
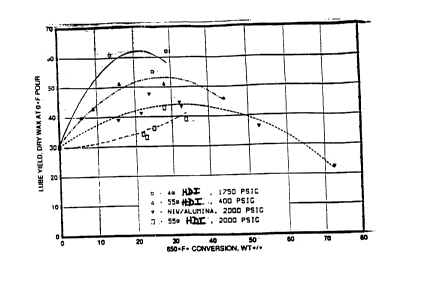
The results are shown in Table 8 below and in Figures 1
and 2.
Ex.~~uElg_~
The aluminosilicate zeolite beta catalyst of
2o4Joos
_29_
Example 3 was charged to the reactor and presulfided as
described in Example 4 above and then used to
hydroisomerize the same slack wax feed under the following
conditions:
LHSV, hr-1 l.0
H2, Flow Rate, SCF/Bbl
(n.l.l.-1) 2000 (356)
Total Pressure, psig
(kPa abs) 400 and 2000 (2857 and 13881)
The temperature was varied from 650 to 750oF
(about 345 to 400oC) to give differing levels of wax
conversion from about 5 to 45 percent, as discussed below.
The results are given in Table 8 below and in Figures 1
and 2.
.E~~E~,~_l:
This Example illustrates the use of an amorphous
catalyst in a single stage high pressure hydroprocessing
operation.
A NiW/A1203 hydrocracking catalyst with the
properties shown in Table 7 was used.
2045096
-30-
TABLE 7
~_rope~ties of I~ij~j~~2Q~_~~~~y,~~
Pore Volume cc/g 0.453
Surface area, m2/g 170
Nickel, wt% 4.6
Tungsten, wt% 23.8
Real Density, g/cc 4.238
Particle Density, g/cc 1.451
The catalyst was charged to a downflow reactor
and sulfided as described in Example 4 above. The
catalyst was also fluorided using o-flouortoluene as a
dopant (25 ppm) in the feed. Hydrogen was fed to the
reactor together with the same slack wax described in
Example 4 in cocurrent downflow under the following
conditions, again varying temperature from 700 to 780oF
(about 370 to 415oC) to vary conversion from about 5 to 75
percent, under the following reaction conditions:
LHSV, hr-1 1.0
H2 Flout Rate, SCF/Bbl
(n.l.l.-1) 7500 (1335)
Total Pressure, psig
(kPa abs) 2000 (13881)
The lobe yields and properties of the resulting
lobes are shown in Table 8 below and in Figures 1 and 2.
-31- 204500f;
TABLE 8
.L~.~~_Y~~~.sd~~I~~_PsQB~~
~.~~D7e~~~TQ~ 4 ~ 4
~~~~ly~~ -~_~~L~~~__ _~~~_~~L~~~~_ _~Ii~dL~l~n~nsa_
Pressure, psig400 1750 400 1750 2000
kPa 2857 12159 2857 12159 13881
Lube yield, 55-58 61 51 41 46
wt%
RV,l00oF, cS 5.8 6.0 5.8 7.0 5.0
Lube V.I. 135-137133-134127 121 142
Figures I and 2 compare the yields and V.I. data
as a function of the slack wax conversion, which is
defined here as the new amount of feed converted to 650oF-
(343oC-). Yield is determined by the amount of 650oFt
material remaining after solvent dewaxing to achieve a OoF
(-l8oC) pour point product.
The results summarized in Table 8 and shown in
Figures 1 and 2 show that slack wax can be processed over
a low acidity catalyst such as Pt/zeolite beta at high
pressure without the yield and V.I. penalties_incurred
with a comparable but more acidic catalyst. These results
show that the low acidity Pt/zeolite beta catalyst of
Example 2 (4a) produces the highest yield for processing
the raw slack wax, as shown by Example 4: the 4a
Pt/zeolite beta catalyst produces as much as 15 percent
more tube than the amorphous NiW/A1203 catalxat used in
Example 6 and 10 to 20% more lube than the h~,gher acidity
55a Pt/zeolite beta catalyst used in Example 5.
Increasing the operating pressure of the
-32- 2045096
hydroisomerization results in a significant yield loss in
the case of the higher acidity Pt/zeolite beta catalyst
used in Example 5, but results in a yield increase for the
low acidity Pt/zeolite beta catalyst used in Example 4.
Product V.I. is not as strongly affected by pressure with
the low acidity Pt/zeolite beta as it is with the higher
acidity Pt/zeolite beta catalyst.