Note: Descriptions are shown in the official language in which they were submitted.
~ -1- F ~ ~ 6 g g '~ 4
METHOD FOR REPROi~UClNG CONIFERS
- BY SOMATIC EMBRYOGENESIS
BACKGROUND OF THE INVENTION
The present ~nvention is a method for reproduclng coniferous
10 plants by somatic em~ g~ is using the techniques of plant tissue
culture. It is especially suited for producing large clones of superior
Douglas-fir selections useful for reEorestation.
Loblolly pine (Pinus taeda L.), its closely related southern
pines, and Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) are prob-
15 ably the most important commercial species of temperate North Americantimber trees. Since the early 1940s, when serious privt~te reforestation
- efforts began, literally billions of one and two year old nu- ,~r~ ~.own
trees have been planted on cut-over or burned forest lands. For many
years these seedling trees were grown using naturally produced seed from
20 cones collected as a part time effort of individuals seeking to supple-
ment their incomes. As early as 1957 forest get~eticists began to plant
seed orchards using either seed or gr~fted scions obtained from superior
trees. These trees were selected for such inheritable characteristics
as rapid growth, straightness of bole, wood density, etc. Now in both
25 th~ southern pine and Douglas-fir regions the bulk of the seed is pro-
duced from selected trees grown in seed orchards, some of them now sec-
ond and third generation orchards.
Despite the fact that the orchards were stocked with superior
trees, pollination often cannot be carefully controlled and frequently
30 the seed trees are fertilized by wild pollen of unknown characteristics.
For this reason, the characteristics of the progeny produced by sexual
reproduction have not been as predictable as hoped and genetic gain
could not be attained as rapidly as desired.
Beginning about 1960, techniques were developed for reproduc-
35 ing some species of plants by tissue culture. These were predominatelya~ 5 ~tnd usually ornamental house plants. The method employed
use of a suitable explant or donor tissue from a desirable plant. This
;:
A
. .~ .
~ ~ n ~
wogl/05854 206996~ PCI/US90/06057
was placed on a series of culture media in which nutrients and growth
hormones were carefully controlled from step to step. The usual pro-
gression was growth froln the explunt lo a callus. The callus was placed
on a budding medium where adventitious buds formed. These, in turn,
5 were separated, elongated, and rooted ~o ultimately form plantlets. A
plantlet has the nature of a seedling but is genetically identical to
the explant donor plant.
Gymnosperms in general, and most forest tree species in par-
ticular, proved to be much more dirficult to reproduce by tissue cul-
10 ture. It was not until about 1975 that Douglas-fir was successrully
reproduced by organogenesis. Loblolly pine was successfully reproduced
about two years later.
Culture by organogenesis is tedious and expensive due to the
large amount of delicate manual handling necessary. It was soon recog-
15 nized that embryogenesis was potentially a much more desirable methodfrom the standpoints of quantity of plantlets produced, cost, and poten-
tial genetic gain. Work on emt,~ of forest species began in the
late 1970s. U.S. Patent 4,217,730 to El-Nil describes one early attempt
at somatic embryogenesis of Douglas-fir. This approach was lAter set
20 aside because advanced stage embryos and plantlets could not be readily
obtained. However, other workers entered the field in increasing num-
bers and progress has been rapid even if it has not until the present
time reached the commercial stage. A brief review of some of the most
important work will follow. This is intended to be representative and
25 is not fully inclusive of all the work in the field. Literature cita-
tions in the text are given in abbreviated form. Reference should be
m~de to the bibliography at the end of the specification for full cita-
tions of the literature noted herein.
The natural embryogeny of gymnosperms is described in great
30 detail by Singh (1978). Conifer-type embryogeny is one of four types
noted for gymnosperms. This includes virtually all of the important
forest species except Sequoia. Singh notes that the immature seeds
typically contain more than one embryo. Most commonly this seems to
occur when a single zygote forms multiple embryos, a phenomenon called
35 "cleavage polyembryony". As the seed matures one embryo becomes domin-
ant while the others are suppressed. The ability to form multiple
embryos from a single zygote forms the basis for most of the present
embryog~nic processes for mulliplying conifers. However, Douglas-fir is
WO91/05354 2~ PCI/US90/06057
-3-~ =.
arl exception. Most typically only a single embryo will be present
throughout the formation and maturation of a seed. This may account for
at least some of the difficulty ~,.~,.. ;~..c~ to date in multiplying Doug-
las-fir by somatic eml,-yut~
Bourgkard and Favre ~1988) describe what is the apparently
successful production of plantlets by somatic embryogenesis of Sequoia
~ senpervirens. As a historic note, this was one of the first forest tree
species successfully r~ 1 by U.t;dl~Or,~
Hakman and her coworkers have concentrated on Norway spruce
(Picea abies)t apparently with some success. In a paper by Hakmao,
Fowke, von Arnold, and Eriksson (1985) the authors describe the produc-
tion of "embryos" but not plantlets. Hakman und von Arnold (1985) do
suggest that they have successfully obtained plantlets. This latter
paper is interesting for its comments on the variability within the
species and the poor success with many of the seed sources used for
e~.plants. The authors suggest that this variability may be due to the
pll~;olvt~l~dl condition of the source material. However, other workers
have noted great differences in behavior between recogni~ed genotypes of
the species.
l~agmani and Bonga (1985) describe cmb.yvg~ from megagam-
etophytes or Larix decidua by tissue culture. The archegonia, pro-
embryos, or embryos with their suspensors were removed prior to culture.
Some of the resulting embryos produced in culture were stated to have
further advanced to become plantlets established in soil. The ploidy of
these plants was not investigated.
Successful production of small quantities of plantlets has now
been reported for loblolly pine. Teasdale, Dawson, and Woolhouse (1986)
showed the criticality of proper mineral nutrients for cell suspension
cultures of loblolly pine. The article by Becwar, Wann, nnd NflgmAni
(1988) is enlightening for the differences shown in performance between
different families (or genotypes). Three families out of the ten tried
accsunted for most of their success. Even so, They appeared unable to
grow ~vlyH~v~ embryos. A companion paper by Nagmani ~nd Becwar
(1988) showed development of Pinus taeda to the precotyledonary stage.
35 In an earlier paper, Gupta and Durzan (1987) described their success in
taking loblolly pine to the plantlet stage by embryogenesis. However,
only one genotype was successfully taken to the plantlet stage and only
one converted plant was produced. The authors note thr n~d for
-
~ ~ 206~6 4
~improYed con~rersion r~tes~ as well ~s other informstion before the
process can be considered commercially practical.
Sugar pine ~Pinas lambertiana) has also been cultured to the
plantlet stage as reported bg Gupta and Durzan (1986). The authors note
S a Yery low 1-2~i conYersion of embryos into piantlets.
- The ebove researchers appear to be the only ones who haYe
f,reY;vu:,ly achieYed success in producing Douglas-fir plantlets (Durzan
and Gupta 198~). Again, the success ratlo appears to be very low and
they haYe obtained only two converted plsnts from a single genOtyi~e.
In ~nitev states Patent No. 4,957,866 i~;r,iued Septe~'ver
~.8,1990, we desc~ibed an
ImproYed method for .~,.vviu~ g conlferous species by somatic embryo-
genesis. An ~ntermediate high osmoticant culture medium wes used to
genèrate strong iate stage p,o~ri.vl~v:" prior to the d.i~', I.~C~ of cot-
15 yledonery embryos in a medium containing absc~sic acid. The methods
disclosed were of particular effectiYeness in somatic polyembryogenesis
of loblolly pine.
ActiYated charcoal has been widely used before In tissue cul- s
ture media where it is believed to function as an adsorbent for tox~c
25 r~etabolic products and undesirable amounts of residual hormones.
Abscisic ac~d has also been recognized as being a useful plant hormone
in cultures inducing conlfer embryogenis; e.g., Boulay, Gupta, i~rog-
strup, and Durzen (1988). The combination of these two materiels hes
been used by e number of workers, generelly with indifferent or negstiYe
30 results. Johansson~ Andersson, and Ericksson (1982) cultured anthers ol
several ornsmentel plant species using a two phese liquid oYer solid
medium in which the agsrified solid phase contained actiYated chercoel.
The chercosl eppeered to be useful for ebsorbing small umounts endoge-
nous abscisic acid. In a related paper Johansson (1983), tested the
35 e~fects of charcoel es sn edsorbent of meteriels inhib~ting the initie-
tion of emb. ~o~.r..,is. In a test intended as a model, he added exo-
genous ABA in emounts Yerying by orders of magnitude between 10 9 ?~i and
10~3 ~ to medis with end without sctiYsled charcoel in the solid portion
;
20-6~996:4 i
WO91/058~4 ; ~ 3~ PCltUS90/06057
_5_
of 8 two phase medium. His conclusion was that initiation was complet-
ely inhibited for all of the test species at Af~A concentrstions above
10-6 M, when no charcoal was used, and 10 4 ~1 when charcoal was present.
Thus, charcoal was seen as an effective mAterial for removing inhibitory
5 amounts of ABA and other undesirable materials such as phenolics.
~ ;iY and Gadasi (1986) studied embryogenesis in several geno-
types of cucumber (Cucumis sativus L.). They us~d liquid cultures as
well as the two layer technique with activated charcoal in the solid
layer of the medium and low (0.4 ~uM) levels of abscisic acid in the
10 liquid layer. In the liquid cultures abscisic acid by itself only
slightly improved embryo formation and was significantly more effective
th~n the combination of abscisic acid with activated charcoal. Plantlet
development in the liquid over solid cultures was slightly improved by
th~ combination of the two Materials.
Buchheim, Colburn, and Ranch (1989) suggest that exogenous
abscisic acid and activated charcoal would probably not be a very useful
combination of ingredients in a culture medium because of adsorption of
the abscisic acid by the charcoal with subsequent loss of its biological
effectiveness.
Since the importance of the osmotic environment within a
developing seed is known (Yeung and 8rown 1982), it has been assumed by
others that the osmotic potential of the media during a culturing pro-
cess could have an important effect (e.g., Raghavan 1987). Lu and
Thorpe (1987), using white spruce (Picea glauca), noted that increasing
the osmolarity of a medium and reducing the auxin concentration enhanced
development and maturation of somatic embryos. They observed that more
embryos developed on media containing 6% than on those with 9% sucrcse
and that similar results were obtained when sorbitol replaced 3 % of the
sucrose in the medium. Sorbitol is known to be only poorly metabolized
SO presumably its effect was osmotic rather than as a carbon source for
the developing embryos. Quite in contrast to their findings, HakmAn snd
von Arnold (1988), using the same species and a combination of abscisic
acid and sucrose in a development medium, found a very sharp falloff in
success in going from 3% to 4% sucrose.
Becwar and Feirer (1989) note work involving the trsnsfer of a
loblolly pine embryonal ...u~ mass to development media containin~
10 uM abscisic acid with 3-6% sucrose. Ilow~ver, they reported no
details of their experimental protocol and only tha~ th~ media "promot~d
WO 91/05854 ~ r~ ~ PCI/US90/06057
- -6-
embryo developmen-t". While a report (Becwar et si.) is noted as being
in press it hss spparently not yet been published.
Finer, Kreibel and Becwsr (1989), studying eastern white pine
(Pinus strobus L.), inttiated snd mslntsined cultures on medla wlth a 3%
S sucrose level. Further embryo developmment W85 then attempted on 8
medium with 1-12~i sucrose combined with a high concentrstion of sbscisic
scid snd varging amounts of glutsmine. Best results were found with 50
mM glutsmine, 38 uM abscisic acid, and 6% sucrose. However, the number
of embryos formed under any of the conditions wss not high And, ss of
10 the time of reporting, none hsd been successfully germinsted snd con-
verted into plsnts.
Schuller and Reuther (1989), in the sbstrsct of a psper,
describe the study of seversl sugsrs snd soluble stsrch ss csrbohydrste
sources for the culture of Abies slbs. They note thst develoement wss
1~ obtsined only on 8 medium using soluble stsrch snd lactose. No detsils
were given snd appsrently no somstic embryos were developed to the cot-
yledonsry stsge.
Von Arnoid (1987) investigsted carbohydrste level of the init-
istion medium for Norwsy spruce. Sucrose wss vsried between about 1-396
20 with successful initistion being obtsined st the higher level on hslf
strength medium. By replscing 8 portion of the sucrose with sorbitol
she showed thst the poorer results on full strength medium were not due
to incressed osmotic pressure.
Von Arnold snd Hskman (198a) took 8 Norwsy spruce embryogenic
25 csllus and trsnsferred it to 8 modified~ intermedi~t~ medium prior to
full embryo development. The intermediate medium contsined sbscisic
~cid snd from 1-3X sucrose. The higher sucrose levels, slong with the
sbscisic scid, resulted in incressed freqùency of sdvsnced stage pro-
embryo development.
The potentisl for schievlng genetic gsin using somstic embryo-
genesis is recognized ss being very grest. However, the problems to
dste hsve been so overwhelming thst commercisl spplicQtion has seemed
ressonsbly close st=hsnd only for Norwsy spruce and, to 8 lesser e~tent,
loblolly pine using the methods described in our psrent applicstions.
3~ Successful embryogenesis of Douglss-fir hss been much more elusive.
Until the present time, while some converted trees hsve been obtsined,
the percentsge of success hss been fsr below thst of the two previously
nsmed species. Possible commercisl production ot Douglas-fir replsnting
~ 9 g ~ 4
--7--
stock by embryogenesis has I~ inPd no more than a fond
hope in the minds of the people working in the f ield.
BRIEF DESCRIPTION OF THE DRAWINGS
The figures show various stages of plant embryog-
enesis in which:
Figure 1 shows early stage proembryos.
Figure 2 shows late stage proembryos.
Figure 3 depicts cotyledonary stage embryos.
Figure 4 shows a plantlet ready for transfer to
soil .
Figure 5 shows the variation in behaviour in
tissue culture of various genotypes of a single coniferous
species.
Figure 6 is a photomicrograph of a clump of
Douglas-f ir early proembryos .
Figure 7 is a photomicrograph of embryos after
singulation .
Figure 8 is a photomicrograph of a clump of
unsingulated Douglas-f ir cotyledonary embryos . -
Figure 9 is a photomicrograph of high quality
Douglas-f ir cotyledonary embryos .
Figure 10 is a graph showing typical levels of
osmotic potential and abscisic acid concentration during
the culture of Douglas-f ir .
SUM~RY OF THE INVENTION
The present invention is a method of reproducing
selected plants by somatic embryogenesis using tissue
culture techniques. The method is particularly suitable
for reproducing woody gymnosperms of the order Coniferales.
It is PCrP~iAlly well suited for generating large clones of
superior forest trees for reforestation, including, species
- within the families Pinaceae, Cupressaceae, and Taxodia-
F 201;
-7a-
ceae. Mo6t or all species within the genera Abies, Pinus,
Plcea~ ~suqa, Pseudotsuqa, Thuia, Juniperis, Larix, and
Sequoia are believed to be well suited for multiplication
by the present method. The present method is most es-
5 pecially useful for reproducing Douglas-fir (Pseudotsuqa
menziesii (Mirb. Franco. )
The method is particularly advantageous in that
it enables greater quantities and more robust somatic
10 embryos to be produced. This results in higher numbers of
embryos that can be successfully converted into plants
growing in soil. Costs per plant can be significantly
reduced over prior known tissue culture methods. In
addition, use of the method generates embryos that can be
15 retained for extended periods of time in cold storage
without transferring them from a development medium.
A number of terms are known to have differing
r--nin~s when used in the literature. The following
20 definitions are believed to be the ones most generally used
in the field of botany and are consistent with the usage of
the terms in the present specif ication .
"Auxins" are plant growth hormones that promote
25 cell division and growth.
"Cytokin;ns" are plant growth hormones that
affect the organization of dividing cells.
"Callus" is generally considered to be a growth
of unorganized and either unconnected or loosely connected
plant cells generally produced from culturing an explant.
"Embryogenic callus" is a translucent white
mucilagenous mass that contains early stage proembryos
attached to suspensors. This is also referred to as an
,'~s "embryonal-suspensor mass" or "ESM" by some investigators.
WO 91/05854 2 ~ gl~9~ PCI/US90/06057
-8-
A "proembryo" is 8 cell or group of cells having the potential
to become a plant but lacking defined mer~stematic organ primordia.
An "eQrly stage proembryo" is u mass generally of 1 - 10 cells
with dense cytoplasm and Idrge nuclei that have the potential of forming
5 a plant. The early stage proembryo is normally found as a head assoc-
iated at the end of a long thin-walled suspensor cell (FIG. 1).
A "late stage proembryo" is a proembryo with a smooth embry-
oral head of at least about 100 cells associated with multiple suspensor
cells. The late stage proembryo is a very robust advanced proembryo
10 (FIG. 2).
A "cotyledonary embryo", sometimes simply referred to as an
"embryo", has a well defined elongated bipolar structure with latent
meristem with cotyledonQry primordia at one end and a potential radicle
at the opposite end. The cotyledonary structure frequently appears as a
15 small "crown" at one end of the embryo (FIGS. 3 and 9). A cotyledonary
somatic embryo is analogous to a developed zygotic embryo.
An "explant" is a piece of tissue taken from a donor plant for
culturing.
A "meristem" or "meristematic center" is a group of tissue
forming cells capable of further development into plant organsj e.g.,
shoots and roots.
An "osmoticant" or "osmoticum" is a chemical material used for
controlling the osmotic potential of a solution. In the present context
the solution would be a culture medium.
A "plantlet" is a plant asexually reproduced by tissue culture
(FIG. 4).
A "converted embryo" is an embryo that has germinated and
been established as a plant growing in soil
"Somatic embryogenesis" is the process using tissue culture
techniques for generating multiple embryos from an explant. The embryos
from a given tissue source are presumed to be genetically identical.
The present method comprises a multistage culturing process.
A suitable explant is first placed on an induction or initiation culture
medium. This usually will contain relatively high qusntities of growth
35 hormones including at least one auxin and frequently one or more cyto-
kinins. However, growth hormones at this initial stage are not alwsys
necessary or desirable for induction of early stage proembryos. A num-
ber of sour~es of explants may ultimately prov~ to be satisfsctory for
WO91/05854 2069~ PCltUS90~06057
-9~
culturing. These include, but are not limited to, tissue froln cotyle-
dons, hypocotyls, epicotyls, buds, meristematic centers for buds or
roots, and seed embryos. Zygotic embryos remoYed from seeds are pr~-
sently preferred. In particular, for species which in the past hav~
5 proved to be very difficult or impossible to propagate by somatic
emb.~yv~ the embryos from immature seeds may be preferred. In the
case of Douglas-fir, an embryo selected between the time that an apical
dome begins to form but before the first appearance of cotyledon pri-
mordia appears to be optimum.
lD The first stage or induction medium will normally be one of
those well known from past work which contain a balanced concentration
of inorganic salts and organic nutrient materials, with plant growth
hormones included as noted above. Auxins are normally present in con-
centrations which may initially be as high as about 600 ~UMtL, more typi-
15 cally not exceeding about 500 ,uM/L. Cytokinins, if present, may initi-
ally be as high as 500 uM/L. The plant growth hormones may include at
least one auxin and one cytokinin in a combined initial concentrrtion
not exceeding about 1100 uM/L, more typically not exceeding about
900 uM/L The particular auxins and cytokinins used and their exact
20 concentrations, or whether they are used at all, will depend somewhat
oIl the species being cultured and even on the particular genotype within
that species. This is something that cannot be easily predicted but can
be readily determined experimentally. These very high levels of growth
hormones assume the presence in the medium of an adsorbent material,
25 such as activated charcoal. Where charcoal is not present the levels of
growth hormones would normally be much lower than those just noted.
Culturing during this stage may be carried out in the dark,
under very low light conditions, or in full light until an embryogenic
mass forms. Lighting conditions will depend in large part on the compo-
30 sition of the particular medium selected. This embryogenic mass hasbeen described by various other names by r.~e~ . who have reported
it in the past; e.g., embryogenic callus (Hakman and von Arnold 1985) or
embryonal-suspensor mass (Durzan and Gupta 198~). It has the appearance
of a whitish, translucent, mucilagenous mass containing early stage pro-
35 embryos which are readily apparent by low power light microscopy. Intl~e case of Douglas-fir the presence of activated charcoal or a similar
adsorbent in the initiation medium appears to be quite advantageous. It
was noted earlier that Douglas-fir does not experience polyemb~yony ss
-
WO 91/0~854 2 ~ PCT/US90/06057
-10-
do most other coniferous species. The reasons for this are not well
understood but one hypothesis suggests that Douglas-fir seeds contsin a
high endogenous level of abscisic acid which prevents polyembryony.
Activated charcoal in the initiation medium may remove this endogenous
5 A~3A, as well as other undesirable metabolic byproducts, to allow poly-
embryony to occur in vitro. Because the charcoal will also gradually
remove growth hormones over time the initial concentrations of these
materials are necessarily higher than might otherwise be the case. The
preferred induction medium for Douglas-fir will preferably contain an
10 auxin or auxins in amounts of about 400-600 uM/L and a cytokinin or
cytokinins in the a~nount of about 240-500 ~uM/L.
Early stage proembryos from the first culture may be directly
transferred to a late proembryo development culture medium having s~g-
nificantly reduced plant growth hormones and, ~or some species, Q higher
15 concentration of osmoticunts. However, they are preferably first sub-
cultured in a maintenance medium of similar or slightly higher osmotic
potential than the induction medium for multiplication. This
multiplication med~um will also usually have the concentration of plant
hormones significanfry reduced below that of the induction medium. 13y
Z0 "significantly reduced" is meant lowered by a factor which may typically
be one whole order of magnitude. In the case of Douglas-fir it may be
two full orders of msgnitude. No hormone ~dsorbent is usually necessary
or desirable at this time. The osmotic pOtentiQI of the induction ~nd
maintensnce medium will most often not exceed ~bout 160 mM/kg.
Z5 The composition and use of the late proembryo development
culture medium is Important to the success of the present process. It
differs from the induction medium by hAving a similar level of plant
growth hormones to those present in the maintenance and multiplication
medium However, ~or many species such as Pinus taeda and Pseudotsuga
menziesii, the late proembryo development media should have a concentra-
tion of osmoticants that is significantly raised above that of the
induction or multiplication media. The optimum osmoticant levels at
each stage will usually differ for each species and often for individual
genotypes within a species ~or loblolly pine the osmotic level should
35 typically be of the magnitude of at least 200 mM/kg and preferably about
240 m M/kg or even higher. ~owever, lower levels of about 1~0 m M/kg
mintmum will suffice for most genotypes of Douglas-fir. The key advan-
tage of this osmotic "pulse" is th~t proembryo quality and/or si~e can
WO 9~/05854 ~ PCr/US90/06057
be significantly improYed. Some species such as Picea abi~s, which are
relatively easy eO reproduce, may not generally require this raised
osmotic level, or it may only be necessary for some genotypes. In these
cases late proembryo deYelopment may usually be achieved without a
5 change in medium composition from the maintenance and multiplication
medium.
Incubation at this stage is usually carried out in the dark or
in greatly reduced light until robust late stage proembryos have formed.
These may then be l~<,..ar~.,v~ to an embryo development medium which
10 preferably lacks auxins and cytokinins entirely.
Many investigators refer to cotyledonary embryo development
simply as a "development" stage and that usage will be understood herein
unless the word "development" is otherwise qualified.
Douglas-fir requires an intermediate step between the late
lS proembryo development stage and cotyledonary embryo development stage
wllich is not necessary for other species. The proembryos tend to form
in tight clumps or clusters (FIG. 6) which must first be singulated
- before going to the development stage. This singulation is carried out
in a liquid shake culture which lacks auxins and cytokinins but has
20 exogenous abscisic acid as a necessary new hormone. The level of
osmotic potential is also reduced from that of the late stage proembryo
development medium. ABA will typically be within the range of S-15 ppln
(20-60 uM/L) with osmotic potentia~ levels in the range of 130-140
mM/kg It is most desirable when transfers to fresh media are made that
25 the initial ABA level of the fresh medium should not be higher than the
final level of the medium at the end of the preceeding culture period.
Tilis will ensure a continuously dropping level of ABA during the singul-
ation period. The singulated late stage proembryos (FIG. 7) can then be
1- r~ to a cotyledonary embryo development medium. If the embryos
30 are not singulated they will develop into a tight clump of cotyledonary
embryos which cannot be readily separated and are useless for furth~r
germination (FIG 8.).
Especially when Douglas-fir is being cultured, the osmotic
potential of the development medium should be shflrply raised above that
35 of any of the preceeding media. Initial levels may be in th~ 300-350
mM/kg range but these should be increased to levels of at least about
4~0 mM/kg as development proceeds. If development is stsrted at levels
around 300-350 mM/kg, the osmotic level may be increased during d~v~lop-
-.
? ~ iP f~
wo g~o~ 2 0 6 9 9 ~ ~ PCI/US90/060~7
-12~
ment by a complete medium change, ~ partial change in which some old
medium is replaced, or by adding an appropriate form, such as a solu-
tion, of osmoticants to the medium without replacement of any of the
original medium. Any of these changes msy be considered a transfer to a
"new" medium. It is preferred that the osmotic leveis at the end of the
development period should be at least about 450 mM/kg although with some
genotypes lower levels are acceptable. These higher levels tend to
prevent deterioration and callusing of the embryos.
Osmotic potential is best controlled by a combinDtion of
osmoticants. One of these should be a readily metabolized carbohydrate
energy source, preferably a sugar such as sucrose, glucose, fructose,
maltose, or galactose. Sucrose is a preferred ingredient but should be
present in amounts only in the range of 2-3%. The other is a poorly
metabolized osmotlcant of which sorbitol, lactose, or a polyalkylene
glycol would be examples. In a solid development medium, a combination
of sorbitol, lactose and polyethylene glycol has proved very eEfective.
Polyethylene glycol alone, in concentrations of 20-3096 of the medium,
- has worked very well in liquid development media. While the salts andorganic components of the medium make a small contribution to the osmol-
ality, the osmotic potential is primarily controlled by the energy-
providing sugar an=d the other osmoticants. It is within the scope of
the invention to use one combination of osmoticants at the beginning of
development and transfer to a medium having a different combination at
some point during the development stage.
For ~any species a supply of exogenous abscisic acid is a
desirable component in the development medium. This is always used in
combination with an ~dsorbent, such as activ~ted charcoal. The adsor-
bent should be present in a sufficlent amount and form to slowly reduc~
the abscisic acid and remove metabolic waste products. It shouid not be
present in such a high concentration as to deplete the abscisic acid in
a very short time; e.g., in a matter of days. The combination of
abscisic acid with the adsorbent will usually require a higher initial
concentration of abscisic acid than would be the case if no adsorbent
was present in the medium. In the particular case of Douglas-fir, and
perhaps other species as well, the level of exogenous abscisic acid
should be generally continuously lowered over time from the 5-15 ppm
normally found necessary at the beginning of the singulation step to a
level ~erhaps of about 1-2 ppm, or even to ~ero, at the end of th~
WO 91/05854 2 0 6 9 ~ 6 ~ i PCr/US90/060~7
-13-
development stage. Accurate measurements of abscisic acid presenf in
the development system have not yet been made due to the extreme diffi-
culties of analyzing the medium.
In some cases when Douglas-fir is being cultured, sufflcient
~ S abscisic acid will be carried oYer wfth the medium associated with the
embryos from the singulation step so that no additional ABA is needed in
the development medium. In other cases, the level of endogenous ABA
after singulation is sufficiently high so that no exogenous ABA need be
present at all. The terms "sufficient" or "having an adequ~te supply
10 o~" should be considered broad enough to encompass all of these situa-
tions. A small amount of activated charcoal, usually in the range of
about 0.02-0.04% still appears to be necessary in the development medium
to effect the continuing reduction in ABA that began with the singula-
tion treatment. Reduction of ABA to low levels at the end of the devel-
15 opment stage seems to help continue late embryo development andmaturation and also reduces the tendency of precocious germination of
the embryos.
Following embryo development the embryos (FIG.9) may be placed
directly on a germination medium for conversion into plantlets. Alter-
20 natively, they may be converted into artificial seeds by any of a numberof published processes.
An advantage of the present process was the discovery that the
more robust somatic embryos produced by the use of the abscisic acid-
adsorbent combination could be readily stored for extended periods of
2s tillle. ''~everal genotypes of at least two coniferous species (Pinus taeda
and Picea abies) have now been stored without loss of vitality for three
months at 4~-5~C without removing them from the development medium.
P~eudotsu~a menziesii has been stored for oYer one month. This has not
been believed possible with any degree of success before the present
30 invention
The germination medium has no hormones, a lowered organic
nitrogen content, and a reduced level of osmoticants. After a suffic-
ient time in darkness followed by light, or a 16 hour light and 8 hour
dark photoperiod, the cotyledonary embryos will have developed into
35 RIQntlets Douglas-fir does not require an initial dark period although
a one week dark period is useful for Norway spruce. The time period for
germination will be about 1-2 months. The resulting plantlets will have
a well developed radicle and cotyledonary structure with a growing ~pi-
.-.
~O91/05854 ~ 9~ -i t PCI/US90/06057
-14-
cotyl and are ready for planting in soil.
The present invention is most particul~rly concerned w~th thecomposition of the cotyledonary embryo development medi~ snd method of
its use. For Douglas-fir, it has been found that a very high osmotic
5 level in combination with a diminishing level of exogenous abscisic acid
is essential. This combin~tion gives greatly improved numbers and qual-
ity of somatic embryos that are not subject to precocious germinstion.
It is an object of the present invention to produce coniferous
plantlets by somstic emtJIy~l6...~Ji~
It is another object to produce a large clone of a genetically
selected forest specles for reforest~tion using the methods of somatic
emt,.~y~,6..~ , and plant tissue culture.
It is a further object to provide a method of somatic embryo-
genesis that will dependably and consistently provide conlferous plant-
lets in large quantities.
It is yet another object to provide a method of somatic
embryogenesis that can dependably snd consistently reproduce large
clones of selected individuals of forest species that heretofore have
not been successfully reproduced by this method.
zo rt is still s further object to provide a method whereby
superior genotypes of coniferous trees can be multiplied by tissue cul-
ture in the large quantities needed for reforestation.
It is also an object to provide a method that will produce
somatic embryos in large quantities with improved robust morphology for
converston into plantlets.
It is a particular object to provide a method and suitsble
culture media for somatic embryogenesis of Douglas-fir that produces
robust somatic embryos with ~ high percentage of conversion to plants
growing in soil.
It still ~nother object to provide a method that generates
robust somatic embryos capable of withstanding extended periods of cold
storage.
These and many other objects will become readily apparent to
those skilled in the art by resding the following detailed description,
t~ken in conjunction with the drawings.
-
~ -Is- ~2~g6 4
.
s
.
20 DETAILED DESCRIPTION OF THE PREFERRED EM~ODIMENTS
The process of the present invent~on is not limited to sny
single culture medium or to the use of spec~fic growth hormones. Any of
a ~lumber of well known media, such as that of Murashige and Skoog
(1962), may be used. Howeverl the present inventors have found the
2~ basal medium described in Table 1 to give excellent results, partic-
ularly when used for culturing loblolly pine ~Pinus taeda). The basal
medium is modified for each of the various culturing stages as shown in
Table 2. Simiiar media ~.li~ preferred for Norway spruce (Picea
abies) are g5ven in Tables 4 and 5.
.. :
.
WO91/058~4 20~64 '~ PCI/US9O/06057
-16-
Ta
Pinus Taeda B~s~l Medium (Modified 1/2 P6 Bssal S~lts )
Constituent Concentr~tion, mg/L
NH4NO3 603.8
KNO3 909.9
KH2PO4 136.1
Ca(NO3)2 4H2O 236.2
MgSO 7H O 246.5
Mg(NO3)2 6H2O 256.5
: . MgC12'6H20 50.0
1~1 4.15
H3BO3 15.5
MnS04'H2o 10.5
ZnSO4 7H2O 14.4
NaMoO4 2H2O 0.125
CuSO4 5H2O 0.125
CoCI 6H O 0.125
FeSO 7H O 6.95
Na2EDTA 9.33
Sucrose 30,000.
myo-lnositol 1,000.
Casamino acids 500.0
L-Glutamine 1000.0
Thiamine HCI 1.00
Pyridoxine HCI 0.50
Nicotinic acid 0.50
Glycine 2 00
Agar+ 6,000.
pH adjusted to 5.7
According to Teasdale, Dawson, and Woolhouse (1986) as modified
+ Used if a solid medium is desired
WO91/0'~54 2d69gl6~; Pcr/US90/06057
-17-
Tsble 2
Composition of Media for Different St~ge Treatments
BMl -- Induction Medium
BM + 2,4-D (50 uM) + KIN (20 ~uM) + BAP (20 uM)
BM2 -- Maintenance and Multiplication Medium
BM + 2,4-D (5 uM) + KIN (2 uM) + BAP (2 yM)
BM3 -- Late Proembryo Development Medium
BM2 + 9000 mg/L myo-inositol
B~4 -- Embryo DeYelopment Medium
8M + 4.0 to 8.0 mg/L abscisic acid
15 . B.\q5 --Germination Medium
BM modified by reducing sucrose to 20,000 mg/L, myo-inositol
to 100.0 mg/L, glutamine to 200.0 mg/L, and cusamino acids to
0.0 mg/L
A number of abbreviAtions are used in the following text.
These ~re in common use in the field of tissue culture.
BAP-- N6-benzylamir ;~e (or N6~ .yL~d~ e)~ a cytokinin
KIN -- klnetin (6-furfurylaminopurine), also a cytokinin
2,4-D -- 2,4-dichlol ~ ~ yllcetic acid, an auxin.
NAA -- 2-Naphthylacetic acid (Naphthalene-2-acetic acid)
ABA -- Abscisic acid
It will be understood by those skilled in the art that other
pl~nt growth hormones can be substituted for those just not~d. As
examples, IAA (indole-3-acetic acid), IBA (indole-3-butyric acid), and
30 NAA (naphthalene-2-acetic acid) are effective auxins and 2-lP (N6-iso-
pentenylaminopurine) Qnd zeatin are frequently used as cytokinins.
As an aId in comparing the present work with other published
data, the following table of conversions from weight to molar concentra-
tions might be useful.
1 uM/L_ 1 mg/L
BAP 0.225 rnglL 4.44 ~uM/L
KIN 0.215 4.65
2,4-D 0.221 4.52
NAA 0.816 5.38
40 ABA 0.264 3.78
,
..
-18~ 1 6 g g ~ i~
~ United st~tes Patent No. 4,957, 866 1ssued ~ f' ' lr~,
1990, we pointed out the importance Or the control of osmotic poten-
tial of the media used in the various cu~uring stQges. A l~rge group
of chemicQI msterials are suiteble Qs osmoticQntS. In generQI these are
highly water soluble polyhydroxylated molecules thQt include either
s~mple or complex sugsrs, hexitols, and cyclitols. The cyclitols are
normally six carbon ring compounds thQt are h~dliyd~ yl6ted~ The most
readily Qvailable cyclitol is _yo-inositol but any of the other eight
stereoisomeric forms, such as scyllo-inositol are believed to be quite
10 suitQble. Among the sugars, sucrose and glucose are known to be very
effective but many others should prove to be equQlly useful. Sorbitol
(3-glucitol), D-mannitol, and galactitol (dulcitol) are straight chsin
sugQr alcohols suitQble as osmoticants. Lactose is e SUgQr effective as
an osmoticant. Other materials suitable Qs osmoticQnts mQy include
15 glycol ethers such Qs poly(ethylene glycol) Qnd poly(propylene glycol)
and their respective monomers.
LOBLOLLY PINE CULTURE
Exa m ple
The following schedule of treQtments hQs been very success-
fully used for the growth of plQntlets by somQtic embr~og~r.csia of
loblolly pine (Pinus tQedQ). ExplQnts were immQtUre embryos dissected
from seeds 4 to 5 weeks after fertilizQtion. Seeds were obtained from t
cones supplied by a W~J_.hC~ CompQny seed orchard locQted at WQsh-
25 ington, North Carolina. The cones were stored Qt 4~C until used.
ImmediQtely before remoYdl of the immQture embryos the seeds were steri-
lized using Q modified method of GuptQ Qnd DurzQn (1885). Briefly, this
involves Qn initial washing and detergent treatment followed by Q first
sterilizQtion in 30~ H2O2 Qnd a second in diluted 10% v/v household
30 bleQch. The QdditionQI HgCl2 treQtment used by GuptQ and Durzan WQS not
found to be necessary to ensure sterility. The eXplQntS were thoroughly
wQshed with sterile distilled water after each treatment.
StaFe I - Induction Sterile dissected embryos were plQced on
Q solid BM1 culture medium Qnd held in Qn environment Qt 22~-25~C with Q
35 24 hour dQrk photoperiod for Q time of 3-5 weeks. The length of time
depended on the pQrtiCUlQr genotype being cultured. At the end o~ this
time Q white mucilQgenous mass hQd formed in associQtion with the orig-
inQI explQnts. This appears to be identicQI with thQt described by
2~ ~9 9 ~
WO 91/05854 PCI/US90/06057
-19-
Gupta and Durzan (1987). Microscopic examination revealed numerous
early stage proembryos associated with the mass. These are generally
characterized as having a long thin-walled suspensor associated with a
small head generally hsving less than 10 individual cells, each with
~ 5 dense cytoplasm and large nuclei. Early proembryos are illustrated in
FIG. 1.
~ Osmolality oE the induction medium may in some instances be as
hiFh as 200 mM/kg. Normally it will be below 1~5 mM/kg and, more typi-
c~lly, about 160mM/kg or even lower. The osmolality of the medium
10 described above was 158 mM/kg.
Stage 11 - Maintenance and Multiplication Early stage pro-
embryos removed from the masses generated in the induction stage were
pl~ced on a BM2 medium. This differs from the induction medium in that
the growth hormones (both auxins and cytokinins) were reduced by a full
15 order of magnitude. The temperature and photoperiod were again 22~-25~C
with 24 hours in the dark. Osmolality of this medium will typically be
similar or identical to that of the induction medium. In the present
example it was identical. Proembryos developed in this stage were
similar in appearance to those from Stage 1 and were subcultured every
20 12-15 days on BM2 medium.
Stage 111 - Late Staf~e Proembryo Development Early stage
proembryos from either Stage I or Stage ll,-preferably the latter, were
placed on a BM3 solid medium. This medium has the same growth hormone
concentration as BM2, however, the osmoticant was raised to e much
25 higher concentration. In this case the osmoticant, myo-inositol, was at
a concentration of 10,000 mg/L or 1% on a w/v basis. Osmotic potenti~l
was measured as 240 mM/kg. Temperature and photoperiod were the same as
for Stages I and 11. After 3 or 4 subcultures of about 12-lS days each,
very robust late stage proembryos hsd formed. These ~ure characterized
30 by smooth eribryonal heads generally having in the r.~:bl-L~ od of over
lQ0 individusl cells with multiple suspensors, as exemplified in FIG. 2.
Osmotic potential of the l~te proembryo development medium should usu-
ally fall within the range of about 200-400 m M/kg for Pinus tueda. Most
typically it should be in the neighborhood of about 1.5 times higher
35 than that of the induction or multipliction media. As was noted ear-
lier, the requirements for elevation of osmotic potential at this stage
will vary for different species.
:
2 0 6~
WO91/05854 ~ PCI/US90/060~i7
-20-
AlternatiYely, the Stage 11 and/or Stage 111 proembryos could
be cultured for late proembryo development in suspension in a liquid
medium of similar composition to BM3 but Iscking the agar. In this case
subcultures could be msde every 7-8 days.
It is preterred that early stsge proembryos brought into Stage
Ill culture should have a Stage 11 subculturing for rapid multiplication
of the particular clone. However, on occasions where time may be of
greater importance than quantity, early stage proembryos from Stage I
may be taken directly into Stage 111.
Stage IV - Embryo Development The late stage proembryos from
Stage Ill culture were transferred to a solid BM4 medium. This medium
either lacks growth hormones entirely or has them present only at very
low levels and has the same lower level of osmoticants as Stages I and
II. However, abscisic acid (5-(1-hydroxy-2,6,6-trimethyl-4-oxo-2-cyclo-
15 hexen-1-yl)-3-methyl-2,4-pentadienoic acid) had been included here as a
necessary material for further development. A critical aspect of the
present invention is the further inclusion of an adsorbent material in
this medium. The adsorbent may be chosen from a number of chemical
materials having extremely high surface area and/or controlled pore size
Zo such as activated charcoal, soluble and insoluble forms of poly(vinyl
pyrrolidone), activated alumina, silica gel, molecular sieves, etc. The
adsorbent will normally be present in a concentration of about 0.1-5
g/L, more generally about 0.25-2.5 g/L. The contribution of the adsorb-
ent appears to be complex and is not well ~ r~ o~1 Adsorbent mater-
Z5 ials, especially activated charcoal, haYe been widely used in the pastin various culture media. However, the particular combination of activa-
ted charcoal with relatively large amounts of abscisic acid in a late
stage somatic embryo development medium is believed to be entirely new.
The prevalling wisdom found in the literature clearly teaches away from
30 use of this combination, especially at this point in the process.
The osmotic potential of this medium will generally be no
greater than about 175 mM/kg. In the present case it was measured as
168 mM/kg. As before, development was carried out in complete darkness
at a temperature of 22~-25~C. Development time was 4-6 weeks after
35 which elongated cotyledonary embryos 4-5 mm long were present. These
appeared as represented in FIG. 3.
WO 91/05854 2 û 6 9~8~ p~/US90/06057
-21 - ~ =
Stage V - Germination Cotyledonary embryos from Stage IV were
pl,lced on solid BM5 medium for germination. This is a basal medium
lacking growth hormones which has been modified by reducing sucrose,
m o-inositol and organic nitrogen. After about 6-8 weeks under environ-
5 mental conditions of 23~-25~C and a 16 hour light/8 hour dark photo-
period the resulting plantlets were Approximately 20 mm in length and
had a well deYeloped radicle and hypocotyl and green cotyledonary struc-
tu~e and epicotyl. The young plsntlets are shown in PIG. 4.
Because of the reduced carbohydrate concentration, the osmotic
10 potential of the germination medium is further reduced below that of the
development medium. It will normally be below about lS0 mM/kg and was,
in the present example, about 100 mM/kg.
Stage Vl - Plant Frowth Plantlets from Stage V were removed
from the culture medium and planted in a soil comprising equal parts of
15 peat and fine perlite.
To the present time, three distinct genotypes of Pinus taeda
ha~e been successfully cultured through Stage V. Some of the plantlets
ha ~e already been successfully transferred to soil and these are growing
with good vigor. Two additional genotypes are being multiplied in Stage
20 ll prior to Stage Ill treatment. In work that preceeded that just
described, all five genotypes when cultured without the Stage 111 high
osnnoticant treatment ultimately browned and died in Stage IV. Stated
differently, the method failed completely when early stage Pinus taeda _ =
proembryos from Stage Il were taken directly into Stage IV, as is taught
25 in the prior art.
While inorganic salts and pure simple organic chemicals gener-
ally behave similsrly in culture media regardless of suppiier, there are
occasions when this is not the case for the more complex materials.
Without intending endorsement of any product over available alterna-
30 tives, chemicals from the following suppliers were used throughout theexperiments to be described in the examples. Agar was obtained from
Di~co Laboratories, Detroit Michigan. Where specified as "tissue cul-
tuI~e agar" the supplier was Hazleton Biologics, Inc., Lcnexa, iiansas.
Casamino acids, a casein hydrolysate, was also supplied by Difco i abor-
35 atories. Activated charcoal was obtained from Sigma Chemical Company,St. Louis Missouri, as their grade NuC-4386.
WO91/05854 20~ PCr/US90/06057
--22--~
Example 2
The combination of ABA and activated charcoAI in the Embryo
Development Medium has proved to be very effective not only with Pinus
taeda but with other important conifer species such~ as Picea abies and
Pseudotsuga menziesii. Tn the following experiments the~ Loblolly Pine
Basal Media of Tables 1 and 2 were used~ .In the Embryo Development
Medium the ABA was adjusted as described in Table 3 and Activated char-
coal was included in a concentration of 2.0 g/L. All of the ingredients
except the abscisic~ acid were combined, autoclaved, and cooled to 50~-
60~C. A filter sterilized solution Pf ABA was then added and mixed.
After 10 minutes the medium was poured into petri dishes.
Late stage proembryo cells of two loblolly pine genotypes,
grown as described in the first example, were settled from a suspension
culture, the supernatant liquid poured off, and 1 - 1.5 mL of the set-
tled cells were plated on the solid Embryo Development Medium in S cm
dishes. These cultures were incubated in the dark at about 22~C for six
weeks. Control cultures having 2 and 4 mg/L ABA without activated
- charcoal were also prepared. The following results were obtained.
Table 3
Medium Composition Embryos Produced
ABA, mg/L Activated Charcoal, g/L Genotype A Genotype B
25 2.0 0.0 2.5
4.0 0.0 5.5 --
20.0 2.0 0 0
40.0 2.0 2 2
60~0 2.0 4 3
30 80.0 2.0 10 4.5
100.0 2.0 8.5 2
The embryos produced on the charcoal containing media were of
better morphology with a well developed cotyledonary structure but
35 without evidence oE germinating precociously when compared to those
grown without activated charcoal in the medium. The media described
here are not represented as being optimized for the species or any
genotype.
WO 91/05354 2 ~ 6 ~ ~ 6 ~'t~ PCI~US90/06057
~ -23- = ~ = ~
~ORl''AY SPRUCE CULTURE ~ _
Exa m ple 3
Some coniferous species sre relatiYely easier to propagate by
somatic embryogenesis than others. Coastal redwood, Sequoia semper-
S virens, is considered be be a relatively easy species while Norway
spruce, Picea abies, is usually thought to be of only moderate diffi-
culty. Most members of the genus Pinus as well as Douglas-fir, Pseudo-
tsuga menziesii, are regarded as very difficult. This has posed a major
challenge to researchers since the latter two genera include a major
10 percentage of the worlds most economically import~nt timber species.
Even though past ~ rcl~ have reported success with somatic embryo-
genesis of several pines and of Douglas-fir, others in the field have
frequently not been able to duplicate the work of these competent inves-
tigators. There are probably several reasons for this. Most certainly,
15 one of them is over optimism on the part of researchers who have
achieved and reported early stage embryogenesis or embryo-like struc-
tures but who later have not been able to succeed in producing signifi-
cant numbers of cotyledonary embryos or plantlets. Another is the great
differences in performance between different genotypes within a given
20 species. Picea abies is a case in point. As noted earlier it is usu-
nlly regarded as a species of only moderate difficulty to reproduce by
somatic emvlyv~ wia using present state-of-the-art technology. ~low-
ever, there are some genotypes of Picea abies that haven proven intract-
able to all previous efforts. Most researchers have limited themselves
25 to working with only one or two genotypes that are known from past
experience to give good results.
Our method has resulted in successful production of late stage
proembryos and cotyledonary embryos on 23 of the 26 genotyp~s of Picea
abies that have been investigated to date. This sample includes a
30 considerable number of previously intractable genotypes. FIG. 5 shows
the maximum yield of embryos per culture plate for 19 genotypes grown on
the same nonoptimized culture (Medium No. 2 as describ~d in Exampl~ 6).
Seven of these are from non-select wild seed and twelve are select seed
from known half-sib orchard fr~milies. The enormous differences in
3s bellaYior constituting two full orders of magnitude, especially within
the non-select seed, are immediately apparent. As has been noted
~ eallier, similar results have be~n obtain~d with Pinus taeda, although
nol all genotypes h~ve b~n proc~ss~d to the later stages of treatment
! '
WO 91/05854 2 0 6 ~ 9 6 ~-24- PCI/US90/060~7
a[ the present time.
While the plsnt growth hormone usages noted in Table 2 are
near optimum for loblolly pine, different concentrations and mixtures
may prove more suitable for other species. It is fairly well estab-
5 lished that growth hormones are usually necessary in Stages 1-111,
although some workers have apparently achieved early stage proembryos
using growth hormone-free media. However, even when initially cultured
on hormone-free media, these early stage proembryos were then transfer-
red to cultures having the usual growth hormones. These hormones may in
10 some instances be a single auxin or a mixture of auxins with or without
one or more cytokinins. As a general rule the total concentration of
all growth hormones should be below about 250 ,uMIL, preferably below
about 100 uM/L in the St~ge I medium. These cQncentrations should be
reduced about tenfold in the Stage 11 and Stage 111 media.
The folrowing tables show preferred media for culture of
Norway spruce by somatic embryogenesis.
.
2~6~9~
WO 91/058~4 r ~ ~ ~ 3 ~; PCr/usgo/o6057
-25-
Table 4
Picea Abies Basic Culture Media __
Constituent Concentration, mg/L
- ~ A(l) B(2)
5 BASAL SALTS
NH4NO3 -- 206.3
KCI 372.5
RNO3 50.0 2340.0
KH2P~4 85.0 85.0
MgS04 ?H2~ 160.0 185.0
CaC12 6H2~ 220.0 220.0
-~1 0.415 0.415
H3BO3 3.10 3.10
MnSO4-H2O 8.45 8.45
ZnSO4-7H2O 4.30 4.30
NaMoO4 2H2~ 0.125 0.125
CuSO ~5H O 0.0125 0.0125
CoC12-6H O 0.0125 0.0125
FeSO ~7H O 13.90 13.93
Na2EDTA 18.65 18.63
ORGANIC A~DITIVES
Sucrose 10,000. 30,000.
myo-lnositol 50.0 1000.0
Casamino acids -- 500.0
z5 I~Glutamine 750.0 450.0
Thiamine-HCI 0.05 1.00
Pyridoxine-HCI 0.05 0.50
Nicotinic acid 0.25 0.50
Glycine -- = 2.00
L-Asparagine 50.0
pH 5.8 5.7
(1) Institute of Paper Chemistry medium (~erhagen and Wann 1989)
(2) Gupta and '~urzan medium BM3 (1986b).
,
WO 91/05854 2 0 6 9 9 6 4 -26- PCI/US90/06057
Tabie 5
Composltion of Picee Abies Media for Different StaFe Treatments
BMI -- Induction~Medium
BMA~1) + NAA(3) (10.8uM) + BAP(4) (4.4uM) + 7.0g/L Difco agar.
BMM -- Mainte~nce and (M5)ultiplication Medium
BMB + 2,4-D (5 uM) + BAP (2uM) + KIN(6) (2~uM).
6.0 g/L Difco agar added if solid medium is desired.
10 BMD --Colyle~ y Embryo Development Medium
BMB + 40.0 mg/L Arginine + 100 mg/L Asparagine + 6.0 g/L
Tissue Culture Agar + Abscisic acid (as specified) + Adsorbent
(e.g., activated charcoal) (as specified). KNO3 is reduced to
1170mg/L in basal salts.
BMG --Germination Medium
BMB with KNO3 reduced to 1170 mg/L, myo-lnositol reduced to
100 mg/L, Sucrose reduced to 20.0 g/L, and L-Glutamine and
Casamino acids removed. 2.5 g/L of Adsorbent and 6.0 g/L of
Tissue Culture Agar are added.
(1)Basic medium A from Table 4
(2)Busic medium B from Table 4
(3)2-Naphthylacetic acid (NaphthAl~ne-2-acetic acid)
25 (4)N6-8en~ylaminopurine
(5)2,4-Di ~ ~,L,h~ .y~lcetic acid
(6)Kinetin
Example 4
3Q The following screening experiment was made as a comparison
between embryo development stage cultures contAining only abscisic acid
as a hormone additive with cultures containing a mixture of abscisic
ac~d and activated charcoal. Mature Picea abies seed embryo explants
were cultured on an Initiation Medium and Maintenance Medium as
35 described in Tables 4 and 5 . Explants were incubated in light of an
intensity approximately 50 ~uEm 2sec 1 In this case the Induction
Medium BMI had a relatively low carbohydrate content with a resulting
low osmolarity of about 62 mM/kg. After an early stage embryogenic mass
had developed, it was transferred to a solid and later to A liquid
40 liquid Maintenance and Multiplication Medium BMU hQving a higher osmol-
ar~ty of about 158 mM/kg. In this case the proembryos had attained a
sufficiently late stage of development without the need for further
culturing on a very high osmotic potential Late Proembryo Development
Medium. These proembryos were settled and washed twice with liquid
~ _z7_ r 2 0 6 9 g ~ 4
Embryo Development Mediurn BMD of Table S to which 10 mg/L of abscisic
acid had been sdded. The washed cells were then drained on polyester
pads. Approximately 2 ml: of the wsshed cells were transferred to solid
Embryo Oevelopment Medium B'.~D on 50 mm petri dishes. The growing celis
S were transferred twice at two week intervals to fresh medis of the same
composition. Culture room conditions were ebout 23-24~C in dqrkness
throughout the experiment. The following table shows the compositions
of the media used and the results obtained.
Table 6
Medium Comr~qi~ion BMD Cotyledoifary Embryos,
ABA, m~/L Activated Charcoal, ~/L Average Yield/2 mL
2.0 0 18;6
15 5.0 0 20.0
7.5 0 13.0
10.0 0 12.9
25.0 0 14.7
25.0 2.S 2.0
20150.0 2.5 4.1 _
100.0 2.5 16.1
250.0 2.5 0.1
The ABAlcharcoal media did not in general produce as many
25 cotyledonQry embryos in this experiment as the media with ABA alone.
However, it was noted that the embryos produced on the media containin~
charcoal were frequently larger and of superior morphology to those
cultured on the media containing only ABA. This experiment showed that
emt)ryos could be successfully cultured on media hav~ng relatiYely high
30 concentrations of ABA and activated charcoal. Further, there was an
indication that these embryos would have superior strength to those
cultured on media containing only ABA.
It was noted during the experiment that development and mass growth
on the charcoal containing media was so rapid that 10-14 days after
35 starting the experiment most growth had stopped and the mass containing
the embryos appeared dry. This suggested that aQailable liquid was
absorbed during early growth and may have become limiting.
The above experimenl was carried out on three additional
genotypes of Plcea abies. In these tests no preliminary washes with ABA-
40 containing medium were given. Results were variable. One genotypeproduced no cotyledonary embryos under any cond~tions. Another produced
an average of 10.2 embryos on th( m~dia without charcoal and only 0.4
,
.
f*
.
wo gl/05854 ~ A ?~ " PCr/US90/06057
~ 2~69964 -28- ~
eMbryos on the charcoal containing media. The third genotype produced
an average of only 1.7 embryos on the ABA only media and 2.0 embryos on
the charcoal containing media.
It should be noted that the above reported experiments were of
5 a preiiminary screening nature only and do not represent optimi~ed
conditions. They were primarily made to see if the use of activated
charcoal in the cotyledonary embryo development stage would be advuntag-
eous.
Example 5
In a followup experiment to that just described, five geno-
types of Picea abies were cultured as described above using a solid
co~yledonary development medium containing 100 mg/L ABA and 2.5 g/L
activated charcoal. This time the liquid BM~q medium containing the
15 proembryos was simply settled and the supernatant liquid poured off.
Then 2 mL of the settled cells were pipetted onto the surface of the
solid medium without washing or further draining in order to provide
additional water for the system.
Embryos were visible within two weeks and harvestable by 3 1/2
20 weeks. One genotype produced 34 robust cotyledonary embryos per mL of
settled cells. Another produced 5.3 embryos per mL. The other geno-
types produced few or no embryos.
160 cotyledonary embryos of the best performing genotype were
transferred to a germination medium, incubated three weeks in the dark,
25 ~ sf~ d to fresh media and moved to the light. About 25 % of these
embryos began epicotyl development after five weeks.
Again, it should be noted that no optimization of the ABA-
charcoal ratio had been attempted.
Example 6
Another set of experiments was made under conditions similsr
to the previous eYample in which activated ch~rcoal in the cotyledonary
embryo development medium was varied between 0 and 2.5 g/L and ABA
varied between 5 and 10D mg/L. Four genotypes of Picea abies was used
35 in the eYperiment. At least five replicates were made at each test
condition.
Media compositions are given in the following table.
-
WO 91/~;854 - 2 ~ 6 9 g 6 ~ ~ PCr/US90/06057
-zq- \ ~
Table 7 _ _ _
Medium ABA, Charcosl, AverAFe Embryos/mL, Genotype
No. m~/L i~/L _ B _ _ D
100 2.5 46 0 0.5 13.2
2 50 1.25 102 0 1.8 9.5
3 10 0.25 76 0 0.6 20.4
4 10 0.10 18 0 0.2 5.4
10 5 5 0,0 0 0 0 0
Medium No. 2 contAining 50 mg/L ABA and 1.25 g/L activated
cllarcoal appeared to be significantly better than the others from the
standpoints of number and vigor of embryos formed.
In a further modification of this experiment the best perform-
ing genotype above (Genotype A) WAS trensferred onto the medium sup-
ported on laborAtory filter paper. The experiment was repiicated using
the same culture media as Above. As is seen in the following table, the
results of cultures grown on agar medium alone and on filter paper
20 supported on Agar media are generslly compArable. This opens up the
pQssibility of direct mAss trAnsfer to germinAtion or other media with a
consfderable savings in hAndling required. It further opens the possib-
ility of a system in which either the charcoal or ABA is locat~d on the
filter pAper with the other component being in the medium.
2~
TAble 8 ~ . =
Medium ABA, Charcoal, Avera~e Embryos/mL,
30 No. ~ mg/L g/L Directly on Agar On i'ilter Paper
100 2.5 46 43
2 50 1.25 ~ 102 93
3 10 0.25 75 55
4 10 0.10 17 30
35 5 5 oo 0 0
Example 7
A comparison was mAde using four methods of culturing PiceA Abies
at the embryo development stage. The first method used Embryo Develop-
40 ment Media containing both ABA and activated charcoal. One subset was a
- replication of the Medium 2 composition from the previous example thAt
gaYe the best results. Another subset used reduced amounts Or both ABA
and Ac~ivsled ch~rco~i. in th~ second method the charcoAI WAS omitted
from the culture medium but th~ lAte stAge proembryos were first coAted
WO91/05854 2~6~a~ PCI/US90/060~7
-30- ,~
with Qctiv~ted charco~l by rinsing them with a chsrcosl-containing
liquid medium. The third method ~irst cultured the late stflge pro-
embryos on 8 medium contsining only sctivsted charcosl followed by cul-
turing on a medium containing only ABA. ~inslly, cultures were made on
5 ABA-containing medium without any charcosl. In one subset of this
method the growing embryos were transferred three times to fresh medium.
In the other subset the embryos remsined on the original medium the
entire culturing period.
Two new genotypes of Picea abies not tested in the eQrlier
10 examples were used for the present set of experiments. Results are
given below. The compositions of all the medis used are given in the
Table 9. Late stage proembryos, cultured ss in the l~st three examples,
were used for all trials.
Table 9
15 Medium No. _ _ Composition
BMD (Tnble ) + 15 mg/L ABA + û.75 g/L activated charcoal
2 BMD + 50 mg/L ABA + 1.25 g/L activated charcoal
3 BMM without hormones or agar + ~.5 g/L activated charcoAI
4 BMD + 10 mg/L ABA
BMD + 15 mg/L ABA
6 BMD + 10 g/L activated charcoal
7 BMD + 5 mg/L ABA
PROCEDURES ~ RESULTS
25 First Method -- ABA/Activated Chsrcoal Medium
1.0 mL of settled late stage proembryos was pipetted directly
onto replicate 5 cm plates of media 1 and 2 above and cultured in the
dsrk at about 22 ~C for six weeks.
Embryos Produced Genotype A Genotype B
Medium 1 54.0 26.3
Medium 2 83.0 92.5
These embryos had improved apical domes, hypocotyl region ~nd
35 root primordia when compared with embryos cultured on a development
medium using ABA alone. Also a greater number of embryos were produced
using the charcoal containing m~dia. Ultimately the germin~tion rate
and successful growth into plantlets W85 also incre~sed ov~r a con~rol
WO 91/0~;854 2 0~ ~ ~ &~ PCI/US90/06057
-31-
group grown without Qctivated charcoal.
Second Method -- Activated Chsrcoal Coated Embryos on ABA Medium
Settled late stage proembryos were suspended in \qedium 3
5 above, settled, and then 1.0 mL was pipetted onto replicate 5 cm plates
of media 4 and 5 above. Most of the activated charcoal in the rinse
medium was retained with the settled proembryos. These were also cul-
tured in the dark for six weeks at about 22~C
~ Embryos Produced Genotype A Genotype B
Medium 4 0.0 1.0
Medium 5 11.8 35.8
These embryos atso had the improved morphology noted for those
15 produced by the first method.
Third Method -- Activated Charcoal Medium then ABA Medium
1.0 mL of settled late stage proembryos was pipetted directly
onto replicate 5 cm plates of medium 6 (activated charcoal only). These
20 were cultured for one week at 22~C in the dark then were trQnsferred to
medium 7 (ABA only) for five weeks. No cotyledonary embryos Nere
produced for either genotype.
Fourth Method -- ABA Medium With and Without Transfers
25 = 1.0 mL of settled late stage proembryos was pipetted directly
onto replicate 5 cm plates of medium 7 (ABA only). These were cultured
in the dark at about 22~C. One subset was maintained on the original
medium for the entire six week period. The other subset was Iransferred
to fresh medium of the same composition at the end of the second and
30 fourth weeks, then maintained on the last medium until the end of the
test.
Embryos Produced Genotype A Genotype B
l~ot transferred 0.0 0.0
35 -. Transferred 2.0 3.3
The few cotyledonllry embryos that developed were of poorer
quality than those de~eloped using the first and second methods. They
- _~
WO91/OS854 ~ 9~ PCI/US90/06057
--32--
did not show the prominent apicQI domes And had a shorter, orten bulg-
ing, shape.
It is very evident that the combination of ABA and activated
charcoal in the Embryo Development Medium is highly advantageous from
5 the standpoint of both numbers and quality of embryos produced. It
appears that the charcoal is most e~fective when it is uniformly
dispersed throughout the medium. However, it is also advantageous when
it is localized around the growing embryos. It is believed that the
charcoal could also be localized on the upper surface of the medium with
10 similar good results. For example the activated charcoal could be on a
filter paper or other type porous membrane which might also be used as a
support surface ~or the growing embryos. It should be noted here that
none of the media compositions used are represented as being optimized
for any of the genotypes employed.
To the present time, using the procedures just outlined, about
3800 plantlets of Picea abies have been produced from 20 different
genotypes of the spec~es and established in soil.
The use of the combined ABA-activated charcoal systems gave an
additionul entirely, ted advantage. Somatic embryos generated on
20 these media could be stored for extended periods of time at 4~-5~C while
remaining on the Embryo Development Medium. A number of genotypes of
both Pinus taeda and Picea abies have now been stored for_over three _
months without any evident deterioration or loss of vigor. Embryos of
PseudotsuFa menziesii have been stored only for shorter periods at the
25 present time but they too seem to have retained full vigor. These
stored embryos develop normally into plantlets when moved to a rooting
medium~ Storage temperatures from just above the freezing point of the
embryos (approximately 0~C) to about 10~C appear to be very satisfac-
tory. It would appear that the temperature needs to be lowered only
30 sufficiently to essentially inhibit metabolic action within the embryos.
Storage is preferebly done in the dark or on low light conditions.
Prior to the present time long term storage usually required desiccation
(e g., see Buchheim, Colburn and Elanch 1989) or alginate gel encapsula-
tion (e.g., Gupta and Durzan 198~).
-
WO 91/058S4 2 0 6 ~ ~q~ ,PCI~/US90/060S7
33-
DOUGLAS FIR CULTUFE _ _
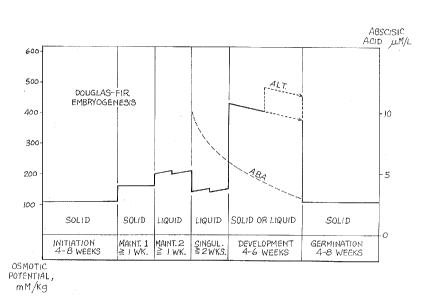
- A number of observations in the 12boratory led to the hypo-
thesis that considersbly raised osmotic levels would be advantegeous in
the embryo development stage of Douglas-fir, and perhaps other difficult
S to culture species as well. Very high osmotic levels would tend to
gradually remove moisture from the developing embryos. This might pro-
vide an analogous situation to the later stages of embryo development
experienced in the formation of a natural seed. Prior to this time
Douglas-fir cultures used a development medium containing 2-3% sucrose
10 and having an osmotic potential in the 130-160 mM/kg range. The result-
ing somatic embryos were generally of poor quality and the conversion
rate to rooted plants was low. The experiments outlined in the follow-
ing examples were designed to test the above hypothesis and to optimize
conditions if results were ~II;OUI~~ g.
lS ~ : ~ As t~oted in the background discussion, the embryogeny of
Douglas-fir is quite different from trees such as the spruces or pines.
One of these differences is seen when early stage proembryos are placed
in or on a late stage proembryo development medium. Instead of single
Iflte stage embryos, Douglas-fir develops tight clumps of these embryos,
20 as is shown in the photomicrograph of Figure 6. Upon further develop-
ment into cotyledonary embryos, these clumps remain united snd the
resulting product is useless for further conversion (Figure ~). This
phenomenon had apparently been recognized earlier by Durzsn and Gupta
(1987) who, while they did not discuss it specifically, transferred
25 their embryonal-suspensor masses to a liquid shake culture containing
0.5 ~uM abscisic acid. They note that under the influence of ABA, indi-
vidual bipolar embryos were produced which were then tr~nsferred to a
development medium without ABA. The present method utilizes a liquid
sllake culture with reduced osmotic level and added abscisic acid between
30 late proembryo development and cotyledonary embryo d~velopment to
achieve the necessary singulation.
A reformulated basal culture medium hfls been developed by the
present inventors specifically to give more successful initiation and
multiplication of Douglas-fir. Preferred media compositions are given
3S in the following tables. A number of ingredients, such as those that
affect the level and balance betwe~n organic and inorgenic nitrogen, are
varied in quantity depending on the respons~ of individual genotypes.
This response cannot b~ reAdil~ predict~d and m~dia optimization must
WO 91/05854 2 ~ 6 9 9 ~ ~ P~/US9ff/06057
--34--
largely be achieved by a combination of in~uition and ~rial and error.
Tsble 10
Pseudotsu~ Menziesli Basic Culture Media
Constituent Concentration, mg/L
WTC(I) BMG(2)
BASAL SALTS
NH4NO3 -- 206.3
10 KNO3 v~ries(l) 1170.0
CACl2 6H2O 200.0 220.0
Ca(N03)2 2H20~ varies(l) -- --
KH2PO4 340.0 85.0
MgSO4 7H2O 400.0 185.0
15 MnSO4~H2o 20.8 8.45
znSO4 7H2O 8 0 4.30
CuSO4~sH2o 0.024 0.013
FeSO4 7H2O 27.85 13.93
Na2EDTA 37.25 18.63
20 H3BO3 5 3.10
NaMOO4~2H2o 0.20 0.125
CoC12 6H2O 0.025 0.0125
Kl 1 00 0.42
AIC13 0.02
ORGANIC ADDITIVES
myo-lnositol Y~ries(l) 100.0
Thiamine-HCI 1.00 1.00
Nicotinic acid 0-50 0-50
30 PyridoYine- HCI 0.50 0.50
Glycine 2.00 2.00
L-Glutamine Yaries 450.0
Casem~no acids 500.0
Sucrose varies 20,000
35 pH 5.7 5.7
(1) Usage varies according to culturing stage and g~notype.
(2) Modified Gupta and Durzan medium EM3 (1986b)- M~dium B 'SG ~
application Seri~l No. 426,331.
-
WO 91/05854 2 ~ 6!~ , p~" J~ o"~
-3~-
~o o o O . o o
b.o' '- '~ - I j ~ I j j j j o o
'' o ' o
~ _ ~ o o ~ '
bD j o I C' o i I I , ~ I O
~ o 10 o ~
o
bD' O C~ -- O I ~ i i i i , i I I C
o o
ID-- -- -- ~ O O
~o ~ _
C _ o .,. i o I ~ ~ ~ I j o ,
cq ~ _ O O O ~
o In
o
~:o o o o ~ _
b.O ~ j O U~ I o l ,, u, c'' l O O , E '~
_ I _ I In ~ I a.
o --
-- E ~-- c
o _ '~
O ~ ,l, C ~, l, Cl_ y ~ . C ~
~r o ~ c --
~O91/05854 20~i PCI/US90/06057
-36-
It will be seen by reference to the mediu compositions thatthe features of the earlier inventions described in our parent applica-
tions have been advantageously retained. A raised osmotic pulse is
still advantageous for good quality late proembryo development. This
5 level will differ somewhat between species and even between genotypes
within each species. Similarly, the cotyledonary embryo development
medium should cont~in the same combination of abscisic acid and acti-
vated charcoal found so desirable with Norway spruce and loblolly pine.
The examples that follow represent steps in the evolutionary
10 process of formulating a Douglas-fir development medium that represents
the best mode known at present for culturing this species by somatic
embryogenesis. These examples are all directed to the cotyledonary
development stage. The steps prior to that time are similar to those
used for loblolly pine and Norway spruce with the exceptions of the now
essential embryo singulation stage and somewhat reformulated media.
These earlier steps will be briefly outlined.
A preferred explant for Douglas-fir is an immature zygotic
embryo. Best results have been realized with embryos selected iust
prior to the development of an apical dome up to the time just before
cotyledon primord~a become Yisible. The cones are split longitudinally
and seeds isolated from young ovuliferous scales. Seeds are sterilized
by first being agitated in 10 % Liqui-Nox laboratory cleaner (Alconox,
Inc, New York, New York) with a small additional amount of liquid sur-
factant for about 10 minutes. They are then rinsed in running tap water
for 3û minutes. At this time they are transferred to a sterile hood and
agitated in 2096 HgO2 for 10 minutes. Following five rinses in sterile
deionized water the seed coat is split and the female gametophyte
removed. This is split on one side and the embryo teased out while
still remaining attsched to the gametophyte by the suspensor. An
explant so prepared is placed on the Stage I solid initiation medium in
a 50 m m petri dish. The explants are incubated in the dark from 4-8
weeks. Success in forming an embryonal-suspensor mass (ESM) containing
proembryos varies from about 1-796 depending on a number of variable
factors which presently are not well understood.
All stages of culture are carried out at temperatures which
may vary between about 20-25~C. Temperature is not generAlly critical
and may, on occasion be varied so as to fall outsid~ this range.
The embryonal-suspensor masses containing early stag~ pro-
WO 91/0~854 ~ ~ ~' ~~ PCr/US90/06057
embryos are transferred to a solid Stage 11 maintenance and multiplica-
tion medium containing greatly reduced plant growth hormones and prefer-
at~ly a somewhat raised osmotic level. Again, culturing is carried out
in the dark with subcultures made at no greater than about two week
5 intervals. The clone can be maintained at this stage for long periods
of time.
Early stage proembryos from the multiplication step are trans-
ferred to a liquid Stage Ill second maintenance medium Having a signifi-
cantly raised osmotic level. This corresponds to the raised osmotic
10 pulse found so beneficial for loblolly pine. It is similarly advanta-
geous for Douglas-fir and Norway spruce. However, a slightly lower
osmotic level of at least about 170 mM/kg will usually suffice for
Douglas-fir although some genotypes may require levels as high as 240
mM/kg. Myo-inositol, which will normally be around 5000 mg/L, may need
15 to be adjusted somewhat depending on the needs of the particular geno-
type in order to obtain optimum results. Culture is carried out in the
dark and is periodically subcultured, usually weekly. nobust late stage
proembryos having 100 or more cells will develop during this time.
Following late proembryo development, the cultures are trans-
20 ferred to a Stage IV.liquid medium for the singulation step referred toearlier. This has a reduced osmotic level and is free of auxins and
cytokinins. Abscisic acid is a newly added hormone in an initial amount
in the range of about 5-15 ppm, more usually the lower level. Cultures
are again carried out in the dark. From two to four subcultures are
25 made on a weekly basis. The level of exogenous abscisic acid will drop
somewhat during each subculture. It is important that the level of
abscisic acid at the beginning of a new subculture not be significantly
higher than the level at the end of the previous subculture. This will
result in an essentially continuous drop in ABA level over the singula-
30 tion period. At this time th~ embryos are ready to begin development tocotyledonary embryos. They are transferred to either a solid or liquid
medium with an effective abscisic acid level which again is not signifi-
cantly higher than that at the end of the final singulation subculture
Most typically this will be about 5-~ ppm effective ABA for cultures on
3~ solid medium but it may be lower. In some cases it is not necessary to
add exogenous ABA to the development medium since a sufficient amount
will be carried over with the residual m~dium accompanying the embryos
w~len the transfer is mad~ from ~he ~ast singulation stag~. However, it
:
=
- 206996~ -
WO 91/05854 ~ " PCI/US90/060
is always necessary for some activated charcoal to be present in the
development medium. It has been found preferable for Douglas-fir to
carry out development cultures entirely in the dark.
Example 8
A basal Douglas-fir development medium was modified by addit-
ion of 1, 2, or 3% myo-inositol, 2% sucrose, 2% sorbitol, or 2% mannitol
to determine the effect of these osmoticants on embryo development. The
above noted sucrose and _yo-inositol was in addition to that normally
lO present in the development medium. All media contained û.5 % activated
charcoal and 5 ppm abscisic acid and were gelled with 5 g/L tissue cul-
ture agar. Each culture plate received 1 m L of settled singulated
cells. Five genotypes were cultured using triplicate plates for most
genotypes. While successful development was not obtained on all geno-
15 types, a clearly superior response was achieved on the media containing
2 % sorbitol.
It is known that activated charcoal is an effective adsorbent
of plant hormones in culture media. For example, we showed earlier that
charcoal can effectively reduce over time the level of exogenous
20 abscisic acid evailable to developing embryos. Until the present, the
rate and magnitude of this effect has not been well known.
ABA concentration in liquid media can be studied using known
analytical methods that determine ABA concentration by measurement of
ultraviolet absorption. Nitrate is un interfering ion so the liquid
25 mediA for the foL'owing tests were made up without any inorganic nitro-
gen present. Present studies h~ve shown that when ABA is added to a
charcoal-containing liquid medium there is an imm~diate drop in the
level of available ABA. As would be expected, with a constant amount of
activated charcoal present, the effect is more pronounced when only
30 small quantities of ABA are added. In the following tests, stirred
~orway spruce liquid development media with nitrstes omitted were sAm-
pled at various times after addition of ABA. All media contained û.075%
activated charcoal. The ABA added increased in 5 ppm steps from 5 to 25
ppm total addition.
Wo 91/0~,854 ~ ~ j! .' '
~' -39-
T~ble 12
Percentage of Originally Added Abscisic Acid Remaining Available
S - ABA Added, Time after ABA Addition, minutes _-
ppm 0 1 5 10
39~ 35 16 9
- 10 50 34 17 13
41 24 19
5~ 44 25 20
- 25 68 46 29 24
It is evident from the above data that when ABA levels are
15 discussed in a charcoal-containing system only available or free ABA
levels should be considered.
Not surprisingly, it was also found that activated charcoal of
different grades and sources adsorbed ABA at different rates. In a
companion experiment to the above, ch~rcoal from four suppliers was
20 tested. An ABA solution of known concentration was added to a liquid
medium with stirring and measurements of free ABA were made over a 24
hour period. In all cases 50 ppm ABA was added. Charcoal concentration
was 0.12596. About 250 mL of medium was made up in a 400 mL beaker. The
medium was stirred for 10 minutes and then the charcoal was al~owed to
25 settle and the supernatant liquid was sampled at the indicated times.
As was also the case with the first example, all suspended charcoal was
immediately filtered from the liquid samples before further analysis.
Results were as follows.
Table 13 ~ :
Amount of Originally Added Abscisic Acid RemaininF Available, ppm
Charcoal Time after ARA Addition
35Source 0 min 5 min 10 min 30 min 2i hours
~ . . .
A 27 11 9 9 6
B 31 20 17.5 17.5 12.5
C 41 30.5 30 29.5 = 25
40D 44 44 43 43 34
It is evident that different sources and/or grades of charcoal
behave in very different manners in regard to adsorption rates of
abscisic acid. Thus the type or brand of activ~ted charcoal should be
45 p--cisely specifi~d if consistcnt resuI~s ar~ to b~ expected. Charcoal
WO91/05854 20699~-'t:.~a~ PCI/IJS90/060~i7
-40-
A was used in the preceeding example and throughout all of the work
described herein. It is availAble from Sigma Chemical Co., St~ Louis,
Missouri as their CAtAlog No. C-4381i And is described as HCI washed.
This is not intended to be sn endorsement of this product over others
that would undoubtedly be equally suitable but merely sets the speclfic
identify of the product used in the examples.
Measurement of A8A in solid media presented a much more diffi-
cult problem thAn meAsurement in e liquid system. Normally it would
involve a tedlous And complex extr~ction process. However, the extrac-
tion method would not be suitAble in an environment where the A8A con-
centration was chAnging rapidly. The following method has been devel-
oped and hes proved very suitable. A smull amount of tritium labeled
abscisic acid is added to the normal abscisic acid used for making up
the media. 3H labeled A8A is available from AmershAm Corp., Arlington
ileights, Illinois. After pouring the plates And Allowing the media to
solidify and cool, a quAdrAnt (1/4 circle) of A 42.4 mm diameter filter
pAper is plAced on the surface of the gelled medium for Approximately 10
- seconds. In this period of time the filter pAper will imbibe about
0.43-0.4~ g of liquid from the medium. The moist paper is removed with
tweezers And plAced in A viAI suitable for counting in a scintillation
counter. All sAmples Are normalized to A pickup of 0.45 g of liquid
medium. The Amount of radioactivity on the filter pAper cAn be can be
relAted to the totAI Amount of avAilable or free Abscisic acid in the
medium.
It was Assumed thAt rAtes of A8A Adsorption by activated char-
coAI would be different in solid And liquid media. The following exper-
iment WAS designed to show free ABA in a development medium solidified
by 3% Gelrite Gellan Gum. Gelrite gum is A microbially produced hetero-
polysAcchAride and is AvAilable form Chemical Dynamics Corp., South
PlAinfield, New Jersey. One medium was made using 0.125% activated
chArcoal. An equivAlent medium was made without chArcoAI for compar-
ison. The media were formulated with the gellant and sterilized by
AUtoCIAving. After cooling to e bout 55-60~C, a filter sterili~ed solu-
tion equivalent to 40 ppm of A8A in the medium was added. The A8A
35 solution included An Appropriate Aliquot of the tritiated A8A. The
mixture wAS then stirred for either I minute or 10 minutes berore being
pipetted into 50 m m petri dishes and sllowed to cool. Abscisic acid
content of the gelled medium w~s measared 2 hours and 24 hours after
WO 91/058~4 2 0 6 9 9 6 4: Pcr/us90/o6057
4 1 -
pouring and agAin after 5 days. ~esults were as follows.
Table 1 4
Abscisic Acid Availability Over Time in Solid Culture Media ~ _
Time of Stirring Effective ABA Concentration, ppm
~ Measurement Time, min With Charcoal Without Charcoai
-10 2 hrs 10 9.0 -40
2 hrs 1 14.5 -40
24 hrs 10 5.~ -40
24 hrs 1 6.6 -40
days 10 4.2 -40
The short term drop in abscisic acid in the charcoal contain-
ing samples is again quite dramatic. i~lo loss of ABA was seen in the
medium without activated charcoal. It is clearly evident that in any
charcoal containing medium it is the effective or free amount of
20 abscisic acid, and undoubtedly other hormones as well, that must be
considered. The amount of added hormone is meaningful only when all
other parameters are defined.
Osmotic potential of the various media is measured using a
Wescor 5500 Vapor Pressure Osmometer. This is available from Wescor,
Z5 Inc., Logan, Utah. Osmotic potential of liquid media is measured by
placing a 6.5 mm circle of filter paper on the sample tray of the
instrument and adding a measured 10 ~uL of medium. For solid media, the
fll~er paper circles are placed on the surface of the gelled media where
they imbibe a sufficient amount of liquid for measurement.
Exa m ple 9 ~ ~ -
Further experiments carried out on the basis of the results
described in the previous example confirmed the beneficial effects of 2% =~
sorbitol used in the development media. However, sorbitol concentra-
35 tions in the 3-4% range, while giving lower embryo yields in terms of
numbers, did appear to improve quality. The embryos were more similar
in appearance to zygotic embryos.
It had been observed elsewhere that the use of a gelling
material other than agar in the development cultures improved embryo
40 yield and quallty. In the following tests tissue culture agar had been
replaced with Gelrite 5ellan Gurr,. Its use as s m~dium g~llant in tissuc
culture is not new, aithouFh it is not belleved to have been used before
.
20~96~
WO91/05854 t ~ ' ' '' - PCI/US9~/tK057
-42-
in a medium similar to the present embryo development medium. As one
hypothesis for its superior performance, Its faster gelling rate, com-
p~red w~th ~gar, is believed to reduce the initial adsorption r~te of
J~bscisic acid by the activated charcoal present in the medium.
Experiments were then carried out in which from 2-6% sorbitol
was used in a basal Douglas-fir development medium in combination with
0.3% Gelrite gum. Other variable components were 2500 ppm KNO3, 750 ppm
I~glutamine, S ppm abscisic acid, and 0.05% activated charcoal. The
media were mixed one minute after the addition of abscisic acid and
poured into petri dishes. Embryos were plated approximately 24 hours
later. Pive genotypes were used with four replicates per genotype per
treatment. Summary results are given in the following table.
Table 15
Osmotic Average Number of Somatic Embryos Formed
Sorbttol, Potential, Genotypes
%mM/kF 738 742 735 676 733
~ 2277 0~8 6.0 14.5 0.0 0.3
20 3 341 12.0 4.0 18.3 2.5 0.0
4402 30.3 19.5 19.5 4.0 0.0
4.5 426 26.8 9.0 9.0 2.0 0.3
5471 37.7 26.3 22.0 2.8 0.0
6523 7.8 15.5 22.5 0.0 0.0
Z5
Depending on genotype, it appears that in combination with
Gelrite gum, sorbitol is beneficial as an osmoticant at concentrations
~t least as high as 6%. Both yield and quality were Improved. Embryos
were compact and yellow and more similar to zygotic embryos than those
30 developed previously. Osmotic levels above 400 mM/kg were the highest
investigated to that time. The performance in this higher range tends
to support the earlier stated hypothesis.
Exa m ple 10
Sorbitol is known to be poorly metabolized by embryos in cul-
ture. Its effect noted above is believed to be primarily osmotic.
However, it also apparently presents a favorable chemical environment as
well since the other osmoticant materials screened earlier (Example 8)
showed definitely inferior performance. Subsequent to the work just
reported, polyethylene glycol (PEG), with an average molecular weight
of ~bout 8000, was evaluated snd found to h~ a superior osmotic~nt. It
WO91/05854 2 ~ 699 6 4; ,1 ;~ P,~/US90,06057
--4 3--
is assumed tha~ there is no metabolism of the PEG by the developing
plant and the reasons for its superior performance, compared with other
materials, is not entirely clear. Very high levels of PEG, up to about
30%, have been found useful in liquid media. When more than about 12%
5 PEG is used in a solid medium there is a tendency to inhibit gelation.
Polyethylene or polypropylene glycols of other molecular weights are
believed to be equally useful. Upper and lower limits of molecular
weight which are useful have not yet been determined.
In order to determine the optimum level of PEG a Stage V liq-
lO uid development medium having 2500 ppm KNO3, 1000 ppm L-glutamine, and
0.1% Activated charcoal but no abscisic acid was made up with polyethyl-
ene glycol 8000 percentages varying between 15 and 3û% (15û,û00-300,000
mg/L?. A 40 X 4û mm polyester pad was dipped into each medium so as to
pick up about 5-5.5 mL of the medium which was kept continuously mixed
15 to keep the charcoal in uniform suspension. The pads were cut from
polyester batting having a thickness of about 4 mm and a basis weight of
about 150 g/m2. Each saturated pad was placed in a 120 mm petri dish.
Singulated l)ouglas-fir late stage proembryos were settled and
excess medium removed. The cells were rinsed with an equal volume of
20 the development medium of similar composition to that on which they were
to be placed. The rinse liquid did not contain any charcoal or PEG but
did contain 2.5 ppm ABA.. The cells were again settled and half of the
supernatant liquid removed (l/4 of the total volume). The cells were
again resuspended in the remaining liquid and 1.5 mL was placed on the
25 medium saturated polyester pad. The effect of the contained liquid
transferred with the cells was to dilute the osmoticant contained within
the pad. This dilution is taken into account in determining the effec-
ti~e osmotic level after dilution. The only exogenous ABA supplied was
that which was transferred with the cells from the rinse liquid. Table
16 whlch ~llow~ ~ experimen~al ~on~itions an~ resulls.
WO91/0~8~4 2069~e(~ PCI/US90/06057
-44-
T~ble 16
Initial Osmolality Finul Osmo-
PEG in Osmol~lity after Dil- Embryo Embryo lality
S Medium, % m ~ J ution, mM/kg Yield Quality mM/kg
15.0 315 -- ~ 50+ Note 1
17.5 372 -- 50+ Note 1 ---
20.0 465 -- 100+ Note 1
10 22.5 543 -- 100+ Note 1
25.0 657 450 100+ Note 2 415
26.0 731 490 100+ Note 2 505
27.0 798 621 100+ Note 2 578
28.0 861 -- 100+ Note 3 ---
15 29.0 93I -- 100+ Note 3 ---
30.0 101I -- 200+ Note 3 ---
~l) Measured before transfer of cells.
Note 1. Embryos were slightly green and small with swollen
hypocotyls.
Note 2. Best quality embryos, yellow in color and with well
developed cotyledons. An elongated hypocotyl region
similar to zygotic embryos.
Note 3. Good quality embryos but about half the size of embryos
produced in 25-27% PEG
Embryos produced using 25-27% polyethylene glycol are shown in
the photomicrograph of Figure 9~ These have a close resemblance to
30 zygotic embryos and have a high rate of germination and conversion to
plants.
In some instances there has been evidence of nutrient exhuus-
tion when the pad system is used with liquid medium. This hus been
overcome by using a pad-on-pad culture which effectively doubles the
35 amount of medium present.
Exa m ple l l
A serles of experiments was made to compare the effectiveness
of solid development media containing vurious ternary combin~tions of
40 polyethylene glycol 8000, sorbitol, snd lactose all having a similar
osmolality of about 450 mM/kg. These were compared with a control med-
ium having only sorbitol and with snother having sorbitol and polyethyl-
ene glycol 8000. Stage 5 medium lsee Tables 10 and 11~ was used with
2500mgtL KNO3, 75~0 mg/L L-glutamine, 30 mg/L initial abscisic ucid, and
45 0.125% Sigma activated churcoal. The osmoticunts, in addition to the 2
sucrose in the basal medium, wer~ as follows.
WO91/05854 ~~ '9 9c6~; PCI/US90/06057
-45-
Table 17
Medium Sorbitol, PEG, Lactose, % Osmolality, Osmolar Ratio
No.' % % % mM/kg S:P:L
4656 3.0 0.0 0.0 386 1:0:0
4982 3.5 6.0 0.0 439 7:1:0
4984 2.0 11.0 0.0 422 1:1:0
4983 3.0 6.0 1.0 438 6:1:1
10 4985 2.0 8.0 2.0 445 2:1:1
~4986 1.25 10.0 2.5 458 3:3:3
4987 0.0 12.0 4.0 447 0:1:1
The media were made by combining all ingredients except abscisic
15 acid and heat sterilizing. The temperature was reduced to about 60-65~C
and filter sterilized A8A was added with stirring for 10 minutes before
plates were poured and cooled. Medium 4984 gelled somewhat prematurely
but produced useable plates. About 24 hours later 1 mL of settled cells
(from Stage 4) of five genotypes was plated onto the test media. After
20 four weeks the cultures were examined and cotyledonary embryos counted
alld graded. The following average numbers of well deYeloped embryos per
plate were obt~ined. One genotype (703) did not produce embryos in uny
of th~ media tested here.
~5 ~ Table 18
Medium Genotype Genotype Genotyp~ Genotype
~lo. 735 711 742 _ 676
30 4656 9.8 0.8 5.5 4.3
4982 47.0 31.5 0.3 8.0
4984 71.3 61.3 5.7 17.7
4983 36.0 74.3 4.0 17.3
4985 100.0 143.3 ~ 27.3 9.0
35 4986 136.0 162.8 1.3 10.3
4987 125.0 91.3 21.0 18.3
It is evident that the best results were obtain~d from media 4985,
4986 and 4987 which were either tern~ry combinations or binary combinat-
40 ic~ns of polyethylene glycol 8000 and lactose without any sorbitol. Theeffect of medium composition on performance of individual genotypes
appears most markedly with Genotype 742. However, the trial on Medium
I'~o. 4986 should be replicated with this genotype before firm ~ '
as to its perform~nce are drawn.
~ s ~: ~
WO91/05854 ~ ~jt~ r~ PCI/US90/06057
Example 12
After long periods o~ time on a development medium containing
polyethylene glycol Douglas-fir embryos often show signs of deteriora-
tion over time. It has not been clear whether this deterioration was
5 due to nutrient depletion, a decrease in osmotic levels, or a negative
response to PEG. The following study was made in order to determine the
effect on this deterioration by making a medium and osmoticant change
during development. Cultures were made using the liquid medium-
polyester pad system described in Example 10.
A development medium was made us~ng 25D0 mg/L KNO3, 750 mg/L
L~lutamine, 260 g/L polyethylene glycol 8000, and 0.1% activated char-
coal. ~o abscisic acid was included in the medium. The polyester pads
were dipped into this medium, as described in Example 10, and picked up
about 5.5 g of the medium.
A rinse medium was made in similar fashion to the above with
the exception that the PEG and charcoal were omitted and 2.5 ppm of ABA
was included. Embryos and associated cells from the final singulation
medium were settled and the supernatant liquid removed. One volume of
the rinse medium was added and mixed well. The cells and embryos were
20 again settled and half of the supernatant liquid was removed. The
embryos and remaining liquid were again mixed and 1.5 mL was pipetted
onto the pads. Five genotypes were used with four replicates per
treat men t.
Osmotic level of the medium over the cells after the rinse was
25 about 200 mM/kg whereas the development medium before plating the cells
was about 490 mM/kg. Duplicate osmolality readings were taken from the
media on the pads at 3 and 4 weeks after th-e initial plating.
To attempt to find an answer to the questions posed above,
after three weeks 2.0 mL of the original development medium, now diluted
30 with the transferred rinse medium, was pipetted from three groups of
plates (5 genotypes X 4 replicates X 3 sets) and replaced with an equiv-
alent amount of fresh medium made up as follows. The original medium
was kept on one set (Set 1) (5 genotypes X 4 replicates) as a control.
~one of the replacement media contained activated charcoal.
35 Sufficient charcoal remained with the original meli~ still on the pads.
206g~
WO 91/05854 ~ t ~, PCI/US90/06057
-47 -
__
- Set 2 lleplacement Medium -- Original 260 g/L PEG replaced with
221 g/L PEG~ Osmolality 512 mM/kg.
Set 3 Replacement Medium -- Original 260 g/L PEG replaced with
61 g/L sorbitol. Osmolality 502 m M/kg.
Set 4 Replacement Medium -- Original 260 g/l PEG replaced with
- 120 g/L lactose. Osmolality 516 mM/kg.
Table 19
Osmolality, 3 Weeks After
Genotype Initial plating, mM/kg
735 401
711 430
15 ~ 742 632
703 510
676 389
Table 20
~ --~
Somatic Embryos Developed for Different Genotypes(1)
Set ~o. 735 711 742 703 676
87 101 17 32 0
25 --- 2 - 148 118 18 84
3 164 148 11 104 0
4 202 130 26 126 0
(1) Average of 4 plates counted 12 days after medium replacement.
Quality was evaluated as follows:
Set 1 -- Small und declining embryos.
Set 2 -- Somewhat larger and better appearing embryos.
Set 3 -- Large, smoother embryos.
Set 4 -- Largest, smoothest, and best sppearing embryos.
While this test is still in progress, it appears that replace-
ment of PEG with lactose or sorbitol midw~y during development will be
advantageous. Osmolality has not yet been measured after the replace-
40 ment medium was added but is expected to be in the range of 450 mM/kg or
ab3ve at the end of the development period.
Example 13
It appears possible to develop Dougl~s-fir embryos without any
45 exogenous abscisic acld in the development medium environment. Good
2p~
WO 91/05854 . . ~ - : PCI/US90/06057
-48-
quality embryos have been formed even when no ABA w~s added to the
medium during makeup and little or none csrried along with the embryos
and assochted ceLs from the singulation step. HoweYer, u small amount
of actiYated charcoal; e.g., 0.02~, appears to be essential. This may
be due to a high level of endogenous ABA within the embryos at the end
5 of singulation, although analytical work has not yet been done to con-
firm this. In the following experiment three solid media were made up.
Each had 1000 mg/L of L-glutamine, 2500 mg/L of KNO3, and 150 g/L of
polyethylene glycol 8000.
Table 21
Medium l~lo.
2 3
15 Activated charcoal, % 0.02 0.04 0.04
Abscisic acid, mg/L -- ~ 8 8
Sorbitol, % 2 2
Three genotypes (7L1, 735, 771) of Douglas-for singulated
20 embryos were used in the trials. These were not washed but 1.5 mL was
transferred in the singulation medium to the culture plates. A low
level of ABA would have been present at the end of singulation. ~ive
replicates were made at each test condition. After four weeks over lO0
embryos had formed on each medium for each genotype. After five weeks
25 the embryos formed on the medium without ABA showed some elongation
indicative of premature germination. This was not observed for the
embryos formed on the medis containing ABA.
It would appear that an osmotic level above about 450 mM/kg
may be a threshold value for Douglas-fir embryo development media ir the
30 best yield and quality are to be obtained. This leYel is one measured
at the end of the development period rather thsn the beginning.
Ideally, it is believed advantageous for the osmotic level to rise some-
what over the period of development. It is possible to control osmotic
level during development by periodic medium changes. To facilitate this
35 without ~ b.~hCC to the developing embryos they may be supported on a
material such as filter paper which is placed directly on either 8 solid
culture medium or on the saturated pad of a liquid medium.
Similarly, it appears inportant to hsve a level of available
exogenous abscisic acid that drops essentially continuously rrom the
40 initial usage at the beginning Or the singulation stage to the end of
WO 91/0!;8~4 2 0 6 9 9 ~ ~ t- Pcr/usgo/o6o57
~, -49-
the development period. An initial level At the beginning of singulA-
tion of About 5-15 ppm Appears suitabie. This will decrease to Q low
level at the end of the development stage. Exact measurements have not
been possible at the end of development due to the limitations of avail-
S able Analytical techniques.
Following embryo development the somatic embryos may be
retained for some period of time in cold storage. They may be converted
into artificial seeds for field or nursery plsntlng. Alternatively,
they may be placed immediately on a germination medium such as Medium
10 BMG (TAble 10) for converslon into plantlets prlor to planting in soil.
Figure 10 contalns somewhat idealized curves for Douglas-fir
showing the osmotic levels and abscisic acid level at each of the var-
ious stages from initiation through germination. It hQs been observed
that the osmotic ~evel will increase somewhat during each llquld shake
15 stage of late proembryo development and singulation. The opposite phen-
omenon seems to occur during development, probably due to utillzatlon of
sucrose and other nutrients. Taking this drop into account is necessary
in adjusting the initial osmotic level of the development media. Here
the solid portion of the curve represents the normal course of osmotic
20 level if no media changes are made. The dotted portion shows how
osmotic level can be increased if one or more transfers to fresh media
are made.
To date 446 converted seedlings from 13 genotypes of Douglas-
fir are growing in soil.
25 =~ ~-lt should be recognized that there is not one single set of
culturing conditions that will be suitable for achieving somatic embryo-
genesis of all species or for all genotypes within a species. Tissue
culture as a whole is a highly unpredictable science. This statement
has even greater applicability to somatic em~ . Adjustments in
30 the mineral and plant hormone constituents or the culture media must
frequently be made depending on the particular species and genotype
being cultured. This applies to each of the various stuges of culturing
from explants to plantlets. These adjustments are considered to be
within the routine experimental capability or those skilled in the art
35 of tissue culture. The important discovery of the present inventlon is
the use of A combination of abscisic Acid and an Adsorbent such as
activated charcoal durinF the growth of late stage proembryos to cotyle-
donary embryos. This has given results thAt are far superior in terms of
WO 91/05854 2 ~ i . PCrtVS90/06057
_5~_
success and consistency thQn any process reported heretofore. The pro-
cess has been successfully applied to all of the several species and
many genotypes of coniferous plants studied to date and appears to be of
general use for all coniferous species.
It will be understood that many Yariations can be made in the
procedures described for the various culturing stages while still
remaining within the spirit of the present inYentiOn. It is the inten-
tion of the inventors that such variations should be included within the
scope of their invention if found defined within the following claims.
BIBLIOGRAPH Y
Abo El-Nil, Mostafa
1980 Embryogenèsls of gymnosperm forest trees. U.S. Patent
4,217,730.
15 Becwart M.R. and R. P. Feirer
1989 Factors regulating loblolly pine Pinus taeda L.) somatic
embryo development. Institute of Paper Chemistry Report,
Southern Forest Tree Improvement Conference, Raleigh, N.C., June
1989.
20 Becwar, M.R., S.R. Wann, and R. Nagmani
1988 A survey of initiation frequency of embryogenic callus
among ten families of P~nus taeda ~loblolly pine). Abstracts,
4th International Conifer Tissue Culture Work Group, August 8-
12, 1988, Saskatoon, Saskatchewan, Canada.
25 Boulay, M. P., P. K. Gupta, P. Krogstrup, and D. J. Durzan
1988 Developtnent of somatic embryos from cell suspension cultures
of Norway spruce (Picea abies Karst.). Plant Cell Reports
7: 134-137.
Bourgkard, F. and J. M. Favre
1988 Somatic embryos from callus of Sequoia sempervirens. Plant
Cell Reports 7: 445-448.
Buchheim, Julie A., Susan M. Colburn, and Jerome P. Ranch
1989 Maturation of soybean somatic embryos and the transition to
plantlet growth. Plant Physiology 89: 768-775.
35 Durzan, D.J. and P.K. Gupta
1987 Somatic embryogenesis and polyembryogenesis in Douglas-fir
cell suspenslon cultures. Plant Science 52: 229-235
.. . ... . .
WO 91/05854 2 0 6~ Pcr/USgo/06057
51 - ~
Finer, John J., Howard ~. Kriebel, and Michael R. Becw~r
1989 Initiation of embryogenic callus and suspension cultures of
eastern white pine (Pinus strobus L.). Plant Cell Reports 8:
203-206.
5 Gupta, Pramod K. and Don J. Durzan
1985 Shoot multiplication from mature trees of Douglas-fir
- (Pseudotsuga menziesii) and sugar pine (Pinus lambertiana).
Plant Cell Reports 4: 177-179.
1986a Somatic polyemb-~yuK~ from callus of mature sugar pine
embryos. Bio/ l ~_h.. ~ v 4: 643-645.
1986b Plantlet regeneration via somatic embryogenesis from
subculturea callus of mature embryos of Picea abies (Norway
spruce). In Vitro Cellular and DeYelopmental Biology 22: 685- ~ _ -
688.
1987 Biotechnology of somatic polyembryogenesis and plantlet
regeneration in loblolly pine. Bio/-~ l.r.ol~icy 5: 147-151.
Hakman, Inger and Sara von Arnold
1985 Plantlet regeneration through somatic eml-. y~,b~ 3is in Picea
abie3 (Norway spruce). Journal of Plant Physiology 121: 149-lS8.
zo 1987 Somatic eml,l ~uK~ . and plant regeneration from suspension
cultures of Picea glauca (White spruce). Physiologia Plantarum
72: 579-587.
Hakman, Inger, Larry C. Fowke, Sara von Arnold, and Tage Eriksson
1985 The development of sorlatic embryos in tissue cultures init-
iated from immature embryos of Picea abies (Norway spruce).
Plant Science 38: 33-35.
Johansson, Lars
1983 Effects of activated charcoal in anther cultures. Physio-
logia Plantarum 59: 397-403.
30 Johansson, Lars, Barbro Andersson, and Tage Eriksson
98i Improvement of anther culture technique: activated charcoal
bound in agar medium in combination with liquid medium and
elevated C02 concentration. rll~ ,;Olo~ ;~ Plantarum 54- a4-30.
Lu, Chin-Yi, and Trevor A. Thorpe~5 - 1987 Somatic embryogenesis and plantlet regeneration in cultured
immature embryos of Picea glauca. Journal of Plant Physiology
128: 297-302.
WO 91/05854 2 0 6 9 9 ~ 4 rcr/us90/06os7
Mur~shige, Toshio ~nd Folke Skoog
1962 A revised medium for rapid growth and bioassays with tobacco
tissue cultures. r~ ;OIOi i~ PlantMrum 15 473-493.
Nagmani, R. and J.M. Bonga.
1985 Eml" ~,8.. ~.~ in subcultured callus of Larix decidua. Canad-
ian Journal of Forest Research 15: 1088-1091.
Nagmani, R. and M. R. Becwar
1988 Factors affecting somatic embryo development in loblolly
pine. Abstracts, 4th International Conifer Tissue Culture Work
Group, August 8-12, 1988, Saskatoon Saskatchewan, Canada.
Raghavan, V. N.
1987 Experimental Em~ . p 1û0, McMillan, New York.
Schuller, Astrid and Gerhard Reuther
1989 Response of Abies alba embryonal-suspensor mass to various
c~.l,vI,yd,~,te treatments. Somatic Cell Genetics Workin~ Party S2-
04-07 and NATO Advanced Research Workshop on Woody Plant Biology,
Institute of Forest genetics, Placerville California, October 15-
19, 1989 (Abstract).
Singh, Hardev
1978 "Embryo" in Embryology of Gymnosperms, Chapter 11?
Gebruder Borntrager, Berlin.
Teasdale, Robert D., Pamela A. Dawson, and Harold W. Wooihouse.
1986 Mineral nutrient reguirenents of a loblolly pine. (Pinus
taeda cell suspension culture. Plant Physiology 82:942-945.
25 Verhagen, S. and S. R. Wann
1989 Norway Spruce Somatic Embryqgenesis: High Frequency
Initiation From Light-Cultured Mature Embryos. Plant Cell Tissue
and Organ Culture 16:103-111.
Von Arnold, Sara
1987 Improved efficiency of somatic embryogenesis in mature
embryos of Picea abies (L?) Karst. Journal of Plant Physiolo~y
128: 233-244.
Von Arnold, Sara and Inger Hakman
1988 Regulatlon of somatic embryo development in Picea abies by
abscisic acid (ABA). Journal of Plant Physiolo~y 132:164-169.
Yeung, Edward C. and D. C. W. Brown
1982 The osmotic environment of developing embryos of Phaseolus
vulgaris. Z. Pfa~ y~iol. Bd. 106 S.: 149-156.
.. . .. ... _ . .. .. . _ . . .. .. . ..... .... . . .. .. . . .
WO 91/05854 2 ~ 6 9 9 6 ~ Pcr/Usgo/060s7
Ziv, Meira and Geula Gadasi
1986 Enhanced emb.~v~ sis and plant regeneration ~rom cucumber
(Cucumis sativus L.) calLus by activated charcoal in
sol d/liquid doubl ayer cultures. Plant Science 47: 115_122.