Note: Descriptions are shown in the official language in which they were submitted.
~156065
~ WO 94/21642 PCTIUS94/02692
.
MACROCYCLIC AMIDE AND UREA IMMUNOMODULATO~.S
This application is a continuation-in-part of copending United States Patent
Application Serial No. 08/149,419, filed November 9, 1993, which is a continuation-in-part
of co-pending United States Patent Application Serial No. 08/032,958, filed March 17, 1993,
which is a continn~tinn-in-part of co-pending Tnt~rn~tional Patent Application No.
PCT/US92/07600, filed September 8, 1992, which is a con~-nll~tic-n-in-part of United States
Patent Application Serial No. 07/iS5,208, filed September 5, 1991 and now abandoned.
Field of the Invention
The present invention relates to novel chemical compounds having
immunomodulatory activity, and in particular to macrolide immunosuppressants. More
particularly, the invention relates to semisynthetic analogs of ascomycin and FK-506, to
means for their preparation, to pharmaceutical compositions containing such compounds and
to methods of tre~tment employing the same.
Back~round of the Invention
The compound cyclosporine (cyclosporin A) has found wide use since its introduction
in the fields of organ transplantation and immunomodulation, and has brought about a
significant increase in the success rate for transplantation procedures. Undesired side-effects
associated with cyclosporine, however, such as nephrotoxicity, have led to a continued search
for immunosuppressant compounds having improved efficacy and safety.
Recently, several classes of macrocyclic compounds having potent
immunomodulatory activity have been discovered. Okuhara et al., in European Patent
Application No. 184162, published June 11, 1986, disclose a number of macrocyclic
compounds isolated from the genus Streptomyces. ~mmllno~,u~ essallt FK-506, isolated
from a strain of S. tsukubaensis, is a 23-membered macrocyclic lactone represented by
formula la, below. Other related natural products, such as FR-900520 (lb) and FR-900523
(lc), which differ from FK-506 in their alkyl substituent at C-21, have been isolated from S.
hygroscopicus yakushimnaensis. Yet another analog, FR-900525, produced by S.
tsukubaensis, differs from FK-506 in the replacement of a pipecolic acid moiety with a
proline group.
FR-900520, also known as ascomycin, has been previously disclosed by Arai et al. in
U.S. Patent No. 3,244,592, issued April 5, 1966, where the compound is described as an
antifungal agent. Mon~.gh ~n, R.L., et al ., on the other hand, describe the use of ascomycin
as an immunosuppressant in European Patent Application No. 323865, published July 12,
1989.
-
-
WO g4/21642 PCT/US94/02692
2$~60~5
Although the immuno~u~cssive a,ctivity of FK-506 has been clinically confirmed,
its toxicity in m~mm~l~ has lim,te~ s utiiity. The activity of FK-506 has, however,
~ru-~-pLed efforts to discover novel analogs of FK-type compounds which possess superior
plope.Lies. These efforts include the isolation of new rtir...c.lLaLion products, the microbial
transforrnation of existing chemical çntiti-os, the chemic~l mo-lification of these macrocycles,
and the synthesis of hybrid species derived from smaller synthetic fragments.
HO~
132
CH30
/~W
~N ~0
o~ ~L.
H07~0
OCH3
OCH3 (1)
l(a): FK-506 R = CH2CH=CH2; n=1
1(b): FR-900520 R = CH2CH3; n=l
1(c): FR-900523 R = CH3; n=1
1(d): FR-900525 R = CH2CH=CH2; n=0
Fermentation products of FK-type compounds include C-21-epi derivatives of FK-
506; a 31-demethylated derivative of FK-506; 31-oxo-FK-506; and compounds derived from
FK-506, FR-900523 and FR-900525 which are characterized by the introduction of hydroxy-
protecting groups, formation of a double bond by elimin~tinn of water between carbons 23
and 24, oxidation of the hydroxy group at carbon 24 to the ketone, and reduction of the allyl
side-chain at carbon 21 via hydrogenation. Other pukli~he~ derivatives include those derived
from FK-506 and FR-900520 where the lactone ring is contracted to give a macrocyclic ring
containing two fewer carbons.
Several microbial transformations of FK-type compounds at carbon 13 have been
published, such as the microbial demethylation of FR-900520 to form the bis-demethylated
~ WO94121C42 2I~S6û65 PCTIUS94l02692
.
13,31 -dihydroxy ring-rearranged derivative of FR-~900520; the microbial monodemethylation
of FK-506 and FR-900520, respectively; and the microbial demethylation of FR-900520 at
C-31, as well as a number of other macrocyclic microbial transformation products.
Numerous chemic~l morlif~calinns of the FK-type compounds have been aU~ tc;d.
These include the preparation of small synthetic fragmsnt~ of FK-type derivatives; a tllerm~l
rearrangement of a variety of derivatives of FK-506 which expands the macrocyclic ring by
two carbons; and modifications which include methyl ether and aryl ether formation at C-32
and/or C-24, oxidation of C-32 alcohol to the ketone, and epoxide formation at C-9.
Although some of these modified compounds exhibit immunosuppressive activity, the
need remains for macrocyclic immunosuppressants which do not have the serious side effects
frequently associated with immunosuppressant therapy. Accordingly, one object of the
invention is to provide novel semisynthetic macrolides which possess the desiredimmunomodulatory activity but which minimi7e undesired side effects.
Another object of the present invention is to provide synthetic processes for the
preparation of such compounds from starting m~ttori~l~ obtained by ferm~ntation, as well as
chemical interme~i~tes useful in such synthetic processes.
A further object of the invention is to provide pharmaceutical compositions
containing, as an active ingredient, one of the above compounds. Yet another object of the
invention is to provide a method of treating a variety of disease states, including post-
transplant tissue rejection and auloi,~ml"~e. disfunction.
Surnrnary of the Invention
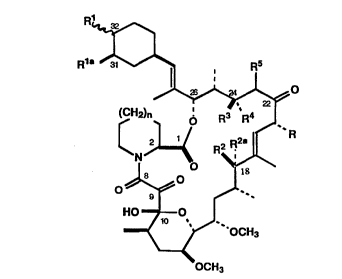
The present invention is directed to compounds having the formula:
Wo 94/21642 `- ~ PCT/US94/02692 1--
?.~$ 6~6~
,. ~,~
~ .
R1a ~ ~ ~
~
(CH2)n ' 3 ~ 4
~ R
HO ~ O
_< ~ OCH3
OCH3 (I)
and pharmaceutically-acceptable salts, esters, amides and prodrugs thereof, wherein n, R, Rl,
Rla~ R2, R2a, R3, R4 and R5 are specifically defined, which possess immunosuppressive,
~ntimiçrobial, antifungal, antiviral, ~ntiinfl~mm~tory and antiproliferative activity, as well as
the ability to reverse chemotherapeutic drug resistance; to pharmaceutical compositions
comprising a compound of the invention in combination with a pharmaceutically-acceptable
carrier; to processes for the preparation of these compounds; to synthetic intermediates useful
in the ~l~al~tions of these and other immnnt)modulator derivatives of ascomycin; to
methods of form~ ting pharmaceutical compositions comprising these compounds; and to a
method of immunomodulatory treatm~.nt of a human or veterinary subject in need of such
tre~tment by the ~mini~tration of a th~ ;ulically-effective amount of a novel compound
according to the present invention.
Detailed Description of the Invention
The instant invention is directed to novel compounds described by ~e general
formula (1):
21$6065
~ Wo 94/21642 PCTIUS94/02692
-, ' ` , .
R1
R1a ~ I R5
(CH2)n~~R
o~(O
HO O
--~ OCH3
OCH3 (I),
and pharmaceutically-acceptable salts, esters, amides and prodrugs thereof, wherein:
n IS zero or one;
R is hydrogen, methyl, ethyl, allyl, propyl, 2-hydroxyethyl, cyclopropylmethyl, 2-oxopropyl
or 2-ethanal;
Rl and Rla are selected such that one of R1 and Rla is hydrogen, -(Cl-C6- aLkyl)oxy or
hydroxy, and the other is chosen from the group consisting of:
(I) -O(CH2)jC(O)Rl2, where j is one-to-five, and
R12 iS:
(A) hydroxy;
(B) -oR13, wherein R13 is:
(i) -(Cl-Clo-aLIcyl);
(ii) -(cyclo-C3-Cg-aLkyl);
(iii) -(cyclo-C3-Cg-aLkyl-Cl -C3-aLcyl);
(iv) aryl-(CI-C6-aLkyl)-, where aryl is as defined below, wherein the
zero, one, two or three substihlçnt.~ on the aryl group, each
designated R301, are independently selected from the group
consisting of:
(a) -(Cl -to-C7-aL~yl);
WO g4121642 ;~ PCT/US94/02692 ~
z~,$6~6S
(b) -(C2-to-C6-aL~enyl);
(c) halogen;
(d) -(CH2)mNR8R9, where m is zero-~o-six, and NR8R9 is
either a nitrogen atom attached to R8 and R9, wherein R8
and R9 are indepe~idçntly selected from the group
consistn~ig l)f:
(1) hydrogen;
(2) -R400, where R400 is selected from the group
consisting of:
a. mod-aryl, as defined below, wherein the one,
two, or three substituents, each designated
R302, are independently selected from the
group consisting of:
1 . -(Cl-to-C7-aL~cyl);
2. -(C2-to-C6-aLkenyl);
3. halogen;
4. -(CH2)mNR18R19,where misas
defined above and NR18Rl9 is
either a nitrogen atom attached to
R18 and R19, wherein R18 and Rl9
are independently selected from the
group consisting of hydrogen, -(C1-
C6-aL~yl), unsubstituted aryl-, and
unsubstituted aryl-(Cl-C6-aL~cyl); or
NR18Rl9 may be a 3-to-
7-membered heterocyclic ring
compri~ing ring carbon atoms, the
nitrogen atom shown, and zero, one
or two additional heteroatoms
independently selected from the
group consisting of -O-,-NH-,
-N(Cl-to-C6-alkyl) and -S(O)s-,
wherein s is zero, one or two;
5. -CN;
6. -CHO;
7. mono-, di-, tri-, or perhalogenated
-Cl-C6-aL~cyl, as defined below;
~ wo g4,2l642 2 1 S 6 0 6 5 PCT/US94/02692
8. -S(O)SR18, where s and R18 are as
defined above;
- 9. -C(O)NR18Rl9, where NR18Rl9 is as
defined above;
10. -(CH2)mOR18, where m and R18 are
as defined above;
11. -CH(oR16)(oR17), where R16 and
R17 are independently chosen from
-(Cl-to-C3 aLkyl) or, taken together,
R16 and R17 form an ethylene or
propylene bridge;
12. -(CH2)mOC(O)R18, where m and
R18 are as defined above;
13. -(CH2)mC(O)OR18, where m and
R18 are as defined above;
14. -OR10, where R10 is: (i)
-PO(OH)O-M+, wherein M+ is a
proton or a positively charged
inorganic or organic counterion, as
defined below, (ii) -SO3-M+,
wherein M+ is as defined above,
(iii) -C(O)(CH2)mC(O)O-M+,
wherein m and M+ are as defined
above;
15. -NO2;
16. -N3;
17. -(C2-to-C6-alkynyl);
18. -C-C-Si(CH3)3; and
19. guanidino substituted by hydrogen;
-(Cl-C6-aL~yl); unsubstituted aryl;
(Cl-Cg-alkyl)-C(O); unsubstituted
aryl-S(0)2; (Cl-C6-alkyl)-OC(O)-;
unsubstituted aryl-(Cl-C6-alkyl)-
OC(O); unsubstituted aryl-OC(O);
or (Cl-C6-alkyl)-SO2-; or
taken together, any two adjacent R302
substituents in a di- or trisubstituted
mod-aryl group form a 5-, 6- or 7-
- 7 -
Wo 94t21642 PCTIUS94/02692 ~
?,,~,~i6Q6S
membered carbocyclic ring or a 5-, 6- or
7-membered heterocyclic ring wherein
the ~ing atoms consist of carbon atoms
~and one or two heteroatoms
independently selected from the group
con~i~ting of -O-,-S(O)s-, where s is as
defined above, and -NR18-, where R18 is
as defined above;
b. -Q-mod-aryl, where mod-aryl is as defined
below and substituent(s) R302 is/are as
defined above, and the divalent radical -Q-
is selected from the group consisting of:
1. -(Cl -to-C6-aL~yl)-;
2. -(C2-to-C6-aL~enyl)-;
3. -(C2-to-C6-aL~ynyl)-;
4. -(CH2)mO-, wherein m is as defined
above;
5. -O(CH2),n-, wherein m is as defined
above;
6. -N(R18)C(O)-, wherein R18 is as
defined above;
7. -C(O)N(R18)-, wherein R18 is as
defined above;
8. -S(O)s-, wherein s is as defined
above;
9. -N(R18)-, wherein R18 is as defined
above;
10. -N(R18)S(O)t-, wherein ~ is one or
two, and R18 is as defined above;
11. -S(O)tN(R18)-, wherein t and R18
are as defined above;
12. -C(O)-;
13. -NN-;
14. -CHN-;
15. -NCH-;
16. -ONCH-; and
17. -CHNO-;
~ WO 94/21642 2 1 5 6 0 6 S ~IUS94/02692
c. -mod-Het, as defined below" wherein the one,
two, or three substit~lent~, each design~ted
R302, are independently selected, and are
as defined above;
d. -Q-mod-Het, where Q is as defined above;
e. -biaryl, as defined below;
f. -Q-biaryl, where Q is as defined above;
g. -mod-aryl-Q-mod-aryl, where Q is as defined
above;
h. -mod-aryl-Q-mod-Het, where Q is as defined
above;
i. -mod-Het-Q-mod-aryl, where Q is as defined
above;
j. -mod-Het-Q-mod-Het, where Q is as defined
above;
k. -mod-Het-mod-aryl;
1. -mod-aryl-mod-Het; and
m. -mod-Het-mod-Het;
(3) -(C! l-to-c6-alkyl);
(4) substituted-Cl-to-C6-aLkyl, as defined below;
(5) -(C3-t-C6-aLkenYl);
(6) substituted-C3-to-C6-alkenyl, as defined below;
(7) -(C3-to-C~-aLIcynyl);
(8) substituted-C3-to-C6-aLkynyl, as defined below;
(9) -(cyclo-C3-to-Clo-aL~cyl);
(10) substituted-cyclo-C3-to-Clo-aLkyl, as defined
below;
(1 1) -(cyclo-C4-to-CIo-aLkenyl);
(12) sllbstit-lte-l-cyclo-C4-to-Clo-aLkenyl~ as defined
below;
(1 3) -(bicyclo-C6-to-Clo-aL~cyl);
(14) substituted-bicyclo-C6-to-Clo-aL~yl, as defined
below;
(15) -(bicyclo-C6-to-Clo-aLkenyl);
(16) substituted-bicyclo-C6-to-Clo-aLkenyl, as defined
below;
(17) -(bicyclo-C6-to-Clo-alkenyl)-cl-to-c6-alkyl; and
Wo 94/21642 PCrlUS94/02692 ~
?"~S6~6S
(18) s~bstit~lt~l-bicyclo-c6-to-clo-aL~enyl-cl-t
aLkyl, as de~med below; or
-NR8R9 may be a 3- to 7-membered heterocyclic ring,
where the ring consists of carbon atoms, the nitrogen
. atom shown, and zero, one or two additional
heteroatoms independently selected from the group
con.~i~tin~ of -O-,-S(O)s-, wherein s is as defined
above, and -NR8-, wherein R8 is as defined above;
(e) -CN;
(f) -CHO;
(g) mono-, di-, tri-, or perhalogenated -Cl-C6-aLkyl;
(h) -S(O)SR8, where s and R8 are as defined above;
(i) -C(O)NR8R9, where NR8R9 is as defined above;
(j) -(CH2)mOR8, where m and R8 are as defined above;
(k) -CH(oR16)(oR17), where R16 and Rl7 are as defined
above;
(1) -(CH2)mOC(O)R8, where m and R8 are as defined above;
(m) -(CH2)mC(O)OR8, where m and R8 are as defined above;
(n) -OR10, where R10 is as defned above;
(o) -NO2;
(p) -N3;
(q) -R400, as defined above;
(r) -S(o)tNR8R9, where t and NR8R9 are as defined above;
(s) -NR8S(o)tR9, where t, R8 and R9 are as defined above;
(t) -(C2-to-C6-aLlcynyl);
(u) -C~C-Si(CH3)3; and
(v) gn~nitlinosub~ u~tdbyhydrogen;-(Cl-C6-aL~yl);-mod-
aryl; (Cl-Cg-aL~cyl)-C(O)-; mod-aryl-S02-; (Cl-C6-aL~cyl)-
OC(O)-; mod-aryl-(Cl-C6-alkyl)-OC(O); mod-aryl-
OC(O)-; or (Cl-C6-aL~yl)S02-;or
taken together, any two adjacent R301 substituents in a di- or
trisubstituted aryl group form a 5-, 6- or 7-membered
carbocyclic ring or a 5-, 6- or 7-membered heterocyclic ring
wherein the ring atoms consist of carbon atoms and zero, one
or two heteroatoms independently selected from the group
consisting of -O-,-S(O)s-, where s is as defined above, and
-NR8-, where R8 is as defined above;
- 10-
I WO 94/21642 215 6 0 6 5 PCT/US94/02692
with the proviso that each R301 substituent or each ring formed by
two adjacent R301 groups may comprise no more than twenty non-
hydrogen atoms;
(v) aryl-, as defined below and substituent(s) R301 is/are as
defined above;
J (Vi) Het-, as define~d below;
(vii) heterocyclic-, as defined below;
(viii) mono-, di-, tri-, or per-halogenated-Cl-C6-aL~yl-;
(ix) -(cyclo-Cs-Clo-aLI~enyl);
(x) -(cyclo-Cs-Clo-aL~cenyl-Cl-C3-aLIcyl);
(xi) -(bicyclo-C6-C12-alkenyl);
(xii~ -(bicyclo-C6-C12-alkenyl-Cl-C3-alkyl);
(C) -NRl4Rl5, wherein NRl4Rl5 is either a nitrogen atom attached to R14
and R15, wherein Rl4 and Rl5 are independently selected from the group
consisting of:
(i) hydrogen;
(ii) -R400, as defined above;
(iii) -(Cl -to-Clo-aL~yl);
(iv) sub-Cl-to-Clo-aL~cyl, as defined below;
(v) -(cyclo-C3-to-Clo-aL~yl);
(vi) sub-cyclo-C3-to-Clo-aL~yl, as defined below;
(vii) -(cyclo-C3-to-Clo-aL~yl-CI-to-C3-aL~cyl);
(viii) sub-cyclo-C3-to-Clo-aL~yl-Cl-to-C3-aL~yl, as defined below;
(ix) -(C3-to-Clo-aL~enyl);
(x) sub-C3-to-Clo-aL~cenyl, as defined below;
(xi) -(cyclo-C4-to-Clo-aL~enyl);
(xii) sub-cyclo-C4-to-C1o-aL~cenyl, as defined below;
(xiii) -(cyclo-C6-to-Clo-alkyl-C3-Cs-alkenyl);
(xiv) sub-cyclo-C6-to-C1o-aL~yl-C3-Cs-aLI~enyl, as defined below;
(xv) -(C3-to-Clo-aL~cynyl);
(xvi) sub-C3-to-C1o-aLkynyl, as defined below;
(xvii) -(cyclo-C6-to-Clo-aLI~yl-C3-Cs-aL~ynyl);
(xviii) sub-cyclo-C6-to-C1o-aL~yl-C3-Cs-alkynyl, as defined below;
(xix) -(bicyclo-C6-to-Clo-aL~yl);
(xx) sub-bicyclo-C6-to-C1o-alkyl, as defined below;
(xxi) -(bicyclo-C6-to-Clo-aL~enyl);
(xxii) sub-bicyclo-C6-to-Clo-alkenyl, as defined below;
(xxiii) -aryl;
- 11-
.s6~GS - PCT/US94/02692
(x~civ) -Het; and
(xxv) R6, where R6 is sçle~ted from the group consisting of:
(a) hydrogen;
(b) -(Cl-to-Clo-alkyl);
(c) mod-Cl-to-C1o-alkylt as defined below;
(d) -(C3-to-C1o-alkeny~
(e) mod-C3-to-C~g-aL~cenyl, as defined below;
(f) -(C3-to-C1o alkynyl);
(g) mod-C3-to-Clo-aL~ynyl, as defined below;
(h) -(cyclo-C3-to-Clo-aL~cyl);
(i) mod-cyclo-C3-to-Clo-aL~yl, as defined below;
(j) -(cyclo-C4-to-Clo-alkenyl);
(k) mod-cyclo-C4-to-Clo-alkenyl, as def ned below;
(1) -(bicyclo-C6-to-Clo-aL~yl);
- (m) mod-bicyclo-C6-to-Clo-alkyl, as defined below;
(n) -(bicyclo-C6-to-C1o-aL~cenyl);
(o) mod-bicyclo-C6-to-C1o-alkenyl, as defined below;
(p) -R8, as defined above;
(q) -aryl; and
(r) -Het; or
-NRl4Rl5 may be a 3- to 7-membered heterocyclic ring, where the ring
consists of carbon atoms, the nitrogen atom shown, and zero, one or two
additional heteroatoms independently selected from -O-, -S(O)s-, wherein s is
as defined above, and -NR8-, wherein R8 is as defined above, which ring is
nn.~ubstit~lted or substituted with from one-to-five compatible radicals
independently selected from the group consisting of:
(i) R6, as defined above;
(ii) -(CH2hT~OR6,where m and R6 are as defined above;
(iii) -(CH2)mNR6R7, where m is as defined above and NR6R7 is either
a nitrogen atom attached to R6 and R7, wherein R6 is as defined
above and R7 is independently selected from the group ~lefining
R6, or
-NR6R7 may be a 3- to 7-membered heterocyclic ring, where the
ring consists of carbon atoms, the nitrogen atom shown, and zero,
one, or two additional heteroatoms independently selected from
the group consisting of -O-, -S(O)s-, wherein s is as defined
above, and -NR8-, wherein R8 is as defined above, which ring is
- 12-
~ WO 94/21642 2~ 5 6 0 6 5 ~S94/02692
., . y , .
unsubstituted or substituted with from one-to-six compatible
radicals independently selected from the group consisting of:
(a) -R8, as defined above;
(b) -(CH2)mOR8, wherein m and R8 are as defined above;
(c) -S(O)SR8, wherein s and R8 are as defined above;
(d) ~ g(O?~NR8R9, wherein t and NR8R9 are as defined
abov~,
(e) -(CH2)mNR8R9, wherein m and NR8R9 are as defined
above;
(f) -SO3H;
(g) =NOR8, wherein R8 is as defined above;
(h) -R400, as defined above;
(i) -aryl;
(j) -Het; and
(k) -R399, wherein R399 is selected from the group
consisting of:
(1) hydroxyl;
(2) -C(O)OH;
(3) -C(O)OR8, where R8 is as defined above;
(4) -(cyclo-C3-to-C7-aL~yl);
(5) oxo;
(6) thiooxo;
(7) epoxy;
(8) halogen;
(9) -CN;
(10) -N3;
(1 1) -N2;
(12) -OR10, where R10 is as defined above;
(13) -S(O)tNR8R9, where t and NR8R9 are as
defined above;
- (14) -NR8S(O)tR9, where t, R8 and R9 are as
defined above;
(15) -CH(oR16)(oR17), where R16 and R17 are as
defined above; and
(16) guanidino substituted by hydrogen, -(C1-C6-
alkyl); aryl; (C1-C6-aL~cyl)CO-; aryl-SO2-; (Cl-
C6-allcyl)OC(O)-; aryl-(Cl-C6-aL~cyl)OC(O)-;
aryl-OC(O)-; or (C1-C6-aLkyl)-SO2-;
WO g4/21642 PCTtUS94/02692
2~,S606S , .
(iv) -C(O)OR6, where R6 is as defined above;
(v) -sO3H;
(vi) -S(O)SR6, where s and R6 are as defined above;
(vii) -S(o)tNR6R7, where t and NR6R7 are as defined above;
(viii) =NOR6, where R6 is as defined above;
(ix) -aryl;
(x) -Het;
(xi) -R399, as defined above; and
(xii) -R400, as defined above;
(D) -aryl;
(E) Het-;
(E~) mono-, di-, tri-, or per-halogenated-Cl-C6aL~yl;
(G) -N(R8)NR14Rl5, where R8 and NRl4Rl5 are as defined above;
(H) -Si(Rll)3, where each Rll is independently -(Cl-C6-aL~yl), aryl-(Cl-C6-
aL~yl)-, or aryl;
(I) -OSi(Rll)3, where each Rll is independently as defined above;
(J) -Sn(Rll)3, where each Rll is independently as defined above;
(K) -P(Rll)2, where each Rll is independently as defined above;
(L) -Rl4, where Rl4 is as defined above; or
(M) halogen;
(II) -O(CH2)mS(O)SR12, where m, s and R12 are as defined above;
(III) -O(CH2)jCN, where j is as defined above;
(IV) -o(cH2)ic(=NoRl4)Rl2~ where j, R12 and Rl4 are as defined above;
(V) -o(cH2)jc(=N+(o-)Rl4)Rl2~ where j, R12 and Rl4 are as defined above, with the
proviso that R14 may not be hydrogen;
(VI) -o(cH2)ic(=NoRl4)Rl5~ where j, R14 and R15 are as defined above;
(VII) -o(cH2)jc(=N+(o-)Rl4)Rl5~ where j, R14 and R15 are as defined above, with
the proviso that Rl4 may not be hydrogen;
(VIII) -oc(o)o(cH2)ic(o)NRl4Rl5~ where j and NR14R15 are as defined above;
(IX) -o(cH2)jNR6c(o)oRl4~ where j, R6 and R14 are as defined above;
(X) -o(cH2)jNR6c(o)NRl4Rl5~ where j, R6 and NRl4Rl5 are as defined above;
(XI) -o(CH2)jNR6C(o)NR7NR14Rl5, where j, R6, R7 and NRl4Rl5 are as defined
above;
(XII) -O(CH2)jNR6C(O)R14,where j, R6 and R14 are as defined above; and
(XIII) -o(cH2)iNR6c(o)oc(o)Rl4~ where j, R6 and Rl4 are as defined above;
- 14-
~ WO 94/21642 2 1 5 6 0 6 ~ PCT/US94102692
R2 and R2a are independently hydrogen, halogen, or -oR14, wh~eill R14 is as defined above,
or one of R2 and R2a may be hydroxy, when the other of R2 or R2a is hydrogen, or R2 and
R2a taken together is oxo or thiooxo;
R3 and R4 are chosen, when R5 is hydrogen, such that one of R3 and R4 is hydrogen and the
other is selected from hydrogen, hydroxy, -OCOR8, where R8 is as defined above, or
-OSi(R11)3, where each R11 is independently as defined above, or one of R3 and R4 is joined
with non-hydrogen R5 to form a C-23/C-24 bond, with the other being hydrogen, hydroxy,
-OCOR8, where R8 is as defined above, or -OSitR11)3, where each R11 is independently as
defined above;
R5 is hydrogen, or taken together with either R3 or R4, forms a C-23/C-24 bond.
The compounds of the invention are subject to the proviso that, when R2 and R2a are both
hydrogen and R la is methyloxy, then it is understood that R1 may not be defined as,
(I)-O(CH2)jC(O)R20, where j is as defined above and R20 is selected from
(A) hydroxy;
(B) -OR21 wherein R2l is loweralkyl, cycloaLl~yl, cycloalkylalkyl or qualified-
arylalkyl, where qualified-arylalkyl is defined below; and
(C) -NR22R23, wherein NR22R23 is either:
a nitrogen atom attached to R22 and R23, where R22 is selected from
hydrogen, loweralkyl, qualified-arylalkyl, cycloaL~yl and cycloaLkylaLkyl;
and R23 is selected from hydrogen, loweralkyl, qualified-arylalkyl,
cycloalkyl, cycloalkylaLkyl, aminoalkyl, hydroxyalkyl, carboxyalkyl, and
thioloweralkyl;
or a saturated heterocyclic ring, where taken together, R22 and R23 are
-(CH2)q~ wherein q is two to five;
or is morpholino; or
(II) ~o(cH2)ic(o)N(R24)(cH2)mcH(R25)c(o)R2o~ where j, m and R20 are as defined
above and R24 is selected from hydrogen, loweralkyl, qualified-arylalkyl,
cycloalkyl and cycloalkylalkyl; and R25 is selected from hydrogen, loweralkyl,
hydroxyloweralkyl, carboxyaL~cyl, thioloweralkyl, thioaL~oxyaLkyl,
guanidinoaLkyl, aminoalkyl, qualified-arylaL~yl and, if m is other than zero, amino
or amidoalkyl;
or taken together, R24 and R25 are -(CH2)p-, wherein p is two to five; or
m) -o(cH2)ic(o)N(R24)(cH2)mcH(R25)-c(o)N(R26)(cH2)mlcH(R27)-c(o)R
where j, m, R20, R24 and R25 are as defined above, and ml is zero to six, R26 isselected from hydrogen, loweralkyl, qualified-arylalkyl, cycloalkyl and
- 15-
~,~,S606S PCTlUS94t0~692
cycloalkylalkyl; and R27 is selected from hydrogen, loweralkyl,
hydroxyloweralkyl, carboxyaL~yl, thioloweralkyl, thioalkoxyaL~yl,
g~-~nitiino~lkyl, aminoalkyl, qualified-arylalkyl and, if ml is other than zero,amino or ~mido~lkyl;
or taken together, R26 and R27 are -(CH2)p-, wherein p is as defined above; or
(IV) -o(CH2)jC(o)N(R24)(cH2)mcH~R2s)-c(o)N(R26)(cH2)mlcH(R27)-
C(O)N(R28)(CH2)m2CH(R29)C(O)R20~where j, m, ml, R20, R24, R25 R26 and
R27 are as defined above, and m2 is zero to six, R28 is selected from hydrogen,
loweralkyl, qualified-arylalkyl, cycloalkyl and cycloaLkylaLkyl; and R29 is
selected from hydrogen, loweralkyl, hydroxyloweralkyl, carboxyaLkyl,
thioloweraLkyl, thioalkoxyalkyl, guanidinoalkyl, aminoaLkyl, qualified-arylaLkyland, if m2 is other than zero, amino or ~midoalkyl;
or taken together, R28 and R29 are -(CH2)p-, wherein p is as defined above.
It is understood that when a variable, such as m, s, t, M+, Q, R6, R7, R8, R9, R10, R11,
R13 R14 R15 R16, R17, R18, Rl9, R301, R302, R399, R400, alkyl, alkenyl, alkynyl, aryl, Het,
mod-aryl, mod-Het, or the like, occurs more than once in a formula, its value is chosen
independently at each occurance. It is further understood that the present application is not
cl~iming substitllents or substitution patterns that are impractical or unreasonable to prepare.
Preferred compounds according to the present invention are represented by formula
(II):
A~~
CH30 ' R5
~
O
H~O
'OCH3
OCH3 (II),
wherein n, R, R2, R2~, R3, R4 and R5 are as defined above and A is selected from among:
- 16-
~ WO 94121642 2 1 5 6 0 6 5 ~.IUS94102692
R14 H
R1~J~ R13O J~ nd , wherej, R12
R13, R14 and R15 are as defined above.
More ~re~.led compounds according to the present invention are represented by
formula (III):
, ~ ~
CH30 ~ ' R5
~
~ R
HO O
OCH3
OCH3 (Ir[)7
wherein n, R, R2, R2a, R3, R4 and R5 are as defined above and B is selected from:
O O R14 H
R ~NJ~ R ~NJ~ 6 ~N~N~
,
Aryl ~NJ~ ~N (CH2)m --NJ~
R14 R15 R6
and where j, m, R6,
Wos4/21642 Pcrlus94l02692
~,~S6~6S
Rl4 and R15 are as defined above, and aryl is as defined below.
Most preferred compounds according to the present invention are represented by
formula (IV):
D~ "~3
CH30 ~ ' R5
~
(CH2)n 0 R3 R4 J
$~ ~ R
HO O
_4 `OCH3
OCH3 (IV).
wherein n, R, R2, R2a, R3, R4 and RS are as defined above and D is selected from:
O R6 H
~N ~ 7~N ~N ~
R7 and O where j, R6 and R7 are as defined
above.
Preferred compounds according to the invention are the compounds of:
Formula II, wherein R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; and A=-OCH2C(O)-O-
CH2-[( 1 R)-(+)-alpha-pinen- 1 0-yl)]; . t
Formula II, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oSi(CH3)3; and A=-
OCH2C(O)-O-CH2-[(lR)-(+)-a-pinen-10-yl)];
~ wo g4,2lC42 2 1 5 6 0 6 5 ~IUS94102692
- ~.
Formula II, wherein R= ethyl; n= 1; R2=R2a-R3=RS=H; R4=oH; and A=-OCH2C(O)-O-
CH2-(4-nitrophenyl);
Formula II, whelein R= ethyl; n= 1; R2=R2~-R3=RS=H; R4=oH; and A=
-OCH2C(O)OC2H5;
Formula II, wherein R= ethyl; n= 1; R2=R2a=R3=R5=H; R4=oH; and A=
-OCH2C(O)OCH2C6H5;
Formula II, wherein R= ethyl; n= 1; R2=R2a=R3=RS=H; R4=oH; and A= -OCH2C(O)OH;
Formula II, wherein R= ethyl; n= 1; R2=R2a=R3=RS=H; R4=oH; and A=
-OCH2C(O)OCH2-(9-fluorenyl);
Formula II, wherein R= ethyl; n= 1; R2=R2a=R3=H; A= -ocH2c(o)ocH2ph; and R4 and
RS taken together form a bond; and
Formula II, wherein R= ethyl; n= 1; R2=R2a=R3=R4=RS=H; and A= -OCH2C(O)OH;
Formula II: R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; A= -OCH2C(O)OCH2CC13;
More preferred compounds according to the invention are the compounds of:
Formula m, wherein R= ethyl; n= 1; R2=R2a-R3=RS=H; R4=oH; and B= -OCH2C(O)-
NHR15, where R15--CH(CH3)CONHCH(CH3)CONHCH(CH3)CO2H (all chiral centers in
R15 are R configuration);
Formula m, wherein R= ethyl; n= 1; R2=R2a=R3=R5=H; R4=oH; and B= -OCH2C(O)-
N(CH2CH2N(CH3)(CH2CH20H))-phenyl;
Formula m, wherein R= ethyl; n= 1; R2=R2a=R3=RS=H; R4=oH; and B= -OCH2C(O)-
N(CH2CH2N(CH3)2)-phenyl;
Formula III, wherein R= ethyl; n= 1; R2=R2a=R3=RS=H; R4=oH; and B= -OCH2C(O)-
NHNHC02CH3;
Forrnula m, wherein R= ethyl; n= 1; R2=R2a=R3=RS=H; R4=oH; and B= -OCH2C(O)-
N(CH2CH20H)-NHC02CH3;
- 19-
~ ,? ~IUS94102C91
Formula m, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and B= -OCH2C(O)-
N(CH2CH2CH20COCH2CH2C02H)(4-fluorophenyl);
Formula m, wherein R= ethyl; n= l; R2=R2a=R3=R~=H; R4=oH; and B= -OCH2C(O)-
HN(CH2)sNH-dansyl;
Formula m, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and B
-OCH2C(O)N(phenyl)CH2CH2CH20H; and
Formula m, wherein R= ethyl; n= 1; R2=R2a=R3=RS--H; R4=oH; and B=-OCH2C(O)-
NH(6-carbomethoxymethylm~;l.;ap~u,ine hydrazidyl);
Most preferred compounds according to the invention are the compounds of:
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=RS=H; R4=oH; and D= -OCH2C(O)-
NH-benzyl;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
N(CH3)-benzyl;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH2;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3--R5=H; R4=oH; and D= -OCH2C(O)-
NH-CH3;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
N(CH3)2;
Formula IV, wherein R= ethyl; n- l; R2=R2a=R3=R5--H; R4=oH; and D= -OCH2C(O)-
NH-ethyl;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
N(CH3)-ethyl;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-CH2CH2CH3;
- 20 -
~ WO g4/21642 2 1 S 6 0 6 5 ~IUS94102692
Formula IV, whc.ein R= ethyl; n= 1; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
N(CH3)-CH2CH2CH3;
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-CH(CH3)2;
Formula IV, wheleill R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
N(CH3)-CH(CH3)2;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-cyclopropyl;
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-CH2CH2CH2CH3;
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
N(CH3)-CH2CH2CH2CH3;
Forrnula IV, wherein R= ethyl; n= 1; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-cH2cH(cH3)2;
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
N(CH3)-CH2CH(CH3);
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=R5--H; R4=oH; and B= -OCH2C(O)-
NHNH-CO-(4-pyridyl);
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-cyclobutyl;
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-CH2CH2CH2CH2CH3;
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
N(CH3)-CH2CH2CH2CH2CH3;
Wo 94/~ ,56~)65 PCT/US94/02692
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-CH2CH2CH(CH3)2;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R~=~, R4=OlH; and D= -OCH2C(O)-
N(CH3)-CH2CH2CH(CH3)2;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; and D= -OCH2C(O)-
NH-cyclopentyl;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-CH2CH2CH2CH2CH2CH3;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; and D= -OCH2C(O)-
N(CH3)-CH2CH2CH2CH2CH2CH3;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; and D= -OCH2C(O)-
NH-cyclohexyl;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NR6R7, where R6 and R7. taken together= -CH2CH2OCH2CH2-, thus forming a six-
membered ring incorporating the nitrogen to which they are attached;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; and B= -OCH2C(O)-
NH(4-morpholinyl);
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-CH2CH20H;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-CH2CH2CH20H;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; and D= -OCH2C(O)-
NH-CH2CH2CH2CH20H;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; and D= -OCH2C(O)-
NH-CH2CH2CH2CH2CH20H;
- 22-
~ wo g4,2l642 2 1 5 6 0 6 5 - ~IUS94102692
Formula IV, wherein R= ethyl; n= l; R2=R2a-R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-CH2CH2NH2;
Formula IV, wherein R= ethyl; n= l; R2=R2a-R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-CH2CH2CH2NH2; ' ,
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-cH2cH2cH2cH2NH2;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-cH2cH2cH2cH2cH2NH2;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-CH2C02CH2Ph;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-CH2C02H;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-CH2CH2C02CH2Ph;
Formula IV, wherein R= ethyl; n= l; R2=R2a---R3=R5--H; R4=oH; and D= -OCH2C(O)-
NH-CH2CH2C02H;
Formula IV, wherein R= ethyl; n= l; R2=R2a-R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-(R)-CH(CH3)CO2H, and has an R con~lguration;
Formula IV, wherein R= ethyl; n= l; R2=R2a-R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-(S)-CH(CH3)C02H;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-3-phenyl-phenyl;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
N(CH2CH20H)(3-phenyl-phenyl);
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
N(CH2(3 -pyridyl))2;
- 23 -
WO s4t21642 ~,~$6~6$ . . ~ PCTIUS94/02692
Forrnula IV, wherein R= ethyl; n= 1; R2=R2a=R3=RS=H; R4=oH; and D= -OCH2C(O)-
N(cyclohexyl)2;
Formula IV, wherein R= ethyl; n= 1; R2=R2~R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH(4-thiomorpholinyl);
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=RS=H; R4=oH; and D= -OCH2C(O)-
NH(4-CF3-phenyl);
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH(4-F-phenyl);
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; and D= -OCH2C(O)-
NH(4-(4-morpholino)-phenyl);
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=R5=H; R4=OEI; and D= -OCH2C(O)-
NH(4-HO-phenyl);
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-3-pyridyl;
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=RS=H; R4=oE~; and D= -OCH2C(O)-
NH-4-pyridyl;
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH-2-pyridyl;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
N(CH3)-CH2CH20H;
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-(L-
prolinocarboxamide);
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-(D-
prolinocarboxamide);
- 24-
~ WO g4/21642 2 I S 6 0 6 5 ~ us94lo2692
Formula IV, wht~ R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-(L-
prolinol);
Formula IV, wllcleill R= ethyl; n= l; R2sR2a-R3=R5=H; R4=oH; and D= -OCH2C(O)-(D-
prolinol);
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH(3-(phenylethynyl)phenyl);
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
N(CH2CH2CH20H)(4-fluorophenyl);
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; and D=
-ocH2c(o)NR6R7~ where R6 and R7, taken together, form the diradical, -CH2CH2
C(OCH2CH20)CH2CH2-;
Formula IV, wherein R= ethyl; n= l; R2=R2a-R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH(3-fluorophenyl);
Forrnula IV, wherein R= ethyl; n= l; R2=R2a-R3=RS=H; R4=oH; and D= -OCH2C(O)-
NH(3-hydroxy-phenyl);
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D=
-oCH2C(o)NR6R7, where R6 and R7, taken together, form the diradical, -CH2CH2-NCH3-
CH2CH2-;
Formula IV, wherein R= ethyl; n= l; R2=R2a--R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH{6-(1,4-benzodioxanyl) };
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH(3,4-methylenedioxy-phenyl);
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2C(O)-
NH- 1 -naphthalenyl;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; D= -OCH2C(O)R12; and
R12 = NR6R7; where R6 and R7 taken together = -cH2cH2cH2cH2-~ thus forming a five
membered ring incorporating the nitrogen to which they are attached;
- 25 -
WO g4/21642 PCT/US94/02692 ~
60~
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=R5=H; R4=oH; D= -OCH2C(O)R12; and
R12 = NR6R7; where R6 and R7 taken together = -CH2CH2CH2CH2CH2-, thus forming a
six membered ring incorporating the nitrogen to which they are attached;
Formula IV, wherein R= ethyl; n= i, R2--R2a=R3=RS=H; R4=oH; and D= -OCH2
C(O)NH-CH2CH2C6H5;
Formula IV, wherein R= ethyI; n= 1; R2=R2a=R3=R5=H; R4=oH; and D= -OCH2
C(O)N(CH3)-CH2CH2C6H5;
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=RS=H; R4=oH; and D= -OCH2 C(O)-
NHC6H5;
Folmula IV, wherein R= ethyl; n= 1; R2=R2a=R3=RS=H; R4=oH; and D= -OCH2
C(O) -NH(cH2)2N(cH2cH2)2o;
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=RS=H; R4=oH; and D= -OCH2
C(O) -NH(cH2)3N(cH2cH2)2o;
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=RS=H; R4=oH; and D= -OCH2
C(O) -NH(cH2)2N(cH3)2;
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=RS=H; R4=oH; and D= -OCH2
C(O) -NH(cH2)3N(cH3)2;
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=RS=H; R4=oH; and D= -OCH2 C(O)-
(S)-NHCH(CH2C6HS)C02cH2Ph;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; and D= -OCH2 C(O)-
(S)-NHCH(CH2C6H5)C02H;
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3--RS=H; R4=oH; and D= -OCH2 C(O)-
(R)-NHCH(CH2C6HS)C02cH2Ph;
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=RS=H; R4=oH; and D= -OCH2 C(O)-
(R)-NHCH(CH2C6H5)C02H;
- 26 -
21S606S
WO 94/21642 PCr/US94102692
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=RS=H; R4=oH; and D= -0CH2
C(O)-HN(CH2)2SH;
Formula IV, wherein R= ethyl; n= l; R2=R2a-R3=RS=H; R4=oH; and D= -OCH2 C(0)-
HN(CH2)3SH;
., ,
Formula IV, wherein R= ethyl; n= 1; R2=R2a=R3=RS=H; R4=oH; and D= -OCH2C(0)-
NH-2-naphthyl;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R4=RS=H; and D= -ocH2c(o)-NR6R7
where R6 and R7, taken together, are the diradical, -CH2CH20CH2CH2-;
Formula IV, wherein R= ethyl; n= l; R2=R2a--R3=RS=H; R4=oH; and D= -OCH2C(0)-
NH {4-(H2NS02)-phenyl };
Formula IV, wherein R= ethyl; n= l; R2=R2a--R3=RS=H; R4=oH; and D=
-ocH2c(o)NR6R7~ where R6 and R7, taken together, form the diradical, -CH2CH2-
N(CH2CH20H)CH2CH2-;
Formula IV, wherein R= ethyl; n= l; R2=R2a--R3=RS=H; R4=oH; and D= -0CH2C(0)-
N(CH3)phenyl;
Formula IV, wherein R= ethyl; n= l; R2=R2a-R3=RS=H; R4=oH; and D= -OCH2C(0)-
N(cH2cH2oH)2;
Formula IV, wherein R= ethyl; n= l; R2=R2a-R3=RS=H; R4=oH; and D= -OCH2C(0)-
N(CH3)CH2CH2CH2N(CH3)2;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; and D= -0CH2C(0)-
NH-CH(CH20H)2;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; and D= -OCH2C(0)-
NH { 3-(CF3)-phenyl };
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; and D= -0CH2C(0)-
N(CH2CN)2;
- 27 -
W0 94/Z~ S6~5Si P~rltlS94/1~2692
Formula IV, wh~ l R= ethyl; n= l; R2---R2~-R3=R5---H; R4=oH; and D= -OCH2-
C(o)NR6R7, where R6 and R7, taken together, form the diradical, -CH2CH2-;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; and D= OCH2-NH(Co)NR6R7; where R6 and R7, taken togethe~:, are the diradical, -cH2cH2ocH2cH2-;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; and D= OCH2-NH(CO)NH-phenyl;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; and D= OCH2-
NH(CO)NH-CH2CH2CH20H;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; and D=OCH2C(O)-
NR6R7, where R6 and R7, taken together, form the diradical, -CH2CH2S02CH2CH2-;
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; and D= OCH2C(O)-NH-
CH2CH2-(4-F-phenyl);
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; and D= OCH2C(O)-
NH(4-Cl-phenyl);
Formula IV, wherein R= ethyl; n= l; R2=R2a--R3=RS=H; R4=oH; and D= OCH2C(O)-
NH(4-(OCH3)-phenyl);
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; and D= OCH2C(O)-
NH(4-CH3 -phenyl);
Formula IV, wherein R= ethyl; n= l; R2--R2a=R3=R5=H; R4=oH; and D= OCH2C(O)-
NH(3,4-C12-phenyl);
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=RS=H; R4=oH; and D= OCH2C(O)-
NH(3-I-phenyl);
Formula IV, wherein R= ethyl; n= l; R2=R2a=R3=H; R4 and RS, taken together, form a
bond; and D--OCH2C(0)~4-(morpholinyl)};
Formula IV, wherein R= ethyl; n= l; R2=R3=R5=H; R2a=R4 =OH; and D= OCH2-
C(0)NH(3-fluoro-phenyl); and
- 28 -
~ WO 94/21642 PCTIUS94/02692
215606S i
Formula IV: R= ethyl; n= l; R2=R2a=R3=R5=H; R4=oH; D= -oCH2C(o)NR6R7; R6_
(CH2)2N(CH2CH2)20; R7=-CH2CH20H.
As used throughout this Specification and Claims, the following terms have the
mç~ning~ specified:
"Acyl", as used herein, refers to an aryl or alkyl group, as defined below, appended to
the rçm~inA~-r of the molecule via a carbonyl group. Examples include, but are not limited
to, acetyl, pivaloyl, benzoyl, and the like.
"Acylamino" refers to an acyl group, as defined above, except that it is appended to
the rçm~in~lçr of the molecule via an amino group. Examples include, but are not limited to,
acetylamino, pivaloylamino, benzoylamino, and the like.
"Acylguanidino" refers to an acyl group, as defined above, except that it is attached to
the rem~inder of the molecule via a nitrogen of a gll~niAino radical in one of three ways:
HN(acyl)C(NH)NH- or H2NC(NH)N(acyl)- or (acyl)NC(NH2)HN-.
"Alkenyl" refers to straight- or branched-chain groups of a specified number of
carbon atoms cont~ining at least one carbon-carbon double bond incl~lAing, but not limited to
ethenyl, l-~rolJellyl, 2-propenyl, 2-methyl-1-plul)ellyl, 1-butenyl, 2-butenyl, and the lilce.
"AL~oxy", "aL~ylether" and "loweraL~oxy" refer to an aL~yl group, as defined below,
attached to the rçm~inder of the molecule through an oxygen atom. Examples include, but
are not limited to, methoxy, ethoxy, isopropoxy, n-butoxy, sec-butoxy, isobutoxy, tert-butoxy
and the like.
''ALIcoxycarbonyl'' refers to an aL~oxy group, as defined above, except that it is
attached to the rem~inclçr of the molecule via a carbonyl group. Examples include, but are not
limited to, methyloxycarbonyl, ethyloxycarbonyl, tert-butylo~ycall,onyl,
cyclohexyloxycarbonyl, and the like.
"Alkoxycarbonylamino" refers to an aL~coxycarbonyl group, as defined above, except
that it is attached to the rem~incl-or of the molecule via an amino group. Examples include,
but are not limited to, methyloxy-carbonylamino, tert-butyloxycarbonylamino, and the like.
"Alkoxycarbonylguanidino" refers to an alkoxycarbonyl group, as defined above,
except that it is attached to the rem~inA~r of the molecule via a nitrogen of a gll~niAinn
radical in one of three ways: HN(aL~oxycarbonyl)C(NH)HN-,
H2NC(NH)N(aL~oxycarbonyl)- or (aL~coxycarbonyl)NC(NH2)HN-.
"ALkyl" refers to a straight- or branched-chain group of a specified number of carbon
atoms including, as a~,~fiate, but not necess~rily limited to, methyl, ethyl, n-propyl,
isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, and the like.
- 29-
WO 94/211i42 6S ~ ~ r PCT/US94102692
"AL~ylamino" refers to a group having the structure -NH-(alkyl), where the aLkylportion is as defined above, including, for example, methylarnino, ethylamino,
isopropylamino, and the like.
"AL~ylsulfonyl" refers to an alkyl group, as defined above, except that it is attached to
the rt-m~inder of the molecule via a sulfur dioxide di~adical. Examples include but are not
limited to, m~th~nesulfonyl, camphorsulfonyl and the like.
"ALkylthioether" and "thioaLkoxy" refer to an aLkyl group, as previously defined,
except that it is attached to the rern~intler of the molecule via a sulfur atom. Examples
include, but are not limited to, thiomethoxy, thioethoxy, thioisopropoxy, n-thiobutoxy, sec-
thiobutoxy, isothiobutoxy, tert-thiobutoxy, and the like.
"Alkynyl" refers to straight- or branched-chain groups of a specified number of
carbon atoms co~ g at least one carbon-carbon triple bond, including, but not limited to
acetylenyl, plupa~yl, and the like.
".Amido~lkyl" refers to a group having the structure -NRlOlC(O)R102 appended to the
rçm~ind~r of the molecule via an aLkyl group, as previously defined, wherein RlOl and R10Z
are independently hydrogen, alkyl, aryl, arylalkyl, or halosubstituted alkyl, or RlOl and R102
taken together, may optionally be -(CH2)aa-, where aa is an integer of from 2-to-6.
"Aminoalkyl" refers to a group having the structure -NR103Rlo4 appended to the
r~.m~in(lçr of the molecule via an aL~yl group, as previously ~lçfin~l, wherein R103 and R104
are independently hydrogen, alkyl, qualified-aryl or qualified-arylaL~yl, or R103 and R104,
taken together, may optionally be -(CH2)bb-, where bb is an integer of from 2-to-6.
"Aryl", as used herein, refers to mono-, di-, tri- or tetracyclic aromatic groups,
charged or uncharged, the rings of which are comprised of from 3-to-7 carbon atoms.
Examples of aryl include, but are not limited to, phenyl, 1- or 2-naphthyl, azulenyl, fluorenyl,
(1, 2)-dihydronaphthyl, (1,2,3,4)-tetrahydlonaphthyl, indenyl, indanyl and the like, which
are unsubstituted or substituted with from one, two or three independently-selected
substitllçnt~, R301, as defined above.
~ Arylalkoxy" and "arylaLkylether" refer to an arylaL~yl group, as defined below,
attached to the parent molecular moiety through an oxygen atom. Examples inchlcle, but are
not limited to, benzyloxy, 2-phenethyloxy, l-naphthylmethyloxy, and the like.
"ArylaLl~oxycarbonyl" refers to an arylaL~oxy group, as defined above, except that it is
attached to the r~.m~inrlçr of the molecule via a carbonyl group. Examples include, but are
not limited to, benzyloxycarbonyl, 9-fluorenylrnethyloxycarbonyl, and the like.
"ArylaLI~oxycarbonylamino" refers to an arylalko~yc~l onyl group, as defined above,
except that it is attached to the rem~in~lcçr of the molecule via an arnino group. Examples
include, but are not limited to, benzyloxycarbonylamino, 9-
fluorenylmethyloxycarbonylamino, and the like.
- 30-
wo g4,2l642 2 1 5 6 0 6 5 ~ s94~02692
"Arylalkoxycarbonylguanidino" refers to an arylalkoxycarbonyl group, as defined
above, except that it is ~ ched to the rçm~inll~o.r of the molecule via a nitrogen of a
gn~ni~linn radical in one of three ways: HN~arylalkoxycarbonyl)C(NH)HN-,
H2NC(NH)N(arylaL~oxycarbonyl)- or (arylalkoxycarbonyl)NC(NH2)HN-.
"Arylalkyl" refers to an aryl group, as previously dçfinçd, except that it is attached to
the rem~inder of the molecule via an alkyl group.
"Arylalkylamino" refers to a group having the structure -NH-(arylalkyl), where the
arylalkyl portion is as previously definç i, except that it is ~ hed to the r~m~inder of the
molecule via an amino group. Examples include benzylamino, l-phenylethylamino, and the
like.
"Arylalkylthioether" and "thioarylalkoxy" refer to an arylalkyl group, as previously
defined, except that it is attached to the rem~indçr of the molecule via a sulfur atom.
"Arylamino" refers to an aryl group, as defined above, except that it is attached to the
rem~in~l~r of the molecule via an amino group. Examples include, but are not limited to,
anilino, naphthylamino, and the like.
"Arylether" and "aryloxy" refer to an aryl group, as previously defined, attached to
the parent molecular moiety through an oxygen atom. Examples include, but are not limited
to, phenoxy, l-naphthoxy, 2-naphthoxy, and the like.
"Aryloxycarbonyl" refers to an arylo~yglou~, as defined above, except that it isattached to the rçm~ind~r of the molecule via a carbonyl group. Examples include, but are
not limited to, phenyloxycarbonyl, and the like.
"Aryloxycarbonylamino" refers to an arylo~yc~bollyl group, as defined above,
except that it is attached to the r~m~indçr of the molecule via an amino group. Examples
include, but are not limited to, phenylu~ycallJonylamino, and the like.
"Aryloxycarbonylguanidino" refers to an aryloxycarbonyl group, as defined above,except that it is attached to the rem~inder of the molecule via a nitrogen of a guanidino
moiety in one of three ways: HN(aryloxycarbonyl)C(NH)HN-,
H2NC(NH)N(aryloxycarbonyl)- or (arylu~yc~bonyl)NC(NH2)HN-.
"Arylsulfonyl" refers to an aryl group, as defined above, except that it is attached to
the r~m~inder of the molecule via a sulfur dioxide group. Examples include, but are not
limited to p-toluenesulfonyl, benzenesulfonyl, and the like.
"Arylsulfonylguanidino" refers to an arylsulfonyl group, as defined above, except that
it is attached to the rçm~ind~r of the molecule via a guanidino group in one of three ways:
HN(arylsulfonyl)C(NH)HN- or H2NC(NH)N(arylsulfonyl)- or (arylsulfonyl)NC(NH2)HN-.
"Arylthioether" and "thioaryloxy" refer to an aryl group, as defined above, except that
it is attached to the rem~ind~r of the molecule via a sulfur atom.
- 31 -
'~ ~ 't
o g4~21~2,~S~6S PCTIUS94/02692
"Biaryl" refers to a mod-aryl group, as defined below, which carries as a substituent
another independently selected mod-aryl g~oup, such that the two are connected by a single
carbon-carbon bond.
"Carboxamido" refers to an amino group attached to the rem~in~er of the moleculevia a carbonyl group, and having the formula H2~C(O)-.
~ Carboxyalkyl" refers to a carboxyl group, -C02H, appended to the ren-~inder of the
molecule via an aLlcyl group, as previously ~lefin~
" Connt~.riQn", as used herein, refers to a positively-charged atom or molecularspecies, with a net charge of +1, which includes, but is not li~ted to Li+, Na+,Ca(OC(O)CH3)+, MgCl+, K+, NH4+, (n-butyl)4N+, andthelike.
"Cycloalkenyl" refers to cyclic groups of 5-to-10 carbons po~.sessing one or more
carbon-carbon double bonds including, but not limited to, cyclopentenyl, cyclohexenyl,
1,3,3-trimethylcyclohexenyl, and the like, in which the point of attachment may occur at any
available valency on the carbocylic ring.
~ CycloaL~yl" refers to s~t~lr~te~ cyclic groups of 3-to-8 carbons including, but not
limited to, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, and the like.
"CycloaL~ylalkenyl" refers to cycloaLkyl, as defined above, except that it is attached to
the remainder of the molecule via an aL~enyl group, as defined above.
~ CycloalkylaL~yl" refers to a cycloalkyl group, as defined above, except that it is
attached to the r.o.m~in~l~.r of the molecule via an aL~yl group. Examples include, but are not
limited to, cyclohexylmethyl, cyclohexylethyl, and the like.
"CycloaL~ylaLl~ynyl" refers to cycloaL~yl, as defined above, except that it is attached
to the rem~ind~r of the molecule via an aL~cynyl group, as defined above.
"Guanidino" refers to a group of the structure -NR105C(-NRl06)NHRlo7 or
-NC(NHRl06)NHRlo7~ wherein R105, R106~ and R107 are independently selected from
hydrogen, (Cl-to-C6-aL~yl), mod-Het-, as defined below, mod-heterocyclic, as defined
below, aminoaLI~yl, as defined above, and mod-aryl, as defined below, or ~ltern~tively, R106,
and R107, taken together, may optionally be -(CH2)CC-, where cc is an integer of from 2-to-6.
"Halo" and "halogen" refer to an atom selected from fltlnrinç, chlorine, bromine and
iodine.
"Het-", as used herein, refers to any aromatic 5-, 6- or 7-membered monocyclic ring
or a bi- or tri-cyclic group comprising fused five- or six-membered rings having ring carbon
atoms and between one and three heteroatoms independently selected from oxygen, sulfur
and nitrogen, wherein (i) each S-membered ring has 2 double bonds and each 6- or 7-
membered ring has 3 double bonds, (ii) the nitrogen and sulfur heteroatoms as well as the
carbon atoms may optionally be oxi~li7ed, (iii) the nitrogen heteroatom may optionally be
quaternized, (iv) any of these rings may be fused to a benzene ring, and (v) any carbon or
heteroatom with suitable valence may bear a ~Ubstihlçnt~ R301, as defined above. Any two
- 32 -
2156065
WOg4121642 ~ TIUS94/02692
~ r
adjacent R301 substit~lent~ in a di-, tri-, tetra- or penta-substituted Het group may form a 5-,
6- or 7-mPmb~o.red ring consisting of carbon atoms and zero, one or two heteroatoms
independently selected from the group consisting of -O-, -S(O)s-, where s is as defined
above, and -NR8-, where R8 is as defined above. Het groups include, but are not limited to,
pyrrolyl, pyrazolyl, cytosinyl, thiocytosinyl, imidazolyl, pyridyl, pyrazinyl, pyrimidinyl,
pyridazinyl, x~nthçnyl, xanthonyl, xanthopterinyl, oxazolyl, thiouracilyl, isoxazolyl, indolyl,
quinolinyl, uracilyl, urazolyl, uricyl, thiazolyl, isothiazolyl, isoquinolinyl, thyminyl,
ben7imi~7olyl, benzothiazolyl, benzoxazolyl, furyl, thienyl, benzothienyl, and the like.
"Heterocyclic" as used herein, except where otherwise specified, refers to any non-
aromatic 5-, 6- or 7-membered monocyclic ring or a bi- or tri-cyclic group comprising fused
five- or six-membered rings, having ring carbon atoms and between one-and-three
heteroatoms independently selected from oxygen, sulfur and nitrogen, wherein (i) each 5-
membered ring has O or 1 double bond and each 6-membered ring has O to 2 double bonds,
(ii) the nitrogen and sulfur helt;loalo-l-s as well as the carbon atoms may optionally be
oxidized, (iii) the nitrogen heteroatom may optionally be qll~terni7ed~ (iv) any of these rings
may be fused to a benzene ring, and (v) any carbon or heteroatom with suitable valence may
bear a substihlçnt R301, as defined above. Any two ~dj~c~nt R301 substituents in a di-, tri-,
tetra- or penta-substituted heterocyclic group may form a 5-, 6- or 7-membered ring
consisting of ring carbon atoms and zero, one or two heleluato...s independently selected
from the group con~i~ting of -O-, -S(O)s-, where s is as defined above, and -NR8-, where R8
is as defined above. R~l~se~ /e heterocycles include, but are not limited to, aziridinyl,
thiomorpholine, thiomorpholine-oxide, thiomorpholine dioxide, and pyrrolidinyl,
pyrazolinyl, pyrazolidinyl, imidazolinyl, imid~7Qlidinyl, piperidinyl, piperazinyl,
oxazolidinyl, isoxazolidinyl, morpholinyl, thiazolidinyl, and isothiazolidinyl, and the like.
"Heterocyclic aIkyl" refers to a heterocyclic group, as defined above, except that it is
attached to the rem~inder of the molecule via an aL~cyl group, as previously de~ln~cl.
"Heterocyclic alkylether" refers to a heterocyclic alkyl moiety, as defined above,
except that it is attached to the rem~inder of the molecule via an oxygen atom.
"Heterocyclic alkenyl" refers to a heterocyclic group, as defined above, except that it
is attached to the rem~inder of the molecule via an alkenyl group, as previously defined.
"Heterocyclic alkylthioether" refers to a heterocyclic alkyl moiety, as defined above,
except that it is attached to the rem~inrler of the molecule via a sulfur atom.
"Heterocyclic alkynyl" refers to a heterocyclic group, as defined above, except that it
is attached to the rem~intlPr of the molecule via an alkynyl group, as previously defined.
"Heterocyclic ether" refers to a heterocyclic moiety, as defined above, except that it is
attached to the rçm~ind~.r of the molecule via an oxygen atom.
"Heterocyclic thioether" refers to a heterocyclic moiety, as defined above, except that
it is attached to the rçm~in(i~.r of the molecule via a sulfur atom.
- 33 -
WO 94/21642?,~,S6~6S . PCT/US94/02692
"HydroxyaL~yl" refers to an -OH appended to an aL~cyl group, as defined above.
"Hydroxy-~rot~eling group" refers to those groups which are known in the art to
protect a hydroxyl group against undesirable reactions during synthetic procedures and to be
selectively removable inclll~ing, but not limited to, methylthiomethyl, tert-
butylrlimethylsilyl, tert-butyldiphenylsilyl, acyl subsS~ t~.~ with an aryl group, where acyl
and aryl are defined above, and the like.
"Leaving group" refers to an aL~cyl-, alkenyl-, or aryl-substituent, where aL~yl, aL~cenyl,
and aryl are as defined above, which in a reaction becomes cleaved to either produce a site of
unsaturation or to introduce another substituent.
"Mod-aryl", as used herein, refers to an aryl group, as defined above, except that the
aryl group is unsubstituted or substituted with from one-to-three independently selected
substituents, R302, rather than R301, where R302 is as defined above. Any two adjacent R302
substituents in a di- or tri-substituted mod-aryl group may form a 5-, 6- or 7-membered
carbocyclic ring or 5-, 6- or 7-membered heterocyclic-ring where the ring atoms are carbon
atoms and one or two heteroatoms independently selected from the group consisting of -O-,
-S(O)s-, where s is as defined above, and -NR18-, where R18 is as defined above."Mod-Cl-to-Clo-aL~yl", as used herein, refers to a -(Cl-to-Clo-alkyl) group
substituted with from one-to-six radicals selected from:
(1) R8, as defined above;
(2) -(CH2)mOR8, where m and R8 are as defined above;
(3) -S(O)SR8, where s and R8 are as defined above;
(4) -S(O)tNR8R9, where t and NR8R9 are as defined above;
(5) -(CH2)mNR8R9, where m and NR8R9 are as defined above;
(6) -SO3H;
(7) =NOR8, where R8 is as defined above;
(8) -R399, as defined above;
(9) -R400, as defined above;
(10) -aryl; and
(1 1) -Het.
"Mod-C3-to-Clo-alkenyl", as used herein, refers to a-(C3-to-Clo-aLI~enyl) group
substituted with from one-to-six radicals selected from:
(1) -R8, where R8 is as defined above;
(2) -(CH2)mOR8, where m and R8 are as defined above;
(3) -S(O)SR8, where s and R8 are as defined above;
(4) -S(O)tNR8R9, where t and NR8R9 are as defined above;
(5) -(CH2)mNR8R9, where m and NR8R9 are as defined above;
(6) -SO3H;
(7) =NOR8, where R8 is as defined above;
- 34-
WOg4/21642 2IS606S ~CTIUS94102692
(8) -R399, where R399 is as defined above;
(9) -R400, where R400 is as defined above;
(10) -aryl; and
(11) -Het. ~ ~
"Mod-C3-to-Clo-aL~ynyl", ase~séd herein, refers to a -(C3-to-Clo-aL~ynyl) group
substituted with from one-to-six radicals selected from:
(1) -R8, where R8 is as defined above;
(2) -(CH2)mOR8, where m and R8 are as defined above;
(3) -S(O)SR8, where s and R8 are as defined above;
(4) -S(o)tNR8R9~ where t and NR8R9 are as defined above;
(5) -(CH2)mNR8R9, where m and NR8R9 are as defined above;
(6) -SO3H;
(7) =NOR8, where R8 is as defined above;
(8) -R399, where R399 is as defined above;
(9) -R400, where R400 is as defined above;
(10) -aryl; and
(1 1) -Het.
"Mod-cyclo-C3-to-Clo-aLIcyl", as used herein, refers to a -(cyclo-C3-to-Clo-aL~yl)
group substituted with from one-to-six radicals selected from:
(1) -R8, where R8 is as defined above;
(2) -(CH2)mOR8, where m and R8 are as defined above;
(3) -S(O)SR8, where s and R8 are as defined above;
(4) -S(O)tNR8R9, where t and NR8R9 are as defined above;
(S) -(CH2)mNR8R9, where m and NR8R9 are as defined above;
(6) -SO3H;
(7) =NOR8, where R8 is as defined above;
(8) -R399, where R399 is as defined above;
(9) -R400, where R400 is as defined above;
(10) -aryl; and
(1 1) -Het.
"Mod-cyclo-C4-to-Clo-aLkenyl", as used herein, refers to a -(cyclo-C4-to-Clo-
- aL~cenyl) group substituted with from one-to-six radicals selected from: (1) -R8, where R8 is as defined above;
(2) -(CH2)mOR8, where m and R8 are as defined above;
(3) -S(O)SR8, where s and R8 are as defined above;
(4) -S(O)tNR8R9, where t and NR8R9 are as defined above;
(5) -(CH2)mNR8R9, where m and NR8R9 are as defined above;
(6) -SO3H;
- 35 -
WO94/21642 2~S606S PCT/US94/02692
(7) =NOR8, where R8 is as defined above;
(8) -R399, where R399 is as defined above;
(9) -R400, where R400 is as defined above;
(10) -aryl; and
(1 1) -Het.
"Mod-bicyclo-C6-to-Clo-aL~cyl", as used h~ein, refers to a -(bicyclo-C6-to-Clo-aL~yl)
group substituted with from one-to-six r~ selected from:
(1) -R8, where R8 is as defined above;
(2) -(CH2)mOR8, where m and R8 are as defined above;
(3) -S(O)SR8, where s and R8 are as defined above;
(4) -S(O)tNR8R9, where t and NR8R9 are as defined above;
(5) -(CH2)mNR8R9, where m and NR8R9 are as defmed above;
(6) -SO3H;
(7) =NOR8, where R8 is as defined above;
(8) -R399, where R399 is as defined above;
(9) -R400, where R400 is as defined above;
(10) -aryl; and
(1 1) -Het.
"Mod-bicyclo-C6-to-Clo-aL~enyl", as used herein, refers to a -(bicyclo-C6-to-Clo-
aLkenyl) group sllbstit~lted with from one-to-six radicals selected from:
(1) -R8, as defined above;
(2) -(CH2)mOR8, where m and R8 are as defined above;
(3) -S(O)sR8, where s and R8 are as defined above;
(4) -S(O)tNR8R9, where t and NR8R9 are as defined above;
(5) -(CH2)mNR8R9, where m and NR8R9 are as defined above;
(6) -SO3H;
(7) --NOR8, where R8 is as defined above;
(8) -R399, is as defined above;
(9) -R400t is as defined above;
(10) -aryl; and
(1 1) -Het.
"Mod-Het-", as used herein, refers to a Het group, as defined above, except that the
Het group may bear one or more substituents R302, rather than R301, where R302 is as defined
above or any two adjacent R302 substituents in a di-, tri-, tetra- or penta-substituted mod-Het
group may form a ~-, 6- or 7-membered ring consisting of ring carbon atoms and zero, one or
two ring heteroatoms independently selected from the group consisting of -O-, -S(O)s-, where
s is as defined above, and -NR18-, where R18 is as defined above.
- 36 -
~ wo g4,2l642 2 I S 6 0 6 S ~ ~US94102692
"Mod-heterocyclic" as used herein, refers to a heterocyclic group, as defined above,
except that the heterocyclic group may bear one or more substituent~ R302, rather than R301,
where R302 is as defined above or any two adjacent R302 substitue.nt~ in a di-, tri-, tetra- or
penta-substituted mod-heterocylic group may form a 5-, 6- or 7-membered ring consisting of
carbon atoms and zero, one or two heteroatoms independently selected from the group
con~i~ting of -O-, -S(O)s-, where s is as defined above, and -NR18-, where R18 is as definéd
above.
"Monoalkylamino" and "dialkylamino" refer respectively to one and two alkyl
groups, as defined above, except that they are appended to the rem~inder of the molecule via
an amino group. Examples include, but are not limited to, methylamino, isopropylamino,
dimethylamino, N,N-methylisopropylamino, and the like.
"MonocycloaL~cylamino" and "dicycloalkylamino" refer respectively to one and tw~cycloalkyl groups, as defined above, except that they are appended to the remainder of the
molecule via an amino group. Examples include, but are not limited to, cyclohexylamino,
bis-(cyclohexyl)amino, and the like.
"Mono-halogenated alkyl", "di-halogenated aL~yl" or "tri-halogenated alkyl" refer to
alkyl groups, as defined above, of specified and co~yalible length, respectively substituted
with one, two, or three halogen atoms, as defined above.
"N-alkylcarboxamido" refers to an alkylamino group, as defined above, except that it
is appended to the rem~in~ler of the molecule via a carbonyl group and has the formula
HN(alkyl)C(O)-.
"N-arylcarboxamido" refers to an arylamino group, as defined above, except that it is
appended to the rem~inder of the molecule via a carbonyl group and having the formula
HN(aryl)C(O)-.
"Naturally occuring amino acid" and "standard amino acid" refer to an amino acidselected from the ~roup con~i~ting of ~l~nine, arginine, ~p~r~gine, aspartic acid, cysteine,
glut~mine, glutamic acid, glycine, hi~tirline, isoleucine, leucine, lysine, methionine,
phenyl~l~nine, proline, serine, threonine, tryptophan, tyrosine and valine.
"N,N-dialkylcarboxamido" refers to dialkylamino group, defined above, except that it
is appended to the rçm~indçr of the molecule via a carbonyl group and has the formula
N(alkyl)(alkyl')C(O)-.
"N,N-diarylcarboxamido" refers to two independently selected aryl groups, as defined
above, except that they are appended to the rem~inder of the molecule via a =NC(O)- group,
exemplified by the formula N(aryl)(aryl)C(O)-.
"N-t~-rmin~l p,ole-;~ing group" refers to those groups known in the art to protect the
N-terminll~ against undesirable reactions during synthetic procedures or to prevent the attack
of exopeptidases on the final compounds or to increase the solubility of the final compounds
and includes, but is not limited to acyl, acetyl, pivaloyl, tert-butylacetyl, tert-
- 37 -
wo 94/21642 ~ 25 S 6 ~ 6~ PCT/US94/02692 ~
butyloxycarbonyl (Boc), benzyloxycarbonyl (Cbz), benzoyl groups, and the like. Other such
groups are described by Gross, E. and Meienhofer, J. in The Peptides. Volume 3; Ac~(lernic
Press, 1981.- -
"Oxo" refers to an oxygen atom forming a~ar~onyl group.
"Per-halogenated aL~cyl" refers to alkyl ~groups, as defined above, of specified length,
substituted with halogen atoms, as defined above, at every available valency.
"Qualified-aryl" as used herein refers to substituted and un~ubstitllted carbocyclic
aromatic groups including, but not limited to, phenyl, 1- or 2-naphthyl, fluorenyl, (1,2)-
dihydronaphthyl, (1,2,3,4)-tetrahydronaphthyl, indenyl, indanyl and the like, optionally
substituted with 1, 2 or 3 substit~lents independently selected from halo, nitro, cyano,
-(Cl-to-Clo-aL~yl), alkoxy and halosubstituted aL~yl.
"Qn~lifie~-arylaL~yl" refers to a qualified-aryl group, as previously defined, attached
to the rçm~indçr of the molecule via an alkyl group.
"Sub-Cl-to-Clo-alkyl", as used herein, refers to a-(Cl-to-Clo-alkyl) substituted with
from one-to-six radicals independently selected from the group consisting of:
(a) R6, where R6 is as defined above;
(b) -(CH2)mOR6, where m and R6 are as defined above;
(c) -NR6R7, where NR6R7 is as defined above;
(d) -C(O)OR6, where R6 is as defined above;
(e) -SO3H;
(f) -S(O)SR6, where s and R6 are as defined above;
(g) -S(o)tNR6R7, where t and NR6R7 are as defined above;
(h) =NOR6, where R6 is as defined above;
(i) -R399, where R399 is as defined above;
(j) -R400, where R400 is as defined above;
(k) -aryl, and
(1) -Het.
"Sub-cyclo-C3-to-Clo-alkyl", as used herein, refers to a -(cyclo-C3-to-Clo-alkyl)
substit~lte-l with from one-to-six radicals independendy selected from the group consi~ting
of:
(a) R6, where R6 is as defined above;
(b) -(CH2)mOR6, where m and R6 are as defined above;
(c) -NR6R7, where NR6R7 is as defined above;
(d) -C(O)OR6, where R6 is as defined above;
(e) -SO3H;
(f) -S(O)sR6, where s and R6 are as defined above;
(g) -S(o)tNR6R7, where t and NR6R7 are as defined above;
(h) =NOR6, where R6 is as defined above;
- 38 -
2156065
WO g4/21642 ~ PCr/US94102692
(i) -R399, where R399 is as defined above;
(j) -R400, where R400 is as defined above;
(k) -aryl, and
(1) -Het.
"Sub-cyclo-C3-to-Clo-alkyl-CI-to-C3-alicyl", as used herein, refers to a -(cyclo-
C3-to-Clo-aL~yl-Cl-to-C3-alkyl) s~lbs*t(lted with from one-to-six r~ al~ independently
selected from the group consis*~ng of:
(a) R6, where R6 is as defined above;
(b) -(CH2)mOR6, where m and R6 are as defined above;
(c) -NR6R7, where NR6R7 are as defined above;
(d) -C(O)OR6, where R6 is as defined above;
(e) -SO3H;
(f) -S(O)SR6, where s and R6 are as defined above;
(g) -S(o)tNR6R7, where t and NR6R7 are as defined above;
(h) =NOR6, where R6 is as defined above;
(i) -R399, where R399 is as defined above;
(~) -R400, where R400 is as defined above;
(k) -aryl, and
(1) -Het.
"Sub-C3-to-Clo-alkenyl", as used herein, refers to a -(C3-to-Clo-alkenyl) subs*tuted
with from one-to-six radicals independently selected from the group consis*ng of:
(a) R6, where R6 is as defined above;
(b) -(CH2)mOR6, where m and R6 are as defined above;
(c) -NR6R7, where NR6R7 are as defined above;
(d) -C(O)OR6, where R6 is as defined above;
(e) -SO3H;
(f) -S(O)SR6, where s and R6 are as defined above;
(g) -S(o)tNR6R7~ where t and NR6R7 are as defined above;
(h) =NOR6, where R6 is as defined above;
(i) -R399, where R399 is as defined above;
(j) -R400, where R400 is as defined above;
(k) -aryl, and
(1) -Het.
"Sub-cyclo-C4-to-Clo-alkenyl", as used herein, refers to a -(cyclo-C4-to-Clo-aL~cenyl)
substituted with from one-to-six radicals independently selected from the group consisting
of:
(a) R6, where R6 is as defined above;
(b) -(CH2)mOR6, where m and R6 are as defined above;
- 39-
WO g4/21642 ?,~S6~6~ PCT/US94/02692
(c) -NR6R7, where NR6R7 are as defined above;
(d) -C(O)OR6, where R6 is as defined above;
(e) -S03H;
(fl -S(O)SR6, where s and R6 are as defined above;
(g) -S(o)tNR6R7, where t and ~7 are as defined above;
(h) =NOR6, where R6 is as def~ned above;
(i) -R399, where R399 is as defined above;
(j) -R400, where R400 is as defined above;
(k) -aryl, and
(1) -Het.
"Sub-cyclo-C6-to-Clo-alkyl-C3-Cs-alkenyl", as used herein, refers to a -(cyclo-
C6-to-Clo-alkyl-C3-Cs-aL~enyl) substituted with from one-to-six r~ic~l~ independently
selected from the group consisting of:
(a) R6, where R6 is as defined above;
(b) -(CH2)mOR6, where m and R6 are as defined above;
(c) -NR6R7, where NR6R7 are as defined above;
(d) -C(O)OR6, where R6 is as defined above;
(e) -SO3H;
(f) -S(O)SR6, where s and R6 are as defined above;
(g) -S(o)tNR6R77 where t and NR6R7 are as defined above;
(h) =NOR6, where R6 is as defined above;
(i) -R399, where R399 is as defined above;
(~) -R400, where R400 is as defined above;
(k) -aryl, and
(1) -Het.
"Sub-C3-to-Clo-alkynyl", as used herein, refers to a -(C3-to-Clo-alkynyl) substituted
with from one-to-six radicals independently selected from the grollp consisting of:
(a) R6, as defined above;
(b) -(CH2)mOR6, where m and R6 are as defined above;
(c) -NR6R7, as defined above;
(d) -C(O)OR6, where R6 is as defined above;
(e) -SO3H;
(f) -S(O)SR6, where s and R6 are as defined above;
(g) -S(o)tNR6R7~ where t and NR6R7 are as defined above;
(h) =NOR6, where R6 is as defined above;
(i) -R399, as defined above;
(i) -R400, as defined above;
(k) -aryl, and
- 40 -
215606S
O g4/21642 - . - PCT/US94/02692
(1) -Het.
"Sub-cyclo-C6-to-Clo-aLkyl-C3-Cs-aL~ynyl", as used herein, refers to a -(cyclo-
C6-to-Clo-alkyl-C3-Cs-aL~ynyl) substituted with from one-to-six radicals independently
selected from the group consisting of
(a) R6, as defined above;, i
(b) -(CH2)mOR6, where m and R6 are as defined above;
(c) -NR6R7, as defined above;
(d) -C(O)OR6, where R6 is as defined above;
(e) -SO3H;
(f) -S(O)SR6, where s and R6 are as defined above;
(g) -S(o)tNR6R7, where t and NR6R7 are as defined above;
(h) =NOR6, where R6 is as defined above;
(i) -R399, is as defined above;
(j) -R400, is as defined above;
(k) -aryl, and
(1) -Het.
"Sub-bicyclo-C6-to-Clo-aL~cyl", as used herein, refers to a -(bicyclo-C6-to-Clo-aL~yl)
substituted with from one-to-six radicals independently selected from the group con.ci.~ting
of:
(a) R6, as defined above;
(b) -(CH2)mOR6, where m and R6 are as defined above;
(c) -NR6R7, as de~lned above;
(d) -C(O)OR6, where R6 is as defined above;
(e) -SO3H;
(f) -S(O)SR6, where s and R6 are as defined above;
(g) -S(o)tNR6R7, where t and NR6R7 are as defined above;
(h) --NOR6, where R6 is as defined above;
(i) -R399, where R399 is as defined above;
(j) -R400, where R400 is as defined above;
(k) -aryl, and
(1) -Het.
"Sub-bicyclo-C6-to-Clo-aL~enyl", as used herein, refers to a -(bicyclo-C6-to-Clo-
alkenyl) substituted with from one-to-six radicals independently selected from the group
- consisting of:
(a) R6, as defined above;
(b) -(CH2)mOR6, where m and R6 are as defined above;
(c) -NR6R7, as defined above;
(d) -C(O)OR6, where R6 is as defined above;
- 41 -
WO 94/21642~ 2~,5 6 0 6S PCTIUS94/02692
(e) -SO3H;
(f) -S(O)sR6, where s and R6 are as defined above;
(g) -S(o)tNR6R7, where t and NR6R7 are as defined above;
(h) =NOR6, where R6 is as defined above;
(i) -R399, is as defined above;
(j) -R400, is as defined above;
(k) -aryl, and
(1) -Het.
"Substituted-bicyclo-C~-to-Clo-alkenyl", as used herein, refers to a -(bicyclo-
6-to-Clo-alkenyl) group substituted with from one-to-three radicals selected from:
halogen; -OH; (Cl-C6-alkyl)NH-; di(Cl-C6-alkyl)N-; -CO2H; -CONH2; -SH;
(Cl-C6-alkyl)S-; (Cl-C6-alkyl)O-; (Cl-C6-alkyl)OC(O)-;mod-aryl-(Cl-C6-
alkyl)OC(O)-; (Cl-C6-alkyl)OC(O)NH-; (Cl-C6-aL~yl)C(O)NH-; mod-aryl-
(Cl-C6-aL~yl)OC(O)NH-; mod-aryl-OC(O)NH-; (Cl-C6-alkyl)CO-guanidino;
mod-aryl-(S02)-guanidino; (Cl-C6-aL~yl)OC(O)-guanidino; H2N-; mod-aryl-
(Cl-C6-alkyl)OC(O)-gl-~ni(linn; mod-aryl-OC(O)-guanidino; (Cl-C6-
alkyl)NHC(O)-; di(Cl-C6-alkyl)NC(O)-; mod-aryl-NHCO-; di(mod-
aryl)NCO-; -OSO2Rll; oxo; epoxy; mod-aryl-O-; mod-aryl-S-; mod-aryl-(Cl-
C6-alkyl)O-; mod-aryl-(Cl-C6-alkyl)-S-; mod-Het-O-; mod-Het-S-; mod-Het-
(Cl-C6-alkyl)O; mod-Het-(Cl-C6-alkyl)S-; mod-auyl; mod-Het-; -SO3H;
-S(O)tNH2; -S(O)tNHRll; -S(O)tNRllRll, where both Rll's are
independently selected; and-S(O)sRll; wherein guanidino, mod-aryl, oxo,
epoxy, mod-Het-, s, t and Rll are as defined above.
"Substituted-bicyclo-C6-to-Clo-aL~yl", as used herein, refers to a -(bicyclo-C6-to-Clo-
aLl~yl) group substituted with from one-to-three radicals selected from:
halogen; -OH; (Cl-C6-alkyl)NH-; di(Cl-C6-alkyl)N-; -CO2H; -CONH2; -SH;
(Cl-C6-aL~yl)S-; (Cl-C6-alkyl)O-; (Cl-C6-alkyl)OC(O)-;mod-aryl-(Cl-C6-
aL~yl)OC(O)-; (Cl-C6-alkyl)OC(O)NH-; (Cl-C6-aL~yl)C(O)NH-; mod-aryl-
(Cl-C6-alkyl)OC(O)NH-; mod-aryl-OC(O)NH-; (Cl-C6-aL~yl)CO-guanidino;
mod-aryl-(SO2)-gu~ni~linn; (Cl-C6-aLcyl)OC(O)-guanidino; H2N-; mod-aryl-
(Cl-C6-alkyl)OC(03-guanidino; mod-aryl-OC(O)-guanidino; (Cl-Cs-
aL~yl)NHC(O)-; di(Cl-C6-aL~yl)NC(O)-; mod-aryl-NHCO-; di(mod-
aryl)NCO-; -OSO2Rll; oxo; epoxy; mod-aryl-O-; mod-aryl-S-; mod-aryl-(Cl-
C6-aL~yl)O-; mod-aryl-(Cl-C6-aL~cyl)-S-; mod-Het-O-; mod-Het-S-; mod-Het-
(Cl-C6-alkyl)O; mod-Het-(Cl-C6-aL~yl)S-; mod-aryl; mod-Het-; -SO3H;
-S(O)tNH2; -S(O)tNHRll; -S(O)tNRllRll, where both Rll's are
independently selected; and -S(O)sRll; wherein guanidino, mod-aryl, oxo,
epoxy, mod-Het-, s, t and Rll are as defined above.
- 42 -
~ WO 94/21642 215 6 0 6 5 PCT/US94/02692
"Substituted-C3-to-C6-aL~enyl", as used herein, refers to a -(C3-to-C6-aL~enyl) group
substituted with from one-to-three radicals selected from:
halogen; -OH; (Cl-C6-aLIcyl)NH-; di(Cl-C6-alkyl~N-; -C02H; -CONH2; -SH;
(Cl-C6-alkyl)S-; ~CI-C6-aL~yl)O-; (Cl-C6-alkyl)OC(O)-;mod-aryl-(Cl-C6-
aLcyl)OC(O)-; (Cl-C6-alkyl)OC(O)NH-; (Cl-C6-aL~yl)C(O)NH-; mod-aryl-
(Cl-C6-alkyl)OC(O)NH-, mod-aryl-OC(O)NH-; (Cl-C6-aL~yl)CO-guanidino;
mod-aryl-(S02)-guanidlno; (Cl-C6-aL~yl)OC(O)-guanidino; H2N-; mod-aryl-
(Cl-C6-aL~yl)OC(O)-guanidino; mod-aryl-OC(O)-guanidino; (Cl-C6-
aL~yl)NHC(O)-; di(Cl-C6-alkyl)NC(O)-; mod-aryl-NHCO-; di(mod-
aryl)NCO-; -OSO2Rll; oxo; epoxy; mod-aryl-O-; mod-aryl-S-; mod-aryl-(Cl-
C6-aL~yl)O-; mod-aryl-(Cl-C6-aL~yl)-S-; mod-Het-O-; mod-Het-S-; mod-Het-
(Cl-C6-alkyl)O; mod-Het-(Cl-C6-aL~yl)S-; mod-aryl; mod-Het-; -SO3H;
-S(O)tNH2; -S(O)tNHRll; -S(O)tNRllRll, where both Rll's are
independently selected; and -S(O)SRll; wherein guanidino, mod-aryl, oxo,
epoxy, mod-Het-, s, t and Rll are as defined above.
"Substituted-Cl-to-C6-alkyl", as used herein, refers to a -(Cl-to-C6-aL~yl) group
substituted with from one-to-three radicals selected from:
halogen; -OH; (Cl-C6-alkyl)NH-; di(Cl-C6-aL~yl)N-; -CO2H; -CONH2; -SH;
(Cl-C6-aL~cyl)S-; (Cl-C6-aL~yl)O-; (Cl-C6-alkyl)OC(O)-;mod-aryl-(Cl-C6-
aL~cyl)OC(O)-; (Cl-C6-aL~cyl)OC(O)NH-; (Cl-C6-aL~yl)C(O)NH-; mod-aryl-
(Cl-C6-alkyl)OC(O)NH-; mod-aryl-OC(O)NH-; (Cl-C6-aLlcyl)CO-guanidino;
mod-aryl-(S02)-guanidino; (Cl-C6-aL~yl)OC(O)-guanidino; H2N-; mod-aryl-
(Cl-C6-alkyl)OC(O)-guanidino; mod-aryl-OC(O)-guanidino; (Cl-C6-
aLcyl)NHC(O)-; di(Cl-C6-alkyl)NC(O)-; mod-aryl-NHCO-; di(mod-
aryl)NCO-; -OSO2Rll; oxo; epoxy; mod-aryl-O-; mod-aryl-S-; mod-aryl-(Cl-
C6-aL~cyl)O-; mod-aryl-(CI-C6-aL~yl)-S-; mod-Het-O-; mod-Het-S-; mod-Het-
(Cl-C6-aL~yl)O; mod-Het-(Cl-C6-aL~yl)S-; mod-aryl; mod-Het-; -SO3H;
-S(O)tNH2; -S(O)tNHRll; -S(O)tNRllRll, where both Rll's are
independently selected; and -S(O)SRll; wherein guanidino, mod-aryl, oxo,
epoxy, mod-Het-, s, t and Rll are as defined above.
"Substituted-C3-to-C6-aL~cynyl", as used herein, refers to a -(C3-to-C6-alkynyl) group
substituted with from one-to-three radicals selected from:
halogen; -OH; (Cl-C6-alkyl)NH-; di(Cl-C6-aL~yl)N-; -C02H; -CONH2; -SH;
(Cl-C6-alkyl)S-; (Cl-C6-aL~yl)O-; (Cl-C6-alkyl)OC(O)-;mod-aryl-(Cl-C6-
aL~yl)OC(O)-; (Cl-C6-alkyl)OC(O)NH-; (Cl-C6-alkyl)C(O)NH-; mod-aryl-
(Cl-C6-alkyl)OC(O)NH-; mod-aryl-OC(O)NH-; (Cl-C6-aL~yl)CO-guanidino;
mod-aryl-(S02)-guanidino; (Cl-C6-aL~yl)OC(O)-guanidino; H2N-; mod-aryl-
(Cl-C6-aLI~yl)OC(O)-guanidino; mod-aryl-OC(O)-guanidino; (Cl-C6-
- 43 -
Wo 94/21C42 2~.~6~6 PCT/US94/02692
aL~cyl)NHC(O)-; di(Cl-C6-aL~yl)NC(O)-; mod-aryl-NHCO-; di(mod-
aryl)NCO-; -OSO2Rll; oxo; epoxy; mod-aryl-O-; mod-aryl-S-; mod-aryl-(Cl-
C6-alkyl)O-; mod-aryl-(Cl-C6-aL~yl)-S-; mod-Het-O-; mod-Het-S-; mod-Het-
(Cl-C6-aL~yl)O; mod-Het-(Cl-C6-a~lyl)S-; mod-aryl; mod-Het-; -SO3H;
-S(O)tNH2; -S(O)tNHRll; -S(O)tNRllRll, where both Rll's are
independently selected; and -S(O)SRll wherein guanidino, mod-aryl, oxo,
epoxy, mod-Het-, s, t and Rll are as aefined above.
"Substituted-cyclo-C4-to-Clo-aL~enyl", as used herein, refers to a -(cyclo-C4-to-Clo-
aL~cenyl) group substituted with from one-to-three radicals selected from:
halogen; -OH; (Cl-C6-alkyl)NH-; di(Cl-C6-alkyl)N-; -C02H; -CONH2; -SH;
(Cl-C6-alkyl)S-; (Cl-C6-aLkyl)O-; (Cl-C6-alkyl)OC(O)-;mod-aryl-(Cl-C6-
aL~yl)OC(O)-; (Cl-C6-alkyl)OC(O)NH-; (Cl-C6-aL~yl)C(O)NH-; mod-aryl-
(Cl-C6-alkyl)OC(O)NH-; mod-aryl-OC(O)NH-; (Cl-C6-aL~yl)CO-guanidino;
mod-aryl-(S02)-guanidino; (Cl-C6-a1~cyl)0C(O)-guanidino; H2N-; mod-aryl-
(Cl-C6-alkyl)OC(O)-guanidino; mod-aryl-OC(O)-guanidino; (Cl-C6-
aL~yl)NHC(O)-; di(Cl-C6-aL~yl)NC(O)-; mod-aryl-NHCO-; di(mod-
aryl)NCO-; -OSO2Rll; oxo; epoxy; mod-aryl-O-; mod-aryl-S-; mod-aryl-(Cl-
C6-aL~yl)O-; mod-aryl-(Cl-C6-aikyl)-S-; mod-Het-O-; mod-Het-S-; mod-Het-
(Cl-C6-aL~yl)O; mod-Het-(Cl-C6-alkyl)S-; mod-aryl; mod-Het-; -SO3H;
-S(O)tNH2; -S(O)tNHRll; -S(O)tNRllRll, where both Rll's are
independently selected; and -S(O)SRll; wherein guanidino, mod-aryl, oxo,
epoxy, mod-Het-, s, t and Rll are as defined above.
"Substituted-cyclo-C3-to-Clo-aL~yl", as used herein, refers to a -(cyclo-C3-to-Clo-
aL~yl) group substituted with from one-to-three radicals selected from:
halogen; -OH; (Cl-C6-alkyl)NH-; di(Cl-C6-alkyl)N-; -C02H; -CONH2; -SH;
(Cl-C6-aL~yl)S-; (Cl-C6-aL~yl)O-; (Cl-C6-alkyl)OC(O)-;mod-aryl-(Cl-C6-
alkyl)OC(O)-; (Cl-C6-aL~yl)OC(O)NH-; (Cl-C6-alkyl)C(O)NH-; mod-aryl-
(Cl-C6-alkyl)OC(O)NH-; mod-aryl-OC(O)NH-; (Cl-C6-alkyl)CO-guanidino;
mod-aryl-(S02)-guanidino; (Cl-C~-aL~yl)OC(O)-guanidino; H2N-; mod-aryl-
(Cl-C6-aLcyl)OC(O)-guanidino; mod-aryl-OC(O)-guanidino; (Cl-C6-
aL~cyl)NHC(O)-; di(Cl-C6-aL~yl)NC(O)-; mod-aryl-NHCO-; di(mod-
aryl)NCO-; -OSO2Rll; oxo; epoxy; mod-aryl-O-; mod-aryl-S-; mod-aryl-(Cl-
C6 aL~yl)O-; mod-aryl-(Cl-C6-aL~yl)-S-; mod-Het-O-; mod-Het-S-; mod-Het-
(Cl-C6-aLlcyl)O; mod-Het-(Cl-C6-aL~yl)S-; mod-aryl; mod-Het-; -SO3H;
-S(O)tNH2; -S(O)tNHRll; -S(O)tNRllRll, where both Rll's are
independently selected; and -S(O)sRll; wherein guanidino, mod-aryl, oxo,
epoxy, mod-Het-, s, t and Rll are as defined above.
-- 44 -
WO 94/21642 ^ PCT/US94/02692
2156065
"Substituted-bicyclo-C6-to-Clo-aLkenyl-Cl-to-C6-aL~yl", as used herein, refers to a
-(bicyclo-C6-to-Clo-aLcenyl)-Cl-to-C6-aLkyl group substituted with from one-to-three
radicals selected from: ~
halogen; -OH; (Cl-C6-alkyl)NH-; di(Cl-C6-aL~yl)N-; -C02H; -CONH2; -SH;
(Cl-C6-alkyl)S-; (Ci-~6-aLkyl)O-; (Cl-C6-aLkyl)OC(O)-;mod-aryl-(Cl-C6-
aLcyl)OC(O)-; (Cl-C6-aLkyl)OC(O)NH-; (Cl-C6-alkyl)C(O)NH-; mod-aryl-
(Cl-C6-alkyl)OC(O)NH-; mod-aryl-OC(O)NH-; (Cl-C6-aLkyl)CO-guanidino;
mod-aryl-(SO2)-guanidino; (Cl-C6-aLyl)OC(O)-guanidino; H2N-; mod-aryl-
(Cl-C6-aLkyl)OC(O)-guanidino; mod-aryl-OC(O)-guanidino; (Cl-C6-
aLkyl)NHC(O)-; di(Cl-C6-aLkyl)NC(O)-; mod-aryl-NHCO-; di(mod-
aryl)NCO-; -OSO2Rll; oxo; epoxy; mod-aryl-O-; mod-aryl-S-; mod-aryl-(Cl-
C6-alkyl)O-; mod-aryl-(Cl-C6-aL~yl)-S-; mod-Het-O-; mod-Het-S-; mod-Het-
(Cl-C6-aLcyl)O; mod-Het-(Cl-C6-aLkyl)S-; mod-aryl; mod-Het-; -SO3H;
-S(O)tNH2; -S(O)tNHRll; -S(O)tNRllRll, where both R11's are
independently selected; and -S(O)sR11; wherein guanidino, mod-aryl, oxo,
epoxy, mod-Het-, s, t and Rll are as defined above.
"Thioalkoxyalkyl" refers to a thio~lk-~xy group, as defined above, except that it is
attached to the rem~indt~r of the molecule via an aLkyl group.
"ThiolaL~yl" refers to an aLkyl group, as defîned above, substituted with an -SH group.
"Thiooxo" refers to a sulfur atom forming a thiocarbonyl group.
"Unsubstituted aryl" refers to mono-, di-, tri- or tetracyclic aromatic groups, charged
or uncharged, the rings of which are comprised of from 3-to-7 carbon atoms. Examples of
unsubstituted aryl include, but are not limited to, phenyl, 1- or 2-naphthyl, azulenyl,
fluorenyl, (1, 2)-dihydronaphthyl, (1,2,3,4)-tetrahydronaphthyl, indenyl, indanyl and the like,
which are solely substituted by hydrogen.
"Pharmaceutically-acceptable salts, esters, amides and prodrugs" refers to thosecarboxylate salts, amino acid addition salts, esters, amides and prodrugs of the compounds of
the present invention which are, within the scope of sound m~lical judgement, suitable for
use in contact with the tissues of hllm~nc and lower ~nim~l~ without undue toxicity, irritation,
allergic response, or the like, commensurate with a reasonable benefitlrisk ratio, and effective
for their inten~ed use, as well as the zwitterionic forms, where possible, of the compounds of
the invention. The term "salts" refers to the relatively non-toxic, inorganic and organic acid
addition salts of compounds of the present invention, which may be prepared in situ during
the final isolation and purification of the compounds or by separately reacting the purified
compound in its free base form with a suitable organic or inorganic acid and isolating the salt
thus formed. Representative salts include the hydrobromide, hydrochloride, sulfate,
bisulfate, phosphate, nitrate, acetate, oxalate, valerate, oleate, p~lmit~te, stearate, laurate,
- 45-
WO 94/21642 ?,~S6~6S PCT/US94/02692
borate, bçn7Oate, lactate, phosphate, tosylate, citrate, m~l.o.~te fumarate, succinate, tartrate,
naphthylate, mesylate, glucoheptonate, lactiobionate and laurylsulphonate salts and the like.
These may include cations based on the aLkali and aLk~line earth metals, such as sodium,
lithillm, potassium, calcium, m~gne~ium and the lil~e, as well as nontoxic ammonium,
qu~t~-rn~ry ammonium and amine cations incIud~ig, but not limited to, ammonium,
tetramethylammonium, tetraethylammonium, methylamine, dimethylamine, L.im~Lllylamine,
triethylamine, ethylamine, and the like (see, for example S. M. Berge, et al., "Pharrnaceutical
Salts," J. Pharm. Sci., 66: 1-19 (1977)).
Examples of pharmaceutically-acceptable, non-toxic esters of the compounds of this
invention include Cl-to-C6-alkyl esters wherein the alkyl group is a straight or branched
chain. Acceptable esters also include Cs-to-(~7-cycloaLkyl esters as well as arylaLkyl esters
such as, but not limited to benzyl. Cl-to-C4 aL~yl esters are preferred. Esters of the
compounds of the present invention may be prepared according to conventional methods.
Conversely, non-toxic esters of alcoholic moieties on the compounds of the invention may be
constructed by condensing these alcohols with Cl-to-C6-aLkyl carboxylic acids, Cl-to-C6-
alkyl dicarboxylic acids or aryl-carboxylic acids. Examples of such esters include, but are
not limited to acetyl, benzoyl or hemi-succinyl.
Examples of pharmaceutically-acceptable, non-toxic amides of the compounds of this
invention include amides derived from ammonia, primary Cl-to-C6-aLcyl amines andsecondary di-Cl-to-C6-aLkyl amines. In the case of secondary amines the amine may also be
in the form of a ~-or- 6 membered heterocycle con~ -g one nitrogen atom. Amides
derived from ammonia, Cl-to-C3-aLkyl ~linlaly amides and di-Cl-to-C2-alkyl secondary
amides are preferred. Amides of the compounds of the invention may be prepared according
to conventional methods.
"Prodrug" refers to compounds that are rapidly transformed in vivo to yield the parent
compound of the above formula, for ex~mr-le, by hydrolysis in blood. A thorough discussion
is provided in T. Higuchi and V. Stella, "Pro-drugs as Novel Delivery Systems", Vol 14 of
the A.C.S. Symposium Series, and in Bioreversible Carriers in Drug Design, ed. Edward B.
Roche, American Pharmaceutical Association and Pergamon Press, 1987.
Prodrugs of compounds of the present invention may be prepared by suitable
methods. For those compounds in which the prodrug moiety is an amino acid or peptide
functionality, the condensation of the drug's amino group with amino acids and peptides may
be effected in accordance with conventional condensation methods such as the azide method,
the mixed acid anhydride method~ the DCC (dicyclohexylcarbodiimide) method, the active
ester method (p-nitrophenyl ester method, N-hydroxysuccinic acid imide ester method,
cyanomethyl ester method and the like), the Woodward reagent K method, the DCC-HOBT
(1-hydroxy-benzotriazole) method and the like. Classical methods for amino acid
- 46 -
WO 94/21642 2 1 ~ 6 0 6 ~ ~ruS94/02692
con-len~fion reactions are described in "Peptide Synthesis" Second Edition, M. Bodansky,
Y.S. Klausner and M.A. Ondetti (1976).
As in conventional peptide synthesis, branched chain amino and carboxyl groups at
alpha and omega positions in amino acids may be protected and deprotected if necessary.
The protecting groups for amino groups which can be used involve, for example,
benzyloxycarbonyl (Z or Cbz), o-chlorobenzyloxycarbonyl ((2-Cl)Z)), p-
nitrobenzyloxycarbonyl (Z(NO2)), p-methoxybenzyloxycarbonyl (Z(OMe)), t-
amyloxycarbonyl (Aoc), isobornealoxycarbonyl, ~ m~ntyloxycarbonyl (Adoc), 2-(4-
biphenyl)-2-propyloxy carbonyl (Bpoc), 9-fluorenyl-methoxycarbonyl (Fmoc),
methylsulfonylethoxy carbonyl (Msc), trifluoroacetyl, phthalyl, formyl, 2-
nitrophenylsulfonyl (Nps), diphenylphosphinothioyl (Ppt), dimethylphosphino-thioyl (Mpt),
and the like.
The examples for protecting groups for carboxyl groups involve, for example, benzyl
ester (OBzl), cyclohexyl ester, 4-nitrobenzyl ester (OBzlNO2), t-butyl ester (OtBu), 4-
pyridylmethyl ester (OPic), and the like.
In the course of the synthesis of certain of the compounds of the present invention,
specific amino acids having functional groups other than amino and carboxyl groups in the
branched chain such as arginine, cysteine, serine and the like may be protected, if necessary,
with suitable protecting groups. It is preferable that, for example, the guanidino group (NG)
in arginine may be protected with nitro, p-toluenesulfonyl (Tos), benzyloxycarbonyl (Z),
ac~m~ntyloxycarbonyl (Adoc), p-methoxybenzenesulfonyl, 4-methoxy-2,6-dimethyl-
benzenesulfonyl (Mts) and the like; the thiol group in cysteine may be protected with benzyl,
p-methoxybenzyl, triphenylmethyl, acetamidomethyl, ethylc~balllyl, 4-methylbenzyl
(4-MeBzl), 2,4,6-trimethylbenzyl (Tmb) and the like; and the hydroxy group in serine may
be protected with benzyl (Bzl), t-butyl, acetyl, tetrahyd,opylanyl (THP), and the like.
Numerous asymmetric centers exist in the compounds of the present invention.
Except where otherwise noted, the present invention coll~t;,l,plates the various stereoisomers
and nli~lulc;s thereof.
The potent immunomodulatory activity which compounds of the instant invention
demonstrate, in common in vitro biological assays, in~lic~te that these compounds possess
immunosuppressive, antimicrobial, antifungal, antiviral, ~ntiinfl~mm~tory, and
antiproliferative activity, and possess the ability to reverse chemotherapeutic drug resistance.
As agents which block T-cell activation, a prerequisite for HIV proliferation, the compounds
are useful as prophylactics for the prevention of HIV replication. While the compounds of
the invention would be useful when used alone, combination therapy with other
immunosuppressants, such as, FK506, rapamycin, cyclosporin A, picibanil, mycophenolic
- 47 -
WO 94/21642 . ~ 606 ~k ;, PCTtUS94/02692
acid, azathioprine, prednisolone, cyclophosphamide, brequinar and leflunomide, would also
be expected to be beneficial.
As immuno~.u~piessants, the compounds of the present invention are useful when
~rimini~t~red for the prevention immllne-m~ ted tissue ~r organ graft rejection. Examples
of transplanted tissues and organs which suffer from these effects are heart, kidney, liver,
mt~.dnll~ ossium, skin, cornea, lung, pancreas, intestinum tenue, limb, muscle, nervus,
duodenum, small-bowel, pancreatic-islet-cell, and the like; as well as graft-versus-host
e~es brought about by medulla ossium transplantation. The regulation of the immllne
response by the compounds of the invention would also find utility in the treatment of
autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus,Hashimoto's thyroiditis, multiple sclerosis, myasthenia gravis, type I diabetes, uveitis,
allergic encephalomyelitis, glomerulonephritis, and the like; and further infectious diseases
caused by pathogenic microorganisms, such as HIV. In the particular cases of HIV-1, HIV-2
and related retroviral strains, inhibition of T-cell mitosis would suppress the replication of the
virus, since the virus relies upon the host T-cell's proliferative functions to replicate.
Further uses include the treatment and prophylaxis of infl~ tc,ly and
hyperproliferative skin diseases and cutaneous manifestations of immunologically-mç~ tcA
illnesses, such as psoriasis, atopical cierm~titi~ contact clermzltiti~ and further eczematous
derm~titi~es, seborrhoeis dermatitis, Lichen planus, Pemphigus, bullous pemphigoid,
Epidermolysis bullosa, urticaria, angioedem~, vasculitides, erythemas, cutaneouseosinophilias, Lupus erythem~tosus, acne, diseases or syndromes resulting in hair loss
including but not limited to Alopecia areata. Further instances where a compound of the
invention would be useful include various eye diseases (autoimml-ne and otherwise) such as
keratoconjunctivitis, vernal conjunctivitis, keratitis, herpetic keratitis, conical cornea,
dystrophia epithelialis corneae, corneal leukoma, ocular pemphigus, Mooren's ulcer, Scleritis,
Graves' opthalmopathy, Vogt-Koyanagi-Harada syndrome, sarcoidosis, etc.; reversible
obstructive airway tli~e~e, which includes conditions such as asthma (for example, bronchial
asthma, allergic asthma, intrinci~ ~thm~, extrinsic asthma and dust asthma), particularly
chronic or inveterate asthma (for example, late asthma and airway hyper-responsiveness),
bronchitis and the like; infl~mm~*on of mucosa and blood vessels such as gastric ulcers,
vascular damage caused by ischemic diseases and thrombosis. Moreover, hyperproliferative
vascular diseases such as intimal smooth muscle cell hyperplasia, restenosis and vascular
occlusion, particularly following biologically- or mechanically-meAi~ted vascular injury can
be treated or prevented by the compounds of the invention.
Other treatable conditions would include but are not limited to ischemic bowel
~i~e~es, infl~mm~tory bowel diseases, necrotizing enterocolitis, intestinal lesions associated
with thermal burns and leukotriene B4-m~i~tt-d diseases; intestinal inflammations/allergies
such as Coeliac diseases, proctitis, eosinophilic gastroenteritis, mastocytosis, Crohn's disease
- 48 -
WO 94/2164Z 215 ~ ~ 6 5 1 ~ J PCT/US94/02692
and ulcerative colitis; food-related allergic diseases which have sy,-~tu"~atic manifestation
remote from the gastro-int~stin~l tract (e.g., migraine, rhinitis and eczenla); renal diseases
such as~interstitial nephritis, Goodpasture's syndrome, hemolytic-uremic syndrome and
diabetic nephropathy; nervous diseases such as multiple myositis, Guillain-Barre syndrome,
Meniere's ~ e~.~e, polyne-lriti.~, multiplejr~euritis, mononeuritis and radiculopathy; endocrine
(ii.ce~es such as hyperthyroidism and :3asedow's disease; hematic diseases such as pure red
cell aplasia, aplastic ~n~o.mi~, hypoplastic anemia, idiopathic thrombocytopenic purpura,
autoimmune hemolytic ~nerni~ agranulocytosis, pernicious ~nemi~, megaloblastic anemia
and anerythroplasia; bone ~ e~es such as osteoporosis; respiratory diseases such as
sarcoidosis, fibroid lung and idiopathic in~el~lilial pneumonia; skin disease such as
dermatomyositis, leukoderma vulgaris, ichthyosis vulgaris, photoallergic sensitivity and
cutaneous T cell lymphoma; circulatory diseases such as arteriosclerosis, atherosclerosis,
aortitis syndrome, polyarteritis nodosa and myocardosis; collagen diseases such as
scleroderma, Wegener's granuloma and Sjogren's syndrome; adiposis; eosinophilic fasciitis;
periodontal disease such as lesions of ging*a, periodontium, alveolar bone and substantia
ossea dentis; nephrotic syndrome such as glomerulonephritis; male pattern aleopecia or
alopecia senilis by preventing epilation or providing hair germination and/or promoting hair
generation and hair growth; muscular dystrophy; Pyoderma and Sezary's syndrome;
Addison's disease; active oxygen-me(li~te~ diseases, as for example organ injury such as
ischemia-reperfusion injury of organs (such as heart, liver, kidney and digestive tract) which
occurs upon preservation, transplantation or ischemic disease (for example, thrombosis and
cardiac infraction): intestinal diseases such as endotoxin-shock, pseudomembranous colitis
and colitis caused by drug or radiation; renal diseases such as ischemic acute renal
insufficiency and chronic renal in~ufficiency; pulmonary diseases such as toxinosis caused by
lung-oxygen or drug (for example, paracort and bleomycins), lung cancer and pulmonary
emphysema; ocular (li~e~çs such as cataracta, siderosis, retinitis, pigmentosa, senile macular
degeneration, vitreal scarring and corneal aL~ali burn; dermatitis such as erythema
multiforme, linear IgA ballous ~lç. ., .~l; l ;.c and cement clerm~titic; and others such as
gingivitis, periodontitis, sepsis, pancreatitis, ~i~e~es caused by environmP.nt~l pollution (for
example, air pollution), aging, carcinogenis, metastasis of carcinoma and hypobaropathy;
disease caused by hi.ct~mine or leukotriene-C4 release; Behcet's disease such as int~.stin~l-,
r vasculo- or neuro-Behcet's disease, and also Behcet's which affects the oral cavity, skin, eye,
vulva, articulation, epididymis, lung, kidney and so on. Furthermore, the compounds of
- the invention are useful for the treatment and prevention of hepatic disease such as
immunogenic diseases (for example, chronic autoimmune liver diseases such as the group
consisting of autoimmllnP hepatitis, primary biliary cirrhosis and sclerosing cholangitis),
partial liver resection, acute liver necrosis (e.g., necrosis caused by toxin, viral hepatitis,
shock or anoxia), B-virus hepatitis, non-A/non-B hepatitis, cirrhosis (such as alcoholic
- 49 -
WO 94121642 2~5 ~ ;S PCT/US94/02692
cirrhosis) and hepatic failure such as fulmin~nt hepatic failure, late-onset hepatic failure and
"acute-on-chronic" liver failure (acute liver failure on chronic liver diseases), and moreover
are useful for various Ai~e~es because of their useful activity such as augmention of
chemotherapeutic effect, preventing or treating activity of ~cytomegalovirus infection,
particularly HCMV infection, anti-infl~mm~tory actiYl~y, and so on.
Additionally, some compounds also posse~s^FK-506 antagonistic properties, and are
thus useful in the tre~tm~nt of immunodepression or a disorder involving immunodepression.
Examples of disorders involving immunodepression include AIDS, cancer, senile Aem~.nti~,
trauma (including wound healing, surgery and shock), chronic bacterial infection, and certain
central nervous system disorders. The immunodepression to be treated may be caused by an
overdose of an immunosuppressive macrocyclic compound, for example derivatives of 12-
(2-cyclohexyl-1-methylvinyl)-13, 19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo~22.3.1.0
4-9] octacos-18-ene such as FK-506, or rapamycin. Overdosing of such meAic~nts by patients
is quite common upon their re~li7ing that they have forgotten to take their medication at the
prescribed time and can lead to serious side effects.
A further situation in which the compounds of the present invention may be used to
treat immunosuppression is in vaccination. It is sometimes found that the antigen introduced
into the body for the acquisition of; ~ y from disease also acts as an
immunosuppressive agent, and therefore antibodies are not produced by the body and
immnnity is not acquired. By introducing a compound of the present invention into the body
(as in a vaccine), the undesired immunosuppression may be overcome and hll~ lllily
acquired.
The compounds of the present invention may also find utility in the
chemosen~iti7~tion of drug resistant target cells. Cyclosporin A and FK-506 are known to be
effective modulators of P-glycopi~tGin, a substance which binds to and inhibits the action of
~nti~ncer drugs; by inhikiting P-glycopr-)tGill, they are capable of increasing the sensitivity
of multidrug resistant (MDR) cells to chemotherapeutic agents. It is believed that the
compounds of the invention may likewise be effective at overcoming resistance expressed to
clinically useful ~ntit--mour drugs such as S-fluorouracil, cisplatin, metho~lG,~ale, vincristine,
vinblastine and adriamycin, colchicine and vincri~tin~.
Further, it has recently been shown that the steroid receptor-associated heat shock
proteins, hspS6 or hspS9, belong to the FKS06 family of immunophilin proteins. The ability
of a steroid receptor-associated heat shock protein to bind the immunosuppressive macrolide
FKS06 may suggest that the steroid recGl~tor and immunophilin signal transduction pathways
are functionally interrelated. The combined treatment of compounds of the present invention
and low concentrations of a steroid ligand (eg. progesterone, dexamethasone) may result in a
significant enhancement of target gene expression over that seen in response to ligand alone.
- 50 -
WO94/21642 _ 2156D~i~i; . PCT/US94l02692
Thus, the compounds of the present invention may potentiate steroid-m~li~ted
transactivation .
Aqueous liquid compositions of the present invention may be particularly useful for
the treatment and prevention of various diseases of the eye such as au~oi~ llulle diseases
(including, for example, conical cornea, keratitis, dysophia epithelialis corneae, leukoma,
Mooren's ulcer, sclevitis and Graves' ophth~lmopathy) and rejection of corneal
transplantation .
When used in the above or other l~ l"-æ~t~, a therapeutically-effective amount of one
of the compounds of the present invention, meaning a sufficient amount of the compound to
treat a particular disorder, at a reasonable benefit/risk ratio, may be employed in pure form
or, where such forms exist, in pharmaceutically acceptable salt, ester or prodrug form.
Alternatively, the compound may be ~lmini~tered as pharmaceutical compositions
containing the compound of interest in combination with one or more pharmaceutically-
acceptable excipients. It will be understood, however, that the total daily usage of the
compounds and compositions of the present invention will be decided by the attending
physician within the scope of sound medical judgement.
The specific therapeutica7lly-effective dose level for any particular patient will depend
upon a variety of factors including the disorder being treated and the severity of the disorder;
activity of the specific compound employed; the specific composition employed; the age,
body weight, general health, sex and diet of the patient; the time of ~lmini~tration~ route of
~minictration, and rate of excretion of the specific compound employed; the duration of the
treatment; drugs used in combination or coincidental with the specific compound employed;
and like factors well known in the me~ l arts. For example, it is well within the skill of the
art to start doses of the compound at levels lower than required to achieve the desired
therapeutic effect and to gradually increase the dosage until the desired effect is achieved.
The total daily dose of the compounds of this invention a-lmini~tered to a human or
lower animal may range from about 0.001 to about 10 mg/kg of patients body mass/day. For
purposes of oral ~tlmini~tration, more preferable doses may be in the range of from about
0.005 to about 3 mg/kg/day. If desired, the effective daily dose may be divided into multiple
doses for purposes of ~-lmini~tration; consequently, single dose compositions may contain
such amounts or submultiples thereof to make up the daily dose.
The pharmaceutical compositions of the present invention comprise a compound of
the invention and a pharmaceutically-acceptable carrier or excipient, meaning a non-toxic
solid, semi-solid or liquid filler, diluent, encapsulating m~t~ri~l or form~ tinn auxiliary of
any type, which may be ~mini~t~red orally, rectally, parenterally, intracisternally,
intravaginally, intraperitoneally, topically (as by powders, ointments, drops or tr~n~clerrnal
patch), bucally, or as an oral or nasal spray. The term "parenteral" as used herein refers to
~ 2~S6~6S PCT/US94/02692
modes of ~fimini~tration which include intravenous, intramuscular, inlla~eli~oneal,
intrasternal, subcutaneous and intraarticular injection and infusion.
Ph~rm~elltical compositions of this invention for pdlellLtlal injection comprisepharm~euti~lly-acceptable sterile aqueous or nonaqueou~ solutions, dispersions,
suspensions or emulsions, as well as sterile powders for reconstitu~ion into sterile injectable
solutions or dispersions just prior to use. Examples of suitable aqueous and nonaqueous
carriers, ~ e.ntc, solvents or vehicles include water, ethanol, polyols (such as glycerol,
propylene glycol, polyethylene glycol, and the like), carboxymethylcellulose and suitable
n~ib~lules thereof, vegetable oils (such as olive oil), and injectable organic esters such as ethyl
oleate. Proper fluidity may be m~int~ine~l, for example, by the use of coating materials such
as lecithin, by the m~inten~nce of the required particle size in the case of dispersions, and by
the use of surfactants.
These compositions may also contain adjuvants such as preservative, wetting agents,
emulsifying agents, and dispersing agents. Prevention of the action of microorganisms may
be ensured by the inclusion of various antibacterial and antifungal agents, for example,
paraben, chlorobutanol, phenol sorbic acid, and the like. It may also be desirable to include
isotonic agents such as sugars, sodium chloride, and the like, Prolonged absorption of the
injectable pharmaceutical form may be brought about by the inclusion of agents which delay
absorption, such as ~ minllm monostearate and gelatin.
In some cases, in order to prolong the effect of the drug, it is desirable to slow the
absorption of the drug from subcutaneous or illLIa~lluscular injection. This may be
accomplished by the use of a liquid suspension of crystalline or amorphous m~teri~l with
poor water solubility. The rate of absorption of the drug then depends upon its rate of
dissolution which, in turn, may depend upon crystal size and crystalline form. Alternatively,
delayed absorption of a parenl~ ly ~mini~tered drug form is accomplished by dissolving or
suspending the drug in an oil vehicle.
Injectable depot forms are made by forming microencapsule matrices of the drug in
biodegradable polymers such as polylactide-polyglycolide, poly(orthoesters) and
poly(anhydrides). Depending upon the ratio of drug to polymer and the nature of the
particular polymer employed, the rate of drug release can be controlled. Depot injectable
formulations are also prepared by entrapping the drug in liposomes or microemulsions which
are compatible with body tissues.
The injectable formulations may be sterilized, for example, by filtration through a
bacterial-retaining filter, or by incorporating ~t~rili7ing agents in the form of sterile solid
compositions which can be dissolved or dispersed in sterile water or other sterile injectable
medium just prior to use.
Solid dosage forms for oral ~lmini~tration include capsules, tablets, pills, powders,
and granules. In such solid dosage forms, the active compound is mixed with at least one
- 52-
WO 94/21642 2 1 S 6 0 6 5 ! PCT/US94/02692
inert, ph~rm~celltic~lly-acceptable excipient or carrier, such as sodium citrate or dicalcium
phosphate and/or a) fillers or e7~tenclers such as starches, lactose, sucrose, glucose, m~nnit
and silicic acid, b) binders such as, for example, carboxymethylcellulose, ~lgin~tes" gelatin,
polyvinylpyrrolidone, sucrose, and acacia, c) hllmect~nt~ such as glycerol, d) disintegrating
agents such as agar-agar, c~lcinm carbonate, potato or tapioca starch, alginic acid, certain
silicates, and sodium carbonate, e) solution retarding agents such as paraffin, f) absorption
accelerators such as q~-~t~rn~ry ammonium compounds, g) wetting agents such as, for
example, cetyl alcohol and glycerol monostearate, h) absorbents such as kaolin and bentonite
clay, and i) lubricants such as talc, calcium stearate, magnesium stearate, solid polyethylene
glycols, sodium lauryl sulfate, and ~ luucS thereof. In the case of capsules, tablets and pills,
the dosage form may also comprise buffering agents.
Solid compositions of a similar type may also be employed as fillers in soft and hard-
filled gelatin capsules using such excipients as lactose or milk sugar as well as high
molecular weight polyethylene glycols and the like.
The solid dosage forms of tablets, dragees, capsules, pills, and granules may beprepared with coatings and shells such as enteric coatings and other coatings well known in
the pharmaceutical formnl~ting art. They may optionally contain opacifying agents and can
also be of a composition that they release the active ingredient(s) only, or preferentially, in a
certain part of the intestin~l tract, optionally, in a delayed manner. Examples of embedding
compositions which can be used include polymeric substances and waxes.
The active compounds can also be in micro-encapsulated form, if a~lopliate, withone or more of the above-mentioned excipients.
Liquid dosage forms for oral ~lminictration include pharmaceutically-acceptable
emulsions, solutions, suspensions, syrups and elixirs. In addition to the active compounds,
the liquid dosage forms may contain inert diluents commonly used in the art such as, for
example, water or other solvents, solllbili7ing agents and emulsifiers such as ethyl alcohol,
isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl ben7o~te7 propylene
glycol, 1,3-butylene glycol, dir"~;lhyl form~mi~e, Oi'lS (in particular, cottonseed, groundnut,
corn, germ, olive, castor, and sesame Oi'lS), glycerol, tetrahydrofurfuryl alcohol, polyethylene
glycols and fatty acid esters of sorbitan, and mi~lulGs thereof.
Besides inert diluents, the oral compositions can also include adjuvants such asr wetting agents, emulsifying and suspending agents, sweetening, flavoring, and perfuming
agents.
Suspensions may contain, in addition to the active compounds, suspending agents as,
for example, ethoxylated isostearyl alcohols, polyoxyethylene sorbitol and sorbitan esters,
microcrystalline cellulose, aluminum metahydroxide, bentonite, agar-agar, and tragacanth,
and 1l ixlul~s thereof.
- 53 -
WO 94/21642 $6~6S PCT/US94/02692
Topical ~rimini~tration incllldes ~lmini~tration to the skin or mucosa, including
surfaces of the lung and eye. Compositions for topical ~minictration, including those for
inhalation, may be plepaled as a dry powder which may be pressurized or non-pres~ulized.
In non-pressurized powder compositions, the active ingredient in finely divided forrn may be
used in ~ ixl-"e with a larger-sized ph~rn~-~utically-acceptable inert carrier comprising
particles having a size, for example, of up to 100 micrometers in ~i~m~t-or. Suitable inert
carriers include sugars such as lactose. Desirably, at least 95% by weight of the particles of
the active ingredient have an effective particle size in the range of 0.01 to 10 miclo"leLe~
~ lternz~tively, the composition may be pressurized and contain a compressed gas,
such as nitrogen or a liquified gas propellant. The liquified propellant mefiillm and indeed
the total composition is preferably such that the active ingredient does not dissolve therein to
any substantial extent. The ples~,ulized composition may also contain a surface active agent,
such as a liquid or solid non-ionic surface active agent or may be a solid anionic surface
active agent. It is preferred to use the solid anionic surface active agent in the form of a
sodium salt.
A further form of topical ~-lmini~tration is to the eye, as for the treatment of immllne-
meAi~ted conditions of the eye such as auloll~illllllue ~i~e~es, allergic or infl~"""~loly
conditions, and corneal transplants. The compound of the invention is delivered in a
pharmaceutically acceptable ophthalmic vehicle, such that the compound is m~int~inecl in
contact with the ocular surface for a sufficient time period to allow the compound to
penetrate the corneal and intern~l regions of the eye, as for example the anterior chamber,
posterior chamber, vitreous body, aqueous humor, vitreous humor, cornea, iris/cilary, lens,
choroid/retina and sclera. The ph~rm~ceutically-acceptable ophth~lmic vehicle may, for
example, be an ointment, vegetable oil or an encapsulating m~teri~l.
Compositions for rectal or vaginal ~I",i~ tion are preferably suppositories which
may be prepared by mixing the compounds of this invention with suitable non-irritating
excipients or carriers such as cocoa butter, polyethylene glycol or a suppository wax which
are solid at room temperature but liquid at body temperature and therefore melt in the rectum
or vaginal cavity and release the active compound.
Compounds of the present invention may also be ~-lmini~tered in the form of
liposomes. As is known in the art, liposomes are generally derived from phospholipids or
other lipid substances. Liposomes are formed by mono- or multi-lamellar hydrated liquid
crystals that are dispersed in an aqueous medium. Any non-toxic, physiologically-acceptable
and metabolizable lipid capable of forming liposomes can be used. The present compositions
in liposome form can contain, in addition to a compound of the present invention, stabilizers,
preservatives, excipients, and the like. The IJre~lled lipids are the phospholipids and the
phosphatidyl cholines (lecithins), both natural and synthetic. Methods to form liposomes are
- 54-
21S6065
WO 94/21642 PCT/US94/02692
known in the art. See, for example, Prescott, Ed., Methods in Cell Biology~ Volume XIV,
Ac~delnic Press, New York, N.Y. (1976), p. 33 et seq.
The compounds of the invention may be prepared using one or more processes. The
starting m~teri~l~ for use in these processes are preferably one of the macrolides i~ol~tecl
from culture media obtained in accordance with known methods by fermentation of
microorgani~m~ of the genus Sll~pL~Ilyces. which are disclosed in European Patent
Application No. 0184162. Samples are available from the Ferment~tion Research Tn~tit~lte;
Tsukuba, Ibaraki 305, Japan under the provisions of the Budapest Treaty, under deposit No.
FERM BP-927. This strain has been redeposited on April 27, 19~9 with the Agricultural
Research Culture Collection Tntern~tional Depository, Peoria, Illinois 61604, USA under the
provisions of the Budapest Treaty, under deposit No. NRRL 18488. The macrolide FR-
900520 (European Patent Application 0184162), also known as ascomycin, may be prepared
in accordance to the p~lbli~he~ methods of (i) H. H~t~n~k~, M. Iwami, T. Kino, T. Goto and
M. Okuhara, FR-900520 and FR-900523, Novel immunosuppressants isolated from A
streptomyces. I. Taxonomy of the producing strain. J. Antibiot., 1988. XLI(11), 1586-1591;
(ii) H. H~t~n~k~, T. Kino, S. Miyata, N. Inamura, A. Kuroda, T. Goto, H. Tanaka and M.
Okuhara, FR-900520 and FR-900523, Novel immunosuppressants isolatedfrom A
streptomyces. II. Fermentation, isolation and physico-chemical and biological
characteristics. J. Anhibiot.~ 1988. XLI(11), 1592-1601; (iii) T. Arai, Y. Koyama, T. Suenaga
and H. Honda, Ascomycin, An Anhfungal Anhibiotic. J. Anhibiot., 1962. 15(231-2); and (iv)
T. Arai in U.S. Patent No. 3,244,592. One or more of the processes discussed below may be
then employed to produce the desired compound of the invention.
Such processes comprise:
(a) producing a compound of formula I, which contains a CH-OLg group, by
selective activation of a selectecl CH-OH group in a corresponding compound wherein -OLg
is a leaving group which is easily displaced by nucleophilic attack;
(b) producing a compound of formula I, which contains a CH-N3 group, by selective
displacement of an -OLg group in a corresponding compound;
(c) producing a compound of formula I, which contains a CH-NH2 group, by
selective reduction of a CH-N3 group in a corresponding compound;
(d) producing a compound of formula I, which contains a R'-NR"COR group, by
- selective acylation of a R'-NR"H group in a corresponding compound wherein R is selected
from hydrogen, aryl, arylalkyl, alkyl, Het, heterocyclic, heterocyclic-alkyl, cycloalkyl, and
cycloalkylalkyl such that R' and/or R" represent(s) a radical derived from formula I; or R' and
R" are R14 and R15 respectively, as defined above, and R represent(s) a radical derived from
formula I;
094/21642 2~S6~ PCT/US94/02692
(e) producing a compound of formula I, which contains a CH-NRlR2 group, by
selective alkylation of a CH-NH2 group in a corresponding compound wherein R1 and R2 are
independently selected from hydrogen, ary~ ~rylalkyl, alkyl, heterocyclic, heterocyclic aL~yl,
Het, Het-aL~cyl, cycloalkyl, and cycloaL~ylaL~yl;
(f) producing a compound of formula I, which contains a CH-NHC(o)NH-R14
group, by selective urea or thiourea formation from a CH-NH2 group in a corresponding
compound, wherein R14 is as defined above; or
producing a compound of formula I, which contains a CH-NHC(o)NR14R15 group,
wherein R14 an R15 are as defined above, by selective formation of a CH-N=C=O group, and
addition of an amine HNR14R15;
(g) producing a compound of formula I, which contains a CH-NH-SO2R group, by
selective sulfonylation of a CH-NH2 group in a corresponding compound wherein R is
selected from aryl, arylaL~yl, aLIcyl, cycloaLkyl, cycloalkylalkyl, Het, Het-alkyl, heterocyclic
alkyl and heterocyclic;
(h) producing a compound of formula I, which contains a CH-NH-C(=O)OR group,
by selective carbamate formation from a selected CH-NH2 group in a correspondingcompound wherein R is selected from aryl, cycloalkyl, cycloalkyl aL~yl, alkyl, heterocyclic
alkyl, Het, Het-alkyl, heterocyclic and arylaL~yl;
(i) producing a compound of formula I, which contains a CH-NH-C(=NH)NH2
group, by selective gn~ni(linil~m formation from a CH-NH2 group in a corresponding
compound;
(j) producing a compound of formula I, which contains a CH-NH-SR group, by
selective sulfenylation of a CH-NH2 group in a corresponding compound wherein R is
selected from aryl, arylalkyl, alkyl, cycloaL~yl, cycloalkylalkyl, heterocyclic aL~yl and Het,
Het-aL~cyl, heterocyclic;
(k) producing a compound of formula I, which contains a CH-X group, by selectivehalogenation of a CH-OH group in a corresponding compound wherein X is selected from
chlorine, bromine, fluorine and iodine,
(1) producing a compound of formula I, which contains a CH-P(O)(OR)2 group, by
selective phosphonic acid ester formation of a CH-X group in a corresponding compound
wherein R is independently selected from alkyl, arylalkyl, and aryl;
(m) producing a compound of formula I, which contains a CH-O-P(O)(OR)2 group,
by selective phosphorylation of a CH-OH group in a corresponding compound wherein R is
independently selected from hydrogen, alkyl, arylaL~yl, and aryl;
(n) producing a compound of formula I, which contains a CH-S-R group, by
selective thioether formation from a CH-OH group in a corresponding compound wherein R
is selected from cycloaL~yl, cycloaL~yl alkyl, Het, Het-alkyl, heterocyclic, heterocylic alkyl,
aL~cyl, arylalkyl, and aryl;
- 56-
2156065
wo 94/21642 PCTtUS94/02692
(o) producing a compound of formula I, which contains a CH-O-C(=S)-OR group, by
selective aryl- or aL~yloxythiocarbonylation of a CH-OH group in a corresponding compound
wherein R is selected from cycloaL~cyl, cycloa'lkyl aL~cyl, Het, Het-aLkyl, heterocyclic,
heterocylic aLI~yl, aLkyl, arylaLI~yl, and aryl;
(p) producing a compound of formula I, which contains one or more CH-O-R groups,by selective ether formation of one or more CH-OH groups in a corresponding compound
wherein R is selected from cycloalkyl, cycloalkylalkyl, Het, Het-aL~cyl, heterocyclic,
(heterocylic)alkyl, (heterocyclic)aL~enyl, (heterocyclic)alkynyl, alkyl, arylaL~yl, aryl,
loweraL~oxycarbonylaL~cyl, arylalkoxycarbonylalkyl, arylalkylcarbonylalkyl,
triaL~cylsilylcarbonylaL~yl, trialkylstannylcarbonylalkyl, amidocarbonylalkyl,
aL~ylamidocarbonylaL~cyl, diaLkylamidocarbonyla'lkyl, arylamidocarbonylaL~cyl and
heterocyclicamidocarbonylaL~yl;
(q) producing a compound of formula I, which contains a CH-
(substituted)phth~limi~e group, by selective cyclic imide formation using a CH-NH2 group in
a corresponding compound;
(t) producing a compound of formula I, which contains a CH2 group, by selective
deoxygenation of a CH-O-C(=S)-OR group in a corresponding compound;
(u) producing a compound of formula I, which contains a C(OH)=CH-C(=O) or a
C(=O)-CH2-C(=O) group, by selective oxidation of a CH(OH)-CH2-C(=O) group in a
corresponding compound;
(v) producing a compound of formula I, which contains a C(=O)-CRlR2-C(=O)
group, by selective alkylation of a C(OH)=CH-C(=O) or a C(=O)-CH2-C(=O) group in a
corresponding compound, wherein Rl and R2 are independently selected from hydrogen,
aryl, cycloaLIcyl, cycloaL~yl aL~cyl, aL~yl, heterocyclic aLlcyl, Het, heterocyclic and arylalkyl,
but both cannot be hydrogen;
(w) producing a compound of formula I, which contains a C(=O)-CXlX2-C(=O)
group, by selective halogenation of a C(OH)=CH-C(=O) or a C(=O)-CH2-C(=O) group in a
corresponding compound wherein Xl and X2 are independently selected from fluorine,
chlorine, bromine and iodine;
(x) producing a compound of formula I, which contains a C(=O)-CH(OH)-C(=O)
group, by selective oxidation of a C(OH)=CH-C(=O) or a C(=O)-CH2-C(=O) group in a
corresponding compound;
(aa) producing a compound of formula I, which contains a C(=CH-R)-CH2-C(=O)
group, by selective olefination of a C(OH)=CH-C(=O) or a C(=O)-CH2-C(=O) group in a
corresponding compound wherein R is selected from aL~cyl, aryl and arylaL~yl;
(bb) producing a compound of formula I, which contains a C(OCOR)=CH-C(=O)
group, by selective O-acylation of a C(OH)=CH-C(=O) or a C(=O)-CH2-C(=O) group in a
- 57 -
WO 94/21642 PCT/US94/02692
~ ,~.S6~GS
corresponding co.l~ou,ld wherein R is selected from aryl, cycloaL~yl, cycloaL~yl aLkyl, aL~yl,
heterocyclic aLkyl, Het, Het-alkyl, heterocyclic and aryla~yl;
(cc) producing a compound of formula I, whiçh contains a C(NH-R)=CH-C(=O)
group, by selective amination of a C(OH)=CH-(;; ~=O) or a C(=O)-CH2-C(=O) group in a
corresponding compound wherein R is selected from alkylamine, arylalkylamine, arylamine
and amino acid derivatives;
(dd) producing a compound of formula I, which contains C(O)-C(=CH-R)-C(=O)
group, by selective aL~cylidene formation of a C(OH)=CH-C(=O) or a C(=O)-CH2-C(=O)
group in a corresponding compound wherein R is selected from hydrogen, aryl, cycloalkyl,
cycloalkyl alkyl, alkyl, heterocyclic alkyl, Het, Het-aL~yl, heterocyclic and arylaL~cyl;
(ee) producing a compound of formula I, which contains a carbon-carbon double
bond, by elimin~tion of HL from a corresponding compound, where L is a leaving group;
(ff) producing a compound of formula I, which contains a quinoxaline,
benzoquinoxaline, pyrazino[2,3-d]pyrid~7ine, pyrido[3,4-b]pyrazine, or a pteridine by
condensation of a 1,2-dicarbonyl or masked 1,2-dicarbonyl groups of a corresponding
compound with an applo~liate aromatic diamine;
(gg) producing a compound of formula I, which contains one or more hydroxyl
groups, by selective reduction of one or more C=O groups of a corresponding compound;
(hh) producing a compound of formula I, which contains one dihydro-
benzo[l,5]thiazepine, by reaction of an alpha,beta-unsaturated ketone of a corresponding
compound with an ~ .,pliate 2-aminothiophenol;
(ii) producing a compound of formula I, which contains one or more carbonyl
groups, by selective oxidation of one or more hydroxyl groups of a co~ onding compound;
(jj) producing a compound of formula I, by selective reaction of one of the carbonyl
groups of a corresponding compound and dithiols;
(kk) producing a compound of formula I, which contains an oxime group, by
selective reaction of one of the carbonyl groups of a corresponding compound with hydroxyl
amine or O-aL~cylated hydroxyl ~mines;
(11) producing a compound of formula I, which contains a pyrazole system, by
condensation of a 1,3-dicarbonyl group of a corresponding compound and ~ropliatehydrazines;
(mm) producing a compound of formula I, which contains a substituted pyrimidine
system, by condensation of the 1,3-dicarbonyl group of a corresponding compound with
appropriate amidines, g~ ni~iines, isoureas, ureas and thioureas;
(nn) producing a compound of formula I, which contains a furan system, by reaction
of the 1,3-dicarbonyl group of a corresponding compound with al)prop,iate diazoacetic esters
or diazomethyl ketones;
(oo) producing a compound of formula I, which contains an isoxazole system, by
condensation of the 1,3-dicarbonyl group of a corresponding compound with hydroxyl
amine;
(pp) producing a compound of formula I, which contains a pyridine system, by
condensation of the 1,3-dicarbonyl group of a corresponding compound with appropriate
malonic acid derivatives or cyanoacetic derivatives;
(qq) producing a compound of formula I, which contains a benzo[1,5]thiazepine,
benzo[1,5]oxazepine or benzo[1,5]diazepine system, by condensation of the 1,3-dicarbonyl
group of a corresponding compound with a appropriate 2-aminothiophenols, 2-aminophenols,
and 1,2-aromatic diamines;
(rr) producing a compound of formula I, which contains a keto-substituted furan
system, by reaction of the 1,3-dicarbonyl group of a corresponding compound with
appropriate aldehydes, and enol ethers;
(ss) producing a compound of formula I, which contains a substituted phenyl group,
by C-arylation of a 1,3-dicarbonyl group of a corresponding compound with appropriate 1-
halo-2-nitro-aromatics;
(uu) producing a compound of formula I, which contains a 2-isoxazoline, by nitrile
oxide 1,3-dipolar cycloaddition to an enone;
(zz) producing a compound of formula I, which contain either a beta-hydroxy ketone
or an alpha,beta-enone, by reductive hydrolysis of a corresponding 2-isoxazolin and
subsequent separation of the two compounds;
(eee) producing a compound of formula I, which contains a hydrazone, by selective
hydrazone formation with a corresponding ketone;
(hhh) producing a compound of formula I, which contains an allylic alcohol, by
selective reduction of a corresponding enone;
(iii) producing a compound of formula I, which contains an epoxide, by selective
addition of the carbene arising from diazomethane across an activated carbonyl;
(jjj) producing a compound of formula I, which contains a carboxylic acid, by
selective ester cleavage in a corresponding compound;
(kkk) producing a compound of formula I, which contains a substituted or
unsubstituted carboxamide, by selective condensation of the corresponding amime with a
corresponding carboxylic acid;
(III) producing a compound of formula I, which contains a 24R-hydroxyl substituent,
by selective inversion of the naturally occuring 24S configuration;
(mmm) producing a compound of formula I, which contains an alkyloxycarbonyl
hydrazone, by selective condensation of an alkyl carbazate with a corresponding compound
of formula I, having a ketone;
-59-
D0 ~5
WO g4/21642 ', PCT/US94/02692
(ppp~ producing a compound of formula I, which contains one thiazole, by
conde.n~tion of an alpha substituted carbonyl or an alpha substituted m~ ed carbonyl group
of a corresponding compound with an ~plo~liate thioamide, thiourea or with dithiocd~bal,-ic
acid derivatives, where the alpha substituent L is.a le~vlng group;
(qqq) producing a compound of form~a.I, which contains one imirl~7ole~ by
condçn~tion of an alpha substituted carbonyi or an alpha substituted m~ed carbonyl group
of a corresponding compound with an a~plo~liate amidine, isourea or guanidine, where the
substituent L is a leaving group;
(rrr) producing a compound of formula I, which contains one oxazole, by
con~lçn.~tion of an alpha substituted carbonyl or an alpha substituted masked carbonyl group
of a corresponding compound with an appro~liate amide, where the substituent L is a leaving
group;
(sss) producing a compound of formula I, which contains a tertiary alcohol, by
selective addition of a Grignard reagent or an organometallic reagent to a carbonyl moiety of
a corresponding compound;
(ttt) producing a compound of formula I, which contains one pyrrole, by cyclization
of an appropliate gamma-amino alpha hydlo~y carbonyl or a m~ked gamma-amino alpha
hydroxy carbonyl of a corresponding compound prepared by process (sss);
(uuu) producing a compound of formula I, which contains one pyrazine, by
conden.c~tion of a 1,2-dicarbonyl or m~kç~l 1,2-dicarbonyl group of a corresponding
compound with an appropliate 1,2-(li~mine in the presence of an oxidizing agent;(vvv) producing a compound of formula I, which contains one pyridine, by
condçn~tion of a l,S-dicarbonyl group prepared by process (sss) of a corresponding
compound with ammonia;
(www) producing a compound of formula I, which contains one pyricl~7.ine, by
condensation of a 1,4-dicarbonyl group prepared by process (sss) of a corresponding
compound with hydrazine;
(xxx) producing a compound of formula I, which contains a 1~2-thiocarbonate, by
reacting a 1,2-diol of a collcs~onding compound with thiocarbonyl-liimirl~7.ole or an
ap~ro~liately activated thiocarbonate;
(yyy) producing a compound of formula I, which contains a 1,2-carbonate, by
reacting a 1,2-diol of a corresponding compound with carbonyl~iimid~7ole, triphosgene,
phosgene or an al,~lopriately activated carbonate;
(zzz) producing a compound of formula I, which contains a 1,2-phosphonate group,by reacting a 1,2-diol of a corresponding compound with an ~lu~liate aL~oxyphosphonyl
dichloride;
(aaaa) producing a compound of formula I, which contains an olefin, by reduction of
a 1,2-thiocarbonate prepared by process (xxx) of a corresponding compound;
- 60-
wo 94121642 2 1 5 6 0 6 5 PCT/US94/02692
(bbbb) producing a compound of formula I, which contains a CH2 group, by
selective reduction of a 1,2-dicarbonyl or masked 1,2-dicarbonyl group of a corresponding
compound;
(cccc) producing a compound, of formula I, which contains an indole group, by
selective reduction and conclen.~1ion of a 2-~o-nitrophenyl)-1,3-diketone [~r~aled by
process (ss)] of a corresponding compound;
(dddd) producing a compound of formula I, which contains a substituted triazole
group, by cycloaddition of a CH-N3 group in a corresponding compound with applop,iate
acetylene analogues;
(eeee) producing a compound of formula I, which contains a substituted pyrrole
group, by reaction of a CH-NH2 group in a corresponding compound with ~plupiiatedicarbonyl compounds;
(ffff) producing a compound of formula I, which contains one ethanalyl group, first
by selective oxidation of the double bond of an allyl group to a vicinal diol, followed by
oxidative cleavage of the diol in a corresponding compound;
(gggg) producing a compound of formula I, which contains one carboxymethyl
group, by selective oxidation of an ethanalyl group in a corresponding compound;(hhhh) producing a compound of formula I, which contains one alkyl carboxymethylgroup, by esterification of a carboxymethyl group in a corresponding compound;
(iiii) producing a compound of formula I, which contains one cycloplo~yllllethylgroup, by selective cyclol,lu~anation of the double bond of an allyl group in a corresponding
compound;
(ii.U) producing a compound of formula I, which contains one pyrrole, by reaction of
a 1,4-dicarbonyl group with amines in a corresponding compound;
(kkkk) producing a compound of formula I, which contains one furan, by cyclization
of a 1,4-dicarbonyl group in a corresponding compound;
(1111) producing a compound of formula I, which contains one methyl ketone, by
selective oxidation of the double bond of an allyl group in a corresponding compound;
(nnnn) producing a compound of formula I, which contains a hydrazide, by reduction
of the corresponding hydrazone;
(oooo) producing a compound of formula I, which contains an amine, by reduction of
the corresponding oxime;
(pppp) producing a compound of formula I, which contains an alpha,beta-saturatedketone, by reduction of the corresponding alpha,beta-unsaturated enone;
(qqqq) producing a compound of formula I, which contains an isoxazoline, by
treatment of a beta-hydroxy oxime with a dehydrating reagent;
(rlTr) producing a compound of formula I, which contains an beta-hydroxy carbonyl,
by treatment of a carbonyl with a base in the presence of another carbonyl moiety;
- 61 -
Wo 94/21642 Pcr/us94/02692 ~
~,s6a6S
(ssss) producing a compound of formula I, which contains a cyclic imine, by
tre~tment of an enone system with a glycine imine in the presence of base resulting in first
Michael addition at the beta-carbon and subsequent imine formation upon aqueous workup;
(tttt) producing a compound of formula I, whic~ contains a substituted pyrrole, by
e~t of an enone with a glycine imine in the pre,sence of an al,plu~,iate catalyst to
induce a 1,3-dipolar cycloaddition;
(uuuu) producing a compound of formula I, which contains a beta-keto carbox-ylicacid, ester or amide, by decomposition with light or heat of an alpha diazoketone;
(vvvv) producing a compound of formula I which contains a ketone, a product of
decarboxylation of a beta-keto carboxyliç acid, by heating;
(yyyy) producing a compound of formula I, which contains a -CHoC(o)oR40o~ by
selective mod-aryl-, mod-Het-, or alkyloxy-carbonylation of a -CHOH group in a
corresponding compound;
(zzzz) producing a compound of formula I, which contains an allylic hydroxyl group,
by selective oxidation of an allylic methylene group in a corresponding compound.
In process (a), suitable reagents for activation of an alcohol include acetic anhydride,
trifluorometh~neslllfonic anhydride (triffic~anhydride), methanesulfonyl chloride (mesyl
chloride), p-toluenesulfonyl chloride (tosyl chloride), triffuoroacetic anhydride,
triffuoroacetyl chlori-le, methoxysulfonyl fllloride (magic methyl), o-nitrobenzenesulfonyl
chloride, l-methyl-2-fluoropyri-lini-lm salt and the like.
The activation may be carried out in a solvent which does not adversely affect the
reaction (e.g., diethylether, dichlorometh~ne, tetrah~dlofuldll, chlorofo~ or N,N-
dimethylform~mide or a mix ~Ul`C; thereof). The reaction may require cooling or heating,
depending on the activation method chosen. Further, the reaction is preferably conducted in
the presence of an organic or inorganic base such as an ~lk~line earth metal (e.g. calcium,
etc.), aL~ali metal hydride (e.g. sodium hydride, etc.), alkali metal hydroxide (e.g. sodium
hydroxide, pot~sinm hydroxide, etc.), aLlcali metal carbonate (e.g. sodium carbonate,
pot~c~illm carbonate, etc.), alkali metal hydrogen carbonate (e.g. sodium hydrogen carbonate,
pot~sillm hydrogen carbonate, etc.), aLk-ali metal alkoxide (e.g. sodium methoxide, sodium
ethoxide, pot~inm tert-butoxide, etc.), alkali metal alkanoic acid (e.g. sodium acetate, etc.),
trialkylamine (e.g. triethylarnine, etc.), pyridine compounds (e.g. pyridine, lutidine, picoline,
4-N,N-dimethylaminopyridine, etc.), quinoline, and the like, preferably in the presence of
organic bases such as triethylamine or pyridine.
The activation may also be carried out using a starting material having an opposite
configuration at a carbon center. In this situation, the following two additional steps are
required to yield a starting material having an epimeric hydroxyl moiety, i.e. (l) the alcohol
is oxidized to its corresponding ketone, (2) the obtained ~etone is reduced under selective
- 62-
Wo 94/21642 2 I ~ 6 0 6 5 PCT/US94/02692
conditions. Both chiral centers having either [R]- or [S]-configuration can be obtained
selectively and sepa~ ly.
In process (b), suitable azide reagents include well-established alkali metal azides
such as sodium or lithium azides (Nal!13 or LiN3) in the ~rese,lce or absence of crown ethers,
more reactive tetraalkylammonium azides (D~ni.~hef~ki, S. J.; DeNinno, M. P.; Chen, S.-H. J.
Am. Chem. Soc. 1988, 110, 3929), tetramethylgll~nitlinium azide, (Papa, A. J. J. Org. Chem.
1966, 31, 1426), a copper-assisted azide reacti,on (Yamamoto, Y.; Asao, N. J. Org. Chem.
1990, 55, 5303) and a hydrogen azide-amine system (Saito, S.; Yokoyama, H.; Ishikawa, T.;
Niwa, N.; Moriwake, T. Tetrahedron Lett. 1991, 32, 663; Saito, S.; T~k~h~hi, N.; Ishikawa,
T.; Moriwake, T. Tetrahedron Lett. 1991, 32, 667). The azide displacement reaction may be
carried out in a solvent which does not adversely affect the reaction (e.g. chlorofol,n,
dichlorometh~ne, tetrahydrofuran, pyridine, dimethylsulfoxide, N~-dimethylformamide,
hexamethylphosphoramide, etc. or a llli~Ul~. thereof). The reaction may be conducted above,
at, or below ambient ~l~p~ ture.
In process (c), the reduction may be carried out catalytically using hydrogen. Suitable
catalysts include, but are not limited to platinum catalysts (e.g. pl~tinllm oxide, platinum
black), p~ riillm catalysts (e.g. palladium oxide, p~ linm on charcoal, palladium black,
palladium hydroxide on charcoal, p~ lillm on calcium carbonate poisoned with lead,
palladium on barium carbonate with quinoline), nickel catalysts (e.g. nickel oxide, Raney
nickel), rhodium catalysts (e.g. rhodium on ~hlmin~). Reduction may also be carried out
using metal reducing reagents (see Review; Scriven, E. F. V.; Turnbull, K. Chem Rev. 1988,
88, 321; Patai, S., Ed., "The ~hemistry of the Azido Group," Interscience Publishers, New
York, 1971; Scriven, E. F. V., Ed., "Azides and Nitrenes Reactivity and Utility," Academic
Press, Inc., New York, 1984) such as sodium borohydride under phase-transfer conditions,
borohydride supported on an ion exchange resin, lithium ~ll"~ l" hydride and the like,
furthermore, 1,3-prop~n~ithiol-triethylamine method (Bayley, H.; Staudring, D. N.;
Knowles, J. R. Tetrahedron Lett. 1978, 3633), triphenylphosphine (Vaultier, M.; Knouzi, N.;
Carrie, R. Tetrahedron Lett. 1983,24, 763), and sodium tellurium hydride (Suzuki, H.;
Takaoka, K. Chem Lett. 1984, 1733).
The reduction may be carried out in a solvent which does not adversely affect the
reaction (e.g., alcohols, water, acetone, dichlolullle~lane, tetrahydrofuran, pyridine or N,N-
dimethyl~o. - l ,~" ,i-le or a n~ ule thereof). The reaction may be conducted above, at, or below
ambient temperature.
In process (d), suitable N-acylations may be carried out using the methods of
symmetric carboxylic acid anhydrides, carboxylic acid halides, mixed carbonic-carboxylic
anhydrides, active esters (p-nitrophenylester, trichlorophenyl ester, pentafluorophenyl ester,
N-hydroxysuccinimide, cyanoethyl, 2,2,2-trichloroethyl and the like), and carboxylic acid
with suitable conclen~ing reagents such as DCC (N,N-dicyclohexylcarbodiimide and its
- 63 -
WO 94/21642 ~ 2~S6~S ~ ~ PCT/US94/02692
related conclen~ing agents), DCC-HOBt (N,N-dicyclohexylcarbodiimide-l-
hydroxybenzotriazole), Woodward reagent K method, N,N-carbonyl~liimi-1~7ole and
phosphonium cont~ining reagents (e.g. bcnzolliazolyloxytris[dimethylamino]-phosphonium
hexafluorophosphate, N,N-bis[2-oxo-3-ox-~7oli~1inyl]phosphoro~i~mi~ic chloride,
diethylphosphorobromi-l~te, diphenylphosphoryl azide, bromo
tris[dimethylamino]phosphnnillm ht-x~flllorophos~hate, and the like). Suitable reagents for
amide formation include, but are not limited to fo~myl derivatives, acetyl halides
(chloroacetyl, trichloroacetyl, o-nitrophenylacetyl, o-nitrophenoxyacetyl, acetoacetyl,
[N'-dithiobenzyloxycarbonylamino]acetyl and the like), and substituted propionyl derivatives
(3-phenylpropionyl, isobutyryl, picolinoyl, and the like). Other groups may be found in
volume 3 of The Peptides Gross, E. and Meinhofer, J. Ac~clemic Press,1981 and Protective
Groups in Organic Synthesis Greene, T. W. John Wiley & Sons, New York, Chapter 7, 1981.
Typically used coupling conditions are described by Gross, E.; Meinhofer, J. "The Peptides"
vol. 3, Academic Press, 1981. The N-acylation may be carried out in a solvent which does
not adversely affect the reaction (e.g. acetone, dichloromethane, chloroform, tetrahydrofuran,
N,N-dimethylform~mide, dimethylsulfoxide, diethylether, and the like, or a mixture thereof).
The reaction may be conducted above, at, or below ambient temperature. Alternatively,
metal salts may be formed from the desired amines and then conden~ecl with an ester which
may or may not be activated. These salts may be formed by treafment of the neutral amine
with triaL~yl~lnminllm~ (See J. I. Levin, E. Turos, S. M. Weinreb Synthet c Communications
1982, 12, 989-93), Sn[N(Si(CH3)3)]2 (See W. Wang, E. J. Roskamp J. Org. Chem. 1992, 57,
6101-3), or grignard reagents. For other methods see A. Solladie-Cavallo, M. Bencheqroun
J. Org. Chem. 1992, 57, 5831-4 as well as footnotes 2, 3, 4, 5, 6 and 7 therein.In process (e), N-aLkylations may be carried out using aldehydes or ketones-followed
by reduction of the initially formed i.,.i..i,.." ion {The following reagents can be used for the
reduction; sodium cyanoborohydride-boron 1riflllnn-1e or the reducing reagents cited in
process (c) }, corresponding halides in the presence of bases listed in process (a), or lithium
dialkyl cuprate (King, F. E.; King, T. J.; Muir, I. H. M. J. Chem. Soc. 1946, 5; Yamamoto,
H.; Maruoka, K. J. Org. Chem. 1980, 45, 2739). Suitable reagents for N-aLlcylation include,
but are not limited to benzyl halide, 3,4-dimethoxybenzyl halide, nitrobenzyl halide, di(p-
methoxyphenyl)methyl halide, triphenylmethyl halide, and the like. Other groups may be
found in volume 3 of The Peptides, Gross, E. and Meinhofer, J. Academic Press, 1981 and
Protect.ve Groups in Organic Synthesis, Greene, T. W. John Wiley & Sons, New York,
Chapter 7, 1981. The N-aLkylation may be carried out in a solvent which does not adversely
affect the reaction (e.g., acetone, dichloromethane, tetrahydloful~n, pyridine or N,N-
dimethylform~mide or a n~ urc thereof). The reaction may be conducted above, at, or below
ambient tcmperature.
- 64-
215606,5
94/21642 ~ ~ PCTtUS94102C92
In process (f), urea formation may be carried out from the following reactions;
reaction with silicon tetraisocyanate or silicon tetraisothiocyanate (Neville, R. G.; McGee, J.
J. Can. J. Chem. 1963, 41, 2123), reaction with N,N-carbonyl-ii;mirl~7Ole or N,N-
thiocarbonyl~iimi-l~7Ole~ followed by N-s~lkstitl-ted prill.~y or secondary amines or
ammonia (Staab, H. A.; Wendel, K. Org. Synth. 1968, 48, 44), and reaction with phosgene or
thiophosgene in the presence of tert-amine, followed by N-substituted primary or secondary
amines or ammonia. The ureido formation may be carried out in a solvent which does not
adversely affect the reaction (e.g. acetone, toluene, dichloromethane, tetrahy&ufuldn,
pyridine, N,N-dimethyLform~mide, etc. or a n~i~ thereof). The reaction may be conducted
above, at, or below ambient temperature.
Alternatively, urea formation may be carried out by first forming an acyl azide by
activating a carboxylic acid in the molecule with a chloroformate, such as isobutyl
chloroformate, in the presence of a tertiary amine, such as N-methyl-morpholine or N-
methyl-piperidine, and treating with an azide source, such as sodium azide, hydrazoic acid,
trimethylsilylazide, or tetramethylgu~ni-linium azide. The acyl azide may also be formed
directly using diphenylphophorylazide in the presence of a tertiary amine. The reaction
is then heated at from 40 C to 100 C for 0.5 to 6 hours, whereupon the amine
HNR14Rl5 is added at a temperature at from 23 C to 100 C. The reaction is conducted in
an inert organic solvent such as diethyl ether, tetrahydrofuran, 1,4-dioxane, chlorofol"l,
methylene chloride, benzene, or toluene.
In process (g), N-sulfonylation may be carried out using substituted sulfonylhalides in
the presence of suitable tert-amines such as triaLkylamine, pyridine, and the like (Remers, W.
A.; Roth, R. H.; Gibs, G. J.; Weiss, M. J. J. Org. Chem. 1971, 36, 1232). Suitable reagents
include, but are not limited to b--nzenes--lfonyl halide, p-methyoxybenzenesulfonyl halide,
2,4,6-trimethylbenzenesulfonyl halide, tolllen~sulfonyl halide, benzylsulfonyl halide, p-
methoxybenzylsulfonyl halide, trifluo,ul,æLllylsulfonyl halide, phenacylsulfonyl halide, and
the like. Some other reprçsçnt~tive groups may be found in volume 3 of The Peptides, Gross,
E. and Meinhofer, J. Ac~lçmic Press, 19gl and Protective Groups in Organic Synthesis,
Greene, T. W. John Wiley & Sons, New York, Chapter 7, 1981. The N-aryl- or
aLkylsulfonylation may be carried out in a solvent which does not adversely affect the
reaction (e.g., acetone, dichloromethane, tetr-ahydlorulall, pyridine or N,N-
dimethyl~o~ . . .icle or a n~ibs~ule thereof). The reaction may be conducted above, at, or below
ambient temperature.
In process (h), N-carbamate forrnations may be carried out using common protecting
groups for amino group such as, but not limited to methylcarbamates (cyclopropylmethyl, 9-
fluorenylmethyl, and the like), substituted ethylcarbamates (2,2,2-trichloroethyl, 2-
phosphonoethyl, 2-methylthioethyl, and the like), substituted propyl and isopropylcarbamates
(1,1-dimethylpl.p~,lyl, 1-methyl-1-(4-biphenylyl)ethyl, tert-butyl, phenyl,p-nitrobenzyl, 8-
- 65 -
21S6~65 `'
~` PCT/US94/02692
quinolyl, N-hydroxypiperidinyl, benzyl, dimetho~yl ellzyl, 9-anth~ yl~ lllyl~ m~ntyl,
cyclohexyl, tert-amyl, cinnamoyl, isobutyl, N'-p-phenylaminothiocarbonyl, N'
piperidinylcarbonyl, diphenylmethyl, and the like). F~ ~aLions of N-carbamates and other
groups may be found in volume 3 of The Peptides, G~oss, E. and Meinhofer, J. Academic
Press, 1981 and Protect~ve Gro~ps in Organ~ Synthesis, Greene, T. W. John Wiley & Sons,
New York, Chapter 7, 1981. The N-c~l~ ate formation may be carried out in a solvent
which does not adversely affect the reaction (e.g., acetone, dichloromethane, tetrahydrofuran,
pyridine or N,N-dimethyl~" ~ icle or a I~ ule thereof). The reaction may be conducted
above, at, or below ambient temperature.
In process (i), N-gll~ni~ lm formation may be carried out using several common
reagents such as l-guanyl-3,5-dimethylpyrazole (Salvadori, S.; Sarto, G. P.; Tomatis, R. Eur.
J. Med. Chem. Chim. Ther. 1983, 18, 489), O-methylisourea (Van Mspen, J. W.; Tesser, G.
I.; Nivard, R. J. F. Int. J. Peptide Protein Res. 1977, 9, 193), and thiourea sulfonylate
(Maryanoff, C. A.; Stanzione, R. C.; Plampin, J. N.; Mills, J. E. J. Org. Chem. 1986, 51,
1882). The N-guzlni~lininm formation may be carried out in a solvent which does not
adversely affect the reaction (e.g., acetone, dichloroll~ hane, tetrahydrofuldn, pyridine or
N,N-dimethylform~micle or a ~ ure thereof). The reaction may be conducted above, at, or
below ambient temperature.
In process (j), N-sulfçn~micles may be prepared from an amine and a sulfenyl halide
(Davis, F. A.; Nadir, U. K. Org. Prep. Proc. Int. 1979, 11, 33; Kobayashi, T.; Iino, K.;
Hiraoka, T. J. Am. Chem. Soc. 1977, 99, 5505; Zervas, L.; Borovas, D.; Gazis, E. J. Am.
Chem. Soc. 1963, 85, 3660). Suitable reagents include, but are not lilnited to benzenesulfenyl
halide, o-nitrobP.n7enesulfenyl halide, 2,4-dinitrosulfenyl halide, pentachlorobenzenesulfenyl
halide, 2-nitro-4-methoxybtq.n7e.n-o.sulfenyl halide, triphenylmethylsulfenyl halide, and the
like. Other groups may be found in volume 3 of The Pept.des, Gross, E. and Meinhofer, J.
~c~ciçmic Press, 1981 and Protective Groups in Organic Synthesis, Greene, T. W. John
Wiley & Sons, New York, Chapter 7, 1981. The N-suLfenylation may be carried out in a
solvent which does not adversely affect the reaction (e.g., acetone, dichloromethane,
tetrahy.lloru,all, pyridine or N,N-dimethylform~mi-le or a lllib~UlG thereof). The reaction may
be con~uct~l above, at, or below amhient temperature.
In process (k), suitable halogenation reagents include, but are not limited to
triphenylphosphine with halogens (Verheyden, J. P. H.; Moffatt, J. G. J. Am. Chem. Soc.
1964, 86, 2093; Bergman, R. G. ibid., 1969, 91, 7405; Hrubiec, R. T.; Smith, M. B. J. Org.
Chem., 1983, 48, 3667), triphenylphosphine with cyanogen halides (Horner, L.; Oediger, H.;
Hoffm~nn, H. Annalen Chem. 1959, 626, 26), triphenylphosphine with carbon tetr~h~ es
(Hooz, J.; Gilani, S. S. H. Can. J. Chem. 1968, 46, 86; Chem. Commun. 1968, 1350),
triphenylphosphine with NBS (N-bromosuccinimide)(Schweizer, E. E.; Creasy, W. S.; Light,
K. K.; Shaffer, E. T. J. Org. Chem. 1969, 34, 212), and triphenylphosphine with
- 66 -
2156~65
WO 94/21642 ^ PCr/US94l02692
hexachloroacetone (Magid, R. M.; Stanley-Fruchey. O.; Johnson, W. L. Tetrahedron ~ett.
1977, 2999; Magnid, R. M.; Stanley-Fruchey, O.; Johnson, W. L.; Allen, T. G. J. Org. Chem.
1979, 44, 359). The halogenation may also be accomplished by other reagents such as mono-
or tri-alkylsilyl halides with or without sodium halides (Olah, G. A.; Husain, A.; Singh, B. P.;
- Mehrota, A. K. J. Org. Chem. 1983, 48, 36~7; Balme, G.; Fournet, G.; Gore, J. Tetrahedron
Lett. 1986, 27, 1907), polymer bound trimethylsilyl derivatives (Cainelli, G.; Contento, M.;
Manescalchi, F.; Plessi, L.; Panunzio, M. Synthesis 1983, 306; Imamoto, T.; M~t~umnto, T.;
Kusumoto, T.; Yokoyama, M. Synthesis 1983, 460), N,N-dichlorophosphoramidic dichloride
(Chem. Lett. 1978, 923), phosphorus trih~ e-zinc halide (Anderson, Jr. A. G.; Owen, N. E.
T.; Freenor, F. J.; Erickson, D. Synthesis 1976, 398), diethylaminosulfur trifluoride
(Middleton, W. J. J. Org. Chem. 1975, 40, 574), triphenoxyphosphonium alkyl halide
(Rydon, H. N. Org. Synth. 1971, 51, 44; Verheyden, J. P. H.; Moffatt, J. G. J. Org. Chem.
1972, 37, 2289), and the like.
The halogenation may be carried out in a solvent which does not adversely affect the
reaction (e.g., acetone, dichloromethane, tetrahydloful~n, pyridine or N,N-
dimethylform~mi-le or a n~i~ule thereof). The reaction may be conducted above, at, or below
ambient ten~ lul~.
In process (1), phosphonic acid ester formation may be ca~Tied out using Michaelis-
Arbuzov reactions (Bhattacharya, A. K.; Thyagarajan, G. Chem. Rev. 1981, 81, 415; Bauer,
G.; Haegele, G. Angew. Chem. Int. Ed. Engl. 1977, 16, 477).
The phosphonic acid ester formation may be carried out in a solvent which does not
adversely affect the reaction (e.g., acetone, dichloromethane, tetrahydlorul~n, pyridine or
N,N-dimethylform~mi~le or a Il~i~s~we thereof). The reaction may be conducted above, at, or
below ambient temperature.
In process (m), phosphorylation may be carried out using, but is not limited to the 2-
halo-2-oxo-1,3,2-dioxaphospholane-triethylamine reaction (Chandrarakumar, N. S.; Hajdu, J.
J. Org. Chem. 1983, 48, 1197). The phosphorylation may be carried out in a solvent which
does not adversely affect the reaction (e.g., benzene, toluene, acetone, dichloromethane,
tetrahydlorulall or N,N-dimethylform~ le or a ~ tule thereof). Further, the reaction is
preferably conducted in the presence of organic or inorganic bases, as described in process
(a), preferably in the presence of organic bases such as triethylamine, pyridine etc. The
reaction may be conducted above, at, or below ambient temperature, more preferably from 0
to50 C.
In process (n), thioether formation may be carried out using, but is not limited to aryl-
or aL~ylmercaptan in the presence of suitable tert-amines such as trialkylamine, pyridine, and
the like. The reaction may also be carried out by a metal-catalyzed thioether formation
(Guindon, Y; Frenette, R; Fortin, R.; Rokach, J. J. Org. Chem. 1983, 48, 1357), alkali metal
salts of aryl- or alkylmercaptans with a compound of formula I which contains CH-OLg
- 67 -
f:
,. r~
WO 94/21642 ~ ~ PCT/us94/02692
~,S6~6S
group (OLg lS the leaving group). The alkali metal may be selected from sodium, pot~ m,
lithillm, and ce-~illm The thioether formation may be carried out in a solvent which does not
adversely affect the reaction (e.g. acetone, dichlorom~th~ne, tetrahy~orul~n, pyridine, N,N-
dirnethylror~ " .ifle, etc. or a I~ Ule thereof). The reaction may be conducted above, at, or
below ambient lempel~Lule.
In process (o), aryl- or aL~yloxythiocarbonylation may be carried out using aryl- or
aL~yloxythiocarbonylchloride or correspondin~ halides in the presence of suitable tert-amines
such as trialkylamine, pyridine, and the like. The aryl- or aLkylthiocarbonylation may be
carried out in a solvent which does not adversely affect the reaction (e.g. acetone,
dichloromtqthS~ne, tetrahy~orulan, pyridine, N,N-dimethylform~mide etc. or a llU~Ule
thereof). The reaction may be conducted above, at, or below ambient temperature.In process (p), ether formation may be carried out using, for example, aryl-, arylalkyl-
, (heterocyclic)aL~yl-, (heterocyclic)alkenyl-, (heterocyclic)alkynyl-,
loweralkoxycarbonylalkyl-, arylalkoxycarbonylalkyl-, arylalkylcarbonylaL~yl-,
triaL~ylsilylcarbonylalkyl-, trialkyl-stannylcarbonylalkyl-, amidocarbonylalkyl-,
alkyl~midoc~rbonylalkyl-, diaL~ylamido-carbonylalkyl-, arylamidocarbonylalkyl-,
alkylamidocarbonylalkyl-, heterocyclicamido-ca-rbonylalkyl-~ heterocyclic or aL~cylh~lides in
the presence of KY-zeolite (Onaka, M.; Kawai, M.; Izumi, Y. Chem. Lett. 1983, 1101),
polymeric m~t.ori~l~ (Kimura, Y.; Kirs7en.~7tejn, P.; Regen, S. L. J. Org. Chem. 1983, 48,
385), nickel-catalysis (Camps, F.; Coll, J.; Moreto, J. M. Synthesis 1982, 186; Y~m~hit~
Synthesis 1977, 803), arylalkyl-O-p-toluenesulfonate (Dewick, P. M. Synth. Commun. 1981,
11, 853), pot~ lm or sodium ~lkc xidçs (Bates, R. B.; Janda, K. D. J. Org. Chem. 1982, 47,
4374), pyridine or other bases (Chem. Lett. 1978, 57), tetraaL~yla,l,l,loniu", halide (Miller, J.
M.; So, K. H.; Clark, J. H. Can. J. Chem. 1979, 1887), mcl~;uly perchlorate (McKillop, A.;
Ford, M. E. Tetrahedron 1974, 30, 2467), silver triflate or silver oxide (Kuhn, R.; Low, I.;
Trischmann, H. Chem. Ber. 1957, 90, 203. Croon, I.; T indb~rg, B. Acta Chem. Scand., 1959,
13, 593) or a phase transfer catalyst (McKillop, A.; Fiaud, J.-C.; Hug, R. P. Tetrahedron
1974, 30, 1379). The ether formation may also be carried out with dialkyl- or
diarylphosphoric acid in the presence of p-toluenesulfonic acid (K~hm~n, Y. J. Org. Chem.
1972, 37, 912), with diazo compounds with tin(~) chloride (Christensen, L. F.; Broom, A. D.
J. Org. Chem. 1972, 37, 3398), or with 2,2,2-trichloroalkanols in the presence of base
(Corey, E. J.; Link, J. O. J. Arn. Chem. Soc. 1992, 114, 1906; Corey, E. J.; Link, J. O.
TetrahedronLett. 1992, 33, 3431). Additionally, ether formation may be accomplished with
a suitable trichloroacetimi-l~te in the presence of an acid catalyst (Wessel, H. P.; Iversen, T.;
Bundle, D. R. J. Chem. Soc. Perk Trans. 1985, l, 2247.) The ether formation may be carried
out in a solvent which does not adversely affect the reaction (e.g. acetone, dichloromethane,
tetrahy~oful~n, pyridine, N,N-dimethylform~mi~le, ether, cyclohexane, etc. or a IlU~Ule
thereof). The reaction may be conducted above, at, or below ambient temperature.
- 68 -
~ wo 94/21C42 2 1 S 6 0 6 S ~usg
More specifically, O-aL~ylation may be carried out using bromoacetic acid
derivatives, iodoacetic acid derivatives, trifluor~ c~ esulfonyloxy acetic acid derivatives,
chloro- bromo- or ioc~omtoth~nesulfonic acid derivatives, chloro- bromo- or
iodoacetyltrimethylsilane and the like in the presence of an a~p~u~fiate base such as
triethylamine, potassium fluoride or silver(I) oxide. The reaction is performed in an inert
solvent such as N,N-dimethylr~ mid~, ace~ e or dichl~)~u~ Lhane~ preferably between
-50 C and 80 C. Alternatively, alkylation car~ be carried out using aL~cyl-, or arylaL~yl-
diazoacetates in the presence of a metal catalyst, for example Rh(OAc)2 in an inert solvent
such as dichloromethane preferably between -20 C and 80 C.
In process (q), N-cyclic imide formations may be carried out using phthalic anhydride
(Sasaki, T.; Minamoto, K.; Itoh, H. J. Org. Chem. 1978, 43, 2320), o-methoxycarbonyl-
benzoyl chloride with triaL~ylamine (Hoogwater, D. A.; Reinhoudt, D. N.; Lie, T. S.;
Gunneweg, J. J.; Beyerman, H. C. Recl. Trav. Chim. Pays-Bas. 1973, 92, 819), orN-
ethoxycarbonylphth~limi~e (Nefkens, G. H. L.; Tesser, G. I.; Nivard, R. J. F. Recl. Trav.
Chim. Pays-Bas. 1960, 79, 688). Other groups and reagents may be found in volume 3 of
The Peptides, Gross, E. and Meinhofer, J. Academic Press, 1981 and Protective Groups in
Organic Synthesis, Greene, T. W. John Wiley & Sons, New York, Chapter 7, 1981. The N-
cyclic imide formation may be carried out in a solvent which does not adversely affect the
reaction (e.g. acetone, dichloromethane, tetrahy~lr~rul~n, pyridine, N,N-dimethylform~mi~e7
etc. or a mixture thereof). The reaction may be conducted above, at, or below ambient
temperature.
In process (t), deoxygenation may be carried out using, but is not limited to
phenoxythiocarbonyl derivative with tributyltin hydride and 2,2-azobis-2-methylpropionitrile
(AIBN) (Robins, M. J.; Wilson, J. S.; Han~.~ke, F. J. Am. Chem. Soc. 1983, 105, 4059;
Barton, D. H. R.; McCombie, S. W. J. Chem. Soc., Perkin Trans.l 1975, 1574), or a
phenyldithiocarbonyl derivative with tributyltin hydride and AIBN (Hayashi, T.; Iwaoka, T.;
Takeda, N.; Ohki, E. Chem. Pharm. Bull. 1978, 26, 1786). The deoxygenation may be carried
out in a solvent which does not adversely affect the reaction (e.g. acetone, dichloromethane,
tetrahydrofuran, pyridine, N,N-dimethylr ~ e~ etc. or a ~ e thereof~. The reaction
may be conducted above, at, or below ambient temperature.
In process (u), suitable oxidizing reagents include activated diaL~yl sulfoxides (e.g.
dimethylsulfoxide, methylethylsulfoxide) (Mancuso, A. J.; Swern, D. Synthesis 1981, 165),
organo chromates [e.g. pyridinium chlorochromate (Corey, E. J.; Suggs, J. W. Tetrahedron
Lett. 1975, 2647; Corey, E. J.; Boger, D. L. TetrahedronLett. 1978, 2461), pyridinium
dich,ull.ate (Corey, E. J.; Schmidt, G. Tetrahedron Lett. 1979, 5, 399), Collins reagent
(Collins, J. C.; Hess, W. W.; Frank, F. J. Tetrahedron Lett. 1968, 3363)],
~ upylammonium perruthenate ((~rifflth, W. P.; Ley, S. V.; Whitcombe, G. P.; White, A.
D. Chem. Commun. 1987, 1625; Griffith, W. P. AldrichimicaActa. 1990,23, 13), and the
- 69-
i~S6~6S PCT/US94/02692
like. The oxidation may be carried out in a solvent which does not adversely affect the
reaction (e.g. acetone, dichloromethane, tetrahyclf oruldn, pyridine, N,N-dimethylform~mi~e7
etc. or a mi~Lu,G thereof). The reaction may be conducted above, at, or below ~mbient
temperature.
In process (v), suitable alkylating reagents ~lude, but are not limited to aldehydes
and ketones in the presence of reducing agent~ (~bowcha~, D. M.; Smith, F. X.
Tetrahedron l ett. 1983,24, 4951), alkyl-, aryl, or arylalkyl halides (Shono, T.; K~himl-r?~,
S.; Sawd~ d, M.; Soejima, T. J. Org. Chem. 1988, 53, 907). In the case that the reaction is
conducted in the presence of an organic or inorganic bases such as an alkaline earth metal
(e.g. calcium, balium, m~gnesillm, thallium etc.), an alkali metal hydride (e.g. sodium
hydride, lithium hydride, etc.), an alkali metal hydroxide (e.g. sodium hydroxide, potassium
hydroxide, etc.), an aLkali metal carbonate (e.g. sodium carbonate, pot~ m carbonate, etc.),
an aL~cali metal hydrogen carbonate (e.g. sodium hydrogen carbonate, potassium hydrogen
carbonate, etc.), an alkali metal aLI~oxide (e.g. sodium methoxide, sodium ethoxide, thallium
ethoxide, potassium tert-butoxide, etc.), an alkali metal alkanoic acid (e.g. sodium acetate,
etc.), a triaL~cylamine (e.g. triethylamine, trimethylamine, etc.), or a pyridine compound (e.g.
pyridine, lllti~line, picoline, 4-N,N-dimethylaminopyridine, etc.), quinoline, and the like. The
alkylation may be carried out in a solvent which does not adversely affect the reaction (e.g.
acetone, dichlol.,.,~eLI~ne tetrahydrofuran, pyridine, N,N-dimethyl~~ le, etc. or a
n~i~ thereof). The reaction may be conducted above, at, or below ambient temperature.
In process (w), suitable halogenation reagents include, but are not limited to halogens
treated by irr~ tion (sun lamp) for several hours (Heffner, R.; Safaryn, J. E.; Joullie, M. M.;
Tetrahedron Lett. 1987, 28, 6539) or oxalyl chloride (Evans, D. A.; Dow, R. L.; Shih, T. L.;
Takecs, J. M.; Zahler, R. J. Am. Chem. Soc. 1990, 112, 5290). The halogenation may be
carried out in a solvent which does not adversely affect the reaction (e.g. acetone,
dichlolu~ ne, tetrahydlofuldn, pyridine, N,N-dimethylfnrm~mici~, etc. or a mixture
thereof). The reaction may be conducted above, at, or below ambient l~ ,r~ e.
In process (x), suitable oxidation reagents include, but are not limited to
oxodiperoxymolybdenum(pyridine)-1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone
(Anderson, J. C.; Smith, S. C. SYNLE7T 1990, 2, 107) and
oxodiperoxymolybdenum(pyridine)-hexamethylphosphoramide (Vedejs, E. J. Am. Chem.Soc. 1974, 96, 5944; Vedejs, E.; Engler, D. A.; Telschow, J. E. J. Org. Chem. 1978, 43, 188).
The oxidation may be carried out in a solvent which does not adversely affect the reaction
(e.g. acetone, dichloromethane, tetrahydrofuran, pyridine, N,N-dimethylformamide, etc., or a
n~i~Lult; thereof). The reaction may be conducted above, at, or below ambient temperature.
In process (aa), suitable olefination reagents include, but are not limited to Wittig
reagents (Maecker, M., Org. React. 1965,14, 270; Johnson, A. W., " Ylid Chemistry,"
Academic Press, New York, 1966) and CH2I2-Zn-TiCk~ [or Ti(NEt2)4] reagent (Hibino, J.;
- 70 -
~ WO 94/21642 2 1 S 6 0 6 5 ~S94l02692
Okazoe, T.; Takai, K.; Nozaki, H. Tetrahedron Lett. 1985,26, 5579; Okazoe, T.; Hibino, J.;
Takai, K.; Nozaki, H. ibid. 1985,26, 5581). The carbonyl ole~ln~tion may be carried out in a
solvent which does not adversely affect the reaction (e.g. acetone, dichloromethane,
tetrahyLufuldn, pyridine, N,N-dimethylform~mi~e, etc., or a n~ixlule thereof). The reaction
may be conducted at room temperature.
In process (bb), suitable O-acylation reagents include, but are not limited to aL~cyl,
aryl, or arylalkyl acyl halides (Lakhvich, F. A.; Khlebnicova, T. S.; Akhrem, A. A. Synthesis
1985, 8, 784). The O-acylation may be carried out in a solvent which does not adversely
affect the reaction (e.g. acetone, dichloromethane, tetrahy~lloruldn, pyridine, N,N-
dimethylru~ -icle7 etc., or a ~ Lul~ thereof). The reaction may be conducted above, at, or
below ambient lelllp~ldlult;.
In process (cc), suitable amination reagents include, but are not limited to amino acid
derivatives and lower alkyl, aryl, or arylalkyl amines (Winkler, J. D.; Hershberger, P. M.;
Springer, J. P. Tetrahedron Lett. 1986, 27, 5177). The reaction may be carried out in
refluxing in benzene, toluene or a solvent which does not adversely affect the reaction (e.g.
tetrahydrofuran, pyridine, N,N-dimethylform~mi~le7 etc., or a nli~sLule thereof). The reaction
may be conducted at room temperature.
In process (dd), the alkylidene formation may be carried out using, but is not limited
to aldehydes and ketones with active methylene compounds. (Schonberg, A.; Singer, E.
Chem. Ber. 1970, 103, 3871; Chatterjee, S. J. Chem. Soc. B, 1969, 725). The alkylidene
formation may be carried out in a solvent which does not adversely affect the reaction (eg.
acetone, dichlorometh~ne7 tetrahydrofuran, pyridine, N,N-dimethylform~mide, etc., or a
mixture thereof). The reaction may be conducted under cooling to he~tin~.
In process (ee), L may be hydroxy, or a good leaving group (halogen, tosylate,
mesylate or triflate, for example). When a precursor compound contains a C(OH)-CH2-C=O
group, the elimin~tion of H2O may be carried out in a solvent which is inert under the
reaction conditions (e.g. toluene) with a trace of acid (e.g. toluenesulfonic acid), at a
temperature selected from 50 to 100 C. When the precursor compound contains a good
leaving group, ~he elimin~tion may be carried out in the presence of a base (e.g. triethyl
amine or potassium carbonate), at a lt;lll~eld~ G selected from 0 to 100 C.
In process (ff), suitable ~ mines include phenylene diamine and substituted 1,2-phenyl ~i~mines, 2,3-diaminopyridine, 3,4-diaminopyridine, 4,5-diaminopyri-i~7.ine, 4,5-
diaminopyrimi~ine and their acid salts, preferably in the presence of tertiary amines (e.g. N-
methylmorpholine). Suitable solvents include methanol, ethanol, propanol, acetonitrile, 2-
butanone and N,N-dimethylform~mi~e7 and a reaction temperature selected from 50 to 100
C.
In process (gg), suitable reagents include sodium borohydride, zinc in acetic acid,
sodium triacetoxyborohydride in acetic acid, lithium trialkoxyaluminum hydride in
- 71 -
WO94/21642 2~56~6S PCT/US94/0202
tetrahyclluful~n, potassium or lithium tri-sec-butylborohydride in tetrahydrofuran, and
borane/t-butylamine complex in a solvent such as methanol or ethanol. The reduction may be
conducted at -70 C to room te~ eialulc.
In process (hh), suitable 2-aminothiophenols inc,lude substituted 1,2-
aminothiophenols, preferably in the presence of tertiary amine (e.g. N-methylmorpholine).
Suitable solvents include methanol, ethanol and r~ opanol; and the reaction may be
conducted at a temperature selected from 50 to 100 C.
In process (ii), the reagent to be used in this reaction may include di(lower)alkyl
sulfoxide (e.g. dimethyl sulfoxide, ethyl methyl sulfoxide, propyl methyl sulfoxide, isobutyl
methyl sulfoxide, butyl methyl sulfoxide, isobutyl methyl sulfoxide, hexyl methyl sulfoxide,
etc). This reaction is usually conducted in the presence of oxalyl chloride, acid chlorides,
lower aL~anoic anhydride such as acetic anhydride in a conventional solvent that does not
adversely inflllence the reaction such as dichlorom-qth~nç, acetone, ethyl acetate,
tetrahydlurul~n, pyridine, N~N-dimethyl~olln~ ç~ etc., followed by the addition of a tertiary
amine (e.g. triethyl amine). The reaction may be conducted at -70 C to room temperature.
In process (jj), the dithiols are lower aL~yl dithiols (e.g. ethanedithiol, prop~neflithiol
or butanedithiol) and 1,2-aryl dithiols (e.g. 1,2-ben7çnç lithiol) in the presence of a Lewis
acid (e.g. boron trifluoride etherate or l~nth~nnm trichloride) in a conventional solvent that
does not adversely inflllence the reaction such as dichlurollleLllane, tetrahydlorul~n or ether.
The reaction may be conducted at -70 C and room temperature.
In process (kk), suitable oxygen-mbs~ d amines include hydroxyl amine, O-
alkylhydroxyl amines, and O-arylalkyl hydlu~yl ~mines, for example O-benzyl hydroxyl
amine. Suitable solvents include those that do not adversely affect the reaction, for example
ethanol or methanol. The reaction is preferably carried out with one equivalent of hydroxyl
amine, and at a temperature of 25 to 100 C, more preferably at the reflux temperature of the
solvent.
In process (11), suitable hy lr~7ines include aL~ylhydrazines (e.g. butylhydrazine),
arylhydrazines (e.g. phenylhydrazine), acylhydrazines (e.g. acetylhydrazine), semicarbazides
(e.g. t-butyloxycarbonyl hydrazine) and sulfonyl hy~ 7;lles (e.g. tosyl hydrazine) in a
conventional solvent that does not adversely affect the reaction such as tetrahydrofuran,
methanol or ethanol. The reaction may be conducted at 20 to 100 C.
In process (mm), 2-substitutions on the pyrimitline may be hydrogen, aL~yl, aryl,
hydroxyl, aL~oxy, thiol, amino, alkylamino, arylamino, acylamino, carbamylamino, and
sulphonylamino groups. The appro~liate pyrimi-line cont~inin~ compounds may be prepared
according to the methods described in " The Chemistry of Heterocyclic Compounds, Vol.16,
supplementlI, Chapter II, pp 21-60", D. J. Brown, John Wiley & Sons, 1985.
In process (nn), the furan cont~ining compounds may be prepared according to themethod described by Paulissen, R., et. al. in Tetrahedron Lett. 1974, 607.
~ WO g4/21642 215 G 0 6 S ~ . ~"S94/02692
,
In process (oo), one equivalent of hydroxyl amine hydrochloride and tertiary amine
(e.g. N-methylmorpholine) in a conventional solvent that does not adversely affect the
reaction such as tetrahydf ufu~dll, methanol, ethanol or iso~-opallol is used to prepare the
compound. The reaction is conducted at 20 to 100 C.
- In process (pp), the pyridine containing compounds may be prepared according to the
liLt;laLu~ Osman, A. N.; Ismail, M. M.; Barakat, M. A. ~ev. Rourn. Chim. 1986, 31, 615-
624; Ried W.; Meyer, A., Ber. Deutsch. Chem. Ges. 1957, 90, 2841; Troschutz, R.;Troschultz, J.; Sollhubt~ , M. Arch Pharm. 1985, 318, 777-781.
In process (qq), a substituted 2-aminothiophenol, a 2-aminophenol or an aromatic 1,2-
diamine is used in a conventional solvent that does not adversely affect the reaction such as
tetrahyd,oru,a,l, ethanol, isc,prupallol, acetonitrile or N,N-dimethylforrn~icle. The reaction
may be conducted at 20 to 100 C.
In process (rr), the keto-substituted furan CO~ irlg compound may be prepared
according to the li~ ule: Williams, P. H. et al, J. Am. Chem. Soc. 1960, 82, 4883; E. J.
Corey et al., Chem. Lett. 1987, 223.
In process (ss), suitable l-halo-2-ni~oalulllalics may be substituted 1-fluoro-2-
nitrobenzene, o-fluoro-niLIopylidines, or o-bromo-nitro-naphth~lene, etc. The arylation may
be carried out in a solvent which does not adversely affect the reaction (e.g. tetrahydrofuran,
N,N-dimethylfu,",~."icle, dimethoxyethane, diglyme, etc.).
The base used to generate the anion may be isopropyl magnesium chloride, lithiumdiisopropyl amine or sodium hydride. The reaction may be conducted at a temperature
selected from -70 C to 100 C.
In process (uu), a nitrile oxide may be formed either by oxidation of an aldoxime or
dehydration of a nitro compound as described in the following references or li~ cited
therein: (1) Torssell, K. G. B. "Mtrile Oxides, Nitrones and Nitronates in Organic
Synthesis"; VCH Publishers: New York, 1988, p 64; (2) Kim, J. N.; Ryu, E. K. Synthetic
Communications 1990, 20, 1373; (3) Chow, Y. L.; Shy, Y. Y.; Bakker, B. H.; Pillay, K. S.
Heterocycles 1989, 29, 2245. The nitrile oxide is placed in the presence of an alpha,beta-
unsaturated enone in an inert solvent to yield an 2-isoxazolines. Any isomers may
subsequently be chomatographically separated.
In process (zz), an isox~7oline may be transformed to the corresponding beta-hydroxy
ketone using but is not limited to molybenum hçx~c~rbonyl in wet acetonitrile according to:
Baraldi, P. G.; Barco, A.; Benetti, S.; Manfredini, S.; Simoni, D. Synthesis 1987, 276.
Alternatively, Ti3+ may be employed to attain N-O bond cleavage: Das, N. B.; Torssell, K. B.
G. Tetrahedron 1983, 39, 2227. Additionally, Raney-nickel may also selectively cleave the
N-O bond without reducing the imino functionality as described in the following reference
and li~ ule cited therein: Torssell, K. G. B. "Nitrile Oxides, Nitrones and Nitronates in
Organic Synthesis"; VCH Publishers: New York, 1988, p 16 and 290. During the course of
- 73 -
o g4l2l642 ~ o ~ PCr/US94/02692
this transformation, a ~i~nific~nt amount of dehydration occurs to produce alpha-beta
n~atllr~t~l enones which may be separated from the beta-hydloxy ketones.
In process (eee), an aryl- or aL~ylsulfonyl hydrazone may be formed by tre~tment of a
ketone with an aryl- or aL~ylsulfonyl hydrazide in the presence of an acid catalyst in a solvent
suitable for the reaction such as methanol or~thanol at temperatures ranging from ambient to
the reflux temperature of the solvent.
In process (hhh), an allylic alcohol may be produced by selective reduction of an
alpha-beta unsa~ ted enone. This is accomplished with but not limited to sodium
borohydride in the presence of cerium(III) chloride heptahydrate in a suitable solvent such as
methanol at or near 0 C.
In process (iii), an epoxide may be produced on the central carbonyl of a tricarbonyl
moiety by but not limited to excess diazomethane as described in: Fisher, M. J.; Chow, K.;
Villalobos, A.; D~nich~fcky, S. J. J. Org. Chem. 1991, 56, 2900-2907.
In process (jjj), liberation of the ester to the acid may be achieved by the cleavage of a
suitably substituted ester function. Such a functional group may be benzyl, 2,2,2-
trichloroethyl, 9-fluorenylmethyl and the like. These are cleaved by methods well known to
those skilled in the art.
In process (kkk), con~ nc~tion of an amine with the acid may be performed using the
mixed or symm.otrical anhydride of said acid, or an ester of the acid, preferably activated,
such as the ester derived from hydroxybenzotriazole, or the corresponding acylcyanide,
acylimicl~7ole, or acylazide of the aforementioned acid.
In process (111), selective protection of the 32-hydroxyl moiety may be achieved using
one of a variety of triaL~ylsilyl groups. This then leaves exposed a lone secondary alcohol on
C-24 for selective inversion, which may be accomplished by activation of the 24-hydroxy as
a mesylate, tosylate, etc., followed by inversion with a suitable nucleophile such as water,
benzoic acid, formic acid, etc. On the other hand inversion of the unactivated 24-hydroxy
group may be achieved using well described Mitsunobu conditions. Liberation of the silyl
ether and inverted C-24 acylated hydroxy (if carboxylic acids are used as the nucleophile) is
accomplished using methods well known to those skilled in the art. ~ltern~tively, inversion
may be accomplished without protection of the 32-hydroxyl group if ascomycin, FK506, or
similar compounds are treated with diethylaminosulfur trifluoride (DAST) in an inert solvent
such as methylene chloride.
In process (mmm), con-ien~ti()n of an alkyloxy or substituted aLclyoxy carbonyl
hydrazine with ascomycin, FK506, similar compounds, or a suitable derivative thereof
wherein the C-22 is available as a reactive center, including but not limited to a carbonyl, is
performed in an inert solvent such as methanol, ethanol, 2-propanol, etc., in the presence of a
catalyst which may be an acid such as formic acid, p-toluenesulfonic acid, or
camphorsulfonic acid.
- 74-
2156~6~
WO 94/21642 PCl'/US94l02692
., ;,
In process (ppp), L may be a hydroxyl group, or a good leaving group (halogen,
tosylate, nitrobenzenesulfonate, mesylate or triflate, for example).
The condensation may be carried out in a solvent which does not adversely affect the
reaction (e.g. isopropanol, acetonitrile, dioxane, N,N-dimethylf~rm~mi-le, tetrahydrofuran,
etc.). The reaction may be carried out in the p~esence of base (e.g. triethylamine, 4-
methylmorpholine or magnesium carbonate, etc.), at a Le~ e~ e selected from 0 to 100 C.
The ~Lo~riate thiazole containing compound may be prepared according to Hantzsch's
s~nthesis described by: Katritzky, A.R.; Rees, C.W. "Comprehensive Heterocyclic
Chemistry"; Pergamon Press: Oxford, 1984, Vol. 6, Part 4B, p.294-299.
In process (qqq), L may be a hydroxyl group, or a good leaving group (halogen,
tosylate, nitrobenzenesulfonate, mesylate or triflate, for example).
The condensation may be carried out in a solvent which does not adversely affect the
reaction (e.g. isopropanol, t-butanol, acetonitrile, dioxane, N,N-dimethylform:~mide,
tetrahyd,vruldn, etc.). The reaction may be carried out in the presence of base (e.g.
triethylamine, 4-methylmorpholine, potassium carbonate or m~gnesium carbonate, etc.), at a
temperature selected from 0 to 100 C.
Suitable ~mit1ines include fnrm~mi~ine, alkyl~mi~lines, aryl~nnidines and
alkylisoureas. Suitable guanidines include N-arylgu~ni-lines, N-acylated gll~nitlin~s and N-
sulfonylated guanidines.
In process (rrr), L may be a hydroxyl group, or a good leaving group (halogen,
tosylate, nitrobenzenesulfonate, mesylate or triflate, for example).
The conden~tion may be carried out in a solvent which does not adversely affect the
reaction (e.g. isopropanol, t-butanol, aceLoniL~ile, dioxane, N,N-dimethylform~micle,
tetrahydrofuran, etc.). The reaction may be carried out in the presence of a base (e.g.,
triethylamine, 4-methylmorpholine, potassium carbonate or magnesium carbonate), at a
temperature selected from 0 to 100 C.
The amides are primary amides such as form~mide, alkylacylamides and
arylacyl~mides.
In process (sss), the organometallic reagent may be a Grignard reagent, an
alkyllithium, or an aryllithium reagents.
The selective addition may be carried out in a solvent which does not adversely affect
the reaction (e.g., hexanes, ether, tetrahy~ufulall, dimethoxyethane or 2-methoxyethyl ether).
The reaction may be carried out in the presence of cerium (III) at a temperature selected from
-100CtoO C.
In process (ttt), the gamma amino alpha hydroxy carbonyl or a masked gamma aminoalpha hydroxy carbonyl of a corresponding compound prepared by process (sss) may have
substitutions (e.g. aL~cyl, aryl groups, etc.) at the alpha and/or beta positions. Furthermore, the
amino group may have N-aL~yl or aryl substitutions.
- 75 -
WO g4/21642 ~,~$ 6~6$ . PCT/US94/02692
The con~lenc~tiQn may be carried out in a solvent which does not adversely affect the
reaction (e.g. isopropanol, t-butanol, acelon;l.;le, dioxane, N,N-dimethylform~mide,
tetrahy~uful~ll, etc.). The reaction may be carried out in the presence of a base (e.g.
triethylamine, 4-methylmorpholine, pot~cci~m carbonate or m~gnecillm carbonate, etc.), at a
tU~G selected from 0 to 100 C.
In ,ulOCGSS (UUU), the reaction is generally c~ied out in two steps: first the
con-lçnc~tion of an alpha ~lik~tQne or a m~çd alpha diketone with an 1,2-~ mino~lk~nç
gives a dih~ulJyldLine. Once the dill~/Lùpy~ e has been prepared, it may be oxidized by
air in thepresence of Pd/C, PtO2 or other catalysts. Metal oxides (e.g. MnO2 or CuO) may
also be used for the aru~ ;on.
The cnn(lenc~tion and oxidation may be carried out in a solvent which does not
adversely affect the reactions (e.g. isopropanol, ~CctOhil/ ;le, dioxane, ben7e~e, toluene, etc.).
The reaction may be carried out in the presence of drying agent such as m~;,.r~ l sulfate or
molecular sieves at a lenl~ a~ sGle.,lcd from 0 C to 100 C.
In process (vvv), a l,S-dicarbonyl group or a m~.cked l,S-dicarbonyl group plep;~ed
by process (sss) may have s~lbstit~*onc (e.g. alkyl, aryl groups etc.) at the alpha and/or beta
pocitionc. The con(lenc~tioll may be carried out with anhydrous ~mnloni~ in a solvent which
does not adversely affect the reactions (e.g. liquid ammonia, iso~ allol, acelonitlile,
~1iox~ne, benzene, tolu~ne, ctc.). The reaction may be carried out at a ~c~ , selected
from ~0 C to 100 C.
In process (ww~,v), a l,~dic~l,onyl group or a m~Cl~ l,~dicarbonyl group ~ ,d
by process (sss) may have s~lbstit~ltions (e.g. aLkyl, aryl groups, etc.) at the alpha position.
The condeT-~tion and oxidation may be carried out with anhydrous hydrazine in a
solvent which does not adversely affect the reactions (e.g. iso~ uallol, acetonitrile, dioxane,
ben7e~e, toluene, etc.). The reaction may be carried out in the presence of a drying agent
such as m~g~r~s~ sulfate or molecular sieves at a ~CIll~ Lul~; selected from 0 C to 100 C.
In process (xxx), the thioc~l,onalG formation may be carried out in a solvent which
does not adversely affect the reaction~ (e.g. tolllene, acetone, methylene clllori~le~
tetrahy.lluru,dll or pyridine, etc.). The reaction may be carried out in the presence of a base
such as triethyl~mine, pyridine, di~ lylaminopyridine and sodium carbonate at a
t.,lll~.,ldlUlG s~olected from 0 C to 100 C. The thiocarbonylating reagent may be
N,N'-thiocarbonyl-rliimitl~7Ole, N,N'-thiocarbonylbis(2-pyridone), thiophosgene, or
O-phenylthiochlol orol"~aL~.
In process (yyy), the carbonate formation may be carried out in a solvent which does
not adversely affect the reactions (e.g. toluene, acetone, butanone, nnethylene chloride,
tetrahyd,ufuldn or pyridine etc.). The reaction may be carried OUt ill the presence of a base
such as triethylamine, pyridine, dimethylaminopyridine and sodium carbonate at atc~ dLu~G selected from 0 C to 100 C.
- 76 -
~ wo g4~2lc42 2 1 5 6 0 6 ~ ~us94~02692
The carbonylating reagent may be N,N'-carbony~ mid~7Qle~ N,N'-carbonyl-bis-
(2-pyridone), phosgene, triphosgene, ethyl chloroformate, ethyl trichloroacetate, or o-
phenylchlororol"late.
In process (zzz), the cyclic phosphonate formation may be carried out by first reactinga diol from a selected compound with phosphorous trichloride followed by the addition of an
a~lupiiate alcohol and amine. The alcohol used may be an alkyl alcohol, or an aryl alcohol.
The amine used may be primary or secondary. ~Itern~tively, the cyclic phosphonate
formation may be carried out by directly reacting the diol from a corresponding compound
with an a~lopliate aLIcoxyphophoryl dichloride.
The phosphonate formation may be carried out in a solvent which does not adversely
affect the reactions (e.g. carbon tetrachloride, chloroform, methylene chloride, toluene,
tetrahydrofuran, etc.). The reaction may be carried out in the presence of a base such as
triethylamine, pyridine, dimethylaminopyridine, and sodium carbonate at a ~en,~e,dture
selected from 0 C to 100 C.
In process (aaaa), the reduction of thiocarbonate may be carried out in a solvent
which does not adversely affect the reactions (e.g., toluene or tetrahyd"~ru,dn) at a
temperature selected from 0 C to 100 C.
The reducing agent used may be trimethylphosphite, triethylphosphite,
triaL~ylphosphite or tri-n-butyltin hydride.
In process (bbbb), the reduction of a 1,2-dicarbonyl group of a corresponding
compound may be carried out in a solvent which does not adversely affect the reactions (e.g.,
methanol, ethanol, ethanol, pyridine or N,N-dimethylform~mi-le).
The reducing agents used may be tin ~m~lg~m, ~lnminllm ~m~lg~m with hydrogen
chloride in ethanol, or may be hydrogen sulfide in pyridine or N,N-dimethylform~mi(le.
In process (cccc), the reduction and conclçns~tion of a 2-(o-nitrophenyl)-1,3-diketone
of a corresponding compound may be carried in a solvent which does not adversely affect the
reactions (e.g. ethanol, tetrahy~ofuldn, ethyl acetate or benzene, etc.).
The reducing agents used may be hydrogen gas over Pd/C, or Pt/C, zinc dust with
ammonium chloride, zinc dust with hydrochloric acid at a temp.,.dture selected from 0 C to
100 C.
In process (dddd), triazole formation may be carried out using, but is not limited to an
azide derivative with suitable acetylene analogues include diethylacetylene dicarboxylate,
dimethylacetylene dicarboxylate, methyl cyanoacetylenecarboxylate, and the likes. The
reaction may be conducted above, or below ambient temperature, more preferably from
0 to ~0 C.
In process (eeee), pyrrole formation may be carried out using, but is not limited to
amine compounds with 1,4-dicarbonyl analogues, such as acetonylacetone, and the likes.
Suitable solvents include methanol, ethanol, n-propanol, isopropanol, acetonitrile and
Wos4/21642~ j6a6~ ~- PCT/US94/02692
N,N-dimethylform~mi~le. The reaction may be conducted above, or below ambient
temperature, more preferably from 50 to 100 C.
In process (ffff), suitable reagents for vicinal hydroxylation include osmium
tetraoxide, potassium perm~ng~n~t~, and iodine in conjunction with silver acetat~. Osmium
tetroxide is preferably used with a regener~ting agent such as hydrogen peroxide, ~lk~line. t-
butyl hydroperoxide or N~ ,Lhylnlorpholine-N-oxide, and a solvent that does not adversely
affect the reaction, for example diethyl ether or tetrahy(llofu-an. pot~c~ m pçrm~ng~n~te is
preferably used in mild conditions, for example ~lk~line aqueous solution or suspensions.
Co-solvents such as t-butanol or acetic acid may also be used. Iodine-silver acetate under
'wet' conditions yields ci-diols. Preferably, iodine is used in aqueous acetic acid in the
presence of silver acetate. Iodine-silver acetate under 'dry' conditions yields trans-diols.
Here, the initial reaction is carried out in the absence of water, and final hydrolysis yields the
diol. In each case, the oxidation is preferably carried out at a temperature of 0 to 100 C.
Suitable reagents for the oxidative cleavage of the vicinal diol include lead
tetraacetate, phenyliodoso acetate, periodic acid or sodium metaperiodate. Suitable solvents
for the first two reagents include benzene and glacial acetic acid. The second two reagents
are preferably used in aqueous solution. The reaction is preferably carried out at a
temperature of 0 to 100 C.
In process (gggg), suitable reagents for the oxidation of an aldehyde of the
corresponding compound may include silver oxide, chromic acid and pot~inm
perm~ng~n~te In the presence of a variety of catalysts, oxygen may also be used in
converting an aldehyde to a carboxylic acid of a corresponding compound. The catalysts
may be p~ lm or pl~tintlm oxide. The air oxidation may be carried out in a solvent which
does not adversely affect the reaction (e.g., ethanol, water, acetonitrile, aqueous acetone or
pyridine) at a temperature of 0 to 100 C.
In process (hhhh), esters of a corresponding carboxylic acid may be ~r~d,cd under
neutral conditions at room ~e---l,~,.aLur~ by the reaction of the carboxylic acid with alcohols in
the presence of molar amounts of activating reagents such as triphenyl phosphine and diethyl
azodicarboxylate, carbofliimitles, N,N'-carbonylrliimicl~7ole and 1-methyl-2-halopyrir~inillm
iodide. Esters may also be formed by reacting the corresponding carboxylic acid with
diazo~lk~nes in a solvent which does not adversely affect the reaciton (e.g., ether,
tetrahydrofuran or methylene chloride) at a temperature of from 0 to 100 C.
In process (iiii), the cyclopropanation of the allyl group of a corresponding compound
may be carried out with ~ 7o~lk~nes in a solvent which does not adversely affect the
reaction (e.g., ether, methylene chloride or tetrahyclrorL~an) in the presence of a catalyst such
as pall~inm (II) acetate. The temperature of the reaction is of -15 to 5 C.
In process (jjjj), a pyrrole ring may be produced by reacting a 1,4-dicarbonyl group of
a corresponding compound with ammonia, or a substituted amine such as benzylamine or 2-
- 78 -
2156065
WO g4/21642 f'CTlUS94/02692
aminoethanol. Suitable solvents include those which do not adversely affect the reaction
(e.g., methylene chlc)ri~e, tetrahy~lluruldn or dioxane). The reaction is preferably carried out
at a ~e,-,peld~ule of 0 to 100 C.
In process (kkkk), the cyclization of a l,~dicarbonyl group ûf a cûrresponding
compound may be carried out in the presence ûf a catalytic amount of acid (e.g., acetic acid
or arylsulfonic acid). The reaction may be carried out in a solvent which does not adversely
affect the reaction (e.g., methylene chloride, ether, benzene or toluene). The reaction is
preferably carried out at a temperature of 0 to 60 C.
In process (1111), suitable reagents include air, a palladium (II) halide (e.g. palladium
(II) chloride), in conjunction with a cuprous halide (e.g. cupper (I) chloride). Suitable
solvents include those that do not adversely affect the reaction (e.g. DMF and water). The
reaction is preferably carried out at a temperature of 0 to 100 C.
In process (nnnn), suitable reducing agents include but are not limited to sodium
cyanoborohydride, lithium aluminum hydride, borane-pyridine, or hydrogen in the presence
of such catalysts as Raney nickel, pl~finllm, platinum oxide, ûr palladium. An acidic
environment may promote the reduction in some cases, and acids such as hydrochloric acid
or p-toluenesulfonic acid may be added for this purpose. The reduction may be carried out in
a solvent which does not adversely affect the reaction (e.g. ethanol, ethyl acetate).
In process (oooo), reduction of an oxime to the corresponding amine may be
accomplished with but not limited to hydrogenation with a suitable catalyst such as p~ rlillm
on carbon in a solvent inert to the reaction conditions (e.g. ethanol) at tempeld~ules ranging
from 0 to 100 C.
In process (pppp), reduction of an enone to the corresponding saturated ketone may
be accomplished with but not limited to hydrogenation with a suitable catalyst such as either
p~ illm on carbon or rhodium on ~ min~ in a solvent inert to the reaction conditions (e.g.
methanol, ethanol, isoprùpanoL et'nyl acetate) in a Le-l-~eld~ule range from -78 to 100 C.
In process (qqqq), isox~7.oline formation may be accomplished by, but not limited to
the following sets of reaction conditions involving a beta-hydroxy oxime. One possible
method is to treat the beta-hydroxy oxime with Martin's sulfurane dellydldLillg reagent at or
near room temperature in a solvent inert to the reaction conditions such as methylene
chloride. Alternatively, the beta-hydroxy oxime may be treated with p-toluenesulfonyl
- chloride in a solvent such as pyridine at temperatures ranging from 0 to 100 C.
In process (rrrr), an intramolecular aldol reaction may be accomplished by, but is not
limited to treatment of a carbonyl with a base such as potassium or sodium hydride in a
solvent which is inert to the reaction conditions (e.g. tetrahydrofuran or N,N-
dimethylfo~ licle) at a temperature range from -78 to 150 C.
In process (ssss), a cyclic imine may be formed by, but is not limited to treatment of
an alpha,beta-unsaturated enone with the sodium enolate of a glycine ester irnine in an inert
- 79 -
WO 94/21642 2lS606S PCT/US94/02692
solvent such as tetrahydl.,rul~l in a ~e~ GldLule range from -78 to 100 C. Upon aqueous
workup, the imine hydrolyzes and spontaneously cyclizes to form the cyclic imine.
In process (mt), a substituted pyrrole may be formed by but is not limited to a
1,3-dipolar cycloaddition between an alpha~beta-lln~ ~ enone with a glycine ester imine
in the presence of a suitable catalyst such as lithiu~ bromide and triethylamine in a solvent
inert to the reaction conditions (e.g. tetrahyd~ n) at or near room L~,.n~ dture.
In process (uuuu), alpha diazoketones can be decomposed by exposure to UV light or
by heating. Wolff rearrangements often ensue yielding beta-keto carboxylic acids when run
in a solvent n~i~lult; conL~ g water, beta-keto esters when run in a solvent containing an
alcohol, or beta-keto amides when run in a solvent col-t~h~ g ammonia, a lJlilll~y or a
secondary amine.
Moreover, in process (wvv), if a beta-keto carboxylic acid is formed, decarboxylation
can occur spontaneously or by heating.
In process (yyyy), aryl-, heterocyclic-, or aL~yloxycarbonylation may be carried out
using aryl-, heterocyclic-, or alkyl- chloroformate in the presence of amines like
triethylamine, diisopropylethylamine, pyridine and the like. ~ltPrn~tively, the reaction may
be carried out by reacting the corresponding aryl-OH, heterocyclic-OH or alkyl-OH with
-CHOC(O)Cl or -CHOC(O)-~-nitrophenyl) in a corresponding compound in the presence of
amine base. The reaction may be carried out in a solvent which does not adversely affect the
reaction (e.g. acetone, dichlorometh~ne, tetrahydlorulan, pyridine and N,N-
dimethylform~mi-le, or a m,~ e thereof). The reaction may be conducted above, at or
below ambient temperature.
In process (zzzz) allylic oxidations may be carried out using sele.nillm dioxide with or
without a co-oxidant, such as tert-butyl hydroperoxide, in an inert solvent such as
tetrahyd~ufulan, ether, ethylacetate, water, or a combination thereof. The reaction may be
conducted at room temperature to 100 C.
The compounds of the present invention are formed by modification of FR-900520
(ascomycin) or one of its congeners (such as FK-506, etc.) by aL~ylation of the C-32-
hydroxyl group with optional modifications exercised at C-18 and/or C-21 and/or C-23
and/or C-24. The compounds, processes and uses of the present invention will be better
understood in connection with the following examples, which are inten~ as an illustration
of and certainly not a limitation upon the scope of the invention. Both below and throughout
the specification, it is inten/le~ that citations to the lit~ratule are expressly incorporated by
reference.
- 80-
~ WO 94/21642 2 1 5 6 0 6 S ~IUS94/0~692
Example 1: Formula I: R= ethyl: n= 1: R2-R2a_3_--=~4-oH: Rla-OCH3: Rl_
-OCH~C(O)OC~H5 (R-Configuration).
A solution of asco,.,yc;ill (0.5 g, 0.63 mmol) in dichlorometh~n~ (10 mL) containing
rhodium(II)acetate dimer (3 mg) was refluxed while ethyl ~ 7O~cet~te (66 uL, 0.63 mmol) in
dichlorom~th~n~ (1 rnL) was added dropwise. After complete addition the reaction was
refluxed for 30 millu~es and additional ethyl diazoacetate (132 uL, 1.26 mmol) in
dichloromethane (1.5 mL) was added dropwise with reflux continning 30 minutes after
complete addition. Solvent was removed in vacuo and the residue puri~led by HPLC on silica
gel eluting with hexane:acetone (3:1). Fractions cont~ining desired product were pooled,
concentrated, dissolved in CC14, and concentrated to constant weight under high vacuum to
give the desired product (274 mg) as an oil in 50% yield. IR (CDC13) 3500, 2930, 1742,
1700, 1645, 1452 cm~1; 13C NMR (125 MHz) delta 9.4, 11.7, 14.1, 14.2, 15.8, 16.2, 20.5,
21.1, 24.2, 24.6, 26.3, 27.6, 30.3, 30.8, 32.7, 32.9, 33.6, 34.6, 36.4, 39.2, 39.7, 43.1, 48.7,
54.7, 56.3, 56.6, 56.9, 57.2, 60.6, 68.5, 70.1, 72.9, 73.7, 75.2, 77.2, 82.8, 83.6, 97.0, 123.1,
129.6, 132.4, 138.7, 164.7, 169.0, 171.1, 196.1, 213.5; MS (FAB) m/z: M+K = 916; Anal.
calc'd. for C47H75NO14-1.0 CC14: C, 54.70; H, 7.33; N, 1.36. Found: C, 54.42; H, 7.22; N,
1.26.
Example 2: Formula I: R= ethvl: n= 1: R-- 2a-R--R--H: R4-oH: Rla-OCH~: Rl_
-OCH~C(O)OCH~C6H~ (R-Configuration).
The prior procedure was modified using benzyl diazo~cet~te instead of ethyl ~ 7O~cetate.
Ascomycin (0.5 g) provided title compound (0.1 g) in 20% yield. mp. 65-72 C; IR (CDC13)
3510, 293Q, 1740, 1695, 1642, 1450 cm~l; 13c NMR (125 MHz) delta 9.4, 11.7, 14.1, 15.8,
16.2, 20.4, 21.1, 24.1, 24.5, 26.3, 27.6, 30.3, 30.8, 32.7, 32.8, 34.4, 34.5, 36.3, 39.2, 39.6,
43.1, 48.6, 53.4, 54.6, 56.3, 56.6, 57.1, 66.3, 68.5, 70.1, 72.8, 73.6, 75.1, 76.8, 82.7, 83.6,
96.9, 123.0, 128.3, 128.4, 128.5, 129.5, 132.3, 135.6, 138.7, 164.7, 168.9, 171.0, 196.2,
213.4; MS (FAB) m/z: M+H-H2O = 922, M+K = 978. Anal. calc'd. for Cs2H77NO14: C,
66.43; H, 8.26; N, 1.49. Found: C, 66.12; H, 8.14; N, 1.41.
Example 3: Formula I: R= ethyl: n= 1: R--=~3-R5-H: R4-oH: Rla-OCH3: Rl_
-OCH_C(O)OH (R-Configuration).
The resultant product of Example 2 (25 mg, 0.03 mmol) and 10% Pd/C (3 mg), were placed
in a flask and the vessel was flushed with nitrogen for 10 min. Methanol (250 uL) was added
via syringe, and the reaction stirred under a hydrogen atmosphere (1 atm) for 45 min. The
mixture was filtered, the catalyst washed with additional methanol (1 mL), and the solvent
removed in vacuo. The resulting residue was partitioned between ethyl acetate (5 mL) and
water (5 mL), the organic layer was dried (MgSO4), filtered and concentrated to constant
- 81 -
Wo 94/21642 PCT/US94/02692
2l56~6~ ~ ''
weight, thus producing the title compound (23 mg) as a white powder. MS f~FAB) m/z: M+K
= 888.
F~xample 4: Formula I: R= ethyl: n= 1: R-2-R2a-R~R~-H: R4-oH: Rla-OCH_: R1-
-OCH~C(O)R12 (R-Configuration): R~ ~ benzyl.
The product of Example 3 (0.50 g, 0.59 mmo~l~ was dissolved in dichlolomt;Lllane (5 mL) and
the solution cooled to 0C. 4-Dimethylaminopyridine (DMAP) (14.4 mg, Ø118 mmol) was
added followed by 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDAC)
(206 mg, 1.07 mmol) then benzylamine (128.3 uL, 1.18 mmol). Tl1e reaction was warmed to
room temperature and stirred overnight. Ethyl acetate (150 mL) was added and the organic
phase washed with lN hydrochloric acid (HCl) (2 x 150 mL), saturated bicarbonate solution
(2 x 150 mL), and then brine (2 x 150 mL). The organic layer was dried using anhydrous
sodium sulfate(Na2SO4), filtered, and solvent removed in vacuo to give 395.7 mg yellow
foam. The crude product was purified by HPLC (30 x 300 mm silica column) eluting with
2: 1 hexane/acetone. Fractions Cont~ining product were combined and solvent removed in
vacuo to give the title compound (273.2 mg, 49% yield) as a white solid: MS (FAB) m/z:
(M+K)=977.
Example 5: Formula I: R= ethyl: n= 1: R~R2a--R3_5-H: R4-oH: R1a-ocH3: R1-
-OCH~C(O)R12 (R-Configuration): R12- NR__15 R14- CH3: R~ benzyl.
The resultant product of Example 3 (0.8 g, 0.94 mrnol) was dissolved in THF (3 mL) and the
solution cooled to 0C before adding N-methylmorpholine (103.4 uL, 0.94 mmol) followed
by isobutyl chloroformate (122.2 uL, 0.94 mmol). The resnlting suspension was stirred for
20 minutes at 0 C after which N-methylbenzylamine (243 uL, 1.88 mmol) was added.
Stirring was continued overnight as the ice melted. The reaction ~ e was loaded onto
silica (40 mL) in a fritted funnel then eluted with dichloromethane (100 mL), 2: 1
hexane/acetone (200 mL), 1:1 hexane/acetone (200 mL), and acetone (100 rnL). Fractions
cont~ining product were combined and solvent removed in vacuo to give 0.64 g yellow foam.
The crude product was further purified by HPLC (30 x 300 mrn silica column) eluting with
1.5:1 hexane/acetone to provide the title compound (523 mg, 58% yield) as a white foam.
MS f~FAB)m/z: (M+K)=991.
Example 6: Formula I: R= ethyl: n= 1: R2_2a_3_5-H. R4-oH: Rla-OCH3: R1--OCH2CfO)R12 fR-Configuration): R~ NR__15~14_15_ H.
The crude isolate ~om Example 81 (1.5 g, ~1.2 mmol ) was dissolved in THF (4 mL) and the
solution cooled to 0C before adding N-methylmorpholine (129.4 uL, 1.2 mmol) followed by
isobutyl chloroformate (152.8 uL, 0.59 mrnol). The resulting suspension was stirred for 20
minutes at 0C after which ammonium hydroxide (14.8M, 159.2 uL, 2.4 rnmol) was added.
- 82 -
~ WO 94/21642 2 1 5 6 0 6 5 PCr/US94l02692
Stirring was continued overnight as the ice melted. The reaction Il~ib~Lule was loaded onto
silica (80 mL) in a fritted funnel then eluted with dichloro,nçth~ne (200 mL), 2:1
hexane/acetone (400 mL), 1:1 hexane/acetone (400 mL), and acetone (200 mL). Fractions
cont~ining product were combined and solvent removed in vacuo to give 358 mg yellow
foam. The crude product was further purifled by RP-HPLC (Rainin Dynamax 41.4 mm
phenyl column) eluting with a gradient of 20% methanoVwater and acG~onillile to provide the
title compound (188.7 mg, 19% yield) as a white foam. MS (FAB)m/z: (M+K)=887.
Example 7: Formula I: R= ethyl: n= 1: R--R2--R~-R$-H: R4-oH: Rla-OCH3: Rl-
-OCH2C(O)R12 (R-Configuration) R12- NR__15~ R14- H R15- CH~.
The product of Example 3 is activated as in Example 5 and then treated with methylamine
instead of N-methylbenzylamine to provide the title compound.
Example 8: Formula I: R= ethyl: n= 1: R2-R~R3_5-H, R4-oH: Rla-OCH3: Rl-
-OCH2C(O)R12 (R-Configuration); R12- NR___~14_15_ CH3.
The product of Example 3 is activated as in Example 5 and then treated with dimethylamine
instead of N-methylbenzylamine to provide the title compound.
Example 9: Formula I: R= ethyl: n= 1: R2_2_R3-RS-H: R4-oH: Rla-OCH3: Rl-
-OCH~C(O)R12 (R-Configuration) R12- NR__15~ R14- H R15- ethyl
The product of Example 3 is activated as in Example 5 and then treated with ethylamine
instead of N-methylbenzylamine to provide the title compound.
Example 10: Formula I: R= ethyl: n= 1: R2-R2a-R3-RS-H: R4-oH: Rla-OCH3: Rl-
-OCH2C(O)R12 (R-Configuration) R12- NR14_15~ R14- CH;~: R15- ethyl
The product of Example 3 is activated as in Example 5 and then treated with N,N-methyl,ethylamine instead of N-methylbenzylamine to provide the title compound.
Example 11: Formula I: R= ethyl: n= 1: R2--R~R3-R5-H. R4-oH: Rl~OCH3: Rl-
-OCH~C(O)R12 (R-Configuration): R12-- NR__15 Rl_ H: R-15- -CH2CH2CH3.
The product of Example 3 is activated as in Example 5 and then treated with propylamine
instead of N-methylbenzylamine to provide the title compound.
Example 12: Formula I: R= ethyl: n= 1: R--R~R3-RS-H: R4-oH: Rla-OCH3: Rl-
-OCH2C(O)R12 (R-Con~lguration): R12- NR--_~ R14- CH3: R15--CH~CH~CH3.
The product of Example 3 is activated as in Example S and then treated with
N,N-methyl,propylamine instead of N-methylbenzylamine to provide the title compound.
- 83-
Wo g4/21642 ~ us94/02692 ~
~,~S6~S ~ ' ~
F.xample 13: Formula I: R= ethyl: n= 1: R2_2a-R3=~5-H: R4-oH: Rla-OCH~: Rl_
-OCH~C(O)R12 (R-Confi~uration): R12- NR14B15 R14- H: R15- -CH(CH3)~.
The product of Example 3 is activated as in Example S and then treated with 2-aminopropane
instead of N-methylbenzylamine to provide the title compound.
Fxample 14: Formula I: R= ethyl: n= 1: R-- 2a-R3_S-H: R4-oH: Rla-OCH~: Rl_
-OCH~C(O)R12 (R-Configuration): R12- NR--~--: R14- CH~. R-15--CH(CH3)~
The product of F.x~mple 3 is activated as in Example S and then treated with N,N-methyl, 2-
propylamine instead of N-methylbenzylamine to provide the title compound.
Example 15: Formula I: R= ethyl: n= 1: R-- 2a-R--RS-H: R4-oH: Rla-OCH~: Rl_
-OCH~C(O)R12 (R-Configuration): R12- NR--_--: R14- H: R15- cyclopro~yl.
The product of Example 3 is activated as in Example S and then treated with
cyclopropylamine instead of N-methylbenzylamine to provide the title compound.
F.xample 1~: Formula I: R= ethyl: n= 1: R 2a_ -R--H: R4-oH: Rla-OCH~: Rl_
-OCH~C(O)R12 (R-Confi~uration): R12- NR--B--R14- H: R15- -CH_CH~CH~CH~,
The product of Example 3 is activated as in Example 5 and then treated with n-butylamine
instead of N-methylbenzylamine to provide the title compound.
F.xample 17: Formula I: R= ethyl: n= 1: R 2a--R3_S-H: R4-oH: Rla-OCH~: Rl_
-OCH~C(O)R12 (R-Confi~uration): R12- NR--_--R14- CH~: RlS--CH~CH~CH?CH~s
The product of Example 3 is activated as in Example S and then treated with
N,N-methylbutylamine instead of N-methylbenzylamine to provide the title compound.
Fxample 18: Formula I: R= ethyl: n= 1: R 2a_3_5-H: R4-oH: Rla-OCH~: Rl_
-OCH~C(O)R12 (R-Confi~uration): R12~--R--; R14- H: RlS- -CH~CH(CH3)~.
The product of Example 3 is activated as in Example S and then treated with isobutylamine
instead of N-methylbenzylamine to provide the title compound.
F.xample 19: Formula I: R= ethyl: n= 1: R2-R2a-R-- S=~4-oH: Rl~OCH3: Rl_
-OCH~C(O)R12 (R-Configuration): R12- NR--_--~ R14- CH3: R15--CH~CH(CH3)~ ~
The product of Example 3 is activated as in Example S and then treated with
N,N-methyl,isobutylamine instead of N-methylbenzylamine to provide the title compound.
- 84-
~ WO 94/21642 21~ 6 0 6~ PCT/US94/02692
Fxample 20: Formula I: R= ethyl: n= 1: R--R~R3_5-H. R4-oH: Rla-OCH~: R1_
-OCH~C(O)R12 (R-Configuration): R12- NR--R--R14- H: R15- cyclobutyl.
The product of Example 3 is activated as in Example 5 and then treated with cyclobutylamine
instead of N-methylbenzylamine to provide the title compound.
Example 21: Formula I: R= ethyl: n= 1: R-- 2a-R3-R5=~4-oH: R-la-OCH~: Rl=
-OCH~C(O)R12 (R-Configuration): R12- NR--_--R14- H: R15_
-CH ~CH~CH ~CH2CH3.
The product of Example 3 is activated as in Example 5 and then treated with pentylamine
instead of N-methylbenzylamine to provide the title compound.
Example 22: Formula I: R= ethyl: n= 1: R-- 2a-R3_5-H. R4-oH: R1a-OCH~: Rl_
-OCH~C(O)R12 (R-Configuration): Rl--NR--_--R14-CH~: R15=
-CH_CH~CH~CH~CH~
The product of Example 3 is activated as in Example 5 and then treated with
N,N-methyl,pentylamine instead of N-methylbenzylamine to provide the title compound.
Example 23: Formula I: R= ethyl: n= 1: R--R~ 3_5=~4-oH: Rla-OCH~: Rl_
-OCH2C(O)R12 (R-Confi~uration): R12- NR--R--R14- H: R15- -CH2CH~CH~(CH~
The product of Example 3 is activated as in Example 5 and then treated with
3-methylbutylamine instead of N-methylbenzylamine to provide the title compound.
Fxample 24: Formula I: R= ethyl: n= 1: R--R2a-R3-R5-H: R4-oH: R1a-OCH3: R1_
-OCH~C(O)R12 (R-Configuration): R12- NR--_--~ R14- CH~: R15-
-CH?CH~CH~(CH3)~,
The product of Example 3 is activated as in Example 5 and then treated with N,N-methyl,3-
methylbutylamine instead of N-methylbenzylamine to provide the title compound.
Example 25: Formula I: R= ethyl: n= 1: R--R2a-R3-R5-H. R4-oH: R1a-OCH~: Rl-
-OCH~C(O)R12 (R-Configuration): R12- NR--B--: R14- H: R15- cyclopentyl.
The product of Example 3 is activated as in Example 5 and then treated with
cyclopentylamine instead of N-methylbenzylamine to provide the title compound.
Example 26: Formula I: R= ethyl: n= 1: R~-R~ 3_--H: R4-oH: R1a-OCH~: Rl-
-OCH7C(O)R12 (R-Configuration): R12- NR--_--R14- H: R15-
-CH_CH~CH~CH~CH~CH3._ _ _
The product of Example 3 is activated as in Example 5 and then treated with n-hexylamine
instead of N-methylbenzylamine to provide the title compound.
- 85-
WOg4/21642 PCrlUS94/02692
~S6~S
Example 27: Formula I: R= ethyl: n= 1: R2-R2a-R3_5-H: R4-oH: R1a-ocH3; Rl-
-OCH2C(O)R12 (R-Configuration): R12_14_15 R14- CH3: R15-
-CH?CH2CH2CH2CH~CH~.
The product of Example 3 is activated as in Example S and then treated with
N,N-methyl,hexylamine instead of N-methylbenzylamine to provide the title compound.
Example 28: Formula I: R= ethyl: n= 1: ~2-R~R3_5-H: R4-oH: Rla-OCH~: Rl-
-OCH2CfO)R12 (R-Configuration) Rl~ NR--R15 R14- H R15- cyclohexyl.
The product of Example 3 was activated as in Example 5 and then treated with
cyclohexylamine instead of N-methylbenzylamine to provide the title compound. MS (FAB)
m/z: M+H = 931.
Example 29: Formula I: R= ethyl: n= 1: R2-R~R3-R5--H: R4-oH: Rla-OCH3: Rl-
-OCH?C(O)R12 (R-Configuration): R12- NR14R15: where R--and R15 taken together=
-CH~CH~OCH2CH2-. thus forming a six membered ring incorporating the nitrogen to which
they are attached.
The product of Example 3 was activated as in Example 4 and then treated with morpholine
instead of benzylamine to give the title compound. MS (FAB)m/z: (M+K)=957.
Example 30: Formula I: R= ethyl: n= 1: R2_~R3-R5-H: R4--OH: R-la-OCH3: Rl-
-OCH?C(O)R12 (R-Configuration): R12- NR__15~ R14- H: R1~ -CH~.CH~OH.
The product of Example 3 was activated as in Example 4 and then treated with
2-aminoethanol instead of benzylamine to give the title compound. MS (FAB)m/z:
(M+K)=93 1 .
Example 31: Formula I: R= ethyl: n= 1: R2_2a-R3-R5-H-~ R4-oH: Rla-OCH~,: Rl-
-OCH?C(O)R12 (R-Configuration): R12- NR14_15~ R14- H: R15- -CH~CH2CH~OH.
The product of Example 3 was activated as in Example 4 and then treated with
3-aminopl-,panol instead of benzylamine to give the title compound. MS (FAB)m/z:(M+K)=945.
Example 32: Formula I: R= ethyl: n= 1: R-- ~ 3_5-H: R4-oH: Rla-OCH3: Rl_
-OCH2C(O)R12 (R-Configuration): R-12- NR__15 R14- H: R15-
-CH?CH~CH?CH?OH.
The product of Example 3 was activated as in Example 5 and then treated with
4-aminobutanol instead of N-methylbenzylamine to give the title compound. MS (FAB)m/z:
(M+K)=959.
- 86-
~ wo g4,2l642 2 1 5 6 0 6 ~ ~/US94l02692
Example 33: Formula I: R= ethyl: n= 1: R_R~R~R~H. R4-oH: Rla-OCH3: Rl-
-OcH?c(o)Rl2(R-configuration) R12-NR__15 R14_H R15_
-CH2CH?CH~CH2CH20H.
The product of Example 3 is activated as in Example 5 and then treated with
5-hydroxypentylamine instead of N-methylbenzylamine to provide the title compound.
Example 34: Formula I: R= ethyl: n= 1: R ~ ~R5-H. R4-oH: R~OCH3: Rl-
-OCH~C(O)R12(R-Configuration) R-l2-NR--_--R14-H R15--CH~CH~NH?
The product of Example 3 is activated as in Example 5, and then treated with
1,2-diaminoethane instead of N-methylbenzylamine to provide the title compound.
Example 35: Formula I: R= ethyl: n= 1: R_R~R3-R-5-H: R4-oH: Rla-OCH3: Rl-
-OCH2C(O)R12 (R-Configuration): Rl2- NR--R15 Rl--H: R15- -CH~CH~CH~NH~.
The product of Example 3 is activated as in Example 5, and then treated with
1,3-diaminopropane instead of N-methylbenzylamine to provide the title compound.
Example 36: Formula I: R= ethyl: n= 1: R~ ~R3_5-H. R4-oH: Rla-OCH3: Rl-
-OCH~C(O)R12 (R-Configuration): R 12___15~ R14_ H: R15_
-CH~CH?CH~CH2NH2.
The product of Example 3 is activated as in Example 5, and then treated with
1,4-diaminobutane instead of N-methylbenzylamine to provide the title compound.
Example 37: Formula I: R= ethyl: n= 1: R2 R2-a-R3_5-H. R4-oH: R-la-OCH3: Rl-
-OCH~C(O)R12 (R-Configuration) R 12- NR14_15; R~ H R15_
-CH2CH~CH2CH'~CH~NH2-
The product of Example 3 is activated as in Example 5, and then treated with
1,5-diaminopentane instead of N-methylbenzylamine to provide the title compound.
Example 38: Formula I: R= ethyl: n= 1: R2-R~ 3-R5-H: R4-oH: R-la-OCH~- Rl-
-OCH~C(O)R12 (R-Configuration): R-12- NR__15 R14- H: R15- -CH~CO~CH~Ph
The product of Example 3 was activated as in Example 4 and then treated with glycine
benzyl ester instead of benzylamine to give the title compound. MS (FAB)m/z:
(M+K)=1035.
- 87 -
i~S6~ PCT/US94/02692
Example 39: Formula I: R= ethyl: n= 1: R--R2a_3-R5--H: R--O~[: Rla--OCH~,: Rl_
-QCH~C(O)R12 (R-Configuration~: R12- NR--B--R14- H: R15- -CH~CO~H.
The title compound was synthesized in the manner described in Example 3 substituting the
product from Example 38 for the product from Example 2. MS (FAB)m/z: (M+K)=945.
Example 40: Formula I: R= ethyl: n= 1: R 2a-R3_5-H: R4-oH: Rla-OCH ~,: Rl-
-OCH~C(O)R12 (R-Confi~uration): R12- NR--B--: R14- H: R-15- -CH~CH~CO~CH~Ph.
The product of Example 3 was activated as in Example 4 and then treated with beta-alanine
benzyl ester instead of benzylamine to give the title compound. MS (FAB)m/z:
(M+K)=1049.
F~xample41 FormulaI: R=ethyl: n= 1: R-- 2a-R--R--H: R4-oH: Rla-OCH~: Rl_
-OCH~C(O)R12(R-Configuration) R--NR--_--R14-H R15- CH~CH~CO~H
The title compound was synthesized in the manner described in Example 3 substitutin~ the
product from Example 40 for the product from Example2. MS (FAB)m/z: (M+K)=959.
Example 42: Formula I: R= ethyl: n= 1: R 2a=~3=1~_H: R4-oH: Rla-OCH~: Rl-
-OCH2C(O)R12 (R-Configuration): R12- NR--_--~ Rl--H: R15- -CH(CH~,)CO~H (~
confiyuration) .
The product of Example 3 is activated as in Example 5, and then treated with
(R)-2-aminol,lo~alloic acid instead of N-methylbenzylamine to provide the title compound.
Example 43: Formula I: R= ethyl: n= 1: R-- 2a-R3-R--H: R4-oH: Rla-OCH~,: Rl_
-OCH~C(O)R12 (R-Configuration): R12- NR___~14_ H: R15- -CH(CH~)CO~H (S
configuration) .
The product of Example 3 is activated as in Example 5, and then treated with
(S)-2-aminopropanoic acid instead of N-methylbenzylamine to provide the title compound.
Fxample 44: Formula I: R= ethvl: n= 1: R-- 2a-R-3-R---H: R4-oH: Rla-OCH~: Rl_
-OCH~C(O)R12 (R-Configuration): R 12_ NR--_--R14- H: R15_
-CH(CH3)CONHCH(CH3)CONHCH(CH3)CO2H (all chiral centers in R--are R
configuration).
The product of Example 3 is activated as in Example 5, and then treated with D-alaninyl-D-
alaninyl-D-alanine instead of N-methylbenzylamine to provide the title compound.
- 88 -
~ WO 94/21642 2 1 5 6 0 6 5 PCTIUS94/02692
F.xample 45: Formula I: R= ethyl: n= 1 R2-R2a_3=~5-H: R4-oH: Rla-OCH~: Rl_
-OCH~C(O)R12 (R-Configuration): R12- NR--R--R14- H: R15- 3-phenyl-phenyl.
The product of Example 3 is activated as in Example 5 and then treated with
3-biphenylamine instead of N-methylbenzylamine to give the title compound.
Example 46: Formula I: R= ethyl: n= 1: R =B2a-R3-R--H~ R4-oH: Rla-OCH~- Rl-
-OCH~C(O)R12 (R-Configuration): R12___--: R14--CH~CH~oH: R15- 3-phenyl-
phenyl.
The product of Example 3 is activated as in Example 5 and then treated with N,N-(ethanol-2-
yl)-(3-biphenyl)-amine instead of N-methylbenzylamine to give the title compound.
Example 47: Formula I: R= ethyl: n= 1: R-- 2a-R--R5-H: R4-oH: Rla-OCH~: Rl_
-OCH~C(O)R12 (R-Configuration): R12- NR--_--R14_
-CH_CH~N(CH~)(CH~CH~OH): R15-phenvl._
The product of Example 3 is activated as in Example 5 and then treated with N-phenyl-
N'-methyl-N'-(ethanol-2-yl)-ethyl(li~mine instead of N-methylbenzylamine to give the title
compound.
Example 48: Forrnula I: R= ethyl: n= 1: R2_2a-R3-R--H: R4-oH: Rla=OCH~: Rl_
-OCH~C(O)R12 (R-Configuration): R12- NR--R--R14-CH~CH~N(CH~)~: R15_
phenyl.
The product of Example 3 is activated as in Example 5 and then treated with N-phenyl-N',N'-
dimethyl-ethyl~ mine instead of N-methylbenzylamine to give the title compound.
Example 49: Formula I: R= ethyl: n= 1: R---R2a_3-R--H: R4-oH: R1a-OCH~: Rl_
-OCH~C(O)R12 (R-Configuration): R12- NR--_--. R14- CH~3-pyridyl): R15- CH~(3-
pyridyl).
The product of Example 3 was activated as in Example 5 and then treated with
3,3'-dipipicolylamine instead of N-methylbenzylamine to give the title compound. MS
(FAB) m/z: M+K = 1069.
Example 50: Formula I: R= ethyl: n= 1: R--R2a_3_--H: R4-oH: R1a-OCH~: R1--
-OCH~C(O)R12 (R-Configuration): R12___--R14-cyclohexyl: R15- cyclohexyl.
The product of Example 3 is activated as in Example 5 and then treated with
dicyclohexylamine instead of N-methylbenzylamine to give the title compound.
Example 51: Formula I: R= ethyl: n= 1: R--R2a--R3_--H: R4-oH: R1a-OCH~: Rl_
-OCH~C(O)R12 (R-Confi~uration): R12- NR--R--R14- H: R15- NH-(4-mor~holinyl).
- 89-
~,~S 6 PCT/US94/02692 ~
The product of Example 3 was activated as in Example 5 and then treated with 1 equivalent
of 4-aminomorpholine and 0.1 equivalents of 4-dimethylaminopyridine instead of N-
methylbenzylamine to give the title compound. MS (FAB) m/z: M+K = 972.
Example 52: Formula I: R= ethyl: n= 1: R2--R2a_3--R-5--H: R4-oH: R1a-ocH3: Rl-
-OCH~C(O)R12 (R-Conf1guration): R12- NR14_15 R14- H: R15- 4-thiomorpholinyl.
The product of Example 3 was activated as in Example 5 and then treated with
thiomorpholine instead of N-methylbenzylamine to give the title compound. MS (FAB) m/z:
M+K = 973.
Example 53: Formula I: R= ethyl: n= 1: R2_2a-R3-R-5-H: R4-oH: R1a-oCH3: Rl_
-OCH2C(O)R12 (R-Configuration): R12-- NR__15 R14- H: R~ 4-CF3-phenyl.
The product of Example 3 was activated as in Example 4 and then treated with
4-aminobenzotrifluoride instead of benzylamine to give the title compound. MS (FAB)m/z:
(M+K)=103 1 .
Example 54: Formula I: R= ethyl: n= 1: R2-R2a-R3-R-5-H: R4-oH: R1a-OCH3: Rl-
-ocH2c(o)Rl2(R-Confguration) Rl~ __15~R14_H Rl-5-4-F-phenyl
The product of Example 3 was activated as in Example 4 and then treated with
4-fluoroaniline instead of benzylamine to give the title compound. MS (FAB)m/z:
(M+K)=98 1 .
Example 55: Formula I: R= ethyl: n= 1: R2_2 L ~R5-H. R4-oH: Rla-OCH3: Rl-
-OCH?C(O)R12 (R-Configuration): R12___15 R14_ H: R15- 4-(4-morpholino)-
phenyl.
The product of Example 3 was activated as in Example 4 and then treated with
4-morpholinoaniline instead of benzylamine to give the title compound. MS (FAB)m/z:
(M+K)=1048.
Example 56: Formula I: R= ethyl: n= 1: R~--R2~-R3_5-H~ R4-oH: Rla-OCH3: Rl-
-OCH2C(O)R12 (R-Configuration): R 1 --NR__15~ R 14- H: R~ 4-HO-phenyl.
The product of Example 3 was activated as in Example 5 and then treated with
p-aminophenol instead of N-methylbenzylamine to give the title compound. MS (FAB)m/z:
(M+K)=979.
Example 57: Formula I: R= ethyl: n= 1: R2--R~R3-R5--H. R4-oH: R~OCH3: R1--
-OCH2C(O)R12(R-Confi~uration) Rl2-NR__15 R14_H R15-3-pyridyl
- 90-
~ Wo 94/21642 2 1 5 6 0 6 5 ~IUS94102692
The product of Example 3 was activated as in Example 5 and then treated with
3-aminopyridine instead of N-methylbenzylamine to give the title compound. MS (FAB)m/z:
(M+K)=964.
Example 58: Formula I: R= ethyl: n= 1: R2-R~ 3-R5-H: R4-oH: R1a-OCH~: R1-
-OCH~C(O)R12 (R-Configuration): R12_14_15 R14- H R15- 4-pyridyl
The product of Example 3 was activated as in Example 5 and then treated with
4-aminopyridine instead of N-methylbenzylamine to give the title compound. MS (FAB)m/z:
~M+K)=964.
Example 59: Formula I: R= ethyl: n= 1: R2_~ 3-R5-H: R4-oH: Rla-OCH3: R1_
-OCH2C(O)R12 (R-configuration) R 12_ NR__15 R14- H R15- 2-pyridyl
The product of Example 3 was activated as in Example 5 and then treated with
2-aminopyridine instead of N-methylbenzylamine to give the title compound. MS (FAB)m/z:
(M+K)=964.
Example 60: Formula I: R= ethyl: n= 1: R2-R2a-R3-R5-H: R--OH: R1a-oCH3: R1-
-OCH~C(O)R12 (R-Configuration): R12= NR14_15 R14- CH~: R15- CH?CH~OH.
The product of Example 3 was activated as in Example 5 and then treated with
2-(methylamino)ethanol instead of N-methylbenzylamine to give the title compound. MS
(FAB)mlz: (M+K)-945.
Example 61: Formula I: R= ethyl: n= 1: R2_2a-R3-R5-H: R4-oH: R1a-OCH3: Rl_
-OCH~.C(O)R12 (R-Configuration): R12___15 R14- H: R~ NHCO~CH3
The product of Example 3 is activated as in Example S and then treated with methylcarbazate
instead of N-methylbenzylamine to give the title compound.
Example 62: Formula I: R= ethyl: n= 1: R~R2a-R3-RS-H: R4-oH: R1a-OCH3: R1-
-OCH~C(O)R12 (R-Confi~uration): R12- L-prolinocarboxamide.
The product of Example 3 is activated as in Example S and then treated with
L-prolinecarboxamide instead of N-methylbenzylamine to give the title compound.
Example 63: Formula I: R= ethyl: n= 1: R2_~R3-R5-H: R4-oH: R1a-OCH3: Rl-
-OCH~C(O)R12 (R-Configuration): R 12- D-prolinocarboxamide.
The product of Example 3 is activated as in Example S and then treated with
D-Prolinecarboxamide instead of N-methylbenzylamine to give the title compound.
- 91 -
wo g4/21642 6~ PCT/US94102692 ~
Fxample 64: Formula I: R= ethyl: n= 1: R 2a-R3_5-H: R4-oH: R1a-OCH~: Rl_
-OCH~C(O)R12 (R-Configuration): R12- L-prolinol.
The product of Example 3 is activated as in Example S and then treated with L-prolinol
instead of N-methylbenzylamine to give the title compound.
Ex~mple 65: Formula I: R= ethyl: n= 1: R--R~ S-H: R4-oH: R1a-OCH~: R1_
-OCH~C(O)R12 (R-Configuration): R12- D-prolinol.
The product of Example 3 is activated as in Example S and then treated with D-prolinol
instead of N-methylbenzylamine to give the title compound.
F.xample 66: Formula I: R= ethyl: n= 1: R--R2a-R3_--H: R4-oH: R1a-OCH~: R1_
-OCH7C(O)R12 (R-Confiyuration): R12- NR8NR-_- R8- -CH~CHtOH: R6- H.
R--CO~CH~
The product of Example 3 is activated as in Example S and then treated with N-(ethanol-
2-yl)-N'-carbomethoxy-hydrazine instead of N-methylbenzylamine to give the titlecompound.
Example 67: Formula I: R= ethvl: n= 1: R-- 2a-R-- --H: R4-oH: R1a-OCH~: R1_
-OCH~C(O)R12 (R-Configuration): R12- NR--R--R14-H: R15- 3-
(phenylethynyl)phenyl.
The product of Example 3 is activated as in Example S and then treated with
3-phenylethynylaniline instead of N-methylbenzylamine to give the title compound.
F.x~mple 68: Formula I: R= ethyl: n= 1: R 2a-R3__H: R4-oH: Rla-OCH~: Rl_
-OCH~C(O)R12 (R-Confi~uration): R12- NR--R--R14- CH~CH~CH~OH: R15- 4-
fluorophenyl.
The product of Example 3 is activated as in Example 5 and then treated with 3-(4-
fluoroanilino)-l-propanol instead of N-methylbenzylamine to give the title compound.
F.xample 69: Formula I: R= ethvl: n= l: R-- 2a-R--R--H: R4-oH: R1a-OCH~: R1_
-OCH?C(O)R12 (R-Configuration): R12- NR--_--R14-
CH7CH?~ CH7OCOCH~CH~CO?H: R15- 4-fluorophenyl.
The product of Example 68 is treated with succinic anhydride, as published in Tetrahedron
Letts. 1989, 30, 5045-48, to give the title compound.
Example 70: Formula I: R= ethyl: n= 1: R-- 2a-R-- --H: R4-oH: R1a-OCH3: Rl_
-OCH_C(O)R12 (R-Configuration): R12- NR--_--~ R--and R15 are taken together as the
following diradicah -CH~CH~C(OCH~CH~O)CH~CH~-.
- 92-
~ wo g4,2l642 2 1 5 6 0 6 ~ PCrlUS94102692
The product of Example 3 was activated as in Example 5 and then treated with 1,4-dioxa-
8-azaspiro[4.5]decane instead of N-methylbenzylamine to give the title compound. MS
(FAB) m/z: M+K = 1013.
Example 71: Formula I: R= ethyl: n= 1: R2-R2a-R3_5-H: R4-oH: R1a-OCH3: Rl-
-OCH2C(O)R12 (R-Configuration): Rl~: R6- H: R7--Co-(4-
pyridyl). !.,'
The product of Example 3 was activated as in Example 5 and then treated with isonicotinic
acid hydrazide instead of N-methylbenzylamine to give the title compound. MS (FAB) m/z:
M+K = 1007.
Example 72: Formula I: R= ethyl: n= 1: R 2~ 3-R~H: R4-oH: R~OCH3: Rl_
-OCH?C(O)R12 (R-Configuration): R 12_14_15 R14=~15- 3-fluorophenyl.
The product of Example 3 was activated as in Example 5 and then treated with
m-fluoro~ni1ine instead of N-methylbenzylamine to give the title compound. MS (FAB) m/z:
M+2K-H = 1045.
Example 73: Formula I: R= ethyl: n= l: R~R~ 3-R5-H~ R4-oH: RLa=OCH3: Rl-
-OCH~C(O)R12 (R-Configuration): R_ NR14B15 Rl4- H: R-15- 3-hydroxy-phenyl.
The product of Example 3 was activated as in Example 5 and then treated with
m-aminophenol instead of N-methylbenzylamine to give the title compound. MS (FAB) m/z:
M+K = 979.
Example 74: Formula I: R= ethyl: n= 1: R ~ 2a-R3-R$-H: R4-oH: RL=OCH3: R 1 -
-OCH2C(O)R 1 2 (R-Configuration): R 12- NR14_15 R_ and R 15 are taken together as the
following diradical: -CH2CH?-NCH3-CH~CH2-.
The product of Example 3 was activated as in Example 5 and then treated with
N-methylpi~ ~ine instead of N-methylbenzylamine to give the title compound. MS (FAB)
m/z: M+H = 932.
Example 75: Formula I: R= ethyl: n= 1: R -R2a_~ 5-H. R4-oH: RL=OCH3: Rl_
-ocH~c(o)RL(R-configuration) Rl2_L4_15 R14_H R15=
6-(1 ,4-benzodioxanyl)-.
The product of Example 3 is activated as in Example 5 and then treated with
1,4-benzodioxan-6-amine instead of N-methylbenzylamine to give the title compound.
- 93-
WO 94/21642 6~6S PCT/US94/02692
F,xample 76: Formula I: R= ethyl: n= 1: R--R2a-R3-R5=~4-oH: Rla-OCH~: Rl_
-OCH~C(O)R12 (R-Confi~uration): R12- NR14B--~ R,14- H: R15- 3.4-methylenedioxy-
phenyl-.
The product of Example 3 is activated as in Example 5 and then treated with
3,4-(methylenedioxy)-aniline instead of N-methylbenzylamine to give the title compound,.
F,xample 77: Formula I: R= ethyl: n= 1: R--R~R3_5=~4-oH: Rla-OCH~: Rl_
-OCH~C(O)R12 (R-Configuration): R12- NR--R--;B14_ H: R15- l-naphthalenyL
The product of Example 3 is activated as in Example 5 and then treated with
l-naphthylamine instead of N-methylbenzylamine to give the title compound.
F,xample 78: Formula I: R= ethyl: n= 1: R2_~R3-R5-H: R4-oH: Rla-OCH~: Rl =
-OCH~C(O)R12 (R-Configuration): R1_ NR14R15: where R--and R15 taken together =
-CH~CH~CH2CH?-~ thus forming a five membered ring incorporating the nitrogen to which
they are attached.
The product of Example 3 is activated as in Example 5 and then treated with pyrrolidine
instead of N-methylbenzylamine to provide the title compound.
F,xample 79: Formula I: R= ethyl: n= 1: R---R2a-R3_--H. R4-oH: Rla-OCH3: Rl _
-OCH~C(O)R12 (R-Configuration): R12 - NR14B15: where R--and R15 taken together =-CH~CH?CH~CH~CH~-. thus forming a six membered ring incorporating the nitrogen to
which they are attached.
The title compound was isolated as a by-product of the reaction described in Example 6. The
product was purified by HPLC (30 x 300 mm silica column) eluting with 5:4
acetoni~rile/dichloromethane. Fractions conlailli,lg pure product were combined and solvent
removed in vacuo to give the title compound as a white foam (215.5 mg, 23% yield). MS
(FAB)m/z: (M+K)=955.
F,xample 80: Formula I: R= ethyl: n= 1: R--R2a-R3_5-H: R4-oH: Rla-OCH~: Rl-
-OCH~C(O)OCH~-(,9-fluorenyl) (R-Configuration).
Ascomycin (10 g, .012 mol) was dissolved in distilled CH2C12 (50 ml). Rhodium (II) acetate
dimer (100 mg) was added and the n~i~ c; cooled to 0 C. 9-Fluorenylmethyl diazoacetate
(3.35 g, .012 mol) was dissolved in CH2C12 (10 mL) and the solution added to the reaction
via syringe pump at a rate of approximately 0.5 mL/hour. Addition was complete in
approximately 24 hours. The reaction was stirred at 0 C for an additional 24 hours then
loaded onto silica (230-400 mesh, 400 g) and the solven~ evaporated by airflow in the hood.
The adsorbed silica was layered over fresh silica (800 g) in a 1 L fritted glass funnel. The
silica plug was eluted with the following solvents: CH2C12 (2 L), 3: 1 CH2C12/CH3CN (4
- 94-
2156065
WO 94/21642 PCrlUS94/02692
L), 2:1 CH2C12/CH3CN (3 L), and 1:1 CH2C12/CH3CN (3 L). Fractions COnl~il~illg product
were combined and concenlld~ed in vacuo to give 7.32 g yellow foam. Fractions con~il~ g
ascomycin were combined and conce~ al~d in vacuo to give 3.30 g green foam (contains
some catalyst). The product was further purified by HPLC (silica gel, 230-400 mesh, 50 x
500 mm column) eluting with 3.5: 1 hexane/acetone at a flow rate of 80 mVmin. Fractions
containg purest m~tenAl were combined and conc~ ed in vacuo to give the title compound
as a white foam (4.0 g, 45% yield based on recovered ascomycin).
IR (KBr) 3440, 1740, 1710 (sh), 1650 cm~l; MS (FAB) m/z 1066 (M+K).
Anal. calcd. for CsgHglNO14-0.7 H2O: C, 68.08; H, 7.98; N, 1.35. Found: C,
68.11; H, 7.88; N, 1.51.
Example81: FormulaI: R=ethyl:n=1:R 2a_3-R5-H R4-oH Rla-ocH~ Rl_
-OCH2C(O)OH (R-Confi,~uration).
The resultant product of Example 80 (5.10 g, 5 mmol) was dissolved in CH2C12 (45 mL),
whereupon piperidine (5 ml) was added. The solution was stirred at room temperature for 2
hours then transferred to a s~al~loly funnel, diluted with additional CH2Cl2 (100 mL), then
washed with lN HCl (2 x 100 mL) and brine (2 x 100 mL). The organic layer was dried
(Na2S04), filtered, and the solvent removed in vacuo to give 5.08 g of a Il~ e of the title
compound and N-(9-fluorenylmethyl)piperidine. MS (FAB) mtz 888 (M+K), 926 (M+2K-H).
Example 82: Formula I: R= ethyl: n= 1: R2_2a__R5-H: R4-oH: Rla-OCH~: Rl_
-OCH~C(O)R 12 (R-Confi~uration): R 12_ NR--_--R15- -CH~CH~C6H~.
The product of Example 81 (100 mg, .118 mmol) was dissolved in dichloromethane (1 mL)
and the solution cooled to 0 C. HOBT-H20 (21.6 mg, .142 mmol) was added followed by
EDAC (27.1 mg, .142 mmol) then phenethylamine (26.7 ,uL, .212 mmol). The reaction was
warmed to room ~tlllp~ e and stirred overnight. Dichloromethane (10 mL) was added
and the organic phase washed with lN HCl (2 x 20 mL), saturated bicarbonate solution (2 x
20 mL), and then brine (2 x 20 mL). The organic layer was dried (Na2SO4), filtered, and
solvent removed in vacuo to give 87.5 mg yellow foam. The crude product was purified by
HPLC (20 x 300 rnm silica column) eluting with 2: 1 hexane/acetone. Fractions containing
product were combined and solvent removed in vacuo to give the title compound (49.6 mg,
44% yield) as a white solid: 93-105 C (mp); IR (KBr) 3435, 1740, 1700, 1650 cm~l; MS
(FAB) m/z 953 (M+H), 991 (M+K).
Anal. calcd. for C53H80N2ol3: C, 66.78; H, 8.46; N, 2.94. Found: C, 67.13; H,
8.33; N, 3.04.
- 95-
WO 94/21642 PCT/US94/02692 ~
'2,~S6~6S
Example 83: Formula I: R= ethyl: n= 1: R2_2a-R3_5-H: R--OH: Rla-OCH~: Rl-
-OCH~C(O)R12 (R-Configuration): R12_NR--_--: R14- CH~: R15- -CH~CH C_H~
Example 82 was repeated substituting N,N-methyl, 2-phenylethyl amine for
2-phenylethylamine to provide the title compound. MS (FAB) m/z: M+K = 1005.
Fxample 84: Formula I: R= ethyl: n= 1 ~35-H: R4-oH: Rla-OCH~: Ri_
-OCH~C(O)R12 (R-Configuration): R1~ -HN(CH_)5NH-dansyl.
The title compound was synthesized in the manner described for Example 82 substituting
dansyl cadaverine for 2-phenylethylamine. IR (KBr) 3420, 1740, 1700, 1645 cm~l; MS
(FAB) m/z 1205 (M+K).
Anal. calcd. for C62H94N4O15S: C, 63.78; H, 8.12; N, 4.80. Found: C, 63.43; H,
8.25; N, 4.48.
Example85: FormulaI: R=ethyl: n= 1: R---R2a-R--R--H: R4-oH: Rla-OCH3: Rl_
-OCH~C(O)R12 (R-Configuration): R12--HNC_H~.
The title compound was synthesized in the manner described for Example 82 substituting
aniline for 2-phenylethylamine. 112-120 C (mp); IR (KBr) 3440, 3400 (sh), 3300 (sh),
1740, 1700, 1650, 1540, 1500 cm~l; MS (FAB) m/z 963 (M+K).
Anal. calcd. for C51H76N2O13: C, 66.21; H, 8.28; N, 3.03. Found: C, 66.11; H,
8.15; N, 3.23.
Example 86: Formula I: R= ethyl: n= 1: R--R2a-R3-R5. H: R4-oH: Rla-OCH~: R1_
-OCH~C(O)R12 (R-Configuration): R12- -HN(CH~)~N(CH~CH~)~O.
The title compound was synthesized in the manner described for Example 5 substituting 2-(4-
morpholino)-ethylamine for N-methylbenzylamine. MS (FAB) m/z: M+H = 962.
Example 87: Formula I: R= ethyl: n= 1: R--R2a-R3--R--H: R4-oH: Rla--OCH~: Rl_
-OCH~C(O)R12 (R-Confi~uration): R12- -HN(CH~)~N(CH~CH~ O.
The title compound was synthesized in the manner described for Example 82 substituting
3-(4-morpholino)-propylamine for 2-phenylethylamine. MS (FAB) m/z: M+K = 1014.
Example 88: Formula I: R= ethyl: n= 1: R-- 2a-R3-R5-H: R4-oH: Rla-OCH~: Rl_
-OCH~C(O)R12 (R-Configuration): R12--HN(CH~)~N(CH~
The title compound was synthesi7ed in the manner described for Example S sub~ u~ing
2-dimethylamino-ethylamine for N-methylbenzylamine. MS (FAB) m/z: M+H = 920.
Fxample 89: FormulaI: R=ethyl: n= 1: R 2a=~3__H R4-oH: Rla-OCH~: Rl_
-OCH~C(O)R12 (R-Con~lguration): R12--HN(CH~)~N(CH~
- 96-
WO 94/21642 2 1 5 ~ ~ 6 5 PCT/US94/02692
The title compound was synthesi7ed in the manner described for Example 82 substituting
3-dimethylamino-propylamine for 2-phenylethylamine. MS (FAB) m/z: M+H = 934.
Example 90: Formula I: R= ethyl: n= 1: R2-R~R3-R5-H: R4-oH: Rla-OCH3: Rl-
'' -OCH~,C(O)R12 (R-Configuration): R12- (S)-HNCH(CH2C5H5)CO?CH?Ph.
The title compound was synthesi7ed in the manner described for Example 82 substituting
L-phenyl~l~nine benzylester for 2-phenylethylamine. MS (FAB) m/z 1125 (M+K).
Example 91: Formula I: R= ethyl: n= 1: R2-R~-R3_5-H: R4-oH: R1a-OCH3: Rl_
-OCH2C(O)R12 (R-Configuration): R12- (S)-HNCH(CH~C6H5)CO~H.
The title compound was synthesized in the manner described in Example 3 substituting the
product from Example 90 for the product from Example 2. IR (CDC13) 1740, 1700(sh),
1645 cm~1; MS (FAB) m/z 1035 (M+K).
Example 92: Formula I: R= ethyl: n= 1: R~R~-R3-R5-H: R-=OH: R-la-OCH~: R1_
-OCH2C(O)R12 (R-Configuration): R12- (R)-HNCH(CH~CH5)CO~,CH2Ph.
The title compound is synthesi7e~ in the manner described for Example 82 substituting
D-phenylalanine benzylester for 2-phenylethylamine.
Example 93: Formula I: R= ethyl: n= 1: R~R~ 3-R5-H: R--OH: R1a-OCH3: R1_
-OCH~C(O)R12 (R-Configuration): R12- (R)-HNCH(CH2C6Hs)CO2H.
The title compound is synthesized in the manner described in Example 3 substituting the
product from Example 92 for the product from Example 2.
Example 94: Formula I: R= ethyl: n= 1: R2_~R3_5 H: R4-oH: R1a-OCH~: R1_
-OCH~C(O)R12 (R-Configuration): R12---HN(CH2)~SH.
The product of Example 3 (1.2 g, 1.4 mmol) was dissolved in THF (4.5 mL) and the solution
cooled to 0 C before adding N-methylmorpholine (155.1 uL, 1.4 mmol) followed byisobutyl chloroformate (122.2 uL, 1.4 mmol). The resulting suspension was stirred for 20
minutes at 0 C then 2-aminoethanethiol hydrochloride(320.8 mg, 2.8 mmol) was added.
The n~i~sL~ e was stiIred for 3 h at room temperature before addition of more N-methylmorpholine (387.8 uL, 3.5 mmol). The reaction was stirred overnight, loaded onto
silica (40 mL) in a fritted funnel, then eluted with dichloromethane (100 mL), 1: 1
hexane/acetone (200 mL), followed by acetone (100 mL). Fractions cont~ining product were
combined and solvent removed in vacuo to give 0.83 g yellow foam. The crude product was
further purified by HPLC (30 x 300 mm silica column) eluting with 1.25: 1 hexane/acetone to
provide the title compound (320 mg, 25% yield) as a white foam. MS (FAB)m/z:
(M+K)=947.
- 97-
Wo 94/~ 56~ PCT/US94/02692
Example 95: Formula I: R= ethyl: n= 1: R2-R~R3-R~-H: R--OH: R-l a-OCH3: R 1_
-OCH2C(O)R12 (R-Configuration): R12- -HN(CH~)3SH.1
The title compound is synthesi7ed in the manner described f'or Example 94 substituting
3-amino-propanethiol for 2-aminoeth~nethiol. e
Example 96: Formula I: R= ethyl: n= 1: R~R~R3-R5-H. R--QH: Rla-OCH3: Rl-
-OCH2C(O)OC2Hs (R-Configuration).
Silver (I) oxide (926 mg, 4.0 mmol) was added to ascomycin (791 mg, 1.0 mmol) dissolved
in acetonitrile (0.8 mL) and ethyl iodoacetate (828 ,uL, 7.0 mmol). Mixture was stirred at
room ~enlpcla~ulc for 3 days, removed volatiles in vacuo, and isolated product by
chromatography on silica gel as described in Example 1. Spectral data were identical to
those obtained for the product of Example 1.
Example 97: Formula I: R= ethyl: n= 1: R2-R2a-R3-R~H: R_=OH: R~OCH3: Rl_
-OCH~C(O)R12 (R-Configuration): R12- NR__15 R14- H: R-15- 2-naphthyl
The product of Example 3 is activated as in Example S and then treated with
2-naphthylamine instead of N-methylbenzylamine to give the title compound.
Example 98: Formula I: R= ethyl n= 1 R2-R2a-R3-H: Rla_ocH3 Rl_
-OCH~C(O)OCH~Ph (R-Configuration): R- and R5 taken together form a bond.
The product of Example 2 (4.7g, 5.0 mmol) was dissolved in toluene (7.5 rnL) andtriethylamine (5 mL). At 0 C methanesulfonic anhydride (2.58g, 15.0 mmol) and 4-
dimethylamino-pyridine (180mg, 1.5 mmol) were added all at once. The reaction ~ Lule
was then stirred at ambient temperature for 18h, concenL.~led to constant weight invacuo, and
then filtered rem~ining residue through silica gel (70-230 mesh, 40 mL) eluting with hexane
(1: 1) until no more desired product eluted. The eluent was concentrated to dryness and
further purified by HPLC on a 30xSOOmrn column (230-400 mesh SiO2), eluting withhexane:acetone (4: 1). Fractions cont~ining desired product were pooled and concentrated
invacuo to provide the title compound (2.2 g). MS (FAB)m/z: (M+K)=960.
Example 99: Formula I: R= ethyl: n= 1: R2-R~-R3-R--R5-H: Rla-OCH3: Rl_
-OCH2C~O)OH (R-Configuration).
The product of Example 98 (2.2g, 2.4 mmol) was dissolved in EtOH (150 mL), to which was
added 10% palladium on carbon (0.22g). After sh~king under hydrogen (1 atm) atmosphere
for S hours, the catalyst was filtered from the Il~ixLule and the solution was concentrated
under reduced pressure. The crude m~t~ l is purified by chromatography as follows. A
coarse fritted 350 mL Buchner funnel was charged with 230-400 mesh SiO2 (175 rnL). The
- 98 -
~ wo g4,2l642 2 1 5 6 0 6 ~ ~/US~4l02692
silica bed was wetted with CH2Cl2:i-PrOH (10:1 with 0.5% AcOH), and tamped to constant
volume, whereupon a circle of filter paper was placed over the bed. A solution of the crude
product in CH2C12:i-PrOH (10:1 with 0.5% AcOH) was then carefully loaded to the top of
the column. The pad was eluted with lL of the same collecting 50 mL fractions throughout.
Thç title product eluted in fractions 4 and 5, which concentrated down to 924 mg. MS (FAB)
m/z: M+K = 872.
Example 100: Formula I: R= ethvl: n= 1: R 2a_3-R4_5-H: Rla-OCH~,: Rl_
-OCH~C(O)R12 (R-Confi,~uration): R12-NR14R15~ where R--and R15 are taken to,eether
as the diradical: -CH~CH2OCH2CH2-.
The product of example 99 is activated as in example 5 and treated with morpholine instead
of N-methylbenzylamine to give the title compound.
Example 101: Formula I: R= ethyl: n= 1: R 2a-R3_5--H: R--OH; Rla=OCH~: Rl_
-OCH?C(O)Rl2 (R-Configuration): R12- NR--_--R14-H: R15- 4-(H~NSO~)-phenyl-.
The product of Example 3 is activated as in Example 5 and then treated with sulfanilamide
instead of N-methylbenzylamine to give the title compound.
Example 102: Formula I: R= ethyl: n= l: R2__=R3_5-H: R4-oH: Rla-OCH3: Rl-
-OCH~C(O)R12 (R-Configuration): R12- NR--_--R--and R15 are taken to,~ether as the
followin~ diradical: -CH2CH~N(CH~CH20H)CH~CH2-.
The product of Example 3 is activated as in Example 5 and then treated with
N-(2-hydroxyethyl)-piperazine instead of N-methylbenzylamine to give the title compound.
Example 103: Formula I: R= ethyl: n= 1: R-- 2a-R3---R5-H: R4-oH: Rla-OCH3: Rl-
-OCH2C(O)R12 (R-Confi~uration): R12____~ R14 =CH3: R15-phenyl.
The product of Example 3 was activated as in Example 5 and then treated with N-
methylaniline instead of N-methylbenzylamine to give the title compound. MS (FAB) m/z:
M+K = 977.
Example 104: Formula I: R= ethyl~ n= 1: R 2a-R3_5-H: R4-oH: Rla=OCH~: R-l_
-OCH2C(O)R12 (R-Confi~uration): R 12_ NR__--~ R--= R15 -CH~CH~OH.
The product of Example 3 was activated as in Example 5 and then treated with N,N-bis-
(2-hydroxyethyl)-amine instead of N-methylbenzylamine to give the title compound. MS
(FAB) m/z: M+K = 975.
99
WO g4/21642 PCT/US94/02692 ~
~,$~9G~
Ex~m~le 105:?Formula I: R= ethyl: n= 1: R2--R2a~3-R--H: R4-oH: Rla-OCH~: Rl_
-OCH~C(O)R12 (R-Configuration): R12- NR--R--R14-CH~ R~
-CE~CH ~CH ~N(CH~
The product of Example 3 was activated as in Example 5 and then treated with N,N'-methyl-
(3-dimethylaminopropyl)-amine instead of N-methylbenzylamine to give the title compound.
MS (FAB) m/z: M+H = 948.
~3xample 106: Formula I: R= ethyl: n= 1: R =~kR3-R--H: R4-oH: Rla-OCH~: Rl_
-OCH~C(O)R12 (R-Configuration): R12- NR--_--~ R14~henvl R15 =-CH~CH~CH~OH.
The product of Example 3 was activated as in Example 5 and then treated with N,N-
phenyl-(3-hydroxypropyl)-amine instead of N-methylbenzylamine to give the title
compound. MS (FAB) m/z: M+K = 1021.
Example 107: Pormula I: R= ethyl: n= 1: R-- 2a-R--R--H: R4-oH: Rla-OCH3: R1_
-OCH~C(O)R12 (R-Configuration) R1 NR--_--R14_H RlS_ CH(CH~OH)~
The product of Example 3 was activated as in Example 5 and then treated with serinol instead
of N-methylbenzylamine to give the title compound. MS (FAB) m/z: M+K = 961.
F.xample 108: Formula I: R= ethyl: n= 1: R--R2a-R3-R5-H: R4-oH: Rla-OCH3: R1_
-OCH~C(O)R12 (R-Configuration): R12- NR--_--~ Rl---H: R15--3-(CF~)-phenyl.
The product of Example 3 was activated as in Example 5 and then treated with 3-
trifluoromethylaniline instead of N-methylbenzylamine to ~ive the title compound. MS
(FAB) m/z: M+K = 1031.
F.xample 109: Formula I: R= ethyl: n= 1: R2_2a-R_R--H: R4-oH: Rla-OCH~: Rl_
-OCH~C(O)R12(R-Configuration):R12-NR--_--R14_15-CH~CN
The product of Example 3 was activated as in Example 5 and then treated with
iminodiacetonitrile instead of N-methylbenzylamine to give the title compound. MS (FAB)
m/z: M+K = 965.
Example 110: Formula I: R= ethyl: n= 1: R--R~-R--R5-H: R4-oH: Rla-OCH~: Rl_
-OCH~C(O)R12 (R-Configuration): R12-NR--_--: R--andRlSaretakentogetherasthe
followin~ diradical: -CH~CH~-.
The product of Example 3 was activated as in Example 5 and then treated with aziridine
instead of N-methylbenzylamine to give the title compound. MS (FAB) m/z: M+K = 913.
- 100-
~ WO 94/21642 21 5 B ~ ~ S PCT/US94/02692
Fxample 111: FormulaT: R=ethyl: n= 1: R2-R2a-R3_--H: R4-oH: Rla-OCH3: Rl_
-OCH~NH(CO)NR--R15 (R-Confiyuration): R--and R15 are taken together as the
following diradical: -CH~CH~OCH7CH2-.
The product of Example 3 (0.5g, 0.58 mmol) in THF(6 mL) was stirred together with N-
methylpiperidine (146 uL, 1.2 mmol) and diphenylphosphorylazide (258 uL, 1.2 mmol) at
ambient le"lp~ ture for S n~ ules, then at reflux for 3h. The stirring solution was cooled to
ambient temperature and treated with morpholine (157 uL, 1.8 mmol) for 60h. The Il~ixlule
was purified by HPLC on a column 20 x 300 mm (YMC l5u, 60 ~ spherical SiO2) eluting
with a step gradient of hexane:acetone (1:1) then hexane:acetone (2:3), to provide 440 mg
(0.47 mmol) of pure titlecompound. MS (FAB)m/z: (M+K)=972.
Example 112: Formula I: R= ethyl: n= 1: R--R2a-R---R---H: R4-oH: Rla-OCH~: Rl_
-OCH~NH(CO)NR--B15 (R-Configuration): R--=H; R15-phenyl.
The product of Example 3 was activated as in Example 111 and then treated with aniline
instead of morpholine to give the title compound. MS (FAB) m/z: M+K = 978.
Example 113: Formula I: R= ethyl: n= 1: R2-R2a_3-R--H: R4-oH: R~OCH~: Rl_
-oCH~NHC(o)R14 (R-Configuration): R14-,phenyl.
The product of Example 3 is activated as in Example 111 and then treated with benzoic acid
instead of morpholine, wl,elt;u~on the ~ c is heated. Purification by chromatography on
silica gel provides the title compound.
Example 114: Formula I: R= ethyl: n= 1: R2-R2a-R--R--H: R--OH: R-la-OCH~: Rl_
-OCH~C(O)OCH~C6H~ Configuration).
Foamed ascomycin (50g, 63 mmol, crystalline m~ten~l completely dissolved in methylene
chloride then concentrated to a dry foam) and benzyl iodoacetate (104g, 378 mmol, 6 eq)
were mixed together, then dissolved in ac~ul~ ile (24 rnL) by stirring with an overhead
mixer until homogeneous. The solution was cooled to 0 C whereupon Ag2O (58.4g, 252
mmol, 4 eq) was added portionwise over 15 rninutes (ca. 15 additions). After complete
addition and mixing (5 minl1tes after last addition), the ice bath was removed and the reaction
allowed to stir at ambient temperature for 7 days. Diethyl ether (125 mL) was added to the
reaction mixture and this was then poured over silica gel (70-230 mesh, 400 rnL), mixed and
allowed to air dry over night. A 3L coarse fritted Buchner funnel was charged with silica gel
(70-230 mesh, 2L) and the adsorbed silica carefully layered over the fresh bed, followed by a
filter paper disk. The column was eluted with CH2cl2 (4L), CH2cl2:cH3cN (9: 1, 10L),
CH2Cl2:CH3CN (3:1, 8L), CH2Cl2:CH3CN (1:1, 2L), and acetone (4L), collecting lL
fractions throughout. Desired product eluted in fractions 14-20 to provide a compound that
was identical to the title product of Example 2 (25.6g, 27.3 mmol) as a pale yellow foam.
- 101 -
WO g4/21642 ,~56~6$ PCT/US94/02692
Example 115: Formula I: R= ethyl: n= 1: R2_~L-R3_~-H: R4-oH: Rla-OCH~: Rl-
-oCH~NH(Co)NR14Rl$ (R-Configuration): R 14 =H: R15--CH2CH2CH;~OH.
The product of Example 3 was activated as in Example 111 and then treated with 3-
amino~ru~dllol instead of morpholine to give the title compound. MS (FAB) m/z: M+K.=
960.
Example 116: FormulaI: R=ethyl: n= 1: R2_~R3_5-H: R4-oH: R1a-OCH3: Rl-
OCH~C(O)R12(R-Configuration):R12-NR--_1~14-H R15 6-
carbomethoxymethylmercal)lo~ ine hydrazid-yl
The product of Example 3 was activated as in Example 5 and then treated with 6-
carbomethoxymethylmelca~ol,u,ine hydrazide instead of N-methylbenzylamine to give the
title compound.
Example 117: FormulaI: R= ethyl: n= 1: R2-R2a-R3-R5-H: R4-oH: Rla-OCH~; Rl-
OCH~C(O)R12 (R-Configuration): R~NR__1~ R--and R15 are taken to~ether as the
following diradical: -CH2CH?SO2CH~CH2-
The product of Example 3 was activated as in Example 5 and then treated with
thiomorpholine sulfone instead of N-methylbenzylamine to give the title compound. MS
(FAB) m/z: M+K = 1005.
Example 118: Formulal: R= ethyl: n= 1: R2-R2a-R3-R5-H: R--OH: Rla-OCH3: Rls
OCH~C(O)R12 (R-Configuration): R12-NR__--R14-H: R15-CH~CH;2-(4-F-phenyl)
The product of Example 3 was activated as in Example 5 and then treated with 4-
fluorophenethylamine instead of N-methylbenzylamine to give the title compound. MS
(FAB) m/z: M+K = 1009.
Example 119: FormulaI: R= ethyl: n= 1: R~-R2a-R3-R5-H. R--OH: Rla-OCH3: Rl-
OCH?C(O)R12(R-Configuration):R12=NR--_--R14-H:R~5-~-Cl-phenyl
The product of Example 3 was activated as in Example 5 and then treated with 4-
chloroaniline instead of N-methylbenzylamine to give the title compound. MS (FAB) m/z:
M+K= 997.
Example 120: FormulaI: R= ethyl: n= 1: R2-R2a-R3_5-H. R--OH: Rla-OCH~: R~
OCH2C(O)R12 (R-Configuration): R12-NR__15 R14--H: R-15-4-(OCH3)-phenyl
The product of Example 3 was activated as in Example 5 and then treated with 4-
methoxyaniline instead of N-methylbenzylamine to give the title compound. MS (FAB) m/z:
M+K = 993.
- 102-
21~6~
WO 94/21642 PCT/US94/02692
Example 121: FormulaI: R= ethyl: n= 1: R-2_2a-R3-R5-H~ R4-oH: R la-OCH3: R 1-
OCH~C(O)R12 (R-Configuration) R12 __15 R14=~15_~ CH~ phenyl
A solution of p-toluidine (1.03 g, 9.6 mmol) in dry THF (1 mL) was added dropwise to a
stirred solution of ethylmagnesium bromide (9.6 mmol) in dry THF (9.6 mL) at 0C. The
mi~ c was stirred for 15 minlltes then cooled to -78 C before the addition of a solution of
the product of Example 114 (750 mg, 0.8 mmol) in dry THF (2 mL). The ~ Lulc was stirred
for 1 hour then added dropwise to a stirring biphasic mi~ c of lN HCl (75 mL) and EtOAc
(75 mL). The Illi~UlC was transferred to a sep~ y funnel, the organic layer washed with
lN HCl (75 mL), saturated NaHCO3 solution (2 x 75 mL) and brine (2 x 75 mL), dried
(Na2SO4), filtered, and concentrated in vacuo to give 0.93 g yellow oil. The crude m~t~
was purified by chromatography to give 385 mg of the title compound as a white foam. MS
(FAB) m/z: M+K = 977.
Example 122: FormulaI: R= ethyl: n= 1: R~R~L 3-R5-H: R4-oH: Rla-OCH~: Rl-
OCH~C(O)R12 (R-Configuration): R1 NR__15 R14-H: R15-3~4-Cl~-phenyl
The product of Example 114 was treated as in Example 121 using 3,4-dichloroaniline instead
of p-toluidine to give the title compound. MS (FAB) m/z: M+K = 1031.
Example 123: FormulaI: R= ethyl: n= 1: R~ ~a-R3-R5-H. R4-oH: R~OCH3: Rl-
OCH2C(O)R12(R-Confi~uration): R12- __--R14-H: R15-3-I-phenyl
The product of Example 114 was treated as in Example 121 using 3-iodoaniline instead of p-
toluidine to give the title compound. MS (FAB) m/z: M+K = 1088.
Example 124: FormulaI: R= ethyl: n= 1: R~-R2~R3_5-H: R4-oH: Rla-OCH3: R-l-
OCH~C(O)R12 (R-Confi~uration): R12-0-CH~-r(lR)-(+)-alpha-pinen-10-yl)l
(a) A three-neck 2L roundbottom flask equipped with an overhead stirrer was charged
with diethylether (800 mL), chloroacetyl chloride (40 mL, 0.5 mol) and (lR)-(-)-nopol (85.3
mL, 0.5 mol). At 0 C, triethylamine (69.5 mL, 0.5 mol) was added dropwise over 15
minutes. After stirring at 0 C for 1 hour, the n~ Lulc was warmed to ambient temperature
and stirred for 18h. The ~Ili;S~lllC was vacuum filtered through a Buchner funnel and the
white cake was extracted with ether (2 x 200 mL). The filtrates were then washedsequentially with 0.5N HCl (500 mL), water (500 mL) and brine (500 mL). After drying the
organics (Na2SO4), the mixture was filtered and conGenL~ated to a lite tan oil (104 g). The
resultant nopol chloroacetate was sufficiently pure to process in the next step.(b) Sodium Iodide (20.1 g, 134 mmol) was refluxed in acetone (55 mL) for 5 minutes
and cooled to room temperature. Nopol chloroacetate from step (a) (5.85 g, 24.17 mmol)
was added and the reaction was stirred for 30 minutes. The solvent was removed in vacuo
- 103-
wo 94/21642 ~ ~ 5 1~ a ~ 5 PCT/US94102692 ~
and the resultin~ slurry was partitioned belwcen water (30 mL) and ethyl acetate (20 mL).
The aqueous portion was extracted with additional ethyl acetate (20 mL) and the combined
organics were washed sequentially with sa~ ted sodium bicarbonate (30 mL) and 10%
sodium bisulfite (30 mL); dried (sodium sulfate) and concenl,~ted in vacuo to an amber oil (7
g). The resulting nopol iodoacetate was sufficiently pure to us~e in the next step.
(c) Ascomycin (2.5 g, 3.16 mmol) was foamed in a round bottom flask (See Example114). To it was added the nopol iodoacetate from step (b) (5.70 g, 17.1 mmol, 5.4 eq)
followed by ace~onillile (1.5 mL). After a homogeneous solution was obtained, it was cooled
to 0 C and silver(I) oxide (3.13 g, 13.4 mmol) was added portionwise (15 min). The
solution was brought to room temperature by gradual melting of the ice and was stirred for 8
days. The reaction was diluted in diethyl ether and poured onto silica gel (70-230 mesh, 20
mL) and allowed to air dry. The adsorbed silica was layered on fresh silica (70-230 mesh,
100 mL) and eluted with methylene chloride (150 mL); methylene chlonde:acetonitrile (9:1,
450 mL); (3: 1, 300 mL); (1: 1, 200 mL); acetone (200 mL). 50 mL fractions were collected.
Fractions 11-17 contained desired crude product which was further purified by HPLC on
silica eluting with 3:1 hexane:acetone (3:1). Isolated pure title compound (0.4 g). MS (FAB)
m/z: M+K = 1036.
Example 125: FormulaT: R= ethyl: n= 1: R~R2a-R3-R5-H: R--OSi(CH~)~: Rla-OCH~:
R 1 --OCH2C(O)R 12 (R-Configuration): R 12-O-CH?-r( lR)-(+)-a-pinen- 10-yl)l
The product of example 124 (0.200 g, 0.201 mmol) was dissolved in dry DMF (2 mL).
Imidazole (0.054 g, 0.80 mmol) was added. TMS-Cl (0.051 mL, 0.401 mmol) was added and
the reaction was stirred at room temperature overnight. The reaction llli~lllC was partitioned
between water (10 mL) and ethyl acetate (20 mL). The organics were washed with water (10
mL), brine (20 mL) dried (sodium sulfate) and concentrated to a ~aint yellow film (0.225 g).
MS (FAB) m/z: M+K= 1108.
Example 126: Formnl~T: R= ethyl: n= 1: R2-R2a-R3-H: R_ and R5 taken to~ether form a
bond: Rla-OCH3: Rl--OCH2C(O)R12 (R-Con~l~uration): R~(morpholinyl)
Morpholine (0.104 mL, 1 g/mL, 1.19 mmol) was dissolved in acelonillile (1.5 mL,
dried over sieves). Trimethyl ~1~.".;"1..~ (0.60 mL of a 6 M solution in hexanes, 1.2 mmol)
was added and the solution was stirred at room temperature for 5 min~ltes, then cooled to
0 C. A solution of the product from Example 125 (0.225 g, 0.210 mmol) in acetonitrile
(1 mL, dried over sieves) was added dropwise and the reaction was stirred for 3 hours. The
reaction n)i~UlC was diluted in ethyl acetate (15 mL) and HCl (0.25 N, 15 mL). A thick
white gel formed at the interface. The organics were washed with water (15 mL) and the
aqueous portion was extracted with additional ethyl acetate (15 mL). The combined organics
were washed with brine (15 mL), dried (Na2SO4), and concentrated to a yellow oil which
- 104-
~ wo g4t2l642 2 1 5 6 0 6 ~ ~S94/02692
was purified by HPLC on silica gel and eluted with hexane:acetone (1.5: 1). Yielded title
compound: 34 mg, 17%; MS (FAB) m/z: M+K = 939. Yield product of example 29: 42
mg,22%.
Example 127: FormulaI: R= ethyl: n= 1: R2_3-R5-H: R~R4 =OH: Rla-OCH~ Rl-
OCH2C(O)R 12 (R-Configuration): R 12--NH-(3-fluoro-phenyl)
The product of example 72 (1.0 g, 1.06 mmol) in THF (10 mL) and water (1 mL) wastreated with sel~.nillm dioxide (0.18 g, 1.59 mmol) and t-butylhydrop~;roxide (1.4 mL of a 3M
solution in 2,2,4-trimethylpentane, 4.24 mmol). The n~ix lur~ was stirred at ambient
temperature for 4 days, whereupon additional t-butylhydroperoxide solution (1.4 mL, 4.24
mmol) was added. After 2 days the reaction mixture was heated to 40 C for 24h, then 70 C
for 48h. The solution was concentrated invacuo and purified by HPLC on silica gel eluting
with hexane:acetone (2:1). Yielded title compound: 0.18 g, 18%. MS (ESI) m/z: M+Na =
963.
Example 128: FormulaI: R= ethyl: n= 1: R2-R2a-R3-R$ H: R4-oH: Rla-OCH3: R~
OCH~C(O)R12 (R-Configuration): R12-O-CH2-r4-nitrophenyll
(a) A three-neck 2L roundbottom flask equipped with an overhead stirrer was charged
with diethylether (800 mL), chloroacetyl chloride (40 mL, 0.5 mol) and 4-nitrobenzylalcohol
(76.5 g, 0.5 mol). At 0 C, triethylamine (69.5 mL, 0.5 mol) was added dropwise over 15
minutes. After stirring at 0 C for 1 hour, the n~LulG was warmed to ambient temperature
and stirred for 18h. The Illi~Ule was vacuum filtered through a Buchner funnel and the
white cake was extracted with ether (2 x 200 mL). The filtrates were then washedsequentially with 0.5N HCl (500 mL), water (500 mL) and brine (500 mL). After drying the
organics (Na2S04), the n~ib~ule was filtered and concellll~t~d to a lite tan solid (74.8 g). The
crude product was le~,ly~lli7ed from diethylether (71-72 C)
(b) Sodium Iodide (39.5 g, 260 mmol) was refluxed in acetone (104 mL) for 3
minutes and cooled to room temperature. 4-Nitrobenzyl chloro~cet~te from step (a) (10.7 g,
47 mmol) was added and the reaction was stirred for 30 minlltes. The solvent was removed
in vacuo and the resulting slurry was partitioned between water (50 mL) and ethyl acetate (50
mL). The aqueous portion was extracted with ~d~iti-)n~l ethyl acetate (50 mL) and the
combined organics were washed sequentially with saturated sodium bisulfite (2 x 50 mL) and
brine (50 mL). The organics were dned (sodium sulfate) and concentrated in vacuo to pure
product (15.6 g).
(c) Ascomycin (5 g, 6.3 mmol) was foamed in a round bottom flask (See Example
114). To it was added the 4-nitrobenzyl iodoacetate from step (b) (15.6 g, 48.6 mmol, 7.7 eq)
followed by acetonitrile (2.5 mL). After a homogeneous solution was obtained, it was cooled
to 0 C and silver(I) oxide (5.9 g, 25.6 mmol) was added portionwise (15 min). The solution
- 105-
2~6a~S , ,
WO94t21642 PCT/US94/02692
was brought to room le~ ature by gradual melting of the ice and was stirred for 5 days.
The reaction was diluted in diethyl ether (25 mL), poured onto silica gel (70-230 mesh, 40
mL) and allowed to air dry. The adsorbed silica was layered on fresh silica (70-230 mesh,
200 mL) and eluted with methylene chloride (500 mL); methylene chloride:acetonitrile (9:1,
400 mL); (6:1, 300 mL); (3:1, 1000 mL); (1:1, 500 mL); (1:2, 300 mL). 100 mL fractions
were collected. Fractions cont~inin~ desired product (CH2C12:CH3CN 3:1) were pooled and
conce~ led invacuo to provide the title compound (2.86 g, 2.9 mmol). Ascomycin was
recovered in the later fractions (1.59 g, 2.0 mmol). MS (ES~).m/z: M+Na = 1007.
Example 129: Formula I: R= ethyl: n= 1: R2-R2a-R3-R5-H: R4-oH: Rla-OCH~: Rl_
-OCH2C(O)R12 ~R-Confi~uration): R12- -NR__15 R14--(CH?~N(CH~CH~)70: R15_
CH~CH~OH.
The title compound was syntheci7ecl in the manner described for Example S substituting
N,N-r2-hydroxyethyl] r2-(4-morpholino)-ethyl]amine for N-methylbenzylamine. MS (FAB)
m/z: M+H = 962.
Example 130: Formula I: R= ethyl: n= 1: R;~RZ~R3_5-H; R4-QH: Rla-OCH~; Rl_
-OCH2C(O)R12 (R-Confi~uration): R12--OCH2CCl~.
Example 128 was repeated substituting 2,2,2-trichloroethanol for p-nitrobenzylalcohol.
Example 131: In Vivo Assay of Biolo~ical Activity
The immunosuppressant activity of the compounds of the present invention was
determined using the human mixed lymphocyte reaction (MLR) assay described by Kino, T.
et al. in Transplantation Proceedings, XIX(5):36-39, Suppl. 6 (1987). The results of the
assay, shown below in Table 1, demonstrate that the compounds tested are effective
immunomodulators at sub-micromolar and, in some instances, sub-nanomolar concentrations.
- 106-
~ WO 94/21642 2I 5 60 6S PCT/US94/02692
Table 1
Ex. ~IC50 (M)
0.9 x 10-9
27.6 x 10-9
3140.0 x 10-9
40.5x 10-9
6O.lxlO-9
290.7 x 10-9
311.1 x 10-9
39172.6 x 10-9
510.3 x 10-9
530.2 x 10-9
540.02 x 10-9
560.01 x 10-9
570.10 x 10-9
580.15 x 10-9
590.05 x 10-9
710.1 x 10-9
720.08 x 10-9
730.04 x 10-9
740.06 x 10-9
820.3 x 10-9
830.4x 10-9
850.5 x 10-9
871.1 x 10-9
88O.lxlO-9
890.5 x 10-9
990.9 x 10-9
1111.8 x 10-9
112O.lxlO-9
It is understood that the foregoing detailed description and accompanying examples
are merely illustrative and are not to be taken as limit~tiQns upon the scope of the invention,
which is defined solely by the appended claims and equivalents thereof. Variations and
modifications of the disclosed embor~imentc will be a~pal~nt to those skilled in the art. Such
variations and modifications, including without limitation those relating to the chemical
structures, substituents, derivatives, intermediates, syntheses, form~ tions and/or methods of
use of the invention, may be made without departing from the spirit and scope thereof.
- 107-