Note: Descriptions are shown in the official language in which they were submitted.
W 0 96117871 PGT/US95/15171
~" ;ir'ty ~
TITLE OF TF.E INVENTION
PLASMA CONCENTRATE ANC
TISSUE SEALANT COMPOSITIONS
BACKGROUND OF THE INVENTION
Field of the Invention:
The present invention concerns compositions, methods and
apparatuses for making concentrated plasma and tissue sealant,
for sealing tissue, for rapid separation of higher molecular
weight components in mixtures, for rapid separation of blood
plasma and for rapid separation of particulate components in
mixtures. The present invention is particularly applicable to .
preparation and use of autologous tissue sealant.
Discussion of the Baekcxround:
Fibrin Glues aad Sealaats
Various substances have been tried to meet the need for-a
suitable tissue adhesive for use in surgical procedures.
Comple=ely synthetic materials such as cyanoacrylate have been
tried and keen found wanting. Natural fibrin glues and sealants
~ made from blood components were recognized early in the
development of this technology. Surgical "fibrin sealants"
(sometimes called "fibrin glues") made up of human fibrinogen
activated by bovine thrombin are used extensively in Europe.
WO 96117871 , PCTIU595115171
z I ~z~~z
-2-
Such fibrin sealants have been shown to be superior to synthetic
adhesives or traditional surgery in many situations. In addition
they reduce the need for blood transfusions. Academic and
surgical opinion on fibrin sealants is very favorable. A recent
review says:
Fibrin sealants are the most successful tissue adhesives to
date. They have many advantages over adhesive technologies
such as cyanoacrylates and marine adhesives in terms of
biocompatibility, biodegradation and hemostasis. There are
several-commercial products in Europe but none in the United
States due to the current regulatory stance against pooled
plasma blood products (Sierra, 1993; vide infra).
In current practice, fibrin sealant is made by isolating a
concentrate of human fibrinogen, fibronectin and factor XIII;-
usually by cryopracipitation, and combining it immediately before
use with bovine.(or sometimes human) thrombin. The thrombin
converts the fibrinogen.to fibrin which rapidly gels to farm a
transient hemostatic plug, which is then stabilized by factor
XIII. Fibronectin provides adhesion for cells repopulating the
~0 clot and tissue_ The most common method of application of fibrin '
sealant is mixing of concentrated fibrinogen from pooled human ,
blood with bovine thrombin and calcium immediately before use.
~
WO 96!17871 PCTlUS95115171
'" 21
82862
-3-
Fibrin sealant is not available in the U.S. commercially,
but is in Europe (TISSEEL°, TISSUCOL°/Immuno;
BERIPLAST°/Behring). Many papers have been published on its use.
Use of fibrin-sealant in the United States is limited to
preparation within the clinic outside FDA control. The reasons
for FDA reluctance-to approve these products in the United States
are:
virus transmission (e. g., HIV, hepatitis) from pooled
human bloods and
~ immunological reactions to bovine thrombin2, for
example thrombin and factor V inhibitors or foreign
body reactions'.
These FDA concerns are so serious that the FDA has not
approved any fibrin sealant product, despite strong interest from
surgeons and very favorable comparative studies in the
literature
These circumstances have led to much attention being given
to methods to isolate an autologous counterpart of the fibrinogen
containing component in the TISSUCOL system in a practical
manner. These efforts are discussed in the reviews cited below.
The following from a review by Thompson' shows that the value of
stat autoiogous fibrinogen is much anticipated:
W0 96117871 PCT/US95115171
.a,A~~,,,l~;. 21 g2~62
-4 -
Fibrin glue is composed of two separate solutions of
fibrinogen and thrombin. When mixed together, these agents
mimic the last-stages of the clotting cascade to form a
fibrin clot. Fibrin glue is available in Europe but is not
commercially available in the U.S.; therefore, investigators
have extemporaneously compounded their own fibrin glue.
Fibrinogen can be obtained from pooled, single-donor, and
autologous blood donors and is usually isolated by the
process of cryoprecipitation... The safest preparations use
the patient's own blood to prepare fibrin glue... Use of...
autologous blood results in minimal risk of disease
transmission, but it requires anticipated use as in an
elective procedure. The use of autologous blood usually is
not possible in trauma or an emergency surgical procedure.
Siedentop5 describes a number of-approaches to the
precipitation of fibrinogen from plasma in the context of the
proposed use of this material as the fibrinogen furnishing
comooner_t of a fibrin glue. Four methods were suggestedc
precipitation with ethanol, use of unfractionated plasma,
~0 cryoprecipitation, and precipitation with ammonium sulfate.
Epstein6 suggests the use of a fibrinogen preparation.from
autologous plasma obtained using polyethylene glycol ,
precipitation. A system for preparing autologous tissue adhesive
WO 96!17871 PGTICTS95115171
;, 2 i 82862
using a relatively complex system based on ethanol precipitation
has been described by Weis-Fogh'. Because none of these methods
has yet produced a clearly superior autologous sealant, research
in the past five years has continued on several approaches
including plasma sealante, ethanol precipitation' and rapid
cryoprecipitationl~: Prior patents are discussed in later
sections, Autologous Precipitate Sealants and Autologous Plasma
Sealants.
None of these methods is readily adaptable for convenient
use of an autologous plasma fraction as an adhesive which can be
prepared quickly during the surgical procedure. All of the
approaches suggested for preparation of the fibrinogen containing
fraction for this purpose are too time-consuming and complex to
be finished in a short enough time to be accomplished during the
surgery. Also, in some procedures, such as cryoprecipitation,
special equipment, such as refrigerated centrifuges, is required.
While the prior art approach is to prepare the composition in
advance, this immediately imposes the necessity for additional
procedures for identification and retrieval of the samples
matched with the patient, and the concomitant opportunity for
error, besides the inconvenience to the patient, who must then
arrange time for an additional medical appointment. And, of
~ course, this practice is not possible when the surgery is
conducted on an emergency basis.
WO 96117871 PCTlUS95115171
..,r,~.i,
.~ .'!'t' ~' i't i, .
v i. . ;. ; ,
..~ s.: v ..,a n.i t .»
-6-
Several useful reviews on surgical glues generally and on
fibrin glues particularly have been published (Sierra, D. H.
"Fibrin sealant adhesive systems: a review of their chemistry,
material properties and clinical applications." J Biomater Appl 7
(4 1993): 309-52;- Wiseman, David M., David T. Rovee, and Oscar M.
Alverez. "Wound Dressings: Design and Use." In Wound Healing:
Biochemical & Clinical Aspects, ed. I.-Kelman Cohen, Robert F.
Diegelmann, and William J. Lindblad. 562-SSD. 1st ed., Vol.
Philadelphia: W. B. Saunders Company, 1992). A review of devices
used by surgeons discusses fibrin glue in context of both
hemostatic agents and adhesive agents (Edlich, RichardF., George
T. Rodeheaver, and John G. Thacker. "Surgical Devices in Wound
Healing Management." In Wound Healinaw Biochemical & Clinical-
Aspects, ed. I. Kelman Cohen, Robert F. Diegelmann, and William
J. Lindblad. 581-6D0_-1st ed., Vol. Philadelphia: W. B. Saunders
Company, 1992). A review of the role of fibrin and fibrinogen in
blood coagulation has also been published (Jackson, C. M. and Y.
Nemerson. "Blood coagulation." Annu Rev Biochem 49 (811 1980):
765-8111.--Other reviews, in reverse chronological order, r
include: -
(1) Lerner, R. and N. S. Binur. "Current status of surgical
adhesives." J Surcr Res 48 (2 1990): 165-81.
R'O 96117871 PGT/US95/15171
' ~. ,; . .-
'::~=~r~:~:~~\'~;: 2182862
,: .J ..S :~ t . .
-
(2) Gibble, J. W. and P. M. Ness. "Fibrin glue: the perfect
operative sealant?" Transfusion 30 (8 1990).: 741-7.
(3) Thompson, D. F., N. A. Letassy, and G. D. Thompson. "Fibrin
glue: a review of its preparation, efficacy, and adverse
effects as a topical hemostat." DYuct Intell Clin Pharm 22
(12 1988): 946-52.
Biological Precipitate Sealaats
The vast majority of prior methods for preparing blood
derived glues or sealants use fibrinogen precipitated (usually
cryoprecipitated) from pooled plasma, a method first published in
19721'. Most biological sealant patents are improvements on
cryoprecipitation. Most go through a lyophilization step before
application. A few touch on plasma, but none mentions a
composition derived from concentrated plasma.
A composition from cryoprecipitation method was patented by
Immuno AG12. Since-the basic method had been published
previously, the composition claims were precisely descriptive.
Exemplary Claim: 1. A lyophilized tissue adhesive of
mammalian protein origin which comprises fibrinogen,
~ 20 albumin, factor 1CIII, cold-insoluble globulin and
plasminogen-activator inhibitor or plasmin inhibitor wherein
W0 96117871 PCTlUS95115171
2182862
_a_
the fibrinogen is present in at least 33% by weight, the
ratio of factor XIII to fibrinogen, expressed in units of
factor XIII per gram of fibrinogen is at least 80; and
fibrinogen and albumin are present in a ratio of-33 to 90:5
to 40.
Several inventions have improved on early cryoprecipitation
methods. A variation of cryoprecipitation is claimed to produce
material superior forpatients with blood coagulation
disordersl'. Stroetmann overcame problems by mixing fibrinogen,
thrombin and protease inhibitor in lyophilized form for
administration as a powder". Behring found a way to improve-
solubility of (poorly soluble) cryoprecipitatels. French
scientists have improved the traditional cryoprecipitation by
using a double cold ethanol precipitationls. Epstein has a
patent application for precipitation with polyethylene glycol
(only the sister (device) patent has issued)". Cryolife has
crafted a patent with a broad exemplary claimle (quoted in
note), but all the claims are limited..by use of a precipitation
step.
'0 Sierra's group has patented compositions with collager_ added
to fibrinogen. -The notion is that collagen improves the
mechanical properties of the sealant'9. Related approaches are ~
addition of hyaluronic acid to make the solution more-viscous
WO 96117871 PCTIUS95115171
w .,;. ,, . ~ 21828b2
_g_
before-gelation (patent application)'° and addition of silk
fibroin for mechanical strength~l
A disclosed World patent application shows that Baxter is
using fibrinogen affinity chromatography to get around the
problems with precipitationa'. A disclosed European patent
application shows that Sa_uibb scientists have developed a method
eliminating the need for thrombin2'. They use fibrin made by
passing fibrinogen through a column with immobilized snake-venom;
chaotropic agents prevent gelation. According to this work,
"(f)ibrin I monomer -is preferred because it can, in contrast to
fibrinogen, readily be converted to fibrin polymer without the
use of thrombin or factor XIII." From blood, the method yields
60-90°s fibrin monomer. It is activated by calcium and a pH
change.
The majority of biological patents-assume a precipitation
step in the manufacture of the tissue sealant.
Autologous Precipitate Sealants
As discussed above, it is widely recognized that autologous
blood products are superior for safety and biocompatibility
reasons alone. Columbia University has patented the use of
autologous fibrin glue", but the claims are restricted to
cryoprecipitate. A European patent application makes similar
claims=, but again the claims are restricted to cryoprecipitate.
WO 96/17871 PCTIU595115171
a~:c~~~~~,
,.~" 212862
-lo-
Autologous Plasma Sealants
Alterbaum has patented using small quantities,of patient ,
plasma to make tissue glue's, but the plasma is not concentrated
and the claims are restricted to use of flat pack centrifugation
for isolation of the plasma.
1. A method for use in the autologous preparation of
fibrin glue,wherein a patient's blood is separated in a
centrifuge having cylindrical cups pivotally mounted to a
rotor to obtain plasma and wherein the plasma is separated
in the centrifuge to produce concentrated fibrinogen,
comprising the steps of: (a) transferring the blood or _
plasma into a substantially flat packet; (b) fixing the
packet containing the blood or plasma in a recess of a
substantially cylindrical insert fixture assembly; (c)
inserting said cylindrical-shaped insert fixture assembly in
a cup of the centrifuge having a-complementary cylindrical
shape so that the insert fixture assembly, is held snugly
within said centrifuge cup; (d) centrifuging the blood or
plasma contained within said packet fixed in said insert
>0 fixture assembly held in said centrifuge cup to separate the
blood or plasma into components; and (e) forming fibrin glue
from one of said separated components. .
W 0 96117871 PCTIUS95I15171
~'v~vc,~,~;t.~; 2182862
-11-
Although no claim mentions or suggests concentrating the
' plasma, the patent which appears to be most similar to the
present invention (Dennis Galanakis, SUNY Stony Brook) covers
unconcentrated plasma glue":
1. A method of treating with autologous mammalian
plasma fibrin to affect hemostasis, comprising the steps of:
(a) obtaining a sample of blood from said animal; (b)
substantially immediately separating the whole plasma from
said blood obtained in-step (a); and (c) contacting said
whole plasma resulting from step (b) with thrombin in a
physiologically acceptable solution at a rate and in a
volume at the site of treatment to provide fibrin
coagulation at said site.
Applicators
Application offibringlue requires mixing the two
comDOnents under controlled conditions because gelation can be
very rapid. The earliest applicator patents are assigned to
Immuno AG. The principal claims revolve around different syringe
diameters to limit_ dilution of the fibrinogen component by using
I20 a smaller-volume ofmore highly concentrated thrombin2B and the
use of medical gas to keep the syringe applicator/mixer clear of
gelz9. Galanakis described an application in which the two
W 0 96117871 PCTIIlS95115171
C
2182x62
-12-
liquids do not mix internally but are applied side-by-side from
tubes that run in parallel all the way-to the point of
application.z' Very similar claims were made previously by
Micromedics'°. Corus Medical has an aerosol device driven by
pressurized gas". The Cryolife approach is to prevent gelation
in a single-component fibrin glue by pH inhibition of
thrombin". Gordon Epstein has patented the use of.a third
suction tip to dry the surface before application".
Plasma Separators
Haemonetics' "bowl" technology allows separation of plasma
continuously by a method which differs-from the methods of the
present invention disclosed below". Several patents address
isolation of platelets from-blood using centrifuge sedimentation
methods" or-red cell barriers'S, but none claims an approach
similar to the ones described herein including sintered (porous)
plastic or in which the cells pass through a barrier, leaving
platelet-rich plasma behind. -
Dextrans
Pharmacia has basic patents on dextranomers'6 and on their
'0 use in wound healing." Interestingly, concentration of
proteins (including fibrinogen) in the vicinity of the wound by
differential absorption of water and electrolytes is identified
W0 96117871 PGTfUS95/15171
-. ,. : ..- : ~ -~ 218 2 8 6 2
~...w twf: ,i ~,
-13-
as a benefit of the technology, but the claims are limited to
methods in which the dextranomer beads are applied to the wound.
Other Patents of Iatereat
Fibrin sealants have been claimed as a component in a
composition to repair cartilage's, to help in eye surgery°9 and
with heat-melted collagen for "tissue welding'°." Another
approach has been to use fibrinogen/thrombin powder to coat a
biodegradable fiber to stop puncture bleeding°1, or in a
collagen sponge or mat with powdered fibrin sealant'2. Addition
of collagenase to fibrin sealant to improve nerve healing has
been patented by Wehling". Pharmacia has patented the use of
hyaluronic acid to prevent adhesions"
Summary of Curreat Uae of Fibrin Sealaata in the United States
Although wounds heal naturally, medicine seeks to improve
and speed the wound healing process. The body's blood clotting
and tissue repair mechanisms are complex cascades progressing
from blood proteins (e. g. fibrinogen) to specialized repair cells
(fibroblastsi. Sutures, surgical staples or surgical adhesives
hold the damaged tissues together so that healing can progress.
~20 Surgical "fibrin sealants," sometimes called "fibrin glues," made
up of human fibrinogen activated by bovine thrombin are used
extensively in Europe. Such fibrin sealants have been shown to
W0 96117871 PCT/US95/15171
;.:j;t.,::r;rt~ X1$2862
,..
-14-
be superior to synthetic adhesives or traditional surgery in many
situations. In addition they reduce the need for blood
transfusions. Academic surgical opinion on fibrin sealants is
very favorable (see the reviews cited above).
In current practice, fibrin sealant is made by isolating a
concentrate of human fibrinogen, fibronectin and factor XIII,-
usually by cryoprecipitation, and combining it immediately before
use with bovine thrombin. The thrombin converts the fibrinogen
to fibrin which then gels rapidly to form a transient hemostatic
plug. The most common method of application of fibrin sealant is
by mixing of concentrated fibrinogen from pooled human blood with
bovine thrombin and calcium immediately before use.
Fibrin sealant is not available in the U.S. commercially,
but is in Europe. The FDA is reluctant to approve these products
in the United States because of the potential for virus
transmission (e. g., HIV, hepatitis) from pooled human blood. and
the potential for immunological reactions. These FDA concerns
are so serious that the FDA has not approved any fibrin sealant
product, despite strong interest from surgeons and very favorable
comparative studies in the literature_ .
1. Hennis, H: L., W. C. Stewart, and E. K. Deter. "Infectious '
disease risks of fibrin glue [letter]." ~hthalmic Surer 23 (9
1992): 640.
WO 96117871 PCTlUS95/15171
~.
»" ~ ~ ,
1~,~» ~:~F i\ .
-,_~'..' ~,, 2182852
-15-
2. Berguer, R., R. L. Staerkel, E. E. Mooze, F. A. Moore, W. B.
Galloway, and M. B. Mockus. "Warning: fatal reaction to the use
of fibrin glue in deep hepatic wounds. Case reports." J Trauma 31
(3 1991): 408-11.
Berruyer, M., J. Amiral, P. Ffrench, J. Belleville, O. Bastien,
J. Clerc, A. Kassir, 5. Estanove, and M. Dechavanne.
"Immunization by bovine thrombin used with fibrin glue during
cardiovascular operations. Development of thrombin and factor V
inhibitors." J Thorac Card~ovasc Sura 105 (5 1993): 892-7.
3. Sanal,- M. "Does fibrin glue cause foreign body reactions?
(letter]." Eur J Pediatr Sura 3 (3 1993): 190.
Sanal, M., H. Dogruyol, A. Gurpinar, and 0. Yerci. "Does fibrin
glue cause foreign body reactions?" Eur J Pediatr Sura 2 (5
1992): 285-6.
4. Thompson, D. F., Letassy, N. A., and Thompson, G. D., Fibrin
glue: a review of its preparation, efficacy, and adverse effects
as a topical hemostat. Drua Intell Clin Pharm, 1988. 22(12): p.
946-52_
5. Siedentop,-K. H., D. M. Harris, and B. Sanchez. "Autologous
fibrin tissue adhesive." Larvnaoscope 95 (9 Pt 1 1985): 1074-6.
6. EDStein, G. H., R. A. Weisman, S. Zwillenberg, and A. D.
Schreiber_ "A new autologous fibrinogen-based adhesive for
otologic surgery." Ann Otol Rhinol La~~~maol 95 (1 Pt 1 1986):
40-5.
7. Weis-Fogh, U. S. "Fibrinogen prepared from small blood
samp_les for autologous use in a tissue adhesive system." Eur Surcr
Res 20 (5-6 1988): 381-9.
8. Hartman, A. R., D. K. Galanakis, M. P. Honig, F. C. Seifert,
and ~.E.Anagnostopoulos. "Autologous whole plasma fibrin gel.
Intraop=_rative procurement." tech Surcr 127 (3 1992): 357-9.
9. Kjaergard, H. K., Fogh Us Weis, and J. J. Thiis. "Preparation
of autciogous fibrin glue from pericardial blood." ArLn Thorac
ur 55 (2 1993): 543-4.
Kjaergard, H. K., U. S. Weis-Fogh, H. Sorensen, J. Thiis, and I.
Rygg. "A simple method of preparation of autologous fibrin glue
by means of ethanol." ~~cr Gvnecol Obstet 175 (1 1992): 72-3.
WO 96117871 PCTIUS95115171
282862
-. , c
~ ;~ ~s '~ ~8 ~ _~ ,.
-16-
10. Casali, B., F. Rodeghiero, A. Tosetto, B. Palmieri, R.
Immovilli, C. Ghedini, and P. Rivasi. "Fibrin glue from
single-donation autologous plasmapheresis." Transfusion 32 (7 ,
1992): 641-3.
Moretz, W, Jr., J Shea Jr.; J. R. Emmett, and J Shea. "A simple
autologous-fibrinogen glue for otologic surgery." Otolarvngol
~iea~ Neck Sura 95 (1 1986): 122-4.
11. Matras, Helene, H. P. Dinges, H. Lassmann, and B. Mamoli.
"Zur nahtlosen interfaszikularen Nerventransplantation im
Tierexperiment." Wein Med Woschtr 122 (37 1972): 517-523.
First clinical results: Kuderma, H. and Helene Matras. "Die
klinische Anwendung der Klebung von Nervenanastomosen mit
Gerinnungssubstanzen bei der Rekonstruction verletzter peripherer
Nerven." Wein Klin Wochenschr-87 (15 1975): 495-501.
12. Schwarz, O., Linriau, Y., Loblich, F., and Seelich, T.,
Tissue adhesive; freeze dried wound healing agent containing
Fibrinogen, albumin, factor XIII, globulin, and plasminogen
blocking Agent. US Patent 4,414,976 (1983); Schwarz, O., Linnau,
Y., Loblich, F., and Seelich, T., Tissue adhesive; factor viii,
proteins, fibrinogen, globulin, albumin and plasmin inhibitor: US
Patent 4,377,572 (1982); Schwarz, O., Linnau, Y., Loblich, F.,
and Seelich, T., Tissue adhesive; fibrinogen, factor XIII,
albumin, plasmin inhibitor. US Patent 4,362,567 (1982); Schwarz,
O., Linnau, Y., Loblich, F., and Seelich, T., Tissue adhesive;
blood proteins; surgery. US Patent 4,298,598 (1981); assigned to
Immuno AG.
13. Martinowitz, U. and Bal, F., Improved tissue glue prepared
by using cryoprecipitate. EP (application) Patent 534,178 (1993)
Assigned to Octapharma AG.
14. Stroetmann, M., Enriched plasma derivative for advancement
of wound closure and healing. US Patent 4,427,650 (1984);
Stroetmaan, M., Fibrinogen-containing dry preparation,
manufacture and use thereof. US Patent.4,442,655 (1984);
Stroetmann, M., Enriched plasma derivative for enhancement of
wound closure and coverage. US Patent 4,427,651 (1984); assigned
to Serapharm Michael Stroetmann DE_
15_ Fuhge, P., Heimberger, N., Stohr, H.-A., and Burk, W.,
Readily Dissolvable Lyophilized Fibrinogen Fozmulation. US Patent
4,650,678 (1987) Assigned to Behringwerke Aktiengesellschaft.
WO 96117871 PGT/US95/15171
iS::;~~j t ~:.
:.z.-, , ~ 2182862
,.-'~~~CC
-17-
16. Burnouf-Radosevich, M. and Burnouf, T., Concentrate of
thrombin coagulable proteins, the method of obtaining same and
therapeutical use thereof. US Patent 5,260,420 (1993) Assigned to
Centre Regional de Transfusion Sanguine de Lille.
17. Epstein, G. H., Method and apparatus for preparing
fibrinogen adhesive from whole blood. US Patent 5,226,877' (1993).
18. Morse, B. S., Carpenter, J. F., Turner, A. D., and Cryolife,
I., Preparation of fibrinogen/factor XIII precipitate. US Patent
5,030,215 (1991)..Assigned to Cryolife. Claim 1: A system for
collecting a blood coagulation factor comprising a first
container means for receiving whole blood, said first container
means having an upper end, a lower end, at least one inlet post,
add at least one outlet port; first conduit means for conveying
whole blood to said first container means, said first conduit
means having an end thereof coupled to a said inlet port of said
first container means; second container means for receiving
plasma which has been separated from red blood cells in said
first container means, said second container means having an
upper end, the container means having a first, relatively wide
diameter portion adjacent to said upper end thereof, a second
relatively narrow portion defined below said first portion for -
receiving a blood coagulation factor precipitate from the plasma
within said second container means and a third relatively wide
portion defined below said second, relatively narrow portion so
that said second, relatively narrow portion defines a relatively
narrow passage for precipitate blood coagulation factor from said
first portion to said third portion; and second conduit means
having-a first end thereof coupled to a said outlet port of said
first container means and a second end thereof coupled to a said
inlet port of said second container means for conveying plasma
from said first contairier means to said second container means.
19. Sierra, D. H., Brown, D. M., and Luck, E. E., Surgical
adhesive material.US Patent 5,290,552 (1994) Assigned to Matrix
Pharm IncfProject Hear.
20. Wadstroem, J., Tissue treatment composition comprising
fibrin or fibrinogen and biodegradable and biocompatible polymer.
WO Patent 92/22,312-('_992) .
21. Iwatsuki, M. and Hayashi, T., Silk-fibroin and
human-fibrinogen adhesive composition.- US Patent 4,818,291 (1989)
Assigned to Ajinomoto Co Inc.
W0 96/17871 PCT1U595/15171
~ i
~; C~ U. ~~~r ~_ .a
282862
-18-
22. Tse, D. C., Alpern, M., Enomoto, S. T., Garanchon, C. M.,
Liu, S. L., Mankarious, S. S., and Thomas, W. R., Topical
fibrinogen complex. WO Application Patent 05067 (1993) Assigned
to Baxter Int Inc. -
23. Edwardson,- P. A. D., Fairbrother, -J. E., Gardner, R. S.,
Hollingsbee, D. A., and Cederholm-Williams, S. A., Fibrin sealant
compositions and method for utilizing same. EP (Application)
Patent 592,242 (1993) Assigned to Squibb.
24. Roae, E. and Dresdale, A., Method of preparing a
cryoprecipitated suspension and use thereof. US Patent 4,928,603
(1990) Assigned to Columbia University: Claim 1: A method of
preparing a cryoprecipitated suspension containing fibrinogen-=and
Factor XIII useful as a precursor ih the preparation of a fibrin
glue which consists essentially of: (a) freezing fresh frozen
plasma from a single donor which has been screened for bloo3 -
transmitted diseases at about -80* C. for at least 6 hours, (b)
raising the temperature of the frozen plasma so as to form a
supernatant and a cryoprecipitated suspension containing
fibrinogen and Factor XIII, and (c) recovering the
cryoprecipitated suspension.
Rose, E. and Dresdale, A., Fibrin adhesive prepared as a
concentrate from single donor fresh frozen plasma. US Patent -
4,627,879 (1986) Assigned to Columbia University. Summary:
Prodn. of afibrin glue comprises preparing a cryoprecipitated
suspension contg. fibrinogen and Factor XIII by freezing fresh
frozen plasma from a single donor which has been screened for
blood transmitted diseases at -80 deg. C for at least 6 hrs.,
raising the temp:- to form a supernatant and cryoprecipitated
suspension contg.-fibrinogen and Factor XIII, and recovering the
suspension. A defined vol: of the suspension is applied to the
desired sits and a compsn. contg. thrombin is applied to cause
the fibrinogen in the suspension to be converted to the
fibrinogen glue, which then solidifies.as a gel. -
25. weis-Fogh, U., A Method and an Apparatus for preparing
tissue repair promoting substances- WO (Application) Patent
8802259 (1388).
26. Alterbaum, R., Method and apparatus for use in preparation
of fibrinogen from a patient's blood. US Patent 4,714,457 (1987).
Claim 1~ A method for usein the autologouspreparation of fibrin
glue wherein a patient's blood is separated in a centrifuge -,
having cylindrical cups pivotally mounted to a rotor to obtain
plasma and where=n the plasma is separated in the centrifugeto
produce concentrated fibrinogen, comprising the steps of; (a)
WO 96117871 PGTlUS95/15171
;.
2i82~62
,,
-19-
transferring the blood or plasma into a substantially flat
packet; (b) fixing the packet containing the blood or plasma in a
recess of a substantially cylindrical insert fixture assembly;
(c) inserting said cylindrical-shaped insert fixture assembly in
a cup of the centrifuge having a complementary cylindrical shape
so that the insert fixture assembly is held snugly within said
centrifuge cup; (d) centrifuging the blood or plasma contained
within said packet fixed in said insert fixture assembly held in
said centrifuge cup to separate the blood or plasma into
components; and (e) forming fibrin glue from one of said
separated components.
27. Galanakis, D. K., Method of preparing autologous plasma
fibrin and application apparatus therefore. US Patent 5,185,001
(1993) Assigned to (Tniv New York State Res Found.
28. Eibl, J., Habison, G., Redl, H., and Seelich, T.,
Arrangement for applying a tissue adhesive. US Patent 4,735,616
(1988) Assigned to Immuno AG.
29. Redl, H. and Habison, G., Apparatus for Applying a tissue
adhesive. US Patent 4,631,055 (1986) Assigned to Immuno AG.
30. Miller, C. H., Altshuler, J. H., and Arenberg, I. K., Fibrin
glue delivery system. US Patent 4,874,368 (1989) Assigned to
Micromedics.
31. Avoy, D. R., Fibrinogen dispensing kit. US Patent 4,902,281
(1990) Assigned to Corus Med Corp.
32. Morse, B. S., McNally, R. T., and Turner, A. D., Fibrin _ ___.
sealant delivery method. US Patent 5,219,328 (1993) Assigned to
Cryolife. _
33. Headley, T. D., Plasmapheresis centrifuge bowl; disposable.
US Patent 4,983,158 (1991) Assigned to Haemonetics Corp; Pages,
3D E., Disposable centrifuge bowl for blood processing. US Patent
4,943,273 (i990); Latham, A., Jr., Apparatus for separating blood
into components thereof. US Patent 4,303,193 (1981); Latham, A.,
Jr., Process for pheresis procedure and disposable plasma. US
Patent 4,204,537 (198D); Latham, A., Jr., Process for pheresis
,35 procedure and disposable pheresis bowl therefor. US Patent
4,059,1D8 (1977); assigned to Haemonetics.
34. Pall, D. B. and Gsell, T. C., Method for obtaining
platelets. US Patent 5,258,126 (1993) Assigned to Pall Core;
Pall, D. B. and Gsell, T. C., Blood collection and processing
40 system. US Patent S,lOD,564 (1992) Assigned to Pall Corp.
WO 96117871 PGTIUS95115171
z ~ sz86z
.~_~-~~~ t
-20-
35. Pall, D: B., Gsell, T. C., Matkovich, V. I., and Hormann,
T., System and method for processing biological fluid. US Patent
5,217,627 (1993) Assigned to Pall Corp; Pall, D. B., Gsell, T.
C., and Muellers, B. T., Method for processing blood for human
transfusion. US Patent 5,152,905 (1992) Assigned to Pall Corp;
Eldegheidy, M. M., Automatic liquid component separator. US
Patent 4,639,316 (1987) Assigned to Becton Dickinson & Co.
36. Process for the Manufacture of Hydrophilic High Molecular
Weight Substances from Dextran Substances. GB Patent 854,715
(1965); Gelotte, E. B. and Soderquist, B. G. F., fHydroxy
compound copolymers dextran-epichlorhydrinJ Verfahren zur
Herstellung von Substitutionsprodukten von Mischpolymerisaten.- DD
Patent 56,103 (1965); Beil, W., Hoeppner, A., Hlclff, H. J., and
Beil, H., (Verfahren zur Herstellung von-hochmolecularen
hydrophilen Vernetzungsproducten von Polysaccharides oder deren
Derivaten oder von Polyvinylalkohol in Form von Gelkoernern) High
mol. wt. hydrophilic copolymer of a hydroxy group-contag.,
non-ionic polymer is obtd. in the form of gel grains by reacting
the polymer, in the presence. DE Patent 1,443,359 (1962);
assigned to Pharmacia.
37. Rothman, U.-S. and Jacobsson, S. A., Method for cleansing
fluid discharging skin surfaces, wounds and mucous membranes and
means for carrying out the method; dry particles of
water-insoluble swellable polymer. US Patent 4,537,767 (1985)
Assigned-to Pharmacia AB; Rothman, U. S. and Jacobsson, S. A.,
Method for cleansing fluid discharging skin surfaces, wounds and
mucous membranesand means for carrying out the method. US Patent
4,225,580 (1.98D) Assigned to Pharmacia AB.
38. Hunziker, E. B., Method and compositions for._the treatment
and repair of defects or lesions in cartilage. US Patent
5,206,023 (1993).
39. Sarfarazi, F., SaYfarazi method of closing a corneal
incision. US Patent 5,190,057 (1993); 0'Donnell, F., E., Jr.,-
Mammen, E., and Nalbandian, R.- M., Intraocular lens implant and
method cf locating and adhering within the posterior chamber. US
Patent 5,002,571 (1991) .
40. Sawyer, P. N., Collagen welding rod material for use -in
tissue welding. US Patent 5,156,613 (1992) Assigned to Interface
Biomedical Laboratories.
41. Sakamoto, I., Unigame, T., and Takagi, K., Hemostatic agent.
US Patent 4,655,211 (1987) Assigned to Unitika KK.
W O 96117871 PCT/US95/15171
2182852
-21-
42. Zimmermann, E. and Schiele, U., Agent for sealing and
healing wounds. US Patent 4,453,939 (1984) Assigned to
Hormon-Chemie Munch; Stemberger, A., Gewebeverklebarre kollagene
Wundauflage. EP Patent 102,773 (1983) Assigned to Dr. Ruhland
Nachf. GmbH.
43. Wehling, P., Method of enhancing the regeneration of injured
nerves and adhesive pharmaceutical formulation therefor. US
Patent 5,279,825 (1994) Assigned to Advanced Biofactures;
Wehling, P., Method of enhancing the regeneration of injured
nerves and adhesive pharmaceutical formulation therefor;
supplying collagenase to the zone of nerve injury during
regeneration. US Patent 5,173,295 (1992) Assigned to Advanced
Biofactures. _.
44. Linablad,-G. and Buckley, P., Composition and method for
prevention of adhesions between bodytissues. US Patent 5,190,759
(1993) Assigned to Kabi Pharmacia AB.
W0 96117871 PCTIUS95115171
212862
-22-
yr~nr~tARy OF THE INVENTION
Accordingly, one object of the present invention is to
provide a novel method and apparatus for rapidly preparing a -
plasma concentrate from blood containing fibrinogen, prothrombin
(the inactive precursor of thrombin) and ether-blood proteins,
and platelets (thrombocytes) (citrate, heparin or other
anticoagulant may be present in an amount-sufficient to prevent
initiation of clotting).
A further object of the present invention is to provide a
novel method and apparatus for rapidly preparing a plasma
concentrate-from a patient's own blood (autologous tissue
sealant).
A further object of,the present invention is to provide a
novel method and apparatus for rapidly preparing a concentrate
from a patient's own blood while in the operating room (stet
autologous tissue sealant).
A further object of the present invention is to provide a
composition with physical, chemical and biological properties
superior to plasma-protein precipitate derived-tissue sealants.
.p BRIEF DESCRIPTION OF THE DRAWINGS
A more complete appreciation of the invention and many of
the attendant advantages thereof will-be readily obtained as-the
same becomes better understood by,reference to the following
W 0 96117871 PCT/US95/15171
~~ y 1 i T ~l
:i:~.
_23- ~1$~862
detailed description when considered in connection with the
accompanying drawings, wherein:
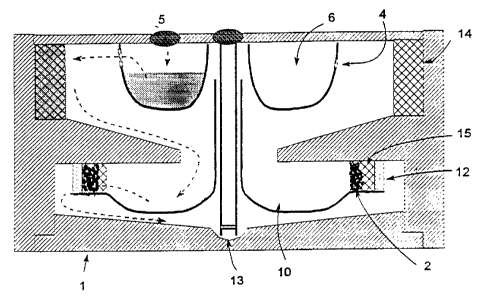
Fig. 1 shows an embodiment of a disposable cartridge in
accordance with the present invention, employing plasma
separation by open cell hydrophobic foam and plasma concentration
by open cell foam-with beads held-thereto by electrostatic force;
Fig. 2 shows an embodiment of a disposable cartridge in
accordance with the present invention, employing plasma
separation by sintered plastic barrier and plasma concentration
by beads distributed in a trough;
Fig. 3 shows-an embodiment of a disposable cartridge in
accordance with the present invention, employing plasma
separation by annular chamber and plasma concentration by open
cell foam with beads held thereto by electrostatic force;
Fig. 4 shaws an embodiment of a disposable cartridge in
accordance with the present invention, employing plasma
separation by felt mat and plasma concentration by beads
impregnated into plastic discs;
Fig. 5 depicts a single enlarged concentrator bead in a
solution of low malecular weight and macromolecule components
(e.g., plasma) in accordance with the present invention; and
Fig. 6 shows an embodiment of a disposable cartridge in
accordance with the present invention after centrifugation and
during syringe withdrawal of the plasma-concentrate;
WO 96117871 PCTIUS95115171
r y Yo .(' ~ ~ i l~.' ~ f'
S~ ;t~;.a ~A ..r i.. ~ y -24
Fig. 7 shows an embodiment of the applicator in accordance
with the present invention employing coaxial needles to allow
mixing of the two components;
Fig. 8 shows an embodiment of the applicator in accordance
with the present invention employing an activator cartridge to
allow application of single component fibrin sealant; and
Fig. 9 graphically shows the relationship between the
concentration of proteins and their molecular weight, for a
protein solution or suspension concentrated 3X with beads having
a molecular weight cutoffof.l0 kDa in. accordance with the
present invention.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
An inexpensive device with a disposable cartridge for
preparation of tissue sealant is disclosed. The device is
particularly applicable to stat preparation of autologous tissue
sealant. The disposable cartridge may fit in the palm ofthe
hand and is h~~-metically sealed to eliminate possible exposure to
patient blood and ensure sterility. Methods of sealing tissue in
which the tissue sealant is applied immediately after mixing
platelet-rich plasma concentrate (from the device? with a
solution of calcium and thrombin or in which the tissue sealant
is applied immediately after physical or immobilized enzyme ,
activation are disclosed..
W 0 96117871 PCTlUS95115171
,.
I .~
2~828~2
-25-
Preparation in the operating room of Scc sealant from SOcc
patient blood x-eauires Less than 15 minutes and only one simple
operator-step. There is no risk of tracking error because
processing can be done in the operating room. Additional
chemicals used in the plasma sealant and preparation thereof may
be limited to anticoagulant (for example, citrate) and calcium
salts (e. g., chloride).
Adhesive and tensile strengths are comparable to pooled
blood fibrin sealants that are available in Europe commercially.
Antifibrinolytic agents (such as aprotinin) are not necessary
because the tissue sealant contains high concentrations of
natural inhibitors of fibrinolysis from the patient's blood. The
tissue sealant- also contains patient platelets and additional
factors not present in available fibrin.sealants that promote
wound healing.
The present (autologous) tissue sealant gels rapidly as it
is mixed with calcium (and optionally bovine or human thrombin)
while being applied to the wound. This sealant contains only
FDA-approved. or approvable components. In the present invention,
a "tissue sealant" contains the components found in blood that
contribute to clot formation, tissue adhesion and wound healing
(preferably all such components in blood), and thus, is
distinguished from prior "fibrin sealants" which typically
contain only cryoprecipitated proteins and no platelets.
WO 96117871 PCTIUS95115171
~a~S~i': ~1$~862
-,
.. -26-
The present invention concerns a method of making platelet-
rich plasma (PRP) concentrate, which can then be combined with
calcium/thrombin to make tissue sealant. The resulting gel is a
composition that has never before been described in the
scientific or-patent literature..
Referring now to the drawings, wherein like reference
numerals designate identical or corresponding parts throughout
the several views, and more particularly to--Figure 1 thereof,in
a preferred embodiment of the present invention, citrated plasma
is processed in two stages in a disposable centrifuge cartridge.
In a first stage, platelet-rich plasma is separated from cells
when centrifugal force causes red and white cells to lodge
irreversibly in a first separator (e. g., a hydrophobic open cell
foam). In a second stage, the platelet-rich plasma is
concentrated by contact with a concentrator (e. g., dextranomer
beads) that absorbs water, electrolytes and small proteins,
leaving a platelet-rich plasma concentrate. Thus, the present
method cf making platelet-rich plasma comprises the steps of~
(a) separating plasma and platelets from whole blood;
(b) contacting the plasma and platelets with a
concentrator to provide the concentrated platelet-rich plasma;
and
(c) separating the concentrated platelet-rich plasma
from the concentrator. -
W 0 96117871 PCTYUS95115171
2182862
-27-
Preferably, the cartridge is disposable and sealed (e. g.,
see housing 1 in each of Figs. 1 through 4), making contamination
impossible. Where the method is performed in a sealed cartridge,
the separating step ta) above may be conducted, for example, in a
first chamber of the cartridge which is in fluid communication
with the blood fill port (inlet). The plasma and platelets may
then be contacted with a concentrator (e. g., in a second chamber
of the cartridge in fluid communication with the first chamber)
sufficiently to concentrate the plasma. An advantage of the
sealed, disposable cartridge is that it can be used in the
operating room, thus eliminating the possibility of tracking
errors and contamination.
In a further embodiment, the method is one for making
autologous concentrated platelet-rich plasma, in which the method
above for making concentrated platelet-rich plasma further
comprises, prior to the separating step (a), collecting blood
from a patient and aiding an effective mount of citrate to
inhibit coagulation (in accordance with standard methods), and
injecting a standard quantity of blood (e.g., 50cc) into the
blood fill port (inlet) 5 of a cartridge 1.
Plasma is separated from blood by a combination of
conventional centrifugation technology and inventions for ease of
separation of the packed cells from the plasma. Centrifuges
which are capable ofspinning at variable speeds up to 3,000-
WO 96!17871 PCTIUS95I15171
~~L ~ f' ~ ~ 2182862
-28-
10,00D rpm in either direction and-which are capable o~ being
controlled by a microprocessor (i.e., for spinning at
predetermined rates of rotation for predetermined lengths of _.
time) are known and are easily adapted; if necessary, for use
with the presentapparatus for separating and concentrating
plasma. Following injection of the whole blood, the operator
starts the microprocessor-controlled.centrifuge by push-button.
All remaining steps until removal of the concentrate may be
automatic.
y Although cells are conventionally separated from plasma-by
their property of packing into a cell mass during centrifugation
(then typically aspirated), the cartridges are designed to ef..fect
rapid separation of the plasma into the next, concentrator
chamber. In one embodiment (Fig. 1), red and white cells remain
trapped in a hydrophobic open cell foam 14. In another
embodiment (Fig. 4) red and white cells remain trapped in a felt
annulus 7. In a third embodiment (Fig. 2) the cells pass through
sinuous pathways in a sintered annulus 8 and remain trapped
outside. In a fourth embodiment (Fig: 3) red and white cells
'0 migrate through a small gap 16 and remain trapped in an outer
annulus by the force of gravity when the centrifuge stops.
Optionally, the plasma may be contacted with dextranomer
beads that have been bonded by electrostatic force with an open,
hydrophobic cellular foam 15 (in accordance with the apparatus of
WO 96117871 PCTlUS95/15171
~ ; .: :'t''-~, ~' ~'r
-29_
Figs. 1 and 3). When wetted the beads lose their electrostatic
attraction and begin to swell. Alternatively, the plasma may be
contacted with free dextranomer beads 2, e.g., in accordance with
the apparatus of Fig 2. The centrifuge may rotate briefly
clockwise then counterclockwise a few times at a speed sufficient
to evenly distribute the free dextranomer beads 2 outside the
concentrator cup lip 3.
The centrifuge may then spin at from 1,000 to 10,000 rpm,
preferably 3,000 rpm, for one to ten minutes to separate
platelet-rich plasma from cells. First, the whole blood passes
through a pore-4 in the blood inlet 6 into the separation
chamber. Cells may become trapped in a matrix 14 (as shown in
Fig. 1), enmeshed in a matrix 7 (as shown in Fig. 4), or pass
through sintered hydrophobic plastic or other porous material S
(as shown in Fig. 2), leaving platelet-rich plasma in the
chamber.
Th=_ centrifuge may then be stopped for 15-90 seconds. Under
the force of gravity, the platelet-rich plasma passes through an
annulus (or through a hydrophilic filter 9) into the second
chamber 10 (the lower chamber in Figs. 1 through 4). This
chamber may also be identified as a first concentrator chamber.
In the first concentrator chamber, platelet-rich plasma
comes into contact with the concentrator (e.g., dextranomer or
polyacryiamide), either bonded by electrostatic force with an
WO 96117871 PCTYUS95115171
'~~~3 ~~'~ ~' 2182862
-3D-
open, hydrophobic cellular foam 15 (Figs. 1 and 3) or free beads
2 (Fig. 2) or beads embossed onto discs) 11 (Fig. 4).
Dextranomer and polyacrylamide concentrators are commercially
available (as SEPHADEX from Pharmacia and as BIO-GEL P from Bio-
Rad Laboratories, respectively). Alternatively, instead of
dextranomer or polyacrylamide absorbent beads, other
concentrators, such as SEPHADEX moisture or water absorbants
(available from Pharmacia), other polyacrylamide absorbants,
silica gel, zeolites, dextramines, alginate gel, starch, cross-
~ linked agarose, etc., in the form of beads or discs are also
acceptable concentrators.
In essence, any material that absorbs water (i.e., which is
sufficiently dry to permit water absorption) and which does not
denature the proteins essential to blood clotting (e. g.,
fibrinogen and factor XIII) can be used as a concentrator, as can
appropriately-selected membranes, which may also employ an
osmoticgradient. Further, where the-centrifuge has autoclave-
like capabilities (i.e., the ability to rotate its contents at
elevated temperatures and/or atmospheric pressures other than 1
D atm), mechanical concentrators, such as an elevated temperature
(e. g., a temperature of 30-80°C, preferably 35-60°C) or a
reduced
pressure/vacuum (e.g., 0.1-500 Torr, preferably 1-200 Torr) may
serve as a concentrator. However, beads employed as described
both above and hereunder are ahe preferred concentrator.
WO 96117871 PCT/US95/15171
~afl'~? ~ 218286
-31-
To maximize results, initial slow rotation of the centrifuge
aids mixing (and thus contact) of the concentrator and the
plasma. "Slow rotation" refers to a centrifuge speed of
from 20 rpm to 500 rpm, preferably from 100 rpm to 300 rpm.
The porosity and hydrophobicity of the porous wall 7, S or
14 are such that the low centrifugal force generated is
sufficient to drive plasma up the wall but is not sufficient to
overcome surface tension, and the plasma remains with the
compartment at the inner surface.
The beads 2 swell with water, ions, and low molecular weight
proteins 51 (see Fig. 5). The size of the pores 52 in the
concentrator bead 2 is chosen so that the higher molecular weight
proteins 53 become concentrated in the space around the beads.
Any commercially available bead concentrators for concentrating
proteins having a molecular weight above a certain preselected
molecular weight cutoff, value may be used.
As those of ordinary skill in protein chromatography
understand, a "molecular weight cutoff" refers to pores in a
matrix (e. g., SEPHADEX) which are distributed about a mean size.
Moieties (e. g., proteins) more than, for example, 25-50% smaller
than mean size diffuse into the bead rapidly, and moieties more
than, for example, 25-50% larger than mean size do not diffuse
into the bead. Proteins having a size within, for example, 25-
50% of the mean size diffuse into the bead slowly (depending on
W0 96/17871 PGTIUS95I15171
~~8~~?~ 2182862
-32-
the particular bead selected). Thus, a bead having a molecular
weight cutoff of 10 kDa concentrates proteins according to
molecular weight as shown graphically in Figure 9.
Consequently, in the present application, "high molecular
weight compounds" refer to those compounds having a molecular
weight greater than the preselected molecular weight cutoff value
of the concentrator. Examples of high molecular weight proteins
in plasma may include fibrinogen and factor XIII, depending on
the selected molecular weight cutoff. Preferred concentrator
0 materials include those beads or materials having a molecular
weight cutoff value of 100 kDa, preferably 30 kDa and more
preferably 5 kDa.
It is important to note that tha platelet-rich plasma
concentrate contains all components of the original plasma, in
i5 either the original concentration (ions and compounds with
molecular weight less than the concentrator cutoff) or at a
greater concentration than naturally-occurring plasma (e. g.,
platelets and compounds with molecular weight more than the -
concentrator cutoff). The protein concentrate contains the same
20 ion concentrations as the unconcentrated plasma.
The quantity of beads is chosen so that the platelet rich
plasma is concentrated by a desired amount, e.g., 3X (the
approximate level of concentration achieved by a predetermined
volume of fluid surrounding close-packed spheres in the same
W 0 96/17871 PCT/US95/15171
-2182862
.,, ;
-33-
volume). When using DEERISAN (trademark, Johnson and Johnson),
approximately 2.6 gm is required per l0cc plasma to achieve 3-
fold concentration.
After absorption of water and low molecular weight solutes
into the concentrator, the rate of rotation is increased (e. g.,
to 1,000-10,000 rpm, preferably to about 3,OOD rpm), forcing the
concentrated platelet-rich plasma out through a filter 12 into an
outer compartment. The centrifuge may then be stopped, and under
the force of gravity, concentrated platelet-rich plasma may be
collected in a bottom well 13. The concentrated platelet-rich
plasma may be left for extended periods (e. g., hours) without
spontaneous gelation orother deleterious effect.
Concentrated platelet-rich plasma, ready for use, may then
be removed into a syringe 16 as in Fig. 6.
Thus, the present invention also concerns a method of
sealing tissue, comprising the steps of contacting the present
concentrated plasma with an activator (either a thrombin/calcium
solution or disrupted platelets as described below) to provide an
activated plasma concentrate, and applying the activated plasma
concentrate to tissue in need of sealing.
In one embodiment of the method of sealing tissue, a
separately-prepared solution containing amounts of thrombin and
calcium effective to coagulate the concentrated plasma may be
combined with the-concentrated plasma (in accordance with methods
WO 96117871 PCT/US95115171
:_ z ~ szs~~
-34-
described in the literature for cryoprecipitated fibrinogen).
Once combined, the solution and concentrated plasma rapidly gel
to form a tissue sealant. An embodiment with features superior
to any previously described is shown in Fig. 7. In the barrel of
the syringe a cylindrical chamber 31 containing thrombin and -
calcium solution is surrounded by an annular chamber 32
containing concentrated platelet-rich plasma. Plunger 30 causes
the solutions to-exit-the apparatus through a coaxial needle, 33
(thrombin) and 35 (concentrated plasma), and mix at the tip 36.
An optional sleeve-34 ensures proper mixing, and may be retracted
to expose premature gel for easy removal.
In a further optional embodiment, a needle tip designedto
activate fibrinogen may be used to apply a single-component
concentrate (i.e., the concentrated plasma) without the use of
added thrombin. In this embodiment, calcium is added to the=
concentrate immediately prior to or during its application to
tissue in need of sealing. Fig. S shows a syringe 21 and
applicator needle tip 23 separated by activator cartridge 22
through which platelet-rich plasma passes for activation by
'0 immobilized proteolytic enzyme, e.g., bovine thrombin.
In a further optional embodiment, a needle tip designed to
disrupt platelets may be used to apply a single-component
concentrate (i.e., the concentrated plasma) without the us=_ of
thrombin. In this embodiment, calcium is added to the
VVO 96117871 PCT/US95l15171
~"~. y i i' E :a ~ ~-,
2i82~~~
-35-
concentrate immediately prior to or during its application to
tissue in need of sealing. Fig. S shows a syringe 21 and
applicator needle tip 23 separated by activator cartridge 22
through which platelet-rich plasma passes for activation by,
e.g., nylon wool.
The preaent invention also concerns an apparatus for
concentrating platelet-rich plasma, comprising:
an inlet,
a first chamber in fluid communication with the inlet,
containing a first separator for separating plasma and platelets
from whole blood, thus forming platelet-rich plasma,
a second chamber in fluid communication with the first
chamber, containing a concentrator for concentrating the
platelet-rich plasma and a second separator for separating the
concentrated platelet-rich plasma from the concentrator, and
an outlet for withdrawing the concentrated platelet-
rich plasma.
In a further embodiment, the first separator comprises an
open cell hydrophobic foam which traps red blood cells and white
blood cells therein under surface tension force.
In a further embodiment, the first separator comprises a
porous plastic wallwhich allows passage of red blood cells and
white blood cells therethrough under a centrifugal force, but
W0 96117871 PCTYUS95/15171
i
~.".~-~'~ rF ~ : 218 28 6 2
-36-
does not permit passage of red blood cells and white blood cells
in the absence of the centrifugal force.
In a further embodiment, the first separator comprises an
annulus at the top of the first chamber that allows red blood
cells and white blood cellsto pass into an outer annulus and be
separated from plasma, maintained by gravity when the centrifuge
stops.
In a further embodiment of the apparatus, the concentrator
may be embedded onto a paper disc, or onto a plurality of discs
(of any commercially available material), or may stick
electrostatically or by otherforces to an open cell matrix.
Many designs of a preferred embodiment of the present
cartridge are envisioned (for example, Figs 1 through 4). The
designs of the first and second chambers are largely independent,
and may be combined into a single cartridge at will. All four
designs shown have at least one separation chamber in fluid
connection with at least one concentration chamber.
The most preferred embodiment, Fig. 1, uses open cell ..
hydrophobic foam 14 for separation of.plasma from cells and -
'0 effecLS plasma cbncentration by beads=held by electrostatic force
in an open cell foam 15. Another preferred embodiment, shown in
Fig. 2, uses plasma separation by sintered plastic barrier 8 and
plasma concentration by beads-distributed in a trough 2. Another ,
preferred embodiment, shown in Fig. 3, uses plasma separation by
WO 96117871 PCT/US95/15171
2182862
-~.~.; ~;~
,'r,~,::Y ~~_
. .. W
-37-
annular chamber 16 and plasma -concentration by beads held by
electrostatic force-within open cell foam I5. Another preferred
embodiment, shown in Fig. 4, uses plasma separation by felt mat 7
and plasma concentration by beads impregnated into plastic discs
12.
Each separator can be used with each concentrator. The four
separators may be combined with the three concentrators to give
twelve possible arrangementsfor the disposable cartridge.
A. Open Cell Hydrophobic Foam Plasma Separator and Dextranomer
Bead in Open Cell Hydrophobic Foam Concentrator
In Figures 1, the ready cartridge is shown in the right half
and the path of the liquid is shown by the dashed arrows on the
left half. The entire chamber is radially symmetric except the
septum 5 for the whole blood inlet. The receiving cup 6 holds
bloori until centrifugal force causes blood to pass through the
filter.4_ In the separation chamber, centrifugal force causes
cells to passinto the open cell hydrophobic foam 14 and
accumulate therein; continuing centrifugal force causes cells to
migra~a into the annulus of open cell-foam and platelet rich
plasma to remain within the separation chamber. The open cell
hydrophobic foam 14 isa honeycomb-like hydrophobic material
which allows fluids and small-particles to flow freely (e. g.,
open call polyurethane foam). Like a wetted sponge, the foam
W0 96117871 PCTlUS95115171
~~ ''; (1 C' t?~ a ~'
.: ~./ i~ .a ~i i ,.,~.
~18Z862
-38-
holds liquid against a certain head of pressure due to surface
tension forces. Thus, blood cells or other suspended _
particulates remain entrapped within the foam when the centrifuge
stops and separated-platelet-rich plasma drains from the surface
under the force of gravity. Foam can be either rigid or flexible
and can be formed into-the appropriate annular shape for the
device by molding or die-cutting. The,parts are sized so that
the packed cell (e. g., erythrocyte and-leukacyte) layer is fully
contained within the outer open cell foam chamber, which retains
the cells when the centrifuge stops.
Concentration comes about when platelet-rich plasma contacts
concentrator (e. g., dextranomer) beads 2 in the concentration
chamber 10. These beads may be a molecular sieve used in
chromatography (SEPHADEX, available from Pharmacies; BIO-GEL P,
available from Bio-Rad Laboratories) and debriding (DEBRISAN,
available from J&J), or may comprise silica gel, zeolites,
dextramines, alginate gel, starch, cross-linked agarose, etc.
DEBRISAIQ appears similar to SEPHADEX G-25, and both are available
commercially.
The beads-are held by static electric forces onto the
surface of open cell hydrophobic foam 15. The open cell
hydrophobic foam 15 is a. honeycomb-like hydrophobic material.
After the platelet-rich plasma has entered the concentration
chamber-10, the cartridge 1 is gently rotated to mix platelet-
W 0 96117871 PGT/US95I15171
~~. ~, ~r~;~.~ ~,;;~, _ 2 ~ 8 2 8 6 2
-39-
rich plasma with concentrator beads 2. As the fluid penetrates
the open foam 15, the beads lose their affinity for the foam and
begin to swell. Foam 15 may be made from the same material as
foam 14 (e. g., open cell polyurethane), but may have a different
porosity. The porosity of foams 14 and 15 is selected
empirically on the basis of the sizes andlor types of particles
to be retained (e.g., cells by foam 14 and concentrator beads by
foam 15). In commercially available open cell, three-dimensional
polyurethane foams, porosity is known to be correlated to density
(which is measured in, e.g., weight per cubic foot).
After a few minutes (e. g., 1-10 min., preferably 3-5 min.),
the concentration is complete. Note that concentration by
approximately three times corresponds to fluid surrounding close-
packed spheres; thus, three times concentration is optimal.
.5 Concentration of more than three times is possible, but to
achievebest results, a second concentration chamber should be
used for further concentration of plasma concentrated in a first
chamber: Thus, concentration o~-nine times is possible for a
three-chambercartridge containing two concentration chambers.
When concentration is complete, the centrifuge increases its
rate o~ rotation and the increased centrifugal forces cause
concentrated platelet-rich plasma to pass through the hydrophobic
annulus 12. Sintered plastic 12 (e.g., POREX) is comprised of
small part'_cles of plastic fused. to create a porous structure.
WO 96117871 PCT/US95115171
:~8~,~ ~ ~ 2182862
-40-
Fluids and small particulates can flow freely through the
tortuous pathways defined by the spaces between the fused plastic
particles when the material is wet. If the plastic is not
specially formulated or treated, the sintered plastic will be
hydrophobic and when in the dry state will afford resistance to
influx of water, more greatly as the size of the pathways is
reduced. - This property of "entry pressure" allows the wetting of
the surface of a hydrophobic porous wallwithout substantial
entry of fluid into the material. At greater pressure, i.e.,
when centrifugal force is increased, this resistive force is
overcome and fluid breaks through the barrier to enter and pass
through the porous wall.
The centrifuge stops, and concentrated platelet-rich plasma
runs to the bottom under the force of gravity and is ready to be
removed through filter 13 as in Fig 6.
Finally, notethat the.concentrator cup 10 is shaped like an
inverted funnel to vent the lower chamber to the upper chamber
allowing displacement of air from the lower chamber by plasma.
B. Hydrophobic Porous Wall Plasma Separator and Free
Dextranomer-Bead Concentrator
In Figure 2, the ready cartridge"is shown in the right half
and the path of the liquid is shown by the-dashed arrows on the
left half. The entire chamber is radially symmetric except the
W 0 96117871 PCT/ITS95115171
~~~~~:.~~v,
._,... . "
'~ ' 2182862
,:i j i,
-41-
septum 5 for the whole blood inlet. The receiving cup 6 holds
blood until centrifugal force causes blood to pass through the
filter 4. In the separation chamber, centrifugal force causes
cells to pass through the sinuous channels of filter 8 and
accumulate in the outer annulus. The filter 8 (e. g., SOj1 POREX)
is comprised of small particles of plastic fused to create a
porous structure. Fluids and small particulates can flow freely
through the tortuous pathways defined by the spaces between the
fused plastic particles when the material is wet. If the plastic
is not specially formulated or treated, the sintered plastic will
be hydrophobic and when in the dry state will afford resistance
to influx of water, more greatly as the size of the pathways is
reduced. This property of "entry pressure" allows the wetting of
the surface of a hydrophobic porous wall without substantial
entry of fluid into the material. At greater pressure, i.e.,
when centrifugal force is increased (e.g., at 1000-10,000 rpm or
higher), this resistive force is overcome and fluid breaks
through the barrier to enter and pass through the porous wall.
This leaves plasma and platelets in the chamber. The parts are
sized so that the packed cell (e. g., erythrocyte and leukocyte)
layer is fully contained within the filter and outer annulus.
When the cen~rifuge stops and the platelet-rich plasma passes
through the hyarophilic funnel filter 9, the force of gravity is
WO 96117871 PCTIUS95/15171
~~:~~'C8 f ~ . 2182~i62
-42-
insufficient to cause cells to pass through the filter 8 and back
into the separation chamber. -
Hydrophilic funnel filter 9 separates the upper and lower
compartments so that the loose beads cannot tumbleinto the upper
compartment during shipping and handling. This material might,
for example, be similar to that used for filter 8. In order that
entry pressure not be so high as to-preclude free drainage of-
plasma (by gravity) into the lower compartment after separation
from blood cells, however, this material would preferably be of
effective porosity minimally sufficient to ensure Entrapment of
beads in the concentrator chamber 10. The material would also
most desirably be treated in a manner such as to render-it more
hydrophilic, (e. g., plasma or corona treated or blended or coated
with surfactant) in order to further-reduce-.entry pressure.
Concentration comes about when platelet-rich plasma contacts
concentrator-beads 2 in the concentration chamber 10. These
beads may be a molecular sieve used in chromatography (SEPHADEX,
available from Pharmacia;- BIO-GEL P, available from Bio-Rad
Laboratories) and debriding (DEBRISAN, available from J&J), or
may comprise silica gel, zeolites, dextramines, alginate gel,
starch, cross-linked agarose, etc. DEBRISAN appears similar to
SEPHADEX G-25, and both are available commercially.
The beads are sealed into the chamber, but may become
unevenly distributed during shipment. A hydrophilic filter 9
W0 96117871 PCTIC1S95/I5171
. .. 2 ~ 82862
-43-
keeps the beads in the concentrator chamber 10. Immediately
after the addition of whole blood, the cartridge may be gently
rotated.and counter-rotated a few times to evenly distribute the
beads behind the lip 3.
After the platelet-rich plasma has entered the concentration
chamber 10, the cartridge 1 is gently rotated to mix platelet-
rich plasma with concentrator beads 2. For best results, it is
important to keep the slurry in motion because of the tennency
for protein to locally concentrate around each bead, retarding
absorption. After a few minutes (e.g., 1-10 min., preferably 3-5
min.), the concentration is complete. Plote that concentration by
approximately three times corresponds to fluid surrounding close-
packed spheres; thus, three times concentration is optimal.
Concentration of more than three times is possible, but to
achieve.best results, a second concentration chamber should be
use3. Thus, concentration of nine times is possible for a three-
chamber cartridge containing two concentration chambers.
When concentration is complete, the centrifuge increases its
rate of rotation and theincreased centrifugal forces cause
concentrated platelet-rich plasma to pass through the hydrophobic
annulus. Finally, the centrifuge stops, and concentrated
platelet-rich plasma runs to the bottom under the force of _
gravity and is ready to be removed.
WO 96117871 PCTIUS95115171
'~~~~~.uH~
212862
-44-
Finally, note that the concentrator cup 10 is shaped like an
inverted funnel to vent the lower chamber to the upper chamber
allowing displacement of air from the lower chamber by plasma.
C. Annular Chamber Plasma Separator and Dextranomer Bead in
Open Cell-Hydrophobic Foam Concentrator
In Figure 3; similar-to Figure 1 in many respects, the ready
cartridge is shown in the right half and the path of the liquid
is shown by the dashed arrows on the left half. The entire
chamber is radially symmetric except the septum 5 for the whole
blood inlet. The receiving cup 6 holds blood until centrifugal
force causes blood to pass through the filter 4. In the
separation chamber, centrifugal force causes cells to pass above
the lip 16 into an outer annular chamber and accumulate therein;
continuing centrifugal force causes cells to migrate into the.
outer annulus and platelet rich plasma to remain within the
separation chamber. The--parts are sized so that the packed cell
(e. g., erythrocyte and leukocyte) layer is fully contained within
the outer annular chamber, which retains the cells when the
centrifuge stops. -When the centrifuge stops, the platelet-rich
plasma flows info-the concentrator chamber 10.
Concentration comes about when platelet-rich plasma contacts
concentrator beads 2 in-the concentration chamber 10. These
beads are a molecular sieve used in chromatography (SEPHADEX,
W 0 96117871 PCT/US95/15171
~~ ~ '~ 2182862
~_; .-. ~, .,, _
-45-
available from Pharmacia; BIO-GEL P, available from Bio-Rad
Laboratories) and debriding (DEBRISAN, available from J&J), or
may comprise silica gel, zeolites, dextramines, alginate gel,
starch, cross-linked agarose, etc. DEBRISAN appears similar to
SEPHADEX G-25, and both are available commercially.
As in Figure 1, the beads are held by static electric forces
onto the surface of open cell hydrophobic foam 15. The open cell
hydrophobic foam IS is a honeycomb-like hydrophobic material.
After the platelet-rich plasma has entered the concentration
chamber 10, the cartridge 1 is gently rotated to mix platelet-
rich plasma with concentrator (dextranomer) beads 2. As the
fluid penetrates the open foam 15, the beads lose their affinity
for the foam and begin to swell. For best results, it is
important to keep the slurry in motion because of the tendency
for protein to locally concentratetaround each bead, retarding
absorption. After several minutes, the concentration is
comnlete_ Note that concentration by approximately three times
corresponds-to fluid surrounding close-packed spheres; thus,
three times concentration is optimal. Concentration of more than
three times is possible, but to achieve best results, a second
concentration chamber should be used. Thus, concentration of
nine times is possible for a three-chamber cartridge containing
two concentration chambers.
W0 96117871 PCT/US95115171
;;~8~~g=~ X218286
-46-
When concentration is complete, the centrifuge increases its
rate of rotation and the increased centrifugal forces cause
concentrated platelet-rich plasma to pass through the hydrophobic
annulus 12. Sintered plastic 12 is made of POREX, comprised of
small particles of plastic fused to create a porous structure_
Fluids and small particulates can flow freely through the
tortuous pathways defined by the spaces between the fused plastic
particles when the material is wet. If the plastic is not
specially fariciulated or treated, the sintered.plastic will be
hydrophobic and when in the dry state will afford resistance to
influx of water, more greatly as the size of the pathways is
reduced. This property of "entry pressure" allows the wetting of
the surface of ahydrophobic porous wall without substantial
entry of fluid into the material. At greater pressure, i.e.,-
when centrifugal-force is increased ie.g., at 1000-10,000 rpm or
higher), this resistive force is overcome and fluid breaks -
through the barrier to enter and pass through the porous wall.
The centrifuge stops, and concentrated platelet-rich plasma
runs to the bottom and is ready to be removed through filter 13
0 as in Fig 6.
Finally-, note that the concentrator cup 10 is shaped like ar_
inverted funnel-to vent the lower chamber to the upper chamber
allowing displacement of air from the=lower chamber by plasma.
W 0 96117871 PCTIUS95/15171
i~ ~
~;y~~~P~r.F.T-SL ~:.1
,_ 2182862
-47-
D. Fibrous Matrix Plasma Separator and Dextranomer-Impregnated
Disc Concentrator
Figure 4 is similar to the previous figures in many
respects. Like Figure 1, the ready cartridge is shown in the
right half and the path of the liquid by the dashed arrows on the
left half. The entire chamber is radially symmetric except the
septum 5 (the whole blood inlet).
Separation is done by driving cells into a felt or foam mat
7. The parts are sized so that the packed cell (e. g.,
erythrocyte and leukocyte) layer is fully contained within the
felt or foam filter, which retains the cells when the centrifuge
stops: -
In this design, even distribution of the dextranomer beads
is assured by using plastic discs embossed with dextranomer
beads. This can be done either with heat or solvent softening of
the plastic followed by dipping in dextranomer beads or affixing
beads to the M astic with adhesive. As the beads swell they
detach from the discs. Separation of the concentrated platelet-
rich plasma is done as in the above description of the present
method cf making concentrated platelet-rich plasma.
WO 96117871 PCTIUS95115171
~8~,8 '~ 2 i 82862
-48-
E. Method of Manufacture of the Disposable Cartridge
The various rigid plastic components comprising the
cartridge are manufactured by injection molding or by pressure-
or vacuum-forming, assembled and sealed by conventional methods
(e.g., either by adhesives or welding).- From Figures 1 through 4
it will be apparent to one, skilled in the art of plastics
fabrication that manufacture is relatively simple and
inexpensive. Thefinal assembled product is packaged, radiation
sterilized and ready for distribution.
F. Plasma Concentrate, Platelet-rich plasma Concentrate and
Tissue Sealant
The present invention also concerns a plasmaconcentrate,
prepared by the present process. The present plasma concentrate
may be platelet-rich, platelet-poor or platelet-free (i.e., the
platelet concentration is. not limited), but is preferably
platelet-rich. The concentration of platelets can be controlled
by the sedimentation rate, in accordance with known procedures
and/or known platelet sedimentation behavior. Preferably, the
plasma concentrate is autologous (administered-to the patient-
from whom the whole blood was taken).
In one embodiment, the present plasma concentrate comprises
platelets, from 5 to 40D mg/ml of fibrinogen, from 0.5 to 35
mg/ml of fibronectin, and a physiologically acceptable carrier
W 0 96117871 PCT/US95/15171
.~~_.,t=r_. ~. 2182862
v, ,~ .u...~=.z. ! ~.~~,.
-49-
comprising water and physiologically acceptable inorganic and
organic ions in a physiologically acceptable concentration. In a
preferred embodiment, the plasma concentrate is prepared from
whole blood, and platelets are concentrated at least one-and-one-
half times relative to the concentration of platelets in
uncancentrated plasma from the same whole blood.
The present plasma concentrate may further contain a
compound selected from the group consisting of an antibiotic, a
collagen fleece, collagenase, hyaluronic acid, a wound-healing
factor,-zinc ions in an amount effective to decrease coagulation
time of--said plasma, and a biologically acceptable dye. A
preferred biologically acceptable dye is disulphine blue,
contained in an amount sufficient to visibly detect the plasma
concentrate during its application to tissue. In a preferred
embodiment, the plasma concentrate further comprises a
pharmaceutically active compound, particularly an antibiotic to
reduce the probability of infection by extraneous microorganisms.
The present invention also concerns a tissue sealant,
comprising the present plasma concentrate and an activator.
Preferably, the plasma concentrate in the present tissue sealant
is platelet-rich plasma concentrate.
In one embodiment o~ the present tissue sealant, the
activator is a mixture of thrombin and calcium in amounts
sufficient to permit gelation of the plasma concentrate. In a
WO 96117871 PCTYUS95115171
~18~862
'.' ,. ,. .~ ~,~ s ;;.
-so-
further embodiment, the activator is autologous thrombin and
platelets.
G. Apparatus for and Method of Mixing Two-Component Fibrin
Sealant
Parallel syringes with the two components te.g.,
concentrated platelet-rich plasma and calcium/thrombin solution)
converge on a coaxial needle with mixing of the components atthe
application site, thus avoiding premature gelation; sae Fig. 7.
In the barrel of the syringe a cylindrical chamber 31 containing
thrombin and calcium solution is surrounded by an annular chamber
32 containing concentrated platelet-rich plasma. Plunger 30
causes the solutions to exit the apparatus through coaxial
needles and mix at the tip 36. An optional sleeve 34 ensures
proper mixing, and may be retracted to expose premature gel for
easy removal.
H. ~ispensar/Activator for Platelet-Rich Plasma Concentrate
Most users of fibrin sealants have resorted to the use of
bovine thrombin to induce gelation. In a preferred embodiment of
the present invention, it is desirable to avoid excessive use of
bovine proteins in the final preparation. Thus, a method and
apparatus employing a small amount of bovine or other - _
thrombogenic enzyme to trigger the autocatalytic activation of
WO 96/17871 PCT/US95/15171
,n
.,,~;;,.~,F, ~ 21$862
-51.-
the coagulation process is envisioned. Fig. 8 shows a syringe 21
and applicator needle tip 23 separated by activator cartridge 22
through which platelet-rich plasma passes for activation by
immobilized thrombin to convert fibrinogen to fibrin.
In a further optional embodiment, a needle designed to
disrupt platelets may be used to apply a single-component
concentrate (i.e., the concentrated plasma) without the use of
thrombin. Fig. 8 shows a syringe 21 and applicator needle tip 23
separated by activator cartridge 22 through which platelet-rich
plasma passes for activation by, e.g., nylon wool. In this
embodiment, calcium is added to the concentrate immediately prior
to or during its application to tissue in need of sealing.
A porous membrane or other material with high exposed
surface-tovoid volume ratio can be derivitized with thrombin or
IS . other thrombogenic or thrombin-like enzyme by known methods. The
derivatized -membrane is fitted between syringe and applicator
needle.
In a separate but related embodiment, glass wool, nylon wool
or cailagen sponge or another material may be used in a similar
2D configuration to cause eversion of the platelets, known to
initiate coagulation.
In a separate but related embodiment, concentrated plasma
may be kept cold to prevent gelation. After application, the
heat of the living tissue will initiate gelation.
W0 96/17871 PCTIUS95I15171
S; ri (~
212862
. ~r :~; ;.:~, .. f ,..'.,.~
A big problem with prior precipitation methods is- that the
fibrinogen must be reconstituted. This- is difficult and may take .
hours, and only a relatively low concentration is achievable.
Thus not only does the present invention allow more rapid
availability, a surgeon can decide that "this one is a bleeder!'
and have sealant in 15 minutes. Previous technology rea_uires
that the surgeon order-fibrin glue before the procedure begins.
The present concentrated platelet-rich glasma can be left_in
the device until needed, for hours if necessary, without
spontaneous gelation.
Other autologous precipitation methods require that blood be
drawn days in advance to allow time so that there is time for
processing. Besides the inconvenience, this procedure allows the
possibility of tracking errors and thus infection.
Generally, a- proteolysis inhibitor such as aprotinin is
added to the fibrinogen component of fibrin glues. (Academic
opinion is actually divided on whether-this is necessary; need
has never been proven rigorously.) The present methods for
producing concentrated PRP retain-the natural inhibitors which
are evidently lost in precipitation steps.
Fibrinogen from fresh plasma produces a mechanically
superior gel (based on the general properties of proteins damaged
by precipitation). Platelets (contained in the present plasma
concentrate) are useful for wound healing (many references.
W O 96117871 PCT/US95/15171
~~~~~:~.~~~s x2182862
-53-
available). Thus, an advantage of the present technology is that
it provides better wound healing than conventional fibrin glues,
which do not contain platelets.
Surgeons, particularly in the U.S., are likely to use bovine
thrombir_ to "activate" the present tissue sealant. This is not a
regulatory problem because bovine thrombin is available and is
used routinely in surgery. However, the present invention
provides a dispenser that activates prothrombin present in the
concentrated platelet-rich plasma and/or that disrupt platelets
so that clotting is initiated by one or more components in the
concentrated plasma.
The present tissue sealant and autologous tissue sealant do
more than merely seal the wound and adhere the damaged
structures. In particular, the present autologous tissue sealant
contains the patient's own living thrombocytes and wound healing
factors, and thus, it nurtures the healing process. Other
advantages of the present tissue sealant include the presence of
attachment factors which (i) improve adhesion to damaged tissue
and (__) promote cellular infiltration. In the present
autologous-tissue sealant, which is made from the patient's own
blood, important advantages include the ease, speed and
convenience of preparation (it may be prepared in the operating
~ room immediately before use). Furthermore, the optional absence
of bovine thrombin in the present autologous tissue sealant
WO 96117871 PCTlUS95115171
~~s~~~ ~ ~ 218262
-54-
avoids immune system and foreign body reactions and eliminates
disease transmission (i.e., from the bovine thrombin source).
I. Separator-and Method for Separating Particulates from
Suspensions
In a further aspect-of the present invention, a separator
and a general method for separating particulates from suspensions
is envisioned. Separating particulates from biological fluids
(such as blood and plasma) is a common problem in the laboratory.
A disposable -cartridge containing a separation chamber (i.e., the
first chamber in the apparatus used for preparing the present
concentrated plasma) allows cellular and dense particle
separation -- basically anything that moves in response to
centrifugal force.: _ _ ___
Thus, the present apparatus for separating particulates from
a liquid suspension comprises:
an inlet,
a first chamber in fluid communication with the inlet,
containing a first separator for separating particulates from the
liquid suspension, thus forming a particulate-free liquid, and
an outlet for caithdrawing the liquid.
A "particulate-free liquid" is one which is substantially
free of insoluble particles, preferably those insoluble particles
having an average size.above a pre-selected-value_(e.g., from 500
WO 96117871 PCT/US95l15171
is~ l ~.
s ;, ~::''~, ~...~ : _,
2182862
-ss-
to 20 ~., or any value for which filters nr-other devices are
available for removing such insoluble particles; e.g., 250 u, 100
80 p, 50 u, etc.).
J. Concentrator and Method for Concentrating Macromolecule
Solutions
In an even further aspect of the present invention, a
concentrator and method for concentrating macromolecule solutions
based on the design of the concentrator (or second) chamber used
in the apparatus for preparing the present concentrated plasma is
envisioned. SEPHADEX (trademark, Pharmacia), for example, is
rarely used for-concentrating macromolecule solutions. The
device may not concentrate to dryness, but is useful for
concentration of proteins (or any high molecular weight
solution), preferably by a factor of three times or a multiple of
three times, depending on the number of concentrator chambers in
the apparatus.
Thus, the present apparatus for concentrating a solution of
a substance having a molecular weight greater than a
predetermined value (i.e., macromolecule solution) comprises.
an inlet,
a chamber in fluid communication with the inlet,
containing a concentrator for concentrating the solution and a
separatcr for separating the solution from the concentrator, and
WO 96117871 PCTIUS95115171
a ~ y. '~ p
-56-
an outlet for withdrawing the concentrated solution.
Other features of the invention will become apparent in the
course of the following descriptions of-exemplary embodiments
which are given for illustration of the invention and are not
intended to be limited thereof.
EXAMPLE 1
Using a cartridge as-shown in either Figures 1, 2,- 3 or 4,
and following the procedure below, a concentrated platelet-rich
plasma and tissue sealant can be prepared:
I. Collect blood from patient using standard citrate
anticoagulant.
II. Inject a standard quantity of blood (e.g., SOcc) in to the
blood fill port of the disposable-cartridge. The cartridge
is sealed making contamination impossible. It will be used
in the operating room making tracking errors impossible.
III. The operator then starts the microprocessor-controlled
centrifuge by push-button. All remaining steps until
removal of the concentrate are automatic.
IV. (Optional step for designs incorporating free-concentrator
beads, Figure--2) The centrifuge -rotates briefly clockwise
then counterclockwise a few times at a speed sufficient to
WO 96/17871 PCT/US95/ISI7I
.2182862
,. ;
-57-
evenly distribute the free. dextranomer beads outside the
concentrator cup lip.
V. The centrifuge spins at 3;000 rpm for one to ten minutes to
separate platelet-rich plasma from cells. The whole blood
passes through a pore in the blood receiver chamber into the
separation chamber.
VI. The centrifuge stops for 15-90 seconds. Under-the force of
gravity the platelet-rich plasma passes into the lower
chamber, or concentrator chamber, (optionally by going
through a through a hydrophilic filter, appropriate when
free beads are present in the concentrator chamber as in
Figure 2).
VII. In the concentrator chamber platelet-rich plasma comes into
contact with beads bonded by electrostatic force to an open
cell foam (cartridge of Figures 1 and 3), free concentrator
beads (cartridge of Figure 2), or beads embossed onto discs
(cartridge of Figure 4). Slow rotation of the centrifuge
aids mixing. The porosity and hydrophobicity of the porous
wall are such that the low centrifugal force generated is
noL sufficient to overcome surface tension and the plasma
remains within-the compartment at the inner surface. The
beads swell with water, ions and low molecular weight
proteins (i.e., those proteins having a molecular weight
below the cutoff value). The pore size of the beads is so
W0 96117871 PCTIUS95115171
~~~ ~u~~~iri i~~~x
-58-
chosen that the proteins become concentrated in the space
around the beads. The quantity of beads is chosen so that
the platelet-rich plasma is concentrated the desired amount,
e.g., 3X. When using DEBRISAN, approximately 2.6 gm is
required perlDcc plasma to achieve 3-fold concentration--
The protein concentrate contains the same ion concentrations
as the unconcentrated plasma.
VIII. The rate of rotation is increased;forcing the concentrated
platelet-rich plasma out through the filter into the outer
compartment.
IX. The centrifuge stops, and under the force of gravity
concentrated platelet-rich plasma collects in the bottom.
The concentrated platelet-rich plasma may be left for
extended periods (hours) without spontaneous gelation.
X. Concentrated platelet-rich plasma is removed into a syringe.
XI: (Optional.) Thrombin/calcium solution is prepared
separately.
XII. Using a-variety of methods described in the literature for
cryoprecipitated fibrinogen,- the two solutions arE combined
and rapidly gel to form-the TISSUE SEAi~ANT_
XIII. tOptional; substitute for XI and XII.) Using a needle
tip designed to disrupt platelets, a single-component
concentrate may be applied without the use of bovine
thrombin.
WO 96!17871 PGT/US95/ISI71
2182862
-59-
A comparison of the properties of the present tissue sealant
and prior tissue sealant prepared from cryoprecipitated fibrin is
shown in the Table below:
TABLE: Concentrations of Tissue Sealant Compositions
Blood Precipitate Fibrin Autologous
Component Glue Concentrated PRP (3X)
per reconstitution identical to patient
Ions solution plasma
3X plasma
Platelets none concentration
only proteins that all plasma proteins
cryoprecipitate; at normal (less than
Proteins fibrinogen, factor 5 kDa) or 3X normal
XIII, fibronectin, (more than 5 kDa)
albumin, plasminogen concentrations
Albumin 10-25 mg/ml 100-165 mg/ml
Factor XIII 75 ~.g/ml 30-60 Pg/ml
Fibrinogen 70-110 mg/ml 5-14 mg/m1
Fibronectin 2-9 mg/ml 0.5-1.2 mg/ml
Plasminogen 20-60 ~Cg/ml 600 ~Cg/ml
Aprotinin -- 1,500 KIU/ml 0 KIU/ml
WO 96117871 PCTIU695115171
~au~ ~ ~ ~ ~ ~ sz~6z
-60-
The present tissue sealant also appears to show similar
tensile strength,to that of cryoprecipitated fibrin tissue
sealant. The adhesive strength provided by the present tissue
sealant is believed to be superior to that of,cryoprecipitated
fibrin tissue sealant because fibrinogen and other important
proteins are not denatured by the present process.
A key advantage of the present invention is the presence of
platelets. Adhesion of the platelets to collagen III fibrils
leads to platelet aggregation, promoted by 5-hydroxytryptamine
0 and epinephrine released from-the platelets. Other growth and
healing factors are released by the platelets. Platelet-derived
growth factor is a mitogen for fibroblasts and smooth muscle
cells. Platelet.factors stimulate neovascularization, especially
when the area is anoxic. Platelets also make fibrin more
.5 resistant to mechanical shear forces and to fibrinolysis.
It is possible that for certain applications lesser numbers
of platelets may be desirable. The invention may be adjusted to
achieve this outcome. Because of their low mass compared with
other blood cells, a preponderance of- platelets is preserved in
20 the final products when conditions are chosen to minimally
separate the bulk of said more massive cells from whole blood.
For example, a 4-inch diameter cartridge, 1 inch in height and '
fitted with a sintered plastic wall as in Fig. 2 will separate
most of the blood cells from 50cc of_whole blood when centrifuged
W 0 96117871 PCTlUS95115171
2182862
-61-
for 3minutes at 2,ODD rpm. By increasing the speed to 5,000 rpm
and the time to 15 minutes, most of the platelets are also
sedimented through the porous walls to yield plasma depleted of
platelets. Such platelet depleted plasma may be desirable if,
for example, it were desired that the plasma or concentrate
derived therefrom should be processed by filtration through a
sterile submicron membrane to render the final product suitable
for storage for an extended time (days).
Obviously, numerous modifications and variations of the
present invention are possible in light of the above teachings.
It is therefore to be understood that within the scope of the
appended claims, the invention may be practiced otherwise than as
specifically described herein.
45. See, for example, DelRossi, A. J., A. C. Cernaianu, R. A.
Vertrees, C. J. blacker, S. J. Fuller, J. Cilley Jr., and W. A.
Baldino. "Platelet--rich plasma reduces postoperative blood loss
after cardiopulmonary bypass." J Thorac Cardiovasc Surer 100 (2
1990): 281-6