Note: Descriptions are shown in the official language in which they were submitted.
2190418
S NEURON A~D ~EURA:L TUMOR GRO~TH REGULATORY SYSTEM,
ANTIBODIES T~IERETO AND USES TH~ ,OF
BACKGROUNI)
Following trauma in the adult central nerYous system ~CNS) of mamn~, injured neurons do not
r~gcn~_te theu tr ~e~ted axons. An i"lpo~ l ba~er to regeneration is the axon growth
~nhibitory activity that is present in CNS myelin and that is al50 associaeed with the pla~ma
nlemblane of oli~sodEndrocytes, lhe cell~ that ~ynthe~i~e myelin in the CNS (see Schwab, ef a~,
Alm. Rev Neuro~ci., 16, 56~-595, 1993 for review). The growth inhibitory prope~ s of c~s
myelin haYe been d~ ted in a number of different laboratories by a wide valiety of
techniques, i 'lvdj~g plating neurons on myelin su~ t~6 or cryostat sel;tion~ of white matter,
and obsa~alions of axon contact w~th mature rligrld~n-lrocytes (Schwab et aL, lg93) Therefore,
iL i~ well d~cumented that adult neur~ns cannot extend neurites over CNS myelin in vitro.
It has also been well docllrnpnted that removing myelin in viw improves the succe~s of
~g~ c~ e gro~th over the natiw terrain of the GNS. Re~ tion occurs aPler irradiation of
n~ ol~l rats, a procedure that kills oligodendrocytes a~d prevents the appearance of myelin
proteins (Savio and Schwab, Neurobiolog~r~ 87~ 4130 4133, 1990). A~er such a procedure in rats
and co-l-bil~d with a corticospinal trait lesion, some corticospinal axons re~row lon~ d;,.~ c~
bcyond the le!~ions. Also, in a chick model of spinal cord repair, tbe onset of m~ -,n
co~rela~es w~th a loss of its r~6ene~..ti~e ability of cut axons (Reir~tead? et a/" Proc. Nat. Acad.
SCi. ~USA)? 89? 11664-tl668~ 19~). The removal of myelin with anti-~al ~c -ebroside and
c ~ le.~ ent ill the embryonic chick spinal cord e~ctend~ the pern~issive period for axonal
30 regenera~ion. These c~e~ en~ demonstrate a good correlation between myelination and the
failure of axon~ to re8enerate in the CNS.
Until recently the identity of specific prote~ns i,l,?ol L~l~ for the inhibitoty activity rern~ined
elusive, althou~h they have been sought ~ince 1988 (Schw~b et aL, 19~3). One conlponenl of
SENT BY~ 15-96; 8:13PM; 6135637~71~ 16046694351;#11
2 1 9 OL 1 8
S the myelin-derived inhibitors as myelin~ ,ociated ~l luLein (MAC~) has been idcntifed
(McKerracher et al., Neuron, 1~., 229-246 and 805-811, 1g94). This finding was at first ~,urpris~ng
because MAG does not have the bioc~ a' plupcl l;es or disbiblltion of the myelin-der~ved
inhl~itor r~t~d by Schwab el ~l~, (1~3).
10 There have been ~ome ~ ~ecl~ion~ of the ~ p~ s ofthe non-MAG inhibitor in myelin7 based
on the work of Martin Schwab (reviewed in detail by Schwab e~ ~., 1993). lt was rep~. led to be
Pttrihl~t~d to two dil~r~lll protans of 35 kDa and 250 KDa. Myelin- denve~ growth inhibitory
acti~ity wa~ also Icpol Itd to be a property of ~,NS myelin but not PNS myelin. Tt has since been
dete~ned that PNS has inllibilory activity, but the inhibitory activity i~ m~sked by lanlinin (David
e~l., ~, 594-602, l995).
Schwab ha~ sought to determine the identity of the myclin-denved ir~ibitors of neunte
oll~growlh7 and his findin~s have been ~;A~ y re~r~ewed (Schwab ef al., l ~93). Schwab
determined a possible mol~cular wei~ht of the growth inhibitory proteins in the follounng way
20 Myelin proteins were separated by SDS PAGE under denaturing c~ditiol~c~ the ~el was cut into
slices and pro~ we~e eluted from the ~lices and inserted into ~lpoe~llles. The liposome~ were
tested ffir irlhibito~y ach~tity. Regions ofthe gel corresponding to 2~0 kDa and 35 Id~a were
idçntified as most inhibitory, and heat de~L-u7_d the inhibitory activity. The los~ of açtivity with
heat ~ested that the activity was due to a protein that required native col~l .llation. Why this
2~ putative protein retain~ biological activity a~er the denaturing conditioll~ of SDS-PAGE remain a
mystely The cvidcnce to claim tbe 250 kI~a and 35 kDa proteins as the ma or myeUn inl-ll lul ~ is
weak.
The e~idence for the 250 kDa and 3s kDa proteins as myelin-derived inh.bilo~ comes mainly
30 ~om the work of S~hwab with their IN-l antibody. Schwab raised monoclonal antibodies to the
inhibitory proteins eluted ~om gels and cloned one mon~rlonal antibody, called IN-1, whiçh is a
low-affinity IgM. It ha~ ~een used to characterize the myelin-derived inbibition. The antibody is
~pOI Ld~l to bind to the 3 ~ kDa and 250 kDa proteinsl but the We~tern blots indicate that it lacks
sl.e~ife;~y and that many ~ it~ bands are al~o reco~nized (Caroni and Schwab, Neuron7 L
SENT BY~ 15-96; 8:14PM; 6135637671~ 16046694351;#12
219041~
S X5-~, Ig881. The immunoprecipita~ion data p~ ed m the same ~l~hlirnliQn was g~ven in
tabular form rather than by showing the gels, as a rigorous analy~is requ~res, and these data
cannot be ea~ily evaluated. However, applieat;v~ of the antibody to vanous in vi~ro prepar tions
has been shown to partiatly blo~k the inhibitory pr~ nies of myelin. Also, the ~l~plir~lion ofthis
antibody in viw allows a small number of co, lica~inal axons to elongate long d~ ~s aftcr
CI~S injury (Schnell and Schwab, Nature, ~, 269-272, l99O, Schnell et aL, Nature, 367~ 170-
173, 1994). l!loreover, raphe spinal serotonergic neuron~ also regenerate, and the~e is
improvement in some aspe~ts of locomotor r~ ;on (r ~man e~ al., Nature~ 498, 1995).
Ihe.~r~r~ the evidence to da~e cu~ljls tkat blocking the myelin-derived inhibitors of neurite
oul will be an impor~nt componcnt of any the~a~culic strateE y to irnprove regeneration in
15 the adult CNS. Because the proteins idenli~;ed by the h,ltil.odi~6 have not been identified, the
on~ of myelin tha~ block axon gro~lvth7 in ad~litiQn to MAG, remain un~ ~olu ~ It has been
noted that both MAG and the new i~hibitor arretin, tbat is dG3e,il,ed herein7 appear to be acidic
protein~. Th~,..,f~.t;, to date, the identity of the non-MAG inhibitory components of mye~in remain
unknown, and the prote;ns that the IN-1 antibo~y ~ux,-: c9 remain uncharaclerized.
While ~e f~nd~s of MAG a~ an inhibitor of neuri~e outgrowth were s~ ..;sil~ other
laboratories have now substantiated our in vi~o documentation that MAG is an important
myelin~erived intlibitor of neurite growth (Muk~opadhay et ~ leuron, l 3, 757-7677 1 ~4;
Schafer e~ al., Neuron7 In pres~, 1996; DeBellard, Mol. Cell Neuro~ci., 77 7616-762~, 1996) Tbe
contribu~don of MAG has also been e~amined in ~rvc~, and the results indicate th~t other ~rowth
inhibitory proteins in myelin ~AiSt (Li et al., J. Ne~rosci. R0B., In press, 1996). In these s~udies it
}~ been shown tha~ some difrtl~nces occur in axon extension after lesions in MAG null mutant
mice, a finding that d~e~ from that reported for a ~irnilar stlldy of a dill~e~ line of
MAG~fi(~t mice (Bart~ch eJ al., Eur. J. Nellro~i., ~, 907-916, 19~5; B~rtsch et al., Neuron,
~ 7~-1381? 1~5 ). In both cases, however, the re~ults ~om the studie~ of MAG knock out
mice injured Y) the CNS are less ~..s~ic than ~ d with ll_.t~ with the ~-1 antibody
(Bartsch etal" 1995 - see below), ~~ ~ the non-MAG inhibitors that remain in CNS myelin
form an important bamerto ~ n~l~tion; indeed their eAp~ r in th~ ab~ence of ~AG
CA~ ,s;,;on may have been upre&~ a~ d duling CNS development.
SENT BY~ 15-96; 8:14PM; 6135637671~ 16046694351;#13
2~90418
5 Data ha~ ed that MAG lnay not be acting alone. To date, the pl~ece of another protein
had not been shown nor were it~ properties known. The present il~C~Ijoll has, for the first timc,
de~ 3~ ,d the presence and pr~.p~. I;es of such a protein.
Tenascins
~our l~cml~ of the tenascin family ha~e been i~lentified and characterized: tenascin-C,
tenascin-R, t~n~;~rX and tenascin-Y (Bristow e~ al., Cell Biol., 122~ 265-27~, 19g3; l~rickson,
H.P., J. Cell Biol, 120, lU79-1081, 1993). Tenascin-X and teD~scin~Y are not prominent in the
nervous system. Tenascin-C is important in the dev~ Fment of the nervous system and it is the
best characterized nlember of thi~ protein family. It i~ generated by alternative splicing (Weller et
al., J. Cell Biol., 11~, 355-362, l991; Sriramsrao and Bourdon, Nud. Acids Res., ~1. 347-362,
1993) and the variants are e~pr~3~d both in the ner~ous system ~nd in several non-neural tissues.
Tenascin-C has been s~-~r~ed to be involved in neuron-~lia adhesi-~e and migratory e~rents and
to promote axon o~ u~~ er injury of peripheral ner~res.
Tenascin-R (I~-R), has a modular structure similu to TN-C, previously desi~nated 31 - 160/180
and janu8in ;n rodents, or restriction in chicken (Pesheva et aL, J. Cell Biol., 109, 176~-1778,
1989, ~uss e~ a~., J. Neurosci. l~es., ~ 29g-307, 1991, and 3. Cell Biol., 1~0, 1237- 1249, I g93).
Tenasc~n-R is 1~ y ~ ed by oligoden~lrocytes during the onset and early phases of
myeIin formation and remains ~lete c~ e in myelin-~onning oligodendrocytes in the adult, and is
~5 also c* - ~e~ by neuron~ ~esheva eJ al., 1989; ~uss el al., l9g3~. Tenascin-R has been shown
to be in~olvcd in pr~-mot;on of neurite outgrowth and mor~hol~c~l polanzation of~ neurons when pre3~l~t~d as a uni~orm sub~trate ~Locbter and Schachner, l
Neurosci., 1 3t 398~ 1000, 1993; Lochter et al., ~ur. J. Neurosci., ~, ~97~ 4). When
offered as a sharp ~ubstratc boundary with a neurite u~l~owlh conducive molecule, tenascin-R is
repellent for growth cone ~dvance (Taylor ef al., J. Neurosci. Res., ~, 347-362, l 993; Pe~h~va et
al., 1993)
Tena~ins are not thollght to be an ll..p.~ component ofthe myelin-derived -'- t ~ ti~rity
because they ~adc the specific myelin di9tribution, they are not ~ icl~to the C~S, and their
SENT BY~ 15-96; 8:14PM; 6135637671~ 16046694351;#14
2190418
5 molecular weight differs ~om the presumptive proteins identified by Schwab. However, studie~
have inticated that both tena~cin R and tenascin C are minor inhibitory cor"pol~e.lts of
octb~glucoside extracts of myelin.
Chondroitin SuLfate Proteo~ s (CS~Gs)
10 P~v~Eo~ ans (PGs) are ~ S that are fiound predan~nantly on the cetl ~urface and in the
extraccllular maSr~; they are cov~lently bound to ~ carbohydrate~, called
~sssmi~n~lyca~. Gly~s"~ noglycans (GAG~) are polylTers of ~:~ac~ c repeats, which
are mostly highly sulphated and nG~~ ly charged. The main ~ s3a~llinoglycan~ in PG~ are
chondroi~n sulfate, de.,.latan sulfa~e, heparan sll~ate, and keratan suLfate. (Ruoslahti, ~., Ann.
1~ ~ev. CeD Biol., 4, 22~-255, 1988). The number of GAG c}uins can vary from one to over one
hundred.
~ot~gl~csns are known to be illlpol ~a ~ for the d~lo~)...en~ and re~ tioll of the nervous
system, but they have not been considered to be myelin proteins or form patt ofthe g,rowth
20 inhibitory activity of myelin. Moreover, proteo~lycans have not been reported to be recogni~ed by
the IN-l ~ntibody orto fonn a major growlh inhibitory eu~ on~,.-L of white matter re~ions ofthe
CNS.
Chondroitin sulfate proteo~cans (CSPGs) conctitute the major population of PGs ~n the CNS.
25 The dilr~ t patt~ of localization and d~ ,lopll.enlal expression of CSPC~s throu~,hout the
ner~ous system implicate them in diverse roles in de~,~lop..~ t and in r~gc~e.alion. After injuries
in the adult CNS, CSPG~ are thought to be important in the formation ofthe ~lial scar. They
h~ve b~n implicated as both positive and negative m~ 7 tc ~ of axonal ~rowth. Recent
observation~ indicate that DSD-l-PG, a neu~l chondroitin sulfate pl.~t~o~rCan, plol~ol~s neurite
o~llg~wlll of embryonic day 14 I~e3~,~ccph-' - and embryonie day 18 hippocampal neurons from
r~t ~ sner et a~., J Neuroçhem, ~4, 1004-lOl~, 1994). However, ~G2, an integral r.l~.n~l~ne
CSPGs tA~re~8ed on the surface of glial progenitor cellsl inhibits neunte ~rowth. The NG2
pro~ yCan al50 inhibits neuri~e growth a~er di~r~tion with cho-~ oi~ ase A~C, indicating that
the inhibitory activity i5 a ,v--~pt~ ofthe core protein and not the covab~y a~tached chondroitin
SENT BY~ 15-96; 8:15PM; 6135637671~ 16046694351;#15
~ 219041~
sulfa~e glycosam~no~csn chains (Dou au~d Levine, J. Neurosci., 14, 7616-762B, 1994), but for
many other tlrpes of CSPGs the inhibitory activity resides in the gl~s m;noglycan~ Chon~l~ .,it~i~
sul~le p~oteo~lycan immu~lore flCtil,~ity is i..cr~d ah[er cere~ral cortical ~ n et al., J.
Neuro~ 11, 3398-3411, l 991~, spinal (Pil.d~ol~ et a~ ev. Biol., l ~, 34~8, 1993) and optic
nerve bsions (Brittis et aL, Sciencc, ~, 733-736, 1992). ~n vitro studies indicate that CSPG
immun~.z -tiVity on astro&ytes increases when they are plated on monolayers of leptomeningeal
cells (:Ne~ and navid, Glia, In press, 19g7). Similar incr~3~ in CSPG immunoreacti~,rity ha~e
been ~ L~d on Sch~4ann cells co-cultured with ~stl~rle~ mi~r and E~ng, Glia, 14~ 14~-
152, l99~). This hi~hly ~ulfated proteoglycan which is a potent inhibitor of neurite growth in
vitro ~Snow e1 al., Neurol, 1~ 30, I990), has been ~hown to be involved in th~ I.li~.~l~tiation of developing retinal ",~ q~' c :- cells, and by acting as an inhibhory sub~rate selves
to a~ vp.~lely guide ~ic - cell axons toward tl~e optic disc (Brittis and SilYer, Proc. Nat.
Acad. Sci. USA, ~ 2, 7535J-7542, 1992). McKeon et al., J. Neuro~, I 1, 3398-3411, 1991) have
~o, l~d that astrocytes han~ested ~om the site of cerebral cortical lesions express increa~ed
.d~ of CSPG, which reduces neurite ~rowlh on tllese cells in vitro. The e A~ s~;dn of CSPG
~0 on the surfaoe of a subset of cultured astrocytes has also been shown to correlate ur~th their
reduc~d ca~acity to 8UppOl't neurite ~rowth (Me~ners et al., J. Neurosci., 1 ~, 8096-8108, 1995).
The coIlapse of the ~owth cone is an ~mportant rc~;)ol~ of the ~rowing exon to inhibitory cues
in the en~ir~ ,ulL Collapse ofthe iam~ rn i8 5(j~ 5 followed by retra~tion oftheneurite ~Kapfhammer and Raper, J. Neurosci., 7, 201-212, 1987; Raper and Grunewald, E~cp.
Neurol., 109, 70-74, 1990, Bandtlow el al" J. Neurosci., 10, 3837-384X, 1~0). Many ~7~ .o~Jsly
cbaracterized inhibitory molecules ~und in the de.~p~,~, nervous sy~,tem have been shown to
caus~e growth cone coll~pse in vitro (Davies e~ al., ~euron, 4, 11-20, 1990; Stahl et al., Neuron,
5, 73~-74~" 1990; Bandtl~vv ef c~ ~0; Keynes e~ ~1" Ann. N.Y. A~ad. SGi. 633, 562, 19~1;
Luo et a~., Ce~, 75, 217-221, 1 ~93). Such coll~r~i~ activity ha~, been observed previously in the
adult chicken brain and shown to bind to PNA, and be ~csrciated with ~lyL;op~uteins with
er~lsr wei~hts o~48 and 55 kDa ~Keynes e~ al., 1991). Others, such a~, ~he 33 k~a inhibitor
in the developing chicken tectum also binds to PNA (Stahl e~al, l99~). Re~ e proteoglycans
are a very L~ R das~ of proteins w~h diverse b ~logir-' ac~ ies it is essentiql that
individual, i~ . d proteins be considered. Relevant to the present inverltion are the
35 pr~leo~c~3 pho~.rho~,a~ and verB~ because the protein of the present invention~ arretin, has
SENT BY~ 15-96; 8:15PM; 6135637671~ 16046694351;#16
~ 21 9041 8
5 common immunological eplilopcs with the~e proteins.
?~
r~ ~ ~h~9~ a-~ is a proteo~lycan in brain I ~o~i ,~ by the 3~ anbbody (Maurel e~ al., ~roc. Nat.
Acad. Sci. USA, 91~ 2512-2516, 1g94), and by the 6B4 antibody (~eda et~L, Neurosci., ~,
23-3~, 19~5). Pho, ' lLC7 - is a splice variant of a l~cepto~-type protein tyro~ine ph~bl~ t~
5~ltho~gh pl~o 1~ it~elf lacks the phospha~ase dom~ins~ It is a protein with an a~pdlG~It
molecular weight of ~p.u~inately SOO kDa, h~ving a core glycoprct ~ of a~ ately 400
kDa. The HNK-I monoclonal antibody reco~s a 3-,m'p~ ed ca~bohydrate epitope, and this
~pitope is strongly r~,.,se.ltLd in p~ plu.:: from 7-day brain, but not in adult brain (Rauch et
a~., J. Biol. Chem., ~, l478~-14B01~ 1991). In de\~elopl~nt phosphaçan is imml~nost~ined on
radial glia and on neuron~ (Maeda eF al., l 9~5~ and generally it is ~.~re~b~d in both whitc matter
and ~rey matter region~ (Mcycr-Puttlitz, ef a~., J. Comp. Neurol. 366? 4~-54, 1~6). and
therefore, unlike the myelin inhibitor~, it i~ not localized on}y to white matter areas. It appear~ to
be ~ynthe~ized only by a~troglia (Engel ef d~, 19~6).
~er~i~an.
~ sic~n? a CSPG oliginally isol~ted from rblubl~, also called PG-M, has an appa~e ll
molecular weight of appr~l.a~ely 900 kDa~ with a core prote~n of ~p~u,ul~&tely300 to 400 kPa
~Braunewell ef al., E~r. J. ~eurosci.~ 7, 792-804, 1995; NaAo el a~, 1994~. Versican belon~s to a
25 fannly of a~grega~ing CSPGs; other ~ l,e.~ of the family ~nclude the cartilage~derived aggrecan,
and two PGs ~A~JIe__~d in the nervous system, neurocan and brev~ca~ our~ Zintmermann and
Zim..l~r~ ann, J. Biol. Chem, ~, 32992-32998, lg94). Versican is widely .li~t~ Jle~ in adult
human tissues, a~sociated with conn~ e tissue of various organs, in ce~tain muscle tissues,
epithelia, and in central and penpheral nervous tissues. Four versican isoforms a~e known (~o,
Vl, VZ, V3), derived by alternati~e splicing. They ~~ary in ~lc~ ted mass ~om appr~ a~ely
370 kDa (Vo~ to appro~;"-nl ly 72 kDa (V3). Tt has been Qu~gruted that the a~sociation of
sican ~pres~ion with cell migration and proliferation m v~vo and ;ts &dh ;:~n inhibitory
prope~ties in ~ritro point to patho~ogical yroc~es such as tumon~enesis and ~
(Bode-l s.sr e~ lAa et al., Histol. ~ Cyto., 44, 303-312, 1996; Naso e~ al,. J. Biol. Chcm., ~2,
32999-33008, 1gg4).
'~_
SENT BY~ 15-96 ; 8:15PM; 6135637671~ 16046694351;#17
~190418
-
S Other CSPG~ related to versican are brevican (Mr app~ , 14~ kDa~ and neurocan (~ >
300 kDa~. Neither of these is known to be c~r~ d by oli"o~ r~ytes and are therefore not
L~A to be pre~en:t in CNS myelin (En~Sel ~t a~., J. Comp. Ncurol. 366, 3443, 1996; Yamada
etc~l., J. Biol. Chem., ~, 10119-101267 1994).
-
~0 Another CSPG family n~ that i5 not related to either ~ Cdn or phosphacan, is NG2.ou~h it is CAIJ~3B~d ~y 02A pro~enitor cells in the developmg rat nervous system, it has no
a~pa ~_nt homology to alTetin-relevan~ GSPG'~, and has an Mr ~.,ploAJn,~tely 400-~00 kDa wi :h a
core protein of approximately 300 kDa (~Ishiyama ef al., J. Cell Biol., 11~, 3~9~371, 1991).
Neurobl~toma
Nel~r~ tlo~ arises from neuroectoderm and C;Ont~ ,p~ tic sympathetic ganglion cells
(reviewed in Pinkel and Howarth~ l9g5, ln: 1\ ledical Oncology, Calabrese, P., Ro3~n~.4 S. A.,
and Schein, P. S., eds., l~ n, N.Y., pp. 122~1257). One i,lt~ i~ aspe~t of
20 r..,.~ 3~ma is that it ha~ one ofthe hi~hest rates of spontaneous re~ ;on among human
tumors (Everson, 1964, Ann. N.Y. Acad. Sci. 114:721-735) and a correlation exists b~ ,cn such
re~res~ion aïl~ nuturalion of beni~n ~anglioneuroma (1301ande, 1977, Am. J. Dis. Child.
122:12-~4). Neuro~ m- cells have been found to retain the ca~acity for mor~Jholo~cal
maturation in culture. The tumors may occur ~ .h~.~ along the ~ F theti~ chain, with ~0% of
25 such tumors originating in the adrenal m~
~'L~ blastoma a~ects predorm~nantly preschool aged ~hildren and is the most COn~Q~On
extra~ranialsolid~umorinchildhood,Gonstitl~ 6.5%ofpediatric~e~lqcmsOnehalf~eles~than two ye~rs of agc upon ,1;~ f~t~ ~ti.~es are evida-t in 60% ofthe patients at
30 ~ s~nti~tion ~sually involving the bones, bone marrow, liver~ or skin. The pr~--h~ a~rlnlJt(j~lls
may be related to the primary tumor (spinal coral co...pre~~lc n, abdominal mass), mP.t~ct~tic
tumor (bone pain) or metabolic e~ects of sub~t~nr~ such as catP~holaminP~s or vasoactive
polypeptides secreted by the tumor (e.g. hypertension, dia~rhea). E~p~ e~ evidence indicates
that an altered ~e~"on~ to NG~ i~ associated with neuroblastoma (Sonoenfield and Ishii, 1982, 1.
l~euro~ci. Re~. 8:375-391) NGF ~tim~ te~ neurite ~ul~uw~h in one-half ofthe neuroblastoma
SENT BY~ 15-96; 8:16PM; 6135637671~ 16û46694351;#18
2190418
_. .
5 cell line~ tested; the other hall'was ~ it-~e. However, NGF neither reduced the gro~th rate
nor enhanced sunival in any nc~.lbl~stoma cell line.Present the~pies for neurob!scto~3 involve
surgery and/or che~otlle.~,y. Radiation the-rapy is used for incomplete tumor re~p~rwes to
chemotherapy. T}lere is a 70-100% suIv~val rate in individual~ with localized tumor~, but only a
20% ~urviYal rate in tho~e with metas¢atic disease even w~th multiagent che.llolhela~. It appear~
10 tha~ patients les9 than one yea~ hav~ a better progno~8 (70%) than older children.
This background ~ ~.c ~ ion is providcd for the purpose of making known information bel;eved
by the applicant to b~ of pogsible lele~ance to the pre~ent invention. No all" -~ Ln is necessarily
h~te ~~k~l, nor should be construed, that any ofthe ~le~li..,6 infolmation constit~teg prior ~t
lS against the present in~ention. ~ub' ~vqti~s referred to throughn~t the ~ n are hereby
;.~co.~orated by refierence in their e.~ in this application.
SUl!llHARY OF T~l;E I~VENTION
The present invention relates to a neuron and neural tumor growth regulatoly systerïl, antibodies
directed again~t dle componenls of thi~ ~ystem and disgrnstir~ lhe.apeulic~ and rese~Gh uses for
each ofthese aspects. The concept of a ~ystem is used to denote the filn~ r~1qtinn~hip
between the ~ene~ (for the regu}~to~y factors and the recel~tol~), the;r enco~ protein-regulatory
25 factors which regulate neuron growth (pârticu~ y neurite growth), and the r~ which are
activated by the protein. The fullc~ ' relatinnship allows one to u~e one component to identif~r
and d~t~ another. For eY~np~e, ha~ing i~PntifiPd the protein co"-ponenl (factor or
r~c~tor), one can use ~echnique~ well known in the a~t to identify the ~ene.
30 In accor~ with the present invention, a prote~n has now been identM~ arretin, a~ one of the
molecular colUp~ clll~ involved in contact-mediated gro~th inhibition on myelin This protein
ha~ an apparent mole~A~~ weight of appro~mately 70 kDa. Given the punfied protein,
procedures for obtainu~g the other pa~t~ of the system are well known to those skilled in the art to
purify the other components to the system For exElmple, the protcin can be used in very standard
35 techniques to obtain the a~no acid soqu~nee which can be u8ed to obtain probes for nucleic acid
SENT BY ~ 15-96 ; 8: 16PM ; 6135637671~ 16046694351; #19
~90418
5 sequ~nreCv encoding arretin. Alternalively~ arretin protein may be tagBed for use as a repo, ll, to
detect receptors of arretul~ which are then vequenced and used to obtain probes for the nucleic
acid seqlJ~ eacodin6 arretin roceptolv Moreover, the production of antibodies to each ol'
these components i9 ~IvO standard procedure.
10 The present invention further relatev to arretin r~e~,tol~ and G~ thereof a~v well as the
nucleic acid 3~qu .çe~ coding for such arretin receptors and ~ c-~c, and their therapeutic and
~;aen~ ;e uses. Su~ n~e,j which fimction as either a~onists or a~agon;Yt~ to alTetin receplol~
are also e.ln;,;onGd and w~thin the scope of the present invention.
15 The present inv~tion filrther relates to the nucleic acid sequence~ wding for arretin and its
rs, in addition to their therapeutic and diagnostic uses.
ln accordance witb another aspect of the present invention, there is provided the use of arretin for
the regulation of growth of neurons and neural tumors.
In a further aspect ofthe present invention, there io, provided a method for inl~ib;~ growth of
ncural tumorr" co. ~r~ ;5 "P the steps of ;ntroducing into the ~rou th environment of the neurons a
growth inhibiting amount of arretin, r~S.~ t~ theseof, or an ar~etin agonist.
25 In yet a fi rther aspect of the present invention, arr~in can be used to design sn}all m(~ es to
block neurite o.ll~uwth and neural tumor growth. These small molecules will be usefill to block
gro~th in situations invol~ Tant spr~ul~lg, epilep~y, or n~et~st~ci~
A fiurther c.l-bodin~e.,l involves a method of s~p~. ~O~ ; the inhibition of neuron gruwth,
30 co~ , the steps of delivering to the nerve growth envirolb~ tibodies directed a~ainst
arretin in an amount ef~ective to rever~e said inhibition.
another a~pect of the pre~ent invention arretin can be used to design alltagon;ot agents that
~..ppr~ the ~rretin-neuronal growth regulato~r system. These 8ntagolli8t a~ents can be u~d to
35 promote a:con ,cgrow~- and recovely ~om traunu or neurode~c~aLi~e disease.
1(~
SENT BY~ 15-96; 8:16PM; 6135637671~ 16046694351;#20
~190418
In accord~nce w~th another aspect of thc prc~nt inven~on, there i5 provided an assay method
usefill to identify ar~etin anlag~ni~; agents that ~yy,~ inhibition of n euron growth, comprising
the step~ of
a) cultu~ng neurons on a growth ~ e substrate tbat i,.co~ alcs a ~rowth-inlf~bilin~
amount of arretin; and
10 b) exposing the cultured neurons of step a) to a calldidate arTetm antagonist agent in an
a~nount and for a period ~ nt l)lUs~ to permit growth ofthe neurons;
thereby identi~ rretin anl~go.~ the c~nrl~ of step b) which elicit neunte outgrowth
from tbe cultured neuron~ of ~tep a).
15 In accordance with another aspect of Ihe pre~ent inv~n~ion, there i~ provided a method to
suppress the inhl~itian of neuron, complising the steps of deliver~g, ta the nerve growth
environment, an arretin anta~onist in an amount ~ to reverse said inhibition.
In another er~bodiment, the nucleic acids ~ncoding arretin and/or it~e I e~eplor can be u~ed in
20 anti~en~e tec~iques and therapies.
A~retin inhikits neurite olltgrowth in nerve cells and nel.,.,t'-Atoma cells. Such inhibitory protein
COlllpli3~9 a 70,000 d~lton m~'e~ weight protein and analo3~s, derivatives, and fragmRnt~
thereof. Arretin atld its related proteins proteins may be used in the treatment of patients with
2~ malignant tllmors which include but are not limited to ~ -nm~ and nenre tis~sue tumors (e.g.,
neuroblastoma). The ~bsence of the arretin prote~n~ can be d;sy.nnsti~ for the 1~ eeence of a
malignant tumor such ~s those n~c~ tic to the brain (e.~., gliobl~ ). The present inventiol1
also relates to ~nt~gonists of arretin, in~lu~lin~ but not limit-ed to, antibodie~. Such ant;bodies can
be u~ed to neutrali~e the neu~ite growth inhibitory ~ctors for regeneral;~e repair after trauma,
3~ degei~e~ion, or inflammation. In a fi~rther specific embodument~ monoclonal antibody may be
~sed to ~lUll.~)t~ re~eneration of nerve fibers over long rl;otr~r~ followin~ spin~l cord damage.
~ar~ous other objects and adv~ntages of the present invention will be~ome apparent from the
detailed dP~rtio-l of the invention.
1~
SENT BY~ 15-96; 8:17PM; 6135637671~ 16046694351;#21
21 9041 8
5 BRlEF DESC,'~II llON OF T~E DRAWINGS
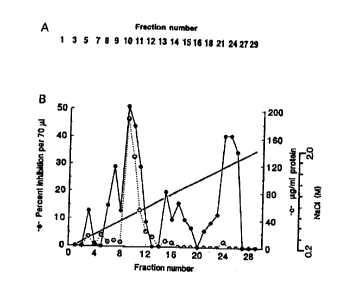
Ei~;ure 1 Anqlysi~ of ~rowth inhibition a~cr separation of myelin proteins by I)EAE anion
- "~ Lh~ n I U~ aphJ~ .
1~ Western blot~ of column ~actions probed with anti-~AG ~-)libo~ly
10 B. Neurite growth inlu~ition and protein profile present in the column firaction shown in A
Fi~ure 2. Ido~tirl~,dtion of 70 kDa c~...pon~nl~ in ~EAE chromatographic fraction~ ~om C~S
myelin as chondroitin sulphate pr~teoglycans. Myelin exlracts (lane l )l DEAE chromatographiç
~actions 10, 25, and 32 (lanes 2, 3, and 4) were s~ ted to SDS-PAGE (6-16% aclylamide
gradient) under reducing conditions~ alld d~ ted by silver staining (A) and Western blots with
anti-MAG (~ anti-T~-C ~C), ant;~ R (D~, and anti-CS 473 antibo~ (E) The position and
molec;ular weight in kDa of marlcer protein~ is in~ ~ted
Figure 3. Westem blot analy8is of PNA afflnity y~ Li~n of the 70 kDa CSPGs from I)EAE
chr~n.atographic lio.cliol.s 20-34. A. Pooled l~AE c~romatographic fiaction$ 20-34 (lane 1),
~actions 2 and C ~lanes 2 and 3) of Hepes bu~er wash, fracti~m~ 2 and 6 ~lanes 4 and 5) of hi~h
salt buf~er wa~h, and ~a~tions ~, 4, 6, and 8 (lanes 6, 7, 8, and 9) were sllbjected to SDS-PAGE
(6-16% aclylamide gradient3 under reducing conditions~ and detected by We~tern blots with
anti-CS 473 antibody. B Pooled DEAE chlc ~ raphic ~actions 20-34 (lane 1), flow-throu~Bh
of PNA ~ity eolumn (lane 2)~ ~action 2 (lane 3) and pooled eluate (lane 4) we~e ', :ed to
SDS-PAGE7, (6-1b% acrylamide ~radient) under reducing c~ru1itiQn~ and d~e~ by We~tem
blots with anti-MAG antibodies. The position and mo~ r weight in Id)a of marlcer proteins is
indieated.
Figure 4. Idc.llirlc~.Lion ofthe 70 kDa co.~pon~ phc~p~- ~an and ver$ican-related molecvles.
A and B. We~em blot analysis with 3F8 pol~vlonal anti-phosp'~ (A) and with polyclonal
antibodies a~ t re~ombinant versican ~B). Practions 20, 22, 24, 26, 28, 30, 32, and 34 (lanes
1-8) from l:)~AE chromstophy were c~bje~ted to SDS-PAGE (~-16% acrylamide gradient) under
reducing condition~ C, D, and E Western blot analy~i~ with 473 anti-CS antibody (C), 3F8
3~ polyclonal anti-phospl~ D) and polyclonal anti-reco,~ l ver~ican (e). Myelin extracts
SENT BY~ 15-96; 8:17PM; 6135637671~ 16046694351;#22
2l9~4l8
_
(lane l ), pool~d l~E~AE chromatographic fractions 20-34 ~lane 2~, pooled flow-thl ou~h from th~
PNA afEinity column ~lane 3), and pooled eluates from PNA affinity column (lane 4~ were
subjected t~ SDS-PAGE (6-16% acrylanude gradient) as in A and B. The position and molecular
weight in l~l)a o~marker protems i~ tic~led
Figure ~. Analysis ofthe 70 kl)a CSPGs after chondroitinase ABC tteatment. Pooled eluates from
the PNA affinity column (lane 1) and cl-on~ se ABC treated pooled eluates from PNA
affinity column (lanc 2) wcrc subjected to SDS-PAGE (6-16% acryla~nide g~adient) under
redllcing conditions ant d~t~led by aIs~ido bladc stain~ng ~A) and by Western blots unth
polyclonal anti-phns~ h~ran 3F8 (B). A bands at 28 kDa (in A lane 1) is PNA (artif~ ly
1~ eluted). Two bands above 72 lcDa (in A lane 2) are chondron~nase As¢. The po~ition and
mok -ular wei~ht in kDa of marker proteins is in~ t~l
Figure 6. Determination of cell-~ype e*,l ~s~;on of the 70 k~:)a CSPGs. Total membrane proteins
(1 oa ~ g) ~om brain (lane l), myelin (lane 2), oli~od~d~ oeyles (tane 3), astrocytes (lane 4),
cerebellar neurons (lane 5), hippocampal neurons (}ane 6)l NG 108- 1 ~ cells llane 7), and L-cells
(lane 8) were ~e~ted to SDs-PAc~E (6-l6% acrylan~de ~radient) under reducin~ conditions
and cletected by We~tern blots with pol~clonal aIIti-p~or,~:la. ~. r 3F~. The po~ition and molecular
weight in IcI~a of marker proteins is indicated.
Figure 7. lnh1~itory e~ects ofthe 70 kl:~a CSPGs on ne~ite ou~lowlh ~om cerebellar neurons.
Cel ~ellar neurons were plated as single cell suspensions on the 70 kDa CSPGs ~arretin) and
other gubstrates applied to PO:RN-treated nitrocellulose su~stlales. Cells were ~ for 24 h
before fixatioP and staining with toluidine blue. Error bars indicate standard deviation. Coating
col~enl~ Glions were a~out 50 nM (I :2~ dilution) und 10 nM (1: 125 ditution) for a~retin and
dena~red arretin (1~1) and 10 nM for laminin. Bars repre~ent percent neurons with neurites
(mean + SD).
Pigure 8. Inhibitory effiects of the 70 kDa CSPGs on neulite o~ Nth ~Qm hippocampal
n~ lippocampal neurons were plated ~s single cell s~p~n~ion~ o~ the 70 kDa CSPCs(arretin) and other su~lrales applied to PORN-treated tissue culture plastic. C.ells were
SENT BY~ 15-96; 8:17P~; 6135637671~ 16046694351;#23
2190418
.~
5 maintained for 24 h before fu~ation and st~ining urith tnl~ e ~lue. Error bars indieate ~f ~ d
deviation. Coating ~ allOI~S were about 50 nM (1 2S dilution) and 10 nM (1: 125 dilution)
fior arretin and dena~ured arretin (DN) and 10 nl~ or l~ninin. Bars Icple~ d percenl neuruns
with n~tes (mean i SD).
Figure 9. inhibitory effect~ of the 70 kDa CSPGs on neurite ~u~.~vvth ~om NG108- 15 cells.
l~G108 cell5 were plated as sin~le cell suspen~ions on the 70 kDa CSPGs (arretin; inhib.p) and
ot~er ~ub~trates applied to PLL-t~eated tiasue ~ re pla~tic~ Cell~ were mainta~ned for 24 h
before fixation ~nd staining -with toluidine blue. Coating concentrations were about 50 nM (I :5
dilution) for arretin (;nhib.p~ and denature arretin (denat. inhib.p) and 10 nM for laminin (~M).
15 Bars l~lt;se.~l neurons uith neurites (% growth~. PLL= polylysine.
DETAILED DESC RIPTION OF THE INVENIION
Fnr the purpose of the present invention the followin~ terms are defined below.
The term, neurite growth regulatory factor, refiers to either a~retin or its l~ceptor.
"Agoni8~' refers to a pharm~e~ltic~l agent ha~ing bicl~ical activity of inhl~iting the neurite
outg~owth of neurons cultured on a permissi~.~e ~ubstrate or inhibitin~ the ~ .,.dLion of
25 rlsm~ged neurons. lt would be desirable to ulhibit neuron growth in cases of epilepsy,
neuroblastoma, and n~ ron.~s, a disea~e ~tate in a ~ nl ' which includes neurite ollL~owll- or
other neural ~rowth of an ~--Ol ~l sort which causes pa~n at the end of an amputated limb.
Antago~t~ ~hich may be u~ed in accordance with the present inven~ion include without
limitation a arretin fra~ment, an analog of arretin of the arretin l~J ubll~cnt, a de~vative of either
30 arretin, the arretin ~llellt or 8aid analog, an anti-idiotypic arret~n antibody or a bindir~
~a~ment ther~of7 ~retin ectodonlain and a pha~ uli~i agent.
'L~nt9~ -u~t" refers to a pha.- :?r:~tical agent which in accordance with the ptesent invention
which inhibit~ at lea~t on biologi~ activity normally associate with a~retin, that i~ bloslrin~ c)r
3~ y the inhibition of neuron growth. hnt~onist~ which may be used in acc~ with
IL~
SENT BY~ 15-96; 8:18PM; 6135637671~ 16046694351;#24
2190418
5 the pre~ent invention include without lim~tation a arretin antibody or a binding fragment of said
antl~ody, a arretin fia~ment, a deri~ative of ~rretin or of a arretin fr~ment, an analo~ of arret~n or
of a arretin rl .y,.l.e.ll or of said derivative, and a pharmaceutical a~ent, and is further ch~l ~ctP. ;,~d
by the ~ v.~y o~ g arretin .~ dted inhibition of neurite outgrowth.
10 The agonist or ~I.t~,ol~ of arretin in ~c~rdance ~4ith tbe pre~ent i..~ tion is not limited to
arretin or it~ derivative~, but al90 includes the lhe. ~p~ulic application of ~1 agents, referred herein
as pharmP c - .ti.~ql agents, which alter the hiol~r~' activ,ity of the nellronal receptor for arretin
such that growth of neurons or their axon i~ ~u~ wbcd. The reeeptor can be identified with
knowtechnologie~bythoseskilledintheatt~Mason,(1~94)(,~t~rr. ~inL,4:1158~ l)andits
15 ~SO~ 5!n with arretin or rlag~ thereof can be detern~ined. The neuronal rec~plor for arretin
may or may not be the same a~. cell surface n~r~ tbat recogt~ze and bind ar~etin in an
edhe~;on as~ay (Kelm et al,, (t994) Curr. B~ol., 4:965-972). Once the a~ive arretin-reco~Snition
domain ofthe receptor(s) is/are known, a~,.upl~te peptides or their analogs can be deci,~tled and
prepared to serve a~ agonist or anl~gon;~ of the arretin-receptor intetaction.
Thc term "effective amount" or "growth-inhibiting amo~mt" refers to the amount of
phar-- - '~t;,C 1~ agent I ~u..~,d to produce a desired agor~ist or antagoni~.t effect of the arrelin
biological activity, The preci~ effiective arnount will vary with the nature of pll~rll Dcc;~nr .l agent
~qed and may be detern~ned by one or ordinary skill in ~he art with only routine eA~ iOn.
2~
A.~. used herein, the terms "a~etin biologic~l activity" refers to cellular events L~ ,c.~d by arretin,
b~ng of either biochemical or b 'opk~i~l nature, The follow~nK list is ndded~ ~thout
lirnit~tion, ~hich dic~l~se- some ofthe known acti~ities s~sor;~ted w~th contact-Tnedi~te~l growth
1 ~, lt- of neurite outgrowth, ~ ;c n to neuronal ~ells, and promotion of neurite out ~routh
30 from new born dorsal root ganglion neurons
Usc of the pl~a~e "s~lb~tu .~1 ;Ally pure" or "isolated" in the present ~ir~ l~iu-- and claims as a
modifier of DNA, RNA, polypeptides or proteins means that the DNA, RNA, polypeptide~ or
pr~teins so d~signated have been separated ~om their in vivo cellular environi\~el-t. As a result
35 ofthis separation and pur;fication, tbe 6ub~ y pure DNAs, R~As, polypeptides and proteins
SENT BY~ 15-96; 8:18PM; 6135637671~ 16046694351;#25
2190418
5 are usefill in ways that the non-separated, impure ~NAs, RNAs, poly~,cplides or pr~teins are not.
A~ used herein, the term "biologically active", or lef .~ e to the biological ~tivity of arretin or,
or polypeptide fi~nent thereof, refèrs to a polypeplide that is able to produce one of the
fimctional chara~e ;..~;es exhibited by arretin or its rec~p~ors d~lil,ed herein In nne
10 embodiment, ~ g~cally active proteins are those that de.l,onstlate inhibitory growth activities
central nefvous ~ystem neurons. Such aeti~Jity may be assayed by any metbod known to those of
skill in the art.
Ba8ed on the present e.,;dence that arretin is a growth inhibitory protein in myelin, the means ~xist
15 to identii3r a~ents and therapies that ~pp~ arretin-r,.c~a~ed inhibition of nerve growth
Further, one can exploit the ~ourth inllibiting properties of arretin, or arretin agonists, to suppress
d~s..~ nerve growth. Without thc clitical finding that arretin ha~ grow~ inkil)ilory properties,
the~e ~l,al~;ies would not be de~eloped.
20 The d~ption of the present invenlion comprising a neuron a~d neural tumor growth regulatory
sy~tem ~an be divided into the following section~ ~olely for the purpose of dc3cliption: (1)
-'- on pwifi~ation and charaetçri7~tinn of arretin; (2) production of arretin-related deriv~tive~,
analogs, and p~,Jtid~,~, (3) alTetin art~ni~t~ and assay methods to identi~ alTetin pntag~ni~t~;
(4)Cl~~ rizationofa~Te~in~c~pt~r~ nrlec~ clomngofgene~orgcne fr~ nt~
2~ encoding alTetin and its re~"tur~; (6) ~,el~;on of arretin related deriva~ive~, analogs, and
p~ P~ (7~ pr~ c~io" of antibodie~ against the col,~r~n~ ofthe a~Tetin ~routh regulat~ry
systen~, (ie. ar~etin, it~ receptors, and the nucleic acid ~equPnr~P~ ~oding for these proteins); (~) the
diagno9tic, ther~peutic and resea~ch uses for each of these cG~ and the antibodies directed
thereto.
3~
1. ~sola~oJt, Punhcat~on, ana' Charactenzafion of Arr~n
The present invent~on relates to CNS myelin associated ~nhibitory proteins of neurite growth and
-16-
SENT BY~ 15-96; 8:18PM; 6135637671~ 16046694351;#26
2190418
5 receptors of CNS myelin associated inhibitory proteins of neurite growth. The CNS myelin
associated inhl~itory proteins ofthe invention may be isolated by first isola~ing myelin and
subsequent pu.;[;~lion i' ~,fiUG.. lsolation prvcedures which may be employed are described
more fully in the gections which follow. Altematively, the CNS myelin associated inhibitory
proteins may be obtained from a reçombinant e.~ ystem. Pr~cedurcs for the iso!~ l or
10 and purification of ~ e;)lols for the CNS myelin ~sociated inhibitory proteins are described
below.
Isolati~n and Purification of Arretin Proteins
1~ Arretin proteins can be isol~ted ~om the CNS myelin of higher vertebrates inGluding, but not
limite~ t~, birds or mammals (both human and nonhuman suçh as bovine, rat, porcine~ chick, etc.).
Myelin can be obtained from the optic nen~e or from central nervous system tissue that includes
but is not limited to spinal cords or brain stems. The tissue may be hon~o,E~. .;,.~d using procedures
11~ 9 ~ ~ed in the art (Colman et al., 1982? J. Cell Biol. 95.598 G08). The myelin fraction can be
20 isolated s~aseyu~ly aiso using procedures fl~ (Colnun et al., 1982, supra)
n one embodiment of the invenlion~ the CNS myelin associated inhibitory proteins can be
solubilized in deter~ent (for e.g., see McKv.l~cll~ et al l 1994). The solubilized proteins can
~lb3~.~ y be purified by various procedures known in the a~, including but not limited to
2~ chromatography (e.g., ion exchan~e, affinity, and sizing chromatography), cenl~;fi~g~t;on~
el~vl~oph~l~lic pr~e~iu,~ olubility, or by any other standard technique for the
pllr~cation of proteins ln one aspe~vt, the solubilized proteins can be subjected to one dimen~iar
el~l~o~.h~ OJs, f~ .. vd by i~oeleel.;c fnr.llcc;~ and elut;on from the focus~in~ gel. Gel~luted
prote~ c~n be acetun~ ed rediOsolved in 10% formic acid and chr~ ,graphed on a
30 C~subr4 re~er6e phase ED~LC column.
Alternativelyl the CNS myelin associated inhibitory proteins may b~ isolated and purified using
imml-aological procedures. For example, in one embodiment ofthe invention, the proteins can
-17-
SENT BY~ 15-96; 8:19PM; 6135637671~ 16046694351;#27
2190418
fi~st be soh~bilized u~ing d~t~ d (e.g., ~onidet P~O.TM, sodium dw~,holate). The proteins
may then be isolated by immu.~op, ~e;pitation with antibodies. Altematively, the CNS myelin
inhibitory proteins may be i801ated using i...,...~ effinity chromato~ ~IJhf in which the
proteins a~e applied to an antibody colunm in
s~' .h:1:7~ form.
a. Pro~ct~'on af Am~n~R~c~ Der~bves, ,4~Jo~s, and Pep~
The production and use of derivatives, analogs, and peptida~ rela~ed to arretin are also envisioned,
and within the scope of the pre~ent invention and include molecules anhgo~ tic to neur~te growth
15 regul~t~Ty factors (for eYAmp'~, and not by way of limitation, anti-idiotype antibodies) Such
derivatives7 analogs, or pepti~e~ which have the desired inhibitory activ~ty can be used, for
ex~rnple, in the tre~ment of n~r~bl~tornA- Derivatives, analogs, or peptides related to a neurite
growth regulatory ~ctor can be tested for the degired activity by sssays for no~ si~e
le effects For ~Y~mple, procedures such a~ the assay for nonperm~ ..e~s in which the
20 effect of the va~ious tl -~Atinn produc~s on the spreading of 3T3 cells on a polylysine coated
ti~ue culture dish is ob~erved
The neunte growth regulatory factor-relzted derivatives, analogs, and peptides of the invention
ean ~e produced by vanous methods known in the art. The manipulations which re~ult in their
25 production can occur at the gene or protein level For f ~ c, a cloned neur~te grourth
regulato~ ctor gene can be mo~ by any of numerous strategies kno~n in the art ~Maniatis,
et al, 1~82, h~ culqr Cloning, A Laboratory Manual, Cold Spring ~ bor Laboratory, Cold
Spring Harbor, N.~f ) A given n~rite growth re~ulatory factor sequence can be ~leaved at
appropria~e sites with r~trietinn ~ndonllclcasc(~ to ~.,q...atic m~ r~ ~n~ if desired,
30 iCol~t~ and ligated in VitlO. In the production of a gene ~neoding a derivative, r--lc~le~ or
peptide related to a neurite growth regulatory factor, care should be taken to ensure that the
moclified gene remains within the same translational reading ~ame as the neurite grow~h
1d10.y faator, uninterrupted by trar~cl~til~n~l Stop signals, ~n the ~ene region where the desire~
SENT BY~ 15-96; 8:19PM; 6135637671~ 16046694351;#28
~1~0418
5 neurite grou~th regulatory f~ctor ~cific a~tivity i~ encoded.
Additionally, a given neurite growth re~?ulatory factor gene can be mutaIed in ~itro or in vivo, to
create andlor destroy t~ laeion, initiation, and/or termination ~ences, or to crea~e variations
in coding reg~ons andlor form new ~ ;elion endonuclease ~ites or destroy ~.e .;~;n.e ones, to
10 filcilitate fi~ther in vitro modification. Any tecbnique for ml~t~genes~s known in the art can be
used, including but not litn~ted to, in vitro site-directed ml~t~g~nP~is (~utchinson~ et al., 1978~ J.
Biol. Chem. 253:6551), use of TAB® Iinkers (Pharmacia)? etc.
15 3. Anre~n Anto~o~usrs ~nd Aswy Uctholls to Id~nt fy Am~n An~a~oni~
In one embodiment suitable a~ alrctin antagonist r--~id~l ~ are developed col~p~ , fra~n~ntR
analogs and derivatives of arretin. Such candidates may illlelr~re with arretin-mediated ~srourth
in}~ibition a~ co,n~tili~re but non-filnçti~ ' mimics of endo~enolls a~etin. From the amino acid
20 B~ ~uçn''~ of arretin and from the cloned DNA coding for it, it will be appreciated that a~retin
~grn~ntS can be produced either by peptide ~ynthesi~ or by ~ bh~ant DNA ~ ,rcssion of
either a lmll~lcd domain of arretul, or of intact arre~n could be pl.,p~red using ~tandard
recominant proGedu.~q, that can then be digeste~ enzymically in either a ra,ndom or a site-select~ve
manner. Analog~ of arretin or a~etin ~ can be generated ~19O by I ~c~ a~ll DNA
techniques or by peptide synthesi~, ~nd will mcorporste one or more, e g. 1-5, L~ or I:)-amino asid
substitutions. Derivative~ of arretin, arretin ~ Pnt~ and arretin analo~s can be ~nerated by
chemical reaction ofthe parent substance to ~.co~ te the desired deriYa~izing group, such as
N-te~i~PI, C-tern~nal and intra-re~idue modii~ying groups that have the effect of n~lCl~ing or
st~b~ g the __~lit- --~ ortarget amino aads within it
Ln specific en~bodiments of the invention, . ~ arretin a~t~nist~ include those that are
derived ~om ~ detar -u t .. of the filnctionally ac~ive reg~on(s) of arr~n. The antibodies
mentioned abpve and any others to be pr~ ed against e~ilu~ in arre~in, when found to be
-19-
SENT BY~ 15-96; 8:19PM; 6135637671~ 16046694351;#29
2190~18
5 filnGtion-b' ~ in in vi~ro assays, can be used to map the active re~gions of the polypeptide as
has been l'.,,)ull~d for other prote~ns (for e~ample, ~e Fahrig et al., (1993) Europ., J. Ne~,os~i.,
5: 1118-1126; Tropalc et al., (1994~ J. N~., oche,..., 62: 854-862~. Thus, it can bc determined
which regions of ar~etin are reco~zed by n.,~ -' r~eptol~ andJor are involved ~n inhibition of
neurite oulgrow~l. When those are l~own, synthetic pcpt;des can be prepared to be a~ayed as
10 c~ndidate r ~Eoni~ of the arretin effiect. Derivative~ of these can be prepared, including tho~e
with ~elected amino acid sub~ tir.~s to pro~ide desirable plop~.li~ to enhanGe their
err~L~fell~:ss as antagoni8t8 of t~e arretin çandidate functional regions of arretin can also be
dete~m~ned by the preparation of altered fotms of t}le arretin domains usin~ r~u~ ant DNA
technolo~e~ to produce deletion or - -- lion mutants that can be ~ .,~od in valious cell types
15 as chim~eric protein~ that contain the Fc portion of inlml~no~l( b~llin G (Kelm et al., (1~4) ~ r~.
Biol., 4: 96~-972) Alternatively, candidate m~tant forms of arretin can be eA~ ssed on cell
Qur~aces by transfection of ValiOU8 cultured cell types. All of the above forms of a~retin, and
form~ that may be generated by technolo~ie~ not limited to the above, wl be tested for the
pr~"~ of fi~rr,tiQnol regions that inhlbit ~r s~.~)p..,ss n~te ou~ uwth, and c~ sed to
20 de~i~n and prepare pe~tide~ to ser~e as nnt~gollist~.
In a~ ~ ~ rd~e with an a~pect of the inYention, the arretin ant~onist i5 fi4~ dlCd as a
pharmaceutical con~ t;nn which co~t~inc the arretin ~t~.g~rist in an amount ef~ective to
&I~)pr~ Tetin-m~ipted inhibition of nen.~e growth, in combination with a suitable
25 phs~naceutical carrier. Such compo~itions are u~ l, in accordance with another aspect of the
iO.I, to suppress arrctin-inhibited nerve growth in pa~ient~ diagnosed with a vanety of
l~"r~ o-d~r, conJilion~ and ailments ofthe PNS and the CNS where ~ to
increase neurite ~ ~,n~ , grouth, or r~ n is desired, e.~., in patients wi~ nemous
system dam~e. Patien~ su~er~ng ~om tra ~ c disorders (; ~ ' diT~ but not limited to spinal
30 cord injuries, spinal ~ord lesion~, sur~ical nerve lesions or other CNS pathway lesions) dama~e
secondary to ~ rction, inrecl;nl, ~Apo3ure to toxic a~ents, mali~r~ eop~
.~..d u...e~, or patients with variuus types of d~nel ali.~, disorders of the central nerYous sy~tem
(Cutler, ~1987) In: ~cien~ificAmericcnl~ec~ic~nes, vol. 2, S~i~ntific Amer~can Inc.~ N Y ~ pp. I 1-
-2~
SENT BY~ 15-96; 8:20PM; 6135637671~ 16046694351;#30
21904t8
5 l l l- 13) can b~ treate~ ~nth such arretin 8-~t~'li5~ of such disorders include but are
not linlited to Strokes, ~l~h~ .~e~'9 di~ease, ~own's ~yndrome, Creutzfieldt-Jacob disease, kuru,
(~erstman-Straussler ~;yndrome, scrapie, tranc s~itl~ mink ~n~ephal~pathy, Hun~ington's disease~
Riley-Day familial d~ ~ Itnnom a, multiple systen~ atrophy, amyl~1t, up! ic lateral sclerosis or Lou
Gehrig's disease, pro~r~3i-~ s.~ ar palsy, P~,L~ disease and the like The arretin
0 allta~OlliSt5 may be used to promote the regeneration OrC~s pathways, fiber systems and tra~s.
Administra~on of antibodies directed to an epitope of arre~n, or the binding portion thereof, or
cells secrelil~g ~uch antibodies can also be used t~ inhibit arretin r.-... ~ in p~tie~t~ In a
particular eml:oJ; ..~ of the ~ tion, tbe arretin ~ g~Jr ~ is used to promote the l~ge~e~alion
of ne~e fibers over lon~ di~tance~ foll~winx spinal cord dam~ge.
ln another cmbodiment, the invention provides an a~ay method adapted to identif~r arretin
antagomsts, ~at is agents that block or suppress the gro~th-inhibiting al;tion of arretin. In its
most convenient form, tbe assay is a ti~sue cutture assay that ~ su~ neu~ite out-growth as a
convenient end-point~ and accordingly uses nerve cells that extend nellrites when grown on a
2~ ~IIL~ subslrate. Nerve cells suitable in this reE ~rd include neuroblastoma cells ofthe NG10
lineage, such as NG108-15~ as well a~ other neuronal cell lines such as PC12 cells (A nelican
Type Cul~re CoUection, 12301 Parklawn Drive, RockYille, MD 20852 USA, ATCC ~cce~iion
~0. CRL 1721), human neuro~ u~ cells, and primary cultures of CNS or PNS neurons taken
from emb~yonic, postnatal or adult animais. The nerve cells, for in~.ta~ce about 103 cell~
25 n~c;rowell or equivalent, are cultured on a growth pem~issive ~bstra~el such as polylysine or
lamin~n, that is over-layed with a growth~inhibit~ng amount of arretin. The arretin incorporated in
the culture is suitab~ myelin-extracted arretin, ~ltho~gl~ forms of arre~in vther than endo~enolls
form~ ~an ~e u~ed provided they e~bibit the arretin p~ope~ ly of inhibiting neuron growth when
added to a wbstrate that iB o~he.~. ;~ growth permissiv~.
In thi~ assay) candidate arretin ~nt~g~ni~, I.e., compounds that block the growth-inhibiting effect
of arretin, al e added to thc arretin-conta~nin~g ti~sue culture prefer~bly i~ amount ~lffi~ier t tu
neutra}i~ the arretin growth-inhibiting activity, that is be~ween 1.~ and 15 ~g of arretin
SENT BY~ 15-96; 8:20PM; 6135637671~ 16046694351;#31
21qO418
S a~tag -nicte per well contaWng a density of l000 ~Gl0~-15 cells/well cultured for 24 hr. in
DlllbeCCO'S mil~ s9e1't~ m~i'lm Afta cullu.i.~3 for a period Y~lffl~ient for neurite
oulgr~wl}~, e.g. 3-7 days, the culture is evaluated for neurite outgrowth, ~d arretin anta~onists
~re thereby revealed ag those GL~ wbich elicit neurite outgrowth. Desirably, c~ndidates
selected as ar~etin ~ njj~ are those which elicit neurite outgrowth to a statistically ~;g~
extent? e.g., in at least 50%, more desirably at least ~ , e.g. 70%, per 1,000 cultured neurons.
Other assay tests that could be u~ed include without limitation the following: I) The growth cone
collapse assay that is used to as~s growlh inl~bitory activity of collapsin (Raper, J.A., and
Ka~ ...-w, J.P., (1990) Neuron, 2 2 l -29; I.un et al., (l 9~3) Cell, 75:217-227) and of various
lS olher inhl~itory ".oleeu - (Igarashi, l~. et al.l ~1~3) ~cience, 25~ 77-79) ~.he.~y the test
sub~tance is added to the allture medium and a loss of elaborate 8rowth cone morphology is
~cored. 2) The use of patterned sub~tra~cs to as~ess sul)~llal~ ~,.ef~.cllce (~lalter, J. et al., (19~7)
Deve~opment, 101 ~ -913, Stahl et al., (Ig90) Neuron, 5 :73~-743) or a~ arlc e of test
s~lb~llah~ (Ethell~ O W. et al., (1993) De~. Brain Res., 72: 1-~). 3) The expres~ion of
re~ombinant protein~ on a heterologous cell surface~ and the t~n~reGI~d cells are used in cn-
culture ~ t~. The ability of the neurons to extend neurites on the transfected cells i5
as3c~3ed (l~ukhopadhyay et al., (l 994) Neuron, 13 757-767). 4) The u~e of sections of lissu~,
such as ~ections of CNS white matter, to a~sess ~"olo~l that may modulate growth inhibition
(Carbonetto et al., (1987) ~ Neuro~cience, 7:610-~20; Savlo, T. and Schwab, M.E., (1989) f.
Ne~lrosci., 9: l 126-1133). 5) Neurite retraction assays whereby test substrates are app~ied to
d,~el~iated neural cellB for their ability to induce or inhibit the retraction of previously Pxtpn~
neuntes a~k et al (1994) J. ~ell Bio., 126:801-810; Sudan, H.S. et al., (1992) lVeuron,
8:363-375; ~m~h~i~, N. (1~93) J. Neuroc~em., 61 :340-342). ~ The repul~ic~ of cell-cell
inLe~ ns by cell a~Jt;on assays (Kelm, S. et al., (1~94) Curre~tBiolo,~, 4:965-~72;
Brady-Kainay, S. et al., (1gg3) .J. Cell ~iol., 4:961-972). 7) The use of nitrocellulose to prepare
su~sXa~s for growth a~says to assess the ability of neural cells to extend neurites on the test
slra~ , C. and l~emn on, V., (1987,~PiVAS7 84.7753-7757, Dou, C-L and Le-r~ne,
J.~L~ (19g4) J. ~ew~ ence, 14 7616-762~).
~22-
SENT BY~ 15-96; 8:20PM; 6135637671~ 16046694351;#32
21 9041 8
. .
S Usefi~l arretin anta~ol~is~s include ~ntibodie~ to arretin and the binding fr~ nt~ ofthose
odies~ ~ ~t hod;e$ which are either mo~e~toral or polyclonal can be produced which
reGog~ e arretin and its variou~ epitopes us~ng now routine procedures. ~or thc raising of
ant~oody, various host animals can be; ~.n ~ d by inJection with arretin or ~agment thereof,
includin~ but not lim~ted to rabbit$, mice, rats, etc. Valious ad~uvants may be used to increase the
10 immlmolo~cal rc3,)0llse, depending on the ho~t specie~, and including but not limited to Freund's
(complete and ;~ul ~,!ete), mineral gels such as aluminum hydro~de~ surface acti~re substances
such a~ l~ ol~ in, pluronic polyols, poly~ions, pe?tidcs, oil emlllci~ln~ keyhole limpet
hemocya~ns, dilul.it-ophellol, and potentially usefill human ad~uvants such as BCG (Bacille
C~l ' ~ I" Guerin).
4. Is~ion and Purifi~rzlinn of ReccptorsforArre~n
Re~ t..r~ for arretin can be i~ola~ed from cells whose atl;achment, spreadin~, growth and/or
motility is ;..I~;~in d by arretin. Such cells include but are not limited to fibroblasts and neurons. In
20 a ~"~f~ l embodiment, neurons are used as the 50UICC for isola~ion and purification of the
recept4.~
In one em~G~i"~ t, receptors to arretin may be isolated by affinity cl~lG~l~lography of neuronal
plasma membrane fractions, in which a myelin associated inhibitory prot;ein or pepbdc fragment
25 thereofis ~obiLized to a solid support Alte..,~tiv~ly, ~ Lor cDNA may be isolated by
~A~ r cloning using purified arretin a~ a ligand for the seJection of receptor v~ g
clones.
Altematively, arrelin protein may be tagged for use a~ a r~po, l~r eO detoct receptors of arretin,
30 U5i~ techniques that are well known in the art. There a~e many dilE~rent types af tags that may
be employed xuch as ~ouresccnce radic~ ve tags.
SENT BY~ 15-96; 8:20PM; 6135637671~ 16046694351;#33
2~90418
-
. Molecu~ ~oning of Ge~es or Gene Fr~gmen~s Enco~ng Anehn and Its ~e. ~
Any mammalian cell can potentially serve as the nucleic acid source for the molecular cloning of
the genes encoding a~retin or its reeeptors. The DNA may be otlained by st~ d procedures
known in the art from cloned DNA (e.g., a DNA "libra~y"), by chemical synthesis, by ~NA
10 clonin~, or by the ~loning of g~nn,.. - DNA, or fi~nents thereof, pur~fied from ~he de~ired
mammalian cell. (See, for exunple, Mania~is et al., 1982, hlol~ Ci~ning A Laboratory
Manual, Cold Spring Harbor l,~u~ y, Cold Sprin~ Harbor, N Y.; Glover, D. M. (ed.), 19~,
DNA Cloning: A Praç~ical Approach, ~L Pres~, Ltd., Oxford, U. K., Vol. 1, Il.) Clones deriYed
~om ~enomic ONA nuy contain re~sulatory and intron ONA regions, in addition to cod~n~
15 regions; clones derived from cDNA will contain only exon se~ue~c~ hatever the source, a
given neurite gro~ regulatory f~etor gene should be molecularly cloned into a suitable veetor
for propagation ofthe gene.
Tn the mol~ qr cloning of a neurite growth regulatory factor gene from ~senomic l )NA, DNA
20 r.~ re 5~,~e.~led, some. of which will encode the desired neurite growth regulatory factor
gene. Tbe DNA may be cleaved at specific s~tes us~ng various restnction e,~ s. ~Itematively~
one rnay use l)N~se in the presence of manganese to f, ~ ~t the DNA, or the DNA can be
physically sheared, as for example, by sonication. The linear DNA ~ynP.nt~ 4an then be
~eparated a~cordil~, to size by standard technique~, including but not limXed to, a.~arose and
25 poly~crylamide gel 414e~vpl~0re~is and column chromato~aphy.
On4e the DNA ~ 1Y are generated, i~e~ ;n of the specific DNA fra~ment ~ a
neurite growth re~ulatoIy factor gene may be accompli~hed in a number of way~. For eAallll~le, if
an amount of A ncurite grnwt~ r~ - ry factnr gene or its specific ItNA, or a r~n~ellt thereof,
30 is available and can be punfied and labeled, the ,~ Lh~d DNA fr~gr~t~ may be ~creene~ by
nucleic acid h~ ion to the labeled probe (13enton and Davis, 1977, Science 196:180;
Grunstein anq ~Pcs~ Ig75, Proc. Natl. Acad. Sci. U.S.~. 72:3961-3965). ~or ~ . le, in a
.~,E~ ,d em~odiment, a portion of a neur~te grov~h regulatory factor ~nino acid sequen~e can be
-2~
SEI~T BY~ 15-96 ; 8:21PM; 6135637671~ 16046694351;#34
21 9041 8
-
S uc t to deduce the DNA sequence, which PNA c~U~nr~ can then be sy..t~ ~d A an
oligon~ id~ for u~e as a hyl>ii~ jon probe. Alternatively, if a punfied neurite growth
regutatory factor probe i unavailable, nudeic a~d fra~tions cnriched in neurlte growth regulatoTy
factor may be used a~. a probe, a~. ~n initial sel~ction procedure. It is also possible to identif~r an
appropr~ate neurite growth regulatory factor~ncoding r~gn~eq,1 by restriction enzyme
lO di~estion~s) and compari90n of l'~ e..t sizes with those l~ d acGor.l;ll~ to a known
l~cu;.~ map if such is available. Further gelection on the bu.is ofthe propc.lies ofthe ~ene, or
the physicat, chemical, or immu"olo~,i~t properties of its eA~I ~3ed product, as desclibed above,
c~n be employed afler the ini~ial selection.
15 Aneunte gr~w~ reglJlato~r factor gene can also be id~ fied by mRNA se'eetinn us~ng nucleie
acid h~ t;on followed hy in vitro translation or translation in Xenopu6 oocytes. Ln an
exan~ple of the latter procedure, oocytes are 1njected with total or size fractionatecl CNS mRNA
populations, and the membrane-associated translation products are scr~ned in a functional assay
(3T3 cell spreading). ~d3~ ic,n ofthe RNA with co~ 1 ..t~/ DN~ ~cD~A) pools leading
20 to the absçnce of eAI~re~d inhibitory factors indica~e~ ~ presence of the desired cDNA.
R~uc t;or nf pool ~ze will finally lead to i~i~tion of a single cDNA clone. In an alternative
pro~hll~, DNA fi;.4.,..~ can be used to isolate complementary mRNAs by hybri~ation Sllch
DNA fi .~ "~ 1!. may ~e~lese..l available, purified neurite growth regulator~ factor DNA, orDNA
that has been e.ll;ched for neurite ~rowth re~ulatory factor s~ ences 1~ ~o~.~ r ~.a sn
25 analysis or fil"etiol~l assays of the in vitro translation products of the isolated rnRNAs identi~es
the m-RNA and~ ~el;~re, the cDNA ~ ~ ,".~ that contain neur~te ~owth re~;ulatory factor
~equences. An example of such a rulleliOllD~ assay involves an assay for nG~p~ i5;,;Vene~s in
which the effect of the various translation produc~s on the spreadiny of 3T3 cell8 on a polyly~ine
coated tissue culture dish is observed. In f d~litioll~ specific mRNAs may be selected by adsorption
30 of polysomes i~lated ~om cells to i..~.lob-'i7~A antibodies ~.ec.ir~cdlly directed again~t a neurite
growth regul~,tory factor protein A r~ lir~ ' e ~ neurite growth regulatoîy factor cDNA can he
o~tl~ d u6ing the sele~ted mRN~ (~om the ~ bed ~ e~) as a templ~te The
radiolabeled ~RNA or cDNA may then be used as a probe to identifi~ the neurite growth
-25-
SENT BY~ 15-96 ; 8:21PM; 6135637671~ 16046694351;#35
21 904 1 8
-
5 ~ ~to~y factor DNA L~,..~ om among other ~enomic DNA fragm~ntR Alternatives t~isolating the neurite growth regulatoly factor genomic DNA include, but are not limited to,
chemic~lly sy~the~izin~ the gene sequence itself ~om a known sequenr-e or ma~n~ cDNA to the
mRNA which encodes the neurite grourth regulatoty ~actor gene. Other methods are possible and
within the scope ofthe invention Ihe iden~ified and iaolated E~ene or cDNA can then be inserted
10 into an appropriate clomng vector. A large number of veGtor-host systems known in the art may
be used. Possible vectors include, but are not limited to, cosmid~, pla~nid~ or mnrlifi~d vimses,
but the ve~tor system must be compatible with the host cell used. Such ve~tors in~lude, but are
not limited to, ba~i~phag~ such as lambda derivative~, or plasmids ~ucb as pBg322 or pUC
plasmid derivatives. E~ecombinant m~'eeules can be introduced into host cells via ll~Lsf~r.ll~tion,
1~ trans~ection, ;,~f~ct;ol~ ~le.lropo-ation, etc.
In an alternative embodimen~, the neurite growth regulatory factor gene may be id~nli~ed and
isolated a~er i~ ion into a suitable clol~ing vector, ~n a "sh~t gun" ~pprc arb En.;ch....,.4 for a
~ven neu~ite growth regulalory ~ctor gene, for examph, by size fir~tion~tion or subtract;on of
20 cDNA specific to low neurite growth regulatoly fa~or producers, can be done before insertion
inlo the cloning vector. In another ombodimont, DNA may be inserted into an expression vector
system, and the recombinant e~)r.~:~ on vector con1 ni - ~lL a neurite growth regulatory factor ~erle
may then be d ~ ~ by ~ ' assays for the neunte growth regulatory factor protein.
2~ The neurite growth re~ulat~.~/ façtor gene is in~erted into a don~n~ vector which can be used to
transfiorm, l~ ,,r~l, or infect ~ propl;ate host cells so that many copies ofthe gene sequences are
generatedr This can be accomplished by ligating the DNA f~ug~ t into a dol~ing vecto~ wllich
has comp~ taly cohesive telmini. ~Iowever, if the ~ !ementary re~triction sites used to
~agment the ~NA are not preSent in the ci~ning vector, the end~ of the DNA mr' ~ may be
30 enzSmatically rnn~;fi~ Altern~ively, any site desired may be pr~Juced by ligating nucleotide
~ ~5 ~inker~) onto the DNA t~nini; these ligated linlcers may cOI~l~Jl;3~ specific chen~ically
synthe~ized oli~o-~--rl~oli~ec 0-~0d~ ,lion endonuclea~e reCQ~n~ ~qu~nc~s. In an
alten~ative me~thod, the cleaved vector and neu~ite growth r~ 'atc ly factor gene may be modi~Sed
SENT BY~ 15-96 ; 8:22PM; 6135637671~ 16046694351;#36
2~904l8
-
5 by ho~opolyme~ic tailing. ld~tification of the cloned neurite ~rowth re~ ctor ~ene can
be accûmplished in a number of ways based on ~e ylup~. Li~,s of the DNA it~ , or alternatively,
on the phy~icall immunological~ or fimt;tional prope~ lie9 of its ~ ~., ded prote;n, For ~ .. p'e, the
I:~NA itself may be rlrs~l~ by plaque or colony nucleic acid hybridization to labeled probes
(Benton, W, and Davis, R., 1917, Science 19~:180; Grunstein~ M. and ~ ne~, D., 1~75, Proc.
N'atl. Acad. Sci. U.S,A. 72 3961). Altematively, the ~l~,~.ce of a neurite growth regulatory
f~ctor Rene may be detecte~ by a~ays based on ~ ,s of its eAlJl~d product. F~r ~A~Jmp1C,
c[)NA clone~, or DNA clones which hybrid-select the proper mRNA~, can be selected which
produce a prote;in that inhibits in vitro neurite ou~lowlh lf an antibody to a neurite growth
regulatory factor is available, a neunte growth regulatory factor protein may be itl~ntifi~d by
15 binding of labeled antibody to the putatively neurite growth regulato~ ctor-s~ynth~i7i~ clone~,
in a~ ELISA (h~u~e l;'lked imnll.nosorbent assay)-type pr~cedure. In specific embodiments,
ullllation of host cells with recombinant DNA m~clllP~ that ...col~o,2~le an i~olated n~urite
grow~ regulatory factor gene, cDNA, or Sy~ Ai ~ NA sequence enables generation of
multiple copies of the gene. Thus, the gene may be o~tained in large quantities by gro~ing
2û tran~formsnts, isolating the recombinant DNA mo~ from the transrolll.d.lts and, when
- ~ e eso~~y, retrieving the inserted gene ~om the isolated r ocom~inant DN~ If the ultimate goal is
to insen the gene into virus expression vectors such as vaccinia virus or a~enovirus, the
recombinant DNA rnolecule that incol ~o~ atcs a neunh growth r~gulatory faGtor ~ene can be
mo~lifie~ ~o that the gene i~ flanked ~y viruR ~uenG~3 that allow for gel~etic recombination in
25 cells infected with the viru~ so that the yene can be in~erted into the viral ,genome. A~er the
neunte ~,rowth re~ulatory faGtor DNA-co,.l~ 8 clone has been identified, grown, and harve~ted,
its D~A insert may be ¢haracteri~ed a~ dc~l ib~d hereill. When the genetic sh~¢ture of a neurite
growth regulatot~r fa~tor ,gene is kno~vn, it is possible to manipulate the structure for ûptimal use
in the p~esent ~ve,lltiOn. ~or e ~ , promoler DNA m~y be ligated 5' of a neurite ~rowth
30 regulatory f~tor coding seqllenc~, in addition to or repl~ce~ ofthe native promoter to proYide
for increased expre~ion ofthe protein. Many rïlaniF~ nn~ are po~ible, and within the scope of
the pre~ent in~ention.
SENT BY~ 15-96; 8:22PM; 6135637671~ 16046694351;#37
2190418
5 }~,~ of the Cloned Ne2-riJe G'rawth ~egu~a~ory Fac~4r Genes.
The n~ otid~ seqU~rp codin~ for a neur~te growth regulatory factor protein or a po~tion
thereof, can be inserted into an app.~.;ate e~pre-~3ion vector, i.e, a vector which contains the
necessary dements for the tra~L~cnption and translation ofthe in~erted protein-çoding ~eq~l~ce.
10 The ne~sary tr~s~ Jtional and lrar. 1~ iOl si~nala can also be 5~ r ~ by thc native neurite
growth regulatory factor 8ene and/or its ~anking regions. A valiety of host-vector systems may be
utilized to express the protein-coding sequence. These include but are not limited to mammalian
cell ~ystems infected with viruC (e.g., vaccinia vilus, adenovirus, etc.); in~sect cell ~ystems infected
with viru~ (e g., baculoviru~); microor~anisms such as yeast cOI ~a ~ yeast vectors, or bacteria
15 transformed ~nth bacteriophage DNA, plasmid DNA, or cosn~id DNA. The e~pr~sion el~,l.,c.~t~
of these vectors vary in their bL~ h~ and ~I.e- fi. ~ ;f i Depending on tbe host-vector system
utilized, any one of a number of suitable ll~n~c(i~ ~r and translation elements nlay be used.Any
ofthe mf~ho~lc prev~ously described for the in3c.l~oll of DI~A G~ nto a vector may be used
to construct exprexsion vector~ c 'a ~ a chimeric gene ~n~ ~t; ~ of appropl;dte
20 L~h~ ional/tran~tior~l control si~nals and the prote~n coding 3e~IU~ D~ TheDe melhods may
include in vitro ~~co.~ ant O~A and synthetic technique~ and in ~ivo r~cc...~k;.. I;ullS ~genetic
reoonlbination).
Expression vectors containirlg n~te growth regulatory factor gene in~ s can be identffied by
25 three general app~c~hes (a) DNA-DNA hybridization, (b) pre~enoe or ab~ence of "nurker" gene
filn~tio~ and (c) ~,~ e~on of inse~ted ~equences. In the first ap~lo~cl1, the pr~3cnce of a
foreiE~n gene inserted in an eA~ ;c n vector can be ~letected by DNA-DNA hybridi~ati~n using
probes ~ .nl r;~ erce~ that are h~ logous to an in~erte~ neurite growth regulatory factor
gene. ln the ~econd _, F ~ , the ri~ n~ v~lorA~osl System c~n ~e i~l~ntified and ~elected
based upontbe pr~ence or ab~ence of cerlain "I~ ." gen~ functions ~e.g., t~ymidine kina~e
actiYity~ rwis;tance to antibiotic~, tral.~f~,rlllALiol~ Fl~ ~nc ~,e, occ.l~lriol body for nation in
baculovirus, otc.) caused by the insertion of foreign genes in the vector. For example, if a given
neurite growth regulatory factor gene i~ inser~ed within the marker ~ene se.lue.~ce ofthe vector,
-28-
SENT BY~ 15-96; 8:22PM; 1 5637671~ 16046694351;#38
L~90418
5 recomb~ts contai~ the neurite growth re~ulatory factor insert can he i~ntified by the
ab~ence ofthe marker ~ene fim~ir~ ~ the third approach, rec~m~ nt ~ A~JIcSsio~l vectors can
be id~ -t;fir~ by assaying the ~rei~n gene product e,.~,~d by the r~.nbi~ Sucb assays can
be based on the physical, imn~ ~.e,lo~ cal, or fiunctional propc. lie~ of a given neurite growth
regulatory factor gene product.
Once a particular recombinant DNA m~e~ c is identified and i~lqteA seve~al m~tllod~ known ~n
thc ~t may be used to propagate it. Once a ~uitable hos~ system and growth conditions are
established, recomb~nant t;~.~,D;..OI~ vectors can be propa~ated and p~ctJal~d in quantity. As
prcviollDly P~p~ ~ the eA~ ,;,;on vectors which can be used include, but are not limited to, the
15 followmg vectors or their deriva~res: human or an~mal viruses such a~ virus or
adenovin~; insect viruse3 such ~s baculo~rirus; yeast vectors; bacteriophage vectors (e.g.
lambda)~ and pla~mid and cosmid DNA vector~, to name but a fe~.
In addition, a host cell strain may be chosen which m~d ~ 5 the e~pJ ~;on of the inserted
20 sequ~nce~, or m- ~lifif~ and proces es the gene product in the specific fashion desired. ~xpre~sion
~om certain promoters can be eh~ ~l in the pl es ~nc e of certa~n induGers, thus, eA~,r~i.;on of the
genetically ~ ,r~:d neurile growth re~ulatory factor protein may be controlled. Furthe~more,
di~.~l ho8t cells have characten~tic and speci~c me~hanisms for the translational and
post-translat;onal processing and mndific~ltiQn (e.g., ~us~1ation, cleavage) of proteins.
25 Appro~te cell lin~s or host systems can be chosen to ensure the desired - c d : ~ jOl~ and
pr~ ~ ofthe foreign prote~n ~A~ d. For example, expression in a t~lPriAl system can be
used to produce an ~ ,ob~'a~ed core protein product. ~ ,~;on in yea~t will produce a
~l~cG~lated product. E~xpression in mqmrn~ n (e.g. COS) cell~ can be used to ensure "nati~e"
~Iycosylation of the heterologous ncurite growth r~,g ~ ~or protein. Fu~ ." ere, di~re"~
30 vectorJhost e~p,~;on system~ may effiect proccss;ng reactions such as proteolytic cleavages to
di~cr~i~l extents.
Idenhf~c~tion and PuriJ~ca~ion of ~he Expressed Gene Prod~Jct
-2~-
SENT BY~ 15-96; 8:23PM; 6135637671~ 16046694351;#39
~9~418
Once a ~ anl which ~I~,DDeS a given ne~rite growth regulatory factor gene is identi~ed,
the ~ene product can be purified ~nd ~zed a~ d~c.;bcd above. The amino acid ~uenr~e Of
arretin and its r~epLor protein can be de~lced from lhe nucleotide ~uel ~e of the cloned gene,
the protein~ or a ~agment thereof, to be synthesized by standard chemical methods
knn~vn in the art (e.g., see ~lunlcapiller; et al., 1984, Nat~re 310:10~-111). In particular
10 embodiments ofthe present invention~ such neurite growth regulatoly factor proteins, whethcr
produeed by recomb.nant DNA tec~niques or by chernical synthetic methods, include but are not
limited to thosc contai~ing altered seq~ n~ ~C in which rull4~ y equivalent amino acid residues
are ~bstituted for residues within the se~uenGe resulting in a silent change. For ~,x~ one or
more amino acid residues within the s~ e~-ce can be ~ mlPd by another amino acid of a
15 similar polarity which acts as a fiJn(~ n~l equivalent, resulting in a silent alteration. SUb8ti~u~P~ for
an amino acid within the ~equ~ may bc selected from other .l.cMbel ~ of the clas~ to which the
amno acid belongs. For exalnple, the nonpolar (L~.uph~ ) am~no ~cids include alanine, leucine,
isoleucine, valine, proline, phenylalanine, tryptophan, and methionine. The polar neutral amino
acids include glycine, ~erine, l1 le: - -e, cysteine, tyrosine, asparag~ne, and glutamine. The
20 po~itively ~harged (basic) amino acids include arginine, lysine, and h;~;J; e The n~atively
charged (acidic) amino acids inçlude a~partic acid and glutamic acid. Also inr~ led within the
SGope of the illvention are neurite growth reg,ul~ory factor protein~ which are difF~ i.,tially
modified during or aflcer translation, e.~., by glycosylation, proteolytic .,le~,~,e, etc.
25 Ch~ u~t" .~l~on of tfie Neuri~e, Growth K~ u,,~ Factor Genes
The ~mJcture of a given neurite growth re~sulatoly faclor gene can be analyzed by va~ou~
I,lF,lh~ own in the art.
30 The cloned DNA or cDNA coll.u~5~ to a g~ven neurite ~rowth re~ulatory faGtor gene can be
analy~d by ~ s ;,-~hldin~, but not limited to Southern h~r;~ (Southern, 1975, J Mol.
Biol. ~8:S03-S17), Northem hyl~ ion ~Alwine, et al., 1~77, Proc. Natl. Acad. Sci. U.S.A.
74:~350-535~; Wahl, et al., 19g7, Meth Enzymol. 152:572-581), re~triction endonuclease
-30-
S~T BY~ 15-96; 8:23PM; 6135637671~ 16046694351;#40
2~90418
m~ppi~5 (Maniatis, et al, l ~82, ~r'e ~ - loning, A Laboratory ~Anuql~ C~ld Sp~ H~bor
Laboratory, Cold Spring Harbor, N.Y.), and DNA sequ~nce ~nalysis. D~ seq~ ana~ysis can
be ~e.~.~ by ary teclmiques known in the art in~ i~ but not limited to the method of
Maxam and Gilb~ (1980, Meth. Enzymol. 65:499-560), the San~3er dideoxy method (Sanger, et
al., 1977, Proc. N~tl. Acad. Sci. U.S.A. 74:~463-~7), or use of an ~ o,~l~l l)NA sequenator
(e.~., App~ed 13io3~ ~t~.~, ~oster City, Cali~).
6. ProducJion of An~" A~mnst the Components of tJ~c Arrehn Crowth Regulatory
Systcm
1 ~ Antibodies can be produced which, ~,co~ e neuri~e growth regulatory factors or related
protein~. Such antibodies ~an be polyclonal or monoclonal.Various procedl~res known in the art
may be used for the productin~ of polyclonal ~l~ib~ e~ to epitopes of a given neunte grou~th
regulatory fi~ctor. For the piod~ n of antibody, various host animals can be imm.~ni7ed by
injection with a neurite grnwth regulatory factor proteul, or a synthetic protein~ or Ll~.lenl
thereo~, inClutli~ but nDt limited to rabbit~/ mice, rats, etc. Various adjuvants may be used to
increase the inun~lnologir-ol re~ponse, d~peu~l;",~ on the host species, and inc~ ~ but not limited
to Freund's (~ompletc and incc - ,~l~e), minaral gels such as rdur~num hydroxide, sur~ace active
substances such as lysc'e-ith;1l, pluronic polyols, po~anions, pl~p~;~k5, oil emulsion~, keyhole
limpet hemocyanins, dinitrophenol, and potent;olly usefill human adjuvants such as BCG (bacille
Calmette-C~uerin1 and ~;oly~v~ iunl par~um. A monoclonal antibody to an epitope of a
neurite growth re6ulatory fa~tor can be p-ep~d by using any t~Ghr que which pro~ide~ for the
prod~lction of antibody l~lecules by continuous cell lines in culture. These include but are not
limitet to the hybndoma technique onginally descnbed by Kohler and l~ tein (1975, Nature
~5~:4~5~7), and the more recent human B cell hybridoma teel~n;q~e (Kozbor et al, 19~3,
Immunology Tod~y 4:72) and EBV-hybr~doma technique (Cole et al., 1985, Mf-noclor~l
Antibodies and Cancer Therapy, Alan R Liss, lnc., pp. 77-96) In a particular embodiment7 the
procedure df s~ ibed . may be used lo obtain mouse mr-- ~ona~ antibo~ies which recogr;7e
arreti~ and its ~ plor~.
-31-
SENT BY~ 15-96; 8:23PM; 6135637671~ 16046694351;#41
2t~O418
5 The n~onoclr-~a~ antl~odies for therapeutic use may be human monocl~na~ antibod;es or chimeric
human-mou~e (or other ~pecie~) monor' ~ r ' antibodies ~nan mono~lonA' antibodie~ may be
made by any of n,~ us te~ n:ques known in the an (®.q., Teng et al, 1983, Proc Natl.
Acad. Sci. U.5.~ 8~:7308-7312, Kozbor et al., 1983~ nunology Today 4:7~-79; Olsson ct al.,
1982, Meth. Enymol. g2:3- 16). Chim~ic antibody mr~ may be prep~ ~d ~onta - n~ a
10 mouse ~Itigel~ binding domain w~th human con~tant regions (Momson et al., lg84, Proc. Natl.
Ac~d. Sci U.S ~ 81:6851, Takeda et al., 1985, Nature ~14:45Z). A mo'ecl~1qr clone of an
antibody to a ncuritc growth rc~ulatory factor epitope can be prepared by kno\;vn techniques.
Recombinant DNA m~hodolo~y (sce e.~., Maniatis et al., 1982, Molecular Cloning, A
l~abo~atory Manual, Cold Spnng, ~Iarbor Laboratory, Cold Sprin~ Harbor, N.~.) may be u~ed to
15 construct nuclelc acid ~qu~nrf~ which encode a ~ ~el~n~' antibody olecule, or antigen bind~n~
reg~on thereo~
A monodonal antibody to an epitope of arretin can be prv?ar~d by using any technique which
pro~ides for the production of ~libody l,.ole ~1 by cor.1;~ ~o~.c cell lines in culture. These
2~ mclude but are not li~nited to the ~ybridoma te v~ 1 ~ue origtnally de~ il,ed by Koler and Mil~tein
(~1975) Na~e, 256:49~97), and the more recent hum~n B cell hybridoma technique (Kozbor
el al., (lg83) ~mm 201O~f ~y, 4:7V ~nd E~V-hyl).idu,~ ue (Cole e~ al., ~1985) In
l~f~ .7~Anti~ ies and Cancer T7zerapy, Alan R Liss, Inc, p~ 77-96). In a partiwlar
embodiment, the ~ d~,il~ed by Nobile-Orazio et al. ((1984) Neurolo~y, 34:133~1342)
2~ may bc used to obtain antibodies which recog~ econ~ ant Arre~n (for exarnple of
techniques, see Attia S et al., (1993) .J. Neurochem., 61 718-726)
The mono~4~ antibodies for therapeutic use may be hum~n monodonal antibodie~ or cl~ e~;c
huTn~n-mouse (or other ~pe~;ies) monoclon~l antibodies. Hum~n monoclonal antibodies may be
30 made by any of numerous technique~ known in the art ~e.g. Tan et al., (1983) Proc. Natl. Acad.
. r~ .A~t 80: 7308-7312; Kozbor et al, ~19X3~ Imnzunolo~y Tod~y, 4: 72-79; ~JIsson e1 al.,
(1982) A~eth FnzymoL, 92: 3-16). Chimeric ~libody m~'e~ s may be pr~ d c~ aining a
mouse antigen-binding domain with human cont~ct region~ ~Morri~ion et al., (19B4)~roc. I~atl.
-32-
S~T BY~ 15-96; 8:23PM; 6135637671~ 16046694351;#42
21 9041 8
._
Acud Sci. U.S.A., 81. G851, Takeda et al., (19~5) Nature, 314: 452).
A molecular clone of an antibody to ~ Arretin epitope can be p~ r ~ -~d by known techniques.
Re~ambinant DNA m~th~dolo~y may be u8ed to con~,l nudeic acid 5e~UenCeS which encode a
...ono~ -' antibody m~ , nr antigen bindinB region thereof ~see e.L., Maniatis et al., (1982)
10 In Molec~ r Clonin~, A Labo~afory Mant~ Cold Spnn~s Harbor Laboratory, Cold Spring
l~bor, N.Y.).
For use, arretin antiboch,r molecules may be puri~ed by known techniques, sllch as
r ~al ~ rption ~r irnmuDc -~Ir~ity ~ o,l...to~raphy, chro.lloto~phic methods such as HPLC
15 (high performance liquid Ghromatography), or a combination thereof, etc.
Arretin antibody ~ ~me ~Is w~ich contain the idiotype orthe nlol~ 'e c~ul be ~,ener~ted by known
technique~. For eYa~nFle, sucb ~ ~~~ include but are not limited to: the F (ab')2 fi-a~,rment
which can be ploduced by pep~in digestion ofthe antibody molecule; the Fab, ~g~ ntc which
~0 cen be ~i~.GL~d by reducing the dis~ de bridge~ of the F (ab')2 fragment, and the two Fab or
Fab fi~ n~s which can be ~ l~ by treating the antibody mf 'nr...'E with papiin and a
reducin~ agent.
Mr:lc 4'~ona' a ltJbodies known to reacl with human a~etin mEly bc tcgtcd for their ~5~1n~ee to
2~ serve as ar~etin anhgonist~ (Nobile-Orazio et al., (19B4) Neurology, 34: 1336-1342; Dobe,~on et
al., (1985)N~.v~x~i ~m. Res., 10: 499-Sl3)
Ar~ibody moIecuIes may be p~ ed by known technique~, e.g., immun~?bs~ tion or
-nn~f~inity chromatography, c~ n.dtographic m ethods sllch as HPLC (high performanGe
30 liquid chromatography)~ or a co,..bi~ ;ol- thereof, etc.Antibody fraE7n~ntc which contain the
idiotype of the .: o~ e can be genc;,nted hy known tech,l;qu~;.. For example7 such fi~g~.ontc
include but are not limited to the F(ab') ~ub 2 ~agment which can be produced by pepsin
~fi~ ofthe antibody nlole :~'e; the Fab, r ..~,.. G~ which can be generated by l~hlc.,~L~ the
S~NT BY~ 15-96; 8:24PM; 6135637671~ 16046694351;#43
~ 2 904 1 8
n~e bridges of the F(ab')2 r, u~l~e~)l., and the 2 Fab or Fab ~agments which can be
generated by treatir~ the antibody molecule with papain and a reducing agent.
7. D~agnosti~ empe~tic and ~t~sef/rch Uses for eack of ~l~e Comyonen~s and the
Antibo& D~ ed Thereto
Arretin, its ,~Jtors, analogs, derivative8, and subsequen~es thereof, and anti-inhibitoly protein
~ntiha 'ie~ or peptides have uses in c~ .ctie,s Such m~ can be used in assays such as
immunoassays to dete~t, pro~gnose, ~l;a~os~, or rnonitor various condilio~ diseases, and
d~sorder~ neurite growth c~ S;on~ invasiYeness~ and re~eneration. In one embodiment
1~ ofthei~ t;on, thesem~er~U~- ms,ybeusedforthedia~nogisof ~1i3~ cie~ Alte.lla~ y~ the
CNS nlyelin associated inhibitory proteins, analo~, derivatives, and s~lbs~qu~nces thereof and
antibodies the~eto may be used to monitw therapies for di~eases an~ condi~ionc which ultiln~tely
result in n~ve damage; such diseases and conditions ~nclude but are not limited to CNS traunla,
(e.g spinal cord injuries), ;nfarction, infection, mali~nancy; exposure to toxic agenls, nutntional
20 d~fir;~ /, palun~: p~ ;c syndromes, and do6~ e nerve di~ es (inç~ i~ but not limited
to ~ 'v disease, Parkin~on's disease, TT.~ Chorea, a~ ,up~ic latoral s~l~osi,~,
pl~D81G~v;~ _ supra-nuclear palsy, and other cl~ ~n~ In a ~pecific elnl~od;,l.ern, such molecules
may be used to dee~ct an increa~e in neurite OU~I OWLIl as an indicatûr of CNS fiber 1 ~ne. ~ion.
~or example, in specific embodi~ , the absence ofthe CNS myelin associated inl~bitoly
25 proteins in a patient saTnple containing CNS myelin can be a diagno~tic marker for the presence of
a malignancy, including but not limited to glioblastoma, neurobla~toma, and n~l&nG.I.a, or
condition involving nerve g~owth, in~ ,ness, or regeneration in a patient. In a particular
~nbodiment, the ab~ence of the inhibitory proteins can be det~r-i by ~eans of an in~m~1n~ sy
in which the l~ck of any binding ~o anti-inhibitory protein antibodies is observe~. ~he
3~ imn~llno~syg wllich can ~e u~ed include but are not limited to eon ti re and non-co~ ti1h/e
assay systems us~ng t~hniq~l~ such a~ radioin~nunoassays, ELISA (~rne linked
i~..n~ l assay), "sanduich" imm1mea~o~ys, precipitation ~ nc, gel diffi~sion
precipitation reactions, immunodiffilsion assays, ~gglutina~ion assays, complement-fKation assays,
-34-
SENT BY~ 15-96; ~:24PM; 6135637671~ 16046694351;#44
~_ 21904l8
5 immunoradiometric assays, nuurescent im~ .o~C~ , protein A immunoassays,
.n~...r.el~c~rot,hol~sis a~ays, and imm~lnohiotor.1~ y on ti~sue se~ , to n~me but a t'ew.
In accordance with another aspect o~the invention, arretin and related compounds that retain the
arretin p~~ y of inhibiting neurone growth (herein re~erred to as arretin 'lg~ t~) are used
I 0 therapelllicaîly to treat CO!~ ;0~'~ in which ~upplet~ ~- of undesirable neuronal growth is desired.
These include for cxample the ~ t of tumors of nelve tisslle and of conditions re~
from ~nc~llt~ o!' - d ner~e sproutulg such a~ is a~uciated with epilep~ and in tbe spin~l cord after
nen~e inJuly. In one enlbod~ t patients with G~lobl~c~m~ ant p~rticularlywith neuropathies
a~iated with cirwlating arre~n antibody, ~an be treated with arretin or alTetin agon~st
Usefi~il for nerve ~jrowth suppressiion are pl-,i. ~~P~ al c~.. po,~ ;on.. that contain, in an amoLlnt
e~ecti~e to ~iuppress nerve growth~ eilher arretin or a arretin a~o~ist in co...binAIio,~ with an
acceptable car~er. Arre~in can be obtained either b~ extraction from myelin as ~es;~ d abo~e
or, more practically, by recombinant l)NA expre~sion o~ATretin F,noo~ ; DNA, for example, in
the manner '~ tedi for MAG by A~tia S.~ e~ al., J. Neurochem ,~, 718-726, 19~3. Usefill
arretin a~om~ts are tho~e co~npounds which~ when added to the ~ ~is~ive substrate d ~ ;b~
above, suppre~s the ~rowth of neuronal cells. Par~icularly usefill Arretin ~olt;cts are those
compounds which cause a statistically si~ifi~nt red~ction in the number of neuronal cells that
extend neur~te~, relative to control cclls not eYro~ to the agonist. Candidate Arretin a~onists
2~ include r~5.,5,v ~t~ of A~etin that incorl)o.al~ the ecLodolnain, indudin~ the ectodomainpe~ se and
other N- andlor C-terminally tn ncated fia~sments of Arretin or the ectodomain? as well as analo~,s
the~eofin ~,vhich an~ino acids, e.g. from I to 10 ~.idues, are su~Ph~tP~ particularly
conserva~ively, and derivatives of Arretin or Arretin fr~..~ ; in ~hich the N- andJor C-tern~inal
re~idues are du;~ by chAn~ical L'~' ' V groups, Such Arretin agor~st8 can also include
30 al~ti-idiotype~ of AITetin antibodies and their binding fi~rnents.
In ~pecif~c en~1JO~I1C~ of the in~ention, candidate Arretin agonists include specific regions of
the Arretin ~leçul~, and analog~ or derivatives ofthese. rrhese ean be i~,~ ed by using the
SENT BY~ 15-96 ; 8:24PM; 6135637671~ 16046694351;#45 2190418
-
5 ~ame te~hn~'~g;es dw~.il,ed above for identi~cation o~Arretin regions that selve as in}~ibitors of
neurite uulgr~lh
The hTetin rdate~ derivatiYes, analogs, and ~ of the invention can be produced by
various n vlhod~ known in the art. The manipulations which result in their prod~ctinn can occur
10 at the gene or protein level. Por example, Arretin e arCh~i~ DNA can be modified by any of
mlmerous strategies known in the art (Maniatis e~ ~1., Molecular Cloning, A Laboratory Manual,
Cold Spring Har~or Woratory, Cold Spnn~ Harbor, N.Y., 1982), such a~ by cleavage at
appropri~te sites with reshicti~n ~n~nnucle~ce(s)~ ~b; cted to enzymatic Ino~ifi~t;on~ if desired,
ted, and liga~ed ~ o
A~ditionally, .the Arretin~ncoding gene can be r~ ted in-vifro or in-vivo for i~ ce in the
manner applied fro produçtic~ ofthe ectodon~ , to create and/or destroy translation,; ;l;a~io~
and/or t~tion sequpnces~ or to create varia~ions in coding reg~ons andlor form new
re~riction end~n~ e ~ites or de~troy pr~- ;J~ B ones, to facilitate further in-vi~ro
20 modific~tion. Any technique for m~t~ne4is known in the art c~b be u~ed, incllldin~ but not
limited to, in-vitto site directed m~ n~,~;s (H~tc~ nP-l)n~ eJ al., ~. siol~ Chem., ~, ~S51, 1978),
use o~TAB'M linkers (Pharmaaa), etc.
For deli~rery of A~retin, Arretin agol~ist or Arretin pnt~g~)mst~ vaIious known delivery sy~tems Gan
25 be used, sllch a~ encapsulation in lipos...vs or semipermeable ~ ncs, eAIJ..,ss;on in suitably
tran~formed or transfection glial cells, ol;cor'e ndroglial cells, fibroblasts, etc. a- c . .ii~.g to the
procedure kno~n to those skilled in the are (LindYall et al., Curr. Opinion Neurobiol., ~, 752-75~,
19g4). Linkage to ligands suçh as antibodie~ can be u~ed to t~r~get delivery to myelin and to other
ll.v,_p ~ relevult sitcs "~viw. Methods of ~ntroduction include, but are not limited to,
30 intradelmal, intramuscular, int~peritoneal, intravenous, s~lhal~ancQus, oral, and ;.ltr : --' route~,
and tranJfu~;~n into ventricle~ or a site of operation (e.g. for spinal cord le~ions) or tumor
removal. Likewise, cells ~l~itii~g Arretin Pt~t~ t arvti~ity, f~r example, and not by u~ay of
lin~it,q~ior, I~JI..;dolna cell~ ç ~ uldl~d in a suitable ~i~lc~ -' me...~r~e n~y be implanted in a
-36-
SENT BY~ 15-96; 8:25PM; 6135637671~ 160466943~1;#46
2190418
patient 80 a~ to pro~ide a cr~ .l nu~ Y source of Alredn inhibitor.
In another specific embodiment, ligands which bind to alretin or its rGc~vl ~ c~ be used in
imaginB techniques. For example, small peptidpQ (e.g., inhibitory protein receptor fjaf~n~nti)
wbich bind to the innibitory proteina, and which are able to penetrate tnrough the blood-brain
10 barrier, when labeled appropriately, can be used for imaging techniques such as PET (po~itron
G~ '&~ tomo~raphy) diagnosis or scintigraphy detection~ under co~ tinl~s nomnv~sive to the
patient.
Neurite growth '~;' I ly factor genes, DNA, cDNA, and RNA~ ~nd related nucleic ~ud
15 seql~snr~ ubsequences, inr1~ldil~g eo- ~ ~ement~ çqu~nrPs, can a~o be used inhybridization a~says. The neurite gro~vth innibitory factor nueleic acid scqu~ s, or subseqllences
thereof comprisin~ about at least 15 m~ otideY~ can be used as h~lidi~tion probes.
Hybri~1i7~tion a~ays can be uged to detect, proEnose, dia~nose, or monitor conditions, disorder$
or disea~e state~ a-~ ~ ci~te~ with changes in neurite growth inhibitory f~tor e~les~;on as
20 desclil~d supra ~or ~AU.~lpi-, total RNA in myelin, e.g., on biopsy tissue sections, ~om a patient
can bea~sayed for the ~le~~,nce of neurite ~rourth ~nhibito~y factor mRNA, wherc the amount of
neuritc growth inhibitory factor rnR~lA is indic~tive of the level of inhibition of nellr~te out~ro~th
activity in a ~iven patient.
25 ~ ic U.~es vf Arre~in
CNS myelin as~ociated inhl~itory proteins of the present invention can be therapeuticaliy usefiul in
the treatment of patients with nnali~ t tumors including, but not limited to ~]fin01~ or tumors
of nerve tissue (e.g. neuroblastoma) In one emb~nenl, patients w~th naul~bl~.t~.~.a can be
30 trea~ed with a~retin or analo~, denYatives, or sllbs~çnees thereof, and the human fu~-c~;o,lal
equiv~lents ~ereof, ~vhich are mhibitors of neurite extension.
ln an altemative embodiment, derivatives, analogsl or 9ubsequences of CN~ myelin inhibitoly
-37-
SENT BY~ 15-96 ; 8:25PM; 6135637671~ 16046694351;~47
21~0418
-
5 proteins which inhibit the nat;ve inhibitory protein fimction can be used in r~,~ where an
increase in neurite extension, ~owth, or l. gene~ation is desired, e.~., in patients with nervou~
system damage. Pstienta ~uffering from traumatic disorders (in~ e but not lin~il;ed to spinal
cord injuries, spinal cord lesions, or other CNS pathway lesions), surgical nerve lesions, damage
secondary to infarction, infection~ expo~ure to toxic agents, malignancy, pal~lc~pl~lic
10 s~ nl~s, or patients with variou~ types of degenerative d;~o~e.s of the central nervou~ ~ysteln
(Cutler, 1987, In: ScientiSc American M~iic.i~-P~ v. 2, Scientific American Inc., N.Y., pp.
11-13) can be treated with such inhibitory protein allta~n~ F.Y~ ~n~ of such disorders
include but are not limited to Alzheimer's [~i~ease, Parkinsons' Di~ease, Huntington's Chorea.
.Jt,ophic lateral ~clero~is, pr~ eD~hre ~upranude~r palsy and other dementias. Such
15 a~ gu.. ~ may be llsed to p,o. ..: the r~ .,e.~io-- of CNS path~,Yays, fiber systems and tracts.
Administration of antibodies directed to an epitope o~ (or the binding porti~n thereo~, or cells
~ccret,.lg such as antibodies) can aiBo be uscd to inhibit arretin protein filn~ion in patients. In a
particular embodiment ofthe inven~on, antibodies &rected to arretin may be used to p~omote the
re~eneration of nerve fiber~ over long distances following spinal cord damage.
Various delivery systems are known and can be us~d fo~ teGvery of arre~in, rela~ed molecules, Ot
ant~bodies thereto, e.~, encapsulation in lipo~omes or sem~p~ ble membranes, e~l~ss;~n by
bacteria, etc. L~nkage to ligands such as antibodies can be used to target myelin associated
protein-rel~ted l, al~ ~'e ~ to therap~uti~ ally desirabie ~ites in vivo. Methods of introduction
2~ include but are not limited to intrade~ t~ s~ r, ;~ape~iloneal~ intraYenous,
subcutaneous, oral, and intranasal route~ and infusion into ~ icl~ or a ~ite of operation (e.g.
for spinal cord lesions) or tumor rernoval. Likewise, cells secreting CNS myelin inhibito~r protein
srhg(~ t activity, for example, and not by way of lim;tatiQn, l~ ido~ cells, enr~r~ tpd ;n a
~uitable biolo~l ~ c may be ;mpl~ted in a p~tient so as to providc a cont;nuous ~ource
30 of anti-CNS nlyelin inhibiting protein antibodies.
In n~ ti , any method which results in dc-,- t~d ~ynthesis of arretin or its receptor~ may be
used to din~nish their biological function. For e~cample, and not by way of limitation, agents toxic
-38-
SENT sY ;11-15-96; 8:25PM; 6135637671~ 16046694351;#48
2 1 904 1 8
5 to the cells which ~nll~e,~e arretin andlor its receptor~ (e~g. o!i~d~ndrocytes) may be used to
d~$G the c- ~centra~on of inhl~itoly proteins to promote reg~ne.~l;on of neuron~.
~r~etin ~ f,~r~
10 A~etin ,~ceplor~ a~ well ~8 analogs, derivatives, and ~Ibs~n~nees thereof, and anti-l~eplol
antibodies have u~es in .I;s~nG~;cs. Theae mnlc~ es of the inv~ntion can be used in ~ssays such
as imm~l Q~Q~IayS or binding ~ssay~ to detect, plognoae, diagno~e, or monitor various con~itinn~,
di~eases, and disord~.~ flfl~dCI;ng neurite growth, extensionl invasion, and regeneration. For
example, it is possible that a lower le~el of expres~ion of these . ~ptor~ may be ~e~e~ d in
1 C7 vur~oua di~orders ~ ~~ ~ with ellh~nf ed neurite sl~rou~l~; and pla~tici~r o~ 5. n~l~tioi~ such
ose i~volving nerve damage, infar~tion, degenerative nerve diseacPs, or ma~ rie5. The
CNS myelin ~~ 1C~ inhibito~y protein l~eptors, ~ulaIog~, derivatives, and ~1b~qu~nc~
thereof may al~o be used to mon~tor therapies for disease~ and di~orders which ultimately result in
nerYe dama~7e, which inchlde but are not limited to CNS trauma (e.g. spinal cord injuries), stroke,
20 d~ h~liYe nerve ~ s~s, and for malig ~ e5.
The assays which can be used include but are not limited to those dc~libed abo~re.
Arretin ~plo~ gene~ and related nucleic aeid sequences and ~JJQs~u~n~es7 including
25 conlplementaly SÇ~lellr~, Call also be used in h~ r~ a~says, to detect, prognose, di~gno~,
or monitor conditions, d;~Grde- b, or di~ea~e ~tates associated with changes in neurite growth
inhibitory f~or receptor eAp~ n
Arre~in R~c~
Arretin rc~p~or~ or ~a~,Jn .~ thereof, and antibodies thereto, q~n be therapeutically usefill in the
treat~ent of patient~ with nelvou~ systetn darnage incl~di~ but not lim~ted to that resulting ~om
CNS trauma ~e.g., spinal cord injurie~), intàrction, or de~ e.~tive d;~dc~ ofthe central
-39-
SENT BY~ 15-96; 8:26PM; 6135637671~ 16û46694~51;#49
2 1 904 1 8
5 ner~ous systen~ which inclu~e but are not limited to ~t-hei; .~ s di~ e, Pa~ ,on'~ disease,
T~ o"'~ Chorea, ~ rul~ophic lateral ~clerosis, or ~.u~ s~e s.lpia~ ~r palsy. Pore~ample, in one embodiment, arretin l ~iC41)to~ b, or ~ ,sequ~ .,r~ or analogs thereof which contain
the inhibito~y protein bindin~ site, can be adn ini~tered to a patient to "col~ t~ out" bindin~ of
the inhibito[y protein~ to their natural receptor, and to thus promote nerve ~rowth or regeneration
10 in the patient. In an alternative embodiment, alltilJc " ~ E to the inhibitory protcin rc;ceplol (or the
binding portion thereof or ~ells ~c~el~.,~ anlil)o~es bmding to the r~ptor) csn be adm~nistered
to a patient in order to pre~rent receptor fimction ~}d thu~ promote n en~e ~rowth or l~Ene,~tion
in the patient. Patients in ~hom such a the~py m~y be desired include but are not limited to those
u~ith nerve damage, strolce, or degenerative disorders of the ccntral nervous system a~ deg~ ed
15 ~upra.
Vario~ls delivery ~ystems are known and can be used for delivery of arretin receptors, related
l clecll'.e~7 or ~ntibodies thereto, e.F., enc~s~ tion in lipowl~, expression by be.cten~, etc.
Linkage to lig~nds such as antibodies can be used to target alTetin-related mnle c~l to
20 ~ .a~tic~lly de~irable sites in vivo. ~[ethods of introduct.ion include but are not limited to
intrade~mal, intramuscular, intraperi ~ al? in~lnvellous, subcutaneous, oral, intranasal routes, and
i~fiusion into ventricles or a si~e of tumor removsl
The present invention is directed to genes and their encoded proteins whictl re~ulate ne,~r~te
. ~5 ~rowth and the diagnostic and therapeutic uses of such proteins. The protein~ of the presenl
invention (arretin and its receptor~) include proteins a~sociale~ with central nervous system
myelin wilh l~hly nonpe~n"s substrate p~ up~,Lej, termed herein neurite growth inhibitory
f~ctors.
30 ~he present invention is ~11$0 directed to antibodie~ to and peptide ~ragments and derivatives of
the neu~ite growth ir~ibitory proteins and their th~rapeutic and diagr o~tic uses. These aMtibodies
or peptide~ qn be used in the treatment of nerve damage resuhing from, e.g., trauma (e.~., spinal
cord inruries), stroke, degenerative di~u, .ler~ of ehe central nervous ~y~tem, etc. In particular,
-4~
SEI~T BY~ 15-96; 8:26PN; 6135637671~ 16046694351;#50
2190418
._
5 anP~odie~ to uretin proteins may be used to pr~n~ regeneration of nerve fibers In a specific
embodiment of the invention, monoclonal ~ntibodie~ directed to allretin andJor its receptors may
be used to ~,ur,l~te the regeneration of nerve ~bers over lon~ ~I ct~ e,9 following spinal cord
damage.
10 The present invention is dcsc.il,~d in filrther detail in the f llu~g non-limitin~ examples. It is lo
be und~t~od that the exunples dese- ;I.ed below are not meant to limit the ~cope ofthe present
Lon. It is eA~ccled that numerous ~ariants will be obvious to the person skilled in the art to
which the present invention pertains, without any departure ~om the ~pirit of ~he present
invention. ~he appended claims, properly constIued, form the only limitation upon the ~eopc of
15 the present invention.
I~AMPLES
20 Exarnple I: I~olation and chara~terizalion of a novel neurite grow~h inhibitoly
molecule from n~ammalian central nen~ous system m~relin
Animals.
25 ICR mice and Wistar rat embryos ~ere obtained ~om the animal facilities at Charle$ RiYer.
1~.? 1 ~
The ~ol'o~ lectin~ were purchased ~om Sigma: Maclura pomifera (osage orange), Arachis
l~ypogaea (PNA), Ulex europaeus (gorse), p - - L~ ~ulg~is PHA-L ~red kidney bean), Triticum
30 vul~aris (~he~t ~erm), and Concanavalin A (ja~k bean). Laminin from EHS sarcol~a?
Poly-L~rn~thine (PORN~, Poly-L-lysine (PLL), Chor.d,u;~ a~e ABC (chondroitin ABC lyase?
E.C. 4.2.2.4. ~oln Proteus Y~ a[is, ~,otease-~ee), hepa~inase and PI~A a~rose beads were ~lso
cl~d ~om Sigma. Horseradish pero~id~ conju~ated 5eC~ / antibodies to
~1 -
SENT BY~ 15-96; 8:26PM; 6135637671~ 16046694351;~51
21904t8
5 rabbit, rat or mouse IgG and 1~ were purchased from Amersl~n and ~ackson Labs.
Antl~odie~.
r~noclQnal antibody 473-HD is a mou~e TgM against a chondroi~ p~ tR epitope on mou~e
brain p-v~do~.,ans ~aissner eJ al., J. Cell Biol, ~0 783-79~, 1994). Rabbit polyclonal
10 anti-versicul antibodies ~ere ~5~..era~ ainst recombinantly e~, o ~ human ver~ican filsion
prote~n~. We used monoclonal anti-L~ antibody (412) ~om rat (lCruse et al., Nature, ~, 146-
148, 1985) and polyclonal anPbody 3F8 a~ainst phosphacan (En~el el ~1., J. Comp. ~eurol., 366,
34~3, 19~6, ~teyer-Puttlitz et al., J. Comp. Neurol., ~, 44-54, l 99~).
15 Multiple neurite grnwth inhibito~y activities are present In ext~acts of CNS rnyelin aiter l;)EAE
chromatography. We have previously shown that two peak~ of nwrite growth ;nhibitory activity
are pre~ent ~n fractions of myelin e~c~acts followinE~ D~AE chromato~ph,r ~McKe~racher et al.,
Neuron, .!~o 805-811, 19g4). The lar~est ofthese peaks i~ associated with the earlier ~actic)ns
eluted offthe DEAE~ çolumn by a 0.2 to 2 M ~radient. A ~b~Pntisl proportivll ofthe inhibitory
~0 ~ctivity ~n this peak is associated with myetin-associated ~opr~t~. ~AG) The i~lh~L~ory
activit~ in column frsction~ was a~sayed by an in vitro bioassay u~ing a n~ r _ l cell line
(NG108-15). The~e results ~ugge~t that molecule~s) other than MA~ o contribute to the
inhibitory acti~rity associated with ~S myelin (Pig 1).
2~ T~ n of a chondroit~n sul~e proteogly~ associated with CNS myelin
In addition to ~AG and the NI3~1250 inhibitoly mr~ es ~ d with myclin (~cK.,. l tlc,
eta~, 1994; M~11rhoF~~l~yay etaL? Neuron, ~, 757-767, 1994; Schwab eta~ ~. Rev
ros~ ~, 56~-5~5? 19~3), throe extra~ellular matr~x"~ nan~ely, tenascin-C (l~N-C~,
tenascin-R (TN-R) and cbor..lfoi~ sulfate proteo~lyean~ (CSP~s~ that are di~tributed in many
30 CNS and non~CNS tissues are also known to have neu[ite growth i~ ito~y activity (Schachner ef
a~, 1994). ~Ve lhe.~ore investi~atecl which of these inhibitory tn~ les are found in the two
inhibitory peaks ebl~ln d aflcer l:~EAE cbromatography of CNS myelin extract~. DEAE ~olumn
chromatogr~phic fractions that c4..~ d the first (fiaction~ 10) and ~econ~ ction 26)
~2-
SENT BY~ 15-96; 8:27PM; 6135637671~ 16046694351;#52
2~ 9041 8
_
5 inhibito~y peaks were ~ubjected to SDS-PAGE on a ~16% polyaclylarnide gradient gel under
rc~u~ g conditions. These gels were eithe~ silver ~tained (~ig. 2A) or Westen~ blotted with
anti-MAG, TN~, l~N-R, and a monocl~nq~ iluo~y against ~,hondroilil, sul~ate (mAb 473) (Fig.
~-E~. The silver stained gels (2,A) ~howed any bands. Anti-MAG antibody recogri7~s a loo
kDa band that is highly enridled in ~actionlO but is much wealcer in L ~tic - 26 and 32 (Fig 2B).
The intensity of the 200 and 220 kDa bands labelbd ~nth anti-TN~C w~s similar to that ofthe
MAG a~tibody, i.e., er~iel.ed in fiaction 10 (Fig. 2C). However, the 160 and l 80 kDa b~nds
~oh~ d by the an~i-TN-R ar,libody were present only in tbe total myelin extract and in fiaction
10 ~ig. 2D). lnte;e~ti..~ly, the anti-CS mA~ 473, ~cogniLed 70 k~a band and a ~ tly ~mall
n~inor band in ~actions 26 and 32 but not in the octylglucoside extr~ct of myelin and/or in fi action
15 10. This shows that these co ~ eal~ can only be d~teet~ ~unochemically a~er s~lbst~nti~
e..,;ch.~ uring the pu-i~c~liol) steps. These eA~.el.n~ents show that M~G~ and TN-R
may conll ibule to the inhibilvry effects of the first peak, and that MAG~ C and the 70 kOa
CSPG band~ may conllibule to the second inhibitory peak. Western blots of sa}nples of bra~n
membranes probed ~ith mab 412 that ~C~r-'7f f~ the ~1 epitope indicate~ that this
20 carbohydrate epitope ;s not found in the 70 kDa CSPG components ~data not sbown).
Enzymt*ic h~hol~ with chondroitinase ABC and heparil~a~
Protein~ were treated with ~,hondroili,.a3e ABC (0.02 U/ml) in 50 mM Tl is ~ te (pH 8 0~ for
2.5 h at 3rc in the ~.~,3ence of prolease ir~ibitors (5 rnM berlzamidine, 1 n~ i~doacet~n~ide and
2~ ~ mM p-to~l-L~ly~ine chlor~-l.le~h;l ketone, sodium s~lt). Hep~rinase dig~ion was done
a~cording to the manufacturer'~ ~nstructions.
Pulifi~liun of Arre~n.
Preparation of myclin extract~ and their ~actionation by DE~AE chromatography have been
30 d ~S nhod (Mc~erracher ef aL, l 9947 see Fig. l). For filrther purification by lectin afflmity
ChrOmatO~ Y7 PNA-COnjU~ated agarO~ beads (1.2 ml) Were USCd. I~EAE~ d~OI~tOgraPI1ie
~aCtiOn~ nUrI1ber 20 tO 34 (2 ml eaCh) Wae POOIed (abOUt 30 ml), d;lUted W;th 3 VOIUme Of ~I2O,
and loaded on the PN~ ;~dluSe column. The ~OW-thrOUgh Wa~7 ~lG~I t~ree times, and the
~3-
SENT BY~ 15-96; 8:27PM; 6135637671~ 16046694351;#53
2~90418
colullm was s~b8equPntly vvashod ~vit~l 12 ml Hepes buffer (pH7.5, 0.08% Sodillm azide, 10 mM
~epe~, 0.15 ml~rf NaCl, 0.1 mM Ca~, and 0.01 nlM Mn~+), followed by 12 ml of a high salt buffer
(pU7. 57 2 M NaCI, and 20 mM Triethanolamine). Thc column was eluted wi~ 20 ml of eludon
buffer (2 M NaCI, 20 mAI Trith~not~mir~p pH7.5~ and 0.5 M D-g~ to~e). Appropri~tely pooled
~actions were dialysed a~ainst 1000 ml of H2O at 40~C, Iyophilised, and dissolved in 1 ml of H20,
suGh that the fin~ ce, ~ion ~4as abo~t 0.1~ M NaCI, 1.6 mM Trith~no~ ne~ pH7.5, and
0.04 M D-~alactose. Sarnples were aliquoted, and stored ~t -70~c. The protein profile was
determined by SDS-P~GE on grudicnt gels (6 to 16% polya~rylamide) (Laemmli, U.K., Nature,
~, 68~685, 1970~, and by Western bhts (Towbin et aL, Proc~ Nat, Acad. Sci. USA., 76, 43~0-
4354, 1979). Protein concentra~ions we~e e~timated accordin~ to Bradford (1976).
Rea~tivity of Arretin ~Nith le~tins.
Proteins ~ d to l,.e...~. ~es were bloGked ~~vith 2% bovine semm albumin (B~S) ~n lBS
buffer (20 mM Tris-HCI, ~00 mM NaCI, pH7.~) for 1 4 and mcubated separately with 6g/ml of
or biotin~onjugated lectins for 2 h. The membr~ w~e washed w~th TTBS (20
Mm Tns-HCl, 500 m~ NaCI, 0.0~% Tween-20, pH7.~) for ~h and complexes werc ~tecte~l by
13CL (DIJ PONT) or the AP-~C (~CTOR) Kit a~ B to the manufa~re~s in~tructions.
As positive controls for lectin bindin~ several ~ugars, in~ ling ~lq~to,se, ~ oa~ gluGo~ e~
galaceownine, fuco~e, and mannose (at ~0 mglml), were applied as spot~ on nitro~ellulose.
25 Purification by Lectin affinity ~h~ to~phy
To filrther puri~r the 70 k~a CSPG c( n~lle~t~ ~om DI~ actions containin~ the
second inhl~itory pe~k, we ~ n~d the ability ofthe co~ )on~ to bind the followin~
lectins: Maclura p~ a (osage orange), Arachi~ hypogaea (PNA), Ulex c~ o~ellc uea 1 ~go~se
or filrze). Phase~ vul~ans (PHA-L), Triticum ~ulgaris (w' ~ "I ag~lutinin) and
30 Concanavalin A (Con-A). Nitrocellulose ~ 4S electro b~otted with pooled DEAl~ ~actions
20 to26 ~er protein separa~ion by SDS-PAGE~ ~ere probed with the various lect~ns. All thc
lectins ex~ept Con-A bound only to the 70 kDa bands ~no~ shown~.
44-
SENT BY~ 15-96 ; 8:27P~; 6135637671~ 16046694351;#54
~190418
-
S We next tested whethe~ the 70 kOa ~om~cnts could be pu~ified by bindingto lectm. For this7 PNA-coniugated agarose beads were chosen. Frac~ions 20 to 26 obtained
from DEAF, column ~h~n~tography of bovine C~S myelin extracts were pooled and incubated
with PNA-conjugated beads in an T7PPF~ rtube. Af[e~ washing the beads, the proteins
bound to the PNA-beads were separated by SDS-PAG~, eleclr-JI h~ ically blotted onto
10 nitrocellulose ...~l~le and probed wilh anti-MAG, TN~ and the 473 antibodies. As expected
only the 70 kDa bands were recog~i7ed by the mAb 473. No la~eling was ul~s_. v~;d with the
other two antibodie4 inA - ~ that PNA lectin can be u~ to scpa~a~e the 80 kDa m~ e
from MAG and TN-C (not shown)
15 A tw~step purification ofthe 70 kDa COn~Oll~.ltS wa~ th~r~,fo-~ ~tt~mpte~ Octylglucoside
extracts of bovine C~IS myelin were passed though a I)EAE column, and the material eluted by a
NaCI ~radient, and ~ t;on~ 20-34 v~ere poolcd. The pooled L -tinns were then subjected ~o
PNA-afflnity ehl4..,~to~aphy. The material elu~d fiom the PNA column was sepa.~t~ on a
SDS-PAGE~ gradient gel (~l 6% acrylamide) under reducing conditions. The gels were then
stained with sil~er, or ~estern blotted an~ probed with anti-MAG, l'N-C and 473 ar~ odies. A
70 kDa doublet was seen a~er Amido black staining (Fig. sA~. This major bant was recogni~d
only by the 473 anti-CS antib~dy (Fig. 3A), but not by ~nti-MAG (~ig. 3B) or anti-T~-C
antibodies (not shown). The n~inor component just below the ~nyor band ~va~ not visible in this
preparation.
The 70 l~Da colllponel~ are novel phosphocan-versican-relate~ e5 We filrther
~re~t;~t~ whether the 70 kl:)a CSPG band~ purified ~om CNS myelin ~hared ~ pes with
other known CSPGs. On Westem blo~s of the DEAE ~ .atographic ~actions the 70 kl)a
CSPGs also reacted w-th polyclonal antibodies against phrsFh~ ~n~ rc~ versican ~ig.
4A and B). I~o~h these antibodie~ plus the 473 anti-C~S recognized the 70 kDa PNA affinity
purified CSPC~s (Fig. 4C, D, E)r A~er .I,on.ll ~ - oce ABC ~ , the major 70 kDa CSPGs
was found to have an app4rc.ll Mr of 50 kDa (Pig. 5A) which did not react with the anti-CS mAb
473 (not 8hown), but did react with anti-phosphacan ~Fig 5B~ and anti~ Sin~e native
45-
SENT BY~ 15-96; 8:27PM; 6135637671~ 16046694351;#55
21904t8
phosphacan has a molecular weight of 500-600 kDa (cote protein 400 kDa), an~ versican is a
very lar~e prote~ ed., with a nlolecul~r ~ei~ht of 900 kDa (core protein 400 kl)a), the 70 kDa
CSPGs that we haYe i~ofated ~om C~NS myelin aRE novel phosphoc~nh~ ~ related CSPGs.
We c~ll these protein~? arretin ~colle~tively) The 2 bands may r ,~ l 2 isoforms, or the smaller
c~ may be ~n altered version ofthe lar~er, due to degradation.
The 70 Id)a CSPG components are ~pi~.,ed b~y ol;godçn~l~ocyte~ To detellnine which CNS cell
types express the 70 l~a CSPGs isolated from CNS myelin ~ t4, total ~ ~e proteins
~om c'isnd~ te8, a~trocytes, neurons? grey matter were separated by Sl:)S-PAGE and
Western blotted onto nitrocellulose ~ L.4ne and probed with polyclonal ~ntibod;es a~ainst
phc~ a~ and recombinant versican. Both polyclonal a-lLil~e~ recog~ed a 70 I~)a doublet
tbat i~ highly e~ ~d ~n nligodc~ ~te~ but only poorly, if at all~ in the other cell~ (Fig 6~,
indica~ing that the 70 kDa co.-~l ol~e~ that we purified fro~ CNS myelin are eA~ sed mainly by
oli~od~ o~tes. The weakly imm~1no~t~ining bands may or may not be the same as the 70 kDa
alTe~n.
The 70 kDa CSPGs inhibit neurite ~rowth. Se~eral lines of e~idence show tl~at CSP~;s can act as
either po8itivo or negative modulators of axo-l growth as de~cnbed above. The present invention
therefore involved a test tha~ examined effects ofthe ?o kDa myelin~derived CSPGs in modulating
neunte ~rowth ~om rat hippocampal and cer~ le cell curons. The 70 kDa CSPC;s
inhibited neurite gro~h ~om t~e~n~ rat cerebellar and hippocampal neurons ~igs. 7 and 8), as
well as *om cu}tured NG108-15 cell~ ~iB. 9). This inhibitoly acti~Jity was lo~t a~er heat
denaturation. These result indicate thal novel ".J~,Iin ~e~ociated 70 k~a CSPG~ are ;nllib:to,~ of
neurite growt~, and are li~ely to be largely respon~ible for the activity associated with ehe second
in~bitory peak in ~ac~ons obt~;ned af~er DEAE ~-?~ on of CNS myelin e~ctracts. The presen~
30 invention ~ .q;~ these new ~~I bit~rs collectively termed as arretin.
~s9ay9 for repulsion of growth cones and cell bodies.
Ti~ e culture dishes (Becton DiçLin~n~ with 24 wells were coated with metlunol~ hi
~6-
SENT BY~ 15-96; 8:2~PM; 6135637671~ 16046694351;#56
2~9'J41B
... .
5 nitrocellulose nc~; rdi~g to T 9~ ,~,r~- Ir and T 4~mmon (1 ~87) and air-dried in a stelile hood. For
as~ays addressing the e~e~ ~f alretin on ~rowth cones, nitrocellulose and poly-L-lysine (PLL
O.Ol~/o) coated dishes were ll~ed a~ des_llLed (Xiao etal., Neurosci., ~ 766-78~, 19g6~. The
di~hes were washed three times with PBS and dried in a sterile hood. Diffierent test proteins
(arretin, denahlred (80~c for 30 min) arretin, I'N-~, and lan~l~in), each at ~ ntrations of 2 nM,
10 IOnM, and ~On~4 were applied in dupticate a~ ~.S ~ I single ~pots to the dishes and inr'lb~ted
overni~ht at 37~c in a humidified aln~osy~
Detelmination of ~ubstrate coatin~ effic~e~c~ wa~ been described by Xiao et al., 1996. Before
plating the NG108 cells or cere~ellar nellrnns, the dishes we~e w~shed ~nth Ca ~+- and M~2+-~ee
15 Hanlcs' b-'-ncc~ 5a1t ~olution (CMF-HBss). Explants ~rere ~ d from cerebclla of 6 to
7-day-old mice and maintained in a chemically defined medium [Fischer et a~., J. Neurosci., 6,
605~12, 1986; Fi~cher, G., Neurosci. Lett, 28, 325-329, 1982). Explants were alluwed to ~row
neurite~ for 72 h and then hxed with glutaraldehyde in PBS at a final concentration of 2.5%.
A~er fixation, cultures were stained with 0.5% toluidine blue (Si~ma) in 2.5% sodium carbonate7
washed five times with water and air dried. All ~ e...2le.~ were p~lf~r~nod at lea~t three times.
Assay for neuri~ olll~owlll. Hippocampal neurons from 18- to Ig-day-old rat emb-yos were
prepared as d~ iled (K~lhn~ler et al., ~atllrel ~, 728-730, 198~; Lochter e~ a~., J. Cell Biol.,
113, 1 1 S9-1171, l991; DolTies e~ aL, 1995 '~). For the assays on neurite outgrowlh~ hippocampal
neuron~ were maintained in chemically defined medium (l~oU~B7~et et al., A~. Rev. Cell Biol.,
12~ 4gS-~04, 1988; Loçhter and Schachner, I. Neurosci., 1;~, 39~6~000, 1993; Xiao et Cl/.,
1~6).
Briefly, 96 well plat~s (Nunc) were pl~tt~ e~ with Sg/ml poly-L-orl~lL;I c (PORN) for 1 to ~
hours at 3rc~ washed twice witll water and air-dned. Protein8 at cc~n~ ~-tt ~tions of 2 r~I, 10 nM,
and 50 nM were coated on the dried surface~ over~ight at 37~c in a humidified ~ eJ~h~
De.t~rnnlation of substrate coalin~ efficiency as de~c-il~c~ Xiao et a~ . The pla~es were
washed three times with C~ HBSS and hippocampal neuron~ p~ a.~;d ~om 18- to 1~-day-old
~7-
SEI~T BY~ 15-96; 8:2~P~; 6135637671~ 16046694351;#57
2190418
S ratembryos(KeilhaucretaL, lg85;Lochteretal., 1991,Dorri~setal., 1995)wereplatedata
density of 3/000 cells per well in lOO ~ I a chemically defined medium (Rousselet ef ~1., lg88;
Lochter and Schachner, l ~3, Xiao et al., 1996). After 12 h, cells were f~xed without a
precedin~ washing step by gentle addition of 25~fo glutaraldehyde to a final c~l~r~ntration of 2.~%.
~fter fLxation, cultures were stained with i ~ in~ blue and morphol~eical palan~e~e.~ were
~ fied with an IBAS ima~e analysis sy~tem. ~or ~ hon~eLIic analysis, only cells without
conta~t with other cells were evaluated. Neuntes were dcfined ~s tho~e processes with a length of
al lea~t one cell body .I;~ eler. The total neulitc len~h per cell ~,vas determined by analysin~ 50
cell~ ~n each oftwo wells. To determine the number of cells ~qth n~rit~q, 100 neurons in each of
two wells were cour~ed per eA~.i~ l. Raw d~ta ~om a~ least three i~de~e~ yerime"l~
1 ~ were analyzed by ANOVA and by the Newman-Keuls te~t with P c 0 OS and P c 0.01 being
considered significant or highly ci~ifi~nt7 respectively. All ~raphs con~prise data denved ~om at
lea~,t three in~l~pe~ A
From ~e foregoin~ des~ription7 one skilled in the art can easily asce~ain the r --~nlial
characteristic~, oftl~ invention, and without departin~ ~om the spirit and s~ope thereof, can
make variou~ chan~es and modific~tinn~ ~o the il~ tion to adapt it to various u~ages and
conditions. Such changes and modifications are ~ perly, equitably, and ~ ded to be within the
filll ran~e of equivalence oftlle following claim~.
-48-