Note: Descriptions are shown in the official language in which they were submitted.
W096/00396 r~
~1 2 1 92~55
IMPROVED METHODS EOR T~E D~ NATION
AND ADuu~ OF PROLA~TIN 3AI~Y REY~Mc
FIELD OF THE ~Nv~ ~N
This invention relates to improved methods for
the reduction in a subject, vertebrate animal or human, of
body fat stores, and r-eduction of at least one of insulin
resistance~ hyperinslll;n~iA, hyperlipidemia, hyperglyce-
mia, and other metabolic diseases, especially those associ-
ated with Type II diabetes. In particular the present
invention is directed to methods for: (i) normalizing the
daily prolactin rhythms of a human or vertebrate animal;
(ii) diagnosing aberrant daily prolactin rhythms of a human
or vertebrate animal; and (iii) determining the appropriate
adjustments that need to be made to normalize such aberrant
prolactin rhythms. Such adjustmgnts include the daily
administration to the subject of at least one of a prolac-
tin stimulator-and/or a prolactin inhibitor at a predeter-
mlned time of day (if only one is administered) or atdifferent predetermined times of day (if both are adminis-
tered~. This therapy typically results in the long-term
adjustment of~aberrant or abnormal prolactin rhythms so
that they ccnform to or simulate normal prolactin cycles,
at which point the therapy may be discontinued, while the
adjustment pcrmits.
This invention is also directed to improved
~ methods for adjusting the neural oscillator (or oscilla-
tors) of which the daily prolactin rhythm is an expre-ssion
~ ~o or marker.
The adjustment of daily prolactin rhythms and the
daily rhythms of key neural oscillators results in reduc-
W096/00396 2 1 9 2 8 5 5 P~ 3~-1
tion and control over an extended time period of various
metabolic or other disorders.
BACRGRO~lND OF TIIE T~V:~ION
Obesitsr and Li~id Metabolism Disorders
Bodv Fat Store5
In humans obesity can be defined as a body weight
exceeding 20~ of the desirable body weight for individuals
of the same sex, height and frame (Salans, L.B , in E
crinoloqv & Metabolism, 2d Ed., McGraw-Hill, New York 198~,
pp. 1203-1244; see also, R.H. Williams, Textbook Qf En~-
crinoloqv, 1974, p~. 904-916). In other animals= (or also
in humans) obesity can be determined by body weight pat-
terns correlated with prolactin profiles given that members
of a species that are young, lean and '~healthy" (i.e., free
of any disorders, not just metabolic disorders~ h~ave daily
plasma prolactin level profiles that folrow a regular
pattern with little or no standard deviatio~. - ==
Obesity, or exce~s~fat deposits, correlate with
and may trigger the onset of various lipid metabolism
disorders, e.g. hypertension, Type II diabetes, atheroscle-
rosis, etc.
Even in the absence of clinical obesity ~accord-
ing to the above definition) the reduction of==body fat
stores (notably visceral fat stores) in man especially on
a long-term or permanent basis~would be of significant
benefit, both cosmetically and physiologically.
The reduction of body fat stores in domestic
animals (as well as pets) especially on~ a~ long-term:=or
permanent basis would also obviously be~ o~f considerable
economic benefit to man, particularly since farm animals
supply a major portion of man's~diet; and the animal fat
may end up as ~e novo fat deposits in man.
Whereas controlled ~iet and exerci~se can proauce
modest results in the reduction of body fat deposits, prior
to the cumulative work of the present inventors (including
~096/00396 2 1 9 2 8 5 5 }~ ',.g ~ ~1
the prior co-pending patent applications and issued U.S.
patents referred to below), no truly effective or practical
treatment had been found for c~ntrolling obesity or other
lipid metabolism disorders. = == ~
Hyperlipoproteinemia is a condition in which the
concentration of one or more of cholesterol- or triglycer-
ide-carrying lipoproteins (such as chylomicrons, very low
density lipoproteins or VLDL and low-density lipoproteins
or LDL)~in p~lasma exceeds a normal~limit. This upper limit
is generally defined as the ninety-fifth percentile of a
random population. Elevated levels of these substances
have also been positively correlated with atherosclerosis
and the often resulting cardiac infarction, or "heart
attack", which accounts for approxima~tely one-half of all
deaths in the United States. Strong clinical evidence has
been presented which correlates a reduction in plasma
lipoprotein concentration with a reduced risk of athero-
sclerosis (Noma, A., et al., Atherosclerosis 49:1, 1983;
Illingworth, D. and Conner, W., in EndocrinoloqY & Metabo-
lism, McGraw-Xill, New York 1987). Thus, a significant
amount of research has been devoted to finding treatment
methods which reduce levels of plasma cholesterol and
triglycerides.
Another subset of the ~lasma lipoproteins found
in vertebrates are high density lipoproteins, or HDL. HDL
serve to remove free cholesterol=from the plasma. A high
HDL conce~tration as a percentage of total plasma choles-
terol has been associated with a reduced risk of athero-
sclero~is and heart disease. Thus HDL are known in the lay
press as "good" cholesterol. Therefore, therapeutic
strategies involve attempts both to reduce plasma LDL and
VLDL content (that is, reduce total plasma cholesterol),
and to increase the XDL fraction of total plasma cholester-
ol Several lines of research indicate that si~ply in-
creasing HDL is of benefit even in the absence of LDL or
VLDL reduction: Bell, G.P. et al., Atherosclerosis 36 47-
54, 1980; Fears, ~., Biochem. Pharmacol, 33:21g-228, 1984;
W096/00396 2 1 9 2 8 5 5 P~ ~ s ~
Thompson, G., sr.~Hear~ J. ~I 585-~a8, 1989; slackburn, H.
N.E.J.M. 309:426-428, 1983.
Current therapies -for hyperlipoprot~in~m'~s
include a low fat diet and:elimination ~of aggravating
factors such as sedentary lifestyle. If the hyperlipoprot-
einemia is secondary (i.e. incident to e g. a deficiency of
lipoprotein lipase or LDL receptor, -various~~endocrine
pathologies, alcoholism, renal disorders, hepatic disor-
ders) then control of the underlying disease is also
central to treatment Hyperlipoproteinemias are also
treated with drugs, which usually alter~the levels of
particular components of the total plasma cholesterol, as
well as reduce the total plasma~lipid component. Among~the
recently introduced drugs to treat hyperlipoproteinemia is
lovastatin (MEVACOR~) which selectively inhibits an enzyme
involved in cholesterol productIon, 3-hydroxy-3-methylglut-
aryl coenzyme A (HMG-CoA) reductase. This drug specifical-
ly reduces total cholesterol and can cause a modest (5-10~)
increase in HDL concentrations. However, benefits from
these therapies vary from subject to subject.
M~ V~L7 use of the HMG-CoA en~yme inhibitor is
sometimes accompanied by side effects such as liver toxici-
ty, renal myoglobinuria, renal shutdown, and lenticular
opacity. The risk~of such side~effects necassita~es close
monitoring of the patients (e.g., liver=function is tested
monthly).
Another drug prescribed against hyperlipoprotein-
emia is clofibrate. The effectiveness of clofibrate also
varies from subject to subject and its use is often accom-
panied by such side effects as~nephrotic syndromes, myal-
gia, nausea and ah~o~;n~l pain.8 5
Diabetes
Diabetes, one of the most insidious of the major
diseases, can strike suddenly or Iie undiagnosed for years
while attacking the blood vessels and nerves. Diabetics,
as a group, are far more often afflicted with blindness,
heart disease, stroke, kidney disease, hearing 1088,
W096/00396 21 92855 P~,lU ,.
~ 5
gangrene and impotence. One third of all visits to physi-
cians are occasioned by this disease~and its complications,
and diabetes and its complications are a leading cause of
untimely death i~ the ~nited ~tates and in the Western
world.
Diabetes adversely af~ects the way the body uses
sugars and starches which, during digestionr are converted
into glucose Insulin, a hormone produced by the pancreas,
makes the glucose available to the body~s cells for energy.
Im muscle, adipose (fat) ~nd connective tissues, insulin
facilitates the entry of glucose into the cells by an
action on the cell membranes The ingested glucose is
normally converted in the liver to ~o~ and ~O (5Q~); to
glycogen (5~); and to fat (30-4Q~l, the latter being stored
in fat depots. Fatty acids from the adipose tissues are
circulated, returned to the liver fo~ re-synthesis of
triacylglycerol and metabolized to ketore bodies for
utilization by the tissues The fatty acids are also
metabolized by other organs. Fat formation is a major
pathway for carbohydrate utilization.
The net effect of insulin-is to promote the
storage and use of carbohydrates, protein and fat. Insulin
deficiency is a common and serious pathologic co~dition in
man. In insulin-dependent (IDDM or Type I) diabetes the
pancreas produces little or no insulin, and insulin must be
injected daily for the survival of the diabetic. In
noninsulin-dependent (NIDDM or Type II) diabetes the
pancreas retains the ability to produce insulin and in fact
may produce higher than normal amounts of insulin, but the
amount of insulin is relatively insufficient, or less than
fully effective, due to ~Pll~ r resistance to insulin.
In either form of diabetes there are widespread
abnormalities. In most NIDDM subjects, the fundamental
defects to:w~ich the abnormalities can be tr~ced are (1~ a
reduced e~try of glucose into various ~peripheral~ tissues
and (2) an ;n~r~A~ liberation of glucose into the circu-
lation from the liver. There is therefore an extracellular
W096/00396 21 928~5 }_I/U~
glucose excess and an intracellular glucose de~iciency.
There is also a decrease in the entry of amino acids into
muscle and an increase in lipolysis. Hyperlipoproteinemia
is also a complication of diabetes. The cumulative ef~ect
of these diabetes-associated abnormalities is se~ere blood
vessel and nerve damage. ~
No effective treatment has been found for con-
trolling either:hyperinsulinemia or insulin resistance
prior to the work of the present inventors. Hyperinsuline-
mia is a higher-than-normal level of insulin in the blood.
Insulin resistance:can be defined as a state in which a
normal amount of insulin produces a subnormal biologic
response. In insulin-treated patients with ~iabetes,
insulin resistance is considered to be present whenever the
therapeutic dose of insulin exceeds the secretory rate of
insulin in normal persons. Insulin resistance is also
associated with higher-than-normal levels of insulin i.e
hyperinsulinemia -= when normal or elevated level~ of blood
glucose are present.
The principal unit of biological time measure-
ment, the circadian or daily rhythm, is present at all
levels of organization. Daily rhythms have been reported
for many hormones inclusive of the adrenal steroids. e.g.,
the glucocortico- steroids, notably cortisol, and prolac-
tin, a hormone secreted by the pituitary. In an early
article, discussing the state-of-the-art at that time, it
was reported that "Although correlations have been ~ade
between hormone rhythms and other rhythms, there is little
direct evidence that the time of the daily presence or peak
level of hormones has important=physiological relevance.~
See Tem~oral SYnerqism of Prolactin and A~ren~l Steroi~ by
Albert H. Meier, ~eneral and Comparative Endocrinology.
Supplement 3, l9~~ Copyright 19~2 by Academic Press, Inc.
The article reports that the peak concentration of prol~c-
tin occurs at different times of day in lean and fat
animals. The article then describes~avian physiological
responses to prolactin injections given daily for several
W0 96100396 2 ~ 9 2 ~ ~ 5 ~ Sl
~ 7
days. These responses include increases and decreases~in
body fat stores, dependent on the ~ime of.day of the
injection_ Eurthermore the time of day when prolactin
injections promote loss of body fat coincides with the time
o~ day when prolaotin is greatest in lean birds Addition-
ally, the.time when prolactin injections promote gain of
body fat coincides with the time when prolactin is greatest
in obese birds. Prolactin was thus found to stimulate
fattening only when injected at certain times o~ the day,
and time of the response to prolactin was found to differ
between lean animals and fat animals.
In an article titled ~;rc.-~iAn and Seasonal
Variation of Plasma Insulin and Cortisol Concentrations in
the Svrian Hamster, Mesocricetus Auratus by Christopher ~_
de Souza and Albert H. Meier, Chronobiology International,
Vol. 4. No. 2. pp 141-151, 1987, there is reported a study
of circadian variations of plasma insulin and cortisol
concentrations in scotosensitive and scotorefractory Syrian
hamsters r-;nt.;n~ on short and long periods of daylight
to determine possible seasonal changes in their daily
rhythms. The baseline concentration of insulin was found
to be higher in female than in male scotosensitive hamsters
on short daylight periods. These differences, it is
reported, may account for the observed heavy fat stores in
female hamsters kept on short daylight periods. The plasma
concentrations of both cortisol and insulin varied through-
out the day for the groups of animals tested, but were not
equivalent. The circadian variation of insulin and corti-
sol di~fered markedly with sex, seasonal condition and day
length. Neither the daily feeding pattern or glucose
concentration varied appreciably with seasonal condition,
or daylight. The time of day, or the season, it is report-
ed, do not appear to a~~ect_the ~nn7~ntrations in glucose
levels. It is postulated that the daily rhythms of cortisol
and insulin are regulated by different neural pacemaker
systems, and that changes in the phase relations of circa-
dian systems account in part ior seasonai changes in body
W096/00396 2 1 9 2 ~ ~ 5 F~~
fat stores. ~he circadian rhythms of prolactin and the
glucocorticosteroid hormones, e.g., cortisol, ~ave t'hus
been perceived as having important though far ~from fully
understood roles in regulating daily and seasonal changes
in body fat stores~and in the organization and integration
of total animal metabolism. See ~;rça~;~n Hormo~e Rh~thms
in ~i~id Requlation by Albert H. Meier and John T. Burns,
Amer. Zool. 16-649-659 (1976) : :
In our prior co-pending U.S. Patent Application
Serial NoS 192,33~, filed May 10, 1988 ~~we have disclosed
and claimed methods for resulating lipid metabolïsm di-sor-
ders by administering prolactin (or both prolactin and a
glucocorticosteroid ("G~ into the bloodstream of an
animal or human on a timed daily basis in an=amount and for
a period of time sufficient to modify and reset'the neural
phase oscillation of the prolactin daily rhythm which then
increases insulin sensitivity. The prolactin (or,prolactin
and glucocorticosteroid) injections are~timed to' create a
peak in the subject's daily prolactin= (or bot~prolactin
and glucocorticosteroid~ profIle that ~coinci:des in time
with the peak prolactin (or prolactin and ~GC peaks,
respectively) of a lean, insuIin=sensitive human to in-
crease insulin sensitivity and reduce body fat stores.
Injections of the same agent(s) are timed towards the peak
prolactin time of an obese subject to achieve fat gain,' if
desired.
In our cQ=pending prior United States Patent
Application Serial No. 463,327,:filed on January 10, ~990
(and its continuation-in-part Serial No.~07~813,I25, fi'led
on December 23, lg91 '(publishea~aa'W0 93/12793 on July 8,
1993)) we have disclosed and c~aimed a method of modifying
and resetting the neural phase oscillations of:the brain
which controls both prolactin and GC in an obese animal (or
human) by administering a dopam:ine ago~ist~at a predeter-
mined time 'of day such thatIthe prolactin (and/or ~GG)
peak(s) of the o~ese animal ~or human) will :be phase-
shifted~to occur at the time that it occurs Ithey occur) in
W096l00396 2 1 9 2 8 5 5 I ./~ . ,
~ g
a lean animal (or human), with the result that at least one
of body fat stores, body weight, hyperinsulinemia, or
hyperglycemia will be reduced and~or insulin sensitivity
will be increased
In our-co-pending prior United States Patent
Application Serial No 719,745, filed June 24, 1991 (pub-
lished as ~O 93/00092 on January 7, 1993~ we have disclosed
and claimed ~nh~nr~ methods for modifying and resetting
the neural phase oscillations of the brain which control
prolactin levels comprising both (a) administering to the
subject a ~p~minP agonist just a~ter the time at which the
normal prolactin profile peaks to reduce prolactin levels
to the low ~'day'~ levels and ~b) administering to the
subject a prolactin stimulator at a time just before the
prolactin level peaks in normal subjects with the objective
of causing the subject~s prolactin ~rofile to mimic in
shape and time the profile o~ a lean human not s~ffering
from one or more of aforementioned metabolic disorders.
United States Patent Application Serial Mo
719,745, also discloses and claims the further administra-
tion of ~ thyroid hormone to subjects that are being
treated with the dopamine agonist and prolactin stimulator,
especially to those subjects that are chronically or
seasonally hypothyroid.
In co-pending United States Patent Application
Ser No. 995,292, filed on December 22, 1992 (International
Application Number PCT/US93/12701) we have disclosed and
claimed certain improved methods for diagnosing aberrant
prolactin rhythms, determining adjustments to be made to
abnormal prolactin rhythms, and normalizing abnormal
prolactin rhythms
In co-pending United States Patent Application
Serial No 171,897, ~iled on ~ecember 22, 1993 ~Interna-
tional Application No PCT/US94/14994~ we disclose and
claim an accelerated release composition ~r~nt~ining a
prolactin inhibitor that is rluickly released into the blood
stream of the subject
W096/00396 2 1 ~ 2 8 5 ~ r~ 03~l
In co-pen~ing United States Patent Application
Serial No. 178,56g, filed on January 07, 1994 (Internation-
al Application No. PCT~US95/006~3~ we disclose and claim a
method of treating various lipid metabolism disorders
involving the synergistic ~ombination of diet and the
administration of a prolactin inhibitor. ~ ~
Various aspects of the present inventio~ have not
been claimed in the foregoing applications. In addition,
various i~.-pl~V! ts to and advantageous refinements of the
administration protocol and its determination have now been
made which increase the effe~tivenes6 of the treatment_
SnMM~RV OF TW~ INV~NTIQN
One aspect of this invention relates to a method
for normalizing the daily prolactin profile of a first
vertebrate subject in need of such treatment which compris-
es the steps of
comparing the prolactin profile of the first
vertebrate subject to the prolactin profile of other verte-
brate subjects having a normal prolactin profile~ and
adjusting the prolactin profile of the first
vertebrate subject to cause the profile of said first
subject to generally conform to (or simulate) the prolactin
profile of said second subjects.
A variation of this~aspect :of ~the =invention
involves (a) comparing only a set of at ~east two ~prefera-
bly at least 4, typically 4-6) prolactin levels of the
first subject measured at time points during at least two
(preferably at least three) key lntervals of the day to the
corresponding prolactin levels of healthy subjects at the
same time points during the day, and (b) adjusting the
prolactin levels of the first subject: to conform to ~or
simulate) the corresponding healthy prolactin levels.
As an e~ample, prolactin levels of the first
subject can be measured at least twice during each of an
early morning key interval and an early evening key inter-
val and preferably at least one or preferably at least two
~VO 96100396 2 ~ 9 2 8 5 5 I ~ 5 1 ~ ~1
~ 11
additional prolactln levels may be measured during a night
interval
A second aspect o~ this invention relates to a
method for evaluating the daily prolactin profile of a
human subject, which comprises the~steps of:
(a) comparing the prolactin profile of~ said
subject, said profile having been determined over a contin-
uous 24-hour period, to a predetermined standard prolactin
profile for healthy human subiects; and
(b) determining whether said~human subject has
an abnormal daily prolactin profile by ascertaining whether
one of the following obtains: (i) at any time point during
waking hours the subject's prolactin level is higher than
l Standard Error of the Mean (SEM) above the mean prolactin
level of healthy subjects at the same time point (or of a
standard waking hours mean prolactin level or a standard
prolactin profile); and (ii) at any time point during
sleeptime the subject~s prolactin level is lower than 1 SEM
below the mean prolactin level of normal (healthy) individ-
uals at the same time point.
Preferably, the time points compared will be
actual prolactin measurement points (i.e. the prclactin
levels of the subject being tested will be blood prolactin
levels and not extrapolations) and, most preferably, the
deter~in~ti~n will be based on two prolactin measurement
points, instead of just one; alternatively, the determina-
tion will be preferably based on~ wh~ether one measured
prolactin level is higher than 2 SEM during waking hours,
or lower than 2 SEM during sleeptime.
A variation of this seco~ aspect of the inven-
tion involves: (a) comparing only a set of at least two
prolactin levels of said~human (measured:at predetermined
time points during each of at least two key intervals of
the day) to the corresponding prola~ctin levels (e.g. in a
prolactin profile) for healthy humans at the same time
points during the day; and (b) determining whether said
human subject has an abnormal prolactin profile by ascer-
W096/00396 2 1 q 2 8 5 5
12
taining whether one of the ~ollowi~g obtains: ~i) at~any
of said time points that fall during waklng ~ours,~the
subject~s prolactin level is higher than 1 SEM above~the
corresponding mean prolactin level of healthy subjects=~or
above the corresponding mean level of a standar:d~prolactin
profile) at the same time point; and (ii) at any of said
predetermined time points that fall during sleeptime, the
subject's prolactin level is lower than 1 SEM,below ,the
corresponding night time level of healthy ~su~jects lor
below the correspondlng night~time level of a stanaard
prolactin profile) at~the same time point.
A third aspect o~ this invention reIates to a
method for determining whether adjustment will be re~uired
to "normalize~ or generally conform to or approach an
abnormal prolactin profile or level to a standard ~ or
healthy) prolactin profile or level, the method=co~mpris:ing:
(a) collecting a plurality of bIood sampIes~from:a, subiect
over a c~t;n~l~us time period, the collection of said
samples being made at predetermined tim=e intervals (or at
predetermined time points) within said time period; E~b)
assaying the prolactin content of each of said samples;~(c)
plotting the prolactin content of: ea~ck of saia samples
against the time at which said sample was collected during
said time period to generate a plurality of data points
(i.e. correlating the prolactin content with the=time); (d~
generating a prolactin profile by connecti=ng (or=o,therwise
fitting a curve through) said data points; and (e~ compar-
ing the prolactin profile to a predetermined:normal prolac-
tin profile. Steps (cl and (d) ~ogether constitute the
step of expressing the prolactin content as a function of
time to generate the prolactin profile of the su~ject.:
A variation of this third aspect of the~invention
involves measuring only key prolactin levels to generate
the data points, omitting step (d), and,:as ~step ~e),
comparing each data point to the corresponding,prolac~tin
level at the same time point on the predetermined stanaard
W096l00396 2 ~ 928 55 r~ 5 ~l
.
13
~ prolactin profile, or to a prolactin level of healthy
subjects at the same time point.
A fourth aspect of this~invention relates to a
method for determining adjustments that will cause an
abnormal daily prolactin profile of a patient to confsrm to
or approach a normal indiYidual's daily prolactin profile,
which comprises: (a) comparing the prolactin profile of the
patient to a predetermined standard prolactin profile for
healthy (normal) subjects; (b) detqrmining that at least
one of the following obtains: (i) the prolactin level of
said patient at any time point (preferably any prolactin
measurement points, most preferably any two prolactin
measurement points) during waking hours exceeds the corre-
sponding prolactin level at the sa~e time point of normal
~healthy) individuals by at least 1 Standard Error of the
mean (SEM); and (ii) the prolactin level of.such patient at
any time point (preferably any prolactin measurement point,
most preferably any two prslactin measurement points)
during sleeptime is below the corresponding prolactin level
of normal healthy individuals at the same time point by at
least one SEM; (c) determining the time at which to admin-
ister at least one member selected from the group consist-
ing of a prolactin inhibitor and a prolactin stimulator to
said subject; and (d) selecting the amount of said prolac-
tin stimulator and/or inhibitor to a.djust a subject~saberrant prolactin level so that the subiect~s prolactin
profile conforms to or approaches the prolactin profile of
normal (healthy) individuals. Again it is preferred that
if the determination is based on or,e prolactin measurement
point being aberrant, the variation from the normal profile
be at l~ast 2 SEM.
A variation sn this four~h ilspect of the inven-
tion involves making the determination required in step (b)
and the selection required in step (d) based on a compari-
son of key prolactin levels of the patient at predeterminedtime points during key intervals of~the day (or night) with
W096/00396 2 1 9 2 8 5 5 r~
o
14
the corresponding ~healthy~ or ~normal" prolaotin level~ at
the same time points.
3RIEF DESCRIPTION OF T~E ~R~Tt--.C
The present invention is further described with
respect to the annexed drawings in which: =~
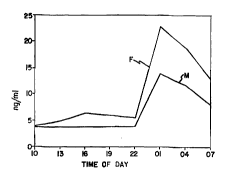
Fig. 1 is a graph of average ~plasma prolactin
levels (nglml~ for healthy individuals (males: "M" curve;
females: "F" curvç) v. time of~day (hours). ~
Fig. 2 is a superimposition~of a~male patient~s
abnormal base-level (pre-therapy) prolactin profile (thin
dotted grey line) and post treatment improved prolactin
profile (thin black line), and the standard normal prolac-
tin profile ~thick grey line), all in graph f~orm. (Prolac-
tin units are in nanograms per ml, and time of day is in
hours.)
Fig. 3 is the same type of graphical superimposi-
tion as Fig. 2 for another male=patient.
Fig. 4 is an illustrative superimposition ~of
various types of typical human aberrant prolactin profiLes
(a) - (d).
DET~TT~n DES~RTPTION OF ~ INv~t~TION ~_~
All patents, patent applications:~and literature
references cited herein~are in~orporate~ by reference~-in
their entirety as if their disclosures were physically
present in the present specification. In case of conflict,
however, the present disclosure~controls.
It has recently been discovered that metabolic
disorders such as the foregoing are associated with aber-
rant patterns in the daily levels (and~~fluctuations) ~o~
prolactin and neural oscillators such as those associated
with dopamine and serotonin. Healthy (normal) subjects,
i.e., lean members of a species not suffering from such
metabolic abnormalities have highly predictable daily
prolactin profiles, which in humans arç characterized by a
low and relatively constant prolactin level during the
W0 96100396 2 1 9 2 8 5 5
~ ~,
waking hours ~day) followed by a sharp rise to a peak
durirg sleep ~night) and subsequent more gradual tapering
down to the waking hours level by mornin~. Conversely,
"healthy" individuals have prolactin profiles that are at
within 1 SEM of the standard prol~ctin profile M or F of
Fig. 1 ~depending on whether they are male or female),
preferably for at least two prolactin levels measured at
different times or within 2 SEM of the standard prolactin
profile tor at least one measured proIactin level.
~ In the present context ~'lean" means not obese and
not abnormally underweight. Ir. turn, an obese human is~
defined as a human whose body weight is over twenty percent
above the ideal body weight for a given population ~R.H.
Williams, Te~tbook of Endocrinology, 1974, pp. 904-gl6).
An abnormally underweight human is anyone at least 10
below his/her ideal bodyweight. Ideal boay weight (IBW)
can be determined by using the Metropolitan Life Insurance
Compary standard age/height~weight charts.
A prolactin profile of a~subject is obtained by
collPrt;ng blood samples from the subject at timed inter-
vals during a consecutive time period (preferably at 1 - 3
hour intervals over approximately a I2 -24 hour period),
assaying each blood sample for prolactin content, plotting
the time of blood sampling against the r~uantity of prolac-
tin present in each sample to generate a data poirt foreach sample, and rnnnPrt; nr- the data points (or other~ise
fittirg them into a curve) to form the prolactin profile.
Alternatively (for the vast majority of subjects)
a set of only a few prolactin levels~need be obtained by
rn11Prt;nr blood samples from the subject at spaced apart
times at least once and preierably at least twice during
each oi at least two key intervals of a 24-hour period.
One such key interval is early morning (06:00 - 09:00);
another is early evening (16:00 - 20:00~. Spaced apart
samples may be collected for ~exampIe at 07:00 and oo:oo
during the first interval and at 16:00 and 20:00 during the
second interval. It is desirable to collect at least one
W096/00396 2 1 9285 5 ~ s
16
additional sample at night, during a third inter~al between
23:00 and 02:00,:~e~g.~at 23:00_or at 24 00 -One or more
additional optional~prolactin measurements can be taker:at
yet another interval, e.g., a late evening interval from
20:00 - 23:00. Prolactin levels:taken during ~ey intervals
will be referred to as ~key prolactin levels"
This alternative method is preferr~d in clinical
practice to obtaining 24 hour prolactin pro~iles for
several reasons. First, such a procedure r whïch avoids
overnight hospital or blood~center stay, is~ sïgnificanfly
less costly than 24 hour blood:draws. Second, the reduced
number of blood samples drawn is preferred by patients as
it is significantly less disruptive to their daily sched-
ules. The key prolactin levels are used as indicators of
the subject's prolactin profile,~ an~d as~subs~titutes there-
of.
Although females generally have higher~prolactin
levels and sharper peaks than~maIes, the: shape of ~the
normal prolactin profile for both sexes=.ls ~uarltatively
similar and does not vary appreciably from no~mal individu-
al to normal individual (of the same s~exJ within the same
species.
By contrast, individuals who suffer from one or
more metabolic disorders have aberrant (often: highly
aberrant) daily prolactin profiles ~and levels. These
prolactin profiles and levels not only diffe~ substantially
from the norm but they can also differ-from one another
The vast majority (at least about 30~) of:pa-
tients with at least one of the foregoing::metabolic disor-
ders have abnormal daily prol:actin~ profiles that ~all
generally in one~of four 24-hour patterns represented
schematically in Figure 4: :
(a) a flat (peak-free~ high level::(in this
context "high" means higher than the "day~ or waking hours
prolactin level);
WO9~0039G 17 2 1 9 2 8 5 S
(b) a flat (peak-free). low level (in this
context '~low" means as low as or lower than the "day~ or
waking hours prolactin level);
(c) a peak during the "day" and a low level at
"night" (in this context, "low" means low as compared to
the normal average sleeptime p~olactin value between 0100
and 0400~; and
(d) a peak during the "day" and a second peak at
~night".
The alternative method of me,asuring key prolactin
levels i~ early morning and late e~verlin~ would diagnose
patients with abnormalities of type~ra), (c) and (d). If
a subject has an abnormal prolactin profile of type (b)
above~ that subject would have to give one or two night
blood samples which would not avoid overnight stay at a
hospital or blood center,
"Waking hours" or "day~ means the period of time
at which in normal (healthy) humans (not working night
shifts or alternate shifts~ prolactin levels are relatively
invariant and low (between 07:00 h and 22:Q0 h).
As employed herein the term ~sleeptime~ or
~night" means the period of time which in normal humans
prolactin level rises to a peak (between 01:00 and 04:00)
and then tapers off
The normal average prolactin levels between the
hours of 01:00 and 04:00 are between 8.0 and 14.0 nanogram-
s/ml for males and between 14.0 and 26.0 nanograms/ml for
females. See Figs 1-4.
"Prolactin inhibitor" shall include substances
which directly or indirectly inhibit prolactin in a
subject (vertebrate animal or human). Nonlimiting examples
of prolactin inhibitors include ~prolactin inhibiting
~p~mi n~ agonists such as dopamine and certain ergot-
related prolactin-inhibiting compounds
Nonlimiting examples of prolactin inhibiting
dopamine agonists are 2-bromo-alpha-ergocriptinei 6-methyl-
8 beta-carbobenzyloxy-aminoethyl-10-alpha-ergolinei 8-
W096t00396 2 1 9 2 8 5 5 r~
18
acylaminoergolines,:: ~re 6-methyl-o-alpha-(N-acyl)ami~o-s-
ergoline and 6-methyl-8 alpha-~N-phenylacetyl)amino-9-
ergoline; ergocornine; 9,1~=dihydroergocornlne; D-~-halo-6-
alkyl-8-substituted ergolines, e.g., D-2-bromo-6-methyl-8-
cyanomethylergoline; and lisuride. Moreover, the non-toxic
salts of the prolactin-inhibiting ergot-related compounds
formed from pharmaceutically acceptable acids are also
useful in the practice of this invention Bromocriptine,
or 2-bromo-alpha-ergocryptine, has been found particularly
useful in the practice of this inYention.
"Prolactin stimulator" shall include substances
which directly or indirectly stimulate prolactin. Nonlimi-
ting examples of prolactin~sti~mulators ~include~=dopamine
antagonists such as metoclopramide, haloperidol,~pimoziae,
phenothi~in~, domperidone, sulpirider chlorpromazine=and
serotonin agonists, i.e 7 MAO inhibitors, e:g., pargyline,
synthetic morphine analogs, e.g., methadonel antiemetics,
e.g., metoclopramide, antipsychotics, e.g., estrogens and
others, e.g., tryptophan and 5-hydroxy-tryptophan, mela~to-
nin, fluoxitane, and dexfenflllorAminP. Moreover/ the non-
toxic salts of the foregoing ~rolactin ~st=imula=ting com-
pounds formed from pharmaceutic~ally acceptabie acids are
also useful in the practice of this invention. Metoclopra-
mide has been found particularly useful in the practice_of
this invention.
~ Prolactin modulator' shall refer to either
prolactin st;mnl~tmrs, prolactin inhibitors, or both.
DEVEr~PM~NT OF T~E "NORMAL" PROLACTIN PROFI~E AND REY
~EVELS
A statistically significant number of healthy and
young (20-35 years of age) humans are selected as follows:
All su~jects are healthy and on normal diurnal
work/ rest schedule (nQ night g~uards or~other night-shift
workers). They are then divided inEo Ewo different groups
according to sex. ~11 subjects must normally sleep betw=een
about 23:00 and about 07:00). The i~dividuals are healthy
~W096~0396 2 ~ 9 2 ~ 5 ~ SI
~' 19
in the sense that they are free of physiologic disorders or
pathologies. In particular, they are not obese (based on
standard age/size/weight tables); are known to have normal
plasma levels o~ insulin; and are euthyroid i.e , have
normal levels of plasma thyroxin, triiodothyronine, free-
thyroxin and TSH, and suffer from no malignancies or
autoimmune disorders or gene~ically transmitted diseases.
A statistically signi~icant number shall mean at
least 3, the smallest sampling number for which statistical
formulas generally have any meaning. However, a sampling
number of at least 6 is generally preferred (at least 10 is
more preferred) because this amount of sampling generally
reduces the standard error (SE) of prolactin determina-
tions. :~ ~
Blood is collected from each subject at 1-3 hour
intervals over a 24-hour period and, diurnal plasma levels
of -prolactin, total triiodothyronine (T3) total thyroxin
(T4), free-T4=and thyroid stimulating hormone (TSH) are
measured for each subject for e g., blood is collected over
a 24-hour period at suitable time intervals (e.g., every 1
to 3 hours), typically starting at lD:00 am and finishing
the next morning at 8:00 am).
Suitable sampling techniques and assay procedures
are well-known to those skilled in the ~ield and can be
selected from pnhli~ procedures, e.g., Linkowski, P. et
al., J. Clin. Endocrinol. Metab. 61:429-438, 1985; Van
Cauter and Copinschi, "Circadian and Episodic Variations~,
Martinus Nyhoff, The Hague Netherlands, pp. 1-25, 1981.
During sampling, all s~bjects must co~sume the
same diet and ~;nt~;n the same sleeptime schedule
Prolactin data are plotted against time of.day and a normal
prolactin curve is developed ' n~ i ng SEM. Mathematical
expressions can also be developed to describe the curves
and the area under the curves.
The result of a normal 24 hour profile for males
approximately 30 years of age is shown as the "M" curve in
Figure 1. ~The result for ~emales approximately 30 years of=:
W096/00396 : 2 1 9 2 8 5 5 r~
age i8 the "F" curve. The SEM for prolactin during waking
hours for males is l.Q to 2.0 nanograms per ml and:appro~i-
mately 3.0 nanograms per ml during sleeptime. For females
the SEM for prolactin during waking hours is be~tween l_Q
and 3.0 nanograms per ml. and between 3.0 and 6.0 nanogrxms
per ml. during sleeptime.
Alternatively, the curves of Fig. l can be used
as the standard prolactin profile (as weIl as for the
standard prolactin levels at times corresponding to key
prolactin measurements); or only a set of prolactin levels
can be measured in healthy subjects at key intervals during
the day, e.g., at least two in the early morning,~at least
two in the early evening and optionally one or two at
night. ~ -
_ ~ :
M ~TLON OF DI~RNAL ~ORNONE
PROFILE ~ND/OR KE~ PROLACTIN ~EVELS
OF "AFFECT~n" S~3~EC~S
The procedures described above~can be used to
develop a 24 hour prolactin profile (or a set of key levels
of prolactin during certain key intervals) for individuals
under clinical evaluation for therapy according to tbe
pre~ent invention. Individuals that are:expected to have
an abnormal daily prolactin rhythm include those having
been diagnosed as afflicted with at least one of the
~ollowing conditions: obesity (i.e., more than 20~ over-
weight, based on age, frame sl'ze and sex characteristics
using tables such as the Metropolitan Life Insurance
Company tables for standard weight for height and age~,
insulin-resistance, hyperglycemia, hyperinsulinemia,
hyperlipidemia, or Type II diabetes. However, the present
evaluation is not limited to such subjects.
The subjects to be considered for therapy should
be euthyroid, i.e., hav~ a total T3 level between about 9o
and about 180 ng/dl, total T4 between about 4.5 and about
12 g/dl, and free-~4 between about 0.7 and about 1.9 g/dl.
If not, T4 and/or T3~ shouId be:administered (preferably in
the morning hours) priorrto or concurrently with prolactin
~W096l00396 2 1 ~2 85~ r~"~ c I
~ 21
modulator administration. In other words, it is preferable
to adjust the prolactin profile of an=individual if his/her
levels of T4 and/or T3 are ~ormal. Thus~ prior to adjust-
ing the prolactin pro~ile or lev~ls ~of a subiect through
administration of a prolactin stimulator, or inhibitor, or
both, it is preferred to determine that the subject is
euthyroid. If the thyroid hormone levels in a subject are
below normal (as determined with reference to the above
normal values) T3 and/or T4 are, preferably, first adminis-
tered to the .subject until the plasma levels of thesehormones are normal and prolactin stimulatory or inhibitory
treatment is then begun. The thyroid hormone amount
administered is the dosage required to bring the patient to
a euthyroid condition; usuaIly between about 25 and about
150 mcg per patient per day. If nrrrCc~ry to maintain
adequate levels of T3/T4 throughout the period of adminis-
tration of prolactin stimulator and~or inhioitor, T3/T4 can
be continued along with the ~prol ctin modulator therapy
agents. Hyperthyroid subject should also receive treatment
(via use of antithyroid agents or thyroid ablation) prior
to prolactin modulator treatment.
Once a diurnal prolactin level profile has been
developed for an individual, the profiIe is compared to.the
"normal" profile (e.g., the one generated as described in
the previous section or to Fig. 1). A determination can
then be made based on the following general criteria:
1. From about 07:00 h to about 22:00 h, i.e.,
during the flat and low ~evel portion of the '~normal~
prolactin profile, at a given time,point in the prolactin
profile of the subject the (blood) prolactin value must not
be higher than one SEM above the mean at the same time
point (or higher than 2 SEM above the mean at one time
point, or higher than one SEM above the mean at two differ-
ent time points). This rule applies to exclude from a
conclusion of ~normal" all indivi~ual prclactin profiles
that do not meet the above criteria, either because their
W096l00396 2 l 9 2 8 5 5 . ~u ~ ~1
22
prolactin is too high duri~-g the day or because their
prolactin is too low during sleeptime, or both.
2. From about 22:0Q h till about 07:00 h, i.e.,
during the rapid change (and sl eptime peak) of the normal
daily prolactin profile, the indlvidual's prolactin profile
must first have a peak at about the sa~e time~or within two
to six hours after=~sleep initiation as the '~normal~ prolac-
tin peak for~subjects in the same category (usually about
01:00) and must aIso be within one SEM of the normal
healthy prolactin prQfile (preferably for two prolactin
readings or alternatively within two SEM for at least one
prolactin reading).~
It should be noted that, based on clini--al
experience of the.present inventors, adjusting~high day
time prolactin levels to normal or near normal values
effects a significant i~ sv~ t in the pathologies of the
patients treated even if, as a re-sult of the treatment, the
night time prolactin peak is lessened. Therefore., consis-
tent with this observation, obser~ance of criterion 1 above
takes priority over observance of criterion 2.
If only a set of key prolactin~easurements are
made for the subject being tested, the above criteria ~ e
applied as follows: if at any of the ''day~ time points at
which key prolactin measurements have been made for the
subject his/her prolactin level is higher than 1 SEM (and
preferably 2 SEM) above the mean day time normal prolactin
level at the same time point, then the subject has an
abnormal day prolactin profile or rhythm; and if at any
~night" time point for which a key prolastin level has been
measured the prolactin level of the subject=-~taken during
the key interval of 23:Q0 - 02:00) is lower than 1 SEM (and
preferably 2 SEM) below the mean .ileeptime normal prolactin
level at the same time point, then the subject has an
abnormal night prolactin profile or rhythm.
To determine if a subject has an aberrant prolao-
tin profile the bedtime on the subject's prolactin profile
should ideally be coincident with the bedtime on the
~W096l00396 2 ~ 9 2 ~ 5 5 r~
23
profile o~:normal subjects. If this is not the case, the
profile o~ the subject and the profile of normal individu-
als can be superimposed and one or the other ca~ be shifted
so that the sleep initiati~n time of~the subject to be
tested coincides with the sleep initiation time of normal
healthy subjects.
"~r~ TION OF rRR~TM~T FOR AN AFFECTED SUBJECT
The information ~prolactin profile and set of key
prolactin levels) generated as described above is used to
(a~ identify the patients that are in need of an adjustment
in their prolactin proiile and (b) to ~r~rmi ne the type
and extent of adjustment re~uired. In general, those
individuals ~hat are obese, hyperinsulinemic, hyperlipid-
emic, hyperglycemic and/or diabetic display abnormalprolactin profiles (or key prolactin levels) as compared to
healthy individuals. Simply stated, by comparing a subjec-
t~ 8 prolactin profile (or key prolactin levels) with the
standard prolactin profile, or corresponding healthy
(normal) set of prolactin levels, it is possible to identi-
fy individuals afflicted with the abnormaL conditions
discussed abo~e By adjusting the abnormal prolactin
proiile of such individuals ~either by administration of a
prolactin ;n~;hit~r or a prolactin stimulator, or both) at
the appropriate time of day and in the appropriate dosage
(amount) it is possible to adjust such individuals~ prolac-
tin profile to~ con~orm (or at least approach) a normal
profile. ~he amount and timing of=administration of such
dosages can be determined based upon information contained
in the prolactin profiles (or key prolactin levels) dis-
cussed above. _
An adjusted profile approaches a normal or
healthy profile if all or a portion of the abnormal profile
moves in the correct direction by at least 2 ng/ml. For
example, if a human subject~s abnormal prolactin level is
18 ng/ml between 07:00 and 10:00 and (after adjustment) it
is reduced to 16 ng/ml during the same time period, the
W096/00396 24 1~~
ad~usted profile approaches~the healthy=profile. It is
thus important to reduce the area under the daytime prolac-
tin curve (typically by at least about 20~) and to avoid
prolactin peaks during the day.
The treatment determination has three~ aspects:
(a) choice of effect desired (i.e., choice of prolactin
stimulator to i~crease prolactin levels, or ~prolactin
inhibitor to reduce prolactin levels, or-both to increase
prolactin at night and to reduce prolactin during the day);
(b~ timing of (each) dose of administration; and Ic) amount
of (each) dose to be administered. For example, generally
if the patient is initially diagnosed as requiring a
prolactin inhibitor, he might initially receive 0 ~-1.6 mgs
per day of (pr2ferably) accelerated.-release bromocriptine
l'i in a sinyle dose (or in divided doses). The dosage might
be adjusted after about 4 weeks Df treatment e.g., at that
time a fresh prolactin profile (or a s-et of key prolactin
levels) would be taken and e.g., if the patient still had
a high prolactin level he would receive an increased dosage
of (preferably) an accelerated release prolactin inhibitor
at the appropriate time of day. The time Df administratlon
might also be adjusted (~r~n~;n~ on the patient's current
prolactin profile or ~ey prolactin levels), e.g., the time
of bromocriptine administration would be changed.
The comparison of ~the individual prolactin
profile (or levels) to the normal prolactin profile (or
levels) will determine (a) and ~b) in tEe preceding para-
graph, as follows: If at any point during the ~'day~ prolac-
tin level is too high (whether or not it has a peak),
administration of a prolactin inhibitor is required.
If the "night'~ prolactin profile lacks a peak or
if its peak is not sufficiently pronounced, administration
of a prolactin stimulator is required.
The preferred prolactin inhibitor (or dopamine
agonist) is an accelerated release inhibitor, in particular
an accelerated release somposition c~nt~;ning bromocriptine
W096l00396 2 ~ ~2855 r~l~u~
~ 25
as set ~orth in co-pendlng U.S. Patent Application Serial.
No. 08/171,897.
The preferred prolactin stimulator is metoclopra-
mide.
Whether a 24-hour prolactin profile is generated
for a subject to be treated, or only key prolactin levels
are measured, the following more specific guidelines will
generally be followed to ;niti~lly determine bromocriptine
administration timing, for a period of treatment of approx-
imately 26 weeks:
a) Week 1 to Week 6. Fir~t ~Qsa~e: If any one
of a patient's 07:00, 08:00, 16:00 or~ 19:Q0 prolactin
levels is e~ual to or higher than 5.0 ng/ml for males or
7.0 ng/ml for females, then 0.8 mg of accelerated release
bromocriptine is administered at 06:00 daily.
Second Doaa~e: Beginning in week 3, a second
dosage containing 0.8 mg of accelerated release bromocript-
ine is also administered at 10:30 daily.
b) Week 7 to Week 12. First dosaQe: If any one
of the oi.00, 08:00, 16:00, or 19:0~ prolactin values is
still e~ual to or higher than 5.0 ng/ml for males or 7.0
ng/ml for females, then 1 6 mg of accelerated release
~vllv~iptine are administered at 06:00. Otherwise, Q.8 mg
of :accelerated release bromocriptine is administered at
06--00 daily.
Second Do~aae: In addition, if the 19:00 prolac-
tin level is less than or eQual to 1.5 ng/ml for males or
females then the second dosage of~0.8 mg of accelerated
release bromocriptine is administered at 08:30 daily
instead of at 10:30. If the 19~ 00 prolactin level is
higher than 1.5 ng/ml for males and females, then the
second dosage ~nt;nll~q to administered at 10:30 daily.
If the 19:00 prolactin I~vel is less than 1.o
ng/ml for males and females, then there is no administra-
tion of second dosage.
W096~0396 2 1 92855
' 26
c) Week 1~ ~o Week 26. For bot~ first and
second dosages the rules are the same set forth for Weeks
7 - 12, subject to the following:
(i) If either the 16:00 or l9enO
prolactin level is equal to or higher' than 5.0 ng/ml for
males or 7.=0 ng/ml for females, ~hen'a~dd an -ad;tional Q a
mg of accelerated; release ~bromocriptine to the first
dosage, unless the patient is already receiving 2.4 mg of
bromocriptine in total. In that case, add the additional
0.8 mg of accelerated release bromocriptine~to the second
dosage;
(ii) If the 19:00 prolactin level is
lower than 1.5 ng/ml for males or females, then the second
dosage time is adjusted by administering it 2 hours earli-
er; and
(iii) If each of the oa oo, 16:00 and19:00 prolactin levels is less than 1.0 ng/ml for males or
females, then subtract 0.8 mg of accelerated release bromo-
criptine from the ~econd dosage, or, if there is no second
dosage, then subtract 0.8 mg of accelerated release bromoc-
riptine from the ~irst dosage. In the vast majority of
patients, the first dosage must contain a minimum of 0.8~mg
of accelerated reIease bromocriptine.
The time and amount schedules given above are
intended as guideli~es ior bromocriptine administr'ation and
those skilled in the art can further adjust the precise
timing and amount of bromocriptine administration based on
the actual prolactin profile or~key prolactin levels of a
patient to be treated For example, if a patient does not
respond (or does not respond adequately) to~a given dosage
or dosages ~e.g. o.a mg) it (or they) can be increased
(e.g. to 1.6:mg).
When needed, metoclopramide ~generally daily
dosage range is 0.5 - 5. n mg/person; preferred daily dosage
range is 0.5 - 2.0 mg/person) ~can be administered once
about one hour be~ore bedtime. A prolactin stimulator in
general is not administered un~ess the average prolactin
W096t00396 . 2 ~ 92855 r~u~ ~1
.
27
level o~ the patlent between Ol:OO a~d 04:Qo is at least 1
SEM (preterably based on two data points or.at l~east 2 5EM
if based on only one data point) lower than 8.0 ng/ml for
males or 14 ng~ml for females Eurthermore, when the day
time prolactin is too high a substantial clinical benefit
will be realized by adjustment of the day time prolactin
levels and the administration of a prolactin stimulator
will not be re~uired in most cases ~although it may be
desirable).
In general, the time at which an inhibitory agent
other than bromocriptine, or stimulatory agent other than
metoclopramide, is to be administered to a patient can be
determined by ascertaining the time between administration
of the agent and the time at which the agent exerts its
maximum biological (i.e., stimulatory or inhibitory)
e~fect. The time at which a stimulator has its maximum
stimulating effect, (or when an inhibitor has its maximum
inhibition effect) can be determined by administering the
stimulatory or inhibitory drug to a patient with a known
prolactin profile and then calculating the time that
elapses between administration of the drug and exertion of
the maximum effect on movement leither inkibition or
stimulation) of the patient's (known) prolactin profile.
In fine tuning a subject's medication administra-
tion schedule, the rebound effect that administration of a
prolactin inhibitor during the day might have in sleeptime
prolactin levels of this subject should be taken into
account. Conversely, the rebound effect that administra-
tion of a prolactin sti~ tnr might have on prolactin
levels during ~the subject's waXing hours should also be
considered ~n this manner, and using the methodology of
the foregoing g~ l;nPq, the time of administration for a
particular inkibitor or stimulator çan be determined using
routine expeYimental procedures.
The precise time of modulator administration that
will yield the most effective results in terms of efficacy
of treatment in a given patient will depend upon the
W096100396 2 1 9 2 8 5 5 r~
2B
activity, pharmacokinetics, and bioavailability of a
particular modulator, physi~logical condi~ions ~oi ~he
patient (including age, disease~type and~stage, physical
condition, responsiveness to a given dosage and modulator~,
route oi administration, etc. ~owever, the above guide-
lines can be used as the basis for determining the optimum
ti~e of administration.
The foregoing are applicable ior setting initial
therapy regimens. _In general a patient receives between
about 3 and about 100 micrograms of bromocriptine per
kilogram of body weight per day, and prefexably between
about 10 and 40 mi~,u~la,r,~ per kg of body weight per day.
The exact dosage of: prolactin inhibitor tor prolactin
etimulator) required to achieve the optimum effect in terms
lS of prolactin adjustmeht must be adjusted for each patient
based upon the patient's drug sensitivity (i.e., response
to drug) age, disease state and stage and physical condi-
tion. The patient is periodical~y reevaluated by measuring
prolactin levels at predetermined intervals~during a 24-
hour period, preferably (or shorter period if necessary),
the first such reevaluation typically occurring at the end
of four weeks from the onset of= therapy, and subseguent
reevaluations occurring every 4 to 3 weeks during therapy
and then every 3 months thereafter. Typical daily dosages
of bromocriptine for humans on~a:per patient basis are~0.2
- lS mg, preferably 0.8 - 3 mg.
Prolactin stimulators are noxmally administered
within about 1 hour prior-to retiring fox the patient~s
normal sleep period.
Adjustments to the amount(s) of~drug(s)~ adminis-
tered and possibly to the time of administration may be
made as described above based c~ these reevaluations. ~
Generally, adjustment of timing and amount~of
drug(s) is not considered, necessary if the sleeptime
prolactin peak during therapy is higher than normal as long
as the peak value occurs at the right time, and the slopes
wo96l003s6 2 1 928 ~5
of the peak are sharp (with normal values at each side of
the normal peak).
The efficacy of a particular regimen on a
particular patient and the adjustments (in dosage and
timing) required, if any, can be determined by comparing
the patient's re-ev~ t;rn prolactin profile or reevalua-
tion key prolactin levels with the standard profile (or the
"healthy~ key profile levels~. ~
In treating vertebrates, generally, dosages of
the acc~lerated release prolactin inhlbitor (bromocriptine)
and/or stimulator (metoclopramide~, respectively, are each
given, generally once a day, generally over a period
ranging from about 10 days to about 180 days, but treatment
can continue in~Pf;n;tely (if necessary or desired) for
months or even years. The preferred prolactin inhibitor
(accelerated release bromocriptine) is given daily at
dosage levels ranging from about 3 micxograms to about lO0
micrograms, preferably from about 10 micrograms to about 40
micrograms, per~ kg. of body weight, and the prolactin
stimulator (metoclopramide) is given daily at dosage levels
ranging from~ about 5 micrograms to about 240 micrograms,
preferably from about 5 mi~L~yLdrrs to about lO0 micrograms,
per kg. of body weight per day to modify, or alter, the
prolactin profile (or the key prolactin level) and contin-
ued for a time sufficient to reset the circadian plasmaprolactin rhythm, at which time treatment may be discontin-
ued. If ~he subject suffers a relapse, treatment may be
resumed.
In treating humans, in partic~lar, the prolactin
ir~ibitor (accelerated release bromocriptine) is generally
given at daily dosage level~ ranging from about 3 micro-
grams to about lO0 micrograms, preferably from about 10
micrograms to about 40 micrograms, Fer kg. of body weight.
The prolactin stimulator metoclopramlde is y-enerally given
at daily dosage levels ranging from about 5 micrograms to
about 50 micrograms, preferably from about 5 micrograms to
about 20 micrograms, per kg. of body weight per day. (Per
W096l00396 21 92~5 p~ oSI ~
person dally dosages range of metoclopramide are~typlcally
0.5 to 5.0 mg; preferably 0.5 to 2.0 mg.l Such treatment
(using one~or both types of drugs) i5 typically~continued
over a period o~ time ranging from about 10 days to usu~lly
about 180 days, resulting in modification and resetting~of
the lipid and glucose metabolism of the patient to that oi
a lean (i.e., normal) healthy person, at which time treat-
ment may be discontinued. For some patients (e.g. patients
in particularly poor physical condition, or those of an
advanced age) it may not be possible to reset the~ir prolac-
tin rhythm withi~ the above time periods and such patients
may require a longer, or even r~nt;nl~rus, treatment with
prolactin stimulators and/or i~hibitors ~he dosage and
timing informatio~ set forth above is designed for~bromocr-
iptine and metoclopramide and will have to be altered for
other agents using ~the dosage and timing methodology
disclosed herein.
In the practice of this invention,~ a prolactin-
inhibiting compound, and a prolactin stimulator are admin-
istered daily to a subject preferably orally, or by subcu-
taneous, intravenous or intramuscular;iniection:_ Dermal
delivery systems e.g., skin patches, as well as supposito-
ries and other well-known systems for administration~of
pharmaceutical agents can also be employed.
sOdy fat deposits, inclusive of adipose/ arterîal
wall and plasma fat, of an obese person will be reduced,
leveled out and generally maintained ~(after the treatments
of the present invention are disc~nt;nl,r~) at that of a
normal (lean) person, over an r~tr~d period of time.~ A
subject that exhibits the effects of insulin resistance,
hyperlipidemia or hyperinsulinemia and/or hyperglycemia, or
both insulin resistance and hyperinsulinemia andlor hyper-
glycemia, treated with the prolactin inhibitor and/or a
prolactin stimulator at the ~appropriate times of day
discussed~above, will become more sensitive tQ insulin
(i.e., will have a lower insulin resistance), and the
effects of hyperinsn7;nrmi~ ~and/or hyperglycemia and
W096l00396 2 1 9 2 8 ~ 5 r~
31
related ab~ormal metabolic values will be reduced on a long
term basis. Treatment generally lasts between about 10 and
about 18D aays on average in humans The administration of
the prolactin inhibitor ~or dopamine agonist) and/or
prolactin stimulator in this manner will thus reset the
phase relations~ of=the two neural oscillations and their
various circadian expressions to alter metabolism on a long
term basis (e g., several years), if not permanently~ In
other words, the result of the timed daily dosages of the
prolactin inhibitor ~or dopamine agonist) and/or prolactin
stimulator will be a long term reversal of the major
pathologies generally associated with the development of
Type II diabetes. Using the methods of=the present inven-
tion, the levels of body fat stores, plasma insulin concen-
trations (including in patients oral hypoglycemic medica-
tions), insulin resistance, hyperglycemia, and blood
pressure or all of these pathologies can be reduced on a
long term basis by such treatmènt, or treatments, from the
high levels often found in obese, hyperinsulinemic, hyper-
lipidemic and/or hyperglycemic persons to approach or
conform to the much lower and much more desirable levels
found in normal persons with normal insulin levels.
The following are non-limiting working examples
of diagnosis, regimen determination and therapy according
to the present invention:
Case StudY 1: ~13001Q)
Subject: male; 52 yrs; 294 lbs; 5 ft 6 in.
Pretreatment ~atholoqy: ,
(a) Obesity: 191~ IBW (based on a standard Table,
e.g., the standard table of Metropolitan Bife.Insurance Co.
NY, ~Y available from the company);
(b) Type II diabetes: fasting plasma glucose 164
mg; percent glycosylated hemoglobin 9.4~ (measured by
affinity chromatography)i The normal morning fasting
plasma glucose is between 80 and 12~ mg percent (mg ~).
W096/00396 2 1 q 2 8 5 5
32
The subject's 24-hour base (pre-therapy) prolac-
tin profile is shown graphically aE the dotted grey line~in
Figure 1 It shows that the subject's prolactin~was too
high throughout the day and early evening, At 07:00, it
was 13.2 ng/ml and at 08:00,-ll~a~ng~ml; ~at 16:~-it was
9 9 ng/ml and at 19:~v0 it was 12'2 ng/ml The subject was
administered accelerated release bromocriptine as follows:
Weeks 1 and 2: 1 6 mg at 05:00;'weeks 3 and~4: 0 8 mg at
05:00 and an adaitional o:a mg at'10:0~; ~reeks 5 -~ 18: 1 6
mg at 05:00 A ~~reevaluation)' prolactinl oro'~ile was
generated for the subject after 13 weeks and i~s graphically
Ehown in Fig 2 as the thin black line_ The ~ey prolactin
values were 07:00 11 6 ng/ml; 08:00 9 5 ng/ml; 16:00 3~3
ng/ml and l9:a0 6 5 ng/ml. ~ ~
The i , L~V. ts observed in this patient's
prolactin levels after 12 weeks of treatment included
significantly lower levels throughout most of the day and
early evening
The clinical benefits of treatment to this
patient included reduction in glycosylated hemoglobin from
a starting level of 9 4~ to 7~ 6~,after 18 weeks of treat-
ment. The patient lost 18 pounds of body fat'::over 18
weeks; plasma cholesterol was reduced from 229 mg ~ to 180
mg ~; triglycerideE were reduced from 186~mg ~O to,1,,22 mg ~;
fasting glucose was reduced from~164 mg ~ to 133 mg ~; and
oral glucoEe tolerance test results improved, over the
treatment period
Case Studv 2: (130007)
Subject: male; 53 yrs; 203 lbos;~5 ft. 1~ in
Pretreatment ~atholoqv: : ,
(a) Obesity: 130~ IBW;
(b) Type II diabetes: fasting pIaEm=a glucose 16a
mg ~, glycosylated hemoglobin~7~5~
On initial evaluation, the subject had the
prolactin profile shown in Figure 3 aE~ the dotted grey
line. The day levels ~particularly the 07:=00 level) are
W096l00396 21 9 2 8 5 5 r ~ Sl
too high: at 7:00, 18.2 :ng/ml; at 8:00, 15.0 ng/ml; at
16:00, 8.1 ng/ml; and at 19:00, 8.8 ng/ml. The night time
peak is also somewhat delayed. The patient was adminis-
tered accelerated release bromocriptine as~follows- Weeks
l and 2: 1.6 mg at 08-3Q; Weeks 3 and 4: 1.6 mg at 08:Q0;
Weeks 5 and 6: 0.8 mg at 08:30i Weeks 7 - 18: 1.6 mg at
05:00.
The second profile (the thin black line) taken
after 18 weeks of treatment shows that the subject~s
prolactin profile was very sensitive to bromocriptine. The
day time levels were significantly reduced. However, the
night time levels were also reduced below normal Prolac-
tin levels were as follows: at 7:00, 1.9 ng/ml; at 8:00,
1.7 ng/ml; at 16:00, 0.9 ng/mli and~at 19:0Q, 1_5 ng/ml.
The clinical benefits of treatment to this
patient included reduction in glycosylated hemoglobin from
a starting level of 7.5~ to 6.1~. The patient lost 23
pounds of body fat; the patient's OGTT test showed consid-
erable improvement, with the area under the glucose curve
declini~g by approxi~ately 30~; and fasting glucose was
reduced from 168 mg ~ to 133 mg ~, over the course of
treatment.
The data show that metabolic states are regulated
at least to a clinically significant degree in part by an
interaction of circadian neuroendocri~e rhythms. This
hypothesis proposes that the daily rhythms of cortisol and
prolactin are individual expressions of two separate
circadian systems and that the phase relations of these two
systems can be reset. Thus, in a hamster model it has been
found that the 0-hour relation resets the circadian oscil-
lations into a pattern that maintalns the lean, insulin
sensitive state and the 12-hour relation permits retention
of a pattern that maintains the obese, insulin resistant
state. Another important addition of the present study is
that the effects:o~ timed injections of a prolactin inhib-
iting dopamine agonist, or other prolactin inhibiting
compound, are long lasting. Apparently once resetr the
W096/00396 2 ~ 92~55 ~ J5 ~l
34 ~
phase relation ~f the_t~o clrcadian oscillations~ tends ~o
maintain its altered pattern
Changes in the phase relations oi two~circadian
neuroendocrine oscillations are eYidenced ~y changes in the
phase relations of their ~ir~ n expressio~s This
expectation is fulfilled respecting plasma glucocorticost=e-
roid and prolactin rhythms. In=several species~examined~
the phase relations of the two hormone rhythms differ:~in
lean and fat animals.
The phase relation between the circadian rhythm
of plasma insulin concentration and the~rhythm of lipogenic
responsiveness to.insulin is shown to differ in=lean and
fat animals. Whereas the daily interval of :lipogenic
responsiveness remains near light onset, the phase of the
insulin rhythm varies markedly. The peak concentration of
insulin, e.g., occurs ~ear light onset =in obese female
hamsters held on short day-lengths. Tha=t is, the daily
peaks of the lipogenic stimulus (i e., insulin) and the
lipogenic response~to insulin co~incide in fat animals and
not in lean animals.
It is apparent that various modifications and
changes can be made without departing from the spirit and
scope of this invention.