Note: Descriptions are shown in the official language in which they were submitted.
CA 02211844 1997-07-28
WO 96/26673 1 PCTlUS96/00670
This invention relates to the field of medical monitoring devices generally
and
has particular application in the field of chronic or long term patient
monitoring
particularly for pressure sensing. There are many situations in which a
patient requires
long term monitoring and when it may be desirable to implant a sensor for
monitoring
within the patient's body. Such a sensor needs to be checked against an
external
reference for accuracy on an ongoing basis. This is particularly important in
the area
of pressure sensing where an implanted pressure sensor within the body is
subjected not
only to the changes in pressure within the body and the location at which the
sensor is
implanted but also to the ambient pressure or barometric pressure in which the
patient
is located. For example, if the patient were to be riding in a car up a
mountain or
travelling up or down a large building in an elevator the local barometric
pressure
around the patient would change affecting the physiological measurements of
the
changes in pressure registered by the implanted pressure sensor/monitor
device.
Because of these problems, long term absolute pressure monitoring of patients
through implantable sensors has not been done before.
There is in the acute or short term pressure sensor field two types of devices
which have been employed generally in hospitals and critical care facilities.
A Millar catheter contains a silicon-based pressure sensor at the tip of a
flexible
catheter which is inserted into the patient's body at the point where pressure
is to be
measured (for example the right ventricle or pulmonary artery). Readings from
the
Millar-type sensor are corrected for atmospheric ambient changes using a
barometer in
the same room.
A second system for measuring internal pressures consists of a fluid-filled
catheter with a diaphragm at one end and a pressure sensor at the other end.
the
diaphragm is placed at the point inside the body where pressure is to be
measured. The
internal pressure is transmitted up the catheter through the incompressible
fluid and is
measured by the pressure sensor outside the body. The effects of barometric
pressure
are eliminated by placing the two ends of the catheter at the same height
above the
floor.
CA 02211844 1997-07-28
WO 96/26673 2 PCT/US96100670
Barometric pressure readings are of course widely available through various
devices. Recently, watch companies have even begun to supply small barometric
pressure sensors in association with their watches. For example, Avocet
provides a
watch (the "AOU-OZ Alpine Vertech) which will describe the height at which one
is
located above sea level based on the pressure reading at that location, anal
has other
functions related to mountain climbing that use the on-board pressure and
temperature
sensors.
Chronically implanted dynamic sensors have been reported but they cannot
measure a baseline or DC pressure (gauge pressure). They do not have a
frequency
response at DC and therefore do not maintain a stable zero point. They can and
do
provide for good waveforms of dynamically changing pressures, but the absolute
value
of the pressure measure is unknown.
Numerous low cost barometric pressure measuring circuits exist in the art.
Some are illustrated in the proceedings of the Second Annual Portable by
Design
Conference sponsored by Electronic Design Magazine (Feb.-13/17-1995) Santa
Clara,
CA.
There has developed a need to monitor pressure or other metrics with a device
implanted into the body of a patient that can be corrected for by changes to
barometric
pressure reference or to an external measurement of the same metric. This
invention
particularly addresses the problem of allowing for changing external ambient
conditions
(particularly pressure) and adjusting for their effects on implanted
monitoring sensors.
~um_marv of the Invention
A preferred form of the invention is a system consisting of implanted sensor,
external reference and apparatus to combine the readings. The readings can be
used to
adaptively reconfigure or change the functioning of the implanted device,
automatically
or with medical oversight through human analysis of displays or printouts of
long term
readings. The invention employs a wearable or otherwise external barometric
pressure
sensor and monitoring apparatus to produce and store a set of barometric
measurements
or pressure readings associated with particular times. A temperature corrected
reading
may be preferred. This external pressure sensor may be mounted in a housing
carried
by an individual. He or she could wear it for example on the wrist or in a
belt, or in a
CA 02211844 2000-10-25
66742-623
3
purse, so that it will go where the individual goes and
therefore be subject to the same ambient pressure environment
that the individual is subjected to. Within the housing, a
sensor reads the ambient barometric pressure and these pressure
readings are coordinated with time such that when they are read
out these readings or measurements can be paired with
measurements made by an internal/implanted body sensor tracking
the pressures within the body of the patient at approximately
those same times.
In its presently preferred form it would be most
useful to have the data from the implantable device telemetered
to a programmer or other similar computer device which would
coordinate data regarding pressure taken from the implanted
device with the data developed and recorded by the external
sensor. This recorded and coordinated data can then be used by
the programmer (or other computerized device) to generate
physiologic measurement readings, or in other words, to
calibrate or adjust the implanted device readings. This
provides an absolute value of the internal measurement that is
adjusted to compensate for any biasing of external forces by the
environment. These adjusted readings can then be used by a
physician to provide further therapy for the patient, whether by
changing the functioning of the implanted device or by use of
drug or other therapies. The readings could also be used to
produce histograms of patient health with respect to the
measured parameter which may be used for many purposes that
would naturally occur to the reader. The corrected measurements
or readings could also be used to automatically change the
functioning of the implanted device if desired. This last
potential use of this invention can make the implanted apparatus
associated with the implanted sensor responsive to changing
patient conditions with respect to the measured parameter over
66742-623
CA 02211844 2000-10-25
3a
time or to adaptively select between available therapies that
can be accomplished by the implanted device when enough data has
been gathered.
The external and implanted sensors may be implemented
in many ways. In one embodiment, the implanted and/or external
sensors may each have one or more of,the following elements: a
memory for storing sensor data, a clock, a sensor for generating
measurement values, a processor for processing sensor data, and
a clock driven program to control processor operations. The
apparatus that combines the readings of the external and
implanted sensors, otherwise referred to as the "coordinating
programmer", or simply the "programmer", may include multiple
communication channels established to communicate with both the
external and implanted sensors.
A form of the invention that does not necessarily
require historical data would have the implanted device
communicate directly with the external reference sensor device
to coordinate the readings without the use of a "programmer"
computer. In such a configuration direct communications would
need to be established between the internal and external
devices, and the external device would be best configured in a
wearable housing.
In summary, the invention is a system for determining
the absolute value of an implanted medical device sensor
measurement where said implanted sensor measurement values are
subject to significant biasing by external forces in the
patient's environment, said system comprising: a) an implanted
sensor and measurement device having a memory for storing sensor
data, a clock, a sensor for generating measurement values and a
processor, so arranged and disposed that the memory holds
CA 02211844 2000-10-25
66742-623
3b
representations of sensor measurement values as coordinated by
the processor, said processor coordinating the enabling of the
sensor to generate and the memory to hold each new measurement
value at appropriate times in accord with a predetermined
processor run and clock driven program, b) an external sensor
and measurement device having a memory for storing sensor data,
a clock, a sensor for generating measurement values and a
processor, so arranged and disposed that the memory holds
representations of sensor measurement values as coordinated by
the processor, said processor coordinating the enabling of the
sensor to generate and the memory to hold each new measurement
value at appropriate times in accord with a predetermined
processor run and clock driven program.
In one aspect there is further provided a coordinating
programmer apparatus for initiating timing of paired programs in
said implanted and external devices by way of two communications
channels establishable between said programmer and said external
device and between said programmer and said internal device.
In another aspect there is further provided a
coordinating program in said implanted device which, by way of
two communications channels establishable between said implanted
and said external device periodically corrects the internal
measurement for said external conditions based on the
measurement data transferred by said external device to said
internal device.
In yet another aspect there is further provided a
coordinating program in said external device which, by way of a
communications channel establishable between said implanted and
said external device periodically corrects the internal
measurement for said external forces based on the measurement
CA 02211844 2000-10-25
66742-623
3c
data transferred by said external device to said internal
device.
According to another aspect the invention provides a
process of producing a chronic histogram of measurement readings
from within a living body comprising: measuring over a long term
at specified intervals within said long term, an internally
sensed parameter with an implanted sensor device that measures
said sensed parameter in absolute terms and recording said
measurements with reference to the time they are taken so as to
produce a measurement value for each interval, at a time
contemporaneous with said long term, and at intervals
approximately contemporaneous with said specified intervals,
measuring an external and ambient parameter near the living body
containing said implanted sensor device and recording said
ambient parameter measurements with reference to the time they
are taken so as to produce a measurement value for each
interval, coordinating the measurements for each approximately
specified interval by subtracting the value of the ambient
measurement for a given one of the specified intervals from the
absolute measurement value from said implanted sensor for the
same specified interval, the result of such subtraction being an
approximation of the internal measurement for each said
specified interval.
CA 02211844 1997-07-28
WO 96/26673 4 PCT/US96/00670
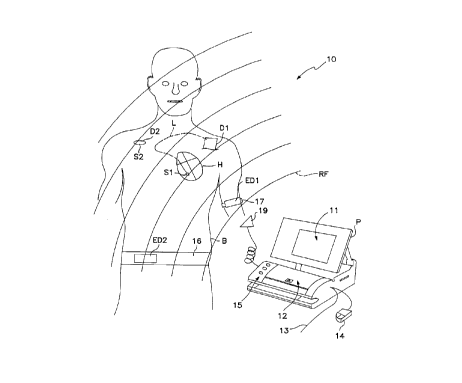
Fig. 1 is an illustration of a human body having implanted and wearable
devices
in association with a programmer.
Fig. 2 is heuristic block diagram of preferred embodiment device
communications configurations.
Fig. 3 is a perspective diagram with a cutaway showing parts and configuration
for an external device in accord with a preferred embodiment of this
invention.
Fig. 4 is a representational diagram of an implantable device as used in
accord
with a preferred embodiment of this invention.
Fig. 5 is a flow diagram for use in describing the coordination of event times
as
in a preferred embodiment of this invention.
Fig. 6 is a paired chronic time diagram for use in describing the function of
a
preferred embodiment of this invention.
Fig. 7 is a simplified block/circuit diagram in accord with a preferred
embodiment of the invention.
For purposes of description, refer first to Fig. 1 in which preferred
embodiments of the system 10 is described in detail as follows.
The human body B may have various implanted devices having sensors which
react or are affected by the ambient environment in which the patient's body B
is
located. For purposes of illustration, two such devices D1 and D2 are shown.
Device
D1 illustrates an implantable device which could be, for example, a cardiac
stimulator
or other device, having at least one lead connected to a sensor S1 which is
implanted
within the heart H. In the presently most preferred embodiment, this sensor SI
would
be a ventricular pressure sensor which can be quite useful for diagnostics and
for
determining long term patient cardiac care.
An alternative device D2 (which at the present time would not be assumed to be
in the same body B as device D1), has associated with it a sensor S2. External
devices
such as ED1 and ED2 are also illustrated here connected to belts 17 and 16,
respectively. It is left to the imagination of the reader of ordinary skill in
this art as to
where other implantable devices of the kind DX and external devices of type
EDX may
CA 02211844 1997-07-28
WO 96!26673 5 pCTlFIS96100670
be located. Such placement configuration is only limited for this invention by
the
claims as set forth at the end of this specification.
Also in system 10 is the programmer device P. This device is primarily a
general purpose computer system with an RF transmitterlreceiver that can
communicate
with the implanted devices DX and the external devices EDX. (Wave RF shown
here
emanating from the programmer P.) In some presently preferred embodiments, a
communications pad such as pad 19 may be connected directly to the programmer
P to
be located in close association wrath the implanted device so as to minimize
the
interference associated with telemetry between the programmer P and the
implanted
device DX. Also, a separate communications port or channel can connect the
external
device to the programmer if desired, rather than using an RF connection.
As is known, various methods for programming and modifying a program by a
user through the external programmer P have developed including a light pen
13,
mouse input device 14, keyboard (under panel 12), function keys 15 and touch
sensitive
screen 11.
Referring now to Fig. 2, the communications system for system l0a is described
in a heuristic block diagram. The components of such a system include a
programmer
Px(20), a implanted device Dx(21) and a external device EDx(22).
Communications
pathways exist between the programmer 20 and implanted device 21 as
illustrated by
broken line B; between the programmer and the external device 22 as
illustrated by
broken line A; and the external device and internal device as illustrated by
broken line
C. Not all of these communication pathways are required at all times. In
accord with
the presently preferred embodiment of this invention using an absolute
pressure sensor
as the implanted device sensor, no communication pathway exists between the
implanted device 21 and the external sensor device 22. Therefore the diagram
would
not include broken line C for such a configuration.
Because the presently preferred embodiment does not include a communications
pathway C, the coordination of the timing of events between the recording of
pressure
sensing in device 21 and device 22 is accomplished in the programmer 20 by
means of
an initialization and uplink procedure described in detail later with
reference to Fig. 5.
The preferred communications pathway A may be a direct hard wired link between
the
CA 02211844 1997-07-28
WO 96/26673 6 PCT/US96/00670
programmer and the external device, say for example using a RS 232 configured
cable.
In almost all situations, it will be difficult not to use RF as the
communications
pathway between the implanted device 21 and the external devices 20 or 22.
However,
it is not outside the scope of this invention to employ communications devices
such as
those described by Funke in U.S. Patent No. 4,987,897, describing a body buss
in
which the entire human body is used as a communications pathway for electrical
signals
between devices within and external to but associated electrically with the
body of the
patient. Other communications pathways including optical based means may be
established as needed or desired by the designer.
Referring now to Fig. 3, an external device EDx is illustrated having a
housing
30 having internal thereto a circuit board 31, a battery 33, and a cable for
connector 32
connecting the circuit board to the programmer type device (like P of Fig. 1)
when
plugged in. In the presently preferred embodiment, this connector is an RS 232
cable.
The circuit board has associated with it certain main components including a
barometric
pressure sensor 34, and Analog to Digital (A/D) convertor 35, a memory 36, a
processor 37 and a clock 38. These components are interconnected electrically
via the
circuit board 31 to provide signal pathways to perform the functions described
herein.
The presently most preferred sensor for use as a sensor 34 is available from a
Luca Nova Sensor, Fremont, California and it is a silicon-based diaphragm
pressure
sensor with a sensitivity of t .OSmm Hg. A pressure sensor of similar
sensitivity
should be mounted in the implanted device to generate easily convertible
readings but
any reasonable sensor pair may be used with appropriate correction algorithms
as can
be easily surmised by one of ordinary skill in this field.
Memory requirements for memory 36 depend upon the length of 'time and the
size of the data word to be stored relative to the measurements being taken
and, also
depends upon the number of measurements that will be kept. These
determinations
may be made on an individual basis depending on the needs of the patient. For
"
example, if a six month period of monitoring is to be undertaken before the
calibration
and reconciliation of the two sensors is to be accomplished, and measurements
are
taken and stored once every hour the memory size minimum would be 24 hours x
31
days x 6 months x number of bits per measurement datum. Compression
techniques,
CA 02211844 1997-07-28
WO 96!26673 7 PCTliTS96/00670
averaging and other algorithms may be used as well to reduce the amount of
storage
required. Generally less than 200k bytes is needed for most applications of
pressure
sensing.
In general, the battery 33 powers the components on the circuit board
including
at least those mentioned previously. The clock 38 provides a reference point
so that the
sensor 34 data converted by the A/D converter 35 can be stored in memory in a
way
that is associated (in a time related way) with the way memory is used to
record sensor
measurements by the implanted device. An on-board processor 37 can be employed
to
execute various algorithms for manipulating or encoding the data as desired by
the user.
A connector 32 allows for the transfer of data from the memory to an off the
board
programmer or other computer although RF or other connections (not shown) can
be
made instead for this purpose.
Referring now to Fig. 4, in which an implantable device is illustrated having
associated lead L and sensor S for connection in this case to the ventricular
area of the
right ventricle of a patient's heart. The body 81 of the implantable device 80
contains
an A/D converter 85 connected to the lead L for converting analog signals from
sensor
S for processing by processor 87 and for storage in the memory 86 and
associated time
related coordination data facilitated and enabled by the timing signals from a
clock 88.
Figure 5 is a flowchart 60 having three primary components, one for the
programmer designated P (51), one for the implantable device labelled ID (52)
and a
third for the external device, ED (53).
In general, the programmer can cause the internal and external sensing devices
to be initialized at the same time by initialization step 54 which requires
communication
links to the internal device (42) and the external device (41). Initialization
may take
many forms as would be known to one of ordinary skill in this art. The
presently
preferred initialization method uses the on-board clock systems of each of the
three
devices to coordinate a real time, related time and date indicator. Thus, at a
specific
time and date both the implantable device and the external device begin
recording their
respective pressure measurements. The programmer (51) is then disconnected
from the
external sensing device (57) while the initialized recording sessions of the
implantable
CA 02211844 1997-07-28
WO 96126673 8 PCT/US96/00670
device (52) and the external device (53) to run their course. The progranuner
may be
used with additional systems or perform other tasks once disconnected.
In blocks 58 and 59, the internal and external devices start at the same time
labeled here as to.
While the easiest implementation would be to simply have both implantable and
external device processors record into memory the same type of measurement
data at
the same time, there are numerous reasons for which users of these devices
would
prefer to have complementary rather than identical strategies for recording
data.
For example, in block 62 it may be advantageous to record both the maximum
and minimum ventricular pressure as well as the 75 % , 50 % and 25 % levels of
pressure
during a single heart beat. For the same time period perhaps only one pressure
reading
need be recorded during the execution of block 63 by the external device. This
time-
paired set of readings extending through a test period would generate a most
useful
histogram for determining exactly what is occurring vis-a-vis the ventricular
pressure
for this patient. When the period of time for which recording is required has
ended,
the internal and external devices preferably go into wait states 65 and 66
during which
time they await a signal from a device such the programmer S 1 indicating that
it is
ready to receive data (i.e. in block 61) through communications channels 43
and 44.
At such time, data is output to the programmer 51, i.e. through blocks 67 and
68.
Within the programmer 51, the data is compared in steps 64 and appropriate
adjustments made so that a report can be generated in step 69. This may be
simply a
visual display on the screen of the programmer or a set of data that may be
interpreted
by another computerized machine downstream or a printed report, depending upon
the
desires of the doctor, his patient or the laboratory doing the work.
Figure 6 describes in a diagrammatic form the collection of data by the
internal
and external sensor devices. In the graph 40 the external device recording
line is
identified as EDx and the internal device recording line as Dx. At the time of
the
beginning of this graph, identified by line 72, and for the interval 45, the
doctor sets
the patient up with the external and internal devices via the programmer so
that
initialization occurs such as described with reference to Fig. 5. At time to
both devices
record for the first time those measurements each device has been programmed
to
CA 02211844 1997-07-28
WO 96!26673 9 PCT/U596/00670
make. Each tick mark (identified as 47, and 48, respectively) along lines 70
and 71
indicate another measurement. The normal spacing for an internal 46 may be
adjusted
by the doctor or researcher according to his desire for data.
Depending on a given clinical use of the implanted monitor, (acute, medium
term, long term) the storage interval in the monitor could be (but is not
limited) 2
seconds to an hour.
The storage (or recording) interval in the external pressure recorder would
preferably be a constant value dependent on expected rate of changes in
ambient
pressure, for example, a constant storage rate of one time per minute.
Since the storage rates in the two devices are in the present embodiment not
the
same, it is necessary for the programmer or report generator to correctly
merge the two
data sets.
One solution is as follows:
If the storage interval in the implanted monitor is less the than the
storage interval in the external device, the single value closest in time from
the
external device is correlated with a number of samples from the implanted
device.
If the storage internal in the implanted monitor is greater than that in the
external device the programmer or report generator will average a range of
samples from the external device closest in time to the data from the
implanted
device to correlate with the data from the implanted device.
Also, under some circumstances, the two data sets may not start or end at
exactly at the same time. In this situation, the programmer or report
generator must
only process those records where the data sets overlap temporally.
An example of such a situation follows:
The implanted monitor is set to record for four weeks. The external
pressure recorder is started at the same time but the patient does not come to
the
clinic for six weeks. The data in the implanted device may have "wrapped"
such that only the last four weeks worth of data is available. (That is, the
capacity of on-board implanted memory is fully stuffed in four weeks and the
data which follows writes over the earliest data - a kind of wraparound
storage.)
CA 02211844 1997-07-28
WO 96/26673 1~ PC7C/US96/00670
In this case the first two weeks of data in the external device must be
skipped.
If, on the other hand, implanted memory is "frozen" at the end of the first
four
weeks, the last two weeks of external data would not even be collected
internally and so the last two weeks of external data should be discarded.
Thus, an extremely flexible system has been described. It allows for
programming at the initiation of recording or prior thereto; of identical or
different
strategies that are complementary as between internal and external devices.
The
internal and external devices each have sensors which record and report their
measurements. At the end of the recording period the sensor device memory can
be
discharged into a programmer that will interpret the internal sensor output in
light of
the external reference sensor readings.
Referring now to Fig. 7 in which a circuit block diagram for the preferred
embodiment external reference device is shown as Circuit 100, it should be
noted that
the circuit includes pressure sensor 34, temperature sensor 81, analog to
digital
coverter 35, microprocessor 37, battery 33, and battery-power stealing and
battery
circuit 93, a clock 38, and an RS 232 controller block 83. They function as
follows:
Power can be supplied and generally is by the battery 33. But in low power
conditions, a power stealing circuit is employed ( in block 93) which allows
power to
be taken from the RS232 line that carries the RTS (ready to send) and DTR
(data
terminal ready) signals. These RTS and DTR signals are set to one or high--
meaning
there is a voltage in the standard RS232 protocol when the bus is in use. The
off
device part of the bus is indicated by reference numeral 32b and the on-device
part of
the bus by 32a. An RS232 controller 83 provides for data transfers between the
RS232
bus and the microprocessor 37 across line 89a, in preferred embodiment as a
serial bit
stream of data and protocol.
In the preferred embodiment, an on-board clock 38 is distributed through the
microprocessor to the other chips on the circuit board to coordinate timing.
The power stealing or battery power circuit 93 provides power to the power
controller 95 which the microprocessor 37 can control via line 86 to save
power by
shutting of memory when not required.
CA 02211844 1997-07-28
WO 96/26673 11 PCT/US96/00670
Shown in this diagram also is OR gate 96 attached to the "analog-to-digital"
(also called A/D, A-D or A to D) converter 35. An OR gate per se is certainly
not
required and this illustration is used merely to indicate that the A to D
converter 35 of
the preferred embodiment accepts one of the three illustrated inputs at a
time. Line
101, is one of these inputs and is connected through block 93 to the battery
33 to
provide a battery level for the A-D converter 35 to test the battery when
microprocessor 37 indicates across line 88 that such is required. The I/O line
88 is also
used to communicate the data from the A/D converter 35.
The pressure sensor 34 typically produces a pair of signals, the difference of
which indicates the barometric pressure reading before the difference of these
signals
are generally attracted before an amplifier 82 before being provided as input
2 in an A
to D converter such as AID 35. This is typically called an "instrumentation
amp" .
In the preferred embodiment a circuit is used including a FET switch to switch
from the temperature sensor output from temperature sense circuit 81 to the
battery
output 101 and vice versa. Nevertheless, there are many forms of multiplexing
which
could be employed to provide for three inputs to an A-D conversion and all
should be
covered within the scope of this invention when used in accord with the
teachings
hereof.
In general then, the A-D converter will typically read and record data from
the
selected A-D input. Then the data will be read out into the microprocessor and
processed into memory as described with reference to 5 and 6. If the circuit
is
constructed as in accord with the most preferred embodiment herein described,
the
reading of the temperature sense output can be recorded then the battery check
input be
read and recorded and the initial reading be subtracted out to derive the
offset indicative
of the battery voltage level at that time.
If it is desired to have all processing done in either the external or
implanted
sensor device, a direct communication channel could be established between
them and
adjustments can be made to the medically relevant output of such an implanted
device
responsive to such data. For such an embodiment a separate programmer would
not be
required and coordination of data between the devices would be direct.
However, the
CA 02211844 1997-07-28
WO 96/26673 12 PC7C/US96/00670
presently most preferred embodiment assumes no communications between the
implanted and the external sensor devices.
Alternative embodiments such as the use of an external pulse oximeter and
internal oxygen measuring device or of an implanted temperature sensor and an
external
one could be employed by one of ordinary skill in the art without undue
experimentation from the disclosure above.
Anomalous changes due to extreme varieties (such as a change within 1 minute
of 15 mmHg) monitored by one of the sensors can be ignored, assuming
appropriate
safeguards. So, for example, a power surge that give a false reading may be
left out of
the data set subject to programmer interpretation.
Venous pressure by a pressure sensor associated with an implantable device
could be located in the vein or other location of interest as an obvious
adaption of this
invention. Accordingly, this invention should not be limited except as set
forth in the
following claims.