Note: Descriptions are shown in the official language in which they were submitted.
~
~ CA 02238341 1998-OS-21
1
PROCESS AND EQUIPMENT FOR TREATMENT OF
4~TASTE PLASTICS
FIELD OF THE INVENTION
This invention relates to a process and equipment
for treatment of waste plastics, particularly for those
waste plastics such as polypropylene (PP), polyethylene
(PE), and polystyrene (PS) without any cleaning and
pretreatment operation.
BACKGROUND OF THE INVENTION
With the rapid development of plastic industry,
plastic articles are getting increasingly important in
industrial production and involving in every field of our
daily life. More and more waste plastics come out with
the abundant applications of plastics. Because the waste
plastics are almost non-decompositionable in natural
condition, it becomes a serious problem to our survival
environment. So it becomes very important to solve the
pollution problem in our environment caused by the waste
plastics, and to get them recycled and utilized.
So far, various methods to treat waste plastics have
been proposed. Generally, catalytic and thermal cracking
may be carried out under the action of catalyst. A
method of treating waste plastics in U.S. Patent
4,851,601 includes disintegration of waste plastics, and
thermal cracking of the disintegrated waste plastics in a
vessel. Then the gas product of the thermal cracking is
further cracked with catalysts, such as ZSM-5 with
medium-sized pore diameter. Finally, the resulting
products are separated by a conventional method. A
~
~ CA 02238341 1998-OS-21
' 2
method of rapidly converting waste plastics into a high
quality oil is disclosed in JP-A-5-345894, which includes
thermal cracking of waste plastics at 200-.700°C, and then
catalytic cracking at 230650°C with catalysts.
A method of treating waste plastic films is also
disclosed in JP-A-62-OI5240, which includes thermal
cracking at a high temperature, condensing the gas
products in a primary condenser, separating the gas and
liquid phase, and liquefying the gas to obtain light and
heavy oil in a secondary condensing stripping column.
There are some existing defects in the above
mentioned technologies:
1. Pretreatment operation is needed for starting or
raw materials. Thus, extra time, labor and energy are
needed. It will cause operation difficulty, especially
in winter time.
2. The process of thermal cracking and catalytic
cracking are proceeded separately at repeated high
temperature. Hence, the energy consumption will be large
due to the very high temperature needed in the process.
3. The catalysts have low efficiency at the low
temperature. Serious carbonization of raw material will
occur at the high temperature, and the oil recovery is
low due to the high loss of dry gas.
4. The product obtained has a low stability against
oxidation, being easy to be oxidized to form gummy
material, and cannot be stored for a long time.
5. The capacity of such processing units are too
small to get an economic efficiency.
CA 02238341 1998-OS-21
3
This invention is aimed at the treatment of waste
plastics that will overcome the defects of the existing
technologies. It is simple in process, stable in
operation, satisfactory in catalyst quality, easy in
maintenance and has a long operational cycle.
It is also an objective of the present invention to
provide a whole set of equipment to realize the process
of this invention.
SUMMARY OF THE INVENTION
Accordingly to this invention, the process for
treating waste plastics includes the steps of:
(1) adding directly waste plastics into a reactor
together with a proportional amount of a catalyst;
(2) heating the waste plastics added in the reactor
at a temperature of about 280°C to about 480°C to generate
a liquid phase and a gas effluent;
(3) condensing the gas effluent in a condenser to
obtain a condensate, and sending non-condensable gas of
the gas effluent to a heating furnace for burning as
fuel ;
(4) transmitting the condensate from the condenser
through an oil-water separator to obtain an oil phase
product, said oil phase product being brought into a
mixing tank, and adding 3-8%(Wt.) of the catalyst
mentioned in step (1) in the mixing tank under the room
temperature, to improve the stability of the oil phase
product against oxidation;
(5) refining the oil phase product obtained from
step (4) to produce gasoline, diesel oil, and other
hydrocarbon fractions.
' ' CA 02238341 1998-OS-21
4
The related equipment of this invention for
treatment of waste plastics includes an automatic
hydraulic solid feeder, a reaction vessel or rector, a
settler, a series of condenser, a vacuum discharge device
for discharging solid residue, a rectification tower, a
tower reboiler, a mixing tank, a final product tank.
BRIEF DESCRIPTION OF THE DRAWING
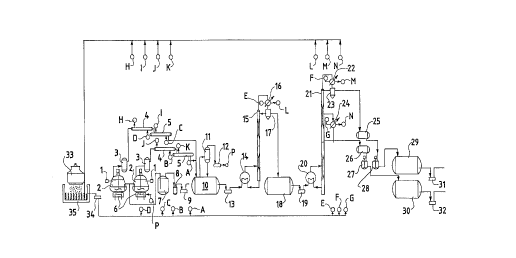
Figure 1 is the schematic diagram for implementation
of the equipment of this invention.
With reference to the Figure, this invention is
explained in detail.
A method of treating waste hydrocarbon plastics of
this invention comprises the steps of:
(1) adding directly waste plastics into a reaction
vessel or rector together with a proportional amount of a
catalyst at the same time;
(2) heating the added waste plastics at a
temperature of about 280°C to about 480°C to generate a
liquid phase product and a gas effluent;
(3) condensing the gas effluent generated from the
reactor, after setting off solid impurities therefrom, in
a condenser to obtain a condensate, and sending non-
condensable gas for burning in a heating furnace;
(4) separating the condensate from water in a
settler by vaporization and transmitting the separated
condensate to a rectification tower, in which light
fractions are obtained from the top of the rectification
tower, and the heavy fractions are from the middle part
of the rectification tower;
' ' CA 02238341 1998-OS-21
' 5
(5) transmitting the light and heavy fractions
obtained from step (4) into a mixing tank separately, and
under the room temperature adding the catalyst mentioned
in step (1) in an amount of 3 to 8% by weight of the two
fractions fed, in order to improve the stability of a
mixed product against oxidation; and
(6) refining the mixed product from step (5) to
obtain final products.
According to this invention, the catalyst used
comprises a silica carrier and a mixture of active
components having the following formula:
AaBl,AI~MdNaeCa fFegOX
Where A is selected from the group consisting of
potassium, barium, phosphorus, vanadium, chromium and
rare earth elements and their mixture, and B is selected
from the group consisting of molybdenum, nickel,
germanium, platinum and their mixture, and M is tungsten,
wherein a is from 25.00 to 26.35%; b is from 36_00 to
37.05%; c is from 7.20 to 9.00%; d is from 1.14 to 1.55%;
a is from 1.75 to 2.15%; f is from 2.40 to 2.80%; g is
from 2.42 to 3.20%; and x is the sigma weight of oxygen
atom needed to the chemical bonding valences of the
various components in the catalyst, which is based on the
total weight of the catalyst; and wherein the content of
silica carrier in the catalyst is from 20 to 35% by
weight.
According to this invention, the waste plastics used
as raw or starting materials include waste PP, PE and PS,
except waste PVC (polyvinyl chloride).
According to this invention, the feeding equipment
can be a hydraulic piston type, and can be manually or
CA 02238341 1999-12-22
i
6
automatically controlled according to various
requirements. The heating device can use any fuels, such
as; coal, electricity or oil according to different
conditions.
In this invention, the temperature for the liquid
phase in the reaction vessel should be controlled from
about 280 to about 480C. The gas effluent generated from
catalytic cracking are a mixture of hydrocarbons Cl~Czo
during the complete period of gas production. The
temperature of the gas phase varies with the feeding
process continuously from about 90C to about 300C. The
best quality of the product is obtained when the
temperature is 195C 30C, and the largest flow rate of
the product per unit time is obtained when the
temperature is 230C 20C. The optimal temperature is
controlled at 195C 30C.
In this invention, the condensate is a mixture of
liquid hydrocarbons CS-.Czo, with the distribution of
alkane accounting 30 to 38%, alkene 45 to 48%, cyclane 10
to'15%, and aromatic hydrocarbon 15 to 23%. The
condensation is proceeded in the condenser series,
including a primary condenser and a secondary condenser.
The gas effluent from the reactor enters into the
settler. Most of solid impurities carried by the gas
phase settle on the bottom of the settler under the
influence of 40# Intalox stainless steel packing. Then,
the clean gas enters into the tube side of the primary
condenser, with its temperature ranging from atmospheric
temperature to 300°C. Then the gas and liquid mixture
enters into the shell side of the secondary condenser
* Trademark
CA 02238341 1999-12-22
i
~ 7
that ensures enough. heat exchanging areas to condense the
mixture of hydrocarbon vapors.
According to this invention, the non-condensable
components C1-.C4 in the gas mixture that is generated in
the reactor will be collected in the tail gas collector_
The collected gas will be sent to the furnace of the
reactor by a Nash-Hytor pump to prevent back-fire and
eliminate the pollution problem. The catalyst is
proportionally fed into the reactor with the successive
addition of raw material, and undertaken the catalytic
cracking reaction, including decomposition, isomerization
and hydrogen-transfer reactions . * Trademark
The treatment in the mixing tank is to make the
unstable fraction of the condensate, which is mainly
unstable alkene such as dime, to undertake
isomerization, aromatization and hydrogen transfer
reactions, thereby converting it into a product which is
stable against oxidation.
In the mixing tank, new catalyst should be used, and
operated at an atmospheric temperature. ,The amount of
the new catalyst is from 3 to 8~ of the weight'of the
condensate mentioned above. The operation cycle of
catalyst is from 10 to 25 with the optimal value of 15.
The catalyst used in the reactor may be those
discharged from the mixing tank, while its total amount
will be correspondingly increased.
According to.this invention, the refinement may be
proceeded in the rectification tower. For example, the
to'nier can be equivalent to 8 theoretical plates, with a
reFlux ratio of 4, using a structured tower packing (such
as~~a protruded corrugated packing). Thus, gasoline and
CA 02238341 1999-12-22
,,
.' 8
diesel oil, which are fuel oils, can be produced
respectively. Moreover, a,further refining separation
from the condensate can be proceeded to acquire more
valuable components of hydrocarbons, such as olefins and
aromatic hydrocarbons.
When the condensate is refined to get gasolir~e and
diesel oils, the rectification tower should be equivalent
to16 theoretical plates, with a reflux ratio of 5, using
25mm Intalox stainless steel tower packing.
When the condensate is refined to get gasoline and
diesel oil in the fractionating tower, there should be
separated stream lines for discharge, reflux, storage and
treatment, rather than the batchwise method to obtain
both fractions.
II When the condensate is refined to get gasoline and
diesel oil, the initial distilling temperature for
gasoline is 31°C with the ending point of 200°C. Its
amount approximately constitutes 52 to 58% of the total
amount of the hydrocarbon mixture. Its octane number is
78(MONj, or 86 to 88(RON). The quality meets with the
National Standard for 70# gasoline (MON 70#). The
distillation temperature range of the diesel oil is 200-
360°C, and its amount constitutes 42 to 48%. Its quality
meets with the National Standard for minus 15# diesel
oil . * Trademarks
The obtained gasoline and diesel oil are reserved in
a head tank separately, then sent respectively into their
mixing tanks to be further treated to improve their
stability. The treated product will be of high
stability.
CA 02238341 1998-OS-21
9
The equipment of this inventio-n is shown in the
Figure, and explained in detail below.
As shown in the Figure, the equipment includes a
reaction vessel or rector 2, which has a cone-shaped
body. A feeding nozzle is provided at the upper part of
the vessel 2, while a solid residue discharging nozzle is
at its lower part. The feeding nozzle is connected with
an automatic hydraulic feeder 1 to perform continuous
feeding. The solid residue discharging nozzle is
connected with a vacuum discharging system 7, 8, 9, so
that residues after reaction can be removed
automatically. When the reaction vessel is heated, the
raw or starting material will be transformed from a solid
to a liquid state with the increasing temperature.
Further, when the liquid is being converted into a gas
phase under the action of the catalyst, the gas generated
will be condensed into a mixture of liquid hydrocarbons
through the condenser series 4 and 5, before which the
dust impurities carried by the gas should be pre-
separated in the settler 3. The mixture of liquid
hydrocarbons thus condensed is treated to remove water
and other solid impurities in the tank 10. Then, the
treated mixture is sent by an oil pump 13 to the heater
14 to be vaporized. The vapor enters the stripping tower
15 and be condensed in the condenser 16. A mixture of
liquid hydrocarbons will be obtained, wherein the water
will be removed through the separator 17 and the mixture
is thus stored in the tank 18. The mixture of liquid
hydrocarbons in the tank 18 enters the heater 20 through
the oil pump 19, and goes into the rectification tower
21. The mixture is separated into gasoline and diesel
CA 02238341 1998-OS-21
oil fractions according to the different temperatures
needed. The gasoline vapor enters the condenser 22 from
the top of the tower and condensed into a liquid phase.
The water layer is removed in the separator 23. The oil
5 layer forms the reflux flow and gasoline distillate,
which enters into the head tank 25, then into the mixing
tank 28 where it is treated with the catalyst as
mentioned above. The treated gasoline is stored in the
final storage tank 29. The final gasoline product is
10 output through the oil pump 31. The diesel oil fraction
enters into the condenser 24 from the middle part of the
rectification tower 21, and then through the head tank
26, enters into the mixing tank 27, where the diesel oil
is treated with catalyst as mentioned above. The treated
oil is sent to the storage tank 30. The final diesel oil
product is output through the oil pump 32. Non-
condensable components Cl~C4, generated from the catalytic
cracking in the reactor 2 is collected in the tail gas
collector 11 and the water is scrubbed with the glass
packing layer in it. Through the Nash-Hytor pump 12, the
non-condensable components are and sent into the heating
furnace 6 to be burnt. Cooling medium in this process is
circulating water, which is circulated through the water
cooling tower 33, and water pool 35. It is then sent by
the water pump 34 to all heat exchangers, where A-G are
the cooling water supplying lines, H-N the recycling
lines.
This invention predominates in this field now,
compared with similar process developed domestically or
abroad. It has the advantage of the stable operation,
' CA 02238341 1998-OS-21
11
simple technology, excellent performance of the catalyst
and long operation period without breakdown.
This process and related equipment are based on the
intrinsic property of raw materials. It can treat
various waste plastics except PVC and other plastics
containing chlorine. This process can eliminate
environmental pollution caused by the waste plastics, and
obtain useful fuel oil products or other hydrocarbon
fractions. Hence it is a feasible and satisfactory
technology to eliminate "white pollution" problem.
This invention has the unique characteristics as
follows:
1. The catalyst used in this invention has wide
suitability and catalytic activity, at a relatively low
temperature for different raw materials.
2. In this process, the product obtained from the
catalytic cracking of waste plastics has a satisfactory
distribution of hydrocarbons of gasoline, diesel oil and
other fractions. The content of olefins is relatively
high, so that it is regarded to have high unstability
theoretically and easy to form gummy substances, but in
fact because of the excellent performance of the catalyst
used in this invention, it restrains the content of the
unstable unsaturated hydrocarbons in the treated product,
and upgrade the stability against oxidation of final
products.
3. In this invention, a uniquely designed mixing
tank is used to give further particular treatment of
product with an excellent catalyst, which improves the
stability of the product and makes the product to be
stored easily and stably.
' ' CA 02238341 1998-OS-21
12
Further explanation to this invention are presented
by examples as follows:
PREFERRED EMBODIMENT OF THE PRESENT INVENTION
Example 1:
100 Kg of catalyst contains 22% silica by weight as
a carrier. The remaining part includes active components
with the following formula:
AaB~,Al ~MdNaeCa fFegOX
Where A represents potassium and barium, B represents
molybdenum, M is tungsten. a is 26.25% (Wt.); b is
37.05%; c is 7.20%; d is 1.14%; a is 1.75%; f is 2.40%; g
is 2.42%; x is the sigma weight of oxygen atom needed to
the chemical bonding valences of various components in
the catalyst. The above catalyst and 5000 Kg uncleaned
waste agricultural plastic films are added into a 5 liter
reactor continuously. The mixture is heated gradually.
The temperature is controlled between 337 and 389°C,
causing the catalytic cracking reaction. The temperature
of vapor in the upper part of the reactor is controlled
between 210 and 267°C. The temperature of vapor entering
the condenser is controlled from 91 to 124°C. The solid
impurities carried by the vapors generated from the
reactor are removed in the settler, obtaining liquid and
non-condensable gas products with a yield of 84.3%.
After the water in condensate is removed by the
separator, the condensate is vaporized and goes to the
rectification tower, to get gasoline fractions from the
top of the tower and diesel oil fractions from the middle
of the tower. The gasoline and diesel oil are
CA 02238341 1998-OS-21
13
respectively transferred into their mixing tanks and
treated at an atmospheric temperature, with added
catalyst of an amount of more than 3~ by weight to the
gasoline and diesel oils, to improve the stability of the
product. After a complete cycle of reaction, there is no
carbonization remained in the reactor, and the impurities
carried by the raw material and catalyst can be drained
out by a vacuum suction from the residue discharging
nozzle. Results are presented in table 1 below.
Example 2:
The process of treatment for waste plastic films is
the same as mentioned in example l, but with different
reaction conditions shown in table 1. See table 1 for
the results.
Example 3:
The process of treatment for waste plastic films is
the same as mentioned in example l, but with different
reaction conditions shown in table 1. See table 1 for
the results.
Table 1
Example 1 2 3
Amount of raw material fed (Ton) 5 5 5
Amount of catalyst (~) 2 1.5 1
Reaction time (hr) 11 8 9
Temperature of liquid phase (C) 337-389 341-384 315-376
Temperature of vapor phase (C) 91-124 89-110 83-131
Temperature of top (C) 210-267 231-273 209-275
Yield (~) 84.3 84.6 82.8
These examples mentioned above are just some non-
restrictive description for this invention. For skillful
technical persons in this field, various modifications
and variation to this invention can be made under proper
conditions.
' ' CA 02238341 1998-OS-21
14
INDUSTRIAL APPLICATION
The method and equipment for treating waste plastics
of the present invention provides for stable operation,
simple processing, good performance of catalyst, less
trouble during operation, and is of strong applicability
in the industry.