Note: Descriptions are shown in the official language in which they were submitted.
CA 02251041 1998-10-07
WO 97/37607 1 PCT/US97/05714
LINEAR ABLATION DEVICE AND ASSEMBLY
BACKGROUND OF THE INVENTION
This invention generally relates to the detection and elimination
of cardiac arrhythmia and particularly atria) fibrillation.
Atria) fibrillation is the disorganized depolarization of a patient's
atrium with little or no effective atria) contraction. This condition may be
chronic or intermittent, and It affects.up to 2 million or more people in
the United States alone. For atria) fibrillation refractory to conventional
drug therapy, it has been conventional practice to make incisions in the
atria) wall, to surgically segregate the tissue thereof, to discontinue the
atria) fibrillation. The atria) segments formed by the surgical
segregation are electrically isolated and too small to allow the
fibrillation to continue. However, the surgical technique is quite
traumatic and is unacceptable to a large fraction of those patient's
experiencing atria) fibrillation or flutter.
Avital in PCT 95/15115 discloses the use of high frequency
electrical energy with a specific intravascular electrophysiological (EP)
device to form linear ablations within a patient's atria) chamber to
provide results similar to the surgical segregation techniques in
terminating atria) fibrillation but with significantly reduced trauma.
However, the Avita! device cannot always be readily placed a
desired location within the patient's atria) chamber and it does not
CA 02251041 1998-10-07
WO 97/37607 2 PCTJITS97/05714
always provide the necessary contact between the ablating electrodes
on the device and atria) tissue at the desired location to generate linear
lesions of a requisite length when RF electrical energy is emitted from
the electrodes.
What has been needed is an ablation device and an assembly
of such a device with a delivery system which can be readily
manipulated within a patient's atria) chamber to generate effective
linear lesions at any desired location within the atria) chamber. The
present invention satisfies these and other needs.
SUMMARY OF THE INVENTION
This invention is directed to an elongated EP device and to an
assembly therewith including a delivery system for the EP device,
which is suitable for forming effective linear ablations within a chamber
of a patient's heart. The lesions from such linear ablations are
particularly suitable for eliminating or minimizing atria) fibrillation and
flutter by isolating sections of the patient's atria) wall.
In a broad sense the assembly of the invention comprises a
delivery system which includes a delivery sheath with an inner lumen
extending therein, an elongated open distal section, an elongated
support member coextensive at least in part with the elongated open
distal section and an elongated E~~ device disposed within the inner
CA 02251041 1998-10-07
WO 97/37607 3 PCT/US97/05714
lumen of the delivery sheath and fixed by its distal end within the distal
portion of the delivery sheath. The EP device of the assembly has a
plurality of electrodes on the distal section thereof which may be used
for both sensing or ablating. The outer maximum dimension of the
distal section of the EP device, particularly the outer maximum
dimensions of the electrodes, are generally less than about 1.65 mm (5
Fr.), preferably less than about 1.3 mm (4 Fr.). The electrodes of this
size provide a much more efficient transfer of electrical energy to the
contacting tissue and there is apparently considerably less energy loss
to the surrounding fluid contacting the exposed electrode and therefore
there is much less power required to form an effective lesion.
Moreover, the electrodes of the present invention provide more narrow
and better defined lesions than prior devices while still maintaining
adequate lesion depth to electrically isolate the heart sections in a
desired fashion.
With the distal tip of the EP device secured within the distal tip of
the delivery sheath, longitudinal movement of the EP device slidably
disposed within the inner lumen of the delivery sheath causes the distal
portion of the EP device to arcuately extend out and away from the
open distal section of the delivery sheath. The supporting member in
the distal portion of the delivery sheath provides support to the distal
end of the EP device and ensures that the distal portion of the EP
CA 02251041 1998-10-07
WO 97/37607 4 PCT/L1S97/05714
device effectively engages the inner surface of the patient's heart
chamber along a length thereof so that high frequency ( e.g. RF)
electrical energy emitted from the electrodes provide effective linear
ablation of heart tissue within the patient's heart chamber. Additionally,
the electrodes may be used for the collection of electrical signals from
the surface of the heart chamber either before the formation of the
lesion is formed, to locate the desired region within the patient's heart
for treatment, or after the lesion is formed to determine the
effectiveness of the lesion in terminating the arrhythmia.
Effective detection of electrical activity is desirable in order to
accurately locate the arrythmogenic site so that the linear ablation can
be performed for effective lesion formation at a required depth within
the wall to isolate the arrythmogenic site.
In a presently preferred embodiment of the EP device, the distal
portion thereof has an inner lumen extending therein with a core
member disposed within the inner lumen. The wall of the distal section
is formed at least in part of individually insulated electrical conductors
which are electrically connected to individual electrodes on the distal
section. Preferably the electrical conductors are braided. A plurality of
polymer strands formed of nylon, DACRON~) (Dupont) and the like
may also be braided either with the electrical conductors as they are
braided or braided separately on the exterior of the tubular member
CA 02251041 1998-10-07
WO 97/37607 5 PCT/US97/05714
formed by the braided conductors. The proximal ends of the electrical
conductors are electrically connected to electrical connectors which
facilitate transmission of high frequency electrical energy from a source
thereof to individual electrodes (if an extracorporeal electrode is used)
or individual pairs of electrodes. The core member is preferably
provided with one or more jackets, which are electrically insulating if an
electrode is provided on the distal end of the EP device and the core
member is used to transmit electrical current to the electrode on the
distal end. This design allows for a iow profile and flexibility, yet it is
sufficiently strong to ensure effective contact between a length of the
electrode section and the region of the patient's endocardium where
the linear ablation is to occur and an effective formation of an
arrhythmia terminating lesion. The EP device may be used by itself or
with the assembly described above.
In one presently preferred embodiment of the assembly of the
invention, the supporting member of the delivery sheath is a metallic
ribbon which has an elongated flat surface facing the elongated
opening in the distal section of the sheath. It may be made from high
strength materials such as stainless steel, pseudoelastic NiTi alloys in
an austenite phase (unstressed). The support member is preferably
manually shaped into a curved or angled condition to facilitate entry of
the distal extremity of the assembly within the patient's heart chamber,
CA 02251041 1998-10-07
WO 97/37607 6 PCT/US97/05714
particularly the right atrium, and the proper positioning of the extended
distal section of the EP device against the inner surface of the heart
chamber. When the distal end is straightened to be introduced into the
patient, the austenite phase of the support member then may convert
at least in part to a stress-induced martensite phase but upon release
of the stress the martensite phase transforms back to the austenite
phase.
The inner radius of the extended distal portion of the EP device
can be controlled by the length of the elongated opening in the delivery
sheath and the distance the EP device is spaced from the support
member. The effective length of the elongated open distal section can
be controlled by the longitudinal location of the distal end of a second
sheath disposed about the exterior of the first sheath. As the distal end
of the second sheath extends distally, the effective length of the
elongated open distal section is shortened and the radius of curvature
of the distal section of the EP device is correspondingly decreased.
These and other advantages of the invention will become more
apparent from the following detailed description and the accompanying
exemplary drawings.
CA 02251041 1998-10-07
WO 97/37607 7 PCT/US97/05714
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is an elevational view, partially in section, of an assembly
embodying features of the invention.
Fig. 2 is a transverse cross-sectional view of the assembly
shown in Fig. 1 taken along the lines 2-2.
Fig. 3 is a transverse cross-sectional view of the assembly
shown in Fig. 1 taken along the lines 3-3.
Fig. 4 is an efevational view, partially in section, of an EP device
suitable for use with the assembly shown in Figs. 1-3.
Fig. 5 is a transverse cross-sectional view of the EP device
shown in Fig. 4 taken along the lines 5-5.
Fig. 6 is a longitudinal cross-sectional view of an alternative
embodiment similar to that shown in Fig. 1 wherein a lumen is provided
to deliver fluid to the distal extremity of the assembly.
Fig. 7 is a transverse cross-sectional view of the assembly
shown in Fig. 6 taken along the lines 6-6.
Fig. 8 is a longitudinal cross-sectional view of an alternative
embodiment similar to that shown in Fig. 6 with a lumen extending from
the proximal end of the assembly to the distal end of the assembly.
Fig. 9 is a transverse cross-sectional view of the assembly
shown in Fig. 8 taken along the lines 9-9.
CA 02251041 1998-10-07
WO 97/37607 8 PCT/US97/05714
Fig. 10 is a transverse cross-sectional view of the assembly
shown in Fig. 8 taken along the lines 10-10.
Fig. 11 is an elevational view, partially in section, of another
alternative embodiment wherein the delivery sheath is provided with
electrodes for sensing and/or ablation.
Fig. 12 is a transverse cross-sectional view of the embodiment
shown in Fig. 11 taken along the lines 12-12.
Fig. 13 is an elevational view of another embodiment wherein
the EP device of the assembly is provided with an inner lumen for
delivery of fluid.
Fig. 14A is a transverse cross-sectional view of the embodiment
shown in Fig. 13 taken along the lines 14-14.
Fig. 14B is a transverse cross-sectional view of an alternative
embodiment of that shown in Fig. 13 taken along the lines 14-14.
Fig. 15 is an elevational view, partially in section, of a distal
section of an alternative embodiment wherein the EP device is
provided with an inner lumen for passage of fluid coolant.
Fig. 16 is a transverse cross-sectional view taken along the lines
16-16.
Fig. 17 is an elevational view, partially in section, of a distal
section of an alternative embodiment wherein an outer sheath is
disposed about the assembly which is longitudinally movable to control
CA 02251041 1998-10-07
WO 97/37607 9 PCTlUS97/05714
the effective length of the elongated opening in the distal section of the
delivery sheath.
Fig. 18 is an elevational view, partially in section, of an
alternative embodiment wherein a longitudinally movable flush sheath
is provided about the EP device of the assembly to delivery fluid to
desired locations on the distal section thereof.
Fig. 19 is a transverse cross-sectional view of the embodiment
shown in Fig. 18 taken along the fines 19-19.
Fig. 20 is an elevational view, partially in section, of an
alternative EP device designed for use without being secured to a
delivery system.
Fig. 21 is a transverse cross-sectional view of the embodiment
shown in Fig. 20, taken along the lines 21-21.
Fig. 22 is an elevational view, partially in section of an
alternative EP device similar to the EP device shown in Fig. 20 which
has a plurality of thermocouples within the distal section to detect
temperature during the procedure.
Fig. 23 is a transverse cross-sectional view of the embodiment
shown in Fig. 22, taken along the lines 23-23.
CA 02251041 2004-12-16
DETAILED DESCRIPTION OF THE INVENTION
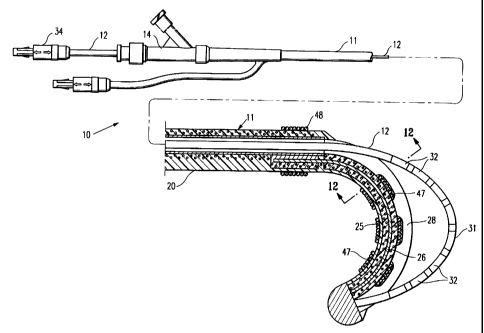
Figs. 1-3 schematically depict a mapping/ablation assembly 10
embodying features of the invention which generally comprises a
delivery sheath 11 and an elongated EP device 12 slidably disposed
5 within the inner lumen 13 of the delivery sheath 11 with the distal end
of the EP device secured within the sheath 11. An adapter 14 is
provided on the proximal end.of the delivery sheath 11 with a
hemostatic valve 15 on the proximal end of the central arm 16 of the
adapter and with a flush port 17 in the proximal end of the side arm 18.
10 The delivery sheath 11 has a proximaa shaft section 20 which is
formed of a braided tubular structure 21 with a polymer impregnate 22
incorporated therein. The braided structure 21 ~ may be formed of high
strength filaments 23 (e.g. 6 x 6 strands) such as stainless steel wire
with a typical diameter of about 0.003 inch (0.08 mm). The polymer
impregnate is preferably a thermoplastic polyurethane such as PEBAX*
6333. An inner lining 24 of high strength polymer material such as
polyimide may be provided which extends to the start of the distal
section 25 of the delivery sheath 11.
A supporting ribbon 26 extends through the distal section 25
with the proximal extremity thereof about 5 to about 15 mm being
secured to the braided tubular structure 21 by suitable means such as
solder or adhesive 27 within the wall of the proximal shaft section .G.
* Trademark
CA 02251041 1998-10-07
WO 97/37607 11 PCT/US97/05714
The supporting ribbon 26 is generally about 6 to about 20 cm in total
length and has a rectangular transverse cross-section of about 0.003-
0.007 inch by 0.01-0.03 inch. The distal extremity of the supporting
ribbon 26 is secured to the distal end of the delivery sheath 11 in a
similar fashion. As shown in Figs. 1 and 3, the braided tubular
structure 21 extends into the distal section 25 of the delivery sheath 11
disposed about the supporting ribbon 26.
The distal section 25 of the delivery sheath 11 has an elongated
opening 28 which allows the distal section 30 of the EP device 12 to be
extended out and away from the distal section 25 of the delivery sheath
11 when an axial compressive force is applied to the proximal extremity
of the EP device which extends out of the patient during the procedure.
The length of the elongated opening 28 is generally the same length as
the distal section 25, i.e. about 3 to about 20 cm. The width of the
elongated opening 28 generally is greater than the diameter of the
distal section 31 of the EP device 12 to allow for the ready outward
movement of the EP device.
The EP device 12, as shown in Figs. 1 and 4-5 includes a
proximal shaft section 30 and a distal shaft section 31. The distal shaft
section 31 has a plurality of mapping/ablation electrodes 32 with each
_ of the electrodes electrically connected to separate electrical
conductors 33 (shown in Figs. 4-5). The electrodes 32 are preferably
CA 02251041 2004-12-16
12
not larger than about 1.5 mr'n (4 Fr.), usually less than 1.3 mm (3.5 Fr.)
in outer transverse dimensions. The electrode length may vary from
about 1 to about 6 mm, preferably about 1 to about 3 mm, and the
interelectrode spacing may vary from about 0.5 to about 4 mm,
preferably about 0.5 to about 2 mm. The electrodes 32 may be in the
form of metallic cylindrical bands, helical coils, arcuate bands or
ribbons and the like. The only portion of the electrodes 32 which need
exposure are those. surfaces which are to be in contact with the inner
surface of the heart chamber to detect electrical activity or effect a
linear ablation.
A suitable EP device 12 shown in detail in Figs. 4 and 5, has
proximal and distal shaft sections 30 and 31, an electrical connector 34
on the proximal end of the device and eight electrodes 32 on the distal
section 31 which are electrically connected to insulated electrical
conductors as in United States Patent 5y509,411. Core member 35 extends
to the distal lend of the device which is secured to the distal end of coil
36 by suitable material such as a gold-tin solder (80% Au-20%S~ The
coil 36 is preferably a 90% Pt-10% Ir wire about 0.005 inch in diameter.
Polymide tubing 37, about 0.001 inch thick, jackets the core member 35
proximal to the coil 36 which is in turn covered with a fluropolymer
tube 38 such as THV
* Trademark
CA 02251041 2004-12-16
13
2006 which is available from 3M. The braided electrical conductors 33
are formed of 36 AWG copper wire with each conductor having a
pofyimide insulating coating of about 0.0005 inch thick (0.013 mm). An
equivalent number of polyester fibers 39 (e.g. Dacron~ from Dupont)
are braided with the electrical conductors 33. The braided structure
formed by the electrical conductors 33 and the polyester strands 39 are
covered by an additional tluoropolymer jacket or coating 40, preferably
THV 200g made by 3M. The electrodes 32 are helical coils which are
preferably formed from 90% Pt-10% lr wire about 0.005 inch ( 0.13
mm) in diameter.
The overall length of the delivery sheath 11, excluding the
adapter 14, is about 110 to about 130 cm and the outer diameter is
about 0.06 to about 0.08 inch (1.5-2.0 mm). The inner lumen 13 is
slightly larger than the outer diameter of the EP device 12 and
generally is about 0.035 to about 0.055 inch (0.9-1.4 mm). The EP
device 12 has a working length of about 110- 155 cm and a total length
of about 135 to about 175 including the electrical connector 34.
The assembly of the invention may be introduced into the
patient's vascular system, e.g., the femoral vein, percutaneously or by
way of a cut-down, advanced therein and through the inferior versa
cava until the distal section 25 is disposed within the right atrium. The
supporting ribbon 26 in the distal shaft section 31 is shaped into a
* Trademark
CA 02251041 1998-10-07
WO 97/37607 14 PCT/CTS97/05714
curved configuration so that it assumes the curved configuration when
unrestrained within the heart chamber. With the supporting ribbon
acting as a supporting surface, a compressive force is applied to the
proximal extremity of the EP device which extends out of the patient to
urge the device in the distal direction, causing the distal shaft section
31 of the EP device 12 to bow outwardly away from the distal section of
the delivery sheath 11 and the support ribbon 26 therein. Torquing the
proximal section 30 of the delivery sheath 11, which extends out of the
patient during the procedure, will cause the distal section 25 thereof to
be rotatably displaced within the atrial chamber and allow the EP
device 12 to be bowed outwardly in a wide variety of directions so
electrical activity can be detected in a linear fashion and heart tissue
can be linearly ablated at a number of locations within the chamber.
When sensing electrical activity essentially all of the electrodes 32 can
be simultaneously employed, but, when performing a linear ablation,
the typical procedure is to direct the RF current to one or two
electrodes at the most distal end of the EP device to perform the first
ablation and then continue proximally one or two electrodes at a time
until a linear ablation of desired length is obtained in the atrial chamber.
This reduces the overall power requirements for the assembly.
The electrodes 32 heat up due to the conductive heat transfer
from the tissue being ablated and it is preferred to bath the electrodes
CA 02251041 1998-10-07
WO 97/37607 15 PCT/US97/05714
with cooling fluid during the procedure to minimize the formation of
thrombus. While not shown in the drawings, thermocouples,
thermistors or other temperature sensing means may be incorporated
into the wall of the EP device 12 to detect the temperature of the
electrodes or device wall. The flow of cooling fluid may be controlled to
bathe the distal shaft section 31 of the EP device 12 based upon the
temperature sensed by the temperature sensing means.
After the ablation, the electrodes 32 can be employed to detect
electrical activity to ensure that the ablation has been effective in
terminating the fibrillation or flutter. The electrodes 32 are much
smaller in diametrical dimensions than prior ablation electrodes which
are usually about 1.5 mm or larger. Surprisingly, it has been found that
the much smaller electrodes of the present invention provide effective
ablation through the atrial wall without the power requirements of the
prior electrodes. The elongated lesion formed by the linear ablation
with the smaller electrodes, while much thinner than lesions formed
with the prior larger electrodes, is quite effective in segregating heart
tissue so as to terminate the fibrillation or flutter. Typically, the
elongated lesion formed with the device of the present invention is
about 3 to about 12 mm, usually about 5 to about 10 mm, in width.
Figs. 6 and 7 illustrate an alternative embodiment to that shown
in Figs. 1-3 wnerein a second lumen 41 is provided within the distal
CA 02251041 1998-10-07
WO 97/37607 16 PCT/US97/05714
section of the delivery sheath in order to pass flushing or cooling fluids
to the distal extremity of the sheath. The spacing between the exterior
of the EP device 12 and the inner surface of the inner lumen 13 of the
delivery sheath 11 is minimized at location 42 so that a significant
portion of fluid passing through the inner lumen 13 will pass through
port 43 into the inner lumen 41. A discharge port 44 is provided in the
distal end of the delivery sheath 11 for discharge of fluid from the inner
lumen 41.
Figs. 8-10 illustrate another embodiment similar in function to
that shown in Figs. 7-8 which has a second lumen 45 extending the
length of the delivery sheath 11 which is in fluid communication with a
second side arm 46 of the adapter 14. The other portions of the
embodiment are similar to the embodiment shown in Figs. 7-8 and are
similarly numbered.
Figs. 11-12 depict yet another embodiment similar in most
regards to that shown in Fig. 1 except that the delivery sheath 11 is
provided with a plurality of electrodes 47 on the distal section 25 and at
least one electrode 48 on the proximal shaft section 20. In this
embodiment, the surtace of the electrodes 47 on the inside of the
curved distal section 25 need to be exposed. The electrodes 47 and
48 may be helical coils as shown or cylindrical tubes or arcuate ribbon
or bands provided on the inside curve of the distal section 25.
CA 02251041 1998-10-07
WO 97/37607 17 PCT/US97/05714
Individual electrical conductors (not shown) may be incorporated into
the braided tubular structure 21 and electrically connected by their
distal ends to the electrodes 47 and 48 and by their proximal ends to
one or more electrical connectors configured to be electrically
connected to a high frequency electrical energy source.
Another alternative embodiment of the invention is shown in
Figs. 13, 14A and 14B wherein the EP device 12 is provided with an
inner lumen 49 for fluid delivery. An adapter 50 is secured to the
proximal end of the EP device 12 to facilitate introduction of fluid to the
70 inner lumen 49. In Fig. 14A the lumen 49 is off set from the electrical
conductors 51 which are braided about the core 52, whereas, in Fig.
14B the lumen 49 is formed by the braided conductors 51 within a
polymer matrix 53. The embodiment of Fig. 14B does not have a core
member 52 as in Fig. 14A. A discharge port 54 is provided in the distal
end of the EP device 12 which is in fluid communication with the inner
lumen 49.
Alternative electrode details are illustrated in Figs. 15 and 16
where the electrodes 32 are formed by a pair of inner and outer coils
55 and 56 which are secured together at each end by solder, adhesive
or the like. The electrodes 32 are cooled by fluid flowing through the
inner lumen 49. The coils may be expanded in the longitudinal
direction to allow passage of fluid therethrough. A passageway (not
CA 02251041 1998-10-07
WO 97/37607 ~ $ PCT/US97/05714
shown) must be provided through the wall of the EP device to facilitate
the passage of fluid. A single coil may be used for each electrode
rather than a pair of coils 55 and 56 as shown.
In some instances it is desirable to change the curvature of the
distal shaft section 31 of the EP device 12 when the distal end of the
device is within the heart chamber to provide a better fit between the
distal shaft section 31 and the inner surface of a heart chamber. To
facilitate such changes, an outer sheath 57 may be provided about the
exterior of the delivery sheath to effectively shorten the elongated
opening 28 in the distal section 25 of the delivery sheath 11 as shown
in Fig. 16. By shortening the elongated opening 28 the radius of
curvature is reduced, as shown in phantom in Fig. 16. Fluid may be
passed through the inner lumen 58 of the sheath 57 to cool the
electrodes 32 during delivery of RF electrical energy. A variety of other
means may be employed to effectively shorten the elongated opening
28.
Figs. 17 and 18 illustrate another method of cooling the
electrodes 32 on the distal section of the EP device 12 where a
flushing sheath 59 is slidably disposed about the EP device. In this
embodiment, the sheath 59 can be longitudinally moved along the shaft
of the EP device to expose one or more electrodes 32. Fluid passing
over the exposed electrodes) while electrical energy is being delivered
CA 02251041 1998-10-07
WO 97/37607 19 PCT/US97/05714
will cool the electrodes sufficiently to avoid thrombus formation.
Usually, electrical energy is not directed to the entire array of
electrodes at the same time due to the rather large power requirements
for such delivery. Electrical energy is preferably delivered to one or two
of the most distal electrodes while fluid is delivered thereto until the
lesion of desired length is formed. The sheath 59 is then pulled
proximally to expose additional electrodes 32, electrical energy is
delivered to one or two additionally exposed electrodes while cooling
fluid flows out of the distal end of the sheath 59. This procedure
continues sequentially delivering electrical energy to the more proximal
electrodes until a linear ablation of the desired length is formed in the
wall of the patient's heart. The individual electrodes 32 may be used to
detect electrical activity after each individual ablation and after the
entire linear ablation procedure has been completed to determine if the
fibrillation or flutter has been terminated.
Figs. 20 and 21 illustrate an embodiment of an EP device which
may be used by itself to form linear ablations within a patient's heart
chamber, particularly the atrial chamber. It is for the most part similar
to the embodiment shown in Figs. 4 and 5 except for the absence of
the coil on the distal tip of the device and the corresponding parts are
similarly numbered. The catheter 30 has a distal shaft section 31
which is provided with a plurality of electrodes 32 formed by helical
CA 02251041 2004-12-16
coils of conducive metallic material such as an alloy of 90% platinum
and 10% iridium. A core member 35 formed of 304 stainless steel is
disposed within the catheter 30 and is provided with a first insulating
coating or jacket 37 which is preferably formed of polyimide and an
,~.
5 outer jacket 38 formed of a fluoropolymer such as THV 2006.
Electrical conductors 33 are braided onto the outer jacket 38 and
polymer strands 39 (e.g. ~Dacron) are braided onto the braided
conductors 33. The ends 60 of the metallic coils fom~ing the electrodes
32 are electrically connected to individual conductors 33 by a suitable
10 electrically conducting solder such as 80Au%-209~oSn. The insulation
on the electrical conductors 33 is removed at the site where the ends
60 of the coil contact the electrical conductors 33. A rounded electrode
61, formed of suitable material such as platinum, is provided at the
distal tip of the catheter 30 which is secured to the tapered end of the
15 core member 35 by a suitable electrical conducting solder 62 (800
Au20%Sn). An electrical conductor 63 is provided to direct electrical
current to the electrode 61. Suitable insulation 64 (e.g. Pebax~4033)
separates the electrode 61 from the electrode 32 and the braided
electrical conductors 33. The distal shaft section is flexible enough to
20 be advanced through the patient's vasculature to the patient's heart
chamber and yet it has sufficient strength to be pressed against the
* Trademark
CA 02251041 1998-10-07
WO 97/37607 21 PCT/US97/05714
patient's endocardium to provide contact to effectively form the
elongated lesion.
A similar embodiment is shown in Figs. 22 and 23 which has
thermocouples 65 located between the outer jacket 38 and the braided
conductors 33. The device is otherwise similar to the prior embodiment
and is provided with the same reference numbers. As shown best in
Fig. 23, the thermocouples 65 are preferably T type thermocouples
joining a copper thermocouple conductor wire 66 and Constantan
thermocouple conductor wire 67. Preferably, a thermocouple 65 is
provided beneath each electrode 32 so that the electrical power
delivered to each electrode can be controlled by a suitable device (not
shown) to control the temperature in a desired manner.
In the embodiments shown in Figs. 20-23 the electrodes are
about 2 to about 4 mm in length, typically about 3 mm, and have an
interelectrode spacing of about 1 to about 3 mm, typically about 2 mm.
The distal shaft section 31 is less than about 5 French (mm), preferably
less than 4.5 French (mm). The length of the distal shaft section 31
having electrodes 32 is about 10 to about 50 cm and preferably about
30 to about 45 cm. Both embodiments of Figs. 20-23 may be
employed with the assembly described in the previously discussed
embodiments.
CA 02251041 1998-10-07
WO 97/37607 22 PCT/US9?/05714
The electrical connector 34 on the proximal ends of the
embodiments described herein may be commercially available
electrical connectors such as Part No. PAB-M08-GLA39J or PAB-
M08-TLA39J for an eight pin connector or Part No. PAB-M08-GLA39A
for a connector with a greater number of pins, e.g. 9-16. The above
connectors are available from Lemo USA, Inc. in Santa Rosa, CA.
Suitable connectors for accessory cables connectable to the above
connectors include PRB-M08-GLL65J for eight pin connectors and
PRB-M08-G1165A for connectors with more than eight pins. The latter
connectors are also available from the same source.
While the invention has been described herein in terms of
certain preferred embodiments directed to the detection and treatment
of atrial fibrillation and flutter, those skilled in the art will recognize
that
the invention may be employed in a wide variety of procedures where
an elongated narrow lesion is to be formed. Moreover, although
individual features of embodiments of the invention may be shown in
some of the drawings and not in others, those skiffed in the art will
recognize that individual features of one embodiment of the invention
can be combined with any or all the features of another embodiment.
A variety of modifications and improvements may be made to the
present invention without departing from the scope thereof.