Note: Descriptions are shown in the official language in which they were submitted.
CA 02283790 1999-09-09
WO 98!43710 PCT/US98/06161
IMPROVED MULTI-LAYER GOLF BALL
UTILIZING SILICONE MATERIALS
Cross References to
Related Applications
The present application claims priority from U.S.
provisional patent Application Serial No. 60/042,117 filed March
28, 1997; and is a continuation-.in-part application of U.S patent
Application Serial No. 08/870,585, filed June 6, 1997, which is
a file wrapper continuation of Serial No. 08/566,237, filed
November 9, 1995, which is a continuation-in-part of serial No.
08/542,793, filed October 13, 1995, which is a continuation-in-
part of Serial No. 08/070,510, filed June 1, 1993.
Field of the Invention
The present invention relates to golf balls and, more
particularly, to improved golf balls comprising multi-layer
covers which have a hard inner layer and a relatively soft outer
layer. The improved golf balls utilize one or more silicone
layers and/or a silicone core. The improved multi-layer golf
balls provide for enhanced distance and durability properties
while at the same time offering the "feel" and spin
characteristics associated with soft balata and balata-like
covers of the prior art.
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
Backcrround of the Invention
Traditional golf ball covers have been comprised of
balata or blends of balata with elastomeric or plastic materials.
The traditional balata covers are relatively soft and flexible.
Upon impact, the soft balata covers compress against the surface
of the club producing high spin. Consequently, the soft and
flexible balata covers provide an experienced golfer with the
ability to apply a spin to control the ball in flight in order
to produce a draw or a fade, or a backspin which causes the ball
to "bite" or stop abruptly on contact with the green. Moreover,
the soft balata covers produce a soft "feel" to the low handicap
player. Such playability properties (workability, feel, etc.~
are particularly important in short iron play with low swing
speeds and are exploited significantly by relatively skilled
players.
Despite all the benefits of balata, balata covered golf
balls are easily cut and/or damaged if mis-hit. Golf balls
produced with balata or balata-containing cover compositions
therefore have a relatively short lifespan.
As a result of this negative property, balata and its
synthetic substitutes, trans-polybutadiene and transpolyisoprene,
have been essentially replaced as the cover materials of choice
by new cover materials comprising ionomeric resins.
Ionomeric resins are polymers containing interchain
ionic bonding. As a result of their toughness, durability and
flight characteristics, various ionomeric resins sold by E._I.
DuPont de Nemours & Company under the trademark "Surlyn°" and
more recently, by the Exxon Corporation (see U. S. Patent No.
-2-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
4,911,451) under the trademarks "Escor~" and the trade name
"Iotek", have become the materials of choice for the construction
of golf ball covers over the traditional "balata"
' (transpolyisoprene, natural or synthetic) rubbers. As stated,
the softer balata covers, although exhibiting enhanced
playability properties, lack the durability (cut and abrasion
resistance, fatigue endurance, etc.) properties required for
repetitive play.
Ionomeric resins are generally ionic copolymers of an
olefin, such as ethylene, and a metal salt of an unsaturated
carboxylic acid, such as acrylic acid, methacrylic acid, or
malefic acid. Metal ions, such as sodium or zinc, are used to
neutralize some portion of the acidic group in the copolymer
resulting in a thermoplastic elastomer exhibiting enhanced
properties, i.e. durability, etc., for golf ball cover
construction over balata. However, some of the advantages gained
in increased durability have been offset to some degree by the
decreases produced in playability. This is because although the
ionomeric resins are very durable, they tend to be very hard when
utilized for golf ball cover construction, and thus lack the
degree of softness required to impart the spin necessary to
control the ball in flight. Since the ionomeric resins are
harder than balata, the ionomeric resin covers do not compress
as much against the face of the club upon impact, thereby
producing less spin. In addition, the harder and more durable
ionomeric resins lack the "feel" characteristic associated with
' the softer balata related covers.
As a result, while there are currently more than fifty
(50) commercial grades of ionomers available both from DuPont and
-3-
CA 02283790 1999-09-09
WO 98143710 PCT/US98106161
Exxon, with a wide range of properties which vary according to
the type and amount of metal cations, molecular weight,
composition of the base resin (i.e., relative content of ethylene
and methacrylic and/or acrylic acid groups) and additive
ingredients such as reinforcement agents, etc., a great deal of
research continues in order to develop a golf ball cover
composition exhibiting not only the improved impact resistance
and carrying distance properties produced by the "hard" ionomeric
resins, but also the playability (i.e., "spin", "feel", etc.)
characteristics previously associated with the "soft" balata
covers, properties which are still desired by the more skilled
golfer.
Consequently, a number of two-piece (a solid resilient
center or core with a molded cover) and three-piece (a liquid or
solid center, elastomeric winding about the center, and a molded
cover) golf balls have been produced by the present inventor and
others to address these needs. The different types of materials
utilized to formulate the cores, covers, etc. of these balls
dramatically alters the balls' overall characteristics. In
addition, multi-layered covers containing one or more ionomer
resins have also been formulated in an attempt to produce a golf
ball having the overall distance, playability and durability
characteristics desired.
This was addressed by Spalding & Evenflo Companies,
Inc., the assignee of the present invention, in U. S. Patent No.
4,431,193 where a multi-layered golf ball is disclosed. In the
'193 patent, a multi-layer golf ball is produced by initially
molding a first cover layer on a spherical core and then adding
-4-
CA 02283790 1999-09-09
WO 98/43710 PCT/iJS98/06161
a second layer. The first layer is comprised of a hard, high
flexural modulus resinous material such as type 1605 Surlyn° (now
designated Surlyn° 8940). Type 1605 Surlyn° (Surlyn°
8940) is a
. sodium ion based low acid (less than or equal to 15 weight
percent methacrylic acid) ionomer resin having a flexural modulus
of about 51,000 psi. An outer layer of a comparatively soft, low
flexural modulus resinous material such as type 1855 Surlyn° (now
designated Surlyn° 9020) is molded over the inner cover layer.
Type 1855 Surlyn° (Surlyn° 9020) is a zinc ion based low acid
(10
weight percent methacrylic acid) ionomer resin having a flexural
modulus of about 14,000 psi.
The '193 patent teaches that the hard, high flexural
modulus resin which comprises the first layer provides for a gain
in coefficient of restitution over the coefficient of restitution
of the core. The increase in the coefficient of restitution
provides a ball which serves to attain or approach the maximum
initial velocity limit of 255 feet per second as provided by the
United States Golf Association (U.S.G.A.) rules. The relatively
soft, low flexural modulus outer layer provides essentially no
gain in the coefficient of restitution but provides for the
advantageous "feel" and playing characteristics of a balata
covered golf ball. Unfortunately, however, while a ball of the
'193 patent does exhibit enhanced playability characteristics
with improved distance (i.e. enhanced C.O.R. values) over a
number of other known multi-layered balls, the ball suffers from
poor cut resistance and relatively short distance (i.e. lower
C.O.R. values) when compared to two-piece, single cover layer
balls. These undesirable properties make the ball produced in
_5_
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
accordance with the '193 patent unacceptable by today's
standards.
The present invention is directed to new multi-layer
golf ball compositions which provide for enhanced coefficient of
restitution (i.e, enhanced resilience or carrying distance)
and/or durability properties when compared to the multi-layer
balls found in the prior art, as well as improved outer cover
layer softness and durability. As such, the playability
characteristics (i.e., "feel", "click", "spin", etc.) are not
diminished.
These and other objects and features of the invention
will be apparent from the following summary and description of
the invention, the drawings and from the claims.
Summary of the Invention
The present invention provides, in one aspect, a golf
ball comprising a core, a cover layer, and at least one interior
layer surrounding the core. The core and/or the interior layer
include one or more silicone materials. The silicone materials
are silicone polymers, silicone fluids, sililcone elastomers, and
silicone resins.
In another aspect, the present invention provides a
golf ball comprising a core, an inner cover layer molded on the
core, an outer cover layer molded on the inner cover layer, and
at least one interior layer between the core and the outer cover
layer. The core and/or the interior layers) include a silicone
material. The inner cover layer is formed from a high acid
ionomer including at least 16o by weight of an alpha, beta-
unsaturated carboxylic acid. The outer cover layer is formed
-6-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
from a relatively soft polymeric material such as low flexural
modulus ionomer resins and non-ionomeric thermoplastic
elastomers.
In yet another aspect, the present invention provides
a multi-layer golf ball comprising a spherical core, an inner
cover layer molded over the core, an outer cover layer molded
over the inner cover layer, and an interior layer disposed
between the core and the outer cover layer. The core and/or
interior layers) comprise a silicone material. The inner and
outer cover layers have particular properties. The inner cover
layer comprises an ionomeric resin including at least 16o by
weight of an alpha, beta-unsaturated carboxylic acid and has a
modulus of from about 15,000 to about 70,000 psi. The outer
cover layer comprises a blend of sodium or zinc salt of a
particular copolymer and a particular unsaturated monocarboxylic
acid, and a sodium or zinc salt of a certain terpolymer, acrylic
acid and a particular unsaturated monomer of the acrylate ester
class. The outer cover layer has a modulus of from about 1,000
to about 30,000 psi.
In yet another embodiment, the present invention
provides a multi-layer golf ball comprising a spherical core, an
inner cover layer molded over the dual core, an outer cover layer
molded over the inner cover layer, and at least one interior
layer between the core and the outer cover layer. The core
and/or the interior layers) comprise a silicone material. The
inner cover layer comprises an ionomeric resin including 17o to
25% of an alpha, beta-unsaturated carboxylic acid, and has a
modulus of from about 15,000 to about 70,000 psi. The outer
_7_
ICA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
cover layer comprises a non-ionomeric thermoplastic selected from
the group consisting of polyester elastomer, polyester urethane,
and polyester amide. The outer cover layer has a modulus in the
range of 1,000 to 30,000 psi.
In a further aspect, the present invention also
provides a golf ball comprising a core comprising a silicone
material, an inner cover layer molded on the core and an outer
cover layer molded on the inner cover layer. The inner cover
layer comprises a high acid ionomer including at least 16% of an
alpha, beta-unsaturated carboxylic acid. The outer cover layer
comprises a relatively soft polymeric material of low flexural
modulus ionomer resins and non-ionomeric thermoplastic
elastomers.
In yet another aspect, the present invention provides
a multi-layer golf ball comprising a spherical core including a
silicone material, an inner cover layer molded over the spherical
core, and an outer cover layer molded over the inner cover layer.
The inner cover layer comprises an ionomeric resin including at
least 16% of an alpha, beta-unsaturated carboxylic acid. The
outer cover layer comprises a relatively soft polymeric material
of low flexural modulus ionomer resins and non-ionomeric
thermoplastic elastomers.
Moreover, the present invention provides a multi-layer
golf ball comprising a core component comprising silicone
material, an inner cover molded over the core, and an outer cover
layer molded over the inner cover. The inner cover layer
comprises an ionomeric resin including at least 160 of an alpha,
beta-unsaturated carboxylic acid and has a modulus of 15,000 to
-8-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
70,000 psi. The outer cover layer comprises a blend of a sodium
or zinc salt of a certain copolymer and a sodium or zinc salt of
a terpolymer. The outer cover has a modulus in the range of
' 1,000 to 30,000 psi.
In yet another aspect, the present invention provides
a multi-layer golf ball comprising a spherical core comprising
a silicone material, an inner cover layer molded over the
spherical core, and an outer cover layer molded over the inner
cover layer. The inner cover layer comprises from about 17% to
about 250 of an alpha, beta-unsaturated carboxylic acid and has
a modulus of from about 15,000 to about 70,000 psi. The outer
cover layer comprises a particular non-ionomeric thermoplastic
and has a modulus in the range of about 1,000 to 30,000 psi.
In still another aspect, the present invention provides
a golf ball comprising a core including a silicone material, an
inner cover layer molded on the core, an outer cover layer molded
on the inner cover layer, and at least one interior layer between
the core and the outer cover layer. The inner cover layer
comprises a high acid ionomer including at least 160 of an alpha,
beta-unsaturated carboxylic acid. The outer cover layer
comprises a relatively soft polymeric material such as low
flexural modulus ionomer resins and non-ionomeric thermoplastic
elastomers.
In yet a further aspect, the present invention provides
a multi-layer golf ball comprising a core including a silicone
material, an inner cover layer molded over the core, an outer
' cover layer molded over the inner cover layer, and at least one
_g_
ICA 02283790 1999-09-09
WO 98/43710 PCT/US98106161
interior layer of a silicone material. The inner and outer cover
layers feature aspects as noted above.
These and other objects and features of the invention
will be apparent from the following detailed description.
Brief Description of the Drawings
FIG. 1 is a cross-sectional view of a preferred
embodiment golf ball in accordance with the present invention
comprising a core and a cover having an inner layer and an outer
dimpled layer;
FIG. 2 is a diametrical cross-sectional view of the
golf ball illustrated in FIG. 1 having a core and a cover made
of an inner layer and an outer layer having dimples;
FIG. 3 is a partial cross-sectional view of another
preferred embodiment golf ball in accordance with the present
invention having an interior layer comprising a silicone
material;
FIG. 4 is a partial cross-sectional view of another
preferred embodiment golf ball in accordance with the present
invention having two interior layers, at least one of which
comprises a silicone material;
FIG. 5 is a partial cross-sectional view of yet another
preferred embodiment golf ball in accordance with the present
invention having three or more interior layers, at least one of
which comprises a silicone material;
FIG. 6 is a partial cross-sectional view of another
preferred embodiment golf ball in accordance with the present
invention having a core comprising a silicone material;
-10-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98106161
FIG. 7 is a partial cross-sectional view of another
preferred embodiment golf ball in accordance with the present
invention having a core comprising a silicone material and at
least two interior layers; and
FIG. 8 is a partial cross-sectional view of yet another
preferred embodiment golf ball in accordance with the present
invention having a core comprising a silicone material and three
or more interior layers.
Detailed Description of the Preferred Embodiments
The present invention is directed to golf balls
comprising improved multi-layer cover compositions in combination
with one or more silicone interior layers and/or a silicone core.
The novel multi-layer golf ball covers of the present
invention include a first or inner layer or ply of a high acid
(greater than 16 weight percent acid) ionomer or ionomer blend
and second or outer layer or ply comprised of a comparatively
softer, low modulus ionomer, ionomer blend or other non-ionomeric
thermoplastic elastomer such as polyurethane, a polyester
elastomer such as Hytrel° polyester elastomer of E.I. DuPont de
Nemours & Company, or a polyesteramide such as the Elf Atochem
S.A. Pebax° polyesteramide. Preferably, the outer cover layer
includes a blend of hard and soft low acid (i.e. 16 weight
percent acid or less) ionomers.
It has been found that the recently developed high acid
ionomer based inner cover layer, provides for a substantial
increase in resilience (i.e., enhanced distance) over known
multi-layer covered balls. The softer outer layer provides for
desirable "feel" and high spin rate while maintaining respectable
-11-
ICA 02283790 1999-09-09
WO 98/43710 PCTIUS98/06161
resiliency. The soft outer layer allows the cover to deform more
during impact and increases the area of contact between the club
face and the cover, thereby imparting more spin on the ball. As
a result, the soft cover provides the ball with a balata-like
feel and playability characteristics with improved distance and
durability. Consequently, the overall combination of the inner
and outer cover layers results in a golf ball having enhanced
resilience (improved travel distance) and durability (i.e. cut
resistance, etc.) characteristics while maintaining and in many
instances, improving the ball's playability properties.
The combination of a high acid ionomer or ionomer blend
inner cover layer with a soft, relatively low modulus ionomer,
ionomer blend or other non-ionomeric thermoplastic elastomer
outer cover layer provides for excellent overall coefficient of
restitution (i.e., excellent resilience) because of the improved
resiliency produced by the inner cover layer. While some
improvement in resiliency is also produced by the outer cover
layer, the outer cover layer generally provides for a more
desirable feel and high spin, particularly at lower swing speeds
with highly lofted clubs such as half wedge shots.
Two principal properties involved in golf ball
performance are resilience and hardness. Resilience is
determined by the coefficient of restitution (C.O.R.), the
constant "e" which is the ratio of the relative velocity of two
elastic spheres after direct impact to that before impact. As
a result, the coefficient of restitution ("e") can vary from 0
to 1, with 1 being equivalent to an elastic collision and 0 being
equivalent to an inelastic collision.
-12-
CA 02283790 1999-09-09
WO 98/43710 PCTIUS98/06161
Resilience (C.O.R.), along with additional factors such
as club head speed, angle of trajectory and ball configuration
(i.e., dimple pattern) generally determine the distance a ball
' will travel when hit. Since club head speed and the angle of
trajectory are factors not easily controllable by a manufacturer,
factors of concern among manufacturers are the coefficient of
restitution (C.O.R.) and the surface configuration of the ball.
The coefficient of restitution (C.O.R.) in solid core
balls is a function of the composition of the molded core and of
the cover. In balls containing a wound core (i.e., balls
comprising a liquid or solid center, elastic windings, and a
cover), the coefficient of restitution is a function of not only
the composition of the center and cover, but also the composition
and tension of the elastomeric windings. That is, both the core
and the cover contribute to the coefficient of restitution.
In this regard, the coefficient of restitution of a
golf ball is generally measured by propelling a ball at a given
speed against a hard surface and measuring the ball's incoming
and outgoing velocity electronically. As mentioned above, the
coefficient of restitution is the ratio of the outgoing velocity
to the incoming velocity. The coefficient of restitution must
be carefully controlled in all commercial golf balls in order for
the ball to be within the specifications regulated by the United
States Golf Association (U.S.G.A.). Along this line, the
U.S.G.A. standards indicate that a "regulation" ball cannot have
an initial velocity (i.e., the speed off the club) exceeding 255
feet per second. Since the coefficient of restitution of a ball
is related to the ball's initial velocity, it is highly desirable
-13-
~CA 02283790 1999-09-09
WO 98/43710 PCTlUS98/06161
to produce a ball having sufficiently high coefficient of
restitution to closely approach the U.S.G.A. limit on initial
velocity, while having an ample degree of softness (i.e.,
hardness) to produce enhanced playability (i.e., spin, etc.).
The hardness of the ball is the second principal
property involved in the performance of a golf ball. The
hardness of the ball can affect the playability of the ball on
striking and the sound or "click" produced. Hardness is
determined by the deformation (i.e., compression) of the ball
under various load conditions applied across the ball's diameter
(i.e., the lower the compression value, the harder the material).
As indicated in U.S. Patent No. 4,674,751, softer covers permit
the accomplished golfer to impart proper spin. This is because
the softer covers deform on impact significantly more than balls
having "harder" ionomeric resin covers. As a result, the better
player is allowed to impart fade, draw or backspin to the ball
thereby enhancing playability. Such properties may be determined
by various spin rate tests.
Accordingly, the present invention is directed, at
least in part, to an improved multi-layer cover which produces,
upon molding each layer around a core (preferably a solid core)
to formulate a multi-layer cover, a golf ball exhibiting enhanced
distance (i.e., resilience) without adversely affecting, and in
many instances, improving the ball's playability
(hardness/softness) and/or durability (i.e., cut resistance,
fatigue resistance, etc.) characteristics.
Another important feature of the present invention golf
balls is the use of one or more interior layers of a silicone
-14-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
composition. In addition to or instead of such silicone layers,
the present invention golf balls may also comprise a core of a
silicone composition. These silicone materials and their
incorporation into the present invention golf balls are described
in greater detail below.
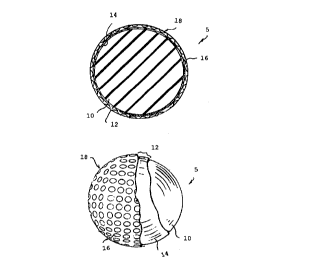
Figures 1 and 2 illustrate a preferred embodiment_golf
ball 5 in accordance with the present invention. The golf ball
5 comprises a multi-layered cover 12 disposed about a solid core
10. The present invention also provides a method for making
same.
The multi-layered cover 12 comprises two layers: a
first or inner cover layer or ply 14 and a second or outer cover
layer or ply 16. The outer layer 16 defines a plurality of
dimples 18. The inner layer 14 is comprised of a high acid (i.e.
greater than 16 weight percent acid) ionomer resin or high acid
ionomer blend. Preferably, the inner layer is comprised of a
blend of two or more high acid (i.e. at least 16 weight percent
acid) ionomer resin neutralized to various extents by different
metal cations. The inner cover layer may or may not include a
metal stearate (e. g., zinc stearate) or other metal fatty acid
salt. The primary purpose of the metal stearate or other metal
fatty acid salt is to lower the cost of production without
affecting the overall performance of the finished golf ball.
Inner Cover Layer
The inner layer compositions include the high acid
ionomers such as those recently developed by E. I. DuPont de
Nemours & Company under the trademark "Surlyn°" and by Exxon
-15-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98106161
Corporation under the trademark "Escor~" or tradename "Iotek",
or blends thereof. Examples of compositions which may be used
as the inner layer herein are set forth in detail in U. S. Patent
No. 5,688,869, incorporated herein by reference. Of course, the
inner layer high acid ionomer compositions are not limited in any
way to those compositions set forth in that '869 patent. For
example, the high acid ionomer resins recently developed by
Spalding & Evenflo Companies, Inc., the assignee of the present
invention, and disclosed in the '869 patent, may also be utilized
to produce the inner layer of the multi-layer cover used in the
present invention.
The high acid ionomers which may be suitable for use
in formulating the inner layer compositions of the subject
invention are ionic copolymers which are the metal, i.e., sodium,
zinc, magnesium, etc., salts of the reaction product of an olefin
having from about 2 to 8 carbon atoms and an unsaturated
monocarboxylic acid having from about 3 to 8 carbon atoms.
Preferably, the ionomeric resins are copolymers of ethylene and
either acrylic or methacrylic acid. In some circumstances, an
additional comonomer such as an acrylate ester (i.e., iso- or n-
butylacrylate, etc.? can also be included to produce a softer
terpolymer. The carboxylic acid groups of the copolymer are
partially neutralized (i.e., approximately 10-75%, preferably 30-
70%? by the metal ions. Each of the high acid ionomer resins
which may be included in the inner layer cover compositions of
the invention contains greater than about 16% by weight of_ a
carboxylic acid, preferably from about 17% to about 25% by weight
-16-
CA 02283790 1999-09-09
WO 98!43710 PCTIUS98/06161
of a carboxylic acid, more preferably from about 18.5% to about
21.5 % by weight of a carboxylic acid.
Although the inner layer cover composition preferably
' includes a high acid ionomeric resin and the scope of the patent
S embraces all known high acid ionomeric resins falling within the
parameters set forth above, only a relatively limited number of
these high acid ionomeric resins have recently become
commercially available.
The high acid ionomeric resins available from Exxon
under the designation "Escor°" and or "Iotek", are somewhat
similar to the high acid ionomeric resins available under the
"Surlyn°" trademark. However, since the Escor°/Iotek ionomeric
resins are sodium or zinc salts of polyethylene-acrylic acid)
and the "Surlyn~" resins are zinc, sodium, magnesium, etc. salts
of polyethylene-methacrylic acid), distinct differences in
properties exist.
Examples of the high acid methacrylic acid based
ionomers found suitable for use in accordance with this invention
include Surlyn° AD-8422 (sodium cation), Surlyn~ 8162 (zinc
cation), Surlyn° SEP-503-1 (zinc cation), and Surlyn~ SEP-503-2
(magnesium cation). According to DuPont, all of these ionomers
contain from about 18.5 to about 21.5% by weight methacrylic
acid.
More particularly, Surlyn° AD-8422 is currently
commercially available from DuPont in a number of different
grades (i.e., AD-8422-2, AD-8422-3, AD-8422-5, etc.) based upon
differences in melt index. According to DuPont, Surlyn° AD-8422
offers the following general properties, as set forth below in
-17-
CA 02283790 1999-09-09
WO 98/43710 PCTIUS98/06161
Table 1, when compared to Surlyn~ 8920, the stiffest, hardest of
all of the low acid grades (referred to as "hard" ionomers in
U.S. Patent No. 4,884,814).
TABLE 1
General Properties n~ Ionomers
of Sur ~
LOW ACID HIGH ACID
(15 wto Acid) (>20 wto Acid)
SURLYN~ SURLYN~ SURLYN~
8920 8422-2 8422-3
IONOMER
Cation Na Na Na
Melt Index 1.2 2.8 1.0
Sodium, Wt% 2.3 1.9 2.4
Base Resin 60 60
MI 60
MP1, C 88 86 85
FPl, C 47 48.5 45
COMPRESSION MOLDED PLAQUE PROPERTIES
Tensile Break,
psi 4350 4190 5330
Yield, psi 2880 3670 3590
Elongation, 0 315 263 289
Flex Mod,
K psi 53.2 76.4 88.3
Shore D
hardness 66 67 68
1 DSC second heat, l0°C/min heating rate.
2 Samples compression molded at 150°C annealed 24
hours at 60°C. 8422-2, -3 were homogenized at
190°C before molding.
In comp ring Surlyn~ 8920 to Surlyn° 8422-2 and Surlyn°
8422-3, it is noted that the high acid Surlyn~ 8422-2 and 8422-3
ionomers have a higher tensile yield, lower elongation, slightly
higher Shore D hardness and much higher flexural modulus.
-18-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
Surlyn~"' 8920 contains 15 weight percent methacrylic acid and is
59o neutralized with sodium.
In addition, Surlynm SEP-503-1 (zinc cation) and
' Surlyn° SEP-503-2 (magnesium cation) are high acid zinc and
magnesium versions of the Surlyn~ AD 8422 high acid ionomers.
As shown in Table 2, when compared to the Surlyn~ AD 8422 high
acid ionomers, the Surlyn SEP-503-1 and SEP-503-2 ionomers can
be defined as follows:
TABLE 2
Other Surlyn~ Ionomers
Surlyn° Ionomer Ion Melt Index Neutralization %
AD 8422-3 Na 1.0 45
SEP 503-1 Zn 0.8 38
SEP 503-2 Mg 1.8 43
Furthermore, Surlyn~ 8162 is a zinc cation ionomer
resin containing approximately 20% by weight (i.e. 18.5-21.5%
weight) methacrylic acid copolymer that has been 30-70%
neutralized. Surlyn~ 8162 is currently commercially available
from DuPont.
Examples of the high acid acrylic acid based ionomers
suitable for use in the present invention also include the Escor~
or Iotek high acid ethylene acrylic acid ionomers produced by
Exxon. In this regard, Escort or Iotek 959 is a sodium ion
neutralized ethylene-acrylic neutralized ethylene-acrylic acid
copolymer. According to Exxon, Ioteks 959 and 960 contain from
about 19.0 to about 21.0% by weight acrylic acid with
approximately 30 to about 70 percent of the acid groups
neutralized with sodium and zinc ions, respectively. AS set
-19-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
forth in Table 3, the physical properties of these high acid
acrylic acid based ionomers are as follows:
TABLE 3
General Prot~erties of Escor~ Ionomers
PROPERTY ESCOR~ (IOTEK) 959 ESCOR~ (IOTEK)
960
Melt Index, g/10 min 2.0 1_g
Cation Sodium Zinc
Melting Point, F 172 174
Vicat Softening Point, F 130 131
1 ~ Tensile ~ Break, psi 4600 3500
Elongation C~ Break, % 325 430
Hardness, Shore D 66 57
Flexural Modulus, psi 66,000 27,000
Furthermore, as a result of the development by the
inventors of a number of new high acid ionomers neutralized to
various extents by several different types of metal rations,
such as by manganese, lithium, potassium, calcium and nickel
rations, several new high acid ionomers and/or high acid
ionomer blends besides sodium, zinc and magnesium high acid
ionomers or ionomer blends are now available for golf ball
cover production. It has been found that these new ration
neutralized high acid ionomer blends produce inner cover layer
compositions exhibiting enhanced hardness and resilience due
to synergies which occur during processing. Consequently, the
metal ration neutralized high acid ionomer resins recently
produced can be blended to produce substantially harder inner
cover layers for multi-layered golf balls having highe-r-
C.O.R.'s than those produced by the low acid ionomer inner
cover compositions presently commercially available.
-20-
,.
CA 02283790 1999-09-09
WO 98/43710 PCT/IJS98106161
More particularly, several new metal ration
neutralized high acid ionomer resins have been produced by the
inventors by neutralizing, to various extents, high acid
copolymers of an alpha-olefin and an alpha, beta-unsaturated
carboxylic acid with a wide variety of different metal ration
salts. This discovery is the subject matter of U.S. Patent
No. 5,688,869, incorporated herein by reference. It has been
found that numerous new metal ration neutralized high acid
ionomer resins can be obtained by reacting a high acid
copolymer (i.e. a copolymer containing greater than 16o by
weight acid, preferably from about 17 to about 25 weight
percent acid, and more preferably about 20 weight percent
acid), with a metal ration salt capable of ionizing or
neutralizing the copolymer to the extent desired (i.e. from
about 10% to 90%).
The base copolymer is made up of greater than 16% by
weight of an alpha, beta-unsaturated carboxylic acid and an
alpha-olefin. Optionally, a softening comonomer can be
included in the copolymer. Generally, the alpha-olefin has
from 2 to 10 carbon atoms and is preferably ethylene, and the
unsaturated carboxylic acid is a carboxylic acid having from
about 3 to 8 carbons. Examples of such acids include acrylic
acid, methacrylic acid, ethacrylic acid, chloroacrylic acid,
crotonic acid, malefic acid, fumaric acid, and itaconic acid,
with acrylic acid being preferred.
The softening comonomer that can be optionally
included in the invention may be selected from the group
consisting of vinyl esters of aliphatic carboxylic acids
-21-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
wherein the acids have 2 to 10 carbon atoms, vinyl ethers
wherein the alkyl groups contains 1 to 10 carbon atoms, and
alkyl acrylates or methacrylates wherein the alkyl group
contains 1 to 10 carbon atoms. Suitable softening comonomers
S include vinyl acetate, methyl acrylate, methyl methacrylate,
ethyl acrylate, ethyl methacrylate, butyl acrylate, butyl
methacrylate, or the like.
Consequently, examples of a number of copolymers
suitable for use to produce the high acid ionomers included in
the present invention include, but are not limited to, high
acid embodiments of an ethylene/acrylic acid copolymer, an
ethylene/methacrylic acid copolymer, an ethylene/itaconic acid
copolymer, an ethylene/maleic acid copolymer, an
ethylene/methacrylic acid/vinyl acetate copolymer, an
ethylene/acrylic acid/vinyl alcohol copolymer, etc. The base
copolymer broadly contains greater than 16% by weight
unsaturated carboxylic acid, from about 30 to about 83% by
weight ethylene and from 0 to about 40% by weight of a
softening comonomer. Preferably, the copolymer contains about
20o by weight unsaturated carboxylic acid and about 80o by
weight ethylene. Most preferably, the copolymer contains
about 20% acrylic acid with the remainder being ethylene.
Along these lines, examples of the preferred high
acid base copolymers which fulfill the criteria set forth
above, are a series of ethylene-acrylic copolymers which are
commercially available from The Dow Chemical Company, Midland,
Michigan, under the "Primacor" designation. These high acid
-22-
~ ~
CA 02283790 1999-09-09
WO 98!43710 PCT/US98/06161
base copolymers exhibit the typical properties set forth below
in Table 4.
TABLE 1
Typical Properties of Primacor
Eth~rlene-Acrylic Acid Copolymers
GRADE PERCENT DENSITY MELT TENSILEFLEXURA VICAT SHORE
D
ACID , glcc INDEX, YD. L SOFT HARDNES
ST PT
g/lOmin(psi) MODULUS (C) S
(psi)
ASTM D-792 D-1238 D-638 D-790 D-1525 D-2240
5980 20.0 0.958 300.0 - 4800 43 50
5990 20.0 0.955 1300.0 650 2600 40 40
5981 20.0 0.960 300.0 900 3200 46 48
5983 20.0 0.958 500.0 850 3100 49 45
5991 20.0 0.953 2600.0 635 2600 38 40
The Melt values obtainedaccordingto ASTM 238, 0C.
Index are D-1 at
19
Due to the high molecular weight of the Primacor
5981 grade of the ethylene-acrylic acid copolymer, this
copolymer is the more preferred grade utilized in the
invention.
The metal cation salts utilized in the invention are
those salts which provide the metal cations capable of
neutralizing, to various extents, the carboxylic acid groups
of the high acid copolymer. These include acetate, oxide or
hydroxide salts of lithium, calcium, zinc, sodium, potassium,
nickel, magnesium, and manganese.
Examples of such lithium ion sources are lithium
hydroxide monohydrate, lithium hydroxide, lithium oxide-and
lithium acetate. Sources for the calcium ion include calcium
hydroxide, calcium acetate and calcium oxide. Suitable zinc
-23-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
ion sources are zinc acetate dihydrate and zinc acetate, a
blend of zinc oxide and acetic acid. Examples of sodium ion
sources are sodium hydroxide and sodium acetate. Sources for
the potassium ion include potassium hydroxide and potassium
acetate. Suitable nickel ion sources are nickel acetate,
nickel oxide and nickel hydroxide. Sources of magnesium
include magnesium oxide, magnesium hydroxide, magnesium
acetate. Sources of manganese include manganese acetate and
manganese oxide.
The new metal ration neutralized high acid ionomer
resins are produced by reacting the high acid base copolymer
with various amounts of the metal ration salts above the
crystalline melting point of the copolymer, such as at a
temperature from about 200° F to about 500° F, preferably from
about 250° F to about 350° F under high shear conditions at a
pressure of from about 10 psi to 10,000 psi. Other well known
blending techniques may also be used. The amount of metal
ration salt utilized to produce the new metal ration
neutralized high acid based ionomer resins is the quantity
which provides a sufficient amount of the metal rations to
neutralize the desired percentage of the carboxylic acid
groups in the high acid copolymer. The extent of
neutralization is generally from about 10% to about 90%.
As indicated below in Table 2 and more specifically
in the Examples in U.S. Patent No. 5,688,869 a number of new
types of metal ration neutralized high acid ionomers can be
obtained from the above indicated process. These include new
high acid ionomer resins neutralized to various extents with
-24-
....
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
manganese, lithium, potassium, calcium and nickel rations. In
addition, when a high acid ethylene/acrylic acid copolymer is
utilized as the base copolymer component of the invention and
this component is subsequently neutralized to various extents
with the metal ration salts producing acrylic acid based high
acid ionomer resins neutralized with rations such as sodium,
potassium, lithium, zinc, magnesium, manganese, calcium and
nickel, several new ration neutralized acrylic acid based high
acid ionomer resins are produced.
-25-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
TABLE 5
Wt-% Wt-% Melt Shore
D
Formulation Cation Salt NeutralizationIndex C.O.R.Hardness
NO.
1(NaOH) 6.98 67.5 0.9 .804 71
2(NaOH) 5.66 54.0 2.4 .808 73
3(NaOH) 3.84 35.9 12.2 .812 69
4(NaOH) 2.91 27.0 17.5 .812 (brittle)
5(MnAc) 19.6 71.7 7.5 .809 73
6(MnAc) 23.1 88.3 3.5 .814 77
1 0 7(MnAc) 15.3 53.0 7.5 .810 72
8(MnAc) 26.5 106 0.7 .813 (brittle)
9(LiOH) 4.54 71.3 0.6 .810 74
10(LiOH) 3.38 52.5 4.2 .818 72
I1(LiOH) 2.34 35.9 18.6 .815 72
1 5 12(KOH) 5.30 36.0 19.3 Broke 70
13(KOH) 8.26 57.9 7.18 .804 70
14(KOH) 10.7 77.0 4.3 .801 67
15(ZnAc) 17.9 71.5 0.2 .806 71
16(ZnAc) 13.9 53.0 0.9 .797 69
17(ZnAC) 9.91 36.1 3.4 .793 67
18(MgAc) 17.4 70.7 2.8 .814 74
19(MgAc) 20.6 87.1 1.5 .815 76
20(MgAC) 13.8 53.8 4.1 .814 74
21(CaAC) 13.2 69.2 1.1 .813 74
2 5 22(CaAc) 7.12 34.9 10.1 .808 70
Controls: 50/50 Blend of Ioteks 8000/7030 C.O.R.=.810/65 Shore D Hardness
DuPont High Acid Surlyn~ 8422 (Na) C.O.R.=.811/70 Shore D Hardness
DuPOnt High Acid Surlyn~ 8162 (Zn) C.O.R.=.807/65 Shore D Hardness
Exxon High Acid Iotek EX-960 (Zn) C.O.R.=.796/65 Shore D Hardness
-26-
T.~
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
TABLE 5 (continued)
Wt-% Wt-% Melt
Formulation Cation Salt Neutralizatio Index C.O.R.
No. n
23(Mg0) 2.91 53.5 2.5 .813
rJ 24(Mg0) 3.85 71.5 2.8 .808
25(Mg0) 4.76 89.3 1.1 .809
26(Mg0) 1.96 35.7 7.5 .815
Control for Formulations 23-26 is 50/50 Iotek 8000/?030,
C.O.R.=.814, Formulation 26 C.O.R. was normalized to that control accordingly
TABLE 5 (continued)
Wt-~ Wt-% Melt
Formulation Cation Salt NeutralizatioIndex C.O.R.Shore D
No. n Hardness
27(NiAc) 13.04 61.1 0.2 .802 71
1 5 28(NiAC) 10.71 48.9 0.5 .799 72
29(NiAc) 8.26 36.7 , 1.8 .796 69
30(NiAC) 5.66 24.4 7.5 .786 64
Control for FormulationNos. 27-3050/50 8000/7030,
is Iotek C.O.R.=.807
When compared to low acid versions of similar cation
neutralized ionomer resins, the new metal cation neutralized
high acid ionomer resins exhibit enhanced hardness, modulus
and resilience characteristics. These are properties that are
particularly desirable in a number of thermoplastic fields,
including the field of golf ball manufacturing.
When utilized in the construction of the inner layer
of a multi-layered golf ball, it has been found that the new
acrylic acid based high acid ionomers extend the range of
hardness beyond that previously obtainable while maintaining
the beneficial properties (i.e. durability, click, feel, etc.)
of the softer low acid ionomer covered balls, such as balls
produced utilizing the low acid ionomers disclosed in U.S.
Patent Nos. 4,884,814 and 4,911,451.
-27-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
Moreover, as a result of the development of a number
of new acrylic acid based high acid ionomer resins neutralized
to various extents by several different types of metal
cations, such as manganese, lithium, potassium, calcium and
nickel cations, several new ionomers or ionomer blends are now
available for production of an inner cover layer of a multi-
layered golf ball. By using these high acid ionomer resins,
harder, stiffer inner cover layers having higher C.O.R.s, and
thus longer distance, can be obtained.
More preferably, it has been found that when two or
more of the above-indicated high acid ionomers, particularly
blends of sodium and zinc high acid ionomers, are processed to
produce the covers of multi-layered golf balls, (i.e., the
inner cover layer herein) the resulting golf balls will travel
further than previously known multi-layered golf balls
produced with low acid ionomer resin covers due to the balls'
enhanced coefficient of restitution values.
For example, the multi-layer golf ball taught in
4,650,193 does not incorporate a high acid ionomeric resin in
the inner cover layer. The coefficient of restitution of the
golf ball having an inner layer taught by the '193 patent
(i.e., inner layer composition "D" in the Examples) is
substantially lower than the coefficient of restitution of the
remaining compositions. In addition, the multi-layered ball
disclosed in the '193 patent suffers substantially in
durability in comparison with the present invention.
-28-
CA 02283790 1999-09-09
WO 98/43710 PCTlUS98/06161
Outer Cover Layer
With respect to the outer layer 16 of the multi-
layered cover of the present invention, the outer cover layer
is comparatively softer than the high acid ionomer based inner
layer. The softness provides for the feel and playability
characteristics typically associated with balata or balata-
blend balls. The outer layer or ply is comprised of a
relatively soft, low modulus (about 1,000 psi to about 10,000
psi) and low acid (less than 16 weight percent acid) ionomer,
ionomer blend or a non-ionomeric thermoplastic elastomer such
as, but not limited to, a polyurethane, a polyester elastomer
such as that marketed by DuPont under the trademark Hytrel~,
or a polyester amide such as that marketed by Elf Atochem S.A.
under the trademark Pebax°. The outer layer is fairly thin
(i.e. from about 0.010 to about 0.050 in thickness, more
desirably 0.03 inches in thickness for a 1.680 inch ball), but
thick enough to achieve desired playability characteristics
while minimizing expense.
Preferably, the outer layer includes a blend of hard
and soft (low acid) ionomer resins such as those described in
U. S. Patent Nos. 4,884,814 and 5,120,791, both incorporated
herein by reference. Specifically, a desirable material for
use in molding the outer layer comprises a blend of a high
modulus (hard) ionomer with a low modulus (soft) ionomer to
form a base ionomer mixture. A high modulus ionomer herein is
one which measures from about 15,000 to about 70,000 psi as
measured in accordance with ASTM method D-790. The hardness
-29-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
may be defined as at least 50 on the Shore D scale as measured
in accordance with ASTM method D-2240.
A low modulus ionomer suitable for use in the outer
layer blend has a flexural modulus measuring from about 1,000
to about 10,000 psi, with a hardness of about 20 to about 40
on the Shore D scale.
The hard ionomer resins utilized to produce the
outer cover layer composition hard/soft blends include ionic
copolymers which are the sodium, zinc, magnesium or lithium
salts of the reaction product of an olefin having from 2 to 8
carbon atoms and an unsaturated monocarboxylic acid having
from 3 to 8 carbon atoms. The carboxylic acid groups of the
copolymer may be totally or partially (i.e. approximately 15-
75 percent) neutralized.
The hard ionomeric resins are likely copolymers of
ethylene and either acrylic and/or methacrylic acid, with
copolymers of ethylene and acrylic acid being the most
preferred. Two or more types of hard ionomeric resins may be
blended into the outer cover layer compositions in order to
produce the desired properties of the resulting golf balls.
As discussed earlier herein, the hard ionomeric
resins introduced under the designation Escor° and sold under
the designation "Iotek" are somewhat similar to the hard
ionomeric resins sold under the Surlyn° trademark. However,
since the "Iotek" ionomeric resins are sodium or zinc salts of
polyethylene-acrylic acid) and the Surlyn° resins are zinc or
sodium salts of polyethylene-methacrylic acid) some distinct
differences in properties exist. As more specifically
-30-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
indicated in the data set forth below, the hard "Iotek" resins
(i.e., the acrylic acid based hard ionomer resins) are the
more preferred hard resins for use in formulating the outer
layer blends for use in the present invention. In addition,
various blends of "Iotek" and Surlyn° hard ionomeric resins,
as well as other available ionomeric resins, may be utilized
in the present invention in a similar manner.
Examples of commercially available hard ionomeric
resins which may be used in the present invention in
formulating the outer cover blends include the hard sodium
ionic copolymer sold under the trademark Surlyn~8940 and the
hard zinc ionic copolymer sold under the trademark
Surlyn~9910. Surlyn~8940 is a copolymer of ethylene with
methacrylic acid and about 15 weight percent acid which is
about 29 percent neutralized with sodium ions. This resin has
an average melt flow index of about 2.8. Surlyn~9910 is a
copolymer of ethylene and methacrylic acid with about 15
weight percent acid which is about 58 percent neutralized with
zinc ions. The average melt flow index of Surlyn~9910 is
about 0.7. The typical properties of Surlyn~9910 and 8940 are
set forth below in Table 6:
-31-
ICA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
TABLE 6
Tvpical Properties of Commercially Available
Hard
Surlyn Resins Suitable for Use in the Outer
Layer Blends of
the Present Invention
ASTM D 8940 9910 8920 8528 9970 9730
Cation Type Sodium Zinc Sodium
Sodium Zinc Zinc
Melt flow index,
gms/10 min. D-1238 2.8 0.7 0.9 1.3 14.0 1.6
Specific Gravity,
1 g/cm' D-792 0.95 0.97 0.95 0.94 0.95 0.95
0
Hardness, Shore D D-2240 66 64 66 60 62 63
Tensile Strength,
(kpsi), MPa D-638 (4.8) (3.6) (5.4) (3.2} (4.1}
(4.2)
33.1 24.8 37.2 29.0 22.0 28.0
Elongation, % D-63B 470 290 350 450 460 460
Flexural Modulus,
(kpsi) MPa D-790 (51) (48) (55) (32} (28) (30)
350 330 380 220 190 210
Tensile Impact (23C)
20 KJ/mz (ft.-lbs./in2) D-182251020 1020 865 1160 760 1240
(485) (485) (410) (360) (590)
(550}
Vicat Temperature, CD-1525 63 62 58 73 61 73
Examples of the more pertinent acrylic
acid based
hard ionomer resin suitab le for use in the resent outer cover
p
25 composition sold under th e "Iotek" tradename by the Exxon
Corporation include Iotek 4000, Iotek 4010, Iotek 8000, Iotek
8020 and Iotek 8030. The typical properties of these and
other Iotek hard ionomers suited for use in formulating the
outer layer cover composi tion are set forth below in Table
7:
-32-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
TABLE 7
Typical Properties of Iotek Ionomers
Resin ASTM
Properties MethodUnits 4000 4D10 8000 8020 8030
S Cation type zinc zinc sodiumsodiumsodium
Melt index D-1238g/10 2.5 1.5 D.8 1.6 2.8
min.
Density D-1505kg/m' 963 963 954 960 960
Melting Point D-3917C 90 90 90 87.5 87.5
Crystallization D-3417C 62 64 56 53 55
Point
Vicat Softening D-1525C 62 63 61 64 67
Point
% Weight Acrylic 16 11
Acid
% of Acid Groups
1 cation neutralized 30 40
5
Plaque ASTM
Properties MethodUnits 4000 4010 8000 8020 8030
(3 mm thick.
compression molded)
Tensile at break D-638MPa 24 26 36 31.5 28
Yield point D-638MPa none none 21 21 23
Elongation at D-638% 395 920 350 410 395
break
1% Secant modulusD-638MPa 160 160 300 350 390
Shore Hardness D-2240-- 55 55 61 58 59
D
2 Film Properties
5
(50 micron film
2.2:1
Blow-uv ratio) 4000 4010 8000 802D 8030
Tensile at Break D-882MPa 41 39 42 52 47.4
MD
TD D-882MPa 37 38 38 38 40.5
3 Yield point MD D-882MPa 15 17 17 23 21.6
~
TD D-882MPa 14 15 15 21 20.7
Elongation at
Break
MD D-882% 310 270 260 295 305
TD D-882% 360 340 280 340 345
3 1% Secant modulusD-882MPa 2I0 215 390 380 380
5 MD
TD D-882MPa 200 225 380 350 345
Dart Drop Impact D-1709g/micron12.4 12.5 20.3
-33-
ICA 02283790 1999-09-09
WO 98143710 PCT/US98/06161
Resin ASTM
Properties Method Units 7010 7020 7030
Cation type zinc zinc zinc
Melt Index D-1238 g/10 min. 0.8 1.5 2.5
Density D-1505 kg/m' 960 960 960
Melting Point D-3417 oC 90 90 90
Crystallization
Point D-3417 oC -- -- --
1 0 Vicat Softening
Point D-1525 oC 60 63 62.5
%Weight Acrylic Acid -- -- --
% of Acid Groups
1 5 Cation Neutralized -- -- --
Plaque ASTM
Properties Method Units 7010 7020 7030
(3 mm thick,
compression molded)
2 0 Tensile at break D-638 MPa 38 38 38
Yield Point D-638 MPa none none none
Elongation at break D-638 % 500 420 395
1% Secant modulus D-638 MPa -- -- --
Shore Hardness D D-2240 -- 57 55 55
25 Comparatively, soft ionomers are used in formulating
the hard/soft blends of the outer cover composition. These
ionomers include acrylic acid based soft ionomers. They are
generally characterized as comprising sodium or zinc salts of
a terpolymer of an olefin having from about 2 to 8 carbon
30 atoms, acrylic acid, and an unsaturated monomer of the
acrylate ester class having from 1 to 21 carbon atoms. The
soft ionomer is preferably a zinc based ionomer made from an
acrylic acid base polymer in an unsaturated monomer of the
acrylate ester class. The soft (low modulus) ionomers have a
35 hardness from about 20 to about 40 as measured on the Shore D
scale and a flexural modulus from about 1,000 to about 10,000,
as measured in accordance with ASTM method D-790.
-34-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98106161
Certain ethylene-acrylic acid based soft ionomer
resins developed by the Exxon Corporation under the
designation "Iotek 7520" (referred to experimentally by
differences in neutralization and melt indexes as LDX 195, LDX
196, LDX 218 and LDX 219) may be combined with known hard
ionomers such as those indicated above to produce the outer
cover. The combination produces higher C.O.R.s at equal or
softer hardness, higher melt flow (which corresponds to
improved, more efficient molding, i.e., fewer rejects) as well
as significant cost savings versus the outer layer of multi-
layer balls produced by other known hard-soft ionomer blends
as a result of the lower overall raw materials costs and
improved yields.
While the exact chemical composition of the resins
to be sold by Exxon under the designation Iotek 7520 is
considered by Exxon to be confidential and proprietary
information, Exxon's experimental product data sheet lists the
following physical properties of the ethylene acrylic acid
zinc ionomer developed by Exxon:
-35-
ICA 02283790 1999-09-09
WO 98/43710 PCTlfJS98/06161
TABLE 8
Physical Properties of Iotek 7520
Property ASTM Method Units Typical Value
Melt Index D-1238
g/10 min. 2
Density D-1505 kg/m3 0.962
Cation Zinc
Melting Point D-3417 C 66
Crystallization
Point D-3417 C 49
Vicat Softening
Point D-1525 C 42
Plaque Properties (2 mm thick pression
Com Molded
Placrues)
Tensile at Break D-638 MPa 10
Yield Point D-638 MPa None
Elongation at Break 0 760
D-638
1% Secant Modulus D-638 MPa 22
Shore D Hardness D-2240 32
Flexural Modulus D-790 MPa 26
Zwick Rebound ISO 4862 0 52
De Mattia Flex
Resistance D-430 Cycles >5000
In addition, test collected
data by the
inventors
indicates that Iotek have Shore D hardnesses of
7520 resins
about 32 to 36 (pe r ASTM D-2240),melt flow indexes of 310.5
g/10 min (at 190oC . per ASTM D-1288), flexural modulus
and a
of about 2500-3500 psi (per ASTM D-790).
Furthermore,
testing
by an independent testing laboratory
by pyrolysis
mass
spectrometry indic ates that Iotek
7520 resins
are generally
-36 -
CA 02283790 1999-09-09
WO 98/43710 PCTlUS98/06161
zinc salts of a terpolymer of ethylene, acrylic acid, and
methyl acrylate.
Furthermore, the inventors have found that a newly
developed grade of an acrylic acid based soft ionomer
available from the Exxon Corporation under the designation
Iotek 7510, is also effective, when combined with the hard
ionomers indicated above in producing golf ball covers
exhibiting higher C.O.R. values at equal or softer hardness
than those produced by known hard-soft ionomer blends. In
this regard, Iotek 7510 has the advantages (i.e. improved
flow, higher C.O.R. values at equal hardness, increased
clarity, etc.) produced by the Iotek 7520 resin when compared
to the methacrylic acid base soft ionomers known in the art
(such as the Surlyn 8625 and the Surlyn 8629 combinations
disclosed in U.S. Patent No. 4,884,814}.
In addition, Iotek 7510, when compared to Iotek
7520, produces slightly higher C.O.R. values at equal
softness/hardness due to the Iotek 7510's higher hardness and
neutralization. Similarly, Iotek 7510 produces better release
properties (from the mold cavities) due to its slightly higher
stiffness and lower flow rate than Iotek 7520. This is
important in production where the soft covered balls tend to
have lower yields caused by sticking in the molds and
subsequent punched pin marks from the knockouts.
According to Exxon, Iotek 7510 is of similar
chemical composition as Iotek 7520 (i.e. a zinc salt of a
terpoloymer of ethylene, acrylic acid, and methyl acrylate)
but is more highly neutralized. Based upon FTIR analysis,
-37-
ICA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
Iotek 7520 is estimated to be about 30-40 wt.-% neutralized
and Iotek 7510 is estimated to be about 40-60 wt.-
neutralized. The typical properties of Iotek 7510 in
comparison of those of Iotek 7520 are set forth below in Table
9:
TABLE 9
Physical Properties of Iotek 7510
in Comparison to Iotek 7520
IOTEK 7520 IOTEK 7510
MI, g/10 min 2.0 0.8
Density, g/cc 0.96 0.97
Melting Point, °F 151 149
Vicat Softening Point, °F 108 109
Flex Modulus, psi 3800 5300
Tensile Strength, psi 1450 1750
Elongation, % 760 690
Hardness, Shore D 32 35
It has been determined that when hard/soft ionomer
blends are used for the outer cover layer, good results are
achieved when the relative combination is in a range of about
90 to about 10 percent hard ionomer and about 10 to about 90
percent soft ionomer. The results are improved by adjusting
the range to about 75 to 25 percent hard ionomer and 25 to 75
percent soft ionomer. Even better results are noted at__
relative ranges of about 60 to 90 percent hard ionomer resin
and about 40 to 60 percent soft ionomer resin. Specific
formulations which may be used in the cover composition are
-38-
CA 02283790 1999-09-09
WO 98/43710 PCTIUS98/06161
included in the examples set forth in U. S. Patent No.
5,120,791 and 4,884,814. The present invention is in no way
limited to those examples.
Moreover, in alternative embodiments, the outer
cover layer formulation may also comprise a soft, low modulus
non-ionomeric thermoplastic elastomer including a polyester
polyurethane such as B.F.Goodrich Company's Estane~ polyester
polyurethane X-4517. According to B.F.Goodrich, Estane~ X-
4517 has the following properties as set forth below in Table
10:
TABLE 10
Properties of Estane~ X-4517
Tensile 1430
100% 815
200% 1024
3000 1193
Elongation 641
Youngs Modulus 1826
Hardness A/D 88/39
Bayshore Rebound 59
Solubility in Water Insoluble
Melt processing temperature >350oF (>177oC)
Specific Gravity (H20=1) 1.1-1.3
Other soft, relatively low modulus non-ionomeric
thermoplastic elastomers may also be utilized to produce the
outer cover layer as long as the non-ionomeric thermoplastic
elastomers produce the playability and durability
characteristics desired without adversely effecting the
enhanced travel distance characteristic produced by the_ high
acid ionomer resin composition. These include, but are not
limited to thermoplastic polyurethanes such as: Texin
thermoplastic polyurethanes from Mobay Chemical Co. and. the
-39-
i
CA 02283790 1999-09-09
WO 98/43710 PCTlUS98/06161
Pellethane thermoplastic polyurethanes from Dow Chemical Co.;
Ionomer/rubber blends such as those in Spalding U.S. Patents
4,986,545; 5,098,105 and 5,187,013; and, Hytrel polyester
elastomers from DuPont and pebax polyesteramides from Elf
Atochem S.A.
Silicone Interior Layers and/or Core
The present invention golf ball as previously noted,
further comprises one or more interior layers comprising one
or more silicone compositions. The present invention golf
ball may also, in addition to these silicone interior layers,
comprise a core or core layers) comprising one or more
silicone materials. The terms "silicone composition" and
"silicone material" as used herein are interchangeable for
purposes of this application and comprise silicone polymers,
silicone fluids, silicone elastomers, and silicone resins,
each of which are described in detail below. It will be
understood that these various silicone materials are
distinguishable from silica, as is used as a filler agent, as
described in U.S. Patents 5,387,637; 3,756,607; and 2,764,572,
all of which are herein incorporated by reference.
The term silicone as referred to herein denotes a
synthetic polymer (RnSi0,4-nli2) m. where n = 1-3 and m z 2 . A
silicone contains a repeating silicon-oxygen backbone and has
organic groups R attached to a signficant proportion of the
silicon atoms by silicon-carbon bonds. In commercially
available silicones, most R groups are methyl, longer alkyl,
fluoroalkyl, phenyl, vinyl, and a few other groups are
-40-
._....~ .._.r..
CA 02283790 1999-09-09
WO 98143710 PCT/US98106161
substituted for specific purposes. Some of the R groups can
also be hydrogen, chlorine, alkoxy, acyloxy, or alkylamino,
etc. These polymers can be combined with fillers, additives,
and solvents to result in products generally termed as
silicones.
Silicones have an unusual array of properties.
Chief among these are thermal and oxidative stability and
physical properties that are minimally affected by
temperature. Other important characteristics include a high
degree of chemical inertness, and resistance to weathering.
These features are such that silicone materials are well
suited for incorporation into golf balls in accordance with
the present invention. The molecular structure of suitable
silicones can vary considerably to include linear, branches,
and cross-linked structures.
Like carbon, silicon has the capability of forming
covalent compounds. Silicon hyrides (silanes) up to Si6H14 are
known. The Si-Si chain becomes thermally unstable at about
this length, however, so that polymeric silanes are unknown.
The siloxane link:
-Si-O-Si-
is more stable, and is the one predominantly found in
commercial silicone polymers. Unlike carbon, silicon does not
form double or triple bonds. Thus silicone polymers a.re
usually formed only by condensation-type reactions.
-41-
SUBSTITUTE SHEET (RULE 26)
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
Silicone polymers are made from organosilicon
intermediates prepared in various ways from elemental silicon,
which is typically produced by reducing quartz in an electric
furnace.
The intermediate "monomers" of silicone polymers are
compounds of the type SiRnX4_" where R is an alkyl or aryl group
and X is a group which can be hydrolyzed to -SiOH, such as
chlorine or alkoxy. The intermediates are generally made by a
direct synthesis in which the R and X groups are attached
simultaneously to the silicon by a high-temperature reaction
of a halide with silicon in the presence of a metal catalyst.
The chief reaction is, for example,
2CH3C1 + Si ~ Si (CH3) 2C12
but a number of side reactions may occur.
Silicone polymers are typically produced by
intermolecular condensation of silanols, which are formed from
the halide or alkoxy intermediates by hydrolysis:
- SiCl +H20 -j - SiOH + HC1
-SiOH + HOST- ~ -Si-O-Si- + Hz0
The desired siloxane structure is obtained by using silanols-
of different functionality, the alkyl (R) groups in the
intermediate being unreactive. _
-42-
SUBSTITUTE SHEET (RULE 26)
CA 02283790 1999-09-09
WO 98/43710 PCT/US98106161
The three commercially important classes of silicone
polymers for use in the preferred embodiment golf balls
include silicone homopolymers, silicone random copolymers, and
silicone-organic (block) copolymers. Polydimethylsiloxanes
(PDMS) constitute by far the largest volume of homopolymers
commerically produced:
CH3
-(Si-O)2-
CH3
PDMS is usually the principal component of the random
copolymers and the principal siloxane component of most
tsilicone-organic copolymers.
The most common silicones are the trimethylsiloxy-
terminated polydimethylsiloxanes. These polymers, as well as
variations with silanol, vinyl, or hydride end groups, form
the building blocks of many silicone fluid-based products and
of most cured silicone elastomers. The properties of
polydimethylsiloxanes are typically modified by substitution
of methyl groups on the silicon atom by hydrogen, alkyl,
phenyl, or organofunctional groups.
Silicone fluids are low polymers typically produced
by the hydrolysis reaction mentioned above, in which a
predetermined mixture of chlorosilanes is fed into water with
agitation. In many cases, the cyclic tetramer predominates in
the resulting mixture. Many silicone fluids are manufactured
commercially, including dimethyl, methyalkyl, and dimethyl-
diphenyl copolymers and silicone-polyether copolymers. These
compounds are typically used as cooling and dielectric fluids,
-43-
SUBSTITUTE SHEET (RULE 26)
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
in polishes and waxes, as release and antifoam agents, and for
paper and textile treatment. In view of their relatively low
viscosity and fluid nature, these compounds are less preferred
for use as the silicone materials in the present invention as
compared to silicone polymers, and as described below,
silicone elastomers and silicone resins. However, it is
contemplated that silicone fluids may be utilized in the
present invention golf balls.
Silicone elastomers are high-molecular-weight linear
polymers, usually polydimethysiloxanes. Cross-linking
silicone polymers of appropriate molecular weight provides
elastomeric properties. Fillers increase strength through
reinforcement, and extending fillers and additives, eg.
antioxidants, adhesion promoters, and pigments, can be
utilized to provide specific properties.
Many curing (cross-linking) systems have been
developed commercially for silicone elastomers. Different
commercially available silicone elastomers are conveniently
distinguished by their cure system chemistries and can be
catagorized by the temperature conditions needed for proper
cure. Most compositions are based on polydimethylsiloxanes:
CH 3
RO- ( S i-O ) z-R
~'' CH3
R is determined by the cure system chemistry. It can be -
hydrogen, an organic radical, or a silyl radical. The silyl
radicals can contain single or multiple reactive groups. like
-44-
SUBSTITUTE SHEET (RULE 26)
r i . ...
CA 02283790 1999-09-09
WO 98/43710 PCTlUS98/06161
vinyl or alkoxy. Small amounts of reactive functionality are
sometimes prsent in the chain in (copolymerized) units such as
(CH.,CH)(CH3)SiO. The value of x varies mainly with the type of
product. For room-temperature-vulcanizing RTV products, x is
in the 200-1,500 range; for heat-cured products, x is
approximately 3,000-11,000.
Silicone elastomers can be cured in several ways:
a. By free-radical crosslinking with, for example,
benzoyl peroxide, through the formation of
ZO ethylenic bridges between chains;
b. By crosslinking of vinyl or allyl groups
attached to silicon through reaction with
silylhydride groups:
R R" R R"
-S i-CH=CHz + H-S i- ~ -S i-CH2-CHz-S i
R' R"' R' R"'
or
c. By crosslinking linear or slightly branched
siloxane chains having reactive end groups such
as silanols. In contrast to the above
reactions, this yields Si-O-Si crosslinks.
The latter mechanism forms the basis of the curing
of room-temperature vulcanizing (RTV) silicone elastomers.
These are available as two-part mixtures in which all three
essential ingredients for the cure (silanol-terminated---
polymer, cross-linking agent such as ethyl silicate, and
catalyst such as a tin soap) are combined at the time the two
-45-
SUBSTITUTE SHEET (RULE 26)
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/Ob161
components are mixed, and as one-part materials using a
hydrolyzable polyfunctional silane or siloxane as crosslinker,
activated by atmospheric moisture.
Silicone elastomers are preferably reinforced by a
finely divided material such as silica to more readily achieve
properties for the silicone material as utilized in the
interior layers) or core. Specifically, the reinforcing
fillers for silicone elastomers may be finely divided silicas
made by fume or wet processes. The fume process provides the
highest degree of reinforcement. Accordingly, the particle
size is small. The particle diameter should be about the
length of a fully extended polymer chain, i.e., about l~,m, for
semireinforcement and about 0.01-0.05 ~,m for strong
reinforcement. Fine particle size does not necessarily
provide good reinforcement because finely divided fillers tend
to agglomerate and are hard to disperse. This tendency can be
countered by treating the filler to give it an organic or a
silicone coating before mixing it with polymer.
Hexamethyldisilazane, [(CH3)3Si]ZNH, is sometimes used as a
coupling agent. Treating the silica particles with hot vapors
of low molecular weight cyclic siloxanes reduces agglomeration
and prevents premature crepe hardening.
Nonreinforcing fillers, such as iron oxide or
titanium dioxide, may be utilized to stabilize or color the
resulting silicone material or to decrease the cost per unit
volume.
Thus fillers of many different chemical compositions
with a broad range of particle sizes and physical properties
-46-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
are suitable for use with silicone elastomers when utilized in
the present invention golf balls. The particular fillers)
selected primarily depend upon the desired end use properties
of the silicone material in the golf balls. The mechanism of
reinforcement has not been unequivocally determined and may
indeed vary from one filler or polymer type to another.
However, particle size is of prime importance for the strength
of the elastomer compound after cure. Effective reinforcement
is generally provided by silica particles having a specific
gravity of about 2 and a range of about 20-400 m2/g specific
surface area.
Nonreinforcing fillers may also be used merely as
extenders. The particle size of such fillers ranges from
submicro-meter to about 10/.~m. These fillers may not improve
physical properties, but can be incorporated in significant
amounts without adversely affecting strength of the resulting
silicone material. Manufacture of these extenders does not
require the specialized technology necessary for extremely
fine particle fillers, but the selected extenders must meet
rigorous requirements of thermal stability, low volatile
content, and chemical purity.
Silicone elastomers differ in several important ways
from most organic elastomers. The most striking difference is
the degree to which the strength of the final compound depends
on the reinforcement conferred by the incorporation of
fillers. Typical unfilled silicone gums, when cross-linked,_
are weak and soft, with tensile strengths on the order of 0.34
MPa (50 psi?. Compounding with suitably reinforcing fillers
-47-
i
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
may increase the tensile strength as much as 50-fold. The
selection of the filler is therefore extremely important for
applications where strength is required. These differences in
polymer-filler interactions and physical property requirements
make fillers suitable for silicone elastomers different from
those used for natural and synthetic rubber compounding.
The preferred filler types for silicone compounds
used in the present invention golf balls include finely
divided silicas prepared by vapor-phase hydrolysis or
oxidation of chlorosilanes, dehydrated silica gels,
precipitated silicas, diatomaceous silicas, and finely ground
high assay natural silicas; fumed titania, alumina, and
zirconia. Pigment-grade oxides especially ferric oxides, are
extensively used as fillers for high temperature compounds in
oxidizing environments. The iron oxide stabilizes the polymer
against atmospheric oxidation and preserves the elastomeric
characteristics, especially resilience and deformability,
after exposure to temperatures above 300°C. Carbon blacks
have had limited application because of their high content of
adsorbed volatiles, which can lead to void formation during
cure. Other types of fillers include calcium carbonate,
clays, siicates, and aluminates. Fibrous fillers improve tear
resistance at the expense of elongation, and hollow glass or
plastic microspheres reduce the specific gravity. Fillers and
their effects on heat-cured rubber properties are shown in
Table 11.
-48-
._.._..._w.,a_~_.. ~
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
TABLE 11
Fillers Suitable for Silicone Polymers
Reinforcementproduced
in
Particle Size silicone gums
Mean Surface Tensile
Diameter, Area, Strength, Elongation,
Filler ~m m~ MPa R
Reinforcing
fumed silica 0.03 silica 9.1-6.9 200-350
acetylene black 0.015-0.02 aerogel 4.1-12.4 200-600
Semireinforcing and nonreinforcing0.095 110-150 4.1-6.2 200-350
-
flux-calcined diatomaceous 175-200
silica
calcined diatomaceous silica1.5 78-85 2.7-5.5 75-200
1 calcined kaolin 1-5 2.7-5.5 75-200
0
precipitated calcium carbonate1-5 < 5 2.7-5.5 75-200
ground silica 0.03-D.OS < 5 2.7-9.1 100-300
ground silica S-10 < 5 0.7-2.8 200-300
ground silica 1-10 32 0.7-2.8 200-300
1 zinc oxide 5 0.7-2.B 200-300
5
iron oxide 0.3 1.9-3.5 100-30D
zirconium silicate < 1 1.4-3.5 100-300
titanium dioxide 3.0 2.8-4.1 100-300
0.3 1.9-3.5 300-900
Some silica or other oxide-filled silicone
20 eiastomers tend to "structure," i.e., to form an elastic mass
before cure, impeding normal processing operations such as
molding and extrusion. Intensive working of the compound with
a rubber mill or other mixer may be necessary to restore
plasticity. To minimize this tendency, plasticizers and
25 process aids may be incorporated into the compounds. The most
commonly used additives are monomeric or oligomeric
organosilicon compounds. High surface silica filler is
treated with a silicon derivative to minimize the buildup of
structure. The structuring tendency is associated with
30 hydrogen bonding between the siloxane polymers and silanol
groups on the filler surface. The extent of hydrogen bonding
is a function of the concentration of surface silanol and
varies with the type and method of preparation of the filler.
-49-
CA 02283790 1999-09-09
WO 98!43710 PCT/US98/06161
Surface silanol concentration can be related to the total
surface area as determined by absorption methods. Sufficient
treating agent can be added to react completely with or be
hydrogen bonded to the silanol groups present and yield a
nonstructuring rubber compound. In an early method, the
filler is treated with chlorosilanes or other reactive
silanes, and the HC1 or other reaction products are removed by
purging the filler mass with an inert gas. Cyclic siloxane
oligomers may be used to treat filler for silicone elastomers.
The extremely high surface silicas used as fillers
present the same storage and handling problems as conventional
fluffy carbon blacks. Typical bulk densities for fumed
silicas typically range from about 32 to about 80 kg/m3. They
can be increased to 160-240 kg/m3 by mechanical compaction and
deaeration.
Oligomers of polydimethylsiloxane can be polymerized
in the presence of fillers. Uncatalyzed base compounds for
both RTV and heat-curing elastomers can be made in this way.
However, optimal properties still depend on conventional
compounding.
Related to silicone elastomers, room temperature
vulcanizing (RTV) silicone elastomers are often available as
uncured rubbers with liquid or paste like consistencies. They
are based on polymers of intermediate molecular weights and
viscosities, e.g., 100-1,000,000 mm2/s at 25°C. Curing is
based on chemical reactions that increase molecular weights
and provide cross-linking. Catalysts may be utilized to
ensure cure control. The RTV silicone rubbers are typically
-50-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
available in two modifications. The cure reactions of one-
component products are triggered by exposure to atmospheric
moisture. Those of two-component products are triggered by
mixing the two components, one of which consists of or
contains the catalyst.
Commercially available one-component RTV rubbers are
typically made by mixing polymers, fillers, additives, curing
agents, and catalysts. The mixture is packaged to protect it
from moisture, which may trigger cure. The time required for
cure depends on the curing system, temperature, humidity, and
thickness of the silicone layer or core component. Under
typical ambient conditions the surface can be tack free in
about 30 minutes, while a 0.3-cm thick layer cures in less
than one day. As cure progresses, strength develops slowly
for about three weeks.
The original viscosity of these RTV materials
depends principally on that of the polymer components and the
filler loading. Filler and original polymer properties and
cross-link density affect the ultimate strength of the fully
cured elastomer. Most commercially available products are
based on polydimethylsiloxanes. Polymers with substituents
other than methyl modify and improve certain properties; e.g.,
trifluoropropyl groups improve solvent resistance. Some
products are compounded with fillers and additives to be
pourable, and others to be thixotropic. Silica-filled
polydimethylsiloxane systems, lacking pigments and other
additives, cure to form translucent rubbers. Since the
specific gravity of silicas, generally about 2.2, exceeds that
-51-
'CA 02283790 1999-09-09
WO 98/43710 PCTIUS98/06161
of siloxanes, generally about 1.0, the specific gravity of
the RTV rubbers depends on the filler loading. Physical
properties of similar cured acetoxy RTV formulations are shown
in Table 12.
TABLE 12
Physical Properties of RTV Rubbers
Durometer
Hardness, Tensile Strength,
Specific Gravity' Shore A 1~a Elongation,
1.18 45 2.4 180
1.30 50 3.1 140
1 0 1.33 50 3.9 200
1.37 55 3.8 120
1.45 60 4.5 110
1.45 60 5.2 160
1.98 65 4.8 110
' With increasing filler loading.
Formulations with different curing systems, polymer
molecular weights and structures, cross-link densities, and
other characteristics offer a broad spectrum of product
properties. For example, one-component products are available
with elongations as high as 10000. Typical properties of
representative cured RTV silicone rubbers are shown in Tables
13 and 14.
TABLE 13
Thermal Pro perties Cured SiliconeElastomers
of
2 5 One Component Two Components
Construction Adhesive Molding
Proyerty General pose Sealant Sealant Co~mound
Pur
3 0 Hardness, Shore A, 22 50 - -60
durometer 30
Tensile Strength, MPa 2.4 1.0 3.4 5.5
Elongation, $ 900 850 200 220
Tear Strength, J/cmz 0.80 0.35 0.52 1.75
-52-
_.. ,
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
'TABLE 14
Thermal Properties of Cured Silicone Elastomers
Property Typical Rancte
Useful temperature range, °C -110 to 200
with thermal stabilizers -110 to 250
Thermal conductivity, w/(m~K) 1.7-3.9
Coefficient of thermal expansion, per °C 3.5 x 10-5
The one-component RTV silicone rubbers are in some
instances, preferred for use in the present invention golf
balls, particularly for one or more interior layers. Such
layers rnay be formed by encapsulating the core with an RTV
silicone rubber material. Many formulations provide self-
bonding to most metals, glass, ceramics, concrete, and
plastics. For example, bonds to aluminum with >1.38 MPa (200
psi) shear strength and 0.35 J/cm2 (20 lbf/in.) tear strength
are obtainable. Bonding can be improved by applying a primer
to the substrate. These primers are solutions of reactive
silanes or resins that dry (cure) on the substrate, leaving a
modified silicone bondable surface. Bond strength develops as
the RTV cure progresses.
The two-component RTV silicone rubbers are
commercially available in a wide range of initial viscosities,
from as low as an easily pourable 100-mmz/s material to as high
as the stiff paste like materials of over 1,000,000 mmz/s at
25°C. Curing system, polymer molecular weight and structure,
cross-link density, filler, and additives can be varied and
combined, giving a group of products whose properties cover a
-53-
ICA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
wider range than that: encompassed by the one-component
products. The highest strength RTV rubbers are provided by
two-component RTV technology. On the other hand, products
that cure to a mere gel are also available. Unfilled resin-
s reinforced compositions can provide optical clarity. Polymers
with phenyl, trifluoropropyl, cyanoethyl, or other
substituents can be used with, or in place of,
polydimethylsiloxanes for low temperature-, heat-, radiation-,
and solvent-resistant elastomers.
In one-component formulations that rely for cure on
the reaction between a reactive cross-linking agent and
atmospheric moisture, the ingredients must be thoroughly
dried, or a drying step must be included in the compounding
cycle. As more filler is added during compounding, the
resistance to mixing tends to peak until "wetting-in" is
reached. The moisture-sensitive cross-linking agent is
usually added last. However, this step can be performed
separately. When the uncatalyzed base compound and cross-
linking agent are mixed, the effective viscosity sometimes
passes through a maximum. As the early chemical interactions
are resolved, a typical consistency is obtained. Allowance
for elevated effective in-process viscosities must be made
when mixing equipment is specified. Silica-reinforced
uncatalyzed base compounds harden (develop structured on--
storage, and the addition of catalyst should not be delayed.
-54-
CA 02283790 1999-09-09
WO 98143710 PCT/US98/06161
For two-component formulations, each park may
contain varying proportions of filler and polymer. The second
part contains the curing catalyst and possibly the cross-
linking agent and pigments. By proper design of the compound,
the proportions of first and second parts to be used may be
adjusted for convenient handling and metering. Typically,
from about 1 to 20 parts of the first part are typically used
per part of the second.
Many commercially available two-component RTV
elastomers can be advantageously cured at 50-150°C, depending
on the product and intended use, but RTV is characteristic.
Hydrosilation-curing RTV compositions can be modified with
inhibitors to become heat-curing systems.
Unlike RTV compositions, most heat-curing silicone
rubbers are based on high molecular weight polymer gums.
Gums, fillers, and additives can be mixed in dough mixers or
Banbury mills. Catalysts are added on water-cooled rubber
mills, which can be used for the complete process in small-
scale operations.
Silicone rubbers are commercially available as gums,
filler-reinforced gums, dispersions, and uncatalyzed and
catalyzed compounds Dispersions or pastes may be stirred
with solvents such as xylene. The following types of gums are
commercially available: general purpose (methyl and vin-y-1),
high and low temperature (phenyl, methyl, and vinyl), low
compression set (methyl and vinyl), low shrink
-55-
ICA 02283790 1999-09-09
WO 9$143710 PCT/US98/06161
(devolatilized), and solvent resistant (fluorosilicone);
properties are shown in Table 15.
The tensile strength of cured dimethylsilicone
rubber gum is only about 0.34 MPa (50 psi). Finely divided
silicas are used for reinforcement. Other common fillers
include mined silica, titanium dioxide, calcium carbonate, and
iron (III) oxide. Crystallizing segments incorporated into
the polymer also serve as reinforcement. For example, block
copolymers containing silphenylene segments,
l0 f (CH3) ZSiC6H4Si (CH3) 20~~, may have cured gum tensile strengths of
6.8-18.6 MPa (1000-2700 psi).
TABLE 15
Properties of Silicone Gums
Williams
Density d25, Plasticity
Tie g~~3 T~, C (ASTM D926)
(CH3) 2Si0 0.98 - 123 95-125
CH3 (C6H5) Si0 0. 98 - 113 135-180
CH3 (CF3CHZCH2) Si0 1 .25 - 65
Consistencies of uncured rubber mixtures range from
a tough putty to a hard deformable plastic. Those containing
reinforcing fillers tend to stiffen, i.e., develop structure,
on storage. Additives, such as water, diphenylsilanediol,
dimethylpinacoxysilane, or silicone fluids, inhibit
stiffening. - --
The properties of fabricated rubber depend on the
gum, filler, catalyst, additives, and solvents and their
-56-
._
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
proportions. A high filler content increases hardness and
solvent resistance and reduces elongation. The properties
also depend on the thoroughness of mixing and the degree of
wetting of the filler by the gum. The properties change as
cure progresses and are stabilized by devolatilization. The
properties may also be affected by the environment and aging.
Before being used, silicone rubber mixtures are
preferably freshened. Catalyst is added, and the mixture is
freshly milled on rubber mills until the components band into
smooth continuous sheets that are easily worked. Specific or
custom mixtures are prepared by suppliers for particular
product applications. A formula is designed to achieve some
special operating or processing requirement, and formulations
are classified accordingly as set forth below in Table 16.
-57-
ICA 02283790 1999-09-09
WO 98/43710 PCT/US98I06161
h
H °°
m ~~ a7 a~ ,-, M M
I
0 o M ..a o0
N
H
O O O UT O N
lD l0 lfl .-i CO M
N N N M N N
d
H
~
U
ro
a d ~ ~
m .
a~
m
~ U
do
r O
-1 0
U
u, a
k~N o u~ o 0 0 0
f ro ~ ~ I I I I
~ I -
I
, ~
,
, y n o 0 0 0 0
p ,-Irl N ~ N N
U
~
W
t0
O
r1 0 0 0 0 o u7 0
U
~ 0 0 0 0 o N o
-rl
~ C' c~ l0 ~f1~ N I~
doI
~ ~ ~
H O O 00 O O O
r~ '-i .-1N r-I'-1117
M M O
O O l0 O
e~l ~
b~
ro
~ r r
J-J O ~ I 1 I I I 1 I
m O ~ ~ ~ m
Q) Ei ~, c u'7v c W O~
~J
H
a
m
a
+t
ro
m 0 0 0 0 0 0 0
.~
~
a ' m o m oo ~ u7
a
1 I I 1 I 1
0 0 X170 0 0 0
c tf1N c tf7tf7sT
N N
O 1~
b
1~ 27 S'-I
N Y.IO E
UJ N Oa ~ 10
O. ~ G -a
a a~ a~ ~ a7 ro w
a m o v +~ a~
ro n m ~ ~ ~ ~
l
. .~ - .
u
- _ _.
V L.IU7 3 CP t0 tn t7~
C Ul O -.-IU N C
O. >a ~-I.G 1N N -a '
CL -O N C
.i ~ v a~ G ~ +~ ro
ro o ~ ~ ro c v~ 'u
S-IU N N ~ N
H H N > ,C b
C 3 .~.~~ s-~~ O~ +~
a~ o x x -.~o -.~ a~
c~ a w w ~ v~ x H
En o
_ ~g _
SUBSTITUTE SHEET (RULE 26)
,. _
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
Silicone rubbers are cured by several mechanisms.
For hydrosilation cure, high molecular weight polymers (gums)
with vinyl functionality are combined with fluid hydride-
functional cross-linking agents. The catalyst, such as a
soluble platinum compound, is added with an inhibitor to
prevent cure initiation before heating.
Silicone rubber is usually cured by heating the
reinforced polymer with a free-radical generator, e.g.,
benzoyl peroxide.
Cure is also effected by gamma or high energy
electron radiation, which causes scission of all types of
bonds, including Si-O; the important cure reactions and those
involving Si-C and C-H. Hydrogen, methane, and ethane evolve,
and bridges between chains are formed by recombination of the
radicals generated. These bridges include Si-CHZ-Si,Si-Si, and
perhaps Si-CHZCHZ-Si. An absorbed dose of 770-1300C/kg (3 x
106 to 5 x 106 roentgen) is typically required for effective
cure. Radiation cure can be used for thick sections, but high
energy electrons penetrate to a depth of only a few
millimeters.
Freshly mixed silicone rubber compounds are usually
molded at 100-180°C and 5.5-10.3 MPa (800-1500 psi). Under
these conditions, thermal cure can be completed in minutes.
The molds are usually lubricated with a 1-2 wt~ aqueous
solution of a household detergent. Final properties can be
developed by oven curing or by continuous steam vulcanization.
_59_
CA 02283790 1999-09-09
WO 98/43710 PCTIUS98/06161
For bonding silicone rubber to other materials, such
as an interior layer or core in the golf balls of the present
invention, primers are preferably used, including silicate
esters, silicone pastes, silicone resins, or reactive silanes.
After evaporation of solvent and setting or cure of the primed
surface, the rubber compounds are applied and cured under
pressure. Self-bonding silicone rubber stocks require no
primer.
Silicone rubber is compounded in dough mixers,
Banbury mixers, two-roll rubber mills, various types of
change-can mixers, and continuous compounders. Large vertical
Banbury mixer systems are used for high volume semicontinuous
production of dry (but not overly tacky) compounds; tackiness
can create problems in unloading. The basic process
requirements are similar in nearly all applications: addition
of gums, fillers, process aids, pigments, and catalysts in the
prescribed order; breakdown of agglomerates in the fillers;
uniform dispersion of filler in the gum; and control of
temperature and, in some cases, pressure for retention or
removal of volatile ingredients and prevention of premature
cure.
The properties of cured silicone elastomers are
temperature dependent. For example, Young's modulus decreases
from about 10,000 to 200 MPa (145 x 10" to 2.9 x 104 psi)--
between -50 and 25°C and remains fairly constant to 260°C.
Tensile strength decreases from approximately 6.9 MPa (1000
-60-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
psi) at 0°C to 2.1 MPa (300 psi) at 300°C. The thermal
conductivity of silicone rubber is usually about 1.5-4 W/(m~K)
and increases with increasing filler content.
Silicone rubber (gum) films are permeable to gases
and hydrocarbons; they are about 10-20 times as permeable as
organic polymers. Water diffuses through lightly cross-linked
gum as monomer, dimer, and trimer, with diffusion coefficients
of 1.5, 3.6, and 3.1 x 10-S, respectively, at 65°C. Silicone
rubber compounds are also permeable to gases. Cross-linking
and fillers reduce permeability.
Solvents diffuse into silicone rubber and swell,
soften, and may result in weakening of the rubber. The degree
of swelling depends on the solvent and has been correlated
with the solubility parameters of solvent and rubber. The
correlation is improved if electrostatic interactions are
considered.
Silicone elastomers appear completely hydrophobic to
liquid water. Aqueous solutions interact with silicone rubber
with varying effects. Water itself has little effect,
although at higher temperatures it causes softening and
weakening. If the rubber is heated with water in a sealed
environment, it is converted to a sticky polymer.
In contrast to the silicone fluids and elastomers,
silicone resins contain Si atoms with no or only one organic
substituent. They are therefore crosslinkable to harder and
stiffer compounds than the elastomers, but many must be
-61-
ICA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
handled in solution to prevent premature cure. They are, in
fact, usually made by hydrolysis of the desired chlorosilane
blend in the presence of a solvent such as mineral spirits,
butyl acetate, toluene, or xylene. These materials are
usually cured with metal soaps or amines.
As noted, silicone resins are highly cross-linked
siloxane systems. The cross-linking components are introduced
as trifunctional or tetrafunctional silanes in the first stage
of manufacture or processing. For example, a solution of
CH3SiC13, (CH3) z, SiCl2, C6HSSiCI3, and (C6H5) zSiCl2 or CH3 (C6H5) SiClz
in toluene is hydrolyzed to form a complex copolymer mixture,
which remains in solution in toluene. The aqueous
hydrochloric acid is separated, and the resin solution is
washed and heated in the presence of a mild condensation
catalyst to adapt (body) the resin to the proper viscosity and
cure time. It is finally adjusted to specifications by
distilling or adding solvents. The properties of the resins
depend on the choice of chlorosilanes, the degree of cure, and
the processing conditions.
The chlorosilanes for a particular resin formulation
determine its characteristics. Monomethyl-, dimethyl-,
monophenyl-, diph~yl-, methyl-phenyl-, monovinyl-, and
methylvinylchlorosilanes, together with silicon tetrachloride,
are typical chlorosilanes. Prediction of specific resin-
properties as a function of composition is difficult since
processing and cure influence the final molecular
-62-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
configuration and related characteristics. However, some
generalizations can be made: trifunctional siloxy units
produce harder, less flexible resins, which are frequently
immiscible with organic polymers; difunctional siloxy units
increase softness and flexibility, and phenylsiloxanes are
more miscible with organic polymers than methylsiloxanes and
produce resins that are less brittle and have superior thermal
resistance. Alkyl groups other than methyl also increase the
compatibility with other organic materials. The effects of
silanes on the properties of a film are shown in Table 17.
Properties of these silanes vary considerably. Some resins
are soft and flexible, and others are hard and glassy.
Processing conditions vary from hydrolysis in strong acid to
dilute acid or buffered aqueous systems. Alkoxysilanes can
also be used to avoid acid conditions. Solvent, temperature,
concentration, and catalyst for bodying and curing affect the
result.
TABLE 17
Effe ct of Silane s on Propertie s
the
of Silicon e Resin Films
ProoertY CH S, C~HtSiCI, (CHI?SiCl2(C,:H~)~SiCt,CH.,(C~HS)SiCI,
iCt~,
Hardiness increase increase decreasedecrease decrease
Brittleness increase great increasedecreasedecrease decrease
2 5 Stiffness increase increase decreasedecrease decrease
Toughness increase increase decreasedecrease decrease
Cure Speed much fastersome increaseslower much slowerslower
Tack decrease some decreaseincreaseincrease increase
- --
Most silicone resin products require heat and
catalysts for curing. During the life of the product, curing
-63-
CA 02283790 1999-09-09
WO 98!43710 PCTIU598/06161
continues, and properties change with time. For this reason,
silicone resins exhibiting this characteristic are generally
less preferred than silicone elastomers and rubbers described
herein.
Silicone resins are cured through the formation of
siloxane linkages by the condensation of silanols. This is a
continuation of the overall condensation process by which the
resin is prepared. As condensation continues, the rate
decreases because of lower silanol concentration, increased
steric hindrance, and reduced mobility. For final cure,
therefore, the reaction must be accelerated by heat and
catalyst. Even so, some silanols remain, and slow cure
continues for the life of the resin. The reaction is
reversible, and water must be removed from the system to
permit a high degree of cure. Many substances catalyze
silanol condensation, including acids and bases; soluble
organic salts of lead, cobalt, tin, iron, and other metals and
organotin compounds, e.g., dibutyl tin dilaurate, or
N,N,N',N'-tetramethylguanidine salts.
Silicone resins based on hydrosilation cure have
also been developed. These materials cure by addition
reactions and are similar in composition to hydrosilation-
curing elastomers, however are generally more highly cross-
linked.
Silicone resins change little on exposure to
humidity, heat, and sunlight. Weather resistance is also
exhibited by silicone--organic copolymers and blends, provided
the silicone content is high enough.
-64-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
A variety of commercially available silicone resins
may be utilized in the preferred embodiment golf balls. For
example, silicone resins can be obtained from Dow Corning
Corp., Midland, MI; GE Silicones, Waterford, NY; Gelest Inc.,
Tullytown, PA; blacker Silicones, Adrian, MI; and Shin-Etsu
Chemical Co., Ltd, Tokyo 100, Japan.
A particularly preferred commercial supplier of
silicone resin is Shin-Etsu. Shin-Etsu offers a two-
component, high strength molding compound under the
designation KE 1300, that provides excellent resin resistance
and will not shrink when cured at room temperature.
KE 1300 features high tear strength. It is ideal
for intricate molds, or applications where tearing or ripping
of a mold is a concern. KE 1300 is available in a T
(translucent) and a white version. Properties and mold life
will be the same with both. The translucent version (KE
1300T) is very useful for applications where visual sighting
of the master or where identification of voids is needed.
KE1300T and KE 1300 (white) are preferred whenever resistance
to attack by epoxies, polyesters and urethanes and high tear
strength in a medium modulus material is required.
Whenever Catalyst 1300L-3 is used to cure KE 1300 or
KE 1300T, a lower modulus material results without seriously
effecting tear strength. This is appropriate for those
applications where demolding is a problem due to deep
undercuts or thick cross-sections.
Other suitable silicone resins available from Shin-
Etsu are set forth below in Table 7. The noted KE 1402, SES
-65-
'CA 02283790 1999-09-09
WO 98/43710 PCTIUS98/06161
412, KE 10 are all condensation cure products. 'The noted
and
KE 1300T, KE 1300, KE 1330ST, KE 13105,
KE 1600, and KE 1604
are alt addition cure products. These
addition cure products
can be is desired. For
heat
accelerated
if
a
faster
cure
example, a heat cure for 2 hours at 60C can be performed at
1
hour 85C.
at
TABLE 18
General
Characteristics of
Commercially Available Silicone Compositions
Product Color Description Pot Life Catalyst
(Hrs)
KE 1402 Pink Low durometer, high 1.5 CAT 1402
strength inhibition
resistant
SES 412 White Medium durometer, low 0.5 CAT RM
viscosity, general
purpose
KE l0 Off-white High durometer, low 1.0 CAT RA
viscosity, general
purpose
1 KE 1300TTranslucent Low durometer, 1.5 CAT 1300L-3
5
high strength
KE 1300TTranslucent Medium durometer, 1.5 CAT 1300
high strength
KE 1300 White Medium durometer, 1.5 CAT 1300
high strength, opaque
KE 1310STTranslucent Premium strength, 2.0 CAT 1310
longest mold life
KE 1310SWhite Premium strength, 2.0 CAT 1310
longest mold life,
opaque
2 KE 1600 Off-white Medium durometer, 2.0 CAT 1300
0
general purpose
KE 1604 Blue High durometer, 2.0 CAT 1604
general purpose
KE 1604 Off-white High durometer, 2.0 CAT 1604T
general purpose,
neutral color
-66-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
TABLE iB (cont.)
Physical
Properties of Commercial~rAvailable
Silicone Compositions
Product Initial HardnessTensile 8longa-Linear Tear Specific
Mixed Durometer Strength tion Shrinkage StrengthGravity
Viscosity (Shore-A)(psi) t (t) (ppli)
(poise)
KE 1902 600 25 600 400 0.4 120 1.10
SES 412 100 40 355 160 0.2 45 1.30
KE 10 300 55 480 150 0.1 45 1.15
KE 1300T 1000 30 630 400 <0.1 110 1.11
1 KE 1300T 1000 40 800 300 <0.1 125 1.11
0
KE 1300 1000 40 800 300 <0.1 125 1.11
KE 1310ST840 40 850 340 <0.1 190 1.07
KE 13105 840 40 850 340 <0.1 140 1.07
KE 1600 1700 50 1000 200 <0.1 80 1.26
1 KE 1604 1000 60 1100 170 <0.1 95 1.26
KE 1604 1000 60 1100 170 <0.1 95 1.26
TABLE 18 (cont.)
Curing
Properties of
20 Commerciall y Available
Silicone Resins
Product Base Curing
Agent Ratio Cu ring Hr/'C
by WT.
KE 1402 10:1 24/25
SES 100:0.5 24/25
412
KE 10 100:2.5 24/25
2 KE 1300T 10:1 24/25
5
KE 1300T 10:1 24/25
KE 1300 10:1 24/25
KE 1310ST10:1 24/25
KE 13105 10:1 24/25
3 KE 1600 10:1 24/25
0
KE 1604 10:1 24/25
KE 1604 10:1 24/25
-67-
'CA 02283790 1999-09-09
WO 98/43710 PCT/US98106161
When utilizing a two part, addition cure, silicone
resin, typical properties of the components and cured
compositions are as follows:
TABLE 19
Typical Pr~erties of Two Part, Silicone Resins
Part A Part B Mixed A/B
Appearance Milky-white Milky-White Milky-White
Translucent Translucent Translucent
Specific Gravity, 1.08 + 0.04 1.08 +0.04 1.08 +0.04
Q 25
Viscosity, Q 25 500-1,OOOP 1-50P 500 t250P
Cured Properties
(Cure Condition: 15
min. O 150°C):
Hardness, Shore 00 70115
Tensile Strength, psi 450~150
1 5 Elongation, 0 5001150
Preferably, the silicone material utilized in the
preferred embodiment golf balls exhibits, upon curing, a Shore
00 hardness of from about 55 to about 100; a tensile strength
0 of from about 300 psi to about 600 psi; and an elongation of
from about 350% to about 6500.
As noted, the present invention golf balls may
comprise one or more interior layers comprising one or more
silicone materials. Referring to Figure 3, a preferred
25 embodiment golf ball 20 is illustrated comprising a core 22
formed from a material as described herein, and an interior _
layer 24 formed from one or more silicone material(s). The
interior layer 24 is disposed between the core 22 and an outer
-68-
T...._.... 1.
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
layer 26. The outer layer 26 may be in the form of the
previously described multilayer cover 12.
In another preferred embodiment, the present
invention provides a golf ball 30 as shown in Figure 4. The
golf ball 30 comprises a core 32, formed from a material as
described herein, and two inner layers, such as 34 and 36.
Either or both of the inner layers 34 and 36 may be formed
from a silicone material. The golf ball 30 may further
comprise an outer layer 38 similar to the most multilayer
cover 12.
In yet another preferred embodiment, the present
invention provides a golf ball 40 as shown in Figure 5. The
golf ball 40 comprises a core 42, formed from a material as
described herein, and three or more inner layers such as
layers 44, 46, and 48, that may be formed from a silicone
material. The golf ball 40 may further comprise an outermost
layer 49 similar to the previously described multilayer cover
12.
Although not wishing to be bound to any particular
dimensions, the present inventors have determined that the one
or more silicone layers preferably have the following
dimensions and characteristics. When utilized in conjunction
with a core of at least about 1.20 inches in diameter or
greater, the total thickness of the silicone layers is at
least about 0.020 inches or greater. The golf balls may
utilize one or more silicone layers, however it is preferred_
to provide at least two or more. If the silicone layers are
used in combination with one or more layers of a non-silicone
-69-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
composition, it is preferred that the thickness of the non-
silicone layers be at least about 0.020 inches or greater.
Examples of such non-silicone materials include, but are not
limited to, relatively hard, resilient materials such as
ionomers, nylons, thermoplastic urethanes, and hytrels for
instance. The minimum total thickness of all layers within
the preferred embodiment golf balls is about 0.040 inches.
The preferred total thickness of all the silicone layers is
about 0.050 inches.
As previously noted, the preferred embodiment golf
balls of the present invention may further comprise a core
comprising a silicone composition. Such material is
preferably selected from the previously noted silicone
materials. A particularly preferred core composition is based
upon blends of ionomers as described herein and a commercially
available silicone rubber, Dow Corning Silastic rubber WC-50.
Silastic WC-50 comprises a low level of vinyl groups and has a
specific gravity of about 1.15 and a brittleness temperature
of about -39°C.
Referring to Figures 6 - 8, several additional
preferred embodiment golf balls are illustrated comprising
cores including a silicone material and one or more inner
layers comprising materials described herein. Figure 6
illustrates a preferred embodiment golf ball 50 comprising a
core 52 including a silicone material, an inner layer 54, and
an outer cover 56. The outer cover 56 may be in the form of_
the previously described multilayer cover 12.
-70-
~ .
CA 02283790 1999-09-09
WO 98/43710 PCT/U898/06161
The invention also provides another preferred
embodiment golf ball 60 illustrated in Figure 7 comprising a
core 62 formed from a silicone material, a first inner layer
64, a second inner layer 66, and an outer cover 68. The outer
cover 68 may be in the form of the previously described
multilayer cover 12.
Figure 8 depicts another preferred embodiment golf
ball 70 comprising a core 72, a plurality of inner layers 74,
76, and 78, and an outer cover 79. The core 72 comprises a
silicone material. The outer cover 79 may be in the form of
the previously described multilayer cover 12.
The core has a preferred set of characteristics as
follows. The silicone core is preferably from about 1.10
inches to about 1.60 inches in diameter. When utilizing a
silicone composition core, the mantle (or one or more interior
layers? thickness is from about 0.020 inches to about 0.145
inches. And, the cover thickness is from about 0.020 inches
to about 0.145 inches. The ball diameter is preferably from
about 1.68 inches to about 1.75 inches or more in diameter.
When utilizing a silicone core, the golf ball preferably
includes at least two or more layers. The mantle and/or cover
layers may be formed from a relatively hard resilient
materials such as for example, ionomers, nylons,
polyurethanes, polyester elastomers, etc.
Moreover, the present invention provides golf balls
having both a core formed from a silicone material and one ox
more inner layers formed from a silicone material. The
-71-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
configuration or structure of such balls may be as depicted in
Figs. 1-8.
In preparing golf balls in accordance with the
present invention, a hard inner cover layer is molded (for
instance by injection molding or by compression molding) about
a core (preferably a solid core). A comparatively softer
outer layer is molded over the inner layer. The conventional
solid core is about 1.545 inches in diameter, although it can
range from about 1.495 to about 1.575 inches. Conventional
solid cores are typically compression molded from a slug of
uncured or lightly cured elastomer composition comprising a
high cis content polybutadiene and a metal salt of an a, Vii,
ethylenically unsaturated carboxylic acid such as zinc mono or
diacrylate or methacrylate. To achieve higher coefficients of
restitution in the core, the manufacturer may include fillers
such as small amounts of a metal oxide such as zinc oxide. In
addition, larger amounts of metal oxide than those that are
needed to achieve the desired coefficient are often included
in conventional cores in order to increase the core weight so
that the finished ball more closely approaches the U.S.G.A.
upper weight limit of 1.620 ounces. Other materials may be
used in the core composition including compatible rubbers or
ionomers, and low molecular weight fatty acids such as stearic
acid. Free radical initiators such as peroxides are admixed
with the core composition so that on the application of heat
and pressure, a complex curing cross-linking reaction takes _
place.
-72-
r i
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
The inner cover layer, such as layer 14 of the
multilayer cover 12, which may be molded over the core or
another interior layer, is about 0.100 inches to about 0.010
inches in thickness, preferably about 0.0375 inches thick.
The outer cover layer, such as layer 16 of the multilayer
cover 12, is about 0.010 inches to about 0.050 inches in
thickness, preferably 0.0300 inches thick. Together, the
core, the inner cover layer and the outer cover layer combine
to form a ball having a diameter of 1.680 inches or more, the
minimum diameter permitted by the rules of the United States
Golf Association and weighing about 1.620 ounces.
Additional materials may be added to the cover
compositions (both inner and outer cover layer) of the present
invention including pigments (For example, Ultramarine Blue
sold by Whitaker, Clark and Daniels of South Plainsfield,
N.J.) (see U.S. Patent No. 4,679,795); and pigments such as
titanium dioxide, zinc oxide, barium sulfate and zinc sulfate;
optical brighteners; and UV absorbers; antioxidants;
antistatic agents; and stabilizers. Further, the cover
compositions of the present invention may also contain
softening agents, such as plasticizers, processing aids, etc.
and reinforcing material such as glass fibers and inorganic
fillers, as long as the desired properties produced by the
golf ball covers are not impaired.
As previously described with regard to silicone
elastomers, it may, in some instances, be preferred to
incorporate one or more filler agents in the one or more inner
layers comprising silicone materials. It may also be
-73-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/061b1
desirable to incorporate such agents in a silicone core.
Incorporating such agents may serve to reinforce that
resulting silicone composite material, and/or alter other
physical properties of the layer and/or core. The use of such
agents may serve to increase, or in some cases, decrease, one
or more of the following properties: hardness, strength,
rigidity, elasticity, and density. With regard to increasing
the density of a silicone material utilized in the present
invention golf balls, it is particularly preferred to
l0 incorporate such agents, particularly those having a
relatively high density, in a silicone layer in order to
increase the weight and moment inertia of the ball. Examples
of suitable filler and/or weighting agents include, but are
not limited to, particulate silica; fumed silica; particulate
aluminum silicate or other similar materials; carbon black
or graphite in fiber or powder form; boron in powder or salt
form; Kevlar in fiber form; cotton flock; nylon flock; glass
in nearly any form; ceramic materials in nearly any form;
Cermet, i.e., ceramic-metal, materials in any form; Hi-Sil;
and metals in any form. Other compounds may be used such
as calcium carbonate, various clays, and plastics such as
ground polypropylene. Regarding the use of metals, nearly any
metal, preferably in fine particulate form, may be utilized.
Examples of suitable metals include aluminum, magnesium,
beryllium, iron, titanium, tungsten, copper, zinc, and alloys
or oxides thereof. Examples of such alloys include brass or_
bronze. It is also contemplated to utilize other materials as
filler, weighting, or reinforcing agents such as metal
-74-
. r. ~
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
stearate salts, silicon carbide, ceramic whiskers, and
combinations thereof. Furthermore, the present inventors have
identified several preferred metallic compounds and
combinations of materials for incorporation in the one or more
silicone material layers and/or core. These preferred
combinations include, but are not limited to: beryllium
oxide, aluminum oxide, titanium dioxide and graphite powder,
titanium and ceramic, and combinations thereof.
The various cover composition layers of the present
invention may be produced according to conventional melt
blending procedures. In the case of the outer cover layer,
when a blend of hard and soft, low acid ionomer resins are
utilized, the hard ionomer resins are blended with the soft
ionomeric resins and with a masterbatch containing the desired
additives in a Banbury mixer, two-roll mill, or extruder prior
to molding. The blended composition is then formed into slabs
and maintained in such a state until molding is desired.
Alternatively, a simple dry blend of the pelletized or
granulated resins and color masterbatch may be prepared and
fed directly into the injection molding machine where
homogenization occurs in the mixing section of the barrel
prior to injection into the mold. If necessary, further
additives such as an inorganic filler, etc., may be added and
uniformly mixed before initiation of the molding process. A
similar process is utilized to formulate the high acid ionomer
resin compositions used to produce the inner cover layer.
The golf balls of the present invention can be produced,
at least in part, by molding processes currently known in the
-75-
CA 02283790 1999-09-09
WO 98/43710 PCT/I1S98I06161
golf ball art. Specifically, the golf balls can be produced
by injection molding or compression molding the inner cover
layer about wound or solid molded cores to produce an
intermediate golf ball having a diameter of about 1.50 to 1.67
inches, preferably about 1.620 inches. The outer layer is
subsequently molded over the inner layer to produce a golf
ball having a diameter of 1.680 inches or more. Although
either solid cores or wound cores can be used in the present
invention, as a result of their lower cost and superior
performance, solid molded cores are preferred over wound
cores.
In compression molding, the inner cover composition is
formed via injection at about 380°F to about 450°F into smooth
surfaced hemispherical shells which are then positioned around
the core in a mold having the desired inner cover thickness
and subjected to compression molding at 200°F to 300°F For
about 2 to 10 minutes, followed by cooling at 50°F to 70°F For
about 2 to 7 minutes to fuse the shells together to form a
unitary intermediate ball. In addition, the intermediate
balls may be produced by injection molding wherein the inner
cover layer is injected directly around the core placed at the
center of an intermediate ball mold for a period of time in a
mold temperature of from 50°F to about 100°F. Subsequently,
the outer cover layer is molded about the core and the inner
layer by similar compression or injection molding techniques
to form a dimpled golf ball of a diameter of 1.680 inches or_
more.
-76-
CA 02283790 1999-09-09
WO 98143710 PCT/US98/06161
Molding or otherwise forming the silicone layers and/or
core rnay further entail additional considerations such as
follows. A silicone mantle could be applied directly over a
core, either a core comprising a silicone composition or as
otherwise described herein, or sandwiched between two or more
non-silicone layers. There are several considerations or
practices that may be followed in a preferred technique for
molding a core and/or one or more layers comprising a silicone
material.
A vessel which is pressure-rated and of adequate size to
degas the desired amount of silicone material is preferably
employed. A vacuum system is used to pull or otherwise remove
air induced during the mixing cycle from the material. This
process insures a void-free molded component.
An oven can be used to accelerate the cure rate of the
silicone material. Oven temperature should not exceed 200'C
(396'F). Most silicone molded materials should not be exposed
to elevated temperatures for more than 2 hours.
Certain chemicals, curing agents, plasticizers and
materials can inhibit cure. The most common are: organo-tin
and other organo-metallic compounds; silicone rubber
containing organo-tin catalyst; sulfur, polysulfides,
polysulfones and other sulfur-containing materials; amines,
urethanes, and amine containing materials; and unsaturated
hydrocarbon plasticizers.
Should a substrate or material be a possible cause of
inhibition, it is best to test a small sample for
compatibility with the elastomer. The presence of liquid or
_77_
CA 02283790 1999-09-09
WO 98/43710 PCT/US98106161
uncured product at the interface between the suspect substrate
and the cured elastomer would indicate cure inhibition.
After molding, the golf balls produced may undergo
various further processing steps such as buffing, painting and
marking as disclosed in U.S. Patent No. 4,911,451.
The resulting golf ball produced from the high acid
ionomer resin inner layer and the relatively softer, low
flexural modulus outer layer provide for an improved multi-
layer golf ball which provides for desirable coefficient of
restitution and durability properties while at the same time
offering the feel and spin characteristics associated with
soft balata and balata-like covers of the prior art.
Additional details of the chemistry and processing of
silicone materials are provided in "Encyclopedia of Polymer
Science and Engineering," Vol. 15, Second Edition, pages 204-
308, by B. Hardman and A. Torkelson, herein incorporated by
reference.
The present invention is further illustrated by the
following examples in which the parts of the specific
ingredients are by weight. It is to be understood that the
present invention is not limited to the examples, and various
changes and modifications may be made in the invention without
departing from the spirit and scope thereof.
Examples
Several intermediate balls (cores plus inner cover
layers) were prepared in accordance with molding procedures
described above. The inner cover compositions were molded
around 1.545 inch diameter cores weighing 36.5 grams such that
_78_
CA 02283790 1999-09-09
WO 98143710 PCT/US98/06161
the inner cover had a wall thickness of about 0.0675 inches,
with the overall ball measuring about 1.680 inches in
diameter.
The cores utilized in the examples were comprised of the
following ingredients: high cis-polybutadiene, zinc
diacrylate, zinc oxide, zinc stearate, peroxide, calcium
carbonate, etc. The molded cores exhibited Riehle
compressions of about 60 and C.O.R. values of about .800. A
representative formulation of the molded cores is set forth
below:
TABLE 20
Representative Formulation For Molded Core
MATERIAL
BR-1220 (high cis-polybutadiene) 70.70
1 Taktene 220 (high cis-polybutadiene) 29.30
5
React Rite ZDA (zinc diacrylate) 31.14
Zinc Oxide 6.23
Zinc Stearate 20.15
Limestone 17.58
2 Ground Flash 20.15
0
(20-40 Mesh)
Blue Masterbatch .012
Luperco 231XL
or Trigonox 29/40 ,gg
2 Papi 94 .50
5
'Blue Masterbatch consists of unknown compositions used only For internal
identification
purposes and has no effect on physical properties.
The inner cover compositions designated herein as
30 compositions A-E utilized to formulate the intermediate--balls
are set forth in Table 8 below. The resulting molded
intermediate balls were tested to determine the individual
_79_
CA 02283790 1999-09-09
WO 98/43710 PCTIUS98/06161
compression (Riehle), C.O.R., Shore C hardness, spin rate and
cut resistance properties. These results are also set forth
in Table 8 below.
The data of these examples are the average of twelve
intermediate balls produced for each example. The properties
were measured according to the following parameters:
Coefficient of restitution (C.O.R.) was measured by
firing the resulting golf ball in an air canon at a
velocity of 125 feet per second against a steel plate
positioned 12 feet from the muzzle of the canon. The
rebound velocity was then measured. The rebound
velocity was divided by the forward velocity to give a
coefficient of restitution.
Shore hardness was measured in accordance with ASTM test
2240.
Cut resistance was measured in accordance with the
following procedure: A golf ball is fired at 135 feet
per second against the leading edge of a pitching
wedge wherein the leading edge radius is 1/32 inch,
the loft angle is 51 degrees, the sole radius is 2.5
inches and the bounce angle is 7 degrees.
The cut resistance of the balls tested herein was
evaluated on a scale of 1 to 5. The number 1
represents a cut that extends completely through the
cover to the core. A 2 represents a cut that does not
extend completely through the cover but that does
break the surface. A 3 does not break the surface of
the cover but does leave a permanent dent. A 4 leaves
only a slight crease which is permanent but not as
severe as 3. A 5 represents virtually no visible
indentation or damage of any sort.
The spin rate of the golf ball was measured by
striking the resulting golf balls with a pitching
wedge or 9 iron wherein the club head speed is about
105 feet per second and the ball is launched at an
angle of 26 to 34 degrees with an initial velocity of
about 110 to 115 feet per second. The spin rate was
measured by observing the rotation of the ball in
flight using stop action Strobe photography.
Initial velocity is the velocity of a ball when
struck at a hammer speed of 143.8 feet per second in
accordance with a test as prescribed by the U.S.G.A.
-80-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
As will be noted, compositions A, B and C include high
acid ionomeric resins, with composition B further including
zinc stearate. Composition D represents the inner layer (i.e.
Surlyn 1605) used in U.S. Patent No. 4,431,193. Composition E
provides a hard, low acid ionomeric resin.
The purpose behind producing and testing the balls of
Table 21 was to provide a subsequent comparison in properties
with the multi-layer golf balls of the present invention.
-81-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98106161
Table 21
Molded Intermediate Golf Balls
Ingredients of
Inner Cover
Compositions A B C D E
Iotek 959 50 50 -- -- --
Iotek 960 50 50 -- -- --
Zinc Stearate -- 50 -- -- --
Surlyn 8162 -- -- 75 -- --
Surlyn 8422 -- -- 25 -- _-
Surlyn 1605 -- -- -- 100 --
Iotek 7030 -- -- -- -- 50
Iotek 8000 -- -- -- -- 50
Properties of
Molded Inter-
mediate Balls
Compression 58 58 60 63 62
C.O.R. .811 .810 .807 .793 .801
Shore C Hardness 98 98 97 96 96
Spin Rate (R.P.M. )7,367 6,250 7,903 8,337 7,956
Cut Resistance 4-5 4-5 4-5 4-5 4-5
As shown in Table 21 above, the high acid ionomer resin
inner cover layer (molded intermediate balls A-C) have lower
spin rates and exhibit substantially higher resiliency
characteristics than the low acid ionomer resin based inner
cover layers of balls D and E.
Multi-layer balls in accordance with the present
invention were then prepared. Specifically, the inner cover
compositions used to produce intermediate golf balls from
Table 21 were molded over the solid cores to a thickness of
about 0.0375 inches, thus forming the inner layer. The
diameter of the solid core with the inner layer measured about
1.620 inches. Alternatively, the intermediate golf balls of
Table 21 were ground down using a centerless grinding machine
to a size of 1.620 inches in diameter to produce an inner
cover layer of 0.0375 inches.
-82-
SUBSTITUTE SHEET (RULE 26)
CA 02283790 1999-09-09
WO 98/43710 PCT/US98106161
The size of 1.620 inches was determined after attempting
to mold the outer cover layer to various sizes (1.600",
1.610", 1.620", 1.630" and 1.640") of intermediate (core plus
inner layer) balls. It was determined that 1.620" was about
the largest "intermediate" ball (i.e., core plus inner layer)
which could be easily molded over with the soft outer layer
materials of choice. The goal herein was to use as thin an
outer layer as necessary to achieve the desired playability
characteristics while minimizing the cost of the more
expensive outer materials. However, with a larger diameter
final golf ball and/or if the cover is compression molded, a
thinner cover becomes feasible.
With the above in mind, an outer cover layer composition
was blended together in accordance with conventional blending
techniques. The outer layer composition used For this portion
of the example is a relatively soft cover composition such as
those listed in U.S. Patent No. 5,120,791. An example of such
a soft cover composition is a 45% soft/55o hard low acid
ionomer blend designated by the inventor as "TE-90". The
composition of TE-90 is set forth below in Table 22 as
follows:
TABLE 22
Outer Cover Layer Composition TE-90
Iotek 8000 22.7 weight
Iotek 7030 22.7 weight %
Iotek 7520 45.0 weight %
White MB1 9.6 weight % -_.
White MB consists of about 23.77 weight percent Ti02; 0.22
weight percent Uvitex OB, 0.03 weight percent Santonox R, 0.05
weight percent Ultramarine blue and 75.85 weight percent Iotek
7030.
-83-
'CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
The above outer layer composition was molded around each
of the 1.620 diameter intermediate balls comprising a core
plus one of compositions A-D, respectively. In addition, for
comparison purposes, Surlyn° 1855 (new Surlyn° 9020), the
cover composition of the '193 patent, was molded about the
inner layer of composition D (the intermediate ball
representative of the '193 patent). The outer layer TE-90 was
molded to a thickness of approximately 0.030 inches to produce
a golf ball of approximately 1.680 inches in diameter. The
resulting balls (a dozen balls For each example) were tested
and the various properties thereof are set forth in Table 9 as
follows:
Table 23
Finished Balls
1 Ingredients: 1 2 3 4 5
5
Inner Cover A H C D D
Composition
Outer Cover TE-90 TE-90 TE-90 TE-90Surlyn~
Composition 9020
2 Properties
0 of
Molded Finished
Balls:
Compression 63 63 69 70 61
C.O.R. .784 .778 .780 .770 .757
2 Shore C Hardness88 88 88 88 89
5
Spin (R.P.M.) 8,825 8,854 8,814 8,9908,846
Cut Resistance3-4 3-4 3-4 3-9 1-2
As it will be noted in finished balls 1-4, by creating-a
multi-layer cover utilizing resins in
the high acid
ionomer
30 the inner cover the hard/soft low acid ionomer resin
layer and
-84-
~.
CA 02283790 1999-09-09
WO 98/43710 PCT/US98106161
in the outer cover layer, higher compression and increased
spin rates are noted over the single layer covers of Table 8.
In addition, both the C.O.R. and the Shore C hardness are
reduced over the respective single layer covers of Table 8.
This was once again particularly true with respect to the
multi-layered balls containing the high acid ionorner resin in
the inner layer (i.e. finished balls 1-5). In addition, with
the exception of prior art ball 5 (i.e. the '193 patent),
resistance to cutting remains good but is slightly decreased.
l0 As noted above, the prior art ball of the '193 patent suffers
substantially in durability (as well as in resiliency) in
comparison to the balls of the present invention.
Furthermore, it is also noted that the use of the high
acid ionomer resins as the inner cover material produces a
substantial increase in the finished balls overall distance
properties. In this regard, the high acid ionomer resin inner
covers of balls 1-3 produce an increase of approximately 10
points in C.O.R..over the low acid ionomer resin inner covers
of balls 4 and about a 25 point increase over the prior art
balls 5. Since an increase in 3 to 6 points in C.O.R. results
in an average increase of about 1 yard in distance, such an
improvement is deemed to be significant.
Several other outer layer formulations were prepared and
tested by molding them around the core and inner cover layer
combination to form balls each having a diameter of about 1.6B
inches. First, B.F.Goodrich Estane~ X-4517 polyester _
polyurethane was molded about the core molded with inner layer
cover formulation A. DuPont Surlyn~9020 was molded about the
-85-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
core which was already molded with inner layer D. Similar
properties tests were conducted on these golf balls and the
results are set forth in Table 24 below:
Table 24
Finish Balls
Ingredients: 6 7
inner Cover Layer
Composition A D
Outer Cover Layer
1 0 Composition Estane~ 4517 Surlyn~ 9020
Properties of
Molded Finished
Balls:
Compression 67 61
1 5 C.O.R. .774 .757
Shore C Hardness 74 89
Spin (R.P.M.) 10,061 8,846
Cwt Resistance 3-4 1-2
The ball comprising inner layer formulation D and
20 Surlyn~ 9020 identifies the ball in the Nesbitt 4,431,193
patent. As is noted, the example provides For relatively high
softness and spin rate though it suffers from poor cut
resistance and low C.O.R. This ball is unacceptable by
today's standards.
25 As for the Estane° X-4517 polyester polyurethane, a
significant increase in spin rate over the TE-90 cover is
noted along with an increased compression. However, the
C.O.R. and Shore C values are reduced, while the cut
resistance remains the same. Furthermore, both the Estane~ X-
30 4517 polyester polyurethane and the Surlyn° 9020 were
relatively difficult to mold in such thin sections.
-86-
r i
CA 02283790 1999-09-09
WO 98/43710 PCTIUS98/06161
In yet another series of experiments, golf ball cores
comprising a silicone material were formed in accordance with
the present invention. Shinetsu X-832-071-1 silicone material
(base and catalyst) was obtained and a silicone molding
material was prepared. The material was degased for
approximately 7 to 10 minutes. The flowable material was then
transferred into hemispherical, or nearly so, molding
cavities. The molding cavities are teflon coated. An excess
of material was deposited into each molding cavity to form a
positive miniscus. The molds, filled with silizone molding
material, are placed in an oven for about 4 to about 6 minutes
until the skins form on the silicone material. Each of the
molded silicone hemispheres are then joined to another
corresponding silicone molded hemisphere. Registraiton and
placement of the molded halves may be controlled by
conventional clamping assemblies. While compressed together,
the molded assembly is returned to the oven for approximately
minutes. After sufficient curing, the molded assembly is
cooled by immersion or spraying with cool water. The Shore 00
20 hardness of the resulting molded core was 98.
A silicone layer, molded about a core, was formed as
follows in accordance with the present invention. Shinetsa X-
832-071-1 silicone molding material was appropriately prepared
and degassed. The flowable material was transferred into
25 hemispherical, teflon coated molding cavities. Each mold is
filled until about one-third full. The partially filled molds
are placed in an oven and heated until a akin forms upon the
silicone molding material. The molds are then removed from
_87_
CA 02283790 1999-09-09
WO 98/43710 PCTIUS98/06161
the oven. A preformed core, either conventional or a silicone
core as described herein, is then appropriately positioned
within each mold, centered and partially contacting the
skinned silicone material. The core is preferably pressed
downward into the mold until the silicone material raises,
preferably to the top (or brim) of the hemispherical molding
cavity. Another partially filled mold containing skinned
silicone material, is then placed over the core and other mold
half. Conventional clamping assemblies may be employed to
ensure proper registration of the halves. The resulting
assembly is then placed in an oven for approximately 25
minutes until the silicone layer is sufficiently cured. The
molded product is removed and cooled with water. The Shore 00
hardness was 100.
In yet another series of experiments, golf balls in
accordance with the present invention were prepared as set
forth below and in Table 25.
Silicone base and catalyst were thoroughly mixed and
then degassed for 7-10 minutes. Mixed material was poured
into 1.40" diameter half shells and these were place in an
oven to skin over. They were then removed and the two halves
were clamped together to form a whole core and placed back in
the oven for final cure. After curing, a 1.57" diameter
mantle layer and core was formed via injection molding and
then balls were molded in two sizes, 1.71" and 1.72". Static
data was measured after each stage and is listed below.
-88-
CA 02283790 1999-09-09
WO 98/43710 PCT/US98/06161
TABLE 25 '
Multi-Lakrer Golf Ball With Silicone Rubber Core
Core Example A Example B
Material Shinetsu X-832-071-1 Shinetsu X-832-071-1
Size 1.400" 1.400"
Weight 25.2 g 25.2 g
Shore 00 82 82
100" Drop Rebound 54" 54"
Mantle or Interior
Layer
Materials
Iotek 1002 50 pph 50 pph
Iotek 1003 50 pph 50 pph
Size 1.57" 1.57"
Thickness 0.085" 0.085"
Weight 34.5 g 34.5 g
Comp *1 *1
COR 643 643
Shore D 70-71 70-71
2 Molded Balls
0
Materials
Iotek 1002 38 pph 38 pph
Iotek 1003 52.6 pph 52.6 pph
TG MB 9.4 pph 9.4 pph
2 Size 171" 1.72"
5
Cover Thickness 0.070 0.075"
Weight 43.2 44.1
Comp 90 90
COR 728 731
3 Shore D 70 70
0
The invention has been described with reference to the
preferred embodiment. Obviously, modifications and
alterations will occur to others upon reading and
understanding the proceeding detailed description. It-is
_89_
CA 02283790 1999-09-09
WO 98143710 PCT/US98/06161
intended that the invention be construed as including all such
modifications and alterations insofar as they come within the
scope of the appended claims or the equivalents thereof.
-90-
r ,