Note: Descriptions are shown in the official language in which they were submitted.
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
Fused 1 2 4 Thiadiazine nPrivatives tt,Pir PrPi~a~atic,~
The present invention relates to fused 1,2,4-thiadiazine derivatives, to
methods for their
preparation, to compositions comprising the compounds, to the use of these
compounds
as medicaments and their use in therapy e.g. in the treatment of diseases of
the central
nervous system, the cardiovascular system, the pulmonary system, the
gastrointestinal
system and the endocrinological system.
BACKGROUND OF T E INVENTION
Potassium channels play an important role in the physiological and
pharmacological
control of cellular membrane potential. Amongst the different types of
potassium channels
~ 5 are the ATP-sensitive (K,,~-) channels which are regulated by changes in
the intracellular
concentration of adenosine triphosphate. The KA-~-channels have been found in
cells from
various tissues such as cardiac cells, pancreatic cells, skeletal muscles,
smooth muscles,
central neurons and adenohypophysis cells. The channels have been associated
with
diverse cellular functions for example hormone secretion (insulin from
pancreatic beta-
2o cells, growth hormone and prolactin from adenohypophysis cells),
vasodilation (in smooth
muscle cells), cardiac action potential duration, neurotransmitter release in
the central
nervous system.
Modulators of the KAY-channels have been found to be of importance for the
treatment of
25 various diseases. Certain sulphonylureas which have been used for the
treatment of non-
insuiin-dependent diabetes mellitus act by stimulating insulin release through
an inhibition
of the KqTp -channels on pancreatic beta-cells.
The potassium channel openers, which comprise a heterogeneous group of
compounds,
3o have been found to be able to relax vascular smooth muscles and have
therefore been
used for the treatment of hypertension.
In addition, potassium channel openers can be used as bronchodilators in the
treatment of
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
2
asthma and various other diseases.
Furthermore, potassium channel openers have been shown to promote hairgrowth,
and
have been used for the treatment of baldness.
Potassium channel openers are also able to relax urinary bladder smooth muscle
and
therefore, can be used for the treatment of urinary incontinence. Potassium
channel
openers which relax smooth muscle of the uterus can be used for treatment of
premature
labor.
By acting on potassium channels of the central nervous system these compounds
can be
used for treatment of various neurological and psychiatric diseases such as
Alzheimer,
epilepsia and cerebral ischemia.
Further, the compounds are found to be useful in the treatment of benign
prostatic
hyperplasia, erectile dysfunction and in contraception.
Compounds of the present invention, which inhibit insulin secretion by
activating
potassium channels of the beta-cell can be used in combination with other
compounds
which may be used to treat non-insulin dependent diabetes mellitus and insulin
dependent
diabetes mellitus. Examples of such compounds are insulin, insulin
sensitizers, such as
thiazolidinediones, insulin secretagogues, such as repaglinide, tolbutamide,
glibenciamide
and glucagon like peptide ( GLP1), inhibitors of a-glucosidases and hepatic
enzymes
responsible for the biosynthesis of glucose.
Recently, it has been shown that Diazoxide (7-chloro-3-methyl-2H-1,2,4-
benzothiadiazine
1,1-dioxide) and certain 3-(alkylamino)-4H-pyrido[4,3-a]-1,2,4-thiadiazine 1,1-
dioxide
derivatives inhibit insulin release by an activation of KA,p-channels on
pancreatic
beta-cells (Pirotte B. et al. 6iochem. Pharmacol, 47, 1381-1386 (1994);
Pirotte B. et al., J.
Med. Chem., ~, 3211-3213 (1993}. Diazoxide has furthermore been shown to delay
the
onset of diabetes in BB-rats ( Vlahos WD et al. Metabolism 40, 39-46 (1991)).
In obese
zucker rats diazoxide has been shown to decrease insulin secretion and
increase insulin
receptor binding and consequently improve glucose tolerance and decrease
weight gain
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
3
(Alemzadeh R. et al. Endocrinol. ~, 705-712, 1993). It is expected that
compounds
which activate KA,.P-channels can be used for treatment of diseases
characterised by an
overproduction of insulin and for the treatment and prevention of diabetes.
EP 618 209 discloses a class of pyridothiadiazine derivatives having an alkyl
or an
alkyfamino group in position 3 of the thiadiazine ring. These compounds are
claimed to be
agonists at the AMPA-glutamate receptor.
In J. Med. Chem. 1980, 23, 575-577 the synthesis of 4(5)-amino-and
formylaminoimidazo-
5(4) carboxamide and their properties as agents of chemotherapeutic value are
described.
Especially, the compounds 3-aminoimidazo[4,5-a]-1,2,4-thiadiazine 1,1-dioxide
and N-
benzoylaminoimidazo[4,5-a]-1,2,4-thiadiazine 1,1-dioxide are shown.
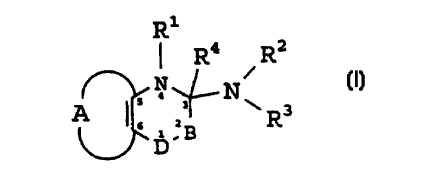
DESCRIPT10N OF THE INVENTION
The present invention relates to fused 1,2,4-thiadiazine and fused 1,4-
thiazine derivatives
of the general formula I:
Ri
R4 Ra
N
4
6 ~ :B R
D
(! )
wherein
B represents >NR$ or >CRSRs, wherein R5 and R6 independently are hydrogen;
hydroxy;
C,_6-alkoxy; or C,~-alkyl, C~.6-cycloalkyl, CZ_s-alkenyl or C2.~-alkynyl
optionally mono- or
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
4
polysubstituted with halogen; or RS and R° together represent one of
the bonds in a double
bond between the atoms 2 and 3 of formula I;
D represents - S(=O)z- or -S(=O)-; or
D-B represents -S(=O)(R')=N-
wherein R' is C,.s-alkyl; or aryl or heteroaryl optionally mono- or
polysubstituted with
halogen, hydroxy, C,~-alkoxy, aryloxy, arylalkoxy, vitro, amino, C,~-monoalkyl-
or
~ 0 dialkylamino, cyano, acyl, or C,_s-alkoxycarbonyl;
R' is hydrogen; hydroxy; C,~ alkoxy; or C,_s-alkyl, C~.s-cycloalkyl, CZ.s-
alkenyi or CZ_s-
alkynyl optionally mono- or polysubstituted with halogen and R4 is hydrogen;
or R'
together with RS represent one of the bonds in a double bond between the atoms
2 and 3
of formula I; or R' together with R' represent one of the bonds in a double
bond between
the atoms 3 and 4 of formula I;
RZ is hydrogen; hydroxy; C,~-alkoxy; or C,_s-alkyl, Cps-cycloaikyl, CZ_6
alkenyl or Cz.s-
alkynyl optionally mono- or polysubstituted with halogen;
R3 is R8; -ORs; -C(=X)Re; -NR8R9; bicycloalkyl, aryl, heteroaryl, arylalkyl or
heteroarylalkyl
optionally mono- or polysubstituted with halogen, hydroxy, C,~-alkoxy,
aryloxy, arylalkoxy,
vitro, amino, C,.s-monoalkyl- or dialkylamino, cyano, oxo, acyl or C,_6
alkoxycarbonyl; or
aryl substituted with C,~-alkyl;
wherein Rs is hydrogen; C~.s-cycloalkyl or (C3s-cycloalkyl)C,.~ alkyl, the
C~.s-cycloalkyl
group optionally being mono- or polysubstituted with C,_s-alkyl, halogen,
hydroxy or C,~-
alkoxy; a 3-6 membered saturated ring system comprising one or more nitrogen-,
oxygen-
or sulfur atoms; or straight or branched C,_,e-alkyl optionally mono- or
polysubstituted with
halogen, hydroxy, C,_s-alkoxy, C,_s-alkylthio, C~6 cycloalkyl, aryl, aryloxy,
arylalkoxy, vitro,
amino, C,~- monoalkyl- or dialkylamino, cyano, oxo, formyl, acyl, carboxy,
C,_s-alkoxy
carbonyl, or carbamoyl;
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
XisOorS;
R9 is hydrogen; C,.6-alkyl; C2_B-alkenyl; C~-cycloalkyl optionally mono- or
polysubstituted
5 with C,~-alkyl, halogen, hydroxy or C,.~-alkoxy; or
Re and R9 together with the nitrogen atom form a 3-12 membered mono- or
bicyclic
system, in which one or more of the carbon atoms may be exchanged with
nitrogen,
oxygen or sulfur, each of these ring systems optionally being mono- or
polysubstituted
1 o with halogen, C,_6-alkyl, hydroxy, C,_s-alkoxy, C,_s-alkoxy-C,_6-alkyl,
vitro, amino, cyano,
trifluor~omethyl, C,_s-monoalkyl- or dialkylamino, oxo; or R3 is
CP
C
C Cn p O r'
/ Cn
N N
Rio
wherein n,m,p independently are 0,1,2,3 and R'° is hydrogen; hydroxy;
C,_s-alkoxy; C~,6-
cycloalkyl optionally mono- or polysubstituted with C,_6-alkyl, halogen,
hydroxy or C,_6-
alkoxy; C,.~-alkyl, CZ_6-alkenyl or CZ_6-alkynyl optionally mono- or
polysubstituted with
halogen; or
R2 and R3 together with the nitrogen atom forms a 3-12 membered mono- or
bicyclic
system, in which one or more of the carbon atoms may be exchanged with
nitrogen,
oxygen or sulfur, each of these ring systems optionally being mono- or
polysubstituted
with halogen, C,_6-alkyl, hydroxy, C,~-alkoxy, C,_6-alkoxy-C,_6-alkyl, vitro,
amino, cyano,
trifluoromethyl, C,~-monoalkyl- or dialkylamino or oxo;
A together with carbon atoms 5 and 6 of formula I represents a 5 or 6 membered
hetero-
cyclic system comprising one or more nitrogen-, oxygen- or sulfur atoms, the
heterocyclic
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
6
systems optionally being mono- or poiysubstituted with halogen; C,_,2-alkyl;
C~-cycloaikyl;
hydroxy; C,_6 alkoxy; C,~-alkoxy-C,_s-alkyl; vitro; amino; cyano; cyanomethyl;
perhalome-
thyl; C,~-monoalkyl- or dialkylamino; suffamoyi; C,~ alkylthio; C,_6-
alkylsulfonyl; C,.~-
alkylsulfinyl; C,_6-alkylcarbonyiamino; arylthio, arylsulfinyl, arylsulfonyi,
the aryl group
optionally being mono- or polysubstituted with C,~-alkyl, halogen, hydroxy or
C,_6-alkoxy;
C,~-alkoxycarbonyl; C,_s-alkoxycarbonyl-C,~-alkyl; carbamyl; carbamyl- methyl;
C,_s-
monoalkyl- or dialkyfaminocarbonyl; C,~-monoalkyl- or
dialkylaminothiocarbonyl; ureido;
C,_6-monoalkyl- or dialkylaminocarbonylamino, thioureido; C,~-monoalkyl- or
dialkylami-
nothiocarbony!- amino; C,_6-monoalkyl- or dialkylaminosulfonyl; carboxy;
carboxy-C,_6-
~ o alkyl; acyl; aryl, arylalkyl, aryloxy, the aryl group optionally being
mono- or poiysubstituted
with C,_6-alkyl, halogen, hydroxy or C,~-alkoxy; (1,2,4-oxadiazoi-5-yl)- or
(1,2,4-oxadiazol-
3-yl)-C,_6 alkyl the oxadiazolyl group optionally being substituted with C,_6-
alkyl or C~-
cycloalkyl; or a 5 - 6 membered nitrogen containing ring, optionally
substituted with phenyl
or C,_s-alkyl;
provided that A together with carbon atoms 5 and 6 of formula I do not form a
pyridine ring
and that the following compounds 3-aminoimidazo[4,5-e]-1,2,4-thiadiazine 1,1-
dioxide and
3- (benzoylamino)imidazo[4,5-a]-1,2,4-thiadiazine 1,1-dioxide are not
included;
2o or a salt thereof with a pharmaceutically acceptable acid or base.
Within its scope the invention includes all optical isomers of compounds of
formula I, some
of which are optically active, and also their mixtures including racemic
mixture thereof.
The scope of the invention also includes all tautomeric forms of the compounds
of formula
The salts include pharmaceutically acceptable acid addition salts,
pharmaceutically
acceptable metal salts or optionally alkylated ammonium salts, such as
hydrochloric,
3o hydrobromic, hydroiodic, phosphoric, sulfuric, trifluoroacetic,
trichloroacetic, oxalic, malefic,
pyruvic, malonic, succinic, citric, tartaric, fumaric, mandelic, benzoic,
cinnamic, methane-
sulfonic, ethane sulfonic, picric and the like, and include acids related to
the pharmaceuti-
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
7
cally acceptable salts listed in Journal of Pharmaceutical Science, ~, 2
(1977) and
incorporated herein by reference, or lithium, sodium, potassium, magnesium and
the like.
The term "C,.s-alkoxy" as used herein, alone or in combination, refers to a
straight or
branched monovaient substituent comprising a C,.6-alkyl group linked through
an ether
oxygen having its free valence bond from the ether oxygen and having 1 to 6
carbon
atoms e.g. methoxy, ethoxy, propoxy, isopropoxy, butoxy, pentoxy.
The term "C,_6-alkylthio" as used herein, alone or in combination, refers to a
straight or
branched monovalent substituent comprising a tower alkyl group linked through
a divalent
sulfur atom having its free valence bond from the sulfur atom and having 1 to
6 carbon
atoms e.g. methylthio, ethylthio, propylthio, butylthio, pentylthio,
The term "Cz_6-alkenyl" as used herein refers to an unsaturated hydrocarbon
chain having
~ 5 2-6 carbon atoms and one double bond such as e.g. vinyl, 1-propenyl,
allyl, isopropenyl,
n-butenyl, n-pentenyl and n-hexenyl.
The term "C~6-cycloalkyl" as used herein refers to a radical of a saturated
cyclic hydrocar
bon with the indicated number of carbons such as cyclopropyl, cyclobutyl,
cyclopentyl, or
cyclohexyl.
The term "C2.6-alkynyl" as used herein refers to unsaturated hydrocarbons
which contain
triple bonds, such as e.g. -C---CH, -C--__CCH3, -CHzC=CH,
-CHZCH2C--_CH, -CH(CH3)C=CH, and the like.
The term "C,_6-alkoxy-C,.6-alkyl" as used herein refers to a group of 2-12
carbon atoms
interrupted by an O such as e.g. CH2-O-CH3, CH2-O-CHZ-CH3, CHZ-O-CH(CH3)2 and
the
like.
3o The term "halogen" means fluorine, chlorine, bromine or iodine.
The term "perhalomethyl" means trifluoromethyl, trichloromethyl,
tribromomethyl or
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
8
triiodomethyl.
The terms "C,~-alkyl", "C,_,z-alkyl" and "C,_,e-alkyl" as used herein, alone
or in combination,
refers to a straight or branched, saturated hydrocarbon chain having the
indicated number
of carbon atoms such as e.g. methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-
butyl, isobutyl,
tert-butyl, n-pentyl, 2-methylbutyl, 3-methylbutyl, 4-methylpentyl, neopentyl,
n-hexyl, 1,2-
dimethylpropyl, 2,2-dimethylpropyl, 1,2,2-trimethylpropyl and the like. The
term "C,_,e-alkyl"
as used herein also includes secondary C~6-alkyl and tertiary C~6-alkyl.
~ 0 The term "C,_6-monoalkyiamino" as used herein refers to an amino group
wherein one of
the hydrogen atoms is substituted with a straight or branched, saturated
hydrocarbon
chain having the indicated number of carbon atoms such as e.g. methyfamino,
ethylamino,
propylamino, n-butylamino, sec-butylamino, isobutylamino, tert-butylamino, n-
pentylamino,
2-methylbutylamino, n-hexylamino, 4-methylpentylamino, neopentylamino, n-
hexylamino,
2,2-dimethylpropylamino and the like.
The term "C,_6-dialkylamino" as used herein refers to an amino group wherein
the two
hydrogen atoms independently are substituted with a straight or branched,
saturated
hydrocarbon chain having the indicated number of carbon atoms; such as
dimethylamino,
N-ethyl-N-methylamino, diethylamino, dipropylamino, N-(n-butyl)-N-methylamino,
di(n-
pentyl)amino, and the like.
The term "aryl" as used herein refers to a monovalent substituent comprising a
C,_6-alkyl
group linked through a carbonyl group; such as e.g. acetyl, propionyl,
butyryl, isobutyryl,
pivaloyl, valeryl, and the like.
The term "C,~-alkoxycarbonyl" as used herein refers to a monovalent
substituent
comprising a C,_6-alkoxy group linked through a carbonyl group; such as e.g.
methoxycar-
bonyl, carbethoxy, propoxycarbonyl, isopropoxycarbonyl, n-butoxycarbonyl, sec-
butoxycarbonyl, tert-butoxycarbonyl, 3-methylbutoxycarbonyl, n-hexoxycarbonyl
and the
like.
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
9
The term "3-12 membered mono- or bicyclic system" as used herein refers to a
monovalent substituent of formula -NRZR' or -NReRs where RZ and R3, or RB and
R9
together with the nitrogen atom form a 3-12 membered mono- or bicyclic system,
in which
one or more of the carbon atoms may be exchanged with nitrogen, oxygen or
sulfur, such
as 1-pyrrolidyl, piperidino, morpholino, thiomorpholino, 4-methylpiperazin-1-
yl, 7-
azabicyclo[2.2.1]heptan-7-yl, tropanyl and the like.
The term "3-6 membered saturated ring system" as used herein refers to a
monovalent
substituent comprising a monocyclic saturated system containing one or more
hetero
atoms selected from nitrogen, oxygen and sulfur and having 3-6 members and
having its
free valence from a carbon atom, e.g. 2-pyrrolidyl, 4-piperidyl, 3-
morpholinyl, 1,4-dioxan-2-
yl, 5-oxazolidinyl, 4-isoxazolidinyl, or 2-thiomorpholinyl.
The term "bicycloalkyl" as used herein refers to a monovalent substituent
comprising a
~ 5 bicyclic structure made of 6-12 carbon atoms such as e.g. 2-norbornyl, 7-
norbomyl, 2-
bicyclo[2.2.2]octyl, and 9-bicyclo[3.3.1 ]nonanyl.
The term "aryl" as used herein refers to phenyl, 1-naphthyl, or 2-naphthyl.
The term "heteroaryl" as used herein, alone or in combination, refers to a
monovalent
substituent comprising a 5-6 membered monocyclic aromatic system or a 9-10
membered
bicyclic aromatic system containing one or more heteroatoms selected from
nitrogen,
oxygen and sulfur, e.g. pyrrole, imidazole, pyrazole, triazole, pyridine,
pyrazine,
pyrimidine, pyridazine, isothiazole, isoxazole, oxazole, oxadiazole,
thiadiazole, quinoline,
isoquinoline, quinazoline, quinoxaline, indole, benzimidazole, benzofuran,
pteridine, and
purine.
The term "arylalkyl" as used herein refers to a straight or branched saturated
carbon chain
containing from 1 to 6 carbons substituted with an aromatic carbohydride; such
as benzyl,
phenethyl, 3-phenylpropyl, 1-naphtylmethyl, 2-(1-naphtyl)ethyl and the like.
The term "aryloxy" as used herein refers to phenoxy, 1-naphthyloxy or 2-
naphthyloxy.
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
The term "arylalkoxy" as used herein refers to a C, ~-alkoxy group substituted
with an
aromatic carbohydride, such as benzyloxy, phenethoxy, 3-phenylpropoxy, 1-
naphthylmethoxy, 2-(1-naphtyi)ethoxy and the like.
5
The term "heteroarylalkyl" as used herein refers to a straight or branched
saturated
carbon chain containing from 1 to 6 carbons substituted with a heteroaryl
group; such as
(2-furyl)methyl, (3-furyl)methyl, (2-thienyl)methyl, (3-thienyl)methyl, (2-
pyridyl)methyl, 1-
methyl-1-(2-pyrimidyl)ethyl and the like.
The term "C,_6-alkylsulfonyl" as used herein refers to a monovalent
substituent comprising
a C,_6-alkyl group linked through a sulfonyl group such as e.g.
methylsulfonyl, ethylsul-
fonyl, n-propylsulfonyi, isopropylsulfonyl, n-butylsulfonyf, sec-
butylsulfonyl, iso-
butylsulfonyl, tert-butylsulfonyl, n-pentylsulfonyl, 2-methylbutylsulfonyl, 3-
methylbutylsulfonyl, n-hexylsulfonyl, 4-methylpentylsulfonyl,
neopentylsulfonyi, n-
hexylsulfonyl and 2,2-dimethylpropylsulfonyl.
The term "C,_6-monoalkylaminosulfonyl" as used herein refers to a monovalent
substituent
comprising a C,_6-monoalkylamino group linked through a sulfonyl group such as
e.g.
methylaminosulfonyl, ethylaminosulfonyl, n-propylaminosulfonyi,
isopropylaminosulfonyl,
n-butylaminosulfonyl, sec-butylaminosulfonyl, isobutylaminosulfonyl, tert-
butylaminosulfonyl, n-pentylaminosulfonyl, 2-methylbutylaminosulfonyl, 3-
methylbutylaminosulfonyl, n-hexylaminosulfonyl, 4-methylpentylaminosulfonyl,
neopenty-
laminosulfonyl, n-hexyiaminosulfonyl and 2,2-dimethylpropylaminosulfonyl.
The term "C,_6-dialkylaminosulfonyl" as used herein refers to a monovalent
substituent
comprising a C,-6-dialkylamino group linked through a sulfonyl group such as
dimethylami-
nosulfonyl, N-ethyl-N-methylaminosulfonyl, diethylaminosulfonyl,
dipropylaminosulfonyl, N-
(n-butyl)-N-methylaminosulfonyl, di(n-pentyl)aminosulfonyl, and the like.
The term "C,~-alkylsulfinyl" as used herein refers to a monovalent substituent
comprising a
straight or branched C,~-alkyl group linked through a sulfinyl group (-S(=O)-
); such as e.g.
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
11
methylsulfinyl, ethylsulfinyl, isopropylsulfinyl, butylsulfinyl,
pentylsulfinyl, and the like.
The term "C,~-alkylcarbonylamino" as used herein refers to an amino group
wherein one of
the hydrogen atoms is substituted with an acyl group, such as e.g. acetamido,
propiona-
mido, isopropylcarbonylamino, and the like.
The term "(C~-cycloalkyl)C,~-alkyl" as used herein, alone or in combination,
refers to a
straight or branched, saturated hydrocarbon chain having 1 to 6 carbon atoms
and being
monosubstituted with a C~-cycloalkyl group, the cycloalkyl group optionally
being mono- or
~ 0 polysubstituted with C,.~-alkyl, halogen, hydroxy or C,.~ alkoxy; such as
e.g. cyclopropylme-
thyl, (1-methylcyclopropyl)methyl, 1-(cyclopropyl)ethyl, cyclopentyimethyl,
cycfohexylmethyl,
and the like.
The term "arylthio" as used herein, alone or in combination, refers to an aryl
group linked
through a divalent sulfur atom having its free valence bond from the sulfur
atom, the aryl
group optionally being mono- or polysubstituted with C,~-alkyl, halogen,
hydroxy or C,~-
alkoxy; e.g. phenylthio, (4-methylphenyl)- thin, (2-chlorophenyl)thio, and the
like.
The term "arylsulfinyl" as used herein refers to an aryl group linked through
a sulfinyl group (-
S(=O)-), the aryl group optionally being mono- or polysubstituted with C,~-
alkyl, halogen,
hydroxy or C,~-alkoxy; such as e.g. phenylsulfinyl, (4-chlorophenyl)sulfinyl,
and the like.
The term "arylsulfonyl" as used herein refers to an aryl group linked through
a sulfonyl
group, the aryl group optionally being mono- or polysubstituted with C,.~-
alkyl, halogen,
hydroxy or C,~-alkoxy; such as e.g. phenylsulfonyl, tosyl, and the like.
The term "C,.~-monoalkylaminocarbonyl" as used herein refers to a monovalent
substituent
comprising a C,.~-monoalkylamino group linked through a carbonyl group such as
e.g.
methylaminocarbonyl, ethylaminocarbonyl, n-propylaminocarbonyi,
isopropylaminocarbonyl,
3o n-butylaminocarbonyl, sec-butylaminocarbonyl, isobutylaminocarbonyl, tert-
butylaminocarbonyl, n-pentylaminocarbonyl, 2-methylbutylaminocarbonyl, 3-
methylbutylamino-carbonyl, n-hexylaminocarbonyl, 4-methylpentylaminocarbonyl,
neo-
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
12
pentylaminocarbonyl, n-hexylaminocarbonyl and 2-2-dimethylpropylaminocarbonyl.
The term "C,.~-dialkylaminocarbonyl" as used herein refers to a monovalent
substituent
comprising a C,~-dialkylamino group linked through a carbonyl group such as
dimethylami-
nocarbonyl, N-ethyl-N-methylaminocarbonyl, diethylaminocarbonyl,
dipropylaminocarbonyl,
N-(n-butyl)-N-methylaminocarbonyl, di(n-pentyl)aminocarbonyl, and the like.
The term "C,.~-monoalkylaminocarbonylamino" as used herein refers to an amino
group
wherein one of the hydrogen atoms is substituted with a C,.~-
monoalkylaminocarbonyl group,
e.g. methylaminocarbonylamino, ethylaminocarbonylamino, n-
propylaminocarbonylamino,
isopropylaminocarbonylamino, n-butylaminocarbonylamino, sec-
butylaminocarbonyiamino,
isobutylaminocarbonylamino, tert-butylaminocarbonylamino, and 2-
methylbutylaminocarbonylamino.
~ 5 The term "C,~-dialkylaminocarbonylamino" as used herein refers to an amino
group wherein
one of the hydrogen atoms is substituted with a C,~-dialkylaminocarbonyl
group, such as
dimethylaminocarbonylarnino, N-ethyl-N-methylaminocarbonylamino, diethyla-
minocarbonylamino, dipropylaminocarbonylamino, N-(n-butyl)-N-
methylaminocarbonylamino, di(n-pentyl)aminocarbonylamino, and the like.
The term "5- or 6-membered heterocyclic system" as used herein refers to: a
monocyclic
unsaturated or saturated system containing one, two or three hetero atoms
selected from
nitrogen, oxygen and sulfur and having 5 members, e.g. pyrrole, furan,
thiophene,
pyrrofine, dihydrofuran, dihydrothiophene, imidazole, imidazoline, pyrazole,
pyrazoline,
oxazole, thiazole, isoxazole, isothiazole, 1,2,3-oxadiazole, furazan, 1,2,3-
triazole, 1,2,3-
thiadiazole or 2,1,3-thiadiazoie; an aromatic monocyclic system containing two
or more
nitrogen atoms and having 6 members, e.g. pyrazine, pyrimidine, pyridazine,
1,2,4-
triazine, 1,2,3-triazine or tetrazine; a non-aromatic monocyclic system
containing one or
more hetero atoms selected from nitrogen, oxygen and sulfur and having 6
members, e.g.
3o pyran, thiopyran, piperidine, dioxane, oxazine, isoxazine, dithiane,
oxathine, thiazine,
piperazine, thiadiazine, dithiazine or oxadiazine.
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
13
The term "5- or 6-membered nitrogen containing ring" as used herein refers to
a monova-
lent substituent comprising a monocyclic unsaturated or saturated system
containing one
or more nitrogen atoms and having 5 or 6 members, e.g. pyrrolidinyl,
pyrrolinyl, imida-
zolidinyl, pyrazolidinyl, pyrazolinyl, piperidyl, piperazinyl, pyrrolyl, 2H-
pyrrolyl, imidazolyl,
pyrazolyl, triazolyl, pyridyl, pyrazinyl, pyrimidinyl, pyridazinyl,
morpholino, thiomorpholino,
isothiazolyl, isoxazolyl, oxazolyl, oxadiazolyl, thiadiazolyl, 1,3-dioxolanyl,
and 1,4-
dioxolanyl.
In a preferred embodiment of the invention the general formula of formula I is
selected
from
Ri
R4 Ra
N
5 ~--
A
6 D=N\RS R
(la)
wherein
R' and RS independently are hydrogen; hydroxy; C,.6-alkoxy; or C,_6-alkyl, C~6-
cycfoalkyl,
C2~-afkenyi or C2_6-alkynyl optionally mono- or polysubstituted with halogen
and R4 is
hydrogen; or
R4 together with R5 represent one of the bonds in a double bond between the
atoms 2 and
3 of formula I and R' is as defined above; or
R° together with R' represent one of the bonds in a double bond between
the atoms 3
and 4 of formula I and RS is as defined above;
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
14
D represents -S(=O)2- or -S(=O)-.
In another preferred embodiment of the invention the general formula of
formula I is
selected from
R1
I R4 R2
N~
S 4 ~Nw
1;N R
D
(Ib}
wherein
R' is hydrogen; hydroxy; C,_6-alkoxy; or C,_6-alkyl, C~-cycloalkyl, CZ_s
alkenyl or CZ_s-
~ 0 alkynyl optionally mono- or polysubstituted with halogen and R4 is
hydrogen; or
R4 together with R' represent one of the bonds in a double bond between the
atoms 3 and
4 of formula 1;
D represents -S(=O)R'=
wherein R' is C,_6-alkyl; or aryl or heteroaryl optionally mono- or
polysubstituted with
halogen, hydroxy, C,_6-alkoxy, aryloxy, arylalkoxy, nitro, amino, C,_6-
monoalkyl- or
dialkylamino, cyano, acyl or C,_6-alkoxycarbonyl.
In another preferred embodiment of the invention the general formula of
formula l is
selected from
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
R1
I R4 R2
N
N~ 3
I6lz RS R
Rs
(Ic)
wherein
5 R', RS and Rs independently are hydrogen; hydroxy; C,~-alkoxy; or C,~-alkyl,
C~.6-
cycloalkyl, CZ_6-alkenyl or CZ_6-alkynyl optionally mono- or polysubstituted
with halogen and
R4 is hydrogen; or
R4 together with RS represent one of the bonds in a double bond between the
atoms 2 and
10 3 of formula I and R' and R6 are as defined above; or
R° together with R' represent one of the bonds in a double bond between
the atoms 3 and
~ of formula I and RS and Rs are as defined above;
15 D represents -S(=O)2- or S(=O).
Preferably, the general formula of formula I is (la).
In another preferred embodiment of the invention D is -S(=O)Z-.
In another preferred embodiment of the invention R' is selected from hydrogen,
C,_s-alkyl,
C~6-cycloalkyl or CZ_s-alkenyl. Preferably R' is hydrogen or C,_s-alkyl.
In another preferred embodiment of the invention R' together with R°
represent one of the
bonds in a double bond between the atoms 3 and 4 of formula 1.
In another preferred embodiment of the invention R° together with RS
represent one of the
bonds in a double bond between the atoms 2 and 3 of formula I.
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
16
In another preferred embodiment of the invention RZ is selected from hydrogen,
hydroxy,
C,~-alkyl, C36-cycloalkyl or C2~-alkenyl. Preferably RZ is hydrogen or
C,~alkyl.
In another preferred embodiment of the invention R' is selected from Re,-ORe, -
NReR9 or
aryl, the aryl group optionally being substituted with C,_s-alkyl; wherein RB
is hydrogen; C~
s-cycloalkyl; (C~6 cycloalkyl)C,~-alkyl; a 3-6-membered saturated ring system
comprising
one, two or three nitrogen-, oxygen- or sulfur atoms; or straight or branched
C,_,e-alkyl
optionally substituted with halogen, hydroxy, C,_6-alkoxy, C,~-alkylthio, C~-
cycloalkyl or
aryl; R9 is hydrogen, C,_6-alkyl or C36-cycloalkyl; or RB and R9 together with
the nitrogen
atom form a 4 - 6 membered ring, preferably 1-pyrrolidyl, piperidine or
morpholino.
In yet another preferred embodiment of the invention R' is selected from
secondary C~-
alkyl, tertiary C~-alkyl, C~-cycloalkyl or (C~-cycloalkyl)methyl optionally
mono- or
polysubstituted with C,~-alkyl, halogen, hydroxy or C,~ alkoxy. Preferably R'
is selected from
isopropyl, 1-methylpropy(, 2-methylpropyl, tert-butyl, 1,1-dimethylpropyl, 1,2-
dimethyipropyl,
1,2,2-trimethylpropyl, 2,3-dimethylbutyl, 1-ethylpropyl, 1-ethyl- 2-
methyipropyl, 1-ethyl-2,2-
dimethylpropyl, 2,3,3-trimethylbutyl, 2-methylbutyl, 1,5-dimethylhexyl, 3-
methylbutyl, 3-
methylhexyl, cyclopropyl, 1-methylcyclopropyl, cyclobutyl, cyclopentyl,
cyciohexyl, cyclopro-
pylmethyl, 1-(cyclopropyl)ethyl cyclobutylmethyl, cyclopentylmethyl or
cyclohexylmethyl.
In a further preferred embodiment of the invention RZ and R3 together with the
nitrogen
atom forms a six membered ring, optionally substituted in the 2-position with
a C,_6-alkyl
group, preferably selected from methyl, ethyl or isopropyl. Preferably the six
membered
ring is a piperidine, piperazine, morpholine or thiomorpholine ring.
In another preferred embodiment of the invention R' is selected from C,_s-
alkyl, phenyl or
pyridyl.
In another preferred embodiment of the invention A forms together with carbon
atoms 5
and 6 of formula I a 5 membered heterocyclic system containing one hetero atom
selected
from nitrogen and sulfur, a 5 membered heterocyclic system containing two
hetero atoms
CA 02294830 1999-12-30
WO 99103861 PCT/DK98/00288
17
selected from nitrogen, oxygen and sulfur, a 6 membered aromatic heterocyclic
system
containing two or three nitrogen atoms, a 6 membered non-aromatic heterocyclic
system
containing one or two hetero atoms selected from nitrogen, oxygen and sulfur;
the
heterocyclic systems optionally being mono- or disubstituted with halogen;
C,_,z-alkyl; C~.6-
cycloalkyl; cyano; cyanomethyl; perhalomethyl; sulfamoyl; C,~-alkylthio; C,_6-
alkylsulfonyl;
C,_6-alkylsulfinyl; arylthio, arylsulfinyl, arylsulfonyl, the aryl group
optionally being mono- or
polysubstituted with C,_6-alkyl, halogen, hydroxy or C,~-alkoxy; C,_6-
alkoxycarbonyl-C,_6-
alkyl; carbamylmethyl; carboxy-C,~-alkyl; aryfoxy; (1,2,4-oxadiazol-5-yl)- or
(1,2,4-
oxadiazol-3-yl)C,_s-alkyl, the oxadiazolyl group optionally being substituted
with C,.6-alkyl
or C~6-cycloalkyl; acyl; or a 5 - 6 membered nitrogen containing ring,
optionally substituted
with phenyl or C,_s-alkyl.
Preferably, A forms together with carbon atoms 5 and 6 a thieno[3,2-a]- or
pyrrolo[3,2-a]-
ring, thiophene, imidazole, thiazole, pyrazole, isoxazole or isothiazole, a
pyrazino[2,3-a]-,
~ 5 a pyrimido[4,5-a]-, a pyrimido[5,4-a]-, a pyridazino[4,5-a]- or a
pyridazino[4,3-a]-ring,
thiopyran, piperidine, dioxane , oxazine or dithiane.
Preferred compounds of the invention are:
7-Cyano-3-isopropylamino-6-methyl-4H-thieno[2,3-a]-1,2,4-thiadiazine 1,1-
dioxide
7-Cyano-6-methyl-3-propylamino-4H-thieno[2,3-a]-1,2,4-thiadiazine 1,1-dioxide
6-Chloro-3-isopentylamino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide
6-Chloro-3-(1-methylheptyl)amino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-
dioxide
6-Chloro-3-(1-ethylpentyl)amino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide
6-Chloro-3-(2-methylbutyl)amino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide
6-Chloro-3-(1-methylhexyl)amino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide
6-Chloro-3-cyclopentylamino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide
6-Chloro-3-cyclohexylmethylamino-4H-thieno[3,2-e]-1,2,4-thiadiazine 1,1-
dioxide
Ethyl 3-(6-chloro-1,4-dihydro-1,1-dioxothieno[3,2-a]-17~g,2,4-thiadiazin-3-
ylamino)butanoate
3-(6-Chloro-1,4-dihydro-1,1-dioxothieno[3,2-e]-1~,6,2,4-thiadiazin-3-
ylamino)butanoic acid
6-Chloro-3-(3-hydroxy-1-methylpropyl)amino-4H-thieno[3,2-a]-1,2,4-thiadiazine
1,1-
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
18
dioxide
(R)-6-Chloro-3-(1-phenylethyl)amino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-
dioxide
(S)-3-sec-Butylamino-6-chloro-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide
6-Chloro-3-isopropyiamino-4H-thieno[2,3-a]-1,2,4-thiadiazine 1,1-dioxide
6-Chloro-3-cyclopentylamino-4H-thieno[2,3-a]-1,2,4-thiadiazine 1,1-dioxide
6-Bromo-3-isopropylamino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide
3-isopropylamino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide
6-Fluoro-3-isopropylamino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide
3-Cyclobutylamino-5,6-dimethyl-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide
3-Cyclopentylamino-5,6-dimethyl-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide
3-Isopropylamino-6,7-dimethyl-4H-thieno[2,3-a]-1,2,4-thiadiazine 1,1-dioxide
3-Cyclobutylamino-6,7-dimethyl-4H-thieno[2,3-a]-1,2,4-thiadiazine 1,1-dioxide
3-Cyclopentylamino -6,7-dimethyl-4H-thieno[2,3-a]-1,2,4-thiadiazine 1,1-
dioxide
5-Chloro-3-isopropylamino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide
5-Chloro-3-propylamino-4H-thieno[3,2-ej-1,2,4-thiadiazine 1,1-dioxide
5-Chioro-3-cyclopentylamino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide
5-Chloro-6-methyl-3-isopropylamino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-
dioxide
6-chloro-3-isopropylamino-5-methyl-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-
dioxide
6-chloro-3-cyclopentylamino-5-methyl-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-
dioxide
6-Fluoro-3-propylamino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide
6-Fluoro-3-cyclopentylamino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide
5-Ffuoro-3-propylamino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide
5-Fluoro-3-isopropylamino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide.
3-Isopropylamino-7-methyl-4H-thieno[2,3-a]-1,2,4-thiadiazine 1,1-dioxide
6-Chloro-3-cyclobutylamino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide
6-Chloro-3-(2-hydroxyethyl)amino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-
dioxide
(t)-3-exo-Bicyclo[2.2.1]hept-2-ylamino-6-chloro-4H-thieno[3,2-a]-1,2,4-
thiadiazine 1,1-
dioxide
(R)-6-Chloro-3-(2-hydroxypropyl)amino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-
dioxide
6-Bromo-3-isopropylamino-4H-thieno[3,2-e]-1,2,4-thiadiazine 1,1-dioxide
5,6-Dibromo-3-isopropylamino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide6-
Chloro-3-
cyclohexylamino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
19
6-Chloro-3-(furan-2-ylmethyl)amino-4H-thieno(3,2-a]-1,2,4-thiadiazine 1,1-
dioxide
6-Chloro-3-(1-ethylpropyl)amino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide
6-Bromo-3-cyclopentylamino-4H-thieno[3,2-ej-1,2,4-thiadiazine 1,1-dioxide
6-Chloro-3-(2-methylallyl)amino-4H-thieno[3,2-e)-1,2,4-thiadiazine 1,1-dioxide
or
6-Cyano-3-isopropylamino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1, i -dioxide.
The compounds of the present invention interact with the potassium channels
and hence
act as openers or blockers of the ATP-regulated potassium channels, which make
them
useful in the treatment of various diseases of the cardiovascular system, e.g.
cerebral
~ 0 ischemia, hypertension, ischemic heart diseases, angina pectoris and
coronary heart
diseases; the pulmonary system; the gastrointestinal system; the central
nervous system
and the endocrinological system.
Since some KATP-openers are able to antagonize vasospasms in basilar or
cerebral
7 5 arteries the compounds of the present invention can be used for the
treatment of vaso-
spastic disorders such as subarachnoid haemorrhage and migraine.
The compounds of the present invention may also be used for the treatment of
diseases
associated with decreased skeletal muscle blood flow such as Raynauds disease
and
20 intermittent claudication.
Further, the compounds of the invention may be used for the treatment of
chronic airway
diseases, including asthma, and for treatment of detrusor muscle instability
secondary to
bladder outflow obstruction and therefore for kidney stones by aiding their
passage along
25 the urethra.
The present compounds could also be used for treatment of conditions
associated with
disturbances in gastrointestinal mobility such as irritable bowel syndrome.
Additionally
these compounds can be used for the treatment of premature labour and
dysmenorrhea.
Potassium channel openers hyperpoiarize neurons and inhibit neurotransmitter
release
and it is expected that such compounds can be used for the treatment of
various diseases
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
of the central nervous system, e.g. epilepsia, ischemia and neurodegenerative
diseases,
and for the management of pain.
Further, potassium channel openers promote hairgrowth, therefore, the
compounds of the
5 present invention can be used for the treatment of baldness.
Potassium channel openers also relax urinary bladder smooth muscle, thus, the
compounds of the present invention can be used for the treatment of urinary
incontinence.
1 o In diseases such as nesidioblastosis and insulinoma in which a
hypersecretion of insulin
causes severe hypoglycemia the compounds of the present invention can be used
to
reduce insulin secretion. In obesity hyperinsulinemia and insulin resistance
is very
frequently encountered. This condition could lead to the development of
noninsulin
dependent diabetes (NIDDM). It is expected that potassium channel openers, and
hence
~ 5 the compounds of the present invention, can be used for reducing the
hyperinsulinemia
and thereby prevent diabetes and reduce obesity. In overt NIDDM treatment of
hyperinsulinemia with potassium channel openers, and hence the present
compounds,
can be of benefit in restoring glucose sensitivity and normal insulin
secretions.
2o In early cases of insulin dependent diabetes (IDDM) or in prediabetic
cases, potassium
channel openers and hence the present compounds can be used to induce
pancreatic cell
rest which may prevent the progression of the autoimmune disease.
The potassium channel openers of the present invention can be administered in
combination with an immunosuppressant or with an agent like nicotinamide,
which will
reduce autoimmune degeneration of beta-cells.
Combining beta-cell rest with a treatment protecting the beta-cells against
cytokine
mediated beta-cell impairment/cytotoxicity is another aspect of this
invention.
3o Insulin requiring or Type 1 diabetes (IDDM) as well as late onset IDDM
(also known as
type 1.5. e.g. non-insulin-requiring Type 2 (NIIDM) patients with
autoreactivity against
beta-cell epitopes that later turns insulin requiring) have circulating
autoreactive mono-
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
21
cytes/lymphocytes that homes to the isletslbeta-cells and releases their
cytokines. Some
of these cytokines (e.g. interleukin-1b (IL-1b) , tumour necrosis factor a
(TNFa) and
interferon g (IFNg)) are specifically toxic to the beta-cells, e.g. through
the induction of
nitric oxide (NO) and other free radicals. Inhibition of this cytotoxicity,
e.g. by co-
y administring nicotinamide (NA), a derivative hereof or other cytokine
protective com-
pounds to the prediabeticldiabetic patients treated with the PCO compound is
an example
of this aspect. Nicotinamide belongs to the B-vitamin family and is derived
from nicotinic
acid by amidation of the carboxyl group. It processes none of nicotine's
pharmacological
properties. NA is converted into NAD+, which acts as a coenzyme for proteins
involved in
1 o tissue respiration. NA has been proposed to influence several of the
putative intracellular
molecular events following immune attack on the beta-cells. Animal experiments
and early
non-blinded experiments in humans have indicated a protective role of this
compound
against IDDM as well as in cytokinelimmune mediated beta-cell destruction.
Yet another aspect of this application concerns the use of a PCO compound
alone or in
15 combination with the inhibitor of cytokine/immune mediated beta-cell
impairment , in
transplantation, e.g. islet transplantation into diabetes patients. The use of
one or both of
these treatments may reduce the risk of rejection of the transplanted
islets/beta-
cells/engineered beta-cells/pancreas.
20 Compounds of the present invention which act as blockers of KATP -channels
can be used
for the treatment of NIDDM.
Preferably, the compounds of the present invention may be used for treatment
or
25 prevention of diseases of the endocrinological system such as
hyperinsulinaemia and
diabetes.
Accordingly, in another aspect the invention relates to a compound of the
general formula
I or a pharmaceutically acceptable acid addition salt thereof for use as a
therapeutically
3o acceptable substance, preferably for use as a therapeutically acceptable
substance in the
treatment of hyperinsulinaemia and treatment or prevention of diabetes.
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
22
Furthermore, the invention also relates to the use of the inventive compounds
of formula I
as medicaments useful for treating hyperinsulinaemia and treating or
preventing diabetes .
in yet another aspect, the present invention relates to methods of preparing
the above
mentioned compounds. The methods comprises:
a) reacting a compound of formula II:
R1
R4
N~ Z
~o D (II)
wherein A, B, D, R' and R4 are as defined above and Z is a leaving group such
as alkoxy,
alkylthio, halogen, preferentially chloro, bromo, iodo, trimethylamino, or
methylsulfonyl with
a compound of formula III:
R2
i
HN
3
R
(III)
wherein Rz and R' are defined above to form a compound of the general formula
l;
b) reacting a compound of formula IV:
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
23
R1
I
N\/X
A I .B
(I
wherein R' is hydrogen and A, B, D and X are as defined above, or B is NH and
R', A, D
and X are as defined above, with the compound of formula III, or a suitable
salt thereof in
the presence of P205 and a high boiling tertiary amine or a suitable salt
thereof, to form a
compound of the general formula I;
c) reacting a compound of the formula IV:
R1
N\/X
A ~I
.B
(IV)
~ 5 wherein R' is hydrogen and A, B, D and X are as defined above or B is NH
and R', A, D
and X are as defined above, with a compound of the formula III, or a suitable
salt thereof
in the presence of titanium tetrachloride and a solvent with which it may form
a complex,
like e.g. tetrahydrofuran, or a mixture of toluene and anisole, to form a
compound of the
general formula 1;
d) reacting a compound of formula V
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
24
NHR1
A
SOzNHz
M
wherein R' and A are as defined above, with a compound of formula VI
R3NC0 (VI)
wherein R3 is as defined above, to form a compound of the general formula I
wherein D is
SOz, B is >NRS, Rz is H, and R° and R5 together form a bond;
e) reacting a compound of the formula V
NHR 1
A
SO2NHz V
( )
wherein R' and A are as defined above, with a compound of formula VII
R3NHC(=O)CI (VII)
wherein R3 is as defined, to form a compound of the general formula I wherein
D is SOz, B
is >NRS, R2 is H, and R4 and RS together form a bond;
f) reacting a compound of the formula V
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
NHR1
A I
SOzNH2
(V)
5 wherein R' and A are defined as above, with a compound of formula VIII
Y
C
HaN/ \~a
(VIII)
io
wherein Y is NH or S, or a suitable salt thereof, to form a compound of the
general formula
I, wherein D is SO2, B is >NR5 , R° and RS together form a bond, and RZ
and R3 are H;
g) reacting in the presence of a base a compound of formula IX
H
N.Rii
A
,,S , NHz
0 0 (IX)
20 or a suitable salt thereof, wherein R" is R' or EtOC(=O), wherein R' and A
are defined as
above, with a compound of formula X
R3N=C=S (X)
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
26
wherein R' is as defined above, to form an adduct which may have either of the
two
structures XI or XII or be a mixture of the two
H
N.Rii
A
,rrH~NH-R3
OS IfO
S
(Xi)
Rli
N_ /NH-R3
I ~S
isv~z
O O
(XII)
either of which by ring-closure, e.g. by treatment with phosgene in a suitable
solvent,
forms a compound of the general formula I, if R" is R' , wherein D is S{=O)2 ,
B is >NR5 ,
Rz is H, and R4 and RS together form a bond, and a compound of the general
formula XIII
if R" is EtOC(=O);
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
27
O~o.~
~N NH~R3
A
"N
S
.. ,.
O O (X111)
h) hydrolyzing and subsequently decarboxylating a compound of the general
formula XIII
O~O~
~N' NH-R3
A
,N
S
,. ,~
O O
(X111)
to form a compound of the general formula I, wherein D is S(=O)2 , B is >NRS ,
R' and RZ
are H, and R' and RS together form a bond, e.g. by heating the starting
compound in
aqueous base.
The starting materials are either known compounds or compounds which may be
prepared in analogy with the preparation of known compounds or in analogy with
known
methods as described by e.g Huang B.-S., et al., J. Med. Chem., ~, 575-7
(1980),
Ofitserov V. I. et al., Khim. GeterotsikL Soedin., 1119-22 (russ.) (1976),
Topliss J. G., U.S.
3,641,017 (1972), Kotovskaya S. K. et al., Khim.-Farm. Zh., ~, 54-57 (russ.)
(1979),
2o Meyer R. F., J. Heterocycl. Chem., ~, 407-408 (1969) and Hattori M., Yoneda
M., and
Goto M., Bull. Chem. Soc. Jap., ,4~, 1890-1 (1973), Williams T.R. and Cram
D.J., J. Org.
Chem., 38, 20-26 (1973), Barnes A.C., Kennewell P.D. and Taylor J.B., J. Chem.
Soc.
Chem. Commun., 1973, 776-777, Stoss and Satzinger, Chem. Ber., 109, 2097
(1976),
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
28
Kresze G., Hatjiissaak A., Phosphorus Sulfur, 29, 41-47 (1987), Dillard R.D.,
Yen T.T.,
Stark P., Pavey D.E., J. Med. Chem., 23, 717-722 (1980).
PIHARMACOLOGICAL METHODS
The ability of the compounds to interact with potassium channels can be
determined by
various methods. When patch-clamp techniques (Hamill O.P., Marty A., Neher E.,
Sakmann B. and Sigworth F.J., PliigersArch., ~1_, 85-100 (1981)) are used the
ionic
current through a single channel of a cell can be recorded.
l0
The activity of the compounds as potassium channel openers can also be
measured as
relaxation of rat aorta rings according to the following procedure:
A section of rat thoracic aorta between the aortic arch and the diaphragm was
dissected
~ 5 out and mounted as ring preparations as described by Taylor P.D. et al ,
Brif J. Pharma-
col, ~1, 42-48 (1994).
After a 45 min. equilibration period under a tension of 2 g, the preparations
were con-
tracted to achieve 80% of the maximum response using the required
concentration of
20 phenylephrine. When the phenylephrine response reached a plateau, potential
vasodila-
tory agents were added cumulatively to the bath in small volumes using half
log molar
increments at 2 min intervals. Relaxation was expressed at the percentage of
the
contracted tension. The potency of a compound was expressed as the
concentration
required to evoke a 50% relaxation of the tissue.
Relaxation of rat aorta rings
Example EC50 micro M
4 2.8
6 20.5
In the pancreatic b-cell the opening of the KATP-channels can be determined by
measuring
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
29
the subsequent change in the concentration of cytoplasmic free Caz'
concentration
according to the method of Arkhammar P. et al. , J. Biol. Chem., ?~, 5448-5454
(1987).
~~b+ efflux from a f3-cell lin -
The RIN 5F cell line was grown in RPMI 1640 with Glutarnax I, supplemented
with 10
fetal calf serum (from GibcoBRL, Scotland, UK) and maintained in an atmosphere
of 5
COZ I 95 % air at 37°C. The cells were detached with a Trypsin-EDTA
solution (from
GibcoBRL, Scotland, UK), resuspended in medium, added 1 mCi/ml B6Rb' and
replated
into microtiter plates (96 well cluster 3596, sterile, from Costar
Corporation, MA, USA) at a
density of 50000 cells/well in 100 pl/well, and grown 24 hours before use in
assay.
The plates were washed 4 times with Ringer buffer (150 mM NaCI, 10 mM Hepes,
3.0 mM
KCI, 1.0 mM CaCl2, 20 mM Sucrose, pH 7.1 ). Eighty ~,I Ringer buffer and 1 ul
control- or
~ 5 test compound dissolved in DMSO was added. After incubation 1 h at room
temperature
with a lid, 50 pl of the supernatant was transferred to PicoPlates (Packard
Instrument
Company, CT, USA) and 100 pl MicroScint40 (Packard Instrument Company, CT,
USA)
added. The plates were counted in TopCount (Packard Instrument Company, CT,
USA)
for 1 minlwell at the'ZP program.
The calculation of ECS° and Em~ was done by SIideWrite (Advanced
Graphics Software,
lnc., CA, USA) using a four parameter logistic curve: y = (a-d)/(1+(xlc}b)+d,
where a = the
activity estimated at concentration zero, b = a slope factor, c = the
concentration at the
middle of the curve and, d = the activity estimated at infinite concentration.
EC5° = c and
Em~= d, when the curve is turned of at infinite concentrations.
Increased Rb-efflux in rin 5F cells
Examale EC50 micro M
4 5.5
6 31
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
The compounds according to the invention are effective over a wide dose range.
In
general satisfactory results are obtained with dosages from about 0.05 to
about 1000 mg,
5 preferably from about 0.1 to about 500 mg, per day. A most preferable dosage
is about 1
mg to about 100 mg per day. The exact dosage will depend upon the mode of
administra-
tion, form in which administered, the subject to be treated and the body
weight of the
subject to be treated, and the preference and experience of the physician or
veterinarian
in charge.
The route of administration may be any route, which effectively transports the
active
compound to the appropriate or desired site of action, such as oral or
parenteral e.g.
rectal, transdermal, subcutaneous, intravenous, intramuscular or intranasal,
the oral route
being preferred.
Typical compositions include a compound of formula I or a pharmaceutically
acceptable
acid addition salt thereof, associated with a pharmaceutically acceptable
excipient which
may be a carrier or a diluent or be diluted by a carrier, or enclosed within a
carrier which
can be in form of a capsule, sachet, paper or other container. In making the
compositions,
conventional techniques for the preparation of pharmaceutical compositions may
be used.
For example, the active compound will usually be mixed with a carrier, or
diluted by a
carrier, or enclosed within a carrier which may be in the form of a ampoule,
capsule,
sachet, paper, or other container. When the carrier serves as a diluent, it
may be solid,
semi-solid, or liquid material which acts as a vehicle, excipient, or medium
for the active
compound. The active compound can be adsorbed on a granular solid container
for
example in a sachet. Some examples of suitable carriers are water, salt
solutions, alco-
hols, polyethylene glycols, polyhydroxyethoxylated castor oil, gelatine,
lactose, amylose,
magnesium stearate, talc, silicic acid, fatty acid monoglycerides and
diglycerides,
pentaerythritol fatty acid esters, hydroxymethylcellulose and
polyvinylpyrrolidone. The
formulations may also include wetting agents, emulsifying and suspending
agents, preser-
ving agents, sweetening agents or flavouring agents. The formulations of the
invention
may be formulated so as to provide quick, sustained, or delayed release of the
active
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
31
ingredient after administration to the patient by employing procedures well
known in the
art.
The pharmaceutical preparations can be sterilized and mixed, if desired, with
auxiliary
agents, emulsifiers, salt for influencing osmotic pressure, buffers and/or
coloring sub-
stances and the like, which do not deleteriously react with the active
compounds.
For parenteral application, particularly suitable are injectable solutions or
suspensions,
preferably aqueous solutions with the active compound dissolved in
polyhydroxylated
castor oil.
Tablets, dragees, or capsules having talc andlor a carbohydrate carrier or
binder or the
like are particularly suitable for oral application. Preferable carriers for
tablets, dragees, or
capsules include lactose, corn starch, and/or potato starch. A syrup or elixir
can be used in
~ 5 cases where a sweetened vehicle can be employed.
Generally, the compounds are dispensed in unit form comprising from about 1 to
about
100 mg in a pharmaceutically acceptable carrier per unit dosage.
A typical tablet, appropriate for use in this method, may be prepared by
conventional
tabletting techniques and contains:
Active compound 5.0 mg
Lactosum 67.8 mg Ph.Eur.
Avicel~ 31.4 mg
Amberlite~ 1.0 mg
Magnesii stearas 0.25 mg Ph.Eur.
EXAMPLES
The process of preparing the compounds of formula I is further illustrated in
the following
examples which, however, are not to be construed as limiting.
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
32
,EXAMPLE 1
7-Cyano-3-iso~r_o~ylamino-6-methyl-4H-thieno~2.3-e]-1.2.4-thiadiazine 1.1-
dioxide
a) 4-Cyano-2-hydrazinocarbonxl-5-meth-thiophene-3-sulfonamide
Methyl 4-cyano-5-methyl-3-sulfamoyl-thiophene-2-carboxylate (2.9 g), prepared
from
1 o methyl 3-amino-4-cyano-5-methyl-thiophene-2-carboxylate in analogy with
known
procedures, e.g. F. Junquera et al. , Eur.J.Med.Chem. 23, 329 (1988), was
suspended in
ml of ethanol. Hydrazine monohydrate (2 ml) was added and the mixture was
stirred for
1 h at room temperature and then evaporated. The oily residue (3.3 g) was
triturated with
10 ml of water and the precipitating crystals were collected by filtration,
washed with water
and dried to give the title compoundas yellow crystals (yield 1.62 g); m.p.
186-91 °C; 'H-
NMR (DMSO-dfi), 8(ppm):7.25 (br, 5H, NH/NHZ), 2.51 (s, 3H, CH3).
b) 4-Cyano-5-methyl-3-sulfamoyl-thiophene-2-carbonyl azide
A solution of sodium nitrite (0.47 g) in 5 ml of water was added dropwise to a
stirred
solution of 4-cyano-2-hydrazinocarbonyl-5-methyl-thiophene-3-sulfonamide (1.6
g) in 38
ml of 1 M hydrochloric acid at 0 °C. The resulting mixture was stirred
for 30 min at 0 °C
and then filtered. The filter cake was washed with water and dried in vacuum
to give 1.35
g of the title compound; 'H-NMR (DMSO-ds), 8(ppm): 7.75 (br, 1 H, NHZ), 2.75
(s, 1 H,
CH3).
c) 4-C~ano-2-ethoxvcarbonylamino-5-met~yrl-thiophene-3-sulfonamide
4-cyano-5-methyl-3-sulfamoyl-thiophene-2-carbonyl azide (1.35 g) was added in
small
portions over 5 min to 50 ml of abs. ethanol at reflux temperature. The
resulting solution
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
33
was refluxed for 5 min and then evaporated. The residue crystallized when
triturated with
20 ml of ethyl acetate. The crystals were filtered off, rinsed with etyl
acetate and dried to
give the title compound; 'H-NMR (DMSO-ds), 8(ppm): 9.45 (s, 1 H, NH), 7.82
(br, 2H, NHZ),
4.23 {q, 2H, CHZ), 2.51 (s, 3H, CH3), 1.25 (t, 3H, CH3).
d) - 4- n a o- I- - h' -N'-' ! hi
A mixture of 4-cyano-2-ethoxycarbonylamino-5-methyl-thiophene-3-sulfonamide
(0.50 g),
potassium carbonate (0.36 g) and isopropyl isothiocyanate {300 ~I) in 10 ml of
dry acetone
was heated at 55 °C for 18 h and then evaporated to dryness. The
residue was dissolved
in 10 ml of water, and pH was adjusted to 2 by dropwise addition of 1 M
hydrochloric acid.
The precipitate was filtered off, rinsed with a small amount of water and
dried to give 0.34
g of the title compound; m.p. 169-171 °C.
e) h I 7- -i I in -6- I - hien -1 4- i i in
~arboxvlate 1 1-dioxide
To a stirred solution of N-(4-cyano-2-ethoxycarbonylamino-5-methyl-3-
thienyisulfonyl}-N'-
isopropylthiourea (0.3 g) and triethylamine (320 wl) in 10 ml of dry THF at 0
°C was added
750 ul of a 20% solution of phosgene in toluene. The mixture was stirred at 0
°C for 1 h
and then evaporated to dryness. The residue was triturated with 10 ml of
water, filtered
off, rinsed on the filter with water and dried to give 0.24 g of crude title
compound; m.p.
116-119 °C. The product was used for the next step without
purification.
f) 7- n -3-i r I m' h I- - hi 4- i in 1- io i a
A mixture of ethyl 7-cyano-3-isopropylamino-6-methyl-4H-thieno[2,3-a]-1,2,4-
thiadiazine-
4-carboxylate 1,1-dioxide (0.15 g) and 5 ml of 1 M aqueous sodium hydroxide
was stirred
at room temperature for 1 h. Then the mixture was filtered and pH was adjusted
to 1-2 by
dropwise addition of 4M hydrochloric acid. After stirring for 30 min the
precipitate was
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
34
filtered off, rinsed with a small amount of water and dried to give the title
compound; m.p.
m.p. 235-238 °C; 'H-NMR (DMSO-ds), 8(ppm): 11.35 (s, 1 H, NH), 7.55 (br
d, 1 H, NH),
3.98 - 3.77 (m, 1 H, CH), 2.50 (s, 1 H, CH3), 1.15 (d, 6H, CH3).
EXAMPLE 2
7-C~rano-6-methyl-3-~ro~yrlamino-4H-thienof2 3-el-1 2 4-thiadiazine 1 1-
dioxide
a) N-(4-Cvano-2-ethoxycarbonylamino-5-methyl-3-thienyrlsulfony~-N'-
ro~ylthiourea
The title compound was prepared from 4-cyano-2-ethoxycarbonylamino-5-methyl-
thiophene-3-sulfonamide and propyl isothiocyanate in analogy with the
synthesis de-
scribed in Example 1-d; m.p. 167-168 °C.
b) Ethyl 7-cyano-6-methyl-3-hrQpylamino-4H-thieno[2 3-el-1 2 4-thiadiazine-4-
carbox5rlate
1,1-dioxide
The title compound was prepared by ring closure of N-(4-cyano-2-
ethoxycarbonyfamino-5
methyl-3-thienylsulfonyl)-N'-propylthiourea in analogy with the synthesis
described in
Example 1-e; m.p. 175-179 °C.
c) 7-Cvano-8-methyl-3- r~o~ylamino-4H-thienoj2 3-a]-1 2 4-thiadiazine 1 1-
dioxide
The title compound was prepared by hydrolysis and subsequent decarboxylation
of ethyl
7-cyano-6-methyl-3-propylamino-4H-thieno[2,3-e]-1,2,4-thiadiazine-4-
carboxylate 1,1-
dioxide in analogy with the synthesis described in Example 1-f; m.p. 293-98
°C; 'H-NMR
(DMSO-ds), 8(ppm): 11.6 (s, 1 H, NH), 7.65 (br, 1 H, NH), 3.14 (dd, 2H, CHZ),
2.58 (s, 1 H,
CH3), 1.65 - 1.4 (m, 2H, CHz), 0.89 (t, 3H, CH3).
CA 02294830 1999-12-30
WO 99/03861 PC'T/DK98/00288
Ir- I I 4- i 1-ix'
5
a) N-(3-Amino-5-chloro- -tniAny S~ I y1)~' f met Ib~yl, i
Potassium tert-butoxide (0.49 g, 4.4 mmol) was added to a solution of 3-amino-
5-
chlorothiophene-2-sulfonamide hydrochloride (0.5 g, 2.0 mmol) in dry N,N-
10 dimethylformamide (5 ml) with stirring on an ice bath. After 10 min, 3-
methylbutyl isothio-
cyanate (0.31 g, 2.4 mmol) was added dropwise to the resulting suspension, and
the
mixture was stirred for 3.5 h at 0 to 20 °C. Most of the solvent was
evaporated at 40°C,
and the residue was taken up in 25 ml of water, treated with decolourising
charcoal, and
filtered. Acidification of the filtrate with acetic acid to pH 3-4 and
filtration afforded 0.21 g
15 (29%) of the title compound; mp 114-115°C decomp., 'H-NMR (DMSO-ds):
s 0.85 (d, 6H,
2 X CH3), 1.40 (q, 2H, CHZ), 1.50 (m, 1 H, CH), 3.45 (q, 2H, CHz), 6.45 (br,
2H, NHZ), 6.65
(s, 1 H, H-4), 8.30 (br t, 1 H, NH), 11.3 (br s, 1 H).
b) hlor - - 3- I 'n -a - 4- i i 1 1-d'oxi
Phosgene (0.242 ml, 20 % in toluene) was added dropwise to a solution of N-(3-
amino-5-
chloro-2-thienylsulfonyl)-N=(3-methyibutyl)thiourea (0.153 g, 0.42 mmol) and
dry triethy-
lamine (0.118 ml, 0.85 mmol) in dry tetrahydrofuran {3 ml) with stirring at
0°C. The mixture
was stirred for 2 h at 0°C, and evaporated to dryness. Crystallisation
was obtained from
ethyl acetate, and the precipitate was isolated by filtration, washed with
water and dried
affording 38 mg (27%) of the title compound. mp 230-231.5°C, 'H-NMR
(DMSO-ds): 8
0.90 (d, 6H, 2 X CH3), 1.40 (q, 2H, CHZ), 1.60 (m, 1 H, CH), 3.20 (q, 2H,
CHZ), 7.05 (s, 1 H,
H-5), 7.25 (br s, 1 H, NH), 10.95 (s, 1 H, NH). MS: M/e 307(M+)
EXAMPLE 4
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
36
6-Chloro-3-{~1-methvlheptyl)amino-4H-thienoj~,2-a]-1 2 4 thiadiazine 1 1
dioxide
a) N-(3-Amino-5-chloro-2-thienyrl I ~yrl)-N'-i(1-methyrlheprl~thiourea
The title compound was prepared from 3-amino-5-chlorothiophene-2-sulfonamide
hydrochloride and 1-methylheptyl isothiocyanate by a procedure analogous to
the
procedure described in example 3-a; a syrup was obtained (yield 29%), 'H-NMR
(DMSO-
ds): b 0.90 (t, 3H, CH3), 1.10 (d, 3H, CH3), 1.25 (m, 8H), 1.47 (m, 2H, CHZ),
4.25 (p, 1 H,
1 o CH), 6.5 (br s, 2H, NH2), 6.65 (s, 1 H, H-4), 7.95 (br , 1 H, NH), 11.2
(br s, 1 H).
b) 6-Chloro-3-(1-methyihepyl)amino-4H-thieno~(3 2-~]-1 2 4 thiadiazine 1 1
dioxide
The title compound was prepared from N-(3-amino-5-chloro-2-thienylsulfonyl)-N'-
(1-
~ 5 methylheptyl)thiourea by a procedure analogous to the procedure described
in example 3-
b (yield 71 %); mp 179-181 °C, 'H-NMR (DMSO-ds): 8 0.85 (t, 3H, CH3),
1.15 (d, 3H, CH3),
1.30 (m, 8H}, 1.45 (m, 2H, CH2), 3.75 (p, 1 H, CH), 7.05 (s, 1 H, H-5), 7.10
(br s, 1 H, NH),
10.65 (s, 1 H, NH). MS: M/e 349 (M+).
EXAMPLE 5
6-Chloro-3-l1-eth~~penty)amino-4H-thieno[~e~-1 2 4-thiadiazine 1 1 dioxide
a) N-(3-Amino-5-chloro-2-thien_yrlsulfonyl)-_IV'-(1-ethyll entyl thiourea
The title compound was prepared from 3-amino-5-chlorothiophene-2-sulfonamide
hydrochloride and 1-ethylpentyl isothiocyanate by a procedure analogous to the
procedure
described in example 3-a; a syrup was obtained (yield 36%), 'H-NMR (DMSO-ds):
8 0.8 (2
q, 6H, 2 x CH3), 1.2 (m, 4H), 1.5 (m, 4H), 4.20 (sextet, 1 H, CH), 6.55 (br ,
2H, NHZ), 6.65
(s, 1 H, H-4), 7.85 (br d , 1 H, NH), 11.3 (br s, 1 H, NH).
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
37
b) 6-Chioro-3-(1-ethyl en yl)amino-4H-thieno[3.2-a]-1.2.4-thiadiazine 1.1-
dioxide
The title compound was prepared from N (3-amino-5-chloro-2-thienylsulfonyl)-N'-
(1-
ethylpentyl)thiourea by a procedure analogous to the procedure described in
example 3-b
(yield 35%); mp 165-167.5°C,'H-NMR (DMSO-ds): 8 0.85 (2 q, 6H, 2 x
CH3), 1.25 (m,
4H), 1.45 (m, 4H), 3.65 (m, 1 H, CH), 7.0 (br, 1 H, NH), 7.05 (s, 1 H, H-5),
10.65 (br s, 1 H,
NH). MS: MJe 335 (M+).
EXAMPLE 6
6-Chloro-3-(2-methyrlbuty[)amino-4H-thieno[3 2-el-1 2 4-thiadiazine 1 1-
dioxide
a) N j3-Amino-5-chloro-2-thiens Ist uifon,y~,~-N'-(2-methyrlbutyrl)~thiourea
The title compound was prepared from 3-amino-5-chlorothiophene-2-sulfonamide
hydrochloride and 2-methylbutyl isothiocyanate by a procedure analogous to the
proce-
dure described in example 3-a (yield 28%); mp 116.5-118 °C, 'H-NMR
(DMSO-d6): 8 0.8
(2 d, 6H, 2 x CH3), 1.10 (m, 1 H), 1.30 (m, 1 H), 1.65 (m, 1 H), 3.40 (m, 2H +
HDO, CHZ),
6.45 (br , 2H, NHZ), 6.65 (s, 1 H, H-4), 8.25 (br t , 1 H, NH), 11.3 (br s, 1
H).
b) 6-Chloro-3-(2-met Ibuty~a~]no-4H-thieno[3 2-a]-1 2 4-thiadiazine 1 1-
dioxide
The title compound was prepared from N-(3-amino-5-chloro-2-thienylsulfonyl)-N'-
(2-
methylbutyl)thiourea by a procedure analogous to the procedure described in
example 3-b
(yield 49%); mp 232-234 °C, 'H-NMR (DMSO-ds): 8 0.85 (2 d, 6H, 2 x
CH3), 1.15 (m, 1 H),
1.40 (m, 1 H), 1.60 (m, 1 H), 3.10 (m, 2H, CHz), 7.05 (s, 1 H, H-5), 7.25 (br,
1 H, NH), 10.85
(br s, 1 H, NH). MS: Mle 307 (M+).
Analysis: talc. For C,oH,4CIN3O2S2 C 39.02 H 4.58 N 13.65, found C 38.98 H
4.72
N 13.40
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
38
EXAMPLE 7
6-Chloro-3-l1-methvlhexyllamino-4H-thiencZj~P~]-1 2 4-thiadiazine 1 1 dioxide
a) N-l3-Amino-5-chloro-2-thienylsulfony~-~1-methylhexyl)thiourea
The title compound was prepared from 3-amino-5-chlorothiophene-2-sulfonamide
hydrochloride and 1-methylhexyl isothiocyanate by a procedure analogous to the
proce-
dure described in example 3-a; a syrup was obtained (yield 43%), 'H-NMR (DMSO-
ds): 8
0.85 (t, 3H, CH3), 1.10 {d, 3H, CH3), 1.25 (m, 6H), 1.45 (m, 2H), 4.25 (m, 1
H, CH), 6.50 (br
2H, NHZ), 6.65 (s, 1 H, H-4), 7.93 (br, 1 H, NH), 11.3 (br s, 1 H, NH).
b) 6-Chloro-3-(1-methylhexyllamino-4H-thienoj3 2-e] 1 2 4 thiadiazine 1 1
dioxide
The title compound was prepared from N-(3-amino-5-chloro-2-thienylsulfonyl)-N'-
(1-
methylhexyl)thiourea by a procedure analogous to the procedure described in
example 3-
b (yield 63%); mp 158-162 °C, 'H-NMR (DMSO-ds): 8 0.85 (t, 3H, CH3),
1.15 (d, 3H, CH3),
1.25 (m, 6H), 1.45 (m, 2H), 3.75 (m, 1 H, CH}, 7.05 (s, 1 H, H-5), 7.15 (br s,
1 H, NH),
10.75 (br s, 1 H, NH). MS: M/e 335 (M+).
EXAMPLE 8
6-Chloro-3-(cvclopenty)~amino-4H-thieno(3 2-a]-1 2 4-thiadiazine 1 1 dioxide
a) N-l3-Amino-5-chloro-2-thienyrlsulfony~"-1 N'-c~~clo~pentylthiourea
The title compound was prepared from 3-amino-5-chlorothiophene-2-sulfonamide
hydrochloride and cyclopentyl isothiocyanate by a procedure analogous to the
procedure
described in example 3-a (yield 46%); 'H-NMR (DMSO-ds): 8 1.30 - 1.70 (m, 6H),
1.90 (m,
2H), 4.40 (sextet, 1 H, CH), 6.55 (br , 2H, NHZ), 6.65 (s, 1 H, H-4), 8.15 (br
d, 1 H, NH), 11.2
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
39
(br s, 1 H, NH).
b) 6-Chloro-3-(cyclo2entyl)amino-4H-thieno(3.2-e~ 1,~,,~~hiadiazine 1 ~1-
dioxide
The title compound was prepared from N-(3-amino-5-chloro-2-thienylsulfonyl)-N'-
cyclopentylthiourea by a procedure analogous to the procedure described in
example 3-b
(yield 57%); mp 291-292 °C, 'H-NMR (DMSO-de): 8 1.40 - 1.70 (m, 6H,
CH3), 1.90 (m,
2H), 3.95 (sextet, 1 H, CH), 7.05 (s, 1 H, H-5), 7.3 (br, 1 H, NH), 10.70 (br
s, 1 H, NH). MS:
M/e 305 (M+).
o- I - h' i in
a) N-(3-Amino-5-chloro-2-thier~,ylsulfonyl)-N'-,~yclohexylmethyithiourea
The title compound was prepared from 3-amino-5-chlorothiophene-2-sulfonamide
hydrochloride and cyclohexylmethyl isothiocyanate by a procedure analogous to
the
procedure described in example 3-a; a syrup was obtained (yield 8%), 'H-NMR
(DMSO-
ds): 8 0.95 (m, 2H), 1.25 (m, 3H), 1.70 (m, 6H), 3.45 (d, 2H, CHZ), 4.45 (br ,
HDO + NH),
6.65 (s, 1 H, H-4).
8-Chloro-3-(cyclohe,~imeth»[)amino-4H-thienoC~2-a]-1 ~~,4-thiadiazine 1 1-
dioxide
The title compound was prepared from N-(3-amino-5-chloro-2-thienylsulfonyl)-N'-
cyclohexylmethylthiourea by a procedure analogous to the procedure described
in
example 3-b (yield 68%); mp.> 200 °C decomp., 'H-NMR (DMSO-ds): 8 0.90
(m, 2H),
1.15 (m, 3H}, 1.70 (m, 6H), 3.05 (t, 2H, CHZ), 7.05 (s, 1 H, H-5), 7.25 (br ,
1 H, NH), 10.95
(br s, 1 H, NH). MS: Mle 333 (M+).
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
EXAMPLE 10
Ethvl 3-l6-chloro-1 4-dihyrdro-1 1-dioxothieno j3 2-~J,-1 ~6 2,4 thiadiazin 3
~rlamino)~butanoate
5
a) Ethvl 3-{3j(3-amino-5-chlorothi n 2 yr~sulfonvllthiourei Q}butannatA
The title compound was prepared from 3-amino-5-chlorothiophene-2-sulfonamide
hydrochloride and ethyl 3-isothiocyanatobutyrate by a procedure analogous to
the
10 procedure described in example 3-a (yield 93%); 'H-NMR (DMSO-ds): b 1.18
(d, 3H, CH3),
1.20 (t, 3H, CH3), 2.61 (m, 2H, CHZ), 4.08 (q, 2H, CH2), 4.58 (m, 1 H, CH),
6.46 (br s, 2H,
NHZ), 6.65 (s, 1 H, H-4), 8.34 (br d, 1 H, NH), 11.35 (br s, 1 H).
b) ~thv1 3-l6-chloro-1 4-dihydro-1 1-dioxothieno[3 2-~,] 1 ~,s 2 4 thiadiazin
3
~ 5 ylamino)butanoate
The title compound was prepared from ethyl 3-{3[(3-amino-5-chiorothien-2-
yl)sulfonyljthioureido}butanoate by a procedure analogous to the procedure
described in
example 3-b except that the product was purified by column chromatography
(yield 71 %);
20 mp 151-155°C (ethyl acetate); 'H-NMR (DMSO-d6): 8 1.18 (t, 3H, CH3),
1.20 (d, 3H, CH3),
2.57 (m, 2H, CHZ), 4.07 (q, 2H, CHZ), 4.17 (m, 1 H, CH), 7.05 (s, 1 H, H-5),
7.25 (br, 1 H,
NH), 10.99 (s, 1H, NH); MS: m/e 351/353 (M+); (C"H"N3ChO,S2 ~ 0.2 ethyl
acetate) calc.
C 38.36 H 4.26 N 11.37, found C 38.35 H 4.18 N 11.58.
EXAMPLE 11
3-(6-Chloro-1.4-dihvdro-1 1-dioxothieno[3,2-e~-1~.6 2 4 thiadiazin 3
vlaminolbutanoic acid
Ethyl3-(6-chloro-1,4-dihydro-1,1-dioxothieno[3,2-a]-1h6,2,4-thiadiazin-3-
ylamino)butanoate (0.5 g. 1.42 mmol) was hydrolyzed to the acid by stirring in
5 ml of 2 N
sodium hydroxide for 2 h at room temperature. The solution was treated with
decolorising
charcoal, filtered and acidified with 4 M hydrochloric acid to pH 2. The
resulting precipitate
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
41
was isolated by filtration, washed with water and dried to give 294 mg (64 %)
of the title
compound; m.p.218-223°C; 'H-NMR (DMSO-ds): 8 1.19 (d, 3H, CH3), 2.49
(m, 2H, CHz),
4.10 (m, 1 H, CH), 7.06 (s, 1 H, H-5), 7.25 (br, 1 H, NH), 10.99 (s, 1 H, NH),
12.38 (br s, 1 H,
OH); MS: m/e 305/307 (M - Hz0)'; (CgH~°N3CI,O4S2) calc. C 33.39 H 3.11
N 12.98, found
C 33.62 H 3.11 N 12.81.
EXAMPLE 12
~ 0 6-Chloro-3-(3-hydroxy-1-meth~~progyl)amino-4H-thienoj3 2-a]-1,~ 4-
thiadiazine 1 1-
i xi
Toluene (5 ml) was cooled to 10°C and lithium aluminum hydride (114 mg,
3 mmol) was
added followed by 0.53 ml of tetrahydrofuran. Ethyl 3-(6-chloro-1,4-dihydro-
1,1-
dioxothieno[3,2-e]-1~.6,2,4-thiadiazin-3-ylarnino)butanoate (352 mg, 1 mmol)
was added to
the cooled solution and the mixture was stirred for 2 h at 0°C and then
at room tempera-
ture over night. The mixture was cooled on an ice bath and 10 ml of water was
added
dropwise followed by 5-10 ml of 20 % sulfuric acid. The mixture was extracted
with ether
(3 x 30 ml) and the organic phase was washed with water, dried and evaporated
to
2o dryness. The crude product was mixed with the solid that was formed in the
aqueous
phase and finally purified by column chromatography on silica with ethyl
acetate/methanol
(9:1) affording 120 mg (39 %) of the title compound; m.p.199-203°C; 'H-
NMR (DMSO-dfi):
8 1.14 (d, 3H, CH3), 1.65 (m, 2H, CHZ), 3.48 (m, 2H, CHZ), 3.90 (m, 1 H, CH),
4.60 (br s,
1 H, OH), 7.06 (s, 1 H, H-5), 7.17 (br, 1 H, NH), 10.86 (s, 1 H, NH); MS: m/e
309/311 (M');
(C9H,ZN3CI,O3S2 ~ 0.15 ethyl acetate) calc. C 35.70 H 4.12 N 13.01, found C
35.7 H 4.1
N 13.1.
EXAMPLE 13
(Rl-6-Chloro-3~1-ohen~ylethy~amino-4H-thienoj3 2-el-1 2 4-thiadiazine 1 1-
dioxide
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
42
a) 1R1-N-l3-Amino-5-chloro-2-thienylsmulfonyl -LN'-(1;~~henyl~f ~,1, j ~rPa
The title compound was prepared from 3-amino-5-chlorothiophene-2-sulfonamide
hydrochloride and D-a-methylbenzyl isothiocyanate by a procedure analogous to
the
procedure described in example 3-a (yield 96% impure product); 'H-NMR (DMSO-
ds): 8
1.46 (d, 3H, CH,), 5.36 (quint, 1 H, CH), 6.45 (br s, 2H, NHZ), 6.64 (s, 1 H,
H-4), 7.2-7.4 (m,
5H, ArH), 8.53 (br d, 1 H, NH), 11.3 (br s, 1 H).
b) (R)-6-Chloro-3-l1-ohenvlethy)amino-4H-thieno[3 2 e~ 1 2 4 thiadiazine 1,1
dioxide
The title compound was prepared from crude (R)-N-(3-Amino-5-chloro-2-
thienylsulfonyl)-
N'-(1-phenylethyl)thiourea by a procedure analogous to the procedure described
in
example 3-b except that the product was purified by column chromatography on
silica with
dichloromethane/methanol (19:1 ), (yield 17%); mp 218-220°C (ethyl
acetate); 'H-NMR
(DMSO-ds): 8 1.48 (d, 3H, CH3), 4.97 (quint, 1 H, CH), 7.10 (s, 1 H, H-5), 7.2-
7.4 (m, 5H,
ArH), 7.73 (br, 1 H, NH), 10.81 (s, 1 H, NH); MS: mle 341/343 (M+);
(C,3H,ZN3CI,OZS2) talc.
C 45.68 H 3.54 N 12.29, found C 45.83 H 3.55 N 12.04.
EXAMPLE 14
(Sl-3-sec-Butvlamino-6-chloro-4H-thieno[3 2-a]'-1 2 4 thiadiazine 1 1 dioxide
A solution of 3,6-dichloro-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide (257
mg, 1.0
mmol) and (S)-(+)-sec-butylamine (0.31 ml, 3.0 mmo!) was stirred in 10 ml of
abs ethanol
for 4 days at 80°C in a sealed flask. The cooled solution was
concentrated in vacuo and
the residue was stirred with water (10 ml) followed by adjustment to pH 2 with
4M
hydrochloric acid. The initially formed gummy product was crystallized by
stirring at 0°C.
3o The precipitate was isolated by filtration, washed with water, and
recrystallised from ethyl
acetate/methanol followed by drying in vacuo at 30°C over night to give
181 mg (62 %) of
the pure title compound; mp 228-230°C (ethyl acetate); 'H-NMR (DMSO-
d6): 8 0.88 (t,
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
43
3H, CH3), 1.14 (d, 3H, CH3), 1,49 (m, 2H, CHZ), 3.68 (m, 1 H, CH), 7.08 (s, 1
H, H-5), 7,13
(br, 1 H, NH), 10.73 (br s, 1 H, NH); MS: m/e 293!295 (M+); (C9H,ZN3CI,OZSz)
calc. C 36.79
H 4.12 N 14.30, found C 36.9 H 4.1 N 14.2.
EXAMPLE 15
6-Chloro-3-iso r~opylamino-4H-thien~,[2 3-e)-1 2 4-thiadiazine 1 1-dioxide
a) N-(5-Chloro-3-(iso r~opyrlthiocarbamoy,[am f y~~lc~~hen-2-y~]acet_amj~Je
Potassium tert-butoxide (135 mg, 1.2 mmol) was added to a solution of N-(5-
chloro-3-
sulfamoylthiophen-2-yl)-acetamide(255 mg, 1.0 mmol) in dry N,N-
dimethylformamide (5
ml) with stirring on an ice bath. After 5 min, isopropyl isothiocyanate (0.128
ml, 1.2 mmol)
was added dropwise and the solution was stirred for 30 min at 0°C and
then at room
temperature for 3 h. A further amount of potassium tert-butoxide (135 mg, 1.2
mmol) was
added to the solution and stirring was continued at room temperature for 1 h.
The solvent
was evaporated at <50°C, and the residue was taken up in 10 ml of water
and the
2o aqueous solution was adjusted to pH 2 with 4 M hydrochloric acid. The
resulting precipi-
tate was isolated by filtration, washed with water and dried to give 274 mg
(77%) of crude
title compound; 'H-NMR (DMSO-ds): b 1.12 (d, 6H, 2 x CH3), 2.28 (s, 3H,
COCH3), 4.21
(m, 1 H, NCH), 7.15 (s, 1 H, H-4), 8.34 (br d, 1 H, NH), 10.26 (s, 1 H, NH).
b) 6-Chloro-3-iso r~o~yrlamino-4H-thienQ[2 3-el-1 2 4-thi~dlazine 1 1-dioxide
Phosgene (0.416 ml, 20 % in toluene) was added dropwise to a solution of N-[5-
chloro-3-
(isopropylthiocarbamoyl)sulfamoylthiophen-2-yl]acetamide(261 mg, 0.73 mmol)
and dry
triethylamine (0.209 ml, 1.5 mmol) in dry tetrahydrofuran (5 ml) with stirring
at 0°C. The
3o mixture was stirred for 1 h at 0°C, and evaporated to dryness. The
residue was triturated
with 10 ml of water, and the precipitate was isolated by filtration, washed
with water and
finally deacetylated by stirring in 2 ml of 2 N sodium hydroxide for 90 min at
room
temperature. The solution was acidified to pH 2 with 4 M hydrochloric acid and
the
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
44
precipitate was filtered off and recrystallised from ethyl acetate with
decolorising charcoal
affording 44 mg (21 %) of the pure title compound; mp 272-274°C (ethyl
acetate); 'H-NMR
(DMSO-ds): 8 1.16 (d, 6H, 2 x CH3), 3.85 (m, 1 H, NCH), 7.23 (s, 1 H, H-7),
7.48 (br d, 1 H,
NH), 11.12 (s, 1 H, NH); MS: m/e 279/281 (M+).
6-Chloro-3-cvclol~ylamino-4H thienoj2.3 e] 1 2 4 thiadiazine 1 1 ioxide
a) N-f5-Chloro-3-l _vctr,r,Antvlthiocarbamoyl)sulfamoyl hio hp en 2
ylJ2icetamide
The title compound was prepared from N-(5-chloro-3-sulfamoylthiophen-2-yl)-
acetamide
and cyclopentyl isothiocyanate by a procedure analogous to the procedure
described in
example 15 a (yield of crude product: 93%); 'H-NMR (DMSO-ds): 8 1.3-2.0 (m,
8H,
(CHZ)4), 2.28 (s, 3H, CH3), 4.32 (sext, 1 H, CH), 7.16 (s, 1 H, H-4), 8.48 (br
d, 1 H, NH),
10.23 (br s, 1 H, NH).
b) 6- hlor -3- o lam~n -4 - i n 4-thi diazin 1 1- i x'
The title compound was prepared from N-(5-chloro-3-
{cyclopentylthiocarbamoyl)sulfamoylthiophen-2-ylJacetamide by a procedure
analogous to
the procedure described in example 15 b (yield 32%); mp 280-282°C
(aqueous ethanol);
'H-NMR (DMSO-ds): 8 1.4-2.0 {m, 8H, (CHz)4), 3.96 (sext, 1 H, CH), 7.23 (s, 1
H, H-7),
7.62 (br, 1 H, NH), 11.09 (s, 1 H, NH); MS: m/e 3051307 (M+).
EXAMPLE 17
-Bromo-3-is r I m~no-4 - hi -1 2 4- hi di zin 1 1- i i
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
Bromine (0.12 ml, 2.3 mmol) was added dropwise to a solution of 6-chloro-3-
isopropylamino-4H-thieno[3,2-e]-1,2,4-thiadiazine 1,1-dioxide (280 mg, 1.0
mmol) in 10 ml
of acetic acid and the mixture was stirred for 24 h at 100°C in a
sealed flask. The cooled
mixture was evaporated to dryness and the residue was triturated with water to
give a
5 solid, which was recrystallised from ethanollwater (1:1 ) affording 118 mg
(39 %) of the title
compound contaminated with 10 % of the starting material; mp ca. 279°C
(aqueous
ethanol); 'H-NMR (DMSO-ds): b 1.16 {d, 6H, 2 x CH3), 3.86 (m, 1 H, CH), 7.14
(s, 1 H, H-
5), 7.18 (br, 1 H, NH), 10.74 (s, 1 H, NH); MS: mle 3231325 (M+)
EXAMPLE 18
3-Iso~r_opylamino-4H-thieno'[3 2-e~ 1,2 4-thiadiazine 1 1-diox~e
Powdered potassium hydroxide (128 mg, 2.28 mmol) was added to a solution of 6-
chloro-
3-isopropylamino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide (319 mg, 1.14
mmol) in 25
ml of methanol and the mixture was hydrogenated at room temperature and
atmospheric
pressure for 3 days with 150 mg of 10 % palladium on carbon. The catalyst was
removed
by filtration and washed with ethanol and water. The combined filtrate was
acidified with 4
M hydrochloric acid and evaporated to dryness. The residue was recrystallised
from
waterlethanol and finally from ethyl acetate with decolorising charcoal
affording the title
compound contaminated with starting material, which could be removed by silica
gel
column chromatography ; 'H-NMR (DMSO-ds): 8 1.16 (d, 6H, 2 x CH3), 3.88 (m, 1
H, CH),
6.95 (d, 1 H, H-5), 7.03 (br d, 1 H, NH), 7.88 (d, 1 H, H-6), 10.70 (br s, 1
H).
EXAMPLE 1
3-Isooroavlamino-7-methyl-4H-thieno[2 3-a]-1 2 4-thiadiazine 1 1-dioxide
a) Cyanomethanesulfonamide
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
46
Dry THF (200 ml) was saturated with ammonia at -10 °C. Then a solution
of cya-
nomethane-sulfonyl chloride (13.9 g; prepared according to Sammes et al,
J.Chem.Soc.,
1971, 2151-5155) in 10 ml of dry THF was added in small portions with stirring
over 20
min at -10 °C. After the addition the temperature was rised to 0
°C and the reaction
mixture was filtered. Removal of the solvent from the filtrate and
purification of the residue
by column chromatography on silica gel eluted with ethyl acetate gave the
title compound
as beige crystals; m.p. 97-99 °C; IR (KBr), v (cm-'): 3316, 3328 (N-H),
2266 (C---N), 1371,
1151 (SOZ).
b) 2-Amino-4-metal-thiolahene-3-sulfonamide
A mixture of cyanomethanesulfonamide (1.0 g), 2,5-dihydroxy-2,5-dimethyl-1,4-
dithiane
~ 5 (0.75 g), and triethylamine (100 ~I) in absolute ethanol (7 ml) was heated
at 45-50 °C
under nitrogen for 3 h. Then the solvent was evaporated and the residue
partitioned in
water (30 ml) and ethyl acetate (50 ml). The aqueous phase was extracted with
4 x 50 ml
of ethyl acetate. The combined ethyl acetate phases were dried over sodium
sulfate and
evaporated. The residue was purified by column chromatography on silica gel
eluted with
2o ethyl acetate. The title compound was obtained as a yellow solid, yield
0.65 (41%); m.p.
142-144 °C;'H-NMR (CD30D), 8 (ppm): 7.19 (s, 1 H, C(5)I-n, 4,88 (br, 4
H, SOZNHZ+
HZO), 4.71 (s, 2 H, NHZ), 2.41 (s, 3 H, CH3); MS: 192 (M' ), 112 (M+ -
SOzNHz), 72 (CH3-
CZHS' )
c) N-(2-Amino-4-methyl-3-thieflsulfony~-N~-isoprowlthiourea
A mixture of 2-amino-4-methyl-thiophene-3-sulfonamide (0.30 g), potassium
carbonate
(0.32 g), and isopropyl isothiocyanate (272 ~I) in dry acetone (5 ml) under
nitrogen was
heated at 50-55 °C overnight. Then the solvent was evaporated and the
residue was
dissolved in water (15 ml); pH was adjusted to 2 by addition of 4 M
hydrochloric acid, and
the mixture was stirred for 30 min. The precipitated crystals were filtered
off, rinsed with a
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
47
small amount of water and dried to give 0.24 g (yield 54%) of the title
compound; m.p.
118-120 °C.
d) 3-IsoRropyrlamino-7-methy -4H-thieno'(2.3-a]-1 2,,4-thiadiazine 1 ~1-
dioxide
A 20 % solution of phosgene in toluene (715 ~I) was added to a stirred and
cooled solution
of N-(2-amino-4-methyl-3-thienylsuffonyl)-N'-isopropylthiourea (0.22 g) and
triethylamine
(313 wl) in dry THF (5 ml) the temperature being kept below 5 °C. After
stirring for 1.5 h
the mixture was evaporated and triturated with 10 ml of water. The precipitate
was
~ 0 collected by filtration and dried to give 0.14 g of crude product. The
crystals were heated
at 70 °C with 5 ml of ethyl acetate and the resulting mixture was
cooled to 0 °C for 10 min
and then filtered. The filter cake was rinsed with a minute amount of ethyl
acetate and
dried to give 0.087 g (yield 45 %) of the title compound as tan crystals; m.p.
152-154 °C;
'H-NMR (DMSO-ds): 8 (ppm): 7.10 (br d, 1 H, NH), 6.61 (s, 1 H, N(4)H), 6.52
(s, 1 H,
C(6)H), 3.89 (m, 1 H, CH), 2.32 (s, 3 H, CH3), 1.18 (d, 6 H, CH3).
EXAMPLE 20
6-Chloro-3-c~rclobyyrlamino-4H thieno~'~ 2 P] 1 2 4 thiadiazine 1 1 ioxide
A solution of 3,6-dichloro-4H-thieno(3,2-e]-1,2,4-thiadiazine 1,1-dioxide (257
mg, 1.0
mmol) in cyclobutylamine (1.0 ml) was stirred for 18 h at 90°C in a
sealed flask. The
cooled solution was concentrated in vacuo and the residue was stirred with
water (10 ml)
at 0°C followed by adjustment to pH 2 with 4M hydrochloric acid. The
product was isolated
by filtration, washed with water, and recrystallised from methanol/ethyl
acetate followed by
drying in vacuo at room temperature to give 155 mg (53 %) of the pure title
compound; mp
315-317°C dec.; 'H-NMR (DMSO-ds): 8 1.58-1.75 (m, 2H), 1.89-2.05 (m,
2H), 2.19-2.30
{m, 2H), 4.16 (m, 1 H), 7.06 (s, 1 H), 7.62 {br s, 1 H), 10.83 (br s, 1 H);
MS: m/e 291 /293
(M+); (C9H,°N3CI,OZSz) talc C 37.05 H 3.45 N 14.40, found C 37.18 H
3.48 N 14.19.
EXAMPLE 21
6-Chloro-3-(2-hydrox~ethyl)amino-4H thieno~(3 2 e] 1 2 4 thiadiazine 1 1
dioxide
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
48
A solution of 3,6-dichloro-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide (206
mg, 0.8
mmol) in ethanolamine (1.0 ml) was stirred for 18 h at 100°C in a
sealed flask. The cooled
solution was concentrated in vacuo and the residue was stirred with water (5
ml) at 0°C
followed by adjustment to pH 2 with 4M hydrochloric acid. The product was
isolated by
filtration, washed with water, and recrystallised from ethanol/water to give
135 mg (60 %)
of the pure title compound; mp 260-261 °C dec.; 'H-NMR (DMSO-ds): 8
3.26 (distorted q,
2H), 3.50 (t, 2H), 4.85 (br s, 1 H), 7.07 (s, 1 H), 7.20 (br s, 1 H), 10.9 (br
s, 1 H); MS: m/e
281/283 (M'); (C,HeN3CI,O3S2} calc C 29.84 H 2.86 N 14.91, found C 30.13 H
2.84 N
14.79.
EXAMPLE 22
(t)-3-exo-Bicvclof2 2 1]kept-2-ylamino-6-chloro-4H-thieno[3 2 eJ 1 2 4
thiadiazine 1 1
xi a
A solution of 3,6-dichloro-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide (206
mg, 0.8
mmol) in exo-2-aminonorbomane (1.0 ml) was stirred for 20 h at 100°C in
a sealed flask.
The mixture was stirred with water (10 ml} at 0°C followed by
adjustment to pH 2 with 4M
hydrochloric acid. The product was isolated by filtration, washed with water,
and recrystal-
lised from ethyl acetate/methanol to give 168 mg (63 %) of the pure title
compound; mp
323-324°C dec.; 'H-NMR (DMSO-ds): 8 1.05-1.55 (m, 7H), 1.68-1.77 (m, 1
H), 2.18-2.28
(m, 2H), 3.51 (m, 1 H), 7.09 (s, 1 H), 7.2 (br s, 1 H), ca.10.5 (br s, 1 H).;
MS: mle
331/333(M'); (C~zH,4N3CI,OZS2) calc C 43.43 H 4.25 N 12.66, found C 43.67 H
4.26 N
12.55.
EXAMPLE 23
R)-6-Chloro-3-l2-hvdroxy~py~)amino-4H-thieno[3 2-a]-1 2 4-thiadiazine 1 1
dioxide
A solution of 3,6-dichloro-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide (200
mg, 0.78
mmol) in (R)-(-)-1-amino-2-propanol (1.0 ml) was stirred for 18 h at
100°C in a sealed
flask. The cooled solution was stirred with water (3 ml) at 0°C
followed by adjustment to
pH 2 with 4M hydrochloric acid. The product was isolated by filtration, washed
with water,
and dried in vacuo at room temperature to give 170 mg (74 %) of the pure title
compound;
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
49
mp 210-211 °C .; 'H-NMR (DMSO-ds): 8 1.08 (d, 3H), 3.0-3,1 (m, 1 H),
3.15-3.25 (m, 1 H),
3.72-3.82 (m, 1 H), 4.91 (br s, 1 H), 7.09 (s, 1 H), 7.14 (br s, 1 H), 10.95
(br s, 1 H).; MS: m/e
2971295{M'); (CBH,oN3C1,03Sz~ 0.5 H20) talc C 31.53 H 3.64 N 13.79, found C
31.57 H
3.58 N 13.58.
EXAMPLE 24
6-Bromo-3-iso~ropylamino-4H-thieno,[3 2-a]-1 2 4-thiadiazine 1 1-dioxide
~ 0 Bromine (1.26 ml, 25 mmol) was added dropwise to a solution of 6-chloro-3-
isopropylamino-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,1-dioxide (2.3 g, 8.2
mmol) in 25 ml of
acetic acid and the mixture was stirred for 48 h at 100°C in a sealed
flask. The cooled
mixture was evaporated to dryness and the residue which consisted of two major
products
was triturated with water to give a solid, which was recrystallised from ethyl
ace-
tate/methanol with decolorising charcoal to afford 538 mg (20 %) of the title
compound. An
analytically pure sample was obtained by preparative hplc on a Source RPC 15
column
using acetonitrile/water (20:80) with 0.1 % TFA as eluent; mp 282-
283°C; 'H-NMR {DMSO-
ds): 8 1.16 (d, 6H), 3.86 (m, 1 H), 7.14 (s, 1 H), 7.18 (br, 1 H), 10.74 (s, 1
H); MS: m/e
3231325 (M'); (CeH,°N3Br,O2S2); talc C 29.64 H 3.11 N 12.96; found C
29.49 H 3.04 N
12.59.
EXAMPLE 25
5 6-Dibromo-3-isolaropvlamino-4H-thien~3 2-a]-1 2 4-thiadiazine 1 1-dioxide
The mother liquor from the crystallisation of 6-brorno-3-isopropylamino-4H-
thieno[3,2-a]-
1,2,4-thiadiazine 1,1-dioxide described above was evaporated to dryness and
the residue
was purified by chromatography on silica with dichloromethanelmethanol (95:5).
Recrys-
3o tallisation from ethyl acetate gave 270 mg (8%) of pure title compound; mp
250-251 °C; 'H-
NMR {DMSO-d6): 8 1.18 (d, 6H), 3.86 (m, 1 H), 7.18 (br, 1 H), 10.31 (s, 1 H);
MS: mle
405/4031401 (M+); (CeH9N3BrzO2S2); talc C 23.84 H 2.25 N 10.42; found C 24.14
H 2.18
N 10.25.
EXAMPLE 26
6-Chloro-3-cyciohexylamino-4H-thienof3 2-a]-1 2,4-thiadiazine 1 1-dioxide
CA 02294830 1999-12-30
WO 99/038b1 PCT/DK98/00288
a) N-(3-Amino-5-chloro-2-thienylsulfonyl)-N'-(cyrclohexy~ ' ~rAa
The title compound was prepared from 3-amino-5-chlorothiophene-2-sulfonamide
5 hydrochloride and cyclohexyi isothiocyanate by a procedure analogous to the
procedure
described in example 3-a (yield 78%); 'H-NMR (DMSO-ds): 8 1.1-1.9 (m, 10H),
4.0 (m,
1 H), 6.45 (br s, 2H), 6.66 (s, 1 H), 8.05 (br d, 1 H), 11.2 (br s, 1 H).
b) 6Chloro-3-cvclohexvlamino-4H-thienoj3 2-a]-1 2 4-thiadiazine 1 1 dioxide
The title compound was prepared from N-(3-Amino-5-chloro-2-thienylsulfonyl)-N~-
(cyclohexyl)thiourea by a procedure analogous to the procedure described in
example 3-b
(yield 66%); mp 282-284°C (ethyl acetate/methanol); 'H-NMR (DMSO-ds): 8
1.1-1.9 (m,
1 OH), 3.55 (m, 1 H), 7.08 (s, 1 H), 7.19 (br, 1 H), 10.73 (br s, 1 H); MS:
m/e 321 /319 (M');
15 (C"H,4N3CI,OZS2) calc. C 41.31 H 4.41 N 13.14, found C 41.66 H 4.45 N
12.99.
EXAMPLE 27
6-Chloro-3-lfuran-2-ytmethy~amino-4H thieno[3 2 e] 1 2 4 thiadiazine 1 1
dioxide
a) N-(3-Amino-5-chloro-2-thienylsulfonyl)-N'-(furan-2 ylmethyl)thiourea
The title compound was prepared from 3-amino-5-chlorothiophene-2-sulfonamide
hydrochloride and furfuryl isothiocyanate by a procedure analogous to the
procedure
described in example 3-a (yield of crude product: 92%).
b) -Chlor - - f r n-2- Im h in -4 - n -a - 4- i i i 1 - i ' a
The title compound was prepared from crude N-(3-Amino-5-chloro-2-
thienylsulfonyl)-N'-
(furan-2-ylmethyl)thiourea by a procedure analogous to the procedure described
in
example 3-b except that the product was purified by column chromatography on
silica with
dichloromethane/methanol (19:1), (yield 11%); mp 224-225°C; 'H-NMR
(DMSO-d6): 8 4.41
(d, 2H), 6.33 (m, 1 H), 6.41 (m, 1 H), 7.05 (s, 1 H), 7.62 (br s, 1 H}, 7.75
{br t, 1 H), 11.2 (br s,
1 H); {C,oHeN3CI,O3S2); calc C 37.80 H 2.54 N 13.22; found C 37.87 H 2.51 N
13.10).
CA 02294830 1999-12-30
WO 99/038b1 PCT/DK98/00288
51
To a solution of 6-bromo-3-isopropylamino-4H-thieno[3,2-a]-1,2,4-thiadiazine
1,1-
dioxide (243 mg, 0.75 mmol) in dry N,N-dimethylformamide (2 ml) was added
copper(I) cyanide (135 mg, 1.5 mmol) and the mixture was heated at 150°
C for 2 h
under nitrogen. The dark mixture was allowed to cool to room temperature and
water
was added. The suspension was made basic by the addition of 1 N sodium
hydroxide,
filtered and the filtrate was acidified by the addition of 4 M hydrochloric
acid. The
resulting precipitate was purified by preparative hplc on a Source RPC 15
column
using acetonitrile/water (20:80) with 0.1 % TFA as eluent to afford 4 mg (2%)
of the
pure title compound; LC-MS: m/e 271 (IM + 1 )+)
Bromine (0.12 ml, 2.3 mmol) was added dropwise to a solution of 6-chloro-3-
cyclopentylamino-4H-thieno[3,2-a]-1,2,4-thiadiazine (305 mg, 1.0 mmol) in
acetic acid
(10 ml) and the mixture was stirred for 21 h at 100 °C in a sealed
flask. The cooled
mixture was evaporated to dryness and the residue was triturated with water to
give
a solid, which was recrystallised from ethyl acetate and ethanol affording the
title
compound (166 mg, 47%) contaminated with 33% of the starting material.
Purifica-
tion by preparative HPLC gave the title compound (65 mg, 18%) contaminated
with
3% of the starting material. 'H-NMR (DMSOI: 8 10.7 (s, 1 H, NH); 7.35 (br s, 1
H,
NH); 7.13 (s, 1 H, H-7); 4.00 (sextet, 1 H); 1.9 (m, 2H); 1.7 to 1.4 ppm (m,
6H).
Decomp.: 287-294 °C
EXAMPLE 30
6-Chloro-3-(2-methvlallyllamino 4H thienof3 Pl 1 ? 4 thiadiazine 1 1 dioxide
A solution of 3,6-dichloro-4H-thieno[3,2-a]-1,2,4-thiadiazine 1,i-dioxide (128
mg, 0.5
CA 02294830 1999-12-30
WO 99/03861 PCT/DK98/00288
52
mmol) in methallylamine (0.5 ml) was stirred for 48 h at 90°C in a
sealed flask. The
cooled solution was concentrated in vacuo and the residue was stirred with
water (3
ml) followed by adjustment to pH 2 with 4M hydrochloric acid. The product was
isolated by filtration and washed with water, to give 92 mg (64 %) of the pure
title
compound; mp 224-226 °C (dec.); 'H-NMR (DMSO-ds): b 1.72 (s, 3H), 3.75
(d, 2H1,
4.83 (s, 2H), 7.05 (s, 1 H), 7.45 (br s, 1 H), 11.0 (br s, 1 H).