Note: Descriptions are shown in the official language in which they were submitted.
CA 02315055 2000-06-16
" ' ~ ~ ~ ~ ~ ~ ~ ~ ~. : PCT/EP98/08582
06-04-20I)0 , , , , , , ~ ~ ~ ~ ~ ~
~ ~ ~ a . . . a . ..i. . ~ i i
. . . . . . ~ . . ~ .
.~ s. ~. . ~. ..
1
Benzo[c]quinolizine derivatives and their use as 5a-reductases inhibitors.
The present invention refers to fully and partially saturated benzo[c]-
quinolizine
derivatives of general formula (I)' their pharmaceutically acceptable salts or
esters, processes for their preparation and composition for pharmaceutical and
s agricultural use containing them.
Field of the invention
The present invention refers to benzo[c]quiraolizine derivatives of general
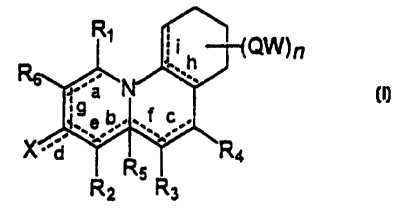
formula (I)
-1~( a~~n
l .h J
to
9 a'b'1 f c
a _...,_ ,.
- ~ -
Rs (I)
wherein: . RZ R3 .
R" R2, R3, R,, Re, same or different, are chosen in the group consisting of:
H,
C,$alkyl, CZ.~alkenyl, C;,$alkynyl, cycloalkyl, aryl, heterocycle, halogen,
CN,
azide, NRR', C,.~alkylamino, arylamino, C,~alkyloxy, aryloxy, COOR, CONRR',
C(=O)R wherein R and R',. same or different, are chosen in the group
consisting
of: H, C,.~alkyl, cycloalkyl, aryl, heterocyGe, arylC,-0alkyl;
zo R5 is chosen in the group consisting of: H, C,$alkyl, C,.~alkylaryl, COOR,
CN,
aryl, heterocycle, C,.~alkyl-heterocycle; C,.alkyl-heterocycle-ribose-
phosphate
X is chosen in the grcwp consisting of: O, C(=O)R, COOR, N02, CONR'R
wherein R and R' are as above defined;
Q is chosen in the group consisting of: simple bond, C,~alkyl, CZ~alkenyl, C2.
25 8alkynyl, cycloalkyl, CO, CONR, NR, wherein R is as above defined;
W is chosen in the group consisting of: H, C,.~alkyl, CZ$ alkenyl, C2-
0alkynyl,
cycloalkyl, trifluoromethyl, C,~alkoxy, C,.~ alkoxy-C,.~alkyl, arylC,.~alkyl,
aryl,
aryloxy, arylamino, C,$alkylcarbonyl, arylcarbonyl, arylcarboxyl,
arylcarboxyamide, halogen, CN, NRR', C,-0alkylamino, .heterocycle wherein.the
so groups alkyl, aikenyl, alkynyl, cycloalkyl, aryl, heterocycle, can be
substitued; n
is an integer comprised between 1 and 4;
AMENDED SHEET
~
CA 02315055 2000-06-16
~ ~ ~ ~ ~ ~ ~ ~ ~ " .' ~. : PCT/EP98/08582
06-04-2000 ~ . . . . .... . .. _ . ..
~ . i . ~ . . 7 . ~... . . ~ . .
' 1 . ~ ~ 1 ~ . i ~ . . 1
.1 .. .. . ~. ..
2
the symbol means that the corresponding bonds a, b, c, d e, f, g, h and i
can be a simple or a double bond; with the proviso that when b or f are a
double bond then the group R5 is absent;
their pharmaceutically acceptabie~ salts or esters, their process of
preparation
s and their use as inhibitors of steroid 5a-reductases.
. State of the art
The enzyme known a5 steroid 5a-reductase (hereinafter indicated as 5a-
reductase) is a system formed by two iso-enzymes (type I and type II or 5aR-I
and 5aR-II respectively) which converts testosterone into dihydrotestosterone,
to the most powerful androgen circulating in the body.. The type I iso-enzyme
(5aR-I) is mainly present in liver and, skin while the type II iso-enzyme (5aR-
II) ~ '
is mainly present in the prostate tissue and in the male sexual organs and its
activity is essential in the fetal developping process for the differentiation
of the
external sexual organs. The production of dihydrotestosterone is associated .
~s with some pathologies which are widely diffused as for example benign
prostate
hypertrophy, prostate cancer, baldness and acne in men and hirsutism in
women. More particularly iso-enzyme I plays a role in the pathologies
regarding -
the skin while iso-enzyme-II is involved in prostate pathologies. In the
recent
years a lot of international searchers have tried to isolate new compounds
Zo capable of inhibiting the 5a-reductase enzyme in order to treat the above
said
pathologies, especially, if possible, acting selectively on only one of the
two iso-
enzymes. Inhibitors of 5a-reductase, and also, of the iso-enzymes 5aR-I and
5aR-II were already described [see for example J.Med.Chem. 36, 4313-15
(1993), J.Med.Chem. 37, 3871-74 (1994), ~ J.Med.Chem. 40, 1112 (1997)
25 J.Med.Chem. 40, 3466 (1997)]; for example finasteride was used 'with
success
in the treatment of benign prostate hypertrophy.
In EP-703 221, EP-591 582, EP-591 583, EP-532 190 and EP-531 026
benzoquinoline-3-ones as 5a.-reductase inhibitors are reported while WO
94121614 describes substituted 3-phenanthridinone derivatives having the
same action.
AMENDED SHEET
. CA 02315055 2000-06-16
:. .: . ", , ~ ' .' ~ . : pCT/EP98/08582
06-04-20~D0 . . . a .... . ..
s ~ . .s . . : ..... . : ii
. . . ., . . ~ , . ..
~ : .. .. .. . .. .:-
2a
Journal of the Chemical Society, Perkin Transaction 1, vol 3, 1979 pages 584 -
590, describes i.a. a benzo[c]quinolizine (see compound 8), without indicating
any use thereof.
It is therefore evident the importance of developping new compounds capable
of inhibiting the action of the 5a-reductase enzyme and in particular capable
of
acting selectively on SaFt-I iso-enzyme which, as said, is responsible, of
widely
diffused pathologies having an high impact as baldness in men and hirsutism in
women.
AMENDED SHEET
CA 02315055 2000-06-16
06-04-2Gi)0 : i .: i !', , ~ ' i.. ~ ~ PCT/EP98/08582
i ~ i i ~ i a.~ i i i : . .
. i ~ i . . . . , ~~~, ~ i , ~
1 ~ i i ~ ~ . 1 ~ i 1 i i
~ 1 , i, 1. i~ i ii fi~
3
Therefore the invention refers also to a method for the treatment of
pathologies
related to 5a-reductase enzymes and in particular for the treatment of acne, .
baldness, prostatic cancer and prostatic hypertrophy in men and hirsutism in
women. Moreover it was also found, and it is another object of the present
invention, that the compound of formula (I) can inhibit steroid 5a-reductase
enzymes in plants and therefore ~ can selectively regulate the .plant growth
in
light and dark condition . The compounds according to the present invention
can be used as phyto-pharmaceuticals in agriculture permitting to improve the
morphogenesis and development of commercially useful plants or as herbicides
capable of inhibiting the growth ~of infesting plants. The compounds can
therefore be used in agricultural compositions for regulating the plant growth
in
particular those which are distributed on the seeds andlor the plants to
treat.
Detailed description of the invention
The present invention refers to new compounds capable of inhibiting the 5a-
reductase enzyme, either selectively in respect of 5aR-I and 5aR-II or on both
the iso-enzymes, useful for the treatment of the pathologies mediated by the
s enzyme or for agricoltural uses as plant growth regulators or herbicides.
The products according to the invention have general formula
R, I 1
;;i h -~-f-QW)n
to ~9 a iv
I~._ a b.~1~.~ c
'd
2
(I)
is wherein the substituents R,, R2, R3, R4, R5, Re, X,~~Q,1N, n and the ~
symbol -
--- are as above defined.
According to the present invention with group C,$alkyl, C2~ alkenyl and C2_
8alkinyl are indicated linear or branched alkyl radicals as for example:
methyl,
AMENDED SHEET
~
CA 02315055 2000-06-16
:. .: .".." ."-. : PCT/EP98/08582
06-04-2600
. . . . . . . . .... . . . .
~ . . ~ : ._ ._
_ . : .. .. .: . .._ :.-
3a
ethyl, propyl, isopropyl, butyl, pentyl, hexyl, heptyl, octyl, ethylene,
propene,
butene, isobutene, acetylene, propine, butine ecc. .
With cycloalkyl are indicated: cyclopropane, cyclobutane, cyclopentane,
cyclohexane, cycloheptane, cyclooctane, norbornane, canphane, adamantane.
s With aryl are indicated: phenyl, biphenyl and naphtyl.
AMENDED SHEET
CA 02315055 2000-06-16
WO 99/33828 PCT/EP98/08582
4
Heterocycle means in particular: saturated or aromatic heterocycles containing
one or more N atoms, more particularly: pyridine, imidazole, pyrrole, indole,
triazoles, pyrrolidine, pyperidine.
Phosphate means the anion of mono-, di- or triphosphoric acid
s Halogen means: fluorine, chlorine, bromine, iodine.
The substituents of the above said group W are preferably: halogen, OR,
phenyl, NRR', CN, COOR, CONRR', C,$alkyl (wherein R and R' are as above
defined).
In particular, according to the present invention compounds of formula (I) are
io preferred wherein:
R5 = H, C,$alkylaryl, COOK, CN, aryl, heterocycle, C,.~alkyl-heterocycle; or a
group C,$alkyl-heterocycle-ribose-phosphate
X = O, COOH
Q = simple bond, CU, CONR, NR (wherein R is as above defined) W = H, F, CI,
is Br, Me, t~butyl, C,$alkoxy, 2,5-dimethylhexyl, trifluoromethyl, 2,5-(di-
trifluoromethyl)-phenyl, 4-methoxy-phenyl, 4-fluoro-phenyl, phenyl, phenyl-C,_
ealkyl, C,~ alkylcarbonyl, phenylcarbonyl.
n=1 and2
R,, R2, R3, R4, Re = H, Me, CN, phenyl, COOR, CONRR' (wherein R and R' are
ao as above defined). Among the pharmaceutically acceptable esters and salts
according to the present invention the following can be mentioned:
hydrochloride, sulphate, citrate, formiate, phosphate.
Preferred compounds according to the present invention are:
2,3,4,4a,5,6,6a,7,8,9,10,10a-dodecahydro-(1 H)..benzo[c]quinolizin-3-one;
zs 8-chloro-2,3,4,4a,5,8~,6a,7,8,9,10,10a-dodecahydro-(1I~-benzo[c]quinolizin-
3-
one;
2,3,4,4a,5,6,6a,7,8,9,10,10a-dodecahydro-8-methyl-(1 h~-benzo[c]quinolizin-3-
one;
2,3,4,4a,5,6,6a,7,8,9,10,10a-dodecahydro-4-methyl-(1 i-~-benzo[cJquinolizin-3-
30 one;
2,3,4,4a,5,6,6a,7,8,9,10,10a-dodecahydro-1-methyl-(1 h~-benzo[c]quinolizin-3-
one;
CA 02315055 2000-06-16
WO 99/33828 PCT/EP98/08582
2,3,5,6,6a,7,8,9,10,1 Oa-decahydro-(1 H)-benzo[c]quinolizin-3-one;
8-chloro-2,3,5,6,6a,'7,8,9,10,1 Oa-decahydro-(1 H)-benzo[c]quinolizin-3-one;
2,3,5,6,6a,7;8,9,10,1 Oa-decahydro-8-methyl-(1 H)-benzo[c]quinolizin-3-one;
2,3,5,6,6a,7,8,9,10,10a-decahydro-4-methyl-(1 H)-benzo[c]quinolizin-3-one;
2,3,5,6,6a,7,8,9,10,1 Oa-decahydro-1-methyl-(1 H)-benzo[c]quinolizin-3-one;
(4aa,6a(i,1 Oaa)-3,4,5,6,6a,7,8,9,10,1 Oa-decahydro-{4aH)-benzo[c]quinoli-zin-
3-
one ;
(4a(i,6a~,10aa~3,4,5,6,6a,7,8,9,10,10a-decahydro-(4aH)-benzo[c]quinoli-zin-3-
one ;
l0 3,4,5,6,6a,7,8,9,10,10a-decahydro-(4aH}-benzo(c]quinolizin-3-one;
8-chloro-3,4,5,6,6a, i',8,9,10,1 Oa-decahydro-(4aH)-benzo[c]quinolizin-3-one;
3,4,5,6,6a,7,8,9,10,1 Oa-decahydro-8-methyl-{4aH)-benzo[c]quinolizin-3-one;
3,4,5,6,6a,7,8,9,10,1 Oa-decahydro-4-methyl-(4aH)-benzo[c]quinolizin-3-one;
3,4,5,6,6a,7,8,9,10,1 Oa-decahydro-1-methyl-(4aH~benzo[c]quinolizin-3-one;
~5 8-chloro-2,3,5,6,6a,T,8,9,10,10a-decahydro-4-methyl-(1H)-benzo[c]quinolizin-
3-
one;
2,3,5,6,6a,7,8,9,10,10a-decahydro-4,8-dimethyl-(1 H~benzo[c]quinolizin-3-one;
8-chloro-2,3,5,6,6x,7,8,9,10,1 Oa-decahydro-1-methyl-(1 H)-benzo[c]quinolizin-
3-
one;
zo 2,3,5,6,6a,7,8,9,10,10x-decahydro-1,4-dimethyl-(1H)-benzo[c]quinolizin-3-
one;
8-chloro-3,4,5,6,6a,7,8,9,10,10x-decahydro-4-methyl-(4aH)-benzo[c]quinolizin-
3-one;
3,4,5,6,6x,7,8,9,10,1 Oa-decahydro-4,8-dimethyl-(4aH~benzo[c]quinolizin-3-
one;
25 8-chloro-3,4,5,6,6a,7,8,9,90,,10x-decahydro-1-methyl-(4aH)-
benzo[c]quinolizin-
3-one;
3,4,5,6,6a,7,8,9,10,11Ja-decahydro-1,8-dimethyl-(4ah~-benzo[c]quinolizin-3-
one;
2,3,5,6,6a,7,8,9,10,10x-decahydro-5-methyl-(1 H)-benzo(c)quinolizin-3-one;
30 8-chloro-2,3,5,6,6a,7,8;9,10,10x-decahydro-5-methyl-(1H)-benzo[c]quinolizin-
3-
one;
2,3,5,6,6a,7,8,9,10,10x-decahydro-5,8-dimethyl-( 1 H)-benzo[c]quinolizin-3-
one;
CA 02315055 2000-06-16
WO 99/33828 PCT/EP98/08582
2,3,5,6,6a,7,8,9,10,10a-decahydro-4,5-dimethyl-(1 H~benzo[c]quinolizin-3-one;
2,3,5,6,6a,7,8,9,10,1 Oa-decahydro-1,5-dimethyl-(1 h~-benzo[c]quinolizin-3-
one;
3,4,5,6,6a,7,8,9,10,1 Oa-decahydro-5-methyl-{4af~-benzo[c]quinolizin-3-one;
8-chloro-3,4,5,6,6a,7,8,9,10,1 Oa-decahydro-5-methyl-(4aH~benzo[c]quinolizin-
3-one;
3,4,5,6,6a,7,8,9,10,10a-decahydro-5,8-dimethyl-(4al-~-benzo[c]quinolizin-3-
one;
3,4,5,6,6a,7,8,9,10,1 Oa-decahydro-4,5-dimethyl-(4aH~benzo[c]quinolizin-3-
one;
l0 3,4,5,6,6a,7,8,9,10,10a-decahydro-1,5-dimethyl-{4aH~benzo[c]quinolizin-3-
one;
8-chloro-2,3,5,6,6a,T,8,9,10,10a-decahydro-4,5-dimethyl-(1 H~
benzo[c]quinolizin-3-one;
2,3,5,6,6a,7,8,9,10,1 Oa-decahydro-4,5,8-trimethyl-(1 H}-benzo[c]quinolizin-3-
1 s one;
8-chloro-2,3,5,6,6a, i',8,9, 9 0,1 Oa-decahydro-1,5-dimethyl-(1 I-~-
benzo[c]quinoiizin-3-one;
2,3,5,6,6a,7,8,9,10,10a-decahydro-1,4,5 trimethyl-(1H~benzo[c]quinolizin-3-
one;
ao 8-chloro-3,4,5,6,6a,7,8,9,10,10a-decahydro-4,5-dimethyl-(4ah~-
benzo[c]quinolizin-3~-one;
3,4,5,6,6a,7,8,9,10,11 Oa-decahydro-4,5,8-trimethyl-(4ahl~benzo[c]quinolizin-3-
one; .
8-chloro-3,4,5,6,6a,7,8,9,10,1 Oa-decahydro-1,5-dimethyl-{4aH~
zs benzo[c]quinolizin-3~-one;
3,4,5,6,6a,7,8,9,10,1 Oa-decahydro-1,5,8-trimethyl-(4aH~benzo[c]quinolizin-3-
one;
2,3,5,6,6a,7,8,9,10,'I Oa-decahydro-6-methyl-(1 l~-benzo[c]quinolizin-3-one;
8-chloro-2,3,5,6,6a,7,8,9,10,1 Oa-decahydro-6-methyl-(1 H)-benzo[c]quinolizin-
3-
3 0 one;
2,3,5,6,6a,7,8,9,10,10a-decahydro-6,8-dimethyl-(1 Hrbenzo[c]quinolizin-3-one;
2,3,5,6,6a,7,8,9,10,1Oa-decahydro-4,6-dimethyl-(1H~benzo[c]quinolizin-3-one;
CA 02315055 2000-06-16
WO 99/33828 PCT/EP98/08582
2,3,5,6,6a,7,8,9,10,1 Oa-decahydro-1,6-dimethyl-(1 H)-benzo[c]quinolizin-3-
one;
3,4,5,6,6a,7,8,9,10,1 Oa-decahydro-6-methyl-(4aH)-benzo[c]quinolizin-3-one;
8-chloro-3,4,5,6,6a,T,8,9,10,1 Oa-decahydro-6-methyl-(4aHj-benzo[c]quinolizin-
3-one;
s 3,4,5,6,6a,7,8,9,10,10a-decahydro-6,8-dimethyl-(4aH)-benzo[c]quinolizin-3-
one;
3,4,5,6,6a,7,8,9,10,1 Oa-decahydro-4,6-dimethyl-(4aH)-benzo[c]quinolizin-3-
one;
3,4,5,6,6a,7,8,9,10,1 Oa-decahydro-1,6-dimethyl-(4ahn-benzo[c]quinolizin-3-
lo one;
8-chloro-2,3,5,6,6a, 7,8,9,10,1 Oa-decahydro-4,6-dimethyl-(1 H)-
benzo[c]quinolizin-3--one;
2,3,5,6,6a,7,8,9,10,1 Oa-decahydro-4,6,8-trimethyl-(1 H)-benzo[c]quinolizin-3-
one;
is 8-chloro-2,3,5,6,6a,7,8,9,10,10a-decahydro-1,6-dimethyl-(1H)-
benzo[c]quinolizin-3--one;
2,3,5,6,6a,7,8,9,10,1 Oa-decahydro-1,4,6-trimethyl-(1 Hrbenzo[c]quinolizin-3-
one;
8-chloro-3,4,5,6,6a, 7,8,9,10,10a-decahydro-4,6-dimethyl-(4aH)-
z o benzo[c]quinolizin-3~-one;
3,4,5,6,6a,7,8,9,10,11 Oa-decahydro-4,6,8-trimethyl-(4ahn-benzo[c]quinolizin-3-
one;
8-chloro-3,4,5,6,6a, i',8,9,10,1 Oa-decahydro-1,6-dimethyl-(4aH)_
benzo[c]quinolizin-3--one;
z s 3,4,5,6,6a,7,8,9,10,110a-decahydro-1,6,8-trimethyl-(4aH)-
benzo[c]quinolizin-3-
one;
2,3,5,6,6a,7,8,9,10,1 Oa-decahydro-5,6-dimethyl-(1 H}-benzo[c]quinolizin-3-
one;
8-chloro-2,3,5,6,6a, i',8,9,10,10a-decahydro-5,6-dimethyl-(1 /~-
benzo[c]quinolizin-3-one;
30 2,3,5,6,6a,7,8,9,10,110a-decahydro-5,6,8-trimethyl-(1H}-benzo[c]quinolizin-
3-
one;
CA 02315055 2000-06-16
WO 99/33828 PCT/EP98/08582
2,3,5,6,6x,7,8,9,10,1 Oa-decahydro-4,5,6-trimethyl-(11-x-benzo[c]quinolizin-3-
one;
2,3,5,6,6a,7,8,9,10,10x-decahydro-1,5,6-trimethyl-(11-x-benzo[c]quinolizin-3-
one;
3,4,5,6,6a,7,8,9,10,10x-decahydro-5,6-dimethy!-(4al-~-benzo[c]quinolizin-3-
one;
8-chioro-3,4,5,6,6x,7,8,9,10,1 Oa-decahydro-5,6-dimethyl-(4aH)-
benzo[c]quinolizin-3.-one;
3,4,5,6,6x,7,8,9,10,1 Oa-decahydro-5,6,8-trimethyl-(4aM-benzo[c]quinolizin-3-
one;
3,4,5,6,6a,7,8,9,10,10x-decahydro-4,5,6-trimethyl-(4aH)-benzo[c]quinolizin-3-
one;
3,4,5,6,6a,7,8,9,10,10x-decahydro-1,5,6-trimethyf-(4aH)-benzo[c]quinolizin-3-
one;
is 8-chioro-2,3,5,6,6a,'1,8,9,10,10x-decahydro-4,5,6-trimethyl-(1H)-
benzo[c]quinolizin-3-one;
2,3,5,6,6a,7,8,9,10,10x-decahydro-4,5,6,8-tetramethyl-{1 H)-benzo[c]quinolizin-
3-one;
8-chloro-2,3,5,6,6a,'7,8,9,10,10x-decahydro-1,5,6-trimethyl-(1 hf7-
ao benzo[c]quinolizin-3-one;
2,3,5,6,6~,7,8,9,10,'t 0a-decahydro-1,4,5,6-tetramethyl-(1 H)-
benzo[c]quinolizin-
3-one;
8-chloro-3,4,5,6,6a,'T,8,9,10,1 Oa-decahydro-4,5,6-trimethyl-(4aH)-
benzo[c]quinolizin-3-one; ,
2s 3,4,5,6,6a,7,8,9,10,10x-decahydro-4,5,6,8-tetramethyl-{4aH)-
benzo[c]quinolizin-3-one;
8-chloro-3,4,5,6,6a,7,8,9,10,10x-decahydro-1,5,6-trimethyl-(4ah~-
benzo[c]quinolizin-3-one;
3,4,5,6,6x,7,8,9,10,1 Oa-decahydro-1,5,6,8-tetramethyl-(4aH)-
benzo[c]quinolizin-3~-one;
5,6,6x,7,8,9,10,1 Oa-octahydro-(3H)-benzo[c]quinolizin-3-one;
8-chloro-5,6,6a,7,8,9,10,10x-octahydro-(3H~benzo[c]quinolizin-3-one;
CA 02315055 2000-06-16
WO 99/33828 PCT/EP98/08582
9
5,6,6x,7,8,9,10,1 Oa-~octahydro-8-methyl-(31~-benzo[c]quinolizin-3-one;
5,6,6x,7,8,9,10,1 Oa-~octahydro-4-methyl-(3H~benzo[c]quinolizin-3-one;
8-chloro-5,6,6x,7,8,9,10,1 Oa-octahydro-4-methyl-(3H~benzo[c]quinolizin-3-one;
5,6,6x,7,8,9,10,1 Oa--octahydro-4,8-dimethyl-(3H)-benzo[c]quinolizin-3-one;
2,3,5,6,7,8,9,10-octahydro-{1 H)-benzo[c]quinolizin-3-one;
8-chloro-2,3,5,6,7,8,9,10-octahydro-{1 H~benzo[c]quinolizin-3-one;
2,3,5,6,7,8,9,10-octahydro-8-methyl-(1 H)-benzo[c]quinolizin-3-one;
2,3,5,6,6x,7,8,9-octahydro-(;1 H~benzo[c]quinolizin-3-one;
8-chloro-2,3,5,6,6a,'t,8,9-octahydro-(1 H~benzo[c]quinolizin-3-one;
~0 2,3,5,6,6a,7,8,9-octahydro-8-methyl-(1H)-benzo[c]quinolizin-3-one;
4a-benzyl-3,4,5,6,6x,7,8,9,10,1 Oa-decahydro-{4aH)-benzo[c]quinolizin-3-one;
4a-~benzyl-8-chloro-3,4,5,6,6a,7,8,9,10,10a-decahydro-(4aH)-
benzo[c]quinolizin-3-one;
4a-benzyl-3,4,5,6,6a,7,8,9,10,10x-decahydro-8-methyl-{4aH~
is benzo(c]quinolizin-3-one;
4a-benzyl-3,4,5,6,6x,7,8,9,10,1 Oa-decahydro-4-methyl-(4aH)-
benzo[c]quinolizin-3-one;
4a-benzyl-3,4,5,6,6x,7,8,9,10,1 Oa-decahydro-1-methyl-(4aH~
benzo[c]quinolizin-3-one;
ao 3,4,5,6,6a,7,8,9,10,10x-decahydro-4a-{4-pyridyl)methyl-(4aH)-
benzo[c]quinolizin-3-one;
8-chloro-3,4,5,6,6x,7,8,9,10,9 Oa-decahydro-4a-(4-pyridyl)methyl-(4aH~
benzo[c]quinolizin-3-one;
3,4,5,6,6x,7,8,9,10,'1 Oa-decahydro-8-methyl-4a-{4-pyridyl)methyl-(4aH)-
25 benzo[c]quinolizin-3-one;
3,4,5,6,6x,7,8,9,10,'1 Oa-decahydro-4-methyl-4a-{4-pyridyl)methyl-(4xH~
benzo[c]quinolizin-3-one;
3,4,5,6,6x,7,8,9,10,'1 Oa-decahydro-1-methyl-4a-(4-pyridyl)methyl-(4aH~
benzo[c]quinolizin-3-one;
3o Dodecahydro-benzo[c]quinolizin-3-ones and decahydro-benzo[c]quinolizin-3-
ones according to the present invention, wherein the double bonds i and h are
absent, can be prepared as shown in Scheme 1, according to the general
CA 02315055 2000-06-16
WO 99/33828 PCT/EP98/08582
to
preparation of benzo[cJquinolizine-3-ones already reported in the patent WO
97129107; in particular, for example, starting from compounds of formula 2
R4
,C40Me
R3
(aWln
(2)
wherein R3, R4, W, C.f and n are as above defined.
The compounds 2 are commercialy available or can be prepared according to
known techniques.
As it can be seen from the Scheme 1 the preparation of the compounds
according to the invention involves the cyclization of the ester 2 to the
enamide
15 3 by heating at 1:?0°C compounds 2 in formic acid in the presence of
ammonium hydrogencarbonate. The enamide 3 is reduced to the traps-fused
amide 4 for example with sodiumcyanoborohydride at pH 4, followed by the
protection of the amide-group with a protecting group, for example tert-
butoxycarbonyl (t-Boc), to give compound 5; compound 5 is reduced to
ao compound 6, for example (when Rb is H) with sodium borohydride in ethanol
(pH 4), particularly good yields are obtained when the reduction is performed
with LiEt3BH in TH'F at -78°C, followed by addition of HCI 2N anhydrous
solution in ethanol up to pH 4. The so obtained compound fi is thereafter
react~:d with a silyloxydiene 8, produced "in situ" starting from vinyl-
ketones 7
25 (wherein R,, RZ and Re are as above defined) with a silylating agent as
trimethylsilyltrifluorometansulphonic anhydride (TMSOTf) and thereafter
hydrolized, for example in sodium hydrogencarbonate, to give the compounds
of formula (I) wherein X = O. The possible introduction of the double bonds
and
the transformation of the group X in one of the other groups mentioned above
3o can be easily performed according to known techniques starting from the
corresponding compound of formula (I) obtained as indicated. For example the
introduction of the double bonds in position a orland b, can be performed by
CA 02315055 2000-06-16
WO 99/33828 PCT/EP98/08582
11
reaction of dichlorodicyanoquinone (DDQ) with the corresponding
silylenolethers or by oxidation with mercuric acetate of the saturated
corresponding compound obtained as described above.
According to a different embodiment of the present invention it is possible to
s obtain directly the double bond in position "a" by performing the reaction
between products 6 and 8 (wherein R, is OCH3 and R2 and Re are H) in the
presence of TiCl4 or TMSOTf as Lewis acids (product 8 as above defined is in
this case a commercially available product) : Acting in this way it is also
possible to direct the stereochemical outcome of the hydrogen atom in position
4a (R5= H) in the final compound. In particular when using TiCl4 the compound,
wherein the above said hydrogen atom is on the same side of the hydrogen in
position 10a, is obtained while using TMSOTf the above said hydrogen atom is
on the opposite side on respect to the hydrogen in position 10a.
The transformation of group X can be performed via the corresponding
is enoltriflates and their carbonylation in the presence of palladium
diacetate,
triphenylphosphine and the suitable nucleophilic reagent (alcohol, amine,
nitro-
group).
The compounds according to the present invention wherein the double bonds i
or h and b are present, can be prepared as shown in Scheme 2, for example
ao starting from the above said compounds of formula 2.
The key step of the: process is the thermal rearrangement-cyclization of the
isoxazoline-5-spirocyclopropane 14 to final product 1. This process has been
already applied for the synthesis of other nitrogen bridgehead polycylic
compounds as reported in J. Org. Chem. 1988, 53, 2426 and in J. Med. Chem.
2 5 1997, 40, 1112.
As it can be seen from the Scheme 2 the preparation of the compounds
according to the invention involves protection of the carbonyl of compound 2
(wherein R3 and R4 are as above defined) as a ketal, for example with
ethylenglicole under acid catalysis, followed by the selective reduction of
the
3o ester group in compound 9 to aldehyde 10, for example by DIBAL at -78
°C.
The transformation of the aldehyde 10 to oxime 11, made for example by
CA 02315055 2000-06-16
WO 99/33828
PCT/EP98J08582
12
reaction with hydroxylamine hydrochloride in pyridine, is followed by
cycloaddition to methylenecyclopropane 12 (wherein R,, R2, Re are as above
defined) of the in situ generated nitrite oxide by reaction of oxime 11 with
sodium hypochlorite and triethylamine. The isoxazoline-5-spirocyclopropane 13
s is then deprotected under acid catalysis and submitted to thermal
rear-angement in boiling DMF for 3-6 hrs to give compounds 1.
Octahydrobenzo[c)quinolizin-3-ones of formula 1, wherein R,, R2, R3, R4, Re
are
H, QW is H or -CH2CONHtBu (at position 8), n = 1 and both the double bonds b
and h (or i) are present can be prepared for example starting from compound 2
wherein R3, R4, are H and QW is H or 5-(N-t butyl)acetamido and n = 1.
Example 1
Preparation of methyl 3-[2-(1,3-dioxolan-2-yl)cyclohexyl]propanoate.
[compound 9 wherein (QW)" = H, R3 = R,, = H)
In a flask provided with a Dean-Stark apparatus, methyl ester 2 (20.0 g, 109
is mmol), ethylenic glycol (60 mL, 1.08 mot) and p-TsOH (0.8 g, 5 mmol) were
dissolved in toluene (550 mL) and the resulting solution was heated under
reflux. After 4 h the reaction was complete and the mixture was washed with
NaHC03 2 N, water' and dried over Na2S04. After filtration and evaporation of
the solvent, a crudE: yellow oil was obtained. This was purified by
distillation
a o under reduced pre;>sure, affording pure 9 [15.9 g, 64%, by 127-130
°C (2
mbar)].
Pl~..~
Preparation of 3-[2-(1,3-dioxolan-2-yl)cyclohexyl)propanal [compound 10
wherein (QW)~ = H, R3 = R, = H)
25 To a solution of 9 ('15.7 g, 69.1 mmol) in toluene (220 mL) cooled at -78
°C,
DIBAL-H (1.2 M solution in toluene, 116 mL, 135 mmoi) was slowly added
during 3 h. After 3 h of stirring, the mixture was poured into water (110 mL)
and
allowed to warm to room temperature. After filtration on a Celite layer, the
organic phase was .dried over Na2S04. After filtration and evaporation of the
3o solvent the residual crude oil was purified by chromatography (petroleum
ether-
EtOAc, 2:1, R,0.30), affording pure aldehyde 10 as oil (6.6 g, 48%).
CA 02315055 2000-06-16
WO 99133828 PCT/EP98108582
13
x I 3
Preparation of 3-[2-~(1,3-dioxolan-2-yl)cyclohexyl]propanal oxime [compound 11
wherein (QW)~ = H, R3 = R4 = HJ.
A solution of aldehyde 10 (6.12 g, 31.0 mmol) and NH20H~HCI (2.76 g, 40.0
s mmol) in pyridine (120 mL) was stirred for 2 h at room temperature. The
mixture
was extracted with Et20 and the organic layer washed with water and dried
over Na2S04. After filtration and evaporation of the solvent the crude oil
obtained was purified by chromatography (petroleum ether- EtOAc, 1.5:1, R,
0.5). Recrystallization from Et20-petroleum ether gave pure oxime 11 (4.02 g,
l0 61 %, mp 74-75 °C) as a 1:1 mixture of E,Z diastereoisomers.
Example 4
Preparation of 6-[2-[2-(1,3-dioxolan-2-yl)cyclohexyl]ethyl]-4-oxa-5-
azaspiro[2.4jhept-5-ene [compound 13 wherein (QW)~ = H, R, = R2 = R3 = R4 =
Re = HJ.
is Liquid methylenecyclopropane [compound 12 wherein R, = R2 = Re = H] (5 mL)
was transferred by .a double-tipped needle into a solution of oxime 11 (4.02
g,
18.8 mmol) and Et3N (226 mg, 2.23 mmol) in CH2CIz (35 mL) cooled at -60
°C.
The mixture was allowed to warm to 0 °C and NaClO (8% solution, 54
mL) was
slowly added in 3.5 h. The solution was stirred for 21 h, then the phases were
ao separated, the aqueous layer was extracted with CH2CI2 (3 x 25 mL) and the
combined organic layers were dried over Na2S04. After filtration and
evaporation of the solvent, crude 13 (4.89 g, 73%) was obtained and used
without purification in the next reaction.
Example 5
25 Preparation of 6-[2-(2-oxocyclohexyl)ethylj-4-oxa-5-azaspiro[2.4]hept-5-ene
[compound 14 wherein (QW)~ = H, R, = R2 = R3 = R4 = Re = H].
Isoxazoline 13 (3.64 g, 13.7 mmol) and p-TsOH (392 mg, 2.23 mmol) were
dissolved in acetone (90 mL) and water (30 mL) and the resulting solution was
stirred at room temperature for 7 days. The product was extracted with CH2CI2,
3o the organic phase washed with NaHC03 (2 N) and dried over NaZS04. After
filtration and evaporation of the solvent, a yellow crude oil (2.36 g) was
obtained. This was purified first by chromatography (CH2CI2-EtOAc, 12.5:1, R,
CA 02315055 2000-06-16
WO 99/33828 PCT/EP98/08582
14
0.35) and then by recrystallization from Et20-petroleum ether, affording pure
isoxazoline 14 (1.43 g, 47°/'°, mp 109 °C).
E~x r~r ple 6
Preparation of 2,3,5,6,7,8,9,10-octahydro-(1i-~-benzo[c]quinolizin-3-one
s [compound 1 wherein (QW)~ = H, R, = R2 = R3 = R4 = RB = H and h = double
bond ].
and 2,3,5,6,6a,7,8,9-octahydro-(3H}-benzo[c]quinolizin-3-one [compound 1
wherein (QW)~ = H, R, = R~ = R3 = R4 = Re = H and i = double bond ].
Isoxazoline 14 (476 mg, 2.15 mmol) dissolved in dry DMF (50 mL) was heated
to under reflux for 3 h. After distillation of the solvent, a yellow crude oil
(470 mg)
was obtained, containing a mixture of rearrangement products. This oil was
purified by chromatography (CH2CI2-MeOH, 20:1 ), affording pure 1 (163 mg,
37%, R, 0.36, oil) as 10:1 mixture of the two isomers having the double bond
in
position h or i respectively.
is xa I 7
Preparation of methyl 3-[[2-(1,3-dioxolan-2-y1~5-(N-t
butyl)acetamido]cyclohexyl]]propanoate [compound 9 wherein (QW)= 5-(N-f
butyl)acetamido n = 1, R3 = R4 = H]
Prepared as in example 1. Starting from compound 2 [wherein (QW)= 5-(N-t
Zo butyl)acetamido n =: 1, R3 = R4 = H] (32.14 g, 108 mmol), crude ketal 9
(22.2 g,
60%) was obtained as an oil. A portion (100 mg) of this crude oil was purified
by
chromatography (CHZCI2-MeOH, 30:1, 1 % Et3N, R, 0.31, oil), affording 9 as a
mixture of cis and traps isomers.
E~ m
25 Preparation of 3-[[2-(1,3-dioxolan-2-yl)-5-(N-f
butyl)acetamido]cyclohexyl]]-
propanal oxime [compound 11 wherein (QW)= 5-(N-t-butyl)acetamido n = 1, R3
=R4=H]
A solution of ketal [compound 9 wherein (QW)= 5-(N-t-butyl)acetamido n = 1, R3
= R4 = H] (22.1 g, 64.7 mmol) in toluene (500 rnL) was cooled at -78
°C; DIBAL
3 o H (solution 1 M in toluene, 288 mL) was then slowly added in 4 h and the
resulting solution was stirred for 3 h. After addition of water -(260 mL), the
mixture was allowed to warm to room temperature, extracted with CH2CIz (4 x
CA 02315055 2000-06-16
WO 99/33828 PCT/EP98/08582
200 mL) and the organic layer dried over Na2S04. After filtration and
evaporation of the solvent a crude oil (17.2 g) was obtained, used without
purification for the next step.
Then, under stirring, to a solution of distilled oxalyl chloride (10.9 mL, 125
s mmol) in CH2CI2 (2'70 mL), cooled at -60 °C, DMSO (15 mL, 211 mmol)
was
added, followed by slow addition (25 min) of a solution of the above crude oil
in
CHZCI2 (260 mL). After 15 min, Et3N (56 mL) was slowly added in 15 min. After
5 min stirring, the mixture was warmed to room temperature and washed with
water (535 mL); after separation of the phases, the aqueous one was extracted
to with CH2CI2 (3 x 250 mL) and the combined organic layers were dried over
Na2S04. After filtration and evaporation of the solvent, the aldheyde
[compound
10 wherein (QW)= ;i-(N-t butyl)acetamido n = 1, R3 = R4 = Hj was obtained as a
crude oil (14.6 g), used without purification for the next reaction.
A solution of this aldheyde (14.6 g) in pyridine (210 mL) was added to a
is solution of NH20H~HCI (13.7 g, 196.9 mmol) in pyridine (107 mL) and the
resulting mixture was stirred at room temperature for 20 h. The mixture was
poured into CH2CI2 (800 mL) and washed with water; after separation of the
phases, the aqueous one was extracted with CH2CI2 (3 x 200 mL) and the
combined organic layers were dried over Na2S04. After filtration and
ao evaporation of the solvent, crude oxime [compound 11 wherein (QW)= 5-(N-f
butyl)acetamido n =~ 1, R3 = R4 = H] (11.3 g) was obtained. This was purified
by
chromatography eluting with CHCI3-MeOH, 50:1, 1 % Et3N, and then with CHCI3-
MeOH, 3:1, 1 % Et3N (R, 0.32), affording pure oxime [compound 11 wherein
(QW)= 5-(N-t butyl)acetamido n = 1, R3 = R4 = H] (7.41 g, 35%, oil) as a 1:1
as mixture of E/Z diastereoisomers
xm
Preparation of 6-[2-[2-(1,3-dioxolan-2-yl}-5-(N-f butyl)acetamidojcyclo-
hexyl]ethyl]-4-oxa-5-azaspiro[2.4]hept-5-ene [compound 13 wherein (QW)= 5-
(N-t butyl)acetamido n = 1, R, = R2 = R3 = R4 = Re = H]
3 o Prepared as example 4. Starting from the above prepared oxime [compound 11
wherein (QW)= 5-(N-t butyl)acetamido n = 1, R3 = R4 = H] (7.40 g, 22.6 mmol),
isoxazoline [compound 13 wherein (QW)= 5-(N-f butyl)acetamido n = 1, R, = R2
CA 02315055 2000-06-16
WO 99/33828 PCT/EP98/08582
16
= R3 = R4 = Rg = H] (4.96 g, 58%) was obtained as a crude oil used without
purification in the next reaction.
Example 10
Preparation of 6-[2-[2-oxo-5-[(N-f butyl)acetamido]cyclohexyl]ethyl]-4-oxa-5-
s azaspiro[2.4]hept-5-ene [compound 14 wherein (QW)= 5-(N-t-butyl)acetamido n
=1,R,=R2=R3=R"=Rg=H].
Crude isoxazoline 13 [wherein (QW)= 5-(N-t butyl)acetamido n = 1, R, = R2 = R3
= R, = Re = H] (4.92 g, 13.1 mmol) was dissolved in acetone {150 mL) and
H2S04 (1.7 M solution in acetone, 9.8 mL) was slowly added, under vigorous
to stirring, at room temperature. When the reaction was complete, Na2C03 was
added up to pH 7; after filtration and evaporation of the solvent, crude 14
was
obtained. This was purified by chromatography, eluting with CH2Cl2-MeOH, 60:1
and then 20:1 (R, 0.28), affording pure 14 as an oil [compound 14 wherein
(QW)= 5-(N-t butyl)acetamido n = 1, R, = R2 = R3 = R4 = Re = H] (1.45 g, 33%)
i5 as a mixture of cis and traps isomers.
Exam la a 11
Preparation of 2,3,5,6,7,8,9,10-octahydro-(1t~-8-(N-f-Butyl)acetamido-
benzo[c]quinolizin-3-one [compound 1 wherein (QW)= 8-(N-t-butyl)acetamido n
_~ 1, R, = R2 = R3 = R4 = R.s = H and h = double bond] and 2,3,5,6,6a,7,8,9-
z o octahydro-(1 M-8-(N-~f Butyl)acetamido-benzo[c]quinoiizin-3-one [compound
1
wherein (QW)= 8-(N-t-butyl)acetamido n = 1, R, = R2 = R3 = R4 = Re = H and i =
double bond].
A solution of isoxazoline [compound 14 wherein (QW)= 5-(N-t-butyl)acetamido
n = 1, R, = R2 = R3 ~= R4 = Rs = H] (947 mg, 2.83 mmol) in DMF (109 mL) was
a5 heated under reflux for 3 h. After distillation under reduced pressure of
the
solvent, a crude oil containing a mixture of rearrangement products was
obtained. Chromatographic separation (CHZCI2-MeOH, 25:1, 1 % NH3) afforded
pure 1 {161 mg, 18°~0, R, 0.32, oil) as 10:1 mixture of the two isomers
having the
double bond in position h or i respectively.
CA 02315055 2000-06-16
WO 99/33828 PCT/EP98/08582
1~
Exam Ip a 12
Preparation of (+/-){4aa,6aji,10aa}-3,4,5,6,6a,7,8,9,10,10a-Decahydro-(4aM-
benzo[c]quinolizin-3-fine [compound 1 wherein (QW)~ = H, R, = R2 = R3 = R4 =
Re = H and a = double bond ].
s and (+/-~)(4a[3,6aj3,10aa)-3,4,5,6,6a,7,8,9,10,10a-Decahydro-(4aH)-
benzojc]quinolizin-3-one [compound 1 wherein (QW)" = H, R, = R2 = R3 = R4 =
Re = H and a = double bond]
The (+/-) frans-fused N Boc-amide 5, [wherein (QW)~ = H, R3 = R4 = H
].prepared according to known methods, was reduced to compound 6 [wherein
to (QW)~ = H, R3 = R4 == H ] according to the following procedure : A solution
of 5,
{4.1 mmol in 12 mL of THF) was cooled to - 78°C, and a 1 M solution of
LiEt3BH
in THF (8.2 mL) was slowly added. After 15 min of stirring at -78°C, 2
N HCI in
anhydrous EtOH was added dropwise until pH 3.5-4 was reached, immediately
followed by addition of 18 mL of ethanol. The mixture was left to warm at 0
°C
~s and after 30 min was diluted with CH2CI2. After the usual work-up the
product
was purified by flash-column chromatography and obtained in 80% yield as a
sticky oil.
To a solution of compound 6 [wherein (QW)~ = H, R3 = R4 = H ] (500 mg, 1.76
mmol in 10 mL of CH2C12) at 0°C were added dropwise 1-methoxy-3
a o trimethylsilyloxy-1,3-butadiene [compound 8, wherein R, = MeO, RZ= H, Rg =
H]
(608 mg, 3.53 mmol), NEt3 (0.5 mL, 3.53 mmol) and TMSOTf (4.4 mmol, 0.85
mL), the mixture was left to warm to r.t. under stirring for 30 rnin. Then the
mixture was treated with NaHC03 (satd) for 24 under stirring. Usual work-up
and purification by flash column chromatography afforded the 4ab isomer (+/-)
25 (4aj3,6aji,10aa)-3,4,:i,6,6a,7,8,9,10,10a-decahydro-(4a1~-
benzo[c]quinolizin-3-
one [compound 1 wherein (QW)~ = H, R, = R2 = R3 = R4 = Re = H and a =
double bond] in 20% yield as an oil
The preparation of 4aa isomer was done as follows
To a solution of compound 6 [wherein (QW)~ = H, R3 = R, = H ] (200 mg, 0.71
3o mmol in 5 mL of CH2CI2) and 1-methoxy-3-trimethylsilyloxy-1,3-butadiene
[compound 8, wherein R, = MeO, R2= H, Re = H] (244 mg, 1.42 mmol), at
0°C
was added dropwise TiCl4 (0.155 mL, of a 2M solution in CH2CI2) and the
CA 02315055 2000-06-16
wo 99r~3s2s Pc~r~~srosssi
is
mixture was left to warm to r.t. under stirring for 1 h. Then the mixture was
treated with NaHCO3 (satd) for 30 min under stirring. Usual work-up and
purification by flash column chromatography afforded the 4aa isomer (+/-)
(4aa,6ap,1 Oaa~3,4,5,6,6a,7,8,9,10,10a-decahydro-(4a1-n-benzo[c]quinolizin-3-
s one [compound 1 wherein (QW)~ = H, R, = Rz = R3 = R4 = Rs = H and a =
double bond] in 16% yield as oil.
Activity Test
The inhibition potency of the prepared compounds in respect of the iso-
enzymes 1 and 2 of 5a-reductase was determined using cellular systems (for
to example CHO cells) expressing human iso-enzymes 2 and 1. The samples are
incubated in the presence of testosterone labelled with tritium and thereafter
the quantity of labelled dihydrotestosterone formed in the absence and in the
presence of the inhibitor is measured. The compounds showed high inhibiting
power of 5a-reductase enzyme (in particular of iso-enzyme 1 ) with an
inhibition
~s higher than 50% at the concentration of 10 -100 nM.
For example the 10:1 mixture of 2,3,5,6,7,8,9,10-octahydro-(1 H}-
benzo[c]quinolizin-3-one [compound 1 wherein {QW)~ = H, R, = R2 = R3 = R4 =
Re - H and h -- double bond ] and 2,3,5,6,6a,7,8,9-octahydro-(3H~
benzo[c]quinolizin-3-one [compound 1 wherein (QW}~ = H, R, = R2 = R3 = R4 =
ao R6 = H and i = double bond], prepared according the example 6, was as
selective inhibitor towards type 1 isoenzyme, having an ICS value of 58 nM,
whereas the ICS towards the type 2 isoenzyme was not determinable.
For the therapeutical administration the compounds according to the invention
are prepared in the form of pharmaceutical compositions containing the active
25 principle and the organic or inorganic excipients suitable for the oral,
parenteral
or topic administratian of the compositions. The pharmaceutical compositions
can thererfore be in the solid form (dragees, suppositories, creams,
ointments),
liquid form (solutions, suspensions, emulsions) and can possibly contain the
stabilizers, conservatives, humectants, emulsifier, buffers or salts used for
so equilibrating the osmotic pressure which are commonly used in the art.
Generally the administration of the compounds is performed according to the
CA 02315055 2000-06-16
WO 99/33828 PCT/EP98/08582
19
modalities and quantities observed for the known agents used for the same
purposes and takinc,~ into consideration the age and conditions of the
patients.
CA 02315055 2000-06-16
WO 99/33828 PCT/EP98/08582
r
t0
M
0
V o 0
z = ~ . o
m
.r
0
m
m ~
w ,
er O
y
E
cL ~ ~ v
r,
O
Z
U
= II
m =
a
z
~m
CA 02315055 2000-06-16
WO 99/33828 PCT/EP98/08582
21
Z
M
J
Q
m_
D
O
m
N VZ
ZW
r ~ --
e~ ~ D L
0
O
s
N
m
t
N z O v
O ~
H