Note: Descriptions are shown in the official language in which they were submitted.
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
1
METHOD AND APPARATUS FOR
PREVENTION OF ATRIAL TACHYA,RRHYTHMIAS
Background of the Invention
The present invention relates generally to the field of implantable
stimulators
and more particularly to cardiac pacemakers and implantable anti-arrhythmia
devices.
It has been proposed to reduce the incidence of tachyarrhythmias in the
ventricle by using multiple site pacing. For example, in U.S. Patent No.
3,937,226,
issued to Funke, multiple electrodes are provided for location around the
ventricles. In
response to a sensed depolarization following a refractory period, at any of
the
electrodes, all electrodes are paced. All electrodes are similarly paced in
the absence
of sensed depolarizations for a period of 1000 ms. U.S. Patent No. 4,088,140
issued to
Rockland et al discloses a similar device, in which a pacing pulse is
delivered only to
a single electrode in response to a failure to sense during a 1000 ms period,
and
delivery of pacing pulses to multiple electrodes is triggered in response to
sensed
2 0 depolarizations occurnng between 150 and 500 ms following delivery of a
previous
sensed depolarization or pacing pulse. 'U.S. Patent No. 4,354,497, issued to
Kahn
adds sensing electrodes adjacent the septum of the heart and delivers pacing
pulses to
multiple electrodes spaced around the ventricles in response to sensed
depolarizations
at the ventricular electrodes which are not preceded by depolarizations sensed
at the
2 5 septum electrodes. Multi-site pacing in the ventricles has also been
proposed to
improve hemodynamic function, as in U.S. Patent No. 4,928,688, issued to
Mower.
The Funke, Kahn, Rockland and Mower patents are all hereby incorporated herein
by
reference in their entireties.
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
2
Multi-site atrial pacing has also been proposed as a mechanism for reducing
the incidence of atrial tachyarrhythmias. For example, multi-site pacing for
arrhythmia prevention is discussed in U.S. patent No. 5,584,867, issued to
Limousin
et at, U.S. Patent No. 5,683,429 issued to Mehra and U.S. patent No.
5,403,356,
issued to Hill et al. and in the article "Prevention of Atrial
Tachyarrhythmias Related
to Advanced Inter-atrial Block by Permanent Atrial Resynchronization", by
Mabo, et
al, published in Pace. Vol. 14, Apr. 1991, Part II, p 648. The Limousin, Mehra
and
Hill et al. patents are hereby incorporated herein by reference in their
entireties.
Pacing methodologies employing only a single pacing site have also been
proposed for prevention of tachyarrhythmias. For example, U.S. Patent No.
4,941,471
issued to Mehra discloses a single site rate stabilization pacing method for
use in the
ventricles. An improvement to this pacing methodology is disclosed in U.S.
Patent
No. 5,545,185 issued to Denker et al, and further improvements are disclosed
in U.S.
Patent No. 5,814,085 issued to Hill, and U.S. Patent Application Serial No.
08/764,568, filed on December 16, 1996 by Peterson et al. An additional atrial
overdrive arrhythmia prevention pacing mode which is disclosed in U.S. Patent
No.
5,713,929, issued to Hess et al. The Mehra, Hill, Hess et al. and Denker
patents, as
well as the Peterson et al. application are all hereby incorporated herein by
reference
2 0 in their entireties.
Summary of the Invention
The present invention is directed toward preventing the occurrence of atrial
or
2 5 ventricular tachyarrhythmias by means of a pacemaker having the capability
of
delivering tachyarrhythmia prevention pacing therapies at single or at
multiple
locations within the atria and/or ventricles. The present invention
accomplishes this
desired goal by means of control and timing circuits and methods of operation
which
provide for optimization of the delivered pacing therapy by choosing which
therapy,
3 0 which electrodes and which pacing sites are employed, from among those
available
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
3
for tachyarrhythmia prevention pacing. The timing and control circuitry
includes
means for tracking the occurrences of tachyarrhythmias over defined extended
time
periods, such as days, weeks or months.
Pacing at multiple sites may be accomplished by delivering pacing pulses
through separate electrode pairs, each pair located adjacent a different site
within the
atria or within the ventricles, may be accomplished by delivering pacing
pulses
between electrodes located adjacent different sites within the atria or within
the
ventricles or may be accomplished by delivering pulses between individual
electrodes
in the atria or ventricles and remote indifferent electrodes. The device may
employ
single or mufti-site pacing in the atria, the ventricles, or in both the atria
and the
ventricles, with separate prioritized lists of therapies and/or electrodes and
polarities
programmed for the atria and the ventricles and may employ separate counts or
durations of occurrences of tachyarrhythmia in the atria and the ventricles in
order to
induce switching of the electrode configurations used to pace the atria and
the
ventricles, independent of one another.
In some embodiments, in response to detection of a predetermined number of
occurrences of tachyarrhythmias within a defined extended time period and/or
2 0 detection of a predefined cumulative duration of tachyarrhythmias within
the defined
extended time period, the selection of pacing therapy and/or interconnection
of the
electrodes available for pacing is modified to disable a tachyarrhythmia
prevention
therapy and/or to change the tachyarrhythmia prevention therapy. With each
subsequent detection of a defined number and/or cumulative duration of
2 5 tachyarrhythmias within the a defined extended time period, the device may
switch to
another available therapy and/or set of electrodes and pacing sites until an
effective
tachyarrhythmia therapy is selected or until all available therapies have been
determined to be ineffective.
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
For example, in a device embodied in the form of a mufti-site atrial pacemaker
having electrodes positioned to stimulate at two different locations within
the atria,
the device may initially employ first and second electrodes in contact with
atrial tissue
to stimulate at both locations using the pacing method disclosed in the above-
cited
Mehra et al '429 patent, and then, in response to detection of a defined
number of
occurrences and/or a defined cumulative duration of tachyarrhythmia within the
defined extended time period, the pacemaker may employ the second electrode,
in
conjunction with an indifferent electrode to stimulate only the second
location using
the pacing method disclosed in the above-cited Mehra '471 patent.
In some preferred embodiments of the invention, upon detection of a defined
number of occurrences of tachyarrhythmias and/or a defined cumulative total
duration
of tachyarrhythmias in the atria or the ventricles within a defined extended
time
period, the device checks to see if there is an available therapy and/or set
of electrodes
and polarities which offer the opportunity of reducing the frequency or
duration of
tachyarrhythmias. In these embodiments, a therapy and/or an associated set of
electrodes and polarities not previously employed will, by definition, be
considered as
offering the possibility of reducing the frequency or duration of
tachyarrhythmias. In
addition, the device may record information with regard to the frequency and
2 0 durations of occurrences of tachyarrhythmia in conjunction with a
particular therapy
and/or associated set of electrodes and polarities, and may consider any
previously
employed therapies and/or sets of electrodes and polarities as offering an
opportunity
for reducing the frequency or duration of tachyarrhythmias if the associated
recorded
information indicates reduced frequency or duration of tachyarrhythmias as
compared
2 5 to the electrodes and polarities currently being employed.
In other preferred embodiments of the invention the device automatically
determines whether any tachyarrhythmia prevention therapy is desirable and, if
so
which therapy and/or set of electrodes and polarities will be initially
employed, based
3 0 upon the frequency and durations of occurrences of tachyarrhythmia in
conjunction
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
with the particular therapies and/or associated sets of electrodes and
polarities
available. In these embodiments the device may first determine whether
tachyarrhythmias in the absence of tachyarrhythmia prevention therapies and/or
multi-
site pacing occur during a first extended time period with a frequency
sufficient to
5 warrant employment of a tachyarrhythmia prevention therapy. If so, the
device
thereafter may sequentially apply each of the available therapies and/or sets
of
electrodes and polarities for a second extended time period to determine which
results
in the lowest incidence of tachyarrhythmias. The device may select the therapy
and/or
set of electrodes and polarities associated with the lowest incidence of
tachyarrhythmias, provided that the incidence of tachyarrhythmias is lower
than with
no tachyarrhythmia therapy delivered. In such devices, after initial selection
of the
most effective tachyarrhythmia prevention therapy andlor electrode sites and
polarities, the device may continue to employ the selected settings for a
third extended
predetermined time period, for example a defined number of months, and on
expiration of this extended time period repeat the process of determining
whether a
tachyarrhythmia prevention therapy is desirable and if so, which therapy is
desirable.
In order to more closely tie the anti-arrhythmia therapies and/or electrodes
and
polarities employed by the device to the incidence of tachyarrhythmias, the
device
2 0 may focus on tachyarrhythmias which are initiated following delivery of a
pacing
pulse. For example, the device may check stored information on sensed and
paced
events to determine whether a pacing pulse initiated the short: inter-
depolarization
period which initiated the tachyarrhythmia, and apply the detected arrhythmia
to the
tachyarrhythmia count or cumulative tachyarrhythmia duration measurement only
if
2 5 the detected tachyarrhythmia is initiated following a pacing pulse.
In addition to or as an alternative to selection between different arrhythmia
prevention pacing modes, a device according to the present invention may also
operate to provide optimized parameters of a selected anti-arrhythmia pacing
mode.
3 0 For example, in the context of a rate stabilization pacing mode as
described in the
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
6
above cited Mehra and Denker patents, or in the context of an atrial overdrive
pacing
mode as described in the above cited Hess et al patent, the value of the
increment
added to the duration of a previously measured R-R interval to define the next
escape
interval may be adjusted. In an analogous fashion, the delay time, if any,
between
pacing pulses delivered at multiple pacing sites or between sensed
depolarizations at
one pacing site and the delivery of a pacing pulse to a second pacing site may
also be
optimized as a function of the monitored result of the therapy provided.
In order to optimize the parameters of the anti-arrhythmia pacing therapy
provided, the device monitors a specific metric associated with the success of
the
therapy in a manner analogous to that described above in conjunction with
selection
between arrhythmia prevention pacing modes. In this context, for example,
frequency
of occurrence of atrial or ventricular beats, occurrence of atrial or
ventricular
tachyarrhythmias, and the like may be monitored and compared to a desired
defined
endpoint condition, with operational parameters of the pacing mode presently
in effect
adjusted in an attempt to cause the measured metric to converge on the desired
endpoint.
The desired endpoint may be defined as a range which may have only an upper
2 0 bound, only a lower bound or both upper and lower bounds. More than one
measured
metric may be employed to determine success of the arrhythmia prevention
pacing
mode. A measured metric falling outside of a defined endpoint range rnay
trigger a
change in the pacing mode to more aggressive or less aggressive parameter
settings.
The time period over which the metric is monitored may extend for a few hours
up to
2 5 several weeks. For example, if the defined metric is frequency of atrial
fibrillation,
the defined metric range might be less than a physician programmed number
occurrences of atrial fibrillation over a two day time period. In this case,
the device
would adjust the parameters of a provided antiarrhythmia mode, for example,
adjust
the increment provided in conjunction with the atrial overdrive pacing
modality
30 described in the above Hess patent, until the measured frequency of
occurrences of
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
7
atrial fibrillation per two day period was within the endpoint range.
Alternatively, if
the metric being measured is.frequency of occurrence of PACs, the defined
metric
might be a defined range of PACs per hour, determined by the physician to
represent
an acceptable range of occurrences of PACs. In this embodiment, the
aggressiveness
of the atrial arrhythmia prevention pacing modality employed may be increased
in
response to the number of occurrences of PACs being in excess of the defined
endpoint range, while the aggressiveness of the therapy might be decreased in
response to an occurrence of less than the defined endpoint range of
occurrences, in
order to avoid over-treating the patient. In response to the number of PACs
per hour
falling within the defined range, the device would leave the parameter
settings of the
arrhythmia prevention pacing modality unchanged.
In some embodiments, the same monitored metric or metrics employed to
optimize the parameters of an arrhythmia prevention pacing modality may also
be
employed to disable the arrhythmia prevention pacing modality in effect or to
trigger
the switch to an alternative pacing prevention modality, as substitute for or
in addition
to the various mechanisms described above for selecting arrhythmia prevention
pacing
modalities. For example, in response to adjustment of the arrhythmia
prevention
pacing modality to its most aggressive parameters (the parameters believed
most
2 0 likely to prevent occurrences of arrhythmias), in conjunction with a
failure of the
measured metric to fall within the defined variance from the desired endpoint
for the
measured metric, the device may disable the arrhythmia prevention pacing
modality
presently under way or trigger a switch to an alternative available arrhythmia
prevention pacing modality.
Brief Description of the Drawings
Figure 1 is a drawing illustrating a mufti-site atrial pacemaker according to
the
3 0 present invention.
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
Figure 2 is a drawing illustrating a mufti-site atrial, single site
ventricular
pacemaker according to the present invention.
Figure 3 is a drawing illustrating a single site atrial, mufti-site
ventricular
pacemaker according to the present invention.
Figure 4 is a drawing illustrating a mufti-site atrial, mufti-site ventricular
pacemaker according to the present invention.
Figure 5 is a block functional diagram of a first embodiment of a cardiac
pacemaker appropriate for use in practicing the present invention.
Figure 6 is a block functional diagram of a second embodiment of a cardiac
pacemaker appropriate for use in practicing the present invention.
Figure 7 is a block functional diagram of a third embodiment of a cardiac
pacemaker appropriate for use in practicing the present invention.
2 0 Figure 8 is a functional flowchart, illustrating the basic operation of a
pacemaker according to according to a first set of preferred embodiments of
the
invention.
Figure 9 is a functional flowchart, illustrating the basic operation of a
2 5 pacemaker according to according to a second set of Preferred embodiments
of the
invention.
Figure 10 is a functional flowchart, illustrating the basic operation of a
pacemaker according to according to a third set of preferred embodiments of
the
3 0 invention.
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
9
Figure 11 is a functional flowchart, illustrating the basic operation of a
pacemaker according to according to a fourth set of preferred embodiments of
the
invention.
Figure 12 is a functional flow chart illustrating the mechanism by which the
present invention may optimize the parameters of a selected arrhythmia
prevention
pacing mode.
Figures 13, 14 and 15 represent alternative mechanisms for adjusting the
parameters of an arrhythmia prevention pacing modality, in the context of the
functional flow chart of Figure 12.
Figure 16 is a partial functional flow chart illustrating a mechanism which
may be employed in conjunction with the functional flow charts of Figures 12
through
1 S, for disabling or changing the arrhythmia prevention pacing mode presently
in
effect.
Detailed Description of the Preferred Embodiments
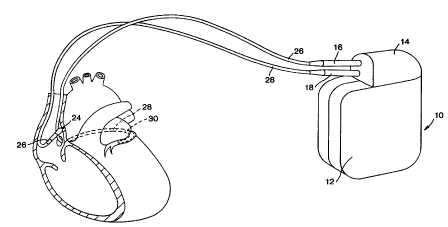
2 0 Figure I illustrates an implantable pacemaker 10 according to the present
invention and an associated lead set. The pacemaker comprises a hermetically
sealed
enclosure 12 containing the pacemaker's circuitry and power source and
carrying a
connector block or header 14 into which the connector assemblies 18 and 16 of
two
pacing leads 20 and 22 have been inserted. Pacing lead 20 is a coronary sinus
lead,
2 5 and carries two electrodes 28 and 30 located thereon, adapted to be
positioned
adjacent the left atrium, within the coronary sinus/great vein of the
patient's heart.
Lead 22 is a right atrial pacing lead carrying a distal, screw-in electrode 24
and a
proximal ring electrode 26.
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
In conjunction with practicing the present invention, the pacemaker may
employ the electrodes on the.various leads in a variety of combinations. Mufti-
site
pacing may be accomplished by simultaneously delivering pacing pulses to the
right
atrium using electrodes 24 and 26, with electrode 24 serving as the pacing
cathode
5 and to the left atrium using electrodes 28 and 30, using either of
electrodes 28 and 39
as the pacing cathode. Alternatively, mufti-site pacing may be accomplished by
delivering pacing pulses between electrodes 24 and 30 or between electrodes 24
and
28, with either of the two chosen electrodes serving as the cathode, in order
to
stimulate the right and left atria simultaneously by using electrode 24 and
either of
10 electrodes 28 and 30 as pacing cathodes and a conductive portion of the
enclosure 12
as a remote anode. Alternatively, the right atrium may be stimulated without
stimulation of the left atrium by employing electrodes 24 and 26 or by
employing
electrode 24 in conjunction with a conductive portion of the housing of the
device
enclosure 12 to accomplish unipolar pacing. Similarly, pacing of the left
atrium may
be accomplished without corresponding pacing of the right atrium by pacing
between
electrodes 28 and 30 or by pacing between either of electrodes 28 and 30 and a
conductive portion of the housing 12.
In conjunction with the present invention, it is preferable that the device 10
be
2 0 configured to allow the physician to program a prioritized list of
tachyarrhythmia
prevention pacing therapies and/or pacing sites and electrode configurations
therein,
for sequential application by the device 10. For example, in the context of a
device as
illustrated in Figure 1, the physician may request that the device 10
initially delivers
pacing pulses to the right and left atria between electrodes 24 and 30 as part
of a first
2 5 arrhythmia prevention therapy, with electrode 24 being a cathodal
electrode, delivers
bipolar pacing pulses in the left atrium employing electrodes 28 and 30 as
part of a
second arrhythmia prevention therapy, with electrode 30 being a cathodal
electrode,
and delivers bipolar pacing in the right atria employing electrodes 24 and 26
as part of
a third arrhythmia prevention therapy, with electrode 24 acting as a cathodal
30 electrode. The first arrhythmia prevention therapy may, for example, simply
be bi-
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
11
atrial bradycardia pacing, while the second and third therapies may, for
example, also
include rate stabilization pacing as in the above-cited Mehra'471 patent.
The device in some embodiments may operate as follows. Following
programming, the device employs electrodes 24 and 30 to simultaneously pace
both
the right and left atria. Over the course of a defined extended time period of
weeks or
months, the device detects a defined number and/or cumulative duration of
tachyarrhythmias according to preset criteria. For example, a. tachyarrhythmia
may be
defined as a high atrial rate maintained for a minimum period of time. In
response to
the number and/or cumulative duration of detected tachyarrhythmia episodes
equaling
a preset value, the device preferably checks to see if there are any available
electrode
configurations which offer the opportunity of reducing the frequency of
occurrence or
the durations of atrial tachyarrhythmias. Because the next electrode
configuration on
the physician-defined list has not been tried, pacing the left atrium using
electrodes 28
and 30 is then employed by the device. If the device detects the required
number of
occurrences and/or cumulative duration of tachyan hythmias during a subsequent
defined time period, the device will determine that the third electrode
configuration,
pacing the right atrium by means of electrodes 24 and 26, is untried, and will
employ
this electrode configuration. On detection of the required number of
occurrences or
2 0 cumulative duration of tachyarrhythmias, the device will then compare the
number of
tachyarrhythmias detected and the time period over which the tachyarrhythmias
were
detected for each of the three electrode configurations, and choose the
electrode
configuration associated with the lowest incidence of tachyarrhythmias.
Operation of
the device in this fashion continues, with the choice of electrode
configuration altered
2 5 automatically in response to an increase in the frequency of occurrence or
cumulative
duration of tachyarrhythmias using the previously selected electrode set, as
compared
to historical measurements of the frequency and/or duration of arrhythmias in
conjunction with the other electrode combinations.
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
12
In an alternative set of embodiments, using the same prioritized list of
arrhythmia prevention therapies, the device may operate for a, first period in
the
absence of any particular arrhythmia prevention therapy and/or alternate
electrode
configuration. For example the device may operate as a conventional AAI,
bradycardia pacemaker, pacing at a single site in the right atrium. The device
operates
in this fashion for a first defined period of time, for example extending over
a period
of days or weeks, and monitors the number of detected tachyarrhythmia episodes
and/or the durations of detected atrial tachyarrhythmias. If the frequency or
duration
of detected atrial tachyarrhythmias detected during this time period is less
than a first
preset threshold, the device may determine that specialized arrhythmia
prevention
pacing therapies and/or mufti-site pacing or a combination of the two are not
required.
If, however, during the first defined time period, a number or total duration
of
atrial tachyarrhythmias exceeding the first threshold are detected, the device
may
activate the first available tachyarrhythmia prevention therapy and/or
alternate
electrode configuration for a second defined period of time, typically less
than the first
defined period, again monitoring the frequency and/or durations of detected
atrial
tachyarrhythmias, followed by sequentially activating the second and third
tachyarrhythmia prevention therapies and/or electrode configurations for the
second
2 0 defined extended time period and determining the frequency and/or
durations of atrial
tachyarrhythmias. After delivering all available tachyarrhythmia prevention
therapies
and/or employing all available electrode configurations, the device may
compare the
relative frequencies and/or durations of atrial tachyarrhythmias to determine
which
therapy and/or electrode configuration results in the lowest incidence of
2 5 tachyarrhythmias, and enabling that therapy and/or set of electrodes and
polarities,
providing that the therapy and/or electrode configuration provides a reduced
incidence
of tachyarrhythmias compared to conventional single site bradycardia pacing as
measured during the first time period.
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
13
As an preferred alternative to employing each of the available tachyarrhythmia
prevention therapies and/or alternative electrode configurations for defined
time
periods and comparing the frequencies or cumulative durations of
tachyarrhythmias
detected therein, the device may instead continue operation in each of the
available
therapies and/or alternative electrode configurations until the earliest of
the expiration
of the defined second time intervals or the meeting of a defined
tachyarrhythmia
duration and/or frequency threshold. The relative incidences or durations of
tachyarrhythmias per unit time may then be compared to chose the most
desirable
therapy and/or electrode configuration. Using this method, the time required
to check
the various available therapies and/or electrode configurations may be
substantially
reduced. Similarly, the initial operation of the device without use of
tachyarrhythmia
prevention therapies and/or alternate electrode configurations may preferably
continue
until the earliest of the expiration of the defined first time interval or the
meeting of a
defined tachyarrhythmia duration and/or frequency threshold, to reduce the
time
required to determine whether tachyarrhythmia prevention therapies and/or
alternate
electrode configurations are desirable.
In a simplified version of this embodiment of the invention, the device may be
provided with only a single tachyarrhythmia prevention pacing therapy and/or a
single
2 0 alternate electrode configuration. In this embodiment, the device merely
compares the
frequency andlor duration of tachyarrhythmias during application of the
arrhythmia
prevention therapy and/or alternate electrode configuration with the frequency
and/or
duration of tachyarrhythmia incidences in the absence of arrhythmia prevention
therapy and/or alternate electrode configuration, enabling application of the
therapy
2 5 and/or alternate electrode configuration only if they result in a reduced
incidence of
tachyarrhythmias.
In the event that the operation of the device and the selection of an initial
arrhythmia prevention therapy is provided as described above, the device may
30 subsequently operate according to the methodology set forth in conjunction
with the
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
14
previously described embodiments of the invention, periodically changing the
tachyarrhythmia prevention therapy and/or the associated set of electrodes and
polarities in response to the increase in the levels of tachyarrhythmias,
selecting an
alternative therapy and/or set of electrodes and polarities which provide a
lower
incidence of tachyarrhythmias, if available. Alternatively, the device may
define a
third extended time period, significantly longer than the first and second
extended
time periods. On expiration of this third time period, the device may repeat
the
sequence of operations described above to again determine whether
tachyarrhythmia
prevention therapies and/or alternate electrode configurations -are desirable
and if so
which should be employed.
Figure 2 illustrates an alternative embodiment of a pacemaker according to the
present invention. Here the pacemaker 40 of Figure 2 generally corresponds to
the
pacemaker 10 of Figure l, with the addition of ventricular pacing
capabilities. The
pacemaker comprises a sealed hermetic enclosure 42 containing the pacemaker's
circuitry and power source and a connector block 44 which receives the
connector
assemblies 46, 48 and 50 of three pacing leads 52, 54 and 56. Leads 52 and 54
correspond to leads 20 and 22, respectively, of Figure 1, and carry atrial
pacing
electrodes 58, 60, 62 and 64. Lead 56 is a ventricular pacing lead carrying a
helical
2 0 electrode 68 imbedded in the right ventricle of the heart and a, ring
electrode 66. A
device according to Figure 2 may employ mufti-site atrial pacing in
conjunction with
ventricular pacing, using pacing modalities such as DDD, DVI and DDI pacing.
Figure 3 is a second alternative embodiment of the pacemaker employing the
2 5 present invention. In this embodiment of the present invention, pacemaker
80
corresponds generally to the pacemaker 40 in Figure 2, including a hermetic
enclosure
82 containing the pacemaker's circuitry and power source and a connector block
84
receiving the connector assemblies 86, 88 and 90 of leads 92, 94 and 96,
respectively.
In this embodiment, the pacemaker is configured to provide mufti-site
ventricular
3 0 pacing in conjunction with atrial. sensing or pacing, so that mufti-site
ventricular
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
pacing rnay be employed in conjunction with known pacing modes such as VDD,
DDD, DVI and DDI. Leads 92, 94 and 96 correspond to leads 52, 54 and 56,
respectively illustrated in Figure 2, and carry pacing electrodes 98, 100,
102, 104, 106
and 108. In the case of lead 92, it has been advanced further into the
coronary
5 sinus/great vein than leads 20 and 52 of Figures l and 2, respectively, so
that
electrodes 102 and 104 are positioned adjacent the left ventricle of the
heart. The set
of leads provided in Figure 3 thus gives the opportunity to provide multi-site
pacing in
the ventricles of the heart, by pacing the right ventricle using the
electrodes 106 and
108 and pacing the left ventricle using the electrodes 102 and 104 or by
pacing
10 between electrodes 108 and either of the electrodes 102 and 104 or by
pacing between
electrode 98 and an uninsulated portion of the housing 82 and pacing between
electrode 102 or 104 and an uninsulated portion of the housing 82. Pacing of
the right
ventricle alone without concurrent pacing of the left ventricle is possible
using
electrodes 106 and 108 or using electrode 108 in conjunction with an
uninsulated
15 portion of the housing 82. Similarly, delivering pacing pulses to the left
ventricle only
is possible using electrodes 102 and 104 together or by using either of
electrodes 102
or 104 in conjunction with a conductive portion of housing 82. Operation of
the
device to select the desired pacing locations within the right and left
ventricles and the
desired electrode configuration may be accomplished in an analogous fashion to
that
2 0 described above in conjunction with Figure I in the context of mufti-site
atrial pacing.
Figure 4 illustrates an additional embodiment of a pacemaker according to the
present invention. Pacemaker 120 corresponds generally to Pacemakers 10, 40
and 80
in Figures 1, 2 and 3, respectively, and includes a hermetic enclosure 122
containing
2 5 the battery and circuitry of the pacemaker and a connector block 124
receiving the
connector assemblies 126, 128 and 130 of pacing leads 132, 134 and 136. Pacing
leads 132 and 134 correspond to pacing leads 90 and 92 of Figure 3, and carry
pacing
electrodes 138, 140, 150 and 152. Lead 136 is provided with four electrodes
142, 144,
146 and 148, allowing pacing of the left atrium using electrodes 142 and 144
and
3 0 pacing of the left ventricle using electrodes 146 and 148. In this
embodiment of the
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
16
invention, the device may select between pacing locations in the atria and the
ventricle
and may select among electrode configurations for accomplishing pacing in
either or
both of the atria and/or either or both of the ventricles in accordance with
the basic
mechanism described in conjunction with Figure 1 for use in selecting
electrodes to be
used in atrial pacing.
Figure 5 is a block diagram of a first embodiment of a pulse generator
appropriate for use in conjunction with the present invention. The block
diagram
illustrated is particularly useful for pacemakers as illustrated in Figure 1,
directed
towards mufti-site pacing of the atria. Similarly, the device illustrated in
Figure 5 is
also suitable for mufti-site pacing of the ventricles, by themselves. The
pacemaker
,includes a microprocessor 200 which controls operation of the device based on
programming stored in read-only memory 202, communicated to the microprocessor
200 by means of data/address bus 208. The timing and control circuitry 206,
under
microprocessors control, specifies the times of delivery of pacing pulses
using the two
pacing pulse amplifiers 2 10 and 212 and communicates occurrences of sensed
events
using sense amplifiers 222 and 224. Information with regard to the operation
of the
pacemaker including information as to the numbers and times of occurrences of
tachyarrhythmias employed in the present invention is accomplished by means of
2 0 random access memory 204.
Microprocessor 200 operates as an interrupt driven device, under software
control, responsive to expiration of timers within timer/control circuitry 206
and in
response to occurrence of sensed events, detected by sense amplifiers 222 and
224.
Telemetry circuit 230 in conjunction with antenna 232 allow communication
between
the device and an external programmer, by means of which the physician can
program
a desired list of electrode configurations into memory 204. The general
operational
methodology of this device may correspond to any of the numerous available
microprocessor controlled cardiac pacemakers, for example, as disclosed in
U.S.
Patent No. 4,404,972 issued to Gordon et al., U.S. Patent No. 4,830,006 issued
to
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
17
Haluska et al. or U.S. Patent No. 4,407,288 issued to Langer et al., all
incorporated
herein by reference in their entireties. In particular, the operation of the
device may
generally correspond to that described in U.S. Patent No. 5,411,524 issued to
Mehra et
al, also incorporated herein by reference in.its entirety.
The device illustrated in Figure 5 differs from the operation of the devices
in
the above described patent and in the above cited references disclosing multi-
site
pacing patents by means of a provision of a switch matrix 226 which operates
under
control of microprocessor 200 via data/address bus 208 according to the
methodology
of the present invention. Switch matrix 226 operates to interconnect the
electrodes
214, 216, 218 and 220 with the pacing pulse generators 2 10 and 212 and with
the
sense amps 222 and 224 in any desired combination or configuration.
For example, electrodes 214 and 216 may correspond to electrodes 24 and 26
of Figure 1, while electrodes 218 and 220 may correspond to electrodes 28 and
30 of
Figure 1. Electrode 228 may correspond to the housing of the device. In
conjunction
with operation of the device to simultaneously pace both atria, switch matrix
226 may
couple pulse generator 210 with electrodes 214 and 216 and may couple pulse
generator 212 with electrodes 218 and 220 to provide for pacing of both the
right and
2 0 left atria. Alternatively, pulse generator 2 10 might be coupled to
electrodes 214 and
220, with the pacing pulse delivered therebetween in order to accomplish
simultaneous pacing of both atria. The device may similarly be employed to
pace only
one atrium using only electrodes 214 and 216 and either of the two output
amplifiers
222 or 224 or to pace the other of the two atria using electrodes 218 and 220
and
2 5 either of the pulse generators 210 and 220. Similarly, the device may be
employed to
pace either one or both of the ventricles of a patient's heart, by locating
electrodes 214
and 216 adjacent one ventricle and electrodes 218 and 220 adjacent the other
ventricle.
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
18
Microprocessor 200, under control of programming stored in read only
memory 302 also serves to implement the various tachyarrhythmia detection
functions
required by the device and to control the timing and delivery of pacing pulses
in both
conventional bradycardia pacing modalities and according to the physician's
specified
prioritized list of arrhythmia prevention pacing therapies. Arrhythmia
detection
mechanisms may correspond to any of those employed in prior art implantable
anti-
arrhythmia devices including anti-tachycardia pacemakers, implantable
cardioverters
and implantable defibrillators. Examples of arrhythmia detection methodologies
appropriate for use in conjunction with the present invention include those
described
in U.S. Patent No. 5,755,736 issued to Gillberg et al., U.S. Patent No.
5,545,186
issued to Olson et al, U.S. Patent No. 5,730,141 issued to Fain et al. and
U.S. Patent
No. 5,379,776 issued to Murphy et al., all incorporated herein by reference in
their
entireties. It should be understood that any of the various known and
available
arrhythmia detection methodologies may be employed in conjunction with the
present
invention.
Arrhythmia prevention pacing therapies which may be implemented by
microprocessor 200 and associated programming may, for example, correspond to
those disclosed in U.S. Patent No. 3,937,226 issued to Funke, U.S. Patent No.
4,354,497 issued to Kahn, U.S. Patent No. 5,683,429 issued to Mehra, U.S.
Patent No.
4,941,471 issued to Mehra, U.S. Patent No. 5,545,185 issued to Denker, U.S.
Patent
No. 5,713,929 issued to Hess et al., U.S. Patent No. 5,158,079 issued to Adams
et al.
and U.S. Patent No. 5,403,356 issued to Hill, some of which pacing modalities
may
also be delivered using multi-site pacing electrode systems, all of which are
2 5 incorporated herein by reference in their entireties. The multi-site
pacing therapies
employed in conjunction with the present invention should also be understood
to
include conventional bradycardia pacing therapies, delivered to multiple sites
within
the atria and/or ventricles, which are also sometimes valuable in preventing
the
occurrences of some tachyarrhythmias, as well as arrhythmia prevention pacing
modalities as discussed above, delivered to multiple sites. Additional mufti-
site pacing
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
19
therapies which may be employed include, for example, those described in U.S.
Patent
No. 4,928,688 issued to Mower et al., U.S. Patent No. 5,720,768 issued to
Verboven-Nelissen, U.S. Patent No. 5,584,868 issued to Salo et al., U.S.
Patent No.
5,243,978 issued to Duffin and U.S. Patent No. 5,267,560 issued to Cohen, all
also
incorporated herein by reference in their entireties
Corresponding sets of arrhythmia detection methods and mufti-site and
arrhythmia prevention pacing methods should be understood to be defined by the
microprocessors and associated programming provided in the pacemakers
illustrated
in Figures 6 and 7 below, constrained to the extent necessary by the available
number
of input amplifiers and pulse generator output circuits included in the
pacemakers.
Figure 6 is an embodiment of a pacemaker which may be employed to provide
mufti-site atrial pacing in conjunction with ventricular pacing or mufti-site
ventricular
pacing in conjunction with atrial pacing, corresponding to those illustrated
in Figures
2 and 3. Microprocessor 300, ram 304, ROM 302, data/address bus 308,
timing/control circuitry 3 06, output amplifiers 3 10 and 3 12 and input
amplif ers 3
22 and 324, as well as switch matrix 326 all correspond generally to
microprocessor
200, rarn 204, ROM 202, data/address bus 208, timing/control 206, output
amplifiers
2 0 2 10 .and 212, input amplifiers 222 and 224 and switch matrix 226 of
Figure 5.
Telemetry circuit 330 in conjunction with antenna 332 allows communication
between the device and an external programmer, by means of which the physician
can
program a desired list of electrode configurations into memory 304. Operation
of the
device differs from that illustrated in Figure 6 in that it is provided with
programming
2 5 stored in ROM 302 which allows microprocessor 300 to operate
timing/control
circuitry 302 to provide dual pacing modes such as DDD, DVI, VDD, DDI, and the
like, using amplifier 3 10 to pace one or more of the atria, amplifier 3 12 to
pace one
or more of the ventricles, sense amplifier 322 to sense atrial depolarizations
and sense
amplifier 324 to sense ventricular depolarizations.
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
If mufti-site atrial pacing is desired in conjunction with ventricular pacing,
for
example, electrode 314 may be placed adjacent the right atrium, electrode 316
may be
placed adjacent the left atrium and electrodes 318 and 320 may be placed in
the right
ventricle. Electrode 328 may correspond to the housing of the device. Pacing
of both
5 atria may be accomplished by coupling atrial pacing amplifier 3 10 to pace
between
electrodes 314 and 316, while pacing of the right atrium individually maybe
accomplished by coupling output amplifier 3 10 to electrodes 314 and 328 and
pacing
of the left atrium individually may be accomplished by coupling amplifier 3 10
to
electrodes 310 and 328. Pacing of the ventricle maybe accomplished by coupling
10 electrodes 318 and 320 to amplifier 312.
In the case in which mufti-site ventricular pacing is desired in conjunction
with atrial pacing, this may be accomplished by placing electrodes 314 and 316
in the
right atrium, electrode 318 in the right ventricle and electrode 320 adjacent
the left
15 ventricle. Atrial pacing may be accomplished by coupling amplifier 3 10 to
electrodes
314 and 316, while pacing of right and left ventricles simultaneously may be
accomplished by coupling pulse generator 312 to electrodes 218 and 320 and
pacing
therebetween. Pacing of the right ventricle individually may be accomplished
by
coupling amplifier 312 to electrodes 318 and 328 while pacing the left
ventricle
2 0 individually may be accomplished by coupling amplifier 312 to electrodes
320 and
328. In the event that mufti-site pacing is desired in both the atria and the
ventricles,
electrodes 314 may be located in the right and left atria respectively, and
electrodes
318 and 320 located in the right and left ventricles respectively, and a
switch matrix
employed as described above to select pacing in either one or both of the
atria or the
2 5 ventricles.
Figure 7 is a block diagram of a pacemaker which may accomplish any of the
pacing modalities discussed above. Pacemaker in Figure 7 corresponds generally
to
that in Figure 5, with the exception that separate output amplifiers are
provided for
3 0 each of the right and left atria and each of the right and left ventricle,
in conjunction
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
21
with separate electrode pairs to be applied to each of the right and left
atria and right
and left ventricles. Microprocessor 400, Ram 404, ROM 402, data/address but
408,
timinglcontrol circuitry 406, pulse generators 410 and 412, sense amplifiers
422 and
424 and switch matrix 426 correspond generally to microprocessor 300, ram 304,
ROM 3 02, data/address bus 3 08, timing/control circuitry 3 06, pulse
generators 3 10
and 312, amplifiers 322 and 324 and switch matrix 326 of Figure 6. Telemetry
circuit
442 in conjunction with antenna 444 allows communication between the device
and
an external programmer, by means of which the physician can program a desired
list
of electrode configurations into memory 304. The device of Figure 7 differs in
the
addition of two additional amplifiers 428 and 430 and the addition of
additional
electrodes 432, 434, 436 and 438 and by means of the correspondingly increased
number of switches required for switch matrix 426 to allow interconnection of
the
four output amplifiers to the electrode pairs to allow for pacing of one or
both of the
ventricles or the atria. For example, electrodes 414 and 416 may be located in
the
right atrium, electrodes 418 and 420 located adjacent the left atrium,
electrodes 432
and 434 may be located in the right ventricle, and electrodes 436 and 438
located
adjacent the left ventricle, to provide a pacemaker corresponding to that
illustrated in
Figure 4. Electrode 440 may correspond to the housing of the device. Pulse
generators
420, 43 0, 410 and 412 may be selectively connected via switch matrix 426 to
allow
2 0 for pacing of one or both of the right and left atria and right and left
ventricles, using
either electrode pairs located adjacent each chamber or by pacing between
electrodes,
one located adjacent each chamber or by pacing between electrodes located
adjacent
each chamber and the enclosure of the device.
2 5 Figure 8 is a functional flow chart illustrating a first method of
operation of a
device according to a first embodiment of the present invention, which may
correspond structurally to the devices of any of figures I - 7. Figure 8
illustrates a
subset of the program stored within the memory of the device, controlling
operation of
the microprocessor, and reflects the operation of the device following sensing
of an
30 atrial or ventricular depolarization, depending upon the particular
implementation of
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
22
the invention. A device according to the present invention may employ the
methodology illustrated in Figure 8 following sensed atrial events if atrial
pacing is
available, following sensed ventricular events if ventricular pacing is
available or
following both atrial and ventricular events if atrial and ventricular pacing
are both
available.
In response to sensing of a depolarization at 500, the microprocessor records
the type of depolarization sensed and the period separating the sensed
depolarization
from previous depolarizations of the same chamber and of other chambers, in
order
that the stored information rnay be employed to determine whether a
tachyarrhythmia
is currently underway. The device checks at 504 to determine whether a
tachyarrhythmia is underway. For example, the presence of a tachyarrhythmia
may be
confirmed by persistence of a rate in the chamber being sensed above a defined
threshold, extending for at least a defined period of time. Alternatively, any
other
known tachycardia or tachyarrhythmia detection algorithm may be employed,
including those set forth in the U.S. patents cited above.
In the event that tachyarrhythmia is not detected at 504, the device checks at
506 to determine whether the extended time period over which occurrences of
2 0 tachyarrhythmia are monitored, as discussed above, has expired. The
extended time
period is preferably at least several days and more preferably at least
several weeks. If
the timer has expired, the timer is reset at 5 10 and the count of occurrences
of
tachyarrhythmias is correspondingly reset at 5 10 and the device returns at 5
0 8 to
pacing the atria and/or ventricles using the electrode configuration
previously
2 5 selected.
In the event that tachyarrhythmia is detected at 504, the device may
optionally
check at 512 to determine whether the event which preceded the first short
period of
the detected tachyarrhythmia was a paced event in the chamber in which the
3 0 tachyarrhythmia is detected. If so, a count of detected occurrences of
tachyarrhythmia
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
23
is incremented at 514. If not, the device checks to see if the extended time
period is
expired at 506, as discussed above. Alternatively, step 512 may be omitted,
and the
device may increment the count of occurrences of tachyarrhythmia each time the
tachyarrhythmia is detected, irrespective of whether the tachyarrhythmia is
preceded
by a delivered pacing pulse in the chamber in which the tachyarrhythmia is
detected.
It is believed that inclusion of the step illustrated at 512 may be preferable
in some
patients, in that it tends to more accurately identify tachyarrhythmias which
might
have been initiated by delivery of cardiac pacing pulses.
At S 16 the incremented count of tachyarrhythmia occurrences is checked
against a preset threshold, and, if the count has not exceeded the preset
threshold, the
microprocessor checks at 506 to determine whether the extended time period has
expired, and proceeds as described above. If, however, the count of detected
tachyarrhythmias at S 16 does exceed the defined threshold, the microprocessor
records information at 518 with regard to the frequency of occurrence and/or
duration
of tachyarrhythrnias, the present tachyarrhythmia prevention therapy and the
present
electrode configuration of the device. For example, the microprocessor may
record in
random access memory associated therewith, the therapy presently in effect,
the
particular electrodes employed to pace the chamber in which the
tachyarrhythmia is
2 0 detected, the polarities of the electrodes and the time span over which
the required
number of tachyarrhythmia occurrences was detected. As discussed above, this
information may later be employed in order to determine whether the existing
arrhythmia prevention therapy and/or electrode configuration. is performing
better
than other available therapies and/or electrode configurations.
The extended time period and the tachyarrhythmia count are reset at 520, and
at 522 the microprocessor checks to determine whether a therapy and/or an
electrode
configuration is available which offers the opportunity of a reduced incidence
of
tachyarrhythmias. By definition, untried therapies and/or electrode
configurations that
3 0 are on the list of configurations programmed into the device by the
physician are
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
24
considered to offer the possibility of a reduced incidence of
tachyarrhythmias.
Alternatively, the previously attempted arrhythmia prevention therapies and/or
electrode configurations which have, based on information recorded by the
microprocessor, provided a lower incidence of tachyarrhythmias are also
considered
to offer the possibility of a reduced incidence of tachyarrhythmias. In the
absence of
untried therapies and electrode configurations, the therapy and/or electrode
configuration having the lowest incidence of tachyarrhythmia per unit of time
will be
employed. At 524, the arrhythmia prevention therapy and/or electrode
configuration
and/or selected pacing sites are modified as appropriate, and the device
continues to
operate using its newly selected therapy and/or electrode configuration. If
the
historical information stored by the microprocessor with regard to other
therapies and
electrode configurations does not indicate the opportunity for a lower
incidence of
tachyarrhythmias, the device continues to operate at 508 using the present
therapy and
electrode configuration and continues to monitor occurrences of
tachyarrhythmia. By
the mechanism described above, as the arrhythmic substrate of the heart
changes over
time, the pacemaker automatically has the ability to optimize the
tachyarrhythmia
prevention therapy, the pacing site or sites employed by the pacemaker and/or
the
electrode configurations employed at the various pacing sites.
2 0 Figure 9 is a functional flow chart illustrating an alternative method of
operation of a pacemaker according to the present invention, which may
correspond
structurally to the devices of any of figures I - 7. Like the method of
operation
illustrated in Figure 8, this portion of the software stored in the read-only
memory of
the device is entered at 600 in response to a sensed atrial or ventricular
depolarization,
2 5 and the associated information concerning the depolarization is recorded
at 602. At
604, the microprocessor checks to determine whether a tachyarrhythmia has been
initiated. If so, the microprocessor optionally checks at 606 to determine
whether the
detected tachyarrhythmia was preceded by a paced beat, as discussed above in
conjunction with Figure 8. If so, the time of onset of the tachyarrhythmia is
recorded
30 at 614. As discussed above, the device may alternatively record the onset
of a
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
tachyarrhythmia regardless of whether it was preceded by a paced beat or not.
In
either case, the device then checks at 608 to determine whether the extended
time
period has expired at 608. If so, the device resets the extended time period
and the
total duration of tachyarrhythmia episodes. at 6 10. In either case, the
device then
5 continues operation at 612 using the electrodes configuration previously
employed.
In the event that tachyarrhythmia is not detected at 604, or in the event that
tachyarrhythmia was previously detected at 604 and continues to be underway,
the
device checks at 618 to determine whether termination of the tachyarrhythmia
has
10 been detected. If not, the device checks at 608 to determine whether the
extended time
period has expired and continues as described above. If, on the other hand,
termination of the tachyarrhythmia has been detected, the device records the
termination at 620 and employs the difference between the time of onset and
the time
of termination to update a cumulative measurement of total duration of
15 tachyarrhythmia episodes at 622.
At 624, the microprocessor compares the total duration of tachyarrhythmia
episodes with a preset threshold value Y. If the total duration of
tachyarrhythmia
episodes accumulated does not exceed Y, the device returns to check to
determine
2 0 whether the extended time period has expired at 608 and continues as
described
above. If, however, the total duration of detected tachyaxrhythmia episodes
exceeds
the preset threshold, information regarding the anti-arrhythmia pacing
therapies and/or
electrode configuration presently in effect in conjunction with information
concerning
the detected tachyarrhythmias is recorded at 626, including the amount of time
2 5 required in order to detect the specified total cumulative duration of
tachyarrhythmia
episodes. This information is used as described above in comparing the therapy
and/or
electrode configuration presently in effect to other available therapies
and/or electrode
configurations. The extended time period and the measurement of total duration
of
tachyarrhythmia episodes is reset at 628, and the device checks as discussed
above, to
3 0 determine whether a therapy and/or electrode configuration offering the
opportunity
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
26
for reduced frequency of tachyarrhythmias is available. If so, the pacemaker
select the
new therapy and/or electrode configuration at 632, and the device continues
operation
at 612 using the newly selected therapy and/or electrode configuration. If,
based on
the historical information recorded by the microprocessor, the other available
therapies and/or electrode configurations do not appear to offer the
likelihood of a
reduction in the overall duration of tachyarrhythmia, the device returns to
its previous
operation using the arrhythmia prevention therapy and electrode configuration
presently in effect at 612.
Figure 10 is a functional flow chart illustrating a third method of operation
of
a pacemaker according to the present invention which may correspond
structurally to
the devices of any of figures 1 - 7. In this embodiment, the pacemaker is
provided
with a single arrhythmia prevention therapy and/or a single alternate
electrode
configuration, for example, multi-site pacing using a conventional bradycardia
pacing
mode, rate stabilization pacing at single or multiple sites as described above
or any of
the other arrhythmia prevention pacing modalities described in the patents
cited
above. In this embodiment, the device simply determines whether or not
delivery of
the arrhythmia prevention therapy and/or use of an alternate electrode
configuration is
appropriate, based upon the frequency and/or duration of occurrences of
arrhythmias
2 0 while the arrhythmia prevention therapy is present as compared to the
frequency or
duration of occurrences of arrhythmias while the arrhythmia prevention therapy
is
disabled.
The operation of the device according to these embodiments of the invention is
2 5 initialized at 700, followed by resetting of the extended time period T I
at 702. The
extended time period in this case typically extends for at least a period of
several days.
During this time period, the device monitors a parameter "X" associated with
detected
tachyarrhythmias which may be, for example, cumulative duration of detected
tachyarrhythmias, frequency of occurrence of tachyarrhythmias, or frequency or
3 0 duration of detected tachyarrhythmias following pacing pulses as described
above in
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
27
conjunction with Figures 8 and 9. Alternatively, a combination of these
parameters
may be employed, as discussed below. The device continues to monitor the value
of
the selected tachyarrhythmia parameter until either time period T1 expires at
710 or
until the value of the monitored parameter exceeds a threshold X2 at 716,
indicative
of a specified frequency or duration of detected tachyarrhythmias. On
expiration of T
I, the device checks to determine whether the value Xi of the measured
tachyarrhythmia parameter exceeds a defined threshold X1 which is set to be
less than
X2 as described above. If not, the device determines that the incidence of
tachyarrhythmias is not sufficient to justify use of the arrhythmia prevention
therapy
and/or alternate electrode configurations, the tachyarrhythmia prevention
therapies
and/or alternative electrode configurations are disabled at 706 and the device
exits to
normal bradyarrhythmia pacing functions at 708.
If, on the other hand, the measured value Xi of the tachyarrhythmia parameter
exceeds XI during time period TI or exceeds X2, prior to the expiration of
time period
T I, the time (T) since initiation of the extended time period and
tachyarrhythmia
parameter value (Xi) are stored at 714. The arrhythmia prevention therapy
and/or
alternative electrode configuration is set on at 718, and the extended time
period is
reset at 720 to again correspond to a second extended time interval T2, which
may be
shorter than T1. In a fashion analogous to that described above, the
microprocessor
waits until either the time period T2 expires at 722 or until the measured
value Xp of
the tachyarrhythmia parameter exceeds a defined threshold X3, which may be
less
than X2, at 724, and following either event, stores the time (Tp) since
resetting of the
extended time period X2 and the measured value (Xp) of the arrhythmia
parameter at
2 5 726. At 728, the microprocessor compares the frequency or duration of
tachyarrhythmia per unit time during use of the therapy and/or alternate
electrode
configuration (XplTp) with the frequency or duration of tachyarrhythmias in
the
absence of the therapy (Xi/Ti). If the frequency or duration of arrhythmias is
not
reduced during delivery of the arrhythmia prevention therapy and/or
alternative
3 0 electrode configuration, the arrhythmia prevention therapy and/or
alternative electrode
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
28
configuration is disabled at 732 and the device exits to normal bradycardia
pacing at
734. If, on the other hand, the incidence of tachyarrhythmias is reduced
during use of
the arrhythmia prevention therapy and/or alternative electrode configuration,
the
therapy and/or alternative electrode configuration is enabled at 730 and the
device
exits the initialization program at 734. If multiple tachyan:hythmia
parameters are
monitored, they may be weighted and combined with one another and compared to
a
single threshold value or each monitored parameter may have its own threshold.
If
multiple thresholds are employed, the device may determine that the required
level of
arrhythmias been detected in response to one, some or all of the thresholds
being met.
Figure 11 is a functional flowchart illustrating a more elaborate method of
operation of the present invention, in which multiple arrhythmia prevention
therapies
and/or electrode configurations are included in a pacemaker which may
correspond
structurally to the devices of any of figures 1 - 7. As described above,
available
operative modes may include single site arrhythmia prevention pacing
therapies,
mufti-site pacing employing traditional bradycardia pacing modes and
specialized
mufti-site arrhythmia prevention pacing therapies, not corresponding to
convention
bradycardia pacing modes. Operation of the device initially corresponds to
that
described to that in Figure 10. Operation of the device according to the
present
2 0 embodiment is initialized at 800, with the extended time period T I
initiated at 802.
During TI the device monitors the parameter "X" associated with the occurrence
of
tachyarrhythmias, which, as described above may be frequency or duration of
tachyarrhythmias, until either expiration of T1 at 8 10 or until the present
value (Xi)
of the monitored parameter exceeds a defined threshold (X2) at 816.
Alternatively, a
2 5 combination of these parameters may be employed, as discussed above in
conjunction
with Figure 10. If on expiration of T1, the value of the monitored
tachyarrhythmia
parameter X, does not exceed a second threshold Xl lower than X2, the device
determines that arrhythmia prevention therapies and/or alternative electrode
configurations are not required at 806 and the device returns to operating at
a
3 0 conventional bradycardia pacemaker at 808. If the value Xi of the measured
CA 02340911 2001-02-16
WO 00/0920b PCT/US99/18442
29
tachyarrhythmia parameter exceeds X1 on expiration of T1, or exceeds X2 prior
to
expiration of T1, the device stores the elapsed time (T) since the initiation
of the
extended time period and the value (Xi) of the measured parameter.
In this embodiment, it is envisioned that the physician has provided a
prioritized list of a number "n" of tachyarrhythmia prevention therapies
and/or
electrode configurations, which the device will sequentially employ for the
defined
extended time periods to measure the relative frequency of occurrences or
durations of
tachyarrhythmias therein. As discussed above, these therapies and electrode
configurations may differ from one another in terms of the timing of the
delivery of
pulses, the conditions for delivery of pulses and the locations and polarities
of the
electrodes according to any of the various patents cited above dealing with
arrhythmia
prevention therapy. The therapy and/or electrode configuration "n" to be
evaluated is
set equal to 1 (the first therapy and/or electrode configuration on the list)
at 818, and
that therapy and/or electrode configuration is enabled at 820. The timer is
reset at 822
to define the extended time period T2 as described above, during which the
arrhythmia parameter "X" is monitored, precisely as discussed above. On
expiration
of time period T2, or on the value (Xn) of the monitored parameter exceeding
the
higher threshold X3 at 828, the device stores the time (Tn) since initiation
of the
2 0 extended time period T2 and the value (Xn ) of the monitored parameter
associated
with the currently enabled arrhythmia prevention therapy and/or electrode
configuration at 830 so that these values may be employed to later select the
most
desirable operation of the device. The device then checks at 832 to determine
whether
n is greater than in, indicating that the therapy in effect is the last
therapy and/or
2 5 electrode configuration on the list. If not, n is incremented at 834 so
that the next
successive therapy and/or electrode configuration may be activated at 820.
This process continues until corresponding values for elapsed time (T) and the
monitored arrhythmia parameter (Xn) have been gathered for each of the in
available
30 arrhythmia prevention therapies and/or electrode configurations. At this
point, the
CA 02340911 2001-02-16
WO 00/09206 PC'T/US99/18442
device at 836 compares the duration or frequency of arrhythmias per unit time
at 836
and selects the therapy and/or electrode configuration having the lowest
frequencies of
occurrence or durations of arrhythmia detected per unit time. The
initialization
sequence is exited at 838 and the device continues to operate in the selected
5 tachyarrhythmia prevention mode. Following selection of an initial therapy
and/or
electrode configuration, the device may operate as described in conjunction
with
Figures 8 or 9 above, if desired. It should be understood in conjunction with
the
selection function that in the event that none of the in tachyarrhythmia
prevention
therapies and/or alternate electrode configurations provide a reduced
frequency or
10 duration of tachyarrhythmias per unit time, the device will disable all of
the
tachyarrhythmia prevention therapies and return to normal bradycardia pacing
at 83 8.
As in the embodiment described in conjunction with Figure 10, if multiple
tachyarrhythmia parameters are monitored, they may be weighted and combined
with
15 one another and compared to a single threshold value or each monitored
parameter
may have its own threshold. If multiple thresholds are employed, the device
may
determine that the required level of arrhythmias been detected in response to
one,
some or all of the thresholds being met.
2 0 In the event that the operation of the device and the selection of an
initial
arrhythmia prevention therapy is provided as described above in conjunction
with the
embodiment of Figure 11, the device may subsequently operate according to the
methodology set forth in conjunction with the previously described embodiments
of
the invention as described in Figures 8 and 9, periodically changing the
2 5 tachyarrhythmia prevention therapy and/or the associated set of electrodes
and
polarities in response to the increase in the levels of tachyarrhythmias,
selecting an
alternative therapy and/or set of electrodes and polarities which provide a
lower
incidence of tachyarrhythmias, if available. Alternatively, after either
selecting a
tachyarrhythmia prevention therapy or alternate electrode configuration or
30 determining that neither is needed as described above in conjunction with
the
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
31
embodiments of Figures 10 and 11, the device may define a third extended time
period, significantly longer than the first and second extended time periods.
On
expiration of this third time period, the device may repeat the sequence of
operations
described above to again determine whether tachyarrhythmia prevention
therapies
and/or alternate electrode configurations are desirable and if so which should
be
employed.
Figure 12 is a functional flow chart illustrating a mechanism by which a
device according to the present invention may optimize the specific parameters
of a
selected arrhythmia prevention pacing modality. The device may operate
according to
the flow chart of Figure 12 after selecting an optimal arrhythmia prevention
pacing
modality according to the mechanisms described in Figures 1-11, discussed
above.
Alternatively, the device may operate according to the flow chart of Figure 12
in
conjunction with the operation of the device according to the flow charts of
Figures 1-
11 above. For example, the device may attempt to optimize a parameter or
parameters
of each selected arrhythmia pacing prevention modality evaluated by the device
according to Figures 1-11, so that the evaluation of the arrhythmia prevention
pacing
modality may take into account the best of available settings for specific
pacing
modality. In such circumstance, device would preferably continue to operate
2 0 according to Figure 12, after selection of a preferred one of the
available arrhythmia
prevention pacing modalities.
At 900, the arrhythmia prevention pacing modality is selected, either by
programming or by the mechanisms described above in conjunction with Figures 1-
2 5 11. Initial settings of the parameters of the arrhythmia prevention pacing
modality,
for example, the increment to be added to preceding V-V, V-R, R-V or R-R
intervals
in the context of a rate stabilization algorithm as described in the above
cited Mehra
or Denker patents, or adjustment of a decrement to sensed P-P or A-P intervals
or
increment to paced A-A intervals in the context of an atrial overdrive pacing
modality
30 as described in the above Hess patent, or an adjustment to the time delay
between
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
32
delivery of pacing pulses at different pacing sites in the context of multi-
site pacing
algorithms as described above. In addition, at 904, the value of the defined
endpoint
range is set and the value of an optional counter "n", used in conjunction
with features
described below is set to 1. The defined endpoint range may be, for example, a
predefined maximum number of premature atrial or ventricular events per a unit
time,
a range extending between defined maximum and minimum numbers of occurrences
of arrhythmias such as atrial fibrillation per unit time, or the like. At 906,
the device
begins timing a time interval T1, during which the device will evaluate the
performance of the selected antiarrhythmia pacing modality, at the initial
parameter
settings. During this time period, the metric to be measured, e.g., premature
beats,
occurrences of arrhythmias, or the like, is monitored at 908. On expiration of
the
interval T 1 at 910, an endpoint En corresponding to the measured metric is
calculated.
For example, if time interval T 1 extends over several days, and the measured
metric
is premature beats per hour, the calculated endpoint may reflect the average
occurrences of premature beats per hour over the entire duration of time
interval T1 or
may reflect the average rate of occurrences of premature beats per hour over
the later
portion of time period T1. After calculation of E", its value is compared to
the defined
endpoint range, and the relationship between the calculated endpoint E" and
the
defined endpoint range is employed to adjust the setting of one or more
parameters of
2 0 the arrhythmia prevention pacing modality at 914. At 916, the value "n" of
the
optional counter is incremented, and a new time interval T1 is initiated at
906.
Figure 13 illustrates one of the simplest mechanisms by which a device
according to the present invention may adjust the parameters of an arrhythmia
2 5 prevention pacing modality. In this context, it is assumed that the
desired endpoint
range is defined by a value which the physician feels is the upper bound of an
acceptable level of occurrences of arrhythmic events. If the measured endpoint
E" is
greater than the upper bound of the defined endpoint range, the parameter
settings of
the arrhythmia prevention pacing modality are determined to be unacceptable,
and the
30 parameters are adjusted in a defined direction in order to make the
prevention pacing
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
33
modality more aggressive, i.e., more likely to prevent occurrences of
arrhythmia. For
example, in the context of adjustment of an atrial overdrive pacing mode as in
the
above cited Hess et al. patent, the decrements to the measured A-P or P-P
interval
may be increased, in order to decrease the relative number of occurrences of
spontaneous beats. In the event that the value of En is already less than the
upper
bound of the defined endpoint range, the parameters of the arrhythmia
prevention
pacing modality are determined to be acceptable, and are not adjusted.
Figure 14 illustrates a somewhat more complex mechanism for adjusting the
parameters of an arrhythmia prevention pacing modality, in which the desired
endpoint range has defined non-zero upper and lower bounds. At 922, the device
compares E" to the upper bound of the desired endpoint range, in the fashion
described in conjunction with block 918 of Figure 13, and performs a
corresponding
adjustment of the arrhythmia prevention pacing parameters at 924. However, in
this
case, if the measured value of E~ is less than the lower bound of the defined
endpoint
range at 926, the device adjusts the parameters of the arrhythmia prevention
pacing
modality in the opposite direction at 928, decreasing the aggressiveness of
the therapy
in order to avoid over-treating the patient.
2 0 For example, if the measured metric is occurrence of premature ventricular
depolarizations, and the arrhythmia prevention pacing modality is rate
stabilization
pacing as described in conjunction with the above Denker and Mehra patents, a
number of premature ventricular beats in excess of the desired endpoint range
may
trigger a decrease in the increment added to a preceding R-R, V-V, R-V or V-R
2 5 interval to define the next subsequent pacing interval. The result would
be an
increased ability to prevent occurrences of premature ventricular
depolarizations, but
at the cost of an increased number of delivered pacing pulses. Conversely, in
the
event that the number of premature ventricular beats is less than the defined
endpoint
range, the value of the increment added to the preceding measured R-R, V-R, R-
V or
3 0 V-V interval may be increased, increasing the likelihood of spontaneous
ventricular
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/I8442
34
beats and decreasing the number of required delivered pacing pulses. In this
fashion,
the device can optimize the parameters of the arrhythmia prevention pacing
modality
to reflect a balance between reduction in occurrences of premature beats and
the
increased current drain associated with increased delivery of cardiac pacing
pulses.
A similar mechanism can be employed in the context of a device which
delivers pacing pulses to multiple sites within the atria or ventricles of the
heart, in
order to induce a more simultaneous depolarization and prevent occurrences of
premature beats or arrhythmias. For example, as discussed above in conjunction
with
devices as pacing electrodes and sense amplifiers associated with multiple
sites within
the atria or associated with multiple sites within the ventricles may be
provided. In
such devices, delivery of a pacing pulse at one site responsive to a sensed
depolarization at another site or delivery of a pacing pulse at one site in
response to
delivery of a pacing pulse at another site may occur after expiration of a
defined
25 relatively short escape interval, as discussed in U.S. Patent No. 4,928,688
issued to
Mower, and incorporated herein by reference in its entirety. In this case, the
longer
the interval separating the sensing or pacing at a first location and the
subsequent
delivery of a pacing pulse at a second location, the more likely it is that
the pacing
pulse at the second location will be inhibited. In the context of a device
operating in
2 0 this fashion, the device may monitor frequency of occurrences of
arrhythmias, and in
response to a measured endpoint E~ corresponding to frequency of occurrence of
arrhythmias exceeding the defined endpoint range, the duration of the escape
interval
between sensing or pacing at one site and subsequent delivery of a pacing
pulse at a
second site may be decreased, in order to provide a more aggressive anti-
arrhythmia
2 5 pacing modality. Conversely, if the frequency of occurrences of
arrhythmias E~ is
less than the defined endpoint range at 926, the escape interval between a
sensed or
paced event at a first location and subsequent delivery of a pacing pulse at a
second
location may be increased, reducing the number of delivered pacing pulses.
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
Figure 15 illustrates a parameter adjustment methodology similar to that in
Figure 14, with the additional capability of responding to an increase in the
measured
metric as reflected by the most recently measured endpoint En, as compared to
a
previously measured endpoint En_~, allowing for an increase in the
aggressiveness of
5 the arrhythmia prevention pacing therapy even in the circumstance in which
the
resulting endpoint En lies within the desired endpoint range. In this flow
chart,
functional blocks 930, 932, 934 and 936 correspond precisely to functional
blocks
922, 924, 926 and 928, respectively in Figure 14. In addition to the
functionality of
the mechanism illustrated in Figure 14, responsive to a determination that the
10 measured endpoint E" is within the defined endpoint range, the device
checks at 936
to determine whether the most recently measured endpoint E" is greater than
the
previously measured endpoint E"_1 plus a defined delta. If so, the
aggressiveness of
the arrhythmia prevention therapy is incremented at 940, in the same fashion
as if the
measured endpoint En was outside the desired endpoint range at 930.
Figure 16 illustrates the mechanism by which the measured metric, as reflected
by the endpoint E" may be employed to terminate a arrhythmia prevention pacing
modality which has proved itself to be incapable of meeting the defined level
of
performance required. Following a determination that the measured endpoint E"
2 0 exceeds the defined endpoint range at 918, 922 or 930 (Figures 14, 1 S, 16
respectively), the device checks to see whether the adjusted parameters of the
arrhythmia prevention pacing modality are at their maximum level of
aggressiveness
at 942, and whether they have been at the level of maximum aggressiveness for
a
series of "X" T1 measurement periods. If not, the device proceeds to adjust
the
2 5 parameters of the arrhythmia prevention pacing modality as described in
conjunction
with Figures 13-15 discussed above. If so, the device checks to determine
whether the
values of En have been in excess of the defined endpoint range for a preceding
series
of "Y" T1 measurement periods. If not, the device continues to adjust the
parameters
as described in conjunction with Figures 13-15. In the event that the device
has been
3 0 operating with its arrhythmia prevention pacing parameters set at the most
aggressive
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
36
settings for a preceding series of measurement periods T1 and has been
unsuccessful
in reducing the value of the measured endpoint En to an acceptable level for
the
preceding y T 1 intervals, the device determines that the arrhythmia
prevention pacing
modality presently in effect is unlikely to be successful in achieving its
desired result
and disables the arrhythmia prevention pacing modality in effect at 946 or
triggers a
switch in alternative arrhythmia prevention pacing modality. Selection from
the
available pacing modalities may be made according to the descriptions in
Figures 1-
11, discussed above, and the device exits at 948 to an appropriate point
within the
software associated with selection between available arrhythmia prevention
modes.
Following selection of a new arrhythmia prevention pacing modality, the device
may
operate as described in the flow chart of Figure 12, employing a newly defined
desired
endpoint range, newly defined initial parameters, and the like associated with
the
newly selected arrhythmia prevention pacing modality.
One specific embodiment of a device operating as described in conjunction
with Figures 12 et seq. may be implemented as follows. The pacemaker may be
configured to operate in the atrial overdrive pacing modality described in the
above-
cited Hess et al . patent. In this pacing mode, the device responds to
occurrences of
atrial depolarizations by setting the next subsequent atrial escape interval
equal to the
2 0 previous A-P or P-P interval, minus a programmed decrement (-delta) and
continues
to pace at this escape interval until either the occurrence of a sensed atrial
depolarization or delivery of a defined number (plateau step) of sequential
atrial
pacing pulses at the new escape interval. If a sensed atrial depolarization
occurs
before completion of the plateau step, a new, shortened atrial escape interval
is
2 5 calculated as described previously. If the plateau step is completed, the
atrial escape
interval is increased by a programmed increment (+delta), and the device paces
at the
new escape interval until either an atrial depolarization is sensed or the new
plateau
step is completed. This process continues, bounded by programmed upper and
lower
rates to provide atrial overdrive pacing at a rate which is generally only
slightly above
3 0 the intrinsic atrial rate.
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
37
In conjunction with the present invention, the device may operate as follows.
the overdrive pacing mode is initially turned on with nominal values of +delta
of SO
msec, -delta of 20 msec, and plateau steps of 10 beats. Endpoint ranges of one
or more
measured metrics are defined by physician programming such that one or more
corresponding measured endpoints (E") must fall above the defined range to
cause
adjustment of the pacing mode parameters to more aggressive settings. For
example,
the endpoint ranges of mean PACs/day <= 200 and AF episodes/day <= 2 may be
defined. In this embodiment, a defined mean ventricular rate range of <= 85
bpm may
also be defined and may be employed to trigger adjustment to a less aggressive
set of
pacing parameters and prevent adjustment of the pacing mode parameters to more
aggressive settings . The observation period T1 may be set to 24 hours.
During the first 24 hours of data is collected with the pacing mode set at
nominal values. For example, the measured endpoints (En) might be: mean
PACs/day
= 5000 and AF/day = 10, with mean V rate = 72 bpm. Due to one or both of the
PAC/day and AF/day values exceeding the defined acceptable ranges, the pacing
parameters are adjusted to be more aggressive. For example, the following
changes
to the algorithm values may be: +delta = 60 msec (increased by 10 msec), -
delta = 10
2 0 msec (decreased by 10 msec.), and plateau steps = 20 beats (increased by
10 beats).
Any one of these changes may be made or all of the changes may be made
simultaneously for increasing aggressiveness of the pacing mode. During a
newly
initiated 24 hour T1 period, data is collected with the pacing parameters at
the new
settings. The new measured endpoints might be: mean PACs/day =100, AF/day = 0,
2 5 mean V rate = 75 bpm. All of these values are within the defined ranges
and hence
the parameter values remain as set for this period.
Alternatively, assuming that the data is same as above for PACs and AF, but
the V rate is 90 bpm. Then the pacing parameters may be made less aggressive,
for
example by decreasing the +delta value, increasing the -delta value and/or
decreasing
3 0 the plateau step. This process may continue until the pacing mode is
programmed off
CA 02340911 2001-02-16
WO 00/09206 PCT/US99/18442
38
by the physician. Alternatively, this process may be self terminating in the
event that
acceptable endpoint measurements are not obtained. For example, the device may
continue attempting to optimize the pacing mode until the device has remained
at the
most aggressive or least aggressive settings for a defined number (1 or more)
of
successive T1 periods during which the device did not have endpoints within
the
defined ranges, after which the device itself disables the atrial overdrive
pacing mode.
An additional embodiment of a device operating as described in conjunction
with Figures 12 et seq. may be implemented as follows. The pacemaker may be
configured to operate in the atrial rate stabilization pacing modality
referred to as
atrial rate stabilization pacing, described in the above-cited Hill et al.
patent. In this
pacing mode, the device responds to occurrences of atrial depolarizations or
delivered
atrial pacing pulses by setting the next subsequent atrial escape interval
equal to the
previous A-A, A-P, P-A or P-P interval, plus a programmed increment (%delta),
bounded by programmed upper and lower rates. % delta is calculated as a
programmed percentage of the preceding measured interval between atrial
events.
Operation of a device according to the invention employing atrial rate
stabilization pacing as described in the above-cited Hill et al. patent may be
as
follows. An endpoint range of <=200 PAC's/day and an observation period T1 may
be set to 3 days. The nominal % delta for atrial rate stabilization may be set
to 25% of
the preceding measured A-A, A-P, P-A or P-P interval. If the measured endpoint
is
2000 PACs/day, then a more aggressive value for %delta = 20%, 15%, etc. may be
employed thereafter. If the measured endpoint was 100 PACs/day, then the
2 5 parameters of the AS algorithm would not be adjusted. The device may also
be
configured to automatically terminate atrial rate stabilization pacing if
ineffective to
reduce PAC's in a manner analogous to that described above in conjunction with
the
atrial overdrive pacing mode.
CA 02340911 2001-02-16
WO 00/09206 PGT/US99/18442
39
An alternative or additional metric for controlling atrial rate stabilization
pacing may be a measurement of RR variability to trigger changes in %delta.
For
example, if the RR variability was 30% of the mean R-R interval or of the
median
R-R interval over a certain observation period (T1), increases in the
aggressiveness of
the atrial rate stabilization pacing mode could be disabled and the %delta
could be
increased to a less aggressive value of 40% to minimize the amount of atrial
pacing
into sinus arrhythmia. This change in RR variability endpoint range may serve
as a
safety mechanism for the atrial rate stabilization pacing mode in the same way
as the
mean V rate serves to prevent excessive average ventricular rates in the
atrial
overdrive pacing mode, as discussed above.
While it is believed that for practical purposes, commercial implementations
of devices employing the present invention will generally take the form of
microprocessor controlled pacemakers as described above, the invention and its
associated functions may also readily be practiced by means of a pacemaker
based on
full custom digital integrated circuitry as widely practiced in the pacing
industry, or
the form of a device fabricated of commercially available discrete components
and
circuits, so long as basic functions set forth above are preserved. Therefore,
the
disclosed embodiments should be considered exemplary, rather than limiting
with
2 0 regard to the claims that follow.
In conjunction with the above disclosure, we claim: