Note: Descriptions are shown in the official language in which they were submitted.
CA 02352974 2001-05-28
WO 00/35530 PCT/US99/29925
TITLE
INSERTION SETS WITH MICRO-PIERCING MEMBERS FOR USE WITH MEDICAL DEVICES
AND METHODS OF USING THE SAME
RELATED APPLICATIONS:
This application claims priority on U.S. provisional application Serial No.
60/112,691 filed December 18, 1998, and entitled "Insertion Sets With Micro-
Needles And Methods Of Using The Same", which is here specifically
incorporated by reference.
FIELD OF THE INVENTION
This invention relates to insertion sets for use with medical devices and,
in particular embodiments, to insertion sets that use micro-piercing members
for
use with infusion pumps, test apparatuses, drug delivery systems and/or
sensors.
BACKGROUND OF THE INVENTION
Traditionally, medications have been delivered by injection with a single,
fine gauge needle or through an intravenous infusion set with a catheter.
2o However, the administration of an injection with a needle or an intravenous
infusion through a catheter is often accompanied by a small amount of pain or
discomfort as the needle or catheter is inserted and withdrawn from the
injection
or infusion site. This often acts as a deterrent to compliance with a medical
regimen as patients seek to avoid the pain or discomfort. To overcome this
drawback, finer needles or catheters have been used. However, the finer
needles
and catheters still irritate the skin and associated nerve endings, causing
some
discomfort and pain, and deternng patient compliance.
As an alternative to overcome these drawbacks, drug delivery systems
have been developed that deliver the medication by infusion into subcutaneous
3o tissue using an infusion set with a soft cannula. However, the soft cannuia
of the
infusion set is still inserted into the skin with a needle to prevent kinking
of the
CA 02352974 2001-05-28
WO 00/35530 PCT/US99/29925
soft cannula. This, while less traumatic than some other injections, still
causes
some, although small, discomfort and irritation from the insertion and removal
of
the needle. One attempt to greatly reduce discomfort and pain has involved the
use of automatic insertion devices. But there is still the possibility of some
minor
irritation since the needle and soft cannula can contact nerves in the
subcutaneous
tissue.
Another alternative to overcome some of these drawbacks has been the
use of transdermal patches to transfer medications through the skin. This
method
avoids piercing the skin. However, this method of introducing medication
Io through the skin is very limited, since only a few medications are easily
passed
through the outer skin layers and most will not be passed through the skin
surface
in sufficient volumes or rates without piercing the skin.
To ovcrcomc this drawback of slow mcdication transfcr, silicon micro-
needles have been proposed that would pierce the skin to a very minor depth at
a
distance that does not contact nerve cells and avoids any introduction of
pain.
However, although this experimental technique is promising there has been no
practical application proposed to deliver the medication through these solid
micro-needles. One example of typical silicon micro-needles is shown in Fig.
1,
and described in "Break Throughs - Technology - Microneedles", Discover
2o magazine, October 1998 (pages 22 and 23), and "Microfabricated
Microneedles:
A Novel Approach to Transdermal Drug Delivery", Journal of Pharmaceutical
Sciences, Volume 87, Number 8, August 1998 (Pages 922-925), which are
attached hereto as part of this specification and incorporated by reference.
In other medical devices, bodily characteristics are determined by
obtaining a sample of bodily fluid. For example, diabetics often test for
blood
glucose levels. Traditional blood glucose determinations have utilized a
painful
finger prick using a lancet to withdraw a small blood sample. This results in
discomfort from the lancet as it contacts nerves in the subcutaneous tissue.
The
pain of lancing and the cumulative discomfort from multiple needle pricks is a
strong reason why patients fail to comply with a medical testing regimen.
-2-
CA 02352974 2001-05-28
WO 00/35530 PCTNS99/29925
Although non-invasive systems have been proposed, or are in development, none
to date have been commercialized, which are effective and provide accurate
results.
SUMMARY OF THE DISCLOSURE
It is an object of an embodiment of the present invention to provide an
improved insertion set, which obviates for practical purposes, the above
mentioned limitations.
In accordance with an embodiment of the present invention, an insertion
set for essentially painless insertion through tissue of a patient includes a
substrate, a plurality of micro-piercing members and a control structure. The
plurality of micro-piercing members are coupled to the substrate to form a
patch.
In addition, the micro-piercing members have a predetermined length to pierce
the tissue to a predetermined depth to interact with the tissue of the
patient. The
control structure is within the insertion set for directing and controlling
the flow
of fluid relative to the substrate and the plurality of micro-piercing members
of
the insertion set. In addition, the insertion set may include or utilize
methods or
structures for maintaining the insertion set on the tissue for a predetermined
period of time. Preferably, the predetermined length of the at least one micro-
piercing member is long enough to pierce the tissue, and yet short enough to
avoid contacting the nerves in the tissue. Still further embodiments of the
present
invention include a light controlling structure within the insertion set for
controlling the entry of light relative to the substrate and the at least one
micro-
piercing member of the insertion set. Some embodiments include a fluorescent
analyte detection compound (or other detection compound) to detect the level
of
an analyte in the tissue, while other embodiments of the insertion set are an
infusion set for infusing a liquid into the tissue. Other embodiments of an
insertion set are a combination of an infusion set and a sensor set to perform
both
functions.
3o In a further embodiment of the present invention, an insertion set for
_3_
CA 02352974 2001-05-28
WO 00/35530 PCT/US99/29925
essentially painless insertion through tissue of a patient includes a
substrate, a
plurality of micro-piercing members, and a light controlling structure. The
plurality of micro-piercing members are coupled to the substrate to form a
patch.
In addition, the micro-piercing members have a predetermined length to pierce
s the tissue to a predetermined depth to interact with the tissue of the
patient. The
light controlling structure is within the insertion set for controlling the
entry of
light relative to the substrate and the plurality of micro-piercing members of
the
insertion set. In addition, the insertion set may include or utilize methods
or
structures for maintaining the insertion set on the tissue for a predetermined
t 0 period of time. Preferably, the predetermined length of the at least one
micro-
piercing member is long enough to pierce the tissue, and yet short enough to
avoid contacting the nerves in the tissue. Still further embodiments of the
present
invention include a light controlling structure within the insertion set for
controlling the entry of light relative to the substrate and the at least one
micro-
15 piercing member of the insertion set. Some embodiments include a
fluorescent
analyte detection compound (or other detection compound) to detect the level
of
an analyte in the tissue, while other embodiments of the insertion set are an
infusion sct for infusing a liquid into the tissuc. Othcr cmbodimcnts of an
insertion set are a combination of an infusion set and a sensor set to perform
both
20 functions.
According to another embodiment of the invention, an insertion set for
insertion through a material includes a substrate and at least one micro-
piercing
member. The at least one micro-piercing member is coupled to the substrate to
form a patch. In addition, the at least one micro-piercing member has a
25 predetermined length to pierce the material to a predetermined depth to
interact
with the material. In particular embodiments, the insertion set also includes
a
control structure within the insertion set for controlling the flow of fluid
relative
to the substrate and the at least one micro-piercing member of the insertion
set.
In addition, the insertion set may include or utilize methods or structures
for
3o maintaining the insertion set on the material for a predetermined period of
time.
-4-
CA 02352974 2001-05-28
WO 00/35530 PCT/US99/29925
Preferably, the predetermined length of the at least one micro-piercing member
is
long enough to pierce the material, and yet short enough to avoid contacting
contact sensitive elements in the material. Still further embodiments of the
present invention include a light controlling structure within the insertion
set for
controlling the entry of light relative to the substrate and the at least one
micro-
piercing member of the insertion set. Some embodiments include a fluorescent
analyte detection compound (or other detection compound) to detect the level
of
an analyte in the material, while other embodiments of the insertion set are
an
infusion set for infusing a liquid into~the material. Other embodiments of an
to insertion set are a combination of an infusion set and a sensor set to
perform both
functions.
In another further embodiment of the present invention, a self lancing test
strip for essentially painless analysis of an analyte in the tissue of a
patient
includes a substrate, a plurality of micro-piercing members, a control
structure,
and an analyte strip. The plurality of micro-piercing members are coupled to
the
substrate to form a patch. In addition, the micro-piercing members have a
predetermined length to pierce the tissue to a predetermined depth to interact
with
the tissue of the patient. The control structure is within the insertion set
for
controlling the flow of fluid relative to the substrate and the plurality of
micro-
2o piercing members of the insertion set. Also, the analyte strip is coupled
to the
substrate to receive fluid from the control structure of the insertion set. In
further
embodiments, the insertion set may include or utilize methods or structures
for
maintaining the insertion set on the tissue for a predetermined period of
time.
Preferably, the predetermined length of the at least one micro-piercing member
is
long enough to pierce the tissue, and yet short enough to avoid contacting the
nerves in the tissue. Some embodiments include a fluorescent analyte detection
compound (or other detection compound) to detect the level of an analyte in
the
tissue. Other embodiments of an insertion set are a combination of an infusion
set and a sensor set to perform both functions.
Other features and advantages of the invention will become apparent from
-5-
CA 02352974 2001-05-28
WO OOI35530 PCTNS99/29925
the following detailed description, taken in conjunction with the accompanying
drawings which illustrate, by way of example, various features of embodiments
of
the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
A detailed description of embodiments of the invention will be made with
reference to the accompanying drawings, wherein like numerals designate
corresponding parts in the several figures.
Fig. 1 is a perspective view of silicon micro-needles of the type that may
1o be used in embodiments of the present invention.
Fig. 2 is a perspective view of an insertion set in accordance with a first
embodiment of the present invention.
Fig. 3 is a perspective view of an insertion set in accordance with a second
embodiment of the present invention.
15 Fig. 4 is a cross-sectional view of the insertion set as shown along the
line
4-~ in rig. 3.
Fig. 5 is a cross-sectional view of the insertion set shown in Fig. 3 and an
encapsulating covering to secure the insertion set to the skin.
Fig. 6 is a cross-sectional view of an insertion set in accordance with a
2o third embodiment of the present invention.
Fig. 7a is a cross-sectional view of an insertion set in accordance with a
fourth embodiment of the present invention.
Fig. 7b is an enlarged, partial cross-sectional view of the insertion set as
shown in the circle 7b of Fig. 7a.
25 Fig. 8 is a cross-sectional view of an insertion set in accordance with a
fifth embodiment of the present invention.
Fig. 9 is a cross-sectional view of an insertion set in accordance with a
sixth embodiment of the present invention.
Fig. 10 is a cross-sectional view of an insertion set in accordance with a
3o seventh embodiment of the present invention.
-6-
CA 02352974 2001-05-28
WO 00/35530 PCTNS99/29925
Fig. 11 is a perspective view of a test strip in accordance with an eighth
embodiment of the present invention.
Fig. 12A is a cross-sectional view of the test strip as shown along line 12-
12 in Fig. 1 I .
Fig. 12B is a cross-sectional view of an alternative embodiment of the test
strip shown in Fig. 12A.
Figs 13a and 13b are top plan views of an insertion sets in accordance
with an embodiment of the present invention that are combinations infusion and
sensor sets.
1o Fig. 14 is a cross-sectional view of an insertion set in accordance with
another embodiment of the present invention.
Fig. 15 is a cross-sectional view of an insertion set in accordance with a
further embodiment of the present invention.
Fig. 16 is a cross-sectional view of an insertion set in accordance with a
still further embodiment of the present invention.
Fig. 17 is a partial bottom plan view of a capillary structure for a layer in
the insertion set shown in Fig. 16.
Fig. 18(a) is a perspective view of an open encapsulating test strip in
accordance with an additional embodiment of the present invention.
Fig. 18(b) is a perspective view of a closed encapsulating test strip in
accordance with the embodiment of Fig. 18(a).
Fig. 19 is a cross-sectional view of an insertion set in accordance with yet
another embodiment of the present invention.
Fig. 20 is a cross-sectional view of an insertion set in accordance with still
yet another embodiment of the present invention.
Fig. 21 is a perspective view of a flexible insertion set in accordance with
a further embodiment of the present invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
3o As shown in the drawings for purposes of illustration, the invention is
CA 02352974 2001-05-28
WO 00/35530 PCTNS99/29925
embodied in an insertion set such as an infusion set, sensor set, medical
device.
combination devices, or the like, with micro-piercing members. Further
embodiments of the insertion sets or medical devices may utilize biodegradable
implants, capsules, impregnated threads (with medications or the like) with
the
micro-piercing members. In addition, the insertion sets may be coated with
medications, or other agents, that inhibit infection and/or promote healing of
the
insertion site. Preferred embodiments of the insertion sets are for
transcutaneous
placement of the insertion set in subcutaneous tissue just below the stratum
corneum, but above the level where nerves are present. However, in alternative
1 o embodiments, the insertion set may be inserted to deeper depths in the
subcutaneous tissue or into other subdermal tissues where the use of micro-
piercing members is advantageous. In addition, still further embodiments may
be
used to place the insertion sets in other types of tissue, such as muscle,
lymph,
organ tissue or the like, and used in animal tissue. The embodiments may also
be
used in other applications to sample other fluid flows, such as manufacturing,
semiconductor fabrication, chemical synthesis, or the like. Further
embodiments
of the invention are for infusion fluids other than medications, such as
vitamins.
hormones, drugs, proteins, peptides, suspensions, emulsions, gels, saline or
the
like.
2o In preferred embodiments, the insertion sets include at least one micro-
piercing member attached to a substrate to pierce the tissue during insertion.
In
particular embodiments, the micro-piercing member is a micro-metal needle. In
alternative embodiments, the micro-needle may be hollow, solid, grooved, or
the
like. In further alternative embodiments, the micro-piercing member may be
made out of other materials, such as ceramic, plastic, etched metals, crystals
embedded on a surface, fibers (such as glass or carbon), ceramics, glass,
composites, silicon, biodegradable, hydrophilic substances, substances that
soften
and/or change once in contact with the body and/or bodily fluids, or the like.
In
other alternative embodiments, the insertion sets may include more than one
3o micro-piercing member. For example, a single insertion set may include a
micro-
_g_
CA 02352974 2001-05-28
WO 00/35530 PGT/US99/29925
piercing member for an infusion portion and another micro-piercing member for
a
separate sensor portion, or the like. Alternatively, the insertion sets may
include a
plurality of micro-piercing members on a small patch or substrate, such as a
series
of hollow (or grooved) micro-needles (such as from silicon, plastics, metal or
the
like) for infusion of a medication or a series of solid micro-needles for
sensor
applications (such as from silicon, plastics, metal or the like), which micro-
needles are used to penetrate the skin. Preferred embodiments of the micro-
piercing member have a length on the order of 100 prn. However, longer lengths
such as 200 ~m or shorter lengths such as 50 p,m may be used. Other lengths
may
1o also be used, with the selection being dependent on the type of tissue to
be
penetrated, the depth of nerve tissue, condition of the patient, type of
medication,
the type of body characteristic to be determined, number of micro-piercing
members, the size of the insertion set, or the like. The above features may be
combined in various configurations to achieve a set with desired
characteristics.
In particular embodiments, the micro-piercing members (or needles) have
a circular cross-section. However, in alternative embodiments, the micro
piercing members may have other cross-sections, such as square, rectangular,
triangular, polygonal, oval, ellipsoid or the like. In preferred embodiments,
a
substrate and micro-piercing members form a rectangular patch. However, in
alternative embodiments, the substrate and micro-piercing members form
different shape patches, such as square, triangular, polygonal, oval
ellipsoid, or
the like. Advantages to the use of micro-piercing members and a substrate
structure include a larger surface area for infusion, fluid collection and/or
sensing
a characteristic, painless insertion, and extremely low profile. The above
features
may be combined in various configurations to achieve a set with desired
characteristics.
Preferably, the substrate structure forming the patch is sized between I/8"
to 1/16" square. However, in alternative embodiments, the substrate structure
forming the patch is sized smaller or can be considerably larger (upwards of
3o several inches square) with the selection of size being dependent on the
type of
-9-
CA 02352974 2001-05-28
WO 00/35530 PCTNS99/29925
medication to be infused, the characteristic to be determined, the patient
condition, the amount of time the insertion set is to remain in position,
and/or the
like. For instance as shown, but not limited to, in Figs. 13a and 13b, an
insertion
set 140 or 142 includes a rigid or flexible substrate 144 that holds at least
one
sensor 146 to determine a characteristic and at least one infuser 148 to
infuse a
liquid. If the substrate 144 is rigid, the insertion set 140 and 142 are worn
most
effective on large surface areas, such as the abdomen, back or the like. If
the
substrate 144 is flexible, the insertion set could be worn around a wrist,
arm, leg
or the like. In particular embodiments, the sensor 146 and the infuser are
separated by several inches if medication is being infused. However, if a
calibration fluid is being infused to calibrate the sensor 146, the infuser
148 may
be adjacent, combined with, or relatively close to the sensor 146. In another
embodiment, as shown in Fig. 21, a plurality of micro-needle patches 147, that
are generally rigid, are placed on a larger contoured and/or flexible patch
149 to
15 provide large surface areas for detection and/or infusion of fluids.
In particular embodiments, the insertion set is maintained in position at
the insertion site on the tissue with an adhesive overdressing. In other
embodiments, an adhesive patch (or under-dressing) is placed on the tissue
prior
to insertion of the insertion set, or is used in addition to an overdressing.
In still
20 other embodiments, the insertion set has wings (or a flange) surrounding
the
periphery of the insertion set, which have an adhesive that attaches the
insertion
set to the tissue. This can be augmented by an overdressing and/or an under-
dressing. In yet other embodiments, the substrate surface between the micro-
piercing members may have an adhesive that attaches the insertion set to the
25 tissue. This can also be augmented by wings (or a flange), an overdressing
and/or
an under-dressing. In alternative embodiments, the insertion set may also be
attached by sutures, staples, clamps, glue, or the like. In particular
embodiments,
the micro-piercing members are coated with an anti-microbial substance that
tends to inhibit infection occurnng around the perforation made in the skin.
3o Further embodiments include a healing agent, such as Vitamin E, anti-
-lo-
CA 02352974 2001-05-28
WO 00/35530 PC'T/US99/29925
inflammatory agents, such as Dexamethasone, or the like, that promotes healing
and/or minimizes scaring after removal of the insertion set with the micro-
needles.
As discussed above, preferably, silicon is used to form the micro-piercing
s members (or needles) and substrates. The micro-piercing members and
substrate
structure can be formed in silicon through the use of silicon wafer technology
such as photolithography, chemical etching, vapor deposition, DREI, laser
drilling, and/or the like. In alternative embodiments, metals, ceramics,
plastics,
or the like, are used to form the micro-piercing members and substrate
structure.
1 o Such materials include, but are not limited to, specially engineered
polymer
materials designed for deep photo etching using MEMS (Micro Electro
Mechanical Systems) processing techniques, or the like. Methods which can be
used for creating the structure in ceramics, metal, or plastic include
molding,
thermoforming, laser drilling, chemical etching and/or the like. Plastics that
can
15 be used for the micro-piercing members and substrate structure include, but
are
not limited to, PEEK (polyetheretherketone) and LCP (Liquid Crystal Polymer),
polycarbonates or the like. PEEK and LCP are particularly strong when formed
with thin cross-sections and lend themselves to conventional molding
techniques.
Plastics may be molded (depending on their flow characteristics) or more
viscous
2o plastics could require a combination of molding and laser drilling/chemical
etching or thermoforming with laser drilling/chemical etching. LCP is a unique
plastic that has both amorphous and crystalline segments that form the
plastic.
The micro-piercing members and substrate could be formed in such a way that
the
crystalline segments line up in a particular direction. Then, the amorphous
25 segment may be removed using chemical etching leaving the segments (rods,
needles or micro-piercing members) of crystalline material exposed. This could
also be done in glass filled plastics. In preferred embodiments, the micro-
piercing members and the substrate are formed from the same material, either
as
an integral unit or separately and later connected. However, in alternative
3o embodiments, the micro-piercing members and the substrate may be formed
from
CA 02352974 2001-05-28
WO 00/35530 PCT/US99/29925
different materials.
In particular embodiments that are either formed from a single piece of
material or formed from multiple materials, it is advisable to coat the
substrate
and micro-piercing members with a material that helps maintain the structural
integrity of the insertion set and minimizes breakage, fracture and/or Ioss of
micro-piercing members once the insertion set is inserted or during withdrawal
of
the insertion set. For instance, the insertion set and micro-piercing members
could be coated with a thin layer (i.e., a few microns) of parylene, plastic
or the
like.
1o In particular embodiments, the micro-piercing members and substrate
structure are generally optically opaque to light and electromagnetic
radiation. In
other embodiments, the micro-piercing members and substrate structure may have
transmissions in ranges or bands for particular purposes, or may be optically
transparent to light and electromagnetic radiation that enable the insertion
sets to
15 be used as described in more detail below. In other embodiments, as shown
in
Fig. 14, the insertion set 1 SO may include "rods" or light pipes 152 that are
included in the substrate 154 to direct light to the piercing members 156. In
preferred embodiments, the light pipes 152 are formed as separate elements out
of
Si02, A1203, glass, plastic, or the like, and are connected to the substrate
154 by
2o the use of anodic bonding. In alternative embodiments, the piercing members
156 are formed as the light pipes 152. In addition, the insertion set may be
formed from a single piece of Si02, AI203, glass, plastic, or the like, and
are
etched to form the substrate and micro-piercing members.
In preferred embodiments, the micro-piercing members (or needles) are
25 solid, and access to the insertion site openings, formed by penetration of
the
micro-piercing members, is through holes drilled in the supporting substrate
of
the micro-piercing members. Fluids can be drawn out of these holes by
capillary
action or active suction. Fluids can also be introduced to the insertion site
by
pumping or capillary action that is biased to flow medication through the
holes
30 and through the insertion openings formed by penetration of the micro-
piercing
-12-
CA 02352974 2001-05-28
WO 00/35530 PCTNS99/29925
members. In alternative embodiments, the micro-piercing members (or needles)
are hollow and permit fluid to be withdrawn or provided to the openings formed
by the micro-piercing members at the insertion site through the interior of
the
micro-piercing members. In further alternatives, the holes may be formed in a
part of the micro-piercing members (i.e., on one side of the member - rather
than
through the exact center) and a part of the substrate. This would simplify
manufacturing and avoid very thin tips that might break off when a hole is
formed
through the exact center of the micro-piercing member. In other embodiments,
the use of holes may be avoided by the use of porous materials such as porous
to sintered titanium, porous polyethylene or other such materials. This would
permit medications or other fluids to permeate through the substrate to the
tissue
or from the tissue to the back of the insertion set. It could also simplify
manufacturing issues associated with forming holes in either the micro-
piercing
members and/or the substrate.
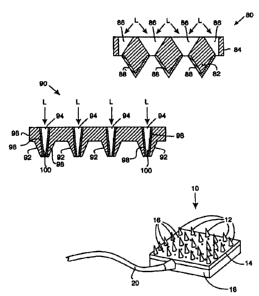
As illustrated in Fig. 2, an insertion set 10 is formed by a plurality of
solid
micro-piercing members 12 (or needles) attached to a substrate 14. In
preferred
embodiments, the micro-piercing members 12 are formed integral with the
substrate 14 or formed separately and attached to the substrate 14. The
substrate
14 is formed with holes 16, or the holes 16 are drilled, adjacent the micro-
2o piercing members 12. The back of the substrate 14 is covered by a fluid
delivery
chamber 18, which is in turn coupled to an infusion supply tube 20. Medication
is then pumped to the medication chamber 18 and dispersed out the holes 16 in
the substrate 14 to permeate into the openings formed in the tissue by the
penetration of the micro-piercing members 12 in the tissue. In alternative
embodiments, the insertion set 10 may be utilized with a sensor and
characteristic
monitor, in which fluid is drawn off and supplied to the sensor.
Figs. 3 and 4 illustrate an insertion set 30 in accordance with a second
embodiment of the present invention that includes an array of micro-piercing
members 32 (or needles) formed on a substrate 34. The micro-piercing members
32 are formed with holes 36 passing through the micro-piercing members and the
-13-
CA 02352974 2001-05-28
WO 00/35530 PCT/US99/29925
substrate. For example, silicon could be used as the materials, and the micro-
piercing members 32 and substrate 34 structure are perforated. Next a fluid
flow
connector 38 is attached to the back 40 of the substrate 34 structure. The
fluid
flow connector 38 is attached to infusion tubing 42 which is attachable to a
pump
(not shown) to provide fluid communication with the holes 36. The holes 36 do
not need to precisely exit the tip 44 (or ends) of the micro-piercing members
32.
In fact, it may be advantages to have the holes 36 slightly offset to produce
"half
needles" or the like with deeper penetration, and which then have the
medication
flow down the sides of the micro-pieicing members 32 into the tissue. In
1o alternative embodiments, the insertion set 30 may be utilized with a sensor
and
characteristic monitor, in which fluid is drawn off and supplied to the
sensor.
Fig. 5 illustrates an alternative embodiment that uses the insertion set 30
shown in Figs. 3 and 4 without the infusion tubing 42 and/or fluid flow
connector
40 or the insertion set 10 shown in Fig. 2. The insertion set 30 containing
the
micro-piercing members 32 and the substrate 34 structure is encapsulated in an
encapsulation material 50 and secured to the tissue by an adhesive 50. The
encapsulation material 50 may be coupled to infusion tubing 42 and an infusion
pump (not shown). In particular embodiments, the encapsulation material 50 can
form a pressurized reservoir 54 that contains medication, or other fluid, that
is
2o slowly infused into the tissue through the openings in the substrate
structure.
Preferably, the medication, or other fluid, is loaded into the reservoir 54
after
insertion of the insertion set to minimize issues of leakage during assembly,
storage and transport. In alternative embodiments, the encapsulation material
50
may be a component of an infusion pump that pumps the medication, or other
fluid, into the user, such as a wrist watch device, or the like mounted over
the
encapsulation material 50. In other embodiments, the encapsulation material 50
may form a negative pressure reservoir to draw off fluid from the tissue. In
other
embodiments, a suction device (not shown) may be attached to the encapsulation
material 50 , where for example, a user uses a valve structure to vent nir and
then
3o apply suction to the interior of the encapsulation material 50 forming the
reservoir
-14-
CA 02352974 2001-05-28
WO 00/35530 PCT/US99/29925
54 to draw off the fluid. The drawn off fluid could be used to determine
bodily
characteristics with a built in sensor or drawn off fluid could be supplied to
a
remote sensor. Preferably, the negative pressure is created in the reservoir
54
after insertion of the insertion set to minimize issues of leakage during
assembly,
storage and transport. In alternative embodiments, the encapsulation material
50
may contain hydrophilic or wicking material (instead of or in addition to the
negative pressure) to draw off fluid from the tissue. In further embodiments,
the
encapsulation material 50 may be divided into sub-regions, in which one region
provides fluid to the tissue and the other region withdraws fluid from the
tissue.
In still further embodiments, the encapsulation material 50 rnay be used with
ioriphoretic medication devices or the like. For example, these types of
devices
would work more efficiently, since the outer layer of the tissue is already
penetrated and fluid flow is easier to facilitate.
As discussed above, embodiments of the insertion sets can be created in
chemically etched metals, such as titanium or stainless steel. Also, high
strength
plastics or composite structures can be used. For example, as shown in Fig. 6,
an
insertion set 60 in accordance with third embodiment of the present invention
utilizes hollow carbon or glass fibers that form the micro-piercing members 62
(or needles). The micro-piercing members 62 are imbedded in another matrix
2o material to form the substrate 64 to create the insertion set 60. In one
embodiment, LCP plastic (described above) is a good candidate for forming an
insertion set 60 having this structure. In alternative embodiments, ceramics
or
sintered metals are also suitable for forming the insertion set 60.
Figs. 7a and 7b illustrate an insertion set 70 in accordance with a fourth
embodiment of the present invention. The insertion set 70 includes micro-
piercing members 72 (or needles) that have an outer surface 74 coated with a
photo-reactive substance or compound 76 that optically changes, fluoresces, or
the like, or other suitable compounds that detect changing properties in the
presence of a bodily fluid analyte, such as glucose or the like. The compounds
3o can also be used to detect the level of an analyte that has been ingested,
injected
-t5-
CA 02352974 2001-05-28
WO 00/35530 PCTNS99/29925
or placed inside the body, such as marker substances, or the like. For
example,
possible compounds, including but not limited to, produce a fluorescent change
in
the presence of a bodily fluid analyte are disclosed in U.S. Patent No.
5,503,770
issued April 2, 1996 to James et al. and entitled "Fluorescent Compound
Suitable
For Use In The Detection Of Saccharides"; U.S. Patent No. 5,512,246 issued
April 30, 1996 to Russell et al. and entitled "Method and Means for Detecting
Polyhydroxyl Compunds"; U.S. Provisional Application Serial No. 60/007,51 S to
Van Antwerp et al. and entitled "Minimally Invasive Chemically Amplified
Optical Glucose Sensor"; and U.S. Patent Application Serial No. 08/752,945 to
1o Van Antwerp et al. and entitled "Detection of Biological Molecules Using
Chemical Amplification", all of which are herein incorporated by reference.
Other compounds using Donor Acceptor fluorescent techniques may be used,
such as disclosed in U.S. Patent No. 5,628,310 issued May 13, 1997 to Rao et
al.
and entitled " Method and Apparatus to Perform Trans-cutaeous Analyte
is Monitoring"; U.S. Patent No. 5,342,789 issued August 30, 1994 to Chick et
al.
and entitled "Method and Device for Detecting and Quantifying Glucose in body
Fluids"; and U.S. Patent No. 5,246,867 issued September 21, 1993 to Lakowicz
et al. and entitled "Determination and Quantification of Saccharides by
Luminescent Lifetimes and Energy Transfer", all of which are herein
incorporated
20 by reference.
In the illustrated embodiment, the micro-piercing members 72 are coated
with the fluorescent material 76 and a substrate 78 is drilled with holes 79
that
permit the passage of light L to illuminate the sides of the micro-piercing
members 72 to induce a fluorescent reaction in the coated material 76 in the
2s presence of the analyte. The strength (or intensity) of the florescence
from the
coated material is used to determine the amount of analyte present in the
bodily
fluid (such as interstitial fluid, blood or the like). In alternative
embodiments,
lifetime measurements of the fluorescence may be used. The use of exterior
coated micro-piercing members 72 is preferred for near continuous monitoring
3o applications, since it is easier for bodily fluids to flow around and be
replenished
-16-
CA 02352974 2001-05-28
WO 00/35530 PCT/lJS99/29925
around the outside of the micro-piercing members 72. In other embodiments, a
second fluorescent compound (not shown) is used as a reference signal and may
be placed at one or more locations around the substrate 78. Still further
embodiments, may be utilized with an infusion set to determine the level of
medication, or fluid being absorbed to determine proper flow rates.
As discussed, preferred embodiments utilize fluorescent compounds to
determine a bodily characteristic. However, alternative embodiments may use
other electro-chemical reactions, such as, for example, in diabetes testing,
the
compounds could be those currently used in conventional blood glucose meters
or
1 o glucose sensors that use interstitial fluid with glucose oxidase sensors
such as
those disclosed in U.S. Patent No. 5,391,250 issued February 21, 1995 to
Cheney,
II et al. and entitled "Method of Fabricating Thin Film Sensors", which is
herein
incorporated by reference. Other compounds for the detection of viral loads
(such
as in HIV, hepatitis or the like), cholesterol levels, or other analytes may
also be
used. In addition, optical analyte materials that measure a change in optical
properties of the materials that are sensitive to IR, visible or other forms
of
radiation may be used.
Fig. 8 illustrates an insertion set 80 in accordance with a fifth embodiment
of the present invention. The insertion set 80 contains a plurality of coated
2o micro-piercing members 82 (or needles) on a substrate 84 similar to that
shown in
Figs. 7a and 7b. However, in this embodiment, the holes 86 in the substrate 84
are more conical to allow better illumination of the sides of the coated micro-
piercing members 82. A preferred method for forming conical holes 86 is the
use
of back side etching of the substrate 84, which would be easier than laser
drilling.
This allows the light L to more directly impinge on the fluorescent compound
88
(or other suitable detection compound), and minimizes reliance on reflection
off
the tissue. In alternative embodiments, the holes may be cylindrical, like in
the
earlier embodiments, but formed at an angle to illuminate one side of the
micro-
piercing members 82. This simplifies manufacturing of the substrate 84, since
3o more conventional manufacturing methods, such as laser drilling may be
used.
_ 17_
CA 02352974 2001-05-28
WO 00/35530 PCTNS99/29925
In another alternative embodiment, the substrate 84 and/or micro-piercing
members 82 are formed from optically transparent materials that permit the
light
to pass through the substrate 84 and the micro-piercing members 82 to
illuminate
the fluorescent compound (or other suitable detection compound). This would be
advantageous, since it would obviate the need to drill light transmitting
holes. It
would also possibly be more acceptable for continuous sensing, since the holes
would not be come clogged with bodily fluids and the fluid around the micro-
piercing members 82 would not tend to easily "dry out." As shown in Fig. 15,
an
insertion set 160 is formed without holes in the substrate and/or through the
to micro-piercing members 164. The substrate 162 and micro-piercing members
164 are formed from a transparent material, such as Si02, A1203, glass,
plastic, or
the like, to permit light L to pass through to the substrate 162 and micro-
piercing
members 164 to a coating 166, similar to that described above in the
embodiments of Figs. 7a-8.
15 Fig. 9 illustrates an insertion set 90 in accordance with a sixth
embodiment of the present invention, in which the micro-piercing members 92
(or needles) are formed with the holes 94 passing through the micro-piercing
members 92 and a substrate 96. In this embodiments, the interior surface 98 of
the hollow micro-piercing members 92 is coated with a fluorescent compound
2o 100 (or other suitable detection compound). This permits easier exposure of
the
fluorescent compound 100 to light L and minimizes the effects of insufficient
illumination or distortion through the substrate 96. This embodiment tends to
be
more ideally suited for discrete measurements, since it would require
ancillary
structure to make the fluid flow from the tissue continuously over long
periods of
25 time. This embodiment (as well as the embodiments as shown in Figs. 7a-8),
could also be used with a fluid delivery system and used to detect back flow
of
bodily fluids (such as interstitial fluids, blood, or the like), which would
indicate
a blockage in the infusion supply tubing, or a compound could be used to
determine the presence of bacteria and infection developing under the
insertion
3o set 90. The coating compound could also be used to detect other
contaminates in
_~ 8_
CA 02352974 2001-05-28
WO 00/35530 PCTNS99/29925
the fluid flow stream from the infusion supply.
Fig. 10 illustrates an insertion set 110 in accordance with a seventh
embodiment of the present invention. This embodiment utilizes micro-piercing
members 112 and a substrate 114 similar to that shown in Fig. 2 (although this
embodiment could easily utilize the hollow micro-piercing member structure
shown in Fig. 3). In this embodiment, the micro-piercing members 112 penetrate
the tissue, and then the holes 116 in the substrate I 14 draw off the
interstitial
fluid (or other liquid or fluid) by capillary action to a layer of material
118 that
contains a fluorescent compound, or the like (as discussed above) that
responds to
I o the presence of an analyte in the interstitial fluid (or other liquid or
fluid). The
layer of material 118 may use capillary action to distribute the interstitial
fluid (or
other liquid or fluid) throughout the layer of material 118. In operation, the
interstitial fluid (or other liquid of fluid) is pulled from the site by
capillary action
and wets the fluorescent compound which is then analyzed by a sensor to
determine the concentration of the analyte.
Figs. 11 and 12A illustrate a self lancing test strip 120 in accordance with
an eighth embodiment of the present invention. The self lancing test strip 120
uses solid (or hollow) micro-piercing members 122 (or needles) and holes 123
on
a substrate 124 coupled via an adhesive or wicking material 126 to an analyte
strip 128 that contains a compound that reacts to the presence of an analyte
in
bodily fluid (such as interstitial fluid, blood or the like) withdrawn from
the fluid.
In further embodiments, the wicking material or adhesive layer 126 may be
omitted and the substrate 124 would be directly coupled to the analyte strip
128.
In particular embodiments, a fluorescent compound and detection method is used
as described above. However, in alternative embodiments, other electro-
chemical
reactions, such as, for example, in diabetes testing the compounds could be
those
currently used in conventional blood glucose meters or glucose sensors that
use
interstitial fluid with glucose oxidase sensors such as those disclosed in
U.S.
Patent No. 5,391,250 issued February 21, 1995 to Cheney, II et al. and
entitled
"Method of Fabricating Thin Film Sensors", which is herein incorporated by
-19-
CA 02352974 2001-05-28
WO 00/35530 PCTNS99/29925
reference. Other compounds for the detection of viral loads (such as in HIV,
hepatitis or the like), cholesterol levels, or other analytes may also be
used.
Preferably, the self lancing test strip harvests interstitial fluid painlessly
from the skin for an intermittent reading of the analyte level as-a-
replacem~tn-
conventional finger sticks used to determine glucose levels, cholesterol
levels or
the like. In the preferred illustrated embodiment, the user taps the self
lancing
test strip I20 with the micro-piercing members 122 against the skin to pierce
the
upper layer and then the interstitial fluid is released from the skin and
pulled by
capillary action through the holes 123 ~in the substrate 124. Alternatively, a
1o flexible dome 300 and vent hole 302 are positioned over the skin
penetrating
portion of the self lancing test strip 120 to create a negative pressure on
the side
opposite the micro-piercing members 122 to assist in drawing fluids through
the
holes 123 in the substrate 124, as shown in Fig. 12B.
The self lancing test strip remains on the skin for a period of time
15 sufficient to withdraw the interstitial fluid, with the time being
determined based
upon the condition of the user's skin, the temperature, the environmental
conditions surrounding the tissue, the type of fluid being withdrawn, the
number
of micro-piercing members122, the number of holes 123, the size of the
substrate
124, or the like. The interstitial fluid is drawn into the wicking and/or
adhesive
2o layer 126 to evenly wet the compound in the analyte strip 128 above it. The
self
lancing test strip 120 is then inserted into a meter (not shown) for analyzing
the
interstitial fluid using conventional tests, or the fluorescent tests
described above.
Alternatively, the self lancing test strip 120 can be left in place on the
skin (or
tissue), and a test meter can be used to periodically measure the analyte,
without
25 the need to remove the self lancing test strip from the skin.
Preferably, the analyte layer 128 is placed down on any optical device to
minimize scratching or abrasion of the optical device by the micro-piercing
members 122. In alternative embodiments, the micro-piercing members are
hollow and draw the interstitial fluid to the regent through the interior of
the
3o micro-piercing members. In further embodiments, the micro-piercing members
-20-
CA 02352974 2001-05-28
WO 00/35530 PCTNS99/Z9925
and substrate are formed out of a porous materials to facilitate transfer of
the
bodily fluid. This may obviate the need for holes in the substrate and/or
micro-
piercing members.
Figs. 16 and 17 illustrate a variation of the embodiments shown in Figs.
10-12, in which an insertion set 170 contains a layer of micro channels 172
between the substrate 174 and the analyte material 176. In preferred
embodiments, the micro-channels are "v" shaped and formed from etching of the
material forming the layer of micro-channels. Also, as shown in Figs. 16 and
17,
the holes 178 in the substrate 174 line' up with the intersections 180 of the
1o channels 182 in a first direction and the channels 184 in a second
direction. The
channels may be at right angles, oblique, acute, or the like to each other.
Preferably, the channels are etched to a few microns depth to promote
capillary
action to draw the fluid to a collection reservoir 186 that concentrates the
collected fluid to provide stronger readings. This allows fluid to be
collected over
a wide area in small quantities to give strong concentrated indications in a
much
smaller area. In alternative embodiments, the micro-channels may be formed on
the opposite side of the substrate to improve the diffusion of the collected
fluid in
the analyte material. In further alternative embodiments, the micro-channels
may
be formed on both sides of the substrate.
Figs. 18(a) and 18(b) illustrate a self lancing test strip 190 similar to the
embodiment shown in Figs. 11, 12A and 12B. The embodiment includes a fold-
over encapsulating tip 192 to cover the micro-piercing members 194 after use
of
the test strip 190. This avoids or minimizes the possibility of bio-hazard
contamination after use of the test strip 190. In preferred embodiments, the
fold-
over encapsulating tip includes an adhesive 196 and is folded over to cover
the
exposed micro-piercing members 194 after the test. In alternative embodiments,
the fold-over tip, may be stiff enough to be bent away from the micro-piercing
members 194 when the test strip 190 is used to avoid premature or accidental
contact with the micro-piercing members 194. Then after use, the stiff fold-
over
3o tip springs back to cover the micro-piercing members 194. In further
-21-
CA 02352974 2001-05-28
WO 00/35530 PCTNS99/29925
embodiments, the interior surface of the fold-over tip that contacts the micro-
piercing members 194 includes a reflective agent to improve the optical
characteristics of the test strip 190, if a reading is taken from the opposite
side.
Fig. 19 is a cross-sectional view of another insertion set 200 in accordance
with an embodiment of the present invention. In this embodiment, the holes 202
(or channels) in the substrate 204 and/or micro-piercing members 206 are
filled
with a hydrophilic material 208 that draws out the fluid from beneath the
skin.
The hydrophilic material 208 facilitates getting the fluid more quickly and
easily
to an analyte detection compound 210. In addition, the hydrophilic material
208
to tends to minimize the ability of the analyte detection compound to contact
or
migrate into the tissues of the user.
Fig. 20 is a cross-sectional diagram showing the use of an optically
transparent substrate 212 and micro-piercing members 214 to permit light L to
be
introduced directly under the skin 215 to illuminate an implanted optical
analyte
15 material 216 to more easily determine the optical changes of the optical
analyte
material 216. The advantage is that the optical transparent substrate 212 and
micro-piercing members 214 provide a shorter light path distance through the
skin 215, which lowers the amount of total diffusion and absorption of light
in the
skin (or tissue).
20 While the description above refers to particular embodiments of the
present invention, it will be understood that many modifications may be made
without departing from the spirit thereof. The accompanying claims are
intended
to cover such modifications as would fall within the true scope and spirit of
the
present invention.
25 The presently disclosed embodiments are therefore to be considered in all
respects as illustrative and not restrictive, the scope of the invention being
indicated by the appended claims, rather than the foregoing description, and
all
changes which come within the meaning and range of equivalency of the claims
are therefore intended to be embraced therein.
-22-