Note: Descriptions are shown in the official language in which they were submitted.
i
., 29974-2
CA 02369000 2002-O1-07
1
FLUIDIC CONNECTION SYSTEM AND METHOD
This application is a divisional of Canadian
patent application Serial No. 2,049,948 filed April 3, 1990
in the name of David S. Zamierowski.
Background of the Invention
Field of the Invention.
The present invention relates generally to fluidic
connection systems, and in particular to systems for
draining liquids from and introducing liquids to patients.
Description of the Relevant Art.
Various types of fluidic connection systems have
heretofore been devised to meet the requirements of
particular applications. In the medical field, fluidic
connection systems find many applications, including wound
dressings and systems for introducing fluids to and removing
fluids from patients.
Wound dressings are typically applied over various
types of wounds to promote healing and to reduce the risk of
infection. Although various types of dressing materials
have been successfully employed, membranes comprising semi-
permeable materials are often preferred because they can
increase patient comfort and lower the risk of infection.
Semi-permeable membranes generally pass moisture vapors, but
are generally impervious to liquids. Thus, they can promote
healing by permitting a wound site to "breathe".
CA 02369000 2002-O1-07
2
1 However, a problem can arise with semi-permeable
2 membranes when they are placed over draining wounds because
3 they tend to retain fluid. For example, surgical wounds
4 often tend to drain for a post-operative period of about
forty-eight hours. The fluid that can accumulate under such
6 a semi-permeable membrane during a draining period can
7 macerate the underlying tissue, cause infection and
8 otherwise inhibit healing. A procedure for alleviating this
9 problem involves periodically piercing the membrane,
IO draining the accumulated fluids and resealing the membrane
11 opening. However, such a procedure is time-consuming for
12 health care professionals and, unless it is conducted at
13 relatively frequent intervals, can be relatively ineffective
14 in dealing with the problems associated with trapped fluid
accumulation. Other procedures which involve opening or
16 changing wound dressings tend to have problems associated
17 with exposing a wound to a greater- risk of infection and can
18 be uncomfortable for patients.
19 Another disadvantage with many previous wound dressings
is that they are not designed to accommodate the
21 introduction of various liquid medications, such as
22 antibiotics and growth factor solutions. The application of
23 growth factor solutions may be particularly important in the
24 regeneration of skin graft donor sites.
Catheters are another type of fluidic connection system
26 with medical applications. They are commonly used for
27 withdrawing fluids from or introducing fluids to patients°
28 bodies. For example, urethral catheters are inserted into
29 the bladder through the urethra fo:r withdrawing urine.
Typical applications for urethral catheters include patients
CA 02369000 2006-02-24
29974-2
3
who are incontinent or have otherwise lost voluntary control
of their bladder functions, e.g. a paraplegic with a spastic
bladder condition. However, patients fitted with urethral
catheters are often subjected to risks of bladder and
urinary tract infections.
To avoid some of these infection risks, condom
catheters have been devised which typically include a body
for placement over the penis and a bellows-type distal end
for resisting kinks and for connection to a drain tube.
However,;condom catheters are susceptible to slippage and
can be difficult to maintain in place unless they are taped
to the patient's penis. Furthermore, there can be
difficulties in effectively draining sudden surges of urine,
which often back up and cause leakage problems.
Heretofore there has not been available a fluidic
connection system and method with the advantages and
features of the present invention.
Summary of the Tnvention
According to one aspect the invention provides a
condom catheter for attachment to a penis, which includes:
(a) a membrane including: (1) an inner, skin contact
surface: (2) an outer surface; (3) a first panel including
an edge strip and a perimeter; (4) a second panel including
an edge strip and a perimeter; (5) a seam extending
transversely across said membrane and comprising said panel
edge strips attached together; and (6) a tube opening
extendinct throuah said seam between said membrane inner and
outer surfaces; (b) adhesive on said membrane inner contact
surface; and (c) tube means including a proximate end
extending through said tube opening and a distal end, said
CA 02369000 2006-02-24
- 30467-1D
4
tube means including a passage extending between and open at
said ends.
According to another aspect the invention provides
a method of catheterizing a penis, which comprises the steps
of: (a) adhering a membrane comprising first and second
panels each including a skin contact surface, an outer
surface and a perimeter with an edge to the penis; (b)
forming a seam, the seam having opposite ends and extending
transversely across said membrane by adhesively engaging
said panel contact surfaces along strips adjacent to said
edges thereof; (c) providing an opening open at said panel
edges and at said contact surface between said strips and
intermediate said opposite ends of the seam; (d) extending a
sheath with a proximate end having an opposed pair of
longitudinally-extending slits forming an opposed pair of
tabs through said tube opening; (e) positioning a mouth open
to a passage of said sheath and formed between said tabs at
said seam adjacent to said skin contact surfaces; and (f)
placing said tube mouth distal to and approximately in
alignment with the meatus of said penis.
Objects and Advantages of the Preferred Embodiments
The principle objects and advantages of the
present invention include: to provide a wound dressing; to
provide such a dressing which promotes the evacuation of
drainage fluids; to provide such a dressing which permits
the introduction of liquid medications; to provide such a
dressing which includes a semi-permeable membrane for
releasable, adhesive attachment to the skin surface
surrounding a wound; to provide such a dressing which
protects against infection; to provide such a dressing which
CA 02369000 2002-O1-07
29974-2
promotes healing; to provide such a dressing which is
economical to manufacture, efficient in operation, capable
of a long operating life and particularly well adapted for
the proposed usage thereof; to provide a wound treatment
5 method; to provide a fluidic connection system and method;
to provide such a connection system and method which are
adaptable to various applications; to provide such a
connection system and method which can be utilized as a
CA 02369000 2006-02-24
30467-1D
6
1 condom catheter; to provide such a connection system and
2 method which are suitable far securing percutaneous tubing;
3 to provide such a connection system which infrequently
4 requires changing; and to provide such a connection system
and method which promote patient comfort, reduce risk of
6 infection, are usable with catheters of various
7 configurations and which are easy to apply and use.
8 Other objects and advantages of this invention will
9 become apparent from the following description~taken in
conjunction with the accompanying drawings wherein are set
11 forth, by way of illustration and example, certain
12 embodiments of this invention.
13 The drawings constitute a part of this specification
14 and include exemplary embodiments of the present invention
and illustrate various objects and features thereof.
16
17 Brief Deacri tion of the Drawings
18
19 Fig. 1 is a top perspective view of a wound dressing
embodying the present invention.
21 Fig. 2 is an enlarged, vertical, cross-sectional view
22 of the dressing taken generally along line 2-2 in Fig. 1.
23 Fig. 3 is a top plan view of the dressing.
24 Fig. 4 is an enlarged, fragmentary, bottom perspective
view of the dressing, particularly showing a proximate end
26 of the tube.
27 Fig. 5 is an enlarged, fragmentary, top perspective
28 view of the dressing, particularly showing a tube closure
29 clip.
CA 02369000 2002-O1-07
7
1 Fig. 6 is an enlarged, fragmentary, vertical, cross-
a sectional view of the dressing, parti<:ularly showing the
3 tube connected to a vacuum source.
4 Fig. 7 is an enlarged, fragmentary, vertical, cross
sectional view of the dressing, particularly showing a
C resealable injection port mounted on a distal end of the
'1 tube .
8 Fig. 8 is a top perspective view of a wound dressing
9 comprising a first modified embodimeni~ of the present
invention.
11 Fig. 9 is a top plan view of a wo and dressing
12 comprising a second modified embodiment of the present
1:3 invention with an intermediate materi<il layer between the
14 wound site and a cover membrane.
1.5 Fig. 10 is an enlarged, fragmentary, vertical, cross-
lfi sectional view of the second modified wound dressing
17 embodiment, taken generally along line 10-10 in Fig. 9.
18 Fig. 11 is a perspective view of a fluidic connection
19 system comprising a third modified embodiment of the present
invention, shown in combination with a drain conduit and
2:L fluid connection vessel for use as a condom catheter and
2:Z urine collection system.
23 Fig. 12 is a top plan view of th<= connection system
24 being applied as a condom catheter.
Fig. 13 is an enlarged, vertical,, cross-sectional view
2b of the connection system taken genera:Lly along line 13-13 in
27 Fig. 12.
28 Fig. 14 is an enlarged, fragmentary, vertical, cross-
2!3 sectional view of the connection system, particularly
showing a funnel end of an inner conduit.
CA 02369000 2002-O1-07
8
1 Fig. i5 is a top plan view of. the connection system.
2 Fig, 16 is a side elevations)- view of the connection
3 system.
4
Detailed Description of the Preferred Embodiments
6
7 I. Introduction and Environment
8
As required, detailed embodiments of the present
invention are disclosed hereins however, it is to be
11 understood that the disclosed embodiments are merely
12 exemplary of the invention, which may be embodied in various
13 forms. Therefore, specific structural and functional
14 details disclosed herein are not to be interpreted as
limiting, but merely as a basis for the claims and as a
16 representative basis for teaching ~Dne skilled in the art to
17 variously employ the present invention in virtually any
18 appropriately detailed structure.
19 Certain terminology will be u:;ed in the following
description for convenience and rei=erence only and will not
21 be limiting. For example, the words "upwardly",
22 "downwardly", "rightwardly" and "lE~ftwardly" will refer to
23 directions in the drawings to which reference is made. The
24 words "inwardly" and "outwardly" will refer to directions
,25 toward and away from, respectively, the geometric center of
26 the structure being referred to. Said terminology will
27 include the words specifically mentioned, derivatives
28 thereof and words of similar import.
29
CA 02369000 2002-O1-07
1 Referring to the drawings in more detail, the reference
2 numeral 10 generally designates a wound dressing embodying
3 the present invention. The dressing 10 is adapted for
4 protecting and treating a variety of wounds, such as that
shown at 12. 6aithout limitation on the generality of the
6 useful applications of the present invention, the dressing
7 10 may be applied over burns, cuts, scrapes and ulcers of
8 various types, e.g. diabetic, decubit.us, peripheral
9 vascular disease, venous stasis and trauma ulcers.
Skin ulcers are a common problem among many diabetics,
11 and are often brought on by poor blood circulation and nerve
12 damage associated with diabetes. The treatment of such
1~ ulcers often involves grafting skin from a relatively
14 healthy donor site to an ulcerous wound site. Split
thickness surgical skin graft techniques may be employed to
lE~ obtain skin grafts from donor sites that can then heal
1T spontaneously. . Full thickness skin grafts, on the other
lE~ hand, generally require closure of they donor site. It will
1u be appreciated from the following description that the wound
dressing and treatment method of the present invention is
21 particularly well adapted for the protection and
22 regeneration of skin graft donor sites, by providing a single
23 dressing which facilitates both fluid drainage and growth
24 factor introduction"
The wound site 12 is surrounded by healthy skin 16. A
26 fibrin layer 18 forms at the wound site 12 from fibrinogen
27 by the action of thrombin and the clotting of blood (Figs.
28 2 and 6). Surgical wounds, including those associated with
29~
20149-602 CA 02369000 2002-O1-07
a
skin grafts, normally drain fluid. The fluid drainage from a
surgical wound is generally heaviest during a post-operative
period of about forty-eight hours.
II. Wound Dressing 10
5 The wound dressing 10 generally comprises a cover
membrane 22 with an interior portion 24 surrounded by a
perimeter 26. The membrane 22 includes a skin contact surface
28 with an adhesive coating 30. The membrane 22 preferably
comprises a breathable semi-permeable material characterized by
10 an ability to pass moisture vapors and an imperviousness to
liquids. The adhesive coating 30 should likewise be semi-
permeable. Such membrane materials are commercially available,
an example being material referred to as "Tagoderm"*~ Which is
available from the 3M (Minnesota Mining and Manufacturing)
Company of St. Paul, Minnesota. Other semi-permeable materials
are available and can be successfully employed with the present
invention. A protective backing 23 is placed over the adhesive
coating 30 on the membrane skin contact surface 28 until the
membrane 22 is ready for application.
The membrane 22 comprises a pair of panels 19 with
inner, upturned edges 20 which can be adhesively joined
together to form a seam 21 which extends transversely across
the membrane 22 and projects generally upwardly therefrom. The
panels 19 can be secured together at the seam 21 by the
adhesive coating 30 to form the seam 21.
A tube or sheath 34 includes a proximate end 36
located under the membrane 22 and a distal or free end 38. The
tube 34 can be inserted through the seam 21 which forms an
Trade-mark
20149-602 CA 02369000 2oo2-oi-o~
11
opening 32 between the panel edge strips 20 at approximately
the centre of the membrane 22. A relatively short length o'f
the tube 34 adjacent to its proximate end 36 is shown under the
membrane 22, but greater lengths of the tube 34 could be placed
under the membrane 22. As shown in Fig. 4, the tube proximate
end 36 is open, and adjacent to the proximate end 36 an opening
is formed. Preferably the tube opening 39 projects downwardly,
i.e. away from the membrane skin contact surface 28. The short
length of the tube 34 which is located under the membrane 22
can be releaseably secured to the skin contact surface 28 by
the adhesive coating 30, preferably with the tube opening 39
facing downwardly.
The tube 34 can comprise, for example; a flexible,
plastic tube of the type that is commonly used as a protective
sheath for protection of sterility for percutaneous intravenous
catheter placement. Such sheaths are commercially available
from Aero International, Inc. of Reading, Pennsylvania.
At its distal end 38, the tube 34 is adapted for: 1)
closure with a variety of suitable closure devices: 2)
connection to various active and passive fluid collection
devices for draining and evacuating fluid from the wound site;
and 3) connection to various fluid source devices for actively
and passively introducing fluid to the wound site.
Fig. 5 shows s bifurcated clip 240 for releaseably
closing and sealing the distal end 238 of tube 134, which is
folded upon itself as shown.
CA 02369000 2002-O1-07
I2
1 Fig. 6 shows a vacuum tube end 91 inserted in the tube
2 distal end 381and secured therein by ties or ligatures 43.
3 The vacuum tube 41 fluidically communicates with a suction
4 or vacuum source 42 for actively draining fluid from the
wound site. The suction or vacuum source 42 may comprise a
6 relatively simple, hand-actuated bulb or bellows, or it may
7 comprise a more sophisticated motorized pump which can be
8 actuated at predetermined time intervals or in response to
9 wound site conditions such as an accumulation of fluid under
the membrane 22.
11 Fig. 7 shows an injection porn 44 sealed to the tube
12 distal end 38 by a band 45. The injection port 44 includes
13 a sleeve 47 which can extend into i:he tube 34 to protect it
14 from needle puncture. The injection port 44 can be of the
type which is designed for reuse and which automatically
16 reseals after being punctured by a syringe needle. It will
17 be appreciated that a wide variety of devices can be
18 employed for connecting the tube distal end 38 to various
19 liquid medication sources.
21 III. Treatment He~thod
22
23 According to the treatment method of the present
24 invention, the protective backing 23 is removed from the
,25 membrane contact surface 28 to expose the adhesive coating
26 30 and the membrane 22 is placed over a wound site 12 with
27 its contact surface 28 down. The membrane perimeter 26 is
28 pressed against the healthy skin 16 surrounding the wound
29 site 12 to preferably form a relatively liquid-tight
adhesive bond therebetween. Various adhesive preparations
CA 02369000 2002-O1-07
13
1 are commercially available for supplementing the bonding
2 action of the adhesive coating 30 in bonding the membrane
3 contact surface 28 to the healthy ski 16, The membranes 22
4 may be provided in various sizes to accomodate wounds of
different sizes. A sufficiently large membrane 22 should
6 normally be selected to provide ample overlap of the
7 perimeter 26 over the healthy skin 16 to insure a good band
8 therebetween.
9 The tube distal end opening 39 may be placed directly
over the approximate center of the wound site 12, or it may
11 be placed eccentrically or at a depending location with
12 respect to the wound site 12. A dependent or lower position
13 for the opening 39 with respect to the wound site 12 may be
14 preferred to facilitate fluid drainage. The dressing l0 may
be applied promptly after a wound is inflicted, e.g.
16 immediately after the graft removal procedure and a skin
17 graft operation. To reduce the risk of infection, it may be
18 advisable to promptly cover the open 'wound site 12. The
19 wound dressing 10 may be kept in a sterile package until it
is needed. Such sterile packages and packaging techniques
21 are well known, Far example, ethylene oxide may be used to
22 sterilize the dressing ZO prior to pl;~cement in a suitable
23 sterile package. The protective backing 23 is removed from
24 the membrane 22, thereby exposing its adhesive-coated
contact surface 28.
26 With the membrane 22 thus secured, a chamber 46 is
27 formed between the wound site 12 and ithe membrane contact
28 surface 28, and is surround by the meanbrane perimeter 26.
29 The chamber 46, fluidically communicates with the membrane
opening 32. In an evacuation mode of operation, such as
CA 02369000 2002-O1-07
14
1 might be desirable for forty-eight hours or so after removal
2 of a split-thickness skin graft at ai donor site, fluid 20
3 which accummulates in the chamber 4E. is communicated through
4 the opening 32 and thence through the tube 34 far collection
and disposal. In a passive evacuation mode of operation,
6 the fluid 20 is evacuated through capillary action, or by
7 gravity with the opening 32 at a deF~endent, lower location
8 in relation to the wound site 12. ~~uch a capillary, passive
9 drainage action may be sufficient for draining the wound
site 12 in many situations. Alternatively, an active
11 evacuation mode of operation involves attaching the tube 34
12 to the suction/vacuum source 42 whereby the fluid 20 is
13 positively drawn from the wound site l2~and the chamber 46.
14 Such an active evacuation mode of operation may be preferred
when the dressing 10 is used in connection with a
16 hydrophilic colloidal material (hydr~ocolloid), as will be
17 explained in more detail hereinafter.
18 It may be desirable to operate the wound dressing 10 in
19 an introduction mode of operation whereby medications such
as antibiotics and growth factor solutions are introduced to
21 the wound site 12. In this mode of operation, the tube
22 distal end 38 is connected to a liquid solution source,
23 which may comprise a syringe or any of various liquid
24 containers for passive, gravity-induced introduction.
Various adaptors, valves and injection needle ports are
26 available for fluidically coupling t:he tube 34 to a wide
27 variety of liquid solution sources. For example, many such
28 connectors and adaptors are available from Aero
29
CA 02369000 2002-O1-07
1 International, Inc. of Reading, Pennsylvania. Such
2 connecting devices are commonly used in connection with the
3 intravenous introduction of various liquid solutions.
4 In an active introduction mode of operation, solutions
5 may be pumped through the tube 34 ixato the chamber 46 for
6 application to the wound site 12.
7 The evacuation and introduction treatment steps can be
8 timed and sequenced as necessary to achieve the treatment
9 objectives. For example, treatment of a skin graft donor
10 site may involve fluid withdrawal and drainage for about two
11 days immediately following the skin graft operation,
12 followed by treatment steps comprising the introduction of
13 antibiotics andjor growth factor solutions to the wound
14 site. The evacuation and introduction steps can be
15 alternated, and the intervals between such steps can be
16 progressively increased or decreased as necessary to
17 facilitate healing. As the wound heals, progressively
18 smaller amounts of fluid will ooze tiherefrom and the
19 frequency and duration of the drainay a operations can be
correspondingly reduced and finally c9iscontinued altogether.
21 It will be appreciated that the wound dressing and
22 treatment method of the present invexition are broadly
23 concerned with introducing fluid to wound sites and
24 evacuating fluid therefrom. The fluid introduction and
evacuation procedures described herein can be performed
26 indefinitely without having to change the dressing 10. The
27 tube 34 cooperates with the membrane 22 to permit the same
28 dressing 10 to be used for both proced ores, which may be
2~
CA 02369000 2002-O1-07
1
1 alternated as often as necessary. Infection risks and
2 patient discomfort can be reduced by minimizing wound
3 dressing changes.
4 The removal of toxins and bao teria from wounds is an
important aspect of the fluid drainage phase of the healing
6 process. The wound dressing of the present invention
7 facilitates removal of serum and other secretions to
8 minimize the risk of infecting the wound site and macerating
9 the tissue thereat. Growth factor solutions can be
important in promoting healing, and antibiotics can be
11 important in preventing and treating infection. Hence, a
12 comprehensive wound treatment can be implemented with the
13 wound dressing and treatment method of the present
14 invention.
The wound dressing 10 can be employed to irrigate a
15 wound whereby fluid is introduced and then removed.
17 The operation of the wound dressing 10 is largely a
18 matter of fluid mechanics, and the function of the wound
19 dressing 10 would probably be determined by such factors and
variables as: 1) fluid viscosity; f.) permeability of the
21 membrane 22; 3) cross-sectional area of the tube 34 and the
22 area of its opening 39; 4) the integrity of the seal around
23 the membrane perimeter 26; 5) the drawing power of the
24 suction or vacuum source 42; 6) coagulation of the serum or
.25 other fluid; 7) the area of the fluid collection chamber 46;
26 8) the length of the tube 34; and 9) gravity and the
27 relative positions of various components. Naturally,.
28 varying one or more of these factors or variables could
29 change the operation of the system. It is anticipated that,
applying such well-known principles of fluid mechanics, all
CA 02369000 2002-O1-07
1 of the wound dressing components could be properly sized and
2 designed. For example, the tube ope=ning 39 could be
3 enlarged, or multiple openings coulc9 be provided to increase
4 the rate of fluid flow into the tubs 34. The rate of fluid
flow can further be increased by locating the tube distal
6 end 38 at a dependent area within the chamber 46, i.e. below
7 the level of most of the wound site 12. The tube 34 can
8 extend downwardly to a collection s~.te below the level of
9 the wound site 12 to facilitate gravity drainage.
It is further anticipated that some fluids will resist
11 drainage because of their viscositie~s or because they tend
12 to coagulate. Drainage of such fluids can be effected by
13 irrigating the wound site 12.
14
IV. First Modified 8mboc3iment 110
1 6
17 Figure 8 shows a wound dressing 110 comprising a
18 first modified embodiment of the present invention wherein a
19 relatively small membrane 122 is provided and functions as a
patch for a larger wound cover 115 with an opening 117 for
21 receiving a distal end 138 of a tube 134. The primary wound
22 cover 115 is selected to cover a wound site 112, and is
23 placed thereover in the normal fashion. The wound dressing
24 110 can be placed on the primary wour.~d cover 115 in a
location chosen to enhance fluid introduction and/or
26 evacuation. For example, to enhance the evacuation of fluid
27 by gravity, it may be desirable to form the opening 117 at a
28 relatively low position of the wound site 112. Thus, fluid
29 will tend to flow to the tube 134 by .gravity. To facility
3~~ the introduction and distribution of :Fluid, it may be
20149-602 ~ 02369000 2002-O1-07
18
desirable to locate the wound dressing 110 at a relatively high
position on the wound cover 115. In fact, two or more wound
dressings 110 could be placed on a single, primary wound cover
115, with a lower wound dressing 110 being provided for fluid
evacuation and an upper wound dressing 110 being provided for
fluid introduction.
In the practice of the treatment method of the
present invention, the wound dressing 110 provides for
considerable flexibility in locating the wound dressing 110 in
an appropriate location on the wound site 112. After the
primary wound cover 115 is positioned, the opening 117 is
formed at the chosen location and the wound dressing 110 may be
applied, much like a patch, with the tube distal end 138
extending through the primary wound cover opening 117. It will
be appreciated that wound dressings 110 may be changed as
needed without changing the primary wound cover 115.
V. Second Modified Embodiment 210
A wound dressing 210 comprising a second modified
embodiment of the present invention is shown in Figs. 9 arid 10
and includes an intermediate layer of material 250 between a
wound site 212 and a cover membrane 222. The intermediate
material layer 250 can comprise a variety of materials with
varying properties such as: 1) absorbency; 2) wicking or
capillary action; and 3) surface contact action. The
intermediate material layer is primarily located in a chamber
246 formed between the wound 212 and the membrane 222.
CA 02369000 2002-O1-07
19
1 As a first example of an intermediate material layer
2 250, several hydrophilic colloid materials (i.e.
3 hydrocolloids) are available which would tend to absorb
4 fluids. For example, Envisan wound cleaning pads and paste
are available from Marion Laboratories, Inc. of Kansas City,
6 Missouri and comprises spherical, hydrophilic Heads of
7 Dextranomer, 0.1 to 0.3mm in diameter; polyethylene glycol
8 3000 in the pad; polyethylene glycol 600; and water QS
9 enclosed in a polyamide net bag in the pad or available in a
metal foil packet for the paste. The Envisan dextraminer
11 beads function to absorb fluid and facilitate healing by
12 drawing fluid from the wound. Excess fluid can be drained
13 from the intermediate material layer 250 to prolong its
14 effectiveness. Other hydrocolloids are commercially
available and may be employed with the wound dressing 210 of
16 the present invention, e.9. dextranimers available under the
17 trademark "Debrisan".
18 Alternatively, the intermediate material layer 250 can
19 comprise a mesh or sheet of synthetic material which is
generally nonabsorbent and would tend to wick fluid from the
21 wound site 212 to a tube distal end 238. For example, rayon
22 available under the trademark Owens non-adherent surgical
23 dressing from the Davis & Geck division of American Cyanamid
24 Company of Danbury, Connecticut could be used to form such
an intermediate material layer 250, and material available
26 from Marion Pferrell Dow, Inc. of Kansas City, Missouri under
27 the trademark "Envinet" could also be employed. Such
28 materials may be referred to as "surface active°', i.e.
2~9 promoting fibrin sealing on the wound surface. Such
materials can also satisfy a capillary purpose whereby fluid
CA 02369000 2002-O1-07
1 is wicked from the wound for collection in the chamber 246
2 and ultimately for drainage. with many such materials, a
3 balance is struck between surface action and capillary
4 action, i.e. one such function is often maximized at the
5 expense of the other. For example, Owens rayon is generally
6 considered to be relatively surface active, but may provide
7 less capillary action than other materials. Envinet mesh,
8 on the other hand, provides greater capillary action, but
9 may provide less surface action as compared to the rayon
10 material.
11 Other materials that can be used for the intermediate
12 material layer 250 include polyurethane foam and
13 polyurethane mesh.
14 The wound dressing 210 can be used according to methods
15 for use with the other wound dressings 10 and 110, and
16 includes the additional step of placing the intermediate
17 material layer 250 over the wound site 212. It will be
18 appreciated that there may be a number of materials suitable
19 for the intermediate layer 254 to achieve various
20 objectives.
21 A closure patch 251 is provided for placement over the
22 tube distal end 238 and is adapted for securing it in a
23 folded configuration to the membrane 222. The closure patch
24 251 can be used in conjunction with a bifurcated clip 240 as
shown in Figs. 9 and 10, and permits convenient access to
26 the tube distal end 238 for coupling it to various devices
27 such as those described herein, allowing future reuse of the
28 tube or intermittent function. Alternatively, the tube can
29
CA 02369000 2002-O1-07
21
1 be severed at the surface of the meambrane, allowing the
2 closure patch 251 or a similar patch of the same material as
3 the wound dressing 10 to permanentl;Y seal the tube site.
4
Vy. Third Modified Embodiment 31~
6
7 A fluitlic connection system 3117 comprising a third
8 modified embodiment of the present invention is shown in
9 Figs. 11-16. Without limitation on the generality of useful
applications of the fluitlic connection system 310, it is
1I shown in connection with a urine collection system 312 and
12 functions as a condom catheter. The connection system 310
13 generally includes a membrane assembly 314 and a tube
14 assembly 316.
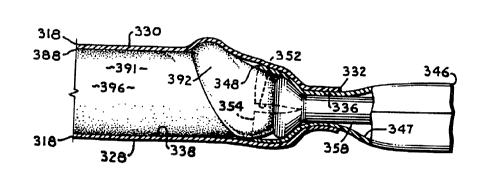
The membrane assembly 314 includes a membrane 318 with
16 an inner or skin contact surface 320, an peter surface 322,
17 a perimeter 324 and an interior portion 326. As shown in
18 Fig. 11, the membrane 318 comprises first and second panels
19 328, 330.
The panels 328, 330 include inner contact surfaces 329,
21 outer surfaces 331, perimetet-s 333, and edges 335 with edge
22 strips 332 which are joined together in opposing relation to
23 form a seam 334 extending transversely across the membrane
24 318 between opposite sides of its perimeter 324. A tube
opening 336 extends through the seam 334 approximately in
26 the middle thereof and is open at the membrane inner and
2~ outer surfaces 320, 322m
28 An adhesive layer 338 substantia:Lly covers the membrane
inner surface 320 and releasably secures a two-piece
protective backing 340 (e. g. paper, p7!_astic or some other
CA 02369000 2006-02-24
30467-1D
Z2
1 suitable material). The backing 340 can form a transverse
2 seam 342 with a pair of unattached edge strips 344 adapted
3 to be grasped for pulling off the backing 340. The membrane
4 318 of the fluidic connection system 310 can comprise a
semi-permeable material.
6 The tube assembly 316 includes an outer tube or sheath
7 346 with proximate and distal ends 348, 350 and a passage
8 347 extending therebetween. The proximate end 348 extends
9 through the tube opening 336 and has a bifurcated
configuration with a pair of longitudinally-extending,
11 opposed slits 352 forming an oppoeed pair of tabs 354 each
12 placed against a respective panel contact surface 320 (Fig.
13 11). The tabs 354 can be secured to the respective panel
Z4 inner surfaces 320 by the adhesive 338 thereon. However,
for many applications of the connecting system 310 it may be
16 preferrable for the tabs 354 not to have adhesive on them.
17 The tabs 354 form a mouth 356 open at the outer tube
18 proximate end 398 and located adjacent to the membrane inner
19 surface 320. The outer tube or sheath 346 can comprise a
flexible, collapsible., impervious material.
21 The tube assembly 316 also includes an inner tube or
22 conduit 358 with a proximate end 360 including a funnel 362,
23 a distal end 364, and a conduit bore 366 extending between
24 and open at the conduit ends 360 and 364.
An annular connector/seal band 372 receives the conduit
26 358 and includes enlarged-diameter end flanges 374 with a
27 reduced-diameter, annular channel or waist 376 therebetween.
28 The connector or seal band 372 can be integrally farmed with
29 the conduit and thus permanently fixed in position thereon,
or, alternatively, the band 372 can slideably receive the
CA 02369000 2002-O1-07
23
1 conduit for adjustable repositioning. Preferrably the band
2 372, in either configuration, forms a relatively fluid-tight
3 seal on the conduit 358. The band 372, like the funnel 362,
4 can be slid through the sheath passage 347 for placement
proximal to the sheath distal end 350 (Fig. 15). Helt or
6 tie means 378 can be provided for sea:lingly fastening the
7 sheath 346 to the band 372. ~s shown in Fig. 15, the
8 belt/tie means 378 can comprise ligatures 380, which can be
9 wrapped around the sheath 346 for tiglhtening it against the
band channel 376. Belt/tie means 378 can comprise other
11 suitable fasteners, such as strips with hook-and-loop
12 fasteners (i.e. fasteners available u~'der the trademark
13 "Velcro"), rubber or elastic bands, plastic coated wire
14 twist ties, etc. Multiple bands 372 can be used for
connecting and sealing the sheath 346 and the conduit 358.
16 The conduit distal end can project distally from the
17 sheath distal end 350 (Fig. 15) for connection to tubing 382
1B by a suitable tubing connector, such as the multi-diameter,
19 double male-ended ("Christmas Tree") connector 384 shown in
Figs. 11 and 15. The tubing 382 can :Lead to a suitable
21 fluid collection vessel 386, which can be positioned remote
22 from the patient.
23
24 QII. Applications and O~~eration
26 The fluidic connector 310 can be utilized for a variety
27 of fluidic connection applications without the inner tube or
28 conduit assembly 358. For example, the fluidic connection
2~ system 310 can function as a wound dressing which operates
in a manner similar to the wound dressings 10, 110 and 210
CA 02369000 2002-O1-07
24
1 described above. In such applications the membrane 318 can
2 comprise a semi-permeable, plastic, film adherrent dressing
3 sheet, such as those commercially available under the trade
4 names "Op-Site°', "Tagaderm", and "Bio-Occlusive"" The outer
tube or sheath 346 can be utilized as a two-way conduit for
6 draining wound exudate and for introducing liquids to the
7 wound. The liquids introduced could comprise, for example,
8 aqueous solutions for irrigating the wound and growth
9 factors for promoting healing. Epidermal growth factor
("EGF") is available from Vicron. Platelet derived growth
11 factor is available from the Curate~ch Corporation. Such
12 growth factors can accelerate healing and re-
13 epithelialization of wound sites. Without limitation on a
14 wide variety of wounds that can be treated with such
dressings, they are particularly suitable for partial
16 thickness wounds, such as skin graft donor sites. Drainage
17 and liquid application can be alternated without having to
18 intermittently change the membrane 318. Frequent dressing
19 changes can be painful to skin wound patients and burdensome
to health care personnel.
21 As with the previously described application of the
22 wound dressing 110 shown in Fig. 8, the cannection system
23 310 can be applied at any desired location on a larger patch
24 or membrane, and can be used in various multiple
"25 combinations, if desired. For example, one connection
26 system 310 can be used for introducing fluids, and another
27 connection system 310 can be used fear draining fluids, with
28 both connection systems 310 operating simultaneously if
29 desired.
CA 02369000 2002-O1-07
1 To promote efficient drainage, the connection system
2 310 can be located at a dependent part of a larger dressing.
3 Alternatively, mechanical suction equipment can be connected
4 for promoting drainage.
5 Another application of the fluidic connection system
6 310 is placement over percutaneous catheters, drain tubes,
7 etc. Such tubes present infection risks where they
8 penetrate the skin surface, and can require frequent
9 application of antibiotics to reduce the risk of infection.
10 Percutaneous tubes are often sutured in place at the stab
11 wound locations where they penetrate the skin, and the
12 sutures are further susceptible to infection and can cause
13 swelling and patient irritation. The connection system 310
14 can be placed over such a percutaneous drain tube or
15 catheter site, with the tubing extending through the sheath
16 346 in the manner of the conduit 358. The tubing can be
17 secured, for example with one or mare bands 372, to protect
18 against traction forces which might otherwise tend to pull
19 the tubing loose. By utilizing a semi-permeable, breathable
20 material for the membrane 318, the skin surrounding a
21 percutanious tubing entry site can be protected against
22 maceation.
23 For use as a condom catheter in .a urine collection
24 system 312, the backing 340 can be removed from the first
25 panel 328, which is then adhesively secured to the ventral
26 side 389 and the lateral sides 391 of a flaccid penis 388
2'7 with the urethra orifice or meatus 39~D directed at the
28 sheath mouth 356 and the sheath tabs :354 placed on the top
2~ and bottom of the glans or penile head 392 (Fig. 13). The
CA 02369000 2002-O1-07
26
1 second panel 330, with the backing 340 removed, can then be
2 adhered to the penile dorsal side 394 and to the first
3 panel 328.
The procedure described abom=_ provides a relatively
secure attachment of the connection system 310 to the penis
6 388, since the penile shaft 396 and the penile head 392
7 provide substantial areas of attachment. The attachment can
8 further be enhanced by prestretch9.ng the flaccid penis to
9 provide maximum contact area.
The connection system 310 described above can be
11 utilized as a complete condom catheter by fluidically
12 connecting the sheath distal end 350 to a suitable urine
13 vessel, for example with a band such as that shown at 45 in
14 Fig. 7. Alternatively, the inner tube or conduit 358 can
then be inserted through the sheath passage 347 in its open,
16 distal end 350. The funnel 362 can be placed against the
17 glens 392 over the meatus 390, and can be secured in this
18 position by fastening the sheath 346 to the band 372 with
19 the sheath 346 slightly in tension and the conduit 358
slightly an compression. The funnel 362 can comprise a
21 moldable plastic material, and its end can be rolled or
22 flanged as shown in Fig. 14 far patient comfort. However,
23 in operation the funnel 362 is not required to form a fluid-
24 tight seal with the glens 392, and it is anticipated that
~25 the funnel 362 may slip distally away from the glens 392.
26 The funnel 362 cooperates with the connection system 310 to
27 direct a surge of urine into the conduit 358. Urine which
28 escapes the funnel 362 can collect in an interstitial space
29 397 between the sheath 346, the conduit 358, the glens 392
and the band 372. Urine in the interstitial space 397 can
CA 02369000 2002-O1-07
27
1 drain to the funnel 362 for evacuation through the conduit
2 358. Placement and sizing of the sheath 346, the conduit
3 358 and the band 372 can be adjusted to vary the volume of
4 the interstitial space 397.
The procedure for applying the connection system 310
6 can be varied according to the conditions of particular
7 patients and the preferences of persons applying it.
8 Properly adherred to a patient, the collection system 310
9 should be functional for a relatively long period of time,
and a semi-permeable membrane mate vial can be utilized to
11 enhance patient comfort.
12 Other useful applications of the connection system 310
13 include placement over circumferentially injured limbs and
14 phalanges for draining exudates andi~or introducing liguids.
For example, an injured hand could be treated by securing
16 the connection system 310 at the wrist, a forearm could be
17 treated by adhering the fluitlic connection system 310 at
18 the elbow, etc.
1g Yet another application is for accessory connections
whereby various fluid devices and connectors could be
21 combined in systems attached to patients for appropriate
22 treatment and diagnostic procedures. Such additional
23 accessories include Jackson-Pratt and Blake suction tubin
9
24 devices, Y-connectors, sampling ports, "Injectaport"
devices, fluid pumps and various fluid reservoirs. Hand-
26 actuated bulbs could be placed in they tubing, and valvin
9
27 could be placed where it is needed.
28 ~ further application of the fluitlic connection s stem
Y
2g 310 would involve placing the membrane 318 over an
intermediate material layer 250 as de;5cribed in connection
CA 02369000 2002-O1-07
za
1 with the wound dressing 210 compr:Lsing a second modified
2 embodiment of the present invention (Figs. 9 and 10). An
3 inner tube or conduit such as that. shown at 358 could then
4 be extended through the sheath 34E~, secured thereto by a
band or bands 372, and the inner tube or conduit distal end
6 364 could be placed beneath the intermediate material layer
7 250 adjacent to the tissue surface at the wound site 212, or
8 the conduit distal end 364 could be embedded within the
9 intermediate material layer 250. The inner tube or conduit
358 could then be used for draining exudate from or
11 introducing fluids to the wound 212.
12 It is to be understood that while certain forms of the
13 present invention have been illustrated and described
14 herein, it is not to be limited to the specific forms or
arrangement of parts described and shown.
16
17
18
19
21
22
23
24
26
27
28
29