Note: Descriptions are shown in the official language in which they were submitted.
CA 02369425 2011-07-27
COMPUTER ARCHITECTURE AND PROCESS OF PATIENT GENERATION
Related Applications
This application claims priority from U.S. provisional patent application
entitled COMPUTER
ARCHITECTURE AND PROCESS FOR PATIENT GENERATION, EVOLUTION, AND
SIMULATION FOR COMPUTERBASED TESTING SYSTEM USING BAYESIAN NETWORKS AS
A SCRIPTING LANGUAGE, Serial No. 60/127,850, to SumnerIl, et a]., filed April
5, 1999.
Field of the Invention
The present invention is generally related to a computer architecture and
process for patient
generation, evolution, and simulation, and more particularly to a computer
architecture and process for
patient generation, evolution, and simulation for a computer based testing
system using belief networks
and/or causal probabilistic networks as a scripting language.
Background of the Related Art
Medical certifying organizations have traditionally relied upon paper and
pencil cognitive
examinations as a method for the assessment of the candidate's medical
knowledge. Traditional formats
such as multiple choice questions have well-defined operating characteristics
and reliability for
examining cognitive knowledge capabilities. See, for example, Stocking ML, An
alternative method
for scoring adaptive tests, Research Report RR-94-98, 1994.
However, these tools generally measure in only cognitive knowledge. These
methods provide
only primitive ability to assess a candidate's problem-solving abilities. See,
for example, Stillman PL,
Swanson DB, Ensuring the clinical competence of medical school graduates
through standardized
patients, Arch Int Med 1978, Vol. 147, pages 1049-52.
Several organizations have previously experimented with computer-delivery of
clinical content
and evaluation. In the late 1960s and 1970s. the Ohio State University
developed a self-directed
Independent Study Program which utilized a "Tutorial Evaluation System," for
conveying curriculum
content. See, for example, Weinberg AD, CAI at the Ohio State University
College of Medicine,
Comput Biol Med 1973, Vol. 3, pages 299-305; Merola AJ, Pengov RE, Stokes BT,
Computer-supported
independent study in the basic medical sciences in: DeLand EC (ed).
Information Technology in Health
Science Education, Plenum Press, New York, 1973.
CA 02369425 2011-07-27
2
Co-synchronously Dr. Octo Barnett's laboratory at the Massachusetts General
hospital began
developmentofclinical simulations. See, for example, Barnett GO, The use of a
computer-based system
to teach clinical problem-solving, Computers in Biomedical Research, Academic
Press, New York 1974;,
Vol.4, pages 301-19; Barnett GO, Hoffer EP, Famiglieti KT, Computers in
medical education: present
and future, Proceedings ofthe Seventh Annual Symposium on Computer
Applications in Medical Care,
IEEE Press, Washington, DC 1983, pages 11-13. The clinical
simulations used the MUMPS language.
At approximately the same time, investigators at the University of Illinois
developed a
simulation model known as (Computer-Associated Simulation of the Clinical
Encounter, or "CASE").
See, for example, Harless WG, Farr NA, Zier MA, et al., MERIT - an application
of CASE, Deland EC
(ed), Information Technology in Health Science Education, Plenum Press, New
York 1978, pages
565-69. This system was at one time considered by the American
Board ofinternal Medicine (ABIM) as at least one component ofa recertification
process. Friedman RB,
A computer program for simulating the patient-physician encounter, J Med Educ
1973, Vol. 48, pages
92-7. Research supported by the ABIM demonstrated that a
computerized examination system appeared feasible in professional
evaluation/certification settings.
Reshetar, RA, et al., An Adaptive Testing Simulation for a Certifying
Examination, presented at the
Annual Meeting of the American Educational Research Association, San
Francisco, CA, April, 1992.
Stevens and colleagues have also demonstrated the feasibility of using
computer-based systems
for testing problem-solving ability in undergraduate medical school curriculum
applications. See, for
example, Stevens RH, et al, Evaluating Preclinical Medical Students by Using
Computer-Based
Problem-Solving Examinations, Academic Medicine 1989, Volume 64, pages 685-87.
Sittig and colleagues have also examined the utility of computer-base
instruction
in teaching naive users basic computer techniques such as "drag and drop" and
other computer
operations. See, for example, Sittig DF, Jiang Z, Manfre S, et al., Evaluating
a computer-based
experiential learning simulation: a case study using criterion-referenced
testing, Comput Nurs; 1995,
Vol. 13, pages 17-24.
We have determined that the above described medical assessment processes
suffer from two
weaknesses: 1) test development requires re-generation of an examination with
new material on a
recurring (usually annual) basis; 2) although multiple choice questions
demonstrate reliable performance
in measuring cognitive knowledge, the use of this format for assessing
clinical problem solving has not
been supported by the literature. Another system was developed at the
University of Wisconsin. This
project served asthe nidus forthe Computer-Based Examination (CBX) developed
by theNational Board
of Medical Examiners (NBME). See, for example, Friedman RB, A computer program
for simulating
CA 02369425 2011-07-27
3
the patient-physician encounter, J Med Educ 1973,.Vol. 48, pages 92-7; Clyman,
Stephen G., Orr, Nancy
A., Status Report on the NBME's Computer-Based Testing, Academic Medicine
1990, Vol. 65, pages
235-41. NBME's CBX development project has been in evolution for
over a decade, and has demonstrated validity in examining professional degree
candidates. See, for
example, Solomon DJ, Osuch JR, Anderson K, et al., A pilot study of the
relationship between experts'
ratings and scores generated by the NBME's computer-based examination system,
Academic Medicine
1992, Vol. 67, pages 130-32,
However, we have determined that the CBX model suffers from the problem that
the clinical
simulations are "hard-wired" in computer source code which must be re-coded
for each new examination.
Once the simulation has been used widely, the examination contents are no
longer secure, necessitating
continuous cycles of new simulation development.
The expert system literature describes the evolution in evaluation and
training systems. Early
artificial intelligence/expert system work concentrated on "rules of thumb" or
heuristics to represent
problem-solving strategies identified by domain experts. See, for example,
David JM, Krivine JP,
Simmons R., Second generation expert systems: a step forward in knowledge
engineering, in: David JM,
Krivine JP, Simmons R. Second Generation Expert Systems, Springer Verlag, New
York, NY 1993,
pages 3-23. We have determined that these rule-based systems were
necessarily constrained to narrow domains, and that the knowledge contained in
the rules was difficult
to validate- Id.
In. addition, early expert systems suffered from rapidly declining performance
when exposed to
circumstances outside narrowly defined domains. See, for example, Davis R.
Expert systems: where
are we and where do we go from here, Al Magazine, 1983, Vol. 3, pages 3-22;
Simmons R. Generate,
Test and Debug: A paradigm for combining associational and causal reasoning,
in: David M, Krivine
JP, Simmons R., Second Generation Expert Systems, Springer Verlag, New York,
NY 1993, pages
79-92, We have determined that this phenomenon occurred at least
in part due to interactions among the many rules needed to define a domain.
Recent work indicates that
the robustness of such systems is enhanced by providing knowledge of different
types. See, for example,
Simmons R, Davis R., The roles of knowledge and representation in problem
solving, In: David M,
Krivine JP, Simmons R., Second Generation Expert Systems, Springer Verlag, New
York, NY 1993,
pages 27-45,
We have further determined that experts generally not only relate to one
dimension ofknowledge
when defining a rule, but also rely upon expansive knowledge ofhow systems
work (i.e., physiology and
pathophysiology in the medical domain) in performing real-world problem-
solving. See, for example,
Davis R., Expert systems: where are we and where do we go from here, Al
Magazine, 1983, Vol. 3,
pages 3-22. This realization has led to re-thinking regarding structure
CA 02369425 2011-07-27
4
of knowledge-based systems to reflect the tasks such a system should
accomplish, the methods the
system should use to accomplish the tasks, and the knowledge required to
support these methods. See,
for example, David JM, Krivine JP, Simmons R., Second generation expert
systems: a step forward in
knowledge engineering, In: David JM, Krivine JP, Simmons R., Second Generation
Expert Systems,
Springer Verlag, New York, NY 1993, pages 3-23.
We have also determined that knowledge-acquisition for such systems entails
development of
a model for the domain and instantiation (i.e., encode and enter needed
information into the system's data
structure) ofthe model with information acquired from knowledge donors. See,
for example, David M,
Krivine JP, Simmons R., Second generation expert systems: a step forward in
knowledge engineering,
In: David M, Krivine JP, Simmons R., Second Generation Expert Systems,
Springer Verlag, New York,
NY 1993, pages 3-23; Breuker J, Weilenga B., Models of expertise in knowledge
acquisition, In: Gida
and Tasso (eds), Topics in Expert System Design: Methodologies and Tools,
North Holland Publishing,
1989,
To obviate the above described weaknesses, we have determined that it is
desirable to provide
a computer-based testing project which will: 1) instantiate medical knowledge
as object-oriented data
structures known as knowledge base of family medicine; 2) utilize the medical
knowledge structures to
create realistic clinical scenarios (simulated patients); and 3) assess the
candidate's clinical problem
solving ability as the effective intervention in the clinical progress of the
simulated patient through the
selection of various actions made available by the testing system.
Applicants have recognized a need for a method and system for evaluating or
educating a user
using belief networks or causal probabilistic networks, such as Bayesian
networks, to describe health
state evolution, medical finding reveal structures, and/or management plan
critiques.
Applicants have also recognized a need for an expert system for facilitating a
user in the
treatment of an actual patient using belief networks or causal probabilistic
networks, such as Bayesian
networks, to describe health state evolution, medical finding reveal
structures, and/or management plan
critiques.
Summary of the Invention
The computer-based testing system described herein represents knowledge at
multiple levels of
complexity. For example, reactive airways disease is represented as a series
of health states: Normal
(Non-reactive) Airways, Reactive Airways-Mild, Reactive Airways-Moderate, and
Reactive
Airways-Severe. Each health state contains identifiers which relate the
particular health state to
precedents and antecedents (e.g., Normal Airways serves as the precursor
health state for Mild Reactive
airways disease, and Mild, Moderate and Severe Reactive Airways Disease
represent target health states
from the Normal circumstance.)
CA 02369425 2011-07-27
Each health state in turn has associated findings, and specific findings. For
example, the Normal
Airways state, the Finding "Shortness of Breath" is instantiated with the
Specific Finding "No shortness
of breath." Similarly, other Findings such as Respiratory Function and Severe
Asthma Attack Frequency
are instantiated with corresponding normal Specific Findings (Normal
Respiratory Functions, and No
5 Severe Attacks.) This representation transports to each new health state in
a manner which we have
determined to be analogous to diagnosis. See, for example, Genesereth M.,
Diagnosis using hierarchical
design models, Proc. -National Conference on Al, 1982. The
computer-based testing system of the present invention partitions knowledge
into fundamental types:
Health States, Agents, Findings, Specific Findings and Patterns describe
system behaviors and
characteristics. Courses-of-Action describe human activities which modify and
evaluate the health state
information and characteristics described in the model. Subdivision of
knowledge types in this manner
facilitates the knowledge acquisition process. This subdivision also promotes
multiple levels of
knowledge abstraction, which enhances the system's ability to represent
varying levels of complexity.
For example, in the Computer-Based Testing System, a pattern such as incidence
is further
sub-divided into sub-patterns such as incidence in females versus males, and
incidence in various
racial/ethnic groups.
Multiple levels of abstraction and types of knowledge impose a substantial
knowledge
acquisition challenge. Knowledge acquisition includes several possible
methodologies, including direct
questioning of domain experts/protocol analysis, see, for example, Ericsson
KaA, Simon HA, Protocol
Analysis; Verbal Reports as Data, MIT Press. Cambridge, MA 1984,
psychometric methods, see, for example, Kelly GA, The Psychology of Personal
Constructs, Norton
Press, New York, NY 1955, and ethnographic methods, Suchman LA,
Trigg RH, Understanding Practice: Video as a Medium for Reflection and Design,
In: Greenbaum, J,
Kyng M (eds)., Design at Work: Cooperative Design of Compute Systems, Lawrence
Earlbaum
Associates 1991, pages 65-89.
Advantageously, the Computer-Based Testing System of the present invention has
included a
blend of these approaches. Direct questioning has been used in querying family
practice physicians
regarding their knowledge of and approaches to specific knowledge domains
(such as osteoarthritis).
Additionally, knowledge acquisition has included access to appropriate
scientific literature, which
functionally serves to provide an ethnographic assay of actual practice.
Knowledge acquisition has also
entailed protocol analysis, both in terms ofanalyzing specific physicians'
problem solving methodologies
and incorporating explicit clinical processes such as those presented in
published clinical guidelines (a
specific example here is the otitis media with effusion guideline developed by
the Agency for Health
Care Policy and Research).
CA 02369425 2011-07-27
6
To facilitate development of such a system, the present invention is divided
into three
components: the knowledge base, the patient simulation generator, and the
presentation system. The
knowledge base has been designed and represented as a series of entity-
relationships. The model has
several fundamental entities: Patient, Health States, Findings, Courses of
Action, and Agents. These
entities have relationships of INTERACTS-WITH, CONTACTS, IS_RELATED, EXHIBITS,
HAS,
EXPOSED_TO, LEADS_TO, ASSOC_WITH, LINKS_TO, USES, IDENTIFY, MANAGE, ALTER,
REVEAL, and EVALUATE.
FIG. I describes an overall or conceptual view of the entities and
relationships included in the
model. Rectangles indicate entities between entities in the model. Hexagons
indicate relationships.
Solid lines indicate Medical Knowledge Relationshins (e.g., a course of action
such as treatment with
non-steroidal anti-inflammatory agents can modify specific findings such as
pain in the patient with
osteoarthritis.) Dotted lines indicate Simulation/Evolution relationships
which define how a particular
domain simulation has proceeded.
The patient simulation generator of the present invention relies upon a series
of generation
methods to instantiate patients for presentation to the
certification/recertification candidate. The
processes function to evolve the patient forward (to reflect progression of
the disease process and
response to interventions) and backward in time (to create a past history for
the patient.) To accomplish
these tasks, the system utilizes processes for:
1. Content specification - these processes define the scope of the simulation
2. Patient generation:
Past History ("backward" generation)
Present and Future History ("forward" generation)
3. Simulation processes (in addition to patient generation):
Interface processes (for presentation of the patient findings developed from
generation
processes.)
Book-keeping processes (for keeping track of candidates' responses and patient
evolution)
The patient generation process proceeds on the basis of a specific health
state identifier (coded
in the database as a name and SNOMED code) passed to the process at the start
of the simulation. The
SNOMED International structured vocabulary is a versatile nomenclature for
describing medical ideas.
See, for example, Cote RA, Rothwell DJ, Palotay JL, Beckett RS, Brochu L,
editors, SNOMED
International: The systematized nomenclature of human and veterinary medicine,
3rd ed. Northfield, Ill,
College of American Pathologists, 1993, This nomenclature allows
one to make inferences from the codes used to represent each idea. For
instance, the code F-37022
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
7
represents "retrosternal chest pain." The first character, "F," indicates that
the code is from a broad class
of ideas called functions. The next to digits, "37," indicate that the code
involves a refinement of the
code F-37000, "chest pain, not otherwise specified." Similarly, code F-37020
specifies "precordial chest
pain." The code F-37022 implies that retrosternal chest pain is a kind of
precordial chest pain, which is
a kind of chest pain, which is a kind of function.
The generation process produces a complete patient description which reflects
the EXHIBITS,
HAS, INTERACTS-WITH, EXPOSED-TO, IS-RELATED, and CONTACTS relationships
described
earlier. These generated entity relations are stored as a collection of
records referred to as the "White
Board" data structures. The information in these records serves as input to
the patient evolution process,
which in turn evolves the patient's health status and medical/personal
characteristics as a function of the
passage of time or physician/examinee intervention.
The original patient generation process is generally called once at the
session's start; the system
calls the evolution processes repeatedly in response to time progression and
physician action.
The first phase of patient generation entails development of the patient's
history outline. This
outline describes the series of health states and risk factors the patient
experienced to reach the current
health state, TS. To develop TS, the system first calls the procedure
GenderRace, which establishes the
patient's sex and racial/ethnic origin. Next, the system establishes the
patient's age and age at onset
through the OnsetAge procedure. The CreatePerson process then assigns the
patient a birth date and
name.
Once the patient's age, sex, racial/ethnic origin, and age at onset of the
condition have been
established, the OutlineFirstStep procedure defines the precursor states and
risk factors which serve as
the substrata for evolving the patient to the current time and target health
state. The OutlineGeneralStep
procedure is then called iteratively until the patient has arrived at the
current TS. These processes are
described in greater detail below.
Logical and procedural knowledge in the database described as "reasoning
elements" (RE) (for
example, Bayesian network describing a generation method, Bayesian network
describing a treatment
plan, and the like), included in the generation methods described above,
"shape selectors" which describe
distributions for the n patterns by which health states evolve (patterns in
turn are specified by findings
and subpatterns), and courses of action (COA) which represent possible further
diagnostic and
management strategies which candidates might select.
The patterns and subpatterns are represented as probability distributions
(discrete and continuous
as appropriate for particular finding) specified through the knowledge
acquisition process. At the
beginning of a simulation, random number generation is used to select a
"master percentile" (MP) which
then serves as the reference for selecting particular patterns, findings and
subpatterns from the
appropriate specified distributions. These selected patterns are queried to
provide description of specific
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
8
findings such as hyperglycemia in response to physician/examinee requests for
information which are
in the form of "courses of action" for a particular health state (e.g.,
hyperglycemia as a manifestation of
diabetes.)
Once presented with the patient description (age, race, sex, clinical
findings), the candidate then
selects appropriate COA's for further evaluation and/or management of the
patient's health state.
Selection of an interventional COA invokes pattern modifiers which evolve the
patient's health state by
implementing shape modifiers. These modifiers act upon the initially selected
health state patterns to
redefine the patient's health state or findings (e.g., a COA of insulin
administration would alter the
hyperglycemic finding specified in the health state descriptions for diabetes
mellitus.)
As mentioned earlier, COA's also include options for further
testing/diagnostic procedures. For
example, the candidate might choose to select a glycosylated hemoglobin
evaluation; the COA process
would access the pattern for glycosylated hemoglobin instantiated at the
beginning of the simulation but
which might not be reported unless specifically asked for by the candidate.
A COA can modify the health state in which a patient exists at one point in
time. When the
candidate selects such a COA, the simulated patient evolves to a new health
state patterns associated with
the new health state in the knowledge base. In order to avoid "state
explosion", health states closely
associated with each other are represented as parallel health states not as
combined health state entities.
For example, the initially generated patient for a case of osteoarthritis
might demonstrate mild
osteoarthritis. However, other health states, such as obesity, might influence
the progress of the patient's
arthritis from mild to moderate or severe disease. To avoid combinatoric
health state explosion, we have
implemented a concept of parallel networks of health states. In this
representation, a newly-generated
patient will exhibit instantiated health state patterns for the primary domain
(in this case osteoarthritis)
and for the parallel health states (obesity in this example) which influence
the primary health state's
progress.
As shown in FIG. 2, osteoarthritis can progress over time from the normal
state to mild,
moderate or severe osteoarthritis. For this particular illness, progress
occurs in one direction only;
osteoarthritis does not regress once developed, but can stabilize at a
particular degree of severity.
Obesity represents a parallel health state which can influence the progression
of osteoarthritis. Mild,
3 0 moderate, and severe obesity can influence this progress at different
rates: the model permits
representation of greater impact for more severe obesity states. Notice also
that obesity can regress (e.g.,
severe obesity can revert to moderate obesity, etc.).
Any one of a number of health states might exist which could progress
independently of
osteoarthritis. For example, the patient who has osteoarthritis will
frequently utilize non-steroidal
anti-inflammatory drugs (NSAID's) for treatment. These agents can improve the
symptoms of
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
9
osteoarthritis, but also impact on the parallel state of peptic ulcer disease.
Treatment with NSAID's can
induce an ulcer, which can then evolve either on the basis of
physician/examinee intervention for it,
and/or for the course and treatment for other parallel health states, and time
with the course and treatment
of osteoarthritis.
The computer based testing system's fidelity depends upon access to a rich
representation of
health state-specific knowledge. This knowledge consists, as described above,
in more detail below. The
template includes a NAME for the health state and an associated SNOMED code.
The template also
includes specific descriptions of the FINDINGS, PATTERNS and SUBPATTERNS for
these
FINDINGS. The patterns and subpatterns are stored as a series of time and
value pairs. As an example
of such patterns, consider the example of Reactive Airways Disease (RAD). One
finding of interest is
the prevalence of RAD as a function of age, sex, and race. The prevalence for
this finding appears in the
knowledge base as collection of graphs illustrating the population prevalence
conditioned on age, sex
and race. Likewise, data such as acute exacerbation rates are represented as
event rate distributions. The
subpatterns also include information describing how various treatment
modalities will modify the
exacerbation rate and other pertinent findings such as peak expiratory flow
rates and symptoms such as
shortness of breath.
The present invention provides a prototypical process for developing domain-
specific
knowledge. The template for each domain includes, for example, the following
hierarchy:
HEALTH STATE: {name assigned by the knowledge donor, e.g., "Normal Airway
Reactivity"}
SNOMED CODE: {appropriate SNOMED code}
PREVALENCE: {age-sex-race specific prevalence; represented as pattern}
INCIDENCE: {age-sex-race specific incidence; represented as pattern}
FINDING: {general name for set of findings, e.g., "Asthma Attack Frequency" in
reactive
airways disease}
Specific
Finding: {description of specific instance of a FINDING; e.g., for the FINDING
of asthma attack
frequency, one specific finding is "No Attacks", associated with "Normal
Airway
Reactivity" I
Each HEALTH STATE affects multiple FINDINGS, which in turn have Specific
Findings
appropriate for that FINDING in that HEALTH STATE. Data such as incidence,
prevalence, and attack
rates are represented as PATTERNS (graphical functions which support the
patient generation simulation
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
processes). The information is collected in paper template form, and then
transferred into
computer-readable format using, for example, any standard Knowledge
Acquisition (KA) tool to enter
the information into an object-oriented database.
The KA "front end" may be developed, for example, in the Visual Basic and
Visual C++
S programming environments. Courses-of-Action (COA), such as further
evaluation and/or management
strategies, are entered using a standard editor that creates text files
describing appropriate
evaluation/management steps to support the simulation processes. The COA
editor may also be designed
under the Microsoft Visual environments mentioned earlier.
The knowledge acquisition step includes the following subcomponents:
10 A. Health state specification
B. Enumeration of FINDINGS for the health state, and agreement among the
development team
members
C. Population of templates with knowledge
D. Entry of health state knowledge into knowledge base using KA tool and/or
direct high level
pseudo-coding
E. Debugging, including generating multiple simulations, to test system
stability/credibility
F. Validation including review of generated cases by representative groups of
family physicians
2 0 It is a feature and advantage of the present invention to: (1) allow
testing at remote sites and
convenient times; (2) uniformly test an expanded range of important family
practice activities, with
fewer questions on exotic problems; (3) adapt tests to examinees' responses or
needs; and (4) create
reasonable questions at test sites to simplify administrative, economic, and
especially security issues.
It is another feature and advantage of the present invention to provide an
approach that does not
incur high maintenance costs and produces efficient and affordable scenarios
for a computer-based
testing system.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
11
It is another feature and advantage of the present invention to provide a
formal model of family
medicine to achieve a relevant and realistic implementation of a computer
based examination.
It is another feature and advantage of the present invention to provide an
examination that does
not require replacement with new questions in order to preserve security of
the certification process.
It is another feature and advantage of the present invention to provide a
computer based testing
system that may measure problem-solving capabilities.
It is another feature and advantage of the present invention to provide a
computer based testing
system that relies upon a knowledge base of family practice which contains
"patterns" and "subpatterns"
which depict in probabilistic terms disease/condition incidence, prevalence,
evolution over time, and
response to interventions.
The present invention is based, in part, on our discovery that prior computer
based testing
systems suffer from various problems, including the problem that the clinical
simulations are
"hard-wired" in computer source code or static data structures which must be
re-coded or reinstantiated
for each new examination. Accordingly, in prior art computer based testing
systems, once the simulation
has been used widely, the examination contents are no longer secure,
necessitating continuous cycles of
new simulation development.
The present invention is also based, in part, on our realization that the
computer based testing
system needs to be capable of efficiently generating new patient cases for
each candidate examined, and
capable of effectively testing a candidate's problem-solving ability. We have
discovered that the above
may be accomplished using a knowledge base of family practice which contains
"patterns" and
"subpatterns" which depict in probabilistic terms disease/condition incidence,
prevalence, evolution over-
time, and response to interventions.
To achieve the above features and advantages, as well as other features and
advantages that will
be apparent from the detailed description provided below, a computer
implemented simulation and
evaluation method simulates interventions to a patient by a user, and
evaluates the interventions
responsive to predetermined criteria and the interventions. The method
includes defining a test area to
evaluate the user on at least one predetermined criterion, selecting genetic
information of the patient
CA 02369425 2001-10-03
WO 00/60431 PCT/US00/08942
12
responsive to the test area, and generating a patient history responsive to
the test area and the genetic
information. The method also includes receiving at least one intervention
input by the user, and
evaluating the user responsive to the intervention and predetermined criteria.
In accordance with another embodiment of the invention, a computer system and
computer
readable tangible medium is provided that stores the process thereon, for
execution by the computer.
In accordance with another embodiment of the invention, a computer readable
tangible medium
is provided that stores an object including the entity relationship model
thereon, for execution by the
computer.
It is another feature and advantage of another embodiment of the instant
invention to include a
method for evaluating or educating a user. The method includes the following
sequential, non-sequential,
or sequence-independent steps. Plurality of parallel health state networks are
generated, for example, by
a user or a computer. One or more first Bayesian networks, which describe each
of the parallel health
state networks generated by a user or a computer. One or more second Bayesian
networks, which
describe rates of progression within and/or between the parallel health state
networks, and describe task
factors that affect the rates of progression, generated by a user or a
computer. One or more third Bayesian
networks which support reveal structures to limit display of patient test data
to patient test data
specifically requested by the user, are generated by a user or a computer. One
or more fourth Bayesian
networks which support plan critiques of queries of and treatment prescribed
by the user, are generated
by a user or a computer.
A knowledge base is scripted by the computer from the one or more first
Bayesian networks and
the one or more second Bayesian networks. A model patient, at least in part,
is instantiated by the
computer from the scripted knowledge base. A course of action or a query for a
specific medical finding
concerning the model patient is received by the computer from the user
responsive to the instantiated
model patient. If the query is received, the specific medical finding is
displayed by the computer to the
user based at least in part on the one or more third Bayesian networks, and
repeating the receiving step.
The model patient is evolved by the computer in accordance with the parallel
health state
networks and responsive to the received course of action. The receiving,
displaying, and evolving steps
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
13
are repeated by the computer until the user has completed treatment of the
model patient. An optimum
combination of treatment and queries based, at least in part, on the one or
more fourth Bayesian networks
and the instantiated model patient is generated by the computer. The query and
the treatment by the user
is evaluated by the computer in comparison to the generated optimum
combination of treatment and
queries.
Optionally, the parallel health state networks describe primary networks
defining disease
evolutions, secondary networks defining risk factors affecting progression
through a particular or given
primary network of the plurality of primary networks, and/or tertiary networks
defining causal
probabilistic medical complications attributed to one or more stages in the
primary network and/or
medical complications attributed to management of the one or more stages.
It is another feature and advantage of the instant invention to provide a
computer readable
medium including instructions being executed by a computer. The instructions
instruct the computer to
execute an educational or testing system for physicians. The instructions
include the following
sequential, non-sequential, or sequence-independent steps. One or more first
belief networks which
describes parallel health state networks are accessed, for example, by a
computer. A knowledge base,
at least in part, is scripted from the one or more first belief networks by
the computer. A model patient,
at least in part, is instantiated by the computer from the scripted knowledge
base. Optionally, for the
computer readable medium, the parallel health state networks describe primary
networks defining disease
evolutions, secondary networks defining risk factors affecting progression
through a primary network
of the plurality of primary networks, and/or tertiary networks defining causal
probabilistic medical
complications attributed to at least one stage in the primary network and/or
medical complications
attributed to management of the one or more stages.
Optionally, the instructions further include one or more second belief
networks, which describe
rates of progression within and/or between the parallel health state networks,
and describe task factors
that affect the rates of progression, are accessed by the computer or the
user. Optionally, one or more
third belief network, which supports reveal structures to limit display of
patient test data to patient test
data specifically requested by the user, are accessed, for example, by the
computer. Optionally, one or
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
14
more fourth belief networks which support plan critiques of queries of and
treatment prescribed by the
user, are accessed by the user or the computer. Optionally, the scripting step
includes scripting the
knowledge base by the computer, at least in part, from the one or more second
belief networks.
Optionally, a course of action or a query for a specific medical finding
concerning the model patient is
received by the computer from the user responsive to the instantiated model
patient. If the query is
received, the specific medical finding is displayed by the processor to the
user based at least in part on
the at least one third network, and the receiving step is repeated by the
processor.
Optionally, the model patient is evolved by the computer in accordance with
the parallel health
state networks and responsive to the received course of action. Optionally,
the receiving, displaying, and
evolving steps are repeated by the computer until the user has completed
treatment of the model patient.
Optionally, an optimum combination of treatment and queries is generated by a
processor based on the
one or more fourth belief networks and the instantiated model patient.
Optionally, the query and the
treatment by the user are evaluated by the computer in comparison to the
generated optimum
combination of treatment and queries.
It is another feature and advantage of the instant invention to include a
system for evaluating or
educating a user. The system includes means for scripting a knowledge base
from at least one first belief
network and at least one second belief network. The system includes means for
instantiating a model
patient, at least in part, from the scripted knowledge base. The system
includes means for receiving a
course of action or a query for a specific medical finding concerning the
model patient from the user
responsive to the instantiated model patient. The system includes means for
displaying, if the query is
received, the specific medical finding to the user based at least in part on
at least one third belief network,
and activating the receiving means.
The system includes means for evolving the model patient in accordance with
the at least one
first belief network and the at least one second belief network and responsive
to the received course of
action. Optionally, the system includes means for communicating with the
receiving means, the
displaying means, and the evolving means until the user has completed
treatment of the model patient.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
Optionally, the system includes means for generating an optimum combination of
treatment and
queries based on at least one fourth belief network and the instantiated model
patient. Optionally, the
system includes means for evaluating the query and the treatment by the user
in comparison to the
generated optimum combination of treatment and queries.
5 Optionally, the system includes means for generating parallel health state
networks describing
primary networks defining disease evolutions, secondary networks defining risk
factors affecting
progression through a primary network of the plurality of primary networks,
and/or tertiary networks
defining causal probabilistic medical complications attributed to at least one
stage in the primary network
and/or medical complications attributed to management of the at least one
stage.
10 Optionally, the system includes means for generating the at least one first
belief network which
describes each of the plurality of parallel health state networks. Optionally,
the system includes means
for generating the at least one second belief network which describes rates of
progression within and/or
between the parallel health state networks, and describes task factors that
affect the rates of progression.
Optionally, the system includes means for generating the at least one third
belief network which support
15 reveal structures to limit display of patient test data to patient test
data specifically requested by the user.
Optionally, the system includes means for generating the at least one fourth
belief network which
supports plan critiques of queries of and treatment prescribed by the user.
It is another feature and advantage of the instant invention to include an
expert system. The
expert system includes a processor. The expert system also includes a computer-
readable medium storing
instructions executable by the processor.
The instructions include the following sequential, non-sequential, or sequence-
independent steps.
Parallel health state networks are accessed by the processor and which
describe primary networks
defining disease evolutions, secondary networks defining risk factors
affecting progression through a
primary network of the plurality of primary networks, and/or tertiary networks
defining causal
probabilistic medical complications attributed to at least one stage in the
primary network and/or medical
complications attributed to management of the at least one stage.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
16
One or more first belief networks, which describe each of the plurality of
parallel health state
networks, are accessed by the processor. One or more second belief networks,
which describe rates of
progression within and/or between the parallel health state networks, and
describe task factors that affect
the rates of progression, are accessed by the processor. One or more third
belief networks, which support
reveal structures to limit display of patient test data to patient test data
specifically requested by the user,
are accessed by the processor. One or more fourth belief networks, which
support plan critiques of
queries of and treatment prescribed by the user, are accessed by the
processor. Patient data for an actual
patient is received by the processor by user input.
A virtual patient having characteristics consistent with the received patient
data and based, at
least in part, on the at least one first belief network and the at least one
second belief network is
instantiated by the processor. A query for a specific medical finding
concerning the actual patient, or a
course of action responsive to at least one normal or abnormal health state of
the plurality of health states
of the virtual patient is generated by the processor. The normal or abnormal
health state corresponds to
at least part of the received patient data. The specific medical finding from
the user, if a query therefor
is generated.
The virtual patient is evolved by the processor in accordance with the at
least one first belief
network and/or the at least one second belief network, and responsive to the
received specific medical
finding and/or the generated course of action. Optionally, the instructions
for the expert system further
includes repeating the generating, receiving, and evolving instruction steps
until the user has dispensed
treatment of the actual patient based on the generating course of action.
Optionally, the instructions
further include storing the evolved virtual patient for subsequent access by
the user, and repeating the
generating, receiving, evolving, repeating, and storing instruction steps upon
each subsequent access by
the user at least until the treatment of the actual patient is completed.
It is another feature and advantage of the instant invention to include a
system for educating or
evaluating a user. The system includes a model patient generator including a
knowledge base scripted
from one or more first causal probability networks, one or more second causal
probability networks. The
one or more first causal probability networks describe each parallel health
state network of a plurality
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
17
of parallel health state network. The one or more second causal probability
networks describe at least one
rate of progression within and/or between the parallel health state networks,
and which describe at least
one task factor that affects the one rate of progression. The patient
generator instantiates, upon user input,
a model patient in a whiteboard, at least in part, from the scripted knowledge
base. The patient generator
receives a course of action or a query for a specific medical finding
concerning the model patient from
the user responsive to the instantiated model patient. The whiteboard
optionally displays, if the query
is received, the specific medical finding to the user based, at least in part,
on at least one third belief
network, which supports patient health state reveal structures. The whiteboard
evolves the model patient
in accordance with the plurality of parallel health state networks and
responsive to the received course
of action.
It is another feature and advantage of the instant invention to include a
system communicatable
with a computer network. The system includes a server communicatable with a
user via the computer
network. The server is in communication with a processor and a computer-
readable medium storing
instructions executable by the processor. The instructions include the
following sequential,
non-sequential, or sequence-independent instruction steps. Parallel health
state networks are accessed
by a user or the processor and which describes primary networks defining
disease evolutions, secondary
networks defining risk factors affecting progression through a primary network
of the plurality of
primary networks, and/or tertiary networks defining causal probabilistic
medical complications attributed
to one or more stage in the primary network and/or medical complications
attributed to management of
the one or more stage.
One or more first belief networks, which describe each of the plurality of
parallel health state
networks, are accessed by the user or the processor. One or more second belief
networks which describe
rates of progression within and/or between said plurality of parallel health
state networks, and to describe
task factors that affect the rates of progression, are accessed by the user or
the processor. One or more
third belief networks, which supports plan critiques of queries of and
treatment prescribed by the user,
are accessed by the user or the processor.
CA 02369425 2001-10-03
WO 00/60431 PCT/US00/08942
18
Patient data for an actual patient are received by user input to the
processor. A virtual patient,
having characteristics consistent with the received patient data and based, at
least in part, on the one or
more first belief networks and the one or more second belief networks, is
instantiated by the processor.
A query to the user for a specific medical finding concerning the actual
patient, or a course of action
based, at least in part on the virtual patient and the one or more third
belief networks are generated by
the processor. The specific medical finding is received by the processor from
the user responsive to the
generated query. The virtual patient is evolved by the processor in accordance
with the one or more first
belief network and/or the one or more second belief network, and responsive to
the received specific
medical finding.
Optionally, the instructions of expert system further include repeating the
generating, receiving,
and evolving instructions steps until the user has dispensed treatment of the
actual patient based on the
generating course of action. Optionally, the evolved virtual patient is stored
by the processor for
subsequent access by the user. Optionally, the generating, receiving,
evolving, repeating, and, storing
instructions are repeated by the computer upon each said subsequent access by
the user at least until the
treatment of the actual patient is completed.
It is another feature and advantage of the instant invention to include a
system communicatable
with a computer network. The system includes a server communicatable with a
user via the computer
network. The server is in communication with a processor and a computer-
readable medium storing
instructions executable by the processor.
The instructions include the following sequential, non-sequential, or sequence-
independent
instruction steps. Optionally, parallel health state networks are accessed by
the processor or a user and
which describe primary networks defining disease evolutions, secondary
networks defining risk factors
affecting progression through a primary network of the primary networks,
and/or tertiary networks
defining causal probabilistic medical complications attributed to one or more
stages in the primary
network and/or medical complications attributed to management of the one or
more stages.
One or more first belief networks which describe each of the plurality of
parallel health state
networks are accessed. One or more second belief networks, which describe
rates of progression within
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
19
and/or between said plurality of parallel health state networks, and describe
task factors that affect the
rates of progression, are accessed. One or more third belief networks, which
support reveal structures
to limit display of patient test data to patient test data specifically
requested by the user, are accessed.
One or more fourth belief networks, which supports plan critiques of queries
of and treatment prescribed
by the user, are accessed. A knowledge base is scripted by the processor or a
user from the one or more
first belief networks and the one or more second belief networks.
A model patient is instantiated by the processor, based, at least in part,
from the scripted
knowledge base. A course of action or a query for a specific medical finding
concerning the model
patient is received by the processor from the user responsive to the
instantiated model patient. If the
query is received by the processor, the specific medical finding is displayed
by the processor to the user
based at least in part on the one or more third belief networks, and repeating
the receiving instruction.
The model patient is evolved by the processor in accordance with at least one
of the one or more first
belief networks and the one or more second belief networks and responsive to
the received course of
action.
Optionally, the instructions further include repeating by the processor the
receiving, displaying,
and evolving instructions until the user has completed treatment of the model
patient. Optionally, an
optimum combination of treatment and queries is generated by the processor
based on the one or more
fourth belief networks and the instantiated model patient. Optionally, the
query and the treatment by the
user is evaluated by the processor in comparison to the generated optimum
combination of treatment and
queries.
It is a feature and advantage of another embodiment of the instant invention
to include a
knowledge base module for an educational or testing system or an expert
system. The knowledge base
module includes one or more first causal probability networks, which describe
each parallel health state
network of a plurality of parallel health state networks. The module includes
one or more second causal
probability networks, which describe one or more rates of progression within
and/or between the parallel
health state networks, and which describe one or more task factors that affect
the one or more rates of
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
progression. The module also includes one or more third causal probability
networks, which describe
plan critiques including peer-accepted courses of action for addressing the
parallel health state networks.
It is a feature and advantage of another embodiment of the instant invention
to include a
computer network appliance. The network appliance includes a thin client
programmably connected via
5 a computer network to a single web hosting facility. The single web hosting
facility includes a server
communicatable with a user via the computer network. The server is in
communication with a processor
and a computer-readable medium storing instructions executable by the
processor.
The instructions include the following sequential, non-sequential, or sequence-
independent
instruction steps. Parallel health state networks describing primary networks
defining disease evolutions,
10 secondary networks defining risk factors affecting progression through a
primary network of the plurality
of primary networks, and/or tertiary networks defining causal probabilistic
medical complications
attributed to at least one stage in the primary network and/or medical
complications attributed to
management of the at least one stage are accessed by the user or the
processor.
One or more first belief networks, which describe each of the parallel health
state network, are
15 accessed by the user or the processor. One or more second belief networks,
which describe rates of
progression within and/or between said plurality of parallel health state
networks, and describe task
factors that affect the rates of progression, are accessed by the user or the
processor. One or more third
belief networks, which support reveal structures to limit display of patient
test data to patient test data
specifically requested by the user, are accessed by the user or the processor.
One or more fourth belief
20 networks, which supports plan critiques of queries of and treatment
prescribed by the user, are accessed
by the user or the processor.
A knowledge base from the one or more first belief networks and/or the one or
more second
belief networks is scripted by the processor. A model patient, at least in
part, is instantiated from the
scripted knowledge base by the processor. A course of action or a query for a
specific medical finding
concerning the model patient is received by the processor from the user
responsive to the instantiated
model patient. If the query is received, the specific medical finding is
displayed by the processor to the
user based in part on the one or more third belief networks, and repeating the
receiving instruction step.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
21
The model patient is evolved by the processor in accordance with the parallel
health state networks and
responsive to the received course of action.
Optionally, the instructions for the computer network appliance repeating the
receiving,
displaying, and evolving instruction steps until the user has completed
treatment of the model patient.
Optionally, the instructions include generating an optimum combination of
treatment and queries based
on the one or more fourth belief networks and the instantiated model patient,
and evaluating the query
and the treatment by the user in comparison to the generated optimum
combination of treatment and
queries.
It is a feature and advantage of another embodiment of the instant invention
to include a
computer network appliance. The computer network appliance includes a thin
client programmably
connected via a computer network to a single web hosting facility. The single
web hosting facility
includes a server communicatable with a user via the computer network. The
server is in communication
with a processor and a computer-readable medium storing instructions
executable by the precessor.
The instructions include the following sequential, non-sequential, or sequence-
independent
instruction steps. Parallel health state networks describing primary networks
defining disease evolutions,
secondary networks defining risk factors affecting progression through a
primary network of the plurality
of primary networks, and/or tertiary networks defining causal probabilistic
medical complications
attributed to at least one stage in the primary network and/or medical
complications attributed to
management of the at least one stage, are accessed by the user or the process.
One or more first belief
networks, which describes each of the parallel health state networks, are
accessed by the user or the
processor. One or more second belief networks, which describe rates of
progression within and/or
between said plurality of parallel health state networks, and describe task
factors that affect the rates of
progression, are accessed by the user or the processor. One or more third
belief networks which support
plan critiques of queries of and treatment prescribed by the user, are
accessed by the user or the
processor.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
22
Patient data for an actual patient are received by user input. A virtual
patient having
characteristics consistent with the received patient data and based, at least
in part, on the one or more first
belief networks and/or the one or more second belief networks are instantiated
by the processor.
A query to the user for a specific medical finding concerning the actual
patient, or a course of
action based, at least in part on the virtual patient and the one or more
third belief networks are generated
by the processor. The specific medical finding is received by the processor
from the user responsive to
the generated query. The virtual patient is evolved by the processor in
accordance with the one or more
first belief networks and/or the one or more second belief networks, and
responsive to the received
specific medical finding.
The instructions for the optionally expert system further include repeating
the generating,
receiving, and evolving instruction steps until the user has dispensed
treatment of the actual patient based
on the generating course of action, and storing the evolved virtual patient by
the processor for subsequent
access by the user. Optionally, the generating, receiving, evolving,
repeating, and storing instruction
steps are repeated by the processor upon each said subsequent access by the
user at least until the
treatment of the actual patient is completed.
It is a feature and advantage of another embodiment according to the instant
invention to include
a system communicatable with a computer network. The system includes a server
communicatable with
a user via the computer network. The server is in communication with a
processor and a
computer-readable medium storing instructions executable by the processor.
The instructions include the following sequential, non-sequential, or sequence-
independent steps.
Parallel health state networks, describing primary networks defining disease
evolutions, secondary
networks defining risk factors affecting progression through a primary network
of the plurality of
primary networks, and/or tertiary networks defining causal probabilistic
medical complications attributed
to at least one stage in the primary network and/or medical complications
attributed to management of
the at least one stage, are accessed by the processor or a user.
One or more first belief networks, which describe each of the plurality of
health states in parallel
networks, are accessed by the processor or a user. One or more second belief
networks, which describe
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
23
transitions between health states within parallel networks, and describe task
factors that affect the rates
of progression, are accessed by the processor or a user. One or more third
belief networks, which support
reveal structures to limit display of patient test data to patient test data
specifically requested by the user,
are accessed by the processor or a user. One or more fourth belief networks,
which support plan critiques
of queries of and treatment prescribed by the user, are accessed by the
processor or a user.
A knowledge engineer, such as a developer or a standard data mining process,
scripts a
knowledge base by specifying one or more first belief networks and/or the one
or more second belief
networks. A model patient is instantiated by the processor based, at least in
part, from the scripted
knowledge base. A course of action or a query for a specific medical finding
concerning the model
patient is received by the processor from the user responsive to the
instantiated model patient. If the
query is received, the specific medical finding is displayed by the processor
to the user based at least in
part on the one or more third belief networks, and repeating the receiving
instruction step. The model
patient is evolved by the processor in accordance with the one or more first
belief networks and/or the
one or more second belief networks and responsive to the received course of
action.
Optionally, the instructions further include repeating the generating,
receiving, and evolving
instruction steps until the user has dispensed treatment of the actual
patient, at least in part, based on the
generated course of action, and storing the evolved virtual patient by the
processor for subsequent access
by the user. Optionally, the generating, receiving, evolving, repeating, and
storing instruction steps are
repeated by the processor upon each subsequent access by the user at least
until the treatment of the
actual patient is completed.
It is a feature and advantage of another embodiment of the instant invention
to include a method
for educating or evaluating a user. The method includes the following
sequential, non-sequential, or
sequence-independent steps. A virtual patient is instantiated for display to
the user, for example, by a
computer. The virtual patient includes a number of health states. A query is
received from the user for
a medical finding concerning the instantiated virtual patient. Optionally,
responsive to the received
query, a specific medical finding is generated at least in part from a first
causal probabilistic network
defining a health state reveal structure corresponding to the instantiated
virtual patient. Optionally,
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
24
responsive to the received query, an indication of an inappropriate query is
generated based, at least in
part, on a second causal probabilistic network defining a medical practice
management plan. By way of
illustration, the medical practice management plan includes healthcare
provider or medical
insurance-approved medical finding queries.
It is a feature and advantage of another embodiment of the instant invention
to include a method
for educating or evaluating a user. A virtual patient is instantiated by a
computer for display to the user.
The virtual patient includes a plurality of health stages. A query for a
medical finding concerning the
instantiated virtual patient is received by the computer. Responsive to the
received query, a specific
medical finding is generated by the computer, at least in part, from a first
causal probability network
defining a health state reveal structure corresponding to the instantiated
virtual patient. Responsive to
the received query, an indication of an inappropriate query, based, at least
in part, on a second causal
probability network defining a medical practice management plan. Responsive to
the received course of
action, an indication of an inappropriate course of action by the computer
based, at least in part, on the
second causal probability network. Optionally, the medical practice management
plan includes healthcare
insurer approved medical finding queries. Advantageously, such a method is
used to familiarize doctors
new to a health care plan with management approved medical tests and/or
medical procedures.
There has thus been outlined, rather broadly, the more important features of
the invention in order that
the detailed description thereof that follows may be better understood, and in
order that the present
contribution to the art may be better appreciated. There are, of course,
additional features of the invention
that will be described hereinafter and which will form the subject matter of
the claims appended hereto.
In this respect, before explaining at least one embodiment of the invention in
detail, it is to be
understood that the invention is not limited in its application to the details
of construction and to the
arrangements of the components set forth in the following description or
illustrated in the drawings. The
invention is capable of other embodiments and of being practiced and carried
out in various ways. Also,
it is to be understood that the phraseology and terminology employed herein
are for the purpose of
description and should not be regarded as limiting.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
As such, those skilled in the art will appreciate that the conception, upon
which this disclosure
is based, may readily be utilized as a basis for the designing of other
structures, methods and systems for
carrying out the several purposes of the present invention. It is important,
therefore, that the claims be
regarded as including such equivalent constructions insofar as they do not
depart from the spirit and
5 scope of the present invention.
Further, the purpose of the foregoing abstract is to enable the U.S. Patent
and Trademark Office
and the public generally, and especially the scientists, engineers and
practitioners in the art who are not
familiar with patent or legal terms or phraseology, to determine quickly from
a cursory inspection the
nature and essence of the technical disclosure ofthe application. The abstract
is neither intended to define
10 the invention of the application, which is measured by the claims, nor is
it intended to be limiting as to
the scope of the invention in any way.
The above objects of the invention, together with other apparent objects of
the invention, along
with the various features of novelty which characterize the invention, are
pointed out with particularity
in the claims annexed to and forming a part of this disclosure. For a better
understanding of the
15 invention, its operating advantages and the specific objects attained by
its uses, reference should be had
to the accompanying drawings and descriptive matter forming a part hereof,
wherein like numerals refer
to like elements throughout, and in which there is illustrated preferred
embodiments of the invention.
Brief Description of the Drawings
FIG. I is a diagram describing an overall or conceptual view of the entities
and relationships in
20 the model used in the computer based examination system of the present
invention;
FIG. 2 is a diagram describing the progression of osteoarthritis over time
from the normal state
to mild, moderate or severe states of osteoarthritis;
FIG. 3 is a detailed diagram of the family medicine model, including the major
entities, relations
and modifying relations;
25 FIG. 4 is a flowchart of the overall process for the computer based
examination system of the
present invention;
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
26
FIG. 5 is a flowchart of the history outline process which generates the
patient history in the
computer based examination system of the present invention;
FIG. 6 is a flowchart of the history generation process which finds values for
the patient history
in the computer based examination system of the present invention;
FIG. 7 is a flowchart providing an overview of the stochastic process in
accordance with another
embodiment of the computer based examination system of the present invention;
FIG. 8 is a flowchart illustrating a first step in tracing previous health
conditions to generate past
medical history of the patient for the stochastic process of the computer
based examination system of the
present invention;
FIG. 9 is a flowchart illustrating a second step in tracing previous health
conditions to generate
past medical history of the patient for the stochastic process of the computer
based examination system
of the present invention;
FIG. 10 is an illustration of the entity-relationship model data structure
stored in the white board
database when patients are not pre-generated;
FIG. II is an illustration of a modified entity-relationship model data
structure stored in the
white board database when patients are not pre-generated;
FIG. 12 is an illustration of parallel network structures for the computer
based examination
system of the present invention; FIGs. 13-14 are detailed flowcharts of the
process of the
computer based examination or assessment system of the present invention;
FIG. 15 is an illustration of a main central processing unit for implementing
the computer
processing in accordance with a computer implemented embodiment of the present
invention;
FIG. 16 illustrates a block diagram of the internal hardware of the computer
of FIG. 15;
FIG. 17 is a block diagram of the internal hardware of the computer of FIG. 16
in accordance
with a second embodiment;
FIG. 18 is an illustration of an exemplary memory medium which can be used
with disk drives
illustrated in FIGs. 15-17.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
27
FIG. 19 is an illustration of a relational diagram of the Bayes networks and
other supporting
structures;
FIG. 20 is an illustration of an example using a Bayes network to describe
osteoarthritis;
FIG. 21 is an illustration of an example using a Bayes network to generate a
report when a user
submits a medical finding query;
FIG. 22 is an illustration of examples of disease evolution described by
parallel health state
networks;
FIG. 23 is an illustration of an example of interactions between parallel
health state networks;
FIG. 24 is an illustration of an example showing the relationships between
entities in a health
state;
FIG. 25 is an illustrative flow chart outlining operation of an embodiment of
the instant
invention;
FIG. 26 is an illustrative flow chart showing operation of another embodiment
of the instant
invention;
FIG. 27 is an illustrative flow chart showing operation of another embodiment
of the instant
invention; and
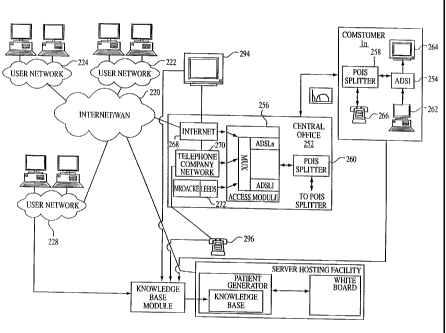
FIG. 28 is an illustration of a computer network architecture.
Notations and Nomenclature
The detailed descriptions which follow may be presented in terms of program
procedures
executed on a computer or network of computers. These procedural descriptions
and representations are
the means used by those skilled in the art to most effectively convey the
substance of their work to others
skilled in the art.
A procedure is here, and generally, conceived to be a self-consistent sequence
of steps leading
to a desired result. These steps are those requiring physical manipulations of
physical quantities.
Usually, though not necessarily, these quantities take the form of electrical
or magnetic signals capable
of being stored, transferred, combined, compared and otherwise manipulated. It
proves convenient at
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
28
times, principally for reasons of common usage, to refer to these signals as
bits, values, elements,
symbols, characters, terms, numbers, or the like. It should be noted, however,
that all of these and
similar terms are to be associated with the appropriate physical quantities
and are merely convenient
labels applied to these quantities.
Further, the manipulations performed are often referred to in terms, such as
adding or comparing,
which are commonly associated with mental operations performed by a human
operator. No such
capability of a human operator is necessary, or desirable in most cases, in
any of the operations described
herein which form part of the present invention; the operations are machine
operations. Useful machines
for performing the operation ofthe present invention include general purpose
digital computers or similar
devices.
The present invention also relates to apparatus for performing these
operations. This apparatus
may be specially constructed for the required purpose or it may comprise a
general purpose computer
as selectively activated or reconfigured by a computer program stored in the
computer. The procedures
presented herein are not inherently related to a particular computer or other
apparatus. Various general
purpose machines may be used with programs written in accordance with the
teachings herein, or it may
prove more convenient to construct more specialized apparatus to perform the
required method steps.
The required structure for a variety of these machines will appear from the
description given.
Best Mode for Carrying Out the Invention
The computer-based testing system described herein represents knowledge at
multiple levels of
complexity.
The computer-based testing system of the present invention partitions
knowledge into
fundamental types: Health States, Agents, Findings, Specific Findings,
Patterns and Sub-patterns
describe system behaviors and characteristics. Courses-of-Action describe
tasks and methods used to
apply, modify, and evaluate the health state information and characteristics
described in the model.
Subdivision of knowledge types in this manner facilitates the knowledge
acquisition process. This
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
29
subdivision also promotes multiple levels of knowledge abstraction, which
enhances the system's ability
to represent varying levels of complexity.
For example, reactive airways disease is represented as a series of health
states: Normal
(Non-reactive) Airways, Reactive Airways-Mild, Reactive Airways-Moderate, and
Reactive
Airways-Severe. Each health state contains identifiers which relate the
particular health state to
precedents and antecedents (e.g., Normal Airways serves as the precursor
health state for Mild Reactive
airways disease, and Mild, Moderate and Severe Reactive Airways Disease
represent target or sequential
successor health states from the Normal circumstance.)
Each health state in turn has associated findings, and specific findings. For
example, in the
Normal Airways state, "Asthma Attack Frequency" appears as a Finding which is
instantiated with the
Specific Finding "No attacks." Similarly, other Findings such as Respiratory
Function and Severe
Asthma Attach Frequency are instantiated with corresponding normal Specific
Findings (Normal
Respiratory Functions, and No Severe Attacks.) This representation transports
to each new health state
in, what we have determined to be somewhat analogous to diagnosis.
Advantageously, the Computer-Based Testing System ofthe present invention in
the knowledge
acquisition process uses direct questioning in querying family practice
physicians regarding their
knowledge of and approaches to specific knowledge domains (such as
osteoarthritis). Additionally,
knowledge acquisition has included access to appropriate scientific
literature, which functionally serves
to provide an ethnographic assay of actual practice.
Overview of Testing/Recertification Process
The testing and/or recertification process, for example, unfolds as follows.
After initial
certification, examinees initiate recertification software on workstations on
computer systems. The
examinee begins recertifying at any convenient time and could suspend the
examination at the conclusion
of any simulated patient encounter. The software of the present invention
presents a patient by using
text, illustrations, still pictures, and video. The examinee questions and
examines the simulated patient,
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
reaches conclusions about the situation, and suggests treatment options. The
simulated patient may
express preferences about these options.
After receiving a treatment plan, the patient leaves, maybe follows the plan,
and perhaps later
returns for follow-up. In the meantime, the examinee sees other simulated
patients. To discourage
5 cheating, the software offers so many cases that a diplomate observing
another examinee recertify gains
little advantage with regard to test content.
The present invention maintains records ofthe information gathered, the
hypotheses entertained,
and the recommendations made for each patient. After monitoring performance on
several similar cases
(for instance, cases involving diagnosis and management of adult-onset
diabetes mellitus), the program
10 draws conclusions about the physician's ability to handle this class of
problems. If competence has been
demonstrated, the class of problems may be removed from further consideration
for several years. Until
competence has been demonstrated, the physician receives feed-back on specific
areas for improvement
and continues to see cases from this class of problems.
The testing and/or recertification process could eventually become a
continuous learning
15 experience at the office or home. Some recertification activities might
qualify as continuing medical
education, partially offsetting the time needed to recertify. Examinees could
anticipate failure to
recertify and take corrective measures years before actually failing.
The present invention provides an approach that does not incur high
maintenance costs to
maintain efficient and affordable examinations. The present invention also
provides a formal model of
20 family medicine to achieve a relevant and realistic implementation of this
kind of computer-based
examination.
In general, a model describes the kinds of information that could be collected
regarding a topic.
For instance, a model of a mailing address should include at least a name,
street address, apartment
number, city, state, and ZIP code. A database built upon this model could list
these items for each entry.
25 Not every item in the model should be described for every entry in the
database; many addresses have
no apartment number. Incomplete database entries still provide useful
information; even if a street
address is missing, the city to search can be found.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
31
Finally, the model limits what the database could do; it could not easily list
first names. A model
of diagnostic medicine of the present invention includes diseases, historical
and examination data, and
links between diseases and data. These models represent knowledge that
physicians apply to uncertain
or imprecise cases. The address example suggests a list of simple
observations, called a database. A
diagnostic program uses a collection of more abstract information, such as a
statistical summary of a
database, to draw inferences about a single case. The program and its
information are often called a
knowledge base.
We have determined that a well-designed formal model supports automatically
created case
simulations, reducing the long-term cost of writing cases by hand and
improving security. The formal
model of the present invention considers that medicine is full of diagnostic
complexities including
disease interaction. Thus, diabetes could change the severity of pain
experienced during an acute
myocardial infarction. With this information, the knowledge base of the
present invention is able to
support a realistic simulation process - a simulated diabetic having an acute
myocardial infarction will
experience a specific discomfort. The present invention attempts to carefully
define interactions for a
number of health states that constitute the bulk of family medicine.
We have further determined that diagnosis and patient management are
inextricably linked to
time. Time receives relatively little attention in many knowledge bases and is
often summarized very
succinctly. For instance, a knowledge base might describe "chest pain lasting
more than 30 minutes" as
a symptom of acute myocardial infarction. This knowledge base could
misinterpret 29 minutes of chest
pain as evidence against acute myocardial infarction, and 2 years of chest
pain as an indicator of acute
myocardial infarction. The present invention also supports the related
concepts of continuity of care and
observation.
In addition to these problems, family physicians deal with a host of issues
that we have
determined are not routinely modeled in diagnostic software. Most of these
issues reflect the
overwhelming importance of patient management in family medicine.
First, family medicine occurs in a social context that is often ignored in
computer-generated
simulations. Knowledge bases do not model social interactions or family
structure.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
32
Second, family practice patients arrive with attitudes shaped by experience,
and physicians must
adjust their strategies to cope with those attitudes. Adjustments range from
changing interview style to
altering treatments. Variability in patient attitudes limits the likelihood
that there exists one best answer
for groups of patients with similar medical conditions.
Third, family physicians emphasize helping patients improve the length and
quality oftheir lives.
Family physicians spend considerable time reassuring worried patients,
alleviating symptoms, and
preventing the onset or progression of disease.
We have determined that the final testing and/or recertification problem,
evaluating the responses
of diplomates, also requires a model of what family physicians do. All
dichotomous evaluations,
especially pass-fail tests, use arbitrary standards. The challenge is to set
standards using generally
agreeable and meaningful criteria. The present invention provides the
flexibility to determine to whom
the criteria should be agreeable - certainly to diplomates, but perhaps also
to patients, insurers, or other
customers. Specifying these customers will help establish meaningful criteria
for certification decisions.
For instance, diplomates have an interest in maintaining respected
credentials, patients want
effective care, insurers desire low costs, and public health advocates have an
interest in clinical
guidelines. It is not at all clear how to respond to these diverse interests.
The present invention delivers
flexible models to describe the consequences of family practice activities, as
seen by various parties, so
that board certification remains a pertinent process regardless of changes in
the health care system.
We have determined that a model is needed to describe the scope of family
medicine in
epidemiologic terms, while including the information about individual
variation that differentiates
individualized patient care from public health. The model will be the
foundation of a family practice
knowledge base storing data about family medicine. The model also supports
other applications of
benefit to family physicians. Specific software applications might involve
medical records, structured
vocabularies, medical reference tools, decision support systems, and
continuing education programs.
Data structures to describe the activities of family physicians include a
series of entity-relation
diagrams. In an entity-relation diagram, entities usually represent things
(nouns). The relations (verbs)
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
33
illustrate how the entities interact. For instance, an entity-relation diagram
of an address list might have
an entity called "person," and an entity called "place," connected by a
relation called "is at." One could
read this diagram, "person is at place." The person entity would store
people's names, the place entity
would store addresses, and the "is at" relation would describe when and why
this person is at that place.
Thus, a person could now live at one place, previously live at another place,
and continuously work at
the first place. One person, two places, and three "is at" relations describe
this address history. This
address model is flexible and realistic.
We have determined that an important class of events exist in the model of
family medicine,
which we call "modifying relations," or modifiers. In database terms,
modifiers are relations between
traditional relations. Modifiers extend the conventional entity relation
diagram and provide a means of
managing statistically dependent events.
Model Structure
The family medicine model includes the major entities, relations and modifying
relations shown
in detail FIG. 3. Formal concepts in the model are capitalized throughout the
text. The model
emphasizes diagnostic and management issues, variability in populations, and
time. It describes
consequences of anatomic and physiologic processes, but largely omits anatomic
and physiologic
reasoning as such. It is also capable of describing interpersonal
relationships and is expendable to
include an explicit representation of families or communities.
Modifiers (for example, Bayesian network from a Lead to relation, Bayesian
network describing
risk factors for progression, and the like) are relations that might change
values in other relations.
Dynamic entities and relations contain information relevant to patient
simulations. Dynamic information
for an individual patient is derived from data in other dynamic and static
entities and relations. The
dynamic Record entity has relations mirroring the Population's relations.
Static entities and relations
contain the best available medical knowledge, similar to data in medical
literature.
The following major entities appear in the design: Populations, Records,
Health States, Findin Qs,
Courses of Action, and Agents of Change.
CA 02369425 2011-07-27
34
Populations represent real humans; their relations should precisely describe
all data that
physicians consider. Populations can be large groups with a shared
characteristic, such as white males
or single-parent families. An individual patient is a Population of I; a
pregnant woman is a Population
of 2; a nuclear family with 2 children and 2 parents is a Population of 4.
Records model beliefs about people; a Record's relations summarize inferences
about a
Population. If a parent brings an infant to the office, this design represents
the infant as a Population,
the parent as another Population, and the parent's description of the infant
as a Record. The physician
can obtain historical information about the infant from two sources: the
physician's medical Record of
the infant, and the parent's Record of the infant. The physician can obtain
current objective information
by examining the infant as a Population. The data linked to Populations are
absolutely precise, but can
be observed, if at all, only during medical encounters. Records summarize the
history of those real data
imprecisely and potentially inaccurately.
Populations have Records ofthemselves, modeling a patient's self-image and
memories. As with
other Records, a patient's self-Record summarizes historical information with
variable accuracy and
might be the physician's only source of some historical information.
A Population is primarily a list of relations with other entities. A Record
not only lists relations
with other entities, but also defines encounters during which these relations
were discovered. A Record
can contain conflicting data acquired at different encounters.
Health States include all normal health states; classic disease presentations;
early, subtle, or late
disease presentations; and somedisease combinations. Health States also
include groups of Health States
with shared characteristics, such as cardiovascular diseases and diseases of
glucose intolerance. The
SysteMetrics Corporation publishes Disease Staging Clinical Criteria, which
define numerous stages in
the development of diseases. See, for example, Gonella JS, Louis DZ, Gozum ME,
editors, Disease
staging clinical criteria, 4th ed. Ann Arbor, Mich: MEDSTAT Systems, 1994.
Each of these stages represents a distinct Health State entity in this design.
The SysteMetrics
staging of diabetes mellitus defines stage 1.1 as asymptomatic diabetes, stage
1.2 as symptomatic
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
diabetes, stage 1.3 as type I diabetes mellitus, and stage 2.1 as diabetes
with end-organ damage. Each
of these stages defines at least one Health State by the presence of specific
objective criteria.
Stage 2.1 might be divided into a group of Health States representing each
damaged end organ.
To represent multiple end organ damage, one might simply superimpose these
states.
5 Findings include genetic, physiologic, symptomatic, physical, and test-
generated data, and
clusters of such data. For instance, musculoskeletal chest pain could be a
Finding. This Finding could
be an example of a Finding called chest pains, which would represent all kinds
of chest pains. Chest
pains could be an example of a still larger Finding called symptoms. Findings
are defined by a collection
of one or more Features, whose current value can be described by a number on a
scale. One Feature
10 pertinent to pain is severity, which might be described on a 10-point
scale.
Structures called Patterns describe the possible values of each Feature over
time. A Pattern
typically lists a series of values and corresponding percentiles at several
points in time. Pediatric growth
charts are the most widely used real example of Patterns. A blank growth chart
illustrates at least the
following observations: (1) Normal birth weights vary within a narrow range.
(2) Weight increases
15 relatively rapidly in the first few months and years. (3) The absolute
variation in weight (e.g., the
difference between 90th and 10th percentile weights) increases after birth.
(4) Most people reach a fairly
constant weight by early adulthood. A pattern listing 10th and 90th percentile
weights for people at age
0, 1 year, 2 years, and so on, illustrates the same concepts.
Growth charts also predict future values from past information. A child at the
50th percentile
20 for weight now is expected to stay near the 50th percentile. If this child
later reaches the 5th percentile
of weight, the expected pattern is absent. The ensuing diagnostic evaluation
is an effort to account for
the deviation by finding a weight Pattern that explains all observations.
These concepts extend easily
to many other values, such as temperature. People have an average temperature
of about 37 C, but some
are a little cooler and some a little warmer. Normal temperature fluctuates
within a narrow range during
25 a lifetime, and most deviations from that range are considered abnormal.
Another example would be ST segments on a electrocardiogram. Following an
acute myocardial
infarction, ST segments usually rise by varying amounts, fall, and return to
normal. The ST segment
CA 02369425 2011-07-27
36
deviation from base line varies with time and can be described by a Pattern,
similar to the variation in
weights of growing children.
Many values change in predictable ways. Patterns might have cycles, sub-
Patterns, and
sub-sub-Patterns to describe these changes. The average value of a variable
often changes during a
lifetime, while the instantaneous value depends on a combination of annual,
lunar, and circadian cycles.
For instance, a nonpregnant 20-year-old woman should experience predictable
lunar and circadian
temperature fluctuations.
Sub-Patterns also describe consequences of other events, such as taking a
drug. For instance,
a dose of acetaminophen might lower a fever for 4 hours. A fever responsive to
acetaminophen could
be modeled by a high-temperature Pattern with a sub-Pattern indicating 4 hours
of normal temperatures
following acetaminophen doses. A person experiencing this fever and taking
acetaminophen every 4
hours maintains a normal temperature. A physician observing this temperature
Pattern would need to
halt the acetaminophen to distinguish between a normal temperature and fever
responsive to
acetaminophen.
Sub-Patterns characterize Features and therefore Findings. For instance, one
of the chest pain
Finding&.might be "crushing substernal chest pain relieved by rest or
nitroglycerin and exacerbated by
exertion." This description implies a Finding with a designated location, a
"crushing" Feature with some
pattern, and 3 sub-Patterns describing the effect of rest, nitroglycerin, and
exercise. The clinical
appearance of simulated patients with this Finding might still vary, depending
on the allowed variation
in sub-patterns. For instance, pain might be more quickly relieved by
nitroglycerin than rest or vice
versa.
Finally, Patterns include Shape Selectors that help maintain consistency
between variables.
Shape Selectors are an example of Reasoning Elements, for example, small
programs loosely based on
the structure of Arden syntax medical logical modules. See, for example;
Johansson BG. Wigertz OB,
An Object oriented approach to interpret medical knowledge based on the Arden
syntax, Proc Annu
Symp Comput AppI Med Car, 1992, pages 52-56.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
37
Reasoning Elements define variables; assign their values from data about the
simulation; use
loops, "if...then" statements, equations, and random numbers to reach
conclusions; and finally produce
some output. In Findings, the Shape Selector produces one percentile curve to
represent the values of
a Feature in an individual patient. For instance, although pediatric growth
charts allow considerable
variation in normal height and weight, one child will exhibit a precise series
of values for both height
and weight. Height will closely track one percentile curve, as will weight.
The percentile of the height
curve often limits the possible percentiles of the weight curve: healthy
children at 95th percentile height
rarely exhibit 5th percentile weight. Most children follow a weight percentile
equal to the height
percentile 20. The weight Shape Selector can use this equation to restate the
familiar height-weight
growth chart.
Patterns model time and are one approach to interrelated medical observations.
Time affects
most numeric values in the model. Consequently, Patterns appear in nearly
every entity and relation.
Patterns describe the incidence of diseases at different ages, the likelihood
of diseases progressing with
time, and concentrations of drugs.
Courses of Action (COA) represent people's activities. Not only can these
activities be medical,
such as taking a blood pressure or performing a coronary artery bypass graft,
but they can also include
attending school, working, asking and answering questions, and following
advice.
Populations invoke Courses of Action to decide when to visit a physician, how
to answer
questions, and whether to follow advice. Therefore, Courses of Action may
advantageously be written
to include missed appointments, lying to physicians, and ignoring physician
advice. These actions could
even depend on aspects of the physician's conduct, such as how the physician
chooses to obtain
information.
Courses of Action have complex internal structures. A Course of Action
organizes Step, which
gather, process, and modify information about Populations or Records. For
example, a Step might be
to obtain a blood pressure from a person. Each Step uses a Reasoning Element
to accomplish its tasks.
In the case of obtaining a blood pressure, the Reasoning Element would
determine and report the
simulated patient's systolic and diastolic blood pressure.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
38
A group of Steps that can occur in any sequence is called a Batch. For
example, when checking
both right and left arm blood pressures, the order in which the arms are
checked is probably unimportant,
so these can be distinct Steps within a Batch. The Course of Action lists a
series of Batches that must
be executed in sequence, and describes any mandatory delays between Batches.
For example, to check orthostatic blood pressures, recumbent pressures would
be obtained in one
Batch. The patient would sit or stand in a second Batch. After a short
mandatory delay, sitting or
standing pressures would be obtained in a third Batch. Courses of Action also
describe possible earnings,
costs, pleasure, and discomfort that motivate people to seek or avoid
activities.
Agents include physical, chemical, biological, behavioral, and social events
capable of
influencing health States or Findings. These Agents can be therapeutic,
injurious, or both. Agent
descriptions include data about intake, metabolism, and excretion, as
applicable. For instance, a
long-acting steroid is a chemical agent. Following intramuscular injection,
the steroid will have
predictable local and systemic concentration Patterns as the chemical
dissipates from the injection site.
The steroid might be metabolized to other compounds and excreted. Exposure to
Agents normally occurs
during a Course of Action, as this example illustrates.
The model of Agents describes their recognition, their presence, and the
presence of metabolites
or byproducts. Other parts of the model, such as the sub-Patterns of Findings,
describe the effects of
Agents.
Table 1 lists relations shown in FIG. 3. The Health States Lead to Health
States relation
describes how diseases evolve, and is therefore, critical for simulations.
Preventive medicine scenarios
might use this relation to generate patients who would benefit from screening.
Case management
problems can use this relation to model both the past and evolving history of
a patient.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
39
Table 1. Relations Between Entities
Population Contacts Population
Population Related to Population
Population Interacts with Courses of Action
Population Exposed to Agents of Change
Population Has Health States
Population Exhibits Findings
Agents of Change Cause Health States
Health States Lead to Health States
Findings Associated with Health States
Findings Link to Findings
Course of Action use Agents of Change
Courses of Action Identify Agents of Change
Courses of Action Treat Health States
Courses of Action Alter Findings
Courses of Action Reveal Findings
Courses of Action Evaluate Findings
Note: These relations link entities in the model together.
Unlike traditional knowledge bases, this relation links Findings (with their
Patterns) to a Health
State, rather than linking a range of Finding values to a Health State.
Sensitivity and specificity are
represented as age dependent Patterns, rather than constants. The sensitivity
of a Finding will be lower
and the specificity higher in this model than in traditional knowledge bases.
The Findings Link to Findings relation describes causal associations between
Finding Patterns,
such as "severe cough causes abdominal muscle pain." This relation contains
data about causality,
mechanisms, and temporal constraints. This relation facilitates reasoning
about Findings.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
The Courses of Action Treat Health States relation illustrates means of curing
Health States or
preventing their progression. Treatments therefore modify probabilities in a
lead to relation.
Courses of Action have three relations with Findings. The first, Alter,
implies changing a
Feature Pattern by invoking a sub-Pattern. For example, giving acetaminophen
could alter a fever. The
5 second relation, Reveal, links examining Courses of Action to the Findings
they produce. For instance,
a procedure called "taking a blood pressure" reveals systolic blood pressure.
The third relation, Evaluate,
links a Finding to a Course of Action that might be used to investigate its
cause. This relation would link
a Finding of systolic hypertension to a Course of Action describing its work-
up.
The Population Contacts Population relation traces transmission of
communicable Agents and
10 potentially beliefs. Population Is Related to Population describes
biological and social relations and the
history of those relations, and traces transmission of genetic Agents. These
two relations allow
descriptions of arbitrarily defined families, with arbitrarily harmonious
interactions.
The Population Interacts with Courses of Action relation describes why the
Population began
the Course of Action, what the Courses of Action cost interested parties, and
how comfortable the
15 Population was during the Courses of Action. This model allows a patient to
remember an unpleasant
experience and resist having it repeated. Because Courses of Action can
include negative (buying a
therapy) or positive (receiving a paycheck) change in wealth, this relation is
also capable of being used
to model patients' economic inability to follow medical advice.
The Population Exposed to Agents of Change relation describes perceptions
about the exposure,
20 knowledge of exposure, and the course of Action responsible for the
exposure. This relation can describe
exactly how an Agent was distributed in, metabolized by, and excreted from
this Population.
The Population Has Health States relation includes the preceding Health State,
a list of Findings
attributable to the Health State, and the age at onset, diagnosis, and
evolution of the Health State. Health
States affect different individuals in. different ways, and treatment often
depends on the patient's
25 impairments and perceptions. Consequently, a patient's beliefs about
disease progression and perceptions
of a Health State belong in the Has relation.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
41
The Population Exhibits Findings relation has similar perception attributes.
Perceptions can be
divided into Dysutility and concern. Dysutility indicates a trade-off a
patient would accept to return to
normal. Concern indicates a trade-off a patient would accept for full
reassurance that a Finding or Health
State does not portend future Dysutility. For instance, a patient with a minor
left-sided chest pain might
rate its current Dysutility as $5 ("I would spend $5 to relieve this pain for
today."), and the concern as
$100 ("I would spend $100 for assurance that nothing serious caused this
pain."). If the pain persists
unchanged, both of these values might decline as the patient learns to cope
with the discomfort and
becomes confident that the symptom has no prognostic importance. Thus,
patients can have changing
attitudes about stable conditions. Patients would typically seek medical care
when provoked to so by a
Dysutility or concern.
Records have the same relations as Populations, except that the details are
always more
ambiguous, inaccurate, or both. For instance, a patient might have influenza
starting December 15, while
his Record of himself indicates that he developed influenza between December
10 and December 13.
The patient's Record of himself is both ambiguous (there are 4 possible days
of onset) and incorrect
(none of the days is December 15).
We have further determined that the data described in the Lead to, Associated
with, and Link to,
relations often change with medical interventions or other events. Modifiers
describe events that cause
a permanent variation in the expected history of these relations. For
instance, an event might make
evolution to another Health State more or less likely (regular low-dose
aspirin reduces the risk of acute
myocardial infarction), or could permanently alter the likelihood of
exhibiting a finding (cardiac
transplant prohibits myocardial ischemic pain). The dashed lines in FIG. 3
show Modifiers. The
following examples illustrate some modifiers (for example, Bayesian network
from a Lead to relation,
Bayesian network describing risk factors for progression, and the like).
Population Interacts with Courses of Action modifies Health States Lead to
Health States. An
appendectomy alters the progression of acute appendicitis to appendiceal
rupture. For example,
life-span-altering interventions always modify a Lead to relation.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
42
Population Exhibits Findings can modify Health States Lead to Health States.
For example,
being overweight increases chances of developing a deep vein thrombosis or
pulmonary embolism.
Population Has Health States can modify Health States Lead to Health States.
Diabetes
accelerates the onset of cardiovascular disease.
Population Has Health States can modify Findings Associated with Health
States. Diabetic
neuropathies diminish pain associated with myocardial infarction or extremity
injuries.
Modifications of these relations account for many benefits ascribed to
receiving medical care.
Other benefits can occur when medical interventions temporarily decrease the
severity o Findings.
The model described herein is intended to be a highly structured and realistic
representation of
family medicine that will guide the design of the family practice knowledge
base and support the
generation and evaluation of recertification examinations. In this model, the
following are strong
assumptions: (1) Health States are discrete and distinguishable on the basis
of associated Findings,
which are also discrete and distinguishable on the basis of the Patterns of
their Features. (2) After
choosing a percentile curve in a Pattern to represent some value, the
percentile does not change
substantially. (3) Changes in Patterns (e.g., the probability of one Health
State evolving to another) can
be described for important combinations of risk factors, interventions, and
time of occurrence. (4)
Transitions from one Pattern to another can be estimated by simple means. (5)
Modifying relations do
not have important interactions with one another. (6) Highly developed
anatomic and physiologic
models are not necessary, because associations between Findings provide the
same information.
Although the model should have clear places to store nearly all interesting
facts about family
practice, test generation does not require a comprehensive description of all
facts used in family practice.
The proposed test generates plausible problems from a set of data
intentionally skewed to generate
interesting (i.e., discriminating) cases. The present invention provides the
flexibility to avoid
controversial questions by controlling skewed data. For instance, if the
management of borderline
diabetes is controversial, the present invention allows editing of the family
practice knowledge base so
that diabetics' fasting blood glucose levels are always markedly elevated. The
family practice knowledge
base would then be incapable of creating a borderline diabetic.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
43
The diagram of the model illustrated in FIG. 3 reflects many family medicine
concepts, and
therefore, helps students, physicians and others understand the process at
work in family medicine. For
instance, the diagram illustrates that Populations have biological and social
relations. Populations exist
in Health States, which evolve into new, sometimes undesired Health States.
A major goal of family medicine is to retard or stop undesirable evolutions
and promote
desirable evolutions. Stopping one undesirable evolution could, however,
result in a different
undesirable evolution. In addition, physicians who treat symptoms will Alter
Findings, but do not
necessarily Treat Health States. Altering Findings usually changes current
quality of life, whereas
treating Health States usually changes future quality and quantity of life.
Because Findings occur in the
context of Health States, we have determined that physicians contemplate what
Health States might be
responsible for Findings, rather than Alter the Finding without considering
future quality of life. The
only tools available for these causes are Courses of Action. Physicians
prescribe Courses of Action, but
only patients Interact with Courses of Action. For example, the prescription
does not guarantee that the
patient follows the correct Course of Action. Agents( e.g., drugs) make a
difference only when used in
the context of a Course of Action.
The model's details provide further insights for students. First, time is an
extremely important
element of primary care. Patterns become more distinctive as time passes,
simplifying diagnosis. The
total risk of going from one Health State to another increases with time,
increasing the value of early
interventions. Second, patients have variable and evolving attitudes about
Health States, Findings, and
Courses of Action. The goal of medicine might not be to adhere to an endorsed
Course of Action, but
to optimize each patient's perception of his or her quality of life. To reach
this goal, physicians adjust
Courses of Action to accommodate individuals' attitudes. Third, the importance
of time and attitude in
optimizing the quality of a patient's lifetime suggests that continuity of
care might help some patients.
The scope of family practice and the importance of protocols, time, individual
variations and
attitudes, and rationales distinguishes the content of the family practice
knowledge base. That is,
advantageously, some differential diagnosis of internally generated cases is
possible using the model.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
44
In this model, differential diagnosis largely depends on establishing the
presence of Findings,
which in turn depends on establishing the presence of Patterns and sub-
Patterns of Features. Except in
rare cases of pathognomonic values, confidence in the presence of a Pattern
will increase with the
passage of time.
We have also determined that the structure of an interface to medical
reference systems might
be enhanced using the model. Current reference systems use the structure of
medical publications and
lists of abstracted subject headings to facilitate searches through very large
databases. These searches
can yield large numbers of extraneous citations, especially for novice users.
The model suggests an alternative indexing strategy, as well as a graphical
search interface. For
instance, one could view a query interface similar to FIG. 3. To request a
query about the effect of
insulin treatment on the development of retinopathy in diabetic patients, one
selects diabetes from an
unrestricted list of Health States. The Lead to allows the user to select
diabetic retinopathy from a list
of diseases restricted to diabetic sequelae. The Modifier specifies which
Course of Action or Agent of
Change to consider. The computer delivers a list of references mentioning
insulin in a diabetes Leads
to diabetic retinopathy relation. Searching for a particular relation between
two entities improves the
efficiency of searches usually performed by naming the entities.
Overview of Patient Generation/Evolution Processes
We describe here an overview of processes used in the
certification/recertification system. The
processes are divided into four main groups:
1. Patient generation processes:
= history outline processes
= history generation processes
2. Simulation processes
= Presentation interface processes
= Patient evolution processes
CA 02369425 2001-10-03
WO 00/60431 PCT/US0O/08942
Patient generation processes are called once and produce the subject for the
examination session.
Simulation processes may be called repeatedly several times. The patient
generation process presents
the patient to the examinee, collect the examinee's responses and queries, and
evolve the patient. See
FIG. 4 for a pictorial overview of the system.
5 For the patient generation process, we assume that the area for the
simulation - a specific object,
sayA, from the class AREA - and a health state, say H, from the primary
network of the areaA are given.
For example, A may be the area of the adult onset diabetes and Hmay be the
health state of symptomatic
diabetes.
The patient generation process consists of two phases:
10 1. history outline, and
2. history generation.
The goal of the history outline phase is to generate a progression of health
states and risk factors
traversed by the patient on the way from the normal condition to the specified
health state H. It starts
with a call to the procedure that establishes sex and race of the patient
being generated (referred to as
15 procedure GenderRace). The next step establishes the age of onset of H(call
to procedure OnsetAge).
The goal of the next step is to select the precursor state for the target
state in the simulation as
well as risk factors (circumstances) that will affect the patient under
construction. This will be
accomplished by a call to the procedure OutlineFirstStep.
The next procedure, OutlineGeneralStep, is called iteratively until the normal
health state is
20 reached. In each iteration, it finds the precursor health state as well as
its onset time. When the normal
health state is reached, the history outline phase is complete. See FIG. 5 for
a flowchart of this process.
The GenderRace procedure generates sex and race of the patient under
construction.
CreatePerson creates a basic description of the person. We select last, first
and middle names,
and age of the person, as well as two basic demographic findings: sex and
race. These last data are
25 stored as EXHIBITS tuples (since demographic findings are treated as
findings).
The OutlineFirstStep procedure generates the precursor state for the target
health state for the
simulation, and its onset age. In addition, it selects circumstances to which
the simulated patient has
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
46
been subject. This procedure also creates an object HS_path, stored on the
white board and containing
the sequence of HAS instances for the precursors of TS, starting with the
normal health state and ending
with TS. This sequence will be used later in the history generation phase.
The Generating history outline, and more specifically, the OutlineGeneralStep
procedure,
generates the complete path of precursors of the target health state. It
starts in the normal health state
and terminates in the target health state TS (of course, the last but one
state on the path has already been
generated by OutlineFirstStep procedure).
History Generation
The history generation phase finds values that are established in each case
when they differ from
normal (normal values are derived from the defaults maintained in the
knowledge base). The general
outline of this phase is given in FIG. 6.
The reasoning element, called generation method, describing how a given health
state or a risk
factor determines a finding, plays an important role in this phase. The
generation method either provides
a description of all relevant basic features at all relevant sites (for normal
states), or determines which
basic features at what sites need to be adjusted and by what specific
findings. The main input for this
phase is the list of associated objects attached to the object P of type
PERSON (the object of the
simulation).
The history generation process looks at all associated objects and modifies
values of patterns
describing relevant basic features so that the detailed description of the
patient is consistent with the
health state history as created in the earlier phase. Therefore, in this phase
we focus on describing
findings and their basic features. To this end, we look at all health states
represented by HAS instances.
We sort them according to their onset times. This results in a list in which
all states normal in their areas
precede all the abnormal states. The reason for this is that all normal states
start at time 0. For each of
these normal states we will run its generation method. This creates a list of
finding names and site names
to which the findings pertain, and defines the domain of all findings for
which specific descriptions are
created.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
47
Next, for every finding, the patterns of its basic features are instantiated.
We obtain these
patterns from "normal" specific finding belonging to the finding in question.
To select specific curves,
we use a percentile value. This value will generally be selected from, for
example, the range [0.15, 0.85]
uniformly at random. Each time we need to use this value to select a specific
pattern, we modify it, for
example, by a randomly selected number from the range [-0.05,0.05]. In this
fashion the modified value
is, for example, in the range [0.10,0.90].
After all normal states are processed, patterns of all basic features of all
relevant findings are
instantiated for life. From now on, when processing other health states these
patterns are modified. The
idea is to run the generation method for a health state. As a result we get a
list of sites and basic features
which must be modified as well as specific findings where the new patterns can
be found. If only some
sites for the finding are generated, only those sites need to be modified. To
modify the patterns, we use
patterns captured by the appropriate specific finding. Again the basic
percentile is varied and used in
the selection. The selected pattern is then superimposed on the existing
pattern (its values replace the
old values starting with the onset age for the health state).
The generation method associated with the health state H, generates the list
of relevant findings
with additional information on sites and specific findings. That is, for each
finding we maintain the list
of sites and with each of those we associate the list of all basic features
(names) corresponding to the
finding. Finally, these basic features are described by their patterns.
The PatientDescription procedure selects HAS instances. It then arranges them
according to
onset times, generally earliest first. In this process, the procedure invokes
the generation method
procedures for each health state, thus creating EXHIBITS tuples describing
findings associated with
health states.
The InitPt Description (Initialize Patient Description) procedure initializes
the list PATIENT
FINDINGS, which contains all findings relevant to the primary health state as
well as all secondary
(modifying) health states. It creates all corresponding EXHIBITS instances and
attaches them to the list
associated objects. All these findings are initialized to their normal values.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
48
After the call to InitPtDescription, the domain of findings, sites and basic
features, which
subsequently will be modified, is defined. CreatePtDescription scans the list
of HAS instances and
adjusts findings so that the resulting patterns are consistent with the
history of health states.
Patient Evolution
As explained earlier, we assume that data required by the processes is stored
in the entity
relationship model, white board (WB) and in the area of memory local to
patient generation and
evolution processes. This local memory will be denoted as LM. We start the
evolution phase with the
patient fully described and stored in the WB. An equivalent description exists
in LM. Several HAS
instances describe continuing health states (one ofthem -primary). After the
assessment phase (requiring
physical examination and history taking) the examinee proposes treatment
consisting of one or more
courses of action. These courses of action may alter some of the health states
the patient is currently in.
All selections made by the examinee are gathered in a table coa list.
LEAD_TO data describes probabilistic information on progress from one health
state to another.
This data depends on modifiers. At present, we use a small generic set of
modifiers: "fast progress,"
"moderate progress" and "slow progress." For each of these modifiers, and for
an edge in the health state
network between a precursor health state PS, and the target health state TS,
the entity relationship model
contains an estimate of the flow along that edge.
Courses of action are represented in WB by a table which describes their
structure in terms of
elementary courses of action. We will describe this structure below. In
addition, each course of action
contains a reasoning element. This reasoning element, given an edge (a pair
(PS,TS)) and a set of other
current health states (as modifying events), computes one of these three
modifiers. Flows on the edges
starting in the current health state are used in the selection process. Once
the selection is made, duration
risk stored in the appropriate LEAD_TO tuple is used to determine the onset
time for the selected health
2 5 state.
The following structure is used to represent a course of action COA in WB. The
data is stored
in a table with, for example, four columns (additional columns may be
necessary later for evaluation
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
49
purposes). The first column is labeled ECOA (elementary course of action). It
lists all concrete
elementary courses of action that might be used in a construction of COA. The
second column describes
the type of the corresponding elementary course of action. ECOAs of the same
type are identified by
the same integer in the second column. The third column contains one of five
boolean operators: none
(NOR), single (XOR), at least one (OR), some but not all (NAND), all (AND).
All members of a type
are assigned the same operator in column 3. The fourth column contains weights
which are used in the
matching process.
One of the courses of action listed with every health state is called TIME. It
describes the effects
of no specific action by the examinee and serves as a default course of
action.
The evolution phase is accomplished by the procedure called Evolve. Evolve has
three input
parameters: patient P, patient's age T, and the list coa_list of COAs selected
by the examinee. Evolve
starts by creating the list of patient P continuing health states. This is
accomplished by the procedure
called SelectPresentHas. SelectPresentHas selects from the list of P
associated objects those HAS
instances that represent continuing health states. It arranges selected HAS
instances in a list.
For each health state PS described by the list of selected HAS instances, we
then identify in all
the courses of action that are relevant to PS. It gathers all those courses of
action that are in relation
MANAGE with the health state PS, in the list called, for example, coas.
At this time, the closest COA, among those found relevant to PS, to the
examinee selection
(described, recall, by the list coa list) is chosen. For the course of action,
say COA, target states are
created for PS, corresponding modifiers and flows. This data is used for
evolution.
These steps are repeated for each health state PS. When the process is
completed, all successor
health states are represented by means of the corresponding HAS instances. The
evolution step is
completed with a call to CreateDescription procedure. It generates
descriptions of specific findings
corresponding to the health states.
Stochastic Process For Patient History Generation
The present invention provides a method to automate authoring of major events
in simulated
medical histories. We have designed a knowledge base with temporal
descriptions of the incidence and
CA 02369425 2011-07-27
prevalence ofhealth conditions and plausible intervals between health
conditions. Each health condition
is part of a small sequence of related and mutually exclusive health
conditions. Many of these small
networks exist in parallel.
We have determined that a patient's overall health can be described by a
vector indicating the
5 patient's current health condition in each network. A patient's location in
one network often affects
timing of transitions in other networks. The knowledge base advantageously
uses modifiers (for
example, Bayesian network from a Lead to relation, Bayesian network describing
risk factors for
progression, and the like) to describe the influence of these and other risk
factors, as well as
interventions, on incidence and transition times. A stochastic history
outlining algorithm uses these data
10 to construct a lifetime and recent medical history whereby a patient might
develop a specified vector of
health conditions.
The present invention generates a large number of plausible history outlines.
The present
invention automates the authoring of major events in the lives of simulated
patients. The present
invention applies a Monte Carlo process to multiple stochastic trees, to
generate large numbers of
15 plausible case outlines. Further automated embellishment of these outlines
yields complete, usable
simulated case histories.
Previous efforts to simulate patients from data have used sensitivity
information stored in a
diagnostic database, or Quick Medical Reference , to stochastically create a
description of findings in
a patient with a disease. See, for example, Bergeron B. Iliad: A Diagnostic
Consultant and Patient
20 Simulator, MD Computing 1991, Vol. 8, pages 46-53; Miller RA, Masarie FE,
Myers JD, "Quick
Medical Reference(QMR)" for diagnostic assurance, MD Computing 1986, Vol. 5,
pages 34-49.
However, we have determined that these simulations lack rich
historical details and may generate implausible combinations of events. See,
for example, Sumner W.,
A review of Iliad and QMR for primary care providers, Archives of Family
Medicine 1993, Vol. 2, pages
25 87-95.
Some simulations generate patient details from a complete and precise
mathematical model of
pathophysiology. See, for example, Valdivia TD. Hotchkiss J, Crooke P, Marini
J., Simulating the
CA 02369425 2011-07-27
51
clinical care of patients: A comprehensive mathematical model of human
pathophysiology, Proc l 9th
Annu Symp Comput Appl Med Care. 1995, page 1015. This elegant
approach is feasible in intensive medical care and some restricted organ
systems, but primary care
problems are not so well understood at present, and therefore require
empirical description.
Accordingly, we have also developed a process for generating detailed patient
histories
culminating in a specified set of simulated health problems. The first segment
of the algorithm creates
an outline ofthe medically important events in a patient's life, including the
patient's age at the onset and
termination of different health conditions or exposures to biologically active
agents. The second segment
of the algorithm yields a detailed description of continuously defined facts
about the patient, such as
physical and chemical characteristics, morphology, function, and sensations
throughout life.
The history outlining algorithm essentially creates paths through temporally
reversed
Monte-Carlo processes, casting major events in a patient's history while
guaranteeing that the history
ends with specified medical conditions. See generally, Rubinstein RY,
Simulation and the Monte Carlo
Method, New York, NY, John Wiley and Sons Inc.; 1981, This process
is applied to a set of stochastic disease history models, each describing the
evolution of one health
problem.
A knowledge base stores these models, along with standard modifiers that
calculate temporal
constraints on disease progression, conditioned on comorbidities and
treatments. This algorithm is
capable of generating many plausible cases in a short period of time preceding
an examination.
The "Health condition Leads To Health condition" cycle is the central
component in the
generation of a patient history. A health condition is a named collection of
facts, which usually have
prognostic implications. Typically, the facts that connote a health condition
have a specified degree of
variation from normal ranges, and are thought to arise from a common
underlying cause. A health
condition can usually be considered to be located at one or more body
structures where that underlying
cause is present.
Health conditions uses patterns and subpatterns to predict their prevalence
and incidence,
conditioned on factors such as sex and race. Prevalence and incidence are
provided in a widely used
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
52
structure called shape, which plots a value over time. In this situation, time
indicates the simulated
patient's age.
A health condition uses a generation method reasoning element to establish the
facts pertaining
to its instantiation. These facts may include events like drinking alcohol or
driving cars, but most facts
are specific instantiations of more generic medical concepts, such as symptoms
or laboratory values, in
specified body parts. For instance, the generic concept of "synovial fluid
glucose level" might be
instantiated as "normal" in "both knees." Shapes describe exactly how a value
in this instantiation may
reasonably evolve or fluctuate over time.
Two special classes of health conditions exist. First, normal health
conditions are incident only
at birth (or conception, depending on testing goals). Second, "Alive" is a
health condition whose
prevalence shows the proportion of a cohort that survives to any age. The age
specific prevalence and
incidence of all other health conditions are defined as the percentage of
living individuals at that age who
experience or acquire the condition, respectively.
The-leads to relation connects one health condition (the precursor) to another
(the target), and
describes possible time intervals required for evolution from the precursor to
the target. A Pattern
describes a probability density function (pdf) of these time intervals,
conditioned on comorbidities,
treatments, and other risk factors. This duration pdf provides a time
constraint mechanism. For instance,
a duration pdf for the progression of mild to moderate knee osteoarthritis,
given obesity, might indicate
a probability density of zero in the first five years following the onset of
mild osteoarthritis, a uniform
probability density from year five to year twenty, and then a probability of
zero. This implies that all
simulated obese patients develop moderate osteoarthritis between five and
twenty years after the onset
of mild osteoarthritis, and forbids simulated onsets at other times.
The modifiers of a Lead to relation also provide time constraints for risk
factors. This allows
the model to represent the concept that obesity must exist for a period of at
least 10 and up to 40 years
for this duration pdf to apply.
Finally, the Lead to relation provides information about how quickly and
completely to convert
from the findings typical of the precursor to findings typical of the target.
For instance, if each knee
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
53
osteoarthritis stage is a health condition, and each stage has a typical
degree of joint space narrowing,
then the transition from one stage to another should be accompanied by more
narrowing of the joint
space. The Lead to relation can indicate that this narrowing occurs over
years, and that the narrowing
is nearly complete when the simulation asserts that the latter osteoarthritis
stage is present.
A series of Lead to relations connect health conditions into small networks
illustrating
evolutionary sequences of events. These networks often suggest a disease
staging scheme, such as (Stage
0) No Knee Osteoarthritis, (Stage 1) Mild Knee Osteoarthritis, (Stage 2)
Moderate Knee Osteoarthritis,
and (Stage 3) Severe Knee Osteoarthritis.
We call this sequence a parallel health condition network. It is "parallel" to
many other networks
of health conditions that exist simultaneously in a person. In general, a
parallel health condition network
lists transitions that occur among an exhaustive set of mutually exclusive
health conditions occurring in
one body part. For instance, the left knee of a patient exists in one of the
health conditions in the
osteoarthritis network. The right knee also exists in one of these conditions,
but not necessarily the same
condition found in the left knee. The patient simultaneously exists with one
condition in a gastric ulcer
network, a weight network, and numerous other networks.
A simulated patient's overall medical condition is therefore a vector, V,
listing the current health
condition from each parallel network at each involved site. A case specifies
vector V0, indicating the
health conditions instantiated at the initial presentation of a simulated
patient, and sufficient information
to create a history of vectors culminating in V0.
Most of the parallel networks in any given case are inactive. These define an
initial, usually
normal, (stage 0) condition of the parallel network. Most cases contain a few
active parallel networks.
Active networks presenting at stage I or higher represent active medical
problems. Active networks
presenting at stage 0 represent potential problems, such as complications
resulting from an active
problem or its treatment. The examinee's task is generally to identify and
respond to active networks in
advanced stages, while minimizing disease progression in active networks at
stage 0.
Active networks can be divided into two categories. A case usually focuses on
care for a primary
network "P" (for instance, osteoarthritis of the knees). A comorbid network
"C" usually includes health
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
54
conditions that influence, or are influenced by, the stage of evolution of a
primary network. For instance,
obesity is a risk factor for osteoarthritis, and osteoarthritis may worsen
obesity by limiting exercise.
Comorbid networks that do not interact with the primary network in any
important manner may serve
as distractors.
For instance, an episode of urethritis might be irrelevant to osteoarthritis,
but suggests Reiter's
syndrome as an alternative explanation for knee pain with an effusion. An
active, stage 0 comorbid
network provides opportunities for complications. For instance, a simulated
osteoarthritis patient
presenting with a "No gastric ulcer" health condition could advance to
"Gastric ulcer" after receiving
steroidal nonsteroidal anti-inflammatory drugs.
When an active parallel network describes a chronic condition, acute
exacerbations may be
expected with some of the health conditions in the network. An exacerbation
network "E" is a parallel
network describing acute flares of illness that occur during a more chronic
health condition. For
instance, flares of knee pain with effusions may occur in patients with
chronic osteoarthritis. In
principle, health conditions within an exacerbation network can have their own
exacerbations. The
simulation process of the present invention allows exacerbation networks to
contain cycles, unlike
primary and comorbid networks.
A simulated patient's medical history is the sum of the events culminating in
the case defining
vector, V0. The case provides sufficient information to create many plausible
histories, but does not store
histories per se. Consider a case defined to culminate in severe bilateral
knee osteoarthritis and morbid
obesity. The relative sequence of events on the primary and comorbid networks
are not necessarily
constrained. Obesity might be required to occur before the onset of mild
osteoarthritis. However, the
onset of morbid obesity could occur before or after the onset of moderate
osteoarthritis.
The cartesian product of two active, linear parallel health condition
networks, P and C, yields
a two dimensional web of health condition combinations. This product re-
establishes the complexity
avoided by the parallel network simplification, and calls attention to
interactions between P and C. A
vertex in this web is composed of the ith health condition in P and the jth
health condition in C, and is
represented by the vector Vo = (P;,Cj). Evolution can be assumed to occur in
only one dimension at a
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
time. If evolution in both networks can occur simultaneously in life, one can
be assumed to occur first,
and the other a moment later for purposes of the model. That is, the set of
vectors V-, = {(P;_,, Cj)}; (P;,
Cj-,)) are immediate precursors of vector VO, but (P;-,, Cj-,) is not.
Similarly, the set of vectors V_2
includes (P;-,, C), (P;-,, C,-,), and (P;, C,-2).
5 Three kinds of interaction are possible in the web formed by networks P and
C. First, the
networks may be completely independent, so that evolution along one dimension
has no implications for
evolution in the other. Second, progression through one network may depend on
the concurrent
condition of an independent network. For instance, the incidence of early
osteoarthritis conditions is
dependent on the presence of obesity. Finally, mutually dependent networks
create a web in which
10 progression through each network depends on the concurrent condition of the
other network. For
instance, a realistic simulation of a severe osteoarthritis history might
require modeling a "vicious cycle"
where obesity accelerates osteoarthritis, which in turn accelerates obesity.
The cartesian product of N parallel health condition networks similarly yields
an n-dimensional
web of health condition combinations, with potentially complex interactions.
Data acquisition for these
15 webs is a daunting task, but might be simplified by (1) limiting the number
of dimensions, (2) ignoring
improbable health condition combinations, particularly when describing vicious
cycles, and (3) assuming
independence for some kinds of test cases even when dependence exists in
reality.
Stochastic Process History Outlining Process
20 The goal is to produce patient care scenarios for recertifying diplomates
to manage. The data
described above allow automatic generation of such cases, starting from a case
specification. The case
is composed of primary network P, and comorbid health condition network C.
Network P is composed
of health conditions Po, ...P, and "lead to" relations PLo_>,,... PLn-,_,,.
Network C is composed of health
conditions Co,...C,n and "lead to" relations CL,_,,...CL,n-,-m=
25 Chronic health condition Pi in network P has acute flares described by
parallel network E.
Network E is composed of conditions E0,... Eq and "lead to" relations EL,-,,,
EL,-,p, ...ELF-,->q, ELq->q-,.
The normal condition of network E is E0, and the network may cycle through Eõ
up to X times.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
56
The vector Vo (P;, Cj, Ek) summarizes the health conditions required at the
presentation of the
case. Health conditions P; and Ek may be incident or prevalent at
presentation. Incident health conditions
would typically require both diagnosis and management, while prevalent health
conditions would often
be known diagnoses, and require only management decision. Health condition CC
is usually prevalent.
The first step assigns the sex, race, and other genetically determined facts
to the prospective
patient. If P, is an incident health condition in the simulation, the
incidence pattern for health condition
P;, is conditioned on sex and race. Sex and race are assigned by obtaining the
area under the incidence
curves for male and female patients of each race. The simulator makes a
weighted random selection of
the patient's sex on the basis of the results.
In the weighted random selection process, a series of positive values is
normalized to one by
dividing each value in the series by the sum of the series. The resulting
series defines a probability
distribution. To select an item according to this probability distribution,
the interval from zero to one
is divided into consecutive subintervals of lengths equal to the corresponding
probability the series. A
random number from zero to one is generated from the uniform distribution. The
interval to which it
belongs defines the selected item.
Because the incidence or prevalence of some illnesses, such as knee
osteoarthritis, can increase
dramatically with age, some correction to approximate the absolute number of
cases occurring at each
age may be useful, depending on the goals of the simulation. To obtain
absolute numbers of incident or
prevalent cases at each age in a cohort, the incidence or prevalence at each
age is multiplied by the
fraction of the cohort in that age interval. Formula l illustrates this
calculation, and the general
procedure for multiplying two shapes.
Formula 1. Absolute prevalence of health conditions as a function of age:
Absolute prevalence(P;, n) = prevalence(P;, n) * prevalence(Alive, n)
Where prevalence (health condition, n) = the prevalence of health condition at
age n years.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
57
Similarly, the joint absolute prevalence of P; and Cj can be calculated by
multiplying the absolute
prevalence of P; by the prevalence of C; in each age interval. Although the
prevalence of either or both
health conditions may be explicitly conditioned on the presence of the other,
knowledge acquisition
efforts are unlikely to capture such dependencies. Calculating the joint
prevalence reduces the chance
of creating an unsolvable history, for instance by creating a prevalent case
of P. at an age where C; does
not exist, regardless of the prevalence produced in knowledge acquisition. A
weighted random selection
of an age of presentation can be made from the product of the age specific
prevalence of all representing
health conditions, and the special condition "Alive."
Often, either P; or Ek is an incident health condition, and the age of onset
of the presenting health
condition vector, Vo = (P;, Cj, Ek), is determined by the preceding step. In
addition, the immediately
preceding health condition vector, V_,, must be (P;_,, C;, Ek) if P; is
incident, because any other vector
would make P; prevalent rather than incident at age N. More commonly, Ek is
incident and vector V_,
must be (P;, Cj, Ek_,). Alternatively, if Vo consist only of P; prevalent
health conditions, then the age of
onset of Vo is unknown. In general, health condition vectors contain a mixture
of conditions with known
ending times (e.g., precursors of incident conditions in V0) and unknown
ending times (e.g., prevalent
conditions in Vo).
Assume that P; is an incident health condition at age N. The interesting
vector is therefore V_,
_ (P;_,, Cj, Ek), because health condition P;., evolved to P; at age N. One
possible precursor of vector V_,
is (Pi-2, C,, Ek) which would evolve to vector V_, at the age of onset of
health condition P;_,.
The age of onset of P;_, is constrained in part by the age specific incidence
of P;_,, and N. The
incidence of health condition P;_,, conditioned on race and sex yields the
number of new cases per year
per number of persons at risk, in each year from birth to age N. Because the
simulated patient must
belong to a cohort of individuals who lived until age N, corrections to obtain
an absolute incidence are
usually not important.
The age of onset of health condition P;_, is further constrained by the
plausible duration of P;_,.
For instance, if P;_, always progresses to P; within ten years, then a case of
P;_, must have begun between
ages (N - 10) and N. The "lead to" relation PL _,, provides a duration pdf,
conditioned on pertinent facts
CA 02369425 2001-10-03
WO 00/60431 PCT/US0O/08942
58
representing some known modifier. The duration pdf is a probability
distribution function defining
probabilities of evolution to Pi time intervals subsequent to the development
of Pi_,. The duration pdf is
truncated at the time equivalent to the age of presentation, N (assuming that
Pi could not have begun
before birth), and reversed in time. The reversed duration pdf indicates at
age 0 the probability that a
transition from Pi-, to Pi would take N years, the simulated patient's entire
life. In the year before
presentation, at age N-1, the reversed duration pdf shows the probability that
the transition would occur
after exactly one year.
For each year from birth to the age of onset of Pi, the incidence of health
condition Pi-, and the
reversed duration pdf are multiplied to obtain a weighting factor for the
onset of Pi_, in that year. These
weights are used to make a random weighted selection of one year to propose as
the age of onset for the
health condition Pi-,. This age represents one proposal for the age of onset
of
V-,=(Pi Cj, Ek)=
Formula 2. Weight (Wõ) for establishing the onset of health condition Pi-, at
age n:
Wn = Incidence(P1-,, n) * DurationPDF (Pi-,, N-n)
Where:
N = age of onset of health condition Pi
DurationPDF (health condition, x) = probability that health condition evolves
to its
successor during the time interval x- I to x years after its onset.
In general, this procedure is repeated for each health condition with an onset
time after birth (or
conception) in the currently interesting vector, V-,. The result is a proposed
list of ages of onset for a
subset of vectors in the set V-2. The next step proposes ages of onset for the
remaining vector in V-,.
Assume that health condition Cj is a prevalent condition in a simulated
patient presenting at age
N. Assume that the annual incidence of Cj is constant from age N-3 to N, and
that Cj is equally likely
to evolve to Cj+, in 1, 2, or 3 years. The duration pdf from the "lead to"
relation CLj-,j-, is therefore
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
59
uniform over years 0 to 3. Consequently, G beginning at age N-3 is as likely
to continue to age N-2 as
to age N-1, but will not be prevalent at age N in either case. Conversely,
most cases of C; beginning at
age N-1 would be prevalent at age N. To accommodate the uncertainty regarding
the onset time of Ci+,,
the duration pdf is reversed in time(as in the previous step), then converted
to a cumulative probability
function. The highest cumulative probability occurs just before the age of
presentation.
Formula 3. Reversed cumulative probability (RCP) of duration of health
condition C;:
RCP(n) = E (DurationPDF(CLj_,j+,, N - y))
y=0 ton
Where:
N = age at presentation
y = a number of years between 0 and n.
For each year from birth to the age of presentation, the incidence and
reversed cumulative
probability of duration are multiplied to obtain a weighting factor for the
onset of Cj in that year, a
random weighted selection chooses the year to propose as the age of onset for
the health condition Ci.
This age represents a second proposal for the age of onset of (P;1, Cj, Ek).
Formula 4. Weight (Wõ for selecting age n for the onset of health condition
Cj:
Wn = Incidence(C, n) * RCP(n)
At this point, the simulator has completed these steps. It found vector Vo to
have a single
possible predecessor, V_,. Each health condition listed in V., could have been
the last to develop,
therefore the simulator proposed a plausible age of onset for each. The
simulator used one of two
algorithms to calculate age of onset of each condition, depending on whether
or not it could identify the
age at which the condition ended.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
Each proposed age corresponds to a change in one element in vector V.,. The
collection of
vectors produced by these single health condition changes is the set V.2.
Consequently, selecting the
health condition to change specifies which member of the set V_, is part o the
history of this simulation.
Although only one vector in V_2 will appear in the history of this simulation,
all of the health conditions
5 in V_, will be traced back to birth through vectors from sets V_3, V_4, etc.
The question is not whether
each condition has a history, but when events occurred.
A safe strategy is to instantiate the vector from V_2 occurring at the latest
age, along with any
facts that had been tentatively proposed with that age and vector. If two or
more vectors from V_2 share
the latest moment in age, one may be selected at random. The history
generation step is repeated with
10 the instantiated vector from V.2 replacing V_, as the focus of attention.
The "lead to" relations, such as PLL_,_,;, may need to instantiate modifiers
in order to produce a
duration pdf.
Some modifiers might be defined by a history of a health condition in an
active network.
Instantiations of health conditions in active networks create additional
temporal constraints for these
15 conditions. These constraints typically dictate that a comorbid health
condition, C., is present at a point
in time (e.g. at age N, the moment of transition from P;_, to P;), for a
period of time (e.g. at least five but
not more than ten years), or both (e.g. for the past two to four years). These
conditions can be evaluated
for logical compatibility with incidence data and the case. For instance, the
instantiation of a modifier
may require that C. is present at the moment of transition from P;_, to P;. If
xyj and Cj is part of the target
20 vector VO, then this instantiation can not apply in this simulation. The
probability of a modifier requiring
C,,,j is therefore zero. A slightly different constraint indicating that CX is
concurrent with P;_, for five to
ten years, where x =j-1, may be logically possible.
Note that the outlining algorithm will select this instantiation only if the
onset of P;_, is proposed
for an older age than the onset of Cj. The simulator can therefore be required
to add C; at an older age
25 than the onset of P;_,. It is important to reconcile this age of onset of
Cj with incidence data for Cj, before
the tentative instantiation.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
61
The simulation algorithm does not require that exacerbation networks reach any
particular health
condition prior to changes in their parent conditions. For instance, health
condition P; may permit
exacerbations to reach condition Ek, while health state P;_, only allows
exacerbations to reach condition
Ek_2. The simulation algorithm may suggest that Ek developed before P;_,,
creating an intermediate vector
such as V; = {P;_,, .. Ek_,}, which is in turn instantly preceded by V;2 =
{P;_,, .. Ek_2}. The simulated
medical history would indicate that the patient developed P; and Ek
simultaneously.
FIG. 7 is a flowchart providing an overview of the stochastic process. In FIG.
7, the stochastic
process begins with defining a test area or subject area to be tested in Step
S2. In Step S4, the sex, race,
and other genetically determined facts are assigned to the prospective
patient. In Step S6, the past
medical history of the patient is generated, by proposing concurrent histories
for each of the health
conditions. In Step S8, the case history that will be accessible to the
examinee is generated for use in
the examination.
In Step 510, the examinee or physician encounters the patient at a
predetermined stage that is
suitable for the examination. The examinee makes a decision as to whether
treatment or intervention is
appropriate, and either performs the treatment or not. The patient is
optionally evolved in Step S12 in
accordance with the examinee's decision and actions performed in Step S 10,
and the examinee may be
optionally tested again in Step S 10.
Stochastic Process History Outlining Example
Consider an examination of the management of osteoarthritis. Among several
cases in this area
is one describing a patient with an acute flare of osteoarthritis of the knee.
The case presents with
established grade II chronic osteoarthritis, obesity, and No Gastric Ulcers.
No other networks are active
in this case. The health conditions in parallel networks are:
P: Grade 0 Knee Osteoarthritis (OA), Grade I Knee OA, Grade II Knee OA, Grade
III Knee OA
C: Normal weight, Obesity, Morbid Obesity
C*: No Gastric Ulcer, Grade I gastric ulcer
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
62
The health conditions Grade I Knee OA and Grade II Knee OA are associated with
exacerbation
networks:
Egrade-II: Baseline Knee OA, Acute Flare of Knee OA
Egrade-I: Baseline Knee OA
The presenting vector is
Vo = {P3, E2, C2, C', }
_ {Grade II Knee OA, Acute Flare of Knee OA, Obesity, No Gastric Ulcer}
The "lead to" relations required for history generation are PL,_12, PL2_13,
PL3_14; EL,_,,, EL2_11; and
CL,_12. The "lead to" relations required for evolution are PL3_,4; EL2_,,;
CL2_11, CL2_13, and C*L,.12.
The normal health condition in the Egrade_õ exacerbation network, Baseline
Knee OA, may be
instantiated twice. The Acute Flare of Knee OA health condition is incident,
and all other conditions are
prevalent.
Age-specific prevalence data aboutthe presenting health condition in the
primary network, Grade
II Knee OA, conditioned on sex, race, and other essentially predetermined and
generally permanent
patient characteristics are provided.
The probability of generating a white female patient, given a case of Grade II
Knee OA is
asserted to be 63%, the fraction of all OA cases found to occur in white
females.
When sex and race are selected, the state of the prevalence node is defined.
The prevalence node
supplies the prevalence of Grade II Knee OA in white females as a shape
defined by the points {(0 years,
0%); (25 years, 0%); (35 years, 0.2%); (60 years, 5%); (100 years, 45%)}. The
prevalence of Grade II
Knee OA at any specific age is found by linear interpolation, so that the
prevalence at age 20 is zero, and
the prevalence at age 80 is 25%. The rapid rise in prevalence from age 60 to
100 suggests a high
probability of generating a very old patient, because these data do not
reflect the scarcity of very old
people.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
63
To correctly simulate the age distribution of patients, an absolute prevalence
is calculated using
formula 1. Assume that the prevalence of the special condition "Alive" for
white females is a roughly
sigmoid curve with a median survival around 78 years, such as {(0 years,
100%); (1 week, 99.9%); (1
year, 99.8%); (15 years, 99.5%); 20 years, 99.2%); (50 years, 95%); (60 years,
85%); (80 years, 30%);
(90 years, 8%); (99 years, 05%); (100 years, 0%)}.
Formula I produces absolute prevalence weights including the points {(0 years,
0); (2 years,
0%); (35 years, 0.2%); (50 years, 2.9%); (60 years, 4.25%); (80 years, 7.5%);
(90 years, 2.8%); (99 years,
0.2%); (100 years, 0%)}. The peak absolute prevalence (8.77%) of Grade II Knee
OA therefore occurs
at age 73 rather than age 100, and absolute prevalence is skewed toward
younger patients, so that the
median age of prevalent cases is 71. The product of the Alive and Grade II
Knee OA prevalence is
similarly multiplied by the prevalence ofthe Obesity and No Gastric Ulcer
conditions. This could further
skew the age distribution away from the elderly as obesity, a risk factor for
death at relatively young
ages, is less prevalent in older patients.
Finally, the incidence of Acute Flare of Knee OA is obtained, if it is
available. Since this health
condition is part of an exacerbation network, it might be safely assumed to be
equally likely to occur at
any age where its parent, Grade II Knee OA, is present, if the incidence of Ek
is not specified. In this
case, no further adjustment to the prevalence product produced above is
required.
In general, the incidence shape for an incident health condition can be
multiplied by the product
of the prevalence shapes obtained above. One year is chosen at random from the
resulting distribution
in a weighted random selection process. We will assume that the process
selects age 70 for this patient's
presentation. This means that a white woman with a history of Grade II Knee
OA, Obesity, and No
Gastric Ulcer, presents at age 70 with an acute flare of her osteoarthritis.
The next process generates the past medical history of the patient, by
proposing concurrent
histories for each of the health conditions in the presentation vector Vo
{Grade II Knee OA, Acute Flare
of Knee OA, Obesity, No Gastric Ulcer}. The first step in this process traces
health condition transitions
as illustrated in FIG. 8.
CA 02369425 2001-10-03
WO 00/60431 PCT/US0O/08942
64
As illustrated in FIG. 8, the Acute Flare of Knee OA is incident, so that its
precursor, Baseline
Knee OA, must be present in vector V_,={Grade II Knee OA, Baseline Knee OA,
Obesity, No Gastric
Ulcer}. The age of onset of V_, and the preceding vector V_2 are obtained
simultaneously by predicting
when each element of V_, might have developed, and asserting that the last
predicted change did occur.
Grade II Knee OA, an element of vectors Vo and V_,, will eventually evolve to
Grade III Knee
OA. A history generating relation, Grade II Knee OA leads to Grade III Knee
OA, describes how long
this might take, perhaps 5 to 10 years. If this relation posits a shorter
interval between these conditions,
then the simulation is constrained to produce patients with a recent onset of
Grade II Knee OA. If the
history generating relation posits a longer interval, then patients may have a
long established
osteoarthritis condition.
Grade II Knee OA is prevalent in vector V0, presenting at age 70, and with no
more than 10 years
allowed for evolution to Grade III knee OA, the earliest age at which the
grade II condition could have
appeared is 60 years. If so, this patient remained a longer time than usual in
Grade II Knee OA, and the
transition to Grade III Knee OA is expected shortly. The patient is most
likely to have developed Grade
II Knee OA between age 65 and 70, among a cohort in which no one would have
progressed to Grade
III Knee OA by age 70. If the incidence of Grade II Knee OA rises from age 60
to 70, the product of the
reversed cumulative PDF and the incidence shapes will be further skewed
towards later ages. We will
assume that age 65 years is randomly selected from this product.
A similar procedure produces an age of onset for obesity. A history generating
relation, Obesity
leads to Morbid Obesity, describes the length of transitions, perhaps 10 to 25
years. Obesity is prevalent
in V0, so a reversed cumulative PDF is multiplied by the incidence of Obesity,
and an onset age between
45 and 60 is proposed.
The No Gastric Ulcer element in Vo is a stage 0 condition, which might evolve
to stage I at some
time. Since the incidence of stage 0 conditions is always between 0 and 100%
at birth, but is always 0%
after birth, so that the duration PDF is irrelevant to the selection of the
age of onset, as long as the
reversed cumulative duration PDF is non-zero at birth.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
Finally, the Acute Flare of Knee OA condition has a known onset time, at age
70. The history
generating relation, Baseline Knee OA leads to Acute Flare of Knee OA,
describes the duration of
Baseline Knee OA, perhaps 3 to 12 months. If the duration of acute flares is
very short, and there are
no other conditions in the exacerbation network, then this PDF also describes
the periodicity of flares,
5 given the presence of Grade II Knee OA. If specific incidence data for the
acute flare condition are not
available, the incidence of the parent condition for the exacerbation network
(Grade II Knee OA) can be
substituted. The product of the reversed (but not cumulative) duration PDF and
the incidence supplies
a distribution from which to select an age of onset, for instance 69 years, 7
months. Since this is the
oldest age proposed, it is selected and instantiated. Step 2 of this process,
illustrated in FIG. 9, is
10 analogous to Step I described above, and therefore, no additional
discussion is described herein.
Finding Generation for Stochastic Process
Finding generation adds detailed descriptions of patients' features to the
outline generated in the
steps above. Beginning with a healthy newborn patient (or embryo) of the
specified sex and race, the
15 finding generation process assigns values of specific findings expected in
healthy individuals. These
may change when the patient develops a new health condition at the age
selected by the outlining
process.
The patient's detailed features are generated using modeling instructions
stored as Reasoning
elements with health conditions. Specific findings associated with normal
health are created in a
2 0 sequence indicated by these instructions. Each Specific finding is
initially defined from the onset of life
until age 100. For instance, the patient's height is derived from a randomly
generated percentile and a
set of shapes resembling a pediatric growth chart extended to age 100. The set
of shapes used may be
conditionally dependent on the sex, race, and any other established facts
about the patient.
The finding generation process should generally create dependent findings,
e.g., knee pain,. after
25 generating the findings upon which they depend, e.g., joint space
narrowing. Careful selection of
findings to represent may reduce some dependencies. For example, the model in
general is more robust
if height and body mass index are considered to be independent findings, and
weight is not calculated
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
66
until explicitly requested during a simulation. Therefore, the model in
general is more robust if height
and body mass index are considered to be independent findings, and weight is
not calculated until
explicitly requested during a simulation. Most findings are instantiated as a
series of pairs of values and
ages. Values at other ages may be found by linear interpolation.
Findings may vary with predictable circadian, lunar, and annual rhythms,
described by shape
subpatterns. Shape subpatterns can be combined with a shape to produce
fluctuations on realistic
temporal scales.
Finding distortions illustrate events having temporary effects on the shape of
some value. For
instance, a temperature shape during a febrile illness might be 39 C, with a
distortion pattern indicating
a I C drop for four hours following administration of acetaminophen. The
exact temperature reported
at a given time would depend on the current value of the lifetime temperature
shape and whether the
patient consumed acetaminophen in the last four hours.
After determining patterns for all findings present at a point in time, the
simulator proceeds
forward in time to the next health condition vector. The simulator updates
findings for the new situation.
This loop continues until the computer has described the findings of the
patient in the final health
condition vector.
Using Pre-Generated Patients
In accordance with one design of the present invention, when the computer
based examination
system generates and evolves a random patient, it cannot reuse the patient
information if the patient is
evolved once. That is, every time the examination is executed, we need to
generate a patient to continue
the test. Not only does the process of generating a patient take tremendous
time, but also the evolved
patient cannot generally be tested again in the future.
In accordance with another design of the invention, the patient is pre-
generated, evolved and
stored in the Whiteboard database. The presentation system can test the
patient in countless time if
wanted. Furthermore, different physicians can test the same patient at the
same time.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
67
FIG. 10 is an illustration of the entity-relationship model when patients are
not pre-generated,
and FIG. 11 presents the modified entity-relation diagram of the modified
Whiteboard database when
the patient is pre-generated. Each node represents a status of a patient with
parallel health states. For
example, when a patient is generated, he or she is located at node 1, the
patient might be evolved to
several status located at node 2, 3, 4..., etc. Therefore, a patient can have
many nodes.
Many nodes can share same EXHIBITS and HAS. For instance, when a patient is
evolved to a
severe knee problem, we first take out the most updated EXHIBITS of the
previous node, modify it and
then write it to the new node, and at the same time generate a new EXHIBITS
for the new node. The
new node will point to the EXHIBITS prior to the most updated EXHIBITS of the
previous node. If
nodes are in the same content area, they also share the same FINDINGS and
PATTERNS, but their
shapes are different, which can be found in table Pattern_Shape.
Since different physicians can use the same patient for the test at the same
time, the
corresponding action contents needs to be given for each physician. Therefore,
every time a patient has
a new node, we also generate the patient's action contents. When the physician
gets to the patient with
the specific node, the action contents are copied to physician_actions tables.
The table ACTIONS, HEALTHSTATE and ACTION_HEALTHSTATE are pre-generated, and
a corresponding utility integrated with pre-generating COA is created.
Accordingly, the evolution
process for pre-generated patients is, for example, as follows:
a) Based on the parallel health states of the patient at the specific node,
fetch all corresponding
actionID from action health state.
b) Based on the possible target of each actionlD, construct all combinations
that lead to
different parallel health states.
c) Create a new node for each possible action combination.
d) Copy the SHAPE from old node to the new node.
e) Construct a tuple in table NodeToNode where the action combination, old
nodelD and new
nodelD will be stored.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
68
Generating Patients with Parallel Health State Networks
A detailed description of parallel health state networks is now described. We
have determined
that parallel health state networks provide a model with a reasonable
biological basis, more easily
defined data, greatly improved reuse potential, and a better segmented
implementation. Evolution of
synergistic health problems (e.g., vicious cycles) are managed using
structures from the original data
model. A working patient generation process is creatable using the parallel
network model.
We have determined that the number of conglomerate health states expands
combinatorially, and
the incidence and duration of these conglomerate health states is often a
matter of speculation or is
redundant with previously stored information.
We have also determined that a parallel network approach improves on the
accessibility and
reusability of health state data, while retaining the ability to handle the
dependencies inherent in
synergistic cycles.
Humans are composed of inter-dependent cells organized into tissues and
organs. Some tissues
directly or indirectly control the state of cells in other organs through
mechanical, neurohumoral, or other
processes.
An individual's health reflects the current health of all of these cells.
Therefore, a very high
resolution model of the life of a human body might describe the histories of
the cells comprising the
body, including their dependency on other cells. In clinically recognizable
processes, the cells
comprising one tissue share similar structure, function, and health with many
of their immediate
neighbors. Their health may diverge rapidly from the health of the cells in
other tissues. Therefore, a
model concentrating on the histories of tissues retains considerable
resolution.
Each tissue can be imagined to evolve on its own standard schedule unless some
local insult
occurs, or an insult to another tissue alters the schedule. The normal tissue
schedules proceed in parallel.
For instance, bone, Islets of Langerhans, nephrons, and retinal tissue all
gain and lose function at
predetermined rates. If bone loses function (strength), a local pathological
parallel process (fracture)
becomes more likely. If Islet cells lost function (insulin secretion), distant
pathological parallel
CA 02369425 2001-10-03
WO 00/60431 PCT/USO0/08942
69
processes in nephrons and retinas become more likely or progress more rapidly
(diabetic nephropathy
and retinopathy).
Without parallel networks, distractors, such as randomly appearing colds or a
history of
appendicitis might require many conglomerate states. Also, information
collected for one disease
domain might have to be completely replicated in other domains (for instance,
obesity descriptions would
occur in osteoarthritis, diabetes, hypertension, combinations of the above,
and independently).
We have also determined that many therapeutic complications are acute site-
specific illnesses
superimposed on an antecedent illness. On the other hand, some problems
interact in synergistic cycles:
Osteoporosis increases the likelihood of fractures, and immobility (following
a fracture) increases the
rate of progression of osteoporosis. Consequently, many of the most
interesting disease processes are
intertwined with others. In a network of conglomerate health states, these
dependencies can be explicitly
described at nodes and along edges between nodes. In a parallel network model,
the interacting networks
must be aware of each other.
This view of health and function, we have determined, suggests a definition of
parallel health
state networks: A parallel health state network for a tissue describes a
collection of clinically
discoverable and mutually exclusive states in which that tissue may exist, and
possible transitions
between states. For example, the normal development of a tissue, described
from a person's birth to
death, is one distinct state in a network.
Physically separated cells ofthe same tissue type may exist in very different
states. For instance,
the left and right knee joints are susceptible to pathologically
indistinguishable osteoarthritic changes,
but one knee may exhibit more advanced changes than the other. Therefore,
parallel networks require
identification of involved sites.
A parallel network is, not coincidentally, a disease staging scheme. Parallel
networks for chronic
diseases are typically restatements of familiar staging concepts (e.g., Stage
0 or no. disease, followed by
Stage I or mild disease, and so on). The parallel network illustrates these as
sequential stages, even in
acute processes such as ankle sprains or burns. A third degree burn is always
preceded by a second
degree burn, if only for the briefest moment of time.
CA 02369425 2001-10-03
WO 00/60431 PCT/US00/08942
Parallel networks alter knowledge acquisition and storage requirements, as
well as patient
generation algorithms, when compared to conglomerate health state models.
Diagnoses previously
combined in a conglomerate state become distinct states in different parallel
networks. The
conglomerate health state of the body is described by a vector indicating the
current status of all parallel
5 networks.
Illustrations of their disease domains help medical experts understand the
scope of their
knowledge acquisition task. Initially intricate domain models were decomposed
into much less
threatening parallel networks. FIG. 12 illustrates parallel network
structures. The simplest network is
a collection of one or more static states, typical of genetic (e.g., Down's
syndrome) and some congenital
10 conditions (e.g., anencephaly). The progressive network is a series of
states with no cycles, typical of
degenerative illnesses such as osteoarthritis. The reversible network
illustrates chronic but reversible
conditions, such as essential hypertension and weight disorders. In the injury
network an acute insult
evolves to either recovery or a chronic condition with a later recovery.
Injury networks describe many
infectious diseases and trauma.
15 The addiction network illustrates that a person may abstain from, use,
abuse, or become addicted
to something; in the current model, a previously addicted person can only be
addicted or recovering, but
cannot return to abstinence, use or abuse. The surgical intervention overlay
illustrates that new states
can be added to the above networks using irreversible therapies such as
radiation or surgery.
Parallel networks of three types are identified. The primary network contains
the diseases that
20 define the domain, such as diabetes mellitus. The second type of network
contains a risk factor for
progression through the primary network, such as obesity. The third type of
network includes
complications attributed to states in the primary network or its management,
such as retinopathy.
We have also determined that the following information is used to create
parallel networks: 1)
how long a risk factor should exist before it could influence a transition
between states in a primary
25 network, 2) the time required for transitions in the primary network, given
different combinations of risk
factors, and 3) the number of passes an individual patient should be allowed
to make through a cycle
(e.g., from acute injury to recovery back).
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
71
The data model objects originally intended to store risk factors included a
"Person HAS Health
State" relation, which identified a health state, its onset and duration. In
addition, HAS relations
indicates a preceding HAS relation to support tracing of medical histories.
These attributes are adapted
to describe parallel synergistic networks.
The patient generation process uses a weighted random process to select all
times and events,
starting with an age of onset for a health state on the primary network. Risk
factors are selected next.
Unlike the conglomerate health state patient generation algorithm, any
diagnoses associated with altered
risk must be described in a parallel network. The plausible range of duration
for each risk factor is stored
in a HAS relation, and used in selecting its onset age. If the risk factor
evolves independently of the
primary network, the HAS relation does not indicate a preceding HAS, and the
algorithm creates the risk
factor history using default assumptions in its parallel network. If the
primary network does interact with
the risk factor, the preceding HAS relations provide time constraints that
promote plausible concurrent
evolution of the primary and risk factor networks.
The original history generation algorithms are used within independently
evolving parallel
networks. Consequently, the system continues to support conglomerate health
states described as a
parallel network. In contrast to the conglomerate health state model, the
parallel network technique may
require explicit and separate generation of the histories of the primary
network and any number of risk
factors.
Computer Implemented Process
The process of the computer based examination or assessment system is
described in detail in
connection with FIGs. 13-14. The computer implemented process includes the
overall concept that the
physician is presented with an examination, and the process generates multiple
instances of patients.
These generated patients represent clinical scenarios that a physician would
have to go through to
administer proper treatment. These scenarios are stored in a white board
database which stores both the
database implementation (i.e. the patients stored in data structures), as well
as computer codes which
operate from base structures including information on physician.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
72
There are three basic actors in the computer based examination system:
physician, white board
and patient generator. The physician/examinee initiates the white board action
by logging in. Once the
examinee logs in, then the white board makes one or more requests to the
patient generator. The white
board generally provides the patient simulator with the basic testing area.
The patient generator then
starts the process of generating the patient and evolving backwards, and
optionally forwards in time for
pre-generated patients. Thus, the computer based examination system includes
separate programming
objects in the general C++ programming sense for physician, the patient and
the white board.
In general, the physician/examinee pre-registers to take the examination, and
provides (or the
system already has stored) detailed background information on the physician,
areas of weaknesses, prior
examination information, and the like. Thus, the physician logs-in to the
computer based examination
system in Step P2, and the system validates the physician in accordance with
predetermined criteria, e.g.,
user ID, password, correct examination, and the like.
The physician/examinee is either presented with an optional list(s) of subject
areas for
examination or mandatory subject areas for examination in Step P4, responsive
to information stored in
the whiteboard database via requests thereto in Step P12. Alternatively, the
examination areas might be
hidden, and the examinee might be told that this is a diabetic problem, with
certain management issues.
The examinee may optionally have a series of selections, whether it is in
terms of individual patients or
they could be in specific areas.
In some instances, the examinee may be provided a patient with some specific
statements about
the patient. The computer implemented process may optionally determine whether
the physician has
been examined before. If the answer is yes, then the physician might require,
for example, five of fifteen
specific subject areas for the examination, of which one or more would be
available for testing.
In addition, prior performance of the physician may also be considered using a
pre-stored or
generated physician profile via Step P6, and requests to the prior physician
performance via Step P8.
The specific exam content is then requested in Step P10 responsive to at least
one of physician profile,
prior performance, content areas. Accordingly, one or more of prior
performance, the physician profile,
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
73
the content of the examination, are used to provide a selection list of the
physician to choose from in Step
P14.
Depending on the above information, the patient generator process is then
initiated to create a
patient for the examination in Step P16. The patient generation process may be
performed in Step P18
in real-time for each patient, or may be pre-generated as described above.
Under the real-time scenario,
the selection of a problem area in Step P14 translates into a target health
state or area.
For example, if the problem area selected was diabetes, the target health
state in the knowledge
base would be diabetes. Using the target health state, there are generally a
plurality of health states
associated therewith. The computer implemented process then optionally
randomly selects one of these
areas as a precursor health state in Step P20. For example, a mild case of
diabetes may be the precursor
health state for normal health state of diabetes.
The selection of the precursor health state is based on, or calculates, onset
age in Step P22 via
incidence data in Step P24. The history generation computer process is a
mechanism that sets up a
reasonable beginning time and ending time for the patient that is being
presented. The computer process
chooses a target health state, precursor information, sex and race from the
target health state, and
establishes the age of the patient. The computer process then moves backwards
in time to establish onset
age when the condition occurs, and proceeds backwards in time all the way to
the normal state. Next,
the process moves forward in time to determine potential subsequent health
states for the patient based
on a variety of possible interventions performed by the examinee. Thus, the
process has two stages.
Depending on the precursor information/health state, information such as the
sex and race, along
with disease prevalence in Step P34, mortality data Step P36 and incidence
information in Step P24, are
used to select the specific sex and race for the simulation.
The mortality data is based on sex and race. The sex and race is selected from
the health state,
incidence or prevalence data and sex and race specify mortality data. For
example, if the health problem
that is presented to the examinee is new to the patient, then it is incident
(e.g., a recently broken bone).
Alternatively, if the health problem is an old established problem such as
long term diabetes, the health
problem is prevalent. Thus, the incidence and prevalence is inserted into the
patient case history over
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
74
and over again depending on the particular problem. Accordingly, a pre-
determined decision is generally
made as to what types of disease are to be tested, prevalent disease or
incident disease.
The sex and race selection uses the disease prevalence and mortality data in
the sex and race
selection process. The mortality data and disease prevalence are used to
establish the reasonable ages
and sexes, and also ages of on[-]set. For example, this mechanism prevents
generation of pregnant
males, 14 year old Type-II diabetics, and the like.
For example, the computer system determines, or is instructed as described
previously, that the
problem area is arthritis. The sex, race, and age of the patient are
determined, for example, at the point
in time where treatment may be necessary. The patient history is then
generated back through the
process/time to establish onset times of the various different health states.
That is, from, for example,
the point in time where the arthritis is severe, the patient history is
generated at a point when the arthritis
was mild, and back to when the arthritis was substantially normal.
When the sex and race selection process is completed via the combination of
sex and race
selection in Step P38 and onset age calculation in Step P40, a patient has
been generated at a specific
point in time with a specific health state problem and the characteristics of
that problem. Thus, the
computer process has generated the patient, moved backwards in time from the
disease onset age all the
way back to normal. For example, if the computer process started with a mild
condition for a specific
disease, the computer process goes backward one time interval to normal from
mild. If the computer
process begins with moderate, the computer process will move backward in time
from moderate.
As a result of the computer process, a patient template is also generated in
Step P26 using the
onset age determination in P40 and sex and race selection in Step P38. In
addition, the generated patient
is given a name in Step P28, and age including a date of birth in Step P30.
The physician/examinee is
then provided with the history of the patient for use in diagnosing or
prescribing treatment for the
generated patient. The patient history includes, for example, age of the
patient, race and sex. Up to
this point in the computer process, the patient is created. From this point of
the examination/computer
process and forward, the patient and physician's interaction with that patient
determine both the
information provided to the physician/examinee, as well as potential evolution
of the patient. Changes
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
in the patient's characteristics is a function of physician's action or
inactions using the evolutionary
process described below.
The evolutionary process is performed using the knowledge base structure or
entity relationship
model described in detail above. The knowledge base structure has been
separated from the white board
5 structures described above for administrative purposes, but alternatively
may also be combined therein.
The knowledge base represents all the information that does not necessarily
have to go with the patient
for purposes of presentation to the examinee. The knowledge base includes
information used to create
the patient and provide instances of information.
However, separating the knowledge base from the white board structure has the
advantage that
10 the computer generated patients do not require as much data to be
transported therewith. Accordingly,
a separate structure is created called the white board structure. The white
board structure advantageously
includes the information required to generate the patients and to present the
patients to the
physician/examinee. The white board structure includes information containing
patient description and
all the findings that are typically generated that are not necessarily related
to the problem, for example,
15 blood pressure, blood glucose, and the like.
That is, the white board structure provides all information that is generally
available to the
examinee, such as information satisfying examinee queries on prior history,
laboratory tests, and the like.
In addition, when pre-generated patients are used, all findings associated
with the patient including all
pre-generated evolutionary states are also stored in the white board data
structure.
20 For example, if the patient had moderate arthritis, the patient may
generally transition to two
other health states: severe arthritis, or mild arthritis. Thus, in one
embodiment of the invention, the
computer process pre-generates the possible health states for the patient.
According to this
embodiment where the patient is pre-generated, the process of evolving a
patient may, in some
circumstances, be more computationally efficient than to generate the patients
dynamically. Thus, for
25 pre-generated patients described above in detail, all possible states are
generated ahead of time and then
used by the white board structure in accordance with the pre-generated state
when activated or selected
by the examinee.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
76
The white board accesses the patient template in Step P42, and generates the
patient record in
Step P46, responsive to requests initiated by the white board to the patient
history information in Step
P44. The patient record is not generally reviewable by the examinee, except on
individual requests by
the examinee in Step P48. The examinee requests information from the patient
record in Step 48 which
provides the examinee the physical view of the patient. For example, the
patient's blood pressure may
be stored in the patient record for retrieval by the examinee. Other examples
of information stored in
the patient record include chief complaint, past medical history, past patient
behavior or compliance
information.
The white board will also generate examinee actions and patient interventions
in Step P52 by
reviewing and evaluating the physician intervention in Step P50, responsive to
the patient record. The
examinee actions and patient interventions contribute to the patient evolution
conditions used in the
patient evolution process described above in detail.
Whether the patient is pre-generated or not, the computer process/patient
generator generates the
initial patient, and subsequently evolves the patient, and subsequently
presents same to the examinee.
The patient is generated by the patient generator accessing the patient
evolution conditions in Step P54,
the target health state in Step P56, and any existing parallel health states
in Step P58. The patient is
evolved by the patient generator in Step P60 to the evolved health state,
which may become the target
health state in Step P62.
At this point we have a patient on the white board presented with a particular
health state, which
typically is the form of a chief complaint. From this time on, the
examinee/physician takes control of
the process, and nothing is going to happen in the computer based examination
system unless the
examinee/physician does something, unless the health state is time dependent
and able to advance to
another state automatically, such as by inaction on the part of the examinee.
For example, if the health state is an acute problem, such as a heart attack,
there may be a time
dependency built in that is going to force some action of the physician within
a specific time before the
patient experiences another heart attack. In this example, the examinee using
the computer based
examination system may dismiss the patient, the patient will walk out of the
doctor's office/hospital, and
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
77
the examinee would receive notification that the patient just showed up in the
emergency room with a
problem.
Alternatively, ifthe examinee is too slow in diagnosing an illness, the
inability to treat the patient
in a short period of time may also result in the patient progressing to a
different health state. For
example, a patient that has a heart attack might progress to a more serious
state if the examinee does not
perform corrective measures very quickly while the patient is, for example, in
the hospital. In general,
allowing time to elapse without intervention is an intervention choice along
with the other active
interventions that an examinee might choose.
In orderto determine the target health state, the "iterate until normal
reached" process is initiated
via Step P64 which sets one or more pre-cursor health states to the target
health state. The "iterate until
normal reached" process iterates in Step P66 until the normal health state is
reached backward from the
target health state. For example, if a mild health state is selected, the
precursor health state is normal.
The "iterate until normal reached" process also establishes one or more
optional parallel health states in
Step P68. Precursor parallel health states are then generated as needed in
Step P70, which are then used
to contribute to the patient history in Step P72.
The computer based examination system ensures that the age of onset for the
various parallel
health states is reasonable. Thus, the process of generating precursor health
states for the parallel health
state is a multi-dimensional process of monitoring health states to be
consistent, to prevent unreasonable
scenarios, time frames, and the like. If the parallel health states are
related, they have to be related to
each other sufficiently enough so that the evolution of health states makes
sense.
The parallel health states are also used to establish the findings in Step
P74, which contribute
to the patient history in Step P76. While the above steps have been described
in, more or less, a
sequential manner, it should be clear that the various steps described herein
may be performed in parallel,
independently, and/or non-sequentially, as needed or for computational
efficiency.
Advantageously, the computer implemented process includes the capability of
utilizing parallel
health states as part of the patient generation process, which is described
above in detail. As part of the
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
78
generation process, a decision is generally made to include or exclude those
particular parallel (e.g.,
morbid or co-morbid) health states along with the original state of the
disease.
We have determined that sometimes health problems tend to be concurrent, but
they are not
generally defined as being necessarily interrelated. The computer based
examination system provides
the feature of handling a plurality of health states, either related or not
related to each other. For
example, there tend to be lots of people with diabetes and high blood
pressure. Accordingly, we define
these two health states as related to each other. Alternatively, the plurality
of health states may be
considered to be substantially independent and still within the scope of the
computer based examination
system of the present invention.
The present invention further provides the feature of dealing with parallel
health states
substantially or completely independent of each other to permit dependent or
independent management
decisions. For example, a person that has diabetes and high blood pressure
generally requires slightly
different management decisions than a person who just has high blood pressure
or a person who just has
diabetes. For example, the physician/examinee may prescribe a more expensive
anti-hypertensive drug
if the patient has both diabetes and high blood pressure because of potential
complications unique to the
combination of health states. Thus, the computer based examination system may
be used to determine
whether the examinee has made the appropriate management decision.
Alternatively, the computer based
system may be used to collect various responses from different well recognized
physicians to establish
a minimum level of care for insurance companies, health care organizations,
other physicians, and the
like.
The present invention also provides the feature of providing distractions when
attempting to
diagnose the disease/illness. That is, the patient may include symptoms and/or
indications that might
be related to the problem seemingly presented to the examinee, but, in fact,
these indications distract the
examinee along an inappropriate path, such as excessive testing, over-
prescribing medications, and the
like. Accordingly, we have also determined that distraction makes a good
argument for having parallel
problems.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
79
At this point and time, the computer based examination system selects an area
for examination,
and is in the process of working backwards in time. The process iterates from
precursor health state
down to the normal health state, where at each precursor
state the process considers potential co-morbid problems. Both the precursor
or subsequent health states
are the primary problem, and the parallel health states generate findings. The
findings are a part of the
patient history. For example, a finding of obesity might be a change in
weight. The process moves
backwards while at the same time looking at potential parallel health states
have been substantiated. The
history of findings are generated at the white board level.
Now if the physician takes some action at this point in time that causes
patient evolution (that
is, the physician causes some action which the white board is checking at this
point and time), the white
board matches the action up against something that is going to cause patient
evolutionary health state
change. The white board then makes a request to the patient generator to
evolve the health state.
If the full patient has been generated on the white board, then the patient
generator is replaced
with the white board itself to provide a pre-evolved patient from memory. If,
however, the knowledge
base is linked for a dynamic situation, then the patient generator dynamically
evolves the patient. In
either situation, the evolved health state becomes the target health state at
this time. For example, the
health state has evolved from mild to moderate arthritis, or from mild obesity
to moderate obesity.
The computer implemented process also includes the possibility of treating
patients with
management health issues that do not generally become totally normal (e.g.,
long term diabetes, arthritis,
and the like), as well as health conditions that may return to completely
normal (e.g., broken bone, and
the like).
In fact, we have determined that it is particularly likely that patients will
revert to normal
conditions when the patient experiences an exacerbation health state/condition
for the computer based
examination system. For example, we have determined that an exacerbation
condition can have, for
example, mild, moderate and severe states. If the patient has a moderate
exacerbation, there is a chance
that the patient experienced a mild exacerbation before evolving to the
moderate state. There is also a
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
chance that the patient had a severe exacerbation, is now recovering, and may
return to the normal state
a few minutes later.
In summary, the computer based examination system utilizes three actors, the
white board the
physician and the patient generator. The examinee initiates the whole process
by logging in. The
5 examinee logs into the white board, and the white board accesses various
information to determine
whether the examinee is valid. If the examinee is valid or verified, the white
board looks up the
examinee's profileto determine anybackground specifics on the examinee, such
as specific areas needing
improvement, past examination results, and the like.
The white board then determines or is provided the exam content, and then
contacts the patient
10 generator. The patient generator begins the generation process, selects the
disease or subject area, and
controls the actual combinations of health states and co-morbid health states
via a case structure. The
case structure controls both the presenting health state as well as the co-
morbid health state. The case
structure filters the generation process and makes a predetermination to
eliminate predetermined
impossible situations, or difficult or unimportant situations that are not to
be used in the testing. The case
15 structure indicates that even though a specific health state or parallel
health state is in the knowledge base
and even potentially legitimate, the case structure will not present that
problem. Thus, the case structure
simply controls which of the health states will be presented to the examinee,
and which of the co-morbid
health states, and possibly flare states will also be presented to the
examinee simultaneously or
sequentially.
20 The white board then retrieves the patient template including, for example,
the patient history,
the chief complaint, the assessment test, and the like. From this time on, the
examinee performs some
action by either requesting data which is controlled by the white board or by
causing, directly or
indirectly, some action to take place. Once an action is performed, the
patient may be evolved to the next
health state by the patient evolution process.
25 Both the request of information and the review and evaluation of the
examinee's actions or
intervention are generally handled by the white board for convenience, but
multiple control mechanisms
may also be used. If the white board sees there has been a change in health
state for the patient, then the
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
81
white board would then go to patient evolution process to initiate the
evolution, and request the patient
generator to provide information regarding the evolved patient.
The patient evolution information may optionally be pre-generated for
computational efficiency.
That is, even when patients are created dynamically, some predetermined
evolution information maybe
ready for use by the computer based examination system based on the potential
of the possible evolution
periods/health states. For example, if the target health state was moderate,
the computer based
examination system may have predetermined onset time of moderate, and
therefore, know the time for
mild, normal, severe, and the like. In effect, the white board database/object
optionally includes a
complete possible look at the future appearance, as well as the past
characteristics for this particular
patient at a particular health state.
The underlying goal of the computer based examination system is that the
evolutionary process
is generally the same as the patient generation process. Both processes are
generally the same, just the
generation process has more steps to generate the patient. In the evolutionary
situation, the computer
based examination system deals with multiple possible health state successors
in different parallel
networks. The zero state, or state where the examination begins, generally has
a primary health state like
moderate arthritis, possibly a flare state such as an acute swelling in the
knee, and comorbid states such
as overweight.
To generate the patient history, the computer process take the moderate
arthritis, the flared up
knee, and the overweight condition and looks backwards in time to determine
the most recent precursor
state. For example, the precursor of the moderate arthritis could be mild, the
precursors of the flare could
be baseline or normal, and the precursor for the overweight condition could be
normal weight. The
computer process sets the patient's current age, for example, as age 50, and
now moves backward in time.
For example, for a 50 year old person with moderate arthritis, it is likely
that the arthritis began
5-10 years ago. With respect to the flared knee, it is likely that this
condition began within the last
couple of days. For the overweight condition, it is likely that the 50 year
old person began this condition
CA 02369425 2001-10-03
WO 00/60431 PCT/US00/08942
82
10-15 years ago. Therefore, there is a 5-10 year interval (arthritis), a 3 day
interval (flared knee), and
a 10-15 year interval (overweight).
The computer process moves backward in time to that last change that should
have occurred. In this
situation, the first precursor health state is the flared knee which occurred
3 days ago. The clock then
gets reset, and the next earliest precursor health state is determined. The
whiteboard generally throws
away all the previous information that was used to generate the later
precursor health state, and
recalculates the next earliest precursor health state until all precursor
health states are generated to the
normal condition for all conditions/diseases.
Switching to the forward version, the patient evolution process, the computer
process looks
forward in time instead of backwards in time. Therefore, considering the above
example, there may be
a change in 3 days (knee flare), another change in 5-10 years (arthritis), and
another change in 10-15
years (overweight). The next change that will occur will be in 3 days. That is
the evolutionary process
which, similar to the patient history generation process, recalculates onset
times for each subsequent
health state. Thus, the main difference between the history generation process
and the patient evolution
process is the data being applied to each process.
The computer based examination system may also be used to determine whether
specific
physicians are practicing cost effective medicine for use by, for example,
insurance companies. The
system can provide objective criteria for treating patients by defining
episodes of care for isolated
problems. For example, the computer system can indicate approximately the
amount of money to spend
on a patient with a heart attack with no other concurrent problems, for an
asthmatic patient per year, and
the like. The computer based examination system and process provides a "flight
simulator" where
the physician can practice specific preferred forms of treatment, as
appropriate. For example, if the
patient has a heart attack, the examinee/physician should generally prescribe
aspirin for long term usage,
but many do not. Thus, the computer based examination system may also be used
as a training system
so that the examinees rehearse a desirable behavior such as prescribing
aspirin after heart attacks. The
computer based examination system can therefore also be used to increase
desirable behavior when the
physician interacts with a real patient.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
83
Consequently, if a particular physician or group of physicians are determined
to be expensive
for an insurance company or health care group, and the computer based system
shows that the physicians
are likely to provide appropriate care, then it is possible that this
physician or group of physicians have
a particularly expensive patient population, and should therefore not be
faulted. Further, the computer
based examination system may also be adapted to receive the specific data
collected by the physician and
interventions associated therewith, to further verify that the practice is
delivering the appropriate
services. Thus, the computer based examination system, may be used to
determine whether a particular
practice is delivering services within predetermined guidelines.
FIG. 15 is an illustration of a main central processing unit for implementing
the computer
processing in accordance with a computer implemented embodiment of the present
invention. The
procedures described above may be presented in terms of program procedures
executed on, for example,
a computer or network of computers.
Viewed externally in FIG. 15, a computer system designated by reference
numeral 40 has a
central processing unit 42 having disk drives 44 and 46. Disk drive
indications 44 and 46 are merely
symbolic of a number of disk drives which might be accommodated by the
computer system. Typically
these would include a floppy disk drive such as 44, a hard disk drive (not
shown externally) and a CD
ROM indicated by slot 46. The number and type of drives varies, typically with
different computer
configurations. Disk drives 44 and 46 are in fact optional, and for space
considerations, may easily be
omitted from the computer system used in conjunction with the
process/apparatus described herein.
The computer also has an optional display 48 upon which information is
displayed. In some
situations, a keyboard 50 and a mouse 52 may be provided as input devices to
interface with the central
processing unit 42. Then again, for enhanced portability, the keyboard 50 may
be either a limited
function keyboard or omitted in its entirety. In addition, mouse 52 may be a
touch pad control device,
or a track ball device, or even omitted in its entirety as well. In addition,
the computer system also
optionally includes at least one infrared transmitter 76 and/or infrared
receiver 78 for either transmitting
and/or receiving infrared signals, as described below.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
84
FIG. 16 illustrates a block diagram of the internal hardware of the computer
of FIG. 15. A bus
56 serves as the main information highway interconnecting the other components
of the computer. CPU
58 is the central processing unit of the system, performing calculations and
logic operations required to
execute a program. Read only memory (ROM) 60 and random access memory (RAM) 62
constitute the
main memory of the computer. Disk controller 64 interfaces one or more disk
drives to the system bus
56. These disk drives may be floppy disk drives such as 70, or CD ROM or DVD
(digital video disks)
drive such as 66, or internal or external hard drives 68. As indicated
previously, these various disk drives
and disk controllers are optional devices.
A display interface 72 interfaces display 48 and permits information from the
bus 56 to be
displayed on the display 48. Again as indicated, display 48 is also an
optional accessory. For example,
display 48 could be substituted or omitted. Communication with external
devices, for example, the
components of the apparatus described herein, occurs utilizing communication
port 74. For example,
optical fibers and/or electrical cables and/or conductors and/or optical
communication (e.g., infrared, and
the like) and/or wireless communication (e.g., radio frequency (RF), and the
like) can be used as the
transport medium between the external devices and communication port 74.
In addition to the standard components of the computer, the computer also
optionally includes
at least one of infrared transmitter 76 or infrared receiver 78. Infrared
transmitter 76 is utilized when the
computer system is used in conjunction with one or more of the processing
components/stations that
transmits/receives data via infrared signal transmission.
FIG. 17 is a block diagram of the internal hardware of the computer of FIG. 15
in accordance
with a second embodiment. In FIG. 17, instead of utilizing an infrared
transmitter or infrared receiver,
the computer system uses at least one of a low power radio transmitter 80
and/or a low power radio
receiver 82. The low power radio transmitter 80 transmits the signal for
reception by components of the
process, and receives signals from the components via the low power radio
receiver 82. The low power
radio transmitter and/or receiver 80, 82 are standard devices in industry.
FIG. 18 is an illustration of an exemplary memory medium which can be used
with disk drives
illustrated in FIGs. 15-17. Typically, memory media such as floppy disks, or a
CD ROM, or a digital
CA 02369425 2011-07-27
video disk will contain, for example, a multi-byte locale for a single byte
language and the program
information for controlling the computer to enable the computer to perform the
functions described
herein. Alternatively, ROM 60 and/or RAM 62 illustrated in FIGs. 16-17 can
also be used to store the
program information that is used to instruct the central processing unit 58 to
perform the operations
5 associated with the process.
Although processing system 40 is illustrated having a single processor, a
single hard disk drive
and a single local memory, processing system 40 may suitably be equipped with
any multitude or
combination of processors or storage devices. Processing system 40 may, in
point of fact, be replaced
by, or combined with, any suitable processing system operative in accordance
with the principles of the
10 present invention, including sophisticated calculators,and hand-held,
laptop/notebook, mini, mainframe
and super computers, as well as processing system network combinations of the
same.
Conventional processing system architecture is more fully discussed in
Computer Organization
and Architecture, by William Stallings, MacMillan Publishing Co. (3rd ed.
1993); conventional
processing system network design is more fully discussed in Data Network
Design, by Darren L. Spohn,
15 McGraw-Hill, Inc. (1993), and conventional data communications is more
fully discussed in Data
Communications Principles, by R.D. Gitlin, J.F. Hayes and S.B. Weinstain,
Plenum Press (1992) and in
The Irwin Handbook ofTelecommunications, by James Harry Green. Irwin
Professional Publishing (2nd
ed. 1992).
Alternatively, the hardware configuration may be arranged according to the
multiple instruction
20 multiple data (MIMD) multiprocessor format for additional computing
efficiency. The details of this
form of computer architecture are disclosed in greater detai I in, for
example, U.S. Patent No. 5,163,131;
Boxer, A., Where Buses Cannot Go, IEEE Spectrum, February 1995, pp. 41-45; and
Barroso, L.A. et al.,
RPM: A Rapid Prototyping Engine for Multiprocessor Systems, IEEE Computer
February 1995, pp.
26-34.
25 In alternate preferred embodiments, the above-identified processor, and in
particular
microprocessing circuit 58, may be replaced by or combined with any other
suitable processing circuits,
including programmable logic devices, such as PALs (programmable array logic)
and PLAs
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
86
(programmable logic arrays). DSPs (digital signal processors), FPGAs (field
programmable gate arrays),
ASICs (application specific integrated circuits), VLSIs (very large scale
integrated circuits) or the like.
The many features and advantages of the invention are apparent from the
detailed specification,
and thus, it is intended by the appended claims to cover all such features and
advantages of the invention
which fall within the true spirit and scope of the invention. Further, since
numerous modifications and
variations will readily occur to those skilled in the art, it is not desired
to limit the invention to the exact
construction and operation illustrated and described, and accordingly, all
suitable modifications and
equivalents may be resorted to, falling within the scope of the invention.
For example, while the above discussion has separated the various functions
into separate
functionality, the functions may be combined, physically and/or logically, and
various functions may be
combined together. While combining various functions may make implementation
details more
cumbersome, nevertheless, the functions described herein may still be
accomplished to advantageously
provide some or all of the benefits of the invention described herein.
As an additional example, the foregoing discussion focused exclusively on
medical applications
of the current invention. Advantageously, the invention applies equally well
to creating simulations of
other complex systems, particularly complex systems in which an empiric
description is easier to obtain
than a comprehensive mathematical description. The concepts in the invention
correspond to generic
concepts that apply to complex systems in general. The labels in the current
invention and the generic
concept are listed in the table below.
The Population (or Person or Simulated Patient) concept represents any complex
system.
Consider a nuclear power plant. All breeder reactors form a population of
breeder reactors, and each
individual breeder reactor is an independent complex system within that
population. The Record concept
again reflects the knowledge of the system held by either people or computers.
The breeder reactor may
have its own Record of itself stored in a computer that supervises its
operations. The public media and
the Department of Energy will maintain other Records regarding the plant. Any
of these records may
contain inaccuracies.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
87
The Health State concept corresponds to a generic System State. As with Health
States, the
System States often apply to specific parts of the plant. The Body Site
concept corresponds to a generic
Physical Component, such as the plant (like the body), the core (like the
heart), and pipes (like the throat,
blood vessels, or urethra). Each System State will apply to some of these
components. For instance, it
may be reasonable to describe the integrity of any pipe by naming its System
State from a group of Pipe
leak states. Obviously, one pipe may be leaking or ruptured while another pipe
is intact, exactly as we
have found with Health States occurring at Body Parts. A System State might
include an error in a
supervising computer's code, leading the complex system to respond
inappropriately in some situation.
This roughly corresponds to mental illness manifested by maladaptive behavior.
The Lead to relation again connects System States into parallel networks. Lead
to relations again
contain Modifiers which describe events that make transitions between System
States more or less rapid.
For instance, an earthquake might cause a fatigued pipe to twist, leak, and
finally rupture, just as a sports
injury can cause an ankle tendon to stretch, tear, and finally rupture.
Findings again represent observable facts about the Complex System, such as
the temperature
of a reactor's core, the water level of the core, or the flow rate of water
through a pipe. System States
will be defined primarily by the Specific Findings present. The exact Findings
required will be provided
by a generation method, such as a Bayesian network that reproduces experts
logic about the clusters of
Findings required to classify a Physical Component of a Complex System as
existing in a particular
System State. The simulation program asserts that the System State required
for the simulation is
present, then solves for all unknown nodes in this Bayesian network.
Courses of action again represent activities by humans, another external
system, or the system
itself. Generally, these will be efforts to restore or maintain equilibrium of
the system, or to intentionally
prepare the systems for a change of State. For instance, preparing the breeder
reactor for a scheduled
shutdown and maintenance is a course of action similar to preparing a patient
for surgery. Agents again
represent inputs to the system that influence its Findings or progression,
such as cooling rods, water, fuel,
or repairs to computer code.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
88
Thus, we believe that the current invention has broad applicability beyond the
domain of medical
simulations. It is especially likely to be useful when the behavior of a
system is so complex that an
understanding of the system defies mathematical description. For instance,
this invention is not well
suited to simulating the flight of an airplane, which is fully described by
physical laws. However, it
might be excellent for simulating maintenance of the airplane, which is likely
to reflect obscure design
decisions and even unknown, but empirically observed, interactions between
design decisions.
Label in this Generic concept Nuclear power plant
invention example
Population Complex system Nuclear power plant
Record Record Press releases,
DOE documentation
Health State System State Overheated core,
Leaking pipe
Body site Physical comp. Plant, Core, Pipe
Lead To Lead to Intact pipe leads to
Leaking pipe
Modifier Modifier Bayesian network
describing how age and
Earthquake modify the
pipe lead to
Finding Finding Core temperature, Water
level
Gener. method Generation method Bayesian network describing an intact nuclear
plant
Course of Act. Course of Action Manual shutdown, automated shutdown
Agent Agent Carbon rod, Water, Uranium, Earthquake
The Empiric Simulation Program
The American Board of Family Practice (ABFP) is developing a computer-based
recertification
process based on an Empiric Simulation Program (ESP) that produces new cases
from an editable
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
89
knowledge base. This approach could yield practically endless numbers of cases
at an affordable cost
per case, while maintaining a high level of security. To maintain an
affordable cost per case, it is
mandatory that the ESP not embed medical knowledge in code, that the ESP
allows some stochastic
variation between cases, and that large chunks of the knowledge base are
reusable. However, to identify
acceptable performance, the system must provide means to compare a candidate's
actions with those
deemed appropriate by a relevant group of peers. This requires that the
knowledge base store conditional
logic.
Medical diagnosis and management is replete with conditional and probabilistic
logic that defies
simple models or deterministic description. As an example, patents suffering
from osteoarthritis (OA)
may experience the disease starting early in life, following joint trauma or
other articular diseases, or late
in life, especially following years of excessive weight bearing. The rate of
progression of the disease has
not been described, but it can be slowed by weight loss. Knee joint
destruction may occur in the lateral
compartment, but more frequently occurs in the medial compartment. Joint pain
correlates poorly with
objective findings. Patients who seek care because of joint pain should
usually be treated with
acetaminophen first. Ifacetaminophen is inadequate, non-steroidal anti-
inflammatory drugs can be added
or substituted. However, some of these drugs might accelerate joint
destruction. Furthermore, the entire
class of drugs is more hazardous in the presence of gastric ulcers, renal
disease, hypertension, bleeding
disorders, and asthma. The gastric ulcer hazard can be mitigated by either
misoprostel or omeprazole,
but probably not by H-2 blockers or sucralfate. Intra-articular steroid
injections are useful for acute
exacerbations or if other therapies fail. Knee joint replacement is an option,
but replacement joints last
about IO years. Finally, the advent of new treatment options, such as COX-2
inhibitors or injectable
hyalin, could completely alter recommended care of OA almost overnight.
Probabilistic and/or conditional logic thus pervades every aspect of OA,
including incidence and
prevalence, disease progression, physical findings, symptoms, recommenced
management, and response
to treatment. Nevertheless, the diagnosis and management of OA is relatively
simple from a clinicians
perspective. Many common problems are considerably more complex. Consequently,
we expect many
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
medical problem domains to contain at least as much conditional and
probabilistic knowledge as the OA
domain.
Early Scripting Efforts
5 The need for scripts was therefore recognized by Applicants at an early
stage of development.
We had simultaneously begun to divide medical knowledge into parallel networks
comprises of health
states (stages of a well described disease process) linked by "leads to"
relations (describing the rate of
progression from state to stage.) Health states were typically defined by
asserting findings such as
"normal height" or "normal hematocrit," but the ESP would eventually have to
provide an actual height
10 and hematocrit. The range of normal values varies with age, sex and race.
We also distinguished a
concept of activities that reveal data about a simulation (e.g. questions and
lab tests), and a concept of
management criteria. Thus, we had five distinct concepts that seemed to hold
the vast majority of
conditional logic: Health state definitions, Lead to descriptions, Specific
Finding definitions, Revealing
queries, and Plans for care. Scripts would be needed for each of these.
15 Fortunately, we determined these scripts could be written to be independent
of any particular
simulation, and therefore could be reusable. For instance, a health state
definition could be completely
independent of the process that lead to the health state. Whether OA developed
quickly because of overt
trauma or slowly because of obesity, the same script would describe associated
findings. Similarly, a
script that predicted the rate of progression could indicate that greater
weight produces faster progression,
20 and that direct trauma produces very fast progression.
Our first scripting approach was inspired by the scripting language of The
Medical Record
(TMR).' TMR used scripts to inspect medical records and alert physicians to
actions they might take.
Those scripts typically consisted of lines containing a conditional statement,
an action to take if the
statement were true, and an action to take if the statement were false. The
lines were executed in
25 sequence, unless a GOTO statement sent the program to a specified line.
Logical loops, such as an
instruction to vaccinate for tetanus every ten years, could be implemented
using GOTO statements. In
our implementation, we developed an interpreted language with a few standard
queries to extract data
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
91
from the simulation, commands to write information from the knowledge base to
the simulation, and
operators to manipulate information within the script.
This scripting language was rapidly implemented for the first prototype of the
simulator,
demonstrated at the American Board of Medical Specialties meeting on computer-
based testing in
Chicago, March 21-22, 1996. It was easily extensible, but very difficult to
write and almost impossible
to proofread or explain to physicians. In addition, the process of parsing and
interpreting the script was
an important performance bottleneck. In implementation, the scripts we created
had very consistent
logical flows related to their tasks. The scripts were monotonously similar.
Sets of Conditions
The consistent scripting requirements allowed us to replace interpreted data
extracting queries
and data writing commands with new classes of objects called Conditions.
Condition subclasses currently
include Performance, describing an examinee's previous evaluations;
FindingValue, for acquiring
continuous values such as height or hematocrit; and Relational, for inspecting
and establishing
relationships between patients and other entities. For instance, a relational
condition may indicate that
a patient Has a Health State, was Exposed to a disease causing Agent, or
Exhibits a Specific Finding.
A Condition used as a query may return either a Boolean or continuous value.
Relational and
Performance Conditions typically yield Boolean values, in effect answering
questions such as, "Has the
patient had knee OA for longer than 5 years?" FindingValue Conditions
typically yield continuous
values, in effect answering questions such as, "What is the patient's height
now?" A Condition used as
a command is a template for writing new information to the simulation. These
typically establish some
concept that persists until succeeded by another concept. For instance, a
Condition would establish that
a patient "Has glucose intolerance starting now and lasting indefinitely."
This Condition would persist
until succeeded by the Condition that the patient "Has type II diabetes
mellitus."
We also designed a class called Sets to replace some of the probabilistic
information previously
embedded in scripts. A Set contains several Conditions, and indicates how many
ought to be present for
the Set to be logically true. Sets support subset concepts such as exactly N
Conditions(N>O),between
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
92
N and M Conditions(M>N_0),and at least N Conditions. Thus, Sets allow a
succinct means of asking
whether a simulated patient has any number of arthritic diseases, or asking
whether the patient has been
prescribed exactly one non-steroidal anti-inflammatory drug, or stating that
the patient must have at least
2 and possibly four cartilaginous abnormalities.
Bayesian Networks
We implemented Conditions and Sets in a new model in 1996, but still needed a
mechanism for
organizing these concepts, and in particular for creating dependencies. We
could use Sets to define the
state of a node in a graph. We briefly experimented with using tree
structures, in which a tree node could
have multiple states. Each state would be defined by a Set of Conditions,
linked to other arbitrary
information (such as multimedia), assigned a probability, and point to another
tree node. To use a tree,
the ESP would inspect the root node and determine whether any of the states
were already established
or impossible in the simulation. It would stochastically select one of the
remaining possible states, then
follow the corresponding branch of the tree, It would repeat this process
until it reached a terminal node.
The terminal node and the path to it would provide the information the ESP
needs to create a plausible
patient, critique a physician's management strategy, or produce a laboratory
report conditioned on the
nuances of a simulation. The ESP would perform these tasks in time
proportional to the greatest depth
of the tree, or better.
Although the tree approach was technically feasible, many practical problems
soon became
evident. The first problem was the frequent need to nearly duplicate part of a
branch with slight changes.
For instance, the root node in a tree that implements OA stages might inspect
the simulation for the
current stage of OA, then produce findings conditioned on that result. Each of
its branches will describe
joint space narrowing in a probabilistic way, with some overlap. Mobility and
pain nodes might depend
on both the stage of OA and the joint space narrowing. Thus the tree has very
redundant looking branches
with only slight changes in probabilities. These trees are therefore hard to
inspect.
Although Bayes nets are NP hard to solve precisely, they have several well
known advantages.
First, most of our node and state concepts could be reused immediately.
Second, a Bayes net will almost
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
93
never represent the same concept in two separate nodes; conditional
probability tables replace the
separate branches required in the tree structure. Third, other groups are
actively developing analytic
techniques that allow very rapid approximation of Bayes net solutions. Fourth,
companies such as Norsys
Software Corp. (www.norsys.com) are developing affordable software packages
such aNeticarmTM and
can provide a well documented, correctly functioning application programming
interface to developers.
Finally, Bayes nets can compactly represent very complicated information, and
allow knowledge editors,
supervisors, and external reviewers to interactively explore a model by
setting the states of nodes and
inspecting updated probabilities.
METHODS
The knowledge base was revised to accommodate Bayesian networks, for example,
represented
as NeticarmTM files, node states defined with Sets of Conditions, and
additional network and node details
required by the ESP. A production quality knowledge acquisition effort was
initiated for OA and diabetes
mellitus, and other diseases that predispose to or complicate these diseases.
We began additional
knowledge acquisition efforts in otitis media, depression, hypothyroidism,
abnormal Pap smear
management, and hypothyroidism.
The use of Bayesian networks as a scripting language has been partially tested
by
implementation of an object oriented database which was populated with data
about OA and obesity.
Expressiveness was tested in the other domains. We were actively programming
an ESP that will rely
almost entirely on Bayes nets and Sets of Conditions to describe conditional
and probabilistic
information in family medicine, and preparing to enter hypothyroidism data in
an object oriented
database.
RESULTS
Current structure
Figure 19 illustrates classes in the relevant portion of the final knowledge
base structure. Lines
indicate that one class is associated with another class. An asterisk, "*,"
indicates a one to many
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
94
relationship, while a number indicates an exact number of associations. For
instance, a Health State is
associated with 3 Bayes nets, which provide incidence, prevalence, and disease
descriptions. Arrows
indicates ISA relationships. The lightly shaded classes require a means of
expressing probabilistic and
conditional information, once provided by a scripting language.
The Bayes Net, Bayes Node, and State structures are replicated in part in the
NeticaTM file. This
design requires that we maintain very strict name consistency between the
knowledge base and NeticaTM
file. The Property may contain a time function (e.g., incidence or prevalence
as a function of age), a
multimedia reference (e.g., a sound to play), simple text, or a function of
properties of other nodes. The
seven most important Relational Conditions are listed.
Expressive verification
The knowledge acquisition effort has used these data structures to record all
of the conditional
dependencies found to date. Space prohibits reproduction of Bayesian nets in
this specification, but the
following fragments illustrate the use of Bayes nets and supporting structures
in place of scripts.
Figure 20 illustrates a simplified OA generating Bayes net. The network was
built as a roughly
physiologic model of the development of OA, assuming that cartilaginous
deformities and destruction
cause joint space narrowing, accompanied by sclerosis and subchondral cyst
formation (not shown), and
leading to gross deformities and loss of mobility. Pain is a variable feature,
but probably must be present
in a test case (otherwise, how would the doctor's attention be drawn to the
joint?). The mild narrowing
state of the joint space node is defined by a Set containing one Condition,
EXHIBITS the Specific
Finding, mild joint space narrowing, which is itself defined as a joint space
of 4 to 6 mm for the knee.
The stage I state of the osteoarthritis stage node is similarly defined by a
Set containing one Condition,
HAS mild OA. The conditional probability tables for this node are arranged so
that certain combinations
of joint space narrowing and deformity define mild OA. Other combinations may
define other stages,
or be declared impossible (e.g. severe deformity without joint space narrowing
might be impossible in
this context).
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
Two interesting benefits of this approach are first, that we can use a single
Bayes net to describe
all five stages of OA, and second, through the logical magic of Bayes theorem,
we can now invert a
Bayes net built from a perspective of classifying stages of OA, and use it to
generate descriptions of OA.
We assert that the patient HAS any stage of OA, update probabilities
throughout the network, and start
5 stochastically assigning states to indeterminate nodes. With each
assignment, we write new information
to the simulation, e.g., that the patient EXHIBITS mild joint space narrowing.
We can test the Bayes net
by experimenting with it from both perspectives, e.g., beginning with an
assumption of cartilage damage
to see what stage of OA results, or beginning with OA to see what other
findings result.
Bayes nets supporting Leads to structures are conceptually very similar to
Health State
10 generating Bayes nets, with two important differences. First, the
Conditions usually describe task factors
for slower or more rapid progression, rather than features of a disease. For
instance, an OA Lead To is
likely to ask whether the patient HAS Obesity, or to assert that the patient
is EXPOSED to some remedy.
Second, the goal of the Lead to structure is to produce a rate of progression,
which is not
specified anywhere else in the simulation. (In contrast, the Health State
generator has a goal of creating
15 a description consistent with an asserted disease).
Figure 21 illustrates a Bayes net that could produce a report when an examinee
requests a
Revealing x-ray test. The only simulation data used in this report is the
joint space, a value indirectly
modified by the Bayes net in Figure 20. Now we have a continuous Bayes node
acquiring its value from
a Condition that extracts the current joint space from the simulation. Note
that there are no requests to
20 determine whether any Specific Findings or Health States are present.
Revealing queries should therefore
be reusable across simulations. Also, the accuracy of the test can be built
into the Bayes net representing
that test. Another test, such as a magnetic resonance image, could have
different size errors.
Subjective queries are much more complex, but still possible to construct
using the same
approach. The primary complication is that subjective responses are uniquely
elaborate in temporal
25 detail, yielding statements such as, "the pain has been coming going for
weeks, but now it is worse than
ever."
CA 02369425 2001-10-03
WO 00/60431 PCT/US00/08942
96
Management Plan critiques are similar to Reveals, except that most of the
Conditions inspect
prescriptions and queries made by the examinee, and the resulting report is a
critique of a physicians'
strategy. Our experience to date confirms the expectation that Bayes nets
support inferences about
actions and plans.
Limitations
The current knowledge base design requires the additional validation of
supporting a working
simulator. We can not yet prove that the concepts presented here will work in
the simulator we are
programming. Our knowledge acquisition experience has uncovered one instance
in which we thought
that the data source for a continuous Bayes node (Hemoglobin AC levels) should
be another Bayes
network. This raised the specter of solving recursive (or accidentally
cyclical) NP hard problems to
produce a simulation or answer a question. We expect that wary knowledge
editing can prevent such
problems.
Bayesian networks, with appropriate supporting structures, are capable of
representing important
concepts in family medicine and seem likely to replace other scripting options
in a simulation program
that we hope will produce recertification tests in the future. We will soon
demonstrate whether we are
able to produce realistic simulations using this scripting language.
Computer-based testing holds promise as a technology that could add
educational content to the
testing process while yielding different, and perhaps more important,
information about examinees than
paper-based tests. Some computer-based tests use traditional multiple choice
item formats. Other tests
simulate patient care experiences. Some elegant simulation programs generate
patient data from systems
of equations, but most outpatient medical problems still require empiric
description. Some programs
embed the logic of the simulation in 2,3 code, although reuse and knowledge
maintenance may be
difficult.
The American Board of Family Practice (ABFP) is developing a computer-based
recertification
process based on an editable knowledge base. This empiric simulation project
(ESP) could yield
practically endless numbers of high quality cases at an affordable cost per
case. Variability in case
CA 02369425 2001-10-03
WO 00/60431 PCT/USO0/08942
97
presentations should help the ABFP maintain a secure test. Conversely,
modeling decisions which restrict
the details of case histories may reduce security.
The ESP development team designed an entity-relationship model of medical
concepts and
algorithms to create patient simulations from the data model. These algorithms
create patient histories
and evolve patients during simulated medical care. The central concept in the
history generation
algorithm is that patients with some health states evolve to experience other
health states.
The assumptions underlying early ESP algorithms and data models were similar
to those of a
Monte Carlo process. A simulated patient would have partially completed a path
through a Monte Carlo
network. A physician's management decisions would influence the remainder of
the path. Nodes along
this path represented the patient's overall health during a period of time,
that is, all simultaneous medical
problems are represented in a single Monte Carlo node. Arcs between nodes
represent the patient's
transitions between conglomerate health states. Other common decision modeling
techniques, such as
Markov processes and decision trees, employ similar models of health states.
The Department of Family Medicine at Duke University and the affiliated
Cabarrus Family
Medicine Program conducted knowledge acquisition experiments for a variety of
problems common in
family practice. These included alcohol abuse, ankle sprains, diabetes
mellitus, hypertension,
osteoporosis, otitis media, peptic ulcer disease, pregnancy, reactive airway
disease, and smoking. These
domains involve addictions and behavioral problems, acute illness; acute
illness superimposed on chronic
predisposing illness; and non-systemic illnesses. The ESP development team
advised the domain experts,
and simultaneously modeled osteoarthritis of the knee and normal health.
These experiments demonstrated many serious difficulties with the conceptual
model. First, to
obtain variable histories required modeling many nodes in a Monte Carlo
simulation. In several domains
a chronic progressive systemic illness (e.g., osteoporosis) combined with
recurrent acute site-specific
exacerbations or complications (e.g., fractures of various bones). The
original model implied the need
for a large number of conglomerate health states, for instance to define
multiple paths from "Normal
health" to "Ex-smoker with severe osteoporosis and healed second left hip
fracture." The number of
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
98
conglomerate health states can expand quickly, and data required to define
these conglomerate health
states (e.g., age specific incidence) is often speculative and redundant.
Second, identical information may be collected in several testing domains. For
instance, highly
redundant obesity descriptions would appear in tests of osteoarthritis,
diabetes, and hypertension.
Third, relations between health problems are unclear. Conglomerate health
states do not
compartmentalize disease processes, obscuring whether domain experts consider
hyperthyroidism or
nicotine addiction as direct precursors of osteoporosis, or risk factors, or
distracters.
Fourth, modeling one therapeutic complication adds many nodes and arcs.
Therapeutic
complications are typically new illnesses superimposed on any of several
antecedent conglomerate health
states. For instance, a patient in any of the osteoporosis nodes might develop
uterine cancer while taking
unopposed estrogen. The number of nodes required in the Monte Carlo model may
double, with an equal
number of new arcs. Historical distracters, such as randomly appearing colds
or a history of appendicitis
might require still more conglomerate states.
Finally, a computer-based test needs to specify the anatomy of disease, so
that it. can correctly
present findings to the examinee. In some diseases the anatomy is erratic. A
typical osteoarthritis patient
will have joints afflicted to different degrees.
Thus, Monte Carlo modeling techniques have an appealing ability to generate
multiple temporal
sequences of events. However, the ABFP's need for finer anatomic detail,
reusable information, and
manageable knowledge acquisition and maintenance required some revision of the
Monte Carlo
approach.
METHODS
The ESP model was revised to define Parallel Networks of Health States, while
discarding
conglomerate health states. A Parallel Network includes a sequence of
distinguishable, mutually
exclusive Health States. These typically reflect the medical literature's
descriptions of stages of
progression or severity of a disease. If the literature does not provide a
staging definition for a disease,
Health States can usually be defined as absent, mild, moderate, and severe.
CA 02369425 2001-10-03
WO 00/60431 PCT/US00/08942
99
A parallel health state network connects these health states with "Leads To"
objects, e.g., mild
disease leads to moderate disease. A Leads To object associates specific
collections of risk factors and
treatments with a fuzzy rate of progression from the preceding to succeeding
Health States. The risk
factors may be Health States from other Parallel Networks, activities (e.g.,
work, play, and habits), and
familyhistory. Treatments may be interventions prescribed by the examinee, or
some simulated previous
provider.
Separate collections of Leads To objects manage history generation and
evolution. In history
generation, the ESP creates a life history and context for the examinee's
encounter with the simulated
patient. The examiner may want an unremarkable story compatible with many
simulated medical
problems, or a story that is virtually pathognomonic. In evolution, an
efficient test might routinely
simulate rapid progression of disease or complications of the examinee's
treatments, regardless of the
likelihood of these events in practice.
Each Parallel Network defined in a simulation imposes its Health States on one
or more anatomic
sites, which evolve simultaneously. For instance, a rheumatoid arthritis
simulation could name a single
Parallel Network and all of the joints affected. An osteoarthritis simulation
might use two copies of a
knee osteoarthritis Parallel Network, applying one to each knee. Different
presenting Health States at
each knee and independent evolution of the knees would be typical of
osteoarthritis. Systemic diseases
involve the entire body of a simulated person.
Health States may recursively contain Parallel Networks representing more
acute exacerbations
of the parent Health State. For instance, moderate osteoarthritis may include
a Parallel Network
describing transitions between baseline and flare Health States. A simulated
patient cycling between
these Health States will display or recount episodes of worsening arthritis
symptoms.
The algorithms for history generation and evolution were adapted from Monte
Carlo techniques.
A request for a simulation identifies the presenting Health State in each
Parallel Network. Using
incidence and prevalence information, the age, sex and race of the simulated
patient are selected. The
time of the next (or, in history generation, the preceding) event in each
Parallel Network is predicted. In
history generation, this may require assertions regarding the activities of
the simulated patient. The
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
100
temporally closest event from all of the Parallel Networks is instantiated. In
history generation, the
process of predicting the most recent preceding Health State change proceeds
backward through time
until no further transitions are defined by the Parallel Networks. In
evolution, this process of predicting
the next event continues until one of the events initiates another encounter
with the physician.
The revised ESP model was tested by additional knowledge acquisition
experiments,
implementation of a PoetTM object oriented database and supporting algorithms,
and generation of
simulated osteoarthritis cases. The database was used to generate cases of
osteoarthritis of the knee with
obesity as a risk factor, and gastric ulcers induced by non-steroidal anti-
inflammatory drugs prescribed
without misoprostel.
RESULTS
Knowledge acquisition
Simple illustrations of their medical domains helped content experts
understand the scope of
their knowledge acquisition tasks. Initially intricate domain models were
decomposed into much less
threatening Parallel Networks. Figure 22 illustrates common Parallel Network
structures. The simplest
network is a collection of one or more static states, typical of genetic
(e.g., Downs syndrome) and some
congenital conditions (e.g., anencephaly). The progressive network is a series
of states with no cycles,
typical of degenerative illnesses such as osteoarthritis. The reversible
network illustrates chronic but
reversible conditions, such as essential hypertension and weight disorders. In
the injury network an acute
insult evolves to either recovery or a chronic condition with a later
recovery. Injury networks describe
many infectious diseases and trauma. The addiction network illustrates that a
person may abstain from,
use, abuse, or become addicted to a substance. In the scheme shown here, a
previously addicted person
can only be addicted or recovering, but cannot return to abstinence, use or
abuse. The surgical
intervention overlay illustrates that new states can be added to the above
networks using irreversible
therapies such as radiation or surgery. Domain experts adapted these networks
to their needs by
eliminating unwanted nodes and arcs, or replacing nodes with another network.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
101
Domain experts began with a primary Parallel Network to sketch the diseases
defining their
domain, such as stages of diabetes mellitus. Parallel Networks of comorbid
conditions were identified
in most domains, typically including risk factors for progression through the
primary network, such as
obesity. Most domains included one recursive layer of Parallel Networks
representing exacerbations of
Health States in the primary Parallel Network. Most domains also identified
one or more Parallel
Network representing complications of Health States in the primary network,
such as retinopathy, or of
treatment of primary Health States, such as gastric ulcers.
Experts were asked to estimate 1) how long a risk factor should exist before
it could influence
a transition between states in a primary network, 2) the time required for
transitions in the primary
network, given different combinations of risk factors, and 3) the number of
passes an individual patient
should be allowed to make through a cycle (e.g. from acute injury to recovery
and back). Although these
data were often non-existent in the literature, domain experts could
comfortably estimate a range of
values from clinical experience. Although the data to gather remained imposing
in volume and
dauntingly quantitative, Parallel Networks in the revised ESP model appeared
to successfully guide
segmentation of data into intellectually plausible sets.
Data model and algorithm implementation
The osteoarthritis experiment continued with development of an object oriented
database
structured after the ESP model. The database was populated with information
about four stages of
osteoarthritis, three weight conditions, and 2 ulcer states.
The algorithms mentioned above were implemented, but without support for acute
exacerbations
or multiple Parallel Network copies afflicting different anatomic sites.
Conditional probabilities were
managed with a simple scripting language. The scripting language has since
been replaced by Bayesian
networks.
Instantiation of the model confirmed the expected difficulty in authoring a
family of cases with
the same underlying disease process, but different details in presentation. In
particular, giving attention
to conditional probabilities slows knowledge acquisition considerably.
Memories of individual clinical
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
102
cases were helpful in authoring a narrowly defined simulation, but much more
attention was required to
produce Health States generation methods and Leads To objects that were robust
to changing
assumptions about sex, race, and obesity. In spite of these difficulties, data
entry in a data base founded
on Parallel Networks was accomplished.
Experimental Verification
The prototype ESP simulator generated a series of patients for demonstration
at the American
Board of Medical Specialties meeting on computer-based testing in Chicago,
March 21-22, 1996.
Approximately 30 patients were generated and stored over a four day period,
including several during
the meeting. Each patient generation required about 20 minutes. After
generating a variety of male and
female patients, data in the knowledge base were skewed to generate middle
aged overweight white
females. These patients were typically 55 to 65 years old and complained of
recently worsening pain in
one or both knees. Patients had been morbidly obese for 1 to 3 years prior to
presentation, and had at
least a 5 year history of mild arthritis in the affected knees.
Their health problems began with either obesity or mild osteoarthritis 10 to
30 years prior to
presentation.
During the demonstration, most history and laboratory requests returned graphs
of values over
the simulated patient's lifetime, enabling viewers to see how variables such
as weight, uric acid, or
osteophyte numbers had changed since birth. These graphs demonstrated
concurrent histories of
worsening osteoarthritis and obesity.
Demonstration patients were managed interactively. Patients managed with high
doses of
nonsteroidal anti-inflammatory drugs without misoprostel would develop ulcers
sometime during a 2
year follow up period. Weight loss was also possible. Optimal management of
weight and prescription
of strengthening exercises would slow the inexorable progression of knee
osteoarthritis, but progression
from moderate to severe knee arthritis would inevitably occur within 10 years.
DISCUSSION
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
103
The simulations demonstrated that the prototype system could generate patients
with plausible
medical histories; appropriate symptoms, signs, and laboratory values; and
could evolve patients over
time. The separation of data controlling osteoarthritis, obesity, and ulcer
histories and presentations
suggests that these components would be reusable with modest modification, if
any, in new disease
domains. Substantially different osteoarthritis simulations could be produced
by replacing a few history
controlling Lead To objects.
Limitations
We are currently developing a simulator with acute exacerbations, past medical
interventions,
and use of multiple copies of one Parallel Network's data. The new model and
algorithms replace simple
scripts with Bayesian networks. Although the next generation simulator is not
yet functional, no fatal
conceptual difficulty is evident.
The knowledge acquisition problem for the ESP model remains daunting. One
vexing problem
is that the history generation algorithms reqQire solutions to multiple
temporal constraints. These
constraints may not always have a solution, and it is not yet clear how to
react if a history generating step
fails, or how to guarantee temporal solutions while reusing data.
The Cartesian product of N parallel networks creates an N dimensional grid
whose nodes
represent conglomerate health states. This grid is a complex Monte Carlo model
with many low
probability paths that would never have been considered in an explicit Monte
Carlo model. Conditional
probabilities within Parallel Network's Leads To objects could provide a means
of pruning the
N-dimensional space. This mechanism may not work, as it places further burdens
on knowledge
acquisition and reusable object design.
These limitations must be considered in context. In the absence of
mathematical models of the
diseases of interest, the ABFP requirements for secure tests, realistic
temporal and clinical features, and
defensible credentialing decisions, complex data is an inevitable feature of a
computerized problem
generation process.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
104
Parallel Networks facilitate some aspects of knowledge acquisition for a
patient simulation
knowledge base, and appropriate algorithms support generation of patients. The
data required are
relatively reusable, in contrast to data explicitly describing global health.
Further experimentation is
required to demonstrate that this approach remains tractable with more complex
scenarios. Parallel
Networks may have application in other endeavors that traditionally describe
global health, such as
decision analysis.
Background Information for Knowledge Development
Medical certifying organizations have traditionally relied upon paper and
pencil cognitive
examinations to measure certification candidates' suitability for board
certification. Traditional formats
such as multiple choice questions have well-defined operating characteristics
and reliability for
examining cognitive knowledge capabilities. They provide, however, only
primitive ability to assess a
candidate's problem-solving capabilities. Additionally, traditional testing
strategies rely upon a
continuous process of item development; once used, the items in an examination
must be replaced with
new questions in order to preserve security of the certification process. Each
examination represents a
product that the certifying organization can use only once. The presently used
medical certification
process thus suffers from two weaknesses: 1) test development requires re-
generating an examination
with new material on a recurring (usually annual) basis; 2) although multiple
choice questions
demonstrate reliable performance in measuring cognitive knowledge, this format
doesn't measure
adequately problem-solving capabilities.
Several organizations have experimented with computer-delivery of clinical
content and
evaluation. In the late '60's and 70's, the Ohio State University developed a
self-directed Independent
Study Program that utilized a "Tutorial Evaluation System" or TES for
conveying curriculum content.
About the same time, Dr. Octo Bamett's laboratory at the Massachusetts General
Hospital began
development of clinical simulations using the MUMPS language. Investigators at
the University of
Illinois developed a simulation model known as Computer Associated Simulation
of the Clinical
Encounter, or "CASE'). Research supported by the American Board of Internal
Medicine demonstrated
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
105
that a computerized examination system appeared feasible in professional
evaluation/certification
settings. Stevens and colleagues also demonstrated the feasibility of using
computer-based systems for
testing problem-solving ability in undergraduate medical school curriculum
applications. Additionally,
Sittig and colleagues examined the utility of computer-based instruction in
teaching native users basic
computer techniques such as "drag and drop" and other computer operations.
These efforts suggest that
computer-based testing techniques will similarly transport to the computer-
native medical certification
candidate.
Another system with special relevance to ABFP's efforts was developed at the
University of
Wisconsin. This project served as the nidus for the Computer-Based Examination
(CBX) developed by
the National Board of Medical Examiners (NBME). NBME's CBX development project
has been in
evolution for over a decade, and has demonstrated validity in examining
professional degree candidates.
The CBX development experience suggests that clinical computer simulations
with automated scoring
algorithms can produce professional certification examinations at reduced cost
compared to traditional
methods. However, the CBX model suffers from one major drawback: the clinical
simulations are
"hard-wired" in computer source code which must be re-coded for each new
examination. Once the
simulation has been used widely, the examination contents are no longer
secure, necessitating continuous
cycles of new simulation development.
To circumvent these weaknesses, ABFP embarked upon a computer-based testing
project which
will 1) generate new patient cases for each candidate examined, and 2) test a
candidate's problem-solving
ability. The system relies upon a knowledge base of family practice that
represents in probabilistic terms
disease/condition incidence, prevalence, evolution over time, and response to
interventions.
Discussions with other certification organizations (other specialty boards,
professional
organizations) have emphasized the potential need and market for knowledge-
based systems in training
and evaluation contexts. The expert system literature affirms this evolution
in evaluation and training
systems. Early artificial intelligence/expert system work concentrated on
"rules of thumb" or heuristics
to represent problem-solving strategies identified by domain experts.
Instances of these rule-based
systems demonstrated that they were necessarily constrained to narrow domains,
and that the knowledge
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
106
contained in the rules was difficult to validate. Research has also indicated
that experts relate only one
dimension of knowledge when defining a rule, but also rely upon expansive
knowledge of how systems
work (i.e., physiology and pathophysiology in the medical domain) in
performing real-world
problem-solving. This realization has led to re-thinking regarding structure
of knowledge-based systems
to reflect the tasks such a system should accomplish, the methods the system
should use to accomplish
the tasks, and the knowledge required to support these methods.
Knowledge-acquisition for such systems entails development of a model for the
domain and
instantiation (ie, encoding and enter needed information into the system's
data structure) of the model
with information acquired from knowledge "donors". The model structure
necessarily drives the
knowledge acquisition effort. ABFP's computer based testing system under
development at ATL while
not an expert system per se, represents knowledge at multiple levels of
complexity. For example, reactive
airways disease is represented as a series of health states: Normal (Non-
reactive) Airways, Reactive
Airways-Mild, Reactive Airways-Moderate, and Reactive Airways-Severe. Each
health state contains
identifiers which relate the particular health state to precedents and
antecedents (eg, Normal Airways
serves as the precursor health state for Reactive Airways-Mild which precedes
Reactive
Airways-Moderate, which in turn leads to Reactive Airways-Severe.) Each health
state in turn is
associated with specific versions of universally observable findings. For
example, a Finding called
"Asthma Attack Frequency" is universally observable, although most people
enjoy a Normal Airways
health state and its associated frequency of asthma attacks of No Attacks
(e.g. a Specific Finding
indicating 0 attacks/month, indefinitely). Similarly, the Finding "Shortness
ofBreath" is instantiated with
the Specific Finding "No shortness of breath" in the Normal Airways state.
Likewise, other Findings such
as Respiratory Function and Severe Asthma Attack Frequency are instantiated
with corresponding
normal Specific Findings (Normal Respiratory Functions, and No Severe
Attacks.) This representation
of Findings with Health State-specific instances of Specific Findings provides
re-usable structure which
transports to each new health state. Such reusability has been identified as a
characteristic which
contributes to the robustness of a knowledge-based system.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
107
Another example will illustrate further the relationship between Findings and
Specific Findings.
Consider the growth curve charts we use in assessing child development. A
Finding associated with
growth is Height, a universal property of individuals. Normal Height would
represent a Specific Finding
which describes the range of heights associated with normal growth. Growth
charts for Boys and Girls
would then be described as Patterns which define the normal probability
distributions for growth in boys
and girls. At the start of a simulation, a percentile (e.g., 29th percentile)
would be selected for the
patient's growth characteristics. Then a Pattern for the particular patient is
instantiated using the 29th
percentile curve from the appropriate gender growth chart. How does the
examinee learn about the
patient's height? A Reveal Course of Action (COA) is initiated (the mechanics
of this aren't important
to understand at this point) to obtain the patient's current value for Height
Finding.
Simmons and Davis have identified the importance of the distinction between
actual knowledge
and representation of knowledge. Knowledge describes the attributes of a
health state; representation
consists of the symbols and language used to encode the knowledge in the
testing or expert system.
Sinunons and Davis have additionally identified that knowledge of multiple
types is needed for robust
performance. These authors have partitioned knowledge into three fundamental
types: knowledge about
tasks, knowledge about methods, and knowledge about models of system behavior.
These types
correspond to those included in ATI's Computer based Testing system. Findings,
Specific Findings,
Patterns and Sub-pattens describe system behaviors and characteristics.
Courses-of Action describe tasks
and methods used to apply, modify, and evaluate the health state information
and characteristics
described in the model. As also indicated by Simmons and Davis, subdivision of
knowledge types in this
manner facilitates the knowledge acquisition process. This subdivision also
promotes multiple levels of
knowledge abstraction, which enhances the system's ability to represent
varying levels of complexity.
For example, in the Computer-based Testing system, a Pattern such as incidence
is further subdivided
into sub-pattens such as incidence in females versus males, and incidence in
various racial/ethnic groups.
To facilitate development of such a system, the developers divided the system
development task
into three components: the knowledge base, the patient simulation generator,
and the presentation
system.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
108
The knowledge base has been designed and represented as a series of entity-
relationships. The
model has several fundamental entities: Patient, Health States, Findings,
Courses of Action (COA), and
Agents. These entities have relationships of INTERACTS-WITH, CONTACTS, IS -
RELATED,
EXHIBITS, HAS, EXPOSED - TO, LEADS - TO, ASSOC-WITH, LINKS-TO, USES, IDENTIFY,
MANAGE, ALTER, REVEAL, and EVALUATE.
Referring to Figure 1, which describes the entities and relationships included
in the model,
rectangles indicate relationships between entities in the model. Hexagons
indicate entities. Solid lines
indicate Medical Knowledge Relationships (e.g., a course of action such as
treatment with non-steroidal
anti-inflammatory agents can modify Specific Findings such as pain in the
patient with osteoarthritis).
Dotted lines indicate Simulation/Evolution relationships which define how a
particular domain
simulation can proceed.
The model depicted in the figure has been published in the Journal of the
American Board of
Family Practice, and presented to national audiences.
The patient simulation generator relies upon a series of generation methods to
create patients for
presentation to the certification/recertification candidate. These algorithms
function to evolve the patient
forward (to reflect progression of the disease process and response to
interventions) and backward in
time (to create a past history for the patient.)
The patterns which describe patient progress and characteristics are defined
as probability
distributions (discrete and continuous as appropriate for particular finding)
during the knowledge
acquisition process. At the beginning of a simulation, a random number
generator selects a master
percentile" (MP) which then serves as the reference for selecting particular
patterns from the appropriate
specified distributions. Properties of these patterns are then presented to
the candidate as findings for a
particular health state (e.g., the current glucose level as a manifestation of
diabetes.) Once presented with
the patient description (age, race, gender, clinical findings), the candidate
then selects appropriate COA's
for further evaluation and/or management of the patient's health state.
Selection of an interventional COA disturbs the simulation in one or both of
two ways. First, it
may cause the simulated patient to change health states, e.g., by removing a
appendix, the patient may
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
109
proceed from a health state called "appendicitis" to one called "post
appendectomy." Second, the
intervention may initiate a pattern representing a temporary perturbation of
some finding. For instance,
administering acetaminophen to a febrile otitis media patient may not cause a
change in health state - an
infection may still exist, and the fever it induces will return in a few
hours. Nevertheless, acetaminophen
administration will reduce the fever for a short time. This perturbation might
be represented in the
knowledge base in the Temperature Finding, Fever Specific Finding, Antipyretic
perturbation, with a
four hour duration temperature change initiated by any antipyretic. When the
examinee requests a
temperature, the COA that reveals the current temperature must combine
information about the
underlying temperature and all antipyretic drugs administered in the last four
hours.
The distinction between changing health states and perturbing findings is
necessarily artificial
(health states are just collections of findings), and the decision to model a
particular process one way or
another may often depend on testing goals, and subsequent decisions about how
finely to model health
states. In general, very fine distinctions between health states should result
in more interventions that
change health states, while coarsely defined health states may require more
perturbations in Findings.
A COA can modify the health state in which a patient exists at one point in
time. When the
candidate selects such a COA , the simulated patient may evolve to a new
health state on the basis of
patterns specified for health state evolution in the knowledge base. The
knowledge for a particular health
domain is stored as a parallel health state network. For example, the
initially generated patient for a case
of osteoarthritis will demonstrate some stage of osteoarthritis. However,
other health states such as
obesity might influence the progress of the patient's arthritis from mild to
moderate and moderate to
severe disease. In the parallel networks of health states representation, a
newly-generated patient will
display findings consistent with a health state in the primary domain (for
example, osteoarthritis) and
in the parallel health states ( e.g., obesity) which influence the primary
health state's progress. As shown
in the following figure, osteoarthritis can progress over time from the normal
state to inild, moderate or
severe osteoarthritis.
For this particular illness, progress occurs in one direction only;
osteoarthritis doesn't regress
once developed, but can stabilize at a particular degree of severity. Obesity
represents a parallel health
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
110
state which can influence the progression of osteoarthritis. Mild, moderate,
and severe obesity can
influence this progress at different rates: the model permits representation
of greater impact for more
severe obesity states. Notice also that obesity can regress (severe obesity
can revert to moderate obesity,
etc.) Similarly, other parallel health states might exist which could modify
progression of osteoarthritis.
For example, the patient who has osteoarthritis will frequently utilize
nonsteroidal anti-inflammatory
drugs (NSAID's) for treatment. These agents can improve the symptoms of
osteoarthritis, but also impact
on the parallel state of peptic ulcer disease, ie treatment with NSAID's can
induce an ulcer, which will
then evolve in parallel with the course and treatment of osteoarthritis.
Initial experience with this
representation indicates that these modifier-relationships are not well-
defined in the medical literature
and constitute a research area for further development.
The simulation system's fidelity depends upon access to a rich representation
of health
state-specific knowledge. This knowledge consists of Findings obtained from
physician
"knowledge-donors" working from templates provided by the Assessment
Technologies, Inc.
development team. The template includes a NAME for the health state and an
associated SNOMED code.
The template also includes specific descriptions of the Findings, and Patterns
for these Findings. The
patterns are stored as distributions; these distributions are obtained from
the medical literature where
available, and from physician expert opinion where such published data don't
exist.
The development team doesn't expect the knowledge groups to provide these
distributions but
rather to indicate the relationships between health states, how parallel
states influence each other
qualitatively (e.g., increase, decrease, or stay the same), and possible
sources of information about the
relevant probabilities.
The knowledge model has evolved over the past six months to include extensive
use of belief
networks (also called Bayesian networks). Belief networks provide a graphical
process for describing
the relationships between entities in a health state. For example, some set of
characteristics (family
history, age, gender, racial origin/ethnicity, body weight) influence the
development of impaired glucose
tolerance.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
111
Figure 24 illustrates these relationships: Family History, Gender, Race,
Weight, and Age, all of
which influence the development of impaired glucose tolerance. The raw
pictorial doesn't say how they
influence IGT, but rather that they "influence" the development of IGT. In the
background, we
incorporate probabilistic information which describes these relationships
quantitatively, but would expect
the knowledge development group to provide only semiquantitative guidance
(e.g., a person whose
mother has diabetes has twice the likelihood of developing IGT compared to an
individual who has no
such family history.) We intend to fill in the more specific quantitative
probabilities on the basis of data
in the literature where available; if such information does not exist, we will
have to rely on expert
opinion.
How The Knowledge Development Process Will Work
What will we need from the knowledge team in order to generate the information
required in our
system? The team should proceed in a step-wise fashion to address the
following
issues:
How is the health state defined? (e.g., What criteria do we use to define the
presence of impaired
glucose tolerance or diabetes mellitus?)
What population/s do/es the condition affect (should the system emphasize a
particular
population group?)
What are the commonly accepted stages of the disease process?
What demographic/patient characteristics, risk factors, and behaviors
influence a patient's
movement from one stage to another? (e.g., obesity's influence on hypertension
and diabetes.)
How do particular characteristics vary within a given stage of illness (e.g.,
what blood pressure
ranges would we expect in Stage 1, 11, etc., for hypertension) How should
these relationships
appear in the Bayesian network format? What therapeutic modalities
(pharmacologic,
nonpharmacologic) exist to modify the progression and/or severity of the
disease process? (e.g.,
magnitude of effect of weight loss on blood pressure in Stage I, Stage 11,
etc; how much will
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
112
weight loss lower blood pressure? How much will weight loss decrease the
likelihood of
progressing from Stage Ito Stage 11 hypertension?)
What guidelines exist to describe optimal management plans for the disease
process (e.g., JNC
VI for hypertension)
For a given health state, what management and diagnostic concepts should we
emphasize in
creating the knowledge base (we cannot reproduce Harrison's in the knowledge
base, nor should
we - the system has to be good enough, not necessarily exhaustive)
What parallel health states should the model reflect for the primary disorder
in questions (e.g.,
for osteoarthritis, obesity and peptic ulcer disease might affect disease
progress and treatment,
respectively)
What multimedia resources will we need to represent adequately the clinical
findings associated
with the health states?
How is the health state defined?
The group should identify the criteria (physiologic, clinical, demographic,
etc) which define the
disease process, and which distinguish the various stages of the disorder. To
whatever extent possible,
we should rely on nationally accepted criteria as published in the peer-review
literature and highly
regarded textbooks.
What populations does the condition affect (should the system emphasize a
Particular population group?)
We might encounter health states or diseases for which the ABFP wants to
emphasize how the
disorder affects certain groups. We will attempt to have one family physician
ABFP director on each of
the knowledge teams to provide the Board's perspective in this regard.
What are the commonly accepted states of the disease process?
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
113
The ABFP has used for many years the Disease Staging system produced by
Systemetrics
(originally developed at Jefferson Medical College). For disease processes
which don't have commonly
accepted staging criteria, we should use the Systemetrics system. However, for
those disorders which
have nationally accepted staging criteria, we should use those instead. For
example, the INC VI has
described the following stages for hypertension: Optimal, Normal, High-normal,
Stage 1, Stage 2, and
Stage 3.
What demographic/patient characteristics, risk factors, and behaviors
influence a patient's movement
from one stage to another? (e.g., obesity's influence on hypertension and
diabetes)?
Issues such as age, gender, family history, body habitus, behaviors,
occupational exposures, etc,
affect the likelihood that an individual's disease will progress (or regress)
from one stage to another. We
will need information regarding 1) what are the important risk factors, 2)
what is the magnitude of these
factors' impact on the disorder's progress, and 3) what is the approximate
time frame for these changes?
How do particular characteristics vary within a given stage of illness (e.g.,
what blood pressure ranges
would we expect in Stage I, II, etc., for hypertension)?
This relates to the stage descriptions alluded to above; however, individuals
within a given
disease stage will also exhibit some variability, ie patients within Stage I
of hypertension will
demonstrate a frequency distribution of systolic and diastolic blood pressures
within the stage definition.
These values might define normal distributions, uniform distributions, or some
totally skewed dispersion.
The group might not know the exact shape of these curves, but, to the extent
possible, should indicate
qualitatively what general configuration we should anticipate. Staff at A.T.I.
will generate these
distributions from literature sources.
How should these relationships appear in the Bayesian network format?
As noted earlier, the model uses Bayesian networks extensively to depict
relationships, effects
of therapy, progression of disease, choice of therapy, calculation of drug
doses, and results of diagnostic
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
114
testing. As the group identifies health states, the members should enumerate
the demographic
characteristics which influence state transitions, attributes which influence
selection oftherapy, etc. Each
disease domain will include literally dozens of these structures. Although the
team will not be expected
to construct and develop fully each of these networks, the knowledge engineer
will need guidance in
what networks to develop. Again, we do not expect the team members to become
facile in the creation
of these networks; however, once developed, the team will have the opportunity
to observe the behavior
of these structures to confirm that they behave as intended.
What therapeutic modalities (pharmacologic, nonpharmacologic) exist to modify
the progression and/or
severity of the disease process? (e.g., magnitude of effect of weight loss on
blood pressure in Stage I,
Stage II. etc, how much will weight loss lower blood pressure? How much will
weight loss decrease the
likelihood of progressing from Stage Ito Stage II hypertension?)
We will need information regarding optimal recommended therapies,
pharmacologic and
nonpharmacologic, which we would expect family physicians to employ in
managing the particular
health state. Additionally, we need some indication about how the therapy
affects the disease process.
For example, does weight loss decrease blood pressure, and by how much (large,
moderate, small
amount)?
What Guidelines exist to describe optimal management plans for the disease
process (e.g., JNC VI for
hypertensions?
To whatever extent possible, we want the system to reflect well-done and
broadly-accepted
clinical guidelines. For some of the domains, no such documents exist and we
will have to create our own
"guideline" as we develop the health state. For others, such as hypertension,
fairly extensive and accepted
guidelines exist (e.g.,. JNC VI), and the system should reflect these
guidelines as closely as possible.
Also, we should attempt to utilize ABFP reference guides for those domains for
which the Board has
produced these documents.
CA 02369425 2001-10-03
WO 00/60431 PCT/US00/08942
115
For a given health state, what management and diagnostic concepts should we
emphasize in creating the
knowledge base (we can't reproduce Harrison's in the knowledge base, nor
should we - the system has
to be good enough, not necessarily exhaustive)?
We will attempt to include a family physician ABFP Board of Directors member
on each team
to provide Board input into health state emphases. In developing diabetes
mellitus and osteoarthritis, we
frequently find ourselves saying, "That's an issue for ABFP to decide." For
example, in assessing a
candidate's ability to manage osteoarthritis of the hand, do we want to
investigate the candidate's ability
to interpret a hand radiograph, or rather do we want to know how the candidate
uses the information that
a patient's x-ray demonstrates stigmata of osteoarthritis? The first question
deals with a psychomotor
skill (radiograph interpretation), while the second question assesses the
candidate's cognitive knowledge
regarding therapy for osteoarthritis. Having an ABFP Board member on each
committee should help
provide ABFP input into such decisions. Nevertheless, committee member input
may be highly valuable
to the Board, and we encourage members to contemplate these issues: What are
the critical commissions
and omissions in care plans for these patients? What are the simplest
approaches to improving length and
quality of life? What are the common mistakes in clinical care? What are the
new insights into
appropriate clinical care? What are likely to be the testable concepts related
to this health state domain?
What parallel health states should the model reflect for the primary disorder
in question?
For osteoarthritis, what other health conditions might influence the progress
and/or management
of the arthritis? For example, obesity certainly has an impact on the progress
of osteoarthritis.
Additionally, the presence of peptic ulcer disease will have a substantial
impact on therapeutic options.
Extended use of NSAID's could influence renal function. In this context,
obesity, peptic ulcer disease,
and renal function represent parallel health states: conditions which coexist
and interact with
osteoarthritis.
What multimedia resources will we need to represent adequately the clinical
findings associated with the
health staters?
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
116
One of the advantages of computer-based testing is the ability to present a
variety of media to
the candidate: sound, video, still-photographs and graphics can all enhance
the system's appearance and
provide the ability to assess psychomotor skills in real time. For each of the
health states, what media
should we acquire and how should we use these resources? For example, as we
develop a module on
heart failure, do we need third heart sounds and chest x-rays? Media represent
a typical
cost-effectiveness question in assessment: these resources cost substantial
amounts. Does the information
gained from presenting the media justify the acquisition cost? There's no easy
answer to this, but we need
to keep in mind that, for the most part, we will have to purchase a lot of
this material. Obviously, we can
use photographs currently in an item bank. Optionally, video and sounds come
from external sources.
Are there testable concepts that require a physical model to test in
sufficient detail (performing a
sigmoidoscopic examination, suturing)?}
Further, as indicated herein, the present invention may be applied across a
broad range of
programming languages that utilize similar concepts as described herein. The
present invention may also
be used in a distributed environment/architecture, optionally using thin
client technology.
Figure 21 is an illustration of the architecture of the combined internet,
POTS, and ADSL
architecture for use in the present invention in accordance with another
embodiment. In Fig. 21, to
preserve POTS and to prevent a fault in the ADSL equipment 254,256 from
compromising analog voice
traffic 226, 296 the voice part of the spectrum (the lowest 4 kHz) is
optionally separated from the rest
by a passive filter, called POTS splitter 258, 260. The rest of the available
bandwidth - from about 10
kHz to 1 MHz - carries data at rates up to 6 bits per second for every hertz
of bandwidth from data
equipment 262, 264, 294. The ADSL equipment 256 then has access to a number of
destinations
including significantly the Internet 268, and other destinations 270, 272.
To exploit the higher frequencies, ADSL makes use of advanced modulation
techniques, of
which the best known is the discrete multitone (DMT) technology. As its name
implies, ADSL transmits
data asymmetrically - at different rates upstream toward the central office
252 and downstream toward
the subscriber 250.
CA 02369425 2001-10-03
WO 00/60431 PCT/USO0/08942
117
Cable television providers are providing analogous Internet service to PC
users over their TV
cable systems by means of special cable modems. Such modems are capable of
transmitting up to 30
Mb/s over hybrid fiber/coal systems, which use fiber to bring signals to a
neighborhood and coax to
distribute it to individual subscribers.
Cable modems come in many forms. Most create a downstream data stream above 50
MHz (and
most likely 550 MHz) and carve an upstream channel out of the 5-50-MHz band,
which is currently
unused. Using 64-state quadrature amplitude modulation (64 QAM), a downstream
channel can
realistically transmit about 30 Mb/s (the oft-quoted lower speed of 10 Mb/s
refers to PC rates associated
with Ethernet connections). Upstream rates differ considerably from vendor to
vendor, but good hybrid
fiber/coax systems can deliver upstream speeds of a few megabits per second.
Thus, like ADSL, cable
modems transmit much more information downstream than upstream.
The internet architecture 220 and ADSL architecture 254, 256 may also be
combined with, for
example, user networds 222, 224, and 228. As illustrated in this embodiment,
users may access or use
or participate in the administration, management computer assisted program in
computer 240 via various
different access methods. In this embodiment, the various databases 230, 232,
234, 236 and/or 238 are
accessible via access to and/or by computer system 240, and or via
internet/local area network 220. These
databases may optionally include objective criteria for evaluating the
corporate governance
characteristics for ranking the corporation.
For example, environmental data is generally publicly available which
indicates a corporation's
compliance history, outstanding violations or potential violations, and the
like. Similarly, standard legal
and/or regulatory and/or administrative and/or business databases may be
consulted to obtain additional
information on corporate governance techniques, potential for government
intervention, shareholder
participation and/or customer loyalty. All this data may then be collected and
analyzed to determine the
overall attributes of the corporate, shareholder, government, and customer
agents, for input into the'
simulation. Alternatively, the individual data may be used and input into the
simulation, and the
simulation may digest or process the data individually or collectively as part
of the simulation.
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
118
In accordance with this embodiment, workstation 240 optionally includes
modules 242, 246, 248,
and 250 for individually handling the operations/simulation of the different
agents. Alternatively, one
module or a different number of modules may be used for processing the agent
relationships, processes,
and or interactions.
Alternatively, users may access or use or participate in the simulation
program for decision
making, indexing, ranking, and the like, via various different access methods
as well. The above
embodiments are only to be construed as examples of the various different
types of computer systems
that may be utilized in connection with the computer assisted and/or
implemented process for decision
making, indexing, ranking, with respect to corporate governance.
Of course, another result of the simulation is identifying companies for
investment purposes, and
actually investing in these companies. Further, the actual investments may be
done manually and/or
electronically, and optionally over the internet.
According to another embodiment, the instant invention includes a method for
evaluating or
educating a user, such as a physician, for example, as shown in Figure 25. In
Step S 100, parallel health
state networks, for example, describing disease evolution in various
medical/domains or health states are
generated by a computer or a user. In Step S110, a knowledge base is scripted
by a user or a computer
using belief networks and/or causal probabilistic networks, such as Bayesian
networks, and based, at
least in part, on the generated parallel health state networks. In step S 120,
the computer instantiates a
model or virtual patient, at least in part, from the scripted knowledge base
and displays to the user one
or more non-normal health states of the model or virtual patient. In step
S130, the user inputs one or
more courses of action to address the one or more non-normal health states.
Alternatively, the user inputs
a query to the computer for a specific medical finding, for the patient such
as would be obtained by
running a medical test or examination on the patient. In such an event, the
computer provides to the user
the requested medical finding and returns to step S 130. In step S 140, the
computer evolves the mode]
or virtual patient based, at least in part, on the generated parallel health
state networks and the user input.
In step 5150, the computer determines whether the user has completed treatment
of to patient. if not,
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
119
method flow returns to step S130. Otherwise, in step S160, the user's inputted
courses of action and
queries are evaluated relative to accepted norms of medical practice by the
computer.
By way of example, Figure 26 shows additional or alternative steps for
evaluating a user. In step
S200, decision variables, which can be controlled, such as courses of action,
and utility variables, which
are to be optimized, such as health states of medical domain in parallel
networks are generated by a user
or a computer. In step 5210, a decision network based on the belief networks
or causal probability
networks, the decision variables, and the utility variables, is generated to
determine an optimum
treatment for the instantiated model or virtual patient. In step S220, the
computer compares the generated
optimum treatment with the courses of action and queries inputted by the user.
The method flow then
proceeds to step 150 as discussed above.
In another embodiment, the instant invention includes an expert system. By way
of example, the
expert system is a stand-alone unit. Alternatively, as shown by way of example
in Figure 21, the expert
system is communicatable with a user via a computer network. The computer
network includes, for
example, POTS for a dial-up expert system and/or the Internet or WAN for a Web-
accessible expert
system.
In one embodiment of the expert system, the instant invention includes a
program having
instructions for executing the expert system. By way of example, in
Instruction S300, the computer
receives patient data for an actual by user input. In Instruction S310, the
computer instantiates a virtual
patient having characteristics consistent with the received patient data and
based, at least in part, on one
or more belief networks and/or causal probabilistic networks describing
disease or health state evolution.
In Instruction S320, the computer generates a query to the user for a specific
medical finding concerning
the actual patient, or a course of action based, at least in part, on the
instantiated virtual patient and the
one or more belief networks or causal probabilistic networks. In Instruction
S330, the computer receives
the specific medical finding from the user responsive to the generated query.
In Instruction 5340, the
computer evolves the instantiated virtual patient in accordance with the above-
mentioned belief networks
and/or causal networks and the received specific medical finding and/or the
generated course of action.
In Instruction S350, the computer determines whether the user has dispensed
complete treatment of the
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
120
actual patient based, at least in part, on the generated courses of action,
for example, for a given medical
visit or encounter. If not, method flow is returned to Instruction S320. In
Instruction S360, the computer
stores the volved virtual model for subsequent access by the user. In
Instruction S370, the computer
and/or the user repeat Instructions S320-S370, for example, for each
subsequent medical visit or
encounter until treatment of the actual patient is completed.
In another embodiment, the instant invention includes a standard thin client
or other standard
client workstation that is programmably connected via a computer network to an
expert system, such as
described above. Alternatively, or in addition, the thin client or other
standard client workstation is
programmably connected via a computer network to an educational or testing
system, such as described
above.
In another embodiment, the instant invention includes a knowledge base module
describing, for
example, disease or health state evolution by way of belief networks or causal
probabilistic networks,
such as Bayesian networks. Advantageously, the knowledge base module enables a
user or educator to
update a knowledge base with current medical beliefs and practices. By way of
example, earlier it was
believed that ulcers were caused by certain foods and/or stress levels. Recent
studies indicate that at least
some ulcers are caused by bacteria, which should, of course, be treated by an
appropriate antibiotic. Such
a treatment would not have been recommended or accepted by a knowledge base
that only reflected the
earlier understanding of ulcers. As another example, advances in laboratory
test or scanning, which
become accepted in the general medical community, are advantageously included
in the knowledge base
module. For instance, such advances are included in reveal structures or
management plan critiques,
which are described using belief networks or causal probabilistic networks,
such as Bayesian networks.
In another embodiment of the instant invention, the instant causal
probabilistic expert systems
have medical applications. An example of a medical application includes
determining optimal antibiotic
selections for an actual patient based at least in part on the patients
clinical characteristics and one or
more parallel causal probabilistic or belief networks describing health
states. Another example of a
medical application includes determining a specific chemotherapy regimen among
several possible
CA 02369425 2001-10-03
WO 00/60431 PCT/USOO/08942
121
regimens for treating a cancer based, at least in part on, the patient's
clinical characteristics and one or
more parallel belief or causal probabilistic networks describing health
states.
In an another embodiment ofthe instant invention, the instant causal
probabilistic expertsystems
have non-medical applications. An example of a non-medical application
includes determining credit
worthiness for loan approval of an applicant in the financial industry based,
at least in part, on the
applicant's suitability characteristics and one or more parallel causal
probabilistic or belief networks
describing personal financial states. The personal financial states include
financial states relative to
balances and payment history, of for example, car loans, home mortgages,
credit cards, student loans,
business loans, total asset value, cash flow from one or more income sources,
and total liabilities.
Another example of a non-medical application includes determining optimal oil
drilling sites based, at
least in part, on one or more parallel causal probabilistic or belief networks
describing one or more
wildcatters' analysis for identifying potential sites for oil drilling.
The many features and advantages of the invention are apparent from the
detailed specification,
and thus, it is intended by the appended claims to cover all such features and
advantages of the invention
which fall within the true spirit and scope of the invention. Further, since
numerous modifications and
variations will readily occur to those skilled'in the art, it is not desired
to limit the invention to the exact
construction and operation illustrated and described, and accordingly, all
suitable modifications and
equivalents may be resorted to, falling within the scope of the invention.