Note: Descriptions are shown in the official language in which they were submitted.
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
TREATMENT OF TISSUE BY APPLICATION OF ENERGY AND DRUGS
Background of the Invention
1. Field of the Invention
This invention relates to treating body tissue, particularly to treating
body tissue by altering the shape, density, relative geometry or tension of
that body
tissue using energy or substances deployed from an interstitial location in
the body.
2. Related Art
Urinary incontinence results from a number of factors. Increasing age,
injury from childbirth and related stresses can cause the relative tone of the
bladder
and accessory muscles to weaken, which, in turn, causes an impaired ability to
retain
urine. Weight gain and overall deterioration of muscle tone can cause
increased
abdominal pressure which overcomes sphincter resistance. Nerve pathways that
cause the "urge" to urinate can become hyperactive. The relative tension of
the
urethra can change with age, causing poor urinary control. Injury to the
detrusor
muscles or to the trigone area also results in impaired urinary continence.
These factors do not usually occur by themselves. The typical patient
usually presents with two or more of them. Therefore, it is desirable to
provide a
treatment that can address many of these factors.
Given the complex etiology and varied causal factors, the ideal
treatment for urinary incontinence requires a device that can perform many
different
functions. For example, a treatment for female urinary incontinence might rely
upon
some or all of the following: ( 1 ) reshaping the bladder to alter the
urethrovesical
angle and resuspend the bladderneck, (2) manipulation of the detruser muscles,
(3)
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
mapping and modulating nervous pathways responsible for urinary urgency, (4)
reducing strain on the bladderneck by changing the structural geometry, (5)
shrinking
discrete and non-discrete areas of the bladder by creating thermal lesions,
(6) three-
dimensional modeling of tissue by adding bulk so as to achieve better closure
(7)
strengthening the structural integrity of a tissue by providing a pattern of
scar tissue
and (7) application of pharmaceutical agents both as a curative and to promote
healing post treatment.
The use of a catheter to apply radio frequency (RF) and other types of
energy to ablate tissue in the body (such as heart muscle tissue) is known in
the art of
cardiac treatment. However, known systems using RF and other types of energy
are
still subject to several drawbacks.
A first problem in the known art involves providing a device that can
perform all of the aforementioned functions. While known systems can perform
one
or more of these functions, nothing in the related art is capable of
performing all of
these functions. Patients are frequently required to return for multiple
treatments
until a cure is finally effected.
A second problem in the known art involves identification, modulation
and/or stimulation of nerves in the targeted tissue. Known systems do not
provide for
protection of sensitive nerves during treatment or allow nerves to be
identified and
stimulated. This is particularly problematic because many tissue disorders,
especially
those involving tone or contractile ability of a sphincter, arise from
afferent and
efferent nerves are either under-stimulated or over-stimulated.
A third problem in the known art involves providing a treatment surface
that can reach all of the desired treatment areas, such as the entire surface
of the
detrusor muscles. While the use of a catheter to deploy energy is known, none
is
disposed to flexibly adapt to the interior shape of an organ so as to provide
optimal
uniform treatment.
2
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
A fourth problem in the known art involves removal of tissue and
substances used in treatment. Known systems do not provide for removal of
excess
substances used in treatment such as cooling fluids, collagen or bulking
substances.
Similarly, known systems do not provide for removal of substances that hinders
or
otherwise obstructs the healing process such as pus, purulent discharges,
suppuration
and pockets of infection.
A fifth problem in the known art involves directing and positioning the
electrodes in the body cavity or orifice. Difficulties in accurately
positioning the
electrodes in the target orifice detract from treatment. Frequently, unhealthy
tissue
remains untreated while healthy tissue is compromised. Difficulties in
directing and
positioning the electrodes are particularly problematic because one of the
goals of
treatment is to minimize collateral damage to healthy tissue and to completely
treat
diseased tissue.
A sixth problem in the known art involves minimizing thermal injury to
the patient. Some known systems rely upon simultaneous application of energy
and
infusion of a cooling liquid into the targeted area for treatment. While such
infusion
of liquid minimizes thermal injury to the patient, it is not applicable to all
parts of the
body. For example, infusion of cooling liquids into an internal body cavity
such as a
bladder, uterus, or stomach can rupture the targeted organ or cause osmotic
imbalance
within the tissue.
A seventh problem in the known art involves difficulty in the
simultaneous use of complimentary technology. Known systems do not provide for
optimal, simultaneous use of auxiliary tools for visualization, monitoring pH
and
pressure or drug administration.
A eighth problem in the known art is that it can be difficult to block the
flow of bodily fluids and gases into an area of the body where tissue ablation
is taking
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
place. Bodily fluids can dissipate and detrimentally absorb the energy to be
applied
to the tissue to be ablated. Dissipation of bodily fluids detracts from the
goal of
treatment of diseased tissue.
Accordingly, it would be advantageous to provide a method and
apparatus for treatment for body structures, especially internal body
structures
involving unwanted features or other disorders, that does not require
relatively
invasive surgery, and is not subject to other drawbacks noted with regard to
the
known art. This advantage is achieved in an embodiment of the invention in
which a
relatively minimally invasive catheter is inserted into the body, a variety of
different
treatments of the body structures is applied using electrodes and a cooling
element,
and the unwanted features or disorders are relatively cured.
Summary of the Invention
The invention provides a method and system for treating disorders of
the genito-urinary tract and other disorders in other parts of the body. A
particular
treatment can include one or more of, or some combination of ablation, nerve
modulation, three-dimensional tissue shaping, drug delivery, mapping,
stimulating,
shrinking (by creation of a pattern of thermal lesions) and reducing strain on
structures by altering the geometry thereof and providing bulk to particularly
defined
regions.
The particular body structures or tissues can include one or more of, or
some combination of regions, including the bladder, esophagus, vagina, penis,
larynx,
pharynx, aortic arch, abdominal aorta, thoracic aorta, large intestine, small
intestine,
sinus, auditory canal, uterus, vas deferens, trachea and all associated
sphincters.
In one aspect of the invention, a catheter is deployed in the body. It
may enter the body via a natural orifice, a stoma, or a surgically created
opening that
4
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
is made for the purpose of inserting the catheter. Insertion may be
facilitated with the
use of a guide wire or a generic support structure or visualization apparatus.
In second aspect of the invention, the treatment can include application
of energy and substances to effect changes in the target tissue. Types of
energy that
can be applied include radiofrequency, laser, microwave, infrared waves,
ultrasound
or some combination thereof. Types of substances that can be applied include
pharmaceutical agents such as analgesics, antibiotics and anti-inflammatory
drugs,
bulking agents such as biologically nonreactive particles, cooling fluids or
dessicants
such as liquid nitrogen for use in cryo-based treatments.
Brief Description of the Drawings
Figure 1 is a block drawing of a system for treatment of female urinary
incontinence using a first device.
Figure 2 is a process flow drawing of a method for treatment of female
urinary incontinence using a first device.
Figure 3 is a block drawing of a system for treatment of female urinary
incontinence using a second device.
Figure 4 is a process flow drawing of a method for treatment of female
urinary incontinence using a second device.
Figure 5 is a block drawing of a system for treatment of female urinary
incontinence using a third device.
Figure 6 is a flow drawing of a method for treatment of female urinary
incontinence using a third device.
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
Detailed Description of the Preferred Embodiment
In the following description, a preferred embodiment of the invention is
described with regard to preferred process steps and data structures.
Embodiments of
the invention can be implemented using general-purpose processors or special
purpose processors operating under program control, or other circuits, adapted
to
particular process steps and data structures described herein. Implementation
of the
process steps and data structures described herein would not require undue
experimentation or further invention.
System Elements
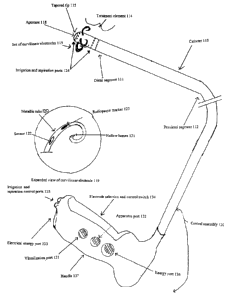
Figure 1 is a block drawing of a system for treatment of female urinary
incontinence using a first device.
A system 100 includes a catheter 110, a treatment element 114, a
control assembly 130 and a shielding element 140. In an alternative
embodiment, the
shielding element 140 is not present.
The Catheter
The catheter 110 includes a distal segment 111 and a proximal segment
112. The distal segment 111 and proximal segment 112 form one continuous
piece.
Two or more lumens 113 (not shown) run the entire interior length of the
catheter
110 and are coupled to the control assembly 140. It is through these lumens
113 that
energy is conducted and flowable substances are exuded.
The distal segment 111 includes a treatment element 114 and a tapered
tip 115. In a preferred embodiment, the tapered tip 115 is rigid so as to
allow easy
insertion into a urethra. In other preferred embodiments, the tapered tip 115
may be
6
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
of varying degrees of flexibility depending where it in the body it is
deployed. In
alternative embodiments, the catheter 110 may be introduced into the target
tissue
using an introducer sheath 116 or a guide wire 117 (not shown). The most
distal end
of the tapered tip 115 includes an aperture 118. Substances that flow through
the
lumens 113 may be applied to the tissue through this aperture 118.
In a preferred embodiment, the distal segment 111 is disposed for
insertion into a cavity of the body such as a female urethra and bladder. In
alternative
embodiments, the cavity may include one or more of, or some combination of the
following:
Any portion of the bronchial system, the cardiovascular system, the
genito-urinary tract, the lymphatic system, the pulmonary system, the vascular
system, the locomotor system, the reproductive system or other systems in the
body;
Any biological conduit or tube, such as a biologic lumen that is patent
or one that is subject to a stricture;
Any biologic operational structure, such as a gland, or a muscle or other
organ (such as the colon, the diaphragm, the heart, a uterus, a kidney, a
lung, the
rectum an involuntary or voluntary sphincter);
Any biologic structure, such as a herniated body structure, a set of
diseaseed cells, a set of displastic cells, a surface of a body structure,
(such as the
sclera) a tumor, or a layer of cells (such as fat, muscle or skin).
Any biologic cavity or space or the contents thereof, such as a cyst, a
gland, a sinus, a layered structure, or a medical device implanted or inserted
in the
body;
7
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
The Treatment Element
The treatment element 114 includes a set of curvilinear electrodes 119
and three sets of irrigation and aspiration ports 124.
The electrodes 119 contained in the set of electrodes are evenly spaced
around the tapered tip 115. Each electrode 119 includes a metallic tube 120
defining
a hollow lumen 121 and is disposed so that it curves away from the tapered tip
115
and has a barbed end, much like a fishhook. Being arced in this direction
allows the
device to be inserted easily into an orifice without causing unintended tissue
damage.
Once the device is inserted, the barbed ends of electrodes 119 grab the tissue
of the
bladderneck and upper urethra in a claw-like manner and bunch it together.
Energy is
delivered through the electrodes to the bunched tissue, causing shrinkage to
occur in
the area surrounding the treatment element 114. This three dimensional shaping
improves continence by improving the structural integrity of the tissue.
In a preferred embodiment, there are four electrodes 119. Other
preferred embodiments may have more or less than four electrodes. Each
electrode
119 is coupled to at least one sensor 122 capable of measuring such factors as
temperature, conductivity, pressure, impedance and other variables. In a
preferred
embodiment, each electrode is also coupled to a radiopaque marker 123 for use
in
fluoroscopic visualization.
In a preferred embodiment, the electrodes 119 can be operated
separately or in combination with each other. Treatment can be directed at a
single
area or several different areas of a bladder or other orifice by operation of
selective
electrodes. Different patterns of submucosal lesions, mucosal lesions,
ablated,
bulked, plumped, desiccated or necrotic regions can be created by selectively
operating different electrodes. Production of different patterns of treatment
makes it
possible to remodel tissues and alter their overall geometry with respect to
each other.
8
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
Each electrode 119 can be disposed to treat tissue by delivering one or
more of, or some combination or any of the following in either a unipolar or
bipolar
mode:
~ Radiofrequency (RF) energy, such as RF in about the 300 kilohertz
to 500 kilohertz range;
~ Chemical treatments, such as acids, antibiotics, enzymes,
radioactive tracers or other bioactive substances;
~ Infrared energy, such as from an infrared laser or diode laser;
~ Microwave energy, such as electromagnetic energy in about the 915
megahertz or 2.45 gigahertz range;
~ Sonic energy, including ultrasound;
~ Photodynamic therapy (PDT)
~ Non-infrared laser energy
~ Cryothermia
In addition to treating tissues by delivering energy, the set of electrodes
119 are disposed to deliver at least one flowable substance to the area of the
body
where treatment is to take place. In a preferred embodiment, the flowable
substance
includes water which aids in cooling of body structures during RF application.
However, in alternative embodiments, the deliverable flowable liquids include
other
substances, including saline, anesthetic drugs, anti-inflammatory agents,
chemotherapeutic agents, systemic or topical antibiotics, collagen and
radioactive
substances such as labeled tracers. In one alternative embodiment, saline is
used to
increate the local conductivity of tissue, enhancing the penetration of RF
energy so as
to create larger lesions. The saline can be delivered through the needle
electrode
submucosally so as to achieve greatest effect.
Three rings of irrigation and aspiration ports 124 circle the distal end of
the catheter 110. Each ring contains numerous irrigation and aspiration ports
124,
evenly distributed around the width of the catheter. One ring of irrigation
and
aspiration ports 124 lies between the aperture 118 and the set of electrodes
119; the
9
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
other two rings of irrigation and aspiration ports 124 are located on the
proximal side
of the electrodes 119. Application of positive pressure makes irrigation and
cooling
of tissues is possible. Alternatively, application of negative pressure causes
the
tissue to be uniformly conformed around the treatment element 114, thereby
achieving the most optimal therapeutic value of the energy and substances.
The Control Assembly 130
The control assembly 130 includes a visualization port 13 l, an
apparatus port 132, an electrical energy port 133, an electrode selection and
control
switch 134, one or more irrigation and aspiration control ports 135, an
therapeutic
energy port 136 and a handle 137.
The visualization port 131 can be coupled to visualization apparatus,
such as fiberoptic device, flouroscopic device, an anoscope, a laparoscope, an
endoscope or other type of catheter.
The apparatus port 132 can be coupled to other medical devices that
may be useful during treatment such as a pH meter, a pressure monitor, drug
administration apparatus, or other device used to monitor or treat the
patient.
In a preferred embodiment, devices coupled to both the visualization
port 131 and the apparatus ports 132 are controlled from a location outside
the body,
such as by an instrument in an operating room or an external device for
manipulating
the inserted catheter 110.
In an alternative embodiment the apparatus port 132 may be coupled to
devices that are implanted or inserted into the body during a medical
procedure. For
example, the apparatus port 132 may be coupled to a programmed AICD
(artificial
implanted cardiac defibrillator), a programmed glandular substitute (such as
an
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
artificial pancreas) or other device for use during surgery or other medical
procedures.
The electrical energy port 133 includes a conductive element such as an
electrical adapter that can be coupled to a source of alternating or direct
current such
as a wall socket, battery or generator.
The electrode selection and control switch 134 includes an element that
is disposed to select and activate individual electrodes 119.
The irrigation and aspiration control ports 135 can be coupled to a
pump or other apparatus to deliver fluid through the aperture 118 or apply
suction
through the set of irrigation and aspiration ports 134.
The therapeutic energy port 136 includes a receptor port for coupling to
a source of any of the following types of therapeutic energy:
~ Radiofrequency (RF) energy, such as RF in about the 300 kilohertz
to 500 kilohertz range;
~ Chemical treatments, such as acids, antibiotics, enzymes,
radioactive tracers or other bioactive substances;
~ Infrared energy, such as from an infrared laser or diode laser;
~ Microwave energy, such as electromagnetic energy in about the 915
megahertz to 2.45 gigahertz range;
~ Sonic energy, including ultrasound;
~ Photodynamic therapy (PDT)
~ Non-infrared laser energy
~ Cryothermia
The handle 137 is disposed for manipulated by medical or veterinary
personnel and can be shaped for being held in the hand. The visualization port
131,
11
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
the apparatus port 132, the electrical energy port 133, the electrode
selection and
control switch 134 and the one or more irrigation and aspiration control ports
135 and
the therapeutic energy port 136 are all mounted in the handle 137 to allow for
easy
operation.
The Shielding Element
The shielding element 140 lies on the proximal side of treatment
element 114 and is disposed to isolate the treatment area. It can also help
position the
catheter 110 in the body. For example, in a preferred embodiment in which the
catheter 110 is inserted into the urethra, the shielding element 140 can
prevent the
catheter 110 from being inserted further into the urethral canal and prevent
substances
used in treatment from escaping. In an alternative embodiment, the shielding
element 140 is optional.
Figure 2 is a process flow drawing of a method for treatment of female
urinary incontinence using a first device.
A method 200 is performed by a system 100, including a catheter 110
and a control assembly 140. Although the method 200 is described serially, the
steps
of the method 200 can be performed by separate elements in conjunction or in
parallel, whether asynchronously, in a pipelined manner, or otherwise. There
is no
particular requirement that the method 200 be performed in the same order in
which
this description lists the steps, except where so indicated.
At a flow point 200, electrical energy port 133 is coupled to a source of
electrical energy. The patient has voided and is positioned on a treatment
table, in an
appropriate position such as horizontal, jackknife or lithotomy. Due to the
potential
for inducing pain, the area surrounding the urinary meatus may be pretreated
with a
topical anesthetic before insertion of the catheter 110; depending upon the
circumstances, a muscle relaxant or short term tranquilizer may be indicated.
The
12
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
position of the patient and choice of pharmaceutical agents to be used are
responsive
to judgments by medical personnel.
At a step 201, the patient's external genitalia and surrounding anatomy
are cleansed with an appropriate agent such as Betadine, or benzalkonium
chloride
At a step 202, the visualization port 131 is coupled to the appropriate
visualization apparatus, such as a flouroscope, an endoscope, a display screen
or
other visualization device. The choice of visualization apparatus is
responsive to
judgments by medical personnel.
At a step 203, the apparatus port 132 is coupled to an external medical
device such as a pH meter, a pressure gauge, or other such equipment. The
choice of
apparatus is responsive to judgments by medical personnel.
At a step 204, the therapeutic energy port 136 is coupled to a source of
any of the aforementioned types of therapeutic energy.
At a step 205, the tapered tip 115 is well lubricated and introduced into
the urethral meatus in an upward and backward direction, in much the same way
a
Foley catheter 110 is introduced.
In a step 206, the catheter 110 is threaded through the urethra until the
treatment element 114 is at the further reaches of the trigone region. An
introducer
sheath 116 or guidewire 117 may also be used to facilitate insertion.
In a step 207, the position of the catheter 110 is checked using
visualization apparatus coupled to the visualization port 131. The position of
the
treatment element 114 is adjusted, if necessary, so that the electrodes 119
have
grabbed onto the tissue and are bunching it together. This apparatus can be
continually monitored by medical professionals throughout the procedure.
13
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
In a step 208, irrigation and aspiration control port 135 is manipulated
so as to exude a cooling liquid such as sterile water, saline or glycerin from
the
aperture 118 into the lower region of the bladder. This cooling fluid lowers
the
relative temperature of the targeted tissues and prevents collateral thermal
damage.
In alternative embodiments, other devices may be coupled to the apparatus port
132
to chill the cooling fluid or to cause sonic cooling, gas expansion, magnetic
cooling
or others cooling methodologies. The choice of cooling fluid or methodology is
responsive to judgments by medical personnel.
In a step 209, electrodes 119 are selected using the electrode selection
and control switch 134. In a preferred embodiment, all electrodes are deployed
at
once. In another preferred embodiment, electrodes may be individually
selected.
This step may be repeated at any time prior to step 217.
In a step 210, suction apparatus is coupled to the irrigation and
aspiration control ports 135 so that suction may be effected through the
irrigation and
aspiration ports 124. The tissue surrounding the treatment element 114 may be
aspirated so as to conform it to the treatment element 114. The aspiration
also
removes excess cooling fluid that was supplied in step 209.
In a step 211, the therapeutic energy port 136 is manipulated so as to
cause a release of energy from the electrodes 119. The duration and frequency
of
energy are responsive to judgments by medical personnel. This release of
energy
creates a pattern of lesions in the mucosal and submucosal tissues of the
trigone
region. The affected area shrinks and is relatively strengthened, so as to
better retain
urine. Alternatively, a different method of treatment can be effected by
partially or
completely ablating nerves responsible for the sensation of urinary urgency.
In a step 212, the catheter 110 is repositioned so that the treatment
element 114 is closer to the bladder neck. Prior to repositioning the catheter
110, the
14
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
electrodes 119 are either retracted or covered by the introducer sheath 116 to
prevent
unintended damage to tissue while the catheter is being moved.
In a step 213, the energy port 137 is manipulated so as to cause a
release of energy from the electrodes 119. The duration and frequency of
energy are
responsive to judgments by medical personnel. This release of energy creates
another
pattern of lesions in the mucosal and submucosal tissues of the trigone area.
The
affected tissue shrinks and is relatively strengthened, so as to better retain
urine. By
creating a selective pattern of lesions in various areas as in steps 211 and
215, the
three-dimensional modeling of the trigone area can be affected. Alternatively,
a
different method of treatment can be effected by partially or completely
ablating
nerves responsible for the sensation of urinary urgency.
In a step 214, the catheter 110 is repositioned for a final time so that the
treatment element 114 is immediately adjacent to the bladder neck. Prior to
repositioning the catheter 110, the electrodes 119 are either retracted or
covered by
the introducer sheath 116 to prevent unintended damage to tissue while the
catheter is
being moved.
In a step 215, the energy port 137 is manipulated so as to cause a
release of energy from the electrodes 119. The duration and frequency of
energy are
responsive to judgments by medical personnel. This release of energy creates
another
pattern of lesions in the submucosal and mucosal tissues around the bladder
neck.
The affected tissue shrinks and is relatively strengthened, so as to better
retain urine.
Taken together with the lesions, created in step 211, and 213, the trigone
area has
been completely remodeled so that the bladder has shrunk and resuspended
itself.
The relative pressure on the bladder neck is relieved. The scar tissue created
by
application of the energy is stronger and better able to resist abdominal
pressure on
the sphincter.
15
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
In a step 216, the irrigation and aspiration control port 135 is
manipulated so as to stop the flow of cooling liquid from the aperture 118.
In a step 217, pharmaceutical agents may be locally administered by
manipulating the irrigation and aspiration control ports 135. These agents may
help
include lubricants, anesthetics, anti-spasmodics, anti-inflammatories,
antiobiotics or
other agents as deemed appropriate by the judgment of medical personal. This
step
may occur any time prior to withdrawal of the catheter 110, to either pretreat
tissue or
post treat tissues.
In a step 218, the catheter 110 is withdrawn from the urethra.
Figure 3 is a block drawing of a system for treatment of female urinary
incontinence using a second device.
A system 300 includes a catheter 310, a microporous treatment balloon
320, a control assembly 330 and a shielding element 340 (not shown). In an
alternative embodiment, the shielding element 340 is not present.
The Catheter 310
The catheter 310 includes two or more lumens 311 (not shown) and a
translation member 312. The two or more lumens 311 and translation member 312
traverse the entire interior length of the catheter 310. The catheter 310 and
lumens
311 are coupled at a distal end to a treatment balloon 320; they are coupled
at a
proximal end to a control assembly 330. The translation member 312 is coupled
to
the distal end of the treatment balloon 320; it is coupled at the proximal end
to a
control assembly 330.
In a preferred embodiment, the catheter 310 and treatment balloon 320
are introduced into cavity of the body, such as a female urethra and bladder
using an
16
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
introducer sheath 313 or a guide tube 314. In alternative embodiments, the
cavity
may include one or more of, or some combination of the following:
~ Any portion of the bronchial system, the cardiovascular system, the genito-
urinary
tract, the lymphatic system, the pulmonary system, the vascular system, the
locomotor system, the reproductive system or other systems in the body;
~ Any biological conduit or tube, such as a biologic lumen that is patent or
one that
is subject to a stricture;
~ Any biologic operational structure, such as a gland, or a muscle or other
organ
(such as the colon, the diaphragm, the heart, a uterus, a kidney, a lung, the
rectum
an involuntary or voluntary sphincter);
~ Any biologic structure, such as a herniated body structure, a set of
disease4d cells,
a set of displastic cells, a surface of a body structure, (such as the sclera)
a tumor,
or a layer of cells (such as fat, muscle or skin).
~ Any biologic cavity or space or the contents thereof, such as a cyst, a
gland, a
sinus, a layered structure, or a medical device implanted or inserted in the
body;
The Microporous Treatment Balloon 320
The microporous treatment balloon 320 is comprised of a relatively
flexible and heat resistant material such as Kevlar, polyurethane, polyvinyl
chloride
(PVC), polyamide, PET, nylon or other materials. The shape of the balloon can
be
manipulated by varying the degree of inflation and the amount of tension
placed on
the translation member 312. By varying the degree of inflation and the tension
on the
translation member, the surface of the treatment balloon can be brought in
contact
with the entire interior surface of the muscles, including the detruser
muscles and the
top of the bladder. In this way, it is possible to treat the entire organ
simultaneously.
17
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
The treatment balloon 320 also includes a flexible basket-like structure
321 and a set of surface electrodes 322. The basket-like structure 321 has
horizontal
and vertical members that completely encompass the balloon 320. The set of
surface
electrodes 322 are evenly distributed on all the members of the basket-like
structure
321. Each electrode 322 includes a sensor 323 to measure temperature,
pressure,
impedance, flow, nervous activity, pH, conductivity or other property of the
tissue or
treatment. Each surface electrode 322 is also coupled to a radiopaque marker
324 for
use in fluoroscopic visualization.
In an alternative embodiment, the surface electrodes 322 and sensors
323 are embedded directly into the exterior surface of the microporous
treatment
balloon 320. In this preferred embodiment, the basket-like structure 321 is
optional.
In both the preferred and alternative embodiments, the electrodes 322
can be operated separately or in combination with each other. Treatment can be
directed at a single area, several different areas, or the entire interior of
a bladder or
other orifice by operation of selective electrodes. Different patterns of
submucosal
lesions, mucosal lesions, ablated, bulked or plumped, desiccated or necrotic
regions
can be created by selectively operating different electrodes. Production of
different
patterns of treatment makes it possible to remodel tissues and alter their
overall
geometry with respect to each other.
Each electrode 322 can be disposed to treat tissue by delivering one or
more of, or some combination or any of the following in either a unipolar or
bipolar
mode:
~ Radiofrequency (RF) energy, such as RF in about the 300 kilohertz
to 500 kilohertz range;
~ Chemical treatments, such as acids, antibiotics, enzymes,
radioactive tracers or other bioactive substances;
~ Infrared energy, such as from an infrared laser or diode laser;
18
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
~ Microwave energy, such as electromagnetic energy in about the 915
megahertz or 2.45 gigahertz range;
~ Sonic energy, including ultrasound;
~ Photodynamic therapy (PDT)
~ Non-infrared laser energy
~ Cryothermia
In addition to treating tissues by delivering energy, the set of electrodes
322 and the micropores in the balloon 320 are disposed to deliver at least one
flowable substance to the area of the body where treatment is to take place.
In a
preferred embodiment, the flowable substance includes sterile water, which
aids in
cooling and hydration of body structures. In other preferred embodiments, the
flowable substance includes saline with a concentration of less than about 10%
NaCI,
which locally enhances tissue conductivity, resulting in a selective areas of
ablation
or creation of thermal lesions at or below the surface of the tissue. However,
in
alternative embodiments, the deliverable flowable liquids include other
substances,
including anesthetic drugs, anti-inflammatory agents, chemotherapeutic agents,
systemic or topical antibiotics, collagen and radioactive substances such as
labeled
tracers. In other alternative embodiments, the sensors on the electrodes are
used for
mapping the foci or pathways of electrical activity in the bladder, the
bladderneck or
urethra. This information is used to guide delivery of energy.
In other alternative embodiments, the balloon 320 is not microporous.
In this alternative embodiment, electrodes 322 or other energy delivery
devices may
be mounted upon or proximate to a surface of the balloon.
The Control Assembly 330
The control assembly 330 includes a visualization port 331, an
apparatus port 332, an electrical energy port 333, an electrode selection and
control
19
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
switch 334, one or more irrigation and aspiration control ports 335, an
therapeutic
energy port 336 and a handle 337.
The visualization port 331 can be coupled to visualization apparatus,
such as a fiberoptic device, a flouroscopic device, an anoscope, a
laparoscope, an
endoscope or other type of catheter.
The apparatus port 332 can be coupled to other medical devices that
may be useful during treatment such as a pH meter, a pressure monitor, drug
administration apparatus, or other devices used to monitor or treat the
patient.
In a preferred embodiment, devices coupled to both the visualization
port 331 and the apparatus ports 332 are controlled from a location outside
the body,
such as by an instrument in an operating room or an external device for
manipulating
the inserted catheter 310.
In an alternative embodiment the apparatus port 332 may be coupled to
devices that are implanted or inserted into the body during a medical
procedure. For
example, the apparatus port 332 may be coupled to a programmed AICD
(artificial
implanted cardiac defibrillator), a programmed glandular substitute (such as
an
artificial pancreas) or other device for use during surgery or other medical
procedures.
The electrical energy port 333 includes a conductive element such as an
electrical adapter that can be coupled to a source of alternating or direct
current such
as a wall socket, battery or generator.
The electrode selection and control switch 334 includes an element that
is disposed to select and activate individual electrodes 322.
20
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
The irrigation and aspiration control ports 335 can be coupled to a
pump or other apparatus to inflate or deflate the balloon and deliver fluids
through the
micropores of the treatment balloon 320.
The therapeutic energy port 336 includes a receptor port for coupling to
a source of any of the following types of therapeutic energy:
~ Radiofrequency (RF) energy, such as RF in about the 300 kilohertz
to 500 kilohertz range;
~ Chemical treatments, such as acids, antibiotics, enzymes,
radioactive tracers or other bioactive substances;
~ Infrared energy, such as from an infrared laser or diode laser;
~ Microwave energy, such as electromagnetic energy in about the 915
megahertz or 2.45 gigahertz range;
~ Sonic energy, including ultrasound;
~ Photodynamic therapy (PDT)
~ Non-infrared laser energy
~ Cryothermia
The handle 337 is disposed for manipulated by medical or veterinary
personnel and can be shaped for being held in the hand. The visualization port
331,
the apparatus port 332, the electrical energy port 333, the electrode
selection and
control switch 334 and the one or more irrigation and aspiration control ports
335 and
the therapeutic energy port 336 are all mounted in the handle 337 to allow for
easy
operation.
The Shielding Element 340
The shielding element 340 lies on the proximal side of the microporous
treatment balloon 320 and is disposed to isolate the treatment area. It can
also help
position the catheter 310 in the body. For example, in a preferred embodiment
in
21
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
which the catheter 310 is inserted into the urethra, the shielding element 340
can
prevent the catheter 310 from being inserted further into the urethral canal
or bladder
and prevent substances used in treatment from escaping. In an alternative
embodiment, the shielding element 340 is optional.
Figure 4 is a process flow drawing of a method for treatment of female
urinary incontinence using a second device.
A method 400 is performed by a system 300 including a catheter 310, a
treatment balloon 320 and a control assembly 330. Although the method 400 is
described serially, the steps of the method 400 can be performed by separate
elements
in conjunction or in parallel, whether asynchronously, in a pipelined manner,
or
otherwise. There is no particular requirement that the method 400 be performed
in
the same order in which this description lists the steps, except where so
indicated.
At a flow point 400, electrical energy port 333 is coupled to a source of
electrical energy. The patient has voided and is positioned on a treatment
table, in an
appropriate position such as horizontal, jackknife or lithotomy. Due to the
potential
for inducing pain, the area surrounding the urinary meatus may be pretreated
with a
topical anesthetic before insertion of the catheter 310; depending upon the
circumstances, a muscle relaxant or short term tranquilizer may be indicated.
The
position of the patient and choice of pharmaceutical agents to be used are
responsive
to judgments by medical personnel.
At a step 401, the patient's external genitalia and surrounding anatomy
are cleansed with an appropriate agent such as Betadine, or benzalkonium
chloride.
At a step 402, the visualization port 431 is coupled to the appropriate
visualization apparatus, such as a flouroscope, an endoscope, a display screen
or
other visualization device. The choice of visualization apparatus is
responsive to
judgments by medical personnel.
22
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
At a step 403, the apparatus port 332 is coupled to an external medical
device such as a pH meter, a pressure gauge, or other medical equipment. The
choice
of apparatus is responsive to judgments by medical personnel.
At a step 404, the therapeutic energy port 336 is coupled to a source of
any of the aforementioned types of therapeutic energy.
In a step 405, suction, inflation or fluid infusion apparatus is coupled to
the irrigation and aspiration control ports 335 so that the treatment balloon
may be
later be inflated and deflated and substances may be administered.
At a step 406, the most distal end of the treatment balloon 320 is
lubricated and introduced into the urethral meatus in an upward and backward
direction, in much the same way a Foley catheter is introduced. The choice of
lubricant is responsive to judgments by medical personnel. In a preferred
embodiment, the balloon 320 is completely deflated during insertion.
In a step 407, the catheter 310 is threaded through the urethra until the
microporous balloon 320 has completely passed the bladderneck and is entirely
in the
bladder. An introduces sheath 313 or guidetube 314 may also be used to
facilitate
insertion.
In a step 408, the position of the catheter 310 is checked using
visualization apparatus coupled to the visualization port 331. This apparatus
can be
continually monitored by medical professionals throughout the procedure.
In a step 409, the irrigation and aspiration control port 335 is
manipulated so as to inflate the microporous treatment balloon 320. In a
preferred
embodiment, the treatment balloon 320 is inflated with a cooling liquid such
as sterile
water, saline or glycerin. This cooling fluid lowers the relative temperature
of the
targeted tissues that are in physical contact and prevents collateral thermal
damage.
23
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
In alternative embodiments, other devices may be coupled to the apparatus port
132
to chill the cooling fluid or cause sonic cooling, gas expansion, magnetic
cooling or
others cooling methodologies. The choice of cooling fluid or methodology is
responsive to judgments by medical personnel.
In a step 410, electrodes 322 are selected using the electrode selection
and control switch 334.
In a step 411, the translation member 312 is manipulated to alter the
shape of the most distal end of the balloon so as to bring the distal end of
the balloon
in optimal physical contact with the top of the bladder.
In a step 412, individual nerves within the bladder are identified using
sensors 323. This step is optional.
In a step 413, the therapeutic energy port 336 is manipulated so as to
cause a release of energy from the electrodes 322. The duration and frequency
of
energy are responsive to judgments by medical personnel. This release of
energy
creates a pattern of lesions in the mucosal or submucosal tissues of the
bladder or
portions thereof. The affected area shrinks and is relatively strengthened, so
as to
better retain urine.
In a step 414, the therapeutic energy port 336 is manipulated so as to
cause a release of energy from the electrodes 322 that is directed at the
nerves that
were identified in step 412. Manipulation and modulation of these nerves may
directly or indirectly affect incontinence related to an uncontrolled urge to
urinate.
This step is optional.
In a step 415, bulking agents such as organic microspheres, collagens,
silicone, PVC and other organic breathable and unbreathable polymers are
exuded
from selected electrodes 322 positioned near the base of the bladder. The type
of
24
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
microspheres and bulking substances and the locations where they are exuded
are
responsive to judgment by medical personnel. These bulking agents can be used
to
strengthen these structures so as to prevent incontinence caused by stress.
In a step 416, pharmaceutical agents may be locally administered by
manipulating the irrigation and aspiration control ports 335. These agents may
help
include lubricants, anesthetics, anti-spasmodics, anti-inflammatories,
antiobiotics or
other agents as deemed appropriate by the judgment of medical personal. This
step
may occur any time prior to withdrawal of the catheter 310, to either pretreat
tissue or
post-treat tissues.
In a step 417, the irrigation and aspiration control port 335 is
manipulated so as to reverse the flow of cooling liquid into the microporous
treatment
balloon 320 and cause it to deflate.
In a step 418, the catheter 310 is withdrawn from the urethra.
Figure 5 is a block drawing of a system for treatment of female urinary
incontinence using a third device.
A system 500 includes a catheter 510, treatment element 520, a control
assembly 530 and a shielding element 540. In an alternative embodiment, the
shielding element 540 is not present.
The Catheter 510
The catheter 510 includes two or more lumens 511, a translation
member 512 and a tapered tip 513. The lumens 511 and translation member 512
run
the entire interior length of the catheter 510. The proximal end of the lumens
511 is
coupled to the control assembly 530; the distal end of the lumens 511 is
coupled to
the treatment element 520. It is through these lumens 511 that energy is
conducted
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
and flowable substances are exuded. The proximal end of the translation member
512 is coupled to the control assembly 530; the distal end of the translation
member
512 is coupled to the taper tip 513.
In a preferred embodiment, the tapered tip 513 is rigid so as to allow
easy insertion into a urethra. In other preferred embodiments, the tapered tip
513
may be of varying degrees of flexibility depending where it in the body it is
deployed.
In alternative embodiments, the catheter 510 may be introduced into the target
tissue
using an introducer sheath 514 or a guide wire 515.
In a preferred embodiment, the tapered tip 513 is disposed for insertion
into a cavity of the body such as a female urethra and bladder. In alternative
embodiments, the cavity may include one or more of, or some combination of the
following:
Any portion of the bronchial system, the cardiovascular system, the
genito-urinary tract, the lymphatic system, the pulmonary system, the vascular
system, the locomotor system, the reproductive system or other systems in the
body;
Any biological conduit or tube, such as a biologic lumen that is patent
or one that is subject to a stricture;
Any biologic operational structure, such as a gland, or a muscle or other
organ (such as the colon, the diaphragm, the heart, a uterus, a kidney, a
lung, the
rectum an involuntary or voluntary sphincter);
Any biologic structure, such as a herniated body structure, a set of
diseased cells, a set of displastic cells, a surface of a body structure,
(such as the
sclera) a tumor, or a layer of cells (such as fat, muscle or skin);
26
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
Any biologic cavity or space or the contents thereof, such as a cyst, a
gland, a sinus, a layered structure, or a medical device implanted or inserted
in the
body.
The Treatment Element 520
The treatment element 520 includes a set of umbrella-like struts 521, a
set of electrodes 522, a set of irrigation and aspiration ports 525 and a set
of sensors
526.
The set of umbrella like struts 521 are several centimeters long. One
end of the struts 521 is not attached to any part of the device. The other end
of the
strut 521 is coupled to the distal end of the translation member 512 at the
tapered tip
513 in such a way that when tension is applied to the proximal end of the
translation
member 512, the umbrella-like struts 521 open up in much the same way as an
umbrella.
A set of electrodes 522 are evenly distributed on the outer surface of
each strut 521. Each free-floating end of a strut 521 includes at least one
electrode
522. Each electrode 522 includes a metallic tube 523 defining a hollow lumen
524.
In a preferred embodiment, the set of electrodes 522 are needle electrodes;
other
preferred embodiments include surface electrodes or a combination of needle
electrodes and surface electrodes.
Each electrode 522 is coupled to at least one sensor 526 capable of
measuring such factors as temperature, conductivity, pressure, impedance and
other
variables. In a preferred embodiment, each electrode 522 is also coupled to a
radiopaque marker 527 for use in fluoroscopic visualization.
In a preferred embodiment, the electrodes 522 can be operated
separately or in combination with each other. Treatment can be directed at a
single
27
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
area or several different areas of a bladder or other orifice by operation of
selected
electrodes. Different patterns of submucosal lesions, mucosal lesions,
ablated,
bulked, plumped, desiccated or necrotic regions can be created by selectively
operating different electrodes. Production of different patterns of treatment
makes it
possible to remodel tissues and alter their overall geometry with respect to
each other.
Each electrode 522 can be disposed to treat tissue by delivering one or
more of, or some combination or any of the following in either a unipolar or
bipolar
mode:
~ Radiofrequency (RF) energy, such as RF in about the 300 kilohertz
to 500 kilohertz range;
~ Chemical treatments, such as acids, antibiotics, enzymes,
radioactive tracers or other bioactive substances;
~ Infrared energy, such as from an infrared laser or diode laser;
~ Microwave energy, such as electromagnetic energy in about the 915
megahertz or 2.45 gigahertz range;
~ Sonic energy, including ultrasound;
~ Photodynamic therapy (PDT)
~ Non-infrared laser energy
~ Cryothermia
In addition to treating tissues by delivering energy, the set of electrodes
522 are disposed to deliver at least one flowable substance to the area of the
body
where treatment is to take place. In a preferred embodiment, the flowable
substance
includes sterile water which aides in cooling and hydration of body
structures. In
another preferred embodiment, the flowable substance includes saline with a
concentration of less than about 10% NaCI. Saline is used to increate the
local
conductivity of tissue, enhancing the penetration of RF energy so as to create
larger
lesions. The saline can be delivered through the needle electrode submucosally
so as
to achieve greatest effect.
28
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
However, in alternative embodiments, the deliverable flowable liquids include
other
substances, including anesthetic drugs, anti-inflammatory agents,
chemotherapeutic
agents, systemic or topical antibiotics, collagen and radioactive substances
such as
labeled tracers.
A set of irrigation and aspiration ports 525 are also evenly distributed
on the outer surface of each strut 521. Each free-floating end of a strut 521
also
includes at least one irrigation and aspiration port 525. Suction can be
applied
through these ports so as to bring the targeted tissue in closer physical
proximity to
the electrodes 522. The irrigation and aspiration ports 525 can also be used
to
administer cooling fluids in such a way as to minimize thermal damage. Drugs,
bulking agents and other flowable substances can be infused through the
irrigation
and aspiration ports 525.
The Control Assembly 530
The control assembly 530 includes a visualization port 531, an
apparatus port 532, an electrical energy port 533, an electrode selection and
control
switch 534, one or more irrigation and aspiration control ports 535, an
therapeutic
energy port 536 and a handle 537.
The visualization port 531 can be coupled to visualization apparatus,
such as a fiberoptic device, a flouroscopic device, an anoscope, a
laparoscope, an
endoscope or other type of catheter.
The apparatus port 532 can be coupled to other medical devices that
may be useful during treatment such as a pH meter, a pressure monitor, drug
administration apparatus, or other device used to monitor or treat the
patient.
In a preferred embodiment, devices coupled to both the visualization
port 531 and the apparatus ports 532 are controlled from a location outside
the body,
29
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
such as by an instrument in an operating room or an external device for
manipulating
the inserted catheter 510.
In an alternative embodiment the apparatus port 532 may be coupled to
devices that are implanted or inserted into the body during a medical
procedure. For
example, the apparatus port 532 may be coupled to a programmed AICD
(artificial
implanted cardiac defibrillator), a programmed glandular substitute (such as
an
artificial pancreas) or other device for use during surgery or other medical
procedures.
The electrical energy port 533 includes a conductive element such as an
electrical adapter that can be coupled to a source of alternating or direct
current such
as a wall socket, battery or generator.
The electrode selection and control switch 534 includes an element that
is disposed to select and activate individual electrodes 522.
The irrigation and aspiration control ports 535 can be coupled to a
pump or other apparatus to deliver fluid through the irrigation and aspiration
ports
525 or electrodes 522 or to apply suction through the set of irrigation and
aspiration
ports 525.
The therapeutic energy port 536 includes a receptor port for coupling to
a source of any of the following types of therapeutic energy:
~ Radiofrequency (RF) energy, such as RF in about the 300 kilohertz
to 500 kilohertz range;
~ Chemical treatments, such as acids, antibiotics, enzymes,
radioactive tracers or other bioactive substances;
~ Infrared energy, such as from an infrared laser or diode laser;
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
~ Microwave energy, such as electromagnetic energy in about the 915
megahertz or 2.45 gigahertz range;
~ Sonic energy, including ultrasound;
~ Photodynamic therapy (PDT)
~ Non-infrared laser energy
~ Cryothermia
The handle 537 is disposed for manipulated by medical or veterinary
personnel and can be shaped for being held in the hand. The visualization port
531,
the apparatus port 532, the electrical energy port 533, the electrode
selection and
control switch 534 and the one or more irrigation and aspiration control ports
535 and
the therapeutic energy port 536 are all mounted in the handle 537 to allow for
easy
operation.
The Shielding Element 540
The shielding element 540 lies on the proximal side of treatment
element 520 and is disposed to isolate the treatment area. It can also help
position the
catheter 510 in the body. For example, in a preferred embodiment in which the
catheter 510 is inserted into the urethra, the shielding element 540 can
prevent the
catheter 510 from being inserted further into the urethral canal and prevent
substances
used in treatment from escaping. In an alternative embodiment, the shielding
element 540 is optional.
Figure 6 is a process flow drawing of a method for treatment of female
urinary incontinence using a third device. Although the method 600 is
described
serially, the steps of the method 600 can be performed by separate elements in
conjunction or in parallel, whether asynchronously, in a pipelined manner, or
otherwise. There is no particular requirement that the method 600 be performed
in
the same order in which this description lists the steps, except where so
indicated.
31
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
A method 600 is performed by a system 500 including a catheter 510, a
treatment element 520 and a control assembly 530.
At a flow point 600, electrical energy port 533 is coupled to a source of
electrical energy. The patient has voided and is positioned on a treatment
table, in an
appropriate position such as horizontal, jackknife or lithotomy. Due to the
potential
for inducing pain, the area surrounding the urinary meatus may be pretreated
with a
topical anesthetic before insertion of the catheter 510; depending upon the
circumstances, a muscle relaxant or short term tranquilizer may be indicated.
The
position of the patient and choice of pharmaceutical agents to be used are
responsive
to judgments by medical personnel.
At a step 601, the patient's external genitalia and surrounding anatomy
are cleansed with an appropriate agent such as Betadine, or benzalkonium
chloride.
At a step 602, the visualization port 531 is coupled to the appropriate
visualization apparatus, such as a flouroscope, an endoscope, a display screen
or
other visualization device. The choice of visualization apparatus is
responsive to
judgments by medical personnel.
At a step 603, the apparatus port 532 is coupled to an external medical
device such as a pH meter, a pressure gauge, or other medical equipment. The
choice
of apparatus is responsive to judgments by medical personnel.
At a step 604, the therapeutic energy port 536 is coupled to a source of
any of the aforementioned types of therapeutic energy.
In a step 605, suction, inflation or fluid infusion apparatus is coupled to
the irrigation and aspiration control ports 535 so that cooling fluids and
phamnacological agents may be administered.
32
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
At a step 606, the tapered tip 513 is lubricated and introduced into the
urethral meatus in an upward and backward direction, in much the same way a
Foley
catheter is introduced. The choice of lubricant is responsive to judgments by
medical
personnel. In a preferred embodiment, the treatment element 520 is completely
closed to facillitate insertion.
In a step 607, the catheter 510 is threaded through the urethra until the
treatment element 520 has completely passed the bladderneck and is entirely in
the
bladder. An introducer sheath 513 or guidetube 514 may also be used to
facilitate
insertion.
In a step 608, the position of the catheter 510 is checked using
visualization apparatus coupled to the visualization port 531. This apparatus
can be
continually monitored by medical professionals throughout the procedure.
In a step 609, the irrigation and aspiration control port 535 is
manipulated so as to exude a cooling fluid. In a preferred embodiment, the
cooling
fluid may include sterile water, saline or glycerin. This cooling fluid lowers
the
relative temperature of the targeted tissues that are in physical and prevents
collateral
thermal damage. In alternative embodiments, other devices may be coupled to
the
apparatus port 532 to chill the cooling fluid or cause sonic cooling, gas
expansion,
magnetic cooling or others cooling methodologies. The choice of cooling fluid
or
methodology is responsive to judgments by medical personnel.
In a step 610, tension is applied to the translation member 512 to cause
extension of the struts 522. Extension of the struts 522 brings the electrodes
522 into
physical proximity with the walls of the bladder.
In a step 61 l, the irrigation and aspiration control ports 535 are
manipulated so as to apply suction through the irrigation and aspiration ports
525 and
bring the walls of the bladder in even closer proximity to the treatment
element 520.
33
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
In a step 612, electrodes 522 are selected using the electrode selection
and control switch 534. In a preferred embodiment, all electrodes are
selected. In
another embodiment, individual electrodes may be deployed.
In a step 613, individual nerves within the bladder are identified using
sensors 526. This step is optional.
In a step 614, the therapeutic energy port 536 is manipulated so as to
cause a release of energy from the electrodes 522. The duration and frequency
of
energy are responsive to judgments by medical personnel. This release of
energy
creates a pattern of lesions in the mucosal and/or submucosal tissues of the
bladder or
portions thereof. The affected area shrinks and is relatively strengthened, so
as to
better retain urine.
In a step 615, the therapeutic energy port 536 is manipulated so as to
cause a release of energy from the electrodes 522 that is directed at the
nerves that
were identified in step 613. Manipulation and modulation of these nerves may
directly or indirectly affect incontinence related to an uncontrolled urge to
urinate.
This step is optional.
In a step 616, bulking agents such as organic microspheres, collagens,
silicone, PVC and other organic breathable and unbreathable polymers are
exuded
from selected electrodes 522 into tissues near the base of the bladder. The
type of
microspheres and bulking substances and the locations where they are exuded
are
responsive to judgment by medical personnel. These bulking agents can be used
to
strengthen these structures so as to prevent incontinence caused by stress.
This step
is optional.
In a step 617, pharmaceutical agents may be locally administered by
manipulating the irrigation and aspiration control ports 535. These agents may
help
34
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
include lubricants, anesthetics, anti-spasmodics, anti-inflammatories,
antiobiotics or
other agents as deemed appropriate by the judgment of medical personal. This
step
may occur any time prior to withdrawal of the catheter 510, either to pre-
treat tissue
or post-treat tissues.
In a step 618, the irrigation and aspiration control port 535 is
manipulated so as to reverse the flow of cooling liquid.
In a step 619, tension is applied to the translation member 512 to cause
the umbrella like struts 521 to collapse and close around the catheter 510.
In a step 620, the catheter 510 is withdrawn from the urethra.
CA 02384866 2002-03-13
WO 01/22897 PCT/US00/26831
Generality of the Invention
The invention has substantial generality of application to various fields
for biopsy or treatment of medical conditions. These various fields include,
one or
more of, or a combination of, any of the following (or any related fields):
As noted above, the invention can be used in any area of the body,
including the biologic systems and locations noted herein. The invention can
be used
for the general purpose of reducing, plumping, or reshaping body structures,
tissues,
or regions of the body otherwise empty (or filled with biologic substances).
For examples, the invention can be used in one or more of, or some
combination of, the following:
o In the head and neck, such as the cheeks, eyes, sinuses, middle ear,
nostrils,
inner ear, Eustachian tubes, pharynx, larynx, or other structures;
o For the purpose of reforming damaged body parts, for the purpose of
reshaping misshapen body parts, dilating occluded tissues, or for cosmetic
effects;
or
~ For the purpose of replacing the volume filled by body parts that are
missing,
whether due to congenital defect, infection, or surgery.
Alternative Embodiments
Although preferred embodiments are disclosed herein, many variations
are possible which remain within the concept, scope, and spirit of the
invention, and
these variations would become clear to those skilled in the art after perusal
of this
application.
36