Note: Descriptions are shown in the official language in which they were submitted.
CA 02390852 2002-05-08
WO 01/37919 PCT/US00/42056
CRYOMEDICAL DEVICE
FOR PROMOTING ANGIOGENESIS
s FIELD OF THE INVENTION
This invention relates to vascular growth, and more particularly to an
apparatus
for using extremely cold temperatures to promote angiogenesis.
BACKGROUND OF THE INVENTION
to Angiogenesis relates to the formation of blood vessels in living tissue.
Not
surprisingly, vascular growth or the lack thereof significantly affects living
tissue. In
adults, the body's network of blood vessels is stable. However, under certain
circumstances, such as physical injury, the body causes new blood vessels to
grow.
Various drug and gene therapies are under study that show promise in
amplifying
~s angiogenesis where it naturally occurs, and promoting it where it does not
otherwise
occur. Drug and gene therapy, however, can not only be difficult to localize,
but the
mechanisms by which they operate are also poorly understood and may cause
unwanted side effects. It would therefore be desirable to provide an
alternative
method of promoting vascular growth.
2o The present invention provides a tip for a cyrocatheter, in which a surface
defines an inner volume such that the inner volume provides a cryogenic
expansion
area. An angiogenic stimulant lumen is positioned within the inner volume. The
angiogenic stimulant lumen is fluidly isolated from the cryogenic expansion
area. A
tissue penetrating element is in fluid communication with the angiogenic
stimulant
2s lumen and is outwardly protrudable from the surface. The tissue penetrating
element
defines an angiogenic stimulant passage and an aperture for permitting
angiogenic
stimulant to exit tissue penetrating element.
1
CA 02390852 2002-05-08
WO 01/37919 PCT/US00/42056
SUMMARY OF THE INVENTION
The present invention provides a cryomedical device in which a device body
has a cryogenic cooling surface and a reservoir for an angiogenic stimulant.
An
apertured tissue penetrating element is in fluid communication with the
reservoir and
is coupled to the device body. The angiogenic stimulant is a drug or other
fluid which
stimulates vascular growth. In other words, the present invention seeks to
obtain a
balance between minimizing trauma to tissue while maximizing angiogenic
response.
BRIEF DESCRIPTION OF THE DRAWINGS
A more complete understanding of the present invention, and the attendant
advantages and features thereof, will be more readily understood by reference
to the
following detailed description when considered in conjunction with the
accompanying drawings, wherein:
FIG. 1 is a schematic illustration of an embodiment of a cryosurgical system
in
is accordance with the invention;
FIG. 2 is a sectional view of a heart muscle showing placement of the catheter
of FIG. 1;
FIG. 3 illustrates the tip region of one embodiment of the catheter in
accordance with the invention;
2o FIGS. 5-8 illustrate alternative tip configurations for cyro-angiogenic
treatment;
FIGS. 9-11 illustrate alternative tip configurations in association with
additional treatment structures;
FIG. 12 illustrates a device which is arranged to cryogenically cool tissue
and
2s inject an angiogenic stimulant therein;
FIG. 13 illustrates a shielded device which is arranged to cryogenically cool
tissue and inject an angiogenic stimulant therein:
FIG. 14 illustrates an angiogenic stimulant reservoir provided as part of a
controller;
2
CA 02390852 2002-05-08
WO 01/37919 PCT/US00/42056
FIG. 15 illustrates an angiogenic stimulant reservoir provided as part of a
catheter handle;
FIG. 16 illustrates a device body having a collapsible reservoir;
FIG. 17 illustrates a device body having a collapsed reservoir after an
actuator
s has been operated;
FIG. 18 illustrates a tissue penetrating element having a plurality of
apertures
on one hemisphere;
FIG. 19 illustrates an enlarged view of the tissue penetrating element of FIG.
18;
to FIG. 20 illustrates an alternate arrangement of a tissue penetrating
element
having a plurality of apertures at an end portion thereof:
FIG. 21 illustrates another alternate arrangement of a tissue penetrating
element having a plurality of apertures distributed substantially along the
entire
length;
is FIG. 22 illustrates a tissue penetrating element used in conjunction with a
device body having a rounded tip;
FIG. 23 illustrates a tissue penetrating element used in conjunction with a
device body having a pointed tip;
FIG. 24 illustrates a tissue penetrating element used in conjunction with a
2o device body having a metallic ring as the tip;
FIG. 25 illustrates a device having a tissue penetrating element and stylets;
FIG. 26 illustrates a device body having a movable penetration element in a
retracted state; and
FIG. 27 illustrates a device body having a movable penetration element in an
2s extended state.
DETAILED DESCRIPTION OF THE INVENTION
FIG. 1 is a schematic illustration of a cryosurgical system in accordance with
the invention that can be employed as described below to promote angiogenesis.
The
CA 02390852 2002-05-08
WO 01/37919 PCT/US00/42056
system includes a supply of cryogenic or cooling fluid 10 in communication
with the
proximal end 12 of a flexible catheter 14. A fluid controller 16 is interposed
or in-
line between the cryogenic fluid supply 10 and the catheter 14 for regulating
the flow
of cryogenic fluid into the catheter in response to a controller command.
Controller
s commands can include programmed instructions, sensor signals, and manual
user
input. For example, the fluid controller 16 can be programmed or configured to
increase and decrease the pressure of the fluid by predetermined pressure
increments
over predetermined time intervals. In another exemplary embodiment, the fluid
controller 16 can be responsive to input from a foot pedal 18 to permit flow
of the
to cryogenic fluid into the catheter 14. One or more temperature sensors 20 in
electrical
communication with the controller 16 can be provided to regulate or terminate
the
flow of cryogenic fluid into the catheter 14 when a predetermined temperature
at a
selected point or points on or within the catheter is/are obtained. For
example a
temperature sensor can be placed at a point proximate the distal end 22 of the
catheter
is and other temperature sensors 20 can be placed at spaced intervals between
the distal
end of the catheter and another point that is between the distal end and the
proximal
end. The cryogenic fluid can be in a liquid or a gas state.
An extremely low temperature can be achieved within the catheter, and more
particularly on the surface of the catheter by cooling the fluid to a
predetermined
2o temperature prior to its introduction into the catheter, by allowing a
liquid state
cryogenic fluid to boil or vaporize, or by allowing a gas state cryogenic
fluid to
expand. Exemplary liquids include chlorodifluoromethane, polydimethylsiloxane,
ethyl alcohol, HFC's such as AZ-20 (a ~0--50 mixture of difluoromethane &
pentafluoroethane sold by Allied Signal), and CFC's such as DuPont's Freon.
2s Exemplary gasses include nitrous oxide, and carbon dioxide.
The catheter 14 includes a flexible member 24 having a thermally-transmissive
region 26 and a fluid path through the flexible member to the thermally-
transmissive
region. A fluid path is also provided from the thermally-transmissive region
to a point
external to the catheter, such as the proximal end 12. Although described in
greater
4
CA 02390852 2002-05-08
WO 01/37919 PCT/US00/42056
detail below, exemplary fluid paths can be one or more channels defined by the
flexible member 24, and/or by one or more additional flexible members that are
internal to the first flexible member 24. Also, even though many materials and
structures can be thermally conductive or thermally transmissive if chilled to
a very
s low temperature and/or cold soaked, as used herein, a "thermally-
transmissive region"
is intended to broadly encompass any structure or region of the catheter 14
that readily
conducts heat. For example, a metal structure exposed (directly or indirectly)
to the
cryogenic fluid path is considered a thermally-transmissive region 26 even if
an
adjacent polymeric or latex catheter portion also permits heat transfer, but
to a much
to lesser extent than the metal. Thus, the thermally-transmissive region 26
can be
viewed as a relative term to compare the heat transfer characteristics of
different
catheter regions or structures. A thermally-transmissive region or element is
not
intended to encompass a structure that is excited by RF or other energy source
to a
point where it begins to radiate heat, for example.
is Furthermore, while the thermally-transmissive region 26 can include a
single,
continuous, and uninterrupted surface or structure, it can also include
multiple,
discrete, thermally-transmissive structures that collectively define a
thermally-
transmissive region that is elongate or linear. Alternatively, the thermally-
transmissive region can be at a single focal location, such as the distal tip
of the
2o catheter. Additional details of the thermally-transmissive region 26 and
the thermal
transfer process are described in greater detail below.
In exemplary embodiments of the invention, the thermally-transmissive region
26 of the catheter 14 is deformable. An exemplary deformation is from a linear
configuration to an arcuate configuration and is accomplished using mechanical
2s and/or electrical devices known to those skilled in the art. For example, a
wall
portion of the flexible member 24 can include a metal braid to make the
catheter
torqueable for overall catheter steering and placement. Additionally, a wire
or cable
can be incorporated with, or inserted into, the catheter for deformation of
the
thermally transmissive region 26.
CA 02390852 2002-05-08
WO 01/37919 PCT/US00/42056
The cryogenic system of FIG. 1 is better understood with reference to its use
in
an operative procedure as shown in FIG. 2. Following the determination of a
proposed treatment site within a heart muscle 28 for example, the catheter 14
is
directed through a blood vessel 30 to a region within the heart. The thermally-
s transmissive region 26 is placed proximate to the tissue to be treated. The
thermally-
transmissive region of the catheter may be deformed to conform to the
curvature of
the tissue before, during, or after placement against the tissue. The
controller 16
allows or causes cryogenic fluid to flow from the cryogenic fluid supply 10 to
the
fluid path in the catheter 14 and thence to the thermally-transmissive region
26 to treat
to the desired area. In one embodiment a first conduit is concentric within a
second
conduit and cooling fluid travels to a thermally-transmissive region proximate
a
closed distal end of the catheter through a first conduit (fluid path) and is
exhausted
from the catheter through the second conduit (fluid path).
Referring specifically to the embodiment depicted in FIG. 3, multiple
is thermally-transmissive elements 34 are integral with a distal portion of a
catheter
having a thermally transmissive tip 32. Thermocouples 35 can be associated
with one
or more of the elements 34 and the tip 32. Additional details of cryocatheter
construction are found in United States Patent Nos. 5, 899,898 and 5,899,899
to
Arless, which are incorporated herein by reference.
2o FIGS. 4-8 illustrate alternative embodiments of the thermally transmissive
region 26. To the right of each drawing is an illustration of the general
shape of the
region treated by each embodiment as seen in plan view and as seen in cross-
section.
More specifically, FIG. 4 depicts a rounded tip, such as element 32 of FIG. 3.
FIG. 5
shows a flat, paddle-like tip; and FIG. 6 shows a needle tip suitable for
penetrating
2s tissue. FIG. 7 illustrates a non-segmented linear tip. FIG. 8 shows a tip
with a
separate channel 36 within the tip or beside it to inject drugs directly in
the tissue,
such as vascular endothelial growth factor (VEGF), before, during or after
cooling
tissue as described below. The drugs) can be deposited on the surface of the
tissue or
injected into the tissue.
6
CA 02390852 2002-05-08
WO 01/37919 PCT/US00/42056
Angiogenesis can be promoted in selected tissue with the above described
devices by injuring tissue over a selected area. For example, to treat
ischemia, a
cryocatheter as described above, is placed on the heart tissue to be treated.
The
cooling or thermally transmissive region 26 of the catheter is chilled to a
temperature
s of -20 ° C to -80 ° C for five seconds to five minutes and
then allowed to warm or
thaw to a temperature in the range of 0 ° C to body temperature. This
step is then
repeated one or more times. In an exemplary procedure. four or more three or
more
injuries are made tissue at regular intervals ~-15 mm apart, wherein the
tissue is
injured to a depth of about 3.0 mm or more to provide a relatively wide and
deep
to treated tissue zone. Treatment zone geometry can be varied widely by
varying pip
Geometry, using tips such as shown in FIGS. 4-8, as well as by varying freeze
rate.
thaw rate, freeze time, and ultimate temperature. A greater or fewer number of
treatment zones can be created as desired to stimulate a greater or lesser
area.
Freezing the tissue initiates an inflammatory response which triggers an
angiogenic
~s process, leading to new blood vessel growth. However, the ability to dose
the injury
minimizes tissue necrosis while maximizing angiogenic response.
Another way to minimize tissue damage, but to trigger an angiogenic reaction,
is to apply high frequency electrical or microwave energy to the tissue while
cooling
the catheter/tissue interface with a cryogenic fluid alternative method.
2o The depth to which tissue is injured can be a significant factor in
promoting
angiogenesis. Thus, .FIG. 9 depicts a cryocatheter 14 with supplemental
structures
that can increase injury depth, such as one or more thermally conductive
wires, needle
or stylets 38. In an exemplary embodiment the stylets 38 are stainless steel
wires less
than 0.050 inches in diameter. Although the stylets 38 can be integrated with
the
25 catheter 14, they can be independent therefrom. For example, FIG. 9 shows a
cryocatheter 14 disposed within a guide lumen 40 that is part of a shaft
portion 42 of
the catheter 14. The stylets 38 are disposed within the guide lumen 40 and are
axially
movable therein. A control mechanism 44 is provided at the proximal end of the
catheter that allows the stylets to be advanced and/or retracted individually
or in
3o unison.
7
CA 02390852 2002-05-08
WO 01/37919 PCT/L1S00/42056
In an exemplary procedure, the catheter 14 is guided to an area of tissue to
be
treated. Then the one or more stylets 38 are advanced so as to penetrate the
tissue to a
selected depth. The catheter 14 is cooled, as described above, and one or more
iceballs form on and near the tissue that is proximate the thermally
transmissive
s region 26. The stylets 38, which are in or exposed to the one or more
iceballs,
conduct cold/remove heat from a region of tissue well below the surface.
Regarding the procedure described with respect to FIG. 9, it should be noted
that not only is a deep region of tissue traumatized by extremely cold
temperature, but
the tissue is also mechanically traumatized by the stylets 38. The combination
of
~o trauma mechanisms is believed to be especially effective in promoting
angiogenesis.
Another combined trauma device is shown in FIG. 10, wherein a rotatable,
ball, wheel or cylinder 46 is secured to the tip 32 of the catheter 14. The
cylinder 46
is provided with sharp or rough surface features, such as spikes 48. A
thermally
conductive support structure 50 conducts heat/cold from the tip 32 to the
spikes 48.
Is An introducer/guide catheter 52 is provided to assist with catheter
placement. Thus,
in an exemplary procedure, the catheter is positioned near tissue as described
above,
and the tip 32 is cooled. The cold is conducted to the cylinder 46 and to the
spikes 48
and thence to the tissue against which the cylinder is pressed and rotated. In
alternative procedures, the tissue is traumatized before or after a cooling
cycle, or
2o between cool/thaw/cool cycles. Thus. angiogenesis is stimulated by physical
and
temperature trauma.
Yet another way of providing combination trauma is described with respect to
FIG. 11, wherein a catheter 14 is shown within a guide sheath 54, and is
slidable
therein. The catheter 14 includes a roughened cooling tip 56. In an exemplary
2s procedure using this catheter, the catheter is placed and the tip is cooled
to create an
iceball 58. After a region of tissue 60 has frozen and has become part of or
joined to
the iceba1158, the catheter tip 56 is pulled away from the tissue, thereby
tearing the
region of tissue 60 from the remaining tissue. The roughness of the tip 56
helps to
prevent the iceball from separating from the catheter when the catheter is
pulled away
3o from the tissue. The sheath 54 can be pushed against the tissue during
treatment to
8
CA 02390852 2002-05-08
WO 01/37919 PCT/US00/42056
shield the tip from surrounding tissue, to help localize the thermal
treatment, and to
help detach the iceball/frozen tissue from the non-frozen tissue.
According to another aspect, angiogenic growth stimulation can be facilitated
by delivery of an angiogenic stimulant such as a drug or other fluid directly
into the
s tissue. This injection can occur before, during or after cryoablation has
been
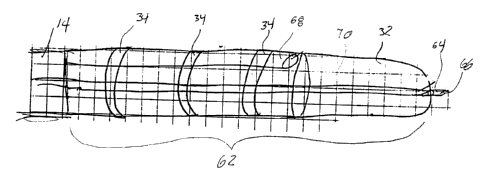
performed on the tissue area. FIG. 12 shows an example of a device which is
arranged to cryogenically cool tissue and inject an angiogenic stimulant
therein. A
device body 62 includes one or more cryogenic cooling surfaces such as
thermally-
transmissive elements 34 and a thermally-transmissive tip 32. Of course,
various
to arrangements for providing cryogenic cooling surfaces can be provided as
discussed
above. A tissue penetrating element 64 is coupled to the device body 62 and
includes
an aperture 66 through which the angiogenic stimulant is delivered to the
tissue. The
device body 62 also includes a cryogenic lumen 68 through which a cooling
fluid is
delivered to the device body 62. The tissue penetrating element 64 is coupled
to a
Is reservoir (not shown) containing the angiogenic stimulant via an angiogenic
stimulant
lumen 70.
In operation, the tissue penetrating element 64 is inserted into the tissue,
such
as myocardial tissue, and the angiogenic stimulant delivered through the lumen
70,
through the aperture 66 and into the tissue. As noted above, the angiogenic
stimulant
2o delivery can occur before, during or after a cryogenic cooling cycle.
FIG. 13 shows another arrangement for the cryomedical device in which a
shield 72 is coupled to the device body 62. The shield 72 is preferably non or
minimally thermally transmissive such that it shields the tissue from
cyroablation.
The tissue penetrating element 64 can be made of a highly thermally conductive
25 material such as a metal which is cryogenically cooled along with the
thermally
transmissive elements of the device body, thereby providing the ability to
cryoablate
inner portions of tissue. Further, by cooling the tissue penetrating element
64, the
cooling operation temporarily affixes the device body to the tissue, providing
for
increased reliability during cryoablation and/or angiogenic stimulant
delivery.
9
CA 02390852 2002-05-08
WO 01/37919 PCT/L1S00/42056
The cryomedical device of the present invention includes a reservoir in fluid
communication with the tissue penetrating element, such as tissue penetrating
element
64. FIG. 14 shows an angiogenic stimulant reservoir 74 provided as part of the
controller 16. FIG. 15 shows the angiogenic stimulant reservoir 74 provided as
part
s of a
catheter handle 76. The handle 76 provides a way for the operator to insert
catheter
14 into the patient, by controlling movement of the catheter within body
lumen. The
handle 76 can also be equipped with switches, valves and the like which allow
the
operator to control the actuation of cryogenic fluid into catheter 14 and
control
to delivery of the angiogenic stimulant into the tissue.
In the case where the angiogenic stimulant reservoir is provided as part of
the
controller 16, a lumen such as an angiogenic stimulant lumen 70 couples the
reservoir
74 to the tissue penetrating element 64. The lumen 70 is also used to couple
the
angiogenic stimulant reservoir 74 to the tissue penetrating element 64 in the
case
is where the reservoir 74 is provided in the catheter handle 76 (see FIG. 15).
In each of these cases, an actuator is used to respond to the operator's
control to
force the angiogenic stimulant through the lumen 70 to the tissue penetrating
element
64 for injection into the tissue. The actuator can take the form of an
electric motor,
pneumatic or hydraulic pressurizing device, a mechanical spring driven element
or
2o any other form by which fluid is pumped through a lumen.
The angiogenic stimulant reservoir can also be provided within the device
body. FIG. 16 shows a example of a device body 62 having a collapsible
reservoir 78
with an angiogenic stimulant 80 contained therein. The collapsible reservoir
78 is
preferably made of any material suitable for biomedical use which can house an
2s angiogenic stimulant. For example, the collapsible reservoir 78 can be made
of an
impermeable membrane, polymer and the like. The device body 62 also includes
within its confines an actuator 82, which when activated, applies compressive
pressure to collapse the reservoir 78, thereby forcing the angiogenic
stimulant fluid 80
through the tissue penetrating element 64. The actuator 82 includes a spring
84
and/or a shaft 86 coupled to a pressure plate 88. The shaft 86 can be
manipulated by
CA 02390852 2002-05-08
WO 01/37919 PCT/L1S00/42056
the operator via the handle 76 as can the spring 84 to move the pressure plate
88
toward and away from the distal end of the device body 62. The pressure plate
88
applies pressure to collapse the reservoir 78 against a back plate 90 which is
fixed to
the inner surface of the device body shell. In the alternative, the back plate
90 can be
s eliminated and the collapsible reservoir 78 instead pressed directly against
the distal
end 92 of the device body 62.
FIG. 17 shows an example of a collapsed reservoir 78 after the actuator 82 has
been operated. As shown, the angiogenic stimulant 80 is expulsed from the
aperture
66. It is contemplated that the tissue penetrating element can be arranged to
inject the
to angiogenic stimulant in a number of different ways. FIG. 18 shows a tissue
penetrating element 94 having a plurality of apertures 96 inserted into tissue
97.
Instead of, or in addition to, the aperture being positioned at the distal end
of tissue
penetrating element 94, such as is the case with aperture 66 in FIG. 12, the
tissue
penetrating element 94 includes a plurality of apertures 96 positioned
substantially
is along one side of the tissue penetrating element 94.
FIG. 19 illustrates an enlarged view of the tissue penetrating element 94 in
which the apertures 96 are positioned along an upper side of the tissue
penetrating
element 94. In other words, in the case of a tubular-shaped tissue penetrating
element
94, the apertures 96 are preferably arranged in the same hemisphere around the
tissue
2o penetrating element 64.
FIG. 20 shows still another arrangement of a tissue penetrating element. As
shown in FIG. 20, a tissue penetrating element 98 is arranged with a plurality
of
apertures 100 at an end portion thereof. As such, one portion of the tissue
penetrating
element 98, for example, an end portion closest to the device body 62, has no
2s apertures therethrough, while the opposite end contains all of the
apertures 100. It is
contemplated that the apertures 100 can be placed at the end portion proximal
to the
device body 62 and the distal portion of tissue penetrating element 98
equipped with
no apertures.
FIG. 21 shows still another arrangement of a tissue penetrating element. As
3o shown in FIG. 21, a tissue penetrating element 102 includes a plurality of
apertures
11
CA 02390852 2002-05-08
WO 01/37919 PCT/US00/42056
104 distributed substantially along the entire length of the tissue
penetrating element
102.
The tissue penetrating elements described herein can be used in conjunction
with any suitable device body. For example, FIG. 22 shows the tissue
penetrating
s element 64 used in conjunction with a device body 62 having a rounded tip
106
similar to that shown in FIG. 4. FIG. 23 shows the device body 62 equipped
with a
pointed tip 108 such as that shown in FIG. 6. Of course, any suitable tip
shape can be
used in conjunction with the tissue penetrating element 64.
FIG. 24 shows a metallic ring 110 as the tip. The metal ring 110 can be used
to
verify contact with the tissue by obtaining an electric impulse therefrom and
can be
used to cool the tissue. In addition, the metal ring 110 can be used to
partially shield
the tissue penetrating element 64.
FIG. 25 shows an example of device having a tissue penetrating element 64
and stylets 38. As discussed above, stylets 38 are thermally conductive and
is cryogenetically treat the tissue 97.
It is further contemplated that the tissue penetrating element can be movable
such that it can be retracted substantially fully within the device body 62 or
extended
to protrude from the device body 62 as necessary during deviceoperation. For
example. FIG. 26 shows an example of a device body 62 into which the tissue
2o penetrating element 64 is retracted into retraction area 106. The retractor
can take the
form of a spring or shaft operated by the device user (not shown) which is
coupled to
the tissue penetrating element. As shown in FIG. 26, the retractor can be a
toroidal
balloon 108. When the device body 62 is warm, the balloon 108 remains inflated
retracting the tissue penetrating element 64 which is fixed to a retraction
base 110.
2s By retracting the tissue penetrating element 64 into the device body 62,
the tip portion
of the device body 62 is available for use as a cryogenic ablation instrument
without
any portion protruding into the tissue.
The balloon 108 is preferably filled with a gas or fluid which, when cooled,
compresses. FIG. 27 shows an example of a retractable tip extended to its
maximum
3o penetrating position. When the device body 62 is cooled via the injection
of a
12
CA 02390852 2002-05-08
WO 01/37919 PCT/US00/42056
cryogenic gas or fluid via the cryogenic lumen 68, the balloon shrinks,
thereby pulling
the retraction base 110 toward the retraction area 106, resulting in the
extension of the
tissue penetrating element 64 outward and into the tissue 97.
In the alternative, the retraction base 110 can be spring loaded (not shown)
to
s force the tissue penetrating element 64 outward into an extended position
when the
device body 62 is cooled.
The present invention, therefore, advantageously provides a device which
combines cryogenic ablation with the injection of an angiogenic stimulating
fluid to
facilitate vascular growth.
to A variety ef modifications and variations of the present invention are
possible
in light of the above teachings. It is therefore understood that, within the
scope of the
appended claims, the present invention may be practiced otherwise than as
specifically described hereinabove. All references cited herein are expressly
incorporated by reference in their entirety.
1J