Note: Descriptions are shown in the official language in which they were submitted.
CA 02402322 2002-09-26
WO 01/75364 PCT/US00/08585
1
Method and Apparatus for a Catalytic Firebox Reactor
Background of the Invention
This invention was made with government support under DE-
FG02-95ERS2054 awarded bv the U.S. Department of Energy. The U.S.
government has certain rights in this invention.
Field of the Invention
The present invention relates to catalytic reactor design. More
specifically, the invention is a catalytic reactor for converting a
fuel/oxidant
mixture into a fuel/oxidant/product mixture and heat. The reactor emplovs an
exothermic catalvtic reaction channel cooled bv numerous cooling channels,
where the cooling fluid is a portion of the ultimate fuel/oxidant/product
mixture. A further refinement in the invention incorporates geometric
changes in the exothermic and/or cooling portions of the reactor to provide
streamwise variation of the velocity of the fluids in the reactor.
Brief Description of the Related Art
Highly exothermic catalytic reactors with internal cooling are well
known. While they have varying applications, the reactors are typified by
exothermic reactions within the catalytic portion of the reactor and a cooling
means to control the temperature within the catalytic portion to avoid a
material failure, either of the substrate or the catalyst. Cooling in these
reactors
can be accomplished by a number of means, including placing the catalyst in a
backside-cooled relationship with the cooling agent. A backside-cooling
arrangement is particularlv suitable for catalvtic reactions that are both
rapid
and highlv exothermic, such as catalvtic combustion. In this arrangement, the
catalvst substrate (typicallv a metal foil) is coated with an oxidation
catalvst on
only one side, the opposite side (or backside) remaining free of oxidation
catalvst. The substrate is shaped and assembled, before or after catalvst
coating,
to create separate channels for exothermic reaction (in the channels coated
with
oxidation catalvst) or for cooling (in the channels free of oxidation
catalyst).
CA 02402322 2002-09-26
WO 01/75364 PCT/US00/08585
2
Fluid passing through the cooling channels removes a portion of the heat
generated in the exothermic reaction channels.
An early example of a backside-cooled catalytic reactor for use in a
catalvtic combustion svstem is presented in US Patent 4,870,824 to Young et
al.
The '824 patent teaches the basic method of splitting a given fuel/air mixture
flow into catalytic and non-catalytic passages. The '824 patent teaches the
use of
a ceramic substrate with multiple parallel channels, generallv of the same
shape
and size, in which the walls which border and define each catalyti.: channel
are
coated with an oxidation catalyst on the sides facing the catalytic channel,
but
are not coated with an oxidation catalyst on the sides facing adjacent non-
catalytic channels. Bv this method, the percentage of total reactants
catalyzed in
the reactor is no greater than the percentage of catalytic channels. The
average
temperature rise through the reactor is thus limited. In addition, the wall
temperatures of catalytic channels bordering adjacent non-catalytic channels
are
controlled through the use of backside cooling.
Refinements of the basic structure taught by Young et al. are
shown in US Patents 5,250,489 and 5,512,250 to Dalla Betta et al. In the '489
patent a metal substrate is used for improved heat conduction to the backside
cooling fluid, and for greater resistance to thermal shock. Aluminum-
containing steels are cited as being preferred. The '489 patent also teaches
the
use of non-similar shape and/or size channels, so that the flow split between
catalytic and non-catalytic channels can be varied while retaining
approximately
half catalytic channels and half non-catalytic channels. Despite these
changes,
the fundamental structure claimed by Young et al., namely a multitude of
catalvtic channels and adjacent non-catalytic channels, is retained.
The '250 patent further refines the structure claimed by the '824
and '489 patents. In the '250 patent, Dalla Betta et al. teach a structure in
which
periodic alterations in channel shape provide different wall heat transfer
rates
in the catalytic channels and non-catalytic channels. Again, however, the
fundamental structure claimed by Young et al., namelv a multitude of catalytic
channels and adjacent non-catalytic channels, is retained. Furthermore, while
the '489 and '250 patents to Dalla Betta et al. teach catalvtic and non-
catalytic
channels of different shape and tortuositv, the average channel properties
(over
CA 02402322 2002-09-26
WO 01/75364 PCT/USOO/08585
~
some finite length) are not varied in the longitudinal direction, so that the
catalvtic reactors taught are effectively one- or two-dimensional in terms of
channel flow properties such as bulk heat transfer coefficient, velocity, or
average cross-sectional shape or area.
In general, the prior art backside-cooled catalytic reactors include a
multitude of catalvtic channels, where each individual catalvtic channel is in
essence a separate catalvtic reactor. As a result, variations in fuel/air
ratio from
channel to channel (due to imperfect premixing, for exa:nple) can lead to
different degrees of combustion and heat release in different channels.
Likewise, variations in inlet temperature from channel to channel can also
lead
to variations in combustion behavior in different channels. Rate of reaction,
catalyst light-off length, and maximum gas or surface temperature can all be
affected by the temperature and fuel/air ratio at a channel inlet. In
addition,
manufacturing tolerances may result in unequal physical properties of
different
channels. Properties which may vary include channel size, wall thickness,
catalyst or washcoat thickness, and catalyst loading; each of these may affect
combustion behavior. In essence, multiple catalytic channels can produce
widely varying degrees of catalytic combustion.
Because there is no mixing between separate catalytic channels in
the prior-art backside-cooled reactors, the reactors suffer the above-
mentioned
disadvantages of sensitivity to premixing (fuel/air ratio) aiid sensitivitv to
inlet
temperature uniformity. Given that all real systems have some level of gas-
stream non-uniformity, these sensitivities translate to a narrowed operating
range.
It has now been found that structures and methods that provide
an un-partitioned exothermic catalytic reaction channel and multiple cooling
channels offer superior performance. The un-partitioned exothermic catalytic
reaction channel allows for continual mixing of the fuel/oxidant stream within
the channel leading to a more uniform combustion and a wider operating
range.
In addition, the structure of the present invention is more flexible,
facilitating cross-stream area changes in the streamwise or longitudinal
direction, since there is no constraint that walls contact each other to form
CA 02402322 2002-09-26
WO 01/75364 PCT/US00/08585
4
multiple catalvtic channels. Thus, the invention can be used to vary the bulk
fluid properties in the streamwise or longitudinal direction via cross-stream
area changes. In particular, it mav be desirable to reduce the velocity of the
fuel/air mixture after it has entered the exothermic catalytic reaction
channel,
to provide greater residence time for reaction within the reactor, while
maintaining sufficient velocity at the reactor inlet to prevent flashback to
the
fuel/oxidant mixture upstream of the reactor.
Summary of the Invention
The present invention, a catalytic firebox reactor, is a catalytic
reactor employing an un-partitioned exothermic catalytic reaction channel and
multiple cooling conduits passing through the exothermic catalytic reaction
channel. An oxidation catalyst is deposited on the exterior surfaces of the
conduits within the exothermic catalytic reaction channel. This placement of
the catalyst allows the oxidation catalyst to be backside cooled by any fluid
passing through the cooling conduits. Backside cooling means that at each
location where an oxidation catalyst is deposited on one surface of a wall no
oxidation catalyst is deposited on an adjacent or opposite surface in contact
with
the cooling fluid, and a portion of the heat generated by reaction on the
oxidation catalyst is conducted through the substrate to the adjacent or
opposite
surface that is in direct contact with the cooling fluid.
The catalytic firebox reactor can be made in numerous
configurations with the following common elements. A casing forms the outer
boundary, which can be of any shape. The reactor casing can be a single
fabricated component, or can consist of two or more components (such as a
mixing duct and a conduit support duct) joined together. Two or more
conduits are placed within the casing such that one fluid stream can traverse
an
un-partitioned channel, the exothermic catalvtic reaction channel, defined by
the interior surface of the casing and the exterior surfaces of the conduits,
and a
number of separate fluid streams can traverse the passages defined by the
interior surfaces of the conduits, without mixing occurring between fluid in
the
exothermic catalytic reaction channel and fluid in the conduits' interior
passages. A heat transfer relationship exists between the fluid in the
CA 02402322 2002-09-26
WO 01/75364 PCT/US00/08585
exothermic catalvtic reaction channel and the fluids in the conduits' interior
passages.
The exothermic catalvtic reaction channel is the region between
the conduits' exterior surfaces and the reactor casing's interior surface,
5 beginning immediately downstream of the most downstream conduit inlet and
ending immediately upstream of the most upstream conduit outlet (note that
the most downstream conduit inlet is upstream of the most upstream conduit
outlet). This region forms an un-partitioned channel because an intentional
gap is made between adjacent conduits for some portion of the conduits'
length,
within the exothermic catalytic reaction channel. The term "un-partitioned"
means that any two points within the channel can be connected by a path
contained entirely within the channel. The terms "upstream" and
"downstream" refer to the location of imaginary cross-stream surfaces, so that
points which are not on the same streamline can have a definite upstream-
downstream relationship. "Cross-stream" means perpendicular to the flow, so
that streamlines of the flow always intersect a cross-stream surface
perpendicularly. Thus, a first point is said to be upstream of a second point
if
the cross-stream surface on which the first point lies is upstream of the
cross-
stream surface on which the second point lies.
The intentional gaps between adjacent conduits allow the
exothermicallv catalvzed fluid to mix as the fluid catalvticallv reacts, so
that
non-uniformities in fluid velocity, fluid composition, and fluid temperature
are ameliorated before the fluid exits the reactor. Incidental leakage of the
exothermically catalyzed fluid between adjacent conduits is not considered an
intentional gap. Rather, the intentional gaps must be of sufficient size that
fluid mav freely pass through the gaps. Specifically, this means that the sum
of
the flow areas through all of the gaps within the exothermic catalytic
reaction
channel must be greater than the channel's minimum cross-stream flow area.
The cross-stream flow area of the exothermic catalytic channel, at anv given
streamwise location, is the area over which exothermicallv catalvzed fluid
passes through an imaginary cross-stream surface within the channel. For flow
through a gap between adjacent conduits, the flow area is defined as the
minimum area within the exothermic catalvtic reaction channel of an
CA 02402322 2002-09-26
WO 01/75364 PCT/USOO/08585
6
imaginary surface which passes through the axial (streamwise) centerlines of
both conduits and which extends between a straight line connecting the two
conduit inlets and a straight line connecting the two conduit outlets. While
it
is possible to have intermediate support structures within the exothermic
catalytic reaction channel which locally close off one or more gaps between
adjacent conduits, these support structures are open to the passage of fluid
from
the upstream portion of the reactor to the downstream portion of the reactor,
and do not significantly reuuce the continual mixing of fluid within the
exothermic catalytic reaction channel.
Oxidation catalyst is deposited on at least a portion of the conduits'
exterior surfaces within the exothermic catalvtic reaction channel. The
oxidation catalvst provides the exothermic reaction surface which supports the
exothermic oxidation of fuel within the exothermic catalytic reaction channel.
Catalyst coating may be applied to all of the conduits, or to only some of the
conduits (leaving some of the conduits un-coated). In general, it is preferred
that no oxidation catalyst is applied upstream of the most downstream conduit
inlet, so that catalyzed fluid cannot enter any of the cooling conduits.
The conduits can be made in numerous configurations
(cylindrical, fluted, externally finned, internally finned, internally
partitioned,
of round, lobed, polygonal, elliptical, or other cross-sectional shapes,
straight or
twisted, and so on), and can be arranged in numerous configurations within the
casing (square-packed, close-packed, braided or spiraled, crisscrossed, and so
on)
using conduits of equal or unequal cross-sectional size, and of equal or
unequal
length. In all configurations, an intentional gap exists between adjacent
conduits for some portion of the conduits' length, so that fluid in the
exothermic catalvtic reaction channel may pass between adjacent conduits,
therebv allowing an un-partitioned exothermic catalvtic reaction channel to
exist within the reactor casing. A conduit mav contain a single cooling
passage,
as in a simple tube for example, or a conduit may contain more than one
cooling passage. For example, two parallel straight tubes may be joined to
form
a single conduit containing two cooling passages. Alternativelv, a divider
mav;
be inserted in a single tube to create a conduit containing two cooling
passages.
Three or more cooling passages mav also be formed in a single conduit, if
CA 02402322 2002-09-26
WO 01/75364 PCT/US00/08585
7
desired. In all configurations, however, oxidation catalyst is applied only to
the
conduits' exterior surfaces, and the conduits must be configured with an
intentional gap between adjacent conduits such that a single exothermic
catalytic reaction channel is formed.
The present invention is also a method for using a backside-cooled
catalvst structure, wherein the velocities of the exothermicallv catalyzed
stream
and the cooling stream may be separately varied as the streams pass through
the
reactor, and in particular where the velocity of the exothermically catalyzed
stream is reduced, after the stream enters the exothermic catalvtic reaction
channel, bv a streamwise increase in the cross-sectional (cross-stream) area
of
the exothermic catalvtic reaction channel. The term "streamwise" means in the
direction of the flow. The catalytic firebox structure can be configured to
accomplish this variation of velocity by a streamwise reduction in the cross-
stream area of the cooling conduits, resulting in a streamwise increase in the
cross-stream area of the exothermic catalytic reaction channel, if the wall
thickness between the cooling conduits and the exothermic catalytic reaction
channel remains unchanged, and if the total cross-stream area of the reactor
(including both the cooling conduits and the exothermic catalytic reaction
channel) remains unchanged. Other means for reducing the velocity of the
exothermically catalyzed stream area also possible, such as limiting the
entrance
flow area to the exothermic catalvtic reaction channel bv a baffle, plate, or
other
structure.
In the present invention, the cooling fluid and the exothermically
catalyzed fluid comprise portions of an ultimate combustion mixture. As a
fuel/oxidant mixture passes through the exothermic catalvtic reaction channel
it catalytically reacts. The heat of reaction is added to both the fluid in
the
exothermic catalytic reaction channel and the fluid passing through the
cooling
conduits. After discharge of the fluids from the exothermic catalvtic reaction
channel and from the cooling conduits, the fluids are allowed to mix such that
a single combined flow is created.
CA 02402322 2006-11-17
7a
In accordance with an aspect of the present invention, there is provided a
catalytic firebox reactor comprising:
a casing having an inlet and an outlet, and an interior surface and an
exterior
surface, the casing interior surface defining an interior chamber;
at least two conduits, each conduit having an inlet and an outlet, and an
interior surface and an exterior surface, the conduits retained within the
interior
chamber, the conduit exterior surfaces and the casing interior surface forming
an exothermic catalytic reaction channel having an exit, the exothermic
catalytic
reaction channel exit and the conduit outlets proximately located such that a
first fluid upon exiting the conduit outlet is in contact with a second fluid
that
has exited the exothermic catalytic reaction channel, and
an oxidation catalyst deposited within the exothermic catalytic reaction
channel
on at least a portion of at least one of the conduit exterior surfaces, the
oxidation catalyst being backside cooled from the conduit interior surface by
the
first fluid.
In accordance with another aspect of the present invention, there is
provided a catalytic firebox reactor comprising:
a casing having an inlet and an outlet, and an interior surface and an
exterior
surface, the casing interior surface defining an interior chamber;
at least two conduits, each conduit having an inlet and an outlet, and an
interior surface and an exterior surface, the conduits retained within the
interior
chamber, the conduit exterior surfaces and the casing interior surface forming
an exothermic catalytic reaction channel having an exit, the exothermic
catalytic
reaction channel exit and the conduit outlets proximately located such that a
first fluid upon exiting the conduit outlet is in contact with a second fluid
that
has exited. the exothermic catalytic reaction channel, wherein the conduits
are
retained within the casing by a securing structure comprising a first retainer
that is open to the passage of fluid connected to said casing upstream of the
conduit inlets, and a second retainer that is open to the passage of fluid
connected to said casing downstream of the conduit outlets wherein at least
one
of any two adjacent conduits has at least one locally expanded cross-section
whereby when aggregated with the adjacent conduit creates the exothermic
catalytic reaction channel; and
an oxidation catalyst deposited within the exothermic catalytic reaction
channel
on at least a portion of at least one of the conduit exterior surfaces.
CA 02402322 2006-11-17
7b
In accordance with another aspect of the present invention, there is
provided a catalytic firebox reactor comprising:
a casing having an inlet and an outlet, and an interior surface and an
exterior
surface, the casing interior surface defining an interior chamber;
at least two conduits, each having an inlet, an outlet, an interior surface
and an
exterior surface, the conduits retained within the interior chamber, the
conduit
exterior surfaces and the casing interior surface forming an exothermic
catalytic
reaction channel having an exit, the exothermic catalytic reaction channel
exit
and the conduit outlets proximately located such that a first fluid upon
exiting
the conduit outlet is in contact with a second fluid that has exited the
exothermic catalytic reaction channel, wherein the conduits are retained by a
single attachment, whereby the conduits are free to expand axially, wherein at
least one of any two adjacent conduits has at least one locally expanded cross-
section such that when aggregated with adjacent conduits the conduits are
laterally positioned within the interior chamber, and the securing means is a
bundle created by connecting adjacent conduits at a single attachment location
and connecting the bundle to said casing; and
an oxidation catalyst deposited within the exothermic catalytic reaction
channel
on at least a portion of at least one of the conduit exterior surfaces.
In accordance with another aspect of the present invention, there is
provided a catalytic firebox reactor comprising:
a casing having an inlet and an outlet and defining an interior chamber;
a first retainer positioned in the interior chamber connected to said casing,
the
first retainer being open to the passage of fluid;
a second retainer positioned within said interior chamber downstream of the
first retainer connected to said casing, the second retainer being open to the
passage of fluid;
at least two conduits, each conduit having an inlet and an outlet and an
interior
surface and an exterior surface, the conduits positioned longitudinally within
the interior chamber, the conduits connected to the retainers, the conduit
exterior surfaces and the casing interior surface forming an exothermic
catalytic
reaction channel having an exit, the exothermic catalytic reaction channel
exit
and the conduit outlets proximately located such that a first fluid upon
exiting
the conduit outlet is in contact with a second fluid that has exited the
exothermic catalytic channel; and
CA 02402322 2006-11-17
7c
an oxidation catalyst deposited within the exothermic catalytic reaction
channel
on at least, a portion of at least one of the conduit exterior surfaces, the
oxidation catalyst being backside cooled from the conduit interior surface by
the
first fluid.
In accordance with an aspect of the present invention, there is provided a
catalytic firebox reactor comprising:
a casing having an inlet and an outlet and defining an interior chamber;
at least two conduits with a nominal cross-section, each conduit having an
inlet
and an outlet and an interior surface and an exterior surface, the conduits
positioned within the interior chamber, each conduit inlet having a first
expanded cross-section and a second expanded cross-section, the first and
second cross-sections being sufficient to create an exothermic catalytic
reaction
channel having an exit, the exothermic catalytic reaction channel defined by
the
interior surface of said casing and the conduits exterior surfaces, the first
expanded cross-sections connected to one another creating a bundle the bundle
connected to said casing, and the exothermic catalytic reaction channel exit
and
the conduit outlets proximately located such that a first fluid upon exiting
the
conduit outlet is in contact with a second fluid that has exited the
exothermic
catalytic channel; and
an oxidation catalyst deposited within the exothermic catalytic reaction
channel
on at least a portion of at least one of the conduit exterior surfaces, the
oxidation catalyst being backside cooled from the conduit interior surface by
the
first fluid.
In accordance with another aspect of the present invention, there is
provided a catalytic firebox reactor comprising:
a casing having an inlet and an outlet and defining an interior chamber;
an inlet retainer having an upstream face and a downstream face, the inlet
retainer connected to said casing within the casing inlet;
an outlet retainer having an upstream face and a downstream face, the outlet
retainer connected to said casing within the casing outlet;
at least two conduits, each conduit having an inlet and an outlet and an
interior
surface and an exterior surface, the conduits positioned longitudinally within
the interior chamber, the conduits extending through the inlet retainer and
the
outlet retainer, the conduits positioned by the inlet retainer and the outlet
retainer, the conduit exterior surfaces and the casing interior surface
forming an
CA 02402322 2006-11-17
7d
exothermic catalytic reaction channel having an exit, the exothermic catalytic
reaction channel exit and the conduit outlets proximately located such that a
first fluid upon exiting the conduit outlet is in contact with a second fluid
that
has exited the exothermic catalytic channel; and
an oxidation catalyst deposited within the exothermic catalytic reaction
channel
on at least a portion of at least one of the conduit exterior surfaces, the
oxidation catalyst being backside cooled from the conduit interior surface by
the
first fluid.
In accordance with another aspect of the present invention, there is
provided a catalytic firebox reactor comprising:
a casing having an inlet and an outlet and defining an interior chamber;
at least two conduits having an inlet and an outlet and an interior surface
and
an exterior surface, the conduits positioned longitudinally within the
interior
chamber;
a support plate being open to the passage of fluid positioned within said
casing
inlet connected to said casing, the conduits passing through the support plate
and connected thereto within the interior chamber, the conduit exterior
surfaces
and the casing interior surface forming an exothermic catalytic reaction
channel
having an exit, the exothermic catalytic reaction channel exit and the conduit
outlets proximately located such that a first fluid upon exiting the conduit
outlet
is in contact with a second fluid that has exited the exothermic catalytic
channel; and
an oxidation catalyst deposited within the exothermic catalytic reaction
channel
on at least a portion of at least one of the conduit exterior surfaces.
In accordance with another aspect of the present invention, there is
provided a method for creating a more reactive fuel/oxidant mixture, said
method comprising:
generating a fuel/oxidant mixture; passing a second portion of the
fuel/oxidant
mixture into cooling channels of a catalytic reactor;
simultaneously passing a first portion of the fuel/oxidant mixture into the
catalytic reactor at an entrance velocity greater than the flame propagation
velocity and into contact with exothermic reaction surfaces, the surfaces
being
in a backside cooled relationship with the cooling channels;
reducing the velocity of the first portion while in the catalytic reactor;
CA 02402322 2006-11-17
7e
increasing the velocity of the second portion while in the cooling channels;
and
mixing the first portion and the second portion after exiting the catalytic
reactor.
In accordance with another aspect of the present invention, there is
provided a method for creating a more reactive fuel/oxidant mixture, said
method comprising:
passing a first fuel/oxidant mixture into a single exothermic catalytic
reaction
channel of a catalytic reactor, the exothermic catalytic reaction channel
having
an oxidation catalyst deposited therein;
simultaneously passing a second fuel/oxidant mixture into cooling conduits of
the catalytic reactor, the conduits passing through the exothermic catalytic
reaction channel; and
combining the cooling conduit effluent and reaction channel effluent.
CA 02402322 2002-09-26
WO 01/75364 PCT/US00/08585
8
Brief Description of the Drawings
Figure 1 illustrates the basic catalytic firebox reactor.
Figure 2 illustrates a second embodiment of a catalytic firebox
reactor employing expanded conduit end-sections in conjunction with
retainers.
Figure 3 is a cross-sectional view of the catalvtic firebox reactor of
Figure 2 taken immediately downstream of the conduit entrance looking
upstream.
Figure 4 is a cross-sectional view of the catalytic firebox of Figure 2
taken in approximately the center of the reactor looking upstream.
Figure 5 illustrates a catalytic firebox reactor employing a retainer
strategy that does not require expanded conduit end sections.
Figure 6 is a cross-section of the catalytic firebox reactor shown in
Figure 5 taken just downstream of the conduit entrances looking upstream.
Figure 7 illustrates a catalytic firebox reactor using localized
expansion of the conduits to retain the conduits within the reactor.
Figure 8 illustrates a catalytic firebox reactor of close-packed
configuration with expanded-end conduits.
Figure 9 illustrates a close-packed configuration of a catalytic
firebox reactor with intermediate conduit expansion.
Detailed Description of the Preferred Embodiments
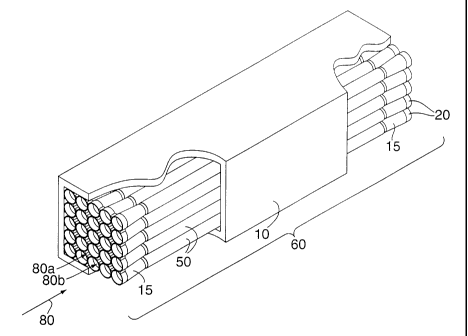
As shown in Figure 1, the catalytic firebox reactor is a structure
comprised of an exothermic catalytic reaction channel 60 penetrated by
multiple
cooling conduits 20 for backside cooling of oxidation catalyst 50 supporting
the
reaction within the channel. In the operation of this reactor a portion of a
fuel/oxidant mixture 80, 80a, enters the exothermic catalytic reaction channel
60
and is exothermically catalvticallv reacted on oxidation catalvst 50, while
simultaneously a portion the fuel/oxidant mixture 80, 80b, enters the multiple
cooling conduits 20 and cools the catalyst 50. The exothermically catalvzed
fluid
80a then exits the exothermic catalvtic reaction channel 60 and mixes with the
cooling fluid 80b exiting the multiple cooling conduits, creating a heated
combustible fuel/oxidant/ product mixture.
CA 02402322 2002-09-26
WO 01/75364 PCT/US00/08585
9
Figure 1 depicts a catalvtic firebox reactor emploving an exothermic
catalvtic reaction channel with an interstitial entrance and exit strategy.
The
reactor is comprised of a casing 10 into which conduits 20 are placed. An
oxidation catalyst 50 is deposited on the exterior surfaces of the conduits 20
within the exothermic catalytic reaction channel 60. The conduits are expanded
at their inlets and outlets. In the depiction shown in Figure 2 the reactor is
fabricated from a square-packed arrangement of parallel, equal-length,
cylindrically-shaped conduits 20. In the configuration shown, the expanded
sections 15 perform the dual function of creating a single un-partitioned
exothermic catalytic reaction channel, by providing a gap between the
conduits,
and creating an entrance into (and exit from) the exothermic catalvtic
reaction
channel bv creating a series of interstices between adjacent expanded end-
sections. A similar configuration could be created by expanding the conduits
near, but not at, their inlets and outlets (an example of this is shown later,
in
Figure 8). In the operation of this configuration a single fuel/air mixture
stream enters the casing and is split between the exothermic catalytic
reaction
channel and the multiple conduits' cooling passages. At the exit of the
reactor
the catalyzed stream and the cooling streams mix to create a single heated,
partially reacted, fuel/air/product mixture stream.
In the configuration shown in Figure 1, the expanded sections 15 at
the ends of each conduit are cvlindrical (round), and are concentric with the
conduit centerlines. Other cross-sectional shapes of the expanded sections are
also possible, including ellipses, squares, hexagons, octagons, lobed shapes
("clover-leaf"), and so on. In general, the term "expanded" means that at some
axial location the maximum diameter of the conduit is greater than the
maximum diameter of the conduit at other axial locations.
Figure 2 shows another embodiment of a catalytic firebox reactor
emploving expanded conduit end-sections 15 in conjunction with a retainer
strategy. In this embodiment the conduits 20 are expanded at the inlets and
outlets and positioned within casing 10. The expanded sections position the
conduits 20 laterally within the casing 10. A first retainer 33 is positioned
at or
near the conduit inlets and a second retainer 34 is positioned at or near the
conduit outlets. The retainers, 33 and 34, longitudinally position conduits 20
CA 02402322 2002-09-26
WO 01/75364 PCT/US00/08585
within the casing. Figure 3 is a cross-section of this reactor taken
immediately
downstream of the conduit entrances, looking upstream, showing that the
expanded end-sections position the conduits 20 laterally within the casing 10
and provide interstices for fluid to enter the exothermic catalvtic reaction
5 channel. Figure 4 is a cross-section of this reactor taken in the middle
looking
upstream and shows that the expanded sections create a single un-partitioned
exothermic catalytic reaction channel by providing gaps between the conduits
20. The retainers 33 and 34 are shown as honeycombs. For this case, the cell
size of the honeycomb is no larger than the expanded conduit ends, so that the
10 conduits are not able to pass through the honeycomb cells. As in the
configuration of Figure 1, oxidation catalyst 50 is deposited on the exterior
surfaces of the conduits 20 within the exothermic catalytic reaction channel
60.
Figure 5 shows another configuration of the reactor employing a
retainer strategy. In this reactor the conduits 20 are inserted in a first
retainer 36
and a second retainer 37, and may or may not have expanded end-sections. The
conduits mav be attached to the first retainer 36, the second retainer 37, or
both,
by, for example, welding or brazing or press fit. If attachment at only one
end is
preferred, the conduits may slide freely through one of the retainers; for
example, the conduits may be welded to the first retainer 36, but may slide
freely
through the second retainer 37. Like the honeycombs shown in Figure 2, the
retainers in Figure 5 have passages that are open to the passage of fluid, as
shown in Figure 6. Passages 70a permit fluid to enter the exothermic catalytic
reaction channel 60 while passages 70b (not shown) permit the fluid to exit.
Passages 71a permit fluid to enter the conduits' 20 while passages 71b (not
shown) permit the fluid to exit. Oxidation catalyst 50 is deposited on the
exterior surfaces of conduits 20 within the exothermic catalytic reaction
channel
60. A variation of the embodiment shown in Figure 5 may incorporate onlv
one retainer. In this case, the conduits pass through a single retainer 36,
and
mav be attached to the retainer bv, for example, welding or brazing or press
fit.
Figure 7 shows a similar configuration to that shown in Figure 5,
but shows a different method of retaining the conduits. In Figure 7, the
conduits 20 are inserted through a first retainer 36 and a second retainer 37,
but
are not attached to the retainers. Instead, each conduit passes through the
CA 02402322 2002-09-26
WO 01/75364 PCT/USOO/08585
11
retainers and is expanded at each end, to a size which does not permit the
expanded conduit ends 15 to pass through the retainers. Thus, no attachment
to the retainers is required, and the conduits may slide freely through the
retainers until an expanded end contacts a retainer. The retainers are
attached
to the reactor casing 10. As in Figure 5, the retainers in Figure 7 have
passage
70a and 70b that permit fluid to enter and exit the exothermic catalvtic
reaction
channel 60, respectively. Oxidation catalyst 50 is deposited on the exterior
surfaces of conduits 20 within the exothermic catalytic reaction channel 60.
A close-packed configuration of expanded-end conduits is shown
in Figure 8. In this close-packed arrangement, the conduits 20 are arranged
within casing 10 with the conduit centerlines lying on an imaginary grid
consisting of adjacent equal-size equilateral triangles, so that each conduit
is in
direct contact with those adjacent at the expanded end-sections. The imaginary
grid lines in this close-packed case consist of three sets of parallel,
equally-spaced
lines, each set of lines being oriented at a 60-degree angle from each other
set of
lines. In the example shown in Figure 8 the conduits are attached to one
another forming a bundle and conduits adjacent to the casing are attached to
the casing by, for example, welding or brazing. Oxidation catalyst 50 is
deposited
on the exterior surfaces of conduits 20 within the exothermic catalytic
reaction
channel 60.
Figure 9 illustrates another close-packed configuration of conduits
20. In this example, the expanded conduit sections 15 are located near, but
not
at, the tube ends. Thus, the conduits are supported and located laterally in
the
same manner as in Figure 8, but the inlet face to the reactor differs between
the
two cases. In particular, since the conduits 20 are expanded at their inlets
in
Figure 8 but are not expanded at their inlets in Figure 9, the ratio of the
inlet
area of the conduits 20 to the total reactor cross-stream area (defined bv the
casing 10 size) is greater in Figure 8 than in Figure 9. In the example shown
in
Figure 9, the conduits are attached to one another at their expanded sections,
and conduits adjacent to the casing are attached to the casing at their
expanded
sections, bv for example welding or brazing. Oxidation catalyst 50 is
deposited
on the exterior surfaces of conduits 20 within the exothermic catalytic
reaction
channel 60.
CA 02402322 2002-09-26
WO 01/75364 PCT/US00/08585
12
Comparing the reactor configurations shown in Figures 1, 8, and 9,
it is clear that in all cases shown a single fuel/air mixture stream enters
the
casing and is split between the exothermic catalytic reaction channel and the
multiple conduits' cooling passages (note, however, that this is not required;
separate fuel/air mixture streams may enter separate portions of the reactor,
if
proper manifolding is provided). The percentage of the initial fuel/air
mixture
stream which enters the exothermic catalytic reaction channel is approximately
determined by the effective flow area of the cooling passages within the
cooling
conduits versus the effective flow area of the exothermic catalytic reaction
channel, since the total pressure drop from reactor inlet to reactor exit is
the
same across each flow path. While there are many variables which determine
the effective area of each flow path, the effective areas are in part
determined by
the cross-stream area at the entrance to a passage or channel. Thus, for
similar
size, shape, and length conduits, a greater percentage of the flow will enter
the
exothermic catalytic reaction channel in the configuration of Figure 1 (square-
packed) than in the configuration of Figure 8 (close-packed), and a greater
percentage of flow will enter the exothermic catalytic reaction channel in the
configuration of Figure 9 (expanded near, but not at, the conduit ends) than
in
the configuration of Figure 8 (expanded at the conduit ends).
As stated above the invention requires that the fluid exiting the
exothermic catalvtic reaction channel exit and the fluid exiting the conduit
outlets must come into contact to permit some degree of mixing to form the
ultimate combustion mixture. To accomplish this, the exothermic catalytic
reaction channel exit and the conduit outlets must be proximately located.
Proximately located means that the exits are spatially located to permit the
fluids exiting the exit and the outlets to come in contact so some degree of
mixing is possible prior to ultimate combustion. Figures 1, 2, 5, 8 and 9 show
the closest possible streamwise proximate locations of the exothermic
catalytic
reaction channel exit and the conduit outlets, occurring essentially at the
same
streamwise location. Figure 7 shows a configuration where the conduit outlets
are downstream of the exothermic catalvtic reaction channel.
Figures 1 through 9 illustrate several strategies for retaining the
conduits 20 within casing 10. The invention does not require symmetrv in
CA 02402322 2002-09-26
WO 01/75364 PCT/US00/08585
13
securing the upstream and downstream ends of the conduits and the different
securing structures illustrated could be combined in a single embodiment. For
example, the conduits 20 could be secured at the upstream end by a retaining
structure and at the downstream end by an expanded conduit structure. In
addition, it must be realized that the casing and the conduits may operate at
different temperatures, resulting in different amounts of thermal expansion.
Thus, in the configuration shown in Figure 2 for example, clearance may be
provided between the conduits and the upstream or downstream retainers, to
allow for thermal growth of the conduits. In the configuration shown in Figure
1, the conduits may be secured (by welding, for example) to each other and to
the casing only at one end, as for example at the inlet or upstream end, with
the
other end free to move longitudinally so that differential thermal growth of
the
conduits may be accommodated. If a clearance is allowed between the conduits
and the retainer(s), or if differential thermal growth between adjacent
conduits
is expected, the conduits' expanded sections (if employed) should be of
sufficient axial length that lateral support and positioning of the conduits
is
provided even when adjacent tubes move in opposite axial directions to the
maximum extent allowed by the clearance space or the expected difference in
thermal growth. If the conduits penetrate or pass through the retainers, as in
Figures 5 and 7, respectively, and if they are laterally positioned by the
retainers,
the expanded sections need not provide lateral support to the conduits. In
this
case, it may also be advantageous to allow the conduits to slide freely
through at
least one of the retainers, to allow for thermal expansion of the conduits.
Conduits 20 can be fabricated from metal, preferablv a high-
temperature-tolerant allov suitable for the application, which provides good
heat transfer from the oxidation catalyst 50 to the cooling fluid contacting
the
interior surface of conduits 20. Conduits 20 may have any wall thickness.
Generally, thinner walls will be weaker and less resistant to long-term
oxidation, while thicker walls will result in increased pressure drop through
the reactor. The preferred wall thickness is between 0.05 mm and 2 mm, with
wall thicknesses between 0.1 mm and 1 mm being most preferred. If expanded
sections are emploved minor variations in wall thickness are acceptable.
Casing
CA 02402322 2002-09-26
WO 01/75364 PCT/US00/08585
1-1
is also fabricated from high-temperature tolerant materials. Wall thickness
is based upon the application.
Oxidation catalyst 50 is applied to the exterior of conduits 20 within
the exothermic catalvtic reaction channel. While the entire exterior of
conduits
5 20 could be catalyst coated, as a practical matter catalyst coatings should
not be
applied where the channels touch one another or the casing 10. This allows
close fabrication and assembly tolerances, without concern for variable
coating
thickness, and allows for welding or brazing of metal-to-metal contact points,
if
desired.
10 The reactor can also incorporate streamwise variation of the fluid
velocities. Specificallv, the velocitv of the fluid in the exothermic
catalytic
reaction channel can be decreased after the fluid enters the channel by
streamwise geometric changes in the reactor wherein the channel's entrance
flow area is less than the channel's cross-stream flow area at some streamwise
location where catalyst 50 is deposited. The channel's entrance flow area is
defined as the channel's cross-stream flow area immediately downstream of the
most downstream conduit inlet. If the conduit wall thicknesses are
approximately constant, streamwise changes in flow velocity can be produced by
providing, for example, either contracted sections of the conduits 20,
expanded
sections of the casing 10, or a combination of both. In a reactor employing
expanded-end conduits 20 (or, equivalently, contracted center sections), as in
figures 1, 2, or 8, the cross-stream area of conduits 20 decreases just after
the
cooling flow enters the cooling passages, where the expanded conduit sections
taper down to the nominal conduit size in the central portion of the reactor.
As
a result, the cooling flow velocity is increased to a value greater than its
entrance velocity. Conversely, because the casing 10 is of constant cross-
sectional size, the cross-stream flow area of the exothermic catalvtic
reaction
channel increases just after the fuel/oxidant mixture enters. As a result, the
flow velocitv in the exothermic catalytic reaction channel is decreased to a
value
less than its entrance velocity.
The gas flow velocity entering the exothermic catalvtic reaction
channel should exceed the minimum required to prevent flashback into the
fuel/oxidant stream upstream of the reactor if the fuel/oxidant mixture
CA 02402322 2002-09-26
WO 01/75364 PCT/US00/08585
entering the exothermic catalvtic reaction channel is within the limits of
flammability. The laminar flame propagation velocity (velocity of a laminar
deflagration wave) is typically less than 1 m/s for hydrocarbon fuels in air,
but
the turbulent flame propagation velocitv may exceed 10 m/s and may approach
5 30 m/s for highly turbulent flow. To prevent flashback, the gas flow
velocity
should exceed 10 to 30 m/s at gas turbine engine conditions, or more if a
safety
margin is allowed. Because catalvst light-off becomes increasingly difficult
with
increasing velocity, it is desirable to reduce the velocitv of the fuel/air
stream
once it has entered the exothermic catalytic reaction channel, bv for example
a
10 streamwise variation of cross-stream area as discussed above. In the
preferred
embodiment of the present invention, the flow velocitv of the fuel/air stream
over the exothermic reaction surface (oxidation catalvst) in the exothermic
catalytic reaction channel is nominally 30 m/s or less. This reduction in
velocity is achieved by a streamwise increase in the cross-stream area for
flow
15 over the exothermic reaction surface. Streamwise changes in cross-stream
area
are fixed by the geometry of the reactor, and do not change in time.
The velocity of the cooling stream should exceed the maximum
flame propagation velocity (nominally 10 to 30 m/s or more) at the exit of the
cooling conduits, if the cooling fluid exiting the cooling passages is within
the
limits of flammability, to prevent flashback from a downstream combustion
chamber. If expanded conduit outlets are employed, it is also very important
that the downstream increase in cross-stream area of the cooling conduits 20
is
sufficiently gradual that recirculation of the cooling flow does not occur, so
that
there is no possibility of flashback or flameholding in the downstream
expanded conduit section. Typically the cone angle for an axi-symmetric
diffuser section should not exceed approximately 8 to 10 degrees for good
pressure recovery and minimal recirculation. If continued backside cooling is
a
consideration, the angle should be especially shallow (less than 4 or 5
degrees
for an axi-symmetric section) to ensure that there is no local separation of
the
cooling flow and concurrent loss of cooling effectiveness.
Also, when the fuel/air ratio in the cooling channels is within the
limits of flammability, it is preferred that the cross-stream area for flow
through
the cooling channels is not increased after the cooling stream enters conduits
CA 02402322 2002-09-26
WO 01/75364 PCT/US00/08585
16
20. The cross-stream area of the cooling passages may in fact be decreased to
increase the cooling flow velocity for greater resistance to flashback or pre-
ignition in the cooling portion of the reactor. The minimum residence time for
pre-ignition to occur in conduits 20 is dependent upon fuel tvpe, fuel/air
ratio,
temperature, and pressure, and can be measured experimentally or calculated
on the basis of elementarv chemical reaction rates, if known. Gas temperatures
in the cooling portion of the reactor may rise to near the material limit of
the
catalyst and substrate (near 1200 Kelvin for precious metal catalvsts),
resulting
in very short ignition delay times, possibly as short as 2 ms for natural gas
in air
at pressures near 10 atm. To prevent pre-ignition in the cooling portion of
the
reactor, the gas residence time in the cooling portion of the reactor should
be
less than the ignition delay time. Increasing the cooling flow velocity within
the cooling passages reduces the residence time of the cooling fluid within
the
reactor, and reduces the cooling fluid's propensity for pre-ignition.
Similarly,
decreasing the velocity of the fluid in the exothermic catalytic reaction
channel
provides increased residence time for reaction in a given length reactor,
while
the cooling fluid's residence time within the same length reactor remains at a
smaller value, allowing a reduced propensity for pre-ignition of the cooling
fluid.
Regardless of the specific catalytic firebox reactor configuration, the
reactor should be capable of providing good contact between the oxidation
catalvst and the fuel/oxidant mixture in the exothermic catalvtic reaction
channel. For catalytic combustion applications, it is preferred that the
reactor be
sized such that the reaction of the fuel/oxidant mixture in the exothermic
catalvtic reaction channel should proceed more than 50% of the way to
completion before exiting. For fuel-lean mixtures, this means that more than
50% of the fuel entering the channel should be consumed. Most preferably,
more than 75% of the fuel entering the exothermic catalytic reaction channel
should burn before exiting.
The percentage of reaction completed in the exothermic catalytic
reaction channel depends both upon the flow rate of the fuel/oxidant mixture
through the exothermic catalytic reaction channel and upon the physical
characteristics of the catalvtic firebox reactor. The chemical composition of
the
CA 02402322 2002-09-26
WO 01/75364 PCT/USOO/08585
17
fuel/oxidant mixture mav also affect the percentage of reaction completed,
particulariv if the rate of chemical reaction is significantlv limiting when
compared to the rate of mass transfer to the catalyst surface.
With regard to percentage of reaction completed, important
phvsical characteristics within the exothermic catalytic reaction channel
include
the rate of mass transfer to the oxidation catalvst surface, the ratio of
oxidation
catalvst surface area to reaction channel volume, and the activity of the
oxidation catalyst.
In a square-packed conduit configuration (parallel conduits
bundled such that the conduit centerlines are arranged on an imaginary square
grid), as shown in Figure 5, the conduits are preferably between 2 mm and 8
mm in diameter, and between 75 mm and 400 mm in length, for a catalytic
combustion application in which the average velocity of the fuel/air mixture
entering the reactor is between 20 m/s and 100 m/s. A close-packed reactor
would have conduits with dimensions in approximately the same ranges as
those for the square-packed reactor.
The fuel may comprise Cl to C20 hydrocarbons, Cl to C20
hydrocarbon oxygenates, and blends thereof. Suitable gaseous fuels include
natural gas, methane, and propane. Suitable liquid fuels include gasoline,
kerosene, No. 1 heating oil, No. 2 heating oil, and conventional aviation
turbine fuels such as Jet A, Jet B, JP-4, JP-5, JP-7, and JP-8. "Hvdrocarbon"
not
onlv refers to organic compounds, including conventional liquid and gaseous
fuels, but also to gas streams containing fuel values in the form of compounds
such as carbon monoxide, organic compounds, or partial oxidation products of
carbon containing compounds. If the fuel is a liquid, it should be vaporized
or
atomized before mixing with oxidant or while being mixed with oxidant.
The exothermic catalytic portion of the catalytic firebox reactor may
have as an active ingredient precious metals, group VIII noble metals, base
metals, metal oxides, or anv combination thereof. Elements such as zirconium,
vanadium, chromium, manganese, copper, platinum, palladium, osmium,
iridium, rhodium, ruthenium, cerium, cobalt, nickel, iron, and the like may be
used. Platinum and palladium are preferred, palladium being especially
preferred for use with natural gas or methane fuels. The oxidation catalvst
mav
CA 02402322 2002-09-26
WO 01/75364 PCT/USOO/08585
18
be applied directlv to the substrate of the reactor, or may be applied to an
intermediate bond coat or washcoat composed of alumina, silica, zirconia,
titania, magnesia, other refractorv metal oxides, or anv combination thereof.
The washcoat mav be stabilized bv the addition of additives such as lanthanum,
cerium, barium, chromium, or other materials. Alumina washcoats are
preferred for use with natural gas or methane fuels.
The catalvst-coated substrate may be fabricated from any of various
high temperature materials. High temperature metal alloys are preferred,
particularly alloys composed of iron, nickel, and / or cobalt, in combination
with
aluminum, chromium, and/or other alloying materials. High temperature
nickel alloys are especially preferred. Other materials which may be used
include ceramics, metal oxides, intermetallic materials, carbides, and
nitrides.
Metallic substrates are most preferred due to their excellent thermal
conductivity, allowing effective backside cooling of the catalyst.