Some of the information on this Web page has been provided by external sources. The Government of Canada is not responsible for the accuracy, reliability or currency of the information supplied by external sources. Users wishing to rely upon this information should consult directly with the source of the information. Content provided by external sources is not subject to official languages, privacy and accessibility requirements.
Any discrepancies in the text and image of the Claims and Abstract are due to differing posting times. Text of the Claims and Abstract are posted:
| (12) Patent: | (11) CA 2403741 |
|---|---|
| (54) English Title: | FUEL-CELL VEHICLE WITH ULTRAVIOLET AMMONIA CRACKER |
| (54) French Title: | VEHICULE A PILES A COMBUSTIBLE MUNI D'UNE UNITE DE CRAQUAGE D'AMMONIAC PAR RAYONNEMENT ULTRAVIOLET |
| Status: | Deemed expired |
| (51) International Patent Classification (IPC): |
|
|---|---|
| (72) Inventors : |
|
| (73) Owners : |
|
| (71) Applicants : |
|
| (74) Agent: | |
| (74) Associate agent: | |
| (45) Issued: | 2011-11-22 |
| (22) Filed Date: | 2002-09-27 |
| (41) Open to Public Inspection: | 2004-03-27 |
| Examination requested: | 2007-05-30 |
| Availability of licence: | N/A |
| (25) Language of filing: | English |
| Patent Cooperation Treaty (PCT): | No |
|---|
| (30) Application Priority Data: | None |
|---|
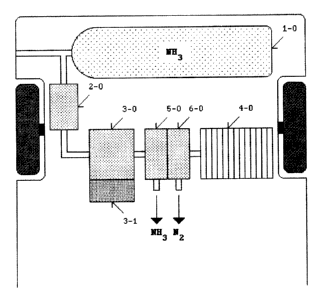
~ A fuel-cell vehicle comprises an ammonia tank, a gas regulator, an
ultraviolet ammonia cracker, and a fuel-cell stack. The ammonia tank
stores anhydrous ammonia fuel in liquid form under pressure. The gas
regulator vapourises liquid ammonia in the ammonia tank into gaseous
ammonia (NH3). The ultraviolet ammonia cracker produces gaseous hydrogen
(H2). The ultraviolet ammonia cracker comprises an ultraviolet light
source for generating electromagnetic radiation in the ultraviolet (UV)
region of the spectrum. The fuel-cell stack generates electrical power
from an electrochemical reaction between gaseous hydrogen (H2) and
gaseous oxygen (O2) in order to drive a plurality of electric motors for
vehicle propulsion. The ultraviolet light source is capable of
dissociating gaseous ammonia (NH3) into gaseous nitrogen (N2) and gaseous
hydrogen (H2) according to formula: 2 NH3 -> N2 + 3 H2. This invention
relates to fuel-cell vehicles, and the principal use of the invention is
for ground transportation.
Un dispositif de pile à combustible comporte un réservoir d'ammoniac, un régulateur de gaz, un dispositif de craquage de l'ammoniac aux ultraviolets ainsi qu'un assemblage de piles à combustible. Le réservoir d'ammoniac renferme un carburant d'ammoniac anhydre sous forme liquide sous pression. Le régulateur de gaz vaporise l'ammoniac liquide présent dans le réservoir en ammoniac gazeux (NH3). Le dispositif de craquage de l'ammoniac aux ultraviolets produit de l'hydrogène gazeux (H2). Ce dispositif de craquage comprend une source de rayonnement ultraviolet permettant de générer un rayonnement électromagnétique dans la région du spectre correspondant au rayonnement ultraviolet (UV). L'assemblage de piles à combustible génère de l'énergie électrique par une réaction électrochimique entre l'hydrogène gazeux (H2) et l'oxygène gazeux (O2); cette énergie permet de faire fonctionner divers moteurs pour la propulsion des véhicules. La source de rayonnement ultraviolet est capable de dissocier l'ammoniac gazeux (NH3) en azote gazeux (N2) et en hydrogène gazeux (H2) selon l'équation suivante : 2 NH3 -> N2 + 3 H2. L'invention a trait à des dispositifs de pile à combustible et sa principale application est le transport terrestre.
Note: Claims are shown in the official language in which they were submitted.
Note: Descriptions are shown in the official language in which they were submitted.
For a clearer understanding of the status of the application/patent presented on this page, the site Disclaimer , as well as the definitions for Patent , Administrative Status , Maintenance Fee and Payment History should be consulted.
| Title | Date |
|---|---|
| Forecasted Issue Date | 2011-11-22 |
| (22) Filed | 2002-09-27 |
| (41) Open to Public Inspection | 2004-03-27 |
| Examination Requested | 2007-05-30 |
| (45) Issued | 2011-11-22 |
| Deemed Expired | 2013-09-27 |
There is no abandonment history.
| Fee Type | Anniversary Year | Due Date | Amount Paid | Paid Date |
|---|---|---|---|---|
| Application Fee | $150.00 | 2002-09-27 | ||
| Maintenance Fee - Application - New Act | 2 | 2004-09-27 | $50.00 | 2003-12-23 |
| Maintenance Fee - Application - New Act | 3 | 2005-09-27 | $50.00 | 2003-12-23 |
| Maintenance Fee - Application - New Act | 4 | 2006-09-27 | $50.00 | 2005-12-19 |
| Maintenance Fee - Application - New Act | 5 | 2007-09-27 | $100.00 | 2007-01-10 |
| Request for Examination | $400.00 | 2007-05-30 | ||
| Maintenance Fee - Application - New Act | 6 | 2008-09-29 | $100.00 | 2008-01-11 |
| Maintenance Fee - Application - New Act | 7 | 2009-09-28 | $100.00 | 2009-01-14 |
| Maintenance Fee - Application - New Act | 8 | 2010-09-27 | $100.00 | 2010-01-01 |
| Maintenance Fee - Application - New Act | 9 | 2011-09-27 | $100.00 | 2011-01-02 |
| Final Fee | $150.00 | 2011-09-08 |
Note: Records showing the ownership history in alphabetical order.
| Current Owners on Record |
|---|
| SUNATORI, GO SIMON |
| Past Owners on Record |
|---|
| None |