Note: Descriptions are shown in the official language in which they were submitted.
CA 02452145 2003-12-05
METHOD FOR PRODUCING PULP AND LIGNIN
Technical Field
This invention pertains to a method for producing pulp (comprising cellulose)
and
lignin from lignocellulosic material such as wood chips.
Background
Wood is a composite material comprising cellulose, lignin and hemicellulose.
Cellulose is the strong, fibrous component which consists of long chains of
glucose
molecules and which is used to make paper. Lignin is a phenolic polymeric
matrix
which holds the cellulose fibres together. Hemicellulose is the component com-
prised of short, branched chains of glucose and other like molecules.
In general terms, pulping processes involve converting wood chips (or other
suitable vegetative material) into a fibrous form in order to produce pulp.
Pulp
refers to cellulose fibres or material containing cellulose fibres which may
be used
in the production of paper or paper containing products.
The two main types of pulping processes in use today are mechanical pulping
and
chemical pulping though there are other pulping processes, such as chemi-
thermal
mechanical pulping, which are a combination thereof.
Mechanical pulping involves the physical separation of individual fibres of
the wood
by forcing debarked logs and hot water between large rotating steel discs with
teeth
that tear the wood apart, or alternatively, by pressing logs against
grindstones.
Generally, pulps produced by mechanical pulping are of lower quality than
pulps
produced according to chemical pulping, and thus are used to produce newsprint
and cardboard type products.
On the other hand, chemical pulping involves subjecting wood to heat and chemi-
cals in order to dissolve the lignin and hemicellulose binding materials,
thereby
separating the cellulosic fibres. Kraft pulping is an example of a chemical
pulping
process, and involves involves cooking wood chips in a pressurized vessel
known as
a digester in the presence of hot caustic soda (NaOH) and sodium sulfide
(Na2S).
Kraft pulping is a variation of the soda process where only NaOH is used to
break
down and remove the lignin. Relative to the soda process, the addition of the
CA 02452145 2003-12-05
-2-
sodium sulfide in the Kraft process assisted in dissolving the lignin with
less
damage to cellulosic fibres.
In Kraft pulping, the digestion process dissolves the lignin that "glues" the
cellu-
losic fibers to each other in the wood. The resultant solution containing the
dissolved ligneous material is referred to as a "black liquor" . After
digestion, the
cellulose-containing pulp is separated from the black liquor and washed. At
this
stage, the resultant colored slurry of cellulose fibres is referred to as
"brownstock" .
The color of the slurry is the result of some residual lignin remaining within
the
slurry with the cellulosic fibres. If desired, a further step of bleaching the
slurry
can be carried out to remove some additional components of the lignin, thereby
brightening the pulp. Typically, the pulp is bleached in multiple stages with
various combinations of oxygen, hydrogen peroxide and sodium hydroxide.
Chelation stages can be utilized in the bleaching process to remove transition
metals
in the pulp which may otherwise interfere with peroxide bleaching.
One disadvantage with Kraft pulping is that it is carried out under extreme
condi-
tions which adversely affect yields. Kraft pulping typically involves
pressures of
approximately 120 p.s.i., temperatures of approximately 160 - 180 °C
and initial pH
values over 12 in the digestion stage. In part, these reaction conditions are
required
by the fact that reactions carried out in Kraft pulping are oxidation
reactions. These
harsh conditions result in a loss of some cellulose and the destruction of
much of
natural form lignin from the wood chips.
Lignin typically is not recovered as an end-product in typical Kraft pulping
pro-
cesses. Instead, in Kraft pulping processes the black liquor is typically
condensed
in recovery boilers in the process of recovering chemicals such as sodium
hydroxide
for reuse. Solids such as lignin are burned as fuel to run the pulp mill
utilizing the
Kraft process. It would be advantageous to recover as much lignin as possible
in a
pulping process since lignin is a valuable component in its own right, having
a wide
range of industrial applications such in the manufacture of fertilizers,
asphalt
emulsifiers, soil stabilizers, wood products (such as plywood and particle
board),
oil well drilling fluids and dispersing agents for preparing concrete.
Summary of Invention
CA 02452145 2003-12-05
-3-
In one aspect the invention provides for methods for producing pulp and lignin
from
lignocellulosic material, the pulp comprising cellulose. The methods comprise:
(a)
contacting the lignocellulosic material with an aqueous acid solution to
impregnate
the lignocellulosic material, the aqueous acid solution comprising from about
10%
to about 40 % by weight of the acid; (b) heating the lignocellulosic material
in two
stages, the first heating stage being carried out for a period of time which
is
sufficient to depolymerize lignin within the lignocellolosic material without
substan-
tially degrading the cellulose or lignin in the ligncellulosic material, the
second
heating stage being carried out at or above the boiling point of the acid to
distill off
the acid; (c) contacting the lignocellulosic material with an aqueous alkaline
solution
under heat to solubilize lignin in the alkaline solution, leaving a black
liquor; (d)
removing the pulp from the black liquor; (e) adding sufficient acid to the
black
liquor to precipitate the lignin; and (fj removing the lignin from the liquor.
The acid in step (a) may be nitric acid. The aqueous acid solution in step (a)
may
comprise, without limitation, from about 10 % to about 30 % , from about 15 %
to
about 30 % , from about 20 % to about 25 % , or from about 10 % to about 15 %
, by
weight of the acid.
The lignocellulosic material may be contacted with the aqueous acid solution
in step
(a) for at least 30 minutes, including without limitation, from about 12 hours
to
about 24 hours. The lignocellulosic material may be contacted with the aqueous
acid solution in step (a) under heat. The lignocellulosic material may be
contacted
with the aqueous nitric acid solution in step (a) at a temperature from about
50 °C to
below the boiling point of the nitric acid.
The temperature during the second heating stage of step (b) may be, without
limitation, from about 73 °C to below 100 °C or from about 90
°C to about 95 °C.
The temperature during the first heating stage of step (h) may be, without
limita-
tion, up to about 75 °C, from about 50 °C to about 75 °C,
from about 60 °C to about
70 °C, from about 50 °C to about 60 °C, or from about 70
°C to about 75 °C. The
first heating stage in step (b) may be carried out for at least 15 minutes.
The aqueous alkaline solution may comprise sodium hydroxide or potassium
hydroxide or a combination of sodium hydroxide and potassium hydroxide. The
aqueous alkaline solution may comprise an amount of alkali solute which is at
least
the normal equivalent of the nitric acid in the aqueous acid solution in step
(a). The
CA 02452145 2003-12-05
-4-
aqueous alkaline solution may comprise an amount of alkali solute which is at
least
the molar equivalent of the nitric acid in the aqueous acid solution in step
(a).
The acid used in step (e) may be sulfuric acid. The amount of acid added in
step (e)
may be at least the molar amount of the alkali in the aqueous alkaline
solution in
step (c). The amount of acid added in step (e) may be at least the normal
amount of
the alkali in the aqueous alkaline solution in step (c).
The black liquor may cooled from the temperature in step (c) before the acid
is
added in step (e). The temperature of the black liquor when the acid is added
in
step (e) may be, without limitation, up to about 75 °C, from about 5
°C to about 75
°C, from about 5 °C to about 50 °C, from about 25
°C to about 50 °C, or from about
50 °C to about 75 °C.
Any aqueous acid solution not absorbed by the lignocellulosic material in step
(a)
may be removed prior to heating the lignocellulosic material in step (b). The
methods may comprise collecting any aqueous acid solution which is removed
prior
to step (b) and recycling the collected aqueous acid solution for use in step
(a), and
comprising collecting any acid which is distilled off in step (b) and
recycling the
collected acid for use in step (a).
The starting moisture content of the lignocellulosic material may be from
about
% to about 55 % by weight of water. The method may comprise contacting the
lignocellulosic material with water before step (a) to increase the moisture
content
25 in the lignocellulosic material. The starting lignocellulosic material may
comprise,
without limitation, wood chips, wood shavings, sawdust, pieces of rye, wheat,
hemp and combinations of two or more of the foregoing. The starting
lignocellulosic material may comprise undigested lignocellulosic material
previously
subjected to the method.
An amber liquor may be left following the removal of the lignin, and the
method
may comprise processing the amber liquor after the lignin has been removed,
for
example, to produce unicellular proteins or alcohols or both. Any water which
is
produced as a reaction byproduct in one or more of the steps in the method may
be
collected and recycled for use in the method.
CA 02452145 2003-12-05
-5-
Steps (a) and (c) and the first heating stage in step (b) may each carried out
at a
temperature from about 50 °C to about 75 °C. One or more of the
heating of the
impregnated lignocellulosic material in step (b), the contacting the
lignocellulosic
material with the aqueous alkaline solution in step (c) and the adding the
acid in step
(e) may be carried out with agitation. The methods may be carried out at atmo-
spheric pressure.
The pulp removed in step (d) may be washed, pressed, bleached and dried.
Similarly, the lignin removed in step (f) may be dried. Any residual liquor
that is
removed from the pulp by pressing may be collected and added to the black
liquor
prior to adding the acid in step (e). Similarly, any residual liquor which is
removed
from the lignin during drying may be collected and added to the liquor after
step
(f), and wherein the liquor is processed after the lignin has been
precipitated and
removed.
Enough acid may be added to the solution in step (e) to lower the pH of the
solution
to an acidic pH.
Without limitation, step (c) may be carried out at a temperature up to about
75°C,
from about 5 °C to about 75 °C, from about SO °C to about
75 °C, from about 20 °C
to about 50 °C, from about 30 °C to about 40 °C, or from
about 40 °C to about 50
°C.
Step (a) may comprise immersing the lignocellulosic material in the aqueous
acid
solution in step (a), or spraying the lignocellulosic material with aqueous
nitric acid
solution.
In another aspect the invention provides for methods for producing pulp and
lignin
from lignocellulosic material, the pulp comprising cellulose, the methods
comprise:
(a) contacting the lignocellulosic material with an aqueous nitric acid
solution to
impregnate the lignocellulosic material, the aqueous nitric acid solution
comprising
from about 10 % to about 40 % by weight of the nitric acid; (b) heating the
impreg-
nated lignocellulosic material in two stages, the first heating stage being
carried out
a temperature from about 50 °C to about 75 °C for a period of
time which is
sufficient to depolymerize lignin within the lignocellolosic material without
substan-
tially degrading the cellulose or lignin in the ligncellulosic material, the
second
heating stage being carried out at or above the boiling point of the nitric
acid to
CA 02452145 2003-12-05
-6-
distill off the nitric acid; (c) contacting the lignocellulosic material with
an aqueous
alkaline solution at a temperature from about 50 °C to about 75
°C to solubilize
lignin in the alkaline solution, leaving a black liquor, the aqueous alkaline
solution
comprising an amount of alkali which is at least the normal amount of the
nitric acid
in the aqueous acid solution in step (a); (d) removing the pulp from the black
liquor; (e) cooling the black liquor and then adding an acid to the black
liquor to
acidify the solution to precipitate the lignin; (f) removing the lignin,
leaving an
amber liquor; and (g) processing the amber liquor to produce unicellular
proteins or
alcohols or both, wherein any aqueous nitric acid not absorbed by the
lignocellulosic material in step (a) is removed and collected following step
(a) prior
to heating the lignocellulosic material in step (b) and then recycled for use
in step
(a), and wherein any nitric acid which is distilled off is collected prior to
contacting
the lignocellulosic material with the alkaline solution in step (c) and then
recylcled
for use in step (a), and wherein the heating of the lignocellulosic material
in step
(b), the contacting the lignocellulosic material with the aqueous alkaline
solution in
step (c) and the addition of the acid in step (e) are each carried out with
agitation.
In yet another aspect the invention provides for methods for producing pulp
and
lignin comprising the steps of contacting lignocellulosic material with an
acid and,
after removing any acid not absorbed by the lignocellulosic material, heating
the
lignocellulosic material at a temperature up to about 75 °C to effect
the acid-
catalyzed hydrolytic depolymerization of the lignin in the lignocellulosic
material
without substantially degrading the cellulose or lignin in the lignocellulosic
mate-
rial, the acid-contacting and heating steps being carried out before the
lignocellulosic material is digested in an alkaline liquor, the pulp being
removed
following the digestion of the lignocellulosic material in the alkaline
liquor, the
lignin being removed after being precipitated out with the addition of an acid
to the
black liquor produced following the digestion of the lignocellulosic material
in the
alkaline liquor, wherein the pulp comprises cellulose. The acid-contacting
step may
comprise immersing the lignocellulosic material in an aqueous solution of the
acid,
and wherein the acid is nitric acid and the aqueous solution comprises from
about
10 % to about 40 %o by weight of nitric acid. The lignocellulosic material may
be
heated, after depolymerizing the lignin but before digesting the
lignocellulosic
material, at a temperature above the boiling point of the acid in order to
distill off
the acid.
CA 02452145 2003-12-05
In yet another aspect the invention provides methods for treating
lignocellulosic
material comprising: (a) contacting the lignocellulosic material with an
aqueous acid
solution to impregnate the lignocellulosic material, the aqueous acid solution
comprising from about 10% to about 40% by weight of the acid; (b) heating the
lignocellulosic material in two stages, the first heating stage being carried
out for a
period of time which is sufficient to depolymerize lignin within the
lignocellolosic
material without substantially degrading the cellulose or lignin in the
ligncellulosic
material, the second heating stage being carried out at or above the boiling
point of
the acid to distill off the acid, wherein any aqueous acid solution not
absorbed by
the lignocellulosic material in step (a) is removed prior to heating the
lignocellulosic
material in step (b); (c) contacting the lignocellulosic material with an
aqueous
alkaline solution under heat to solubilize lignin in the alkaline solution,
leaving a
black liquor; and (d) removing the pulp from the black liquor, the pulp
comprising
cellulose.
In yet another aspect the invention provides methods for producing pulp and
lignin
from lignocellulosic material, the pulp comprising cellulose, the methods
compris-
ing: (a) contacting the lignocellulosic material with an aqueous nitric acid
solution
to impregnate the lignocellulosic material, the aqueous acid solution
comprising
from about 10 % to about 40 % by weight of the nitric acid; (b) heating the
lignocellulosic material in two stages, the first heating stage being carried
out for a
period of time which is sufficient to depolymerize lignin within the
lignocellolosic
material without substantially degrading the cellulose or lignin in the
ligncellulosic
material, the second heating stage being carried out at or above the boiling
point of
the acid to distill off the acid, wherein any aqueous acid solution not
absorbed by
the lignocellulosic material in step (a) is removed prior to heating the
lignocellulosic
material in step (b); (c) contacting the lignocellulosic material with an
aqueous
alkaline solution under heat to solubilize lignin in the alkaline solution,
leaving a
black liquor; (d) removing the pulp from the black liquor; (e) adding
sufficient acid
to the black liquor to precipitate the lignin; and (f) removing the lignin
from the
liquor.
Further aspects of the invention and features of specific embodiments of the
invention are described below.
Brief Description of Drawings
CA 02452145 2003-12-05
Figure 1 is an illustration of the mechanism of acid-catalyzed hydrolysis of
an ester;
and
Figure 2 is a schematic illustration of a method according to the present
invention.
S
Description
Throughout the following description specific details are set forth in order
to
provide a more thorough understanding of the invention. However, the invention
may be practiced without these particulars. In other instances, well known
elements
have not been shown or described in detail to avoid unnecessarily obscuring
the
present invention. Accordingly, the specification and drawings are to be
regarded
in an illustrative, rather than a restrictive, sense.
Most existing pulping processes involve high pressure and/or high temperature
conditions or concentrated chemicals. Not only do these factors increase
costs, but
they also reduce the yield and purity of cellulose-containing pulp and lignin
end-
products since the cellulose and lignin can be degraded or destroyed during
the
pulping processes. The present invention addresses these deficiencies by
utilizing
the acid catalyzed hydrolysis of lignocellulosic material in methods for
producing
pulp and lignin from lignocellulosic material. The hydrolysis reaction allows
the
controlled de-polymerization of lignin within the starting lignocellulosic
material,
utilizes much lower concentrations of pulping chemicals and lower temperatures
than existing Kraft pulping processes and may be carried out at atmospheric
pressure. These modest reaction conditions not only result in reduced costs
relative
to existing Kraft pulping processes, but also increase the yield and purity of
the
cellulose-containing pulp and lignin since there is less degradation and and
destruc-
tion of these components during the pulping process relative to existing Kraft
pulping processes.
The invention includes methods involving contacting lignocellulosic material
with
an acid, preferably nitric acid, in order to impregnate the lignocellulosic
material
and then heating the lignocellulosic material to de-polymerize the lignin
within the
lignocellulosic material prior to digesting the lignocellulosic material in an
alkaline
solution to solubilize lignin in the alkaline solution, leaving a black liquor
(compris-
ing the solubilized lignin) and pulp. The pulp is then removed and processed
(e.g.
pressing, washing, bleaching, drying). The solubilized lignin is precipitated
out of
CA 02452145 2003-12-05
-9-
the black liquor with the addition of an acid and then removed for processing.
The
remaining solution can be further processed to produce other useful by-
products
such as unicellular proteins, alcohols or both.
As used herein, "lignocellulosic material" refers to any material which
contains
cellulose and lignin and, without limitation, includes pieces or particles
(for
example wood chips, wood shavings, sawdust) from any type of tree (for example
pine, oak, maple, fir, spruce) and other vegetative material (for example rye,
wheat
hemp) and combinations thereof.
As used herein, "impregnation" refers to the absorption of an impregnating
material
by lignocellulosic material. For example, impregnating wood chips can be accom-
plished by immersing or soaking the wood chips in a sufficient volume of the
impregnating material (e.g. nitric acid solution) to saturate all or part of
the wood
chips. Other methods such as spraying could also be used to accomplish the
impregnation.
The separation of lignin and cellulose in their natural forms has proven
difficult in
practice. Many existing pulping processes are inefficient, with valuable
cellulose
and lignin being degraded, modified or destroyed during pulping, or less than
optimal separation between cellulose and lignin being achieved in the pulping
process. The difficulty in separating lignin and cellulose arises from the
inherent
instability and fragile nature of pure lignin. Lignins are formed by removal
of water
from sugars to create aromatic structures and these reactions are
irreversible. There
are many possible monomers of lignin, and the types and proportions depend on
the
source in nature. Some typical monomers of lignin are shown below:
CA 02452145 2003-12-05
-10-
CH3 H~=p CH~H
CHI CH C=~
CHI CH HC~H
~,''.
CH ~ CH ~ Hp,
H H3 ~H~
(I) (u)
The hydroxyl (-OH) groups, either the hydroxyl groups on the chains or the
hydroxyl groups on the aromatic rings, can react with each other or with the
aldehyde or ketone groups. A ether linkage is formed when a hydroxyl group
reacts
with another hydroxyl group, a hemiacetal is formed when a hydroxyl group
reacts
with an aldehyde and a ketal is formed when a hydroxyl group reacts with a
ketone.
An early stage in the condensation of various monomers to form lignin is shown
below, wherein there are several groups shown that can react further:
CA 02452145 2003-12-05
-1~_-
~IV~
Some monomers will simply extend the polymer while others will establish
cross-linking. The shaded monomer has three of its functional groups linked to
other monomers, so it is starting a branch or cross-link. Lignin molecules are
three-
dimensional and heavily cross-linked. A typical lignin molecule can have a
molecu-
lar weight of about 15,000 amu. Lignin molecules are susceptible to harsh
chemical
concentrations and temperature exposure. A lignin polymeric matrix may be
broken down at temperatures as low as 100°C. Lignin is unstable, light
sensitive,
and breaks down into acid compound as it ages, and its presence in paper items
contributes to their degradation over time.
As mentioned above, the present invention utilizes the acid-catalyzed
hyrolysis of
lignocellulosic material. The lignin within lignocellulosie material includes
ester
functional groups. Figure 1 illustrates the mechanism of the acid-catalyzed
hydroly-
CA 02452145 2003-12-05
-12-
10
sis of an ester. The rate of ester hydrolysis is very slow because their
leaving
groups are basic. The rate can be increased by the presence of a catalyst,
such as an
acid. In such an acid catalyzed reaction alt organic reactants, intermediates,
and
products are positively charged. The following steps take place within the
hydroly-
sis of the ester:
(A) An acid/base reaction is illustrated. Since there is only a weak
nucleophile
and a poor electrophile the ester needs to be activated. The acid protonates
the ester carbonyl, making it more electrophilic.
(B) The oxygen atom in the water molecule functions as the nucleophile, attack-
ing the electrophilic carbon atom in the C = O. Electrons move towards the
oxonium ion, thereby creating a tetrahedral intermediate.
(C) Another acid/base reaction is illustrated. The oxygen atom in the water
molecule is deprotonated to neutralize the charge.
(D) Another acid/base reaction is illustrated. The -OCH3 must leave, but first
must be converted into a more stable group by being protonated.
(E) The electrons from the adjacent oxygen atom assist the formation of the
leaving group, a neutral methanol molecule CH30H.
(F) Another acid/base reaction. The oxonium ion is deprotonated, which exposes
the carbonyl C=O carboxylic acid product and regenerates the acid catalyst.
The carboxylic acid product obtained in (F) is a de-polymerized form of
lignin.
Because water (H20) and methanol (CH30H) have approximately the same basicity,
it will be equally easy for the tetrahedral intermediates to collapse to
reform the
ester as it would be for the formation of the carboxylic acid. Hence, at
equilibrium
both the ester and the carboxylic acid will be obtained. However, an excess of
water will shift the equilibrium towards the formation of the carboxylic acid
product, de-polymerized lignin. Thus, methods of the present invention will
render
higher yields with the addition of water and, for this reason, it is possible
to use
lignocellulosic material which has a relatively high moisture content (e.g.
"green"
wood chips) or which has been previously contacted with water in order to
increase
CA 02452145 2003-12-05
-13-
the moisture content prior to impregnating the lignocellulosic material with
the acid.
The ability to use lignocellulosic materials with a relatively high moisture
content in
the present invention is an advantage compared to existing Kraft type pulping
processes. In many existing processes it is necessary to dry wood chips prior
to
pulping in order to remove or reduce moisture to meet stringent requirements.
In
fact, chips are preferred in many existing processes on a BDU (bone dry unit)
basis.
Requiring a drying step adds expense to such processes. The present invention
overcomes this disadvantage since "wet" starting materials can be used, thus
removing the need to dry the wood chips or other starting materials. Moreover,
as
explained above in relation to Figure 1, since excess water drives the
depolymerization reaction to favor the formation of a carboxylic acid instead
of
collapsing to reform an ester, additional moisture in the chips improves
yields in
methods according to this invention.
Following depolymerization of lignin, the lignocellulosic material is digested
in the
presence of an alkali such as sodium hydroxide (NaOH) and/or potassium
hydroxide
(KOH). The positive cations (K+ or Na+ or another suitable ion) then attacks
the
exposed -OH on the lignin product (carboxylic acid) bumping out the hydrogen
canon (H+). The hydrogen cation then bonds with the negatively charged
hydroxyl
ion to form water. The lignin is then water-soluble and drops into solution,
thereby
separating the dissolved lignin from the cellulose-containing pulp. Due to
more
modest temperature, pressure and reactant concentrations involved, the lignin
and
cellulose produced are subject to less degradation than products obtained
through a
Kraft type process, thereby providing increased yield and purity.
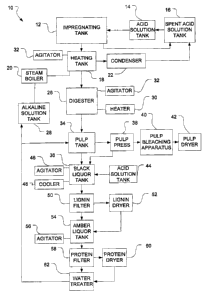
Figure 2 is a schematic illustration of a method 10 according to the present
inven-
tion. Lignocellulosic material, such as wood chips (not shown), is used as the
starting raw material in an impregnating tank 12. The wood chips are prepared
by
chipping wood into a convenient size, for example, chips of roughly 0.5 - 2.0
inches in diameter. This can be done with a standard mill. Other sizes and
shapes
of lignocellulosic material can be used. Given the hydrolysis involved in the
method, preferably "green" wood chips are used, or in other words, chips
having a
starting moisture content of approximately 30 - 55 % , or even higher, by
weight of
water. A pre-treatment stage could be utilized wherein the wood chips were
immersed or otherwise contacted with water prior to being placed in the
impregnat-
ing tank in order to increase the moisture content within the chips up to or
beyond
CA 02452145 2003-12-05
-14-
30 - 55 % by weight of water, though this step is not necessary to produce
lignin,
pulp and other ancillary products according to this invention. Impregnating
bone
dry chips with an acid solution could be done, but this will result in higher
con-
sumption rates of the acid impregnant, thereby increasing costs.
Wood chips are loaded into impregnating tank 12 where they are contacted with
an
acid in order to impregnate the wood chips with the acid. The acid is
preferably
provided within an aqueous solution, and in such a case, the acid impregnates
the
wood chips as part of the solution. That is, the wood chips absorb both acid
and
water. According to one aspect of the invention, the aqueous solution
comprises
from about I O % to about 40 % by weight of the acid. The invention also
includes
utilizing other, narrower acid concentration ranges within this range. For
example,
the aqueous acid solution may comprise from about 15 % to about 30 % , from
about
10 % to about 25 % , from about 10 % to about 15 % , or from about 20 % to
about
IS 25 % , by weight of the acid.
The aqueous acid solution is provided to impregnating tank 12 from acid
solution
tank 14 in a sufficient volume to immerse the wood chips. The acid used is
preferably nitric acid, but those skilled in the art will appreciate that
other acids
could instead be used within the scope of the present invention. Nitric acid
is used
herein for illustration purposes.
The wood chips are impregnated by allowing them to soak in the aqueous nitric
acid
solution in the impregnating tank 12. The impregnation time may last for as
little
as 30 minutes or less and may extend for any length of time before the
degradation
of the wood chips begins to occur. For example, impregnation may last from
about
2 hours and 45 minutes to about 48 hours. It is contemplated that the
impregnation
time may fall within a narrower time ranges such as, for example, from about
12 to
about 24 hours and ranges within this range.
Heating the chips during the impregnation step may decrease the impregnation
time.
For example, impregnation of the wood chips could occur from about 30 minutes
or
less to about 13 hours with the addition of heat, or up to 48 hours or longer,
without the addition of heat. Heating during impregnation can be achieved by
any
number of well known techniques that could be used to heat the wood chips
within
impregnating tank 12. For example, the exterior of impregnating tank could be
heated with steam to heat the tank 12. After sufficient time has passed to
complete
CA 02452145 2003-12-05
-15-
impregnation, any excess nitric acid solution is removed from the wood chips,
by
drainage for example, and passed to spent solution tank 16. Any collected
excess
nitric acid solution may then be recycled for use in the impregnating tank 12.
Preferably any excess nitric acid solution is filtered and purified using
conventional
techniques before being reconstituted back up to strength. Filtration and/or
purification could be accomplished by passing any spent nitric acid solution
from
spent acid solution tank 16 to a conventional filtration and/or purification
apparatus
(not shown) before the solution is passed to acid solution tank 14. However,
it may
not be necessary to filter and purify the excess nitric acid solution if there
are not
significant amounts of contaminants present.
The impregnated wood chips are then passed to a heating tank 18. This transfer
can
be accomplished by gravity feed, if impregnating tank I2 is located above the
heating tank 18, or by a suitable transfer mechanism if not. The impregnated
chips
are heated within heating tank 18. The exterior of heating tank 18 is heated
with
steam produced by steam boiler 20, thereby heating the chips within heating
tank
18. Many other known apparatus or techniques could alternatively be used to
heat
heating tank 18. Heating of the chips in heating tank 18 may be carried out in
two
heating stages; the first heating stage to depolymerize the lignin within the
wood
chips without substantially degrading the cellulose or lignin, and the second
heating
stage to distill off the acid following depolymerization of the lignin. The
distilled
off nitric acid is recycled for use in the impregnation stage. In particular,
the
distilled off nitric acid is collected and passed to a condenser 22 and then
to spent
solution tank 16 to be reconstituted to a desired strength and then passed
back to
nitric acid tank 14 for eventual use in impregnating tank 12.
The nitric acid attacks the cross-links or ester groups of the lignin
polymeric matrix
during the first heating stage. The first heating stage is carried out for a
period of
time which is suff cient to depolymerize the lignin within the wood chips
without
substantially degrading the cellulose or lignin. When nitric acid is used as
the
impregnating acid, preferably the first heating stage is carried out at a
temperature
up to about 75 °C, but above the freezing point of the aqueous acid
solution
It is within the scope of the invention for the first heating stage to be
carried out
within a narrower temperature range, for example from about 50 °C to
about 75
°C, from about 50 °C to about 60 °C, from about 70
°C to about 75 °C, or from
about 60 °C to about 70 °C, when nitric acid is used as the
impregnating acid. It is
desirable to maintain these more modest temperatures (relative to Kraft
pulping
CA 02452145 2003-12-05
-16-
temperatures) in order to prevent the unwanted degradation and destruction of
the
lignin and cellulose within the wood chips, thus providing improved product
yield
and purity.
Preferably heating tank 18 is fitted with an agitator 24 to provide agitation
during
the heating of chips within heating tank 18. Aggressive agitation by agitator
24
helps to both bring about a homogeneous temperature within heating tank 18 and
to
begin breaking apart the fibers in the wood chips.
During the second heating stage, the temperature is then brought above the
boiling
point of the acid for a sufficient time (for example, 30 minutes) to distill
off the
nitric acid. It is important to note that the temperature of the boiling point
of the
acid in this stage may depart slightly from the temperature that one would
expect
the acid to boil. This discrepancy results from various factors including the
interaction of the acid with the wood, the moisture content within the wood,
and,
for nitric acid, the formation of azeotropes with water. For example, whereas
some
scientific literature establishes the boiling point of nitric acid to be
83 °C, the inventor has observed boiling for nitric acid with wood
chips as low as
73 °C. Thus, it is preferred for the temperature of the second heating
stage within
heating tank 18 be at least about 73 °C where nitric acid is utilized.
Similarly, it is
also preferred to maintain the temperature during the second heating stage
below the
boiling point of water, 100 °C, to avoid the accidental distilling off
of wood chip
moisture, which would initiate the burning of the wood chips. Thus, according
to
one aspect of the invention, the second heating stage could be carried out
from
about 73 °C to below 100 °C. Again, it is within the scope of
the invention to
utilize narrower ranges, for example from about 90 °C to about 95
°C, within this
larger range.
The amount of time for the first and second heating stages will vary depending
upon
a number of factors, including the particular temperatures involved. For
example,
the first heating stage may be carried out for as little as 15 minutes, and
could be
carried out for a longer period of time.
Following the heating in the heating tank 18, the wood chips are then
transferred to
a digester 26. There the wood chips are digested by being contacted with a
caustic
solution, or in other words, an adueous alkaline solution wherein the solute
is an
alkali such as sodium hydroxide, potassium hydroxide, or a combination of
these.
CA 02452145 2003-12-05
-17-
Those skilled in the art will appreciate that other bases having similar
chemical
properties as these bases could be used. The wood chips are contacted with the
aqueous alkaline solution under heat to solubilize the lignin in the solution.
The
resultant solution is darker in color due to the solubilized lignin and is
thus referred
to as black liquor. This digestion step thereby separates the lignin and the
black
liquor from the pulp. The alkaline solution is provided to digester 26 from
caustic
solution tank 28. The concentration of the alkaline solution is such to just
make the
lignin water soluble. This is accomplished by the positive cations (K~ or Na+
or
another suitable ion) attaching to the exposed cleavage sites on the lignin
monomers
and the lignin then dissolving into the water.
The temperature during the digestion stage is preferably up to about 7S
°C, but
above the freezing point of the aqueous alkaline solution. It is however
within the
scope of the invention to increase the temperature above 7S °C up to
the boiling
1S point of the alkaline solution. For example, where the alkaline solution
comprises
an aqueous sodium hydroxide solution, it is possible for the temperature
during the
digestion step to be from about 7S °C to about 9S °C. It is also
within the scope of
the invention for the digestion stage to be carried out within a narrower
temperature
range, for example, from about S °C to about 7S °C, from about
SO °C to about 7S
°C, from about 20 °C to about SO °C, from about 30
°C to about 40 °C, or from
about 40 °C to about 50 °C. Heat is provided to digester 26 by
heater 30, which
may be any conventional apparatus or arrangement for providing heat to the
digester 26.
2S Digester 26 may be fitted with an agitator 32 to provide agitation during
the
digestion of the chips. Aagitation helps to bring about a homogeneous
temperature
within the digester 26 and to break up the wood chips. Agitation also
increases the
rate that the cation (e.g. K+, Na+, etc.) binds to the exposed lignin cleavage
sites
thus minimizing any possible caustic action on the cellulose.
For cost and yield reasons, it is desirable to use as little alkali as
possible within the
caustic solution in the digestion of the wood chips. In one aspect of the
invention,
the alkaline solution may include an amount of alkali which is at least the
normal
equivalent of the nitric acid in the solution used in the impregnation stage.
Adding
3S excess alkali will increase costs by requiring more acid to be added at a
later step.
Further, the alkali concentration in the aqueous alkaline solution should be
main-
CA 02452145 2003-12-05
-18-
tained below 17.5 % by weight of the solution since beta and gamma cellulose
dissolve at this level.
Following digestion, the resultant pulp (containing cellulose) and black
liquor
(containing solubilized lignin) is transferred to pulp tank 34 . The black
liquor is
removed, for example by drainage, and transferred to black liquor tank 36. The
cellulose-containing pulp is then processed according to the user's needs.
Typically, processing would involve washing, pressing, bleaching and drying
the
pulp following the separation from the black liquor. For example, in Figure 2,
the
pulp would be pressed in a pulp press 38 to remove any residual black liquor
(which would also be transferred to black liquor tank 36), and then bleached
and
dried with bleaching apparatus 40 and dryer 42. Bleaching apparatus 40 and
dryer
42 could be any conventional known apparatuses for these purposes.
The lignin is precipitated out of the black liquor in black liquor tank 36
after the
addition of an acid from acid solution tank 44. Preferably the acid added is a
mineral acid, such as sulfuric acid. The acid added to the black liquor strips
off
the cation from the caustic solute, thereby precipitating out the lignin and a
caustic
salt (e.g. where sulfuric acid is used, the salts NaZS04, KZS04, or other
similar salts
depending upon the alkali solute used in digestion, would result). Addition of
the
acid to the black liquor lowers the pH of the liquor until it is just slightly
acidic.
The acid may be added to the black liquor within an aqueous solution. For cost
and
yield reasons, it is advantageous if the acid solution is as dilute a solution
as
possible. Black liquor tank 36 may be fitted with an agitator 46 to provide
agitation
during the addition of the acid from tank 44. The contents of black liquor
tank 36
may be cooled, for example by cooler 48, in order to faciliate the
precipitation of
lignin. Cooler 48 can be any known apparatus for cooling purposes. The black
liquor may be cooled from the digestion temperature before the addition of the
acid.
Preferably the temperature is up to about 75 °C, but above the freezing
point of the
black liquor. Other narrower temperature ranges could be used at this step
such as
from about 5 °C to about 75 °C, 5 °C to about 75
°C, from about 50 °C to about 75
°C, or from about 25 °C to about SO °C.
The precipitated lignin can then be removed from the resultant amber liquor
(which
is lighter in color than the black liquor due to the removal of the lignin)
and
processed using any conventional technique. For example, the resultant amber
liquor and lignin slurry may be passed from black liquor tank 36 through a
lignin
CA 02452145 2003-12-05
-19-
filter 50, with the lignin then being transferred to a lignin dryer 52 and
dried at
temperatures that will not degrade the lignin. The resultant lignin contains
natural
form lignin.
The filtrate passing through lignin filter 50 is the amber liquor, which is
then
transferred to an amber liquor tank 54. Any residual amber liquor removed
during
the drying of lignin in lignin dryer 52 is also transferred to amber liquor
tank 54, as
illustrated in Figure 2. Amber liquor is an aqueous solution containing
starches,
sugars, and other minerals and compounds found in plants that are not
cellulose or
lignin. In amber liquor tank 54, the amber liquor can be processed to yield
various
other products of value including alcohols, unicellular proteins or both. For
example, the amber liquor could be used as a starting culture for various
bacteria to
produce various products, such as animal feeds and alcohols. Amber liquor tank
54
may be fitted with an agitator 56 to provide agitation if desired. Any
proteins
produced during the processing of the amber liquor may be removed by
filtration
(via protein filter 58) and dried (via protein dryer 60). Suitable apparatus
modifica-
tions and/or additions could be made to accommodate alcohol processing and/or
the
production of other desired end products. The final filtrate can be recovered
and
treated in water treater 62 to remove any contaminants, thereby allowing the
recycling of water within various steps in the method, for example in reuse in
washing in pulp tank 34, preparation of the alkaline solution in caustic
solution tank
28 and in steam boiler 20. Any recovered water could also be used in steam
boiler
20 or in the preparation of the aqueous acid solutions in acid solution tank
14
and/or acid solution tank 44.
Without limitation, the present invention provides the following benefits:
1) Wet starting materials can be used. Since water is essential to the
hydrolysis
of the lignocellulosic material, this removes the need to dry the starting
materials prior to the pulping process, thus resulting in lower costs relative
to certain existing pulping methods.
2) The method of the present invention does not require added pressure, but
may be carried out at atmospheric pressure. This reduces costs relative to
certain existing pulping methods, as does the fact that the method of the
present invention does not require the addition of heat at temperatures
approaching those used in typical Kraft type pulping processes.
CA 02452145 2003-12-05
-20-
3) Weak concentrations of strong acids and strong bases may be used, thereby
minimizing raw material costs and degradation of final products.
4) The acid catalyst can be recovered and recycled for reuse, allowing im-
proved cost efficiency. These factors also allow a closed system, minimize
pollution, and therefore environmental impact. Very little pollution is
caused by methods according to this invention.
5) Only small amounts of chemicals are needed to bring back to strength each
recovered chemical before being reused in the method. Moreover, unlike
recovery stages in certain Kraft type processes, external energy (and the
resultant expense) is not required during the recovery of chemicals in
methods according to the present invention.
6) Water used in the method may be recovered in saleable byproducts (such as
alcohols and animal feeds) treated and reused or vented as steam, with
suitable apparatus and process modifications. The vented steam could be
used in providing energy for the method, thereby eliminating even this small
loss of water.
7) The method is flexible in terms of starting materials. The ability to
process
a wide variety of lignocellulosic material without retooling any apparatus or
changing the methods involved gives flexibility in pulp production. Cur-
rently, mills are typically designed to produce specific pulp types and
utilize
specific wood species as raw materials. Furthermore, most Kraft mills
require chips meeting stringent quality specifications to remain economically
viable. The present method may utilize not only any number of differently
sized chips, but also sawdust, and also chips that would be considered
"green" and unusable by current pulp mill standards. The flexibility pro-
vided by methods of this invention eliminates or simplifies the need for chip
mills designed to produce chips of stringent standards, thereby offering the
potential for lower operating and capital costs.
8) The yield of alpha cellulose is high using the method, while the method
also
allows for a high yield of lignin, which is a valuable component itself.
CA 02452145 2003-12-05
-21 -
9) Aside from cellulose and lignin, other useful and potentially valuable by-
products may be recovered using methods of this invention. For example,
the amber liquor is suitable for fermentation of unicellular protein following
precipitation and removal of lignin. The protein can be used in animal feeds
or for research purposes.
10) Methods of the invention may be applied to different sized and configured
apparatuses, thereby improving the flexibility of use.
11) Mills utilizing a method of the present invention will be highly
efficient,
with lower operating costs than mills using typical Kraft pulping processes.
The following examples are presented by way of illustration and not by way of
limitation.
Example 1
This example comprises 19 trials which were run to illustrate the yields of
pulp and
lignin obtained using methods according to this invention, and also to
illustrate the
recovery of the acid used in the impregnation stage.
The starting lignocellulosic material in Trials 1 - 18 comprised 200 grams of
a
mixture of hardwood and softwood wood chips obtained from a sawmill and chip
mill operation in Kelowna, British Columbia, Canada. 600 grams of wood chips
were used as the starting material in Trial 19. All of the different species
of trees
that the chips originated from were not ascertained, but at least some of the
chips
came from Ponderosa Pine, Douglas Fir, Maple, Oak and Spruce.
The moisture content of the wood chips was between 35 - 50 % water by weight.
The moisture content was calculated by drying separate samples of the wood
chips
(which were not subjected to the steps of the method) and measuring the weight
difference in the wood chips following the drying step.
The acid used in the impregnating step was nitric acid (HN03). Nitric acid
solu-
dons were prepared by diluting an amount (chosen depending upon the strength
of
solution desired) of 70% (w/w) nitric acid with distilled water. In each
trial, 1 L
of an aqueous nitric acid solution was used in the impregnating step, with the
CA 02452145 2003-12-05
-22-
exception that Trials 17 and 18 each used 750 mL, and Trial 19 used 2250 mL. A
2500 mL beaker sealed with laboratory film was used as the impregnating tank.
Following the impregnation step, excess nitric acid solution was removed from
the
wood chips before they were heated in the heating step. The heating step was
carried out in a 2000 ml round bottom triple neck boiling flask, which was
fitted
with a distillation setup so that nitric acid distilled out in the heating
step would be
collected.
In order to calculate the efficiency of the method in recovering the nitric
acid,
samples from the excess acid solution collected following the impregnating
step and
from the distilled nitric acid which was collected during the heating step
were each
titrated using a 10 % (w/w) % solution that was prepared by dissolving 100 g
NaOH
in distilled water and topped up in a 1 L volumetric flask. Titration of the
excess
nitric acid solution collected from after the impregnation step permitted the
calcula-
tion of the number of moles of nitric acid recovered after the impregnating
step,
which in turn permitted the calculation of the number of moles of nitric acid
which
were absorbed by the wood chips, since the moles of nitric acid in the initial
1 L
solution was known. After titrating the nitric acid that was distilled out and
collected from the heating step, it was possible to calculate the total moles
of nitric
acid recovered in the method. By comparing this number to the amount of moles
in
a particular starting nitric acid solution allowed the calculation of the
percentage of
starting nitric acid which was recovered by the method. Also, it was found
that red
fuming nitric acid (RFNA) and nitrogen dioxide (NOZ) formed during the heating
step and these vapors were lost to the atmosphere. These vapors could have
been
retained with better equipment, and if that were the case, the amount of
nitric acid
actually recovered would have greatly increased. To account for this, the
amount
of mass lost from the heating tank was determined by weighing the tank before
and
after the heating stage, with the difference which was lost as RFNA and NO2.
On
the assumption that this amount could be recoverable as HN03 with appropriate
equipment modifications, it is then possible to calculate what the total moles
of
HN03 which would be recoverable using the method by adding the amount actually
recovered with the amount lost as RFNA and NOZ, and expressing this as a
percent-
age of the starting moles of HN03. This is shown in Tables 1-5 for Trials 1 -
19.
All titrations used 2 drops phenolphthalein as an indicator.
CA 02452145 2003-12-05
- 23 -
Following the heating step, the chips were digested in a round stainless steel
tank
with a 9.283 liter capacity (22.86 cm diameter, 11.43 cm height). The alkaline
solution used in digestion was prepared by diluting an amount of 2.5 molar
sodium
hydroxide (NaOH) solution, 2.5 molar potassium hydroxide (KOH) solution, or a
combination thereof, with distilled water. Various volumes of the initial 2.5
molar
solutions were added to make the final alkaline solution used in digestion and
various volumes of the final alkaline solution prepared were used for the
various
trials. The moles of each alkali solutes) and the total volume of the aqueous
alkaline solution prepared for Trials 1 - 19 are found in Tables 1 - 5.
In each of Trials 1 - 19 it took about 10 minutes or less to digest
approximately
95 % or more of the wood chips. In certain trials, a portion of the chips
remained
undigested, and the amounts are indicated in Tables 1 - 5 for such trials, as
are the
total digestion time for each trial.
Following the digestion step, the black liquor (containing solubilized lignin)
was
vaccum filtered in a buchner funnel without filter paper to remove the pulp,
which
was washed with distilled water and then air-dried at room temperature and
weighed. The black liquor was transferred to a 2500 mL beaker, where the
lignin
was precipitated with the addition of a sulfuric acid (HZS04) solution. The
sulfuric
acid solution was prepared by diluting an volume (depending upon the
concentration
sought) of pure sulfuric acid in a 1 L volumetric flask with distilled water.
In most
cases, the temperature of the black liquor was allowed to cool from the
digestion
step for the lignin precipitation step. The various temperatures are shown in
Tables
1 - 5.
The lignin was vacuum filtered in a buchner funnel without filter paper and
thus
removed from the amber liquor. The lignin was air-dried at room temperature
and
weighed. The lignin was not however dried and weighed for Trials 17 and 18. In
these trials, the lignin did precipitate and was, by visual inspection, found
to have a
similar relative amount and appearance as the lignin produced in Trials 1 -
16.
As set out below, heat was applied during the heating and digestion steps and
in
five trials, during the impregnation step. Where so indicated, the heat was
supplied
in various steps by using a heating mantle made by Glas-Col Apparatus Co., Cat
10
0-410, having 465 total watts and 110 volts. The mantle controller was a
Powerstat
variable autotransformer, Type 3PN116B, made by The Superior Electric Co. of
CA 02452145 2003-12-05
-24-
Bristol, Conn. Agitation was provided during the digestion step by a cold
steel
impeller which was 8 inches in diameter with 1/2 inch separation between 45
°
offset blades. A 18 volt DEWALTT"' XRP cordless drill was used to power the
agitator. Agitation was provided during the lignin precipitation step by
manual
stirring using a glass stir rod for approximately 30 - 45 seconds in each
trial.
The conditions and results of Trials 1 - 19 are found in Tables 1 - 5.
Table 1
CA 02452145 2003-12-05
- 25 -
Triall Trial2 Trial3Trial4
IMPREGNATION STEP
HN03 (w/w) 15 24.15 25 30
Impregnating Time (hours) 24 48 48 13
HEATING STEP
Time to reach 50 C (min.) 55 41 40 30
Time between 50 - 75 C (min.) 76 91 30 75
Time above 75 C (min.) 59 19 35 32
Minimum HN03 Distillation Temp.85 81 81 73
(C)
Distillation time (min.) 59 19 35 32
DIGESTION STEP
Alkaline Solution
moles NaOH 0.425 0.625 0 0.435
moles KOH 0 0.125 0.625 0.19
Vol. of Alkaline Solution (mL)2670 3300 3000 3000
Digestion Temp. (C) 60 63 50 70
Digestion Time (min.) 30 50 40 25
Amount of Undigested Chips 0 0 5.4 0
(g)
LIGNIN PRECIPITATION STEP
% HzS04 Solution added to black20 10 10 20
liquor
(v/v)
Vol. of Acid Sofn added to 35 140 140 75
black liquor
(mL)
Temp. during Addition of Acid 25 45 45 25
(C)
RESULTS
Pulp recovered (g) 92.1 104.6 99.6 94.5
Pulp recovered (%) 46.05 52.3 49.8 47.25
Lignin recovered (g) 41.4 42.1 3 9.8 43.1
Lignin recovered (%) 20.7 21.05 19.9 21.55
HN03 recoverable (%) 98.44 93.2 91.7 77.7
CA 02452145 2003-12-05
-26-
Table 2
Trial Trial Trial Trial 8
s 6 ?
IMPREGNATION STEP
HN03 (w/w) 15 15 11.5 15
Impregnating Time (hours) 24 21 48 13, at 75-80C
HEATING STEP
Time to reach 50 C (min.) 15 19 31 35
Time between 50 - 75 C (min.)20 90 65 25
Time above 75 C (min.) 45 12 15 20
Minimum HN03 Distillation 83 81 83 83
Temp.
(C)
Distillation time (min.) 45 12 15 20
DIGESTION STEP
Alkaline Solution
moles NaOH 0.55 0 0.25 0.3125
moles KOH 0 0.625 0.3 0.3
Vol. of Alkaline Solution 3220 3250 3220 3245
(mL)
Digestion Temp. (C) 50 50 60 70
Digestion Time (min.) 30 60 15 30
Amount of Undigested Chips 7 8.6 14.2 7
(g)
LIGNIN PRECIPITATION STEP
HZS04 Solution added to 10 10 10 10
black
liquor (v/v)
Vol. of Acid Sol'n added 90 132 150 125
to black
liquor (mL)
Temp. during Addition of 40 32 34 61
Acid (C)
RESULTS
Pulp recovered (g) 92.2 90.1 88.6 101.4
Pulp recovered (%) 46.1 45.1 44.3 50.7
Lignin recovered (g) 37.9 39.1 38.3 42.2
Lignin recovered (%) 19 19.6 19.2 21.1
HN03 recoverable (%) 93.4 92.1 93.4 88.1
CA 02452145 2003-12-05
-27-
Table 3
Trial9 Tria110 TrialllTria112
IMPREGNATION STEP
HN03 (w/w) 15 20 15 24.15
Impregnating Time (hours} 17 at 2:45 31 30
75-80C at
75C
HEATING STEP
Time to reach 50 C (min.) 35 19 37 41
Time between 50 - 75 C 24 85 32 105
(min.)
Time above 75 C (min.) 22 12 114 30
Minimum HN03 Distillation 83 75 80 83
Temp.
(C)
Distillation time (min.) 22 12 20 30
DIGESTION STEP
Alkaline Solution
moles NaOH 0.625 0.625 0 0.3125
moles KOH 0 0 0.625 0.3125
Vol. of Alkaline Solution 3000 3000 2500 2250
(mL)
Digestion Temp. (C) SO - 63 70 55 60
Digestion Time (min.) 30 15 15 20
Amount of Undigested Chips0 20.3 24.4 0
(g)
LIGNIN PRECIPITATION STEP
HZS04 Solution added to 10 10 20 10
black
liquor (v/v)
Vol. of Acid Sofn added 150 150 65 125
(mL)
Temp, during Addition of 29 38 27 31
Acid (C)
RESULTS
Pulp recovered (g) 85.6 87.1 77.1 103.5
Pulp recovered (%) 42.8 43.6 38.6 51.75
Lignin recovered (g) 38.9 43.9 33.9 44.1
Lignin recovered (%) 19.5 22 17 22.05
HN03 recoverable (%) 93.4 92.61 93.8 65.05
CA 02452145 2003-12-05
-28-
Table 4
Trial Trial Trial Tria116
l3 l4 l5
IMPREGNATION STEP
HN03 (w/w) 20 30 15 15
Impregnating Time (hours) 13 15 12 14
HEATING STEP
Time to reach 50 C (min.) 19 23 14 6
Time between 50 - 75 C (min.)95 55 48 57
Time above 75 C (min.) 15 20 35 18
Minimum HN03 Distillation 81 80 81 79
Temp.
(C)
Distillation time (min.) 15 20 45 25
DIGESTION STEP
Alkaline Solution
moles NaOH 0.625 0 0.5 0.5
moles KOH 0 0.625 0 0
Vol. of Alkaline Solution 2500 2750 2700 2700
(mL)
Digestion Temp. (C) 55 61 73 41
Digestion Time (min.) 20 25 10 13
Amount of Undigested Chips 4.9 11.6 28.7 102.3
(g)
LIGNIN PRECIPITATION STEP
HzS04 Solution added to black10 10 20 20
li-
quor (v/v)
Vol. of Acid Sofn added to 150 150 110 130
black li-
quor (mL)
Temp. during Addition of 26 36 18 S
Acid (C)
RESULTS
Pulp recovered (g) 96.4 87 71.2 35.2
Pulp recovered (%) 48.2 43.5 35.6 17.6
Lignin recovered (g) 47 38.6 27.2 17.8
Lignin recovered (%) 23.5 19.3 13.6 8.9
HN03 recoverable (%) 91.1 90.6 83.7 84.1
CA 02452145 2003-12-05
-29-
Table 5
Trial 17 Trial 18 Trial
19
IMPREGNATION STEP
HN03 (w/w) 15 15 15
Impregnating Time (hours)1:25 at 50 - 2:10 at 50 12
55 C - 55 C
HEATING STEP
Time to reach 50 C 12 10 20
(min.)
Time between 50 - 75 60 (held between55 (held between35
C 70 - 75 C) 60 - 70 C)
(min.)
Time above 75 C (min.)11 22 27
Minimum HN03 Distillation83 82 83
Temp. (C)
Distillation time (min.)11 15 32
DIGESTION STEP
Alkaline Solution
moles NaOH 0.5 0.475 1.2625
moles KOH 0 0 0
Vol. of Alkaline Solution2500 2690 3050
(mL)
Digestion Temp. (C) 70 63 83
Digestion Time (min.) 10 3 10
Amount of Undigested 31.6 37.8 166
Chips
(g)
LIGNIN PRECIPITATION
STEP
HZS04 Solution added 20 20 20
to
black liquor (v/v)
Vol. of Acid Sofn added130 115 300
to
black liquor (mL)
Temp. during Addition 7 10 32
of
Acid (C)
RESULTS
CA 02452145 2003-12-05
-30-
Pulp recovered (g) 71 6?.6 169.3
Pulp recovered (%) 35.5 33.8 28.2
Lignin recovered (g) Not Weighed Not Weighed 72.5
Lignin recovered (%) Not Weighed Not Weighed 12.1
HN03 recoverable (%) 80.2 83.2 78.8
Example 2
This example comprised a trial that was similar to Trials 1 - 19 in example 1
except
that the starting lignocellulosic material was 150 grams of shavings and
sawdust from
Hemlock, Oak and Pine tree species. Impregnation was done for 30 minutes in
15%
(w/w) HN03 solution under heat of 50 °C. The impregnated shavings and
sawdust
were heated for 60 minutes between 50 - ?5 °C, and then for 10 minutes
over 80 °C.
Following the heating step, the shavings and sawdust were contacted with an
alkaline
solution having 1.25 moles of NaOH and 2600 mL total volume at a temperature
of 52
°C for 7 minutes. Following pulp removal, lignin was precipitated with
the addition
of 20 % (v/v) HZS04. The pulp and lignin were not weighed, but a visual
inspection
showed satisfactory appearance and yield for each, proving that the method of
this
invention can be carried out with relatively small pieces of lignocellulosic
material
such as shavings and sawdust.
Example 3
The pulp samples produced in Trials 5, 7 and 2 from example 1 were
subsequently
analyzed to determine the relative amounts of alpha cellulose, beta cellulose
and
gamma cellulose therein. The results are found in Table 6 below.
35
CA 02452145 2003-12-05
-31-
Table 6
Trial S Trial 7 Trial 2
Alpha Cellulose ( % 81. 3 79.6 79.2
)
Beta Cellulose ( % 4.4 3 .7 7.5
)
Gamma Cellulose (%) 14.3 16.7 13.3
In addition to the methods herein, the present invention also includes pulp
produced
according to the methods herein, as well as paper products comprising
cellulose from
pulp produced according to the methods herein. Similarly, the present
invention also
includes lignin produced according to the methods herein, as well as numerous
other
products and compositions comprising lignin produced according to the methods
herein, including for example, fertilizers, asphalt emulsifiers, soil
stabilizers, wood
products (such as plywood and particle board), oil well drilling fluids and
dispersing
agents for preparing concrete.
As will be apparent to those skilled in the art in the light of the foregoing
disclosure, many alterations and modifications are possible in the practice of
this
invention without departing from the scope thereof. Accordingly, the scope of
the
invention is to be construed in accordance with the substance defined by the
following claims.