Note: Descriptions are shown in the official language in which they were submitted.
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
PERVAPORATIVELY COOLED. CONTAINERS
Background of the Invention
Field of the Invention
This invention relates to a device and method of construction of a container
or closure used
to cool a liquid by means of pervaporation.
Description of the Related Art
Evaporative cooling of both dwellings and water originated in Ancient Egypt
and
subsequently spread eastward through the Middle-East and Iran, to the north of
India, westward
across north Africa to southern Spain and other regions suffering from a hot
and dry climate. In the
initial use of this process non-glazed clay pots were used for centuries for
the storage of water with
the added side benefit of cooling the liquid water contents by absorbing and
wicking the water to
the outer clay surface followed by the evaporation of the water from this
surface. Unfortunately,
evaporation directly from the outer clay surface eventually lead to scale
formation and reduced
cooling efficiency as the minerals build up on this surface reducing the
liquid permeability and
lowering the liquid vapor pressure.
Other methods based on heat transfer reduction from the environment to the
liquid have
been used. Methods that have been used include vacuum and air gap thermoses,
and foam
insulative jackets. Additional devices using ice, frozen cold packs or sticks
have been used to
compensate for heating by surrounding environment and the return of the liquid
in the container to
ambient temperature. In all these cases the design of the system necessitates
that either the liquid
contents, a separate chamber and /or the shell of the bottle be cooled which
can lead to excessive
weight issues in addition to a liquid volume displacement loss in the
container. In all of these
methods the temperature of the liquid will equilibrate and eventually return
to the ambient
temperature.
Pervaporation (PV) is defined as a combination of matrix vapor permeation and
evaporation. From 1987 on, membrane pervaporation has gained wide acceptance
by the chemical
industry for the separation and recovery of liquid mixtures (Chemical
Engineering Progress, pp.
45-52, July 1992). The technique is characterized by the introduction of a
barrier matrix between a
liquid and a gaseous phase. A liquid is in intimate contact with one side of
the matrix. Mass
transfer of vapor occurs selectively to the gas side of the matrix resulting
in the loss of liquid or the
loss of select volatile liquid components and the loss of evaporative latent
heat. The process is
termed pervaporation because of the unique combination of vapor "permeation"
through the porous
matrix and the liquid to vapor phase change "vaporization". Without heat added
to the liquid, the
temperature falls due to the latent heat of vaporization until an equilibrium
temperature is reached
-1-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
where the heat absorbed from the environment is equal to the latent heat lost
due to liquid
evaporation at the matrix surface or within the pores.
U.S. Patent Number 5,946,931 illustrates the use of an evaporative cooling
PTFE
membrane device using a stream of fluid in a laminar flow profile above a
membrane in order to
cool an attached device or environment. U.S. Patent Number 4,824,741
illustrates the use of a
pervaporative cooling matrix to cool the surface of the plate of an
electrochemical cell. The moist
plate may be made from uncatalyzed PTFE-bonded electrode material, a suitable
porous sintered
powder, porous fibers, or even a porous polymer film. U.S. Patent 4,007,601
demonstrates the use
of evaporative cooling in a circulating porous hollow heat exchanger to obtain
a cooled fluid.
Summary of the Invention
Disclosed herein is a simplified pervaporative cooling system for beverage and
liquid
containers that does not use any mechanical pumps to supply liquid to the
pervaporative matrix
surface and does not rely on a vacuum to enhance the cooling efficiency as in
the above prior art.
A container is defined as any apparatus or enclosure that holds liquid whether
it is open or closed
to the external environment. In one embodiment, this approach utilizes a
pervaporative matrix that
preferably fornls part of the container body or housing and comprises between
5 to 100% of the
total surface area of the container. The liquid contents of the container are
then cooled directly at
the surrounding liquid/membrane interface due to the latent heat of
evaporation of the water. The
resulting liquid vapor is lost through the matrix to the surrounding
environment or to a collector or
trap such as may comprise an absorbent material. Preferred containers include
bottles, jars,
carboys, and pouches. The containers may, in some embodiments, be fabricated
into larger
structures, including housings, dispensers, and garments.
In one embodiment, there is provided a pervaporatively cooled container,
comprising a
container body comprising one or more walls, wherein at least a portion of
said one or more walls
comprises a pervaporative matrix, said matrix comprising a porous hydrophobic
material, wherein
said matrix allows for the passage of small quantities of molecules of a
volatile liquid vapor
through the matrix, the evaporation of which cools the container, including
any contents of the
container. In one embodiment, there is provided a pervaporatively cooled tube
or straw,
comprising an elongate hollow tubular structure comprising an outer
pervaporative layer
comprising a hydrophobic material coextensive with a porous internal layer
comprising a
hydrophilic material, the internal layer defining a lumen through which a
liquid can pass. In one
embodiment, the tubular structure is formed from a hydrophobic porous tube in
which the inner
surface of the tube has been chemically treated to be hydrophilic, thus
forming the internal layer.
In one embodiment, there is provided a cooling jacket for a container,
comprising a jacket
body comprising an outer layer comprising a hydrophobic porous material; and
an inner layer
_2_
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
coextensive with said outer layer and in fluid communication with said outer
layer, said inner layer
being adapted to hold a volatile liquid wherein said jacket body is shaped to
allow the .inner layer
to contact at least a portion of a container.
W a preferred embodiments, the containers and cooling jaclcets may further
comprise a
regenerable or disposable outer layer, directly adjacent to or in contact with
the pervaporative
layer, comprising a desiccant, absorbent material or other substance that
absorbs or adsorbs the
moisture or other fluid resulting from pervaporation.
In one embodiment, there is provided a cooling garment comprising at least two
layers: an
outer layer comprising a pervaporative material comprising a hydrophobic
pervaporative laminate;
an optional middle layer comprising a thin support liquid barrier layer for
the pervaporative layer;
and an inner layer; wherein the outer layer is in fluid communication with a
body of coolant liquid,
and the inner layer is in thermal contact with the wearer of the garment. The
wearer of the garment
is cooled by the pervaporation of the coolant liquid through the pervaporative
material of the outer
layer. In a preferred embodiment, the cooling garment is incorporated or
integrated into a piece of
clothing such as a protective garment or suit. The garment may further
comprise a tube in fluid
communication with the body of coolant liquid which allows the wearer of the
garment to orally
consume coolant liquid, preferably water. In a preferred embodiment, the
garment further
comprises a regenerable or disposable outer layer comprising a desiccant or an
absorbent material
that absorbs the moisture or other fluid resulting from pervaporation.
In preferred embodiments, one or more of the following may also be present:
the garment
is in thermal contact either by direct contact with the skin or contact
through a piece of fabric or
material, such fabric or material being worn by the wearer of the garment
and/or being part of the
garment itself; the outer layer is pleated to increase surface area for
pervaporation; the middle layer
is a barrier to potentially hazardous biological or chemical materials; and
the inner layer comprises
patterned or serpentine regions formed by a heat sealing process.
In a related embodiment, the garment may further comprise or be in fluid
communication
with a reservoir holding additional coolant liquid. The coolant can be fed
into the interstices
formed between the pervaporative matrix and the middle layer from the
reservoir by gravity or by
wicking. Preferred coolant liquids comprise water, alcohols, and blends
thereof.
In related embodiments, containers such as bottles or backpacks comprising
pervaporative
material, as described below, are also provided.
Brief Descriution of the Drawines
Figures lA and 1B illustrate a bottle in plan and exploded view in which a
generally planar
porous matrix may be wrapped around or pushed over a bottle body as a
cylinder.
-3-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
Figure 2 shows a partially exploded view of a multilayered structure according
to one
embodiment comprising a thin membrane layered between two macroporous layers
Figures 3A, 3B, 3C and 3D, illustrates plan and cut away views of embodiments
in which
support ribs enhance the rigidity of a porous matrix.
Figure 4 shows a container comprising an outer porous insulative layer. This
sleeve
reduces direct radiative warming of the inner bottle surface, yet allows for
the pervaporative flux
and loss of latent heat.
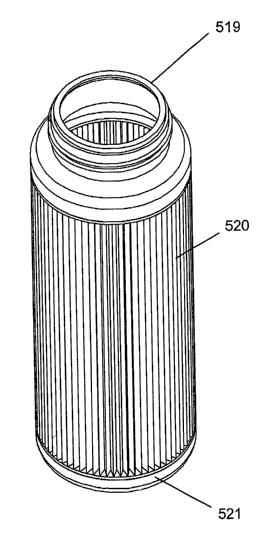
Figure 5 illustrates one embodiment of container comprising a pleated matrix
which serves
as a method for increasing the effective cooling surface area of the
container. This allows for a
higher surface area and quicker liquid cool down time for the container.
Figures 6A and 6B show one embodiment of a container in plan and cutaway view
comprising an adjustable sleeve to limit the extent of pervaporative flux and
liquid loss from the
container. This sleeve preferably also reduces direct radiative warming of the
inner bottle surface,
yet allows for the pervaporative flux and loss of latent heat.
Figure 7 illustrates a cross section of a two-layer pervaporative sleeve
comprising a sponge
or sponge-like material that can be used with a container.
Figure 8 shows a cutaway view of another embodiment of pervaporative cooling j
acket that
is used on a central housing containing a liquid, such as a carbonated
beverage.
Figure 9 is a graph of time versus cooling pertaining to pervaporative cooling
equilibrium
using a variety of porous matrices.
Figure 10 illustrates one embodiment of a pervaporatively-cooled drinking cup.
Figures 11A, 11B and 11C illustrate one embodiment of pervaporative cooling
storage
container (e.g. a cooler) having a pervaporative body shell and pervaporative
lid.
Figure 12 illustrates a preferred liquid dispensing reservoir comprising a
pervaporative
matrix.
Figure 13 illustrates one embodiment of a hydration backpack comprising a
pleated
pervaporatively-cooled reservoir filled with liquid.
Figure 14 illustrates a pervaporatively-cooled drinking pouch in an optional
porous webbed
strap on holder. In addition an internally wettable pervaporatively-cooled
tube is shown, which can
be used for immediately-chilled drinking or dispensing in connection with the
illustrated pouch or
with other containers.
Figure 15 illustrates a pervaporatively cooled jacket according to one
embodiment.
The figures illustrate preferred embodiments and are intended to be merely
exemplary and
representative of certain embodiments. To that end, several figures contain
optional features that
need not be included in any particular embodiment of the invention, and the
shape, type, or
-4-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
particular configuration of container or closure illustrated should not be
taken as limiting on the
invention.
Detailed Descr~tion of the Preferred Embodiments
Disclosed herein are containers and enclosures that use pervaporative cooling
to cool a
liquid or item residing in such container or enclosure. In preferred
embodiments, the containers
are comprised of porous vent materials, also called porous matrices. In one
embodiment the
container forms part of a pervaporative cooling garment.
Porous matrices may be made of any of a wide variety of materials, including,
but not
limited to, plastics, elastomers, metals, glass, and ceramics. Combinations of
plastics, elastomers,
metals, glasses, or ceramics may also be used. The combinations may be
intimate, such as from
blending of two or more components to become co-sintered, or may be layered,
such as from
laminate structures derived from two or more materials. Combinations of
different plastics,
elastomers, metals, glasses, or ceramics can also be co-sintered or fabricated
into laminate
structures for use in pervaporative containers. Preferred plastics for porous
vent materials include,
but are not limited to thermoplastic polymers, thermoset elastomers, and
thermoplastic elastomers.
Preferred thermoplastic polymers include, but are not limited to, low density
polyethylene (LDPE),
linear low density polyethylene (LLDPE), medium density polyethylene (MDPE),
high-density
polyethylene (HDPE), ultra-high molecular weight polyethylene (UHMWPE),
polypropylene (PP)
and its copolymers, polymethylpentene (PMP), polybutylene terephthalate (PBT);
polyethyleneterephthalate (PET), polyethyleneterephthalate glycol modified
(PETG),
polyetheretherketone (PEEK), ethylenevinylacetate (EVA),
polyethylenevinylalcohol (EVOH),
polyacetal, polyacrylonitrile (PAID, poly(acrylonitrile-butadiene-styrene)
(ABS),
poly(acrylonitrile-styrene-acrylate) (AES), poly(acrylonirile-ethylene-
propylene-styrene) (ASA),
polyacrylates, polymethacrylates, polymethylmethacrylate (PMMA),
polyvinylchloride (PVC),
chlorinatedpolyvinylchloride (CPVC), polyvinyldichloride (PVDC) fluorinated
ethylenepropylene
(FEP), polyvinylfluoride (PVF), polyvinylidinefluoride (PVDF),
polytetrafluoroethylene (PTFE),
polyester, cellulosics, polyethylenetetrafluoroethylene (ETFE),
polyperfluoroalkoxyethylene
(PFA), nylon 6 (N6), polyamide, polyimide, polycarbonate,
polyetheretherlcetone (PEEK),
polystyrene (PS), polysulfone, and polyethersulfone (PES). Preferred thermoset
elastomers include
styrene-butadiene, polybutadiene (BR), ethylene-propylene, acrylonitrile-
butadiene (NBR),
polyisoprene, polychloroprene, silicone, fluorosilicone, urethanes,
hydrogenated nitrile rubber
(HNBR), polynorborene (PNR), butyl rubber (IIR) to include chlorobutyl (CIIIR)
and bromobutyl
(BIIR), fluoroelastomers such as Viton~ and Kalrez~, Fluorel TM, and
chlorosulfonated
polyethylene. Preferred thermoplastic elastomer (TPE) categories include
thermoplastic olefins
(TPO) including those commercially available as Dexflex~ and Indure~;
elastomeric PVC blends
-S-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
and alloys; styrenic block copolymers (SBC) including styrene-butadiene-
styrene (SBS), styrene-
isoprene-styrene (SIS), styrene-ethylene/butylene-styrene (SEBS), and styrene-
ethylene-propylene-
styrene (SEPS), some commercially available SBCs include those sold under the
trademarks
Kraton~, Dynaflex~, and ChronopreneTM; thermoplastic vulcanizate (TPV, also
lrnown as
dynamically vulcanized alloys) including those commercially available under
the trademarks
Versalloy~, Santoprene~, and Sarlink~; thermoplastic polyurethane (TPU)
including those
commercially available under the trademarks ChronoThane~, VersollanTM, and
Texrin~;
copolyester thermoplastic elastomers (COPE) including those commercially
available as Ecdel~;
and polyether block copolyamides (COPA) including those commercially available
under the
trademark PEBAX~. Preferred metals for porous materials include stainless
steel, aluminum, zinc,
copper and its alloys. Preferred glass and ceramics for porous materials
include quartz,
borosilicate, aluminosilicate, sodiumaluminosilicate, preferably in the form
of sintered particles or
fibers derived from said materials.
A preferred method of making macroporous plastic is by a process called
sintering,
wherein powdered or granular thermoplastic polymers are subjected to the
action of heat and
pressure to cause partial agglomeration of the granules and formation of a
cohesive macroporous
sheet or part. The macroporous material comprises a network of interconnected
macropores that
form a random tortuous path through the sheet. Typically, the void volume or
percent porosity of a
macroporous sheet is from 30 to 65°1o depending on the conditions of
sintering although it may be
greater or lesser than the stated range depending on the specific method of
manufacturer. Due to
the adjustment of chemical or physical properties , the surface tension of a
macroporous matrix
can be tailored to repel or absorb liquids , but air and vapors can readily
pass through. For
example, U.S. Patent No. 3,051,993 to Goldman, herein incorporated by
reference in its entirety,
discloses the details of making a macroporous plastic from polyethylene.
Porous plastics, including macroporous plastics, suitable for making a
pervaporatively-
cooled container in accordance with preferred embodiments, can be manufactured
in sheets or
molded to specification and is available for purchase from a number of
sources. Porex Corporation
(Fairburn, Georgia, U.S.A.) is one such source, and provides porous plastic
under the trademark,
POREX~. Porous plastic sold under the name POREX~ can be purchased in sheets
or molded to
specification from any one of the thermoplastic polymers previously described.
The average
porosity of such POREX~ materials can vary from about 1 to 350 microns
depending on the size
of polymer granules used and the conditions employed during sintering.
GenPore~ (Reading,
Pennsylvania, U.S.A.) is another manufacturer of porous plastic products, with
pore sizes ranging
from 5 to 1000 microns. MA Industries Inc. (Peachtree City, Georgia, U.S.A.)
also manufactures
porous plastic products. Porvair Technology Ltd (Wrexham North Wales, U.K.) is
another
-6-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
manufacturer of porous products supplying both porous plastic (range of 5 to
200um pore size
under brand name VyonTM) and porous metal media (under brand name Sinterflo~).
The basic size, thickness and porosity of the plastic chosen to make a
pervaporative matrix
may be determined by calculating the amount of vapor that must pass through
the vent in a given
period of time (flow rate) and the heat transfer rate from the environment
back into the liquid. The
flux rate (flow rate per unit area) of a given macroporous plastic varies
depending on factors
including the pore size, percent porosity, and cross sectional thickness of
the matrix and is
generally expressed in terms of volume per unit time per unit area. To achieve
a sufficient degree
of pervaporative cooling, the flow rate of vapor through the matrix should be
such that the
thermodynamic heat removed from the liquid initially at room temperature due
to vaporization is
greater than the heat absorbed from the environment. During the pervaporative
process the
container liquid temperature cools until the heat loss of the liquid due to
vaporization of the liquid
contents through the matrix matches the heat gain from the surrounding
environment.
In common usage, "Macroporosity" generally refers to the overall void volume
of a
material or its macrostructure. The terns "Macroporous" is generally used to
classify a material's
individual pores that are considered large in size. The term "Microporosity"
generally refers to the
individual pore sizes or distribution of pore sizes that constitute the
microstructure of a porous
material. The term "Microporous" is generally used to classify a material's
individual pores that
are considered small in size. For purposes of the disclosure herein, pore size
(diameter) is
classified according to the International Union of Pure and Applied Chemistry
(ILTPAC)
Subcommittee of Macromolecular Terminology, definitions of terms drafted on
February 26, 2002.
This standard divides pore size classification into three categories:
Microporous (< 0.002pm),
Mesoporous (0.002 to O.OSOpm) and Macroporous (>O.OSOpm). Also for the
purposes of this
disclosure herein, void volume will be discussed in terms of the "Percent
Porosity" of the material.
Both macroporous as well as mesoporous materials with pore sizes of 0.05 qm or
less can be used
for pervaporative cooling. Preferred methods for fabrication include casting
or stretching
membranes of such materials.
Preferred porous materials include those in which the pores on opposite
surfaces (what will
become the interior and exterior surfaces) are interconnected such that the
two sides are in
communication with each other. Such interconnections are preferably not,
however, straight
through as to create a single cylindrical tube through which material passes;
instead a network of
pores creates a tortuous path.
For a single layer pervaporative matrix, the porous materials are preferably
macroporous
with pore sizes greater than or equal to O.OS~.m, preferably about 0.1 to
SOO~m, and about 0.5 to
lOpm, including 0.25, 0.5, 1, 5, 15, 20, 40, 60, 80, 100, 150, 200, 250, 300,
350, 400, and 450 ~,m.
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
In one embodiment, the matrix materials used in conjunction with the
pervaporative containers are
between 0.1 and 100~,m, preferably between 0.5 and 75~m. The percent porosity
(percent open
area) of the materials are preferably about 10 to 90%, preferably 30 to 75% or
50 to 70%, including
20%, 40%, 60%, and 80%. The thickness of the porous materials preferably
ranges from 0.025 to
7mm, including between 1 and 3mm. Preferred thiclrness for matrix materials
used in
pervaporative containers are about 0.05 to Smm and about 0.1 to 3.Omm,
including 0.2, 0.3, 0.5,
0.7, 1.0, 1.25, 1.5, 1.75, 2.0, and 2.Smm. Other embodiments may have values
for the above
parameters that are above or below those set forth above. For single layer
matrices, it is preferred
that the material be hydrophobic or have a hydrophobic coating. For the values
set forth in this
paragraph, as well as elsewhere in the specification, the stated ranges
include as the values
contained in between the values specifically mentioned. In other embodiments,
materials can have
one or more properties having values lying outside the disclosed ranges.
The matrix material can be derived from plastic, elastomers, glass, metal, or
combinations
thereof. Some preferred matrix materials, including thermoplastic polymers,
thermoset elastomers,
thermoplastic elastomer, metals, glass and ceramics are as detailed above.
Matrix materials may be
purchased from commercial sources, or they may be made according to a variety
of techniques.
U.S. Patent No. 4,076,656 to White et al. details one technique in which
porogens are added to
molten or dissolved materials, which can be leached out with a solvent, or
extracted with
supercritical fluids after the material sets and is in its final form. U.S.
Patent No. 5,262,444 to
2.0 Rusincovitch et al. details another technique to create porous material by
introducing porogens that
evolve into gases after processing a material, to leave behind a porous
structure. These patents are
hereby incorporated by reference in their entireties.
Although many pervaporative matrix materials discussed herein are hydrophobic,
oleophobic pervaporative materials may also be used when the pervaporation
liquid is an organic
liquids such as alcohol. Commodity plastic materials such as nylon,
polysulfone, and the
cellulosics, are available in hydrophilic grades. These hydrophilic materials
can be milled into
particles and sintered using techniques known to those familiar in the art to
produce hydrophilic
porous materials with high liquid flux rates. Porous hydrophilic plastic,
including macroporous
plastic can be manufactured in sheets or molded to specification and is
available for purchase from
a number of sources, including Porex Corporation. Porous hydrophilic fiber
materials can range in
pore size from 20 to 120~,m with percent porosity ranging from 25 to 80 for
the pore volume.
Moreover, hydrophobic porous materials can be rendered hydrophilic by one or
more treatment
processes familiar to those skilled in the art including, but not limited to,
plasma etching, chemical
etching, impregnation with wetting agents, or application of hydrophilic
coatings. In addition, a
masking process can be used in conjunction with one or more treatment
processes to selectively
_g_
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
pattern a hydrophobic porous material with regions of hydrophilicity with high
liquid flux rates, if
desired.
For example, multilayered porous constructs containing two or more layers of
porous
material. Thin layers can be laminated to malee thicker layers using
techniques familiar to those in
the art. Multilayered constructs may be used to obtain a mechanical and
physically superior matrix
as previously observed in our tests. For instance, combining a sintered
macroporous matrix of
polyethylene with a thin layer of expanded PTFE on the liquid side of the
container increases the
hydrophobicity and liquid breakthrough pressure of water from 5 psi to over 30
psi, yet the layered
matrix still maintains a similar pervaporative flux to that obtained using
porous polyethylene by
itself. Thickness of laminates preferably ranges from about 0.025 to 7000pm
with average pore
sizes, percent porosity and other properties preferably as described above.
Pervaporative matrix materials may also be derived from porous materials made
from
blends. In a preferred embodiment, the porous materials comprise a fluorinated
resin, including,
but not limited to, polyvinylfluoride (PVF), polyvinylidinefluoride (PVDF),
polytetrafluoroethylene (PTFE), polyethylenetetrafluoroethylene (ETFE),
fluorinated ethylene
propylene (FEP), polyperfluoroalkoxyethylene (PFA), and/or fluorinated
additives such as Zonyl~,
blended with selected polyolefm or other resins, preferably those selected
from the series of
polyethylenes (LLDPE, LDPE, MDPE, HDPE, UHMWPE) polypropylene, polyesters,
polycarbonates, ABS, acrylics, styrene polymethylpentene (PMP), polybutylene
terephthalate
(PBT); polyethyleneterephthalate (PET), polyetheretherketone (PEEK),
ethylenevinylacetate
(EVA), polyacetal, poly(acrylonitrile-butadiene-styrene) (ABS),
poly(acrylonitrile-styrene-
acrylate) (AES), poly(acrylonirile-ethylene-propylene-styrene) (ASA),
polyesters, polyacrylates,
polymethacrylates polymethylmethacrylate (PMMA), polyvinylchloride (PVC),
polyvinyldichloride (PVDC) nylon 6 (N6), polyamide, polyimide, polycarbonate,
polystyrene, and
polyethersulfone (PES). Elastomers may also be used alone or in blends.
Preferred elastomers
include those of the thermoset type such as styrene-butadiene, polybutadiene
(BR), ethylene-
propylene, acrylonitrile-butadiene (NBR), polyisoprene, polychloroprene,
silicone, fluorosilicone,
urethanes, hydrogenated nitrile rubber (HNBR), polynorborene (PNR), butyl
rubber (1TR) to
include chlorobutyl (CIIR) and bromobutyl (BIIR). The resulting blends,
including sintered blends,
have porous structures with varying amounts of porosity, flexibility and
mechanical strength
determined predominately from the non-PTFE or other non-fluorinated resin, and
high water
intrusion pressures determined predominately from the fluorinated resin due to
its preferential
migration to the pore surface during the sintering process. The percent
porosity, pore size, and
thickness are preferably as noted above. Blended matrix materials may be
purchased from
commercial sources, or they may be made according to a variety of techniques.
U.S. Patent No.
-9-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
5,693,273 to Wolbrom details a process of cosintering to produce multi-
porosity porous plastic
sheets that can be derived from two or more polymeric resin materials and U.S.
Patent No.
5,804,074 to Takiguchi et al. et al. details a process to produce a plastic
filter by cosintering two or
more polymeric resins in a molding process to produce filter parts. Both of
these patents are
hereby incorporated by reference into this disclosure in their entirety.
Pervaporative Cooling
In preferred embodiments, a simplified pervaporative cooling system for
containers is
presented that does not use any mechanical pumps to supply liquid to the
pervaporative matrix
surface and does not rely on a vacuum to enhance the cooling efficiency. The
present approach
utilizes a pervaporative matrix that forms part of the container, preferably
the housing of the
container, and comprises between about 5 to 100% of the total surface area of
the container,
including about 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, and 90% of the total
surface area.
The liquid contents of the container are preferably cooled directly at the
surrounding liquid/matrix
interface due to the latent heat of evaporation of the liquid, such as water
or a water/dissolved solid
mixture or solution, in the container. In an alternate embodiment, a
pervaporative sleeve or
housing is used to cool a body such as a drinking vessel or container in
contact with the sleeve.
The resulting liquid vapor is lost to the surrounding environment or to an
absorbent material
through the matrix. In most containers, natural convection and conductive heat
transfer within the
liquid are predominant heat transfer mechanisms responsible for cooling the
liquid contents of the
container. Depending upon the dimensions and other properties of the
container, the cooling may
be substantially uniform throughout the container.
The liquid contents of the pervaporative container or sleeve acts as a
coolant. Preferably
the liquid volume loss is marginal; for example, in one embodiment, the liquid
volume loss of
approximately 15% over a 24 hour time period even with significant external
air circulation. Due
to the high latent heat of vaporization of water (583 cal/g at 75°F),
for example, approximately
seven times as much weight in ice would be required to maintain the same
temperature drop as a
loss of water due to vaporization. An added benefit of the porous matrix in
addition to
pervaporative cooling is in venting any pressure differential developed in the
container due to the
release of carbonation from a beverage or due to the consumption of the
contents.
Referring now to the drawings, there is shown in Figures lA and 1B one
embodiment of a
vented pervaporative cooling container formed in accordance with this
invention. The wall 501 of
the container is formed at least in part of pervaporative matrix. This vapor
permeable matrix can
be from about 5 to 100% of the total surface area of the container.
Approximately 100% coverage
is achieved if the entire cap and housing (comprising the top 500 walls 501
and bottom 502) are
made from porous matrix material. In one preferred embodiment, the
pervaporative surface area is
-10-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
greater than about 30% of the total container surface and provides a
substantial amount of
pervaporative flux to effectively cool the contained liquid below ambient
temperature and maintain
a subambient liquid temperature.
In one embodiment, as shown in Figure 2, a multilayered construct comprising
two or more
layers may be used. In one embodiment three layers of porous material 503, 504
and 505 are used
to obtain a multilayer or laminate matrix. In one embodiment, a sintered
macroporous matrix of
polyethylene 505 with a thin layer of expanded PTFE 504 on the liquid side of
the container
increases the hydrophobicity and liquid intrusion pressure but helps to
maintain a similar
pervaporative flux and good mechanical stability as is obtained using porous
polyethylene by itsel.
In addition, a third layer of porous polyethylene 503 forming a sandwich with
the expanded PTFE
in the middle 504 provides a scratch resistance surface close to the inside of
the container making it
dishwasher safe and substantially preventing or reducing the soft expanded
PTFE layer from being
damaged. In related embodiments, laminates can comprise greater or fewer than
three layers
and/or different porous matrix materials.
In an alternate embodiment, the inner layer 503 comprises a pervaporative
matrix or
laminated matrix, middle layer 504 comprises a thermally insulative material
with pores or other
open spaces to allow passage of the vapor, and outer layer 505 comprises a
desiccant or absorbent
material.
A preferred orientation of the matrix is where a higher liquid intrusion
membrane faces the
inside of the container and the porous matrix support is exposed to the air
outside of the container.
Thicknesses for these porous materials in a preferred embodiment are in the
range from about
1/1000" (0.025 mm) to 1/4" (6.4 mm). The porous matrices can also provide
structural rigidity,
scratch resistance, and/or mechanical integrity to the walls of the container.
In a preferred embodiment, a membrane or thin layer of material with a small
pore size
(<10 ~,m) can be selected from a group of highly hydrophobic materials such as
expanded
polytetrafluoroethylene (ePTFE) and laminated in between thicker highly porous
supports such as
sintered polyethylene, which allow for a substantial pervaporative flux. If
only two layers are used,
Each of these layers can vary in thickness from a monoatomic surface treatment
to 1/4" (6.4 mm) in
thickness or greater for a foam insulation or porous composite. Porous ceramic
materials including
molecular sieves (zeolites) or porous polymer films (CSP Technologies -
Auburn, Alabama) and
organic matrices such as activated carbon can be used to substantially prevent
or reduce odors from
the environment from contaminating the liquid contents of the pervaporative
cooling device or
container.
In a preferred embodiment, a layered construct comprises five layers: an inner
ePTFE
layer, a porous polypropylene, a thermally insulative urethane foam layer, a
ceramic such as zeolite
-11-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
and a thin nonporous polyolefin or polyester outer wrap. This device can be
used to maintain a
pervaporative cool within the device in a humid environment. Upon absorption
of the vapor
released from the liquid, the zeolite or other desiccant transfers the heat
directly or indirectly into
the environment while the insulated liquid contents within the pervaporative
sleeve are cooled.
The outer two layers comprising zeolite and a nonporous film may be disposable
or regenerable
such as by drying in an oven.
Except for any surface treatments that may be applied directly to the porous
matrices in the
constructs, the porosities of the matrices or composite are preferably
maintained between about 10
to 95%. This provides for structural support within the matrix and enhances
the available
pervaporative surface area and hence the overall cooling rate of the
container. The pore size of the
matrix can also have an effect with Knudsen diffusion predominating below a
pore size of 200 nm,
effectively decreasing the vapor permeation rate and extending the liquid to
vapor transition and
cooling zone to the air/vapor surface of the material. In accordance with one
embodiment,
preferred pore sizes include those between about 0.5 ~m to 30 Vim, which are
larger than the
Knudsen diffusion range. The liquid intrusion pressure decreases substantially
above a 100 ~m
pore size, making the use of a single layer of macroporous material less
desirable in some
instances. If a combination of a membrane and a macroporous support are used,
then larger pore
sizes in the macroporous support become more desirable than in the absence of
the combination.
As shown in Figures 3A, 3B, 3C, and 3D ribs 508 and 514 may be added to the
inside
and/or outside walls of the container to enhance the structural rigidity of
the container, prevent or
reduce damage to the pervaporative matrix 507 and 513 and/or provide a
handhold 514. Figures
3C and 3F show a sports version of the ribbed design with a narrowed neck 512.
The embodiment in Figure 4 comprises a layer of open cell porous insulator 518
may be
i
added to the outside surface of the container to allow for relatively
unimpeded vapor diffusion out
of the system but reduced convective and radiative heat flow from the
surrounding environment
through to the inner container walls 517 and into the liquid. A beneficial
feature of this insulator
518 is that it aides as an additional structural support, provides a hand grip
on the container and to
reduce or prevent damage to the matrix 517. As used herein, "pleated" includes
rippled surfaces
and other configurations for increasing surface area. Pleated matrices include
those in which the
entire surface is pleated, or in which one or more portions are pleated and
others are left smooth.
Use of a pleated membrane or pleated porous sintered matrix 520, as shown in
Figure 5,
can enhance the pervaporative cooling of the container since the rate of
pervaporative cooling is a
direct function of the surface area of the container.
Pervaporative containers and garments may comprise an adjustable or movable
sleeve on
the outside of the pervaporative matrix to allow for selective covering or
uncovering of some or all
-12-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
of the pervaporative material. Covering some of the pervaporative material
reduces the vapor flux
rate is while still maintaining some pervaporative cool. Covering all
substantially stops the
pervaporation and can serve as a type of "on-off ' switch for the container or
garment.
For example, sleeves 524 and 525, as shown in Figures 6A and 6B can be
provided as a
means to reduce the exposed porous surface area 527 and overall evaporative
cooling rate of the
container and hence reduce the liquid vaporization rate and cooling rate
allowing for greater
control of the temperature of the container contents. Reduced cooling may be
desired in some
situations such as when the absolute pressure, relative humidity and/or
ambient temperature are
low. As shown in Figure 6B, there is preferably a separation or gap 530
between one or more
portions between the container and the surrounding sleeve. The gap can serve
as an insulating
region and/or as a region of buoyant natural connective flow of vapor,
allowing for the
maintenance of pervaporative cooling and the minimization of radiative heat
transfer to the liquid
contents 529 of the container. The inner sleeve 524 on the outside of the
porous matrix 523 of the
container is preferably attached to the pervaporative matrix at least at the
top 522 and bottom 526
portions of the container housing, especially if such portions are non-porous.
In one embodiment, some or all of a pervaporative garment or container may
comprise a
pervaporative sponge which both holds water within the body of the sponge and
also serves to
provide cooling by pervaporation. One preferred embodiment is a two-layer
pervaporative sponge
having an inner sponge comprising a hydrophilic material and an outer
hydrophobic layer attached
thereto. In this configuration, the inner sponge can be soalced with water or
another vaporizable
liquid prior to use and the porous hydrophobic top layer substantially
prevents or reduces the
leakage of the pervaporative liquid at the outer surface of the pervaporative
matrix. The liquid
provides a heat transfer path through the wet matrix directly to the inner
container wall surface.
Figure 7 illustrates a two-layer pervaporative sponge 533 that can be used on
glasses,
bottles and containers. This configuration allows the inner sponge layer 534
to be soaked with
water or another vaporizable liquid and a porous hydrophobic top layer 535
substantially prevents
or reduces the leakage of the liquid coolant at the outer surface of the
pervaporative matrix 535.
The liquid provides a heat transfer path through the wet matrix directly to
the inner container wall
surface 532.
Figure 8 shows an alternate configuration in which a cooling jacket 542
holding water or
another pervaporative fluid 541 is filled through the port 543 and used to
cool the contents of an
enclosed container housing 539. The housing comprises one or more sections of
pervaporative
matrix 537 and optionally comprises one or more ribs 538 to enhance structural
strength. The
liquid contents 540 within the enclosed central housing 539 can thus be
sealed, substantially
preventing or reducing the loss of liquid volume or carbonation within this
region. In addition, the
-13-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
pervaporative cooling efficiency of the container is not dependent on the
nature of the enclosed
liquid; it depends only on the volatility, heat of vaporization, ionic
strength (tonicity) and solute
content of the water or liquid 541 used to fill the surrounding housing. As
shown in Figure 7 the
cooling jacket may also be made of a detachable sleeve consisting of an outer
hydrophobic
pervaporative layer 535 and an Timer porous liquid holding or absorbing layer
534.
Figure 10 illustrates a pervaporatively-cooled drinking cup similar in
function to the
pervaporative bottles shown in Figures 1 A, 1B, 2, 3 A and 3B. As soon as
liquid is poured into the
cup the porous matrix 555 allows the liquid to pervaporatively chill. The
bottle housing and
support ribs 556 provide structural support and insulation.
These types of cooling jackets 533 and 542 can also be used in a similar
configuration as a
food cooler to reduce and maintain the temperature of the contents 568 below
ambient. In one
embodiment, as shown Figures 11A, 11B and 11C, baffles 560, 565 and 573 can be
used on the
cooler to protect the pervaporative matrix 566, and to aide in the mechanical
rigidity and handling
of the storage container. Both the lid 558, 572 and bottom 563, 559 portions
of the cooler can be
filled with water or another pervaporative liquid 567 and 575 through the
liquid fill and drain ports
561 and 576. The inside of the lid 574 and the bottom 569 portions of the
container are preferably
made of a nonporous material.
Figure 12 illustrates a chilled water dispenser comprising of a high capacity
water bottle
579, such as a 5 or 10 gallon bottle, and a pervaporatively chilled liquid
dispensing reservoir 580.
As the liquid is replenished in the reservoir 580 from the bottle 579, the
pervaporative matrix 581
surrounding the reservoir chills the liquid prior to being dispensed from one
or more port valves
583. Alternately, one valve can be used for chilled water and one valve can be
used for hot water.
Pervaporative cooling reduces or eliminates the need for an electrical
chilling mechanism such as a
refrigerant compressor. The plastic housing 582 of the reservoir 580 provides
mechanical support
for the pervaporative matrix 581.
In one embodiment, a pervaporative container may comprise one or more straps
so as to
allow the container to be carried on the body. The container may be worn in
any manner, including
but not limited to, being strapped around the torso or a limb or worn in the
form of a backpaclc or
purse. Potential market applications of this technology fit within the scope
of pervaporatively
cooled sports equipment to optimize athlete performance. Figure 13 shows one
embodiment of a
pervaporatively cooled hydration pack 585. The pack comprises a body 588
comprising
pervaporative matrix 591, which in one embodiment is ribbed to provide greater
pervaporative
surface area. The pack is filled with pervaporative fluid through the fill
drain port 587 and can be
carried by means of one or more straps 586. A drinking tube 589 is in fluid
communication with
the interior of the pack is preferably included to allow the carrier to
conveniently drink the fluid.
-14-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
Pervaporatively-cooled hydration pack, including backpack-type
wearable/carryable containers
may be constructed by forming at least a portion of the bladder component of
any of a variety of
hydration packs as are known in the art and are available commercially (e.g.
CamelBak, Petaluma,
CA; HydraPalc, Berlceley, CA) with a pervaporative material such as by heat
sealing, adhesive
andlor stitching techniques.
In one embodiment, a hydration pack 585 comprises a laminate of at least two
layers: (1)
an outer layer 591comprising a pleated or nonpleated pervaporative layer
comprising a
hydrophobic pervaporative laminate; (2) a support layer 593 including a,
preferably, thin support
layer for the pervaporative layer 591 which acts as a liquid barrier. In some
embodiments, such as
for extended operations, water is fed by gravity or by wicking from a liquid
holding reservoir 588
down into the interstices 592 formed between the pervaporative matrix 591 and
the middle layer
593.
An optional third layer preferably comprises insulation and directly touches
the skin (or is
in thermal contact with the skin through clothing) and provides a thermal
barrier between the user
and the hydration pack. This layer may be continuous or have a bumped pattern
(e.g. fluted,
pleated, scalloped) to allow the passage of air between the user and the
hydration pack. An
optional third or fourth layer comprises a desiccant or absorbent material.
Figure 14 shows a pervaporatively-cooled drinking pouch 594 in an optional
webbed strap-
on holder 599. The holder may comprise materials other than webbing; it need
only be able to hold
the pouch and preferably not substantially interfere with pervaporation. A
pervaporative pouch
such as this can, for example, be strapped into a belt loop using securing
straps 600 or attached to
the side of an existing belt. The webbing 601 allows a free path for the
porous pouch matrix 595 to
pervaporate. The pouch 594, in one preferred embodiment, comprises three main
parts: 1) the
pervaporative body 595 comprising a pervaporative matrix, 2) a fill port 596
and 3) a
pervaporatively chilled drinlcing tube 597, 602 and a valued spout 598. The
body 595 may be made
substantially entirely or in part of pervaporative matrix. The pervaporatively
cooled drinleing tube
602, in one embodiment, comprises an outer pervaporative hydrophobic layer
604, which
substantially prevents or reduces liquid leakage and pervaporative cooling,
and an internal liquid
wettable layer 605. Once liquid is introduced through the center 603 of this
layered construct 602
the liquid penetrates into the hydrophilic material producing a liquid lock
605 which substantially
substantially prevents or reduces air form entering the center of the tube 603
through the porous
matrix 604. The liquid trapped in the hydrophilic matrix 605 is free to
pervaporate through the
outer hydrophobic matrix 604. This combination of hydrophilic 605 and
hydrophobic 604 matrices
in a tube format 602 provides the benefit of delivering chilled drinking water
directly from the
internal tube volume 603 when placed in combination with a pervaporatively
cooled reservoir 594
-15-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
or with a non-pervaporatively cooled reservoir, in an alternate embodiment.
One simple method of
manufacture of such a device 602 is to plasma treat the center of a
hydrophobic porous PTFE tube.
Alternatively, the drinking tube may be made of non-pervaporative materials.
In some embodiments, the pervaporative container is in the form of a
lightweight liquid
s filled (preferably water-filled) pervaporative cooling garment that serves
as a simple personal
microclimate cooling system to relieve heat stress in individuals wearing
protective clothing, in
normal or elevated ambient temperature conditions. This type of cooling
garment . can be
manufactured into protective clothing, such as chemically or biologically
protective suits or Nomex
fire suits, to form a part of such clothing or it may be worn in conjunction
with such protective
garments. Alternatively, the garment can be worn under a layer of body armor.
A cooling garment according to preferred embodiments, can be used for many
purposes,
including, but not limited to, fire and rescue personnel, military personnel,
and hazardous
(chemical and/or biological) materials workers, as well as for sports
enthusiasts who could increase
their endurance by releasing more heat from their bodies during sporting
activities. Pervaporative
garments can also lower the amount of infrared radiation given off by the
wearer. In preferred
embodiments, water or a combination of water and ethanol (preferably about 5
to 15%) as a
pervaporative coolant source is used to allow the device to be substantially
non-hazardous and
provide an additional functionality such as an extra pouch for pervaporatively-
chilled drinking
water for the wearer. Chilled drinking water can also lessen the heat load on
an individual wearing
a protective suit or clothing or engaging in sporting activities, especially
those requiring endurance.
Although non-hazardous and/or potable coolants are preferred, any liquid
capable of providing
pervaporative cooling functionality may be utilized, including methanol,
isopropanol, non-potable
water, and other liquids and solvents. Preferably, the coolant chosen is
compatible with the
materials) it contacts within the garment.
In one preferred embodiment, a pervaporative cooling garment is in the form of
a jacket or
vest. The pervaporative garment may be worn alone or it may be worn
incorporated or integrated
into another article of clothing or garment, such as a protective suit. When
incorporated or
integrated into another garment, the pervaporative garment preferably
comprises the innermost
layers so as to be in close contact (i.e. in thermal contact) with the wearer.
The pervaporative
cooling garment may be in direct contact with the skin or it may be in contact
with other clothing
worn by the wearer. In some embodiments, the pervaporative garment comprises a
layer of fabric
or material covering some or all of the portion of the pervaporative matrix
which is directed toward
the inner portion of the garment (i.e. the portion that touches or is in
thermal contact with the
wearer).
-16-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
Although the discussion regarding pervaporative garments is in terms of a vest
or jacket
having a particular configuration, this discussion should not be construed to
limit the disclosed
invention. The principles discussed herein provide for a variety of
pervaporatively cooled
garments, including jackets, hats, belts, pants, leggings, and structures that
encase one or more
parts of the body, such as a wrap for a leg or arm (or a portion thereof), or
the necle.
Figure 15 shows a design of one preferred embodiment of a jacket 608. The
jacket may be
worn alone or the jacket or vest may be hidden under clothing or protective
clothing such as a
chemical suit, Nomex fire suit or body armor.
In a preferred embodiment, the jacket comprises a laminate of three or four
layers:
(1) an optional regenerable or disposable outer layer 610 comprising a
desiccant or an
absorbent material that absorbs the moisture or other fluid resulting from
pervaporation
(2) an outer layer comprising a pervaporative layer 611, preferably pleated,
comprising a
pervaporative laminate, preferably hydrophobic in nature;
(3) a middle layer 613 comprising a thin support layer for the pervaporative
layer that may
also act as a liquid barrier, and in some embodiments, a barrier to
potentially hazardous biological
or chemical materials. For extended operations, in one embodiment, water or
other cooling fluid
can be fed by gravity or by wicking from a liquid holding reservoir 616 such
as on the shoulders of
the j acket down into the interstices 612 formed between the pervaporative
matrix and the middle
layer; and
(4) an inner layer 615 that is in contact with the skin, directly or through a
piece of fabric
or material, such fabric or material being part of the jacket itself and/or a
separate item worn by the
wearer. The inner layer preferably comprises patterned or serpentine regions
formed by a heat
sealing process. In one embodiment, there is provided a simplified jacket
comprising only layers 2
and 4 above.
[0073] The fluid may be placed in the jacket through the port 607 on the
jacket. In a
preferred embodiment, the space 614 between the inner and middle layers forms
an air bladder
which, when inflated via a mouthpiece 618, provides insulation from the liquid
in the cooling
jacket. When the air bladder is collapsed via the terminal mouth piece on the
air hose, the liquid
layer comes into thermal contact with the skin through the stacking of the
middle and inner layers
and this provides on demand cooling. In another embodiment, a segregated water
reservoir in the
jacket is sandwiched between the middle and an inner insulative layers to
provide a cool source of
drinking water. Optionally, the reservoir may comprise a collapsible bag to
prevent water from
sloshing around which may create undue or undesirable noise. In other
embodiments, the garment
may comprise a drinking tube 617 to allow the wearer to consume the liquid in
the jacket.
-17-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
If a pervaporative garment not having an outer desiccant/absorbent layer is
worn under
clothing, protective or otherwise, it is preferred that such clothing be
permeable to the
pervaporative fluid or that the clothing have vents, pores or other openings
to allow for passage of
the pervaporative fluid.
In some embodiments, the pervaporative garment further comprises a regenerable
or
disposable outer layer comprising a desiccant or an absorbent material that
absorbs the moisture or
other fluid resulting from pervaporation. Suitable desiccants or absorbent
materials for aqueous
pervaporative fluid include, but are not limited to, ammonium sulfate,
molecular sieves and
polyacrylic acid. The outer desiccant/absorbent layer can be discarded
following use or it may be
regenerated such as by application of heat and/or reduced pressure. In a
preferred embodiment, the
absorbent/desiccant layer absorbs at least about 3-4 times its weight in
water. The process of
absorbing water in the layer is preferably endothermic or at least minimally
exothermic. In
preferred embodiments, this layer provides a high degree of absorbency,
dimensional stability
and/or minimizes heating due to water vapor hydration in this layer. As will
be readily understood
by those skilled in the art, a desiccant or absorbent layer may be used in
combination with any
pervaporative container described herein. When a pervaporative garment of this
type is used in
combination with or incorporated or integrated into another garment, there is
no need for pores,
vents, openings and the like in the other garment, although they may be
present if desired. In a
related embodiment, at least one surface of the outer desiccant/absorbant
layer comprises a material
which is chemically resistant and/or substantially impervious to chemical
and/or biological agents
to provide additional protection to the wearer.
The following is brief look at the thermodynamic feasibility of such a
construction.
Assuming an average water vapor pervaporative flux through a porous matrix of
4* 10-6 g*cm-2*s-
1 at 75°F in still air from Table 1 and assuming the water vapor flux
is doubled at 95°F gives 8* 10-
6 g*cm-2*s-las the flux. If the enthalpy of vaporization at 95°F is
2400 J/g, then the energy
dissipation per unit area of the matrix is 1.9*10-2 Watts*cm-2. In order to
achieve a power
dissipation of 25 Watts approximately 1500 cm2 or 1.5 ft2 of available matrix
surface area needs to
be used in the construction the hydration pack. Use of a pleated membrane or a
pleated porous
sintered matrix to'enhance the pervaporative cooling power, since
pervaporative cooling power is a
direct function of the porous surface area of the jacket. In order to cool for
4 hours at this rate
approximately 150 mL of water will be spent in the process. Thereby a little
under 0.5 lbs. of
water will be used in the process. It would seem reasonable that a water
filled jacket like this may
be made to weigh approximately 3 lbs or less.
-18-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
As will be understood by those skilled in the art, the various layer
configurations in the
embodiments of jacket, pouch, and backpaclc discussed above are
interchangeable, as they are
interchangeable with other container configurations disclosed herein.
A preferred orientation of multilayer or multifunctional matrix according to
one
embodiment is where the higher liquid intrusion matrix surface faces the
inside of the garnZent and
the matrix supporting backing is exposed to the air outside of the garment.
Thicknesses for these
porous materials in a preferred embodiment are in the range from about 1/128"
(0.2 mm) to 1/8"
(3.2 rnm). In one embodiment, layered composites of membranes and
pervaporative matrices are
selected to provide both a high liquid intrusion pressure at the liquid/matrix
interface using a thin
highly hydrophobic material with a small pore size such as expanded
polytetrafluoroethylene
(ePTFE) laminated in between thicker highly porous supports such as sintered
polyethylene, which
allow for a substantial pervaporative flux.
Methods of Manufacture
Several processes are available for the manufacture of pervaporative
containers or the
pervaporative matrix portion of a pervaporative garment including, but not
limited to, sintering
sub-millimeter size plastic beads in a mold cavity to directly form the
pervaporation wall; thermal
or ultrasonic lamination or welding of one or more pieces of pervaporative
matrix together or to a
suitable frame; insert molding whereby one or more sheets or a cylinder of the
porous matrix is
inserted into the cavity of a mold and a thermoplastic polymer is injection
molded directly around
the inserts) to form the desired composite having porous matrix portions; heat
sealing; attaching
components using adhesives; and/or stitching techniques may also be used to
assemble all or part
of a pervaporative garment or container.
Multilayered constructs containing two or more layers of porous material may
be used to
obtain a mechanical and physically superior matrix. For instance, combining a
sintered
macroporous matrix of polyethylene with a thin layer of expanded PTFE on the
liquid side of a
container increases the hydrophobicity and liquid breakthrough pressure of
water from 5 psi to over
psi, yet the layered matrix still maintains a similar pervaporative flux to
that obtained using
porous polyethylene by itself.
Figures lA and 25B show the construction of a preferred embodiment of a
pervaporative
30 container with a wall portion 501 comprising pervaporative matrix. The wall
501 is fixed to the
top 500 and bottom 502 portions of the container by a process such as by
insert molding, thermal or
ultrasonic welding, adhesive joining; or other suitable means. Insert
injection molding may also be
used to attach the matrix to the other portions of the container. The top of
the bottle 500 illustrated
in this example allows for a threaded fit and can be used with a vented bottle
cap. The top 500 and
-19-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
bottom 502 portions of the container may be made by any suitable method,
including injection
molding, vacuum forming, and the like.
Figures 3A and 3B show a ribbed configuration for a thin pervaporative matrix
507 for
which additional structural support 508 is desired. The ribs 508 give the
container wall both
structural integrity and a ridged surface for a firmer hand grasp on the
container. The ribs 508 can
be placed on the outside, inside and/or one or more sides of the pervaporative
matrix. The ribs 508
are preferably injection molded by insert molding onto the pervaporative
matrix 507.
Alternatively, ribs 508 can be sealed to a porous matrix 507 or porous matrix
507 can be sealed to a
ribbed container shell 508 by ultrasonic, thermal or adhesive means, among
others.
Figures 3C and 3D demonstrate a sports version of this container which allows
the
container to be fixed securely in a holding bracket by the bottle neck 512.
The mouth of the bottle
511 allows for the use of various closures, including a snap lid and threaded
closure.
Figure 4 shows a thermally insulating, hydrophobic open cell foam layer 518
that allows
water vapor to move through the open cell structure, but impedes the
connective and radiative
heating of the container contents. Table 1 demonstrates that the thermally
insulating matrix
reduces the liquid loss rate while maintaining a substantial pervaporative
cool. In a preferred
embodiment, the insulating foam 518 is placed or taken off of the bottle as an
elastic sleeve.
Increases in pervaporative cooling efficiency can be achieved by increasing
the surface
area of the matrix in contact with the liquid by pleating the matrix. Figure 5
shows a pleated
container body 520 that allows for a greater pervaporative surface area to be
exposed per
contained liquid volume. This configuration allows for a decrease in the time
taken to
pervaporatively cool the container volume. A container having this
configuration can be made by
insert molding or by potting both ends with adhesive to a bottom 521 and top
519 container
elements or with molten plastic.
Figures 6A and 6B demonstrate a rotating sleeve 525 on the outside of the
matrix body
523. As the outer sleeve 525 rotates past the inner sleeve 524, a set of
vertical slits 527 is formed,
which open and close to allow variable exposure to the pervaporative matrix
523, thereby reducing
the vapor flux rate but still maintaining an adequate pervaporative cool. A
vertical slip sleeve,
whose slits are adjusted vertically instead of by rotation, may also be used
in a configuration of this
type. The inner and outer sleeves 524 and 525 are made of a substantially
nonporous material such
as plastic or metal that does not allow water vapor to pass. Figure 6B
illustrates the annular sleeve
which helps to maintain a very thin gap 530 between the porous matrix 523 and
the inner
stationary sleeve 524. This gap 530 is useful as a shield to substantially
prevent or reduce direct
conductive and radiative heat transfer to the porous matrix 523 of the main
container body. In
addition, this spacing 530 allows for vapor flux out of this annular region
527. The sleeves 524
-20-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
and 525 may also be used over a pleated pervaporative surface 520 such as
shown in Figure 5.
Again, the sleeves 524 and 525 can be placed on the outside of the container
by sliding them onto
the outside of the container. The inner sleeve 524 may be attached or sealed
in place.
Figures 7 and 8 show a jacketed embodiments of pervaporative containers. As
shown in
Figure 7 the cooling jacket may be made of a detachable sleeve consisting of
an outer hydrophobic
pervaporative layer 535 and an inner porous liquid holding or absorbing layer
534. In the
embodiment of Figure 8, the outside jacket 541 is filled through special ports
543 with water or
other volatile fluids 541 and the contents of the inner liquid container 540
are maintained at a sub
ambient temperature. One advantage to this configuration lies in that a
carbonated beverage can be
stored in this container without losing carbonation. In addition, a liquid
with a low propensity to
pervaporate, such as a liquid high in electrolytes or sugar can be placed in
the inner chamber 540 of
the container while distilled water or other easily pervaporated liquid 541 is
placed in the outside
chamber to obtain an adequate temperature drop.
Another embodiment for a sponge 533 or jacketed 542 pervaporative
configuration as
shown in Figures 7 and 8 is for the use of an oleophobic pervaporative matrix
which retains organic
liquids such as alcohol. In such a configuration the outside jacket 533, 542
are be filled with
ethanol, and serves as the pervaporative coolant 534, 541.
Figure 10 illustrates a pervaporatively-cooled drinking cup similar in
function to Figures
lA, 1B, 2, 3A and 3B. Assembly may be performed wrapping a planar
pervaporative matrix
around or pushing the matrix 555 over the cup body 556 as a cylinder and
attaching the material by
adhesive, potting, thermal welding or ultrasonic welding. Insert molding may
be used to directly
attach the material into the bottle frame and walls.
Figures 1 lA, 11B and 11C show a configuration for producing a cooling
container for the
storage of beverages and foodstuffs 568. In this configuration the lid 558,
the cooler walls 559 and
564 or preferably both the lid 572 and the walls 559 and 564 contain a liquid-
filled pervaporative
jacket 566 and 578. The container may further comprise one or more layers of
insulation. The
container can be used to store foods and beverages 568 at sub-ambient
temperatures for several
days at a time. In one embodiment, assembly of the cooler body 563 is
performed by placing the
planar pervaporative matrix 566 inside the case body 564 and attaching the
material by adhesive,
potting, thermal welding or ultrasonic welding. Alternatively, insert molding
is used to directly
attach the material 566 into the frame and walls 564.
One proposed solution for heat stress relief is based on the idea of
pervaporation. A
chilled hydration pack or other cooling garment utilizing a pervaporative
cooling mechanism, such
as this would fmd applications not only in the military as a personal cooling
system but also for a
sports enthusiast who could increase their endurance by releasing more heat
from their bodies
-21-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
during a race. Using water or a combination of water and ethanol (preferably
about 5 to 15%) as a
pervaporative coolant source allows such a device to be non-hazardous and
provide an additional
functionality such as an extra pouch for pervaporatively-chilled drinking
water. Chilled drinking
water would also lessen the heat load on an individual wearing a protective
suit or clothing.
The pervaporative hydration pack described herein will follow a design similar
to the
pervaporative beverage cooling bottles, which were previously designed. A
comparison of the
cooling efficiency using pervaporative cooling (2400 J/g) versus the heat of
fusion (335 J/g) plus
the warming of the liquid (105 J/g) to room temperature (77 °F) reveals
that pervaporative cooling
is five times more efficient on a mass basis than using ice. Tables 1, 2, and
3 provide data to show
what happens to the pervaporative cooling bottles under different conditions
of wind speed and
matrix composition at room temperature and a relative humidity of 30 to 40%.
Figure 14 shows one embodiment of a pervaporatively-cooled drinking pouch 594,
shown
in an optional webbed strap-on holder 599. In one embodiment, a strap is
attached directly to the
body 595 and no holder, webbed or otherwise, is used. A pervaporative pouch
such as this can be
worn over the shoulder, strapped into a belt loop or another portion of the
body using securing
straps 600 or similar attachment devices or attached to the side of an
existing belt. In one
embodiment, the webbing 601 issewn from nylon netting and the straps 600 are
Velcro, a
Nylon/Velcro Composite, or other natural or synthetic material. The various
portions of the pouch
porous matrix 595 can be assembled by thermal sealing, thermal welding,
ultrasonic welding or
adhesive lamination, or other methods discussed herein in relation to other
containers. In one
embodiment, a pervaporatively cooled drinking tube 602 comprises an outer
pervaporative
hydrophobic layer 604, which substantially substantially prevents or reduces
liquid leakage and
pervaporative cooling, and an internal liquid wettable layer 605. Once liquid
is introduced through
the center 603 of this layered construct 602 the liquid penetrates into the
hydrophilic material
producing a liquid lock 605 which prevents or substantially reduces the amount
of air entering the
center of the tube 603 through the porous matrix 604. The liquid trapped in
the hydrophilic matrix
605 is free to pervaporate through the outer hydrophobic matrix 604. This
combination of
hydrophilic 605 and hydrophobic 604 matrices in a tube format 602 provides the
benefit of
delivering chilled drinking water directly from the internal tube volume 603.
As noted above, this
tube may be used with a pervaporative or non-pervaporative pouch, or it may be
used with other
containers, both pervaporative and non-pervaporative. One method of
manufacture of a
pervaporative tube 602 is to plasma treat the center of a hydrophobic porous
PTFE tube rendering
the inner portion of the tube 605 hydrophilic.
Operation of a Pervaporatively Cooled Device
-22-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
Preferred designs for pervaporative cooling devices are simple and can be
operated under
ambient conditions to cool and/or maintain the coolness of fluid or solid
contents of the container
without the weight and portability limitations associated with mechanical
pumping or the need for
the application of an external mechanical vacuum to increase the pervaporative
cooling rate. In a
preferred embodiment, the radial dimensions of a container of the type in
Figure 1A are large
enough such that connective mixing by natural convection of liquid contents is
obtained. This is
because, in some cases, the thermal conductivity of the liquid alone may not
be high enough to
effectively maintain a generally uniform temperature distribution throughout
the container. When
the liquid at the inner walls of the container are cooled, this reduces the
density of the liquid at the
inner walls as compared to that in the center. Because of this density
difference, the cooler liquid
flows down the inside walls of the container to the bottom of the container
where it is entrained
back up into a circulatory pattern within the middle of the container in a
process called natural
convection, as opposed to forced convection. When the cooling rate is high
enough, connective
eddies break off from the side of the container and enhance the mixing rate.
These phenomena and their occurrence can be predicted using a combination of
calculated
dimensionless parameters, namely the Grashof Number (parameter for fluid
buoyancy in a
gravitational field) and the Prandlt Number (parameter that describes the
thermal and capacitive
nature of the liquid). The combination of these two parameters leads to the
calculation of the
Nusslet Number (an overall heat transfer parameter). Natural convection within
a pervaporative
container will enhance the cooling efficiency and the cooling rate of the
device by allowing for
connective heat transfer through the buoyant fluid in lieu of thermal
conduction through the same
liquid medium.
Table 1 presents endpoint pervaporative cooling data at a relative air
humidity of 30% to
41% and different ambient air velocities and the effect of a porous insulative
matrix. Tables 2 and
3 present endpoint water pervaporative cooling data at different relative
humidities and in the
shade (Table 2) or in the presence of direct solar irradiation (Table 3). The
pervaporative materials
are PTFE (polytetrafluoroethylene) or sintered UHMWPE (ultra high molecular
weight
polyethylene). X-7744, X-6919, and 402HP are all UHMWPE materials of various
porosity, pore
size and thickness as outlined in the tables.
Table 1
Matrix PorositPore ThicknesLiquiFlux Cool Cool Cool
at at
Material y size s (mm) d (g (F) 2 mph 5 mph
(um) Loss cm2/s)x106 (F) (F)
(%/hr
Control None None 1.5 0.0 0.0 0.0 0.0 0.0
1
(PE)
-23-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
Control None None 1.5 0.0 0.0 0.0 0.0 0.0
2
(PE)
PVDF 75% 0.5 0.1 0.4- 1.9-7.6 12.7 14.3 14.8
3.0
TJIIMWP 35-50%7 0.6 0.3- 1.2-6.6 10.6 12.6 13.0
E 1.0
PVDF 75% 13.5 5.1 0.4- 2.0-6.5 12.1 11.5 10.7
w/foam 1.9
insulation
UHMWP 35-50%20 5.6 0.3- 2.2-5.2 9.8 10.5 11.2
E w/foam 0.8
insulation
Table 2
Shade I RH 38.6%
175F ~ Temperature
Pervaporative
Cool
Matrix Material
Porosity Pore
Size (pm) Thickness
(mm) (F) (F)
Control #1 (PE) None None 1.5 72.2
Control #2 (PE) None None 1.5 71.9 -
X-7744 35 to 7 0.6 63.6 8.4
50%
X6919 35 to < 15 1.6 65.1 6.9
50%
402HP 40 to 40 0.6 63.4 8.7
45%
402HP 40 to 40 1.3 64.7 7.3
45%
Supported PTFE 75% > 50 0.3 63.4 8.7
Table 3
Full Sun I RH
41.0% I77F (Shaded
Sensor) Temperature
Pervaporative
Cool
Matrix Material
Porosity Pore
Size (pm) Thickness
(mm) (F) (F)
Control #1 PE None None 1.5 93.6
Control #2 PE None None 1.5 93.3
X-7744 35 to 7 0.6 71.3 22.2
50%
X6919 35 to < 15 1.6 73.1 20.4
50%
402HP 40 to 40 0.6 73.1 20.4
45%
402HP 40 to 40 1.3 73.7 19.7
45%
Supported PTFE 75% > 50 0.3 73.1 20.4
Table 1 sets forth endpoint water pervaporative cooling data at different
ambient air
velocities and the effect of a 1/16 " open-cell porous urethane insulative
matrix at a relative
humidity of 30%. Tables 2 and 3 set forth endpoint water pervaporative cooling
data at different
relative humidities and in the dark or in the presence of direct solar
irradiation. The pervaporative
materials in all three tables are PTFE or sintered UHMWPE (ultra high
molecular weight
polyethylene).
Additional enhancements in cooling efficiency may be seen with the container
as the
outside relative humidity drops and if the container is placed in direct
sunlight. The lower external
humidity increases the vapor concentration gradient, and the externally
applied heat raises the
liquid temperature and vapor pressure, which lead to a rise in the
pervaporative flux. Depending
on ambient conditions, the geometry and materials selection of the container,
this process can
-24-
CA 02488438 2004-12-02
WO 03/102480 PCT/US03/17544
maintain a sub-ambient cool in the container of 22°F below ambient
temperature. See Table 3.
The time to attain this cooled temperature for a liquid volume of 700 ml is
around 2 hours as
demonstrated in Figure 9 for a variety of pervaporative matrices and
combinations thereof.
One preferred embodiment of evaporative cooling container includes a single or
combined
porous matrix having a pervaporative layer thiclcness of about 0.025 mm (0.001
in.) to 10 mm
(0.394 in.). Additionally, to increase the efficiency of the pervaporative
process, the matrix
preferably has qualities such that it is minimally thermally conducting. It is
preferable that the
matrix does not substantially impede vapor diffusion, such that, in one
embodiment, a pore size
above about 100 nm is preferred. Preferred surface porosities of the matrix
are between about 15
and 90% including about 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80,
and 85%. A porous
matrix with a low thermal conductivity, such as a porous perfluorinated
Styrofoam, an expanded
porous matrix, or an open cell porous matrix made from hollow fused particles,
can help to
substantially prevent or reduce undue heat transfer from the surroundings into
the container.
The various methods and techniques described above provide some of the
numerous ways
to carry out the invention. Of course, it is to be understood that not
necessarily all objectives or
advantages described may be achieved in accordance with any particular
embodiment described
herein or with any other single embodiment. Thus, for example, those skilled
in the art will
recognize that the methods may be performed and/or the articles made in a
manner that achieves or
optimizes one advantage or group of advantages as taught herein without
necessarily achieving
other objectives or advantages as may be taught or suggested herein.
Furthermore, the skilled artisan will recognize the interchangeability of
various features
from different embodiments. Similarly, the various features and steps
discussed above, as well as
other known equivalents for each such feature or step, can be mixed and
matched by one of
ordinary skill in this art to perform methods in accordance with principles
described herein.
Although the invention has been disclosed in the context of certain
embodiments and
examples, it will be understood by those skilled in the art that the invention
extends beyond the
specifically disclosed embodiments to other alternative embodiments and/or
uses and obvious
modifications and equivalents thereof.
-25-