Note: Descriptions are shown in the official language in which they were submitted.
CA 02498904 2005-03-11
WO 2004/033594 PCT/US2003/033321
-1-
ENHANCED LUBE OIL YIELD BY LOW HYDROGEN
PRESSURE CATALYTIC DEWAXING OF PARAFFIN WAX
FIELD OF THE INVENTION
[0001] This invention relates to a process for catalytically dewaxing paraffin
containing hydrocarbons. More particularly, this invention relates to the
production of lube base oils having a pre-determined or pre-selected pour
point
by catalytically dewaxing a paraffin containing feed at low hydrogen partial
pressures.
BACKGROUND OF THE INVENTION
[0002] The production of lube base oils by hydroprocessing paraffin contain-
ing feeds is well known, e.g., hydroisomerization or hydrocracking of the feed
to
produce lube base oils. These processes are catalytic and are usually carried
out
at relatively high hydrogen pressures, e.g., > 3549 kPa (500 psig) hydrogen
partial pressures. Catalytic dewaxing is a form of hydroprocessing and
involves
paraffin isomerization and some hydrocracking in the production of lube base
oils.
[0003] Hydrogen has always been used in the hydroprocessing, i.e.,
isomerization, cracking, dewaxing, of paraffins to produce lube base oils.
Hydrogen is believed to be important for promoting extended catalyst life by
e.g., reductive coke removal; see, for example U.S. Patent 4,872,968.
Catalytic
dewaxing is, essentially, the conversion of n-paraffins to branched paraffins.
That is, the conversion of waxy molecules to molecules exhibiting better flow
properties, particularly at lower temperatures. The hydrogen partial pressures
usually employed in catalytic dewaxing processes range from about 1480 kPa
(200 psig) to about 6996 kPa (1000 psig) or more, e.g., see U.S. Patent
CA 02498904 2005-03-11
WO 2004/033594 PCT/US2003/033321
-2-
5,614,079 with hydrogen pressures in the higher end of this range being
preferred - for reasons of catalyst life.
[0004] U.S. Patent 5,362,378 discloses hydrogen partial pressures of 597-
1599 kPa (72-2305 psig) for use with large pore zeolite beta. This patent does
not mention catalyst life or TTR, i.e., temperature increase required,
necessary
for maintaining product specifications, such as pour point or cloud point.
Large
pore zeolite beta is typically not classified as a dewaxing catalyst, but as
an
isomerization catalyst, and products produced utilizing such catalysts in
accordance with U.S. Patent 5,362,378 would need to be dewaxed in order to
achieve the low pour and cloud points obtained from the instant process.
[0005] We have now surprisingly found that a particular combination of
features allows for conducting catalytic dewaxing at low hydrogen pressures of
less than 3549 kPa (500 psig) and conditions that are selective to
hydroisomerization, with little or no hydrocracking, good lube yield, and
without
sacrificing catalyst life, the product having low pour and cloud points.
SUMMARY OF THE INVENTION
[0006] According to the present invention, a feed containing at least 80 wt%
n-paraffins is catalytically dewaxed in the presence of a catalyst comprising
a
molecular sieve with a one dimensional pore structure having an average
diameter of 0.50 nm to 0.65 nm, and a metal dehydrogenation component, at
hydrogen partial pressures of less than 3549 kPa (500 psig). The difference
between the maximum diameter and the minimum diameter of the pores is
preferably <_ 0.05 nm. By using these process conditions, the catalyst
deactivation rate as defined hereafter is maintained at a low level of less
than
16.7 K (30°F)/year.
CA 02498904 2005-03-11
WO 2004/033594 PCT/US2003/033321
-3-
[0007] The molecular sieve is, for example, ZSM-23, ZSM-35, ZSM-4~,
ZSM-22, SSZ-32, zeolite beta, mordenite and rare earth ion exchanged
ferrierite.
[0008] The dehydrogenation component is usually a metal component, prefer-
ably manganese, tungsten, vanadium, zinc, chromium, molybdenum, rhenium,
Group VIII metals such as nickel, cobalt, or noble metals such as platinum and
palladium.
[0009] Catalyst deactivation rate is reported herein as TIR; that is "tempera-
ture increase required" for maintaining a pre-determined pour point (of prefer-
ably less than -12°C) or cloud point. The catalyst deactivation rate is
determined
by the difference in the initial temperature and the temperature at the end of
a
specified period of time, sufficient to maintain the pour point or cloud point
target.
DESCRIPTION OF THE DRAWINGS
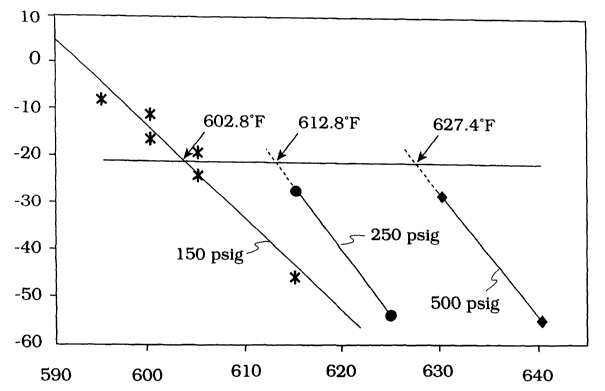
[0010] Figure 1 is a plot of pour point, °C (ordinate) against
temperature, °C
(°F) (abscissa) showing that catalytic activity increases with
decreasing hydrogen
pressure.
[0011] Figure 2 is a plot of % conversion (ordinate) against pour point,
°C
(abscissa) showing that selectivity to isomerization increases with decreasing
hydrogen pressure.
[0012] Figure 3 is a plot of average reactor temperature, °C
(°F) (ordinate)
against days on stream (abscissa) and shows a deactivation rate by regression
CA 02498904 2005-03-11
WO 2004/033594 PCT/US2003/033321
-4-
when producing a lube base oil of -21°C pour point at a hydrogen
partial
pressure of 1135.5 kPa (150 psig).
[0013] Figure 4 is a plot of temperature, °C (ordinate) against days on
stream
(abscissa) at 1825 kPa (250 psig) hydrogen pressure to meet a diesel cloud
point
of -15°C.
[0014] Figure 5 is a plot similar to Figure 4 to meet a -21°C wide cut
lube
base oil pour point.
[0015] Figure 6 is a plot of reactor temperature, °C (°F)
(ordinate) against
days on stream (abscissa) to meet a -21°C pour point for a 371-
510°C
(700-950°F) isomerate.
[0016] Figure 7 is a plot of reactor temperature, °C (°F)
(ordinate) against
days on stream (abscissa) to meet a +8°C cloud point for a
510°C+ (950°F+)
isomerate.
[0017] For the particular set of features described herein, reducing hydrogen
partial pressure results in increased catalyst activity, and increased
isomerization
yield. That is, the increase in activity is almost entirely an increase in
isomerization activity, and little hydrocracking occurs. Nevertheless, while
decreasing hydrogen partial pressure would normally result in decreased
catalyst
life, the features of this invention show that catalyst life is not
sacrificed.
[0018] For purposes of this invention, the pour point is determined by ASTM
D-5950, the cloud point is determined by ASTM D-5773 and the pore
parameters of the molecular sieve are determined by X-ray diffraction.
CA 02498904 2005-03-11
WO 2004/033594 PCT/US2003/033321
DETAILED DESCRIPTION OF THE INVENTION
[0019] The feed that is employed in this invention is a paraffin containing
feed that contains at least 80 wt% n-paraffins, more preferably greater than
90 wt% n-paraffins, still more preferably greater than 95 wt% n-paraffins and
still more preferably 98 wt% n-paraffins. The feed generally boils in the
range
221°C+ (430°F+), preferably 232°C+ (450°F+), more
preferably 232-649°C
(450-1200°F) (minor amounts, e.g., less than 10% of 649°C+
(1200°F+) material
may be present). Preferably, the feed contains at least 90 wt% n-paraffins and
boils in the range above 221 °C (430°F).
[0020] The feed is preferably low in unsaturates, that is, low in both
aromatics and olefins. Preferably, the unsaturates level is less than 10 wt%,
preferably less than 5 wt%, more preferably less than 2 wt%. Also, the feed is
relatively low in nitrogen and sulfur, e.g., less than 200 ppm of each,
preferably,
less than 100 ppm of each, more preferably less than 50 wppm of each. Where a
Fischer-Tropsch derived feed is employed, there is no need to pre-sulfide the
catalyst, and indeed, pre-sulfiding should be avoided.
[0021] Most preferably, the feed is the product of a Fischer-Tropsch reaction
that produces essentially n-paraffins, and still more preferably the Fischer-
Tropsch process is conducted with a non-shifting catalyst, e.g., cobalt or
ruthenium, preferably a cobalt containing catalyst. The advantages of using a
Fischer-Tropsch product as a feedstock reside in the high n-paraffin content
and
low heteroatom content of this feed.
[0022] The catalyst employed in the catalytic dewaxing step comprises a
molecular sieve with one dimensional pore structure and a metal dehydrogena-
tion component having an average diameter of 0.50 nm to 0.65 nm, and,
CA 02498904 2005-03-11
WO 2004/033594 PCT/US2003/033321
-6-
preferably, the difference between the maximum diameter and the minimum
diameter is <_0.05 nm. This includes molecular sieves such as ZSM-23, ZSM-35,
ZSM-22, SSZ-32, zeolite beta, mordenite and rare earth ion exchanged
ferrierite.
Preferably, a ZSM-48 catalyst is used containing a metal dehydrogenation
functionality, preferably supplied by the presence of platinum or palladium or
both platinum and palladium, preferably platinum. Other zeolites structurally
equivalent to ZSM 48, such as EU-2, EU-lland ZBM-30 may also be employed.
ZSM-48 is particularly preferred. The use of catalysts based on these
molecular
sieves makes it possible to obtain low pour point lubricants in high yield at
low
pressure (less than 3549 kPa, 500 psig), and the process is characterized by
low
catalyst deactivation rates of less than 16.7 K (30°F)/ year.
[0023] The molecular sieves are well known in the art. They are for example
described in J. Schlenker, et al., Zeolites 1985, vol. 5, November, 355-358.
[0024] ZSM-48 is characterized by the X-ray diffraction pattern shown in
Table 1 below. The material is further characterized by the fact that it
exhibits a
single line within the range of 11.8~0.2 Angstrom units. The presence of a
single line at the indicated spacing structurally distinguishes ZSM-48 from
closely related materials such as ZSM-12 (described in U.S. Patent No.
3,832,449) which has two lines, i.e., a doublet, at 11.8~0.2 Angstrom units,
and
high silica ZSM-12 (described in U.S. Patent No. 4,104,294) which also has a
doublet at the indicated spacing.
CA 02498904 2005-03-11
WO 2004/033594 PCT/US2003/033321
_7_
Table 1
Characteristic lines of ZSM-48 (calcined, Na Exchanged Form)
d(A) Relative Intensity
(I/Io)
11. 80.2 S
10.20.2 W-M
7.20.15 W
4.20.08 VS
3.90.08 VS
3.60.06 W
3.10.05 W
2.850.05 W
[0025] The values were determined by a standard technique, i.e., radiation
was K-alpha doublet of copper, and diffractometer equipped with a
scintillation
counter. The peak heights, I, and the positions as a function of two times
theta,
where theta is the Bragg angle, were determined by a compactor. From these the
relative intensities, 100 I/Io, where Io is the intensity of the strongest
line or
peak, arid d(obs.), the interplanar spacing in A corresponding to the recorded
lines, were calculated. Table 1 gives the intensities in terms of the symbols
W=weak, S=strong, VS=very strong, M=medium, and W-M=weak to medium
(depending on the cationic form). Ion exchange of the sodium ion with other
cations reveals substantially the same pattern with some minor shifts in
interplanar spacing and variation in relative intensity. Other minor
variations
can occur depending on the silicon to aluminum ratio of the particular sample,
as
well as any subsequent thermal treatment.
[0026] ZSM-48 and methods for its preparation are described in U.S. Patent
Nos. 4,375,573; 4,397,827; 4,448,675; 4,423,021; and 5,075,269. The method
CA 02498904 2005-03-11
WO 2004/033594 PCT/US2003/033321
_g_
of preparation described in U.S. Patent No. 5,075,269 is particularly
preferred.
This method is for preparing a catalyst particularly suitable for the
catalytic
dewaxing process.
[0027] The dehydrogenation component is preferably a noble metal, most
often palladium or platinum, or both platinum and palladium. Platinum is the
most preferred. The dehydrogenation component is most often present in an
amount of 0.01 to 5.0 wt°lo, preferably 0.1 to 1.5 wt%, based on the
catalyst total
weight. Such component can be exchanged into the catalyst or the molecular
sieve, impregnated thereon, or physically intimately admixed therewith. Such
component can be impregnated in or onto the molecular sieve, such as, by
treating the molecular sieve with metal-containing ion. In the case of
platinum,
suitable platinum compounds include chloroplatinic acid, platinous chloride
and
various compounds containing the platinum tetra-ammonia complex.
[0028] The compounds of the metals used to prepare the catalyst according to
the present invention can be divided into compounds in which the metal is
present in the cation of the compound and compounds in which it is present in
the anion of the compound. Both types of compounds which contain the metal
in the ionic state can be used. In the case of platinum, a solution in which
platinum metals are in the form of a canon or cationic complex, e.g.,
Pt(NH3)4C12, is particularly useful.
[0029] Prior to its use, the catalyst is usually at least partially
dehydrated.
This dehydration step is typically conducted to remove water from the
catalyst.
Excess water may cause steaming of the support material, leaching or migration
of the metals, contamination of the products or other undesirable reactions.
Dehydration can be done by heating to a temperature in the range of from
100°C
to 600°C in an inert atmosphere, such as air, nitrogen, etc., and at
atmospheric or
CA 02498904 2005-03-11
WO 2004/033594 PCT/US2003/033321
-9-
subatmospheric pressures for between 1 and 48 hours. Dehydration can also be
performed at lower temperature merely by placing the catalyst in a vacuum. The
molecular sieve catalyst is formed in a wide variety of particle sizes.
Generally
speaking, the particles can be in the form of a powder, a granule, or a molded
product, such as extrudate having a particle size sufficient to pass through a
2
mesh (Tyler) screen and be retained on a 400 mesh (Tyler) screen. In cases
where the catalyst or the molecular sieve is molded, such as by extrusion, it
can
be extruded before drying or dried or partially dried and then extruded:
[0030] It may further be desired to incorporate the molecular sieve with a
matrix material which is resistant to the temperatures and other conditions
employed in the dewaxing process herein. Such matrix materials include active
and inactive materials and synthetic or naturally occurring zeolites as well
as
inorganic materials such as clays, silica and/or metal oxides e.g., alumina.
The
latter may be either naturally occurring or in the form of gelatinous
precipitates,
sols or gels including mixtures of silica and metal oxides. Use of a material
in
conjunction with the molecular sieve, i.e., combined therewith, which is
active,
may enhance the conversion and/or selectivity of the catalyst herein. Inactive
materials suitably serve as diluents to control the amount of conversion in a
given process so that products can be obtained economically and orderly
without
employing other means for controlling the rate of reaction. Frequently,
molecular sieves have been incorporated into naturally occurring clays, e.g.,
bentonite and kaolin. These materials function, in part, as binders for the
catalyst. It is desirable to provide a catalyst having good crush strength
since in
a petroleum refinery the catalyst is often subject to rough handling which
tends
to break the catalyst down into powder-like materials which cause problems in
processing.
CA 02498904 2005-03-11
WO 2004/033594 PCT/US2003/033321
-10-
[0031] Naturally occurring clays which can be composited with molecular
sieve include the montmorillonite and kaolin families which include the
sub-bentonites, and the kaolins commonly known as Dixie, McNamee, Georgia
and Florida clays, or others in which the main mineral constituent is
halloysite,
kaolinite, dickite, nacrite or anauxite. Such clays can be used in the raw
state as
originally mined or initially subjected to calcination, acid treatment or
chemical
modification.
[0032] In addition to the foregoing matrix materials, the molecular sieve can
be composited with a porous matrix material such as silica-alumina, silica-
magnesia, silica-zirconia, silica-thoria, silica-beryllia, silica-titania, as
well as
ternary compositions such as silica-alumina-thoria, silica-alumina-zirconia,
silica-alumina-magnesia and silica-magnesia-zirconia. The matrix can be in the
form of a cogel. Mixtures of these components can also be used. The relative
proportions of the finely divided molecular sieve and the matrix material may
vary widely. Generally the molecular sieve content ranges from 1 to 90 percent
by total weight of the catalyst, and more usually from 2 to 80 percent.
[0033] One of the preferred catalysts according to the present invention is an
alumina bound ZSM-48 molecular sieve, preferably containing 10-90 wt%
zeolite crystals and up to 2 wt% platinum. These preferred catalysts have the
advantages of exhibiting very low deactivation after prolonged use in dewaxing
Fischer Tropsch derived wax.
[0034] In general, reaction conditions for dewaxing may vary widely even
when the hydrogen partial pressures are maintained at low levels. Thus, start
of
run temperatures may vary between 288-343°C (550-650°F). End of
run
conditions can be defined by the nature of the product being produced, for
example, when predetermined color specifications can no longer be met (an
CA 02498904 2005-03-11
WO 2004/033594 PCT/US2003/033321
-11-
indication of catalyst deactivation), or when the predetermined pour point or
cloud point can no longer be obtained, or the selectivity to isomerization is
reduced as evidenced by an increase in methane yield due to hydrocracking. In
general, however, end of run temperatures should be less than 427°C
(800°F),
preferably less than 399°C (750°F), more preferably less than
385°C (725°F).
Reaction temperatures may for instance range from 288°C
(550°F) to about
427°C (800°F). Reaction temperatures ranging from 288 to
385°C provide
particularly good results.
[0035] According to a preferred embodiment of the present invention,
hydrogen partial pressure is maintained as low as reasonably possible without
sacrificing desired catalyst life. Catalyst life may be longer or shorter
depending
on desired results and severity of the dewaxing process, i.e., higher severity
obtained by increasing temperature or decreasing feed velocity, or both.
However, at end of run conditions the catalyst must be either rejuvenated or
replaced, if rejuvenation is no longer possible. In either case the unit must
be
shut down and valuable operating time is lost. Because the process of the
invention gives lower catalyst deactivation rates, the unit can be kept on-
stream
for an extended period of time.
[0036] In the process of the present invention, the catalyst deactivation rate
is
preferably less than 13.9 K (25°F)/year, more preferably less than 11.1
K
(20°F)/year, and still more preferably less than 5.6 K
(10°F)/year. Such catalyst
deactivation rate at dewaxing conditions most often allows the process of the
present invention to be carried out, while still meeting a predetermined pour
point of less than -12°C, for a period of at least six months,
preferably at least
twelve months, more preferably at least 18 months, and still more preferably
for
at least 24 months, or longer, for example, greater than 30 months or greater
than
36 months without catalyst replacement.
CA 02498904 2005-03-11
WO 2004/033594 PCT/US2003/033321
- 12-
[0037] With the preferred process according to the invention, the catalyst's
temperature increase required for meeting a pre-determined pour point of -21
°C
is less than 16.7°C (30°F)/year, more preferably less than
14°C (25°F)/year, still
more preferably less than 11°C (20°F)/year, and still more
preferably less than
5.6°C (10°F)/year.
[0038] Catalyst deactivation is believed to be a result of coke formation on
the surface of the catalyst, the coke covering or blocking access to the
catalytic
metal, as well as blocking the pores of the zeolite. The catalyst may be
regenerated by known methods including hot hydrogen stripping, coke removal
by oxygen treatment or a combination of hydrogen stripping and oxygen
treatment. Briefly, hydrogen stripping can be carried out with hydrogen or a
mixture of hydrogen and an inert gas such as nitrogen, at isomerization
reaction
temperatures for a period of time sufficient to allow the catalyst to regain
at least
about 80%, preferably at least about 90% of its original lined out activity.
Oxygen treatment can be carried out at calcining conditions, e.g., using air
at
temperatures from 500°C to 650°C, again for a period of time
sufficient to allow
the catalyst to regain at least 80%, preferably at least 90% of initial lined
out
activity after subsequent reduction.
[0039] The catalyst life requirements can be satisfied with hydrogen partial
pressures of less than 3549 kPa (500 psig), preferably less than 2859 kPa
(400 psig), more preferably positive hydrogen partial pressures greater than
101.325 kPa (0 psig) and less than 2859 kPa (400 psig), most preferably at
hydrogen partial pressures ranging from 791 - 2859 kPa (100-400 psig), such as
791 - 2515 kPa (100-350 psig), and still more preferably at about 1136 -
2515 kPa (150-350 psig).
CA 02498904 2005-03-11
WO 2004/033594 PCT/US2003/033321
-13-
[0040] In the process of the invention, the feed is contacted under
hydrodewaxing conditions including a hydrogen partial pressure of less than
about 3549 kPa (500 psig) with the catalyst, and the process temperature is
adjusted (increased) whenever a pre-determined pour or cloud point is not met.
A pour point of less than -12°C is preferred, and a pour point of about
-18°C or
less is more preferred.
[0041] At a hydrogen partial pressure of less than 3549 kPa (500 psig) and a
pour point of -12°C or less, a typical deactivation rate is less than
16.7K
(30°F)/year. Preferably at a hydrogen partial pressure of less than
3549 kPa (500
psig) and a pour point of about -18°C or less, a typical deactivation
rate is less
than 16.7 K (30°F)/year. In a most preferred embodiment, at 1136 - 2515
kPa
(150-350 psig) hydrogen partial pressure and a pour point of about -
21°C or less,
a typical deactivation rate is less than 8.3 K (15°F)/year.
[0042] In general, other gases may be present that will not interfere with the
reaction. Such other gases may be nitrogen, methane, or other light
hydrocarbons (that may be produced during the reaction). Total pressure may
range up to 13790 kPa (2000 psi), preferably 690 -13790 kPa (100-2000 psi),
more preferably 1034 - 6895 kPa (150-1000 psi), still more preferably 1034
3447 kPa (150-500 psi). Hydrogen can make up 50-100% by volume of total
gas, preferably 70-100% by volume, more preferably 70-90% by volume. At the
low hydrogen partial pressures recited herein, small amounts of olefins and
aromatics may be produced, and hydrofinishing, at well known conditions, may
be necessary to remove these components.
[0043] The liquid hourly space velocity is generally between about 0.1 and
about 10 volume of feed per volume of catalyst per hour, and preferably is
generally between about 0.5 and 4. The hydrogen to feed ratio is generally
CA 02498904 2005-03-11
WO 2004/033594 PCT/US2003/033321
-14-
between about 17.8 and about 1781, and preferably between about 142.5 and
about 712.5 liter of hydrogen per liter of feed at standard conditions of
101.325 kPa and 15.5°C.
[0044] Alpha Value is an indication of the catalytic cracking activity of the
catalyst compared to a standard catalyst and provides a relative rate constant
(rate of normal hexane conversion per volume of catalyst per unit time). The
value is based on the activity of a silica-alumina cracking catalyst taken as
an
Alpha of 1 (rate constant = 0.016 sec 1). The test for Alpha Value is
described
in U.S. Patent No. 3,354,078 and in the Journal of Catalysis. vol. 4, p. 527
(1965); vol. 6, p. 278 (1966); and vol. 61, 395 (1980). The Alpha Value of the
catalyst used in the present invention prior to metal loading is preferably in
the
range of about 10 to about 50.
[0045] According to a particular embodiment the product of the dewaxing
reaction is further subjected to a hydrorefining reaction. Such reaction
consist of
contacting the catalyst with a hydrofinishing catalyst, containing an active
metal
component sufficient to saturate a desired portion of olefins and aromatics
which
may be present, as is well known in the art.
[0046] The products obtained with the process according to the present
invention exhibit particularly good properties. Also, the process according to
the
invention allows for the production of a low pour point lube product with a
remarkable low yield of low value cracked fuel products while still showing
good activity maintenance.
[0047] The following examples serve to illustrate this invention:
CA 02498904 2005-03-11
WO 2004/033594 PCT/US2003/033321
-15-
EXAMPLE 1
[0048] This example shows the benefits in lube base oil yield obtained as
hydrogen partial pressure is reduced from 3549 - 1136 kPa (500 to 150 psig).
The following unit conditions and process variables were studied with ZSM-48
using a wide cut Fischer-Tropsch feed, i.e., 221 °C+ (430°F+)
feed.
[0049] Catalytic dewaxing was carried out in a downflow reactor simulating a
trickle bed reactor immersed in a sand bath to maintain isothermal reactor
conditions. The reactor contained 80 cc of an unsulfided ZSM-48 catalyst
containing 35% alumina matrix with 0.6 wt% Pt based on total weight diluted
with glass beads. Conversion of a 221 °C+ (430°F+) wax obtained
from a cobalt
slurry catalyzed Fischer-Tropsch process was controlled by temperature.
[0050] The process was operated at temperatures ranging from 304-338°C
(580-640°F) with reactor hydrogen pressures, at the reactor exit of
1136-
3549 kPa (150-500 psig). The hydrogen treat gas rate was 320.6-445 liter of
hydrogen per liter of feed at standard conditions of 101.325 kPa and
15.5°C, and
the liquid hourly space velocity was 1.25v1v/hr.
[0051] The liquid product was fractionated by 15/5 distillation unit and the
following fractions were recovered: IBP/160°C (320°F),
160°C/371°C
(320/700°F), and 371 °C+ (700°F+). The 371 °C+
(700°F+) fraction was analyzed
fox pour and cloud points, and kinematic viscosity and viscosity index; the
160°C/371 °C (320/700°F) fraction was analyzed for cloud
point.
[0052] In Figure 1, lines A, B, and C refer to hydrogen pressures of 1136,
1825 and 3549 kPa (150, 250 and 500 psig). At a pre-determined pour point of
CA 02498904 2005-03-11
WO 2004/033594 PCT/US2003/033321
-16-
-21°C, catalytic activity increases with decreasing operating pressure,
as shown
in Table 2 below.
Table 2
Operating H2 Pressure, psig/kPa Temperature required for -21°C
P.P.
500/3549 (comparative) 627.4
250/1825 612.8
150/1136 602.8
[0053] The invention is based, inter alia, on the finding that the kinetics of
the
dewaxing process described herein is negative second order in hydrogen, so
that
the yield will increase with a reduction in hydrogen partial pressure, but,
surprisingly and contrary to common belief, by using specific conditions and
catalysts, the catalyst deactivation rate was kept remarkably low.
[0054] Selectivity to Tubes increased with decreasing hydrogen pressure. In
Figure 2, where lines A, B, and C again refer to hydrogen pressures of 1136,
1825 and 3549 kPa (150, 250 and 500 psig). The Tubes yield, (i.e., 1-
conversion),
at a -21°C pour point is shown for each pressure in Table 3, below.
Table 3
Operating H~, Pressure, kPalpsig Lubes Yield, at -21°C P.P., %
3549/500 (comparative) 66.7
1825/250 73.9
1136/150 77.7
[0055] The data surprisingly show that catalyst activity and Tube selectivity
increased at lower pressure. Consequently, overall Tube yield increased.
CA 02498904 2005-03-11
WO 2004/033594 PCT/US2003/033321
-17-
[0056] Nevertheless, the prevailing wisdom is that catalyst life decreases
substantially as hydrogen pressure decreases, thereby leading to shortened on
stream periods and longer down times. To determine the effect of reduced
hydrogen pressure on catalyst life (and the rate of catalyst deactivation)
another
experiment was conducted over a period of 70 days at 1136 kPa (150 psig)
hydrogen pressure and producing lube base oil of -21°C pour point. By
regression, the deactivation rate was 11.7K (21°F)/year, by two point
activity
check the deactivation rate was 14.4 K (26°F)/year.
[0057] Consequently, operating at a very low hydrogen pressure results in a
good deactivation rate, and clearly shows that hydrogen pressures of less than
3549 kPa (500 psig), preferably less than 2859 kPa (400 psig), more preferably
less than 1136 kPa (150 psig), e.g., 963 kPa (125 psig), or less than 791 kPa
(100
psig), e.g., about 619 kPa (75 psig), will benefit both selectivity to
isomerization
and increased lube base oil yield while maintaining deactivation rates of less
than about 16.7 K (30°F)/year, or preferably less than about 13.9 K
(25°F)/year,
and more preferably less than about 8.3 K (15°F)/year.
EXAMPLE 2
[0058] The reactor described in Example 1 was operated with a 221 °C+
(430°F+) wide cut Fiseher-Tropsch wax feed to study the operation of a
dewaxing unit at 1825 kPa (250 psig). The catalyst of Example 1 was used, as
well. The hydrogen treat gas rate was 445.3 liter of hydrogen per liter of
feed
(2500 SCF/bbl) at standard conditions of 101.325 kPa and 15.5°C. The
liquid
hourly space velocity was 1Ø Temperature was adjusted to meet Tube pour
point or diesel cloud point. When operated to meet a diesel cloud point of -
15°C,
the deactivation rate was less than 1 K/year (1.8°F/year). The results
are shown
in Figure 4.
CA 02498904 2005-03-11
WO 2004/033594 PCT/US2003/033321
-18-
[0059] Operation of this unit to meet a -21°C wide-cut lube pour point
resulted in a deactivation rate of about 3 K/year (5.4°F/year). The
results are
shown in Figure 5.
EXAMPLE 3
[0060] ' The same feed as used in Example 1 was hydroisomerized and the
isomerate was distilled into two fractions: (i) 371 °C-510°C
(700-950°F) light
cut, and (ii) a 510°C+ (950°F+) heavy cut. Each fraction was
processed in the
reactor described in Example 1 and conditions described in Example 2 to meet a
-21°C pour point and a cloud point of +8°C, respectively. Each
fraction was run
for four (4) months. The results are shown in Figures 6 and 7; Figure 6
showing
a deactivation rate (by regression) for fraction (i) of about 1.1 K
(2°F)/year,
Figure 7 showing a deactivation rate (by regression) for fraction (ii) of
about
1.1 I~ (2°F)/year.