Note: Descriptions are shown in the official language in which they were submitted.
CA 02523847 2011-02-09
VENTILATOR AND METHODS FOR TREATING HEAD TRAUMA
AND LOW BLOOD CIRCULATION
BACKGROUND OF THE INVENTION
[0003] This invention relates generally to the field of intracranial and
intraocular pressures.
More specifically, the invention relates to devices and methods for decreasing
intracranial,
intraocular and systemic arterial pressures and increasing systemic vital
organ perfusion, such
as those resulting from a traumatic head injury and other injuries.
100041 Head trauma and shock are generally regarded as the leading cause of
morbidity and
mortality in the United States for children and young adults. Head trauma
often results in
swelling of the brain. Because the skull cannot expand, the increased
pressures within the
brain can lead to death or serious brain injury. While a number of therapies
have been
evaluated in order to reduce brain selling, including use of hyperventilation
and steroids, an
effective way to treat intracranial pressures remains an important medical
challenge.
Similarly, multi-organ injury associated with head trauma and other vital
organ damage is
associated with increased pressures within the brain and decreased vital organ
perfusion.
These patients have an extremely high mortality rate and similarly remain a
major medical
challenge.
BRIEF SUMMARY OF THE INVENTION
0005] In one embodiment, the invention provides a device for decreasing
intracranial or
intraocular pressures and increasing systemic blood pressures and organ
perfusion. The
CA 02523847 2005-10-27
WO 2004/096109
PCT/US2004/012294
device comprises a housing having an inlet opening and an outlet opening that
is adapted to
be interfaced with a person's airway. The device further includes a valve
system that is
operable to regulate respiratory gas flows through the housing and into the
person's lungs
during spontaneous or artificial inspiration. For a person who requires
artificial inspiration,
the valve system can be attached to a vacuum source. The valve system assists
in lowering
intrathoracic pressures during spontaneous inspiration and in non-breathing
patients when not
actively delivering a breath to continuously or intermittently lower pressures
in the venous
blood vessels that transport blood out of the head to thereby reduce
intracranial or intraocular
pressures and increase systemic blood pressures In addition, the invention
lowers the
pressures within the left and right heart, when positive pressure ventilations
are not being
provided, thereby helping to increase the efficiency of heart function. The
invention can
therefore be used to treat patients suffering from a number of disease states
including but not
limited to those suffering from elevated intracranial pressures, intra-ocular
pressures,
circulatory collapse, and cardiac arrest, and heart failure.
[0006] Such a device may also be used to facilitate movement of cerebral
spinal fluid. In so
doing, intracranial pressures may be further reduced. Such a device may
therefore be used to
treat those suffering from head trauma associated with elevated intracranial
pressures as well
as those suffering from heart conditions that increase intracranial pressures.
[0007] In one aspect, the valve system is configured to open to permit
respiratory gasses to
freely flow to the person's lungs when the negative intrathoracic pressure
reaches a pressure
in the range from about -2 cmH20 to about -20 cmH20 in order to reduce
intracranial or
intraocular pressures. In this way, the negative intrathoracic pressure is
lowered until a
threshold pressure is reached, at which time the valve opens. The cycle may be
repeated
continuously or periodically to repetitively lower intrathoracic pressures.
The device may
include means for compressing the chest to improve blood circulation in
patents in or with
low blood circulation or cardiac arrest. The compression could be accomplished
with an
automated chest compression, a circumferential vest, and the like. This would
improve blood
flow to the heart and brain in patients with low blood circulation.
[0008] The device may also include means for causing the person to
artificially inspire
through the valve system. For example, the device may utilize an electrode, an
iron lung
cuirass device, a chest lifting device, a ventilator or the like.
[0009] In another embodiment, the device may comprise a means to reduce
intrathoracic
pressure by applying a vacuum within the airway. The vacuum may be adjusted in
terms of
2
CA 02523847 2005-10-27
WO 2004/096109
PCT/US2004/012294
frequency, amplitude, and duration. This results in a decrease in intracranial
pressure in
proportion to the degree of vacuum applied. Hence, intracranial pressures may
be reduced
simply by manipulating airway pressures to reduce intrathoracic pressures. In
addition, the
vacuum created within the thorax enhances venous blood flow back to the heart,
thereby
simultaneously increasing cardiac output and systemic vital organ perfusion.
[0010] The device may further include a mechanism for varying the level of
impedance of
the valve system. This may be used in combination with at least one
physiological sensor
that is configured to monitor at least one physiological parameter of the
person. In this way,
the mechanism for varying the level of impedance may be configured to receive
signals from
the sensor and to vary the level of impedance of the valve system based on the
signals.
Examples of sensors that may be used include those that measure respiratory
rate,
intrathoracic pressure, intratracheal pressure, blood pressure, heart rate,
end tidal CO2,
oxygen level, intracranial perfusion, and intracranial pressure.
[0011] In one aspect, a coupling mechanism may be used to couple the valve
system to the
person's airway. Examples of coupling mechanisms include a mouthpiece, an
endotracheal
tube, and a face mask.
[0012] A wide variety of valve systems may be used to repetitively decrease
the person's
intrathoracic pressure. For example, valve systems that may be used include
those having
spring-biased devices, those having automated, electronic or mechanical
systems to occlude
and open a valve lumen, duck bill valves, ball valves, other pressure
sensitive valve systems
capable of opening a closing when subjected to low pressure differentials
triggered either by
spontaneous breathing and/or external systems to manipulate intrathoracic
pressures (such as
ventilators, phrenic nerve stimulators, iron lungs, and the like).
[0013] In another embodiment, the invention provides a method for decreasing
intracranial
or intraocular pressures. According to the method, a valve system is coupled
to a person's
airway and is configured to at least periodically reduce or prevent
respiratory gases from
flowing to the person's lungs. With the valve system coupled to the airway,
the person's
negative intrathoracic pressure is repetitively decreased to in turn
repetitively lower pressures
in the venous blood vessels that transport blood out of the head. In so doing,
intracranial and
intraocular pressures are reduced. Such a method also facilitates movement of
cerebral spinal
fluid. In so doing, intracranial pressures are further reduced. As such, this
method may also
be used to treat a person suffering from head trauma that is associated with
elevated
3
CA 02523847 2005-10-27
WO 2004/096109
PCT/US2004/012294
intracranial pressures as well as those suffering from heart conditions that
increase
intracranial pressures, such as atrial fibrillation and heart failure.
[0014] The person's negative intrathoracic pressure may be repetitively
decreased as the
person repeatedly inspires through the valve system. This may be done by the
person's own
efforts (referred to as spontaneous breathing), or by artificially causing the
person to
repeatedly inspire through the valve system. For example, the person may be
caused to
artificially inspire by repeatedly stimulating the phrenic nerve, by
manipulating the chest with
an iron lung cuirass device, by generating negative pressures within the
thorax using a
ventilator, by applying a vacuum within the thorax that can be regulated by
the valve system,
by applying a high frequency ventilator that supplies oscillations at a rate
of about 200 to
about 2000 per minute, or the like.
[0015] In another aspect, the level of impedance of the valve system may be
fixed or
variable. If variable, at least one physiological parameters of the person may
be measured,
and the impedance level may be varied based on the measured parameters.
[0016] To couple the valve system to the airway, a variety of techniques may
be used, such
as by using a mouthpiece, an endotracheal tube, a face mask or the like.
Further, the
respiratory gases may be prevented from entering the lungs through the valve
system until a
negative intrathoracic pressure in the range from about 0 cmH20 to about -25
cmH20 is
achieved, at which time the valve system permits respiratory gases to flow to
the lungs.
[0017] In another embodiment, the invention provides a method for treating a
person
suffering from head trauma associated with elevated intracranial pressures.
According to the
method, a positive pressure breath is delivered to the person. Following the
positive pressure
breath, respiratory gases are extracted from the person's airway to create an
intrathoracic
vacuum. In turn, this lowers pressures in the venous blood vessels that
transport blood out of
the head to thereby reduce intracranial pressures. The steps of delivering
positive pressure
breaths and extracting respiratory gases are repeated to continue the
treatment.
[0018] In one aspect, the delivery of the positive pressure breaths and the
extraction of
gases are performed using a mechanical ventilator. The respiratory gases may
be extracted
with a constant extraction or a pulsed extraction.
[0019] In a further aspect, the breath may be delivered for a time in the
range for about 250
milliseconds to about 2 seconds. Also, the breath may be delivered at a rate
in the range from
4
CA 02523847 2005-10-27
WO 2004/096109
PCT/US2004/012294
about 0.1 liters per second to about 5 liters per second. In another aspect,
the vacuum may be
maintained at a pressure in the level from about 0 mmHg to about -50 mmHg. The
vacuum
may be maintained with a negative flow or without any flow. The time that the
positive
pressure breath is supplied relative to the time in which respiratory gases
are extracted may
be in the range from about 0.5 to about 0.1.
[0020] A variety of equipment may be used to extract the respiratory gases
including
mechanical ventilators, phrenic nerve stimulators, ventilator bags, a vacuum
attached to the
airway device, iron lung cuirass devices and the like. In some cases, a
threshold valve may
also be coupled to the person's airway. The threshold valve may be configured
to open when
an adult's negative intrathoracic pressure exceeds about -3 cmH20. For
pediatric cases, the
valve may open when the pressure exceeds about -2 cmH20 to about -5 cmH20. In
this
way, when the person inhales, the negative intrathoracic pressure may be
lowered.
[0021] A variety of schemes may be used to deliver and extract respiratory
gases. For
example, respiratory gases may be extracted to achieve a pressure of about -5
mmHg to about
-10 mmHg and then kept generally constant until the next positive pressure
breath. As
another example, the positive breath may be slowly delivered and the
intrathoracic pressure
may be rapidly lowered to a pressure of about -10 mmHg to about -20 mmHg and
then
gradually reduced towards about 0 mmHg. As a further example, the
intrathoracic pressure
may be slowly lowered to a pressure of about -20 mm Hg.
[0022] In a further embodiment, the invention provides a device for lowering
intrathoracic
pressures. The device comprises a housing having an interface that is adapted
to couple the
housing to the person's airway. A vacuum source is in fluid communication with
the housing
for repeatedly extracting respiratory gases from the person's lungs and airway
to create and
periodically maintain a negative intrathoracic pressure. A vacuum regulator is
used to
regulate the extraction of respiratory gases from the patient's lungs and
airway. Also, a
positive pressure source is in fluid communication with the housing for
intermittently
supplying positive pressure breaths to the person. Such a device may be used
to treat a
variety of ailments, such as head trauma associated with elevated intracranial
pressures, low
blood pressure, low blood circulation, low blood volume, cardiac arrest and
heart failure.
[0023] In some cases, a switching mechanism may be used to stop the extraction
of
respiratory gases during delivery of a positive pressure breath. A variety of
switching
mechanisms may be used, such as mechanical devices, magnetic devices, and
electronic
5
CA 02523847 2005-10-27
WO 2004/096109
PCT/US2004/012294
devices. Also, a variety of vacuum sources may be used to extract the
respiratory gases,
including a mechanical ventilator, a vacuum with vacuum regulator, a phrenic
nerve
stimulator, an extrathoracic vest, a ventilator bag, and an iron lung cuirass
device, a suction
line, a venturi device attached to an oxygen tank and the like.
[0024] To regulate the vacuum, a threshold valve may be placed in fluid
communication
with the person's airway. The threshold valve may be configured to open when
the person's
negative intrathoracic pressure reaches about -3 cm H20 to about -20cm H20 to
permit
respiratory gases to flow into the person's airway. Also, a variety of
pressure sources may be
used to deliver a positive pressure breath, such as a mechanical ventilator, a
hand held bag
valve resuscitator, mouth-to-mouth, or a means to provide intermittent
positive pressure
ventilation.
BRIEF DESCRIPTION OF THE DRAWINGS
[0025] Fig. 1 is a flow chart illustrating one method for reducing
intracranial and
intraocular pressures according to the invention.
[0026] Fig. 2 is a perspective view of one embodiment of a facial mask and a
valve system
that may be used to reduce intracranial and intraocular pressures according to
the invention.
[0027] Fig. 3 is a perspective view of the valve system of Fig. 2.
[0028] Fig. 4 is a cross sectional side view of the valve system of Fig. 3.
[0029] Fig. 5 is an exploded view of the valve system of Fig. 3.
[0030] Fig. 6 is a schematic diagram of a system for reducing intracranial and
intraocular
pressures according to the invention.
[0031] Fig. 7 is a series of graphs illustrating the lowering of intracranial
pressures in an
animal study.
[0032] Fig. 8 is a series of graphs illustrating the lowering of intracranial
pressures in
another animal study.
[0033] Fig. 9A is a schematic diagram of a person's brain under normal
conditions.
[0034] Fig. 9B illustrates the brain of Fig. 9A after increased swelling.
6
CA 02523847 2005-10-27
WO 2004/096109
PCT/US2004/012294
[0035] Fig. 10 shows three graphs illustrating the effect of lowering
intrathoracic pressure
on intracranial pressure and right atrial pressure.
[0036] Fig. 11 is a flow chart illustrating another method for reducing
intracranial and
intraocular pressures according to the invention.
[0037] Figs. 12A-12C show three graphs illustrating patterns for delivering a
positive
pressure breath and extracting respiratory gases according to the invention.
= [0038] Figs. 13A and 13B schematically illustrate one device that may be
used to lower
intrathoracic pressures with a non-breathing patient according to the
invention.
[0039] Figs. 14A and 14B illustrate another device that may be used to lower
intrathoracic
pressures with a non-breathing patient according to the invention.
[0040] Figs. 15A and 15B illustrate one embodiment of a threshold valve system
that may
be used with the device of Figs. 14A and 14B.
DETAILED DESCRIPTION OF THE INVENTION
[0041] In a broad sense, the invention provides devices and techniques for
lowering
intracranial and intraocular pressures. Such devices and techniques may be
particularly
helpful with patients who have suffered a traumatic brain injury and those
with low blood
flow states and low blood pressure. One way to lower the pressure within the
head but
maintain or increase systemic pressures is by using a valve system that is
coupled to a
person's airway and that is used to lower intrathoracic pressures. In so
doing, the valve
systems may be used to accelerate the removal of venous blood from the brain,
thereby
decreasing intracranial and intraocular pressures. At the same time, the
systemic pressures
increase due to enhancement of venous return to the heart. Other techniques
may be used as
well, such as by creating a vacuum intermittently within the thorax. By
reducing intracranial
pressures, movement of cerebral spinal fluid is also enhanced. In so doing,
intracranial
pressures are further reduced thereby providing further treatment for those
suffering from
head trauma. In some cases, the valve systems may also be used to treat the
brain function in
a person suffering from a heart condition (atrial fibrillation, heart failure,
cardiac tamponade,
and the like) that results in elevated intracranial pressures. Such heart
conditions may
include, for example, atrial fibrillation or heart failure. By reducing
intracranial pressures,
cerebral spinal fluid movement and translocation is increased to help improve
brain function.
7
CA 02523847 2011-02-09
[0042] Intracranial pressures are regulated by the amount the cerebral
perfusion pressure,
which is determined by the arterial blood pressure to the head, the pressures
within the skull,
and the pressures within the venous system that drains blood flow from the
brain. The
devices and methods of the invention may be used to enhance the egress of
venous blood out
of the brain, thereby lowering intracranial pressures. The devices and methods
can be used in
patients that are breathing spontaneously and those that require assisted
ventilation. To do so,
the devices and methods may be used to augment the intrathoracic vacuum effect
each time a
patient inhales (or in the case of a non-breathing patient, each time the
pressure within the
chest is manipulated to fall below atmospheric pressure), thereby lowering the
pressures in
the thorax and in the venous blood vessels that transport blood out of the
brain. The vacuum
effect is transduced back into the brain, and as a result, intracranial
pressures are lowered
with each inspiratory effort. This in turn causes more venous blood to flow
out of the head
than would otherwise be possible, resulting in lower intracranial pressures
and lower
intraocular pressures. In addition, circulation to the vital organs is
increased as the increase
in venous return to the heart each time a negative intrathoracic pressure is
generated results in
an increase in cardiac output and improved vital organ perfusion. As such,
this invention
may be used to help patients suffering from low cardiac output states and low
blood pressure.
[0043] To prevent or impede respiratory gases from flowing to the lungs, a
variety of
impeding or preventing mechanisms may be used, including those described in
U.S. Patent
Nos. 5,551,420; 5,692,498; 6,062,219; 5,730,122; 6,155,257; 6,234,916,
6,224,562 and
6,776,156, and in U.S. Patent Publication No. 2004/00164285. The valve systems
may be
configured to completely prevent or provide resistance to the inflow of
respiratory gases into the
patient while the patient inspires. For valve systems that completely prevent
the flow of
respiratory gases, such valves may be configured as pressure responsive valves
that open after a
threshold negative intrathoracic pressure has been reached.
[0044] For example, the resistance to the inflow of respiratory gases may be
set between
about 0 cm H20 and about -25 cm H20 and may be variable or fixed. More
preferably, the
valve system may be configured to open when the negative intrathoracic
pressure is in the
8
CA 02523847 2005-10-27
WO 2004/096109
PCT/US2004/012294
range from about -2 cmH20 to about -20 cmH20. In addition, the valve system
may used
continuously or on a variable basis. For example, the valve system may be used
for every
other spontaneous breath.
[0045] Although not intended to be limiting, specific kinds of impedance
valves that may
[0046] In the past, such threshold valve systems have been used to increase
the venous
preload on the heart and to increase cardiac output, stroke volume and blood
pressure because
of the augmented effects of the intrathoracic vacuum on the subsequent cardiac
contraction.
In contrast, the techniques of the invention function by facilitating the
removal of blood from
[0047] With the valve system coupled to the person's airway, the negative
intrathoracic
pressure may be enhanced by inspiring through the valve system. If the person
is
spontaneously breathing, the person may simply breath through the valve
system. If the
9
CA 02523847 2011-02-09
pressure is below or negative with respect to the pressure in the peripheral
venous
= vasculature. Upon contraction of the respiratory muscles, the patient
will typically "gasp".
These techniques may be performed alone, or in combination with a valve
system.
[00481 Among the respiratory muscles that may be stimulated to contract are
the
diaphragm, the chest wall muscles, including the intercostal muscles and the
abdominal
muscles. Specific chest wall muscles that may be stimulated to contract
include those that
elevate the upper ribs, including the scaleni and stemocleidomastoid muscles,
those that act
to fix the shoulder girdle, including the trapezii, rhomboidei, and levatores
arigulorum
scapulorum muscles, and those that act to elevate the ribs, including the
serrati antici majores,
and the pectorales maj ores and minores as described generally in Leslie A.
Geddes,
"Electroventilation - A Missed Opportunity?", Biomedical Instrumentation &
Technology,
July/August 1998, pp. 401-414.
Of the respiratory muscles, the two hemidiaphragms and intercostal muscles
appear to be the greatest contributors to inspiration and expiration. The
respiratory muscles
may be stimulated to contract in a variety of ways. For example, the diaphragm
may be
stimulated to contract by supplying electrical current or a magnetic field to
various nerves or
muscle bundles which when stimulated cause the diaphragm to contract. Similar
techniques
may be used to stimulate the chest wall muscles to contract. A variety of
pulse trains, pulse
widths, pulse frequencies and pulse waveforms may be used for stimulation.
Further, the
electrode location and timing of pulse delivery may be varied. In one
particular aspect, an
electrical current gradient or a magnetic field is provided to directly or
indirectly stimulate
the phrenic nerve.
100491 To electrically stimulate the inspiratory motor nerves, electrodes are
preferably
placed on the lateral surface of the neck over the point where the phrenic
nerve, on the chest
surface just lateral to the lower sternum to deliver current to the phrenic
nerves just as they
enter the diaphragm, on the upper chest just anterior to the axillae to
stimulate the thoracic
nerves, in the oral pharyngeal region of the throat, or on the larynx itself.
However, it will be
appreciated that other electrode sites may be employed. For example, in one
embodiment the
respiratory muscles are stimulated by a transcutaneous electrical impulse
delivered along the
lower antero-lat margin of the rib cage. In one embodiment, inspiration is
induced by
stimulating inspiratory muscles using one or more electrodes attached to an
endotracheal tube
or pharyngeal tube. To stimulate the diaphragm, the phrenic nerve may be
stimulated in the
neck region near C3-C7, such as between C3, C4 or C5, or where the phrenic
nerves enter the
CA 02523847 2011-02-09
=
diaphragm. Alternative techniques for stimulating diaphragmatic contraction
include
magnetic field stimulation of the diaphragm or the phrenic nerve. Magnetic
field stimulation
may also be employed to stimulate the chest wall muscles. Electrical field
stimulation of the
diaphragm or the chest wall muscles may be accomplished by placing one or more
electrodes
on the skin, preferably in the vicinity of the neck or the lower rib cage
(although other
locations may be employed) and then providing an electrical voltage gradient
between
electrodes that induces transcutaneous current flow to stimulate the
respiratory muscles to
contract. Still further, subcutaneous electrodes may also be used to stimulate
respiratory
muscle contraction. Other techniques are described in U.S. Patent No.
6,463,327.
[0050] The valve systems may have a fixed actuating pressure or may be
variable so that
once a desired negative intrathoracic pressure is reached, the resistance to
flow may be
lessened. Further, the valves of the invention may be configured to be
variable, either
manually or automatically. The extent to which the resistance to flow is
varied may be based
on physiological parameters measured by one or more sensors that are
associated with the
person being treated. As such, the resistance to flow may be varied so that
the person's
physiological parameters are brought within an acceptable range. If an
automated system is
used, such sensors may be coupled to a controller which is employed to control
one or more
mechanisms that vary the resistance or actuating pressure of the inflow valve
as generally
described in the references that have been identified herein.
[0051] Hence, the valve systems of the invention may also incorporate or be
associated
with sensors that are used to detect changes in intrathoracic pressures or
other physiological
parameters. In one aspect, the sensors may be configured to wirelessly
transmit their
measured signals to a remote receiver that is in communication with a
controller. In turn the
controller may use the measured signals to vary operation of the valve systems
described or
incorporated by reference herein. For example, sensors may be used to sense
blood pressure,
pressures within the heart, intrathoracic pressures, positive end expiratory
pressure,
respiratory rate, intracranial pressures, intraocular pressures, respiratory
flow, oxygen
delivery, temperature, blood pH, end tidal CO2, tissue CO2, blood oxygen,
cardiac output or
the like. Signals from these sensors may be wirelessly transmitted to a
receiver. This
information may then be used by a controller to control the actuating pressure
or the a a
resistance of an inflow valve as described in the references identified
herein.
11
CA 02523847 2005-10-27
WO 2004/096109
PCT/US2004/012294
[0052] The techniques for reducing intracranial pressures may be used in a
variety of
settings. For example, the techniques may be used in person's who are
spontaneously
breathing, those who are not breathing but whose hearts are beating, and those
in cardiac
arrest. In the latter case, the techniques may use some means to create a
vacuum
intermittently within the thorax during the performance of CPR. This could be
by using a
valve system or some other type of pressure manipulation system. Further, such
systems may
be used in other settings as well, including when the person is breathing.
[0053] Fig. 1 is flow diagram illustrating one method for reducing
intracranial or
intraocular pressures. As shown in step 10, the process proceeds by coupling a
valve system
to the person's airway. Any kind of coupling mechanism may be used, such as by
a
mouthpiece, an endotracheal tube, a face mask, or the like. Further, any of
the valve systems
described or incorporated herein by reference may be used. In step 20, the
person's negative
intrathoracic pressure is repetitively decreased (either artificially or by
spontaneous
breathing). Examples of techniques to artificially reduce the negative
intrathoracic pressure
include use of an iron lung cuirass device, a ventilator that is capable of
generating negative
pressures, a ventilator that is capable of providing high frequency
oscillations at a rate of
about 200 to about 2000 per minute, a phrenic nerve stimulator, or the like.
As the person's
negative intrathoracic pressure is repeatedly decreased while the valve system
is coupled to
the airway, the pressures in the venous vessels that transport blood out of
the head are also
lowered. In so doing, intracranial and intraocular pressures are reduced.
[0054] As shown in step 30, various physiological parameters of the person may
optionally
be measured. Examples of such parameters include respiratory rate,
intrathoracic pressure,
intertracheal pressure, intracranial pressure, intracranial blood flow,
intraocular pressure,
blood pressure, heart rate, end tidal CO2, oxygen saturation, and the like.
Further, as shown
in step 40, the valve system's actuating threshold level may optionally be
varied based on the
measured physiological parameters. This may be done to maximize the amount of
blood
drawn out of the brain or simply to monitor the patient's condition to insure
that the patient
remains stable.
[0055] Fig. 2 illustrates one embodiment of a facial mask 100 to which is
coupled a valve
system 200. Mask 100 is configured to be secured to a patient's face so as to
cover the mouth
and nose. Mask 100 and valve system 200 are examples of one type of equipment
that may
be used to lower intrathoracic pressures and thereby lower intracranial and
intraocular
12
CA 02523847 2005-10-27
WO 2004/096109
PCT/US2004/012294
pressures. However, it will be appreciated that other valve systems and other
coupling
arrangements may be used including, for example, those previously referenced.
As such the
invention is not intended to be limited to the specific valve system and mask
described below.
[0056] Referring also to Figs. 3-5, valve system 200 will be described in
greater detail.
Valve system 200 includes a valve housing 202 with a socket 204 into which a
ball 206 of a
ventilation tube 208 is received. In this way, ventilation tube 208 may rotate
about a
horizontal axis and pivot relative to a vertical axis. A respiratory source,
such as a ventilation
bag, may be coupled to tube 208 to assist in ventilation. Disposed in
ventilation tube 208 is a
filter 210 that is spaced above a duck bill valve 212. A diaphragm holder 214
that holds a
diaphragm 216 is held within housing 202. Valve system 200 further includes a
patient port
218 that is held in place by a second housing 220. Housing 220 conveniently
includes tabs
222 to facilitate coupling of valve system 200 with facial mask 100. Also held
within
housing 220 is a check valve 224 that comprises a spring 224a, a ring member
224b, and an
o-ring 224c. Spring 224a biases ring member 224b against patient port 218.
Patient port 218
includes bypass openings 226 that are covered by o-ring 224c of check valve
224 until the
pressure in patient port 218 reaches a threshold negative pressure to cause
spring 224a to
compress.
[0057] When the patient is actively ventilated, respiratory gases are forced
through
ventilation tube 208. The gases flow through filter 210, through duck bill
valve 212, and
forces up diaphragm 214 to permit the gases to exit through port 218. Hence,
at any time the
patient may be ventilated simply by forcing the respiratory gases through tube
208.
[0058] During the exhalation phase of a breathing cycle, expired gases flow
through port
218 and lift up diaphragm 214. The gases then flow through a passage 227 in
ventilation tube
208 where they exit the system through openings 229 (see Fig. 3).
[0059] During the inhalation phase of a breathing cycle, valve system 200
prevents
respiratory gases from flowing into the lungs until a threshold negative
intrathoracic pressure
level is exceeded. When this pressure level is exceeded, check valve 224 is
pulled downward
as springs 224a are compressed to permit respiratory gases to flow through
openings 226 and
to the patient's lungs by initially passing through tube 208 and duck bill
valve 212. Valve
224 may be set to open when the negative intrathoracic pressure is in the
range from about 0
cm H20 to about ¨25 cm H20, and more preferably from about ¨2 cm H20 to about
¨20 cm
H20. Hence, the magnitude and duration of negative intrathoracic pressure may
be enhanced
13
CA 02523847 2005-10-27
WO 2004/096109
PCT/US2004/012294
during patient inhalation by use of valve system 200. Once the intrathoracic
pressure falls
below the threshold, recoil spring 224a again close check valve 224. In this
way, pressure
within the venous blood vessels that transport blood out of the brain are also
lowered. In so
doing, more blood is drawn out of the brain to reduce intracranial and
intraocular pressures.
[0060] Any of the valve systems described herein may be incorporated into a
treatment
system 300 as illustrated in Fig. 6. System 300 may conveniently include
facial mask 100
and valve system 200, although any of the valve systems or interfacing
mechanisms
described herein or the like may be used. Valve system 200 may conveniently be
coupled to
a controller 310. In turn, controller 310 may be used to control the impedance
level of valve
system 200 in a manner similar to any of the embodiments described or
incorporated herein.
The level of impedance may be varied based on measurements of physiological
parameters,
or using a programmed schedule of changes. System 300 may include a wide
variety of
sensors and/or measuring devices to measure any of the physiological
parameters described
herein. These sensors or measuring devices may be integrated within or coupled
to valve
system 200 or facial mask, or may be separate.
[0061] For example, valve system 200 may include a pressure transducer for
taking
pressure measurements (such as intrathoracic pressures, intracranial
pressures, intraocular
pressures), a flow rate measuring device for measuring the flow rate of air
into or out of the
lungs, or a CO2 sensor for measuring expired CO2.
[0062] Examples of other sensors or measuring devices include a heart rate
sensor 330, a
blood pressure sensor 340, and a temperature sensor 350. These sensors may
also be coupled
to controller 310 so that measurements may be recorded. Further, it will be
appreciated that
other types of measuring devices may be used to measure various physiological
parameters,
such as oxygen saturation and/or blood levels of 02, blood lactate, blood pH,
tissue lactate,
tissue pH, blood pressure, pressures within the heart, intrathoracic
pressures, positive end
expiratory pressure, respiratory rate, intracranial pressures, intraocular
pressures, respiratory
flow, oxygen delivery, temperature, end tidal CO2, tissue CO2, cardiac output
or the like.
[0063] In some cases, controller 310 may be used to control valve system 200,
to control
any sensors or measuring devices, to record measurements, and to perform any
comparisons.
Alternatively, a set of computers and/or controllers may be used in
combination to perform
such tasks. This equipment may have appropriate processors, display screens,
input and
14
CA 02523847 2005-10-27
WO 2004/096109
PCT/US2004/012294
output devices, entry devices, memory or databases, software, and the like
needed to operate
system 300.
[0064] A variety of devices may also be coupled to controller 310 to cause the
person to
artificially inspire. For example, such devices may comprise a ventilator 360,
an iron lung
cuirass device 370 or a phrenic nerve stimulator 380. Ventilator 360 may be
configured to
create a negative intrathoracic pressure within the person, or may be a high
frequency
ventilator capable of generating oscillations at about 200 to about 2000 per
minute.
[0065] Example 1
[0066] The following is a non-limiting example illustrating how intracranial
pressures may
be lowered according to the invention. In this example, 30 kg pigs were
anesthetized with
propofol. Using a micromanometer-tipped electronic Millar catheter inserted
below the dura,
intracranial pressures were measured continuously in the spontaneously
breathing pigs.
Intrathoracic pressures (ITP) were recorded using a Millar catheter placed in
the trachea at
the level of the carina. After stabilizing the pigs blood pressure, heart
rate, and ventilation
rate, intracranial pressures (ICP) and intrathoracic pressures were recorded,
with 0 cmH20
inspiratory impedance and then with inspiratory impedances of 5,10,15, and 20
cm H20.
Inspiratory impedance was achieved using an impedance threshold valve (ITV) as
described
in Figs. 2-5.
[0067] At base, the intracranial pressure was approximately 8/4 mmHg. With
increasing
amounts of inspiratory impedance, the intracranial pressure was lowered
proportionally as
shown in Figure 7. The intracranial pressure was 6/-2 mmHg when the pig
breathed through
an impedance of 20 cm H20. These findings were observed in multiple pig
studies and were
reproducible. Next, the Millar catheter was inserted 3 cm into the pig's
brain. The
intracranial pressure increased secondary to the trauma associated with the
insertion of the
probe. The intracranial pressure increased to 25/22 mmHg at the new baseline.
Next, the
impedance threshold valve was evaluated at different levels of resistance
(Fig. 8). Again,
there was a decrease in intracranial pressure proportional to the degree of
inspiratory
impedance.
[0068] Example 2
[0069] In this example, intracranial pressures were increased in the setting
of recovery from
cardiac arrest. The example used a pig model with ventricular fibrillation for
6 minutes
CA 02523847 2011-02-09
followed by cardiopulmonary resuscitation for 6 minutes, followed by
defibrillation.
Spontaneous breathing resulted in an up to 50% decrease in intracranial
pressures when the
animals breathed through an inspiratory impedance of 10 cm H20 using a valve
system
similar to Example 1.
[0070] In all examples above, the intrathoracic pressure decreased relative to
the rest of the
body, creating a suction effect that reduced the pressure in the venous blood
vessels draining
the brain, thereby reducing intracranial pressures.
[0071] The invention further provides techniques and devices for reducing
intracranial
pressure (ICP) by facilitating movement of cerebral spinal fluid (CFS). There
are a number
of causes of increased ICP including: head injury, ischemia, osmolar
imbalance, cerebral
edema, tumors, complications of dialysis, infections, stroke, hypertensive
crises. Each can
result in a slow, and in some cases, an acute rise in the ICP. The solid
matter of the brain
contents makes up about 80-85% of the material enclosed by the skull. Cerebral
blood
volume accounts for 3-6% and CSF for 5-15%. See, Anesthesia, Third Edition
Editor, Ron
Miller. Chapter authors: Shapiro and Drummond. Chapter 54 (1990);
CSF moves within the brain from its site of
production to its site of reabsorption in the brain in an unimpeded manner
under normal
physiological states. Since the contents in the brain are practically
incompressible, a change
in volume of any one of the three major components (brain matter, blood
volume, CSF
volume) results in a reciprocal change in one or both of the other brain
components. When
the volume of the brain expands, secondary to an increase in the non-CSF
component(s),
some of the CSF is forced to other locations, including through the foramen
magnum (hole in
skull connecting skull to space where the spinal cord is located) and into the
CSF fluid space
surrounding the spinal cord. When the non-CSF components expand in volume or
size, the
intracranial pressure rises. Normal ICP levels are 10-15 mmHg when supine. At
levels
greater than 15-20 mmHg, damage to the brain can occur secondary to
compression and
resultant tissue ischemia (lack of adequate blood flow). A reduction in ICP
levels can be
achieved by a number of clinical interventions including water restriction,
diuretics, steroids,
=
hyperventilation, a reduction of cerebral venous pressure, hypothermia, CSF
drainage, and
surgical decompression.
[0072] Increased ICP results in reduced CSF fluid movement and translocation.
CSF fluid
production generally remains constant (about 150 ml/day) despite elevated ICP.
CSF fluid
16
CA 02523847 2011-02-09
reabsorption is can& slowed by elevated ICP. By using the valve systems
described herein,
central venous pressures may be reduced. In turn, this results in a decrease
in ICP and results
in an increase in CSF fluid movement or translocation and reabsorption. This
results in a
further reduction in ICP.
[0073] The valve systems of the invention may be used in spontaneously
breathing
individuals, in patients ventilated with negative pressure ventilation or in
patients ventilated
with a ventilator that causes a decrease in central venous pressures for at
least a portion of the
respiratory cycle. Each time the intrathoracic pressure is reduced with the
valve systems of
the invention, there is a concomitant reduction in ICP and an increase in the
movement of
CSF. In other words, there is an increase in the difference between the peak
and trough of the
ICP wave form when using the valve systems. The sinusoidal movement occurs in
spontaneously breathing people because of the change in pressure in the thorax
that is
transmitted to the brain via the venous blood vessels. The normally
fluctuating CSF
pressures (the pressure increases and decreases with each inspiration) are
altered by the valve
systems. More specifically, the valve systems create a lower trough value
thereby creating an
overall created change in the ICP with each inspiration. In the non-breathing
patient, a
similar effect can be produced with the valve systems when used with a variety
of ventilator
devices, including an iron lung, a phrenic nerve stimulator (such as those
described in U.S.
Patent Nos. 6234985; 6224562; and 6312399, a suction cup on the chest that is
used to
periodically expand the chest and the like.
100741 Increased CSF fluid movement results in an overall improved metabolic
state for the
brain. This is shown schematically in Figs. 9A and 9B. In Fig. 9A, the brain
400 is shown
under normal conditions. The brain 400 is surrounded by CSF 402 which is
produced at a
site 404. The CFS in turn is surrounded by the skull 406. Blood enters brain
400 through an
artery 408 and exits through a vein 410. Vein 410 also includes a site 412 of
CFS drainage.
Shown in Fig. 9A is an arrow showing the direction of CFS flow when draining.
Extending
from brain 400 is the spinal cord 414 that is surrounded by the foramen magnum
416.
[0075] In Fig. 9B, the brain 400 is significantly swollen which reduces the
space 402 where
the CFS is located. The swelling of the brain 400 can cause blockage of CSF to
the spinal
cord 414 as shown by arrow 418. Also, movement of CSF to site 412 is reduced
to hinder
movement of CSF out of the skull 406.
17
CA 02523847 2005-10-27
WO 2004/096109
PCT/US2004/012294
[0076] By treating the elevated ICP associated with all of the conditions
noted above using
the valve systems described herein, brain swelling can be reduced. In so
doing, CFS
movement and fluid translocation is increased under those same conditions.
This results in a
further decrease in intracranial pressure as the CSF is able to relocate.
[0077] Referring now to Fig. 10, the effects of contracting the atria of the
heart on ICP will
be described. As shown, contraction of the atria results in a phasic movement
in ICP. This
can be most clearly demonstrated during cardiac ventricular fibrillation. In
that setting, the
atria often beat spontaneously and the pressure of each contraction and
relaxation waveform
is transmitted immediately to the brain and is reflected in nearly identical
fluctuations in ICP.
The inventor has discovered that the fluid systems (venous blood vessels and
CSF) are so
closely linked, that subtle changes in the heart rhythm result in immediate
changes in CSF
pressure. Thus, in some patients with significant heart rhythms, or
significant heart failure,
the rise in right heart pressures as a result of these conditions results in
an increase in ICP.
4 Such rises in ICP can lead to a decrease in cerebral perfusion, since
cerebral perfusion is
determined by the pressure of the blood entering the brain (mean arterial
pressure) minus the
pressure of the blood leaving the brain (ICP and central venous pressure). Use
of the valve
and intrathoracic vacuum systems described herein will result in a decrease in
intrathoracic
pressure. As shown in Fig. 10, the downwardly pointing arrows represent the
timing of each
inhalation through the valve system. In the baseline state, before the onset
of atrial
fibrillation, each inspiration (small arrows) results in a reduction in ITP, a
reduction of right
atria pressure, a reduction in central venous pressures, and then an immediate
reduction in
ICP. With the onset of atrial fibrillation, the intracranial pressure rises
and the sinusoidal
pattern of ICP amplitude changes becomes dampened. As soon as the animal
begins to
inspire through an inspiration impedance of -10 cm 1120 there is an immediate
decrease in
intrathoracic pressure (ITP), an immediate decrease in right atrial (RA)
pressures, and an
immediate decrease in intracranial pressure (ICP) along with the restoration
of a sinusoidal
fluctuation in ICP with each inspiration. With elevated ICP, inspiration
through the impeding
means results in a decrease in ICP, increased cerebral spinal fluid flow, and
a decrease in
cerebral ischemia secondary to increased cerebral perfusion. As such, the
valve systems can
used in patients with heart rhythms, such as atrial fibrillation, or patients
with heart failure
who have increased ICP in order to reduce their ICP, increase CSF fluid
movement and
translocation, and ultimately help them to improve their brain function.
18
CA 02523847 2011-02-09
[0078] Hence, the amount of inspiratory resistance, or the amount of negative
intrathoracic
pressure generation (which may be generated using a variety of techniques) can
be controlled
or regulated by feedback from measurement of ICP, blood pressure, respiratory
rate, or other
physiological parameters. Such a system could include a closed loop feedback
system.
[0079] Fig. 11 is a flow chart illustrating another method for treating a
person suffering
from head trauma associated with elevated intracranial pressures. In so doing,
it will be
appreciated that such techniques may also be used to treat those suffering
from low blood
pressure or those in cardiac arrest, among others. The techniques are
particularly useful in
cases where the person is not breathing, although in some cases they could be
used for
breathing patients as well.
[0080] In a broad sense, when treating a person suffering from head trauma, a
person's
intrathoracic pressure is lowered to decrease intracranial pressures. In turn,
this assists in
reducing secondary brain injury. As shown in step 500, equipment may be
coupled to the
person to assist in lowering the person's intrathoracic pressure. A wide
variety of equipment
and techniques may be used to decrease the intrathoracic pressure, including
using a
mechanical ventilator capable of extracting respiratory gases, such as the one
described in
U.S. Patent No. 6,584,973, a phrenic nerve or other muscle stimulator (with or
without the
use of an impedance mechanism, such as those described in U.S. Patent Nos.
5,551,420;
5,692,498; 6,062,219; 5,730,122; 6,155,257; 6,234,916 and 6,224,562) such as
those
described in U.S. Patent Nos. 6234985; 6224562; 6312399; and 6463327, an iron
lung
device, a thoracic vest capable of pulling outward on the chest wall to create
an intrathoracic
vacuum similar to the effect of an iron lung, a ventilatory bag. For breathing
patients, a
threshold valve as described above and this is set to open when about 5 cmH20
is generated
during an inhalation may be used to enhance the person's negative
intrathoracic pressure.
[0081] When the person is not breathing, a positive pressure breath is
delivered to the
person as illustrated in step 502. This may be done with a mechanical
ventilator, a
ventilatory bag, mouth to mouth, and the like. This is followed by an
immediate decrease in
intrathoracic pressure. This may be done by extracting or expelling
respiratory gases from
19
CA 02523847 2005-10-27
WO 2004/096109
PCT/US2004/012294
the patient's lungs as shown in step 504. Any of the techniques described
above may be used
to lower the intrathoracic pressure. Such a reduction in intrathoracic
pressure also lowers
central venous pressure and intracranial pressure.
[0082] The vacuum effect during the expiratory phase may be constant, varied
over time or
[0083] As shown in step 506, the process of delivering a positive pressure
breath and then
immediately lowering intrathoracic pressures may be repeated as long as
necessary to control
intracranial pressures. Once finished, the process ends at step 508.
depending upon a particular application. These may be applied in a variety of
waveforms
having different durations and slopes. Examples include using a square wave,
biphasic
(where a vacuum is created followed by positive pressure, decay (where a
vacuum is created
and then permitted to decay), and the like. Three specific examples of how
this may occur
CA 02523847 2005-10-27
WO 2004/096109
PCT/US2004/012294
immediately reversed to a negative pressure of about -10 mmHg. This pressure
is kept
relatively constant until the end of the expiratory phase where the cycle is
repeated.
[0085] In Fig. 12B, the positive pressure is more slowly applied. When
reaching a pressure
of about 10 to about 15 mmHg, the pressure is rapidly reversed to a negative
pressure of
about -20 mmHg. The negative pressure gradually declines to about 0 mmHg at
the end of
the expiratory phase. The cycle is then repeated. Hence, in the cycle of Fig.
12B, the
positive pressure is reduced compared to the cycle in Fig. 12A, and the
negative pressure is
initially lower, but allowed to gradually increase. The technique is designed
to help reduce a
possible airway collapse.
[0086] In Fig. 12C, the positive pressure is brought up to about 20 mmHg and
then
immediately brought down to about 0 mmHg. The negative pressure is then
gradually
increased to about -20 mmHg toward the end of the expiratory phase. This cycle
is designed
to help reduce a possible airway collapse.
[0087] Figs. 13A and 13B schematically illustrate one embodiment of a device
500 that
may be used to lower intrathoracic pressures in a non-breathing patient.
Device 500
comprises a housing 502 having an interface opening 504 that may be directly
or indirectly
coupled to the patient's airway using any type of patient interface. Housing
502 also includes
a vacuum source interface 506 that may be in fluid communication with any type
of device or
system capable of producing a vacuum. Also coupled to housing 502 is a means
to regulate
the vacuum, such as a pressure responsive valve system 508. Device 500 further
includes a
ventilation interface 510 that may be used to provide a breath to the patient,
if needed, when
the vacuum is not applied.
[0088] In this embodiment, the vacuum may be provided by essentially any type
of a
vacuum source, and the regulator may comprise an impedance valve, such as
those described
in U.S. Patent Nos. 5,551,420; 5,692,498; 6,062,219; 5,730,122; 6,155,257;
6,234,916;
6,224,562; 6,234,985; 6,224,562; 6,312,399; and 6,463,327 as well as others
described
herein. To supply a breath, a variety of ventilation sources may be used, such
as, for
example, a bag valve resuscitator, that is coupled to interface 510. Device
500 may further
include a mechanism 512 to inhibit the vacuum when delivering a breath to the
patient from
the bag valve resuscitator. Once the breath is delivered, mechanism 512
operates to permit
the vacuum within the thorax to be reapplied. The mechanism 512 used to turn
off and on the
vacuum source can include a slider switch that moves to close off the branch
in housing 500
21
CA 02523847 2005-10-27
WO 2004/096109
PCT/US2004/012294
having the vacuum source as illustrated in Fig. 13B. However, other types of
switches or
mechanisms may be used. In some cases, the vacuum source may have a controller
that is
configured to shut off the vacuum when the breath is administered so that
mechanism 512 is
not needed. Also, a controller and appropriate sensors could be used to sense
when the breath
is delivered and stopped so that mechanism 512 may be appropriately operated
by the
controller. After the breath is delivered, mechanism 512 moves back to the
position
illustrated in Fig. 13A so that the vacuum may be supplied to the patient.
When the vacuum
reaches a threshold amount, regulator 508 operates to maintain the level of
vacuum at about
the threshold amount.
[0089] Figs. 14A and 14B illustrate another embodiment of a device 530 that
may be used
to treat a patient. Device 530 operates using similar principles as device 500
illustrated in
Figs. 13A and 13B. Device 530 comprises a housing 532 having a patient
interface 534 that
may be coupled to the patient's airway and a vacuum interface 536 that may be
coupled to a
vacuum source. Housing 532 also includes a ventilation interface 538 through
which a
positive pressure breath may be supplied. Also coupled to housing 532 is a
vacuum regulator
540 that regulates the amount of vacuum supplied to the patient. One example
of a flow
regulator that may be used is described below with references to Figs. 15A and
15B.
However, it will be appreciated that any of the flow regulators described
herein may be used.
Disposed within housing 532 is a flow control device 542 that is used
orchestrate gas flows
through housing 532. Flow control device 542 comprises a cylindrical member
544 that may
slide within housing 532 and includes a flow path 546 that permits gas flow
between
interfaces 534 and 536 when flow control device 542 is in the position
illustrated in Fig. 14A.
Conveniently, a spring 548 or other biasing mechanism is used to hold flow
control device
542 in the home position illustrated in Fig. 14A. Flow control device 542 also
includes a
flow path 550 illustrated by the arrow in Fig. 14A to permit gas flows between
regulator 540
and interface 536. Hence, when in the home position, a vacuum may be supplied
through
interface 536 which lowers the person's intrathoracic pressure. If the vacuum
becomes to
great, gas flows are permitted through regulator 540 to lower the amount of
vacuum.
[0090] As illustrated in Fig. 14B, flow control device 542 also includes a
flow path 552 that
passes from interface 538 to interface 534. This permits a positive pressure
breath to be
supplied to the patient through interface 538. More specifically, as gasses
are injected
through interface 538, they flow into flow control device 542 causing it to
move within
housing 532 and compress spring 548. In so doing, flow path 546 closes as it
becomes
22
CA 02523847 2005-10-27
WO 2004/096109
PCT/US2004/012294
blocked by housing 532. Flow path 550 also closes, leaving only flow path 552
opened to
permit the respiratory gases to flow to the patient. When the positive
pressure breath stops,
spring 548 forces flow control device back to the home position where the
vacuum is once
again supplied to the patient.
[0091] Hence, when a vacuum is applied from interface 536, air is pulled out
of the patient
through interface 534 until the cracking pressure of the impedance valve 540
is reached. At
that point air passes through impedance valve 540 from the ventilation source
at interface
538, thereby setting the limit of the vacuum achieved in the patient. When
positive pressure
ventilation is delivered from the ventilation source at interface 538, the
internal slider switch
cylinder 542 moves downward to close off the vacuum source, allowing for
delivery of a
positive pressure volume to provide a breath to the patient. Flow control
device 542 may
include a cup-shaped opening 556 which helps to move the device 542 along with
minimal
force applied. Once the breath has been delivered, and there is no positive
force delivered
from the ventilation source to the device 542, spring 548 pushes upwards, re-
exposing the
patient to the vacuum source.
[0092] Device 530 may also include an optional pressure pop-off regulator 560.
In the
event that the vacuum source is too great, the pop-off regulator 560 opens
allowing for
pressure relief above the desired vacuum pressure. The pop-off regulator 560
may be
configured to open for pressures greater than about 20 to about 100 mmHg.
[0093] Although the devices illustrated in Figs. 13 and 14 are shown with
mechanical
switching mechanisms, others may also be used, such as magnetic, electronic,
or electrical.
Other kinds of possible switches include a ball valve, flapper valve, fish
mouth valve, or
other mechanical means as well as electric or electronic valving systems,
including a
solenoid, to allow for temporary inhibition of the vacuum once the positive
pressure breath is
delivered from the ventilation source. Additional regulators can also be used
on the vacuum
source to limit the flow or force of the vacuum. For example, the vacuum
source could be
configured to provide a constant vacuum once a threshold level has been
achieved. In
addition, the vacuum regulator and impedance valves 508 and 530 may be
variable or set at a
fixed level of impedance. The vacuum source may also be a suction line or come
from a
venture device attached to an oxygen tank that could both provide oxygen to
the patient and a
vacuum source. Further, the invention is not limited to using an impedance
valve, as shown,
to regulate the vacuum. Multiple switching and regulating means may be used
instead. The
23
CA 02523847 2005-10-27
WO 2004/096109
PCT/US2004/012294
-
ventilation source is similarly not limiting and may include sources such as
mouth-to-mouth,
a bag-valve resuscitator, an automatic ventilator, and the like.
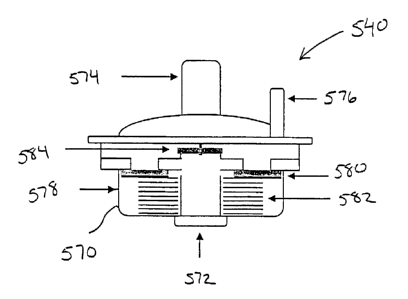
[0094] Figs. 15A and 15B illustrate flow regulator 540 in greater detail.
Regulator 540
comprises a housing 570 having a patient port 572 and a ventilation port 574.
Optionally, a
supplemental oxygen port 576 may also be provided. Gas may flow through
housing 570
(between ports 572 and 574) through one of two flow paths. The first flow path
is blocked by
a one way check valve 578 that comprises a check valve gasket 580 and a spring
582. The
second flow path is blocked by a diaphragm 584.
[0095] In operation, a vacuum is experienced at patient port 572 as the vacuum
source
draws a vacuum at port 536 (See Fig. 14A). When the vacuum reaches a threshold
level,
spring 582 compresses to move gasket 580 downward, thereby creating a flow
path as
illustrated in Fig. 15B. As the vacuum is pulled, diaphragm 584 closes to
prevent air from
flowing through the other flow path. Gasket 580 remains spaced apart from the
opening as
long as the vacuum is at the threshold level. In this way, regulator 540 is
able to maintain the
vacuum at a constant level.
[0096] When ready to ventilate the patient, the vacuum is stopped and
respiratory gases are
injected into port 574 and/or port 576. These gasses lift diaphragm 584 to
permit the gases to
flow to the patient.
Example 3
[0097] Example 3 is another non-limiting example illustrating how intracranial
pressures
and intrathoracic pressures may be lowered and systolic arterial pressure may
be increased
according to one aspect of the invention. In this example, 30 kg pigs were
anesthetized with
propofol. Using a micromanometer-tipped electronic Millar catheter inserted 2
cm below the
24
CA 02523847 2005-10-27
WO 2004/096109
PCT/US2004/012294
objectives, methods, results, and conclusions describing these novel
cardiopulmonary-cranial
interactions are summarized below.
[0098] An objective of this example was to evaluate the acute use of a novel
inspiratory
impedance threshold device (ITD) attached to a controlled but continuous
vacuum (CV)
source to decrease intrathoracic pressure (ITP) and intracranial pressure
(ICP) but
simultaneously increase mean arterial pressure (MAP), coronary perfusion
pressure (CPP)
and cerebral perfusion pressure (CerPP) in an apneic pig model of sequential
insults of
cardiac arrest and fixed-bleed hemorrhage hypotensive shock. This animal model
is
associated with both elevated ICP after cardiac arrest and significant
hypotension after
hemorrhage.
[0099] This example used 6 female farm pigs (28-32kg) that were anesthetized
with
propofol, intubated and ventilated to maintain normocarbia and 02 saturation
>90%.
Ventricular fibrillation was induced and followed by 6 min of no treatment, 6
min of standard
CPR, and then defibrillation. After return of spontaneous circulation and
while ventilated
mechanically at 10 breaths/min, 35% of blood volume was removed with a rate of
60 cc/min.
Five min later ITD-CV was applied for 5 min along with positive pressure
ventilation with
100% oxygen at a rate of 10 bpm. The ITD-CV was then removed and positive
pressure
ventilation at a rate of 10 breaths/min was reapplied. Hemodynamic parameters
and arterial
blood gases were assessed before, during, and after ITD-CV application.
Statistical analysis
was performed with a paired t-test and ANOVA to compare +/- ITD-CV use.
[0100] The results are summarized in the Table below. As shown, by regulating
thoracic
pressures, use of the ITD-CV causes an instantaneous decrease in ITP and ICP
as well as a
rapid rise in MAP and a marked increase in CerPP. Hence, the ITD-CV may be
used to treat
hypotension, shock, and cerebral hypertension.
Table
Before ITD-CV During ITD-CV After ITD-CV p value
ITP 0.5 0.1 -12.0 1.1 0.1 0.2
0.001
MAP 46.7 5.2 54.7 7.7 38. 4.1 0.03
3
ICP 14.1 3.9 6.1 4.5 15. 3.9 0.001
4
CerPP 32.7 4.2 48.6 5.9 23. 4.5 0.01
0
CA 02523847 2005-10-27
WO 2004/096109
PCT/US2004/012294
CPP 40.1 4.5 58.4 7.7 31. 3.4 0.008
1
[0101] The invention has now been described in detail for purposes of clarity
and
understanding. However, it will be appreciated that certain changes and
modifications may
be practiced within the scope of the appended claims.
26