Note: Descriptions are shown in the official language in which they were submitted.
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
METHOD OF COMPUTER-ASSISTED LIGAMENT BALANCING AND
COMPONENT PLACEMENT IN TOTAL KNEE ARTHROPLASTY
FIELD OF INVENTION
The invention relates generally to computer-assisted surgical (CAS)
systems and methods of their use. More specifically, the invention relates to
instrumentation, systems, and processes for proper positioning, and alignment
of
the prosthetic knee components and proper balancing of soft tissues, including
any necessary surgical release or contraction, of the knee ligaments, during
computer-assisted total knee replacement (TKR) surgery.
BACKGROUND
Computer-assisted surgical systems use various imaging and tracking
devices and combine the image information with computer algorithms to track
the
position of the patient's anatomy, surgical instruments, prosthetic
components,
virtual surgical constructs such as body and limb axes, and other surgical
structures and components. The computer-assisted surgical systems use this
data to make highly individualized recommendations on a number of parameters,
including, but not limited to, patient's positioning, the most optimal
surgical cuts,
and prosthetic component selection and positioning. Orthopedic surgery,
including, but not limited to, joint replacement surgery, is one of the areas
where
computer-assisted surgery is becoming increasingly popular.
During joint replacement surgery, diseased or damaged joints within the
musculoskeletal system of a human or an animal, such as, but not limited to, a
knee, a hip, a shoulder, an ankle, or an elbow joint, are partially or totally
replaced with long-term surgically implantable devices, also referred to as
joint
implants, joint prostheses, joint prosthetic implants, joint replacements, or
prosthetic joints.
Knee arthroplasty is a procedure for replacing components of a knee joint
damaged by trauma or disease. During this procedure, a surgeon removes a
portion of one or more knee bones forming the knee joint and installs
prosthetic
1
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
components to form the new joint surfaces. In the United States alone,
surgeons
perform approximately 250,000 total knee arthroplasties (TKAs), or total
replacements of a knee joint, annually. Thus, it is highly desirable to
improve this
popular technique to ensure better restoration of knee joint function and
shortening the patient's recovery time.
The structure of the human knee joint is detailed, for example, in
"Questions and Answers About Knee Problems" (National Institute of Arthritis
and
Musculoskeletal and Skin Diseases (NIAMS) Information Clearinghouse National
Institutes of Health (NIH), Bethesda, MD, 2001). The human knee joint includes
essentially four bones. The lower extremity of the femur, or distal femur,
attaches
by ligaments and a capsule to the proximal tibia. The distal femur contains
two
rounded oblong eminences, the condyles, separated by an intercondylar notch.
The tibia and the femur do not interlock but meet at their ends. The femoral
condyles rest on the condyles of the proximal tibia. The fibula, the smaller
shin
bone, attaches just below the tibia and is parallel to it. The patella, or
knee cap, is
at the front of the knee, protecting the joint and providing extra leverage. A
patellar surface is a smooth shallow articular depression between the femoral
condyles at the front. Cartilage lines the surfaces of the knee bones,
cushions
them, and minimizes friction. Two C-shaped menisci, or meniscal cartilage, lie
between the femur and the tibia, serve as pockets for the condyles, and
stabilize
the knee. Knee ligaments connect the knee bones and cover and stabilize the
joint. The knee ligaments include the patellar ligament, the medial and
lateral
collateral ligaments, and the anterior (ACL) and posterior (PCL) cruciate
ligaments. The medial collateral ligament (MCL) provides stability to the
inner
(medial) part of the knee. The lateral collateral ligament (LCL) provides
stability
to the outer (lateral) part of the knee. The anterior cruciate ligament (ACL),
in the
center of the knee, limits rotation and the forward movement of the tibia. The
posterior cruciate ligament (PCL), also in the center of the knee, limits
backward
movement of the tibia. Ligaments and cartilage provide the strength needed to
support the weight of the upper body and to absorb the impact of exercise and
activity. Tendons, such as muscle, and cartilage are also instrumental to
joint
stabilization and functioning. Some examples of the tendons are popliteus
2
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
tendon, which attaches popliteus muscle to the bone. Pes anserinus is the
insertion of the conjoined tendons into the proximal tibia, and comprises the
tendons of the sartorius, gracilis, and semitendinosus muscles. The conjoined
tendon lies superficial to the tibial insertion of the MCL. The iliotibial
band
extends from the thigh down over the knee and attaches to the tibia. In knee
flexion and extension, the iliotibial band slides over the lateral femoral
epicondyle.
The knee capsule surrounds the knee joint and contains lubricating fluid
synovium.
A healthy knee allows the leg to move freely within its range of motion
while supporting the upper body and absorbing the impact of its weight during
motion. The knee has generally six degrees of motion during dynamic
activities:
three rotations (flexion/extension angulations, axial rotation along the long
axis of
a large tubular bone, also referred to as interior/exterior rotation, and
varus/valgus angulations); and three translations (anterior/posterior,
medial/lateral, and superior/inferior).
A total knee arthroplasty, or TKA, replaces both the distal femur and the
proximal tibia of the damaged or diseased knee with artificial components made
of various materials, including, but not limited to, metals, ceramics,
plastics, or
their combinations. These prosthetic knee components are attached to the
bones, and the existing soft tissues are used to stabilize the artificial
knee.
During TKA, after preparing and anesthetizing the patient, the surgeon makes a
long incision along the front of the knee and positions the patella to expose
the
joint. After exposing the ends of the bones, the surgeon removes the damaged
tissue and cuts, or resects, the portions of the tibial and femoral bones to
prepare
the surfaces for installation of the prosthetic components.
To properly prepare femoral surfaces to accept the femoral and tibial
components of the prosthetic knee, the surgeon needs to accurately determine
the position of and perform multiple cuts. The surgeon may use various
measuring and indexing devices to determine the location of the cut, and
various
guiding devices, such as, but not limited to, guides, jigs, blocks and
templates, to
guide the saw blades to accurately resect the bones. After determining the
3
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
desired position of the cut, the surgeon usually attaches the guiding device
to the
bone using appropriate fastening mechanisms, including, but not limited to,
pins
and screws. Attachment to structures already stabilized relative to the bone,
such as intramedullary rods, can also be employed. After stabilizing the
guiding
device at the bone, the surgeon uses the guiding component of the device to
direct the saw blade in the plane of the cut.
After preparation of the bones, the knee is tested with the trial
components. Soft-tissue balancing, including any necessary surgical release or
contraction of the knee ligaments and other soft tissues, is performed to
ensure
proper post-operative functioning of the knee. Both anatomic (bone-derived
landmarks) and dynamic or kinematic (ligament and bone interactions during the
knee movement) data may be considered when determining surgical cuts and
positioning of the prosthetic components. After ligament balancing and proper
selection of the components, the surgeon installs and secures the tibial and
femoral components. The patella is typically resurfaced after installation of
the
tibial and femoral component, and a small plastic piece is often placed on the
rear side, where it will cover the new joint. After installation of the knee
prosthesis, the knee is closed according to conventional surgical procedures.
Post-operative rehabilitation starts shortly after the surgery to restore the
knee's
function.
In order to ensure proper post-operative functioning of the prosthetic knee,
proper positioning, and alignment of the prosthetic knee components and proper
balancing, including any necessary surgical release or contraction, of the
knee
ligaments, during total knee replacement (TKR) surgery is necessary. Improper
positioning and misalignment of the prosthetic knee components, and improper
ligament balancing commonly cause prosthetic knees to fail, leading to
revision
surgeries. This failure increases the risks associated with knee replacement,
especially because many patients requiring prosthetic knee components are
elderly and highly prone to the medical complications resulting from multiple
surgeries. Also, having to perform revision surgeries greatly increases the
medical costs associated with the restoration of the knee function. In order
to
prevent premature, excessive, or uneven wear of the artificial knee, the
surgeon
4
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
must implant the prosthetic device so that its multiple components articulate
at
exact angles, and are properly supported and stabilized by accurately balanced
knee ligaments. Thus, correctly preparing the bone for installation of the
prosthetic components by precisely determining and accurately performing all
the
required bone cuts, and correct ligament balancing are vital to the success of
TKR.
Traditionally, the surgeons rely heavily on their experience to determine
where the bone should be cut, to select, align and place the knee prosthetic
components, and to judge how the knee ligaments should be contracted or
released to ensure proper ligament balancing. With the advent of computer-
assisted surgery, surgeons started using computer predictions in determining
surgical cutting planes, ligament balancing, and selection, alignment and
positioning of the prosthetic components. In the conventional TKR methods,
anatomical (bone-derived landmarks) and dynamic or kinematic (ligament and
bone interactions during the knee movement) data are usually considered
separately when determining surgical cuts and positioning of the components of
the prosthetic knee. Generally, conventional methods are either excessively
weighted toward anatomical landmarks on the leg bones or soft tissue balancing
(such as adjustment of lengths and tensions of the knee ligaments). Often,
only
femoral landmarks are considered when determining femoral component
positioning, and only tibial landmarks are considered when determining tibiaf
component positioning. In the conventional techniques, irreversible bone cuts
in
the knee are usually made prior to considering the dynamic balance of the
surrounding soft tissue envelope.
One conventional method of determining the femoral resection depth is
anterior referencing, which is primarily focused on placing the femoral
component
in a position that does not notch or stuff anteriorly. The method largely
ignores
the kinematics of the tibio-femoral joint. Another conventional method,
posterior
referencing of the femoral resection depth uses the posterior femoral condyles
as
a reference for resection, but also ignores the dynamic tissue envelope. As an
additional drawback, varus and vaigus knee deformities affect the resection
depth
determination by anterior and posterior referencing.
5
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
Determining the resection depth based on the surrounding soft tissue
envelope is also problematic. If the resection determination is made before
the
envelope is adequately released, the resection may be inappropriately placed
and, likely, excessive. Generally, ignoring the important anatomical landmarks
can result in significant malrotation of the femoral component with respect to
the
bony anatomy.
Conventional anatomical methods of determining femoral component
positioning employ the anatomical landmarks such as epicondylar axes,
Whiteside's line, and the posterior condyles. By using these anatomical
landmarks and ignoring the state of the soft tissue envelope around the knee,
the
methods encounter certain limitations. For example, the epicondylar axes rely
on
amorphous knee structures and, thus, are not precisely reproducible.
Typically,
several sequential determinations of the epicondylar axis produce differing
results. Exposing the condyies to determine the epicondylar axis requires
significant tissue resection and increases risks to the patient and healing
time.
Whiteside's line is based on the orientation of the trochlea and is also not
precisely reproducible. Furthermore, the line is not correlated with the bony
anatomy and ligaments of the tibio-femoraf joint in either flexion or
extension.
While easily reproduced, resection of the femur parallel to the posterior
femoral condyies is potentially inaccurate because it ignores the dynamic
status
of the surrounding soft tissue envelope. Further, the deformity and wear
pattern
of the arthritic knee is incorporated into the decision. For example, varus
knees
typically have significant cartilage wear in the posterior portion of the
medial
femoral condyle, while the lateral femoral condyle often has a normal
cartilage
thickness posteriorly. This results in excessive rotation of the femoral
component
upon placement. Knees with vaigus maialignment and lateral compartment
arthrosis typically have full-thickness cartilage loss in the lateral femoral
condyle,
and under-development, or hypoplasia of the condyle. The use of posterior
referencing to determine femoral component rotation typically results in
excessive
internal rotation of the femoral component.
6
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
Determining femoral component rotation based on the surrounding soft
tissue envelope is attractive because resection of the femur perpendicular to
the
tibia at 900 of flexion with the ligaments under distraction assures a
rectangular
flexion gap. However, this method ignores the anatomy of the femur and the
extent of the ligament release. For example, if the knee is severely varus and
is
inadequately released, then the medial side will remain too tight, which
results in
excessive external rotation of the femoral component. The opposite problem
arises due to inadequate released knees with vaigus-flexion contractures.
Several systems and methods of computer-assisted ligament balancing
are known. One system and method compares the kinematics of the trial
prosthetic joint components installed in a knee joint with the kinematics of
the
normal joint, and provides the surgeon with the information allowing the
balancing
of the ligaments of the installed prosthetic joint. A video camera registers
and a
computer determines the position and orientation of the trial components with
respect to each other and the kinematics of the trial components relative to
one
another, identifies anomalies between the observed kinematics of the trial
components and the known kinematics in a normal knee, and then suggests to
the surgeon which of the ligaments should be adjusted to achieve a balanced
knee. Essentially, the femur and the tibia are cut first, and the knee
kinematics
are checked after the irreversible bone cuts are made and trial prosthetic
components are installed. The method is not suitable for prediction of the
optimal
bone cuts based on the combination of the anatomic and the kinematic data, and
does not employ the combination of such data in prosthetic component
positioning and ligament balancing. Furthermore, the method requires the use
of
the video camera to acquire the images of the installed trial components and
employs complex "machine vision" algorithm to deduce the position of the
prosthetic components and other landmarks from the images.
Another known method of computer assisted ligament balancing provides
for ligament balancing prior to femoral resection and prosthetic component
positioning, but relies on using a tensor that is inserted between the femur
and
the tibia and separates the ends of the tibia and the femur during kinematic
testing. The method relies extensively on visual images and surgeon judgment
in
7
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
ligament alignment, selection of the implant geometry and size, and
determination of the femoral resection plane, and prosthetic component
positioning.
There is an unrealized need for improved systems and methods for
computer-assisted soft-tissue balancing, component placement, and surgical
resection planning during TKA. Particularly, the field of computer assisted
TKA
needs improved methods and systems that consider and correlate both
anatomical landmarks and dynamic interactions of the knee bones and soft
tissues. Systems and methods are also desired that incorporate soft tissue
balancing and component placement algorithms for quantitative assessment of
the anatomical and dynamic aspects of the human knee and provide
recommendations on soft tissue balancing, component selection and/or
placement, and propose bone resection planes based on iterative convergence of
the anatomical and the dynamical factors. Preferably, the desired systems and
methods comprise a logic matrix for quantitative assessment of the state of
the
knee's soft tissues. Systems and methods are also needed that allow for
prosthetic component selection and/or placement, soft tissue balancing, and
resection planning in a variety of combinations and sequences, based on the
patient's need and the surgeon's preference. There is also a need in the
systems
and methods that allow for component selection and/or placement, soft tissue
balancing, and resection planning prior to making any surgical cuts.
In general, there is a need for systems and methods that are flexible and
allow the surgeon to operate in accordance with the patient's need and the
surgeon's own preferences and experience, that do not limit the surgeon to a
particular surgical technique or method, and that allow for easy adaptation of
the
existing surgical techniques and method to computer-assisted surgery, as well
as
for the improvement of and development of new surgical techniques and
methods. The field of computer-assisted surgery is in need of the improved
systems and methods for computer-assisted soft-tissue balancing, component
placement, and surgical resection planning during TKA that are versatile,
provide
reliable recommendations to the surgeon, and result in improved restoration of
the knee function and patient's recovery as compared to the conventional
8
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
methods. Some or all, or combinations of some, of these needs are met in
various systems and processes according to various embodiments of the
invention.
SUMMARY
The aspects and embodiments of the present invention provide improved
systems, methods and processes for computer-assisted soft tissue balancing,
including ligament balancing, such as release or contraction of knee
ligaments,
determining surgical cuts, and selection and/or positioning or placement of
the
components of the prosthetic knee during TKR. The improved methods,
systems, and processes consider and correlate anatomical landmarks and
dynamic interactions of the knee bones and soft tissues. The improved methods,
systems and processes resolve several problems related to the prosthetic knee
component positioning and soft-tissue balancing during computer-assisted TKR.
The improved methods, systems and processes are flexible and versatile,
provide reliable recommendations to the surgeon, and improve restoration of
the
knee function and patient recovery.
In one aspect, certain embodiments of the invention provide a system for
use by a surgeon in the course of computer-assisted total arthroplasty on a
patient's knee. The system comprises:
at least one first fiducial associated with a femur or a femoral prosthetic
component;
at least one second fiducial associated with a tibia or the tibial prosthetic
component;
a tracking functionality capable of tracking position and orientation of the
at least
one first fiducial and the at least one second fiducial;
a computer, wherein the computer is
adapted to receive and store information from the tracking functionality on
the position and orientation of the at least one first fiducial and thus at
9
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
least one the femur or the femoral prosthetic component, and the at least
one second fiducial and thus at least one of the tibia or the tibial
prosthetic
component,
adapted to receive and store information acquired during kinematic testing
of the knee on the position and orientation of the at least one first fiducial
and thus the at least one of the femur or the femoral prosthetic
component; and the at least one second fiducial and thus the at least one
of the tibia or the tibial prosthetic component;
adapted to store in memory a logic matrix for assessing kinematics of the
knee by comparing to the logic matrix the information acquired during the
kinematic testing of the knee, and
adapted to provide recommendations on soft tissue balancing based on
comparison to the logic matrix of the information obtained during the
kinematic testing.
The system may further comprise:
an imager for obtaining at least one image of the tibia or the femur, wherein
the
computer is adapted to receive from the imager and store at least one image of
the tibia, the femur, the tibial prosthetic component, or the femoral
prosthetic
component; and
a monitor adapted to receive information from the computer in order to display
the at least one image of the tibia, the femur, the tibial prosthetic
component, or
the femoral prosthetic component.
The system may further comprise surgical instruments associated with one
or more fiducials and adapted for navigation and positioning at the knee. The
fiducials associated with the instruments are tracked by the tracking
functionality.
Real or schematic images of the instruments may be displayed on the monitor.
The systems, methods, and processes provided herein may be adapted to
beneficially use the images of the body parts, surgical instrumentations and
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
items, and prosthetic components. Nevertheless, unlike in the existing
methods,
continuous image acquisition and "machine vision" algorithms are not required
for
operation of the systems, methods and processes according to certain aspects
and embodiments of the present invention. The methods, systems, and
processes provided herein are generally adapted to derive the position and
orientation of the relevant landmarks and structures by establishing
appropriate
coordinate systems and tracking the fiducials in relation to the coordinate
systems. This advantageously simplifies the operation of the systems, methods
and processes of the present invention and releases processing capacity for
other operation.
The system may further comprise prosthetic components associated with
one or more fiducials and adapted for navigation and positioning at the knee.
The fiducials associated with the prosthetic components are tracked by the
tracking functionality. Real or schematic images of the prosthetic components
may be displayed on the monitor. The computer may be further adapted to store
in memory information on various types of prosthetic components, such as their
size and mode of positioning, and to provide recommendations to the surgeons
on component selection and positioning based on the patient data.
The system may further comprise at least one cutting jig or cutting guide
for positioning at the femur or the tibia, wherein the cutting jig is
associated with
one or more fiducials and the position and orientation of the fiducial
associated
with the cutting jig is trackable by the computer for navigation and
positioning of
the cutting jig at the femur. The position of the cutting jig or cutting guide
may be
adjustable in at least one degree of rotational or at least one degree of
translational freedom. The cutting jig or cutting guide may be adapted for
performing several surgical cuts.
In another aspect, certain embodiments of the invention provide a method
of computer-assisted total arthroplasty on a patient's knee. The method
comprises:
11
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
registering with a computer at least one first fiducial associated with the
femur or
the femoral prosthetic component; and at least one second fiducial associated
with the tibia or the tibial prosthetic component;
tracking position and orientation of the at least one first fiducial and the
at least
one second fiducial with a tracking functionality;
using the computer adapted to receive signals and store information from the
tracking functionality on the position and orientation of the at least one
first
fiducial and thus at least one of the femur or the femoral prosthetic
component;
and the at least one second fiducial and thus at least one of the tibia or the
tibial
prosthetic component;
assessing performance of the knee using kinematic testing of the knee in six
degrees of spatial freedom;
using the computer to compare information from the tracking functionality
obtained during the kinematic testing, and
using the computer to provide recommendations on soft tissue balancing of the
knee based on the comparison with the logic matrix.
The method may further comprise:
using an imager for obtaining at least one image of a tibia or a femur,
wherein the
computer is adapted to receive from the imager and store the at least one
image
of the tibia, the femur, the femoral prosthetic component, or the tibial
prosthetic
component; and
using a monitor adapted to receive information from the computer to display
the
at least one image of the tibia, the femur, the tibial prosthetic component,
or the
tibial prosthetic component.
The method may further comprise registering with the computer and
navigating and positioning at the knee of the surgical instruments associated
with
one or more fiducials. The method may further comprise registering with the
12
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
computer and navigating and positioning at the knee of prosthetic components
associated with one or more fiducials. The method may further comprise
registering with the computer and navigating and positioning at the femur,
using
the images displayed on the monitor, of a cutting jig or a cutting guide
associated
with one or more fiducials.
Other aspects and embodiments of the present invention extend to an
improved versatile and flexible computer algorithm for controlling a computer
used during computer-assisted surgery on a patent's knee. When controlling the
computer, the algorithm assesses the state of the knee based on the kinematic
testing and provides recommendations on soft tissue balancing. The algorithm
also allows selection or prosthetic component size, prosthetic component
positioning, or planning of surgical cuts, or any combination thereof. The
algorithm is adaptable to the patient's needs and the surgeon's preferences
and
does not limit the surgeon to a particular surgical technique or sequence of
steps.
The algorithm is easily adaptable to the existing surgical techniques and
methods.
Flexibility and versatility are important advantages of certain methods,
systems and processes provided by the embodiments of the present invention,
unlike existing methods that require the surgeons to perform according to
strictly
pre-determined procedures and are often limited to a subset of situations that
arise in the process of TKA. In contrast, the embodiments of the present
invention allow the surgeon to pivot more easily than the conventional
methods,
taking into account personal preferences, patient's need, and computer
generated recommendations.
One embodiment of the invention provided herein is an improved system
and method of computer-assisted soft tissue balancing in a knee during total
knee arthroplasty, wherein the method considers and correlates both the
anatomical landmarks and the dynamic interaction of the knee bones and
ligaments. The method advantageously considers both femoral and tibial
landmarks. According to some embodiments of the provided method, prosthetic
component size, positioning, and surgical cuts can be planned before any
13
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
irreversible bone cuts are made, although the system and method are adaptable
for soft tissue balancing in patients after the surgical cuts are performed,
or after
the prosthetic components are installed. The method facilitates minimally
invasive, small-incision TKR by providing recommendation on optimal surgical
cuts and component positioning and reducing the need in revision surgeries.
The system and method register and consider the anatomical landmarks
and the dynamic data from the knee in flexion and extension under one or more
kinematic tests, such as varus/valgus, AP drawer, and rotation tests. A knee
is
considered properly balanced when cutting planes advised by the anatomical
methods and cutting planes advised by dynamic methods converge. When the
anatomic and the dynamic recommendations differ, more soft tissue balancing
may be provided, after which the anatomic and the dynamic recommendations
may change. This is an iterative process.
An embodiment of a method of computer-assisted soft tissue balancing in
a knee during total knee arthroplasty is provided. Essentially, the method
establishes a rectangular gap between tibia and femur in both flexion and
extension without distorting the anatomy of the knee. It is perfectly
conducted
after the surgeon exposes the bones, and performs any preliminary osteophyte
(bony excrescence at the joint margin, such as those seen in osteoarthritis)
resections and ligament release. The method employs the following steps
performed with computer assistance:
1. Establishing femoral and tibial coordinate systems by tracking at least
one fiducial associated with a femur and at least one fiducial
associated with a tibia;
2. Establishing in a computer memory a femoral resection plane
perpendicular to a mechanical axis of the femur (an anatomical femoral
resection plane), and a proposed tibial resection plane perpendicular to
a mechanical axis of the tibia.
3. Placing the knee under distraction in flexion and extension in at least
one of varus/vaigus, AP drawer, or rotation tests, and establishing, in
14
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
flexion and extension, in a computer memory femoral resection planes
perpendicular to the long axis of the tibia.
4. Comparing the femoral resection planes perpendicular to the long axis
of the tibia (dynamic resection planes) to the femoral resection planes
perpendicular to the mechanical axis of the femur (anatomical
resection planes), whereby the state of the ligament balance of the
knee is represented in flexion and extension by an angle formed
between the femoral anatomical resection plane and the femoral
dynamic resection planes in flexion and extension.
5. Using the computer to provide recommendations to the surgeon on
adjustment of soft tissue leading to the decrease of the angle formed
between the femoral anatomical resection plane and the femoral
dynamic resection planes in flexion and extension.
6. Adjusting the soft tissues; and
7. Repeating the steps 4-6 until the anatomical and the dynamic planes
converge.
The method may further comprise the steps of placing a distal femoral
cutting jig at the femur and resecting the femur based at the recommended
converged planes.
Various embodiments of the present invention are better understood in
reference to the following schematic drawings that are provided herein for
illustrative purposes and are in no way limiting. The embodiments of the
present
invention may differ from the provided schematic illustrations.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a schematic representation of an operation of a data input
devices during computer assisted surgery.
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
Figure 2 shows a knee during computer assisted TKA after preliminary
osteophyte resection and ligament release.
Figure 3 is a schematic representation of improved soft tissue balancing
algorithm according to a preferred embodiment of the invention.
Figure 4 is a schematic representation of anatomical landmarks used in
kinematic assessment of the knee, wherein the extended knee is shown in the
anterior/posterior direction.
Figure 5 is a schematic representation of anatomical landmarks used in
kinematic assessment of the knee, wherein the extended knee is shown in the
medial/lateral direction. Comment above
Figure 6 is a schematic representation of anatomical landmarks used in
kinematic assessment of the knee, wherein the flexed knee is shown in the
anterior/posterior direction. Comment above
Figure 7 is a schematic representation of anatomical landmarks used in
kinematic assessment of the knee, wherein the flexed knee is shown in the
medial/lateral direction. Comment above
Figure 8 is a schematic representation of anatomical and dynamic
resection planes in a knee at full extension.
Figure 9 is a schematic representation of anatomical and dynamic
resection planes in a flexed knee.
DETAILED DESCRIPTION
Various aspects and embodiments of the present invention provide
improved systems, methods and processes of soft tissue balancing, determining
surgical cuts, and positioning of the components of the prosthetic knee during
computer-assisted TKA. During installation of a prosthetic knee, systems
according to certain embodiments of the present invention advantageously
assess and provide feedback on the state of the soft tissues in a rage of
motion,
16
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
such as under varus/vaigus, anterior/posterior and rotary stresses, and can
suggest or at least provide more accurate information than that obtainable by
the
conventional methods about soft tissue adjustments, including, but not limited
to
the recommendations on which ligaments the surgeon should release or contract
in order to obtain correct balancing, alignment and stability of the knee
joint.
Systems, methods and processes according to various aspects and
embodiments of the present invention can also provide recommendations on
implant size, positioning, and other parameters relevant to achieving optimal
kinematics of the knee joint. As used herein, the term "kinematics" means the
pattern of motion having six degrees of freedom. More particularly, the term
"kinematics" in reference to a knee joint is used to denote the motion, or
articulation, of the knee joint in six degrees of freedom. Systems and
processes
according to various embodiments of the present invention can also include
databases of information or logic matrixes regarding tasks such as soft tissue
balancing, in order to provide suggestions to the surgeon based on performance
the knee in kinematic tests.
The tests, such as varus/valgus knee distraction, AP drawer test, or axial
rotation are known. Tests which are presently unknown can be included in
systems and processes according to the invention in the future. When the knee
is distracted in the course of kinematic testing, a physical spacer or tensor,
such
as an inflatable balloon, a hydraulic bag, a mechanical device, or any other
physical tensor or spacer, may be applied to the to the knee to achieve the
degree of tension that is the closest to the normal knee tested this way. For
example, for AP drawer test, the spacer is applied to the medial side to
achieve a
desired degree of tension. The physical spacer is typically adapted to be
locked
or stabilized in any desired position. The spacer may comprise a measurement
scale to allow a reading of the gap obtained, and may be adapted to feed the
information to the computer functionality for display and/or use as desired.
Nevertheless, it is one advantage of the present invention over the existing
methods that the use of the spacers and tensors is optional and is based on
the
surgeon's consideration and patient's need.
17
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
Computer-Assisted Surgical Systems
In one aspect, certain embodiments of the present invention provide a
computer-assisted surgical system for use by a surgeon during TKA. Generally,
computer-assisted surgical systems use various imaging and tracking devices
and combine the image information with computer algorithms to track the
position
of the patient's anatomy, surgical instruments, prosthetic components, virtual
surgical constructs such as body and limb axes, and other surgical structures
and
components. Some of the computer-assisted surgery systems use imaging
systems based on CT scans and/or MRI data or on digitized points on the
anatomy. Other systems align preoperative CT scans, MRIs, pr other images
with intraoperative patient positions. A preoperative planning system allows
the
surgeon to select reference points and to determine the final implant
position.
Intraoperatively, the computer-assisted surgery system calibrates the patient
position to that preoperative plan, such as by using a "point cloud"
technique,
conventional kinematic techniques, and/or a robot to make bone preparations.
Other systems use position and/or orientation tracking sensors, such as
infrared
sensors acting stereoscopically or otherwise, to track positions of body
parts,
surgery-related items such as implements, instrumentation, trial prosthetics,
prosthetic components, and virtual constructs or references such as rotational
axes which have been calculated and stored based on designation of bone
landmarks.
As used herein, the term "position and orientation" denotes a position of an
object in three-dimensional space with respect to all six degrees of freedom
relative to a known coordinate system. When the object, such as a body part or
a
prosthetic component, is a solid member, and because the position and
orientation of the fiducial marks associated with the targets are fixed, by
knowing
the position and orientation of the fiducials in space, the position and
orientation
of all surfaces on the object is also known. If the position and orientation
of both
femoral and tibial prosthetic components is known with respect to a single
reference system, the position and orientation of the components relative to
one
another may be determined. Prosthetic components can be navigated relative to
each other in an absolute fashion, that is the computer assumes that the
trials are
18
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
positioned perfectly, and the gaps between the components are tracked relative
to each other without the need for landmarking and without fiducials applied
to
the tibia and the femur. Additional landmarking, for example, for validation
purposes, can be additionally be performed (for example, relative to the
location
of head of the femur and center of the ankle) to determine that the components
were placed as desired.
Processing functionality, whether standalone, networked, or otherwise,
takes into account the position and orientation information as to various
items in
the position sensing field (which may correspond generally or specifically to
all or
portions or more than all of the surgical field) based on sensed position and
orientation of their associated fiducials or based on stored position and/or
orientation information. The processing functionality correlates this position
and
orientation information for each object with stored information regarding the
items, such as a computerized fluoroscopic imaged file of a bone, a wire frame
data file for rendering a representation of an instrumentation component,
trial joint
prosthesis or actual joint prosthesis, or a computer generated file relating
to a
rotational axis or other virtual construct or reference. The processing
functionality
then displays position and orientation of these objects on a screen or
monitor, or
heads-up display or otherwise. The surgeon may navigate tools,
instrumentation,
prosthetic components, actual prostheses, and other items relative to bones
and
other body parts to perform a surgery more accurately, efficiently, and with
better
alignment.
The computer-assisted surgical systems use the position and orientation
tracking sensors to track the fiducial or reference devices associated with
the
body parts, surgery-related items such as implements, instrumentation, trial
prosthetics, prosthetic components, and virtual constructs or references, such
as
limb rotational axes calculated and stored based on designation of bone
landmarks. Any or all of these may be physically or virtually associated with
any
desired form of mark, structure, component, or other fiducial or reference
device
or technique that allows position or orientation, or both, of the associated
item to
be sensed and tracked in space, time, or both. Fiducials can be single markers
or reference frames or arrays containing one or more reference elements.
19
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
Reference elements can be active, such as energy emitting, or passive, such as
energy reflective or absorbing, or any combination thereof. Reference elements
may be optical, employ ultrasound, or employ any suitable form of
electromagnetic energy, such as infrared, micro or radio waves. In general,
any
other suitable form of signaling may also be used, as well as combinations of
various signals. To report position and orientation of the item, the active
fiducials,
such as microchips with appropriate field or a position/orientation sensing
functionality, and a communications link, such as a spread-spectrum radio
frequency link, may be used. Hybrid active/passive fiducials are also
possible.
The output of the reference elements may be processed separately or in concert
by the processing functionality.
To locate and register an anatomical landmark, a CAS system user may
employ a probe operatively associated with one or more fiducials. For example,
the probe may be is triangulated in space relative to two sets of fiducials.
The
one or more fiducials provide information relating the landmark via a
tracking/sensing functionality to the processing functionality. To indicate
input of
a desired point to the processing functionality, one or more devices for data
input
are commonly incorporated into the computer-assisted surgery systems. The
data input devices allow the user to communicate to the processing
functionality
to register data from the probe-associated fiducials.
A CAS system user may input data to the computer functionality by a
variety of means. Some systems employ a conventional computer interface,
such as a keyboard or a computer mouse, or a computer screen with a tactile
interface. In some systems, the user presses a foot pedal to indicate to the
computer to input probe location data. Others use a wired keypad or a wireless
handheld remote. The probe may also interact with arrays, sensors, or a
patient
in such a way as to act like an input device.
During surgery, CAS systems employ a processing functionality, such as a
computer, to register data on position and orientation of the probe to acquire
information on the position and orientation of the patient's anatomical
structures,
such as certain anatomical landmarks, for example, a center of a femoral head.
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
The information is used, among other things, to calculate and store reference
axes of body components such as in a knee or a hip arthroplasty, for example,
the axes of the femur and tibia, based on the data on the position and/or
orientation of the improved probe. From these axes such systems track the
position of the instrumentation and osteotomy guides so that bone resections
position the prosthetic joint components optimally, usually aligned with a
mechanical axis. Furthermore, the systems provide feedback on the balancing of
the joint ligaments in a range of motion and under a variety of stresses and
can
suggest or at least provide more accurate information than in the past about
the
ligaments that the surgeon should release in order to obtain correct
balancing,
alignment and stability of the joint, improving patient's recovery. CAS
systems
allow the attachment of a variable adjustor module so that a surgeon can
grossly
place a cutting block based on visual landmarks or navigation and then finely
adjust the cutting block based on navigation and feedback from the system.
CAS systems can also suggest modifications to implant size, positioning,
and other techniques to achieve optimal kinematics. Instrumentation, systems,
and processes according to the present invention can also include databases of
information regarding tasks such as ligament balancing, in order to provide
suggestions to the surgeon based on performance of test results as
automatically
calculated by such instrumentation, systems, and processes.
CAS systems can be used in connection with computing functionality that
is networked or otherwise in communication with computing functionality in
other
locations, whether by PSTN, information exchange infrastructures such as
packet
switched networks including the Internet, or as otherwise desired. Such remote
imaging may occur on computers, wireless devices, videoconferencing devices or
in any other mode or on any other platform which is now or may in the future
be
capable of rending images or parts of them produced in accordance with the
present invention. Parallel communication links such as switched or unswitched
telephone call connections or Internet communications may also accompany or
form part of such telemedical techniques. Distant databases such as online
catalogs of implant suppliers or prosthetics buyers or distributors or
anatomical
archives may form part of or be networked with the computing functionality to
21
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
give the surgeon in real time access to additional options for implants which
could
be procured and used during the surgical operation.
In some aspects and embodiments, the present invention relates to a
system for use by a surgeon during TKA, comprising: a tracking functionality
adapted to track position and orientation of at least one fiducial attached to
a
knee bone; a computer adapted to receive information from the tracking
functionality in order to track position and orientation of the fiducials, and
instruments for release and contraction of the knee ligaments. The system may
further comprise a tensor for applying tension to the knee ligaments after
resection of the patients' femur or tibia. The computer is adapted to store a
logic
matrix with the various kinematic parameters of the knee. The computer is
programmed to compare the patient's knee kinematic data obtained by the
surgeon during kinematic testing with the parameter stored in the logic matrix
and
to issue the recommendations to the surgeon regarding release or contraction
of
the knee ligaments. The computer may also be adapted to store the data on the
anatomical landmarks, the data relating to the three dimensional position and
orientation of the knee prosthetic components, and the data on the potential
or
existing surgical resection planes. The computer may also be adapted to
calculate virtual surgical constructs, such as the surgical resection planes
or the
axes, based on the data stored in the memory.
Minimally Invasive Surgery
In one more aspect, the embodiments of the present invention provide a
computer-assisted surgical system for TKA that is particularly useful,
although not
limited to, minimally invasive surgical applications. The term "minimally
invasive
surgery" (MIS) generally refers to the surgical techniques that minimize the
size
of the surgical incision and trauma to tissues. Minimally invasive surgery is
generally less intrusive than conventional surgery, thereby shortening both
surgical time and recovery time. Minimally invasive TKA techniques are
advantageous over conventional TKA techniques by providing, for example, a
smaller incision, less soft-tissue exposure, improved collateral ligament
balancing, and minimal trauma to the extensor mechanism (see, for example,
22
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
Bonutti, P.M., et al., Minimal Incision Total Knee Arthroplasty Using the
Suspended Leg Technique, Orthopedics, September 2003). To achieve the
above goals of MIS, it is necessary to modify the traditional implants and
instruments that require long surgical cuts and extensive exposure of the
internal
knee structures. Minimally invasive techniques are advantageous over
conventional techniques by providing, for example, a smaller incision, less
soft-
tissue exposure, and minimal trauma to the tissues. To achieve the above goals
of MIS, it is necessary to modify the traditional surgical techniques and
instruments to minimize the surgical cuts and exposure of the patient's
tissues.
System and methods for use by a surgeon during TKA
In one aspect, the invention provides a system for use by a surgeon in the
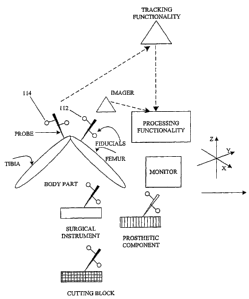
course of computer-assisted total arthroplasty on a patient's knee. Figure 1
is a
schematic view showing one embodiment of a system according to the present
invention. According to this embodiment, the system is used to perform a knee
surgery, particularly total knee arthroplasty. In reference to Figure 1, the
system
comprises a fiducial associated with the femur or the femoral prosthetic
component; a fiducial associated with the tibia or the tibial prosthetic
component;
a tracking functionality capable of tracking position and orientation of the
femoral
and the tibial fiducial. The system can track various body parts, such as
tibia and
femur, or prosthetic components, to which fiducials are implanted, attached,
or
otherwise associated with physically, virtually, or otherwise. In the
embodiment
shown in Figure 1 fiducials are structural frames, at least some of which
comprise
reflective elements, LED active elements, or both, for tracking using a
tracking
functionality, comprising one or more stereoscopic position/orientation
sensors,
such as infrared sensors. The sensors are adapted for sensing, storing,
processing and/or outpufiting data relating to position and orientation of the
fiducials and, thus, components with which they are associated.
The system according to this embodiment of the present invention also
comprises a computer comprising a processing functionality generally adapted
to
receive and store information from the tracking functionality on the position
and
orientation of the femoral fiducial (112) 'and the tibial fiducial (114). In
the
23
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
embodiment shown in Figure 1, the computer may include a processing
functionality, a memory functionality, an input/output functionality, on a
standalone or distributed basis, via any desired standard, architecture,
interface
and/or network topology. In this embodiment, computer functionality is
connected to a monitor, on which graphics and data may be presented to the
surgeon during surgery. The screen may comprise a tactile interface so that
the
surgeon may point and click on screen. The system may also comprise a
keyboard interface, a mouse interface, a voice recognition functionality, a
foot
pedal, or any other functionality for imputing information, wired or wireless,
or any
combination or modification of the functionalities. Such functionalities allow
the
system's user, such as, but not limited to, a nurse or a surgeon, to control
or
direct the functionality, among other things, to capture position/orientation
information.
Items such as body parts, virtual surgical constructs, prosthetic
components, including trial components, implements, and/or surgical
instrumentation may be tracked in position and orientation relative to body
parts
using fiducials. Computer functionality can process, store, and output various
forms of data relating to position, configuration, size, orientation, and
other
properties of the items. When they are introduced into the field of tracking
functionality, computer functionality can generate and display separately or
in
combination with the images of the body parts computer-generated images of
body parts, virtual surgical constructs, trial components, implements, and/or
surgical instrumentation, or other items for navigation, positioning,
assessment or
other uses.
To perform TKA according to aspects and embodiments of the present
invention, surgically related items, as well as body parts, items of the
anatomy
and virtual surgical construct are registered, which means ensuring that the
computer know which body part, item, or constructs corresponds to which
fiducial
or fiducials, and how the position and orientation of the body part, item, or
construct is related to the position and orientation of its corresponding
fiducial.
Registration of body parts may occur in conjunction with acquisition of
images,
which can be obtained together with position and/or orientation information
24
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
received by, noted and stored within the computer functionality. Registration
of
body parts may also occur independently from acquisition of images. The
images may aid the user in designating various anatomical landmarks. For
example, the center of the femoral head may be designated with the purpose of
establishing the mechanical axis of the leg. The center of rotation can be
established by articulating the femur within the acetabuium to capture a
number
of samples of position and orientation information, from which the computer
may
calculate the center of rotation. The center of rotation can also be
established by
using the probe and designating a number of points on the femoral head and
thus
allowing the computer to calculate the center. Graphical representations and
schematics, such as controllably sized circles displayed on the monitor and
fitted
by the surgeon to the shape of the femoral head can also be used to designate
the center of the femoral head. Nevertheless, the systems according to the
aspects and embodiments of the present invention do not necessarily rely on
images to designate the anatomical landmarks and surgical axes. Other
techniques for determining, calculating or establishing points or constructs
in
space can be used in accordance with the present invention.
Before or after registering the body parts, the surgical items may also be
designated by instructing the computer to correlate the data corresponding to
a
particular fiducial or fiducials with the data need to represent a particular
surgical
item. The computer then stores identification, position and orientation
information
relating to the fiducial or fiducials correlated with the data for the
registered
surgical item. Upon registration, when sensor tracks the item, the monitor can
show the item, moving and turning properly positioned and oriented, relative
to
the body part which is also being tracked. The user may navigate the shown
item.
Similarly, various virtual surgical constructs may be registered, such as the
mechanical axis of the leg that passes through the rotational center of the
hip and
the rotational center of the ankle, the mechanical axis of the femur that
passes
through the rotational center of the hip and the center of the femoral
condyles, or
the mechanical axis of the tibia, that passes through the rotational center of
the
ankle and the center of the tibial plateau. Using the images and/or the probe,
the
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
surgeon can select and register in the computer the center of the femoral head
and ankle in orthogonal views on a touch screen. The surgeon then uses the
probe to select any desired anatomical landmarks or references at the
operative
site of the knee or on the skin or surgical draping over the skin. These
points are
registered in three-dimensional space by the system and tracked relative to
the
fiducials on the patient anatomy, which are preferably placed
intraoperatively.
Registering points using actual bone structure is one preferred way to
establish the axis, but other methods can be employed, such as a cloud of
points
approach by which the probe is used to designate multiple points on the
surface
of the bone structure, as can moving the body part and tracking movement to
establish a center of rotation as discussed above. Once the center of rotation
for
the hip, the center of rotation of the ankle, the condylar components or the
tibial
plateau are registered, the computer is able to calculate, store, and render,
or
otherwise use the data related to these anatomical landmarks.
One aspect of the present invention ensures that the prosthetic
components are positioned for the best possible balance of soft tissues in the
knee. Another aspect of the present invention ensures that the prosthetic
components of the correct size and type are chosen to achieve the best
possible
balance of soft tissues in the knee. Thus, the methods, systems and processes
of the present invention may be adapted to provide recommendations on the
prosthetic component type and size, as well as on its positioning. If needed,
additional components or parts may be installed to improve the position of the
implant. Such need may particularly arise during revision surgeries, when
significant portions of the bony anatomy have been removed. Pre-calibrated
trial
prosthetic components, such as trial prosthetic components adapted for
calibration can be utilized in the systems and processes according to the
embodiments of the present invention. Calibration ensures that accuracy of the
stored in the computer memory data on the geometry of the component, and its
position and/or orientation relative to the associated one or more fiducials.
Figure 2 shows an exposed human knee (200) in a surgical field after the
osteophyte resection and the preliminary ligament release. The user registers
26
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
the anatomical landmarks by using a probe (202) comprising fiducials (204) and
associated with the distal femur (206).
Figure 3 schematically represents the improved soft-tissue balancing
algorithm according to certain embodiments of the preset invention. During
operation of these improved systems, methods and processes according to these
aspects and embodiments of the present invention, the user, such as a surgeon,
commands the computer to retrieve the soft-tissue balancing algorithm, also
referred to as advanced ligament balancing algorithm, ligament balancing
algorithm, or ALB. It is to be understood that the term "ligament balancing"
as
used herein may refer to testing and adjustment of the soft tissues of the
knee,
including, but not limited to, ligaments, tendons, and knee capsule soft
tissues.
Upon retrieval of the algorithm by the computer, the surgeon enters his or her
profile and preferences into the computer memory, or commands the computer to
retrieve a profile from its memory. The algorithm takes into account the
stored
profile and preferences when providing recommendations and feedback on soft
tissue balancing.
The surgeon then selects the appropriate option for soft tissue balancing.
In a preferred embodiment, the algorithm provides at least the following
options:
soft tissue balancing and prosthetic component placement in a knee, wherein
the
tibial or femoral, or both, bone cuts have previously been performed, such as
after prosthetic implant installation or during revision surgery; navigation
of bony
resections in a knee followed by component placement and soft tissue
balancing;
and soft tissue balancing, component placement, and bony resection planning in
a knee.
In one embodiment, described herein in reference to Figure 3, the user
employs the system and method provided herein for soft tissue balancing. For
example, the user employs the balancing algorithm in a knee where the surgical
cuts have been performed. The trial prosthetic components can also have been
selected and installed utilizing conventional surgical methods. When using the
balancing algorithm for ligament and soft tissue balancing and prosthetic
component placement in a knee where the tibial or femoral, or both, bone cuts
27
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
have been performed, the surgeon establishes femoral and tibial coordinate
systems, inputs or invokes from computer memory the implant and surgical data,
such as, but not limited to, implant type, size, the operated on side of the
patient.
In this embodiment, one or more fiducials can be associated with the
prosthetic
components, such as a femoral trial prosthetic component, a tibial trial
prosthetic
component, or both. In this case, the femoral and tibial coordinate systems
are
defined, at least in part, by the prosthetic component geometry. The surgeon
can
also establish surgical axes using existing anatomical landmarks. One such
axis
is the mechanical axis of the leg that passes through rotational centers of
the hip
and the ankle center. Various procedures are known and may be employed to
establish the mechanical axis. Using the existing anatomical landmarks allows
the system to determine the position and orientation of the surgical
components
in relation to the existing landmarks and provides the beneficial information
for
verification and/or adjustment of the prosthetic component placement. Using
the
navigated trial components can eliminate the need for fiducial placement on
the
the femur and the tibia, thus eliminating the stress-concentrations caused by
fiducial fixation. The navigational algorithm is invoked for computer-assisted
navigation of the prosthetic components and surgical instruments at the knee.
The system uses the known location, such as, but not limited to, full
extension,
neutral rotation, and neutral rollback, to acquire knee gap data prior to
kinematic
testing. The surgeon then performs kinematic testing at the flexed and
extended
knee. The kinematic tests include but are not limited to, varus/vaigus
rotation,
anterior/posterior drawer, and internal/external rotation. The tests are
conventional in the field of orthopedic surgery and are performed according to
the
accepted in the field guidelines. Other tests can also be used. The computer
registers the anatomical reference points at the distal femoral and proximal
tibial
surfaces, and calculates the kinematic parameters based on the relative
positions
of the reference points.
In another embodiment, the systems and methods provided herein allow
the user, such as a surgeon, to navigate surgical cuts after anatomical
landmarking is performed, and balance the soft tissues after the cuts have
been
made. In further reference to Figure 3, when using the advanced soft tissue
28
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
balancing algorithm for navigation of bony resections in a knee, followed by
component placement and ligament and tissue balancing, the surgeon
establishes femoral and tibial coordinate systems and the surgical references
using the existing anatomical landmarks at the distal femur and proximal
tibia.
For example, tibial and femoral fiducials are applied the tibia and the femur,
the
head of the femur is identified, the center of the ankle is identified, and
other
landmarking is performed as desired, such as determination of rotational axes,
to
establish the anatomical parameters used in determining bony cuts for
prosthetic
component placement. The navigational algorithm is invoked to navigate the
surgical instruments, cutting jigs and guides, and prosthetic components. The
surgeon performs the resections, selects and navigates prosthetic components,
and places them at the knee. Following component placement, the surgeon
performs kinematic testing at the flexed and extended knee. The kinematic
tests
include but not limited to, varus/vaigus rotation, anterior/posterior drawer,
and
internal/external rotation. The computer registers the anatomical reference
points
at the distal femoral and proximal tibial surfaces, and calculates the
kinematic
parameters based on the relative positions of the reference points.
The embodiment of the system and method provided herein can be
adapted to employ any number of instruments to navigate the surgical space for
ligament and soft tissue balancing. Non-navigated prosthetic components,
including trial prosthetic components, also commonly referred to as trials,
spacer
blocks, and tensioners can also be used, particularly, but not limited to,
during
testing and logic matrix comparison. Navigated trial components can be used,
providing an additional advantage of confirming the location of the trials
relative
to the cuts. Navigated cutting blocks could remain in place, or a lock feature
could be employed so that the system is able to determine where the cuts are
relative to the instruments in the space. If non-navigated instruments are
used,
prior to testing the system can acquire knee gap data for a known position,
for
example, but not limited to, full extension, neutral rotation, and neutral
rollback.
In further reference to Figure 3, when using the ligament balancing
algorithm for a ligament and soft tissue balancing, component placement, and
surgical resection planning in a knee, the surgeon establishes femoral and
tibial
29
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
coordinate systems and the surgical references using existing anatomical
landmarks. The navigation algorithm is invoked to navigate the surgical
instruments used in soft tissue balancing. The surgeon performs kinematic
testing at the flexed and extended knee. The kinematic tests include but not
limited to, varus/valgus rotation, anterior/posterior drawer, and
internal/external
rotation. The computer registers the anatomical reference points at the distal
femoral and proximal tibial surfaces, and calculates the kinematic parameters
based on the relative positions of the reference points.
The embodiments of the system and method provided herein compare
data acquired during the kinematic testing of the patient's knee to baseline
kinematic data. This comparison is referred to as a logic matrix or logic
chart,
schematically illustrated in Table 2. As stated earlier, surgeon traditionally
rely on
their judgment during soft tissue balancing and often use subjective measures
to
balance the knee joint. The aspects of the present invention provide an
objective
assessment of the state of the balance of the knee by determining the gaps
between the femur and the tibia at full flexion and full extension and at
intervals in
between as desired during diagnostic varus/valgus, AP drawer, and rotational
tests. The software analyzes the gap data, determines how the gap is shaped
(rectangular versus trapezoid), and compares the gap shape to a logic matrix.
For example in the case of varus/valgus testing, if the gap data, or the
distances
between the medial and the lateral femur and tibia, are below the thresholds
stored in the logic matrix, the system reports a normal knee balance and
indicates that no soft tissue needs to be balanced. However, if the gap
distances
on the medial and/or lateral side exceed the threshold values stored in the
logic
matrix, then system directs the user's attention to the compartment that
appears
to be imbalanced and suggest that the user evaluates those soft tissue
structures. For example, after the user has acquired data from AP drawer,
varus/valgus, and rotational testing, the software indicates that the knee
appears
to be tight medially in flexion only, and that the user should evaluate the
anterior
medial collateral ligament and perform releases deep or superficially as
appropriate.
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
Figures 4-7 schematically illustrate a human knee in extension
(Figures 4 and 6) and flexion (5 and 7) the kinematic parameters and variables
registered and/or calculated during kinematic testing and the anatomical
reference points used in the calculation of the parameters. For ease of
description, the knee (400), comprising femur (402), tibia (404) and fibula
(406) is
shown with respect to Cartesian coordinates. In Figures 4 and 6 (a view in the
anterior-posterior direction), the x- and y-axes lie in a horizontal plane,
and the z-
axis extends vertically. In Figures 5 and 7 (a view in the medial-lateral
direction),
the y- and z-axes lie in a horizontal plane, and the x-axis extends
vertically.
Thus, dx represents the distance in x direction (medial/lateral); dy
represents the
distance in y direction (proximal/distal); dz represents the distance in z
direction
(anterior/posterior). However, it will be appreciated that this method of
description is for convenience only and is not intended to limit the invention
to
any particular orientation. Likewise, unless otherwise stated, terms such as
"top,"
"bottom," "upper," "lower," "left," "right," "front," "back," "proximal,"
"distal,"
"medial," "lateral," "inferior," "superior" and the like are used only for
convenience
of description and are not intended to limit the invention to any particular
orientation. The anatomic reference points and the kinematic parameters, or
variables, used during soft tissue balancing, include, but are not limited to,
those
listed in Table 1.
Table 1
Kinematic variables
Variable Description
ri internal rotation
re external rotation
fa flexion angle
lfce lateral femoral condyle tangent point in extension
mfce medial femoral condyle tangent point in extension
It lateral tibial tangent point in extension and flexion
mt medial tibial tangent point in extension and flexion
plfc posterior lateral femoral condyle tangent point in
flexion
31
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
pmfc posterior medial femoral condyle tangent point in
flexion
le distance from lfce to It (in extension)
me distance from mfce to mt (in extension)
If distance from plfc to It (in flexion)
mf distance from pmfc to mt (in flexion)
It is to be understood that the reference points used in the assessment of
the kinematic parameters do not have to be repeatedly registered and/or
tracked
during the kinematic testing. Once the patient's tibia and femur are
registered by
or known to the computer-assisted surgical systems, the system tracks the one
or
more fiducials associated with the tibia and the femur, the femoral or tibial
prosthetic components, or any combination thereof, respectively, and deduces
the location of the reference points from the information on the
position/orientation of the tibia and the femur. The position and orientation
of the
reference points relative to the corresponding fiducials may be initially
saved in
the computer memory by inputting their location with an appropriate probe.
Alternatively, the position and orientation of the reference point may be
deduced
from the position of the tracked fiducials based on the tibial and femoral
surface
data stored in the computer memory.
Table 2 (A and B) schematically shows an embodiment of a logic matrix
used for assessment of the state of the knee based on the kinematic testing
according to one embodiment of the invention. It is to be understood that
Table 2
is divided into parts A and B for ease of representation only. Other
information
can also be added or deleted to or from the matrix, and the information can be
included in the matrix in any desired format, with any desired arrangement of
cells, and any desired context and format of information in these. In any
event,
the logic matrix according to the embodiment generally relates the results of
the
kinetic testing in a knee (columns D through I), their causes (column C) , and
associated conditions (column A). As shown in columns D through I of Table 2
(A and B), the computer assesses and/or compares the kinematic parameters
that are registered and calculated during the kinematic tests listed in row 1,
32
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
columns D through I. Using the criteria shown in columns D though I, rows 2
through 22, the computer evaluates the results of the kinematic tests against
the
logic matrix Based on the relationships in the logic matrix, the computer
outputs
the causes (column A) and the soft tissues needing adjustments (column C). The
computer can output specific instructions, if desired, such as to release a
certain
ligament, or other action. These instructions can also be included in the
matrix if
desired. The logic matrix may be expanded or otherwise changed as desired
and/or as more surgical data are collected, in order to incorporate various
parameters and criteria, associated causes and conditions, kinematic tests,
and
so on. Based on the causes and conditions identified by the computer, the
surgeon adjusts the soft tissues, and repeats the testing cycle, followed by
the
comparison to the logic matrix. The iterative cycle of the kinematic testing,
comparison to the logic matrix and ligament balancing by the surgeon continues
until reasonable convergence of the results of the kinematic testing with the
desirables kinematic properties stored in the computer memory. This process
preferably results in the improved balance of the knee joint. It is to be
appreciated that the general principles of the iterative convergence methods
and
their limitations are well known and are employed in certain embodiments of
the
present invention. For example, the selection of the convergence criteria,
assessment of the relative errors, and avoidance of the local optima are
routinely
addressed in the field of the iterative convergence methods and are attended
to
as relevant and according to the conventional procedures.
When improved balance of the knee joint is achieved, the surgery may be
completed according to the conventional methods and surgical data summary
may be stored in the computer memory, for example, for archival purposes. The
data may also be used intraoperatively to provide recommendations to the
surgeon on the optimal resection planes and the surgeon may perform resections
de novo, followed by component selection and placement, or improve on the
preliminary resections based on the recommendations provided by the system.
33
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
Table 2
Logic matrix
A.
A B C D E F
1 Flexion/ Varus/valgus Varus/varus
Condition # Cause Extension extension flexion
angle
2. ight PCL 1 ight PCL dy (me) = dy (Ie) dy (mf) = dy (If)
medial dy (mf) > dy(me)
extension gap = Medial flexion
lateral extension gap = lateral
gap lexion gap and
lexion gaps >
extension gaps-
lift off around
PCL
3. ight medially 2 nterior dy (me) = dy (le) dy (If) > dy (mf)
in flexion MCL medial lateral flexion
Loose medially extension gap = gap > medial
in extension lateral extension lexion gap
gap
. Balanced in 3a Posterior a> 10 dy (me) = dy (le) dy (mf) = dy (If)
lexion MCL lexion medial dy (If) > dy (le)
contraction extension gap = medial flexion
ight in lateral extension gap = lateral
extension gap texion gap, and
iexion gap is
bigger than
extension gap
5. 3b Medial a> 10 dy (me) = dy (le) dy (mf) = dy (If)
posterior lexion medial dy (If) > dy (le)
capsule contraction extension gap = medial flexion
lateral extension gap = lateral
gap lexion gap, and
lexion gap is
bigger than
extension gap
6. ight medially 4a nterior a> 10 dy (me) < dy (le) dy (mf) < dy (If)
in flexion MCL lexion medial medial flexion
contraction extension gap < gap < lateral
ight medially lateral extension lexion gap
in extension gap
34
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
A B C D E F
1. Flexion/ Varus/valgus Varus/varus
Condition # Cause Extension extension flexion
angle
7. 4b Posterior dy (me) < dy (le) dy (mf) < dy (If)
MCL medial medial flexion
extension gap < gap < lateral
lateral extension lexion gap
gap
8. 4c Medial dy (me) < dy (le) dy (mf) < dy (If)
posterior medial medial flexion
capsule extension gap < gap < lateral
lateral extension lexion gap
gap
9. 4d Semime- dy (me) < dy (le) dy (mf) < dy (If)
mbranos-us medial medial flexion
and pes extension gap < gap < lateral
anserinus lateral extension lexion gap
ap
10. ight popliteus 5 Popliteus
endon endon
11. Compensatory 6 Iliotibial dy (me) > dy (le)
lateral release band medial
- extension extension gap >
only lateral extension
gap
12. Compensatory 7 LCL and dy (me) > dy (le) dy mf > dy (If)
lateral release popliteus medial medial flexion
- flexion and tendon extension gap > gap > lateral
extension lateral extension lexion gap
gap
13. Tight laterally 8a Popliteus dy (me) > dy (le) dy mf > dy (If)
in flexion endon medial medial flexion
extension gap > gap > lateral
Tight laterally lateral extension lexion gap
in extension gap
14. 8b LCL dy (me) > dy (le) dy mf > dy (If)
medial medial flexion
extension gap > gap > lateral
lateral extension lexion gap
gap
15. 8c Posteralate dy (me) > dy (le) dy mf > dy (If)
ral corner medial medial flexion
of capsule extension gap > gap > lateral
lateral extension lexion gap
gap
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
A B C D E F
Flexion/ Varus/valgus Varus/varus
Condition # Cause Extension extension flexion
angle
16. ight laterally 8d Popliteus dy (me) > dy (le) dy ( mf) > dy (If)
in flexion endon medial dy (le) < dy (If)
extension gap > medial flexion
Tight laterally lateral extension gap > lateral
in extension gap lexion gap and
(tighter in lateral extension
extension than gap < lateral
in flexion) lexion gap
17. Balanced in 9a Iliotibial dy (le) < dy (me) dy (If) = dy (mf)
lexion band lateral extension lateral flexion
gap < medial gap = medial
Tight laterally extension gap lexion gap
18. in extension 9b Lateral dy (le) < dy (me) dy (If) = dy (mf)
posterior lateral extension lateral flexion
capsule gap < medial gap = medial
extension gap lexion gap
19. Tight laterally 10 Popliteus dy (me) = dy (le) dy (If) < dy (mf)
in flexion a tendon medial lateral flexion
Balanced in extension gap = gap < medial
extension lateral extension lexion gap
gap
20. 10 LCL dy (me) = dy (le) dy (If) < dy (mf)
b medial lateral flexion
extension gap = gap < medial
lateral extension lexion gap
gap
21. 10 Posterolate dy (me) = dy (le) dy (If) < dy (mf)
c ral corner medial lateral flexion
of capsule extension gap = gap < medial
lateral extension lexion gap
ap
22. Deficient PCL 11 PCL
36
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
B.
A B C G H
AP drawer AP drawer
Condition # Cause extension flexion Rotation
2. ight PCL 1 ight PCL dz (me) = dz (mf)>O >TBD
dz (le) dz (mf) > dz (If)
posterior medial femoral
medial rollback is
rollback in posterior, TBD
extension = alue
posterior determines how
lateral ar beyond
rollback in midline, and
extension medial rollback
> posterior
lateral rollback
3. ight medially 2 nterior dz(me)=dz(dz (mf)> O>TBD ri (me) < re (le)
in flexion MCL le) dz (mf) > dz (If) Internal rotation
Loose medially posterior medial femoral about the medial
in extension medial rollback is complex is <
rollback in posterior, TBD external rotation
extension = alue about the lateral
posterior determines how complex in
lateral ar beyond extension
rollback in midline, and
extension medial rollback
> posterior
lateral rollback
Balanced in 3a Posterior dz(le)=dz( dz(le)<dz(If)
lexion MCL me) dz(me)< dz(mf)
posterior lateral and
ight in medial medial rollback
extension rollback = in extension are
posterior less than lateral
lateral and medial
rollback in rollback in
extension lexion
5. 3b Medial dz (le) = dz dz(le)<dz(lf)
posterior (me) dz(me)<dz(mf)
capsule posterior lateral and
medial medial rollback
rollback = in extension are
posterior less than lateral
lateral and medial
rollback in rollback in
extension lexion
37
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
A B C G H I
Condition # Cause AP drawer AP drawer Rotation
extension flexion
6. ight medially 4a nterior dz(me)<dz( dz(mf)<dz(If) ri (mf) < re (If)
in flexion MCL le) posterior medial internal rotation
posterior rollback < about medial
ight medially medial posterior lateral side in flexion <
in extension rollback < rollback in external rotation
posterior lexion about the lateral
lateral side
rollback in
extension
7. 4b Posterior dz (me) < dz (mf) < dz (If) ri (mf) < re (If)
MCL dz (le) posterior medial internal rotation
posterior rollback < about medial
medial posterior lateral side in flexion <
rollback < rollback in external rotation
posterior lexion about the lateral
lateral side
rollback in
extension
8. 4c Medial dz (me) < dz (mf) < dz (If) ri (mf) < re (If)
posterior dz (le) posterior medial internal rotation
capsule posterior rollback < about medial
medial posterior lateral side in flexion <
rollback < rollback in external rotation
posterior lexion about the lateral
lateral side
rollback in
extension
9. 4d Semime- dz (me) < dz (mf) < dz (ff) ri (mf) < re (If)
mbranos- dz (le) posterior medial internal rotation
us and pes posterior rollback < about medial
anserinus medial posterior lateral side in flexion <
rollback < rollback in external rotation
posterior lexion about the lateral
lateral side
rollback in
extension
10. ight popliteus 5 Popliteus ri (mf) > re (If)
tendon tendon internal rotation
about medial
side > external
rotation about
lateral side
11. Compensatory 6 Iliotibial
lateral release band
- extension
38
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
A B C G H I
Condition # Cause AP drawer AP drawer Rotation
extension flexion
only
12. Compensatory 7 LCL and
lateral release popliteus
- flexion and endon
extension
13. ight laterally 8a Popliteus dz(me)>dz( dz(mf)>dz(If) ri(me)>re(le)
in flexion endon le) posterior medial internal rotation
posterior rollback > about the medial
ight laterally medial posterior lateral side > external
in extension rollback > rollback in rotation about
posterior lexion he lateral side
lateral
rollback in
extension
14. 8b LCL dz(me) dz dz (mf) > dz (If) ri (me) > re (le)
(le) posterior medial internal rotation
posterior rollback > about the medial
medial posterior lateral side > external
rollback > rollback in rotation about
posterior lexion he lateral side
lateral
rollback in
extension
15. 8c Posteralate dz (me) > dz (mf) > dz (If) ri (me) > re (le)
ral corner dz (le) posterior medial internal rotation
of capsule posterior rollback > about the medial
medial posterior lateral side > external
rollback > rollback in rotation about
posterior lexion he lateral side
lateral
rollback in
extension
16. ight laterally 8d Popliteus dz (me) > dz (mf) > dz (If) ri (me) > re (le)
in flexion tendon dz (le) dz (le) < dz (If) internal rotation
posterior posterior medial about the medial
ight laterally medial rollback > side > external
in extension rollback > posterior lateral rotation about
(tighter in posterior rollback in he lateral side
extension than lateral lexion and
lexion) rollback in posterior lateral
extension rollback in
extension >
posterior lateral
rollback in
39
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
A B C G H I
Condition # Cause AP drawer AP drawer Rotation
extension flexion
lexion
17. Balanced in 9a Iliotibial dz (le) < dz dz (If) = dz (mf) ri (le) < re (me)
lexion band (me) posterior lateral internal rotation
posterior rollback = about lateral
ight laterally lateral posterior medial side < external
in extension rollback < rollback in rotation about
posterior lexion medial side
medial
rollback in
extension
18. 9b Lateral dz (le) < dz z(If) = dz (mf) ri (le) < re (me)
posterior (me) posterior lateral internal rotation
capsule posterior rollback = about lateral
lateral posterior medial side < external
rollback < rollback in rotation about
posterior lexion medial side
medial
rollback in
extension
19. ight laterally 10 Popliteus dz (le) = dz dz (If) < dz (mf)
in flexion a endon (me) posterior lateral
posterior rollback <
Balanced in lateral posterior medial
extension rollback = rollback in
posterior lexion
medial
rollback in
extension
20. 10 LCL dz (le) = dz dz (If) < dz (mf)
b (me) posterior lateral
posterior rollback <
lateral posterior medial
rollback = rollback in
posterior lexion
medial
rollback in
extension
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
A B C G H I
AP drawer AP drawer
Condition # Cause Rotation
extension flexion
21. 10 Posterolate dz (le) = dz dz ()f) < dz (mf)
c ral corner (me) posterior lateral
of capsule posterior rollback <
lateral posterior medial
rollback = rollback in
posterior lexion
medial
rollback in
extension
22. Deficient PCL 11 PCL dz (If) < 0 ri (me) < re (le)
dz (mf) < 0 internal rotation
medial and about medial
lateral condyles side < external
are displaced rotation about
negatively (i.e., lateral side
anteriorly)
For navigating surgical instrument, prosthetic components, and other
items, the systems and processes according to an embodiment of the present
invention can invoke and employ various navigational algorithms, either
commercially available or proprietary. In one embodiment illustrated in Figure
3,
the proprietary "AchieveCAS" TKA software is used in this capacity.
As illustrated in Figure 1, systems according to some embodiments of the
present invention may also comprise an imager for obtaining at least one image
of the tibia, the femur, the tibial prosthetic component, or the femoral
prosthetic
component, wherein the computer is adapted to receive from the imager and
store the at least one image of the tibia, the femur, the tibial prosthetic
component, or the femoral prosthetic component; and a monitor adapted to
receive information from the computer in order to display the at least one
image
of the tibia, the femur, the tibial prosthetic component, or the femoral
prosthetic
component.
Systems according to some embodiments may further comprise surgical
instruments associated with one or more fiducials and adapted for navigation
and
positioning at the knee using the images displayed on the monitor. The systems
may further comprise prosthetic components associated with one or more
41
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
fiducials and adapted for navigation and positioning at the knee using the
images
displayed on the monitor. The systems may further comprise at least one
cutting
jig or cutting block for positioning at the femur, wherein the cutting jig is
associated with one or more fiducials and the position and orientation of the
fiducial associated with the cutting jig is trackable by the computer for
navigation
and positioning of the cutting jig at the femur. The cutting jig or block may
be
adjustable and/or multi-purpose.
The systems and processes according to aspects and embodiments of the
present invention can be adapted the variety of the surgical techniques and
surgeon's preferences. The systems and processing according to the
embodiments of the present invention employ surgeon profiles so that the
surgeon can retrieve his or her surgical setup or profile from the computer
memory. However, the user, such as the surgeon, can change the setup before,
after or during the surgery to incorporated desired changes needed based on
,15 surgical anatomy, and/or anomalies specific to a patient, or a prosthetic
device.
This system provides objective measures assess the soft-tissue balancing
within
TKA by applying a logic matrix to the data acquired during the static
assessment
and the kinematic testing of the knee joint. The systems and processes are
flexible and can be adapted to the technique employed by the surgeon. The
systems can also be used to verify implant trial placement when using
conventional surgical TKA techniques. The logic matrix is programmable and can
be adapted to the individual needs of the surgeon. For example, the system can
be adapted to allow the surgeon to modify the default threshold values, and
add
to or delete information from the logic matrix. Some embodiments of the
invention can also provide a method of computer-assisted total arthroplasty on
a
patient's knee using the above-described systems and processes.
A Soft-tissue Balancing Algorithm Based on the Convergence of the Anatomical
Landmarks and the Dynamic Interaction of the Knee bones and Ligaments
In one embodiment of the present invention, the systems, methods and
processes employ a soft-tissue balancing algorithm that advantageously
considers and correlates both the anatomical landmarks and the dynamic
42
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
interaction of the knee bones and ligaments, an important advantage over the
existing methods that are generally excessively weighted towards either
anatomical or dynamic factors. The algorithm also advantageously considers
and correlates both femoral and tibial landmark, an advantage over the
existing
methods that commonly consider only femoral or only tibial landmarks. The
method establishes a rectangular gap between tibia and femur in both flexion
and
extension without distorting the anatomy of the knee. According to some
aspects
and embodiments of the method, prosthetic component size, positioning, and
surgical cuts can be planned before any irreversible bone cuts are made,
although the system and method are adaptable for ligament balancing in
patients
after the surgical cuts are performed, or after the prosthetic components are
installed. It is to be understood that the method is performed with the
computer
assistance and in the context of computer-assisted surgical systems and
methods as described elsewhere herein. Consideration of the anatomy,
kinematics, coordinate systems, and of real and/or virtual surgical
constructs,
such as axes and planes generally involves storage of data in computer memory
and calculations optimally performed with the aid of a computer. A computer-
assisted surgical system according to some embodiments of the present
invention employs computers programmed with the algorithms for performing the
steps necessary for carrying out the method.
With reference to Figures 2, and 8-9, the method can be used as follows.
The surgeon exposes the knee in a conventional manner, and performs
preliminary osteophyte resection and ligament release. The anterior cruciate
ligament may be divided, if present, and/or the posterior cruciate ligament
may be
resected at the surgeon's discretion. The distal femoral anatomy is registered
by
the imager and digitized and the proposed position of the femoral component
based on the traditional anatomical landmarks, such as a posterior condylar or
epicondylar rotation and posterior condylar measured resection are registered.
Figure 2 shows an exposed human knee (200) in a surgical field after the
osteophyte resection and the preliminary ligament release. The surgeon
establishes femoral and tibial coordinate systems by, for example, registering
the
navigational landmarks for the end of the respective bones. The navigation
43
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
instrument (202) on the distal femur (206) tracks the position of the femur
relative
to the tibial coordinate system. The femur is distracted in flexion and
extension.
As shown in Figure 8, with the knee (800), comprising femur (804), tibia
(808) and fibula (810),
in extension, a proposed distal femoral resection plane perpendicular to
the mechanical axis (802) of the femur (804) in varus/vaigus (PDFRP; an
anatomical femoral resection plane) is established, and a proposed tibial
resection plane (PTRP) perpendicular to the mechanical axis (807) of the tibia
(808) in varus/vaigus is established. Using a navigation instrument on the
distal
femur shown in Figure 2 (204), tracking its position relative to the tibial
coordinate
system, distal femoral resection plane is established that is perpendicular to
the
long axis of the tibia (DFRPPT, a dynamic resection plane). Using the
anatomical landmarks the femoral resection plane perpendicular to the tibia
(DFRPPT) is compared to the proposed femoral resection plane perpendicular to
the mechanical axis of the femur (PDFRP). The state of the soft tissue balance
of the knee is represented in extension by the angle 0 formed between the
femoral anatomical resection plane and the femoral dynamic resection planes in
extension.
In one embodiment, the final femoral resection level is not determined until
after the soft tissues are balanced. To perform the resection using computer-
assisted navigation, the pins are placed in the distal femur for positioning
of a
distal femoral cutting jig at a known angle to the mechanical axis of the
femur.
As shown in Figure 9, when the knee (800) is flexed, a proposed posterior
femoral resection plane perpendicular to the mechanical axis (802) of the
femur
(804) is established (MRP; an anatomical femoral resection plane), a proposed
tibial resection plane (PTRP) perpendicular to the mechanical axis of the
tibia
(806) in varus/valgus distraction, and posterior femoral resection plane
perpendicular to the mechanical axis of the tibia (PFPPT, a dynamic resection
plane) are established. Using the anatomical landmarks, PFPPT is compared in
to MRP. The state of the soft tissue balance of the knee (800) is represented
in
44
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
flexion by the angle ~ formed between the femoral anatomical resection plane
and the femoral dynamic resection plane.
In flexion and extension, if the anatomical and the femoral resection
planes agree, they are approximately parallel and the angles ~ and 0 are close
to
0. The resection gap in the knee is then approximately rectangular in both
flexion
and extension. If not, more soft tissue balancing, such as ligament release
and
contraction, is necessary. Based on the angle, the system establishes if the
ligaments need further adjustment, and provide necessary recommendations to
the surgeon on ligament balancing. For example, as shown in Figure 8, the
medial side of the knee is tight and the planes are at a non-zero angle 0.
Based
on the calculated angle 0, the system employing the provided method suggests
that the medial side is tight in extension and may need further released. Upon
soft tissue adjustment, the state of the knee is reassessed. The distance A
between the tibial and the femoral resection planes preferably allows for
placement of the tibial tray, plastic femur, and bone cement.
The iterative cycle of knee assessment and ligament balancing is
performed until the anatomical and the dynamic planes converge. It is to be
appreciated that convergence does not necessarily mean coincidence, and that
the known principles of the iterative convergence methods and their
limitations
are utilized in the embodiments of the present invention.
The bones can be resected at the recommended converged planes, or an
existing surgical plane may be assigned to the algorithm. Due to the fact that
ligament balancing and surgical planes prediction according to certain aspects
and embodiments of the method occur prior to resection of the leg bones, the
method facilitates minimally invasive, small-incision TKR. The adjustable
and/or
multifunctional cutting jigs or blocks can be used in conjunction of the
method of
the present application.
The method can be adapted to various special circumstances. For
example, in case of significant flexion constructure, preliminary distal
femoral and
posterior femoral cuts may be necessary to remove posterior osteophytes and
CA 02594874 2007-07-16
WO 2006/078236 PCT/US2005/001354
ensure adequate posterior capsule release. In general, the preliminary
resection
may be shallow enough so as not to determine the final surgical cutting planes
in
accordance with the provided method and algorithm. The method can be
adapted to particular prosthetic systems and methods of installation thereof.
For
example, certain available knee prosthetic components are adapted for
placement at pre-determined angles to the tibial and femora axes. Such
features
of the prosthetic systems are easily incorporated into the provided method by
assigning appropriate parameters.
The foregoing discloses preferred embodiments of the present invention,
and numerous modifications or alterations may be made without departing from
the spirit and the scope of the invention.
The particular embodiments of the invention have been described for
clarity, but are not limiting of the present invention. Those of skill in the
art can
readily determine that additional embodiments and features of the invention
are
within the scope of the appended claims and equivalents thereto. All
publications
cited herein are incorporated by reference in their entirety. The entire
content of
U.S. Provisional Patent Application Serial No. 60/536,901 entitled "A New
Method
of Computer-Assisted Ligament Balancing and Component Placement in Total
Knee Arthroplasty" filed on January 16, 2004, is incorporated herein by this
reference.
46