Note: Descriptions are shown in the official language in which they were submitted.
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
METHOD FOR MAKING A NON-TOXIC DENSE MATERIAL
Field of the Invention
This invention relates to methods and compositions for making material useful
as
shot, bullets and the like. More specifically, this invention relates to
methods and
compositions, for making non-toxic dense material for use in shot, bullets,
milling media,
wear media, blasting media, hard tools and the like.
Background of the Invention
A variety of materials and methods have been proposed or are in use as shotgun
shot and rifle or pistol bullets, hereinafter referred cumulatively as "shot."
Because of its
toxic nature, lead has been banned or criticized for use as shot. Lead has
been banned in
the United States and Canada for use in shotgun shells for hunting waterfowl.
Lead has
also been banned on all U.S. Federal Refuges when hunting game animals or
training dogs.
Generally, the reasons for the bans and criticism are that when waterfowl
ingest pellets, the
lead is retained and ground up in the bird's gizzard and poisons the bird. The
principle
substitute for lead is steel, but the density of steel is only about 7.9
grams/cc, as
compared to 11.4 grams/cc for lead or 11 grams/cc for commonly used lead
alloys.
Because of the lower density the effectiveness of steel is greatly diminished
when
compared to lead. One advantage of steel is that it is ferromagnetic, making
it easily
detectable by law enforcement with a simple hand held magnet. Several
manufacturers
have announced that they are working on finding a substitute for lead. These
include
1
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
Federal's Iron-Tungsten or Tungsten, Environmental's and Remington's Hevishot,
Kent's
Tungsten-Matrix and Bismuth shot, made by The Bismuth Cartridge Company.
Federal's Iron-Tungsten or Tungsten claims a density of about 10.4 grams/cc,
although it has been measured at closer to 10.2 grams/cc. Iron-Tungsten uses a
powder
metallurgy method of making a pellet from tungsten, iron and/or ferro-tungsten
powders.
The process is described in U.S. Patents 5,831,188, 5,527,376, 5,905,936,
5,713,981 and
6,270,549. The process involves pressing each individual pellet in a press
with a binder of
some sort to hold the pellet together in the "green" state. The pressing
operation leaves a
band on the pellet that must be removed. After a presintering operation, the
pellet is then
typically ground or rolled to make it truer (more round) and to eliminate or
minimize the
band. Firing the pellet at about 1500 degrees C in hydrogen gas then follows
to densify
and strengthen the pellet. Further treatment may well include additional
grinding and/or
the application of a rust inhibitor. This method tends to be expensive due to
the high
firing temperature, the individual pressing of pellets and the followed
rounding steps. This
individual pressing of pellets further limits the pellets to larger sizes.
Environmental and Remington claim that Hevishot has a density of about 12
grams/cc. The process of making Hevishot is generally described in US Patent
No.
6,270,549. Hevishot uses a process of making shot from molten metal similar to
the
method used historically to make lead shot by dropping molten lead through a
screen
inside a tower, as the lead falls it becomes round and is quenched in water.
Because
Hevishot is generally made of an iron, tungsten, nickel and sometimes a
manganese alloy it
melts at a much higher temperature, about 1637 degrees C, than lead which
melts at about
2
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
327 degrees C. Therefore, the process is modified to accommodate the much
higher
temperatures. The process modifications include dropping the molten metal
through a
ceramic sieve and, in place of the shot tower, into a high velocity stream of
air or gas which
helps break up the molten droplets of metal alloy and allows the surface
tension to form
round or rounded droplets that are then quenched in water. Unfortunately,
Hevishot does
not tend to be very round and frequently has two or more spheres attached to
each other
of different sizes or can be hollowed out and tend not to be of uniform size.
Kent's Tungsten-Matrix is claimed to have a density of 10.8 grams/cc, but
depending on the shot size measured between 10.3 and 10.7 grams/cc. This shot,
described in U.S. Patent No. 6,216,598, uses tungsten powder held together by
a polymer.
Tungsten powder is much more expensive than FeW (ferro-tungsten) powder.
Because of
the polymer, the resulting shot is comparatively weak and can deform during
the shooting
process. Also, the process typically requires that the pellets be formed
individually be
pressing from a polymer sheet, filled with tungsten powder, with opposing
rolls.
Bismuth has also been proposed. However, with a density of 9.8 grams/cc
Bismuth,
although relatively easy to manufacture, can be excessively brittle, unless
alloyed with tin
(Sn) in small amounts. Sn has a density of 7.3g/cc, so any addition of Sn
lowers the
density of the alloy.
A number of other shot materials and processes have been proposed. Generally,
however, these prior techniques have not made use of carbon to increase the
density of the
shot and to reduce the firing temperature required, and the associated energy
and
production costs.
3
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
Although these references may not actually qualify as "prior art," the reader
is
referred to the following U.S. patent documents for general background
material. Each of
these patents is hereby incorporated by reference in its entirety for the
material contained
therein.
U.S. Patent No. 1,847,617 describes hard alloys that include the addition of
chromium and cobalt.
U.S. Patent No. 2,119,876 describes shot used primarily in shot shells
designed for
target shooting and game hunting and in air rifles.
U.S. Patent No. 2,183,359 describes a method of manufacture of heavy metallic
material, composed of tungsten, nickel and copper.
U.S. Patent No. 3,372,021 describes a tungsten addition agent for use in the
manufacture of steel.
U.S. Patent No. 3,623,849 describes powder metallurgy products using sintered
refractory compound materials and the method for producing such materials.
U.S. Patent No. 3,952,657 describes a cartridge that includes a projectile,
which is
inserted in a plastic shell receiving a propellant charge, and the cartridge
is expelled out of
a barrel by propellant gases.
U.S. Patent No. 3,987,730 describes iron and lead-containing composite metal
shot.
U.S. Patent No. 4,027,594 describes shot pellets that are formed from finely
divided
powder adhered together in pellet form by a thermoplastic polymeric material
decomposable in the acid environment of the digestive tract of waterfowl.
4
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
U.S. Patent No. 4,167,904 describes a shot compressor device for use in
shotgun
cartridges.
U.S. Patent Nos. 4,200,456 and 4,297,133 describe a method and member for
adding a treating agent for molten metal.
U.S. Patent No. 4,292,877 describes an ammunition loader with hoppers for shot
and/or powder and a slideable bar for measuring.
U.S. Patent No. 4,316,414 describes a fuse apparatus that arms itself in
flight and is
especially adapted for use in training shells.
U.S. Patent No. 4,714,023 describes a non-toxic wildlife shot pellet for
shotgun
shells and the like that comprises a lead shot pellet with a coating of nickel-
phosphorous
alloys.
U.S. Patent No. 4,754,684 describes a method and device for cutting a shotgun
shell for shooting in a shotgun.
U.S. Patent No. 4,784,690 describes a low-density tungsten alloy article and
the
method for producing the article.
U.S. Patent No. 4,841,866 describes a tracer shotgun shell that includes an
improved tracer element and a single integral wad member.
U.S. Patent No. 4,854,240 describes a two-stage, shaped charge projectile
having a
rear principal charge and a front secondary smaller charge with an initiator-
fuse assembly.
U.S. Patent No. 4,856,408 describes a modification or replacement for shot and
powder loading systems having wad jammer tubes with telescopic loading
funnels.
5
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
U.S. Patent No. 4,949,644 describes non-toxic wildlife shot pellets for
shotgun
shells that are formed from bismuth or bismuth alloy.
U.S. Patent No. 4,960,563 describes a process for the product of a heavy
tungsten-
nickel-iron alloy.
U.S. Patent No. 5,279,787 describes a high-density projectile and the method
of
making the same from a mixture of low density and high-density metal powders.
U.S. Patent No. 5,335,578 describes a retrofitting shell feeding attachment
for
shotgun shell reloading machines.
U.S. Patent No. 5,399,187 describes a composite lead-free bullet, comprising a
heavy constituent selected from the group of tungsten, tungsten carbide,
carballoy and
ferro-tungsten and a second binder constituent consisting of either a metal
alloy or a
plastic blend.
U.S. Patent No. 5,442,989 describes a method of making the casing for
frangible
armor piercing incendiary projectiles.
U.S. Patent No. 5,512,080 describes a Fe-based alloy powder adapted for
sintering,
a Fe-based sintered alloy, and a process for producing the Fe-based sintered
alloy.
U.S. Patent No. 5,527,376 describes a shot pellet or small arms projectile
that
comprises 40-60% by weight of tungsten and 60-40% by weight of iron formed by
sintering tungsten containing powders.
U.S. Patent No. 5,623,118 describes a shot shell wad that may comprise a
powder
cup and a shot cup connected by first and second shot cup support members.
U.S. Patent No. 5,666,634 describes alloy steel powders for sintered bodies.
6
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
U.S. Patent No. 5,713,981 describes a high specific gravity, lead free shot
shell
pellets that are produced by preparing an iron-tungsten alloy, melting the
alloy, pouring
the alloy and allowing the alloy to fall by gravity through a gaseous medium
to form drops.
U.S. Patent No. 5,714,573 describes a melt-processable lactide polymer
composition, process for manufacturing these compositions and articles made
from these
compositions.
U.S. Patent No. 5,719,352 describes a low toxicity shot or pellets for shotgun
cartridges or the like that comprises finely divided molybdenum and tungsten
particles in a
polymer matrix.
U.S. Patent No. 5,728,349 describes a material primarily for sport shooting
ammunition, both pellet ammunition and ball ammunition, including at least the
materials
zinc and bismuth.
U.S. Patent No. 5,760,331 describes a projectile made by combining two
different
metals in proportions calculated to achieve a desired density, without using
lead.
U.S. Patent No. 5,814,759 describes a composite lead-free bullet that
comprises a
heavy constituent selected from the group of tungsten, tungsten carbide,
carballoy and
ferro-tungsten and a second binder constituent consisting of either a metal
alloy or a
plastic blend.
U.S. Patent No. 5,831,188 describes methods of making high specific gravity
shotgun shot and small arms projectiles from melts containing primarily
tungsten and iron.
U.S. Patent No. 5,861,572 describes a universal shotgun shell wad that may be
used
to assemble a variety of shotgun shells with a wide variety of shot and powder
loadings.
7
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
U.S. Patent No. 5,870,989 describes an abrasion resistant valve seat made of
sintered alloy for internal combustion engines.
U.S. Patent No. 5,874,689 describes a one piece shot cup designed especially
for
use in protecting the bore of a shotgun barrel.
U.S. Patent No. 5,905,936 describes generally rough sphere-shaped work pieces
made of fragile material that are ground into more uniform spheres.
U.S. Patent No. 5,913,256 describes a non-lead environmentally safe projectile
and
explosive container.
U.S. Patent No. 5,922,832 describes melt-processable lactidue polymer
compositions.
U.S. PatentNo. 5,932,828 describes a reloader with snap-in tools.
U.S. Patent No. 5,963,776 describes a projectile, such as a bullet, made by
combining two different metals in proportions calculated to achieve a desired
density,
without using lead.
U.S. Patent No. 5,970,878 describes a combination of shot sleeve and a shot
cup
base form a universal shot wad that precisely fixes an adjustable volume.
U.S. Patent No. 5,997,805 describes a manufacturing method for the production
of
high density, high carbon, and sintered powder metal steels.
U.S. Patent No. 6,048,379 describes a high-density composite material that may
act
as a replacement for lead in applications where the high density of lead is
important, but
where the toxicity of lead is undesirable.
U.S. Patent No. 6,092,467 describes a flare apparatus that includes a shell
base.
8
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
U.S. Patent No. 6,102,820 describes an auto-tensioner that inhibits the
generation
of heat in an insert bearing made of a synthetic resin.
U.S. Patent No. 6,112,669 describes a lead-free projectile made from a
composition
containing about 5-25% by weight of tungsten and more than about 97% by weight
tungsten plus iron.
U.S. Patent No. 6,128,846 describes an improved shotgun choke tube.
U.S. Patent No. 6,139,658 describes a metal matrix alloy that comprises
carbon,
titanium and a matrix material.
U.S. Patent Nos. 6,149,705 and US 6,174,494 B1 describe non-lead,
environmentally safe projectiles and a method for making the same.
U.S. Patent No. 6,1 58,351 describes a ferromagnetic bullet that is lead free.
U.S. Patent No. 6,159,226 describes a process for producing a sintered powder
metal body.
U.S. Patent No. US 6,193,927 B1 describes a high density forming process with
ferro
alloy and prealloy.
U.S. Patent No. US 6,202,561 B1 describes a shot shell that is comprised of
shot
pellets of different densities and materials.
U.S. Patent No. US 6,209,180 B1 describes a composite shot for shot shells
that
includes a ferrous metal core and a non-toxic coating having a density greater
than lead.
U.S. Patent No. US 6,216,598 B1 describes shot for shotgun cartridges made
from
finely divided particles of dense metal such as a mixture of tungsten and
molybdenum,
bound by a matrix.
9
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
U.S. Patent No. US 6,263,797 B1 describes a flare apparatus that includes a
shell
case.
U.S. Patent No. US 6,270,549 B1 describes a ductile, high-density, non-toxic W-
Ni-
Mn-Fe alloy compositions and methods of manufacture.
U.S. Patent No. US 6,358,298 Bl describes an iron-graphite composite powder
having a microstructure that comprises carbon clusters embedded in a ferrous
matrix.
U.S. Patent No. US 6,439,124 Bi describes a lead-free projectile suitable for
use as
a bullet to be fired from a pistol or rifle or as a slug to be fired from a
shotgun.
U.S. Patent No. US 6,475,262 B1 describes a method of forming a component by
sintering an iron-based powder mixture.
U.S. Patent No. US 6,495,631 B1 describes a melt-processable lactide polymer
compositions, processes for manufacturing these compositions, and articles
made from
these compositions.
U.S. Patent No. US 6,514,307 B2 describes a sintered iron-based powder metal
body with outstanding lower re-compacting load and having a high density and a
method
of manufacturing an iron-based sintered component.
U.S. Patent No. US 6,517,774 B1 describes a high-density composite material
that
may act as a replacement for lead in applications where the high density of
lead is
important, but where the toxicity of lead is undesirable.
U.S. Patent No. US 6,527,880 B2 describes ductile medium and high density, non-
toxic shot and other articles and a method for producing the same.
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
U.S. Patent No. US 6,533,836 B2 describes an iron-based mixed powder for use
in
powder metallurgy and die filling.
U.S. Patent No. US 6,536,352 B1 describes a frangible bullet comprising powder
particles of one metal bonded together by another metal wherein the metals
have
substantially different melting points or an alloying metal is diffused
between the metal
particles are manufactured by compacting the metal particles and heating under
conditions
to create brittle bonds.
U.S. Patent No. US 6,537,489 B2 describes high-density products and method for
the preparation thereof.
U.S. Patent No. US 6,551,375 B2 describes ammunition using non-toxic metals
and
binders.
Summary of the Invention
It is desirable to provide a method for producing non-toxic materials, which
can be
used for shot and/or bullets, which are more dense than steel, bismuth or
lead. It is
particularly desirable to provide an economical method for producing such non-
toxic
materials.
Accordingly, it is an object of one embodiment of this invention to provide a
method for the manufacture of a dense material that is non-toxic.
It is another object of one embodiment of this invention to provide a method
for the
manufacture of non-toxic material having a density of 9 to 11.8 g/cc and in
some
embodiments even higher, up to and including densities of as much as 1 5 g/cc.
11
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
Another object of one embodiment of this invention is to provide a method for
the
manufacture of a more dense than lead material that is economically produced.
A still further object of one embodiment of this invention is to provide a
method for
the manufacture of a more dense than lead material that has a relatively low
sintering
temperature.
It is a further object of one embodiment of this invention to provide a method
for
the manufacture of a more dense than lead material that uses relatively less
expensive
materials.
It is a still further object of one embodiment of this invention to provide a
method
for the manufacture of a more dense than lead material that is compatible with
a wide
range of pellet sizes.
Another object of one embodiment of this invention is to provide a method for
the
manufacture of a more dense than lead material that does not necessarily
require grinding
or shaping, although grinding and shaping may be useful, but not necessary, in
some
applications.
A further object of one embodiment of this invention is to provide a method
for the
manufacture of a dense non-toxic material, which in some embodiments is
provided with
an uneven dimpled surface.
A still further object of one embodiment of this invention is to provide a
method for
the manufacture of a dense non-toxic material that includes sintering a
different density
material such as tungsten powder to prevent sticking of the material to
itself.
12
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
Another object of one embodiment of this invention is to provide a method for
the
manufacture of a dense non-toxic material that uses carbon as a sintering aid.
It is an object of one embodiment of this invention to provide a method for
the
manufacturing of a dense non-toxic material that, in some embodiments, uses
sintering in
tungsten powder to increase the density by tungsten incorporated into the
surface and
alter the surface characteristics of the material.
It is another object of one embodiment of this invention to provide a method
for the
manufacturing of a dense non-toxic material that, in some embodiments, employs
sintering in SiC, tungsten, ferro niobium and/or tungsten-carbide powder of a
large mesh
size to prevent sticking of the pellets together during sintering.
It is a further object of one embodiment of this invention to provide a method
for
the manufacturing of a dense non-toxic material that, in some embodiments,
includes
Boron Nitride (BN) powder or spray to prevent sticking.
It is a still further object of one embodiment of this invention to provide a
method
for the manufacturing of a dense non-toxic material that, in some embodiments,
includes
non=wetting, high melting point material to prevent or reduce sticking of the
resulting
pellets.
Another object of one embodiment of this invention is to provide a method for
the
manufacturing of dense non-toxic material that, in some embodiments, lowers
the
sintering temperature of FeW by the addition of a much higher melting
temperature
material.
13
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
A further object of one embodiment of this invention is to provide a method
for the
manufacturing of dense non-toxic material that, in some embodiments, provides
for the
varying of the strength and frangibility of the resulting material by varying
the time and
temperature of the sintering stage as well as the composition.
A still further object of one embodiment of this invention is to provide a
method for
the manufacturing of dense non-toxic material that, in some embodiments,
provides the
proper strength and frangibility to milling media made from these
compositions.
Another object of one embodiment of this invention; is to provide a method for
the
manufacture of a dense non-toxic material that does not require compression or
pressure
to form into a generally round shape.
It is another object of one embodiment of this invention to provide a method
for the
manufacture of a dense non-toxic material that is useful in the manufacture of
hard tools.
It is a further object of some embodiments of this invention to provide a
method for
the manufacture of dense non-toxic material that retains magnetic properties.
A still further object of some embodiments of this invention is to provide a
method
for the manufacture of shot of varying sizes with "tailored" densities to
enhance the
trajectory control of the shot pellets.
Additional objects, advantages and other novel features of this invention will
be set
forth in part in the description that follows and in part will be apparent to
those skilled in
the art upon examination of the following or may be learned with the practice
of the
invention. The objects and advantages of this invention may be realized and
attained by
means of the instrumentalities and combinations particularly pointed out in
the appended
14
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
claims. Still other objects of the present invention will become readily
apparent to those
skilled in the art from the following description wherein there is shown and
described
present preferred embodiments of the invention, simply by way of illustration
of the best
modes currently known to carry out this invention. As it will be realized,
this invention is
capable of other different embodiments, and its several steps, details, and
specific
components, dimensions and materials, are capable of modification in various
aspects
without departing from the invention. Accordingly, the drawings and
descriptions should
be regarded as illustrative in nature and not as restrictive.
Brief Description of the Drawings
The accompanying drawings incorporated in and forming a part of the
specification,
illustrate embodiments of the present invention. Some, although not all,
alternative
embodiments are described in the following description.
In the drawings:
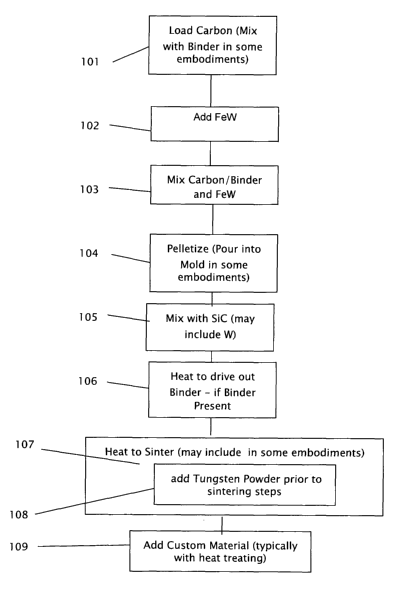
Figure 1 is a flow chart of the present steps of the method of this invention.
Reference will now be made in detail to the present preferred embodiments of
the
invention, examples of which are illustrated in the accompanying drawings.
Detailed Description
This invention is a material with a density adapted to be less than, equal to,
or
greater than lead as desired for the particular use, but without the toxicity
of lead, which is
appropriate for use as shotgun shot, bullets and the like, and the method of
making such
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
material. While the components of the present embodiment of this invention
include
tungsten (W), iron (Fe) and carbon (C) along with a binder (which may
typically be Acrawax,
Polyvinyl Alcohol (PVA), or Paraffin), a wide variety of alternative materials
and/or specific
configurations of the materials, some of which are detailed and described in
the following
description, can be substituted without departing from the concept of this
invention.
Alternative binders include, but are not limited to: bees wax; oils including
oil from light oil
to tar; organic binding materials including sea weed, sugars, molasses, corn
syrup, maple
syrup, refiners syrups and the like; flour from wheat, rice, potatoes, corn
and the like;
cheeses; gums; wood dust including saw dust, shavings, fibers and the like;
cotton;
synthetic fiber; rubbers; cements; starches; ductile inorganic materials
including metals
such as Al, Sn, Cu, Fe, Ni, Nb, V, Ti, clays, cements; silicon based materials
including
silicone oils, waxes and greases; soaps including dry soap, liquid soap and
stearates; and
other like materials that have the ability to help powders to stay together,
even for a short
time or even just marginally, can be used as a binder. The components and the
method of
manufacture are designed to minimize cost.
This invention specifically addresses the problem of using toxic materials,
such as
lead, in hunting or likewise in a manner which tends to leave toxic materials
in the
environment in a manner which can adversely affect the health of waterfowl and
other
animals, ground water and the like. Because this invention uses economical
materials,
generally lower heating temperatures and shorter heating time periods, and
produces a
product in a pelletized form, without the requirement of pressing, machining
or selecting
which can greatly increase the ultimate cost of the produced product, this
invention directly
16
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
addresses the need for an economically viable toxic-free solution for shot,
bullets and the
like. The density of the material used in a shot and bullet product is
directly related to the
effectiveness (range, predictability and take-down power) of the product. The
greater the
density of the bullet material, the more effective the shot or bullet. Because
rifle barrels
have a twist designed for lead bullets, it can be desirable to provide a high
density core to
a non lead bullet, which is designed to bring the total density of the bullet
to
approximately that of a lead bullet, thereby reducing or eliminating the need
for
modification of the bullet cross-section or in the twist of the barrel to
maintain bullet
accuracy when fired. The product of this invention meets these needs by using
a tungsten
compound in combination with typically a carbon source mixed and formed in a
manner
appropriate to facilitate the above desired product features. In the present
embodiment
FeW (ferrotungsten) powder and graphite (carbon) is used to reach densities at
about 1 1.8
g/cc at processing temperatures at about 1 150 C maintained typically for less
than about
fifteen minutes. Throughout this description FeW is defined by the applicant
to mean any
iron tungsten alloy in any suitable proportion. Variations, both higher and
lower, in density
of the product are achieved with alternative temperatures, compositions and
heating
periods, and therefore produce variations in frangibility and strength.
Typically and
presently preferably the processing of the method of this invention is
performed in a
Hydrogen, Argon or otherwise protective atmosphere.
Carbide compositions in this invention can be produced from ferroalloys by
attrition, the result then blended with carbon and fired at temperatures lower
than those
necessary for forming carbides of the primary metal. Examples of such carbide
17
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
compositions include, but are not necessarily limited to, FeW, FeTi, FeCr and
other
ferroalloys that are suitable for alloying with steel and/or other alloys that
contain iron and
carbon. Cutting tools that contain carbides, such as those made by cementing
carbides
together with cobalt, nickel or iron can also benefit from the addition of
carbided or
carburized ferroalloys. The carbiding or carburizing of the master ferroalloy,
before the
addition of other alloys or cemented carbide tools, can provide an accurate
method of
controlling the ratios of carbon and of the alloy additive. This control can
be helpful in
maintaining the desired properties of the final product.
During the development of this invention the inventor determined that a very
dense
compact can be made by sintering Nickel (Ni) and Manganese (Mn) with Tungsten
powder
(W) at temperatures below 1200 C. In some instances and embodiments densities
greater
than 14 g/cc have been achieved. Using a combination of Ni, Mn, W and FeW
densities in
excess of 12.5 g/cc have been achieved. NiMn alloys have particular advantages
in this
process for making dense non-toxic material, including: Ni and Mn together
form a low
melting eutectic, at about 1030 C to 1040 C with about 40-60% Ni in the NiMn
alloy. Ni
melts at about 1453 C and Mn at about 1244 C so either element alone does not
sinter at
the low temperature of the eutectic. By varying the quantities of Ni and Mn
the hardness of
the alloy is also varied, from too soft to measure to a value in the 80's on
the Rockwell A
scale. In other words, the ratio between the amounts of Ni and Mn in the alloy
can affect
the hardness from softer than many steels to as hard as some softer carbides.
The range
of density achieved in the composition can also be adjusted by selecting the
amounts of Ni
and Mn used. Using the NiMn sintering agent permits parts to be formed
mechanically or
18
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
machined to a final shape or dimension with standard metalworking techniques,
i.e.,
pressing, rolling, cutting and the like. FeW-C parts when used as an
alternative, typically
require grinding and other processes necessary for hard and brittle materials.
Since NiMn
acts to hold the other materials in the composition, hardness, toughness,
formability and
even the magnetic properties can be regulated by the way in which the powders
are
assembled and sintered. For example, the FeW-C composition is non-magnetic and
the
FeW, W and NiMn composites are also non-magnetic if the particle sizes in the
powders are
kept small. However, when larger FeW particles are used the magnetic
properties of the
large particles is retained, so long as the particles are merely held together
and are not
allowed to react with the other constituents. Therefore, a non-magnetic or a
magnetic
product can be produced from either the NiMn, FeW, W, or FeW-C composition.
The NiMn-
W composition will not yield a magnetic product.
For some applications it is desirable to have magnetic properties in the
product of
the process of this invention. One of these applications is the use of the
product as shot in
shotshells for hunting waterfowl. It is probable that the components of the
material of the
various present embodiments of this invention are sufficiently non-toxic to be
used as shot
in shotshells as non-toxic shot. One of the common qualities of non-toxic shot
is that it
may be easily determined in the field, by non-destructive and non-invasive
techniques,
that the shot is non-toxic. A simple means that has been developed to make
this
determination is the use of a magnet. Steel, a common non-toxic shot and some
other
non-toxic shot have been demonstrated to be magnetic. Naturally, the magnetic
19
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
properties of nearly any product can be altered by such means as coating the
product with
a magnetic coating, such as BiMn, AICuMn, which is both magnetic and non-
toxic.
An alternative method of providing magnetic properties to the product is in
the
method of manufacture. For example, Tungsten (W); Manganese (Mn); Carbon (C);
Nickel-
Manganese (NiMn); and Iron-Tungsten-Carbide (FeWC) are all non-magnetic, while
Iron-
Tungsten (FeW) and Iron (Fe) and Nickel (Ni) are all magnetic. When FeW is
reacted with C,
the FeW ceases to be magnetic. Similarly, when Ni reacts with Mn it is no
longer magnetic
above 30 to 40% Mn. If an alloy of Ni and Mn is used where the Ni is about 60%
or more
the alloy will be magnetic and this magnetism will be retained in the final
product. Any of
the products of this present invention can be made magnetic by not reacting
the magnetic
component, that is, by using particles of FeW or Ni that are large enough so
that although
they are part of the composition, then do not completely react and therefore
retain their
magnetic qualities. By carefully choosing the desired particle size of the FeW
and/or Ni, a
degree of magnetism can be retained in or can be removed from the final
product. The
density of FeW is high so there is generally no sacrifice of overall product
density when FeW
is used as the chosen material for magnetism, and in fact the use of FeW can
enhance or
increase the overall density of the resulting product. While coatings may
decrease to some
degree the overall density of the product, the use of FeW or other similar
material that is at
least as dense, than the desired final product, does not tend to reduce the
final product's
density. Other large particle magnetic materials, that do not tend to be
changed by the
sintering steps, such as Fe, Ni and the like, can also be used to provide
magnetism.
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
The following is a brief summary of various elements and compounds that the
inventor has determined can be used in various ways and with a variety of
results in the
process and product of this invention. This list is not intended to be
exhaustive but rather
only to be exemplary of the wide variety of materials that may be employed in
this
invention.
Nickel (Ni) can be applied by electrolysis and is commonly known and used in
this
manner although electrolysis may not necessarily be the best way to apply Ni
to shot to
magnetize the shot. Another alternative and probably better way of applying Ni
is to place
the shot in a rotating mill with soft Ni powder such as Ni 123 and rotate the
mill until the
Ni powder covers and adheres to the surface of the shot. This method of
applying Ni
works equally well for applying Ni to any rough or cylindrical surface for
which applying Ni
to the surface is desirable. The present product will accept Ni coating in
this way and thus
provide the desired magnetic qualities required for detection of non-toxic
shot in the field
with a simple magnet. Ni may also be coated on the shot by applying a slurry
of Ni powder
and then drying and sintering at a temperature below the point where the Ni
would
combine with the substrate to lose its magnetic property, but simply sintering
to the
surface thereby retaining the magnetic qualities.
Bismuth-Manganese (BiMn) can be used as both a coating and as an inherent
material. As a coating the Bi is typically added with a low temperature dip
after the product
is finished. Although Bi is not magnetic itself, it will tend to combine with
the Mn in the
product for the requisite alloy. Testing has been performed and has determined
that BiMn
alloys in the range of 1 part Bi and 3 parts Mn to 3 parts Bi and 1 part Mn
produces a
21
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
magnetic part, therefore close control of the composition is not generally
necessary to
assure that the product retains magnetic qualities. Bi has a low melting point
of about
271 C, which facilitates the application of the Bi to the product. Simply
dipping the
product in melted Bi and/or reheating can be done relatively easily. Bi also
provides some
corrosion protection, even though the product itself is generally quite
corrosion resistant.
Surface coatings of BiMn can be applied in much the same manner as Ni powder
if the BiMn
is made into a powder first, then rolled and used as is or sintered onto the
surface at lower
temperatures than are normally required in processing. BiMn can also be used
inherent to
the product composition to sinter or "glue" the denser materials, such as FeW,
W and the
like, together. This use of BiMn also facilitates the magnetic quality of the
product. A
present drawback to the extensive use of Bi is its high cost. However, if the
cost of Bi
decreases and availability increases, this could be an attractive ingredient
for the product
composition. Since the alloy, BiMn, would typically be about 80% by weight Bi
the cost of
the Bi could be driving factor in the determination of its use. The Mn content
makes the
sintering to good strength more feasible than with just Bi alone and the Bi is
not magnetic,
while the alloy BiMn is. BiMn also appears to wet to almost anything it
contacts and
therefore can be a problem as a sintering agent, since it will tend to stick
to nearly
everyth i n g.
Aluminimum-Copper-Manganese (AlCuMn) is also a low melting point alloy and can
be applied to the product by dipping, spraying or other similar means to
provide a coating
on the product. This alloy further has the advantage than Mn is already likely
to be present
in the base material, so the Cu and Al can be the coating. Cu and Mn make a
good
22
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
combination for sintering and can be altered with a small amount of Al to
provide the
desired magnetic properties. AICuMn can also be applied in much the same
manner as
described in relation to Ni powder if made into a powder first, then rolled
and used as it or
sintered onto the surface at lower temperatures than is typically required in
standard
processing. The CuMn alloy does not provide as high a density as NiMn, but is
sufficient
for most applications, such as shot, projectiles, fishing weights and the
like.
Iron (Fe) and Chromium (Cr) can also be applied as a powder to the surface,
either
with adhesive or sintered on to the product to provide the desired magnetic
properties.
The amount of Fe and Cr that should be used depends on the desired magnetism
and the
resulting density. Fe, steel, Ni, Cr and other magnetic materials can also be
applied as
coatings, although for some with difficulty. Fe and steel are not corrosion
resistant unless
they are in turn coated, as some steel shot presently is. Ni has been coated
onto the final
product as a powder with separate steps, following the primary sintering, of
coating with a
Ni powder, which has some kind of binder, such as sugar water, to attach the
powder to
the sintered article, followed by another sintering step to sinter the Ni
metallurgically. This
second sintering step can be eliminated if the binder is sufficiently strong
to keep the Ni or
other magnetic material in place.
Copper-Manganese (CuMn), Manganese-Nickel (MnNi) and Copper-Nickel-
Manganese (CuNiMn) all have melting points below or well below 1200 C, thus
making
processes using these alloys into a relatively less expensive and more
economical realm for
manufacturing. Other factors, such as the sintering-aid effect previously
described as part
of the FeW-C mix can also be important in the effectiveness of this process.
Mn has been
23
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
found to provide this sintering-aid effect when it is included in the
composition, such as in
CuMn, MnNi and CuMnNi alloys. Mn is also beneficial for not only lowering the
melting
point of the composition but also for assisting the sintering step and
densification,
whenever Mn is present and if the proportions of Mn is higher the effect is
magnified. Cu
appears to have the opposite effect and when Cu is increased, the composition
sintering-
aid effect is reduced, to the point where if Cu is the only component,
sintering is minimal
or almost nonexistent even though the melting point of Cu is well below the
processing
temperatures. No sintering-aid effect has been found for Ni, since the melting
point of Ni
is well above the typical processing temperatures. Mn alone, among the studied
elements,
melts well above the processing temperatures but aids sintering and
densification, so that
the full benefit of Mn is made available at the process temperatures when Mn
is alloyed
with other elements that lower the melting temperatures below 1200 C, and
typically much
below 1200 C. Other elements that Mn alloys well with are Tin (Sn) and Zinc
(Zn).
Tin-Zinc (SnZn) and Manganese-Tin-Zinc (MnSnZn) are alloys that have very low
melting points and are not easy to make into powders. Although powders may not
always
be necessary at the low temperatures if wetting is good or excellent. SnZn
does not wet as
well without the Mn, so small amounts of Mn are preferably added to the alloy
for wetting
and sintering. 4% Mn added to the composition helps wetting at 1200 C, but
does not
typically increase the density values significantly because there was very
little increase in
sintering. Greater amounts of Mn are desirable for increasing the sintering
and has been
demonstrated with 10% Sn and 80% Mn compositions. Larger amounts of Mn can
reduce
24
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
the retained magnetism unless sintering temperatures, and sometimes densities,
are
lowered or particle size of the magnetic materials are increased.
The following are some examples of compositions, processing temperatures and
atmospheres and resulting products of this invention. These examples are not
intended to
be exhaustive but rather only present examples of various embodiments of the
composition of this invention. These examples are presented by showing the
approximate
quantity of each major constituent, the firing (sintering) temperature, the
atmosphere
preferred, the sintering time, the resulting hardness in Rockwell Hardness
Units (RHA),
scale A, the product density, and whether the product is magnetic. For the
approximate
quantity of the constituent the values provided have been rounded to the
nearest
percentage. These percentages are not intended to imply that no other
constituent
elements, alloys, compounds or compositions are included, rather they describe
the
relative quantities of the major constituents pertinent to the objective of a
non-toxic dense
material. Other various components may and generally are included in the final
product as
trace elements or contaminants. Mesh sizes are provided where pertinent using
standard
US sieve mesh sizes. With this tabular description additional comments and
notes are
provided to give other specific information related to the particular
embodiment.
Example 1- An example of the use of Tin.
Ex# Sn Mn W FeW Firing Temp Atmos Time Dnsty Mag
1 -8.3 -16.6 -41.7 -33.2 1200 C Ar 1 hr 11.5 slightly
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
Example 2 - FeW powder is mixed with fine carbon powder (graphite)
Ex# FeW C Firing Temp Atmos Time Hardness Density Mag
2 -98.6 -1.4 1175'C H 15 m 67 11.7 no
High hardness is provided because of the carbides formed at the temperatures
where the sintering takes place, some WC can be formed in the reaction
sintering as the
bond between the Fe and W is broken by C.
Examples 3-6 - Ni, Mn and -100 to +400 mesh, in a ration of about 1/2/4 with W
powder added as noted in each.
Ex# Ni Mn FeW W Firing Temp Atmos Time Hrd Dnsty Mag
3 -10 -20 -40 -30 1200 C Ar 15m 27 10 10.0 no
4 -8.3 -16.6 -33.2 -41.7 1200 C Ar 15m 41 18 10.9 no
5 -7.1 -14.2 -28.4 -50 1200 C Ar 15m 31 7 11.6 no
6 -6.3 -12.6 -25.2 -56.3 1200 C Ar 15m 45 5 11.9 no
These hardness ranges go from well below that which is accepted hardness for
steel
shot and other "hard" shot to about the same as is found in such steel shot.
The relatively
large variation in hardness in these examples shows the variation possible
with a multiple
phase product.
26
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
Examples 7-10 - Ni, Mn and -200 mesh FeW powder, in a ratio of about 1/2/4,
with W powder added as noted in each example.
Ex# Ni Mn FeW W Firing Temp Atmos Time Hrd Dnsty Mag
7 -10 -20 -40 -30 1200 C Ar 15m 46 6 10.6 no
8 -8.3 -16.6 -33.2 -41.7 1200 C Ar 15m 46 9 11.1 no
9 -7.1 -14.2 -28.4 -50 1200 C Ar 15m 47 3 11.7 no
-6.3 -12.6 -25.2 -56.3 1200 C Ar 15m 47 0 12.0 no
These examples are somewhat softer than that typically used in the market for
5 "hard" shot.
Example II - Ni, Mn and W powders used to make a dense sintered product. The
powders are mixed and fired.
Ex# Ni Mn W Firing Temp Atmos Time Hrd Dnsty Mag
11 -6.6 -15.4 -80.0 1200 C Ar 15m 53 2 14 no
10 The density of this example is very high compared to related product
compositions.
The density can be varied by the addition of more or less of the lower density
Ni and Mn, in
other manufacturing processes these types of higher densities typically
require expensive,
hot pressing and/or high temperature manufacturing techniques (above 12000C) .
The
particle sizes of the W powder can also be modified to control the hardness
and density,
where the larger the W particle size, leads to lower hardness and increased
density. The
27
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
larger particle W powder also tends to lower the porosity both open and closed
for the
resulting product.
Examples 12-15 - Examples of the effect of the particle size of W on hardness,
the
finer the W particles, the harder the resulting product. In this table the
tungsten size is
average particle size in pm.
Ex# Ni Mn FeW W/size Firing Temp Atmos Time Hrd Dnsty Mag
12 -5.5 -12.6 -25.2 -56.7/40 1200 C Ar 15m 41 3 12.3 no
13 -5.5 -12.6 -25.2 -56.7/20 1200 C Ar 15m 42 7 12.2 no
14 -5.5 -12.6 -25.2 -56.7/12 1200 C Ar 15m 48 8 12.4 no
-5.5 -12.6 -25.2 -56.7/6 1200 C Ar 15m 60 4 12.4 no
As can be seen in these examples (Examples 12-15) there is not much effect on
the
hardness until the particle size approaches 12um and finer, with a profound
effect at 6pm
10 average particle size. Showing that hardness can be controlled, within a
range, by the
particle size of W.
Examples 16-24 - Magnetic enhanced products.
15 It is possible, in various embodiments of this invention, to enhance or
reduce
magnetic qualities of the resulting product by the use of careful selection of
particle sizes
and the metals chosen as the sintering medium. Metals such as Mn and Ni or C
in FeW-C
28
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
composition, react with the FeW to cause the FeW to lose the ferromagnetic
property that is
naturally possesses. Other metals, such as Cu, Zn and Sn can be used in
sintering with a
reduced effect on the magnetic properties of the FeW or other magnetic
additions because
of the reduced reaction with magnetic materials. For this reason Ni and Mn can
be used to
sinter and obtain high densities with no magnetism if such is desired and can
be mixed
with Cu, Sn and/or Zn to achieve the same results, although Cu appears to
reduce the
sintering/densification and the resultant densities to some degree. However,
if Cu, Sn and
Zn and their alloys are used the magnetic properties of the resulting product
are largely
unaffected and their magnetism is retained. Since the NiMn alloy composition
provides
higher densities, it can also be desirable to use NiMn as the sintering
medium, and to
thereby retain the magnetic properties of the product. Hence, the magnetic
properties of
the FeW or other magnetic material in the product mix can be retained even if
the sintering
medium reacts with the magnetic material by using a particle size that does
not react
completely but is still sintered densely. Among the several advantages of
using a larger
particle size FeW are: (1) Since FeW is quite hard and not very friable it is
much easier to
obtain a larger, particle size and therefore is therefore is generally less
costly to grind to a
large size. (2) The density of FeW is about 13.9 g/cc and, if it is used in a
larger size,
contributes to the overall density by the reduction of porosity or potential
porosity. (3)
Larger particle sizes tend to reduce the average hardness of the product. This
can be an
advantage for any use such as projectiles or shot where there is a concern for
barrel wear
or scarring. (4) Lower temperature processing reduces the interaction of the
sintering
medium with the magnetic component leaving more of the component unaltered and
29
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
thereby contributing to the retention of magnetism in the product. These
factors are
demonstrated by the following examples 16-24.
In Examples 16-18 the mesh size of the FeW is +200.
Ex# Ni Mn FeW W Firing Temp Atmos Time Dnsty Mag
16 -7.7 -20.5 -41.0 -30.8 1200 C Ar 15m 10.8 yes
17 -6.4 -17.0 -34.0 -42.6 1200 C Ar 15m 11.4 yes
18 -4.8 -12.7 -25.4 -57.1 .1200 C Ar 15m 12.1 yes
The magnetic qualities of the product are proportional to the non-magnetic
components, namely, NiMn is non-magnetic, if the Ni is lower than 60%, as is
W, so that
portion of unreacted FeW is the magnetic component, and the magnetism is
therefore
reduced proportionally.
In Examples 19-22 the objective is to not retain any ferromagnetic properties.
The
mesh size of the FeW is -200.
Ex# Ni Mn FeW W Firing Temp Atmos Time Hrd Dnsty Mag
19 -7.7 -20.5 -7.7 -30.8 1200 C Ar 15m 50 10.7 no
-6.4 -17.0 -20.5 -42.6 1200 C Ar 15m 50 11.5 no
21 -5.5 -14.5 -30.8 -50.9 1200 C Ar 15m 50 12.0 no
22 -4.8 -12.7 -41.0 -57.1 1200 C Ar 15m 50 12.4 no
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
In Examples 23-24 are shown other examples for retaining magnetism to a higher
degree with larger ferromagnetic particles. The FeW particle sizes are from
+100 mesh to
-20 mesh.
Ex# Ni Mn FeW W Firing Temp Atmos Time Hrd Dnsty Mag
23 -8.3 -16.6 -33.3 -47.7 1200 C Ar 15m 47 11.7 yes
24 -7.2 -14.2 -28.5 -50.0 1200 C Ar 15m 45 12.3 yes
Each of these examples (23 and 24) were highly ferromagnetic and would be
easily
detected by a magnet in nondestructive field testing of a shotshell for
magnetic shot.
Other tests with still larger FeW particle sizes, showed that the retained
magnetism
increases with the particle size until the particles were too large and
allowed open porosity.
The presently known optimal size for the FeW particles is found to be between
greater than
200 mesh and less than 10 mesh, although mixtures of large mesh size particles
can still
provide low porosity and high density. Where magnetism is desired with a
porous
structure, this can be readily obtained with a large mesh sizes, even up to
and exceeding
10 mesh.
Examples 25-32 demonstrate the use of CuMn. Cu was previously considered to be
eliminated from use because of known toxicity. More recent tests have shown
that the
toxicity to waterfowl of Copper is dependent on the residence time in the
gizzard and the
otherwise in the bird. Government approval has been granted to use Cu
containing shot so
31
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
tests have been conducted using CuMn, because of the low melting point of such
alloys
and the ability of Mn to act as a sintering aid.
Ex# Cu Mn FeW W Firing Temp Atmos Time Mag
25 -20.0 -10.0 -40.0 -30.0 1200 C Ar 15m no
26 -16.0 -8.0 -32.0 -41.6 1200 C Ar 15m no
27 -14.0 -7.0 -28.0 -50.0 1200 C Ar 15m no
28 -13.0 -6.5 -26.0 -56.2 1200 C Ar 15m no
Only a small amount of sintering was observed with little or no shrinkage.
Ex# Cu Mn FeW W Firing Temp Atmos Time Dnsty Mag
29 -10.0 -20.0 -40.0 -30.0 1200 C Ar 15m 10.2 no
30 -8.3 -16.6 -33.2 -41.7 1200 C Ar 15m 10.8 no
31 -7.1 -14.2 -28.4 -50.0 1200 C Ar 15m 11.4 no
32 -6.3 -12.6 -25.2 -56.3 1200 C Ar 15m 11.8 no
Reversing the ratio of Cu and Mn increases the density, probably due to the
higher
Mn or the lower Cu. The densities are lower than when Ni is used in place of
Cu at the
same ratios even though Ni and Cu densities are quite close at 8.96 g/cc for
Cu and 8.90
g/cc for Ni.
32
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
Examples 33-36 shows examples CuSn as a sintering aid.
Ex# Cu Mn FeW W Firing Temp Atmos Time Dnsty Mag
33 -10.0 -20.0 -40.0 -30.0 750 C Ar lhr porous yes
34 -8.3 -16.6 -33.2 -41.7 750 C Ar lhr porous yes
35 -7.1 -14.2 -28.4 -50.0 750 C Ar lhr porous yes
36 -6.3 -12.6 -25.2 -56.3 750 C Ar 1 hr porous yes
With no shrinkage this composition can be used to fire near net shape of the
mold
used. This composition has application where no shrinkage is important and
little or no
density increase from the formed part upon firing.
Example 37 is an example of Sn10Zn sintering. This example is sintered at 1200
C
for 1 hour in Ar. Zinc is known to be especially toxic to waterfowl, so this
example
composition may have application only in non-hunting uses, although the dwell
time in the
digestion system for ingested shot may be short enough to make this a viable
application,
especially with low levels of Zn in the alloy. This alloy has a very low
melting point at
198.5 C and there are no intermetallics formed, which may harden the alloy
more than the
initial introduction of Zn. This alloy did not wet the W and FeW component
powders so no
sintering took place and the alloy is assumed to have limited effectiveness,
unless a
wetting flux such as flux is applied. Fluxes are available for no lead
solders, which this
alloy is similar to, and may work with the alloy. A Sn10Zn4Mn alloy was also
used to see if
the Mn additive can solve the wetting problem in the SnlOZn alloy described
above. The
result was that the wetting did occur but that no significant sintering
occurred. The result
33
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
for this composition was similar to that of CuSn, in that it can be used for
making a solid
low strength piece at a near net forming shape.
Examples 38-43 are of the use of the alloy MnSn, which has a low melting
point, as
does MnSnCu and MnZn.
Ex# Sn Mn Cu Zn FeW W Temp Atm Time Dnsty Mag
38 -8.3 -16.6 0 0 -33.3 -41.7 1200 C Ar lhr 1.5 some
39 -4.7 -16.6 -4.7 0 -33.3 -41.7 1200 C Ar lhr 11.0 yes
40 -4.3 -17.4 0 0 -34.8 -60.9 1200 C Ar lhr 11.4 some
41 -3.7 -14.8 0 0 -29.6 -51.8 1200 C Ar lhr 11.9 yes
42 0 -17.4 0 -4.3 -34.8 -60.9 1200 C Ar lhr porous no
43 0 -16.0 0 -4.0 0 -80.0 1200 C Ar lhr porous no
The magnetism in the samples with Sn and the additional magnetism in the
sample
with Cu provides additional compositions of interest. Particular effects of
several of these
low melting alloys have been observed. Cu, Sn and to a certain degree Zn, none
of which
have much solubility in or reactivity with Fe or W, appear to inhibit
sintering or
densification while many compositions containing Mn have densification
enhanced and the
more Mn in the composition, the more sintering is provided, so long as the
melting point
of the mixture or alloy is below the sintering temperature of 1200 C, chosen
for the
economics of production.
34
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
Examples 44-59 show the use of additional alloys for sintering dense
materials.
Ex# Cu Mn W Temp Atm Time Dnsty
44 -33.3 -16.6 -50.0 1200 C Ar 15m 9.5
45 -22.2 -11.1 -66.6 1200 C Ar 15m 10.9
46 -16.6 -8.3 -75.0 1200 C Ar 15m 11.2
47 -13.3 -6.6 -80.0 1200 C Ar 15m 11.9
Examples 44-47 shows compositions with lower densities than are shown in
examples 48-51, but show that the selection of density is selectable by
adjusting the
Cu/Mn ratio.
Ex# Cu Mn W Temp Atm Time Dnsty
48 -16.6 -33.3 -50.0 1200 C Ar 15m 10.2
49 -11.1 -22.2 -66.6 1200 C Ar 15m 11.9
50 -8.3 -16.6 -75.0 1200 C Ar 15m 12.3
51 -6.6 -13.3 -80.0 1200 C Ar 15m 13.2
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
Examples 52-5 5 show the use of CuMnW(FeW).
Ex# Cu Mn W FeW Temp Atm Time Dnsty
52 -10.0 -20.0 -30.0 -40.0 1200 C Ar 15m 10.2
53 -9.1 -18.2 -45.4 -36.4 1200 C Ar 15m 10.8
54 -7.7 -15.4 -53.8 -30.8 1200 C Ar 15m 11.4
55 -6.6 -13.2 -60.0 -26.6 1200 C Ar 15m 11.8
Examples 56-59 show the use of powders mixed and then fired.
Ex# Cu Mn Temp Atm Time Dnsty
56 -80.0 -20.0 1200 C Ar 15 m 10.2
57 -66.6 -33.4 1200 C Ar 15m 10.8
58 -57.1 -42.9 1200 C Ar 15m 11.4
59 -50.0 -50.0 1200 C Ar 15m 11.8
These results are obtained even though Cu is more dense, 8.96 g/cc, than Mg at
7.43 g/cc. The alloys in these examples are less dense than the lighter of the
two
components when mixed at a 50-50 weight percentage. This shows that when used
as a
sintering alloy for W and W plus FeW, the higher Mn content, the less dense
the resulting
metal and the higher the sintered density. This shows the "sintering aid"
effect of the Mn,
since even thought the CuMn alloy is less dense with higher Mn sintered
product with W is
denser.
36
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
Examples 60-63 show compositions with Mn, Ni and Graphite (C) without W, only
FeW.
Ex# Mn Ni C FeW Temp Atm Time Dnsty Mag
60 -6.0 -1.9 -1.2 -82.7 1200 C H 15m 10.2 no
61 -5.9 -2.0 -1.4 -82.5 1200' C H 15m 10.8 no
62 -5.8 -2.1 -1.6 -82.3 1200 C H 15m 11.4 no
63 -5.9 -2.0 -1.8 -82.1 1200 C H 15m 11.8 no
This demonstrates the present most economical composition that still provides
corrosion resistance and a high density.
Example 64 shows a composition with the use of oxides of FeW.
Ex# Oxide C FeW Temp Atm Time Dnsty Mag
64 -17.2 -4.0 -78.7 1200 C H 15m 11.5 no
The use of oxides provides similar results to the mixes without oxides. This
is
extremely important to the use of starting materials as they can be either FeW
as a
compound or the burned oxides of FeW. The easiest way to reduce the particle
size to a
good range for manufacture may be to "burn", or oxidize the FeW in air or
oxygen at high
temperature and then screen and add the burned material to the process, either
as the
total component or mixed with metallic FeW in the chosen proportions. The
advantages
are lower cost materials and ease of production.
37
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
Referring now specifically to figure 1, which details the present steps of the
processing method of this invention. Carbon is loaded 101 for use in the
composition. In
some present preferred embodiments this step 101 includes mixing the loaded
carbon with
a binder in approximately equal proportions by weight, alternatively more or
less binder
may be used to accommodate the manufacturing technique and to improve even
carbon
distribution. In some alternative embodiments of the invention other
materials, including
iron, manganese, nickel, and/or chromium, typically in a micro powder form,
are also
mixed with the carbon and binder, as a part of this step 101. For the purposes
of the
disclosure, carbon or carbon-like material is defined as a composition useful
as a sintering
aid with tungsten, including a carbon composition, such as carbon black,
graphite, nano
tubes and related carbon forms, diamond, charcoal, hydro carbon and the like
and other
good sintering aid materials such as tin, bismuth, aluminum and the like.
A sintering aid is an element, compound or the like that when added to a
powder to
be sintered, aids the sintering process such that some desired physical
property is
attained. Sometimes a sintering aid may become a part of the final product and
other
times it may vaporize off, or otherwise be eliminated, after it has acted as
an aid. In some
embodiments the sintering aid may be reduced or eliminated in a pre-sinter or
post-sinter
step. In some embodiments, the sintering aid is incorporated into the sintered
piece. A
property commonly attained with a sintering aid is higher density achieved
with lower
processing (sintering) temperatures, shorter sintering time or both. Another
sometimes
desirable property is the drawing together of particles and the enhancement of
densification in all directions, to reduce or avoid slumping and distortion.
This property
38
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
can be achieved with lithium compounds as a sintering aid. Another sometimes
desirable
properties are the maintenance of a designed shape of the final product
through
production, along with densification at lower sintering temperatures and
shorter firing
times. These properties can be achieved with the addition of carbon as a
sintering aid.
Reactive sintering can be employed in this invention. Reactive sintering is a
method
of sintering whereby some or all of the components chemically react, thereby
resulting in a
sintered product that has improvements or enhancements. An example of this
reactive
sintering is the carbon/FeW reaction, in which the carbon reacts with the FeW
alloy and
becomes a part of the final product during sintering. It is presently believed
by the
inventor, that this reaction contributes to the densification of the product
at lower
temperatures and with shorter firing times, that is, the reactive sintering
acts also as a
sintering aid. When the reaction of carbon with oxides of FeW occurs, there is
some
reactive sintering along with the chemical reaction that occurs. In one
reaction, the carbon
reacts with the oxygen that is combined with the FeW and thereby producing a
carbon
monoxide and/or carbon dioxide gas, while the excess carbon reacts with the
FeW that has
been left behind. The reactions in this example occur almost simultaneously,
thereby likely
enhancing both the reaction sintering and sintering aid effects. A combination
of these
effects is likely to occur in this invention whether the starting material is
ferroalloy or
oxidized ferroalloy.
The reactive sintering, which can but need not always be used in this
invention, is
different from melting or liquid phase formation in a sintering step. Often a
liquid or
slushy phase may be formed during sintering, that may or may not aid the
sintering step.
39
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
Sometimes there is merely slumping that does not necessarily densify, and does
not tend
to hold the shape of the part being sintered. In most sintering operations it
is desirable to
hold the shape of the product, although substantial shrinkage often
accompanies firing and
densification. If the molten phase assists densification like a sintering aid
by causing
densification in all dimensions equally without slumping or distortion of the
original shape,
then the molten phase itself can be considered a sintering aid.
With regard to sectional density, it is known that typically the sectional
density of
round objects changes with the diameter of the object as a function of object
volume.
Round shotshell shot has a sectional density that decreases with decreasing
size of the
shot. Accordingly, for example, if #2 shot and #6 shot are fired from the same
gun at the
same initial velocity, the #6 shot will slow down faster than the #2 shot.
This difference
can be important for accurate firing at long ranges. This invention can make
different
densities of shot and can therefore be used to maintain consistent density
regardless of
shot size and can therefore minimize or eliminate the differences in
deceleration of
different shot sizes. This advantage can have particular importance when
loading different
sizes of shot in the same shotshell, because with this invention, despite the
differences in
size, all the shot pellets will travel at the same speed and the pattern will
tend to stay
together. As the pattern stays together, the long range effectiveness is
improved.
Alternatively, in some commercial loadings, two or more different sizes of
shot are used
specifically to cause the pattern to "string", that is, to lengthen out as it
travels from the
bore with the larger shot leading to the small shot. In some dual shot size
loads, the larger
shot is placed behind the small shot and may cause the larger shot to push
through the
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
smaller and disrupt the shot pattern. While pattern disruption or "stringing"
of the pattern
may be the objective in this type of load, with the density control of this
invention this
disruption or "stringing" can be minimized or enhanced in a controlled manner
according
to the shot pattern characteristics desired.
Presently, the preferred form of carbon is in the form of graphite powder,
although
alternative carbon sources or in some cases tin (Sn) can be substituted for
the graphite in
the mixture. The present binder is a binding composition such as Acrawax,
Polyvinyl
Alcohol (PVA), Paraffin or the like, although alternative binders as
previously described can
be substituted without departing from the concept of this invention. FeW is
added 102 to
the mixture of carbon and binder, presently in proportions of approximately
97% by weight
FeW; 1.5% by weight of carbon and 1.5% by weight of binder. In alternative
embodiments
the iron in the FeW composition is replaced with one or more of Nickel;
Manganese; Cobalt;
Copper; Silver; Gold; Gallium; Germanium; Chromium; Vanadium; Nickel, Niobium;
Molybdenum and the like, although generally not in sufficient quantities so as
to lower the
density of the final product. Currently, the FeW is provided in a mesh-200
powder form, or
with an average particle size of 10 microns, with about 20% of the particles
being
substantially larger. Although FeW is typically provided in mesh sizes of 2"
by down, 3/4"
by down, 1/4" by down or in a range, such as minus 2" plus 1" or minus 3/4"
plus 1/4", the
FeW is presently brought down to the desired size through the use of attrition
mills, ball
mills, jet mills, jaw crushers, hammer mills or any other customary technique
for reducing
the size of a material. After being reduced in size, a variety of techniques,
including
screens, gas classifiers and the like, can be used to assure that the
corrector optimum size
41
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
distribution is achieved. Blending can be used as necessary to achieve the
desired average
particle size. An alternative technique for reducing the size of the FeW, or
similar material,
is to oxidize the material in an appropriate furnace so that it changes form
and is more
easily attritted, ball milled, jet milled or other wise reduced in average
particle size. This
oxidation process provides partially or completely oxidized FeW, which can
then be
classified for blending or mixing to the appropriate proportions. Moreover,
this oxidized
FeW, or other material, can also then be formed into a desired shape or loaded
into a mold
that will provide the desired shape after having been mixed with an
appropriate amount of
carbon and then fired in a hydrogen furnace. In such a furnace, the carbon and
the
hydrogen will then reduce the FeW oxide, and facilitate the sintering to the
desired density.
Since oxides have a larger volume than the FeW, or other substituted
materials, there will
be greater composition shrinkage, a characteristic that can be used to reach a
desired
density. The combination of carbon/binder and FeW is mixed 103 to an
approximately
uniform mixture. The resulting mixture is pelletized 104, presently using
standard
pellitization techniques well known in the art. In alternative embodiments,
rather than
pellitizing, the resulting mixture is poured into a mold to be molded, pressed
or
compacted into the desired shape and/or is extruded. The pelletizing technique
presently
used in this invention involves mixing the powders (typically FeW and carbon-
like
materials) with a binder material, and then processing the mixed material in a
machine for
rolling, tumbling or the like in order to cause the powders to adhere to each
other and to
grow into spheres, pellets, rods and the like. This process is also used to
homogenize
powder mixes. A wide variety of pellet sizes are possible using this
pelletizing technique.
42
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
This preferred pelletizing technique uses no compaction or pressure to form
the pellets;
rather it is similar to rolling a snowball until it is large enough to make a
snowman.
In the molding alternative, the molding is accomplished by mixing the FeW
powder
and the Carbon-like material (typically graphite) and then pouring the
resulting mixture
into a mold of the desired shape for firing. The mixture in its mold is then
fired. This
firing of the mixture can be accomplished with one or more steps as necessary
to achieve
the desired result. For example, "green" material can be fired at just enough
time and
temperature to strengthen it sufficiently so that it can be further shaped
before final firing,
typically without the mold. This final firing stage may be done in the
tungsten powder or
in SiC grit or in graphite. The designation of SiC is not intended to indicate
any particular
proportion of the composition, nor is the designation of graphite intended to
limit the
forms of carbon.
The pressure or compaction alternative technique involves pressing or
compacting
the mixed powder (typically FeW and graphite, usually with a binder) in a die
to form the
desired shape, that is a product such as round pellets, bullets, milling media
and the like.
The green ("unfired") pressing or compacted product is then typically fired,
similar to the
molded product, to either a complete or an intermediate stage, where further
shaping can
be accomplished with a stronger partially fired pellet, bullet or the like.
The extruded technique is another method of forming or making pellets, bullets
and bullet cores, rods, milling media and the like. Mixed powders, with or
without a
binder, are forced through a die, which can be shaped and sized to give the
desired cross
section, and can be of nearly any desired length. When the desired length is
reached the
43
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
extrusion is sheared off and is processed further to shape the product into
the desired
shape. Again, the firing steps can include one or more firings as desired to
further work or
shape the product prior to final firing. The extrusion die can have a single
or multiple
openings. Extrusion, although not previously used in the manufacture of
shotgun pellets
or bullets, is well known in the production of products from ceramics to
metals, to plastics
and to foods. When extrusion is used to make the bullet cores, typically a
follow on step of
"swaging" is preformed to finalize the shape and dimensions of the bullet,
and/or to add a
jacket to the core. In this invention, the extrusion technique will typically
be used on a
FeW, graphite and binder mixture. The extruded product may be rolled or shaped
after
shearing to specific desired lengths to complete the shaping of the pellet to
the desired
shape.
A Silicon-Carbide (SiC) composition is mixed 105 into the pellitized product
to
more evenly distribute the heat that is applied in the following steps. As an
alternative, the
SiC composition mixing step 105 can be substituted with step of mixing in
tungsten
powder, ferro-tungsten powder, tungsten-carbide powder or ferro niobium powder
during
sintering. Heat of approximately 600 C is applied 106 to the pelletized (or
molded)
mixture for about fifteen minutes to drive out the binder, if such binder is
present. In
some alternative embodiments, the choice of binder or the lack of a binder may
make this
step unnecessary. An application of additional heat of approximately, in one
embodiment
of 11 50 C for approximately fifteen minutes is applied 107 to sinter the
entire product into
the pelletized form. These heating steps 106, 107, can include, in some
embodiments,
heating in a protective or reducing atmosphere. In the present embodiment it
is desirable
44
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
the temperature is maintained at or below 1200 C, because such lower
temperatures
dramatically reduce the production cost. Also, by sintering at a temperature
at or below
1200 C high densities with a smooth finish and little or no porosity may be
achieved. The
inventor has found that the addition of Mn or Ni with Carbon will enable the
lowering of the
sintering temperature to below 1200 C, may reduce the tendency of the
resulting product
to rust, but may also lower the resulting density to about 10.4 g/cc. However,
the addition
of both Mn and Ni combined with lowering the sintering temperature to about 1
100 C has
been shown to retain magnetic properties of the product and to maintain
densities in the
11.7 to 12.2 g/cc range. The sintering with carbon, presently in a graphite
form, is used
instead of melting because the pellet is first made and then is hardened,
while melting
requires higher processing temperatures and accordingly a high resulting
manufacturing
cost. In an alternative embodiment, tungsten powder, tungsten carbide,
manganese nickel,
ferro niobium and/or silicon carbide is added 108 as part of the sintering
step 107 and
thereafter sintered to reduce the likelihood of the final material to stick to
itself. In some
embodiments of this invention, this sintering step 107 is enhanced with small
quantities of
manganese. This sintering in of tungsten or tungsten carbide powder further
increases the
density of the resulting material and permits the alteration of the surface
characteristics of
the resulting material, as well as improving the heat distribution during
sintering.
Relatively large tungsten, tungsten carbide, ferro niobium or silicon carbide
particle sizes
are preferred for the added 108 powder because of the improved heat
distribution
characteristics of larger particles. For example, one present embodiment of
the produced
material is provided with a dimpled surface. In another embodiment of this
invention, the
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
sintering step also includes the sintering in of SiC powder in a relatively
large mesh size. In
alternative embodiments, both the temperatures, compositions and heating times
are
modified to produce product with different densities, strength, toughness or
friability, or
when alternative substitute materials are used. For example, if Sn is used
instead of
carbon, a sintering temperature of about 1050 C to 1200 C is appropriate. In
this tin
alternative, it is preferred to add tin prior to sintering and again when
molten during
sintering. Tin (Sn) or tin alloys can also be added after sintering to fill
voids and increase
density and to provide corrosion protection. After cooling, the resulting
product is a pellet
composed of a composition of matter consisting essentially of iron-carbide-
tungsten
(FeXCYWZ), or in alternative embodiments, iron-tungsten-tin (FeXWySnZ) or iron-
carbide-
tungsten-tin (FeWCXWYSnZ).
In some alternative embodiments the sintering 107 includes a pre-sinter step
followed by some machining (which may include grinding, drilling, rounding
etc.) followed
by a final sinter step to finalize the formation of the product. Also, in some
alternative
embodiments, the porosity as well as the density of the resulting product may
be
controlled through the addition 109, typically after the sintering step 107
with possible
heat treating, of an additional material. Materials such as metals, plastics
and the like, can
be used to increase or decrease the final density of the product. Tin or other
corrosion
resistant metals, plastics, paints and the like can be added to increase
corrosion resistance.
Ductile materials, like teflon, can also be added to cushion or reduce the
surface hardness
of the final product. In some embodiments of this invention, after sintering
107 color is
added to provide a technique of identifying the shot, for example #4 is blue,
#6 is red, etc.,
46
CA 02598122 2007-07-26
WO 2007/086852 PCT/US2006/002826
or color can be used to correspond to a weight, density, shape or size. A
variety of surface
coatings can also be added to make a slick, sticky or rough surface as
desired. In some
embodiments, a variety of additional materials, selected from the above may be
combined
in this 109 step to create a desired combination effect or use where a non-
toxic material
can be useful or required.
As noted above, in alternative embodiments, where it is desirable to retain
the
magnetic properties of the resulting pellet(s) the firing (sintering)
temperature is lowered
from typically about 1200 degrees C to about 1 100 degrees C and the firing
time can be
reduced by about 15 minutes. The lower temperature and/or reduced firing time
can
provide sintering without a complete reaction of the MnNi (which individually
were typically
added during the mixing step 103, although alternative could have been added
during the
mix with SiC step 105) with the FeW. In testing, the resulting pellet product
retains its
magnetic properties and has a density of between 11.7 and 12.3 g/cc.
While the invention has been described with respect to certain specific
embodiments, compositions and steps, it will be appreciated that many
modifications and
changes may be made by those skilled in the art without departing from the
invention. It is
intended, therefore, by the appended claims to cover all such modifications
and changes as
may come within the true spirit and scope of the invention.
47