Note: Descriptions are shown in the official language in which they were submitted.
CA 02628003 2008-04-30
DESCRIPTION
ELECTROSTATIC IMAGE DEVELOPING TONER, TONER KIT AND
IMAGE FORMING APPARATUS
Technical Field
The present invention relates to electrostatic image developing
toners, containing a polyester resin as a binder resin, that are utilized as
dry
toners to develop electrostatic images or magnetic latent images in
electrophotographic, electrostatic recording or electrostatic printing
1o processes; and also toner kits and image forming apparatuses.
Background Art
Polyester resins have been used as binders in the art in order to
improve low temperature toner fixability (Patent Literatures 1 and 2). In
order to improve the low temperature toner fixability still further, the
molecular mass and/or the glass transition temperature Tg should be
lowered with respect to the resins, however, which typically leading to poor
blocking resistance of toners under high temperature and high humidity
conditions. Such resins are also problematic as to reduce charging capacity
of developers since the toners adhere firmly to carriers, developing sleeves,
etc. Moreover, the reduction of charging capacity tends to be pronounced
with time in particular under high temperature and high humidity
conditions or low temperature and low humidity conditions with large image
1
CA 02628003 2008-04-30
areas. As such, toners and/or image forming apparatuses have been
demanded that can output stably high quality images under a wide variety of
operating conditions meanwhile being substantially non-problematic under
usual operating conditions.
A binder containing a charge controller or a charge control agent is
proposed in order to improve charging ability or charge stability and to
prevent background smear (Patent Literature 3). However, the charge
controller typically exhibits a low temperature fixability inferior to that of
polyester resins, thus is likely to deteriorate the low temperature fixability
of
polyester resins. It is therefore necessary for the toner to improve the low
temperature fixability still more that the charge controller should disperse
uniformly into the toner and represent a sufficient charging property in less
amount.
Developers are typically used in electrophotographic, electrostatic
recording or electrostatic printing processes in a way that a developer
firstly
attaches to a photoconductor on which an electrostatic image is formed in a
developing step, then the developer is transferred from the photoconductor to
a recording medium such as paper in a transfer step and fixed on the
recording medium in a transfer step. The developers for developing
electrostatic images on the surfaces with latent images are usually
two-component developers containing a carrier and a toner or one-component
developers containing a magnetic or non-magnetic toner and no carrier. In
2
CA 02628003 2008-04-30
the processes as regards the two-component developers, the toner particles
tend to attach the carrier surface to degrade the developer, and one-sided
consumption of toners decreases the toner concentration in the developers,
which requires to maintain a certain ratio between toner and carrier by
means of large-size developing devices. On the other hand, the apparatuses
or devices have been downsized by virtue of advanced function of developing
rollers as regards the one-component developers.
In recent years, automation and coloring have been popularized still
further in offices, such that various graphs by means of personal computers,
images taken with digital cameras, or pictorial drafts read by scanners are
printed and copied on a number of papers for personal presentation, for
example. Images to be output by printers typically contain a complicated
configuration including solid images, line images and halftone images even
in one draft, thus are demanded in various manners along with high
reliability.
Conventional electrophotographic processes on the basis of
one-component developers are classified into magnetic one-component
developing processes by use of magnetic toners and non-magnetic
one-component developing processes by use of non-magnetic toners. In the
magnetic one-component developing processes, which have been recently in
practical use for numerous small-size printers etc., a magnetic toner that
contains a magnetic material such as magnetites is supported by a developer
3
CA 02628003 2008-04-30
bearing member with a magnetic field-generating unit therein, and the toner
is thin-layered by means of a layer thickness-control member and developed
subsequently. However, most of the magnetic materials are of colored or
black, which affording a deficiency that the coloring is difficult.
On the other hand, in the non-magnetic one-component developing
processes, a toner supply roller etc. is urged to contact with a developer
bearing member thereby to supply a toner on the developer bearing member
that electrostatically supports the toner, which is then thin-layered by means
of a layer thickness-control member and developed, by virtue of the
non-magnetic property of toners. The processes may advantageously be
compliant to colorizing due to the absence of color magnetic materials, and
the apparatuses may be small-sized still more and of low cost due to the
absence of magnets in developer bearing members, thus have been recently
in practical use for small-size full-color printers etc.
The two-component developing systems may maintain stably the
charging ability and the transportability even under prolonged usage and be
easily compliant with high-speed developing devices, since a carrier is
employed as a means for charging and transporting, the toner and the
carrier is sufficiently stirred inside a developing unit and then transported
to
a developer bearing member before the developing.
In contrast, there remain currently many problems to be solved in
the one-component developing processes. That is, problems in charging or
4
CA 02628003 2008-04-30
transporting tend to occur under prolonged usage or high speed in the
one-component developing processes due to the absence of the charging and
transporting means such as carriers. Specifically, when the toner is
transported on the developer bearing member followed by thin-layering the
toner by means of the layer thickness-control member before the developing
in the one-component developing processes, toners of low or inverse charging
tend to generate in a rate more than that of the two-component developing
processes since the contacting or the frictional charging period is
significantly
shorter between the toner and the developer bearing member, the layer
lo thickness-control member or the frictional electrification.
In non-magnetic one-component developing processes, toners or
developers are transported typically by at least one toner transporting
member and electrostatic latent images on the latent image are developed by
use of the transported toner. In the processes, the layer thickness of the
toner should be as thin as possible on the surface of the toner transporting
member. This is applicable to two-component developers with carriers
having a very small diameter. When one-component developers and toners
with a high electric resistance are employed together with, the layer
thickness of the toner should also be as thin as possible in particular, since
the toners are to be charged by developing units. In cases where the toner
layer is thick, the toner layer is likely to be charged at only around its
surface
and far from uniformly charging over the entire toner layer. Therefore,
5
CA 02628003 2008-04-30
toners are required to exhibit a rapid charging velocity and an appropriate
charging amount.
As such, charge control agents and additives are conventionally
added to toners in order to stabilize the charging ability. The charge control
agent controls and maintains the frictional charge amount of toners. The
charge control agents of negative electricity are exemplified by mono azo
dyes; metal salts of salicylic acid, naphthoic acid and dicarboxylic acids;
metal complex salts of dicarboxylic acids; diazo compounds; and boron
complex compounds. The charge control agents of positive electricity are
exemplified by quaternary ammonium salts, imidazole compounds,
nigrosines and azine dyes.
However, some of these charge control agents are of chromatic color
and inadequate for color toners. In addition, some of these charge control
agents have a poor compatibility with binder resins and those on toner
surface, which mostly contributing to the charging, tend to separate from the
surface and fluctuate the charging ability of toners, or may
disadvantageously smear developing sleeves or cause filming on
photoconductors.
Therefore, there conventionally arises a troublesome phenomenon
that initial appropriate images degrade gradually to cause background
smear or unclearness. In cases of continuous color copy along with
supplying toners in particular, long term usage cannot be achieved since the
6
CA 02628003 2008-04-30
charge amount of toners decreases and the initial tone of images significantly
alters, such that no more than several thousand sheets of copy bring about
premature exchange of process cartridges of an imaging unit, which leading
to a large environmental load and bothersome processing of users.
Moreover, heavy metals in almost all process cartridges are causing a social
safety issue in recent years.
In order to solve the problems described above, resin charge-control
agents are proposed that improve the compatibility with binder resins,
clarity of fixed toner images and environmental safety. The resin
1o charge-control agents may afford stable charging ability/clarity due to
appropriate compatibility with binder resins. However, the charge control
agents are inferior in the charge amount/charging rate compared to toners
containing mono azo dyes, metal salts or metal complex salts of salicylic
acid,
naphthoic acid or dicarboxylic acids. When the added amount of the resin
charge-control agent increases, the charging ability may be improved but the
toner fixability such as low temperature fixability or offset resistance is
likely
to degrade. Moreover, these compounds tend to exhibit excessively large
environmental stability or moisture resistance with respect to their charge
amount, which possibly resulting in background smear or fog (Patent
Literatures 4 to 7).
As such, copolymers are proposed that are proposed from monomers
having an organic acid salt such as a sulfonic acid salt group and aromatic
7
CA 02628003 2008-04-30
monomers having an electron attracting group. However, these copolymers
represent an insufficient dispersion into the binder resins, and the effects
on
suppressing the fluctuation of toner charge amount or preventing the filming
on developing sleeves or photoconductors are insufficient as regarding a
prolonged period, although the charge amounts are sufficient by virtue of the
moisture absorbability and tackiness derived possibly from monomers
containing the organic acid salt such as the sulfonic acid salt group (Patent
Literatures 8 to 11).
In addition, such copolymers are proposed, formed of monomers
containing an organic acid salt like a sulfomc acid salt group, aromatic
monomers containing an electron- attracting group, and styrene or polyester
monomers, in order to enhance the compatibility with binder resins such as
styrene resins and polyester resins, however, providing insufficient effects
on
maintaining the charge amount or preventing the filming on developing
sleeves or photoconductors. In particular, the charge control agents are
typically unsatisfactory in combination with polyester or polyol resins as
used for a color toner binder resin that are usually desirable in terms of
coloring property and intensity.
There have been such a technical trend that the apparatuses are
small-sized, high-speed, and cost-lowered along with the printer market
expanding; and currently, the apparatuses are demanded for higher
reliability and longer life, toners are required to maintain their properties
for
8
CA 02628003 2008-04-30
a long period; however, the resin charge-control agents are less likely to
maintain their charge control effect thus to blur or foul the developing
sleeves or layer thickness-control members such as blades and rollers,
consequently decreasing charging ability of toners and causing filming on
photoconductors.
The small-sized, high-speed apparatuses necessarily lead to
developing processes with lower amounts of developers and shorter periods,
which requiring developers having an excellent initial charging property. A
variety of developing systems have been proposed for both of one-component
1o developers and two-component developers; non-magnetic one-component
development is desirable for printers by virtue of small-sizing or
weight-saving ability and absence of carriers. In the developing systems,
the toner amount on developing rollers is adjusted by way of forcibly
frictioning and attaching toners on developing rollers or by means of blades
since such properties are poor as toner-supplying ability onto the developing
rollers and toner-sustaining ability on the developing rollers. As a result,
there arise such problems as filming tendency of toners onto the developing
rollers, shorter lifetime of the developing rollers and unstable charge amount
of toners, and these problems possibly disturb adequate development.
Accordingly, color toners for the non-magnetic one-component development
are often unsatisfactory in thermal resistance of toner binder resins in
addition to usually necessary properties for conventional color toners, thus
9
CA 02628003 2008-04-30
are likely to cause toner filming on the developing rollers.
Furthermore, Patent Literatures 1 to 4 describes Examples that
show poor charge amount and charging velocity. When the added amount of
resin charge-control agents for the countermeasure is increased, the charging
ability may be improved but the toner fixability such as low temperature
fixability or offset resistance is likely to be deteriorated. Moreover, these
compounds tend to exhibit excessively large environmental stability or
moisture resistance in their charge amount, which possibly resulting in
background smear or fog.
Furthermore, the proposals in Patent Literatures 8 to 11 may assure
a sufficient charge amount due to moisture absorbability or adhesive
property, however, there remain such problems as insufficient dispersion into
toner binders, unsatisfactory suppression of charge fluctuation and
insufficient effect on preventing filming onto sleeves and photoconductors.
In forming images by electrophotographic processes, a latent image
is electrostatically formed on an image bearing member of photoconductive
materials etc., then charged toner particles are attached to the electrostatic
latent image to form a visible image, followed by transferring the toner
image onto a recording medium like papers and fixing thereof to produce an
output image. In recent years, electrophotographic copiers and printers are
changing rapidly from monochrome to full-color systems, and the full-color
market has been expanding.
CA 02628003 2008-04-30
In forming color images by full-color electrophotographic processes,
typically, color toners of three elementary colors of yellow, magenta and cyan
or four colors adding black thereto are duplicated to reproduce every color.
In order to produce clear full-color images with excellent color
reproducibility,
therefore, the surface of fixed toner images should be somewhat smoothed to
decrease optical diffraction, and it is also important that pigments are
uniformly dispersed into toners and the dispersed pigments maintain the
finely dispersed condition without re-coagulating.
In order to reproduce the color of human skin in particular, it is
1o required that the color is expressed by a subtractive mixing process
through
overlapping a yellow toner and a magenta toner, thus an optimum
combination from yellow pigments, magenta pigments and resins for
dispersion matrix has been investigated as a subject matter.
Patent Literature 12, for example, discloses a magenta toner for
developing electrostatic images, in which the toner is prepared by way of
dissolving a toner composition, containing a polyester resin modified to form
a urea bond, into an organic solvent to form a solution, which then undergoes
a polyaddition reaction, then the dispersion liquid is removed for the solvent
and rinsed, and the toner contains at least a colorant of a specific compound.
In addition, Patent Literature 13 discloses a magenta toner for
electrophotography containing at least a binder resin and a colorant, in
which the toner contains a naphthol pigment having a certain structure as
11
CA 02628003 2008-04-30
51216-9
the colorant, and the tone has a shape factor SF1 of 110 to 140 and a volume
average particle diameter of 2 to 9 gm.
However, these proposals may be far from recovering by themselves
the poor color reproducibility due to pigment re-agglomeration in toners, and
thus the color reproducibility of images is currently far from accurate
reproduction as for human shin color in particular.
Patent Literature 1: Japanese Patent Application Laid-Open (JP-A)
No. 62-178278
Patent Literature 2: JP-A No. 4-313760
Patent Literature 3: Japanese Patent Publication (JP-B) No. 7-062766
Patent Literature 4= JP-A No. 63-88564
Patent Literature 5= JP-A No. 63-184762
Patent Literature 6= JP-A No. 03-56974
Patent Literature 7= JP-A No. 06-230609
Patent Literature 8: JP-A No. 08-30017
Patent Literature 9: JP-A No. 09-171271
Patent Literature 10: JP-A No. 9-211896
Patent Literature 11= JP-A No. 11-218965
Patent Literature 12: JP-A No. 2004-77664
Patent Literature 13: JP-A No. 2003-215847
Disclosure of Invention
12
CA 02628003 2008-04-30
It is an object of the present invention to provide an electrostatic
image developing toner that is excellent in blocking resistance as well as low
temperature fixability under high temperature and high humidity conditions,
free from background smear, and far from lowering charging ability of
developers due to firm deposition of toner ingredients onto carriers or
developing sleeves with time even under high temperature and high
humidity conditions or low temperature and low humidity conditions and
also under outputting with large image areas, thus outputting stably high
quality images.
It is another object of the present invention to provide an
electrostatic image developing dry-toner that can control and maintain stably
the frictional charge amount of the toner, keep stably the frictional charging
ability, be excellent in transportability, developing ability, transferring
ability
and storage stability, and be free from abnormal images caused by deposition
onto photoconductors.
It is another object of the present invention to provide a
one-component and a two-component developer each utilizing the
electrostatic image developing toner and an image forming apparatus
utilizing at least one of the developers.
It is another object of the present invention to provide a toner kit for
developing latent electrostatic images, which is free from re-agglomeration of
pigments once-dispersed into resins and the related inferior color
13
CA 02628003 2008-04-30
reproducibility, thus can appropriately represent a color reproducibility of
yellow and magenta, and also red in a subtractive mixing process.
The present inventors have been investigated vigorously to solve the
problems described above and have found that the problems may be solved
by a toner binder of a polycondensation polyester resin produced under a
specific catalyst and a toner having a particle diameter and a particle
diameter distribution each controlled in a certain range, or by use of a
specific charge control agent.
The present invention has been made based on the findings
to described above; the problems described above can be solved by the
invention
as follows:
<1> A toner, comprising a colorant and a binder resin,
wherein the binder resin comprises a polyester resin that is prepared
by a polycondensation reaction in the presence of at least a
titanium-containing catalyst expressed by General Formula (I) or (II),
the toner has a volume average particle diameter of 2.0 m to 10.0
m and a ratio Dv/Dn of 1.00 to 1.40, in which Dv represents a volume
average particle diameter and Dn represents a number average particle
diameter,
T i (-X) m (- 0 H) n General Formula (I)
0 = T i (-X) P (- ci R) q General Formula (II)
14
CA 02628003 2008-04-30
in General Formulas (I) and (II), X represents a residue of a
mono-alkanolamine of 2 to 12 carbon atoms or a polyalkanolamine from
which a hydrogen atom of one hydroxyl group is removed; other hydroxyl
group(s) and still other hydroxyl group(s), within the polyalkanolamine
molecule that has a directly bonding Ti atom, may polycondense to form a
ring structure; other hydroxyl group(s) and still other hydroxyl group(s) may
polycondense intermolecularly to form a repeating structure; and the
polymerization degree is 2 to 5 in a case of forming the repeating structure;
R represents one of a hydrogen atom and alkyl groups of 1 to 8
carbon atoms that may have 1 to 3 ether bonds; "m" is an integer of 1 to 4;
"n"
is an integer of 0 to 3; the sum of "m" and "n" is 4; "p" is an integer of 1
or 2;
"q" is an integer of 0 or 1; the sum of "p" and "q" is 2; and in a case that
"m"
and "p" is 2 or more, the respective Xs may be identical or different each
other.
<2> The toner according to <1>, wherein the polyester resin
comprises at least a species of polyester resin that is prepared by a
polycondensation reaction in the presence of a titanium-containing catalyst
expressed by General Formula (I) or (II), and X in General Formulas (I) and
(II) represents a residue of a dialkanolamine or a trialkanolamine from
which a hydrogen atom of one hydroxyl group is removed.
<3> The toner according to <1> or <2>, wherein the polyester resin
comprises at least a species of polyester resin that is prepared by a
CA 02628003 2008-04-30
polycondensation reaction in the presence of a titanium-containing catalyst
expressed by General Formula (I) or (II), in which "m" or "p" is 2 or more,
and
all of Xs are an identical group.
<4> The toner according to any one of <1> to <3>, wherein the
polyester resin comprises at least a species of polyepoxide-modified resin.
<5> The toner according to any one of <1> to <4>, wherein the
polyester resin comprises substantially no THE insoluble matter, the content
of the ingredients having a molecular mass of 500 or less is no more than 4%
by mass in the molecular mass distribution based on gel permeation
1o chromatography, and a main peak exists within a range of 3000 to 9000 in
the molecular mass distribution.
<6> The toner according to any one of <1> to <5>, wherein the
binder resin represents an endothermic peak within a range of 60 C to 70 C
under the measurement using a differential scanning calorimeter (DSC).
<7> The toner according to any one of <1> to <6>, wherein the
binder resin has a ratio Mw/1\4n of 2 to 10, in which Mw represents a mass
average molecular mass and Mn represents a number average molecular
mass.
<8> The toner according to any one of <1> to <7>, wherein the
binder resin has an acid value of 10 mgKOH/g or less.
<9> The toner according to any one of <1> to <8>, wherein the
binder resin represents a temperature within a range of 95 C to 120 C at
16
CA 02628003 2008-04-30
which the apparent viscosity comes to 103 Pa=s measured by a flow tester.
<10> A toner kit, comprising the toner according to any one of <1>
to <9>,
wherein the toner kit comprises a yellow toner, a magenta toner and
a cyan toner,
the magenta toner comprises an organic pigment expressed by the
following Structural Formula (1), and the yellow toner comprises an organic
pigment having two units per molecule each expressed by Structural
Skeleton (A) and no halogen atom;
H3CO / C I
OCH3 H-N
H O 0
N Structural Formula (1)
H N
0 /
CH3
O
H N Structural Skeleton (A)
N N -
O Fi
in the Structural Formula (1) and Structural Skeleton (A),
=C=N-NH- may be =CH-N=N-.
<11> The toner kit according to <10>, wherein the organic pigment,
having two units per molecule each expressed by Structural Skeleton (A) and
no halogen atom, is an organic pigment expressed by Structural Formula (2)
17
CA 02628003 2008-04-30
or (3).
CH3
O
"N NN /-\
O H H3C
H N OCH2CH2O O
o H NH Structural Formula (2)
H
0 0-
H
N. H3C0
COOCH3
O
H-N
O N
O H
H3C-N N CH3
H O Structural Formula (3)
N O
N-H
/ ` O
H3000C
OCH3
<12> An image forming apparatus, comprising:
a latent electrostatic image bearing member,
a latent electrostatic image forming unit configured to form a latent
electrostatic image on the latent electrostatic image bearing member,
at least three developing units configured to develop a visible image
to using the toner kit according to <10> or <11>,
a transfer unit configured to transfer the visible image onto a
recording medium, and
a fixing unit configured to fix the transferred image on the recording
medium.
18
CA 02628003 2008-04-30
Brief Description of Drawings
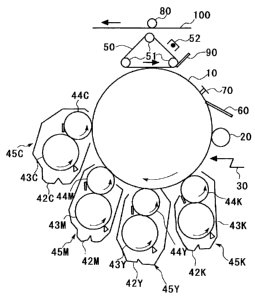
FIG. 1 is a schematic constitutional view of a developing device of an
inventive image forming apparatus.
FIG. 2A is a schematic view of toner shape to explain the shape
factor SF-1.
FIG. 2B is a schematic view of toner shape to explain the shape
factor SF-2.
FIG. 3A is a schematic view of toner shape to explain the shape
factors SF-1, SF-2.
FIG. 3B is a schematic view of toner shape to explain the shape
factors SF-l, SF-2.
FIG. 3C is a schematic view of toner shape to explain the shape
factors SF-1, SF-2.
FIG. 4 shows exemplarily an embodiment of an inventive image
forming apparatus.
FIG. 5 shows exemplarily another embodiment of an inventive image
forming apparatus.
FIG. 6 is a schematic view that shows exemplarily a contact charger
used in an inventive image forming apparatus.
FIG. 7 is a schematic view that exemplarily shows a color-image
forming apparatus of tandem system.
FIG. 8 is a schematic view that exemplarily shows a color-image
19
CA 02628003 2008-04-30
forming apparatus of tandem system with an intermediate transfer.
FIG. 9 is a schematic view that exemplarily shows an entire
configuration of an image forming apparatus of tandem indirect image
transfer system.
FIG. 10 is a schematic view that exemplarily shows an image
forming apparatus of tandem indirect transfer system with an inventive
process cartridge.
FIG. 11 a graph that plots the values measured for a* and b* in
L*a*b* color specification system with respect to the toners of Examples 75
to 78 and Comparative Examples 26 to 29.
FIG. 12 a graph that plots the values measured for a* and b* in
L*a*b* color specification system with respect to the toners of Examples 75,
78 and Comparative Examples 26, 27.
FIG. 13 is a partially enlarged view of FIG. 12.
FIG. 14 a graph that plots the values measured for a* and b* in
L*a*b* color specification system with respect to the toners of Examples 76,
77 and Comparative Examples 28, 29.
FIG. 15 is a partially enlarged view of FIG. 14.
Best Mode for Carrying Out the Invention
Toner
The toner according to the present invention comprises a colorant
CA 02628003 2008-04-30
and a binder resin, and also optional other ingredients.
The binder resin contains at least a polyester resin that is prepared
by a polycondensation reaction in the presence of at least a
titanium-containing catalyst expressed by General Formula (I) or (II).
The titanium-containing catalyst is a compound expressed by
General Formula (I) or (II) and may be two or more compounds thereof,
T i (-X) m (-0 H) n General Formula (I)
C3 = T i (-- X) P (- C7 F) General Formula (II)
in General Formulas (I) and (II), X represents a residue of a
mono-alkanolamine of 2 to 12 carbon atoms or a polyalkanolamine thereof
1o from which a hydrogen atom of one hydroxyl group is removed; other
hydroxyl group(s) and still other hydroxyl group(s), within the
polyalkanolamine that directly bonds to a Ti atom, may polycondense to form
a ring structure; other hydroxyl group(s) and still other hydroxyl group(s)
may polycondense intermolecularly to form a repeating structure. In cases
of repeating structures, the polymerization degree is 2 to 5;
R represents one of a hydrogen atom and alkyl groups of 1 to 8
carbon atoms that may have 1 to 3 ether bonds;
"m" is an integer of 1 to 4; "n" is an integer of 0 to 3; the sum of "m"
and "n" is 4; "p" is an integer of 1 or 2; "q" is an integer of 0 or 1; the
sum of
"p" and "q" is 2; in case that "m" and/or "p" is 2 or more, the respective Xs
21
CA 02628003 2008-04-30
may be identical or different each other.
In General Formulas (I) and (II) above, X represents a residue of a
mono-alkanolamine of 2 to 12 carbon atoms or a polyalkanolamine thereof
from which a hydrogen atom of one hydroxyl group is removed; the number
of nitrogen atoms, i.e. the total number of primary, secondary and tertiary
amines, is preferably 1 or 2, more preferably 1.
The monoalkanolamine may be properly selected depending on the
application; examples thereof include ethanolamine and propanolamine.
The polyalkanolamine may be properly selected depending on the
to application; examples thereof include dialkanolamines such as
diethanolamine, N-methyldiethanolamine and N-butyldiethanolamine;
trialkanolamines such as triethanolamine and tripropanolamine; and
tetraalkanolamines such as N,N,N',N'-tetrahydroxyethylethylenediamine.
In cases of polyalkanolamines, there exists at least one hydroxyl
group in addition to the hydroxyl group for the residue to form Ti-O-C bond
with a Ti atom; the hydroxyl group(s) and other hydroxyl group(s), within the
polyalkanolamine that directly bonds to a Ti atom, may polycondense to form
a ring structure; or the hydroxyl group(s) and other hydroxyl group(s) may
polycondensate intermolecularly to form a repeating structure. In cases of
repeating structures, the polymerization degree is 2 to 5. In cases where
the polymerization degree is above 5, the catalytic activity tends to be
lower,
which may increase the amount of oligomers and deteriorate blocking
22
CA 02628003 2008-10-21
51216-9
resistance of toners.
X may be a residue of dialkanolamines in particular diethanolamme
or a residue of tialkanolamines in particular triethanolamine, particularly
preferable is the residue of triethanolamine.
R represents one of a hydrogen atom (H) and alkyl groups of 1 to 8
carbon atoms that may have 1 to 3 ether bonds. Examples of the alkyl
groups of 1 to 8 carbon atoms include methyl group, ethyl group, n-propyl
group, isopropyl group, n-butyl group, n-hexyl group, n-octyl group,
beta-methoxyethyl group and beta-ethoxyethyl group. Among these, R is
preferably hydrogen atom or alkyl groups of 1 to 4 carbon atoms having no
ether bond, more preferably, hydrogen atom, ethyl group or isopropyl group.
In General Formula (I) above, "m" is an integer of 1 to 4, preferably 1
to 3; "n" is an integer of 0 to 3, preferably 1 to 3; the sum of "m" and "n"
is 4.
In General Formula (II) above, "p" is an integer of 1 or 2; "q" is an integer
of 0
or 1; the sum of "p" and "q" is 2. Xs maybe identical or different each other
in case that "m" and/or "p" is 2 or more.
Examples of the titanium-containing catalyst expressed by General
Formula (I) include titanium dihydroxybis(triethanolaminate), titanium
trihydroxytriethanolaminate, titanium dihydroxybis(diethanolaminate),
titanium dihydroxybis(monoethanolaminate), titanium
dihydroxybis(monopropanolaminate), titanium dihydroxybis(N-
methyldiethanolaminate), titanium
23
CA 02628003 2008-10-21
i 1
51216-9
dihydroxybis(N-buthyldiethanolaminate) and reaction products
of these compounds with N,N,N',N'-tetrahydroxy
ethylethylenediamine or intermolecular polycondensation
products of these compounds.
Examples of the titanium-containing catalyst
expressed by General Formula (II) include
titanylbis(triethanolaminate), titanylbis(diethanolaminate),
titanylbis(monoethanolaminate),
titanylhydroxyethanolaminate,
titanylhydroxytriethanolaminate,
titanylethoxytriethanolaminate,
titanylisopropoxytriethanolaminate, and intramolecular or
intermolecular polycondensation products of these compounds.
Among these, preferable are titanium
dihydroxybis(triethanolaminate), titanium
dihydroxybis(diethanolaminate),
titanylbis(triethanolaminate), polycondensation products
thereof, and combinations of these compounds particularly
preferable is titanium dihydroxybis(triethanolaminate).
These titanium-containing catalysts may be readily
synthesized by reaction of commercially available titanium
dialkoxybisalcoholaminates (by DuPont Co.) at 70 C to 90 C
in the presence of water.
The amount of the titanium-containing catalyst is
preferably 0.0001 to 0.8% by mass based on the resulting
polycondensation product in view of polymerization activity,
more preferably 0.0002 to 0.6% by mass, still more
preferably 0.0015 to 0.55% by mass.
24
CA 02628003 2008-04-30
The titanium-containing catalyst may be combined with other
esterification catalysts in an appropriate non-harmful range. Examples of
the other esterification catalysts include tin-containing catalysts such as
dibutyltin oxide; antimony trioxide; titanium-containing catalysts other than
the titanium-containing catalysts such as titanium alkoxides, potassium
titanyl oxalate and titanium terephthalate; zirconium-containing catalysts;
germanium-containing catalysts; alkaline (earth) metal catalysts such as
carboxylates of alkaline metals and alkaline earth metals, lithium acetate,
sodium acetate, potassium acetate, sodium benzoate and potassium
1o benzoate; and zinc acetate. The amount of the other catalysts is preferably
0 to 0.6% by mass based on the resulting polymer. The amount of no more
than 0.6% by mass may lead to less coloring of the polyester resin thus is
desirable for color toners. The content of the titanium-containing catalyst in
the entire catalyst is preferably 50 to 100% by mass.
Binder Resin
The polycondensed polyester resin of the binder resin may be
polycondensate of polyester resins (AX) between polyols and polycarboxylic
acids or modified polyester resins (AY) by reaction of AX and polyepoxides
(c).
These AX and AY may be used alone or combinations of two or more.
The polyol may be diols (g) or trivalent or more polyols W. The
polycarboxylic acid may be dicarboxylic acids (i) or trivalent or more
polycarboxylic acids (j). These may be combinations of two or more.
CA 02628003 2008-04-30
The polyester resin (AX) or (AY) may be those shown below, and
these may be used in combination.
(AXi) : linear polyester resins prepared from (g) and (i);
(AX2) : nonlinear polyester resins prepared from (g) and (i) along
with (h) and/or (j);
(AY1) : modified polyester resins by reaction of (AX2) with (c).
The diol (g) is preferably those having a hydroxyl value of 180 to
1900 mgKOH/g. Specific examples are alkylene glycols of 2 to 36 carbon
atoms such as ethylene glycol, 1,2-propylene glycol, 1,3-propylene glycol,
1,4-butylene glycol and 1,6-hexanediol; alkyleneether glycols of 4 to 36
carbon atoms such as diethylene glycol, tiethylene glycol, dipropylene glycol,
polyethylene glycol, polypropylene glycol and polybutylene glycol;
cycloaliphatic diols of 6 to 36 carbon atoms such as 1,4-cyclohexane
dimethanol and hydrogenated bisphenol A; adducts of cycloaliphatic diols
described above with alkylene oxides of 2 to 4 carbon atoms such as ethylene
oxide (EO), propylene oxide (PO) and butylene oxide (BO) (added mole
number: 1 to 30); adducts of bisphenols such as bisphenol A, F and S with
alkylene oxides of 2 to 4 carbon atoms such as EO, PO and BO (added mole
number: 2 to 30).
Among these, preferable are alkylene glycols of 2 to 12 carbon atoms,
adducts of bisphenols with alkylene oxides, or combinations thereof,
particularly preferable are adducts of bisphenols with alkylene oxides,
26
CA 02628003 2008-04-30
alkylene glycols of 2 to 4 carbon atoms, or combinations of two or more
thereof. The hydroxyl value may be measured in accordance JIS K 0070, for
example.
The trivalent or more polyols (h), i.e. 3 to 8 valence or more, are
preferably those having a hydroxyl value of 150 to 1900 mgKOHIg. Specific
examples are aliphatic polyvalent alcohols of 3 to 36 carbon atoms and 3 to 8
or more valences such as alkane polyols and intra- or inter-molecular
dehydration products like glycerin, triethylolethane, trimethylolpropane,
pentaerythritol, sorbitol, sorbitan, polyglycerin and dipentaerythritol;
saccharide and derivatives thereof like simple sugar and methyl glucoside;
adducts of aliphatic polyvalent alcohols with alkylene oxides of 2 to 4 carbon
atoms such as EO, PO and BO (added mole number: 1 to 30); adducts of
trisphenols such as trisphenol PA with alkylene oxides of 2 to 4 carbon atoms
such as EO, PO and BO (added mole number: 2 to 30); and adducts of
novolac resins such as phenol novolacs and cresol novolacs having an average
polymerization degree of 3 to 60 with alkylene oxides of 2 to 4 carbon atoms
such as EO, PO and BO (added mole number: 2 to 30).
Among these, preferable are aliphatic polyvalent alcohols of 3 to 8 or
more valences and adducts of novolac resins with alkylene oxides (added
mole number: 2 to 30), particularly preferable are adducts of novolac resins
with alkylene oxides.
Preferably, the dicarboxylic acid (i) has an acid value of 180 to 1250
27
CA 02628003 2008-04-30
mgKOH/g; specific examples thereof include alkane dicarboxylic acids of 4 to
36 carbon atoms such as succinic acid, adipic acid and sebacic acid; alkenyl
succinic acids such as dodecenylsuccinic acid; cycloaliphatic dicarboxylic
acids of 4 to 36 carbon atoms such as dimer acids like linoleic acid dimer;
alkene dicarboxylic acids of 4 to 36 carbon atoms such as maleic acid, fumaric
acid, citracomc acid and mesaconic acid; and aromatic dicarboxylic acids of 8
to 36 carbon atoms such as phthalic acid, isophthalic acid, terephthalic acid
and naphthalenedicarboxylic acid. Among these, particularly preferable are
alkene dicarboxylic acids of 4 to 20 carbon atoms and aromatic dicarboxylic
acids of 8 to 20 carbon atoms. The compounds (i) may be acid anhydrides or
esters of lower alkyls of 1 to 4 carbon atoms, derived from those described
above, such as methyl esters ethyl esters and isopropyl esters.
The trivalent or more polycarboxylic (j) (i.e. 3 to 6 valences or more)
is preferably those having an acid value of 150 to 1250 mgKOH/g; specific
examples thereof include aromatic polycarboxylic acids of 9 to 20 carbon
atoms such as trimellitic acid and pyromellitic acid; and vinyl polymers of
unsaturated carboxylic acids having a number average molecular mass of
450 to 10000 by gel permeation chromatography (GPC) such as
styrene/maleic acid copolymers, styrene/acrylic acid copolymers,
alpha-olefin/maleic acid copolymers and styrene/fumaric acid copolymers.
Among these, preferable are aromatic polycarboxylic acids of 9 to 20 carbon
atoms, in particular trimellitic acid and pyromellitic acid. The trivalent or
28
CA 02628003 2008-04-30
more polycarboxylic (j) may be acid anhydrides or esters of lower alkyls of 1
to 4 carbon atoms, derived from those described above, such as methyl esters,
ethyl esters and isopropyl esters.
The compounds (g), (h), (i) or (j) may be respectively copolymerized
with aliphatic or aromatic hydroxycarboxylic acids (k) of 4 to 20 carbon atoms
or lactones (1) of 6 to 12 carbon atoms.
The hydroxycarboxylic acid (k) is exemplified by hydroxystearic acid
and aliphatic acids of hydrogenated castor oil; the lactone (1) is exemplified
by
caprolactone.
Examples of the polyepoxide (c) include polyglycidyl ethers such as
ethylene glycol diglycidyl ether, tetramethylene glycol diglycidyl ether,
bisphenol A diglycidyl ether, bisphenol F diglycidyl ether, glycerin
tridiglycidyl ether, pentaerythritol tetraglycidyl ether and glycidyl-
etherified
phenol novolac (average polymerization degree: 3 to 60); and diene oxides
such as pentadiene oxide and hexadiene oxide. Among these, preferable are
polyglycidyl ethers, in particular ethylene glycol diglycidyl ether and
bisphenol A diglycidyl ether.
The number of epoxy groups is preferably 2 to 8 per molecule of the
polyepoxide (c), more preferably 2 to 6, and still more preferably 2 to 4. The
epoxy equivalent of the polyepoxide (c) is preferably 50 to 500; more
preferably, the lower limit is 70, still more preferably 80; more preferably,
the
upper limit is 300, still more preferably 200. The number of epoxy groups
29
CA 02628003 2008-04-30
and the epoxy equivalent within the ranges may provide appropriate
developing ability as well as fixing ability; more preferably, the number of
epoxy groups and the epoxy equivalent are within the preferable ranges at
the same time.
The reactant ratio of the polyol and the polycarboxylic acid is
preferably 2/1 to 1/2 in terms of the equivalent ratio [OH]/[COOH], more
preferably 1.5/1 to 1/1.3, still more preferably 1.3/1 to 1/1.2. It is
preferred
that the specific compounds of the polyol and the polycarboxylic acid are
selected such that the glass transition temperature Tg of the resulting
polyester toner binder is 40 C to 90 C considering the molecular mass.
The binder resin is typically required for different properties between
full-color and monochromic applications, which also leading to different
designs for the polyester resins. That is, full color images are required for
high gloss, which requesting a low-viscosity binder resin, and monochromic
images are demanded for hot offset resistance instead of the gloss, which
requesting a high-modulus binder resin.
The (AX1), (AX2) and (AY1) described above and also combinations
thereof are preferable in order to form high gloss images suited for full-
color
copiers etc. From the viewpoint that the polyester resin is preferably of
lower viscosity, the content of (h) and (j) is 0 to 20% by mole based on the
total of (g) to (j) by mole number, more preferably 0 to 15% by mole, still
more
preferably 0 to 10% by mole.
CA 02628003 2008-04-30
The (AX2) and (AY1) described above and also combinations thereof
are preferable in order to form images with hot offset resistance suited for
monochromic copiers etc. From the viewpoint that the polyester resin is
preferably of high modulus, the polyester resin is preferably prepared by
both of (h) and (j) in particular. The content of (h) and (j) is preferably
0.1 to
40% by mole based on the total of (g) to (j) by mole number, more preferably
0.5 to 25% by mole, still more preferably 1 to 20% by mole.
In cases of polyester resins for full-color, the temperature at which
the complex viscosity coefficient il* being 100 Pa-s (TE) is preferably 90 C
to
170 C, more preferably 100 C to 165 C, still more preferably 105 C to 150 C.
The TE of no higher than 170 C may bring about sufficient gloss, and the TE
of no lower than 90 C may lead to appropriate storage stability at high
temperatures.
The temperature TE can be determined by way of measuring the
complex viscosity coefficient rl* while changing the resin temperature using a
commercially available measurement device for dynamic viscoelasticity after
melting-kneading a resin block at 130 C, 70 rpm for 30 minutes using a
laboblast mill.
The insoluble matter into tetrahydrofuran (THF) of polyester resins
for full-color is no more than 10% by mass in view of glossiness, more
preferably no more than 5% by mass.
The insoluble matter or soluble matter into THE can be measured by
31
CA 02628003 2008-04-30
the following processes.
A sample 0.5 g is precisely weighed into a 200 mL Meyer flask with a
stopper, to which 50 mL of THE is added and the mixture is stirred under
reflux for 3 hours, then the insoluble matter is filtered off using a glass
filter.
The content of the THE insoluble matter is calculated from the mass ratio of
the sample and the matter filtered-dried at 80 C for 3 hours. The molecular
mass described later is determined by use of the filtrate as the THE soluble
matter.
In cases of polyester resins for monochrome, the temperature at
1o which the storage modulus Gbeing 6000 Pa (TG) is preferably 130 C to
230 C, more preferably 140 C to 230 C, still more preferably 150 C to 230 C.
The temperature TG can be determined by way of measuring the
storage modulus while changing the resin temperature using a commercially
available measurement device for dynamic viscoelasticity after
melting-kneading a resin block at 130 C, 70 rpm for 30 minutes using a
laboblast mill.
In cases of polyester resins for monochrome, the temperature at
which the complex viscosity coefficient rl* being 1000 (TE) is preferably 80 C
to 140 C in view of low temperature fixability and high temperature storage
stability, more preferably 90 C to 135 C, still more preferably 105 C to
130 C.
The polyester resin for monochrome preferably contains 2 to 70% by
32
CA 02628003 2008-04-30
mass of the THE insoluble matter, more preferably 5 to 60% by mass, still
more preferably 10 to 50% by mass. The THE insoluble matter of no less
than 2% by mass may lead to appropriate hot offset resistance, and no higher
than 70% by mass thereof may lead to favorable low temperature fixability.
The peak top molecular mass Mp of the polyester resin is preferably
1000 to 30000 for monochrome as well as full-color, more preferably 1500 to
25000, still more preferably 1800 to 20000. The peak top molecular mass
Mp of no less than 1000 may lead to appropriate high temperature storage
stability and proper powder flowability, and no higher than 30000 thereof
may enhance milling ability of toners and thus bring about proper
productivity.
It is also preferred in the inventive toner containing a toner binder
resin of polyester resins that the content of ingredients having a molecular
mass of no more than 1500 is 1.8% by mass or less, more preferably 1.3% by
mass or less, and still more preferably 1.1% by mass or less. The content of
ingredients, having a molecular mass of no more than 1500, of 1.8% by mass
or less may lead to more proper storage stability.
The peak top molecular mass Mp, the number average molecular
mass, and the content of ingredients having a molecular mass of no more
than 1500 may be measured for THE soluble matter of polyester resins or
toners using GPC under the following conditions.
Apparatus: HCL-8120, by Tosoh Co.
33
CA 02628003 2008-04-30
Column: TSK gel GMHXL (two),
TSKge1 Multipore HXL-M (one)
Measuring temperature: 40 C
Sample solution: 0.25% solution in THE
Injecting solution amount: 100 l
Detecting device: refractive index
Standard: polystyrene
The molecular mass, which corresponding to the highest peak on the
resulting chromatogram, is referred to as "peak top molecular mass" (Mp).
1o The ratio of peak area, corresponding to matters less than the molecular
mass of 1500, may represent the ratio existing as low molecular mass
matters.
The acid value of the polyester resin is preferably 0.1 to 60 mgKOH/g
for monochrome as well as full-color, more preferably 0.2 to 50 mgKOH/g,
still more preferably 0.5 to 40 mgKOH/g. The acid value of 0.1 to 60
mgKOH/g may bring about appropriate charging ability.
The hydroxyl value of the polyester resin is preferably 1 to 70
mgKOHIg for monochrome as well as full-color, more preferably 3 to 60
mgKOH/g, still more preferably 5 to 55 mgKOH/g. The hydroxyl value of 1
to 70 mgKOH/g may bring about appropriate environmental stability.
The glass transition temperature of the polyester resin is preferably
40 C to 90 C for monochrome as well as full-color, more preferably 50 C to
34
CA 02628003 2008-04-30
80 C, still more preferably 55 C to 75 C. The glass transition temperature
Tg of 40 C to 90 C may favorably bring about high temperature storage
stability and low temperature fixing ability.
The glass transition temperature Tg of the polyester resin may be
measured in accordance with DSC method defined in ASTM D 3418-82 using
DSC20 SCC/580 by Seiko Instruments Inc., for example.
The polyester resin for the binder resin may be produced by a process
similar as conventional processes for producing polyesters; under such
conditions as in inert gas atmosphere like nitrogen gas in the presence of
titanium-containing catalysts at reaction temperature of preferably 150 C to
280 C, more preferably 160 C to 250 C, still more preferably 170 C to 240 C,
for example. The reaction period is preferably 30 minutes or longer, more
preferably 2 to 40 hours from the view point of assuring the polycondensation
reaction. The atmosphere may be effectively reduced to 1 to 50 mmHg, for
example, in order raise the reaction velocity at the end stage of the
reaction.
The process for producing the linear polyester resin (AX1) is
exemplified by heating a diol (g) and a dicarboxylic acid (i) to 180 C to 260
C
to undergo dehydration and condensation under normal or reduced pressure
in the presence of a titanium-containing catalyst of 0.0001 to 0.8% by mass
based on the mass of the resulting polymer and other optional catalysts
thereby to prepare (AX1).
The process for producing the nonlinear polyester resin (AX2) is
CA 02628003 2008-04-30
exemplified by heating a diol (g), a dicarboxylic acid (i) and a trivalent or
more polyol (h) to 180 C to 260 C to undergo dehydration and condensation
under normal or reduced pressure in the presence of a titanium-containing
catalyst (a) of 0.0001 to 0.8% by mass based on the mass of the resulting
polymer and other optional catalysts thereby to prepare (AX2). The (j) may
be reacted with the (g), (i) and (h) at the same time.
The process for producing the modified polyester resin (AY1) is
exemplified by adding a polyepoxide (c) to the polyester resin (AX2) and
allowing a molecule-extending reaction of the polyester at 180 C to 260 C
1o thereby to prepare the (AY1).
The acid value of (AX2) to react with (c) is preferably 1 to 60
mgKOHIg, more preferably 5 to 50 mgKOH/g. The acid value of no less
than 1 mgKOH/g may eliminate the possibility of (c) not to react and thus to
degrade the resin properties, and the acid value of no more than 60
mgKOH/g may bring about proper thermal stability of the resin.
The amount of (c) to prepare (AX1) is preferably 0.01 to 10% by mass
based on (AX2), more preferably 0.05 to 5% by mass in view of low
temperature fixability and hot offset resistance.
The polycondensation polyester resin is preferable in the present
invention for the binder resin of full-color toners in view of coloring
properties and image intensity. Color images typically result in thicker
toner layers due to multiple overlapping of toner layers, which leading to
36
CA 02628003 2008-04-30
cracks or defects on images due to insufficient strength and/or diminishing
appropriate gloss. As such, the polyester resin is employed for maintaining
appropriate gloss and excellent strength.
It is preferred for the polyester resin in the binder resin in particular
that there exists substantially no THF-insoluble matter, the content of the
ingredients having a molecular mass of 500 is no more than 4% by mass in
the molecular mass distribution of gel permeation chromatography, and one
peak exists within a molecular-mass range of 3000 to 9000. The THE
insoluble matter tends to decrease the glossiness and transparency, thus
high quality images are difficult in OHP sheets. It is preferable for the
inventive toner to prevent filmings on blades or sleeves such that the content
of the ingredients having a molecular mass of 500 is no more than 4% by
mass in the molecular mass distribution of the binder resin, and the ratio of
mass average molecular mass (Mw) to number average molecular mass (Mn)
is 2 < Mw/Mn < 10. The content of more than 4% by mass of the ingredients
having a molecular mass of 500 tends to bring about smearing the blades or
sleeves under prolonged usage and to induce filming.
The molecular mass of the binder resin in the inventive toner may be
measured based on gel permeation chromatography by way of conditioning a
column within a heat chamber at 40 C, flowing THE into the column at
lmL/min at the temperature as the solvent, and injecting a THE sample
solution, prepared from a toner at a sample concentration of 0.05 to 0.6% by
37
CA 02628003 2008-04-30
mass, in an amount of 200 L. The THE insoluble matter in the THE
sample solution is removed by a 0.45 gm filter for liquid chromatography
before injection thereof.
The molecular mass distribution of samples is calculated from a
relation between logarithmic values of a calibration curve formed from a
number of monodispersion polystyrene standards and a counted number.
The polystyrene standards for the calibration curve are those having a
molecular mass of 6x 102, 2. lx 103, 4x 103, 1.75x 104, 5. lx 104, 1. lx 105,
3.9x 105,
8.6x 105, 2x 106, 4.48x 106 by Pressure Chemical Co. or Tosoh Co., or the
like,
preferably at least about 10 polystyrene standards are utilized. The
detector is a refractive index (RI) detector. The existence of THE insoluble
matters in the binder resin may be judged at preparing the THE sample
solution for measuring the molecular mass distribution. That is, it is judged
that substantially no THE insoluble matters exist as long as the filter being
not clogged when a filter unit of 0.45 m is attached to a syringe and a
liquid
is extruded from the syringe.
It is preferred in the present invention that the binder resin
represents an endothermic peak at 60 C to 70 C under the measurement
using a differential scanning calorimeter (DSC). The endothermic peak of
below 60 C may affect the toner storage ability and cause problems such as
toner solidification within cartridges or hoppers. On the other hand, the
endothermic peak of below 60 C may affect the toner productivity and cause
38
CA 02628003 2008-04-30
problems such as low feeding ability at milling processes. The endothermic
peak in the differential scanning calorimeter may be read as a main
maximum peak in the endothermic curve using, for example, Rigaku
THRMOFLEX TG8110 (by Rigaku Co.) under a temperature-rising rate of
10 C/min.
It is preferable as described above that the ratio of mass average
molecular mass (Mw) to number average molecular mass (Mn) is 2 < Mw/Mn
< 10 in the polyester resin. The ratio Mw/Mn of above 10 may bring about
images with less gloss of the fixed toner and far from high quality images.
to On the other hand, ratio Mw/Mn of below 2 may bring about low productivity
in milling processes of toner production and smearing of blades or sleeves
under prolonged usage, and thus inducing the filming.
It is preferred in the polyester resin that the acid value is no more
than 10 mgKOHJg when a resin charge-control agent described later is
employed. It is known that the charging ability and the acid value
represent a proportional relation in the polyester resin, and that the higher
acid value leads to larger negative-charging ability of the resin and also
affects the environmental properties at charging. That is, when the acid
value is higher, the charge amount is larger under low temperature and low
2o humidity conditions, and the charge amount is lower under high
temperature and high humidity conditions. The change of the charge
amount due to the environmental conditions may enlarge the changes of
39
CA 02628003 2008-04-30
background smear, image concentration and color reproducibility, thus
making difficult to maintain high quality images. In general, the acid value
of above 20 mgKOH/g may possibly increase the charge amount and
deteriorate the environmental fluctuation.
When the polyester resin is employed in the inventive toner, the
resistance of the toner particles may be controlled by the resin charge-
control
agent, hydrophobic silica, hydrophobic titanium oxide etc. Therefore, the
charge control effect of the resin charge-control agent, hydrophobic silica,
or
hydrophobic titanium oxide may be disturbed when the acid value of the
1o polyester resin is above 10 mgKOHJg. The acid value of the polyester resin
employed in the present invention is preferably no more than 10 mgKOH/g,
more preferably no more than 5 mgKOH/g.
It is preferred that the polyester resin represents a temperature
within 95 C to 120 C at which the apparent viscosity comes to 103 Pa-s
measured by a flow tester. When the temperature is below 95 C, the hot
offset tends to occur at fixing processes, and the temperature of above 120 C
may result in insufficient gloss. The temperature, at which the apparent
viscosity comes to 103 Pa-s, may be measured using a flow tester CVI'-500 (by
Shimadzu Co.) under conditions of load 10 kg/cm2, orifice size 1 mm by
length 1 mm, and temperature-rising rate 5 C/min, and reading the
temperature corresponding to the apparent viscosity of 103 Pa-s.
Resin Charge-Control Agent
CA 02628003 2008-04-30
When a monomer containing a sulfonic acid salt group is added as a
monomer of the resin charge-control agent, the resin charge-control agent
may improve the negative-charging effect. On the other hand, the
environmental stability or temperature/humidity stability of the toner tends
to degrade due to the moisture-absorbing property, thus it is popular in the
art that an aromatic monomer with an electron- attracting group is utilized
for a copolymer. However, when the toner is used for a long term over
several ten thousands of sheets, smears or photoconductor filmings appear
on the developing sleeves or layer thickness-control members such as blades
1o and rollers, the charge stability of toners or high quality images cannot
be
maintained sufficiently, and the productivity decreases, even though several
thousands of sheets cause substantially no problem.
In order to address these deficiencies, the inventive toner employs a
copolymer that is formed from (1) a monomer containing a sulfonic acid salt
group, (2) an aromatic monomer containing an electron attracting group, and
(3) a monomer of a (meth)acrylic acid ester, or a copolymer formed from (1) to
(3) and also (4) an aromatic vinyl monomer, as the resin charge-control agent,
for the purpose of a binder resin for full-color toner in addition to
polyester
resins that are favorable in terms of coloring properties and image intensity,
thereby, an electrostatic image developing toner is provided that may exhibit
excellent charging stability and environmental stability, that are far from
smearing the developing sleeves or layer thickness-control members such as
41
CA 02628003 2008-04-30
blades and rollers, that may appropriately form thin films, that may free
from photoconductor filmings, and that may maintain high image quality
and high productivity.
The resin charge control agent is defined in terms of molecular mass
distribution as for the content of molecular mass of no more than 1x103.
The ingredients having a molecular mass of no more than lx 103 are lower
molecular mass compounds, copolymers, ionomers, residual monomers etc.;
these ingredients possibly inhibit to generate charging and fluctuate the
charging under the influence of temperatures and humidities. These
1o ingredients also affect its safety such as skin stimulation and fish
poison.
The ingredients having a molecular mass of no more than lx103 in a content
of 10% by mass or more may make the charging property unstable under the
significant influence of temperatures and humidities.
These inventive effects are estimated due to the following reasons:
the combination of the monomer containing a sulfomc acid salt group and the
aromatic monomer containing an electron attracting group may enhance the
negative-charge effect. The monomer of a (meth)acrylic acid ester and also
the aromatic vinyl monomer may still enhance the environmental charge
stability and increase the resin hardness, which leading to desirable milling
property and effectively preventing the photoconductor filmings without
smearing the developing sleeves or layer thickness-control members such as
blades and rollers.
42
CA 02628003 2008-04-30
In addition, the low molecular mass ingredients as well as the
combination of monomers in the resin charge control agent may bring about
an electrostatic image developing toner having an adequate dispersing
ability and a sharp distribution of charge amount desirable for long term
charge stability and high image quality, in the combination with a polyester
resin that is favorable in terms of coloring properties and image intensity as
a binder resin for full-color toners.
The monomer containing a sulfomc acid salt group in the resin
charge-control agent is exemplified by aliphatic monomers containing a
sulfomc acid salt group and aromatic monomers containing a sulfonic acid
salt group. Examples of the aliphatic monomers containing a sulfonic acid
salt group include alkaline metal salts, alkaline earth metal salts, amine
salts, and quaternary ammonium salts of vinylsulfonic acids,
allylvinylsulfonic acids, 2-acrylamide-2-methylpropanesulfomc acid,
methacryloyloxyethylsulfonic acid, or perfluorooctanesulfonic acid.
Examples of the aromatic monomers containing a sulfonic acid salt group
include alkaline metal salts, alkaline earth metal salts, amine salts, and
quaternary ammonium salts of styrenesulfonic acid, sulfophenyl acrylamide,
or sulfophenyl itaconic imide. The metal salts of heavy metals like nickel,
copper, zinc, mercury and chromium are undesirable in terms of safety.
Examples of the aromatic monomers containing an electron
attracting group in the resin charge control agent include substituted
43
CA 02628003 2008-04-30
styrenes such as chlorostyrene, dichlorostyrene, bromostyrene, fluorostyrene,
nitrostyrene and cyanstyrene; substituted phenyl(meth)acrylates such as
chlorophenyl(meth)acrylate, bromophenyl(meth)acrylate,
nitrophenyl(meth)acrylate and chlorophenyloxyethyl(meth)acrylate;
substituted phenyl(meth)acrylamides such as chloophenyl(meth)acrylamide,
bromophenyl(meth)acrylamide and nitrophenyl(meth)acrylamide;
substituted phenylmaleimides such as chlorophenylmaleimide,
dichlorophenylmaleimide, nitrophenylmaleimide and
nitrochlorophenylmaleimide; substituted phenylitaconimides such as
chlorophenylitaconimide, dichlorophenylitaconimide, nitrophenylitaconimide
and nitrochlorophenylitaconimide; and substituted phenylvinyl ethers such
as chlorophenylvinyl ether and nitrophenylvinyl ether. Among these,
phenylmaleimide and phenylitaconimide substituted by a chloride or nitro
group are particularly preferable in view of charging ability and filming
resistance.
Examples of the (meth)acrylic acid ester monomer in the resin
charge control agent include methyl(meth)acrylate, ethyl(meth)acrylate,
propyl(meth)acrylate, n-butyl(meth)acrylate, isobutyl(meth)acrylate,
stearyl(meth)acrylate, dodecyl(meth)acrylate and
2-ethylhexyl(meth)acrylate.
Examples pf the aromatic vinyl monomer in the resin charge-control
agent include styrene, vinyltoluene, and alpha-methylstyrene.
44
CA 02628003 2008-04-30
It is preferred in the resin charge-control agent that the amount of
the monomer containing a sulfomc acid salt group is 1 to 30% by mass based
on the entire mass of the resin charge-control agent, more preferably 2 to
20% by mass. In cases where the amount of the monomer containing a
sulfonic acid salt group is less than 1% by mass, the initial charging
property
and/or the saturated charge amount is insufficient, possibly influencing
images. In cases where the amount is above 30% by mass, the
environmental stability degrades at the charging step, the charge amount is
lower at high temperature and high humidity conditions, the charge amount
is higher at low temperature and low humidity conditions, thus the charge
stability of toners or high quality images cannot be maintained sufficiently.
Moreover, smears or photoconductor filmings tend to appear on the
developing sleeves or layer thickness-control members such as blades and
rollers, and the productivity in kneading-milling steps of toner production
tends to decrease.
The amount of the aromatic monomer containing an electron
attracting group is preferably 1 to 80% by mass based on the entire mass of
the resin charge control agent, more preferably 20 to 70% by mass. When
the amount of the aromatic monomer containing an electron attracting group
is less than 1% by mass, the charge amount is insufficient, and background
smear or toner scattering is likely to occur; and when the amount is above
80% by mass, the monomer exhibits poor dispersibility into toners to widen
CA 02628003 2008-04-30
the charging distribution of toners, which leading to background smear, toner
scattering and insufficient high quality images.
The amount of the acrylic ester monomer and/or methacrylic ester
monomer is preferably 10 to 80% by mass based on the resin charge control
agent, more preferably 20 to 70% by mass. When the amount of the acrylic
ester monomer and/or methacrylic ester monomer is below 10% by mass, the
environmental stability is insufficient in the charging step, the milling
ability
is insufficient at kneading-milling steps in the toner production, smears on
the developing sleeves or layer thickness-control members such as blades
and rollers or photoconductor filmings cannot be sufficiently prevented; when
the amount is above 80% by mass, the initial charging property and/or the
charge amount is insufficient, possibly influencing images.
The amount of the aromatic vinyl monomer is preferably 0 to 30% by
mass based on the entire mass of the resin charge control agent, more
preferably 3 to 20% by mass. When the amount of the aromatic vinyl
monomer is above 30% by mass, the resin charge control agent comes to hard,
which leading to a wide charging distribution, background smear, toner
scattering in the processes, and also inferior toner fixability in particular
poor coloring property at mixing color toners.
The aromatic monomer in the resin charge control agent may be
phenylmaleimide or phenylitacommide substituted by chloride or a nitro
group as described above. The resin charge control agent may fluctuate its
46
CA 02628003 2008-04-30
volume resistivity possibly due to residual matters of catalysts,
polymerization inhibitors, or solvents at the monomer production processes,
which sometimes adversely influences on the intended toner charging
amount. Therefore, there may cause problems in initial charging ability or
charging to a saturated level with respect to toners that contain a resin
negative-charge control agent.
As such, it is preferred in the present invention that the volume
resistivity of the resin charge control agent is 9.5 to 11.5 Log ohm-cm, more
preferably 10.0 to 11.0 Log ohm-cm. In cases where the volume resistivity of
the resin charge control agent is below 9.5 Log ohm-cm, toners on developing
rollers may initially take an insufficient charge amount, which possibly
causing background smear or toner scattering. In cases where the volume
resistivity of the resin charge control agent is above 11.5 Log ohm-cm, toners
on developing rollers may initially take a sufficient charge amount, however,
charge up tends to appear with time, which possibly leading to nonuniform
toner thin layers on developing rollers under one-component developing
systems to generate color streaks or irregularities on images. In cases of
two-component developing systems, the image density often decreases, and
background smear or toner scattering is likely to occur.
The volume resistivity of the resin charge control agent may be
measured in accordance with JIS K6911. Specifically, the resin charge
control agent is size-controlled with a mesh and conditioned at 23 C and 50%
47
CA 02628003 2008-04-30
RH. The sample of 3 g is molded at pressure 500 kg/cm2 using an automatic
pressure molding machine to prepare a disc-like test piece of 2 mm thick by 4
cm diameter. The test piece is placed on a dielectric loss tester (TR-10C, by
Ando Electric Co.) after measuring precisely the thickness with a micrometer,
and the volume resistivity is measured with applying an alternative voltage
of frequency 1 kHz.
It is preferred in the resin charge control agent that the temperature
corresponding to the apparent viscosity of 104 Pa=s by a flow tester is 85 C
to
110 C. In cases where the temperature is below 85 C, the dispersibility of
1o the resin charge control agent is inappropriate in toners, which possibly
decreasing the charge amount and also leading to inferior storage stability
and agglomeration or solidification; moreover, fixation tends to occur in
kneading, milling, or classifying production steps, which deteriorating the
productivity. On the other hand, in cases where the temperature is above
110 C, the monomer exhibits poor dispersibility into toners to widen the
charging distribution of toners, which leading to background smear or toner
scattering in the systems. Moreover, toner fixability, in particular the
coloring property, degrades at overlapping color toners. The temperature, at
which the apparent viscosity comes to 104 Pa-s, may be measured by using a
flow tester CFT-500 (by Shimadzu Co.) under conditions of load 10 kg/cm2,
orifice of diameter 1 mm by length 1 mm and temperature-rising rate
5 C/min, and reading the temperature corresponding to the apparent
48
CA 02628003 2008-04-30
viscosity of 104 Pa=s.
The mass average molecular mass of the resin charge control agent
is preferably 5x103 to 1x105. In cases where the mass average molecular
mass is below 5x103, the dispersibility of the resin charge control agent is
inappropriate in toners, which possibly decreasing the charge amount and
also leading fixation in milling steps during production processes including
kneading, milling, or classifying steps, which further deteriorating the
productivity. On the other hand, in cases where the mass average
molecular mass is above lx105, the monomer exhibits poor dispersibility into
toners to widen the charging distribution of toners, which leading to
background smear or toner scattering in the systems, or inferior toner
fixability of coloring properties.
It is also preferred in the resin charge control agent that the mass
amount of ingredients having a molecular mass of no more than 1x103 is no
more than 10% by mass, more preferably no more than 6% by mass. The
ingredients having a molecular mass of no more than lx103 are lower
molecular mass compounds, copolymers, ionomers, residual monomers etc.;
these ingredients possibly inhibit to generate charging and fluctuate the
charging under the influence of temperatures and humidities; moreover,
these ingredients also affect its safety such as skin stimulation and fish
poison.
It is also preferred that the following relation is satisfied: 0.9 < T1/T2
49
CA 02628003 2008-04-30
< 1.4, in which T1 represents the temperature at which the inventive binder
resin has an apparent viscosity of 103 Pa-s measured by a flow tester, and T2
represents the temperature at which the resin charge control agent has an
apparent viscosity of 104 Pa=s measured by the flow tester.
The dispersibility of the charge control agent into the binder resin is
an important factor to decide the charging ability of toners. In accordance
with the present invention, a combination of a specific binder resin and a
specific resin charge control agent may lead to a toner with an appropriate
charging ability and an excellent initial charging property. On the other
1o hand, it is apparent as described above that the dispersibility or
compatibility between the binder resin and the resin charge control agent
affects the charging ability. The present inventors have found the optimum
range in terms of the apparent viscosity measured by a flow tester and the
dispersibility of binder resins and resin charge control agents. In cases
where T1/T2 is below 0.9, the apparent viscosities of the binder resin and the
resin charge control agent are similar, which leading to a dissolved condition
between the binder resin and the resin charge control agent, resulting in an
insufficient saturated charge amount and inferior initial charging property.
In cases where T1/T2 is above 1.4, the apparent viscosities of the binder
resin
and the resin charge control agent are excessively different, which leading to
inferior dispersibility of the resin charge control agent, resulting in
initial
background smear and decrease of the charge amount with time. In
CA 02628003 2008-04-30
addition, proper charging ability may be attained and filmings are unlikely
to generate by way of defining the constitutional monomers, apparent
viscosity thereof, and viscosity ratio of apparent viscosities of dispersed
binder resins.
The amount of the resin charge control agent is preferably 0.1 to 20%
by mass based on the toner particles, more preferably 0.5 to 10% by mass.
In cases where the amount is below 0.1% by mass, the initial charging and
the charge amount are insufficient, which possibly influencing images like
background smear and dusts. On the other hand, in cases where the
amount is above 20% by mass, the poor dispersibility widens the charging
distribution, which possibly leading to background smear or toner scattering
in the systems.
The additives utilized in the inventive toner are exemplified by
hydrophobic-treated silica having a primary particle diameter of 0.01 to 0.03
gm and hydrophobic-treated specific titanium oxide having a primary
particle diameter of 0.01 to 0.03 m and a specific surface area of 60 to 140
m2/g, in cases a resin charge control agent is utilized. When these additives
are employed along with the polyester resin and the resin charge control
agent, the toner may be obtained with a stable charging ability.
When the hydrophobic-treated silica having a primary particle
diameter of 0.01 to 0.03 pm is attached to the surface of the base toner, the
toner may take the necessary flowability and charging ability, resulting in
51
CA 02628003 2008-04-30
appropriate developing ability on developing rollers and therefrom to
photoconductors. The amount of the silica is preferably no less than 2.1
parts by mass based on 100 parts by mass of the base toner. Consequently,
the toner may be made into uniform thin layers on developing rollers,
irregularity may be significantly improved for the thin layers, and also white
streaks due to toner fusion onto developer coating blades may be prevented
due to stirring by developing rollers for a long period. In cases where the
silica amount is less than the range, the toner flowability may be
insufficient
for supplying a necessary amount of toner to developing rollers, or the charge
amount of the toner may be less than the necessary level. Moreover, the
toner may be made into nonuniform thin layers on developing rollers, which
possibly inhibiting uniform developments and images or generating white
streaks due to toner fusion onto developer coating blades.
In addition, by virtue of attaching a hydrophobic-treated titanium
oxide having a primary particle diameter of 0.01 to 0.03 m and a specific
surface area of 60 to 140 m2/g onto the surface of the base toner, the
charging
ability of the toner may be stabilized, in particular the initial charging
property is improved and the charge up is prevented. The amount of the
titanium oxide is preferably 0.4 to 1.0 part by mass based on 100 parts by
mass of the base toner. When the amount is less than 0.4 part by mass, the
development of the toner may be insufficient due to excessively high charging
ability of the toner, and when the amount is above 1.0 part by mass, the
52
CA 02628003 2008-04-30
toner may scatter from developing rollers or cause background smear due to
excessively low charging ability of the toner.
The term "base toner" means the particles on the way of production
that contain at least a binder resin, colorant, and resin charge control other
than additives.
The inventive toner binder resin (A) may contain optional other
resins in addition to the polycondensation polyester resins described above.
Examples of the other resins include styrene resins such as
copolymers of styrene and alkyl(meth)acrylate and copolymers of styrene and
diene monomers; epoxy resins such as ring-opening polymers of bisphenol A
diglycidyl; and urethane resins such as polyadducts of diols and/or trivalent
or more polyols and diisocyanates.
Preferably, the mass average molecular mass of the other resins is
1000 to 2,000,000. The amount of the other resins is preferably 0 to 40% by
mass in the toner binder resin (A), more preferably 0 to 30% by mass, still
more preferably 0 to 20% by mass.
In cases where two or more species of polyester resins are used in
combination, or at least one species of polyester resin and at least one
species
of other resin are combined, these may be powder-mixed or melted-mixed, or
may be mixed in toner production processes.
The temperature for melting and mixing is preferably 80 C to 180 C,
more preferably 100 C to 170 C, still more preferably 120 C to 160 C.
53
CA 02628003 2008-04-30
Lower mixing temperatures below the range may result in insufficient
mixing and nonuniform mixture. When two or more species of polyester
resins are mixed, excessively high mixing temperatures may deteriorate
resin properties necessary for toner binder because of averaging through an
ester exchange reaction.
The mixing period in the melting and mixing step is preferably 10
seconds to 30 minutes, more preferably 20 seconds to 10 minutes, still more
preferably 30 seconds to 5 minutes. When two or more species of polyester
resins are mixed, excessively long mixing periods may deteriorate resin
properties necessary for toner binder because of averaging through an ester
exchange reaction.
The mixing device at the melting and mixing step may be batch
mixing devices such as reaction vessels and continuous mixing devices.
Continuous mixing devices are suited for uniformly mixing at an appropriate
temperature for shorter periods. The continuous mixing devices are
exemplified by extruders, continuous kneaders, three rollers, etc. Among
these, extruders and continuous kneaders are preferable. In cases of
powder mixing, conventional mixing conditions and devices are available.
As for the mixing conditions of powder mixing, the mixing
temperature is preferably 0 C to 80 C, more preferably 10 C to 60 C; the
mixing period is preferably no shorter than 3 minutes, more preferably 5 to
60 minutes. Examples of the mixing device include Henschel mixers,
54
CA 02628003 2008-04-30
Nautor mixers, banbury mixers, etc. Among these, Henschel mixers are
preferable in particular.
The electrostatic image developing toner contains at least (A) a
binder resin and (B) a colorant, and optionally (C) a release agent, (D) a
charge control agent, and (E) a fluidizer, etc.
Colorant
The colorant may be properly selected from conventional dyes,
pigments, and magnetic powders; examples thereof include carbon black,
nigrosine dyes, iron black, Naphthol Yellow S, Hansa Yellow (10G, 5G, G),
1o cadmium yellow, yellow iron oxide, yellow ocher, chrome yellow, Titan
Yellow,
Polyazo Yellow, Oil Yellow, Hansa Yellow (GR, A, RN, R), Pigment Yellow L,
Benzidine Yellow (G, GR), Permanent Yellow (NCG), Vulcan Fast Yellow (5G,
R), Tartrazine Lake, Quinoline Yellow Lake, anthracene yellow BGL,
isoindolinone yellow, colcothar, red lead oxide, lead red, cadmium red,
cadmium mercury red, antimony red, Permanent Red 4R, Para Red, Fire
Red, parachlororthonitroanihne red, Lithol Fast Scarlet G, Brilliant Fast
Scarlet, Brilliant Carmine BS, Permanent Red (F2R, F4R, FRL, FRLL,
F4RH), Fast Scarlet VD, Vulcan Fast Rubine B, Brilliant Scarlet G, Lithol
Rubine GX, Permanent Red F5R, Brilliant Carmine 6B, Pigment Scarlet 3B,
Bordeaux 5B, Toluidine Maroon, Permanent Bordeaux F2K, Helio Bordeaux
BL, Bordeaux 10B, BON Maroon Light, BON Maroon Medium, eosine lake,
Rhodamine Lake B, Rhodamine Lake Y, Alizarin Lake, Thioindigo Red B,
CA 02628003 2008-04-30
Thioindigo Maroon, Oil Red, quinacridone red, Pyrazolone Red, Polyazo Red,
Chrome Vermilion, Benzidine Orange, Perynone Orange, Oil Orange, cobalt
blue, cerulean blue, Alkali Blue Lake, Peacock Blue Lake, Victoria Blue Lake,
metal-free phthalocyanine blue, Phthalocyanine Blue, Fast Sky Blue,
Indanthrene Blue (RS, BC), indigo, ultramarine, Prussian blue,
Anthraquinone Blue, Fast Violet B, Methyl Violet Lake, cobalt violet,
manganese violet, dioxazine violet, Anthraquinone Violet, chrome green, zinc
green, chromium oxide, viridian, emerald green, Pigment Green B, Naphthol
Green B, Green Gold, Acid Green Lake, Malachite Green Lake,
Phthalocyamne Green, Anthraquinone Green, titanium oxide, zinc white,
lithopone, magnetite, iron black and combinations thereof.
The amount of the colorant selected from dyes or pigments is
preferably 1 to 15% by mass based on the toner, more preferably 3 to 10% by
mass.
The amount of the colorant selected from magnetic powders is
preferably 1 to 70% by mass based on the toner, more preferably 15 to 70%
by mass, still more preferably 30 to 60% by mass, particularly preferably 2 to
30% by mass.
The colorant for use in the present invention may be a master batch
prepared by mixing-kneading a pigment with a resin. Examples of binder
resins for use in the production of the master batch or in kneading with the
master batch are, in addition to the aforementioned modified and unmodified
56
CA 02628003 2008-04-30
polyester resins, polystyrenes, poly-p-chlorostyrenes, polyvinyltoluenes, and
other polymers of styrene and substituted styrenes; styrene-p-chlorostyrene
copolymers, styrene -propylene copolymers, styrene-vinyltoluene copolymers,
styrene-vinylnaphthalene copolymers, styrene-methyl acrylate copolymers,
styrene-ethyl acrylate copolymers, styrene-butyl acrylate copolymers,
styrene-octyl acrylate copolymers, styrene-methyl methacrylate copolymers,
styrene-ethyl methacrylate copolymers, styrene-butyl methacrylate
copolymers, styrene -acrylonitrile copolymers, styrene-vinyl methyl ketone
copolymers, styrene-butadiene copolymers, styrene-isoprene copolymers,
styrene-acrylonitrile-indene copolymers, styrene-maleic acid copolymers,
styrene-maleic ester copolymers, and other styrenic copolymers; poly(methyl
methacrylate), poly(butyl methacrylate), poly(vinyl chloride), poly(vinyl
acetate), polyethylene, polypropylenes, polyesters, epoxy resins, epoxy polyol
resins, polyurethanes, polyamides, polyvinyl butyral), poly(acrylic acid)
resins, rosin, modified rosin, terpene resins, aliphatic or alicyclic
hydrocarbon
resins, aromatic petroleum resins, chlorinated paraffins, and paraffin waxes.
Each of these resins can be used alone or in combination.
Release Agent
A wax having a low melting point of 50 C to 120 C may be used for
the release agent (C); the wax effectively works on between fixing rollers and
toner surfaces as a release agent, which effects hot offset resistance even
without coating a release agent such as lubricants onto the fixing rollers.
57
CA 02628003 2008-04-30
Examples of the wax include vegetable waxes such as carnauba wax,
cotton wax, sumac wax and rice wax, animal waxes such as bees wax and
lanoline; mineral waxes such as ozokerite and ceresin; and petroleum waxes
such as paraffin, micro crystalline and petrolatum.
Besides these natural waxes, there are synthetic hydrocarbon waxes
such as Fischer-Tropsch wax, polyethylene wax; and synthetic waxes such as
of ester, ketone, and ether. Further, it is also possible to use aliphatic
amides such as 12-hydroxystearic acid amide, stearic acid amide, phthalic
anhydride imide and chlorinated hydrocarbons; low-molecular-weight
1o crystalline polymers including homopolymers such as poly-n-stearyl
methacrylate and poly-n-laurylmethacrylate and copolymers such as
n-stearyl acrylate-ethylmethacrylate copolymer; and crystalline polymers
having a long alkyl group in its side chain.
More specifically, the release agent (C) is exemplified by carnauba
waxes (C1), Fischer-Tropsch waxes (C2), paraffin waxes (C3) and polyolefin
waxes (C4).
Examples of (Cl) include natural carnauba waxes and free aliphatic
acid carnauba waxes.
Examples of (C2) include petroleum Fisher Tropsch waxes (Paraflint
H1, Paraflint H1N4, and Raffint C105, by Schumann Sasol Co.), natural gas
Fisher Tropsch waxes (FT100, by Shell MDS Co.), and separated ad
crystallized products thereof such as MDP-7000 and MDP-7010 (by Nippon
58
CA 02628003 2008-04-30
Seiro Co.).
Examples of (C3) include petroleum paraffin waxes such as paraffin
wax HNP-5, HNP-9 and HNP-11 (by Nippon Seiro Co.). Examples of (C4)
include polyethylene waxes such as Sunwax 171P and Sunwax LEL400P (by
Sanyo Chemical Industries Ltd.) and polypropylene waxes such as Biscol
550P and Biscol 660P (by Sanyo Chemical Industries Ltd.).
Among these waxes, carnauba waxes and Fischer-Tropsch waxes are
preferable, carnauba waxes and petroleum Fischer-Tropsch waxes are more
preferable.
These waxes may act as a release agent and provide excellent low
temperature fixability with toners.
The amount of the release agent (C) is preferably 0 to 15% by mass
based on the toner, more preferably 1 to 10% by mass.
Charge Control Agent
The charge control agent (D) may be conventional ones; examples
thereof include nigrosme dye, triphenylmethane dye, chrome-contained
metal-complex dye, molybdic acid chelate pigment, rhodamine dye, alkoxy
amine, quaternary ammonium salt such as fluoride-modified quaternary
ammonium salt, alkylamide, phosphoric simple substance or compound
thereof, tungsten itself or compound thereof, fluoride activator, salicylic
acid
metallic salt, and salicylic acid derivative metallic salt. Specifically,
Bontron 03 of a nigrosine dye, Bontron P-51 of a quaternary ammonium salt,
59
CA 02628003 2008-04-30
Bontron S-34 of a metal containing azo dye, Bontron E-82 of an oxynaphthoic
acid metal complex, Bontron E-84 of a salicylic acid metal complrex, and
Bontron E-89 of a phenol condensate (by Orient Chemical Industries, Ltd.);
TP-302 and TP-415 of a quaternary ammonium salt molybdenum metal
complex (by Hodogaya Chemical Co.); Copy Charge PSY VP2038 of a
quaternary ammonium salt, Copy Blue PR of a triphenylmethane derivative,
and Copy Charge NEG VP2036 and Copy Charge NX VP434 of a quaternary
ammonium salt (by Hoechst Ltd.); LRA-901, and LR-147 of a boron metal
complex (by Japan Carlit Co., Ltd.), copper phtalocyamine, perylene,
quinacridone, azo pigment, and other high-molecular weight compounds
having a functional group, such as sulfonic acid group, carboxyl group, and
quaternary ammonium salt. Among the charge control agents, those
capable of controlling toners to a negative polarity are preferable.
The amount of the charge control agent depends on the type of
binder resins, optional additives, and methods for manufacturing; preferably,
the amount is 0.1 to 10 parts by mass based on 100 parts by mass of binder
resin, more preferably 0.2 to 5 part by mass. When the amount is more
than 10 parts by weight, toner-charge properties are excessive, which lessens
the effect of the charge control agent, increases in electrostatic attraction
force with developing rollers, and degrades developer fluidity and image
density.
Examples of the charge control agents preferable for the present
CA 02628003 2008-04-30
invention are the resin charge control agents described above,
bis[1-(5-chloro-2-hydroxyphenylazo)-2-naphtolat]chromic (III) acid, nigrosine,
perfluoroalkyltrimethylammonium iodine, polyhydroalkanoate and those
expressed by General Formulas (III), (IV), and (V).
[cH3c}3 C V COO- Z u++
General Formula (III)
C (CH3)3
2
S03 E)
C4H9 +}+
C4H9 General Formula (N)
- N - CH2
C4H9 H
C2H5
1
Rf - CF = CH - CH2 - N' - CH3 B(Phenyl)4- General Formula (V)
C2H5
Rf = CsFõ - CõF23
The charge control agent is preferably the copolymers containing a
quaternary ammonium salt group formed from the monomer expressed by
General Formula (VI) in a content of 65 to 97% by mass and the monomer
expressed by General Formula (VII) in a content of 3 to 35% by mass and
having a mass average molecular mass of 2000 to 10000.
61
CA 02628003 2008-04-30
Rt
- C CH2
General Formula(VI)
R4
1 I
C00- R3 - N - 95- C83 S03 a General Formula(VI()
I -C~
R6
in General Formulas (VI) and (VII) described above, R1 is a hydrogen
atom or a methyl group, R2 is a hydrogen atom or a methyl group, R3 is an
alkylene group, and R4, R5 and Rs are each an alkyl group.
In addition, compounds expressed by General formula (VIII) or (IX)
are also preferable as the charge control agent.
O2N no
0 N-N R02N carrit-no
o ~Q a+ General Formula(VllI)
HN0C NO2
-~~ NO2
62
CA 02628003 2008-04-30
GL Q e
~+/~ HEN
0 -g--@ [a1(NH4+)+b1(Na)+c1(H+)l
Fe
j X a
a NON
o c i General Formula (IX)
in General Formulas (VIII) and (IX), al is a number of 0.8 to 0.98, bl
is a number of 0.01 to 0.19, cl is a number of 0.01 to 0.19, and al+bl+ci=1.
The amount of the charge control agent is preferably 0.01 to 20% by
mass based on the toner, more preferably 0.1 to 15% by mass.
Fluidizer and Toner External Additive
Inorganic fine particulates for the inventive toner added as a
fluidizer (E) of an external additive are exemplified by silica, alumina,
titanium oxide, barium titanate, magnesium titanate, calcium titanate,
strontium titanate, iron oxide, copper oxide, zinc oxide, tin oxide, silica
sand,
clay, mica, tabular spar, diatomite, chromium oxide, cerium oxide, colcothar,
antimony trioxide, magnesium oxide, zirconium oxide, barium sulfate,
barium carbonate, calcium carbonate, silicon carbide, silicon nitride, etc.
Among these, preferable are metal oxides, metal nitrides and metal carbides,
in particular those external additives having a number average particle
diameter of 8 to 80 nm or 120 to 300 nm. Among the inorganic fine particles
described above, preferable are silica, alumina, titanium oxide, in particular
silica and titanium oxide. It is preferred for the charging ability and
63
CA 02628003 2008-04-30
flowability of toners that the external additive comprises titanium oxide
having a number average particle diameter of 5 to 40 nm in terms of the
primary particles.
The amount of the inorganic fine particles as the external additive is
preferably 0.01 to 5% by mass based on the base toner.
In order to control precisely the flowability of toners, not only control
of production conditions to produce the additives but also crushing or milling
and screening of the resulting products are important. It is also important
how to attach the additives to toner surface and the attaching conditions.
The external additives may be used in combination with inorganic
fine particles or hydrophobic-treated inorganic fine particles. Preferably,
there exist two species of fine particles on the toner surface, such that one
is
low diameter inorganic fine particles having an average particle diameter of
hydrophobic-treated primary particles of 1 to 20 nm, more preferably 6 to 15
nm (BET surface area: 100 to 400 m2/g), and another is high diameter
inorganic fine particles having an average particle diameter of
hydrophobic-treated primary particles of 30 to 150 nm, more preferably 90 to
130 nm (BET surface area: 20 to 100 m2/g). Preferably, the low diameter
inorganic fine particles are of silica or titanium oxide, more preferably the
both; preferably, the large diameter inorganic fine particles are of silica;
preferably, the silica is of wet processes such as sol-gel processes; more
preferably, medium diameter inorganic fine particles, preferably of silica,
also
64
CA 02628003 2008-04-30
exist on the toner surface, of which the average particle diameter being 20 to
50 nm (BET surface area: 40 to 100 m2/g).
The inorganic fine particles may be selected from conventional ones
including silica fine particles, hydrophobic silica; fatty acid metal salts
such
as zinc stearate and aluminum stearate; metal oxides such as titania,
alumina, tin oxide and antimony oxide; and fluoropolymers.
Particularly preferable additive is hydrophobic-treated silica, titania,
titanium oxide and alumina fine particles. Examples of the silica fine
particles include HDKH2000, HDKH2000/4, HDKH2O50EP, HVK21,
HDKH1303 (by Hochst Co.), R972, R974, RX200, RY200, R202, R805 and
R812 (by Nippon Aerosil Co.). Examples of the titania fine particles include
P-25 (by Nippon Aerosil Co.), STT-30, STT-65C-S (by Titanium Industries
Ltd.), TAF-140 (by Fuji Titanium Industry, Co.), MT-150W, MT-500B,
MT-600B and MT-150A (by Tayca Co.). Examples of the
hydrophobic-treated titanium oxide fine particles include P-805 (by Nippon
Aerosil Co.), STT-30A, STT-65S-S (by Titanium Industries Ltd.), TAF-500T,
TAF-1500T (by Fuji Titanium Industry, Co.), MT-100S, MT- 100T (by Tayca
Co.), and ITS (by Ishihara Sangyo Kaisha Ltd.)
The hydrophobic-treated oxide fine particles of silica, titania or
alumina may be produced by treating the hydrophilic fine particle with
silane coupling agents such as methyltriethoxysilane and
octyltriethoxysilane. In addition, silicone oil-treated oxide fine particles
or
CA 02628003 2008-04-30
inorganic fine particles are available, which are treated with a silicone oil
with heating as required.
Examples of the silicone oil include dimethyl silicone oil,
methylphenyl silicone oil, chlorophenyl silicone oil, methylhydrogen silicone
oil, alkyl-modified silicone oil, fluorine-modified silicone oil,
polyether-modified silicone oil, alcohol-modified silicone oil, amino-modified
silicone oil, epoxy-modified silicone oil, epoxy-polyether-modified silicone
oil,
phenol-modified silicone oil, carboxyl- modified silicone oil, mercapto-
modified
silicone oil, acrylic or methacrylic-modified silicone oils, and
alpha- methylstyrene -modified silicone oils.
The inorganic fine particles are exemplified by silica, alumina,
titanium oxide, barium titanate, magnesium titanate, calcium titanate,
strontium titanate, iron oxide, copper oxide, zinc oxide, tin oxide, silica
sand,
clay, mica, wollastonite, diatomaceous earth, chromium oxide, cerium oxide,
iron oxide red, antimony trioxide, magnesium oxide, zirconium oxide, barium
sulfate, barium carbonate, calcium carbonate, silicon carbide and silicon
nitride. Among these, silica and titanium dioxide are preferable in
particular. The added amount is preferably 0.1 to 5% by mass based on the
toner, more preferably 0.3 to 3% by mass.
The average particle diameter of primary particles of the inorganic
fine particles is preferably no larger than 100 nm, more preferably 3 to 70
nm.
In cases where the diameter is less than the range, the inorganic fine
66
CA 02628003 2008-04-30
particles tend to be embedded into toners to hide the effective performance;
and when the diameter is larger than the range, the photoconductor surface
is likely to be damaged nonuniformly.
The other external additives or fluidizers are exemplified by polymer
fine particles of polystyrenes, methacrylate copolymers or acrylate
copolymers produced through soap-free emulsion, suspension or dispersion
polymerization; polycondensation products such as silicones,
benzoguanamine and nylon; and polymer particles of thermosetting resins.
These fluidizers may be possibly surface-treated to enhance the
1o hydrophobicity thereby to maintain the flowability and/or charging property
even under high humidity conditions; examples of the treating agents are
silane coupling agents, silylation agents, silane-coupling agents having alkyl
fluorides, organo-titanium coupling agents, aluminum coupling agents,
silicone oil, and modified silicone oil
The toner may also contain a cleaning aid to assist the cleaning of
developers remaining on photoconductors or primary transferred bodies;
examples of the cleaning aid include fatty acid metal salts such as zinc
stearate, stearic acid calcium and stearic acid; and polymer fine particles
produced through soap-free-emulsion polymerization such as
polymethylmethacrylate fine particles and polystyrene fine particles. Those
polymer fine particles preferably have a narrower particle diameter
distribution and a volume average particle diameter of 0.01 m to 1 m.
67
CA 02628003 2008-04-30
In addition, the toner may further contain, as the other additives,
fluoropolymers, polyolefins of low molecular mass; metal oxides such as
aluminum oxide, tin oxide and antimony oxide; conductivity enhancer such
as carbon black and tin oxide; and surface-treated products thereof. These
additives may be used alone or in combination; the amount is preferably 0.1
to 10 parts by mass based on 100 parts by mass of the toner.
The charge control agent and the release agent may be melted and
kneaded with a master batch and/ or binder resin or may be dissolved into an
organic solvent and dispersed.
The charge control agent and the release agent may be added
externally to the toner by wet processes using solvents or water and optional
active agents besides dry processes using Henschel mixers or Q mixers.
In the mixing process of the external additives, a dry mixing may be
carried out while dispersing and coating the external additive onto toner
surface by way of stirring a mixture of a toner material and the additive
using mixers. In such a process, it is important that the additive of
inorganic or resin fine particles is attached uniformly and firmly onto the
toner material in view of higher durability. For the purpose, such conditions
are typically important, as blade shape of mixers, rotation frequency, mixing
period, mixing times, external additive amount, toner material amount,
surface properties of toner material like irregularity, hardness and
viscoelasticity.
68
CA 02628003 2008-04-30
The wet processes may apply inorganic file particles on toners in
liquid media. This process may be carried out after toner particles are
produced in water and the used surfactants are washed away. Excessive
surfactants are removed through solid-liquid separating processes, then the
resulting cake or slurry is dispersed again into aqueous media. The
inorganic fine particles are added and dispersed into the slurry;
alternatively,
the fine particles may be dispersed previously into the aqueous water.
When a reverse-polarity surfactant is added into the aqueous media, the
inorganic fine particles may attach the surface of toner particles more
efficiently. In cases where the inorganic fine particles are
hydrophobic-treated and hardly dispersible into aqueous media, an
additional small amount of alcohols may decrease the surface tension thus
make the inorganic fine particles more wettable and dispersible. The
reverse-polarity surfactant is then added gradually into the aqueous media
with stirring. The amount of the reverse-polarity surfactant is preferably
0.01 to 1% by mass based on the solid content of toner particles. The
addition of the reverse-polarity surfactant may neutralize the charge of the
inorganic fine particle dispersion in the aqueous media, which allowing the
inorganic fine particles to coagulate and attach onto the toner surface. The
amount of the inorganic fine particles is preferably 0.01 to 5% by mass base
on the solid content of toner particles.
The inorganic fine particles, attaching to the toner surface, may be
69
CA 02628003 2008-04-30
then fixed on the toner surface through heating the slurry thereby be
prevented from the separation. Preferably, the heating of the slurry is
carried out at higher than Tg of the resin in the toner, and/or after drying
while preventing agglomeration thereof.
The inventive toner may be incorporated a metal stearate as a
lubricant in order to reduce friction coefficient of photoconductor surface
and
to improve cleaning ability. Preferably, the metal stearate is zinc stearate.
Toner Production Process
The inventive toner for developing electrostatic images may be
produced through conventional milling and polymerizing processes,
specifically, air-flow milling, mechanical milling, emulsion-agglomeration,
and suspension-polymerization processes; substantially any processes may
derive the inventive effects.
In conventional kneading-milling processes to produce toners, the
constitutional ingredients of toners are dry-mixed, and melted-kneaded, then
finely milled by use of jet mills etc., followed by air-classifying, thereby
toners
may be produced with a volume average particle diameter of 2 to 10 m.
The volume average particle diameter may be determined by Coulter
counter (article name: Multitizer III, by Beckman Coulter, Inc.).
The processes for producing the inventive toner may be by
conventional ones; specifically, the inventive toner may be produced by a
process that comprises a step of mechanically mixing toner ingredients such
CA 02628003 2008-04-30
as a binder resin, a charge control agent and colorant, a step of melting and
kneading the mixture, a step of milling, and a step of classifying. The
powders other than those adapted to milling or classifying steps may be
recycled to the step of mechanically mixing or melting-kneading.
The powders (by-product) other than those adapted to milling or
classifying steps mean fine or coarse particles that are out of desirable
particle diameters after milling steps followed by a melting-kneading step or
out of desirable particle diameters after the following classifying steps. The
amount of the byproduct is preferably 1 to 20 parts by mass based on 100
1o parts by mass of the essential ingredients in the melting-kneading step.
The mixing step to mechanically mix the toner ingredients such as
binder resins, colorants, resin charge control agents, and other charge
control
agents or the mixing step to mechanically mix the toner ingredients such as
binder resins, colorants and resin charge control agents with by-products
may be carried out under usual conditions using conventional mixers with
rotatable blades.
After the mixing step, the mixture is put into a melting kneader to
melt and knead. The melting kneader may be mono-axis or two-axis
continuous kneaders or batch kneaders with roll mills; preferable examples
thereof include KTK type two-axis extruder (by Kobe Steel, Ltd.), TEM type
two-axis extruder (by Toshiba Machine Co.), two-axis extruder (by KCK Co.),
PCM type two-axis extruder (by Ikegai Ltd.), and Co-kneader (by Buss Co.).
71
CA 02628003 2008-04-30
It is important that the melting-kneading step is carried out under
appropriate conditions far from cutoff of molecular chains in binder resins.
Specifically, the melting-kneading temperature is adjusted referring to the
softening point of the binder resin; when the temperature is excessively
lower than the softening point, the cutoff will be significant, and
excessively
high temperature results in poor dispersion.
The kneaded product is milled after the step of melting-kneading.
Preferably, the material is roughly milled then finely milled in the milling
step. Preferable milling processes are exemplified by making the materials
collide with a plate by means of jet air, making particles collide each other
by
means of jet air, or pulverizing by use of a narrow gap between mechanically
rotating rotors and stators. After the milling step, the milled product is
classified in an air flow by use of centrifugal force, thereby to produce a
developer having a predetermined particle diameter of 5 to 20 m, for
example. In order to improve the flowability, storage stability,
developing property, and transferring property of toner, inorganic fine
particles such as hydrophobic silica fine particles may be further added and
mixed to the resulting toner base particles. The external additives may be
mixed using conventional powder mixers, preferably, the mixers are
equipped with a jacket etc. to adjust the inside temperature. The load
history on the additives may be changed by intermediate or gradual
additions of external additives, or rotation number, rolling rate, rolling
time,
72
CA 02628003 2008-04-30
temperature, etc., or a high load is firstly applied and then a weak load is
applied, or vice versa. Examples of the mixing equipments include V-type
mixers, rocking mixers, Loedige mixers, Nauta mixers, and Henschel mixers.
The inventive toner with the inventive toner binder resin may be
employed as a two-component developer for electrostatic latent images by
way of mixing with carrier particles of ferrites etc. optionally coated with
magnetic powders such as of iron, nickel, ferrite and magnetite; glass beads
and/or resins such as acrylic resins and silicone resins. The inventive toner
may form electrostatic latent images by fractioning with charging blades or
other members in place of carrier particles.
Then the latent images are fixed by conventional heat roll-fixing
processes on supports such as paper and polyester films.
In recent years, the particle diameter of toners has been reduced still
more to form highly precise images. One way to reduce the diameter may
be on the basis of conventional mixing, melting and miffing processes,
however, these processes lead to considerably expensive cost from the
viewpoint of energy and yield and also may be limited to reduce the diameter
still further in view of a minimum limit attainable by milling processes.
For the countermeasure, toner production processes have been
proposed on the basis of suspension polymerization, emulsion polymerization,
dispersion polymerization processes, etc.
The toner for inventive image forming apparatuses is produced by
73
CA 02628003 2008-04-30
dispersing a polyester prepolymer with a nitrogen-containing functional
group, a polyester resin, a colorant, and a release agent into an organic
solvent to prepare a toner material liquid, then which is subjected to
crosslinking or extending reaction in an aqueous solvent. The polyester
resin is an inventive polycondensation polyester resin. The constitutive
materials and production process of these toners will be explained in the
following.
Modified Polyester
The toner of the present invention comprises a modified polyester (i)
as a binder resin. A modified polyester indicates a polyester in which a
combined group other than ester bond may reside in a polyester resin, and
different resin components are combined into a polyester resin through a
covalent bond, ionic bond or the like. Specifically, a modified polyester is
one where a functional group such as an isocyanate group or the like, which
reacts with a carboxylic acid group and a hydrogen group, is introduced to a
polyester end and further reacted to an active hydrogen-containing
compound to modify the polyester end.
Examples of the modified polyester (i) include a urea modified
polyester which is obtained by a reaction between a polyester prepolymer (A)
having an isocyanate group and amines (B). Examples of the polyester
prepolymer (A) having an isocyanate group include a polyester prepolymer,
which is a polycondensation polyester of a polyvalent alcohol (PO) and a
74
CA 02628003 2008-04-30
polyvalent carboxylic acid (PC) and having an active hydrogen group, is
further reacted with a polyvalent isocyanate compound (PIC). Examples of
the active hydrogen group involved into the above-noted polyester include a
hydroxyl group (an alcoholic hydroxyl group and a phenolic hydroxyl group),
an amino group, a carboxyl group, and a mercapto group. Among these
groups, an alcoholic hydroxyl group is preferable.
The urea-modified polyester may be formed in the following manner.
Examples of the polyvalent alcohol compound (PO) include divalent alcohols
(DIO), and trivalent or more polyvalent alcohols (TO), and any of a divalent
1o alcohol (DIO) alone and a mixture of a divalent alcohol (DIO) with a small
amount of a polyvalent alcohol (TO) are preferable. Examples of the
divalent alcohol (DIO) include alkylene glycols such as ethylene glycol,
1,2-propylene glycol, 1,3-propylene glycol, 1,4-bytandiol, and 1,6-hexanediol;
alkylene ether glycols such as diethylene glycol, triethylene glycol,
dipropylene glycol, polyethylene glycol, polypropylene glycol, and
polytetramethylene ether glycol; alicyclic diols such as 1,4-cyclohexane
dimethanol, and hydrogenated bisphenol A; bisphenols such as bispheonol A,
bisphenol F, and bisphenol S; alkylene oxide adducts of the above-noted
alicyclic diols such as ethylene oxide, propylene oxide, and butylene oxide;
and alkylene oxide adducts of the above-noted bisphenols such as ethylene
oxide, propylene oxide, and butylene oxide. Among the above mentioned, an
alkylene glycol having carbon number of 2 to 12 and an alkylene oxide
CA 02628003 2008-04-30
adduct of bisphenols are preferable, and an alkylene oxide adduct of
bisphenols and a combination of the adduct with an alkylene glycol having a
carbon number of 2 to 12 are particularly preferable. Examples of the
trivalent or more polyvalent alcohol (TO) include a polyaliphatic alcohol of
trivalent to octavalent or more such as glycerine, trimethylol ethane,
trimethylol propane, pentaerythritol, and sorbitol; and trivalent or more
phenols such as trisphenol PA, phenol novolac, and cresol novolac; and
alkylene oxide adduct of the trivalent or more polyphenols.
Examples of the polyvalent carboxylic acid (PC) include a divalent
carboxylic acid (DIC) and a trivalent or more polyvalent carboxylic acid (TC),
and any of a divalent carboxylic acid (DIC) alone and a mixture of a divalent
carboxylic acid (DIC) with a small amount of a polyvalent carboxylic acid
(TC) are preferable. Examples of the divalent carboxylic acid (DIC) include
alkylene dicarboxylic acids such as succinic acid, adipic acid, and sebacic
acid; alkenylen dicarboxylic acids such as maleic acid and fumaric acid;
aromatic dicarboxylic acids such as phthalic acid, isophthalic acid,
terephthalic acid, and naphthalene dicarboxylic acid. Among these divalent
carboxylic acids, an alkenylen dicarboxylic acid having a carbon number of 4
to 20 and an aromatic dicarboxylic acid having a carbon number of 8 to 20
are preferable. Examples of the trivalent or more polyvalent carboxylic acid
(TC) include an aromatic polyvalent carboxylic acid having a carbon number
of 9 to 20 such as trimellitic acid, and pyromellitic acid. A polyvalent
76
CA 02628003 2008-04-30
carboxylic acid (PC), an acid anhydride from among the polyvalent carboxylic
acids or a lower alkyl ester such as methyl ester, ethyl ester, and isopropyl
ester may be reacted with a polyvalent alcohol (PO).
The ratio of a polyvalent alcohol (PO) to a polyvalent carboxylic acid
(PC), defined as an equivalent ratio [OH]/[COOH] of a hydroxyl group [OH]
to a carboxyl group [COOH], is typically 2/1 to 1/1, preferably 1.5/1 to 1/1,
and more preferably 1.3/1 to 1.02/1.
Examples of the polyvalent isocyanate compound (PIC) include
aliphatic polyvalent isocyanates such as tetramethylen diisocyanate,
hexamethylen diisocyanate, and 2,6-diisocyanate methyl caproate; alicyclic
polyisocyanates such as isophorone diisocyanate, and cyclohexyl methane
diisocyanate; aromatic diisocyanates such as tolylene diisocyanate, and
diphenylmethane diisocyanate; aromatic aliphatic diisocyanates such as
a,a,a'a'-tetramethyl xylylene diisocyanate; isocyanates; a compound in which
the above noted polyisocyanate is blocked with a phenol derivative, an oxime,
caprolactam, and the like; and a combination of two or more elements
thereof.
The ratio of a polyvalent isocyanate compound (PIC), defined as an
equivalent ratio [NCO]/[OH] of an isocyanate group [NCO] to a hydroxyl
group [OH] of a polyester having a hydroxyl group, is typically 5/1 to 1/1,
preferably 4/1 to 1.2/1, and more preferably 2.5/1 to 1.5/1. When
[NCO]/[OH] is more than 5, low-temperature image fixability is often poor.
77
CA 02628003 2008-04-30
When a urea modified polyester is used in the molar ratio of [NCO] is less
than 1, the urea content of ester becomes lower, which making hot-offset
resistance insufficient.
The component content of polyvalent isocyanate compound (PIC) of a
polyester prepolymer having an isocyanate group (A) is typically 0.5 to 40%
by mass, preferably 1 to 30% by mass, and more preferably 2 to 20% by mass.
When less than 0.5% by mass, hot-offset resistance is insufficient and there
appear a disadvantage in the compatibility between hot storage resistance
and low-temperature image fixability. On the other hand, when it is more
than 40wt%, low-temperature image fixability tends to be poor.
The number of isocyanate groups contained per one molecular of
polyester prepolymer having isocyanate group (A) is typically 1 or more,
preferably 1.5 to 3 in average, and more preferably 1.8 to 2.5 in average.
When the number of isocyanate groups is less than 1 per one molecular of
polyester prepolymer, the molecular weight of the urea modified polyester
becomes lower, which making hot-offset resistance poor.
Examples of amines (B) to be reacted with the polyester prepolymer
(A) include a divalent amine compound (B1), a trivalent or more polyvalent
amine compound (B2), an aminoalcohol (B3), an amino mercaptan (B4), an
amino acid (B5), and a compound in which the amino group of B1 to B5 is
blocked (B6).
Examples of the divalent amine compound (B1) include aromatic
78
CA 02628003 2008-04-30
diamines such as phenylene diamine, diethyl toluene diamine, 4,4'-diamino
diphenyl methane; alicyclic diamines such as 4,4'-diamino-3,3'-dimethyl
dicyclohexyl methane, diamine cyclohexane, and isophorone diamine; and
aliphatic diamines such as ethylene diamine, tetramethylene diamine, and
hexamethylene diamine. Examples of the trivalent or more polyvalent
amine compound (B2) include diethylene triamine and triethylene tetramine.
Examples of the aminoalcohol (B3) include ethanol amine, and
hydroxyethylaniline. Examples of the amino mercaptan (B4) include
aminoethyl mercaptan and aminopropyl mercaptan. Examples of the
1o amino acid (B5) include aminopropionic acid, aminocaproic acid, and the
like.
Examples of the compound, in which the amino group of B1 to B5 is blocked
(B6), include a ketimine compound obtained from the above-noted amines of
Bl to B5 and ketones such as acetone, methyl ethyl ketone, and methyl
isobuthyl ketone and oxazolidine compound, and the like. Among these
amines (B), a divalent amine compound B1 and a mixture of B1 with a small
amount of a trivalent or more polyvalent amine compound (B2) are
preferable.
The ratio of amines (B), defined as an equivalent ratio [NCO]/[NHx]
of isocyanate group [NCO] in a polyester prepolymer having isocyanate
group (A) to amine group [NHx] in amines (B), is typically 1/2 to 2/1,
preferably 1.5/1 to 1/1.5, and more preferably 1.2/1 to 1/1.2. When
[NCO]/[NHx] is more than 2 or less than 1/2, the molecular weight of urea
79
CA 02628003 2008-04-30
modified polyester becomes lower, which making hot-offset resistance
degrade.
In addition, the urea modified polyester may include a urethane
bond as well as a urea bond. A molar ratio of the urea bond content to the
urethane bond content is typically 100/0 to 10/90, preferably 80/20 to 20/80,
and more preferably 60/40 to 30/70. When a molar ratio of the urea bond is
less than 10%, hot-offset resistance may degrade.
The modified polyester (i) used in the present invention is
manufactured by one-shot methods or prepolymer methods. The weight
average molecular weight of the modified polyester (i) is typically 10000 or
more, preferably 20000 to 10,000,000, and more preferably 30000 to
1,000,000. The molecular weight peak is preferably 1000 to 10000, and
when less than 1000, it is hard to undergo an elongation reaction and the
toner elasticity is low, which making hot-offset resistance poor. When the
molecular weight peak is more than 10000, it may cause degradation of
fixability and may bring hard challenges in manufacturing in yielding toner
fine particles and in toner grinding. The number average molecular weight
of the modified polyester (i) when used together with an unmodified polyester
(ii), which will be hereafter described, may be a number average molecular
weight which is easily obtained to be used with the above-noted weight
average molecular weight. When a modified polyester (i) is used alone, the
number average molecular weight is typically 20000 or less, preferably 1000
CA 02628003 2008-04-30
to 10000, and more preferably 2000 to 8000. When the number average
molecular weight is more than 20000, low-temperature image fixability and
glossiness when used in a full-color device become poor.
In cross-linking and/or elongation reactions of a polyester prepolymer
(A) and amines (B) in order to obtain a modified polyester (i), a reaction
stopper may be used as required to control the molecular weight of a urea
modified polyester to be obtained. Examples of the reaction stopper include
a monoamine such as diethyl amine, dibutyl amine, buthyl amine, and lauryl
amine, and a compound in which the above-noted elements are blocked.
The molecular weight of the resulting polymer can be measured by
means of gel permeation chromatography (GPC), using a tetrahydrofuran
(THF) solvent.
Unmodified Polyester
In the present invention, not only the modified polyesters but also
unmodified polyesters (ii) may be included together with the modified
polyester (i) as binder resin components. The unmodified polyester (ii) in
combination with a modified polyester (i) is preferred to the modified
polyester (i) alone, because low-temperature image fixability and glossiness
may be improved when in a full-color device. Examples of the unmodified
polyester (ii) include a polycondensation polyester of a polyvalent alcohol
(PO) and a polyvalent carboxylic acid (PC), and the like, same as in the
modified polyester (i) components. Preferable compounds thereof are also
81
CA 02628003 2008-04-30
the same as in the modified polyester W. As for the unmodified polyester
(n), in addition to an unmodified polyester, it may be a polymer which is
modified by a chemical bond other than urea bonds, for example, it may be
modified by a urethane bond. It is preferable that at least a part of modified
polyester (i) is compatible with part of an unmodified polyester (ii), from
the
aspect of low-temperature image fixability and hot-offset resistance. Thus,
it is preferable that the composition of the modified polyester (i) is similar
to
that of the unmodified polyester (ii). A weight ratio of a modified polyester
(i) to an unmodified polyester (ii) when an unmodified polyester (ii) being
included, is typically 5/95 to 80/20, preferably 5/95 to 30/70, more
preferably
5/95 to 25/75, and still more preferably 7/93 to 20/80. When the weight ratio
of a modified polyester (i) is less than 5%, it makes hot-offset resistance
degraded and brings about disadvantages in compatibility between heat
resistant storage properties and low-temperature image fixability.
The molecular weight peak of the unmodified polyester (ii) is
typically 1000 to 10000, preferably 2000 to 8000, and more preferably 2000 to
5000. When the molecular weigh peak of the unmodified polyester (ii) is
less than 1000, hot storage stability may degrade, and when more than
10000, low-temperature image fixability may degrade. The hydroxyl value
of the unmodified polyester (ii) is preferably 5 mgKOH/g or more, more
preferably 10 to 120 mgKOH/g, and still more preferably 20 to 80 mgKOH/g.
When the value is less than 5 mgKOH/g, it brings about disadvantages in the
82
CA 02628003 2008-04-30
compatibility between hot storage stability and low-temperature fixability.
The acid number of the unmodified polyester (ii) is preferably 1 to 5
mgKOH/g, and more preferably 2 to 4 mgKOH/g. Since a wax with a high
acid value is used, as for the binder, the binder is easily matched with the
toner used in a two-component developer, because such a binder leads to
charging and a high volume resistivity. The glass transition temperature
(Tg) of the binder resin is typically 35 C to 70 C, and preferably 55 C to 65
C.
When less than 35 C, the hot storage stability degrades, and when more
than 70 C, low temperature fixability becomes insufficient. The toner of the
present invention shows a proper hot storage stability even with a low glass
transition temperature, compared to a toner made from conventional
polyesters, because a urea modified polyester easily exists on the surface of
particles of the toner base to be obtained. The glass transition temperature
(Tg) can be measured using a differential scanning calorimeter (DSC).
The toner may be properly selected in terms of the shape, size, etc.
depending on the application; preferably, the toner has the flowing volume
average particle diameter, ratio of volume average particle diameter to
number average particle diameter (volume average particle
diameter/number average particle diameter), average circularity, shape
factors SF-1 and SF-2, glass transition temperature, agglomeration degree,
volume resistivity and apparent density.
Preferably, the inventive toner has a volume average particle
83
CA 02628003 2008-04-30
diameter of 2.0 to 10.0 m, preferably 3.0 to 7.0 m, more preferably 3.0 to
5.0 gm. The ratio of (Dv/Dn) is 1.00 to 1.40, preferably 1.00 to 1.30, more
preferably 1.00 to 1.20, wherein Dv means a volume average particle
diameter and Dn means a number average particle diameter.
In general, toners of smaller particle diameters may deposit precisely
over electrostatic images. However, volume average diameters smaller than
the range in cases of two-component developers may lead to toner fusion on
the surface of magnetic carriers under prolonged stirring in developing
apparatuses and poor charging ability of the magnetic carriers. On the
other hand, the toner having a volume average particle diameter over the
inventive range may make difficult to take high-resolution and high quality
images, and also the particle diameter of toner often fluctuates along with
inflow and outflow of toners.
Further, narrower particle diameter distribution of toners may lead
to uniform charge distribution, high quality images with less background fog,
and higher transfer rate. However, Dv/Dn above 1.40 undesirably tends to
broaden the charge distribution to decrease the resolution.
Preferably, the content of fine particles of no larger than 4 m is 0 to
20% by number, and the content of coarse particles of no larger than 12.7 m
is 0 to 3% by number.
The average particle diameter and the particle diameter distribution
of toners can be measured using Coulter Counter TA-II, and Coulter
84
CA 02628003 2008-04-30
Multisizer II (by Beckman Coulter, Inc.). In the present invention, Coulter
Counter TA-II model was used with connecting an interface (by The Institute
JUSE) and a personal computer (PC9801, by NEC Co.) which outputs
number distributions and volume distributions.
Preferably, the inventive toner has a shape factor SF-1 of 100 to 180,
more preferably 100 to 150. The shape factor SF-2 is preferably 100 to 180,
more preferably 100 to 160.
FIGs. 2A and 2B and FIGs. 3A to 3C are schematic views of a toner
particle to explain shape factors SF-1 and SF-2. The shape factor SF-1
io represents a circular level of toner shape, which is calculated from
Equation
(1), in which the maximum length MXLNG (see FIG. 2A) of the toner image
projected on two-dimensional plane is squared, then divided by the area
value of AREA and multiplied by 100ri/4.
SF-1= [(MXLNG)2/AREA]x(100n/4) Equation (1)
The SF-1 value of 100 corresponds to exact sphere, the larger is the
SF-1 the shape is more different from exact sphere.
The shape factor SF-2 represents an irregularity of toner shape,
which is calculated from Equation (2), in which the peripheral length PERI of
the toner image projected on two-dimensional plane is squared, then divided
by the area value of AREA and multiplied by 100/4n.
SF-2= [(RERI)2/AREA]x(100/4n) Equation (2)
The SF-2 value of 100 corresponds to non-irregular shape of toner
CA 02628003 2008-04-30
surface, the larger is the SF-2 the more irregular is the surface shape.
When the toner shape comes to sphere, the contact area between
toner particles or between toner particles and photoconductors comes to
narrow like a spot contact; consequently, the adsorptivity comes to lower
between toner particles, the flowability comes to higher, the adsorptivity
comes to lower between toner particles and photoconductors, and the
transfer rate comes to higher. On the other hand, SF-1 and SF-2 preferably
have a somewhat higher value from the viewpoint that spherical toner
particles easily enter into a space between cleaning blades and
photoconductors. In addition, excessively large values with respect to SF-1
and SF-2 tend to bring about lower image quality due to higher toner
scattering on images, thus SF-1 and SF-2 are preferred to be no more than
180.
Specifically, SF-1 and SF-2 were determined by way of taking
pictures using a scanning electron microscope S-800 (by Hitachi, Ltd.) and
analyzing the pictures using an image analyzer Luzex AP (by Nireco Co.).
It is preferred for stable color reproducibility in the present invention
that the toner is of spindle shape, and the spindle shape may be defined by a
long axis rl, a short axis r2, and a thickness r3 (rl > r2 > r3), the ratio
r2/r1
is 0.5 to 1.0, and r3/r2 is 0.7 to 1Ø
FIGs. 3A to 3C schematically show the toner shape. When a toner
having an approximately spherical shape, as shown in FIG. 3, is defined by a
86
CA 02628003 2008-04-30
long axis r1, a short axis r2, and a thickness r3 (rl > r2 > r3), it is
preferred in
the present invention that the ratio of short axis to long axis (r2/rl) is 0.5
to
1.0 (see FIG. 3B), and the ratio of thickness to short axis (r3/r2) is 0.7 to
1.0
(see FIG. 3C). The ratio r2/rl of below 0.5 may result in poor dot
reproducibility and low transfer efficiency and be far from high quality
images due to departing from spherical shape. The ratio r3/r2 of below 0.7
may be far from higher transfer efficiencies like those of spherical toners
due
to almost flat shape. When the ratio r3/r2 is 1.0, the toner flowability may
be enhanced in particular by virtue of the rotatable shape with a long axis as
1o the rotating axis.
The rl, r2 and r3 were determined from observation of photographs
with various view angles using a scanning electron microscope (SEM).
It is preferred that the toner has an average circularity of 0.94 or
more and below 1.00, more preferably 0.96 to 0.99. The average circularity
of 0.94 or more may favorably lead to excellent dot reproducibility and less
fluctuation of color reproducibility at narrow line images in particular.
Moreover, the proper transfer ability may advantageously bring about high
quality images; the higher average circularity may bring about uniform
development, transfer and distribution with less adhesion of toner
agglomerates at half tone or solid portions. Consequently, uniform
intermediate colors may be reproduced with less color polarization after
superimposing toners as color overlapping. It is difficult to take high
87
CA 02628003 2008-04-30
quality images with sufficient transfer ability and without scattering from
the toner far from spherical shape with an average circularity of less than
0.94. These irregular particles may provide many contacting points with
smooth surface such as of photoconductors, and concentrate charges at
projecting tips, thus exhibit higher adhesive force than relatively spherical
particles due to van der Waals force or mirror image force. Therefore,
spherical particles among irregular particles and spherical particles within
toners are selectively transferred in the electrostatic transfer steps,
resulting
in voids at letter or line images. In addition, residual toners should be
1o removed for the subsequent developing steps, which resulting in such
problems that cleaning devices are necessary or toner yield (the rate of
toners
for image formation) is lower.
It is preferred that the rate of toner particles having an average
circularity of below 0.93 is no more than 30%. Toners with the rate of above
30%, i.e. higher fluctuation of circularity, are undesirable since the
charging
velocity or level comes to broad and the distribution of charge amount is
broad.
The average circularity of the toner is a value obtained by optically
detecting toner particles, and the circumferential length of a circle that has
an area equivalent to the projection area of the toner is divided by a
circumferential length of an actual toner particle; specifically, the average
circularity of the toner is measured using a flow particle image analyzer
88
CA 02628003 2008-04-30
(FPIA-2000, by Sysmex Corp.). Pure water of 100 to 150 mL is poured into
a vessel, to which 0.1 mL to 0.5 mL of a surfactant and 0.1 to 9.5 g of a
sample are added. The suspension with the sample is dispersed for about 1
to 3 minutes using an ultrasonic device to adjust the concentration into 3000
to 10000 / L then to measure the shape and the distribution of the toner
sample.
The agglomeration degree of toners is preferably 1% to 25%, more
preferably 3% to 15%. The measurement of the agglomeration degree is
carried out as follows using a powder tester (by Hosokawa Micron Co.) as the
1o measuring device, the attachment parts are set on a vibrating table
according to the following procedures.
(i) vibro-shoot
(ii) packing
(iii) space ring
(iv) screens (three types) upper > middle > lower
(v) pressing bar
The screens are fixed by knob nuts, the vibrating table is operated
with the conditions below:
screen opening (upper) : 75 m
screen opening (middle) : 45 m
screen opening (lower) : 22 gm
vibration amplitude : 1 mm
89
CA 02628003 2008-04-30
sample mass : 2 g
vibrating period : 15 seconds
The agglomeration degree is calculated as follows after the operation.
mass of powder on the upper screen x 1 : (a)
mass of powder on the middle screen x 0.6 : (b)
mass of powder on the lower screen x 0.2: (c)
The total of these three values is defined as the agglomeration degree
(%); i.e. agglomeration degree (%) = (a) + (b) + W.
It is preferred that the toner has a loose apparent density of 0.2 to
0.7 g/mL. The loose apparent density may be measured by a powder tester
PT-S (by Hosokawa Micron Co.).
It is preferred that the toner has a volume resistivity of 8 to 15 Log
ohm-cm, more preferably 9 to 13 Log ohm-cm.
The volume resistivity is measured by way of pressing a toner into a
pellet, the pellet is placed between parallel electrodes with a gap of 2 mm,
then DC 1000 volts is applied between the electrodes, the resistivity is
measured after 30 seconds by a high resist meter (e.g., TR8601, by Advantest
Co.), then the volume resistivity is calculated as a logarithmic value from
the
measured resistivity and the pellet thickness.
It is preferred that the toner has a softening point of 80 C to 180 C,
more preferably 90 C to 130 C. The softening temperature of the toner is
defined as the temperature at which the flow amount comes to the half under
CA 02628003 2008-04-30
the conditions below in a constant temperature-raising rate.
device : flow tester CTF-500D (by Shimadzu Co.)
load : 20 kfg/cm2
die: 1 mmc to 1 mm
temperature- rising rate : 6 C/min
sample mass : 1.0 g
It is preferred that the toner has a glass transition temperature Tg of
35 C to 90 C, more preferably 45 C to 70 C. The glass transition
temperature Tg of the toner may be measured under the following
conditions.
differential scanning calorimeter : Seiko 1D SC100, Seiko 1SSC5040
(disc station)
measuring conditions: temperature range of 25 C to 150 C,
temperature-rising rate of 10 C/min, sampling period of 0.5 second, sampling
amount : 10 mg
Toner Kit
The inventive toner kit comprises the inventive toners of at least a
yellow toner, a magenta toner and a cyan toner. The magenta toner
contains an organic pigment expressed by the following Structural Formula
(1); the yellow toner contains an organic pigment having two units per
molecule each expressed by Structural Skeleton (A) and no halogen atom.
91
CA 02628003 2008-04-30
H3CO / CI
OCH3 H-N
H O 0
N Structural Formula (1)
H N
O \ /
CH3
O
H N Structural Skeleton (A)
N N-
O F-I
in the Structural Formula (1) and Structural Skeleton (A),
=C=N-NH- encompasses =CH-N=N-.
The inventive toner kit, which contains a polyester resin synthesized
in the presence of a novel titanium-containing catalyst and specific yellow
and magenta pigments, may effectively represent color reproducibility of
images, in particular color reproducibility of intermediate red.
The mechanism to improve the color reproducibility is not
necessarily clear, but it is believed that the effective catalytic activity of
the
novel titanium-containing catalyst may achieve a condition of molecular
chain and/or molecular mass distribution adequate for pigment dispersion.
As a result, the energy for the pigment dispersed into the resin on toner
production to re-agglomerate again will be reduced, which makes possible to
maintain the dispersed condition and improves the color reproducibility at
forming images.
92
CA 02628003 2008-04-30
Organic pigments represented by Structural Formula (1) as the
magenta toner are azo lake pigments. The pigments for the magenta toner
have been azo pigments such as azo lake pigments and insoluble azo
pigments; and organic pigments such as quinacridone polycyclic pigments.
Azo pigments include naphthol pigments and oxynaphthoe acid pigments,
and naphthol pigments such as C.I. PR49, C.I. PR68, and C.I. PR 184 have
been used so far among them. The quinacridone pigments have been C.I.
PR122, C.I. PR209, and C.I. PR206. The magenta toner used for the toner
is an oxynaphthoe acid pigment of C.I. PR269 represented by Structural
Formula (1). This pigment reproduces brilliant magenta colors due to the
narrow absorption band at the wavelength of 500 nm to 600 nm.
Specifically, when the ID (image density: -Log reflectivity) is set to
1.00 measured by X-RITE938 densitometer after fixing an image to recording
media such as transfer sheets and film sheets using an observing light D50
(JISZ-8720 (1983)) at a view angle of 2 , "a*" is 55 to 75 and "b*" is -8 to 0
in
the color specification system of L*a*b* (CIE1976). These values are
obtained through the use of uniform measurements in which color density is
measured through a complementary color filter to keep the color density
given to humans at a constant state. When "a*" is less than 55 or "b*" is
less than 0, the color reproducibility degrades at intermediate colors when
mixed with toners with other colors; and when "a*" is more than 75 or "b*" is
more than -8, the amount of the pigment should be increased, which leading
93
CA 02628003 2008-04-30
to higher opacifying power and similarly lower color reproducibility at
intermediate colors when mixed with toners with other colors.
The amount of the magenta toner of the organic pigment expressed
by Structural Formula (1) is preferably 2 to 15% by mass, more preferably 3
to 10% by mass.
The yellow toner contains an organic pigment that contains an
organic pigment having two units per molecule each expressed by Structural
Skeleton (A) and no halogen atom. The organic pigment, having two units
per molecule each expressed by Structural Skeleton (A) and no halogen atom,
1o is preferably one expressed by Structural Formula (2) or (3) below.
CH3
O
HN NN /-\
H / \ O H H3C
N OCH2OH2O O
0 N / \ riN NH Structural Formula (2)
N H
H H 0 0-
NC0
H
H3CO /
COOCH3
0/H-N
O
O N
0 H[
H3C--~' NH O CH3 Structural Formula (3)
N O
N-H
\
H3000C
-4,
The yellow toner contains an organic pigment expressed by
Structural Formula (2) and/or (3), the both are insoluble azo pigments. The
yellow toner has been polycychc organic pigments including acetoacetic acid
94
CA 02628003 2008-04-30
allylid dis-azo pigments, acetoacetic acid imidazolon pigments, quinacridone
pigments and threne pigments. Specifically, acetoacetic acid allylid dis-azo
pigments of C.I. PY13 and C.I. PY17 have been widely used. The yellow
toners employ the organic pigments expressed by Structural Formula (2), i.e.
C.I. pigment yellow 180 disazo organic pigment and/or those by Structural
Formula (3), i.e. C.I. pigment yellow 155 dis-azo organic. These pigments
contain no halogen and reproduce brilliant yellow colors due to a narrow
absorption band at wavelength of 400 to 500 nm.
Specifically, when the ID is set to 1.00 measured by X-RITE938
densitometer after fixing an image to recording media such as transfer
sheets and film sheets using an observing light D50 (JISZ-8720 (1983)) at a
view angle of 2 , "a*" is -2 to -22 and "b*" is 67 to 90 in the color
specification system of L*a*b* (CIE1976). These values are obtained
through the use of uniform measurements in which color density is
measured through a complementary color filter to keep the color density
given to humans at a constant state. When "a*" is less than -12 or "b*" is
less than 67, the color reproducibility degrades at intermediate colors when
mixed with toners with other colors; and when "a*" is more than -2 or "b*" is
more than 90, the amount of the pigment should be increased, which leading
to higher opacifying power and similarly lower color reproducibility at
intermediate colors when mixed with toners with other colors.
The mixture of the magenta toner and the yellow toner allows to
CA 02628003 2008-04-30
reproduce red (R) colors. When the ID is 1.00 measured by X-RITE938
densitometer after fixing an image using an observing light D50 (JISZ-8720
(1983)) at a view angle of 2 , "a*" is set to be 60 to 68 and "b*" is set to
be 45
to 55 in the color specification system of L*a*b*. The respective ranges of
color reproducibility in the L*a*b* color specification system may be adjusted
by the contents of the magenta toner and the yellow toner, the amount of
adhered toner, and the color reproduction range of red colors maybe widened
from skin color to vermillion by virtue of the range. When "a*" is less than
60 or "b*" is less than 45, the color reproducible range is narrow and various
1o intermediate reds cannot be reproduced, and when "a*" is more than 68 or
"b*" is more than 55, the amount of the pigment should be increased, which
leading to higher opacifying power and similarly lower color reproducibility
at intermediate colors.
Reproduction of red colors is important when expressing humans
and other things; however, the red color reproducibility has been poor
compared to photographic papers or sublimation photographs particularly in
cases of higher opacifying power since the reproducible range is narrow and
organic pigments reduce the transparency. As such, the inventive image
forming apparatus may broadly attain red color reproducibility by defining
the color reproducible ranges with respect to organic pigments of both of
magenta toner and yellow toner.
The amount of the organic pigment, having two units per molecule
96
CA 02628003 2008-04-30
each expressed by Structural Skeleton (A) and no halogen atom, is preferably
3 to 20% by mass in the yellow toner, more preferably 5 to 15% by mass.
It is preferred that the cyan toner contains a copper phthalocyanine
pigment.
It is preferred in the present invention that the layer of the magenta
toner is formed under that of the yellow toner. The yellow pigment
expressed by Structural Formula (2) or (3) in the inventive toner typically
exhibits lower opacifying power thus is far from opacifying the underlying
organic pigment. The organic pigments expressed by Structural Formula
(2) or (3) described above have a narrower optical absorption range thus are
far from disturbing the red color reproduction by the underlying magenta
toner. Moreover, the magenta toner, containing the magenta pigment
expressed by Structural Formula (1), under the yellow toner may provide red
color reproducibility in a wide range.
When a wax is incorporated into the toner of the inventive toner kit,
the image surface tends to appear an orange surface, as a result, the rate of
diffuse reflection increases such that the spectral reflectance at wavelength
of 500 to 700 nm is increased in yellow toners, the spectral reflectance at
wavelength of 400 to 500 nm is increased in magenta toners, and the spectral
reflectance at wavelength of 400 to 600 nm is increased in yellow toners. As
such, when reproducing colors by a subtractive color mixing, increase of
reflectance at wavelengths other than to be absorbed may improve the color
97
CA 02628003 2008-04-30
reproducibility.
The inventive toner kit may be favorably applied to image forming
apparatuses that utilize yellow, cyan and magenta toners, and also black
toners.
Developer
When the inventive toner is applied to two-component developers,
the toner is mixed with a magnetic carrier. The amount of the toner is 1 to
parts by mass based on 100 parts by mass of carriers.
The magnetic carrier may be conventional ones such as iron powder,
10 ferrite powder, magnetite powder, resin-coated magnetic carrier and glass
beads having a particle diameter of 20 to 200 gm.
Examples of the coating materials of the resin-coated magnetic
carrier include phenol resins, amino resins, urea-formaldehyde resins,
melamine resins, benzoguanamine resins, urea resins, polyamide resins,
epoxy resins, polyvinyl resins, polyvinylidene resins, acrylic resins,
polymethylmethacrylate resins, polyacrylonitrile resins, polyvinyl acetate
resins, polyvinyl alcohol resins, polyvinyl acetal resins, polyvinyl butyral
resins, polystyrene resins, styrene-acrylic copolymer resins, halogenated
olefin resins such as polyvinyl chloride resins and polyvinylidene chloride;
polyester resins such as polyethylene terephthalate resins and polybuthylene
terephthalate resins; polycarbonate resins, polyethylene resins,
polyfluorocarbon, polyfluorovinylidene resins, polytrifluoroethylene resins,
98
CA 02628003 2008-04-30
polyhexafluoropropylene resins, copolymers of vinylidene fluoride and acrylic
monomers, copolymers of vinylidene fluoride and vinyl fluoride, fluoro
terpolymers such as those of tetrafluoroethylene, and vinylidene fluoride and
other non-fluoride monomers, and silicone resins.
Among these, silicone resin-coated carriers are excellent in view of
carrier lifetime. Electrically conductive powers may be included into the
coating resins as required. Examples of the electrically conductive powers
include metal powders, carbon black, titanium oxide, tin oxide and zinc oxide.
Preferably, these electrically conductive powers have an average particle
diameter of no more than 1 gm since the diameter above 1 gm makes
difficult to adjust the resistivity.
In the two-component developers, the amount of the toner is
preferably 0.5 to 20.0 parts by mass based on 100 parts of carriers.
The inventive toner may be employed as a magnetic toner in
one-component developers without carrier or as non-magnetic toners.
Magnetic Material
The inventive toner may be employed as a magnetic toner with a
magnetic material. The magnetic toner may be prepared by incorporating
magnetic fine particles into the toner particles. The magnetic materials are
exemplified by ferromagnetic metals like iron, nickel and cobalt, alloys and
compounds thereof such as iron oxide including ferrites, magnetites and
hematites; alloys, which contain no ferromagnetic element but exhibit
99
CA 02628003 2008-04-30
ferromagnetism through a appropriate heat treatment, such as Huesler
alloys containing manganese and copper like Mn-Cu-Al and Mn-Cu-Sn; and
chromium dioxide etc.
It is preferred that the magnetic material has an average particle
diameter of 0.1 to 2 gm, more preferably 0.1 to 1 gm, and is uniformly
dispersed as fine particles. The amount of the magnetic material is
preferably 5 to 150 parts by mass based on 100 parts of toner, more
preferably 10 to 70 parts by mass, still more preferably 20 to 50 parts by
mass.
Image Forming Apparatus and Image Forming Method
The image forming method according to the present invention
comprises a latent electrostatic image forming step, a developing step, a
transferring step, and a fixing step and further may include other steps
suitably selected in accordance with the necessity such as a charge
elimination step, a cleaning step, a recycling step and a controlling step.
The image forming apparatus according to the present invention
comprises at least a photoconductor, a latent electrostatic image forming
unit,
a developing unit, a transferring unit, and a fixing unit and may further
comprise other units suitably selected in accordance with the necessity such
as a charge elimination unit, a cleaning unit, a recycling unit and a
controlling unit.
In the latent electrostatic image forming step, a latent electrostatic
100
CA 02628003 2008-04-30
image is formed on a photoconductor.
The latent electrostatic image bearing member (sometimes referred
to as "electrophotographic photoconductor" or "photoconductor") may be
properly selected in terms of material, shape, structure, size or the like,
and
may be suitably selected from conventional ones; the shape of the
photoconductor is preferably drum-like; preferable examples of the material
include amorphous silicon and selenium for inorganic photoconductors and
polysilane and phthalopolymethine for organic photoconductors. Among
these, amorphous silicon is preferable in view of longer operating life.
The latent electrostatic images may be formed, for example, by
charging the surface of the photoconductor uniformly and then exposing the
surface thereof imagewisely by means of the latent electrostatic image
forming unit. The latent electrostatic image forming unit is provided with,
for example, at least a charger configured to uniformly charge the surface of
the photoconductor, and an exposer configured to expose the surface of the
photoconductor imagewisely.
The surface of the photoconductor may be charged by applying a
voltage to the surface of the photoconductor through the use of, for example,
the charger.
The charger may be properly selected depending on the application;
examples thereof include conventional contact chargers which are equipped
with a conductive or semi-conductive roller, a brush, a film, a rubber blade
or
101
CA 02628003 2008-04-30
the like, and non-contact chargers utilizing corona discharge such as
corotoron and scorotoron.
The surface of the photoconductor may be exposed, for example, by
exposing the photoconductor surface imagewisely using the exposer.
The exposer may be properly selected depending on the application;
examples thereof include various types of exposers such as reproducing
optical systems, rod lens array systems, laser optical systems, and liquid
crystal shutter optical systems.
In the present invention, the back light method may be employed in
which exposing is performed imagewisely from the back side of the
photoconductor.
Developing Step and Developing Unit
The developing step is one in which the latent electrostatic image is
developed using the developer of the present invention to form a visible
image.
The visible image can be formed by developing the latent
electrostatic image using, for example, the developer in the developing unit.
The developing unit may be properly selected from conventional ones
in the art; preferable examples thereof include those having at least an
image developing apparatus which houses the developer of the present
invention therein and enables supplying the developer to the latent
electrostatic image in a contact or a non-contact state; preferable example is
102
CA 02628003 2008-04-30
a developing unit with a toner-containing container.
The image developing unit may be of a dry-developing process or a
wet-developing process. It may be a monochrome developing unit or a
multi-color developing unit. Preferred examples thereof include one having
a stirrer by which the developer is frictionally charged, and a rotatable
magnet roller.
In the image developing apparatus, for example, a toner and the
carrier are mixed and stirred, the toner is charged by frictional force at
that
time to be held in a state where the toner is standing on the surface of the
rotating magnet roller to thereby form a magnetic brush. Since the magnet
roller is located near the photoconductor, a part of the toner constituting
the
magnetic brush formed on the surface of the magnet roller moves to the
surface of the photoconductor by electric attraction force. As the result, the
latent electrostatic image is developed using the toner to form a visible
toner
image on the photoconductor surface.
The developer in the developing unit is one that contains the
inventive toner. The developer may be of one-component developer or
two-component developer.
Transferring Step and Transferring Unit
In the transferring step, the visible image is transferred onto a
recording medium, preferably, an intermediate transfer member is used, the
visible image is primarily transferred to the intermediate transfer member
103
CA 02628003 2008-04-30
and then the visible image is secondarily transferred onto the recording
medium. An embodiment of the transferring step is more preferable in
which two or more color toners are used, an embodiment of the transferring
is still more preferably in which a full-color toner is used, and the
embodiment includes a primary transferring in which the visible image is
transferred to an intermediate transfer member to form a composite transfer
image thereon, and a secondary transferring in which the composite transfer
image is transferred onto a recording medium.
The transferring may be performed, for example, by charging a
1o visible image formed on the surface of the photoconductor using a
transfer-charger to transfer the visible image, and this is enabled by means
of the transferring unit. For the transferring unit, it is preferably an
embodiment which includes a primary transferring unit configured to
transfer the visible image to an intermediate transfer member to form a
composite transfer image, and a secondary transferring unit configured to
transfer the composite transfer image onto a recording medium.
The intermediate transfer member may be properly selected from
conventional ones; preferable examples thereof include transferring belts.
The transferring unit (i.e. primary transferring unit and the
secondary transferring unit) preferably includes at least an image-transferer
configured to exfoliate and charge the visible image formed on the
photoconductor to transfer the visible image onto the recording medium.
104
CA 02628003 2008-04-30
The transferring unit may be of one part or two or more parts.
Examples of the image transferer include corona transferers,
transferring belts, transfer rollers, pressure transfer rollers, and adhesion
transfer units. The recording medium may be properly selected from
conventional ones.
In the fixing step, a visible image transferred on a recording medium
is fixed using a fixing apparatus, and the image fixing may be performed
every time each color toner is transferred onto the recording medium or at
the time when individual color toners are superimposed.
The fixing apparatus may be properly selected depending on the
application, and heat-pressure units known in the art are preferably used.
Examples of the heat-pressure units include a combination of a heat roller
and a pressure roller, and a combination of a heat roller, a pressure roller,
and an endless belt.
The heating temperature in the heat-pressure unit is preferably 80 C
to 200 C.
In the present invention, for example, an optical fixing apparatus
known in the art may be used in the fixing step and the fixing unit or instead
of the fixing unit.
In the charge elimination step, the charge is eliminated by applying
a charge-eliminating bias to the photoconductor, and it can be suitably
performed by means of a charge-eliminating unit. The charge-eliminating
105
CA 02628003 2008-04-30
unit may be properly selected from among conventional ones. For example,
charge-eliminating lamps are preferable.
In the cleaning step, a residual electrographic toner remaining on the
photoconductor is removed, and the cleaning can be preferably performed
using a cleaning unit. The cleaning unit may be properly selected from
conventional ones; examples thereof include magnetic brush cleaners,
electrostatic brush cleaners, magnetic roller cleaners, blade cleaners, brush
cleaners, and web cleaners.
In the recycling step, a step eliminated in the cleaning is recycled to
the developing step, and the recycling can be suitably performed by means of
a recycling unit. The recycling unit may be properly selected; examples
thereof include conventional conveying or transporting units.
The control unit is one to control the every step. The control unit
may be properly selected depending on the application; examples thereof
include such instruments as sequencers and computers.
The image forming apparatus in this embodiment comprises a
charging unit, an exposing unit, a developing unit, a transfer unit, and a
cleaning unit in order; and also a paper-feeding unit configured to feed
recording media from a paper-feeding tray, and a fixing device configured to
fix toners onto recording media after separating recording media, on which
toner images being transferred, from the photoconductor. In the image
forming apparatus of this configuration, the surface of the rotating
106
CA 02628003 2008-04-30
photoconductor is uniformly charged by the charging unit then irradiated
laser beams from an exposing unit based on image information to form a
latent image on the photoconductor, to which then toners are deposited to
form images.
On the other hand, the recording media is conveyed from the paper
feeding unit, and transported at a transfer site where the photoconductor
and the transfer unit face each other. The transfer unit applies the charge
of reverse polarity with toner images on the photoconductor, thereby the
toner images on the photoconductor are transferred onto the recording media.
1o Then the recording media is separated from the photoconductor and
conveyed to a fixing device, where the toners are fixed on the recording
media to form images.
FIG. 1 is a schematic constitutional view of developing device 1 of
this embodiment. The developing device 1 employed in the inventive image
forming apparatus will be explained more specifically with reference to FIG.
1. The developing device 1, which being disposed at a side of
photoconductor 8, comprises a non-magnetic developing sleeve 7 that support
a two-component developer (hereinafter, sometimes referred to as
"developer") containing a toner and a magnetic carrier. The developing
sleeve 7 is attached such that a portion thereof is exposed from an opening at
a developing casing in the side of photoconductor 1, and is rotated to arrow
"b" direction by a driving device (not shown). The material of the developing
107
CA 02628003 2008-04-30
sleeve may be one used for conventional devices; examples thereof are
stainless steel, aluminum, non-magnetic materials like ceramics, and coated
materials thereof. The shape of the developing sleeves may also be properly
selected. A magnet roller (not shown) of a magnetic-field generating unit is
disposed inside the developing sleep. The developing unit 1 is equipped
with a rigid doctor 9 as a developer-control member that controls the amount
of the developer supported on the developing sleeve 7.
In addition to the doctor 9, a developer container 4 is disposed at
upstream of the rotating direction of the developing sleeve 7, the first and
the
1o second stirring screws 5, 6 are provide for mechanically stirring the
developer in the developer container 4. Furthermore, a toner supply inlet
23 disposed above the developer container 4, a toner hopper 2 for supplying
toners to developer container 4, and a toner conveying 3 between the toner
supply inlet 23 are provided.
In the developing device 1, the developer in the container 4 is stirred,
and the toner and the magnetic carrier are reversely friction-charged by
rotating the first and the second stirring screws 5, 6. The developer is
supplied to the circumferential surface of the developing sleeve 7 that is
rotating toward arrow "b" direction, the developer is supported on the
circumferential surface of the developing sleeve 7, and conveyed toward the
rotating direction "b". The conveyed developer is then controlled for the
amount by the doctor 9, then the controlled developer is conveyed to the
108
CA 02628003 2008-04-30
developing site where the photoconductor 8 and the developing sleeve 7 face
each other. The toner at the site is electrostatically transferred onto
electrostatic latent images on the surface of the photoconductor, thereby the
electrostatic images are visualized as toner images.
The space of developing gap Gp between the photoconductor 8 and
the developing sleeve 7 is preferably 0.01 to 0.7 mm. In cases where the
space is less than 0.01 mm, it is possibly difficult to convey toners,
decreasing
uniformity of solid images, and in cases where the space is above 0.7 mm, the
initial charging property and stability of developers are unfavorably
lo deteriorated.
Intermediate Transfer Body
An embodiment of the intermediate transfer body will be explained
with reference to FIG. 4. A charging roller 20, an exposing device 30, a
cleaning device 60 with a cleaning blade, a charge eliminating device 70, a
developing device 40 and an intermediate transfer body 50 are disposed
around a photoconductor 10. The intermediate transfer body 50 is
suspended by plural suspension rollers 51, and moves toward the arrow
direction by driving means such as a motor (not shown) in a manner of an
endless belt. One or more of the suspension rollers 51 has an additional role
as a transfer bias roller, which supplies a transfer bias to the intermediate
transfer body, and a power supply (not shown) applies a desired transfer bias
voltage thereto. Additionally, a cleaning device 90 having a cleaning blade
109
CA 02628003 2008-04-30
for the intermediate transfer body 50 is also arranged. Further, a transfer
roller 80 is positioned facing the intermediate transfer body 50 as transfer
means to transfer a developed image to a sheet of support paper 100, which
is the final support material. A power supply (not shown) applies a transfer
bias voltage to the transfer roller 80. Moreover, corona charger 52 as a
charging device is located by the intermediate transfer body 50.
The image developer 40 comprises developing belt 41 as a developing
agent support, a black (hereinafter Bk) developing unit 45K, yellow
(hereinafter Y) developing unit 45Y, magenta (hereinafter M) developing unit
45M, and cyan (hereinafter C) developing unit 45C, the developing units
positioned around the developing belt 41. In addition, the developing belt
41 is configured so that it is suspended by a plurality of belt rollers, and
by
driving means such as a motor or the like (not shown), is advanced to the
direction of the arrow in a manner of an endless belt. The developing belt
41 moves at substantially the same speed as the photoconductor 10 at the
section where the two contact each other.
Since the configurations of the developing units are common, only
the Bk developing unit 45K will be described, and for other developing units
45Y, 45M, and 45C, components that correspond to those in the Bk
developing unit 45K are shown in the figure with the same reference
numbers followed by a letter Y, M, and C, respectively, and their descriptions
are omitted. The developing unit 45K comprises a developing tank 42K
110
CA 02628003 2008-04-30
that contains a solution of developing agent of high viscosity and high
density including toner particles and a carrier liquid component, a scooping
roller 43K that is positioned so that its lower portion is dipped in the
liquid
developing agent within the developing tank 42K, and a applying roller 44K
that receives the developing agent scooped by the scooping roller 43K makes
a thin layer of the developing agent, and applies the developing agent to the
developing belt 41. The applying roller 44K is electrically conductive, and a
power supply (not shown) applies a desired bias thereto.
With regards to the device configuration of the copier of this
embodiment, a device configuration different from one shown in FIG. 4 may
be employed in which a developing unit of each color is located around a
photoconductor 10, as shown in FIG. 5.
Next, the operation of the copier of embodiment will be described.
In FIG. 1, the photoconductor 10 is rotationally driven in the direction of
the
arrow and is uniformly charged by the charging roller 20. Then, the
exposing device 30 uses reflected light from the original document passing
through an optical system (not shown) and forms an electrostatic latent
image on the photoconductor 10. The electrostatic latent image is then
developed by the image developer 40, and a toner image as a visualized
(developed) image is formed. A thin layer of developing agent on the
developing belt 41 is released from the belt 41 in a form of a thin layer by a
contact with the photoconductor in a developing region, and is moved to the
111
CA 02628003 2008-04-30
portion where the latent image is formed on the photoconductor 10. The
toner image developed by the image developer 40 is transferred to the
surface of the intermediate transfer body 50 at a portion of contact (primary
transfer region) of the photoconductor 10 and the intermediate transfer body
50 that is moving at the same speed (primary transfer). In a case when
three colors or four colors are transferred and overlaid, the process is
repeated for each color to form a color image on the intermediate transfer
body 50.
The corona charger 52 is placed in order to charge the overlaid toner
image on the intermediate transfer body at a position that is downstream of
the contact section of the photoconductor 10 and the intermediate transfer
body 50, and that is upstream of the contact section of the intermediate
transfer body 50 and the sheet of support paper 100 with regards to the
direction of the rotation of the intermediate transfer body 50. Then, the
corona charger 52 provides a charge to the toner image the polarity of which
is the same as that of the toner particles that form the toner image, and
gives
a sufficient charge for a good transfer to the sheet of support paper 100.
After being charged by the corona charger 52, the toner image is transferred
at once to the sheet of support paper 100 that is carried in the direction of
the
arrow from a sheet feeder (not shown) by a transfer bias of the transfer
roller
80 (secondary transfer). Thereafter, the sheet of support paper 100 to which
the toner image is transferred is detached from the photoconductor 10 by a
112
CA 02628003 2008-04-30
detaching device (not shown), and fusing is conducted thereto by a fusing
device (not shown). After that, the sheet 100 is ejected from the device. On
the other hand, after the transfer, the cleaning device 60 removes and
retrieves toner particles that are not transferred from the photoconductor 10,
and the charge removing lamp 70 removes remaining charge from the
photoconductor 10 to prepare for the next charging.
The static friction coefficient of the intermediate transfer body is
preferably 0.1 to 0.6, more preferably 0.3 to 0.5. The volume resistance of
the intermediate transfer body is preferably several SZ=cm or more and 103
Q-cm or less. By controlling the volume resistance from several SZ=cm to 103
Q-cm, charging of the intermediate transfer body itself is prevented. It also
prevents uneven transfer at secondary transfer because the charge provided
by charging means does not remain as much. In addition, it is easier to
apply transfer bias for the secondary transfer.
The materials for the intermediate transfer body may be properly
selected depending on the application; examples are as follows:
(1) Materials with high Young's moduli (tension elasticity) used as a
single layer belt, which includes polycarbonates (PC), polyvinylidene fluoride
(PVDF), polyalkylene terephthalate (PAT), blend materials of PC/PAT,
ethylene tetrafluoroethylene copolymer (ETFE)/PC, and ETFE/PAT,
thermosetting polyimides of carbon black dispersion, and the like. These
single layer belts having high Young's moduli are small in their deformation
113
CA 02628003 2008-04-30
against stress during image formation and are particularly advantageous in
that mis-registration is not easily formed when forming a color image.
(2) A double or triple layer belt using the above-described belt having
high Young's modulus as a base layer, added with a surface layer and an
optional intermediate layer around the peripheral side of the base layer.
The double or triple layer belt has a capability to prevent print defect of
unclear center portion in a line image that is caused by the hardness of the
single layer belt.
(3) A belt with a relatively low Young's modulus that incorporates a
1o rubber or an elastomer. This belt has an advantage that there is almost no
print defect of unclear center portion in a line image due to its softness.
Additionally, by making the width of the belt wider than driving and tension
rollers and thereby using the elasticity of the edge portions that extend over
the rollers, it can prevent snaky move of the belt. Therefore, it can reduce
cost without the need for ribs and a device to prevent the snaky move.
Conventionally, intermediate transfer belts have been adopting
fluorine resins, polycarbonates, polyimides, and the like, but in the recent
years, elastic belts in which elastic members are used in all layers or a part
thereof. There are issues on transfer of color images using a resin belt.
Color images are typically formed by four colors toners. In one color
image, toner layers of layer 1 to layer 4 are formed. Toner layers are
pressurized as they pass the primary transfer in which the layers are
114
CA 02628003 2008-04-30
transferred from the photoconductor to the intermediate transfer belt and
the secondary transfer in which the toner is transferred from the
intermediate transfer belt to the sheet, which increases the cohesive force
among toner particles. As the cohesive force increases, phenomena such as
drop outs of letters and dropouts of edges of solid images are likely to
occur.
Since resin belts are too hard to be deformed by the toner layers, they tend
to
compress the toner layers and therefore drop out phenomena of letters are
likely to occur.
Recently, the demand for printing full color images on various types
of paper such as Japanese paper and paper having a rough surface is
increasing. However, sheets of paper having low smoothness tend to form
gaps between the toner and the sheet at transfer and thus leading to
miss-transfers. When the transfer pressure of secondary transfer section is
raised in order to increase contact, the cohesive force of the toner layers
will
be higher, which will result in drop out of letters as described above.
Elastic belts are used for the following aim. Elastic belts deform
according to the toner layers and the roughness of the sheet having low
smoothness at the transfer section. In other words, since the elastic belts
deform to comply with local bumps and holes, a good contact is achieved
without increasing the transfer pressure against the toner layers excessively
so that it is possible to obtain transferred images having excellent
uniformity
without any drop out of letters even on sheets of paper of low flatness.
115
CA 02628003 2008-04-30
For the resin of the elastic belts, one or more can be selected from the
group including polycarbonates, fluorine resins (ETFE, PVDF), styrene
resins (homopolymers and copolymers including styrene or substituted
styrene) such as polystyrene, chloropolystyrene, poly-a-methylstyrene,
styrene-butadiene copolymer, styrene-vinyl chloride copolymer, styrene-vinyl
acetate copolymer, styrene-maleic acid copolymer, styrene-acrylate
copolymers (styrene-methyl acrylate copolymer, styrene-ethyl acrylate
copolymer, styrene-butyl acrylate copolymer, styrene-octyl acrylate copolymer,
and styrene-phenyl acrylate copolymer), styrene-methacrylate copolymers
(styrene-methyl methacrylate copolymer, styrene-ethyl methacrylate
copolymer, styrene-phenyl methacrylate copolymer, and the like),
styrene-a-chloromethyl acrylate copolymer, styrene-acrylonitrile acrylate
copolymer, and the like, methyl methacrylate resin, butyl methacrylate resin,
ethyl acrylate resin, butyl acrylate resin, modified acrylic resins
(silicone-modified acrylic resin, vinyl chloride resin-modified acrylic resin,
acrylic urethane resin, and the like), vinyl chloride resin, styrene-vinyl
acetate copolymer, vinyl chloride-vinyl acetate copolymer, rosin-modified
maleic acid resin, phenol resin, epoxy resin, polyester resin, polyester
polyurethane resin, polyethylene, polypropylene, polybutadiene,
polyvinylidene chloride, ionomer resin, polyurethane resin, silicone resin,
ketone resin, ethylene-ethylacrylate copolymer, xylene resin and
polyvinylbutylal resin, polyamide resin, modified polyphenylene oxide resin,
116
CA 02628003 2008-04-30
and the like.
For the rubber and elastomer of the elastic materials, one or more
can be selected from the group consisting of butyl rubber, fluorine rubber,
acrylic rubber, ethylene propylene rubber (EPDM), acrylonitrilebutadiene
rubber (NBR), acrylonitrile-butadiene-styrene natural rubber, isoprene
rubber, styrene-butadiene rubber, butadiene rubber, ethylene-propylene
rubber, ethylene-propylene terpolymer, chloroprene rubber, chlorosufonated
polyethylene, chlorinated polyethylene, urethane rubber, syndiotactic
1,2-polybutadiene, epichlorohydrin rubber, silicone rubber, fluorine rubber,
polysulfurized rubber, polynorbornen rubber, hydrogenated nitrile rubber,
thermoplastic elastomers such as polystyrene elastomers, polyolefin
elastomers, polyvinyl chloride elastomers, polyurethane elastomers,
polyamide elastomers, polyurea elastomers, polyester elastomers and
fluorine resin elastomers.
The electric conductive agent may be properly selected depending on
the application; examples thereof include carbon black, graphite, metal
powders such as aluminum, nickel, and the like; and electric conductive
metal oxides such as tin oxide, titanium oxide, antimony oxide, indium oxide,
potassium titanate, antimony tin oxide (ATO), indium tin oxide (ITO), and
the like. The metal oxides may be coated on non-conducting particulates
such as barium sulfate, magnesium silicate, calcium carbonate, and the like.
Materials of the surface layer are required to prevent contamination
117
CA 02628003 2008-04-30
of the photoconductor by the elastic material and to reduce the surface
friction of the transfer belt so that toner adhesion is lessened and the
cleanability and secondary transfer property are increased. For example,
one or more of polyurethane, polyester, epoxy resin, and the like is used, and
powders or particles of a material that reduces surface energy and enhances
lubrication such as fluorine resin, fluorine compound, carbon fluoride,
titanium dioxide, silicon carbide, or the like can be dispersed and used. One
or more lubricant materials may be used, alternatively, powders or particles
of different sizes may be employed. In addition, it is possible to use a
1o material such as fluorine rubber that is treated with heat so that a
fluorine-rich layer is formed on the surface and the surface energy is
reduced.
Charging Unit
FIG. 6 is a schematic diagram showing an example of the
image-forming apparatus that equips a contact charger of charging unit.
The photoconductor 140 to be charged as a latent electrostatic
photoconductor is rotated at a predetermined speed of process speed in the
direction shown with the arrow in the figure. The charging roller 160,
which is brought into contact with the photoconductor, contains a core rod
and a conductive rubber layer formed on the core rod in a shape of a
concentric circle. The both terminals of the core rod are supported with
bearings (not shown) so that the charging roller enables to rotate freely, and
the charging roller is pressed to the photoconductor at a predetermined
118
CA 02628003 2008-04-30
pressure by a pressure member (not shown). The charging roller 160 in this
figure therefore rotates along with the rotation of the photoconductor. The
charging roller 160 is generally formed with a diameter of 16 mm in which a
core rod having a diameter of 9 mm is coated with a rubber layer having a
moderate resistance of approximately 100,000 Q=cm.
The power supply (not shown) is electrically connected with the core
rod of the charging roller 160, and a predetermined bias is applied to the
charging roller by the power supply, thereby, the surface of the
photoconductor 140 is uniformly charged at a predetermined polarity and
potential.
The charging device in the present invention may be a
non-contacting unit rather than the contacting unit described above;
preferably, the contact charger is preferable since the generation of ozone is
relatively little.
An alternative electric field is applied to the charging device of the
image forming apparatuses of the present invention. Direct electric field
typically generates a great number of 03 and N03, since the photoconductor
is charged as one polarity. The ozone and nitrogen oxide tend to attach to
the photoconductor and degrade the surface of the photoconductor;
consequently, the surface of the photoconductor is hardened, the abrasion
wear comes to larger, the external additive tends to deposit due to lowered
friction coefficients, resulting in frequent occurrences of filming. On the
119
CA 02628003 2008-04-30
contrary, alternative electric field duplicated with AC may reduce the
generation of ozone etc. and the photoconductor may be charged uniformly.
In particular, the alternative electric field may suppress the ozone-derived
degradation of photoconductor due to the generation of H3O+ having a
reverse polarity.
The configuration of the charging device may be properly selected
depending on specifications of the image forming apparatus; for example, the
configuration may be magnetic brush, fur brush etc. in addition to roller.
The magnetic brush is typically constructed from a charging material of
ferrite particles such as Zn-Cu ferrite, a non-magnetic conductive sleeve for
the support, or a magnetic roll encased therein. The fur blush is formed of a
fur to which such a conductive material is applied as carbon, copper sulfide,
metals, or metal oxides; the fur is wounded or adhered to the other metals or
conductive materials to form a charging device.
Tandem Color Image Forming Apparatus
FIG. 7 is a schematic view that exemplarily shows a color-image
forming apparatus of a tandem system. In the direct transfer system as
shown in FIG. 7, a transfer device 2, serving as a transfer, transfers images
on individual photoconductors 1 sequentially to a sheet "s", serving as a
recording medium, transported by a sheet conveyer belt 3. In the indirect
transfer system as shown in FIG. 8, a primary transfer device 2 sequentially
transfers images on individual photoconductors 1 to an intermediate transfer
120
CA 02628003 2008-04-30
4, and a secondary transfer device 5 transfers the resulting images on the
intermediate transfer 4 to the sheet "s" at once. The transfer device 5,
serving as the transfer, may be a transfer conveyer belt or a roller.
The direct transfer system must comprise a sheet feeder 6 upstream
to the sequentially arrayed photoconductors 1 of the tandem image forming
apparatus T and an image-fixing device 7 downstream thereof. The system
inevitably increases in its size in a sheet conveying direction. In contrast,
in
the indirect transfer system, the secondary transfer mechanism can be
relatively freely arranged, and the sheet feeder 6 and the image-fixing device
7 can be arranged above and/or below the tandem image forming apparatus
T. The apparatus of the indirect transfer system can therefore be
downsized.
In the direct transfer system, the image-fixing device 7 should be
arranged in the vicinity of the tandem image forming apparatus T to prevent
upsizing of the apparatus in a sheet conveying direction. The sheet "s"
cannot sufficiently bend in such a small space between the image-fixing
device 7 and the tandem image forming apparatus T. Accordingly, image
formation upstream to the image-fixing device 7 is affected by an impact,
specifically in a thick sheet, formed when the tip of the sheet "s" enters the
image-fixing device 7 and by the difference between the conveying speed of
the sheet when it passes through the image-fixing device 7 and the conveying
speed of the sheet by the transfer conveyor belt.
121
CA 02628003 2008-04-30
In contrast, in the indirect transfer system, the sheet "s" can
sufficiently bend in a space between the image-fixing device 7 and the
tandem image forming apparatus T. Thus, the image-fixing device 7 does
not significantly affect the image formation.
In the color electrophotographic apparatus of the tandem type as
shown in FIG. 8, a photoconductor cleaning device 8 removes a residual toner
on the photoconductor 1 after transferring and cleans the surface of the
photoconductor 1 for another image forming process. In addition, an
intermediate transfer cleaning device 9 removes residual toners on the
intermediate transfer 4 after the secondary transferring step to thereby clean
the surface of the intermediate transfer 4 for another image-forming process.
The inventive embodiment will be explained with reference to FIG. 9.
FIG. 9 is a schematic view showing an example of an
electrophotographic apparatus of the tandem indirect image transfer system
as an embodiment using the toner and the developer of the present invention.
The apparatus includes a copying machine main body 100, a feeder table 200
on which the copying machine main body 100 is placed, a scanner 300
arranged on the copying machine main body 100, and an automatic
document feeder (ADF) 400 arranged on the scanner 300. The copier main
body 100 includes an endless-belt intermediate transfer 10.
The intermediate transfer member 10 shown in FIG. 9 is spanned
around three support rollers 14, 15 and 16 and is capable of rotating and
122
CA 02628003 2008-04-30
moving in a clockwise direction in the figure.
This apparatus includes an intermediate transfer cleaning device 17
on the left side of the second support roller 15. The intermediate transfer
cleaning device 17 is capable of removing a residual toner on the
intermediate transfer 10 after image-transfer.
Above the intermediate transfer 10 spanned between the first and
second support rollers 14 and 15, yellow, cyan, magenta, and black
image-forming device 18 are arrayed in parallel in a moving direction of the
intermediate transfer 10 to thereby constitute a tandem image forming unit
l0 20.
The apparatus further includes an exposing device 21 serving as an
image-developer, above the tandem image forming unit 20 and a secondary
transfer 22 below the intermediate transfer 10 as shown in FIG. 9. The
secondary transfer 22, shown in FIG. 9 comprises an endless belt serving as
a secondary transfer belt 24 spanned around two rollers 23. The secondary
transfer belt 24 is pressed on the third support roller 16 with the
interposition of the intermediate transfer 10 and is capable of transferring
an
image on the intermediate transfer 10 to a sheet.
An image-fixing device 25 is arranged on the side of the secondary
transfer 22 and is capable of fixing a transferred image on the sheet. The
image-fixing device 25 comprises an endless image-fixing belt 26 and a
pressure roller 27 pressed on the image-fixing belt 26.
123
CA 02628003 2008-04-30
The secondary transfer 22 is also capable of transporting a sheet
after image transfer to the image-fixing device 25. Naturally, a transfer
roller or a non-contact charger can be used as the secondary transfer 22. In
this case, the secondary transfer 22 may not have the capability of
transporting the sheet.
The apparatus also includes a sheet reverser 28 below the secondary
transfer 22 and the image-fixing device 25 in parallel with the tandem image
forming unit 20. The sheet reverser 28 is capable of reversing the sheet so
as to form images on both sides of the sheet.
A copy is made using the color electrophotographic apparatus in the
following manner. Initially, a document is placed on a document platen 30
of the automatic document feeder 400. Alternatively, the automatic
document feeder 400 is opened, the document is placed on a contact glass 32
of the scanner 300, and the automatic document feeder 400 is closed to press
the document.
At the push of a start switch (not shown), the document, if any,
placed on the automatic document feeder 400 is transported onto the contact
glass 32. When the document is initially placed on the contact glass 32, the
scanner 300 is immediately driven to operate a first carriage 33 and a second
carriage 34. Light is applied from a light source to the document, and
reflected light from the document is further reflected toward the second
carriage 34 at the first carriage 33. The reflected light is further reflected
124
CA 02628003 2008-04-30
by a mirror of the second carriage 34 and passes through an image-forming
lens 35 into a read sensor 36 to thereby read the document.
At the push of the start switch (not shown), a drive motor (not
shown) rotates and drives one of the support rollers 14, 15 and 16 to thereby
allow the residual two support rollers to rotate following the rotation of the
one support roller to thereby rotatably convey the intermediate transfer 10.
Simultaneously, the individual image forming device 18 rotates their
photoconductors 40 to thereby form black, yellow, magenta, and cyan
monochrome images on the photoconductors 40, respectively. With the
conveying intermediate transfer 10, the monochrome images are sequentially
transferred to form a composite color image on the intermediate transfer 10.
Separately at the push of the start switch (not shown), one of feeder
rollers 42 of the feeder table 200 is selectively rotated, sheets are ejected
from
one of multiple feeder cassettes 44 in a paper bank 43 and are separated in a
separation roller 45 one by one into a feeder path 46, are transported by a
transport roller 47 into a feeder path 48 in the copying machine main body
100 and are bumped against a resist roller 49.
Alternatively, the push of the start switch rotates a feeder roller 50 to
eject sheets on a manual bypass tray 51, the sheets are separated one by one
on a separation roller 52 into a manual bypass feeder path 53 and are
bumped against the resist roller 49.
The resist roller 49 is rotated synchronously with the movement of
125
CA 02628003 2008-04-30
the composite color image on the intermediate transfer 10 to transport the
sheet into between the intermediate transfer 10 and the secondary transfer
22, and the composite color image is transferred onto the sheet by action of
the secondary transfer 22 to thereby record a color image.
The sheet bearing the transferred image is transported by the
secondary transfer 22 into the image-fixing device 25, is applied with heat
and pressure in the image-fixing device 25 to fix the transferred image,
changes its direction by action of a switch blade 55, is ejected by an
ejecting
roller 56 and is stacked on an output tray 57. Alternatively, the sheet
changes its direction by action of the switch blade 55 into the sheet reverser
28, turns therein, is transported again to the transfer position, followed by
image formation on the back surface of the sheet. The sheet bearing images
on both sides thereof is ejected through the ejecting roller 56 onto the
output
tray 57.
Separately, the intermediate transfer cleaning device 17 removes a
residual toner on the intermediate transfer 10 after image transfer for
another image forming procedure by the tandem image forming unit 20.
The resist roller 49 is generally grounded, but it is also acceptable to
apply a bias thereto for the removal of paper dust of the sheet.
In the tandem image forming apparatus 20, each of the image
forming units 18 comprises drum photoconductor 40, and around the
photoconductor 40 are equipped with charge charger 60, developer 61, first
126
CA 02628003 2008-04-30
transfer unit 62, cleaner 63, charge eliminator 64. Further, developing
agent 65, stirring puddle 68, partition plate 69, toner-concentration sensor
71,
developing sleeve 72, doctor 73, cleaning blade 75, cleaning brush 76,
cleaning roller 77, cleaning blade 78, toner-discharge auger 79, and driving
unit 80 are equipped as shown in FIG. 9.
Process Cartridge
The process cartridge applied from the present invention includes at
least a latent electrostatic image bearing member to carry electrostatic
images, and developing unit for developing by use of the developer to form
1o visible images, and other optional units. The developing unit contains at
least a developer container that contains the inventive toner or the developer
and a developer carrier that carries and transports the toner or the developer
in the developer container, and also a layer-thickness control member to
control the layer thickness of the carrying toner.
FIG. 10 is a schematic view of an image forming apparatus of
tandem indirect transfer system that comprises the process cartridge.
The process cartridge contains integrally at least the photoconductor
302 and the developing unit 304 among the photoconductor 302, charging
unit 303, developing unit 304, and cleaning unit 305 etc., and preferably, the
process cartridge is detachably attached to main bodied of image forming
apparatuses such as copiers and printers.
The inventive electrostatic image developing toner, containing the
127
CA 02628003 2008-04-30
inventive binder resin of the polycondensation polyester resin, may exhibit
excellent blocking resistance and low temperature fixability, provide high
quality images stably with time under such conditions as high temperature
and high humidity, low temperature and low humidity, or outputting larger
area images without such problems as decreasing charging capacity due to
firm adhesion of toners onto carriers or developing sleeves, and also
represent appropriate storage stability, melting-flowability and charging
property. Moreover, the resin properties are adequate even though the
catalyst is other than tin compounds that are environmentally harmful.
In addition, the inventive toner, in particular the toner combined
with the specific charge control agent may be far from background smear
under high temperature and high humidity conditions, exhibit proper
charging ability, less environmental fluctuation and excellent low
temperature fixability, and achieve less environmental load by virtue of the
toner binder prepared from catalyst others than tin catalysts that
biologically
toxic and environmentally harmful.
Moreover, the inventive electrostatic image developing toner, which
containing the polyester resin prepared under a specific titanium-containing
catalyst and the resin charge control agent in a specific ratio, may exhibit a
high charge amount and a sharp charge distribution, excellent initial
charging property and excellent background smear, and be hardly affected by
temperature/humidity change, be free from smears and filmings for long
128
CA 02628003 2008-04-30
usage such as several ten thousand sheets on developing supports like
developing rollers or sleeves and layer-thickness control members like blades
or rollers, and provide efficient productivity due to proper milling ability,
and
far from environmental problems, as such be appropriate for full-color
allocation.
The present invention provide also a one-component developer and
two-component developer that contain the toner, and an image forming
method and an image forming apparatus that utilize the toner.
The present invention will be explained with reference to Examples,
1o to which the present invention will be limited in no way. In the
descriptions
of Examples below, all parts means "parts by mass" and all percentages
means "% by mass".
In the Examples and Comparative Examples, toner properties were
measured in accordance with the following processes.
Measurement of Softening Temperature of Toner
The temperature of a sample material is raised at a constant rate
using a flow tester under the conditions below, the temperature at which half
of the sample material having been flown out is defined as the softening
temperature.
device : flow tester CTF-500D (by Shimadzu Co.)
load : 20 kgf/cm2
die : 1 mmc - lmm
129
CA 02628003 2008-04-30
temperature-rising rate : 6 C/min
sample mass : 1.0 g
Measurement of Particle Diameter of Toner
The particle diameter distribution of toner particles was measured
using Coulter counter TA-11 (by Beckman Coulter, Inc.) as follows:
Initially, 0.1 to 5 mL of a surfactant of alkylbenzene sulfonate is
added as a dispersant into 100 to 150 mL of an aqueous electrolyte solution.
The aqueous electrolyte solution is an about 0.1% NaCl aqueous solution,
which is prepared from ISOTON-II (by Beckman Coulter, Inc.). A sample of
2 to 20 mg was added to the electrolyte solution, which was then
ultrasonically dispersed for 1 to 3 minutes using a ultrasonic dispersing
device, thereafter volume and number of the toner particles are measured by
the Coulter counter TA-I1 using an aperture of 100 gm to calculate the
volume distribution and the number distribution, from which the volume
average particle diameter and the number average particle diameter are
determined.
In order to measure particles having a particle diameter (Pd) of no
less than 2.00 gm to less than 40.30 gm, thirteen channels are used such as
2.00 gm < Pd < 2.52 gm, 2.52 gm < Pd < 3.17 gm, 3.17 gm < Pd < 4.00 gm,
2o 4.00 gm < Pd < 5.04 gm, 5.04 gm < Pd < 6.35 gm, 6.35 gm < Pd < 8.00 gm,
8.00 gm < Pd < 10.08 gm, 10.08 gm < Pd < 12.70 gm, 12.70 gm < Pd < 16.00
gm, 16.00 gm < Pd < 20.20 gm, 20.20 gm < Pd < 25.40 gm, 25.40 gm < Pd <
130
CA 02628003 2008-04-30
32.00 gm and 32.00 m < Pd < 40.30 m.
Measurement of Average Circularity of Toner
The average circularity is measured using a flow-type particle image
analyzer FPIA-2100 (by Sysmex Co.). Specifically, 0.3 mL of a surfactant of
alkylbenzene sulfonate is added as a dispersant into 120 mL of pure water, to
which about 0.2 g of a sample is added. The dispersion containing the
sample is ultrasonically dispersed for about 2 minutes using a ultrasonic
dispersing device, the dispersion concentration is adjusted to 5000/ L then
the shape and the distribution of the toner are measured.
1o Measurement of Shape Factors SF-1 and SF-2 of Toner
SEM images taken using FE-SEM (S-4800, by Hitachi, Ltd.) are
randomly sampled by 300 views, which are inputted into Image Analyzer
LUSEX3 (by Nireco Co.) through an interface and analyzed.
Measurement of Agglomeration Degree of Toner
The agglomeration degree is measured using a powder tester (by
Hosokawa Micron Co.) as the measuring device; attachment parts are set on
a vibrating table according to the following procedures.
(i) vibro-shoot
(ii) packing
(iii) space ring
(iv) screens (three types) upper > middle > lower
(v) pressing bar
131
CA 02628003 2008-04-30
The screens are fixed by knob nuts, the vibrating table is operated
with the conditions below:
screen opening (upper) : 75 m
screen opening (middle) : 45 gm
screen opening (lower) : 22 gm
vibration amplitude : 1 mm
sample mass : 2 g
vibrating period : 15 seconds
The agglomeration degree is calculated as follows after the operation.
mass of powder on the upper screen x 1 : (a)
mass of powder on the middle screen x 0.6 : (b)
mass of powder on the lower screen x 0.2: (c)
The total of these three values is defined as the agglomeration degree
(%); i.e. agglomeration degree (%) = (a) + (b) + W.
Measurement of Glass Transition Temperature Tg
The glass transition temperature Tg of toner is measured under the
following conditions.
differential scanning calorimeter : Seiko 1D SC100, Seiko lSSC5040
(disc station)
measuring conditions: temperature range of 25 C to 90 C,
temperature- rising rate of 10 C/min, sampling period of 0.5 second, and
sampling amount of 10 mg
132
CA 02628003 2008-04-30
Measurement of Volume Resistivity
The volume resistivity is measured by way of pressing a toner into a
pellet, the pellet is placed between parallel electrodes with a gap of 2 mm,
then DC 1000 volts is applied between the electrodes, the resistivity after 30
seconds is measured by a high resist meter (TR8601, by Advantest Co.), then
the volume resistivity is calculated as a logarithmic value from the measured
resistivity and the pellet thickness.
Measurement of Loose Apparent Density
The loose apparent density is measured by a powder tester PT-S (by
lo Hosokawa Micron Co.).
[I] Examples 1 to 12 and Comparative Examples 1 to 4
Evaluation Device
Images to be evaluated are formed by use of evaluation devices A, B,
C, D or E.
Evaluation Device A
Evaluation device A was a tandem full-color laser printer equipped
with a developing unit of a four color non-magnetic two-component system
and a four-color photoconductor (IPSiO Color 8000, by Ricoh Co.) of which
the fixing unit was modified into an oilless fixing unit and tuned. The
printing rate was high-speed printing of 20 to 50 sheets/min ofA4-size.
Evaluation Device B
Evaluation device B was a tandem full-color laser printer equipped
133
CA 02628003 2008-04-30
with a developing unit of a four color non-magnetic two-component system
and a four-color photoconductor (IPSiO Color 8000, by Ricoh Co.), in which
the printer was modified into an intermediate transfer type such that images
were primary-transferred onto an intermediate transfer body and then the
toner images were secondary-transferred onto a transfer material; and the
fixing unit was modified into an oilless fixing unit and tuned. The printing
rate was high-speed printing of 20 to 50 sheets/min of A4-size.
Evaluation Device C
Evaluation device C was a full-color laser copier (IMAGIO Color 2800,
by Ricoh Co.) where a four-color developing unit develops each color image
respectively on one drum-like photoconductor using two-component
developers, the color images are transferred on an intermediate transfer
body sequentially, then four color images are transferred collectively on a
recording medium, in which and the fixing unit was modified into an oilless
fixing unit and tuned.
Evaluation Device D
Evaluation device D was a full-color laser printer (IPSiO Color 5000,
by Ricoh Co.) where a four-color developing unit develops using each
non-magnetic one-component developers respectively on one belt-like
photoconductor, the color images were transferred on an intermediate
transfer body sequentially, then four color images were transferred
collectively on a recording medium, in which and the fixing unit was modified
134
CA 02628003 2008-04-30
into an oilless fixing unit and tuned.
Evaluation of Two-Component Developer
The two-component developer for evaluating images was prepared,
from a ferrite carrier having an average particle diameter of 50 m and
coated with a silicone resin of 0.3 m thick in average, by mixing 100 part of
the carrier and 5 parts of respective color toners uniformly using a tumbler
mixer of tumbling-mixing type to charge them, thereby the development was
produced.
Production of Carrier
Core Material
Cu-Zn ferrite particles *1) 5000 parts
Coating Material
toluene 450 parts
silicone resin (SR2400) *2) 450 parts
amino silane (SH6020) *3) 10 parts
carbon black 10 parts
*1) mass average diameter: 35 m
*2) non-volatile content: 50%, by Toray Dow Corning Silicone Co.
*3) by Toray Dow Corning Silicone Co.
The coating materials were dispersed by a stirrer for 10 minutes to
prepare a coating liquid, and the coating liquid and the core material were
poured into a coating device that coats the coating liquid onto the core
135
CA 02628003 2008-04-30
material while swirling them by use of a rotatable bottom disc and stirring
blade within a fluidized bed. The coated product was heated at 250 C for 2
hours to prepare the carrier.
Evaluation Items
(1) Carrier Loss
After outputting 100,000 sheets of a chart of 50% image area while
controlling image concentration within 1.4 0.2, the charge amount ( c/g) of
developers was compared between before and after the outputting and
evaluated under the following criteria. The charge amount was measured
1 o in accordance with a blow off process.
A: loss of 0% to 30%
B: loss of 30% to 50%
C: loss of 50% or more
(2) Fog
As for respective toners, a chart of image area 50% was output at
temperature 10 C and RH 15% continuously on 100,000 sheets, then the
toner smear on background was visually evaluated using a loupe under the
following criteria.
A: no smear of toner
B: slightly observable smear, substantially no problem
C: some observable smear
D: non-allowable significant smear, problematic
136
CA 02628003 2008-04-30
(3) Toner Scattering
As for respective toners, a chart of image area 10% was output at
temperature 40 C and RH 90% continuously on 100,000 sheets, then the
toner smear within the copier was visually evaluated under the following
criteria.
A: no smear of toner
B: slightly observable smear, substantially no problem
C= some observable smear
D: non-allowable significant smear, problematic
(4) Blocking Resistance (Environmental Preservability)
A toner of 10 g was placed into a glass vessel of 20 mL, then the glass
vessel was tapped 100 times and allowed to stand for 48 hours at
temperature 55 C and RH 80%, followed by measuring a penetrating degree
(Pd) using a needle-penetrating meter. Separately, the toner was placed
into another glass vessel and allowed to stand at low temperature and low
humidity condition of 10 C and RH 15%. The smaller penetrating degree
judged between at high temperature and high humidity condition and at low
temperature and low humidity condition was employed, and evaluated under
the following criteria.
A: 20 mm < Pd
B: 15 mm<Pd< 20 mm
C: 10 mm5 Pd<15mm
137
CA 02628003 2008-04-30
D:Pd <10min
(5) Fixability (Hot Offset Resistance, Low Temperature Fixability)
A solid image was output at a toner amount of 1.0 0.1 mg/cm2 on a
regular paper and a thick paper (type 6200, by Ricoh Co., copy paper <135>,
by NBS Ricoh Co.) using an image forming apparatus (Imagio Neo 450, by
Ricoh Co.) that had been modified into a belt-fixing system. A sample toner
was fixed on the regular paper while changing the temperature of the fixing
belt and the maximum temperature without hot offset was defined as the
upper limit of fixing temperature. The lower limit of fixing temperature
1o was defined as the temperature of the fixing roll at which the residual
rate of
image density after rubbing a fixed image with a pad was 70% or more. It is
typically desirable that the upper limit of fixing temperature is 200 C or
higher and the lower limit of fixing temperature is 140 C or lower.
Synthesis of Titanium-Containing Catalyst
A mixture of 1617 parts of titanium diisopropoxy
bis(triethanolaluminate) and 126 parts of deionized water was poured into a
reactor vessel equipped with a condenser, a stirrer and a nitrogen gas inlet
capable of bubbling a liquid therein, the mixture was heated gradually to
90 C and allowed to react at 90 C for 4 hours (hydrolysis) while bubbling the
liquid with nitrogen gas thereby to prepare titanium dihydroxy
bis(triethanolaluminate).
Other titanium-containing catalysts in Examples below, available for
138
CA 02628003 2008-10-21
51216-9
the present invention, may be prepared in similar synthetic processes.
Example 1
Synthesis of Linear Polyester Resin
Four hundred and thirty parts of an adduct of bisphenol A with 2
moles of PO, 300 parts of an adduct of bisphenol A with 3 moles of P0, 257
parts of terephthalic acid, 65 parts of isophthalic acid, 10 parts of maleic
anhydride, and 2 parts of titanium dihydroxy bis(triethanolaminate) as a
condensation catalyst were poured into a reactor vessel equipped with a
condenser, a stirrer and a nitrogen gas inlet, and the mixture was allowed to
react at 220 C for 10 hours under nitrogen gas flow while distilling away the
water generated in the reaction. Then the reactant was allowed to react
under a reduced pressure of 5 to 20 mmHg, and then taken out when the
acid value came to 5 mgKOH/g. After cooling to room temperature, the
reaction product was milled, consequently, a linear polyester resin AX1-1 was
obtained.
The resulting AXl-1 contained no THF-insoluble matter, and had an
acid value of 7 mgKOH/g, a hydroxyl value of 12 mgKOH/g, a glass transition
temperature Tg of 60 C, a number average molecular mass Mn of 6940, and
a peak top molecular mass Mp of 19100. The rate of the molecular mass of
no more than 1500 was 1.2%.
Synthesis of non-Linear Polyester Resin
Three hundred and fifty parts of an adduct of bisphenol A with 2
139
CA 02628003 2008-10-21
51216-9
moles of EO, 326 parts of an adduct of bisphenol A with 3 moles of PO, 278
parts of terephthalic acid, 40 parts of phthalic anhydride, and 2 parts of
titanium dihydroxy bis(triethanolaminate) as a condensation catalyst were
poured into a reactor vessel equipped with a condenser, a stirrer and a
nitrogen gas inlet, and the mixture was allowed to react at 230 C for 10
hours under nitrogen gas flow while distilling away the water generated in
the reaction. Then the reactant was allowed to react under a reduced
pressure of 5 to 20 mmHg, and cooled to 180 C when the acid value came to 2
mgKOH/g or less, and 62 parts of trimellitic anhydride was added to the
in reactant, then the mixture was allowed to react under normal pressure of
sealed atmosphere for 2 hours. After cooling to room temperature, the
reaction product was milled, consequently, a non-linear polyester resin AXl-1
was obtained.
The resulting AX2-1 contained no THF-insoluble matter, and had an
acid value of 35 mgKOH/g, a hydroxyl value of 17 mgKOH/g, a glass
transition temperature Tg of 69 C, a number average molecular mass Mn of
3920, and a peak top molecular mass Mp of 112010. The rate of the
molecular mass of no more than 1500 was 0.9%.
Synthesis of Toner Binder 1
Four hundred parts of the polyesterAXl-1 and 600 parts of the
polyesterAX2-1 were melted-kneaded using a continuous kneader at a jacket
temperature of 150 C and a residence time of 3 minutes. The melted resin
140
CA 02628003 2008-04-30
was cooled to 30 C over 4 minutes using a steel-belt cooler, then milled to
prepare an inventive toner binder 1.
Production of Toner
Black Toner
water 1000 parts
phthalocyanine green hydrous cake *1> 200 parts
carbon black *2) 540 parts
toner binder 1 1200 parts
*1) solid content: 30%
*2) MA60, by Mitsubishi Chemical Co.
The ingredients described above were mixed by a Henschel mixer to
prepare a mixture containing pigment agglomerates to which water
infiltrates. The mixture was kneaded for 45 minutes using twin rolls of
which the surface being controlled to 130 C, calendered and cooled, then was
crushed by a pulverizer thereby to prepare a master batch of pigment.
toner binder 1 100 parts
master batch described above 8 parts
charge control agent (Bontron E-84) *1) 2 parts
wax (aliphatic acid ester wax) *2) 5 parts
*1) by Orient Chemical Co.
*2) melting point: 83 C, viscosity: 280 mPa=s at 90 C
The ingredients described above were mixed by a mixer, and the
141
CA 02628003 2008-04-30
mixture was melted-kneaded 3 times or more by a two-roll mill, then the
kneaded product was calendered-cooled. Then the mixture was milled
using a jet-mill of collision-plate type (I-type mill, by Japan Pneumatic Mfg.
Co.) and air-classified by swirling flow (DS classifier, by Japan Pneumatic
Mfg. Co.) thereby to obtain black color particles having a volume average
particle diameter of 5.5 m. To the black color particles, hydrophobic silica
(primary particle diameter: 10 nm, HDK H2000, by Clariant Japan K.K.)
was added in an amount of 1.0%, then the mixture was mixed by a Henschel
mixer and passed through a screen having an opening of 50 m to remove
1o agglomerates thereby to prepare a black toner 1. The toner properties are
shown in Table 1-1 and evaluation results are shown in Table 2.
Yellow Toner
water 600 parts
C.I. Pigment Yellow 17 hydrous cake *1> 1200 parts
toner binder 1 1200 parts
*1) solid content: 50%
The ingredients described above were mixed by a Henschel mixer to
prepare a mixture containing pigment agglomerates to which water
infiltrates. The mixture was kneaded for 45 minutes using twin rolls of
which the surface being controlled to 130 C, calendered and cooled, then was
crushed by a pulverizer thereby to prepare a master batch of pigment.
toner binder 1 100 parts
142
CA 02628003 2008-04-30
master batch describes above 8 parts
charge control agent (Bontron E-84) *1) 2 parts
wax (aliphatic acid ester wax) *2) 5 parts
*1) by Orient Chemical Co.
*2) melting point: 83 C, viscosity: 280 mPa-s at 90 C
The ingredients described above were mixed by a mixer, and the
mixture was melted-kneaded 3 times or more by a two-roll mill, then the
kneaded product was calendered-cooled. Then the mixture was milled
using a jet-mill of collision-plate type (I-type mill, by Japan Pneumatic Mfg.
1o Co.) and air-classified by swirling flow (DS classifier, by Japan Pneumatic
Mfg. Co.) thereby to obtain yellow color particles having a volume average
particle diameter of 5.5 gm. To the yellow color particles, hydrophobic silica
(HDK H2000, by Clariant Japan K.K.) was added in an amount of 1.0%, then
the mixture was mixed by a Henschel mixer and passed through a screen
having an opening of 50 m to remove agglomerates thereby to prepare
yellow toner 1. The toner properties are shown in Tables 1-1 and 1-2, and
evaluation results are shown in Table 2.
Magenta Toner
water 600 parts
C.I. Pigment Red 57 hydrous cake *1> 1200 parts
toner binder 1 1200 parts
*1) solid content: 50%
143
CA 02628003 2008-04-30
The ingredients described above were mixed by a Henschel mixer to
prepare a mixture containing pigment agglomerates to which water
infiltrates. The mixture was kneaded for 45 minutes using twin rolls of
which the surface being controlled to 130 C, calendered and cooled, then was
crushed by a pulverizer thereby to prepare a master batch of pigment.
toner binder 1 100 parts
master batch described above 8 parts
charge control agent (Bontron E-84) *1) 2 parts
wax (aliphatic acid ester wax) *2) 5 parts
*1) by Orient Chemical Co.
*2) melting point: 83 C, viscosity: 280 mPa=s at 90 C
The ingredients described above were mixed by a mixer, and the
mixture was melted-kneaded 3 times or more by a two-roll mill, then the
kneaded product was calendered-cooled. Then the mixture was milled
using a jet-mill of collision-plate type (I-type mill, by Japan Pneumatic Mfg.
Co.) and air-classified by swirling flow (DS classifier, by Japan Pneumatic
Mfg. Co.) thereby to obtain magenta color particles having a volume average
particle diameter of 5.5 m. To the yellow color particles, hydrophobic silica
(HDK H2000, by Clariant Japan K.K.) was added in an amount of 1.0%, then
the mixture was mixed by a Henschel mixer and passed through a screen
having an opening of 50 m to remove agglomerates thereby to prepare
magenta toner 1. The toner properties are shown in Tables 1-1 and 1-2, and
144
CA 02628003 2008-04-30
evaluation results are shown in Table 2.
Cyan Toner
water 600 parts
C.I. Pigment Blue 15:3 hydrous cake *1) 1200 parts
toner binder 1 1200 parts
*1) solid content: 50%
The ingredients described above were mixed by a Henschel mixer to
prepare a mixture containing pigment agglomerates to which water
infiltrates. The mixture was kneaded for 45 minutes using twin rolls of
1o which the surface being controlled to 130 C, calendered and cooled, then
was
crushed by a pulverizer thereby to prepare a master batch of pigment.
toner binder 1 100 parts
master batch described above 8 parts
charge control agent (Bontron E-84) *1) 2 parts
wax (aliphatic acid ester wax) *2) 5 parts
*1) by Orient Chemical Co.
*2) melting point: 83 C, viscosity: 280 mPa=s at 90 C
The ingredients described above were mixed by a mixer, and the
mixture was melted-kneaded 3 times or more by a two-roll mill, then the
kneaded product was calendered-cooled. Then the mixture was milled
using a jet-mill of collision-plate type (I-type mill, by Japan Pneumatic Mfg.
Co.) and air-classified by swirling flow (DS classifier, by Japan Pneumatic
145
CA 02628003 2008-04-30
Mfg. Co.) thereby to obtain cyan color particles having a volume average
particle diameter of 5.5 m. To the yellow color particles, hydrophobic silica
(HDK H2000, by Clariant Japan K.K.) was added in an amount of 1.0%, then
the mixture was mixed by a Henschel mixer and passed through a screen
having an opening of 50 m to remove agglomerates thereby to prepare cyan
toner 1. The toner properties are shown in Tables 1-1 and 1-2, and
evaluation results are shown in Table 2. The evaluation was conducted
using an evaluation device A.
Example 2
Synthesis of Linear Polyester Resin
A linear polyester resin AXl-2 was prepared by a similar reaction as
that of Example 1 (AXl-1), followed by cooling to room temperature and
milling except that the polycondensation catalyst was changed into titanyl
bis(triethanolaluminate).
The resulting AXl-2 contained no THF-insoluble matter, and had an
acid value of 8 mgKOH/g, a hydroxyl value of 10 mgKOH/g, a glass transition
temperature Tg of 60 C, a number average molecular mass Mn of 6820, and
a peak top molecular mass Mp of 20180. The rate of the molecular mass of
no more than 1500 was 1.1%.
Synthesis of non-Linear Polyester Resin
A linear polyester resin AX2-2 was prepared by a similar reaction as
that of Example 1(AX2-1), followed by cooling to room temperature and
146
CA 02628003 2008-10-21
a ti
51216-9
milling except that the polycondensation catalyst was changed into titanyl
bis(triethanolaminate).
The resulting AX2-2 contained no THF-insoluble matter, and had an
acid value of 33 mgKOH/g, a hydroxyl value of 14 mgKOH/g, a glass
transition temperature Tg of 70 C, a number average molecular mass Mn of
4200, and a peak top molecular mass Mp of 11800. The rate of the
molecular mass of no more than 1500 was 0.8%.
Synthesis of Toner Binder 2
The inventive toner binder 2 was prepared by powder-mixing 500
1o parts of the polyester AX1-2 and 500 parts of the polyester AX2-2 for 5
minutes using a Henschel mixer.
Preparation of Toner
A toner was prepared and evaluated in the same manner as the
black toner of Example 1 except that the toner binder 2 was used in the toner
resin and the master batch. The toner properties are shown in Tables 1-1
and 1-2, and evaluation results are shown in Table 2. The evaluation was
conducted using an evaluation device A.
Example 3
Synthesis of Modified Polyester Resin
Five hundred and forty-nine parts of an adduct of bisphenol A with 2
moles of propylene oxide, 20 parts of an adduct of bisphenol A with 3 moles of
propylene oxide, 133 parts of an adduct of bisphenol A with 2 moles of
147
CA 02628003 2008-10-21
51216-9
ethylene oxide, 10 parts of an adduct of phenol novolac (average
polymerization degree: about 5) with 5 moles of ethylene oxide, 252 parts of
terephthalic acid, 19 parts of isophthalic acid, 10 parts of trimellitic
anhydride, and 2 parts of titanium dihydroxy bis(triethanolaminate) as a
condensation catalyst were poured into a reactor vessel equipped with a
condenser, a stirrer and a nitrogen gas inlet, and the mixture was allowed to
react at 230 C for 10 hours under nitrogen gas flow while distilling away the
water generated in the reaction. Then the reactant was allowed to react
under a reduced pressure of 6 to 20 mmHg till the acid value came to 2
mgKOH/g or less. Then 50 parts of trimellitic anhydride was added to the
reactant, which was allowed to react under normal pressure for 1 hour
followed by reacting under a reduced pressure of 20 to 40 mmHg, then 20
parts of bisphenol A diglycidyl ether was added to the reactant, followed by
taking out when the softening temperature came to 150 C. After cooling to
room temperature, the reaction product was milled, consequently, a modified
polyester resin AYl-1 was obtained.
The resultingAY1-1 had an acid value of 52 mgKOH/g, a hydroxyl
value of 16 mgKOH/g, a glass transition temperature Tg of 73 C, a number
average molecular mass Mn of 1860, a peak top molecular mass Mp of 6550,
and a THF-insoluble content of 32%; the rate of the molecular mass of no
more than 1500 was 1.0%, which was used as toner binder 3.
Preparation of Toner
148
CA 02628003 2008-10-21
51216-9
A toner was prepared and evaluated in the same manner as the
black toner of Example 1 except that the toner binder 3 was used in the toner
resin and the master batch. The toner properties are shown in Tables 1-1
and 1-2, and evaluation results are shown in Table 2. The evaluation was
conducted using an evaluation device A.
Example 4
Synthesis of non-Linear Polyester Resin
One hundred and thirty-two parts of an adduct of bisphenol A with 2
moles of propylene oxide, 371 parts of an adduct of bisphenol A with 3 moles
of propylene oxide, 20 parts of an adduct of bisphenol A with 2 moles of
ethylene oxide, 125 parts of an adduct of phenol novolac (average
polymerization degree: about 5) with 5 moles of propylene oxide, 201 parts of
terephthalic acid, 25 parts of maleic anhydride, 35 parts of dimethyl
terephthalate and 2 parts of titanyl bis(triethanolaminate) as a
condensation catalyst were poured into a reactor vessel equipped with a
condenser, a stirrer and a nitrogen gas inlet, and the mixture was allowed to
react at 230 C for 10 hours under nitrogen gas flow while distilling away the
water generated in the reaction. Then the reactant was allowed to react
under a reduced pressure of 5 to 20 mmHg, and cooled to 180 C when the
acid value came to 2 mgKOH/g or less, and 65 parts of trimellitic anhydride
was added to the reactant, then the mixture was allowed to react under
normal pressure of sealed atmosphere for 2 hours. After cooling to room
149
CA 02628003 2008-10-21
51216-9
temperature, the reaction product was milled, consequently, a non-linear
polyester resin AX2-3 was obtained.
The resulting non-linear polyester resin (AX2-3) had a softening
temperature of 144 C, an acid value of 30 mgKOH/g, a hydroxyl value of 16
mgKOH/g, a glass transition temperature Tg of 59 C, a number average
molecular mass Mn of 1410, a peak top molecular mass Mp of 4110, and a
THF-insoluble content of 27%; the rate of the molecular mass of no more
than 1500 was 1.0%, which was used as toner binder 4.
Preparation of Toner.
A toner was prepared and evaluated in the same manner as the
black toner of Example 1 except that the toner binder 4 was used in the toner
resin and the master batch. The toner properties are shown in Tables 1-1
and 1-2, and evaluation results are shown in Table 2. The evaluation was
conducted using an evaluation device A.
Example 5
Synthesis of non-Linear Polyester Resin
Four hundred and ten parts of an adduct of bisphenol A with 2 moles
of propylene oxide, 270 parts of an adduct of bisphenol A with 3 moles of
propylene oxide, 110 parts of terephthalic acid, 125 parts of isophthalic
acid,
15 parts of maleic anhydride and 2 parts of titanium dihydroxy
bis(triethanolaminate) as a condensation catalyst were poured into a
reactor vessel equipped with a condenser, a stirrer and a nitrogen gas inlet,
150
CA 02628003 2008-10-21
51216-9
and the mixture was allowed to react at 220 C for 10 hours under nitrogen
gas flow while distilling away the water generated in the reaction. Then the
reactant was allowed to react under a reduced pressure of 5 to 20 mmHg,
and cooled to 180 C when the acid value came to 2 mgKOH/g or less, and 25
parts of trimellitic anhydride was added to the reactant, then the mixture
was allowed to react under normal pressure of sealed atmosphere for 2 hours.
After cooling to room temperature, the reaction product was milled,
consequently, a non-linear polyester resin AX2-4 was obtained.
The resulting AX2-4 contained no THF-insoluble matter, and had an
lo acid value of 18 mgKOHJg, a hydroxyl value of 37 mgKOH/g, a glass
transition temperature Tg of 62 C, a number average molecular mass Mn of
2130, and a peak top molecular mass Mp of 5350. The rate of the molecular
mass of no more than 1500 was 1.3%.
Synthesis of Modified Polyester Resin
Three hundred and seventeen parts of an adduct of bisphenol A with
2 moles of ethylene oxide, 57 parts of an adduct of bisphenol A with 2 moles
of propylene oxide, 298 parts of an adduct of bisphenol A with 3 moles of
propylene oxide, 75 parts of an adduct of phenol novolac (average
polymerization degree= about 5) with 5 moles of propylene oxide, 30 parts of
isophthalic acid, 157 parts of terephthalic acid, 27 parts of maleic
anhydride,
and 2 parts of titanium dihydroxy ' bis(triethanolaminate) as a condensation
catalyst were poured into a reactor vessel equipped with a condenser, a
151
CA 02628003 2008-04-30
stirrer and a nitrogen gas inlet, and the mixture was allowed to react at
230 C for 10 hours under nitrogen gas flow while distilling away the water
generated in the reaction. Then the reactant was allowed to react under a
reduced pressure of 5 to 20 mmHg, and cooled to 180 C till the acid value
came to 2 mgKOH/g or less. Then 68 parts of trimellitic anhydride was
added to the reactant, which was allowed to react under normal pressure for
1 hour followed by reacting under a reduced pressure of 20 to 40 mmHg, then
25 parts of bisphenol A diglycidyl ether was added to the reactant, followed
by taking out when the softening temperature came to 155 C. After cooling
to room temperature, the reaction product was milled, consequently, a
modified polyester resin AYl-2 was obtained.
The resultingAYl-2 had an acid value of 11 mgKOH/g, a hydroxyl
value of 27 mgKOH/g, a glass transition temperature Tg of 60 C, a number
average molecular mass Mn of 3020, a peak top molecular mass Mp of 6030,
and a THF-insoluble content of 35%. The rate of the molecular mass of no
more than 1500 was 1.1%.
Synthesis of Toner Binder 5
Five hundred parts of the AX2-3 and 500 parts of the AY1-2 were
melted-kneaded using a continuous kneader at a jacket temperature of
150 C and a residence time of 3 minutes. The melted resin was cooled to
C over 4 minutes using a steel-belt cooler, then milled to prepare an
inventive toner binder 5.
152
CA 02628003 2008-04-30
Preparation of Toner
A toner was prepared and evaluated in the same manner as the
black toner of Example 1 except that the toner binder 5 was used in the toner
resin and the master batch. The toner properties are shown in Tables 1-1
and 1-2, and evaluation results are shown in Table 2. The evaluation was
conducted using an evaluation device A.
Example 6
A black toner was prepared in the same manner as black toner 1 of
Example 1, except that external additives were mixed in a wet process as
described below, and evaluated in the same manner as Example 1.
Ten parts of black color particles having a volume average particle
diameter of 5.5 m of Example 1 and 2 parts of hydrophobic silica having a
primary particle diameter of 10 nm (HDK H2000, by Clariant Japan K.K.)
were dispersed-mixed in water containing 0.1 % of a surfactant using a
mono-pump. While monitoring the slurry by fluorescent X ray analysis that
the additive amount of the silica came to 1% by mass, a toner was prepared
from the slurry, and passed through a screen having an opening of 50 m to
remove agglomerates thereby to prepare a black toner. The toner properties
are shown in Tables 1-1 and 1-2, and evaluation results are shown in Table 2.
The evaluation was conducted using an evaluation device A.
Example 7
A black toner was prepared in the same manner as black toner 1 of
153
CA 02628003 2008-04-30
Example 1, except that external additives were mixed in the following
process.
In addition to black toner 1, 0.4 parts of zinc stearate was mixed by a
Henschel mixer, then the mixture was passed through a screen having an
opening of 50 m to remove agglomerates thereby to prepare a black toner.
The toner properties are shown in Tables 1-1 and 1-2, and evaluation
results are shown in Table 2. The evaluation was conducted using an
evaluation device A.
Example 8
A black toner was prepared in the same manner as black toner 1 of
Example 1, except that external additives were mixed in the following
process.
In addition to black toner 1, 0.5% by mass of titanium oxide (average
primary particle diameter: 15 nm, STM-150AI, by Tayca Co.) was mixed by a
Henschel mixer, then the mixture was passed through a screen having an
opening of 50 m to remove agglomerates thereby to prepare a black toner.
The toner properties are shown in Tables 1-1 and 1-2, and evaluation
results are shown in Table 2. The evaluation was conducted using an
evaluation device A.
Example 9
A chemical toner was prepared in the following processes and
evaluated in the same manner as Example 1.
154
CA 02628003 2008-04-30
Synthesis of Emulsion of Organic Fine Particles
Six hundred and eighty-three parts of water, 11 parts of sodium salt
of an adduct of sulfonate with methacrylic acid ethylene oxide (Eleminol
RS-30, by Sanyo Chemical Industries Ltd.), 166 parts of methacrylic acid,
110 parts of butylacrylate and 1 part of ammonium persulfate were poured
into a reaction vessel set with a stirring rod and a thermometer, and the
mixture was stirred at 3800 rpm for 30 minutes to prepare a white emulsion,
which was allowed to react at 75 C for 3 hours. Thirty parts of 1%
ammonium persulfate aqueous solution was further added to the reactant,
1o which was then aged at 70 C for 5 hours to prepare an aqueous dispersion
(fine particle dispersion 1) of a vinyl resin (copolymer of methacrylic acid,
butylacrylate, and sodium salt of an adduct of sulfonate with methacrylic
acid ethylene oxide). The volume average particle diameter of the fine
particle dispersion 1 was measured to be 75 nm using LA-920. A part of the
fine particle dispersion 1 was dried to separate the resin content. The glass
transition temperature Tg of the resin content was 60 C and the mass
average molecular mass Mw was 110000.
Preparation of Aqueous Phase
Nine hundred and ninety parts of water, 83 parts of the fine particle
dispersion 1, 37 parts of 48.3% aqueous solution of sodium
dodecyldiphenylether disulfonate (Eleminol MON-7, by Sanyo Chemical
Industries Ltd.), and 90 parts of ethylacetate were mixed and stirred to
155
CA 02628003 2008-10-21
51216-9
prepare an opaque liquid of aqueous phase 1.
Synthesis of Low-Molecular Mass Polyester
Four hundred and thirty parts of an adduct of bisphenol A with 2
moles of PO, 300 parts of an adduct of bisphenol A with 3 moles of PO, 257
parts of terephthoc acid, 65 parts of isophthalic acid, 10 parts of maleic
anhydride and 2 parts of titanium dihydroxy bis(triethanolaminate) as a
condensation catalyst were poured into a reactor vessel equipped with a
condenser, a stirrer and a nitrogen gas inlet, and the mixture was allowed to
react at 200 C for 8 hours under nitrogen gas flow while distilling away the
water generated in the reaction. Then the reactant was allowed to react
under a reduced pressure of 5 to 20 mmHg, and taken out when the acid
value came to 7 mgKOH/g. After cooling to room temperature, the reaction
product was milled, consequently, a low-molecular mass polyester resin 1
was obtained.
The resulting low-molecular mass polyester resin 1 contained no
THF-insoluble matter, and had an acid value of 9 mgKOH/g, a hydroxyl
value of 12 mgKOH/g, a glass transition temperature Tg of 52 C, a number
average molecular mass Mn of 4820, and a peak top molecular mass Mp of
17000. The rate of the molecular mass of no more than 1500 was 0.8%.
Synthesis of Intermediate Polyester
Six hundred and eighty-two parts of an adduct of bisphenol A with 2
moles of ethylene oxide, 81 parts of an adduct of bisphenol A with 2 moles of
156
CA 02628003 2008-04-30
propylene oxide, 283 parts of terephthalic acid, 22 parts of trimellitic
anhydride, and 2 parts of dibutyltin oxide were poured into a reactor vessel
equipped with a condenser, a stirrer and a nitrogen gas inlet, and the
mixture was allowed to react at 230 C for 7 hours under normal pressure
and for 5 hours under a reduced pressure of 10 to 15 mmHg to prepare an
intermediate polyester 1. The intermediate polyester 1 had a number
average molecular mass of 2200, a mass average molecular mass of 9700, a
glass transition temperature Tg of 54 C, an acid value of 0.5 mgKOH/g and a
hydroxyl value of 52 mgKOH/g.
Next, 410 parts of the intermediate polyester 1, 89 parts of
isophoronediisocyanate and 500 parts of ethylacetate were poured into a
reactor vessel equipped with a condenser, a stirrer and a nitrogen gas inlet,
and the mixture was allowed to react at 100 C for 5 hours to prepare
prepolymer 1. The content of free isocyanate was 1.53% by mass in the
prepolymer 1.
Synthesis of Ketimine
One hundred and seventy parts of isophoronediamine and 75 parts of
methylethylketone were poured into a reaction vessel set with a stirring rod
and a thermometer, and the mixture was allowed to react at 50 C for 4.5
hours to prepare ketimine compound 1. The ketimine compound 1 had an
amine value of 417.
Preparation of Master Batch (MB)
157
CA 02628003 2008-04-30
Six hundred parts of water, Pigment Blue 15:3 hydrous cake (solid
content: 50%) and 1200 parts of polyester resin were mixed using a Henschel
mixer (by Mitsui Mining Co.). The mixture was then kneaded for 45
minutes at 120 C using twin rolls, followed by being calendered and cooled,
then was crushed by a pulverizer thereby to prepare master batch 1.
Preparation of Oil Phase
Three hundred and seventy-eight parts of the low-molecular mass
polyester 1, 100 parts of Carnauba wax and 947 parts of ethylacetate were
poured into a reaction vessel set with a stirring rod and a thermometer, and
1o the mixture was heated to 80 C and maintained at 80 C for 5 hours then
cooled to 30 C over 1 hour. Then 500 parts of the mater batch 1 and 500
parts of ethylacetate were introduced into the vessel, the mixture was mixed
for 1 hour to obtain raw material solution 1.
Thereafter, 1324 parts of the raw material solution 1 was transferred
into a container, a pigment and a wax were dispersed into the raw material
solution 1 using a beads mill (Ultra Visco mill, by AIMEX Co.) under the
conditions of liquid feed rate of 1 kg/hr, disc circumferential velocity of 6
m/sec, 0.5 mm zirconia beads of 80% by volume, and three times pass to
prepare a mixture. Then 1324 parts of an ethylacetate solution of 65%
low-molecular mass polyester 1 was added to the mixture, which was then
passed through the beads mill two times under the conditions described
above thereby to prepare pigment-wax dispersion 1. The solid content of the
158
CA 02628003 2008-04-30
pigment-wax dispersion 1 was 50% at 130 C for 30 minutes.
Emulsification and de-Solvent
Seven hundred and forty-nine parts of the pigment-wax dispersion 1,
115 parts of the prepolymer 1 and 2.9 parts of the ketimine compound 1 were
poured into a container to prepare a mixture, which was then mixed at 5000
rpm for 2 minutes using TK homomixer (by Primix Co.), followed by adding
1200 parts of the aqueous phase 1 into the container and mixing at 13000
rpm for 25 minutes using TK homomixer to prepare emulsified slurry 1.
The emulsified slurry 1 was poured into a vessel set with a stirring
rod and a thermometer, then subjected to remove solvents at 30 C for 8 hours,
and aged at 45 C for 7 hours to prepare dispersion slurry 1.
Purification and Drying
One hundred parts of the dispersion slurry 1 was vacuum-filtered,
followed by:
(1) 100 parts of deionized water was added to the filtered cake, the
mixture was mixed using TK homomixer at 12000 rpm for 10 minutes and
then filtered;
(2) 100 parts of 10% sodium hydroxide aqueous solution was added
to the filtered cake (1), the mixture was mixed using TK homomixer at 12000
rpm for 30 minutes and then filtered;
(3) 100 parts of 10% hydrogen chloride aqueous solution was added
to the filtered cake (2), the mixture was mixed using TK homomixer at 12000
159
CA 02628003 2008-04-30
rpm for 10 minutes and then filtered;
(4) 300 parts of deionized water was added to the filtered cake (3),
the mixture was mixed using TK homomixer at 12000 rpm for 10 minutes
and then this procedure was repeated once more to prepare filtered cake 1;
and the filtered cake 1 was dried at 45 C for 48 hours.
Next, a toner base and 1% aqueous dispersion of the fluorine
compound (2) shown below were mixed within a water bath in an amount of
0.1% by mass of the fluorine compound (2) based on the toner base to adhere
or deposit the fluorine compound (2). Then the mixture was dried at 45 C
for 48 hours in an air-circulating dryer and further at 30 C for 10 hours on
shelves, and then screened through a mesh of opening 75 m thereby to
prepare toner base particles 1.
Fluorine Compound (2)
CH3
C9 F'_-r0 CONH 4 CH12-}-,N"-Cff, =I'D
CH3
Next, 100 parts of the toner base particles 1 and hydrophobic silica
having a primary particle diameter of 10 nm (HDK H2000, by Clani ant
Japan K.K.) were mixed by a Henschel mixer (FM20C, by Mitsui Mining Co.)
to prepare a toner under such conditions as three repeating times of rotating
for 20 seconds at circumferential velocity 30 m/sec as well as stopping for 60
seconds.
160
CA 02628003 2008-04-30
The toner properties are shown in Tables 1-1 and 1-2, and evaluation
results are shown in Table 2. The evaluation was conducted using an
evaluation device A.
Examples 10 to 12
Toners were prepared in the same manner as Example 1 using the
black toner of Example 1 and evaluated except that the evaluation devices
were devices B, C or D. The results are shown in Table 2.
Comparative Example 1
A toner was prepared and evaluated in the same manner as Example
1, except that the binder resin used for the black toner of Example 1 was
changed into the resin H2 shown below.
Two hundred and twenty-nine parts of an adduct of bisphenol A with
2 moles of ethylene oxide, 529 parts of an adduct of bisphenol A with 3 moles
of propylene oxide, 208 parts of terephthalic acid, 46 parts of adipic acid
and
2 parts of dibutyltin oxide were poured into a reactor vessel equipped with a
condenser, a stirrer and a nitrogen gas inlet, and the mixture was allowed to
react at 230 C for 7 hours under normal pressure and further under a
reduced pressure of 10 to 15 mmHg for 5 hours. Then 44 parts of trimellitic
anhydride was poured into the reaction vessel and the reactant was allowed
to react at 180 C for 3 hours under normal pressure thereby to prepare a
polyester resin H2. The resulting polyester resin H2 had a number average
molecular mass of 2300, a mass average molecular mass of 6700, a glass
161
CA 02628003 2008-04-30
transition temperature Tg of 43 C and an acid value of 25 mgKOH/g. One
part of dibutyltin was mixed as for the catalyst.
The toner properties are shown in Tables 1-1 and 1-2, and evaluation
results are shown in Table 2. The evaluation was conducted using an
evaluation device A.
Comparative Example 2
A black toner was prepared and evaluated in the same manner as
that of Example 1, except that the particle diameter, the particle diameter
distribution, the content of fine powder and the content of course powder
were adjusted as shown in Table 1-1 by classifying procedures. The toner
properties are shown in Tables 1-1 and 1-2, and evaluation results are shown
in Table 2. The evaluation was conducted using an evaluation device A.
Comparative Example 3
A black toner was prepared and evaluated in the same manner as
that of Example 1, except that the particle diameter, the particle diameter
distribution, the content of fine powder and the content of course powder
were adjusted as shown in Table 1-1 by classifying procedures. The toner
properties are shown in Tables 1-1 and 1-2, and evaluation results are shown
in Table 2. The evaluation was conducted using an evaluation device A.
Comparative Example 4
A black toner was prepared and evaluated in the same manner as
that of Example 1, except that the particle diameter, the particle diameter
162
CA 02628003 2008-04-30
distribution, the content of fine powder and the content of course powder
were adjusted as shown in Table 1-1 by classifying procedures. The toner
properties are shown in Tables 1-1 and 1-2, and evaluation results are shown
in Table 2. The evaluation was conducted using an evaluation device A.
[295]
Table 1-1
Particle Diameter Circurality
Volume Number Content Content of
Average Average Average
Particle Particle of Fine Coarse Dv/Dn Circura- SF-1 SF-2
Diameter Diameter Particles Particles lity
(Dv, Jim) (Dn, pm) (!C 4pm) (12.7gm<)
BT 5.5 4.3 11 0.2 1.28 0.93 150 143
YT 5.6 4.1 15 2.1 1.37 0.94 164 160
Ex. 1
MT 5.7 4.2 8 0.4 1.36 0.91 171 162
CT 5.4 4.0 4 1.2 1.35 0.92 163 149
Ex. 2 BT 7.8 6.5 22 3.5 1.20 0.93 171 143
Ex.3 BT 5.6 4.0 17 0.0 1.40 0.94 170 152
Ex. 4 BT 5.3 4.5 19 1.5 1.18 0.94 168 153
Ex. 5 BT 5.5 4.2 4 0.1 1.31 0.96 159 157
Ex. 6 BT 5.5 4.3 11 0.2 1.28 0.93 150 143
Ex.7 BT 5.5 4.3 11 0.2 1.28 0.93 150 143
Ex. 8 BT 5.5 4.3 11 0.2 1.28 0.93 150 143
Ex. 9 CT 4.7 4.5 2 0.0 1.04 0.98 120 115
Co. Ex. 1 BT 5.5 4.0 19 2.8 1.38 0.93 151 147
Co. Ex. 2 BT 11.1 8.0 11 0.2 1.39 0.93 150 143
Co. Ex. 3 BT 5.5 3.8 24 3.5 1.45 0.93 150 143
Co. Ex. 4 BT 1.8 1.0 70 0.0 1.80 0.91 170 164
BT: black toner, YT: yellow toner, MT: magenta toner, CT: cyan toner
163
CA 02628003 2008-04-30
Table 1-2
Glass
Loose Volume
Agglomeration Apparent Softening Transition
Degree(%) Density Resistivity Point CO Temperature
(g/ml) (Log Sam) CO
11 0.35 11.1 106 53
Ex.1 12 0.34 10.8 105 52
0.32 10.7 106 52
9 0.35 10.6 107 54
Ex. 2 24 0.33 10.5 104 55
Ex. 3 10 0.34 10.4 106 56
Ex. 4 12 0.41 10.7 91 48
Ex. 5 14 0.36 10.6 105 59
Ex.6 22 0.45 11.1 106 53
Ex. 7 24 0.29 11.2 106 53
Ex.8 6 0.38 11.1 106 53
Ex. 9 8 0.38 10.4 85 45
Com. Ex. 1 21 0.31 10.1 84 41
Com. Ex. 2 11 0.35 11.1 106 53
Com. Ex. 3 11 0.35 11.1 106 53
Com. Ex. 4 35 0.21 10.7 105 53
Table 2
Carrier Fog Toner Blocking Fixability
Loss g Scattering Resistance Lower Upper
Limit CO Limit CO
BT B B A B 130 200
Ex.l YT B B B B 130 200
MT B B B B 130 200
CT B A B B 130 200
Ex.2 BT C A A A 140 200
Ex.3 BT B C C B 130 200
Ex.4 BT A A A C 125 190
Ex.5 BT B B B B 145 210
Ex. 6 BT A B C C 130 200
Ex.7 BT B B B A 130 200
Ex.8 BT B A B A 130 200
Ex.9 CT A B B B 120 200
Ex. 10 BT B B C B 130 200
Ex. 11 BT B C C B 130 200
Ex. 12 BT B C C B 130 200
Com. Ex. 1 BT D D D D 150 160
Com. Ex. 2 BT C C D C 145 180
Com. Ex. 3 BT C D D C 130 180
Com. Ex. 4 BT D D D D 130 160
BT: black toner, YT: yellow toner, MT: magenta toner, CT: cyan toner
164
CA 02628003 2008-10-21
51216-9
[II] Examples 13 to 72 and Comparative Examples 5 to 24
Synthesis of Toner Binder A
Synthesis of Linear Polyester Resin
Four hundred and thirty parts of an adduct of bisphenol A with 2
moles of PO, 300 parts of an adduct of bisphenol A with 3 moles of PO, 257
parts of terephthalic acid, 65 parts of isophthalic acid, 10 parts of maleic
anhydride and 2 parts of titanium dihydroxy bis(triethanolaminate) as a
condensation catalyst were poured into a reactor vessel equipped with a
condenser, a stirrer and a nitrogen gas inlet, and the mixture was allowed to
1o react at 220 C for 10 hours under nitrogen gas flow while distilling away
the
water generated in the reaction. Then the reactant was allowed to react
under a reduced pressure of 5 to 20 mmHg, and taken out when the acid
value came to 5 mgKOH/g. After cooling to room temperature, the reaction
product was milled, consequently, a linear polyester resinAX1-1 was
obtained.
The resultingAX1-1 contained no THF-insoluble matter, and had an
acid value of 7 mgKOH/g, a hydroxyl value of 12 mgKOH/g, a glass transition
temperature Tg of 60 C, a number average molecular mass Mn of 6940, and
a peak top molecular mass Mp of 19100. The rate of the molecular mass of
no more than 1500 was 1.2%.
Synthesis of non-Linear Polyester Resin
Three hundred and fifty parts of an adduct of bisphenol A with 2
165
CA 02628003 2008-10-21
51216-9
moles of EO, 326 parts of an adduct of bisphenol A with 3 moles of P0, 278
parts of terephthalic acid, 40 parts of phthalic anhydride and 2 parts of
titanium dihydroxy bis(triethanolaminate) as a condensation catalyst were
poured into a reactor vessel equipped with a condenser, a stirrer and a
nitrogen gas inlet, and the mixture was allowed to react at 230 C for 10
hours under nitrogen gas flow while distilling away the water generated in
the reaction. Then the reactant was allowed to react under a reduced
pressure of 5 to 20 mmHg, cooled to 180 C when the acid value came to 2
mgKOH/g or less, 62 parts of trimellitic anhydride was added, then the
1o mixture was allowed to react under normal pressure of sealed atmosphere
for 2 hours. After cooling to room temperature, the reaction product was
milled, consequently, a non-linear polyester resin AX2-1 was obtained.
The resultingAX2-1 contained no THF-insoluble matter, and had an
acid value of 35 mgKOH/g, a hydroxyl value of 17 mgKOH/g, a glass
transition temperature Tg of 69 C, a number average molecular mass Mn of
3920, and a peak top molecular mass Mp of 11200. The rate of the
molecular mass of no more than 1500 was 0.9%.
Synthesis of Toner Binder A
Four hundred parts of the polyester AXl-1 and 600 parts of the
AX2-1 were melted-kneaded using a continuous kneader at a jacket
temperature of 150 C and a residence time of 3 minutes. The melted resin
was cooled to 30 C over 4 minutes using a steel-belt cooler, then milled to
166
CA 02628003 2008-10-21
51216-9
prepare an inventive toner binder A.
Synthesis of Toner Binder B
Synthesis of Linear Polyester Resin
A linear polyester resin AX1-2 was prepared by a similar reaction as
that of AXl-1 of toner binder A, followed by cooling to room temperature and
milling except that the polycondensation catalyst was changed into titanyl
bis(triethanolaminate).
The resulting AXl-2 contained no THF-insoluble matter, and had an
acid value of 8 mgKOl-l/g, a hydroxyl value of 10 mgKOHlg, a glass transition
temperature Tg of 60 C, a number average molecular mass Mn of 6820, and
a peak top molecular mass Mp of 20180. The rate of the molecular mass of
no more than 1500 was 1.1%.
Synthesis of non-Linear Polyester Resin
A non-linear polyester resin AX2-2 was prepared by a similar
reaction as that of AX2-1 of toner binder A, followed by cooling to room
temperature and milling except that the polycondensation catalyst was
changed into titanyl bis(triethanolaminate).
The resulting AX2-2 contained no THF-insoluble matter, and had an
acid value of 33 mgKOH/g, a hydroxyl value of 14 mgKOH/g, a glass
transition temperature Tg of 70 C, a number average molecular mass Mn of
4200, and a peak top molecular mass Mp of 11800. The rate of the
molecular mass of no more than 1500 was 0.8%.
167
CA 02628003 2008-04-30
Synthesis of Toner Binder B
The inventive toner binder B was prepared by powder-mixing 500
parts of the polyester AX1-2 and 500 parts of the polyester AX2-2 for 5
minutes using a Henschel mixer.
Synthesis of Toner Binder C
Synthesis of Comparative Linear Polyester Resin
The reaction was carried out in the same manner as that ofAXl-1 in
synthesis of toner binder A, except that the polycondensation catalyst was
changed into titanium tetraisopropoxide. There arose such a problem that
the reaction was stopped on the way due to catalysis deactivation and the
distillation of generated water was also stopped, thus 2 parts of titanium
tetraisopropoxide was added four times during the reaction thereby to obtain
a comparative linear polyester resin CAXl-1.
The resulting CAXl-1 contained no THF-insoluble matter, and had
an acid value of 7 mgKOH/g, a hydroxyl value of 12 mgKOH/g, a glass
transition temperature Tg of 58 C, a number average molecular mass Mn of
6220, and a peak top molecular mass Mp of 18900. The rate of the
molecular mass of no more than 1500 was 2.2%.
Synthesis of Comparative non-Linear Polyester Resin
The reaction was carried out in the same manner as that of AX2-1 in
synthesis of toner binder A, except that the polycondensation catalyst was
changed into titanium tetraisopropoxide. The reaction was carried out
168
CA 02628003 2008-04-30
under normal pressure for 16 hours and under a reduced pressure for 8
hours. The reaction velocity was slow, thus 2 parts of titanium
tetraisopropoxide was added three times during the reaction thereby to
obtain a comparative non-linear polyester resin CAX2-1.
The resulting CAX2-1 contained no THF-insoluble matter, and had
an acid value of 34 mgKOH/g, a hydroxyl value of 16 mgKOH/g, a glass
transition temperature Tg of 68 C, a number average molecular mass Mn of
3420, and a peak top molecular mass Mp of 12100. The rate of the
molecular mass of no more than 1500 was 2.1%.
Synthesis of Toner Binder C
Four hundred parts of the polyester CAXI-1 and 600 parts of the
CAX2-1 were melted-kneaded using a continuous kneader at a jacket
temperature of 150 C and a residence time of 3 minutes. The melted resin
was cooled to 30 C over 4 minutes using a steel-belt cooler, then milled to
prepare an inventive toner binder C. The toner binder C was a resin of
intense purplish brown.
Example 13
One hundred parts of the inventive toner binder A, 5 parts of
Carnauba wax (Carnauba wax C1, melting point: 84 C, by S. Kato & Co.), 4
parts of a yellow pigment (toner yellow HG VP2155, by Clariant Co.) and 3
parts of zinc salicylate (Bontron E-84, by Orient Chemical Co.) were
preliminarily mixed using a Henschel mixer (FMIOB, by Mitsui Mining Co.)
169
CA 02628003 2008-04-30
and then kneaded using a two-axis kneader (PCM-30, by Ikegai Ltd.).
The mixture was finely milled using a super sonic jet mill (lab jet, by
Japan Pneumatic Mfg. Co.) and then classified using an air classifier (MDS-I,
by Japan Pneumatic Mfg. Co.) to prepare toner particles having a particle
diameter D50 of 8 m. Then 0.5 part of colloidal silica (Aerosil R972, by
Nippon Aerosil Co.) was mixed with 100 parts of the toner particles using a
sample mill thereby to prepare a toner T13.
Example 14
A toner T14 was prepared in the same manner as Example 13,
except that the zinc salicylate (Bontron E-84, by Orient Chemical Co.) was
changed into a quaternary ammonium salt (Bontron P-51, by Orient
Chemical Co.) and the colloidal silica (Aerosil R972, by Nippon Aerosil Co.)
was changed into H30TA (by Wacker Chemical Co.).
Example 15
A toner T15 was prepared in the same manner as Example 13,
except that the zinc salicylate (Bontron E-84, by Orient Chemical Co.) was
changed into bis[1-(5-chloro-2-hydroxyphenylazo-2-naphtolato)chrome (III)
acid.
Example 16
A toner T16 was prepared in the same manner as Example 13,
except that the zinc salicylate (Bontron E-84, by Orient Chemical Co.) was
changed into ngrosine (Nigrosine Base EX, by Orient Chemical Co.) and the
170
CA 02628003 2008-04-30
colloidal silica (Aerosil R972, by Nippon Aerosil Co.) was changed into H30TA
(by Wacker Chemical Co.).
Example 17
A toner T17 was prepared in the same manner as Example 13,
except that the zinc salicylate (Bontron E-84, by Orient Chemical Co.) was
changed into a fluorine compound (Copy Charge NX VP 434, by Clariant
Japan K.K.).
Example 18
A toner T18 was prepared in the same manner as Example 13,
except that the zinc salicylate (Bontron E-84, by Orient Chemical Co.) was
changed into perfluoroalkyltrimethyl ammonium iodide (FT-310, by Neos
Company Ltd.).
Example 19
A toner T19 was prepared in the same manner as Example 13,
except that the zinc salicylate (Bontron E-84, by Orient Chemical Co.) was
changed into a quaternary ammonium salt-containing styrene/acrylic
copolymer (FCA-77PR, by Fujikurakasei Co.) and the colloidal silica (Aerosil
R972, by Nippon Aerosil Co.) was changed into H30TA (by Wacker Chemical
Co.).
Example 20
A toner T20 was prepared in the same manner as Example 13,
except that the zinc salicylate (Bontron E-84, by Orient Chemical Co.) was
171
CA 02628003 2008-04-30
changed into a Cr azo dye (CCA-7, by AstraZeneca Co.).
Example 21
A toner T21 was prepared in the same manner as Example 13,
except that the zinc salicylate (Bontron E-84, by Orient Chemical Co.) was
changed into a Fe azo dye (T-77, by Hodogaya Chemical Co.).
Example 22
A toner T22 was prepared in the same manner as Example 13,
except that the zinc salicylate (Bontron E-84, by Orient Chemical Co.) was
changed into polyhydroxyalkanoate.
The method for producing the polyhydroxyalkanoate will be shown
below.
Method for Producing Polyhydroxyalkanoate
A colony of agar plate was plated on 200 mL of a medium containing
0.5% polypeptone and 0.1% phenylsulfanylvaleric acid, and cultured in a
shaking flask of 500 mL at 30 C for 30 hours. After the incubation, the
fungus was harvested and rinsed with methanol, followed by freeze-drying.
The dried fungus was sampled, to which acetone was added, the mixture was
stirred for 72 hours to extract polymer. The acetone, containing the
extracted polymer, was filtered and condensed by an evaporator, then
collecting substances deposited-solidified by cold methanol, followed by
vacuum-drying to obtain intended polymer. The mass of the dried fungus
was 215 mg, and the mass of the resulting polymer was 76 mg.
172
CA 02628003 2008-04-30
Example 23
A toner T23 was prepared in the same manner as Example 13,
except that the toner binder A was changed into the toner binder B.
Example 24
A toner T24 was prepared in the same manner as Example 14,
except that the toner binder A was changed into the toner binder B.
Example 25
A toner T25 was prepared in the same manner as Example 15,
except that the toner binder A was changed into the toner binder B.
Example 26
A toner T26 was prepared in the same manner as Example 16,
except that the toner binder A was changed into the toner binder B.
Example 27
A toner T27 was prepared in the same manner as Example 17,
except that the toner binder A was changed into the toner binder B.
Example 28
A toner T28 was prepared in the same manner as Example 18,
except that the toner binder A was changed into the toner binder B.
Example 29
A toner T29 was prepared in the same manner as Example 19,
except that the toner binder A was changed into the toner binder B.
Example 30
173
CA 02628003 2008-04-30
A toner T30 was prepared in the same manner as Example 20,
except that the toner binder A was changed into the toner binder B.
Example 31
A toner T31 was prepared in the same manner as Example 21,
except that the toner binder A was changed into the toner binder B.
Example 32
A toner T32 was prepared in the same manner as Example 22,
except that the toner binder A was changed into the toner binder B.
Example 33
A toner T33 was prepared in the same manner as Example 13,
except that 3 parts of zinc salicylate (Bontron E-84, by Orient Chemical Co.)
was changed into 3 parts of zinc salicylate (Bontron E-84, by Orient
Chemical Co.) and 2 parts of
bis[1-(5-chloro-2-hydroxyphenylazo-2-naphtolato)chrome (III) acid.
Example 34
A toner T34 was prepared in the same manner as Example 14,
except that 3 parts of quaternary ammonium salt (Bontron P-51, by Orient
Chemical Co.) was changed into 3 parts of quaternary ammonium salt
(Bontron P-51, by Orient Chemical Co.) and 2 parts of ngrosine (Nigrosine
Base EX, by Orient Chemical Co.).
Example 35
A toner T35 was prepared in the same manner as Example 15,
174
CA 02628003 2008-04-30
except that 3 parts of
bis[l-(5-chloro-2-hydroxyphenylazo-2-naphtolato)chrome (III) acid was
changed into 3 parts of
bis[1-(5-chloro-2-hydroxyphenylazo-2-naphtolato)chrome (III) acid and 2
parts of zinc salicylate (Bontron E-84, by Orient Chemical Co.).
Example 36
A toner T36 was prepared in the same manner as Example 16,
except that 3 parts of ngrosine (Nigrosine Base EX, by Orient Chemical Co.)
was changed into 3 parts of ngrosine (Nigrosine Base EX, by Orient
Chemical Co.) and 2 parts of quaternary ammonium salt (Bontron P-51, by
Orient Chemical Co.).
Example 37
A toner T37 was prepared in the same manner as Example 17,
except that 3 parts of fluorine compound (Copy Charge NX VP 434, by
Clariant Japan K.K.) was changed into 3 parts of the fluorine compound
(Copy Charge NX VP 434, by Clariant Japan K.K.) and 2 parts of zinc
salicylate (Bontron E-84, by Orient Chemical Co.).
Example 38
A toner T38 was prepared in the same manner as Example 18,
except that 3 parts of perfluoroalkyltrimethyl ammonium iodide (F1,310, by
Neos Company Ltd.) was changed into 3 parts of perfluoroalkyltnim ethyl
ammonium iodide (FT-310, by Neos Company Ltd.) and 2 parts of zinc
175
CA 02628003 2008-04-30
salicylate (Bontron E-84, by Orient Chemical Co.).
Example 39
A toner T39 was prepared in the same manner as Example 19,
except that 3 parts of quaternary ammonium salt-containing styrene/acrylic
copolymer (FCA-77PR, by Fujikurakasei Co.) was changed into 3 parts of
quaternary ammonium salt-containing styrene/acrylic copolymer (FCA-77PR,
by Fujikurakasei Co.) and 2 parts of quaternary ammonium salt (Bontron
P-51, by Orient Chemical Co.).
Example 40
A toner T40 was prepared in the same manner as Example 20,
except that 3 parts of Cr azo dye (CCA-7, by AstraZeneca Co.) was changed
into 3 parts of Cr azo dye (CCA-7, by AstraZeneca Co.) and 2 parts of zinc
salicylate (Bontron E-84, by Orient Chemical Co.).
Example 41
A toner T41 was prepared in the same manner as Example 21,
except that 3 parts of Fe azo dye (T-77, by Hodogaya Chemical Co.) was
changed into 3 parts of Fe azo dye (T-77, by Hodogaya Chemical Co.) and 2
parts of zinc salicylate (Bontron E-84, by Orient Chemical Co.).
Example 42
Atoner T42 was prepared in the same manner as Example 22,
except that 3 parts of polyhydroxyalkanoate was changed into 3 parts of
polyhydroxyalkanoate and 2 parts of zinc salicylate (Bontron E-84, by Orient
176
CA 02628003 2008-04-30
Chemical Co.).
Comparative Example 5
A toner T5' was prepared in the same manner as Example 13, except
the toner binder A was changed into the toner binder C.
Comparative Example 6
A toner T6' was prepared in the same manner as Example 14, except
the toner binder A was changed into the toner binder C.
Comparative Example 7
A toner T7' was prepared in the same manner as Example 15, except
lo the toner binder A was changed into the toner binder C.
Comparative Example 8
A toner T8' was prepared in the same manner as Example 16, except
the toner binder A was changed into the toner binder C.
Comparative Example 9
A toner T9' was prepared in the same manner as Example 17, except
the toner binder A was changed into the toner binder C.
Comparative Example 10
A toner T10' was prepared in the same manner as Example 18,
except the toner binder A was changed into the toner binder C.
Comparative Example 11
A toner T11' was prepared in the same manner as Example 19,
except the toner binder A was changed into the toner binder C.
177
CA 02628003 2008-10-21
51216-9
Comparative Example 12
A toner T12' was prepared in the same manner as Example 20,
except the toner binder A was changed into the toner binder C.
Comparative Example 13
A toner T13' was prepared in the same manner as Example 21,
except the toner binder A was changed into the toner binder C.
Comparative Example 14
A toner T14' was prepared in the same manner as Example 22,
except the toner binder A was changed into the toner binder C.
1o Synthesis of Toner Binder D
Five hundred and forty-nine parts of an adduct of bisphenol A with 2
moles of propylene oxide, 20 parts of an adduct of bisphenol A with 3 moles of
propylene oxide, 133 parts of an adduct of bisphenol A with 2 moles of
ethylene oxide, 10 parts of an adduct of phenol novolac (average
polymerization degree: about 5) with 5 moles of ethylene oxide, 252 parts of
terephthalic acid, 19 parts of isophthalic acid, 10 parts of trimellitic
anhydride, and 2 parts of titanium dihydroxy bis(diethanolaminate) as a
condensation catalyst were poured into a reactor vessel equipped with a
condenser, a stirrer and a nitrogen gas inlet, and the mixture was allowed to
react at 230 C for 10 hours under nitrogen gas flow while distilling away the
water generated in the reaction. Then the reactant was allowed to react
under a reduced pressure of 5 to 20 mmHg till the acid value came to 2
178
CA 02628003 2008-04-30
mgKOH/g or less. Then 50 parts of trimellitic anhydride was added to the
reactant, which was allowed to react for 1 hour under normal pressure and
then under a reduced pressure of 5 to 20 mmHg, 20 parts of bisphenol A
diglycidyl ether was added when the softening temperature came to 105 C,
then the reactant was taken out when the softening temperature came to
150 C. After cooling to room temperature, the reaction product was milled,
consequently, a modified polyester resinAY1-1 was obtained.
The resulting AYl-1 had an acid value of 52 mgKOH/g, a hydroxyl
value of 16 mgKOH/g, a glass transition temperature Tg of 73 C, a number
1o average molecular mass Mn of 1860, a peak top molecular mass Mp of 6550,
and a THF-insoluble content of 32%. The rate of the molecular mass of no
more than 1500 was 2.1%. This resin was used as a toner binder D.
Synthesis of Toner Binder E
Synthesis of Modified Polyester Resin
A comparative modified polyester resin CAYl-2 was prepared in the
same manner as Example 15, except that the polycondensation catalyst was
changed into titanium tetrabutoxide.
The resulting CAY1-2 had a softening temperature of 150 C, an acid
value of 53 mgKOH/g, a hydroxyl value of 17 mgKOH/g, a glass transition
temperature Tg of 71 C, a number average molecular mass Mn of 1660, a
peak top molecular mass Mp of 6340, and a THF-insoluble content of 34%.
The rate of the molecular mass of no more than 1500 was 3.1%. This resin
179
CA 02628003 2008-10-21
51216-9
was used as a toner binder E.
Synthesis of Toner Binder F
Synthesis of non-Linear Polyester Resin
One hundred and thirty-two parts of an adduct of bisphenol A with 2
moles of propylene oxide, 371 parts of an adduct of bisphenol A with 3 moles
of propylene oxide, 20 parts of an adduct of bisphenol A with 2 moles of
ethylene oxide, 125 parts of an adduct of phenol novolac (average
polymerization degree: about 5) with 5 moles of propylene oxide, 201 parts of
terephthalic acid, 25 parts of maleic anhydride, 35 parts of dimethyl
1o terephthalate and 2 parts of titanyl bis(triethanolaminate) as a
condensation catalyst were poured into a reactor vessel equipped with a
condenser, a stirrer and a nitrogen gas inlet, and the mixture was allowed to
react at 230 C for 10 hours under nitrogen gas flow while distilling away the
water generated in the reaction. Then the reactant was allowed to react
under a reduced pressure of 5 to 20 mmHg, cooled to 180 C when the acid
value came to 2 mgKOH/g or less, 65 parts of trimellitic anhydride was
added, then the mixture was allowed to react under normal pressure of
sealed atmosphere for 2 hours. After cooling to room temperature, the
reaction product was milled, consequently, a non-linear polyester resin AX2-3
was obtained.
The resulting non-linear polyester resin AX2-3 had a softening
temperature of 144 C, an acid value of 30 mgKOH/g, a hydroxyl value of 16
180
CA 02628003 2008-10-21
51216-9
mgKOH/g, a glass transition temperature Tg of 59 C, a number average
molecular mass Mn of 1410, a peak top molecular mass Mp of 4110, and a
THF-msoluble content of 27%_ The rate of the molecular mass of no more
than 1500 was 1.0%. This resin was used as a toner binder F.
Synthesis of Toner Binder G
Synthesis of non-Linear Polyester Resin
Four hundred ten parts of an adduct of bisphenol A with 2 moles of
propylene oxide, 270 parts of an adduct of bisphenol A with 3 moles of
propylene oxide, 110 parts of terephthalic acid, 125 parts of isophthalic
acid,
15 parts of maleic anhydride and 2 parts of titanium dihydroxy
bis(triethanolaminate) as a condensation catalyst were poured into a
reactor vessel equipped with a condenser, a stirrer and a nitrogen gas inlet,
and the mixture was allowed to react at 220 C for 10 hours under nitrogen
gas flow while distilling away the water generated in the reaction. Then the
reactant was allowed to react under a reduced pressure of 5 to 20 mmHg,
cooled to 180 C when the acid value came to 2 mgKOHJg or less, 25 parts of
trimellitic anhydride was added, then the mixture was allowed to react
under normal pressure of sealed atmosphere for 2 hours. After cooling to
room temperature, the reaction product was milled, consequently, a
non-linear polyester resin AX2-4 was obtained.
The resulting AX2-4 contained no THF-insoluble matter, and had an
acid value of 18 mgKOH/g, a hydroxyl value of 37 mgKOH/g, a glass
181
CA 02628003 2008-10-21
51216-9
transition temperature Tg of 62 C, a number average molecular mass Mn of
2130, and a peak top molecular mass Mp of 5350. The rate of the molecular
mass of no more than 1500 was 1.3%.
Synthesis of Modified Polyester Resin
Three hundred and seventeen parts of an adduct of bisphenol A with
2 moles of ethylene oxide, 57 parts of an adduct of bisphenol A with 2 moles
of propylene oxide, 298 parts of an adduct of bisphenol A with 3 moles of
propylene oxide, 75 parts of an adduct of phenol novolac (average
polymerization degree= about 5) with 5 moles of propylene oxide, 30 parts of
1o isophthalic acid, 157 parts of terephthalic acid, 27 parts of maleic
anhydride
and 2 parts of titanium dihydroxy bis(triethanolaminate) as a condensation
catalyst were poured into a reactor vessel equipped with a condenser, a
stirrer and a nitrogen gas inlet, and the mixture was allowed to react at
230 C for 10 hours under nitrogen gas flow while distilling away the water
generated in the reaction. Then the reactant was allowed to react under a
reduced pressure of 5 to 20 mmHg, cooled to 180 C when the acid value came
to 2 mgKOH/g or less, then 68 parts of trimellitic anhydride was added to the
reactant, which was allowed to react for 1 hour under normal pressure
followed by under a reduced pressure of 20 to 40 mmHg, then 25 parts of
bisphenol A diglycidyl ether was added when the softening temperature came
to 120 C, the reactant was taken out when the softening temperature came
to 155 C. After cooling to room temperature, the reaction product was
182
CA 02628003 2008-04-30
milled, consequently, a modified polyester resin AY1-2 was obtained.
The resultingAY1-2 had an acid value of 11 mgKOH/g, a hydroxyl
value of 27 mgKOH/g, a glass transition temperature Tg of 60 C, a number
average molecular mass Mn of 3020, a peak top molecular mass Mp of 6030,
and a THF-insoluble content of 35%. The rate of the molecular mass of no
more than 1500 was 1.1%.
Synthesis of Toner Binder G
Five hundred parts of the polyester AX2-3 and 500 parts of the AY1-2
were melted-kneaded using a continuous kneader at a jacket temperature of
150 C and a residence time of 3 minutes. The melted resin was cooled to
30 C over 4 minutes using a steel-belt cooler, then milled to prepare an
inventive toner binder G.
Example 43
A toner T43 was prepared in the same manner as Example 13,
except the toner binder A was changed into the toner binder D.
Example 44
A toner T44 was prepared in the same manner as Example 14,
except the toner binder A was changed into the toner binder D.
Example 45
A toner T45 was prepared in the same manner as Example 15,
except the toner binder A was changed into the toner binder D.
Example 46
183
CA 02628003 2008-04-30
A toner T46 was prepared in the same manner as Example 16,
except the toner binder A was changed into the toner binder D.
Example 47
A toner T47 was prepared in the same manner as Example 17,
except the toner binder A was changed into the toner binder D.
Example 48
A toner T48 was prepared in the same manner as Example 18,
except the toner binder A was changed into the toner binder D.
Example 49
A toner T49 was prepared in the same manner as Example 19,
except the toner binder A was changed into the toner binder D.
Example 50
A toner T50 was prepared in the same manner as Example 20,
except the toner binder A was changed into the toner binder D.
Example 51
A toner T51 was prepared in the same manner as Example 21,
except the toner binder A was changed into the toner binder D.
Example 52
A toner T52 was prepared in the same manner as Example 22,
except the toner binder A was changed into the toner binder D.
Example 53
A toner T53 was prepared in the same manner as Example 13,
184
CA 02628003 2008-04-30
except the toner binder A was changed into the toner binder F.
Example 54
A toner T54 was prepared in the same manner as Example 14,
except the toner binder A was changed into the toner binder F.
Example 55
A toner T55 was prepared in the same manner as Example 15,
except the toner binder A was changed into the toner binder F.
Example 56
A toner T56 was prepared in the same manner as Example 16,
except the toner binder A was changed into the toner binder F.
Example 57
A toner T57 was prepared in the same manner as Example 17,
except the toner binder A was changed into the toner binder F.
Example 58
A toner T58 was prepared in the same manner as Example 18,
except the toner binder A was changed into the toner binder F.
Example 59
A toner T59 was prepared in the same manner as Example 19,
except the toner binder A was changed into the toner binder F.
Example 60
A toner T60 was prepared in the same manner as Example 20,
except the toner binder A was changed into the toner binder F.
185
CA 02628003 2008-04-30
Example 61
A toner T61 was prepared in the same manner as Example 21,
except the toner binder A was changed into the toner binder F.
Example 62
A toner T62 was prepared in the same manner as Example 22,
except the toner binder A was changed into the toner binder F.
Example 63
A toner T63 was prepared in the same manner as Example 13,
except the toner binder A was changed into the toner binder G.
Example 64
A toner T64 was prepared in the same manner as Example 14,
except the toner binder A was changed into the toner binder G.
Example 65
A toner T65 was prepared in the same manner as Example 15,
except the toner binder A was changed into the toner binder G.
Example 66
A toner T66 was prepared in the same manner as Example 16,
except the toner binder A was changed into the toner binder G.
Example 67
A toner T67 was prepared in the same manner as Example 17,
except the toner binder A was changed into the toner binder G.
Example 68
186
CA 02628003 2008-04-30
A toner T68 was prepared in the same manner as Example 18,
except the toner binder A was changed into the toner binder G.
Example 69
A toner T69 was prepared in the same manner as Example 19,
except the toner binder A was changed into the toner binder G.
Example 70
A toner T70 was prepared in the same manner as Example 20,
except the toner binder A was changed into the toner binder G.
Example 71
A toner T71 was prepared in the same manner as Example 21,
except the toner binder A was changed into the toner binder G.
Example 72
A toner T72 was prepared in the same manner as Example 22,
except the toner binder A was changed into the toner binder G.
Comparative Example 15
A toner T15' was prepared in the same manner as Example 13,
except the toner binder A was changed into the toner binder E.
Comparative Example 16
A toner T16' was prepared in the same manner as Example 14,
except the toner binder A was changed into the toner binder E.
Comparative Example 17
A toner T17' was prepared in the same manner as Example 15,
187
CA 02628003 2008-04-30
except the toner binder A was changed into the toner binder E.
Comparative Example 18
A toner T18' was prepared in the same manner as Example 16,
except the toner binder A was changed into the toner binder E.
Comparative Example 19
A toner T19' was prepared in the same manner as Example 17,
except the toner binder A was changed into the toner binder E.
Comparative Example 20
A toner T20' was prepared in the same manner as Example 18,
except the toner binder A was changed into the toner binder E.
Comparative Example 21
A toner T21' was prepared in the same manner as Example 19,
except the toner binder A was changed into the toner binder E.
Comparative Example 22
A toner T22' was prepared in the same manner as Example 20,
except the toner binder A was changed into the toner binder E.
Comparative Example 23
A toner T23' was prepared in the same manner as Example 21,
except the toner binder A was changed into the toner binder E.
Comparative Example 24
A toner T24' was prepared in the same manner as Example 22,
except the toner binder A was changed into the toner binder E.
188
CA 02628003 2008-04-30
Evaluation Process (Positively Charged Toner)
Evaluation Item
(1) Low Temperature Fixability (Peeling Property with Tape)
A developer was prepared by mixing for 5 minutes 4 parts by mass of
a toner and 96 parts by mass of a silicone-coated ferrite carrier (average
particle diameter 100 m, by Kanto Denka Kogyo Co.) using a turbuler mixer.
The developer was input into a copier (Imagio 105, by Ricoh Co.) that had
been modified so as to fix at outside the apparatus, and unfixed images of 2
cm by 12 cm were formed in a toner amount of 0.5 mg/cm2. Then the
unfixed images were fixed at a linear velocity of 1500 mm/sec while raising
the temperature of the fixing roll from 100 C to 250 C stepwise with an
increment of 5 C per step. The fixing paper was RICOPY PPC paper Type
6000 (by Ricoh Co.).
A scotch tape (by Sumitomo 3M Ltd.) was glued on images formed at
respective fixing temperatures and allowed to stand for 3 hours, then the
tape was peeled away and disposed on a white paper. The density of
unfixed images on the tape was measured by X-Rite 938 (by X-Rite Co.); the
difference of the density from that of blank being no less than 0.150 was
evaluated as "unfixed", the temperature at which the difference firstly came
to less than 0.150 was defined as the lowest fixing temperature. The low
temperature fixability was evaluated based on the lowest fixing temperature
in accordance the following criteria.
189
CA 02628003 2008-04-30
Evaluation Criteria
A: lowest fixing temperature < 140 C
B: 140 C < lowest fixing temperature < 150 C
C: 150 C < lowest fixing temperature
(2) Evaluation of Background Smear
Using the toners of Examples and Comparative Examples similarly
as above (1), a solid image was developed on 10000 sheets of paper under
high temperature and high humidity condition by use of a copier. Then a
scotch tape (by Sumitomo 3M Ltd.) was glued on the background portion of
the photoconductor, followed by being peeled away and disposed on a white
paper. The density of background smear on the tape was measured by
X-Rite 938 (by X-Rite Co.); the difference of the density from that of blank
being no less than 0.050 was evaluated as occurrence of background smear,
the difference of less than 0.010 and no less than 0.005 was evaluated as
appropriate resistance for background smear, and the difference of less than
0.005 was evaluated as very appropriate resistance for background smear.
Evaluation Criteria
A: very appropriate resistance for background smear
B: appropriate resistance for background smear
C: occurrence of background smear
Evaluation Process (Negatively Charged Toner)
Evaluation Item
190
CA 02628003 2008-04-30
(1) Low Temperature Fixability (Peeling Property with Tape)
A developer was prepared by mixing for 5 minutes 4 parts by mass of
a toner and 96 parts by mass of a ferrite carrier (F- 150, Powder Tec Co.)
using a turbuler mixer. The developer was input into a copier (Imagio Neo
C385, by Ricoh Co.) that had been modified so as to fix at outside the
apparatus, and unfixed images of 2 cm by 12 cm were formed in a toner
amount of 0.5 mg/cm2. Then the unfixed images were fixed at a linear
velocity of 1500 mm/sec while raising the temperature of the fixing roll from
100 C to 250 C stepwise with an increment of 5 C per step. The fixing
paper was RICOPY PPC paper Type 6000 (by Ricoh Co.).
A scotch tape (by Sumitomo 3M Ltd.) was glued on images of
respective fixing temperatures and allowed to stand for 3 hours, then the
tape was peeled away and disposed on a white paper. The density of
unfixed images on the tape was measured by X-Rite 938 (by X-Rite Co.); the
difference of the density from that of blank being no less than 0.150 was
evaluated as "unfixed", the temperature at which the difference firstly came
to less than 0.150 was defined as the lowest fixing temperature. The low
temperature fixability was evaluated based on the lowest fixing temperature
in accordance the following criteria.
Evaluation Criteria
A: lowest fixing temperature < 140 C
B: 140 C < lowest fixing temperature < 150 C
191
CA 02628003 2008-04-30
C: 150 C < lowest fixing temperature
(2) Evaluation of Background Smear
Using the toners of Examples and Comparative Examples similarly
as above (1), a solid image was developed on 10000 sheets of paper under
high temperature and high humidity condition by use of a copier. Then a
scotch tape (by Sumitomo 3M Ltd.) was glued on the background portion of
the photoconductor, followed by being peeled away and disposed on a white
paper. The density of background smear on the tape was measured by
X-Rite 938 (by X-Rite Co.); the difference of the density from that of blank
being no less than 0.050 was evaluated as occurrence of background smear,
the difference of less than 0.010 and no less than 0.005 was evaluated as
appropriate resistance for background smear, and the difference of less than
0.005 was evaluated as very appropriate resistance for background smear.
Evaluation Criteria
A: very appropriate resistance for background smear
B: appropriate resistance for background smear
C: occurrence of background smear
192
CA 02628003 2008-04-30
Table 3
LTF BSR LTF BSR LTF BSR
Ex. 13 B B Ex. 43 B B Co. Ex. 5 C C
Ex. 14 B B Ex. 44 A A Co. Ex. 6 B D
Ex. 15 A B Ex. 45 A B Co. Ex. 7 B D
Ex. 16 B B Ex. 46 B B Co. Ex. 8 C C
Ex. 17 A B Ex. 47 A B Co. Ex. 9 C D
Ex. 18 B B Ex. 48 B B Co. Ex. 10 D C
Ex. 19 B B Ex. 49 A A Co. Ex. 11 C C
Ex. 20 B B Ex. 50 B B Co. Ex. 12 C D
Ex. 21 A B Ex. 51 A B Co. Ex. 13 C C
Ex. 22 B A Ex. 52 A A Co. Ex. 14 B D
Ex. 23 B B Ex. 53 B A Co. Ex. 15 C D
Ex. 24 B B Ex. 54 A B Co. Ex. 16 D C
Ex. 25 A A Ex. 55 A A Co. Ex. 17 C D
Ex. 26 B B Ex. 56 B B Co. Ex. 18 C C
Ex. 27 B B Ex. 57 A A Co. Ex. 19 C C
Ex. 28 B B Ex. 58 A B Co. Ex. 20 C D
Ex. 29 A B Ex. 59 B A Co. Ex. 21 D C
Ex. 30 B B Ex. 60 A B Co. Ex. 22 C C
Ex. 31 B B Ex. 61 B A Co. Ex. 23 C D
Ex. 32 A A Ex. 62 A A Co. Ex. 24 C C
Ex.33 B B Ex.63 B B
Ex.34 A B Ex.64 B B
Ex.35 B B Ex.65 A A
Ex.36 B B Ex.66 A B
Ex.37 B B Ex.67 B A
Ex.38 A B Ex.68 A A
Ex.39 B B Ex.69 B B
Ex.40 B B Ex.70 A B
Ex.41 A B Ex.71 B B
Ex.42 B A Ex.72 A A
LTF: Low Temperature Fixability
BSR : Background Smear Resistance
The results described above demonstrate that the inventive toners
may exhibit appropriate low temperature fixability and be far from
background smear of toners even under high temperature and high humidity
conditions.
[III] Examples 73, 74 and Comparative Examples 25
Synthesis Example 1
Synthesis of Linear Polyester Resin
193
CA 02628003 2008-10-21
51216-9
Forty hundred and thirty parts of an adduct of bisphenol A with 2
moles of P0, 300 parts of an adduct of bisphenol A with 3 moles of PO, 257
parts of terephthalic acid, 65 parts of isophthalic acid, 10 parts of maleic
anhydride and 2 parts of titanium dihydroxy bis(triethanolaminate) as a
condensation catalyst were poured into a reactor vessel equipped with a
condenser, a stirrer and a nitrogen gas inlet, and the mixture was allowed to
react at 220 C for 10 hours under nitrogen gas flow while distilling away the
water generated in the reaction. Then the reactant was allowed to react
under a reduced pressure of 5 to 20 mmHg and taken out when the acid
to value came to 5. After cooling to room temperature, the reaction product
was milled, consequently, a linear polyester resin AX1-1 was obtained.
Synthesis of non-Linear Polyester Resin
Three hundred and fifty parts of an adduct of bisphenol A with 2
moles of EO, 326 parts of an adduct of bisphenol A with 3 moles of PO, 278
parts of terephthalic acid, 40 parts of phthalic anhydride and 2 parts of
titanium dihydroxy bis(triethanolaminate) as a condensation catalyst were
poured into a reactor vessel equipped with a condenser, a stirrer and a
nitrogen gas inlet, and the mixture was allowed to react at 230 C for 10
hours under nitrogen gas flow while distilling away the water generated in
the reaction. Then the reactant was allowed to react under a reduced
pressure of 5 to 20 mmHg, cooled to 180 C when the acid value came to 2
mgKOH/g or less, 62 parts of trimellitic anhydride was added, then the
194
CA 02628003 2008-10-21
51216-9
mixture was allowed to react under normal pressure of sealed atmosphere
for 2 hours. After cooling to room temperature, the reaction product was
milled, consequently, a non-linear polyester resin AX2-1 was obtained:
Synthesis of Toner Binder TB1
Four hundred parts of the polyester AM -I and 600 parts of the
AX2-1 were melted-kneaded using a continuous kneader at a jacket
temperature of 150 C and a residence time of 3 minutes. The melted resin
was cooled to 30 C over 4 minutes using a steel-belt cooler, then milled to
prepare an inventive toner binder TB1.
The resulting toner binder resin TB1 had a content of 3.5% in terms
of the molecular mass of no more 500, a main molecular-mass peak of 7500, a
glass transition temperature Tg of 62 C, a Mw/Mn ratio of 5.1, and an acid
value of 2.3 mgKOH/g. The temperature was 112 C at which the apparent
viscosity being 103 Pa-s. The resin had substantially no THF-insoluble
matter.
Synthesis Example 2
Synthesis of Linear Polyester Resin
A linear polyester resin AX1-2 was prepared by a similar reaction as
that ofAXl-1 of the synthesis example 1, followed by cooling to room
temperature and milling except that the polycondensation catalyst was
changed into titanyl bis(triethanolaminate).
Synthesis of non-Linear Polyester Resin
195
CA 02628003 2008-10-21
51216-9
A linear polyester resin AX2-2 was prepared by a similar reaction as
that ofAX2-1 of the synthesis example 1, followed by cooling to room
temperature and milling except that the polycondensation catalyst was
changed into titanyl bis(triethanolaminate).
Synthesis of Toner Binder TB2
The inventive toner binder resin TB2 was prepared by
powder-mixing 500 parts of the polyester AXl-2 and 500 parts of the
polyester AX2-2 for 5 minutes using a Henschel mixer.
The resulting toner hinder resin TB2 had a content of 3.0% in terms
to of the molecular mass of no more 500, a main molecular-mass peak of 8000, a
glass transition temperature Tg of 62 C, a Mw/Mn ratio of 4.7, and an acid
value of 0.5 mgKOH/g. The temperature was 116 C at which the apparent
viscosity was 103 Pa-s by the flow tester. The resin had substantially no
THF-insoluble matter.
Synthesis Example 3
Synthesis of Comparative Linear Polyester Resin
The reaction was carried out in the same manner as that ofAXl-1 of
synthesis example 1, except that the polycondensation catalyst was changed
into titanium tetraisopropoxide. There arose such a problem that the
reaction was stopped on the way due to catalysis deactivation and the
distillation of generated water was also stopped, thus 2 parts of titanium
tetraisopropoxide was added four times during the reaction thereby to obtain
196
CA 02628003 2008-04-30
a comparative linear polyester resin CAX1-1.
Synthesis of Comparative non-Linear Polyester Resin
The reaction was carried out in the same manner as that of AX2-1 in
synthesis example 1, except that the polycondensation catalyst was changed
into titanium tetraisopropoxide. The reaction was carried out under normal
pressure for 16 hours and under a reduced pressure for 8 hours. The
reaction velocity was slow, thus 2 parts of titanium tetraisopropoxide was
added three times during the reaction thereby to obtain a comparative
non-linear polyester resin CAX2-1.
1o Synthesis of Comparative Toner Binder Resin CTB1
Four hundred parts of the polyester CAX1-1 and 600 parts of the
polyester CAX2-1 were melted-kneaded using a continuous kneader at a
jacket temperature of 150 C and a residence time of 3 minutes. The melted
resin was cooled to 30 C over 4 minutes using a steel-belt cooler, then milled
to prepare a comparative toner binder resin CTB1. The toner binder CTB1
was a resin of intense purplish brown.
The resulting toner binder resin CTB1 had a content of 5.1% in
terms of the molecular mass of no more 500, a main molecular-mass peak of
9200, a glass transition temperature Tg of 71 C, a Mw/Mn ratio of 4.6, and
an acid value of 10.0 mgKOH/g. The temperature was 117 C at which the
apparent viscosity was 103 Pas by a flow tester. The resin had
substantially no THF-insoluble matter, and was used as a toner binder
197
CA 02628003 2008-10-21
51216-9
CTB 1.
Synthesis Example 4
Synthesis of Modified Polyester Resin
Five hundred and forty-nine parts of an adduct of bisphenol A with 2
moles of propylene oxide, 20 parts of an adduct of bisphenol A with 3 moles of
propylene oxide, 133 parts of an adduct of bisphenol A with 2 moles of
ethylene oxide, 10 parts of an adduct of phenol novolac (average
polymerization degree: about 5) with 5 moles of ethylene oxide, 252 parts of
terephthalic acid, 19 parts of isophthnlic acid, 10 parts of trimellitic
anhydride, and 2 parts of titanium dihydroxy bis(diethanolaminate) as a
condensation catalyst were poured into a reactor vessel equipped with a
condenser, a stirrer and a nitrogen gas inlet, and the mixture was allowed to
react at 230 C for 10 hours under nitrogen gas flow while distilling away the
water generated in the reaction. Then the reactant was allowed to react
under a reduced pressure of 5 to 20 mmHg till the acid value came to 2
mgKOH/g or less. Then 50 parts of trimellitic anhydride was added to the
reactant, which was allowed to react for 1 hour under normal pressure
followed by under a reduced pressure of 20 to 40 mmHg, then 25 parts of
bisphenol A diglycidyl ether was added when the softening temperature came
to 105 C, the reactant was taken out when the softening temperature came
to 150 C. After cooling to room temperature, the reaction product was
milled, consequently, a modified polyester resin AYl-1 was obtained.
198
CA 02628003 2008-04-30
The resulting AY1-1 had a content of 2.8% in terms of the molecular
mass of no more 500, a main molecular-mass peak of 6900, a glass transition
temperature Tg of 64 C, a Mw/Mn ratio of 5.5, and an acid value of 8.1
mgKOH/g. The temperature was 102 C at which the apparent viscosity
was 103 Pa=s by a flow tester. The resin had substantially no THF-insoluble
matter, and was used as a toner binder resin TB3.
Synthesis Example 5
Synthesis of Comparative Modified Polyester Resin
A comparative modified polyester resin CAYl-2 was prepared in the
same manner as synthesis example 4, except that the polycondensation
catalyst was changed into titanium tetrabutoxide.
The resulting CAY1-2 had a content of 6.1% in terms of the
molecular mass of no more 500, a main molecular-mass peak of 10700, a
glass transition temperature Tg of 74 C, a Mw/Mn ratio of 7.2, and an acid
value of 10.6 mgKOH/g. The temperature was 122 C at which the apparent
viscosity was 103 Pa=s by a flow tester. The resin had a THF-insoluble
content of 12%, and was used as a toner binder resin CTB2.
Synthesis Example of Resin Charge Control Agent
Synthesis Example 1
Three hundred and fifty parts of 3,4-dichlorophenylmaleimide and
100 parts of 2-acrylamide-2-methylpropanesulfonic acid were copolymerized
for 8 hours in dimethylformamide (DMF) at a temperature of below the
199
CA 02628003 2008-04-30
boiling point using di-t-butylperoxide as an initiator. Then 500 parts of
n-butylacrylate and 50 parts of styrene were added to the reactant, and the
mixture was graft-polymerized for 4 hours using di-t-butylperoxide as an
initiator, followed by distilling away the DMF under vacuum-drying, thereby
a resin charge control agent 1 was prepared that had a volume resistivity of
10.5 Log SZ=cm and a mass average molecular mass of 1x104, the temperature
was 96 C at which the apparent viscosity was 104 Pa-s, and the content was
6% that corresponding to components having a mass average molecular mass
of lx 103 or less.
Synthesis Example 2
Six hundred parts of m-nitrophenylmaleimide and 100 parts of
perfluorooctane sulfonic acid were copolymerized for 8 hours in DMF at a
temperature of below the boiling point using di-t-butylperoxide as an
initiator.
Then 250 parts of 2-ethylacrylate and 30 parts of styrene were added to the
reactant, and the mixture was graft-polymerized for 4 hours using
di-t-butylperoxide as an initiator, followed by distilling away the DMF under
vacuum-drying, thereby a resin charge control agent 2 was prepared that
had a volume resistivity of 9.5 Log 0=cm and a mass average molecular mass
of 5.5x103, the temperature was 85 C at which the apparent viscosity was
104 Pa-s, and the content was 8% that corresponding to components having a
mass average molecular mass of lx 103 or less.
Synthesis Example 3
200
CA 02628003 2008-04-30
Five hundred parts of 3,4-dichlorophenylmaleimide and 150 parts of
2-acrylamide-2-methylpropanesulfonic acid were copolymerized for 8 hours
in dimethylformamide (DMF) at a temperature of below the boiling point
using di-t-butylperoxide as an initiator. Then 350 parts of n-butylacrylate
and 250 parts of alpha-methylstyrene were added to the reactant, and the
mixture was graft-polymerized for 4 hours using di-t-butylperoxide as an
initiator, followed by distilling away the DMF under vacuum-drying, thereby
a resin charge control agent 3 was prepared that had a volume resistivity of
11.5 Log K2-cm and a mass average molecular mass of 9.6x104, the
temperature was 110 C at which the apparent viscosity was 104 Pa-s, and
the content was 5% that corresponding to components having a mass average
molecular mass of 1x103 or less.
Synthesis Example 4
Four hundred parts of 3,4-dichlorophenylmaleimide and 200 parts of
perfluorooctane sulfonic acid were copolymerized for 8 hours in DMF at a
temperature of below the boiling point using di-t-butylperoxide as an
initiator.
Then 300 parts of n-butylacrylate was added to the reactant, and the mixture
was graft-polymerized for 4 hours using di-t-butylperoxide as an initiator,
followed by distilling away the DMF under vacuum-drying, thereby a resin
charge control agent 4 was prepared that had a volume resistivity of 10.4 Log
S2-cm and a mass average molecular mass of 1.5x104, the temperature was
105 C at which the apparent viscosity was 104 Pa=s, and the content was 6%
201
CA 02628003 2008-04-30
that corresponding to components having a mass average molecular mass of
1x103 or less.
Synthesis Example 5
Four hundred parts of 3,4-dichlorophenylmaleimide and 100 parts of
2-acrylamide-2-methylpropanesulfonic acid were copolymerized for 8 hours
in DMF at a temperature of below the boiling point using di-t-butylperoxide
as an initiator. Then 500 parts of n-butylacrylate and 100 parts of styrene
were added to the reactant, and the mixture was graft-polymerized for 4
hours using di-t-butylperoxide as an initiator, followed by distilling away
the
1o DMF under vacuum-drying, thereby a resin charge control agent 5 was
prepared that had a volume resistivity of 9.3 Log 0=cm and a mass average
molecular mass of 3x104, the temperature was 101 C at which the apparent
viscosity was 104 Pa-s, and the content was 6% that corresponding to
components having a mass average molecular mass of lx103 or less.
Example 73
Colorants were treated by the following formulations.
Yellow colorant formulation:
binder resin TB1 100 parts
C.I. pigment yellow 180 100 parts
Red colorant formulation:
binder resin TB1 100 parts
C.I. pigment red 122 100 parts
202
CA 02628003 2008-04-30
Blue colorant formulation:
binder resin TB1 100 parts
C.I. pigment blue 15.3 100 parts
Black colorant formulation:
binder resin TB1 100 parts
carbon black 100 parts
The materials were respectively mixed in a Henschel mixer, the
mixture was subjected to an air-cooling two-roll mill and melted-kneaded for
minutes. Then the melted-kneaded material was calendered and cooled,
10 followed by coarsely milled by a hammer mill thereby to prepare colorants
treated with binder resins.
Toners were prepared by the following formulations.
Yellow toner formulation:
binder resin TB1 91 parts
15 yellow colorant treated with binder resin TB1 12 parts
resin charge control agent 1 3 parts
Magenta toner formulation:
binder resin TB1 92 parts
red colorant treated with binder resin TB1 10 parts
resin charge control agent 1 3 parts
Cyan toner formulation:
binder resin TB1 94 parts
203
CA 02628003 2008-04-30
blue colorant treated with binder resin TB16 parts
resin charge control agent 1 3 parts
Black toner formulation:
binder resin TB1 90 parts
black colorant treated with binder resin TB1 12 parts
blue colorant treated with binder resin TB12 parts
resin charge control agent 1 3 parts
The materials were respectively mixed in a Henschel mixer, the
mixture was subjected to a roll mill heated to 110 C and melted-kneaded for
30 minutes. Then the kneaded material was cooled, followed by coarsely
milled by a hammer mill and finely milled by an air-jet mill, then fine
powders were removed by an air classifier thereby to prepare toners of
respective colors. TI/T2 was 1.16 in the binder resin TB1 and the resin
charge control agent 1.
The resulting toners were mixed with the following additives based
on 100 parts of respective toners to prepare one-component colorants.
hydrophobic silica 2.5 parts
primary particle diameter: 0.02 m
hydrophobic titanium oxide 0.8 parts
primary particle diameter: 0.015 m, specific surface area: 90
mg/Cm2
The resulting one-component developers were set in a commercially
204
CA 02628003 2008-04-30
available digital full-color printer (IPSiO Color 6500, by Ricoh Co.) and
images were formed. The resulting images were clear and far from defects
like background smear. The developing roller was visually observed and the
toner thin layer was confirmed to be uniform on the roller. The charge
amount on the developing roller by an absorbing process was measured to be
-35 gC/g in the yellow developer, -30 C/g in the magenta developer, -31
gC/g in the cyan developer and -32 C/g in the black developer. Images
were similarly formed under a high temperature and high humidity
condition of 27 C and 80% RH and a low temperature and low humidity
1o condition of 10 C and 15% RH, consequently, excellent images were formed
under both conditions without significant difference. A durability test was
conducted such that a full-color image was formed continuously under
normal temperature, low temperature and low humidity, high temperature
and high humidity, and normal temperature conditions on a total of 40000
sheets, consequently, there appeared no significant difference on fixed
images,
and 40000th image was clear with no background smear.
The developing roller was visually observed and confirmed that the
toner thin layer underwent no significant change on the roller, the charge
amount of developers was stable such as -31 C/g in the yellow developer,
-29 C/g in the magenta developer, -29 gC/g in the cyan developer and -27
C/g in the black developer. No filming was observed on the developing
roller, the blades and the photoconductor.
205
CA 02628003 2008-04-30
Example 74
Colorants were treated by the following formulations.
Yellow colorant formulation:
binder resin TB2 100 parts
C.I. pigment yellow 180 100 parts
Red colorant formulation:
binder resin TB2 100 parts
C.I. pigment red 146 100 parts
Blue colorant formulation:
binder resin TB3 100 parts
C.I. pigment blue 15.3 100 parts
Black colorant formulation:
binder resin TB3 100 parts
carbon black 100 parts
The materials were respectively mixed in a Henschel mixer, the
mixture was subjected to an air-cooling two-roll mill and melted-kneaded for
15 minutes. Then the melted-kneaded material was calendered and cooled,
followed by coarsely milled by a hammer mill thereby to prepare colorants
treated with binder resins.
Toners were prepared by the following formulations.
Yellow toner formulation:
binder resin TB2 91 parts
206
CA 02628003 2008-04-30
yellow colorant treated with binder resin TB2 12 parts
resin charge control agent 2 3 parts
Magenta toner formulation:
binder resin TB2 92 parts
red colorant treated with binder resin TB2 10 parts
resin charge control agent 2 3 parts
Cyan toner formulation:
binder resin TB3 94 parts
blue colorant treated with binder resin TB36 parts
resin charge control agent 3 3 parts
Black toner formulation:
binder resin TB3 90 parts
black colorant treated with binder resin TB3 12 parts
blue colorant treated with binder resin TB32 parts
resin charge control agent 4 3 parts
The materials were respectively mixed in a Henschel mixer, the
mixture was subjected to a two-axis continuous kneader heated to 80 C and
melted-kneaded. Then the kneaded material was cooled, followed by
coarsely milled by a hammer mill and finely milled by an air-flow mill, then
fine particles were removed by an air classifier thereby to prepare toners of
respective colors. T1/T2 was 1.11 in the binder resin TB2 and the resin
charge control agent 2, T1/T2 was 1.15 in the binder resin TB3 and the resin
207
CA 02628003 2008-04-30
charge control agent 3, and T1/T2 was 1.21 in the binder resin TB3 and the
resin charge control agent 4.
The resulting toners were mixed with the following additives based
on 100 parts of respective toners.
hydrophobic silica 2.1 parts
primary particle diameter: 0.02 gm
hydrophobic titanium oxide 1.0 parts
primary particle diameter: 0.015 m, specific surface area: 120
mg/cm2
Two-component developers were prepared by way of blending the
respective toners of 6 parts and a silicone-resin coated carrier of 94 parts.
The resulting two component developers were set in commercially available
digital full-color printer (IPSiO Color 7100, by Ricoh Co.) and images were
formed. The resulting images were clear without background smear. No
problem appeared on images and charging under high temperature and high
humidity condition as well as low temperature and low humidity condition.
A durability test was conducted such that a full-color image was formed on
10000 sheets, consequently, there appeared no problem in images, and there
existed no scattering within the apparatus and no deposition on the
photoconductor.
Comparative Example 25
Colorants were treated by the following formulations.
208
CA 02628003 2008-04-30
Yellow colorant formulation:
binder resin CTB1 100 parts
C.I. pigment yellow 180 100 parts
Red colorant formulation:
binder resin CTB1 100 parts
C.I. pigment red 122 100 parts
Blue colorant formulation:
binder resin CTB2 100 parts
C.I. pigment blue 15.3 100 parts
Black colorant formulation:
binder resin CTB2 100 parts
carbon black 100 parts
The materials were respectively mixed in a Henschel mixer, the
mixture was subjected to an air-cooling two-roll mill and melted-kneaded for
15 minutes. Then the melted-kneaded material was calendered and cooled,
followed by coarsely milled by a hammer mill thereby to prepare colorants
treated with binder resins.
Toners were prepared by the following formulations.
Yellow toner formulation:
binder resin CTB1 91 parts
yellow colorant treated with binder resin CTB1 12 parts
resin charge control agent 1 3 parts
209
CA 02628003 2008-04-30
Magenta toner formulation:
binder resin CTB1 92 parts
red colorant treated with binder resin CTB1 10 parts
resin charge control agent 3 3 parts
Cyan toner formulation:
binder resin CTB2 94 parts
blue colorant treated with binder resin CTB2 6 parts
resin charge control agent 5 3 parts
Black toner formulation=
binder resin CTB2 90 parts
black colorant treated with binder resin CTB2 12 parts
blue colorant treated with binder resin CTB2 2 parts
resin charge control agent 5 3 parts
The materials were respectively mixed in a Henschel mixer, the
mixture was subjected to a roll mill heated to 100 C and melted-kneaded for
minutes. Then the kneaded material was cooled, followed by coarsely
milled by a hammer mill and finely milled by an air-jet mill, then fine
powders were removed by an air classifier thereby to prepare toners of
respective colors. T1/T2 was 1.20 in the binder resin CTB1 and the resin
20 charge control agent 1, T1/T2 was 1.11 in the binder resin CTB1 and the
resin charge control agent 3, and T1/T2 was 1.34 in the binder resin CTB2
and the resin charge control agent 5.
210
CA 02628003 2008-04-30
The resulting toners were mixed with the following additives based
on 100 parts of respective toners to prepare one-component colorants.
hydrophobic silica 2.5 parts
primary particle diameter: 0.02 gm
hydrophobic titanium oxide 0.8 parts
primary particle diameter: 0.015 gm, specific surface area: 90
mg/Cm2
The resulting one-component developers were set in a commercially
available digital full-color printer (IPSiO Color 6500, by Ricoh Co.) and
images were formed. The resulting images were clear and far from defects
like background smear. The developing roller was visually observed and the
toner thin layer was confirmed to be uniform on the roller. The charge
amount on the developing roller by an absorbing process was measured to be
-43 gC/g in the yellow developer, -36 gC/g in the magenta developer, -38
gC/g in the cyan developer and -35 gC/g in the black developer. When
images were formed under a high temperature and high humidity condition
of 27 C and 80% RH, the images included irregularity or mutter. When
images were formed under a low temperature and low humidity condition of
10 C and 15% RH, the images were thin and of low density. When a
durability test was conducted with forming full-color images continuously
under normal temperature, low temperature and low humidity, high
temperature and high humidity, and normal temperature conditions,
211
CA 02628003 2008-10-21
51216-9
problems appeared on the images, such as background smear, dusts and
streaks.
When the developing roller was visually observed at that time,
streaks had occurred circumferentially in the toner thin film on the
photoconductor. The measurement of charge amount of the developers
revealed the degradation such as -28 C/g in the yellow developer, -22 C/g
in the magenta developer, -25 C/g in the cyan developer and -21 C/g in
the black developer.
Synthesis of Titanium- Containing Catalyst
A mixture of 1700 parts of titanium diisopropoxy
bis(triethanolaminate) and 130 parts of deionized water was poured into a
reactor vessel equipped with a condenser, a stirrer and a nitrogen gas inlet
capable of bubbling a liquid therein, the mixture was heated gradually to
90 C and allowed to react at 90 C for 4 hours (hydrolysis) while bubbling the
liquid with nitrogen gas thereby to prepare titanium dihydroxy
bis(triethanolaminate).
Other titanium-containing catalysts in Examples below, available for
the present invention, may be prepared in similar synthetic processes.
Synthesis 1 of Linear Polyester Resin
Forty hundred and thirty parts of an adduct of bisphenol A with 2
moles of P0, 300 parts of an adduct of bisphenol A with 3 moles of PO, 257
parts of terephthalic acid, 65 parts of isophthalic acid, 10 parts of maleic
212
CA 02628003 2008-10-21
51216-9
anhydride and 2 parts of titanium dihydroxy bis(triethanolaminate) as a
condensation catalyst were poured into a reactor vessel equipped with a
condenser, a stirrer and a nitrogen gas inlet, and the mixture was allowed to
react at 220 C for 10 hours under nitrogen gas flow while distilling away the
water generated in the reaction. Then the reactant was allowed to react
under a reduced pressure of 5 to 20 mmHg and taken out when the acid
value came to 5 mgKOH/g. After cooling to room temperature, the reaction
product was milled, consequently, a linear polyester resin AX1-1 was
obtained.
The resultingAX1-1 contained no THF-insoluble matter, and had an
acid value of 7 mgKOH/g, a hydroxyl value of 12 mgKOH/g, a glass transition
temperature Tg of 60 C, a number average molecular mass Mn of 6940, and
a peak top molecular mass Mp of 19100. The rate of the molecular mass of
no more than 1500 was 1.2%.
Synthesis 1 of non-Linear Polyester Resin
Three hundred and fifty parts of an adduct of bisphenol A with 2
moles of EO, 326 parts of an adduct of bisphenol A with 3 moles of PO, 278
parts of terephthalic acid, 40 parts of phthalic anhydride and 2 parts of
titanium dihydroxy bis(triethanolaminate) as a condensation catalyst were
poured into a reactor vessel equipped with a condenser, a stirrer and a
nitrogen gas inlet, and the mixture was allowed to react at 230 C for 10
hours under nitrogen gas flow while distilling away the water generated in
213
CA 02628003 2008-04-30
the reaction. Then the reactant was allowed to react under a reduced
pressure of 5 to 20 mmHg, cooled to 180 C when the acid value came to 2
mgKOHIg or less, 62 parts of trimellitic anhydride was added, then the
mixture was allowed to react under normal pressure of sealed atmosphere
for 2 hours. After cooling to room temperature, the reaction product was
milled, consequently, a non-linear polyester resin AX2-1 was obtained.
The resultingAX2-1 contained no THF-insoluble matter, and had an
acid value of 35 mgKOH/g, a hydroxyl value of 17 mgKOH/g, a glass
transition temperature Tg of 69 C, a number average molecular mass Mn of
3920, and a peak top molecular mass Mp of 11200. The rate of the
molecular mass of no more than 1500 was 0.9%.
Synthesis 1 of Toner Binder
Four hundred parts of the AX1-1 and 600 parts of the AX2-1 were
melted-kneaded using a continuous kneader at a jacket temperature of
150 C and a residence time of 3 minutes. The melted resin was cooled to
30 C over 4 minutes using a steel-belt cooler, then milled to prepare an
inventive toner binder (resin A).
Synthesis 2 of Comparative Linear Polyester Resin
The reaction was carried out in the same manner as that ofAXl-1 of
synthesis example 1, except that the polycondensation catalyst was changed
into titanium tetraisopropoxide. There arose such a problem that the
reaction was stopped on the way due to catalysis deactivation and the
214
CA 02628003 2008-04-30
distillation of generated water was also stopped, thus 2 parts of titanium
tetraisopropoxide was added four times during the reaction thereby to obtain
a comparative linear polyester resin CAX1-1.
The resulting CAX1-1 contained no THF-insoluble matter, and had
an acid value of 7 mgKOH/g, a hydroxyl value of 12 mgKOH/g, a glass
transition temperature Tg of 58 C, a number average molecular mass Mn of
6220 and a peak top molecular mass Mp of 18900. The rate of the
molecular mass of no more than 1500 was 2.2%.
Synthesis 2 of Comparative non-Linear Polyester Resin
The reaction was carried out in the same manner as that ofAX2-1 in
synthesis example 1, except that the polycondensation catalyst was changed
into titanium tetraisopropoxide. The reaction was carried out under normal
pressure for 16 hours and under a reduced pressure for 8 hours. The
reaction velocity was slow, thus 2 parts of titanium tetraisopropoxide was
added three times during the reaction thereby to obtain a comparative
non-linear polyester resin CAX2-1.
The resulting CAX2-1 contained no THF-insoluble matter, and had
an acid value of 34 mgKOH/g, a hydroxyl value of 16 mgKOH/g, a glass
transition temperature Tg of 68 C, a number average molecular mass Mn of
3420 and a peak top molecular mass Mp of 12100. The rate of the
molecular mass of no more than 1500 was 2.1%.
Synthesis 2 of Comparative Toner Binder
215
CA 02628003 2008-10-21
51216-9
Four hundred parts of the CAX1-1 and 600 parts of the CAX2-1 were
melted-kneaded using a continuous kneader at a jacket temperature of
150 C and a residence time of 3 minutes. The melted resin was cooled to
30 C over 4 minutes using a steel-belt cooler, then milled to prepare a
comparative toner binder (resin B). The resin B was of intense purplish
brown.
Synthesis 3 of Linear Polyester Resin
A linear polyester resin AXl-2 was prepared by a similar reaction as
that ofAXl-1 of the synthesis example 1, followed by cooling to room
temperature and milling except that the polycondensation catalyst was
changed into titanyl bis(triethanolaminate).
The resultingAX1-2 contained no THF-insoluble matter, and had an
acid value of 8 mgKOH/g, a hydroxyl value of 10 mgKOH/g, a glass transition
temperature Tg of 60 C, a number average molecular mass Mn of 6820 and a
peak top molecular mass Mp of 20180. The rate of the molecular mass of no
more than 1500 was 1.1%.
Synthesis 3 of non-Linear Polyester Resin
A linear polyester resin AX2-2 was prepared by a similar reaction as
that of AX2-1 of the synthesis example 1, followed by cooling to room
temperature and milling except that the polycondensation catalyst was
changed into titanyl bis(triethanolaminate).
The resulting AX2-2 contained no THF-insoluble matter, and had an
216
CA 02628003 2008-04-30
acid value of 33 mgKOH/g, a hydroxyl value of 14 mgKOH/g, a glass
transition temperature Tg of 70 C, a number average molecular mass Mn of
4200 and a peak top molecular mass Mp of 11800. The rate of the molecular
mass of no more than 1500 was 0.8%.
Synthesis 3 of Toner Binder
The inventive toner binder resin (resin C) was prepared by
powder-mixing 500 parts of the AX1-2 and 500 parts of the AX2-2 for 5
minutes using a Henschel mixer.
Production Example of Toner A
Formulation
resin A 100 parts
magenta pigment (C.I. Pigment Red 269) 5 parts
charge control agent (E-84) *1) 2 parts
*1) by Orient Chemical Co.
Among the ingredients described above, the pigment and the
polyester resin, and also pure water were blended in a mass ratio of 1:1:0.5
and kneaded using twin rolls. The mixture was kneaded at 70 C, then the
water was evaporated by raising the roll temperature to 120 C thereby to
prepare a master batch.
Using the prepared master batch, the ingredients were mixed based
on the formulation described above, melted-kneaded at 50 C for 40 minutes
using twin rolls and cooled, followed by coarsely milled by a hammer mill and
217
CA 02628003 2008-04-30
finely milled by an air-jet mill, then the resulting fine powders were
classified by an air classifier thereby to prepare a base toner having a
volume
average particle diameter D4 of 6.8 gm. In addition, 0.15 part of zinc
stearate (by Sakai Chemical Industry Co.), 1 part of hydrophilic silica (by
Clariant Japan K.K.) and 1 part of hydrophobic titanium oxide (by Tayca Co.)
were added and mixed by a mixer to prepare toner A.
The resulting toner A had a volume average particle diameter Dv of
6.8 gm, a ratio Dv/Dn of 1.38, and shape factors SF-1, SF-2 of 151, 142.
Production Example of Toner B
Toner B was prepared in the same manner as the production
example of toner A except that the magenta pigment was changed into that
shown below.
yellow pigment (C.I. Pigment Yellow 180) 5 parts
The resulting toner B had a volume average particle diameter Dv of
6.8 gm, a ratio Dv/Dn of 1.35, and shape factors SF-1, SF-2 of 150, 141.
Production Example of Toner C
Toner C was prepared in the same manner as the production
example of toner A except that the magenta pigment was changed into that
shown below.
yellow pigment (C.I. Pigment Yellow 155) 5 parts
The resulting toner C had a volume average particle diameter Dv of
6.8 gm, a ratio Dv/Dn of 1.38, and shape factors SF-1, SF-2 of 158, 150.
218
CA 02628003 2008-04-30
Production Example of Toner D
Toner D was prepared in the same manner as the production
example of toner A except that the magenta pigment was changed into that
shown below.
magenta pigment (C.I. Pigment Red 184 (mixture of C.I. Pigment
Red 146 and C.I. Pigment Red 147)) 5 parts
The resulting toner D had a volume average particle diameter Dv of
6.8 m, a ratio Dv/Dn of 1.36, and shape factors SF-1, SF-2 of 150, 142.
Production Example of Toner E
Toner E was prepared in the same manner as the production
example of toner A except that the magenta pigment was changed into that
shown below.
yellow pigment (C.I. Pigment Yellow 17) 5 parts
The resulting toner E had a volume average particle diameter Dv of
6.8 m, a ratio Dv/Dn of 1.35, and shape factors SF-1, SF-2 of 154, 148.
Production Example of Toner F
Toner F was prepared in the same manner as the production
example of toner A except that the magenta pigment was changed into that
shown below.
cyan pigment (C.I. Pigment Blue 15:2) 5 parts
The resulting toner F had a volume average particle diameter Dv of
6.8 m, a ratio Dv/Dn of 1.36, and shape factors SF-1, SF-2 of 151, 145.
219
CA 02628003 2008-04-30
Production Example of Toner G
Toner G was prepared in the same manner as the production
example of toner A except that the resin A was changed into that shown
below.
resin B 100 parts
The resulting toner G had a volume average particle diameter Dv of
6.8 gm, a ratio Dv/Dn of 1.32, and shape factors SF-1, SF-2 of 153, 149.
Production Example of Toner H
Toner H was prepared in the same manner as the production
example of toner B except that the resin A was changed into that shown
below.
resin B 100 parts
The resulting toner H had a volume average particle diameter Dv of
6.8 gm, a ratio Dv/Dn of 1.33, and shape factors SF-1, SF-2 of 159, 148.
Production Example of Toner J
Toner J was prepared in the same manner as the production example
of toner A except that the wax shown below was added.
Carnauba wax 5 parts
The resulting toner J had a volume average particle diameter Dv of
6.8 gm, a ratio Dv/Dn of 1.32, and shape factors SF-1, SF-2 of 152, 145.
Production Example of Toner K
Toner K was prepared in the same manner as the production
220
CA 02628003 2008-04-30
example of toner B except that the wax shown below was added.
Carnauba wax 5 parts
The resulting toner K had a volume average particle diameter Dv of
6.8 m, a ratio Dv/Dn of 1.37, and shape factors SF-1, SF-2 of 151, 149.
Production Example of Toner L
Toner L was prepared in the same manner as the production
example of toner F except that the wax shown below was added.
Carnauba wax 5 parts
The resulting toner L had a volume average particle diameter Dv of
6.8 m, a ratio Dv/Dn of 1.34, and shape factors SF-1, SF-2 of 155, 144.
Production Example of Toner M
Toner M was prepared in the same manner as the production
example of toner A except that the resin A was changed into that shown
below.
resin C 100 parts
The resulting toner M had a volume average particle diameter Dv of
6.8 m, a ratio Dv/Dn of 1.31, and shape factors SF-1, SF-2 of 159, 142.
Evaluation Process
(1) Color Difference in L*a*b* Color Specification System
Using an image forming apparatus, respective image densities at a
100% image-area ratio in monochrome mode of yellow (Y), magenta (M) and
cyan (C) were measured, and intermediate colors of blue (B), green (G) and
221
CA 02628003 2008-04-30
red (R) were measured by color-mixing 50% of yellow (Y), magenta (M) or
cyan (C). The respective image densities were measured using X-Rite 938
(by X-Rite Inc.) in a condition of observable eyespot 2 at observing light
D50
(JIS Z-8720 (1983)), then a* and b* where the image density ID (-Log
Reflectivity) being "1.0" was measured. The results are shown in FIGs. 11
to 15. FIG. 13 is a partially enlarged view of FIG. 12, and FIG. 15 is a
partially enlarged view of FIG. 14.
When toners are overlapped for two or more colors, images are
formed firstly by magenta, followed by cyan, and followed by yellow.
Examples 75 to 78 and Comparative Examples 26 to 29
The toner kits to evaluate toners of Examples 75 to 78 and
Comparative Examples 26 to 29 are shown in Table 4.
Table4
magenta toner yellow toner cyan toner
Ex. 75 toner A toner B toner F
Ex. 76 toner A toner C toner F
Ex. 77 toner J toner K toner L
Ex. 78 toner M toner B toner F
Com. Ex. 26 toner G toner B toner F
Com. Ex. 27 toner A toner H toner F
Com. Ex. 28 toner D toner B toner F
Com. Ex. 29 toner A toner E toner F
The evaluation results are shown in Tables 5, 6 and FIGs. 11 to 15.
In the figure where a*b* is plotted in L*a*b* color specification system, the
wider area enclosed by six colors of Y/RJM/B/C/G indicates that color
reproducibility is more excellent.
222
CA 02628003 2008-04-30
FIGs. 12 and 13 demonstrate that Examples 75 and 78 definitely
represent wider color reproducible area in terms of R and M compared to
Comparative Example 26 and 27, in particular the color reproducible area is
excellently wide for R.
On the contrary, Comparative Example 26 represents a wider color
reproducible area in terms of G/C, however, narrow in terms of R/M.
Comparative Example 27 represents a wide area in terms of M, however,
remarkably narrow in terms of G/Y/R.
As such, it is clear that Example 75 and 78 represent color
reproducibility over entire regions, in particular wide in R.
It is also clear from FIGs. 14 and 15 that Example 76 represents a
wide area particularly in R without sacrificing the other regions, and
Example 77 is not as wide as Example 76 in terms of R but wide in terms of
MB.
Image Evaluation
The toners described above and Cu-Zn ferrite carrier (coated with a
silicone resin, average particle diameter= 40 gm) were blended by 5% and
95% as content to prepare two-component developers, which were used to
develop a draft photograph containing flesh color. The development was
carried out on 1000 sheets with full-color mode of 400 dpi using a modified
copier (Imagio Neo C385, by Ricoh Co.), and the developed images were
evaluated visually by 50 persons and ranked in accordance with the
223
CA 02628003 2008-04-30
following criteria. The results are shown in Table 5.
Sensitive Evaluation for Flesh Color Photography
The evaluation results were ranked under the following five steps
with respect to superiority for flesh color on the basis of human visual
inspection.
The evaluation was such as full marks being 100 points, and the
lowest being 0 point, then the points by 50 persons being averaged.
A: very good, 80 points or higher
B: good, 60 to 79 points
C: ordinary, 40 to 59 points
D: bad, 20 to 39 points
E: very bad, 19 points or lower
Table 5
Sensitive Evaluation for Flesh
Color Photography
Ex. 75 B
Ex. 76 B
Ex. 77 A
Ex. 78 B
Com. Ex. 26 D
Com. Ex. 27 D
Com. Ex. 28 C
Com. Ex. 29 C
224
CA 02628003 2008-04-30
Table 6
a* b*
Y -6.79 88.02
R 64.53 47.35
Ex. 75 M 72.17 -3
B 22.3 -41.01
C -28.85 -50.59
G -58 20.15
Y -3 88.3
R 64 51
Ex. 76 M 72.17 -3
B 23.1 -41.2
C -28.6 -50.4
G -57 25
Y -6.8 88.3
R 62.3 44.2
Ex. 77 M 69 -10
B 20 -46
C -28.5 -50.23
G -56 19
Y -3 88.5
R 63 52
Ex. 78 M 72.12 -3
B 22 -40.8
C -28 -49.9
G -55 26
Y -6.6 89
R 61 44
Com. Ex. 26 M 70.2 -0.2
B 21 -38
C -30 -49
G -58.2 20.3
Y -4 85
R 61 44
Com. Ex. 27 M 72.17 -3
B 22.56 -41.16
C -28.1 -50.6
G -55 20
Y -6.6 88.1
R 62 45
Com. Ex. 28 M 70.2 -0.2
B 20 -38
C -28.75 -50.48
G -58.2 20.3
Y -4 86
R 60 45
Com. Ex. 29 M 72.17 -3
B 22.56 -41.16
C -28.1 -50.6
G -55 20
225
CA 02628003 2008-04-30
Industrial Applicability
The inventive toner may exhibit excellent blocking resistance and
low temperature fixability, provide high quality images stably with time
under such conditions as high temperature and high humidity, low
temperature and low humidity, or outputting larger area images, without
such problems as decreasing charging capacity due to firm adhesion of toners
onto carriers or developing sleeves, therefore, is available as an
electrostatic
image developing toner.
The inventive toner kit may represent wide reproducible regions in
terms of yellow and magenta colors, in particular of intermediate flesh and
red colors, and may also decrease scattering of magenta and yellow toners in
particular, therefore, is available as a kit for developing electrostatic
latent
images.
226