Note: Descriptions are shown in the official language in which they were submitted.
CA 02683004 2009-11-02
50749-18D
1
EXPANDABLE DEVICES FOR TREATMENT
OF FRACTURED OR DISEASED BONE
This is a divisional of Canadian Patent Application
No. 2,222,144 which has a filing date of June 6, 1996, and
claims priority from therein.
This invention relates to improvements in the
surgical treatment of bone conditions of the human and other
animal bone systems and, more particularly, to an inflatable
balloon-like device for use in treating such bone
conditions.
Osteoporosis, avascular necrosis and bone cancer
are diseases of bone that predispose the bone to fracture or
collapse. There are 2 million fractures each year in the
United States, of which about 1.3 million are caused by
osteoporosis, while avascular necrosis and bone cancers are
more rare. These conditions cause bone problems that have
been poorly addressed, resulting in deformities and chronic
complications.
The outcome of many other orthopedic procedures to
treat bone, such as open surgeries involving infected bone,
poorly healing bone or bone fractured by severe trauma, can
also be improved. Currently, bone is prepared to receive
materials such as bone graft or bone substitutes by removing
diseased or injured bone using standard tools, usually made
of metal. Gaps between the patient's remaining bone and the
inserted materials delay or prevent healing.
CA 02683004 2009-11-02
2 -
50749-18D
Therapeutic substances like antibiotics and
bone growth factors have not been applied to bone in a
way that optimizes and maintains their contact with the
desired area of bone. Antibiotics, bone growth factors
and other drugs can prevent complications and hasten
repair. They are currently placed as dry powders or
liquids around the treated bone, or else are formulated
into a gel or a degradable plastic polymer and inserted
into areas with defects (holes in the bone). Delivered
iq in this manner, they can be washed away by blood or other
fluids, either immediately or as their carrier degrades.
Also, the amount of therapeutic substance delivered in a
gel or polymer can be limited by the space provided by
the defect.
BACKGROUND OF THE INVENTION
In U.S. Patents 4,969,888 and 5,108,404, an
apparatus and method are disclosed for the fixation of
fractures or other conditions of human and other animal
bone systems, both osteoporotic and non-osteoporotic.
The apparatus and method are especially suitable for, but
not limited to, use in the fixation of vertebral body
compression fractures, Colles fractures and fractures of
the proximal humerus.
The method disclosed in these two patents
includes a series of steps in which a surgeon or health
care provider can perform to form a cavity in fractured
or pathological bone (including but not limited to
osteoporotic bone, osteoporotic fractured metaphyseal and
epiphyseal bone, osteoporotic vertebral bodies, fractured
osteoporotic vertebral bodies, fractures of vertebral
_ ..:. . __.
bodies"due to' tumors especially round cell tumors,_
avascular necrosis of the epiphyses of long bones,
especially avascular necrosis of the proximal femur,
CA 02683004 2009-11-02
- 3 -
50749-18D
distal femur and proximal humerus and defects arising
from endocrine conditions).
The method further includes an incision in the
skin (usually one incision, but a second small incision
may also be required if a suction egress is used)
followed by the placement of a guide pin which is passed
through the soft tissue down to and into the bone.
The method further includes drilling the bone
to be treated to form a cavity or passage in the bone,
and inserting an inflatable balloon-like device into the
cavity or passage. Inflation of the inflatable device
causes a compacting of the cancellous bone and bone
marrow against the inner surface of the cortical wall of
the bone to further enlarge the cavity or passage. The
inflatable device is then deflated and then is completely
removed from the bone. A smaller inflatable device (a
starter balloon) can be used initially, if needed, to
initiate the compacting of the bone marrow and to
commence the formation of the cavity or passage in the
cancellous bone and marrow. After this.has occurred, a
larger, inflatable device is inserted into the cavity or
passage to further compact the bone marrow in all
directions.
A flowable biocompatible fillingmaterial, such
as methylmethacrylate cement or a synthetic bone
substitute, is then directed into the cavity or passage
and allowed to set to a hardened condition to provide
structural support for the bone. Following this.latter
step, the insertion instruments are removed from the body
and the incision in the skin is covered with a bandage.
While the apparatus and method of the above
patents provide an adequate protocol for the fixation of
bone, it has been found that the compacting of the bone
marrow and/or the trabecular bone and/or cancellous bone
against the inner surface of the cortical wall of the
CA 02683004 2009-11-02
- 4 -
50749-18D
bone to be treated can be significantly improved with the
use of inflatable devices that incorporate additional
engineering features not heretofore described and not
properly controlled with prior inflatable devices in such
patents. It has also been found that therapeutic
substances can be delivered with the apparatus and
methods of the above patents in an unexpected way. It
has been additionally found that the apparatus and
methods of the above patents can be adapted in ways not
previously described to improve open surgeries to fix,
fuse or remove bone, as well as to deliver therapeutic
substances during these surgeries. A need has therefore
arisen for improvements in the shape, construction and
size of inflatable devices for use with the foregoing
apparatus and method, as well as for new methods, and the
present invention satisfies such need.
Prior Techniques for the Manufacture of Balloons for
In-Patient Use
A review of the prior art relating to the
manufacture of balloons shows=that a fair amount of
background=information has-been amassed in the formation
of guiding catheters which are introduced into .
cardiovascular systems of patients through the brachial
or femoral arteries':- However, there is a scarcity of
disclosures relating to inflatable devices used in bone,
and none for compacting bone marrow in vertebral bodies
and long bones.
In a dilatation catheter, the catheter is
advanced into a patient until a balloon is properly
positioned across a lesion to be treated. The balloon is
inflated with a radiopaque liquid at pressures above four
atmospheres to compress the plaque of the lesion to
thereby dilate the lumenof the artery. The balloon can
then be deflated, then removed from the artery so that
, . j.. . . . ._ . . . . . ... . .._.... ., .. _.. ...... ... ..... . .. . . .
.
CA 02683004 2009-11-02
- 5 -
50749-18D
the blood flow can be restored through the dilated
artery.
A discussion of such catheter usage technique
is found and clearly disclosed in U.S. Patent 5,163,989.
Other details of angioplasty catheter procedures, and
details of balloons used in such procedures can be found
in U.S. Patents 4,323,071, 4,332,254, 4,439,185,
4,168,224, 4,516,672, 4,538,622, 4,554,929, and
4,616,652.
Extrusions have also been made to form prism
shaped balloons using molds which require very accurate
machining of the interior surface thereof to form
acceptable balloons for angioplastic catheters. However,
this technique of extrusion forms parting lines in the
balloon product which parting lines are limiting in the
sense of providing a weak wall for the balloon itself.
Patent 5,163,989 discloses a mold and technique
.for molding dilatation catheters in which the balloon of
the catheter is free of parting lines. The technique
involves inflating a plastic member~of tubular shape so
as to press it against the innermolding surface which is
heated. Inflatable devices are molde&into the-desired
size and shape, then cooledand deflated to remove it
from the mold. The patent states that, while the balloon
of the present invention is especially suitable for
forming prism-like balloons, it can also be used;for
forming balloons of a wide variety of sizes and shapes.
A particular improvement in the catheter art
with respect to this patent, namely U.S.:Patent
4,706,670, is the use of a coaxial catheter with inner
and outer tubing formed and reinforced by continuous
helical filaments. Such filaments cross each other
causing the shaft of the balloon to become shorter in
length while the moving portion of the-shank becomes
longer in length. By:suitably balancing the lengths and
CA 02683004 2009-11-02
6 -
50749-18D
the angle of the weave of the balloon and moving portions
of the filaments, changes in length can be made to offset
each other. Thus, the position of the inner and outer
tubing can be adjusted as needed to keep the balloon in a
desired position in the blood vessel.
Other disclosures relating to the insertion of
inflatable devices for treating the skeleton of patients
include the following:
U.S. Patent 4,313,434 relates to the fixation
of a long bone by inserting a deflated flexible bladder
into a medullary cavity, inflating the balloon bladder,
sealing the interior of the long bone until healing has
occurred, then removing the bladder and filling the
opening through which the bladder emerges from the long
bone.
U.S. Patent 5,102,413 discloses the way in
which an inflatable bladder is used to anchor a metal rod
for the fixation of a fractured long bone.
Other references which disclose the use of
balloons and cement for anchoring of a prosthesis include
U.S. Patents 5,147,366, 4,892,550, 4,697,584, 4,562,598,
and 4,399,814:
A Dutch-patent, NL 901858, discloses a means
for fracture repair with a cement-impregnated bag which
is inflated into a preformed cavity and allowed to
harden. -
It can be concluded from the foregoing review
of the prior art that there is little or no substantive
information on inflatable devices used to create cavities
in bone.- It does not teach the shape of the balloon
which creates a cavity-that-best supports the bone when
appropriately filled. It does not teach how to prevent
balloons from being-spherical when inflated, when-this is
desired. Current medical -balloons can compress.bone but
are-too-small and generally have the wrong configuration
CA 02683004 2009-11-02
50749-18D
-7-
and are generally not strong.enough to accomplish
adequate cavity formation in either the vertebral bodies
or long bones of the.body.
U.S. Patents 4,969,888 and 5,108,404 disclose a
checker-shaped balloon for compressing cancellous bone,
but does not provide information on how this balloon
remains in its shape when inflated. It also does not
provide methods to deliver an enhanced supply of
therapeutic agent.
U.S. Patent No. 4,892,550 describes an elastic
balloon for anchoring a metal prosthesis inside of a
bone. U.S. Patent No. 4,313,434 describes a deflatable
bladder to substitute for metal rods which are placed
inside the intramedullary cavity of fractured long bones
(thigh, leg and arm) to keep them together while they
heal.
Thus, the need continues for an improved
inflatable device and methods.for use with fractured
and/or pathological bones..
SUMMARY OF THE INVENTION
The present invention is directed.to a-balloon-
like inflatable device or balloon for use in carrying out
the apparatus and method of the.above-mentioned patents
4,969,888 and 5,108,404, and to new methods for using
these devices, and to new uses of the methods.and
devices.- Such inflatable devices, hereinafter sometimes
referred to:as balloons, have.shapes forcompressing
cance,ll:ous_bone and marrow.(also known as medullary bone
or:trabecular bone) against the inner cortex of bones
whether the bones are fractured or not.
In.particular, the present inventionis.
_directed to a balloon for use in treating a bone
predisposed to,fracture or to collapse. Theballoon
compri:ses an inflatable, non-expandable balloon body for
CA 02683004 2009-11-02
- S -
50749-18D
insertion into said bone.' The body has a predetermined
shape and size when substantially inflated sufficient to
compress at least a portion of the inner cancellous bone
to create a cavity in the cancellous bone and to restore
the original position of the outer cortical bone, if
fractured or collapsed. The balloon body is restrained
to create said predetermined shape and size so that the
fully inflated balloon body is prevented from applying
substantial pressure to the inner surface of the outer
cortical bone if said bone is unfractured or uncollapsed.
Substantial pressure is defined herein as pressure
sufficient to displace the cortical cone beyond its
normal configuration.
In addition to the shape of the inflatable
device itself, another aspect of importance is the
construction of the wall or walls of the balloon such
that proper inflation the balloon body is achieved to
provide for optimum compression of all the bone marrow.
The material of the balloon is also desirably chosen so
as to be able to`fold the balloon so that it can be
inserted quickly and easilyinto a bone using a guide pin
and a canula, yet'cari also withstand high pressures when
infiated. The balloon can also include optional ridges
or indentations which are left in the cavity after the
balloon has been removed, to enhance the stability of the
filler. Also; the inflatable device can be made to have
an optional,' built-in suction catheter. This is used to
remove any fat or flu'id extruded from the bone during
balloon inflation in the bone. Also, the balloon body
can be protected from puncture by the cortical bone or
canula by being covered while inside the canula with an
optional protective sleeve of suitable material, such as
Kevlar or PET or other polymer or substance that can
protect the balloon. A main purpose of the inflatable
device, therefore, is the forming or enlarging of a
CA 02683004 2009-11-02
- 9 -
50749-18D
cavity or passage in a bone, especially in, but not
limited to, vertebral bodies.
In one aspect, the invention provides an
improved balloon-like inflatable device for use in
carrying out a surgical protocol of cavity formation in
bones t.o enhance the efficiency of the protocol, to
minimize the time prior to performing the surgery for
which the protocol is designed and to improve the
clinical outcome. These balloons approximate the inner
shape of the bone they are inside of in order to
maximally compress cancellous bone. They have additional
design elements to achieve specific clinical.goals.
Preferably, they are made of inelastic material and kept
in their defined configurations when inflated, by various
restraints, including (but not limited to) use of
inelastic materialsin the balloon body, seams in the
balloon body created by bonding or f-using separate pieces
of material together, or by fusing or bonding together
opposing sides of the balloon body., woven material bonded
inside or outside the balloonbody,.strings or bands
placed at selected points in the balloon body, and,
stacking balloons of similar or, different sizes-or shapes
on top of each other by gluing or by heat fusing them
together. Optional ridges or indentations created by the
foregoing structures, or added on by bonding additional
material, increases stability of the filler. Optional
suction devices, preferably, placed so that if at least
one hole is in the lowest point of the cavity being
formed, will allow the cavity to be cleaned before
filling.
In another aspect, the invention provides new
uses for these balloons, and new methods for their use.
Balloons can be used to deliver therapeutic substances by
coating the balloons with the therapeutic substance
before inserting the balloon into bone. When coated
CA 02683004 2009-11-02
- 10 -
50749-18D
balloons are inflated in bone, the therapeutic substances
are pressed into the cancellous bone while that bone is
being compressed by the balloon. This allows desired
amounts of the therapeutic substance to be delivered
directly to the site of -therapy in a manner that is
maintained over time. The balloons can also be used
during minimally invasive or open surgeries to provide an
improved space for orthopedic implants, bone graft, bone
substitutes, acrylic cements, bone fillers, bone growth
factors, chemotherapeutic agents, antibiotics or other
drugs. The agents inside the bone can be intended to
treat the bone itself or to serve as a reservoir of drug
for a structure nearby, such as an osteosarcoma.
In yet another aspect of the invention, the
balloons can be used to temporarily provide structural
support for a fractured or diseased bone. In this
embodiment, the fractured or diseased bone can be treated
by inflating the balloon at the treatment site and
leaving it in place until the surrounding cortical bone
heals. In other words, the balloon will take the place
of the biocompatible filling material used in previous
methods to support the fractured or diseased bone. The
invention will include a mechanism for sealing the
inflated balloon,outside of the bone cavity, but within
the patient.. -The sealing mechanism can include a metal
or plastic clip, a check valve activated by unscrewing
theinflation tube, a plug for sealing the inner passage
of the balloon or the.like.. Similar.to previous
embodiments,.the balloon will be delivered into the bone
and inflated to compress the innercancellous bone and
create a cavity therein.. The inflated balloon will then
be sealed, e.g., by inserting a plug within the inflation
opening, the inflation tube will be removed from the
patient, and the percutaneous incision will be closed.
The fluid pressure within the balloon provides sufficient
CA 02683004 2009-11-02
50749-18D
-11-
support for the bone to allow the bone to heal. The
balloon can be left in the bone cavity in the inflated
configuration for an amount of time necessary for the
outer cortical bone to completely or at least partially
heal, usually about 1 day to 3 months and preferably
about 6-8 weeks. In this aspect of the invention, the
balloon is providing at least four functions: (1)
realigning the bones; (2) eliminating or at least
reducing diseased inner cancellous bone; (3)
strengthening the outer cortical bone by providing
additional calcium from the compressed inner cancellous
bone which is incorporated into the outer cortical bone
as it heals; and (4) acting as an internal cast while the
cortical bone heals.
After the cortical bone has healed, the
surgeon can access the balloon through the same or
another percutaneous incision, to deflate the,balloon by
removing the clip,_plug or, in the case of a check.valve,
by screwing the inflationtube back into the balloon. In
many cases, the cortical,bone will.hav,e become
sufficiently strengthened through healing with additional
calcium from the.compressed .can,cellous . In these cases,
the, balloon will be removed from,the bone cavity., The
balloon may include a coating, such as.Gel.foam or..an
antibiotic, onits.outer surface ,to stop bleeding,;
prevent infection, minimize bone growth into the balloon
and/or to~:facilitate separation of.the balloon from the
bone when the balloon is deflated. If, however, the
surgeon determines that the cortical bone is still too
weak (e.g., through a bone density scan or other
measurement), appropriate supporting,material, such as
acrylic cements, bone substitutes, bone fillers or bone
growth factors, can be inserted into :the bone cavity
before removal of the balloon.
CA 02683004 2009-11-02
- 12 -
50749-18D
The methods of the above-mentioned patents and
the improvements herein can be applied anywhere in the
skeleton where there is cancellous and/or trabecular
and/or medullary bone.
Among the various embodiments of the present
invention are the following:
1. A doughnut (or torus) shaped balloon with
an optional built-in suction catheter to remove fat and
other products extruded during balloon expansion.
2. A balloon with a spherical outer shape
surrounded by a ring-shaped balloon segment for body
cavity formation.
3. A balloon which is kidney bean shaped in
configuration. Such a balloon can be constructed in a
single layer, or several layers stacked on top of each
other. This embodiment can also be a square or a
rectangle instead of a kidney bean.
4. A spherically shaped balloon approximating
the size of the head of the femur (i.e. the proximal
femoral epiphysis). 'Such a balloon can also be a
hemisphere.
5. A balloon in the shape of a humpbacked
banana or a modified pyramid shape approximating the
configuration ofthe distal end of the radius (i.e. the
distal radial`epiphysis and metaphysis).
~6. A balloon in the shape of a cylindrical
ellipse to approximate the configuration'of either the
medial half or the lateral half of the proximal tibial
epiphysis. Such a balloon can also be constructed to
approximate theconfiguration of both halves of the
proximal tibial epiphysis.
7. A balloon in the shape of-sphere on a base
to approximate the shape of the proximal humeral
epiphysis and metaphysis with a plug to compress
CA 02683004 2009-11-02
50749-18D
-13-
cancellous bone into the diaphysis, sealing it off. Such
an-embodiment can also be a cylinder.
8. A balloon in the shape of a boomerang to
approximate the inside of the femoral head, neck and
lesser trochanter, allowing a procedure to prevent hip
fracture.
9. A balloon in the shape of a cylinder to
approximate the size and shape of the inside of the
proximal humerus or of the distal radius.
10. A balloon device with an optional
suctional device. and
11. Protective sheaths to act as puncture
guard members optionally covering each balloon inside its
catheter.
The present invention, therefore, provides
improved, inflatable devices for creating or'enlarging a
cavity or passage in a bone wherein the devices are
inserted.into the bone. The configuration of each device
is defined by the surrounding cortical bone and adjacent
internal structures, and is designed to occupy about 70-
90% of the volume of the inside of the bone, although
balloons that are as small as about 40% and as large as
about 99% are workable for fractures. In certain cases,
usually avascular necrosis, the balloon size may be as
small as 10% of the cancellous bone volume of the area of
bone being treated, due to the localized nature of the
fracture.or collapse. The fully expanded size and shape
of the balloon.is limited by additional material in
selected portions of the balloon body whose extra
thickness creates a restraint as well as by either
internal or external restraints formed in the device
including, but not limited to, mesh work, a winding or
spooling of material laminated to portions of the balloon
body, continuous or non-continuous strings across the
inside held in place at specific locations by glue inside
CA 02683004 2009-11-02
- 14 -
50749-18D
or by threading them through to the outside and seams in
the balloon body created by bonding two pieces of body
together or by bonding opposing sides of a body through
glue or heat. Spherical portions of balloons may be
restrained by using inelastic materials in the
construction of the balloon body, or may be additionally
restrained as just described. The material of the
balloon is preferably a non-elastic material, such as
polyethylene tetraphthalate (PET), Kevlar or other
patented medical balloon materials. It can also be made
of semi-elastic materials, such as silicone or elastic
material such as latex, if appropriate restraints are
incorporated. The restraints can be made of a flexible,
inelastic high tensile strength material including, but
not limited, to those described in U.S. Patent 4,706,670.
The thickness of the balloon wall is typically in the
range of 2/1000ths to 25/1000ths' of an inch, or other
thicknesses that can withstand pressures of up to 250-400
psi.
One important goal of percutaneous vertebral
body augmentation of the present invention is to provide
a balloon which can create a cavity inside the vertebral
body whose configuration is optimal for supporting the
bone. Another important goal is to move the -top of the
vertebral body back into place to retain height where
possible, however, both of these objectives must be
achieved without changing the outer diameter of the sides
of the vertebral body, either by fracturing the cortical
wall of the vertebral body or by moving already fractured
bone. This feature could push vertebral bone toward the
spinal cord, a condition which is not to be desired.
The present invention satisfies these goals
through the design of inflatable devices to be described.
Inflating such a device compresses the calcium-containing
CA 02683004 2009-11-02
50749-18D
-15-
soft cancellous bone into a thin shell that lines the
in-side of the hard cortical bone.creating a large cavity.
At the same time, the biological components
(red blood cells, bone progenitor cells) within the soft
bone are pressed out and removed by rinsing during the
procedure. The body recreates the shape of the inside of
an unfractured vertebral body, but optimally stops at
approximately 70 to 9001 of the inner volume. The
balloons of the present invention are inelastic, so
maximally inflating them can only recreate the
predetermined shape and size. However, conventional
balloons become spherical when inflated. Spherical
shapes will not allow the hardened bone cement to support
the spine adequately, because they make single points of
contact on each vertebral body surface (the equivalent of
a circle inside a square, or a sphere inside a cylinder).
The balloons of the present invention recreate the flat
surfaces of the vertebral body by including restraints
that keep the balloon in the desired shape. This
maximizes the contacts between the-..vertebral body
surfaces and the bone cement, whi.ch strengthens the
spine. In.addition,-the volume o-f,bone cement that fills
these cavities creates a thick mantle of cement.(4, mm or
greater), which is required for appropriate compressive
strength.,. Another useful feature, although not required,
are ridges in,the balloons which leave their:imprint in
the lining of compressed cancellous bone. The .resulting
bone cement "fingers" provide enhanced stabili.ty.
,The balloons which optimally compress-
cancellous bone in vertebral bodies are the balloons
listed as balloon types 1, 2 and 3 above. These balloons
are configured to approximate the shape of the vertebral
body. Since the balloon is chosen to occupy.70, to 90% of
the inner volume, it will not exert undue pressure on the
sides of the vertebral body, thus the vertebral body will
. . ,. . . .. . . ~ .. . . .. .. . . . . . . . ....... .. . .
CA 02683004 2009-11-02
- 16 -
50749-18D
not expand beyond its normal size (fractured or
unfractured). However, since the balloon has the height
of an unfractured vertebral body, it can move the to,p,
which has collapsed, back to its original position. Any
number of individual balloons can be stacked, and stacks
containing any of the balloons of types 1, 2 and 3 can be
mixed.in shape and/or size to provide greater flexibility
and/or control.
A primary goal of percutaneous proximal humeral
augmentation is to create a cavity inside the proximal
humerus whose configuration is optimal for supporting the
proximal humerus. Another important goal is to help
realign the humeral head with the shaft of the humerus
when they are separated by a fracture. Both of these
goals must be achieved by exerting pressure primarily on
the cancellous bone, and not the cortical bone. Undue
pressure against the cortical bone could conceivably
cause a-worsening of a shoulder fracture by causing
cortical bone fractures.
The present invention satisfies these goals
through the design of the inflatable devices to be
described. Inflating such a device compressesthe
cancellous-bone against the cortical walls of the
epiphysis and metaphysis of the proximal humerus thereby
creating a cavity. In some cases, depending on the
fracture location, the balloon or inflatable device may
be used to extend the cavity into the proximal part of
the humeral diaphysis.
~.Due to the design of the "sphere on a stand"
-balloon (described as number 7 above), the cavity,made by
this balloon recreates or approximates the shape of the
inside cortical wall of the proximal humerus. The
approximate volumeof the cavity made by the "spherical
on a stand balloon" is~ 70 to 90% that of the proximal
humeral epiphysis and metaphysis, primarily, but not
CA 02683004 2009-11-02
50749-18D
-17-
necessarily exclusive of., part of.the diaphysis. The
shape approximates the.shape of .the humeral head. The
"base" is designed to compress the trabecular bone into a
"plug" of bone in the.distal metaphysis or proximal
diaphysis. This plug of bone will prevent the flow of
injectable material into the shaft of the humerus,
improving the clinical outcome. The sphere can also be
used without a base. Alternatively, the balloon can be
shaped like a fat cylinder, with one end at the top of
the humeral head attached to the catheter and the other
end filling the function of the plug. The cylinder can
also be formed so that the diameter of the end in the
humerus is greater than the diameter of the"end which
functions as the plug.
A primary goal of-percutaneous dis.tal radius
augmentation is to create a cavi,ty.inside.the distal
radius whose configuration is optimal for supporting the
distal radius. Another important goal is to help.fine
tune fracture realignment after t-he fracture"has.been
partially realigned by finger tr.aps.- Both of these goals
must be achieved by exerting pressure."primarily_..on the
cancellous bone and not onthe.cortical bone. Excessive
pressure against the cortical bone could conceivably
cause cortical bone fractures, thus worsening the.
condition. =
The present invention satisfies these.goals
through..the design of inflatable devices either, already
described or to be described.
The design of the "humpbacked banana", or
modified pyramid design (as described as_number-5 above),
approximates the shape of the distal radius and.
therefore, the cavity made by this balloon approximates
the shape of the distal radius:as well. The approximate
volume of the cavity_to be made by this humpbacked banana
shaped balloon is 70 to 90o tha,t of the distal radial
CA 02683004 2009-11-02
- 18 -
50749-18D
epiphysis and metaphysis primarily of, but not
necessarily exclusive of, some part of the distal radial
diaphysis. Inflating such a device compresses the
cancellous bone against-the cortical walls of the
epiphysis and metaphysis of the distal radius in order to
create a cavity. In some cases, depending on the
fracture location, the osseous balloon or inflatable
device may be used to extend the cavity into the distal
part of the radial diaphysis.
A primary goal of percutaneous femoral head (or
humeral head) augmentation is to create a cavity inside
the femoral head (or humeral head) whose configuration is
optimal for supporting the femoral head. Another
important goal is to help compress avascular (or aseptic)
necrotic bone or support avascular necrotic bone in the
femoral head. This goal may include the realignment of
avascular bone back into the position it previously
occupied in the femoral head in order to improve the
spherical shape of the femoral head. These goals must be
achieved by exerting pressure primarily on the cancellous
bone inside the femoral head.
The present invention satisfied these goals
through the:design of Inflatable devices either already
described or to be described.
The design of the spherical osseous balloon
(described as balloon type 4 above) approximates the
shape of the femoral head and therefore creates a cavity
which approximates the shape of the femoral head as well.
(It should be noted that the spherical shape of this
inflatable device also approximates the shape of~the
humeral head and would; in fact, be appropriate for
cavity formation in this osseous location as well.)
Inflating such a device compresses the cancellous bone of
the femoral head against its inner cortical walls in
order to create a cavity. In some cases, depending upon
CA 02683004 2009-11-02
50749-18D
-19-
the extent of the avascular necrosis, a smaller or larger
cavity inside the femoral head will be formed. In some
cases, if the area of avascular necrosis is- small, a
small balloon will be utilized which might create a
cavity only 10 to 150 of the total volume of the femoral
head. If larger areas of the femoral head are involved
with the avascular necrosis, then a larger balloon would
be utilized which might create a much larger cavity,
approaching 80 to 90% of the volume of the femoral head.
The hemispherical balloon approximates the
shape of the top half of the femoral (and humeral) head,
and provides a means for compacting cancellous bone in an
area ofavascular necrosis or small fracture without
disturbing the rest of the head. This makes it easier to
do a future-total joint replacement if required.
Percutaneous hip augmentation is designed to
prevent hip fracture by compacting weak cancellous bone
in the femur where hip fractures occur and replacing it
with appropriate supporting material. A primary-goa1 of
percutaneous hipaugmentation is to:create a cavity
inside the femoral head,femoral neck and lesser .
trochanter:which will compress diseased cancellous bone
.and allow it tobe replaced with appropriate-supporting
material, preventing hip fracture. The cavity created by
the procedure usually extends from.the-femoral head, past
the les=ser trochanter by a defined amount, but generally
not further. The cavity should not expand into the
greater-.:_trochanter, where hip fractures do not'affect the
patient, because this may prevent the balloon-from
expanding into the lesser trochanter, where hip'fractures
do affect the patient. The balloon should compact the
cancellous bone as fully as possible without pushing the
inner cortical bone, which could cause (instead-of
prevent) a fracture.
CA 02683004 2009-11-02
- 20 -
50749-18D
The present invention satisfies these goals by
providing inflatable devices to be described and which
have special features, including their placement on the
catheter, to orient the balloon appropriately.
A primary goal of percutaneous proximal tibial
augmentation is to create a cavity inside the proximal
tibia whose configuration is optimal for supporting
either the medial or lateral tibial plateaus. Another
important goal is to help realign the fracture fragments
of tibial plateau fractures, particularly those features
with fragments depressed below (or inferior to) their
usual location. Both of these objectives must be
achieved by exerting pressure on primarily the cancellous
bone and not the cortical bone. Pressure on the cortical
bone could conceivably cause worsening of the tibial
plateau fracture.
The present invention satisfies these goals
through the design of the inflatable devices to be
described. Inflating such a device compresses the
cancellous bone against the cortical walls of the medial
or lateral tibial plateau in order to create a cavity.
Due to the design of the "elliptical cylinder"
balloon (described as balloon type 6 above) the cavity
made by this balloon recreates or approximates the shape
of thecortical walls of either the medial or lateral
tibial plateaus. The approximate volume of the cavity to
be made by the appropriate elliptical cylindrical balloon
is.50.to 900 of"the proximal epiphyseal bone of either
the medial half or the lateral half of the tibial.
Due to the nature of the injury, disease or
other treatments, it may be preferable to treat a bone
with the devices of this invention during an open
surgical procedure. In addition, a goal of the
percutaneous or open surgery may be to replace the
CA 02683004 2009-11-02
50749-18D
21
diseased or injured bone with materials (such as bone
fillers or certain drugs) which do not flow.
The present invention satisfies these goals
through the systems and methods of this invention described
below.
The invention relates to a system comprising a
first tool sized and configured to establish an access path
through soft tissue to bone having an interior volume
occupied, at least in part, by cancellous bone; an
expandable structure sized and configured for expansion in
cancellous bone, and a second tool sized and configured for
passage through the access path to introduce into the
cancellous bone the expandable structure, which remains
within the cancellous bone upon removal of the second tool.
Other objects of the present invention will become
apparent as the following specification progresses,
reference being had to the accompanying drawings for an
illustration of the invention.
CA 02683004 2009-11-02
50749-18D
21a
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a perspective view of a first embodiment
of the balloon of the present invention, the embodiment
being in the shape of a stacked doughnut assembly;
Fig. 2 is a vertical section through the balloon
of Fig. 1 showing the way in which the doughnut portions of
the balloon of Fig. 1, fit into a cavity of a vertebral
body;
Fig. 3 is a schematic view of another embodiment
of the balloon of the present invention showing three
stacked balloons and string-like restraints for limiting the
expansion of the balloon in directions of inflation;
Fig. 4 is a top plan view of a spherical balloon
having a cylindrical ring surrounding the balloon;
Fig. 5 is a vertical section through the spherical
balloon and ring of Fig. 4;
Fig. 6 shows an oblong-shaped balloon with a
catheter extending into the central portion of the balloon;
Fig. 6A is a perspective view of the way in which
a catheter is arranged relative to the inner tubes for
inflating the balloon of Fig. 6;
CA 02683004 2009-11-02
- 22 -
50749-18D
Fig. 7 is a suction tube and a contrast
injection tube for carrying out the inflation of the
balloon and removal of debris caused by expansion from
the balloon itself;
Fig. 8 is a vertical section through a balloon
after it has been deflated and as it is being inserted
into the vertebral body of a human;
Figs. 9 and 9A are side elevational views of a
canula showing how the protective sleeve or guard member
expands when leaving the canula;
Fig. 9B is a vertical section through a
vertebral bone into which an access hole has been
drilled;
Fig. 10 is a perspective view of another
embodiment of the balloon of the present invention formed
in the shape of a kidney bean;
Fig. 11 is a perspective view of the vertebral
bone showing the kidney shaped balloon of Fig. 10
inserted in the bone and expanded;
Fig. 12 is a top view of a kidney shaped
balloon formed of several compartments by a heating
element or branding tool;
Fig. 13 is a cross-sectional view taken along
line 13-13 of Fig. 12 but with two kidney shaped balloons
that have been stacked.
Fig. 14 is a view similar to Fig. 11 but
showing the stacked kidney shaped balloon of Fig. 13 in
the vertebral bone;
Fig. 15 is a top view of a kidney balloon
showing outer tufts holding inner strings in place
interconnecting the top and bottom walls of the balloon;
Fig. 16 is a cross sectional view taken along
lines 16-16 of Fig. 15;
Fig. 17A is a dorsal view of a humpback banana
balloon in a right distal radius;
CA 02683004 2009-11-02
23 -
50749-18D
Figs. 17B is a cross sectional view of Fig. 17A
taken along line 17B-17B of Fig. 17A;
Fig. 18 is a spherical balloon with a base in a
proximal humerus viewed from the front (anterior) of the
left proximal humerus;
Fig. 18A is a cylindrical balloon viewed from
the front (anterior) of the left proximal humerus.
Fig. 19A is the front (anterior) view of the
proximal tibia with the elliptical cylinder balloon
introduced beneath the medial tibial plateau;
Fig. 19B is a three quarter view of the balloon
of Fig. 19A;
Fig. 19C is a side elevational view of the
balloon of Fig. 19A;
Fig. 19D is a top plan view of the balloon of
Fig. 19A;
Fig. 20 is a spherically shaped balloon for
treating avascular necrosis of the head -of the=femur (or
humerus) as seen from the front (anterior) of the- left
hip;
Fig. 20A is a side view of a hemispheri.cally
shaped balloon for treating avascular necrosis of the
head of the femur (or humerus);
Fig. 21 is a balloon for preventing hip
fracture as seen from the front (anterior) of the. left
hip; and
Figs. 22A-C are schematic~illustrations of a
representative method and system for delivering a
therapeutic substance to a bone according to the present
invention.
CA 02683004 2009-11-02
- 24 -
50749-18D
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
BALLOONS FOR VERTEBRAL BODIES
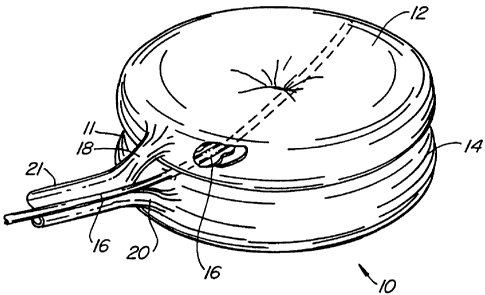
A first embodiment of the balloon (Fig. 1) of
the present invention is broadly denoted by the numeral
and includes a balloon body 11 having a pair of
hollow, inflatable, non-expandable parts 12 and 14 of
flexible material, such as PET or Kevlar. Parts 12 and
14 have a suction tube 16 therebetween for drawing fats
10 and other debris by suction into tube 16 for transfer to
a remote disposal location. Catheter 16 has one or more
suction holes so that suction may be applied to the open
end of tube 16 from a suction source (not shown).
The parts 12 and 14 are connected together by
an adhesive which can be of any suitable type. Parts 12
and 14 are doughnut-shaped as shown in Fig. 1 and have
tubes 18 and 20 which communicate with and extend away
from the parts:12 and 14, respectively, to a source of
inflating liquid~under pressure (not shown). The liquid
can be any sterile biocompatible solution. The liquid
inflates the balloon 10, particularly parts 12 and 14
thereof after the balloon has been insert-ed in a
collapsed condition (Fig. 8) into a bone to be treated,
such as a vertebral bone 22 in Fig. Z. -The above-
mentioned paterits ~4, 969, 888 and 5,108,404 disclose the
use of a guide pin and canula for inserting the balloon
into bone to be'treated when the balloon is deflated and
has been-inserted into a tube and driven bythe catheter
into the cortical.bone1where the'balloon-is inflated.
Fig. 8 shows a deflated balloon 10 being
inserted through a canula 26 into bone. The balloon in
canula 26 is deflated and is forced through the canula by
exerting manual force on the catheter 21 which extends
into=a passage 28 extending into the interior of the
bone. The catheter is'slightly flexible but is
CA 02683004 2009-11-02
- 25 -
50749-18D
sufficiently rigid to-allow the-balloon to be forced into
the interior of the bone where the balloon is then
inflated by directing fluid-into tube 88 whose outlet
ends are coupled to respective parts 12 and 14.
In use, balloon 10 is initially deflated and,
after the bone to be filled with the balloon has been
prepared to receive the balloon with drilling, the
deflated balloon is forced into the bone in a collapsed
condition through canula 26. The bone is shown in
Fig. 2. The balloon is oriented preferably in the bone
such that it allows minimum pressure to be exerted on the
bone marrow and/or cancellous bone if there is no
fracture or collapse of the bone. Such pressure will
compress the bone marrow and/or cancellous bone against
the inner wall of the cortical bone, thereby compacting
the bone marrow of -the bone to be treated and to further
enlarge the cavity in which the bonemarrow is.to-be
replaced by a biocompatible, flowable bone material.
The balloon is then inflated to compact the
bone marrow and/or cancellous bone,-in the cavity and,
after compaction of t.he bone marrow-and/or cancel].ous
bone, the balloon is deflated and removed from the
cavity. While inflation of the balloon and compaction
occurs;- fats and other debris are-;sucked-out of.the space
between and-around parts,12 and 14 by applying a suction '
force tocatheter tube 16. Following this, and following
the -compaction.of the bone mar.row,the balloon is-.
deflated and,pulled out-'of the cavity by applying=a
manual pullingforce to.the catheter tube 21. .
The-second embodiment of the inflatable device
of the present invention is broadly denoted by the
numeral 60 and is shown in Figs. 4 and 5. Balloon 60
includes a central spherical part,62 which is hollow and
which receives an inflating l.iqu.id under - pressure:through
a tube 64. The spherical -part is provided witha
CA 02683004 2009-11-02
- 26 -
50749-18D
spherical outer surface 66 and has an outer periphery
which is surrounded substantially by a ring shaped part
68 having tube segments 70 for inflation of part 68. A
pair of passages 69 interconnect parts 62 and 68. A
suction tube segment 72 draws liquid and debris from the
bone cavity being formed by the balloon 60.
Provision can be made for a balloon sleeve 71
for.balloon 60 and for all balloons disclosed herein. A
balloon sleeve 71 (Fig. 9) is displaceably mounted in an
outer tube 71a and can be used to insert the balloon 60
when deflated into a cortical bone. The sleeve 71 has
resilient fingers 71b which bear against the interior of
the entrance opening 71c of the vertebral bone 22
(Fig. 9A) to prevent tearing of the balloon. Upon
removal of the balloon sleeve, liquid under pressure will
be directed into tube 64 which will inflate parts 62 and
68 so,as to compact the bone marrow within the cortical
bone. Following this, balloon 60 is deflated and removed
from the bone cavity.
Figs. 6 and 6A show several views of a modified
doughnut shape balloon 80 of the type shown in-Figs. 1
and 2, except the-doughnut shapes of balloon 80 are not
stitched onto one another. In Fig. 6, balloon 80 has a
pear-shaped outer=convex surface 82 which is made up of a
first hollow part 84 and a second hollow part 85.- A tube
88 is provided for directing liquid into t.he two parts
along branches 90 and 92 to inflate the parts after the
parts have been inserted into the medullary cavity of a
bone. A catheter tube 16 is inserted into the space 96
between two parts of the balloon 80. An adhesive bonds
the two,parts-84-and 85 together at the interface
thereof.
Fig. 6A-shows the way in which-the catheter
tube 16 is inserted-into the space or opening 96 between
the two parts of the balloon 80.
, . .~. . ... . . .. ... .. ... .. ... .. . . . . .. . .
CA 02683004 2009-11-02
50749-18D
-27-
Fig. 7 shows tube 88 of which, after directing
inflating liquid into the balloon.80, can inject contrast
material into the balloon 80 so that x-rays can be taken
of the balloon with the inflating material therewithin to
determine the proper placement of the balloon. Tube 16
is also shown in Fig. 6, it being attached in some
suitable manner to the outer side wall surface of tube
88.
Still another embodiment of the invention is
shown in Fig. 3 which is similar to Fig. 1 except that it
is round and not,a doughnut and includes an inflatable
device 109 having three balloon units 110, 112 and 114
which are inflatable and which have string-like
restraints 117 which limit the expansion of the balloon
units in a direction transverse to the longitudinal axes
of the balloon units. The restraints are made of the
same or similar material as that of the balloon so that
they have some resilience but substantially no expansion
capability.
A tube system 115 is provided to direct liquid
under pressure into balloon units 110, 112 and 114 so
that liquid can be used to inflate the-balloon units when
placed inside the bone in a deflated state. .Following
the proper inflation and compaction of the bone marrow,
the balloon can be removed by deflating it=and pulling it
outwardly of the bone being treated. The restraints keep
the opposed sides 77 and 79 substantially flat and
parallel with each other.
In Fig. 10, another embodiment of the
inflatable balloon is shown. The device is a.kidney
shaped balloon body 130 having a pair of opposed kidney
shaped side walls 132 which are adapted to be collapsed
and to cooperate with a continuous end wall 134 so that
the balloon 130 can be forced into a bone 136 shown in
Fig. 11. A tube 138 is used to direct inflating liquid
CA 02683004 2009-11-02
- 28 -
50749-18D
into the balloon to inflate the balloon and cause it to
assume the dimensions and location shown vertebral body
136 in Fig. 11. Device 130 will compress the cancellous
bone if there is no fracture or collapse of the
cancellous bone. The restraints for this action are due
to the side and end walls of the balloon.
Fig. 12 shows a balloon 140 which is also
kidney shaped and has a tube 142 for directing an
inflatable liquid into the tube for inflating the
balloon. The balloon is initially a single chamber
bladder but the bladder can be branded along curved lines
or strips 141 to form attachment lines 144 which take the
shape of side-by-side compartments 146 which are kidney
shaped as shown in Fig. 13. A similar pattern of strips
as in'140 but in straight lines would be applied to a
balloon that is square or rectangular. The branding
causes a welding of the two sides of the bladder to occur
`since the material is standard medical balloon material,
which is similar to plastic and can be formed by heat.
Fig. 14 is a perspective view of a vertebral
body 147 containing the balloon of Fig. 12, showing a
double stacked balloon 140 when it is inserted in
vertebral: bone 147.
Fig: 15,is a view similar to Fig. 10 except
-that tufts 155, which are string-like restraints, extend
between and are connected to the side walls 152 of
inflatable device 150 and limit the expansion of the side
walls with respect to each other, thus rendering the side
walls generally parallel with each other. Tube 88 is
used to.fill the kidney shaped balloon'with an inflating
liquid in the manner described above.
The dimensions for the vertebral body balloon
will vary acrossa-broad range. The heights (H, Fig. 11)
of"the vertebral b-ody balloonfor both lumbar and
thoracic vertebral bodies typically range from 0.5 cm to
CA 02683004 2009-11-02
50749-18D
-29-
3.5 cm. The anterior to posterior (A, Fig. 11) vertebral
body balloon dimensions for both lumbar and thoracic
vertebral bodies range from 0.5 cm to 3.5 cm. The side
to side (L, Fig. 11) vertebral body dimensions for
thoracic vertebral bodies will range from 0.5 cm to 3.5
cm. The side to side vertebral body dimensions for
lumbar vertebral bodies will range from 0.5 cm to 5.0 cm.
An optimal vertebral body balloon is stacked with two or
more members of unequal height where each member can be
separately inflated through independent tube systems.
The total height of the stack when fully.inflated should
be within the height ranges specified above. Such a
design allows the fractured vertebral body to be returned
to its original height in steps, which can be easier on
the surrounding tissue, and it also allows the same
balloon to be used in a wider range of vertebral body
sizes.
The eventual selection of the appropriate
balloon for, for instance, a given vertebral body,is
based upon several factors. The anterior-posterior (A-P)
balloon dimension for a given vertebral body,is selected
from the CT scan or plain film x-ray views-of the
vertebral body. The A-P dimension is, measured,.from the
.internal cortical wall of the anterior cortex to the
internal-cortical wall of the posterior cortex of-the
vertebral body. In general, the appropriate A-P balloon
dimension .i.s 5 to 7 millimeters :less- than this measurement. .
The.appropriate side to.side balloon dimensions
for a.given vertebral body is selected from the CT:.scan
or from a plain film x-ray view of the vertebral-body to
be treated. The side to side distance is measured from
the internal cortical walls of ;the side of the vertebral
bone. In general, the appropriate-side to.side. balloon
dimension is 5 to 7 millimeters less than this -
CA 02683004 2009-11-02
- 30 -
50749-18D
measurement by the addition of the lumbar vertebral body
tends to be much wider than side to side dimension then
their A-P dimension.- In thoracic vertebral bodies, the
side to side dimension and their A-P dimensions are
almost equal.
The height dimensions of the appropriate
vertebral body balloon for a given vertebral body is
chosen by the CT scan or x-ray views of the vertebral
bodies above and below the vertebral body to be treated.
The height of the vertebral bodies above and below the
vertebral body to be treated are measured and averaged.
This average is used to determine the appropriate height
dimension of the chosen vertebral body balloon.
BALLOONS FOR LONG-BONES
Long bones which can be treated with the use of
balloons of the present invention include distal radius
(larger arm bone at the wrist), proximal tibial plateau
(leg bone just below the knee), proximal humerus (upper
end of the-arm at-the shoulder), and proximal femoral
head (leg bone in the hip).
Distal Radius Balloon
For the distal radius, a balloon 160 is shown
in the distal radius 152 and the balloon has a shape
which approximates a pyramid but more closely can be
considered the shape of a humpbacked banana in that it
substantially fills the interior of the space of the
distal radius to force cancellous bone 154 lightly
==against the inner surface 156 of cortical bone 158. Note
that the spherical radius balloon discussed above may
also be appropriately sized for the distal radius 152.
The balloon 160-has a lower, conical portion
159 which extends-downwardly into the hollow space of the
distal radius 152, and this conical portion 159-increases
CA 02683004 2009-11-02
50749-18D
-31-
in cross section as a central distal portion. 161 is
approached. The cross section of.the balloon 160. is
shown at a central-location (Fig. 17B) and this location
is near the widest location of the balloon. The upper
end of the balloon, denoted by the numeral 162, converges
to the catheter 88 for directing a liquid into the
balloon for inflating the same to force the cancellous
bone against the inner surface of the cortical bone. The
shape of the balloon 160 is determined and restrained by
tufts formed by string restraints 165. These restraints
are optional and provide additional strength to the
balloon body 160, but are not required to achieve the
desired configuration. The balloon is placed into and
taken out of the distal radius in the same manner as that
described above with respect to the vertebral bone.
The dimensions of the distal radius balloon
vary as follows:
The proximal end of the balloon (i.e. the part
nearest the elbow) is cylindrical in shape and will vary
from 0.5 x 0.5 cm to 1.8 x 1.8 cm.
The length of the distal radius balloon will
vary from 1.0 cm to 12.0 cm.
The widest medial to lateral dimension of the
distal-radius balloon, which occurs..,at or near the distal
radio.-ulnar-joint, will measure from 1.0 cm to 2.5 cm.
The distal anterior-posterior dimension of the
distal ra.diusballoon will varyfrom 0._5 to 3.0 cm.
Proximal Humerus Fracture -Balloon
The selection of the appropriate balloon size
to treat a given fracture of the distal-radius will
depend on the radiological size.of the distal radius and
-thelocation of the fracture.
:. :
In the case of the: proximal: humerus :169; a
balloon 166 shown in Fig. 18 is.spherical and has-a base
CA 02683004 2009-11-02
- 32 -
50749-18D
design. It compacts the cancellous bone 168 in a
proximal humerus 169. A mesh 170, embedded or laminated
and/or winding, may be used to form a neck 172 on the
balloon 166, and second mesh 170a may be used to conform
the bottom of the-base 172a to the shape of the inner
cortical wall at the start of the shaft. These
restraints provide additional strength to the balloon
body, but the configuration can be achieved through
molding of the balloon body. This is so that the
cancellous bone will be as shown in the compacted region
surrounding the balloon 166 as shown in Fig. 18. The
cortical bone 173 is relatively wide at the base 174 and
is thin-walled at the-upper end 175. The balloon 166 has
a'feed tube 177 into which liquid under pressure is
forced into the balloon to inflate it to lightly compact
the cancellous bone in the proximal humerus. The balloon
is inserted into and taken out of the proximal humerus in
the same manner as that described above with respect to
the vertebral bone.
The-dimensions of the proximal humerus fracture
balloon vary=as follows:
The spherical end of the balloon will vary from
1:0 x 1.0 cm to-1:0 x 3.0 cm.
The neck of the proximal humeral fracture
.,balloon willvary from-0.8 x 0.8 cm to 3.0 x 3.0 cm.
The width-of the base portion or.distal portion
of the proximal numeral fracture balloon will vary from
0.5 x 0.5 cm to 2.5 x 2.5 cm.
The length of the balloon will vary from 4.0 cm
to 14:0 cm::.
The selection-of the-appropriate balloon to
treat agiven proximal humeral.fracture depends on the
radiologic size of the proximal humerus.and the location
of the f racture .
CA 02683004 2009-11-02
50749-18D
-33-
Another balloon adapted.for use in the proximal
humerus 169 is the cylindrical balloon 225 shown,in Fig.
18A. Like the feed tube 177 of Fig. 18, cylindrical
balloon 225 has an inflation tube 226 for inserting
liquid therein. 227 shows the site of a typicai shoulder
fracture. The cylinder can have a uniform circumference
or it can be wider at one end than at the other. The
wider end would be attached to the inflating tube 226 to
compact the cancellous bone 168 of the.humeral head 168a.
Appropriate restraints to maintain the shape include
multiple inelastic bands (228 is one of.them) spaced
around.the circumference at regular intervals. For a
cylinder with a uniform width, the restraining bands will
usually have the same diameter. For a cylinder with one
end wider than the other, each band would successively
have a wider diameter.
The length of the balloon is usually the same
as that of the sphere on the base., preferably ranging
from 4-14 cm, with the width usually rangingfrom 0.5 cm
to 2.5 cm. The surgeon uses plain film X-ray of the
humerus to be treated. The r-equired lengthis defined by
measuring the distance from the inner humeral head at the
site of insertion to about 3 cm below the site of,.
fracture. The diameter is at least,0.5 cm smaller than
the inner diameter of the cortex of the humeral shaft (at;
its narrowest point along the balloon's length).
Proximal Tibial Plateau Fracture Balloon
The,tibial fracture is shown-in Fig. 19A in
which a balloon 180 is placed in one side.182 of:a tibia -
183. 7'The balloon, when inflated, compacts the cancellous
bonein the layer 184 surrounding the balloon 180. A
cross:;section of the balloon.is shown in Fig. 1.9C.wherein
the balloon has a pair of opposed sides 185 and 187 which
are interconnected by restraints 188 which can be in the
CA 02683004 2009-11-02
- 34 -
50749-18D
form of strings or flexible members of any suitable
construction. The main purpose of the restraints is to
make the sides 185 and 187 substantially parallel with
each other and non-spherical. A tube 190 is coupled to
the balloon 180 to direct 1-iquid into and out of the
balloon. The ends of the restraints are shown in Figs.
19B and 19D and denoted by the numeral 191. The balloon
is inserted into and taken out of the tibia in the same
manner as that described above with respect to the
vertebral bone. Fig. 19B shows a substantially circular
configuration for the balloon; whereas, Fig. 19D shows a
substantially elliptical version of the balloon.
The dimensions of the proximal tibial plateau
fracture balloon vary as follows:
The thickness or height of the balloon will
vary from 0.5 cm to 5.0 cm.
The anterior/posterior (front to back)
dimension will vary from-1.0 cm to 6.0 cm.
The side to side (medial to lateral) dimension
will vary from 1:0 -cm to 6.0 cm.
The selection of the appropriate balloon to
treat a given tibial plateau fracture will depend on the
radiological size of the proximal tibial and the location
of the fracture.
Femoral Head Balloon
In the case of the femoral head, a balioon 200
is shown as having been inserted-inside the cortical bone
202 of the-femoral head which is thi-n at the outer end
204 of`the-femur and which can increase in thickness at
the lower-end 206 of the femur. The cortical bone
surrounds the cancellous bone 207 and this bone is
compacted by the-inflation of balloon 200. The tube for
directiing liquid for inflation purposes into the balloon
is denoted by the numeral 209. It extends along the
CA 02683004 2009-11-02
50749-18D
-35-
femoral neck and is directed into the femoral head which
is-generally spherical in configuration.. Fig. 20A shows
that the balloon,.denoted by the riumeral 200a, can be
hemispherical as well as spherical, as shown in Fig. 20.
The balloon.200 is inserted into.and taken out of the
femoral head in the same manner as that described with
respect to the vertebral bone. The hemispherical shape
is maintained in this example by bonding overlapping
portions of the bottom, creating.pleats 200b as shown in
Fig. 20A.
The dimensions of the femoral head balloon vary
as follows:
The diameter of the femoral head balloon will
vary from 1.0 cm to up to 4.5 cm. The appropriate size
of the femoral head balloon to be chosen depends on the
radiological or CT scan size of the head of the femur and
the location and size of the avascular necrotic bone.
The dimensions of the hemispherical balloon are.the same
as thethose of the spherical balloon, except that
approximately one half is provided.
Prevention of Hip Fracture
Fig. 21 illustrates a,"boomerang" balloon 210
adapted for preventing hip fracture. When inflated, the
"boomerang" balloon 210 is a cylinder which gradually
bends in the middle, like a boomerang, and extends from
about.0:..5 cm from.the end of the femoral head 211 through
the femoral:neck 212 and down into the proximal femoral
diaphys.is 213 about 5-7 cm past the lesser trochanter
214., _Balloon _210.,preferably maintains its shape by rings-
of inelastic material (215_is one of them). held closer
together on one side by attachment to a shorter inelastic
band 216 running the length of =the side of balloon and
further apart,by attachment to-a longer inelastic band
217.bonded on the opposite side.
CA 02683004 2009-11-02
= - 36 -
50749-18D
After and prior to inflation, balloon 210 is
folded back (shown in dotted lines at 218) against the
inflation tube 219. ` Prior to inflation, the balloon 210
is also rolled up and held against the inflation tube
with loose attachments that break when the balloon is
inflated. To insert the balloon on its inflation tube
into the hip, the surgeon uses a power drill under
radiographic guidance to create a cavity 220 that is
usually 4-6 mm wide starting at the lateral femoral
cortex 221 and proceeding into the femoral head 211.
Inflation of balloon 210 into the greater trochanteric
region 222 instead of down the femoral diaphysis 213 is
not desirable and is prevented by the shape of the
balloon, by its placement and correct orientation (the
deflated balloon facing the lesser trochanter). After
the balloon 210 has been inflated within the cavity 220
(see the dotted lines in Fig. 21), the predetermined size
and shape of the balloon biases the proximal portion of
the balloon downward into the lesser trochanter..
Optionally, a second cavit-y can be drilled down into the
diaphysis, starting from the same entry point or from the
other side.
Patients with bone density in the hip below a
threshold value are at increased risk of hip fracture,
''and`lower densities,create greater-risk. Patierit
selection is done through a bone density scan. "The
balloon length is chosen by the surgeon to extend about
0:5 cm from the endof the femoral head, through-the
femoral neck and into-the proximal femoral diaphysis,
30- -usually about 4-8-cm below the lesser trochanter:. The
balloon diameter is chosen by measuring the inner
cortical diameter of the-femoral neck (the most narrow
' area) and subtracting-0.5 cm. The preferred dimensions
of the-"boomerang balloon are a total length of 10-20 cm
and a diameter of about 1.0-2.5 cm. (A "humpback
CA 02683004 2009-11-02
37 -
50749-18D
banana" balloon with appropriate length may also be
useful in hip fracture,prevention, as long as the
"humpback" width does not.exceed.the allowed femoral neck
dimensions.)
Patients having the.lowest bone densities in
the femoral head may require greater compacting in the
femoral head, which may, for example, be provided by
using two balloons, one after the other: the-"boomerang"
followed by the femoral head balloon -(inserted.at.the
same point and expanded prior to inserting any supporting
material.) Alternatively, the "boomerang" balloon may be
adapted to have a distal portion that approximates the
shapeof the femoral head balloon.
Other Uses, Methods And Balloons
The cavity created by the balloon can be filled
with a medically-appropriate.formulation of a,drug or a
growthfactor. As an example of deliveringa drug, a
typical dose of the antibiotic, gentamicin, to.treat a
local osteomyelitis (bone infection), is 1 gram (although,
the therapeutic range for gentamicin is far greater, from
1 nanogram to 100 grams, depending on the.condition being
treated-and the size of.the area to be covered). A
medically-suitable gelformulated with appropriate gel
materials, such as polyethylene glycol,-can contain 1
gram of...gentamicin in a setvolume.of gel, suc.h as 10 cc.
A balloon with-this volume whose shape-and-size,is
appropriate for the site being treated .(that,is, the
balloon cannot move and therebybreak the cortical bone
when inflated at the-chosen site).can be..used to-compact.
the infected cancellous bone. This creates a space which
can be filled with the antibiotic gel in an open or
minimally.invasive procedure: _This places..and.holds the
required amount of drug right at the site needing
treatment, and protects the drug from being washed away
CA 02683004 2009-11-02
- 38 -
50749-18D
by blood or other fluids. Not only can the dose be
optimized, but additional doses can be applied at later
times without open surgery, enhancing the therapeutic
outcome. If the required cavity for the optimal drug
dose weakens the bone, the bone can be protected from
future fracture with a cast or with current internal or
external metal or plastic fixation devices. The
therapeutic substance put into bone may be acting outside
the bone as well. A formulation containing
chemotherapeutic agent could be used to treat local solid
tumors, localized multiple myeloma or even a nearby
osteosarcoma or other tumor near that bone.
As an alternative, to deliver therapeutic
substances, balloons can be dipped in a medical
formulation (often a dry powder, liquid or gel)
containing a medically-effective amount of any desired
antibiotic, bone growth factor or other therapeutic agent
to-coat the`balloon with the above-mentioned substance
before it is inserted intoa bone being treated.
Optionally, the balloon'can be wholly or partially
inflatedwith air or liquid before thecoating is
performed. Optionally, the coated balloon can be dried
with air or`by othe'r means when the:applied formulation
is wet, such as a liquid or,a gel. The balloon is
.`refolded as required'and either used immediately or
stored,-if appropriate and desired. Coated on the
balloon, therapeutic substances canbe delivered while
cancellous bone is being compressed, or with an
additional-balloon once the cavity is made'..
The methods described above can also be used to
coat Gelfoam or other agents onto the balloon before use.
Inflating the Gelfoam-coated balloon inside bone will
further fill-any cracks in fractured bone not,already
filled-by the compre"ssed cancellous bone.
CA 02683004 2009-11-02
39 -
50749-18D
Figs. 22A-C schematically illustrate one system
and method,for delivering a therapeutic substance to the
bone according to the present invention. As shown in
Fig. 22A, an inflated balloon 229 attached to an
inflating tube 230 is stabilized with a clip 231 that
couples tube 230 to a wire 232. As shown in Fig. 22B, a
measured amount of gel formulation containing the desired
amount of substance 233 is uniformly dispensed from a
container 234,, preferably in thin lines 235, onto the
outer surface of a balloon 236. As shown in Fig. 22C,
the coated balloon 237 is then deflated and allowed to
dry until the get sets. The coated balloon 237 is then
readylor packaging for use by the surgeon. Of course,
the balloon can also be coated without prior inflation.
In addition, the coating substance can be the desired
compound alone in its natural state (solid, liquid or
gas) or in an appropriate formulation, including a dry
powder, an aerosol or a solution. The optional drying
time will, of course, depend on the nature of the
compound and its formulation.
Delivering a therapeutic substance on the
outs:ide.of the balloon used to compact the bone.or with a
second;(slightly.larger) balloon after the.bone.is
compacted, is qualitatively different than putting
formulated.drug into the cavity. When delivered:,while
compressing the bone, the substance becomes incorporated
into the compacted bone. This can -serve as.a-way to
instantly formulate a slow release version of the
substance. It simultaneously allows.the surgeon,to fill
the cavity wi-th an appropriate;:supporting material, like
acrylic bone cement or biocompatible bone substitute, so
no casting-or.metal fixation is required. Such a
combination allows the surgeon; for example,. to .,.
percutaneously fix.an osteoporotic-fracture while
delivering a desired therapeutic substance (like an
CA 02683004 2009-11-02
= - 40 -
50749-18D
antibiotic, bone growth factor or osteoporosis drug) to
the site. Thus,,casts or metal fixation devices are
generally not ever required.
Medically-effective amounts of therapeutic
substances are defined by their manufacturers or sponsors
and are generally in the range of 10 nanograms to 50
milligrams per site, although more or less may be
required in a specific case. Typical antibiotics include
gentamicin and tobramycin. Typical bone growth factors
are members of the Bone Morphogenetic Factor, Osteogenic
Protein, Fibroblast Growth Factor, Insulin-Like Growth
Factor and Transforming Growth Factor alpha and beta
families. Chemotherapeutic and related agents include
compounds such as cisplatin, doxorubicin, daunorubicin,
methotrexate, taxol and tamoxifen. Osteoporosis drugs
include estrogen, calcitonin, diphosphonates, and
parathyroid hormone antagonists.
The balloons described in this invention can be
used in open surgical procedures at the sites discussed
above to provide an improved space for inserting
orthopedic implants, bone graft, bone substitutes, bone
fillers or therapeutic substances. The size and shape
of balloon chosen would be determined by the site being
treated and then by the size, shape or amount of.material
that the surgeon wants to insert into thexemaining bone.
Square and rectangular balloons can be used-at any site
,for the placement of bone substitutes-like-
hydroxyapatites which`are available in those shapes.
Balloons would bemade to match those predetermined
sizes, and the,surgeon would chose the balloon to-fit the
size of material chosen.
To insert materials which do not flow into
the balloon-madecavity, like hydroxyapatite granules or
bone mineral matrix, the surgeon can push-them down a
tube with a long pin whose diameter is slightly more
CA 02683004 2009-11-02
50749-18D
-41-
narrow than the inner diameter of the canula through
procedures which the minimally-invasive procedure is
taking place. During open surgery., the surgeon can
approach the bone to be treated as if the procedure is
percutaneous, except that there,is no skin and other
tissues between the surgeon and the bone being treated.
This keeps the cortical bone as intact as possible. If
the material to be inserted does-not flow and should not
be pushed into the cavity through a canula (as in the
case of the hydroxyapatite block, because that can cause
damage), the surgeon can make the cavity using the
"minimally invasive" approach, then punch a hole using
standard tools (such as a punch, gouge or rasp) into one
side of.the cortical bone to allow insertion of the
block. This same approach can be used for implanting a
metal prosthesis, such as the metal tibial component of a
total knee replacement system.
Different sizes and/or-shapes of balloons may
be used at sites not specified;above, such as the jaw
bones, the midshaft of the arm and leg-bones, the
cervical vertebral bodies, the foot and ankle bones, the
ribs and the.like. Oneof the keys to choosing balloon
.,shape and size in treating or preventing-bone fracture is
the teaching of.this application that, optimally, about
70-90 s...of . the cancellous bone.needs to be compacted in
cases-where the bone disease causing fracture (or the
risk of:fracture) is the loss of cancellous bone-mass (as
in osteoporosis). Compacting less than the optimal 70-
90:0 of-the cancellous bone-at- the-site being treated (or
4*0-99 s as the workable range).may,:leave-too much-of the
diseased cancellous bone at the treated site. -The
diseased cancellous bone remains weak and can later
collapse, causing fracture.despite t.reatment. With this
principle,. the-allowed:shapes and:minimum sizes-for any
chosen bone are.explained and defined. .
CA 02683004 2009-11-02
- 42 -
50749-18D
There are specific exceptions to the 70-9001
rtile, as described in this specification. One is when
the bone disease being treated is localized, as in
avascular necrosis, where local loss of blood supply is
killing bone in a limited area. In that case, the
balloons can be smaller, because the diseased area
requiring treatment is smaller. A second exception is in
the use of the devices to improve insertion of solid
materials in defined shapes, like hydroxyapatite and
components in total joint replacement. In these cases,
the balloon shape and size is defined by the shape and
size of the material being inserted. Another exception
is the delivery of therapeutic substances. In this case,
the cancellous bone may or may not be affected. If it is
not, it is being sacrificed by compacting it to improve
the delivery of a drug or growth factor which has an
important therapeutic purpose. In this case, the bone
with the drug inside is supported while the drug works
and then the bone heals through casting or current
fixation devices.
Another key to choosing balloon shape and size
is'the teaching of this invention that inelastic balloon
restraints are generally required and that inelastic
balloon materials are preferred. These materials safely
and easily preventthe balloon from expanding beyond its
predetermined shape and size which is defined by the
limits of the normal dimensions of the outside edge of
the cancellous bone (which is the inside of the cortical
bone).~ A balloon which is too big, for example, creates
' the risk of immediate fracture, so this~ defines the upper
limits of balloon sizes at each site. With many typical
angioplasty balloons, surgeons usually-rely on monitoring
pressure (insteadof the balloon design features of this
invention) to prevent their balloons from inflating too
much. This requires greater surgical skill than the
CA 02683004 2009-11-02
~ - 43 -
50749-18D
teachings of this application, which are to take an X-ray
of the siteto be treated and measure the important
dimensions as described herein. In addition, inbone
treatment, relying on pressure can result in an inferior
clinical outcome. The surgeon generally will not know in
advance what pressure is required to completely compact
the cancellous bone, because this varies depending on the
thickness of the cancellous bone and the extent to which
it has lost density due to its disease. The surgeon is
likely to underinflate the balloon to avoid the harsh
consequences of overinflation and immediate fracture.
This leaves too much cancellous bone and can lead to
future fracture.
Another teaching of this application is that it
requires maximal pressures equally exerted in all
directions to compress cancellous bone. This is an
inherent property of the balloons drawn in figures in
this application and all the others described in the
specification. If the balloon design does not allow
this, it usually will not compress cancellous bone. The
shape of the cancellous bone to be compressed, and the
local structures that could be harmed if bone were moved
inappropriately, are generally understood by medical
professionals using textbooks of human skeletal anatomy
along with their knowledge of the site and its disease or-
injury.' Ranges of shapes and dimensions are defined by
the site to be treated. Precise dimensions for a given
patient=-are determined by X-ray of the site to be
treated, the therapeutic,goal and safety constraints at
the site. For diseased bone, replacement of the most of
the cancellous bone is usually desired, so a balloon
whose shape and size will compress around 70-900 of the
volume nf the cancellous bone in the treated region will
be chosen. However, balloons that are smaller or larger
may be appropriate,.particularly where. delivery of a
CA 02683004 2009-11-02
, . . - 44 -
50749-18D
therapeutic substance is the main goal. There, the
balloon size could be chosen by the desired amount of
therapeutic substance, keeping in mind that the balloon
should not displace the cortical bone beyond its normal
dimensions.