Note: Descriptions are shown in the official language in which they were submitted.
CA 02731376 2011-02-11
ENHANCEMENT OF FISCHER-TROPSCH PROCESS FOR
HYDROCARBON FUEL FORMULATION
The present invention relates to the modification of the Fischer-Tropsch
sequence of
operations including the Fischer-Tropsch process for the production of
hydrocarbon fuels
in an efficient manner.
In the prior art, the Fischer-Tropsch process has been used for decades to
assist in
the formulation of hydrocarbons. In the last several years, this has become a
concern
giving the escalating environmental concerns regarding pollution together with
the
increasing costs of hydrocarbon exploration and refining. The major producers
in this area
have expanded the art significantly in this technological area with a number
of patented
advances and pending applications in the form of publications.
In the art, advances made in terms of the raw materials that have been
progenitor
materials for the Fischer-Tropsch process, have included, for example, coal-to-
liquid
(CTL), bio-to-liquid (I3TL) and gas-to-liquid (GTL). One of the more
particularly
advantageous features of the bio or biomass to liquid technology is the fact
that it presents
a possibility to not only formulate a less carbon intensive product, but also
make use of
waste biomass materials, such as forestry by products, construction and other
wood waste
products, human waste products, or agriculture feedstocks, byproducts and
waste products.
As is generally known, the Fischer-Tropsch process converts hydrogen and
carbon
monoxide (commonly known as syngas) into liquid hydrocarbon fuels, examples of
which
include synthetic diesel, naphtha, kerosene, aviation or jet fuel and
paraffinic wax. As a
precursory step, the coal, gas or biomass, etc. is thermally gasified using
heat and pressure
to produce the syngas which results in turning the feedstock into hydrogen and
carbon
monoxide. As a result of the Fischer-Tropsch technique, the synthetic fuels
are very
appealing from an environmental point of view, since they are paraffinic in
nature and
substantially devoid of contamination. This is particularly true in the case
of the diesel fuel
synthesis where the synthetic product has ideal properties for diesel engines,
including
extremely high cetane rating >70, negligible aromatics and sulphur content, in
addition to
enabling optimum combustion and virtually emission free operation. Synthetic
diesel
fuels significantly reduce nitrous oxide and particulate matter when compared
with
petroleum based diesel fuel.
1
CA 02731376 2011-02-11
One example of recent advances that have been made in this area of technology
includes the features taught in United States Patent No. 6,958,363, issued to
Espinoza, et
al., October 25, 2005. In the document, Espinoza et al. provide for hydrogen
use in a GI'L
plant.
In essence, the patent teaches a process for synthesizing hydrocarbons where
initially, a synthesis gas stream is formulated in a syngas generator. The
synthesis gas
stream comprises primarily hydrogen and carbon monoxide. The process involves
catalytically converting the synthesis gas stream in a synthesis reaction to
produce
hydrocarbons and water followed by the generation of hydrogen-rich stream in
the
hydrogen generator. The process indicates that the hydrogen generator is
separate from
the syngas generator (supra) and that the syngas generator comprises either a
process for
converting hydrocarbons to olefins, a process for catalytically
dehydrogenating
hydrocarbons, or a process for refining petroleum, and a process for
converting
hydrocarbons to carbon filaments. The final step in the process in its
broadest sense,
involves consumption of hydrogen from the hydrogen-rich stream produced in one
or
more processes that result and increase value of the hydrocarbons or the
productivity of
the conversion of the hydrocarbons from the earlier second mentioned step.
Although a useful process, it is evident from the disclosure of Espinoza et
al. that
there is a clear intent to create olefins such as ethylene and propylene for
petrochemical
use, and aromatics for gasoline production. Additionally, there is a reforming
step
indicated to include the reformation of naphtha feedstock to generate a net
surplus
hydrogen byproduct which is then recombined into the process. The naphtha is
subsequently converted to aromatics for high octane gasoline blend stock.
There is no
specific contemplation and therefore no discussion of effectively destroying
the naphtha
for purposes of enhancing the Fischer-Tropsch process which, in turn, results
in the
significant augmentation of hydrocarbon synthesis.
The Espinoza et al. process is an excellent gas to a liquid process link to
gasoline
production from natural gas using naphtha reformation to make the gasoline
product. In
the disclosure, it was discovered that the excess hydrogen could be used to
enhance the
productivity of conversion.
A further significant advancement in this area of technology is taught by
Bayle et
al., in United States Patent No. 7,214,720, issued May 8, 2007. The reference
is directed to
2
CA 02731376 2011-02-11
the production of liquid fuels by a concatenation of processes for treatment
of a
hydrocarbon feedstock,
It is indicated in the disclosure that the liquid fuels begin with the organic
material,
typically biomass as a solid feedstock. The process involves a stage for the
gasification of
the solid feedstock, a stage for purification of synthesis gas and
subsequently a stage for
transformation of the synthesis gas into a liquid fuel.
The patentees indicate in column 2 the essence of the technology:
"A process was found for the production of liquid fuels starting from a
solid feedstock that contains the organic material in which:
a) The solid feedstock is subjected to a gasification stage so as to convert
said feedstock into synthesis gas comprising carbon monoxide and hydrogen,
b) the synthesis gas that is obtained in stage a) is subjected to a
purification
treatment that comprises an adjustment for increasing the molar ratio of
hydrogen
to carbon monoxide, H2/CO, up to a predetermined value, preferably between 1.8
and 2.2,
c) the purified synthesis gas that is obtained in stage b) is subjected to a
conversion stage that comprises the implementation of a Fischer-Tropsch-type
synthesis so as to convert said synthesis gas into a liquid effluent and a
gaseous
effluent,
d) the liquid effluent that is obtained in stage c) is fractionated so as to
obtain at least two fractions that are selected from the group that consists
of. a
gaseous fraction, a naphtha fraction, a kerosene fraction, and a gas oil
fraction, and
e) at least a portion of the naphtha fraction is recycled in gasification
stage."
Although a meritorious procedure, the overall process does not result in
increased
production of hydrocarbons. The naphtha recycle stream that is generated in
this process is
introduced into the gasification stage. This does not directly augment the
syngas volume to
the Fischer-Tropsch reactor which results in increased volumes of hydrocarbons
being
produced giving the fact that the feedstock is required for the process. To
introduce the
naphtha to the gasification stage as taught in Bayle et al., is to modify the
H2ICO ratio in
3
CA 02731376 2011-11-02
the gasification stage using an oxidizing agent such as water vapour and
gaseous
hydrocarbon feedstocks such as natural gas with the recycled naphtha, while
maximizing
the mass rate of carbon monoxide and maintain sufficient temperature above
1000 C to
1500 C in the gasification stage to maximize the conversion of tars and light
hydrocarbons.
In United States Patent No. 6,696,501, issued February 24, 2004, to Schanke et
al.,
there is disclosed an optimum integration process for Fischer-Tropsch
synthesis and
syngas production.
Among other features, the process increases the conversion of natural gas or
other
fossil fuels to higher hydrocarbons where the natural gas or the fossil fuel
is reacted with
steam and oxygenic gas in a reforming zone to produce synthesis gas which
primarily
contains hydrogen, carbon monoxide and carbon dioxide. The synthesis gas is
then passed
into a Fischer-Tropsch reactor to produce a crude synthesis containing lower
hydrocarbons, water and non-converted synthesis gas. Subsequently, the crude
synthesis
stream is separated in a recovery zone into a crude product stream containing
heavier
hydrocarbons, a water stream and a tail gas stream containing the remaining
constituents.
It is also taught that the tail gas stream is reformed in a separate steam
reformer with steam
and natural gas and then the sole reformed tail gas is introduced into the gas
stream before
being fed into the Fischer-Tropsch reactor.
In the reference, a high carbon dioxide stream is recycled back to an ATR in
order
to maximize the efficiency of the carbon in the process. It is further taught
that the primary
purpose of reforming and recycling the tail gas is to steam reform the lower
hydrocarbons
to carbon monoxide and hydrogen and as there is little in the way of light
hydrocarbons,
adding natural gas will therefore increase the carbon efficiency. There is no
disclosure
regarding the destruction of naphtha in an SMR or ATR to generate an excess
volume of
syngas with subsequent recycle to maximize hydrocarbon production. In the
Schanke et al.
reference, the patentees primarily focused on the production of the high
carbon content
syngas in a GTL environment using an ATR as crude synthesis stream and
reforming the
synthesis tail gas in an SMR with natural gas addition to create optimum
conditions that
feed to the Fischer-Tropsch reactor.
As with the previous art that has been discussed above, this reference
employs, as a
cornerstone technology, catalytic gasification.
4
CA 02731376 2011-02-11
In respect of other progress that has been made in this field of technology,
the art is
replete with significant advances in, not only gasification of solid carbon
feeds, but also
methodology for the preparation of syngas, management of hydrogen and carbon
monoxide in a GTL plant, the Fischer-Tropsch reactors management of hydrogen,
and the
conversion of biomass feedstock into hydrocarbon liquid transportation fuels,
inter alia.
The following is a representative list of other such references. This
includes: US Patent
Nos. 7,776,114; 6,765,025; 6,512,018; 6,147,126; 6,133,328; 7,855,235;
7,846,979;
6,147,126; 7,004,985; 6,048,449; 7,208,530; 6,730,285; 6,872,753, as well as
United
States Patent Application Publication Nos. US2010/0113624; US2004/0 1 8 1 3 1
3;
US2010/0036181; US2010/0216898; US2008/0021122; US 2008/0115415; and US
2010/0000153.
Copious features flow from practicing the technology of this application,
exemplary of which are:
i. high quality diesel product or additive;
ii. high quality diesel and jet fuel with an absence of sulfur;
iii. absence of petroleum by-products or low value feedstocks such as
naphtha;
iv. low emission and clean burning diesel and jet fuel;
v. increased cetane rating with concomitant augmented performance;
and
vi. significant volume output of diesel/jet fuel compared to
conventional processes using a Fischer-Tropsch reactor.
One aspect of the present invention is to provide an improved Fischer-Tropsch
based synthesis process for synthesizing hydrocarbons with a substantially
increased yield.
A further aspect of one embodiment of the present invention is to provide a
process
for synthesing hydrocarbons, comprising the steps of formulating a hydrogen
lean syngas
stream in a non-catalytic thermal partial oxidizing gasification reaction
(POX);
catalytically converting the syngas stream to produce naphtha; recycling
produced naphtha
as a feedstream to a hydrogen generator to formulate/synthesize a hydrogen
rich stream;
5
CA 02731376 2011-02-11
and combining the hydrogen rich stream with the hydrogen lean syngas stream of
step (a)
to enhance the conversion of hydrocarbons.
The present technology provides a very elegant solution to ameliorate the
shortcomings that have been clearly evinced in the prior art references.
Despite the fact
that the prior art, in the form of patent publications, issued patents, and
other academic
publications, all recognize the usefulness of a Fischer-Tropsch process, steam
methane
reforming, autothermal reforming, biomass gasification, naphtha recycle, and
other
processes, the prior art when taken individually or when mosaiced is deficient
a process
that provides the synthesis of a hydrogen rich stream to augment a lean stream
for passage
into a Fischer-Tropsch or suitable reactor for the purpose of enhancing the
production of,
as one example, diesel fuel or aviation fuel. As is well known, the Fischer-
Tropsch process
is particularly useful since the resultant synthetic fuel is "clean" fuel and
does not have the
contamination level typically associated with the same petroleum based fuel.
The present invention amalgamates, in a previously unrecognized combination, a
series of known unit operations into a much improved synthesis route for
production of
synthetic hydrocarbon fuels. This process engages a counter-intuitive step,
namely, the
removal of a production fraction, namely the naphtha, which, despite being a
refined
product, is then effectively destroyed making use of the naphtha as a
feedstock for a
hydrogen generator and then recycled into the Fischer-Tropsch generator. This
keystone
unit operation is propitious since it works in concert with all of the other
precursor
operations which, of their own right, are highly effective, namely the
gasification,
hydrogen generation, and Fischer-Tropsch synthesis operations.
It has been discovered that by employing the naphtha product fraction as a
feedstock to the hydrogen generator, shown in the example and discussed
hereinafter in
greater detail, as a steam methane reformer (SMR) results in a 40% increase in
the volume
of diesel, or as it is more effectively referred to in the art, as syndiesel.
In accordance with a further embodiment of the instant methodology, the
process
may also include an autothermal reforming unit (ATR) operation. As is well
known to
those skilled in the art, autothermal reforming employs carbon dioxide and
oxygen, or
steam, in a reaction with light hydrocarbon gases like natural gas to form
syngas. This is
an exothermic reaction in view of the oxidation procedure. When the
autothermal reformer
6
CA 02731376 2011-02-11
employs carbon dioxide, the hydrogen to carbon monoxide ratio produced is 1:1
and when
the autothermal reformer uses steam, the ratio produced is approximately 2.5:
1.
The reactions that are incorporated in the autothermal reformer are as
follows:
2CH4 + 02 + CO2 -+3 H2 + 3CO + H2O + 1-TEAT.
When steam is employed, the reaction equation is as follows:
4CH4 + 02 + 2H20 + HEAT -' 10H2 + 4CO.
One of the more significant benefits of using the ATR is realized in the
variability
of the hydrogen to carbon monoxide ratio. In the instant technology, an ATR
may also be
considered as a hydrogen generator, as described previously. It has been found
that the
addition of the ATR operation to the circuit in combination with the hydrogen
generation
circuit, shown in the example above as a steam methane reformer (SMR), has a
significant
effect on the hydrocarbon productivity from the overall process.
A major discovery materialized from making use of; for example, light
hydrocarbon gas as byproduct from the Fischer-Tropsch reaction and hydrocarbon
upgrader processing, commonly known as refinery gas, as a feedstock to the ATR
together
with the naphtha recycle as feedstock to the SMR, which results in a
significant increase in
the volume of syndiesel fuel produced. By way of example, by employing the
combination
of SMR and ATR with naphtha recycle, the process is capable of converting 100%
of all
the carbon introduced by the biomass feedstock to syndiesel with a 300%
increase in
production of syndiesel and synthetic jet fuel, as compared to conventional
Fischer-
Tropsch operation and without the production of any hydrocarbon byproducts.
This
obviously has significant economic benefits.
Accordingly, a further aspect of one embodiment of the present invention is to
provide a process for synthesing hydrocarbons, comprising the steps of (a)
formulating a
hydrogen lean syngas stream in a non-catalytic partial oxidation reformer
(POX) reaction;
(b) catalytically converting the syngas stream to produce hydrocarbon
containing at least
naphtha and fuel gas; (c) recycling the naphtha to a hydrogen generator to
form a
hydrogen rich stream; (d) recycling fuel gas from step (b) to a second syngas
generator to
form a supplemental syngas stream; and (e) combining the hydrogen rich stream
and the
supplemental syngas stream with the hydrogen lean stream of step (a) to
enhance the
conversion of hydrocarbons.
7
CA 02731376 2011-02-11
In accordance with a further aspect of one embodiment of the present
invention, a
system for synthesizing hydrocarbons, the system comprising: (a) means for
generating
syngas lean in hydrogen content; (b) means for catalytically converting the
syngas to
produce hydrocarbon containing at least naphtha; (c) a hydrogen generator; (d)
circuit
means for recycling naphtha to the hydrogen generator to form a hydrogen rich
stream;
and (e) circuit means for combining the hydrogen rich stream with the syngas
stream lean
in hydrogen content to provide a blended and enriched hydrogen content stream
for
enhancing hydrocarbon production.
Referring now to the drawings as they generally describe the invention,
reference
will now be made to the accompanying drawings illustrating preferred
embodiments and
in which:
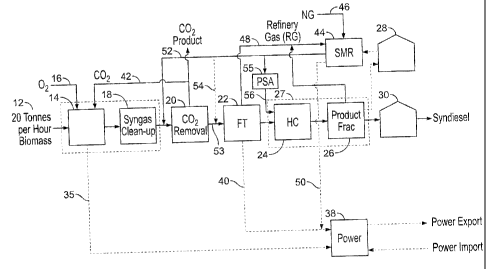
Figure 1 is a process flow diagram of methodology known in the prior art;
Figure 2 is a process flow diagram similar to Figure 1, illustrating a first
embodiment of the present invention;
Figure 3 is a process flow diagram illustrating a further variation of the
instant
technology;
Figure 4 is a process flow diagram illustrating yet another variation of the
present
invention;
Figure 5 is a process flow diagram of a still further embodiment of the
present
invention; and
Figure 6 is a process flow diagram illustrating a still further variation of
the present
methodology.
Similar numerals employed in the figures denote similar elements.
Referring now to Figure 1, shown is a process flow diagram of a circuit for
gasifying biomass with the result being the production of naphtha and
syndiesel. The
process is generally denoted by numeral 10 and begins with a biomass feedstock
12, which
feedstock has been described with examples herein previously. The biomass is
then treated
in a gasifier 14 to which oxygen 16 may be added as required. The gasifier may
be any
suitable gasifier, however, as an example, a gasifier that is useful in this
process is that
which has been patented by Choren Industries GmbH. Details of this gasifier
and the
8
CA 02731376 2011-02-11
process for using the gasifier are disclosed in United States Patent No.
7,776,114, issued
August 17, 2010, to Ruger et al. The Choren gasification process and apparatus
has been
found to be effective in the methodology of the present invention to be
discussed
hereinafter. Generally, as is known from the Choren process, the same
effectively involves
a low temperature pyrolysis stage which is followed by a high temperature
gasification
stage.
Although the Choren gasifier is a highly eligible process and apparatus for
carrying
out the instant technology, it will be well appreciated by those skilled in
the art that any
other suitable gasifier can be integrated into the process without any
compromise in
performance. Table 1 delineates gasifiers useful for syngas production.
TABLE 1
Special Gasifiers for Synthesis Gas Production
Carbo V Entrained CFB Blue Tower FICFB
flow gasifier Gasifier
Total system Low temp. Pyrolysis and Circulation Pyrolysis and Fast internal
gasifier and entrained flow Fluidized Bed reforming gasification
entrained flow gasifier gasifier (CFB)
gasifier
1 s' stage Low temp. Flash Pyrolysis 550-
gasifier at pyrolysis 600 C
400-600 C 500 C
Gasifier Autotherm Autotherm Autotherm Autotherm CFB
entrained flow entrained flow CFB with reforming with gasification
gasifier with 2 gasifier silica sand as ceramic as with 2 zones:
zones: 1300 C and bed, >900 C, heat carrier, Combustion
Combustion >50 bar Palm 950 C, Patm with air
1300-1500 C, 970 C,
Gasification Gasification
800-900 C 900 C
Palm
Gasification O2/air 02 02/H20 H2O H2O
Agent
Gas cleaning Bag filter, wet Wet scrubber, Hot gas filter unknown Filter, wet
scrubber, cooling SO2- with ceramic, scrubber,
S02-removal removal wet scrubber, ZnO
carbon adsorber,
adsorber removal of S
and Cl
9
CA 02731376 2011-02-11
Carbo V Entrained CFB Blue Tower FICFB
flow gasifier Gasifier
Gas* WGS, WGS, WGS, unknown unknown
conditioning C02-removal C02-removal C02-removal
Synthesis gas composition (%vol) after gas cleaning
H2 40.2 (22.1) 27 26.04 53 38-40
CO 39.2 (21.8) 50 29.91 12 22-26
CO2 20.4 (11.4) 14 33.69 25 20-22
CH4 0.1 (0) <0.1 8.8 6 9-11
N2 0.1 (44.7) 6.3 0.17 2 1.2-2
Note: The number in the bracket for Carbo V gasifier is the synthesis gas
composition, when air is
used as gasification agent.
Sources:
(1) Henrich, E. and Dinjus, E. 2003 Das FZK - Konzept zur
Kraftstoffherstellung aus Biomasse,
Der Konigsweg fur eine effiziente Strom- and Kraftstoffbereitstellung,
Leipzig;
(2) Rauch, R. 2002 Zweibett-Wirbelschichtvergasung in Guessing (A) mit 2
MWe1/4,5 MW,;
Konzept, Betrieberfahrung and Wirtschaftlichkeit. 7.Holzenergie-Symposium,
Zurich.
Optional unit, not required in this application.
As is known, the gasifier is useful for synthesizing a hydrogen lean or
deficient
synthesis gas (syngas) stream in a non-catalytic partial oxidation reaction.
The so formed
syngas is then subjected to cleaning operations 18 with subsequent removal of
carbon
dioxide at 20. It is not preferred in this process to include a water gas
shift (WGS) reactor
unit prior to the CO2 removal as all the carbon, primarily as CO is used to
the maximum
production of synthesis liquids product. The process uses the supplemental
addition of
hydrogen to maximize the conversion to syndiesel. The raw syngas is treated in
various
steps of scrubbing units and guard units well known to those skilled in the
art to create a
relatively pure clean syngas suitable for use in a Fischer-Tropsch unit. The
carbon dioxide
removal may also include a compression step (not shown) which is optionally
attributable
to the other processes discussed in forthcoming Figures. The syngas is then
transferred to a
Fischer-Tropsch reactor 22 to produce the hydrocarbons and water. The so
formed
hydrocarbons are then passed on to a hydrocarbon cracking stage 24, a product
fractionating stage 26 with naphtha being produced at 28 as a fraction, as
well as diesel 30
CA 02731376 2011-07-27
as an additional product. The diesel 30 formulated in this process is commonly
known as
syndiesel. As an example, this process as is well known in the art, results in
the
formulation of 701 barrels per day (bbl/day) based on 20 tonnes per hour of
forestry
biomass. As is illustrated in the flow diagram, an external source of hydrogen
32 is to be
supplemented to the Fischer-Tropsch unit 22 and hydrocarbon cracking unit 24
denoted as
streams 36 and 34 respectively. Further, energy 35 from the gasifier,
typically in the form
of steam, may be used to generate power and this is equally true of the
Fischer-Tropsch
reactor 22 creating energy 40. Table 2 establishes a comparison between FT
diesel and
conventional petroleum based diesel.
TABLE 2
Specification of FT-diesel in comparison to conventional diesel
Diesel Fuel Specification FT-Diesel Conventional Diesel
Chemical formula Paraffin C12H26
Molecular weight (kg/kmol) 170-200
Cetane number >74 50
Density (kg/1) at 15 C 0.78 0.84
Higher Heating Value (MJ/kg) at 15 C 44.0 42.7
Lower Heating Value (MJ/1) at 15 C 34.3 35.7
Stoichiometric air/fuel ratio (kg air/kg 14.53
fuel)
Oxygen content (%wt) -0 0-0.6
Kinematic viscosity (mm2/s) at 20 C 3.57 4
Flash point ( C) 72 77
Source: KMITL Sci. Tech. J. Vol. 6 No. 1 Jan. - Jun. 2006, p. 43
11
CA 02731376 2011-02-11
TABLE 3
Typical Specification of FT-Jet Fuel
Typical Product Specification FT Jet Fuel
Acidity mg KOH/g 0.10
Aromatics %vol max <25.0
Sulfur mass% <0.40
Distillation C
50% recovered Min 125 C max 190 C
End Point 270 C
Vapor Pressure kPa max 21
Flash Point C
Density 15 C, kg/m3 750-801
Freezing Point C max -51
Net Heat Combustion MJlkg min 42.8
Smoke Point mm, min 20
Naphthalenes vol% max <3.0
Copper Corrosion 2hr @ 100 C, max rating No l
Thermal Stability
Filter Pressure drop mm Hg, max 25
Visual Tube rating, max <3
Static Test 4hr @ 150 C mg/100ml, max -
Existent Gum mg/100ml, max _
Naphtha can be generally defined as a distilled fraction of the Fischer-
Tropsch FT
hydrocarbon liquids, categorized by way of example with a typical boiling
range of 30 C
to 200 C, and more preferred 30 C to 105 C. The specific naphtha specification
will be
optimized for each application to maximize syndiesel production and partially
or fully
eliminate the naphtha byproduct.
Suitable examples of FT reactors include fixed bed reactors and slurry-bubble
reactors such as tubular reactors, and multiphase reactors with a stationary
catalyst phase.
12
CA 02731376 2011-02-11
For the slurry-bubble reactor, the FT catalyst particles are suspended in a
liquid, e.g.,
molten hydrocarbon wax, by the motion of bubbles of syngas sparged into the
bottom of
the reactor. As gas bubbles rise through the reactor, the syngas is absorbed
into the liquid
and diffuses to the catalyst for conversion to hydrocarbons. Gaseous products
and
unconverted syngas enter the gas bubbles and are collected at the top of the
reactor. Liquid
products are recovered from the suspending liquid using different techniques
such as
separators, filtration, settling, hydrocyclones, and magnetic techniques.
Cooling coils
immersed in the slurry remove heat generated by the reaction. In a fixed bed
reactor, the
FT catalyst is held in a fixed bed contained in tubes or vessels within the
reactor vessel.
The syngas flowing through the reactor vessel contacts the FT catalyst
contained in the
fixed bed. The reaction heat is removed by passing a cooling medium around the
tubes or
vessels that contain the fixed bed. Other possibilities for the reactor will
be appreciated by
those skilled.
In the FT process, H2 and CO combine via polymerization to form hydrocarbon
compounds having varying numbers of carbon atoms. Typically 70% conversion of
syngas
to FT liquids takes place in a single pass of the FT unit. It is also common
practice to
arrange the FT reactors in series and parallel to achieve conversion levels of
90+%. After
the FT separation stage to divert the unconverted syngas and light
hydrocarbons, the FT
liquids are directed to the hydrocarbon upgrader unit denoted as 27. The
upgrader unit
typically contains a hydrocracking step 24 and a fractionation step 26.
Hydrocracking denoted as 24 used herein is referencing the splitting an
organic
molecule and adding hydrogen to the resulting molecular fragments to form
multiple
smaller hydrocarbons (e.g., C10H22 + 1-12 - C4I110 and skeletal isomers +
C6H14). Since a
hydrocracking catalyst may be active in hydroisomerization, skeletal
isomerization can
occur during the hydrocracking step. Accordingly, isomers of the smaller
hydrocarbons
may be formed. Hydrocracking a hydrocarbon stream derived from Fischer-Tropsch
synthesis preferably takes place over a hydrocracking catalyst comprising a
noble metal or
at least one base metal, such as platinum, cobalt-molybdenum, cobalt-tungsten,
nickel-
molybdenum, or nickel-tungsten, at a temperature of from about 550 F to about
750 F
(from about 288 C to about 400 C) and at a hydrogen partial pressure of about
500 psia to
about 1,500 psia (about 3,400 kPa to about 10,400 kPa).
13
CA 02731376 2011-02-11
The hydrocarbons recovered from the hydrocracker are further fractionated 26
and
refined to contain materials that can be used as components of mixtures known
in the art
such as naphtha, diesel, kerosene, jet fuel, lube oil, and wax. The combined
unit consisting
of the hydrocracker 24 and hydrocarbon fractionator 26 are commonly known as
the
hydrocarbon upgrades 27. As is known by those skilled in the art, several
hydrocarbon
treatment methods can form part of the upgrader unit depending on the desired
re -fined
products. The hydrocarbon products are essentially free of sulfur. The diesel
may be used
to produce environmentally friendly, sulfur-free fuel and/or blending stock
for diesel fuels
by using as is or blending with higher sulfur fuels created from petroleum
sources.
Further, useable energy commonly generated as steam from the gasification
stage,
denoted by numeral 35, may be used to generate electric power 38. This is
equally true of
useable energy that can be drawn from the Fischer-Tropsch unit, owing to the
fact that the
reaction is very exothermic and this represents a useable source of energy.
This is denoted
by numeral 40.
Turning now to Figure 2, shown is a preliminary embodiment of the technology
of
the instant invention. As is evinced from Figure 2, many of the preliminary
steps are
common with that which is shown in Figure 1. Table 4 lists a series of biomass
species
with calorific values for the purposes of examples. Table 5 sets forth
component analysis
of examples of biomass.
TABLE 4
Proximate analysis and calorific values of different biomass resources
Biomass Calorific value Ash content (%} Volatile matter Fixed Carbon
Species (Kcal/Kg) (%) (%)
Bagass 3406-4403.6 1.8-22.1 18.2-86.3 7,0-70.8
Bamboo Dust 3632-4731 5.8-16.5 71.6-76.5 9.3-21.0
Coconut coir 4318 17.2 69.6 13.2
Coconut fibre 4332 4.7 82.1 13.2
waste
Coconut shell 3649 1.9 79.9 18.2
Coir pith 4146 9.1 62.4 28.5
Corn cob -- 85.4 2.8 --
14
CA 02731376 2011-02-11
Biomass Calorific value Ash content (%) Volatile matter Fixed Carbon
Species (KcallKg) (ova) (%)
Corn stalks - 8.1 6.8 -
Cotton gin waste -- 88.0 5.4 --
Cotton shell 4360 4.6 72.2 23.3
Groundnut shell 4200-4680 2.3-5.4 72.2-77.9 19.8-22.9
Mustard shell 4126-4320 3.7-9.4 72.5-79.7 13.9-18.1
Mustard stalk 4018-4473 2.6-17.2 60.9-80.0 14.3-21.9
Pine needles 4750 1.5 72.4 26.1
Rice Bran 3950 13.1 75.7 11.2
Rice Husk 3000-3618 13.9-22.4 62.1-68.9 12.7-41.2
Rice straw 3730 15.5 68.3 16.2
(ground)
Sugarcane 4120-4390 4.8-10.9 70.4-77,4 14.9-19.2
leaves
Sweet Sorghum 4124-4230 7,4-7.7 74.0-76.0 16.6-18.3
stalk
Wheat stalk 3912 5.7 78.7 15.6
Wheat straw 4100-4516 6.4-8.0 69.6-80.6 11.7-24.0
Municipal solid 1345-3376 27.5-70.0 25.0-55.1 4.0-17.4
waste
Forestry waste 4000-4500 0.25-3 2-5 66-69
Source: Biomass-Thermo-chemical Characterization, Ed. PVR lyer, TR Rao & PD
Grover,
Biomass Conversion Laboratory, Chemical Engineering Department, lIT Delhi
CA 02731376 2011-02-11
TABLE 5
Sample component analysis of biomass (wt % on dry basis)
Biomass Cellulose Hemi-cellulose Lignin Extractives Ash
Species
Bagasse 33.6-41.3 22.6-27.0 15.0-18.3 13.7-18.4 2.9
Coconut coir 47.7 25.9 17.8 6.8 0.8
Coconut shell 36.3 25.1 28.7 8.3 0.7
Coir pith 28.6 15.3 31.2 15.8 7.1
Corn cob 40.3 28.7 16.6 15.4 2.8
Corn stalks 42.7 23.6 17.5 9.8 6.8
Cotton gin 77.8 16.0 0.0 1.1 5.4
waste
Rice husk 31.3 24.3 14.3 8.4 23.5
Rice straw 30.2-41.36 24.5-22.7 11.9-13.6 5.6-13.1 16.1-19,8
Wheat straw 30.5-40.0 28.9 16.4 7,38-13A 7.0-11.2
Source. Biomass-Thermo-chemical Characterization, Ed. PVR lyer, TR Rao & PD
Grover,
Biomass Conversion Laboratory, Chemical Engineering Department, IIT Delhi
Conveniently, the initial feedstock to the gasifier may be any one of coal,
biomass,
petroleum resids, municipal waste, plastics, wood, demetallized tire scrap,
forestry waste,
waste water byproduct, sewage biomass, livestock waste products, agricultural
byproduct
and waste, carbonaceous material and mixtures thereof.
As is widely appreciated by those of skill, the hydrogen to carbon monoxide
ratio
of the clean syngas leaving the biomass gasifier stage once it has passed the
cleanup stage
18, is generally 1:1. In the embodiment shown in Figure 2, the carbon dioxide
removal
stage 20, at least a portion of the carbon dioxide 42 may be reintroduced into
the gasifier
14 for purposes of controlling the reaction therein. Once the CO2 is removed,
the
procedure follows the unit operations as identified in Figure 1.
As the key difference, one of the most effective procedures in the instant
technology, relates to the fact that once the product fractionation stage has
been completed
and the naphtha 28 formulated, it has been found that by inclusion of the
hydrogen
16
CA 02731376 2011-07-27
generator using naphtha as the primary source, significant results can be
achieved in the
production of the synthetic diesel. This is effected by transferring at least
a portion of the
naphtha fraction created to a hydrogen steam generator 44, shown in the
example as a
steam methane reformer (SMR). This results in the formation of the hydrogen
rich stream
52. This procedure is well known and is perhaps one of the most common and
economic
methods for synthesizing hydrogen. The general reaction is as follows:
Natural Gas + Naphtha + Steam + Heat --+ CO + nH2 + CO2
The steam reformer may contain any suitable catalyst and be operated at any
suitable conditions to promote the conversion of the naphtha hydrocarbon to
hydrogen H2
and carbon monoxide. The addition of steam and natural gas may be optimized to
suit the
desired production of hydrogen and carbon monoxide. Generally natural gas or
any other
suitable fuel can be used to provide energy to the SMR reaction furnace. The
catalyst
employed for the steam reforming process may include one or more catalytically
active
components such as palladium, platinum, rhodium, iridium, osmium, ruthenium,
nickel,
chromium, cobalt, cerium, lanthanum, or mixtures thereof. The catalytically
active
component may be supported on a ceramic pellet or a refractory metal oxide.
Other forms
will be readily apparent to those skilled.
As has been discussed herein previously, it is unusual and most certainly
counter-
intuitive to effectively destroy the naphtha in order to generate a hydrogen
rich stream as
the naphtha is commonly desired as primary feedstock for gasoline production.
Although
this is the case, it is particularly advantageous in the process as set forth
in Figure 2. The
steam methane reformer may be augmented in terms of the hydrogen using natural
gas 46,
or using refinery gas 48 from the Fischer-Tropsch reactor 22 and the
hydrocarbon
upgrader 27. Energy recovered from the SMR 44 in the form of steam may be
distributed
via line 50 for production of electric power 38.
Once the hydrogen rich stream 52 has been formulated in the SMR, the same is
introduced into the syngas stream exiting syngas clean-up stage 18. At this
point, a
hydrogen rich stream from the SMR unit is combined at an optimal rate with a
relatively
lean hydrogen gas stream to generate the optimal Fischer-Tropsch syngas feed.
This
commingled or mixed stream is subject to the carbon dioxide removal and acts
as a
feedstock to the Fischer-Tropsch reactor 22. At the point of entering the
Fischer-Tropsch
reactor 22, the stream has a hydrogen to carbon monoxide ratio of
approximately 1:1 to
17
CA 02731376 2011-09-15
5:1, preferably 2:1 as indicated by numeral 53. Optionally, a portion 54 of
the hydrogen
rich stream 52 may bypass the CO2 removal unit and feed the Fischer-Tropsch
unit
directly at 53. Once the carbon dioxide removal stage 20 has been effected,
the hydrogen
to carbon monoxide ratio is approximately 2 and is then subsequently
introduced into the
Fischer-Tropsch reactor 22 and subjected to the same steps that have been
discussed with
respect to Figure 1. The results are quite substantial, providing the naphtha
recirculation
route and particularly using the naphtha as a feedstock to generate a hydrogen
rich stream,
the result is syndiesel production in great excess to that which is discussed
in Figure 1. As
an example, the syndiesel production by following the methodology of Figure 2
results in
a 977 barrel per day (bbl/day) production based on a 20 tonnes per hour
biomass feed.
Subsequently, a small portion of the hydrogen rich stream 52 may be removed
and
treated on by a common hydrogen purification unit, a typical example is a
Pressure Swing
Adsorption unit (PSA) 55, to create a high quality hydrogen stream 56 for use
in the
hydrocarbon upgrader unit 27.
Figure 3 sets forth a further interesting variation on the overall process
that is set
forth in Figure 2. In this variation, the process results in the formulation
of not only diesel,
but also jet fuel or aviation fuel. The operations common with Figure 2 are
denoted with
similar numerals. In this process variation, a split product is made between
the jet fuel and
diesel fuel. As an example, the split may be 25%:75% between jet fuel and
diesel
production from the fractionator 26. In order to effect this, jet fuel as
indicated by numeral
59 in Figure 3, requires modification of the fractionation unit operation 26.
As will be
appreciated by those skilled in the art, the fractionation unit operation can
be modified for
the jet fuel recovery by adding a suitable side stripper as part of the
fractionation unit
operation 26. In terms of further modifications to the overall process set
forth in Figure 2,
a hydrotreater step 57 should be considered for the hydrocarbon cracking unit.
The
hydrotreater is a method to ensure the stability of the refined products by
addition and
saturation of the product with hydrogen. The jet fuel produced is unique in
that it will be
of very'high purity and free of sulfur compounds, thus coveted as a "Clean
Green"
aviation fuel.
A further variation of the overall process embraced by the technology
discussed
herein is shown in Figure 4. In essence, the process flow of unit operations
shown in
Figure 4 is an amplification of the process as shown in Figure 2 and
essentially augments
18
CA 02731376 2011-09-15
further utilization of carbon and hydrogen to provide an alternate stream for
introduction
into the Fischer-Tropsch reactor 22. This has dramatic consequences on the
production of
syndiesel. As with the previous figures, the similarly denoted unit operations
are common
in Figure 4. From the flow diagram, it is evident that the SMR unit operation
44 Figure 2
is absent in this flow diagram. This unit operation has been replaced with an
ATR
(Autothermal Reformer) unit operation, denoted by numeral 60. Both the naphtha
and the
refinery gas, 62 and 48, respectively, may be combined or separately
transformed in the
ATR unit 60. Utility heating for the ATR may be provided by natural gas 66.
Oxygen may
be introduced at 70. The ATR is useful to produce some hydrogen and carbon
monoxide
syngas which is, of course, useful to introduce into and further enhance the
Fischer-
Tropsch reactor 22. External hydrogen may be used for the hydrocarbon upgrader
27
requirement. The formed syngas from the ATR is denoted by line 68 and is
introduced in
advance of the carbon dioxide removal stage 20. Alternatively, a portion of 68
or the entire
stream 68 may be introduced after the CO2 removal unit 20. Additional carbon
dioxide 69
may also be provided to the ATR to optimize the augmented syngas composition
to the
Fischer-Tropsch unit 22.
Turning to Figure 5, what is shown is yet another variation of the overall
process
according to the present invention combining the benefits of Figures 2 and 4.
In this
embodiment, both the SMR and ATR unit operations are amalgamated into the
generic
circuit with one embodiment of the present invention to convert all the carbon
introduced
as biomass feed to high value syndiesel product. This has dramatic
consequences in terms
of productivity of the diesel as is clear from an output of, as an example,
2,027 barrels per
day (bbl/day) based on 20 tonnes per hour of biomass feed. In this embodiment,
the
refinery gas 48 from the FT unit 22 and the upgrader unit 27 is employed as
feedstock to
the ATR unit 60. Further, naphtha 45 is employed as feedstock to the SMR unit
44 to
generate a hydrogen rich syngas. Further, the refinery gas 48, oxygen 70,
natural gas 46
and carbon dioxide 69 are commingled in optimized proportions and processed
through
the ATR 60 and blended with the SMR 44 hydrogen rich syngas to achieve the
optimum
syngas for combining with stream 53 to the FT unit 22. This effectively
results in a net
increase in carbon monoxide as well as hydrogen for use in the Fischer-Tropsch
reactor
22. As is evident from the flow diagram, the Fischer-Tropsch reactor is
effectively fed
with the hydrogen rich stream generated from the SMR as well as the
supplemental syngas
stream generated from the ATR. The SMR stream and ATR stream are commingled
with
19
CA 02731376 2011-07-27
the hydrogen lean syngas stream exiting cleanup unit operation 18 and
subsequently
introduced into the Fischer-Tropsch reactor 22. As noted above, this has a
very significant
effect on the output of the syndiesel and takes advantage of the effectiveness
of the
naphtha recycle for generating the hydrogen rich stream as well as the ATR
which
contributes hydrogen and carbon monoxide for mixture with the lean gas stream.
The
combination of all these syngas streams can effectively result in the full
transformation of
all the carbon entering the process as biomass being converted to highly
valuable green
syndiesel without by product hydrocarbons.
Referring now to Figure 6, shown is a further variation of the overall process
which is similar to that which is shown in Figure 5, with the exception of the
absence of
gasifier 14 and syngas cleanup operation 18.
While the preferred embodiments of the invention have been shown and
described,
modifications thereof can be made by one skilled in the art without departing
from the
spirit and teachings of the invention. Reactor design criteria, hydrocarbon
processing
equipment, and the like for any given implementation of the invention will be
readily
ascertainable to one of skill in the art based upon the disclosure herein. The
embodiments
described herein are exemplary only, and are not intended to be limiting.