Note: Descriptions are shown in the official language in which they were submitted.
CA 02733852 2011-03-10
ELECTROCHEMICAL METHOD FOR MEASURING CHEMICAL REACTION RATES
Field of the Invention
The present invention relates to the measurement of the progress of a chemical
reaction that generates an
electroactive reaction product that is subsequently detected at an electrode
amperometrically or coulometrically. The
method is useful in applications where it is desirable to follow the progress
of, a chemical reaction, particularly in
sensor applications where the progress of the reaction of an analyte can be
useful in determining the analyte
concentration.
Background of the Invention
Description of the Related Art
In amperometric electrochemistry the current flowing at the electrode can be
used as a measure of the
concentration of electroactive species being reacted electrochemically at.the
working electrode. In coulometry the
current flowing at the electrode is integrated over time to give a total
amount of charge passed which yields a measure
of the amount of electroactive material reacted at the working electrode. The
current flowing (or charge passed at any
time) at the electrode is dependent upon the rate of transfer of the
electroactive species to the working electrode.
When a significant concentration of electroactive species is situated close to
the electrode and an electrical potential is
applied to the electrode sufficient to electrochemically react the
electroactive species at the electrodelsolution
interface, initially a higher current will flow which will diminish with time.
For an isolated electrode, where the
potential applied to the electrode is sufficient to react the electroactive
species effectively instantaneously upon
arriving-at the electrode and the transfer of electroactive species to the
electrode is controlled by diffusion, the current
will follow a curve known in the art as the Cottrell Equation. According to
this equation the current varies inversely
with the square root of time. This yields a current which decays with time as
the electroactive species that reacts at
the electrode becomes depleted close to the electrode and so electroactive
species has to travel from further and
further away to reach the electrode as time progresses.
If in addition to the electrochemical reaction of the electroactive species at
the electrode the electroactive
species is being generated close to the working electrode by a chemical
reaction, the form of the current flowing at the
electrode becomes complex. The electrode reaction tends to decrease the
concentration of electroactive species close
to the working electrode whereas the chemical reaction tends to increase the
concentration of the electroactive
species in this region. The time dependent behavior of these two processes
therefore mix and it is difficult to measure
the chemical reaction kinetics from the current flowing (or charge passed) at
the electrode.
For this reason, in the published literature, the rates of chemical reactions
are not generally measured
electrochemically except in specialized applications using specialized
equipment. An example of such equipment is
known in the art as a rotating ringidisc electrode. This apparatus is only
applicable to relatively fast reaction kinetics
and requires that the electrode be rotated at a known controlled rate with
well. characterized liquid hydrodynamics.
=1=
CA 02733852 2011-03-10
Summary of the Invention
The method provided allows for the extraction of chemical reaction rate
information using a simple
electrochemical method and apparatus.
In a first aspect, a method is provided for measuring a rate of a chemical
reaction between a component of a
liquid sample and a reagent, the reaction producing an electroactive species,
including providing an electrochemical cell
having a working electrode, a counter electrode, and at least one Wall;
substantially immobilizing the reagent in the
electrochemical cell at a site at a minimum distance from the working
electrode, wherein the distance is such that
transfer of the electroactive species from the site to the working electrode
is diffusion controlled; placing the liquid
sample in the electrochemical cell such that the liquid sample is in contact
with the reagent, the working electrode, and
the counter electrode; reacting the component with the reagent to produce the
electroactive species; applying a
potential between the working electrode and the counter electrode, wherein the
potential is sufficient to
electrochemically react the electroactive species at the working electrode;
and measuring the current produced by the
electrochemical reaction at the working electrode to obtain a measure of the
rate of the chemical reaction.
In one aspect of this embodiment, the working electrode and the counter
electrode are sufficiently spaced
such that a product of an electrochemical reaction occurring at the counter
electrode does not reach the working
electrode while the current is measured. The working electrode and the counter
electrode may be spaced apart at a
distance greater than about 500 microns; between about 500 microns and about 5
mm; or between about 1 mm and
about 2 mm. The working electrode and the counter electrode may be situated on
the same plane.
In another aspect of this embodiment, the site and the working electrode are
separated by a minimum
distance ranging from about '10 microns to about 5 millimeters; from about 50
microns to about 500 microns; or from
about 100 microns to about 200 microns.
In another aspect of this embodiment, the counter electrode is capable of
functioning as a combined
counterlreference electrode. The electrochemical cell may further include a
reference electrode.
In another aspect of this embodiment, the working electrode functions as an
anode, and may include
platinum, palladium, carbon, carbon in combination with one or more inert
binders, iridium, indium oxide, tin oxide,
indium in combination with tin oxide, and mixtures thereof.
In another aspect of this embodiment, the working, electrode functions as an
cathode and may include
platinum, palladium, carbon, carbon in combination with one or more inert
binders, iridium, indium oxide, tin oxide,
indium in combination with tin oxide, steel, stainless steel, copper, nickel,
silver, chromium, and mixtures thereof.
In another aspect of this embodiment, the counter electrode includes platinum,
palladium, carbon, carbon in
combination with inert binders, iridium, indium oxide, tin oxide, indium in
combination with tin oxide, steel, stainless
steel, copper, nickel, chromium, silver, and mixtures thereof. The counter
electrode may also include silver coated with
a substantially insoluble silver salt, such as silver chloride, silver
bromide, silver iodide, silver ferrocyanide, and silver
ferricyanide.
-2-
CA 02733852 2011-03-10
In another aspect of this embodiment, the site is situated on the counter
electrode or on the wall.
The site and the working electrode may be situated on the same plane or in a
plane facing and substantially
parallel to the working electrode.
In another aspect of this embodiment, the reagent is contained within a
polymeric matrix attached
to a surface in the electrochemical cell; is chemically tethered to a surface
in the electrochemical cell; is
physically tethered to a surface in the electrochemical cell; or is dried onto
a surface in the electrochemical
cell and exhibits sufficiently low mobility in the liquid sample such that the
reagent does not substantially
migrate while the current is measured.
In another aspect of this embodiment, the method further includes a redox
mediator. The redox
mediator may include ferrocinium, osmium complexes with bipyridine,
benzophenone, and ferricyanide.
In another aspect of this embodiment, the sample may include whole blood. The
component may
include glucose. The reagent may include an enzyme such as PQQ dependent
glucose dehydrogenase, NAD
dependent glucose dehydrogenase, glucose oxidase, lactate dehydrogenase, and
alcohol dehydrogenase.
In another aspect of this embodiment, the potential is preferably between +50
and +500 mV, and
more preferably about +300 mV.
in a second embodiment, a method is provided for measuring a rate of a
chemical reaction between
glucose and PQQ dependent glucose dehydrogenase in whole blood including
providing an electrochemical cell
having a working electrode, a counter electrode, at least one wall, a redox
mediator including ferricyanide
and contained within the electrochemical cell, and a reagent including PQQ
dependent glucose
dehydrogenase, the reagent being substantially immobilized in the
electrochemical cell at a site at a minimum
distance from the working electrode; placing the whole blood sample in the
electrochemical cell such that the
sample is in contact with the reagent, the redox mediator, the working
electrode, and the counter electrode;
reacting the glucose with the PQQ dependent glucose dehydrogenase to produce
reduced PQQ dependent
glucose dehydrogenase, the reduced PQQ dependent glucose dehydrogenase in turn
reacting with the ferricyanide
redox mediator to form ferrocyanide; applying a potential between the working
electrode and the counter
electrode, wherein the potential is sufficient to electrochemically react the
ferrocyanide at the working electrode;
and measuring the current produced by the electrochemical reaction of
ferrocyanide at the working electrode,
wherein the measurement is indicative of the rate of the chemical reaction
between glucose and PQQ
dependent glucose dehydrogenase.
in accordance with a further aspect, there is provided a method for measuring
a rate of a chemical
reaction between a component of a liquid sample and a reagent, the reaction
producing an electroactive
species, comprising:
providing an electrochemical cell having a working electrode, a counter
electrode, and at least one
wall;
-3-
CA 02733852 2011-03-10
substantially immobilizing the reagent in the electrochemical cell at a site
at a minimum distance
from the working electrode, wherein the site is situated on the counter
electrode, wherein the distance is
such that transfer of the electroactive species from the site to the working
electrode is diffusion controlled;
placing the liquid sample in the electrochemical cell such that the liquid
sample is in contact with
the reagent, the working electrode, and the counter electrode;
reacting the component with the reagent to produce the electroactive species;
applying a potential between the working electrode and the counter electrode,
wherein the
potential is sufficient to electrochemically react the electroactive species
at the working electrode; and
measuring the current produced by the electrochemical reaction at the working
electrode to obtain
a measure of the rate of the chemical reaction, the rate of the chemical
reaction being defined by a change
in concentration of the reagent with respect to a change in time.
In accordance with a further aspect, there is provided a method for measuring
a rate of a chemical
reaction between a component of a liquid sample and a reagent, the reaction
producing an electroactive
species, comprising:
providing an electrochemical cell having a working electrode, a counter
electrode, and at least one
wall;
substantially immobilizing the reagent in the electrochemical cell at a site
at a minimum distance
from the working electrode, wherein the site and the working electrode are
situated on the same plane,
wherein the distance is such that transfer of the electroactive species from
the site to the working electrode
is diffusion controlled;
placing the liquid sample in the electrochemical cell such that the liquid
sample is in contact with
the reagent, the working electrode, and the counter electrode;
reacting the component with the reagent to produce the electroactive species;
applying a potential between the working electrode and the counter electrode,
wherein the
potential is sufficient to electrochemically react the electroactive species
at the working electrode; and
measuring the current produced by the electrochemical reaction at the working
electrode to obtain
a measure of the rate of the chemical reaction, the rate of the chemical
reaction being defined by a change
in concentration of the reagent with respect to a change in time.
In accordance with a further aspect, there is provided a method for measuring
a rate of a chemical
reaction between glucose and PQQ dependent glucose dehydrogenase in whole
blood comprising:
providing an electrochemical cell having a working electrode, a counter
electrode, at least one
wall, a redox mediator comprising ferricyanide and contained within the
electrochemical cell, and a reagent
comprising PQQ dependent glucose dehydrogenase, the reagent being
substantially immobilized in the
electrochemical cell at a site at a minimum distance from the working
electrode, wherein the site is situated
on the counter electrode;
-3a-
CA 02733852 2011-03-10
placing a whole blood sample in the electrochemical cell such that the sample
is in contact with the
reagent, the redox mediator, the working electrode, and the counter electrode;
reacting the glucose with the PQQ dependent glucose dehydrogenase to produce
reduced PQQ
dependent glucose dehydrogenase, the reduced PQQ dependent glucose
dehydrogenase in turn reacting
with the ferricyanide redox mediator to form ferrocyanide;
applying a potential between the working electrode and the counter electrode,
wherein the
potential is sufficient to electrochemically react the ferrocyanide at the
working electrode ; and
measuring the current produced by the electrochemical reaction of ferrocyanide
at the working
electrode, wherein the measurement is indicative of the rate of the chemical
reaction between glucose and
PQQ dependent glucose dehydrogenase, the rate of the chemical reaction being
defined by a change in
concentration of the ferrocyanide with respect to a change in time.
In accordance with a further aspect, there is provided a method for measuring
a rate of a chemical
reaction between a component of a liquid sample and a reagent comprising PQQ
dependent glucose
dehydrogenase, the reaction producing an electroactive species, comprising:
providing an electrochemical cell having a working electrode, a counter
electrode, and at least one
wall;
substantially immobilizing the reagent comprising PQQ dependent glucose
dehydrogenase in the
electrochemical cell at a site at a minimum distance from the working
electrode, wherein the site and the
working electrode are situated on the same plane, and wherein the distance is
such that transfer of the
electroactive species from the site to the working electrode is diffusion
controlled;
placing the liquid sample in the electrochemical cell such that the liquid
sample is in contact with
the reagent, the working electrode, and the counter electrode;
reacting the component with the PQQ dependent glucose dehydrogenase reagent to
produce the
electroactive species;
applying a potential between the working electrode and the counter electrode,
wherein the
potential is sufficient to electrochemically react the electroactive species
at the working electrode; and
measuring the current produced by the electrochemical reaction at the working
electrode to obtain
a measure of the rate of the chemical reaction, the rate of the chemical
reaction being defined by a change
in concentration of the electroactive species with respect to a change in
time.
Brief Description of the Drawings
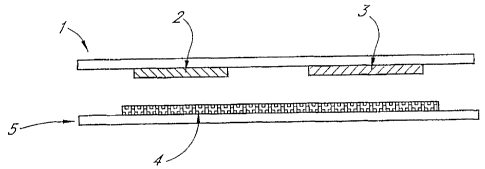
Figure 1 depicts an electrochemical cell wherein the reagent is situated on a
wall of the cell facing
the working electrode.
Figure 2 depicts an electrochemical cell wherein the reagent is situated on
the counter electrode.
-3b-
CA 02733852 2011-03-10
Figure 3 shows current as a function of time for three whole blood samples for
a reaction system
including glucose, PQQ dependent glucose dehydrogenase and potassium
ferricyanide redox mediator. The
three blood samples contain haematocrit levels of 20%, 42%, and 65%,
respectively, where the haematocrit
is the volume percent of red
-3c-
CA 02733852 2011-03-10
blood cells in the sample.
Detailed Description of the Preferred Embodiment
The following description and examples illustrate a preferred embodiment of
the present invention in detail.
Those of skill in the art will recognize that there are numerous variations
and modifications of this invention that are
encompassed by its scope. Accordingly, the description of a preferred
embodiment should not be deemed to limit the
scope of the present invention.
According to the present invention, information relating to the rate of a
chemical reaction that yields at least
one electroactive product can be obtained using an electrochemical cell by
ensuring that the chemical reaction is
localized at a site remote from the electrode used to electrochemically react
the electroactive product (s).
Methods and devices for obtaining electrochemical measurements of fluid
samples are discussed further
in copending U. S. patent application no 091615,691, filed on July 14,2000,
entitled "ANTIOXIDANT SENSOR,"
copending U. S. patent application no 091616,512, filed on July 14,2000,
entitled "HEMOGLOBIN SENSOR," and
copending U. S. patent application no 091616,433, filed on July 14,2000,
entitled "IMMUNOSENSOR".
The site of the chemical reaction is sufficiently removed from the electrode
such that the mass transfer of
the electroactive species from the chemical reaction site to the electrode
effectively controls the current flowing at the
electrode at any time. This arrangement ensures a substantially linear
electroactive species concentration gradient
between the chemical reaction site and the electrode. The concentration of the
electroactive species is maintained at
effectively zero at the electrode by the electrochemical reaction taking place
there. The time course of the magnitude
of this concentration gradient will therefore be substantially determined only
by the time course of the concentration
of the electroactive specie(s) at the chemical reaction site and the diffusion
coefficient(s) of the electroactive reaction
product(s) in the liquid medium. Since the current flowing at the electrode is
proportional to the concentration gradient
of the electroactive specie(s) at the electrode, the time course of this
current will reflect the time course of the
chemical reaction occurring at the remote site. This allows the current
measured at the electrode (or charge passed if
the current is integrated) to be a used as a convenient measure of the rate
and extent of the chemical reaction taking
place.
An example of a suitable method for ensuring that the chemical reaction is
remote from the working
electrode is to immobilize one or more of the reaction components on a solid
surface remote from the electrode. The
reaction componen (s) can be immobilized by incorporating them in a polymeric
matrix that is dried on or otherwise
attached to the solid surface. The reaction component(s) can also be tethered
directly to the solid surface either by
chemical or physical bonding. Alternatively one or more of the reaction
components can simply be dried onto the solid
surface without special immobilization means. In this situation one or more of
the reaction components is sufficiently
low in mobility, in the liquid matrix filling the electrochemical cell, that
it does not migrate substantially from the
position where it was dried during the time period that the electrochemical
current can be usefully monitored to
-4-
CA 02733852 2011-03-10
perform the required measurement. In this context substantial migration means
that the slowest moving component
required for the chemical reaction approaches closely enough to the working
electrode that Cottrell type depletion
kinetics begin to effect the time course of the current flowing at the
electrode,
The range of separation distance between the chemical reaction site and the
working electrode in preferred
embodiments is desirably less than about 1 cm, preferably less than 5 mm, more
preferably between 5,10,50,100,
200, 500 microns and 5 mm, more preferably between 5,10,50,100,200 and 500
microns, and most preferably
between 5,10,50,100 and 200 microns.
As well as the working electrode, at least a counter electrode in contact with
the liquid sample is provided to
complete the electrochemical circuit. Optionally the counter electrode can
function as a combined counter/reference
electrode or a separate reference electrode can be provided. In a preferred
embodiment, the working electrode and
counter electrode are desirably spaced apart at a distance greater than about
300 microns, preferably at a distance
greater than about 500 microns, more preferably at a distance between about
500 microns and 10 mm, more
preferably at a distance between about 500 microns and 1,2,5 mm, and most
preferably between 1 mm and 2,5,10
mm.
The working electrode is constructed of materials that do not react chemically
with any component with
which it will come into contact during use to an extent that interferes with
the current response of the electrode. If
the working electrode is to be used as an anode then examples of suitable
materials are platinum, palladium, carbon,
carbon in combination with inert binders, iridium, indium oxide, tin oxide,
mixtures of indium and tin oxide. If the
working electrode is to be used as a cathode then in addition to the material
listed above other suitable materials are
steel, stainless steel, copper, nickel, silver and chromium.
Examples of materials suitable for the counter electrode are platinum,
palladium, carbon, carbon in
combination with inert binders, iridium, indium oxide, tin oxide, mixture of
indium and tin oxide, steel, stainless steel,
copper, nickel, chromium, silver and silver coated with a substantially
insoluble silver salt such as silver chloride, silver
bromide, silver iodide, silver ferrocyanide, silver ferricyanide.
The site of the chemical reaction can be localized on a bare wall or on the
counter electrode, remote from the
working electrode. The site of the chemical reaction can be on the same plane
as the working electrode or more
preferably in a plane facing and substantially parallel to the working
electrode.
Figure 1 depicts an apparatus suitable for use with one embodiment. In Figure
1, a working electrode 2 and
a counter electrode 3 are disposed on an electrically insulating substrate 1.
On a second substrate 5 is disposed a
layer of chemical reactants 4, where at least one of the reactants is
substantially immobilized on the substrate 5. In
use, the space between substrates 1 and 5 is filled with a liquid containing a
substance which is capable of reacting with
the reagents 4 to produce at least one electroactive species. The products of
the chemical reaction diffuse towards
the working electrode 2 where the electroactive specie(s) are
electrochemically reacted to produce a current. The
magnitude of the current or the charge passed at a particular time, or the
time course of the current or charge passed
-5-
CA 02733852 2011-03-10
can then be used to obtain a measure of the rate or extent of the chemical
reaction occurring at the reactant layer 4.
Figure 2 depicts another embodiment. The numbering of the components in Figure
2 correspond to the
components in Figure 1. In Figure 2 the reactants 4 are disposed on the
counter electrode 3 which is disposed on
a substrate 5 which can be electrically resistive. In this embodiment the
materials of construction of the counter
electrode 3 are inert to reaction with any of the components of the reactants
4 disposed on the electrode 3.
An example of a chemistry and reaction that is suitable for use in a preferred
embodiment is measuring
glucose in whole blood using the enzyme PQQ dependent glucose dehydrogenase
(GDHpgq) and a redox mediator. In
this reaction glucose in the blood reacts with GDHpgq to form gluconic acid.
In the process, the PQQ in the enzyme is
reduced. A mediator, such as potassium ferricyanide, then oxidizes the PQQ in
the enzyme and forms ferrocyanide.
The enzyme in the oxidized form can then react with further glucose. The net
effect of this reaction is to produce two
ferrocyanide molecules for each glucose molecule reacted. Ferrocyanide is an
electroactive species, and so can be
oxidized at an electrode to produce a current. Other suitable enzymes for this
reaction are glucose oxidase (GOD) or
NAD dependent glucose dehydrogenase. For other reactions, lactate
dehydrogenase and alcohol dehydrogenase may
be used. Other suitable redox mediators include ferrocinium, osmium complexes
with bipyridine, and benzophenone.
The reaction of glucose in whole blood with the enzyme can be slow, taking up
to a few minutes to go to
completion. Also, the higher the haematocrit of the blood sample, the slower
the reaction. The haematocrit of the
blood is the volume fraction of red cells in the whole blood sample. In this
example, an electrochemical cell according
to Figure 2 was constructed. A solution containing 50 mg/ml GDHpqq, 0.9 M
potassium ferricyanide and 50 mM
buffer at pH 6.5 was deposited on the counter electrode and the water removed
to leave a dried reactant layer. In this
layer the GDHpqq is large enough to be effectively immobilized on the counter
electrode, whereas the ferricyanide can
mix more evenly throughout the liquid in the electrochemical cell. The blood
sample was introduced into the cell and a
potential of +300 mV immediately applied between the working electrode and the
counter electrode. Although a
potential of +300 mV is most preferred for oxidizing ferrocyanide, the
potential is desirably between +40 mV and
+600 mV, preferably between +50 mV and +500 mV, and more preferably between
+200 mV and +400 mV. In the
cell, the working electrode consisted of a layer of gold sputtered onto a
polyester substrate and the counter electrode
consisted of a layer of palladium sputtered onto a polyester substrate.
The current traces recorded for blood samples of different haematocrits,
showing a faster rate of reaction in
lower haematocrit blood, are given in Figure 3. The number at the end of each
line is the percent haematocrit of the
blood sample used, i.e., 20%, 42%, and 65%, respectively. The glucose level in
each blood sample is approximately
the same, namely 5.4 mM for the 65% haematocrit sample, 5.5 mM for the 42%
haematocrit sample, and 6.0 mM for
the 20% haematocrit sample.
The current shown in Figure 3 can be approximately given by the equation:
i = -FADC/L
where i is the current, F is Faraday's constant (96486.7 C/mole), A is the
electrode area, D is the diffusion coefficient
-6-
CA 02733852 2011-03-10
of the ferrocyanide in the sample, C is the concentration of ferrocyanide at
the reaction site and L is the distance
between the reaction site and the electrode. The reaction rate, given by the
rate of change of C with time is therefore
given by:
dC/dt - -(LIFAD)dildt.
For the reactions depicted in Figure 3, between 6 and 8 seconds for the 20%,
42% and 65% haematocrit samples, the
average dildt was 3.82, 2.14 and 1.32 microamps/second, respectively. The
diffusion coefficients of ferrocyanide for
these samples were 2.0 x 10'8, 1.7 x 10'6 and 1.4 x 10-6 cm2lsec for 20%, 42%,
and 65% haematocrit samples,
respectively. The electrode area was 0.1238 cm2 and L was 125 microns. These
values yield reaction rates of 2.0,
1.3, and 0.99 mMlsecond for the 20%, 42%, and 65% haematocrit samples,
respectively.
The method described above may be used with any suitable electrochemical
system. For example, the
method may be used for measuring oxidants or antioxidants in fluid samples.
The method is applicable to any oxidant
or antioxidant that exists in a usefully representative concentration in a
fluid sample. Antioxidants that may be
analyzed include, for example, sulfur dioxide and ascorbic acid. Oxidants that
may be analyzed include, for example,
chlorine, bromine iodine, peroxides, hypochlorite, and ozone. Water insoluble
oxidants or antioxidants may also be
analyzed if an aqueous form can be prepared, e.g., by using a detergent to
prepare an emulsion of the water insoluble
redox reactive analyte. The method may also be applied to antibodylantigen
reactions or to hemoglobin analysis.
The above description discloses several methods and materials of the present
invention. This invention is
susceptible to modifications in the methods and materials, as well as
alterations in the fabrication methods and
equipment. Such modifications will become apparent to those skilled in the art
from a consideration of this disclosure
or practice of the invention disclosed herein. Consequently, it is not
intended that this invention be limited to the
specific embodiments disclosed herein, but that it cover all modifications and
alternatives coming within the true scope
and spirit of the invention as embodied in the attached claims.
.7.