Note: Descriptions are shown in the official language in which they were submitted.
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
PROCESSES FOR MAKING
ETHYL ACETATE FROM ACETIC ACID
PRIORITY CLAIM
[0001] This application claims priority to U.S. Application Number 12/588,727,
filed October
26, 2009, entitled "Tunable Catalyst Gas Phase Hydrogenation of Carboxylic
Acids," the
entirety of which is incorporated herein by reference.
FIELD OF THE INVENTION
[0002] The present invention relates generally to processes for hydrogenating
acetic acid to
form ethyl acetate or a mixture of ethyl acetate and ethanol and to novel
catalysts for use in
such processes, the catalysts having high selectivities for ethyl acetate.
BACKGROUND OF THE INVENTION
[0003] There is a long felt need for an economically viable process to convert
acetic acid to
ethyl acetate. Ethyl acetate is an important commodity feedstock for a variety
of industrial
products and is also used as an industrial solvent in the manufacture of
various chemicals. For
instance, ethyl acetate can readily be converted to ethylene by subjecting it
to a cracking
process, which can then be converted to a variety of other products. Ethyl
acetate is
conventionally produced from feedstocks where price fluctuations are becoming
more
significant. That is, fluctuating natural gas and crude oil prices contribute
to fluctuations in the
cost of conventionally produced, petroleum or natural gas-sourced ethyl
acetate, making the
need for alternative sources of ethyl acetate all the greater when oil prices
rise.
[0004] Ethanol is another important commodity chemical, which may be used in
its own
right, for example as a fuel, or as a feedstock for forming ethylene, vinyl
acetate, ethyl acetate,
or other chemical products. The hydrogenation of carboxylic acids over
heterogeneous
catalysts to produce alcohols is well reported. For instance, United States
Patent No. 2,607,807
discloses that ethanol can be formed from acetic acid over a ruthenium
catalyst at extremely
high pressures of 700-950 bar in order to achieve yields of around 88%,
whereas low yields of
only about 40% are obtained at pressures of about 200 bar. However such
extreme reaction
conditions are unacceptable and uneconomical for a commercial operation.
1
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
[0005] More recently, even though it may not still be commercially viable it
has been
reported that ethanol can be produced from hydrogenating acetic acid using a
cobalt catalyst at
superatmospheric pressures such as about 40 to 120 bar. See, for example,
United States Patent
No. 4,517,391 to Shuster et al.
[0006] On the other hand, United States Patent No. 5,149,680 to Kitson et al.
describes a
process for the catalytic hydrogenation of carboxylic acids and their
anhydrides to alcohols
and/or esters utilizing a platinum group metal alloy catalyst. The catalyst is
comprised of an
alloy of at least one noble metal of Group VIII of the Periodic Table and at
least one metal
capable of alloying with the Group VIII noble metal, admixed with a component
comprising at
least one of the metals rhenium, tungsten or molybdenum. Although it has been
claimed
therein that improved selectivity to a mixture of alcohol and its ester with
the unreacted
carboxylic acid is achieved over the prior art references it was still
reported that 3 to 9 percent
of alkanes, such as methane and ethane are formed as by-products during the
hydrogenation of
acetic acid to ethanol under their optimal catalyst conditions.
[0007] A slightly modified process for the preparation of ethyl acetate by
hydrogenating
acetic acid has been reported in EP 0 372 847. In this process, a carboxylic
acid ester, such as
for example, ethyl acetate is produced at a selectivity of greater than 50%
while producing the
corresponding alcohol at a selectivity less than 10% from a carboxylic acid or
anhydride
thereof by reacting the acid or anhydride with hydrogen at elevated
temperature in the presence
of a catalyst composition comprising as a first component at least one of
Group VIII noble
metal and a second component comprising at least one of molybdenum, tungsten
and rhenium
and a third component comprising an oxide of a Group IVB element. However,
even the
optimal conditions reported therein result in significant amounts of by-
products including
methane, ethane, acetaldehyde and acetone in addition to ethanol. In addition,
the conversion
of acetic acid is generally low and is in the range of about 5 to 40% except
for a few cases in
which the conversion reached as high as 80%.
[0008] From the foregoing it is apparent that existing processes do not have
the requisite
selectivity to ethyl acetate and/or ethanol, employ highly expensive catalysts
or produce
undesirable by-products such as methane and ethane. Thus, the need exists for
forming ethyl
acetate (and optionally ethanol) at high selectivity using a more economical
catalyst, while
minimizing the formation of undesirable byproducts.
2
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
SUMMARY OF THE INVENTION
[0009] The present invention relates to a process for producing ethyl acetate
at high
selectivities. In one embodiment, the process comprises hydrogenating acetic
acid in the
presence of a catalyst under conditions effective to form ethyl acetate,
wherein the catalyst
comprises a first metal, a second metal and a support, wherein the first metal
is selected from
the group consisting of nickel, palladium and platinum, and is present in an
amount greater than
1 wt%, based on the total weight of the catalyst. The second metal may be
selected from the
group consisting of molybdenum, rhenium, zirconium, copper, cobalt, tin, and
zinc. The first
metal may be present, for example, in an amount of from 0.1 to 10 wt.% and the
second metal
may be present in an amount of from 0.1 to 10 wt.%, based on the total weight
of the catalyst.
[0010] In another embodiment, the present invention relates to a process for
producing ethyl
acetate, comprising hydrogenating acetic acid in the presence of a catalyst
under conditions
effective to form ethyl acetate, wherein the catalyst comprises a first metal,
a second metal and
a support, wherein the first metal is selected from group consisting of nickel
and palladium, and
wherein the second metal is selected from the group consisting of tin and
zinc. In one
embodiment, the first metal is present in an amount from 0.1 to 25 wt.%, based
on the total
weight of the catalyst.
[0011] In another embodiment, the present invention relates to a process for
producing ethyl
acetate comprising hydrogenating acetic acid in the presence of a catalyst
under conditions
effective to form ethyl acetate, wherein the catalyst comprises a first metal,
a support, and at
least one support modifier selected from the group of oxides of Group IVB
metals, oxides of
Group VB metals, oxides of Group VIB metals, iron oxides, aluminum oxides and
mixtures
thereof. As an example, the first metal may be present in an amount from 0.1
to 25 wt%, based
on the total weight of the catalyst. In one aspect, the first metal is
selected from the group
consisting of Group IB, IIB, IIIB, IVB, VB, VIB, VIIB, or VIII transitional
metal, a lanthanide
metal, an actinide metal or a metal from any of Groups IIIA, IVA, VA, or VIA.
As another
option, the first metal may be selected from the group consisting of copper,
iron, cobalt, nickel,
ruthenium, rhodium, palladium, osmium, iridium, platinum, titanium, zinc,
chromium,
rhenium, molybdenum, and tungsten. In another embodiment, the catalyst further
comprises a
second metal (optionally different from the first metal). In embodiments where
a second metal
3
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
is present, the second metal may be different from the first metal and may be
selected from the
group consisting of copper, molybdenum, tin, chromium, iron, cobalt, vanadium,
tungsten,
palladium, platinum, lanthanum, cerium, manganese, ruthenium, rhenium, gold,
and nickel.
Preferably, the first metal is platinum and/or the second metal is tin. In
another preferred
combination, the first metal is palladium and the second metal is rhenium.
Optionally, the
catalyst further comprises a third metal different from the first and second
metals. The third
metal may be selected from the group consisting of cobalt, palladium,
ruthenium, copper, zinc,
platinum, tin, and rhenium and/or may be present in an amount of 0.05 and 4
wt.%, based on
the total weight of the catalyst.
[0012] As noted above, the catalysts may, generally, be suitable for use as a
hydrogenation
catalyst in converting acetic acid to ethyl acetate and at least 10% of the
acetic acid may be
converted during hydrogenation. Also, the hydrogenation may be performed in a
vapor phase
at a temperature of from 125 C to 350 C, a pressure of 10 KPa to 3000 KPa, and
a hydrogen to
acetic acid mole ratio of greater than 4:1. In addition, the catalysts may
have a selectivity to
ethyl acetate of at least 40% and/or a selectivity to methane, ethane, and
carbon dioxide of less
than 4%. In one embodiment, the catalyst has a productivity that decreases
less than 6% per
100 hours of catalyst usage.
[0013] In one embodiment, the support is present in an amount of 25 wt.% to 99
wt.%, based
on the total weight of the catalyst and is selected from the group consisting
of iron oxide, silica,
alumina, silica/aluminas, titania, zirconia, magnesium oxide, calcium
silicate, carbon, graphite,
high surface area graphitized carbon, activated carbons, and mixtures thereof.
As one option,
the catalyst may comprise at least one support modifier selected from the
group consisting of
(i) alkaline earth metal oxides, (ii) alkali metal oxides, (iii) alkaline
earth metal metasilicates,
(iv) alkali metal metasilicates, (v) Group IIB metal oxides, (vi) Group IIB
metal metasilicates,
(vii) Group IIIB metal oxides, (viii) Group IIIB metal metasilicates, and
mixtures thereof
preferably being CaSiO3. In another option the support modifier is selected
from the group
consisting of oxides of Group IVB metals, oxides of Group VB metals, oxides of
Group VIB
metals, iron oxides, aluminum oxides and mixtures thereof. The support
modifier may be
present in an amount of 0.1 wt.% to 50 wt.%, based on the total weight of the
catalyst.
[0014] In another embodiment, the invention relates to a crude product
(optionally obtained
from the hydrogenation of acetic acid, as discussed herein), which comprises
(a) ethyl acetate
4
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
in an amount from 5 to 70 wt.%; (b) ethanol in an amount from 5 to 70 wt.%;
(c) acetic acid in
an amount from 5 to 75 wt.%; (d) water in an amount of from 5 to 50 wt%; and
(e) any other
compounds in an amount less than 10 wt %, wherein all weight percents are
based on the total
weight of the crude product. A preferred crude ethanol product comprises (a)
ethyl acetate in
an amount from 15 to 50 wt.%; (b) ethanol in an amount from 5 to 70 wt.%; (c)
acetic acid in
an amount from 10 to 60 wt.%; (d) water in an amount of from 10 to 45 wt%; and
(e) any other
compounds in an amount less than 6 wt %, wherein all weight percents are based
on the total
weight of the crude product.
BRIEF DESCRIPTION OF DRAWINGS
[0015] The invention is described in detail below with reference to the
appended drawings,
wherein like numerals designate similar parts.
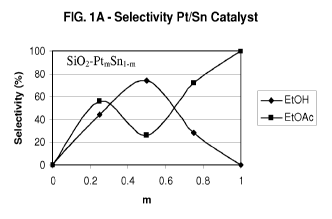
[0016] FIG. 1A is a graph of the selectivity to ethanol and ethyl acetate
using a Si02-Pt.Snl_m
catalyst;
[0017] FIG. 1 B is a graph of the productivity to ethanol and ethyl acetate of
the catalyst of
FIG. IA;
[0018] FIG. 1C is a graph of the conversion of the acetic acid of the catalyst
of FIG. 1A;
[0019] FIG. 2A is a graph of the selectivity to ethanol and ethyl acetate
using a Si02-Re.Pdl_,,
catalyst;
[0020] FIG. 2B is a graph of the productivity to ethanol and ethyl acetate of
the catalyst of
FIG. 2A;
[0021] FIG. 2C is a graph of the conversion of the acetic acid of the catalyst
of FIG. 2A;
[0022] FIG. 3 is a graph of the activity of a catalyst compared to the
productivity of the
catalyst to a mixture of ethyl acetate and ethanol at various temperatures
according to one
embodiment of the invention; and
[0023] FIG. 4 is a graph of the activity of a catalyst compared to the
selectivity of the catalyst
to a mixture of ethyl acetate and ethanol at various temperatures according to
one embodiment
of the invention.
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
DETAILED DESCRIPTION OF THE INVENTION
Introduction
[0024] The present invention relates to processes for producing ethyl acetate
by
hydrogenating acetic acid in the presence of a catalyst. The hydrogenation
reaction may be
represented as follows:
o
2 2H2
C CH2 + 2 H2O
CHs OH CH O/ CH3
Depending on the catalyst and process conditions employed, the hydrogenation
reaction may
produce ethanol in addition to ethyl acetate. Embodiments of the present
invention beneficially
may be used in industrial applications to produce ethyl acetate and/or ethanol
on an
economically feasible scale.
[0025] The processes of the invention employ various catalysts that may be
used to form
ethyl acetate and optionally ethanol. Typically, the catalyst will comprises a
first metal and
optionally one or more of a second metal, a third metal, and optionally
additional metals. The
one or more metals preferably are disposed on a support, such as silica or
titania. In a first
embodiment, the process employs a catalyst that includes a high loading of
nickel, palladium or
platinum. In a second embodiment, the process employs a catalyst that
comprises a first metal
selected from nickel and palladium and a second metal selected from tin and
zinc. In a third
embodiment, the process employs a catalyst that comprises one or more metals
on a support
that has been modified with an acidic support modifier or a redox support
modifier. It has now
been discovered that these catalyst compositions surprisingly and unexpectedly
can be
formulated to be selective for the formation of ethyl acetate, optionally in
combination with
ethanol.
High Loading Nickel, Palladium and Platinum Catalysts
[0026] In a first embodiment, the invention is to hydrogenation processes
using catalysts that
comprise one or more of nickel, palladium or platinum at high metal loadings.
For example,
the catalyst may comprise a first metal selected from the group consisting of
nickel, palladium,
and platinum on a support in an amount greater than 1 wt.%, e.g., greater than
1.1 wt.%, or
greater than 1.2 wt.%, based on the total weight of the catalyst. In terms of
ranges, the amount
6
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
of the first metal on the support preferably is from 1 to 25 wt.%, e.g., from
1.2 to 15 wt.%, or
from 1.5 wt.% to 10 wt.%. For purposes of the present specification, unless
otherwise
indicated, weight percent is based on the total weight the catalyst including
metal and support.
[0027] The metal(s) in the catalyst may be present in the form of one or more
elemental
metals and/or one or more metal oxides. For purposes of determining the weight
percent of the
metal(s) in the catalyst, the weight of any oxygen that is bound to the metal
is ignored. In a
more preferred aspect, the first metal is selected from platinum and
palladium. When the first
metal comprises platinum, it is preferred that the catalyst comprises the
platinum in an amount
greater than 1 wt.%, but less than 10 wt.%, e.g., less than 5 wt.% or less
than 3 wt.%, due to the
availability of platinum.
[0028] In addition to the first metal, the catalyst optionally further
comprises one or more of a
second metal, a third metal or additional metals. In this context, the
numerical terms "first,"
"second," "third," etc., when used to modify the word "metal," are meant to
indicate that the
respective metals are different from one another. If present, the second metal
preferably is
selected from the group consisting of molybdenum, rhenium, zirconium, copper,
cobalt, tin,
and zinc. More preferably, the second metal is selected from the group
consisting of
molybdenum, rhenium, tin and cobalt. Even more preferably, the second metal is
selected from
tin and rhenium.
[0029] Where the catalyst includes two or more metals, one metal may act as a
promoter
metal and the other metal is the main metal. For instance, with a platinum/tin
catalyst,
platinum may be considered to be the main metal and tin may be considered the
promoter
metal. For convenience, the present specification refers to the first metal as
the primary
catalyst and the second metal (and optional metals) as the promoter(s). This
should not be taken
as an indication of the underlying mechanism of the catalytic activity.
[0030] In the first embodiment, when the catalyst includes two or more metals,
e.g., a first
metal and a second metal, the first metal optionally is present in the
catalyst in an amount from
1 to 10 wt.%, e.g., from 1.2 to 5 wt.%, or from 1.5 to 3 wt.%. The second
metal optionally is
present in an amount from 0.1 and 20 wt.%, e.g., from 0.1 to 10 wt.%, or from
0.1 to 5 wt.%.
For catalysts comprising two or more metals, the two or more metals may be
alloyed with one
another or may comprise a non-alloyed metal solution or mixture.
7
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
[0031] The preferred metal ratios may vary somewhat depending on the metals
used in the
catalyst. In some embodiments, the mole ratio of the first metal to the second
metal is from
10:1 to 1:10, e.g., from 4:1 to 1:4, from 2:1 to 1:2, from 1.5:1 to 1:1.5 or
from 1.1:1 to 1:1.1.
[0032] Molar ratios other than 1:1 may be preferred depending on the
composition of the
catalyst employed. It has now surprisingly and unexpectedly been discovered,
for example,
that for platinum/tin catalysts, platinum to tin molar ratios less than
0.4:0.6, or greater than
0.6:0.4 are particularly preferred in order to form ethyl acetate from acetic
acid at high
selectivity, conversion and productivity, as shown in FIGS. IA, lB and 1C.
More preferably,
the Pt/Sn ratio is greater than 0.65:0.35 or greater than 0.7:0.3, e.g., from
0.65:0.35 to 1:0 or
from 0.7:0.3 to 1:0. Selectivity to ethyl acetate may be further improved by
incorporating
modified supports as described herein.
[0033] With rhenium/palladium catalysts, as shown in FIGS. 2A, 2B and 2C,
preferred
rhenium to palladium molar ratios for forming ethyl acetate in terms of
selectivity, conversion
and production are less than 0.7:0.3 or greater than 0.85:0.15. A preferred
Re/Pd ratio for
producing ethyl acetate in the presence of a Re/Pd catalyst is from 0.2:0.8 to
0.4:0.6. Again,
selectivity to ethyl acetate may be further improved by incorporating modified
supports as
described herein.
[0034] In embodiments when the catalyst comprises a third metal, the third
metal may be
selected from any of the metals listed above in connection with the first or
second metal, so
long as the third metal is different from the first and second metals. In
preferred aspects, the
third metal is selected from the group consisting of cobalt, palladium,
ruthenium, copper, zinc,
platinum, tin, and rhenium. More preferably, the third metal is selected from
cobalt, palladium,
and ruthenium. When the third metal is present, the catalyst composition
preferably comprises
the third metal in an amount from 0.05 and 4 wt.%, e.g., from 0.1 to 3 wt.%,
or from 0.1 to 2
wt. %.
[0035] In addition to the metal, the catalysts of the first embodiment further
comprise a
support, optionally a modified support. As will be appreciated by those of
ordinary skill in the
art, support materials are selected such that the catalyst system is suitably
active, selective and
robust under the process conditions employed for the formation of ethyl
acetate or a mixture of
ethyl acetate and ethanol. Suitable support materials may include, for
example, stable metal
oxide-based supports or ceramic-based supports as well as molecular sieves,
such as zeolites.
8
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
Examples of suitable support materials include without limitation, iron oxide,
silica, alumina,
silica/aluminas, titania, zirconia, magnesium oxide, calcium silicate, carbon,
graphite, high
surface area graphitized carbon, activated carbons, and mixtures thereof.
Exemplary preferred
supports are selected from the group consisting of silica/aluminas, titania,
and zirconia. The
total weight of the support in the catalyst, based on the total weight of the
catalyst, preferably is
from 25 wt% to 99 wt%, e.g., from 30 wt% to 98.5 wt%, or from 35 wt% to 98
wt%.
[0036] A preferred silica/alumina support material is KA-160 (Sud Chemie)
silica spheres
having a nominal diameter of about 5 mm, a density of about 0.562 g/ml, in
absorptivity of
about 0.583 g H20/g support, a surface area of about 160 to 175 m2/g, and a
pore volume of
about 0.68 ml/g.
[0037] In one embodiment, the support material comprises a silicaceous support
material
selected from the group consisting of silica, silica/alumina, a Group IIA
silicate such as calcium
metasilicate, pyrogenic silica, high purity silica and mixtures thereof. In
one embodiment silica
may be used as the silicaceous support, and the amount of aluminum, which is a
common
contaminant for silica, may be low, preferably under 1 wt.%, e.g., under 0.5
wt.% or under 0.3
wt.%, based on the total weight of the support. In this regard, pyrogenic
silica is preferred as it
commonly is available in purities exceeding 99.7 wt.%. High purity silica, as
used throughout
the application, refers to silica in which acidic contaminants such as
aluminum are present, if at
all, at levels of less than 0.3 wt.%, e.g., less than 0.2 wt.% or less than
0.1 wt.%.
[0038] The surface area of the support may vary widely depending on the type
of support. In
some aspects, the surface area of the support material, e.g., silicaceous
material, may be at least
about 50 m2/g, e.g., at least about 100 m2/g, at least about 150 m2/g, at
least about 200 m2/g or
most preferably at least about 250 m2/g. In terms of ranges, the support
material preferably has
a surface area of from 50 to 600 m2/g, e.g., from 100 to 500 m2/g or from 100
to 300 m2/g.
High surface area silica, as used throughout the application, refers to silica
having a surface
area of at least about 250 m2/g. High surface area silica/alumina, as used
throughout the
application, refers to silica/alumina having a surface area of at least about
150 m2/g. For
purposes of the present specification, surface area refers to BET nitrogen
surface area, meaning
the surface area as determined by ASTM D6556-04, the entirety of which is
incorporated
herein by reference.
9
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
[00391 The support material, e.g. silicaceous material, also preferably has an
average pore
diameter of from 5 to 100 nm, e.g., from 5 to 30 nm, from 5 to 25 nm or from
about 5 to 10 nm,
as determined by mercury intrusion porosimetry, and an average pore volume of
from 0.5 to 2.0
cm3/g, e.g., from 0.7 to 1.5 cm3/g or from about 0.8 to 1.3 cm3/g, as
determined by mercury
intrusion porosimetry.
[0040] The morphology of the support material, and hence of the resulting
catalyst
composition, may vary widely. In some exemplary embodiments, the morphology of
the
support material and/or of the catalyst composition may be pellets,
extrudates, spheres, spray
dried microspheres, rings, pentarings, trilobes, quadrilobes, multi-lobal
shapes, or flakes
although cylindrical pellets are preferred. Preferably, the support material,
e.g., silicaceous
material, has a morphology that allows for a packing density of from 0.1 to
1.0 g/cm3, e.g.,
from 0.2 to 0.9 g/cm3 or from 0.5 to 0.8 g/cm3. In terms of size, the support
material, e.g.
silicaceous material, preferably has an average particle size, meaning the
diameter for spherical
particles or equivalent spherical diameter for non-spherical particles, of
from 0.01 to 1.0 cm,
e.g., from 0.1 to 0.5 cm or from 0.2 to 0.4 cm. Since the one or more metal(s)
that are disposed
on or within the modified support are generally very small in size, they
should not substantially
impact the size of the overall catalyst particles. Thus, the above particle
sizes generally apply
to both the size of the modified supports as well as to the final catalyst
particles.
[0041] A preferred silica support material is SS61138 High Surface Area (HSA)
Silica
Catalyst Carrier from Saint Gobain NorPro. The Saint-Gobain NorPro SS61138
silica contains
approximately 95 wt% high surface area silica; a surface area of about 250
m2/g; a median pore
diameter of about 12 nm; a total pore volume of about 1.0 cm3/g as measured by
mercury
intrusion porosimetry and a packing density of about 0.352 g/cm3 (22 lb/ft).
[0042] The supports for the first embodiment may further comprise a support
modifier. A
support modifier is added to the support and is not naturally present in the
support. A support
modifier adjusts effects of the acidity of the support material. The acid
sites, e.g. Bronsted acid
sites, on the support material may be adjusted by the support modifier, for
example, to favor
selectivity to ethyl acetate and mixtures of ethyl acetate during the
hydrogenation of acetic
acid. Unless the context indicates otherwise, the acidity of a surface or the
number of acid sites
thereupon may be determined by the technique described in F. Delannay, Ed.,
"Characterization of Heterogeneous Catalysts"; Chapter III: Measurement of
Acidity of
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
Surfaces, p. 370-404; Marcel Dekker, Inc., N.Y. 1984, the entirety of which is
incorporated
herein by reference.
[0043] In some aspects, the support material may be undesirably too acidic for
formation of
ethyl acetate at high selectivity. In this case, the support material may be
modified with a basic
support modifier. Suitable basic support modifiers may be selected, for
example, from the
group consisting of: (i) alkaline earth oxides, (ii) alkali metal oxides,
(iii) alkaline earth metal
metasilicates, (iv) alkali metal metasilicates, (v) Group IIB metal oxides,
(vi) Group IIB metal
metasilicates, (vii) Group IIIB metal oxides, (viii) Group IIIB metal
metasilicates, and mixtures
thereof. In addition to oxides and metasilicates, other types of modifiers
including nitrates,
nitrites, acetates, and lactates may be used in embodiments of the present
invention.
Preferably, the basic modifiers have a low volatility or are non-volatile. Low
volatility
modifiers have a rate of loss that is low enough such that the acidity of the
support modifier is
not reversed during the life of the catalyst. For example, the support
modifier may be selected
from the group consisting of oxides and metasilicates of any of sodium,
potassium, magnesium,
calcium, scandium, yttrium, and zinc, and mixtures of any of the foregoing. A
particularly
preferred basic support modifier is calcium metasilicate (CaSi03).
[0044] In some aspects, the support material is too basic or is not acidic
enough for formation
of ethyl acetate at high selectivity. In this case, the support may be
modified with a support
modifier that adjusts the support material by increasing the number or
availability of acid sites
by using a redox support modifier or an acidic support modifier. Suitable
redox and acidic
support modifiers may be selected from the group consisting of. oxides of
Group IVB metals,
oxides of Group VB metals, oxides of Group VIB metals, iron oxides, aluminum
oxides, and
mixtures thereof. These support modifiers are redox or acid non-volatile
support modifiers.
Preferred redox support modifiers include those selected from the group
consisting of W03,
MoO3, Fe203, and Cr203. Preferred acidic support modifiers include those
selected from the
group consisting of Ti02, Zr02, Nb205, Ta205, and A1203. Without being bound
by theory, it is
believed that an increase in acidity of the support may favor ethyl acetate
formation. However,
increasing acidity of the support may also form ethers and basic modifiers may
be added to
counteract the acidity of the support.
11
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
Catalysts Comprising Nickel or Palladium and Tin or Zinc
[0045] In a second embodiment of the present invention, the invention is to a
hydrogenation
process that employs a catalyst for making ethyl acetate or optionally a
mixture of ethyl acetate
and ethanol, in which the catalyst comprises a first metal selected from the
group consisting of
nickel and palladium, a second metal selected from the group consisting of tin
and zinc, and a
support, optionally a modified support. In contrast to the above-described
first embodiment, in
the second embodiment, lower loadings of the first metal may be employed. For
example, the
catalyst may comprise the first metal in an amount from 0.1 to 10 wt.%, e.g.,
from 0.1 to 5
wt.%, or from 0.1 to 3 wt.%. The second metal preferably is present in an
amount from 0.1 and
20 wt.%, e.g., from 0.1 to 10 wt.%, or from 0.1 to 5 wt.%. The mole ratio of
the first metal to
the second metal preferably is from 10:1 to 1:10, e.g., from 4:1 to 1:4, from
2:1 to 1:2, from
1.5:1 to 1:1.5 or from 1.1:1 to 1:1.1. Optionally, the catalyst of the second
embodiment may
further comprise a third metal as described above in connection with the first
embodiment.
[0046] In the second embodiment, the catalyst includes a support, optionally a
modified
support, as discussed above in connection with the first embodiment. The total
weight of the
support, based on the total weight of the catalyst, for the second embodiment
preferably is from
25 wt.% to 99.9 wt.%, e.g., from 30 wt.% to 97 wt.%, or from 35 wt.% to 95
wt.%.
Catalyst on Acidic or Redox Modified Support
[0047] In a third embodiment of the invention, the hydrogenation process uses
a catalyst that
comprises a first metal and optionally one or more of a second metal, a third
metal or additional
metals, on a support that has been modified with a redox support modifier or
an acidic support
modifier. The total weight of all metals present in the catalyst preferably is
from 0.1 to 25
wt.%, e.g., from 0.1 to 15 wt.%, or from 0.1 1 to 10 wt.%.
[0048] The first metal may be a Group IB, IIB, IIIB, IVB, VB, VIB, VIIB, or
VIII
transitional metal, a lanthanide metal, an actinide metal or a metal from any
of Groups IIIA,
IVA, VA, or VIA. In a preferred embodiment, the first metal is selected the
group consisting of
copper, iron, cobalt, nickel, ruthenium, rhodium, palladium, osmium, iridium,
platinum,
titanium, zinc, chromium, rhenium, molybdenum, and tungsten. Preferably, the
first metal is
selected from the group consisting of platinum, palladium, cobalt, nickel, and
ruthenium. More
preferably, the first metal is selected from platinum and palladium. When the
first metal
12
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
comprises platinum, it is preferred that the catalyst comprises the platinum
in an amount less
than 5 wt%, e.g. less than 3 wt% or less than 1 wt%, due to the limited
availability of platinum.
[0049] The catalyst optionally further comprises a second metal selected from
the group
consisting of copper, molybdenum, tin, chromium, iron, cobalt, vanadium,
tungsten, palladium,
platinum, lanthanum, cerium, manganese, ruthenium, rhenium, gold, and nickel.
More
preferably, the second metal is selected from the group consisting of copper,
tin, cobalt,
rhenium, and nickel. More preferably, the second metal is selected from tin
and rhenium.
[0050] If the catalyst includes two or more metals, e.g., a first metal and a
second metal, the
first metal optionally is present in the catalyst in an amount from 0.1 to 10
wt.%, e.g. from 0.1
to 5 wt.%, or from 0.1 to 3 wt.%. The second metal preferably is present in an
amount from 0.1
and 20 wt.%, e.g., from 0.1 to 10 wt.%, or from 0.1 to 5 wt.%. For catalysts
comprising two or
more metals, the two or more metals may be alloyed with one another or may
comprise a non-
alloyed metal solution or mixture.
[0051] As stated above in the first embodiment, in the third embodiment the
preferred metal
ratios may vary somewhat depending on the metals used in the catalyst. In some
embodiments,
the mole ratio of the first metal to the second metal preferably is from 10:1
to 1:10, e.g., from
4:1 to 1:4, from 2:1 to 1:2, from 1.5:1 to 1:1.5 or from 1.1:1 to 1:1.1.
[0052] Molar ratios other than 1:1 may be preferred for other catalysts. It
has now
surprisingly and unexpectedly been discovered, for example, that for
platinum/tin catalysts,
platinum to tin molar ratios less than 0.4:0.6, or greater than 0.6:0.4 are
particularly preferred in
order to form ethyl acetate from acetic acid at high selectivity, conversion
and productivity, as
shown in FIGS. 1 A, 1 B and 1 C. A preferred Pt/Sn molar ratio for producing
ethyl acetate in
the presence of a Pt/Sn catalyst is from 0.65:0.35 to 0.95:0.05, e.g., from
0.7:0.3 to 0.95:0.05.
Selectivity to ethyl acetate may be further improved by incorporating modified
supports as
described throughout the present specification.
[0053] With rhenium/palladium catalysts, as shown in FIGS. 2A, 2B and 2C,
preferred
rhenium to palladium molar ratios for forming ethyl acetate in terms of
selectivity, conversion
and production are less than 0.7:0.3 or greater than 0.85:0.15. A preferred
Re/Pd molar ratio
for producing ethyl acetate in the presence of a Re/Pd catalyst is from
0.2:0.8 to 0.4:0.6.
Again, selectivity to ethyl acetate may be further improved by incorporating
modified supports
as described throughout the present specification.
13
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
[0054] In embodiments when the catalyst comprises a third metal, the third
metal may be
selected from any of the metals listed above in connection with the first or
second metal, so
long as the third metal is different from the first and second metals. In
preferred aspects, the
third metal is selected from the group consisting of cobalt, palladium,
ruthenium, copper, zinc,
platinum, tin, and rhenium. More preferably, the third metal is selected from
cobalt, palladium,
and ruthenium. When present, the total weight of the third metal preferably is
from 0.05 and 4
wt.%, e.g., from 0.1 to 3 wt.%, or from 0.1 to 2 wt.%.
[0055] In one embodiment, the catalyst comprises a first metal and no
additional metals (no
second metal, etc.). In this embodiment, the first metal preferably is present
in an amount from
0.1 to 10 wt. %. In another embodiment, the catalyst comprises a combination
of two or more
metals on a support. Specific preferred metal compositions for various
catalysts of this
embodiment of the invention are provided below in Table 1. Where the catalyst
comprises a
first metal and a second metal, the first metal preferably is present in an
amount from 0.1 to 5
wt.% and the second metal preferably is present in an amount from 0.1 to 5
wt.%. Where the
catalyst comprises a first metal, a second metal and a third metal, the first
metal preferably is
present in an amount from 0.1 to 5 wt.%, the second metal preferably is
present in an amount
from 0.1 to 5 wt.%, and the third metal preferably is present in an amount
from 0.1 to 2 wt.%.
Where the first metal is platinum, the first metal preferably is present in an
amount from 0.1 to
3 wt.%, the second metal is present in an amount from 0.1 to 5 wt.%, and the
third metal, if
present, preferably is present in an amount from 0.1 to 2 wt.%.
14
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
TABLE 1
EXEMPLARY METAL COMBINATIONS FOR CATALYSTS
First Metal Second Metal Third Metal
Cu Ag
Cu Cr
Cu v
Cu w
Cu Zn
Ni Au
Ni Re
Ni V
Ni W
Ni Zn
Ni Sn
Pd Zn
Pd Co
Pd Cr
Pd Cu
Pd Fe
Pd La
Pd Mo
Pd Ni
Pd Re
Pd Sn
Pd V
Pd W
Pt Co
Pt Cr
Pt Cu
Pt Fe
Pt Mo
Pt Sn
Pt Sn Co
Pt Sn Re
Pt Sn Ru
Pt Sn Pd
Rh Cu
Rh Ni
Ru Co
Ru Cr
Ru Cu
Ru Fe
Ru La
Ru Mo
Ru Ni
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
TABLE 1
EXEMPLARY METAL COMBINATIONS FOR CATALYSTS
First Metal Second Metal Third Metal
Ru Sn
[0056] Depending primarily on how the catalyst is manufactured, the metals of
the catalysts
of the present invention may be dispersed throughout the support, coated on
the outer surface of
the support (egg shell) or decorated on the surface of the support.
[0057] In addition to one or more metals, the catalysts of the third
embodiment of the present
invention further comprise a modified support, meaning a support that includes
a support
material and a support modifier. In particular, the use of acidic or redox
modified supports
surprisingly and unexpectedly has now been demonstrated to favor formation of
ethyl acetate
over other hydrogenation products.
[0058] Examples of suitable support materials include those stated above in
connection with
the first embodiment and without limitation include iron oxide, silica,
alumina, silica/aluminas,
titania, zirconia, magnesium oxide, calcium silicate, carbon, graphite, high
surface area
graphitized carbon, activated carbons, and mixtures thereof. The support
further comprises a
support modifier that, for example, may be selected from the group consisting
of: oxides of
Group IVB metals, oxides of Group VB metals, oxides of Group VIB metals, iron
oxides,
aluminum oxides, and mixtures thereof. These support modifiers are redox or
acidic support
modifiers. Preferred redox support modifiers include those selected from the
group consisting
of W03, MoO3, Fe203, and Cr203. Preferred acidic support modifiers include
those selected
from the group consisting of Ti02, Zr02, Nb205, T a205, and A1203. Preferably,
the support
comprises a support modifier that is an acidic or redox modifier having a low
volatility or is
non-volatile. Low volatility modifiers have a rate of loss that is low enough
such that the
acidity of the support modifier is not reversed during the life of the
catalyst. As indicated
above, the support modifier is added to the support and is not naturally
present in the support.
[0059] The total weight of the modified support, including the support
material and the
support modifier, based on the total weight of the catalyst, preferably is
from 25 wt.% to 99.9
wt.%, e.g., from 30 wt.% to 97 wt.%, or from 35 wt% to 95 wt.%. The support
modifier
preferably is provided in an amount sufficient to increase the number of
active Bronsted acid
sites or availability of those acid sites. In preferred embodiments, the
support modifier is
16
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
present in an amount from 0.1 wt.% to 50 wt.%, e.g., from 0.2 wt.% to 25 wt.%,
from 0.5 wt.%
to 15 wt.%, or from 1 wt.% to 8 wt.%, based on the total weight of the
catalyst. In preferred
embodiments, the support material is present in an amount from 25 wt.% to 99
wt.%, e.g., from
30 wt.% to 97 wt.% or from 35 wt.% to 95 wt.%.
[0060] If desired, the acidic or redox support modifiers described herein in
connection with
the third embodiment of the invention may also be used to modify the supports
of the above-
described first embodiment or the second embodiment.
[0061] Catalysts of the present invention are particulate catalysts in the
sense that, rather than
being impregnated in a wash coat onto a monolithic carrier similar to
automotive catalysts and
diesel soot trap devices, the catalysts of the invention preferably are formed
into particles,
sometimes also referred to as beads or pellets, having any of a variety of
shapes and the
catalytic metals are provided to the reaction zone by placing a large number
of these shaped
catalysts in the reactor. Commonly encountered shapes include extrudates of
arbitrary cross-
section taking the form of a generalized cylinder in the sense that the
generators defining the
surface of the extrudate are parallel lines. As indicated above, any
convenient particle shape
including pellets, extrudates, spheres, spray dried microspheres, rings,
pentarings, trilobes,
quadrilobes and multi-lobal shapes may be used, although cylindrical pellets
are preferred.
Typically, the shapes are chosen empirically based upon perceived ability to
contact the vapor
phase with the catalytic agents effectively.
[0062] One advantage of catalysts of the present invention, in all of the
above embodiments,
is the stability or activity of the catalyst for producing ethyl acetate and
mixtures of ethyl
acetate and ethanol. Accordingly, it can be appreciated that the catalysts of
the present
invention are fully capable of being used in commercial scale industrial
applications for the
hydrogenation of acetic acid, particularly in the production of ethyl acetate.
In particular, it is
possible to achieve such a degree of stability such that catalyst activity
will have rate of
productivity decline that is less than 6% per 100 hours of catalyst usage,
e.g., less than 3% per
100 hours or less than 1.5% per 100 hours. Preferably, the rate of
productivity decline is
determined once the catalyst has achieved steady-state conditions.
Processes for Making the Catalysts
[0063] The catalyst compositions of the first, second and third embodiments of
the present
invention preferably are formed through metal impregnation of the support
and/or modified
17
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
supports, although other processes such as chemical vapor deposition may also
be employed.
Before the metals are impregnated, it typically is desired to form the
modified support, if
necessary, through a step of impregnating the support material with the
support modifier. In
one aspect, the support modifier, e.g., W03 or Ti02, or a precursor to the
support modifier is
added to the support material in an aqueous suspension. For example, an
aqueous suspension
of the support modifier may be formed by adding the solid support modifier to
deionized water,
followed by the addition of colloidal support material thereto. The resulting
mixture may be
stirred and added to additional support material using, for example, incipient
wetness
techniques in which the support modifier is added to a support material having
the same pore
volume as the volume of the support modifier solution. Capillary action then
draws the support
modifier into the pores in the support material. The modified support can then
be formed by
drying and calcining to drive off water and any volatile components within the
support modifier
solution and depositing the support modifier on the support material. Drying
may occur, for
example, at a temperature of from 50 C to 300 C, e.g., from 100 C to 200 C or
about 120 C,
optionally for a period of from 1 to 24 hours, e.g., from 3 to 15 hours or
from 6 to 12 hours.
Once formed, the modified supports may be shaped into particles having the
desired size
distribution, e.g., to form particles having an average particle size in the
range of from 0.2 to
0.4 cm. The supports may be extruded, pelletized, tabletized, pressed, crushed
or sieved to the
desired size distribution. Any of the known methods to shape the support
materials into desired
size distribution can be employed. Calcining of the shaped modified support
may occur, for
example, at a temperature of from 250 C to 800 C, e.g., from 300 to 700 C or
about 500 C,
optionally for a period of from 1 to 12 hours, e.g., from 2 to 10 hours, from
4 to 8 hours or
about 6 hours.
[00641 In a preferred method of preparing the catalyst, the metals are
impregnated onto the
support or modified support. A precursor of the first metal (first metal
precursor) preferably is
used in the metal impregnation step, such as a water soluble compound or water
dispersible
compound/complex that includes the first metal of interest. Depending on the
metal precursor
employed, the use of a solvent, such as water, glacial acetic acid or an
organic solvent, may be
preferred. The second metal also preferably is impregnated into the support or
modified support
from a second metal precursor. If desired, a third metal or third metal
precursor may also be
impregnated into the support or modified support.
18
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
[0065] Impregnation occurs by adding, optionally drop wise, either or both the
first metal
precursor and/or the second metal precursor and/or additional metal
precursors, preferably in
suspension or solution, to the dry support or modified support. The resulting
mixture may then
be heated, e.g., optionally under vacuum, in order to remove the solvent.
Additional drying and
calcining may then be performed, optionally with ramped heating to form the
final catalyst
composition. Upon heating and/or the application of vacuum, the metal(s) of
the metal
precursor(s) preferably decompose into their elemental (or oxide) form. In
some cases, the
completion of removal of the liquid carrier, e.g., water, may not take place
until the catalyst is
placed into use and calcined, e.g., subjected to the high temperatures
encountered during
operation. During the calcination step, or at least during the initial phase
of use of the catalyst,
such compounds are converted into a catalytically active form of the metal or
a catalytically
active oxide thereof.
[0066] Impregnation of the first and second metals (and optional additional
metals) into the
support or modified support may occur simultaneously (co-impregnation) or
sequentially. In
simultaneous impregnation, the first and second metal precursors (and
optionally additional
metal precursors) are mixed together and added to the support or modified
support together,
followed by drying and calcination to form the final catalyst composition.
With simultaneous
impregnation, it may be desired to employ a dispersion agent, surfactant, or
solubilizing agent,
e.g., ammonium oxalate, to facilitate the dispersing or solubilizing of the
first and second metal
precursors in the event the either or both precursors are incompatible with
the desired solvent,
e.g., water.
[0067] In sequential impregnation, the first metal precursor is first added to
the support or
modified support followed by drying and calcining, and the resulting material
is then
impregnated with the second metal precursor followed by an additional drying
and calcining
step to form the final catalyst composition. Additional metal precursors
(e.g., a third metal
precursor) may be added either with the first and/or second metal precursor or
a separate third
impregnation step, followed by drying and calcination. Of course, combinations
of sequential
and simultaneous impregnation may be employed if desired.
[0068] Suitable metal precursors include, for example, metal halides, amine
solubilized metal
hydroxides, metal nitrates or metal oxalates of the desired metal(s). For
example, suitable
compounds for platinum precursors and palladium precursors include
chloroplatinic acid,
19
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
ammonium chloroplatinate, amine solubilized platinum hydroxide, platinum
nitrate, platinum
tetra ammonium nitrate, platinum chloride, platinum oxalate, palladium
nitrate, palladium tetra
ammonium nitrate, palladium chloride, palladium oxalate, sodium palladium
chloride, and
sodium platinum chloride. Generally, both from the point of view of economics
and
environmental aspects, aqueous solutions of soluble compounds of platinum are
preferred. In
one embodiment, the first metal precursor is not a metal halide and is
substantially free of metal
halides.
[0069] In one aspect, the "promoter" metal or metal precursor is first added
to the modified
support, followed by the "main" or "primary" metal or metal precursor. Of
course, the reverse
order of addition is also possible. Exemplary precursors for promoter metals
include metal
halides, amine solubilized metal hydroxides, metal nitrates or metal oxalates.
As indicated
above, in the sequential embodiment, each impregnation step preferably is
followed by drying
and calcination. In the case of promoted bimetallic catalysts as described
above, a sequential
impregnation may be used, starting with the addition of the promoter metal
followed by a
second impregnation step involving co-impregnation of the two principal
metals, e.g., Pt and
Sn.
Hydrogenation of Acetic Acid
[0070] The process of hydrogenating acetic acid to form ethyl acetate or a
mixture of ethyl
acetate and ethanol according to one embodiment of the invention may be
conducted in a
variety of configurations using a fixed bed reactor or a fluidized bed reactor
as one of skill in
the art will readily appreciate using catalysts of the first, second or third
embodiments. In
many embodiments of the present invention, an "adiabatic" reactor can be used;
that is, there is
little or no need for internal plumbing through the reaction zone to add or
remove heat.
Alternatively, a shell and tube reactor provided with a heat transfer medium
can be used. In
many cases, the reaction zone may be housed in a single vessel or in a series
of vessels with
heat exchangers therebetween. It is considered significant that acetic acid
reduction processes
using the catalysts of the present invention may be carried out in adiabatic
reactors as this
reactor configuration is typically far less capital intensive than tube and
shell configurations.
[0071] Typically, the catalyst is employed in a fixed bed reactor, e.g., in
the shape of an
elongated pipe or tube where the reactants, typically in the vapor form, are
passed over or
through the catalyst. Other reactors, such as fluid or ebullient bed reactors,
can be employed, if
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
desired. In some instances, the hydrogenation catalysts may be used in
conjunction with an
inert material to regulate the pressure drop of the reactant stream through
the catalyst bed and
the contact time of the reactant compounds with the catalyst particles.
[0072] The hydrogenation reaction may be carried out in either the liquid
phase or vapor
phase. Preferably the reaction is carried out in the vapor phase under the
following conditions.
The reaction temperature may the range from of 125 C to 350 C, e.g., from 200
C to 325 C,
from 225 C to about 300 C, or from 250 C to about 300 C. The pressure may
range from 10
KPa to 3000 KPa (about 0.1 to 30 atmospheres), e.g., from 50 KPa to 2300 KPa,
or from 100
KPa to 1500 KPa. The reactants may be fed to the reactor at a gas hourly space
velocities
(GHSV) of greater than 500 hr-1, e.g., greater than 1000 hr-1, greater than
2500 hr-1 and even
greater than 5000 hr-1. In terms of ranges the GHSV may range from 50 hr-1 to
50,000 hr- 1, e.g.,
from 500 hr-1 to 30,000 hr-1, from 1000 hr-1 to 10,000 hr-1, or from 1000 hr-'
to 6500 hr-1.
[0073] In another aspect of the process of this invention, the hydrogenation
is carried out at a
pressure just sufficient to overcome the pressure drop across the catalytic
bed at a suitable
GHSV, although there is no bar to the use of higher pressures, it being
understood that
considerable pressure drop through the reactor bed may be experienced at high
space velocities,
e.g., on the order of 5000 hr"1 or 6,500 hr" 1.
[0074] Although the reaction consumes two moles of hydrogen for every two
moles of acetic
acid to produce one mole of ethyl acetate, the actual molar ratio of hydrogen
to acetic acid in
the feed stream may vary from about 100:1 to 1:100, e.g., from 50:1 to 1:50,
from 20:1 to 1:2,
or from 12:1 to 1:1. Most preferably, the molar ratio of hydrogen to acetic
acid is greater than
4:1, e.g., greater than 5:1 or greater than 10:1.
[0075] Contact or residence time can also vary widely, depending upon such
variables as
amount of acetic acid, catalyst, reactor, temperature and pressure. Typical
contact times range
from a fraction of a second to more than several hours when a catalyst system
other than a fixed
bed is used, with preferred contact times, at least for vapor phase reactions,
from 0.1 to 100
seconds, e.g., from 0.3 to 80 seconds or from 0.4 to 30 seconds.
[0076] The acetic acid may be vaporized at the reaction temperature, and then
the vaporized
acetic acid can be fed along with hydrogen in undiluted state or diluted with
a relatively inert
carrier gas, such as nitrogen, argon, helium, carbon dioxide and the like. For
reactions run in
21
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
the vapor phase, the temperature should be controlled in the system such that
it does not fall
below the dew point of acetic acid.
[0077] In particular, the catalysts and processes of the present invention may
achieve
favorable conversion of acetic acid and favorable selectivity and productivity
to ethyl acetate or
mixtures of ethyl acetate and ethanol. For purposes of the present invention,
the term
conversion refers to the amount of acetic acid in the feed that is convert to
a compound other
than acetic acid. Conversion is expressed as a mole percentage based on acetic
acid in the feed.
The conversion of acetic acid (AcOH) is calculated from gas chromatography
(GC) data using
the following equation:
AcOH Conv. (%) =100 * mmol AcOH (feed stream) - mmol AcOH (GC)
mmol AcOH (feed stream)
[0078] For purposes of the present invention, the conversion maybe at least
10%, e.g., at
least 20%, at least 40%, at least 50%, at least 60%, or at least 70% or at
least 80%. Although
catalysts that have high conversions are desirable, such as at least 80% or at
least 90%, a low
conversion may be acceptable at high selectivity for ethyl acetate or mixtures
of ethyl acetate
and ethanol. It is, of course, well understood that in many cases, it is
possible to compensate
for conversion by appropriate recycle streams or use of larger reactors, but
it is more difficult to
compensate for poor selectivity.
[0079] "Selectivity" is expressed as a mole percent based on converted acetic
acid. It should
be understood that each compound converted from acetic acid has an independent
selectivity
and that selectivity is independent from conversion. For example, if 50 mole %
of the
converted acetic acid is converted to ethyl acetate, we refer to the ethyl
acetate selectivity as
50%. Selectivity to ethyl acetate (EtOAc) and mixtures of EtOAc and ethanol
(EtOH) is
calculated from gas chromatography (GC) data using the following equation:
EtOAc Sel. (%) =100 * mmol EtOAc (GC)
Total mmol C (GC) _ mmol AcOH (feed stream)
2
[0080] wherein "Total mmol C (GC)" refers to total mmols of carbon from all of
the products
analyzed by gas chromatograph.
[0081] For purposes of the present invention, the selectivity to ethoxylates
of the catalyst is at
least 60%, e.g., at least 70%, or at least 80%. As used herein, "ethoxylates"
refers converted
22
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
compounds that have at least two carbon atoms, such as ethanol, acetaldehyde,
ethyl acetate,
etc., but excludes ethane. Preferably, the selectivity to ethyl acetate is at
least 40%, e.g., at
least 50% or at least 60%.
[0082] Preferably, the selectivity to mixtures of ethyl acetate and ethanol is
at least 50%, e.g.,
at least 60% or at least 70%. In one embodiment of the present invention, it
is preferred that
ethyl acetate comprises at a major component of the product mixture, e.g., at
least 50 wt%, e.g.
from at least 55 wt% or from at least 60 wt%. In addition to ethyl acetate,
ethanol also may be
formed, for example, at selectivities of at least 20%, e.g. least 30% or at
least 40%. In another
embodiment of the present invention, the process forms ethanol as a major
component, e.g., in
an amount greater than 50 wt%, e.g., at least 55 wt% or at least 60 wt%. In
this aspect, ethyl
acetate may be also be formed, for example, at a selectivities of at least
20%, e.g. at least 30%
or at least 40%. It should be understood that in such mixtures, if desired,
either the ethyl
acetate may be further reacted to form more ethanol, or the ethanol may be
further reacted to
form more ethyl acetate.
[0083] In embodiments of the present invention, it is also desirable to have
low selectivity to
undesirable products, such as methane, ethane, and carbon dioxide. The
selectivity to these
undesirable products preferably should be less than 4%, e.g., less than 2% or
less than 1%.
Preferably, no detectable amounts of these undesirable products are formed
during
hydrogenation. In several embodiments of the present invention, formation of
alkanes is low,
usually under 2%, often under I%, and in many cases under 0.5% of the acetic
acid passed over
the catalyst is converted to alkanes, which have little value other than as
fuel.
[0084] Productivity refers to the grams of a specified product, e.g., ethyl
acetate, formed
during the hydrogenation based on the kilogram of catalyst used per hour. In
one embodiment,
a productivity of at least 200 grams of ethyl acetate per kilogram catalyst
per hour, e.g., at least
400 grams of ethyl acetate or least 600 grams of ethyl acetate, is preferred.
In another
embodiment, a productivity of at least 200 grams of a mixture of ethyl acetate
and ethanol per
kilogram catalyst per hour, e.g., at least 400 grams of a mixture of ethyl
acetate and ethanol or
least 600 grams of ethyl a mixture of ethyl acetate and ethanol, is preferred.
In terms of
ranges, the productivity preferably to ethyl acetate is from 200 to 3,000
grams of ethyl acetate
per kilogram catalyst per hour, e.g., from 400 to 2,500 or from 600 to 2,000.
23
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
[0085] Some catalysts of the present invention may achieve a conversion of
acetic acid of at
least 10%, a selectivity to ethyl acetate of at least 60%, and a productivity
of at least 200 g of
ethyl acetate per kg of catalyst per hour. A subset of catalysts of the
invention may achieve a
conversion of acetic acid of at least 50%, a selectivity to ethyl acetate of
at least 70%, a
selectivity to undesirable compounds of less than 4%, and a productivity of at
least 600 g of
ethyl acetate per kg of catalyst per hour.
Crude Ethyl Acetate Product
[0086] In another embodiment, the invention is to a crude ethyl acetate
product formed by
any of the processes of the present invention. The crude ethyl acetate product
produced by the
hydrogenation process of the present invention, before any subsequent
processing, such as
purification and separation, typically will comprise primarily unreacted
acetic acid, ethyl
acetate and optionally ethanol. In some exemplary embodiments, the crude
product comprises
ethyl acetate in an amount from 5 wt% to 70 wt.%, e.g., from 15 wt.% to 50
wt.%, or from 20
wt.% to 35 wt.%, based on the total weight of the crude product. The crude
product may
comprise ethanol in an amount from 5 wt.% to 70 wt.%, e.g., from 15 wt% to 50
wt.%, or from
20 wt.% to 35 wt.%, based on the total weight of the crude product. The crude
product
typically will further comprise unreacted acetic acid, depending on
conversion, for example, in
an amount from 5 to 75 wt.%, e.g., from 10 to 60 wt.% or from 20 to 50 wt.%.
Since water is
formed in the reaction process, water will also be present in the crude
product, for example, in
amounts ranging from 5 to 50 wt.%, e.g., from 10 to 45 wt.% or from 15 to 35
wt.%. Other
components, such as, for example, aldehydes, ketones, alkanes, and carbon
dioxide, if
detectable, collectively may be present in amounts less than 10 wt.%, e.g.,
less than 6 or less
than 4 wt.%. In terms of ranges other components may be present in an amount
from 0.1 to 10
wt.%, e.g., from 0.1 to 6 wt.%, or from 0.1 to 4 wt.%.
[0087] In a preferred embodiment, depending on the specific catalyst and
process conditions
employed, the crude ethyl acetate product may have any of the compositions
indicated below in
Table 2. Crude mixtures of ethyl acetate and ethanol may have any of the
compositions
indicated below in Table 3.
24
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
TABLE 2
CRUDE ETHYL ACETATE
PRODUCT COMPOSITIONS
Conc. Conc. Conc.
Component (wt.%) (wt.%) (wt.%)
Ethyl Acetate 5-70 15-50 20-35
Acetic Acid 5-75 10-60 20-50
Water 5-50 10-45 15-35
Other <10 <6 <4
TABLE 3
CRUDE ETHYL ACETATE/ETHANOL MIXTURE
PRODUCT COMPOSITIONS
Conc. Conc. Conc.
Component (wt.%) (wt.%) (wt.%)
Ethyl Acetate 5-70 15-50 20-35
Ethanol 5-70 15-50 20-35
Acetic Acid 5-75 10-60 20-50
Water 5-50 10-45 15-35
Other <10 <6 <4
[00881 The raw materials used in connection with the process of this invention
may be
derived from any suitable source including natural gas, petroleum, coal,
biomass and so forth.
It is well known to produce acetic acid through methanol carbonylation,
acetaldehyde
oxidation, ethylene oxidation, oxidative fermentation, and anaerobic
fermentation. As
petroleum and natural gas prices fluctuate becoming either more or less
expensive, methods for
producing acetic acid and intermediates such as methanol and carbon monoxide
from alternate
carbon sources have drawn increasing interest. In particular, when petroleum
is relatively
expensive compared to natural gas, it may become advantageous to produce
acetic acid from
synthesis gas ("syn gas") that is derived from any available carbon source.
United States Patent
No. 6,232,352 to Vidalin, the disclosure of which is incorporated herein by
reference, for
example, teaches a method of retrofitting a methanol plant for the manufacture
of acetic acid.
By retrofitting a methanol plant, the large capital costs associated with CO
generation for a new
acetic acid plant are significantly reduced or largely eliminated. All or part
of the syn gas is
diverted from the methanol synthesis loop and supplied to a separator unit to
recover CO and
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
hydrogen, which are then used to produce acetic acid. In addition to acetic
acid, the process
can also be used to make hydrogen which may be utilized in connection with
this invention.
[0089] United States Patent No. RE 35,377 to Steinberg et al., also
incorporated herein by
reference, provides a method for the production of methanol by conversion of
carbonaceous
materials such as oil, coal, natural gas and biomass materials. The process
includes
hydrogasification of solid and/or liquid carbonaceous materials to obtain a
process gas which is
steam pyrolized with additional natural gas to form synthesis gas. The syn gas
is converted to
methanol which may be carbonylated to acetic acid. The method likewise
produces hydrogen
which may be used in connection with this invention as noted above. See also,
United States
Patent No. 5,821,111 to Grady et al., which discloses a process for converting
waste biomass
through gasification into synthesis gas as well as United States Patent No.
6,685,754 to Kindig
et al., the disclosures of which are incorporated herein by reference.
[0090] Alternatively, acetic acid in vapor form may be taken directly as crude
product from
the flash vessel of a methanol carbonylation unit of the class described in
United States Patent
No. 6,657,078 to Scates et al., the entirety of which is incorporated herein
by reference. The
crude vapor product, for example, may be fed directly to the ethanol synthesis
reaction zones of
the present invention without the need for condensing the acetic acid and
light ends or
removing water, saving overall processing costs.
[0091] Ethyl acetate obtained by the present invention, may be used in its own
right,
polymerized, or converted to ethylene through a cracking process. The cracking
of ethyl
acetate to ethylene is shown below.
0 0
CH2 10 CH2=CH2 +
CH3 0 CH3 CH3 OH
[0092] The cracking may be a catalyzed reaction utilizing a cracking catalyst.
Suitable
cracking catalysts include sulfonic acid resins such as perfluorosulfonic acid
resins disclosed in
United States Patent No. 4,399,305, noted above, the disclosure of which is
incorporated herein
by reference. Zeolites are also suitable as cracking catalysts as noted in
United States Patent
No. 4,620,050, the disclosure of which is also incorporated herein by
reference.
[0093] Any ethanol in the mixtures of the present invention, may be used in
its own right as a
fuel or subsequently converted to ethylene which is an important commodity
feedstock as it can
26
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
be converted to polyethylene, vinyl acetate and/or ethyl acetate or any of a
wide variety of
other chemical products. For example, ethylene can also be converted to
numerous polymer
and monomer products. The dehydration of ethanol to ethylene is shown below.
"~OH O + H2O
[0094] Any of known dehydration catalysts can be employed in to dehydrate
ethanol, such as
those described in copending applications U.S. Application No. 12/221,137 and
U.S.
Application No. 12/221,138, the entire contents and disclosures of which are
hereby
incorporated by reference. A zeolite catalyst, for example, may be employed as
the
dehydration catalyst. While any zeolite having a pore diameter of at least
about 0.6 nm can be
used, preferred zeolites include dehydration catalysts selected from the group
consisting of
mordenites, ZSM-5, a zeolite X and a zeolite Y. Zeolite X is described, for
example, in U.S.
Pat. No. 2,882,244 and zeolite Y in U.S. Pat. No. 3,130,007, the entireties of
which are hereby
incorporated by reference. A zeolite catalyst may be used to concurrently
dehydrate ethanol to
ethylene and decompose ethyl acetate to ethylene in a highly efficient process
of the invention.
[0095] In embodiments where a mixture of ethyl acetate and ethanol is formed,
it may be
desired to further react the mixture in order to enrich the mixture in either
the ethyl acetate or
ethanol. For example, if desired, the ethanol concentration in the mixture may
be increased
through hydrolysis of the ethyl acetate in the presence of an acid catalyst to
make additional
ethanol and acetic acid. The acetic acid then may be recycled back in the
hydrogenation
process.
[0096] The following examples describe the procedures used for the preparation
of various
catalysts employed in the process of this invention.
Examples
Catalyst parations (general)
[0097] The catalyst supports were dried at 120 C overnight under circulating
air prior to use.
All commercial supports (i.e., Si02, Ti02) were used as a 14/30 mesh, or in
its original shape
(1/16 inch or 1/8 inch pellets) unless mentioned otherwise. Powdered materials
were
pelletized, crushed and sieved after the metals had been added. The individual
catalyst
preparations of the invention, as well as comparative examples, are described
in detail below.
27
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
Example 1 - SiO2-CaSiO3(5)-Pt 3)-Sn(1.8)
[0098] The catalyst was prepared by first adding CaSiO3 (Aldrich) to the Si02
catalyst
support, followed by the addition of Pt/Sn. First, an aqueous suspension of
CaSiO3 (< 200
mesh) was prepared by adding 0.52 g of the solid to 13 ml of deionized H2O,
followed by the
addition of 1.0 ml of colloidal Si02 (15 wt% solution, NALCO). The suspension
was stirred
for 2 h at room temperature and then added to 10.0 g of Si02 catalyst support
(14/30 mesh)
using incipient wetness technique. After standing for 2 hours, the material
was evaporated to
dryness, followed by drying at 120 C overnight under circulating air and
calcination at 500 C
for 6 hours. All of the Si02-CaSiO3 material was then used for Pt/Sn metal
impregnation.
[0099] The catalysts were prepared by first adding Sn(OAc)2 (tin acetate,
Sn(OAc)2 from
Aldrich) (0.4104 g, 1.73 mmol) to a vial containing 6.75 ml of 1:1 diluted
glacial acetic acid
(Fisher). The mixture was stirred for 15 min at room temperature, and then,
0.6711 g (1.73
mmol) of solid Pt(NH3)4(NO3)2 (Aldrich) were added. The mixture was stirred
for another 15
min at room temperature, and then added drop wise to 5.0 g of Si02-CaSiO3
support, in a 100
ml round-bottomed flask. The metal solution was stirred continuously until all
of the Pt/Sn
mixture had been added to the Si02-CaSiO3 support while rotating the flask
after every addition
of metal solution. After completing the addition of the metal solution, the
flask containing the
impregnated catalyst was left standing at room temperature for two hours. The
flask was then
attached to a rotor evaporator (bath temperature 80 C), and evacuated until
dried while slowly
rotating the flask. The material was then dried further overnight at 120 C,
and then calcined
using the following temperature program: 25 -> 160 C/ramp 5.0 deg/min; hold
for 2.0 hours;
160 -> 500 C/ramp 2.0 deg/min; hold for 4 hours. Yield: 11.21 g of dark grey
material.
Example 2 - KA160-CaSiO3(8)-Pt 3 -Sn(1.8)
[0100] The material was prepared by first adding CaSiO3 to the KA160 catalyst
support
(Si02-(0.05) A1203, Sud Chemie, 14/30 mesh), followed by the addition of
Pt/Sn. First, an
aqueous suspension of CaSiO3 (< 200 mesh) was prepared by adding 0.42 g of the
solid to 3.85
ml of deionized H2O, followed by the addition of 0.8 ml of colloidal Si02 (15
wt% solution,
NALCO). The suspension was stirred for 2 h at room temperature and then added
to 5.0 g of
KA160 catalyst support (14/30 mesh) using incipient wetness technique. After
standing for 2
hours, the material was evaporated to dryness, followed by drying at 120 C
overnight under
28
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
circulating air and calcinations at 500 C for 6 hours. All of the KA160-CaSiO3
material was
then used for Pt/Sn metal impregnation.
[0101] The catalysts were prepared by first adding Sn(OAc)2 (tin acetate,
Sn(OAc)2 from
Aldrich) (0.2040 g, 0.86 mmol) to a vial containing 6.75 ml of 1:1 diluted
glacial acetic acid
(Fisher). The mixture was stirred for 15 min at room temperature, and then,
0.3350 g (0.86
mmol) of solid Pt(NH3)4(NO3)2 (Aldrich) were added. The mixture was stirred
for another 15
min at room temperature, and then added drop wise to 5.0 g of Si02-CaSiO3
support, in a 100
ml round-bottomed flask. After completing the addition of the metal solution,
the flask
containing the impregnated catalyst was left standing at room temperature for
two hours. The
flask was then attached to a rotor evaporator (bath temperature 80 C), and
evacuated until dried
while slowly rotating the flask. The material was then dried further overnight
at 120 C, and
then calcined using the following temperature program: 25 -+ 160 C/ramp 5.0
deg/min; hold
for 2.0 hours; 160 -> 500 C/ramp 2.0 deg/min; hold for 4 hours. Yield: 5.19 g
of tan-colored
material.
Example 3 - Si02-CaSi03(2.5)-Pt(1.5)-Sn(0.9).
[0102] This catalyst was prepared in the same manner as Example 1, with the
following
starting materials: 0.26 g of CaSiO3 as a support modifier; 0.5 ml of
colloidal Si02 (15 wt%
solution, NALCO), 0.3355 g (0.86 mmol) of Pt(NH3)4(NO3)2; and 0.2052 g (0.86
mmol) of
Sn(OAc)2. Yield: 10.90 g of dark grey material.
Example 4 - SiQ2 + MgSiO3-Pt(1.0)-Sn(1.0)
[0103] This catalyst was prepared in the same manner as Example 1, with the
following
starting materials: 0.69 g of Mg(AcO) as a support modifier; 1.3 g of
colloidal Si02 (15 wt.%
solution, NALCO), 0.2680 g (0.86 mmol) of Pt(NH3)4(NO3)2; and 0.1640 g (0.86
mmol) of
Sn(OAc)2. Yield: 8.35 g. The Si02 support is impregnated with a solution of
Mg(AcO) and
colloidal Si02. The support is dried and then calcined to 700 C.
Example 5 - Si02-CaSiO3(5)-Re(4.5)-Pd(1)
[0104] The Si02-CaSiO3(5) modified catalyst support was prepared as described
in
Example 1. The Re/Pd catalyst was prepared then by impregnating the Si02-
CaSiO3(5) (1/16
inch extrudates) with an aqueous solution containing NH4ReO4 and Pd(N03)2. The
metal
solutions were prepared by first adding NH4ReO4 (0.7237 g, 2.70 mmol) to a
vial containing
12.0 ml of deionized H2O. The mixture was stirred for 15 min at room
temperature, and 0.1756
29
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
g (0.76 mmol) of solid Pd(N03)2 was then added. The mixture was stirred for
another 15 min
at room temperature, and then added drop wise to 10.0 g of dry Si02-
(0.05)CaSiO3 catalyst
support in a 100 ml round-bottomed flask. After completing the addition of the
metal solution,
the flask containing the impregnated catalyst was left standing at room
temperature for two
hours. All other manipulations (drying, calcination) were carried out as
described in Example
1. Yield: 10.9 g of brown material.
Example 6 - Si02-ZnO(5)-Pt(1)-Sn(1).
[0105] Powdered and meshed high surface area silica NPSG SS61138 (100 g) of
uniform
particle size distribution of about 0.2 mm was dried at 120 C in a circulating
air oven
atmosphere overnight and then cooled to room temperature. To this was added a
solution of
zinc nitrate hexahydrate. The resulting slurry was dried in an oven gradually
heated to 110 C
(>2 hours, 10 C/min.) then calcined. To this was added a solution of platinum
nitrate
(Chempur) in distilled water and a solution of tin oxalate (Alfa Aesar) (1.74
g) in dilute nitric
acid (1N, 8.5 ml) The resulting slurry was dried in an oven gradually heated
to 110 C (>2
hours, 10 C/min.). The impregnated catalyst mixture was then calcined at 500 C
(6 hours,
1 C/min).
Example 7 -Ti02-CaSiO3(5)-Pt(3)-Sn(1.8)
[0106] The material was prepared by first adding CaSiO3 to the Ti02 catalyst
(Anatase,
14/30 mesh) support, followed by the addition of Pt/Sn as described in Example
1. First, an
aqueous suspension of CaSiO3 (< 200 mesh) was prepared by adding 0.52 g of the
solid to 7.0
ml of deionized H2O, followed by the addition of 1.0 ml of colloidal Si02 (15
wt% solution,
NALCO). The suspension was stirred for 2 h at room temperature and then added
to 10.0 g of
Ti02 catalyst support (14/30 mesh) using incipient wetness technique. After
standing for 2
hours, the material was evaporated to dryness, followed by drying at 120 C
overnight under
circulating air and calcination at 500 C for 6 hours. All of the Ti02-CaSiO3
material was then
used for Pt/Sn metal impregnation using 0.6711 g (1.73 mmol) of Pt(NH3)4(NO3)2
and 0.4104 g
(1.73 mmol) of Sn(OAc)2 following the procedure described in Example 1. Yield:
11.5 g of
light grey material.
Example 8 - Pt(2)-Sn(2) on High Surface Area Silica.
[0107] Powdered and meshed high surface area silica NPSG SS61138 (100 g) of
uniform
particle size distribution of about 0.2 mm was dried at 120 C in a circulating
air oven
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
atmosphere overnight and then cooled to room temperature. To this was added a
solution of
nitrate hexahydrate (Chempur). The resulting slurry was dried in an oven
gradually heated to
110 C (>2 hours, 10 C/min.) then calcined. To this was added a solution of
platinum nitrate
(Chempur) in distilled water and a solution of tin oxalate (Alfa Aesar) in
dilute nitric acid. The
resulting slurry was dried in an oven gradually heated to 110 C (>2 hours, 10
C/min.). The
impregnated catalyst mixture was then calcined at 500 C (6 hours, 1 C/min).
Example 9 - KA160-Pt(3)-Sn(1.8).
[0108] The material was prepared by incipient wetness impregnation of KA160
catalyst
support (Si02-(0.05) A1203, Sud Chemie, 14/30 mesh) as described in Example 1.
The metal
solutions were prepared by first adding Sn(OAc)2 (0.2040 g, 0.86 mmol) to a
vial containing
4.75 mf of 1:1 diluted glacial acetic acid. The mixture was stirred for 15 min
at room
temperature, and then, 0.3350 g (0.86 mmol) of solid Pt(NH3)4(NO3)2 were
added. The mixture
was stirred for another 15 min at room temperature, and then added drop wise
to 5.0 g of dry
,KA160 catalyst support (14/30 mesh) in a 100 ml round-bottomed flask. All
other
manipulations, drying and calcination was carried out as described in Example
16. Yield: 5.23
g of tan-colored material.
Example 10 - SiO,-SnO2(5)-Pt 1)-Zn 1).
[0109] Powdered and meshed high surface area silica NPSG SS61138 (100 g) of
uniform
particle size distribution of about 0.2 mm was dried at 120 C in a circulating
air oven
atmosphere overnight and then cooled to room temperature. To this was added a
solution of tin
acetate (Sn(OAc)2). The resulting slurry was dried in an oven gradually heated
to 110 C (>2
hours, 10 C/min.) then calcined. To this was added a solution of platinum
nitrate (Chempur) in
distilled water and a solution of tin oxalate (Alfa Aesar) in dilute nitric
acid The resulting slurry
was dried in an oven gradually heated to 110 C (>2 hours, 10 C/min.). The
impregnated
catalyst mixture was then calcined at 500 C (6 hours, 1 C/min).
Example 11 - Si02-Ti02(10)-Pt(3)-Sn(1.8).
[0110] The Ti02-modified silica support was prepared as follows. A solution of
4.15 g
(14.6 mmol) of Ti {OCH(CH3)2}4 in 2-propanol (14 ml) was added dropwise to
10.0 g of Si02
catalyst support (1/16 inch extrudates) in a 100 ml round-bottomed flask. The
flask was left
standing for two hours at room temperature, and then evacuated to dryness
using a rotor
evaporator (bath temperature 80 C). Next, 20 ml of deionized H2O was slowly
added to the
31
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
flask, and the material was left standing for 15 min. The resulting water/2-
propanol was then
removed by filtration, and the addition of H2O was repeated two more times.
The final material
was dried at 120 C overnight under circulation air, followed by calcination at
500 C for 6
hours. All of the Si02-TiO2 material was then used for Pt/Sn metal
impregnation using 0.6711
g (1.73 mmol) of Pt(NH3)4(NO3)2 and 0.4104 g (1.73 mmol) of Sn(OAc)2 following
the
procedure described above for Example 1. Yield: 11.98 g of dark grey 1/16 inch
extrudates.
Example 12 - Si02-W03(10)-Pt(3 -Sn(1.8).
[0111] The W03-modified silica support was prepared as follows. A solution of
1.24 g
(0.42 mmol) of (NH4)6H2W12040 . n H2O, (AMT) in deionized H2O (14 ml) was
added
dropwise to 10.0 g of Si02 NPSGSS 61138catalyst support (SA = 250 m2/g, 1/16
inch
extrudates) in a 100 ml round-bottomed flask. The flask was left standing for
two hours at
room temperature, and then evacuated to dryness using a rotor evaporator (bath
temperature
80 C). The resulting material was dried at 120 C overnight under circulation
air, followed by
calcination at 500 C for 6 hours. All of the (light yellow) Si02-WO3 material
was then used for
Pt/Sn metal impregnation using 0.6711 g (1.73 mmol) of Pt(NH3)4(NO3)2 and
0.4104 g (1.73
mmol) of Sn(OAc)2 following the procedure described above for Example 1.
Yield: 12.10 g of
dark grey 1/16 inch extrudates.
Example 13 - Comparative
[0112] Sn(0.5) on High Purity Low Surface Area Silica. Powdered and meshed
high purity
low surface area silica (100 g) of uniform particle size distribution of about
0.2 mm was dried
at 120 C in an oven under nitrogen atmosphere overnight and then cooled to
room temperature.
To this was added a solution of tin oxalate (Alfa Aesar) (1.74 g) in dilute
nitric acid (IN, 8.5
ml). The resulting slurry was dried in an oven gradually heated to 110 C (>2
hours,
C/min.). The impregnated catalyst mixture was then calcined at 500 C (6 hours,
1 C/min).
Example 14 - Gas Chromatographic (GC) Analysis of the Crude Product Hydro eng
ation
[0113] Catalyst of Examples 1-13 were tested to determine the selectivity and
productivity
to ethyl acetate and ethanol as shown in Table 4.
[0114] In a tubular reactor made of stainless steel, having an internal
diameter of 30 mm
and capable of being raised to a controlled temperature, there are arranged 50
ml of catalyst
listed in Table 2. The length of the combined catalyst bed after charging was
approximately
about 70 mm. The reaction feed liquid of acetic acid was evaporated and
charged to the reactor
32
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
along with hydrogen and helium as a carrier gas with an average combined gas
hourly space
velocity (GHSV), temperature, and pressure as indicated in Table 4. The feed
stream contained
a mole ratio hydrogen to acetic acid as indicated in Table 4.
[0115] The analysis of the products was carried out by online GC. A three
channel
compact GC equipped with one flame ionization detector (FID) and 2 thermal
conducting
detectors (TCDs) was used to analyze the reactants and products. The front
channel was
equipped with an FID and a CP-Sil 5 (20 m) + WaxFFap (5 m) column and was used
to
quantify: Acetaldehyde; Ethanol; Acetone; Methyl acetate; Vinyl acetate; Ethyl
acetate; Acetic
acid; Ethylene glycol diacetate; Ethylene glycol; Ethylidene diacetate; and
Paraldehyde. The
middle channel was equipped with a TCD and Porabond Q column and was used to
quantify:
C02; ethylene; and ethane. The back channel was equipped with a TCD and
Molsieve 5A
column and was used to quantify: Helium; Hydrogen; Nitrogen; Methane; and
Carbon
monoxide.
[0116] Prior to reactions, the retention time of the different components was
determined by
spiking with individual compounds and the GCs were calibrated either with a
calibration gas of
known composition or with liquid solutions of known compositions. This allowed
the
determination of the response factors for the various components.
33
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
Q N d- ~O o0 M ~ N M \O \O N l~ 00 1- r.-
al 00 00 00 00 N M M M "dt 'IT d' l-
U
N M 00 O n 00 N M N O d M M
W) kn r N
0
O .r
9 ~+ M'0 N o0 N 00 N =-+ . M M N O
oON ~I NN NMMMN-'O--+N- .-
U
~^ o 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
OOoN N-N-N-N-N-N- t-o N-N- NO
In In In Vn kn Vn kn In to kn Vn kn kn Vn to kn
N N N ~O ~O ~O ~O ~D ~D \O N C ~O ~O N
Ho OOOOOkO OOv' O k r) tf) N N in Cn r- W) O1 00 kf) N kn r- W) In I )
N N N N N N N N N N N N N N N N
0
U
N `~ o o O o o O O O O o 0 o O O O
0 t) o
o O o 0 0 0 0 0 0 0 0 0 0 0 0 0
NN"t dd'd'~:t ti d d'N~'t d'N
-4 -4 -4 -4 --~ '-+ -+ N -- --~ --~ N
N
O U
66
71 71 7~
kn to tn 4 kn in kn kn to kr) 4 kn O\
ON
00 0 C n U )- , , 00
coo 00
M ski
u O r. .L, N rn V1
X
U M W *, ., - v1g 0o0
a Qy ~ ~/ `^N N N N ~~
00
M in ~~+ n Cn Cn C/] V)
~" a a N
M m cn , cn 0 O O O - O O O
kf)
nl 1
O O O O N N N N y N N M
Cn U Cn C/1 Q Cn ~ \_I ~' 0 Q 0
U o U + U N U Cn Cn Cn Cn Cn E-4
' i i I / 1 I 1 I 1 I
N --~ N N N N N NNNN ~ N N N
O CCCCC~.~.."
rn U1 zn rn v) E~ a a~ a~ a v~ v) olo~ Ci
k O .-+ N M
U W N M d kn \O l- 00 00 00 00 Q1 ,-+ --1 -4
CA 02778814 2012-04-24
WO 2011/053366 PCT/US2010/022949
Example 15
[0117] Vaporized acetic acid and hydrogen were passed over a hydrogenation
catalyst of
the present invention comprising 2 wt % Pt; and 2 wt % Sn on high surface area
silica (NPSG
SS61138) having a surface area of approximately 250 m2/g at a ratio of
hydrogen to acetic acid
of about 160 sccm/min H2: 0.09 g/min HOAc, the hydrogen being diluted with
about 60
sccm/min N2 at a space velocity of about 6570 hr-1 and a pressure of 200 psig
(1379 kPag).
The temperature was increased at about 50 hrs, 70 hrs and 90 hrs as indicated
in FIG. 3 and
FIG. 4. The productivity in grams of the indicated products (ethanol,
acetaldehyde, and ethyl
acetate) per kilogram of catalyst per hour are indicated in FIG. 3, and the
selectivity of a
catalyst for the various products are indicated in FIG. 4 with the upper line
indicating
productivity of or selectivity to ethyl acetate, the intermediate line
indicating ethanol and the
lower line indicating acetaldehyde. It is considered especially significant
that production of,
and selectivity for, acetaldehyde were low. FIGS. 3 and 4 demonstrate that the
relative
insensitivity of the catalyst to changes in temperature make this catalyst
well-suited for use in a
so-called adiabatic reactor in which the temperature may vary substantially
over the catalyst
bed due to the low and uneven rate of heat removal from the reactor.
[0118] While the invention has been described in detail, modifications within
the spirit and
scope of the invention will be readily apparent to those of skill in the art.
In view of the
foregoing discussion, relevant knowledge in the art and references discussed
above in
connection with the Background and Detailed Description, the disclosures of
which are all
incorporated herein by reference. In addition, it should be understood that
aspects of the
invention and portions of various embodiments and various features recited
below and/or in the
appended claims may be combined or interchanged either in whole or in part. In
the foregoing
descriptions of the various embodiments, those embodiments which refer to
another
embodiment may be appropriately combined with other embodiments as will be
appreciated by
one of skill in the art. Furthermore, those of ordinary skill in the art will
appreciate that the
foregoing description is by way of example only, and is not intended to limit
the invention.