Note: Descriptions are shown in the official language in which they were submitted.
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
SELF-SUSPENDING PROPPANTS FOR HYDRAULIC FRACTURING
RELATED APPLICATIONS
[0001] This application claims the benefit of U.S. Provisional Application
Serial No.
61/635,612, filed April 19, 2012, U.S. Provisional Application Serial No.
61/662,681,
filed June 21, 2012, U.S. Provisional Application Serial No. 61/725,751, filed
November
13, 2012 and U.S. Provisional Application Serial No. 61/764,792, filed
February 14,
2013. The entire contents of the above-referenced applications are
incorporated by
reference herein.
FIELD OF APPLICATION
[0002] This application relates generally to systems and methods for
fracturing
technologies.
BACKGROUND
[0003] In the process of acquiring oil and/or gas from a well, it is often
necessary to
stimulate the flow of hydrocarbons via hydraulic fracturing. The term
"fracturing" refers
to the method of pumping a fluid into a well until the pressure increases to a
level that is
sufficient to fracture the subterranean geological formations containing the
entrapped
materials. This process results in cracks and breaks that disrupt the
underlying layer to
allow the hydrocarbon product to be carried to the well bore at a
significantly higher rate.
Unless the pressure is maintained, however, the newly formed openings close.
In order to
open a path and maintain it, a propping agent or "proppant" is injected along
with the
hydraulic fluid to create the support needed to preserve the opening. As the
fissure is
formed, the proppants are delivered in a slurry where, upon release of the
hydraulic
pressure, the proppants form a pack or a prop that serves to hold open the
fractures.
[0004] To accomplish the placement of the proppants inside the fracture, these
particles
are suspended in a fluid that is then pumped to its subterranean destination.
To prevent
the particles from settling, a high viscosity fluid is often required to
suspend them. The
viscosity of the fluid is typically managed by addition of synthetic or
naturally-based
polymers. There are three common types of polymer-enhanced fluid systems in
general
use for suspending and transporting proppants during hydraulic fracturing
operations:
slickwater, linear gel, and crosslinked gel.
1
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
[0005] In slickwater systems, an anionic or cationic polyacrylamide is
typically added as
a friction reducer additive, allowing maximum fluid flow with a minimum of
pumping
energy. Since the pumping energy requirements of hydraulic fracturing are
high, on the
order of 10,000 to 100,000 horsepower, a friction reducer is added to
slickwater fluids to
enable high pumping rates while avoiding the need for even higher pumping
energy.
While these polymers are effective as friction reducers, they are not highly
effective as
viscosifiers and suspending agents. Slickwater polymer solutions typically
contain 0.5
to2.0 gallons of friction reducer polymer per 1000 gallons of slickwater
fluid, and the
solutions have low viscosity, typically on the order of 3 to15 cps. At this
low viscosity,
suspended proppant particles can readily settle out of suspension as soon as
turbulent
flow is stopped. For this reason, slickwater fluids are used in the fracturing
stages that
have either no proppant, proppant with small particle size, or low proppant
loadings.
[0006] The second type of polymer enhanced fluid system is known as a linear
gel
system. Linear gel systems typically contain carbohydrate polymers such as
guar,
hydroxyethylcellulose, hydroxyethyl guar, hydroxypropyl guar, and
hydroxypropylcellulose. These linear gel polymers are commonly added at a use
rate of
10 to 50 pounds of polymer per 1000 gallons of linear gel fluid. These
concentrations of
linear gel polymer result in a fluid with improved proppant suspending
characteristics vs.
the slickwater fluid. The linear gel fluids are used to transport proppants,
at loading
levels of about 0.1 to 1 pound of proppant per gallon of fluid. Above this
proppant
loading level, a more viscous solution is typically required to make a stable
suspension.
[0007] Crosslinked gel is the most viscous type of polymer-enhanced fluid used
for
transporting of proppant. In crosslinked gel systems, the linear gel fluid as
described
above is crosslinked with added reagents such as borate, zirconate, and
titanate in the
presence of alkali. Upon crosslinking of the linear gel fluid into a
crosslinked gel fluid,
the viscosity is much higher and the proppants can be effectively suspended.
The linear
gel and crosslinked gel fluids have certain advantages but they require a high
dose rate of
expensive polymer.
[0008] Modifications of proppant particles could be used advantageously to
improve
their performance in hydraulic fracturing systems. First, if the proppant
particles were
more buoyant, a less viscous suspension fluid could be used, which would still
convey the
particles to the target area but which would be easier to pump into the
formation. Second,
it is desirable that the proppants remain where they are placed throughout the
lifetime of
the well after they have been injected into a fracture line. If changes within
the reservoir
2
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
during well production force the proppants out of position, production
equipment can be
damaged, and the conductivity of the reservoir formation can be decreased as
the
reservoir pores are plugged by the displaced proppants. Third, the proppants
in the system
should be resistant to closure stress once they are placed in the fracture.
Closure stresses
can range from 1700 psi in certain shale gas wells, up to and exceeding 15,000
psi for
deep, high temperature wells. Care must be taken that the proppants do not
fail under this
stress, lest they be crushed into fine particles that can migrate to
undesirable locations
within the well, thereby affecting production. Desirably, a proppant should
resist
diagenesis during fracture treatment. The high pressures and temperatures
combine with
to the chemicals used in frac fluids can adversely affect the proppant
particles, resulting in
their diagenesis, which can eventually produce fine particulate matter that
can scale out
and decrease the productivity of the well over time.
[0009] Current proppant systems and polymer-enhanced fracturing fluids
endeavor to
address these concerns, so that the proppants can be carried by the fracturing
fluids, can
remain in place once they arrive at their target destination, and can resist
the closure
stresses in the formation. One approach to preparing suitable proppants
includes coating
the proppant materials with resins. A resin-coated proppant can be either
fully cured or
partially cured. The fully cured resin can provide crush resistance to the
proppant substrate
by helping to distribute stresses among the grain particles. A fully cured
resin can
furthermore help reduce fine migration by encapsulating the proppant particle.
If initially
partially cured, the resin may become fully cured once it is placed inside the
fracture. This
approach can yield the same benefits as the use of a resin that is fully-cured
initially.
Resins, though, can decrease the conductivity and permeability of the
fracture, even as the
proppants are holding it open. Also, resins can fail, so that their advantages
are lost. Resin-
based systems tend to be expensive and they are still prone to settling out of
suspension.
[0010] In addition, there are health, safety and environmental concerns
associated with
the handling and processing of proppants. For example, fine particulates
("fines"), such
as crystalline silica dust, are commonly found in naturally occurring sand
deposits. These
fines can be released as a respirable dust during the handling and processing
of proppant
sand. With chronic exposure, this dust can be harmful to workers, resulting in
various
inhalation-associated conditions such as silicosis, chronic obstructive
pulmonary disease,
lung cancers, and the like. In addition to these health effects, the fines can
cause
"nuisance dust" problems such as fouling of equipment and contamination of the
environment.
3
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
[0011] Another approach to preparing suitable proppants involves mixing
additives with
the proppant itself, such as fibers, elastomeric particles, and the like. The
additives,
though, can affect the rheological properties of the transport slurry, making
it more
difficult to deliver the proppants to the desired locations within the
fracture. In addition,
the use of additives can interfere with uniform placement of the proppant
mixture into the
fracture site. While there are known methods in the art for addressing the
limitations of
proppant systems, certain problems remain. There is thus a need in the art for
improved
proppant systems that allow precise placement, preserve fracture conductivity
after
placement, protect well production efficiency and equipment life, simplify
hydraulic
fracturing operations, reduce environmental impact, and promote worker health
and
safety. It is further desirable that such improved systems be cost-effective.
SUMMARY
[0012] Disclosed herein are embodiments of a modified proppant, comprising a
proppant particle and a hydrogel coating, wherein the hydrogel coating
localizes on the
surface of the proppant particle to produce the modified proppant. In
embodiments, the
proppant particle comprises sand or comprises a resin-coated substrate. In
additional
embodiments, the proppant particle comprises bauxite, sintered bauxite,
ceramic, or lower
density materials. In embodiments, the modified proppant further comprises an
adhesion
promoter, wherein the adhesion promoter affixes the hydrogel coating to the
resin-coated
substrate. In embodiments, the hydrogel coating comprises a water-swellable
polymer.
In embodiments, the hydrogel coating comprises a polymer selected from the
group
consisting of polyacrylamide, poly(acrylic acid), carboxymethyl cellulose,
hydroxyethyl
cellulose, hydroxypropyl cellulose, guar gum, carboxymethyl guar,
carboxymethyl
hydroxypropyl guar gum, hydrophobically associating swellable emulsion
polymers, and
latex polymers. In embodiments, the modified proppant further comprises a
cationic/anionic polymer pair comprising a cationic polymer and a high
molecular weight
anionic polymer. In embodiments, the cationic polymer is selected from the
group
consisting of poly-DADMAC, LPEI, BPEI, chitosan, and cationic polyacrylamide.
In
embodiments, the modified proppant further comprises a crosslinking agent. The
crosslinking agent can comprise a covalent crosslinker. The covalent
crosslinker can
comprise a functional group selected from the group consisting of an epoxide,
an
anhydride, an aldehyde, a diisocyanate, and a carbodiamide. The covalent
crosslinker can
be selected from the group consisting of polyethylene glycol, diglycidyl
ether,
4
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
epichlorohydrin, maleic anhydride, formaldehyde, glyoxal, glutaraldehyde,
toluene
diisocyanate, and methylene diphenyl diisocyanate, and 1-ethy1-3-(3-
dimethylaminopropyl) carbodiamide. In embodiments, the crosslinking agent
comprises
an organometallic compound. In embodiments, the modified proppant further
comprises
a hydrophobic layer, which can be selected from the group consisting of fatty
acids,
hydrogenated oils, vegetable oils, castor oil, waxes, polyethylene oxides, and
polypropylene oxides. In embodiments, the modified proppant comprises a
chemical
breaker, for example, an oxidative breaker. In embodiments, the modified
proppant
further comprises a delayed hydration additive. The delayed hydration additive
can be
selected from the group consisting of a low hydrophilic-lipophilic balance
surfactant, an
exclusion agent capable of excluding a finishing surfactant, a light ionic
crosslinking
agent, a light covalent crosslinking agent and a monovalent salt charge
shielder. In
embodiments, the modified proppant further comprises an alcohol, which can be
selected
from the group consisting of ethylene glycol, propylene glycol, glycerol,
propanol, and
ethanol. In embodiments, the modified proppant further comprises an anticaking
agent.
In embodiments, the hydrogel coating comprises an additive, which can be a
chemical
additive. In embodiments, the additive is a tracer or a chemical breaker. In
embodiments, the modified proppant contains less fines than a proppant
particle that is
not modified. In embodiments, the hydrogel coating comprises an additive,
which can be
a chemical additive or a tracer.
[0013] The invention additionally encompasses a hydraulic fracturing
formulation
comprising a modified proppant particle described herein.
[0014] Further disclosed herein are formulations that comprise the modified
proppant as
disclosed herein. Also disclosed herein are methods for fracturing a well,
comprising
preparing the hydraulic fracturing formulation as disclosed herein, and
introducing the
hydraulic fracturing formulation into the well in an effective volume and at
an effective
pressure for hydraulic fracturing, thereby fracturing the well.
[0015] Also disclosed herein are methods of manufacturing a modified proppant,
comprising providing a proppant substrate particle and a fluid polymeric
coating
composition; applying the fluid polymeric coating composition on the proppant
substrate
particle; wherein the fluid polymeric coating composition comprises a hydrogel
polymer,
and wherein the hydrogel polymer localizes on the surface of the proppant
substrate
particle to produce the modified proppant. In embodiments the fluid polymeric
coating
comprises a crosslinking species. In embodiments, the method further comprises
the step
5
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
of drying the modified proppant. In embodiments, the manufacturing takes place
at or
near a point of use for the modified proppant. In embodiments, the proppant
substrate
particle comprises sand, which can be obtained at or near the point of use for
the modified
proppant. In embodiments, the method further comprises a step of adding an
alcohol
selected from the group consisting of ethylene glycol, propylene glycol,
glycerol,
propanol, and ethanol during or before the step of applying the fluid
polymeric coating
composition on the proppant substrate particle. In embodiments, the method
further
comprises a step of adding an inversion promoter during or following the step
of mixing
the proppant substrate particles and the fluid polymer coating composition. In
embodiments, the method further comprises the step of adding an anticaking
agent to the
modified proppant.
[0016] Further disclosed herein are methods of manufacturing a hydrogel-coated
proppant, comprising providing a proppant substrate particle and a formulation
comprising a coating precursor, wherein the coating precursor is capable of
forming a
hydrogel coating on the proppant substrate particle by in situ polymerization;
applying
the formulation to the proppant substrate particle; and polymerizing the
coating precursor
in juxtaposition to the proppant substrate particle to form the hydrogel-
coated proppant.
BRIEF DESCRIPTION OF THE FIGURES
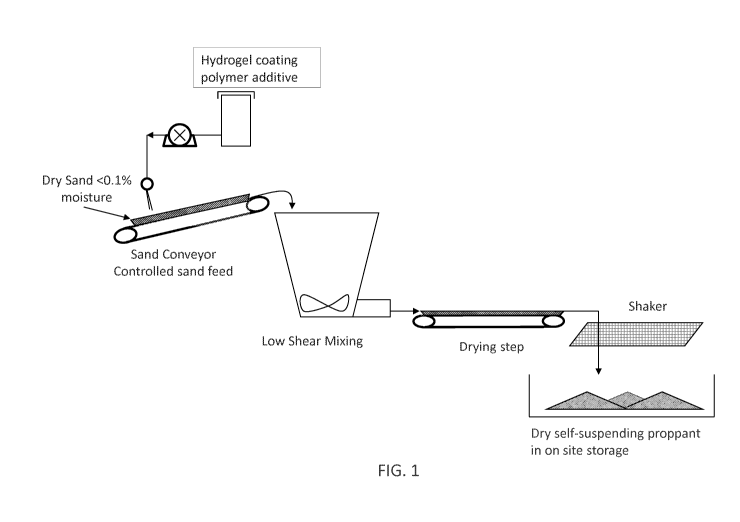
[0017] FIG. 1 is a flow diagram of a manufacturing process for self-suspending
proppants.
[0018] FIG. 2 is a graph of Bed Height (mm) versus Shear Time (min) for SSP,
SSP +
Glycerol and SSP + Ethal.
[0019] FIG. 3 is a graph of Bed Height (mm) versus Shear Time (min) for
samples with
glycerol and without glycerol.
[0020] FIG. 4 is a graph of Bed Height (mm) versus Shear Time (min) for
samples with
glycerol and without glycerol.
DETAILED DESCRIPTION
1. Modified Proppant Particles
[0021] Disclosed herein are systems and methods for forming and using proppant
particles having a hydrogel surface layer to enhance the hydrodynamic volume
of the
proppant particles during fluid transport, creating a more stable proppant
suspension that
resists sedimentation, separation, and screenout before the proppant can reach
the
6
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
intended target destination in the fracture. Further benefits of the hydrogel-
coated
proppants as disclosed herein include lower tendency to erode equipment, lower
friction
coefficient in the wet state, good bonding adhesion with each other after
placement in a
fracture site, resistance to uncontrolled fines formation, and anti-fouling
properties
attributable to the hydrophilic surface. In embodiments, the disclosed systems
for forming
proppant particles can be applied to the types of proppant substrates most
widely used,
e.g., sand and ceramics. In other embodiments, the proppant particles can be
formed
from a variety of substrates, including fibrous materials, as would be
available to those
having ordinary skill in the art. In certain embodiments, the proppant
particles can be
fabricated so that they resist crush or deformation, so that they resist
displacement, and so
that they can be suspended in less viscous fluid carriers for transporting
into the
formation.
[0022] In embodiments, these self-suspending proppants are formed by
modification of
a particulate substrate with a water swellable polymer coating such as a
hydrogel. In
embodiments, the particulate substrate can be modified with the polymer
coating before
the particulate substrate is introduced into the fracturing fluid. In
embodiments, the
amount of hydrogel polymer coating can be in the range of about 0.1 to about
10% based
on the weight of the proppant. In embodiments, the hydrogel layer applied onto
the
surface of the proppant substrate can be a coating thickness of about 0.01% to
about 20%
of the average diameter of the proppant substrate. Upon hydration and swelling
of the
hydrogel layer in the fracturing fluid, the hydrogel layer can become expanded
with
water, such that the hydrogel layer thickness can become about 10% to about
1000% of
the average diameter of the proppant substrate.
[0023] Methods for modification of proppant include spraying or saturation of
a liquid
polymer formulation onto a proppant substrate, followed by drying to remove
water or
other carrier fluids. The drying process can be accelerated by application of
heat or
vacuum, and by tumbling or agitation of the modified proppant during the
drying process.
The heating can be applied by forced hot air, convection, friction,
conduction,
combustion, exothermic reaction, microwave heating, or infrared radiation.
Agitation
during the proppant modification process has a further advantage of providing
a more
uniform coating on the proppant material.
[0024] FIG. 1 shows an example of a manufacturing process for the self-
suspending
proppant using dried sand and a liquid polymer. In the depicted embodiment,
sand is
conveyed into a mixing vessel, and a liquid polymer composition is sprayed via
pump and
7
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
spray nozzles onto the sand along the conveyor belt. The sand and liquid
polymer report
to a low shear mixing vessel, where the ingredients are further blended. After
mixing, the
modified sand containing the liquid polymer is sent to a dryer to remove water
and/or
organic carrier fluids associated with the liquid polymer. After the drying
step, the
modified sand is passed through size classification equipment, such as a
sieve, to remove
over-sized agglomerates. Mechanical mixers, shear devices, grinders, or
crushers can be
used to break up aggregates to allow the material to pass through the
appropriate sized
sieve. The finished material is then stored for shipment or use.
[0025] In embodiments, the sand that is used to produce self-suspending
proppant is pre-
dried to a moisture content of <1%, and preferably <0.1% before being modified
with a
hydrogel polymer. In embodiments, the sand temperature at the time of mixing
with the
liquid polymer is in the range of about 10 to about 200 C, and preferably in
the range of
about 15 to about 60 C.
[0026] In embodiments, the sand is contacted with the liquid polymer
composition by
means of spraying or injecting. The amount of liquid polymer composition added
is in
the range of about 1 to about 20%, and preferably about 2 to about 10% by
weight of the
sand. The sand and liquid polymer are blended for a period of about 0.1 to
about 10
minutes. In a preferred embodiment, the mixing equipment is a relatively low
shear type
of mixer, such as a tumbler, vertical cone screw blender, v-cone blender,
double cone
blender, or ribbon blender. In embodiments, the mixing equipment can be
equipped with
forced air, forced hot air, vacuum, external heating, or other means to cause
evaporation
of the carrier fluids.
[0027] In embodiments, the modified sand containing the liquid polymer is
dried to
remove water and/or organic carrier fluids associated with the liquid polymer.
The dryer
equipment can be a conveyor oven, microwave, or rotary kiln type. In an
embodiment the
drying step is carried out in such a way that the dried, modified sand
contains less than
1% by weight of residual liquids, including water and any organic carrier
fluids
associated with the liquid polymer composition.
[0028] In embodiments, the same equipment can be used to blend the sand with
the
liquid polymer and to dry the blended product in a single processing stage, or
in a
continuous production line.
[0029] In other embodiments, methods for modification of proppant include
synthesis of
a hydrogel coating in situ, or in the presence of the proppant particle,
resulting in a
hydrogel layer encapsulating the surface of the proppant particle. As an
example, the in
8
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
situ synthesis of the hydrogel can be accomplished by combining proppant
particles with
coating precursor monomers and/or macromonomers followed by a polymerization
step.
In other exemplary instances a water-soluble polymer can be dissolved in
monomers,
with or without solvent, followed by polymerization in the presence of the
proppant
particles, resulting in the formation of interpenetrating polymer networks as
a coating on
the proppants. In other exemplary instances, the water-soluble polymer is
dispersed in the
monomers, with or without solvent, and the subsequent polymerization will
result in
proppants encapsulated by a hydrogel consisting of water-soluble polymer
particles
locked up by the newly formed polymer. The monomers or macromonomers used can
be
selected from monomers that result in water-soluble polymers. In other
exemplary
instances, the particles can be encapsulated by non-water soluble polymer that
will then
be modified or hydrolyzed to yield the water-soluble hydrogel coating. As
would be
understood by those of ordinary skill in the art, the encapsulating layer can
be formed by
different polymerization techniques, with or without solvents. The in situ
polymerization
of polymer on the surface of proppant grains can have the advantage of
reducing or
eliminating drying steps.
[0030] By way of example, a water-soluble monomer(s) can be chosen from the
following monomers or salts thereof: acrylic acid, methacrylic acid,
acrylamide,
methacrylamide, and their derivatives, carboxyethyl acrylate,
hydroxyethylmethacrylate
(HEMA), hydroxyethylacrylate (HEA), polyethyleneglycol acrylates (PEG-
acrylates), N-
isopropylacrylamide (NiPA), 2-acrylamido-2-methyl-1-propanesulfonic acid
(AMPS),
sodium salt of styrene sulfonate, vinylsulphonic acid, (meth)allylsulphonic
acid,
vinylphosphonic acid, N-vinylacetamide, N-methyl-N-vinylacetamide, N-
vinylformamide, N-methyl-N-vinylformamide, N-vinylpyrrolidone, N-butyrolactam
or N-
vinylcaprolactam, maleic anhydride, itaconic acid, vinyl acetate,
dimethyldiallylammonium chloride; quaternized dimethylaminoethyl methacrylate
(DMAEMA), (meth)acrylamidopropyltrimethylammonium chloride,
methylvinylimidazolium chloride; 2-vinylpyridine; 4-vinylpyridine, and the
like. The
ratio of ionic to nonionic monomers can be selected to yield hydrogels with
different
charge density. In some instances, for example, it is desirable to have
hydrogels with
higher charge in order to yield coatings with faster hydration or swelling
properties. In
other instances the ionizable monomers can be selected to have higher or lower
ionization
constants to yield hydrogels more or less stable in brine environments. Other
advantageous properties can be imparted by selection of appropriate charge
densities.
9
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
[0031] In embodiments, coating precursors can include polyfunctional monomers
that
contain more than one polymerizable group and that will introduce the
crosslinking or
branching points in the hydrogel. Examples of these monomers are:
pentaerythritol triallyl
ether, PEG-diacrylates and metahcrylates, N,N'-methylenebisacrylamide,
epichlorohydrin, divinyl sulfone, and glycidyl methacrylate. When such
monomers are
used, the crosslinking monomer will be in the range of about 0.001 to about
0.5% of the
total monomer content. In selecting a range for adding crosslinkers, one
should be aware
that adding excessive amounts of crosslinker could form brittle hydrogels that
can
fracture or degrade under pressure, and adding insufficient amounts of
crosslinkers could
form hydrogels that can be easily detached form the surface particle under
extreme
conditions.
[0032] In embodiments, the monomers/macromonomers used are selected from
coating
precursor monomers that that will form a non-water soluble coating. After the
coating is
applied, its further modification will result in the water swellable polymer.
As an
example, a polymeric coating containing hydrolysable groups can be formed, and
subsequent hydrolysis will yield the hydrogel. Examples of monomers that fall
in this
category are esters, anhydrides, nitriles, and amides; for example the ester
monomers
methyl acrylate, t-butyl acrylate can be used. As another example, a monomer
containing
vinyl functionalities can form the hydrogel by different polymerization
techniques with or
without solvents. The polymerization techniques include bulk, suspension,
admicellar,
solution polymerization.
[0033] In other embodiments, coating monomers or precursors can be selected to
form a
self-suspending proppant with a hydrogel comprising a polyurethane or
polyurea. A list
of suitable monomers to form polymers with polyurethane and/or polyurea
functionalities
are: polyols such as ethylene glycol, propylene glycol, glycerin,
trimethylolpropane,
1,2,6-hexanetriol, pentaerythritol, sorbitol, sucrose, a-methylglycoside,
polyoxyalkylenes,
such as PEG, copolymers of PEG-PPG, Pluronics, Tetronics, polyamines such as
JEFFAMINEO polyetheramines. Among the isocyanates there may be mentioned
toluene-diisocyanate, naphthalenediisocyanate, xylene-diisocyanate,
tetramethylene
diisocyanate, hexamethylene diisocyanate, trimethylene diisocyanate, trimethyl
hexamethylene diisocyanate, cyclohexy1-1,2-diisocyanate, cyclohexylene-1,4-
diisocyanate
and the like. Other appropriate polymers can include HYPOLO hydrophilic
polyurethane
prepolymers from Dow, DESMODURO and MONDURO resins from Bayer (2,4'-
diphenylmethanediisocyanate, 4,4'-diphenylmethanediisocyanate, and their
mixtures),
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
and CONATHANEO (polyisocyanate functionalized prepolymers of toluene
diisocyanate
and poly(tetramethylene glycols)) from Cytec, and the like.
[0034] The coating of proppant particle with a polyurethane (PU) hydrogel can
be
carried out by conventional methods. In an embodiment, the coating can be
performed in
bulk without the use of solvents. For example, a typical formulation for a
crosslinked PU
hydrogel can be prepared in a one-step bulk polymerization process using a
diisocyanate,
polyoxyalkylene, and a multifunctional crosslinking agent. In an embodiment,
the
formulation will contain 10 to 80% of a polyoxyalkylene having the
polyoxyalkylene
molecular weight between 200 and 25,000.
[0035] Another method to form the hydrogel layer in situ can be carried out by
dissolving or suspending a water-soluble polymer in a monomer formulation
followed by
polymerization of the monomer. The monomers can be selected form the previous
list of
water soluble monomers. In the case that the water-soluble polymer is
dissolved in the
monomer mixture, the resulting coating will consist in interpenetrating
hydrogel network
of the initial water-soluble polymer and the polymer formed in situ. In the
case where the
water-soluble polymer is suspended in the monomer mixture, the resulting
coating will
consist of a hydrogel coating in which the water soluble particles are locked
up or
entrapped. For example, these particles can be trapped inside the newly formed
hydrogel
coating or they can be bonded to the newly formed polymer. The water-soluble
polymer
can be dissolved or suspended in the monomer formulation in the presence or
absence of
a solvent and the polymerization can be carried out by different techniques.
[0036] Suitable water soluble polymers to be mixed with monomers can be
selected
from the group consisting of polyacrylamide, polyacrylic acid, copolymers of
acrylamide
with acrylic acid salts, polyethyleneglycol, polyvinylpyrrolidone,
polyvinylalcohol,
carboxymethyl cellulose, hydroxyethyl cellulose, hydroxypropyl cellulose, guar
gum,
carboxymethyl guar, carboxymethyl hydroxypropyl guar gum, hydrophobically
associating swellable emulsion polymers, starches, latex polymers, and the
like.
[0037] Another method for modification of proppant particles includes grafting
hydrophilic polymers onto the particle. The grafting of polymer chains onto
the surface of
the particle can be done by reactions such as Huisgen cycloaddition and other
coupling or
addition reactions that can immobilize the polymers onto the particle surface.
[0038] The proppant particle used for these purposes can be selected to have
surface
functional groups such as epoxy, vinyl, amine, hydroxyl, etc. Those groups can
then
react with polymers having groups capable of reacting with the functional
groups on the
11
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
particle surface. For example, proppant particles comprising silica can be
surface
modified by silanes such as aminosilanes, vinylsilanes, epoxysilanes, and the
like.
[0039] In embodiments, the polymers that will react with the functionalized
particle are
hydrophilic linear or branched polymers or copolymers. The polymer can have
one or
more grafting moiety. In embodiments, the polymers can have functional groups
such as
amino, carboxyl or salts thereof, hydroxyl, thiol, acid anhydride, acid
chloride and/or
isocyanate groups which enable covalent binding to the functional groups of
the particle.
Examples of polymers that can be used to react with the functionalized
particle are:
epoxide functionalized PEG, amine functionalized PEG, azide functionalized
PEG,
lo polyethyleneimine, polyacrylic acid, polyvinyl alcohol, and the like.
[0040] In embodiments the resulting hydrogel, in addition to having swellable
properties, can have temperature responsive or pH-responsive properties. The
resulting
swellable properties of the proppant can thus be tuned. This is an added
benefit for
transporting proppant down the wellbore, since temperatures are lower at the
early stages
in which proppant is transported and full swelling behavior is desirable;
higher
temperatures are expected inside the fractures where lower swelling of the
hydrogel layer
is desirable for packing improvement. The monomers used to make the
temperature
responsive hydrogel coated proppants can be selected from N-
isopropylacrylamide
(NiPA), ethylene oxide, propylene oxide, or macromonomers/polymers that
display a
lower critical solution temperature (LCST).
[0041] In an embodiment, the process of converting a substrate such as sand
into a self-
suspending proppant can be conducted at or near the point of use, for example,
at an oil or
gas well site in preparation for hydraulic fracturing. This method has the
advantage of
converting a commodity material with high material handling costs, such as
sand, into a
specialized material that has added features. The sand can be acquired from
local sources
or shipped directly from a sand mining site or warehouse, for modification at
the point of
use. This avoids having to ship sand first into a blending plant and then ship
a second
time from the blending plant to the point of use. In the case of sand, the
shipping costs
can be higher than the material costs, so avoidance of extra shipping is
desirable for
controlling costs.
[0042] Hydrogel polymers that can be used to modify proppants in accordance
with the
systems and methods disclosed herein can be introduced, in embodiments, as oil-
based
emulsions, dispersions, water-based emulsions, latexes, solutions, and
dispersions. In
embodiments, the hydrogel polymer can be an alkali-swellable emulsion, wherein
the
12
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
hydrogel properties of the polymer are not fully developed until the polymer
is contacted
with alkali. In this embodiment, the alkali-swellable emulsion can be coated
onto the
proppant substrate to form a modified proppant, and this modified proppant can
be
suspended in a fracturing fluid in the presence of an alkaline material.
[0043] In embodiments, an additive such as an alcohol selected from the group
consisting of ethylene glycol, propylene glycol, glycerol, propanol, and
ethanol can be
added during or before the step of mixing the proppant substrate particles and
the liquid
polymer coating composition. In embodiments, inversion promoters useful as
additives in
the polymer coating formulations for self-suspending proppants can include
high HLB
surfactants, such as polyethylene oxide lauryl alcohol surfactant, (ETHAL LA-
12/80%
from ETHOX), ethylene glycol, propylene glycol, water, sodium carbonate,
sodium
bicarbonate, ammonium chloride, urea, barium chloride, and mixtures thereof In
embodiments, inversion promoters can serve the function of facilitating the
release of
active polymer ingredients from the internal phase of an oil based emulsion
polymer into
the (typically aqueous) process fluid to be treated. Since this process
converts an oil
continuous polymer into a water continuous environment, it can be
characterized as a
phase inversion.
[0044] In other embodiments, the proppant substrate can be modified with a
polymer
formulation, without the need for a drying step. This can be accomplished by
the use of a
solvent-free polymer formulation, or a curable formulation. In certain
simplified
methods, a dry or liquid polymer formulation can be applied onto the proppant
substrate
via inline mixing, and the modified material thus prepared can be used without
further
processing. The moisture content of the proppant substrate can be modified by
addition
or removal of water, or addition of other liquids, to allow the substrate to
be effectively
coated, handled, and delivered into the fracturing fluid.
[0045] The modified proppants can be further modified with a wetting agent
such as a
surfactant or other hydrophilic material to allow for effective dispersion
into a fracturing
fluid. When the hydrogel-modified proppants are suspended into a fracturing
fluid, they
are considered to be self-suspending if they require a lower viscosity fluid
to prevent the
particles from settling out of suspension.
[0046] The modified proppants can be further modified with an anticaking agent
such as
calcium silicate, calcium carbonate, talc, kaolin, bentonite, diatomaceous
earth, silica,
colloidal silica, or microcrystalline cellulose to improve the flowability and
handling
properties of the modified proppant material. The modified proppants with the
anticaking
13
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
agent can have improved handling properties, such as free-flowing properties,
resistance
to clumping, ease of conveying, ease of metering, and ease of discharging from
a storage
or transport vessel. In embodiments, the modified proppants with the
anticaking agents
can have reduced drying requirements, so that the finished product can be
produced with
a reduced amount of energy, time, and equipment.
[0047] The hydrogel-modified proppants of the invention can advantageously use
a
localized polymer concentration on the proppant surface, in contrast to the
traditional
approach of making the entire fluid medium viscous. This localized hydrogel
layer can
permit a more efficient use of polymer, such that a lower total amount of
polymer can be
used to suspend proppant, as compared, for example, with conventional polymer-
enhanced fracturing fluids such as slickwater, linear gel, and crosslinked
gel. Although
the hydrogel-modified proppants are considered to be self-suspending, they can
be used
in combination with friction reducers, linear gels, and crosslinked gels.
[0048] The hydrogel-modified proppants as disclosed herein can have the
advantage of
delivering friction-reducing polymer into the fracturing fluid, so that other
friction
reducer polymers might not be required or might be required in lesser amounts
when the
hydrogel-modified proppants are used in hydraulic fracturing operations. In
embodiments, some of the hydrogel polymer can desorb from the surface of the
proppant
to deliver friction reducing benefits or viscosity features to the fracturing
fluid. While the
exemplary embodiments herein focus on the use of hydrogel-modified proppants
for
hydraulic fracturing purposes, other uses for hydrogel-modified proppants can
be
envisioned, where their capabilities for water retention or friction reduction
can be
exploited. For example, hydrogel-modified proppants can be used for absorbing
water
from moist environments, forming water-retaining particles that can be removed
from the
environment, carrying with them undesirable moisture. As another example,
hydrogel-
modified proppants can be used in situations where adding water to an
environment
would be advantageous. A hydrogel-modified proppant can be saturated with
water or an
aqueous solution and then used, for example, as a soil remediation additive in
a dry
environment. The hydrogel-modified proppant can be formed from sand or other
substrates that are compatible with the soil, and they can be transported to
the area of
interest in dry form; they then can be saturated with water and used as a soil
amendment.
In other embodiments, hydrogel-modified proppants can be used as a soil
amendment in
dry form, where they can absorb and hold moisture from the environment, from
irrigation,
from rainfall and the like. In these embodiments, the moisture-holding
properties of the
14
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
hydrogel-modified proppant can be used advantageously. In embodiments, the
hydrogel-
modified proppant can be used to reduce erosion of topsoil, seedbeds,
hydroseeding
mixtures, and the like. In embodiments, the hydrogel-modified proppant can be
used as a
vehicle for introducing other compatible agents into the region, for example
into the soil.
Hydrogel-modified proppants can comprise additional formulations that leach
out of or
through the hydrogel layer into the environment, either as the hydrogel
degrades, or as it
absorbs moisture and expands. Examples of these formulations include
fertilizers, seeds,
plant growth regulators, herbicides, pesticides, fungicides, and the like.
Other uses for
hydrogel-modified proppants prepared in accordance with these formulations and
methods can be envisioned that are consistent with their properties described
herein.
[0049] The hydrogel polymer used for preparation of hydrogel-modified
proppants can,
in embodiments, comprise polymers such as a polyacrylamide, copolymers of
acrylamide
with anionic and cationic comonomers, copolymers of acrylamide with
hydrophobic
comonomers, poly(acrylic acid), poly(acrylic acid) salts, carboxymethyl
cellulose,
hydroxyethyl cellulose, hydroxypropyl cellulose, guar gum, alginate,
carrageenan, locus
bean gum, carboxymethyl guar, carboxymethyl hydroxypropyl guar gum,
hydrophobically associating swellable emulsion (HASE) polymers, latex
polymers,
starches, and the like. In embodiments, the hydrogel polymer can be
crosslinked to
enhance the water absorbing and swelling properties of the polymer. The
crosslinkers can
be introduced as an element of the hydrogel base polymer, or they can be
introduced as
chemical modifiers for pre-formed polymers.
[0050] Localizing the polymer around the proppant surface as described herein
can
result in a more effective use of polymer and can prevent proppant from
settling out of a
polymer solution. In embodiments, the polymer layer hydrates around the
proppant
effectively preventing proppant/proppant (interparticle) contact. This can
prevent the
proppant from forming a compact settled bed and can result in a proppant that
is easier to
resuspend in a fracturing fluid. The resuspension properties for the modified
proppants
can be important if the fluid flow is interrupted during hydraulic fracturing
operations. In
this event, when the flow is resumed, it is important that the proppant can be
resuspended
to avoid the loss of proppant or the unintended blockage of a fluid path.
[0051] The polymer surface modifications as described herein can cause an
increase in
the hydrodynamic radius of the proppant particle when the polymer swells. This
can
result in increased drag on the proppant as well as effectively changing the
overall
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
hydrogel/particle density. Both can result in a proppant particle with a
slower settling
rate and superior transport properties.
[0052] In embodiments, polymer pairing or ionic crosslinking can be used to
improve
the hydrogel polymer retention on the surface of the proppant particles. For
example, a
cationic polymer can be deposited onto the proppant as a first layer to "lock
in place" a
second layer containing a hydrogel such as a high molecular weight anionic
polymer. In
embodiments, the cationic polymer can be polydiallyldimethylammonium chloride
(poly-
(DADMAC)), linear polyethylenimine (LPEI), branched polyethylenimine (BPEI),
chitosan, epichlorohydrin/dimethylamine polymer, ethylene dichloride
dimethylamine
polymer, or cationic polyacrylamide. The cationic polymer layer can be placed
on the
proppant either before or after proppant surface modification with the anionic
hydrogel
layer. The ionic interaction can act as a crosslink mechanism to help prevent
the anionic
polymer from desorbing in high shear environments such as going through a pump
or
during pumping down the wellbore. The cationic polymer can also improve
polymer
retention by causing a delay in the hydration and extension of the anionic
polymer chains.
It is believed that less polymer chain extension during the pumping process
will yield
higher polymer retention on the proppant (i.e. less desorption).
[0053] Covalent crosslinking of the hydrogel polymer layer on proppant surface
can
improve the swelling properties of the polymer and the shear tolerance to
prevent
premature release of the hydrogel from the proppant. Covalent crosslinkers can
include
the following functional groups: epoxides, anhydrides, aldehydes,
diisocyanates,
carbodiamides, divinyl, or diallyl groups. Examples of these covalent
crosslinkers
include: PEG diglycidyl ether, epichlorohydrin, maleic anhydride,
formaldehyde, glyoxal,
glutaraldehyde, toluene diisocyanate, methylene diphenyl diisocyanate, 1-ethy1-
3-(3-
dimethylaminopropyl) carbodiamide, methylene bis acrylamide. Covalent
crosslinking of
the hydrogel polymer layer on the proppant surface can effectively create a
swellable
"polymer cage" around the proppant. The covalent bonds prevent the polymer
from
completely desorbing into solution. The slightly insoluble polymer layer is
able to swell
and produce a hydrated polymer layer.
[0054] To further prevent the possible detachment of the hydrogel from the
surface of
the particle, the proppant particle can be treated to impart functionalities
that will also
take part in the polymerization process. For example, sand particles can be
treated with
silanes to yield particles with vinyl functionalities, hydroxyl, epoxy, etc.
16
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
[0055] Delayed/controlled hydration of polymer layer may be desirable to delay
the
hydration of the polymer surface modification during handling of the proppant
and initial
pump-down through the wellbore. Environmental factors, such as humidity and
rain,
could cause premature hydration of the polymeric coating, which would make it
difficult
to effectively meter the proppant dose into the blender during a hydraulic
fracturing
operation. It is also believed that a fully hydrated polymer layer can be more
prone to
desorption under the high shear conditions associated with pumping of a
fracturing fluid
down the tubular. For these reasons, it may be advantageous to engineer a
surface-
modified proppant having slower or delayed hydration properties. In
embodiments,
delayed hydration can be achieved by addition of a low hydrophilic-lipophilic
balance
(HLB) surfactant, exclusion of a high HLB finishing surfactant, ionic
crosslinking,
covalent crosslinking, charge shielding using a monovalent salt, or by
incorporation of a
hydrophobic layer such as a fatty acid, or a fatty alcohol.
[0056] In embodiments, hydrophobic groups can be incorporated into the
hydrogel
polymer to allow for hydrophobic interactions. This method can improve the
salt
tolerance of the hydrogel layer, such that the hydrogel layer remains
swellable even in an
aqueous fluid that contains elevated salt concentrations.
[0057] Also disclosed herein is a method of improving well productivity by
improved
proppant placement using a hydrogel-coated proppant. The hydrogel-coated
proppant can
be more effectively transported into the far end of fractures to enable higher
productivity
of oil and gas from a well. Because the surface-modified proppants disclosed
herein can
be less inclined to settle out of the fluid and easier to resuspend and
transport through the
fracture, it is believed that proppant placement will be more effective. The
ability to
transport proppant further into fractures could significantly increase the
effectiveness of a
fracturing stimulation operation, resulting in a larger of volume of higher
density
fractures. These fracture channels can advantageously allow gas/condensate to
more
easily flow into the wellbore from the reservoir.
[0058] Also disclosed herein is an improved method of proppant placement using
a low
viscosity fluid. The surface modified proppants as disclosed herein utilize
polymers more
effectively to suspend/transport proppant particles. The surface modification
renders the
proppant self-suspending, thereby reducing or eliminating the need for highly
viscous
fluids/gels to transport proppant. Hence, lower viscosity fluids can be used
in
combination with the surface-modified proppant to transport proppant into
fractures. This
would advantageously simplify the formulation of fracturing gels for use with
proppants.
17
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
[0059] Also disclosed herein is a more efficient method of fracturing a well
using less
proppant. Because highly effective proppant placement can be achieved with the
easily-
transportable surface-modified proppants as disclosed herein, it is
anticipated that a
smaller amount of these surface-modified proppants would be required for any
given
fracturing operation, as compared to systems using traditional proppants. With
an
increasing demand for fracturing grade sand/proppants, and a decreasing supply
of
desirably-shaped sand for proppant use, it would be advantageous to provide
systems and
methods such as those disclosed herein where less proppant can be used to
achieve results
comparable to or superior to the outcomes using current techniques.
[0060] After the hydrogel coated proppants of the invention have been pumped
into a
well, the hydrogel layer can be degraded by chemical, thermal, mechanical, and
biological mechanisms. Specifically, the polymeric surface modification on the
proppant
can be broken down with the aid of chemical breakers, for example, ammonium
persulfate or other oxidizers. Controlled breaking of the hydrogel layer upon
reaching a
target temperature or amount of time in the fluid, can be used as a means to
direct the
placement of the proppant in the desired location in fractures. The
degradation of the
hydrogel layer is also beneficial to ensuring the adequate conductivity of the
propped
fracture after completing the hydraulic fracturing operations.
[0061] Also disclosed herein is a method of delivery of additives, for
example, chemical
additives, into the proppant pack, by incorporating the additives into the
hydrogel layer of
the modified proppant. The additives can include chemical additives that can
be
advantageously delivered in the hydrogel layer, for example scale inhibitor,
biocide,
breaker, wax control, asphaltene control, and tracers. In embodiments, these
chemical
additives can be chemically bound to the polymer in the hydrogel layer, for
example by
covalent bonding, ionic bonding, hydrophobic association, hydrogen bonding,
and the
like. After placement in a proppant pack, the desorption, oxidation, or
degradation of the
hydrogel polymer can result in the controlled release of the chemical
additives from the
self-suspending proppant. In embodiments, a hydraulic fracturing operation can
have
multiple stages of fracturing; the proppants injected in each stage can
contain unique
tracers. Analysis of the fluids produced from the fractured well can provide
information
about the relative productivity of each fracturing stage by the presence and
concentration
of the unique tracers corresponding to the stages. In other embodiments,
additives, for
example, particulate additives, can be physically bound or entangled in the
polymer layer.
18
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
[0062] In embodiments, the surface of a proppant particulate substrate can be
coated
with a selected polymer, either as a single layer or as a series of multiple
coating layers.
The coating (either single layer or multilayer) can show switchable behavior
under certain
circumstances. As used herein, the term "switchable behavior" or "switching
behavior"
refers to a change in properties with a change in circumstances, for example,
a change
from one set of properties during the transport phase and another set of
properties inside
the fracture. Switching behavior can be seen, for example, when a particle
demonstrates
hydrophilic properties in the fracturing fluid and adhesive properties when in
place within
the fractures. Such behavior can triggered by circumstances like the high
closing
pressures inside the fracture site so that the outer layer of the coating
rearranges itself to
exhibit more advantageous properties.
[0063] In an embodiment, the coated particle can switch from hydrophilic to
hydrophobic when subjected to the high pressures inside the fractures. In an
exemplary
embodiment, during the transport phase, when the hydrophilic covering of the
particle is
exposed to the water-based fracturing fluid, it will tend to be fully
distended. As a result,
the coating can provide the particle with lubrication in this state,
facilitating its movement
through the proppant slurry. When the particle has been conveyed to its
destination
within the fractures in the formation though, the high pressures there will
overcome the
steric repulsions of the external hydrophilic polymer chains, forcing the
outer layer to
rearrange itself so that the inner layer is exposed. In embodiments, the
switchable inner
layer can be hydrophobic or adhesive, or both. As the inner layer becomes
exposed, its
properties can manifest themselves. If the inner layer has adhesive
properties, for
example, it can fix the particles to each other to prevent their flowback.
This inner layer
can also be configured to capture fines in case the proppant particle fails.
Moreover, the
residual intact hydrophilic groups present in the outer coating can allow easy
flow of oil
through the proppant pack.
[0064] In embodiments, a coated proppant particle can be produced that bears
the
following layers of coating. First, a pressure-activated fixative polymer can
be used to
coat the proppant substrate. This coating layer can be elastomeric, thereby
providing
strength to the proppant pack by helping to agglomerate the proppant particles
and
distribute stress. In addition, this coating layer can encapsulate the
substrate particles and
retain any fines produced in the event of substrate failure. Second, a block
copolymer can
be adsorbed or otherwise disposed upon the first layer of coating. The
copolymer can
have a section with high affinity for the first polymeric layer, allowing
strong interaction
19
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
(hydrophobic interaction), and can have another section that is hydrophilic,
allowing for
easy transport of the proppant in the transport fluid.
[0065] In certain embodiments, a stronger interaction between the first and
second
coating layers may be useful. To accomplish this, a swelling-deswelling
technique can be
implemented. For example, the block copolymer can be adsorbed onto the surface
of the
elastomeric-coated particle. Then, the first coating layer can be swelled with
small
amounts of an organic solvent that allow the hydrophobic block of the
copolymer to
penetrate deeper into the first coating layer and to become entangled in the
elastomeric
coating. By removing the organic solvent, the layered polymeric composite will
deswell,
resulting in a stronger interaction of copolymer with the elastomeric
particle. A method
for swelling-deswelling technique that can be useful is set forth in "Swelling-
Based
Method for Preparing Stable, Functionalized Polymer Colloids," A. Kim et al.,
J. Am.
Chem. Soc. (2005) 127: 1592-1593, the contents of which are incorporated by
reference
herein.
[0066] While the systems described herein refer to a two-layer coating system,
it is
understood that there can be multiple (i.e., more than two) coating layers
forming the
composite proppant particles disclosed herein, with the each of the multiple
coating layers
possessing some or all of the attributes of the two coating layers described
above, or with
one or more of the multiple coating layers providing additional properties or
features.
[0067] The addition of a species capable of crosslinking the swellable polymer
on the
proppant surface can effectively reduce the ability of the polymer layer to
swell
prematurely. Decreased swelling of the polymer can reduce the tendency of the
polymer-
coated proppant to undergo caking during storage in humid conditions. As used
herein,
the term "caking" refers to the formation of clumps or solid masses by
adhesion of the
loose granular material. Caking of proppants during storage is undesirable for
material
handling purposes. Preferably, the crosslinker will not impede
hydration/swelling of the
polymer coating once the polymer-coated proppant is dispersed in an aqueous
fluid, such
as a hydraulic fracturing fluid. In embodiments, the crosslinking species has
the
capability of forming a bond with a carboxyl functional group, an amide
functional group,
or both. Preferably, the crosslinking species forms a bond that can be broken
or removed
under mechanical shear or by the action of a chemical breaker. The
crosslinking species
can be added directly into the polymer used to coat the proppant,
simultaneously added to
the proppant with the polymer while mixing, or added some time after addition
of the
polymer to the proppant but before drying.
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
[0068] The crosslinking species can be chosen from organic compounds
containing
aldehyde, amine, anhydride, or epoxy functional groups. The crosslinking
species can
also be an organometallic compound. Organometallic compounds able to associate
and/or
bond with the carboxyl functional group are an example of a crosslinking
species that
form shear sensitive bonds. In such embodiments, the organometallic compound
is able
to reduce the swelling tendency of the polymer-coated proppant via
crosslinking the
carboxyl groups prior to the introduction of the proppant into a hydraulic
fracturing fluid.
Then, when the crosslinked polymer coating encounters the high shear forces of
pumping
associated with hydraulic fracturing, the crosslink on the polymer can be
degraded,
allowing the polymer is able to swell unhindered when the proppant is
introduced into the
hydraulic fracturing fluid.
[0069] In certain embodiments, a thin, non-hygroscopic coating layer can be
applied to
the surface of a hydrogel-coated proppant to create a barrier preventing the
swellable
polymer layer on adjacent proppant particles from adhering during storage. The
outer
layer utilized can be comprised of compounds that are water-soluble, water
insoluble or
both. In embodiments, the outer layer can be formulated such that it remains
in the solid
phase at temperatures below 40 C and has a melting point in the range of 40 C
to 120 C.
Preferably the outer layer is formulated such that the melting point is low
enough that the
outer layer will be in the liquid phase during the drying process in the
manufacturing of
the polymer coated proppant, yet is high enough that the outer layer will
exist in the solid
phase during storage and transport of the polymer coated proppant.
[0070] In these embodiments, the outer layer acts as a barrier to reduce
caking of the
coated proppant in humid environments. The hydrophobic outer layer can be
added to the
polymer-coated proppant as a finely divided powder or as a liquid. In
embodiments, the
outer layer material can be melted prior to addition to the coated proppant;
in other
embodiments the outer layer material can be added as a solid or waxy material,
which can
melt during the drying process. The solid outer layer can be added to the
proppant
simultaneously with the polymer or can be added at some time after addition of
polymer
but before the drying process. The outer layer can be comprised of fatty
acids,
hydrogenated oils, vegetable oils, castor oil, waxes, polyethylene oxides,
polypropylene
oxides, and the like.
21
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
2. Particulate Substrate Materials
[0071] Composite proppant particles in accordance with these systems and
methods can
be formed using a wide variety of proppant substrate particles. Proppant
particulate
substrates can include for use in the present invention include graded sand,
resin coated
sand, bauxite, ceramic materials, glass materials, walnut hulls, polymeric
materials,
resinous materials, rubber materials, and the like. In embodiments, the
substrates can
include naturally occurring materials, for example nutshells that have been
chipped,
ground, pulverized or crushed to a suitable size (e.g., walnut, pecan,
coconut, almond,
ivory nut, brazil nut, and the like), or for example seed shells or fruit pits
that have been
chipped, ground, pulverized or crushed to a suitable size (e.g., plum, olive,
peach, cherry,
apricot, etc.), or for example chipped, ground, pulverized or crushed
materials from other
plants such as corn cobs. In embodiments, the substrates can be derived from
wood or
processed wood, including but not limited to woods such as oak, hickory,
walnut,
mahogany, poplar, and the like. In embodiments, aggregates can be formed,
using an
inorganic material joined or bonded to an organic material. Desirably, the
proppant
particulate substrates will be comprised of particles (whether individual
substances or
aggregates of two or more substances) having a size in the order of mesh size
4 to 100
(US Standard Sieve numbers). As used herein, the term "particulate" includes
all known
shapes of materials without limitation, such as spherical materials, elongate
materials,
polygonal materials, fibrous materials, irregular materials, and any mixture
thereof
[0072] In embodiments, the particulate substrate can be formed as a composite
from a
binder and a filler material. Suitable filler materials can include inorganic
materials such
as solid glass, glass microspheres, fly ash, silica, alumina, fumed carbon,
carbon black,
graphite, mica, boron, zirconia, talc, kaolin, titanium dioxide, calcium
silicate, and the
like. In certain embodiments, the proppant particulate substrate can be
reinforced to
increase their resistance to the high pressure of the formation which could
otherwise
crush or deform them. Reinforcing materials can be selected from those
materials that are
able to add structural strength to the proppant particulate substrate, for
example high
strength particles such as ceramic, metal, glass, sand, and the like, or any
other materials
capable of being combined with a particulate substrate to provide it with
additional
strength.
[0073] In addition to bare or uncoated substrates, composite hydrogel-coated
proppants
can be formed from substrates that have undergone previous treatments or
coatings. For
example, a variety of resin-coated proppant particles are familiar to skilled
artisans. The
22
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
formulations and methods described above for coating are suitable for use with
coated or
treated proppant particles, including curable and precured resin coated
proppants.
[0074] In one embodiment for treating resin-coated sand, a swellable hydrogel
layer, as
described above, can be applied to the resin-coated sand to improve its
suspension
characteristics. In embodiments, one can include the addition of the species
that acts as an
adhesion promoter to attach the hydrogel to the resin layer. The adhesion
promoters can
be, for example, block co-polymers composed of both hydrophilic and
hydrophobic
monomers. The block co-polymer can be added after the substrate sand is resin-
coated or
at the same time as the resin coating. In addition to block co-polymers,
cationic species
HI can be used such as fatty amines, polyquaternary amines, and cationic
surfactants.
[0075] In certain embodiments, the proppant particulate substrate can be
fabricated as an
aggregate of two or more different materials providing different properties.
For example,
a core particulate substrate having high compression strength can be combined
with a
buoyant material having a lower density than the high-compression-strength
material.
The combination of these two materials as an aggregate can provide a core
particle having
an appropriate amount of strength, while having a relatively lower density. As
a lower
density particle, it can be suspended adequately in a less viscous fracturing
fluid, allowing
the fracturing fluid to be pumped more easily, and allowing more dispersion of
the
proppants within the formation as they are propelled by the less viscous fluid
into more
distal regions. High density materials used as proppant particulate
substrates, such as
sand, ceramics, bauxite, and the like, can be combined with lower density
materials such
as hollow glass particles, other hollow core particles, certain polymeric
materials, and
naturally-occurring materials (nut shells, seed shells, fruit pits, woods, or
other naturally
occurring materials that have been chipped, ground, pulverized or crushed),
yielding a
less dense aggregate that still possesses adequate compression strength.
[0076] Aggregates suitable for use as proppant particulate substrates can be
formed
using techniques to attach the two components to each other. As one
preparation method,
a proppant particulate substrate can be mixed with the buoyant material having
a particle
size similar to the size of the proppant particulate substrates. The two types
of particles
can then be mixed together and bound by an adhesive, such as a wax, a phenol-
formaldehyde novolac resin, etc., so that a population of doublet aggregate
particles are
formed, one subpopulation having a proppant particulate substrate attached to
another
similar particle, one subpopulation having a proppant particulate substrate
attached to a
buoyant particle, and one subpopulation having a buoyant particle attached to
another
23
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
buoyant particle. The three subpopulations could be separated by their
difference in
density: the first subpopulation would sink in water, the second subpopulation
would
remain suspended in the liquid, and the third subpopulation would float.
[0077] In other embodiments, a proppant particulate substrate can be
engineered so that
it is less dense by covering the surface of the particulate substrate with a
foamy material.
The thickness of the foamy material can be designed to yield a composite that
is
effectively neutrally buoyant. To produce such a coated proppant particulate,
a particle
having a desirable compression strength can be coated with one reactant for a
foaming
reaction, followed by exposure to the other reactant. With the triggering of
foam
lo formation, a foam-coated proppant particulate will be produced.
[0078] As an example, a water-blown polyurethane foam can be used to provide a
coating around the particles that would lower the overall particle density. To
make such a
coated particle, the particle can be initially coated with Reactant A, for
example a mixture
of one or more polyols with a suitable catalyst (e.g., an amine). This
particle can then be
exposed to Reactant B containing a diisocyanate. The final foam will form on
the
particle, for example, when it is treated with steam while being shaken; the
agitation will
prevent the particles from agglomerating as the foam forms on their surfaces.
[0079] The crosslinking species can be added directly into the polymer used to
coat the
proppant, simultaneously added to the proppant with the polymer while mixing,
or added
some time after addition of the polymer to the proppant but before drying.
EXAMPLES
Materials
= 30/70 mesh frac sand
= 40/70 mesh frac sand
= Polydiallyldimethylammonium chloride (Aldrich, St. Louis, MO)
= LPEI 500 (Polymer Chemistry Innovations, Tucson, AZ)
= Ethyl Alcohol, 200 Proof (Aldrich, St. Louis, MO)
= Hexane (VWR, Radnor, PA)
= FLOPAM EM533 (SNF)
= Polyethyleneglycol diglycidyl ether (Aldrich, St. Louis, MO)
= Glyoxal, 40wt% solution (Aldrich, St. Louis, MO)
= HFC-44 (Polymer Ventures)
= Carboxymethyl Cellulose, sodium salt (Sigma-Aldrich, St Louis, MO)
24
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
= Ammonium Persulfate (Sigma-Aldrich, St. Louis, MO)
= Ethoxylated lauryl alcohol surfactant (Ethal LA-12/80%) (Ethox Chemical
Co, SC)
= Phenolic resin coated frac sand from a commercial source
= SMA 4000i, from Sartomer
= SMA 2000i, from Sartomer
= Pluronic Surfactant L31, from BASF, Florham Park, NJ
= Pluronic Surfactant L35, from BASF, Florham Park, NJ
= Pluronic Surfactant L81, from BASF, Florham Park, NJ
= ARQUADO 2HT-75, from Sigma Aldrich, St. Louis, MO
= ADOGENO 464, from Sigma Aldrich, St. Louis, MO
= Isopropanol (IPA), manufactured by Sigma Aldrich, St. Louis, MO
= Tetrahydrofuran (THF), manufactured by Sigma Aldrich, St. Louis, MO
= Glycerol, manufactured by Sigma Aldrich, St. Louis, MO
= 30/50 mesh white sand
= Thixcin-R (Elementis Specialties)
= Castor oil (J.T. Baker)
= Stearic Acid Powder (J.T. Baker)
= Tyzor TE (Dorf Ketal)
= Tyzor TEAZ (Dorf Ketal)
[0080] Example 1: Preparation of Inner Polymer Layer
[0081] An inner polymer layer of 100 ppm concentration was prepared on a sand
sample
by adding 200g 30/70 mesh frac sand to a FlackTek Max 100 long jar. To the
sand was
added 85 g tap water and 2 g of a 1% polydiallyldimethylammonium chloride
(PDAC)
solution. The sample was then shaken by hand for approximately 5 minutes,
vacuum
filtered and dried in an oven at 80 C. The sand sample was then removed from
the oven
and used in subsequent testing.
[0082] An identical method was used as described above to formulate a lOppm
inner
polymer layer coating with the exception being that only 0.2 g of a 1% PDAC
solution
were used.
[0083] An identical method was used as described above to formulate an inner
polymer
layer at a maximum polymer loading ("Max PDAC") with the exception that 1 g of
a
20wt% PDAC solution was used. Following treatment the sand was washed with
excess
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
tap water, vacuum filtered and dried in an oven at 80 C. The sand sample was
then
removed from the oven and used in subsequent testing.
[0084] Example 2: Preparation of Inner Polymer Layer
[0085] An inner polymer layer of 100 ppm concentration was prepared on a sand
sample
by dissolving 0.2 g LPEI 500 in 10 g ethanol to form a 2% LPEI 500 solution in
ethanol.
To 70 g ethanol in a 250 mL round bottom flask was added 0.75 g of the 2% LPEI
500
solution. Then 150 g of 30/70 mesh frac sand was added to the round bottom
flask. The
solvent was removed using a rotary evaporator with a 65 C water bath. The
sample was
then removed from the flask and used in subsequent testing.
[0086] Example 3: Preparation of Outer Polymer Layer
[0087] Outer polymer layers were applied to sand samples by mixing sand with
liquid
Flopam EM 533 polymer under different conditions. In one coating method,
polymer
product was added neat. In another coating method the polymer product was
extended by
diluting with hexane. For hexane dilution 10 g polymer was added to 10 g
hexane in a 40
mL glass vial and vortex mixed until homogenous. Polymer was then added to
30/70
mesh frac sand samples of 30 g in FlackTek Max 100 jars. Samples were placed
in a
FlackTek DAC150 SpeedMixer at 2600 rpm for about 25 seconds. Samples were
removed from SpeedMixer and allowed to dry in an oven at 80 C overnight.
[0088] Example 4: Performance of Outer Polymer Layer, Settling Times
[0089] Sand samples prepared in previous example were assessed for performance
in a
settling test. Prior to testing, all sand samples were sieved through a 25
mesh screen.
Settling times were obtained by adding 1 g of sand sample to 100 mL of tap
water in a
100 mL graduated cylinder. The graduated cylinder was then inverted about 8
times and
then the time required for all of the sand to settle at the bottom of the
graduated cylinder
was recorded. Three times were recorded for each sample. Settling times are
reported in
Table 1.
Table 1 Settling Times
Settling Settling
Settling
Sample Inner Outer Layer Treatment
Time! Time2 Time3
Layer Treatment Added (g)
(sec) (sec) (sec)
1 100ppm Flopam 1 34 35 32
PDAC EM533
50:50
2 100ppm Flopam 2 25 25 26
PDAC EM533/
hexane
26
CA 02870730 2014-10-16
WO 2013/158308 PCT/US2013/032435
Settling Settling
Settling
Sample Inner Outer Layer Treatment
Time! Time2 Time3
Layer Treatment Added (g)
(sec) (sec) (sec)
3 100ppm Flopam 3 35 71 60
PDAC EM533
50:50
4 100ppm Flopam 6 24 33 32
PDAC EM533/
hexane
Max Flopam 1 19 21 27
PDAC EM533
50:50
6 Max Flopam 2 17 23 21
PDAC EM533/
hexane
7 Max Flopam 3 29 31 35
PDAC EM533
50:50
8 Max Flopam 6 23 24 25
PDAC EM533/
hexane
9 None Flopam 1 22 22 22
EM533
None Flopam 3 25 54 64
EM533
11 None None 0 10 10 10
[0090] Example 5: Performance of Outer Polymer Layer, Settled Bed Height
[0091] Sand samples prepared in Example 3 with outer polymer layer were also
assessed
by observing the settled bed height in water. In a 20 mL glass vial, 1 g of a
sand sample
5 was added to 10 g tap water. The vials were inverted about 10 times to
adequately wet
the sand treatments. The vials were then allowed to sit undisturbed for about
30 minutes.
A digital caliper was then used to record the height of the sand bed in the
vial. Results
are reported in Table 2.
10 Table 2 Settled Bed Heights
Sample 1 2 3 4 5 6 7 8 9 10 11
Bed Height 13.5 6.9 22.6 8.9 8.9 5.8 11.9 n/a
11.9 22.9 0.8
(mm)
27
CA 02870730 2014-10-16
WO 2013/158308 PCT/US2013/032435
[0092] Example 6: Ionic Crosslink of Outer Polymer Layer
[0093] A 40g 30/70 mesh frac sand sample was treated with an outer polymer
layer by
adding 1.3 g Flopam EM 533 polymer to the 40 g of sand in a FlackTek Max 100
jar and
shaking the jar by hand for 2 minutes. The sand was then sieved through a 25
mesh
screen. To assess polymer retention on sand under shear, tests were conducted
by adding
g of treated sand to 200 g tap water with different levels of PDAC in a 300 mL
glass
beaker. It is believed that the PDAC will interact ionically to stabilize the
polymer layer
on the sand. The slurries were then stirred at 900 rpm with an overhead mixer
using a flat
propeller style mixing blade for 5 minutes. Mixing was then stopped and
samples were
10 allowed to settle for 10 minutes. Viscosity of the supernatant was then
measured using a
Brookfield DV-III+ rheometer with an LV-II spindle at 60 rpm. Bed height of
the settled
sand in the beaker was also recorded using a digital caliper. Results are
reported in Table
3.
Table 3 Polymer Retention
Sample PDAC Conc. (ppm) Visc. (cP) Bed
Height (mm)
12 0 25 4.5
13 60 10 8.6
14 200 2.5 6.3
[0094] Example 7: Covalent Crosslink of Outer Polymer Layer ¨ PEGDGE
[0095] Four samples of 30/70 mesh frac sand were treated with Flopam EM 533 by
adding 0.66 g polymer to 20 g sand in a FlackTek Max 100 jar and shaking by
hand for 2
minutes. Then various amounts of a fresh 1% polyethyleneglycol diglycidyl
ether
solution in deionized water were added to the treated sand samples. The
samples were
again shaken by hand for 2 minutes and then placed in an oven at 100 C for 1
hour.
Samples were then removed from the oven and sieved through a 25 mesh screen.
Bed
heights were measured for the four samples by adding 1 g of the sand sample to
10 g of
tap water in a 20 mL glass vial, inverting the vials approximately 10 times to
adequately
wet the sand and allowing the vials to sit undisturbed for about 10 minutes.
Bed heights
were then measured with a digital caliper. Results are listed in Table 4.
28
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
Table 4 PEGDGE Treated Outer Polymer Layer
Sample 1% PEGDGE (g) Bed Height (mm)
15 0.1 9.3
16 0.2 8.8
17 1.0 6.2
18 0 12.7
[0096] Example 8: Covalent Crosslink of Outer Polymer Layer ¨ Glyoxal
[0097] Four samples of 30/70 mesh frac sand were treated with Flopam EM 533 by
adding 0.66 g polymer to 20 g sand in a FlackTek Max 100 jar and shaking by
hand for 2
minutes. A 1% glyoxal solution in ethanol was formulated by adding 0.25 g 40
wt%
glyoxal to a 20 mL glass vial and diluting to 10 g with ethanol. Then varying
amounts of
the 1% glyoxal solution were added to the treated sand samples, and the
samples were
shaken by hand for 2 minutes and placed in the oven at 100 C for 30 minutes.
The sand
samples were removed from the oven and sieved through a 25 mesh screen. For
settled
bed height measurements 1 g of sand was added to 10 g tap water in 20 mL
vials, inverted
about 10 times and given about 10 minutes to settle. Bed height was measured
with a
digital caliper. Results are listed in Table 5.
Table 5 Glyoxal Treated Outer Polymer Layer
Sample 1% glyoxal (g) Bed Height (mm)
19 0.2 3.8
0.5 3.5
21 1.0 2.7
22 2.0 2.7
[0098] Example 9: Cationic/Anionic polymer treatments
[0099] Three samples of 30 g of 30/70 mesh frac sand were treated with Polymer
Ventures HCF-44 in a FlackTek Max 100 jar. The jar was shaken by hand for 2
minutes.
20 Flopam EM 533 was then added to each of the samples. The jars were again
shaken by
hand for 2 minutes. The samples were then dried at 80 C overnight. The sand
samples
were removed from the oven and sieved through a 25 mesh screen. For settled
bed height
measurements 1 g of sand was added to 10 g tap water in 20 mL vials, inverted
about 10
29
CA 02870730 2014-10-16
WO 2013/158308 PCT/US2013/032435
times and given about 10 minutes to settle. Bed height was measured with a
digital
caliper. Results are given in Table 6.
Table 6 Cationic/Anionic polymer treatment
Sample HCF-44 (g) Flopam EM 533 (g) Bed Height (mm)
23 0 0.45 10.26
24 0.07 0.38 8.08
25 1.0 0.35 5.08
26 1.5 0.30 3.94
[00100] Example 10: Sand coated with macromolecular particles
[00101] A 30 g sample of 30/70 mesh frac sand was added to a FlackTek Max 100
jar. To
the sand, 0.3g of paraffin wax was added. The sample was placed in a FlackTek
DAC 150
SpeedMixer and mixed at 2500 rpm for 2 minutes. After mixing, 1 g of
carboxymethyl
cellulose was added to the sample. The sample was again placed in the FlackTek
DAC
150 SpeedMixer and mixed at 2500 rpm for 1 minute. The sand sample was sieved
through a 25 mesh screen. For settled bed height measurements 1 g of sand was
added to
10 g tap water in a 20 mL vial, inverted about 10 times and given about 10
minutes to
settle. The sand in this sample clumped together immediately and did not
disperse in the
water, and an accurate measurement of bed height could not be obtained.
[00102] Example 11: Modified sand beaker testing
[00103] A 30 g sample of 30/70 mesh frac sand was added to a FlackTek Max 100
jar.
The sand was treated with Flopam EM 533 by adding 0.45g of the polymer to the
jar and
shaking by hand for 2 minutes. The sample was then dried at 80 C overnight.
After
drying, the sample was removed from the oven and sieved over a 25 mesh screen.
After
sieving, four samples were prepared by adding 1 g of the treated sand to 10 g
of tap water
in a 20 mL vial. The vials were inverted about 10 times and left to settle for
10 minutes.
A 10% ammonium persulfate solution was made by adding 2 g of ammonium
persulfate
to 18 g of tap water. Varying amounts of the 10% ammonium persulfate solution
were
then added to the sample vials. The samples were inverted several times to
mix, and then
placed in an oven at 80 C for 1 hr. After 1 hour the samples were removed and
the settled
bed heights were observed. Table 7 shows the results.
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
Table 7 Breaker testing
Sample 10% APS (gm) Sand Suspension
27 0 Suspended
28 180 Settled
29 90 Settled
30 18 Settled
[00104] Example 12: Emulsion Additives
[00105] To determine the effect of emulsion additives on self-suspending
proppant
("SSP") performance, we added glycerol and Ethal LA-12/80% to the emulsion
polymer
EM533 before coating the proppant sand. Three different polymer samples were
made as
follows:
= SSP Polymer: lOg of EM533, no additive
= SSP + glycerol: 9g EM533 and lg of glycerol
= SSP + glycerol + Ethal: 9g EM533 + 0.9g glycerol + 0.1g Ethal LA-12/80%.
[00106] Each of the above samples was vortex mixed for 30s to ensure
homogeneity. To
make the modified proppant, 50g of 40/70 sand was combined with 1.5g of one of
the
polymer samples above and then mixed for 30s. The modified proppant samples
were
evaluated for shear stability in the 1 liter shear test. This test involves
addition of 50
grams of modified proppant to 1 liter of water in a square plastic beaker,
followed by
mixing on a paddle/jar mixer (EC Engineering model CLM-4) at 200 rpm for
different
amounts of time. The sheared samples are then poured into a 1000 mL graduated
cylinder and allowed to settle by gravity for 10 minutes, then the bed height
of the settled
proppant sand is recorded. For comparison, an unmodified proppant sand will
produce a
bed height of 10 mm after any amount of mixing. The self-suspending proppant
samples
will produce a higher bed level vs. unmodified proppant due to the hydrogel
layer
encapsulating the sand grains. Generally, increasing the shear rate or time
can cause the
bed height of self-suspending proppant to decrease as a result of desorption
of the
hydrogel layer from the surface of the modified proppant. For this reason, it
is desirable
for the bed height to be as high as possible in this test, especially after
shear. The results
below show that the addition of glycerol improves the bed height and the shear
stability
of the product. The addition of glycerol and Ethal, while it improves the
initial bed height,
the long term shear stability is slightly decreased. These results are
illustrated in FIG. 2.
31
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
[00107] Example 13: Glycerol and Processability
[00108] This experiment sought to determine the effect of glycerol and other
additives on
the performance of self-suspending proppants (denoted as SSP below). 1 kg of
dry 40/70
sand was added to the bowl of a KitchenAid stand mixer, model KSM90WH, which
was
fitted with the paddle attachment. 3.09 g of glycerol was mixed with 27.84 g
of EM533
emulsion polymer, then the mixture was added to the top of the sand and
allowed to soak
in for 1 minute. At time 0 the mixer was started at speed 1 (72 rpm primary
rotation).
Samples were collected at 1-2 minute intervals and dried for 1 hour at 90 C.
Then, each
sample was subjected to the 1 liter shear test, where 50g of SSP was added to
1 L of water
and sheared at 200 rpm (an approximate shear rate of 550 s-1) for 20 minutes.
After
transferring the water/SSP mixture to a 1 liter graduated cylinder and
settling for 10 min,
the bed heights were recorded. The experiment was repeated with 30.93 g EM533
emulsion polymer alone added to 1 kg of sand. These results are shown in FIG.
3.
[00109] As shown in FIG. 3, the glycerol additive increased bed heights
significantly.
[00110] The difference in performance was even more marked when the experiment
was
repeated at higher mixing speeds. Here the mixer was set to speed 4 (150 rpm
primary
rotation). At low mixing times, the samples were insufficiently mixed, leading
to
incomplete coating of the sand and ready desorption of the polymer from the
surface of
the SSP during the shear test. As mixing time of the coating process increased
so did
performance, until an ideal coating was reached, giving maximum bed height for
that
sample. After that, increasingly worse (lower) bed heights were seen at higher
mixing
times, possibly as a result of abrasion of the coating during extended mixing.
At higher
mixing speeds, this process happened even faster, such that the processing
window for the
emulsion polymer alone was less than 1 minute. With the addition of glycerol
and the use
of a lower mixing speed, this processing window was widened to nearly 15
minutes. In
comparison to the tests with emulsion polymer alone, glycerol caused the
processing
window to widen, indicating that SSP preparation with the glycerol is more
robust. At
the same time, glycerol allowed the polymer emulsion to invert more fully,
leading to
better coatings and increased bed heights. Testing with combinations of
glycerol and the
emulsion polymer EM533 at a higher mixing speed yielded the results shown in
FIG. 4.
[00111] Example 14: Modified proppant with an anticaking agent
[00112] Modified proppant samples were made with and without anticaking agent
for a
comparison. For Sample A, 50g of 40/70 sand was added to a FlackTek jar. 1.5g
of
32
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
EM533 emulsion polymer was added to the sand and the sample was mixed for 30
seconds. After mixing, 0.25g of calcium silicate was added to the sample and
the sample
was mixed again for 30 seconds. The sample was then dried for 1 hour at 85 C.
After
drying, the sample was poured over a 25 mesh screen and shaken lightly for 30
seconds.
The amount of sand that passed through the sieve was then measured. For Sample
B, 50g
of 40/70 sand was added to a FlackTek jar. 1.5g of EM533 emulsion polymer was
added
to the sand and the sample was mixed for 30 seconds. The sample was then dried
for 1
hour at 85 C. After drying, the sample was poured over a 25 mesh screen and
shaken
lightly for 30 seconds. The amount of sand that passed through the sieve was
then
measured. Table 8 shows the results.
Table 8
Total Mass Mass passing % Passing
Sample
Sample, g Sieve, g Sieve
Sample A 50.5 50.16 99.3%
Sample B 50.5 15.71 31.1%
[00113] The results of sieve testing show that the incorporation of an
anticaking agent
was effective at improving the handling properties of the modified proppants.
[00114] Samples A and B were separately added to 1L of water and then sheared
in the
EC Engineering Mixer for 20 minutes at 200 rpm. After shearing, the samples
were
transferred to a 1 L graduated cylinder and left to settle for 10 minutes.
After settling, the
bed heights were measured, showing no significant loss in shear stability as a
result of
incorporating an anticaking agent. Table 9 shows these results.
Table 9
Sample Bed Height, mm
Sample A 56.21
Sample B 59.67
[00115] Example 15: Hydrogel coating of sand by dissolving a water-soluble
polymer in
a monomer formulation followed by polymerization of the monomers
[00116] 2.5 g of a mixture of acrylic acid (Aldrich 147230), poly(ethylene
glycol) methyl
ether acrylate (Aldrich 454990), and polyethylene glycol dimethacrylate
(Aldrich
437441) in a mol ratio: 0.5/0.4/0.1 can be mixed with 7.5 g of polyethylene
glycol
33
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
(Aldrich 202371) and 1 wt% of ammonium persulfate. The solution can be mixed
with
100 g of 30/70 mesh sand under nitrogen and can be allowed to react by
increasing the
temperature to 70 C for 5 hours. Next the solids obtained are washed with
methanol,
vacuum filtered and dried in an oven at 80 C.
[00117] Example 16: Polyurethane hydrogel coating of sand
[00118] 100 g of 30/70 mesh frac sand can be added to a Hobart type mixer and
heated to
120 C. Next 6 g of a polyethyleneglycol (Fluka 81190) will be added and
allowed to mix
for 1 minute. Then 0.53 g of Desmodur N75 from Bayer will be added. After
mixing for 1
more minute, one drop of catalyst 1,4-Diazabicyclo[2.2.2]octane (Aldrich
D27802) will
be added and the mixture will be allowed to react for 5 more minutes. The
obtained solid
is washed with methanol, vacuum filtered and dried in an oven at 80 C.
[00119] Example 17: Hydrogel coating of sand by admicellar polymerization
[00120] 250 g of 30/70 frac sand can be added to 500 ml of a previously
degassed
aqueous solution containing 0.6 mM hexadecyltrimethylammonium bromide (CTAB)
surfactant (equivalent to 2/3 of the critical micelle concentration of CTAB),
and 6 mM
monomer (mixture of acrylic acid/acrylamide in a mol ratio 30/70). Adsorption
of the
CTAB and monomer onto the sand particle can be carried out under gentle
stirring for 24
h at 25 C. Then, 0.6 mM Ammonium persulfate can be added to the reactor and
the
polymerization will take place for 3 h at 80 C. Excess polymer and surfactant
can be
rinsed with several volumes of water and the sample will be dried overnight in
the
vacuum oven at 80 C.
[00121] Example 18: Hydrogel coating of sand by inverse suspension
polymerization
[00122] To a flask can be added 60 ml of DI-water, 6.6 g acrylamide, 3 g of
acrylic acid,
2.4 g of N,N'- methylenebisacrylamide, 0.1 g ammonium persulfate, 2.0 g sodium
chloride and 2 drops of N, N, N', N'-tetramethylethylenediamine. To this
solution can be
added 200 g of 30/70 mesh frac sand and the whole mixture will be kept at
temperature
<10 C. To the mixture can be added 200 ml of cyclohexane and the whole mixture
can
be vigorously stirred under nitrogen. Next the temperature can be increased to
60 C and
the reaction allowed to proceed for 6 hours. The resultant coated particles
can be filtered
and washed with hot water, acetone and dried at 45 C under reduced pressure.
[00123] Example 19: Coating Polymer
[00124] A mixture for coating the proppant was made by mixing SNF Flopam EM
533
and glycerol in a 9:1 ratio. This polymer mixture is used in following
examples.
34
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
[00125] Example 20: Preparation of 40/70 mesh self-suspending proppant ("SSP")
[00126] A sample of 40/70 mesh size SSP was prepared by adding 500 g of 40/70
frac
sand into the bowl of a KitchenAid mixer. 20 g of the coating polymer of
Example 19
was added to the sand. The mixer was turned on at a setting of 1 and the sand
and
polymer mixture mixed for 7 minutes. After mixing, the sample was dried for 1
hour at
85 C. After 1 hour, the sample was removed from the oven and any lumps were
broken
into individual grains.
[00127] Example 21: Preparation of 30/50 mesh self-suspending proppant ("SSP")
[00128] A sample of 30/50 mesh size SSP was prepared by adding 500 g of 30/50
frac
sand into the bowl of a KitchenAid mixer. 20g of the coating polymer of
Example 19 was
added to the sand. The mixer was turned on at a setting of 1 and the sand and
polymer
mixture mixed for 7 minutes. After mixing, the sample was dried for 1 hour at
85 C.
After 1 hour, the sample was removed from the oven and any lumps were broken
into
individual grains.
[00129] Example 22: Reduced fines content of self-suspending proppant ("SSP")
vs. sand
[00130] A stack of standard mesh sieves was prepared with 40 mesh on top, 70
mesh in
the middle, and a pan at the bottom. The tare weight of each clean/dry sieve
was
measured and recorded. 50 g of the 40/70 mesh SSP of Example 20 was added to
the top
of the sieve stack, and the stack was shaken by hand for five minutes. After
shaking, the
stack was disassembled and each sieve was weighed. The mass retained on each
sieve
was calculated as a percent of the original sample mass, and the amount of the
sample
remaining in the pan represents the fines fraction, as defined by a 70 mesh
cutoff. The
procedure was repeated, substituting unmodified 40/70 mesh frac sand for the
40/70 SSP.
The results in Table 10 show the particle size distribution for 40/70 SSP.
Table 11
contains the particle size analysis for unmodified 40/70 frac sand. The
results show that
the amount of material passing the 70 mesh screen is reduced in 40/70 SSP
(1.2% vs.
4.8%). This shows that SSP can contain a reduced amount of fine particulates
than a sand
sample.
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
Table 10: Particle size analysis of 40/70 SSP
Sample: 49.516 g of 40/70 SSP
Mesh Tare, g Final Mass, g Mass Sand
Retained, g Size Distribution
40 118.826 127.685 8.859 17.9%
70 111.136 151.036 39.9 80.6%
Pan 81.501 82.072 0.571 1.2%
Total 49.33 99.6%
Table 11: Particle size analysis of unmodified 40/70 white sand
Sample: 50.974 g of 40/70 White sand
Mesh Tare, g
Final Mass, g Mass Sand Retained, g Size Distribution
40 118.806 118.921 0.115 0.2%
70 111.045 159.465 48.420 95.0%
Pan 81.503 83.935 2.432 4.8%
Total 50.967 100.0%
[00131] Example 23: Friction Reduction
[00132] 1 L of tap water was added to a square beaker and the beaker was
placed in an
EC Engineering CLM-4 Mixer. The mixer was turned on and set to 200 rpm mixing
speed. 120 g of the 30/50 SSP of Example 21 was added to the tap water. The
slurry
mixed for 20 minutes, then was transferred to a 1 L graduated cylinder and
left to settle
for 10 minutes. After settling, the supernatant was collected. This procedure
was repeated
until 2 L of supernatant fluid was collected. The friction reduction of the
collected fluid
was then determined using a flow loop apparatus. The flow loop consists of a
0.12 in (ID)
by 3 ft stainless steel test pipe and a pump that delivers a constant flowrate
of 55 gph.
These conditions correspond to a Reynolds number of 23,000, confirming that
the fluid is
in turbulent flow. The percent friction reduction (%FR) is determined
experimentally by
measuring the pressures at the entrance and the exit of the test pipe at a
constant flow rate.
The following equation to calculate the percent friction reduction: %FR =
100*(1-
(41314Po)), where here AP, is the pressure drop across the test pipe using the
SSP
supernatant fluid and AP is the pressure drop across the test pipe using tap
water. The
pressure values were AP, = 11.8 psi and AP = 38.5 psi, corresponding to a
friction
36
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
reduction (%FR) of 69%. This shows that the SSP contributes significantly to
friction
reduction of the associated fluid, representing a reduction in the pumping
requirements.
[00133] Example 24: Hydraulic conductivity tests
[00134] To model hydraulic conductivity of a simulated proppant pack, 48 g of
30/50
mesh size SSP of Example 21 were mixed into 1 liter of water. Ammonium
persulfate,
was added at 0.1% level and the mixture was heated to 185 F for 1 hour. After
cooling to
room temperature, the mixture was filtered through a 2.25 inch ID column with
a 100
mesh sieve at the bottom, separating the particles from the fluid. The
particles formed a
bed depth of 0.5 inch on the 100 mesh screen, and the flowrates of various
fluids through
the bed were measured by gravity flow. A plain sand bed was constructed in a
similar
manner and the flowrates compared with the SSP derived bed. Using this method,
the
flowrates obtained by SSP (250 mL efflux in 28 seconds) and plain sand (250 mL
efflux
in 25 seconds) were nearly identical, showing that SSP, once treated with
oxidative
breakers such as ammonium persulfate, has no deleterious effect on hydraulic
conductivity of a sand bed or a simulated proppant pack.
[00135] Example 25: Self-suspending proppants ("SSP") with anticaking agents
[00136] In addition to anticaking agents being able to replace a drying step,
they can be
used to generally improve handling qualities for SSP. A number of different
particulate
materials were tested as anticaking agents, as set forth in Table 12 below. To
prepare the
samples for each material, 800 g of 30/50 mesh sand was mixed in a KitchenAid
mixer at
speed 1 (144 rpm) with 32 g of coating polymer of Example 19. 20 g samples
were taken
and blended with a selected anticaking agent in a mixer, with anticaking agent
doses
calculated as a percent of the total sand in the sample. The consistency of
the samples
was observed and recorded as "Appearance before drying," then they were dried
for 1
hour at 85 C. Their consistency was again observed and recorded as "Appearance
after
drying." Samples were then subjected to conditions of 80%-90% relative
humidity at 25-
C for one hour to assess their anticaking properties, and consistency was
observed and
recorded as "Appearance after humidity exposure." Results are shown in Table
12 below,
indicating that the anticaking agent improves the handling properties of the
SSP, where
30 free-flowing is a desirable feature.
37
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
Table 12: Evaluation of SSP samples with added anticaking agents
Appearance
Anticaking Anticaking Appearance Appearance
after humidity
agent dose agent before drying after drying
exposure
0 None Wet Clumpy Sticky
(control)
0.1% Fumed Silica Wet Slightly clumpy
Free-flowing
0.2% Fumed Silica Slightly clumpy Free-flowing
Free-flowing
0.5% Fumed Silica Free-flowing Free-flowing Free-flowing
0.5% Calcium Free-flowing Free-flowing Free-flowing
Silicate
1% Corn Starch Wet Clumpy Clumpy
1% Sodium Wet Clumpy Slightly clumpy
Stearate
0.5% Kaolin Slightly clumpy Free-flowing
Free-flowing
0.5% Bentonite Slightly clumpy Free-flowing
Free-flowing
0.2% Attapulgite Slightly clumpy Free-flowing
Free-flowing
[00137] Example 26: Treating Resin Coated Sand with SMA 4000i
[00138] Resin coated sand was coated with SMA 4000i by adding 25 g of resin
coated
sand into a 250 mL round bottom flask. Separately, 0.25 g of SMA 4000i was
dissolved
in 3.57 g of tetrahydrofuran (THF) to make a 7% solution. 1.43 g of the THF
solution was
then added to the resin coated sand in the round bottom flask. Additional THF
was added
to the round bottom flask until the sand was coved. The THF was then
evaporated off of
the sample using a rotary evaporator.
[00139] Example 27: Treating Resin Coated Sand with SMA 4000i
[00140] Resin coated sand was coated with SMA 2000i by adding 25 g of resin
coated
sand into a 250 mL round bottom flask. Separately, 0.25 g of SMA 2000i was
dissolved
in 3.57 g of THF to make a 7% solution. 0.72 g of the THF solution was then
added to the
resin coated sand in the round bottom flask. Additional THF was added to the
round
bottom flask until the sand was coved. The THF was then evaporated off of the
sample
using a rotary evaporator.
[00141] Example 28: Treating Resin Coated Sand with SMA 2000i
[00142] Resin coated sand was coated with SMA 2000i by adding 25 g of resin
coated
sand into a 250 mL round bottom flask. Separately, 0.25 g of SMA 4000i was
dissolved
in 3.57 g of THF to make a 7% solution. 1.43 g of the THF solution was then
added to the
38
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
resin coated sand in the round bottom flask. Additional THF was added to the
round
bottom flask until the sand was coved. The THF was then evaporated off of the
sample
using a rotary evaporator.
[00143] Example 29: Treating Resin Coated Sand with SMA 2000i
[00144] Resin coated sand was coated with SMA 2000i by adding 25 g of resin
coated
sand into a 250 mL round bottom flask. Separately, 0.25 g of SMA 2000i was
dissolved
in 3.57 g of THF to make a 7% solution. 0.72 g of the THF solution was then
added to the
resin coated sand in the round bottom flask. Additional THF was added to the
round
bottom flask until the sand was coved. The THF was then evaporated off of the
sample
using a rotary evaporator.
[00145] Example 30: Treating Resin Coated Sand with Pluronic L31
[00146] Resin coated sand was coated with Pluronic Surfactant L31 by adding 20
g of
resin coated sand into a small FlackTek jar. 0.025 g of the surfactant was
added to the
resin coated sand. The sample was then mixed using a FlackTek Speedmixer at
800 rpm
for 30 seconds.
[00147] Example 31: Treating Resin Coated Sand with Pluronic L35
[00148] Resin coated sand was coated with Pluronic Surfactant L35 by adding 20
g of
resin coated sand into a small FlackTek jar. 0.025 g of the surfactant was
added to the
resin coated sand. The sample was then mixed using a FlackTek Speedmixer at
800 rpm
for 30 seconds.
[00149] Example 32: Treating Resin Coated Sand with Pluronic L81
[00150] Resin coated sand was coated with Pluronic Surfactant L35 by adding 20
g of
resin coated sand into a small FlackTek jar. 0.025 g of the surfactant was
added to the
resin coated sand. The sample was then mixed using a FlackTek Speedmixer at
800 rpm
for 30 seconds.
[00151] Example 33: Treating Resin Coated Sand with ARQUADO 2HT-75
[00152] Resin coated sand was coated with ARQUADO 2HT-75 by adding 25 g of
resin
coated sand into a 250 mL round bottom flask. Separately, 0.25 g of ARQUADO
2HT-75
was dissolved in 3.57 g of IPA to make a 7% solution. 0.72 g of the IPA
solution was
then added to the resin coated sand in the round bottom flask. Additional IPA
was added
to the round bottom flask until the sand was coved. The IPA was then
evaporated off of
the sample using a rotary evaporator.
39
CA 02870730 2014-10-16
WO 2013/158308 PCT/US2013/032435
[00153] Example 34: Treating Resin Coated Sand with ADOGENO 464
[00154] Resin coated sand was coated with ADOGENO 464 by adding 20 g of resin
coated sand into a small FlackTek jar. 0.025 g of the ADOGENO 464 was added to
the
resin coated sand. The sample was then mixed using a FlackTek Speedmixer at
800 rpm
for 30 seconds.
[00155] Example 35: Coating Polymer Mixture
[00156] 9 g of Flopam EM 533 (SNF) was combined with 1 g of glycerol in a 20
mL vial.
The vial was then mixed for 30 seconds on a vortex mixer.
[00157] Example 36: Hydrogel Coating of Sand Samples
[00158] Sand samples were prepared by placing 20 g of the samples prepared in
Example
26 through Example 34 was added to small FlackTek jars. 0.6 g of the coating
mixture
prepared in Example 35 was added into each the jar. The contents were then
mixed at 800
rpm for 1 minute using a FlackTek speed mixer. The samples were then dried for
30
minutes at 100 C. After drying, 1 g of each sample was added to a 20 mL vial
containing
10 mL of tap water. The vials were mixed gently then left to settle for 10
minutes. After
settling, the bed height was measured to determine polymer hydration. The
results of the
testing are shown in Table 13.
Table 13: Settled bed heights
ExampleAmount on Resin
Additive Bed Height
Number Coated Sand
26 SMA 4000i 4000 ppm 10 mm
27 SMA 4000i 2000 ppm 8 mm
28 SMA 2000i 4000 ppm 8 mm
29 SMA 2000i 2000 ppm 4 mm
Pluronic
30 1250 ppm 8 mm
Surfactant L31
Pluronic
31 1250 ppm 7 mm
Surfactant L35
Pluronic
32 1250 ppm 10 mm
Surfactant L81
ARQUADO
33
2HT-75 2000 ppm 3 mm
ADOGENO
34
464 1250 ppm 2 mm
40
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
[00159] Example 37: Humidity Aging Test (Metal Chelate Crosslinkers)
[00160] Tyzor TE is a triethanolamine titanium chelate 80% solution in
ethanol. Tyzor
TEAZ is a 100% actives triethanolamine zirconium chelate product. These metal
chelates
were dispersed in castor oil at different concentrations and applied to
proppant in a
second addition step during the coating process. Samples of coated proppant
were
prepared by adding 3 g of a blend of Flopam EM533 and glycerol to 100 g of
30/50 mesh
proppant white sand in a FlackTek Max 100 jar. The samples were then mixed in
a
FlackTek Speedmixer at 850 rpm for 30 seconds. Samples were then removed from
the
Speedmixer and in some cases treated with a metal chelate/castor oil blend.
Samples
were then returned to the Speedmixer and mixed at 850 rpm for 30 seconds.
Samples
were then removed from the Speedmixer, transferred to a watch glass, and dried
at 100 C
for 30 minutes in a forced air laboratory oven. After drying, samples were
sieved through
an 18 mesh screen. For humidity aging, 50 g of the formulated samples were
placed in
Max 100 FlackTek jars and left sitting in a humidity chamber for 1 hour. The
relative
humidity of the chamber was kept between 60-70%. After humidification, samples
were
tested in a Carver Press cell (2.25" I.D.) with an applied load of 1,000 lbs
for 30 seconds.
Caking of the samples was visually assessed and compared to the control (no
second
addition). The extent to which samples caked was scored from 1 to 4 with a
score of "1"
indicating a solid cake and a score of "4" indicating a free-flowing, non-
caking material.
Results are shown in Table 14.
Table 14 Caking Results with Metal Chelate Add
Sample Metal chelate Metal Chelate Caking Score
Conc. (ppm)
1 None 0 1
2 Tyzor TE 600 3
3 Tyzor TE 1500 3
4 Tyzor TEAZ 600 2
5 Tyzor TEAZ 900 3
[00161] (Caking Scores for Table 14: "1"-Solid cake that can be handled
without falling
apart, "2"-Mostly solid cake that begins to break as handled, "3"-Cake is
crumbly out of
press cell, "4"-No cake formation).
41
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
[00162] Example 38: Humidity Aging Test (Powder Additives)
[00163] Samples of coated proppant sand were formulated by adding 3 g of a
Flopam
EM533/glycerol blend to 100 g of 30/50 mesh proppant white sand. The samples
were
mixed at 850 rpm for 30 seconds in a FlackTek Speedmixer. Samples were then
removed
from the Speedmixer and in some cases treated with a dry powder. Samples were
then
returned to the Speedmixer and mixed at 850 rpm for 30 seconds to uniformly
distribute
the powder through the sample. Samples were then removed from the Speedmixer,
transferred to a watch glass, and dried at 100 C for 30 minutes in a forced
air laboratory
oven. After drying, samples were sieved through an 18 mesh screen. For
humidity aging
about 50 g of the formulated samples were placed in Max 100 FlackTek jars and
left
sitting in a humidity chamber for 1 hour. The relative humidity of the chamber
was kept
between 60-70%. After humidification, samples were tested in a Carver Press
cell (2.25"
I.D.) with an applied load of 1,000 lbs for 30 seconds. Caking of the samples
was
visually assessed and compared to the control (no second addition). The extent
to which
samples caked was scored from 1 to 4 with a score of "1" indicating a solid
cake and a
score of "4" indicating a free-flowing, non-caking material, as shown in Table
15.
Table 15 Caking Results of Coated Proppant with Powder Additive
Sample Powder Powder Melting Powder Conc. Caking Score
Point ( C) (wt%)
6 None N/A 0.0% 1
7 Thixcin-R 85 0.5% 2
8 Stearic Acid 70 0.6% 3
[00164] (Caking Scores for Table 15: "1"-Solid cake that can be handled
without falling
apart, "2"-Mostly solid cake that begins to break as handled, "3"-Cake is
crumbly out of
press cell, "4"-No cake formation).
[00165] Example 39: Oil-based Additives
[00166] Several oil-based or relatively hydrophobic materials were tested to
determine
their efficacy in decreasing caking in humidified samples of self-suspending
proppant
(SSP). Samples were prepared by mixing 300 g of 30/50 sand, preheated to 45
C, with 9
g of a 10% glycerol/90% Flopam 533 mixture in a KitchenAid mixer at speed 1.
After 1
minute of mixing, the second additive (usually at 0.2% by wt sand) was
introduced and
the mixture was mixed for another minute. The sample was dried under medium
shear
42
CA 02870730 2014-10-16
WO 2013/158308 PCT/US2013/032435
conditions using a heat gun and KitchenAid. The samples were then subjected to
>50%
RH in a humidity chamber for 1 hour. They were then individually tested for
caking
behavior by undergoing the compression test. This consisted of being
compressed at 200
PSI for 30 seconds in a compression cell using a Carver Press, then removed
from the cell
and observed. The resulting cake (See Table 16, Compression Test) was graded
on the
following scale: "1"-Solid cake that can be handled without falling apart, "2"-
Mostly
solid cake that begins to break as handled, "3"-Cake is crumbly out of press
cell, "4"-No
cake formation, as set forth in Table 16.
Table 16 Anti-caking Results of Coated Proppant with Oil Based Additives
rd Addition: 2nd Addition: Compression
Chemical Identity Amount (% of total sand weight) Test
Control - 1
Castor Oil 0.2% 1
Triacetin (mixed into 0.3% 1
polymer)
Grapeseed Oil 0.2% 1
50% Corn Oil mixed with 0.2% 1
50% D400 Jeffamine
Adogen 464 0.2% 3
50% Adogen 464 mixed 0.2% 2
with 50% Castor Oil
10% Adogen 464 mixed 0.2% 1
with 90% Castor Oil
Petroleum Jelly 0.2% 1
Mineral Oil (high 0.2% 1
molecular weight)
Corn Oil 0.2% 1
Dimethyl, phenylmethyl 0.2% 1
siloxane, trimethyl
terminated
Aminopropyl terminated 0.2% 3
poly(dimethyl siloxane)
50% Adogen mixed with 0.2% 2
50% Corn Oil
Arquad 2HT-75 0.2% 3
Adogen 464 0.1% 3
[00167] The sample treated with Adogen 464 barely formed a cake in this test,
even at
lower doses.
43
CA 02870730 2014-10-16
WO 2013/158308
PCT/US2013/032435
EQUIVALENTS
[00168] While specific embodiments of the subject invention have been
disclosed herein,
the above specification is illustrative and not restrictive. While this
invention has been
particularly shown and described with references to preferred embodiments
thereof, it
will be understood by those skilled in the art that various changes in form
and details may
be made therein without departing from the scope of the invention encompassed
by the
appended claims. Many variations of the invention will become apparent to
those of
skilled art upon review of this specification. Unless otherwise indicated, all
numbers
expressing reaction conditions, quantities of ingredients, and so forth, as
used in this
specification and the claims are to be understood as being modified in all
instances by the
term "about." Accordingly, unless indicated to the contrary, the numerical
parameters set
forth herein are approximations that can vary depending upon the desired
properties
sought to be obtained by the present invention.
[00169] While this invention has been particularly shown and described with
references
to preferred embodiments thereof, it will be understood by those skilled in
the art that
various changes in form and details may be made therein without departing from
the
scope of the invention encompassed by the appended claims.
44