Note: Descriptions are shown in the official language in which they were submitted.
- 1 -
SYSTEM AND PROCESS FOR CONVERTING PLASTICS TO PETROLEUM
PRODUCTS
FIELD OF THE INVENTION
The invention relates to a system and process for converting plastics and
other heavy
hydrocarbon solids into retail petroleum products by subjecting the plastics
to melting, pyrolysis,
vapourization, and selective condensation, whereby final in-spec petroleum
products are
produced. The system and process is energy efficient, as fuels generated
during the process are
recycled for use upstream in the process.
BACKGROUND
Plastic materials represent a valuable source of petroleum-based fuels.
Plastics are comprised of
hydrocarbons which, when broken down into their substituent compounds, can be
used as diesel
fuel, gasoline, furnace oil, kerosene, or lower carbon-chain fuels such as
methane, butane and
propane. The recycling of plastic materials to generate fuel is important for
reducing the
dependency on obtaining petroleum using costly and environmentally hazardous
drilling and
extraction means.
Methods of processing plastics into petroleum fuels are known. These are
described in, for
example, US Patent No. 4,851,601; US Patent No. 5,414,169; US Patent No.
5,608,136; US
Patent No. 5,856,599; US Patent No. 6,172,271; US Patent No. 6,866,830; US
Patent No.
7,511,703; US Patent Publication 2009/0062581; US Patent Publication
2010/0018116; Chinese
patent publication CN 101050373; Chinese patent publication CN 1824733;
Japanese patent
publication 07331251; Japanese patent publication 09316459; Japanese patent
publication
11138125; Japanese patent publication 2003301184; Japanese patent publication
2009209278;
Japanese patent publication 2010059329; and PCT
publication
WO 2000/064997. Typical methods use external sources of fuel to melt and
pyrolize plastics into
the substituent compounds. The materials are often separated using
distillation columns and
other means. Generally, previous methods have been inefficient at generating
higher quantity and
quality on-specification petroleum products. They have
often required
CA 2879973 2018-03-01
- 2 -
higher temperatures to effectively crack the hydrocarbon substituents which,
counterproductively, requires more energy input than is generated.
There remains a need, therefore, for a system and method for producing oil
from petroleum-
based products, such as plastics.
This background information is provided for the purpose of making known
information believed
by the applicant to be of possible relevance to the present invention. No
admission is necessarily
intended, nor should be construed, that any of the preceding information
constitutes prior art
against the present invention.
SUMMARY
.. The present system and process attempt to address the problems encountered
with previous
systems and processes for converting plastics to petroleum products.
The present invention provides a system and process for converting plastics
into industrial
quality petroleum fuels. In accordance with one aspect of the present
invention there is provided
a system for processing plastics into one or more petroleum products, the
system comprising: a)
.. a reactor for subjecting the plastics to pyrolysis and cracking
hydrocarbons in the plastics to
produce a plastics vapour comprising hydrocarbon substituents; b) one or more
separation
vessels for separating the plastics vapour into hydrocarbon substitutents
based on boiling points
of the hydrocarbon substituents; c) one or more condensers for condensing the
hydrocarbon
substituents into one or more petroleum products; and d) means for collecting
the one or more
petroleum products.
In accordance with another aspect of the present invention there is provided a
process for
processing plastics into one or more petroleum products, the process
comprising: providing
plastics to a pyrolysis reactor; subjecting the plastics to pyrolysis and
cracking to produce a
plastics vapour, plastics liquids and plastics solids comprising hydrocarbon
substituents;
separating the plastics vapour in a separation vessel to form a first liquid
petroleum product from
a gaseous petroleum product; and condensing the gaseous petroleum product into
a second liquid
petroleum product.
CA 2879973 2018-03-01
- 3 -
Advantageously, the system and process of the present invention provides a
closed loop, which
allows the generation not only of on-specification petroleum products, but
also petroleum fuels
for use within the system and process itself. Further, less input fuel is
required, providing
environmental and cost benefits.
BRIEF DESCRIPTION OF THE FIGURES
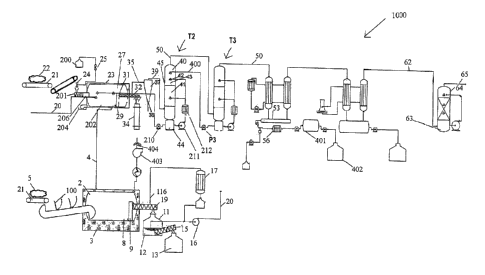
Figure 1 shows an overview of a system in accordance with the present
invention.
Figure 2 shows a premelt system for use in a system and process according to
the present
invention.
Figure 3 shows a pyrolysis reactor for use in a system and process according
to the present
invention.
Figure 4 shows a catalyst tower for use in a system and process according to
the present
invention.
DETAILED DESCRIPTION
In accordance with one aspect of the present invention, there is provided a
system for processing
plastics into one or more petroleum products, the system comprising: a) a
reactor for subjecting
the plastics to pyrolysis and cracking hydrocarbons in the plastics to produce
a plastics vapour
comprising hydrocarbon substituents; b) one or more separation vessels for
separating the
plastics vapour into hydrocarbon substitutents based on boiling points of the
hydrocarbon
substituents; c) one or more condensers for condensing the hydrocarbon
substituents into one or
more petroleum products; and d) means for collecting the one or more petroleum
products.
The present invention also provides a process for processing plastics into one
or more petroleum
products, the process comprising: providing plastics to a pyrolysis reactor;
subjecting the plastics
to pyrolysis and cracking to produce a plastics vapour, plastics liquids and
plastics solids
comprising hydrocarbon substituents; separating the plastics vapour in a
separation vessel to
form a first liquid petroleum product from a gaseous petroleum product; and
condensing the
gaseous petroleum product into a second liquid petroleum product.
CA 2879973 2018-03-01
- 4 -
The entire process is typically performed at atmospheric pressure.
As used herein, "plastics" refers generally to synthetic or semi-synthetic
plastic-based materials,
such as those comprising polymers of high molecular mass, which are derived
primarily from
petroleum and natural gas. Examples include high density polyethylene (HDPE),
low density
polyethylene (LDPE), polypropylene (PP), polyethylene terephthalate (PET),
polystyrene (PS),
polyvinylchloride (PVC), polyurethanes, cellulose- based plastics, and the
like.
As used herein, "petroleum" refers generally to hydrocarbon-based flammable
liquids that are
used as fuels, such as, for example, diesel fuel, naphtha, gasoline, kerosene,
methane, ethane,
propane, butane, and the like.
An overview of a system according to the present invention is generally shown
in Figure 1. The
system comprises components for treating and converting waste plastics to
petroleum products.
It is understood that modifications within the system - such as, for example,
the dimensions of
the individual components, number of components within the system, or types of
materials used
for the components - may be contemplated within the scope of invention.
As shown in the embodiment of Figure 1, the system comprises one or more
conveyor belts 21
for introducing plastics 22 into the system. The plastics feedstock which is
added to the system
can come from any number of sources, such as directly from sanitation trucks
used for collecting
waste plastics from residential or industrial locations. The plastics can
include plastic containers
(such as beverage and food containers), plastic scrap, grocery bags, and the
like, and of different
sizes and shapes. Soft and hard plastics can similarly be processed. Plastics
feedstock containing
contaminants, such as metals, halogenated hydrocarbons and other undesirable
materials, may
also be processed, but larger contaminating items are preferably removed by
hand from the
conveyor belt. The plastics are placed onto the conveyor belt in loose or
packaged baled form,
and can be added directly from a receptacle or into a hopper 200 to facilitate
the process. The
conveyor belts 21 are sufficiently long and wide to accept plastics of a wide
range of sizes. The
one or more conveyor belts 21 lead to a feed 24 which guides the plastics to
feeder 201.
Alternatively, the feed can include a shredder 25 for reducing the plastics
into smaller material.
CA 2879973 2018-03-01
- 5 -
Figure 2 illustrates a pre-melt reactor. Before undergoing pyrolysis, the
plastics 5 can be
transported through a conveyor 100 into a pre-melt reactor 2 (hereinafter
"premelter"). The
premelter 2 is part of a pre-melt system generally shown in Figure 2. The
premelter 2 melts the
plastics feedstock 5 to provide a liquid material to facilitate extraction of
petroleum therefrom. It
can also be used to separate contaminants from the plastics.
The premelter 2 is housed within a heating chamber 3. The premelter 2 is
heated to about 250 C
to 340 C to melt the plastics therein and boil off any undesired contaminants.
Desirably, the heat
for the heating chamber 3 can be provided from hot air that has been used to
heat the reactor (see
below) via a flue gas pipe 4 connected to the heating chamber 3.
The premelter 2 is typically a rotary kiln. At the end of the screw feeder 100
a rotary seal can be
provided attach the screw feeder 100 to the premelter 2. In one embodiment,
the premelter 2 has
a diameter of about 7 feet and a length of about 18-20 feet. However, the
premelter 2 can be
much larger depending on the application and the need for higher throughput,
such as having a
diameter of about 8 feet and length of about 60 feet, for example. Within the
premelter 2, the
plastic feedstock 5 is liquefied to produce liquefied plastics, non-aqueous
vapours (which can
include halides, if present in the feedstock), water vapour, and contaminant
solids, such as
metals. Optionally, a lifter 9 is present in the premelter 2 to shuttle molten
plastic and residue
from the bottom 8 of the premelter 2 for removal thereform. An outlet screw
19, which can be
similar to the screw feeder 100 at the entry to the premelter 2, transports
molten plastic from the
premelter 2. The outlet screw 19 is within a larger pipe sleeve in such a way
that waste solids,
unmelted plastics, and other solid and liquid residue settle to the bottom of
the outlet screw 19,
while residue vapour which is collected in the pipe sleeve 116 and sent to a
residue condenser
17. Condensed vapour and residue are collected from the residue condenser 17.
A residue
removal tank 11 is positioned below the outlet screw 19 to collect the residue
12. Solid residue is
collected in residue barrel 13 via a residue screw 15 and may contain acids or
other vendible
products, or can be discarded. Liquid residue is removed from the residue
removal tank 11 via a
liquid plastics pump 16, and sent to the pyrolysis reactor.
Figure 3 illustrates one embodiment of a pyrolysis reactor (hereinafter
"reactor") in accordance
with the present invention. Liquid plastics from the pre-melt system (if used)
are pumped to the
CA 2879973 2018-03-01
- 6 -
reactor 27 via pipe 20. The liquid plastics are essentially free of halogens,
water and most
contaminants. If no pre-melt system is used, a conveyor 21 similar to
described above can be
used to transport solid (shredded) plastics to a feeder 201 and into reactor
27.
The feeder 201 can be any transport mechanism for shuttling the plastics, but
in one embodiment
is a channel comprising a screw-type feeder. The feeder 201 transports the
nitrogen-laden
plastics into the reactor 27. The feeder can have a hollow- flight screw.
Coolant, such as water, is
sent through the screw as well as through an air jacket around the feeder 201.
The purpose of
both of these is to maintain the plastic feed at a temperature below its
melting point so that it can
be transported into the reactor as a solid, thus reducing gumming residue.
A nitrogen source can be connected to the feeder 201 for supplying nitrogen to
a sealed
intermediate space 206 between the feeder and the reactor. The nitrogen is
used to displace
oxygen so as to minimize the amount of oxygen entering the process, thus
reducing the yield of
undesirable CO2 end product. A slide gate 207 at the entry of the intermediate
space 206 opens
and allows the plastics to enter therein. At the same time, the nitrogen
source supplies nitrogen
into the intermediate space 206. The plastics becomes packed in the
intermediate space 206 and
filed with nitrogen. Once the plastics have been exposed and saturated with
the nitrogen, a door
209 on the opposite end of the intermediate space 206 opens, and the
nitrogenated plastics exit
the intermediate space and enter the reactor.
The reactor 27 is a vessel, ideally large enough to handle large quantities of
plastics, such as
about 2000-5000 lbs of raw plastics, for efficient flowthrough of the
feedstock through to
processing. Ideally, the reactor 27 can have a length of about 22 feet to
about 100 feet, typically
22 feet to about 40 feet, more typically 18 feet. Longer reactors may be
desired to increase the
interior volume, throughput and efficiency. The diameter of the reactor 27 is
typically between
about 3-10 feet, or about 6 feet.
.. The feed rate to the reactor can be controlled to maintain an efficient use
of heat in the reactor
27, the rate of fuel being produced, temperature and pressure of gasses in
reactor 27, and
temperature of gasses downstream, for example. As one example, the feed rate
can be controlled
such that material is fed into the reactor until the reactor cools to below a
target temperature, or
CA 2879973 2018-03-01
- 7 -
within a target temperature range, such as at or below 360 C. A thermocouple
(not shown) can
be added to a side of the reactor 27 to indicate the amount of liquid in the
reactor; with more
liquid present, the temperature is generally lower, and the addition of
plastics to the reactor can
be reduced. The addition or reduction of plastics can be controlled through
manual or automatic
means, such as through a computer- based algorithm or the like.
The reactor 27 can be made of any suitable material, but ideally iron as a
major component. In
one embodiment, the reactor has a shell 23 comprising 99.5% iron and up to
0.5% manganese.
Chromium oxide formed by the reaction protects the iron from rusting. While a
stainless steel
reactor may be used, it can be damaged by various impurities (such as halides,
for example) in
the plastics, requiring it to be replaced more frequently and adding to the
expense of the
operation.
The reactor 27 is effectively a gradient system comprising different zones
therein. The reactor
receives the plastics from the feeder 201 (as raw feedstock 5 or molten
plastic from the premelter
2, if such is used, via pipe 20). The reactor 27 can be of the rotary type
which is rotated during
.. pyrolysis of the plastics so that its internal surfaces are hot enough to
vapourize the liquid/solid
plastics, forming hydrocarbon vapour and carbon black. A catalyst can be mixed
with the liquid
or hard plastic in the reactor 27 to facilitate selective cracking of
hydrocarbons. A catalyst may
be selected according to the desired fuel to be generated from the overall
process. For example,
catalysts such as aluminum oxide or calcium hydroxide can be added to
facilitate the removal of
halogens, such as chlorine. In other embodiments, a Group VIII metal can be
added to the
reaction mixture. This can facilitate a reaction whereby water is broken down,
carbon monoxide,
unsaturated hydrocarbons, and hydrogen gas react to form saturated
hydrocarbons and carbon
dioxide. H2 gas is particularly beneficial for effecting hydrogen saturation
of the fuel
downstream. This reduces the need for more costly additives, such as in
current methods which
require an external H2 source and a platinum catalyst under high pressure (300
psi), to facilitate
hydrogen saturation. Within the reactor 27 is a reaction zone 202 where the
liquid and solids are
vapourized, producing a hydrocarbon stream, and solid residue. The vapourized
hydrocarbons
are cracked to molecules having carbon chains ranging from Cl to about C49 or
C50. Higher
length carbon chains are cracked until they are within the lower range. The
solid residue
comprises primarily carbon black. The reactor operates in a range of about 340
C to 445 C,
CA 2879973 2018-03-01
- 8 -
ideally about 350 C to 425 C, or about 400 C. The heat required for this
temperature can be
obtained with a furnace which uses hydrogen gas, methane and ethane as fuel.
Ideally, the fuel
can be obtained downstream in the process of the current invention. Typically,
this combination
of gases burns at approximately 2,300 F. The hot combustion gases are then
circulated through a
spiral duct that runs around the outside of the reactor. The spiral duct is
typically a spiral
refractory with heat on end and exhaust on the other. The residence time of
the plastics in the
reactor for any desired length of time, such as, for example, between 10
minutes to 1 hour or
more.
The liquefied plastic is moved at a slow rate until reaching the end of
reactor 27. Solids from the
reactor 27 are removed by lifters 29 and chutes 31 inside the reactor 27.
Metal solids form a bed
in within reactor 27. Similar with the outlet 19 on the premelt 2, liquid
passes through the outlet
residue pipe 32 surrounded by a sleeve pipe which collects vapour and sends it
to through vapour
pipe 35 and preventing backflow of the vapour into the reactor 27, while solid
residue settles on
the bottom of the channel and is collected in a residue drum 34. The solid
residue is discarded
using appropriate disposal means in accordance with local regulations. Whereas
larger metal
solids and biomass are typically removed from the feedstock in the premelter
2, finer solids are
removed from the reactor 27.
A cyclone 37 may also be used to remove any of the solid residue from the
reactor discharge.
Hydrocarbon vapour flows out of reactor 27 through pipe 35 to the cyclone 37.
The cyclone 37
removes any entrained particulate matter from the vapour steam. The
particulate matter falls out
through opening 36 into the residue drum 34. Vapour from the cyclone 37 then
heads to a
catalyst tower via a pipe 39. Ideally, a pipe having a 16 inch diameter
(including at the inlet end)
can be used.
The solids-free vapours are then cracked, treated and condensed in one or more
catalyst towers,
such as catalyst towers T2 and T3, shown in Figure 4. The catalyst towers are
separation vessels
which, as a type of reactor, separate vapours into petroleum products based on
the boiling points
of the hydrocarbon constituents. Typically, the catalyst tower T2,T3 is about
20 feet high having
a diameter of about 3 feet, although any size as appropriate can be used. Each
catalyst tower
T2,T3 consists of one, two or more catalyst zones 40,41 separated by one or
more weirs 45 to
CA 2879973 2018-03-01
- 9 -
treat the hydrocarbons and facilitate selective hydrocarbon cracking. Each
catalyst tower T2,T3
produces and blends a petroleum product within a particular specification.
Petroleum products
which are out of specification are pumped either out of the catalyst towers
T2,T3 for further
processing. For example, off-spec petroleum products are collected in
receptacle 44 and are sent
by a pump 210 back to the reactor 27. The off-spec petroleum can also be
collected and pumped
via pump 211, condensed in condenser 212 and added back to the tower at nozzle
42.
Alternatively, these hydrocarbons can be collected and taken out of the system
for use as fuel
(such as furnace oil #4, #5 or heavy diesel, depending on the operation). From
further
downstream catalyst towers, such as T3, off-spec petroleum products are pumped
back to
catalyst tower T2 via pump P3 and pipe (400). The shunting of the different
petroleum products
can also be performed automatically, such as with automatic ball valves
controlled by the
system, for example. The system open or closes the valve, depending on the
amount of
movement of fuels, and any blending of the fuels, between the towers to
achieve the desired fuel
product.
The temperatures of the catalyst towers vary T2,T3, and generally decrease
from upstream to
downstream when multiple towers are used. Heat from the hydrocarbon vapours
maintains the
interior of the towers within a broad range. For example, the temperature in
T2 can be about
400 C, while the temperature in T3 can be up to 320 C. This difference in
temperatures permits
petroleum products of differing boiling points to precipitate from the vapour.
There is also an oil
jacket around each tower (not shown) which has hot oil circulating through it,
which can also
maintain the temperature in each tower within a very narrow range. No other
external heat source
is required to maintain the temperature ranges within the catalyst towers,
other than the heat from
the hydrocarbon vapours. Further, hydrocarbon vapours from a downstream
catalyst tower (such
as T3) which are sent back to an upstream catalyst tower (such as T2) can
change and regulate
the temperature of the upstream tower, and vice versa.
The hydrocarbon vapours pass through the weirs 45 containing a catalyst 43.
These catalysts can
include platinum, the catalysts are composed predominantly of compounds such
as a Group II,
Group VI and/or Group VIII/XIII metal compound sulfides, oxides and
hydroxides, for example,
molybdenum sulfide (MoS2), and a zeolite depending on the fuel to be produced.
Particularly
preferred zeolites are synthetic Y-type zeolite and ZSM-5. Hydrocarbon vapours
meeting
CA 2879973 2018-03-01
- 10 -
specification pass through pipe 50, ideally platinum plated and having a
diameter of about 8
inches, at the top of the reactor and proceed to the next catalyst tower or
hydrocarbon
condensation system, depending on the desired products to be obtained from the
process. The
platinum can act as a catalyst to facilitate the saturation of unsaturated
hydrocarbons before
.. leaving the catalyst towers to be condensed. Hydrogen and highly reactive
low boilers are in the
stream to facilitate saturation.
Hydrocarbons are condensed from heavy to light, depending on the desired
petroleum product,
and each requires its own hydrocarbon condensing system stage. One or more
heat exchangers
are in fluid communication with the catalyst tower T2 (or, if two or more
catalyst towers are
used, the one most downstream T3), to receive vapours coming from the top of
the catalyst
tower(s) and condense the vapours. The temperature in the heat exchanger(s) is
regulated by a
hot oil system coiled around the outside of the heat exchanger 212, which
provides water, hot oil
or air cooling within a very narrow range to ensure the right selection of
products is obtained.
The products flow into and are collected in pipe 53.
The petroleum product passes through a manometer and is further cooled (if
required) by a 3-
phase separator and heat exchanger 56. The petroleum product may be treated in-
line with fuel
additives required to bring the fuel in specification. The additives are
metered into the system
through a pump and flow to the liquid petroleum product, which is blended in
the pipe 53. Fuel
additives can be lubricity additives, antioxidants, and other common industry
fuel additives. An
automation system controls the pump to ensure the proper ratio of fuel
additive and fuel.
Petroleum product in the 3-phase separator 56 is cooled to room temperature
and sent to a
centrifuge (401) and filtered by a simple strainer and into a storage tank
(402).
Multiple condensers and heat exchangers permit different fuels to be
precipitated out of the
hydrocarbon vapour. The temperature of the cooling system is set based on the
hydrocarbon
product the condensation system is intended to condense, and based on the
temperature of the
catalyst towers. For example the temperature is set at 170 C to 180 C for
diesel or heating oil #2,
240 C for heating oil #6, and about 20 C for gasoline. At 230 C, the fuel is
fairly light (i.e., a
mixture of C5-C8 hydrocarbons depending on the degree of blending of the fuels
at higher
temperatures. At 280 C, 60-70% diesel and 30-40% gasoline is obtained, whereas
at 235 C, the
CA 2879973 2018-03-01
- 11 -
ratio is about 60-70% gas and 10-15% diesel. Beyond 280 C, paraffin wax (C20-
C40) is
obtained.
As shown best in Fig. 1, the selective condensation system 1000 condenses
diesel at
approximately 170 C to 180 C, the light naphtha (or gasoline) is condensed at
20 C. The
remaining hydrocarbon low boilers pass through a fuel seal that ensure oxygen
cannot pass back
through the process. The low boilers (ethane, methane, butane, propane, and
hydrogen) are
compressed by a compressor or blower and routed to the furnace to provide
fuel.
Residual vapours then pass through pipe 62 to a petroleum water seal within
tank 64. The
vapours from pipe 62 condenses any water remaining in the vapour stream. This
water condenses
and falls into the bottom of the tank 63. The vapour bubbles through the
water. The water acts as
a seal to exclude oxygen from passing back through the system. The remaining
vapour consists
of hydrogen and hydrocarbons having low boiling points (such as methane,
ethane, butane, and
propane).
Optionally, a pH meter may be connected to the bottom of the tank 63 to
monitor for any halides
.. that got into (or through) the system. The vapour is sent via pipe 65 drawn
to an off- gas
compressor to pressurize the off-gas (to about 1000 psi) for use as fuel for
furnace burners, such
as for heating the premelter 2 and/or the reactor 27, or for other uses, such
as propane for
barbecue tank fuel. No thermal oxidizers, scrubbers, or filters are required
for the flue gas.
Ambient air is mixed with syngas by the burner 204 and burned to provide heat
for the reactor
27. The flue gas exhaust from the furnace indirectly heats both the premelter
2 and the reactor
27, and then is routed to a stack (404) via an exhaust fan (403). Overall,
solid waste plastic is
converted to approximately 86.7% liquid petroleum products, 1-5% residue, and
8% syngas used
to provide fuel for the furnace.
All publications, patents and patent applications mentioned in this
Specification are indicative of
the level of skill of those skilled in the art to which this invention
pertains.
The invention being thus described, it will be obvious that the same may be
varied in many
ways. Such variations are not to be regarded as a departure from the spirit
and scope of the
CA 2879973 2018-03-01
- 12 -
invention, and all such modifications as would be obvious to one skilled in
the art are intended to
be included within the scope of the following claims.
CA 2879973 2018-03-01