Note: Descriptions are shown in the official language in which they were submitted.
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
WOUND DRESSING SEALANT AND USE THEREOF
Embodiments described herein relate to compositions, devices incorporating the
same, apparatuses, kits, their
uses in wound care, and methods for the treatment of wounds and for creating a
main wound dressing portion for
use in wound dressing, for example in advanced wound management, particularly,
but not exclusively, in
negative pressure therapy (TNP therapy).
Different types of wound dressing exist for aiding in the healing process of a
human or animal subject in need
thereof. These include different types of materials, for example, gauze and/or
foam with overlying drapes, and
composites thereof, provided in assembled layers and in a selection of sizes
or shapes, typically square or
rectangular shapes. Advanced wound management dressings address specific wound
therapies by means of
tailored dressing components, particularly, but not exclusively, TNP therapy
dressings incorporate a means for
transmitting negative pressure to the wound, and a fluid-tight drape for
enclosing a negative pressure, provided
as independent components or as a composite dressing.
We have found, as a first problem, that certain wounds and body topography
cannot be adequately dressed
using the existing selection of composite TNP dressings, for example vein
harvest wounds extending the length
of a subject's leg exceed available dressing dimensiors or diabetic foot
ulcers where complex topography
prevents rectangular designs from conforming adequately to tight body
contours..
We have found, as a further problem, that advanced wound therapy and in
particular TNP therapy, cannot
therefore be applied to those wounds which perhaps have the greatest need for
this therapy, but rather
conventional wound care must be relied on.
We have therefore defined a need for improved dress,ngs, more particularly but
not exclusively composite TNP
therapy dressings, which exhibit enhanced adaptability in wound care.
It is an aim of certain embodiments to at least mitigate the above-mentioned
problems. Certain embodiments
disclosed herein relate to improved compositions and their use in combination
with dressings to confer enhanced
adaptability in wound care, preferably in TNP wound care. Dressings applied in
combination with such
compositions may have advantages over traditiondy applied dressings which may
be more difficult to apply,
particularly around wounds such as lengthy incision sites or irregularly
shaped wounds. Dressings applied to
such wounds with use of such compositions may be of comparable effectiveness
to dressings applied in
traditional manner to more regular sized or shaped wounds. Wounds dressed with
such dressings in combination
with such compositions may enable the application of TNP therapy. Also
disclosed are improved methods of use
and systems for use of the compositions in combination with dressings,
preferably in negative pressure wound
therapy.
It is an aim of certain embodiments to provide means to enable composite wound
dressings to more universally
be used on wounds of different shapes or sizes.
It is an aim of certain embodiments to provide a dispensable sealant
composition for a composite wound
dressing which can more universally be used on wounds of different shapes or
sizes.
It is an aim of certain embodiments to provide a devise including such
composition for dispensing in improved
manner to a composite wound dressing to more universally be used on wounds of
different sizes or shapes.
-1-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
It is an aim of certain embodiments to provide a wound dressing kit including
a composite dressing, preferably an
advanced wound management dressing, more preferably a composite TNP therapy
dressing, together with a
sealant composition, adapted to be applied in conjunction at a wound site.
It is an aim of certain embodiments to provide an apparatus in the form of a
composite wound dressing which
can more universally be used on wounds of different shapes or sizes.
It is an aim of certain embodiments to provide a method of treating a wound by
sealing a composite dressing
which can more universally be used on wounds of different shapes or sizes.
In one embodiment, there is provided a dispensable composition for woundcare,
wherein the composition is dispensed into a wound dressing location, said
wound dressing comprising:
a backing layer having an upper surface and a lower surface, otherwise termed
a backing sheet having
two faces, and defining a perimeter configured to be positioned over skin
surrounding a wound site;
an optional wound contact layer;
one or more transmission layers provided directly or indirectly to the lower
backing layer surface, or
otherwise configured to be positioned below the backing layer, or otherwise
positioned at or on one side
of one face of the backing sheet,
or enclosed between the backing layer and the wound contact layer, where
present; and
a port configured to transmit negative pressure through the backing layer for
the application of topical
negative pressure at the wound site
wherein removing a portion of the wound dressing directly enclosing the
transmission layer to create a main
wound dressing portion with one or more exposed portions wherein the
transmission layer is exposed at a
portion thereof,
said exposed portion(s) being the location as hereinbefore defined, the
dispensed composition seals the
exposed portion(s).
The composition is particularly for creating a main wound dressing portion for
use in wound care more
particularly for sealing a trimmable dressing, having a main dressing portion
or cell in fluid (e.g., gas)
communication with additional dressing portions or cells, for use in
woundcare, more particularly for treatment of
a wound site,
The composition may be dispensed into or onto the exposed portion or both. By
this means the dispensed
composition impregnates or envelopes the exposed portion or both.
Preferably the dispensed composition impregnates the exposed portion. This has
the advantage of enhanced
robustness whereby the seal forms part of the dressing.
Alternatively the dispensed composition envelopes the exposed portion. This
more resembles a simple repair
applied to the upper surface of the dressing. Choice of dispensing by
impregnation or enveloping may be
selected according to the nature of the dressing to be sealed, in particular
its laminar structure, and more
particularly the laminar structure at the exposed portion thereof. Optionally
the composition impregnates and
additionally envelopes the sealed portion thereby prcviding a seal operating
at internal surfaces of the
transmission layer and external surfaces of the dressing. Composition may be
dispensed directly at the exposed
-2-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
portion through the exposed face thereof, or indirectly via the backing layer
or backing sheet or via the optional
wound contact layer, thereby internally penetrating the exposed portion.
Some embodiments may further comprise a device comprising the composition, an
apparatus in the form of a
dressing for use with the composition, kits thereof, uses and methods of
therapy. Some embodiments may
further comprise a source of negative pressure configured to supply negative
pressure through the port. Some
embodiments may further comprise retention strips or sealing strips configured
to hold in place or seal the
dressing to skin surrounding a wound.
In another embodiment, a method of creating a main wound dressing portion for
use in dressing or otherwise of
treating a wound comprises:
providing a wound dressing as hereinbefore defined comprising:
a backing layer, otherwise termed a backing sheet;
an optional wound contact layer; and
one or more transmission layers as hereinbefore defined
removing a portion of the wound dressing to create a main wound dressing
portion with one or more
exposed portions;
optionally positioning the main wound dressing portion over a wound and
sealing the main wound
dressing to skin surrounding the wound,
and dispensing a composition as hereinbefore defined to a location comprising
the one or more
exposed portions of the main wound dressing portion thereby sealing the
exposed portion(s); and
optionally applying negative pressure to the wound through the backing layer
of the main wound
dressing portion.
Sealing a wound dressing to skin may be prior to creating a main wound
dressing portion and prior to dispensing
composition or may be subsequent to creating a main wound dressing portion and
prior to or subsequent to
dispensing composition. Accordingly the method may be a method relating to
dressing manufacture for use in
dressing wounds or may be a method relating to dressing wounds. As is used
herein the backing layer
represents a gas impermeable membrane. Also referred to herein as wound cover
or drape. Some examples of
materials suitable for backing layers included thin polyurethane films, which
may optionally be coated with
adhesive. It is also possible that a number of laminates be brought together
to form multi-laminar backing layers,
in such cases the description of the upper and lower surfaces of the backing
layer are taken to mean the upper
and lower surfaces of the complete backing layer.
As is used herein, a dressing also comprises one or more transmission layers
and other layers (such as
absorbent material) positioned beneath the backing layer. For example, one or
more transmission layers or
other layers may be positioned or enclosed between a backing layer and an
optional wound contact layer, for
example, sealed therebetween. The transmission layer(s) may be in turn
positioned between the backing layer
and (optional wound contact layer and) a wound site over which the dressing is
configured to be positioned, for
example sealed therebetween.
A transmission layer as described herein allows transmission of fluid such as
air, and optionally additionally other
gases and liquids, away from a wound site into upper layer(s) of the wound
dressing, the port, and therefrom to a
-3-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
fluid canister if present and/or into a negative pressure pump. A transmission
layer may assist in maintaining an
open air channel to communicate negative pressure over the wound area even
when the dressing is handling
substantial amounts of exudates. The layer should remain open under the
typical pressures that will be applied
during negative pressure wound therapy. Preferably, a transmission layer
remains open over an area
corresponding to the wound site, and thereby ensures that the whole wound site
sees an equalised negative
pressure. Alternatively the transmission layer may comprise one or more
specific air paths which remain open,
such as in and between bridging portions of a wound dressing as described
further below.
A transmission layer may comprise voids or may comprise one or more materials
which transmit fluid, or may be
a combination thereof. The transmission layer may incorporate other functional
materials provided that it is still
capable of transmitting negative pressure, and preferably also liquid fluids.
In some embodiments, the
transmission layer is capable of transmitting wound exudates and other
compositions of matter.
Some examples of materials suitable for a transmission layer include a three
dimensional structure, for example,
a knitted or woven spacer fabric (for example Baltex 7970 weft knitted
polyester), although other materials such
as foam (e.g., reticulated foam), nonwoven materials (e.g., an acquisition
distribution layer as described below)
could of course be used. Alternatively or additionally the transmission layer
may incorporate absorbent material
and absorb liquid drawn away from the wound under the applied negative
pressure.
Some embodiments described herein include a trimmable dressing, having a main
dressing portion or cell in fluid
(e.g., gas) communication with additional dressing portions or cells. As is
used herein, a main dressing portion
represents a portion which has a size or shape or profile or articulation
which is compatible with a wound or
wound site to be dressed. One or more additional portions or cells may be
removed to provide a dressing having
a size or shape or profile or articulation which is to be compatible with a
wound or wound site to be dressed.
Preferably a large surface area, or elongate main dressing portion is provided
to dress a similarly large surface
area or elongate wound; for example portions or cells may =be retained to
provide such a large surface area, or
elongate main dressing portion, or portions or cells may be removed to dress a
correspondingly reduced surface
area or reduced length wound. preferably a shaped main dressing portion is
provided to dress a similarly shaped
wound; or a shaped main portion is provided to dress a wound incorporating or
adjacent a protrusion such as a
device, for example a pin, or such as a body part such as a digit, for example
one or more additional portions or
cells may be conformed to provide a shaped dressing; preferably a profiled
main dressing portion is provided to
dress a similarly profiled wound or wound site, such as a wound located on
complex body topography, for
example one or more additional portions or cells may be conformed to provide a
profiled dressing; preferably an
articulated main dressing portion is provided to dress a similarly articulated
wound or wound site such as a
wound located on a joint, for example one or more additional portions or cells
may be articulated.
A main dressing portion or portions and additional portions or cells as
described herein may be connected by
one or more bridge portions including one or more transmission layers as
described above, in other words a
bridging portion underneath the backing layer, or otherwise positioned at or
on one side of one face of the
backing sheet, the bridging portion comprising at least one material layer
configured to transmit negative
pressure from the first portion through the bridging portion. In some
embodiments, the at least one material layer
in the bridging portion has a smaller dimension or a different material
structure than a corresponding dimension
-4-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
or material structure of the first portion, for example the one or more
transmission layers comprising one or more
bridging portions having a smaller width than adjacent portions of the one or
more transmission layers or than
the main dressing portion, or the one or more transmission layers comprising
one or more bridging portions
having a smaller height than adjacent portions of the one or more transmission
layers or than the main dressing
portion.
As is used herein, an exposed portion of transmission layer represents a
portion which the backing layer is not
configured to enclose and seal against a surface such as a wound site, for
example the backing layer and
optional wound contact layer do not enclose the transmission layer. For
example, a section of transmission layer
and overlying backing layer is absent, whereby the remaining transmission
layer terminates in open-ended
manner, or the backing layer may be partially absent, and additionally the
optional wound contact layer may be
partially absent, at which the transmission layer terminates in open-ended
manner. It may be desired to seal
such exposed portion of transmission layer (and exposed portions of other
layers). As is used herein, sealing
represents sealing in manner to contain fluid, more preferably in manner to
contain negative pressure.
As is used herein, fluid represents liquid and gas. However it is not intended
that "fluid" should encompass
"vapour", a favourable moisture vapour transmission rate (MVTR) being a
requirement of dressings envisaged
herein. The backing layer is impermeable or substantially impermeable to
fluids including wound exudate. The
backing layer is air-tight or substantially air-tight, whereby a negative
pressure may be maintained at a wound
site to which the dressing is applied and sealed with the composition. Wound
exudates and other fluids may be
contained within the wound site and/or dressing and any collection means
associated therewith.
As is used herein, a dispensable composition represents a composition having
viscosity in the range from 5 to
300 Pa.s, preferably 10 ¨ 100 Pa.s. Viscosity (n) is determined in accordance
with DIN EN ISO 3219 : 1994,
Annex B. For some embodiments viscosity is in the range 20 ¨ 80 Pa.s. The
composition parts may be combined
and allowed to partially cure to a suitable viscosity for application, or may
have properties such that a suitable
viscosity reduction is achieved when subject to shear forces during
application. The composition may have flow
properties such that it can be drawn within transmission layer(s) at exposed
portion(s) by the prevailing negative
pressure and then cure. The composition ay for example flow to a distance of
up to 25mm, eg 1mm to 25mm.
The cured sealant preferably retains a degree of flow or conformability, for
example extensibility, such that it can
accommodate the dynamic conditions encountered when on the skin.
As is used herein a TNP system may be operated with any suitable source of
negative pressure, including and
not limited to pumps, springs (SNaP) and any other functional equivalents.
As is used herein, a wound dressing represents a composite wound dressing,
preferably an advanced wound
management dressing tailored to include specific wound therapy provision for
i.a. management of wound
exudates (eg ALLEVYN Gentle Border, DURAFIBER, ALLEVYN Life), infection
management (eg ACTICOAT,
IODOSORB), iv site care (eg IV3000), management of compromised skin about the
wound, TNP (eg RENASYS
F/AB, PICO, KCI Prevena, Kalypto Medical Inc NPD1000 NP Wound Therapy System),
post operative care such
as surgical drapes (eg OPSITE), temporary bioskin dressings (eg BIOBRANE) and
the like, most preferably a
TNP dressing.
-5-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 illustrates an embodiment of a wound treatment system;
Figures 2A-D illustrates the use and application of an embodiment of a wound
treatment system onto a
patient;
Figure 3A illustrates an embodiment of a wound dressing in cross-section;
Figure 3B illustrates another embodiment of a wound dressing in cross-section;
Figure 3C illustrates another embodiment of a wound dressing in cross-section;
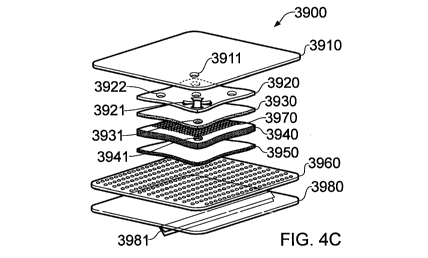
Figures 4A and 4C illustrate an exploded view of an embodiment of a wound
dressing;
Figure 4sB and 4D illustrate a cross sectional view of an embodiment of a
wound dressing;
Figure A1 ¨ A4 and A7 illustrate embodiments of commercially available wound
dressings trimmable for
sealing with the present composition, to size, to profile on complex
topography, or to shape around a fixation
device or for puncture repair;
Figures A5 ¨ A6 illustrate the use and application and sealing of embodiments
of Figures A1 ¨ A4 onto
a patient;
Figures B1 ¨ B2 and C1 ¨ C7 illustrate the spacer nozzle relationship of an
applicator for dispensing
composition to a trimmable dressing;
and C1 ¨ C3 also illustrate the sealing of embodiments of wound dressings; and
C6 also illustrates a
novel nozzle head for an applicator;
Figure 5A illustrates an embodiment of a wound dressing trimmable at a bridge
portion;
Figure 5B illustrates another embodiment of a wound dressing trimmable at a
bridge portion;
Figures 5C and 5D illustrate the use and application and sealing of
embodiments of Figures 5A ¨ 5B
onto a patient;
Figure 6 illustrates an embodiment of a trimmable wound dressing comprising a
plurality of portions or
cells;
Figure 611 illustrates the use and application and sealing of an embodiment of
Figure 6 onto a patient;
Figure 7 illustrates an embodiment of a trimmable T-shaped wound dressing
comprising a plurality of
portions with multiple port attachment sites;
Figure 711 illustrates the use and application and sealing of an embodiment of
Figure 7 onto a patient;
Figure 8 illustrates an embodiment of a trimmable wound dressing with multiple
port attachment sites;
and
Figure 811 illustrates the use and application and sealing of an embodiment of
Figure 8 onto a patient.
Figures 9A and 9B illustrate one embodiment of spacer layer material;
Figures 10A-10D illustrate one embodiment of acquisition distribution layer
material;
Figures 11A and 11B illustrate one embodiment of absorbent layer material;
Figures 12A and 12B illustrate one embodiment of obscuring layer material;
Figure 13 illustrates one embodiment of an adhesive spread on cover layer
material;
Figures 14A-14B illustrate one embodiment of a trimmable dressing having a
reduced height bridging portion;
Figure 15 illustrates an embodiment of a trimmable wound dressing comprising a
plurality of portions or cells;
Figures 16A and 16B illustrate another embodiment of acquisition distribution
layer material; and
Figures 17A through 17C illustrate another embodiment of acquisition
distribution layer material.
-6-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Embodiments disclosed herein relate to compositions, devices, apparatuses,
uses, kits and methods of treating a
wound with reduced pressure, including pump and wound dressing components and
apparatuses. The
apparatuses and components comprising the wound overlay and packing materials,
if any, are sometimes
collectively referred to herein as dressings, unless otherwise indicated or
intimated. Throughout the specification,
the terms sealant and composition are hereinafter used interchangeably unless
otherwise indicated or intimated.
It will be appreciated that throughout this specification reference is made to
a wound. It is to be understood that
the term wound is to be broadly construed and encompasses open and closed
wounds in which skin is torn, cut
or punctured or where trauma causes a contusion, or any other superficial or
other conditions or imperfections on
the skin of a patient or otherwise that benefit from reduced pressure
treatment. A wound is thus broadly defined
as any damaged region of tissue where fluid may or may not be produced.
Examples of such wounds include,
but are not limited to, abdominal wounds or other large or incisional wounds,
either as a result of surgery,
trauma, stemiotomies, fasciotomies, or other conditions, dehisced wounds,
acute wounds, chronic wounds,
subacute and dehisced wounds, traumatic wounds, flaps and skin grafts,
lacerations, abrasions, contusions,
burns, diabetic ulcers, pressure ulcers, stoma, surgical wounds, cosmetic
wounds, trauma and venous ulcers or
the like. Wounds may include readily accessible and difficult to access
wounds, exposed and concealed wounds,
large and small wounds, regular and irregular shaped wounds, planar and
topographically irregular, uneven or
complex wounds, more preferably on a site selected from the torso, limb and
extremities such as heel, sacrum,
axial, inguinal, shoulder, neck, leg, foot, digit, knee, axilla, arm and
forearm, elbow, hand or for sealing a crevice
adjacent or adjoining a wound site, selected from such as sacral cleft, fossa
and the like.
It will be understood that embodiments of the present disclosure are generally
applicable to use in topical
negative pressure ("TNP") therapy systems. TNP therapy sometimes referred as
vacuum assisted closure
V.A.C.(R) or negative pressure wound therapy (NPVVT) using sub-atmospheric
pressure is applicable to a broad
range of wounds such as chronic wounds, incisional wounds, open wounds and
abdominal wounds or the like.
Briefly, TNP assists in the closure and healing of many forms of wound, by
reducing tissue oedema; encouraging
blood flow and granular tissue formation; removing excess exudate and may
reduce bacterial load (and thus
infection risk). In addition, the therapy allows for less disturbance of a
wound leading to more rapid healing. TNP
therapy systems may also assist in the healing of surgically closed wounds by
removing fluid and by helping to
stabilize the tissue in the apposed position of closure. A further beneficial
use of TNP therapy can be found in
grafts and flaps where removal of excess fluid is important and close
proximity of the graft to tissue is required in
order to ensure tissue viability.
During TNP therapy, a suction source such as a vacuum pump or the like is
utilised to create a negative
pressure region. That is to say, a region where an experienced pressure is
below that of the surroundings. The
suction source creates a negative pressure via a dressing or drape positioned
over and sealed about or around
the periphery of the wound. Wound exudate and other potentially harmful
material is enclosed under the dressing
or drape and extracted therefrom.
-7-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
As is used herein, reduced or negative pressure levels such as ¨X mmHg,
represent pressure levels that are
below standard atmospheric pressure, which corresponds to 760 mmHg (or 1 atm,
29.93 in Hg, 101.325 kPa,
14.696 psi, etc.). Accordingly, a negative pressure value of ¨X mmHg reflects
absolute pressure that is X mmHg
below 760 mmHg or, in other words, an absolute pressure of (760¨X) mmHg. In
addition, negative pressure that
is "less" or "smaller" than X mmHg corresponds to pressure that is closer to
atmospheric pressure (e.g., ¨40
mmHg is less than ¨60 mmHg). Negative pressure that is "more" or "greater"
than ¨X mmHg corresponds to
pressure that is further from atmospheric pressure (e.g., ¨80 mmHg is more
than ¨60 mmHg).
The negative pressure range for some embodiments of the present disclosure can
be approximately -80 mmHg,
or between about -20 mmHg and -200 mmHg. Note that these pressures are
relative to normal ambient
atmospheric pressure. Thus, -200 mmHg would be about 560 mmHg in practical
terms. In some embodiments,
the pressure range can be between about -40 mmHg and -150 mmHg. Alternatively
a pressure range of up to -
75 mmHg, up to -80 mmHg or over -80 mmHg can be used. Also in other
embodiments a pressure range of
below -75 mmHg can be used. Alternatively, a pressure range of over
approximately -100 mmHg, or even 150
mmHg, can be supplied by the negative pressure apparatus. In some embodiments
of wound closure devices
described here, increased wound contraction can lead to increased tissue
expansion in the surrounding wound
tissue. This effect may be increased by varying the force applied to the
tissue, for example by varying the
negative pressure applied to the wound over time, possibly in conjunction with
increased tensile forces applied to
the wound via embodiments of the wound closure devices. In some embodiments,
negative pressure may be
varied over time for example using a sinusoidal wave, square wave, and/or in
synchronization with one or more
patient physiological indices (e.g., heartbeat). Canisterless NPVVT (omitting
a dedicated canister to contain
wound exudate) has also been considered using negative pressure values in the
same range as conventional
NPVVT. More preferably -40 to ¨200 mmHg. More preferably -40 to -140 mmHg.
Embodiments address the problem of providing dressings in a range of sizes and
shapes to accommodate
irregularly shaped wounds and body topography, for example vein harvest wound
dressings accommodating
variations in height and leg-length of individuals, which is impractical both
to the manufacturer and to the user.
Embodiments enhance adaptability of existing dressings, and of more recently
introduced multisite dressings
such as trilobes and quadrilobes. Embodiments enable a portion of a dressing
to be removed to create a main
wound dressing of desired size or shape or profile or articulation, and
sealing exposed portion(s) thereof to
contain a negative pressure.
Exposed portion(s) as hereinbefore defined are the result of removing a
portion of the wound dressing, which
may be by any envisaged means, for example cutting the wound dressing or
tearing along a weakened line.
Composite wound dressings may comprise a border for affixing around a wound,
about a central wound contact
portion. The dressing as hereinbefore defined may nclude a backing layer and
wound contact layer of similar
footprint or surface area to the transmission layer or other layers enclosed
therebetween (i.e. a borderless
dressing) or of greater footprint or surface area than the transmission layer
enclosed therebetween (i.e a
bordered dressing). Exposed portion(s) as hereinbefore defined result from
removing a portion of the wound
dressing as hereinbefore defined directly enclosing tne transmission layer,
for example by cutting into or through
the backing layer and wound contact layer and the transmission layer
therebetween.
-8-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
The portion(s) of the wound dressing may be removed to size the main wound
dressing portion for positioning
over a wound as hereinbefore defined, for example an incisional wound, an
elongate leg wound, an arcuate
incisional wound and the like. Similarly the portion(s) of the wound dressing
may be removed to shape the main
wound dressing portion for positioning over a wound as hereinbefore defined,
such as a flap wound, over a
protruding device such as a fixation device or a protruding body part, to
profile the main wound dressing for
positioning over a wound as hereinbefore defined, for example on complex body
topography, or to articulate the
main wound dressing for positioning over a wound as hereinbefore defined for
example on a flexing joint.
Preferably a dressing is skin compatible. Skin compatible as used herein
refers to the ability to apply or reapply a
dressing and in particular a backing layer or wound contact layer to skin and
remove from skin without trauma to
the wearer, and without causing substantial damage to the skin. Skin
compatible materials include adhesive or
non-adhesive materials such as pressure sensitive adhesive (PSA), typically
acrylic, hydrocolloid, silicone and
silicone based materials and other materials as hereirbelow recited. A
particularly well known skin compatible
material comprises silicone or is silicone based, and skin compatible
materials are envisaged having properties
corresponding to silicone or silicone based material.
COMPOSITION AND METHOD FOR DISPENSING
The composition may be selected from a curing system and a non-curing system.
Ideally this is a material that
will not flow substantially from its application site but that during
application is either shear thinning or shape
conformable when subject to load. A curing system may be selected from a
curable one or more parts
composition, for example silicone curing systems which may include one, two or
more part silicone systems and
may include a range of curing mechanisms, epoxy curing systems, cyanoacrylate
curing systems, polyurethane
curing systems, polymeric systems functionalised with silicone chain linking
functional groups, polymeric system
functionalised with polyurethane curing functional groups, drying system such
as an elastomer rendered fluid by
the presence of a volatile solvent, spray on elastomers such as acrylic in
water or'solvent base, UV or light
curing systems.
A non-curing system may be selected from a one or more part composition, for
example a putty, a jelly such as
petroleum jelly, grease such as silicone grease, a gel such as a hydrogel,
organogel or xerogel, a paste, a
colloidal system such as a hydrocolloid.
Preferably the composition dispensed to a location as hereinbefore defined
forms an elastomeric seal.
Curing time of a curable composition is not a limiting feature. A curing
composition usefully has curing time at 23*C
in the range from 0.05 min to 24 hours, eg 0.5 to 20 min, more preferably from
0.5 to 18 min, more preferably from
0.5 to 16 min, most preferably 12 min, most preferably 0.5 to 5 min. Cure time
is manual kinetic as known in the art.
A number of methods are known in the art to monitor the cure of liquid
polymers and in particular RTV-2
silicones, these vary from continuous monitoring across the full cure profile
of the material with instruments such
as scanning vibrating needle curemeters (B.G. Willoughby and K.W. Scott,
Understanding cure with the scanning
vibrating needle curemeter (scanning VNC), RTU2844, Rapra Technology Limited,
Shawbury) or differential
scanning calorimeters (L.M. Lopez, A.B. Cosgrove, J.P. Hernandez-Ortiz, T.A.
Osswald, Modeling the
Vulcanization Reaction of Silicone Rubber, Polym. Eng. Sci., 2007, 47, 675-
683) through to empirical single point
-9-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
determinations typically based on clear physical changes, for example
recording the time taken to reach the gel
point.
During the trials described in the examples it was found that transfer of
uncured sealant from the application site
to other surfaces was a clear disadvantage. For the purpose of defining an
unambiguous single point on the cure
profile, cure time is taken to mean manual kinetic cure time. Manual kinetic
cure time is herein defined as the
cure time (at a specified temperature) at which material is no longer
transferred to skin (i.e. a fingertip) when
subject to a light, brief touch.
Due to the temperature dependence of the cure profile on addition cure RTV
silicones it is important that
comparison between any measurements is carried out at the same temperature and
that the temperature be
reported. Guidance set out in Methods of Test for Surgical Dressings in the
British Pharmacopoeia (BP), 1993,
14th edition, A222, Appendix XX is that the temperature of a regulated
atmosphere is taken as 20 C 2 C.
Within the silicone industry there are many instances where curing parameters
of addition cure RTV-2 silicones
are reported at a nominal temperature of 23 C, this falls in line with
standard test methods for other temperature
dependant properties such as viscosity (when measuring viscosity DIN EN ISO
3219 : 1994 describes a
preferred measurement temperature of 23.0 C 0.2 C), examples of this
include: Pot Life reported by Wacker
Silicones (at 23 C on Technical data sheet for Silpurane 2445 A/B, Version
1.3 & Technical data sheet for
Silpurane 2450 A/B, Version 1.3, Wacker Chemie AG, MOnchen); Maximum Working
Time reported by Bluestar
Silicones (at 23 C on The Silbionee Difference, Silicones for Healthcare
Applications, Bluestar Silicones France
SAS, Lyon) and Pot Life reported by Momentive (defined as the time for initial
viscosity to double at 23 C on
Silicone Gels for Healthcare Applications, 152-053-00E-GL, Momentive
Performance Materials Inc., Columbus).
When considering the temperature of a material applied to skin, it should be
noted that the temperature of skin is
nominally taken as 32 C. In a clinical environment, when a curing RTV-2
silicone is applied as a thin bead, layer
or film in intimate contact with the skin, it has been assumed that the
material will reach thermal equilibrium with
the skin rapidly.
Within the literature other discrete points along the cure profile are
routinely used, of note are: pot life, this
usually indicates the maximum period of time after which the mixed silicones
may still be worked, poured, spread
etc. Where flow is an important requirement pot life is usually quoted as the
time required for the initial viscosity
to double (Bastosil, Processing RTV-2 silicone rubbers, 6020e/06.06, Wacker
Chemie AG, Munchen) and tack
free time, this is an appropriate measure when considering a rubber (by
definition the material must not have any
discernible tack or grab once cured) and may be assessed in a similar way to
manual kinetic.
Preferably the composition has cure time as hereinbefore defined at 23 C in
the range from 0.5 min to 20 min, more
preferably from 0.5 to 18 min, more preferably from 0.E to 16 min, most
preferably 12 min, most preferably 0.5 to 5
min. Cure time is manual kinetic as hereinbefore defined.
Values at 32 C are particularly instructive in the present application,
preferably cure time at 32 C is in the range 0.5
to 10 minutes, more preferably 0.5 to 8 minutes, most preferably in the range
0.5 to 7 minutes.
Tack
-10-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
Tack is hereinbelow measured as maximum force required to separate a probe
from cured composition. However
for the purpose of determining tack-free or low tack time, a touch and lift
test was performed at intervals with the
finger, on controlled samples, and tack free or low tack time determined as
the time at which the sample did not
adhere to and lift with touch.
Preferably tack-free time is in the range from 0.5 to 25 minutes, more
preferably from 0.5 to 22 minutes. Preferably
the composition has tack as hereinbeforedefined at a period in the range from
0,5 minutes to 22 minutes after
combining such as to not adhere items such as paper or clothing which contact
the composition. Finger tack is a
relatively subjective evaluation which can be obtained by touching the surface
of the dispensed composition to
determine the "stickyness" thereof. Descriptive terms such as high H), low (L)
and moderate (M) can then be
attributed as a preliminary measure.
Preferably a composition intended for external seal of the exposed portion
forms a substantially tack free seal in a
period as hereinbefore defined. Preferably a composition intended for internal
seal within the bridging portion forms
a substantially tack free to moderate tack seal.
Viscosity
Preferably as hereinbefore defined, compositions having low viscosity in the
range 11 ¨ 14 Pa.s-1 are of particular
advantage when dispensed internally.
A composition may be shear thinning, to assist application, for example
exhibiting change in viscosities with shear
rate,for example as follows:
Target shear rate (S1) viscosity (eg range) mPa.s-1
1.0 250 (240 ¨ 270)
2.50 80 (65 ¨ 94)
5.00 55 (42 ¨ 63)
10.00 40 (33 ¨ 51)
25.00 20 (20 ¨ 25)
50.00 15 (9 ¨ 21)
100.00 10 (5 ¨ 13)
Shear thinning compositions advantageously revert to their rest viscosity and
remain in place once the dispensing
force or applicator force is removed.
Alternatively a rapid onset of cure stabilises the composition in position.
Preferably compostiion penetrates within
the transmission layer and optional additional layers to a distance of from
1mm to 10mm, for example substantially
5mm. Penetration should not exceed 25mm.
Extensibility
A dressing as hereinbefore defined should approximate as closely as possible
to skin, to minimize discomfort
and to maximize the beneficial effects thereof. W02009/156709 discloses the
properties of skin specifically in
relation to extensibility, which are to be approximate by a dressing.
Preferably the composition cures to a seal
which approximates to the extensibility of the dressing which it seals and/or
the extensibility of the skin of the
wearer. Preferably the composition after curing as a sample with a height of
1mm has extensibility comparable to
or greater than the main dressing portion, bridging portion, trimming
portion(s) or component layers thereof up to
a maximum corresponding to the backing sheet. For 9xample for the backing
sheet the load required to produce
a 20% extension at a rate of extension of 300 mm per minute is in the range of
less than or equal to 1.4 kgf per
-11-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
cm width (kgfcm-1), preferably in the range 0.001 to 1.4 kgf cm-1 expressed
preferably as 0.001 to 14.0 kgf crri2
to produce 20% extension, more preferably in the range 0.001 to 5.0 kgf cm-2.
The extensibility of a typical spacer layer is approximately : 0.08 kgfcm-1
(Direction A) ; 0.07 kgfcm-1 (Direction
B)
The extensibility of a typical superabsorber layer (Laminate EU33 top film,
superabsorbent airlaid (Chemposite
11C/450 airlaid superabsorbent pad, spacer layer Baltex 7970 and perforated Si
wound contact layer is
approximately 0.59 kgfcm-1 (Direction A); 0.78 kgfcm-1 (Direction A).
Preferably extensibility of seal as hereinbefore defined is 0.04 to 3.00, more
preferably preferably 1.00 to 2.50.
Permanent set
Permanent set for the cured seal enclosed within the layers may be
substantially 0. In the case of a seal
enclosed within the layers then permanent set is preferably in the range
comparable to Allevyn dressings.
Tensile strength
In the case of a seal enclosed within the layers the composition benefits from
the support of the dressing and
tensile strength values may be widely variable. In the case of a seal enclosed
within the layers then tensile
strength is preferably in the range comparable to Allevyn dressings.
Elongation at break
In the case of a seal enclosed within the layers the composition benefits from
the support of the dressing and
elongation at break values may be widely variable. In the case of a seal
enclosed within the layers then
elongation at break is preferably in the range comparable to the spacer
materials typically present in dressings.
Spacer elongation at break is 115%. Preferably elongation at break is in the
range 5 ¨ 15%.
Compressibility
Preferably the composition forms a seal having equal or greater
compressibility than the bridging layer. Further
detail is given herein in relation to preferred compressibility of the
bridging layer and bridging layer materials when
subject to negative pressure. Preferably a seal does not protrude above the
exposed portion which is seals,
preferably a protruding seal is compressible. Compressibility is measured
according to penetrometry ASTM 82137,
more prferably is in the range 20 ¨ 500 / 10mm.
It will be clear that viscosity for each of Parts A and B is for the as-
provided components, prior to mixing. Suitably
the components mix to a dispensible viscosity.
Preferably the cured composition has elongation at break as hereinbelow
defined, greater than or equal to 50%.
Preferably the cured composition has tensile strength, as hereinbelow defined,
greater than or equal to 5 kgfcm-2.
Preferably for the cured composition permanent set is in the range 20% to 0%.
-12-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
Preferably the composition is a Silpuran composition as hereinbelow recited,
more preferably is Silpuran 24O0', or
a functional analogue thereof, optionally incorporating viscosity and/or cure
time modifier providing increased
viscosity and reduced cure time. Preferably the composition has translucent
appearance after curing. Preferably the
composition is dispensed within the transmission layer by the first embodiment
method disclosed herein (Figures
C1).
In a first embodiment the composition is dispensing into a location as
hereinbefore defined, the location being
substantially internal to or received within the transmission layer at the
exposed portion as hereinbefore defined.
In this embodiment, composition is required to be dispensed from an outlet of
a dispensing device such as a
static mixer, said outlet being capable of being received within the
transmission layer at the exposed portion as
hereinbefore defined. Suitably a dispenser comprises a syringe or static mixer
comprising a nozzle having an
outlet. Preferably the nozzle is capable of being received within the
transmission layer at the exposed portion in
manner that the location at which composition is to be dispensed is a short
distance within the exposed portion,
for example is up to 25mm distant from the exposed portion, more preferably
from 2mm to 20mm, more
preferably from 3mm to 18mm, for example in the range from 5mm to 12mm.
Composition may be dispensed via
the exposed portion face or via the backing layer or sheet, for example by
injection through the backing layer or
sheet. Dispensing may be before or after trimming a dressing. Dispensing by
this means may be by puncturing
the film either singly or in multiple places as described above. Preferably
the film is punctured with use of one or
more resiliently deformable needles, for example a plastic needle or with use
of one or more limited penetration
depth needles. Such needle minimizes risk of skin puncture. Alternatively the
injection could take place prior to
dressing placement and removal of the dressing handle, thus confining the
sealant to between the backing layer
or sheet and the handle. Dispensing may be prior to trimming the dressing
alongside or through the cured
sealant to leave a sealed edge. Composition may alternatively be dispensed to
intact skin and exposed portion of
the dressing located thereover. Composition so dispensed flows into or is
drawn into the dressing to the
transmission layer thereby sealing.
Composition so dispensed is dispensed to a location deeper into the exposed
portion distanced from its face.
Composition is dispensed as a band returning back to the face of the exposed
portion as the dispensing device
is withdrawn. A seal so generated is more secure, with more comprehensive
blocking of passageways within the
exposed portion of the transmission layer. Such a seal has a lesser likelihood
of presenting leaks when negative
pressure is applied. A suitable nozzle for a dispensing device includes low
aperture nozzles, needles and the
like. Nozzles may be formed from plastic. Such nozzles are disposable and
present no hazard, being non-
perforating to human skin. Such nozzles are currently available for use with
pipette tips.
Composition may be dispensed via multiple point injection at intervals along
the exposed face, for example
through the spaces between the spacer layer struts. Composition may flow to
some extent on initial application,
either or both laterally to the direction of dispensing and advancing and
receding, flow becoming less as
composition hardens or cures. This may aid in providing a continuous lateral
seal, whereby dispensing intervals
along the face of exposed portion may be increased. Nozzle insertion distance
within the exposed portion may
be selected to confine the seal spaced a short distance in from the face of
the exposed portion, or to allow some
spill of composition out of the exposed portion and onto surrounding surfaces
such as a preparation plate or skin.
Advantages of this embodiment include minimizing the amount of composition
required to be dispensed. This in
turn allows use of a lower capacity dispensing device, for example a 5m1 or
10m1 or 15ml or 25m1 syringe or
static mixer. The back pressure encountered on dispensing from a static mixer
increases with the mixer volume,
-13-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
which in tum leads to a decrease in the viscosity which the static mixer is
able to dispense. It is generally
advantageous to this embodiment to deliver composition at as high a viscosity
as possible to ensure that
composition is confined within the exposed portion. A further element in the
total back pressure or resistance
encountered on dispensing composition is the nozzle aperture of static mixer.
For this embodiment, it is desired
to dispense composition from a small aperture nozzle, and this adds to the
back pressure. The advantage that
this embodiment delivers of enabling a relatively small volume syringe or
mixer to be employed, allows greater
freedom to operate a small aperture nozzle.
Finally we have found that a seal generated by dispensing composition
internally to the exposed portion
according to this embodiment, is highly effective. The dressing should be
trimmed, as hereinbefore described,
such that the exposed portion overlies intact skin about a wound, and does not
overly the wound itself. In the
case of a dressing having an adhesive or tacky wound contact layer, such as a
silicone contact layer as
hereinbefore described, the wound contact layer adheres to the skin about the
wound and seals the dressing to
skin about the exposed portion and the dispensed seal. Preferably the exposed
portion is bordered by a border
region at the 2 extremities thereof, for example a border of backing sheet or
layer as hereinbefore defined,
preferably having depth in the range 5mm to 25mm, more preferably 7mm to 25mm,
for example 14mm to
25mm. The wound contact layer is perforated or otherwise porous to allow
transmission of fluids to and from the
wound bed, and this may permit flow of composition onto skin directly proximal
to the internal seal. This may
beneficially enhance the seal between the wound contact layer and skin. In the
event that flow of composition to
skin directly proximal to the internal seal is not desired, composition
suitably has a sufficiently high viscosity to
restrict flow, alternatively the wound contact layer may be non-porous or non-
permeable in the region proximal to
an envisaged exposed portion, for example at a bridging portion or trimmable
portion as hereinbefore defined.
Composition may be dispensed to a location as hereinbefore defined in a
dressing having no obscuring layer, or
having window(s) in obscuring layer at bridging portions or trimmable
portion(s). This allows visual control of
nozzle insertion distance within the exposed portion of composition, of volume
dispensed, and/or of lateral flow
enabling a suitable dispensing interval across the face of exposed portion to
be determined. In the case that no
obscuring layer is present it is preferred that the composition incorporates
ADL as hereinbefore defined as
transmission layer, rather than spacer layer which may pose a risk of
penetrating the backing sheet.
In this embodiment preferably the dressing does rot comprise absorbent layer
such as ADL in the bridging
portion or at the trimmable portion.
In a further embodiment of the composition for dispensing into a location as
hereinbefore defined, the location
comprises the backing layer or backing sheet adjacent the exposed portion,
whereby composition flows across
and covers the exposed portion. In some cases composition flows a short
distance into or is drawn a short
distance within the exposed portion. It may be desired to dispense or smooth
composition at the perimeters or
extremities of the exposed portion for example adjoining a border region, and
for example directed slightly back
along the perimeter or extremity. This has the advantage of advancing
composition a short distance at the
perimeter of the exposed portion, ensuring a total seal and also securing the
seal in place. As composition
hardens or cures, the viscosity typically increases and flow ceases whereby
composition is retained at or in the
dispensing location and forms an effective seal.
This further embodiment places performance requirements on the composition and
the resulting seal, additional
to those of the first embodiment of sealing and mode of dispensing.
Specifically composition requires a
continuous film to be dispensed and formed across the surface of the backing
layer or sheet bridging onto the
exposed portion of any additional layers and the exposed portion of the
transmission layer and bridging onto the
-14-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
skin surface. Therefore composition must be sufficiently viscous and/or
cohesive to form an intact film. Such film
may be thin or may be of appreciable depth and/or thickness of for example
from the order of depth and/or
thickness of the backing sheet to the order of depth and/or thickness of the
dressing or of the component layers
at the exposed portion thereof, for example 1 mm to 5mm. Should such film
rupture or fail prior to setting or
curing of composition then the seal will fail. After setting or curing of an
intact film, the exposed nature of the seal
and its presentation as a film place additional requirements of robustness,
both to external influences and also,
to its ability to retain integrity across interfaces between adjacent layers.
These requirements are likely to be
greater in the case of a thin film. Preferably therefore a seal according to
this further embodiment is
characterized by properties of tensile strength, permanent set, and elongation
at break, optionally also
extensibility, in ranges as hereinbefore defined. In contrast a seal generated
according to the first embodiment,
as hereinabove, is supported in large part by the fabric of the dressing
enclosing the seal, whereby requirements
of tensile strength, permanent set, elongation at break, are significantly
lower, also being enclosed within the
lower extensibility dressing, the requirement for extensibility is
significantly lower than for the further embodiment
as herein.
A seal according to this embodiment may be effective from the backing layer
surface across the exposed portion.
As will be apparent, a seal across the exposed portion alone is susceptible to
failure at the interface of the
backing sheet and exposed portion and any intervening layers.
This further embodiment is likely to be more effective when adopted in
relation to a dressing comprising no
additional layers as hereinbefore defined, thereby better resisting strains
introduced by separation at the
interface of additional layer(s) and transmission layer. Additional layer(s)
if present may beneficially be secured
at their interfaces with each other and with transmission layer, by needling,
stitching and other means as known
in the art.
The further embodiment moreover requires that a seal have low profile and /or
have compressibility greater than
or equal to the surrounding dressing. This is of advantage in minimizing
discomfort to the wearer imposed by a
protruding ridge at the exposed portion of the dressing.
In one embodiment the composition may comprise any polymers that follow a
hydrosilylation reaction. One polymer
(i) preferably contains alkenyl groups, the other (ii) preferably contains Si-
H moieties. The group of siloxane
polymers is based on a structure comprising altemate silicon and oxygen atoms
with various organic moieties
attached to the silicon. Curing can be defined as a treatment that decreases
the flow of an elastonner. This change
is generally brought about by linking reactions between polymer molecules.
Where the silicon hydride (Si-H) moiety
is part of a polysiloxane, it is possible for the alkenyl group to either be
part of a siloxane polymer or otherwise part
of a non-siloxane polymer. The position of the alkenyl functional group is not
critical and it may be either at the
molecular chain terminals or in non-terminal positions along the molecular
chain.
A curing system is preferably apportioned between at least one Part A and at
least one Part B and comprises:
one or more alkenyl-group containing polymers (i) having at least one alkenyl
group or moiety per molecule,
one or more SiH-containing polymers (ii) having at least one Si-H unit per
molecule; and
a catalyst (iii) for curing by addition of alkenyl-containing polymer (i) to
SiH-containing polymer (ii).
A "unit" as herein referred represents a group or moiety or part thereof. A
"moiety" as herein referred is a group of
atoms having further atoms disposed on two or more sides thereabout, ie having
two or more valencies unspecified.
-15-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
A "group" as herein referred represents a group of atoms having further atoms
disposed on one side thereof, ie
having one valency unspecified. Si-H units herein have the same meaning as SiH
units
Polymers (i) and (ii) as hereinbefore defined are fluid-phase polymers
incorporating reactive groups which cross-link
in presence of catalyst to form a copolymer more preferably a cured elastomer.
Suitably Part A comprises catalyst
together with polymer (i), and Part B comprises polymer (ii) optionally
together with any remaining polymer (i).
Suitably polymers, catalyst and optional further components are apportioned in
manner to balance volumes and
viscosities of both Parts. Preferably polymer (i) is an alkenylsiloxane-
containing polymer.
Preferably the Parts are combined and intimately admixed prior to or during to
dispensing.
Suitably the components and Parts mix to a dispensible viscosity.
Polymers (i) and / or (ii) are commercially available or may be obtained by
known techniques. Suitably polymers (i)
and / or (ii) are independently selected from known and novel fluid phase
homopolymeric, and copolymeric
polymers, and their entangled systems and mixtures thereof. The compositions,
in turn, cure to form copolymers,
and may also include their entangled systems and mixtures with other non-
reactive polymers if present in the
composition.
Copolymeric polymers include all hybrids derived from two or more monomeric
species, including alternating,
periodic, statistical, random, block, linear, branched, star, graft and
pendant copolymers. Entangled systems include
interpenetrating networks (IPNs) and semi-interpenetrating networks (SIPNs).
It is also the case that these polymers
can incorporate both organic and inorganic moieties.
Preferably polymers (i) and (ii) are selected from silicones, including
siloxanes and modified siloxanes,
polyurethanes (PU) including polyester and polyether urethanes, elastomeric
polyether polyesters, polyglycolic acid,
poll/acetates such as ethyl vinyl acetate, polyacrylate, polyacid derivatives
of polysaccharides, such as
carboxyalkylcellulose, carboxyalkylchitosan and copolymers thereof, and their
hybrids including copolymers,
entangled systems and mixtures thereof.
The composition may make use of an addition cure reaction between
organohydrogensiloxane units and
organoalkenylsiloxane units. These units may be incorporated into a wide range
of polymeric, copolymeric,
entangled and mixed polymers as hereinbefore defined. Preferred siloxane
polymers (i) and (ii) therefore include
these respective units and are more preferably polyorganosiloxanes. Polymer
(i) is preferably a
polydiorganosiloxane polymer comprising alkenyl-containing units, more
preferably is a
polydiorganoalkenylsiloxane polymer. Preferably polymer (ii) is a
polydiorganosiloxane polymer comprising SiH
units, more preferably is a polydiorganohydrogensiloxane polymer.
Examples of hybrid organic-inorganic polymeric systems that have used both
siloxane and organic units include:
acrylate functionalized siloxane copolymers, which have found use in contact
lenses (US 3,808,178); hybrid grafts
where organic polymers are grafted onto a polysiloxane chain or where
siloxanes are grafted onto organic
polymers, for example in silane graft technology for cross linkable HDPE (US
3,646,155) where hybrid grafts have
been used to allow the cross linking of organic polymers through siloxane bond
formation; hybrid block copolymers
-1 6-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
for example silicone-polycarbonate block copolymers (US 3,274,155); and
copolymers of hybrids of silicone and
ethylene copolymers, cross-linked with vinyl-containing silicone copolymers
which have found use in coating textiles
(US 2005/0100692);
IPNs represent a special class of hybrid polymeric systems, these systems use
a combination of mechanical
entanglement and crosslinking in which one polymer is cured about another;
these include thermoplastics entangled
with platinum catalyzed addition cure silicones such as silicone-urethane IPNs
and semi-IPNs including silicone-
urethane and silicone-polyamide systems which are of general application or
have found specific use in coating
textiles (US 4,714,739, US 7,543,843); hydrophilic components immobilised in a
silicone polymer (US 5,397,848)
which have found use as contact lens material; and silicone polymer cured
about a non-reactive polymer of
comparable adhesion, which have found use in coating textiles (US 7,132,170).
Polymers may also be selected from modified silicones (MS) which find use as
adhesives in catheter tubing and the
like.
Preferred compositions comprise a polydiorganosiloxane polymer (i) and/or (ii)
and/or their respective combinations
with the aforementioned polymers. A composition in which polymers comprise or
consist essentially of
polydiorganosiloxane polymers (i) and (ii) has particular advantages, for
example in applications where low toxicity
is an advantage, preferably in medical or dental applications or in non-
medical or non-dental applications requiring
low toxicity or favorable biocompatibility.
Altematively or additionally polymers (i) and (ii) are as commercially
available (Cavi-CareTm A/B, and the like) or
variants thereof, optimised for viscosity and curing to give a fluid-tight
exposed surface (hereinafter skin-formation or
"skinning") as hereinbefore defined.
Polymer (i) and (ii) may comprise respective alkenyl-containing units and
organohydrogensiloxane units situated
along the length of polymer chains, and/or as polymer chain end-capping units
or a combination thereof. Polymer (i)
in-chain and end-capping alkenyl units preferably comprise alkenyl group or
moiety lek selected from C2-20 alkenyl
optionally substituted or including one or more aryl groups or moieties. RAlk
may comprise terminal or non terminal
unsaturation, and may be of the formula i-l:
(i-I) -ekl- CRAlkl = CRAlk22
in which the groups RAlkl and RA lk2 are independently selected from H, C1_20
alkyl and C5_20 aryl groups and
combinations thereof and a moiety RAm is selected from a single bond, C1-20
alkyl and C5-20 aryl groups and
combinations thereof. One of RAlk2 may be a moiety linking to polymer chain.
More preferably each trek is
independently selected from vinyl, allyl, propenyl, and from terminally and
non-terminally unsaturated butenyl,
pentenyl, hexenyl, heptenyl, octenyl, nonenyl and decenyl groups, most
preferably selected from vinyl and hexenyl
groups.
Preferably polymer (i) comprises a polydiorganosiloxane polymer or copolymer
comprising alkenyl-containing units
of the formula (i-11):
(i-II) = Si ¨ Fek, more particularly of the formula (i-III) and/or (i-IV):
-17-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
(i-III) ¨ ¨ Si R1 RAlk ¨ ¨
(i-IV) ¨ O ¨ Si R12 RAlk
wherein RAH' is as hereinbefore defined and one or more groups R1 are organo
groups suitably independently
selected from alkyl and aryl groups, more preferably C1_20 alkyl and C5-20
aryl groups and combinations thereof, for
example from methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl, octyl, nonyl
and / or decyl groups and moieties.
More particularly polymer (i) is selected from the formula i-V and i-VI:
i-V: Pi ¨ ¨ Si R1 RAH' ¨ ¨ Pi
i-VI ¨ O ¨ Si R12 RAlk wherein Pi denotes the remainder of the polymer
chain which may incorporate same or
different units, and R1 is as hereinbefore defined.
Polymer (i) may also comprise a polyorganosiloxane exhibiting, per molecule,
at least two C2-C6 alkenyl groups
bonded to the silicon and having, for example, a viscosity of between 10 and
300 000 mPa-s, that is to say 0.01 to
300 Pa.s, such that when combined in Part A with further Part A components and
optionally additionally in Part B
with further Part B components, Part A, and Part B as appropriate, is (are) of
viscosity in a range as hereinbefore
defined, which can in particular be formed of at least two siloxyl units of
formula:
YdRoSiO (4-d-e)
2
(11I)
in which:
- Y is a C2-C6 alkenyl such as vinyl, allyl or hexenyl groups, preferably
vinyl,
- R is a monovalent hydrocarbon group with no unfavorable effect on the
activity of the catalyst which is generally
chosen from alkyl groups having from 1 to 8 carbon atoms inclusive, such as
the methyl, ethyl, propyl and 3,3,3-
trifluoropropyl groups, cydoalkyl groups, such as the cyc.lohexyl, cycloheptyl
and cydooctyl groups, and aryl groups,
such as xylyl, tolyl and phenyl,
- d is 1 or 2, e is 0, 1 or 2 and d + e = 1, 2 or 3,
optionally all the other units being units of average formula:
R SiO 4-f
f
2
(IV)
in which R has the same meaning as above and f = c, 1, 2 or 3.
Examples of polymer (i) are, for example, dimethylpolysiloxanes comprising
dimethylvinylsilyl ends,
(methylvinyl)(dimethyl)polysiloxane copolymers comprising trimethylsilyl ends
or (methylvinyl)(dimethyl)polysiloxane
copolymers comprising dimethylvinylsilyl ends.
-1 8-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
A convention accepted in the art for denoting the units cf silicones according
to the number of oxygen atoms
bonded to the silicon is used here. This convention uses the letters M, D, T
and Q (abbreviations for "mono", "di",
"tri" and "quatro") to denote this number of oxygen atoms. This nomenclature
of silicones is described, for example,
in the work by Walter Noll, "Chemistry and Technology of Silicones", Academic
Press, 1968, 2nd edition, on pages
1 to 9.
Polymer (i) may also be a silicone resin bearing at least two alkenyl,
preferably vinyl groups. Such silicone resin
comprising at least two different siloxane units chosen from those of M
siloxane unit of formula R3SiO1o, D
siloxane unit of formula R2Si02/2, T siloxane unit of formula RSiO3/2 and Q
siloxane unit of formula S10.412,
wherein R denotes a monovalent hydrocarbon group, with the conditions that at
least one of these
siloxane units being a T or Q siloxane unit and that at least two of the M, D
and T siloxane units comprises an
alkenyl group.
The silicone resin could be selected from the group consisting of:
- an organopolysiloxane resin of formula MTviQ consisting essentially of:
- (a) trivalent siloxane units Tv' of the formula R'5iO3/2;
- (b) monovalent siloxane units M of the forrrula R3SiO1n, and
- (c) tetravalent siloxane units Q of the formula SiO4/2
- an organopolysiloxane resin of formula MDviQ consisting essentially of:
- (a) divalent siloxane units Dvi of the formula RR'Si02/2;
- (b) monovalent siloxane units M of the formula R3S101/2, and
- (c) tetravalent siloxane units Q of the formula SiO4/2
- an organopolysiloxane resin of formula MDDviQ consisting essentially of:
- (a) divalent siloxane units Dvi of the formula RR'Si02/2;
- (b) divalent siloxane units D of the formula R2SiO212
- (b) monovalent siloxane units M of the formula R35i01/2, and
- (c) tetravalent siloxane units Q of the formula SiO4/2
- an organopolysiloxane resin of formula MvIQ consisting essentially of:
- (a) monovalent siloxane units Mvi of the formula RR2Si0v2;and
- (b) tetravalent siloxane units Q of the fornula SiO4/2, and
- an organopolysiloxane resin of formula MviTv'Q consisting essentially of:
- (a) monovalent siloxane units Mvi of the formula R'R25i01,2;
(b) trivalent siloxane units Tvi of the formula R'SiO3/2, and
- (c) tetravalent siloxane units Q of the formula SiO4/2
wherein R denotes a monovalent hydrocarbon group such as methyl and R' denotes
a vinyl group:
Such resins are well-known branched organopolysiloxane oligomers or polymers
which are commercially available.
They are provided in the form of solutions, preferably siloxane solutions.
Polymer (ii) in-chain and end-capping polyorganohydrogensiloxane units are
preferably selected from the formula H-
I and ii-11:
ii-1 ¨0 - Si R2H ¨ 0 ¨
-19-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
¨0 - Si R22H, more preferably polymer (ii) is selected from formula ii-III and
ii-IV:
P" ¨ - Si R2H ¨ - P"
ii-IV Pi' ¨0 - Si R22H wherein
Pil denotes the remainder of the polymer chain which may incorporate same or
different units and one or more
groups R2 are organo groups suitably independently selected from C1-20 alkyl,
C5-20 aryl and combinations thereof,
for example from methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl, octyl,
nonyl and / or decyl groups.
Polymer (ii) preferably comprises a polyorganohydrogensitoxane ¨
polydiorganosiloxane copolymer, incorporating
one or more units ii-I and/or ii-II :
ii-l¨ O - Si R2H ¨ O -
ii-II ¨0 - Si R22 H and one or more units ii-V and/or ii-VI:
ii-V ¨ - Si R22 - -
.ii-V1 ¨ O - Si R23 wherein R2 is as hereinbefore defined, more preferably
copolymer incorporating
polyorganohydrogensiloxane end-capping units, i.e polymer chains terminate
with the group or moiety ii-VII:
ii-Vll= Si ¨ H, more particularly with the unit of formula ii-II:
¨0 - Si R22 H as hereinbefore defined. Most prefarably polymer (ii) comprises
methylhydrogensiloxane-
dimethylsiloxane copolymers.
Polymer (ii) may also comprises a polyorganosiloxane, exhibiting, per
molecule, at least two hydrogen atoms
bonded to the silicon and preferably at least three ESiH units and having, for
example, a viscosity of between 1
and 5000 mPa.s, that is to say between 0.001 and 5 Pa.s, up to 300 Pa.s as
hereinbefore defined, such that
when combined in Part B with further Part B components, Part B is of viscosity
in a range as hereinbefore
defined, which can in particular be formed of siloxyl units of formula:
H X 1 SiO 4-g-i
2
(V)
in which:
- X is a monovalent hydrocarbon group with no unfavorable effect on the
activity of the catalyst which is
generally chosen from alkyl groups having from 1 to 8 carbon atoms inclusive,
such as the methyl, ethyl,
propyl and 3,3,3-trifluoropropyl groups, cycloalkyl groups, such as the
cyclohexyl, cycloheptyl and cyclooctyl
groups, and aryl groups, such as xylyl, tolyl and phenyl,
- g = 1 or 2, preferably= 1, i = 0, 1 or 2 and g + i = 1,2 or 3,
optionally all the other units being units of average formula:
XISiO 4.j
2
(VI)
in which X has the same meaning as above and j = 0, 1, 2 or 3.
Examples of polymer (ii) are polymethylhydrosiloxanes or
methylhydrodimethylsiloxane copolymers.
In the case that polymers include other units additional 10 1111, ilV, iil and
iill for example, these are suitably not
reactive with the respective polymer at ambient temperature or under
sterilising conditions.
-20-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
Suitably the ratio of silicon-bonded hydrogen atoms provided by (ii) to
silicon-bonded alkenyl moieties provided by
(i) is at least 0.5 :1, preferably 1:1,
Preferably embodiments of the curable composition follow the catalysed
addition cure reaction according to the
following scheme:
Pi ¨RAli1-CRAI"=CRAik22 + P" ¨ SiHR2R2/P¨)fcatarYstj
Pi
4RAlki ...cH RM.! cRAII(22 SiR2R2/P P"
more preferably:
RUP RI R2
pi Si
/H
RAik + Si \
O R2/P
catalyst
R2
\ /RI
OP'
Si \ Si
I
o \ RAI"- CHRAlki CR2
R2/P
wherein integers are as hereinbefore defined and RuP is selcted from Pi and R1
as hereinbefore defined and R2/P is
selected from Pt' and R2 as hereinbefore defined.
The polymers (i) and (ii) and catalyst (iii) may be apportioned in at least
one Part A and at least one Part B in
manner to provide respective Parts A and B which in isolation are not reactive
at ambient temperature, nor under
sterilisation conditions, such as heat or radiation. Apportioning may also be
determined according to volume and
viscosity.
Polymers (i) and (ii) and catalyst (iii) may be apportioned in at least one
Part A and at least one Part B in manner
such that polymer (ii) is absent from Part A and polymer (i) is absent from
Part B or Part B incorporates a trace
amount of polymer (i) represented as molar ratio (Si-H unit or
moiety)/(alkenyl unit or moiety) of greater than or
equal to 2000. Such composition may be sterilised at effective gamma or
radiation dose for example as
disclosed in W02012/069794, the contents of which are incorporated herein by
reference.
The at least one Part A and at least one Part B may be of substantially equal
volume and viscosity or of different
volume and/or viscosity. Part A or Part B may incorporate a suitable viscosity
moderator or diluent, in amount to
increase or reduce volume and /or viscosity. By this means Part A and Part B
having different volume and
=
viscosity may be volume and viscosity matched for improved ease and intimacy
of mixing and dispensing. A
suitable diluent is for example a silicone oil which is available in any
desired viscosity for thickening or thinning
-21-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
effect. Alternatively or additionally at least one Part A and at least one
Part B are sealed in respective
receptacles or on respective supports which are thermally stable at an
elevated temperature of 121 C or more
for a period of up to 28 hours, for example as disclosed in W02012/069793, the
contents of which are
incorporated herein by reference.
The composition may thereby be rendered terminally sterile by being sterilised
in its primary packaging and this
property may be characterised by a Sterility Assurance Level (SAL). The SAL is
defined in ISO 11139:2006 as
the probability of a single viable microorganism occurring on an item after
sterilization. The term SAL takes a
quantitative value, in the format of 1r, where typically n = 3, 4, 5 or 6,
preferably SAL = 10-3 or 10-6.
A catalyst as hereinbefore defined may be any catalyst which is effective in
catalysing the addition curing reaction
as hereinbefore defined, more preferably as hereinabove illustrated. Suitable
catalysts are selected from any known
form of platinum, rhodium, palladium, nickel and like addition curing
hydrosilylation catalysts, for example as
disclosed in US 5,153,231, US 2006/0217016, US 3,928 629 and US 4,529,553 the
contents of which are
incorporated herein by reference.
A platinum catalyst may be selected from platinum black platinum as deposited
on carriers including silica such as
silica gel or carbon such as powdered charcoal, platinic chloride or
chloroplatinic acid and alcohol solutions thereof,
salts of platinic and chloroplatinic acids and platinum complexes such as
platinum/olefin, platinum/alkenylsiloxane,
platinum/beta-diketone, platinum/phosphine and the like. Chloroplatinic acid
may be the hexahydrate or anhydrous
form. A platinum complex may be prepared from chloroplatinic acid and its
hexahydrate, or from platinous chloride,
platinum dichloride, platinum tetrachloride and their neutralised complexes
with divinyltetramethyldisiloxane,
optionally diluted with dimethylvinylsiloxy endcapped porydimethylsiloxane.
A palladium catalyst may be selected from palladium on carbon, palladium
chloride and the like.
A rhodium catalyst may be selected from rhodium chloride and one or more
complexes of rhodium having the
general formula iii-I or iii-II:
(iii-I) RhX3 (SR2)3
(iii-II) Rh2(C0)4X2
wherein each X represents a halogen atom and each R represents an alkyl or
aryl radical or combination thereof
having from 1 to 8 inclusive carbon atoms or the R'3SiQ group in which Q
represents a divalent aliphatic
hydrocarbon radical having from 1 to 6 inclusive carbon atoms and R'
represents an alkyl or aryl radical or
combination thereof having from 1 to 8 inclusive carbon atoms or a (CH3)3Si-
group, not more than one R' per
molecule being (CH3)3Si-. For example rhodium chlorida/di(n-butyl)sulfide
complex and the like.
A nickel catalyst is preferably a zero valent nickel selected from M2Ni( )
such as bis(1,5-cyclo-octadienyl)nickel
(Ni(COD)2) and from MNimG wherein M is a bidentate alkene cyclic hydrocarbon
ring of C842 and G is selected
from monodentate and bidentate phosphorous groups 'laving hydrogen atoms,
substituted or unsubstituted
hydrocarbon radicals or mixtures thereof bonded to the phosphorous atoms of
the phosphorous groups.
The composition may include a catalyst inhibitor. Suitable inhibitors are
known in the art. For example a catalyst
inhibitor may be selected from a polymethylvinylsiloxane cyclic compound and
an acetylenic alcohol, such as
-22-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
methyl butynol for example as in Cavi-Care or disclosed in US 5153231, the
contents of which are incoporated
herein by reference.
Preferably the composition comprises an addition-reaction retardant or a
crosslinking inhibitor chosen, for example,
from the following compounds:
- polyorganosiloxanes substituted with at least one alkenyl that may
optionally be in cyclic form,
tetramethylvinyltetrasiloxane being particularly preferred,
- organic phosphines and phosphites,
- unsaturated amides,
- alkyl maleates, and
- acetylenic alcohols.
These acetylenic alcohols (see FR-A-1 528 464 and FR-A-2 372 874), which are
among the preferred thermal
blockers of the hydrosilylation reaction, have the formula:
(R')(R")C(OH)-CECH
in which formula
- R' is a linear or branched alkyl radical, or a phenyl radical;
- R" is H or a linear or branched alkyl radical, or a phenyl radical; the
radicals R', R" and the carbon atom
alpha to the triple bond possibly forming a ring; and
- the total number of carbon atoms contained in R' and R" being at least 5
and preferably from 9 to 20.
Examples that may be mentioned include:
- 1-ethyny1-1-cyclohexanol;
- 3-methyl-1-dodecyn-3-ol;
- 3,7,11-trimethy1-1-dodecyn-3-ol;
- 1, 1-dipheny1-2-propyn-1-ol;
- 3-ethy1-6-ethy1-1-nonyn-3-ol;
- 2-methyl-3-butyn-2-ol;
- 3-methy1-1-pentadecyn-3-ol.
These a-acetylenic alcohols are commercial products. Such a retardant is
present in a maximum proportion of
3000 ppm relative to the total weight of the polyorganosiloxanes in the
silicone composition. Methyl butynol could
be chosen as in Cavi-Care.
The composition may be non-foamable or may be foamable, comprising (iv) an
expansion or "blowing" agent,
selected from any agent which evolves gas or vapour as part of or during the
curing reaction, for example selected
from H-donors, OH-containing agents, H-bonding agents such as:
- alcohols including methanol, ethanol, n-propanol, isopropanol, n-butanol, 2-
butanol, tert-butanol, n-hexanol, n-
octanol and benzyl alcohol. n-Propanol, n-butanol, n-hexanol and n-octanol are
particularly preferred,
- polyols such as diols including ,4-butanediol, 1,5-pentanediol and 1,7
heptanediol,
- silane or polysilane having at least one silanol group, or
-23-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
- water.
The composition forms, after hydrosilylation, a silicone elastomer which may
be foamed or have gel properties. A
foamed composition does not transmit air through the body of the foam or
through the foam surface or both, for
example incorporates cells closed either by chemical or mechanical means. The
term "silicone gel" denotes a
crosslinked silicone product characterized by a degree of penetration of, for
example, between 20 and 500 tenths of
a mm (measured by ASTM D 2137 penetrometry, weight of the rod and of the cone:
62.5 g).
When the composition is prepared for a silicone gel it may have at least one
nonfunctionalized polyorganosiloxane
comprising:
a) end siloxyl units of type M = (R6)3Si01/2
in which the R6 radicals which are identical or different, correspond to an
optionally substituted linear or branched
C1-C6 alkyl group and/or a substituted or unsubstituted aryl group, and
b) identical or different siloxyl units of type D = (R7)2Si02/2
in which the R7 radicals correspond to the same definition as R6.
The physical properties of these gels are adjusted according to the use by
varying the levels of siloxyl units carrying
Si-alkenyl and SiH functional groups and when it is present by varying the
percentage by weight of
nonfunctionalized polyorganosiloxane, which is well known in the prior art.
To enhance the adhesive properties of a silicone gel, the composition may
further comprise a monofunctional
polyorganosiloxane carrying a single Si-alkenyl group per molecule as taught
by European patent application EP-
1633830-A2.
Further, a composition may also comprise inorganic filler such as reinforcing
or bulking fillers. These fillers can be
provided in the form of very finely divided products, the mean particle
diameter of which is less than 0.1 pm. These
fillers include in particular fumed silicas and precipitated silicas; their
specific surface is generally greater than 10
m2/g and generally lies within the range 20-300 m2/g.
These fillers can also be provided in the form of more coarsely divided
products, with a mean particle diameter of
greater than 0.1 pm. Mention may in particular be made, as examples of such
fillers, of ground quartz, calcium
carbonate, diatomaceous silicas, calcined clay, titanium oxide of the rutile
type, iron, zinc, chromium, zirconium or
magnesium oxides, the various forms of alumina (hydrated or nonhydrated),
boron nitride, lithopone or barium
metaborate; their specific surfaces are generally less than 30 m2/g.
The filler may have a hydrophobic surface, which may be obtained by treating
the filler, e.g. with suitable silanes,
short chain siloxanes, fatty acids or resinous silicone materials.
Hexamethyldisilazane treated fumed silica may be considered, or if
translucence is to be maintained, vinyl "Q"
reinforcing resins may be used. A filler may be hydropiobic. Suitable
materials and processes for rendering the
surface of fillers hydrophobic have been described in the literature, and are
known to the person skilled in the art.
The fillers can also be composed of a mixture of several types of fillers with
different particle sizes.
-24-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
The composition may comprise a thixotropic agent. A thixotropic agent confers
on a composition propertes whereby
it becomes viscous during application and reverts to higher viscosity after
application when no longer being worked.
Thixotropes include fillers such as silica, and certain silicone-based
substances.
A composition may include additional components including other adjuvants,
preservatives including propyl gallate,
extenders, rheology regulators, adhesion promoters or adhesion reducers,
moisture vapor permeability (MVP) or
moisture vapor transmission rate (MVTR) promoters to prevent maceration of
skin having composition applied
thereto, whereby skin can transpire and pass liquid but still function as a
sealant and bacterial barrier, and the like.
Suitably such additional components confer properties as hereinbefore defined
on the composition.
The composition may comprise active agents, which may have any desired
activity for the intended purpose, and
include active pharmaceutical ingredients (API's) and the like.
Antimicrobial agents, biocides and disinfectants may be selected from silver,
in particular nano crystalline silver,
and derivatives including silver complexes and salts such as ionic silvers,
silver zeolite, silver oxide, silver nitrate,
silver acetate, silver chloride, silver sulphadiazine), biguanides including
polyhexamethylene biguanide,
chlorhexidine digluconate and its acetate salts chlorhexidine acetate and
diacetate, manuka honey, peroxides
(e.g. hydrogen peroxide), iodine (e.g. povidone iodine), sodium hypochlorite,
copper, copper complexes; zinc
(e.g. zinc oxide, zinc pyrithione), gold, gold complexes; phosphates, amines,
amides and sulphonamides (e.g.
hexatidine,proflavine. mafenide, nitrofurazone, norfloxacin); antibiotics
(e.g. gentamicin, bacitracin, rifampicin;
alcohols and acids (e.g. ethanol, phenoxy ethanol, mupirocin).
Nutrients, pain killers and other pain management techniques suitably include
analgesics and anasthetics and
may be selected from amethocaine, lignocaine, non-steroidal anti-inflammatory
drugs, anti inflammatories such
as hydrocortisone, paraffin to reduce adherence to tie skin, urea to reduce
dehydration of the skin; buffering
components to promote healing of the skin.
Heamostats may be selected from chitin, chitosan, kaolin; antifibrinolytics
such as amino acids, aminocaproic
acid, tranexamic acid, aminomethylbenzoic acid; Proteinase inhibitors
including aprotinin, alfa1 antitrypsin, C1-
inhibitor, camostat; Vitamin K and other hemostatics including vitamin K,
phytomenadione, menadione;
fibrinogen including human fibrinogen; local hemostatics including absorbable
gelatin sponge, oxidized cellulose,
tetragalacturonic acid hydroxymethylester, adrenalone, thrombin, collagen,
calcium alginate, epinephrine; blood
coagulation factors including coagulation factor IX, II, VII and X in
combination, coagulation factor VIII, factor VIII
inhibitor bypassing activity, coagulation factor IX, coagulation factor VII,
von Willebrand factor and coagulation
factor VIII in combination, coagulation factor XIII, eptacog alfa (activated),
nonacog alfa, thrombin and systemic
hemostatics: etamsylate, carbazochrome, batroxobin romiplostim, eltrombopag.
Active agents may further include combination materials including
superabsorbers, odour management agents,
wovens and non wovens, gellable fibres; growth factors, wound debridements ¨
mechanical, autolytic and
enzymatic; resorbable dressings and micro structure to influence cell
ingrowth; cells, tissue (e.g. autologous
treatments); indicators; dyes and colourants and coloured indicators,
whiteners such as zinc oxide and titanium
oxide.
-25-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
The composition may be in a form that it may be dispensed in any known manner,
such as by pallet knife,
syringe, static mixer, roll-on applicator, spray, wipe, brush, foam, sponge,
non-woven, part integrated or fully
integrated into dressing, or manually applied. An applicator using a sponge is
demonstrated with Chloraprep i.e.
http://www.chloraprep.co.uk. For a two part curing system that requires
mixing, a static mixer such as a double
barreled syringe with a mixing head may be used.
Preferably therefore the curable two part composition is provided as at least
one Part A and at least one Part B
sealed in or on respective receptades or supports suitable for cooperatively
dispensing from a cooperative
dispensing device, preferably sealed in respective barrels or respective
cassettes for a static mixer such as a
double barrel syringe, more preferably provided together with a cooperating
dispensing device such as a static
mixer.
More preferably a syringe with a nozzle to allow insertion of material into
exposed transmission layer, or a
spreader tip to allow spread of material across the severed transmission layer
edge, or a combination thereof
(spreader having plural projecting apertures) may be used (e.g. Double-Syringe
Prefilled Delivery System (L-
System), Medmix Systems Ag, fitted with static mixer and spreader tip
http://www.medmix.ch/L-SYSTEM.html).
For certain embodiments there is a clear advantage in using an applicator with
an integral spreader. Where
material is applied directly to the severed dressing edge, particularly in the
case of an extended severed edge, it
is advantageous to manipulate this material to ensure optimal placement. An
integral spreader minimises cross
contamination when the sealant is manipulated. During the manipulation cross
contamination could relate to:
contamination of the sealant with a microbiological burden, contamination of
the sealant with foreign bodies,
contamination of the sealant with chemicals (such chemicals may have an
influence on the sealant) and
contamination of personnel or equipment with the sealant. For other
embodiments there is advantage in using an
applicator without a spreader, for example for filling body crevices etc.
Where the composition is a curing system chemical contamination may adversely
affect the cure process. For
example where the composition is a platinum catalysed RTV-2 silicone, contact
with latex or nitrile containing
gloves may affect the curing. . The problem caused by the example given has
been documented in the dental
press with regards to the delayed setting of polyvinyl siloxane dental
impression materials when mixed with
certain types of glove (Y. Walid, Z. Al-Ani and R. Gray, Silicone impression
materials and latex gloves. Is
interaction fact or fallacy?, Dent Update, 2012, 39, pp. :39-42).
In a medical setting an integral applicator with spreader therefore overcomes
the obstacle of a clinician being
unable to use a gloved finger (subject to the chemical composition of the
glove) to manipulate the sealant,
overcomes the possibility of using an ungloved finger, thus eliminating direct
hand to patient contact (not only
would this approach be inappropriate for most clinical settings, it would
likely result with transfer of the curing
composition to the clinician's fingertip) and overcomes the requirement to
contaminate any other medical devices
or implements with the curing composition.
Dispensing may be by means of syringe or static mixer having a nozzle head
comprising a combination of
spreader tip with plural nozzles as hereinbefore and hereinbelow defined, for
example a spreader having plural
-26-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
projecting apertures. Preferably a nozzle head comprises 2 to 10 nozzles for
example 3 to 6 nozzles such as 3 or
nozzles. Nozzles preferably have low aperture to dispense sealant to the
interior portion within the exposed
portion of transmission layer. For example the spread of the nozzle head may
match the width of the bridging
portion enabling dispensing on a single insertion. This embodiment of
dispenser for and mode of dispensing
composition benefits from a decreased burden and decreased requirement for
accuracy on the part of the
operator, an increase in mechanical accuracy of dispensing location and
continuous seal formation. It may also
slightly reduce the back-pressure at the syringe allowing the use of higher
viscosity composition. A substantial
border region as hereinbefore defined may contribute to seal integrity.
Preferably a nozzle head has moderate width or spread of head for dispensing
into exposed portion of a
transmission layer on a curve, e.g. a body contour, for example having from 2
to 4 nozzles, such as 2 or 3
nozzles. An alternative multi nozzle head is flexible or deformable in two
locations facilitating dispensing into
exposed portion of a transmission layer on a curve, e.g. a body contour and
/or dispensing into a location having
obstructed access. Such nozzle head comprises a flexible arm or restraint
through which the plural nozzles
emerge. The arm is joined to the main nozzle head and thereby to releasably to
the static mixer, optionally via
flexible tubes. The arm may be bent to conform to an arc. The tubes may
similarly be bent to conform to
generate an angled nozzle. The tubes may beneficially increasing the entry
angle for dispensing.
The flexible arm is typically not elastic, i.e. it retains the shape conferred
for dispensing until bent to return to its
original shape or a different conformation. The flexible arm could be formed
of a deformable polymer or putty or
the like or it could be a mechanical flexible or deformable arm (i.e.
htto://snakeclamo.com/ or
htto://joby.com/gorillaood).
Preferably a dispenser has low profile and can be contained within an
imaginary cone. This dictates the
maximum dimensions that may advantageously be considered in the design of the
dispenser to allow a shallow
entry angle relative to the skin to allow a nozzle to be inserted into an
exposed portion of transmission layer in a
dressing adhered to a patient.
Preferably the sealant is a TNP sealant which generates or enhances a fluid-
tight, preferably an air-tight, seal.
In one embodiment a composition comprises a RTV-2 silicone such as Silpuran
2445mm which may optionally be
modified to have viscosity as hereinbefore defined. Modification of viscosity
is as known in the art and is suitably
by incorporating filler such as for example fumed silica or optionally
translucent filler or resin or reinforcing resin
as hereinbefore defined, to achieve the hereinbefore defined viscosity, or by
combining Parts A and B and
allowing to pre-react to the hereinbefore defined viscosity before
application, or the like. Increasing cure rate is
as known in the art, for example increasing the amount of catalyst or reducing
the amount of catalyst inhibitor
present, if any.
METHOD OF PREPARATION
A further aspect is the preparation of a composition as hereinbefore defined.
Methods for preparing non-curing
or curable compositions as hereinbefore defined in 1 or more Parts are known
in the art. Preferably the method
comprises loading composition or respective parts thereof into an applicator
or cassettes therefore as
hereinbefore defined.
A further aspect is a method for preparing a curable composition as
hereinbefore defined comprising the steps
of:
-27-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
combining polymers (i) and (ii) and catalyst (iii) as hereinbefore defined to
form at least one Part A and at least
one Part B; in manner suitable for cooperatively dispensing, for example for
cooperatively dispensing from a
double barrel syringe.
METHOD OF STERILISATION
A further aspect is a method of sterilising the curable composition as
hereinbefore defined comprising heating
the one or more parts, for example the at least one Part A and at least one
Part B sealed in respective thermally
stable receptacles or supports at an elevated temperature of 121 C or more for
a period of up to 28 hours, or by
irradiating wherein in the case of a 2 part curable composition the polymers
(i) and (ii) and catalyst (iii) are
apportioned in at least one Part A and at least one Part B in manner such that
polymer (ii) is absent from Part A
and polymer (i) is absent from Part B or Part B incorporates a trace amount of
polymer (i) represented as molar
ratio (Si-H unit or moiety)/(alkenyl unit or moiety) of greater than or equal
to 2000 with a radiation source
selected from the group consisting of gamma, x-ray, and e-beam radiation in
effective sterilising dose.
DEVICE
A further aspect is in the form of a device suitable for use in the field of
woundcare, comprising a dispensing
device having one or plural barrel(s) or cassette(s), advancing means and
optional mixing means, said barrel(s)
or cassette(s) comprising the composition as hereinbefore defined, in the case
of a two or more part composition
such that Parts A and B are contained in respective barrels or cassettes, the
device having means for contacting
respective Parts.
Preferably optional mixing means, contacting means and/or advancing means are
provided integral with or
separate from the device. Mixing means may be static or active. The device may
incorporate a dwell chamber for
mixed Parts A and B to partially cure to higher viscosity before being
dispensed.
Preferably the device is disposable comprising integral barrel(s) or
cassette(s).
Preferably the device comprises an applicator for applying composition
comprising means to configure composition
on application, for example comprising an applicator with nozzle or integral
spreader or a combination thereof.
Preferably a device comprises a nozzle head comprising a combination of
spreader tip with plural nozzles as
hereinbefore and hereinbelow defined, for example a spreader having plural
projecting apertures. Preferably a
nozzle head comprises 2 to 10 nozzles for example 3 to 6 nozzles such as 3 or
5 nozzles. Nozzles preferably
have low aperture to dispense sealant to the interior portion within the
exposed portion of transmission layer. For
example the spread of the nozzle head may match the width of the bridging
portion enabling dispensing on a
single insertion. This embodiment of dispenser for and mode of dispensing
composition benefits from a
decreased burden and decreased requirement for accuracy on the part of the
operator, an increase in
mechanical accuracy of dispensing location and continuous seal formation. It
may also slightly reduce the back-
pressure at the syringe allowing the use of higher viscosity composition. A
substantial border region as
hereinbefore defined may contribute to seal integrity.
Preferably a nozzle head has moderate width or spread of head for dispensing
into exposed portion of a
transmission layer on a curve, e.g. a body contour, for example having from 2
to 4 nozzles, such as 2 or 3
nozzles. An alternative multi nozzle head is flexible or deformable in two
locations facilitating dispensing into
exposed portion of a transmission layer on a curve, e.g. a body contour and
/or dispensing into a location having
obstructed access. Such nozzle head comprises a flexible arm or restraint
through which the plural nozzles
-28-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
emerge. The arm is joined to the main nozzle head and thereby to releasably to
the static mixer, optionally via
flexible tubes. The arm may be bent to conform to an arc. The tubes may
similarly be bent to conform to
generate an angled nozzle. The tubes may beneficially increasing the entry
angle for dispensing.
The flexible arm is typically not elastic, i.e. it retains the shape conferred
for dispensing until bent to return to its
original shape or a different conformation. The flexible arm could be formed
of a deformable polymer or putty or
the like or it could be a mechanical flexible or deformable arm (i.e.
httri://snakeclamp.com/ or
htto://joby.com/gorillaood).
Preferably a dispenser has low profile and can be contained within an
imaginary cone. This dictates the
maximum dimensions that may advantageously be considered in the design of the
dispenser to allow a shallow
entry angle relative to the skin to allow a nozzle to be inserted into an
exposed portion of transmission layer in a
dressing adhered to a patient.
In a further embodiment there is provided a novel device as hereinbefore
defined comprising a a nozzle head
comprising a combination of spreader tip with plural nozzles as hereinbefore
and hereinbelow defined, for example
a spreader having plural projecting apertures. Preferably a nozzle head
comprises 2 to 10 nozzles for example 3 to
6 nozzles such as 3 or 5 nozzles. Further features are as hereinbefore
described.
If required these systems may be used together with a suitable skin-compatible
sealant at the perimeter as
defined in PCT GB2012/000866.
A dressing as hereinbefore defined may be any wound dressing, preferably is a
wound dressing having a Si
wound contact surface, more preferably is a TNP dressing, optionally modified
to comprise a perimeter region as
hereinbefore defined. Known TNP dressings include: Smith & Nephew Disposable
Kits for TNP such as Smith &
Nephew, RENASYS-F/AB, Abdominal Dressing Kit; Smith & Nephew, RENASYS-F/P,
Foam Dressing Kit With
Port; Smith & Nephew, RENASYS-G, Gauze Dressing Kit; Smith & Nephew, PICOTM
dressing kit; and KCI Kits
for TNP including, V.A.C.TM GranuFoam Dressings Kits; and the like. Additional
dressings and methods of
treating wounds with negative pressure are disclosed in the following
applications that are hereby incorporated
by reference: U.S. Application Serial No. 13/381,885, filed 30 December 2011
and published as
US2012/0116334; U.S. Application Serial No. 12/886,088, filed 20 September
2010 and published as
US2011/0213287; U.S. Application Serial No. 13/092,042, filed 21 April 2011
and published as
U52011/0282309; U.S. Application Serial No. 12/744,277, filed 20 September
2010 and published as
US2011/0028918; and U.S. Application Serial No. 12/744,218, filed 20 September
2010 and published as
US2011/0054421, also W02011/000622, WO 2011i000621, W02011/135285,
W02011/135286, US7964766
and US 7615036 (all Smith & Nephew) the contents of which are incorporated
herein by reference.
Conventional TNP dressings are applied with a drape placed thereover, of which
the second face is air-tight.
Such dressings can additionally comprise a tissue (wound) contact layer, a
negative pressure distribution and
transmission layer and an optional wound exudate absorbing layer as
hereinbefore defined.
Preferably the composition is dispensed to a composite TNP dressing such as
the PIC0rm dressing. A composite
dressing incorporates an integral air-tight backing layer (also referred to
herein as a wound cover or drape), that
may be made of a gas impermeable membrane and integral TNP therapy layers,
such as one or more negative
pressure transmission or distribution layers, a tissue (wound) contact layer,
an absorbent material layer such as
a wound exudate absorbing layer or acquisition distribution layer (ADL) any of
these optionally including a
-29-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
superabsorbent polymer (SAP), said layers positioned beneath the backing
layer, and the backing layer or
wound cover optionally allows transpiration or liquid evaporation from wound
exudate, as for example with the
PICO TM dressing.
For example, one or more transmission layers or other layers may be positioned
or enclosed between a backing
layer and an optional wound contact layer. The transmission layer(s) may be in
turn enclosed between the
backing layer and (optional wound contact layer and) a wound site over which
the dressing is configured to be
positioned, for example sealed therebetween. The composite dressing may be
supplied together with a number
of adhesive strips comprised of drape material or may omit such strips with
sealing by means of a sealant as
disclosed in PCT/GB2012/000866, the contents of which are incorporated herein
by reference.
The composition may therefore be applied to any dressing which it is desired
to cut to size or shape or to profile
or articulate. Cutting is simply by removing the excess portion and retaining
the required portion including
negative pressure port.
Preferably the composition is dispensed to a trimmable dressing, having a main
dressing portion in fluid (gas)
communication with additional dressing portions or cells. One or more
additional portions or cells may be
removed to provide a dressing having size or shape or profile or articulation
to be compatible with a wound or
wound site to be dressed. Preferably portions or cel s may be retained to
provide a large surface area, or
elongate, dressing to dress a similarly large surface area or elongate wound,
or portions or cells may be
removed to dress a correspondingly reduced surface area or reduced length
wound; preferably one or more
additional portions or cells may be conformed to provide a shaped dressing to
dress a similarly shaped wound or
to dress a wound incorporating or adjacent a protrusion such as a fixation
device, for example a pin, or such as a
body part such as a digit; preferably one or more additional portions or cells
may be conformed to provide a
profiled dressing to dress a similarly profiled wound or wound site, such as a
wound located on complex body
topography; preferably one or more additional portions or cells may be
articulated to dress a similarly articulated
wound or wound site such as a wound located on a joint.
In an advantage the composition may be used to seal a trimmable dressing as
disclosed in U.S. Provisional
Application Serial No. 61/800,040, filed March 15, 2013, titled "WOUND
DRESSING AND METHOD OF
TREATMENT," the contents of which are incorporated herein directly and by
reference.
A trimmable dressing is preferably a wound treatment apparatus for treatment
of a wound site comprising:
a backing layer having an upper surface and a lower surface and defining a
perimeter configured to be
positioned over skin surrounding a wound site;
a transmission layer configured to be positioned below the backing layer; and
one or more ports configured to transmit negative pressure through the backing
layer for the application
of topical negative pressure at the wound site;
wherein the apparatus comprises a plurality of cells or regions separated by
one or more trimmable
portions. Trimmable portion(s) may be bridging portions as hereinbefore
defined.
In some embodiments, the plurality of cells forms a plurality of repeating
negative pressure treatment modules.
In one embodiment, one or more of the modules can be removed and the removed
module(s) can subsequently
be used to provide negative pressure to the wound site. In another embodiment,
one or more modules can be
-30-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
removed and the remaining module(s) can subsequently be used to provide
negative pressure to the wound site.
In further embodiments, the trimmable portions may have a maximum width of 50
mm (or approximately 50 mm),
40 mm (or approximately 40 mm), 30 mm (or approximately 30 mm), 20 mm (or
approximately 20 mm), or even
15 mm (or approximately 15 mm). In some embodiments, the trimmable portion may
be from 10 mm to 20 mm
(or approximately 10 mm to approximately 20 mm). The one or more trimmable
portions may comprise one or
more bridging portions having a smaller width as compared to the width of an
adjacent cell or region. For
example, the bridging portion may have a maximum width of 1/8, 1/4, or 1/3 (or
approximately 1/8, 1/4, or 1/3) of
a width of an adjacent cell or region. The plurality of cells or regions may
comprise an absorbent material, the
absorbent material positioned between the transmission layer and the backing
layer. The one or more trimmable
portions may comprise an absorbent material, the absorbent material positioned
between the transmission layer
and the backing layer. In other embodiments, no absorbent material is
positioned between the transmission
layer and the backing layer. Some embodiments may fisther comprise an
acquisition distribution layer having a
similar footprint to the transmission layer, the acquisition distribution
layer configured to be positioned above the
transmission layer. The apparatus is preferably as further hereinbelow
defined.
Exposed portion(s) as hereinbefore defined are the result of removing a
portion of the wound dressing, which
may be by any envisaged means, for example cutting the wound dressing or
tearing along a weakened line.
Composite wound dressings may comprise a border for affixing around a wound,
about a central wound contact
portion. The dressing as hereinbefore defined may include a backing layer and
wound contact layer of similar
footprint or surface area to the transmission layer or other layers enclosed
therebetween, for example is a
borderless dressing) or of greater footprint or surface area than the
transmission layer enclosed therebetween
(for example is a bordered dressing). Exposed portion(s) as hereinbefore
defined result from removing a portion
of the wound dressing as hereinbefore defined directly enclosing the
transmission layer or other layers, for
example by cutting into or through the backing layer and wound contact layer
and the transmission layer
therebetween.
Embodiments of dressings described herein address the problem of providing
dressings in a range of sizes or
shapes to accommodate irregularly shaped wounds or body topography, for
example vein harvest wound
dressings accommodating variations in height and leg-length of individuals,
the provision of which is impractical
both to the manufacturer and to the user. Embodiments enhance adaptability of
existing dressings, including
more recently introduced multisite dressings such as trilobes and quadrilobes.
Certain embodiments enable a
portion of a dressing to be removed to create a main wound dressing of desired
size or shape or profile or
articulation, and sealing exposed portion(s) thereof to contain a negative
pressure.
The portion(s) of the wound dressing may be removed to size the main wound
dressing portion for positioning
over a wound as hereinbefore defined, for example an incisional wound, an
elongate leg wound, an arcuate
incisional wound and the like. Similarly the portion(s) of the wound dressing
may be removed to shape the main
wound dressing portion for positioning over a wound as hereinbefore defined,
such as a flap wound, about a
protruding device such as a fixation device or a protruding body part, to
profile the main wound dressing for
positioning over a wound as hereinbefore defined, for example on complex body
topography, or to articulate the
main wound dressing for positioning over a wound as I-ereinbefore defined for
example on a flexing joint.
-31-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
The wound treatment apparatus may be rolled into a tape which can be cut along
the one or more bridging
portions. Cutting along the bridging portion may sever adjacent cells.
The composition may alternatively be advantageously dispensed to seal a
dressing for treatment of a wound site
comprising:
a backing layer having an upper surface and a lower surface otherwise a
backing sheet as hereinbefore
termed and defining a perimeter configured to be positioned over skin
surrounding a wound site;
a transmission layer configured to be positioned beneath the backing layer; or
otherwise positioned at
or on one side of one face of the backing sheet and
a plurality of ports configured to transmit negative pressure spaced apart on
the backing layer.
The wound treatment apparatus may be configured to be rolled into a tape. The
plurality of ports each may
comprise an opening in the backing layer covered with a releasable tab. The
transmission layer may comprise
one or more bridging portions having a smaller width than adjacent portions of
the transmission layer. A negative
pressure may be established at a wound site by means of any one of the
plurality of ports, the remainder of
which may remain sealed or may be removed with a section of dressing. The
wound treatment apparatus may be
used in any desired length by cutting between adjacent ports.
In above dressings, the wound treatment apparatus further comprises an
optional wound contact layer, with the
transmission layer(s) positioned between the backing layer and the wound
contact layer. The transmission
layer(s) may be in direct or indirect contact with a lower surface of the
backing layer. In some embodiments, the
one or more transmission layers comprise a first layer comprising a spacer
material configured to vertically wick
fluid. The one or more transmission layers may further comprise a second layer
comprising an acquisition
distribution material configured to horizontally wick fluid, the second layer
positioned above the first layer. One of
the first layer and the second layer, or both, may be present in the one or
more bridging portions. In other
embodiments, the one or more transmission layers comprise an acquisition
distribution material configured to
horizontally wick fluid. In some embodiments, the pert may comprise an opening
in the backing layer. The port
may comprise a port member attached to the backing layer over an opening in
the backing layer. The port
member may be sealed to the upper surface of the backing layer. Some
embodiments may further comprise an
absorbent material between the backing layer and the transmission layer having
a similar footprint to that of the
transmission layer(s). Absorbent material may be present or absent in bridging
portion(s) as hereinbefore
defined. Some embodiments of the one or more transmission layers may further
comprise an acquisition
distribution layer between the backing layer and the optional wound contact
layer and the transmission layer
and/or absorbent layer having a similar footprint to that of the absorbent
material and/or absorbent layer. The
one or more transmission layers may further comprise a spacer material
configured to distribute negative
pressure, the spacer material having a similar footpr nt to the acquisition
distribution material, the spacer material
configured to be positioned beneath the acquisition distribution material.
.Acquisition distribution layer or material
may be present or absent in bridging portion(s) as hereinbefore defined. The
acquisition distribution material may
be provided as the transmission material or layer
The transmission layer (hereinafter layer(s)) may have a rectangular shape
having a longitudinal axis extend
along its length. The transmission layer may comprise one or more bridging
portions centered on the
-32-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
longitudinal axis. The transmission layer may comprise three or more bridging
portions centered on the
longitudinal axis. The one or more bridging portions may also be offset from
the longitudinal axis. The one or
more bridging portions may have a width that is less than 1/3 the width of
adjacent portions of transmission layer.
The one or more bridging portions may have a width that is less than 1/4 the
width of adjacent portions of
transmission layer. The one or more bridging portions may have a width that is
less than 1/8 the width of
adjacent portions of transmission layer. As is used herein, a smaller width
represents a narrowing of or neck or
constriction in transmission layer with respect to adjacent portions thereof.
The transmission layer may have a T-
shape with a bridging portion on each leg of the T. The transmission layer may
have a T-shape with at least one
bridging portion on each leg of the T. The transmission layer may comprise a
plurality of cells each separated by
one or more bridging portions. The transmission layer may comprise a plurality
of cells, and wherein each of the
plurality of cells is connected to at least one adjacent cell by one or more
bridging portions, preferably the
bridges provide for gas communication between adjacent cells.
Some embodiments may further comprise a fluidic connector configured to supply
negative pressure to the port.
Some embodiments may further comprise a source of negative pressure configured
to supply negative pressure
through the port. Negative pressure may be established at a wound site by
means of any one of the plurality of
ports, or by means of multiple ports of the plurality of ports, the remainder
of which may remain sealed or may be
removed with a section of dressing. Some embodiments may further comprise one
or more separate or integral
adhesive strips or sealing strips. Strips are configured to retain and seal
the backing layer to skin surrounding a
wound after the apparatus is cut along or across the one or more bridging
portions, i.e between adjacent cells or
ports. Strips may be comprised of backing layer material, such as polyurethane
or hydrocolloid, or silicone based
material such as OPSITE FLEXIFIX or OPSITE FLEXIFIX Gentle.
In a further aspect there is provided a novel apparatus as hereinbefore
defined. Accordingly there is provided a
wound treatment apparatus for treatment of a wound site comprising:
a backing layer having an upper surface and a lower surface and defining a
perimeter configured to be
positioned over skin surrounding a wound site;
a transmission layer configured to be positioned below the backing layer; and
one or more ports configured to transmit negative pressure through the backing
layer for the application
of topical negative pressure at the wound site;
wherein the apparatus comprises a plurality of cells or regions separated by
one or more trimmable
portions. Features are as hereinbefore defined.
The one or more ports may each comprise an opening in the backing layer
covered with a releasable tab, and
negative pressure may be applied to the backing layer through at least one of
the openings. Some embodiments
may comprise multiple ports configured to transmit negative pressure through
the backing layer, each port
corresponding to a separate negative pressure treatment module. Some
embodiments may further comprise a
wound contact layer configured to be positioned beneath the transmission
layer, the wound contact layer further
configured to seal to the backing layer around the perimeter.
In some embodiments, the plurality of cells may be approximately the same
size, approximately square, and
configured in a grid. In other embodiments, the plurality of cells may be
configured in a T-shape. In other
-33-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
embodiments, the plurality of cells may be configured into a roll. In other
embodiments, the plurality of cells may
be configured in a linear arrangement. In some embod ments, each of the
plurality of cells may be configured
with one of the one or more ports. In other embodiments, at least two of the
plurality of cells may be each
configured with one of the one or more ports. The apparatus may further
comprise a source of negative
pressure connected to some or all of the one or more ports. In some
embodiments, the dressing may comprise
an exposed portion of transmission layer. The exposed portion may be sealed
with a sealant or adhesive
material. The apparatus is preferably as further hereinbefore defined.
In some embodiments, the at least one material layer of the first portion
comprises one or more of a
transmission layer such as reticulated open-cell foam, woven material, non-
woven material, 3D knit fabric, Baltex
7970 weft knitted polyester, acquisition distribution material, DryWeb TDL2,
SlimCore TL4, or the like. The at
least one material of the first portion can additionally or alternatively
comprise an absorbent layer, for example a
superabsorbent pad comprising cellulose fibers and superabsorbent particles,
MH460.101, ALLEVYNTM foam,
Freudenberg 114-224-4, or ChemPositeTM 11C-450. In some embodiments, the
bridging portion comprises at
least one material layer comprising one or more of -eticulated open-cell foam,
woven material, non-woven
material, 3D knit fabric, Baltex 7970 weft knitted polyester, acquisition
distribution material, DryWeb TDL2,
SlimCore TL4, or the like. In some embodiments, the at least one material
layer of the bridging portion should
transmit a negative pressure of at least -40 mmHg against a set point in the
range -60 to -200 mmHg with an air
leak of 50 cc/minute. In some embodiments, the at least one material layer of
the bridging portion should
experience a pressure differential of approximately -25 mmHg or less (that is,
closer to zero) at a set point of -
200 mmHg with an air leak of 50 cc/minute over an approximately 20 mm 1 mm
distance. In other
embodiments, the at least one material layer of the bridging portion should
experience a pressure differential of
approximately -5 mmHg or less (that is, closer to zero) at a set point of -2Q0
mmHg with an air leak of 50
cc/minute over an approximately 20 mm 1 mm distance. In some embodiments,
the at least one material layer
of the bridging portion has a height, in an uncompressed state, of at least 1
mm (or approximately 1 mm), at least
3 mm (or approximately 3 mm), at least 4 mm (or approximately 4 mm), or at
least 5 mm (or approximately 5
mm), and a width of at least 1 mm (or approximately 1 mm), or at least 2 mm
(or approximately 2 mm), at least 3
mm (or approximately 3 mm), at least 4 mm (or approximately 4 mm), or at least
5 mm (or approximately 5 mm).
In some embodiments, the at least one material layer of the bridging portion
has a maximum height, in an
uncompressed state, of 9 mm (or approximately 9 mm) for purposes of being more
easily re-sealable when cut.
In some embodiments in which the dressing is sealed with a sealant, the at
least one material layer can be
resilient to compression such that a height of a sealed portion, in a
compressed state, is substantially the same
as the height of the sealed portion in an uncompressed state. In one
embodiment, the at least one material layer
of the bridging portion comprises a spacer material having a height of at
least 2 mm (or approximately 2 mm) and
a width of at least 1 mm (or approximately 1 mm). In one embodiment, the at
least one material layer of the
bridging portion comprises a reticulated open-cell foam having a height of at
least approximately 5 mm and a
width of at least approximately 3 mm, which, when wet, may experience a
pressure differential of -8.9 (or
approximately -8.9) mmHg. In another embodiment, the at least one material
layer of the bridging portion
comprises an acquisition distribution layer (e.g., SlimCore TL4) having a
height of at least approximately 2 mm
and a width of at least approximately 4 mm. Such dimensions can represent an
uncompressed dimension of the
material layer of the bridging portion. In one embodiment, the at least one
material layer of the bridging portion is
not compressible.
-34-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
We have found that a composition when dispensed to seal a dressing in manner
as hereinbefore defined, may
provide an advantageous seal in relation to an exposed trimmed portion or
bridging portion comprising material
layer(s) which undergo no change or substantially no change in compressibility
on initiation of negative pressure,
i.e. is resilient to or substantially resilient to compression induced by
negative pressure, or which undergo a
substantially similar compression to or lesser compression than the
composition seal, on initiation of negative
pressure, i.e is substantially equally resilient or less resilient than the
composition seal to compression induced by
negative pressure. In particular in relation to a curing or hardening system,
this relative compressibility is in relation
to the cured elastomer or hardened seal. Preferably a composition forms a seal
which is compressible to touch in
relation to material layer(s) which are substantially non-compressible or
compressible to a lesser degree, on
initiation of negative pressure, than the seal. Preferably the one or more
trimmable portions or briding portions
comprise material substantially resilient to the application of negative
pressure, preferably the bridging portion(s)
have height which is substantially unchanged on the application of negative
pressure, preferably having height
which is reduced by less than or equal to 10%, more preferably 8%, most
preferably 5%, on the application of
negative pressure. This ensures a smooth surface to the dressing and minimal
discomfort provided to the wearer, if
negative pressure is applied after sealing the dressing, and also ensures that
the seal remains intact and is not
ruptured if negative pressure is applied before sealing the dressing and is
subsequently temporarily interrupted.
In some embodiments, the bridging portion comprises the same layer(s) as the
first portion. In other
embodiments, the bridging portion comprises fewer layers than the first
portion. In some embodiments, the
layer(s) in the bridging portion have a smaller width than the layer(s) in the
first portion. In some embodiments,
the layer(s) in the bridging portion have a dimension that is smaller than the
layer(s) in the first portion (for
example, the individual or combined height of the layer(s) in the bridging
portion is smaller than the height of the
layer(s) in the first portion. In other embodiments, the layer(s) in the
bridging portion have the same width as the
layer(s) in the first portion. In some embodiments, the bridging portion
connects the first portion to an adjacent
portion having a similar layered construction and/or width as the first
portion. In some embodiments, there are
multiple bridging portions that may connect a first portion to multiple
adjacent portions, or may connect between
multiple adjacent portions.
KIT AND COMPONENTS THEREOF
A further aspect is a kit for use in the field of wound care comprising a
dressing for overlying a wound and skin
thereabout which may be cut to size or shape as hereinbefore defined together
with a composition as
hereinbefore defined.
Some kits comprise a vacuum pump.
In a particular advantage, the kit, sealant composition and/or dressing or
wound cover may be terminally sterile.
Techniques are known for sterilising apparatus, such as dry heat, steam,
radiation and the like. GB1020005.3,
GB 1019997.4 and GB1104512.7 disclose terminally sterilisable 2 part
compositions and methods for their
sterilisation. Methods include heat sterilisation and radiation sterilisation,
in particular gamma, e-beam or x-ray
radiation sterilisation. Preferably the sealant is terminally sterilisable or
sterile and is sterilized prior to dispensing
by heating the first and second parts in a thermally stable receptacle or
support at an elevated temperature of
121 C or more for a period of up to 28 hours, or by irradiating the first and
second parts with a radiation source
-35-
=
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
selected from the group consisting of gamma, x-ray, and e-beam radiation with
a dose that provides an effective
sterility assurance level.
A further aspect is a method for dispensing or releasing, and curing a
composition as hereinbefore defined,
comprising dispensing into a desired location at curing temperature for curing
time.
The composition may be manually mixed and dispensed. Alternatively any form of
dispensing device may be
employed, for example the composition may be dispensed by means of a
cooperative dispensing device
cooperatively dispensing, for example by means of a double barrel syringe, for
example by activating respective
barrels of a double barrel syringe, or loading respective cassettes therefore
and activating.
A further aspect is an elastomer comprising a cured composition as
hereinbefore defined,
METHOD OF USE
A further aspect is a method for dispensing a composition as hereinbefore
defined comprising:
optionally combining Parts A and B of a curable composition as hereinbefore
defined thereby initiating cure;
dispensing composition into a location as hereinbefore defined;
after a suitable period an optionally elastomeric seal is formed.
A further aspect is a method for sealing a woundcare dressing comprising:
cutting a dressing to size or shape;
positioning the dressing overlying a wound and skin thereabout;
optionally combining Parts A and B of a curable composition as hereinbefore
defined thereby initiating cure;
dispensing composition into a location as hereinbefore defined;
after a suitable period an optionally elastomeric seal is formed at the
severed dressing edge.
Preferably the composition is dispensed by means of a syringe for example a
cooperative dispensing device as
hereinbefore defined cooperatively dispensing, for example by means of a
double barrel syringe, for example by
activating respective barrels of a double barrel syringe, or loading
respective cassettes therefore and activating,
preferably wherein the syringe incorporates integral means to configure the
dispensed sealant, for example an
integral spreader head.
METHOD OF TREATMENT
A further aspect is a method for sealing a dressing or for treating a wound
site, of a human or animal subject in
need thereof comprising:
dressing the wound site with a dressing, as hereinbefore defined, exposing a
portion thereof as
hereinbefore defined and
dispensing a composition as hereinbefore defined.
Preferably the method comprises:
providing a wound dressing as hereinbefore defined comprising:
a backing layer; and
a transmission layer positioned beneath the backing layer,
-36-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
removing a portion of the wound dressing to create a main wound dressing
portion with one or more
exposed portions;
positioning the main wound dressing portion over a wound;
sealing the main wound dressing to skin surrounding the wound, and further
sealing further sealing the
one or more exposed portions of the main wound dressing portion; and
applying negative pressure to the wound through the backing layer of the main
wound dressing portion.
In some embodiments of the method, removing a portion of the wound dressing
comprises cutting the wound
dressing across at least one of the one or more bridging portions. At least a
portion of the wound dressing may
comprise pre-cut score marks to facilitate removing of the portion of wound
dressing. The dressing may
comprise a plurality of openings in the backing layer covered with a
releasable tab, and negative pressure may
be applied to the backing layer through one of the openings. The dressing may
comprise a plurality of openings
in the backing layer covered with a releasable tab, and negative pressure may
be applied to the backing layer
through two or more of the openings.
The portions of the wound dressing may be removed to size the main wound
dressing portion for positioning over
an incisional wound. The portions of the wound dressing may be removed to size
the main wound dressing
portion for positioning over an elongate leg wound. The portions of the wound
dressing may be removed to size
the main wound dressing portion for positioning over an arcuate incisional
wound.
In another embodiment, a method of treating a wound is provided, comprising:
providing a wound dressing comprising a backing layer, a transmission layer
beneath the backing layer,
and a plurality of spaced apart openings in the backing layer each covered
with a releasable tab, the wound
dressing configured into a length or roll;
optionally unrolling a portion of the wound dressing from the roll;
removing a portion of the wound dressing from the length or roll, the removed
portion comprising at
least one opening in the backing layer covered with a releasable tab;
positioning the removed portion of the wound dressing over a wound; and
applying negative pressure through at least one opening in the backing layer
after a releasable tab has
been removed.
Preferably dispensing a sealant composition is by means of a device as
hereinbefore defined.
Preferably the dressing is adhered over the wound site with at least an
adhesive underside of the dressing or an
adhesive disposed on at least an underside of the dressing.
Preferably the method further comprises adjusting the position of the dressing
before the composition is
dispensed.
Preferably the sealant is dispensed after dressing the wound site with the
dressing.
Preferably the wound dressing is adapted to contain a negative pressure, the
method additionally comprising
applying negative pressure to the wound site using a source of negative
pressure connected to the wound site.
Preferably applying negative pressure is conducted before and after dispensing
sealant.
-37-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
Preferably applying negative pressure is by means of a portable negative
pressure source in fluid communication
with the wound dressing located over a wound site.
Preferably the method includes monitoring transmitted negative pressure at the
wound against generated
negative pressure.
Preferably a dressing is a combination TNP therapy dressing incorporating
fluid-tight backing layer, functional
wound therapy layers and an integral attachment for a negative pressure
source, preferably a portable and/or
periodic negative pressure source by means of which negative pressure is
applied to the wound site through or
under the backing layer. Preferably an aperture is created into or under the
drape so as to connect the wound site to
the source of negative pressure.
A wound packing material may be located so as to partially or completely fill
the wound site.
Providing the sealant may be by means of dispensing a sealant composition as
hereinbefore defined by the
method as hereinbefore defined.
The method may include monitoring transmitted negative pressure against
generated negative pressure. This
may be used to provide the user with feedback during the dressing application.
Typically NP is monitored at the
pump, or alternatively at end of port.
Preferably the dressing is applied, the NP source activated, pump down
initiated, detecting for alarms indicating
NP loss, rub down dressing to close off any sites of NP loss, apply sealant at
severed edges.
In an advantage, providing the sealant is by means of dispensing a sealant
composition, wherein the
composition is a fluid that when dispensed forms a material capable of making
a substantially fluid-tight seal.
Preferably the method comprises combining at least two pre-polymers to form
the sealant.
Preferably the dressing is part of a portable NPWT system. The exudate is
managed in a portable canister or
withIn the dressing. The negative pressure source is portable or may be
connected intermittently. Preferably the
skin contact layer is an adhesive silicone gel, other adhesive or combination
of adhesive silicone gel and other
adhesive.
Portable composite TNP dressings are commercially available and include
Prevena (KCI), NPD1000 NP wound
Therapy System (Kalypto Medical Inc), PICO (Smith & Nephew), amongst others,
and are more extensively
described in the literature, for example in PCT/GB2011/000629, the contents of
which are incorporated herein by
reference.
Upon the application of negative pressure with the pump, the dressing may in
some embodiments partially
collapse and present a wrinkled appearance as a result of the evacuation of
some or all of the air underneath the
dressing. In some embodiments, the pump may be configured to detect if any
leaks are present in the dressing,
such as at the interface between the dressing and the skin surrounding the
wound site. Should a leak be found,
such leak is preferably remedied prior to continuing treatment.
Treatment of the wound site preferably continues until the wound has reached a
desired level of healing. In
some embodiments, it may be desirable to replace the dressing after a certain
time period has elapsed, or if the
-38-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
dressing is full of wound fluids. During such changes, the pump may be kept,
with just the dressing being
changed.
A further aspect provides the use of a composition, kit or apparatus as
hereinbefore defined for dressing wounds,
preferably for negative pressure wound therapy dressing of wounds as
hereinbefore defined. The sealant
composition, kit and apparatus may be useful for example in sealing medical
dressings, for example in
restraining egress of wound exudate or ingress of air or infection, in
addition to providing a vacuum seal for TNP
application.
Such use includes use on wounds selected from chronic, acute, traumatic, sub-
acute and dehisced wounds, ulcers
(such as pressure or diabetic), partial-thickness burns and flaps and grafts.
These include open, moist, granulating
wounds, preferably surgical wounds such as those resulting from excision of
ulcers, cancerous tissue such as
perianal and perineal wounds and the like. For optimum healing of such wounds,
the wound should be prevented
from closing in on itself and allowing fluids to accumulate, whilst at the
same time allowing the tissue around the
wound to progressively contract, and the wound to shrink. Wound filling
materials in NPVVT therefore function as a
type of "stent", supporting the wound and holding it open.
A sealant composition, kit or apparatus is particularly suited for use in
clean, aseptic or sterile applications.
Preferably the composition, kit or apparatus is rendered sterile, as known in
the art or as hereinbefore defined,
and packaged within barrier means. Further barrier means provide a barrier to
infection, whereby the
composition, kit or apparatus is a double wrapped item, this allows for the
removal of the first layer of sterile
sealed packaging to reveal receptacles or supports such as cartridges for or
incorporated in a syringe, adhesive
strips and the like, which are completely sterile inside and out, facilitating
entry into a sterile environment. The
composition omitting a further barrier means would comprise a non-sterile
external surface of receptacles or
supports and associated barrier means. If it is not possible to sterilise the
composition using standard conditions
for medical apparatus as hereinbefore described, it may not be possible to
take such a composition into a sterile
field.
A sealant for medical dressings may be applied in any known or novel manner.
WO 00/74738 (Guyuron)
discloses use of silicone based RTV-2 compositions to seal wounds i.a to
minimise potential infections. The
sealant may suitably therefore be used by casting on top of the wound and
surrounding skin and allowing to
cure.
A further aspect provides the medical use of a kit, sealant or apparatus as
hereinbefore defined.
Embodiments have one or more of the following advantages:
Allows severed dressing edges to be sealed readily.
Sealing of 3-dimensional dressing perimeters following complex body contours
enhancing the ability to remove
or reduce leaks.
Sealing of custom sized, shaped, contoured, articulated dressings.
Sealing of dressings where the borders conform to body geometries with tight
external radii or are otherwise
subject to high levels of deformation.
-39-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
Sealing of systems where the dressing will be subject to a great deal of
movement (e.g. neck, shoulder,
underarm, elbow, forearm, wrist, hand, groin, knee, ankle, heel, foot).
A number of specific embodiments are given hereinbelow, appropriate for
conventional Advanced Wound
Dressings, conventional NPVVT Drapes/Dressings or PICOTT" and a sealant as
hereinbefore defined. General
references hereinbelow are however not to be construed as limiting to the
specific figure or embodiment which
they are intended to illustrate, rather for the sake of avoiding undue
duplication such description may be present
in the following section although of equal or greater relevance and equally
pertinent to the foregoing.
Figure 1 illustrates an embodiment of a TNP wound treatment system 100
comprising a wound dressing 110 in
combination with a pump 150. As stated above, the wound dressing 110 can be
any wound dressing
embodiment disclosed herein including without limitation dressing embodiment
or have any combination of
features of any number of wound dressing embodiments disclosed herein. Here,
the dressing 110 may be
placed over a wound as described previously, and a conduit 130 may then be
connected to the port 120,
although in some embodiments the dressing 101 may be provided with at least a
portion of the conduit 130
preattached to the port 120. Preferably, the dressing 110 is provided as a
single article with all wound dressing
elements (including the port 120) pre-attached and integrated into a single
unit. The wound dressing 110 may
then be connected, via the conduit 130, to a source of negative pressure such
as the pump 150. The pump 150
can be miniaturized and portable, although larger conventional pumps may also
be used with the dressing 110.
In some embodiments, the pump 150 may be attached or mounted onto or adjacent
the dressing 110. A
connector 140 may also be provided so as to permit the conduit 130 leading to
the wound dressing 110 to be
disconnected from the pump, which may be useful for example during dressing
changes.
Figures 2A-D illustrates the use of an embodiment of a TNP wound treatment
system being used to treat a
wound site on a patient. Figure 2A shows a wound site 200 being cleaned and
prepared for treatment. Here,
the healthy skin surrounding the wound site 200 is preferably cleaned and
excess hair removed or shaved. The
wound site 200 may also be irrigated with sterile saline solution if
necessary. Optionally, a skin protectant may
be applied to the skin surrounding the wound site 200. If necessary, a wound
packing material, such as foam or
gauze, may be placed in the wound site 200. This may be preferable if the
wound site 200 is a deeper wound.
After the skin surrounding the wound site 200 is dry, and with reference now
to Figure 2B, the wound dressing
110 may be positioned and placed over the wound site 200. Preferably, the
wound dressing 110 is placed with
the wound contact layer 2102 over and/or in contact with the wound site 200.
In some embodiments, an
adhesive layer is provided on the lower surface 2101 of the wound contact
layer 2102, which may in some cases
be protected by an optional release layer to be removed prior to placement of
the wound dressing 110 over the
wound site 200. Preferably, the dressing 110 is positioned such that the port
2150 is in a raised position with
respect to the remainder of the dressing 110 so as to avoid fluid pooling
around the port. In some embodiments,
the dressing 110 is positioned so that the port 2150 is not directly overlying
the wound, and is level with or at a
higher point than the wound. To help ensure adequate sealing for TNP, the
edges of the dressing 110 are
preferably smoothed over to avoid creases or folds.
-40-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
With reference now to Figure 2C, the dressing 110 is connected to the pump
150. The pump 150 is configured
to apply negative pressure to the wound site via the dressing 110, and
typically through a conduit. In some
embodiments, and as described above in Figure 1, a connector may be used to
join the conduit from the
dressing 110 to the pump 150. Upon the application of negative pressure with
the pump 150, the dressing 110
may, in some embodiments, partially collapse and present a wrinkled appearance
as a result of the evacuation of
some or all of the air underneath the dressing 110. In some embodiments, the
pump 150 may be configured to
detect if any leaks are present in the dressing 110, such as at the interface
between the dressing 110 and the
skin surrounding the wound site 200. Should a leak be found, such leak is
preferably remedied prior to
continuing treatment.
Turning to Figure 2D, additional fixation strips 210 may also be attached
around the edges of the dressing 110.
Such fixation strips 210 may be advantageous in some situations so as to
provide additional sealing against the
skin of the patient surrounding the wound site 200. For example, the fixation
strips 210 may provide additional
sealing for when a patient is more mobile. In some cases, the fixation strips
210 may be used prior to activation
of the pump 150, particularly if the dressing 110 is placed over a difficult
to reach or contoured area.
Treatment of the wound site 200 preferably continues until the wound has
reached a desired level of healing. In
some embodiments, it may be desirable to replace the dressing 110 after a
certain time period has elapsed, or if
the dressing is full of wound fluids. During such changes, the pump 150 may be
kept, with just the dressing 110
being changed.
Figures 3A-C illustrates cross-sections through a wound dressing 2100 similar
to the wound dressing of Figure 1
according to an embodiment of the disclosure. A view from above the wound
dressing 2100 is illustrated in
Figure 1 with the line A-A indicating the location of the cross-section shown
in Figures 3A and 3B. The wound
dressing 2100, which can alternatively be any wound dressing embodiment
disclosed herein including without
limitation wound dressing 110 or any combination of features of any number of
wound dressing embodiments
disclosed herein, can be located over a wound site to be treated. The dressing
2100 may be placed to as to
form a sealed cavity over the wound site. In a preferred embodiment, the
dressing 2100 comprises a backing
layer 2140 attached to a wound contact layer 2102, both of which are described
in greater detail below. These
two layers 2140, 2102 are preferably joined or sealed together so as to define
an interior space or chamber.
This interior space or chamber may comprise additional structures that may be
adapted to distribute or transmit
negative pressure, store wound exudate and other fluids removed from the
wound, and other functions which will
be explained in greater detail below. Examples of such structures, described
below, include a transmission layer
2105 and an absorbent layer 2110.
As illustrated in Figures 3A-C, a lower surface 2101 of the wound dressing
2100 may be provided with an
optional wound contact layer 2102. The wound contact layer 2102 can be a
polyurethane layer or polyethylene
layer or other flexible layer which is perforated, for example via a hot pin
process, laser ablation process,
ultrasound process or in some other way or otherwise made permeable to liquid
and gas. The wound contact
layer 2102 has a lower surface 2101 and an upper surface 2103. The
perforations 2104 preferably comprise
through holes in the wound contact layer 2102 which enable fluid to flow
through the layer 2102. The wound
contact layer 2102 helps prevent tissue ingrowth into the other material of
the wound dressing. Preferably, the
-41-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
perforations are small enough to meet this requirement while still allowing
fluid to flow therethrough. For
example, perforations formed as slits or holes having a size ranging from
0.025 mm to 1.2 mm are considered
small enough to help prevent tissue ingrowth into the wound dressing while
allowing wound exudate to flow into
the dressing. In some configurations, the wound contact layer 2102 may help
maintain the integrity of the entire
dressing 2100 while also creating an air tight seal around the absorbent pad
in order to maintain negative
pressure at the wound.
Some embodiments of the wound contact layer 2102 may also act as a carrier for
an optional lower and upper
adhesive layer (not shown). For example, a lower pressure sensitive adhesive
may be provided on the lower
surface 2101 of the wound dressing 2100 whilst an upper pressure sensitive
adhesive layer may be provided on
the upper surface 2103 of the wound contact layer. The pressure sensitive
adhesive, which may be a silicone,
hot melt, hydrocolloid or acrylic based adhesive or other such adhesives, may
be formed on both sides or
optionally on a selected one or none of the sides of the wound contact layer.
When a lower pressure sensitive
adhesive layer is utilized may be helpful to adhere the wound dressing 2100 to
the skin around a wound site. In
some embodiments, the wound contact layer may comprise perforated polyurethane
film. The lower surface of
the film may be provided with a silicone pressure sensitive adhesive and the
upper surface may be provided with
an acrylic pressure sensitive adhesive, which may help the dressing maintain
its integrity. In some
embodiments, a polyurethane film layer may be provided with an adhesive layer
on both its upper surface and
lower surface, and all three layers may be perforated together.
A layer 2105 of porous material can be located above the wound contact layer
2102. This porous layer, or
transmission layer, 2105 allows transmission of fluid including liquid and gas
away from a wound site into upper
layers of the wound dressing. In particular, the transmission layer 2105
preferably ensures that an open air
channel can be maintained to communicate negative pressure over the wound area
even when the absorbent
layer has absorbed substantial amounts of exudates. The layer 2105 should
preferably remain open under the
typical pressures that will be applied during negative pressure wound therapy
as described above, so that the
whole wound site sees an equalized negative pressure. The layer 2105 may be
formed of a material having a
three dimensional structure. For example, a knitted or woven spacer fabric
(for example Baltex 7970 weft knitted
polyester) or a non-woven fabric could be used.
A layer 2110 of absorbent material is provided above the transmission layer
2105. The absorbent material,
which comprise a foam or non-woven natural or synthetic material, and which
may optionally comprise a super-
absorbent material, forms a reservoir for fluid, particularly liquid, removed
from the wound site. In some
embodiments, the layer 2100 may also aid in drawing fluids towards the backing
layer 2140.
With reference to Figures 3A-C, a masking or obsuring layer 2107 can be
positioned beneath at least a portion
of the backing layer 2140. In some embodiments, the obscuring layer 2107 can
have any of the same features,
materials, or other details of any of the other embodiments of the obscuring
layers disclosed herein, including but
not limited to having any viewing windows or holes. Additionally, the
obscuring layer 2107 can be positioned
adjacent to the backing layer, or can be positioned adjacent to any other
dressing layer desired. In some
embodiments, the obscuring layer 2107 can be adhered to or integrally formed
with the backing layer.
Preferably, the obscuring layer 2107 is configured to have approximately the
same size and shape as the
-42-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
absorbent layer 2110 so as to overlay it. As such, in these embodiments the
obscuring layer 2107 will be of a
smaller area than the backing layer 2140.
The material of the absorbent layer 2110 may also prevent liquid collected in
the wound dressing 2100 from
flowing freely within the dressing, and preferably acts so as to contain any
liquid collected within the absorbent
layer 2110. The absorbent layer 2110 also helps distribute fluid throughout
the layer via a wicking action so that
fluid is drawn from the wound site and stored throughout the absorbent layer.
This helps prevent agglomeration
in areas of the absorbent layer. The capacity of the absorbent material must
be sufficient to manage the
exudates flow rate of a wound when negative pressure is applied. Since in use
the absorbent layer experiences
negative pressures the material of the absorbent layer is chosen to absorb
liquid under such circumstances. A
number of materials exist that are able to abscrb liquid when under negative
pressure, for example
superabsorber material. The absorbent layer 2110 may typically be manufactured
from ALLEVYNTM foam,
Freudenberg 114-224-4 and/or Chem-Posite Tml 1C-450. In some embodiments, the
absorbent layer 2110 may
comprise a composite comprising superabsorbent powder, fibrous material such
as cellulose, and bonding fibers.
In a preferred embodiment, the composite is an airlaic, thermally-bonded
composite.
An orifice 2145 is preferably provided in the backing Layer 2140 to allow a
negative pressure to be applied to the
dressing 2100. A suction port 2150 is preferably attached or sealed to the top
of the backing layer 2140 over an
orifice 2145 made into the dressing 2100, and communicates negative pressure
through the orifice 2145. A
length of tubing 2220 may be coupled at a first end to the suction port 2150
and at a second end to a pump unit
(not shown) to allow fluids to be pumped out of the dressing. The port may be
adhered and sealed to the backing
layer 2140 using an adhesive such as an acrylic, cyanoacrylate, epoxy, UV
curable or hot melt adhesive. The
port 2150 is formed from a soft polymer, for example a polyethylene, a
polyvinyl chloride, a silicone or
polyurethane having a hardness of 30 to 90 on the Shore A scale. In some
embodiments, the port 2150 may be
made from a soft or conformable material, for example using the embodiments
described below in Figures 3A-B.
Preferably the absorbent layer 2110 and the obscuring layer 2107 include at
least one through hole 2146 located
so as to underlie the port 2150. The through hole 2146, while illustrated here
as being larger than the hole
through the obscuring layer 2107 and backing layer 2140, may in some
embodiments be bigger or smaller than
either. Of course, the respective holes through these various layers 2107,
2140, and 2110 may be of different
sizes with respect to each other. As illustrated in Figures 3A-C a single
through hole can be used to produce an
opening underlying the port 2150. It will be appreciated that multiple
openings could alternatively be utilized.
Additionally should more than one port be utilized according to certain
embodiments of the present disclosure
one or multiple openings may be made in the absorbent layer and the obscuring
layer in registration with each
respective port. Although not essential to certain embodiments of the present
disclosure the use of through
holes in the super-absorbent layer may provide a fluid flow pathway which
remains unblocked in particular when
the absorbent layer 2100 is near saturation.
The aperture or through-hole 2146 is preferably provided in the absorbent
layer 2110 and the obscuring layer
2107 beneath the orifice 2145 such that the orifice is connected directly to
the transmission layer 2105. This
allows the negative pressure applied to the port 2150 to be communicated to
the transmission layer 2105 without
passing through the absorbent layer 2110. This ensures that the negative
pressure applied to the wound site is
-43-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
not inhibited by the absorbent layer as it absorbs wound exudates. In other
embodiments, no aperture may be
provided in the absorbent layer 2110 and/or the obscuring layer 2107, or
altematively a plurality of apertures
underlying the orifice 2145 may be provided.
The backing layer 2140 is preferably gas impermeable, but moisture vapor
permeable, and can extend across
the width of the wound dressing 2100. The backing layer 2140, which may for
example be a polyurethane film
(for example, Elastollan SP9109) or hydrocolloid film, having a pressure
sensitive adhesive on one side, is
impermeable to gas and this layer thus operates to cover the wound and to seal
a wound cavity over which the
wound dressing is placed. In this way an effective chamber is made between the
backing layer 2140 and a
wound site where a negative pressure can be established. The backing layer
2140 is preferably sealed to the
wound contact layer 2102 in a border region 2200 around the circumference of
the dressing, ensuring that no air
is drawn in through the border area, for example via adhesive or welding
techniques. The backing layer 2140
protects the wound from external bacterial contamination (bacterial barrier)
and allows liquid from wound
exudates to be transferred through the layer and evaporated from the film
outer surface. The backing layer 2140
preferably comprises two layers; a polyurethane or hydrocolloid film and an
adhesive pattern spread onto the
film. The film is preferably moisture vapor permeable and may be manufactured
from a material that has an
increased water transmission rate when wet.
The absorbent layer 2110 may be of a greater area than the transmission layer
2105, such that the absorbent
layer overlaps the edges of the transmission layer 2105, thereby ensuring that
the transmission layer does not
contact the backing layer 2140. This provides an outer channel 2115 of the
absorbent layer 2110 that is in direct
contact with the wound contact layer 2102, which aids more rapid absorption of
exudates to the absorbent layer.
Furthermore, this outer channel 2115 ensures that no liquid is able to pool
around the circumference of the
wound cavity, which may otherwise seep through the seal around the perimeter
of the dressing leading to the
formation of leaks.
As shown in Figure 3A, one embodiment of the wound dressing 2100 comprises an
aperture 2146 in the
absorbent layer 2110 situated underneath the port 2150. In use, for example
when negative pressure is applied
to the dressing 2100, a wound facing portion of the port 150 may thus come
into contact with the transmission
layer 2105, which can thus aid in transmitting negative pressure to the wound
site even when the absorbent layer
2110 is filled with wound fluids. Some embodiments may have the backing layer
2140 be at least partly adhered
to the transmission layer 2105. In some embodiments, the aperture 2146 is at
least 1-2 mm larger than the
diameter of the wound facing portion of the port 2150, or the orifice 2145.
A filter element 2130 that is impermeable to liquids, but permeable to gases
is provided to act as a liquid barrier,
and to ensure that no liquids are able to escape from the wound dressing. The
filter element may also function as
a bacterial barrier. Typically the pore size is 0.2pm. Suitable materials for
the filter material of the filter element
2130 include 0.2 micron GOreTM expanded PTFE from the MMT range, PALL
VersaporeTM 200R, and
DonaldsonTM TX6628. Larger pore sizes can also be used but these may require a
secondary filter layer to
ensure full bioburden containment. As wound fluid contains lipids it is
preferable, though not essential, to use an
oleophobic filter membrane for example 1.0 micron MMT-332 prior to 0.2 micron
MMT-323. This prevents the
lipids from blocking the hydrophobic filter. The filter element can be
attached or sealed to the port and/or the
-44-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
backing layer 2140 over the orifice 2145. For example the filter element 2130
may be molded into the port 2150,
or may be adhered to both the top of the backing layer 2140 and bottom of the
port 2150 using an adhesive such
as, but not limited to, a UV cured adhesive.
In Figure 3B, an embodiment of the wound dressing 2100 is illustrated which
comprises spacer elements 2152,
2153 in conjunction with the port 2150 and the filter 2130. With the addition
of such spacer elements 2152,
2153, the port 2150 and filter 2130 may be supported out of direct contact
with the absorbent layer 2110 and/or
the transmission layer 2105. The absorbent layer 2110 may also act as an
additional spacer element to keep the
filter 2130 from contacting the transmission layer 2105. Accordingly, with
such a configuration contact of the filter
2130 with the transmission layer 2105 and wound fluids during use may thus be
minimized. As contrasted with
the embodiment illustrated in Figure 3A, the aperture 2146 through the
absorbent layer 2110 and the obscuring
layer 2107 may not necessarily need to be as large o- larger than the port
2150, and would thus only need to be
large enough such that an air path can be maintained from the port to the
transmission layer 2105 when the
absorbent layer 2110 is saturated with wound fluids.
With reference now to Figure 3C, which shares many of the elements illustrated
in Figures 3A-C, the
embodiment illustrated here comprises the backing layer 2140, masking layer
2107, and absorbent layer 2110,
all of which have a cut or opening made therethrough which communicate
directly to the transmission layer 2105
so as to form the orifice 2145. The suction port 2150 is preferably situated
above it and communicates with the
orifice 2145.
In particular for embodiments with a single port 2150 and through hole, it may
be preferable for the port 2150 and
through hole to be located in an off-center position as illustrated in Figures
3A-C and in Figure 1. Such a location
may permit the dressing 2100 to be positioned onto a patient such that the
port 2150 is raised in relation to the
remainder of the dressing 2100. So positioned, the port 2150 and the filter
2130 may be less likely to come into
contact with wound fluids that could prematurely occlude the filter 2130 so as
to impair the transmission of
negative pressure to the wound site.
Figure 4A illustrates an exploded view of a dressing 3400 for use in negative
pressure wound therapy. Although
this figure illustrates a dressing having one particular shape, the
construction of the layers can be applied to any
of the embodiments identified below, including Figures 5A-8, and any of the
dressing shapes and configurations
described in the patent applications incorporated by reference herein. The
dressing 3400 comprises a release
layer 3480, wound contact layer 3460, a transmission layer 3450, an
acquisition distribution layer 3440, an
absorbent layer 3430, an obscuring layer 3420, and a backing layer 3410. The
dressing 3400 may be connected
to a port. At least the wound contact layer 3460, traismission layer 3450,
absorbent layer 3430, obscuring layer
3420, and backing layer 3410 may have properties as described with respect to
particular embodiments above,
such as the embodiments of Figures 3A-C, as well as or instead of the
properties described below.
The dressing 3400 may comprise a wound contact layer 3460 for sealing the
dressing 3400 to the healthy skin of
a patient surrounding a wound area. Certain embodiments of the wound contact
layer may comprise three
layers: a polyurethane film layer, a lower adhesive layer and an upper
adhesive layer. The upper adhesive layer
may assist in maintaining the integrity of the dressing 3400, and the lower
adhesive layer may be employed for
sealing the dressing 3400 to the healthy skin of a patient around a wound
site. As described above, in some
embodiments with respect to Figures 3A-C, some embodiments of the polyurethane
film layer may be perforated.
-45-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
Some embodiments of the polyurethane film layer anc upper and lower adhesive
layers may be perforated
together after the adhesive layers have been applied to the polyurethane film.
In some embodiments a pressure
sensitive adhesive, which may be a silicone, hot melt, hydrocolloid or acrylic
based adhesive or other such
adhesives, may be formed on both sides or optionally on a selected one side of
the wound contact layer. In
certain embodiments, the upper adhesive layer may cam-prise an acrylic
pressure sensitive adhesive, and the
lower adhesive layer may comprise a silicone pressure sensitive adhesive. In
other embodiments the wound
contact layer 3460 may not be provided with adhesive. In some embodiments, the
wound contact layer 3460
may be transparent or translucent. The film layer of the wound contact layer
3460 may define a perimeter with a
rectangular or a square shape. A release layer 3480 may be removably attached
to the underside of the wound
contact layer 3460, for example covering the lower adhesive layer, and may be
peeled off using flaps 3481.
Some embodiments of the release layer 3480 may have a plurality of flaps
extending along the length of the
layer 3480.
Some embodiments of the dressing 3400 may comprise a spacer or transmission
layer 3450. The transmission
layer 3450 may comprise a porous material or 3D fabric configured to allow for
the passage of fluids
therethrough away from the wound site and into the upper layers of the
dressing 3400. In particular, the
transmission layer 3450 can ensure that an open air channel can be maintained
to communicate negative
pressure over the wound area even when the absorbent layer 3430 has absorbed
substantial amounts of
exudates. The transmission layer 3450 should remain open under the typical
pressures that will be applied
during negative pressure wound therapy as described above, so that the whole
wound site sees an equalized
negative pressure.
Some embodiments of the transmission layer 3450 may be formed of a material
having a three dimensional
structure. For example, a knitted or woven spacer fabric (for example Baltex
7970 weft knitted polyester) or a
non-woven fabric can be used. In some embodiments, the transmission layer 3450
can have a 3D polyester
spacer fabric layer. This layer can have a top layer which is a 84/144
textured polyester, and a bottom layer
which can be a 100 denier flat polyester and a third layer formed sandwiched
between these two layers which is
a region defined by a knitted polyester viscose, cellulose or the like
monofilament fiber. In use, this differential
between filament counts in the spaced apart layers tends to draw liquid away
from the wound bed and into a
central region of the dressing 3400 where the absorbent layer 3430 helps lock
the liquid away or itself wicks the
liquid onwards towards the cover layer 3410 where it can be transpired. Other
materials can be utilized, and
examples of such materials are described in U.S. Patent Pub. No. 2011/0282309,
which are hereby incorporated
by reference and made part of this disclosure. However, the transmission layer
3450 may be optional, and for
example may be optional in embodiments of the dressing 3400 which comprise the
acquisition distribution layer
3440, described below.
Some embodiments may comprise a wicking or acquisition distribution layer
(ADL) 3440 to horizontally wick fluid
such as wound exudate as it is absorbed upward through the layers of the
dressing 3400. Lateral wicking of fluid
may allow maximum distribution of the fluid through the absorbent layer 3430
and may enable the absorbent
layer 3430 to reach its full holding capacity. This may advantageously
increase moisture vapor permeation and
efficient delivery of negative pressure to the wound site. Some embodiments of
the ADL 3440 may comprise
viscose, polyester, polypropylene, cellulose, or a combination of some or all
of these, and the material may be
-46-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
needle-punched. Some embodiments of the ADL 3440 nay comprise polyethylene in
the range of 40-150 grams
per square meter (gsm).
The dressing 3400 may further comprise an absorbent or superabsorbent layer
3430. The absorbent layer can
be manufactured from ALLEVYNTm foam, Freudenberg 114-224-4 and/or Chem-
PositeTm11C-450, or any other
suitable material. In some embodiments, the absorbent layer 3430 can be a
layer of non-woven cellulose fibers
having super-absorbent material in the form of dry particles dispersed
throughout. Use of the cellulose fibers
introduces fast wicking elements which help quickly and evenly distribute
liquid taken up by the dressing. The
juxtaposition of multiple strand-like fibers leads to strong capillary action
in the fibrous pad which helps distribute
liquid.
For example, some embodiments of the absorbent layer 3430 may comprise a
layered construction of an upper
layer of non-woven cellulose fibers, superabsorbent particles (SAP), and a
lower layer of cellulose fibers with 40-
80% SAP. In some embodiments, the absorbent layer 3430 may be an air-laid
material. Heat fusible fibers can
optionally be used to assist in holding the structure of the pad together.
Some embodiments may combine
cellulose fibers and air-laid materials, and may further comprise up to 60%
SAP. Some embodiments may
comprise 60% SAP and 40% cellulose. Other embodiments of the absorbent layer
may comprise between 60%
and 90% (or between about 60% and about 90%) cellu ose matrix and between 10%
and 40% (or between about
10% and about 40%) superabsorbent particles. For example, the absorbent layer
may have about 20%
superabsorbent material and about 80% cellulose fibers. It will be appreciated
that rather than using super-
absorbing particles or in addition to such use, super-absorbing fibers can be
utilized according to some
embodiments of the present invention. An example of a suitable material is the
Product ChemPositeTM 11 C
available from Emerging Technologies Inc (ETi) in the USA.
Super-absorber particles/fibers can be, for example, sodium polyacrylate or
carbomethoxycellulose materials or
the like or any material capable of absorbing many t.mes its own weight in
liquid. In some embodiments, the
material can absorb more than five times its own weight of 0.9% W/VV saline,
etc. In some embodiments, the
material can absorb more than 15 times its own weight of 0.9% W/W saline, etc.
In some embodiments, the
material is capable of absorbing more than 20 times its own weight of 0.9% WNV
saline, etc. Preferably, the
material is capable of absorbing more than 30 times its own weight of 0.9% W/W
saline, etc. The absorbent
layer 3430 can have one or more through holes 3431 located so as to underlie
the suction port.
Some embodiments of the present disclosure may optionally employ a masking or
obscuring layer 3420 to help
reduce the unsightly appearance of a dressing 3400 during use due to the
absorption of wound exudate. The
obscuring layer 3420 may be a colored portion of the absorbent material, or
may be a separate layer that covers
the absorbent material. The obscuring layer 3420 may be one of a variety of
colors such as blue, orange, yellow,
green, or any color suitable for masking the presence of wound exudate in the
dressing 3400. For example, a
blue obscuring layer 3420 may be a shade of blue similar to the shade of blue
commonly used for the material of
medical gowns, scrubs, and drapes. Some embodiments of the obscuring layer
3420 may comprise
polypropylene spunbond material. Further, some embodiments of the obscuring
layer 3420 may comprise a
hydrophobic additive or coating. Other embodiments may comprise a thin fibrous
sheet of 60, 70, or 80 gsm.
-47-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
The obscuring layer may comprise at least one viewing window 3422 configured
to allow a visual determination
of the saturation level of the absorbent layer. The at least one viewing
window 3422 may comprise at least one
aperture made through the obscuring layer. The at least one viewing window
3422 may comprise at least one
uncolored region of the obscuring layer. Some embodiments of the obscuring
layer may comprise a plurality of
viewing windows or an array of viewing windows.
The masking capabilities of the obscuring layer 3420 should preferably only be
partial, to allow clinicians to
access the information they require by observing the spread of exudate across
the dressing surface. An
obscuring layer 3420 may be partial due to material properties allowing wound
exudate to slightly alter the
appearance of the dressing or due to the presence of at least one viewing
window 3422 in a completely
obscuring material. The partial masking nature of the obscuring layer 3420
enables a skilled clinician to perceive
a different colour caused by exudate, blood, by-products etc. in the dressing
allowing for a visual assessment
and monitoring of the extent of spread across the dressing. However, since the
change in colour of the dressing
from its clean state to a state with exudate contained is only a slight
change, the patient is unlikely to notice any
aesthetic difference. Reducing or eliminating a visual indicator of wound
exudate from a patient is likely to have a
positive effect on their health, reducing stress for example.
The obscuring layer 3420 can have one or more through holes located so as to
underlie the suction port. Some
embodiments may have a maltese cross 3421 or other shaped cutout underlying
the suction port, wherein the
diameter of the maltese cross 3421 is greater than the diameter of the port.
This may allow a clinician to easily
asses the amount of wound exudate absorbed into the ayers beneath the port.
The dressing 3400 may also comprise a backing layer, or cover layer 3410
extending across the width of the
wound dressing. The cover layer 3410 may be gas impermeable but moisture vapor
permeable. Some
embodiments may employ a polyurethane film (for example, Elastollan SP9109) or
any other suitable material.
For example, certain embodiments may comprise translucent or transparent 30gsm
EU33 film. The cover layer
3410 may have a pressure sensitive adhesive on the lower side, thereby
creating a substantially sealed
enclosure over the wound in which negative pressure may be established. The
cover layer can protect the
wound as a bacterial barrier from external contamination, and may allow liquid
from wound exudates to be
transferred through the layer and evaporated from the film outer surface.
The cover layer 3410 can have an orifice 3411 located so as to underlie the
suction port. The orifice 3411 may
allow transmission of negative pressure through the cover layer 3410 to the
wound enclosure. The port may be
adhered and sealed to the cover film using an adhesive such as an acrylic,
cyanoacrylate, epoxy, UV curable or
hot melt adhesive. Some embodiments may have a plurality of orifices for the
attachment of multiple ports or
other sources of negative pressure or other mechanisms for distributing fluid.
Figure 4B illustrates a cross sectional view of the would dressing 3400,
displaying an embodiment of the relative
thicknesses of layers of the dressing 3400. In some embodiments, the wound
contact layer 3460 may be flat
and the top film layer 3410 may be contoured over the inner layers of the
dressing 3400. The spacer layer 3450
may be half as thick as the acquisition distribution layer 3440 in some
embodiments. In some embodiments, the
-48-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
absorbent layer 3430 may be about 1.5 times thicker tian the spacer layer
3450. The obscuring layer 3420 may
be about half the thickness of the spacer layer 3450.
Figure 4C illustrates another embodiment of a wound dressing 3900, with the
various layers illustrated in an
exploded view. Although this figure illustrates a dressing having one
particular shape, the construction of the
layers can be applied to any of the embodiments identified below, including
Figures 5A- Figure 8, and any of the
dressing shapes and configurations described in the patent applications
incorporated by reference herein. The
wound dressing may comprise a release layer 3980, wound contact layer 3960, a
transmission layer 3950, an
acquisition distribution layer 3940, an adhesive layer 3970, an absorbent
layer 3930, an obscuring layer 3920,
and a backing layer 3910. At least the wound con:act layer 3960, transmission
layer 3950, absorbent layer
3930, obscuring layer 3920, and backing layer 3910 may have properties as
described with respect to particular
embodiments above, such as the embodiments of Figures 3A-3C, as well as or
instead of the properties
described below.
The dressing 3900 may be connected to a port 3990, as illustrated in Figure 4D
(shown without the release layer
3980). At least the backing layer 3910, obscuring layer 3920, absorbent layer
3930, and acquisition distribution
layer 3940 may have openings underlying the port 3990, and the port 3990 may
comprise a three-dimensional
fabric 3997 and a filter element 3995 overlying the openings. The absorbent
layer 3930 may be configured to
absorb and retain exudate from a patient's wound. Tie absorbent layer 3930
will preferably be constructed from
a material which has good absorbent qualities under negative pressure. The
adhesive layer 3970 may bond an
upper surface of the acquisition distribution layer 3940 to a lower surface of
the absorbent layer 3930. In some
embodiments of a trimmable dressing 3900, other layers may be bonded together
to provide consistency with
respect to layer alignment when the dressing is cut on one or more sides, such
that the layers remain together
when the sides of the dressing are cut, and such that there is not vertical
separation of the layers at the cut
portions.
Figures A1 ¨ A4 illustrate trimming a TNP dressing in various manners. In
Figure A1, the cut line is a simple
truncation of the dressing, exposing the internal transmission layer; In
Figure A2, a hole has been cut to receive
a fixation device or digit; in Figure A3, the dressing has been cut to allow
profiling to a curved body portion; in
Figure A4 the dressing has been articulated to dress a moving joint such as a
knee, and in this case,
transmission layer has been cut away but an amount of border region 2200 has
been retained to assist in
retaining and sealing the dressing at its edge.
Figures A5 ¨ A6 illustrate applying the trimmed dressings and dispensing
sealant at the exposed transmission
layer portions by means of syringe A. In Figure A7. sealant is dispensed via
syringe A to a puncture B in the
backing layer 2140.
Figure B1 illustrates in detail the means of dispensing sealant by syringe A.
The syringe in this case has a nozzle
aperture which allows sealant to be dispensed within the structure of the
transmission layer, in Figure B2 a
suitable relationship of transmission layer height or thickness and syringe
nozzle cross section and aperture is
shown.
Figures C1 ¨ C3 illustrate different modes of dispensing sealant C: C1 -
internally to the transmission layer 2105,
as was shown in Figure B1; C2 and C2a - bridging the exposed portion of
transmission layer 2105 and skin
-49-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
surrounding a wound 200; and C3 ¨ bridging the exposed portion of transmission
layer 2105 and retention strips
laid down on skin surrounding a wound, before the dressing has been applied.
Dressings are shown comprising
backing layer or sheet and transmission layer, being spacer layer, at the
exposed portion 2105. The dressing
may comprise further layer(s) (not shown) as hereinbefore defined at the
exposed portion 2105. The main
dressing portion or module of the dressing pad may contain further layers
(e.g. an absorbent layer above the
transmission layer) not shown. Figure C1 shows one embodiment of sealing and
mode of dispensing of
composition C. In this embodiment syringe A such as a static mixer is located
such that the nozzle penetrates
within the exposed portion 2105. The syringe A nozzle is of cross-section area
suited to be received within the
exposed portion 2105 of transmission layer. Composition C is dispensed
internally to the exposed portion 2105.
Syringe A nozzle is inserted to penetrate a short distance within the exposed
portion 2105, at intervals along the
exposed face, whereby composition C is dispensed intemally as a seal within
the exposed portion 2105.
Composition may flow to some extent on initial application, either or both
laterally to the direction of dispensing
and advancing and receding, flow becoming less as composition hardens or
cures. This may aid in providing a
continuous lateral seal, whereby dispensing intervals along the face of
exposed portion 2105 may be increased.
Nozzle insertion distance within the exposed portion 2105 may be selected to
confine the seal spaced a short
distance in from the face of the exposed portion, or to allow some spill of
composition C out of the exposed
portion and onto surrounding surfaces such as a preparation plate or skin 200.
Advantages of this embodiment include minimizing the amount of composition C
required to be dispensed. This
in tum allows use of a lower capacity syringe or static mixer A. The back
pressure encountered on dispensing
from a static mixer increases with the mixer volume, vilich in turn leads to a
decrease in the viscosity which the
syringe or mixer A is able to dispense. It is generally advantageous to this
embodiment to deliver composition C
at as high a viscosity as possible to ensure that composition C is confined
within the exposed portion 2105. A
further element in the total back pressure or resistance encountered on
dispensing composition C is the nozzle
aperture of syringe A. For this embodiment, it is desired to dispense
composition C from a small aperture nozzle,
and this adds to the back pressure. The advantage that this embodiment
delivers of enabling a relatively small
volume syringe or mixer A to be employed, allows greater freedom to operate a
small aperture nozzle.
Finally we have found that a seal generated by dispensing composition C
internally to the exposed portion 2105,
according to this embodiment, is highly effective. The dressing should be
trimmed, as hereinbefore described,
such that the exposed portion overlies intact skin about a wound, and does not
overly the wound itself. In the
case of a dressing having an adhesive or tacky wound contact layer, such as a
silicone contact layer as
hereinbefore described, the wound contact layer adheres to the skin 200 about
the wound and seals the dressing
to skin 200 about the exposed portion 2105 and the dispensed seal C. The wound
contact layer is perforated or
otherwise porous to allow transmission of fluids to and from the wound bed,
and this may permit flow of
composition C onto skin directly proximal to the internal seal. This may
beneficially enhance the seal between
the wound contact layer and skin 200. In the event that flow of composition to
skin 200 directly proximal to the
internal seal is not desired, composition C suitably has a sufficiently high
viscosity to restrict flow, alternatively
the wound contact layer may be non-porous or non-permeable in the region
proximal to an envisaged exposed
portion, for example at a bridging portion or trimmable portion as
hereinbefore defined.
Figure B1 may be considered, for one purpose, to illustrate dispensing
composition C to a dressing having an
obscuring layer whereby the dispensed seal is obscured. In contrast, the
dispensed seal is shown beneath the
backing layer or sheet in Figure C1. This illustrates that dispensing
composition C to a dressing having no
obscuring layer, or having window(s) in obscuring layer at bridging portions
or trimmable portion(s) allows visual
-50-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
control of nozzle insertion distance within the exposed portion 2105, of
composition C volume dispensed, and of
lateral flow enabling a suitable dispensing interval across the face of
exposed portion 2105 to be determined. In
the case that no obscuring layer is present it is preferred that the
composition incorporates ADL as hereinbefore
defined as transmission layer, rather than spacer layer which may pose a risk
of penetrating the backing sheet.
Figure C2a shows an extension of Figure C2 showing the sealant on the top
film. Parts are referred to using the
same reference numbering as in Figure C2. We have found that an advantageous
feature of a further
embodiment of sealing and mode of dispensing is that the composition C is
dispensed to the backing layer or
backing sheet adjacent the exposed portion 2105, whereby composition C flows
across the exposed portion
2105 totally covering the exposed portion 2105. In some cases composition C
flows a short distance into or is
drawn a short distance within the exposed portion 2105. It may be desired to
dispense or smooth composition C
onto the perimeter of the exposed portion 2105 adjoining border region 2200 as
shown in Figure C2a, and even
directed slightly back along the perimeter (not shown). This has the advantage
of advancing composition C a
short distance at the perimeter of the exposed portion 2105, ensuring a total
seal C and also securing the seal C
in place. As composition C hardens or cures, the viscosity typically increases
and flow ceases whereby
composition C is retained at or in the dispensing location 2105 and forms an
effective seal C.
This further embodiment places performance requirements on the composition C
and the resulting seal,
additional to those of the first embodiment of sealing and mode of dispensing.
Specifically composition C
requires a continuous film to be dispensed and formed across the surface of
the backing layer or sheet bridging
onto the exposed portion of any additional layers and the exposed portion 2105
of the transmission layer and
bridging onto the skin surface 200. Therefore composition C must be
sufficiently viscous and/or cohesive to form
an intact film. Such film may be thin or, as illustrated in Figure C2, C2a and
C3 may be of appreciable depth
and/or thickness of for example from the order of depth and/or thickness of
the backing sheet to the order of
depth and/or thickness of the dressing or of the component layers at the
exposed portion 2105 thereof. Should
such film rupture or fail prior to setting or curing of composition C then the
seal will fail. After setting or curing of
an intact film, the exposed nature of the seal and its presentation as a film
place additional requirements of
robustness, both to external influences and also, to its ability to retain
integrity across interfaces between
adjacent layers. These requirements are likely to be greater in the case of a
thin film. Preferably therefore a seal
according to this further embodiment is characterized by properties of tensile
strength, permanent set, and
elongation at break, optionally also extensibility, in ranges as hereinbefore
defined. In contrast a seal generated
according to the first embodiment, as illustrated in Figwe C1 above, is
supported in large part by the fabric of the
dressing enclosing the seal C, whereby requirements of tensile strength,
permanent set, elongation at break, are
significantly lower, also being enclosed within the lower extensibility
dressing, the requirement for extensibility is
significantly lower than for the further embodiment of Figures C2 and C3.
Figures C2a illustrates the need for the seal of this embodiment to be
effective from the backing layer surface
across the exposed portion 2105. As will be apparent, a seal across the
exposed portion alone is susceptible to
failure at the interface of the backing sheet and exposed portion and any
intervening layers.
The further embodiment illustrated by Figures C2 to C3 is likely to be more
effective when adopted in relation to
a dressing comprising no additional layers as hereinbefore defined, thereby
better resisting strains introduced by
separation at the interface of additional layer(s) and transmission layer.
Additional layer(s) if present may
beneficially be secured at their interfaces with each other and with
transmission layer, by needling, stitching and
other means as known in the art.
-51-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
The further embodiment of Figures 2 and 3 moreover requires that a seal C have
low profile and /or
compressibility greater than or equal to the surrounding dressing. This is of
advantage in minimizing discomfort
to the wearer imposed by a protruding ridge at the exposed portion 2105 of the
dressing.
In Figures C4 and C5 syringe A is illustrated dispensing composition C to
exposed portion 2105 of transmission
layer in dressing, having border region 2200. In Figure C6 syringe A is
illustrated with modest nozzle head
spread for dispensing composition C to profiled exposed portion 2105 of
transmission layer. In Figure C7 syringe
A nozzle head is illustrated having combined spreader tip with plural nozzles,
conformable to dispense to a
profiled exposed portion and/or to dispense with obstructed access to exposed
portion. In Figure C8, an
imaginary cone illustrates maximum dispenser A dimensions to allow a shallow
entry angle to exposed portion
2105 relative to the skin 200.
Figures C4 and C5 illustrate dispensing composition C according to the first
embodiment of sealing and mode of
dispensing of composition C, of Figure C1, using a syringe or static mixer A
having a combination of spreader tip
with plural nozzles as hereinbefore defined (spreader having plural projecting
apertures). Parts are referred to
using the same reference numbering as in Figure C1. A dispenser (A) is
illustrated having a nozzle head
comprising 5 nozzles with small nozzle cross section to deliver sealant to the
interior portion within the exposed
portion 2105 of transmission layer. The spread of the nozzle head matches the
width of the bridging portion
shown enabling dispensing on a single insertion. Figura C4 illustrates
internally dispensing composition C to the
interior portion of the exposed portion 2105 of a bridging portion in the
context of a relatively larger dressing. The
main dressing portion or module of the dressing pad may contain further layers
(e.g. an absorbent layer above
the transmission layer) not shown. Figure C5 may for example illustrate
dispensing to the interior portion of the
exposed portion 2105 of a bridging portion in the context of any shape or
configuration dressing as hereinbefore
defined. This embodiment of dispenser for and mode of dispensing composition
benefits from a decreased
burden and decreased requirement for accuracy on the part of the operator, an
increase in mechanical accuracy
of dispensing location and continuous seal formation. It may also slightly
reduce the back-pressure at the syringe
allowing the use of higher viscosity composition. A substantial border region
2200 is illustrated, which contributes
to seal integrity.
Figure C6 illustrates a multi nozzle head syringe A where the overall width or
spread of the head is kept modest
so as to allow dispensing into exposed portion 2105 of a transmission layer on
a curve, e.g. a body contour.
Figure C7 illustrates an alternative multi nozzle head dispenser A that is
flexible or deformable in two locations
facilitating dispensing into exposed portion 2105 of a transmission layer on a
curve, e.g. a body contour and /or
dispensing into a location 2105 having obstructed access. There is a flexible
arm or restraint (shown in grey) with
four nozzles emerging out of the arm. This is joined to the main body (with
integral static mixer) via four flexible
tubes (also shown in grey).
The end on view illustrates the nozzle ends and flexible arm showing how the
arm may be bent to conform to an
arc. The tubes may similarly be bent (not shown) to conform to generate an
angled nozzle, beneficially
increasing the entry angle for dispensing.
The flexible arm is typically not elastic, i.e. it retains :he shape conferred
for dispensing until bent to return to its
original shape or a different conformation. The flexible arm could be formed
of a deformable polymer or putty or
the like or it could be a mechanical flexible or deformable arm (i.e.
httn://snakeclamp.com/ or
htto://joby.com/gorillaood).
-52-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
Figure C8 illustrates an imaginary cone containing the dispenser A. This shows
the maximum dimensions that
may advantageously be considered in the design of the dispenser A to allow a
shallow entry angle relative to the
skin 200, to allow a nozzle to be inserted into an exposed portion 2105 of
transmission layer in a dressing
adhered to a patient 200.
Figures 5A and 5B illustrate various embodiments of a wound dressing 500 which
may be trimmable at a bridge
portion 530. The dressing 500 may comprise a backing layer 510, an absorbent
layer and/or transmission layer
formed in a main portion 520 and at least one additional portion 540 separated
by a gap 560 and connected by a
bridge portion 530, and a port 550. In some embodiments, the main portion 520,
additional portion 540 and
bridge portion 530 comprise at least a transmission layer such as described
above between an optional wound
contact layer and a backing layer 510. In any or all of these sections, the
dressing 500 may further comprise an
optional absorbent material such as described positioned between the backing
layer 510 and the transmission
layer. In some embodiments, the absorbent layer may have a similar footprint
to the transmission layer. In other
embodiments, the absorbent layer may be located at main portion 520 and at
least one additional portion 540,
but the absorbent layer may not be included in the bridge portion 530. As
illustrated, the dressing has an
elongate, rectangular shape, though other shapes are also contemplated. The
absorbent layer preferably has a
smaller footprint than the backing layer, so that the absorbent layer is
completely surrounded by the backing
layer. It will be appreciated that in some embodiments, the absorbent layer is
an integral, one-piece layer of
material that extends across the main portion 520, the additional portion 540
and in the bridge portion 530.
Some embodiments may be manufactured without the port 550 and may include at
least one area for attaching a
port. For example, the port 550 may simply be an opening in the backing layer
for attaching a separate port
member.
The dressing 500 may also comprise other layers as discussed above with
respect to Figures 3A-4B. For
instance, the dressing 500 may comprise a wound contact layer which may be
sealed to the backing layer 510,
thereby creating an enclosed chamber for the absorbent layer and/or
transmission layer and any other layers
within the dressing. The wound contact layer and backing layer may be sealed
along a perimeter with a certain
distance from the edge of the sealed perimeter to the edge of the absorbent
layer. The wound contact layer and
backing layer may also be sealed together throughout some or all of the area
of a gap 560 between portions of
the inner layers.
The transmission or wicking layer, as described above, may be provided for the
transmission of negative
pressure throughout the dressing and for drawing wound exudate away from the
wound site and into the upper
layers of the dressing 500. Some embodiments of the transmission layer may
comprise the acquisition
distribution layer, as described above with respect to Figure 4A, for lateral
transmission of fluids such as wound
exudate. Some embodiments may employ both a wicking layer and an acquisition
distribution layer. Use of one
or more of these layers may advantageously maintain fluid transmission through
narrow portions of the dressing
such as the bridge portions, and may keep these narrow portions from partially
or completely collapsing under
negative pressure.
As illustrated in Figure 5B, the absorbent layer and/or transmission layer may
comprise a main portion 520 and a
plurality of additional portions 540. The additional portions may be smaller
than or the same size as the main
portion 550. For example, as measured along the longitudinal length of a
rectangular dressing, the length of the
-53-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
additional portions may be smaller than the length of the main portion, and
each additional portion may have the
same length. As illustrated, the main portion 520 is connected to the first
additional portion 540 by one bridge
portion 530 aligned along the center longitudinal axis of the dressing 500,
and each additional portion is
connected to the next additional portion by a similar bridge. The bridge
portion may in Figures 5A and 5B may
also be located off the center axis, for example at the side of the dressing.
Other embodiments may employ a
plurality of bridges for connecting the portions of the dressing. For example,
one embodiment may employ two
bridges to connect adjacent portions, wherein the bridges are located at the
side edges of the adjacent portions
next to the sealed perimeter. Another embodiment may employ two bridges each
located a distance away from
the side edges of the adjacent portions.
In some embodiments the main portion 520 may be a precalculated minimum
length, and some or all of the
additional portions 540 may have lengths that can be removed for custom sizing
of the dressing to a variety of
lengths exceeding the minimum length. The main portion length may be longer
than the additional portion
lengths, or the main portion may have the same length as the additional
portions. Such embodiments may be
advantageous for a long incision such as a leg incision made for a vein
harvest. In an embodiment, the main
portion 520 may be a minimum incision length or minimum leg length, and the
additional portions 540 may be
included in the dressing to achieve a length up to a maximum incision length
or a maximum leg length. In use,
the dressing may be trimmed according to the incision or leg length of the
patient across the bridge portions, for
example at cut line 570 described below. In some embodiments, additional ports
or port attachment sites may
be Located on some or all of the additional portions in order to maintain a
substantially even level of negative
pressure throughout a relatively long dressing.
The bridge portion 530 in Figures 5A and 5B creates a continuous path for
negative pressure delivery between
multiple portions of the dressing. The bridge portion 530 may have a width
that is less than 1/8, 1/4, or 1/3 the
width of adjacent portions of absorbent material andior transmission layer. A
wider bridge portion allows for
greater transmission of negative pressure and fluids such as wound exudate,
however a narrower bridge portion
is advantageous for sealing a dressing trimmed at the bridge portion. Further,
patient comfort may be enhanced
if the bridge portion 530 is wide enough to cover a wound or an incision.
Embodiments of the dressings
described herein may balance these factors according to a variety of purposes
and/or considerations, and
therefore the width of bridge portion 530 may vary. In some embodiments the
bridge portion 530 may be
approximately 15 mm wide, however other embodiments may be 10 mm to 20 mm (or
about 10 mm to about 20
mm) wide or thinner or thicker. In embodiments employing a plurality of bridge
portions, the bridge portions may
all be a uniform width or may have varying widths. In some embodiments, the
bridge portion 530 may comprise
a wound contact layer, a transmission layer (which may be one or both of the
wicking layer or acquisition
distribution layers described above with respect to Figure 4A), and a backing
layer. Some embodiments of the
bridge portion 530 may further comprise an absorben: or superabsorbent layer.
The layers in the bridge portions
530 may be continuous with layers found in the portions 520 or 540 of the
dressing, or they may be discrete
layers positioned side-by-side.
In a dressing applied to a nonplanar surface, the bridge portions may also
advantageously provide enhanced
flexing of the dressing for conforming to the nonplanar surface. Further, the
bridge portions may enhance side
flexing capabilities of the dressing for covering a curved or arcuate
incision. In some embodiments, the location
-54-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
and width of the bridge portions may be selected for both connecting a
plurality of trimmable portions as well as
for flexibility of the dressing.
The dressing 500 may be trimmed at or across the bridge portion 530. Although
the dressing may be trimmed at
any portion, trimming the dressing at bridge portion 530, for example
perpendicular to the length of the dressing,
enables easier sealing as a narrower cross sectional area is exposed, and thus
less area requires sealing after
trimming. In some embodiments, the gap 560 may have the same width as the
distance from the sealed
perimeter edge to the absorbent layer, such that when the dressing is trimmed
along a trim line 570 adjacent to
the additional portion 540 the sealed perimeter around the inner layer(s) is
substantially unchanged. In some
embodiments this width may be approximately 2.5 cm, and in other embodiments
may be any width suitable for
maintaining the seal between the backing layer and the wound contact layer. It
will be appreciated that the
dressing may be trimmed at locations other than the illustrated trim line 570,
which is included for illustrative
purposes only, for example at a trim line in the center of the bridge portion
530 or at a diagonal or curved trim
line.
In some embodiments, the absorbent layer and/or other layers of the wound
dressing may be prescored for
sizing. Other layers, such as the transmission layer or acquisition
distribution layer, may also be prescored. The
backing layer may not be scored, as a through hole may limit the ability of
the backing layer to function as a
bacterial barrier or compromise the ability of the dressing to maintain
negative pressure. Other embodiments
may include a printed or indented pattern on some or all of the layers to
indicate possible trim lines.
After trimming, the dressing 500 may be sealed by an adhesive strip, a piece
of a sealing drape, by another
dressing, or by a sealant. In some embodiments, a retention strip may be
applied at the interface of the dressing
edge and the skin. The retention strips may be applied to cover trimmed
dressing borders. In some
embodiments the retention strips may comprise a pressure-sensitive adhesive on
the lower surface, and in other
embodiments may be applied over a sealant. It will be appreciated that any
other adhesive method or
mechanism may be used to seal the dressing. For example, a sealant may be
applied with a tool such as a
syringe around the trimmed area in order to reseal the chamber of the dressing
or to seal the dressing to a
patient. Some embodiments of the dressing may be self-sealing.
Figures 5C and 5D illustrate removing a portion of d-essing from the dressings
of Figures 5A and 5C thereby
exposing a bridging portion of transmission layer 2105 which can be sealed
before or after applying the dressing
to a wound site, by means of sealant C dispensed from syringe A. In the case
of Figure 5C, the dressing may
simply be suited to a particular ulcer size, whilst in tie case of Figure 5D,
the dressing is admirably suited to
dressing a vein harvest wound running the length of a subject's leg.
Figure 6 illustrates an embodiment of a trimmable wound dressing 600
comprising a plurality of portions or cells
620. The dressing 600 may comprise a sealed perimeter 610 of a backing layer
and a wound contact layer, a
plurality of cells 620, a plurality of bridges 630 cornecting adjacent
portions, and a port member 640. As
described above, the dressing 600 may be trimmed at the bridge portions and
sealed along the trim line. Each of
the cells 620 may include absorbent material and/or a transmission layer as
described above, along with other
optional layers. The bridge portions 630 may comprise a wound contact layer, a
transmission layer (which may
-55-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
be one or both of the wicking layer or acquisition distribution layers
described above with respect to Figure 4A),
and a backing layer. Some embodiments of the bridge portions 630 may further
comprise an absorbent or
superabsorbent layer. The layers in the bridge portions 630 may be continuous
with layers found in the cells
620, or they may be discrete layers positioned side-by-side.
As illustrated, the dressing comprises a 4 x 4 array of cells 620. Other
embodiments may comprise any suitable
array of cells, or may be configured as a long rolled dressing N cells wide.
The cells may be connected by one
or more narrow bridge portions 630 and separated by gaps 650. The backing
layer and wound contact layer
may be sealed together throughout the gaps. By trimming at the bridge portions
630, the integrity of the dressing
may be maintained even as the dressing is significantly resized. For example,
the dressing may be trimmed so
that only one inner cell or a group of inner cells remain, and the layers of
the dressing will not separate due to the
sealing of the backing layer and wound contact layer throughout the area of
the gaps 650.
In some embodiments, the center cells of the dressing 600 may be removed. This
may provide benefits, for
example, when the dressing is used to cover a grafted skin flap or sutured
skin flap. The dressing may be
resized so that the unsutured skin is substantially uncovered by the dressing.
Thus, the removed sections would
otherwise cover the healthy skin of the flap. Cove-ing the healthy skin with
the dressing potentially creates
problem such as exposing the wound to bacteria on :he surface of the flap and
exposing the healthy skin of the
flap to excess moisture. The dressing may also be resized accordingly to cover
circular, curved, or otherwise
irregularly shaped suture lines.
The port member 640 may be located, as illustratec, on a corner cell of the
dressing 600. However, in other
embodiments the port may be located on a different cell. Some embodiments may
employ multiple ports, each
port connected to a different cell. For example, a large dressing or longed
rolled dressing may comprise a port at
an edge cell of every N rows, such every as four rows or five rows. Some
embodiments may, instead of the
illustrated port member 640, comprise a port attachment site or sites.
Figure 611 illustrates removing a portion of dressing from the dressing of
Figure 6 thereby exposing multiple
bridging portions of transmission layer which can be sealed before or after
applying the dressing to a wound site,
by means of sealant C dispensed from syringe A. It can clearly be seen that
the use of bridging portions
dramatically reduces the cross-sectional area of transmission layer that must
be sealed, reducing thereby both
dressing time and risk of leaks.
Figure 7 illustrates an embodiment of a trimmable wound dressing 700
comprising a plurality of portions with
multiple port attachment sites 760. Similar to the dressing 600 described
above, the T-shaped dressing 700
comprises a backing layer and wound contact layer taving a sealed perimeter
710 around a plurality of cells 720
containing absorbent material and/or a transmission layer connected by bridge
portions 730 and separated by
gaps 740. The bridge portions 730 may comprise a wound contact layer, a
transmission layer (which may be
one or both of the wicking layer or acquisition distribution layers described
above with respect to Figure 4A), and
a backing layer. Some embodiments of the bridge portions 730 may further
comprise an absorbent or
superabsorbent layer. The layers in the bridge portions 730 may be continuous
with layers found in the cells
720, or they may be discrete layers positioned side-by-side. The backing layer
and wound contact layer may
-56-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
also be sealed together throughout some or all of the area of the gaps 740. As
described above, the dressing
700 may be trimmed at the bridge portions and sealed along the trim line.
Although the dressing is illustrated as
being T-shaped, this is for illustrative purposes only, and the dressing may
be a variety of branched shapes.
Each branch may comprise one or more cells connected by one or more bridge
portions.
The dressing comprises a plurality of port attachment sites 760. Each
attachment site 760 may be a hole in the
backing layer and may be covered with a removable tab 760. The tab may
comprise a suitable backing material
with a layer of adhesive on some or all of the lower surface. Some embodiments
may comprise a ring of
adhesive sized to surround the hole 750 in the backinc layer. The tab 760 may
be removed so that a port may
be attached to the backing layer over the hole 750 for transmission of
negative pressure into the dressing 700.
In some embodiments, port attachments may be secured at just one port
attachment site. In other embodiments,
port attachments may be secured over a plurality of attachment sites as needed
for transmission of negative
pressure throughout the dressing. Some ports may comprise an adhesive on the
lower surface thereof for
attachment to the dressing. Some embodiments of the dressing may comprise an
adhesive layer for attaching
the port.
Figure 711 illustrates removing a portion of dressing from the dressing of
Figure 7 thereby exposing a bridging
portion of transmission layer which can be sealed before or after applying the
dressing to a wound site, by
means of sealant C dispensed from syringe A. It car clearly be seen that this
form of trimmable dressing is
suitable for difficult to dress wounds which may require some visual
inspection and comparison with dressing
configurations, for example in the case of a flap wound.
Figure 8 illustrates an embodiment of a trimmable wound dressing 800 with
multiple port attachment sites 840.
The dressing comprises a backing layer and wound contact layer having a sealed
perimeter 710, an absorbent
layer 820, a spacer layer 830 below the absorbent layer, and a plurality of
holes 840 in the backing layer covered
by tabs 850. The spacer layer 830 may be one or both of the transmission layer
and acquisition distribution layer
discussed above. It will be appreciated that in some embodiments, only one of
the absorbent layer or spacer
layer may be provided, with the other layer being optional.
The dressing 800 is configured as a roll with port attachment sites 840 spaced
a distance apart along the upper
surface. In some embodiments this distance may be uniform between all port
attachment sites, and in other
embodiments the distance may vary. The dressing roll may be custom sized by
unrolling a length of dressing,
trimming the dressing, sealing the two sides, and attaching a port or ports to
one or more port attachment sites.
In some embodiments, unused port attachment sites 840 may remain sealed by
adhesive tabs 850. In some
embodiments, the spacer layer 830, and optionally the absorbent layer 820, may
comprise a bridge portion or
plurality of bridge portions located between each port attachment site for
ease of sealing a trimmed dressing. It
will be appreciated that any of the dressings described above may be
configured as a trimmable roll with a
plurality of port attachment sites located a distance apart on the roll. For
example, an elongate dressing
configured as a roll may include narrower bridging portions spaced along a
length of the dressing between port
attachment sites to facilitate trimming of the dressing to a suitable size.
-57-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
Figure 811 illustrates removing a portion of dressing from the dressing of
Figure 8 thereby exposing multiple
bridging portions of transmission layer which can be sealed before or after
applying the dressing to a wound site,
by means of sealant C dispensed from syringe A.
Such adaptable, resizable dressings may provide the advantage of reducing the
inventory of dressings that a
hospital or clinic is required to keep. Rather than maintaining a large
inventory of dressings consisting of a
multitude of shapes and sizes for all possible wound or incision sites, a
hospital or clinic may only require one or
several of the dressings described herein which can be modified to suit any
patient needs. Further, it may be
advantageous from a manufacturing perspective to produce adaptable dressings.
Overview of Example Layer Materials
Figures 9A and 9B illustrate one embodiment of spacer layer, or transmission
layer, material which may be used
in any of the dressing embodiments described above, and which may also be used
in any of the port or fluidic
connector embodiments described above. The spacer or transmission material is
preferably formed of a material
having a three dimensional structure, and may have a top layer and a bottom
layer comprising a knit pattern. For
example, a knitted or woven spacer fabric (for example Baltex 7970 weft
knitted polyester) or a non-woven fabric
could be used. The top and bottom fabric layers may comprise polyester, such
as 84/144 textured polyester or a
flat denier polyester. Other materials and other linear mass densities of
fiber could of course be used. In some
embodiments, the top and bottom fabric layers may be the same pattern and the
same material, and in other
embodiments they may be different patterns and/or different materials. The top
fabric layer may have more
filaments in a yarn used to form it than the number of filaments making up the
yarn used to form the bottom
fabric layer, in order to control moisture flow across the transmission layer.
Particularly, by having a filament
count greater in the top layer, that is to say, the top layer is made from a
yarn having more filaments than the
yarn used in the bottom layer, liquid tends to be wicked along the top layer
more than the bottom layer. Figure
9A illustrates one possible knit pattern for a top or bottom fabric layer.
As illustrated in the side view of Figure 9B, between the top and bottom
fabric layers may be a plurality of
filaments. The filaments may comprise a monofilament fiber or a multistrand
fiber, and may be knitted polyester
viscose or cellulose. In some embodiments, a majority of the filaments, by
volume, may extend vertically (that is,
perpendicular to the plane of the top and bottom layers), or substantially or
generally vertically. In another
embodiment, 80%-90% (or approximately 80% to approximately 90%) of the
filaments or more, by volume, may
extend vertically, or substantially or generally vertically. In another
embodiment, all or substantially all of the
filaments, by volume, may extend vertically, or substantially or generally
vertically. In some embodiments, a
majority, 80%-90% (or approximately 80% to approximately 90%) of the filaments
or more, or even all or
substantially all of the filaments, extend upward from the bottom fabric layer
and/or downward from the top fabric
layer, and in some embodiments, such filaments extend over a length more than
half the distance between the
top and bottom fabric layers. In some embodiments, a majority, 80%-90% (or
approximately 80% to
approximately 90%) of the filaments or more, or even all or substantially all
of the filaments, span a distance that
is greater in a direction perpendicular to the top and bottom fabric layers (a
vertical direction) than in a direction
parallel to the top and bottom fabric layers (a horizontal direction). The
orientation of such filaments may
promote vertical wicking of fluid through the spacer layer. In some
embodiments, the ratio of the amount of fluid
wicked vertically through the spacer material to the amount of fluid wicked
laterally across the spacer material
when under negative pressure may be 2:1 or more, or approximately 2:1 or more,
or may be up to 10:1 or more,
or approximately 10:1 or more, in some embodiments. Such filaments may also
keep the top and bottom layers
-58-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
spaced apart when exposed to compressive forces or negative pressure. Some
embodiments of the spacer
layer may have a tensile strength that substantially prevents tearing by
typical force applied by human hands,
and accordingly would need to be severed by other means, such as being cut or
sliced, if implemented in a
trimmable dressing.
Figures 10A-10D illustrate one embodiment of acquisition distribution layer
(ADL) material which may be used in
any of the dressing embodiments described above, and which may also be used in
any of the port or fluidic
connector embodiments described above. To those versed in the art of
acquisition distribution layers it would be
obvious that other ADL materials may be used to achieve a similar effect.
Figure 10A illustrates a backscatter scanning electron microscope (SEM) plan
view of a sample portion of
acquisition distribution layer material at 140x magnification. Figure 10B
illustrates an SEM cross sectional view
at 250x magnification. As illustrated in Figure 10B, a majority of the fiber
volume may extend horizontally (that is,
parallel to the plane of the top and bottom surfaces of the material), or
substantially or generally horizontally. In
another embodiment, 80%-90% (or approximately 80% to approximately 90%) or
more of the fiber volume may
extend horizontally, or substantially or generally horizontally. In another
embodiment, all or substantially all of
the fiber volume may extend horizontally, or substantially or generally
horizontally. In some embodiments, a
majority, 80%-90% (or approximately 80% to approximately 90%) of the fibers or
more, or even all or
substantially all of the fibers, span a distance perpendicular to the
thickness of the ADL material (a horizontal or
lateral distance) that is greater than the thickness of the ADL material. In
some embodiments, the horizontal or
lateral distance spanned by such fibers is 2 times (or about 2 times) or more,
3 times (or about 3 times) or more,
4 times (or about 4 times) or more, 5 times (or about 5 times) or more, or 10
times (or about 10 times) or more
the thickness of the ADL material. The orientation of such fibers may promote
lateral wicking of fluid through the
ADL material. This may more evenly distribute fluid such as wound exudate
throughout the ADL material. In
some embodiments, the ratio of the amount of fluid wicked laterally across the
ADL material to the amount of
fluid wicked vertically through the ADL material under negative pressure may
be 2:1 or more, or approximately
2:1 or more, or may be up to 10:1 or more, or approximately 10:1 or more, in
some embodiments.
Figure 10C is a two dimensional microtomographic cross sectional view of a
compressed portion of a sample of
ADL material which is approximately 9.2 mm long. Figure 10D is an SEM cross
sectional view at 130x
magnification of the compressed portion illustrated in Figure 10C. Such
compressed portions may occur in the
ADL material may occur due to the application of pressure to the material.
Figures 10C and 10D further
illustrate the horizontal network of ADL fibers.
Figures 11A and 11B illustrate one embodiment of absorbent material which may
be used in any of the dressing
embodiments described above. Figure 11A illustrates a three dimensional
microtomographic cross sectional
view of a sample of absorbent material, depicting a fibrous composition
interspersed with superabsorbent
particles. The absorbent material may, for example, be any of the materials
described in U.S. Patent Pub. No.
2012/308780, titled "Absorbent Structure," filed May 25, 2012, the contents of
which are hereby incorporated by
reference in their entirety.
Figure 11B is a cross sectional schematic diagram of an embodiment of the
absorbent material illustrating a
plurality of layers within the absorbent material. The absorbent material may
have a textured layer 4210 on one
side of a fibrous network, the fibrous network defining the bulk of the
absorbent material and comprising layers
-59-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
4220, 4240, and 4250. Superabsorbent particles 4230 may be dispersed
throughout layers 4220, 4240, and
4250. The textured layer 4210, also referred to as the "tissue dispersant
layer" in above portions of this
specification, may be configured to laterally transmit fluid. Though depicted
as the lowermost layer of the
absorbent material, the textured layer 4210 may in some embodiments be
positioned as the uppermost layer of
the absorbent material, and in some embodiments may be positioned as both the
lowermost and uppermost
layers of the absorbent material. The textured layer 4210 may comprise fiat
fibers 20 pm to 50 pm in width, or
approximately 20 pm to approximately 50 pm in width. The textured layer 4210
may comprise 1 to 2 or
approximately 1 to approximately 2 layers of the flat fibers, and the textured
layer 4210 may have an overall
thickness of 0.04 mm, or approximately 0.04 mm.
The bulk of the absorbent material, comprising layers 4220, 4240, and 4250,
may have a thickness of 1.7 mm, or
approximately 1.7 mm, or may have a thickness in the range of 0.5 mm to 5.0
mm, or about 0.5 mm to about 5.0
mm. The bulk of the absorbent material may comprise a mix of two fiber types
arranged in a fibrous network, for
example the cellulosic fiber having a width of 20 pm to 50 pm, or
approximately 20 pm to approximately 50 pm,
and the PE/PET composite fiber, described above with respect to the ADL
material. The superabsorbent
particles 4230 may be irregularly shaped and varied in size, and may have a
diameter of up to 1 mm, or
approximately 1 mm. The superabsorbent particles 4230 may comprise a sodium
acrylate type material. There
may be relatively fewer superabsorbent particles in a portion of the uppermost
surface of the bulk of the
absorbent material (the surface of layer 4250 opposite the textured layer
4210), for example in an uppermost
surface having a thickness of approximately 0.1 mm.
Layer 4220 may be a liquid absorption layer configured to draw liquid upward
through the material towards layers
4240 and 4250. Layer 4240 may be a storage layer configured to hold absorbed
liquid. Layer 4220 may be a
liquid distribution layer configured to apply a "reverse suction" effect to
the liquid storage layer 4240 in order to
inhibit (or substantially inhibit) absorbed liquid from leaking back down
through the lower layers of the absorbent
material, a phenomenon which is commonly known as "back wetting."
Superabsorbent particles 4230 may be distributed primarily within the storage
layer, may extend partially into the
absorption layer 4220 and liquid distribution layer 4250, or may be
distributed evenly (or substantially evenly)
throughout the layers. The layers 4220, 4240, and 4250 may overlap with a
portion of adjacent layers, and may
or may not be separable.
Figures 12A and 12B illustrate one embodiment of obscuring layer material
which may be used in any of the
dressing embodiments described above. Figure 12A illustrates a photographic
plan view of obscuring material,
depicting a material comprising a fibrous network having a reoccurring
regularly spaced criss.cross diamond
pattern. The diamond shaped pattern may, in one embodiment, be 1.2 mm long by
1.0 mm wide, and may have
a thickness of approximately 0.04 mm thick, consisting of fibers that are more
densely packed relative to the
surrounding area of the material. The diamond shaped pattern may increase
structural stability of the fibrous
network of the material, for example serving as "tacking" points. Figure 12B
illustrates a three dimensional
microtomographic perspective view of the compressed diamond pattern and the
surrounding uncompressed
fibers.
-60-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
Some embodiments of the obscuring material may comprise polypropylene spunbond
material. Further, some
embodiments of the obscuring material may comprise a hydrophobic additive or
coating, for example a
hydrophobic wash designed to permeate the fibers of the obscuring material to
make the material substantially
waterproof while permitting vapor permeability. Other embodiments may comprise
a thin fibrous sheet of 60, 70,
or 80 gsm. The fibers of the obscuring material may, in one embodiment,
comprise layers of polypropylene (PP)
fibers having a smooth surface morphology, and the PP fibers may have a
thickness of approximately 25 pm. In
some embodiments, the obscuring material may have a thickness of .045 mm or
about .045 mm, or may have a
thickness in the range of 0.02 mm to 0.5 mm, or about 0.02 mm to about 0.5 mm.
Figure 13 illustrates one embodiment of an adhesive spread on approximately
one square centimeter of a film
material, which may be used as the cover or backing layer in any of the
dressing embodiments or fluidic
connector embodiments described above. The adhesive on the film has been
covered with carbon powder for
ease of illustrating the spread of the adhesive. The adhesive may comprise,
for example, an acrylate type
adhesive, for example K5 adhesive, and may be laid down in a criss cross
pattern. In some embodiments, the
adhesive material may cover approximately 45.5% approximately 1.3% of the
film surface. The pattern and
coverage of the adhesive may vary so long as the configuration is suitable for
desired vapor permeability.
I. Overview of Additional Bridged Dressing Embodiments
Figure 14A (previously Figure 17A) illustrates a plan view of a trimmable
dressing 1600 embodiment wherein the
number of layers present in the bridging portions 1620 of the dressing is less
than in an absorbent pad portion
1630 or a secondary absorbent portion 1650 of the dressing. Figure 14B
(previously Figure 17B) illustrates a
side view of the dressing 1600. Accordingly, the overall height of the
dressing is reduced at the bridging portions
1620 relative to the absorbent pad portions. In some embodiments, the dressing
can also reduce in width at the
bridging portions relative to the absorbent pad portions. The dressing 1600
also includes a port 1640 for delivery
of negative pressure.
The dressing 1600 includes a spacer layer 1662 in the absorbent pad portion
1630 and secondary absorbent
portions 1650. An ADL 1664 extends across the length of the dressing through
the absorbent pad portion 1630,
secondary absorbent portions 1650, and bridging portions 1620. The ADL 1664
satisfies the testing criteria
specified above and is capable of negative pressure transmission through the
bridging portions 1620. In some
implementations of the trimmable dressing 1600, the ADL 1664 may be
constructed from an ADL material that is
easier to cut than a spacer material, and may be accordingly selected for the
bridging portions 1620. In other
embodiments, the spacer layer 1662 may extend across the length of the
dressing in addition to or instead of the
ADL 1664.
In the absorbent pad portion 1630 and secondary a osorbent portions 1650, the
dressing 1600 includes an
absorbent layer 1666 and masking layer 1668. In some embodiments, the masking
layer 1668 may extend
across the bridging portions 1620, and may include holes, windows,
perforations, or other visual indicators for
indicating to a user where to cut the dressing. For example, perforations may
be arranged in a dashed or dotted
line configuration along a location within a bridging potion 1620, revealing a
contrasting color of the ADL 1664
beneath the masking layer 1668 to visually indicate a potential location for
trimming the dressing 1600. This
approach could be extended to include designs and symbology such as the symbol
of a pair of scissors and/or a
dotted line, or notches/chevron on each side of the masking layer, lettering
indicating a "cut here" location, or the
-61-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
like. The interior layers 1662, 1664, 1666, 1668 are positioned between a
wound contact layer 1672 and a top
film layer 1674 that are sealed together around a perimeter 1610, for example
a perimeter of approximately 2.5
cm in some embodiments.
This layer arrangement can provide the advantage of increased flexibility at
the bridging points during wear of the
dressing, easy cutting with scissors (or other means) during fitting and
shaping of the dressing to a wound site,
and easier sealing of cut portions. The reduced height of the bridging
portions provides a smaller gap that needs
to be sealed. Cut or trimmed portions can be sealed with a sealant, a sealing
strip, a flexible adhesive drape, or
other sealing means. In addition, use of different top layers in the absorbent
pad portions compared to the
bridging portions can result in a color coded dressing, making the cutting
locations clear to the user. Such a
dressing can be convenient for use along long incisicn wounds where the length
varies from patient to patient,
for example incisions resulting from abdominoplasty orocedures, as the
dressing can be trimmed according to
specific patient needs.
Referring now to Figure 15 (formerly Figure 21), another embodiment of a
trimmable dressing 2100 is illustrated.
The dressing may comprise, from bottom to top, an optional wound contact layer
(not shown), a transmission
layer and/or ADL over the wound contact layer, a plu-ality of absorbent cells
over the transmission layer and/or,
and a cover layer over the plurality of absorbent cells. As illustrated in
Figure 15, one embodiment of the
dressing includes a border 2105, a generally rectangular transmission layer
2110, a number of absorbent cells
2115, a port 2120, and a conduit 2125 for connection of the dressing 2100 to a
source of negative pressure. The
border 2105 can include a cover layer as described above sealed to the healthy
skin of a patient surrounding a
wound in one example, or can include a cover layer sealed to a wound contact
layer as described above. This
cover layer may extend over the plurality of absorbent cells 2115. The port
2120 and conduit 2125 can be
configured for transmitting negative pressure to the dressing 2100 from a
source of negative pressure when in
use.
The transmission layer 2110 can extend across the entire central pad area, and
can be any material described
herein, or the equivalent, having suitable permeabili:y to gas and liquid at a
minimum height and/or width. By
having the transmission layer 2110 extend across the central pad area rather
than only being placed in bridging
areas, a more comfortable distribution of pressure over the patient's therapy
site can be achieved. Such
pressure distribution can be considered both from the point of view of NPWT
delivery and from the point of view
of protecting friable skin, where (depending on the design of the dressing)
blistering can be caused at pad edges.
Therefore, a continuous transmission layer can, in some embodiments, minimize
the number of pad edges (i.e.
using a continuous lower layer) providing an advantage for pressure
distribution.
A number of absorbent cells 2115 can be included above the transmission layer
2110, and can be any of the
absorbent materials described herein, for example with respect to Figures 3A-
4D and 11A-11B. By cutting the
dressing 2100 along the areas of transmission layer 2110 between adjacent
cells 2115, the dressing 2100 can
be adaptively sized to correspond to the shape of a patient's wound. The
dressing 2100 can be sealed along cut
portions by one or more of re-sealing of the cover fayer and wound contact
layer, through a sealant adhesive,
and sealing strips in various embodiments.
-62-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
Although the absorbent cells 2115 are illustrated as being triangular in
shape, other variations can include
circular, oval, square, rectangular, hexagonal, or other shaped cells.
Further, although the absorbent cells 2115
are illustrated as being discrete portions of absorbent material, in other
embodiments the absorbent cells 2115
can be connected by bridging portions.
II. Overview of Additional Layer Materials
Figures 16A and 168 (formerly 22A and 22E) illustrate an example of Libeltex
DryWeb T28F that can be suitable
for use as acquisition distribution layer material (ADL) material which may be
used in any of the dressing
embodiments described above, and which may also be used in any of the port or
fluidic connector embodiments
described above. To those versed in the art of acquisition distribution
layers, also known as "surge layers," it
would be obvious that other ADL materials may be used to achieve a similar
effect of laterally wicking fluid.
Suitable ADL materials can allow for full capacity use. Such ADL layers may be
composed of multiple fiber types
and be complex in structure and design.
Figure 16A illustrates a backscatter scanning electroi microscope (SEM) plan
view of a sample portion of ADL
material at 70x magnification. As illustrated by Figure 16A, the ADL material
can comprise a number of non-
woven fibers extending at least partially horizontally (that is, parallel to
the plane of the top and bottom surfaces
of the material) for laterally/horizontally wicking fluid through the ADL
material.
Figure 168 illustrates an SEM cross sectional view of the ADL material at
1550x magnification. In the illustrated
embodiment, the ADL material may consist of a mix of multiple fiber types. One
may be a roughly cylindrical
fiber. Another fiber may be a relatively flatter fiber having a centrally-
located negative space. Another fiber may
be a multi-component fiber that has at least one inner core fiber, in some
embodiments three inner core fibers as
in the illustrated sample, and an outer layer surrounding the inner core.
Figures 17A (formerly 23A) 17B (23B) and 17 C (fo-merly 23D) illustrate an
example of Libeltex SlimCore TL4
that can be suitable for use as acquisition distribution layer material.
Figure 17A illustrates an SEM cross
sectional view of a sample portion of ADL material at 50x magnification. The
ADL material can include an upper
layer 2305 and a lower layer 2310 having different densities, lofts, and
thicknesses. For example, the upper
layer 2305 can comprise a more dense, less lofted fiber configuration and can
be approximately 730 pm thick in
some embodiments. The lower layer 2310 can comprise a less dense, more lofted
fiber configuration and can
be approximately 1200 pm thick in some embodiments. Figure 17B illustrates an
SEM plan view of a sample
portion of the denser upper layer 2305 at 70x magnification, Figure 17C
illustrates an SEM plan view of a sample
portion of the more lofted lower layer 2310 at 70x magnification. As
illustrated by Figures 17A-17C, the upper
and lower layers 2305, 2310 of the ADL material can comprise different
densities of a number of non-woven
fibers extending at least partially horizontally (that is, parallel to the
plane of the top and bottom surfaces of the
material) for laterally/horizontally wicking fluid through the ADL material.
As illustrated by Figures 17A-17C, the non-woven fibers of the various
illustrated ADL materials can extend more
in a horizontal direction than in a vertical direction to aid in lateral
wicking of fluids through the material. In some
embodiments, a majority of the fiber volume may extend horizontally or
substantially or generally horizontally. In
another embodiment, 80%-90% (or approximately 60% to approximately 90%) or
more of the fiber volume may
extend horizontally, or substantially or generally horizontally. In another
embodiment, all or substantially all of
-63-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
the fiber volume may extend horizontally, or substantially or generally
horizontally. In some embodiments, a
majority, 80%-90% (or approximately 80% to approximately 90%) of the fibers or
more, or even all or
substantially all of the fibers, span a distance perpendicular to the
thickness of the ADL material (a horizontal or
lateral distance) that is greater than the thickness of the ADL material. In
some embodiments, the horizontal or
lateral distance spanned by such fibers is 2 times (or about 2 times) or more,
3 times (or about 3 times) or more,
4 times (or about 4 times) or more, 5 times (or about 5 times) or more, or 10
times (or about 10 times) or more
the thickness of the ADL material. The orientation of such fibers may promote
lateral wicking of fluid through the
ADL material. This may more evenly distribute fluid such as wound exudate
throughout the ADL material. In
some embodiments, the ratio of the amount of fluid wicked laterally across the
ADL material to the amount of
fluid wicked vertically through the ADL material under negative pressure may
be 2:1 or more, or approximately
2:1 or more, or may be up to 10:1 or more, or approximately 10:1 or more, in
some embodiments.
Features, materials, characteristics, or groups described in conjunction with
a particular aspect, embodiment, or
example are to be understood to be applicable to any other aspect, embodiment
or example described herein
unless incompatible therewith. All of the features disclosed in this
specification (including any accompanying
claims, abstract and drawings), and/or all of the steps of any method or
process so disclosed, may be combined
in any combination, except combinations where at least some of such features
and/or steps are mutually
exclusive. The protection is not restricted to the details of any foregoing
embodiments. The protection extends
to any novel one, or any novel combination, of the features disclosed in this
specification (including any
accompanying claims, abstract and drawings), or to any novel one, or any novel
combination, of the steps of any
method or process so disclosed.
While certain embodiments have been described, these embodiments have been
presented by way of example
only, and are not intended to limit the scope of protection. Indeed, the novel
methods and systems described
herein may be embodied in a variety of other forms. Furthermore, various
omissions, substitutions and changes
in the form of the methods and systems described herein may be made. Those
skilled in the art will appreciate
that in some embodiments, the actual steps taken in the processes illustrated
and/or disclosed may differ from
those shown in the figures. Depending on the embodiment, certain of the steps
described above may be
removed, others may be added. Furthermore, the features and attributes of the
specific embodiments disclosed
above may be combined in different ways to form additional embodiments, all of
which fall within the scope of the
present disclosure.
Although the present disclosure includes certain embodiments, examples and
applications, it will be understood
by those skilled in the art that the present disclosure extends beyond the
specifically disclosed embodiments to
other alternative embodiments and/or uses and obvious modifications and
equivalents thereof, including
embodiments which do not provide all of the features and advantages set forth
herein. Accordingly, the scope of
the present disclosure is not intended to be limited by the specific
disclosures of preferred embodiments herein,
and may be defined by claims as presented herein or as presented in the
future.
Throughout the description and claims of this specification, the words
"comprise" and "contain" and variations of
them mean "including but not limited to", and they are not intended to (and do
not) exclude other moieties,
additives, components, integers or steps. Throughout the description and
claims of this specification, the
singular encompasses the plural unless the context otherwise requires. In
particular, where the indefinite article
-64-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
is used, the specification is to be understood as contemplating plurality as
well as singularity, unless the context
requires otherwise.
Features, integers, characteristics, compounds, chemical moieties or groups
described in conjunction with a
particular aspect, embodiment or example are to be uncle-stood to be
applicable to any other aspect,
embodiment or example described herein unless incompatible therewith. All of
the features disclosed in this
specification (including any accompanying claims, abstract and drawings),
and/or all of the steps of any method
or process so disclosed, may be combined in any combination, except
combinations where at least some of such
features and/or steps are mutually exclusive. Details of any foregoing
embodiments should not be considered to
be limiting unless expressly indicated as such. Embodiments may relate to any
novel one, or any novel
combination, of the features disclosed in this specification (including any
accompanying claims, abstract and
drawings), or to any novel one, or any novel combination, of the steps of any
method or process so disclosed.
Herein either a full stop or comma is used as the decimal marker.
The reader's attention is directed to all papers and documents which are filed
concurrently with or previous to this
specification in connection with this application and which are open to public
inspection with this specification,
and the contents of all such papers and documents are incorporated herein by
reference.
EXAMPLES The following Examples are given as non-limiting illustration.
Compositions
Commercially available RTV-2 Si compositions having 2 Parts incorporating i),
ii) and iii) as defined above, literature
values of physical properties are publicly available:
P1 CaviCareTM (20g), Part A and Part B polymers product code 4563 (Smith &
Nephew). This is a
commercially available open cell foaming in situ dressing. Guide cure time
within 2 minutes
P2 MepisealTM (3m1), Part A and Part B polymers ref. 283100 (Molnlycke)
This is a commercially available dispensable adhesive sealant intended to seal
wound exudates within a wound
area from contacting intact skin. Guide cure time within 20 minutes
P3 Elastosil SC870 Part A and B polymers. This is a commercially available
foaming elastomer, black in
colour. Guide cure time within 90 minutes. (pot life 150s, tack free time 10 -
15 min, density 0.35 ¨ 0.4, Shore
hardness 8 - 12)
P4
Silpuran 2445 A/B Part A and B polymers Ref 60063054 and 60063056 (both
Wacker
Chemie AG). This is a commercially available elastomer for casting as
alignment, shock, damping members etc in
prosthetics. Guide cure time within 25 minutes
P5 Silpuran 2400/18 A/B (Wacker) is an addition-curing RTV-2 silicone
rubber curing to a blue coloured
silicone of low hardness. It has application in flexible moulding applications
for prosthetics. Guide cure time 120 C
for 1 hour.
P6 Silpuran 2111 A/B (Wacker) is a commercially available 2-part, addition-
curing silicone composition
curing to a soft, tacky silicone adhesive. It is suitable for use in wound
dressings. Guide cure time 120 C for 1 hour.
P7 Elastosil SC835 Part A and B polymers. This is a commercially available
foaming elastomer, reddish
brown in colour. Guide cure time within 90 minutes (pot life 240s, tack free
time 10 -15 min, density 0.4 ¨ 0.45,
Shore hardness 20);
-65-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
P8 Silbione RTV 4511 A/B (Bluestar) ¨ this commercially available
composition is modified to reduce cure
time and temperature for application at or around a wound site.
P9 Silbione LSR 4301 A/B (Bluestar) ¨ this commercially available liquid
silicone rubber composition would
not be preferred for application at or around a wound site.
Procedure A
A TNP dressing (PICOTM 15 x 20cm) similar (unwaisted) to that illustrated in
Figure 1 was adhered to an acrylic
or glass plate or aluminium plate, adhesive side down, and connected to a pump
to establish a negative
pressure, as illustrated in Figures 2B and 2C. Once it was established that a
good seal had formed, the pump
was turned off and the end portion of the dressing opposite to the pump end
was removed by slicing, as shown
in Figure A1. Removing the dressing portion exposed the transmission layer and
other internal layers as shown
in Figures 3A ¨ 3C.
Sealant composition was loaded into the cylinders of a static mixer and
dispensed therefrom to coat the exposed
edge of the dressing as in Figure CII, but with considerable overlap of both
the top surface of the dressing
adjacent the exposed edge, and the plate. After 90 minutes the pump was turned
on again to determine whether
a good seal had formed.
Example 1 ¨ CaviCare applied to dressing edge
Procedure A followed as above, applying dressing to acrylic plate. The
composition was dispensed, and cured
within 2 minutes. In this application, the sealed dressing leaked through the
open cells of the foam, although we
have observed that the nature of the foam structure of this composition causes
it to perform differently under
different conditions for example an external load may cause cell collapse with
resulting gas impermeability the
composition could well be optimised to achieve cell closure. Dispensing was
acceptable.
Example 2 ¨ Elastosil applied to dressing edge
Procedure A followed as above, applying dressing to acrylic, glass and
aluminium plates. Adhesion was better
with glass than acrylic and better with aluminium than glass. The composition
wads dispensed and cured and
after 90 minutes the pump was turned on. Leak was significant for sealed
trimmed dressing on acrylic plate and
low level requiring intermittent pump activity, when sealed to glass and
aluminium plates. This was a factor of
adhesion to the plate, not the dressing to which adhesion was acceptable.
Cured sealant was difficult to remove
from acrylic and glass substrates, less so from aluminium.
Example 3 ¨ Si!Duran 2445 applied to dressing edge.
Procedure A followed as above, applying dressing -.o glass plate. The
composition was dispensed and left to
cure overnight. The sealed dressing performed well, with very good seal
established, hardly requiring the pump
to come on once a negative pressure had been established within the sealed
trimmed dressing initially.
Procedure B
Procedure followed as for A above, applying to glass plate, but with injection
of composition directly into exposed
transmission (spacer) layer as illustrated in Figures El, BII and Cl.
Example 4 ¨ CaviCare injected directly into exposed transmission layer
-66-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
Procedure B was followed. The composition did not appear to penetrate the
dressing, and seemed to be blocked
by the dense superabsorbent polymer (SAP) layer. On starting the pump, a very
poor seal was observed, with
continual pump activity unable to achieve required vacuum.
Example 5 ¨ Mepiseal injected directly into exposed transmission layer
Result as for Example 4.
Example 6a ¨ Elastosil SC 870 injected directly into exposed transmission
layer, SAP removed
Composition was observed to penetrate approx 1cm within the transmission
layer. On foaming and cure the
backing layer top surface was observed to lift a little. Good seal, although
edge of dressing lifted where
composition was present in too great quantity.
Example 6b ¨ Si!Duran 2445 injected directly into exposed transmission layer.
SAP removed
As Example 6, no dressing lift, good seal and vacuum established instantly.
Example 7 ¨ Mepiseal iniected directly into exposed transmission layer, SAP
removed
As Example 6b, no dressing lift.
Example 8 ¨ CaviCare injected directly into exposed t-ansmission layer. SAP
removed
As Example 6b, but penetration was 1.5 ¨ 2cm due to foaming action, no
dressing lift. No vacuum due to open
cell structure.
CONCLUSION
Gas impermeable curable sealant dispensed externally to cover and/or
internally to impregnate exposed
transmission layer was surprisingly effective. Seal was absolute, and both
modes were visually effective.
Further samples.
Example 9 ¨ Procedure A as above was conducted on samples as follows:
9.1 - Ostomy paste ¨ Paste block was worked to render supple and pasted to the
dressing edge. Seal was
robust. Disadvantages included bioburden risk due to working before
application, seal height exceeds dressing
height.
9.2 - Adhesive gel strip ¨ Strip is tacky at both faces, one face applied to
dressing gave good seal;
disadvantage of tacky exposed surface, seal degraded over a few days as strip
lost its tack;
9.3 - Shaving gel ¨ The non-foaming gel was of low viscosity, such that a
substantial amount of gel was pulled
into the transmission layer, the dressing stopped delivering negative
pressure, due to the aqueous component of
the gel filling up the superabsorber layer.
9.4 - germolene ¨ The cream was of low viscosity, such that an was pulled into
the transmission layer, the seal
collapsed and negative pressure leakage occurred,
9.5 - SavIon spray ¨ The spray works by forming a liquid film on skin, water
evaporates off to leave the polymer
medication. In this example, the spray was unable to generate a film which
could bridge the interface between
absorber and transmission layer to skin. Two discrete films formed one at the
backing layer surface and one at
the wound model representing skin. There was no seal formed.
9.6 ¨ Elastosil SC835 (Wacker) ¨ a foam seal of appreciable height was formed
at the exposed portion, some
composition was drawn in to the transmission layer, to a depth of 1 ¨ 2mm. The
seal adhered strongly to the
wound model and seemed robust.
9.7 ¨ Elastosil SC870 (Wacker) ¨ a foam seal of appreciable height was formed
at the exposed portion,
comparable to 9.6. This composition was however more viscous to apply and the
resulting seal offers lesser
resilience than 5C835.
-67-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
COMPOSITION MEASUREMENTS
Methods were used as described in W02013076450, the contents of which are
incorporated herein by reference.
Example 10.1 ¨ Viscosity measurement
Viscosity is a measure of the resistance of a fluid to deformation by shear or
tensile stress and gives an
indication of the fluidity of a liquid, suspension or slurry. The viscosity of
a sample is measured using a rotational
viscometer which simultaneously measures shear rate and shear stress. Vaseline
:ID original, Moisturising
shaving gel Nivea Men Originals and Germolene, antiseptic cream were
independently tested on a cone and
plate rheometer, using a 2 steel cone of diameter 60 mm. The samples were
tested across a shear rate range
of 5-15 s-1, at 20 C in accordance with DIN EN ISO :3219: 1994, Annex B. The
viscosity is calculated using the
shear stress at a shear rate of 10 s-1 and is given in Pa.s.
CALCULATIONS
Viscosity (Pa.$) = Shear stress (Pa)
Shear rate (s-1)
Fotdional Force ;
eometer cone
Sarnple
k..77. eometer plebe
= - = .
L . .
Results NB: It was not possible to measure the viscosity of the RENASYS Ostomy
Strip Paste as this was too
viscous and tacky to allow suitable sample construction at the width required
for the instrument.
Table - viscosity
Viscosity at shear rate of 10/s, T 23 C (Pa.$)
Results Mean
Cavi-Care Part A 1.643 1.653 1.648
Cavi-Care Part B 1.773 1.804 1.7885
Mepiseal part from chamber with Text 42.30 41.97 42.135
Mepiseal part from chamber without Text 34.19 36.10 35.145
Silpuran 2450A 79.40 76.32 77.86
Silpuran 2450B 25.20 24.39 24.795
Silpuran 2445A 26.07 20.39 23.23
Silpuran 2445B 11.81 12.01 11.91
Silpuran 2400 1.8
Elastosil 835 Part A 15
Elastosil 835 Part B 15
Elastosil 870 Part A = 50
Elastosil 870 Part B 35
-68-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
Viscosity at shear rate of 10 s-', T 20 C (Pa.$)
Vaseline e original, pure petroleum jelly, Unilever 23.84
Moisturising shaving gel, Nivea Men, Originals, 11.93
Beiersdorf
Germolene, antiseptic cream (Phenol and 14.12
Chlorhexidine Digluconate), Bayer plc
Example 10.2 - Compressibilty measurements
Compressibility was determined as a measure of penetration by a plunger. Using
a Setamatic Penetrometer with
automatic release, timing device and standard 47.5 g plunger. The instrument
was fitted with a hollow plastic
cone with a stainless steel tip of mass 15 g. A dwell time of 60 seconds was
used. *Measurements were
recorded in triplicate (n=3).
Relative mass of parts
Mean penetration /
A B 1/10mm
Silpuran 2400/18 A/B* 50.0% 50.0% 51 (SD 1)
Silpuran 2111 A/B 50.0% 50.0% 200 (SD 3)
Subject to the desired performance requirements of the system these values can
be considered acceptable.
Example 10.3 Extensibility, permanent set, tensile strength, elongation at
break
Extensibility (kgfcml
Results Mean
Cavi-Care 0.04 0.06 0.04 0.07 0.07 0.06
Mepiseal 0.07 0.06 I 0.09 0.07 0.08 0.07
Silpuran 2450 2.60 2.41 2.42 2.25 2.70 2.48
Silpuran 2445 1.20 1.39 1.32 1.39 1.60 1.38
,
Permanent Set (%)
Results Mean
Cavi-Care o o o o o o
Mepiseal 0 o o o o o
Silpuran 2450 o o o o o o
Silpuran 2445 o o o o o 0
Tensile Strength (kgfcm4)
Results Mean
Cavi-Care 0.24 0.27 0.26 0.24 0.29 0.26
Mepiseal 1.64 0.95 1.77 1.69 2.00 1.61
Silpuran 2450 47.64 47.64 55.85 44.37 - 48.87
Silpuran 2445 = 37.74 39.73 39.98 41.16 37.15 39.15
Elastosil 835 450
Elastosil 870 350
_________________________________________________________________ _
, _________________________________________________________
-69-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608
PCT/GB2014/050786
Elongation at Break (/o)
Results Mean
Cavi-Care 92 90 83 73 120 92
Mepiseal 425 283 463 450 466 418
Silpuran 2450 199 204 234 184 - 205
Silpuran 2445 322 332 334 342 302 326
Elastosil SC 870 100
Elastosil 5C835 80
-70-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
Example 11 ¨ Extensibility testing of spacer layer (transmission layer)
Baltex
Reference Description
A Spacer Layer
Laminates made with EU33 top film, Chemposite 11C-450 Air Laid
superabsorber pad, Baltex ref: 7970, batch number TO061Spacer layer
and ALLEVYN Gentle Border wound contact layer (non-sterile and hand
manufactured)
METHOD
SOP/QPM/230 (1003423) Extensibility
RESULTS
Test method A
Results(kgfcm-1) Mean
Extensibility (Direction A) 0.07 0.38 = 0.08 0.08
1003423
Extensibility (Direction B) 0.07 0.08 0.07 0.07
1003423
Test method
Results(kgfcm-1) Mean
Extensibility (Direction A) 0.58 0.61 0.59 0.59
1003423
Extensibility (Direction B) 0.79 0.80 0.76 0.78
1003423
CONCLUSIONS
The spacer layer gave good (ie low) extensibility values. Extensibility was
restricted once the
material was incorporated into a dressing with the superabsorber pad.
Example 12 ¨ Silpuran 2400
Silpuran was subiect to testing according to procedures A and B above. The
results are shown as follows. This
composition has a very long cure time and low viscosity and it was expected
that it would simply run out of
position before curing. In fact the composition demonstrated a surprisingly
effective seal, however showed a
dramatic performance difference depending on dispensing according to presence
or absence of the absorber
layer in Procedure B. The composition gave excellent results when dispensed
according to Procedure B.
Silpuran 2400 was then dispensed internally according to the first embodiment
procedure illustrated in Figure C1.
The composition was retained admirably in the transmission layer, and only a
small volume of composition was
required. This enables use of a small volume syringe with associated back-
pressure advantages. It was
important to use a small bore nozzle to minimize run off, allowing as little
composition as possible to contact skin.
Dispensing again was ineffective using the Procecure A method with
superabsorber layer, but was highly
-71-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
effective for Procedure B with single transmission layer in the trimmed
portion. The transmission layer mav be
spacer or ADL. but with a preference for omitting obscuring layer to monitor
the dispensing and seal formation,
the transmission layer is preferably an ADL- type layer or a combination of
ADL and spacer, minimizing the risk
of spacer layer puncturing the backing layer.
Composition: Silpuran 2400/20, Part A and Part B polymers (Wacker)
Silpuran 2400
Component A
Color translucent
Viscosity (Plate/Cone) DIN EN ISO 3219 1800 mPa s
Density DIN EN ISO 2811 1,00 g/cm3
Component B
Color translucent
Viscosity (Plate/Cone) DIN EN ISO 3219 1800 mPa s
Density DIN EN ISO 2811 1,00 g/cm3
Product data (catalyzed A + B) =
Mix ratio A : B 1 : 1
Color translucent
Pot life at 23 C 21 min
Product data (cured)
Hardness Shore A ISO 868 7
Hardness Shore 00 ASTM 2240 / Type 00 55
Tensile strength ISO 37 2,00 N/mm2
Elongation at break ISO 37 600 %
Tear strength ASTM D 624 B 3,0 N/mm
Curing conditions: 10 min / 100 C
Run 8A
Followed as detailed in procedure A of the patent spec with application of
Silpuran 2400/20.
Dressing before application of Silpuran 2400/20
Dressing immediately after application of Silpuran 2400/20
A torch / flash used to examine the reflection of the silicone and ensure no
pin holes were present in the
composition immediately following application.
Cured composition (90 minutes was allowed to elapse, and cure beyond manual
kinetic point confirmed). Sealant
was not effective as there was no cured sealant across the cut face. Gap
demonstrated
Run 8B
Followed as detailed in procedure B of the patent spec with application of
Silpuran 2400/20.
Dressing before application of Silpuran 2400/20
Dressing immediately after application of Silpuran 2400/20
Cured composition (90 minutes was allowed to elapse, and cure beyond manual
kinetic point confirmed)
Sealant was effective when it had been dispensed into the transmission layer
directly.
It was noted that whilst the spacer layer was more resilient to compression
when the cured silicone was inside it
then the unfilled spacer; by comparison to run 8A where the same sealant had
been applied across the cut face
the profile of the sealant was less pronounced and (subjectively) the rigidity
was significantly less.
The following observations were made:
-72-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
Significantly less sealant was needed when sealing the intemal space of the
transmission layer as opposed to
sealing over the cut end.
The resulting finish was significantly neater when the sealant was applied
into the internal space of the
transmission layer as opposed to sealing over the cut end. The silicone that
flowed onto the wound model came
from the fact that the nozzle aperture was not sufficient to penetrate the
spacer layer deeply and was thus
delivering silicone close to the edge of the cut transmission layer. A
dispensing nozzle with external bore small
enough to penetrate the transmission layer could be inserted into the
transmission layer (to a selected distance)
at the time of dispensing and limit the amount of curing silicone that was
able to travel the distance back out of
the transmission layer.
The absence of an obscuring layer or opaque layer above the bridge in Run 8B
(such as the absorbent layer
present in Run 8A) allowed the user to clearly see the ingress of the sealant
into the bridge during dispensing.
This provided control over where to apply the sealant, how much and allowed
the user to ensure that no air paths
remained in the seal.
The low viscosity of Silpuran 2400/20 was a significant advantage during
dispensing. It flowed well into the
transmission layer and appeared to readily conform to the internal shape of
the transmission layer. A low
viscosity material would also have the added advantage of reducing the back
pressure generated in a dispensing
unit. This could be of particular importance if a dispensing nozzle with
narrow aperture was used (as desired to
penetrate the transmission layer) so that the back pressure generated by the
liquid travelling through the
resultant narrow orifice was reduced. To provide an example of the influence
of back pressure, in a dispenser
comprising a double barrelled syringe with integral mixer it would relate to
the force that the user needed to apply
to the syringe plunger to eject the liquid.
Example 13 Dispenser design
A dispenser with a single nozzle that penetrated the t-ansmission layer could
be an advantage.
A dispenser with multiple nozzles could be an advantage, however, subject to
the width of this head, injecting the
sealant into a dressing following body contours be limited. Therefore either
the width of the mixing head should
be balanced so as to ensure ease of application (wicer better) versus
following body contours (narrower better).
Or the mixing heads should be conformable so as to be moved to shape (ideally
this would stay in position once
moved) to allow the user to approximate the shape.
Thought should be given to the clearance of the dispenser given that it will
be entering the transmission at an
acute angle to the skin.
Results of foregoing examples are illustrated as follows:
Example 6b results
Exp 3 Silpuran 2445
-73-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
r----
,,....--t7-7, ,
''-
'
' - --- ¨ --- ¨ = ..ma,;,?.
..
Example 10 results
Exp 5 run la
Elastosil SC835, Wacker Silicones
Dispensed from Medmix Systems AG, Double-Cartridge Prefilled Delivery System
(S-System), dispenser 25m1
1:1/2:1 med plain, part: DS 24-01-00M; cartridge, 25m1, 1:1, PP natural, med.,
part: CS 025-01-32M; piston A,
25m1, 4:1, PE natural, Si 0-ring, med., part: KSA 25-04-02SM; mixer, DN6.5x16,
1:1/2:1, green , Part: MB 6.5-
16-S; & cap, 1:1/2:1, PE natural, med., Part: VB 200-SM.
V
., '' ., - . . eS,
, ,1 ..1.-.,;:
i.,4 t
= -
`11 'c
= ,.:t.. 7. .-
o = 4 tt.i, V ,..,',7.
.1,
-,,,(7-..
:
. . , . _........
Ex. 5 run lb
- ...
1 ,
1
=
,
= .
Exp 5, run 2.
RENASYS Adhesive Gel Patch, 10 cm x 7 cm, Ref 66801082, Smith & Nephew
', ..' . -... '.... - ,... = , . . .
_ , = . - , . ,,,
=. = .... _. ,,
= _
Exp 5 run 3
RENASYS Ostomy Strip Paste
Component from appropriate RENASYS-G Gauze kits. Smith & Nephew
-74-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
,
I,
Exp 5, run 4
Vaseline original, 100 ml, Pure petroleum jelly (Ingredients: Petrolatum)
Unilever
ofgaryir..,
:-
--
, .
,
.0 -1
. .- i. ,
,
Exp 5 run 5
Moisturising shaving gel, Nivea Men, Originals, 200 ml in pressurised
container
Beiersdorf
= = t. = , =':=?.
-
,
= . . õ ,
, .
= ,
,
=
. , .
= = ..
= . = .
- -
= - ==
,
' = = , ,.
,
'
'
-75 -
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608 PCT/GB2014/050786
= = .
= t:
= - - 1" s'
r" = e.
= kt4 c't, :41;.
=
_ =
Exp 5 run 6
Germolene, antise3tic cream (Phenol and Chlorhexidine Digluconate), 55 g tube
Bayer plc
_
,
.4
_ = ..
=
= .
=
Exp 5 run 7
Savlon, Spray Plaster, antiseptic upon application, 40 ml pressurised
container
Novartis Consumer Health
_
. =
. . _
Example 12 Composition: Silpuran 2400/20, Part A and Part B polymers (Wacker)
Run 8A
Followed as detailed in procedure A of the patent spec with applicatio, of
Silpuran 2400/20.
-76-
SUBSTITUTE SHEET (RULE 26)
CA 02902396 2015-08-25
WO 2014/140608
PCT/GB2014/050786
_ -
Dressin s before a slication of Silpuran 2400/20
'
, 4 s
t
4.4V,
1 =
_
=
Dressins immediatel after application of Silpuran 2400/20
. = .
=
=
=
ELI
. -1Kit
r
Sealant was not effective as there was no cured sealant across the cut face.
Gap demonstrated
Run 8B
Followed as detailed in procedure B of the patent spec with application of
Silpuran 2400/20.
*_1-1
õ
== =
-7
"
Wit
Dressing_before apslica-ion of Silpuran 2400/20
=
' - - -
= ,
=
,
Dressing immediately after application of Silpuran 2400/20
,
-
=
Cured composition (90 minutes was allowed to elapse, and cure beyond manual
kinetic point confirmed).
-77 -
SUBSTITUTE SHEET (RULE 26)