Note: Descriptions are shown in the official language in which they were submitted.
CA 02931899 2016-06-01
PROCESS TO REMEDIATE CONTAMINATED SOILS USING CARBON
DIOXIDE-ASSISTED HYPOCHLORITE OXIDATION
TECHNICAL FIELD
"[he present disclosure relates to the remediation of contaminated soils that
require treatment.
BACKGROUND OF THE DISCLOSURE
Contaminated soils are a concern in urban areas and various processes exist to
remediate these
soils. In urban areas, it is generally necessary to excavate the contaminated
soils. Therefore, a
regional soil treatment centre performs the ex-situ remediation treatment. The
most common
soil contaminants are heavy metals (Cd, Cr, Cu, Ni, Pb, Zn) and hydrocarbon
compounds (C10-
050, HAP). These contaminants are either alone or together in a same soil.
This section
discusses the background art of commercial treatment of hydrocarbon-
contaminated soils.
Commercial-scale treatments can use chemical oxidation in soil piles for the
remediation of
contaminated soils. The treatment uses strong oxidants, such as hydrogen
peroxide, potassium
permanganate, ozone or persulfate salts. In this process, the main problems
are the high oxidant
consumption, the low substrate selectivity and the strong oxidant
ineffectiveness in oxidizing
recalcitrant organic contaminants, namely C25-050 and heavy polycyclic
aromatic
hydrocarbons (HAP). In most cases, these treatments are too lengthy for an
efficient ex-situ
treatment.
One observation regarding existing commercial oxidation treatments is that the
stronger the
oxidant is, the less cost-efficient the oxidation reaction. Finally, the more
severe the
decontamination needs to be, the higher the oxidant consumption per
contaminant mass will be.
Another observation is that the oxidative treatment could necessitate the
dosing of a surfactant.
'[he surfactant eases the release of the hydrocarbon contaminants and
facilitate the remediation
process. The surfactant is loss in the process.
CA 02931899 2016-06-01
SUMMARY OF THE DISCLOSURE
In one aspect, there is provided a soil decontamination process comprising:
.. providing a solution comprising an hypochlorite salt;
providing a slurry comprising said solution and contaminated soils comprising
hydrocarbon-
based compounds, wherein said slurry is maintained at a from 6
to 10 by contacting carbon
dioxide with said slurry; and
recovering soils, wherein said recovered soils are comprising an amount of
hydrocarbon-based
compounds lower than that of said contaminated soils.
BRIEF DESCRIPTION OF THE DRAWINGS
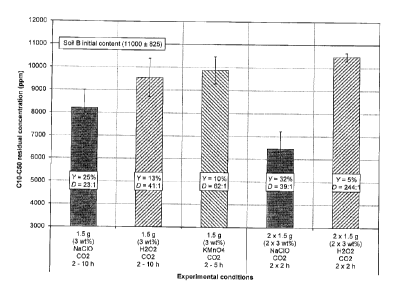
Fig. 1 represents the residual C10-050 concentration in a contaminated soil
following NaCIO,
I-1202 or KMn04 oxidation at 25 C in a single or double batch operation;
Fig. 2 schematically illustrates an embodiment of how this disclosure may be
incorporated in a
soil decontamination process;
Figs. 3A, 3B and 3C illustrate side and front sectional views as well as a top
view of an
embodiment for a treatment centre that may incorporate a soil decontamination
process as
defined herein; and
Fig. 4 illustrates the influence of the NaCIO initial concentration with a CO2
overhead on the
decontamination of a soil comprising C 1 0-050 contaminants.
DETAILED DESCRIPTION OF THE DISCLOSURE
In accordance with the present description there is now provided a
hypochlorite (CIO-) oxidation
process assisted with carbon dioxide to remediate contaminated soils.
In one embodiment, the soil is contaminated with hydrocarbon aliphatic
contaminants ranging
from C10 to C50 alkanes. In this embodiment, the present disclosure addresses
soils with an
initial contamination level that is no more than about 15 000 mg/kg,. In this
embodiment, the
decontamination target is a residual Cl 0-050 content ranging from 300 to 3
500 mg/kg. In one
embodiment, the target C 10-050 content is lower than 3 500 mg/kg, lower than
about or lower
CA 02931899 2016-06-01
than about 700 mg/kg. It is also a target to provide C25-050 amount in similar
ranges in the
decontaminated soils. The legislation determines which decontamination level
to reach.
In a further embodiment, the soil is contaminated with polycyclic aromatic
hydrocarbons
(13AI I). The legislation lists about two dozen carcinogenic PAH including,
for example,
anthracene and phenanthrcne. In this embodiment, the initial contamination
content is up to
5 000 mg/kg with a typical initial contamination content up to 500 mg/kg. In
this embodiment,
the decontamination target is a residual PAH content up to 100 mg/kg. The
legislation
determines which decontamination level to reach for each PAH. PAH may be
present in
contaminated soils in addition to C10-050 or independently present on their
own. A list of PAI-1
may be comprising the following: acenaphtene, acenaphtylene, anthracene, benzo
(a)
anthraeene, benzo (a) pyretic, benzo (b+j+k) fluoranthene, benzo (c)
phenanthrene, benzo (g,h,i)
perylene, chrysane, dibenzo (a,h) anthracene, dibenzo (a,i) pyrene, dibenzo
(a,h) pyrene,
dibenzo (a,l) pyrenc, dimethy1-7,12 benzo (a) anthracene, fluoranthene,
fluorenc, indeno (1,2,3-
.. cd) pyrene, methyl-3 cholanthrene, naphtalene, methyl-1 naphtalene, methyl-
2 naphtalOne,
dimethyl-1,3 naphtalene, trimethy1-2,3,5 naphtalene, phenanthrene, and pyrene.
In both these embodiments, an accredited protocol measures the extent of the
contaminant
oxidation. 'Fhis protocol includes a solvent extraction on a soil sample and a
chromatographic
.. scan (GC-FID or GC-MS) on the extracted solvent phase. The solvent can be,
for example,
hexane or dichloromethane. The solvent extracts part of the natural soil
organic matter even
though it is no contamination. Thus, the solvent extraction reduces the soil
sample mass. This
mass reduction is not proportional and sometimes does not even correlate to
the
decontamination yield. For this reason, it is necessary to compare a
chromatographic scan prior
and alter treatment to measure the oxidation yield.
In one embodiment, the soil is sifted to remove the largest oversized material
which is
inconvenient for the reaction. A trommel removes these oversized materials
(branches, bricks,
etc.) prior to the oxidation treatment. The residual oversized materials which
diameter is greater
than 2.5 mm are not an inconvenience for the reaction.
The reaction suits all soil types (clay, sand, silt, peat, etc.). The easiness
to remediate a
contaminated soil depends on the contamination age and less on the soil type.
3
CA 02931899 2016-06-01
In one embodiment, the present disclosure provides a hypochlorite aqueous
solution, referred to
as a solution, comprising 0.1 wt% to 15 wt%, or 0.1 wt% to 3 wt%, or 1 wt% to
7 wt%, or I
wt% to 3 wt% of one or a mixture of the fbllowing components: sodium
hypochlorite. lithium
hypochlorite or calcium hypochlorite. Sodium hypochlorite and/or lithium
hypochlorite are
preferred and sodium hypochlorite is most preferred. Calcium hypochlorite is
the least preferred
because it is a partially soluble powder difficult to handle.
In one embodiment, the process is comprising adding a second, or further,
amount of the
hypochlorite salt solution before said step of recovering the soils.
Preferentially, an electrolytic
hypochlorite generator provides on-line the addition of hypochlorite.
The contaminated soil mixes with a solution in a soil-to-solution mass ratio
ranging from 1:1 to
1:10, preferably 1:5 or more preferably 1:3 to optimize the mixing and to
minimize the required
quantity of solution. The soil and solution mix is referred to as a slurry.
In another embodiment, carbon dioxide contacts with a slurry as a gas overhead
in a closed
slurry reactor or as a bubbling gas in an open slurry reactor. Flue gas from a
direct combustion
burner or a carbon dioxide cylinder supply the carbon dioxide to the reaction.
Carbon dioxide
increases the hypochlorite reaction rate by maintaining the slurry at the
required p1-1. A
sufficient amount of carbon dioxide contacts the slurry to provide the
required p1.1. The amount
of carbon dioxide can be determined by the skilled person as it will vary
based on the
remediation conditions, contamination and soils. The pH of the slurry will
guide the amount of
carbon dioxide to be used.
In one embodiment, the pH of the slurry (i.e. soil and solution mix) is
maintained from 6 to 10.
Preferably the pH is from 7 to I 0 or more preferably about 8.
In one embodiment, the oxidation reaction is conducted on the soil at a
temperature of 5 to 30
C, preferentially 15 to 25 C. The process can use a heating device to raise
the slurry
temperature to the desired value if necessary.
In another embodiment, the reactor comprises a partially fluidized soil
column, a solution
recirculation loop, a flue gas sparger and a scrubber to neutralize the
hypochlorite smell.
4
CA 02931899 2016-06-01
In another embodiment, the recovered solution is either regenerated into the
original
hypochlorite solution or sent to a water treatment unit. The solution
regeneration takes place in
an electrolytic cell and can be performed while the oxidation proceeds. The
water treatment unit
consists in a reverse osmosis membrane to concentrate the salts. In the
treatment unit, water is
recycled back to the process and hypochlorite and chloride salts are either
discarded or
regenerated into a hypochlorite solution.
One advantage of the present disclosure is that it is possible to keep low and
fairly constant the
specific oxidant consumption. In this regard, NaCIO compares favorably to the
common
oxidants used in soil oxidation processes (Fig. 1). The specific oxidant
consumption is the mass
of oxidant required to oxidize a given mass of contaminant. (e.g. kg oxidant /
kg oxidized
contaminant). Typically, the specific NaCIO consumption stays below 40 kg
NaCIO kg
oxidized Cl 0-050 to decontaminate up to the decontamination target. According
to the prior art,
the specific strong oxidant consumption (e.g. 11207) can increase many folds
as the oxidation
IS reaction proceeds. This increases almost exponentially the oxidation
cost. Therefore, the
advantage of the present disclosure is to keep the oxidation cost proportional
to the mass of
contaminant to oxidize.
A further advantage of the present process is that organochlorides are not
likely produced due to
the neutral-to-basic reaction An alkaline reagent, preferentially sodium
hydroxide, may be
added to the slurry if initial acidic conditions prevail prior to the
oxidation treatment. The
alkaline reagent may also be added to the slurry at the end of the treatment
to ensure a neutral to
basic soil pH.
A further advantage of the present process may also be to free the heavy metal
contamination
from the natural soil organic matter. As a result, this fac,=ilitates the
subsequent heavy metal
decontamination of the resulting soil.
The process of the present disclosure is effective in the ex-situ remediation
of contaminated
soils.
Fig. 2 schematically illustrates how this
disclosure may be incorporated in a soil
decontamination process: soil excavation (1), particle size segregation (2),
preparation of the
oxidative solution (3). oxidation reaction (4), hypochlorous acid recovery
(5), soil dewaterin
5
CA 02931899 2016-06-01
(6) and regeneration of the solution (7). The oxidant regeneration is
preferentially performed on
the residual salt (sodium chloride) recovered in the spent solution or
performed on a fresh
sodium chloride supply.
Soil excavation (section 1)
First, standard analysis (Centre d'expertise et d'analyse environnementale du
Quebec
(CEAEQ), methods MA.200-Met1.2 (heavy metals), MA.400-HAP1.1 (HAP), MA.400-
1-lYD.I .1 (C 10-050)) determine the contamination type and level in a
contaminated land. Then,
the soil on this land is excavated and loaded on trucks to a treatment centre.
The treatment
center can be a permanent centre or a mobile centre. The soil is unloaded from
the trucks on a
dumping pad awaiting for treatment.
Particle size setzre2ation (section 2)
The excavated contaminated soil on the dumping pad contains large
uncontaminated debris and
rocks. A trommel with 1-to-5 inch openings separates these debris and rocks
from the soil
because they are uncontaminated material and inconvenient to carry in the
reaction process.
These debris and rocks are discarded.
At the exit of the trommcl, the soil is once again stacked awaiting for
treatment. At this point,
the soil contains maximum I to 5-inch soil particles. However, contamination
is distributed in
the fine soil particles. The fine particles are the soil particles with an
average diameter less than
2.5 mm. according to the standard analysis protocols previously listed.
Optionally, the soil could
be segregated once again in a different trommel to remove the coarser soil
fraction. The
decision would depend on the soil particle distribution (e.g. a high gravel
proportion). This
coarser fraction is not inconvenient for the reaction.
Preparation of the oxidative solution (section 3)
The oxidative solution comprises 0.1 wt% to 15 wt% of one or a mixture of the
following
components: sodium hypochlorite, lithium hypochlorite or calcium hypochlorite
and is prepared
prior to the soil oxidation. The balance is tap water. A covered reservoir can
be used to stock
this solution. Regeneration of the solution could use the same reservoir.
6
CA 02931899 2016-06-01
Oxidation reaction (section 4)
A loader carries the soil from the dumping pad to the soil oxidation set-up
(Fig. 3). The design
of this set-up rests on the principle that partial soil fluidization causes
the soil and solution to
form a slurry in which the reaction proceeds. The set-up comprises a reaction
section (8), a
solution recirculation section (9) and a solution distribution section (10)
which are all three
located below the ground level. 'Me paragraphs below describes the three
sections of the set-up.
The reaction section comprises a 15 slope (II) and a soil pile (12). The
slope allows the loader
to stack the soil in a soil pile. The loader stacks about 200 tons of soil in
a rectangular pile
whose height is maximum 2-meter. The depth of the reaction section is maximum
6 meters. A
removable wall (13) isolates the soil pile from the slope.
The solution recirculation section (9) shares the same liquid level with the
reaction section (8).
This allows the fluidized soil particles to slow down. Therefore, the
circulating solution carries
only the finest soil particles (clay and silt) and the heavier soil particles
(fine sand) loop in the
reaction section. The section is designed to minimize the required solution-to-
soil ratio while
ensuring an efficient soil mixing.
The solution distribution section (10) is beneath the reaction section. The
section comprises
perforated pipe (14) buried in 0-3/4-inch gravel. "[he number of perforations
shown on Fig. 3 is
for illustration purposes only. The section distributes the solution flow rate
as uniformly as
possible at the entrance of the reaction section. The uniform solution flux
dictates the achievable
level of mixing in the reaction section.
The oxidation reaction takes place when the solution contacts the soil.
To increase the oxidation reaction rate, carbon dioxide is injected as flue
gases or as a pure gas
in the solution recirculation section (9). This carbon dioxide reacts with the
hypochloritc salts to
lower the pH from 10 to 12 down to 6 to 10. Consequently, the hypochlorite
oxidation
potential is increased due to the formation of hypochlorous acid.
7
CA 02931899 2016-06-01
The oxidation reaction time can last from 30 min up to several hours but
preferentially less than
2 hours. The oxidation reaction operates batchwisc, e.g. one pile of soil at a
time. At the end of
the reaction, sodium hydroxide raises the slurry p1-1 to pH 10 to 12 to
neutralize all remaining
hypochlorite odor.
Hypochlorous acid recovery (section 5)
lypochlorous acid is a vapor and can be entrained out of the slurry by
bubbling carbon dioxide
or flue gases. This active oxidation component may be recovered. 'Ibis
recovery can be
performed, for example, in a scrubber in which the gases exiting the soil
column contact with a
6 to 12 caustic solution. This neutralization reaction recovers hypochlorous
acid as a sodium
hypochlorite that can recycle back to the soil oxidation set-up.
Soil dewatering (section 6)
Once the oxidation is complete, the solution circulation stops. The slurry
decants in the reaction
section (8). Two phases form, the aqueous form on top comprising most of the
spent solution
and a soil phase at the bottom. The soil phase has to be dewatered.
The spent solution is pumped to the covered reservoir. The spent solution in
the aqueous phase
on top is pumped from the top. The soil phase which contains part of the spent
solution is
drained from the perforated pipes in the solution distribution section. To do
this, the pump is
either inverted or. preferentially, the spent solution in the reaction section
is syphoned through
the perforated pipes. A sump pit collects the drained spent solution (15). The
soil phase is
dewatered sufficiently to be shoveled out of the reaction section with a
loader.
Regeneration of the solution (section 7)
As described in the previous steps, the spent solution is collected in the
supernatant and
syphoned through the perforated pipes. The spent solution is pumped to the
covered reservoir.
Two options arc possible to regenerate the oxidative solution.
The first option is to use a membrane to recycle the water and discard a
concentrated salt
solution. The recycled water is fed with fresh hypochlorite and the solution
is regenerated. The
8
CA 02931899 2016-06-01
fresh hypochlorite is either bought or generated in-situ using an
electrochemical cell and a fresh
chloride salt supply. This is the less preferred option.
The second option is to regenerate the entire spent solution in an
electrochemical cell.
.. Ilypochlorite, preferentially sodium hypochlorite, can be regenerated from
its corresponding
chloride salt. In the case of sodium hypochlorite, the corresponding salt is
sodium chloride.
Sodium chloride is the oxidation by-product of sodium hypochloritc. In this
option, the
regeneration can also be performed in the oxidation set-up while the oxidation
proceeds. This is
the most preferred option.
1 0
EXAMPLES
The examples illustrate the typical performance of the disclosure and provide
a better
understanding of the disclosure.
Example 1
The procedure was initiated with filling a bucket with about 15 kg of a real C
I 0-050
contaminated calcareous soil. The soil came from the dumping pad in a soil
treatment center.
The soil was identified Soil 13 and was highly contaminated (Fig. 1). Soil B
contained a low
carbonate content. Sun exposition dried the soil for over a week to facilitate
the dry sifting of
the soil. A 2.5-mm sieve sifted the soil to recover the Fines. The fines were
mostly sand with a
low organic content (3 wt% hexane extractible content). Analysis (1CP-MS,
hexane extraction,
GC-MS) quantified the contamination and soil elemental composition on a
homogenized Imes
lot. These analysis determined the initial contamination level. The reaction
in the example used
only these fines. Each reaction used 25 g of the fines from the homogenized
lot.
For each reaction, 25 g of soil fines were put in a glass autoclave. A 20-psi
CO, overhead
prevailed in the autoclave for the CO2-assisted experiments. Each reaction
used 30 ml of
oxidative solution with a 3-wt% oxidant content. The experiments compared the
oxidants
NaC10, KM n04 and 1-1209. Each oxidant was reagent grade and was bought from
Sigma-
Aldrich. The reactions lasted from 2 h up to 10 11.
9
CA 02931899 2016-06-01
At the end of the reaction, filtration with a Whatman #1 paper then recovered
the solids from
the spent solution. The filtration cake was washed and the spent solution was
discarded. The
cake was air-dried at ambient temperature and its hydrocarbon content analysed
according to
procedure MA.400-tlY D. I .1 .
The performance parameters are the oxidation yield (Y) and the specific
oxidant dosage
expressed as g dosed oxidant : g oxidized CIO-050 (D). For soil B, NaCIO
provided the lowest
specific oxidant consumption D compared to KMn04 and H202 (Fig. 1). NaCIO kept
its better
oxidation yield with varying reaction time or double reaction treatment. 11902
was particularly
inefficient even though the soil did not contain a high carbonate content. It
is well known in the
art that carbonates consume uselessly the peroxide free radicals.
Example 2
IS The procedure was initiated with filling a bucket with about 15 kg of a
real C I O-050
contaminated calcareous soil. The soil came from the dumping pad in a soil
treatment center.
The soil was identified Soil A. The soil preparation prior to the oxidation
reaction was
conducted as described in example I. The fines were mostly fine sand and clay
with a high
organic content (5 wt% hexane extractible content). The reaction in the
example used only these
lines. Each reaction used 25 g of the fines from the homogenized lot.
For each reaction, 25 g of soil lines were put in a glass autoclave. A 20-psi
CO2 overhead
prevailed in the autoclave for the Ca)-assisted experiments. Each reaction
used 50 ml of
oxidative solution but the NaCIO content varied from 2.5 wt% to 7.0 wt%. 'the
NaCIO supply
was reagent-grade 10-15wt% NaCIO solution bought from Sigma-Aldrich. Each
reaction lasted
2 h.
At the end of the reaction, filtration with a Whatman I 1 paper then recovered
the solids from
the spent solution. The -filtration cake was not washed and the spent solution
was discarded. The
cake was air-dried at ambient temperature and its hydrocarbon content analysed
according to
procedure MA.400-HYD.1.1.
For this soil, the oxidation yield correlated linearly with the oxidant
content in the solution for
the CO2-assisted reactions (Fig. 4). Consequently, the specific oxidant dosage
(D) was constant.
Example 3
The procedure initiates with a real HAP and heavy-metal contaminated
calcareous soil. The soil
was identified Soil C. The soil preparation prior to the oxidation reaction
was conducted as
described in example 1. The fines were mostly clay with a high organic content
(5 wt% hexane
extractible content). In this example, each reaction used 50 g of the fines
from the homogenized
lot.
For each reaction, 50 g of soil fines were put in a PVC bench-scale fluidized
bed with a similar
hydrodynamic design than the oxidation set-up shown on Fig. 3. In this design,
150 ml of
solution ensured a homogeneous mixing in the reaction zone.
The oxidation reaction used 1-wt% or 3-wt% NaCIO solutions. The NaCIO supply
was a
commercial maintenance product (brand: La ParisienneTm). CO2 bubbled at 10-20
ml/min in the
slurry to lower the reaction pH to 8 before the reaction begins. The pH
lowered to a value from 7
to 8 over the reaction time. The recirculation flow rate was set to maintain a
uniform mixing in
the slurry. Each reaction lasted 2 h.
At the end of each reaction, filtration with a Whatman #1 paper recovered the
solids from the
spent solution. The filtration cake was washed and the spent solution was
discarded. At the very
end of the experiment, a NaOH solution raised the slurry pH to 11 to
neutralize any remaining
hypochlorous acid. The cake was air-dried at ambient temperature and its
hydrocarbon content
analysed according to procedure MA.400-HAP1.1.
Part of the dried cake (25 g) underwent heavy metal extraction. This was to
evaluate the
contribution of the oxidation treatment on the heavy metal extraction yield.
The extraction
treatment consisted in 3 successive washes with 50 ml of a 2-wt%
nitrilotriacetic acid solution
(NTA) at 25 C. Each chclation reaction lasted 2 h. At the end of each
reaction, filtration with a
Whatman #1 paper recovered the solids from the leachate. The filtration cake
was washed and
the leachate and wash water were analyzed for heavy metal content by ICP-MS
(MA.200-
Met.1.2).
11
CA 2931899 2019-02-19
CA 02931899 2016-06-01
For soil C. the oxidation yield correlated with the oxidant concentration in
the oxidation process
with NaC10 (Table 1). The PAH oxidation yield is the overall oxidation yield
of all listed PAH
and is indicative of the yield for each PAH. NaC10 can form sodium complexes
with the soil
organic matter. These complexes adsorb on the soil surface and may limit the
oxidation extent.
Washing away these complexes is a technique that may be used in such case. The
oxidation
protocol also favors the heavy metal extraction by chelation in a subsequent
soil treatment.
Table 1. Enhanced remediation of soil C heavy metal and PAH content by NaC10-
0O2
oxidation.
Soil remediation treatment NTA recovery
oxidation Cu Pb Zn PAH
cake wash
SOM oxidation Heavy metal Wash 1 Wash 2 Wash 3 extraction
oxidation
extraction
3 x I g NTA /
70% 65% 65% 40% 0%
cake
2 x I .3 g (3 wt%)
g
NaCIO-CO, / 25 a 3 NTA /
no 25% 0%
cake
soil C
2 x I g (3 wt%)
3 x I g NTA /
NaCIO-CO, / 25 g yes 87% 84% 84% 40% 43%
cake
soil C
3 x 0.8 g (I wt%)
NaCIO-CO, / 25 g no 0% 0%
soil C
3 x 0.5 g (I wt%)
NaCIO-CO, 25 g yes 0% 0% 0% 0% 22%
soil C
12