Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02271440 1999-OS-11
WO 98I28057 - PCT/ITS97/23558
BIOLOGICAL FLUID PROCESSING
This application claims the benefit of U.S. provisional patent application
60/034,145, filed December 24, 1996, which is incorporated by reference in its
entirety.
Technical Field
This invention relates to processing biological fluids such as blood and blood
components. The invention particularly relates to removing undesirable
material from
the biological fluid and/or minimizing contamination of the biological fluid
with the
undesirable material.
Background of the Invention
Blood consists of a number of components having different characteristics and
uses. Accordingly, blood is typically processed to separate the components to
yield a
variety of valuable blood products . For example, a unit of donated whole
blood can be
processed to separate red cells, usually concentrated as packed red cells
(PRC), platelets,
usually concentrated as platelet concentrate (PC), and plasma. In accordance
with some
processing protocols, blood can be treated to form platelet-rich-plasma (PRP)
or huffy
2 0 coat, before forming PC and/or separating plasma.
The separated components can be stored before being used as a blood product,
particularly before being used as a transfusion product. Illustratively, PC
can be stored
for several days or more, and PRC can be stored for several weeks or more,
before
transfusion into a patient. Moreover, multiple units of some components, e.g.,
PC,
2 5 huffy coat, and/or plasma, can be pooled before producing the final blood
product. Two
or more units can be pooled and transfused without having been stored as
individual
units. Alternatively, units can be pooled and then stored before use.
However, stored and/or non-stored components typically include undesirable
material such as bacteria and/or leukocytes. Bacteria can contaminate the
blood or blood
3 0 component during blood collection and/or storage. One source of bacterial
contamination may be the blood donor's skin, which may contain one or more
varieties
of bacteria, e.g., gram positive bacteria such as Staphylococcus epidermidis,
and S.
1
CA 02271440 1999-OS-11
WO 98/28057 - PCT/US97/23558
aureus, and/or gram negative bacteria. Since swabbing the donor's skin {e. g.
, with
alcohol) prior to venipuncture may be inadequate to assure sterility, the
bacteria may
pass into the blood collection container, and the bacteria may reproduce while
the blood
or blood component is stored. Moreover, since some phlebotomy needles may cut
a disc
of skin when the phlebotomy needle is inserted into the donor, the bacteria-
containing
skin plug can pass with the blood into the blood collection container, and the
bacteria
can reproduce during storage.
Since some blood components (e.g., platelets) are typically stored at ambient
temperatures, the problem of contamination may be magnified, since some
bacteria
reproduce more rapidly at ambient temperatures. The administration of the
bacterially
contaminated transfusion product, particularly when the product contains
massive
bacterial contamination, may have adverse affects on the recipient. As a
result, the
United States prohibits the transfusion of platelet products that have been
stored for
more than 5 days, since platelets stored for 7 days are considered more likely
to have
massive bacterial contamination. Japan and Europe have similar, or even
stricter,
prohibitions. Additionally, fear of contamination is one reason that the
United States
prohibits the transfusion of pooled platelet products unless the platelets are
transfused
within four hours of pooling.
Blood and blood components also contain other undesirable material. In
2 0 particular, blood and blood components contain varying amounts of
leukocytes, which
may also adversely affect the recipient receiving the leukocyte containing
transfusion
product. For example, the administration of leukocyte contaminated transfusion
products has been associated with febrile reactions, alloimmunization, and
Graft Versus
Host Disease. Moreover, the presence of leukocytes during blood component
storage
2 5 may be undesirable, as leukocytes may contain bacteria, and/or have
bacteria attached
thereto, and the bacteria may reproduce as noted above. Additionally, or
alternatively,
the leukocytes can release products that adversely affect the blood components
during
storage, or adversely affect the patient receiving the transfusion.
Some material present in blood is undesirable when the material is present in
3 0 particular transfusion products. For example, platelet-containing
transfusion products
such as platelet concentrate (PC) should be substantially free of red blood
cells. Since
red blood cells are antigenic, the presence of a significant level of red
blood cells in a
2
CA 02271440 1999-OS-11
WO 98/28057 - PCT/LTS97/23558
platelet transfusion product can lead to an adverse immune response by the
patient. The
problem is magnified when multiple units of platelets (typically 4-6 units)
are pooled
before transfusion, since the patient can be exposed to multiple (e.g., 4-6)
sets of red
blood cells, each set of cells having a different antigenicity.
Thus, there is an unaddressed need in the art to minimize the presence of
undesirable material in blood or blood components, particularly in stored
blood
components, more particularly in stored blood products that are pooled before
transfusion into a patient. Additionally, there is a need in the art to reduce
the
likelihood that undesirable material such as bacteria that may be present in
the blood
component can reproduce to a significant level during storage.
The present invention provides for ameliorating at least some of the
disadvantages of the prior art. These and other advantages of the present
invention will
be apparent from the description as set forth below.
Summary of the Invention
Methods and systems according to the present invention provide for minimizing
the presence of undesirable material such as bacteria and leukocytes in
donated
biological fluid to be used for blood products, preferably by providing that a
first portion
of donated biological fluid (which may include bacteria passed from the
donor's skin,
2 0 and/or the donor's skin plug), is collected separately than a second
portion of donated
biological fluid (which is less likely to present a significant risk of
bacterial
contamination). The second portion of donated biological fluid is processed to
separate
one or more blood components of interest (e. g. , plasma, platelets, and/or
red blood
cells) to produce a variety of blood products, and at least one desirable
blood component
2 5 is depleted of leukocytes. Preferably, the leukocyte depletion is carried
in a closed
system.
In some embodiments, the first and second portions of biological fluid are
collected from each of a plurality of sources (e.g., blood donors), and the
plurality of
second portions (or blood components thereof) are subsequently pooled. The
blood
3 0 components can be pooled before, after, or while being depleted of
leukocytes.
In some embodiments, the leukocyte depletion of the biological fluid can be
carried out while minimizing contamination of the collected leukocyte depleted
3
CA 02271440 1999-OS-11
WO 98/28057 - PCT/US97/23558
biological fluid with red blood cells. Accordingly, the collected leukocyte
depleted
biological fluid can be substantially free of red blood cells.
Methods and systems according to the invention also provide for storing the
leukocyte depleted portion of biological fluid while killing and/or preventing
the
reproduction of undesirable material that may be present in the biological
fluid. For
example, the leukocyte depleted portion of biological fluid can be stored in a
container
that has a bacteriocidal or bacteriostatic effect on bacteria that may be
present in the
biological fluid. In one illustrative embodiment according to the invention,
the container
comprises polyvinyl chloride (PVC) plasticized with tri (2-ethylhexyl)
trimellitate
(TOTM), and the leukocyte-depleted biological fluid comprises platelet-
containing fluid
(e.g., platelet concentrate).
Methods and systems according to the invention are particularly suitable for
those
protocols that include pooling of blood components, especially components such
as PC
or huffy coat.
Brief Description of the Drawines
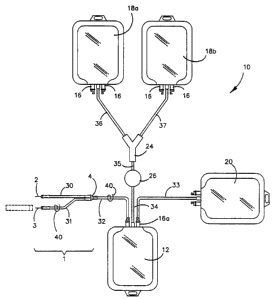
Figure 1 illustrates an embodiment of a system according to the present
invention, allowing the separate collection of a first portion of a biological
fluid, and a
second portion of a biological fluid. The illustrated system includes a
leukocyte
2 0 depletion filter.
Figure 2 illustrates another embodiment of a system according to the present
invention, allowing the separate collection of a first portion of a biological
fluid, and a
second portion of a biological fluid.
Figure 3 illustrates a system for pooling biological fluid from a plurality of
2 5 sources (e.g., a plurality of blood donors). The illustrated system
includes a pooling
assembly interposed between a plurality of biological fluid source containers
and a
biological fluid receiving container. Each biological fluid source container
is suitable
for holding a second portion of a biological fluid, or at least one blood
component
separated from the second portion of biological fluid.
3 0 Figure 4 illustrates embodiments of systems that are especially useful for
processing blood components such as huffy coat. Figure 4A illustrates an
embodiment
of a system for producing huffy coat, and Figure 4B illustrates an embodiment
of a
4
CA 02271440 1999-OS-11
WO 98/Z8057 - PCTIUS97/Z3558
system for pooling huffy coats.
Specific Description of the Invention
In accordance with an embodiment of the invention, a method for processing
biological fluid comprises obtaining a first portion of biological fluid,
obtaining a second
portion of biological fluid, and passing at least one component of the second
portion of
biological fluid through a leukocyte depletion medium.
Embodiments according to the invention comprise processing biological fluid
from a plurality of sources. For example, one embodiment of a method comprises
(A)
obtaining a first portion of biological fluid from a first source of
biological fluid, and
obtaining a second portion of biological fluid from the first source of
biological fluid;
(B) obtaining a fast portion of biological fluid from a second source of
biological fluid,
and obtaining a second portion of biological fluid from the second source of
biological
fluid; and (C) combining at least one component of the second portion of
biological fluid
from the first source of biological fluid with at least one component of the
second
portion of biological fluid from the second source of biological fluid to
produce a pooled
biological fluid. Embodiments of the method can also include leukocyte
depleting at
least one component of the second portions of biological fluid, or leukocyte
depleting the
pooled biological fluid.
2 0 Typically, the biological fluid is processed in a closed system. In some
embodiments, the pooled or unpooled biological fluid is stored for at least
two days
before being used as a transfusion product. In an embodiment, the processed
biological
fluid is stored in a container comprising polyvinyl chloride (PVC) plasticized
with tri
(2-ethylhexyl) trimellitate (TOTM).
2 5 In accordance with embodiment of the invention, a system for processing
biological fluid comprises a phlebotomy device including at least two needles,
wherein at
least one needle is suitable for penetrating the skin of a biological fluid
donor, and a
leukocyte depletion filter assembly in fluid communication with the phlebotomy
device.
Each of the components of the invention will now be described in more detail
3 0 below. Like components have like reference numbers.
Figures 1 and 2 illustrate embodiments of a biological fluid processing system
10
in accordance with the present invention. The exemplary illustrated system 10
includes
5
CA 02271440 1999-OS-11
WO 98/28057 - PCT/US97/23558
a plurality of containers 12, 18a, 18b, and 20, in fluid communication with a
plurality of
conduits 32-37, a plurality of connectors 4 and 24, a filter assembly 26
(Figure 1), and a
phlebotomy device 1. The system 10 also includes at least one, and typically
two or
more flow control devices 40. In some embodiments (not shown), the system
includes at
least one additional filter assembly, e.g., interposed between containers 12
and 20.
Typically, as noted in more detail below, container 12 is suitable for
receiving a unit of
biological fluid, which can be processed to form a supernatant layer including
platelet-rich-plasma, and a sediment layer including red blood cells; or
processed to
form a supernatant layer including platelet-poor-plasma, an intermediate layer
including
the buffy coat, and a sediment layer including red blood cells.
The phlebotomy device 1 illustrated in Figures 1, 2 and 4A comprises a
connector 4, a plurality of conduits 30 and 31, and a plurality of needles 2
and 3. At
least one of the needles is a phlebotomy needle. In an embodiment, needles 2
and 3 are
both phlebotomy needles. The illustrated device 1 can be pre-assembled before
connection to the other components of the system.
Of course, the phlebotomist device can have other configurations, e. g. ,
additional
conduits, connectors, and/or needles.
The phlebotomy device 1 includes at least one needle suitable for penetrating
the
skin of a blood donor, and the device 1 is suitable for obtaining a plurality
of portions of
2 0 biological fluid so that:
( 1 ) at least the first portion is not passed into biological fluid receiving
container
12, and
(2) a subsequent portion is passed into the receiving container 12.
Since it is believed that the first portion of biological fluid presents an
increased
2 5 potential for bacterial contamination, preventing the passage of the first
portion into
receiving container 12 as described below allows a subsequent portion to be
collected in
container 12 that is less likely to present the risk of significant bacterial
contamination.
Using the embodiment illustrated in Figure 1 for reference, needle 2 is
suitable
for penetrating the skin of a blood donor, and needle 3 (which can be
identical to needle
3 0 2) is suitable for penetrating the cap of a blood sampling device such as
a vacutainer
(shown in dotted lines). With flow control device 40 associated with conduit
31 open,
and flow control device 40 associated with conduit 32 closed (to prevent fluid
6
CA 02271440 1999-OS-11
WO 98I28057 - PCT/US97/23558
communication with the biological fluid receiving container 12), such an
arrangement
can allow a first portion of a biological fluid such as blood to pass from the
donor,
through needle 2, conduit 30, conduit 31, and needle 3 into the blood sampling
device.
In some embodiments, this first portion of a biological fluid is collected
essentially free
of anticoagulant in the sampling device.
Subsequently, i.e., after closing the flow control device 40 associated with
conduit 31 and opening flow control device 40 associated with conduit 32, a
second
portion of biological fluid (which is less likely to present the potential for
significant
bacterial contamination) can be passed into container 12. The second portion
can be
further processed, e.g., to separate blood components and to leukocyte deplete
at least
one separated component, as described below.
In some embodiments, blood components are separated from each of a plurality
of second portions (e.g., from different donors), and the blood components are
pooled.
For example, first and second portions of blood can be collected from a
plurality of
blood donors, and each second portion can be processed to produce a unit of
platelet
concentrate (PC). A plurality of units of PC can subsequently be pooled.
A variety of pooling arrangements are suitable for carrying out the invention,
and
the invention is not to be limited thereby. Figure 3 illustrates an embodiment
of a
biological fluid pooling system that can be used in accordance with the
invention. The
2 0 illustrated system 100 includes a plurality of containers 18a, each
suitable for holding a
biological fluid such as PC, in fluid communication with a pooling assembly
141. In the
illustrated embodiment, the pooling assembly 14I includes a network or
plurality of
conduits 140 that converge into a single conduit 60 at outlet or junction 50.
The outlet
or junction 50 of the pooling assembly 141 is in fluid communication with a
receiving or
2 5 transfer container 118a. In the illustrated embodiment, fluid
communication with the
receiving container 118a is provided by a conduit 60. Interposed in the
conduit 60
between the outlet or junction 50 and the container 118a may be at least one
device or
assembly. For example, as shown in the illustrated embodiment, the pooling
system 100
may include a gas inlet 80, a drip chamber 81, a filter assembly 26 such as a
leukocyte
3 0 depletion assembly, and a gas outlet 82. One example of a suitable system
including a
pooling assembly is disclosed in U.S. Patent No. 5,364,526.
Figure 4B illustrates another embodiment of a biological fluid pooling system
7
CA 02271440 1999-OS-11
WO 98/28057 - PCT/ITS97/23558
that can be used in accordance with the invention. The illustrated system
includes a
plurality of containers 12, each suitable for holding a biological fluid such
as huffy coat,
in fluid communication via conduits 160 with a first receiving container 118a
for pooled
huffy coat. The illustrated system in Figure 4B also includes a filter
assembly 2b, such
as a leukocyte depletion assembly, interposed between the first receiving
container 118a
and a second receiving container 118a. Typically, the system also includes an
additional
container 20, e.g., for holding an additive or wash solution.
Figure 4A illustrates an embodiment of a biological fluid processing system 10
than can be used to produce the individual units of huffy coat that can be
pooled using
the system illustrated in Figure 4B. The system illustrated in Figure 4A
includes a
phlebotomy device 1, and a plurality of containers, e.g., containers 12, 18b,
and 20 in
fluid communication via a plurality of conduits 160, as described with respect
to Figure
1. For example, containers 12 in Figure 4B may be comprised of individual
units of
huffy coat from container 12 in Figure 4A.
The containers 12, 18a, 18b, 20, and 118a may be constructed of any material
and shape compatible with a biological fluid. A wide variety of these
containers are
already known in the art. For example, blood collection and satellite bags are
typically
made from plasticized PVC, e.g., PVC plasticized with dioctylphthalate (DOP),
diethylhexylphthalate (DEHP) (e.g., di-(2-ethylhexyl) phthalate)), or
trioctyltrimellitate
(TOTM) (e.g., tri (2-ethylhexyl) trimellitate). Illustrative containers
include, but are not
limited to, those produced in accordance with UK Patent Application GB 2, 301,
822A,
and U.S. Patent Nos. 4,280,497 and 4,670,013.
In an embodiment, at least one of the containers is made from PVC plasticized
with tri 2-ethylhexyl trimellitate (TOTM). Illustratively, the container can
be plasticized
2 5 with at least about 30 weight percent TOTM. Typically, containers
plasticized with
TOTM also include, for example, at least one epoxidized vegetable oil, a metal
soap,
and/or mineral oil. Containers can be plasticized with a blend of
plasticizers, e.g.,
TOTM and dioctylphthalate (DOP). The containers can provide for killing and/or
preventing the reproduction of undesirable material such as microorganisms
and/or
3 0 viruses.
In some embodiments, e.g., involving the storage of a platelet-containing
biological fluid such as platelet concentrate, such bags can provide a
bacteriostatic or
8
CA 02271440 1999-OS-11
WO 98I28057 - PCT/US97/23558
bacteriocidal effect. The bags can provide the effect on bacteria that, for
example, were
present in the blood donor's blood stream before donation, and/or bacteria
that
contaminated the fluid during collection or storage.
One example of a suitable container is a CLX~ bag, available from Medsep
Corporation (Covina, CA).
In some embodiments, at least one of the containers is compatible with a
biological fluid additive and/or preservative solution. Alternatively, or
additionally, at
least one of the containers is compatible with, for example, an antibacterial
and/or
antiviral agent. The agents) can be utilized to kill and/or prevent the
reproduction of
undesirable material such as microorganisms and/or viruses present with
platelets,
plasma, and/or red blood cells. Suitable agents are known in the art.
Exemplary agents
include, but are not limited to, quinolones and their derivatives, e. g. , a
quinolone
carboxylic derivative such as ciprofloxacin.
The conduits 30-37, 140, 60, and 160 used in the instant invention may be
constructed of any material compatible with biological fluid. Preferably, they
may be
composed of a flexible material, such as plasticized PVC, e.g., as described
above with
respect to the blood collection and satellite bags.
The flow control device 40 illustrated in the Figures comprises a clamp, seal,
valve, transfer leg closure, or the like. Systems typically include a
plurality of flow
2 0 control devices, and they can be located within or on the conduits and/or
the containers.
The filter assembly 26 illustrated in Figures 1, 3 , and 4 comprises a housing
including an inlet and an outlet, and defining a flow path between the inlet
and the
outlet, with at least one porous medium interposed between the inlet and the
outlet. In a
more preferred embodiment, the filter assembly 26 comprises a leukocyte
depletion
2 5 device, and the porous medium comprises a leukocyte depletion medium. The
filter
assembly 26 can be suitable for leukocyte depleting an individual unit of
biological fluid
(Figure 1), a plurality of units of biological fluid (Figure 3), or pooled
units of
biological fluid (Figure 4B).
In some embodiments, e.g., involving the leukocyte depletion of
3 0 platelet-rich-plasma (PRP), pooled or non-pooled huffy coat, or transition
zone material,
the leukocyte depletion device comprises a combined leukocyte depletion
medium/red
cell barrier medium.
9
CA 02271440 1999-OS-11
WO 98/28057 - PCTIUS97/23558
Systems according to some embodiments of the invention include a plurality of
filter assemblies 26, e.g., to filter different components of the biological
fluid. For
example, in a variation of the embodiment illustrated in Figure 1, a second
filter
assembly 26 can be interposed between containers 12 and 20. For example, a
second
filter assembly 26 can be used to deplete leukocytes from a red blood cell
containing
biological fluid such as packed red blood cells (PRC).
Exemplary filter assemblies, particularly exemplary leukocyte depletion
devices
and media include but are not limited to those disclosed in U.S. Patent Nos.
5,152,90S,
4,925,572, 4,880,548, 5,399,268, 5,217,627, and 5,100,564, as well as
International
Publication No. WO 93/04763.
Systems according to the invention can be open, or, more preferably, closed.
As
used herein, the term "closed" refers to a system that allows the collection,
processing,
filtration, storage, and preservation of donor blood or blood components
without the
need to enter the system (and risk contamination of the system). A closed
system can be
as originally made, or result from the connection of system components using
what are
known as "sterile docking" devices. Illustrative sterile docking devices are
disclosed in
U.S. Patent No. 4,507,119.
In some embodiments of the invention, the system can include additional
elements or components, such as one or more additional containers, a drip
chamber, at
2 0 least one venting device, e. g . , at least one gas inlet, at least one
gas outlet, and/or at
least one gas collection and displacement loop.
Illustratively, a gas inlet can be disposed upstream of a filter assembly such
as a
leukocyte depletion device, and/or a gas outlet can be disposed downstream of
the filter
assembly. For example, a gas inlet and/or a gas outlet may be used to maximize
the
2 5 recovery of biological fluid in receiving or transfer container 118a.
Using the
illustrative system illustrated in Figure 3 for reference, the gas inlet 80
and the gas outlet
82 may be, respectively, upstream and downstream of the filter assembly 26.
In accordance with the embodiment exemplified in Figure 3, gas inlet 80 is
disposed downstream of the outlet 50 of the pooling assembly l41, and upstream
of a
30 drip chamber 81, which is upstream of the filter assembly 26. Gas outlet 82
is disposed
downstream, interposed between the filter assembly 26 and the receiving or
transfer
container 118a. Alternatively, a gas inlet and/or a gas outlet may be
positioned in a drip
CA 02271440 1999-OS-11
WO 98I28057 - PCT/US97/23558
chamber, a conduit, or the receiving and/or source containers.
The gas inlet and gas outlet each comprise at least one porous element
designed
to allow gas to pass therethrough. The gas inlet and gas outlet should be
chosen so that
the sterility of the system is not compromised. A variety of materials may be
used,
provided the requisite properties of the porous element are achieved. These
properties
include the necessary strength to handle the differential pressures
encountered in use and
the ability to provide the desired filtration capability while providing the
desired
permeability without the application of excessive pressure. In a closed
system, the
porous elements of the gas inlet and the gas outlet should also preferably
have a pore
rating of about 0.2 micrometer or less to preclude bacteria entering the
system.
Preferably, the gas inlet and gas outlet include at least one liquophobic
porous
element. Because the liquophobic porous element is not wettable, or poorly
wettable, by
the biological fluid being processed in the system, gas in the system that
contacts the
liquophobic element will pass through it, while the biological fluid will not.
The gas
outlet may include at least one liquophilic porous element, that allows gas to
pass
through. In an embodiment, the gas outlet includes both a liquophobic membrane
and a
liquophilic membrane, and gas will pass through both membranes until the
Iiquophilic
membrane is wetted by the biological fluid. Additionally, the gas inlet and/or
the gas
outlet may be included in a housing, which may include a cap or closure.
2 0 Exemplary venting devices, including gas inlets, gas outlets, and/or gas
collection and displacement loops, and processes for using them, are as
disclosed in, for
example, International Publication Nos. WO 91/17809 and WO 92/07656, and U. S.
Patent Nos. 5,126,054, 5,364,526, and 5,472,621.
The processing of biological fluid in the context of the present invention may
2 5 take place at any suitable time, which may be soon after donation. For
example, when
the biological fluid is donated blood, it is typically processed as soon as
practicable in
order to maximize the number of components derived and to maximize blood
component
viability and physiological activity. Early processing may more effectively
reduce or
eliminate contaminating factors, including, but not limited to, leukocytes and
3 0 microaggregates. In accordance with the subject invention, the biological
fluid may be
processed within about 24 hours of collection from the donor. The subject
invention
may also include processing biological fluid in accordance with United States
practice,
11
CA 02271440 1999-OS-11
WO 98/280S7 - PCT/US97/23558
wherein the processing of whole blood is generally within 8 hours of
collection from the
donor.
In accordance with the invention, leukocyte depletion can be carried out on
any
blood component at any point in the processing protocol. For example,
leukocyte
depletion can be carried out while separating platelet-rich-plasma (PRP) from
red blood
cells, while pooling platelet concentrate (PC), while filtering pooled or non-
pooled buffy
coat, or while administering PC.
An exemplary embodiment of a method according to the invention can be
described with reference to Figures 1, 2, and 4, which illustrate a phlebotomy
device 1
having first and second phlebotomy needles 2 and 3, wherein the device 1 is in
fluid
communication with multiple containers, e.g., a multiple blood bag system
including a
blood collection bag 12, and one or more satellite bags, e.g., 20, 18a, and
18b.
Typically, blood collection bag 12 contains an anticoagulant, and satellite
bag 20
contains an additive such as a red cell storage solution.
Flow control devices 40 such as clamps (associated with conduits 31 and 32)
are
initially closed, and needles 2 and 3 are initially capped (caps not shown).
A blood donor's arm is prepared for venipuncture in the usual manner, and
needle 2 is uncapped and inserted into the donor's vein. Needle 3 is uncapped
and
inserted into a blood sampling device such as a vacutainer, the clamp 40
associated with
2 0 conduit 31 is opened, and a first portion of biological fluid is passed
into the vacutainer.
In some embodiments, the needle 2 cuts a disc of skin from the donor, and the
skin plug
can pass into the vacutainer.
One or more blood sampling devices can be sequentially filled as desired.
After
the last sampling device is filled, clamp 40 associated with conduit 31 is
closed, and the
2 5 needle 3 is removed from the vacutainer. Clamp 40 associated with conduit
32 is
opened, and a second portion of biological fluid is passed into collection
container 12.
This portion of biological fluid is less likely to present a significant risk
of bacterial
contamination during storage. After a suitable volume of biological fluid is
collected in
the container, flow control device 40 is closed, and needle 2 is removed from
the donor.
3 0 In some embodiments, after needle 2 and/or 3 is uncapped, or removed from
the donor,
the needle is placed in a device such as a phlebotomist protector to minimize
the risk of
accidental needle puncture.
12
CA 02271440 1999-OS-11
WO 98I28057 - PCT/US97/23558
Typically, the unit of biological fluid (i.e., the second portion of
biological fluid)
in the container 12 is processed to separate one or more blood components.
For example, the unit of biological fluid can be processed to form
concentrated
red blood cells and platelet-rich-plasma (PRP), and the PRP is processed to
produce
platelet concentrate (PC) and plasma. Alternatively, the unit of biological
fluid can be
processed to form concentrated red blood cells, huffy coat, and platelet-poor-
plasma
(PPP), and the huffy coat is subsequently processed to produce PC.
Illustratively, the container 12 can be centrifuged to form a sediment layer
including red blood cells, and a supernatant layer including platelets
suspended in
plasma such as platelet-rich-plasma (PRP). In one embodiment, using the
exemplary
system illustrated in Figure 1 for reference, the PRP is passed through a
filter assembly
26 comprising a leukocyte depletion device, and leukocyte-depleted PRP is
collected in
satellite container 18a. In some embodiments, the leukocyte depletion device
comprises
a leukocyte depletionlred cell barrier medium, and the leukocyte-depleted PRP
collected
2 5 in satellite container 18a is substantially free of red blood cells.
Typically, satellite containers 18a and 18b are subsequently separated from
the
rest of the system while maintaining a closed system. The leukocyte-depleted
PRP can
be further processed to form PC and plasma in containers 18a and 18b as is
known in the
art. If desired, the separated red blood cells, PC, and/or plasma can be
stored until
2 0 needed. In an embodiment, the method includes storing the separated
components while
killing and/or preventing the reproduction of bacteria and/or viruses that may
be present
in the fluid. For example, one or more containers, e.g., 12, 20, 18a and/or
18b may
provide a bacteriostatic and/or a bacteriocidal effect. Alternatively, or
additionally, at
least one antibacterial agent and/or antiviral agent can be added to the
containers(s),
2 5 before or after the components are passed into the bag. If desired, the
added agents)
can be removed from the blood components before transfusing the components.
The separated components, e.g., stored or non-stored PC and/or plasma, can be
pooled before further use.
In another illustrative embodiment, the PRP is not filtered. Rather, the PRP
is
3 0 processed to further separate the blood components, and the separated
components can
be filtered, e.g., as illustrated in Figures 2 and 3.
For example, the non-filtered PRP can be processed to produce PC and plasma
13
CA 02271440 1999-OS-11
WO 98I28057 - PCT/US97/23558
using the embodiments of the system shown in Figure 2. Subsequently, the PC
can be
filtered, e. g. , while pooling multiple units of PC (as shown in Figure 3),
or while
administering individual units of PC to a patient (not shown). Of course, the
PC
(individual units or pooled units) can be stored until needed as described
above. For
example, the method can include storing the separated components while killing
and/or
preventing the reproduction of bacteria and/or viruses that may be present in
the fluid.
In accordance with another embodiment of the invention, using the exemplary
system illustrated in Figure 4A for reference, the second portion of
biological fluid can
be collected in the container 12 using phlebotomy device 1 as described above.
Container 12 can be centrifuged to form a sediment layer including red blood
cells, an
intermediate layer including the majority of the platelets (the huffy coat),
and a
supernatant layer including most of the plasma such as platelet-poor-plasma
(PPP).
The layers are separated (e.g., leaving huffy coat in container 12), and the
huffy
coat is processed to produce a platelet-containing blood product such as PC.
If desired,
multiple units of huffy coat can be pooled before producing the platelet-
containing blood
product. Illustratively, units of huffy coat can be pooled using the system
illustrated in
Figure 4B, or using a system similar to that illustrated in Figure 3, with or
without a
filter assembly 26 interposed between the pooling assembly 141 and the
receiving
container 118a. As noted above, these systems can be open or closed.
2 0 With respect to the embodiment illustrated in Figure 4B, individual
containers 12
having huffy coat therein can be placed in fluid communication with each
other,
preferably by sterile docking the upstream conduit 160 from one container to
the
downstream conduit 160 from another container. Typically, the processing
system also
includes at least one receiving or transfer container 118a suitable for
holding the pooled
2 5 units of huffy coat, and a container 20 suitable for holding an additive
solution such as a
wash solution to wash huffy coat from one or more containers 12 (and conduits
therebetween) into the first receiving or transfer container 118a. The huffy
coats can be
pooled as is known in the art.
The pooled huffy coat in container 118a can be centrifuged to form a sediment
3 0 layer including red blood cells and a supernatant layer including
platelets suspended in
plasma. The supernatant layer can be filtered through a filter assembly 26
such as a
leukocyte filter device, which can include a combined leukocyte depletion
medium/red
14
CA 02271440 1999-OS-11
WO 98/28057 - PCT/ITS97/23558
cell barrier medium.
One embodiment of method using the system illustrated in Figure 3 includes
introducing air or gas into source containers 18a prior to passing the
biological fluid
from the containers and through the pooling assembly. For example, air or gas
may be
introduced into the containers 18a through the gas inlet assembly 80 or the
gas outlet
assembly 82, preferably by using a syringe (not shown). The introduced air or
gas is
preferably ambient air or a sterile gas.
Introducing gas into the source containers 18a (Figure 3) may be accomplished
by opening a flow path from the gas inlet 80 or the gas outlet 82 to the
appropriate
container 18a, while closing the flow path to the receiving or transfer
container 118a.
For example, the clamps on the conduits leading to the receiving or transfer
container
l I8a and a11 but one container 18a may be closed, so that when gas is
introduced into the
system, gas in the conduit will enter the open container. In one embodiment,
the
process includes introducing gas sequentially into the containers 18a. The
flow path to
each source container may be closed after gas has been introduced into that
container.
The flow path from the gas inlet 80 or the gas outlet 82 is then closed. The
flow
path to the first container 18a is then opened, and as the biological fluid
passes from the
first container 18a, and flows through the pooling assembly l41 toward
receiving or
transfer container 118a, it displaces the gas that was ahead of the column of
flowing
2 0 biological fluid. If desired, this gas can be exhausted or removed from
the system. The
gas may be vented, a . g . , through an open gas outlet 82. Once the gas has
been vented,
the gas outlet may be inactivated, e.g., to prevent gas from entering the
system. The gas
outlet may include both a liquophobic element and a liquophilic element, which
inactivates the outlet automatically, upon wetting the liquophilic element
with the
2 5 biological fluid.
Once the gas ahead of the biological fluid column has been exhausted and the
flow of biological fluid has stopped, clamps 40 adjacent to the other
containers 18a are
opened, preferably, sequentially, so that biological fluid from the other
containers 18a
may pass through the pooling assembly 14I (Figure 3) toward the receiving or
transfer
3 0 container 118a. The clamp 40 adjacent to the receiving or transfer
container 118a is
opened so that the biological fluid can flow into the container 118a.
Preferably, the
clamp 40 adjacent to the receiving or transfer container 118a is opened before
the clamps
CA 02271440 1999-OS-11
WO 98I28057 - PCT/US97/23558
adjacent to the other source containers are opened.
Initiating the flow of biological fluid from the other source containers also
displaces gas ahead of the other units of biological fluid. Preferably, this
gas may be
collected in drip chamber 81 interposed between the outlet or junction 50 and
the
receiving or transfer container 118a. Passing the biological fluid through a
drip chamber
81 may include collecting gas and/or controlling the rate of flow of the
biological fluid.
The drip chamber 81 is typically inverted until the biological fluid fills the
drip chamber,
at which point the drip chamber is returned to its normal orientation.
In accordance with an embodiment of the invention as illustrated in Figure 3,
the
biological fluid may also be passed through a filter assembly 26 such as a
leukocyte
depletion device interposed between the outlet or junction 50 of the pooling
assembly
141 and the receiving or transfer container Il8a. Preferably, the filter
assembly 26 is
located between the gas inlet 80 and the gas outlet 82.
As the biological fluid passes through the drip chamber 81 and the optional
filter
assembly 26, the gas ahead of the biological fluid may be passed through the
gas outlet
82 as described previously. Pooled biological fluid is then recovered in the
receiving or
transfer container 118a and, in accordance with the invention, the
introduction of air or
gas into the receiving container can be minimized, so the biological fluid is
recovered
without collecting excess air.
2 0 In order to maximize recovery of biological fluid, gas may be introduced
behind
the biological fluid retained in the system. Using the illustrative system
illustrated in
Figure 3 for reference, the gas that was initially introduced into the
containers 18a
through either the gas inlet 80 or the gas outlet 82 will follow the
biological fluid as it
flows through the conduits. This increases the recovery of the biological
fluid, since the
2 5 gas following the biological fluid "chases" the fluid from the conduits.
Furthermore,
after the biological fluid has passed through the pooling assembly into the
receiving or
transfer container 118a and the containers 18a have collapsed, gas may be
introduced
behind the retained biological fluid by opening gas inlet 80. Additional
biological fluid
may then be recovered in the receiving or transfer container 118a.
3 0 Once recovery of biological fluid has been completed, receiving or
transfer
container 118a may be sealed and separated from the system, without the
introduction of
air into the container. Preferably, receiving or transfer container 118a is
heat sealed,
16
CA 02271440 1999-OS-11
WO 98/28057 - PCT/US97/23558
although other methods of sealing are also suitable.
Further embodiments are encompassed by the present invention. For example,
biological fluid can be collected without separating a first portion, and the
biological
fluid can be filtered and placed in a container that has a bacteriocidal or
bacteriostatic
effect on bacteria that may be present in the biological fluid. For example, a
unit of
whole blood can be collected, and centrifuged to form packed red blood cells
and
platelet-rich-plasma (PRP). The PRP can be passed through a leukocyte
depletion
device and the leukocyte depleted PRP, or platelet concentrate (PC) derived
therefrom,
can be placed in a container comprising polyvinyl chloride (PVC) plasticized
with tri
(2-ethylhexyl) trimellitate (TOTM).
A11 of the references cited herein, including publications, patents, and
patent
applications, are hereby incorporated in their entireties by reference.
While the invention has been described in some detail by way of illustration
and
example, it should be understood that the invention is susceptible to various
modifications and alternative forms, and is not restricted to the specific
embodiments set
forth. It should be understood that these specific embodiments are not
intended to limit
the invention but, on the contrary, the intention is to cover a11
modifications,
equivalents, and alternatives falling within the spirit and scope of the
invention.
17