Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02279902 1999-08-06
WO 98/34563 PCTIUS98/02244
METHOD FOR USING TROPOELASTIN AND
FOR PRODUCING TROPOELASTIN BIOMATERIALS
BACKGROUND OF THE INVENTION
This invention relates to a method for using
tropoelastin, and more particularly to a method for
producing tropoelastin biomaterials.
Elastic fibers are responsible for the elastic
properties of several tissues such as skin and lung, as
well as arteries, and are composed of two
morphologically distinct components, elastin and
microfibrils. Microfibrils make up the quantitatively
smaller component of the fibers and play an important
role in elastic fiber structure and assembly.
The most abundant component of elastic fibers is
elastin. The entropy of relaxation.of elastin is
responsible for the rubber-like elasticity of elastic
fibers. In vertebrates elastin is formed through the
secretion and crosslinking of tropoelastin, the 72-kDa
biosynthetic precursor to elastin. This is discussed,
for example, in an article entitled "Oxidation, Cross-
linking, and Insolubilization of Recombinant
Crosslinked Tropoelastin by Purified Lysyl Oxidase" by
Bedell-Hogan, et al in the Journal of Biological
Chemistry, Vol. 268, No. 14, on pages 10345-10350
(1993).
In vascular replacement and repair, the best
current option is to implant autologous veins and
arteries where the obvious limit is the supply of
vessels which can be sacrificed from the tissues they
were intended to service. Autologous vein replacements
for damaged arteries also tend to be only a temporary
CA 02279902 2004-11-25
2
measure since they can deteriorate in a few years in high
pressure arterial circulation.
When autologous graft material is not available, the
surgeon must choose between sacrificing the vessel, and
potentially the tissue it sub-served, or replacing the
vessel with synthetic materials such as DacronTM or Gore-
texTM. Intravascular compatibility indicate that several
"biocompatible polymers", including DacronTM, invoke
hyperplastic response, with inflammation particularly at
the interface between native tissue and the synthetic
implant. Incomplete healing is also due, in part, to a
compliance mismatch between currently used synthetic
biomaterials and native tissues.
Thirty to forty percent of atherosclerotic stenoses
that are opened with balloon angioplasty restenose as a
result of ingrowth of medial cells. Smooth muscle
ingrowth into the intima appears to be more prevalent in
sections of the artery where the internal elastic
lamina(IEL) of the artery is ripped, torn, or missing, as
in severe dilatation injury from balloon angioplasty,
vessel anastomoses, or other vessel trauma that results
in tearing or removal of the elastic lamina.
Prosthetic devices, such as vascular stents, have
been used with some success to overcome the problems of
restenosis or re-narrowing of the vessel wall resulting
from ingrowth of muscle cells following injury. However,
metal stents or scaffolds being deployed presently in
non-surgical catheter based systems to scaffold damaged
arteries are inherently thrombogenic
CA 02279902 1999-08-06
WO 98/34563 PCTIUS98/02244
3
and their deployment can result in catastrophic
thrombotic closure. Metal stents have also been well
demonstrated to induce a significant intimal
hyperplastic response within weeks which can result in
restenosis or closure of the lumen. Optimal arterial
reconstruction would restore the arterial architecture
such that normal vascular physiology and biology would
be re-established thus minimizing acute and long-term
maladaptive mechanisms of vascular homeostasis. Until
relatively recently, the primary methods available for
securing a prosthetic material to tissue (or tissue to
tissue) involved the use of sutures or staples. fibrin
glue, a fibrinogen polymer polymerized with thrombin,
has also been used (primarily in Europe) as a tissue
sealant and hemostatic agent.
Damage to the arterial wall through disease or
injury can involve the endothelium, internal elastic
lamina, medial smooth muscle and adventitia. In most
cases, the endogenous host response can repair and
replace the endothelium, the smooth muscle and the
adventitial layers over a period of.weeks to months
depending upon the severity of the damage. However,
elastin does not undergo extensive post-developmental
remodelling and the capacity for elastin synthesis
declines with age. (see "Regulation of Elastin
Synthesis in Organ and Cell Culture" by Jeffrey M.
Davidson and Gregory C. Sephel in Methods in Enzymology
144 (1987) 214-232. Therefore, once damaged, elastic
fibers are not substantially reformed. Neosynthesis of
elastin in arterial walls subject to hypertension or
CA 02279902 1999-08-06
WO 98/34563 PCT/US98/02244
4
neointimal hyperplasia represents the most significant
example of post developmental elastin synthesis. This
synthesis results in elastic structures mostly composed
of elastin fibrils whose organization is unlike normal
elastin architecture and probably contributes little to
the restoration of normal vascular physiology.
In animal models of intimal hyperplasia or
atherosclerosis it is well accepted that disruption of
the internal elastic lamina is a prerequisite to
reliable production of intimal hyperplasia or
atherogenesis in large animals or primates. see
Schwartz R.S., et al, in an article entitled
"Restenosis After Balloon Angioplasty: Practical
Proliferation Model In Porcine Coronary Arteries" in
Circulation 1990: 82: 2190-2200. This observation is
supported by several lines of evidence that suggest a
role for elastin in the biological regulation of
several cell types. Pathological studies indicate that
elastin provides a secure attachment for endothelial
cells and can act as a barrier to macromolecules such
as mitogens and growth factors preventing these
molecules from entering the media of blood vessels.
Lipids, foamy macrophages, and other inflammatory cells
do not appear to enter the intima as readily when a
substantial and continuous elastin membrane is present
immediately to the endothelium according to Sims, F.H.,
et al, in an article entitled "The Importance of A
Substantial Elastic Lamina Subjacent To The Endothelium
In Limiting the Progression of Atherosclerotic Changes"
in Histopathology (1993) at 23:307-317. In addition,
CA 02279902 1999-08-06
WO 98/34563 PCT1US98102244
it has been shown by Ooyama, Toshiro and Sakamoto that
chemotactic effects of soluble elastin peptides and
platelet derived growth factor are inhibited by
substratum bound elastin peptides. see "Elastase in the
5 Prevention of Arterial Aging and the Treatment of
Atherosclerosis. see "The Molecular Biology and
Pathology of Elastic Tissues" edited by Chadwick, Derek
J. and Jamie A. Goode, John Wiley and Sons Ltd,
Chichester, England (1995). In vitro experiments show
that alpha elastin suppresses the phenotypic transition
(contractile to synthetic) of rabbit arterial SMC by
interacting with a 130 kDa cell surface elastin binding
protein for cell binding sequence VGVAPG. Rabbit
smooth muscle cells adhering to elastic fibers appears
to favor the contractile over the synthetic state which
is identified with restonotic responses to injury. see
"Changes in Elastin Binding Proteins During Phenotypic
Transition of Rabbit Arterial Smooth Muscle Cells in
Primary Culture" by Yamamoto, et al in Experimental
Cell Research 218 (1995) pg.339-345. Similar work by
Ooyama and colleagues has demonstrated that the
phenotypic change of smooth muscle cells from the
contractile to the modified type is significantly
retarded when the cells are grown on elastin coated
dishes.
Until relatively recently, the primary methods
available for securing a prosthetic material to tissue
(or tissue to tissue) involved the use of sutures or
CA 02279902 1999-08-06
WO 98/34563 PCTIUS98/02244
6
staples. Fibrin glue, a fibrin polymer polymerized
with thrombin, has also been used (primarily in Europe)
as a tissue sealant and hemostatic agent.
Laser energy has been shown to be effective in
tissue welding arterial incisions, which is thought to
occur through thermal melting of fibrin, collagen and
other proteins. The use of photosensitizing dyes
enhances the selective delivery of the laser energy to
the target site and permits the use of lower power
laser systems, both of which factors reduce the extent
of undesirable thermal trauma.
The present invention combines the advantages of
tropoelastin-based products with the advantages of
laser welding techniques, and provides a unique method
of tissue repair and replacement. The invention makes
possible tissue prostheses (particularly, vascular
prostheses) that are essentially free of problems
associated with prostheses known in the art.
Arterial replacement or reconstruction using
tropoelastin based biomaterials not only may provide
normal strength and elasticity but also may encourage
normal endothelial re-growth, inhibit smooth muscle
cell migration and thus restore normal vascular
homeostasis to a degree not currently possible with
synthetic grafts.
U.S. 4,589,882 is directed to a method for
producing synthetic elastomeric polypeptide biomaterial
which replicates a portion of the crosslinked
CA 02279902 2004-11-25
7
tropoelastin polypeptide sequence. This synthetic
elastomeric polypeptide biomaterial can be employed in
repairing a natural elastic system of an animal body.
U.S. 4,721,096 and 4,963,489 disclose a three-
dimensional cell culture system in which a living stromal
tissue is prepared in vitro by a framework composed of a
biocompatible, non-living material formed into a three-
dimensional structure having interstitial spaces.
Collagen has been considered for a biodegradable
biomaterials for use as a framework for a three-
dimensional, multi-layer cell culture system (see U.S.
Patent No. 4,721,096 and No. 4,963,489).
An improved three-dimensional cell culture systems
in which metabolic cycling optimizes the formation of
extracellular matrix by cells grown on a three-
dimensional matrix is disclosed in U.S. Patent No.
5,478,739. U.S. Patent No. 5,478,739 reports production
of collagens I, III, and IV, fibronectin, decorin, and
non-sulfated glycosaminoglycans by cells in a three
dimensional culture.
SUMMARY OF THE INVENTION
It is a general object of an aspect of the invention
to provide a method of effecting repair or replacement or
supporting a section of a body tissue using tropoelastin,
preferably crosslinked tropoelastin.
It is a specific object of an aspect of the
invention to provide a tropoelastin biomaterial suitable
for use as a stent, for example, a vascular stent, or as
conduit replacement, for example, as an artery, vein or a
ureter replacement. The tropoelastin biomaterial itself
can also be used as a stent or conduit covering or
coating or lining.
CA 02279902 2004-11-25
8
It is a further object of an aspect of the invention
to provide a tropoelastin graft material suitable for use
in repairing a lumen wall.
It is another object of an aspect of the invention
to provide a tropoelastin material suitable for use in
tissue replacement or repair in, for example, interior
bladder replacement or repair, intestine, tube
replacement or repair such as fallopian tubes, esophagus
such as for esophageal varicies, ureter, artery such as
for aneurysm, vein, stomach, lung, heart such as
congenital cardiac repair, or colon repair or
replacement, or skin repair or replacement, or as a
cosmetic implantation or breast implant.
It is also an object of an aspect of the invention
to provide a method of securing a tropoelastin
biomaterial to an existing tissue with or without the use
of sutures or staples.
The subject invention relates to method for using a
tropoelastin polymer and for producing a tropoelastin
biomaterial. Such methods comprise providing a
tropoelastin monomer and then polymerizing same as
hereinafter described. This will form a tropoelastin
polymer which can be formed into a biocompatible
tropoelastin biomaterial from said tropoelastin polymer
for use in biomedical applications. For example, as
CA 02279902 1999-08-06
WO 98/34563 PCTIUS98/02244
9
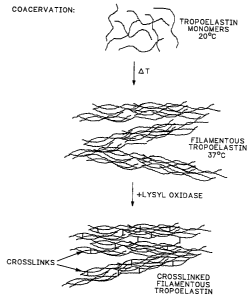
shown in FIG. 1, the tropoelastin mononer can be formed
into a filamentous structure by coacervation using
moderating heating to effect same. Then, the
filamentous tropoelastin is crosslinked using a
crosslinking agent, such as lysyl oxidase, to form a
crosslinked filamentous tropoelastin material.
Finally, the tropoelastin polymer is formed into a
layer of biocompatible tropoelastin biomaterial. It is
this biocompatible tropoelastin biomaterial which can
be used in the hereinafter described biomedical
applications.
The subject invention provides a biocompatible,
tropoelastin biomaterial formed into a three-
dimensional structure. This structure can be used, for
example, in a stromal support matrix populated with
actively growing stromal cells. The stromal support
matrix, which are preferably fibroblasts, can then be
used to provide support, growth factors, and regulatory
factors needed to sustain long-term active
proliferation of cells in culture. A living stromal
tissue can be prepared comprising stromal cells and
connective tissue proteins naturally secreted by the
stromal cells which are attached to and substantially
enveloping a framework composed of a biocompatible,
non-living material formed into three dimensional
structure having interstitial spaces bri.dged by the
stromal cells. The stromal cell systems contemplated
herein are described in the following U.S. patents of
CA 02279902 2004-11-25
Advanced Tissue Sciences, Inc. (formerly Marrow-Tech
Incorporated):
US 5,478,739, US 5,460,939, US 5,443,550, US 5,266,480,
US 5,518,915, US 5,516,681, US 4,963,489, US 5,032,508,
5 US 4,721,096, US 5,516,680, US 5,512,475, US 5,510,254,
and US 5,160,490.
The tropoelastin structure can also have a cellular
lining of human cells. The cells can be derived
autologously, or otherwise, and formed into a lining on
10 one of the major surfaces of the tropoelastin layer.
Preferably, the cells which are employed to form such a
lining are one endothelial cells and/or epithelial cells
and/or urothelial cells.
The tropoelastin structure can also be formed into a
biocompatible lining for mechanical structures to ensure
their continued internal use in a human body. Examples
of this are biocompatible linings for heart valves, heart
implants, dialysis equipment, or oxygenator tubing for
heart-lung by-pass systems.
The subject invention is directed to a method for
producing a tropoelastin biomaterial, typically a
crosslinked tropoelastin material, which is fused onto a
tissue substrate, and the tropoelastin biomaterial
itself. It is also directed to a method for using that
tropoelastin biomaterial, a method for producing a
tropoelastin biomaterial fused onto a tissue substrate,
and a prosthetic device and a method of producing a
prosthetic device including tropoelastin.
CA 02279902 1999-08-06
WO 98/34563 PCT/US98/02244
11
The present invention also relates to a method of
repairing, replacing or supporting a section of a body
tissue using tropoelastin. The method comprises
positioning tropoelastin at the site of the section and
bonding the biomaterial to the site or to the tissue
surrounding the site. The bonding is effected by
contacting the biomaterial and the site, or tissue
surrounding the site, at the point at which said
bonding is to be effected, with an energy absorbing
agent. The agent is then exposed to an amount of
energy absorbable by the agent sufficient to bond the
biomaterial to the site or to the tissue surrounding
the site.
A tissue-fusible tropoelastin biomaterial can be
produced using the method of the present invention
which comprises a layer of tropoelastin biomaterial and
a tissue substrate each having first and second outer
surfaces, and an energy absorbing material applied to
at least one of the outer surfaces. Preferably, the
energy absorbing material penetrates into the
biomaterial. -
The energy absorbing material is energy absorptive
within a predetermined range of light wavelengths
depending on material thickness. The energy absorbing
material is chosen so that when it is irradiated with
light energy in the predetermined wavelength range, the
intensity of that light will be sufficient to fuse
together one of the first and second outer surfaces of
CA 02279902 1999-08-06
WO 98/34563 PCT/US98/02244
12
the tropoelastin biomaterial and the tissue substrate.
Preferably, the first and second outer surfaces of the
tropoelastin biomaterial are major surfaces.
Typically, an energy absorbing material.is
indirectly irradiated by directing the light energy
first through the tropoelastin biomaterial or tissue
substrate and then to the energy absorbing material.
Although the energy absorbing material can be applied
directly to the tissue substrate, it is not the
preferred method because of the difficulty in
controlling penetration into the intertices of the
tissue substrate.
In a preferred method of this invention, the
energy absorbing material comprises a biocompatible
chromophore, more preferably an energy absorbing dye.
In one form of the present invention, the energy
absorbing material is substantially dissipated when the
tropoelastin biomaterial and the tissue substrate are
fused together. In another form of this invention, the
energy absorbing material comprises-a-material for
staining the first or second surface of the
tropoelastin biomaterial. The energy absorbing
material can also be applied to one of the outer
surfaces of the biomaterial by doping a separate
elastin layer with an energy absorbing material and
then fusing the doped separate elastin layer to the
tropoelastin biomaterial. In any case, the energy
absorbing layer is preferably substantially uniformly
applied to at least one of the outer surfaces,
CA 02279902 1999-08-06
WO 98/34563 PCTIUS98/02244
13
typically in a manner wherein the energy absorbing
material substantially covers the entire outer surface
of the tropoelastin biomaterial.
Some of the key properties which effect the method
of the present invention regarding fusing the
tropoelastin biomaterial and tissue substrate include
the magnitude of the wavelength, energy level,
absorption, and light intensity during irradiation with
light energy of the energy absorbing material, and the
concentration of the energy absorbing material. These
properties are arranged so that the temperature during
irradiation with light energy for period of time which
will cause fusing together of one of the first and
second outer surfaces of the tropoelastin biomaterial
and the tissue substrate is from about 40 to 140
degrees C., and more preferably from about 50 to 100
degrees C., but if well localized to the biomaterial
tissue interface, can be as high as 600 degrees C.
Furthermore, the average thickness of_the energy
absorbing material in the preferred method of this
invention is from about 0.5 to 300 microns.
The subject invention is also directed to a
prosthetic device comprising a support member
comprising a stent, a conduit or a scaffold having a
layer of tropoelastin material located on the support
member. In the preferred case, the layer of the
tropoelastin biomaterial completely surrounds the
support member.
CA 02279902 2004-11-25
14
The support member of the prosthetic device is
preferably formed of a metal or a synthetic material.
The metal preferably comprises titanium, tantalum,
stainless steel or nitinolTM. The synthetic material
typically comprises a polymeric material. This polymeric
material is generally selected from a group consisting of
polyethylene terepthalate (DacronTM), Gore-texTM, teflonTM,
polyolefin copolymer, polyurethane and polyvinyl alcohol.
The support member can be formed from a hybrid polymer
comprising a synthetic polymeric material and a natural
polymeric material including fibrin and/or elastin. The
support member can also be formed from a biological
material, preferably from collagen.
The prosthetic device can comprise a layer of
tropoelastin biomaterial. Preferably this layer comprises
a covering, a coating, or a lining for the support
member. The tropoelastin biomaterial can be formed by
polymerization, or formed to a suitable size and shape by
molding. The polymerized tropoelastin biomaterial can
also be further cross-linked using gamma radiation and/or
a cross-linking agent. In one form of the invention,-the
tropoelastin biomaterial is formed into a sheet, and the
sheet is employed as the covering for the support. The
sheet can also be attached to the support by grafting, by
mechanical bonding, or by laser bonding.
CA 02279902 2000-12-06
The prosthetic device of this invention is implantable within an artery, a
vein, an esophagus, an intestine, a colon, a ureter, a liver, a urethra, or a
fallopian tube.
A drug can be incorporated into the layer of tropoelastin material
5 thereby decreasing the need for systemic intravenous or oral medications.
Also, photodynamic therapy drugs ("PTD") which are activated with light can
be employed herein.
In use, a method for producing the prosthetic device of the present
invention comprises first providing a layer of tropoelastin biomaterial and a
io support member comprising a stent, a conduit or a scaffold. Then, the layer
of tropoelastin biomaterial is applied to the support member to form the
prosthetic device. For example, a layer of tropoelastin material can be
located on the support member and can be fused together. This can be
accomplished by applying an energy absorbing material, which is energy
15 absorptive within a predetermined range of light wavelengths, to the
tropoelastin biomaterial in an amount which will cause fusing together
thereof.
Thus, the energy absorbing material is irradiated with light energy in the
predetermined wavelength range with an intensity sufficient to fuse together
the tropoelastin biomaterial on the support member thereby fusing together
the tropoelastin biomaterial on the tissue substrate.
According to an aspect of the present invention, there is provided a
method for producing a tropoelastin biomaterial fused onto a tissue substrate
comprising:
providing a layer of tropoelastin biomaterial having a first and second
outer major surface and a tissue substrate having a first and second outer
major surface; and
applying an energy absorbing material, which is energy absorptive
within a predetermined range of light wavelengths, to a selected one of the
first and second outer surfaces of the tropoelastin biomaterial in an amount
which will cause fusing together of one of the first and second outer surfaces
of the tropoelastin biomaterial and one of the first and second outer surfaces
of the tissue substrate, the energy absorbing material penetrating into the
interstices of the tropoelastin biomaterial;
CA 02279902 2000-12-06
15a
irradiating the energy absorbing material with light energy in the
predetermined wavelength range with an intensity sufficient to fuse together
one of the first and second outer surfaces of the tropoelastin biomaterial and
the tissue substrate; and
fusing together the selected one of the first and second outer surfaces
of the tropoelastin biomaterial and the tissue substrate.
According to another aspect of the invention, there is provided a
method for using an tropoelastin biomaterial comprising:
providing a layer of tropoelastin biomaterial having a first and second outer
io major surface which is tissue-fusible;
providing a tissue substrate having a first and second outer major
surface;
applying an energy absorbing material, which is energy absorptive
within a predetermined range of light wavelengths, to one of the first and
second outer surfaces of the tropoelastin biomaterial in an amount which will
cause fusing together of one of the first and second outer surfaces of the
tropoelastin biomaterial and one of the first and second outer surfaces of the
tissue substrate, the energy absorbing material penetrating into the
interstices
of the tropoelastin biomaterial;
irradiating the energy absorbing material with light energy in the
predetermined wavelength range with an intensity sufficient to fuse together
one of the first and second outer surfaces of the tropoelastin biomaterial and
the tissue substrate; and
fusing together one of the first and second outer surfaces of the
tropoelastin biomaterial and the tissue substrate.
According to another aspect of the invention, there is provided a
method for producing an tropoelastin biomaterial fused onto a tissue substrate
comprising:
providing a layer of tropoelastin biomaterial having a first and second
outer major surface and a tissue substrate having a first and second outer
major surface; and
applying an energy absorbing material, which is energy absorptive
within a predetermined range of light wavelengths, to one of the first and
second outer surfaces of the tropoelastin biomaterial in an amount which will
CA 02279902 2000-12-06
15b
cause fusing together of one of the first and second outer surfaces of
the tropoelastin biomaterial and one of the outer surface of the tissue
substrate, the energy absorbing material penetrating into the interstices of
the
tropoelastin biomaterial;
indirectly irradiating the energy absorbing material by directing the light
energy first through the tropoelastin biomaterial or tissue substrate and then
to the energy absorbing material, the light energy being in the predetermined
wavelength range with an intensity sufficient to fuse together one of the
first
and second outer surfaces of the crosslinked tropoelastin biomaterial and the
io outer surface of the tissue substrate; and
fusing together one of the first and second outer surfaces of the
crosslinked tropoelastin biomaterial and the outer surface of the tissue
substrate and substantially dissipating the energy absorbing material when
the crosslinked tropoelastin biomaterial and the tissue substrate are fused
together.
According to another aspect of the invention, there is provided a
prosthetic device comprising:
a support member comprising a stent, a conduit or a scaffold; and
a layer of a tropoelastin biomaterial located on the support member.
According to another aspect of the invention, there is provided a
method for producing a prosthetic device comprising:
providing a layer of tropoelastin biomaterial and a support member
comprising a stent, a conduit or a scaffold; and
applying the layer of tropoelastin biomaterial to the support member to
form the prosthetic device.
According to another aspect of the invention, there is provided a
method for producing a tropoelastin biomaterial, which comprises:
providing a tropoelastin monomer;
polymerizing the tropoelastic monomer to form a tropoelastin polymer;
3o and
forming a biocompatible tropoelastin biomaterial from the tropoelastin
polymer for use in biomedical applications.
According to another aspect of the invention, there is provided a
method for using a tropoelastin polymer, which comprises:
CA 02279902 2000-12-06
15c
providing a tropoelastin monomer;
polymerizing the tropoelastic monomer to form the tropoelastin
polymer;
forming a biocompatible tropoelastin biomaterial from the tropoelastin
polymer; and
using the biocompatible tropoelastin biomaterial in biomedical
applications.
According to yet another aspect of the invention, there is provided a
tropoelastin biomaterial and tissue composite product, which comprises:
a layer of tropoelastin biomaterial having a first and second outer major
surface;
a tissue substrate having a first and second outer major surface; and
an energy absorbing material, which is energy absorptive within a
predetermined range of light wavelengths, disposed on one of the first and
second outer surfaces of the tropoelastin biomaterial in an amount which will
cause fusing together of one of the first and second outer surfaces of the
tropoelastin biomaterial and one of the first and second outer surfaces of the
tissue substrate,
one of the first and second outer surfaces of the tropoelastin
2o biomaterial and the tissue substrate being fused together by the energy
absorbing material which penetrates into the interstices of the tropoelastin
biomaterial.
According to a further aspect of the invention, there is provided a
method for producing a tropoelastin biomaterial capable of being fused onto a
tissue substrate comprising:
providing a layer of tropoelastin biomaterial having a first and second
outer major surface; and
applying an energy absorbing material, which is energy absorptive
within a predetermined range of light wavelengths, to a selected one of the
first and second outer surfaces of the tropoelastin biomaterial in an amount
which will cause fusing together of one of the first and second outer surfaces
of the tropoelastin biomaterial and an outer surface of the tissue substrate,
the
energy absorbing material penetrating into the interstices of the tropoelastin
biomaterial,
CA 02279902 2004-11-25
15d
the selected one of the first and second outer
surfaces of the tropoelastin biomaterial being capable of
fusing together with the outer surface of the tissue
substrate by irradiating the energy absorbing material
with light energy in a predetermined wavelength range
with an intensity sufficient to facilitate the fusing
together.
According to a still further aspect of the
invention, there is provided a tropoelastin biomaterial
capable of being fused onto a tissue substrate
comprising:
a layer of tropoelastin biomaterial having a first
and second outer major surface; and
an energy absorbing material, which is energy
absorptive within a predetermined range of light
wavelengths, applied to a selected one of the first and
second outer surfaces of the tropoelastin biomaterial in
an amount which will cause fusing together of one of the
first and second outer surfaces of the tropoelastin
biomaterial and an outer surface of the tissue substrate,
the energy absorbing material penetrating into the
interstices of the tropoelastin biomaterial,
the selected one of the first and second outer
surfaces of the tropoelastin biomaterial being capable of
fusing together with the outer surface of the tissue
substrate by irradiating the energy absorbing material
with light energy in a predetermined wavelength range
with an intensity sufficient to facilitate the fusing
together.
According to another aspect of the present
invention, there is provided a method for producing a
tropoelastin biomaterial fused onto a tissue substrate
comprising:
CA 02279902 2004-11-25
15e
providing a layer of biomaterial consisting
essentially of tropoelastin having a first and second
outer major surface and a tissue substrate having a first
and second outer major surface; and
applying an energy absorbing material, which is
energy absorptive within a predetermined range of light
wavelengths, to a selected one of the first and second
outer surfaces of the tropoelastin biomaterial in an
amount which will cause fusing together of one of the
first and second outer surfaces of the tropoelastin
biomaterial and one of the first and second outer
surfaces of the tissue substrate, the energy absorbing
material penetrating into the interstices of the
tropoelastin biomaterial;
irradiating the energy absorbing material with light
energy in the predetermined wavelength range with an
intensity sufficient to fuse together one of the first
and second outer surfaces of the tropoelastin biomaterial
and the tissue substrate; and
fusing together the selected one of the first and
second outer surfaces of the tropoelastin biomaterial and
the tissue substrate.
According to a further aspect of the present
invention, there is provided a method for using a
tropoelastin biomaterial as a tissue-fusible layer,
comprising:
providing a layer of biomaterial consisting
essentially of tropoelastin having a first and second
outer major surface which is for use as a tissue-fusible
material;
providing a tissue substrate having a first and
second outer major surface; and
CA 02279902 2004-11-25
15f
using the tropoelastin biomaterial as a heat fusible
material by applying an energy absorbing material, which
is energy absorptive within a predetermined range of
light wavelengths, to one of the first and second outer
surfaces of the tropoelastin biomaterial in an amount
which will make the tropoelastin biomaterial tissue-
fusible, and which will cause fusing together of one of
the first and second outer surfaces of the tropoelastin
biomaterial and one of the first and second outer
surfaces of the tissue substrate, the energy absorbing
material being applied so that it will penetrate into the
interstices of the tropoelastin biomaterial,
irradiating the energy absorbing material with light
energy in the predetermined wavelength range with an
intensity being sufficient to fuse together one of the
first and second outer surfaces of the tropoelastin
biomaterial and the tissue substrate.
According to another aspect of the present
invention, there is provided a method for producing a
tropoelastin biomaterial fused onto a tissue substrate
comprising:
providing a layer of biomaterial consisting
essentially of tropoelastin having a first and second
outer major surface and a tissue substrate having a first
and second outer major surface;
applying an energy absorbing material, which is
energy absorptive within a predetermined range of light
wavelengths, to one of the first and second outer
surfaces of the tropoelastin biomaterial in an amount
which will cause fusing together of one of the first and
second outer surfaces of the tropoelastin biomaterial and
one of the outer surface of the tissue substrate, the
CA 02279902 2004-11-25
15g
energy absorbing material penetrating into the
interstices of the tropoelastin biomaterial;
indirectly irradiating the energy absorbing material
by directing the light energy first through the
tropoelastin biomaterial or tissue substrate and then to
the energy absorbing material, the light energy being in
the predetermined wavelength range with an intensity
sufficient to fuse together one of the first and second
outer surfaces of the tropoelastin biomaterial and the
outer surface of the tissue substrate; and
fusing together one of the first and second outer
surfaces of the tropoelastin biomaterial and the outer
surface of the tissue substrate and substantially
dissipating the energy absorbing material when the
tropoelastin biomaterial and the tissue substrate are
fused together.
According to a further aspect of the present
invention, there is provided a prosthetic device
comprising:
a support member comprising one of a stent, a
conduit and a scaffold; and
a layer of a biomaterial consisting essentially of
tropoelastin located on the support member.
According to another aspect of the present
invention, there is provided a tropoelastin biomaterial
and tissue composite product, which comprises:
a layer of biomaterial consisting essentially of
tropoelastin having a first and second outer major
surface;
a tissue substrate having a first and second outer
major surface; and
an energy absorbing material, which is energy
absorptive within a predetermined range of light
CA 02279902 2004-11-25
15h
wavelengths, disposed on one of the first and second
outer surfaces of the tropoelastin biomaterial in an
amount which will cause fusing together of one of the
first and second outer surfaces of the tropoelastin
biomaterial and one of the first and second outer
surfaces of the tissue substrate,
one of the first and second outer surfaces of the
tropoelastin biomaterial and the tissue substrate being
fused together by the energy absorbing material which
penetrates into the interstices of the tropoelastin
biomaterial.
According to a further aspect of the present
invention, there is provided a method for producing a
biomaterial consisting essentially of tropoelastin for
fusion onto a tissue substrate comprising:
providing a layer of biomaterial consisting
essentially of tropoelastin having a first and second
outer major surface; and
applying an energy absorbing material, which is
energy absorptive within a predetermined range of light
wavelengths, to a selected one of the first and second
outer surfaces of the tropoelastin biomaterial in an
amount which will cause fusing together of one of the
first and second outer surfaces of the tropoelastin
biomaterial and an outer surface of the tissue substrate,
the energy absorbing material penetrating into the
interstices of the tropoelastin biomaterial,
the selected one of the first and second outer
surfaces of the tropoelastin biomaterial being capable of
fusing together with the outer surface of the tissue
substrate by irradiating the energy absorbing material
with light energy in a predetermined wavelength range
CA 02279902 2004-11-25
15i
with an intensity sufficient to facilitate the fusing
together.
According to another aspect of the present
invention, there is provided tropoelastin biomaterial for
fusion onto a tissue substrate comprising:
a layer of biomaterial consisting essentially of
tropoelastin having a first and second outer major
surface; and
an energy absorbing material, which is energy
absorptive within a predetermined range of light
wavelengths, applied to a selected one of the first and
second outer surfaces of the tropoelastin biomaterial in
an amount which will cause fusing together of one of the
first and second outer surfaces of the tropoelastin
biomaterial and an outer surface of the tissue substrate,
the energy absorbing material penetrating into the
interstices of the tropoelastin biomaterial,
the selected one of the first and second outer
surfaces of the tropoelastin biomaterial being capable of
fusing together with the outer surface of the tissue
substrate by irradiating the energy absorbing material
with light energy in a predetermined wavelength range
with an intensity sufficient to facilitate the fusing
together.
Further objects and advantages of the invention will
be clear from the description that follows.
CA 02279902 1999-08-06
WO 98/34563 PCT/US98/02244
16
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a schematic representation of a reaction
of tropoelastin monomers for producing crosslinked
tropoelastin.
FIG. 2 is a schematic representation of a reaction
of tropoelastin monomers in the presence of fibroblasts
for producing crosslinked fibroblast-tropoelastin
matrix.
FIG. 3 is a schematic representation of a reaction
of tropoelastin monomers in the presence of a preformed
collagen lattice for producing tropoelastin fibrils
supported on the collagen latice structure.
DETAILED DESCRIPTION OF THE INVENTION
Monomer Synthesis
Tropoelastin monomer is the soluble biosynthetic
precursor to elastin. It is formed naturally in
vetebrates. Tropoelastin can be isolated from the
aortas of copper deficient swine by known methods such
as described by E.B. Smith, Atherosclerosis 37 1980)
tropoelastin is a 72-kDa polypeptide which is rich in
glycine, proline, and hydrophobic amino acids. The
exact amino acid composition of tropoelastin differs
from species to species. Any polypeptide moiety that
has art-recognized homology to tropoelastin can be
considered a tropoelastin monomer for the invention.
The tropoelastin can be isolated from mammalian
tissue or produced using recombinant expression
CA 02279902 1999-08-06
WO 98/34563 PCT/US98/02244
17
systems. Furthermore, tropoelastin splice variants
from any species can also be used for the invention.
The following are exemplary descriptions of
methods of producing tropoelastin monomers used in the
invention:
1. Tropoelastin can be extracted from
mammals which have been placed on copper deficient or
lathyritic diets. The deficiency of copper in the
mammalian diet inhibits lysyl oxidase resulting in the
accumulation of tropoelastin in elastin rich tissues.
Copper deficient animals are grown rapidly on a diet
composed largely of milk products and must be kept
isolated from contaminating sources of copper. The
protocol for raising copper deficient swine is detailed
by L.B. Sandberg and T. B. Wolt. Production of
Soluble Elastin from Copper Deficient Swine. Methods
in Enzymology 82 (1982) 657-665. 150 mg of
tropoelastin can be extracted from a 15-kg
copper-deficient swine.
2. In a method similar to copper deficiency
method in No. 1 above, feeding animals chemicals that
effectively inhibit the action of lysyl oxidase
(lathyrogens) also restricts the conversion of
tropoelastin to amorphous elastin. This method
produces similar yields of tropoelastin to
copper-deficient swine. However, the special cages,
water and diet required to raise copper-deficient
animals are not required herein. To induce lathyrisim,
animal diets are supplemented with 0.1% by weight
a-aminoacetonitrile-HC1 and 0.05% a-aminocaproic acid
CA 02279902 1999-08-06
WO 98/34563 PCT/US98/02244
18
as described by Celeste B. Rich and Judith Ann Foster,
Isolation of Soluble Elastin- Lathyrism. Methods in
Enzymology 82 (1982) 665-673.
3. Tropoelastin can also be produced by
mammalian cell culture systems. Short term cultivation
of bovine vascular endothelial cells, nuchal ligament
fibroblasts from cows and sheep, human skin fibro-
blasts, and vascular smooth muscle cells from pigs and
rabbits results in the accumulation of tropoelastin in
the culture medium.
4. Recombinant tropoelastin produced by a
protein expression system is the preferred monomer for
the invention. Recombinant protein technology is the
transfer of recombinant genes into host organisms that
grow and convert nutrients and metabolites into
recombinant protein products. Using this technology,
cDNA encoding tropoelastin can be cloned and expressed
in protein expression systems to produce biologically
active recombinant tropoelastin. Functionally
distinct hydrophobic domains and lysine rich
crosslinking domains are encoded in-separate exons.
This existence of multiple splice variants of
tropoelastin in several species can be attributed to
Cassette-like alternative splicing of elastin pre-mRNA.
Expression of different recombinant splice variants of
tropoelastin can produce proteins with distinct
qualities. In addition, site directed in vitro
mutagenesis can be used to alter the polypeptide
sequence of the naturally occurring gene, thus creating
alternate polypeptides with improved biological
CA 02279902 1999-08-06
WO 98134563 PCT/US98/02244
19
activity and physical properties. Expression of the
full length elastin cDNA clone, cHEL2 and subsequent
purification of recombinant human tropoelastin (rTE)
has been achieved by Joel Rosenbloom, William R.
Abrams, and Robert Mecham. Extracellular Matrix 4: The
Elastic Fiber. The Faseb Journal 7 (1993) 1208-1218.
rTE produced by the methods of Rosenbloom et. al. can
be used for the invention, however, the methods are not
considered to be part of the present invention. In
addition, the invention is not limited to rTE produced
from.the expression of cHEL2. rTE produced from the
expression of any tropoelastin genomic or cDNA can be
used for the invention.
To help overcome the moderate yields of rTE
recovered by Rosenbloom and colleagues, Martin,
Vrhovski and Weiss successfully synthesized and
expressed a gene encoding human tropoelastin in E.
Coli. In constructing the gene they tailored the rare
codon bias of the synthetic sequence to match the known
preferences of E. Coli. rTEtropoelastin produced by
expression of synthetic genes can be used for the
invention.
rTE is used in the invention can be produced in
non-bacterial expression vector systems. Yeast
expression vector systems are well suited for
expressing eukaryotic proteins and tropoelastin is a
potentially excellent candidate for expression in
yeast.
For large scale heterologous gene expression, the
baculovirus expression vector system (BEVS) is
CA 02279902 1999-08-06
WO 98/34563 PCT/US98/02244
particularly advantageous. BEVS has several advantages
over other expression systems for mammalian gene
expression. It is safer, easier to scale up, more
accurate, produces higher expression levels, and is
5 ideal for suspension cultures permitting the use of
large-scale bioreactors. Generation of a recombinant
baculovirus particle carrying a clone of elastin cDNA
coding for an isoform of tropoelastin is achieved
through homologous recombination or site specific
10 transposition and is followed by recombinant
baculovirus infection of insect cells (Sf9 or High
Five) and subsequent recombinant gene expression as
follows:
Elastin cDNA encoding tropoelastin is identified
15 and isolated from a cDNA library. The gene is cloned
into a pFastBac or pFastBac HT donor plasmid using
standard restriction endonucleases and DNA ligase.
Correct insertion of gene is verified by restriction
endonuclease digestion and PCR analysis. The DNA is
20 then transformed into DH10Bac cells which harbor a
bacmid a mini-attTn7 target site and a helper plasmid.
Once cloned into the DH10Bac cells, the elastin gene
undergoes site-specific transposition into the Bacmid.
Transposition results in the disruption of a LacZalpha
gene and colonies containing recombinant bacmids are
white. High molecular weight mini-prep DNA is prepared
from selected E. Coli clones containing the recombinant
bacmid and is used to transfect SF9 or High Five
insect cells using Cel1FECTIN reagent. The insect
cells produce actual baculovirus particles harboring
CA 02279902 1999-08-06
WO 98/34563 PGT/US98/02244
21
the tropoelastin encoding gene. The virus particles
are harvested and are subsequently used to infect
insect cells which produce high yields of the
recombinant protein product, tropoelastin.
Tropoelastin accumulated in elastin rich tissues
by the inhibition of lysyl oxidase through copper
deficiency or lathyrism can be isolated by exploiting
tropoelastin's high solubility in short-chain alcohols.
Modified methods of this alcohol extraction procedure
can be used to purify rTE from expression hosts such as
bacteria, yeast, insect, and mammalian cells in
culture. Methods have been described in detail which
involve precipitation of tropoelastin with n-propanol
and n-butanol. Tropoelastin expressed in insect cells
using the pFastBac HT baculovirus expression system
(Life Technologies, Gaithersburg, MD) can be purified
in a single affinity chromatography step with Ni-NTA
resin. The invention is not limited to any particular
method of tropoelastin isolation or purification.
Polymer Synthesis
In tissue, tropoelastin is naturally crosslinked
by several tetra and bifunctional cross-links to form
elastin. These crosslinks arise through the oxidative
deamination and condensation of lysyl side chains.
Both bifunctional lysinonorleucine and allysine aldol
and tetrafunctional desmosine crosslinks are formed.
Tetrafunctional desmosine crosslinks are a
distinguishing feature of elastin. Tropoelastin can be
converted to a tropoelastin biomaterial by oxidative
deamination of lysyl residues and the subsequent
CA 02279902 1999-08-06
WO 98/34563 PCTIUS98/02244
22
crosslinking of the monomeric moiety catalyzed by the
copper dependent enzyme lysyl oxidase (protein-lysine
6-oxidase).
A primary purpose of the invention is to produce
cross-linked elastic matrices that are identical to or
closely mimic those found naturally in elastic tissue.
It is, therefore, advantageous to crosslink tropo-
elastin monomers with the same bifunctional and
tetrafunctional cross-links found in elastin. However,
the invention is not limited to these naturally
occurring cross-links and any type of cross-link formed
between tropoelastin monomers, whether produced
chemically, enzymatically or radiatively, can be used
for the invention.
Crosslinking tropoelastin with lysyl oxidase will
produce matrices that closely resemble or imitate
naturally occurring ones. Lysyl oxidase
(protein-lysine 6-oxidase) catalyzes the oxidation of
lysine residues to a peptidyl a-aminoadipic
-a-semialdehyde. This aldehyde residue spontaneously
condenses with neighboring aldehydes or a-amino groups
forming interchain or intrachain crosslinkages (Kagan,
1991). Lysyl oxidase from any source can be used so
long as the tropoelastin it is intended to oxidize is a
suitable ligand. Lysyl oxidase is typically extracted
from bovine aorta and lung, human placentas, and rat
lung with 4 to 6 M urea extraction buffers.
Recombinantly produced lysyl oxidase can also be used
to cross-link tropoelastin. Recombinant tropoelastin
(rTE26A) has been cross-linked with lysyl oxidase in
CA 02279902 1999-08-06
WO 98/34563 PCT/US98/02244
23
0.1 M sodium borate, 0.15 M NaCl, pH 8.0 when incubated
for 24 hr at 37 C (Bedell-Hogan, 1993). Another
preferred method of crosslinking tropoelastin is with
y-irradiation. y-irradiation causes formation of free
radicals which can result in crosslink formation. 20
mrad of y-irradiation has been shown to crosslink an
elastin like polypeptide, poly(GLy-Val-Gly-Val-Pro),
into an elastomeric matrix and has increased the
elasticity and strength of a elastin-fibrin
biomaterial. The addition of chemical agents that form
crosslinks when activated with irradiation can also be
used. Sulfur derivatives combined with y-irradiation
been shown to further increase the strength of an
elastin-fibrin biomaterial. Chemical crosslinking
reagents such as glutaraldelhyde may also be used to
cross-link tropoelastin matrices.
A preferred method of organizing tropoelastin
monomers into fibrous structures prior to cross-linking
is by taking advantage of the property of coacervation
exhibited by tropoelastin. Tropoelastin is soluble in
water at temperatures below 37oC, however, upon raising
the temperature to 37oC tropoelastin aggregates into a
filamentous structure called a coacervate. Formation
of tropoelastin coacervates may be a natural step prior
to cross-link formation during elastogenesis in tissue.
Coacervated tropelastin can be crosslinked by lysyl
oxidase under the appropriate conditions to produce
filamentous elastin fibrils. Alignment may be
facilitated by exposure of the tropoelastin coacervates
to a magnetic field prior to crosslinking.
CA 02279902 1999-08-06
WO 98/34563 PCT/US98/02244
24
Collagen is the major structural polymer of
connective tissues. Artificial collagen fibers have
been produced from soluble collagen I extracts. Fibers
such as these can be formed into scaffoldings onto
which tropoelastin can be cross-linked into amorphous
insoluble elastin producing a elastin/collagen
composite (see Fig. 3). The collagen fibers lend form
and tensile strength to the tropoelastin material and
the crosslinked tropoelastin fibrils lend elasticity
thus creating a composite material that very nearly
approximates naturally occurring connective tissue.
Proteoglycans are major constituents of the
extracellular matrix. The addition of Hyaluronic acid,
dermatan sulfate, keratane sulfates, or Chondroitin
sulfates as co-materials may further the strength and
cohesion of the material. In addition, cell function
is in part controlled by the extracellular matrix.
Fibronectin, vitronectin, laminin nad collagen, as well
as various glycosaminoglycans all mediate cell
adhesion. Fibronectin has several roles in the
connective tissue matrix. It has an_organizing role in
developing tissues and it plays a major role in cell
adhesion to the extracellular matrix. Incorporation of
fibronectin as a co-material may improve the cell
adhesion properties of the tropoelastin based
biomaterial. Microfibrils are distributed throughout
the body, and are prevalent in elastic tissues and
fibers. The presence of microfibrils during
polymerization of tropoelastin monomers may help to
organize monomers yielding a material with improved
CA 02279902 1999-08-06
WO 98/34563 PGT1US98/02244
structural organization. Also, microfibrils are known
to sequester calcium ions and are thought to play a
role in protecting tropoelastin from chronic
calcification.
5 Product Synthesis
The utility of tropoelastin based biomaterials may
be further improved by combining them with synthetic or
natural polymer co-materials, forming composites, and
by adding bioactive impregnates.
10 Antibiotics and/or anticoagulants or other agents
can be added to the tropoelastin matrix providing
localized drug therapy and preventing infection. In
surgical repair of abdominal traumatic injuries,
infection represents a major problem particularly when
15 vascular prosthetic implants are used. An tropoelastin
graft with antibiotic incorporation may be ideal
because it avoids sacrifice of an autologous artery or
vein which decreases surgical time and precludes the
necessity to use synthetic prosthetic materials which
20 may be more prone to infection than tropoelastin
grafts. Bioactive impregnates may also include
anti-coagulants (Hirudin), coagulants, anti-
proliferative drugs (Methatrexate), growth factors,
anti-virals, and anti-neoplastics.
25 Small diameter vascular grafts fail at an
unacceptable rate due to their inherent throm-
bogenicity. This problem may be decreased by the
deposition of a living autologous endothelial cell
lining. Autologous endothelial cell transplantation
can accelerate the formation of an immunologically
CA 02279902 1999-08-06
WO 98/34563 PCT/US98/02244
26
compatible, complete endothelial lining using
microvascular endothelial cells derived from the
adipose tissue of a recipient animal (Jarrell, et al.)
in the porcine model the peritoneal fat had been
determined to be optimal for this purpose due to the
predominance of microvascular and endothelial cells.
Following extraction of peritoneal fat, homogenization,
collagenase digestion,'and centrifugal separation,
cells are expeditiously transplanted onto the luminal
surface of crosslinked tropoelastin vascular grafts
using an intra-operative isolation technique combined
with the rapid pressure sodding techniques described by
Jarrell and Williams.
The present invention constitutes a three
dimensional matrix made of elastin or tropoelastin for
use as a framework for a three-dimensional, multi-layer
cell culture system. Populating endogenous biologic
materials such as a tropoelastin matrix with stromal
cells is preferable to populating matrices made of
synthetic biocompatible, non-living materials.
Synthetic biodegradable biomaterials must undergo
enzyme catalyzed degradation or*spontaneous hydrolysis
in order to avoid permanent chronic foreign body
reactions. On the contrary, elastin is a naturally
occurring protein in the extracellular matrix of many
tissues and, therefore, does not illicit a foreign body
reaction. Unlike collagen, elastin undergoes very
little post-developmental remodelling or breakdown and
is a relatively permanent connective tissue structure
during the life of an organism. Tropoelastin
CA 02279902 1999-08-06
WO 98/34563 PCT/US98/02244
27
biomaterials can provide a relatively permanent,
natural support matrix for three dimensional cell
cultures that when implanted acts as a template for
reconstruction of the organs and tissues. In addition
the longevity and integrity of implanted tropoelastin
is regulated in response to the biological needs of the
tissue rather than environmentally induced hydrolysis
or enzymatic degradation of a foreign substance.
Elastin structures constituting a framework for a
three-dimensional, multi-layer cell culture system will
provide intact elastic structures not constructed by
stromal cells populating synthetic matrices. In vivo
elastin production is thought to only occur during
development and ceases during childhood (the only
exceptions being hypertension and restenosis).
Elastogenesis is a complex method and formation of
mature elastic structures not likely to be achieved in
relatively simple in vitro cell culture systems.
However, it has not been reported that such three
dimensional cell culture systems can organize elastin
into coherent fibrous matrices analogous to those found
in elastic tissues. A method by which to produce a
living tissue graft with elastic structure and function
most similar to tissue which is high in elastin content
is by culturing cells in three dimensional frameworks
made of elastin or elastin based biomaterials. This
insures the presence of biologically important elastic
structures in the living tissue grafts.
A method for both organizing tropoelastin fibrils
and providing a support for fibroblast growth is by
CA 02279902 1999-08-06
WO 98/34563 PCT/US98/02244
28
coacervating tropoelastin monomers in solution with
fibroblasts. Tropoelastin monomers mixed with stromal
cells (fibroblasts) in a physiologic buffer aggregate
into fibers (coacervation) upon raising the temperature
of the solution to 37 C. In doing so the fibroblasts
become.trapped in a loose matrix of elastic fibers.
The tropoelastin fibers can be crosslinked either by
including lysyl oxidase in the buffer or a temperature
sensitive recombinant form of lysyl oxidase that, for
example, is inactive at 20 C and active at 37 C or by
culturing the tropoelastin- fibroblast matrix in such a
manner that the fibroblasts secrete natural lysyl
oxidase into the coacervate matrix. The contraction of
the fibroblasts bound to the coacervated tropoelastin
monomers could preferentially align the tropoelastin
fibrils prior to crosslinking.
Sterilization
The tropoelastin biomaterial of the invention is
normally secured to existing tissue. Various
20. techniques for effecting that attachment can be used,
including art-recognized techniques,_ including
suturing, staples and gluing. However, in some cases
it is preferred that the biomaterial be secured using a
tissue welding energy source and an agent that absorbs
energy emitted by that source. Advantageously, the
energy source is an electromagnetic energy source,
such as a laser, and the absorbing agent is a dye
having an absorption peak at a wavelength corresponding
to that of the laser. The tropoelastin biomaterial
and the tissue to be welded have much less absorption
CA 02279902 1999-08-06
WO 98/34563 PCT/US98/02244
29
of light at this wavelength and the effect therefore is
confined to a zone around the dye layer. A preferred
energy source is a laser diode having a dominant
wavelength at about 808 nm and a preferred dye is
indocyanine green (ICG), maximum absorbance 795-805 nm
(see WO 91/04073). Other laser/dye combinations can
also be used. It is preferred that the dye be applied
to that portion of the biomaterial that is to be
contacted with and secured to the existing tissue. The
dye can also be applied to the surface of the structure
to which the tropoelastin biomaterial is to be welded
or secured. The dye can be applied directly to the
biomaterial or the surface of the biomaterial can first
be treated or coated (eg primed) with a composition
that controls absorption of the dye into the
biomaterial so that the dye is kept as a discrete layer
or coating. Alternatively, the dye can be bound to
the troptropoelastin biomaterial so that it is secured
to the surface and prevented from leeching into the
material. The dye can be applied in the form of a
solution or the dye can be dissolved in or suspended in
a medium which then can be applied as a thin sheet or
film, preferably, of uniform thickness and dye
concentration.
Tissue welding techniques employing a soldering
agent can be used. Such techniques are known (WO
91/04073). Any proteinaceous material that thermally
denatures upon heating can be used as the soldering
agent (for example, any serum protein such as albumin,
fibronectin, Von Willebrand factor, vitronectin, or any
CA 02279902 1999-08-06
WO 98/34563 PCT1US98/02244
mixture of proteins or peptides). Solders comprising
thrombin polymerized fibrinogen are preferred, except
where such materials would cause undesirable thrombosis
or coagulation such as within vascular lumens. Solders
5 are selected for their ability to impart greater
adhesive strength between the biomaterial and the
tissue. The solder should be non-toxic and generally
biocompatible.
In accordance with the present invention, the
10 laser energy can be directed to the target site (eg,
the dye) directly from the laser by exposure of the
tissue (eg, during a surgical procedures). In some
cases, i.e. endovascular catheter-based treatments
where open surgical exposure does not occur, the laser
15 energy is directed to the bonding site via optical
fibers. When ICG is used as the dye, targeting media
wavelengths of around 800nm can be used. Such
wavelengths are not well absorbed by many tissues,
particularly blood and vascular tissues, therefore,
20 there will be a negligible effect on these tissues and
thermal effects will be confined to the dye layer. The
biomaterial of the invention similarly has little
optical absorbance in this waveband, as compared to the
energy absorbing dye. Thus, the laser energy can pass
25 through either the biomaterial or the native tissue and
be absorbed by the dye layer as shown in Figure 1. Once
the surgeon has exposed the surface or vessel where the
biomaterial reinforcement or replacement is to be
effected, the dye-containing surface of the biomaterial
30 is placed in contact with the native tissue at the site
CA 02279902 1999-08-06
WO 98/34563 PCTIUS98/02244
31
and laser energy delivered by directing the laser beam
to the desired location. The absorbance of the dye (eg
ICG) layer is ideally previously or concurrently
determined so that the optimal amount of light for
optimal bonding can be delivered. Pressure can be used
to ensure adequate approximation of the tissue and
biomaterial. With a diode laser source, the diode
laser itself, or a condenser or optical fiber based
optical delivery system, can be placed against the
material to ensure uniform light delivery.
In cases where a new elastin lining or
new-internal elastic lamina is required, for example,
following an open surgical endarterectomy, once the
artery has been surgically cleared of the atheroma or
other lesion, the biomaterial is then put in place, dye
side down. The biomaterial can be deployed as a flat
patch or as a tubular segment. A tubular segment can
be hollow or filled with a material that supports the
lumen during placement and that is melted with low
grade heat or dissolved or removed with a variety of
means. When necessary, a small number of surgical
sutures (eg stay sutures) can be used to appose the
edges of the vessel together or to sew the vessel.
Once the biomaterial is in place, the laser energy is
directed through the vessel wall or through the
biomaterial to the absorbing dye, the appropriate laser
energy having been previously determined based upon the
measured absorbance in the biomaterial. Alternatively,
the dye can be applied at the time of the surgery to
the biomaterial or the vessel wall or both and then
CA 02279902 1999-08-06
WO 98134563 PCTIUS98/02244
32
laser energy delivered. In this embodiment, absorbance
can be determined at the time of the surgery to the
biomaterial or the vessel wall or both and then laser
energy delivered or with a feedback device that
assesses the adequacy of the bonding or thermal effect.
In addition to.the above, the biomaterial of the
invention can be used as a patch material for use in
intestinal or colon repairs which frequently do not
heal well with current techniques, particularly when
the patient has nutritional or other problems or when
the patient is in shock, such as in the case of
multiple gunshot wounds or other abdominal injuries
(see Figure 3). The use of such a patch can, for
example, seal off intestinal contents and thereby
reduce the likelihood of peritonitis. In addition, a
patch can be used on a solid organ, such as the liver
or lung, when lacerations have occurred. Similarly,
the biomaterial of the invention can be used to repair
or replace portions of the urinary system i.e., from
the calyces of the kidney on down to the urethra. The
patch can also be used to seal a defect in a cardiac
chamber, such as an atrial septal defect, as well as
bronchial or rectal fistulas. The biomaterial can
also be used as a cerebrovascular patch for an
aneurysm. The biomaterial can be sealed in place with
targeted laser fusion. For applications where direct
exposure is not possible or not desirable, a variety of
catheter or endoscopic systems can be employed to
direct the laser energy to the target site bio-
materials to which the invention relates can be used in
CA 02279902 2004-11-25
33
a variety of other clinical and surgical settings to
effect tissue repair graft. For delivery of biomaterial
in the form of an intravascular stent, the biomaterial
can be pre-mounted upon a deflated balloon catheter. The
balloon catheter can be maneuvered into the desired
arterial or venous location using standard techniques.
The balloon can then be inflated, compressing the stent
(tropoelastin biomaterial) against the vessel wall and
then laser light delivered through the balloon to seal
the stent in place (the dye can be present on the outside
of the biomaterial). The balloon can then be deflated
and removed leaving the stent in place. A protective
sleeve (of plastic or the like) can be used to protect
the stent during its passage to the vessel and then
withdrawn once the stent is in the desired location.
The biomaterial of the invention can also be used as
a biocompatible covering for a metal or synthetic
scaffold or stent. In such cases, simple mechanical
deployment can be used without the necessity for laser
bonding. Laser bonding can be employed, however,
depending upon specific demands, eg, where inadequate
mechanical bonding occurs, such as in stent deployment
for abdominal aortic aneurysms. An alternative catheter-
based vascular stent deployment strategy employs a
temporary mechanical stent with or without a balloon
delivery device.
A further catheter-based vascular stent deployment
strategy employs a heat deformable metal (such as
nitinolTM or other similar type metal) scaffold or stent
CA 02279902 1999-08-06
WO 98/34563 PCT1US98/02244
34
or coating that is incorporated into the catheter
tubing beneath the stent biomaterial. The stent is
maneuvered into the desired location whereupon the
deformable metal of the stent is activated such that it
apposes the stent against the vessel wall. Laser light
is then delivered via an optical fiber based system,
also incorporated into the catheter assembly.
The tropoelastin-based biomaterial can also be
used to replace portions of diseased or damaged
vascular or nonvascular tissue such as esophagus,
pericardium, lung plura, etc. The biomaterial can also
be used as a skin layer replacement, for example, in
burn or wound treatments. As such, the biomaterial
serves as a permanent dressing that acts as a
scaffolding for epithelial cell regrowth. The
biomaterial can include antibiotics, coagulants or
other (drugs desirable for various treatments that
provide high local concentrations with minimal systemic
drug levels. The tropoelastin biomaterial can be
deployed with a dye on the tissue side and then fused
with the appropriate wavelength and laser energy.
In addition to repair of tubular body structures,
the biomaterial of the present invention can also be
used in organ reconstruction. For example, the
biomaterial can be molded or otherwise shaped as a
pouch suitable for use in bladder reconstruction. The
biomaterial of the invention can also be molded or
otherwise shaped so as to be suitable for esophageal
replacement. Again, metal or synthetic mesh could also
be associated with the implant if extra wall support is
CA 02279902 2004-11-25
needed so as to control passage of food from the pharynx
to the stomach. This could be used for stenosis of the
esophagus, repair from acid reflux for erosive
esophagitis or, more preferably, for refurbishing damaged
5 esophageal segments during or following surgery or
chemotherapy for esophageal carcinoma.
For certain applications, it may be desirable to use
the biomaterial of the invention in combination with a
supporting material having strong mechanical properties.
10 For those applications, the biomaterial can be coated on
the supporting material (see foregoing stent
description), for example, using the molding techniques
described herein. Suitable supporting materials include
polymers, such as woven polyethylene terepthalate
15 (DacronTM), teflonTM, polyolefin copolymer, polyurethane
polyvinyl alcohol or other polymer. In addition, a
polymer that is a hybrid between a natural polymer, such
as fibrin and elastin, and a non-natural polymer such as
a polyurethane, polyacrylic acid or polyvinyl alcohol can
20 be used (see Giusti et al, Trends in Polymer Science
1:261 (1993). Such a hybrid material has the advantageous
mechanical properties of the polymer and the desired
biocompatibility of the tropoelastin material. Examples
of other prostheses that can be made from synthetics (or
25 metals coated with the tropoelastin based biomaterial or
from the biomaterial/synthetic hybrids include cardiac
valve rings and esophageal stents.
CA 02279902 1999-08-06
WO 98/34563 PCT/US98/02244
36
The tropoelastin-based prostheses of the invention
can be prepared so as to include drug; that can be
delivered, via the prostheses, to particular body
sites. For example, vascular stents can be produced so
as to include drugs that prevent coagulation, such as
heparin, or antiplatelet drugs such as hirudin, drugs
to prevent smooth muscle ingrowth or drugs to stimulate
endothelial damaged esophageal segments during or
following surgery or chemotherapy for esophageal
carcinoma or endothelial regrowth. Vasodilators can
also be included.
Prostheses formed from the tropoelastin bio-
material can also be coated with viable cells, cells
from the recipient of the prosthetic device.
Endothelial cells, preferably autologous (eg harvested
during liposuction), can be seeded onto the elastin
bioprosthesis prior to implantation (eg for vascular
stent indications). Alternatively, the tropoelastin
biomaterial can be used as a skin replacement or repair
media where cultured skin cells can be placed on the
biomaterial prior to implantation. Skin cells can thus
be used to-coat elastin biomaterial.
All documents cited above are hereby incorporated
in their entirety by reference. One skilled in the art
will appreciate from a reading of this disclosure that
various changes in form and detail can be made without
departing from the true scope of the invention.