Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 1 -
Production Of Butanol From Carbon Monoxide By A Recombinant Microorganism
FIELD
The present invention relates to methods for the production of biofuels by
microbial
fermentation and genetically modified micro-organisms suitable for use in such
methods.
BACKGROUND
Butanol is an important bulk chemical with a wide range of industrial uses
that has worldwide
production of 4.5-5.5 million tonnes per annum. It is used as a precursor for
the production of
acrylate and methacrylate esters (used in coatings, plastics, textiles,
adhesives, etc), glycol ethers
(coatings, electronics) and butyl acetate (paints, ink, coatings, synthetic
fruit flavoring) as well as
butylamines (production of pesticides and pharmaceuticals) and amine resins.
It also has direct
use as a solvent (in ink, dyes, etc), an extractant (for the production of
drugs and natural
substances such as alkaloids, antibiotics, hormones, and vitamins), and in
deicing fluids, cosmetics
and chromatography.
Butanol also has potential as a second generation biofuel, and in this context
is referred to as
Biobutanol (Kopke & Durre, 2010). It has similar properties to gasoline and
superior properties to
ethanol. Specifically, it tias increased mileage due to higher energy density,
it can be mixed with
gasoline in any concentration (while ethanol can only be blended up to 85%)
and is not
hygroscopic or corrosive.
Biofuels for transportation are attractive replacements for gasoline and are
rapidly penetrating
fuel markets as low concentration blends. Biofuels, derived from natural plant
sources, are more
environmentally sustainable than those derived from fossil resources (such as
gasoline), their use
allowing a reduction in the levels of so-called fossil carbon dioxide (CO2)
gas that is released into
the atmosphere as a result of fuel combustion. In addition, biofuels can be
produced locally in
many geographies, and can act to reduce dependence on imported fossil energy
resources.
The vast majority of biofuels are produced via traditional yeast-based
fermentation processes
that use crop derived carbohydrates as the main carbon source and are known as
first generation
biofuels. However, these crops are required for food and many crops also
require high
agricultural inputs in the form of fertilizers. These limitations mean that
first generation biofuels
are considered unsustainable and the greenhouse gas reductions that can be
achieved are
limited. The aim of second generation biofuels is the sustainable use of non-
food parts of current
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 2 -
crops or other industrial waste to reduce greenhouse gas emissions and reduce
dependency on
fossil fuels.
Recent 1-butanol production has been mainly by oxo synthesis (WeiRermel &
Arpe, 2003).
Petrochemicals including crude oil are cracked to form propylene which is used
during oxo
synthesis. However the synthesis process requires use of non-renewable
resources as well as
suffering from being expensive and non-specific in the products formed.
Butanol can also be produced through biological production methods, the most
common being
the Acetone-Butanol-Ethanol (ABE) fermentation which has been used
industrially since 1913
(Kopke & Nirre, 2010). This method has the unwanted by-product of acetone
which is usually
produced at about half the volume of butanol which therefore substantially
reduces the yield.
Additionally, this method of fermentation is limited by the toxicity of
butanol to the micro-
organism which results in growth being almost completely inhibited at such low
butanol
concentrations as 1.5% (Kopke and Diirre 2010). Furthermore ABE fermentation
uses sugar from
corn, starch, cassava and sugar cane as a feedstock. This results in the
undesirable use of arable
land to produce fuel rather than food. It can also exacerbate problems related
to deforestation
and desertification.
Only a few organisms are known to naturally produce butanol and none of these
produce butanol
at a high yield from abundant sources (such as carbon monoxide - CO). Two
organisms known to
naturally produce butanol from CO are Butyribacterium methylotrophicum (which
synthesises
only traces of butanol (Heiskanen et al, 2007)), and Clostridium
carboxidivorans (which produces
low yields of 1-butanol as a by-product to the main fermentation products
ethanol and acetate
(Liou et at, 2005)).
A number of organisms have been genetically modified to produce 1-butanol
including E. coli,
Bacillus subtilis, Saccharomyces cerevisiae, Pseudomonas putida, or
Lactobacillus brevis. However
all of these organisms still rely on sugar as feedstock (Kopke & Diirre,
2010). Despite over 250
Clostridium species being known, only a few are genetically accessible. There
is no natural
competence (uptake of extracellular DNA from the cell's environment) known in
Clostridia and
electrotransformation or conjugation are the only methods available for
transformation. These
issues present significant difficulties in effectively transforming Clostridia
species. Most Clostridia
have one or more restriction/methylation systems to protect against foreign
and phage DNA
which means that transformation is particularly difficult and unpredictable.
CA 02813431 2013-04-18
WO 2012/053905
PCT/NZ2011/000203
301946506_1.DOCX
- 3 -
Bibliographic details of the publications referred to herein are collected at
the end of the
description.
It is an object of the invention to overcome one or more disadvantages of the
prior art, or to at
least provide the public with a useful alternative to known technologies.
SUMMARY OF INVENTION
In accordance with the invention, it has been discovered that a genetically
modified
microorganism is capable of using CO to produce 1-butanol or a precursor
thereof as the main
fermentation product.
In a first aspect, the invention provides a carboxydotrophic acetogenic
recombinant
microorganism which produces 1-butanol and/or a precursor thereof as the main
fermentation
product.
In a related aspect, the invention provides an acetogenic recombinant
microorganism which is
capable of producing 1-butanol and/or a precursor thereof by fermentation from
a substrate
comprising CO at a concentration of greater than approximately 1mM or 0.075g/I
per litre of
fermentation broth.
Preferably, the microorganism comprises exogenous nucleic acids adapted to
express one or
more enzymes in the butanol biosynthesis pathway.
In one embodiment, the one or more enzymes are chosen from the group
consisting:
Thiolase
3-hydroxybutyryl-CoA dehydrogenase
Crotonase/crotonyl-CoA hydratase
Butyryl-CoA dehydrogenase
Electron Transfer Flavoprotein A
Electron Transfer Flavoprotein B
Preferably, the microorganism comprises one or more exogenous nucleic acids
encoding one or
more of the enzymes.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 4 -
Preferably, the one or more nucleic acids encoding the one or more enzymes is
chosen from the
nucleic acids SEQ ID NO. 1 to SEQ ID NO. 6 or functionally equivalent variants
thereof.
Preferably, the microorganism comprises one or more exogenous nucleic acids
encoding each of
Thiolase, 3-hydroxybutyryl-00A dehydrogenase, Crotonase, Butyryl-CoA
dehydrogenase, Electron
Transfer Flavoprotein A and Electron Transfer Flavoprotein B.
Preferably, the microorganism comprises a plasmid encoding one or more of, or
preferably each
of, Thiolase, 3-hydroxybutyryl-CoA dehydrogenase, Crotonase, Butyryl-CoA
dehydrogenase,
Electron Transfer Flavoprotein A and Electron Transfer Flavoprotein B.
In one embodiment, the microorganism comprises one or more exogenous nucleic
acids encoding
each of the enzymes thiolase 3-hydroxybutyryl-CoA dehydrogenase, crotonase /
crotonyl-CoA
hydratase and butyryl-CoA dehydrogenase.
Preferably, the microorganism further comprises an exogenous
phosphotransacetylase/acetate
kinase promoter. Preferably, the promoter corresponds to SEQ_ID No. 7 or a
functionally
equivalent variant thereof.
Preferably, the promoter is contained on a construct encoding one or more of
the enzymes
referred to herein before.
In one embodiment, the microorganism comprises exogenous nucleic acids adapted
to express
one or more of the enzymes chosen from the group consisting of:
Phosphotransbutyrylase;
butyrate kinase;
ferredoxin dependent aldehyde oxidoreductase;
butyraldehyde dehydrogenase;
butanol dehydrogenase;
a bifunctional butyraldehyde dehydrogenase and butanol dehydrogenase.
In one embodiment, the microorganism comprises exogenous nucleic acids adapted
to express
one or more of butyraldehyde dehydrogenase, butanol dehydrogenase and a
bifunctional
butyraldehyde dehydrogenase/butanol dehydrogenase. Preferably, the
microorganism comprises
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 5 -
one or more exogenous nucleic acids encoding one or more of butyraldehyde
dehydrogenase,
butanol dehydrogenase and a bifunctional butyraldehyde dehydrogenase/butanol
dehydrogenase.
In one embodiment, the microorganism comprises exogenous nucleic acids adapted
to express
one or more of Phosphotransbutyrylase, butyrate kinase, ferredoxin dependent
aldehyde
oxidoreductase, and butanol dehydrogenase. Preferably, the microorganism
comprises one or
more exogenous nucleic acids encoding one or more of Phosphotransbutyrylase,
butyrate kinase,
ferredoxin dependent aldehyde oxidoreductase, and butanol dehydrogenase. In
particular
embodiments, the microorganism comprises exogenous nucleic acids adapted to
express each of
Phosphotransbutyrylase, butyrate kinase, ferredoxin dependent aldehyde
oxidoreductase, and
butanol dehydrogenase.
In one embodiment, the one or more nucleic acids encoding the one or more
enzymes is chosen
from the nucleic acids outlined in tables 7 to 10 herein after and
functionally equivalent variants
thereof.
In one embodiment, the microorganism comprises one or more nucleic acid
adapted to express at
least two of the enzymes in the butanol biosynthesis pathway, at least 3, at
least 4, at least 5, at
least 6, at least 7, at least 8, at least 9, at least 10, at least 11, or at
least 12 of the enzymes.
In one embodiment, the microorganism comprises one or more nucleic acid
adapted to express
Thiolase, 3-hydroxybutyryl-00A dehydrogenase, Crotonase/crotonyl-CoA
hydratase, Butyryl-CoA
dehydrogenase, Electron Transfer Flavoprotein A, Electron Transfer
Flavoprotein B, and one or
both of butyraldehyde dehydrogenase and butanol dehydrogenase (or a
bifunctional enzyme).
In one embodiment, the microorganism comprises one or more nucleic acid
adapted to express
Thiolase, 3-hydroxybutyryl-00A dehydrogenase, Crotonase/crotonyl-CoA
hydratase, Butyryl-CoA
dehydrogenase, Electron Transfer Flavoprotein A, Electron Transfer
Flavoprotein B, and at least
one of phosphotransbutyrylase and butyrate kinase and ferredoxin dependent
aldehyde
oxidoreductase and butanol dehydrogenase.
CA 02813431 2013-04-18
WO 2012/053905
PCT/NZ2011/000203
301946506_1.DOCX
- 6 -
Preferably, the microorganism is selected from the group of carboxydotrophic
acetogenic
bacteria. In certain embodiments the microorganism is selected from the group
comprising
Clostridium autoethanogenum, Clostridium ljungdahlii, Clostridium ragsdalei,
Clostridium
carboxidivorans, Clostridium drake!, Clostridium scatolo genes,
Butyribacterium limosum,
Butyribacterium methylotrophicum, Acetobacterium woodii, Alkalibaculum
bacchii, Blautia
producta, Eubacterium limosum, Moore/la the rmoacetica, Moore/la
thermautotrophica,
Oxobacter pfennigii, and Thermoanaerobacter
Preferably, the microorganism is Clostridium autoethanogenum DSM23693.
In one embodiment, the recombinant microorganism of the invention has the
defining
characteristics of the microorganism deposited at the DSMZ (Deutsche Sammlung
fiir
Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany) under the
accession number
DSM24138.
In a second aspect, the invention provides a recombinant methyltransferase
gene according to
nucleotide SECLID NO 27 or a functionally equivalent variant thereof.
In a third aspect, the invention provides a methyltransferase according to SEQ
_ID NO 28 or a
functionally equivalent amino acid variant thereof.
In a related aspect the invention provides a recombinant microorganism
comprising a
methyltransferase gene according to the second aspect. The methyltransferase
gene may be
present on a nucleic acid construct or integrated into the genome of the
microorganism.
In a fourth aspect, the invention provides a nucleic acid comprising SECI_ID
No 1 to 6, or
functionally equivalent variants thereof, in any order.
Preferably, the nucleic acid comprises SEQ_ID No 1 to 6 in the order shown in
figure 2.
Preferably, the nucleic acid further comprises a phosphotransacetylase/acetate
kinase promoter.
Preferably, the promoter corresponds to SEQ _ID No. 7 or a functionally
equivalent variant
thereof.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 7 -
In a fifth aspect, the invention provides an expression construct comprising
one or more nucleic
acid sequences wherein the construct, when expressed in an acetogenic
microorganism, results in
1-butanol and/or a precursor thereof being produced as the main fermentation
product.
Preferably, the one or more nucleic acid sequences encode one or more enzymes
that are part of
the 1-butanol biosynthesis pathway.
Preferably, the nucleic acids are selected from nucleic acids encoding
thiolase, 3-hydroxybutyryl-
00A dehydrogenase, crotonase, butyryl-CoA dehydrogenase, electron transfer
flavoprotein A
and/or electron transfer flavoprotein B.
Preferably, the one or more nucleic acid sequences are selected from SEQ _ID
NO. 1 to SECUD
NO. 6 or functionally equivalent variants thereof.
In one embodiment, the nucleic acids are further selected from nucleic acids
encoding
Phosphotransbutyrylase, butyrate kinase, ferredoxin dependent aldehyde
oxidoreductase,
butyraldehyde dehydrogenase, butanol dehydrogenase, and a bifunctional
butyraldehyde
dehydrogenase/butanol dehydrogenase.
In one embodiment, the nucleic acids are selected from the group of nucleic
acids outlined in
tables 7 to 10 herein after and functionally equivalent variants thereof.
In one embodiment, the expression construct encodes at least 2 enzymes in the
butanol
biosynthesis pathway, at least 3, at least 4, at least 5, at least 6, at least
7, at least 8, at least 9, at
least 10, at least 11 or at least 12 of the enzymes.
Preferably, the expression construct further comprises a
phosphotransacetylase/acetate kinase
operon promoter. In another embodiment, the expression construct comprises
another highly
active promoter such as the promoter of the pyruvate:ferredoxin oxidoreductase
(SEQ_ID No.
48), the Wood-Ljungdahl gene cluster (SEQ_ID No 47), Rnf operon (SEQ_ID No 49)
or the ATP
synthase operon (SEQ_ID No 50). Preferably, the phosphotransacetylase/acetate
kinase operon
promoter corresponds to SEQ_ID No. 7 or a functionally equivalent variant
thereof.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 8 -
In a sixth aspect, the invention provides a methylation construct comprising a
methyltransferase
gene as described herein.
In a seventh aspect, the invention provides a composition comprising the
expression construct of
the fifth aspect and the methylation construct of the sixth aspect.
Preferably, the composition is able to produce a recombinant microorganism
which produces 1-
butanol and/or a precursor thereof as the main fermentation product.
In an eighth aspect, the invention provides a method of producing a
recombinant microorganism
comprising:
a. introduction into a shuttle microorganism of (i) an expression
construct and (ii) a
methylation construct according to the sixth aspect comprising a
methyltransferase
gene;
b. expression of the methyltransferase gene;
c. isolation of one or more constructs from the shuttle microorganism; and,
d. introduction of at least the expression construct into a destination
microorganism;
wherein the expression construct comprises one or more genes encoding enzymes
to be
expressed in the destination microorganism.
In one embodiment, expression of the methyltransferase gene in step b. is
constitutive. In
another embodiment, expression of the methyltransferase gene in step b. is
induced.
In one embodiment, both the methylation construct and the expression construct
are isolated in
step C. In another embodiment, the expression construct is isolated in step C.
In one embodiment, only the expression construct is introduced into the
destination
microorganism. In another embodiment, both the expression construct and the
methylation
construct are introduced into the destination microorganism.
Preferably, the expression construct is as defined in the fifth aspect.
Preferably, the recombinant microorganism produces 1-butanol and/or a
precursor thereof as the
main fermentation product.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 9 -
In a related aspect, the invention provides a method of producing a
recombinant microorganism
comprising:
a. methylation of an expression construct in vitro by a methyltransferase
according to
SEQ_ID No 28 or a functionally equivalent variant thereof
b. introduction of an expression construct into a destination
microorganism;
wherein the expression construct comprises one or more genes encoding enzymes
to be
expressed in the destination microorganism.
Preferably, the expression construct is as defined in the fifth aspect.
Preferably, the recombinant microorganism produces 1-butanol and/or a
precursor thereof as the
main fermentation product.
Preferably, the methyltransferase is produced by expressing a
methyltransferase gene, preferably
according to SEQ_ID No 27 or a functionally equivalent variant thereof, in a
microorganism and
isolating the methyltransferase enzyme.
In a further related aspect, the invention provides a method of producing a
recombinant
microorganism comprising:
a. introduction into the genome of a shuttle microorganism of a
methyltransferase
gene, preferably according to SEQ_ID No 27 or a functionally equivalent
variant
thereof
b. introduction of an expression construct into the shuttle microorganism
c. isolation of one or more constructs from the shuttle microorganism; and,
d. introduction of at least the expression construct into a
destination microorganism;
wherein the expression construct comprises one or more genes encoding enzymes
to be
expressed in the destination microorganism.
Preferably, the expression construct is as defined in the fifth aspect.
Preferably, the recombinant microorganism produces 1-butanol and/or a
precursor thereof as the
main fermentation product.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 10 -
In a further related aspect, the invention provides a method of producing a
recombinant
microorganism comprising:
a. methylation of an expression construct in accordance with the fifth
aspect in vitro by
a methyltransferase
b. introduction of the expression construct into a destination microorganism.
Preferably, the methyltransferase is encoded by a methyltransferase gene as
defined in the
second aspect or a methyltransferase as defined in the third aspect.
Preferably, the recombinant microorganism produces 1-butanol and/or a
precursor thereof as the
main fermentation product.
In a ninth aspect, the invention provides a method of producing a recombinant
microorganism
comprising:
a. introduction of (i) an expression construct according to the fifth aspect
and (ii) a
methylation construct comprising a methyltransferase gene into a shuttle
microorganism;
b. expression of the methyltransferase gene;
c. isolation of one or more constructs from the shuttle microorganism; and
d. introduction of at least the expression construct into a destination
microorganism;
wherein the expression construct comprises one or more genes encoding enzymes
to be
expressed in the destination microorganism.
In one embodiment, expression of the methyltransferase gene in step b. is
constitutive. In
another embodiment, expression of the methyltransferase gene in step b. is
induced.
In one embodiment, both the methylation construct and the expression construct
are isolated in
step C. In another embodiment, the expression construct is isolated in step C.
In one embodiment, only the expression construct is introduced into the
destination
microorganism. In another embodiment, both the expression construct and the
methylation
construct are introduced into the destination microorganism.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 11 -
Preferably, the recombinant microorganism produces 1-butanol and/or a
precursor thereof as the
main fermentation product.
In a tenth aspect, the invention provides a method of producing a recombinant
microorganism
that produces 1-butanol or a precursor thereof as the main fermentation
product comprising:
a. Introduction of (i) an expression construct and (ii) a methylation
construct
comprising a methyltransferase gene into a shuttle microorganism;
b. expression of the methyltransferase gene;
c. isolation of one or more constructs from the shuttle microorganism; and,
d. introduction of at least the expression construct into a destination
microorganism;
wherein the expression construct comprises one or more genes encoding enzymes
to be
expressed in the destination microorganism.
another embodiment, expression of the methyltransferase gene in step b. is
induced.
In one embodiment, both the methylation construct and the expression construct
are isolated in
step C. In another embodiment, the expression construct is isolated in step C.
In one embodiment, only the expression construct is introduced into the
destination
microorganism. In another embodiment, both the expression construct and the
methylation
construct are introduced into the destination microorganism.
Preferably, the methylation construct is as defined in the sixth aspect.
In an eleventh aspect, the invention provides a method of production of 1-
butanol and/or a
Preferably, 1-butanol and/or a precursor thereof is the main fermentation
product.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 12 -
Preferably, the recombinant microorganism is as described in any one of the
eighth to the tenth
aspects.
Preferably, 1-butanol and/or a precursor thereof is produced in a yield of
from approximately
0.075 grams per litre of fermentation broth (g/l) to approximately 20g/I. In
one embodiment, the
yield is from approximately 0.15g/I to approximately 1.54g/I. In other
embodiments, the yield is
approximately 10g/I, approximately 5g/I, or approximately 2g/I. Preferably,
the yield of 1-butanol
is up to the limit at which butanol becomes toxic to the surrounding media.
Preferably, the substrate comprises CO. Preferably, the substrate is a gaeous
substrate
comprising CO. In one embodiment, the substrate comprises an industrial waste
gas. In certain
embodiments, the gas is steel mill waste gas or syngas.
In one embodiment, the substrate will typically contain a major proportion of
CO, such as at least
about 20% to about 100% CO by volume, from 20% to 70% CO by volume, from 30%
to 60% CO by
volume, and from 40% to 55% CO by volume. In particular embodiments, the
substrate comprises
about 25%, or about 30%, or about 35%, or about 40%, or about 45%, or about
50% CO, or about
55% CO, or about 60% CO by volume.
While it is not necessary for the substrate to contain any hydrogen, the
presence of H2 should not
be detrimental to product formation in accordance with methods of the
invention. In particular
embodiments, the presence of hydrogen results in an improved overall
efficiency of alcohol
production. For example, in particular embodiments, the substrate may comprise
an approx 2:1,
or 1:1, or 1:2 ratio of H2:CO. In one embodiment the substrate comprises about
30% or less H2 by
volume, 20% or less H2 by volume, about 15% or less H2 by volume or about 10%
or less H2 by
volume. In other embodiments, the substrate stream comprises low
concentrations of H2, for
example, less than 5%, or less than 4%, or less than 3%, or less than 2%, or
less than 1%, or is
substantially hydrogen free. The substrate may also contain some CO2 for
example, such as about
1% to about 80% CO2 by volume, or 1% to about 30% CO2 by volume.
Preferably, the precursor produced by the method of any of the preceding
aspects is converted to
1-butanol in the presence of phosphotransbutyrylase, butyrate kinase,
ferredoxin dependent
aldehyde oxidoreductase, and butanol dehydrogenase.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 13 -
Preferably, the microorganism produces phosphotransbutyrylase, butyrate
kinase, ferredoxin
dependent aldehyde oxidoreductase, and butanol dehydrogenase both before and
after
introduction of an exogenous nucleic acid.
Preferably, the precursor produced by the method of any of the preceding
aspects is converted to
1-butanol in the presence of butyraldehyde dehydrogenase, butanol
dehydrogenase and/or
a bifunctional butyraldehyde dehydrogenase/butanol dehydrogenase.
Preferably, the microorganism produces butyraldehyde dehydrogenase, butanol
dehydrogenase
and/or a bifunctional butyraldehyde dehyrogenase/butanol deydrogenase before
and after
introduction of an exogenous nucleic acid.
In a twelfth aspect, the invention provides 1-butanol or a precursor thereof
when produced by
the method of the eleventh aspect.
In a thirteenth aspect, the invention provides a shuttle microorganism
comprising a methylation
construct as defined herein.
Preferably, the shuttle microorganism further comprises an expression
construct as defined
herein.
Preferably, the shuttle microorganism is E.coli, Bacillus subtillis or
Lactococcus lactis.
Preferably, the methylation construct of any of the previous aspects comprises
a lac promoter
and the methyltransferase gene and is induced by Isopropyl-P-D-thio-
galactoside (IPTG).
Expression of the methyltransferase could also be controlled by other
inducible promoter systems
such as ara, tet, or T7.
In a fourteenth aspect, the invention provides a nucleic acid having a
sequence chosen from the
group consisting of SEQ_ID NOs 8 to 13.
In a fifteenth aspect, the invention provides a nucleic acid having a sequence
chosen from the
group consisting of SEQ_ID NOs 16 to 23.
In a sixteenth aspect, the invention provides a nucleic acid comprising at
least the nucleic acid
sequence of SEQ ID No. 7 or a functionally equivalent variant thereof, a
nucleic acid construct or
CA 02813431 2013-09-23
WO 2012/053905 PCT/NZ2011/000203
- 14 -
vector comprising same, and microorganisms comprising said nucleic acid or
nucleic acid
construct or vector.
In a seventeenth aspect, the invention provides a nucleic acid which encodes a
methyltransferase
according to SECLID No 28.
In an eighteenth aspect, the invention provides a nucleic acid comprising a
nucleic acid encoding a
polypeptide having the amino acid sequence of a polypeptide chosen from the
group listed in
tables 7 to 10 herein after and functionally equivalent variants of any one or
more thereof.
In a nineteenth aspect, the invention provides a nucleic acid comprising a
nucleic acid chosen
from the group listed in tables 7 to 10 herein after and functionally
equivalent variants of any one
or more thereof.
In a twentieth aspect, the invention provides constructs and microorganisms
comprising a nucleic
acid of the eighteenth or nineteenth aspects of the invention.
In a twenty first aspect, the invention provides a nucleic acid having a
sequence chosen from the
group consisting of SEQ_ID NOs 32 to 38 and 123 to 135.
In a twenty second aspect, the invention provides a polypeptide comprising the
amino acid
sequence of a polypeptide chosen from the group listed in tables 7 to 10
herein after and
functionally equivalent variants of any one or more thereof.
The invention may also be said broadly to consist in the parts, elements and
features referred to
or indicated in the specification of the application, individually or
collectively, in any or all
combinations of two or more of said parts, elements or features, and where
specific integers are
mentioned herein which have known equivalents in the art to which the
invention relates.
BRIEF DESCRIPTION OF THE FIGURES
These and other aspects of the present invention, which should be considered
in all its novel
aspects, will become apparent from the following description, which is given
by way of example
only, with reference to the accompanying figures, in which:
Figure 1 shows the butanol biosynthesis pathway from CO.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 15 -
Figure 2 shows an exemplary expression plasmid encoding genes involved in 1-
butanol
biosynthesis.
Figure 3 shows sequencing results of pMTL85245-thIA-crt-hbd which demonstrate
that the 1-
butanol biosynthesis genes found on the expression plasmid were free of
mutations.
Figure 4a, 4b and 4c show a nucleotide alignment of the C. autoethanogenum
(CAU), C. ljungdahlii
(CU), C. ragsdalei (CRA) and the designed methyltransferase (DMT) genes.
Figure 4d shows an amino acid alignment of the methyltransferases from C.
autoethanogenum
(CAU1+2), C. ljungdahlii (CU), C. ragsdalei (CRA1+2) and the designed
methyltransferase (DMT).
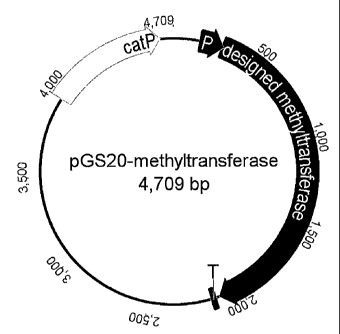
Figure 5 shows an exemplary methylation plasmid of the invention
Figure 6 shows an agarose gel electrophoresis image of isolated plasmid DNA.
Lane 1, 6, 11, 16,
21 and 26 show 100 bp Plus DNA Ladder. Lane 2-5 shows PCR with original
methylated plasmid
mix as template in the following order: ermB, Co/El, thIA, crt. Lane 7-10, 12-
15, 17-20, 22-25 and
27-30 show PCR with isolated plasmids from 4 different clones as template,
each in the following
order ermB, ColE1, thIA, crt. Lane 32-35 shows plasmid prep from 4 different
clones. Lane 36
shows plasmid prep from original C. autoethanogenum DSM23693.
Figure 7 shows HPLC results showing 1-butanol production with C.
autoethanogenum harboring
butanol plasmid pMTL85245-thIA-crt-hbd.
Figure 8 shows an analysis of expression of over 200 genes during a typical
fermentation with
Clostridium autoethanogenum at standard conditions using real-time PCR to
identify appropriate
promoter regions for the expression of heterologous genes.
Figure 9 shows the sequence for SEQ_ID No 1, 2 and 3.
Figure 10 shows the sequence for SECLID No 4, 5 and 6.
Figure 11 shows the sequence for promoter regions encoded by SEQ _ID No 7, 47,
48, 49 and 50.
CA 02813431 2013-04-18
WO 2012/053905
PCT/NZ2011/000203
301946506_1.DOCX
- 16 -
Figure 12 shows the sequence for SECLID No 14
Figure 13 shows the sequence for SECLID No 15
Figure 14 shows the sequence for SEQ JD No 24 and 25
Figure 15 shows the sequence for SEC:LID No 26
Figure 16 shows the sequence for SECLID No 27
Figure 17 shows the sequence for SEQ JD No 28
Figure 18 shows the sequence for SECLID No 29
Figure 19 shows the 16s rRNA gene of C. autoethanogenum (Y18178, GI:7271109)
Figures 20 and 21 show the sequence for SEQ JD No 31
Figure 22 shows Seq. ID 39: Nucleotide acid sequence of bifunctional butanol/
butyraldehyde
dehydrogenase of C. autoethanogenum
Figure 23 shows Seq. ID 40: Nucleotide acid sequence of bifunctional butanol/
butyraldehyde
dehydrogenase of C. autoethanogenum
Figure 24 shows Seq. ID 41: Nucleotide acid sequence of butyraldehyde
dehydrogenase of C.
autoethanogenum; and, Seq. ID 42: Amino acid sequence of butyraldehyde
dehydrogenase of C
autoethanogenum
Figure 25 shows Seq. ID 43: Nucleotide acid sequence of butyraldehyde
dehydrogenase of C.
autoethanogenum; and, Seq. ID 44: Amino acid sequence of butyraldehyde
dehydrogenase of C.
autoethanogenum
Figure 26 shows Seq. ID 45: Nucleotide acid sequence of butyraldehyde
dehydrogenase of C.
autoethanogenum
CA 02813431 2013-04-18
WO 2012/053905
PCT/NZ2011/000203
301946506_1.DOCX
- 17 -
Figure 27 shows Seq. ID 46: Amino acid sequence of butyraldehyde dehydrogenase
of C.
autoethanogenum; and, Seq. ID 119: Nucleotide acid sequence of butanol
dehydrogenase of C.
autoethanogenum
Figure 28 shows Seq. ID 120: Amino acid sequence of butanol dehydrogenase of
C.
autoethanogenum; and Seq. ID 121: Nucleotide acid sequence of butanol
dehydrogenase of C.
autoethanogenum.
Figure 29 shows Seq. ID 122: Amino acid sequence of butanol dehydrogenase of
C.
autoethanogenum; and, Seq. ID 51: Nucleotide acid sequence of butanol
dehydrogenase of C.
autoethanogenum.
Figure 30 shows Seq. ID 52: Amino acid sequence of butanol dehydrogenase of C
autoethanogenum; and, Seq. ID 53: Nucleotide acid sequence of butanol
dehydrogenase of C.
autoethanogenum
Figure 31 shows Seq. ID 54: Amino acid sequence of butanol dehydrogenase of C.
autoethanogenum; and, Seq. ID 55: Nucleotide acid sequence of butanol
dehydrogenase of C.
autoethanogenum
Figure 32 shows Seq. ID 56: Amino acid sequence of butanol dehydrogenase of C.
autoethanogenum; and, Seq. ID 57: Nucleotide acid sequence of butanol
dehydrogenase of C.
autoethanogenum.
Figure 33 shows Seq. ID 58: Amino acid sequence of butanol dehydrogenase of C.
autoethanogenum; and Seq. ID 59: Nucleotide sequence of phosphate
acetyl/butyryl transferase
from C. autoethanogenum; and Seq. ID 60: Amino acid sequence of phosphate
acetyl/butyryl
transferase from C. autoethanogenum.
Figure 34 shows Seq. ID 61: Nucleotide sequence of acetate/butyrate kinase
from C.
autoethanogenum; and Seq. ID 62: Amino acid sequence of acetate/butyrate
kinase from C.
autoethanogenum.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 18 -
Figure 35 shows Seq. ID 63: Nucleotide sequence of aldehyde:ferredoxin
oxidoreductase from C.
autoethanogenum; and Seq. ID 64: Amino acid sequence of aldehyde:ferredoxin
oxidoreductase
from C. autoethanogenum.
Figure 36 shows Seq. ID 65: Nucleotide sequence of aldehyde:ferredoxin
oxidoreductase from C.
autoethanogenum; and Seq. ID 66: Amirio acid sequence of aldehyde:ferredoxin
oxidoreductase
from C. autoethanogenum.
Figure 37 shows Seq. ID 67: Nucleotide acid sequence of bifunctional butanol/
butyraldehyde
dehydrogenase of C. ljungdahlii
Figure 38 shows Seq. ID 68: Amino acid sequence of bifunctional butanol/
butyraldehyde
dehydrogenase of C. ljungdahlii
Figure 39 shows Seq. ID 69: Nucleotide acid sequence of bifunctional butanol/
butyraldehyde
dehydrogenase of C. ljungdahM
Figure 40 shows Seq. ID 70: Amino acid sequence of bifunctional butanol/
butyraldehyde
dehydrogenase of C. ljungdahlii; and Seq. ID 71: Nucleotide acid sequence of
butyraldehyde
dehydrogenase of C. ljungdahM.
Figure 41 shows Seq. ID 72: Amino acid sequence of butyraldehyde dehydrogenase
of C.
ljungdahlii; and Seq. ID 73: Nucleotide acid sequence of butyraldehyde
dehydrogenase of C.
ljungdahlii; and Seq. ID 74: Amino acid sequence of butyraldehyde
dehydrogenase of C.
ljungdahlii.
Figure 42 shows Seq. ID 75: Nucleotide acid sequence of butanol dehydrogenase
of C. ljungdahlii;
and Seq. ID 76: Amino acid sequence of butanol dehydrogenase of C.
ljungdahlii; and Seq. ID 77:
Nucleotide acid sequence of butanol dehydrogenase of C. ljungdahlii.
Figure 43 shows Seq. ID 78: Amino acid sequence of butanol dehydrogenase of C.
ljungdahlii; and
Seq. ID 79: Nucleotide acid sequence of butanol dehydrogenase of C.
ljungdahlii; and Seq. ID 80:
Amino acid sequence of butanol dehydrogenase of C. ljungdahlii.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 19 -
Figure 44 shows Seq. ID 81: Nucleotide acid sequence of butanol dehydrogenase
of C. ljungdahlii;
and Seq. ID 82: Amino acid sequence of butanol dehydrogenase of C.
ljungdahlii; and Seq. ID 83:
Nucleotide acid sequence of butanol dehydrogenase of C. ljungdahlii.
Figure 45 shows Seq. ID 84: Amino acid sequence of butanol dehydrogenase of C.
ljungdahlii; and
Seq. ID 85: Nucleotide sequence of phosphate acetyl/butyryl transferase from
C. ljungdahlii; and
Seq. ID 86: Amino acid sequence of phosphate acetyl/butyryl transferase from
C. ljungdahlii; and
Seq. ID 87: Nucleotide sequence of acetate/butyrate kinase from C.
ljungdahlii.
Figure 46 shows Seq. ID 88: Amino acid sequence of acetate/butyrate kinase
from C. ljungdahlii;
and Seq. ID 89: Nucleotide sequence of aldehyde:ferredoxin oxidoreductase from
C. ljungdahlii;
and Seq. ID 90: Amino acid sequence of aldehyde:ferredoxin oxidoreductase from
C. ljungdahlii.
Figure 47 shows Seq. ID 91: Nucleotide sequence of aldehyde:ferredoxin
oxidoreductase from C.
ljungdahlii; and Seq. ID 92: Amino acid sequence of aldehyde:ferredoxin
oxidoreductase from C.
ljungdahlii.
Figure 48 shows Seq. ID 93: Nucleotide Acid sequence of bifunctional butanol/
butyraldehyde
dehydrogenase from C. ragsdalei
Figure 49 shows Seq. ID 94: Amino Acid sequence of bifunctional butanol/
butyraldehyde
dehydrogenase from C. ragsdalei
Figure 50 shows Seq. ID 95: Nucleotide Acid sequence of bifunctional butanol/
butyraldehyde
dehydrogenase from C. ragsdalei.
Figure 51 shows Seq. ID 96: Amino Acid sequence of bifunctional butanol/
butyraldehyde
dehydrogenase from C. ragsdalei; and Seq. ID 97: Nucleotide Acid sequence of
butyraldehyde
dehydrogenase from C. ragsdalei.
Figure 52 shows Seq. ID 98: Amino Acid sequence of butyraldehyde dehydrogenase
from C.
ragsdalei; Seq. ID 99: Nucleotide Acid sequence of butyraldehyde dehydrogenase
from C.
ragsdalei; and Seq. ID 100: Amino Acid sequence of butyraldehyde dehydrogenase
from C.
ragsdalei.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 20 -
Figure 53 shows Seq. ID 101: Nucleotide Acid sequence of butanol dehydrogenase
from C.
ragsdalei; and Seq. ID 102: Amino Acid sequence of butanol dehydrogenase from
C. ragsdalei; and
Seq. ID 103: Nucleotide Acid sequence of butanol dehydrogenase from C.
ragsdalei.
Figure 54 shows Seq. ID 104: Amino Acid sequence of butanol dehydrogenase from
C. ragsdalei;
and Seq. ID 105: Nucleotide Acid sequence of butanol dehydrogenase from C.
ragsdalei; and Seq.
ID 106: Amino Acid sequence of butanol dehydrogenase from C. ragsdalei:
Figure 55 shows Seq. ID 107: Nucleotide Acid sequence of butanol dehydrogenase
from C.
ragsdalei; and Seq. ID 108: Amino Acid sequence of butanol dehydrogenase from
C. ragsdalei; and
Seq. ID 109: Nucleotide Acid sequence of butanol dehydrogenase from C.
ragsdalei.
Figure 56 shows Seq. ID 110: Amino Acid sequence of butanol dehydrogenase from
C. ragsdalei;
and Seq. ID 111: Nucleotide sequence of phosphate acetyl/butyryl transferase
from C. ragsdalei;
and Seq. ID 112: Amino acid sequence of phosphate acetyl/butyryl transferase
from C. ragsdalei;
and Seq. ID 113: Nucleotide sequence of acetate/butyrate kinase from C.
ragsdalei.
Figure 57 shows Seq. ID 114: Amino acid sequence of acetate/butyrate kinase
from C. ragsdalei;
and Seq. ID 115: Nucleotide sequence of aldehyde:ferredoxin oxidoreductase
from C. ragsdalei;
and Seq. ID 116: Amino acid sequence of aldehyde:ferredoxin oxidoreductase
from C. ragsdalei.
Figure 58 shows Seq. ID 117: Nucleotide sequence of aldehyde:ferredoxin
oxidoreductase from C.
ragsdalei; and Seq. ID 118: Amino acid sequence of aldehyde:ferredoxin
oxidoreductase from C.
ragsdalei.
Figure 59 shows SEQ. ID 136: 16S rRNA gene of Clostridium ljungdahlii
(CP001666.1,
GI:300433347).
Figure 60 shows Gene expression pattern of (A) bifunctional
butanol/butyraldehyde
dehydrogenase (Seq ID 39); (B) butyraldehyde dehydrogenase (Seq. ID 41); (C)
butyraldehyde
dehydrogenase (Seq. ID 45); (D) butanol dehydrogenase (Seq. ID 53); (E)
butanol dehydrogenase
(Seq. ID 57); (F) phosphate acetyl/butyryl transferase (Seq. ID 57); (G)
acetate/butyrate kinase
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 21 -
(Seq. ID 59); (H) aldehyde:ferredoxin oxidoreductase (Seq. ID 63); (01)
aldehyde:ferredoxin
oxidoreductase (Seq. ID 65).
DETAILED DESCRIPTION OF THE INVENTION
The following is a description of the present invention, including preferred
embodiments thereof,
given in general terms. The invention is further elucidated from the
disclosure given under the
heading "Examples" herein below, which provides experimental data supporting
the invention,
specific examples of various aspects of the invention, and means of performing
the invention.
Among others, the closely related microorganisms C. autoethanogenum, C.
ljungdahlii, and C.
ragsdalei are known to be useful for production of ethanol as biofuel from
carbon monoxide. In
order to produce 1-butanol as a biofuel from a gaseous substrate, a universal
transformation
system for these organisms has been developed and production of 1-butanol as
the main
fermentation product from CO has been demonstrated.
The inventors have found that when particular genes encoding proteins in the 1-
butanol
biosynthesis pathway (figure 1) were introduced into acetogenic
microorganisms, such
microorganisms were able to use a gaseous substrate to produce 1-butanol or a
precursor thereof
as the main fermentation product. Although some unmodified microorganisms are
known to
produce 1-butanol, the yield of 1-butanol from CO produced by such unmodified
microorganisms
is very low. As a result, their utility for production of biofuels from
gaseous substrates is
extremely limited due to their low efficiency and a subsequent lack of
commercial viability.
Clostridium autoethanogenum naturally produces ethanol, acetate, 2,3-butandiol
and lactic acid
but is not known to produce 1-butanol.
As shown in figure 1, the Wood-Ljungdahl pathway converts CO to acetyl-CoA.
This compound
may be further converted to 1-butanol in acetogenic microorganisms by the
action of the
enzymes thiolase, 3-hydroxybutyryl-CoA dehydrogenase, crotonase / crotonyl-CoA
hydratase,
butyryl-CoA dehydrogenase, butyraldehyde dehydrogenase and butanol
dehydrogenase. In a
particular embodiment of the invention, the microorganism expresses the first
four enzymes
which may be encoded by the nucleic acid SEQ_ID Nos 1 to 4 or functionally
equivalent variants
thereof. The present invention provides a microorganism that facilitates the
conversion of acetyl-
CoA to 1-butanol by the action of enzymes encoded by recombinant nucleic acids
as well as
naturally occurring enzymes. The invention also provides for the use of
microorganisms
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 22 -
expressing other recombinant nucleic acid sequences which encode enzymes at
other stages in
the Wood-Ljungdahl or butanol biosynthesis pathways. The inventors have also
identified a
number of novel enzymes and nucleic acids.
Since there is no natural competence (uptake of extracellular DNA from the
cell's environment)
known in Clostridia and electrotransformation or conjugation are the only
methods available for
transformation. These issues present significant difficulties in effectively
transforming
Clostridium species. Additionally, the restriction/methylation systems found
in Clostridia protect
against foreign and phage DNA and result in their genetic transformation being
particularly
troublesome. Transformation of several Clostridium strains (C. acetobutylicum
ATCC824, C.
cellulolyticum ATCC35319, C. botulinum ATCC25765, and C. difficile CD3 and
CD6) was shown to
be only possible if DNA is methylated in vivo in E. coli or methylated in
vitro in a specific pattern
prior to transformation (Mermelstein et al, 1993; Herbert et al, 2003; Jennert
et al, 2000; Davis et
al, 2000). However, the determination of the correct methylation pattern is
often not possible
due to unspecific exonucleases, etc. Additionally, many Clostridium species
also possess
restriction systems which digest DNA that is methylated at a specific
("wrong") position.
The abovementioned major hurdles have been overcome by the inventors in
developing the
recombinant microorganisms of the present invention. A novel methylation
system comprising a
novel methyltransferase gene was developed to circumvent the naturally
occurring restriction
barriers present in native acetogenic microorganisms. Accordingly, the
methylation method and
methyltransferase gene of the present invention may be applied to a number of
compatible
microorganisms that have restriction barriers preventing effective
introduction and expression of
desirable recombinant nucleic acids in microorganisms.
Definitions
As referred to herein, "precursors of 1-butanol" include butyryl CoA, butyryl-
phosphate, butyrate,
and butyraldehyde.
As referred to herein, a "fermentation broth" is a culture medium comprising
at least a nutrient
media and bacterial cells.
As referred to herein, a "shuttle microorganism" is a microorganism in which a
methyltransferase
enzyme is expressed and is distinct from the destination microorganism.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 23 -
As referred to herein, a "destination microorganism" is a microorganism in
which the genes
included on the expression construct are expressed and is distinct from the
shuttle
microorganism.
As referred to herein, the term "main fermentation product" is intended to
mean the one
fermentation product which is produced in the highest concentration and/or
yield.
The terms "increasing the efficiency", "increased efficiency" and the like,
when used in relation to
a fermentation process, include, but are not limited to, increasing one or
more of the rate of
growth of microorganisms catalysing the fermentation, the volume of desired
product (such as
alcohols) produced per volume of substrate (such as sugar) consumed, the rate
of production or
level of production of the desired product, and the relative proportion of the
desired product
produced compared with other by-products of the fermentation.
The phrase "substrate comprising carbon monoxide" and like terms should be
understood to
include any substrate in which carbon monoxide is available to one or more
strains of bacteria for
growth and/or fermentation, for example.
The phrase "gaseous substrate comprising carbon monoxide" and like phrases and
terms includes
any gas which contains a level of carbon monoxide. In certain embodiments the
substrate
contains at least about 20% to about 100% CO by volume, from 20% to 70% CO by
volume, from
30% to 60% CO by volume, and from 40% to 55% CO by volume. In particular
embodiments, the
substrate comprises about 25%, or about 30%, or about 35%, or about 40%, or
about 45%, or
about 50% CO, or about 55% CO, or about 60% CO by volume.
While it is not necessary for the substrate to contain any hydrogen, the
presence of H2 should not
be detrimental to product formation in accordance with methods of the
invention. In particular
embodiments, the presence of hydrogen results in an improved overall
efficiency of alcohol
production. For example, in particular embodiments, the substrate may comprise
an approx 2:1,
or 1:1, or 1:2 ratio of H2:CO. In one embodiment the substrate comprises about
30% or less H2 by
volume, 20% or less H2 by volume, about 15% or less H2 by volume or about 10%
or less H2 by
volume. In other embodiments, the substrate stream comprises low
concentrations of H2, for
example, less than 5%, or less than 4%, or less than 3%, or less than 2%, or
less than 1%, or is
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 24 -
substantially hydrogen free. The substrate may also contain some CO2 for
example, such as about
1% to about 80% CO2 by volume, or 1% to about 30% CO2 by volume. In one
embodiment the
substrate comprises less than or equal to about 20% CO2 by volume. In
particular embodiments
the substrate comprises less than or equal to about 15% CO2 by volume, less
than or equal to
about 10% CO2 by volume, less than or equal to about 5% CO2 by volume or
substantially no CO2.
In the description which follows, embodiments of the invention are described
in terms of
delivering and fermenting a "gaseous substrate containing CO". However, it
should be
appreciated that the gaseous substrate may be provided in alternative forms.
For example, the
gaseous substrate containing CO may be provided dissolved in a liquid.
Essentially, a liquid is
saturated with a carbon monoxide containing gas and then that liquid is added
to the bioreactor.
This may be achieved using standard methodology. By way of example, a
microbubble dispersion
generator (Hensirisak et. al. Scale-up of microbubble dispersion generator for
aerobic
fermentation; Applied Biochemistry and Biotechnology Volume 101, Number 3 /
October, 2002)
could be used. By way of further example, the gaseous substrate containing CO
may be adsorbed
onto a solid support. Such alternative methods are encompassed by use of the
term "substrate
containing CO" and the like.
In particular embodiments of the invention, the CO-containing gaseous
substrate is an industrial
off or waste gas. "Industrial waste or off gases" should be taken broadly to
include any gases
comprising CO produced by an industrial process and include gases produced as
a result of
ferrous metal products manufacturing, non-ferrous products manufacturing,
petroleum refining
processes, gasification of coal, gasification of biomass, electric power
production, carbon black
production, and coke manufacturing. Further examples may be provided elsewhere
herein.
Unless the context requires otherwise, the phrases "fermenting", "fermentation
process" Dr
"fermentation reaction" and the like, as used herein, are intended to
encompass both the growth
phase and product biosynthesis phase of the process. As will be described
further herein, in some
embodiments the bioreactor may comprise a first growth reactor and a second
fermentation
reactor. As such, the addition of metals or compositions to a fermentation
reaction should be
understood to include addition to either or both of these reactors.
The term "bioreactor" includes a fermentation device consisting of one or more
vessels and/or
towers or piping arrangement, which includes the Continuous Stirred Tank
Reactor (CSTR),
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 25 -
Immobilized Cell Reactor (ICR), Trickle Bed Reactor (TBR), Bubble Column, Gas
Lift Fermenter,
Static Mixer, or other vessel or other device suitable for gas-liquid contact.
As is described herein
after, in some embodiments the bioreactor may comprise a first growth reactor
and a second
fermentation reactor. As such, when referring to the addition of substrate to
the bioreactor or
fermentation reaction it should be understood to include addition to either or
both of these
reactors where appropriate.
"Exogenous nucleic acids" are nucleic acids which originate outside of the
microorganism to
which they are introduced. Exogenous nucleic acids may be derived from any
appropriate source,
including, but not limited to, the microorganism to which they are to be
introduced, strains or
species of microorganisms which differ from the organism to which they are to
be introduced, or
they may be artificially or recombinantly created. In one embodiment, the
exogenous nucleic
acids represent nucleic acid sequences naturally present within the
microorganism to which they
are to be introduced, and they are introduced to increase expression of or
over-express a
particular gene (for example, by increasing the copy number of the sequence
(for example a
gene)). In another embodiment, the exogenous nucleic acids represent nucleic
acid sequences
not naturally present within the microorganism to which they are to be
introduced and allow for
the expression of a product not naturally present within the microorganism or
increased
expression of a gene native to the microorganism (for example in the case of
introduction of a
regulatory element such as a promoter). The exogenous nucleic acid may be
adapted to integrate
into the genome of the microorganism to which it is to be introduced or to
remain in an extra-
chromosomal state.
It should be appreciated that the invention may be practised using nucleic
acids whose sequence
varies from the sequences specifically exemplified herein provided they
perform substantially the
same function. For nucleic acid sequences that encode a protein or peptide
this means that the
encoded protein or peptide has substantially the same function. For nucleic
acid sequences that
represent promoter sequences, the variant sequence will have the ability to
promote expression
of one or more genes. Such nucleic acids may be referred to herein as
"functionally equivalent
variants". By way of example, functionally equivalent variants of a nucleic
acid include allelic
variants, fragments of a gene, genes which include mutations (deletion,
insertion, nucleotide
substitutions and the like) and/or polymorphisms and the like. Homologous
genes from other
bacteria capable of butyric acid or butanol fermentation may also be
considered as examples of
functionally equivalent variants of the sequences specifically exemplified
herein. These include
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 26 -
homologous genes in species such as Clostridium acetobutylicum, Clostridium
beijerinckii,
Clostridium tetani, Clostridium pasteurianum, Clostridium kluyveri,
Clostridium cellulovorans,
Clostridium perfringens, Clostridium botulinum, Clostridium butyricum strain
DSM10702,
Clostridium tyrobutyricum strain ATCC 25755, Anaerococcus prevotii DSM 20548,
Thermoanaerobacter tengcongensis, Brachyspira pilosicoli, Bacillus megaterium,
Streptococcus
pyogenes and Clostridium saccharoperbutylacetonicum details of which are
publicly available on
websites such as Genbank or NCBI. The phrase "functionally equivalent
variants" should also be
taken to include nucleic acids whose sequence varies as a result of codon
optimisation for a
particular organism. "Functionally equivalent variants" of a nucleic acid
herein will preferably
have at least approximately 70%, preferably approximately 80%, more preferably
approximately
85%, preferably approximately 90%, preferably approximately 95% or greater
nucleic acid
sequence identity with the nucleic acid identified. In a particular
embodiment, the functionally
equivalent variant of the thiolase gene as defined herein may be the atoAB
gene in E. coli
(NC_000913.2; atoA = GenelD: 946719; atoB = GenelD: 946727). Functionally
equivalent variants
of the eftAB gene as defined herein may be found in Tsai and Saier (1995).
It should also be appreciated that the invention may be practised using
polypeptides whose
sequence varies from the amino acid sequences specifically exemplified herein.
These variants
may be referred to herein as "functionally equivalent variants". A
functionally equivalent variant
of a protein or a peptide includes those proteins or peptides that share at
least 40%, preferably
50%, preferably 60%, preferably 70%, preferably 75%, preferably 80%,
preferably 85%, preferably
90%, preferably 95% or greater amino acid identity with the protein or peptide
identified and has
substantially the same function as the peptide or protein of interest. Such
variants include within
their scope fragments of a protein or peptide wherein the fragment comprises a
truncated form
of the polypeptide wherein deletions may be from 1 to 5, to 10, to 15, to 20,
to 25 amino acids,
and may extend from residue 1 through 25 at either terminus of the
polypeptide, and wherein
deletions may be of any length within the region; or may be at an internal
location. Functionally
equivalent variants of the specific polypeptides herein should also be taken
to include
polypeptides expressed by homologous genes in other species of bacteria, for
example as
exemplified in the previous paragraph.
"Substantially the same function" as used herein is intended to mean that the
nucleic acid or
polypeptide is able to perform the function of the nucleic acid or polypeptide
of which it is a
variant. For example, a variant of an enzyme of the invention will be able to
catalyse the same
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 27 -
reaction as that enzyme. However, it should not be taken to mean that the
variant has the same
level of activity as the polypeptide or nucleic acid of which it is a variant.
One may assess whether a functionally equivalent variant has substantially the
same function as
the nucleic acid or polypeptide of which it is a variant using any number of
known methods.
However, by way of example, the methods outlined in 'nu' et al (2008) may be
used to assess
enzyme activity.
"Over-express", "over expression" and like terms and phrases when used in
relation to the
invention should be taken broadly to include any increase in expression of one
or more protein as
compared to the expression level of the protein of a parental microorganism
under the same
conditions. It should not be taken to mean that the protein is expressed at
any particular level.
A "parental microorganism" is a microorganism used to generate a recombinant
microorganism
of the invention. The parental microorganism may be one that occurs in nature
(ie a wild type
microorganism) or one that has been previously modified but which does not
express or over-
express one or more of the enzymes the subject of the present invention.
Accordingly, the
recombinant microorganisms of the invention have been modified to express or
over-express one
or more enzymes that were not expressed or over-expressed in the parental
microorganism.
The terms nucleic acid "constructs" or "vectors" and like terms should be
taken broadly to include
any nucleic acid (including DNA and RNA) suitable for use as a vehicle to
transfer genetic material
into a cell. The terms should be taken to include plasmids, viruses (including
bacteriophage),
cosmids and artificial chromosomes. Constructs or vectors may include one or
more regulatory
elements, an origin of replication, a multicloning site and/or a selectable
marker, among other
elements, sites and markers. In one particular embodiment, the constructs or
vectors are
adapted to allow expression of one or more genes encoded by the construct or
vector. Nucleic
acid constructs or vectors include naked nucleic acids as well as nucleic
acids formulated with
one or more agents to facilitate delivery to a cell (for example, liposome-
conjugated nucleic acid,
an organism in which the nucleic acid is contained).
It should be appreciated that nucleic acids of the invention may be in any
appropriate form,
including RNA, DNA, or cDNA, including double-stranded and single-stranded
nucleic acids.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 28 -
In one aspect the invention provides genetically modified microorganisms
capable of using CO to
produce 1-butanol and/or a precursor thereof as the main fermentation product.
The
microorganism is preferably an acetogenic recombinant microorganism which
produces 1-butanol
and/or a precursor thereof as the main fermentation product. In one particular
embodiment, the
acetogenic recombinant microorganism is capable of producing 1-butanol or a
precursor thereof
by fermentation from a substrate comprising CO at a concentration of greater
than approximately
1mM or 0.075g/I of butanol per litre of fermentation broth.
In one particular embodiment, the microorganism comprises one or more
exogenous nucleic acid
adapted to express or over-express one or more enzymes in the butanol
biosynthesis pathway. In
one embodiment, the microorganism is adapted to express one or more enzyme in
the butanol
biosynthesis pathway which is not naturally present in the parental
microorganism from which it
is derived, or to over-express one or more enzyme in the butanol biosynthesis
pathway which are
naturally present in the parental microorganism.
The microorganism may be adapted to express or over-express the one or more
enzymes by any
number of recombinant methods including, for example, increasing expression of
native genes
within the microorganism (for example, by introducing a stronger or
constitutive promoter to
drive expression of a gene), increasing the copy number of a gene encoding a
particular enzyme
by introducing exogenous nucleic acids encoding and adapted to express the
enzyme, introducing
an exogenous nucleic acid encoding and adapted to express an enzyme not
naturally present
within the parental microorganism.
In certain embodiments, the parental microorganism may be transformed to
provide a
combination of increased or over-expression of one or more genes native to the
parental
microorganism and introduction of one or more genes not native to the parental
microorganism.
Preferably, the microorganism comprises one or more exogenous nucleic acids
encoding one or
more of the enzymes chosen from the group consisting: Thiolase; 3-
hydroxybutyryl-CoA
dehydrogenase; Crotonase/crotonyl-CoA hydratase; Butyryl-CoA dehydrogenase;
Electron
Transfer Flavoprotein A; and, Electron Transfer Flavoprotein B. In one
embodiment, the one or
more nucleic acids encoding the one or more enzymes is chosen from the nucleic
acids SEQ ID
NO. 1 to SEQ ID NO. 6 or functionally equivalent variants thereof.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 29 -
In one embodiment the recombinant microorganism is adapted to express one or
more of the
genes which encode the enzymes thiolase (IUBMB enzyme nomenclature EC:2.3.1.9)
(thIA), 3-
hydroxybutyryl-CoA dehydrogenase (EC:1.1.1.157) (hbd), crotonase / crotonyl-
CoA hydratase
(EC:1.1.1.157) (crt or cch) and/or butyryl-CoA dehydrogenase (EC4.2.1.55)
(bcd). In one
embodiment, the microorganism is adapted to express all of these enzymes. In a
further
embodiment, the genes correspond to one or more of the nucleic acid sequences
selected from
SECI_ID Nos 1 to 4 or functionally equivalent variants thereof. The
recombinant microorganism of
the invention may also contain two electron transferring proteins. In one
embodiment, the
electron transferring proteins are electron transferring flavoproteins
(EC1.3.99.2) (etfAB) encoded
by SEQJD Nos 5 and 6, or functionally equivalent variants thereof. The use of
these electron-
transferring flavoproteins enhances the efficiency of the microorganism in
producing 1-butanol.
The flavoproteins provide a stable complex that is required for the activity
of Bcd.
In one particular embodiment, the microorganism comprises one or more
exogenous nucleic
acids encoding each of Thiolase, 3-hydroxybutyryl-CoA dehydrogenase,
Crotonase, Butyryl-CoA
dehydrogenase, Electron Transfer Flavoprotein A and Electron Transfer
Flavoprotein B.
In one embodiment, the microorganism comprises a plasmid encoding one or more
of, or
preferably each of, Thiolase, 3-hydroxybutyryl-CoA dehydrogenase, Crotonase,
Butyryl-CoA
dehydrogenase, Electron Transfer Flavoprotein A and Electron Transfer
Flavoprotein B.
In one embodiment, the microorganism alternatively or further comprises
exogenous nucleic
acids adapted to express one or more of the enzymes chosen from the group
consisting of:
Phosphotransbutyrylase; butyrate kinase; ferredoxin dependent aldehyde
oxidoreductase (or in
other words aledhyde:ferredoxin oxidoreductase); butyraldehyde dehydrogenase;
butanol
dehydrogenase; a bifunctional butyraldehyde dehydrogenase/butanol
dehydrogenase.
In one embodiment, the microorganism comprises exogenous nucleic acids adapted
to express
one or more of butyraldehyde dehydrogenase, butanol dehydrogenase and a
bifunctional
butyraldehyde dehydrogenase/butanol dehydrogenase. Preferably, the
microorganism comprises
one or more exogenous nucleic acids encoding one or more of butyraldehyde
dehydrogenase,
butanol dehydrogenase and a bifunctional butyraldehyde dehydrogenase/butanol
dehydrogenase.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 30 -
In one embodiment, the microorganism comprises exogenous nucleic acids adapted
to express
one or more of Phosphotransbutyrylase, butyrate kinase, ferredoxin dependent
aldehyde
oxidoreductase, and butanol dehydrogenase. Preferably, the microorganism
comprises one or
more exogenous nucleic acids encoding one or more of Phosphotransbutyrylase,
butyrate kinase,
ferredoxin dependent aldehyde oxidoreductase, and butanol dehydrogenase. In
particular
embodiments, the microorganism comprises exogenous nucleic acids adapted to
express each of
Phosphotransbutyrylase, butyrate kinase, ferredoxin dependent aldehyde
oxidoreductase, and
butanol dehydrogenase.
In one embodiment, the microorganism comprises one or more nucleic acid
adapted to express at
least two of the enzymes in the 1-butanol biosynthesis pathway, at least 3, at
least 4, at least 5, at
least 6, at least 7, at least 8, at least 9, at least 10, at least 11, or at
least 12 of the enzymes.
In one embodiment, the microorganism further comprises an exogenous
phosphotransacetylase/acetate kinase promoter, although other promoters may be
used.
Preferably, the promoter corresponds to SEQ_ID No. 7 or a functionally
equivalent variant
thereof. Preferably, the promoter is contained on a construct encoding one or
more of the
enzymes referred to herein before.
Preferably, the parental microorganism is selected from the group of
carboxydotrophic
acetogenic bacteria. In certain embodiments the microorganism is selected from
the group
comprising Clostridium autoethanogenum, Clostridium ljungdahlii, Clostridium
ragsdalei,
Clostridium carboxidivorans, Clostridium drakei, Clostridium scatolo genes,
Butyribacterium
limosum, Butyribacterium methylotrophicum, Acetobacterium woodii,
Alkalibaculum bacchii,
Blautia producta, Eubacterium limosum, Moore/la the rmoacetica, Moore/la
thermautotrophica,
Oxobacter pfennigii, and Thermoanaerobacter kiuvi.
In one particular embodiment, the parental microorganism is selected from the
cluster of
ethanologenic, acetogenic Clostridia comprising the species C.
autoethanogenum, C. ljungdahlii,
and C. ragsdalei and related isolates. These include but are not limited to
strains C.
autoethanogenum JAI-1T (DSM10061) [Abrini J, Naveau H, Nyns E-.1: Clostridium
autoethanogenum, sp. nov., an anaerobic bacterium that produces ethanol from
carbon
monoxide. Arch Microbiol 1994, 4: 345-351], C. autoethanogenum LBS1560
(D5M19630)
[Simpson SD, Forster RL, Tran PT, Rowe MJ, Warner IL: Novel bacteria and
methods thereof.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
-31 -
International patent 2009, WO/2009/0642001, C. autoethanogenum LB51561
(DSM23693), C.
ljungdahlii PETCT (DSM13528 = ATCC 55383) [Tanner RS, Miller LM, Yang D:
Clostridium ljungdahlii
sp. nov., an Acetogenic Species in Clostridial rRNA Homology Group I. Int J
Syst Bacteriol 1993, 43:
232-236], C. ljungdahlii ERI-2 (ATCC 55380) [Gaddy JL: Clostridium stain which
produces acetic
acid from waste gases. US patent 1997, 5,593,886], C. ljungdahlii C-01 (ATCC
55988) [Gaddy JL,
Clausen EC, Ko C-W: Microbial process for the preparation of acetic acid as
well as solvent for its
extraction from the fermentation broth. US patent, 2002, 6,368,819], C.
ljungdahlii 0-52 (ATCC
55989) [Gaddy JL, Clausen EC, Ko C-W: Microbial process for the preparation of
acetic acid as well
as solvent for its extraction from the fermentation broth. US patent, 2002,
6,368,819], C.
ragsdalei P11T (ATCC BAA-622) [Huhnke RL, Lewis RS, Tanner RS: Isolation and
Characterization of
novel Clostridial Species. International patent 2008, WO 2008/028055], related
isolates such as
"C. coskatir [Zahn JA, Saxena .1, Do Y, Patel M, Fishein S, Datta R, Tobey R:
Clostridium coskatii, sp.
nov., an Anaerobic Bacterium that Produces Ethanol from Synthesis Gas. Poster
SIM Annual
Meeting and Exhibition, San Francisco, 2010], or mutated strains such as C.
ljungdahlii OTA-1
(Tirado-Acevedo 0. Production of Bioethanol from Synthesis Gas Using
Clostridium ljungdahlii.
PhD thesis, North Carolina State University, 2010). These strains form a
subcluster within the
Clostridial rRNA cluster I , and their 16S rRNA gene is more than 99%
identical with a similar low
GC content of around 30%. However, DNA-DNA reassociation and DNA
fingerprinting
experiments showed that these strains belong to distinct species [Huhnke RL,
Lewis RS, Tanner
RS: Isolation and Characterization of novel Clostridial Species. International
patent 2008, WO
2008/028055].
All species of this cluster have a similar morphology and size (logarithmic
growing cells are
between 0.5-0.7 x 3-5 p.m), are mesophilic (optimal growth temperature between
30-37 C) and
strictly anaerobe [Tanner RS, Miller LM, Yang D: Clostridium ljungdahlii sp.
nov., an Acetogenic
Species in Clostridial rRNA Homology Group I. Int J Syst Bacteriol 1993, 43:
232-236; Abrini
Naveau H, Nyns E-J: Clostridium autoethanogenum, sp. nov., an anaerobic
bacterium that
produces ethanol from carbon monoxide. Arch Microbiol 1994, 4: 345-351; Huhnke
RL, Lewis RS,
Tanner RS: Isolation and Characterization of novel Clostridial Species.
International patent 2008,
WO 2008/028055]. Moreover, they all share the same major phylogenetic traits,
such as same pH
range (pH 4-7.5, with an optimal initial pH of 5.5-6), strong autotrophic
growth on CO containing
gases with similar growth rates, and a similar metabolic profile with ethanol
and acetic acid as
main fermentation end product, and small amounts of 2,3-butanediol and lactic
acid formed
under certain conditions. [Tanner RS, Miller LM, Yang D: Clostridium
ljungdahlii sp. nov., an
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 32 -
Acetogenic Species in Clostridial rRNA Homology Group I. Int J Syst Bacteriol
1993, 43: 232-236;
Abrini J, Naveau H, Nyns E-J: Clostridium autoethanogenum, sp. nov., an
anaerobic bacterium that
produces ethanol from carbon monoxide. Arch Microbiol 1994, 4: 345-351; Huhnke
RL, Lewis RS,
Tanner RS: Isolation and Characterization of novel Clostridial Species.
International patent 2008,
WO 2008/0280551. Ind le production was observed with all three species as
well. However, the
species differentiate in substrate utilization of various sugars (e.g.
rhamnose, arabinose), acids
(e.g. gluconate, citrate), amino acids (e.g. arginine, histidine), or other
substrates (e.g. betaine,
butanol). Moreover some of the species were found to be auxotroph to certain
vitamins (e.g.
thiamine, biotin) while others were not.
In one embodiment, the microorganism produces phosphotransbutyrylase, butyrate
kinase,
ferredoxin dependent aldehyde oxidoreductase, and butanol dehydrogenase both
before and
after introduction of an exogenous nucleic acid.
In one embodiment, the microorganism produces butyraldehyde dehydrogenase
and/or butanol
dehydrogenase both before and after introduction of an exogenous nucleic acid.
In one particular embodiment, the microorganism is Clostridium autoethanogenum
DSM23693.
In one embodiment, the recombinant microorganism of the invention has the
defining
characteristics of the microorganism deposited at the DSMZ (Deutsche Sammlung
fur
Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany) under the
accession number
DSM24138.
The one or more exogenous nucleic acids may be delivered to a parental
microorganism as naked
nucleic acids or may be formulated with one or more agents to facilitate the
tranformation
process (for example, liposome-conjugated nucleic acid, an organism in which
the nucleic acid is
contained). The one or more nucleic acids may be DNA, RNA, or combinations
thereof, as is
appropriate.
The microorganisms of the invention may be prepared from a parental
microorganism and one or
more exogenous nucleic acids using any number of techniques known in the art
for producing
recombinant microorganisms. By way of example only, transformation (including
transduction or
transfection) may be achieved by electroporation, conjugation, or chemical and
natural
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1. DOCX
- 33 -
competence. Suitable transformation techniques are described for example in
Sambrook et al,
1989.
In certain embodiments, due to the restriction systems which are active in the
microorganism to
be transformed, it is necessary to methylate the nucleic acid to be introduced
into the
microorganism. This can be done using a variety of techniques, including those
described below,
and further exemplified in the Examples section herein after.
In another aspect, the invention provides a method of producing a recombinant
microorganism
comprising the following steps:
a. introduction into a shuttle microorganism of (i) an expression construct
and (ii) a
methylation construct comprising a methyltransferase gene;
b. expression of the methyltransferase gene;
c. isolation of one or more constructs from the shuttle microorganism; and,
d. introduction of the one or more constructs into a destination
microorganism;
wherein the expression construct comprises one or more genes encoding enzymes
to be
expressed in the destination organism.
In one embodiment, the methyltransferase gene of step B is expressed
consitutively. In another
embodiment, expression of the methyltransferase gene of step B is induced.
The shuttle microorganism is a microorganism, preferably a restriction
negative microorganism,
that facilitates the methylation of the nucleic acid sequences that make up
the expression
construct. In a particular embodiment, the shuttle microorganism is a
restriction negative E. coli,
Bacillus subtillis or Lactococcus lactis.
Once the expression construct and the methylation construct are introduced
into the shuttle
microorganism, the methyltransferase gene present on the methylation construct
is expressed. In
one embodiment, where expression must be induced, induction may be by any
suitable promoter
system although in one particular embodiment of the invention, the methylation
construct
comprises an inducible lac promoter (preferably encoded by SEQ_ID NO 28) and
is induced by
addition of lactose or an analogue thereof, more preferably isopropyl-B-D-thio-
galactoside (IPTG).
Other suitable promoters include the ara, tet, or 17 system. In an alternative
embodiment of the
invention, the methylation construct promoter is a constitutive promoter.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 34 -
In one embodiment the expression construct promoter is a constitutive promoter
that is
preferably highly active under appropriate fermentation conditions. However,
an inducible
promoter could be used. In preferred embodiments, the expression construct
promoter is
selected from the group comprising phosphotransacetylase/acetate kinase operon
promoter,
pyruvate:ferredoxin oxidoreductase (SEQ_ID No. 48), the Wood-Ljungdahl gene
cluster (SEQ_ID
No 47), Rnf operon (SECLID No 49) or the ATP synthase operon ((SEQ_ID No 50).
Preferably, the
phosphotransacetylase/acetate kinase operon promoter corresponds to SEQ_ID No.
7 or a
functionally equivalent variant thereof. Figure 8 shows that expression of
genes operably linked
to these promoters have a high level of expression in Clostridium
autoethanogenum under
standard conditions.
In a particular embodiment, the methylation construct has an origin of
replication specific to the
identity of the shuttle microorganism so that any genes present on the
methylation construct are
expressed in the shuttle microorganism. Preferably, the expression construct
has an origin of
replication specific to the identity of the destination microorganism so that
any genes present on
the expression construct are expressed in the destination microorganism.
Expression of the methyltransferase enzyme results in methylation of the genes
present on the
expression construct. The expression construct may then be isolated from the
shuttle
microorganism according to any one of a number of known methods. By way of
example only,
the methodology described in the Examples section described hereinafter may be
used to isolate
the expression construct.
In one particular embodiment, both constructs are concurrently isolated. The
expression
construct may be introduced into the destination microorganism using any
number of known
methods. However, by way of example, the methodology described in the Examples
section
hereinafter may be used. Since the expression construct is methylated, the
nucleic acid
sequences present on the expression construct are able to be incorporated into
the destination
microorganism and successfully expressed.
In a further embodiment, the invention provides a method of producing a
recombinant
microorganism comprising:
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 35 -
a. methylation of an expression construct in vitro by a methyltransferase,
preferably
according to SEQ_ID No 28 or a functionally equivalent variant thereof; and,
b. introduction of an expression construct, preferably according to the
fifth aspect, into
a destination microorganism;
wherein the expression construct comprises one or more genes encoding enzymes
to be
expressed in the destination microorganism.
It is envisaged that a methyltransferase gene of the invention, preferably
according to SEQ_ID No
27 or a functionally equivalent variant thereof, may be introduced into a
shuttle microorganism
and over-expressed. The resulting methyltransferase enzyme may be collected
using known
methods and used in vitro to methylate an expression construct, preferably,
the expression
construct is as defined in the fifth aspect. The expression construct may then
be introduced into
the destination microorganism for expression. Preferably, the recombinant
microorganism
produces 1-butanol and/or a precursor thereof as the main fermentation
product.
In a further embodiment, the invention provides a method of producing a
recombinant
microorganism comprising:
a. introduction into the genome of a shuttle microorganism of a
methyltransferase
gene, preferably according to SEQ_ID No 27 or a functionally equivalent
variant
thereof;
b. introduction of an expression construct into the shuttle microorganism;
c. isolation of one or more constructs from the shuttle microorganism; and,
d. introduction of at least the expression construct into a destination
microorganism;
wherein the expression construct comprises one or more genes encoding enzymes
to be
expressed in the destination microorganism.
Standard methods are used for the introduction of a methyltransferase gene,
preferably
according to SEQ_ID No 27, into the genome of the shuttle microorganism. The
methyltransferase may be constitutively expressed by the microorganism and
result in the
production of a methyltransferase enzyme, preferably according to SEQ_ID No 28
or a
functionally equivalent variant thereof. An expression construct is
methylated, isolated and
introduced into the destination microorganism which preferably, produces 1-
butanol and/or a
precursor thereof as the main fermentation product.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 36 -
The invention also includes microorganisms comprising a recombinant
methyltransferase gene or
methylation construct as herein described.
The present invention also provides a hybrid methyltransferase gene (SEQ _ID
NO 28) developed
following analysis of methyltransferase nucleic acid sequences and restriction
barrier systems
from C. autoethanogenum, C. ljungdahlii, and C. ragsdalei.
The methyltransferase gene is expressed in a shuttle microorganism which
results in the
production of a methyltransferase enzyme which methylates the sequence of the
expression
construct. The methyltransferase gene may be present on a construct or
integrated into the
genome of the shuttle microorganism. The hybrid methyltransferase gene is
codon optimised for
E. coli and may be incorporated into a methylation construct (figure 5). The
methyltransferase
gene may be codon optimised for use in another species of microorganism where
appropriate, for
example Bacillus subtillus. Methods for codon optimisation are standard and
would be known to
one of skill in the art (Carbone et al, 2003). Also incorporated within the
scope of the invention
are methyltransferase genes that have at least 70%, preferably 75%, preferably
80%, preferably
85%, preferably 90%, preferably 95% or greater nucleic acid sequence identity
to SEQJD NO 28
and express a polypeptide which is able to methylate DNA.
It will be appreciated by one of skill in the art that the methylation method
and methyltransferase
gene will have utility across a range of microorganisms. In one embodiment,
the destination
microorganism is selected from the group comprising Clostridium
autoethanogenum, Clostridium
ljungdahlii, Clostridium ragsdalei, Clostridium carboxidivorans, Clostridium
drakei, Clostridium
scatolo genes, Butyribacterium limosum, Butyribacterium methylotrophicum,
Acetobacterium
woodii, Alkalibaculum bacchii, Blautia producta, Eubacterium limosum, Moore/la
the rmoacetica,
Moore/la thermautotrophica, Oxobacter pfennigii, and Thermoanaerobacter
kiuvi.. In one
particular embodiment, the destination microorganism is selected from the
group consisting
Clostridium autoethanogenum, Clostridium ljungdahlii and Clostridium
ragsdalei. In one particular
embodiment the destination microorganism is Clostridium autoethanogenum
DSM23693.
The invention also provides various nucleic acids or nucleic acid constructs
as outlined in aspects
4, 5, 14, 15, 16, 18, 19 and 21 of the invention herein before described.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 37 -
In another embodiment of the invention, there is an expression construct
comprising one or more
nucleic acids encoding one or more enzymes chosen from Thiolase, 3-
hydroxybutyryl-CoA
dehydrogenase, Crotonase, Butyryl-CoA dehydrogenase and an electron transfer
protein or a
functionally equivalent variant thereof. Preferably, the electron transfer
protein is Electron
Transfer Flavoprotein A or Electron Transfer Flavoprotein B. In a particular
embodiment, both
Electron Transfer Flavoprotein A and Electron Transfer Flavoprotein B are
included on the
expression construct.
Exemplary sequence information for each gene and equivalent enzyme is provided
on GenBank as
detailed in Table 1 herein after. Skilled persons will readily appreciate
alternative genes and
enzymes which may be used. In one embodiment, the enzymes are encoded by the
nucleic acid
SEQ_ID No 1 to 6 which may be present in any order on the construct or in the
order shown in
figure 2. SEQ_ID Nos 8 to 13 and SEQJD Nos 16 to 23 are novel sequences used
to clone and
sequence the genes referred to in the immediately preceding paragraph.
In order to obtain 1-butanol from a precursor the activity of one or more of
butyraldehyde
dehydrogenase (EC1.2.1.10), alcohol dehydrogenase (EC 1.1.1.1),
phosphotransbutyrylase (EC
2.3.1.19), butyrate kinase (EC 2.7.2.7), aldehyde:ferredoxin oxidoreductase
(EC1.2.7.5) and
alcohol dehydrogenase (EC 1.1.1.1) may be required. The alcohol dehydrogenase
of the invention
is a butanol dehydrogenase. In certain embodiments, butyraldehyde
dehydrogenase (EC1.2.1.10)
and alcohol dehydrogenase (EC 1.1.1.1), or phosphotransbutyrylase (EC
2.3.1.19), butyrate kinase
(EC 2.7.2.7), aldehyde:ferredoxin oxidoreductase (EC1.2.7.5) and alcohol
dehydrogenase (EC
1.1.1.1), or a combination of both sets of enzymes is required. In one
embodiment, the
butyraldehyde dehydrogenase and butanol dehydrogenase activity is supplied by
a bifunctional
butyraldehyde dehydrogenase/butanol dehydrogenase. These various enzymes are
shown in the
butanol biosynthesis pathway depicted in figure 1. In some microorganisms
butyraldehyde
dehydrogenase, butanol dehydrogenase, phosphotransbutyrylase, butyrate kinase,
and/or
aldehyde:ferredoxin oxidoreductase are naturally expressed by the
microorganism and therefore
catalyse the conversion of butyryl-CoA to 1-butanol.
Accordingly, in one embodiment, the expression construct comprises nucleic
acids encoding one
or more of phosphotransbutyrylase, butyrate kinase, ferredoxin dependent
aldehyde
oxidoreductase, butyraldehyde dehydrogenase, butanol dehydrogenase, and a
bifunctional
butyraldehyde dehydrogenase/butanol dehydrogenase in addition to or in the
alternative to one
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 38 -
or more of Thiolase, 3-hydroxybutyryl-00A dehydrogenase, Crotonase, Butyryl-
CoA
dehydrogenase and an electron transfer protein.
Examples of appropriate enzymes and amino acid and nucleic acid sequence
information include,
but are not limited to: butyraldehyde dehydrogenase, such as Ald from C.
beijerinckii (ABR35947,
GI:149905114), C. saccharobutylicum (CAQ57983, G1:189310620), or Clostridium
saccharoperbutylacetoniucm (AAP42563, GI:31075383); butanol dehydrogenase,
such as BdhB
from C. acetobutylicum (NP_349891, GI:15896542); bifunctional
butyraldehyde/butanol
dehydrogenase enzyme, such as AdhEl from C. acetobutylicum (NP_149325,
GI:15004865) or
AdhE2 from C. acetobutylicum (NP 149199, GI:15004739), C. beijerinckii.
YP_001307449,
GI:150015195); a phosphotransbutyrylase such as Ptb from C. acetobutylicum
(NP_348368);
butyrate kinase such as Buk from C. acetobutylicum (AAK81015.1);
aldehyde:ferredoxin
oxidoreductase AOR from C. acetobutylicum (NP_348637). Persons of ordinary
skill in the art to
which the invention relates may readily appreciate alternative examples of
appropriate enzymes
of use in the invention. The inventors have also identified a number of novel
enzymes and genes
which may be used in the invention, the details of which are provided herein
after in the
Examples section (in particular see tables 7 to 10). The invention also
encompasses functionally
equivalent variants of these enzymes and genes and their use in methods of the
invention.
The inclusion of one or more of these genes may help avoid co-production of
butyrate
completely, increasing the efficiency of 1-butanol production. The invention
also provides
recombinant microorganisms comprising one or more nucleic acids adapted to
express or
increase expression of one or more of these enzymes.
In one embodiment, the nucleic acid(s) encode an enzyme chosen from the group
of enzymes
listed in tables 7 to 10 herein after and functional equivalents of any one or
more thereof. In a
particular embodiment, the nucleic acids are chosen from the group of nucleic
acids listed in
tables 7 to 10 herein after and functional equivalents of any one or more
thereof.
In one embodiment, the expression construct encodes at least 2 enzymes in the
butanol
biosynthesis pathway, at least 3, at least 4, at least 5, at least 6, at least
7, at least 8, at least 9, at
least 10, at least 11 or at least 12 of the enzymes.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 39 -
Preferably, the expression construct further comprises a suitable promoter as
hereinbefore
described. In one embodiment the promoter is a phosphotransacetylase/acetate
kinase
promoter. Preferably, the promoter corresponds to SEQ_ID No. 7 or a
functionally equivalent
variant thereof.
In a preferred embodiment, the expression construct comprises a nucleic acid
encoding all of said
enzymes. It will be appreciated by one of skill in the art that the expression
construct may
comprise nucleic acids encoding alternative electron transferring proteins.
The genes to be expressed in the recombinant microorganism may be assembled in
the
expression construct under the control of any appropriate promoter. In a
particular embodiment,
the promoter allows for substantially constitutive expression of the genes
under its control. In a
particular embodiment, the promoter is a phosphotransacetylase/acetate kinase
(SECUD NO 7)
promoter. Other promoters which may find use in the invention include those
from C.
autoethanogenum (or C. ljungdahlii). The inventors have also identified a
number of other
promoters that are operably linked to genes that were highly expressed under
typical
fermentation conditions in Clostridium autoethanogenum (figure 8). Analysis of
expression of
over 200 genes during typical fermentation conditions using real-time PCR
identified a number of
appropriate promoters. These include pyruvate:ferredoxin oxidoreductase
(SECLID No. 48), the
Wood-Ljungdahl gene cluster (SEQ _ID No 47), Rnf operon (SEQ _ID No 49) and
the ATP synthase
operon (SEQ _ID No 50). It will be appreciated by those of skill in the art
that other promoters
which can direct expression, preferably a high level of expression under
appropriate fermentation
conditions, would be effective as alternatives to the presently preferred
embodiments.
In one embodiment, the invention comprises a construct, recombinant
microorganism or a
nucleic acid sequence comprising nucleic acid SEQ _ID NOs 1 to 6 in the order
shown in figure 2.
However, it will be appreciated by one of skill in the art that the invention
may still have the
desired utility when the nucleic acid sequences are presented in any order and
with one or more
of the sequences absent.
In another embodiment, the invention comprises a nucleic acid comprising the
promoter
sequence represented by Seq ID No. 7, or a functionally equivalent variant
thereof, construct
comprising said promoter and recombinant microorganisms comprising same.
CA 02813431 2013-04-18
WO 2012/053905
PCT/NZ2011/000203
301946506_1.DOCX
-40 -
It will be appreciated that an expression construct of the present invention
may contain any
number of regulatory elements in addition to the promoter as well as
additional genes suitable
for expression of further proteins if desired. In one embodiment the construct
includes one
promoter. In another embodiment, the construct includes two or more promoters.
In one
particular embodiment, the construct includes one promoter for with each gene
to be expressed.
In one embodiment, the construct includes one or more ribosomal binding sites,
preferably a
ribosomal binding site for each gene to be expressed.
It will be appreciated by those of skill in the art that the nucleic acid
sequences and construct
sequences defined herein may contain standard linker nucleotides such as those
required for
ribosome binding sites and/or restriction sites. Such linker sequences should
not be interpreted
as being required and do not provide a limitation on the sequences defined.
When the expression construct of the invention is expressed in an acetogenic
microorganism, the
microorganism produces 1-butanol or a precursor thereof as the main
fermentation product. It is
envisaged that other genes which encode enzymes catalyzing different steps of
the Wood-
Ljungdahl or butanol biosynthesis pathways may also be incorporated in the
expression construct
in order to produce 1-butanol as the main fermentation product.
It is envisaged that the expression construct and the methylation construct as
defined above may
be combined to provide a composition of matter. Such a composition has
particular utility in
circumventing restriction barrier mechanisms in a wide variety of
microorganisms but in a
preferred embodiment, the recombinant microorganism produced by use of the
composition
produces 1-butanol or a precursor thereof as the main fermentation product.
Nucleic acids and nucleic acid constructs, including expression constructs of
the invention, may be
constructed using any number of techniques standard in the art. For example,
chemical synthesis
or recombinant techniques may be used. Such techniques are described, for
example, in
Sambrook et al (1989). Further exemplary techniques are described in the
Examples section
herein after. Essentially, the individual genes and regulatory elements will
be operably linked to
one another such that the genes can be expressed to form the desired proteins.
Suitable vectors
for use in the invention will be appreciated by those of ordinary skill in the
art. However, by way
of example, the following vectors may be suitable: pMTL80000 shuttle vectors,
pIMP1, 0111750
and the plasmids exemplified in the Examples section herein after.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203,
301946506_1.DOCX
- 41 -
To the extent that the invention provides novel nucleic acids and nucleic acid
vectors, it also
provides nucleic acids which are capable of hybridising to at least a portion
of a nucleic acid
herein described, a nucleic acid complementary to any one thereof, or a
functionally equivalent
variant of any one thereof. Such nucleic acids will preferably hybridise to
such nucleic acids, a
nucleic acid complementary to any one thereof, or a functionally equivalent
variant of any one
thereof, under stringent hybridisation conditions. "Stringent hybridisation
conditions" means
that the nucleic acid is capable of hybridising to a target template under
standard hybridisation
conditions such as those described in Sambrook et al (1989). It
will be appreciated that the
minimal size of such nucleic acids is a size which is capable of forming a
stable hybrid between a
given nucleic acid and the complementary sequence to which it is designed to
hybridise.
Accordingly, the size is dependent on the nucleic acid composition and percent
homology
between the nucleic acid and its complementary sequence, as well as the
hybridisation conditions
which are utilised (for example, temperature and salt concentrations). In one
embodiment, the
nucleic acid is at least 10 nucleotides in length, at least 15 nucleotides in
length, at least, 20
nucleotides in length, at least 25 nucleotides in length, or at least 30
nucleotides in length.
It should be appreciated that nucleic acids of the invention may be in any
appropriate form,
including RNA, DNA, or cDNA, including double-stranded and single-stranded
nucleic acids.
The invention also provides host organisms, particularly microorganisms, and
including viruses,
bacteria, and yeast, comprising any one or more of the nucleic acids described
herein.
The invention provides a method of production of 1-butanol and/or a precursor
thereof by
microbial fermentation comprising fermenting a gaseous substrate comprising CO
using a
recombinant microorganism. In certain embodiments, 1-butanol or a precursor
thereof is co-
produced with another fermentation product (for example, ethanol). In one
embodiment, the 1-
butanol or a precursor thereof is the main fermentation product. In one,
embodiment, the
recombinant microorganism is as herein before described.
In one embodiment, 1-butanol and/or a precursor thereof is produced in a yield
of from
approximately 0.075 grams per litre of fermentation broth (g/1) to
approximately 20g/1. In one
embodiment, the yield is from approximately 0.15g/I to approximately 1.54g/1.
In other
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
-42 -
embodiments, the yield is approximately 10g/I, approximately 5g/I, or
approximately 2g/I.
Preferably, the yield of 1-butanol is up to the limit at which butanol becomes
toxic to the bacteria.
Preferably, the fermentation comprises the steps of anaerobically fermenting a
substrate in a
bioreactor to produce 1-butanol and/or a precursor thereof using recombinant
microorganisms as
described herein.
Where the precursor of 1-butanol is referred to herein it is envisaged that it
may be optionally
converted to 1-butanol in the presence of butyraldehyde dehydrogenase, butanol
dehydrogenase, a bifunctional butyraldehyde dehydrogenase/butanol
dehydrogenase,
phosphotransbutyrylase, butyrate kinase, and/or ferredoxin dependent aldehyde
oxidoreductase.
Preferably, the microorganism produces one or more of these enzymes both
before and after
introduction of a recombinant nucleic acid.
In an embodiment of the invention, the gaseous substrate fermented by the
microorganism is a
gaseous substrate containing CO. The gaseous substrate may be a CO-containing
waste gas
obtained as a by-product of an industrial process, or from some other source
such as from
automobile exhaust fumes. In certain embodiments, the industrial process is
selected from the
group consisting of ferrous metal products manufacturing, such as a steel
mill, non-ferrous
products manufacturing, petroleum refining processes, gasification of coal,
electric power
production, carbon black production, ammonia production, methanol production
and coke
manufacturing. In these embodiments, the CO-containing gas may be captured
from the
industrial process before it is emitted into the atmosphere, using any
convenient method. The
CO may be a component of syngas (gas comprising carbon monoxide and hydrogen).
The CO
produced from industrial processes is normally flared off to produce CO2 and
therefore the
invention has particular utility in reducing CO2 greenhouse gas emissions and
producing butanol
for use as a biofuel. Depending on the composition of the gaseous CO
¨containing substrate, it
may also be desirable to treat it to remove any undesired impurities, such as
dust particles before
introducing it to the fermentation. For example, the gaseous substrate may be
filtered or
scrubbed using known methods.
It will be appreciated that for growth of the bacteria and CO-to-1butanol
fermentation to occur,
in addition to the CO-containing substrate gas, a suitable liquid nutrient
medium will need to be
fed to the bioreactor. A nutrient medium will contain vitamins and minerals
sufficient to permit
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
-43 -
growth of the micro-organism used. Anaerobic media suitable for fermentation
to produce
butanol using CO are known in the art. For example, suitable media are
described Biebel (2001).
In one embodiment of the invention the media is as described in the Examples
section herein
after.
The fermentation should desirably be carried out under appropriate conditions
for the CO-to-
butanol fermentation to occur. Reaction conditions that should be considered
include pressure,
temperature, gas flow rate, liquid flow rate, media pH, media redox potential,
agitation rate (if
using a continuous stirred tank reactor), inoculum level, maximum gas
substrate concentrations
to ensure that CO in the liquid phase does not become limiting, and maximum
product
concentrations to avoid product inhibition.
In addition, it is often desirable to increase the CO concentration of a
substrate stream (or CO
partial pressure in a gaseous substrate) and thus increase the efficiency of
fermentation reactions
where CO is a substrate. Operating at increased pressures allows a significant
increase in the rate
of CO transfer from the gas phase to the liquid phase where it can be taken up
by the micro-
organism as a carbon source for the production of butanol. This in turn means
that the retention
time (defined as the liquid volume in the bioreactor divided by the input gas
flow rate) can be
reduced when bioreactors are maintained at elevated pressure rather than
atmospheric pressure.
The optimum reaction conditions will depend partly on the particular micro-
organism of the
invention used. However, in general, it is preferred that the fermentation be
performed at
pressure higher than ambient pressure. Also, since a given CO-to-butanol
conversion rate is in
part a function of the substrate retention time, and achieving a desired
retention time in turn
dictates the required volume of a bioreactor, the use of pressurized systems
can greatly reduce
the volume of the bioreactor required, and consequently the capital cost of
the fermentation
equipment. According to examples given in US patent no. 5,593,886, reactor
volume can be
reduced in linear proportion to increases in reactor operating pressure, i.e.
bioreactors operated
at 10 atmospheres of pressure need only be one tenth the volume of those
operated at 1
atmosphere of pressure.
The benefits of conducting a gas-to-ethanol fermentation at elevated pressures
has been
described elsewhere. For example, WO 02/08438 describes gas-to-ethanol
fermentations
performed under pressures of 30 psig and 75 psig, giving ethanol
productivities of 150 g/l/day and
369 g/l/day respectively. However, example fermentations performed using
similar media and
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
-44 -
input gas compositions at atmospheric pressure were found to produce between
10 and 20 times
less ethanol per litre per day.
The composition of gas streams used to feed a fermentation reaction can have a
significant
impact on the efficiency and/or costs of that reaction. For example, 02 may
reduce the
efficiency of an anaerobic fermentation process. Processing of unwanted or
unnecessary gases in
stages of a fermentation process before or after fermentation can increase the
burden on such
stages (e.g. where the gas stream is compressed before entering a bioreactor,
unnecessary energy
may be used to compress gases that are not needed in the fermentation).
Accordingly, it may be
desirable to treat substrate streams, particularly substrate streams derived
from industrial
sources, to remove unwanted components and increase the concentration of
desirable
components.
In certain embodiments a culture of a bacterium of the invention is maintained
in an aqueous
culture medium. Preferably the aqueous culture medium is a minimal anaerobic
microbial growth
medium. Suitable media are known in the art and described for example in US
patent no.s
5,173,429 and 5,593,886 and WO 02/08438, and as described in the Examples
section herein
after.
Butanol, or a mixed alcohol stream containing butanol and one or more other
alcohols, may be
recovered from the fermentation broth by methods known in the art, such as
fractional
distillation or evaporation, pervaporation, and extractive fermentation,
including for example,
liquid-liquid extraction. By-products such as acids including butyrate may
also be recovered from
the fermentation broth using methods known in the art. For example, an
adsorption system
involving an activated charcoal filter or electrodialysis may be used.
Alternatively, continuous gas
stripping may also be used.
In certain preferred embodiments of the invention, butanol and by-products are
recovered from
the fermentation broth by continuously removing a portion of the broth from
the bioreactor,
separating microbial cells from the broth (conveniently by filtration), and
recovering butanol and
optionally acid from the broth. Alcohols may conveniently be recovered for
example by
distillation, and acids may be recovered for example by adsorption on
activated charcoal. The
separated microbial cells are preferably returned to the fermentation
bioreactor. The cell free
permeate remaining after the alcohol(s) and acid(s) have been removed is also
preferably
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
-45 -
returned to the fermentation bioreactor. Additional nutrients (such as B
vitamins) may be added
to the cell free permeate to replenish the nutrient medium before it is
returned to the bioreactor.
Also, if the pH of the broth was adjusted as described above to enhance
adsorption of acetic acid
to the activated charcoal, the pH should be re-adjusted to a similar pH to
that of the broth in the
fermentation bioreactor, before being returned to the bioreactor.
In one embodiment of the invention, butanol is recovered from the fermentation
reaction using
extractive fermentation procedures in which butanol is recovered into an oil
phase in the reactor.
Skilled persons would readily appreciate techniques for achieving this
Examples:
The invention will now be described in more detail with reference to the
following non-limiting
examples.
Genetic modifications were carried out using a plasmid containing a synthetic
operon consisting
of a strong, native C. autoethanogenum promoter controlling a thiolase, 3-
hydroxybutyryl-CoA
dehydrogenase, crotonase, butyryl-CoA dehydrogenase, and 2 electron
transferring flavoproteins
genes from C. acetobutylicum (Fig. 1-2). This plasmid was methylated in vivo
using a novel
methyltransferase and then transformed into C. autoethanogenum DSM23693.
Production of 1-
butanol as the main fermentation product was shown on different industrial gas
streams (steel
mill waste gas, syngas).
Construction of expression plasmid:
Standard Recombinant DNA and molecular cloning techniques were used in this
invention and are
described by Sambrook et al, 1989 and Ausubel et al, 1987. DNA sequences. of
butanol
biosynthetic genes of Clostridium acetobutylicum ATCC824 used were obtained
from NCBI (Table
1). The phosphotransacetylase/acetate kinase operon promoter of C.
autoethanogenum
DSM10061 were sequenced and used for expression of target genes (Table 1). RT-
PCR
experiments showed that this promoter is constitutively expressed at a high
level (figure 8).
Table 1: Sources of 1-butanol pathway genes
SEQ_ID
Gene/Promoter GenBank Citation
NO.
Thiolase (thIA) NC_003030 Clostridium acetobutylicum ATCC 824, 1
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
-46 -
complete genome; GI:15896127; GenelD:1119056
3-hydroxybutyryl-CoA dehydrogenase NC_003030 Clostridium acetobutylicum ATCC
824,
2
(hbd) complete genome; GI:15895965; GenelD:1118891
NC_003030 Clostridium acetobutylicum ATCC 824, 3
Crotonase (crt)
complete genome; GI:15895969; GenelD:1118895
NC_003030 Clostridium acetobutylicum ATCC 824,
butyryl-CoA dehydrogenase (bcd) 4
complete genome; GI:15895968; GenelD:1118894
Electron Transfer Flavoprotein A NC_003030 Clostridium acetobutylicum ATCC
824, 5
(etfA) complete genome; GI:15895966; GenelD:1118892
Electron Transfer Flavoprotein B NC_003030 Clostridium acetobutylicum ATCC
824, 6
(etfB) complete genome; GI:15895967; GenelD:1118893
phosphotransacetylase/acetate
Clostridium autoethanogenum DSM10061 7
kinase promoter (P
pro-ack)
Genomic DNA from Clostridium acetobutylicum ATCC824 and Clostridum
autoethanogenum
DSM10061 was isolated using a modified method by Bertram and Durre (1989). A
100-ml
overnight culture was harvested (6,000 x g, 15 min, 4 C), washed with
potassium phosphate
buffer (10 mM, pH 7.5) and suspended in 1.9 ml STE buffer (50 mM Tris-HCI, 1
mM EDTA, 200 mM
sucrose; pH 8.0). 300 I lysozyme (-100,000 U) were added and the mixture was
incubated at 37
C for 30 min, followed by addition of 280 1.11 of a 10 % (w/v) SDS solution
and another incubation
for 10 min. RNA was digested at room temperature by addition of 240 p.1 of an
EDTA solution (0.5
M, pH 8), 20 I Tris-HCI (1 M, pH 7.5), and 10 pl RNase A (Fermentas). Then,
100 p.I Proteinase K
(0.5 U) were added and proteolysis took place for 1-3 h at 37 C. Finally, 600
p.I of sodium
perchlorate (5 M) were added, followed by a phenol-chloroform extraction and
an isopropanol
precipitation. DNA quantity and quality was inspected spectrophotometrically.
Butanol biosynthesis genes and the phosphotransacetylase/acetate kinase
promoter were
amplified by PCR with oligonucleotides in table 2 using iProof High Fidelity
DNA Polymerase (Bio-
Rad Labratories) and the following program: initial denaturation at 98 C for
30 seconds, followed
by 32 cycles of denaturation (98 C for 10 seconds), annealing (50-62 C for
30-120 seconds) and
elongation (72 C for 45 seconds), before a final extension step (72 C for 10
minutes).
Table 2: Oligonucleotides for cloning
OligonucleotideSECLID
Target DNA Sequence (5 to 3')
Name NO.
Ppta-ack Ppta-ack-Notl-F GAGCGGCCGCAATATGATATTTATGTCC 8
Ppta-ack Ppta-ack-Ndel-R TTCCATATGTTTCATGTTCATTTCCTCC 9
ThIA ThIA-Cac-Ndel-F GTTCATATGAAAGAAGTTGTAATAGC 10
ThIA Th I A-Ca c-E co R I-R
CAAGAATTCCTAGCACTTTTCTAGC 11
crt-bcd-etfB-etfA- Crt-Cac-Kpnl-F AAGGTACCTTAGGAGGATTAGTCATGG 12
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
-47 -
hbd operon
crt-bcd-etfB-etfA- Crt-hbd-Cac-Ba m HI-
GAG GATCCG GATTCTTGTAAACTTATTTTG 13
hbd operon
The amplified 498 bp promoter region of the phosphotransacetylase/acetate
kinase operon (P pta-
ack) was cloned into the E. coli-Clostridium shuttle vector pMTL 85141 (Seq.
ID 14; FJ797651.1;
Nigel Minton, University of Nottingham; Heap et al., 2009) using Notl and Ndel
restriction sites
and strain DH5a-T1R (Invitrogen). The created plasmid pMTL85145 and the 1,194
bp PCR product
of the thiolase gene were both cut with Ndel and EcoRl. A ligation was
transformed into E. coli
XL1-Blue MRF' Kan (Stratagene) resulting in plasmid pMTL85145-thIA.
Subsequently, the
amplified 4,764 bp PCR fragment of the crt-bcd-etfB-etfA-hbd operon from C.
acetobutylicum
ATCC 824 was cloned into this vector using Kpnl and BamHI and E. coli ABLE K
(Stratagene),
creating plasmid pMTL85145-thIA-crt-hbd. Finally, the antibiotic resistance
cassette was changed
from chloramphenicol to clarithromycin. Therefore, an ermB cassette was
released from vector
pMTL82254 (Seq. ID 15; FJ797646.1; Nigel Minton, University of Nottingham;
Heap et al., 2009)
using restriction enzymes Pmel and Fsel and exchanged with the catP cassette
of plasmid
pMTL85145-thIA-crt-hbd. The insert of the resulting expression plasmid
pMTL85245-thIA-crt-hbd
(SEQ_ID No. 31 was completely sequenced using oligonucleotides given in table
3 and results
confirmed that the butanol biosynthesis genes were free of mutations (Figure
3).
Table 3: Oligonucleotides for sequencing
Oligonucleotide Name DNA Sequence (5' to 3') SECLID NO.
seq-ThIA-hbd-3562-4162 CAGAGGATGTTAATGAAGTC 16
seq-ThIA-hbd-4163-4763 GCATCAGGATTAAATGACTG 17
seq-ThIA-hbd-4764-5364 ATAGCGAAGTACTTG 18
seq-ThIA-hbd-5365-5965 GATGCAATGACAGCTTTC 19
seq-ThIA-hbd-5966-6566 GGAACAAAAGGTATATCAGC 20
seq-ThIA-hbd-7168-7768 CGGAGCATTTGATAAAGAA 21
seq-ThIA-hbd-7769-8369 GCTGATTGTACATCACTTGA 22
seq-ThIA-hbd-8370-8870 CCAGAATTAATAGCTCAAGT 23
Methylation of DNA:
A hybrid methyltransferase gene fused to an inducible lac promoter was
designed (Seq. ID 28), by
alignment of methyltransferase genes from C. autoethanogenum (SECLID No. 24),
C. ljungdahlii
(SEQ_ID No. 25), and C. ragsdalei (SEQ_ID No. 26) (figure 4a, 4b and 4c).
Expression of the
methyltransferase gene resulted in production of a methyltransferase enzyme
according to
SEQ_ID No. 28. Methyltransferase amino acid sequence alignment data is shown
in figure 4d.
CA 02813431 2013-09-23
WO 2012/053905 PCT/NZ2011/000203
-48 -
The hybrid methyltransferase gene (SECLID No. 27) was chemically synthesized
and cloned into
vector pGS20 (Seq. ID 29; ATG:biosynthetics GmbH, Merzhausen, Germany) using
EcoRI (Fig. 5).
The resulting methylation plasmid pGS20-methyltransferase was double
transformed with the
expression plasmid pMTL85245-thIA-crt-hbd into the restriction negative E.
coli XL1-Blue MRF'
Kan (Stratagene). In vivo methylation was induced by addition of 1 mM IPTG,
and methylated
plasmids were isolated using the PureLinkTM HiPure Plasmid Maxiprep Kit
(Invitrogen). The
resulting methylated plasmid composition was used for transformation of C.
autoethanogenum
DSM23693.
Transformation:
During the complete transformation experiment, C. autoethanogenum DSM23693 and
C.
ljundahlii (DSM13528) were grown in PETC media (Tab. 4) with 10 g/I fructose
and 30 psi steel mill
waste gas (collected from New Zealand Steel site in Glen brook, NZ;
composition: 44% CO, 32% N2i
22% CO2, 2% 1-12) as carbon source at 37 C using standard anaerobic
techniques described by
Hungate (1969) and Wolfe (1971).
Table 4: PETC media (ATCC media 1754)
Media component Concentration per 1.0L of media
NH4C1 1 g
KCI 0.1 g
MgSO4.7H20 0.2 g
NaCI 0.8g
KH2PO4 0.1 g
CaCl2 0.02 g
Trace metal solution 10 ml
Wolfe's vitamin solution 10 ml
Yeast Extract 1 g
Resazurin (2 g/L stock) 0.5 ml
NaHCO3 2g
Reducing agent 0.006-0.008 % (v/v)
Distilled water Up to 1 L, pH 5.5 (adjusted with HCI)
- - .
Wolfe's vitamin solution per L of Stock
Biotin 2 mg
Folk acid 2 mg
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 49 -
Pyridoxine hydrochloride 10 mg
Thiamine.HCI 5 mg
Riboflavin 5 mg
Nicotinic acid 5 mg
Calcium D-(+)-pantothenate 5 mg
Vitamin B12 0.1 mg
p-Amino benzoic acid 5 mg
Thioctic acid 5 mg
Distilled water To 1 L
Trace metal solution per L of stock
Nitrilotriacetic Acid 2 g
Mn504.H20 1 g
Fe (SO4)2(NF14)2.6H20 0.8 g
CoC12.6H20 0.2 g
Zn504.7H20 0.2 mg
CuC12.2H20 0.02 g
NaMo04.2H20 0.02 g
Na2Se03 0.02 g
NiC12.6H20 0.02 g
[¨N¨a-2-W04.2H20 02g.
Distilled water To 1 L
rReducing agent stock per 100 ml of stock
NaOH 0.9 g
Cystein.HCI 4g
Na2S 4g
Distilled water To 100 mL
To make competent cells, a 50 ml culture of C. autoethanogenum DSM23693 and a
50m1 culture
of C. ljundahlii DSM13528 were subcultured to fresh media for 3 consecutive
days. These cells
were used to inoculate 50 ml PETC media containing 40 mM DL-threonine at an
OD600nm of 0.05.
When the culture reached an OD600nm of 0.4, the cells were transferred into an
anaerobic chamber
and harvested at 4,700 x g and 4 C. The culture was twice washed with ice-
cold electroporation
buffer (270 mM sucrose, 1 mM MgC12, 7 mM sodium phosphate, pH 7.4) and finally
suspended in
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 50 -
a volume of 600 I fresh electroporation buffer. This mixture was transferred
into a pre-cooled
electroporation cuvette with a 0.4 cm electrode gap containing 1 g of the
methylated plasmid
mix (and in the case of C. ljundahlii 10 Type 1 restriction inhibitor
(Epicentre Biotechnologies))
and immediately pulsed using the Gene pulser Xcell electroporation system (Bio-
Rad) with the
following settings: 2.5 kV, 600 0, and 25 F. Time constants of 3.7-4.0 ms
were achieved. The
culture was transferred into 5 ml fresh media. Regeneration of the cells was
monitored at a
wavelength of 600 nm using a Spectronic Helios Epsilon Spectrophotometer
(Thermo) equipped
with a tube holder. After an initial drop in biomass, the cells start growing
again. Once the
biomass has doubled from that point, the cells were harvested, suspended in
200 p.I fresh media
and plated on selective PETC plates (containing 1.2 % BactoTM Agar (BD)) with
4 p.g/ I
Clarithromycin. After 4-5 days of inoculation with 30 psi steel mill gas at 37
C, 15-80 colonies per
plate were clearly visible.
The colonies were used to inoculate 2 ml PETC media containing 4 g/p.I
Clarithromycin. When
growth occurred, the culture was upscaled into 5 ml and later 50 ml PETC media
containing 4
g/ I Clarithromycin and 30 psi steel mill gas as sole carbon source.
Conformation of the successful transformation:
C. autoethanogenum: To verify the DNA transfer, a plasmid mini prep was
performed from 10 ml
culture volume using the QIAprep Spin Miniprep Kit (Qiagen). Due to
Clostridial exonuclease
activity (Burchhardt and Niue, 1990), the isolated plasmid DNA from 4 analyzed
clones were
partly degraded and only resulted in a smear on an agarose gel, while a
plasmid isolation from the
original C. autoethanogenum DSM23693 strain didn't result in a signal at all
(Fig. 6). However, the
quality of the isolated plasmid DNA was sufficient to run a control PCR using
4 sets of primers,
covering all relevant different regions of the plasmid (Table 5). The PCR was
performed with
illustra PuReTaq Ready-To-GoTm PCR Beads (GE Healthcare) using a standard
conditions (95 C for
5 min; 32 cycles of 95 C for 30 s, 50 C for 30 s, and 72 C for 1 min; 72 C
for 10 min). PCR of all 4
analyzed transformants resulted in the same signals as with the original
methylated plasmid mix
as template (Fig. 6). As a further control, 1 p.I of each of the partly
degraded isolated plasmids
were re-transformed in E. coli XL1-Blue MRF' Kan (Stratagene), from where the
plasm ids could be
isolated cleanly and verified by restriction digests.
To confirm the identity of the 4 clones, genomic DNA was isolated (see above)
from 40 ml of each
culture and a PCR was performed against the 16s rRNA gene (Tab. 5; Weisberg et
al., 1991) using
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
-51 -
illustra PuReTaq Ready-To-GoT" PCR Beads (GE Healthcare) and standard
conditions (95 C for 5
min; 32 cycles of 95 C for 30 s, 50 C for 30 s, and 72 C for 1 min; 72 C
for 10 min). The
respective PCR products were purified and sequenced. Sequences of all clones
showed at least
99.9% identity against the 16S rRNA gene of C. autoethanogenum (Seq. ID 30;
Y18178,
GI:7271109).
A respective strain was deposited at DSMZ (Deutsche Sammlung fur
Mikroorganismen und
Zellkulturen GmbH, Braunschweig, Germany) under the accession number DSM24138
on 26
October 2010.
C. ljungdahlii: Clostridium ljungdahlii transformants were confirmed using the
same method and
primer sets. Sequencing of the 16S rRNA gene resulted in a 100 % match with
the 16S gene of
Clostridium ljungdahlii (Seq. ID 119; CP001666, GI:300433347).
Table 5: Oligonucleotides for PCR confirmation of plasmid and species
Oligonucleotide Seq ID
Target region DNA Sequence (5 to 3')
Name No.
CCGAATTCGTCGACAACAGAGTTTGATCCTGGCT
16s rRNA gene fD1 135
CAG
CCCGGGATCCAAGCTTACGGCTACCTTGTTACGA
16s rRNA gene rP2 32
CTT
Antibiotic resistance
ermB-F TTTGTAATTAAGAAGGAG 33
cassette (ermB)
Antibiotic resistance
ermB-R GTAGAATCCTTCTTCAAC 34
cassette (ermB)
Insert 1 (thIA) ThIA-Cac-Ndel-F GTTCATATGAAAGAAGTTGTAATAGC 10
Insert 1 (th/A) ThIA-Cac-EcoRI-R
CAAGAATTCCTAGCACi ii iCTAGC 11
Insert 2 (crt-bcd-etfAB-
Crt-conserved-F GCTGGAGCAGATAT 35
hbd)
Insert 2 (crt-bcd-etfAB-
Crt-conserved-R GCTGTCATTCCTTC 36
hbd)
Replication origin (ColE1) ColEl-F CGTCAGACCCCGTAGAAA 37
Replication origin (ColE1) ColE1-R CTCTCCTGTTCCGACCCT 38
1-butanol production:
To demonstrate 1-butanol production from CO as sole energy and carbon source,
PETC media
without yeast extract and fructose were prepared and inoculated with the novel
C.
autoethanogenum and C. ljungdahlii strains harbouring butanol plasmid
pMTL85245-thIA-crt-hbd.
Bottles were pressurized with 30 psi of a CO containing gas stream from two
industrial sources,
steel mill waste gas (collected from New Zealand Steel site in Glenbrook, NZ;
composition: 44%
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 52 -
CO, 32% N2, 22% CO2, 2% H2) and syngas (Range Fuels Inc., Broomfield, CO;
composition: 29 % CO,
45 % H2, 13 % CH4, 12 % CO2, 1 % N2). 1-Butanol production could be
demonstrated on with both
strains and both gas mixes over several subculturing periods. Co-production of
butyrate was
observed as well. Neither 1-butanol nor butyrate were detected in samples of
unmodified strains
of C. autoethanogenum DSM23693 and C. ljungdahlii DSM13528 under the same
conditions.
Analysis of metabolites were performed by HPLC using an Agilent 1100 Series
HPLC system
equipped with a RID operated at 35 C (Refractive Index Detector) and an
Al!tech I0A-2000
Organic acid column (150 x 6.5 mm, particle size 5 m) kept at 60 C. Slightly
acidified water was
used (0.005 M H2SO4) as mobile phase with a flow rate of 0.7 ml/min. To remove
proteins and
other cell residues, 400 I samples were mixed with 100 I of a 2 % (w/v) 5-
Sulfosalicylic acid and
centrifuged at 14,000 x g for 3 min to separate precipitated residues. 10 p.I
of the supernatant
were then injected into the HPLC for analyses.
In serum bottle experiments the highest 1-butanol production was observed in
two static cultures
of C. autoethanogenum harboring butanol plasmid pMTL85245-thIA-crt-hbd. In
these cultures, 1-
butanol was the main fermentation end product observed with 1.54 g/1 (25.66
mM) (Table 6, Fig.
7). The production of the other metabolites was reduced compared to the
original strain C.
autoethanogenum DSM23693, which only produced ethanol, acetate, and 2,3-
butandiol.
Although the carbon flux was shifted towards 1-butanol production, the amount
of total carbon
incorporated into metabolic end products remain almost the same (Table 6). The
slight increase
of 20 % is likely to be the result of an extra reducing equivalents offload by
producing 1-butanol
and butyrate compared to ethanol and respectively acetate. The production of
2,3-butandiol
which usually acts as electron sink, was completely diminished.
Table 6: Metabolite production and carbon balance of C. autoethanogenum
harboring butanol
plasmid pMTL85245-th1A-crt-hbd compared to original C. autoethanogenum
DSM23693
Original C. autoethanogenum
C. autoethanogenum DSM23693
DSM23693 + pMTL85245-thIA-crt-
bcd
P Carbo Product Carbon
Product Carbon
Product Product
Product [g/mo [g/cm n [mmo1/1 [mmo1/1 [mmo1/1
[mmo1/1
I] 3] atoms Eg/IJ [gill
Ethanol 46.08 0.789 2 1.02 28.06 56.11 0.37 10.18 20.35
Acetate 60.05 1.049 2 1.87 29.69 59.37 0.30 4.76 9.52
2,3-
90.12 0.987 4 0.18 2.02 8.09 0 0 0
butandiol
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 53
1-butanol 74.12 0.810 4 0 0 0 1.54
25.66 102.63
Butyrate 88.11 0.960 4 0 0 0 0.31
3.67 14.67
Total 123.58
147.17
1-butanol production was also observed in cultures of C. /jungdah/ii DSM13528
harbouring the
butanol plasmid pMTL85245-thIA-crt-hbd in significant amounts of up to 0.36
g/L (6mM),
although lower compared to C. autoethanogenum DSM23693 carrying the same
plasmid. This
can be explained as C. autoethanogenum DSM23693 is a strain with improved
alcohol production
and correspondingly, the unmodified strain of C. autoethanogenum DSM23693
produces more
ethanol and less acetate than the unmodified strain of C. ljungdahlii DSM13528
(both strains
produce neither butanol nor butyrate).
C. ljungdahlii harbouring the butanol plasmid pMTL85245-thIA-crt-hbd had a
lower 1-
butanol:butryrate ratio than C. autoethanogenum. The ratio of 1-butanol to
butyrate, however,
can be altered by process conditions. This allows production of 1-butanol as
the main
fermentation product, but also production of butyrate as the main fermentation
product in both
strains C. autoethanogenum and C. ljungdahlii. In serum bottle experiments,
molar ratios of 1-
butanol:butyrate between 50:1 to 1:30 were observed with C. autoethanogenum
and between
20:1 and 1:30 with C. ljungdahlii. Cultures which were incubated under shaking
produced
generally higher butyrate and lower 1-butanol levels compared to static
cultures. The
concentration of CO (and H2) in the headspace was found to have an effect on
the 1-
butanol:butyrate ratio as well. In cultures with less CO in the headspace,
butyrate production was
more favoured and could be produced as the main fermentation product.
Correspondingly,
higher 1-butanol titers were observed on the CO-richer steel mill gas (44 %
CO) than on the CO-
leaner syngas (29 % CO) in performed serum bottle experiments. A maximum of
1.08 g/1 (12.8
mM) butyrate was observed with Clostridium autoethanogenum harbouring plasmid
pMTL85245-
thIA-crt-hbd and a level of 1.03 g7L (12.5 mM) with C. ljungdahlii carrying
the same plasmid. This
effect can be explained by the extra carbon going into the system and also the
additional reducing
power generated from CO oxidation by the carbon monoxide dehydrogenase (CODH).
Conversion of butyryl-CoA to butyrate and butanol:
The expression plasmid only contains the genes necessary for production of
butyryl-CoA from
acetyl-CoA. Butyryl-CoA can then be converted directly to butanol by action of
a butyraldehyde
dehydrogenase and butanol dehydrogenase (Fig. 1). A second possibility is that
butyryl-CoA is
converted to butyrate via a phosphotransbutyrylase and butyrate kinase (Fig.
1), in which case
CA 02813431 2013-04-18
WO 2012/053905
PCT/NZ2011/000203
301946506_1.DOCX
- 54 -
ATP is gained via substrate level phosphorylation (SLP). Since operation of
the Wood-Ljungdahl
pathway requires ATP, acetogenic cells rely on ATP from SLP, which is also
reflected in the fact
that every acetogenic bacteria known produces acetate (Drake et al., 2006).
However, the
recombinant cell can now also generate ATP via SLP also by producing butyrate.
Butyrate can then
be further reduced to butyraldehyde via a aldehyde:ferredoxinoxidoreductase
(AOR) (Fig. 1). This
reaction could be driven by reduced ferredoxin, provided by oxidation of CO
via the carbon
monoxide dehydrogenase (CO + Fdred -> CO2 + Fdox), the initial step in the
Wood-Ljungdahl
pathway. Butyraldehyde can then be converted to butanol via a butanol
dehydrogenase (Fig. 1).
Conversion of externally added butyrate to butanol by a culture of C.
autoethanogenum has been
demonstrated (W02009/113878).
Respective genes/enzymes with butyraldehyde dehydrogenase, butanol
dehydrogenase,
phophotransbutyrylase, butyrate kinase, and aldehyde:ferredoxin oxidoreductase
activity have
been identified by the inventors in C. autoethanogenum, C. ljungdahlii, and C.
ragsdalei (Tab. 7-
10). Potential genes and enzymes were predicted by comparison with
characterized genes and
enzymes using BLAST (Altschul et al, 1990), COG (Tatusov et al, 2003), and
TIGRFAM (Haft et al,
2002) databases. Motif scans were performed against PROSITE (Hub o et al.,
2008) Pfam (Finn et
al., 2010) databases. Genomes of C. autoethanogenum, C. ljungdahlii, and C.
ragsdalei contain
several genes encoding enzymes with alcohol and aldehyde dehydrogenase
activity. As indicated
in tables 7 to 10, some of these were found to have high homology of over 70 %
to characterized
butyraldehyde and butanol dehydrogenases from C. acetobutylicum, C.
beijerinckii, or C.
saccharobutylicum, while others have at least in some 40 % identity to these
enzymes. All three
genomes encode exactly one enzyme with Phosphate acetyl/butyryl transferase
activity and one
with Acetate/butyrate kinase activity. C. autoethanogenum, C. ljungdahlii, and
C. ragsdalei each
possess 2 aldehyde:ferredoxin oxidoreductase genes.
Table 7: Genes of C. autoethanogenum potentially conferring butyraldehyde and
butanol
dehydrogenase activity
Sequence Description Identity (protein) to characterized enzymes
Seq. ID 39-40 Bifunctional butanol/ = bifunctional aldehyde/alcohol
dehydrogenase AdhE2 from C. beijerinckii NCIMB
butyraldehyde dehydrogenase 8052
(Identities = 644/861 (75%), Positives = 748/861 (87%), e-value = 0.0)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
ATCC824 (Identities = 594/858 (70%), Positives = 730/858 (86%), e-value = 0.0)
Seq. ID 41-42 Butyraldehyde dehydrogenase = bifunctional
aldehyde/alcohol dehydrogenase AdhE2 from C. beijerinckii NCIMB
8052 (Identities = 367/504 (73%), Positives = 437/504 (87%), e-value = 0.0)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
ATCC824 (354/504 (71%), Positives = 440/504 (88%), e-value = 0.0)
Seq. ID 43-44 Butyraldehyde dehydrogenase = bifunctional
aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
ATCC824 (Identities = 173/352 (50%), Positives = 236/352 (68%), e-value = le-
91)
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 55 -
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. beijerinckii
NCIMB
8052 (Identities = 160/374(43%). Positives = 234/374 (63%), e-value = 5e-87)
= bifunctional aldehyde/alcohol dehydrogenase AdhEl from C. acetobutylicum
ATCC824 (Identities = 158/366 (44%), Positives = 235/366 (65%), e-value = 5e-
82)
= butyraldehyde dehydrogenase Aid from C. beijerinckii NCIMB8052
(Identities = 110/354 (32%), Positives = 184/354 (52%), e-value = 9e-44)
= butyraldehyde dehydrogenase from C. saccharoperbutylacetonicum
(111/354 (32%), Positives = 182/354(52%), e-value = 2e-44)
Seq. ID 45-46 Butyraldehyde dehydrogenase = bifunctional
aldehyde/alcohol dehydrogenase AdhE2 from C. beijerinckii NCIMB
8052 (Identities = 188/477 (40%), Positives = 270/477 (57%), e-value = 9e-84)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
ATCC824 (Identities = 164/428 (39%), Positives = 256/428 (60%), e-value = le-
- 79)
Seq. ID 119- Butanol dehydrogenase = NADPH-dependet butanol
dehydrogenase from C. saccharobutylicum
120 (Identities = 285/388 (74%), Positives =
334/388 (87%), e-value = 7e-177)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
ATCC824 (Identities = 163/396 (42%), Positives = 237/396 (60%), e-value = 4e-
80)
Seq. ID 121- Butanol dehydrogenase = NADPH-dependet butanol
dehydrogenase from C. saccharobutylicum
122 (Identities = 271/388 (70%), Positives =
328/388 (85%), e-value = 3e-168)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
ATCC824 (Identities = 169/403 (42%), Positives = 240/403 (60%), e-value = 3e-
83)
Seq. ID 51-52 Butanol dehydrogenase = bifunctional
aldehyde/alcohol dehydrogenase AdhE2 from C. beijerinckii NCIMB
8052 (246/315 (79%), Positives = 287/315 (92%), e-value = le-153)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
ATCC824 (208/312 (67%), Positives = 260/312 (84%), e-value = 4e-128)
Seq. ID 53-54 Butanol dehydrogenase = NADPH-dependet
butanol dehydrogenase from C. saccharobutylicum
(Identities = 264/388 (69%), Positives = 326/388 (85%), e-value = 5e-163)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. beijerinckii
NCIMB8052 (Identities = 169/410 (42%), Positives = 246/410 (60%), e-value =
5e-22)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
ATCC824 (Identities = 162/402 (41%), Positives = 240/402 (60%), e-value = 2e-
78)
Seq. ID 55-56 Butanol dehydrogenase = NADH-dependent
butanol dehydrogenase BdhA from C. acetobutylicum
ATCC824 (Identities = 161/388 (42%), Positives = 243/388 (63%), e-value = 7e-
92)
= NADH-dependent butanol dehydrogenase BdhB from C. acetobutylicum
ATCC824 (Identities = 155/389 (40%), Positives = 242/389 (63%), e-value = 4e-
85)
Seq. ID 57-58 Butanol dehydrogenase = NADPH-dependet
butanol dehydrogenase AdhE2 from C. saccharobutylicum
(Identities = 156/385 (41%), Positives = 236/385 (62%), e-value = le-72)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
ATCC824 (Identities = 154/412 (38%), Positives = 233/412 (57%), e-value = 8e-
70)
Seq. ID 59-60 Phosphate acetyl/butyryl = phosphate
butyryltransferase from C. acetobutylicum ATCC 824 (Identities =
transferase 85/338 (26%), Positives = 146/338(44%), e-
value = 2e-12)
Seq ID 61-62 Acetate/butyrate kinase = butyrate kinase from C.
acetobutylicum ATCC 824 (Identities = 49/175 (28%),
Positives = 78/175 (45%), e-value 5e-08)
Seq ID 63-64 Aldehyde:ferredoxin = aldehyde:ferredoxin oxidoreductase
from C. acetobutylicum ATCC 824
oxidoreductase (Identities = 183/618 (30%), Positives =
311/618 (51%), e-value = 6e-72)
Seq ID 65-66 Aldehyde:ferredoxin = aldehyde:ferredoxin oxidoreductase
from C. acetobutylicum ATCC 824
oxidoreductase (Identities = 191/633 (31%), Positives =
308/633 (49%), e-value = 2e-70)
Table 8: Genes of C. ljungdahlii potentially conferring butyraldehyde and
butanol dehydrogenase
activity
Sequence Description Identity to characterized enzymes
Seq. ID 67-68 Bifunctional butanol/ = bifunctional
aldehyde/alcohol dehydrogenase AdhE2 from C. beijerinckii NCIMB
butyraldehyde dehydrogenase 8052 (Identities = 644/862 (75%), Positives =
751/862 (88%), e-value = 0.0)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
ATCC824 (Identities = 592/858 (69%), Positives = 729/858 (85%), e-value = 0.0)
Seq. ID 69-70 Bifunctional butanol/ = bifunctional
aldehyde/alcohol dehydrogenase AdhE2 from C. beijerinckii NCIMB
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 56 -
butyraldehyde dehydrogenase 8052 (Identities = 636/860 (74%), Positives =
752/860 (88%), e-value = 0.0)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
ATCC824 (Identities = 585/858 (69%), Positives = 733/858 (86%), e-value = 0.0)
Seq. ID 71-72 Butyraldehyde dehydrogenase = bifunctional
aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
A1CC824 (Identities = 209/429 (49%), Positives = 286/429 (67%), e-value = 4e-
111)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. beijerinckii
NCIMB
8052 (Identities = 196/467 (42%), Positives = 286/467 (62%), e-value = le-102)
= bifunctional aldehyde/alcohol dehydrogenase AdhEl from C. acetobutylicum
ATCC824 (Identities = 193/443 (44%), Positives = 283/443 (64%), e-value = 7e-
100)
= butyraldehyde dehydrogenase Aid from C. beijerinckii NCIMB8052
(Identities = 125/409 (31%), Positives = 206/409 (51%), e-value = 3e-49)
= butyraldehyde dehydrogenase from C. saccharoperbutylacetonicum
(124/409 (31%), Positives = 204/409 (50%), e-value = 2e-48)
Seq. ID 73-74 Butyraldehyde dehydrogenase = bifunctional
aldehyde/alcohol dehydrogenase AdhE2 from C. beijerinckii NCIMB
8052 (Identities = 188/477 (40%), Positives = 270/477 (57%), e-value = 9e-84)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
ATCC824 (Identities = 164/428 (39%), Positives = 256/428 (60%), e-value = le-
79)
Seq. ID 75-76 Butanol dehydrogenase = NADPH-dependet
butanol dehydrogenase from C. saccharobutylicum
(Identities = 285/388 (74%), Positives = 335/388 (87%), e-value = 9e-177)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
A1CC824 (Identities = 164/396 (42%), Positives = 238/396 (61%), e-value = le-
80)
Seq. 10 77-78 Butanol dehydrogenase = NADPH-dependet
butanol dehydrogenase from C. saccharobutylicum
(Identities = 281/388(73%), Positives = 327/388 (85%), e-value = 2e-173)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
ATCC824 (Identities = 169/403 (42%), Positives = 240/403 (60%), e-value = 3e-
83)
Seq. ID 79-80 Butanol dehydrogenase = NADPH-dependet
butanol dehydrogenase from C. saccharobutylicum
(Identities = 264/388 (69%), Positives = 326/388 (85%), e-value = 5e-163)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. beijerinckii
NCIMB8052 (Identities = 169/410 (42%), Positives = 246/410 (60%), e-value =
4e-82)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
ATCC824 (Identities = 162/402 (41%), Positives = 240/402 (60%), e-value = 2e-
78)
Seq. ID 81-82 Butanol dehydrogenase = NADH-dependent
butanol dehydrogenase BdhA from C. acetobutylicum
ATCC824 (Identities = 161/388 (42%), Positives = 243/388 (63%), e-value = 7e-
92)
= NADH-dependent butanol dehydrogenase BdhB from C. acetobutylicum
ATCC824 (Identities = 155/389 (40%), Positives = 242/389 (63%), e-value = 4e-
85)
Seq. ID 83-84 Butanol dehydrogenase = NADPH-dependet
butanol dehydrogenase from C. saccharobutylicum
(Identities = 150/389 (39%), Positives = 233/389 (60%), e-value = 7e-73)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
ATCC824 (Identities = 154/412 (38%), Positives = 233/412 (57%), e-value = 8e-
70)
Seq. ID 85-86 Phosphate acetyl/butyryl = phosphate
butyryltransferase from C. acetobutylicum ATCC 824 (91/340(27%),
transferase Positives = 156/340 (46%), e-value = le-16)
Seq ID 87-88 Acetate/butyrate kinase = butyrate kinase from C.
acetobutylicum ATCC 824 (49/162 (31%), Positives =
77/162 (48%), e-value 5e-08)
Seq ID 89-90 Aldehyde:ferredoxin = aldehyde:ferredoxin oxidoreductase
from C. acetobutylicum ATCC 824 (188/631
oxidoreductase (30%), Positives = 318/631 (51%), e-value = 3e-
11)
Seq ID 91-92 Aldehyde:ferredoxin = aldehyde:ferredoxin oxidoreductase
from C. acetobutylicum ATCC 824
oxidoreductase (Identities = 191/633 (31%), Positives =
308/633 (49%), e-value = 2e-70)
Table 10: Genes of C. ragsdalei potentially conferring butyraldehyde and
butanol dehydrogenase
activity
Sequence Description Identity to characterized enzymes
Seq. ID 93-94 Bifunctional butanol/ = bifunctional
aldehyde/alcohol dehydrogenase AdhE2 from C. beiferinckii NCIMB
butyraldehyde dehydrogenase 8052 (Identities = 645/861 (75%), Positives =
751/861 (88%), e-value = 0.0)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
CA 02813431 2013-0 4 -18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 57 -
ATCC824 (Identities = 591/858 (69%), Positives = 731/858 (86%), e-value = 0.0)
Seq. ID 95-96 Bifunctional butanol/ = bifunctional
aldehyde/alcohol dehydrogenase AdhE2 from C. beijerinckii NCIMB
butyraldehyde dehydrogenase 8052 (Identities = 639/860 (75%), Positives =
752/860 (88%), e-value = 0.0)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
A1CC.2.24 (Identities = 591/85S169%), Positives = 735/858 (86%), e-value =
0.0)
Seq. ID 97-98 Butyraldehyde dehydrogenase = bifunctional aldehyde/alcohol
dehydrogenase AdhE2 from C. acetobutylicum
ATCC824 (Identities = 214/457 (47%), Positives = 294/457 (65%), e-value = Se-
111)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. beijerinckii
NCIMB
8052 (Identities = 200/457 (44%), Positives = 283/457 (62%), e-value = le-103)
= bifunctional aldehyde/alcohol dehydrogenase AdhEl from C. acetobutylicum
ATCC824 (Identities = 198/457(44%), Positives = 289/457(64%), e-value =4e-
101)
= butyraldehyde dehydrogenase Ald from C. beijerinckii NCIMB8052
(Identities = 125/409 (31%), Positives = 206/409 (51%), e-value = 3e-49)
= butyraldehyde dehydrogenase from C. saccharoperbutylacetonicum
(Identities = 123/409 (31%), Positives = 205/409 (51%), e-value = le-48)
Seq. ID 99-100 Butyraldehyde dehydrogenase = bifunctional aldehyde/alcohol
dehydrogenase AdhE2 from C. beijerinckii NCIMB
8052 (Identities = 188/477 (40%), Positives = 270/477 (57%), e-value = 9e-84)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
A1CC824 (Identities = 164/428 (39%), Positives = 256/428 (60%), e-value = le-
79)
Seq. ID 101- Butanol dehydrogenase = NADPH-dependet butanol
dehydrogenase from C. saccharobutylicum
102 (Identities = 285/388(74%), Positives = 335/388
(87%), e-value = 9e-177)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
ATCC824 (Identities = 164/396(42%), Positives = 238/396 (61%), e-value = le-
80)
Seq. ID 103- Butanol dehydrogenase =
NADPH-dependet butanol dehydrogenase from C. saccharobutylicum =
104 (Identities = 281/388 (73%), Positives =
327/388(85%), e-value = 2e-173)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
ATCC824 (Identities = 169/403 (42%), Positives = 240/403 (60%), e-value = 3e-
83)
Seq. ID 105- Butanol dehydrogenase = NADPH-dependet butanol
dehydrogenase from C. saccharobutylicum
106 (Identities = 264/388 (69%), Positives =
326/388(85%), e-value = 5e-163)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. beijerinckii
NCIMB8052 (Identities = 169/410(42%), Positives = 246/410 (60%), e-value =
4e-82)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
ATCC824 (Identities = 162/402 (41%), Positives = 240/402 (60%), e-value = 2e-
78)
Seq. ID 107- Butanol dehydrogenase = NADH-dependent butanol
dehydrogenase BdhA from C. acetobutylicum
108 ATCC824 (Identities = 162/388 (42%), Positives
= 243/388 (63%), e-value = 3e-
92)
= NADH-dependent butanol dehydrogenase BdhB from C. acetobutylicum
ATCC824 (Identities = 155/389(40%), Positives = 242/389 (63%), e-value = 6e-
85)
Seq. ID 109- Butanol dehydrogenase = NADPH-dependet butanol
dehydrogenase from C. saccharobutylicum
110 (Identities = 147/389 (38%), Positives =
227/389 (59%), e-value = 3e-71)
= bifunctional aldehyde/alcohol dehydrogenase AdhE2 from C. acetobutylicum
ATCC824 (Identities = 155/412 (38%), Positives = 233/412 (57%), e-value = 2e-
70)
Seq. ID 111- Phosphate acetyl/butyryl = phosphate butyryltransferase
from C. acetobutylicum ATCC 824 87/325 (27%),
112 transferase Positives = 148/325 (46%), e-value = 2e-16)
Seq ID 113- Acetate/butyrate kinase = butyrate kinase from C.
acetobutylicum ATCC 824 (Identities = 49/162 (31%),
114 Positives = 77/162 (48%), e-value 4e-11)
Seq ID 115- Aldehyde:ferredoxin = aldehyde:ferredoxin oxidoreductase
from C. acetobutylicum ATCC 824
116 oxidoreductase (Identities = 187/633 (30%), Positives =
319/633 (51%), e-value = 3e-74)
Seq ID 117- Aldehyde:ferredoxin = aldehyde:ferredoxin oxidoreductase
from C. acetobutylicum ATCC 824
118 oxidoreductase (Identities = 187/633 (30%), Positives =
302/633 (48%), e-value = le-69)
Gene expression studies
Gene expression studies were performed to confirm successful expression of
introduced Thiolase,
3-hydroxybutyryl-CoA dehydrogenase, Crotonase, Butyryl-CoA dehydrogenase,
Electron Transfer
Flavoprotein A and Electron Transfer Flavoprotein B genes in C.
autoethanogenum harboring
CA 02813431 2013-04-18
WO 2012/053905
PCT/NZ2011/000203
301946506_1.DOCX
- 58 -
butanol plasmid pMTL85245-thIA-crt-hbd. In addition, a selection of putative
butaraldehyde,
butanol dehydrogenase, phosphate acetyl/butyryl transferase acetate/butyrate
kinase,
aldehyde;ferredoxin oxidoreductase genes identified in the genome of C.
autoethanogenum
(Table 7) were also found to be expressed under standard fermentation
conditions (Figure 60).
A sample was harvested by centrifugation (6,000 x g, 5 min, 4 C). RNA was
isolated by suspending
the cell pellet in 100 pi of lysozyme solution (50,000 U lysozyme, 0.5 1.11
10% SDS, 10 mM Tris-HCI,
0.1 mM EDTA; pH 8). After 5 min, 350 1.11 of lysis buffer (containing 10 p.L
of 2-mercaptoethanol)
was added. The cell suspension was mechanistically disrupted by passing five
times through an
18-21 gauge needle. RNA was then isolated using PureLinkTM RNA Mini Kit
(Invitrogen) and eluted
in 100 1.11_ of RNase-free water. The RNA was checked via PCR and gel
electrophoresis and
quantified spectrophotometrically, and treated with DNase I (Roche) if
necessary. Quality and
integrity of RNA was checked using a BioAnalyzer (Agilent Technologies). The
reverse
transcription step was carried out using SuperScript III Reverse Transcriptase
Kit (Invitrogen). RT-
PCR reactions were performed in MyiQ Single Colour Real-Time PCR Detection
System (Bio-Rad
Labratories) in a reaction volume of 15 1.11. with 25 ng of cDNA template, 67
nM of each primer
(Tab. 11), and lx iQ SYBR Green Supermix (Bio-Rad Labratories, Hercules, CA
94547, USA).
Guanylate kinase and formate tetrahydrofolate ligase were used as housekeeping
gene and non-
template controls were included. The reaction conditions were 95 C for 3 min,
followed by 40
cycles of 95 C for 15 s, 55 C for 15 s and 72 C for 30 s. A melting-curve
analysis was performed
immediately after completion of the RT PCR (38 cycles of 58 C to 95 C at 1
C/s), for detection of
primer dimerisation or other artifacts of amplification.
mRNA for all heterologous genes could successfully be detected showing that
the genes are
expressed. The signal for all genes was on a similar level.
Tab. 11: Oligonucleotides for qRT-PCR
OligonucleotideSECLID
Target DNA Sequence (5 to 3')
Name NO.
GnK-F TCAGGACCTTCTGGAACTGG 131
Guanylate kinase
GnK-R ACCTCCCLI ii ICTTGGAGA 132
Formate tetrahydrofolate FoT4L-F CAGGTTTCGGTGCTGACCTA 133
ligase F0T4L-R AACTCCGCCGTTGTATTTCA 134
thIA-RT-F
TTGATGAAATGATCACTGACGGATT 123
Thiolase
thIA-RT-R GAAATGTTCCATCTCTCAGCTATGT 124
3-hydroxybutyryl-CoA hdb-RT-F
CATCACTTTCAATAACAGAAGTGGC 125
CA 02813431 2013-04-18
WO 2012/053905
PCT/NZ2011/000203
301946506_1.DOCX
- 59 -
dehydrogenase hbd-RT-R TACCTCTACAAGCTTCATAACAGGA 126
bcd-RT-F AAAATGGGTCAGTATGGTATGATGG 127
Butyryl-CoA dehydrogenase ________________________________________________
bcd-RT-R TGTAGTACCGCAAACCTTTGATAAT 128
Electron Transfer etfA-RT-F CAAGTTTACTTGGTGGAACAATAGC 129
Flavoprotein A etfA-RT-R GAGTTGGTCTTACAGTITTACCAGT 130
Bifunctional butanol/ adhE-RT-F CGGCTGO:CAAAAGAAATTTICTAGC 137
butyraldehyde
dehydrogenase (Seq. ID 39) adhE=IRT-R CCAGAACTCCGCAGGTCTTITCACCC 138
Butyra Id e hyde Bld1-RT-F GGCAGTAGAAGAAAGCGGAATGG 139
dehydrogenase (Seq. ID 41) Bldl-RT-R AAAGCCTGCATCTCTCTCTAAAACTCC 140
Butyraldehyde B1d2-RT-F TAATGATTTGCTCTCCATCCAAGAATC 141
dehydrogenase (Seq. ID 45)
Bld2-RT-R TCCGATTTCTTCCGCCATACG 142
Butanol Dehydrogenase (Seq. BDH1-RT-F AGCTGTAGTAGTTGTTGGAGGAGGA 143
ID 53) TCC
BDH1-RT-R CACAGACGGATCTGGTTCAACACC 144
Butanol Dehydrogenase (Seq. BDH2-RT-F GAATCTATTCAAC.ri I IAGAGCAAGT 145
ID 57) CACTGG
BDH2-RT-R CAACGGAACTTATTCCAGCMGC 146
Phosphate acetyl/butyryl Pta-RI-F GATGCTTTTTATGAATTGAGAAAGAA 147
transferase (Seq. ID 59) GAAGG
Pta-RT-R TGAAACCAATCCATCTGCATCTCC 148
Acetate/butyrate kinase (Seq. Ack-RT-F TGCAAGATGAAAGTGTTGTAGCAAA 149
ID 61) GG
Ack-RI-R ACTTTGTGGTCTTCCATTGGTTGC 150
Aldehyde:ferredoxin AOR1-RT-F CTTCAACAGGAAACAGATTCGAGAGC 151
oxidoreductase (Seq. ID 63) AOR1-RT-R CCAACACCACCACGTCCTGC 152
Aldehyde:ferredoxin AOR2-RT-F GGTTGGGATATGATAATAGTAGAGG 153
oxidoreductase (Seq. ID 65) ATAAGGC
AOR2-RT-R GTAALI ii ICCCCAAAGCTGTGACG 154
The invention has been described herein, with reference to certain preferred
embodiments, in
order to enable the reader to practice the invention without undue
experimentation. However, a
person having ordinary skill in the art will readily recognise that many of
the components and
parameters may be varied or modified to a certain extent or substituted for
known equivalents
without departing from the scope of the invention. It should be appreciated
that such
modifications and equivalents are herein incorporated as if individually set
forth. Titles, headings,
or the like are provided to enhance the reader's comprehension of this
document, and should not
be read as limiting the scope of the present invention.
CA 02813431 2013-09-23
WO 2012/053905 PCT/NZ2011/000203
- 60 -
The scope of the claims should not be limited by specific embodiments and
examples
provided in the disclosure, but should be given the broadest interpretation
consistent with
the disclosure as a whole.
Throughout this specification and any claims which follow, unless the context
requires otherwise,
the words "comprise", "comprising" and the like, are to be construed in an
inclusive sense as
opposed to an exclusive sense, that is to say, in the sense of "including, but
not limited to".
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 61 -
References:
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local
alignment search tool J Mol
Biol 215: 403-410.
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K
(1987) Current
protocols in molecular biology. John Wiley & Sons, Ltd., Hoboken, NJ.
Bertram J, Durre P (1989) Conjugal transfer and expression of streptococcal
transposons in
Clostridium acetobutylicum. Arch Microbiol 151: 551-557.
Biebel (2001). Journal of Industrial Microbiology & Biotechnology 27,18-26.
Burchhardt G and Diirre P (1990) Isolation and characterization of DNase-
deficient mutants of
Clostridium acetobutylicum. Curr Microbiol 21: 307-311.
Carbone A, Zinovyev A and Kepes F (2003) Codon adaptation index as measure of
dominating
codon bias. Bioinformatics 19: 2005-2015.
Drake HL, Kase' K, Matthies C, Acetogenic prokariotes. In: Dworkin M,
Rosenberg E, Schleifer KH,
Stackebrandt E The Prokaryotes, 3rd edition, vol. 2 (Ecophysiology and
Biochemistry). Springer,
NY: 354-420.
Finn RD, Mistry J, Tate J, Coggill P. Heger A, Pollington JE, Gavin OL,
Gunasekaran P, Ceric G,
Forslund K, Holm L, Sonnhammer EL, Eddy SR, Bateman A (2010) The Pfam protein
families
database. Nucleic Acids Res 38: D211-222.
Haft DH, Selengut DH, White 0 (2003) The TIGRFAMs database of protein
families. Nucleic Acids
Res 31: 371-373.
Heap JT, Pennington OJ, Cartman ST, and Minton NP (2009) A modular system for
Clostridium
shuttle plasmids. J Microbiol Methods 78: 79-85.
Hub o N, Bairoch A, Bulliard V. Cerutti L, Cuche BA, de Castro E, Lachaize C,
Langendijk-Genevaux
PS, Sigrist CJ (2008) The 20 years of PROSITE Nucleic Acids Res 36: D245-249.
Hungate RE (1969) A roll tube method for cultivation of strict anaerobes, in
Norris JR and Ribbons
DW (eds.), Methods in Microbiology, vol. 3B. Academic Press, New York, NY: 117-
132.
Inui, et al. Appl Microbiol Biotechnol (2008) 77:1305-1316
Kopke M, Durre P (2010) Biochemical production of biobutanol, in Luque R,
Campelo J, Clark JH
(Eds.): Handbook of biofuel production - Processes and technologies, Woodhead
Publishing,
Camebridge, UK: 221-257.
Liou 1S, Balkwill DL, Drake GR, Tanner RS (2010) Clostridium carboxidivorans
sp. nov., a solvent-
producing clostridium isolated from an agricultural settling lagoon, and
reclassification of the
acetogen Clostridium scatologenes strain SL1 as Clostridium drakei sp. nov.
Int J Syst Evol
MicrobioL 55: 2085-2091.
CA 02813431 2013-04-18
WO 2012/053905 PCT/NZ2011/000203
301946506_1.DOCX
- 62 -
Mermelstein LD, Papoutsakis ET (1993) In vivo methylation in Escherichia coil
by the Bacillus
subtilis phage CD3TI to protect plasmids from restriction upon transformation
of Clostridium
acetobutylicum ATCC 824. Appl Environ Microbial 59:1077-1081.
Noack S, Kopke M, Durre P (2009) Microbially produced fuels and other
biofuels, in Wright JH,
Evans DA (Eds.): New research on Biofuels, Nova Publishers, Haupage, NY: 17-
30.
Heiskanen H, Virkajarvib I, Viikarib L (2007) The effect of syngas composition
on the growth and
product formation of Butyribacterium methylotrophicum. Enz Microbial Technol
41: 362-367.
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A laboratory
Manual, Cold Spring
Harbour Labrotary Press, Cold Spring Harbour, NY.
Tatusov RL, Fedorova ND, Jackson JD, Jacobs AR, Kiryutin B, Koonin EV, Krylov
DM, Mazumder R,
Mekhedov SL, Nikolskaya AN, Rao BS, Smirnov S, Sverdlov AV, Vasudevan S, Wolf
YI, Yin JJ, Natale
DA (2003) The COG database: an updated version includes eukaryotes. BMC
Bioinformatics 4: 41.
Tsai MH and Saier. Jr. MH (1995) Phylogenetic characterization of the
ubiquitous electron transfer
flavoprotein famliles ETF-a and ETF-13. Res. Microbio.I 146: 397-404.
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991). J Bacterial. 173: 697-
703.
WeiRermel K, Arpe HJ (2003) Industrial organic chemistry, 4th edition, Wiley-
VCH Verlag, GmbH &
Co. KGaA, Weinheim, Germany.
Wolfe RS (1971) Microbial formation of methane. Adv Microb Physiol 6: 107-146.