Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 2967482 2017-05-17
HAND-HELD DEVICE AND COMPUTER-IMPLEMENTED SYSTEM AND METHOD
FOR ASSISTED STEERING OF A PERCUTANEOUSLY INSERTED NEEDLE
TECHNICAL FIELD
The present invention relates to a hand-held device and computer-implemented
systems and methods for real-time assisted steering of percutaneously inserted
needles, such
as may be used during prostate brachytherapy.
BACKGROUND OF THE INVENTION
Prostate brachytherapy is an effective treatment for prostate cancer due to
its
excellent success rates, favorable toxicity profile, and non-invasiveness. In
conventional
prostate brachytherapy, a surgeon inserts a long, flexible needle loaded with
radioactive
seeds through the perineum into the patient's body. With the guidance of a
grid template and
ultrasound images generated by a transrectal probe, the surgeon manually
steers the needle
toward preplanned locations in the prostate gland and then deposits the
radioactive seeds.
The radioactive seeds emit radiation to kill the tumor cells in a short
vicinity of the treated
area, while minimizing radiation exposure to adjacent critical structures.
Accurate radioactive seed placement is critical to effective prostate
brachytherapy. In
practice, however, the needle may not travel on the planned straight path,
resulting in
deviations of actual radioactive seed placement from the planned locations.
Factors
contributing to such imprecision include prostate gland tissue deformation
and/or needle
deflection. In regard to the latter factor, brachytherapy needles typically
have a beveled tip
to facilitate cutting through patient tissue. However, asymmetrical forces act
on the beveled
needle tip, causing it to deflect from the planned straight path. Previous
work has shown that
seeds can be placed with an average absolute accuracy of 5 mm, which is more
than 10% of
the average prostate gland diameter [4]. This substantial error narrows the
scope of
brachytherapy to primarily treating the entire prostate gland for patients
with localized
prostate cancer.
1
CA 2967482 2017-05-17
To address the need for needle targeting accuracy, fully automated robotic
systems
have been developed to automatically insert a needle and control its
trajectory towards target
locations in the prostate (see [5] to [12]). To create the steering effect,
these systems rotate
the needle base to change the orientation of the beveled tip and consequently
the direction of
the resultant force is then used to control the direction of needle
deflection. However, to
date, no such system has been deployed in clinical practice due to
complexities and
e
significant modifications that would be necessary in the procedure and the
operating room.
SUMMARY OF THE INVENTION
An object of the present invention is to provide real-time assistance to a
surgeon to
precisely, efficiently, and intuitively position a needle percutaneously
inserted in a patient to
achieve a target needle trajectory, while allowing the surgeon to maintain at
least partial
manual control over needle insertion.
It will be understood that the present invention is used with a needle
extending axially
between a proximal end and a distal end comprising a beveled needle tip.
In one aspect, the present invention comprises a hand-held device for assisted
steering
of a percutaneously inserted needle. In embodiments, the device comprises:
(a) a handle for manual gripping of the device by a user of the
device;
(b) an actuation unit attached to the handle, the actuation unit
comprising:
(i) a rotary actuator for axially rotating the needle relative to the
handle;
(ii) an axial actuator for inducing axial micro-vibrations of the needle
relative to the handle;
wherein the rotary actuator and the axial actuator are simultaneously operable
to simultaneously axial rotate the needle relative to the handle and induce
axial micro-vibrations of the needle relative to the handle; and
(c) a haptic feedback unit for inducing vibrations in the handle.
2
CA 2967482 2017-05-17
In embodiments of the device, the device may further comprise a sensor unit
comprising at least one sensor attached to the handle for generating, in
response to movement
of the device, an electronic signal indicative of a needle insertion parameter
comprising one
or a combination of the needle position, a needle orientation, a needle axial
rotation angle, a
needle velocity, and a needle acceleration. In embodiments of the device, the
at least one
sensor may comprise one or a combination of an accelerometer and a gyroscopic
sensor.
In another aspect, the present invention comprises a computer-implemented
system
for assisted steering of a percutaneously inserted needle. In embodiments, the
system
comprises:
(a) a hand-held device comprising:
(i) a handle for manual gripping of the device by a user of
the device;
(ii) an actuation unit attached to the handle, the actuation
unit comprising:
(1) a rotary actuator for axially rotating the needle relative to the
handle;
(2) an axial actuator for inducing axial micro-vibrations of the
needle relative to the handle,
wherein the rotary actuator and the axial actuator are simultaneously
operable to simultaneously axial rotate the needle relative to the handle
and induce axial micro-vibrations of the needle relative to the handle;
e
(iii) a haptic feedback unit for inducing vibrations in the
handle;
(b) a sensor unit comprising at least one sensor for generating, in
response to
movement of the device, an electronic signal indicative of a needle insertion
parameter comprising one or a combination of the needle position, a needle
orientation, a needle axial rotation angle, a needle velocity, and a needle
acceleration;
(c) a display device; and
(d) a computer operatively connected to the device and the display
device, the
computer comprising a processor and a memory comprising a non-transitory
computer readable medium storing instructions executable by the processor to
3
CA 2967482 2017-05-17
implement, in real-time, with the insertion of the needle, a method comprising
the steps of:
(i) determining a location of a portion of the needle;
(ii) calculating a needle insertion parameter comprising one or a
combination of a needle position, a needle orientation, a needle axial
rotation angle, a needle velocity, and a needle acceleration, wherein
the calculating is based on an electronic signal from the sensor unit;
(iii) calculating a needle shape and a needle position, wherein the
calculating is based on one or a combination of the determined
location of the portion of the needle and the calculated needle insertion
parameter;
(iv) displaying on the display device one or a combination of the
calculated
needle shape, the calculated needle position, and the calculated needle
insertion parameter;
(v) calculating a
correction to the needle insertion parameter for a target
needle trajectory, wherein the calculating is based on one or a
combination of the calculated needle shape, the calculated needle
position, and the calculated needle insertion parameter, and wherein
the correction to needle insertion parameter comprises at least either a
correction to the needle axial rotation angle or a needle rotation depth
paired with a discrete needle axial rotation angle;
(vi) controlling the rofarY actuator of the device to axially rotate
the needle
by either the correction to the needle axial rotation angle or by the
discrete needle axial rotation angle at the paired needle rotation depth;
(vii) activating the haptic feedback unit of the device to vibrate the handle
of the device in a vibration pattern, wherein the vibration pattern is
determined by a rules database depending on one or a combination of
the calculated needle shape, the calculated needle position, and the
calculated correction to the needle insertion parameter; and
(viii) repeating steps (i) to (vi).
4
CA 2967482 2017-05-17
In embodiments of the system, the sensor of the sensor unit is attached to the
handle
and comprises at least one or a combination of an accelerometer and a
gyroscopic sensor.
In embodiments of the system, the sensor unit comprises a camera for tracking
the
position of the device.
In embodiments of the system, the step of determining the location of the
portion of
the needle comprises processing an ultrasound image of the needle.
e t6
BRIEF DESCRIPTION OF DRAWINGS
In the drawings, like elements are assigned like reference numerals. The
drawings are
not necessarily to scale, with the emphasis instead placed upon the principles
of the present
invention. Additionally, each of the embodiments depicted is but one of a
number of
possible arrangements utilizing the fundamental concepts of the present
invention. The
drawings are briefly described as follows:
Figures IA and 1B are a distal perspective view and a proximate perspective
view,
respectively, of an embodiment of a hand-held device of the present invention.
Figure 2 is a side cross-sectional view of another embodiment of a hand-held
device
of the present invention, similar to the device shown in Figures IA and 1B.
Figure 3A and 3B are an assembled perspective view and an exploded perspective
view, respectively, of an actuation unit of the device shown in Figure 2.
Figure 4 (PRIOR ART) is a depiction of a cantilever compliant beam model of a
needle used to predict needle deflection in a needle-tissue interaction model.
Figure 5 is a series of graphs showing: (a) control variable u(d) as a
function of the
needle rotation depth dr; (b) and (c) the cost functions J penalizing needle
targeting error for
5
CA 2967482 2017-05-17
Case 1 and Case 2, respectively; (d), (e) and (f) hypothetical examples of
candidate rotation
points within the control horizon [d, did and the resultant deflection in [d,
dd.
Figure 6 is a series of graphs showing: (a) and (b) the optimal rotation depth
for Case
1 for df = 130 and df = 150 mm, respectively; (c) and (d), the depth of first
(d1) and second
(d2) rotation that minimizes the cost function for Case 2 for df = 150 mm.
Figures 7A-D show an overview of the needle steering controller, with Figure
7A
showing the block diagram of the needle steering system; in Figure 7B, the RRT
algorithm
evaluates the needle targeting accuracy, for different rotation depths as
shown in Figure 7C;
in Figure 7D, the resultant set of rotation depths.
Figure 8A is a schematic block diagram of an embodiment of the computer-
implemented system of the present invention, in relation to a surgeon, a
patient, a needle, and
a transrectal ultrasound probe.
Figure 8B shows: (a) a schematic depiction of a system for determination of
needle
shape and position using a partial sagittal ultrasound image observation; and
(b) experimental
results for two different phantom tissues.
Figure 8C shows: (a) a schematic depiction of system for determination of
needle
shape and position using a series of transverse ultrasound images; (b) needle
shape
estimation results; and (c) needle tip tracking experimental results.
Figure 9 depicts an experimental set up for testing an embodiment of a
prototype of
the computer-implemented system of the present invention.
Figure 10 is a schematic block diagram of the embodiment of the prototype
system of
Figure 9.
6
CA 2967462 2017-05-17
Figure 11 is a graph showing measured needle-tissue axial insertion forces for
different vibration frequencies applied to the needle shaft in experiments
conducted with the
prototype system of Figure 9.
Figure 12 is a series of graphs showing for experiments with the prototype
system of
Figure 9 with different tissue samples: (top, first panel) the measured needle
tip deflection
and the predicted needle tip deflection using the identified model parameters;
(middle,
second panel) the error between the predicted and measured needle tip
deflection; and
(bottom, third panel) the observed tip force.
Figure 13 shows model fit results thr each tissue sample. The model parameters
are
found by minimizing the difference between the measured and estimated needle
tip
deflection at the depth of 140 mm.
Figure 14 shows the path followed by the needle tip in the X and Y planes
(defined
in Figure 9) during insertion in porcine, bovine, and synthetic tissue and the
average position
of the bevel angle using open loop (a) and closed loop (b) controllers, for
each of the 15
insertions. Only the deflection in the X is controlled.
Figure 15A shows the seed tracking routine in ultrasound images. Figure 15B
shows
the image processing. Ultrasound images captured during a Phase 3 showing the
last
implanted seed to be localized, with the tracking algorithm steps shown
underneath.
Figure 16 shows experimental results of seed deposition following a
hypothetical pre-
planning. The solid gray dot indicates the seed target location. The blue
circle is the position
of the needle tip at the target depth, and the dark square shows the final
position of the
centroid of each seed after the needle is withdrawn.
Figure 17 shows dummy seed displacement from the deposition location during
needle withdrawal in each tissue with open loop (left) and closed loop (right)
needle steering
controllers.
7
CA 2967482 2017-05-17
DETAILED DESCRIPTION
The present invention relates to a hand-held device for real-time assisted
steering a
percutaneously inserted needle, and related computer-implemented systems and
methods.
Any term or expression not expressly defined herein shall have its commonly
accepted
definition understood by a person skilled in the art. As used herein, the
"real time" in
describing a series of steps means that the steps are completed within a time
period that is, in
embodiments, within about 0.1 seconds, 0.2 seconds, 0.5 seconds, 1 second, or
5 seconds or
seconds.
10 Needle and Stylet
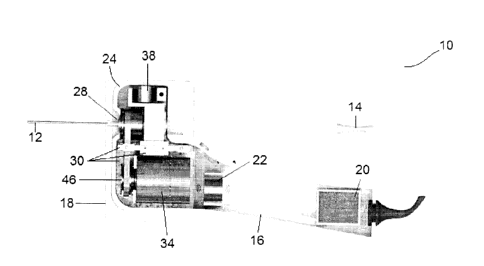
Referring to Figures IA and 1B, it will be understood that the device 10 of
the present
invention may be used with an elongate coriventional brachytherapy needle 12
with a needle
shaft having a needle hub, and extending axially between a proximal end and a
distal end
comprising a beveled needle tip. In an exemplary embodiment, the needle 12 may
be a
standard 18-gauge hollow brachytherapy needle. The needle 12 is loaded with
radioactive
seeds in the needle lumen, and a stylet 14 inserted into the needle lumen. As
is known to
those skilled in the art, a surgeon pushes the device 10 towards a patient to
insert the needle
12 until a target depth is reached, whereupon the surgeon retains the stylet
14 in place while
retracting the device 10 and the needle 12 so that the stylet 14 pushes the
radioactive seeds
from inside the needle 12 out of the needle lumen and deposits them into the
prostate gland.
As the beveled needle tip cuts through tissue, tissue displacement at the edge
of the bevel
needle tip creates a resultant force normal to the needle shaft that causes it
to bend on a
curved trajectory. Hence, changing the orientation of the beveled needle tip
by axial rotation
of the needle base changes the direction of the force applied at the beveled
needle tip,
causing the needle 12 to bend in a different direction. Thus, a proper
combination of needle
translation and axial rotation can force the needle tip to follow a desired
trajectory [15, 16].
Hand-held device
Figures 1A, 1B and 2 show an exemplary hand-held device 10 of the present
invention with a conventional needle 12 and stylet 14. In an exemplary
embodiment, the
device 10 is constructed so as to weigh less than about 0.16 kg (0.35 lb.),
facilitating its use
8
CA 2967482 2017-05-17
e
for conventional needle insertion techniques as described above. In the
exemplary
embodiment, the device 10 comprises a handle 16 with an attached actuation
unit 18, sensor
unit 20, and haptic feedback unit 22, as are described below. In other
exemplary
embodiments, the sensor unit 20 may be partially or wholly detached from the
hand-held
device 10.
Handle
A purpose of the handle 16 is to provide a member that may be manually gripped
by a
surgeon so as to allow the surgeon to maintain manual control over the needle
insertion
depth. In the exemplary embodiment shown in Figures 1A, 1B and 2, the handle
16 is a
substantially cylindrical member having a length of approximately 140
millimeters, and a
diameter of approximately 30 millimeters. The handle 16 may be contoured with
ridges that
engage the surgeon's fingers so as to prevent slipping of the device in the
surgeon's hands.
The handle 16 may also be provided with control knobs. In the exemplary
embodiment, the
handle 16 is 3D-printed from plastic and extends into a monolithically printed
housing 24
that encloses the actuation unit 18, haptic feedback unit 22 and sensor unit
20, thereby
attaching these components to the handle 16. The housing 24 defines an
aperture 26 that
allows for through passage of the needle 12 and the stylet 14. In other
exemplary
embodiments (not shown), the handle 16 may have a different configuration and
be made of
other materials, and manufactured using different processes, known in the art.
Actuation unit
In the exemplary embodiment shown in Figures 3A and 3B, the actuation unit 18
comprises a needle holder 28, support structure 30, a rotary bearing assembly
32, a rotary
actuator 34, a rotary encoder, drivetrain 36, and an axial actuator 38, all of
which are
operatively connected to an electrical povyer source such as an electric power
cord or an
electrochemical battery. It will be appreciated that the configuration of the
actuation unit 18
allows for simultaneous operation of the rotary actuator 34 and the axial
actuator 38 so as to
simultaneously rotate the needle 12 about its axis, and to vibrate the needle
12 in the axial
direction.
9
CA 2967482 2017-05-17
e
Needle holder
A purpose of the needle holder 28 is to attach the needle 12 to the other
components
of the actuation unit 18. In the exemplary embodiment shown in Figures 3A and
3B, the
needle holder 28 comprises a threaded tubular member that extends through the
aperture 26
of the housing 24. In use, the needle 12 is inserted through the needle holder
28 so that the
needle hub 40 is placed within the needle holder 28, whereupon the needle
holder 28 may be
secured to the needle 12 by a quick-snap (and quick-release) mechanism.
Support structure
A purpose of the support structure 30 is to provide a member or members for
attachment of the actuation unit 18 directly or indirectly to the handle 16.
In the exemplary
embodiment, the support structure 30 comprises a pair of plates 42 and
plurality of linear
rails 44. The outward facing surfaces of the plates 42 attach to the inside of
the housing 24. It
will be understood that in Figures 3A and 3B, one of the plates 42 has been
removed to show
the components of the actuation unit 18. The inward facing surfaces of the
plates 42 attach to
miniature linear rails 44, which in turn provide mounting points for the other
components of
the device 10. The needle holder 28 and housing assembly 24 slide on two of
miniature linear
rails 44 such that they can translate axially ¨ that is, in the direction of
needle insertion.
Rotary bearing assembly
A purpose of the rotary bearing assembly 32 is to permit axial rotation of the
needle
holder 28 (and hence the needle 12) relative to the handle 16. In the
exemplary embodiment,
the rotary bearing assembly 32 comprises an outer race, an inner race, and
bearing elements
(concealed from view). The outer race is secured to the support structure 30.
The inner race is
secured circumferentially around the needle holder 28. The bearing elements
permit the inner
race (and hence the needle holder 28) to rotate axially relative to the outer
race (and hence
the support structure 30 and handle 16).
4
Rotary actuator
A purpose of the rotary actuator 34 is to axially rotate the needle 12 in a
controlled
manner. In the exemplary embodiment, the rotatory actuator 34 comprises an
electric motor,
CA 2967482 2017-05-17
a drivetrain 36, and a rotary encoder. A pi-pose of the electric motor is to
convert electrical
energy from the electrical power source to rotation of a motor rotor. In an
exemplary
embodiment, the motor is a DC motor model 26195024SR from FauihaberTM
(Croglio,
Switzerland), having embedded reduction gears with a 33:1 reduction ratio, and
powered by
a L298N PMW drive.
Drivetrain
A purpose of the drivetrain 36 is to transmit rotation of the motor rotor to
axial
rotation of the needle holder 28. In the exemplary embodiment, the drivetrain
36 comprises a
first pulley 46, a second pulley 48, and a belt 50. The first pulley 46 is
attached to the motor
rotor for rotation therewith. The second pulley 48 is attached to the needle
holder 28. The
belt 50 is looped over the first pulley 46 and the second pulley 48 to
transmit rotation of the
first pulley 46 to the second pulley 48. The distance separating the needle 12
and the motor
shafts plus half of the circumference of each pulley 46, 48 gives the length
of the belt 50
around the pulleys 46, 48. In order to allow for simultaneous needle rotation
and some small
longitudinal relative translation of the pulleys 46, 48, a 2 mm clearance is
added to the length
of the belt 50.
Rotary encoder
A purpose of the rotary encoder is to generate electronic signals that are
indicative of
the angular rotational position of the motor rotor (and hence the angular
rotation position of
the needle holder 28 and the needle 12). Rotary encoders are electromechanical
devices that
are well known to persons skilled in the art. A variety of different types of
rotary encoders
may be suitable for use with the device 10. In the exemplary embodiment, the
encoder is an
incremental encoder with 16 pulses per revolution connected to the gear of the
electric model
that permits the angular position of the needle shaft to be measured with 0.1
degree accuracy.
In other embodiments, electromechanical means other than a rotary encoder may
be used to
measure the angular rotational positiou a the needle 12 by detecting the
position of the
motor drive shaft or the needle holder 28. Such devices may include with
limitation, a
synchro transducer, an electrical resolver transformer, a rotary variable
differential
transformer, or potentiometers.
11
CA 2967482 2017-05-17
Axial actuator
A purpose of the axial actuator 38 is to induce high-frequency, axial micro-
vibrations
in the needle 12. As used herein, the term "micro-vibrations" refers to
oscillations that are
less than or equal to about 0.04 millimeters in amplitude. The reason for
inducing micro-
vibrations is that translational friction along the needle shaft can be
reduced by modulating a
vibratory low-amplitude displacement onto a regular needle insertion profile
[17]. This can
make the needle insertion easier for the surgeon and potentially reduce tissue
deformation. In
addition, the micro-vibrations can allow for easy detection of the needle tip
under Doppler
ultrasound imaging [18]. The axial actuator 38 may comprise any electro-
mechanical
transducer that is suitable for converting electrical signals to oscillating
movements that will
generate axial micro-vibrations in the needle holder 28. In an exemplary
embodiment, the
axial actuator 38 is an amplified piezoelectric actuator (APA6OS from Cedrat
TechnologiesTm, Meylan, France), powered by a piezo-electric drive (PDm200,
PiezoDriveTM, Callaghan, Australia).
0 4%
Sensor unit
A purpose of the sensor unit 20 is to generate electronic signals in real-time
that are
indicative of, or may be used to derive, the position, velocity, acceleration
and orientation of
the device 10. In an exemplary embodiment, the sensor unit 20 is attached to
the device 10
and may comprise one or more types of sensors for measuring the position or
motion of the
device 10, including without limitation inertial sensing technologies such as
accelerometers
and gyroscopes. Accelerometers and gyroscopic sensors are well known to
persons skilled in
the art. A variety of different types of accelerometers and gyroscopic sensors
may be suitable
for use with the device 10, including without limitation micro-electronical
systems (MEMS)-
based accelerometers and gyroscopic sensors.
In other embodiments, the sensor unit 20 may be at least partially detached
from the
hand-held device 10. For instance, in the exemplary embodiment of the
prototype system
described in Example 1, the sensor unit 20, implements optical tracking
technology wherein
the senor unit 20 comprises cameras that detect the movement of tracking
markers attached
to the handle 16. Optical sensing technology is well known to person skilled
in the art.
12
CA 2967482 2017-05-17
In other embodiments, the sensor unit 20 may be embedded within the hand-held
device 10. For instance, in the exemplary embodiment of the prototype system
described in
= a
Example 2, a compression/traction sens i
or s embedded in the hand-held device 10 to measure
the axial force applied to the needle base during insertion and withdrawal
(model LSB200 S-
Beam from Futek, Irvine, USA). The force measurements from two 1-DOF force
sensors
during needle insertion and withdrawal may be used to estimate the forces
applied by the
tissue onto the needle tip, such that future needle deflection can be
predicted by a mechanics-
based model and the necessary corrective action taken by the hand-held device
10.
Haptic feedback unit
A purpose of the haptic feedback unit 22 is to induce vibrations in the handle
16 so as
to provide the surgeon with tactile alerts of the need or lack of need for
corrective
maneuvering of the device 10. The haptic feedback unit 22 may comprise any
electro-
mechanical transducer that is suitable for converting electrical signals to
oscillating
movements that will induce vibrations in the handle 16 that can be sensed by
the surgeon.
For example, electro-mechanical transducers used in smart phones to create
vibration alerts
s
may be suitable for use in the haptic feedback unit 22.
Needle ¨ Tissue Interaction Model
An object of the computer-implemented system and method of the present
invention
is to determine control commands to be applied to the device so that the
needle moves along
a target needle trajectory. This requires a needle-tissue interaction model to
predict needle
deflection. It will be appreciated that needle-tissue interaction models other
than the
particular models 1 and 2 described below may be suitable for use with the
present invention
so long as the model allows the calculation of the deflected shape of the
needle. In the
exemplary embodiment, the objective is to develop a model that can be entirely
identified
using only 2D ultrasound images of the needle in tissue, which are often
available in clinical
settings. In prostate brachytherapy, the needle ideally follows a straight
line trajectory.
Hence, and as there is no need to generate 3D trajectories, the model may be
limited to planar
needle deflections. .
13
CA 2967482 2017-05-17
i. Needle ¨ Tissue Interaction Model 1
In order to predict needle deflection during insertion, the needle is modelled
as a
cantilever compliant beam that undergoes forces applied by the tissue as shown
in Figure 4.
According to the Galerkin-Bubnov method [19], beam deflection can be
approximated as the
sum of n candidate shape functions (eigenfunctions), each of which represents
a mode of
vibration. The deflection v(d,z) of a needle at a point z along its shaft and,
for a given
insertion depth d in this case, can be defined as
v(d, Eqi(z)ili(d) (1)
i. .o
where q,(z) is the displacement of the needle (deflection) at each point z
along its shaft and
g(d) is a weighting coefficient (eignenvalue) for each of the n assumed
vibration modes. The
eingenfunctions q,(z) must satisfy the boundary conditions of a cantilever
beam and be
differentiable at least up to the highest order of the partial differential
equations of the beam.
For a cantilever beam of length L, the deflection can be given by [19]
q(z) = ¨1 ¨ sinhOz) ¨ (c(s 4;1)
cush4(z)1]
(12)
t
here
4(:) = (3)
and the constants yi and ki are computed as
?lilt
(11.- 4 eu-li (4)
Ki .), -.1.1111 . ¨ -1(cos -- cosh '10
The values of the constants J3i for a clamped-free beam are 131 = 1.857, 132 =
4.695, 133
= 7.855, 04 = 10.996, and 13, ¨ 1/2) for i > 4 [19]. At this stage the
assumed
displacement functions are entirely parametrized. In the following, it is
demonstrated that the
weighting coefficients g(d) can be given as functions of the needle-tissue
interaction forces
such that the system reaches equilibrium.
a 0
14
CA 2967482 2017-05-17
A. Needle-Tissue Equilibrium
To calculate the weighting coefficients g,(d), a variational method known as
the
Rayleigh-Ritz method is used in which equilibrium of the system is established
using the
principle of minimum potential energy. This approach has been previously
employed to
estimate needle deflection in [20]. In the present invention, the tissue model
accounts for
unlimited number of needle rotations while accounting for tissue displacement.
In addition,
the mathematical approach reduces the model to a simple system of linear
equations, making
it computationally efficient, and enabling it to be parametrized using only
ultrasound images
of the needle during insertion.
The coefficients g(d) must minimize the system potential I1(d) defined by
Bid) U(d) + 1-7(d) (5)
where U (d) is the total stored energy in the system and V (d) is the work
done by
conservative forces. The expressions for the potential energy and the work for
the needle-
tissue system are now derived.
As said earlier, as the needle tip cuts through the tissue, the bevel creates
a resultant
normal force F at the needle tip (see Figure 4). Other forces applied at the
bevel will be
neglected as they mostly induce axial compression of the needle. As the needle
bends, the
work due to F is
1r (
which is added to 11(d) in (5). The bending strain energy stored in the needle
as a result of
deflection is
ub(d) irkd.
dz, (7)
2 0 0,72
where E and 1 are the needle Young's modulus of elasticity and its second
moment of inertia,
respectively.
CA 2967482 2017-05-17
In brachytherapy, the needles are inserted through a guiding template to help
guide
the needle towards a target and to minimize deflection outside tissue. The
target is usually
defined on a straight line from the needle location in the template to a
desired depth in tissue.
The template is modelled as a rigid spring of stiffness Kp >> 0, which has no
thickness. The
spring is connected to the needle shaft at a distance of zt from the needle's
base with zt = L ¨
d ¨ ct, where ct is the distance from the template to the tissue surface (see
Figure 4). The
potential energy stored in the template is
1
(d) = ¨K v(d,zt)2. (8)
" 2 P
As the needle bends, the shaft moves and deforms the surrounding tissue. In
turn, the
compressed tissue applies forces to the needle shaft. Assuming small local
magnitude and
deformation velocity of the tissue, it is reasonable to assume that the tissue
is a purely elastic
medium. Thus, the force applied to the needle at a certain point along the
shaft becomes
proportional to the tissue displacement at that point. If vt(z) is the initial
position of the
uncompressed tissue, the tissue reaction force is K(v(d, z) ¨ vt(z)), where K
is the stiffness of
the tissue per unit length of the needle and vt(z) is the path cut by the
needle tip. Therefore,
the energy due to tissue compression is
trt(d) ¨K v . z) ¨ vild . 11',k, (9)
2 L
As the model essentially compares the current needle shape with the path cut
by the
needle tip, it can automatically account for an unlimited number of needle
rotations,
B. Calculating the Eigenvalues g(d)
Now that all the components of the system potential fl(d) have been defined,
the
weighting coefficients g1(d) can be calculated using the principle of minimum
potential
energy. According to the Rayleigh-Ritz method [21], the coefficients g1(d)
must give 611, = 0
for any values of 6g, where 6 denotes infinitesimal difference. Therefore,
g(d) must satisfy:
(d)
ti _____________________ rb + + ET/ +v) =o (10)
Ogt( d) (d)
16
, a
CA 2967482 2017-05-17
Replacing (6)-(9) in (10) and taking the partial derivative with respect to
g(d) yields
E I ,, d) iii(z)dz
. i 1
( Pi
+ kp Eqi(zOgi(d) th(zt)
(111
L. ( ri
Ik' Eq,,(z),i, (d)) eti(v)(1z
I".: -il I I
¨ K iI,
rt (d. z)qi( z)dz = F.
L =d
where the double dot denotes the second derivative of q,(z) with respect to z.
In order to isolate the weighting coefficients g1(d) in the previous equation,
four
supplementary variables are created and defined as follows:
L I.
op =(z iiii (z)dz, Lep .
f if fii (Z. j lb (Z jdz ,
o L rI
I. (12)
7.0 r----- (ii (-It )(b. (24) , (/), =f
, -rf
After some straightforward manipulation, the previous equation rearranges as
E igi (d)(EI Op +K wit + K)1 - . F (13)
This equation shows that the model has been reduced to a system composed of n
linear equations. This is the closed form solution through which the
coefficients g(d) are
found in order to calculate the needle deflection given in (1).
C. Tip Force Estimator
The proposed model requires only two input parameters, i.e., the tissue
stiffness K
and the force at the needle tip F. The first one can be obtained
experimentally by model
fitting and can be considered to be constant throughout the insertion. To
identify the second
parameter, an observer is developed in order to calculate the force F as the
needle is inserted.
To this end, it is assumed that the deflection of the needle tip can be
acquired from
17
. µ
CA 2967482 2017-05-17
ultrasound images of the needle in tissue, that will be referred to as vL.
Therefore, from (1),
and knowing that q1(L) = 1 Vi, it yields:
= g(d) + 92(d) + gn(d) = (14)
Now, adding this equation to the system of n equations given in (13), results
in a
system of n + 1 expressions with only One`unknown parameters (i.e., the tissue
stiffness K).
Hence, the coefficients g(d) and the force applied at the needle tip at every
insertion depth d,
are given by combining (13) and (14) to form the new system of equations
expressed in
matrix form as follows:
(d)-
k
=(15)
gra(d) LIT 4- KC + õI'
F
where the matrices Co, F, (1) and A are given by
/011 vir, () tJ-
40 = = = !.! = .
I On' = = , /4:7$9.n WIt I = LA.'n,n,
o
.= = 0 . ¨
F= = A = = = : :
¨ = ..`-'7%n a i 0 . . 0 ¨
0 . . . 0 Oi .... 1 0
= :Oil = = = 6n1 vi.]
Notice that all matrices but (Ds in the previous equation are n+1 square
defined. Now,
needle deflection can be calculated for every insertion depth using (1).
ii. Needle ¨ Tissue Interaction Model 2
In order to calculate the force F applied at the needle tip, the needle
steering
apparatus measures the forces applied to the needle's base F,õ that are
necessary to insert and
withdraw it from the tissue. As the needle is pushed into tissue, a force F,
is applied at the
18
e A
CA 2967482 2017-05-17
needle tip, that has transverse and longitudinal components Q, and F,
respectively. These
forces are functions of F, and of the needle bevel angle . As the surgeon
pushes the needle
into the tissue, the measured force at the needle base Fin corresponds to F, =
P + f where f
is the needle-tissue frictional force along the shaft given by f = (bvi)d ,
where v, is the
insertion velocity, and b is the friction coefficient per unit length of the
inserted needle.
When the needle is withdrawn after insertion, the measured force F2
corresponds to friction
only. If the needle is withdrawn with a velocity of v2, the force P can be
found as
r
P = F1¨ F2 (16)
\ 2
It is thereby implied that b is constant during insertion and withdrawal. The
force F is
finally computed as F = P(tan ,
where 15' is the needle bevel angle. Knowing F, one
can determine K by fitting the model such that the estimated needle deflection
i),(K)
matches the measured deflection v, of an inserted needle, at a point i along
its shaft. More
specifically, K is found to minimize
(K)= minE(vi --i;,(K))2 , (17)
z=i
where n is the number of measurements taken.
Once the needle-tissue model parameters are identified, the model can be used
to estimate the
optimal needle rotation depths.
Needle Steering Control Algorithm
An object of the computer-implemented system and method of the present
invention
is to determine control commands to be applied to the device so that the
needle moves along
a target needle trajectory. It will be appreciated that needle steering
control algorithms other
than the particular model 1 and 2 described below may be suitable for use with
the present
invention so long as the model allows for the calculation of the amount by
which the needle
must be axially rotated to reach a desired target. For example, in
embodiments, the needle
steering control algorithm may continuously determine the amount of rotation
required as the
19
CA 2967482 2017-05-17
needle insertion depth varies. Alternatively, the needle steering control
algorithm may
determine the depth(s) at which the needle must be rotated by a discrete
amount (e.g., 180
degrees) by the hand-held device in order to reach a desired target.
i) Needle Steering Control Algorithm 1
In the exemplary embodiment described below, the steering algorithm works in
three
distinct phases as follows to determine the needle rotation depths at which
the needle is
rotated by the discrete amount.
Phase 1 - Observation phase
The ultrasound probe has moved in synchrony with the needle tip up to a
certain
insertion depth d enabling the model to predict both the needle deflection and
the force
applied at the needle tip F' using (15). At this stage, the current needle
shape, the estimated
F', and the path cut by the needle tip are known. This information is used in
Phase 2 in order
to predict the needle deflection as the needle is inserted further into
tissue.
Phase 2 - Prediction phase
Phase 2 predicts the needle deflection for upcoming insertion depths. Unlike
in Phase
I, for causality reasons the force applied at the needle tip cannot be
directly observed, nor
can any image feedback be obtained. Therefore, in order to calculate future
needle
deflections, we use (13) and set F = F'u(d), with F' being the average
estimate from Phase 1.
u(d) is an auxiliary variable to reverse the orientation of the tip force when
the needle base is
axially rotated by 180 degrees at a depth d, 'and given by
,v
ti(d) H (d) 2 E1-.1 }T H (d ¨ dr)
r I (18)
with H(d) being the Heaviside step function, and N number of admissible axial
needle
rotations. As shown in Figure 5(a), u(d) = 1 indicates that the needle bevel
tip is facing up,
causing the needle to deflect downward (as shown in Figure 4), and u(d) = -1
indicates the
e
CA 2967482 2017-05-17
bevel angle is facing down, causing the needle to deflect upwards. The sign of
the tip force F
is then reversed every time the needle passes through a depth dr, with 1 < r
<N and r EN.
Phase 3 - Control phase
Now, the role of the steering algorithm is to find the N needle rotation
depths dr that
minimize a cost function J representing the total needle targeting error
relative to the desired
target/trajectory. For formulating J, let us consider two different procedures
commonly used
in brachytherapy seed implantation.
Case 1: In this experimental scenat'io the needle is loaded with a single
radioactive
seed, which must be deposited at a certain target depth in tissue, called df.
Thus, the needle
tip should reach the target regardless of what trajectory the needle takes.
This case can also
be useful for tissue biopsy. Since in brachytherapy, the needle insertion
point and the target
are typically on the same horizontal line, the cost function essentially
amounts to minimizing
the needle tip deflection at the depth of the target (see Figure 5(b)). Hence,
the cost function
is defined as
it = ly(df, L)1.
(19)
Case 2: As in current low-dose rate (LDR) brachytherapy, several seeds spaced
appropriately can be loaded in the same needle prior to insertion. Once the
needle reaches the
target depth df, the surgeon holds the stylet in place and withdraws the
needle such that all
the seeds are deposited along the prostate length, denoted by /. Ideally and
according to the
dosimetry pre-planning assumptions, this chain of seeds will wind up on the
horizontal line
that connects the target depth to the insertion point in tissue. Thus, the
cost function is
defined as the mean absolute error of tip deflection inside the prostate (see
Figure 5(c)).
Hence, in this case the cost function .12 is defined as
d=di
.12 ¨I E Iv(d, L)I.
d=di (20)
21
CA 2967482 2017-05-17
The optimal depths dr at which the device must rotate the needle are those
that
minimize the cost function for each scenario over a fixed control horizon.
Inspired by Model
Predictive Control (MPC) theory, the control horizon is defined as a moving
window that
starts at the current insertion depth and ends at a pre-defined future depths
(35 mm ahead).
This will correspond to the spatial interval in which the optimization solver
tries to minimize
the cost function. Thereby, we convert the N-variable optimization problem
into a single
variable optimization problem. Optimization is performed by a simulated
annealing
algorithm [22]. This solver provides a fast minimization of a quadratic
function subject to
linear and nonlinear constraints and bounds.
,
Figure 5(d) shows a hypothetical example for Case I, where the needle is first
inserted to a depth d. The controller evaluates the future needle deflection
up to the target
depth df for different rotation depth candidates sitting within the control
horizon [d; dh],
where dh = d + 35 mm. In the current control horizon, the optimal depth for
rotation is
determined by the optimize to be dh. In Figure 5(e), the needle is further
advanced into tissue.
Whenever the updated optimal rotation depth becomes equal to the current
insertion depth,
the needle must be rotated right the way. The optimal rotation depths
calculated using the
proposed algorithm for an 18-gauge standard brachytherapy needle (whose
characteristics
will be given in the next section) inserted in different tissues with
stiffness per unit length K
values ranging from 0.1 x 105 to 10 x 105 Nm-2 and experiencing a force at the
tip of 0.1 <
Fj l< 3.5 N are presented in Figure 6. In Figure 6(a) and Figure 6(b), the
target is a single
point that is at a depth of 150 mm and 130 mm, respectively (Case 1). These
results indicate
that a single needle rotation is required to minimize .11 and reach the
target. For Case 2, the
model predicts that two rotations are necessary to minimize the cost function
.12. The
corresponding depths of the first (d1) and second (d2) rotations are shown in
Figure 6(c) and
Figure 6(d), for a target depth of 150 mm.
ii) Needle Steering Control Algorithm 2
A motion planner computes a large number of needle tip trajectories (plans)
using the
model presented in [33] and selects the best plan. It outputs a set of depths
at which the
needle is axially rotated that brings the needle to the target. The planner
uses the Rapidly
22
CA 2967482 2017-05-17
Exploring Random Tree (RRT) algorithm [34, 35] to calculate the rotation
depths. RRT is an
efficient sampling algorithm to quickly search high-dimensional spaces that
have algebraic
constraints such as the number of allowed needle rotations, by randomly
building a space-
filling tree. Figure 7A shows a block diagram of the closed-loop control
algorithm based on
the online motion planning.
To design the online motion planner we present the needle steering problem in
the
needle configuration space, called C. Assuming the needle moves in a 2D
insertion plane,
the needle workspace is a Euclidean space W = R2. The configuration space (C)
is the
space of all possible control actions (i.e., depth(s) of needle rotation(s)),
whose values
identify the configuration of the needle tip in the workspace. Considering
symmetry of
rotation depths (e.g., rotations at depths of 40 and 80 mm are equal to
rotations at 80 and 40
mm) the configuration space is an n-dimensional simplex, where n is the number
of axial
rotations. For instance, if the maximum allowable number of rotations is 3,
the configuration
space forms a tetrahedron.
The proposed motion planner uses an approximate decomposition of C. Assuming
that the distance between two consecutive rotations is at least 5 mm, C can be
decomposed
into several smaller simplices shown in Figure 7C. This is a valid assumption
since two close
180 axial rotations are equal to one 360 rotation of the needle tip and this
action has no
effect on needle deflection. The disjoint cells in C form a connectivity
graph. The nodes of
this graph are vertices of the cells corresponding to a certain configuration
(i.e., rotation
depths). Assuming that the initial guess for a configuration in C is q, and
the goal
configuration that steers the needle toward the target is qg, planning a
motion for the needle
involves searching the connectivity graph' for a path from cell containing qs
to the cell
containing qg. For this purpose we use the RRT algorithm. In the following a
pseudocode
description of the motion planner algorithm is given.
23
CA 2967482 2017-05-17
AlgOrHIM 1: tifi,,,;(
C d Xõ. :V!
7- 4¨ luiLialize tree (X0,N1
Avhile = 0 A 1' . do
grand +-- Ran d _Cord
q, 4-- Vot t.ex C)
T., ft, 4-- q.õ
pnre,. 4-- Nk.;-.11_.3- -:11+)del [27]
T Add_ Vertex(ivrwJ
T 4-- Add_Edge( (New, qnc.r)
if p,õ,E G thi,n
tractCorLqew)
eni1
The inputs of the RRT are the current depth Xo , the number of allowed
rotations N,
and the computation time available for planning T.õx. A hypothetical example
of tree
generation for N = 2 is shown in Figure 7B. First, the configuration space C
is formed
based on the number of allowed rotations N and the current needle insertion
depth Xo = 0.
The tree is initialized with a first vertex qs located at (0, 0) (see (I) in
Figure 7B). The
algorithm then generates a random candidate 'grand from the N -dimentional
configuration
space C (See Rand_Conf in Algorithm I. and (II) in Figure 7B). Next, Near
Vertex runs
through all the vertices (candidate rotation depths) in C to find the closest
vertex to grand.
New_Conf produces a new candidate configuration qõ,,, on the segment joining
q,,eur to qõ,õd
at a predefined arbitrary distance g from quõ,, (see (HO in Figure 713).
1 5 The
random tree T is expanded b; incorporating qn,õ, and the segment joining it to
(Inearas shown in (VI). Next, needle tip path and targeting accuracy ( A.) are
obtained by
inputting the selected rotation depths in the needle-tissue interaction model
[33]. The
predicted needle shape for various candidate set of rotation depths is shown
in Figure 7C.
When the needle path for the newly added configuration is found to lie in the
target region
24
CA 2967482 2017-05-17
(G), or when the computation times exceeds T. the RRT planner terminates. The
target
region is a closed circle with 1 mm diameter, centred on the desired target
location in W.
The former condition implies that when the estimated needle tip deflection at
the maximum
depth is less than 0.5 mm, the algorithm stops. If the stopping condition is
not met, the
algorithm continues to expand the tree with new vertices as depicted in (V)
and (V/) in
Figure 7B.
Once the algorithm stops, the output q,õa, contains the best set of rotation
depths that
will bring the needle towards G . The RRT expansion procedure results in a
very efficient
exploration of C and the procedure for generating new candidates in RRT is
intrinsically
biased toward regions of C that have not been visited.
In prostate brachytherapy, the ndedle insertion point and the target are
typically on the
same horizontal line. The target is assumed to lie at a depth of 140 mm. In
order to limit
tissue trauma, the total number of needle axial rotations is set to three.
Results of the
simulation of the motion planner in configuration space C and the
corresponding needle
deflection predictions in needle workspace W for an insertion depth of 140 mm
starting at 0
mm are shown in Figures 7C and 7D, respectively.
The RRT has been used for needle steering in [34]. Unlike [34], our search
space is
directly constrained by the possible control inputs and by the number and
depths of rotations.
Therefore, there is no need to solve for the inverse kinematics of the model,
which enables
the optimization problem to be solved faster and makes the solution method
suitable for
online applications.
System
In the exemplary embodiment shown schematically in Figure 8A, the system of
the
present invention comprises the hand-held device 10 of the present invention,
a display
device 52, and a computer operatively connected to the hand-held device 10 and
the display
device 52, in relation to a surgeon, a patient, and a transrectal ultrasound
probe. It will be
CA 2967482 2017-05-17
understood that the operative connections between the computer, the hand-held
device 10,
and the display device 52 may comprise wired connections, wireless connections
or a
combination of wired and wired connections.
Display device
A purpose of the display device 52 is to generate visual representations of
the
information relevant to needle insertion, such as needle shape, needle
position or needle
insertion parameters such as a needle position, a needle orientation, a needle
axial rotation
angle, a needle velocity, and a needle acceleration, wherein the calculating
is based on an
electronic signal from the sensor unit 20 and the ultrasound images. The
information may be
displayed numerically and/or by graphical representations. In exemplary
embodiments, the
display device 52 may comprise one or a combination of a video display screen.
Computer
A purpose of the computer is to control the device and the display device in
accordance with methods of the present invention, for assisting the surgeon to
precisely and
efficiently place the needle. In general, the computer comprises a computer
processor and a
computer memory. In an exemplary embodiment, the computer processor may
comprise a
microprocessor (i.e., a computer processor on an integrated circuit device).
The computer
processor executes the instructions stored on the computer memory to implement
methods of
the present invention. The computer memory is a computer device that comprises
a non-
transitory computer readable medium that stores instructions that are
executable by computer
processor to implement methods of the present invention. In exemplary
embodiments, the
computer memory may comprise volatile memory (i.e., memory that requires power
to
maintain the stored data) as well as non-volatile memory (i.e., memory that
can be retrieved
after power to the computer memory has been cycled on and off). In exemplary -
embodiments, the computer memory may comprise solid-state flash memory,
magnetic
media, and optical media. It will be appreciated that the computer may be
implemented by
one or more general purpose computers, a special purpose computers, or a
combination of
general purpose computers and special purpose computers with appropriate
software or
firmware stored on a variety of non-transitory computer readable media, as
known to persons
26
CA 2967482 2017-05-17
skilled in the art. The computer may be partly or wholly physically integrated
with or
physically discrete from the hand-held device.
The computer may be characterized as having functional modules including a
ultrasound image processing module (USIPM) 54, a needle shape and position
estimation
module (NSPEM) 56, a display module (DM) 58, a needle steering planning module
(NSPM)
60, and a haptic feedback module (HFM) 62. It will be appreciated that these
functional
modules are not physically discrete modules, and may functionally overlap with
each other in
operation.
A purpose of the ultrasound image processing module (USIPM) 54 is to process
an
ultrasound image 64 to determine a location of a portion of the needle 12
within the
ultrasound image 64. A purpose of the needle shape and position estimation
module
(NSPEM) 56 is to calculate a shape and position of the of the needle 12 based
on locations of
known portions of the needle 12 as kterwined by the USIPM 54, and/or needle
insertion
parameters derived from electronic signals generated by the sensor unit 20 of
the hand-held
device 10. When using a transrectal ultrasound probe, it may be desirable to
use a thin, firm
sleeve that minimizes prostate deformation as the probe is moved inside the
rectum.
Alternatively, it may be desirable to use an ultrasound system such as
TargetScanTm
(Envisioneering Medical, Pittsburgh, USA) or the anorectal 3D 2052 ultrasound
probe (BK
Ultrasound Machines, Peabody, Massachusetts, United States), in which the
probe is
stationary inside the rectum, but the transverse imaging plane can be changed.
In an exemplary embodiment as shown in Figure 8B, the needle 12 is imaged in
the
sagittal ultrasound plane, where only a small portion (roughly 40 mm) of the
needle 12 is
visible as shown schematically in Figure 8B. This is input into the needle-
tissue interaction
model and the observation phase of the needle steering control algorithm. This
technique
allows the ultrasound probe to remain stationary during brachytherapy, which
will eliminate
any ultrasound probe induced tissue motion. The points immediately above
"imaged portion
of the needle" in Figure 8B, panel (b), represent the portion of the needle
viewed in the
ultrasound image. The error in predicting the entire needle shape is less than
0.5 mm. This
27
CA 2967482 2017-05-17
technique uses only a frame grabber device connected to an ultrasound imaging
system and
does not require any modification to the clinical ultrasound machine. The
ultrasound images
may be captured or "grabbed" at a rate of approximately 30 to 60 frames a
second.
In an alternative exemplary embodiment as shown in Figure 8C, the needle shape
and
position is estimated using a series of transverse images of the needle 12
obtained at different
depths. An image processing routine that enhances the visibility of the needle
12 and locates
candidate needle points within each of the ultrasound images. Then, using a
random sample
and consensus algorithm, false needle point candidates are removed and the
needle shape is
fit to the remaining inliers as shown in (b). For the case where the
ultrasound probe moves at
the same velocity as the needle during insertion, the success of the real-time
needle
deflection estimation algorithm is shown in (c). Coordination of the velocity
of the
ultrasound probe and the needle 12 may be accomplished by physical connection
between the
ultrasound probe and the hand-held device 10, or controlling movement of the
ultrasound
probe using information derived from the sensor unit 20 of the hand-held
device 10.
A purpose of the display module (DM) 58 is to cause the display device 52 to
show
the needle shape and position, and/or needle insertion parameters determined
by the NSPEM
56.
A purpose of the needle steering planning module (NSPM) 60 is to predict the
needle
trajectory, calculate a correction to one or more needle insertion parameters
for a target
needle trajectory including at least a correction to the needle axial rotation
angle paired with
a needle rotation depth. In an exemplary embodiment, these calculations may be
made in
accordance with the prediction phase and control phase of the needle steering
control
algorithm. Having determined the correction to the needle insertion parameter,
in an
exemplary embodiment, the NSPM 60 controls the rotary actuator 34 of the hand-
held device
10 to axially rotate the needle 12 by the correction to the needle axial
rotation angle at the
paired needle rotation depth.
,
28
CA 2967482 2017-05-17
A purpose of the haptic feedback module (HFM) 62 is to activate the haptic
feedback
unit 22 of the device 10 to vibrate the handle 16 of the device 10 in a
vibration pattern to
provide a tactile alert to the surgeon wi-ien,the NSPM 60 determines that
corrections to the
needle insertion parameters are required or not required. In an exemplary
embodiment, the
vibration pattern is determined by a rules database depending on one or a
combination of the
calculated needle shape, the calculated needle position, and the calculated
correction to the
needle insertion parameter. For example, the rules database may cause the HFM
62 to
activate the haptic feedback unit 22 to generate vibration patterns that are
recognizably
distinct to the surgeon (e.g., vibration patterns characterized by different
durations,
amplitudes, and/or number of vibrations) that correspond to the need or lack
of need for
different corrective needle steering manoeuvres (e.g., accelerate/decelerate
needle insertion;
rotate needle clockwise/counter-clockwise; push needle up/down/left/right;
pause needle
motion; maintain needle motion), or to the arrival of the needle at the target
position.
Applications
As described above, the present invention provides real-time assistance to a
surgeon
to precisely, efficiently, and intuitively position a needle percutaneously
inserted in a patient
to achieve a target needle trajectory, while allowing the surgeon to maintain
at least partial
manual control over needle insertion. In an exemplary embodiment, the
invention may be
used during prostate brachytherapy. However, it will be appreciated that the
invention may
be used for brachytherapy treatments for organs including, but not limited to,
prostate, breast,
cervix, and skin, and tumors in other body sites.
Example 1
The experimental setup used to test the prototype system is shown in Figure 9
in
phantom and ex-vivo biological tissue. A schematic depiction of the prototype
system is
shown in Figure 10. In order to track the position of the handle of the device
in real time, the
3D position of tracking markers added to its left is measured at 20 Hz by a
dual camera
optical motion tracker (BB2-BWHx60 from Claron Tech, Toronto, Canada). The
needle is
inserted by the handheld assistant into a piece of tissue held in a
transparent container
through a standard brachytherapy template grid (model D0240018BK, C.R. Bard,
Covington,
29
CA 2967482 2017-05-17
USA). The grid template is assumed to have a stiffness of Kp = 109 Nm-1. From
the measured
position of the tracking markers and knowing the length of the needle, the
needle insertion
depth is deduced. The needle used throughout the experiments is a 200 mm long
18-gauge
standard brachytherapy needle (Eckect 4 Ziegler Inc., Oxford, USA) with a
Young's
modulus of 200 GPa and a moment of inertia of 7.75 x 10 -14 IT14.
As the needle is inserted in the tissue, a 4DL14-5/38 linear ultrasound probe
connected to a Sonix Touch ultrasound machine (Ultrasonix, Richmond, Canada)
slides
above the tissue to acquire at 30 Hz transverse 2D ultrasound images of the
needle.
Transverse images show a cross section of the needle ensuring that the problem
of probe
alignment found in longitudinal (sagittal) imaging will not be present [23]. A
linear stage
motorized by a DC motor moves the ultrasound probe, while its absolute
position is
measured by a linear potentiometer (LP-250FJ from Midori Precisions, Tokyo,
Japan) in real
time (not visible in Figure 9). The ultrasound imaging plane is initially
placed close to the
needle tip. As the needle is pushed into the tissue, the motorized linear
stage controlled by a
discrete PID (proportional¨integral¨derivative) controller moves in synchrony
with the
needle tip such that the same point of the needle shaft is always visible in
the image. Each
transverse ultrasound image is then processed in order to obtain the current
needle tip
deflection using the algorithm presented in [23]. For safety reasons, the
motorized linear
stage that translates the ultrasound probe is only activated when the needle
is inserted
through the grid template. This is done by establishing a virtual workspace
defined as a 3D
rectangular volume located in front of the grid template. For the hand-held
device to be in
that workspace, the needle must be inserted in the grid template.
25TM
Two computers running Matlab in
xPC real-time mode are used in the
experimental setup as depicted in Figure 10. Computer I receives images
generated by the
ultrasound machine which are captured by a frame grabber, and images of the
tracking
markers obtained by the motion tracker. After processing the images, the
measured needle tip
deflection in the current ultrasound image and the 3D coordinates of the hand
held device are
sent via UDP (user datagram protocol) to Computer II. The current needle tip
position can
then be used in Computer 11 by the needle-tissue model and the steering
algorithm. A PID
CA 2967482 2017-05-17
compensator controls the desired orientation of the needle bevel angle
calculated by the
steering algorithm. The position of the hand-held device is sent to a second
digital PID
compensator that adjusts the horizontal position of the ultrasound imaging
plane. Both
control loops run at 2000 Hz and the communication delay between them is 4 ms.
We performed needle insertion in three different tissues. Tissue 1 and Tissue
2 are
made of industrial gelatin derived from acid-cured tissue (gel strength 300
from Sigma-
Aldrich Corporation, Saint Louis, USA). The mass ratio of gelatin to water in
Tissue 1 and
Tissue 2 is 0.15:1 and 0.2:1, respectively, making Tissue 2 stiffer than
Tissue 1. Tissue 3 is
prepared by embedding a 130 mm long piece of beef tenderloin in the same
gelatin used in
Tissue 2. This tissue presents several layers of fat and muscle, making it
highly non-
homogeneous. The gelatin is meant to create a flat surface to ensure good
acoustic contact
between the ultrasound probe and the biological tissue and to generate a
second thin tissue
layer. In the experiments in Tissue 3, the needle first goes through the
biological tissue.
When the insertion depth is higher than 130 mm, the needle reaches the gelatin
layer. For
each of the three tissue samples and two steering cases, we carried out needle
insertions to
attain two different target depths i.e., 130 mm and 150 mm. This amounts to a
total of 12
different experimental scenarios. For each scenario, 6 needle insertions were
performed,
which yields a total of 72 needle insertions.
The following discussion is divided in three parts. First, we will see the
effects of
longitudinal micro vibrations cause by the piezo-actuator on needle-tissue
friction. Next,
image-based identification of needle tissue interaction model parameters are
described. The
obtained results are used to steer the needle towards pre-determined targets.
A. Effects of Longitudinal Needle Vibration
In order to observe the effects of needle longitudinal vibration on the needle-
tissue
frictional forces, the piezoelectric actuator unit connected to an 18-gauge
brachytherapy
needle is attached to the needle insertion robot presented in [24]. The robot
is controlled to
insert the needle at a constant insertion velocity of 5 mm si through a 40 mm
thick piece of
tissue made of plastisol gel (M-F Manufacturing Co., Fort Worth, USA) with a
Young's
31
CA 2967482 2017-05-17
modulus of 25 kPa. Once the needle tip is placed close the tissue surface, the
robot is
controlled to move the needle towards the tissue a distance of 70 mm, while
the axial
insertion force is recorded by a force sensor.
As the needle tip passes through the tissue, the measured force corresponds to
the
axial needle-tissue cutting force plus the frictional force generated along
the shaft. Inertial
effects are neglected since the needle is driven at a constant velocity. When
the needle tip
exits the tissue, the measured force corresponds to friction only. For each
insertion, the
piezoelectric actuator receives a 5 V in amplitude sinusoidal voltage with a
different
frequency ranging from 0 Hz (no vibration) to 1200 Hz in 200 Hz increments.
The measured
insertion force for each frequency is presented in Figure 11. The results show
that the needle
insertion forces can be reduced up to 48%. No considerable variation in the
insertion force is
observed for frequencies beyond 1200 Hz.
B. Model Parametrization from Ultrasound Images
The first step towards needle steering is to find the model parameters, i.e.,
the tip
force F and the needle tissue stiffness. To this end, three insertions are
performed in each
tissue without axial rotation. From the acquired data, and using the needle
tissue interaction
model, these parameters can be calculated. Next, the optimal depths of
rotation can be
calculated for each experimental scenario as described in the needle steering
control
algorithm above. The model parameters are found following each of the 7 steps
detailed
below.
1) Insert the needle in tissue and record the needle deflection using
ultrasound
images (see the first plot in Figure 12);
2) In the needle-
tissue model, initialize or, whenever appropriate, update the
current needle-tissue stiffness K;
3) Run the observation phase up to 60% of the maximum insertion depth;
4) Calculate the average of the observed force F during the observation
phase
(see the third panel in Figure 12. Due to imaging noise, the first 20 mm are
not
considered);
32
, a
CA 2967482 2017-05-17
5) Using the average force F from Step 4 and the current stiffness K from
Step 2,
run the prediction phase from the end of the observation phase to the
maximum insertion depth;
6) Evaluate the mean squared error between the model predicted and measured
needle tip deflection (see the second panel in Figure 12);
7) Repeat the process from Step 2 until the prediction error in Step 6
reaches a
minimum.
Figure 12 shows the estimated tip deflection for each tissue sample and after
model
parametrization. The prediction error for both phantom tissues is less than
0.2 mm, and
increases to 0.5 mm for the biological tissue. The obtained model parameters
are summarized
in the first two lines of Table I.
TABLE I
IDENTIFIED MODEL PARAMEIERS FOR EACH EXPERIMENTAL SCENARIO
Gelatin gelatin Biolo gic
15%
Stiffness k [N m-2] 0.5x105 1.2x105 1.6x105
Estimatedforce F {N} ¨0.33 ¨0.85 ¨0.73
Nleasured force F [N] ¨0.39 ¨0.96 ¨0.57
For comparison, the third line shows the tip force F measured for each tissue
by means of a
force sensor connected to the needle's base and the procedure described in
[24]. Note that
different combinations of stiffness K and tip force F can lead to the same tip
deflection at a
given depth. The disparity between them can be seen in the path followed by
the needle tip
(i.e., ve(d,z)). Hence, a tissue with high K - F does not necessary have a
high Young's
modulus, but rather will induce the needle to deflect forming a high radius of
curvature
(tending to a straight line). This is the case for the biological tissue as
compared to the gelatin
phantom tissues.
33
GA 2967482 2017-05-17
C. Needle Steering
Updating the model parameters as the needle is inserted requires the
ultrasound probe
to move in synchrony with the needle tip during the procedure [25], [26].
However,
automated ultrasound probe motion is rarely available in operating rooms.
Furthermore,
probe motion during brachytherapy can result in additional deformation of the
prostate gland
[27]. This has been shown to result in anatomic variations of the
preoperatively planned
needle targets [28], [29]. Hence, it is desirable to limit the motion of the
ultrasound probe.
For these reasons, the experiments reported'here assume that the identified
model parameters
are constant during insertion and the steering algorithm does not employ
ultrasound images
during insertion. Six needle insertions are performed for each experimental
scenario using
the hand-held device. Each insertion is done at a new location in tissue to
avoid the influence
of previous insertions on the current one. Table II shows the calculated
optimal depth(s) (i.e.,
dl and d2, if applicable) where the needle rotates by 180 degrees during
insertion.
TABLE Il
MEASURED AVERAGE NEEDLE TIP DEFLECTION (41 1AT A DEPTH dr AND AVERAGE NEEDLE
TIP DEFLECTION (J2 1 BETWEEN dr AND dr - SO FOR EACH
EXPERIMENTAL CONDITION. ALL UNITS ARE IN MILLIMETRES.
Target Tissue Rotation Rotation Cost Ji stand.
Cost ..12 stand. Prediction
Case depth df sample depth di depth d2 fun ct ion J1
deviation fun ct ion J2 deviation error
Gelatin 15% 59 n a. 0.33 0.38 1.06 0.36
0.33
150 Gelatin 20% 56 A.A. 0.27 0.28 1.19 0.32
0.27
1
Biological 48 11.it 0.32 0.32 0.96 0.48
0.32
Gelatin 15% 53 BA. 0.35 0.39 1.02 0.63
0.35
130 Gelatin 20% 50 ILI 0.54 0.21 1.53 0.21
0.54
Biological 42 Ma- 0.77 0.51 0.92 0.37
0.77
Gelatin 15% 50 138 0.42 0.28 0.61 0.53
0.31
150 Gelatin 20% 41 123 0.46 0.34 0.27 0.54
0.13
2 Biological 38 121 0.76 0.31 0.45 0.25
0.37
Gelatin 15% 45 ' 122' 0.55 0.14 0.43 0.50
0.18
130 Gelatin 20% 34 I i 0 0.21 0.15 0.11 0.14
0.29
Biological 39 107 0.42 0.25 0.32 0.38
0.16
Average of J1 in Case 1, and J2 in Case 2 0.43 0.36
Average over 72 insertions 0.44 0.72 0.33
As predicted in the simulations reported in the needle steering control
algorithm, the
higher K, the sooner the needle is rotated. The corresponding measured values
after insertion
of the cost functions J1 (tip deflection at the target depth df) and J2
(average tip deflection
between df -50 mm and df) are summarized in the sixth and eighth columns of
Table II,
respectively. Note that the objective in Case 1 is to minimize the cost
function J1; J2 is only
presented as an indication of the average tip deflection when the needle
approaches the target
34
. .
CA 2967482 2017-05-17
depth. Likewise, in Case 2, the controller only minimizes J2. The error
between the estimated
and measured cost-functions is shown in the last column. For Case 1, this
equals J 1. For Case
2 the predicted cost function J2 is never zero due to the non-holonomic
constrains of needle
steering. Hence, the reported error is the difference between the model
predicted and
measured J2. For Case 1, the average needle tip deflection at the target depth
is 0.43 0.19
mm. The highest average tip deflection is 0.77 mm, observed for biological
tissue, and the
lowest is 0.27 mm obtained in the gelatin tissue. With regards to Case 2, the
average
deflection over the 50 mm preceding the maximum depth is 0.36 0.17 mm, and
the average
e a.
prediction error when compared to the steering algorithm predictions is 0.24
mm. The overall
average error between model predictions and the measured cost functions over
72 needle
insertions is 0.33 17 mm.
D. Discussion
We have evaluated the ability of the hand-held needle steering system to
minimize
needle deflection in two different case studies. The first case intends to
minimize the needle
tip deflection at the maximum depth (quantified by J1). The second case
minimizes the
needle tip deflection over the 50 mm that precede the maximum insertion depth
(quantified
by J2). In Case 1, J1 does not exceed 0.7 mm while .12 can be as high as 1.19
mm. In Case 2,
J2 is reduced to no more than 0.61 mm without affecting J1. Hence, it can be
concluded that
Case 2 also contains Case 1 as a subset, at the cost of only one additional
needle rotation.
Deviations between model prediction and measured results are less than 0.77
mm,
with an average of 0.33 mm. This can be partially attributed to imaging
uncertainties
observed for model parametrization and partially to ground truth. Firstly, the
ultrasound
probe is imaging the needle on average 3 mm behind the needle tip, which in
the worst case
scenario can induce a deflection measurement error of 0.2 mm. Secondly, the
noise present in
ultrasound images may impair ability of the model to capture a small amount of
inherent
variability in the results and thereby lead to non-negligible variations in
the estimated force
F. The latter can be solved by improving the needle tracking algorithm or by
replacing it with
a more accurate measurement modality. Another source of uncertainty arises
from the
operator's susceptibility to involuntarily turn the wrist (rotate) as he/she
uses the hand-held
CA 2967482 2017-05-17
device. This small rotation of the needle's base can lead to a small change in
the orientation
of the needle bevel tip, which is not compensated for in the controller.
In spite of these uncertainties, and with a limited number of model
parameters, the
proposed steering system is able to steer a brachytherapy needle towards a
desired
target/trajectory with satisfactory accuracy. For comparison with other needle-
tissue models,
the nonholonomic model [15] reports an error between the model prediction and
measurements of 1.3 mm. In [30] the average targeting error during steering is
0.46 mm for
different kinematics and mechanics-based models. In [31], a sliding-mode based
closed-loop
=
needle steering algorithm has an accuracy of 0.43 mm. Table III shows a
comparison of our
proposed hand-held device with other reported models and steering algorithms.
TABLE III
COMPARISON WITH OTHER D0CUMEN1ED MODELS (1-2) AND STEERING SYSTEMS (3-7)
Guidance Tissue Number of Targeting Hand-held
parametrization model rotations error
insertion
AbA,y44ict et al. [30] US images, ARFIa soft 2-19 0.46
Wthcatgx et al. [15] Camera ima,c,Tes stiff 1-2 1.30
Rucker et al. [311 Magnetic tracking stiff 1 0,43
er
jçtigetal. [91 CT images soft 2 1.00
Schneider et al. [101 US images rigid 1 2.50
Smith et al. [71 3D US images rigid DA, 0.27
Qicazawa et al. [131 US images stiff 13.01 <1.0
Prototype system US images soft 1-2 0.44
The prototype system shows fairly good accuracy when compared with other
models
and fully automated needle insertion schemes. Ways to improve needle targeting
accuracy
could be found in tracking the needle tip as it is inserted in order to update
the model
parameters on the fly in a closed-loop control scheme. For brachytherapy
applications, this is
only viable as long as the moving parts of the ultrasound probe are not in
contact with the
surrounding tissue. This could be implemented with a thin, firm sleeve in
which a transrectal
ultrasound probe translates, such that when the transducer moves, it does not
deform the
prostate gland and/or adjacent anatomical structures. Another option involves
using an
ultrasound system such as the TargetScan (Envisioneering Medical, Pittsburgh,
USA) in
which the probe is stationary, but the transverse imaging plane can be
translated.
36
e
CA 2967482 2017-05-17
Example 2
Three different tissues were used in these experiments. The first tissue was
made by
encasing a 130 mm long piece of porcine tissue into a mixture of 20% gelatin
derived from
acid-cured tissue (gel strength 300 from Sigma-Aldrich Corporation, USA) per
litre of water.
The gelatin was meant to create a 20 mm layer of tissue through which the
needle was
inserted before reaching the porcine tissue, and also to create a flat surface
in order to ensure
good acoustic contact between the ultrasound probe and the tissue. In the
second tissue, the
porcine layer was replaced with bovine tissue. Hence, the first two tissues
were composed of
two different layers. The third tissue was made of high friction plastisol gel
(M-F
Manufacturing Co., USA) mixed with 20% plastic softener. For each tissue, 15
needle
e
insertions at different locations in the grid template followed by deposition
of a single seed
were performed. The seeds were deposited at a depth of 140 mm. For each
tissue, a set of 15
insertions was performed using an open loop controller (image feedback is not
used), and
another set of 15 implants is performed using a closed-loop needle insertion
controller. This
amounts to a total of 6 different experimental scenarios and 90 seed implants
in total.
Each seed implantation procedure was composed of three phases:
1. Phase 1 - Pre-scan: The needle has not been inserted in the tissue. The
ultrasound
moves with a constant velocity of 8 mm=s up to a depth of 150 mm and returns
to the
initial position. Thereby, all previously implanted seeds and tracks in tissue
left by other
insertions can be identified.
2. Phase 2 - Needle insertion: The ultrasound imaging plane is placed close to
the
needle tip. During insertion, the ultrasound probe moves in synchrony such
that the needle tip
is always visible in the image. Once the,nee,dle reaches the desired depth of
140 mm, the seed
is manually deposited and the needle is withdrawn.
3. Phase 3 - Post-scan: After the needle is withdrawn the tissue is scanned in
order to
identify the position of the seed deposited in Phase 2.
The needle steering controller may be employed in two different ways. In open-
loop
mode, the controller determines 3 optimal rotation depths prior to needle
insertion. In closed-
loop mode, the RRT controller updates the rotation online based on the
measured needle tip
37
CA 2967482 2017-05-17
e
position. The maximum computation time allowed for planning is 1 second, which
was found
to provide good convergence. The needle bevel angle is initially oriented such
that the needle
deflects in a plane that is parallel to the table shown in Figure 9.
Deflection along the vertical
plane is not controlled.
Needle tip tracking is done online as the needle is inserted into the tissue.
Each
transverse ultrasound image is processed in real-time using the algorithm
presented in [36].
Seed localization is done using the information from both the Phase 3 scan,
containing the
implanted seed, and the Phase I scan, which is used to reduce background noise
in the Phase
3 transverse images. Final implanted seed positions are obtained offline after
Phase 3 scan is
completed. Note that when open-loop needle steering is used, the images are
not used as
feedback in the controller but the needle tip is still tracked.
From the final needle tip position in Phase 2, the seed deposition depth is
obtained
and the traverse ultrasound image that contains the seed can be selected from
the Phase 3
scan, which we will denote as 11,3. The original image obtained in Phase 3 is
shown in Figure
15(b). Even with the deposition depth of the seed known, seed localization in
transverse
images is complicated by several factors, the most important of which is that
previous seeds
are present alongside the target seed, as well as the seed not being very
distinct from the
background image noise. An additional complication is that the implanted seed
moves away
from the final needle tip location, found in Phase 2, as the needle is
withdrawn.
The seed tracking algorithm consists of 2 stages, i.e., a pre-processing stage
and the
background noise removal (Figure 15(a)). The first step in the pre-processing
stage is to
define a region of interest (ROI) around.the, final needle tip location, found
in Phase 2, in
that is large enough to capture the seed with moderate motion. Empirically, an
ROI of 100 px
by 100 px is found to be sufficient. The next step is to find the ultrasound
image at the seed
deposition depth captured in Phase 1, which we will call I pl . This image
contains the
previously deposited seeds as well as background noise from the phantom
tissue. In order to
remove the noise and other seeds from the ROI in /,,, the exact same ROI is
taken from
38
CA 2967482 2017-05-17
and the background is removed through a subtraction, such that a cleaner
image, denoted I.,
is created, where =I
43 ¨in I . The image I. is then enhanced through the same contrast
stretching method given in [?], see Figure 15(b).
With the background noise and previous seeds removed from the image, the
target
seed is now quite distinct from the background and so the final step is the
seed segmentation.
A straightforward binary threshold, determined empirically to count any pixel
with an
intensity above 150 (on a scale from 0 to 255). As a final segmentation step
all 4-connected
component objects in the binary image are found and the object with the
largest number of
pixels is chosen as the seed. The seed location is then determined by taking
the x and y
centroids of all of the pixels in the seed's 4-connected object. In the
following sections, the
calibration of the needle steering contrdlleis presented, followed by the
needle steering and
seed implant results.
i) Model Identification
The first step in performing assisted needle steering for accurate seed
deposition is to
calibrate the needle steering controller. To this end, 3 needle insertions
followed by
withdrawals are performed in each tissue at an average velocity of 2 mm = s-1.
The controller
is turned off and the needle insertion/withdrawal force is recorded. For
verification purposes,
the ultrasound probe is following the needle tip. However, in a clinical
scenario the
ultrasound probe could instead be maintained stationary at the maximal
insertion depth to
measure the needle deflection at a single depth. Following the procedure, the
force applied at
the needle tip is identified. The obtained force is input to the needle-tissue
interaction model
[33] and the needle deflection is estimated for various candidate tissue
stiffness values. The
optimal needle-tissue stiffness is the one that minimizes the difference
between the predicted
and observed needle tip deflection at the maximal insertion depth. Figure 13
presents the
results obtained with the identified model parameters. The prediction error is
less than 1 mm
for all tissue samples. The results, including the optimal tissue stiffness,
are summarized in
Table IV.
39
CA 2967482 2017-05-17
e a
Table IV: Idelitified needle tip force (N), tissue st if Inem
(N=mm-2), and average absolute prediction error (nun).
Porcine Bovine Synthetic
tiss ile tissue tiss Lie
Force 1.10 0M7 1.26 0M5 0.78 0.12
Stiffness 72.6 86.5 36.6
Mean error 0.53 0.28 0.83 0.44 0.89 0.62
ii) Seed implant with non-image based needle steering
Knowing all the parameters necessary for estimating the needle tip trajectory,
the
depths of rotation are determined by the controller. Let us first assume that
no image
feedback is available. Therefore, the controller is only used prior to the
needle insertion. The
needle is inserted through the grid template at different locations spaced 5
mm apart as in
current clinical brachytherapy. 15 insertions are performed followed by seed
deposition. The
path followed by the needle tip is shown in Figure 14(a) along with the
orientation of the
needle bevel angle. Over 45 insertions, the average needle targeting accuracy
in the X and
Y directions is 0.93 and 0.62 mm with the highest error occurring in bovine
tissue and the
lowest error observed in porcine tissue. Once the needle reaches the depth of
140 mm, the
seed loaded in the needle shaft is deposited in tissue and the needle is
withdrawn. The final
seed location with respect to the desired hypothetical seed distribution is
shown in Figure
16(a). The gray solid dot indicates the desired seed location, which is
defined as a point in a
2D plane parallel to the grid template at a depth of 140 mm. The final needle
tip location is
shown by the blue circle and the square is centroid of each seed after needle
withdrawal. The
average seed targeting accuracy in the X and Y planes is 0.89 and 0.60 mm,
respectively.
= During needle withdrawal the tissue deforms and moves the seeds by up to
0.30 mm see
(Figure 17). These results are summarized in Table V.
CA 2967482 2017-05-17
e
Table V: Average absolute needle targeting accuracy, seed placement error and
seed
deviation after needle withdrawal, and average depth of needle rotation. Units
in mm.
Nt.,iu IiviitSynt.ho Arel,
Xi, 1' 0.6.9 .0 .1.07 1.05 0.28
0.93
Y 1,1.16 11.:ti tit 0.3$` 0.1j2
= X s4.4.,41 0.,1 0.5t.4j,3 IMI
0.16
2" y wed 0,53 30 11..0J 0.37 0.S1 7,+,;
0.53 O. GO
X JIL e2 0.3-I 0..10 0.37
0,29 .0,21 0.32
0.35 0.17 0.22 +0.23 (l.:H 0.23
(11.3.1.
1 31.1 I
. 2 51.3 1tt.49.1
ion 3 .1011.9 .102.5 118.9
X 0.51. 0.3) .. 0.21; 0.1 0.30 0.57
0.79 .Lit..";2 0.11 0.31 1L40 ir25 0.53
7: X .[ 0.110 0.25 0.21 0.46
0.S4 '; 1 I. I +0.29 0.31 l
X In =i= {),T- 11 2 1 41..11 .. 0.26 0.21.
0.21 11.311
Y O. IT ...1122 0.1.1 i),(.19 11.31
0.31 0.29
9
ion 1. 31E2 12.1 30 +0.3 2 .
Hu. 2 52, 1.143.7 V1.6 -..f_11.9 55.2 =10.3
)n 3 HS.5 +10-1 122 +15,8 !15.12.2
iii) Seed implant with image-based needle steering
Let us now assume that the position of the needle tip can be measured at any
time
during insertion from ultrasound images. As a result, the steering controller
can update the
optimal rotation depths on-line. This is expected to result in an immediate
improvement of
targeting accuracy since the controller replans the path towards the target
given the current
position of the needle tip X0, and the number ii of axial rotations that have
been performed.
The path followed by the needle tip is presented in Figure 14(b). The third
panel shows the
average position of the bevel angle. The absolute needle targeting accuracy in
the X and Y
planes is 0.57 and 0.53 mm, respectively. Considering the deflection along X,
this
corresponds to an improvement of 40% compared to the case without image
feedback. The
final needle tip location at the target. depth, and the final location of the
deposited seeds are
shown in Figure 16(b) . The average deviation from the actual to the desired
seed location is
0.46 and 0.49 mm in the vertical and horizontal planes, respectively. The
second part of
Table V summarizes these results.
41
CA 2967482 2017-05-17
iv. Discussion
e 4
Two different approaches have been proposed to steer a seed-carrying needle
towards
a pre-defined target. In the first approach the needle steering apparatus
rotates the needle
base at optimal depths determined preoperatively. In the second case, the the
current position
of the needle tip is used to update the optimal rotation depths
intraoperatively.
The first method is compatible with a clinical setting where real-time
measurement of
the needle tip cannot be obtained during insertion. To address this limitation
the steering
apparatus is equipped with a force sensor that measures the needle insertion
and withdrawal
forces and estimates the required model parameters using the deflection
measured at a single
depth after insertion. 15 seeds are implanted 5 mm apart in the tissue to form
a hypothetical
seed distribution. The average needle and seed targeting accuracy in the
controlled deflection
direction is 0.93 and 0.89 mm on average, respectively.
The second method uses ultrasound images to measure the needle tip deflection
in
tissue as it is inserted. The controller running at 1 Hz recalculates the
steering manoeuvres
online, such that deviations from the offline predicted path can be corrected.
With this
approach, the average seed placement error is reduced to 0.46 mm. Some
commercially
available ultrasound systems can be employed to follow the needle tip during
insertion.
Examples include the TargetScan from Envisioneering Medical, Overland, USA,
where the
2D axial imaging plane translates within a stationary transrectal probe, and
the 3D-2052
ultrasound probe from B&K Ultrasound. Peabody, USA, where the imaging plane
translates
axially by 70 mm. As an alternative, the Sonalis Ultrasound System from Best
Medical,
Pittsburgh, USA, has a longitudinal array that provides for 140 mm length of
view,
encompassing the bladder, the prostate and the perineum. Hence, the needle can
be observed
during throughout the insertion as long as it does not deflect out of the
imaging plane.
Standards for seed implant quality are typically defined in terms of
quantitative X-ray
Computed Tomography-based postoperaae dosimetric evaluation. Currently,
ultrasound-
based postoperative seed identification cannot be done routinely with any
better than 80%
accuracy [37, 38]. CT-based dosimetry evaluation requires a separate imaging
session to scan
42
CA 2967482 2017-05-17
the patient prostate in order to determine the final location of the seeds.
This assessment is
subject to anatomical variations of the Nostate position and postoperative
edema of the
prostate gland. With the described method, assessment and corrections
regarding seed
implantation errors can be taken during the procedure without the need for
postoperative
imaging.
In summary, we demonstrate the feasibility of a new framework for accurate
radioactive seed implantation and tracking during low dose rate prostate
brachytherapy for
prostate cancer. A hand-held needle steering apparatus controls the deflection
of a seed-
carrying needle during insertion such that the needle tip reaches the desired
target with
minimum deflection. The steering controller evaluates the effects of axial
needle rotations at
different depths on the needle targeting accuracy via a needle-tissue
interaction model.
Optimal rotation depths are determined prior to the procedure and can be
updated as the
needle insertion progresses. The device automatically steers the needle as the
surgeon
manually inserts it in tissue, keeping the surgeon in control of the
procedure. Once the needle
reaches the target, the surgeon can depbsitathe seeds in tissue as in current
clinical practice.
Hence, the proposed framework does not require major modifications to the
operating room
setup. Knowing the final needle tip location prior to seed deposition, a
method is proposed to
track the final seed locations after needle withdrawal, allowing the surgeon
to monitor
implant quality on the fly.
Despite the current clinical individual seed placement uncertainty of 5 mm,
very good
clinical results for brachytherapy can be achieved when the whole prostate
gland is treated.
This is a consequence of the large number of seeds involved in a whole gland
implant
(typically 80 to 100), and the addition of a 3 mm margin around the prostate
to create a
planning target volume to which the treatment dose is prescribed [39]. With
the proposed
system, the average seed placement accuracy is improved to 0.46 mm in tissue
phantoms.
Reducing seed placement error to this order in the clinic can enable accurate
brachytherapy
boost or focal treatment of dominant intra-prostatic lesions rather than
treating the whole
prostate gland. Seeds carrying higher radiation doses can be considered to
reduce the number
of implanted seeds and the targeted areas within the prostate.
43
CA 2967482 2017-05-17
Combined with improved imaging techniques [40], it is possible to identify men
with
low- to intermediate-risk prostate cancer who have low volume focal disease
and who may
be suitable for local therapy. This would result in fewer side effects to the
patient including
reduced urinary problems, rectal symptoms, and improved erectile function
[41]. In addition,
the possibility of post-treatment after focal brachytherapy is expected to be
easier than after
conventional treatment of the whole prostate gland. Among the options for such
treatment, it
is possible to treat remaining regions of the prostate volume with specific
techniques of
external irradiation or salvage surgery [42].
The present invention has been described above and shown in the drawings by
way of
exemplary embodiments and uses, having regard to the accompanying drawings.
The
exemplary embodiments and uses are intended to be illustrative of the present
invention. It is
not necessary for a particular feature of a particular embodiment to be used
exclusively with
that particular exemplary embodiment. Instead, any of the features described
above and/or
depicted in the drawings can be combined with any of the exemplary
embodiments, in
addition to or in substitution for any of the other features of those
exemplary embodiments.
One exemplary embodiment's feature g are not mutually exclusive to another
exemplary
embodiment's features. Instead, the scope of this disclosure encompasses any
combination of
any of the features. Further, it is not necessary for all features of an
exemplary embodiment
to be used. Instead, any of the features described above can be used, without
any other
particular feature or features also being used. Accordingly, various changes
and
modifications can be made to the exemplary embodiments and uses without
departing from
the scope of the invention as defined in the claims that follow.
REFERENCES
All publications mentioned are incorporated herein by reference (where
permitted) to
disclose and describe the methods and/or materials in connection with which
the publications
are cited. The publications discussed herein are provided solely for their
disclosure prior to
the filing date of the present application. Nothing herein is to be construed
as an admission
that the present invention is not entitled to antedate such publication by
virtue of prior
e
44
CA 2967482 2017-05-17
invention. Further, the dates of publication provided may be different from
the actual
publication dates, which may need to be independently confirmed.
[1] (2015) Canadian Cancer society:. Canadian cancer society's steering
committee:
Canadian cancer statistics 2015. [Online]. Available:
http://www.cancer.ca/statistics.
[2] (2015) American cancer society. prostate cancer statistics. [Online].
Available:
http://www.cancer.org/cancer/prostatecancer.
[3] M. Keyes, J. Crook, W. J. Morris, G. Morton, T. Pickles, N. Usmani, and
E.
Vigneault, "Canadian prostate brachytherapy in 2012," Canadian Urological
Association
Journal, vol. 7, no. 1-2, p. 51,2013.
[4] R. Taschereau, J. Pouliot, J. Roy, and D. Tremblay, "Seed misplacement
and
stabilizing needles in transperineal permanent prostate implants,"
Radiotherapy and
Oncology, vol. 55, no. 1, pp. 59-63,2000.
[5] A. Pollack, G. K. Zagars, L. G. Smith, J. J. Lee, A. C. von Eschenbach,
J. A. Antolak,
G. Starkschall, and I. Rosen, "Preliminary results of a randomized
radiotherapy dose-
escalation study comparing 70 gy with 78 gy for prostate cancer," Journal of
Clinical
Oncology, vol. 18, no. 23, pp. 3904-3911,2000.
[6] D. Stoianovici, L. L. Whitcomli, J.1-1. Anderson, R. H. Taylor, and L.
R. Kavoussi,
"A modular surgical robotic system for image guided percutaneous procedures,"
in Medical
Image Computing and Computer- Assisted Intervention - MICCAI 1998. Springer,
1998, pp.
404-410.
[7] W. L. Smith, K. Surry, G. Mills, D. B. Downey, and A. Fenster, "Three
dimensional
ultrasound-guided core needle breast biopsy," Ultrasound in medicine &
biology, vol. 27, no.
8, pp. 1025-1034,2001.
[8] J. A. Cadeddu, D. Stoianovici, R. N. Chen, R. G. Moore, and L. R.
Kavooussi,
"Stereotactic mechanical percutaneous renal access," Journal of Endourology,
vol. 12, no. 2,
pp. 121-125,1998.
[9] G. Fichtinger, T. L. DeWeese, A. Patriciu, A. Tanacs, D. Mazilu, J.
H. Anderson, K.
Masamune, R. H. Taylor, and D. Stoianovici, "System for robotically assisted
prostate biopsy
and therapy with intraoperative ct guidance," Academic Radiology, vol. 9, no.
1, pp. 60-74,
2002.
CA 2967482 2017-05-17
[10] C. M. Schneider, A. M. Okamura, and G. Fichtinger, "A robotic system for
transrectal
needle insertion into the prostate with integrated ultrasound," in Robotics
and Automation,
2004. Proceedings. ICRA'04. 2004 IEEE International Conference on, vol. 1.
IEEE, 2004,
pp. 365¨ 370.
[11] K. Cleary, M. Freedman, M. Clifford, D. Lindisch, S. Onda, and L.
Jiang, "Image-
guided robotic delivery system for precise placement of therapeutic agents,"
Journal of
Controlled release, vol. 74, no. 1, pp. 363-368, 2001.
[12] T. K. Podder, L. Beaulieu, B. Caldwell, R. A. Cormack, J. B. Crass, A.
P. Dicker, A.
Fenster, G. Fichtinger, M. A. Meltsner, M. A. Moerland et al., "{AAPM} and
{GEC-
ESTRO} guidelines for image-guided robotic brachytherapy: Report of task group
192,"
Medical physics, vol. 41, no. 10, p. 101501, 2014.
[13] S. Okazawa, R. Ebrahimi, J. Chuang, S. Salcudean, and R. Rohling,
"Hand-held
steerable needle device," Mechatronics, IEEE/ASME Transactions on, vol. 10,
no. 3, pp.
285-296, June 2005.
[14] R. J. Hendrick, C. R. Mitchell, S. D. Herrell, and R. J. Webster,
"Handheld
transendoscopic robotic manipulators: A transurethral laser prostate surgery
case study," The
International Journal of Robotics Research, p. 0278364915585397, 2015.
[15] R. J. Webster, J. S. Kim, N. J. Cowan, G. S. Chirikjian, and A. M.
Okamura,
"Nonholonomic modeling of needle steering," The International Journal of
Robotics
Research, vol. 25, no. 5-6, pp. 509-525, 2006.
[16] S. Patil, J. Burgner, R. J. Webster, and R. Alterovitz, "Needle
steering in 3-d via rapid
replanning," Robotics, IEEE Transactions on, vol. 30, no. 4, pp. 853-864,
2014.
[17] I. Khalaji, M. Hadavand, A. Asadian, R. Patel, and M. Naish, "Analysis
of needle-
tissue friction during vibration-assisted needle insertion," in Intelligent
Robots and Systems
(IROS), 2013 IEEE/RSJ International Conference on, Nov 2013, pp. 4099-4104.
[18] T. Adebar, A. Fletcher, and A. Okamura, "3-d ultrasound-guided robotic
needle
steering in biological tissue," Biomedical Engineering, IEEE Transactions on,
vol. 61, no. 12,
pp. 2899-2910, Dec 2014.
[19] G. Genta, Vibration dynamics and control. Springer, 2009.
46
CA 2967482 2017-05-17
[20] S. Misra, K. B. Reed, B. W. Schafer, K. Ramesh, and A. M. Okamura,
"Mechanics of
flexible needles robotically steered through soft tissue," The International
journal of robotics
research, 2010.
[21] R. Bhat, "Natural frequencies of rectangular plates using
characteristic orthogonal
polynomials in rayleigh-ritz method," Journal of Sound and Vibration, vol.
102, no. 4, pp.
493-499, 1985. ,
[22] S. Kirkpatrick, C. D. Gelatt, M. P. Vecchi et al., "Optimization by
simulated
annealing," science, vol. 220, no. 4598, pp. 671-680, 1983.
[23] M. Waine, C. Rossa, R. Sloboda, N. Usmani, and M. Tavakoli, "3d shape
visualization of curved needles in tissue from 2d ultrasound images using
ransac," in
Robotics and Automation (ICRA), 2015 IEEE International Conference on, May
2015, pp.
4723-4728.
[24] C. Rossa, R. Sloboda, N. Usmani, and M. Tavakoli, "Estimating needle
tip deflection
in biological tissue from a single transverse ultrasound image: application to
brachytherapy,"
International Journal of Computer Assisted Radiology and Surgery, pp. 1-13,
2015. [Online].
Available: http://dx.doi.org/10.1007/s11548-015-1329-4
[25] G. J. Vrooijink, M. Abayazid, S. Patil, R. Alterovitz, and S. Misra,
"Needle path
= planning and steering in a three-dimensional non-static environment using
two-dimensional
ultrasound images," The International Journal of Robotics Research, pp. 1361-
1374, 2014.
,
[26] M. Abayazid, G. J. Vrooijink, S. Patil, R. Alterovitz, and S. Misra,
"Experimental
evaluation of ultrasound-guided 3d needle steering in biological tissue,"
International journal
of computer assisted radiology and surgery, vol. 9, no. 6, pp. 931-939, 2014.
[27] J. Hong, T. Dohi, M. Hashizume, K. Konishi, and N. Hata, "An ultrasound-
driven
needle-insertion robot for percutaneous cholecystostomy," Physics in Medicine
and Biology,
vol. 49, no. 3, p. 441, 2004.
[28] M. Lachaine and T. Falco, "Intrafractional prostate motion management
with the
clarity autoscan system," Med. Phys. Int, vol. I, no. 1, pp. 72-80, 2013.
[29] J. Schlosser, K. Salisbury, and D. Hristov, "Tissue displacement
monitoring for
prostate and liver igrt using a robotically controlled ultrasound system,"
Medical Physics,
vol. 38, no. 6, pp. 3812-3812, 2011.
47
,
CA 2967482 2017-05-17
[30] M. Abayazid, R. Roesthuis, R. Reilink, and S. Misra, "Integrating
deflection models
and image feedback for real-time flexible needle steering," Robotics, IEEE
Transactions on,
vol. 29, no. 2, pp. 542-553, April 2013.
[31] D. C. Rucker, J. Das, H. B. Gilbert, P. J. Swaney, M. Miga, N. Sarkar,
R. J. Webster
et al., "Sliding mode control of steerable needles," Robotics, IEEE
Transactions on, vol. 29,
no. 5, pp. 1289-1299, 2013.
[32] M. Keyes, D. Schellenberg, V. Moravan, M. McKenzie, A. Agranovich, T.
Pickles, J.
Wu, M. Liu, J. Bucci, and W. J. Morris, "Decline in urinary retention
incidence in 805
patients after prostate brachytherapy: The effect of learning curve?"
International Journal of
Radiation Oncology* Biology* Physics, vol. 64, no. 3, pp. 825-834, 2006.
[33] C. Rossa, M. Khadem, R. Sloboda, N. Usmani, and M. Tavakoli. "Adaptive
quasi-
static modeling of needle deflection during steering in soft tissue." IEEE
Robotics and
Automation Letters 1(2), 916-923, 2016.
[34] S.M. LaValle and J.J. Kuffner. "Randomized kino-dynamic planning. The
International Journal of Robotics Research 20(5), 378-400, 2001.
[35] S. Patil et al. "Needle steering in 3D via rapid replanning." IEEE
Transactions on
Robotics 30(4), 853-864, 2014.
[36] M. Waine, C. Rossa, R. Sloboda, N. Usmani, and M. Tavakoli. "Needle
tracking and
deflection prediction for robot-assisted needle insertion using 2D ultrasound
images." Journal
of Medical Robotics Research 01(01), 1640001, 2016.
[37] B. Han, K. Wallner, G. Merrick, W. Butler, S. Sutlief, and J.
Sylvester. "Prostate
brachytherapy seed identification on post-implant trus images." Medical
Physics 30(5), 898-
900, 2003.
[38] Z. Wei, L. Gardi, D. Downey, and A. Fenster. "Automated localization of
implanted
seeds in 3d trus images used for prostate brachytherapy." Medical Physics
33(7), 2404-2417,
2006.
[39] C. Salembier, P. Lavagnini, P. Nickers, P. Mangili, A. Rijnders, A.
Polo et al.
"Tumour and target volumes in permanent prostate brachytherapy: a supplement
to the
estro/eau/eortc recommendations on prostate brachytherapy. Radiotherapy and
oncology
83(1), 3-10, 2007.
48
CA 2967482 2017-05-17
[40] M. Atri, M. Gertner, M. Haider, R. Weersink, and J. Trachtenberg.
"Contrast-
enhanced ultrasonography for real-time monitoring of interstitial laser
therapy in the focal
treatment of prostate cancer." Canadian Urological Association Journal 3(2),
125-30, 2009.
[41] S. Langley, U. H. Ahmed, B. Al-Qaisieh, D. Bostwick, L. Dickinson, and
F. Veiga et
al. "Report of a consensus meeting on focal low dose rate brachytherapy for
prostate cancer."
BJU International 109(s1), 7-16, 2012.
[42] J.M. Cosset, X. Cathelineau, G. Wakil, N. Pierrat, O. Quenzer, and D.
Prapotnich et
al. "Focal brachytherapy for selected low-risk prostate cancers: a pilot
study." Brachytherapy
12(4), 331-337, 2013.
49