Note: Descriptions are shown in the official language in which they were submitted.
~6~,~15~
The present invent;on relates to embonates of
quinolonecarboxylic acids and of their derivatives, to
process for their preparation and to oral agents con~
taining these compounds for human and veterinary
medicine.
Quinolonecarboxylic ac;dsy their derivatives
and their salts are already known (compare DE-OS tGer-
man Published Specif;cation) 3,033,157). They are
distinguished by outstanding bactericidal properties.
However, o~ing to the bitter taste ~hich some of them
have, there are limits to their oral use in the form
of solutions, suspensions or admixtures to feed or in
drinking water. For example, it is not possible for
these active compounds to be directly administered
orally to livestock with a highly developed sense of
taste, such as, for example~ pigs.
The embonates of quinolonecarboxylic acids and
of their derivatives of the formula tI) have been
found
~ COOH
2~ C~2 x Y~
~C OOH
in ~hich
n represents 1 or 2,
Y represents radicals of the formulae (II~ or
(III)
CO~"2 ~II)
B A
Le A 23 943
.3~9
o
F~C^~
B ~ N~
! ~ ~
~ R
in which
A represents nitrogen or =C-R4,
R4 represents hydrogen, fluorine,
chlorine~ n;tro or methyl,
R6
representS ~5-N N ~ and B also
R ~
represents R when R1 does not
~ N-
denote cyclopropyl, and
R represents hydrogen, a branched or
unbranched alkyl group ~hich has 1 to
4 carbon atoms and can optionally be
substituted by a hydroxyl or methoxy
group,
R6 represents hydrogen, methyl or phenyl,
R7 represents hydrogen or methyl,
R~ represents amino, alkyl- or
dialkylamino having 1 or 2 carbon atoms
in the alkyl group, aminomethyl,
alkyl- or dialkylam;nomethyl having 1
or 2 carbon atoms in the alkyl group,
R represen~s an alkyl radical having
1 to 3 carbon atoms9 cyclopropyl, 2-
fluoroethyl, vinyl, methoxy~ 4-fluoro-
phenyl or methylamino,
R2 represents hydrogen, alkyl having
1 to 6 carbon atoms, and cyclohexyl~
benzyl~ Z-oxopropyl, phenacyl and
Le A 23 943
,35~?
ethoxycarbonylmethyL,
R represents hydrogen, methyl or ethyl,
Z represents oxygen, nitrogen which is
substituted by methyl or phenyl, and =CH2-.
It has also been found that the embonates of
quinolonecarboxyLic acids and of their derivatives of
the formula (I)
~COOH
Yn (I)
~OH
COOH
in which
n represents 1 or 2,
Y represents radicals of the formulae (Il) or
(111) 0
~_' COOR2 ( 11 )
B A N
R
o
~'COOR2 ~ I 11 )
ZJ~R 3
in ~hich
A represents nitrogen or =C-R4~
R4 represents hydrogen, fluorine,
chlorine, nitro or methyl,
~ repreSents -N N- , and ~ also
R
~8
represents ~ ~hen R1 does not
Le A 23 943
,3~
-- 4
denote cyclopropyl~ and
R5 represents hydrogen, a branched or
unbranched alkyl group which has 1 to
4 carbon atoms and can optionally be
substituted by a hydroxyl or methoxy
group,
R6 represents hydrogen, methyl or phenyl,
R7 represents hydrogen or methyl,
R8 represents am;no, alkyl- or
d;alkylamino hav;ng 1 or 2 carbon atoms
in the alkyl group, aminomethylO
alkyl~ or dialkylaminomethyl having 1
or 2 carbon atoms in the alkyl group,
R1 represents an alkyl radical having
1 to 3 carbon atoms, cycLopropyl, 2-
fluoroethyl, vinyl, methoxy, 4-fluoro-
phenyl or methylamino,
R2 represents hydrogen, alkyl having
1 to 6 carbon atoms, and cyclohexyl,
benzyl, 2-oxopropyl~ phenacyl and
ethoxycarbonylmethyl,
R3 represents hydrogen, methyl or
ethyl~
Z represents oxygen, nitrogen which is
subst;tuted by methyl or phenyl, and
-CH2-
are obta;ned by reaction of quinolonecarboxylic acids
and of their derivatives of the formulae (II) or tllI)
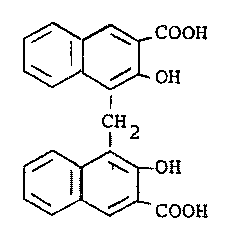
w;th embon;c acid of the formula (IV)
OH
i ~I
r ~j,
I
0
~C~
Le A 23 943
~2~i~13~
-- 5
It has been found, surprisingly, that the new
compounds according to the invention have a neutral
taste compared with other sal~s of quinolonecarboxylic
acids. Thus they are outstandingly suitable for the
preparation and use in agents which can be aclminis-
tered orally in human medicine and veterinary medi-
cineu They are also suitable for the preparation of
medicated animal feed or of preparations ~hich are
administered in the drinking ~ater. Furthermore in
human medicine for the oral administration of these
derivatives in the form of suspensions, syrups, emul-
sions etcO in cases where other pharmaceutical formul-
ations~ for example tablets or capsules, cannot be
used~
The use of the compounds according to the
;nvention in veterinary medicine is part;cularly pre-
ferred.
This ~as all the more surpr;s;ng since ;t has
not been possible to ach;eve an effect on the taste of
qu;nolonecarboxylic acids or of the;r derivatives by
the formation of other salts.
The invention preferably relates to embonates
of quinolonecarboxylic acids and of their derivatives
of the formula (lla)
~OR~
a
B~A~N~
Le A 23 943
` ~2~.35~3
-- 6
in which
R6 R8
8 represents R~-N N- or ~ N-
when R1 does not denote cyclopropyl in the
latter case, and
A, R2, R5, R6, R7 and R8 have the above-
mentioned meaning.
The invention particularly preferably relates
to embonates of quinolonecarboxylic acids and of their
derivatives of the formula (Ila)
~COOR ~ 1 1 a )
E~ A N
in which
R2 represents hydrogen, alkyl having 1 to 4
carbon atoms, and benzyl, 2-oxopropyL, phen-
acyl and ethoxycarbonylmethyl,
R~
B represents R5-N N-
R represents hydrogen, methyl or ethyl,
R6 represents hydrogen or methyl,
R7 represents hydrogen or methyl, and
A has the abovementioned meaning.
Particular mention may be made of embonates of
the following quinolonecarboxylic acids and of their
derivatives:
1-cyclopropyl-6-fluoro-1,4-dihydro--4-oxo-7-
~ (1-pipe~razinyl or 4-methyl- or 4~ethyl-1-piperazinyl-)-
l 25 quinol~ine-3-carboxylic acid, 1-ethyl-6-fluoro-1,4-
dihydro-4-oxo-7-(4-methyl-1-piperazinyl)-quinoline-3-
Le A 23 943
,3~i~
-- 7
carboxylic acid, 1~ethyl-6-fluoro-1,4-dihydro-4-oxo-
7~ piperazinyl)-quinoline-3-carboxylic acid, 1-
ethyl-6-fluoro-1,~-dihydro-4-oxo-7-(1-piperazinyl)-
1,8-naphthyridine-3~carboxylic acid, 9-fluoro-3-
methyl-10-(4-methyL-1-piperazinyl)-7-oxo-2,3-dihydro-
7,4-pyridot1,2,4-de]1,4-benzoxazine-6-carboxylic acid
and the methyl and ethyl esters of these compounds.
The preparation of the embonates of qu;nolone-
carboxylic acids and of their derivatives of the
formula (I~ is preferably carried out by addition of
embonic acid to quinolonecarboxylic acids and their
derivatives of the formuLa (II~ or (III) in a suitable
solvent and, ~here appropriate, heating.
Quinolonecarboxylic acid and embonic acid are
used for this in the ratio ~:1 or 1:2. It is prefer-
able to use for this an excess of embonic acid of
1-10%, preferably 1-5%, relative to the amount of
embonic acid necessary for the abo~ementioned ratios.
It is also possible to react the salts of
am;noqu;nolonecarboxylic acids with strong acids,
such as, for example, hydrochloric acid, methane-
sulphonic acid or formic acid, with alkali metal or
alkal;ne earth metal salts of embonic acid.
Solvents suitable for the reaction which may
be mentioned are inert organic solvents such as
alcohols, for example methanol or ethanol, ethers such
as diethyl ether or diisopropyl ether, glycol ethers
such as glycol monomethyl ether, hydrocarbons such as
petroleum ether, toluene, benzene and xylene, as well
3û as water.
The reaction is carried out at temperatures
of 2û-150C. It is pre~erably carried out at the
boiling psint of the solvent. It is also possible to
carry out the reaction at room temperature~
The active compounds are preferably used in
the form of oral formulations suitable for humans and
Le A 23 943
35~
-- 8
animals. Formulations of this type are:
Oral solutions, concentrates for oral adminis-
tration after dilution, emulsions and suspensions ~or
oral suspension.
Formulations in which the active compound is
incorporated into an ointment base or into an oil-in-
water or water-in-oil emulsion base; solid formulations
such as powder, premixes or concentrates, granules,
pellets, tablets, boli and capsules.
~ral solutions are prepared by dissolution of
the active compound in a suitable solvent and, pos-
sibly, addition of additives such as solubilizers,
acids, bases, buffer substances, antioxidants and pre-
servatives. The solutions are, ~here appropriate,
sterilized by filtration and dispensed into containers.
Solvents ~hich may be mentioned are: physi-
logically tolerated solvents such as water, alcohols,
such as ethanol, butanol, benzyl acohol, glycerol,
propylene glycol and polyethylene glycols, and N-
methyl-pyrrolidone and mixtures thereof.
The active compounds can also, where appropri-
ate, be dissolved in physiologically tolerated vege-
table or synthetic oils.
Solubilizers which may be mentioned are:
solvents uhich promote the dissolution of the active
compound or prevent its precipitation. Examples are
polyvinylpyrrolidone, polyoxyethylated castor oil and
polyoxyethylated sorbitan esters.
Examples o~ preservatives are benzyl alcohol,
trichlorobutanol and p-hydroxybenzoic esters.
Oral solutions are used directly. Concen-
trates are used oralLy after previous ~ilution to the
use concentration.
Emulsions which can be used orally are either
of the ~ater-in-oil type or of the oil-in-wzter type.
They are prepared by dissolution of the active
Le A 23 943
5~1i
compound in one phase and homogenization thereof with
the assistance of suitable emulsifiers and, ~here
appropriate, further auxiliaries such as pigments,
substances promoein9 absorption, preservatives, anti-
oxidants, sunscreen agents and substances increasingthe viscosity.
The following may be mentioned as hydrophobic
phase (oils): paraffin oils, silicone oils, natural
vegetable oils such as sesame oil, almond oil and
castor oil, synthetic triglycerides such as caprylic/
capric acid bigylceride, triglyceride mixtures with
vegetable ~atty acids of chain length Cg_12 or other
specially selected natural fatty acids, partial gly-
ceride mixtures of saturated or unsaturated fatty
acids, which possibly also contain hydroxyl groups,
and mono- and diglycerides of Cg_C10-fatty acids.
Fatty acid esters such as ethyl stearate, di-
n-butyryl adipate, hexyl laurate, dipropylene glycol
pelargonate, esters of a branched fatty acid of medium
chain length with saturated C16-C1g-fatty alcohols,
isopropyl myristate, isopropyl palmitate, caprylic/
capric esters of saturated fatty alcohols of chain
length C12-C1g, isopropyl stearate, oleyl ol~ate,
decyl oleate, ethyl oleate, ethyl lactate, wax-like
fatty acid esters such as artificial duck preen gland
fat~ dibutyl phthalate, diisopropyl adipate, ester
mixtures related to the latter, etc.
Fatty alcohols such as isotridecyl alcohol,
2-octyldodecanol, cetylstearyl alcohol and oleyl
alcohol.
Fatty acids such as, for example, oleic acid
and its mi~tures~
The following may be mentioned as hydrophilic
phase: water, alcohols such as, for example, propylene
glycol, glycerol, sorbitol and their mixtures.
Emulsifiers which may be mentioned are:
Le A 23 943
~2~,35~
- 10 -
surfactants (including emulsifiers and wetting agents)
such as:
1. Hon-ionic emulsifiers, for example polyoxyethylated
castor oil, polyoxyethylated sorbitan monoleate,
sorbitan monostearate~ ethyl alcohol, glycerol
monostearate9 polyoxyethyl stearate and alkylphenol
polyglycol ethers,
2. Ampholytic emulsifiers such as di-Na N-lauryl-~-
iminocdipropionate or lecithin,
3. Anionic emulsifiers such as La lauryl sulphate,
fatty alcohol ether sulphates, mono/dialkyl poly-
glycol ether orthophosphate monoethanolamine salt,
4~ Cationic emulsifiers such as cetyltrimethylammonium
chloride
Further auxiliaries which are suitable are:
substances which increase the viscosity and stabilize
the emulsion, such as carboxymethylcelluLose, methyl-
cellulose and other celluluse and starch derivatives,
polyacrylates, alginates, gelatine, gum arabic, poly-
vinylpyrrolidone, polyvinyl alcohol, copolymers of
methyl vinyl ether and maleic anhydride, polyethylene
glycols and waxes.
Colloidal silica or mixtures of the substances
listed.
Suspensions which can be used orally are pre-
pared by suspension of the active compound in a liquid
vehicle, where appropriate with the addition of ~ur-
ther auxiliaries such as wetting agents, pigments,
substances promoting absorption, preservatives, anti-
oxidants and sunscreen agents.
Liquid vehicles which may be mentioned are all
homogeneous solvents and solvent mixtures.
~etting agents (dispersing agents) which may
be mentioned are:
Surfactants (including emulsifiers and ~etting
agents) such as
Le A 23 943
- 11 -
1. Anionic surfactants such as, for example, Na
lauryl sulphate, fatty alcohol ether sulPhates,
mono/dialkyl polyglycol ether orthophosphate mono-
ethanolamine salt, lignin sulphonates or dioctyl
S sulphosuccinate,
2. Cationic surfactants such as, for example, cetyl
trimethylammonium chloride,
3. Ampholytic surfactants such as, for example, di-
Na N~lauryl-~-iminodipropionate or lecithin
4. Non-ionic surfactants such 35, for example, poly-
oxyethylated castor oil, polyoxyethylated sorbitan
monoleate, sorbitan monostearate, ethyl alcohol,
glycerol monostearate, polyoxyethylene stearate,
alkylphenol polyglycol ethers and Pluronic~.
Other auxiliaries which may be mentioned are
those indicated above.
Semisolid formulations which can be adminis-
tered orally differ from the suspensions and emulsions
described above only by their higher viscosity.
2~ To prepare solid formulations, the active com-
pound is mixed with suitable vehicles, where appropri-
ate with the addition of auxiliaries, and converted
into the desired form.
Vehicles which may be mentioned are all physi-
ologically tolerated solid, inert substances. Allsuch serve inorganic and organic substances. Examples
of inorganic substances are sodium chloride~ carbon-
ates such as calcium carbonate, bicarbonates~ alumin-
ium oxides, silicas, aluminas, precipitated or
colloidal silicon dioxide, and phosphates.
Examples of organic substances are sugars,
cellulose, foodstuffs and feedstuffs such as po~dered
milk, meat-and-bone meals, coarse and fine grain
meals, and starches.
Auxiliaries are preservatives, antioxidants
and pigments, which have already been listed above.
Ie A 23 943
,3~7
- 12 -
Further suitable auxiliaries are lubricants
such as, for example, magnesium stearate, stearic
acid~ talc and bentonites, substances promoting dis-
integration, such as starch or crosslinked polyvinyl-
pyrrolidone, binders such as, for example, starch,gelatine or linear polyvinylpyrrolidone, and dry
binders such as microcrystalline cellulose.
The active compounds can also be encapsulated
in the form of their abovementioned solid or liquid
formulations. It may also be advantageous to use the
active compounds in formulations ~hich release the
active compound in a delayed manner.
The active compounds are preferably adminis-
tered together with the feed and/or the drinking
water.
The feed includes non-compound feedstuffs of
vegetable origin such as hay, roots, grain and grain
byproducts, non-compound feedstuffs of animal origin
such as meat, fats, milk products, bonemeal and fish
products, also the non-compound feedstuffs such as
vitamins, proteins, amino acids, for example DL-
methircines, and salts such as lime and sodium chlor-
ide. The feed also includes supplementary, compound
and mixed feedstuffs. These contain non-compound
~5 feedstuffs in a composition which ensures a balanced
d;et w;th regard to the supply of energy and proteins
and the supply ~ith vitamins, mineral salts and trace
elements.
The concentration of the active compounds in
the feed is normally about 0.01-500 ppm, preferably
0.1-50 ppm.
The ~ctive compounds can be added as such, or
in the form of premixes or feed concentrates, to the
feed.
Premixes and feed concentrates are mixtures of
the active compound with a suitable vehicle. Vehicles
Le A 23 943
,, .
- 13 -
include the non-compound feedstuffs or mixtures
thereof.
Furthermore, they can contain other auxili-
aries such as, for example, substances which regulate
the flow o~ properties and miscibility, such as, for
example, s;lica, bentonites and l;gninsulphonates.
Furthermore, it is possible to add antioxidants such
as BHT, or preservatives such as sorbic acid or cal-
cium propionate.
Concentrates for administration in the drink-
ing water must be formulated so that a clear solution
or a homogeneous suspension is produced on mixing with
the drinking ~ater.
Thus, suitable vehicles are water-soluble
substances (~eed additives) such as sugars or salts
(for example citrates, phosphates, sodium chloride and
Na carbonate).
They can likewise contain antioxidants and
preservatives.
The active compounds can be present in the
formulations alone or mixed with other active com-
pounds, mineral salts, trace elements, vitamins, pro-
teins, pigments, fats or flavourings.
The active compounds act against micro-
organisms which are pathogenic for humans and live-
stock.
These microorganisms ;nclude:
1. Spirochaetaceae (for example Treponema, Leptospira,
Porrelia)
2. Spirillaceae
3. Micrococcaceae (for example Staphylococc; Piotype
A-Fo St. hyicus)
4. Streptococcaceae (for example Streptococcus uberis,
Str. equi, Str. agalactiae~ Str. dysgaLactiae,
Streptococci of Lancefield types A-N)
5. Pseudomonaceae (for example Pseudomonas malei~ Ps.
Le A 23 943
2~i~23~
- 14 -
cepacia., ~s. aeruginosa, Ps. maltophilia),
8ruceLLa such as erucella abort, B. melitensis, B.
suis, Bordetella such as Bordetella bronchiseptica,
Moraxella, Acinetobacter)
6. Enterobacteriaceae (for example Salmonella o~ ~ypes
B-E, Shigella, E. coli, Klebsiella~ Proteus, Citro-
bacter, Edwardsiella, Uaemophilus, Providencia,
Yersina)
7. Vibrionaceae (for example Yibrio such as V;brio
cholerae), Pasteurella such as Pasteurella multo-
cida~ Aeromonas, Actinobacillus, Streptobacillus
8. Bacteoi~aceae (for example Bacteroides, Fusobac-
terium),
9. Erysiphylothix, Listeria such as Listeria monocyto-
jenes
10.Bacillaceae (for exampLe BaciLlus, Clostridiumof types A-D, such as Clostridium perfringens),
Lactobacillaceae and anaerobic Cocci such as for
exampLe Peptostreptococci and Peptococci
11.Coryneform bacteria (for example Corynebacterium
pyogenes)
12.Mycobacteriaceae (for example Mycobacterium bovis,
M. avium, M. tuberculosis)
13.Actinomyceae (for example Actinomyces bovis, A.
israelii)
14.Nocardiaceae (for example Norcardia facinica, N.
asteroides)
15.Rickettsiaceae tfor example Coxiella, Rickettsia)
16.Bartonellaceae (for exampLe aartoneLla)
17.Chlamycliaceae (for example Chlamydia psittaci)
18.Mycoplasmataceae (for example Mycoplasma mycoides,
M. agalactiae, M~ gallisepticum~.
Microorganisms pathogenic for humans and live-
stock can cause, as singLe or mixed infections, mani-
festations of disease in the following organ systems
of the livestock:
Le A 23 943
~ 2~
- 15 -
Lung and trachial cavity, digestive systems
such as stomach and intestine, breast and udder,
genital systems such as uterus, soft tissues such as
skin, muscles~ naiLs, claws and hooves, active and
passive locomotor systems such as bones, muscles,
tendons and joints, urogenital systems such as
kidneys, urethra and ureter, nervous system, auditory
apparatus and eyes.
As already mentioned, the active compounds are
used to combat bacterial diseases of humans and live-
stock. The livestock include:
Mammals, such as, for example, cattle, horses,
pigs, sheep, goats, dogs, cats, camels, fur-bearing
animals such as mink and chinchilla, and animals in
zoos and laboratories, such as, for example, mice
ancl rats;
Birds, such as, for example, geese, chickens,
turkeys, ducks, pigeons, zoo birds, and laboratory
birds such as, for example, parrots and budgerigars;
Fish, such as, for example, carp, trout, sal-
mon, tench and eels, also ornamental fish and aquarium
fish;
Reptiles, such as, for e~ample, crocodiles and
snakes.
The bacterial diseases of livestock include,
for example, pig dysentery; spirochaetosis of poultry,
leptospirosis of cattle, pigs, horses and dogs; Cam-
pylobacter enteritis in cattle; Campylobacter abortion
in sheep and pigs; Campylc,bacter hepatitis of chickens;
skin infections: pyoderma of dogs; otitis externa;
mastitis of cattle, of sheep and of goats; strepto-
coccal mastitis; streptococcal infections of horses,
in pigs and other species; pneumococcal infections of
calves and other species; glanders; conjunctivitis;
enteritis; pneumonia; brucellosis of cattle, sheep and
pigs; rhinitis atrophicans of pigs; salmonellosis of
Le A 23 ~43
- 16 -
cattle, horses, sheep, chickens and other species;
septicaemia; Escherichia coli infection in piglets;
metritis-mastitis-agalactic (MMA) syndrome; Klebsiella
infestions; pseudotuberculosis; infectivus pleuro-
pneumonia; primary pasteurelloses; lameness of foals;
necrobacillosis in cattle and domestic animals; lep-
tospirosis; erysipelas of pigs and other species,
listeriosis; anthrax; clostridioses; tetanus infec-
tions; botulism; infections with Coynebacteria pyo-
genes; tuberculosis of cattle, pigs, poultry and otherspecies; paratuberculosis of ruminants; nocardiosis;
Q fever; ornithosis-psittacosis; encephalomyclitis;
mycoplasmosis of cattle and other livestock, and
enzotic pneumonia of pigs.
Example 1
__
Salt A:
,
(n in Formula (I) represents 1)
A suspension of 62.1 g (0.173 mol) of 1-
cyclopropyL-6-fluoro-1,4-dihydro-4-oxo-7-(4-ethyl-1-
piperazinyl)quinoline-3-carboxylic acid and 67.3 g
(0.173 mol) of embonic acid (pamoic acid, 4,4'-
methylene-bis-(3-hydroxy)-2~naptho;c acid, Fluka No.
45150) in 800 ml of glycol monomethyl ether is re-
fluxed for 30 minutes. The mixture is cooled and the
precipitate is filtered off with suct;on, uashed with
ethanol and dried to constant ~eight in vacuo at 120C.
127.6 9 (~8.6% of theory) of salt A of decomposition
po;nt 232-235C are obtained~
Example 2
Salt ~:
(n in Formula (I) represents 2)
A suspension of 7108 9 (0.2 mol) of 1-cyclo-
propyl-6-fluoro-1,4-dihydro-4-oxo-7-(4-ethyl-1-piper-
azinyl)quinoline-3-c3rboxylic acid (1) and 40 9 ~0.103
mol) of embonic acid (2~ in 5ûO Ql of glycol monomethyl
ether is refluxed for one hour. The mixture is cooled
Le A 23 943
.3~
- 17 ~
and the precipitate is ~iltered off with suct;on,
washed with ethanol and dried at constant weight in
vacuo at 120C. 107.2 9 (96.9X of theory) of salt B
of decomposition point 226-228C are obtained.
S C61H60F2N6o12 tl106)
Calculated: C 66.16 H 5.42 N 7.59 F 3.43
Found: 66.0 5.6 7.5 3.4
The following salts of 1-cyclopropyl-6-fluoro-
1~4-dihydro-4-oxo-7-(4-ethyl-1-piperazinyl)-quinoline-
carboxylic acid are prepared in analogy to the above-
mentioned procedures:
Salts with the bases (NaOH: KOH: Ca(OH)2:
Salts with the acids (HCl: H~r: HN03: CH3S03H:
CH3COOH). All these salts showed a marked bitter taste
~hen a tasting test was carried out.
In contrast, the salts of Examples 1 and 2
showed nc taste.
Le A 23 943
.