Note: Descriptions are shown in the official language in which they were submitted.
3~
54693-3866D
The invention of the present divisional application is
divided out of parent application serial no. 513,089 filed on July
4, 1986.
The invention of the present application relates to rinq
brominated epoxy aromatic compounds of the formula II (as defined
hereinafter) and compositions containing them.
The invention of the parent application relates to ring
brominated hydroxyaromatic compounds of ~he ~ormula I (as de~ined
hereinafter) and to processes for preparing them.
It is known to prepare brominated alkyl phenols. See,
e.g., Can. J. Chem., Volume 61, pages 1045-1052 (1983); and
Russian Chemical Reviews, Volume 32, pages 75-93 (1963).
Brominated tetraalkylhydroxyaromatic compounds having 2 aromatic
rings also have been prepared in the past. Brominated tetraalkyl
biphenols having the benzene rings directly linked have been
prepared from tetraalkyl diphenoquinones; see, e.g., United States
Patents 3,929,908; 3,956,403 and 4,058,570. However, when
brominating compounds wherein the aromatic rings have an alkylene
bridge, the products typically do not have bromine on the aromatic
rings. For example, Bradley and Sanders J. Cnem. Soc., Volume
1962, pages 480-486 (1962) disclose the reaction of 3,3',5,5'-
tetra-t-butylstilbenequinone with HHr to yield a,~~dihromo-4,4'-
dihydroxy-3,3',5,5'-tetra-t-butyldibenzyl. Kharasch and Joshi,
J. Orq. Chem., Volume 22, pages 1435-1438 (1957) disclose the
reaction of bromine ~ith 4,4'-methylenebis(2,6-ditertiarybutyl-
phenol) in the presence of acetic acid to give 1-bromo-1,1-bis-
(3,5-ditertiarybutyl-4-hydroxyphenyl)methane.
1 ' 1
3~.~
64693-3866D
The compound 2,2'-(1,2-ethanediyl)bis~3,5-dibromo-4,6-
dimethylphenol) has been prepared by the hydrogenation of
4',5,6',7-tetrabromo-3',5',6,8-tetramethyl-3,4-dihydrospiro(2H-l-
benzopyran-2,1'-[3,5]cyclohexadien)-2'-one Ann., Volume 548,
pages 48-77 at page 57 (1939); and by the bromination of 2,2'-
(1,2-ethanediyl)bis(4,6-dimethylphenol).
In v:iew of the deficiencies of prior art bromination
methods, it would be desirable to have a simple method for the
preparation of novel ring brominated polymethylene-bridged
di(dialkylhydroxyaromatic) compounds having terminal para hydroxyl
moieties. Such a method would be useful in the preparation of
novel epoxy derivatives of said compounds.
The invention of the parent application concerns a
compound of the formula I:
~ a~ ~ >~ ,~ OH (I)
wherein
n is zero or a positive integer, each X independently is
Br or H with proviso that at least one X is H, each Ra
independently is H or alkyl of up to 12 carbon atoms, and each R
independently is a primary or secondary alkyl moiety of up to 6
carbon atoms.
The invention of the parent application also concerns a
process for the preparation of a compound of formula I in which n,
-- 2
~. .
6~693-3~6
R and R are as hereinbefore defined and X independently is Br or
a
H which process is chaxac~erized in that (a) a tetraalk~l
dihydroxydiaromatic polymethylene-bridged compound of the formula
III:
HO ~ H2 C ~ C 2 ~ ~ (III)
R H H R
wherein
n is zero or a positive integer, each Ra independently
is H or alkyl of up to 12 carbon atoms, and each R independently
is a primary or secondary alkyl moiety of up to 6 carbon atoms is
contacted with a bromination agent in the presence of a reaction
medium and, optionally, a brominated catalyst to form a reaction
mixture; ~b) the temperature of the reaction mixture is then
raised to an elevated temperature until the reaction ls completed,
and (c) the resulting compound of formula I is recovered.
Surprisin~ly, the polymethylene-bridge does not cleave
under bromination conditions, nor do the products contain benzyl
bromine atoms. The ring brominated novel compounds are hiyhly
stable and are useful as chemical intermediates in the preparation
of valuable chemical compounds.
For example, the compounds can be reacted with
epichlorohydrin using known techniques to give the corresponding
epoxy resins, or with polyisocyanates to form polyurethanes, or
can be employed in other reactions requiring reactive hydroxyl
, ~,
4~
64593-3i355D
groups. The compounds are useful as flame retardants due to their
bromine content.
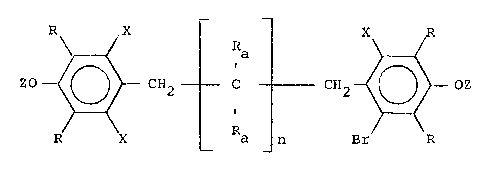
The present invention is directed to novel epoxy
derivatives of ring-brominated polymethylene-bridged di(dialkyl-
hydroxyaromat.ic~ compounds of formula I. The epoxides have
terminal para epo~y moieties and at least one bromine atom meta
relative to at least one of said epoxy moieties and are
represented by the following formula II:
Z0 ~ CH~ C ]~ ; CH2 ~\ OZ (II)
R X Br R
wherein
n is zero or a positive integer, each X independently is
bromine or hydrogen, each Ra independently is hydrogen or alkyl of
up to 12 carbon
64693-3866D
atoms, each R independently is a primary or secondary
alkyl moiety of up to 6 carbon atoms, and each Z
independently is a moiety having a terminal epoxide
moiety. The r;ng-brominated novel epoxy compounds are
highly stable and are useful as chemical intermediates
in the preparation of valuable chemical compounds. For
example, the epoxide compounds can be employed in the
preparation of cured epoxy resins. The epoxide
compounds of Formula II are prepared by contacting
compounds of Formula I with haloalkylene oxide under
reaction conditions.
Preferred TDDPC compounds are represented
generally by the formula:
]
¢ ~ U
wherein n is zero or a positive integer, each Ra
independently is E~ or alkyl of up to 12 carbon atoms,
and each R independently is a primary or secondary alkyl
moiety of up to 6 carbon atoms~ Preferably, n is zero
or a positive integer of up to 12, each Ra independently
is H or alkyl of up to 6 carbon atoms, and each R
independently is alkyl of up to 3 carbon atoms. R most
preferably is methyl, Ra most preferably is H, and n
most preferably is zero. It should be noted that the
process can be employed to put additional bromine atoms
on partially brominated TDDPC compounds.
33,739A-F DIV. I -5-
2~
--6--
A brominating agent is employed. ~hile it may
be possible to employ known brominating agents which are
useful for the bromination of aromatic rings, bromine is
the preferred brominating agent when high purity
products are desired. The amount of bromine to employ
depends upon (1) the amount of bromine in the product
desired, and (2) whether a catalyst is employed. In
general, less bromine is required when a catalyst is
employed. For example, if the dibromo-product is
desired, then stoichiometry would indicate that at least
2 moles of bromine atoms are required per mole of
substrate compound to be brominated. Typically, with a
catalyst, a stoichiometric excess of bromine ranging
from 0 to 25 percent or more is employed; preferably, a
stiochiometric excess ranging from 5 to 15 percent is
employed. Typically, up to 12 moles of bromine are
employed per mole of TDDPC in the production of
tetrabrominated products when operating without a
catalyst. Smaller excesses of bromine typically require
longer reaction times. Similarly, if a brominating
agent is employed which is not bromine, the amount of
said agent to be employed should provide bromine in the
quantities states hereinabove.
A bromination catalyst is optionally employed
in the process. Friedel-Crafts catalyst are preferred,
and are well known. Examples of bromination catalysts
include the halides of metals such as iron, aluminum,
and tin. Examples of preferred catalysts include
aluminum bromide and aluminum chloride, with aluminum
chloride being most preferred. The catalyst is employed
in catalytic quantities. Preferably, the amount of
catalyst employed ranges from 0.1 to 5 weight percent of
33,739A-F DIV. I -6-
catalyst based on the mass of aromatic compound
employed. Larger amounts of catalyst may be employed,
but may be economically impractical. The catalyst may
be employed in a variety of forms.
S
A reaction medium advantageously is employed in
the process. The reaction medium functions to
solubilize the reactants and reaction products, and to
aid in heat transfer. While the amount of reaction
medium employed may range widely, the amount of reaction
medium to be employed generally is indicated by
practical considerations, and typically ranges from
about 8 to about 20 moles of reaction medium per mole of
aromatic compound. Preferably, from 10 to 15 moles of
reaction medium are employed per mole of aromatic
compound. Typical solvents include the halogenated
lower alkanes including the perhalogenated lower alkanes
such as methylene chloride, carbon tetrachloride,
1,2-dichloroethane etc. However, it is to be noted that
carbon tetrachloride is the preferred solvent due to its
physical properties.
The order of addition of the reactants is not
critical. However, according to a preferred process, a
brominating agent is slowly added to a mixture
comprising a reaction medium, a TDDPC7 and, Gptionally,
a bromination catalyst. When the addition of the
brominating agent is complete, the resulting reaction
mixture typically is brought to elevated temperature
until the reaction is completed.
The initial addition temperature, i.e., the
temperature of the reaction mixture during the period of
addition of the brominating agent thereto, typically
33,739A-F DIV. I -7-
--8--
is a temperature at which the reaction mixture is a
liquid. Preferably, the ini-tial addition temperature is
up to 30C. More preferably, the addition temperature
i6 from 20C -to 30C. Most preferably, for the sake of
convenience, ambient temperature is employed.
As stated hereinabove, when the addition of
the brominating agent to the reac-tion mixture is com-
plete, the total reaction mix-ture can be heated -to
elevated temperature in order to assure complete bro-
mination. Typically, the total reaction mixture isheated -to reflux temperature and said temperature is
maintained until the reaction is complet~. Completion
of the reaction can be observed by following the rate
of evolution of hydrogen bromide from the reaction
mixture, i.e., -the reaction is complete when the rate
of hydrogen bromide evolution falls to zero. Ordin-
arily, the reaction will proceed at atmospheric pres-
sure or higher, but subatmospheric pressure can be
employed if desired.
The -total reaction time of from 1 to 100
hours, depending primarily on -the aromatic reac-ting, is
generally adequate for comple-te reaction under -the
conditions of the invention. Typically, a total
reaction time of up to 20 hours will be sufficient to
produce high yields of high assay products. In some
cases, bromination may be comple-te in 3 hours or less.
It is desirable to add the brominating agent to the
reaction mixture at a sufficiently slow rate to mini-
mize loss of bromine and reaction medium, and to permit
the desired low addition temperature to be maintained
under conditions of control and safety.
33,739~ F -8-
3~
g
When the reaction is carried out as described
hereinabove, a brominated TDDPC of Formula I will be
formed. Preferably, at least one X moiety in Formula I
is Br. Most preferably, two or three X moieties in
Formula I are Br.
The reaction mixture resulting from carrying
out the process can be processed by a variety of known
work-up procedures to isolate the brominated products.
The crude reaction mixture, which may contain the
brominated products, excess reaction medium and excess
catalyst, can, for instance9 be subjected to stripping
either at atmospheric pressure or preferably under
reduced pressure to the point of constant weight of the
residue. The crude product which is thus isolated may
be further purified, for instance, by recrystallization
or by digestion with a recovery medium such as acetone,
toluene, or dilute hydrochloric acid. This isolation
method by stripping is fast, simple and gives reliable
yield data and relatively pure product. It ls preferred
to employ a work-up method which neutralizes bromine.
The yield of pure product, i.e., the numerical product
of conversion of TDDPC, selectivity to the desired
product, and purity of the desired product, typically is
at least 50 mole percent. Preferably, the yield is at
least 60 mole percent, and more preferably, the yield is
at least 75 mole percent.
It is generally possible to predict the
product(s~ which will result from application of this
perbromination process under optimum reaction condit-
ions to any particular starting material. The general
33,739A-F DIV. I -9-
~z~
--10--
rule is that every nuclear hydrogen atom of the aromatic
compound will be replaced by a bromine atom if the
reaction is carried to completion, that is, until the
evolution of hydrogen bromide has stopped. This level
of bromination may be reached by proper adjustment or
reaction temperature~ catalyst concentration, if any,
and reaction time. The bromination process is continued
until such time as the sampling indicates that the
desired degree of bromination has been reached, or the
bromination reaction may be continued until e~lolution of
hydrogen bromide has substantially ceased.
As stated hereinabove, the epoxide compounds of
Formula II are prepared by contacting a brominated TDDPC
of Formula I with a haloalkylene oxide under reaction
conditions such that there is formed an epoxy derivative
of a brominated TDDPC.
Typical haloalkylene oxides are presented
generally by the formula
X-C(Rb)2CRb C(Rb)2 (IV)
wherein X is halo, and each Rb independently is H or an
aliphatic or inertly-substituted aliphatic moiety of up
to 25 carbon atoms. Preferably, each Rb is H, and X is
chlorine or bromine, with chlorine being more preferred.
Examples of haloalkylene oxides desirably employed in
the process include chloropropylene oxide, iodopropylene
oxide, methyl epichlorohydrinl methyl epibromohydrin
33,739A-F DIV. I -10-
methyl epiiodohydrin, chlorobutylene oxide,
bromopropylene oxide, and the like of up to 5 carbon
atoms, with chloropropylene oxide (epichlorohydrin)
being preferred. Mixtures of haloalkylene oxides can be
employed.
The reaction conditions employed for the
addition of haloalkylene oxides to hydroxyl-containing
or active-hydrogen-containing compounds are well-known.
See, e.gO, Handbook of Epoxy Resins, by Lee and Neville,
McGraw-Hill (19673; and U.S. Patent 4,284,573. Said
known conditions are advantageously employed in the
preparation of the epoxide compounds. Typically, for
example, from 3 to 50 moles of haloalkylene oxide are
employed per mole of active hydrogen atoms in the
brominated TDDPC, with a preferred amount being from 10
to 25 moles per mole. Larger or smaller amounts can be
employed if desired. The contacting can be performed at
any combination of temperature and pressure at which the
desired reaction will proceed. Typically, the
contacting is performed at elevated temperature.
Preferably, the temperature is from 60C to the boiling
point of the haloalkylene oxide. Ambient pressure is
preferred for the sake of convenience.
A catalyst is optionally employed, and can be
selected from known catalysts for this reaction,
including the wide range of catalysts mentioned in the
references cited previously herein. Examples of
preferred catalysts include, for example,
tetraethylammonium bromide, ethyltriphenyl phosphonium
acetate and the like.
33,739A-F DIV. I
When the brominated TDDPC of Formula I and
haloalkylene oxide of Formula IV are contacted as
described hereinabove, an epoxy resin is produced which
is an epoxy derivative of the brominated TDDPC~
Examples of typical epoxy resins are presented by the
formula:
Ra n
R X ~ Br R
wherein N, X, Ra~ R and Z are as hereinbefore defined.
The Z moieties correspond to the structure of the
haloalkylene oxide employed. For example, when
epichlorohydrin is the haloalkylene oxide, Z is a moiety
of the formula:
-CH2-CH-CH2
The epoxy resins can be cured to form novel
epoxy polymers having surprisingly improved properties.
Thus, in another embodiment, the present invention is a
composition comprising an adduct of (a) a compound of
Formula II; (b) a curing agent ~or epoxy resins; and,
optionally, (a) a curing catalyst. The epoxy resins can
be cured using well~known techniques. The novel cured
resins typically are prepared by heating the polyepoxide
33,739A-F DIV. I -12-
~.-
3~
-13-
compounds with a curing agent, typically at a tem-
perature of from 0C to 300C, and preferably from 25C
to 250C.
As curing agents there can, for example, be
men-tioned: amines or amides such as aliphatic, cyclo-
aliphatic or aromatic primary, secondary and tertiary
amines, for example, monoethanolamine, ethylenediamine,
hexamethylenediamine, trimethylhexamethylenediamine,
diethylenetriamine, triethylenetetramine, tetraethylene-
pentamine, N,N-dimethylpropylenediamine-1,3, N,N-die-
thylpropylenediamine-1,3, bis(4'-amino-3-methylcyclo-
hexyl)methane, 2,2-bis(4'-aminocyclohexyl)propane,
3,5,5-trimethyl-3-(aminomethyl)cyclohexylamine ("iso-
phoronediamine"), N-aminoethylpiperazine, Mannich
bases, such as 2,4,6-tris(dimethylaminomethyl)phe-
nol; m-phenylenediamine, p-phenylenediamine, bis-
(p-aminophenyl)-methane, bis(p--aminophenyl)sulfone
and m-xylylenediamine; adducts of acrylonitrile or
monoepoxides such as ethylene oxide or propylene
oxide to polyalkylenepolyamines such as diethylene-
triamine or triethylenetetramine; adducts of poly-
amines such as excess diethylenetriamine or tri-
ethylenetetramine, and polyepoxides such as diphe-
nylmethane polyglycidyl ethers; ketimines, for
example, from acetone or methyl ethyl ketone and
bis(p-aminophenyl)methane; adducts of monophenols
or polyphenols and polyamines; polyamides, espe-
cially those from aliphatic polyamines, such as
diethylenetriamine or triethylenetetramine and
dimerized or trimerized unsaturated fatty acids
such as dimerized linseed oil fatty acid ("VERS-
AMID"); polymeric polysulfides ("THIOKOL"); dicy-
33,739A-F -13-
-14-
andiamide; aniline-formaldehyde resins; polyhydric
pheno]s, for example, resorcinol, 2,2-bis(4-hydroxy-
phenyl)propane or phenol-formaldehyde resins; boron
trifluoride and its complexes with organic compounds,
such as BF2 ether complexes and BF3 amine complexes,
for example, BF3-monoethylamine complex; acetone-
acetanilide-BF3 complex; phosphoric acid, triphe-
nylphosphite, polybasic carboxylic acids and their
anhydrides, ~or example, phthalic anhydride, tetra-
hydrophthalic anhydride, hexahydrophthalic anhy-
dride, 4-methylhexahydrophthalic anhydride, 3,6-
-endomethylene-tetrahydrophthalic anhydride,
methyl.-3, 6-endomethylene-tetrahydrophthalic
anhydride, (methylnadicanhydride), 3,4,5,6,7,7-
~hexachlor-3,6-endomethylene-tetrahydrophthalic
anhydride, succinic anhydride, adipic anhydride,
azelaic anhydride, sebacic anhydride, maleic anhy-
dride, dodecenyl-succinic anhydride; pyromellitic
dianhydride or mixtures of such anhydrides.
It is particularly advantageous to use
curing agents which in themselves yield molding
materials of good electrical properties, such as
especially cycloaliphatic dicarboxylic acid anhy-
drides such as, for example, ~-te-trahydrophthalic
anhydride or hexahydrophthalic anhydride, or cyclo-
aliphatic polyamines such as, for example, 2,2-bis-
(4'-aminocyclohexyl)propane or "isophoronediamine".
It is furthermore possible to use cure
accelerators during the cure, and in particular
when using polyamides, polymeric polysulfides,
dicyandiamide or polycarboxylic acid anhydrides
33,739A-F -14-
~ 23~
-15-
as curing agents; such accelerators are, for example,
tertiary amines 9 their salts or quaternary ammonium
compounds, for example, 2 9 4,6-tris(dimethylamino-
methyl)phenol, benzyldimethylamines, 2-ethyl-4-
methylimidazole or triamylammonium phenolate; or alkalimetal alcoholates such as, for example, sodium
hexanetriolate.
The expression "cure" as used here denotes the
conversion of the above adducts containing epoxide
groups into insoluble and infusible cross-linked
products, and in particular as a rule with simultaneous
shaping to give shaped articles such as castings 7
pressings or laminates, or to give two-dimensional
structures such as coatings, lacquer films or adhesive
bonds.
If desired, it is possible to add active
diluents such as, for example, styrene oxide,
butylglycidyl ether, isooctylglycidyl ether,
phenylglycidyl ether, cresylglycidyl ether or glycidyl
esters of synthetic highly branch~d mainly tertiary
aliphatic monocarboxylic acids ("CARDURA E"), or
cycloaliphatic monoepoxides such as 3-vinyl-2,4-
dioxaspiro (5,5)-9,10-epoxy-undecane.
The adducts can furthermore be mixed with other
curable diepoxide or polyepoxide compounds. As such
that can, for example, be mentioned: polyglycidyl
ethers of polyhydric alcohols such as 1~4-butane-
diol, polyethylene glycols, polypropylene glycols
33,739A-F DIV. I -15-
-16-
or 2,2-bis(4'-hydroxycyclohexyl)propane; polygly-
cidyl ethers of polyhydric phenols such as 2,2-bis-
(4'-hydroxyphenyl)-propane, 2,2-bis(4'-hydroxy-
-3,5'-dibromophenyl)-propane, bis(4~hydroxyphenyl)-
sulfone, 1,1,2,2-tetrakis(4-hydrox-yphenyl)ethane
or condensation products of formaldehyde wich phe-
nols produced in an acid medium, such as phenol
novolacs or cresol novolacs; polyglycidyl esters
of polycarboxylic acids such as, for example,
phthalic acid diglycidyl ester, tetrahydrophtha-
lic acid diglycidyl ester or hexahydrophthalic
acid diglycidyl ester; triglycidyl isocyanurate,
N,N'-diglycidyl-5,5-dimethyl hydantoin, or amino-
polyepoxides such as are obtained by dehydrohalo-
genation of the reaction products of epihalogeno-
hydrin and primary or secondary amines such as
aniline or 4,4'-diaminodiphenylmethane; also ali-
cyclic compounds containing several epoxide groups,
such as vinylcyclohexene-diepoxide, dicyclopentadi-
enediepoxide, ethylene glycol-bis(3,4-epoxytetra-
hydrodicyclopentadien-8-yl)ether, (3,4-epoxycyclo-
hexylmethyl)-3,4-epoxycyclohexanecarboxylate,
(3',4'-epoxy-6'-methylcyclohexylmethyl)-3,4-epoxy-
-6-methylcyclohexanecarboxylate, bis(cyclopentyl)-
25 ether diepoxide or 3-(3',4'-epoxycyclohexyl)-2,4-
-dioxaspiro-(5,5)9,10-epoxyundecane.
The subject matter of the present inven-
tion therefore also includes curable mixtures which
are suitable for the manufacture of shaped articles
including two-dimensional structures and which con-
tain the so-called "advanced" adducts containing
33,739A-F -16-
3~
-17-
epoxide groups according to the invention, optionally
together with other diepoxide or polyepoxide compounds
and also curing agents for epoxide resins such as
polyamines or polycarboxylic acid anhydrides.
The compounds, or their mixtures with other
polyepoxide compounds and/or curing agents, can
furthermore be mixed, at any state before cure, with
usual modifiers such as extenders, fillers and
reinforcing agents, pigments, dyestuffs, organic
solvents, plasticizers and the like.
As extenders, reinforcing agents, fillers and
pigments which can be employed in the curable mixtures
there can, for example, be mentioned: coal tar,
bitumen, glass fibers, boron fibers, carbon fibers,
cellulose, polyethylene powder, polypropylene powder,
mica, asbestos, quartz powder, slate powder, aluminum
oxide trihydrate, chalk powder, gypsum, antimony
~ trioxide, bentones, silica aerogel ("AEROSIL"),
lithopone, barite, titanium dioxide, carbon black,
graphite, iron oxide or metal powder such as aluminum
powder or iron powder.
The following are, for example, suitable as
organic solvents for modifying the curable mixtures:
toluene, xylene, n-propanol, butyl acetate, acetone,
methyl ethyl ketone, diacetone-alcohol, ethylene glycol,
monomethyl ether, monoethyl ether and monobutyl ether.
33,739A-F DIV. I -17-
-18-
Dibutyl, dioctyl and dinonyl phthal-
ate, tricresyl phosphate, trixylenyl phosphate
and also polypropylene glycols can, for example,
be employed as plasticizers for modifying the
curable mixtures.
Especially for use in the lacquer field,
the new adducts containing epoxide groups can fur-
thermore be partially or completely esterified in
a known manner with carboxylic acids, such as espe-
cially higher unsaturated fatty acids. It is fur-
thermore possible to add other curable synthetic
resins, for example, phenoplastics or aminoplas-
tics, to such lac~uer resin formulations.
It is furthermore also possible to add
other usual additives, for example, agents for con-
ferring thixotropy, flow control agents such as
silicones, cellulose acetobutyrate, polyvinyl
butyral, waxes, stearates and the like (which
are in part also used as mold release agents)
to the curable mixtures.
The cura'ole mixtures can be manufac-
tured in the usual manner with the aid of known
mixing e~uipment (stirrers, kneaders, rollers and
the like).
The curable epoxide resin mixtures
referred to hereinabove can be employed in the
fields of surface protection, the electrical
industry, laminating processes and the building
industry. They can be used in a formulation
33,739A-F -18-
646~3-3865D
which is in each case suited to the particular end use, in the
unfilled or ~illed state, optionally in the form of solutions or
emulsions, as paints, lacquers, sintering powders, compression
molding compositions, dipping resins, casting resins, injection
mold.ing formulations, impregnating resins and adhesives, as tool
resins, laminating .resins, sealing and filling compositions floor
covering compositions and binders for mineral aggregates.
A main ~ield of application lies in the field of
compression molding powders and of sintering powders. Here the
epoxide resin pow~er mixtures can be processed without pressure or
with pressure, according to known processes such as fluidized bed
sinterin~, e:Lectrostatic fluidized bed sintering, spraying,
electrosta-tic spraying, compression molding and the like.
The following Examples and Comparative Experiments are
given to illustrate the invention of both the parent and
divisional application and should not be construed as limiting the
scope. All parts and percentages are by weight unless otherwise
indicated.
ExamPle 1
Preparation of 4,4'-(1,2-Ethanediyl)bis(3-bromo-2,6-
dimethylphenol) (Dibromotetramethvlbisphenol E)
A 20.0 g (0.074 mole) por~ion of tetramethylbisphenol E
was suspended in 75 ml of CC14. A 4.2 ml portion of bromine
(0.082 mole) was added at 23-2~C, and the mixture was heated to
reflux. All of the bromine has
-- 19 --
~,
5~
-20-
reacted by that time. Analysis by gas chromatography
(GC) and NMR indicated the following composition: 42
area percent starting material, 14 area percent mono-
bromotetramethyl-bisphenol E, and 43 area percent
dibromotetramethylbisphenol E. After adding 4.2 more ml
of bromine, the mixture was refluxed for 1.5 hrs and
analyzed by GC; the following composition was obtained: 2
area percent starting material, 7 area percent
monobrominated product, 90 area percent dibrominated
product, and 1 area percent tribrominated material.
Cooling of the slurry to 25C and filtration of the
insoluble solid gave 29.2 g of a brown solid which melts
at 191-194C. Recrystallization from toluene gave a
solid which melts at 194-197C and has the following
composition: 5 area percent monobromo, 93 area percent
dibromo, and 2 area percent tribromotetramethylbisphenol
E. The NMR spectrum is consistent with the proposed
structure: 1H ~R (acetone d6) ~: 2.20 (s, 6H, -CH3),
2.36 (s, 6H, -CH3), 2.86 (s, 4H, -CH2-), 6-88 (s, 2H,
-CH), and 7.35 (s, 2H, -OH).
Example 2
Preparation of 3,5-Dibromo-4-(2-(2-bromo-4-hydroxy-
-3,5-dimethylphenyl)ethYl)-2,6-dimethYlphenol (Tri
bromotetramethylbisphenol E)
A 12.5 g (0.046 mole) portion of tetramethyl-
bisphenol E was suspended in 100 ml of CC14, and 12.0 ml
(0.234 mole) of bromine was added at 23-25C. After
refluxing the mixture for 1.0 hr, the following com-
position was observed: 16 area percent dibromo, 77 area
percent tribromo, and 7 area percent tetrabromotetramethyl-
bisphenol E. The unreacted bromine was removed by
33,739A-F -20-
-21-
distillation. More CC14 was added (50 ml), and the
slurry is cooled to 25C. Filtration of the insoluble
solid afforded 18.2 g of a brown solid which melts at
249-255C. Recrystallization from toluene afforded a
5 gray-brown solid which melts at 257-262C, and has the
following composition: 6 area percen-t dibromo, 78 area
percent tribromo, and 16 area percent tetrabromotetra-
methylbisphenol E. It has the following NMR spectrum:
lH NMR (DMSO d6) ~: 2.12 (s, 3H), 2.28 (s, 9H), 3.20
(s, 4H), and 6.90 (s,lH).
Example 3
Preparation of 4,4'-(1,2-ethanediyl)bis(3,5-dibromo-
-2,6-dimethylphenol) (Tetrabromotetramethylbisphenol E)
A 27.1 g (0.1 mole) portion of tetramethyl-
bisphenol E was suspended in 100 ml of CCl4. A 60 ml
(1.17 mole) portion of bromine was added dropwise while
keeping the temperature below 30C using a water bath
for cooling. Immedia-te evolution of HBr was observed.
The mixture was brought to reflux for 2 hrs. The
excess bromine was removed by distillation with the aid
of 200 ml of CCl4. The mixture was cooled to 25C, and
the insoluble solid was filtered. This afforded 52.0 g
of brown solid which melts at 290-297C. Purification
of the insoluble solid involved suspending it in 100 ml
of acetone, refluxing for 1.0 hr, cooling to 25C, and
filtering the insoluble solid. A white solid was
obtained, 46.0 g, which melts at 295-297C and has the
following composition~ 75 area percent tetrabromo and
25 area percent tribromotetramethylbisphenol E. The 1H
NMR spectrum (DMSO d6) has a small single-t at 2.12 ~
and 2 major peaks, a singlet at 2.26 ~, and a singlet
at 3.20 ~, in a ratio of 3 to 1. This spectrum is
consistent with the proposed structure.
33,739A-F -21-
-22-
Example 4
Bromination Using A Friedel-Crafts Catalyst
A 136 g (0.5 mole) portion of tetramethyl
bisphenol E ls suspended in 1,400 ml of CH2C12. Fol-
5 lowing the addition of 2.0 g of FeC13, 86 ml (1.65
mole) of bromine was added at 20-24C. After refluxing
the mixture for 2.0 hr all of the bromine had reacted.
A portion of the solvent, 300 ml, was removed by dis-
tillation, and the slurry ~7as cooled to 25C. Fil-
tration of the insoluble solid afforded 262 g of a
light brown solid which has the following composition:
8 area percent dibromo, 58 area percent tribromo and 34
area percent tetrabromo tetramethyl-bisphenol E. The
lH NMR spec-trum is consistent with this composition.
Comparative Experiment 1 - No-t an embodiment of the
present invention.
Bromination of Tetramethylbisphenol F.
A 25.6-g portion of tetramethylbisphenol F
(0.1 mole) was suspended in 125 ml of carbon tetra-
chloride, and the slurry was cooled to 5C. A 6-ml
portion of bromine (0.12 mole) was added dropwise, and
the mixture was stirred for 15 minutes. All of -the
bromine reacted. Analysis of the mixture by gas
chroma-tography indicates that >90 percent of the
starting material reacts. The major product formed was
4-bromo-2,6-dimethylphenol, which was identified by
comparison with an authentic sample; a number of other
cleavage products were formed. Addition of 6 more ml
of bromine gave complete cleavage of the tetramethyl-
30 bisphenol F. .
33,739A-F -22-
3~
-23-
Comparative Experiment 2 - No-t an embodiment of the
present invention.
Bromination of Tetramethylbisphenol A.
A 14.2-g portion of tetramethylbisphenol A
(0.05 mole) was suspended in 100 ml of carbon -tetra-
chloride, and the slurry was cooled to 5C. A 3-ml
portion of bromine (0.06 mole) was added dropwise, and
the reaction was analyzed by gas chromatography. More
than 60 percent of the starting material reacted,
forming -two major produc-ts, one of thern being 4-bromo-
2,6-dimethylphenol. After stirring at 25C for two
hours, the insoluble product was filtered, 5.5 g, and
is identified as tetramethylbisphenol A. The carbon
tetrachloride solution has 4-bromo-2,6-dimethylphenol
as the main component, as identified by gas chroma-
tography and nuclear magnetic resonance, and by com-
parison with an authentic sample.
The preceding Examples and Comparative Experi-
ments surprisingly indicate that TDDPC compounds having
a polymethylene-bridge can be brominated on -the aromatic
rings, whereas similar compounds having only one linking
carbon atom do no-t ring-brominate.
Example 5
To a slurry of 25 g of brominated bisphenol E
25 (70 percent tribromo, 18 percent tetrabromo, 12 percent
dibromo) in 250 ml of epichlorohydrin plus 30 ml of
isopropanol at approximately 80C was added 19.6 g of
50 percent weight/weight NaOH in water over a two-hour
period. After the caustic addition, the reaction
30 temperature was maintained at approximately 80C for 2 .
33,739A-F -23-
-24-
hours. After cooling, the reaction mixture was
diluted with approximately 200 ml of CH2Cl2 and
was filtered to remove NaCl. Upon evaporation of
the CH2Cl2, the filtrate yielded white crystals.
Additional material was obtained by rotoevaporation
of the epichlorohydrin/isopropanol. The -total
yield of product was 23.8 g (78 percent).
The initial solid has a melting point
of 197C 200C and an epoxide equivalent weight
of 315.9 g. The second solid has a melting point
of 171C-175C and an epoxide equivalent weight
of 309 g. These data, along with nuclear magnetic
resonance spectra, suggest that the initial solid
is a~mixture of tri- and tetrabromotetramethylbis-
phenol E-diglycidyl ether, while the second solid
is a mixture of di- and tribromotetrame-thylbisphe-
nol E diglycidyl ether.
Comparative Experimen-t 3
~ The diglycidyl ether of tetrabromobis-
phenol A, 6.00 g (available from The Dow Chemical
Company under the name DER~ 5~2), was cured with
1.127 g diaminodiphenylsulfone. The cured resin
is maintained at 265C for 1 hour in a convection
oven. The resin was observed to lose >50 percent
of its weight.
Example 6
The first product of Example 5 was
employed in the procedure of Comparative Experi-
ment 3 as a replacement for -the diglycidyl ether
of tetrabromobisphenol A. The resin lost no
weight after 1 hour. .
33,739A-F -24-
3~
-25-
A comparison of the results of Exam-
ple 6 and Comparative Experiment 3 indicates that
the resin of Example 6 exhibits unexpectedly
improved thermal stability.
33,739A-F -25-