Note: Descriptions are shown in the official language in which they were submitted.
q~
NARROW GAP RETICULATE ELECTRODE
ELECTROLYS I S CELL
Field of the_Invention
This invPntion relates to electrolytic ceIls,
and particularly to cells producing an alkaline cathode
product such as chloralkali generation cells. More
specifically this invention relates to improved cathodes
and cathode assemblies for use in these electrochemical
cells, and to methods for making the~e improved cathodes
and assemblies.
Background of the Invention
Electrolytic cells for tne generation of
chemical reac~ion products are widely employed. One field
particularly where these cells have found widespread use
is in generation of halogens and caustic compounds from
salts of the halogen. In such cells, the halogen is
generally evolved at the anode, while the caustic compound
is evolved adjacent the cathode.
Recently a considerable efort has been directed
towards the development of improved anode configurations
that enable operation of the electrochemical cell more
e~ficiently. These efforts have born fruit in the
development of~such improvements as dimensionally s~able
25~ anodes DSA~3 a proprietary anode coating system of Diamond
Shamrock Corporation. Anode improvements ha~e assisted in
mproving economics in operating chloralkali cells.
: :
: ,, ~
. .. . .
- .
In cells where a separator such as a diaphragm
separates the cell defining anode and cathode
compartments, considerable effort has been devoted to
development of improved separators. Sep~rators based, for
example, upon perfluorocarbon copolymers and having
pendant cation exchange functional groups have been
identified as providing, under certain cell operating
conditions, the opportunity for achieving considerable
economic advantage in operating a cell.
One remaining inefficiency in electrochemical
cell operation is associated with power inefficiencies
having their root in spacing imposed in most conventional
cells between the separator and anodes and cathodes
utilized in the cell. ~. variety of reasons can exist for
the presence of the spacing. One common reason relates,
for example, to gas bubble release difficulties where an
electrode is pressed into a relatively soft separator such
as a diaphragm type separator.
Spacing between anode and cathode in an
electrochemical cell requires electrical current to follow
a current ~pathway through cell electrolyte(s) where
resistance to current pa.ssage can be relatively elevated.
Generally, wider spacings between anode and cathode
require that a more elevated voltage be applied to the
cell to effect the desired electrochemical reaction. This
elevated voltage requirement adds to electrical power
consumption in operating the cell, adding to costs of cell
operation.
A number of proposals exist focused upon
reducing anode cathode spacing within a cell, and thereby
reducing power consumption associated with cell operation.
Reduced anode cathode spacing has been proposed for
application to cells separated by a hydraulically
permeable diaphragm and by a hydraulically impermeable
membrane.
In diaphragm cells, for example, the spacing
between anode and cathode has been reduced until one or
'~
'
-. -
both of the electrodes contacts the diaphragm. Many
diaphragms are fabricated from materials which subject the
diaphragm to swelling in cell environmentO Electrodes
~tilized in such cells are frequently Qf a wire or mesh
construction. Swelling of a diaphragm in contact with
such an electrode can cause partial plugging of apertures
in the electrode leading to poor release of gas bubbles
being generated adjacent the electrode, and restrict
flow of electrolyte from anode to cathode compartment
through the diaphragm. One resulting repercussion can be
an overvoltage at the electrode offsetting power gains
achieved by reducing anode cathode spacing at least in
part.
~n membrane cells, the mer~brane, g~neral'y a
cation exchange material, is normally quite thin, being on
the order of a few mils. In addition such membranes
frequently exhibit substantial dimensional stabilit~,
making placement of electrodes adjacent the membranes
feasible without substantial risk of membrane expansion
plugging apertures in the electrode. However when mesh
electrodes -or those fabricated from wire are placed
adiacent a membrane allowing the electrodes to be within a
few mils of one another, lines of electric flux between
the individual elements of the electrodes do not always
encompass all of the membrane material- separating the
anode and cathode resulting in inefficient use of the
membrane and a correspondill~ increase in voltage drop
attributable less than optimal electrolytic flux through
the membrane~
Additionally, where a grid or mesh type
electrode is contacted with a membrane, gas bubbles tend
to agglomerate within apertures of the grid and these gas
bubbles often lead to an overpotential at the electrode.
In one proposal for a closer anode to cathode
spacing, as shown for example in U.S. Patents 4,253 r 924;
4,253,922; and 4,250,013, a porous perhaps conductive
secondary electrode material is utili~ed to fill,
'~ 3
-- 4
particularly the cathode compartments of the cell, and to
press a primary electrode into contact with a cell
membrane. An interface between the primary electrode and
the porous secondary electrode material.can substantially
contribute to electrode resistance between the two and at
least partially negate advantages otherwise available from
the large electrode surface area potentially presented by
the secondary electrode materials.
A cell configuration wherein a primary foam or
reticulate electrode contacts a cell divider offers
potential for improved economics in the operation of
electrolyte.
- Disclosure of the Invent on
The present invention provides a electrode
assembly for use in electrochemical cells whereln a
separator divides the cell into anode and cathode
compartments. A porous reticulate, generally in the form
of an openly porous foam appearing structure,
substantially fills the electrode compartment, being in
substantial physical contact with the separator.
~urrent collector ls bound intermetallically to the
electrode so that voltage losses associated with the
transfer of electrical current between the electrode and
collector are negligible. Electrolyte distribution means
are provided for introducing electrolyte into and removing
electrolyte from the electrode.
The electrode assembly of the instant invention
is made by substantially filling an electrode compartment
of a cell with the porous electrode material generally of
a foamed nature so that the foam substantially physically
contacts the separatorO The foam, made conductive, is
subjeoted to deposition techniques whereby an electrode
metal is coated upon the foam forming a reticulate
electrode. A current feeder is provided for electrical
contact with the foam and is intermetallically bound to
.
~.
.
the foam electrode in substantlal electrical contact with
the foam or reticulate electrode.
These reticulate electrodes, generally cathodes,
can be made conducti~e by conventional ~echniques such as
carbon impregnation and electroless plating.
El~ctrodeposition can be accomplished by conventional
techniques. These techniques can be applied in situ
within the electrolytic cell, or external to the cell.
One advantage to the method of the instant
invention for making electrode assemblies is that the
steps can be performed interchangeably. That is the~ foam
may be cut to size first, made conductive first, or made
conductive and electroplated before cutting. This
flexibility permits wide options in fabricating cells fcr
a variety of end uses.
With a porous foam or reticulate electrode in
contact with the separator, only the thickness of the
separator need space the anode from ~e ca~ode within the oell,
reducing voltage requirements associated with electrical
current travel through the cell electrolyte. Forced
circulation~of electrolyte through the porous electrode
can assist in suppressing bubble accumulation adjacent
surfaces of the separator in contact with the electrode
and can function to reduce concentration gradients within
the electrode compartment particularly adjacent the
separator. Since concentration gradients and bubble
formation both can contribute to overpotentials, their
reduction can lower voltages required for operating an
electrochemical cell utilizing the electrode assembly of
the instant invention.
The above and other features and advantages of
the inventlon will become apparent from the following
detailed description of the invention made with reference
to the accompanying drawing which together form part of
the specification.
::
''
.
.
-- 6 --
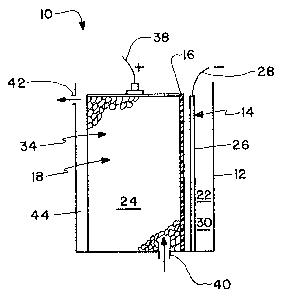
escri~on of the Drawings
Fig. 1 is a cross sectional representation of an
electrochemical cell embodying a cathode assembly of the
instant invention.
Best Embodiment of the Invent:ion
-
Referring to the drawing, Fig. 1 shows an
electrochemical cell 10, in this best embodiment a
chloralkali cell, in cross section. The cell includes a
housing 12, an anode assembly 14, a separator 16, and a
~athode assembly 18.
The housing 12 can be of any uitable or
conventional material relatively chemically inert to
electrochemical contents of the cell. Where, as in this
best embodiment, the electrochemical cell is one for the
generation of chlorine and caustic products from a brine
of an alkali metal halogen~to;~salt, the housing can be
fabricated from a plastic material such as polypropylene.
Generally a cell cover, not shown, is fitted to the upper
portion of the cell during operation.
The cell is divided into anode 22 and cathode 24
compartments by the separator 16. This .separator can be
either of a hydraulically porous nature such as a
diaphragm or be of a hydraulically impervious nature such
as a cation exchange membrane. Where the separator is of
a diaphragm nature, the diaphragm can be one prepared by
any of a variety of well known techniques to yield a
hydraulically permeable separator. Generally such
diaphragm separators include asbestos fibers when
fabricated for use in a chloralkali cell.
Where the cell is divided by a membrane, the
membrane generally separates the compartments 22,24 in a
manner precluding free fluid movement between the
compartments. It is necessary that this membrane transmit
electrical current between the compartments, and therefore
such membranes are generally capable of transmittlng a
particular ion or charged species between the
compartments. Where electrolyte contents of the
. : ..
,
-:
.
-- 7 --
electrochemical cell include aggressive compounds, it is
desirable that the membrane be fabricated from a compound
substantlally resistant to aggressive attack by
electrolyte contained in the compartment~;.
For a chloralkali cell of this best embodiment,
this membrane may be of a suitable or conventional
material resistant to aggressive materials included in
electrolytes contained in eash compartment 22,24. One
much preferred material is a perfluorinated copolymer
having pendant cation exchange functional groups. These
perfluorocarbons are a copolymer of at least two monomers
with one monomer being selected from a group including
vinyl fluoride, hexafluoropropylene, vinylidene fluoride7
trifluoroeth~lene, chlorotrifluorcethylene, perfluoro
(alkylvinyl ether), tetrafluoroethylene and mixtures
thereof.
The second monomer o~ten is selected from a
group of monomers usually containing an SO2F or sulfonyl
fluoride pendant group. Examples of such second monomers
can be generically represented by the formula
CF2=CFRlSO2F. R1 in the generic formula is a bifunctional
perfluorinated radical comprising generally 1 to 8 carbon
atoms but upon occasion as many as 25. One restraint upon
the generic formula is a general requirement for the
presence of at least one fluorine atom on the carbon atom
adjacent the -SO2F group, particularly where the
functional group exists as the -(-SO2NH)mQ form. In this
form, Q can be hydrogen or an alkali or alkaline earth
metal cation and m is the valence of Q. The R1 generic
formula portion can be of any suitable or conventional
configuration, but it ha~ been found preferably that the
vinyl radical comonomer join the R1 group through an ether
linkage.
Such perfluorocarbons, generally are available
commercially such as through E. I. duPont, their products
being known generally as NAFION.~ Perfluorocarbon
copolymers containing perfluoro~3,6-dioxa-4 methyl-7-
, ......................................... .
octenesulfonyl fluoride) comonomer have found particular
acceptance in C12 cells. Where sodium chloride brine is
utilized for making chloralkali products from an
electrochemical cell, it has been found advantageous to
employ membranes having their preponderant bulk comprised
of perfluorocarbon copolymer having pendant sulfonyl
fluoride derived functional groups, and a relatively thin
layer of perfluorocarbon copolymer having carbonyl
fluoride derived functional groups adjacent one membrane
surface.
The anode compartment 22, includes an anode 26,
and an anodic cuxrent feeder 28. The current feeder 28
communicates with a source of electrical current, not
shown. An electrolyte 30 generalLy fills void space
within the anode compartment. Generally this electrolyte
30, or anolyte is a brine of an alkali metal halogen salt
prepared according to well known methods. The
compositions of these brines are generally well known in
: the industry.
The anode 26 is fa~ricated of a suitable or
conventional material suitably resistant to the anolyte
and to halogen compounds bein~ generated within the
electrolytic cell. Typically titanium is utilized having
an applied co~ting of one or more metals or metal oxides
such as ruthenium oxide. DSA ~ anodes, available from
Diamond Shamrock Corporation are well suited for use in a
cell such as is shown in this best embodiment.
The anode 26 can be positioned immediately
adjacent the separator; or at a distance from the
separator. In one equally preferred alternate to this
preferxed embodiment a catalyst such as ruthenium oxide
attaches directly to the separator, a membrane, in contact
with a grid or mesh like current collector.
The cathode compartment 24 includes a cathode
a~sembly 18. The cathode assembly 18 comprises a foam
like reticulate cathode 34 , a cathodic current feeder 38
and an inlet 40 and outlet 42 for electrolyte. The
~e!r4 '
~` ' ' ~ ;
'
- ~ 9
cathode compartment is generally filled with an
electrolyte 42 or catholyte that includes a hydroxide of
the alkali metal included in the halide salt forming the
brine. This catholyte also fills tha~ portion of the
cathode compartment not occupied by the cathode 34.
The cathode is of an openly porous reticulate
nature. While pores can be of any suitable or
conventional size, pores of between 0.013 and 10 milli~leters
are preferred, wi-th pores of between abou-t 0.025 and 5 milli-
i n me-ters being much preferred. By openly porous what is
meant is that the cathode is substantially hydraulically
permeable throughout its structure.
The cathode 34 includes a substrate formed of a
resinous or plastic material such as polyure-thanes,
polyesters, olefin polymers such as polypropylene or
polyeth~lene, or other suitable or conventional materials.
~his substrate is utilized in the form of a foam, and need
not be a rigid foam.
The substrate i5 encapsulated at least in part
by one or more coatings of at least one conductive cathode
metal. These coatings can be applied to the substrate in
~ny suitable or conventional manner such as by
electrodeposltion. For electrodeposition, electrical
conductivity of the resinous or plastic foam substrate
generally is required. The foam substrate generally can
be made conductive by suitable or conventional well known
techniques such as electroless plating, or by impregnation
with a corlductive substance such as carbon.
Application of the coating metal renders the
foam reticulate conductive and thereby suitable for use as
a cathode. While a variety of cathode metals are known,
for purposes of this best embodiment in the context of a
chloralkali cell, nickel and/or copper are preferred.
Particularly nickel appears to function in assisting the
electrochemical reaction at the cathode. Metal coating
upon the substrate need be only sufficiently thick and
ContinUQuS to provide a negligible resistance to
,
' '
:,:
-- 10 --
electrical current flow through the cathode to a point of
electrical current collection.
The reticulate cathode is cc)ntained in the
cathode compartment 24 generally in contact with the
separator. Since the foam reticulate becomes relatively
rigid upon application of the metal coating, it i5 often
preferred that the foam be sized for being received in the
cathode compartment prior to application of the metal~
coating.
The reticulate cathode functions as a primary
electrode within the cell. Electrical current is supplied
to this reticulate primary cathode via the cathodic
current feeder 38. It is much preferred that elec~rlcal
resistance associated with any electrical interconnection
between the reticulate cathode and the current feeder 38
be negligible. Preferably this low electrical resistance
is accomplished by making the connection intermetallic in
nature.
: One method by which the cathodic current feeder
can be attached to the reticulate cathode is by inserting
the current feeder 38 into the foam substrate of the
reticulate cathode 34 prior to application of the coating
metal. -Insertion c~n be accomplished by slitting the foam
suhstrate of the reticulate structure and inserting the
current feeder, heating the current feeder to a
temperature in excess of the melting temperature of the
foam and immexsing the heating feeder into the foam,
coextrusion or forming of the foam with the current feeder
embedded. Intermetallic joining of the current feeder and
reticulate cathode can then be accomplished by
electrodeposition of the metal for coating the foam
substrate while utilizing the current feeder for supplying
electrical current for the electrodeposition of the
coating metal.
The inlet 40 and outlet 42 are arranged to
provide circulation of catholyte through the foam
reticulate cathode. The reticulate cathode being porous,
,~
. .
,
catholyte is relatively readily forced through the cathode
using any suitable or conventional means such as by
p~mping. One or more inlets and/or outlets can be
provided depending upon the size of the cathode, the
degree of circulation desired and other factors.
In operation of the electrolytic chloralkali
cell of this best embodiment, metal ions, usually sodium
ions, traverse the separator from the anode compartment,-
at least partially in response to electrical current
flowing through the cell. These sodium ions react at the
cathode with hydroxyl radicals being produced by the
disassociation of water at the cathode, but remain in
ionic solution. As operation of electrochemical cell
continues,-the concentration of these metal ions adjacent
the separator typically can increase, providing a
concentration gradient resistance to further mi~ration of
metal ions. Overcoming this resistance would ordinarily
require a more elevated cell voltage between the anode and
cathode within the cell, consequently increasing power
requirements for cell operation. Circulation, tending to
reduce this concentration gradient resistance or
overpotential, can avoid an increased power consumption in
cell operation.
The disassociation of water ongoing at the
cathode can produce hydrogen, forming into bubbles. Where
these bubbles adhere to the cathode these bubbles can
effectively reducè the cathode surface available for
electrochemical reaction, resulting in an electrical
resistance or overpotential. Circulation of catholyte
through the reticulate cathode can reduce bubble
adherence, and thereby avoid an elevated operational cell
voltage that might otherwise be required to compensate for
this bubble overpotential.
The volume of catholyte desirably circulated
through the cathode can vary, with generally lower flow
rates being preferred to conserve power. Flow rates of
between about 1 liter per minute per cubic meter of
- 12 -
reticulate cathode and 250 liters per minute. Only a flow
rate sufficient to avoid bubble and concentration
overpotential need be utilized.
It is not necessary that the reticulate cathode
36 fill the cathode compartment 24 entirely. The
reticulate foam cathode need only fill portions of the
cathode compartment adjacent the separator and be in
substantial physical contact with the separator. Where
the reticulate cathode does not fill the cathode
compartment completely, the balance of the cathode
compartment can be filled with a resistant material such
as a foam capable of functioning to bias the reticulate
cathode into contact with the separator. Alternately, a
reticulate- cathode not completely filling the cathode
compartment can be biased into contact with the separator
in any suitable or conventional manner such as by using a
resilient grid.
In one al~ernate of the best embodiment, the
cathodic current collector is first embedded in the foam
substrate of the reticulate cathode. The foam substrate
is fitted t~ the cathode compartment of the cellO Where
the foam substra~e has not previously been rendered
electrically conductive by reason of carbon impregnation
or like process, a preliminary metal coating is applied to
the foam substrate by electroless plating techniques.
Generally this electroless plating can be accomplished
within the confines of the electrolytic cell, and in most
preferréd applications is conducted primarily to impart
conductivity to the foam substrate for subsequent
electrodeposition of cathode metal.
Subsequent metal electrodeposition onto the
reticulate cathode assembly being formed preferably is
conducted within the confines of the electrolytic cell.
Plating solution is introduced into the cathode
compartment, and the cathodic current feeder is connected
to a source of electrical current. Metal ions contained
in the plating solution thereby become deposited upon the
. .,
p ~ ~ ~
- 13 -
reticulate foam substrate using well know platin~
techniq~es.
During electrochemical cell operation this
catholyte metal deposited upon the foam substrate is
5 cathodically protected from the action of aggressive
chemicals present in the catholyte. In a chloralkali cell
where the separator is a cation exchange mPmbrane, metal
ions such as sodium ions traverse the membrane from ~he
anode compartment and react with the hydroxyl radicals
being produced at the reticulate cathode to create a metal
hydroxide solution comprising the catholyte. Hydrogen gas
is evolved. By periodic removal of some catholyte from
the catholyte being circulated through the reticulate
cathode, and replacement with water, metal hydrcxide
concentra~ion within the catholyte can be controlled
within desired, well known preferred limits in the
operation of a membrane chloralkali cell.
Where the reticulate electrode contacts a porous
separator such as a diaphragm, brine containing ions of
the metal flows through the diaphragm to the cathode
compartment~ joining catholyte circulating through the
cathode compartment. Removal of circulating catholyte
compensates in ~ell known manner for spent brine volumes
traversing the separator, and controls metal hydroxide
concentration in the circulating catholyte.
Anodes may be fabricated for use in electrolytic
cells in a fashion identical to the formation of the foam
or reticulate cathode assemblies described supra. Such
foam or reticulate anodes may be utilized in
electrochemical cells and substantial physical contact
with a separator dividing anode and cathode compartments
in the cell. Frequently it is advantageous that anodes
fabricated in accordance with this invention include a
topcoatins or electrocatalytic coating applied after
~ormation of the foam or reticulate anode assembly.
Typical coatings would include DSA ~, or TIR-2~00/
proprietary coatings sys~em manufactured by Diamond
* Trademaxk
~.~ . . ':
- 14 -
Shamrock Corporation and other suitable or conventional
electrode coatings~
The following examples further illustrate the
invention.
EX~LE 1
A nickel reticulate cathode was prepared by
first attaching a nickel current distributor, fabricated
from nickel grid sheet stock, to a conductor bar. The
grid and conductor bar were then heated above the melting
point of polyurethane foam, the foam being sized to occupy
substantially the entirety of the cathode compartment of
an electrolytic cell. The hot conductor bar with grid was
set into the foam and permitted to cool. Wit~ cooling,
foam melted by the heat of the conductor bar and grid
fused to the grid. The resulting cathode assembly was
then plated. Such plated cathodes functioned effectively
in electrolytic cell where the foam contained between 10
and 45 pores per inch ~ppi~.
For plating the cathode assembly was immersed in
a room temperature bath consisting of an aqueous solution
of 10 gram per liter (gpl.) tin chloride and 10 milliliter
(ml) per liter hydrochloric acid (20Be). After 5
minutes, the cathode assemblv was rinsed gently in room
temperature water, and then immersed in an aqueous
solution o~ 0.5 gpl. PdC12 and 10 ml per liter
hydrochloric acid for five minutes. Following an aqueous
rinse, the foam cathode assembly was immersed for 10
minutes at 50C in a mixture comprising one liter of an
aqueous solution of 45 gpl. nickel chloride hexahydrate,
50 gpl. ammonium chloride, 100 gpl. sodium nitrate, 0.5
liters per liter ammonium hydroxide and 27.3 ml of a 450
gpl. aqueous solution of sodium hypophosphite. These
preceding steps deposited an electroless nickel plate upon
the cathode assembly.
Electroless plating was followed by nickel
electrolytic plating. The cathode assembly was immersed
~'
. . .
~ '
- 15 -
in an aqueous solution of 141 gpl. nlckel chloride
hexahydrate, 291 gpl. nickel sulfate hexahydrate and 45
gpl. phosphoric acid, with the solution being adjusted to
a pH of 4.5 to 6.5 using hydrochloric acid. The cathode
assembly was made cathodic to nickel anodes mounted
approximately 3.8 centimeters from the surfaces of the
foam cathode assembly, and plating was conducted at 2.25
volts for 3 hours at 30-40C. Plating is continued until
a coating of 10 microns thickness or greater was
established upon the foam.
Optionally, after 1 1/2 hours of plating, the
polyurethane foam may be ashed by placing the reticulate
in a flame or oven until the foam is burned out. After
rinsing, plating can then be continued.
EXAMPLE 2
A nickel reticulate cathode was prepared in
accordance with Example l, except that after completion of
electroless plating the cathode assembly was immersed in
an aqueous solution of 250 gpl. nickel chloride
hexahydrate; and 50 gpl. zinc chloride. This solution was
maintained at 45C with a pH o~ approximately 4.5,
adjusted by the addition of ECl. Nickel and nickel zinc
anodes were placed in close proximity to surfaces of the
cathode assembly for about one hour and made anodlc to the
cathode assembly at 2.00 volts. The solution was agitated
during the electrolytic plating operation.
Following completion of electrolytic plating,
the cathode assembly was immersed in an aqueous solution
of 200 gpl. sodium hydroxide for one hour at 75C.
EXAMPLE 3
The fabrication steps of Example 1 were repeated
except that electrolytic plating was conducted from an
aqueous solution comprising 265 gpl. cobalt chloride
hexahydrate, 90 gpl. zinc chloride, and 30 gpl. boric acid
at 50C and a pH of 4 maintained by the addition of
~ .
" , ' .
- 16 -
hydrochloric acid. Zinc anodes, mounted approximately an
3.8 centimeters from surfaces of the cathode assembly were
utilized to plate at 0.6 volts zinc onto the cathode
assembly over a period of one hour.
.
EXAMPLE 4
A cathode assembly was prepared in accordance
with Example 1 except that the current clistribution grid~
was fabricated from copper in lieu of nickel. An
~lectroless copper plate was deposited on the polyurethane
foam by immersing the cathode assembly for 5 minutes at
room temperature sequentially into two baths. The first
bath contained 10 gpl. tin chloride and 10 ml per liter
hydrochloric acid (20Be). The second bath contained 0.5
gpl. palladium chloride and 10 ml per liter hydrochloric
acid (2OBe). ~ter each bath a water rinse at room
temperature was conducted. The cathode assembly was then
soaked for 20 minutes at room temperature in commercial
copper electroless plating solution made by mixing 777A
and 777M Cu Electroless Makeup (available from CuTech
Inc.) in a I:1:8 ratio with water and then rinsed gently~
The cathode assembly was then immersed in a
electrolytic plating bath comprising an aqueous solution
of 40 gpl. copper as copper sulfate, 10 gpl. sul~uric acid
and having a pH of 1 maintained by the addition of
sulfuric acid at room temperature. Copper
electrodeposition was conducted by placing copper anodes
3.8 centimeters from surfaces of the cathode assembly,
making the cathode assembly cathodic to these copper
anodes, and passing 1 1/2 volts between them for
approximately 1 hour. Optionally the polyurethane foam
may be ashed in accordance with Example 1 approxima~ely
half-way through the electrodepositionO
Since copper by itself may be excessively
subject to corrosion/attack in a chloralkali cell cathode
~ 2 ~ i~ 5 ~L't'; L ~l
- 17 -
compartment, advantayeously the copper electroplated
cathode assemhly was then nickel plated in accordance with
the electrolytic plating step of Example 1.
EXAMPLE 5
A cathode assembly was prepared by attaching a
current feeder to a current distribution grid fabricated
from nickel in accordance with Example 1. Heated, the~
current distribution grid and current feeder were immersed
into a polyurethane foam in accordance with Fxample
resulting in a foam or reticulate cathode assemhly.
The foam cathode assembly was then electrolessly
plated with copper in accordance with Example 4 and
received an e'ectrolytic copper plating in accordance with
Example 4. An electrolytic nickel plate was then applied
to the foam cathode assembly in accordance with Example l.
An electrolytic deposition of nickel and zinc
was then made to the reticulate cathode assembly in
accordance with Example 2.
~ EXAMPLE 6
A cathode assembly was prepared in accordance
with Example l. Following application of the
electrolytic nickel a palladium oxide-zirconium oxide
coating was applied to the cathode. Application was
accomplished by ball milling a slurry of 10 ml of waterr l
ml of acetic acid, 1.5 grams of palladium chloride
particles and 2 grams of zirconyl nitrate for two hours to
stabilize the palladium chloride and reduce the size of
any non-solubilized material particles~ This slurry was
then brushed onto the nickel pla~ed reticulate cathode
assembly of Example l which had been ashed in accordance
with Example 1. The brush coating and cathode were heated
at 125C for 3 minutes and then cured at 500C for 7
minutes in air thereby converting the palladium chloride
to palladium oxide and the zirconyl nitrate to zirconium
dioxide. Four additional coatings or the slurry were then
.
`;
::
- 18
applied and cured. In alternate preparation techniques,
as much as one-half of the palladium chloride was replaced
by cobalt and/or nickel in preparing the cathode.
ExAMæLE 7
A foam or reticulate cathode c~ssembly was made
in accordance with the steps of Example 1 including the
application of nickel electrolytic plate. Following-
electrolytic plating with nickel, the foam cathode
assembly was immersed in an electrolytic plating bath
comprising one gallon of water, 20 grams sulfamic acid,
and 20 grams of ruthenium as ruthenium sulfamate. The
bath was maintained at between 80 and 100F and
electrodeposition was conducted usi~g platin~m anodes
spaced approximately 3.8 centimeters from surfaces of the
foam cathode assembly. With the foam cathode assembly
made cathodic, current was passed between the platinum
anodes and the foam cathode assembly at a density of 0.15
amps per square centimeter at 1.75 volts.
- EXAMæLE 8
A foam or reticulate cathode assembly was
prepared in accordance with Example 1 including
electrolytic nickel plating. Following the electrolytic
nickel plate, the foam cathode assembly was immersed in an
agitated coating solution containing 5 ml of H3Pt(SO3~2OH
in 150 ml of water adjusted to a pH of 3 by the use of l
normal NaOH, and including approximately 15 ml of a 30%
hydrogen peroxide solution. The foam cathode assembly was
soaked in this peroxlde containing solution for
approximately one hour during which the pH gradually
dropped ~o 1. The pH was then restored to 3 using one
normal caustic and the solution and cathode assembly were
heated to 80C while agitating the peroxide containing
solution until all peroxide bubbling stopped. After
washing, the foam cathode assembly was dried at l-S-150C.
~ .
;
.. ,,;
3~
-- 19 --
EXAMPLE 9
A foam cathode assembly was prepared in
accordance wlth Example 1 including electrolytic nickel
plating. Sintering or ashing was conduc.ted in accordance
with Example 1. Following electrolytic deposition of
nickel, a coating of aluminum was applied by plasma
spraying onto surfaces of the foam cathode assembly. The
assembly was then heat treated at 760C for 8 hours in a
nitrogen atmosphere interdiffusing the nick~l and
aluminum. In actual operation of a chloralkali cell using
such a cathode, the aluminum would be leached from the
interdiffused surface by hot NaOH contained within the
cell. Leaching provides greater surface area on the foam
cathode asse.nbly than a~ailable without interdiffusing and
leaching.
EXAMP~E 10
~ foam or reticulate cathode assembly was
prepared in accordance with Example 1 except that
electrolytic plating was accomplished by immersing the
foam cathode assembly into an aqueous solution of 240 gpl.
ferrous sulfate at a pH of 2.8 to 3.5 and a temperature of
between 32C and 66C. The cathode assembly was made
cathodic to iron anodes available from Armco Steel Company
positioned approximately 3.8 centimeters from the surfaces
of the cathode assembly. Plating was conducted for
between l and 3 hours at between 0.04 amps per square
centimeter and 0.11 amps per square centimeter. The
ferrous sulfate bath was agitated during electrodeposition
to assist in providing a uniform coating upon the foam
cathode assembly.
EXAMPLE 11
A foam or reticulate cathode assembly was
fabricated in accordance with Example 4 and then subjected
to electrodeposition with iron in accordance with Example
10 .
V'3 ~
- 20 -
EXAMPLE 12
A foam or re~iculate cathode assembly was
prepared in accordance with Example 1 including sintering
or ashing intermediate during the ,electrodeposition
op~ration. The cathode assembly was then plasma sprayed
with a mixture of 80% nickel, 10% mo],ybdenum and 10
aluminum. The molybdenum and aluminum were then leached
in hot sodium hydroxide by operation in' a chloralkali~
cell. A cathode assembly having a substantially elevated
10 surface area resulted.
- EXP~IPLE 13
Example 1 was repeated except using as a
starting m~terial polyurethane foam 1.25 centimeters in
15 thickness made by laminating four thicknesses of 0.32
centimeters foam. A structure indistinguishable from the
structure of Example 1 resulted.
EXAMPLE 14
.
Two electrolytic cells were operated in
parallel. ~ne cell was equipped with an anode made in
accordance with E~ample 1. The cathode in this first cell
was a perforated nickel plate The cell included a
separator fabricated from NAFION ~ 295, a product of E. I.
2~ duPont deNemours and Company, a cation exchange material
suitable for use in electrolytic chloralkali cells. The
cell was fed with an aqueous stream of sodium bicarbonate
and sod,ium carbonate in a 1:1 molar ratio.
The second cell was equipped identically with
'30 the first cell except that the cathode in the second cell
was a porous foam or reticulate cathode made in accordance
with Example 1. This second cell operated on the same
feed materials as the first cell.
In both cells, the electrodes were in
substantial physical contact with the separator. The
first cell operated at a voltage of 2~82 volts under
current ~low of 0.15 amps per square centimeter, while the
's~'~ ' .
' ~
.,., ~'.` . ' .
J'~
- 21 -
second cell demonstrated a voltage of 2.63 volts at 0.15
amps pex square centimeter. The first cell demonstrated a
voltage of 3.33 volts at 0.31 amps per square centimeter,
while the secvnd cell demonstrated a vol~age of 3.05 volts
a 0.31 amps per square centimeter. Current efficiency
between the cells was equivalent within experimental
laboratory accuracy. The second cell, because of the
presence of the nickel reticulate or foam cathode`
assembly, operated at a significantly lower voltage which,
in a commercial operation, would result in a lower power
requirement for cell operation. The nickel reticulate or
foam cathode assembly provided an operational advantage of
200 millivolts at 0.15 amps per square centimeter and 280
millivolts-at 0.15 amps per square centimeter.
EXAMPLE 15
__
Two electrolytic cells were operated in
parallel. One cell included an anode fabricated from a
titanium mesh having diamond shaped openings
approximately 0.64 centimeters by 0.32 centimeters and
coated with- a DSA R electrocatalytic coating for the
production of chlorine. DSA R coated titanium mesh is
available from Diamond Shamrock Corporation and generally
includes ruthenium oxide as a surface coating. The
cathode in this first cell was formed from nickel mesh
having diamond shaped apertures also approximately 0.64
centimeters by 0.32 centimeters. The cell included a
separator made from duPont NAFION R 295, both the anode
and cathode being in intimate contact with the NAFION R
separator. The cell compartment defined by the separator
and containing the anode was fed with sodium chloride
brine (170 grams per liter).
The second cell in the para~lel pair was
equipped identically except that the cathode provided was
a foam or reticulate cathode assembly made in accordance
with Exam~le 1. This cathode, as well as the anode in the
second cell, were in intimate contact with the separator.
.,
.. . .
.. . :
:
~ ~ .
J~
- 22 -
The feedstock to this second cell was identical with the
feedstock of the first cell. Both cells were operated at
0.31 amps per square centimeter of membrane surface area
with the first cell requiring 3.84 vol~s and the second
cell at 3.18 volts for electrolysis. Current efficiencies
were equivalent. Use of a reticulate foam electrode
provided the second chlorine generation cell with an
advantage of 660 millivolts at 0.31 amps per square
centimeter.
EXAMPLE 16
Two cells were operated in parallel. One cell
included an anode fabricated from a titanium mesh having a
DSA elec'rocatalytic coa~ing a~plied to the mesh~
Apertures in the mesh were appro~imately 0.32 centimeters
by 0.32 centimeters. The cathode in this first cell was
fabricated from nickel mesh having the same aperture
dimensions. Anode and cathode were in contact with and
separated by a NAFION 290 cation exchange membrane. A
i70 gram per liter sodium chloride brine was fed to a
compartment~ of the cell defined by the separator
containing the anode.
The second cell of this parallel pair included
an identical anode and separator but was fabricated
utilizing a nickel foam or reticulate cathode assembly
made in accordance with Example 1. Feedstock to this
second cell was identical with the feed to the first cell.
While the first cell operated at 3.28 volts generating
cnlorine at a current density of approximately 0.3 amps
per square centimeter measured at the separator, the
second cell achieved an operating voltage of 3.13 volts
operating at an identical current density. Current
efficiencies of the cells were identical.
EXAMPLE 17
Two cells were operated in parallel~ In one
cell the anode was a porous foam reticulate anode
.
, `' `'~ .
.
.
:'`, ' ` '~` '
- 23 -
structure fabricated in accordance with Example 1. The
first cell included a cathode fabricated from perforated
nickel plate. The foam or reticulate anode assembly and
nickel plate were in substantial contact with a
microporous separator fabricated from polypropylene. 300
gram per liter sodium carbonate was fed to this cell.
The second cell contained an identical anode and
separator but included a foam or reticulate cathode
assembly in contact with the separator, the foam or
reticulate cathode being made in accordance with Example
1. An identical feedstock was provided to this second
cell. While the first cell operated at 2.72 volts at a
current density of 0.15 amps per square centimeter, the
second cell achieved a 2.44 operating voltase at an
identical current density. Within experimental error, the
current efficiencies of the two cells were equivalent.
The cell operated using a reticulate cathode achieved a
280 millivolt operatin~ advantage over an identical cell
operated without the reticulate cathode.
~ EXAMPLE 18
Two cells were operated in parallel. The first
cell included a cathode fabricated from perforated nickel
plate in intimate contact with a porous ceramic alumina
separator. The anode in this first cell was a sheet of
titanium metal mesh having apertures of approximately 0.64
centimeters by 0.32 centimeters and coated with TIR2000 R
a Diamond Shamrock proprietary anode coating useful where
it is desired that oxygen be evolved. This first cell was
fed with 300 grams per liter sodium carbonate.
The second cell was operated equipped
identically with the first cell except that the anode in
the second cell was a foam or reticulate anode fabricated
in accordance with Example 1~ The feedstock to this
second cell was identical to that of the first. While the
first cell operated at 3.4 volts at 0.15 amps per square
centimeter, the second cell, using the foam or reticulate
~: ,
,. ~'. . :
- 24 -
anode assembly, operated at 2.72 volts at an identical
current density. Current efficiency of the two cells
within experimental error, was identical. The second
cell, using the foam or reticulate anode assembly in
contact with the separator, achieved a .680 volt operating
advantage over a perforated nickel plate anode in contact
with the separator.
While a preferred embodiment has-been shown and~
described in detail, it should be apparent that various
modifications and alterations may be made without
departing from the scope of the claims following.
:' '.