Note: Descriptions are shown in the official language in which they were submitted.
5~
-- 1 --
Case 5-13861/ZFO/~/DIV
Qui~olone intermediates for the preparation of 1,2,5,6-
Tetrahydro-4H-pyrrolo[3,2,1-ij]-quinollne-4-one.
The present invention relates to nove:L 5-halo-1,2,3-
(1,2-dihydropyrrolo)-4-quinolones which are useful in the
preparation of 1,2,5l6-tetrahydro-4H-pyrrolo[3,2,1-ij]-
quinolin-4~one.
This application is divided from applicant's copending
application Serial No. 424 259 ~iled May 16, 1983 relating
to a process for producing 1,2,5,6-tetrahydro-4H-pyrrolo-
[3,2,1-ij]-quinolin-4-one.
The 1,2,5,6-tetrahydro-4H-pyrrolo[3,2,1-ij]-quinolin-
4-one of aforementioned application Serial No. 424 259
~ corresponds to the formula
:'
T~
~i/ ~ (I)
and is known also under the trivial name of 4-lilolidone.
The compound can be used as a systemic fungicide for the
protection of cultivated plants, for example rice, against
infestation by phytopathogenic microorganisms and thus
against plant diseases caused by these microorganisms
~cp. G.B. Patent Specification No. 1,394,373).
4-Lilolidone has hitherto been produced by an
.
`; intramolecular Friedel-Crafts alkylation from N~
chloropropionyl)-indoline (cp. J. Chem. Soc,:1518,
.~ (1964), G.B, Patent Specification No. 1,394,373 and
~; ~$
.. .. ..
,~ . . ..
, ,
~ ~2546
-- 2 --
J. Agric. Food Chem. 29, 576 (1981). A large excess
of aluminium chloride, high reaction temperatures ar
long reaction times are required în this process.
The process is disadvantageous also in that the heat of
reaction is difficult to remove) and that the separa~ion
of by-products and thP processing of the final product are
very lengthy and complicated. The process is for this
reason unsuitable for a profitable production of
4-lilolidone on a commercial scale.
It was therefore the object of aforementi~ned applica~
tion Serial No. 424 259 to make 4-lilolidone available in
a sirnple and economical manner, in good yields and with a
high degree of purity.
It is suggested according to the aformentioned appli-
cation Serial No. 424 259 that 4-lilolidone be produced by
reacting a haloacetylindoline of the formula II
~ ;
/ \ ~ (II),
o
~E~ -Hal
in which "Hal" is chlorine or bromine, with an addltion
product formed from an N,N-disubstituted formamide of
the formula III o
~C - (III),
in which Rl is Cl-C4-alkyl or phenyl, and R2 is Cl-C4-
aLkyl, and an acid halide to give a 5-halo-1,2,3-(1,2-
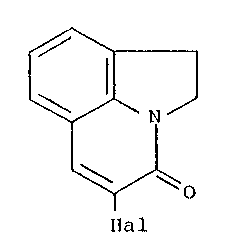
dihydropyrrolo)-4-quinolone of the formula IV
/ ~ (IV),
~al
.
::
.
~', :' .
~2~546
in which 'lHal" has the meaning defined in the foregoing;
and then converting this compoundg by catalytic hydro-
genation9 into 4-lilolidone of the formula I.
The 5 halo-1,2,3-(1,2-dihydropyrrolo)-4-quinolones of
the formula IV are novel compounds and form the subject
matter of the present invention.
The present invention, together with that of appli-
cant's aforementioned application Serial No. 424 259, will
now be described in more detail.
The reaction of a haloacetylindoline of the formula II
with the addition product formed from an N,N-disubstituted
formamide o~ the formula III and an acid halide i9 advan-
tageously performed in an inert solvent. Suitable such
solvents are in particular: halogenated aliphatic or
aromatic hydrocarbons, such as chloroform, dichloromethane,
dichIoroethane, carbon tetrachloride, chlorobenzene~
dichlorobenzenes, toluene and xylene. It is also possible
to use as solvent excess N,N-disubstituted formamide of the
formula III, and especially excess acid chloride.
Preerred solvents are 1,2-dichloroethane, chloroform and
particularly toluene. Especially advantageous is also the
use of excess phosphorus oxychloride as solvent.
Acid halides that can be used are in general those
which are able to react with an N,N-disubstituted formamide
of the formula III to form a Vilsmeier complex. Suitable
acid halides are or example: phosphorus trichloride,
phosphorus tribromide, phosphorus pentachloride, phosphorus
~ ,
~ ~ oxychloride, phosphorus oxybromide, phosgene, carbonyl
`; dibromide~ carbonyl difluoride, oxalyl chloride5 hepta-
~ ~ chlorobutyric acid chloride, thionyl chloride and thionyl
:` ,
. .
.
:, .,, ~
'- ''::, ~, .
.. . .. - .
: .
~2~2546
bromide. Also derivatives of some of the aforementioned
acid chlorides can be used, for example 272,2-trichloro-
1,3-dioxa-2-phosphaindane and 2,2-dichloro-1,3-dioxa
indane, which can be regarded as derivatives of phosphorus
oxychloride and phosgene. Preferred acid halides are
phosphorus oxychloride and phosgene.
Suitable N,N-disubstituted formamides of ~he formula
III are for example: N,N-dimethylformamlde, N,N-diethyl-
formamide, N,N-dipropylformamide, N,N-dibutylformamide,
N-butyl-N-methylformamide and N-methyl-N-phenylformamide
(N-formyl-N-methylaniline).
Preferred N,N-disubstituted formamides of the formula
II are N,N-dlmethylformamide and N-methyl-N-phenyl-
formamide. N,N-~imethylformamide is especially preferred.
The acid halide is used, when the
process is performed in an organic solvent, in an amount
of at least 2 mols per mol of N-haloacetylindoline of the
formula II. Where the process is carried out in an
organic solvent, the acid halide is preferably used in an
amoun~ of 2.5 - 5.0 mols per mol of N-haloacetylindoline
of the formula II.
The reaction of a haloacetylindoline of the formula II
with the addition product formed from an N,N-disubstituted
fonmamide o the formula III and a~ acid halide can be
performed by firstly producing the addition product from
the N,N-disubsti~uted formamide and the acid halide~ and
aterwards adding the haloacetylindoline of the formula II.
The reaction can however also be carried out by adding
the addition produc~ formed from an N,N-disubstituted
formamide of the formula III and an acid halide to ~he
haloa~etylindoLine of the formula II. Furthermore, the
reaction can also be advantageously performed by placing
. .
: :
~'' ~ .'
~, ,
- ' ~-:
..
. .
~2tj25~6
-- 5 --
a mixture of a haloacetylindoline of the formula II and
an N,N-disubs~ituted formamide o the formula III in~o
the reaction vessel, and introducing the acid halide
into this mixture, thus effecting the formation of the
addition produc~ from N,N-disubstituted formamide of
the formula III and the acid halide in situ.
The reaction temperatures are 2S a rule between 40
and 100C. The reaction is generally completed within
a few hours. The reaction is particularly advantageously
performed at temperatures of between 50 and 75C, and at
these temperatures the reaction time is 1-2 hours. Under
these preferred, relatively mild, conditions, the reaction
proceeds in general. with negligible formation of
by-products.
After the reaction of the haloacetylindoline of the
formula II with the addition product formed from an
N,N-disubstituted formamide of the formula III and an
acid halide, the reaction mixture can be further processed
in a simple manner, for example by pouring it into aqueous
sodium hydr~xide solution. There is thus yielded with
the use of excess acid halide, especia~ly phosphorus
oxychloride, an aqueous suspension of the 5-halo-1,2,3-
(1,2-dihydropyrrolo)-4-quinolone of the formula IV, from
which the prsduct is easily obtained by filtration and
drying. If the reaction of the haloacetyLindoline of
thé formula II with the addition product formed from an
N,N dlsubstituted formamide of the formula III and an
acid halide has been performed in the presence of one of
the aforementioned organic solvents, there is obtained,
after ~he pouring of the reaction mixture into aqueous
sodium hydroxide solution, a 2-phase mixture in which the
re~uired 5-halo-1,293-(1,2-dihydropyrrolo)-4-quinolone of
the formula IV is present as solution in the organic phase.
.'~ ` ' .
'. : ' `
~ 2 ~ 5~
On removal of the organic phase, there remains a solution
o~ the desired 5-halo-1,2,3-(1,2-dihydropyrrolo)-4-
quinolone of the formula IV in the employed solvent,
which solution, especially wi~h the use of toluene as
the solvent, can be used directly for the subsequent
catalytic hydrogenatisn.
The catalytic hydrogenation of the 5-halo-1,2,3-
~1,2-dihydropyrrolo)-4-quinolone of the formula IV, which
proceeds with the removal of the 5-halogen, is advan-
tageously performed in an inert organic solvent, and,
for neutralising the formed hydrogen halide, in the
presence of a base. Suitable inert solvents are in
particular: aliphatic and aromatic hydrocarbons, such as
cyclohexane, toluene or xylene, as well as lower aliphatic
carboxylic acids, particularly acetic acid. The bases
used, in the presence of which the catalytic hydrogenation
is carried out, are advantageously hydroxides, carbonates
and hydrogen carbonates of alkali metals and alkaline-
earth metals, and also a~monia or amines. Also alkali
metal acetates, especially sodium acetate, can be used
as bases.
There may be mentioned as further bases, in the
presence of which the catalytic hydrogenation of the
5-halo-1,2,3-(1,2 dihydropyrrolo)-4-quinolones of the
~form-~la IV can be carried out, for example: sodium
hydroxide, potassium hydroxide, ammonia, ~riethylamine
and pyridine.
:
Suitable as catalysts for the catalytic hydrcgenation
~ ~ of the 5-halo-1,2,3~-(1,2-dihydropyrrolo)-4-quinolones of the
- formula IV are noble metals o~ thè group VIII of the
~periodic system, particularly ni~ckel,`palladium and
platinum. The catalysts are used in a very finely divided
.: . .
.. . . . ..
,,
:
~X~ 6
-- 7 --
form, for ex2mple as Raney nickel, or on a carrier, for
example palladium on charcoal, or platinum on charcoal.
The catalytic hydrogenation of the 5-halo-1,2,3-
(1,2-dihydropyrro~o)-4-quinolone of the formula IV is
performed as a rule under norma~ pressure or under a
sLightly elevated pressure. Catalytic hydrogenation is
carried out in practice advantageously under pressures
of 1-10 bar, preferably 3-5 bar.
The temperatures at which catalytic hydrogenation
can be performed are in general between room temperature
and looc. Temperatures of 40-75C have proved to be
particularly advantageous.
After compl,etion o~ hy,drogenation, ur~her processing
of the reaction mixture comprises filteri'ng off the
catalyst and evaporating off the solvent.
It is possible with the process accordin~ to the
aforementioned application Serial No. 424 259 to produce
4-lilolidone, starting with haloacetylindolines of the
formula II, in a yield o~ about 9o % of theory. The process
is easy to carry out, and is therefore well suited also
for production of 4-lilolidone on a commercial scale. The
haloacetylindolines of the formula II, required as starting
material, can be produced in a simple manner, commencing
with indoline, by reaction thereof with haloacetyl halides,
especially haloacetyl chloride, or by reaction of indole
with haloacetyl'chloridej and catalytic hydrogenation of
the N-haloacetylindole obtained.
The process according to the aforementionea application
Serial No. 424 259 is further illustrated by the following
Examples.
, ~ ~
- :
, :,~, ~ ,, .
.
:......... ,-: ~
-- 8 --
Example _
a) Production of 5-chloro-1,2,3-(1,2-dihydropyrrolo)-4-
quinolone
19.55 g (0.1 mol) of N-chloroacetylindoline are added
por~ionwise to a mixture of 150 ml (251.2 g; 1.64 mols~
of phosphorus oxychloride and 20 ml (19.0 g; o.26 mol) of
N,N-dimethylformamide. After the addition of the
N-chloroacetylindoline is completed, the mixture is heated
for 1.75 hours at 70-75C internal temperature. The
unreacted phosphorus oxychloride is afterwards evaporated
off at 40C under reduced pressure. The residue is poured
into cold sodium hydroxide solution (10%), upon which the
pxoduct precipitates. After a stirring time of 1 hour,
the precipitate is filtered off and dried. The yield is
20.05 g (97.8~/o of theory) of 5-chloro-1,2,3-(1,2-dihydro-
pyrrolo) 4-quinolone in the form o a beige powder; melting
point: 190-192C:
IR spectrum (CHC13): 1660, 1640 (C0, C=C)cm 1. Hl-NMR
spectrum (100 MHz, CDC13): 3.33 (broad t, 2H), 4.30
(broad t, 2H), 6.95 - 7.30 (m, 3H), 7.85 ts, lH> ppm.
3C-NMR spectrum (CDC13: 156.6, 141~3, 130.4, 123.7,
116.5, 47.8 and 27.3 (all s), as well as 134.7, 125.1,
123.8 and 122.8 (all d) ppm.
The phosphorus oxychloride which was evaporated off
is pure and can be used again. The N-chloroacetylindoline
required as starting material is produced, in the usual
manner, from indoline and chloroacetyl chloride; melting
point: 129-130C.
; ~ ~b) Production o 4-lilolidone
20.0 g (0.097 mol) of 5-chloro-1,2,3~(1,2-dihydro-
pyrrolo)-4-quinolone are dissolved with 8.0 g of sodium
acetate in 200 ml of glacial acetic acid, and3 after the
addition of 7.0 g of palladium on charcoal (5%), the
', . :
,: .
: .
l.Z~254~
g
mixture is hydrogenated at 70C under 4 bar. The
absorp~ion of hydrogen ceases af~er 3 hours. The catalyst
is filtered off, and subsequently washed on the filter
with glacial acetic acid. The filtrate is concentrated
by evaporation~ and the residue is taken up in ethyl
acetate, washed with water, dried over magnesium sulfate
and conce~ltrated by evaporation. The yield is 15.3 g
(91~/o of theory) of 4-lilolidone in the form of a white
crystalline powder, the entire physical data of which is
in agreement with the relevant data in ~he literature.
Exæm~le 2
a) Production of 5-chloro-1,2,3-(1,2-dihydropyrrolo)-4-
quinolone
19.55 g (0.1 mol) of N-chloroacetylindoline is
introduced portionwise into a solution of 40.0 g to.26 mol)
of phosphorus oxychloride and 19.0 g (o.26 mol) of
N,N-dimethylformamide in 150 ml of chloroform. After the
addition of N-chloroacetylindoline is completed, the
mixture is heated for 24:hours at the reflux temperature.
The reactio~ mixture is afterwards further processed ~y
the injection of (10%) sodium hydroxide solution,
separation of the aqueous phase and removal of the
chloroform by evaporation. There are obtained 12.5 g
(61% of theory) of 5-chloro-1,2,3-(1,2 dihydropyrroLo)-4-
quinolone 7 m.p. 190-192C.
Exam~ Production of 5-chloro-1,2,3-(L,2-dihydro-
pyrrolo)-4-quinolone
40~g (0.4 mol) of phosgene are introduced at 35C into
a soLution of: 14.6 g (0.2 mol) of N,N-dimethylformamide in
70~ml of ~,2-aichloroe~hane. There are subsequently
added portionwise 19.55 g (0.1 mol) of N-chloroacetyl-
indoline~ and stirring is maintained at 65C for 2 hours.
The reaction mixture is then poured onto ice and
.
....
' , , ~ . . ~ -
:. .
~2~i25~
- 10 -
neutralised with sodium hydroxide solution, the 1,2-
dichloroethane is afterwards distilled off, and the
precipitate is iltered off and dried. The yield is
18.9 g (92% of theory) of 5-chloro-1,2,3-(1,2-dihydro-
pyrrolo)-4-quinolone, m.p. 191-192C.
Example 4
19.0 g (o.26 mol) of N,N-dimethylformamîde are added
dropwise at 25-30C, in the course of 1 hour, to 190 g
(2.28 mols) of thionyl chloride. There are subsequently
added 19.55 g (0.1 mol) of N-chloroacetylindoline, and
the mixture is stirred at 60C for 3 hours. The excess
thionyl chloride is afterwards dis~illed off at 40C
under reduced pressure; the residue is stirred up with
200 g of ice and neutralised with sodium hydroxide solution.
The yield after filtration and drying is 8.6 g (42% of
theory) of 5-chloro-1,2,3-(1,2-dihydropyrrolo~-4-
quinolone, m.p. 190-192C.
.
:
:
. ~ :
~:
.
~ ~ .
'
. ~
, ~
..