Note: Descriptions are shown in the official language in which they were submitted.
'~ 2J~ 3
- L -
BACKG_OUND OF THE INVENTION
1. Field of the Invention
Th~s invention relates to cas-ting molten steel.
2. Description of the Prior Art
In normal practice, molten steel produced by any of the
classic processes, for example, the B.O.F., the Q.B.O.P., or the
electric furnace process, usually c:ontains a high level of oxygen.
This degrades the steel. To overcome this, the steel is kllled
by introducing into the mol-ten s-teel deoxldizing ayents, for
ins-tance, silicon, in the -form o-f ferro silicon or aluminum or
both. This is usually performed in a transfer ladle, at tap.
Following deoxidation treatment, the killed molten
steel has a strong affinity for oxygen, which it picks up when
exposed to the atmosphere, during pouring from a furnace, or
casting into ingot molds, into billets, or into slabs. This
results in defects, for example, non-metallic inclusions, in the
resulting steel which can reduce the quality oF the finished
products.
To prevent or to reduce this "oxygen pick-up", various
protective methods have been used. One involves shielding open
cast steel streams between tundish and mold with ceramic tubes.
This has been an established practice for maintaining high
quality -in continuous casting of large bloom and slab sections.
It cannot be applied to smaller bloom and billet sections, how-
ever, because of space limitations. An example of this type of
process is found in Canadian Patent 1,097,881, Thalmann-et al,
March 24, 1981.
- ., . " ~;, : . .~ ~ - , ' : ..
..
:
~L~ ~ 2~3~2~
In another expedient, liquid argon has been poured into
ingot molds. The argon evaporates on contact with the molten
steel and shlelds it from -the atmosphere as it continues to be
poured into the mold. Main drawbacks of this method are that the
storage and transfer of equipment is dif-ficult to adapt to the
hard working condi-tions of the pouring floor, and, the cost o-f
argon in relation to the price of normal grades of steel is high.
The inert gas shrouding of strand cast steel has also
been described in the article "Gas Shrouding of Strand Cast Steel
at Jones & Laughlin Steel Corporation" by Samways, Pollard &
Fedenco, Journal of Metals, October 1974. U.S. patents relating
to this method are 3,908,734, September 30, 1975, 3,963,224, June
15, 1976, and 4,023,614, May 17, 1977z all to Pollard.
Another method uses liquid nitrogen to form a shroud
about the molten steel as it is teemed into a continuous casting
machine. This is described in the brochure entitled "Conspal
Surface Protection", published by Concast AG, Zurich, Switzerland,
March 1977 and in U.S. Pa-ten-t 4,178,980 (1979), L'Air Liquide.
In general, liquid nitrogen has provided a degree of protection
which gives some improvement over other methods. But, handling
this substance under the hard conditions of the pouring floor makes
it difficult to provide continui-ty of Flow, during the operation.
Also, nitrogen has a density close to that of air, reducing
its ability to displace air effectively. Moreover, nitrogen
inerting is not practicable for grades of steel where nitride
formation is undesirable.
.
;.
, Y: .
.
~: . ~ : . . , . - . .
.
:~LZ~2~
In accordance with one aspect of the invention there is
provided a method of forming a gas shroud to protec-t against pick-
up impurities a gravitating stream of mol-ten steel having a sur-
face normally exposed to an atmosphere of air, in a succession of
operat.ions, comprising: Eor a number of opera~ions, in series,
inLerlllittently drawing carbon d.ioxide vapor in increments from the
ullage space oE a supply vessel containing a body of liquid carbon
dioxide and an overlying mass of vapor, as each increment of vapor
is withdrawn, renewing -the vapor by withdrawing and vaporiziny a
corresponding part of the carbon dioxide in the vessel and recycl-
ing i-t into said ul]age space, passing each increment of carbon
dioxide -through a heater -to superheat it above amb;ent temperature,
passing the heated increment of carbon dioxide through a regulat-
ing control valving mechanism and releasing the carbon dioxide as
a gas through restricted dispensing passages at ambient temperature
to form said shroud protecting the molten stee]. surface.
In accordance with another aspect of the inventi.on there
is provided an appara-tus for carrying ou-t a number of shrouding
operations in series, in each operation a stream of steel being
passed from an upper vessel ~o a lower vessel, cGmprising: an
upper vessel having a bottom opening for forming a gravi-tating
stream of s-teel, a pressure vessel for containing a body of liquid
carbon dioxide covered by an atmosphere of carbon dioxide vapor,
a vapor dispensing communication from an upper position in the
vapor space in the vessel leading to the dispensing means, means
for controlling the vapor dispensing communication whereby vapor
of carbon dioxide is dispensed from the vessel in increments
required for each shrouding operation, means for controlling the
gas communication whereby the gas is supplied in amounts suitable
for forming the shroud, means for superheating -the carbon dioxide
leaving the vessel, pressure reducing means and valve r~eans for
controlling the flow of gas to -the dispensing means, means for
controlling the ternperature to which the carbon dioxide is super-
heated, whereby when it is expanded in dispensing, the temperature
is reduced to not less than ambient tempera-ture so -that the
carbon dioxide is dispensed as a gas.
f~
: . !
!,
.
~ ' ' ~ , ' "' ' '"' ~ "
'
1' . . ` ' ' " ' ' .: ' ,
~z~
-- 4 --
The applicants have now found that, surprisingly,
carbon dioxide may be effectively employed to form a gas shield
in protecting molten steel from oxidation from the atmosphere,
for example, in continuous casting, in ingot molding, and in
tapping steel from a Furnace.
Carbon dioxide has been used in shrouding molten me-tal
like lead, zinc, copper, metals with a melting point lower than
the temperature of dissociation of carbon dioxide. From thermo-
dynamic considerations, it would be expected that, on contact of
carbon dioxide with molten steel, the latter would be oxidized
by the dissociation of the gas, because its dissociation tempera-
ture is well below that of molten steel (1600C to 1650C).
However, the applicants have found, unexpectedly, the k;netics
are such that on contact wi-th gravitating streams or molten
steel, while the carbon dioxide at the gas metal interface does
dissociate, d very small amoun-t oF oxygen dissolves in the metal,
and the carbon monoxide Formed serves as a barrier layer at the
gas metal interface. Not only is the oxidation considerably
; reduced to below the level it would reach ~if there were no
barrier from the atmosphere7 but pick-up by the molten steel of
nitrogen and hydrogen (from moisture in the air) is also pre-
vented. The pick-up of dissociated oxygen From the shrouding
gas has been found to be less than about 70 parts per million
and may be as low as 20 to 30 parts. The carbon dioxide
,,
;, .
:: -.:: ;.
': : : : ' - .:
~: . ~ .. ;: . , : .
- 5 -
is thus capable of providing an effective barrier between the
molten steel and the surrounding atmosphere which drastically
reduces the rate of further oxidation, to the point where this
gas can be employed as a most effective shroud to protect molten
steel being transferred from one vessel to another from contamina-
tion by air.
In carrying out the applicants method, carbon dioxide gas
is placed in such quantities and in such proximity to the surface
of molten steel for such a time as to cause dissociation of the
carbon dioxide at a rate which furnishes an atmosphere of carbon
monoxide and gives off a negligible amount of oxygen to be picked
up by the steel, thus providing a barrier which isolates the steel
surface from the surrounding atmosphere and prevents pick-up
therefrom of oxygen, nitrogen or hydrogen.
More specifically, in transferring molten steel from a
higher vessel to a lower one, for example, ln teeming from a ladle
to a mold, an atmosphere of carbon dioxide is formed, in a shroud,
about the liquid stream, near its source, to form a gaseous
blanket which covers the surface of the steel until it solldifies.
In the case of top poured ingot teeming into a mold, the mold is
flushed, in advance, with carbon dioxide to remove the air and
; provide, in the mold, an atmosphere of carbon dioxide into and
through which the steel is teemed. In this way, the oxygen
content of the mold, prior to teeming, may be reduced substantially
to a minimum, for example, to less thao 3% by volume, preferably
not more than 1%.
t :
i
.
.
. ~ . . , .:
:
' ' ,
The flow rate should be not less than equivalent to
about 2.2 cubic meters and preferably as much as 3.~ cubic meters per
minute for flushing a mold having a volume of about 100 cubic
feet. The lapse time between the end of the purge and the start
of the teeming should be kept to a minimum and should not exceed
about 35 seconds, and should preferably be between 20 and 30
seconds to insure that the atmosphere of carbon dioxide is sub-
stantially intact.
The shroud may be formed by providing a ring, with dis-
pensing openings, about the molter steel stream, near its source
at the outlet of the upper vessel, to supply the carbon dioxide
in the proximity of the steel stream in the form of jets which
merge into a blanket which surrounds the moving surface of the
steel streamandis carried along with it. In the case of teeming
into an ingot mold, a dispensing ring may surround the outlet
nozzle of the teeming ladle. A similar arrangement may be
employed, in continuous casting, in the transfer of the steel
from the ladle to the tundish, and from the tundish to the mold.
In transferring steel from a furnace to a ladle in a stream,
appropriate dispensing means may be provided to supply carbon
dioxide in proximity to the stream, to shroud it in an analogous
manner.
BRIEF DESCRIPTION OF THE DRAWINGS
The invention will be illustrated in mcre detail by
reference to the accompanying drawings,~ ustrating preferred~
embodiments, and in which:
.:
.
.
.,
~ ~ ,
' . ,. ~
~ 6 ~2~3~'$
Fig. 1 is a perspective illustration showing the
relationship between the ladle and a succes-
sion of molds, during the carrying out of a
method, according to the invention;
Fig. 2 is a vertical cross-section, partly in eleva-
tion, through a mold, in the course of being
flushed with carbon dioxide, to prepare it for
receiving molten steel from the ladle;
Fig. 3 is an enlarged fragmentary view showing a
corrugated steel stand supporting the bottom
of the mold;
Fig. 4 is a vertical cross-section, partly in eleva-
tion, showing the mold and ladle during an
ingot teeming operation; and
Fig. 5 is a diagram showing the arrangment of pieces
of equipment suitable for supplying carbon
dioxide for carrying out a method according
to the invention, and the fluid connections
between them.
; 20 DESCRIPTION OF PREFERRED EMBODIMENTS
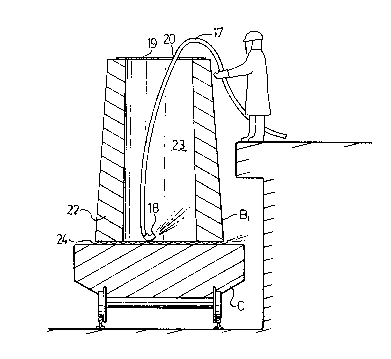
Referring more particularly to the drawings, Fig. 1
i
shows a ladle A containing molten steel being teemed into a
mold B. A layer 12 oF~slag tops the molten steel. Carbon ~ ~
dioxide shroudlng gas is supplied through a dispensing collar ~ `
~; 25 (shown in Fig. 4) through a supply line 15.
A mold BI, waitlng~its turn for receiving molten steel~
from the ladle is shown receiving purging carbon dioxide gas
.,
;
' ~ .
~æ~ 6
through a line 17 and subsequent molds B1 and B2 are awaiting
their turn.
An aluminum foil cap 19 sits on top of each mold.
The cap 19 is ruptured locally to provide an opening for the gas
line.
Fig. 2 shows, in more cletail, the mold B1, in the
course of being flushed with carbon dioxide. The line 17 is
passed through an opening 20 in the aluminum foil cap and
terminates in a nozzle 18 through which carbon dioxide is dis-
pensed into the bottom of the ladle to displace the air and
replace it with an atmosphere of carbon dioxide which is main-
tained untll just before teeming molten metal into that mold.
The mold B1 has a wall 22, enclosing a tapered mold
cavity 23. The bottom of the wall 22 sits on a corrugated metal
stand 24 supported by the deck of a track mounted stool C to
provide a seal between the bottom of the wall 22 and the surface
of the deck of the stool ~, allowing lateral escape of a certain
amount of the carbon dioxide gas. The stool is used to carry
the ingots out of the teeming bay.
Carbon dioxide is flushed into the mold B1, until its
oxygen content is reduced substantially to a minimum. For
example, it has been found possible tu reduce the oxygen con-
tent to less than 3~ and even to not more than 1% by volume. The
`
rate of flow of the flushing gas has to be unexpectedly high to
compensate for the conditions encountered, for example, through
` heat of the mold and leaks beneath the mold at the base and ~
between the top of the mold and the cover. The level of oxygen
, ~ . .
~:
; .
~L~6~1~2~
g
is maintained at substantially a minimum by continuing the flow
of flushing gas just before teeming is started.
The mold B and the ladle A are brought into teeming
position and the teeming operation carried out as will be
described in relation to Fig. 4. A slide gate in the mold B is
opened by remote control allowing the molten steel to pass down
through the outlet passage 25 in the ladle A and passed in the
form of a vertical stream S, past a shroud diffuser 27. The
stream leaving the ladle outlet 27 is circular in cross-section
and of diameter 50 to 100 millimeters and of length between the
outlet and C02 in the mold, which is 45 to 80 centimeters. In
the case of continuous casting, the stream from ladle to tundish
would have a diameter of about 50 millimeters to 100 millimeters
and a length of 30 centimeters to 60 centimeters, whereas the
length of the stream from the tundish to the casting mold would
be from about 30 centimeters to about 45 centimeters.
The diffuser 27 is ~ed with gaseous carbon dioxide
from a line 15, causing a shroud of gas to surround the stream
of molten steel and to be drawn along with it to within the
~ 20 carbon dioxide atmosphere in the mold B. From the time it
; leaves the outlet of the ladle to the time it reaches its des-
tination in the mold, the molten steel is screened from the
atmosphere by a continuous curtain of gas as described above.
Once the mold has been filled, the sllde gate valve of the ladle
is closed to cut off the flow of molten steel and the next mold
B1 and the ladle A brought into register for rece~ving its
supply of molten steel.
~ '
~ .
,~ , . . .
: ~ :
,',
~i2~
- 10 -
The way in which the carbon dioxide is supplied is
important to carrying out a number of shrouding operations one
after the other. To this end, a preferred installation, as
illustrated in Fig. 5, will be described as follows.
Liquid carbon dioxide is stored in an insulated refri-
gerated pressure vessel E at a temperature between about 17 and
18C and at a pressure of 20 kilos per square centimeter.
The vessel E is protected by a safety pressure relief
valve 31, set at 24 kilos per square centimeter. Carbon dioxide
is withdrawn as a vapor, from the ullage space 33 of the vessel
E, through ablock valve 3~. Withdrawal of carbon dioxide vapor
from the vessel E lowers the pressure in the ullage space 33. A
vaporizer 35 is fed from an energy source (electric, hot water
or steam) and is provided to vaporize liquid carbon dioxide and
maintain the pressure within the ullage space 33 as carbon
dioxide is withdrawn through the block valve 34 towards the
point of use. Additional vaporizers 36 and 37 may be added in
parallelto maintain the pressure in the ullage space under con-
ditions of high withdrawal of carbon dioxide vapor through the
~ 20 block valve 34.
I ~ There is also a sensor (not shown) which senses the
~ pressure in the ullage space 33. When the pressure falls below
!`' that~described, then more vapor is supplied to the space 33 to
,~,
restore the pressure. If the tank is left to stand, for any time,
without dispensing vapor the heat increases and thus the pressure.
A refrigerator (not shown) is then activated and the vapor
cooled down.
. ~ :
'~ ~ .. ;~ - ' '
~2~32~
Carbon dioxide vapor passes from the ullage space 33
to the block valve 34, at the pressure of the storage vessel (20
kilos per square centimeter) to an inline heater F, fed from an
external energy source. It is the purpose of the heater F to add
sensible heat to the carbon dioxide vapor so that it is at a
temperature where it may subsequently be expanded without produc-
ing a temperature outside the operating range of the downstream
equipment and which will ultimately dispense carbon dioxide gas
at ambient temperature. The temperature to which the gas is
heated in the heater may be within the range from 100C to 120C.
The carbon dioxide vapor passes, at this temperature,
from the inline heater F through check valves 40 and 41 and block
valves 42 and 43 to pressure-reducing regulators 44 and 45. The
pressure-reducing regulators 44 and 45 are set to a pressure which
will give adequate flow for the downstream requirements.
Flow indicating devices or meters 46 and 47 are pro-
vided and the flow of carbon dioxide is controlled by valves 48
and 49. Pressure gauges or indicators 50 and 51 are interposed
between theregulators 13 and 14 and the respective meters 46 and
47. The temperature of the gas between the regulators 44 and 45
and the flow indicating devices 46 and 47 will be in the range
from about 5C to about`15C.
EXAMPLE
For the purpose of this example, equlpment was employed
substantially as shown in the drawings and operated substantially
as described above. A ladle was employed, having a capacity of
120 tonnes and molds each having a volume of approximately 100
cu. ft. and a capacity of 8 to 9 tonnes so that each 120 tonne
/ ,, ; ~
.. . .
: ' ' ~
'::
~6~2~
- 12 -
heat yielded 6 to 9 ingots. The 1adle had a circular outlet or
nozzle of diameter from 5 to 6.5 cm. Each mold produced ingots
270 cm. tall and had rectangular sections averaging 70 x 160 cm.
The distance from thebottom of the outlet to the top of the
mold was 75 cm. Each mold restecl on a track-mounted stool (base
plate) which is used to carry the solidi~ied ingots out of the
teeming bay.
The ladle was equipped with a perforated ring, just be
low the outlet, capable of forming a protective shroud of carbon
dioxide gas. This ring was connected to a continuous source of
supply of carbon dioxide gas as shown in Fig. 5. In addition,
conventional apparatus was available for flushing the mold with
carbon dioxide gas.
While the steel was in the furnace, the molds were being
prepared for teeming, according to the following procedure. A
strong jet of compressed air was applied to the stool to remove
any loose particles. A coating dispersion, consisting of cement
is dilute phosphoric acid was then applied to the stool. Four
strips of corrugated steel sheet about 6" x 30" x 1/16" were
placed in a square or oblong pattern on the stool to provide a
stand. When the mold was placed in position on the stand, its
weight deformed the corrugation to reduce the chances of molten~
steel leakage tsee delay in Fig. 2).
An oblong well made of light gauge steel sheet measur-
ing approximately 20" x 40" x 50" was placed on the stool inside
the mold to reduce the intensity of splashing when the first
~i molten metal was teemed into the mold. Exothermic "boards"
i ("hot tops") were fixed on the top 12" of the inside of the mold
:
. .
- 13 -
which7 upon contact with the molten steel generate heat that
slows down the rate of cooling at the top of the ingot, thereby
reducing the depth of the "pipe" in the top of this ingot which
must be cropped before subsequent rolling. A cover of aluminum
foil was placed on top of the mold to limit the exposure to atmos-
phere before the mold had been purged with carbon dioxide.
Air was displaced from inside the mold by carbon dioxide
purging at a rate of 2.25 to lZ0 scfm for approximately 3 to 5
minutes before teeming each ingot. An asbestos protected rubber
hose was introduced into the mold throuyh the aluminum foil in
such a way that the diffuser reached as far down as possible, as
illustrated in Fig. 2. The flow of gas was continued until the
air had been expelled from the mold, to the point where the
oxygen concentration in the mold was not more than 1~ by volume.
The flushing continued until just prior to the teemin~ into that
mold, to take care of gas leak between the mold and its stool.
At the start of teeming, the molten steel perforated a
small hole in the aluminum foil, thus reducing the amount of
ambient alr drawn into the mold.
The temperature of the steel in the stream was within
the r~ange from 1~25C to 1650C.
Duriny teeming to each mold, a shroud of carbon dioxide
was formed near the source of the stream, i.e. just below the
bottom of the ladle underneath the nozzle. The shroud formed
about the stream of molten steel was entrained with it and formed
a protective gas barrier from the atmosphere from the time it
left the nozzle to the point of impact in the mold. The flow rate
of carbon d;oxide to the shroud~ was 2.a cubic meters per minute,
,,, "
', ~ ~ ~ `':'::
~ .
:: ::
2~i
- 14 -
The ladle containing the 120 tonnes of steel was
positioned over the already purged first mold and the shroud gas
flow was started. The purge hose had been transferred to the
second mold without interrupting the gas flow.
The slide gate was opened to start teeming. The nozzle,
at times, is blocked by either frozen metal or slag. In either
case, oxygen lancing is required to clear the noz~le.
Although C02 was supplied in liquid form, gaseous C02
was used at both injection points (flushing and shrouding). A
system was therefore employed which ensured a vaporization capa-
bility to provide a flow rate comparable to that of an inert gas,for example, argon. A C02 supply set-up similar to that shown in
Fig. 5 was used.
The first ingot took the least time to fill since the
metal head gradually decreased during teeming. In approximately
3 minutes, the mold was filled and the slide gate was closed (for
about 20-30 seconds) while the overhead crane operator positioned
f
~- the iadle over the second mold. The purging gas hose had meanwhile
been transferred to the next mold and the slide gate was re-
opened to fill the mold that had just been purged. The sequence
was continued until the ladle was emptied of its metal charge.
, It was found that there was no significant increase
" .
over 1% oxygen by volume resulting ln the first 45 seconds after
carbon dioxide purging. The elapsed time between the end of the
purge to the start of teeming averaged not more than 30 seconds
with the exception of the first ingot which took slightly longer9
but less than ~5 seconds, because of the oxygen lancing of the
teeming nozzle.
.
:
i , .
: :
The charge in each mold was allowed to cool, in the
classic way, with a protective flux on the surface, to form a
solid ingot. The molds were then stripped from the ingots.
Quality of Steel
Each ingot was hot rolled into skelp, according to
standard practice, and tested for surface defects. The accept-
able skelp was then rolled into sheet and the sheet made into
spirally welded pipe. The pipe was then subjected to sonic
testing to reveal defects.
Control heats were then carried out, in an identical
manner, using argon and carbon dioxide as shown in the table
below.
The gas flow in the case of carbon dioxide was 2.8
cubic meters per minute and argon 2.8 cubic meters per minute.
Each mold was flushed for about 3 minutes and the stream of
molten metal was protected for the duration of the teeming
operation, about 25 minutes.
A comparison of the results follows in terms of surface
defects on skelp rolled from billets produced.
Rejection rate
Shrouding Mold Flushing % by Weight
Argon Argon ~0.7
Carbon dioxide Carbon Dioxide 0,55
;~ ~ Argon Carbon Dioxide ~ 0.43
:
; :
.
- .-; .~ . . : , . .
.,. ,, :
.. . . . .
- 16 -
Defects by Sonic lest on spiral welded pipe:
Rejection Rate
Shrouding old Flushing % by Weight
Argon Argon 0.4
Carbon dioxide Carbon dioxide 0.15
Argon Carbon Dioxide 0.00
Advantages
Because of the relatively low cost of carbon dioxide
gas and its ready availability~ as compared, for example, with
argon or hydrogen, and the fact that the gas can be generated
locally and supplied continuously makes it a most useful gas when
used as described herein. Carbon dioxide is heavier than air
(1.5:1) as against argon (1.25:1) and will therefore maintain an
effective protection shroud longer than lighter gases because it
will not disperse into the atmosphere as readily.
In carrying out a number of shrouding operations one
after the other, despite the heavy drain on the carbon dioxide
supply and its expansion when dispensed, the expedients described
which differ from that of dispensing other shrouding gas, make it
possible to maintain the gas at a temperature at which the equip-
ment is protected and the carbon dioxide does not freeze.
The amount of oxygen in the starting steel, being
teemed, would depend on the grade of steel and could amount to
400 parts per million to 1,900 parts per million. ,In a normal
teeming operation, without shroudlng, one would expect the oxygen
pick-up in the steel to be between several thousand parts per
million by volume. When the mold is flushed and the steel stream
: ' .
~ ' :: ~ ' ,
. . .;,: , .
- 17 -
is shrouded with carbon dioxide, in accordance with the inven-
tion, the pick-up is no more than 70 ppm and can be as low as
20 to 30 ppm.
.
,
,
: . : , . .
,-: , . ..
. . ." : . . :
: ~ : :. ,
.