Note: Descriptions are shown in the official language in which they were submitted.
~L,Z~
rhe present invent-ion re'lates to 13 d-a'lkyl-'l7 ~-
~3-c~ yloxy-propyl)-cJonanes~ a process -for their marlufacture
an(l l:~hdrrllaceutical preparations containing these c~lllpourlds.
S Accor(l1ng to the present invention there are
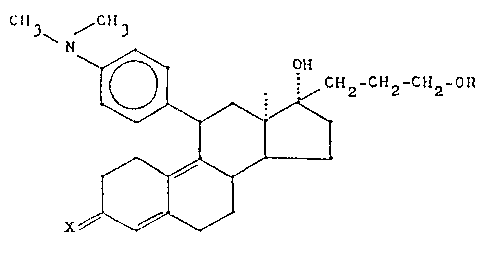
provided 13 ~-alkylgonanes of general Formula I
'10 3~ ~ 3
~ ~ CH2-CH2-CH~ O~
~ ~ ~ tI)
X~V.
where R is an acyl radical with up to 10 C-atoms and X is an
oxygen atom or the N~OH grouping.
13 ~-alkyl-17R -(3-hydroxypropyl)-4,9-gonadiene-3-
oneas w~i~cqh are disclosed in European Patent Application No.
8~73~ have a strong affinity For gestagen recep-tors,
~L~ without themselves having any gestagenic activity. 'I'hey are
compe-titive antagonists of progesterone (antigestagens) and
are sui-table for inducing abortions since they displace the
progesterone necessary for maintenance of pregnancy from the
receptor. The compounds are thus valuable and of interest
: from the point of view of their use for postcoi-tal (p.c.)
control o-F fertility.
~z~
It ilas now been found that their bio-availability
car~ rpris-ir1(J1y~ be considerably enhancec1 by converting
these compounc1s into the l3 ~-alkyl l7 ~-(3-acyloxypropy'1)-
gonar1es of general ~ormu'1a I. In comparison to the 13 ~-
a'1ky1-17~ -(3-hydroxypropyl)-~,9-gonadiene-3-ones they
disp1ay a prolon(Jed duration oF efFectiveness with the same
or greater effect. The -Fact that the new corr1pounds possess
excellent crystallization properties proved to be another
a~vantage. The preparation oF -the pure active substance is
1~ ri1uc11 less diFFicu1t in their case than in the case of '13 q-
dlk)/1--17~ -(3-hydroxypropy'l~-~,9-gonadiene-3-ones.
~~urtiler111ore, they possess excellent chemica'1 sta-
hi'1ity. They can be stored with no diFficulty for a 'long
IS period of time at room ternperature without deco1r1posir1g.
l'he acyl radical R in general forrnula I rnay
contain up to lO C-atoms. Suitable acyl radicals are, for
exanlple, the formyl, acetyl, propionyl, butyryl, isubutyryl,
valeryl, isovaleryl, glycoloyl, cyclopentylactyl, mono-,
di-, trichloroacetyl, benzoyl, trifluoromethylbenzoy'1, and
nicotinoyl radicals. Preferred acyl radicals are the acetyl
and benzoyl radicals.
X stands for an oxygen atorn or the group N ~ OH,
with t-ile hydroxyilnino radical being in the syn or anti posi-
tion.
The cornpounds of general formula I in accordance
with the invention may be prepared by reacting a compound of
formula II
-- 2
S ~ N
2 -C~ 2 -C H 2 - 0~1
I U ~\l~\~L ( I I )
O ~\~
with arl acid chloride or acid anhydride of general formula
Ill or IV respectively
(~ ( I I I )
R-C-Cl
p (IV)
(R-C)20
where R has the above meaning in the presence of a base at a
temperature between 0 and 60C and, when required, sub-
sequently reacting with hydroxylamine hydrochloride in the
presence of a tertiary amine at a temperature between -20
and ~40C.
The preferred bases for esterification are
tertlary amines such as pyridine. The preferred tertiary
amirle during conversion for the preparation of oximes is
pyridine. Further suitable tertiary bases are, for example,
trimethylamine, triethylamine, N,N-dimethylanlino pyridine,
1,5-diazabicycloC4.3.0] nonene-5 (DBN) and 1~5-diazabicyclo
'
~ 2~ 3
[5.'~.0:1 undecene-5 (DBU).
The 13 ~-a'lky'lgonanes o-f genera1 formula I can be
used irl the -form of pharmaceutical preparations. The pre-
paraliorls are nlarlu-factured by the methods genera'1ly known in
galenics by m-ixing with organic or inorganic inert carrier
materia1 sui-table for enteral, percutaneous or parentera1
application.
For humans the dosage of the active inyreclients in
accor(lance with the invention amounts to approxi1llate1y 10 -to
lO00 nl9 per day.
The present invention wlll be further i'1lustratec1
by the Following Examples:
: 30
~:
,
.
solution oE 2.4 g of 11B-(4-dimethylaminophenyl)-17~-
hydro~y-13~-methyl-17B(3-hydroxypropyL)-4,9-gonadiene-
3-one in 7 ml of pyridine and l4 ml of acetanhydride is
stirred for l4 hours at 25 C. This mixture is therl poured
illtO warm water (100 ml) at a temperature of approx. 50 C,
stirred for 15 minutes and the cooled emulsion extracted
with methylene chloride. The methylene chloride phase ls
washed neutral with NaHCO3 solution, dried over Na2SO~
aIld concentrated. Crystalliza-tion of the ~ude product
EroIn ethylacet-lte/dl:lsopropyl ~theryields 2.24 y o 17B~
(3-acetoxypropyl)-11B-(4-dimethyl.amirlophenyl)-17~ hydroxy-
13~-methyl-4~9-yonadiene-3-one with a meltlng polnt of
162 - 164 C.
~aJ25 ~ 436.5 (CEIC13, c = 0.515).
Example 2
0.36 ml of benzoyl chloride in 4 ml of methylene chloride
are dripped, ice-cooled, into a solution of 700 mg of 11B-
(4-dimethylarninophenyl)-17~-hydroxy-13~-methyl-17B-~3-hy~
droxypropyl)-4,9-gonadiene-3-one. The mixture is stirred
for 60 minutes at ~5 C, then poured into saturated NaHCO3
solution and extracted with ethyl acetate. Crystalliza-tion
of the crude product from hexane/diisopropyletheryields
630 mg of 17B-(3-ben~oyloxypropyl)-11B-(4-dimethylamino-
phenyl)-17~-hydroxy-13~-methyl-4,9-gonadiene-3-one with
a melting point of 114 - 116 C.
-- 5
~LZ~
EX~ 3.
~ so'lution of 630 mg of 17~ -(3-acetoxypropyl)-
(4-dilnethylalllinophenyl)-l7c~-hydroxy-l3 q'-methyl-4,9-
gon~diene-3-one in 'lO m'l of pyridine is stirred for 1.5
hours a-t 0C a-Fter the addition of 600 mg of hydroxy'lanline
hyclrochloride. The mixture is subsequently poured into a
mixture of ice water and saturated HN4Cl solution and ex-
tracted with ethyl acetate. AFter chromatography on silica
~ gel with hexane/ethy'l acetate 410 my of' '17~ -(3-acetoxy-
propyl)-ll~ -(4-dinlethyl-alninophenyl)-17~ -hydroxy 'l3 q-
methy'l-4,9-gorlacliene-3-one-anti-oxlrne are obtalned with a
nleltirlg polnt of' 201-204C.
lS l) ~ max ~ 2~8 nln (~ - 27400).
Exanlple 4
. . . ~
Cor,lpositiorl of a tab'let with the preferred corn-
pound 17 a-(3-acetoxypropyl)-11~ -(4-N,N-dimethylamino-
pheny'l)-17 ~-hydroxy-13 ~-methyl-4,9-gonadiene-3-one ~or
oral application.
10.0 mg 17~ -(3-acetoxypropyl)-11 ~-(4 N,N-
dimethylaminopheny'l)-17 ~-hydroxy-13 ~-methyl-4,9-gona-
diene-3-one.
'l40.5 mg lactose
69.5 mg corn starch
2.5 mg polyvinylpyrrolidone 25
2.0 Ing ~Kn~r~H'~ ~4roSil < ~ ~ ~ ~nR~k)
0.5 mg magnesium stearate
225.0 mg
-- 6
., ,