Note: Descriptions are shown in the official language in which they were submitted.
~j
1~i3331
' '
1 PROCESS FOR UPGRADING TAR SAND BITUMEN
Background of the Invention
This invention relates to a method for upgrading tar sand bitumen
for the preparation of useful hydrocarbon products therefrom, such as a
higher quality syncrude essentally free of metals and asphaltenes and with a
much lower moleuclar weight. In particular, the invention relates to a
process for upgrading bitumens derived from tar sands which contain
calloidal mineral contaminants generally referred to as colloidal clay.
Extensive deposits of tar sands, bituminous sands, bituminous
diatomite and similar materials are known to exist throughout the world.
These materials comprise a siliceous matrix of sands, sandstones or
diatomaceous earth which is coated or saturated with relatively high
molecular weight hydrocarbon materials. These deposits are generally
located at or near the earth's surface, although some deposits may be buried
by as much as two thousand feet of overburden. It has been estimated that
the reserves of petroleum products recoverablé from the known deposits of
tar sands would be approximately equivalent to the world-wide reserves
estimated for conventlonal crude oil.
As mined, the tar sands are present in general as agglomerates or
lumps comprising sand, clay, water and viscous hydrocarbonaceous material
called bitumen. While there is no universally accepted defInition of
"bitumen", it may be characterized as that portion of petroleum that exists
in the semi-solid or solid phase in natural deposits. It has been proposed
by the United Nations Institute for Training and Research (UNITAR) that
2S
Bitumens, or natural tars, be defined as the petroleum component which has a
viscosity greater than lO,OOO m~a.s (cp) measured at the conditions in the
deposit and gravity greater than l,OOO kg/m3 (less than 10 API) at
standard condLtions of 15.6C (60F) and a pressure of one atmosphere. The
definition was suggested at the Second International Conference on Heavy
Crude and Tar Sands, held in Caracas, Venezuela on February 7-17, 1982. At
that time it was also noted that a continuously variable spectrum of
1%~333~
1 properties can be found not only geographically between deposits but also
laterally and vertically within a given petroleum occurrence. Accordingly,
the proposed definition employs essentially an arbitrary demarcation between
bitumen and heavy crudes, when the materials are compared on the basis of
these physical properties alone.
Additional distinctions between bitumen and conventional heavy
crude oil may be made on the basis of their chemical compositions. Relative
to most heavy crudes, bitumen has a large asphaltene component. Asphaltenes
are complex, polynuclear hydrocarbons which are insoluble in n-pentane
and/or n-heptane. Due tO their substantial asphaltene content, bitumens
exhibit a high carbon/hydrogen ratio. For the preparation of transportation
fuels, it is generally necessary to reduce the carbon/hydrogen ratio by
addition of hydrogen through catalytic hydrogenation. Bitumen typically
also contains significant amounts of sulfur, nitrogen and metals as
contaminants, often substantially more than most conventional heavy crudes.
The predominating mineral component of the material as mined is in
most cases a fine quartz sand. It is surrounded by bitumen in quantities of
perhaps about S to over 20 weight percent of the total composition. In
addition, tar sands generally also contain colloidal (minus 2 micron)
material, usually referred to as colloidal clay since it contains silica and
alumina, in quantities of from about I to about 50 weight percent of the
total composition.
The bitumen as found in naturally occurring tar sands is not of
great economic value in its crude form. Such bitumen, however, may be
upgraded to hydrocarbons of lower molecular weight, in particular to
hydrocarbons which are liquids at room temperature. Extensive recovery of
tar sand oil has not been seriously considered until relatively recently,
primarily because of the expense of known recovery and upgrading methods in
relation to the cost of preparing the same products from crude petroleum.
The rising costs of crude petroleum production and the depletion of known
-- 2 --
12~33~
1 petroleum reserves, however, have made an efficient and economical processfor the treatment of such tar sand more and more desirable. The vastness of
the known deposits has encouraged many people to look at these raw materials
as a potential source for filling energy and chemical feedstock needs in a
S world of depleting conventional crude oil sources.
...
Several methods have been developed'for purifying tar sands to
provide bitumen concentrates that can be used as feedstock for further
upgrading to produce useful products. The principal purification technique
which has been applied to tar sands in order to concentrate bitumen
therefrom is extraction. In one type of extraction commonly known as the
"hot water" process, advantage is taken of the fact that tar sands produce a
bituminous slurry when mulled with hot water and sodium hydroxide. This
slurry divides into two components upon further dilueion with hot water in a
settling zone. A bituminous froth rises to the-surface of the water and is
withdrawn for further concentration of bitumen, while essentially
bitumen-free sand is discarded as a downward flowing aqueous tailings
stream.
Another known beneficiation process for recovery of bitumen from
tar sand is known as the "cold water" process. This process comprises the
following steps: grinding the ore in the presence of water and a
dispersant; flotation with fuel oil; dilution of the bitumen concentrate
with solvent; and separation of beneficiated bitumen from the sand/water
residue. This process for the preparation of a bltumen concentrate avoids
the requirement of large quantities of heat needed to raise the temperature
of the water in the process described in the preceding paragraph. In the
first stage of preparation, the tar sands as mined are crushed, for example
in a gyratory crusher, to form a coarse ore stock pile. Through the use of
cone crushers~ rod mills and/or ball mills, the latter possibly in closed
circuit with cyclones, a product which is approximately 80% below 150
microns may be prepared. Water and a major portion of the conditioning and
-- 3 --
'
1 2S333~ -
1 flotation reagents used in the process are then added to Eorm a slurry. A
variety of materials may be added to the crushed tar sand ore prior to
conditioning and flotation. Fuel oil or other solvent, in quantities of
about 5 lbso per ton, may be added at this stage. Sodium carbonate (up to
about 10 lb/ton) and/or sodium silicate (up to about 5 lb/ton) may also be
employed. The slurry is then passed to one or more conditioning tanks.
Some conditioning may also be accomplished merely by the flow of slurry
through pipes over extended distances.
The sized and conditioned slurry is then fed to a flotation
circuit, comprising one or more flotation trains. Each of these trains
comprises a rougher/scavenger unlt and a single- or multiple-stage cleaner
circuit utilizing flotation cells. A typical retention time in the
flotation cells is on the order of 15 minutes. Tails from the scavenger
cell are passed to thickeners, to which lime or another suitable flocculant,
in an amount of about 5 lb/ton, is added. Overflow water from the thickener
is recycled back into the circuit. A tailings slurry at about 50-60% by
weight solids is discharged into a tailings pond. Concentrates from the
last stage of the flotation process, containing approximately 25 percent by
weight bitumen, are then suitable for further concentration, for example, by
solvent upgrading. Unfortunately, colloidal clay floats with the bitumen
concentrate and is not effectively removed by flotation.
As currently practiced, bitumen concentrate from the flotation
process is transferred to a mixing vessel where it is combined with at least
one part, and generally several parts, of liquid solvent per part of
bitumen. While the exact amount and composition of the solvent is not
critical, it has been suggested that for maximum effectiveness the solvent
should contain about 20% aromatics. Heretofore, the solvent has been
almost entirely recovered in subsequent steps. It is possible to use the
same fuel oil for solvent upgrading as is used in the flotation process.
The diluted bitumen is pumped into settling or holding tanks, where the
remaining water and sands begin to settle out.
-- 4 --
~6333~ `
1 The final stage of ;he solvent upgrading process comprises the
solvent or diluent recovery stage. This may be a distillation tower or
other mechanism which is used to separate solvent and flotation oils for
recycling to upstream stages of the extraction process. Depending on the
nature of the charge to the solvent upgrading step and the intended use for
the concentrated bitumen, additional separation steps, such as dehydration
or centrifugation may be necessary.
In yet another type of extraction process, tar sand agglomerates
are contacted with a suitable solvent such as a gas-oil boiling range
fraction to produce a solution of bitumen and gas-oil. This solution is
separated from the sand and then passed to a conventional hydrocarbon
conversion unit.
Treatment of tar sands by these beneficiation techniques in order
to separate an enriched bitumen stream from the sand is a substantial
component of the recovery costs, above those mining the crude ore. These
processes generally provide products which contain colloidal clay even after
repeated treatments. Colloidal clay contents of beneficiated bitumen
concentrates typically range from 2500 ppm to 7 weight percent, usually
below 2 weight percent, based on the weight of the bitumen. Since the
colloidal clay forms a stable emulsion with water it cannot be readily
removed from the bitumen recovered from tar sands. The water, which may be
present in amount up to 15~ based on the weight of the bitumen, causes
problems in downstream upgrading processes. For example, water causes
foaming to take place in cokers. Thus, selective mining has been employed
heretofore to minimize the content of clay in bitumen concentrates obtained
from tar sands. Tar sands containing high levels of clay are generally not
exploited. In addition to colloidal clay, the bitumen concentrates
generally contains high levels of sulfur, nitrogen, metals and other
contaminants. The residual colloidal clay and high contaminants level have
also heretofore presented ~ajor problems in the subsequent use of the
recovered product.
-- 5 --
33~
Retort methods similar to those used in the pyrolysis or thermal
cracking of oil shale have also been proposed for the recovery of bitumen
from tar sands. The raw tar sand is contacted with spent sand and fluidized
by reactor off gas at temperatures above about 900F. Volatile products are
flashed off while coke is deposited through thermal cracking. The coke is
burned off in a separate unit at 1200-1400F and the sand recirculated.
Substantial amounts of spent sand, for example 5-10 parts per part of raw
tar sand, are needed for the process. This makes necessary a very large
retort volume per barrel recoverable oil. Serious waste heat and handling
problems also arise with this process, making it of little interest
commercially.
Once the bitumen has been recovered (concentrated) from the tar
sands, two primary bitumen upgrading routes are available: carbon rejection
and hydrogen addition. Carbon rejection upgrades bitumen by removing
asphaltenes, and is examplified by conventional solvent deasphalting,
delayed coking and fluid coking processes. Various modifications of the
basic coking process have also been proposed. For example, U. S. Patent No.
2,905,595 describes a process in which tar sands are subjected to a coking
process to produce coker gas, gasoline and gas oil and a coke-laden sand
stream. The coke-laden sand is contacted with an oxygen-containing gas,
such as air, to effect combustion of coke deposited on the sand grains,
thereby produclng a clean hot sand stream which is recirculated into the
process. According to the preferred method described in this patent,
coke-laden sands are burned and heated in a specially-designed gas lift
furnace. The coke-laden sand is suspended in a plurality of parallel
vertical burning zones and recycled through a furnace zone surrounding these
vertical tubes. This process produces a distillate product directly. The
method essentially employs a recirculated stream of hot solids
simultaneously to vaporize, coke and crack the hydrocarbon fraction.
iL26~33i
1 U. S. Patent No. 3,320,152 describes a process in which tar sand
agglomerates are intrvduced into a feed preparation zone and admixed with
relatively hot contact material in order to drive off water and reduce the
viscosity of hydrocarbon material, thereby providing a fluidizable mixture
~ of sand particles and hydrocarbons. A portion of the fluidizable mixture is
passed through a pressure-developing zone and then introduced into a
reaction zone containing a fluidized bed of solid particulate material.
This reaction zone is maintained under conditions suitable for carrying out
thermal coking of the hydrocarbon material.
U. S. Patent No. 4,082,646 describes a modified direct coking
process in which the combustion stage is divided into two sequential
operations. In the first operation, coke solids produced in a reaction zone
are introduced into a coke burning zone where they are contacted with
combustion air and the minimum amount of supplementary fuel, if any, needed
to burn substantially all tl1e coke. Part of these solids is discarded while
the remainder, required for heating the coking reaction zone, is introduced
into a fuel burner zone. Here the major portion of the supplemental fuel
required to maintain heat balance is combined with air or oxygen to hezt
further the clean solids until their heat content is sufficient to meet the
requirements of the coking reaction zone.
Carbon rejection alone cannot deal with the bitumen upgrading job
in a cost effective manner. This is because an extraordinarily high amount
of either a coke byproduct or an asphalt byproduct is produced. These
by-products necessarily contain hlgh contents of sulfur, metals and ash,
rendering the coke or asphalt relatively valueless. Moreover, the
production of unnecessary coke or asphalt markedly reduces the yield of
lighter, more valuable liquid hydrocarbons. This yield consideration is of
particular importance with respect to tar sand processing, where mining
represents roughly 80% of the total operating costs. Thus, an increase in
usable fuel yield~ from each ton of ore can result in disproportionately
large overall cost savings.
-- 7 --
~3~
1 U. S. Patent No. 4,161,442 describes a process in which high
temperature solids comprising silica are combined with tar sands in a
thermal strippLng operation resericted not materially to exceed incipient
cracking of the petroleum materials. The operating temperature is limited
. to within the range of 600F to 850F, and preferably below 800F. A high
oily residue deposited on the sand is used to generate fuel gas by heating
to a temperature above 1500F with addition of steam or air. Since the
fIuid distillation is operated to minimize cracking, the concentration of
residual oil material on the sand is relatively high, and only those
components which vaporize below the temperatures of incipient cracking are
removed. This process provides only minimal amounts of desirable liquid
hydrocarbon products, because of the low process temperatures employed.
An alternative route for the upgrading of bitumen ls hydrogen
addition. When hydrogen addition is used alone as the upgrading route, the
lS large amounts of hydrogen required to prepare useful products from the
hydrogen-deficient asphaltene molecules raises the cost of the fuel produced
thereby to unaccepable levels. Moreover, nickel, vanadium and asphaltenes
interfere with the hydrogenation and conversion catalysts, shortening run
lengths and requiring a more frequent replacement of catalyst. Any fines
present in the hydrogen addition feedstock not only block the active sites
of the hydrogenation catalyst, thereby reducing its activity, but also lead
to the formation over time of obstructions in the flow path of the feedstock
through the caLalyst bed. This in turn leads to the development of large
pressure gradlents in the system, ultimately resulting in its shutdown.
Combinations of prior art carbon rejection and hydrogen addition processes
would only serve to compound the most undesirable characteristics of each.
Another method for deriving useful hydrocarbon products from
heavier precursors such as bitumen is the method of catalytic cracking.
When catalytic cracking was first introduced in the petroleum industry
during the 1930's, the process constituted a major advance over the earlier
-- 8 --
1 techniques for increasing pressure to charge catalytic cracking units with
heavier crudes and products such as bitumen. Two very effective restraints
have limited the extent to which this has been practical: the coke
precursor content and the metals, especially heavy metals, content of the
_ feed. As these values rise, the capacity and efficiency of the catalytic
cracker are adversely affected.
Polynuclear aromatics, such as asphaltenes, tend to break down
during the catalytic cracking process to form coke. This coke deposits on
the active surface of the catalyst, thereby reducing its activity level. In
general, the coke-forming tendency or coke precursor content of a material
can be ascertained by determining the weight percent of carbon remaining
after a sample of the material has been pyrolyzed. This value is accepted
in the industry as a measure of the extent to which a given feedstock tends
to form coke when treated in a catalytic cracker. One method for making
this evaluation is the Conradson Carbon Test. When a comparison of
catalytic cracking feedstocks is made, a higher Conradson Carbon number (CC)
reflects an increase in the portion of the charge converted to "coke"
deposited on the catalyst. The Conradson Carbon test has been adopted as an
American National Standard and is described in ASTM Method D189. Another
generally accepted method for evaluating coke precursor content is the
Ramsbottom Carbon test, as described in ASTM Method 524. The Conradson
Carbon test, however, is the preferred method for samples that are not
mobile below 90C, such as bitumens.
It has been conventional to burn off the inactivating coke with
air to "regenerate" the active surfaces, after which treatment the catalyst
is returned in cycllc fashion to the reaction stage for contact with and
conversion of additional feedstock. The heat generated in the burning
regeneration stage is recovered and used, at least in part, to supply heat
for vaporization of the feedstock and for the cracking reaction.
_ g _
1 The regeneration stage generally operates under a maximum
temperature limitation in order to avoid heat damage to the catalyst. When
feedstock wth a high CC content is processed, a larger amount of the
feedstock in weight percent is deposited as coke on the catalyst than would
~ be the case with low CC feedstock. When this catalyst is regenerated, the
additional coke leads to high temperatures in the regenerator. At these
higher temperatures, a number of problems arise. The circulation rate of
the catalyst is reduced, often resulting in lower conversion rates.
Incomplete regeneration of the catalyst may also occur, reducing its
catalytic activity. Finally, if the temperature of the regenerator is
sufficiently high, an inactivation of the catalyst takes place. There is
thus a practical limit to the amount of coke which can be burned per unit
time.
As CC of the charge stock is increased, cokeburning capacity
becomes the limiting factor, often requiring a reduction in the rate of
charge to the unit. Moreover, part of the charge is diverted to an
undesired reaction product, thereby reducing the efficiency of the process.
Since bitumen comprises to a great extent hydrogen deficient, high molecular
weight hydrocarbons such as asphaltenes, a direct catalytic cracking of
bitumen would clearly be a highly inefficient method for upgrading for this
reason alone. This is confirmed by Bunger et al., "Catalytic Cracking of
Asphalt Ridge Bitumen", Advances in Chemistry Series, No. 179, "Refining of
Synthetic Crudes". p. 67 (1979). These authors report an inhibited rate of
catalytic cracking, low octane numbers for the gasoline produced and
substantially higher coke make than experienced presently for commercial
gas-oil cracking.
An additional drawback to direct catalytic cracking of bitumen is
the metals content of the feed. Most bitumens contain heavy metals such as
nickel and vanadium. These metals are deposited almost quantitatively on a
catalytic cracking catalyst as the molecules in which they occur are broken
-- 10 --
-
~Z63~1
1 down. The deposits of these metals build up over repeated cracking cycles
to levels which become troublesome. Some of these metals also unfavorably
alter the chemical composition of catalysts. For example, vanadium tends to
form fluxes with certain components of common FCC catalysts, lowering thelr
melting point to a degree that sintering begins at FCC operating
temperatures with resultant loss of catalytic activity.
The heavy metals present in crude oils are also potent catalysts
for the production of coke and hydrogen from the cracking feedstock. The
lowest boiling fractions of the cracked product - butane and lighter - are
processed through fractionation equipment to recover components of value
greater than as fuel for the furnaces. This fraction comprises primarily
propane, butane and olefins of like carbon number. Hydrogen, being
incondensable in the "gas plant", occupies space as a gas in the compression
and fractionation train. As the metals level of the charge stock is
increased, hydrogen production becomes the limiting factor, often requiring
a reduction in the rate of charge to the unit. ~oreover, since bitumen is
already hydrogen deficient, the generation of additional hydrogen therefrom
would be a serious problem.
The sodium content of bitumen also presents problems for a
conventional catalytic cracking system. Sodium reacts with a zeolite
catalyst to produce the inactive form of zeolite. The product bitumen
generally contains at least about 1% water, with significant amounts of
sodium compounds dissolved therein. These sodium compounds comprise
primarily sodium carbonate and sodium hydroxide, which are conventionally
used as conditioning agents in the upgrading of tar sands. These compounds
are deposited on the catalyst as the bitumen is subjected to catalytic
cracking, and can lead to a substantial deactivation of the cataiytic
cracklng catalyst over time, requiring its replacement. Sodium, like
vanadium, also tends to form fluxes with certain FCC cataly~t components.
-- 11 --
33~
1 In addition, all of the known processes for preparing bitumen
concentrates from tar sands provide products contalning at least some
residual clay, generally several percent by weight. This clay ls of a very
fine particle size. Because of the viscosity of the bitumen and the
chemical constitution of the components thereof, it has not been possible to
remove this clay from the bitumen by conventional methods, such as
hydroclone separators or conventional filtration means. This clay,
particularly the clay of finest particle size, introduces additional
complications in hydrogen addition treatments, as noted above.
THE INVENTION
Accordingly, it is an object of an aspect of the invention to provide a method
for deriving a useful hydrocarbon product from tar sands in an economically
acceptable manner.
It is an object of an aspect of the invention to provide a method for
upgrading bitumen derived from tar sands which maximizes the yield of
higher-value middle distillate components, while avoiding the disadvantages
of the known upgrading routes for tar sand bitumen.
It is an object of an aspect of the invention to provide a method
for upgrading a concentrate of bitumen ~hich is not adversely affected by
the content of fine particle size clay and water in the bitumen.
It is an object of an aspect of the invention to provide a ~ethod for
upgrading bitumen which results in a product with reduced Conradson Carbon
number, sulfur and nitrogen, and a minimized content of metals and colloidal
clay.
-12-
~21~333~
An aspect of the invention is as follows:
A process for upgrading a charge of a tar sand bitumen
concentrate containing colloidal clay and water which comprises contacting
said charge in a riser in the presence of a low boiling organic solvent with
finely divided attrition-resistant particles of a hot fluidizable
substantially catalytically inert solid at high temperature and short
contact time which permits vaporization of the high hydrogen containing
components of said bitumen, said period of time being less than that which
induces substantial thermal cracking of said charge, at the end of said time
separating said vaporized product from said fluidizable particles, said
fluidizable particles now bearing a deposit of both combustible solid and
adherent particles of colloidal clay, immediately reducing the temperature
of said vaporized product to minimize thermal cracking and recovering said
product for further refining to produce one or more premium products such as
gasoline, and oassing said particles of inert solid with deposit of
combustibles and colloidal clay to a regenerator provided with cyclones and
high velocity air jets to oxide the combustible portions of the deposit and
to heat said fluidizable particles and to attrite colloidal clay from said
attrition-resistant fluidizable particle, recirculating the heated
fluidizable solid depleted at least in part of colloidal clay to contact
with incoming charge, and recovering clay removed by attrition from the
regenerator.
By way of added explanation, the foregoing and other objects
of the invention may be achieved according to the instant invention using
a selective vaporization system comprising a contactor a burner regene-
rator and an inventory of fluidi~able, essentially catalytically inert.
attrition-resistant contact material in the form of microspheres about 20
to 120 microns in diameter which continuoustly circulates between
the contactor and the regenerator. A bitumen concentrate
-12a-
~L21Ei33~3~
1 derived from tar sand, generally diluted on account of its viscosity with at
least an equivalent amount by weight of solvent, such as fuel oil or
kerosene, or naphtha or light gas oil product from the selective
vaporization system is introduced into the seLective ~porization system,
optionally dispersed with steam or product gas. The combined feed is
contacted for a short time with heated contact material, causing a selective
vaporization of the lighter high hydrogen content components of the bitumen.
A portion of the heavier asphal~enes and most of the components which
contain metals, sulfur and nitrogen remain on the particles of the contact
material. At the vapor exit of the contactor, a quench stream is generally
introduced for rapid cooling of the exiting hydrocarbon products to minimize
subsequent thermal cracking;
The rapid application of heat generated within the contactor of
the selective vaporization system and carried by the fluidizable,
substantially catalytically inert contact material vaporizes most of the
hydrocarbon components of tar sand bitumen. The unvaporized components -
asphaltenes and compounds bearing metals, nitrogen and sulfur - are
deposited on the fluidizable contact material.
Unexpectedly, colloidal clay particles also deposit almost
quantitatively as protuberances on the fluidizable particles of
substantially cataLytically inert contact material instead of being carried
over with the vaporized hydrocarbon components of the bltumen, thereby
removing colloidal clay from the feedstock to downstream processing units
such as hydroprocessing or FCC units. The contact material with its deposit
of colloidal clay, metals and hydrogen deficient hydrocarbon is circulated
into a burner regenerator provided with cyclones and high velocity air jets
where the combustible portion of the materials deposited on the contact
material is oxidized and the deposit of clay is attrited off by design of
cyclones and air distribution to induce attrition and a ball milling action.
The material removed by attrition is recovered in bag houses, cyclones or
scrubbers downstream from the regenerator burner. The heated contact
- 13 -
'
lZ~3~31
1 material exits the burner and is reintroduced into the contactor for further
removal of contaminants from new charge stock. F}esh contact material is
introduced lnto the system and spent contact material withdrawn therefrom on
a continuous or semi-continuous basis in order to maintain a predetermined
~ average metal content upon the circulating contact ~aterial.
It is fortuitous that colloids remaining in the bitumen
concentrate from tars are deposited as aetritable protuberances on the
circulating inventory of attrition-resistant selective vaporizaeion contact
material and that the selective vaporization process can be practiced with
burners (regenerators) equipped with cyclones and air jets which attrite the
protuberances but do not substantially attrite the particles of contact
material. If the colloids accreted on the particles of contact material and
were not removed by attrition the particles of contact material would form a
dense shell which would grow in size. The resulting material will not be
useful in removing metals in a riser unless extremely high addition rate of
fresh contact material was to be praceiced. This is demonstrated by the
following estimation of what would occur if removal of accreted colloids did
not take place in a commercial selective vaporiæation contactor operated
with a feed containing 1 wt % colloidal clay (3.5 ~/clay barrel), 70 ppm Ni
+ V and with fresh contact material (hereinafter ARTCAT~ contact material)
added to control metals level on equilibrium contact material to 30,000 ppm.
If no clay were deposited, 1.2 #/barrel of fresh ARTCAT contact material per
100 pp. Ni ~ V would be needed; hence 0.84 #/barrel of ARTCAT contact
material would be needed in the cited case of feed containing 70 ppm Ni + V.
If 1 wt. % colloidal clay were permitted to accumulate and form a dense
shell the weight of deposited clay per unit weight of fresh ARTCAT would be
4.16#/#ARTCAT contact material (3.5# clay deposited/0.84# ARTCAT material.
In other words, the weight of the contact material would be multiplied by a
factor of about four and the siæe would increase correspondingly to levels
not suitable for use in a riser. Also, we believe that at these levels the
- 14 -
1263313~
deposited colloidal clay would impart undesirable catalytic
cracking properties to contact material originally
substantially catalytically inert.
HPat is rapidly removed from the vaporized bitumen
components in order to minimize destructive molecular
conversion of the non-contaminated, lighter hydrocarbon
material. The selective vaporization process thereby
minimizes thermal conversion of the product to gas, naphtha
or coke. In addition, the hydrogen content of the liquid
product is for the most part preserved.
This selective vaporization process, which is a
modification of the process disclosed in U. S. Patent No.
4,263,128, removes from the feedstock most of those
contaminants which would poison downstream conversion
processes, while retaining those having a high hydrogen
content. The selective vaporization process also shifts
the range of compounds in the feedstock towards the middle
distillate range, thereby reducing residual oils and
molecular weight.
The solid contacting agent is essentially inert in
the sense that it induces minimal cracking of heavy
hydrocarbons by the standard microactivity test conducted
by measurement of amount of gas oil converted to gas,
gasoline and coke by contact with the solid in a fixed bed.
Charge in that test i 0.8 grams of mid-Continent gas oil
of 27 API contacted with 4 grams of catalyst during 48
second oil delivery time at 910F. This results in a
catalyst to oil ratio of 5 at weight hourly space velocity
(WHSV) of 15. By that test, the solid here employed
exhibits a microactivity less than 20, preferably about 10.
The selective vaporization process is operated to
minimize molecular conversion of that portion of t~e
hydrocarbon feedstock which is suitable for later catalytic
cracking or other methods for producing high octane
3s hydrocarbon products. The asphaltenes present in the
bitumen are
- 15 -
~2~333~
1 either converted to lower molecular weight hydrocarbons or deposited on the
contact material. The selective vaporization process also removes
essentially all of the metals (over 90%, and typically over 95%).
In order to disclose more clearly the nature of the present
invention, the following drawing, description and examples illustrating
specific embodiments of the invention are given. It should be understood,
however, that this is done solely by way of example and is intended neither
to delineate the scope of the invention nor the ambit of the appended
claims.
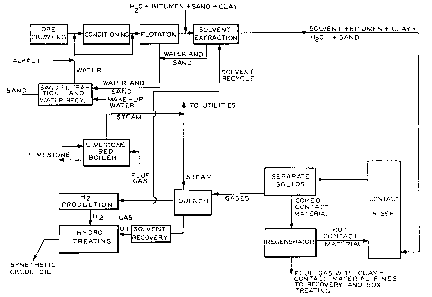
Figure 1 is a schematic diagram of a tar sands bitumen upgrading
process incorporating selective vaporization and utilizing solvent employed
in upgrading the bitumen as diluent for the bitumen in the selective
vaporization contactor.
Figure 2 is a diagramatic sketch of a selective vapor system for
upgrading tar sand bitumen concentrates in a riser/burner system.
Figure 3 contains distillation curves of tar sand bitumen
feedstock and the synthetic crude product obtained therefrom.
DESCRIPTION OF SPECIFIC EMBODIMENTS
In order to cope with the contaminant concentratlon and the
viscosity of tar sand bitumen employed, it is generally desirable to dilute
the feedstock unless sufficient solvent is already present. One
particularly suitable diluent which may be employed in the selective
vaporization process is a clean, light gas oil boiling in the 250 - 600F
range which is produced from tar sand bitumen by the selective vaporization
process. This light gas oil material is repeatedly recycled through the
selective vaporization process as a captive diluent material. This diluent
ls practically devoid of carbon residue or metal. In general, at least one
equivalent by weight of diluent is used per unit bitumen.
~nother suitable diluent for use in the selective vaporization
process is the solvent employed during the purification of the bitumen from
- 16 -
'
~2:~;33
1 water and sand. This solvent may be left in large part in the bitumen,
rather ehan being removed by fractionation as customarily done. This
results in overall energy savings in the production scheme. Solvent can be
allowed to remain, in whole or in part, within the bitumen stream introduced
into the seLective vaporization process. See the accompanying Figure 1.
This allows for a single fractionation of the purified bitumen, rather than
fractionation in two steps - once during the conventional solvent "clean-up"
and again during the selective vaporization process.
Referring to Figure 1, crude tar sand bitumen ore is crushed,
conditioned with alkali (e.g., sodium hydroxide) and water and subjected to
flo~ation to produce as a float product a concentrate of bitumen mixed with
water, sand and clay. The underflow from the flotation cell, a concentrate
of sand and water, is charged to a filter for recovery of water which is
reused in the flotation plant. The float product is then subjected to
solvent extraction, using, for example, fuel oil in an amount roughly equal
in weight to the weight of the bitumen. Without recovering the solvent in
fractionation equipment, as in conventional tar sands bitumen beneficiation,
the solvent diluted mixture of bitumen, clay, water and possibly sand, is
circulated through the contactor riser/regenerator system shown in detail in
Figure 2. The regenerator (burner), discussed below in connection with the
description of Figure 2, operates with cyclones and high velocity air which
attrites clay deposited on the fluidizable particles of hot contact material
circulating in the system. The flue gas from the regenerator therefore
contains attrited clay as well as fines resulting from physical breakdown of
contact material. These fines are recovered in conventional means such as
baghouses after separation from the flue gases which are handled in
equipment suitable to remove oxides of sulfur before discharge to the
atmosphere.
In the process shown in Figure 1, product from the selective
vaporization riser, after quench and fractionation to separate the solvent
?
~2~;~3;~
1 and gas from the syncrude, is passed to a hydrotreating facility to produce
a synthetic crude oil. Solvent liquified and separated after the quench is
recycled to the solvent extraction plant. Flue gases from the regenerator
are processed to remove oxides of sulfur in a limestone bed boiler and steam
recovered during this operation is used to operate utilities. The gas
produced in the selective vaporization riser is used to provide hydrogen for
the hydrotreater.
In general, an initial charge of fluidizable contact material is
made to circulate into the contacting zone, into the burning zone and again
into the contacting zone prior to the introduction of feedstock. A
combustible material, such as what is sometimes referred to as "torch oil',
is charged to the selective vaporization process burning zone to initiate
combustion. This material may be a waste product from a refinery. The heat
of combustion of this material warms the system to the operating temperature
range. Feedstock is then introduced and torch oil injection discontinued.
As ncted earlier, the residual colloidal clay content of bitumen
derived from tar sand has in the past proved to be a major problem in the
subsequent upgrading of these tar sands. One of the advantages of the
instant invention is that these colloids do not have any negative impact
upon the seleceive vaporization process because the colloids are
continuously removed from the system. This bitumen feed may comprise a
crude bitumen concentrate prepared by extraction or one which has been
subjected to some additional treatment, such as solvent upgrading.
For treatment of the initial bitumen charge as well as for use
throughout the selective vaporization process, the calcined kaolin clay
microspheres described in the above-noted U. S. Patent No. 4,263,128 would
be suitable. Other solids of low catalytic activity, low surface area and
similar particle size may also be employed. In general, solids of lo~ cost
are recommended, as it is necessary to discard a portion of the contact
material on a continuous or semi-continuous basis and replace it with fresh
-- 10
1 material to maintain a suitable metals level. In some cases, a portion of
the contact material is residual plus 20 microns sand contained in the
initial bitumen charge and derived from the crude tar sands as mined.
The heat requirements of the system are supplied essentially by
the heat of combustion of the coke deposited on the contact material during
the vaporization process. These requirements include the heat necessary to
bring the various components of the Eeed thydrocarbonaceous material,
entrained water and any sand, etc.) to the contactor temperature and the
heat of vaporization and reaction of the various hydrocarbon feed
1~ components. The regene}ator heat requirements must also be considered.
These include the heat necessary to bring air, contact ma - ial and the
deposited coke to the regeneration temperature. Finally, some allowance
must be made for heat loss to the environment. Through evaluation of these
heat balance requirements of the system, it has been determined that raw
bitumen charge containing optionally up to about 7.5% by weight sand
relative to the bitumen can be treated through the selective vaporization
process with a practical minimum conversion to coke equivalent to about 80%
of the Conradson Carbon value. Moreover, upwards of 300% by weight sand in
the bitumen charge could be accommodated~ albeit with a higher production of
coke. The bitumen charge may also contain substantial amounts of water.
For the limiting case in which the sand content of the feed is minimal and
the conversion to coke is equivalent to 80% of the CC value, at least 14
weight percent of the charge based on the bitumen may be water, and as much
as about one-half of the charge as water can be accommodated with an
acceptable level of coke production.
The selective vaporization process is characterized by short
residence times of the charge in the contactor. As used herein, hydrocarbon
residence time is calculated as length of the contactor from the charge
introduction point to the point of separating solids from vapors divided by
the superficial linear velocity at the solids separation point, thus
_ 19 _
1 assuming that linear velocity is constant along the contactor. The
assumption is not strictly accurate but provides a highly useful
measurement. As so measured, the hydrocarbon residence time will be less
than 5 seconds and preferably less than 3 seconds when applying the process
~ to best advantage. Since some cracking, part1cularly of the deposit on the
inert solid, will take place at the preferred temperatures for bitumens, the
extent to which residence time can be reduced is often limited by
characteristics of the equipment employed. If the equipment permits,
residence times of less than 2 seconds are preferred and residence times of
less than one second are most preferred.
In general, the selective vaporization process is carried out
under temperatures and pressures corresponding to those currently used in
selective vaporization of heavy crudes and distillation residua thereof.
~he contact material is generally heated above about 1100F; the upper
temperature limit is determined by the particular burner employed and rarely
exceeds 1800F. When impacted by the charge, the contact material has in
~ost cases a temperature of at least 800F; temperatures above 850F, and
most particularly in the range of 900-1050F, are preferred. The operating
pressures in the system are preferably as low as possible. This pressure
rarely exceeds 50 psia, and is usually about 20-35 psia.
The instant invention is preferably conducted in a contactor very
similar in construction and operation to the riser reactors employed in
modern fluid catalytic cracking (FCC) units. Bitumen charge prepared
according to the cold or hot water processes described above, diluted with
an equal weight of low boiling hydrocarbon diluent such as kerosene and
containing about 2500 ppm to 7 wt % colloidal clay based on the bitumen, is
introduced at the lower end of a vertical conduit. Unless sufficient
solvent used co refine the upgraded tar sands remains with ~he bitumen,
additional volatile material, such as light hydrocarbon recycled in the
process, steam, gas and/or water, is added in amounts sufficient to decrease
- 20 -
~;~i33~
1 substantially the hydrocarbon partial pressure of the feedstock. The
pressure in the system should be sufficient to overcome pressure drops, and
is generally on the order of 20 to 50 psia. The charge may be preheated in
a heat exchanger or a furnace before introduction to the contactor. This
preheating may be to any desired temperature below thermal cracking
temperature. Typically, the charge may be heated to about 200-800F, and
preferably to about 300-700F. Higher temperatures would induce thermal
cracking of the feed, with the result being increased production of low
valued product.
With reference to the accompanying Figure 2, the feed, optionally
further diluted by light hydrocarbons, steam or the like, rises in the
contactor 1 at high velocity, such as for example 40 feet per second. Hot
inert solid in finely divided form is introduced into the feed from a
standpipe 2 in a quantity sufficient to provide a mixture at a suitably
elevated temperature which causes deposition of all components of high CC
number and high metal content onto the contact material and volatilization
of lighter, hydrogen rich hydrocarbons.
The length of the contactor 1 is such to provide a short residence
time for contact between the feed and the contacting agent. This is
preferably on the order of 3 seconds or less, more preferably about 2
seconds, and most preferably 1 second or less. The residence time, however,
should be sufficiently long to allow for good uniformity of contact between
the feed and the contacting agent, i. e., at least about 0.1 second. The
residence time is calculated on the basis of the vapor residence time
determined from outlet conditions.
At the top of the contactor, e. g., about 3Q to 40 feet above the
point of introduction of contacting agent from standpipe 2, vaporized
hydrocarbons are separated as rapidly as possible from particulate solids
bearing the high CC deposits and metals. This may be accomplished by direct
discharge from the contactor into a large disengaging zone defined by vessel
- 21 -
1 3. It is however, possible that the contactor discharge the product
directly into cyclone separators 4 from which vapors are transferred to
vapor line 5. Entrained solids drop into the disengaging zone by diplegs 6
to a stripper 7. Steam and/or hydrocarbons admitted to scripper 7 by line 8
evaporate traces of volatile hydrocarbons from the solids.
The mixture of steam and hydrocarbons, together with entrained
solids, enters cyclone 9 by opening 10 to disengage the suspended solids for
return to stripper 7 by dipleg 11. As is well known in the art, a plurality
of cyclones 4 and 9 may be used. These cyclones may be multistage, with gas
phase from a first stage cyclone discharging to a second stage cyclone.
The cyclones may be of the stripper cyclone type described in U.S.
Patent No. 4,043,899. In this case, the stripping steam admitted to the
cyclone may be at a relatively low temperature, such as 400-500F, and may
serve to perform part or all of the quenching function presently to be
described. Alternatively, sùperheated steam or gas may be introduced to
keep the products from condensing before an external quench. A system of
preference in the present invention is the vented riser described in Meyers
et al., U.S. Patent Nos. 4,006,533 and 4,070,159.
The vaporized hydrocarbon from cyclones 4 and 9 passing by way of
line 5 is then mixed with cold hydrocarbon liquid introduced by line 12 for
the purpose of quenching thermal cracking. The quenched product is cooled
in condenser 13 and passed through accumulator 14. Gases are removed for
fuel from accumulator 14, and water, if any, is taken from sump 15,
preferably for recycle to the contactor for generation of steam to be used
as an aid in vaporizing charge at bottom of the riser and/or for removing
heat from the burner. Condenser 13 may be advantageously set up as a heat
exchanger to preheat charge to the contactor or to the FCC unit employed
subsequently.
In one embodiment, quenching is advantageously conducted in a
column equipped with vapor-liquid contact zones such as disc and doughnut
- 22 -
~;333~
1 trays and valve trays. Bottoms from such a column quencher could go
directly to catalytic cracking or hydrotreating with overhead passing to
condenser 13 and accumulator 14 or the overhead could be further
fractionated to recover the solvent, recycle streams, and naphtha, gas and
water from accumulator 14.
Certain advantages can be realized in the system by the use of
recycled light hydrocarbons at the bottom of contactor 1 for further vapor
pressure reduction if the solvent is present in amount sufficient to reduce
the viscosity of the bitumen to an acceptable level but is not present in
amount to achieve the desired reduction in hydrocarbon vapor pressure.
Recycle of water from accumulator 14 for this purpose requires that the
effluent of the contactor be cooled to the condensation point of water. In
this water vapor/hydrocarbon vapor system, that temperature would be about
150F. When hydrocarbons are used for pressure reduction and as the
lS stripping medium at line 8 condensation becomes unnecessary when only small
amounts of water are associated with the bitumen. In particular, the use of
hydrocarbon both as diluent and for vapor pressure reduction allows for
efficient recycling of this material. The contactor effluent may be passed
directly to a catalytic cracking reactor. In this case, the contactor also
functions as the catalytic cracking preheae furnace.
The light hydrocarbons chosen to boil below the temperature in
contactor 1 for use both as diluent and as means for vapor pressure
reduction are preferably recycled in the process. While for purposes of
vapor pressure reduction, light hydrocarbons such as naphtha, kerosene
and/or gas oil fractions derived from the process may be employed, the use
of ~he gas fraction derived from the process is preferred. In particular,
the use of these liquid solvents during the separation of bitumen from the
raw tar sand and their retention in the selective vaporization feedstock
leads to an especially efficient system.
The liquid hydrocarbon phase from accumulator 14 is a desalted,
decarbonized and demetallized fraction which, after removal of any entrained
- 23 -
~,:~333i
particulate sand not removed by cyclones 4 and 9, would be satisfactorycharge for catalytic cracking or, where desired, hydrotreating to increase
the hydrogen content. This product of contact in contactor 1 may be used in
part as the quench liquid at line 12. The balance is preferably transferred
directly to a subsequent refining stage via line 16. This product may be
optionally treated with a particulate separation means prior to refining.
In stripper 7, the catalytically inert solid particulate material,
bearing a discontinuous coating of particles of colloidal clay, passes by a
standpipe 17 to the inlet of burner regenerator 18. Most commercial
regeneration unit designs operate with air distributors in the combustor as
a jet at 125 to 400 feet per second (fps). As material is charged
perpendicularly into the regenerator lnto contact with air jets at 125-400
fps the effect will be a combination of a fluid energy mill and a ball
milling action, the latter taking place by particle-to particle contact.
Assuming circulation of fresh ARTCAT contact material at 4#/#feed, there
will be 400# ARTCAT/# clay when operating with a bitumen feed containing 1
wt % clay. This will provide an ample number of collisions to remove
protuberances of deposited colloidal clay before it can build up into a
dense, attrition resistance shell.
This inert contact material also bears a deposit of high CC and
metallic content material. Standpipe 17 discharges to a riser 19 where it
meets a rising column of air introduced into line 19. The spent particles
are mixed with hot inert particles from burner recycle 20, whereby the
mixture is rapidly raised to a temperature for combustion of the deposits
from treating the bitumen, e. g., 1200-1600F. The mixture enters an
enlarged zone 21 to form a small fluidizd bed for thorough mixing and
initial burning of deposits. The flowing stream of air carries the burning
mass through a restricted riser 22 to discharge at 23 into an enlarged
disengaging zone. The hot burned particles, now largely fr~e of combustible
deposit, fall to the bottom of the disengaging æone, burner 18. A portion
- 24 -
~Z~333~
1 of the particles is introduced into recycle 20. Another part enters
standpipe 2 for supply to contactor 1 after steam stripping. Because of the
high temperatures which can be obtained in this type of burner, C0 will burn
to provide a flue gas containing very little of that gas in the presence of
a stoichiometric excess of oxygen. In other types of burners, the
combustion products may contain substantial amounts of C0 which can be
burned for its heating value in C0 boilers of the type commonly used in FCC
units.
In the type of burner shown, the gaseous products of combustion,
containing carbon dioxide, some residual oxygen, nitrogen, oxides of sulfur
and nitrogen, and perhaps trace C0, enter a cyclone 25 to disengage
entrained solids for discharge by dipleg 26. As is known in the art, a
plurality of such cyclones may be used. The clarified gases pass to a
plenum 27 from which flue gas i9 removed by outlet 28.
Although the system ~ust described bears a superficial resemblance
to an FCC unit, its operation is very different from that of an FCC system~
Of greatest significance is the fact that the contactor 1 is operated in
such a manner as to remove from the charge an amount not greatly in excess
of the equivalent of twice the Conradson Carbon value of the feed. This is
achieved by the very low severity cracking due to the inert character of the
solid and the very short residence time at cracking temperature. It is
generally recognized that cracking severity is a function of time and
temperature. Accordingly, increased temperature may be compensated for by
reduced residence time, and vice ver~a. Ideally, no more than 120% of the
CC equivalent is removed from the charge in the form of coke. The practical
lower limit for the selective vaporization of bitumen is about 80% of the CC
equivalent.
The selective vaporization process affords a control aspect not
available to FCC units in the supply of hydrocarbons or steam to the
contactor. When stocks of high CC number are processed, the burner
- 25 -
333~
1 temperature will tend to rise because of increased supply of fuel to the
burner. This may be compensated for by increasing the amount of
hydrocarbons and/or steam supplied to reduce initial pressure of
hydrocarbons in the contactor or by recycling water from the overhead
receiver to be vaporized in the contactor to produce steam.
After transfer via line 16, the hydrocarbon product may be
introduced to the feed line of an FCC reactor operated in the conventional
manner. Because the FCC unit provides a product under normal operations
containing some fines generated through abrasion of the contact material, it
has been generally necessary to employ some means of physical separation to
remove these fines in the FCC unit itself. Accordingly, the charge to the
FCC unit need not be treated to remove entrained mineral particles prior to
charging.
When the products of selective vaporization of bitumen are to be
subjected to a hydrogen addition treatment, removal of most of the entrained
solids should be carried out in order to minimize pore blockage of the
hydrogenation catalyst and blockages in the hydrogen addition unit. It is
particularly advantageous to collect the selective vaporization products
with any entrained fine mineral particles in a settling tank prior to
hydrotreating. The bottoms from this settling tank could be fed directly
into a catalytic cracker, burned in the regenerator or simply removed from
the system. The ligher fraction, referred to as 'clarified oil', is
substantially free of entrained solids; any remaining particles are then
removed by conventional means, such as centrifuging or electronic
separation. These known methods for removal of solids provide hydrotreating
charge containing as little as 500 ppm fines or less.
In some cases> it may be desirable to subject the hydrocarbon
product to a hydrocracking treatment. This for~ of high severity
hydrotreating simultaneously induces molecular conversion, desulfurization
and denitrification. Lt is carried out at much higher pressures than a
- 26 -
~2~333~
1 standard hydrotreating as used to saturate double bonds in the hydrocarbon
product, and generally requires a significantly greater hydrogen input as
well. Material which is to be hydrocracked should also be subjected to a
preliminar~ treatment to remove substantially all of the fines.
Yet another method for production of useful hydrocarbon products
from the selective vaporization product is vacuum distillation in a
so-called "vacuum tower." The bottoms from the tower, generally comprising
materials boiling above 1000F, may be used to prepare heavy fuel oil, such
as Bunker C and No. 6 oils. The fraction boiling at 600-1000F can be
subjected to conventional hydrotreating for further upgrading, or catalytic
cracking to prepare high octane products.
The terms and expressions which have been employed are used as
terms of description and not of limitation~ and there is no intention in the
use of such terms and expressions of excluding any equivalents of the
features shown and described or portions thereof, but it is recognized that
various modifications are possible within the scope of the invention
claimed.
The following examples are given to illustrate certain aspects of
the invention.
Example I
This example demonstrates that removal of colloids deposited on
contact material from tar sands bitumen in a selective vaporiz~.tion process
prior to circulating the contact material to renewed contact with incoming
charge of tar sand bitumen feedstock is desirable.
Solvent-diluted tar sands bitumen were used in a selective
vaporization process carried out in a conventional pilot plant FCC
riser-regenerator system. The regeneration air was introduced through a
fritted air distribution system; consequently, there was no provision to
induce attrition and a ball milling action to remove colloids deposited on
the contact mater~ial (microspheres of calcined kaolin clay) prior to
- 27 -
-
lZ~;~3~
1 recirculating contact material to the contactor. Thus, in the pilot plant
tests, the deposited colloids were able to build up as a dense shell on the
particles of contact material.
The properties of the bitumen prior to dilution with solvent are
set forth in Table I. The chemical composition of the ash in the bitumen is
detailed in Table II.
The chemical, physical and catalytic properties of the equilibrium
bitumen contact material sample used to heat the bitumen are presented in
Table III. For comparison purposes, representative values for fresh contact
material are also included in the tables, along with representative values
for equilibrium contact materials used in selective vaporization of crude
oil fractions.
Comparison of the chemical analyses (Table II and Table III) of
the bitumen treated equilibrium contact material sample and the bitumen ash
clearly indicates that a large fraction of the ash, especially iron,
titanium, calcium and sulfur, has been incorporated into the microsphere
sample. In addition, the surface area and micropore volume of the sample of
contact material used to treat the bitumen were significantly higher than
either fresh or equilibrium contact material which had been used for
selective vaporization of residual fractions of petroleum (resid contact
material sample). These changes in the physical and chemical nature of the
sample are presumed to be responsible for its higher catalytic activity in
MAT test results (Table IV). The high MAT conversion values for the bitumen
treated contact material sample are from high yields of C3 and C4
(primarily olefins), and gasoline. The low yields of Cl and C2 and coke
products suggests that the hydrocarbon products result from acid cracking
rather than thermal cracking or metal dehydrogenation reactions. The high
sulfur content on the regenerated bitumen contact material sample is also
quite unusual. The sulfur is probably present as thermally stable sulfate
compounds. The particle size distribution is typical of equilibrium
- 28 -
12-~;3~33~
1 selective vaporization contact materials (low levels of -40 micron
material).
Several representative SEM photographs were taken, and EDX (energy
dispersive x-ray) analyses of the bitumen sample contact material are
presented in Table V. Only clay-based microspheres were present.
Apparently the ash material was so fine that no large (50-70 micron)
particles were formed. The surface roughness, protrusions and
irregularly-shaped particles which were observed on the microspheres
surfaces are very unusual for an equilibrium contact material and have not
been observed in samples used to treat residual oils. The close match of
the chemical properties of the surface particles on the microspheres and the
bitumen ash clearly indicates that the bitumen ash has deposited on the
exterior surfaces of the contact material during the selective vaporization
process operation.
EXAMPLE 2
The test work described in this example suggests that
selective-attrition will be effective for removal of ash contaminants
deposited on contact material during the upgrading of clay contaminated tar
sand bitumen by selective vaporization.
A sample of the equilibrium calcined kaolin clay contact material
used in the pilot plant test run of Example 1 was subjected to attrition to
determine whether deposited ash could be selectively attrited from the
microspheres of calcined kaolin clay. As shown in Table III, the fresh
contact material (microspheres of calcined kaolin clay) analyzed
approximately 45 wt% A1203, 52 wt% SiO2, 2 wt% TiO2, less than 1%
iron oxide and negligible calcium. The analysis of the equilibrium contact
material including ash deposit also appears in Table III under the legend
8itumen Contact Material Sample and shows appreciably higher levels of
iron, titanium and calcium than were present in the contact material.
Since the run in whlch the ash was deposited on contact material
was carried out in a pilot unit not equipped with means to attempt to
- 29
333~ ,
coneinuously attrite the ash deposit during regeneration, the efEort to
determine the response of the equilibrium material to a high velocity air
jet was carried out in a Roller Attrition test unit. This Roller attrition
test is well known in the FCC industry where it is used to determine the
attrition resistance of samples of fluid cracking catalyst. The Roller test
applies a high velocity air jet to a sample located in a U-tube below a
cyclone. After the application of the high velocity air, the attrited
material which passed through the cyclone was collected in a filter. The
attrited material was recovered and analyzed by SEM/ED~ techniques,
insufficient material being available for complete chemical analysis or to
make a material balance.
The attrited material was found to consist of two general types of
particles, i.e., shell pieces of the order of about 10 microns or less in
size and fine dust. SEM analysis of the microspheres remaining after
~5 removal of attrited material in the Roller unit showed evidence of cracking
and partial removal of the shell. The chemical compositions of the attrited
components, expressed as oxides, are set forth below:
wt.% Shell Pieces Fine Dust
Na20 -- 0.44
MgO 4-95 ~~
A1203 13.83 37.93
SiO2 23.87 51.36
P205 5.09 1.16
S03 4.~5 0.76
Cl 0.14 __
K20 0.63 - 0.57
CaO 20.27 2.94
TiO2 11.16 2.79
Fe203 15.70 2.05
Only qualitative conclusions could be drawn since, as mentioned,
there was not sufficient material to make complete chemical analyses and
- 30 -
~L2~i~33~
1 material balances. However, by comparing the results of the SEM/EDX
investigations with chemical analyses of the contact material before the
deposit of ash (Table III), it appears that the attrited materials were
enriched in calcium, iron and ~itanium. Enrichment was especially evident
~ in the case of the shell pieces. As mentioned, the remaining microspheres
exhibited evidence of cracking and partial removal of the shell. These
results suggest that the ash deposit was selectively attrited by application
of the air jet.
EXA~LE 3
A Utah, USA tar sand bitumen has been treated in an ART process
unit pilot plant to produce an upgraded synthetic crude. This extremely
heavy material had an API of 9.3 and contained 1.2 wt~ mineral matter.
Table VI summarizes expected commercial unit yields based on pilot plant
tests adjusted for heat balance and providing for continual removal by
attrition of deposited colloid.
To illustrate the dramatic change in boiling range which took
place in processing of this tar sand bitumen we have illustrated in Figure 3
the distillation curves of the synthetic crude product and the bitumen
feedstock. Figure 3 shows estimated true boiling point distillation and API
gravity curves of the products and feedstock (distillation only). Most
Interestingly the bitumen feedstock had an initial boiling point of about
482C (900F). The synthetic crude was substantially lighter, 70 Vol. % of
which boiled below the initial boiling point of the bitumen feedstock. Very
important was the fact that the 565C+ (1050F~) portion of the synthetic
crude amounted to only 12 Vol. % of the synthetic crude oil. This fraction,
corresponding to vacuum residum, comprised about 92 Vol. % of the bitumenfeedstock. In fact, comparing the synthetic crude oil which would be
produced from the bitumen with heavy Arabian crude oil indicates that the
synthetic crude, being of much lower contaminant content and having much
more distillate range material, could be of significantly higher value.
- 31 -
~L263331
1 Table I
TAR SANDS BIT~IEN FEED PROPERTIES
API 10
Sulfur 0.4 wt.
Ramsbottom Carbon 16 wt. Z
Nickel 60 wppm.
Vanadium 10 wppm.
Ash ca. 1 wt.
Table II
TAR SANDS BITUMEN ASH CO~1POSITION
CaO 23.5
Fe2O3 15.1
TiO2 14.7
SO3* 11.1
SiO2 10.5
MgO 6.5
Al2O3 5.1
K20 1.1
Na2O
88.5**
*It is believed that the presence of SO3 may be derived from
incomplete decomposition of sulfur compounds in the hydrocarbon portion of
the bitumen.
**It is believed that carbonate was also present in the sample but
was not accounted for in making the analysis.
- 32 -
1:21Ei3~.
Table III
C~IEMICAL AND PHYSICAL PROPERTIES
EQUILIBRIUM SELECTIVE VAPORIZATION CONTACT MATERIALS
Bitumen Equilibrium
Chemical Contact Fresh Resid Contact
Analyses Material Contact Material
(wt.%) Sample Material Sample
LOI 3.54 1.0 0.15
Al2O3 44.03 45.10 44.59
SiO2 47.10 51.72 51.95
Na20 0.51 0.45 0.91
Fe2O3 1.82 0.40 1.10
TiO2 2.71 1.90 1.87
K2O 0.17 0.10 --
CaO 2.19 0.05 --
MgO 0.52 0.03 --
P2O5 0.63 0.45 --
Ni (ppm) 625 -- 1300
V (ppm) 1620 ~~ 2400
Leco C wt.% (as is) 0.02 -- .02
Leco S ~t.% (as is) 1.32 -- --
Mullite Index 7 4 ~~
BET S. A. (m2/g) 16.0 8.6 7.8
N2 Pore Size Dist. .02 .004 --
100 A
100-600 A (cc/g) .04 .05 ~~
Hg Pore Volume 0.20 0.25 0.17
(cc/g) (100-
20,000 A dia)
Particle Size Distribution
Bitumen Fresh Crude
Micron Size:
0-20 2 4
0-40 3 14 6
0-60 12 37 24
0-80 40 59 47
Avg. Part. Size 86 71 82
~micron)
- 33 -
~2~333~
1 Table IV
M~T RESULTS
SELECTIVE VAPORIZATION CONTACT MATERIALS
Bitumen Equilibrium
M'AT Contact Fresh Contact
Yields Material Contact Material
_wt.%) Sample Material Sample
Conversion 23~19 7.0 6.1
-- H 0.13 0.06 0.12
C2 0.56 0.50 0.6g
C3+C4 2.20 0.70 1.1
Cs-421F 18.38 5.0 3.0
421-602~F 23.95 22.41 25.1
602+ 52.86 70.54 68.90
Coke 2.05 0.85 1.30
(Average of 2 runs) (Average of 2 runs) (Average of 2 runs)
Table V
EDX ANALYSIS
BITUMEN SAMPLE CONTACT MATERIAL
15A. Overall Microsphere Composition:
Oxide Component Wt. %
MgO 3.24
A123 10.79
SiO2 30.31
P205 3.41
so3 9.13
CaO 14.60
TiO2 7.76
V25 0.46
Fe23 9-97
CuO 0.31
B. Protuberance Composition:
Oxide Component Wt
MgO 2.41
A12O3 30.42
SiO2 39.71
P2O5 1.63
so3 2.61
K2O 0.12
CaO 3.69
TiO2 1.66
V25 0.37
Cr2O3 0.38
MnO 0.19
- 30 Fe23 16.79
- 34 -
~ "
~2~3~3i
Table VI
TAR SAND BITUMEN YIELDS
Expected commercial unlt yields based on pilot plant tests and
adjusted for heat balance.
- ~ , C2 3.2
LPG 2.7
- Cs~205C 14.1
(Cs-400F)
205C-345C 10.8
(400-650F)
345C+ . 56.4
(650F+)
COKE 12.8
..
- 35 -