Note: Descriptions are shown in the official language in which they were submitted.
- lZ63795
PHOTOPATTERNABLE DIELECTRIC COMPOSITIONS
AND METHODS FOR MAKING AND USING
,
Backgrnund of thP lnvention
The present ;nvention relates to novel photoreslst composi-
tions and methods of making and using such compositions. More
particularly, the present invention provides photol~thographic
methods for making photoresists by reacting a polyamic acid
with a photoreactive compound, applying the photoreactive reac-
tion product to a substrate and allow~ng ~t ~o dry, exposing
the treated substrate while it ~s masked to a source of light
so as to effect crossl~nk~ng of the photoreactive groups, and
heating the resultlng crosslinked coatlng at a temperature
effective for convert~ng the coating to a polyimide. The
present invention also relates to novel polyimide precursor
compositions and methods for making such compositions.
Prior to the present invention polyimides and polysiloxane
imides were obta~ned by effectins reactlon between a carboxylic
acld dianhydrlde and `a diamino compound and/or diaminopolysi-
loxane to obtaln an init~al reaction product havlng the
fonmula, for example,
_ _ _ . , _ _ _ _ , . , .... ... . . ... .. .. . . . _ .... .. .
605I-339/4617L
GLL:mz
O O Q
1 11
_ - N - C ~ - C ~ , C - NH - R - ~
HO - C - ~ ~ C - OH n
R = aliphatic and/or aromatic and/or siloxane
Upon heating at a temperature of about l 50DC to 350C the poly-
meric amido compound cyclized to y~eld an imidized composition
of recurring units of the fonmula, for example,
~ O O o
C 11 lCI ~
~N ~ ~ N - ~
Il 11
O O
R = aliphat~c and/or aromatic and/or s~loxane
This method ~s descr1bed more fully in U.S. Pat. Nos. 3,325,450
and 3,553,282, among other patents and literature references.
( ~ --
~Z6379~;
60SI-339/4617L
GLL:mz
Photores~st materials which are based on a photoreactive
precursor are described by Rubner et al. ln "Production of
Highly-Heat Resistant Film Patterns from Photoreactive Poly-
meric Precursors, Part I, General Principles ~Jan: 1976)~ and
"Production of Highly-Heat Resistant Film Patterns from Photo-
reactlve Polymerlc Precursors, Part 2, Polyimide Film Patterns
(May 1976)". A photoreact~ve polyamide is made by initially
e~fecting reactlon between an aromatic dianhydride, for
example, pyromellitic dianhydride, and allyl alcohol. The
resulting aromatic dicarboxylic acid diester is then converted
to the correspond;ng aromatic dlacid chloride by reaction with
thionyl chloride. The diacid chloride is further reacted with
an aromatic diamine to produce a photoreactive aromatic poly-
IS am~de ester. The aforementloned photoreactlve polyimide
precursor is then applied to a substrate, for example by spln
coating, and exposed to light with the aid of a mask, followed
by developing the treated surface with an organic solvent to
produce a photoreslst. The aromatic patterned polyamide ester
is then heated to convert it to a patterned polyimide.
Although valuable results have been achieved with the
aforementioned photoreactive aromatic polyamide ester, those
skilled in the art recognize that the use of a chlorinating
agent such as thionyl chlorlde to convert the aromat~c dicar-
boxylic acid to the corresponding ac1d chloride prior to the
polymerization reaction with aromatic diamine can result in the
introductlon of residual chlorlde contam~natlon. Such chlorlde
contaminatlon can lnterfere with the u~llity of the resulting
aromatic polyimide as a dlelectric.
3 ~
60SI-339/4617L
GLL:mz
--4--
1 It is, therefore, des~rable to proYide a photosensittve
polyamide acid useful and a photoresist and convertible to a
patterned insulat~ng layer free of chloride contam~nation.
Summary of the Invention
It is one object of the present invention to provide a
method for pattern~ng an insulative polyimide or silicone-poly-
imide layer on a substrate.
It is another object of the present invention to provide a
photosensitive polyam~de acid or silicone-polyamide ac~d.
Another object of the present invent~on is to provide a
method for making photosensitive polyamlde acids and silicone
polyamide acids.
In accordance with the present invPntion a photosensitive
polyamide acid useful as a photoresist and convertible to a
patterned insulating layer free of chloride contamination can
be made by reacting a dianhydr~de of the general formula
D 0
C C
~ \ / \
0 R 0 (1)
C C
Il 11
O O
~LZ63795 60SI-339/4617L
GLL:m~
1 with a diamine of the general fonmula
H2N - Rl - NH2 (2~
to obtain a polyamide ac~d consisting essent~ally of chemically
combined units of the formula
O O ~
~ 11 li \
/ HO - C C - OH
- N C C - N - Rl ¦ (3
1¦ 10
\ H O O H
where R is a tetravalent organic radical or a tetravalent
organosiloxane-containing radical and Rl is a d;valent
organic radical or a divalent organosiloxane-containing radical.
The polyam;de acid of fonmula (3) is further reacted with a
photosensitive acrylate, cinnamate or 2,3-diphenylcyclopropenol
ester ~n order to provide a modified or photoreact~ve polyam~de
acid consistlng essent~ally of chemically combined units of the
fonmula
2 6~3~7~3~i
60SI-339/4617L
GLL:mz
--6--
O O
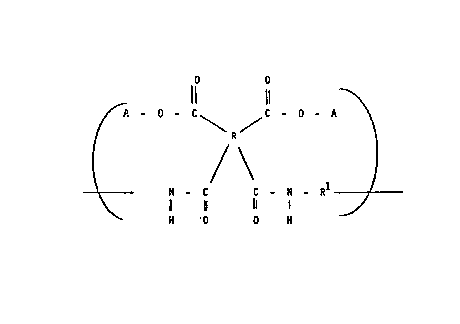
Il 11 ~
~. - O - C\ R ~ ~ - A ~ (4)
/ \ 1
\ N - C C - N - R ~ f-----
11 11 1
H -0 0 H
where R and Rl are as previously defined and A is a photo-
reactive acrylate, c~nnamate or 2,3-d~phenylcyclopropenol ester.
In another aspect of the present invention there is provi-
ded a method for patterning an insulat~ve polylmide or
5s~licone-polyimide layer on a substrate which comprises:
(1) dissolving the photoreactive polyamide acid of fonmula
(4) in an inert solvent;
(2) applying a coating of the solution of fonmula (4) to a
substrate, for example,---by sp~n coating, in the~
10substantial absence of light;
(3) allow~ng the coated substrate to dry;
(4) exposing the coated substrate for a tlme sufficient to
crosslink the desired photoreactive polyamide
molecules;
~26:~7~S
60SI-339
-- 7
(5) developing the resulting crosslinked
polyamide acid coated substrate; and
(6) heating the developed crosslinked polyamide
acid coated substrate at a temperature
effective for converting the polyamide acid
to a polyimide.
Description of the Invention
In accordance with the present invention, a
photosensitive polyamide acid useful as a photoresist
and convertible to a patterned insulating layer free
of chloride contamination is prepared by first
reacting a dianhydride of formula (1) with a diamine
of formula (2) to provide a non-photosensitive
polyamide acid having the general formula (3).
Generally it is not critical what dianhydride and
diamine are used and the skilled artisan can readily
select those most suitable for his contemplated use.
The dianhydride has the general formula (l) where R is
a tetravalent organic radical or a tetravalent
organosiloxane-containing radical. Suitahle
dianhydrides and their method of preparation are
described in U.S. Pat. Nos. 3,553,282 to Holub and
4,030,948 to Berger.
Illustrative of the dianhydrides suitable for use
in the present invention are pyromellitic dianhydride;
2,3,6,7-naphthalene tetracarboxylic acid dianhydride;
3,3',4,4'-diphenyl tetracarboxylic acid dianhydride;
1,2,5,6-naphthalene tetracarboxylic acid dianhydride;
, .,/
~,,
37~S ~
60SI-339/4617L
GLL:mz
2,2',3,3'-dfphenyl tetracarboxylic ac~d dfanhydr~de;
2,2-b~s (3,4-dlcarboxyphenyl)propane d~anhydrfde;
bfs (3,4-dfcarboxyphenyl) sulfone dianhydrfde;
3,4,3,10-perylene tetracarboxylic acfd dfanhydrfde;
bfs (3,4-dicarboxyphenyl) ether d~anhydride;
2,2-bis (2,3-dicarboxyphenyl) propane dfanhydrfde;
l,l-bfs (2,3-d~carboxyphenyl) ethane dianhydride;
bis (3,4-dicarboxypheny7 ~ methane dianhydride;
1 bfs (2,3-dicarboxyphenyl) sulfone dfanhydrfde; and
benzophenone tetracarboxylfc acfd dianhydrfde.
Generally the preferred dfanhydrides of fonmula (1) are
where R is a tetravalent C(6 303 aromatfc radical selected
from
~' .
~ ~
O O
~ IC _ Q
and
~ - R5 -
2~i3 7 9~; C ~
60SI-339/4617L
GLL:mz
1 where Q ~s a divalent radical having the fonmula - ZR6Z -
where Z is selected from -O- and -NH-, and R5 and R6 are
selected from C~2 13) organic radicals.
Also acyclic or cycllc aliphat~c dianhydr~des such as
cyclopentane tetracarboxylic acid dianhydrfde, cyclohexane
tetracarboxylic acid dlanhydride, butane tetracarboxylic ac~d
dianhydride and the like are suitable for use ~n the present
invention.
Organosiloxane-containing dianhydrides are also within the
scope of the present invent~on and preferably have the general
fonmula
o~ 3 ~~s~
o o o
where D is a di-nitrogen or di-oxygen tenminated siloxane.
Dianhydrides of formula (5) can be prepared by reacting trimel-
litic chloride, ~.e.
Il ~ IC - Cl
O O
~26379~
60SI-339/4617L
GLL:mz
-10-
1 with any diamine or dialcohol, preferably having siloxane
linkages. Accordingly, siloxane-containing dianhydrides of the
present invention have formulas such as, for example,
) ~ 3 L C N R3~ S~-R3- N - C~/>~
O O H R R3 H O
S and
\ ~ G - oR3 ~ si-R3- o - C
where R3 is a subst~tuted or unsubstituted hydrocarbon
radical, preferably haYing from 1 to 8 carbon atoms, and n has
a value of from 1 to about 200 and preferably from 1 to about
100.
G G
1~379S
60SI-339/4617L
GLL:mz
The most preferred dianhydr~des are pyromell~tic d~anhy-
dride, benzophenone tetracarboxylic ac~d dianhydr~de and
~ ~0~ 0
CH3
Illustrative of the aminosiloxanes which can be reacted
lS ~ith trimellitic chloride to obtain s~loxane-containing dian-
hydrides are
fH CH
H2~ CH2 ~ S~ - O - S~ CH2 ~ 2 '
CH3 CH3
~Jhere n preferably equals 2 through 6 inclusive and most
preferably equals 3. ~~ ~ ~~~ ~ ~~
l6H5 l6HS
H2N - C6H4 ~ C - li - C6H4 2
C6H5 C6H5
~ c)
1263795
605I-33g/461 7L
GLL:mz
-12-
H2N ~ 5l 0 -- S7 ~ ~ NH2
CBH4F3 C3H4F3
CH3 ~ CIH3 ~ IH3
H2N ~ cH2~ liO t li r 1~ ~ cH2~ NH2
CH3 ~ 3 J x 3
where n i5 aspreviously defined and
I ~ IH
H2N ~ CH2)n- ji- ~ i~-t si ( CH2~n NH2
B ~ C2H4 ~ x P'
2~
B = CH3 and/or phenyl
where n ~s as previously defined. Of course, dialcohol end-
stopped compounds can be used in place of the diamino end-
stopped compounds. In either case, the method for preparing
the siloxane-containing dianhydrides is well known to those
skilled in the arS.
J
2~;3 7 9~i
60SI-339/4617L
GLL:mz
-13-
1 Generally Rl of the dlamlne of formula (2) has the formula
~r
{~}~} Y ~}~ {~}'
0 CH
where Y is -S-, 5 or ~ 3
~ z ~ 3
where Z is CH2, ~H3 or -0-
-C-
~H3
R7 ~ R ~ R7
I \
- R7 - Si 0 Si / 0 Si - P~7 -
R7 R7~ X R7
where R7 is a C(1,13) organic radical and x = 1 to about 200'.
Of course, other variations will be obvious to the skilled artisan.
G CJ
~1263~
60SI-339/4617L
GLL:mz
D1amlnes wh1ch can be reacted w1th the forego1ng dianhy-
dr1des to obtain non-photosens1t1ve polyamide acids are well
known In the art and are also described in U.S. Pat. Nos.
3,553,282 and 4,030,948. D1amines within the scope of formula
(2) include for example,
n-phenylenediam1ne;
p-phenylenediamine;
4,4'-diaminodiphenylpropane;
4,4'-diaminodiphenylmethane;
benzidine;
4,4'-diaminodiphenylsulfide;
4,4'-diaminodiphenyl sulfone;
4,4'-diaminodiphenyl ether;
l,5-diaminonaphthalene;
3,3'-dimethoxybenzidine
2,4-bis IB-amino-t-butyl) toluene;
1,3-diamino-4-isopropylbenzene;
m-xylyened1amine;
p-xylenediamine;
2,2-d1methylpropylenediam1ne;
l,4-cyclohexanediamine; and
bis (3-aminopropyl) sulfide.
This listing of suitable am1nes, as with the previous list-
ing of su1table dianhydrides, is not intended to be exclusive
as those skilled in the art recognize that a complete listing
is not feas1ble. It should be noted that in add1tion to
organ1c diamines, there are also 1nc1uded w1thin the scope of
3~ fonmula (2) di(aminoalkyl)polysiloxanes as disclosed herein-
above for reaction with trimellitic chloride to obtain dian-
hydrides.
G ~ J
~i3Y7~3~;
60SI-339/4617L
GLL:mz
-15-
The react10n product of the dlanhydrlde and the dlamlne ~s
a polyamlde-acid of chem~cally combined unlts of fonmula (3),
i.e.,
~ O O
11 11
/ HO - C C - OH
l \ /
R
-- ~ - C C - N - Rl /
\ I Ol 11
~ H O O H ~
where R ls the tetravalent organic or organos~loxane-containing
radical of the dianhydride and Rl ~s the divalent organic or
organosiloxane-contalning radical of the dlam~ne.
In order to provide a photosensitive or modified polyamide
acid, ~t has been found desirable to react the polyamide ac~d
of formula (3) with a photosensit~ve acrylate, cinnamate or
2,3-diphenylcyclopropenol ester. So that the photosensitlve
compound will reac~ wlth the polyamide acld of fonmula (3) to
provlde the photosensltiYe modifled polyamide ac~d of formula
(4) lt is necessary that the compound have a functlonal group
which will replace the acid hydrogen of the polyamide acid, for
examp1e, an epoxy contaln~ng group such as glycidyl.
G
50SI -339/461 7L
GLL :mz
-1 6-
In order that those skllled ~n the art wlll more clearly under-
stand the react~on between the polyam~de acld and the photo-
sensitlve compound the following ~llustrat~on ~s provided.
O O
HO - C C - OH
~ I 11 F~ I ~7L
H O O H
x
OH O O OH
2 \ ~ 2 - B
N - C C - N - Rl /
1 11 11 1 ~
H O O H x
~Z6379~i 60SI-339/4~17L
GLL:mz
1 4
where R is a divalent Cl 8 radical, B is ~ photosensltive
acrylate, cinnamate or 2,3-diphenylcyclopropenol ester, R and
Rl are as prev10usly defined, and x illustrates that substan-
tially equimolar quantities of reactants are utilized.
Included among the su~table photosenslt~ve compounds are:
glycidyl methacrylate;
glycidyl cinnamate;
glycidyl-2,3-diphenylcyclopropenol
glycidylallylphthalate
glycidylallylether
The most preferred photosensitive compounds are glycidyl
methacrylate, glycidyl cinnamate, and glycidyl-2,3-diphenyl-
cyclopropenol.
Modification of the polyamide acid or silicone-polyamide
acid ~s effected by agitating a mixture of the acid and photo-
sensitive compound in the substantial absence of light and in
the presence of an appropriate organ~c solvent such as N-me~hyl
pyrolidinone, dimethylacetamide, dimethylformamide dimethoxy-
ethane, diethyleneglycoldlmethylether, and dimethylsulfoxide.
.
~2637~;
605I-339/4617L
GLL:mz
Generally the reactiDn mixture should be st~rred at room
temperature for a per10d of from about 8 to about 24 hours.
After modifkation of the polyamide acid with the photo-
sensitive compound, if desired, the resulting modified polymer
can be sensitized wlth an appropriate photoinitiator. There
can be util~zed from about O.lX to about lOX by weight of
photoinitiator based on the we~ght of the mod~fied polyamide
acid. Suitable photoinitiators or sensitizers are, for
example, Michler's ketone, benzophenone, and 2,2-dimethoxy-2-
phenylacetophenone, each with N-methyldiethanolamine.
Such modified or photoreactive polyamide acid compos~t~ons
are useful as photoresists whkh are convert;ble to a patterned
insulating layer in electronic devices, particularly integrated
circuits. Accordingly, the present invention also provides a
method for obtaining an insulative polyimide layer on a sub-
strate such as a silicon wafer from the foregoing photosensi-
t~ve polyamide acid composition.
Initial~y the photosensitive polyamlde acid composition is
dissolved in a suitable ~nert solvent such as tetrahydrofuran,
dimethylformamide, N-methylpyrrolidinone, dimethylsulfoxide and
ethyleneglycoldimethoxyether.----The percent solids ~n -the solu-
tion can range anywhere from about 5 to about 95 percent by
weight sol~ds, but most preferably ranges from 5 to 25 weight
percent solids.
G
i2~
60SI-339/461 7L
GLL:mz
- -19-
The substrate surface, which typically is a s~licon wafer,
must he properly cleaned to ensure adequate resist wetting and
adhesion.
s
There are several methods by which a substrate may be
coated w~th the photores~st. The method chosen w~ll depend on
the need for uniformity and the th1ckness of the coating
deslred~ Spin coating ~s particularly advantageous in the
microelectronics industry where a thin unifonm coating of a
photoresist i5 applied to one side of a small part of the
substrate and where a high degree of uniformity is desirable.
In spin coat~ng the thickness of the resulting film is
controlled by adjusting the spinning speed and the solids
content of the resist solution.
Other methods of applying the photores~st to the substrate
are spray coating, dip coating and roller coating. Spray
coating is probably the best way to apply a thick coat~ng,
however, it results in waste of the photores1st mater~al. Dip
coating provides the most uniform coatings but it is relatively
slow and has llmitations in coating thickness. Roller coat~ng
is a method of uniformly applying very thin coatings to rigid
surfaces.
~5
After applying the photoresist to the substrate it ~s often
desirable, though optional, to oven dry or pre-exposure bake
the photores~st. This bake eliminates residual solvent,
promotes adhesion, and hardens the res1st. Temperature and
time depend upon the specific resist being used, but generally
are from 70-90C and 10-30 minutes, respectively.
f~J
~2~:~9~
60SI-339/4617L
GL~:mz
-20-
The photoresist 1s next exposed using a light source wh~ch
provides optical energy over a wavelength band that is
contained within the reslst absorption spectrum. Several
techniques are known for carrylng out the exposure. The oldest
and prnbably most widely used technique is contact pr~nting in
wh~ch ~he wafer is pushed ~nto ~nt~mate contact w~th a mask and
then the mask ~s f~ooded w~th l~ght. In proxim~ty pr~nt~ng the
mask and wafer are separated by a gap, nominally from lO ts 25
mm. In project~on prin~ing a high quality lens or mirror
system ls used to project the mask ~mage onto the wafer
surface. The purpose of the exposure step is to crosslink the
photoreact~ve portions of the photoreslst that were exposed to
the light while the masked or unexposed portions rema~n
uncrosslinked and hence soluble in selected solvents.
I5 Development of the photores~st ~nvolves contacting the
exposed photoresist with a solvent wherein the crosslinked
polyamide acid is insoluble but the uncrosslinked polyamide
acid is dissolved thereln. Th~s leaves a tough, chemlcally
resistant mask on the surface of the substra~e.
Finally, the developed photoresist ~s heated at a tempera-
ture sufficient to convert the polyamide acid to a poly~mide.
Generally the temperature necessary to effect imidization
ranges from about 200-C to about 300-C and the time req~ired
ranges fnom about 30 to about 60 minutes.
In order that those skilled ~n the art will be better able
to practice the invention, the following example ls given by
way of illustration and not by way of limitation. All parts
are by ~eight.
G C~
Z~3~
60SI~339/4517L
GLL:mz
-Zl-
1 Example
A polymer was prepared by adm~x~ng 0.11 mole (22 grams~ of
oxyd~an~line, 0.03 mole (7.44 grams) of bis-aminopropyltetra-
methyldisiloxane and 150 ml tr~glyme ~n a 500 ml flask. There-
after 74.0 grams ~0.144 mole) of bis-phenol A d~ankydr~de was
added to the m~xture. This was followed by the addit~on of 90
ml tetrahydrofuran. The mixture was st~rred overn~ght and then
0.144 mold (20.5 grams) glycidoxy methacrylate was added. Such
mixture was stirred an addit~onal 24 hours.
A 50 gra~ sample of the result~ng polymer was d~luterd to
10~ sol~ds with tetrahydr~furan. 3~ by we~ght of Irgacure ~51
photoinitiator was then added. The resulting solution was flow
coated on an aluminum substrate. The solvent was removed by
air drying for 30 minutes and thermally drying at 75-85nC for
one hour. The aluminum substrate was partially masked and
exposed for 10 seconds ~N2 atmosphere) to ultraviolet light.
The aluminum substrate was washed w~th tetrahydrofuran. The
unexposed area dissolved in the solvent whereas the exposed
area remained on the alum~num substrate.