Note: Descriptions are shown in the official language in which they were submitted.
- 1 -
AN ELECTRO~E FOR AN ELECTROLYTIC CELL FOR THE RECOVERY
OF METALS FROM METAL BEARING ORES OR CONCENTRATES
AND METHOD OF MAKING SAME
This application is divided from copending Canadian
Patent Application No. 443,033 in which is described
and claimed an electrolytic cell for the recovery
of metal from mineral ores or concentrates. The present
invention is directed to electrodes and a me-thod of
making same, which electrodes are useful in electrolytic
cells for the recovery of metals from mineral ores
and concentrates, particularly electrolytic cells
as described in copending Canadian Patent Application
No. 443,033.
FIELD OF THE INVENTION
This invention relates to an electrode for an
electrolytic cell for treating mineral ores and con-
centrates, and a method of making same.
BACKGROUND OF THE IN~ENTION
The electrolytic cell is of particular importance
in the recovery of copper from copper bearing ores
and concentrates as described in U.S. Patent 4,061,552
and the recovery of lead from lead bearing ores and
~a concentrates as described in U.S. Patent No. 4,381,225.
In these processes not only are electrodes and
electrolyte involved but also two lots of solids,
the metal bearing ore or concentrate and the particulate
metal product. To achieve a maximizing of reaction
with resultant high yield it has been previously believed
the anode and cathode should be in close parallel
relationship.
Also typical of the conventional electrolytic
cell is the use of diaphragm bags surrounding the
cathode. A multiplicity of diaphragm bags is employed
to keep slurry away from the cathodes where clean
metal is required to be deposited. Some problems
experienced in the operation of such a cell include:
1) Clogging of the diaphragm materials with
~; .. .
" ;'"''~ : ~' ` '
-- 2
particles when high hydraulic gradients must be used
in the cell to maintain a uniformity of agitation
of the slurry.
2) Difficulty in trying to maintain large areas
of cloth in parallel planes without distortion, which
is particularly aggravated by high hydraulic gradients
in the cell. In most cases it is undesirable for
the cloth to come in contact with the electrodes.
3) The energy requirements resulting from the
1~ necessity for agitation in the bottom of the cell
to maintain adequate suspension of the mineral between
the bags.
Other problems include:
Difficulties in recovering the metal powder if
it falls off the electrodes onto the cell floor or
the bags, or difficulties and costs in removing and
stripping the electrodes if the metal particulate
adheres strongly.
To overcome these problems it has been known
~0 to introduce additives into the electrolyte which
inhibit the growth of dendrites of metal powder on
the cathode. Further, many attempts have been made
to provide a simple and effective recovery of metal
powder. However the very design of parallel cathode
relationship complicates recovery. In particular,
previously it has not been possible to integrate a
central recovery system, especially with diaphragm
cells, without complex pipework and flushing techniques.
The present invention seeks to mitigate these
disadvantages of recovery of deposited product.
Accordingly, in one aspect of the invention,
there is provided a cathode for use in an electrolytic
cell for the recovery of metal from mineral ores or
concentrates, characterized by a conductive portion,
and a non-conductive covering overlying a portion
of said conductive portion, the non-conductive covering
being comprised of a perforated tubular member formed
of heat shrinkable plastics material which has been
heat-shrunk directly around said cathode to leave only
, .~
.,
,,, ~`,' ' ., ' : '
.
~ 3~
areas of said cathode exposed which are loc~ted below
the perforations in -the tubular member.
The conductive por-tion may be rod shaped, preferably
a tube.
The cathode may be a copper cathode.
According to a second aspect of the invention
there is provided a method of producing a cathode
for use in an electrolytic cell for the recovery of
metal from minerals, ores or concentrates, characterized
l() by providing an elongated conductive member, contacting
and surrounding said elongated conductive member with
a perforated tubular non-conductive covering formed
of heat shrinkable plastic, and heat shrinking said
non-conductive covering so as to leave exposed only
areas of said conductive member which lie below per-
forations of said non-conductive covering.
The invention is diagrammatically illustrated
by way of example, with reference to -the accompanying
drawings:
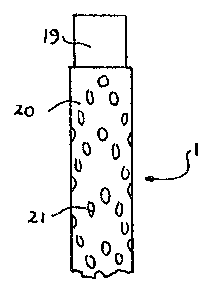
Figure 1 is a view of an electrode coated in
accordance with the invention.
Figure 1 shows the surface of an electrode 1
in the form of a cathode for the deposition of product
of elec-trolysis in an easily detachable form in an
electrolytic cell for treating mineral ores and con-
centrates to remove product in the form of metal powder,
there being a plurality of electrodes in -the cell.
A conduc-tive cathode 19 is partially covered
with a non-conductive material 20 which allows product
to grow from the electrodes 19 only in certain areas
21. One of the most convenient methods of achieving
this effect is by covering rod or pipe electrodes,
which are usually copper, with perforated shrlnkable
plastics tubing or plastics net. The plastics tubing
or net is then heated and shrinks onto the rod or
tube. This causes the product to grow out from the
electrode in small discrete forms which allows it
to be easily detached from the elec~rode (in some
cases assisted by a periodic vibration of the electrode)
~0 and easily
j, . ". ..
;.... . . . .
... .
.
,,
pumped as a slurry.
The foregoing describes the advantages of the
cathode design. The following data shows a chemical
effect achieved by such electrode in an electrolytic
cell.
EXAMPLE
40 kilos of a copper concentrate analyzing 23~
copper and 23.~% iron were added to a cell, as described
in the drawings of applican-ts copending applica-tion
443,033, which contained 1500 1 of electrolyte analyzing
35 g/l copper (total ionic Cu) 6.4 gpl of cupric and
0.5 g/l of iron. The mixture was aerated using 135
l of air per minute and current was passed at a rate
of 700 amps with a voltage of 1.0 V. The cathodes
were gently tapped every 15 to 30 minutes and a small
vibration imparted to the fibreglass frame to allow
the copper powder to travel down the arms in-to the
sloping bot-tom of the central container. From the
lowest point of this container the copper powder was
withdrawn, in slurry form, through a vertical pipe,
as required, to a settling chamber where the copper
powder separa-ted from the electrolyte which was then
passed to a centrifugal pump for transfer back to
the cell. The pH of the mixture in the anolyte com-
partment remained between 2.2 and 3.0 throughout the
test and could be varied slightly by adjusting the
amount of air admitted to the cell. A decrease in
the amount of air admitted to the cell could lower
the pH to the 2.0 to 2.5 pH preferred range. A~ter
3n 10 hours operation the air and current were turned
off and the slurry was filtered and the filter cake
washed and dried. The filter cake analyzed 0.8~ copper
and 24% iron giving a recovery of 97% of the copper
from the mineral with an elec-trolysis powder consumption
of approximately 0.75 kWh per kilo of copper produced.
The sulphur in the chalcopyrite concentrate was almost
completely converted to elemental form and the iron
was converted to an oxide and remained substantially
in the residue. This example illustrates the single
..
.
,, - .
. .. .
: :. . : ~ . .,
,:,~,.............. .
:. ' ' ' ;
~: :.~ , , ,
- 5 -
step conversion of copper concentrates to high purity
metal and elemental sulphur avoiding atmospheric pollution
from sulphur dioxide and using very low energy at
atmospheric pressure and moderate temperatures.
The use of perforated heat shrinkable plastics
material on cathode rods or tubes provides an inexpensive
and efficient means of causing electrolytically deposited
metals, often in the form of powders, to grow out
from the small holes in the shrink plastics material
la at the cathode surface to form "trees" which have
a hi~h stress concentration at the cathode surface.
This ~akes it very simple to cause the metal to separate
from the cathode surface for collection. For example,
a slight vibration imparted to the cathode rods or
tubes will cause deposited metal to separate from
the cathode surface. It is difficult to produce the
effect with flat plates since perforated flat sheets
of plastics material tend to lift off a flat plate
whereas tubular shrink plastics material tends to
hold tightly around rod-like or tubular surfaces.
It will be appreciated that various alterations,
modifications and/or additions may be introduced into
constructions and parts previously described without
departing from the spirit or ambit of the invention
as defined by the appended claims.
- . : : .. ~ .